User login
9-valent, Quadrivalent HPV Vaccines Have Comparable Safety
The 9-valent human papillomavirus vaccine was well tolerated in 16-26 year olds and had a safety profile comparable to that of the quadrivalent vaccine, according to an analysis of seven phase III trials.
“The demonstrated efficacy and favorable safety profile of the 9vHPV vaccine support widespread vaccination programs,” reported Dr. Edson D. Moreira Jr. and his associates in Pediatrics.
Vaccines were given in a three-dose regimen: 15,776 male and female patients received at least one dose of the 9-valent vaccine and 7,391 control subjects received at least one dose of the quadrivalent vaccine.
Frequencies of vaccine-related systemic adverse events such as headache and pyrexia were “generally similar” between the two vaccine groups, the investigators reported.
Compared with the quadrivalent vaccine, the 9-valent vaccine was associated with significantly more frequent adverse events at the injection site, including pain (84% vs. 90%), swelling (29% vs. 40%), and erythema (34% vs. 26%). Most of these reactions in both groups were mild to moderate in intensity, however.
Discontinuations and vaccine-related serious adverse events in the 9-valent vaccine group were rare (0.1% and less than 0.1%, respectively).
Read the full study here (doi:10.1542/peds.2015-4387).
The 9-valent human papillomavirus vaccine was well tolerated in 16-26 year olds and had a safety profile comparable to that of the quadrivalent vaccine, according to an analysis of seven phase III trials.
“The demonstrated efficacy and favorable safety profile of the 9vHPV vaccine support widespread vaccination programs,” reported Dr. Edson D. Moreira Jr. and his associates in Pediatrics.
Vaccines were given in a three-dose regimen: 15,776 male and female patients received at least one dose of the 9-valent vaccine and 7,391 control subjects received at least one dose of the quadrivalent vaccine.
Frequencies of vaccine-related systemic adverse events such as headache and pyrexia were “generally similar” between the two vaccine groups, the investigators reported.
Compared with the quadrivalent vaccine, the 9-valent vaccine was associated with significantly more frequent adverse events at the injection site, including pain (84% vs. 90%), swelling (29% vs. 40%), and erythema (34% vs. 26%). Most of these reactions in both groups were mild to moderate in intensity, however.
Discontinuations and vaccine-related serious adverse events in the 9-valent vaccine group were rare (0.1% and less than 0.1%, respectively).
Read the full study here (doi:10.1542/peds.2015-4387).
The 9-valent human papillomavirus vaccine was well tolerated in 16-26 year olds and had a safety profile comparable to that of the quadrivalent vaccine, according to an analysis of seven phase III trials.
“The demonstrated efficacy and favorable safety profile of the 9vHPV vaccine support widespread vaccination programs,” reported Dr. Edson D. Moreira Jr. and his associates in Pediatrics.
Vaccines were given in a three-dose regimen: 15,776 male and female patients received at least one dose of the 9-valent vaccine and 7,391 control subjects received at least one dose of the quadrivalent vaccine.
Frequencies of vaccine-related systemic adverse events such as headache and pyrexia were “generally similar” between the two vaccine groups, the investigators reported.
Compared with the quadrivalent vaccine, the 9-valent vaccine was associated with significantly more frequent adverse events at the injection site, including pain (84% vs. 90%), swelling (29% vs. 40%), and erythema (34% vs. 26%). Most of these reactions in both groups were mild to moderate in intensity, however.
Discontinuations and vaccine-related serious adverse events in the 9-valent vaccine group were rare (0.1% and less than 0.1%, respectively).
Read the full study here (doi:10.1542/peds.2015-4387).
FROM PEDIATRICS
9-valent, quadrivalent HPV vaccines have comparable safety
The 9-valent human papillomavirus vaccine was well tolerated in 16-26 year olds and had a safety profile comparable to that of the quadrivalent vaccine, according to an analysis of seven phase III trials.
“The demonstrated efficacy and favorable safety profile of the 9vHPV vaccine support widespread vaccination programs,” reported Dr. Edson D. Moreira Jr. and his associates in Pediatrics.
Vaccines were given in a three-dose regimen: 15,776 male and female patients received at least one dose of the 9-valent vaccine and 7,391 control subjects received at least one dose of the quadrivalent vaccine.
Frequencies of vaccine-related systemic adverse events such as headache and pyrexia were “generally similar” between the two vaccine groups, the investigators reported.
Compared with the quadrivalent vaccine, the 9-valent vaccine was associated with significantly more frequent adverse events at the injection site, including pain (84% vs. 90%), swelling (29% vs. 40%), and erythema (34% vs. 26%). Most of these reactions in both groups were mild to moderate in intensity, however.
Discontinuations and vaccine-related serious adverse events in the 9-valent vaccine group were rare (0.1% and less than 0.1%, respectively).
Read the full study here (doi:10.1542/peds.2015-4387).
The 9-valent human papillomavirus vaccine was well tolerated in 16-26 year olds and had a safety profile comparable to that of the quadrivalent vaccine, according to an analysis of seven phase III trials.
“The demonstrated efficacy and favorable safety profile of the 9vHPV vaccine support widespread vaccination programs,” reported Dr. Edson D. Moreira Jr. and his associates in Pediatrics.
Vaccines were given in a three-dose regimen: 15,776 male and female patients received at least one dose of the 9-valent vaccine and 7,391 control subjects received at least one dose of the quadrivalent vaccine.
Frequencies of vaccine-related systemic adverse events such as headache and pyrexia were “generally similar” between the two vaccine groups, the investigators reported.
Compared with the quadrivalent vaccine, the 9-valent vaccine was associated with significantly more frequent adverse events at the injection site, including pain (84% vs. 90%), swelling (29% vs. 40%), and erythema (34% vs. 26%). Most of these reactions in both groups were mild to moderate in intensity, however.
Discontinuations and vaccine-related serious adverse events in the 9-valent vaccine group were rare (0.1% and less than 0.1%, respectively).
Read the full study here (doi:10.1542/peds.2015-4387).
The 9-valent human papillomavirus vaccine was well tolerated in 16-26 year olds and had a safety profile comparable to that of the quadrivalent vaccine, according to an analysis of seven phase III trials.
“The demonstrated efficacy and favorable safety profile of the 9vHPV vaccine support widespread vaccination programs,” reported Dr. Edson D. Moreira Jr. and his associates in Pediatrics.
Vaccines were given in a three-dose regimen: 15,776 male and female patients received at least one dose of the 9-valent vaccine and 7,391 control subjects received at least one dose of the quadrivalent vaccine.
Frequencies of vaccine-related systemic adverse events such as headache and pyrexia were “generally similar” between the two vaccine groups, the investigators reported.
Compared with the quadrivalent vaccine, the 9-valent vaccine was associated with significantly more frequent adverse events at the injection site, including pain (84% vs. 90%), swelling (29% vs. 40%), and erythema (34% vs. 26%). Most of these reactions in both groups were mild to moderate in intensity, however.
Discontinuations and vaccine-related serious adverse events in the 9-valent vaccine group were rare (0.1% and less than 0.1%, respectively).
Read the full study here (doi:10.1542/peds.2015-4387).
FROM PEDIATRICS
Surgery for acute type A dissection shows 20-year shift to valve sparing, biological valves
NEW YORK – A study of an international database of individuals who have had open repair for acute type A aortic dissection (ATAAD) has revealed that in the past 20 years, cardiovascular surgeons have widely embraced valve-sparing procedures, bioprosthetic valves, and cerebral profusion strategies, according to a report here on the latest analysis of the database.
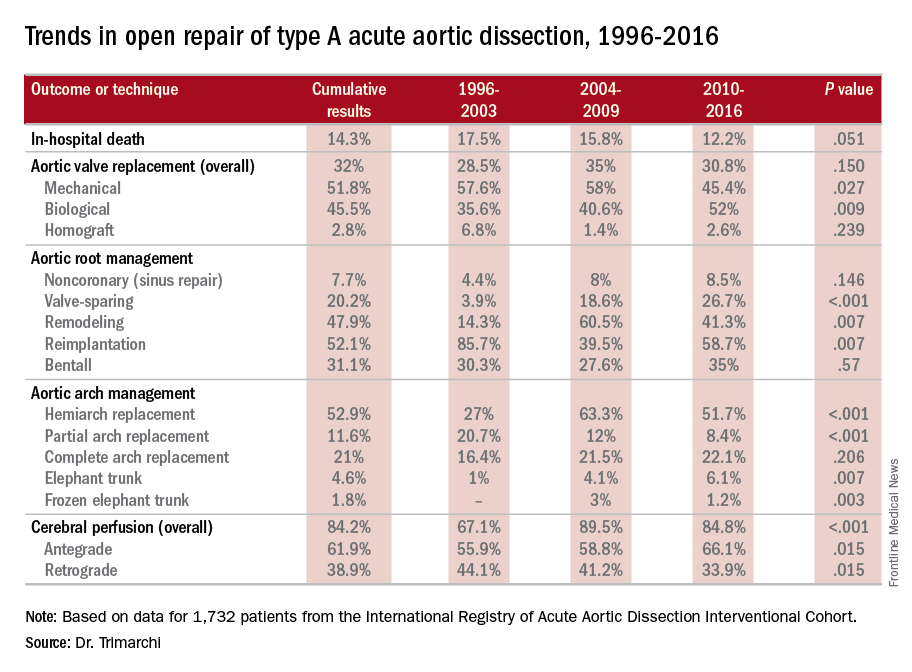
The most telling result is the decline in overall mortality, Santi Trimarchi, MD, PhD, of the University of Milan IRCCS Policlinico San Donato in Italy reported on behalf of the International Registry of Acute Aortic Dissection (IRAD) Interventional Cohort (IVC). The cohort analyzed surgery techniques and outcomes of 1,732 patients who had open repair from 1996 to 2016, clustering results in three time intervals: 1996-2003; 2004-2009; and 2010-2015.
“We noted in the registry that the overall in-hospital mortality rate was 14.3%, and this mortality decreased over time from 17.5% in the first six-year time span to 12.2% in the last six years,” Dr. Trimarchi said.

Among other trends the study identified are greater reliance on biological vs. mechanical valves, an increase in valve-sparing procedures, and steady use of Bentall procedures throughout the study period. “Operative techniques for redo aortic valve repair have been improving over the time, and that’s why we see more frequent use of biologic valves,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
“Cerebral profusion management has been widely adopted,” Dr. Trimarchi said. “Also there is an important trend showing an increasing utilization of antegrade cerebral profusion while we see a negative trend of the utilization of retrograde brain protection.”
Dr. Trimarchi attributed the detail the study generated to the survey form sent to the 26 IRAD-IVC sites around the world. The form measures 131 different variables, he said.
“Using this new specific surgical data form, we think we can address some surgical issues and report better data from the IRAD registry results on acute dissection,” he said. “These analyses have shown there have been significant changes in operative strategy over time in terms of managing such patients, and more importantly, a significant decrease in in-hospital mortality was observed in a 20-year time period.”
Dr. Trimarchi disclosed that he has received speaking and consulting fees and research support from W.L. Gore & Associates and Medtronic. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
NEW YORK – A study of an international database of individuals who have had open repair for acute type A aortic dissection (ATAAD) has revealed that in the past 20 years, cardiovascular surgeons have widely embraced valve-sparing procedures, bioprosthetic valves, and cerebral profusion strategies, according to a report here on the latest analysis of the database.
The most telling result is the decline in overall mortality, Santi Trimarchi, MD, PhD, of the University of Milan IRCCS Policlinico San Donato in Italy reported on behalf of the International Registry of Acute Aortic Dissection (IRAD) Interventional Cohort (IVC). The cohort analyzed surgery techniques and outcomes of 1,732 patients who had open repair from 1996 to 2016, clustering results in three time intervals: 1996-2003; 2004-2009; and 2010-2015.
“We noted in the registry that the overall in-hospital mortality rate was 14.3%, and this mortality decreased over time from 17.5% in the first six-year time span to 12.2% in the last six years,” Dr. Trimarchi said.

Among other trends the study identified are greater reliance on biological vs. mechanical valves, an increase in valve-sparing procedures, and steady use of Bentall procedures throughout the study period. “Operative techniques for redo aortic valve repair have been improving over the time, and that’s why we see more frequent use of biologic valves,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
“Cerebral profusion management has been widely adopted,” Dr. Trimarchi said. “Also there is an important trend showing an increasing utilization of antegrade cerebral profusion while we see a negative trend of the utilization of retrograde brain protection.”
