User login
Adopting high-dose radiation vs. conventional after mastectomy could be ‘game changer’
SAN DIEGO – , according to a new prospective, randomized study.
Side effects and physical well-being scores were similar among 400 women who received the two treatment regimens, and outcomes were similar or slightly better in the higher-dose group, reported Rinaa Punglia, MD, MPH, an associate professor of radiation oncology at Dana-Farber Brigham Cancer Center in Boston and colleagues at the annual meeting of the American Society for Radiation Oncology. In a press statement, Dr. Punglia noted that the outcomes weren’t as impressive as researchers had hoped, but it’s positive that higher doses didn’t cause more side effects.
The use of the higher-dose approach, known as hypofractionation, “resulted in fewer treatment breaks and less financial toxicity” vs. conventional fractionation, Dr. Punglia said at a news briefing. The findings of the FABREC study “support the use of hypofractionated postmastectomy radiation for patients with basic reconstruction.”
According to Dr. Punglia, “postmastectomy radiation therapy is indicated for almost one-third of mastectomy patients and improves the lives of patients who are at an elevated risk for recurrence.” However, “the addition of radiation therapy greatly increases the risk of reconstruction complications.”
The typical radiation treatment period is 5-6 weeks in these patients, a major hardship for patients that can take them away from their families for extended periods of time. The researchers sought to understand whether another approach – hypofractionation over 3-4 weeks – is a better option. The strategy is widely used after breast-conserving surgery, she said, and has been linked to similar cancer outcomes, improved quality of life, and improved breast appearance.
From 2018 to 2021, the researchers recruited 400 patients with stage 0-III breast cancer who were treated with mastectomy and immediately underwent implant-based reconstruction (median age = 47.0, 23-79). None had tumors growing into the chest wall or skin.
The patients, spread nationwide across 16 institutions, were randomized to receive conventional fractionation (n = 201, 25 fractions, 5 days a week for 5 weeks of 200 cGy) or hypofractionation (n=199, 16 fractions, 5 days a week, for about 3 weeks of 266 cGy).
The researchers tracked 385 patients over a median follow-up of 40.4 months. There was no statistically significant difference in distant recurrence (12 in conventional fractionation arm, 11 in hypofractionation arm), death (2 in each arm), local recurrence (1 in each arm), or toxicity in the chest wall area (20 in conventional fractionation arm, 19 in hypofractionation arm). Changes in physical well-being scores, the primary endpoint, were similar after controlling for age.
“We found that younger patients randomized to hypofractionation were less bothered by side effects of treatment at 6 months relative to their counterparts who received conventional fractionation,” Dr. Punglia said.
Treatment breaks were more common in the conventional fractionation arm (7.7%, mean = 3.3 days) vs. the hypofractionation arm (2.7%, mean = 2.8 days, P = .03).
Among 51 patients who took unpaid time off work, those who underwent hypofractionation took fewer mean days off (73.7 days vs. 125.8 days for conventional fractionation, P = .046).
The study is the first of its kind to compare conventional fractionation to hypofractionation in this population in a randomized, phase III study, Dr. Punglia said.
At the news briefing, an independent expert – Lori Pierce, MD, a professor of radiation oncology at the University of Michigan – said the new study is a “game changer.”
The findings about the benefits of hypofractionation “will potentially impact thousands of women,” said Dr. Pierce, former president of the American Society of Clinical Oncology. The shorter course of radiation is more convenient for patients, she said, and reduces hardship.
“Without a doubt, these results should be discussed with all patients who have had mastectomy and implant-based reconstruction,” she said.
In an interview, Bruce G. Haffty, MD, MS, professor and chair of Radiation Oncology at Rutgers Cancer Institute of New Jersey, said the study adds to existing data suggesting that shorter courses of therapy “are probably OK.” The new findings “give people a little more confidence that [short courses are] safe in terms of well-being and toxicity.”
However, the follow-up in the trial is relatively short, he said, and longer-term research will be needed to change the standard of care in these patients. “It’ll be an evolving story over the next 5-10 years,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Punglia has no disclosures; disclosures for other authors were not provided. Disclosure information for Dr. Pierce was not provided. Dr. Haffty is an investigator in a similar study called RT CHARM.
SAN DIEGO – , according to a new prospective, randomized study.
Side effects and physical well-being scores were similar among 400 women who received the two treatment regimens, and outcomes were similar or slightly better in the higher-dose group, reported Rinaa Punglia, MD, MPH, an associate professor of radiation oncology at Dana-Farber Brigham Cancer Center in Boston and colleagues at the annual meeting of the American Society for Radiation Oncology. In a press statement, Dr. Punglia noted that the outcomes weren’t as impressive as researchers had hoped, but it’s positive that higher doses didn’t cause more side effects.
The use of the higher-dose approach, known as hypofractionation, “resulted in fewer treatment breaks and less financial toxicity” vs. conventional fractionation, Dr. Punglia said at a news briefing. The findings of the FABREC study “support the use of hypofractionated postmastectomy radiation for patients with basic reconstruction.”
According to Dr. Punglia, “postmastectomy radiation therapy is indicated for almost one-third of mastectomy patients and improves the lives of patients who are at an elevated risk for recurrence.” However, “the addition of radiation therapy greatly increases the risk of reconstruction complications.”
The typical radiation treatment period is 5-6 weeks in these patients, a major hardship for patients that can take them away from their families for extended periods of time. The researchers sought to understand whether another approach – hypofractionation over 3-4 weeks – is a better option. The strategy is widely used after breast-conserving surgery, she said, and has been linked to similar cancer outcomes, improved quality of life, and improved breast appearance.
From 2018 to 2021, the researchers recruited 400 patients with stage 0-III breast cancer who were treated with mastectomy and immediately underwent implant-based reconstruction (median age = 47.0, 23-79). None had tumors growing into the chest wall or skin.
The patients, spread nationwide across 16 institutions, were randomized to receive conventional fractionation (n = 201, 25 fractions, 5 days a week for 5 weeks of 200 cGy) or hypofractionation (n=199, 16 fractions, 5 days a week, for about 3 weeks of 266 cGy).
The researchers tracked 385 patients over a median follow-up of 40.4 months. There was no statistically significant difference in distant recurrence (12 in conventional fractionation arm, 11 in hypofractionation arm), death (2 in each arm), local recurrence (1 in each arm), or toxicity in the chest wall area (20 in conventional fractionation arm, 19 in hypofractionation arm). Changes in physical well-being scores, the primary endpoint, were similar after controlling for age.
“We found that younger patients randomized to hypofractionation were less bothered by side effects of treatment at 6 months relative to their counterparts who received conventional fractionation,” Dr. Punglia said.
Treatment breaks were more common in the conventional fractionation arm (7.7%, mean = 3.3 days) vs. the hypofractionation arm (2.7%, mean = 2.8 days, P = .03).
Among 51 patients who took unpaid time off work, those who underwent hypofractionation took fewer mean days off (73.7 days vs. 125.8 days for conventional fractionation, P = .046).
The study is the first of its kind to compare conventional fractionation to hypofractionation in this population in a randomized, phase III study, Dr. Punglia said.
At the news briefing, an independent expert – Lori Pierce, MD, a professor of radiation oncology at the University of Michigan – said the new study is a “game changer.”
The findings about the benefits of hypofractionation “will potentially impact thousands of women,” said Dr. Pierce, former president of the American Society of Clinical Oncology. The shorter course of radiation is more convenient for patients, she said, and reduces hardship.
“Without a doubt, these results should be discussed with all patients who have had mastectomy and implant-based reconstruction,” she said.
In an interview, Bruce G. Haffty, MD, MS, professor and chair of Radiation Oncology at Rutgers Cancer Institute of New Jersey, said the study adds to existing data suggesting that shorter courses of therapy “are probably OK.” The new findings “give people a little more confidence that [short courses are] safe in terms of well-being and toxicity.”
However, the follow-up in the trial is relatively short, he said, and longer-term research will be needed to change the standard of care in these patients. “It’ll be an evolving story over the next 5-10 years,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Punglia has no disclosures; disclosures for other authors were not provided. Disclosure information for Dr. Pierce was not provided. Dr. Haffty is an investigator in a similar study called RT CHARM.
SAN DIEGO – , according to a new prospective, randomized study.
Side effects and physical well-being scores were similar among 400 women who received the two treatment regimens, and outcomes were similar or slightly better in the higher-dose group, reported Rinaa Punglia, MD, MPH, an associate professor of radiation oncology at Dana-Farber Brigham Cancer Center in Boston and colleagues at the annual meeting of the American Society for Radiation Oncology. In a press statement, Dr. Punglia noted that the outcomes weren’t as impressive as researchers had hoped, but it’s positive that higher doses didn’t cause more side effects.
The use of the higher-dose approach, known as hypofractionation, “resulted in fewer treatment breaks and less financial toxicity” vs. conventional fractionation, Dr. Punglia said at a news briefing. The findings of the FABREC study “support the use of hypofractionated postmastectomy radiation for patients with basic reconstruction.”
According to Dr. Punglia, “postmastectomy radiation therapy is indicated for almost one-third of mastectomy patients and improves the lives of patients who are at an elevated risk for recurrence.” However, “the addition of radiation therapy greatly increases the risk of reconstruction complications.”
The typical radiation treatment period is 5-6 weeks in these patients, a major hardship for patients that can take them away from their families for extended periods of time. The researchers sought to understand whether another approach – hypofractionation over 3-4 weeks – is a better option. The strategy is widely used after breast-conserving surgery, she said, and has been linked to similar cancer outcomes, improved quality of life, and improved breast appearance.
From 2018 to 2021, the researchers recruited 400 patients with stage 0-III breast cancer who were treated with mastectomy and immediately underwent implant-based reconstruction (median age = 47.0, 23-79). None had tumors growing into the chest wall or skin.
The patients, spread nationwide across 16 institutions, were randomized to receive conventional fractionation (n = 201, 25 fractions, 5 days a week for 5 weeks of 200 cGy) or hypofractionation (n=199, 16 fractions, 5 days a week, for about 3 weeks of 266 cGy).
The researchers tracked 385 patients over a median follow-up of 40.4 months. There was no statistically significant difference in distant recurrence (12 in conventional fractionation arm, 11 in hypofractionation arm), death (2 in each arm), local recurrence (1 in each arm), or toxicity in the chest wall area (20 in conventional fractionation arm, 19 in hypofractionation arm). Changes in physical well-being scores, the primary endpoint, were similar after controlling for age.
