User login
Data Trends 2023: Depression
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
CBT linked to reduced pain, less catastrophizing in fibromyalgia
TOPLINE:
In patients with fibromyalgia, cognitive behavior therapy (CBT) can reduce pain through its effect on pain-related catastrophizing, which involves intensified cognitive and emotional responses to things like intrusive thoughts, a new study suggests.
METHODOLOGY:
- The study included 98 female patients with fibromyalgia (FM), mean age about 42 years, who underwent a baseline neuroimaging assessment and were randomly assigned to CBT (where patients learned to identify negative thoughts and use cognitive restructuring to diminish pain-related distress) or a matched educational intervention (where patients learned about fibromyalgia and chronic pain); both groups had eight weekly individual 60- to 75-minute visits.
- The primary outcome was the pain interference subscale of the Brief Pain Inventory (BPI); secondary outcomes included the BPI pain severity subscale, the Fibromyalgia Impact Questionnaire–Revised (FIQR), and the Pain Catastrophizing Scale (PCS), which includes subscales of rumination, magnification, and helplessness.
- Researchers used functional magnetic resonance imaging (fMRI)-adapted task to investigate the neural circuitry supporting pain catastrophizing.
TAKEAWAY:
- After controlling for baseline values, BPI pain interference scores were significantly reduced, with a larger reduction in the CBT group, compared with the education group (P = .03), which was also the case for FIQR scores (P = .05) and pain catastrophizing (P = .04).
- There were larger reductions in pain-related symptomatology in the CBT group, but they did not reach statistical significance.
- Following CBT treatment, the study showed reduced connectivity between regions of the brain associated with self-awareness, pain, and emotional processing.
IN PRACTICE:
The results “highlight the important role of targeting pain catastrophizing with psychotherapy, particularly for patients reporting high levels of catastrophizing cognitions” write the authors, adding that altered network connectivity identified by the study “may emerge as a valuable biomarker of catastrophizing-related cognitive and affective processes.”
SOURCE:
The study was carried out by Jeungchan Lee, PhD, department of radiology, center for biomedical imaging, Massachusetts General Hospital, Boston, and the Discovery Center for Recovery from Chronic Pain, Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, and colleagues. It was published in Arthritis & Rheumatology.
LIMITATIONS:
Findings were limited to female participants. CBT for chronic pain includes different therapeutic modules, and the study can’t draw definitive conclusions regarding which CBT skills were most beneficial to patients in reducing catastrophizing. Baseline symptom severity was higher for the CBT group, which may complicate interpretation of the findings.
DISCLOSURES:
The study received support from the National Institutes of Health: National Center for Complementary and Integrative Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Center for Research Resources. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with fibromyalgia, cognitive behavior therapy (CBT) can reduce pain through its effect on pain-related catastrophizing, which involves intensified cognitive and emotional responses to things like intrusive thoughts, a new study suggests.
METHODOLOGY:
- The study included 98 female patients with fibromyalgia (FM), mean age about 42 years, who underwent a baseline neuroimaging assessment and were randomly assigned to CBT (where patients learned to identify negative thoughts and use cognitive restructuring to diminish pain-related distress) or a matched educational intervention (where patients learned about fibromyalgia and chronic pain); both groups had eight weekly individual 60- to 75-minute visits.
- The primary outcome was the pain interference subscale of the Brief Pain Inventory (BPI); secondary outcomes included the BPI pain severity subscale, the Fibromyalgia Impact Questionnaire–Revised (FIQR), and the Pain Catastrophizing Scale (PCS), which includes subscales of rumination, magnification, and helplessness.
- Researchers used functional magnetic resonance imaging (fMRI)-adapted task to investigate the neural circuitry supporting pain catastrophizing.
TAKEAWAY:
- After controlling for baseline values, BPI pain interference scores were significantly reduced, with a larger reduction in the CBT group, compared with the education group (P = .03), which was also the case for FIQR scores (P = .05) and pain catastrophizing (P = .04).
- There were larger reductions in pain-related symptomatology in the CBT group, but they did not reach statistical significance.
- Following CBT treatment, the study showed reduced connectivity between regions of the brain associated with self-awareness, pain, and emotional processing.
IN PRACTICE:
The results “highlight the important role of targeting pain catastrophizing with psychotherapy, particularly for patients reporting high levels of catastrophizing cognitions” write the authors, adding that altered network connectivity identified by the study “may emerge as a valuable biomarker of catastrophizing-related cognitive and affective processes.”
SOURCE:
The study was carried out by Jeungchan Lee, PhD, department of radiology, center for biomedical imaging, Massachusetts General Hospital, Boston, and the Discovery Center for Recovery from Chronic Pain, Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, and colleagues. It was published in Arthritis & Rheumatology.
LIMITATIONS:
Findings were limited to female participants. CBT for chronic pain includes different therapeutic modules, and the study can’t draw definitive conclusions regarding which CBT skills were most beneficial to patients in reducing catastrophizing. Baseline symptom severity was higher for the CBT group, which may complicate interpretation of the findings.
DISCLOSURES:
The study received support from the National Institutes of Health: National Center for Complementary and Integrative Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Center for Research Resources. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with fibromyalgia, cognitive behavior therapy (CBT) can reduce pain through its effect on pain-related catastrophizing, which involves intensified cognitive and emotional responses to things like intrusive thoughts, a new study suggests.
METHODOLOGY:
- The study included 98 female patients with fibromyalgia (FM), mean age about 42 years, who underwent a baseline neuroimaging assessment and were randomly assigned to CBT (where patients learned to identify negative thoughts and use cognitive restructuring to diminish pain-related distress) or a matched educational intervention (where patients learned about fibromyalgia and chronic pain); both groups had eight weekly individual 60- to 75-minute visits.
- The primary outcome was the pain interference subscale of the Brief Pain Inventory (BPI); secondary outcomes included the BPI pain severity subscale, the Fibromyalgia Impact Questionnaire–Revised (FIQR), and the Pain Catastrophizing Scale (PCS), which includes subscales of rumination, magnification, and helplessness.
- Researchers used functional magnetic resonance imaging (fMRI)-adapted task to investigate the neural circuitry supporting pain catastrophizing.
TAKEAWAY:
- After controlling for baseline values, BPI pain interference scores were significantly reduced, with a larger reduction in the CBT group, compared with the education group (P = .03), which was also the case for FIQR scores (P = .05) and pain catastrophizing (P = .04).
- There were larger reductions in pain-related symptomatology in the CBT group, but they did not reach statistical significance.
- Following CBT treatment, the study showed reduced connectivity between regions of the brain associated with self-awareness, pain, and emotional processing.