Dr. Trimarchi attributed the detail the study generated to the survey form sent to the 26 IRAD-IVC sites around the world. The form measures 131 different variables, he said.
“Using this new specific surgical data form, we think we can address some surgical issues and report better data from the IRAD registry results on acute dissection,” he said. “These analyses have shown there have been significant changes in operative strategy over time in terms of managing such patients, and more importantly, a significant decrease in in-hospital mortality was observed in a 20-year time period.”
Dr. Trimarchi disclosed that he has received speaking and consulting fees and research support from W.L. Gore & Associates and Medtronic. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
NEW YORK – A study of an international database of individuals who have had open repair for acute type A aortic dissection (ATAAD) has revealed that in the past 20 years, cardiovascular surgeons have widely embraced valve-sparing procedures, bioprosthetic valves, and cerebral profusion strategies, according to a report here on the latest analysis of the database.
The most telling result is the decline in overall mortality, Santi Trimarchi, MD, PhD, of the University of Milan IRCCS Policlinico San Donato in Italy reported on behalf of the International Registry of Acute Aortic Dissection (IRAD) Interventional Cohort (IVC). The cohort analyzed surgery techniques and outcomes of 1,732 patients who had open repair from 1996 to 2016, clustering results in three time intervals: 1996-2003; 2004-2009; and 2010-2015.
“We noted in the registry that the overall in-hospital mortality rate was 14.3%, and this mortality decreased over time from 17.5% in the first six-year time span to 12.2% in the last six years,” Dr. Trimarchi said.

Among other trends the study identified are greater reliance on biological vs. mechanical valves, an increase in valve-sparing procedures, and steady use of Bentall procedures throughout the study period. “Operative techniques for redo aortic valve repair have been improving over the time, and that’s why we see more frequent use of biologic valves,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
“Cerebral profusion management has been widely adopted,” Dr. Trimarchi said. “Also there is an important trend showing an increasing utilization of antegrade cerebral profusion while we see a negative trend of the utilization of retrograde brain protection.”
Dr. Trimarchi attributed the detail the study generated to the survey form sent to the 26 IRAD-IVC sites around the world. The form measures 131 different variables, he said.
“Using this new specific surgical data form, we think we can address some surgical issues and report better data from the IRAD registry results on acute dissection,” he said. “These analyses have shown there have been significant changes in operative strategy over time in terms of managing such patients, and more importantly, a significant decrease in in-hospital mortality was observed in a 20-year time period.”
Dr. Trimarchi disclosed that he has received speaking and consulting fees and research support from W.L. Gore & Associates and Medtronic. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
AT AATS AORTIC SYMPOSIUM 2016
Key clinical point: Operations for acute type A aortic dissection (ATAAD) have seen significant changes in technique over the past 20 years.
Major finding: Use of biological valves increased from 35.6% of procedures to 52% over the study period while reliance of mechanical valves declined from 57.6% to 45.4%.
Data source: Interventional Cohort database of 1,732 patients enrolled in the International Registry of Acute Aortic Dissection database who had open surgery for ATAAD from February 1996 to March 2015.
Disclosures: Dr. Trimarchi disclosed having receive speaking and consulting fees from W.L. Gore & Associates and Medtronic as well as research support from the two companies. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
Teen birth rate continues to decline
The U.S teen birth rate has dropped for another consecutive year, adding to the long-term decline in teen pregnancy, according to a federal report on trends in child health and well being.
In 2014, the teen birth rate was 11 births per 1,000 girls’ aged 15-17 years, down from 12 per 1,000 in 2013. Racial and ethinic disparities in the teen birth rate have also dropped significantly since 1995 – with the difference between the highest and lowest rates dropping from 55 points in 1995 to 17 points in 2014. But substantial disparities persist.
The report also found that the percentages of 10th and 12th-graders in all racial and ethnic groups who binge-drink were the lowest in 2015 since the report started in 1980. The percentage of uninsured children also declined, falling from 7% in 2013 to 5% in 2014. However, there was no improvement in the rate of childhood obesity. During 2011-2014, 19% of children aged 6-17 years were obese.
The annual report is published by the Federal Interagency Forum on Child and Family Statistics, a working group of 23 federal agencies that collect, analyze and report data on conditions and trends related to child and family well-being. The report tracks 41 health and social indicators.
Read the full 2016 America’s Children Report here.
The U.S teen birth rate has dropped for another consecutive year, adding to the long-term decline in teen pregnancy, according to a federal report on trends in child health and well being.
In 2014, the teen birth rate was 11 births per 1,000 girls’ aged 15-17 years, down from 12 per 1,000 in 2013. Racial and ethinic disparities in the teen birth rate have also dropped significantly since 1995 – with the difference between the highest and lowest rates dropping from 55 points in 1995 to 17 points in 2014. But substantial disparities persist.
The report also found that the percentages of 10th and 12th-graders in all racial and ethnic groups who binge-drink were the lowest in 2015 since the report started in 1980. The percentage of uninsured children also declined, falling from 7% in 2013 to 5% in 2014. However, there was no improvement in the rate of childhood obesity. During 2011-2014, 19% of children aged 6-17 years were obese.
The annual report is published by the Federal Interagency Forum on Child and Family Statistics, a working group of 23 federal agencies that collect, analyze and report data on conditions and trends related to child and family well-being. The report tracks 41 health and social indicators.
Read the full 2016 America’s Children Report here.
The U.S teen birth rate has dropped for another consecutive year, adding to the long-term decline in teen pregnancy, according to a federal report on trends in child health and well being.
In 2014, the teen birth rate was 11 births per 1,000 girls’ aged 15-17 years, down from 12 per 1,000 in 2013. Racial and ethinic disparities in the teen birth rate have also dropped significantly since 1995 – with the difference between the highest and lowest rates dropping from 55 points in 1995 to 17 points in 2014. But substantial disparities persist.
The report also found that the percentages of 10th and 12th-graders in all racial and ethnic groups who binge-drink were the lowest in 2015 since the report started in 1980. The percentage of uninsured children also declined, falling from 7% in 2013 to 5% in 2014. However, there was no improvement in the rate of childhood obesity. During 2011-2014, 19% of children aged 6-17 years were obese.
The annual report is published by the Federal Interagency Forum on Child and Family Statistics, a working group of 23 federal agencies that collect, analyze and report data on conditions and trends related to child and family well-being. The report tracks 41 health and social indicators.
Read the full 2016 America’s Children Report here.
Study quantifies volume disparities for ATAD repair in the U.K.
NEW YORK – Mastery is the product of repetition, and it has long been taken for granted that surgeons and centers that perform a high volume of an operation will have better results than those who don’t do the operation as often, but a study out of the United Kingdom has determined just how much better high-volume centers are when it comes to repair of acute type A aortic dissection (ATAD) – and what the in-hospital mortality odds ratio is for lower-volume surgeons.
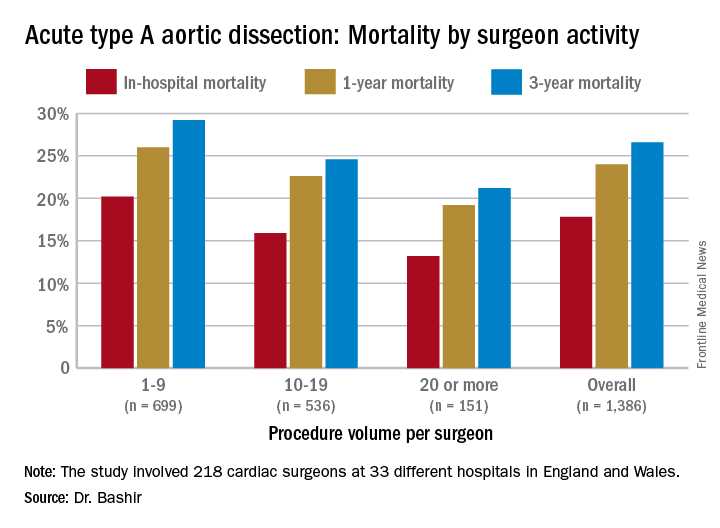
Specifically, that odds ratio is 1.64 (P = .030), Mohamad Bashir, MD, PhD, MRCS, a research fellow at Liverpool Heart and Chest Hospital, said in reporting early results of the study here. Lower-volume surgeons had worse outcomes in 12 of 14 different operative metrics the study evaluated, most notably in-hospital mortality: 20.2% for lower-volume surgeons vs. 15.2% for higher-volume surgeons. “There is an initiative in the U.K. to change the trend,” Dr. Bashir said. Full study results will be published in an upcoming issue of BMJ, he said.
“In-hospital mortality for surgeons who operate on 20 or more procedures is very good at 13.2%, and the same follows for 90-day mortality, one-year mortality and three-year mortality,” Dr. Bashir said.
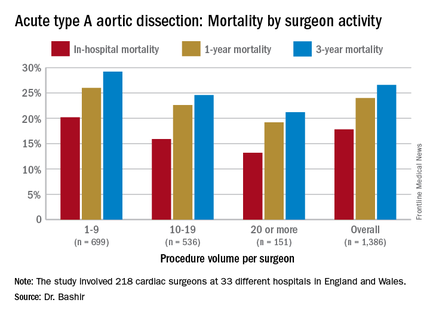
The study evaluated 1,386 ATAD procedures in the National Institute for Cardiovascular Outcomes Research database by 218 different cardiac surgeons at 33 different hospitals in England and Wales from April 2007 to March 2013. That would make the average number of procedures per surgeon 6.4, Dr. Bashir said, but a closer look at each surgeon’s case load reveals some disconcerting trends: almost 80% of the surgeons performed fewer than 10 ATAD repairs in the 6-year span of the study, and 34 surgeons, or about 15%, just did a single procedure in that time. The highest-volume surgeon did 32 procedures. The minimum hospital volume was 8 ATAD operations and the maximum was 103.
The study stratified lower- and higher-volume surgeon groups by characteristics of the patients they operated on. “The differences between these two groups are pretty interesting because we noticed that the lower-volume surgeons are actually operating on patients who are diabetic, who are smokers, who use inotropic support prior to anesthesia and who also have an injection fraction that is significant,” Dr. Bashir said.
In drilling down into those characteristics, people with diabetes made up 6% of the lower-volume surgeons’ cases vs. 3.1% of the higher-volume surgeons’ cases, despite an almost 50-50 split in share of procedures between the two surgeon groups. Current smokers comprised 20.5% of the lower-volume surgeons’ patients vs. 15.5% of their high-volume counterparts’ patients. Operative characteristics in terms of urgency of surgery were similar between the two groups. However Dr. Bashir noted, lower-volume surgeons had longer times for cardiopulmonary bypass, aortic cross-clamping, and circulatory arrest.
The study investigators applied a multivariable logistic regression model to determine predictors of in-hospital mortality for ATAD. “The odds ratio (OR) of mortality for lower-volume surgeons is 1.64, which is statistically significant,” Dr. Bashir said. Odds ratios for other predictors are: previous cardiac surgery, 2.51; peripheral vascular disease, 2.15; preoperative cardiogenic shock, 2.05; salvage operation, 5.57; and concomitant coronary artery bypass procedure, 2.98. For 5-year mortality, the odds ratio was 1.37 for the lower-volume surgeons.
Dr. Bashir laid out how the National Health Service can use the study results. “Concentration of expertise and volume to the appropriate surgeons and centers who perform increasingly more work and more complex aortic cases would be required to change the paradigm of acute type A aortic dissection outcomes in the U.K.,” he said. “It is reasonable to suggest that there should be a national standardization mandate and a quality-improvement framework of acute aortic dissection treatment.”
Dr. Bashir had no financial relationships to disclose.
NEW YORK – Mastery is the product of repetition, and it has long been taken for granted that surgeons and centers that perform a high volume of an operation will have better results than those who don’t do the operation as often, but a study out of the United Kingdom has determined just how much better high-volume centers are when it comes to repair of acute type A aortic dissection (ATAD) – and what the in-hospital mortality odds ratio is for lower-volume surgeons.
Specifically, that odds ratio is 1.64 (P = .030), Mohamad Bashir, MD, PhD, MRCS, a research fellow at Liverpool Heart and Chest Hospital, said in reporting early results of the study here. Lower-volume surgeons had worse outcomes in 12 of 14 different operative metrics the study evaluated, most notably in-hospital mortality: 20.2% for lower-volume surgeons vs. 15.2% for higher-volume surgeons. “There is an initiative in the U.K. to change the trend,” Dr. Bashir said. Full study results will be published in an upcoming issue of BMJ, he said.