“We found that younger patients randomized to hypofractionation were less bothered by side effects of treatment at 6 months relative to their counterparts who received conventional fractionation,” Dr. Punglia said.
Treatment breaks were more common in the conventional fractionation arm (7.7%, mean = 3.3 days) vs. the hypofractionation arm (2.7%, mean = 2.8 days, P = .03).
Among 51 patients who took unpaid time off work, those who underwent hypofractionation took fewer mean days off (73.7 days vs. 125.8 days for conventional fractionation, P = .046).
The study is the first of its kind to compare conventional fractionation to hypofractionation in this population in a randomized, phase III study, Dr. Punglia said.
At the news briefing, an independent expert – Lori Pierce, MD, a professor of radiation oncology at the University of Michigan – said the new study is a “game changer.”
The findings about the benefits of hypofractionation “will potentially impact thousands of women,” said Dr. Pierce, former president of the American Society of Clinical Oncology. The shorter course of radiation is more convenient for patients, she said, and reduces hardship.
“Without a doubt, these results should be discussed with all patients who have had mastectomy and implant-based reconstruction,” she said.
In an interview, Bruce G. Haffty, MD, MS, professor and chair of Radiation Oncology at Rutgers Cancer Institute of New Jersey, said the study adds to existing data suggesting that shorter courses of therapy “are probably OK.” The new findings “give people a little more confidence that [short courses are] safe in terms of well-being and toxicity.”
However, the follow-up in the trial is relatively short, he said, and longer-term research will be needed to change the standard of care in these patients. “It’ll be an evolving story over the next 5-10 years,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Punglia has no disclosures; disclosures for other authors were not provided. Disclosure information for Dr. Pierce was not provided. Dr. Haffty is an investigator in a similar study called RT CHARM.
AT ASTRO 2023
SABR could defer systemic therapy in oligoprogressive breast cancer
SAN DIEGO – Stereotactic ablative body radiotherapy (SABR) appeared to delay the need for changes in systemic therapy in postmenopausal patients with oligoprogressive luminal ER-positive, HER2-negative breast cancer, according to a new phase 2 study.
In the AVATAR trial, patients with one to five metastatic lesions who’d been treated with cyclin-dependent kinase (CDK) 4/6 inhibitors and aromatase inhibitors for at least 6 months underwent SABR. Of those, 47% had event-free survival of more than 6 months, an unexpectedly high figure, reported radiation oncologist Steven David, MBBS, of Peter MacCallum Cancer Center, Melbourne, and colleagues at the annual meeting of the American Society for Radiation Oncology.
“We found surprisingly that SABR delayed a change in therapy by 10 months, which is great for patients. Also, one in three patients had a second round of SABR,” said Dr. David in an interview. “This trial provides the first prospective evidence to delay a change in therapy in this population, and this strategy is ready to go now.”
According to Dr. David, oligoprogressive luminal, ER-positive, HER2-negative, advanced breast cancer cannot be cured. However, patients can live more than 10 years in some cases, and an early treatment – CDK 4/6 inhibitors and aromatase inhibitors – is well tolerated. “Patients can lead a normal life and avoid chemo” as long as those medications keep working.
The goal of the study was to determine if SABR is helpful in these patients. The treatment, which produces highly focused radiation, “has very few side effects and does a great job in eliminating progressing metastases,” Dr. David said.
For the study, researchers recruited 32 subjects at 13 Australian sites. Participants could not have had leptomeningeal disease, previous chemotherapy for metastatic disease, or prior radiotherapy to an oligoprogressing lesion. Most metastases were to bone (n = 44, 71%), node (systemic, n = 8; 13%) and lung (n = 4; 6%).
The patients were treated with SABR, most commonly 24 Gy (n = 25; 43%) and 20 Gy (n = 10; 17%); half had one lesion treated (50%), and 25% had two lesions treated.
The median follow-up was 15.8 months. The median event-free survival was 5.2 months (95% confidence interval, 3.1-9.4 months), with events defined as progression within 6 months or in at least three lesions. Fifteen patients (47%) reached event-free survival of 6 or more months.
Elysia Donovan, MD, MSc, a radiation oncologist at McMaster University, Hamilton, Ont., said in an interview that the new study is thoughtfully designed, although it’s not definitive. “At this point we still do not know the optimal treatment regimen for oligoprogressive breast cancer. The findings of this trial are promising and exciting. However, further randomized trials are required before routine implementation in clinical practice. For now, patients should be considered in a case by case basis with multidisciplinary discussion to determine the optimal systemic therapy regimen at oligoprogression and whether SABR may provide benefit.”
Median modified progression-free survival was 10.4 months, and median progression-free survival was 5.2 months; 31% of patients received SABR for further oligoprogression, and 46% patients remained on CDK4/6 inhibitors and aromatase inhibitors for 12 months. Overall survival was 100%.
A total of 14 patients had grade 1 adverse events, 2 had grade 2 events, and none had grade 3 or higher events; 47% had no treatment-related toxicity.
The strategy “potentially has a place in other cancer types and other breast cancer types,” Dr. David said.
In an interview, Katarzyna Jerzak, MD, MSc, a medical oncologist with Sunnybrook Odette Cancer Center in Toronto, said the findings are promising, although the study is small and the patients are similar. Toxicity was limited, and a 12-month delay in a switch to therapy – reached by 46% – “is very meaningful for patients.” She added that “the positive results should serve as motivation to investigate the strategy further.”
Dr. David said a larger trial called AVATAR 2 is funded and in the works. It will have more patients and more breast cancer subtypes.
The study was funded by the Donald Ratcliffe and Phyllis McLeod Trust. Dr. David disclosed grant/research funding from Roche Genentech, and other authors reported various disclosures including relationships with AstraZeneca, Pfizer, Gilead, and others. Dr. Jerzak disclosed speaker/advisor board/consultant relationships with Amgen, AstraZeneca, Apobiologix, Eli Lilly, Esai, Genomic Health, Gilead, Knight Therapeutics, Merck, Myriad Genetics, Pfizer, Roche, Seagen, and Novartis and research funding from AstraZeneca, Eli Lilly, and Seagen. Dr. Donovan disclosed a Bright Foundation grant for a prospective trial of SABR for oligoprogressive breast cancer.
SAN DIEGO – Stereotactic ablative body radiotherapy (SABR) appeared to delay the need for changes in systemic therapy in postmenopausal patients with oligoprogressive luminal ER-positive, HER2-negative breast cancer, according to a new phase 2 study.
In the AVATAR trial, patients with one to five metastatic lesions who’d been treated with cyclin-dependent kinase (CDK) 4/6 inhibitors and aromatase inhibitors for at least 6 months underwent SABR. Of those, 47% had event-free survival of more than 6 months, an unexpectedly high figure, reported radiation oncologist Steven David, MBBS, of Peter MacCallum Cancer Center, Melbourne, and colleagues at the annual meeting of the American Society for Radiation Oncology.
“We found surprisingly that SABR delayed a change in therapy by 10 months, which is great for patients. Also, one in three patients had a second round of SABR,” said Dr. David in an interview. “This trial provides the first prospective evidence to delay a change in therapy in this population, and this strategy is ready to go now.”
According to Dr. David, oligoprogressive luminal, ER-positive, HER2-negative, advanced breast cancer cannot be cured. However, patients can live more than 10 years in some cases, and an early treatment – CDK 4/6 inhibitors and aromatase inhibitors – is well tolerated. “Patients can lead a normal life and avoid chemo” as long as those medications keep working.
The goal of the study was to determine if SABR is helpful in these patients. The treatment, which produces highly focused radiation, “has very few side effects and does a great job in eliminating progressing metastases,” Dr. David said.
For the study, researchers recruited 32 subjects at 13 Australian sites. Participants could not have had leptomeningeal disease, previous chemotherapy for metastatic disease, or prior radiotherapy to an oligoprogressing lesion. Most metastases were to bone (n = 44, 71%), node (systemic, n = 8; 13%) and lung (n = 4; 6%).
The patients were treated with SABR, most commonly 24 Gy (n = 25; 43%) and 20 Gy (n = 10; 17%); half had one lesion treated (50%), and 25% had two lesions treated.
The median follow-up was 15.8 months. The median event-free survival was 5.2 months (95% confidence interval, 3.1-9.4 months), with events defined as progression within 6 months or in at least three lesions. Fifteen patients (47%) reached event-free survival of 6 or more months.
Elysia Donovan, MD, MSc, a radiation oncologist at McMaster University, Hamilton, Ont., said in an interview that the new study is thoughtfully designed, although it’s not definitive. “At this point we still do not know the optimal treatment regimen for oligoprogressive breast cancer. The findings of this trial are promising and exciting. However, further randomized trials are required before routine implementation in clinical practice. For now, patients should be considered in a case by case basis with multidisciplinary discussion to determine the optimal systemic therapy regimen at oligoprogression and whether SABR may provide benefit.”
Median modified progression-free survival was 10.4 months, and median progression-free survival was 5.2 months; 31% of patients received SABR for further oligoprogression, and 46% patients remained on CDK4/6 inhibitors and aromatase inhibitors for 12 months. Overall survival was 100%.
A total of 14 patients had grade 1 adverse events, 2 had grade 2 events, and none had grade 3 or higher events; 47% had no treatment-related toxicity.
The strategy “potentially has a place in other cancer types and other breast cancer types,” Dr. David said.
In an interview, Katarzyna Jerzak, MD, MSc, a medical oncologist with Sunnybrook Odette Cancer Center in Toronto, said the findings are promising, although the study is small and the patients are similar. Toxicity was limited, and a 12-month delay in a switch to therapy – reached by 46% – “is very meaningful for patients.” She added that “the positive results should serve as motivation to investigate the strategy further.”
Dr. David said a larger trial called AVATAR 2 is funded and in the works. It will have more patients and more breast cancer subtypes.
The study was funded by the Donald Ratcliffe and Phyllis McLeod Trust. Dr. David disclosed grant/research funding from Roche Genentech, and other authors reported various disclosures including relationships with AstraZeneca, Pfizer, Gilead, and others. Dr. Jerzak disclosed speaker/advisor board/consultant relationships with Amgen, AstraZeneca, Apobiologix, Eli Lilly, Esai, Genomic Health, Gilead, Knight Therapeutics, Merck, Myriad Genetics, Pfizer, Roche, Seagen, and Novartis and research funding from AstraZeneca, Eli Lilly, and Seagen. Dr. Donovan disclosed a Bright Foundation grant for a prospective trial of SABR for oligoprogressive breast cancer.