IN PRACTICE:
The results “highlight the important role of targeting pain catastrophizing with psychotherapy, particularly for patients reporting high levels of catastrophizing cognitions” write the authors, adding that altered network connectivity identified by the study “may emerge as a valuable biomarker of catastrophizing-related cognitive and affective processes.”
SOURCE:
The study was carried out by Jeungchan Lee, PhD, department of radiology, center for biomedical imaging, Massachusetts General Hospital, Boston, and the Discovery Center for Recovery from Chronic Pain, Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, and colleagues. It was published in Arthritis & Rheumatology.
LIMITATIONS:
Findings were limited to female participants. CBT for chronic pain includes different therapeutic modules, and the study can’t draw definitive conclusions regarding which CBT skills were most beneficial to patients in reducing catastrophizing. Baseline symptom severity was higher for the CBT group, which may complicate interpretation of the findings.
DISCLOSURES:
The study received support from the National Institutes of Health: National Center for Complementary and Integrative Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Center for Research Resources. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
PET scan at diagnosis may help to predict aneurysm risk in patients with giant cell arteritis
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
What’s right and wrong for doctors on social media
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
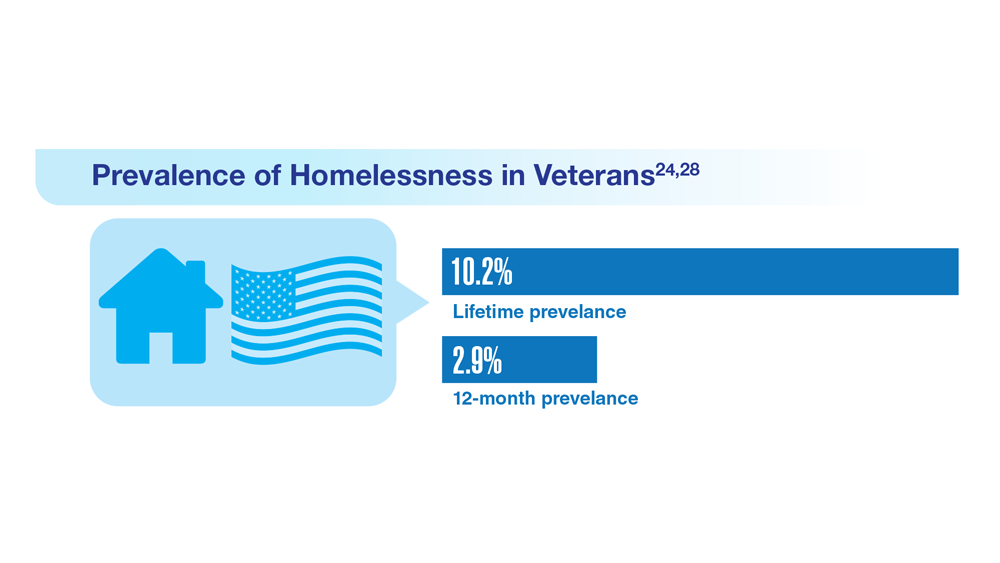
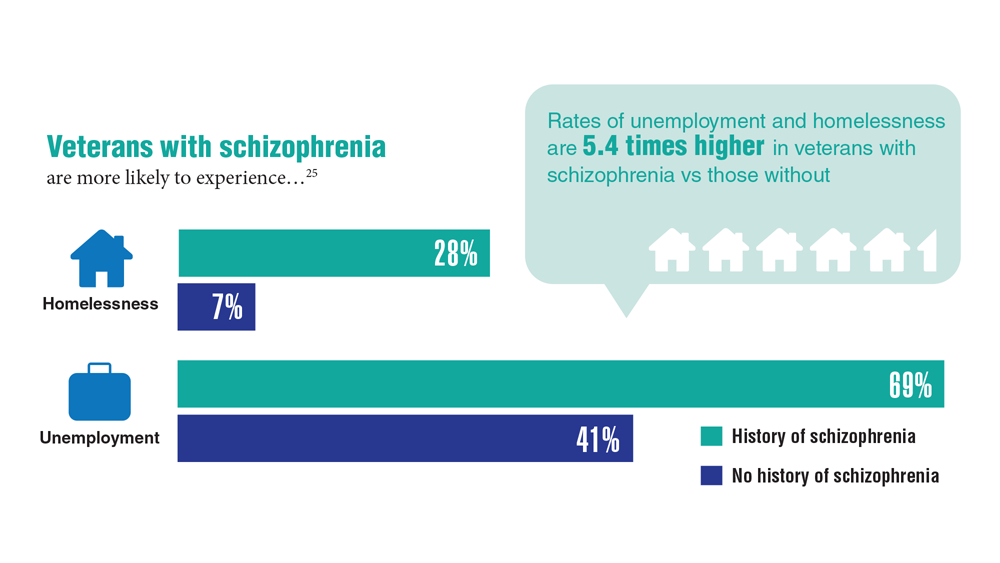
Data Trends 2023: Homelessness
24. Nichter B et al. Psychol Med. 2022;1-11. doi:10.1017/S0033291722000617
25. Lin D et al. BMC Psychiatry. 2022;22(1):458. doi:10.1186/s12888-022-04022-x
26. Jutkowitz E et al. R I Med J (2013). 2021;104(4):20-25. Published 2021 May 3.
27. Holliday R et al. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