“In-hospital mortality for surgeons who operate on 20 or more procedures is very good at 13.2%, and the same follows for 90-day mortality, one-year mortality and three-year mortality,” Dr. Bashir said.

The study evaluated 1,386 ATAD procedures in the National Institute for Cardiovascular Outcomes Research database by 218 different cardiac surgeons at 33 different hospitals in England and Wales from April 2007 to March 2013. That would make the average number of procedures per surgeon 6.4, Dr. Bashir said, but a closer look at each surgeon’s case load reveals some disconcerting trends: almost 80% of the surgeons performed fewer than 10 ATAD repairs in the 6-year span of the study, and 34 surgeons, or about 15%, just did a single procedure in that time. The highest-volume surgeon did 32 procedures. The minimum hospital volume was 8 ATAD operations and the maximum was 103.
The study stratified lower- and higher-volume surgeon groups by characteristics of the patients they operated on. “The differences between these two groups are pretty interesting because we noticed that the lower-volume surgeons are actually operating on patients who are diabetic, who are smokers, who use inotropic support prior to anesthesia and who also have an injection fraction that is significant,” Dr. Bashir said.
In drilling down into those characteristics, people with diabetes made up 6% of the lower-volume surgeons’ cases vs. 3.1% of the higher-volume surgeons’ cases, despite an almost 50-50 split in share of procedures between the two surgeon groups. Current smokers comprised 20.5% of the lower-volume surgeons’ patients vs. 15.5% of their high-volume counterparts’ patients. Operative characteristics in terms of urgency of surgery were similar between the two groups. However Dr. Bashir noted, lower-volume surgeons had longer times for cardiopulmonary bypass, aortic cross-clamping, and circulatory arrest.
The study investigators applied a multivariable logistic regression model to determine predictors of in-hospital mortality for ATAD. “The odds ratio (OR) of mortality for lower-volume surgeons is 1.64, which is statistically significant,” Dr. Bashir said. Odds ratios for other predictors are: previous cardiac surgery, 2.51; peripheral vascular disease, 2.15; preoperative cardiogenic shock, 2.05; salvage operation, 5.57; and concomitant coronary artery bypass procedure, 2.98. For 5-year mortality, the odds ratio was 1.37 for the lower-volume surgeons.
Dr. Bashir laid out how the National Health Service can use the study results. “Concentration of expertise and volume to the appropriate surgeons and centers who perform increasingly more work and more complex aortic cases would be required to change the paradigm of acute type A aortic dissection outcomes in the U.K.,” he said. “It is reasonable to suggest that there should be a national standardization mandate and a quality-improvement framework of acute aortic dissection treatment.”
Dr. Bashir had no financial relationships to disclose.
NEW YORK – Mastery is the product of repetition, and it has long been taken for granted that surgeons and centers that perform a high volume of an operation will have better results than those who don’t do the operation as often, but a study out of the United Kingdom has determined just how much better high-volume centers are when it comes to repair of acute type A aortic dissection (ATAD) – and what the in-hospital mortality odds ratio is for lower-volume surgeons.
Specifically, that odds ratio is 1.64 (P = .030), Mohamad Bashir, MD, PhD, MRCS, a research fellow at Liverpool Heart and Chest Hospital, said in reporting early results of the study here. Lower-volume surgeons had worse outcomes in 12 of 14 different operative metrics the study evaluated, most notably in-hospital mortality: 20.2% for lower-volume surgeons vs. 15.2% for higher-volume surgeons. “There is an initiative in the U.K. to change the trend,” Dr. Bashir said. Full study results will be published in an upcoming issue of BMJ, he said.
“In-hospital mortality for surgeons who operate on 20 or more procedures is very good at 13.2%, and the same follows for 90-day mortality, one-year mortality and three-year mortality,” Dr. Bashir said.

The study evaluated 1,386 ATAD procedures in the National Institute for Cardiovascular Outcomes Research database by 218 different cardiac surgeons at 33 different hospitals in England and Wales from April 2007 to March 2013. That would make the average number of procedures per surgeon 6.4, Dr. Bashir said, but a closer look at each surgeon’s case load reveals some disconcerting trends: almost 80% of the surgeons performed fewer than 10 ATAD repairs in the 6-year span of the study, and 34 surgeons, or about 15%, just did a single procedure in that time. The highest-volume surgeon did 32 procedures. The minimum hospital volume was 8 ATAD operations and the maximum was 103.
The study stratified lower- and higher-volume surgeon groups by characteristics of the patients they operated on. “The differences between these two groups are pretty interesting because we noticed that the lower-volume surgeons are actually operating on patients who are diabetic, who are smokers, who use inotropic support prior to anesthesia and who also have an injection fraction that is significant,” Dr. Bashir said.
In drilling down into those characteristics, people with diabetes made up 6% of the lower-volume surgeons’ cases vs. 3.1% of the higher-volume surgeons’ cases, despite an almost 50-50 split in share of procedures between the two surgeon groups. Current smokers comprised 20.5% of the lower-volume surgeons’ patients vs. 15.5% of their high-volume counterparts’ patients. Operative characteristics in terms of urgency of surgery were similar between the two groups. However Dr. Bashir noted, lower-volume surgeons had longer times for cardiopulmonary bypass, aortic cross-clamping, and circulatory arrest.
The study investigators applied a multivariable logistic regression model to determine predictors of in-hospital mortality for ATAD. “The odds ratio (OR) of mortality for lower-volume surgeons is 1.64, which is statistically significant,” Dr. Bashir said. Odds ratios for other predictors are: previous cardiac surgery, 2.51; peripheral vascular disease, 2.15; preoperative cardiogenic shock, 2.05; salvage operation, 5.57; and concomitant coronary artery bypass procedure, 2.98. For 5-year mortality, the odds ratio was 1.37 for the lower-volume surgeons.
Dr. Bashir laid out how the National Health Service can use the study results. “Concentration of expertise and volume to the appropriate surgeons and centers who perform increasingly more work and more complex aortic cases would be required to change the paradigm of acute type A aortic dissection outcomes in the U.K.,” he said. “It is reasonable to suggest that there should be a national standardization mandate and a quality-improvement framework of acute aortic dissection treatment.”
Dr. Bashir had no financial relationships to disclose.
AT THE AMERICAN ASSOCIATION FOR THORACIC SURGERY AORTIC SYMPOSIUM
Key clinical point: Patients undergoing repair of acute type A aortic dissection (ATAD) by lower-volume surgeons have high mortality in comparison with those undergoing repair by the highest-volume surgeons.
Major finding: In-hospital mortality for ATAD repair was 20.2% for lower-volume surgeons and 15.3% for higher-volume surgeons.
Data source: Analysis of 1,386 ATAD procedures from April 2007 to March 2013 in the National Institute for Cardiovascular Outcomes Research data.
Disclosures: Dr. Bashir reported having no financial disclosures.
Task force affirms value of colorectal cancer screening
Colorectal cancer screening by a variety of methods is worthwhile and recommended for all adults aged 50-75 years, according to the latest recommendations from the U.S. Preventive Services Task Force. The USPSTF statement and summary of evidence were published in JAMA on June 15.
In addition, the USPSTF recommended selective screening for older adults aged 76-85 years, depending on their health status and screening history.
A team of researchers led by Dr. Jennifer S. Lin of Kaiser Permanente in Portland, Ore., reviewed studies on colorectal cancer screening published between Jan.1, 2008, and Dec. 31, 2014, with surveillance continuing through Feb. 23, 2016 (JAMA. 2016 Jun;315:2576-94 [doi: 10.1001/jama.2016.3332]). The USPSTF’s last recommendations on colorectal cancer screening were released in 2008.
“Although CRC screening has a large body of supporting evidence, additional research is still needed to weigh the relative benefits and harms of each test within a program of screening” for average-risk adults, the researchers noted.
The final recommendation statement includes three screening options that carry over from the 2008 guidelines: colonoscopy, sigmoidoscopy, and fecal immunochemical testing (FIT) with occult blood.
Other options now recommended include computed tomographic colonography (CTC), fecal immunochemical tests with DNA (FIT-DNA), guaiac-based fecal occult blood testing (gFOBT), and sigmoidoscopy plus FIT.
Some highlights from the analysis: Four randomized trials including 458,002 patients showed that one-time or two-time screening with flexible sigmoidoscopy was associated with decreased mortality from colorectal cancer, compared with no screening, for an incidence rate ratio of 0.73, the researchers wrote.
In addition, the researchers found that CTC had 73%-98% sensitivity and 89%-91% specificity to detect adenomas 6 mm and larger, compared with colonoscopy in seven studies. However, the risk of harm from low-dose ionizing radiation remains a consideration.
For diagnostic accuracy, colonoscopy showed per-person sensitivity of 89%-98% for adenomas 10 mm or larger, and 75%-93% for adenomas 6 mm or larger, in studies comparing it with CTC or as an adjunct to CTC. However, studies showing applicability to community practices were limited.
Fecal immunochemical tests (FITs) showed sensitivity ranging from 73% to 88% and specificity from 90% to 96%.
Data from five randomized, controlled trials evaluating multiple rounds of biennial screening using gFOBT showed a significant reduction in colorectal cancer mortality, from a relative risk of 0.91 at 19.5 years to a relative risk of 0.78 at 30 years.
Colonoscopy remains the standard by which other tests are assessed, although it has the highest risk of procedural complications, the researchers said. Three new randomized, controlled trials involving screening colonoscopy in average-risk adults scheduled for completion in 2021, 2026, and 2027, may yield more information on incidence and mortality, they added.
The evidence report and review was limited by its focus on average-risk adults; it did not address factors including screening for high-risk adults, availability and access to tests, potential risks of overdiagnosis, and overuse of screening after adenoma detection, the researchers said. In addition, “data are still needed on the differential uptake of and adherence to screening modalities and on continued adherence to repeated rounds of screening and diagnostic follow-up to screening over longer periods,” they said. However, they concluded, “colonoscopy, flexible sigmoidoscopy, CTC, and various stool tests have differing levels of evidence to support their use in CRC screening, ability to detect CRC and precursor lesions, and risk of serious adverse events in average-risk adults.”
The researchers had no relevant financial conflicts to disclose. The research was supported by the Agency for Healthcare Research and Quality under a contract with the U.S. Preventive Services Task Force.
The final recommendation statement is available online at USPSTF.
The recommendation puts emphasis on shared decision making between doctors and patients but does not provide much guidance on how to do it. Few, if any, practices would offer all of the screening tests and let patients choose. More commonly, a practice may offer one or two of the recommended options, and if patients do not accept those recommendations, would move on to other options.

|
Dr. David Lieberman |
Also missing from this document is a discussion of colon cancer prevention and screening. Data are provided in the supplements with regard to reduction in colon cancer incidence but not discussed in the text of the guideline. This could be an important element of shared decision making. Some patients may be willing to accept an option that is more likely to prevent cancer and avert the cost and morbidity of cancer care, even if it means more lifetime colonoscopies.
There is little discussion in the document about screening program quality. Programs that involve several steps may have issues with adherence, which may reduce effectiveness. Therefore, quality metrics ideally should be measured for all screening programs to ensure high-quality delivery of the program and adherence to the programmatic steps.
Dr. David Lieberman is vice president of the AGA Institute and chief, division of gastroenterology and hepatology, Oregon Health and Science University, Portland.
The recommendation puts emphasis on shared decision making between doctors and patients but does not provide much guidance on how to do it. Few, if any, practices would offer all of the screening tests and let patients choose. More commonly, a practice may offer one or two of the recommended options, and if patients do not accept those recommendations, would move on to other options.

|
Dr. David Lieberman |
Also missing from this document is a discussion of colon cancer prevention and screening. Data are provided in the supplements with regard to reduction in colon cancer incidence but not discussed in the text of the guideline. This could be an important element of shared decision making. Some patients may be willing to accept an option that is more likely to prevent cancer and avert the cost and morbidity of cancer care, even if it means more lifetime colonoscopies.
There is little discussion in the document about screening program quality. Programs that involve several steps may have issues with adherence, which may reduce effectiveness. Therefore, quality metrics ideally should be measured for all screening programs to ensure high-quality delivery of the program and adherence to the programmatic steps.
Dr. David Lieberman is vice president of the AGA Institute and chief, division of gastroenterology and hepatology, Oregon Health and Science University, Portland.
The recommendation puts emphasis on shared decision making between doctors and patients but does not provide much guidance on how to do it. Few, if any, practices would offer all of the screening tests and let patients choose. More commonly, a practice may offer one or two of the recommended options, and if patients do not accept those recommendations, would move on to other options.