SAN DIEGO – Stereotactic ablative body radiotherapy (SABR) appeared to delay the need for changes in systemic therapy in postmenopausal patients with oligoprogressive luminal ER-positive, HER2-negative breast cancer, according to a new phase 2 study.
In the AVATAR trial, patients with one to five metastatic lesions who’d been treated with cyclin-dependent kinase (CDK) 4/6 inhibitors and aromatase inhibitors for at least 6 months underwent SABR. Of those, 47% had event-free survival of more than 6 months, an unexpectedly high figure, reported radiation oncologist Steven David, MBBS, of Peter MacCallum Cancer Center, Melbourne, and colleagues at the annual meeting of the American Society for Radiation Oncology.
“We found surprisingly that SABR delayed a change in therapy by 10 months, which is great for patients. Also, one in three patients had a second round of SABR,” said Dr. David in an interview. “This trial provides the first prospective evidence to delay a change in therapy in this population, and this strategy is ready to go now.”
According to Dr. David, oligoprogressive luminal, ER-positive, HER2-negative, advanced breast cancer cannot be cured. However, patients can live more than 10 years in some cases, and an early treatment – CDK 4/6 inhibitors and aromatase inhibitors – is well tolerated. “Patients can lead a normal life and avoid chemo” as long as those medications keep working.
The goal of the study was to determine if SABR is helpful in these patients. The treatment, which produces highly focused radiation, “has very few side effects and does a great job in eliminating progressing metastases,” Dr. David said.
For the study, researchers recruited 32 subjects at 13 Australian sites. Participants could not have had leptomeningeal disease, previous chemotherapy for metastatic disease, or prior radiotherapy to an oligoprogressing lesion. Most metastases were to bone (n = 44, 71%), node (systemic, n = 8; 13%) and lung (n = 4; 6%).
The patients were treated with SABR, most commonly 24 Gy (n = 25; 43%) and 20 Gy (n = 10; 17%); half had one lesion treated (50%), and 25% had two lesions treated.
The median follow-up was 15.8 months. The median event-free survival was 5.2 months (95% confidence interval, 3.1-9.4 months), with events defined as progression within 6 months or in at least three lesions. Fifteen patients (47%) reached event-free survival of 6 or more months.
Elysia Donovan, MD, MSc, a radiation oncologist at McMaster University, Hamilton, Ont., said in an interview that the new study is thoughtfully designed, although it’s not definitive. “At this point we still do not know the optimal treatment regimen for oligoprogressive breast cancer. The findings of this trial are promising and exciting. However, further randomized trials are required before routine implementation in clinical practice. For now, patients should be considered in a case by case basis with multidisciplinary discussion to determine the optimal systemic therapy regimen at oligoprogression and whether SABR may provide benefit.”
Median modified progression-free survival was 10.4 months, and median progression-free survival was 5.2 months; 31% of patients received SABR for further oligoprogression, and 46% patients remained on CDK4/6 inhibitors and aromatase inhibitors for 12 months. Overall survival was 100%.
A total of 14 patients had grade 1 adverse events, 2 had grade 2 events, and none had grade 3 or higher events; 47% had no treatment-related toxicity.
The strategy “potentially has a place in other cancer types and other breast cancer types,” Dr. David said.
In an interview, Katarzyna Jerzak, MD, MSc, a medical oncologist with Sunnybrook Odette Cancer Center in Toronto, said the findings are promising, although the study is small and the patients are similar. Toxicity was limited, and a 12-month delay in a switch to therapy – reached by 46% – “is very meaningful for patients.” She added that “the positive results should serve as motivation to investigate the strategy further.”
Dr. David said a larger trial called AVATAR 2 is funded and in the works. It will have more patients and more breast cancer subtypes.
The study was funded by the Donald Ratcliffe and Phyllis McLeod Trust. Dr. David disclosed grant/research funding from Roche Genentech, and other authors reported various disclosures including relationships with AstraZeneca, Pfizer, Gilead, and others. Dr. Jerzak disclosed speaker/advisor board/consultant relationships with Amgen, AstraZeneca, Apobiologix, Eli Lilly, Esai, Genomic Health, Gilead, Knight Therapeutics, Merck, Myriad Genetics, Pfizer, Roche, Seagen, and Novartis and research funding from AstraZeneca, Eli Lilly, and Seagen. Dr. Donovan disclosed a Bright Foundation grant for a prospective trial of SABR for oligoprogressive breast cancer.
AT ASTRO 2023
False-positive Pap smear may indicate genitourinary syndrome
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY ANNUAL MEETING
What is the future for multicancer early-detection tests?
Suzette Delaloge, MD, MSc, oncologist, breast cancer specialist, and director of the individualized cancer prevention program (Interception) at the Gustave Roussy Institute in Villejuif, France, looks into these “liquid biopsies” and shares her reservations about their potential marketing, especially to the organized care plans.
Question: What are the general principles underpinning these MCED tests?
Suzette Delaloge, MD, MSc: Despite their specificities, the general idea is to detect certain cancer markers in various body fluids (blood, urine, saliva, etc.), for example, molecules released by cancer cells (cytokines, inflammatory proteins, leptin, etc.) or distinctive features of the DNA in tumor cells. In blood, these molecules can be found in plasma or in serum. In urine, it’s more about detecting kidney, bladder, and urinary tract cancers.
Q: What sort of time frame are we looking at for these MCED tests to be used in routine practice?
Dr. Delaloge: They first appeared around 10 years ago. Development of these tests has intensified in recent years. There are numerous research laboratories, both public and private, that are developing different early-detection tests for cancer.
Some of these development processes are about to come to an end and are expected to be in regular, concrete use within 5-10 years. For the most advanced developments, the main biologic material researched and analyzed is DNA from cancer cells. We all have fragments of DNA from dead cells in our plasma (apoptosis), but cancer cells release more of these than others, and most importantly, their DNA has distinctive characteristics. The idea is to develop tests capable of detecting these characteristics.
Liquid biopsies based on genomic biomarkers could make MCED a reality, especially for cancers for which there is no standard screening process. But at this stage of the research, there are limitations, including low sensitivity for detecting stage I cancers in validation studies and an increased risk for overdiagnosis.
Q: What specific set of characteristics are the most advanced approaches based on?
Dr. Delaloge: They’re based on the analysis of DNA methylation, a biological process by which CH3 methyl groups are added to the DNA molecule and that determines gene expression. This phenomenon differs depending on whether the cell is cancerous. Among the tests currently under development making use of this specific characteristic is the Galleri test, which is the most advanced of them all.
A previous British National Health Service study, SYMPLIFY, which was published in 2023 by researchers at the University of Oxford, was conducted in symptomatic patients attending a health center. It offers promising results in a diagnostic situation. It has nothing at all to do with screening here. A large, randomized English study, NHS-Galleri, is underway, this time involving the general population, with the aim of assessing the potential benefit of the same test as screening in 140,000 people between ages 50 and 77 years.
In the SYMPLIFY study, which was carried out in symptomatic patients attending a health center, the Galleri MCED test had a positive predictive value of 75.5%, a negative predictive value of 97.6%, a sensitivity of 66.3%, and a specificity of 98.4%. Sensitivity increased with age and cancer stage from 24.2% at stage I to 95.3% at stage IV. For cases for which a cancer signal was detected in patients with cancer, the prediction of the original site of the cancer by the MCED test was accurate in 85.2% of cases. This large-scale prospective evaluation of an MCED diagnostic test confirms its feasibility in a symptomatic population but is not yet sufficiently accurate to “confirm or rule out the presence of cancer.” According to the authors, “in cases in which the MCED test detects a cancer signal in this context, the probability of a diagnosis of cancer being made is considerably higher and may identify cancers at sites other than those suspected during the initial referral phase, thus reducing delays in diagnosis.” A negative test means a lower likelihood of cancer but not so low that proper investigation can be ruled out. Further tests will be needed to optimize use of a negative predictive value.
Q: Does MCED testing concern all types of cancer?
Dr. Delaloge: The Galleri test is based on full profiling of DNA methylation. This allows for early diagnosis of cancer even before it can be seen on imaging tests. The issue with these tests is that they aren’t that good at early diagnosis of the most common types of cancer (breast, colorectal, cervical, etc.) for which we already have more efficient means such as the fecal immunochemical test for colorectal cancer, mammography, HPV testing, and so on.
These blood tests would thus not be aimed at replacing routine screening but rather at screening asymptomatic individuals or those with nonspecific signs for cancers for which we have few or no screening measures and which are on the rise, such as deep tumors and cancer diagnosed at a late stage, namely pancreas, bile duct, ovarian, esophageal, lung, stomach, etc.
The results from the studies published are promising, but others are underway to confirm the benefit of these MCEDs. The challenge is to identify cancer at an early stage, at a stage where it will be easier to cure the patient and control its growth using treatments that are less onerous for the patient and that have fewer aftereffects but not at the expense of a massive increase in overdiagnosis, as seen with prostate-specific antigen levels in prostate cancer a few years ago!
Q: What would be the focus of these MCED tests?
Dr. Delaloge: We must be alert to the risk for the market development of MCED tests. For now, they are mostly, especially the Galleri test, developed in the general population to screen for types of cancer that could not be detected in any other way but also because it’s the most financially beneficial situation. The designers want to position themselves in the general population, regardless of whether this means they’ll have to test hundreds of people to find one for whom the test is beneficial. What’s more, developing tests in isolation, without considering their place in ad hoc treatment pathways, is not realistic. It’s likely that some of these tests will be marketed within the next 10 years, but the health care systems destined to receive them are not remotely ready to do so.
Q: An even more recent publication, from late July 2023, is even more exciting in relation to early detection of lung cancer using circulating DNA sequencing. What are your thoughts on it?
Dr. Delaloge: Initially overtaken by other technologies in favor of MCED approaches, DNA sequencing as a technique to detect somatic mutations seems to have reentered the competition with this new-generation research. The authors published some very interesting results, especially for stage I lung cancer with a very high sensitivity of 75%. [Editor’s note: A machine-learning model using genome-wide mutational profiles combined with other features and followed by CT imaging detected more than 90% of patients with lung cancer, including those with stage I and II disease.]