28. Koh KA et al. Am J Prev Med. 2022;63(1):13-23. doi:10.1016/j.amepre.2021.12.028
24. Nichter B et al. Psychol Med. 2022;1-11. doi:10.1017/S0033291722000617
25. Lin D et al. BMC Psychiatry. 2022;22(1):458. doi:10.1186/s12888-022-04022-x
26. Jutkowitz E et al. R I Med J (2013). 2021;104(4):20-25. Published 2021 May 3.
27. Holliday R et al. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
28. Koh KA et al. Am J Prev Med. 2022;63(1):13-23. doi:10.1016/j.amepre.2021.12.028
24. Nichter B et al. Psychol Med. 2022;1-11. doi:10.1017/S0033291722000617
25. Lin D et al. BMC Psychiatry. 2022;22(1):458. doi:10.1186/s12888-022-04022-x
26. Jutkowitz E et al. R I Med J (2013). 2021;104(4):20-25. Published 2021 May 3.
27. Holliday R et al. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
28. Koh KA et al. Am J Prev Med. 2022;63(1):13-23. doi:10.1016/j.amepre.2021.12.028
FDA approves first tocilizumab biosimilar
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
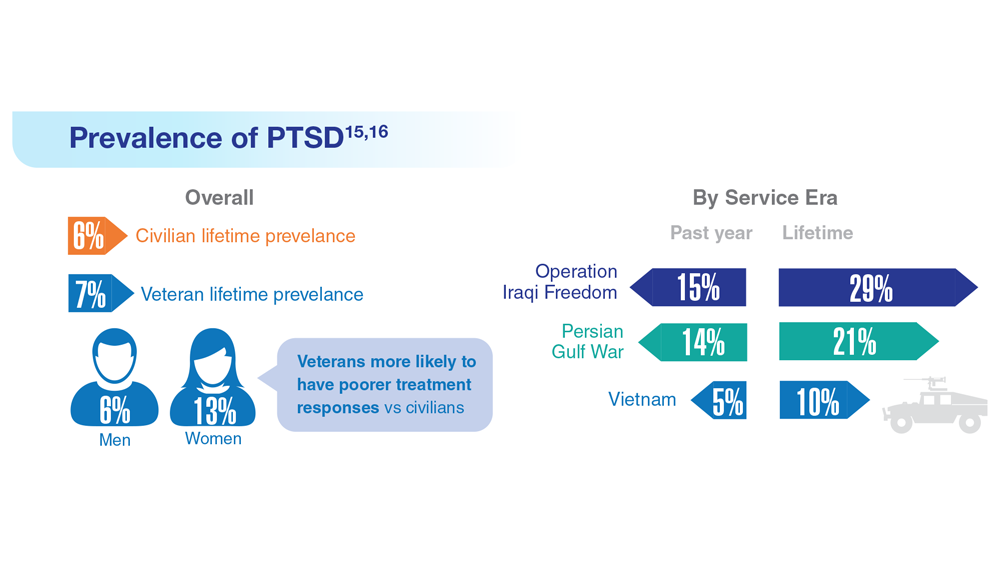
Data Trends 2023: PTSD and Psychedelic Treatments
15. US Department of Veterans Affairs. How common is PTSD in veterans? Updated February 3, 2023. Accessed April 21, 2023. https://www.ptsd.va.gov/understand/common/common_veterans.asp
16. Murphy D, Smith KV. J Trauma Stress. 2018;31(5):753-763. doi:10.1002/jts.22333
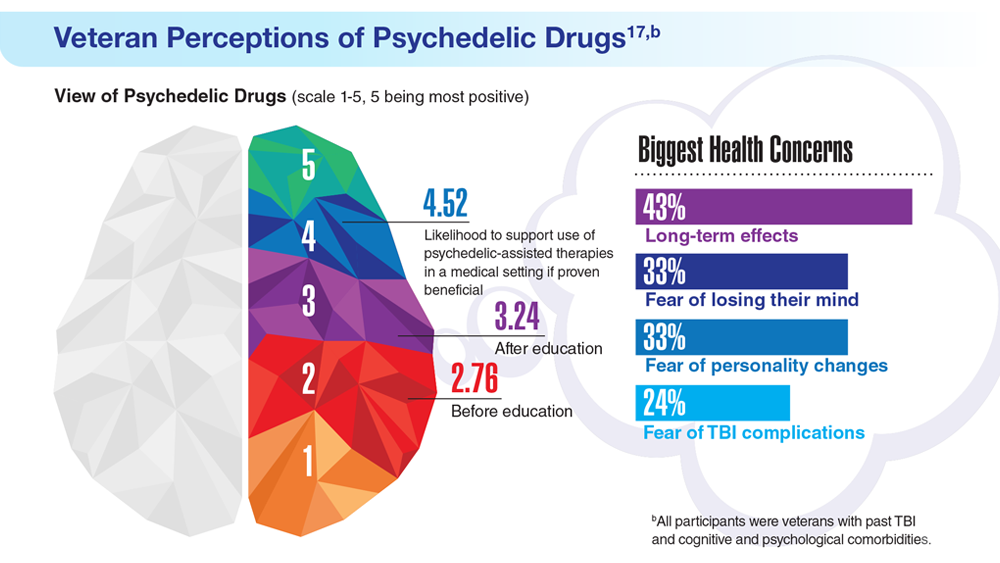
17. Gray JC et al. Mil Med. 2022;usac400. doi:10.1093/milmed/usac400
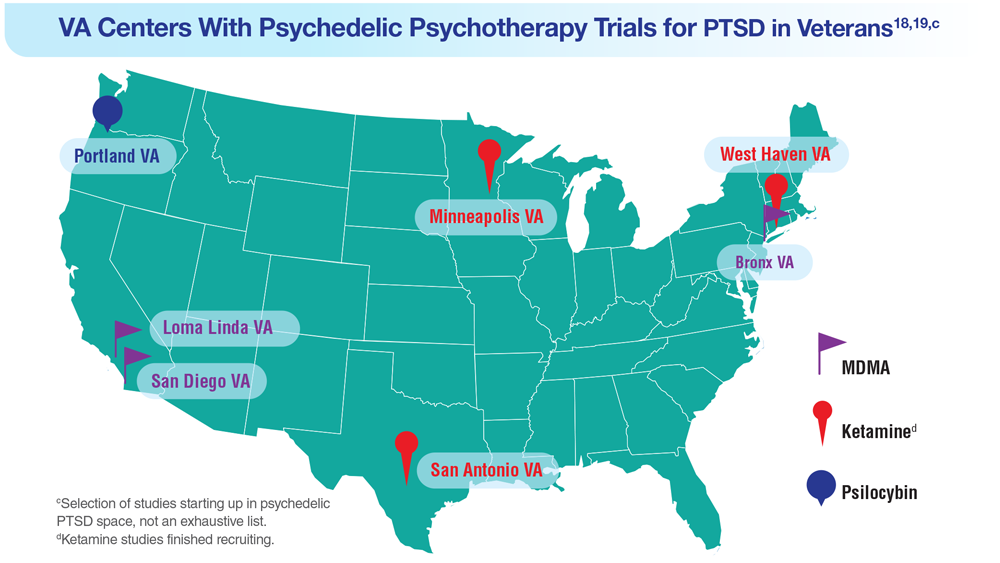
18. Herrington AJ. VA studying psychedelics as mental health treatment for veterans. Forbes. Published June 24, 2022. Accessed April 21, 2023. https://www.forbes.com/sites/ajherrington/2022/06/24/va-studying-psychedelics-as-mental-health-treatment-for-veterans/?sh=149266f6c0d4
19. Search of: Veterans: Ketamine - list results. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=ketamine&term=veterans&cntry=&state=&city=&dist=. Accessed March 23, 2023.