|
Dr. David Lieberman |
Also missing from this document is a discussion of colon cancer prevention and screening. Data are provided in the supplements with regard to reduction in colon cancer incidence but not discussed in the text of the guideline. This could be an important element of shared decision making. Some patients may be willing to accept an option that is more likely to prevent cancer and avert the cost and morbidity of cancer care, even if it means more lifetime colonoscopies.
There is little discussion in the document about screening program quality. Programs that involve several steps may have issues with adherence, which may reduce effectiveness. Therefore, quality metrics ideally should be measured for all screening programs to ensure high-quality delivery of the program and adherence to the programmatic steps.
Dr. David Lieberman is vice president of the AGA Institute and chief, division of gastroenterology and hepatology, Oregon Health and Science University, Portland.
Colorectal cancer screening by a variety of methods is worthwhile and recommended for all adults aged 50-75 years, according to the latest recommendations from the U.S. Preventive Services Task Force. The USPSTF statement and summary of evidence were published in JAMA on June 15.
In addition, the USPSTF recommended selective screening for older adults aged 76-85 years, depending on their health status and screening history.
A team of researchers led by Dr. Jennifer S. Lin of Kaiser Permanente in Portland, Ore., reviewed studies on colorectal cancer screening published between Jan.1, 2008, and Dec. 31, 2014, with surveillance continuing through Feb. 23, 2016 (JAMA. 2016 Jun;315:2576-94 [doi: 10.1001/jama.2016.3332]). The USPSTF’s last recommendations on colorectal cancer screening were released in 2008.
“Although CRC screening has a large body of supporting evidence, additional research is still needed to weigh the relative benefits and harms of each test within a program of screening” for average-risk adults, the researchers noted.
The final recommendation statement includes three screening options that carry over from the 2008 guidelines: colonoscopy, sigmoidoscopy, and fecal immunochemical testing (FIT) with occult blood.
Other options now recommended include computed tomographic colonography (CTC), fecal immunochemical tests with DNA (FIT-DNA), guaiac-based fecal occult blood testing (gFOBT), and sigmoidoscopy plus FIT.
Some highlights from the analysis: Four randomized trials including 458,002 patients showed that one-time or two-time screening with flexible sigmoidoscopy was associated with decreased mortality from colorectal cancer, compared with no screening, for an incidence rate ratio of 0.73, the researchers wrote.
In addition, the researchers found that CTC had 73%-98% sensitivity and 89%-91% specificity to detect adenomas 6 mm and larger, compared with colonoscopy in seven studies. However, the risk of harm from low-dose ionizing radiation remains a consideration.
For diagnostic accuracy, colonoscopy showed per-person sensitivity of 89%-98% for adenomas 10 mm or larger, and 75%-93% for adenomas 6 mm or larger, in studies comparing it with CTC or as an adjunct to CTC. However, studies showing applicability to community practices were limited.
Fecal immunochemical tests (FITs) showed sensitivity ranging from 73% to 88% and specificity from 90% to 96%.
Data from five randomized, controlled trials evaluating multiple rounds of biennial screening using gFOBT showed a significant reduction in colorectal cancer mortality, from a relative risk of 0.91 at 19.5 years to a relative risk of 0.78 at 30 years.
Colonoscopy remains the standard by which other tests are assessed, although it has the highest risk of procedural complications, the researchers said. Three new randomized, controlled trials involving screening colonoscopy in average-risk adults scheduled for completion in 2021, 2026, and 2027, may yield more information on incidence and mortality, they added.
The evidence report and review was limited by its focus on average-risk adults; it did not address factors including screening for high-risk adults, availability and access to tests, potential risks of overdiagnosis, and overuse of screening after adenoma detection, the researchers said. In addition, “data are still needed on the differential uptake of and adherence to screening modalities and on continued adherence to repeated rounds of screening and diagnostic follow-up to screening over longer periods,” they said. However, they concluded, “colonoscopy, flexible sigmoidoscopy, CTC, and various stool tests have differing levels of evidence to support their use in CRC screening, ability to detect CRC and precursor lesions, and risk of serious adverse events in average-risk adults.”
The researchers had no relevant financial conflicts to disclose. The research was supported by the Agency for Healthcare Research and Quality under a contract with the U.S. Preventive Services Task Force.
The final recommendation statement is available online at USPSTF.
Colorectal cancer screening by a variety of methods is worthwhile and recommended for all adults aged 50-75 years, according to the latest recommendations from the U.S. Preventive Services Task Force. The USPSTF statement and summary of evidence were published in JAMA on June 15.
In addition, the USPSTF recommended selective screening for older adults aged 76-85 years, depending on their health status and screening history.
A team of researchers led by Dr. Jennifer S. Lin of Kaiser Permanente in Portland, Ore., reviewed studies on colorectal cancer screening published between Jan.1, 2008, and Dec. 31, 2014, with surveillance continuing through Feb. 23, 2016 (JAMA. 2016 Jun;315:2576-94 [doi: 10.1001/jama.2016.3332]). The USPSTF’s last recommendations on colorectal cancer screening were released in 2008.
“Although CRC screening has a large body of supporting evidence, additional research is still needed to weigh the relative benefits and harms of each test within a program of screening” for average-risk adults, the researchers noted.
The final recommendation statement includes three screening options that carry over from the 2008 guidelines: colonoscopy, sigmoidoscopy, and fecal immunochemical testing (FIT) with occult blood.
Other options now recommended include computed tomographic colonography (CTC), fecal immunochemical tests with DNA (FIT-DNA), guaiac-based fecal occult blood testing (gFOBT), and sigmoidoscopy plus FIT.
Some highlights from the analysis: Four randomized trials including 458,002 patients showed that one-time or two-time screening with flexible sigmoidoscopy was associated with decreased mortality from colorectal cancer, compared with no screening, for an incidence rate ratio of 0.73, the researchers wrote.
In addition, the researchers found that CTC had 73%-98% sensitivity and 89%-91% specificity to detect adenomas 6 mm and larger, compared with colonoscopy in seven studies. However, the risk of harm from low-dose ionizing radiation remains a consideration.
For diagnostic accuracy, colonoscopy showed per-person sensitivity of 89%-98% for adenomas 10 mm or larger, and 75%-93% for adenomas 6 mm or larger, in studies comparing it with CTC or as an adjunct to CTC. However, studies showing applicability to community practices were limited.
Fecal immunochemical tests (FITs) showed sensitivity ranging from 73% to 88% and specificity from 90% to 96%.
Data from five randomized, controlled trials evaluating multiple rounds of biennial screening using gFOBT showed a significant reduction in colorectal cancer mortality, from a relative risk of 0.91 at 19.5 years to a relative risk of 0.78 at 30 years.
Colonoscopy remains the standard by which other tests are assessed, although it has the highest risk of procedural complications, the researchers said. Three new randomized, controlled trials involving screening colonoscopy in average-risk adults scheduled for completion in 2021, 2026, and 2027, may yield more information on incidence and mortality, they added.
The evidence report and review was limited by its focus on average-risk adults; it did not address factors including screening for high-risk adults, availability and access to tests, potential risks of overdiagnosis, and overuse of screening after adenoma detection, the researchers said. In addition, “data are still needed on the differential uptake of and adherence to screening modalities and on continued adherence to repeated rounds of screening and diagnostic follow-up to screening over longer periods,” they said. However, they concluded, “colonoscopy, flexible sigmoidoscopy, CTC, and various stool tests have differing levels of evidence to support their use in CRC screening, ability to detect CRC and precursor lesions, and risk of serious adverse events in average-risk adults.”
The researchers had no relevant financial conflicts to disclose. The research was supported by the Agency for Healthcare Research and Quality under a contract with the U.S. Preventive Services Task Force.
The final recommendation statement is available online at USPSTF.
FROM JAMA
Key clinical point: Colorectal cancer screening is recommended for all adults aged 50-75 years, and several screening methods are supported by evidence-based research.
Major finding: In four randomized trials including 458,002 patients, one- or two-time screening via flexible sigmoidoscopy was associated with decreased mortality from colorectal cancer, compared with no screening, for an incidence rate ratio of 0.73.
Data source: Studies were selected based on searches of MEDLINE, PubMed, and the Cochrane Central Register of Controlled Trials.
Disclosures: The researchers had no relevant financial conflicts to disclose. The research was supported by AHRQ under a contract with the USPSTF.
Guideline tweak addresses conflicting recommendations on BAV
NEW YORK – While overall guidelines for aortic repair surgery have not changed significantly in the past 5 years, guidelines for the timing of surgery in patients with bicuspid aortic valves and enlarged aortas have undergone some updating in an attempt to clear up disparities in different guidelines on when to operate on those patients.
Lars G. Svensson, MD, PhD, chairman of the Cleveland Clinic Heart and Vascular Institute, coauthor of the clarification statement by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (J Thorac Cardiovasc Surg. 2016;151:959-66), reported on the guidelines clarification at the meeting sponsored by the American Association for Thoracic Surgery. He noted that five different clinical guidelines between 2010 and 2014 recommended five different size thresholds for prophylactic aortic root or ascending aortic surgery in the setting of bicuspid aortic valve (BAV), ranging from 4 cm to greater than 5.5 cm. “This created a bit of a quandary and controversy between different guidelines and time periods,” he said.
Dr. Svensson and Loren Hiratzka, MD, medical director of cardiac surgery for TriHealth in Cincinnati, and their colleagues drafted the guideline clarification that makes the following recommendations for aortic root and ascending aorta repair or replacement when patients have BAV (strength of recommendation):
• Surgery is indicated to replace the aortic root or ascending aorta in asymptomatic patients with BAV if the diameter of the aortic root or ascending aorta is 5.5 cm or greater (Class 1).
• Surgical repair is indicated for asymptomatic patients with BAV if the root or ascending aorta diameter is 5 cm or greater in two scenarios: if the patient has an additional risk factor for dissection, such as family history or excessive aortic growth rate; or if the patient is a low surgical risk and has access to an experienced surgeon at a high-volume center (Class IIa).
The guideline update also addresses BAV in patients with Turner syndrome. The 2010 joint guidelines of 10 societies left some questions with regard to surgery in these patients, Dr. Svensson said. The established guidelines included a Class IIb recommendation for imaging of the heart and aorta to help determine the aorta risk in patients with Turner syndrome who had additional risk factors, including BAV, aortic coarctation and/or hypertension, or were planning a pregnancy.
The updated guideline includes Class IIa recommendation that in short-statured patients with Turner syndrome and BAV, measurement of the aortic root or ascending aorta diameter may not predict the dissection risk as well as aortic diameter index greater than 2.5 cm/m2. The updated recommendations also draw on one study that reported that in patients with BAV, a maximum aortic cross-sectional area-to-height ratio of 10 cm2/m or greater was also predictive of aortic dissection. (Ann Thorac Surg. 2015;100:1666-73)
The updated recommendations for open surgery for ascending aortic aneurysm include separate valve and ascending aortic replacement in patients without significant aortic root dilatation or in elderly patients, or in younger patients with minimal dilatation who have aortic valve disease; and excision of the sinuses of Valsalva with a modified David reimplantation when technically feasible in patients with connective tissue disease and others with dilatation of the aortic root and sinuses. For patients in whom the latter procedure is not feasible, root replacement with valved graft conduit would be indicated, Dr. Svensson said.
Dr. Svensson also reported on recent studies that validated recommendations in established guidelines.
Studies of circulatory arrest practices in aortic arch surgery as prescribed by established guidelines showed confirmatory results, he said. “The one point I would make about circulatory arrest is that we found in a fairly large study of 1,352 circulatory arrest patients that we reduced the risk of stroke by 40% when we used the axillary artery with a side a graft,” he said (Ann Thorac Surg. 2004;78:1274-84). His own institution’s clinical trial of 121 patients who received antegrade or retrograde brain perfusion showed rates of 0.8% for each stroke and operative death, he said (J Thorac Cardiovasc Surg. 2015;150:1140-7).
“What was also of interest there was no difference in outcomes with antegrade vs. retrograde brain profusion,” he said. “I think protection of the brain is pretty good if you follow the fundamental principles of brain protection.”
He also reported on a recent study at his institution that documented the benefits of intrathecal papaverine (IP) for spinal cord protection during descending open and endovascular aortic repairs. In 398 aortic repairs from 2001-2009, the rates of spinal cord injury were 23% in the non-IP group vs. 7% in the IP group (P = .07) in a matched cohort.