This research illustrates the difficulty of providing high performance while covering a broad range of cancers. Here, the good results mainly concern lung cancer. Researchers and health care authorities must be alert to ensuring that MCED tests prove themselves in terms of sensitivity and specificity in responding to a medical need and in their impact on specific mortality. This craze for MCED tests must not hinder the development of “single-cancer” technologies that may be much better for detecting specific cancers. This recent publication is interesting in this respect, because this sequencing test seems to be particularly good at detecting lung cancer.
Q: Another approach used in MCED tests is based on analyzing the size of DNA fragments in the blood. Can you explain how this works?
Dr. Delaloge: When cancer is not present, the size of DNA fragments in cells is much more homogeneous. Here also, the benefit of MCED based on this technique rests on the very early detection of cancers that are less common than those for which we already have good screening methods available.
Other approaches, still at the experimental stage, detect certain proteins, certain inflammatory molecules, RNA, etc. But for many researchers, the future will involve pairing tests on the basis of circulating DNA in the blood with the detection of specific molecules indicating the presence of cancer to obtain early screening tests that are even more effective or that possibly even allow us to identify an appropriate treatment at an early stage.
The development of a simple test based on a blood draw that allows us to screen early for all cancers and that would replace all current screening measures is, therefore, not imminent, although it could potentially be on the horizon in years to come. Alongside this, an important issue is the benefit of cancer screening in the general population vs. in a targeted population with a specific risk. The latter option is in development but requires an individualized screening pathway based on blood testing and current screening methods: imaging, etc. It also depends on an individual’s cancer risk profile such as age, personal and family medical history, genetic predisposition, and so on.
According to recent modeling, these multicancer tests could theoretically prevent a minimum of 2,000 deaths from cancer per 100,000 people between ages 50 and 79 years screened per year (17% fewer deaths from cancer per year).
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Suzette Delaloge, MD, MSc, oncologist, breast cancer specialist, and director of the individualized cancer prevention program (Interception) at the Gustave Roussy Institute in Villejuif, France, looks into these “liquid biopsies” and shares her reservations about their potential marketing, especially to the organized care plans.
Question: What are the general principles underpinning these MCED tests?
Suzette Delaloge, MD, MSc: Despite their specificities, the general idea is to detect certain cancer markers in various body fluids (blood, urine, saliva, etc.), for example, molecules released by cancer cells (cytokines, inflammatory proteins, leptin, etc.) or distinctive features of the DNA in tumor cells. In blood, these molecules can be found in plasma or in serum. In urine, it’s more about detecting kidney, bladder, and urinary tract cancers.
Q: What sort of time frame are we looking at for these MCED tests to be used in routine practice?
Dr. Delaloge: They first appeared around 10 years ago. Development of these tests has intensified in recent years. There are numerous research laboratories, both public and private, that are developing different early-detection tests for cancer.
Some of these development processes are about to come to an end and are expected to be in regular, concrete use within 5-10 years. For the most advanced developments, the main biologic material researched and analyzed is DNA from cancer cells. We all have fragments of DNA from dead cells in our plasma (apoptosis), but cancer cells release more of these than others, and most importantly, their DNA has distinctive characteristics. The idea is to develop tests capable of detecting these characteristics.
Liquid biopsies based on genomic biomarkers could make MCED a reality, especially for cancers for which there is no standard screening process. But at this stage of the research, there are limitations, including low sensitivity for detecting stage I cancers in validation studies and an increased risk for overdiagnosis.
Q: What specific set of characteristics are the most advanced approaches based on?
Dr. Delaloge: They’re based on the analysis of DNA methylation, a biological process by which CH3 methyl groups are added to the DNA molecule and that determines gene expression. This phenomenon differs depending on whether the cell is cancerous. Among the tests currently under development making use of this specific characteristic is the Galleri test, which is the most advanced of them all.
A previous British National Health Service study, SYMPLIFY, which was published in 2023 by researchers at the University of Oxford, was conducted in symptomatic patients attending a health center. It offers promising results in a diagnostic situation. It has nothing at all to do with screening here. A large, randomized English study, NHS-Galleri, is underway, this time involving the general population, with the aim of assessing the potential benefit of the same test as screening in 140,000 people between ages 50 and 77 years.
In the SYMPLIFY study, which was carried out in symptomatic patients attending a health center, the Galleri MCED test had a positive predictive value of 75.5%, a negative predictive value of 97.6%, a sensitivity of 66.3%, and a specificity of 98.4%. Sensitivity increased with age and cancer stage from 24.2% at stage I to 95.3% at stage IV. For cases for which a cancer signal was detected in patients with cancer, the prediction of the original site of the cancer by the MCED test was accurate in 85.2% of cases. This large-scale prospective evaluation of an MCED diagnostic test confirms its feasibility in a symptomatic population but is not yet sufficiently accurate to “confirm or rule out the presence of cancer.” According to the authors, “in cases in which the MCED test detects a cancer signal in this context, the probability of a diagnosis of cancer being made is considerably higher and may identify cancers at sites other than those suspected during the initial referral phase, thus reducing delays in diagnosis.” A negative test means a lower likelihood of cancer but not so low that proper investigation can be ruled out. Further tests will be needed to optimize use of a negative predictive value.
Q: Does MCED testing concern all types of cancer?
Dr. Delaloge: The Galleri test is based on full profiling of DNA methylation. This allows for early diagnosis of cancer even before it can be seen on imaging tests. The issue with these tests is that they aren’t that good at early diagnosis of the most common types of cancer (breast, colorectal, cervical, etc.) for which we already have more efficient means such as the fecal immunochemical test for colorectal cancer, mammography, HPV testing, and so on.
These blood tests would thus not be aimed at replacing routine screening but rather at screening asymptomatic individuals or those with nonspecific signs for cancers for which we have few or no screening measures and which are on the rise, such as deep tumors and cancer diagnosed at a late stage, namely pancreas, bile duct, ovarian, esophageal, lung, stomach, etc.
The results from the studies published are promising, but others are underway to confirm the benefit of these MCEDs. The challenge is to identify cancer at an early stage, at a stage where it will be easier to cure the patient and control its growth using treatments that are less onerous for the patient and that have fewer aftereffects but not at the expense of a massive increase in overdiagnosis, as seen with prostate-specific antigen levels in prostate cancer a few years ago!
Q: What would be the focus of these MCED tests?
Dr. Delaloge: We must be alert to the risk for the market development of MCED tests. For now, they are mostly, especially the Galleri test, developed in the general population to screen for types of cancer that could not be detected in any other way but also because it’s the most financially beneficial situation. The designers want to position themselves in the general population, regardless of whether this means they’ll have to test hundreds of people to find one for whom the test is beneficial. What’s more, developing tests in isolation, without considering their place in ad hoc treatment pathways, is not realistic. It’s likely that some of these tests will be marketed within the next 10 years, but the health care systems destined to receive them are not remotely ready to do so.
Q: An even more recent publication, from late July 2023, is even more exciting in relation to early detection of lung cancer using circulating DNA sequencing. What are your thoughts on it?
Dr. Delaloge: Initially overtaken by other technologies in favor of MCED approaches, DNA sequencing as a technique to detect somatic mutations seems to have reentered the competition with this new-generation research. The authors published some very interesting results, especially for stage I lung cancer with a very high sensitivity of 75%. [Editor’s note: A machine-learning model using genome-wide mutational profiles combined with other features and followed by CT imaging detected more than 90% of patients with lung cancer, including those with stage I and II disease.]
This research illustrates the difficulty of providing high performance while covering a broad range of cancers. Here, the good results mainly concern lung cancer. Researchers and health care authorities must be alert to ensuring that MCED tests prove themselves in terms of sensitivity and specificity in responding to a medical need and in their impact on specific mortality. This craze for MCED tests must not hinder the development of “single-cancer” technologies that may be much better for detecting specific cancers. This recent publication is interesting in this respect, because this sequencing test seems to be particularly good at detecting lung cancer.
Q: Another approach used in MCED tests is based on analyzing the size of DNA fragments in the blood. Can you explain how this works?
Dr. Delaloge: When cancer is not present, the size of DNA fragments in cells is much more homogeneous. Here also, the benefit of MCED based on this technique rests on the very early detection of cancers that are less common than those for which we already have good screening methods available.
Other approaches, still at the experimental stage, detect certain proteins, certain inflammatory molecules, RNA, etc. But for many researchers, the future will involve pairing tests on the basis of circulating DNA in the blood with the detection of specific molecules indicating the presence of cancer to obtain early screening tests that are even more effective or that possibly even allow us to identify an appropriate treatment at an early stage.
The development of a simple test based on a blood draw that allows us to screen early for all cancers and that would replace all current screening measures is, therefore, not imminent, although it could potentially be on the horizon in years to come. Alongside this, an important issue is the benefit of cancer screening in the general population vs. in a targeted population with a specific risk. The latter option is in development but requires an individualized screening pathway based on blood testing and current screening methods: imaging, etc. It also depends on an individual’s cancer risk profile such as age, personal and family medical history, genetic predisposition, and so on.
According to recent modeling, these multicancer tests could theoretically prevent a minimum of 2,000 deaths from cancer per 100,000 people between ages 50 and 79 years screened per year (17% fewer deaths from cancer per year).
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Suzette Delaloge, MD, MSc, oncologist, breast cancer specialist, and director of the individualized cancer prevention program (Interception) at the Gustave Roussy Institute in Villejuif, France, looks into these “liquid biopsies” and shares her reservations about their potential marketing, especially to the organized care plans.
Question: What are the general principles underpinning these MCED tests?
Suzette Delaloge, MD, MSc: Despite their specificities, the general idea is to detect certain cancer markers in various body fluids (blood, urine, saliva, etc.), for example, molecules released by cancer cells (cytokines, inflammatory proteins, leptin, etc.) or distinctive features of the DNA in tumor cells. In blood, these molecules can be found in plasma or in serum. In urine, it’s more about detecting kidney, bladder, and urinary tract cancers.
Q: What sort of time frame are we looking at for these MCED tests to be used in routine practice?
Dr. Delaloge: They first appeared around 10 years ago. Development of these tests has intensified in recent years. There are numerous research laboratories, both public and private, that are developing different early-detection tests for cancer.