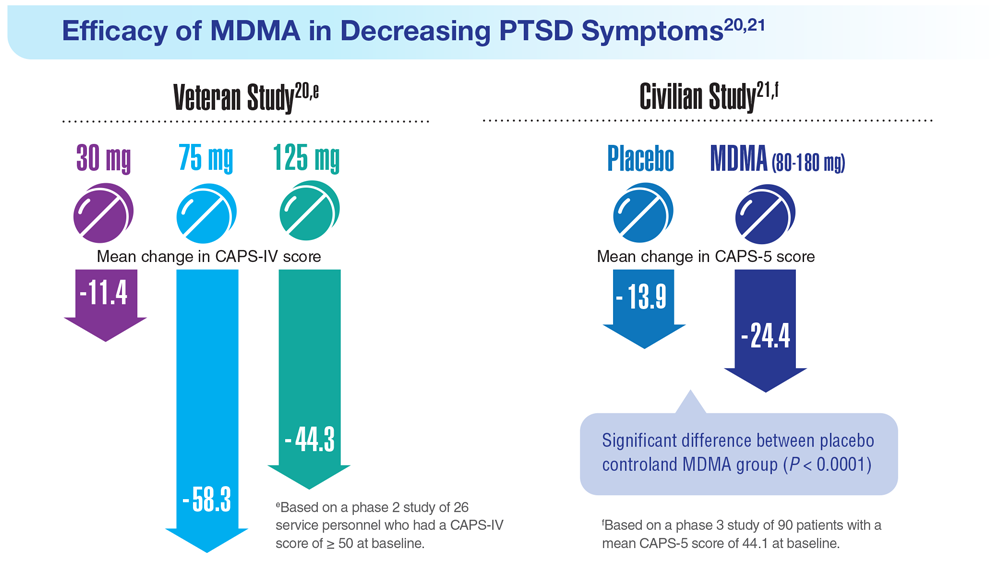
20. Mithoefer MC et al. Lancet Psychiatry. 2018;5(6):486-497. doi:10.1016/S2215-0366(18)30135-4
21. Mitchell JM et al. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
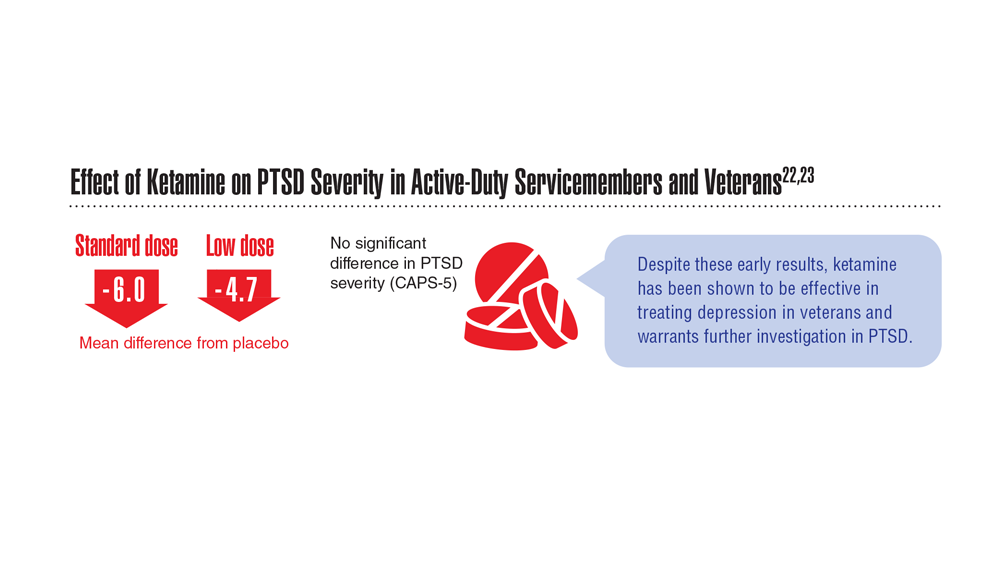
22. Abdallah CG et al. Neuropsychopharmacology. 2022;47(8):1574-1581. doi:10.1038/s41386-022-01266-9
23. Artin H et al. EClinicalMedicine. 2022;48:101439. doi:10.1016/j.eclinm.2022.101439
15. US Department of Veterans Affairs. How common is PTSD in veterans? Updated February 3, 2023. Accessed April 21, 2023. https://www.ptsd.va.gov/understand/common/common_veterans.asp
16. Murphy D, Smith KV. J Trauma Stress. 2018;31(5):753-763. doi:10.1002/jts.22333
17. Gray JC et al. Mil Med. 2022;usac400. doi:10.1093/milmed/usac400
18. Herrington AJ. VA studying psychedelics as mental health treatment for veterans. Forbes. Published June 24, 2022. Accessed April 21, 2023. https://www.forbes.com/sites/ajherrington/2022/06/24/va-studying-psychedelics-as-mental-health-treatment-for-veterans/?sh=149266f6c0d4
19. Search of: Veterans: Ketamine - list results. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=ketamine&term=veterans&cntry=&state=&city=&dist=. Accessed March 23, 2023.
20. Mithoefer MC et al. Lancet Psychiatry. 2018;5(6):486-497. doi:10.1016/S2215-0366(18)30135-4
21. Mitchell JM et al. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
22. Abdallah CG et al. Neuropsychopharmacology. 2022;47(8):1574-1581. doi:10.1038/s41386-022-01266-9
23. Artin H et al. EClinicalMedicine. 2022;48:101439. doi:10.1016/j.eclinm.2022.101439
15. US Department of Veterans Affairs. How common is PTSD in veterans? Updated February 3, 2023. Accessed April 21, 2023. https://www.ptsd.va.gov/understand/common/common_veterans.asp
16. Murphy D, Smith KV. J Trauma Stress. 2018;31(5):753-763. doi:10.1002/jts.22333
17. Gray JC et al. Mil Med. 2022;usac400. doi:10.1093/milmed/usac400
18. Herrington AJ. VA studying psychedelics as mental health treatment for veterans. Forbes. Published June 24, 2022. Accessed April 21, 2023. https://www.forbes.com/sites/ajherrington/2022/06/24/va-studying-psychedelics-as-mental-health-treatment-for-veterans/?sh=149266f6c0d4
19. Search of: Veterans: Ketamine - list results. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=ketamine&term=veterans&cntry=&state=&city=&dist=. Accessed March 23, 2023.