He noted that the clinical guidelines of the American Association for Thoracic Surgery as well as AATS/Society of Thoracic Surgeons joint guidelines are open to input. “If you have areas where you think guideline should be written about, please let me or other members of the committee know,” he said.
Dr. Svensson had no disclosures relevant to his presentation.
NEW YORK – While overall guidelines for aortic repair surgery have not changed significantly in the past 5 years, guidelines for the timing of surgery in patients with bicuspid aortic valves and enlarged aortas have undergone some updating in an attempt to clear up disparities in different guidelines on when to operate on those patients.
Lars G. Svensson, MD, PhD, chairman of the Cleveland Clinic Heart and Vascular Institute, coauthor of the clarification statement by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (J Thorac Cardiovasc Surg. 2016;151:959-66), reported on the guidelines clarification at the meeting sponsored by the American Association for Thoracic Surgery. He noted that five different clinical guidelines between 2010 and 2014 recommended five different size thresholds for prophylactic aortic root or ascending aortic surgery in the setting of bicuspid aortic valve (BAV), ranging from 4 cm to greater than 5.5 cm. “This created a bit of a quandary and controversy between different guidelines and time periods,” he said.
Dr. Svensson and Loren Hiratzka, MD, medical director of cardiac surgery for TriHealth in Cincinnati, and their colleagues drafted the guideline clarification that makes the following recommendations for aortic root and ascending aorta repair or replacement when patients have BAV (strength of recommendation):
• Surgery is indicated to replace the aortic root or ascending aorta in asymptomatic patients with BAV if the diameter of the aortic root or ascending aorta is 5.5 cm or greater (Class 1).
• Surgical repair is indicated for asymptomatic patients with BAV if the root or ascending aorta diameter is 5 cm or greater in two scenarios: if the patient has an additional risk factor for dissection, such as family history or excessive aortic growth rate; or if the patient is a low surgical risk and has access to an experienced surgeon at a high-volume center (Class IIa).
The guideline update also addresses BAV in patients with Turner syndrome. The 2010 joint guidelines of 10 societies left some questions with regard to surgery in these patients, Dr. Svensson said. The established guidelines included a Class IIb recommendation for imaging of the heart and aorta to help determine the aorta risk in patients with Turner syndrome who had additional risk factors, including BAV, aortic coarctation and/or hypertension, or were planning a pregnancy.
The updated guideline includes Class IIa recommendation that in short-statured patients with Turner syndrome and BAV, measurement of the aortic root or ascending aorta diameter may not predict the dissection risk as well as aortic diameter index greater than 2.5 cm/m2. The updated recommendations also draw on one study that reported that in patients with BAV, a maximum aortic cross-sectional area-to-height ratio of 10 cm2/m or greater was also predictive of aortic dissection. (Ann Thorac Surg. 2015;100:1666-73)
The updated recommendations for open surgery for ascending aortic aneurysm include separate valve and ascending aortic replacement in patients without significant aortic root dilatation or in elderly patients, or in younger patients with minimal dilatation who have aortic valve disease; and excision of the sinuses of Valsalva with a modified David reimplantation when technically feasible in patients with connective tissue disease and others with dilatation of the aortic root and sinuses. For patients in whom the latter procedure is not feasible, root replacement with valved graft conduit would be indicated, Dr. Svensson said.
Dr. Svensson also reported on recent studies that validated recommendations in established guidelines.
Studies of circulatory arrest practices in aortic arch surgery as prescribed by established guidelines showed confirmatory results, he said. “The one point I would make about circulatory arrest is that we found in a fairly large study of 1,352 circulatory arrest patients that we reduced the risk of stroke by 40% when we used the axillary artery with a side a graft,” he said (Ann Thorac Surg. 2004;78:1274-84). His own institution’s clinical trial of 121 patients who received antegrade or retrograde brain perfusion showed rates of 0.8% for each stroke and operative death, he said (J Thorac Cardiovasc Surg. 2015;150:1140-7).
“What was also of interest there was no difference in outcomes with antegrade vs. retrograde brain profusion,” he said. “I think protection of the brain is pretty good if you follow the fundamental principles of brain protection.”
He also reported on a recent study at his institution that documented the benefits of intrathecal papaverine (IP) for spinal cord protection during descending open and endovascular aortic repairs. In 398 aortic repairs from 2001-2009, the rates of spinal cord injury were 23% in the non-IP group vs. 7% in the IP group (P = .07) in a matched cohort.
He noted that the clinical guidelines of the American Association for Thoracic Surgery as well as AATS/Society of Thoracic Surgeons joint guidelines are open to input. “If you have areas where you think guideline should be written about, please let me or other members of the committee know,” he said.
Dr. Svensson had no disclosures relevant to his presentation.
NEW YORK – While overall guidelines for aortic repair surgery have not changed significantly in the past 5 years, guidelines for the timing of surgery in patients with bicuspid aortic valves and enlarged aortas have undergone some updating in an attempt to clear up disparities in different guidelines on when to operate on those patients.
Lars G. Svensson, MD, PhD, chairman of the Cleveland Clinic Heart and Vascular Institute, coauthor of the clarification statement by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (J Thorac Cardiovasc Surg. 2016;151:959-66), reported on the guidelines clarification at the meeting sponsored by the American Association for Thoracic Surgery. He noted that five different clinical guidelines between 2010 and 2014 recommended five different size thresholds for prophylactic aortic root or ascending aortic surgery in the setting of bicuspid aortic valve (BAV), ranging from 4 cm to greater than 5.5 cm. “This created a bit of a quandary and controversy between different guidelines and time periods,” he said.
Dr. Svensson and Loren Hiratzka, MD, medical director of cardiac surgery for TriHealth in Cincinnati, and their colleagues drafted the guideline clarification that makes the following recommendations for aortic root and ascending aorta repair or replacement when patients have BAV (strength of recommendation):
• Surgery is indicated to replace the aortic root or ascending aorta in asymptomatic patients with BAV if the diameter of the aortic root or ascending aorta is 5.5 cm or greater (Class 1).
• Surgical repair is indicated for asymptomatic patients with BAV if the root or ascending aorta diameter is 5 cm or greater in two scenarios: if the patient has an additional risk factor for dissection, such as family history or excessive aortic growth rate; or if the patient is a low surgical risk and has access to an experienced surgeon at a high-volume center (Class IIa).
The guideline update also addresses BAV in patients with Turner syndrome. The 2010 joint guidelines of 10 societies left some questions with regard to surgery in these patients, Dr. Svensson said. The established guidelines included a Class IIb recommendation for imaging of the heart and aorta to help determine the aorta risk in patients with Turner syndrome who had additional risk factors, including BAV, aortic coarctation and/or hypertension, or were planning a pregnancy.
The updated guideline includes Class IIa recommendation that in short-statured patients with Turner syndrome and BAV, measurement of the aortic root or ascending aorta diameter may not predict the dissection risk as well as aortic diameter index greater than 2.5 cm/m2. The updated recommendations also draw on one study that reported that in patients with BAV, a maximum aortic cross-sectional area-to-height ratio of 10 cm2/m or greater was also predictive of aortic dissection. (Ann Thorac Surg. 2015;100:1666-73)
The updated recommendations for open surgery for ascending aortic aneurysm include separate valve and ascending aortic replacement in patients without significant aortic root dilatation or in elderly patients, or in younger patients with minimal dilatation who have aortic valve disease; and excision of the sinuses of Valsalva with a modified David reimplantation when technically feasible in patients with connective tissue disease and others with dilatation of the aortic root and sinuses. For patients in whom the latter procedure is not feasible, root replacement with valved graft conduit would be indicated, Dr. Svensson said.
Dr. Svensson also reported on recent studies that validated recommendations in established guidelines.
Studies of circulatory arrest practices in aortic arch surgery as prescribed by established guidelines showed confirmatory results, he said. “The one point I would make about circulatory arrest is that we found in a fairly large study of 1,352 circulatory arrest patients that we reduced the risk of stroke by 40% when we used the axillary artery with a side a graft,” he said (Ann Thorac Surg. 2004;78:1274-84). His own institution’s clinical trial of 121 patients who received antegrade or retrograde brain perfusion showed rates of 0.8% for each stroke and operative death, he said (J Thorac Cardiovasc Surg. 2015;150:1140-7).
“What was also of interest there was no difference in outcomes with antegrade vs. retrograde brain profusion,” he said. “I think protection of the brain is pretty good if you follow the fundamental principles of brain protection.”
He also reported on a recent study at his institution that documented the benefits of intrathecal papaverine (IP) for spinal cord protection during descending open and endovascular aortic repairs. In 398 aortic repairs from 2001-2009, the rates of spinal cord injury were 23% in the non-IP group vs. 7% in the IP group (P = .07) in a matched cohort.
He noted that the clinical guidelines of the American Association for Thoracic Surgery as well as AATS/Society of Thoracic Surgeons joint guidelines are open to input. “If you have areas where you think guideline should be written about, please let me or other members of the committee know,” he said.
Dr. Svensson had no disclosures relevant to his presentation.
AT THE AATS AORTIC SYMPOSIUM 2016
Key clinical point: Various clinical guidelines provided five different recommendations for the timing of aortic repair surgery in patients with bicuspid aortic valves.
Major finding: Recent updates in guidelines provide clarity on when an aortic repair is needed in the setting of aortic bicuspid valve.
Data source: American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.
Disclosures: Dr. Svensson had no relevant financial relationships to disclose.
Olympic Games Create Novel Opportunity to Study Zika Virus
Behind the competition and pageantry of the 2016 Summer Olympics and Paralympics in Rio de Janeiro, researchers at the University of Utah will be busy monitoring a subset of athletes, coaches, and other U.S. Olympic Committee staff for potential Zika virus exposure.
“Of everyone I talk to who’s at risk for Zika virus, their No. 1 question is, what are the risks to my reproductive health?” said the study’s principal investigator Carrie L. Byington, MD, a pediatrician and infectious disease specialist who is codirector of Utah Center for Clinical and Translational Science at the University of Utah in Salt Lake City. “Can I have a healthy baby? How can I protect that opportunity to reproduce? We are dedicated to trying to find some answers.”
In a study funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Dr. Byington and a team of six other clinicians will recruit up to 1,000 athletes, coaches, and other U.S. Olympic Committee (USOC) staff attending the games to complete health surveys and undergo pre- and post-travel periodic antibody testing for Zika virus, a mosquito-borne flavivirus that has emerged in the Americas with local transmission identified in 30 countries and territories as of April 2016, including Brazil. From that group they expect to identify infected individuals. “Hopefully, it’s a very small proportion of the group but we think that we will identify some, because it is going to be impossible to prevent all mosquito exposure, even over the short term,” Dr. Byington said. Those found to harbor Zika virus by antibody testing will be followed for up to 2 years and will be asked to submit self-collected samples of blood, urine, saliva, semen, and vaginal secretions monthly. Affected individuals who wish to conceive after the games will have access to the study personnel, who include four infectious disease specialists, two obstetrician-gynecologists, and a laboratory expert. “We will have monthly testing and direct consultation with them regarding their test results and help them make the best reproductive decisions they can,” Dr. Byington said.
In April 2016, the Centers for Disease Control and Prevention confirmed that fetal infection with Zika virus was the cause of microcephaly and other severe brain anomalies that result in permanent morbidity in surviving infants. According to a description of the current study published by the National Institutes of Health, many questions remain regarding infection with Zika virus, including the duration and potential for sexual or perinatal transmission from body fluids; the short and long-term reproductive outcomes of individuals infected with Zika virus; and the outcomes for infants born to men and women with either symptomatic or asymptomatic Zika virus infection. The researchers consider each study participant as equally susceptible to Zika virus exposure, regardless of his or her sport or role with the USOC. “People will be both indoors and outdoors, and these are indoor-dwelling mosquitoes, so I don’t think we can completely eliminate the risk for any type of traveler,” Dr. Byington said. “We’re very interested in the water venues, but we’re also concerned about standing water outside other venues or hotel rooms.” If a study participant falls ill in Rio de Janeiro with symptoms consistent with Zika virus, USOC medical personnel will send samples of blood, urine, and saliva to the Utah-based research team for confirmatory polymerase chain reaction testing.
The idea for the current study grew out of a pilot trial that Dr. Byington and her associates conducted in 150 individuals affiliated with the USOC who were traveling back and forth to Brazil in preparation for the games during March and April of 2016. It enabled the researchers to develop online web-based tools for consenting, tracking, and returning test results. “It allowed us to do some work with our laboratory facilities for shipping and receiving specimens and processing and running specimens and returning some results,” Dr. Byington said. “That work has been really important. We found that about one-third of our pilot was interested in becoming pregnant very shortly after the games, so that was very important information that we were able to share with the USOC and the NIH. This is a group that is very interested in their reproductive health, which makes an ideal cohort for the study.”