Some of these development processes are about to come to an end and are expected to be in regular, concrete use within 5-10 years. For the most advanced developments, the main biologic material researched and analyzed is DNA from cancer cells. We all have fragments of DNA from dead cells in our plasma (apoptosis), but cancer cells release more of these than others, and most importantly, their DNA has distinctive characteristics. The idea is to develop tests capable of detecting these characteristics.
Liquid biopsies based on genomic biomarkers could make MCED a reality, especially for cancers for which there is no standard screening process. But at this stage of the research, there are limitations, including low sensitivity for detecting stage I cancers in validation studies and an increased risk for overdiagnosis.
Q: What specific set of characteristics are the most advanced approaches based on?
Dr. Delaloge: They’re based on the analysis of DNA methylation, a biological process by which CH3 methyl groups are added to the DNA molecule and that determines gene expression. This phenomenon differs depending on whether the cell is cancerous. Among the tests currently under development making use of this specific characteristic is the Galleri test, which is the most advanced of them all.
A previous British National Health Service study, SYMPLIFY, which was published in 2023 by researchers at the University of Oxford, was conducted in symptomatic patients attending a health center. It offers promising results in a diagnostic situation. It has nothing at all to do with screening here. A large, randomized English study, NHS-Galleri, is underway, this time involving the general population, with the aim of assessing the potential benefit of the same test as screening in 140,000 people between ages 50 and 77 years.
In the SYMPLIFY study, which was carried out in symptomatic patients attending a health center, the Galleri MCED test had a positive predictive value of 75.5%, a negative predictive value of 97.6%, a sensitivity of 66.3%, and a specificity of 98.4%. Sensitivity increased with age and cancer stage from 24.2% at stage I to 95.3% at stage IV. For cases for which a cancer signal was detected in patients with cancer, the prediction of the original site of the cancer by the MCED test was accurate in 85.2% of cases. This large-scale prospective evaluation of an MCED diagnostic test confirms its feasibility in a symptomatic population but is not yet sufficiently accurate to “confirm or rule out the presence of cancer.” According to the authors, “in cases in which the MCED test detects a cancer signal in this context, the probability of a diagnosis of cancer being made is considerably higher and may identify cancers at sites other than those suspected during the initial referral phase, thus reducing delays in diagnosis.” A negative test means a lower likelihood of cancer but not so low that proper investigation can be ruled out. Further tests will be needed to optimize use of a negative predictive value.
Q: Does MCED testing concern all types of cancer?
Dr. Delaloge: The Galleri test is based on full profiling of DNA methylation. This allows for early diagnosis of cancer even before it can be seen on imaging tests. The issue with these tests is that they aren’t that good at early diagnosis of the most common types of cancer (breast, colorectal, cervical, etc.) for which we already have more efficient means such as the fecal immunochemical test for colorectal cancer, mammography, HPV testing, and so on.
These blood tests would thus not be aimed at replacing routine screening but rather at screening asymptomatic individuals or those with nonspecific signs for cancers for which we have few or no screening measures and which are on the rise, such as deep tumors and cancer diagnosed at a late stage, namely pancreas, bile duct, ovarian, esophageal, lung, stomach, etc.
The results from the studies published are promising, but others are underway to confirm the benefit of these MCEDs. The challenge is to identify cancer at an early stage, at a stage where it will be easier to cure the patient and control its growth using treatments that are less onerous for the patient and that have fewer aftereffects but not at the expense of a massive increase in overdiagnosis, as seen with prostate-specific antigen levels in prostate cancer a few years ago!
Q: What would be the focus of these MCED tests?
Dr. Delaloge: We must be alert to the risk for the market development of MCED tests. For now, they are mostly, especially the Galleri test, developed in the general population to screen for types of cancer that could not be detected in any other way but also because it’s the most financially beneficial situation. The designers want to position themselves in the general population, regardless of whether this means they’ll have to test hundreds of people to find one for whom the test is beneficial. What’s more, developing tests in isolation, without considering their place in ad hoc treatment pathways, is not realistic. It’s likely that some of these tests will be marketed within the next 10 years, but the health care systems destined to receive them are not remotely ready to do so.
Q: An even more recent publication, from late July 2023, is even more exciting in relation to early detection of lung cancer using circulating DNA sequencing. What are your thoughts on it?
Dr. Delaloge: Initially overtaken by other technologies in favor of MCED approaches, DNA sequencing as a technique to detect somatic mutations seems to have reentered the competition with this new-generation research. The authors published some very interesting results, especially for stage I lung cancer with a very high sensitivity of 75%. [Editor’s note: A machine-learning model using genome-wide mutational profiles combined with other features and followed by CT imaging detected more than 90% of patients with lung cancer, including those with stage I and II disease.]
This research illustrates the difficulty of providing high performance while covering a broad range of cancers. Here, the good results mainly concern lung cancer. Researchers and health care authorities must be alert to ensuring that MCED tests prove themselves in terms of sensitivity and specificity in responding to a medical need and in their impact on specific mortality. This craze for MCED tests must not hinder the development of “single-cancer” technologies that may be much better for detecting specific cancers. This recent publication is interesting in this respect, because this sequencing test seems to be particularly good at detecting lung cancer.
Q: Another approach used in MCED tests is based on analyzing the size of DNA fragments in the blood. Can you explain how this works?
Dr. Delaloge: When cancer is not present, the size of DNA fragments in cells is much more homogeneous. Here also, the benefit of MCED based on this technique rests on the very early detection of cancers that are less common than those for which we already have good screening methods available.
Other approaches, still at the experimental stage, detect certain proteins, certain inflammatory molecules, RNA, etc. But for many researchers, the future will involve pairing tests on the basis of circulating DNA in the blood with the detection of specific molecules indicating the presence of cancer to obtain early screening tests that are even more effective or that possibly even allow us to identify an appropriate treatment at an early stage.
The development of a simple test based on a blood draw that allows us to screen early for all cancers and that would replace all current screening measures is, therefore, not imminent, although it could potentially be on the horizon in years to come. Alongside this, an important issue is the benefit of cancer screening in the general population vs. in a targeted population with a specific risk. The latter option is in development but requires an individualized screening pathway based on blood testing and current screening methods: imaging, etc. It also depends on an individual’s cancer risk profile such as age, personal and family medical history, genetic predisposition, and so on.
According to recent modeling, these multicancer tests could theoretically prevent a minimum of 2,000 deaths from cancer per 100,000 people between ages 50 and 79 years screened per year (17% fewer deaths from cancer per year).
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Loneliness tied to increased risk for Parkinson’s disease
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The surprising link between loneliness and Parkinson’s disease
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.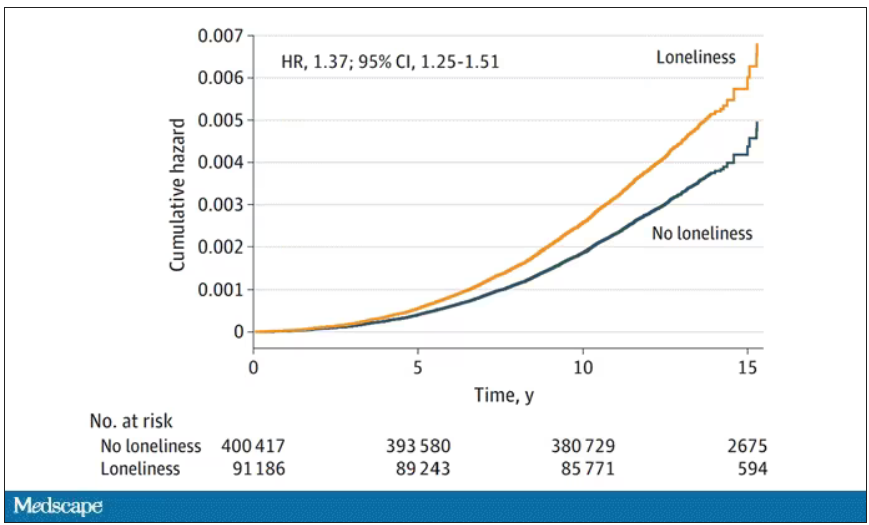
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
From scrubs to screens: Growing your patient base with social media
With physicians under increasing pressure to see more patients in shorter office visits, developing a social media presence may offer valuable opportunities to connect with patients, explain procedures, combat misinformation, talk through a published article, and even share a joke or meme.
But there are caveats for doctors posting on social media platforms. This news organization spoke to four doctors who successfully use social media.
Use social media for the right reasons
While you’re under no obligation to build a social media presence, if you’re going to do it, be sure your intentions are solid, said Don S. Dizon, MD, professor of medicine and professor of surgery at Brown University, Providence, R.I. Dr. Dizon, as @DoctorDon, has 44,700 TikTok followers and uses the platform to answer cancer-related questions.
“It should be your altruism that motivates you to post,” said Dr. Dizon, who is also associate director of community outreach and engagement at the Legorreta Cancer Center in Providence, R.I., and director of medical oncology at Rhode Island Hospital. “What we can do for society at large is to provide our input into issues, add informed opinions where there’s controversy, and address misinformation.”
If you don’t know where to start, consider seeking a digital mentor to talk through your options.
“You may never meet this person, but you should choose them if you like their style, their content, their delivery, and their perspective,” Dr. Dizon said. “Find another doctor out there on social media whom you feel you can emulate. Take your time, too. Soon enough, you’ll develop your own style and your own online persona.”
Post clear, accurate information
If you want to be lighthearted on social media, that’s your choice. But Jennifer Trachtenberg, a pediatrician with nearly 7,000 Instagram followers in New York who posts as @askdrjen, prefers to offer vaccine scheduling tips, alert parents about COVID-19 rates, and offer advice on cold and flu prevention.
“Right now, I’m mainly doing this to educate patients and make them aware of topics that I think are important and that I see my patients needing more information on,” she said. “We have to be clear: People take what we say seriously. So, while it’s important to be relatable, it’s even more important to share evidence-based information.”
Many patients get their information on social media
While patients once came to the doctor armed with information sourced via “Doctor Google,” today, just as many patients use social media to learn about their condition or the medications they’re taking.
Unfortunately, a recent Ohio State University, Columbus, study found that the majority of gynecologic cancer advice on TikTok, for example, was either misleading or inaccurate.
“This misinformation should be a motivator for physicians to explore the social media space,” Dr. Dizon said. “Our voices need to be on there.”