20. Mithoefer MC et al. Lancet Psychiatry. 2018;5(6):486-497. doi:10.1016/S2215-0366(18)30135-4
21. Mitchell JM et al. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
22. Abdallah CG et al. Neuropsychopharmacology. 2022;47(8):1574-1581. doi:10.1038/s41386-022-01266-9
23. Artin H et al. EClinicalMedicine. 2022;48:101439. doi:10.1016/j.eclinm.2022.101439
Sentinel central events prevalent during DISE for obstructive sleep apnea
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
FROM THE AAOH-HNS MEETING
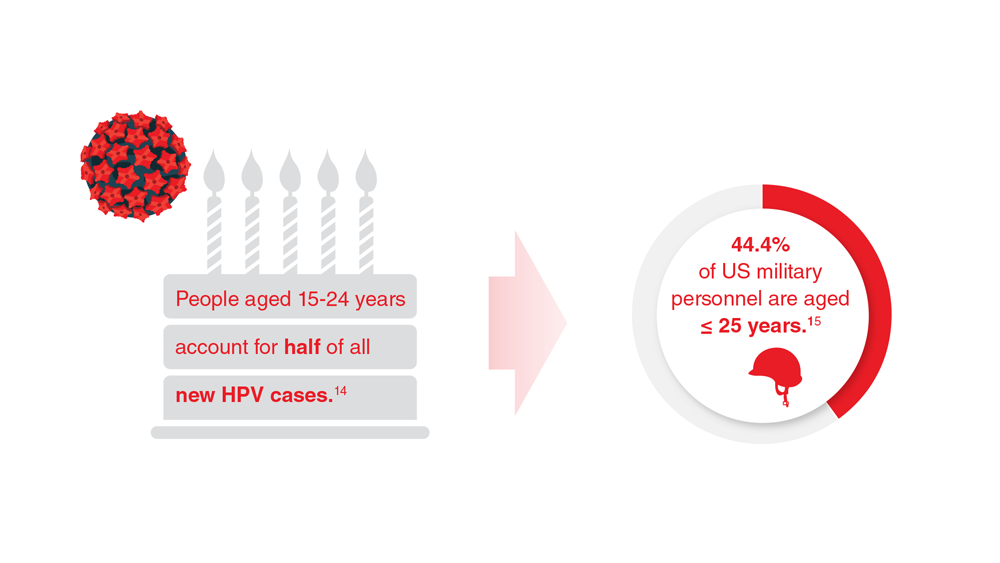
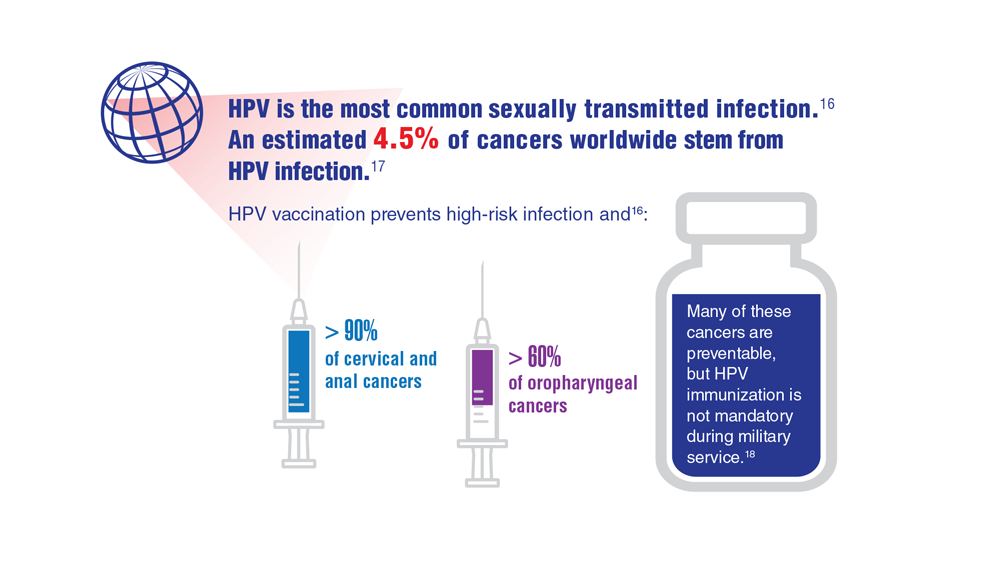
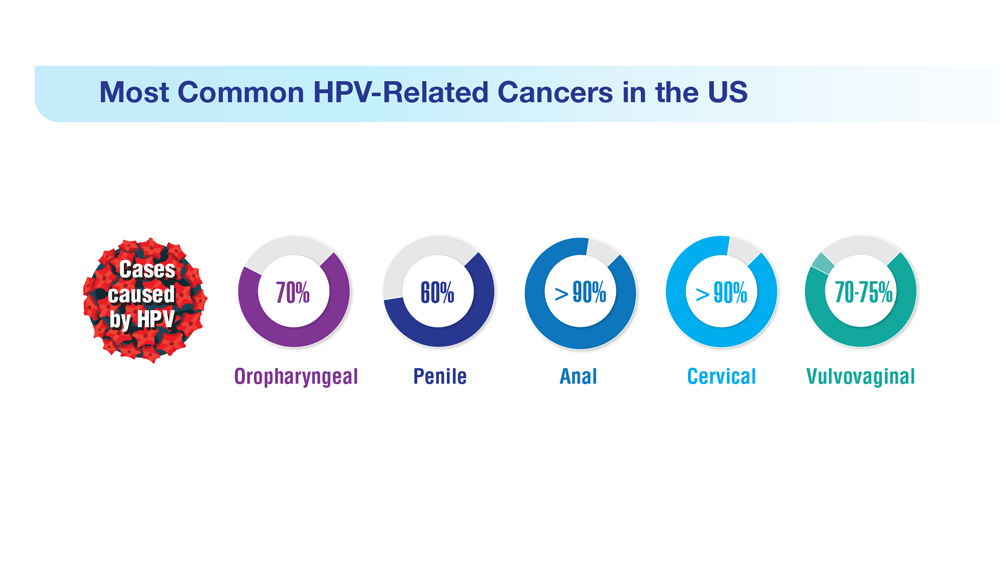
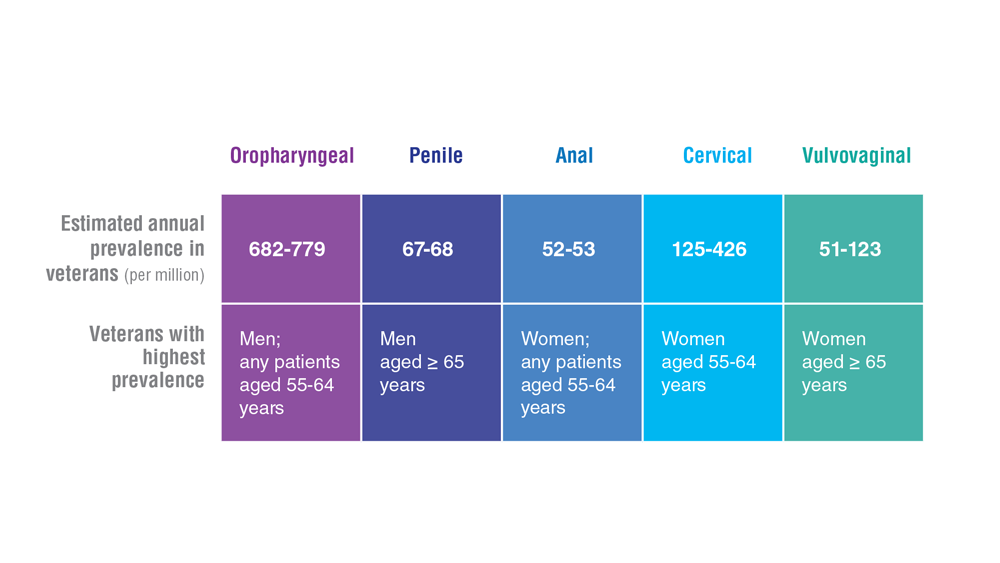
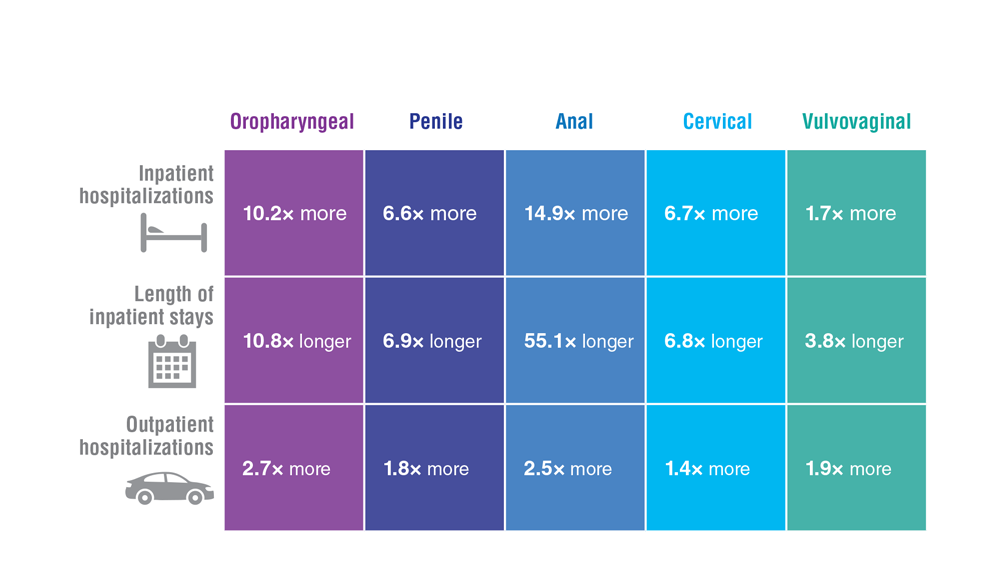
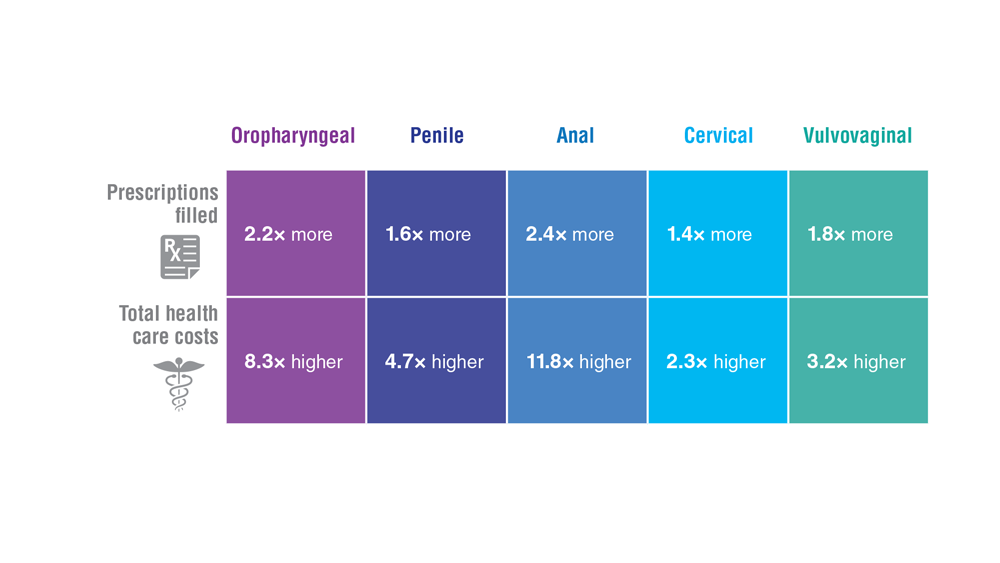
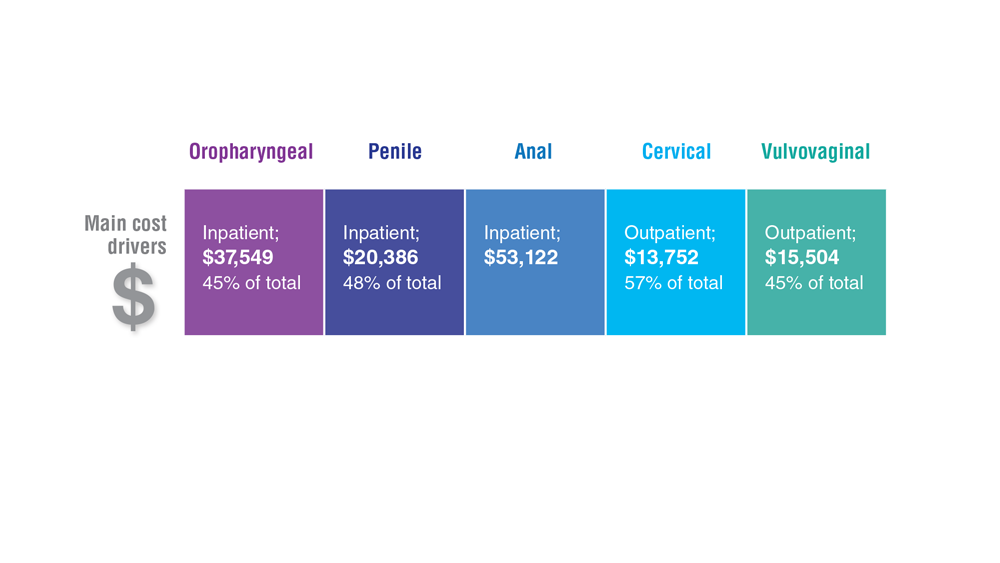
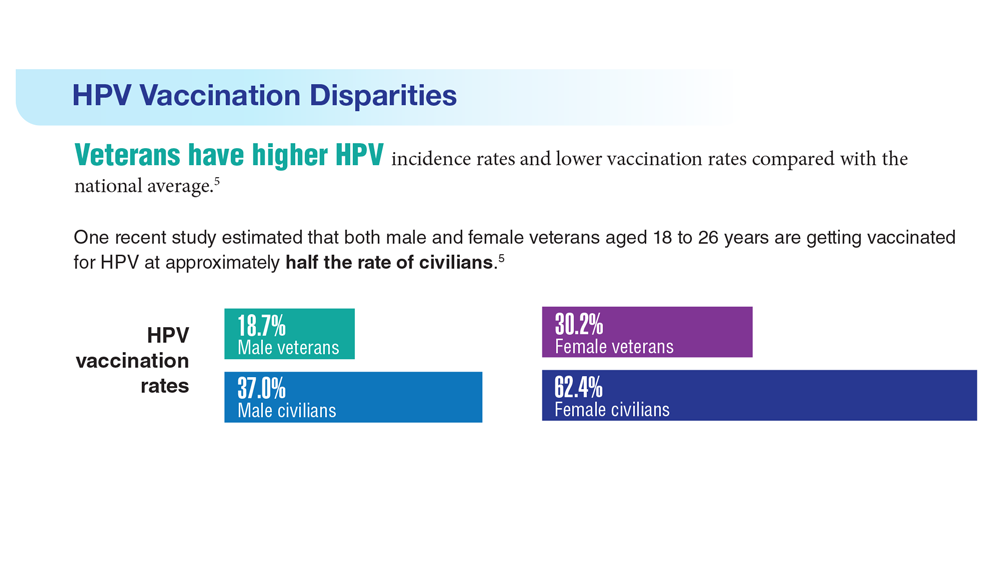
Data Trends 2023: HPV and Related Cancers
- Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018;67(33):918-924. doi:10.15585/mmwr.mm6733a2
- Nsouli-Maktabi H et al. MSMR. 2013;20(2):17-20. Published February 20, 2013. Accessed April 8, 2023. https://pubmed.ncbi.nlm.nih.gov/23461306/
- Zevallos JP et al. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
- Saxena K et al. J Med Econ. 2022;25(1):299-308. doi:10.1080/13696998.2022.2041855
- Chidambaram S et al. JAMA Oncol. 2023;e227944. doi:10.1001/jamaoncol.2022.7944
- Meites E et al. MMWR Morb Mortal Wkly Rep. 2019;68(32):698-702.
- González-Moles MÁ et al. Cancers (Basel). 2022;14(19):4967. doi:10.3390/cancers14194967