David Turok, MD, an ob.gyn. and member of the research team, planned long ago to attend the Olympic Games in Rio as a spectator with his wife and 14-year-old son. He intends to carry out those plans and described the current study as a unique opportunity to better understand the Zika virus. “The need for data on the topic is pressing,” said Dr. Turok, who directs the family planning fellowship at the University of Utah. “People who are Olympic athletes and coaches are probably more likely to plan their lives. We know from a wealth of epidemiologic data that people who plan their pregnancies have better outcomes. This is something that our society has done a really poor job in communicating: the challenges of parenting and the benefits of planning pregnancy and making the most effective methods of contraception available. This study is an opportunity to better our game. There’s probably no better opportunity for prospective evaluation of a group of people who we know are going to have some exposure [to Zika virus]. The known exposure and the known desired outcome make it a unique opportunity.”
The 2016 Summer Olympics will take place in Rio de Janeiro Aug. 5-21, while the Paralympic Games take place Sept. 7-18. Dr. Byington said that she hopes to be able to share preliminary study results with the public sometime in October.
Behind the competition and pageantry of the 2016 Summer Olympics and Paralympics in Rio de Janeiro, researchers at the University of Utah will be busy monitoring a subset of athletes, coaches, and other U.S. Olympic Committee staff for potential Zika virus exposure.
“Of everyone I talk to who’s at risk for Zika virus, their No. 1 question is, what are the risks to my reproductive health?” said the study’s principal investigator Carrie L. Byington, MD, a pediatrician and infectious disease specialist who is codirector of Utah Center for Clinical and Translational Science at the University of Utah in Salt Lake City. “Can I have a healthy baby? How can I protect that opportunity to reproduce? We are dedicated to trying to find some answers.”
In a study funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Dr. Byington and a team of six other clinicians will recruit up to 1,000 athletes, coaches, and other U.S. Olympic Committee (USOC) staff attending the games to complete health surveys and undergo pre- and post-travel periodic antibody testing for Zika virus, a mosquito-borne flavivirus that has emerged in the Americas with local transmission identified in 30 countries and territories as of April 2016, including Brazil. From that group they expect to identify infected individuals. “Hopefully, it’s a very small proportion of the group but we think that we will identify some, because it is going to be impossible to prevent all mosquito exposure, even over the short term,” Dr. Byington said. Those found to harbor Zika virus by antibody testing will be followed for up to 2 years and will be asked to submit self-collected samples of blood, urine, saliva, semen, and vaginal secretions monthly. Affected individuals who wish to conceive after the games will have access to the study personnel, who include four infectious disease specialists, two obstetrician-gynecologists, and a laboratory expert. “We will have monthly testing and direct consultation with them regarding their test results and help them make the best reproductive decisions they can,” Dr. Byington said.
In April 2016, the Centers for Disease Control and Prevention confirmed that fetal infection with Zika virus was the cause of microcephaly and other severe brain anomalies that result in permanent morbidity in surviving infants. According to a description of the current study published by the National Institutes of Health, many questions remain regarding infection with Zika virus, including the duration and potential for sexual or perinatal transmission from body fluids; the short and long-term reproductive outcomes of individuals infected with Zika virus; and the outcomes for infants born to men and women with either symptomatic or asymptomatic Zika virus infection. The researchers consider each study participant as equally susceptible to Zika virus exposure, regardless of his or her sport or role with the USOC. “People will be both indoors and outdoors, and these are indoor-dwelling mosquitoes, so I don’t think we can completely eliminate the risk for any type of traveler,” Dr. Byington said. “We’re very interested in the water venues, but we’re also concerned about standing water outside other venues or hotel rooms.” If a study participant falls ill in Rio de Janeiro with symptoms consistent with Zika virus, USOC medical personnel will send samples of blood, urine, and saliva to the Utah-based research team for confirmatory polymerase chain reaction testing.
The idea for the current study grew out of a pilot trial that Dr. Byington and her associates conducted in 150 individuals affiliated with the USOC who were traveling back and forth to Brazil in preparation for the games during March and April of 2016. It enabled the researchers to develop online web-based tools for consenting, tracking, and returning test results. “It allowed us to do some work with our laboratory facilities for shipping and receiving specimens and processing and running specimens and returning some results,” Dr. Byington said. “That work has been really important. We found that about one-third of our pilot was interested in becoming pregnant very shortly after the games, so that was very important information that we were able to share with the USOC and the NIH. This is a group that is very interested in their reproductive health, which makes an ideal cohort for the study.”
David Turok, MD, an ob.gyn. and member of the research team, planned long ago to attend the Olympic Games in Rio as a spectator with his wife and 14-year-old son. He intends to carry out those plans and described the current study as a unique opportunity to better understand the Zika virus. “The need for data on the topic is pressing,” said Dr. Turok, who directs the family planning fellowship at the University of Utah. “People who are Olympic athletes and coaches are probably more likely to plan their lives. We know from a wealth of epidemiologic data that people who plan their pregnancies have better outcomes. This is something that our society has done a really poor job in communicating: the challenges of parenting and the benefits of planning pregnancy and making the most effective methods of contraception available. This study is an opportunity to better our game. There’s probably no better opportunity for prospective evaluation of a group of people who we know are going to have some exposure [to Zika virus]. The known exposure and the known desired outcome make it a unique opportunity.”
The 2016 Summer Olympics will take place in Rio de Janeiro Aug. 5-21, while the Paralympic Games take place Sept. 7-18. Dr. Byington said that she hopes to be able to share preliminary study results with the public sometime in October.
Behind the competition and pageantry of the 2016 Summer Olympics and Paralympics in Rio de Janeiro, researchers at the University of Utah will be busy monitoring a subset of athletes, coaches, and other U.S. Olympic Committee staff for potential Zika virus exposure.
“Of everyone I talk to who’s at risk for Zika virus, their No. 1 question is, what are the risks to my reproductive health?” said the study’s principal investigator Carrie L. Byington, MD, a pediatrician and infectious disease specialist who is codirector of Utah Center for Clinical and Translational Science at the University of Utah in Salt Lake City. “Can I have a healthy baby? How can I protect that opportunity to reproduce? We are dedicated to trying to find some answers.”
In a study funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Dr. Byington and a team of six other clinicians will recruit up to 1,000 athletes, coaches, and other U.S. Olympic Committee (USOC) staff attending the games to complete health surveys and undergo pre- and post-travel periodic antibody testing for Zika virus, a mosquito-borne flavivirus that has emerged in the Americas with local transmission identified in 30 countries and territories as of April 2016, including Brazil. From that group they expect to identify infected individuals. “Hopefully, it’s a very small proportion of the group but we think that we will identify some, because it is going to be impossible to prevent all mosquito exposure, even over the short term,” Dr. Byington said. Those found to harbor Zika virus by antibody testing will be followed for up to 2 years and will be asked to submit self-collected samples of blood, urine, saliva, semen, and vaginal secretions monthly. Affected individuals who wish to conceive after the games will have access to the study personnel, who include four infectious disease specialists, two obstetrician-gynecologists, and a laboratory expert. “We will have monthly testing and direct consultation with them regarding their test results and help them make the best reproductive decisions they can,” Dr. Byington said.
In April 2016, the Centers for Disease Control and Prevention confirmed that fetal infection with Zika virus was the cause of microcephaly and other severe brain anomalies that result in permanent morbidity in surviving infants. According to a description of the current study published by the National Institutes of Health, many questions remain regarding infection with Zika virus, including the duration and potential for sexual or perinatal transmission from body fluids; the short and long-term reproductive outcomes of individuals infected with Zika virus; and the outcomes for infants born to men and women with either symptomatic or asymptomatic Zika virus infection. The researchers consider each study participant as equally susceptible to Zika virus exposure, regardless of his or her sport or role with the USOC. “People will be both indoors and outdoors, and these are indoor-dwelling mosquitoes, so I don’t think we can completely eliminate the risk for any type of traveler,” Dr. Byington said. “We’re very interested in the water venues, but we’re also concerned about standing water outside other venues or hotel rooms.” If a study participant falls ill in Rio de Janeiro with symptoms consistent with Zika virus, USOC medical personnel will send samples of blood, urine, and saliva to the Utah-based research team for confirmatory polymerase chain reaction testing.
The idea for the current study grew out of a pilot trial that Dr. Byington and her associates conducted in 150 individuals affiliated with the USOC who were traveling back and forth to Brazil in preparation for the games during March and April of 2016. It enabled the researchers to develop online web-based tools for consenting, tracking, and returning test results. “It allowed us to do some work with our laboratory facilities for shipping and receiving specimens and processing and running specimens and returning some results,” Dr. Byington said. “That work has been really important. We found that about one-third of our pilot was interested in becoming pregnant very shortly after the games, so that was very important information that we were able to share with the USOC and the NIH. This is a group that is very interested in their reproductive health, which makes an ideal cohort for the study.”
David Turok, MD, an ob.gyn. and member of the research team, planned long ago to attend the Olympic Games in Rio as a spectator with his wife and 14-year-old son. He intends to carry out those plans and described the current study as a unique opportunity to better understand the Zika virus. “The need for data on the topic is pressing,” said Dr. Turok, who directs the family planning fellowship at the University of Utah. “People who are Olympic athletes and coaches are probably more likely to plan their lives. We know from a wealth of epidemiologic data that people who plan their pregnancies have better outcomes. This is something that our society has done a really poor job in communicating: the challenges of parenting and the benefits of planning pregnancy and making the most effective methods of contraception available. This study is an opportunity to better our game. There’s probably no better opportunity for prospective evaluation of a group of people who we know are going to have some exposure [to Zika virus]. The known exposure and the known desired outcome make it a unique opportunity.”
The 2016 Summer Olympics will take place in Rio de Janeiro Aug. 5-21, while the Paralympic Games take place Sept. 7-18. Dr. Byington said that she hopes to be able to share preliminary study results with the public sometime in October.
Preventing and managing vaginal cuff dehiscence
Vaginal cuff dehiscence, or separation of the vaginal incision, is a rare postoperative complication unique to hysterectomy. Morbidity related to evisceration of abdominal contents can be profound and prompt intervention is required.
A 10-year observational study of 11,000 patients described a 0.24% cumulative incidence after all modes of hysterectomy.1 Though data are varied, the mode of hysterectomy does have an impact on the risk of dehiscence.
Laparoscopic (0.64%-1.35%) and robotic (0.46%-1.5%) hysterectomy have a higher incidence than abdominal (0.15%-0.26%) and vaginal (0.08%-0.25%) approaches.2 The use of monopolar cautery for colpotomy and different closure techniques may account for these differences.
Cuff cellulitis, early sexual intercourse, cigarette smoking, poor nutrition, obesity, menopausal status, and corticosteroid use are all proposed risk factors that promote infection, pressure at the vaginal cuff, and poor wound healing. Although some are modifiable, the rarity of this complication has made establishing causality and promoting prevention challenging.
Prevention
• Preoperatively. Treating bacterial vaginosis, Trichomonas vaginalis, gonorrhea, and chlamydia can decrease the risk of cuff cellulitis and dehiscence.3
• Intraoperatively. Surgeons should ensure adequate vaginal margins (greater than 1 cm) with full-thickness cuff closures while avoiding excessive electrocautery.4 Retrospective data show that transvaginal cuff closure is associated with a decreased risk of dehiscence.5 However, given the lack of randomized data and the difficulty controlling for surgeon experience, gynecologists should use the approach that they are most comfortable with. Though the various laparoscopic cuff closure techniques have limited evidence regarding superiority, some experts propose using two-layer cuff closure and barbed sutures.6-8 Several retrospective studies have found an equivalent or a decreased incidence of cuff dehiscence with barbed sutures, compared with other methods (e.g., 0-Vicryl, Endo Stitch).9,10
• Postoperatively. Women should avoid intercourse and lifting more than 15 pounds for at least 6-8 weeks as the vaginal cuff gains tensile strength. Vaginal estrogen can promote healing in postmenopausal patients.11
Management
Patients with vaginal cuff dehiscence commonly present within the first several weeks to months after surgery with pelvic pain (60%-100%), vaginal bleeding (30%-60%), vaginal discharge (30%), or vaginal pressure/mass (30%).1,7 Posthysterectomy patients with these complaints warrant an urgent evaluation. The diagnosis is made during a pelvic exam.