Break down barriers – and make connections
Mike Natter, MD, an endocrinologist in New York, has type 1 diabetes. This informs his work – and his life – and he’s passionate about sharing it with his 117,000 followers as @mike.natter on Instagram.
“A lot of type 1s follow me, so there’s an advocacy component to what I do,” he said. “I enjoy being able to raise awareness and keep people up to date on the newest research and treatment.”
But that’s not all: Dr. Natter is also an artist who went to art school before he went to medical school, and his account is rife with his cartoons and illustrations about everything from valvular disease to diabetic ketoacidosis.
“I found that I was drawing a lot of my notes in medical school,” he said. “When I drew my notes, I did quite well, and I think that using art and illustration is a great tool. It breaks down barriers and makes health information all the more accessible to everyone.”
Share your expertise as a doctor – and a person
As a mom and pediatrician, Krupa Playforth, MD, who practices in Vienna, Va., knows that what she posts carries weight. So, whether she’s writing about backpack safety tips, choking hazards, or separation anxiety, her followers can rest assured that she’s posting responsibly.
“Pediatricians often underestimate how smart parents are,” said Dr. Playforth, who has three kids, ages 8, 5, and 2, and has 137,000 followers on @thepediatricianmom, her Instagram account. “Their anxiety comes from an understandable place, which is why I see my role as that of a parent and pediatrician who can translate the knowledge pediatricians have into something parents can understand.”
Dr. Playforth, who jumped on social media during COVID-19 and experienced a positive response in her local community, said being on social media is imperative if you’re a pediatrician.
“This is the future of pediatric medicine in particular,” she said. “A lot of pediatricians don’t want to embrace social media, but I think that’s a mistake. After all, while parents think pediatricians have all the answers, when we think of our own children, most doctors are like other parents – we can’t think objectively about our kids. It’s helpful for me to share that and to help parents feel less alone.”
If you’re not yet using social media to the best of your physician abilities, you might take a shot at becoming widely recognizable. Pick a preferred platform, answer common patient questions, dispel medical myths, provide pertinent information, and let your personality shine.
A version of this article first appeared on Medscape.com.
With physicians under increasing pressure to see more patients in shorter office visits, developing a social media presence may offer valuable opportunities to connect with patients, explain procedures, combat misinformation, talk through a published article, and even share a joke or meme.
But there are caveats for doctors posting on social media platforms. This news organization spoke to four doctors who successfully use social media.
Use social media for the right reasons
While you’re under no obligation to build a social media presence, if you’re going to do it, be sure your intentions are solid, said Don S. Dizon, MD, professor of medicine and professor of surgery at Brown University, Providence, R.I. Dr. Dizon, as @DoctorDon, has 44,700 TikTok followers and uses the platform to answer cancer-related questions.
“It should be your altruism that motivates you to post,” said Dr. Dizon, who is also associate director of community outreach and engagement at the Legorreta Cancer Center in Providence, R.I., and director of medical oncology at Rhode Island Hospital. “What we can do for society at large is to provide our input into issues, add informed opinions where there’s controversy, and address misinformation.”
If you don’t know where to start, consider seeking a digital mentor to talk through your options.
“You may never meet this person, but you should choose them if you like their style, their content, their delivery, and their perspective,” Dr. Dizon said. “Find another doctor out there on social media whom you feel you can emulate. Take your time, too. Soon enough, you’ll develop your own style and your own online persona.”
Post clear, accurate information
If you want to be lighthearted on social media, that’s your choice. But Jennifer Trachtenberg, a pediatrician with nearly 7,000 Instagram followers in New York who posts as @askdrjen, prefers to offer vaccine scheduling tips, alert parents about COVID-19 rates, and offer advice on cold and flu prevention.
“Right now, I’m mainly doing this to educate patients and make them aware of topics that I think are important and that I see my patients needing more information on,” she said. “We have to be clear: People take what we say seriously. So, while it’s important to be relatable, it’s even more important to share evidence-based information.”
Many patients get their information on social media
While patients once came to the doctor armed with information sourced via “Doctor Google,” today, just as many patients use social media to learn about their condition or the medications they’re taking.
Unfortunately, a recent Ohio State University, Columbus, study found that the majority of gynecologic cancer advice on TikTok, for example, was either misleading or inaccurate.
“This misinformation should be a motivator for physicians to explore the social media space,” Dr. Dizon said. “Our voices need to be on there.”
Break down barriers – and make connections
Mike Natter, MD, an endocrinologist in New York, has type 1 diabetes. This informs his work – and his life – and he’s passionate about sharing it with his 117,000 followers as @mike.natter on Instagram.
“A lot of type 1s follow me, so there’s an advocacy component to what I do,” he said. “I enjoy being able to raise awareness and keep people up to date on the newest research and treatment.”
But that’s not all: Dr. Natter is also an artist who went to art school before he went to medical school, and his account is rife with his cartoons and illustrations about everything from valvular disease to diabetic ketoacidosis.
“I found that I was drawing a lot of my notes in medical school,” he said. “When I drew my notes, I did quite well, and I think that using art and illustration is a great tool. It breaks down barriers and makes health information all the more accessible to everyone.”
Share your expertise as a doctor – and a person
As a mom and pediatrician, Krupa Playforth, MD, who practices in Vienna, Va., knows that what she posts carries weight. So, whether she’s writing about backpack safety tips, choking hazards, or separation anxiety, her followers can rest assured that she’s posting responsibly.
“Pediatricians often underestimate how smart parents are,” said Dr. Playforth, who has three kids, ages 8, 5, and 2, and has 137,000 followers on @thepediatricianmom, her Instagram account. “Their anxiety comes from an understandable place, which is why I see my role as that of a parent and pediatrician who can translate the knowledge pediatricians have into something parents can understand.”
Dr. Playforth, who jumped on social media during COVID-19 and experienced a positive response in her local community, said being on social media is imperative if you’re a pediatrician.
“This is the future of pediatric medicine in particular,” she said. “A lot of pediatricians don’t want to embrace social media, but I think that’s a mistake. After all, while parents think pediatricians have all the answers, when we think of our own children, most doctors are like other parents – we can’t think objectively about our kids. It’s helpful for me to share that and to help parents feel less alone.”
If you’re not yet using social media to the best of your physician abilities, you might take a shot at becoming widely recognizable. Pick a preferred platform, answer common patient questions, dispel medical myths, provide pertinent information, and let your personality shine.
A version of this article first appeared on Medscape.com.
With physicians under increasing pressure to see more patients in shorter office visits, developing a social media presence may offer valuable opportunities to connect with patients, explain procedures, combat misinformation, talk through a published article, and even share a joke or meme.
But there are caveats for doctors posting on social media platforms. This news organization spoke to four doctors who successfully use social media.
Use social media for the right reasons
While you’re under no obligation to build a social media presence, if you’re going to do it, be sure your intentions are solid, said Don S. Dizon, MD, professor of medicine and professor of surgery at Brown University, Providence, R.I. Dr. Dizon, as @DoctorDon, has 44,700 TikTok followers and uses the platform to answer cancer-related questions.
“It should be your altruism that motivates you to post,” said Dr. Dizon, who is also associate director of community outreach and engagement at the Legorreta Cancer Center in Providence, R.I., and director of medical oncology at Rhode Island Hospital. “What we can do for society at large is to provide our input into issues, add informed opinions where there’s controversy, and address misinformation.”
If you don’t know where to start, consider seeking a digital mentor to talk through your options.
“You may never meet this person, but you should choose them if you like their style, their content, their delivery, and their perspective,” Dr. Dizon said. “Find another doctor out there on social media whom you feel you can emulate. Take your time, too. Soon enough, you’ll develop your own style and your own online persona.”
Post clear, accurate information
If you want to be lighthearted on social media, that’s your choice. But Jennifer Trachtenberg, a pediatrician with nearly 7,000 Instagram followers in New York who posts as @askdrjen, prefers to offer vaccine scheduling tips, alert parents about COVID-19 rates, and offer advice on cold and flu prevention.
“Right now, I’m mainly doing this to educate patients and make them aware of topics that I think are important and that I see my patients needing more information on,” she said. “We have to be clear: People take what we say seriously. So, while it’s important to be relatable, it’s even more important to share evidence-based information.”
Many patients get their information on social media
While patients once came to the doctor armed with information sourced via “Doctor Google,” today, just as many patients use social media to learn about their condition or the medications they’re taking.
Unfortunately, a recent Ohio State University, Columbus, study found that the majority of gynecologic cancer advice on TikTok, for example, was either misleading or inaccurate.
“This misinformation should be a motivator for physicians to explore the social media space,” Dr. Dizon said. “Our voices need to be on there.”
Break down barriers – and make connections
Mike Natter, MD, an endocrinologist in New York, has type 1 diabetes. This informs his work – and his life – and he’s passionate about sharing it with his 117,000 followers as @mike.natter on Instagram.
“A lot of type 1s follow me, so there’s an advocacy component to what I do,” he said. “I enjoy being able to raise awareness and keep people up to date on the newest research and treatment.”
But that’s not all: Dr. Natter is also an artist who went to art school before he went to medical school, and his account is rife with his cartoons and illustrations about everything from valvular disease to diabetic ketoacidosis.
“I found that I was drawing a lot of my notes in medical school,” he said. “When I drew my notes, I did quite well, and I think that using art and illustration is a great tool. It breaks down barriers and makes health information all the more accessible to everyone.”
Share your expertise as a doctor – and a person
As a mom and pediatrician, Krupa Playforth, MD, who practices in Vienna, Va., knows that what she posts carries weight. So, whether she’s writing about backpack safety tips, choking hazards, or separation anxiety, her followers can rest assured that she’s posting responsibly.
“Pediatricians often underestimate how smart parents are,” said Dr. Playforth, who has three kids, ages 8, 5, and 2, and has 137,000 followers on @thepediatricianmom, her Instagram account. “Their anxiety comes from an understandable place, which is why I see my role as that of a parent and pediatrician who can translate the knowledge pediatricians have into something parents can understand.”
Dr. Playforth, who jumped on social media during COVID-19 and experienced a positive response in her local community, said being on social media is imperative if you’re a pediatrician.
“This is the future of pediatric medicine in particular,” she said. “A lot of pediatricians don’t want to embrace social media, but I think that’s a mistake. After all, while parents think pediatricians have all the answers, when we think of our own children, most doctors are like other parents – we can’t think objectively about our kids. It’s helpful for me to share that and to help parents feel less alone.”