- Mazul AL et al. Cancer. 2022;128(18):3310-3318. doi:10.1002/cncr.34387
- Clark E et al. Clin Infect Dis. 2021;72(9):e359-e366. doi:10.1093/cid/ciaa1162
- Rohner E et al. Int J Cancer. 2020;146(3):601-609. doi:10.1002/ijc.32260
- Guiguet M et al. Lancet Oncol. 2009;10(12):1152-1159. doi:10.1016/S1470-2045(09)70282-7
- Abraham AG et al. J Acquir Immune Defic Syndr. 2013;62(4):405-413. doi:10.1097/QAI.0b013e31828177d7
- Massad LS et al. Am J Obstet Gynecol. 2015;212(5):606.e1-e8. doi:10.1016/j.ajog.2014.12.003
- Centers for Disease Control and Prevention. Genital HPV infection – basic fact sheet. Updated April 12, 2022. Accessed April 20, 2023. https://www.cdc.gov/std/hpv/stdfact-hpv.htm
- US Department of Defense. 2021 Demographics: profile of the military community. Accessed April 20, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2021-demographics-report.pdf
- National Cancer Institute. HPV and cancer. Updated April 4, 2023. Accessed May 4, 2023. https://www.cancer.gov/about-cancer/causesprevention/risk/infectious-agents/hpv-and-cancer
- de Martel C et al. Int J Cancer. 2017;141(4):664-670. doi:10.1002/ijc.30716
- Daly CM et al. J Community Health. 2018;43(3):441-447. doi:10.1007/s10900-017-0447-z
- Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? Updated October 3, 2022. Accessed May 4, 2023. https://www.cdc.gov/cancer/hpv/statistics/cases.htm
- Zevallos JP et al. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
- Mashberg A et al. Cancer. 1993;72(4):1369-1375. doi:10.1002/1097-0142(19930815)72:4<1369::AID-CNCR2820720436>3.0.CO;2-L