Broad-spectrum antibiotics are necessary because all vaginal cuff separations or dehiscences expose the peritoneal cavity to vaginal flora. Nonsurgical management is reasonable for small separations – less than 25% of the cuff – if there is no evidence of evisceration.
However, surgically closing all recognized cuff dehiscences is reasonable, given the potential for further separation. A vaginal approach is preferred when possible. Women with vaginal cuff dehiscence, stable vital signs, and no evidence of bowel evisceration can be repaired vaginally without an abdominal survey.
In contrast, women with bowel evisceration have a surgical emergency because of the risk of peritonitis and bowel injury. If the eviscerated bowel is not reducible, it should be irrigated and wrapped in a warm moist towel or gauze in preparation for inspection and reduction in the operating room. If the bowel is reducible, the patient can be placed in Trendelenburg’s position. Her vagina should be packed to reduce the risk of re-evisceration as she moves toward operative cuff repair.
If the physician is concerned about bowel injury, inspection via laparoscopy or laparotomy would be reasonable. However, when bowel injury is not suspected, a vaginal technique for dehiscence repair has been described by Matthews et al.:12
1. Expose the cuff with a weighted speculum and Breisky-Navratil retractors.
2. Sharply debride the cuff edges back to viable tissue.
3. Dissect adherent bowel or omentum to allow for full-thickness closure.
4. Place full-thickness, interrupted delayed absorbable sutures to reapproximate the cuff edges.
Cuff dehiscence is a rare but potentially morbid complication of hysterectomy. Prevention, recognition, and appropriate management can avoid life-threatening sequelae.
References
1. Obstet Gynecol. 2011 Oct;118(4):794-801.
2. JSLS. 2012 Oct-Dec;16(4):530-6.
3. Am J Obstet Gynecol. 1990 Sep;163(3):1016-21; discussion 1021-3.
4. Obstet Gynecol. 2013 Mar;121(3):654-73.
5. Obstet Gynecol. 2012 Sep;120(3):516-23.
6. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):474-80.
7. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):134-8.
8. Obstet Gynecol. 2009 Aug;114(2 Pt 1):231-5.
9. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23.
10. Int J Surg. 2015 Jul;19:27-30.
11. Maturitas. 2006 Feb 20;53(3):282-98.
12. Obstet Gynecol. 2014 Oct;124(4):705-8.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Vaginal cuff dehiscence, or separation of the vaginal incision, is a rare postoperative complication unique to hysterectomy. Morbidity related to evisceration of abdominal contents can be profound and prompt intervention is required.
A 10-year observational study of 11,000 patients described a 0.24% cumulative incidence after all modes of hysterectomy.1 Though data are varied, the mode of hysterectomy does have an impact on the risk of dehiscence.
Laparoscopic (0.64%-1.35%) and robotic (0.46%-1.5%) hysterectomy have a higher incidence than abdominal (0.15%-0.26%) and vaginal (0.08%-0.25%) approaches.2 The use of monopolar cautery for colpotomy and different closure techniques may account for these differences.
Cuff cellulitis, early sexual intercourse, cigarette smoking, poor nutrition, obesity, menopausal status, and corticosteroid use are all proposed risk factors that promote infection, pressure at the vaginal cuff, and poor wound healing. Although some are modifiable, the rarity of this complication has made establishing causality and promoting prevention challenging.
Prevention
• Preoperatively. Treating bacterial vaginosis, Trichomonas vaginalis, gonorrhea, and chlamydia can decrease the risk of cuff cellulitis and dehiscence.3
• Intraoperatively. Surgeons should ensure adequate vaginal margins (greater than 1 cm) with full-thickness cuff closures while avoiding excessive electrocautery.4 Retrospective data show that transvaginal cuff closure is associated with a decreased risk of dehiscence.5 However, given the lack of randomized data and the difficulty controlling for surgeon experience, gynecologists should use the approach that they are most comfortable with. Though the various laparoscopic cuff closure techniques have limited evidence regarding superiority, some experts propose using two-layer cuff closure and barbed sutures.6-8 Several retrospective studies have found an equivalent or a decreased incidence of cuff dehiscence with barbed sutures, compared with other methods (e.g., 0-Vicryl, Endo Stitch).9,10
• Postoperatively. Women should avoid intercourse and lifting more than 15 pounds for at least 6-8 weeks as the vaginal cuff gains tensile strength. Vaginal estrogen can promote healing in postmenopausal patients.11
Management
Patients with vaginal cuff dehiscence commonly present within the first several weeks to months after surgery with pelvic pain (60%-100%), vaginal bleeding (30%-60%), vaginal discharge (30%), or vaginal pressure/mass (30%).1,7 Posthysterectomy patients with these complaints warrant an urgent evaluation. The diagnosis is made during a pelvic exam.
Broad-spectrum antibiotics are necessary because all vaginal cuff separations or dehiscences expose the peritoneal cavity to vaginal flora. Nonsurgical management is reasonable for small separations – less than 25% of the cuff – if there is no evidence of evisceration.
However, surgically closing all recognized cuff dehiscences is reasonable, given the potential for further separation. A vaginal approach is preferred when possible. Women with vaginal cuff dehiscence, stable vital signs, and no evidence of bowel evisceration can be repaired vaginally without an abdominal survey.
In contrast, women with bowel evisceration have a surgical emergency because of the risk of peritonitis and bowel injury. If the eviscerated bowel is not reducible, it should be irrigated and wrapped in a warm moist towel or gauze in preparation for inspection and reduction in the operating room. If the bowel is reducible, the patient can be placed in Trendelenburg’s position. Her vagina should be packed to reduce the risk of re-evisceration as she moves toward operative cuff repair.
If the physician is concerned about bowel injury, inspection via laparoscopy or laparotomy would be reasonable. However, when bowel injury is not suspected, a vaginal technique for dehiscence repair has been described by Matthews et al.:12
1. Expose the cuff with a weighted speculum and Breisky-Navratil retractors.
2. Sharply debride the cuff edges back to viable tissue.
3. Dissect adherent bowel or omentum to allow for full-thickness closure.
4. Place full-thickness, interrupted delayed absorbable sutures to reapproximate the cuff edges.
Cuff dehiscence is a rare but potentially morbid complication of hysterectomy. Prevention, recognition, and appropriate management can avoid life-threatening sequelae.
References
1. Obstet Gynecol. 2011 Oct;118(4):794-801.
2. JSLS. 2012 Oct-Dec;16(4):530-6.
3. Am J Obstet Gynecol. 1990 Sep;163(3):1016-21; discussion 1021-3.
4. Obstet Gynecol. 2013 Mar;121(3):654-73.
5. Obstet Gynecol. 2012 Sep;120(3):516-23.
6. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):474-80.
7. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):134-8.
8. Obstet Gynecol. 2009 Aug;114(2 Pt 1):231-5.
9. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23.
10. Int J Surg. 2015 Jul;19:27-30.
11. Maturitas. 2006 Feb 20;53(3):282-98.
12. Obstet Gynecol. 2014 Oct;124(4):705-8.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Vaginal cuff dehiscence, or separation of the vaginal incision, is a rare postoperative complication unique to hysterectomy. Morbidity related to evisceration of abdominal contents can be profound and prompt intervention is required.
A 10-year observational study of 11,000 patients described a 0.24% cumulative incidence after all modes of hysterectomy.1 Though data are varied, the mode of hysterectomy does have an impact on the risk of dehiscence.
Laparoscopic (0.64%-1.35%) and robotic (0.46%-1.5%) hysterectomy have a higher incidence than abdominal (0.15%-0.26%) and vaginal (0.08%-0.25%) approaches.2 The use of monopolar cautery for colpotomy and different closure techniques may account for these differences.
Cuff cellulitis, early sexual intercourse, cigarette smoking, poor nutrition, obesity, menopausal status, and corticosteroid use are all proposed risk factors that promote infection, pressure at the vaginal cuff, and poor wound healing. Although some are modifiable, the rarity of this complication has made establishing causality and promoting prevention challenging.
Prevention
• Preoperatively. Treating bacterial vaginosis, Trichomonas vaginalis, gonorrhea, and chlamydia can decrease the risk of cuff cellulitis and dehiscence.3
• Intraoperatively. Surgeons should ensure adequate vaginal margins (greater than 1 cm) with full-thickness cuff closures while avoiding excessive electrocautery.4 Retrospective data show that transvaginal cuff closure is associated with a decreased risk of dehiscence.5 However, given the lack of randomized data and the difficulty controlling for surgeon experience, gynecologists should use the approach that they are most comfortable with. Though the various laparoscopic cuff closure techniques have limited evidence regarding superiority, some experts propose using two-layer cuff closure and barbed sutures.6-8 Several retrospective studies have found an equivalent or a decreased incidence of cuff dehiscence with barbed sutures, compared with other methods (e.g., 0-Vicryl, Endo Stitch).9,10
• Postoperatively. Women should avoid intercourse and lifting more than 15 pounds for at least 6-8 weeks as the vaginal cuff gains tensile strength. Vaginal estrogen can promote healing in postmenopausal patients.11
Management
Patients with vaginal cuff dehiscence commonly present within the first several weeks to months after surgery with pelvic pain (60%-100%), vaginal bleeding (30%-60%), vaginal discharge (30%), or vaginal pressure/mass (30%).1,7 Posthysterectomy patients with these complaints warrant an urgent evaluation. The diagnosis is made during a pelvic exam.
Broad-spectrum antibiotics are necessary because all vaginal cuff separations or dehiscences expose the peritoneal cavity to vaginal flora. Nonsurgical management is reasonable for small separations – less than 25% of the cuff – if there is no evidence of evisceration.
However, surgically closing all recognized cuff dehiscences is reasonable, given the potential for further separation. A vaginal approach is preferred when possible. Women with vaginal cuff dehiscence, stable vital signs, and no evidence of bowel evisceration can be repaired vaginally without an abdominal survey.
In contrast, women with bowel evisceration have a surgical emergency because of the risk of peritonitis and bowel injury. If the eviscerated bowel is not reducible, it should be irrigated and wrapped in a warm moist towel or gauze in preparation for inspection and reduction in the operating room. If the bowel is reducible, the patient can be placed in Trendelenburg’s position. Her vagina should be packed to reduce the risk of re-evisceration as she moves toward operative cuff repair.
If the physician is concerned about bowel injury, inspection via laparoscopy or laparotomy would be reasonable. However, when bowel injury is not suspected, a vaginal technique for dehiscence repair has been described by Matthews et al.:12
1. Expose the cuff with a weighted speculum and Breisky-Navratil retractors.
2. Sharply debride the cuff edges back to viable tissue.
3. Dissect adherent bowel or omentum to allow for full-thickness closure.
4. Place full-thickness, interrupted delayed absorbable sutures to reapproximate the cuff edges.
Cuff dehiscence is a rare but potentially morbid complication of hysterectomy. Prevention, recognition, and appropriate management can avoid life-threatening sequelae.
References
1. Obstet Gynecol. 2011 Oct;118(4):794-801.
2. JSLS. 2012 Oct-Dec;16(4):530-6.
3. Am J Obstet Gynecol. 1990 Sep;163(3):1016-21; discussion 1021-3.
4. Obstet Gynecol. 2013 Mar;121(3):654-73.
5. Obstet Gynecol. 2012 Sep;120(3):516-23.
6. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):474-80.
7. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):134-8.
8. Obstet Gynecol. 2009 Aug;114(2 Pt 1):231-5.
9. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23.
10. Int J Surg. 2015 Jul;19:27-30.
11. Maturitas. 2006 Feb 20;53(3):282-98.
12. Obstet Gynecol. 2014 Oct;124(4):705-8.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Medicare's Managed Care Option Trades Off With Patient Preferences
CHICAGO - Medicare enrollees are moving in greater numbers than ever to the program's managed care option as a way to save money. But the tradeoff is much less ability to use their preferred doctors and hospitals.
Seniors can choose between traditional fee-for-service Medicare - which is accepted by most healthcare providers - or a Medicare Advantage plan. The latter encompasses health maintenance organizations (HMOs) or preferred provider organizations (PPOs), which control costs by creating healthcare provider networks that enrollees must use.
In theory, prospective Advantage enrollees can review lists of in-network providers before opting into a plan. But a new study by the Kaiser Family Foundation (KFF) finds that provider data often is very difficult to review, can be out of date and frequently contain inaccurate information.
KFF's review also found shortcomings in the quality of providers in some Medicare Advantage provider networks. One out of every five plans did not include a regional academic medical center - institutions which usually offer the highest quality care and top specialists. And only 40 percent of Advantage provider networks included top-quality cancer centers, as indicated by membership in the National Cancer Institute's network.