If you’re not yet using social media to the best of your physician abilities, you might take a shot at becoming widely recognizable. Pick a preferred platform, answer common patient questions, dispel medical myths, provide pertinent information, and let your personality shine.
A version of this article first appeared on Medscape.com.
Enlarging pink patches after traveling
The patient’s multiple pink, subtly annular patches after recent travel to Lyme-endemic areas of the United States demonstrated a classic manifestation of disseminated Lyme disease. An enzyme-linked immunosorbent assay was positive for Borrelia burgdorferi IgM and IgG antibodies, confirming an acute infection.
While not usually necessary, skin biopsy shows a nonspecific perivascular cellular infiltrate that may be comprised of histiocytes, lymphocytes, and plasma cells. Spirochetes are not typically seen, but they may be identified with antibody-labeled or silver stains.
Lyme disease initially manifests as localized disease with erythema migrans, a targetoid lesion on the skin that appears at the site of the tick bite. This initial stage develops within the first few weeks of the bite and may be accompanied by fatigue and a low-grade fever.
If left untreated, the infection may progress to early disseminated disease, which occurs weeks to months after the initial bite. This second stage of Lyme disease manifests with multiple erythema migrans lesions on additional parts of the body, indicating spirochete dissemination through the bloodstream and lymphatic system. Early disseminated disease may also include borrelial lymphocytoma, Lyme neuroborreliosis, and cardiac conduction abnormalities such as AV block.
The third stage of Lyme disease, late Lyme disease, occurs months to years after an initial infection that has gone untreated. The key feature of this stage is arthritis, which tends to affect the knees and may be migratory in nature. Neurological symptoms such as encephalopathy and polyneuropathies may also develop. A minority of patients with late Lyme disease may develop acrodermatitis chronica atrophicans, a rash that typically occurs on the dorsal hands and feet as blue-red plaques that turn the affected skin atrophic.1
This patient was treated with a 3-week course of oral doxycycline 100 mg twice daily and was referred to an infectious disease specialist for further work-up of systemic symptoms, given the risk for cardiac pathology in disseminated Lyme disease.
Photo courtesy of Le Wen Chiu, MD. Text courtesy of Le Wen Chiu, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1

The patient’s multiple pink, subtly annular patches after recent travel to Lyme-endemic areas of the United States demonstrated a classic manifestation of disseminated Lyme disease. An enzyme-linked immunosorbent assay was positive for Borrelia burgdorferi IgM and IgG antibodies, confirming an acute infection.
While not usually necessary, skin biopsy shows a nonspecific perivascular cellular infiltrate that may be comprised of histiocytes, lymphocytes, and plasma cells. Spirochetes are not typically seen, but they may be identified with antibody-labeled or silver stains.
Lyme disease initially manifests as localized disease with erythema migrans, a targetoid lesion on the skin that appears at the site of the tick bite. This initial stage develops within the first few weeks of the bite and may be accompanied by fatigue and a low-grade fever.
If left untreated, the infection may progress to early disseminated disease, which occurs weeks to months after the initial bite. This second stage of Lyme disease manifests with multiple erythema migrans lesions on additional parts of the body, indicating spirochete dissemination through the bloodstream and lymphatic system. Early disseminated disease may also include borrelial lymphocytoma, Lyme neuroborreliosis, and cardiac conduction abnormalities such as AV block.
The third stage of Lyme disease, late Lyme disease, occurs months to years after an initial infection that has gone untreated. The key feature of this stage is arthritis, which tends to affect the knees and may be migratory in nature. Neurological symptoms such as encephalopathy and polyneuropathies may also develop. A minority of patients with late Lyme disease may develop acrodermatitis chronica atrophicans, a rash that typically occurs on the dorsal hands and feet as blue-red plaques that turn the affected skin atrophic.1
This patient was treated with a 3-week course of oral doxycycline 100 mg twice daily and was referred to an infectious disease specialist for further work-up of systemic symptoms, given the risk for cardiac pathology in disseminated Lyme disease.
Photo courtesy of Le Wen Chiu, MD. Text courtesy of Le Wen Chiu, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The patient’s multiple pink, subtly annular patches after recent travel to Lyme-endemic areas of the United States demonstrated a classic manifestation of disseminated Lyme disease. An enzyme-linked immunosorbent assay was positive for Borrelia burgdorferi IgM and IgG antibodies, confirming an acute infection.
While not usually necessary, skin biopsy shows a nonspecific perivascular cellular infiltrate that may be comprised of histiocytes, lymphocytes, and plasma cells. Spirochetes are not typically seen, but they may be identified with antibody-labeled or silver stains.
Lyme disease initially manifests as localized disease with erythema migrans, a targetoid lesion on the skin that appears at the site of the tick bite. This initial stage develops within the first few weeks of the bite and may be accompanied by fatigue and a low-grade fever.
If left untreated, the infection may progress to early disseminated disease, which occurs weeks to months after the initial bite. This second stage of Lyme disease manifests with multiple erythema migrans lesions on additional parts of the body, indicating spirochete dissemination through the bloodstream and lymphatic system. Early disseminated disease may also include borrelial lymphocytoma, Lyme neuroborreliosis, and cardiac conduction abnormalities such as AV block.
The third stage of Lyme disease, late Lyme disease, occurs months to years after an initial infection that has gone untreated. The key feature of this stage is arthritis, which tends to affect the knees and may be migratory in nature. Neurological symptoms such as encephalopathy and polyneuropathies may also develop. A minority of patients with late Lyme disease may develop acrodermatitis chronica atrophicans, a rash that typically occurs on the dorsal hands and feet as blue-red plaques that turn the affected skin atrophic.1
This patient was treated with a 3-week course of oral doxycycline 100 mg twice daily and was referred to an infectious disease specialist for further work-up of systemic symptoms, given the risk for cardiac pathology in disseminated Lyme disease.
Photo courtesy of Le Wen Chiu, MD. Text courtesy of Le Wen Chiu, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
1. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
Weight loss with semaglutide maintained for up to 3 years
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM EASD 2023
USPSTF should reconsider recommendation to lower mammogram age: Experts
The updated draft recommendation from the U.S. Preventive Services Task Force that would lower the recommended start age for routine screening mammograms by a decade for all average-risk women is not justified, experts argue in a “dissenting view” published in the New England Journal of Medicine.
The proposed change would affect more than 20 million U.S. women, and it’s “hard to see any potential benefits associated with lowering the starting age,” coauthor Steven Woloshin, MD, with Dartmouth Cancer Center, Lebanon, N.H., said in an NEJM podcast.
Back in May, when USPSTF released the draft recommendation, task force member John Wong, MD, with Tufts Medical Center, Boston, said in an interview, “It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women.”
But, according to Dr. Woloshin, there is no recent evidence that mortality from breast cancer is increasing in young women.
In fact, the United States has seen a steady decrease in breast cancer mortality, especially among younger women. Breast cancer mortality among women under 50 “has been cut in half over the past 30 years,” Dr. Woloshin and coauthors explained.
Another wrinkle: The task force did not base its recent recommendation on randomized trial data. In fact, there have been no new randomized trials of screening mammography for women in their 40s since 2016. Instead, the task force relied on statistical models to “estimate what might happen if the starting age were lowered,” Dr. Woloshin and colleagues said.
Relying on a statistical model, however, “is problematic because it has some very optimistic assumptions about the benefit of mammography,” Dr. Woloshin said in the podcast. For instance, the models assume that screening mammography reduces breast cancer mortality by about 25%.
That 25% reduction is “far greater than what’s reported in the meta-analyses of the available randomized trials,” Dr. Woloshin explained. The meta-analyses report about a 16% reduction for all the trials combined and an estimated 13% for trials at low risk of bias. But “even these meta-analyses are likely to overstate the effect of screening since the trials were done before the major advances in treatment.”
In their own calculations, Dr. Woloshin and colleagues found that lowering the screening age to 40 came with a small potential benefit and a substantial risk for harm.
Combing data from the National Cancer Institute, the team reported that the risk for death for women in their 40s from any cause over the next 10 years was about 3% whether or not they received their biennial mammogram.
The risk for death from breast cancer in that time was 0.23% with mammograms – about 2 in every 1,000 women – and 0.31% without. “That’s 1 less breast cancer death per 1,000 women screened for 10 years,” Dr. Woloshin said.
Put another way, with mammography screening, “the chance of not dying from breast cancer over the next 10 years increases from 99.7% to 99.8%,” Dr. Woloshin said.
The benefit is arguably small, while the harms appear quite significant, Dr. Woloshin said. About 36% of women who begin screening at age 40 would have at least one false alarm over 10 years, and almost 7% would have a false alarm requiring a biopsy in that time frame.
Ease or exacerbate racial disparity?
Another argument that the USPSTF highlighted for lowering the screening age: Research indicates that Black women get breast cancer at younger ages and are more likely to die of the disease, compared with White women.
Dr. Woloshin and coauthors, however, also took issue with the view that lowering the screening age could reduce disparities between Black and White women.
“There’s no question that there are substantial differences between Black and White women in terms of breast cancer mortality, but there’s actually very little disparity in breast cancer screening – about 60% of Black and White women in their 40s are screened regularly in the United States,” Dr. Woloshin explained in the podcast.
Therefore, it’s “really hard to imagine” how recommending the same intervention to both groups could possibly reduce the disparity, he said.
“The disparity is not a reflection of screening. It reflects differences in cancer biology,” he added. “Black women are at higher risk for more aggressive, fast-growing cancers that are less likely to be caught by screening and unfortunately are less likely to benefit from treatment.”
Earlier screening would also not address the problems facing poor women, who tend to be disproportionately Black, such as lower quality of available medical services, follow-up delays after abnormal scans, treatment delays, and less use of adjuvant therapy, Dr. Woloshin cautioned.
In Dr. Woloshin’s view, lowering the screening age, which broadens the eligible population, may actually “exacerbate problems contributing to disparity by diverting resources toward expanded screening rather than doing what we know works by ensuring that high-quality treatments are more readily accessible to poor women with breast cancer.”
Reconsider the change?
Because task force recommendations are so influential, Dr. Woloshin and colleagues worry that mammography screening for women in their 40s will probably become a performance measure.