- Agha Z et al. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
- Singh JA et al. J Am Geriatr Soc. 2005;53(1):108-113. doi:10.1111/j.1532-5415.2005.53020.x
- Morgan RO et al. Health Serv Res. 2005;40(5 pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
- National Cancer Institute. Head and neck cancers. Updated May 25, 2021. Accessed May 4, 2023. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet
- Odani S et al. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
- Ames G, Cunradi C. Alcohol use and preventing alcohol-related problems among young adults in the military. Alcohol Res Health. 2004;28(4):252-257.
- Di Credico G et al. Br J Cancer. 2020;123(9):1456-1463. doi:10.1038/s41416-020-01031-z
- Centers for Disease Control and Prevention. HPV-associated cancer risks. Updated October 3, 2022. Accessed May 4, 2023. https://www.cdc.gov/cancer/hpv/statistics/index.htm
- Sandulache VC et al. Head Neck. 2015;37(9):1246-1253. doi:10.1002/hed.23740
- Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018;67(33):918-924. doi:10.15585/mmwr.mm6733a2
- Nsouli-Maktabi H et al. MSMR. 2013;20(2):17-20. Published February 20, 2013. Accessed April 8, 2023. https://pubmed.ncbi.nlm.nih.gov/23461306/
- Zevallos JP et al. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
- Saxena K et al. J Med Econ. 2022;25(1):299-308. doi:10.1080/13696998.2022.2041855
- Chidambaram S et al. JAMA Oncol. 2023;e227944. doi:10.1001/jamaoncol.2022.7944
- Meites E et al. MMWR Morb Mortal Wkly Rep. 2019;68(32):698-702.