NCI-designated cancer centers offer cutting-edge treatments and tend to have greater access to clinical trials. They are especially important for patients with rare and advanced cancers, or other complicating conditions, said Gretchen Jacobson, KFF's associate director of the program on Medicare policy and co-author of the study.
The upshot: Medicare Advantage may be just fine if you are healthy, but problems may crop up if your healthcare needs become more complex and you have very specific healthcare provider preferences.
This year, 31 percent of Medicare enrollees are in Advantage plans, up from 11 percent in 2010. That number is expected to hit 41 percent by 2026, according to a forecast by the Congressional Budget Office.
When you sign up for Advantage, your Part B premium goes to the insurance company providing the plan. The largest providers are UnitedHealthcare, Humana Inc and Blue Cross Blue Shield.
One often hears critics claim that healthcare providers are bailing out of traditional Medicare in large numbers - but that is not actually the case. Last year, 14 percent of Medicare enrollees who were seeking a new primary care doctor reported major problems in finding a physician who would treat them, according to survey data from the Medicare Payment Advisory Commission, an independent congressional agency. Among those seeking a new specialist, 6 percent reported major problems. In both cases, that represents 1 percent of the total Medicare population.
ADVANTAGES, PITFALLS
Advantage plans often offer extra benefits, such as health club memberships, vision care and some limited dental care. Cost-sharing is often lower, and many plans provide prescription drug coverage with no extra premium. "It can be very attractive to many seniors who are living on a fixed budget," Jacobson said.
The trade-off is limited provider networks - and the challenges prospective enrollees face in determining who they are allowed to see for healthcare, and who is off-limits. KFF reviewed 409 Advantage plans, including 307 HMOs and 102 PPOs. Researchers found provider directories often were riddled with errors, omissions and outdated information.
"There's no reason in this era of technology why this needs to be as difficult as it is," Jacobson said. "People should be able to simply tell the system who their doctors are, the illnesses they have, and get a recommendation for a plan that will work for them."
KFF also found that Advantage provider network quality differs significantly. For example, Los Angeles has three NCI-designated cancer centers. Most of the Advantage plans there do not include any of them, but one plan includes all three.
A report last year by the U.S. Government Accountability Office found that the Centers for Medicare & Medicaid Services (CMS), which runs Medicare, needs to improve its oversight of Advantage plans to assure that provider networks are robust. The report also criticized CMS for doing too little to assess the accuracy of Advantage plan provider lists.
Even when Advantage enrollees are able to confirm participation by their healthcare providers, there is no guarantee that will continue. Advantage plans are free to add or drop health providers during the course of an enrollment season.
That became an especially hot issue in 2014 when UnitedHealthcare dropped providers who covered thousands of the insurer's patients, including the prominent Yale-New Haven Hospital system.
Democrats in Congress have proposed legislation that would prohibit Advantage plans from dropping providers without cause during the middle of an enrollment year.
Under current rules, plans must provide 30 days' notice to enrollees when providers are dropped. Enrollees who lose access to a provider can make a midyear plan change only under very limited circumstances. "You can do it only if you are receiving ongoing care from a provider that is terminated," Jacobson said. "Otherwise you need to wait until the next open enrollment period."
The annual enrollment period for Advantage and Part D prescription drug plans are held from Oct. 15 to Dec. 7 each year. At that point, a beneficiary could switch to a different Advantage plan, or shift back to traditional Medicare. But a serious diagnosis in January would leave you hamstrung until the following year.
Said Jacobson: "It can be a roll of the dice."
(The opinions expressed here are those of the author, a columnist for Reuters.)
CHICAGO - Medicare enrollees are moving in greater numbers than ever to the program's managed care option as a way to save money. But the tradeoff is much less ability to use their preferred doctors and hospitals.
Seniors can choose between traditional fee-for-service Medicare - which is accepted by most healthcare providers - or a Medicare Advantage plan. The latter encompasses health maintenance organizations (HMOs) or preferred provider organizations (PPOs), which control costs by creating healthcare provider networks that enrollees must use.
In theory, prospective Advantage enrollees can review lists of in-network providers before opting into a plan. But a new study by the Kaiser Family Foundation (KFF) finds that provider data often is very difficult to review, can be out of date and frequently contain inaccurate information.
KFF's review also found shortcomings in the quality of providers in some Medicare Advantage provider networks. One out of every five plans did not include a regional academic medical center - institutions which usually offer the highest quality care and top specialists. And only 40 percent of Advantage provider networks included top-quality cancer centers, as indicated by membership in the National Cancer Institute's network.
NCI-designated cancer centers offer cutting-edge treatments and tend to have greater access to clinical trials. They are especially important for patients with rare and advanced cancers, or other complicating conditions, said Gretchen Jacobson, KFF's associate director of the program on Medicare policy and co-author of the study.
The upshot: Medicare Advantage may be just fine if you are healthy, but problems may crop up if your healthcare needs become more complex and you have very specific healthcare provider preferences.
This year, 31 percent of Medicare enrollees are in Advantage plans, up from 11 percent in 2010. That number is expected to hit 41 percent by 2026, according to a forecast by the Congressional Budget Office.
When you sign up for Advantage, your Part B premium goes to the insurance company providing the plan. The largest providers are UnitedHealthcare, Humana Inc and Blue Cross Blue Shield.
One often hears critics claim that healthcare providers are bailing out of traditional Medicare in large numbers - but that is not actually the case. Last year, 14 percent of Medicare enrollees who were seeking a new primary care doctor reported major problems in finding a physician who would treat them, according to survey data from the Medicare Payment Advisory Commission, an independent congressional agency. Among those seeking a new specialist, 6 percent reported major problems. In both cases, that represents 1 percent of the total Medicare population.
ADVANTAGES, PITFALLS
Advantage plans often offer extra benefits, such as health club memberships, vision care and some limited dental care. Cost-sharing is often lower, and many plans provide prescription drug coverage with no extra premium. "It can be very attractive to many seniors who are living on a fixed budget," Jacobson said.
The trade-off is limited provider networks - and the challenges prospective enrollees face in determining who they are allowed to see for healthcare, and who is off-limits. KFF reviewed 409 Advantage plans, including 307 HMOs and 102 PPOs. Researchers found provider directories often were riddled with errors, omissions and outdated information.
"There's no reason in this era of technology why this needs to be as difficult as it is," Jacobson said. "People should be able to simply tell the system who their doctors are, the illnesses they have, and get a recommendation for a plan that will work for them."
KFF also found that Advantage provider network quality differs significantly. For example, Los Angeles has three NCI-designated cancer centers. Most of the Advantage plans there do not include any of them, but one plan includes all three.
A report last year by the U.S. Government Accountability Office found that the Centers for Medicare & Medicaid Services (CMS), which runs Medicare, needs to improve its oversight of Advantage plans to assure that provider networks are robust. The report also criticized CMS for doing too little to assess the accuracy of Advantage plan provider lists.
Even when Advantage enrollees are able to confirm participation by their healthcare providers, there is no guarantee that will continue. Advantage plans are free to add or drop health providers during the course of an enrollment season.
That became an especially hot issue in 2014 when UnitedHealthcare dropped providers who covered thousands of the insurer's patients, including the prominent Yale-New Haven Hospital system.
Democrats in Congress have proposed legislation that would prohibit Advantage plans from dropping providers without cause during the middle of an enrollment year.
Under current rules, plans must provide 30 days' notice to enrollees when providers are dropped. Enrollees who lose access to a provider can make a midyear plan change only under very limited circumstances. "You can do it only if you are receiving ongoing care from a provider that is terminated," Jacobson said. "Otherwise you need to wait until the next open enrollment period."
The annual enrollment period for Advantage and Part D prescription drug plans are held from Oct. 15 to Dec. 7 each year. At that point, a beneficiary could switch to a different Advantage plan, or shift back to traditional Medicare. But a serious diagnosis in January would leave you hamstrung until the following year.
Said Jacobson: "It can be a roll of the dice."
(The opinions expressed here are those of the author, a columnist for Reuters.)
CHICAGO - Medicare enrollees are moving in greater numbers than ever to the program's managed care option as a way to save money. But the tradeoff is much less ability to use their preferred doctors and hospitals.
Seniors can choose between traditional fee-for-service Medicare - which is accepted by most healthcare providers - or a Medicare Advantage plan. The latter encompasses health maintenance organizations (HMOs) or preferred provider organizations (PPOs), which control costs by creating healthcare provider networks that enrollees must use.
In theory, prospective Advantage enrollees can review lists of in-network providers before opting into a plan. But a new study by the Kaiser Family Foundation (KFF) finds that provider data often is very difficult to review, can be out of date and frequently contain inaccurate information.
KFF's review also found shortcomings in the quality of providers in some Medicare Advantage provider networks. One out of every five plans did not include a regional academic medical center - institutions which usually offer the highest quality care and top specialists. And only 40 percent of Advantage provider networks included top-quality cancer centers, as indicated by membership in the National Cancer Institute's network.
NCI-designated cancer centers offer cutting-edge treatments and tend to have greater access to clinical trials. They are especially important for patients with rare and advanced cancers, or other complicating conditions, said Gretchen Jacobson, KFF's associate director of the program on Medicare policy and co-author of the study.
The upshot: Medicare Advantage may be just fine if you are healthy, but problems may crop up if your healthcare needs become more complex and you have very specific healthcare provider preferences.
This year, 31 percent of Medicare enrollees are in Advantage plans, up from 11 percent in 2010. That number is expected to hit 41 percent by 2026, according to a forecast by the Congressional Budget Office.
When you sign up for Advantage, your Part B premium goes to the insurance company providing the plan. The largest providers are UnitedHealthcare, Humana Inc and Blue Cross Blue Shield.
One often hears critics claim that healthcare providers are bailing out of traditional Medicare in large numbers - but that is not actually the case. Last year, 14 percent of Medicare enrollees who were seeking a new primary care doctor reported major problems in finding a physician who would treat them, according to survey data from the Medicare Payment Advisory Commission, an independent congressional agency. Among those seeking a new specialist, 6 percent reported major problems. In both cases, that represents 1 percent of the total Medicare population.
ADVANTAGES, PITFALLS
Advantage plans often offer extra benefits, such as health club memberships, vision care and some limited dental care. Cost-sharing is often lower, and many plans provide prescription drug coverage with no extra premium. "It can be very attractive to many seniors who are living on a fixed budget," Jacobson said.
The trade-off is limited provider networks - and the challenges prospective enrollees face in determining who they are allowed to see for healthcare, and who is off-limits. KFF reviewed 409 Advantage plans, including 307 HMOs and 102 PPOs. Researchers found provider directories often were riddled with errors, omissions and outdated information.
"There's no reason in this era of technology why this needs to be as difficult as it is," Jacobson said. "People should be able to simply tell the system who their doctors are, the illnesses they have, and get a recommendation for a plan that will work for them."
KFF also found that Advantage provider network quality differs significantly. For example, Los Angeles has three NCI-designated cancer centers. Most of the Advantage plans there do not include any of them, but one plan includes all three.
A report last year by the U.S. Government Accountability Office found that the Centers for Medicare & Medicaid Services (CMS), which runs Medicare, needs to improve its oversight of Advantage plans to assure that provider networks are robust. The report also criticized CMS for doing too little to assess the accuracy of Advantage plan provider lists.
Even when Advantage enrollees are able to confirm participation by their healthcare providers, there is no guarantee that will continue. Advantage plans are free to add or drop health providers during the course of an enrollment season.
That became an especially hot issue in 2014 when UnitedHealthcare dropped providers who covered thousands of the insurer's patients, including the prominent Yale-New Haven Hospital system.
Democrats in Congress have proposed legislation that would prohibit Advantage plans from dropping providers without cause during the middle of an enrollment year.
Under current rules, plans must provide 30 days' notice to enrollees when providers are dropped. Enrollees who lose access to a provider can make a midyear plan change only under very limited circumstances. "You can do it only if you are receiving ongoing care from a provider that is terminated," Jacobson said. "Otherwise you need to wait until the next open enrollment period."
The annual enrollment period for Advantage and Part D prescription drug plans are held from Oct. 15 to Dec. 7 each year. At that point, a beneficiary could switch to a different Advantage plan, or shift back to traditional Medicare. But a serious diagnosis in January would leave you hamstrung until the following year.
Said Jacobson: "It can be a roll of the dice."
(The opinions expressed here are those of the author, a columnist for Reuters.)