“Our concern is that, rather than fostering informed decisions, clinicians and practices are going to be judged and rewarded and punished based on compliance with this quality metric,” Dr. Woloshin said.
That’s a problem, he noted, “because women should be able to make the decision for themselves rather than having this be a public health imperative, which is imposed by physicians and practices who are incentivized to meet a quality metric.”
The hope, said Dr. Woloshin, is that this prospective piece will help influence the task force to “reconsider the recommendation, because we think that the bottom line is that their models are insufficient to support a new imperative. The benefits are really limited, and there are really common and important harms for healthy women.”
The comment period for the draft recommendation is now closed, and a final decision from the task force is forthcoming.
The research had no funding. Dr. Woloshin has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The updated draft recommendation from the U.S. Preventive Services Task Force that would lower the recommended start age for routine screening mammograms by a decade for all average-risk women is not justified, experts argue in a “dissenting view” published in the New England Journal of Medicine.
The proposed change would affect more than 20 million U.S. women, and it’s “hard to see any potential benefits associated with lowering the starting age,” coauthor Steven Woloshin, MD, with Dartmouth Cancer Center, Lebanon, N.H., said in an NEJM podcast.
Back in May, when USPSTF released the draft recommendation, task force member John Wong, MD, with Tufts Medical Center, Boston, said in an interview, “It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women.”
But, according to Dr. Woloshin, there is no recent evidence that mortality from breast cancer is increasing in young women.
In fact, the United States has seen a steady decrease in breast cancer mortality, especially among younger women. Breast cancer mortality among women under 50 “has been cut in half over the past 30 years,” Dr. Woloshin and coauthors explained.
Another wrinkle: The task force did not base its recent recommendation on randomized trial data. In fact, there have been no new randomized trials of screening mammography for women in their 40s since 2016. Instead, the task force relied on statistical models to “estimate what might happen if the starting age were lowered,” Dr. Woloshin and colleagues said.
Relying on a statistical model, however, “is problematic because it has some very optimistic assumptions about the benefit of mammography,” Dr. Woloshin said in the podcast. For instance, the models assume that screening mammography reduces breast cancer mortality by about 25%.
That 25% reduction is “far greater than what’s reported in the meta-analyses of the available randomized trials,” Dr. Woloshin explained. The meta-analyses report about a 16% reduction for all the trials combined and an estimated 13% for trials at low risk of bias. But “even these meta-analyses are likely to overstate the effect of screening since the trials were done before the major advances in treatment.”
In their own calculations, Dr. Woloshin and colleagues found that lowering the screening age to 40 came with a small potential benefit and a substantial risk for harm.
Combing data from the National Cancer Institute, the team reported that the risk for death for women in their 40s from any cause over the next 10 years was about 3% whether or not they received their biennial mammogram.
The risk for death from breast cancer in that time was 0.23% with mammograms – about 2 in every 1,000 women – and 0.31% without. “That’s 1 less breast cancer death per 1,000 women screened for 10 years,” Dr. Woloshin said.
Put another way, with mammography screening, “the chance of not dying from breast cancer over the next 10 years increases from 99.7% to 99.8%,” Dr. Woloshin said.
The benefit is arguably small, while the harms appear quite significant, Dr. Woloshin said. About 36% of women who begin screening at age 40 would have at least one false alarm over 10 years, and almost 7% would have a false alarm requiring a biopsy in that time frame.
Ease or exacerbate racial disparity?
Another argument that the USPSTF highlighted for lowering the screening age: Research indicates that Black women get breast cancer at younger ages and are more likely to die of the disease, compared with White women.
Dr. Woloshin and coauthors, however, also took issue with the view that lowering the screening age could reduce disparities between Black and White women.
“There’s no question that there are substantial differences between Black and White women in terms of breast cancer mortality, but there’s actually very little disparity in breast cancer screening – about 60% of Black and White women in their 40s are screened regularly in the United States,” Dr. Woloshin explained in the podcast.
Therefore, it’s “really hard to imagine” how recommending the same intervention to both groups could possibly reduce the disparity, he said.
“The disparity is not a reflection of screening. It reflects differences in cancer biology,” he added. “Black women are at higher risk for more aggressive, fast-growing cancers that are less likely to be caught by screening and unfortunately are less likely to benefit from treatment.”
Earlier screening would also not address the problems facing poor women, who tend to be disproportionately Black, such as lower quality of available medical services, follow-up delays after abnormal scans, treatment delays, and less use of adjuvant therapy, Dr. Woloshin cautioned.
In Dr. Woloshin’s view, lowering the screening age, which broadens the eligible population, may actually “exacerbate problems contributing to disparity by diverting resources toward expanded screening rather than doing what we know works by ensuring that high-quality treatments are more readily accessible to poor women with breast cancer.”
Reconsider the change?
Because task force recommendations are so influential, Dr. Woloshin and colleagues worry that mammography screening for women in their 40s will probably become a performance measure.
“Our concern is that, rather than fostering informed decisions, clinicians and practices are going to be judged and rewarded and punished based on compliance with this quality metric,” Dr. Woloshin said.
That’s a problem, he noted, “because women should be able to make the decision for themselves rather than having this be a public health imperative, which is imposed by physicians and practices who are incentivized to meet a quality metric.”
The hope, said Dr. Woloshin, is that this prospective piece will help influence the task force to “reconsider the recommendation, because we think that the bottom line is that their models are insufficient to support a new imperative. The benefits are really limited, and there are really common and important harms for healthy women.”
The comment period for the draft recommendation is now closed, and a final decision from the task force is forthcoming.
The research had no funding. Dr. Woloshin has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The updated draft recommendation from the U.S. Preventive Services Task Force that would lower the recommended start age for routine screening mammograms by a decade for all average-risk women is not justified, experts argue in a “dissenting view” published in the New England Journal of Medicine.
The proposed change would affect more than 20 million U.S. women, and it’s “hard to see any potential benefits associated with lowering the starting age,” coauthor Steven Woloshin, MD, with Dartmouth Cancer Center, Lebanon, N.H., said in an NEJM podcast.
Back in May, when USPSTF released the draft recommendation, task force member John Wong, MD, with Tufts Medical Center, Boston, said in an interview, “It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women.”
But, according to Dr. Woloshin, there is no recent evidence that mortality from breast cancer is increasing in young women.
In fact, the United States has seen a steady decrease in breast cancer mortality, especially among younger women. Breast cancer mortality among women under 50 “has been cut in half over the past 30 years,” Dr. Woloshin and coauthors explained.
Another wrinkle: The task force did not base its recent recommendation on randomized trial data. In fact, there have been no new randomized trials of screening mammography for women in their 40s since 2016. Instead, the task force relied on statistical models to “estimate what might happen if the starting age were lowered,” Dr. Woloshin and colleagues said.
Relying on a statistical model, however, “is problematic because it has some very optimistic assumptions about the benefit of mammography,” Dr. Woloshin said in the podcast. For instance, the models assume that screening mammography reduces breast cancer mortality by about 25%.
That 25% reduction is “far greater than what’s reported in the meta-analyses of the available randomized trials,” Dr. Woloshin explained. The meta-analyses report about a 16% reduction for all the trials combined and an estimated 13% for trials at low risk of bias. But “even these meta-analyses are likely to overstate the effect of screening since the trials were done before the major advances in treatment.”
In their own calculations, Dr. Woloshin and colleagues found that lowering the screening age to 40 came with a small potential benefit and a substantial risk for harm.
Combing data from the National Cancer Institute, the team reported that the risk for death for women in their 40s from any cause over the next 10 years was about 3% whether or not they received their biennial mammogram.
The risk for death from breast cancer in that time was 0.23% with mammograms – about 2 in every 1,000 women – and 0.31% without. “That’s 1 less breast cancer death per 1,000 women screened for 10 years,” Dr. Woloshin said.
Put another way, with mammography screening, “the chance of not dying from breast cancer over the next 10 years increases from 99.7% to 99.8%,” Dr. Woloshin said.
The benefit is arguably small, while the harms appear quite significant, Dr. Woloshin said. About 36% of women who begin screening at age 40 would have at least one false alarm over 10 years, and almost 7% would have a false alarm requiring a biopsy in that time frame.
Ease or exacerbate racial disparity?
Another argument that the USPSTF highlighted for lowering the screening age: Research indicates that Black women get breast cancer at younger ages and are more likely to die of the disease, compared with White women.
Dr. Woloshin and coauthors, however, also took issue with the view that lowering the screening age could reduce disparities between Black and White women.
“There’s no question that there are substantial differences between Black and White women in terms of breast cancer mortality, but there’s actually very little disparity in breast cancer screening – about 60% of Black and White women in their 40s are screened regularly in the United States,” Dr. Woloshin explained in the podcast.
Therefore, it’s “really hard to imagine” how recommending the same intervention to both groups could possibly reduce the disparity, he said.
“The disparity is not a reflection of screening. It reflects differences in cancer biology,” he added. “Black women are at higher risk for more aggressive, fast-growing cancers that are less likely to be caught by screening and unfortunately are less likely to benefit from treatment.”
Earlier screening would also not address the problems facing poor women, who tend to be disproportionately Black, such as lower quality of available medical services, follow-up delays after abnormal scans, treatment delays, and less use of adjuvant therapy, Dr. Woloshin cautioned.
In Dr. Woloshin’s view, lowering the screening age, which broadens the eligible population, may actually “exacerbate problems contributing to disparity by diverting resources toward expanded screening rather than doing what we know works by ensuring that high-quality treatments are more readily accessible to poor women with breast cancer.”
Reconsider the change?
Because task force recommendations are so influential, Dr. Woloshin and colleagues worry that mammography screening for women in their 40s will probably become a performance measure.
“Our concern is that, rather than fostering informed decisions, clinicians and practices are going to be judged and rewarded and punished based on compliance with this quality metric,” Dr. Woloshin said.
That’s a problem, he noted, “because women should be able to make the decision for themselves rather than having this be a public health imperative, which is imposed by physicians and practices who are incentivized to meet a quality metric.”
The hope, said Dr. Woloshin, is that this prospective piece will help influence the task force to “reconsider the recommendation, because we think that the bottom line is that their models are insufficient to support a new imperative. The benefits are really limited, and there are really common and important harms for healthy women.”
The comment period for the draft recommendation is now closed, and a final decision from the task force is forthcoming.
The research had no funding. Dr. Woloshin has no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE