User login
FDA advisory panel unanimously backs biosimilars for Humira, Enbrel
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
Study identifies important predictors for PC/PGL
BALTIMORE – Tumor size and the presence of mutations of the succinate dehydrogenase complex subunit B (SDHB) gene may be reliable indicators of prognosis after surgery for pheochromocytoma and abdominal paraganglioma, investigators in a National Cancer Institute–funded study have reported.
“The staging of pheochromocytoma and abdominal paraganglioma can be difficult, but it is critical for optimal patient care,” Yasmine Assadipour, MD, of the National Cancer Institute, Bethesda, Md., and the George Washington University Hospital, Washington, reported at the annual meeting of the American Association of Endocrine Surgeons.
“Any clinically relevant grading or prognostic system should include SDHB mutation status and primary tumor size as prime features of scoring,” Dr. Assadipour said. “Histologic features such as Ki-67 or mitotic index may not be as useful for prognostic information in patients with pheochromocytoma and abdominal paraganglioma, particularly in the setting of SDHB mutation.”
Dr. Assadipour and her coinvestigators focused their investigation on mutations of the SDHB (succinate dehydrogenase complex subunit B) gene, which codes for one of four subunits comprising a mitochondrial protein.
They also considered primary tumor size, functionality, pathology, surgical approach, and histologic features including Ki-67 index and mitotic index. The study was a retrospective analysis of 84 patients who had surgery for PC [pheochromocytoma] or PGL [paraganglioma] and had germ line genetic testing. Of the 84 patients, 35 patients had sporadic disease and 49 had germ line SDHB mutation. The study analyzed tumor samples for Ki-67/MIB-1 staining and mitotic index.
“In a univariate analysis, SDHB mutation, tumor size and surgical approach were associated with local regional recurrence,” Dr. Assadipour said. “In a multivariate analysis, the only independent risk factors were SDHB mutation status and tumor size; Ki-67 and mitotic index did not have any association with recurrence.”
The researchers found similar results when they looked at distant metastasis. “SDHB mutation, tumor size, abdominal paraganglioma and surgical approach were associated with distant metastasis,” Dr. Assadipour said. “Again, Ki-67 and mitotic index were not.”
In the multivariate analysis, again, only patient SDHB status and tumor size were independently associated with metastasis.”
The incidence of local recurrence in patients with the SDHB mutation was 47.6% vs. 9.1% in those without the gene mutation, Dr. Assadipour said. The rates of distant metastasis showed a similar disparity: 56.5% and 9.1%, respectively.
Patients with the SDHB mutation presented at a younger age than those without the mutation, 33 vs. 49.6 years old. Among the 65 patients who underwent R0 primary tumor resection, those with the SDHB mutation, paraganglioma, and larger tumor size had a shorter disease-free survival, Dr. Assadipour said.
In analyzing tumor size, Dr. Assadipour said two stratifications were studied: evaluating tumors sized 0-3 cm, 3-6 cm and 6 cm and larger; and 0-5 cm and 5 cm and larger. “Tumors over 6 cm had the shortest disease-free survival, and even when we applied the under-5 cm and over-5 cm scale, we clearly saw a difference in disease-free survival,” she said. Ki-67 and mitotic index were not related to disease-free survival.
The presence of a SDHB mutation had a hazard ratio of 16.2, while tumor diameter greater than 6 cm had a HR of 15.4, Dr. Assadipour said. These were the only independent risk factors for local recurrence, distant metastases and shorter disease-free interval found in the study.
During the discussion, Lawrence T. Kim, MD, of the University of North Carolina asked if the researchers found any differences in outcomes related to the surgical approach. “We were unable to identify whether any surgical approach improved or worsened outcomes on multivariate analysis” Dr. Assadipour said.
Thomas J. Fahey, MD, of New York asked what she would recommend for surgical approaches for patients with PC and PGL.
“Our general recommendation is that an adrenal pheochromocytoma that is over 6 cm carries a higher risk of recurrence and distant metastasis so an open approach with lymph node dissection ensuring negative surgical margins should be considered,” Dr. Assadipour said. “For abdominal paragangliomas, unless they are quite small and in a favorable location, we would generally recommend an open approach.”
The study was supported by the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Dr. Assadipour and her coauthors had no financial relationships to disclose.
BALTIMORE – Tumor size and the presence of mutations of the succinate dehydrogenase complex subunit B (SDHB) gene may be reliable indicators of prognosis after surgery for pheochromocytoma and abdominal paraganglioma, investigators in a National Cancer Institute–funded study have reported.
“The staging of pheochromocytoma and abdominal paraganglioma can be difficult, but it is critical for optimal patient care,” Yasmine Assadipour, MD, of the National Cancer Institute, Bethesda, Md., and the George Washington University Hospital, Washington, reported at the annual meeting of the American Association of Endocrine Surgeons.
“Any clinically relevant grading or prognostic system should include SDHB mutation status and primary tumor size as prime features of scoring,” Dr. Assadipour said. “Histologic features such as Ki-67 or mitotic index may not be as useful for prognostic information in patients with pheochromocytoma and abdominal paraganglioma, particularly in the setting of SDHB mutation.”
Dr. Assadipour and her coinvestigators focused their investigation on mutations of the SDHB (succinate dehydrogenase complex subunit B) gene, which codes for one of four subunits comprising a mitochondrial protein.
They also considered primary tumor size, functionality, pathology, surgical approach, and histologic features including Ki-67 index and mitotic index. The study was a retrospective analysis of 84 patients who had surgery for PC [pheochromocytoma] or PGL [paraganglioma] and had germ line genetic testing. Of the 84 patients, 35 patients had sporadic disease and 49 had germ line SDHB mutation. The study analyzed tumor samples for Ki-67/MIB-1 staining and mitotic index.
“In a univariate analysis, SDHB mutation, tumor size and surgical approach were associated with local regional recurrence,” Dr. Assadipour said. “In a multivariate analysis, the only independent risk factors were SDHB mutation status and tumor size; Ki-67 and mitotic index did not have any association with recurrence.”
The researchers found similar results when they looked at distant metastasis. “SDHB mutation, tumor size, abdominal paraganglioma and surgical approach were associated with distant metastasis,” Dr. Assadipour said. “Again, Ki-67 and mitotic index were not.”
In the multivariate analysis, again, only patient SDHB status and tumor size were independently associated with metastasis.”
The incidence of local recurrence in patients with the SDHB mutation was 47.6% vs. 9.1% in those without the gene mutation, Dr. Assadipour said. The rates of distant metastasis showed a similar disparity: 56.5% and 9.1%, respectively.
Patients with the SDHB mutation presented at a younger age than those without the mutation, 33 vs. 49.6 years old. Among the 65 patients who underwent R0 primary tumor resection, those with the SDHB mutation, paraganglioma, and larger tumor size had a shorter disease-free survival, Dr. Assadipour said.
In analyzing tumor size, Dr. Assadipour said two stratifications were studied: evaluating tumors sized 0-3 cm, 3-6 cm and 6 cm and larger; and 0-5 cm and 5 cm and larger. “Tumors over 6 cm had the shortest disease-free survival, and even when we applied the under-5 cm and over-5 cm scale, we clearly saw a difference in disease-free survival,” she said. Ki-67 and mitotic index were not related to disease-free survival.
The presence of a SDHB mutation had a hazard ratio of 16.2, while tumor diameter greater than 6 cm had a HR of 15.4, Dr. Assadipour said. These were the only independent risk factors for local recurrence, distant metastases and shorter disease-free interval found in the study.
During the discussion, Lawrence T. Kim, MD, of the University of North Carolina asked if the researchers found any differences in outcomes related to the surgical approach. “We were unable to identify whether any surgical approach improved or worsened outcomes on multivariate analysis” Dr. Assadipour said.
Thomas J. Fahey, MD, of New York asked what she would recommend for surgical approaches for patients with PC and PGL.
“Our general recommendation is that an adrenal pheochromocytoma that is over 6 cm carries a higher risk of recurrence and distant metastasis so an open approach with lymph node dissection ensuring negative surgical margins should be considered,” Dr. Assadipour said. “For abdominal paragangliomas, unless they are quite small and in a favorable location, we would generally recommend an open approach.”
The study was supported by the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Dr. Assadipour and her coauthors had no financial relationships to disclose.