- González-Moles MÁ et al. Cancers (Basel). 2022;14(19):4967. doi:10.3390/cancers14194967
- Mazul AL et al. Cancer. 2022;128(18):3310-3318. doi:10.1002/cncr.34387
- Clark E et al. Clin Infect Dis. 2021;72(9):e359-e366. doi:10.1093/cid/ciaa1162
- Rohner E et al. Int J Cancer. 2020;146(3):601-609. doi:10.1002/ijc.32260
- Guiguet M et al. Lancet Oncol. 2009;10(12):1152-1159. doi:10.1016/S1470-2045(09)70282-7
- Abraham AG et al. J Acquir Immune Defic Syndr. 2013;62(4):405-413. doi:10.1097/QAI.0b013e31828177d7
- Massad LS et al. Am J Obstet Gynecol. 2015;212(5):606.e1-e8. doi:10.1016/j.ajog.2014.12.003
- Centers for Disease Control and Prevention. Genital HPV infection – basic fact sheet. Updated April 12, 2022. Accessed April 20, 2023. https://www.cdc.gov/std/hpv/stdfact-hpv.htm
- US Department of Defense. 2021 Demographics: profile of the military community. Accessed April 20, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2021-demographics-report.pdf
- National Cancer Institute. HPV and cancer. Updated April 4, 2023. Accessed May 4, 2023. https://www.cancer.gov/about-cancer/causesprevention/risk/infectious-agents/hpv-and-cancer
- de Martel C et al. Int J Cancer. 2017;141(4):664-670. doi:10.1002/ijc.30716
- Daly CM et al. J Community Health. 2018;43(3):441-447. doi:10.1007/s10900-017-0447-z
- Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? Updated October 3, 2022. Accessed May 4, 2023. https://www.cdc.gov/cancer/hpv/statistics/cases.htm
- Zevallos JP et al. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
- Mashberg A et al. Cancer. 1993;72(4):1369-1375. doi:10.1002/1097-0142(19930815)72:4<1369::AID-CNCR2820720436>3.0.CO;2-L
- Agha Z et al. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
- Singh JA et al. J Am Geriatr Soc. 2005;53(1):108-113. doi:10.1111/j.1532-5415.2005.53020.x
- Morgan RO et al. Health Serv Res. 2005;40(5 pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
- National Cancer Institute. Head and neck cancers. Updated May 25, 2021. Accessed May 4, 2023. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet
- Odani S et al. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
- Ames G, Cunradi C. Alcohol use and preventing alcohol-related problems among young adults in the military. Alcohol Res Health. 2004;28(4):252-257.
- Di Credico G et al. Br J Cancer. 2020;123(9):1456-1463. doi:10.1038/s41416-020-01031-z
- Centers for Disease Control and Prevention. HPV-associated cancer risks. Updated October 3, 2022. Accessed May 4, 2023. https://www.cdc.gov/cancer/hpv/statistics/index.htm
- Sandulache VC et al. Head Neck. 2015;37(9):1246-1253. doi:10.1002/hed.23740
- Van Dyne EA et al. MMWR Morb Mortal Wkly Rep. 2018;67(33):918-924. doi:10.15585/mmwr.mm6733a2
- Nsouli-Maktabi H et al. MSMR. 2013;20(2):17-20. Published February 20, 2013. Accessed April 8, 2023. https://pubmed.ncbi.nlm.nih.gov/23461306/
- Zevallos JP et al. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
- Saxena K et al. J Med Econ. 2022;25(1):299-308. doi:10.1080/13696998.2022.2041855
- Chidambaram S et al. JAMA Oncol. 2023;e227944. doi:10.1001/jamaoncol.2022.7944
- Meites E et al. MMWR Morb Mortal Wkly Rep. 2019;68(32):698-702.
- González-Moles MÁ et al. Cancers (Basel). 2022;14(19):4967. doi:10.3390/cancers14194967
- Mazul AL et al. Cancer. 2022;128(18):3310-3318. doi:10.1002/cncr.34387
- Clark E et al. Clin Infect Dis. 2021;72(9):e359-e366. doi:10.1093/cid/ciaa1162
- Rohner E et al. Int J Cancer. 2020;146(3):601-609. doi:10.1002/ijc.32260
- Guiguet M et al. Lancet Oncol. 2009;10(12):1152-1159. doi:10.1016/S1470-2045(09)70282-7
- Abraham AG et al. J Acquir Immune Defic Syndr. 2013;62(4):405-413. doi:10.1097/QAI.0b013e31828177d7
- Massad LS et al. Am J Obstet Gynecol. 2015;212(5):606.e1-e8. doi:10.1016/j.ajog.2014.12.003
- Centers for Disease Control and Prevention. Genital HPV infection – basic fact sheet. Updated April 12, 2022. Accessed April 20, 2023. https://www.cdc.gov/std/hpv/stdfact-hpv.htm
- US Department of Defense. 2021 Demographics: profile of the military community. Accessed April 20, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2021-demographics-report.pdf
- National Cancer Institute. HPV and cancer. Updated April 4, 2023. Accessed May 4, 2023. https://www.cancer.gov/about-cancer/causesprevention/risk/infectious-agents/hpv-and-cancer
- de Martel C et al. Int J Cancer. 2017;141(4):664-670. doi:10.1002/ijc.30716
- Daly CM et al. J Community Health. 2018;43(3):441-447. doi:10.1007/s10900-017-0447-z
- Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? Updated October 3, 2022. Accessed May 4, 2023. https://www.cdc.gov/cancer/hpv/statistics/cases.htm
- Zevallos JP et al. Head Neck. 2021;43(1):108-115. doi:10.1002/hed.26465
- Mashberg A et al. Cancer. 1993;72(4):1369-1375. doi:10.1002/1097-0142(19930815)72:4<1369::AID-CNCR2820720436>3.0.CO;2-L
- Agha Z et al. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
- Singh JA et al. J Am Geriatr Soc. 2005;53(1):108-113. doi:10.1111/j.1532-5415.2005.53020.x
- Morgan RO et al. Health Serv Res. 2005;40(5 pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
- National Cancer Institute. Head and neck cancers. Updated May 25, 2021. Accessed May 4, 2023. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet
- Odani S et al. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
- Ames G, Cunradi C. Alcohol use and preventing alcohol-related problems among young adults in the military. Alcohol Res Health. 2004;28(4):252-257.
- Di Credico G et al. Br J Cancer. 2020;123(9):1456-1463. doi:10.1038/s41416-020-01031-z
- Centers for Disease Control and Prevention. HPV-associated cancer risks. Updated October 3, 2022. Accessed May 4, 2023. https://www.cdc.gov/cancer/hpv/statistics/index.htm
- Sandulache VC et al. Head Neck. 2015;37(9):1246-1253. doi:10.1002/hed.23740
Impact of Liraglutide to Semaglutide Conversion on Glycemic Control and Cost Savings at a Veterans Affairs Medical Center
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2