BALTIMORE – Tumor size and the presence of mutations of the succinate dehydrogenase complex subunit B (SDHB) gene may be reliable indicators of prognosis after surgery for pheochromocytoma and abdominal paraganglioma, investigators in a National Cancer Institute–funded study have reported.
“The staging of pheochromocytoma and abdominal paraganglioma can be difficult, but it is critical for optimal patient care,” Yasmine Assadipour, MD, of the National Cancer Institute, Bethesda, Md., and the George Washington University Hospital, Washington, reported at the annual meeting of the American Association of Endocrine Surgeons.
“Any clinically relevant grading or prognostic system should include SDHB mutation status and primary tumor size as prime features of scoring,” Dr. Assadipour said. “Histologic features such as Ki-67 or mitotic index may not be as useful for prognostic information in patients with pheochromocytoma and abdominal paraganglioma, particularly in the setting of SDHB mutation.”
Dr. Assadipour and her coinvestigators focused their investigation on mutations of the SDHB (succinate dehydrogenase complex subunit B) gene, which codes for one of four subunits comprising a mitochondrial protein.
They also considered primary tumor size, functionality, pathology, surgical approach, and histologic features including Ki-67 index and mitotic index. The study was a retrospective analysis of 84 patients who had surgery for PC [pheochromocytoma] or PGL [paraganglioma] and had germ line genetic testing. Of the 84 patients, 35 patients had sporadic disease and 49 had germ line SDHB mutation. The study analyzed tumor samples for Ki-67/MIB-1 staining and mitotic index.
“In a univariate analysis, SDHB mutation, tumor size and surgical approach were associated with local regional recurrence,” Dr. Assadipour said. “In a multivariate analysis, the only independent risk factors were SDHB mutation status and tumor size; Ki-67 and mitotic index did not have any association with recurrence.”
The researchers found similar results when they looked at distant metastasis. “SDHB mutation, tumor size, abdominal paraganglioma and surgical approach were associated with distant metastasis,” Dr. Assadipour said. “Again, Ki-67 and mitotic index were not.”
In the multivariate analysis, again, only patient SDHB status and tumor size were independently associated with metastasis.”
The incidence of local recurrence in patients with the SDHB mutation was 47.6% vs. 9.1% in those without the gene mutation, Dr. Assadipour said. The rates of distant metastasis showed a similar disparity: 56.5% and 9.1%, respectively.
Patients with the SDHB mutation presented at a younger age than those without the mutation, 33 vs. 49.6 years old. Among the 65 patients who underwent R0 primary tumor resection, those with the SDHB mutation, paraganglioma, and larger tumor size had a shorter disease-free survival, Dr. Assadipour said.
In analyzing tumor size, Dr. Assadipour said two stratifications were studied: evaluating tumors sized 0-3 cm, 3-6 cm and 6 cm and larger; and 0-5 cm and 5 cm and larger. “Tumors over 6 cm had the shortest disease-free survival, and even when we applied the under-5 cm and over-5 cm scale, we clearly saw a difference in disease-free survival,” she said. Ki-67 and mitotic index were not related to disease-free survival.
The presence of a SDHB mutation had a hazard ratio of 16.2, while tumor diameter greater than 6 cm had a HR of 15.4, Dr. Assadipour said. These were the only independent risk factors for local recurrence, distant metastases and shorter disease-free interval found in the study.
During the discussion, Lawrence T. Kim, MD, of the University of North Carolina asked if the researchers found any differences in outcomes related to the surgical approach. “We were unable to identify whether any surgical approach improved or worsened outcomes on multivariate analysis” Dr. Assadipour said.
Thomas J. Fahey, MD, of New York asked what she would recommend for surgical approaches for patients with PC and PGL.
“Our general recommendation is that an adrenal pheochromocytoma that is over 6 cm carries a higher risk of recurrence and distant metastasis so an open approach with lymph node dissection ensuring negative surgical margins should be considered,” Dr. Assadipour said. “For abdominal paragangliomas, unless they are quite small and in a favorable location, we would generally recommend an open approach.”
The study was supported by the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Dr. Assadipour and her coauthors had no financial relationships to disclose.
AT AAES 2016
Key clinical point: SDHB mutation and tumor size may be better predictors of outcomes in patients with pheochromocytoma and abdominal paraganglioma than are other previously identified predictors.
Major finding: The incidence of local recurrence in patients with the SDHB mutation was 47.6% vs. 9.1% in those without the gene mutation.
Data source: Retrospective analysis of 84 patients with PC/PGL evaluated by the Surgical Endocrine Oncology branch at George Washington University Hospital from 1998-2015.
Disclosures: The study was supported by the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Dr. Assadipour and her coauthors reported having no financial disclosures.
CMS: Projected overall growth rate in health spending holding firm
Health spending is projected to grow on average 5.8% from 2015-2025, the same projected rate of grown as announced last year covering 2014-2024, according the CMS Office of the Actuary.
However, health care is projected to make up 20.1% of the economy at the end of the 10-year period, up from 17.5% in 2014, as health spending is projected to grow 1.3 percentage points faster than gross domestic product from 2015-2025. The analysis was published online July 13 in the journal Health Affairs.
Health spending for 2015 is projected to have grown 5.5%, up from 5.3% in 2014, and to have reached $3.2 trillion, driven in part by increased use of health services by newly insured patients. More than 9 in 10 (92%) of U.S. residents are projected to be insured by 2025, according to the actuary’s office.
While national spending per capita is projected to exceed $10,000 for the first time in 2016, spending growth is projected to slow to 4.8%, driven by slowdowns in Medicaid spending after 2 years of rapid growth.
Private health insurance expenditures are expected to grow at a similar rate (5.4%) through 2025, CMS actuaries said.
Medical price inflation slowed to 0.8% in 2015 from 1.4% in 2014. Hospital prices increased by 0.9% while prices for physician services dropped 1.1%, they noted.
Prescription drug spending is projected to grow an average of 6.7% from 2016 to 2025, slowing down from 12.2% in 2014 and 8.1% in 2015 when a number of high-priced specialty drugs, including those treating hepatitis C, were driving higher spending.
Health spending is projected to grow on average 5.8% from 2015-2025, the same projected rate of grown as announced last year covering 2014-2024, according the CMS Office of the Actuary.
However, health care is projected to make up 20.1% of the economy at the end of the 10-year period, up from 17.5% in 2014, as health spending is projected to grow 1.3 percentage points faster than gross domestic product from 2015-2025. The analysis was published online July 13 in the journal Health Affairs.
Health spending for 2015 is projected to have grown 5.5%, up from 5.3% in 2014, and to have reached $3.2 trillion, driven in part by increased use of health services by newly insured patients. More than 9 in 10 (92%) of U.S. residents are projected to be insured by 2025, according to the actuary’s office.
While national spending per capita is projected to exceed $10,000 for the first time in 2016, spending growth is projected to slow to 4.8%, driven by slowdowns in Medicaid spending after 2 years of rapid growth.
Private health insurance expenditures are expected to grow at a similar rate (5.4%) through 2025, CMS actuaries said.
Medical price inflation slowed to 0.8% in 2015 from 1.4% in 2014. Hospital prices increased by 0.9% while prices for physician services dropped 1.1%, they noted.
Prescription drug spending is projected to grow an average of 6.7% from 2016 to 2025, slowing down from 12.2% in 2014 and 8.1% in 2015 when a number of high-priced specialty drugs, including those treating hepatitis C, were driving higher spending.
Health spending is projected to grow on average 5.8% from 2015-2025, the same projected rate of grown as announced last year covering 2014-2024, according the CMS Office of the Actuary.
However, health care is projected to make up 20.1% of the economy at the end of the 10-year period, up from 17.5% in 2014, as health spending is projected to grow 1.3 percentage points faster than gross domestic product from 2015-2025. The analysis was published online July 13 in the journal Health Affairs.
Health spending for 2015 is projected to have grown 5.5%, up from 5.3% in 2014, and to have reached $3.2 trillion, driven in part by increased use of health services by newly insured patients. More than 9 in 10 (92%) of U.S. residents are projected to be insured by 2025, according to the actuary’s office.
While national spending per capita is projected to exceed $10,000 for the first time in 2016, spending growth is projected to slow to 4.8%, driven by slowdowns in Medicaid spending after 2 years of rapid growth.
Private health insurance expenditures are expected to grow at a similar rate (5.4%) through 2025, CMS actuaries said.
Medical price inflation slowed to 0.8% in 2015 from 1.4% in 2014. Hospital prices increased by 0.9% while prices for physician services dropped 1.1%, they noted.
Prescription drug spending is projected to grow an average of 6.7% from 2016 to 2025, slowing down from 12.2% in 2014 and 8.1% in 2015 when a number of high-priced specialty drugs, including those treating hepatitis C, were driving higher spending.
FROM HEALTH AFFAIRS
Primary Cutaneous Dermal Mucinosis on Herpes Zoster Scars
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
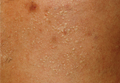
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.
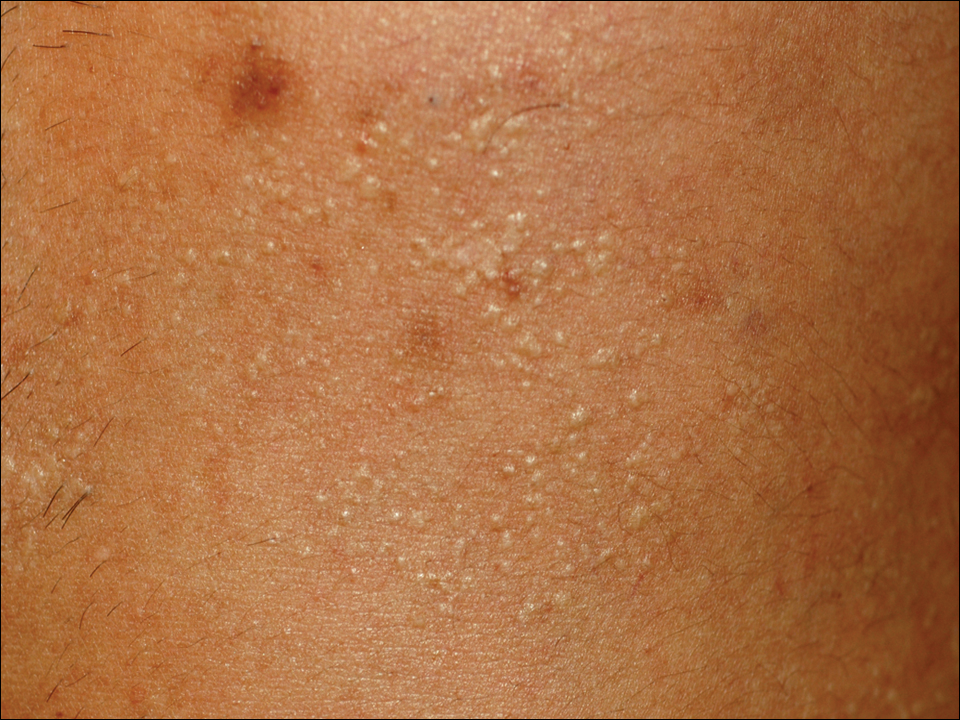
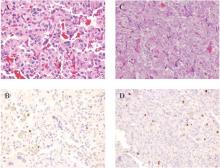
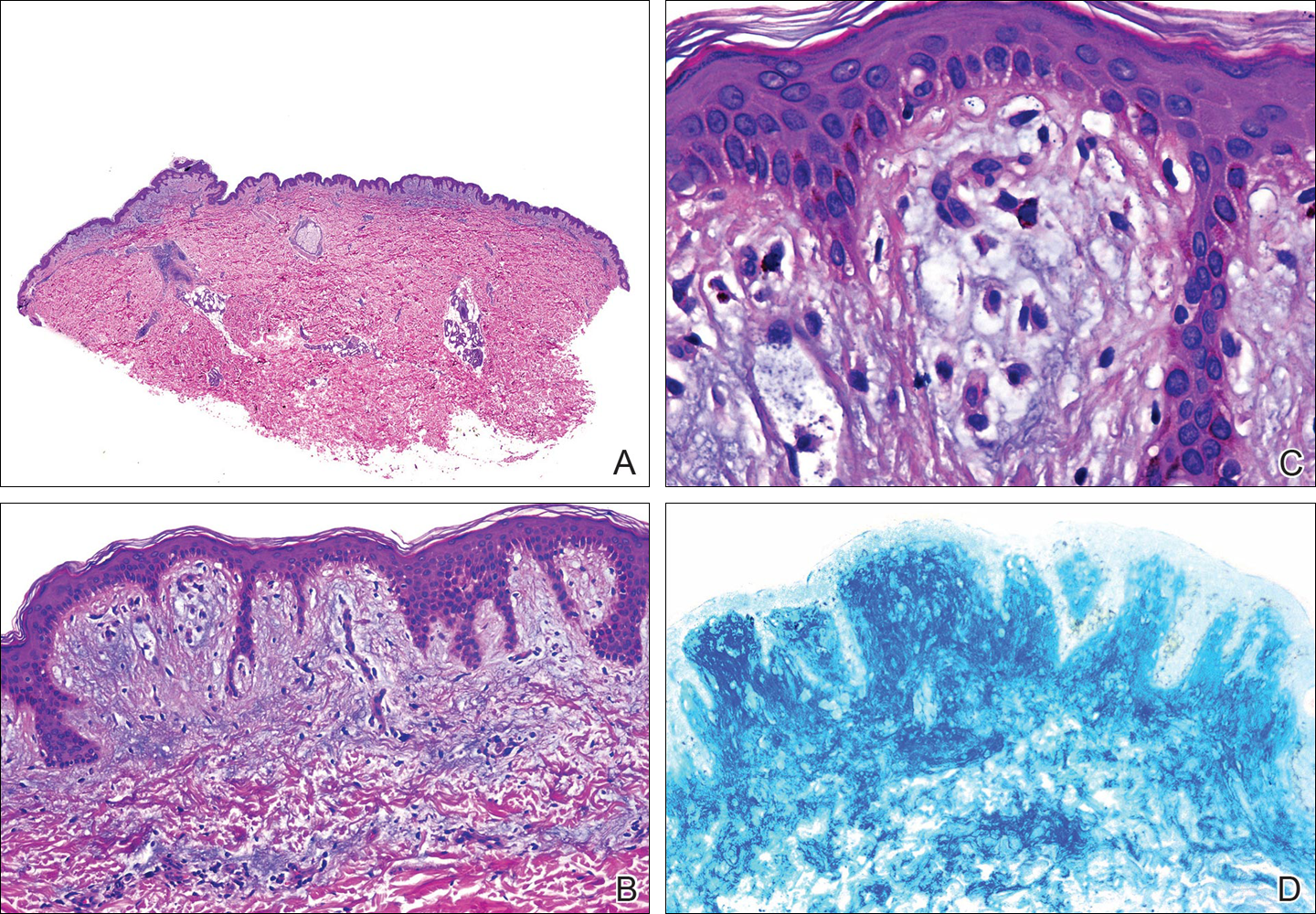
Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.
Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.

Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.

Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
Mucin is an amorphous gelatinous substance that is found in a large variety of tissues. There are 2 types of cutaneous mucin: dermal and epithelial. Both types appear as basophilic shreds and granules with hematoxylin and eosin stain.1 Epithelial mucin (sialomucin) is found mainly in the gastrointestinal tract and lungs. In the skin, it is present in the cytoplasm of the dark cells of the eccrine glands and in the apocrine secretory cells. Epithelial mucin contains both neutral and acid glycosaminoglycans, stains positive with Alcian blue (pH 2.5) and periodic acid–Schiff, is resistant to hyaluronidase, and does not stain metachromatically with toluidine blue. Dermal mucin is composed of acid glycosaminoglycans (eg, dermatan sulfate, chondroitin 6-sulfate, chondroitin 4-sulfate, hyaluronic acid) and normally is produced by dermal fibroblasts. Dermal mucin stains positive with Alcian blue (pH 2.5); is periodic acid–Schiff negative and sensitive to hyaluronidase; and shows metachromasia with toluidine blue, methylene blue, and thionine.
Cutaneous mucinosis comprises a heterogeneous group of skin disorders characterized by the deposition of mucin in the interstices of the dermis. These diseases may be classified as primary mucinosis with the mucin deposition as the main histologic feature resulting in clinically distinctive lesions and secondary mucinosis with the mucin deposition as an additional histologic finding within the context of an independent skin disease or lesion (eg, basal cell carcinoma) with deposits of mucin in the stroma. Primary cutaneous mucinosis may be subclassified into 2 groups: degenerative-inflammatory mucinoses and neoplastic-hamartomatous mucinoses. According to the histologic features, the degenerative-inflammatory mucinoses are better divided into dermal and follicular mucinoses.2 We describe a case of primary cutaneous dermal mucinosis on herpes zoster (HZ) scars as an isotopic response.
Case Report
A 33-year-old man presented to the dermatology department with slightly pruritic lesions on the left side of the chest and back that had appeared progressively at the site of HZ scars that had healed without treatment 9 months prior. Dermatologic examination revealed sharply defined whitish papules (Figure 1) measuring 2 to 4 mm in diameter with a smooth surface and linear distribution over the area of the left T8 and T9 dermatomes. The patient reported no postherpetic neuralgia and was otherwise healthy. Laboratory tests including a complete blood cell count, biochemistry, urinalysis, and determination of free thyroid hormones were within reference range. Serologic tests for human immunodeficiency virus, hepatitis B and C viruses, and syphilis were negative. Antinuclear antibodies also were negative.

Histopathology demonstrated abundant bluish granular material between collagen bundles of the papillary dermis (Figure 2). No cytopathologic signs of active herpetic infection were seen. The Alcian blue stain at pH 2.5 was strongly positive for mucin, which confirmed the diagnosis of primary cutaneous dermal mucinosis.
Topical corticosteroids were applied for 2 months with no notable improvement. The lesions gradually improved without any other therapy during the subsequent 6 months.

Comment
The occurrence of a new skin disease at the exact site of a prior unrelated cutaneous disorder that had already resolved was first reported by Wyburn-Mason3 in 1955. Forty years later, the term isotopic response was coined by Wolf et al4 to describe this phenomenon. Diverse types of skin diseases such as herpes simplex virus,5 varicella-zoster infections,4 and thrombophlebitis4 have been implicated in cases of isotopic response, but the most frequently associated primary disorder by far is cutaneous HZ.
Several benign and malignant disorders may occur at sites of resolved HZ lesions, including granulomatous dermatitis,6 granuloma annulare,7 fungal granuloma,8 fungal folliculitis,9 psoriasis,10 morphea,11 lichen sclerosus,12 Kaposi sarcoma,13 the lichenoid variant of chronic graft-versus-host disease,14 cutaneous sarcoidosis,15 granulomatous folliculitis,16 comedones,17 furuncles,18 erythema annulare centrifugum,19 eosinophilic dermatosis,20 cutaneous pseudolymphoma,21 granulomatous vasculitis,22 Rosai-Dorfman disease,12 xanthomatous changes,23 tuberculoid granulomas,24 acneform eruption,25 lichen planus,26 acquired reactive perforating collagenosis,27 lymphoma,28 leukemia,29 angiosarcoma,30 basal cell carcinoma,31 squamous cell carcinoma, and cutaneous metastasis from internal carcinoma.32 The interval between the acute HZ episode and presentation of the second disease is quite variable, ranging from days to several months. Postzoster isotopic response has been described in individuals with varying degrees of immune response, affecting both immunocompetent12 and immunocompromised patients.14 There is no predilection for age, sex, or race. It also seems that antiviral treatment during the active episode does not prevent the development of secondary reactions.Kim et al33 reported a 59-year-old woman who developed flesh-colored or erythematous papules on HZ scars over the area of the left T1 and T2 dermatomes 1 week after the active viral process. Histopathologic study demonstrated deposition of mucin between collagen bundles in the dermis. The authors established the diagnosis of secondary cutaneous mucinosis as an isotopic response.33 Nevertheless, we believe that based on the aforementioned classification of cutaneous mucinosis,2 both this case and our case are better considered as primary cutaneous dermal mucinosis, as the mucin deposition in the dermis was the main histologic finding resulting in a distinctive cutaneous disorder. In the case reported by Kim et al,33 a possible relationship between cutaneous mucinosis and postherpetic neuralgia was suggested based on the slow regression of skin lesions in accordance with the improvement of the neuralgic pain; however, our patient did not have postherpetic neuralgia and the lesions persisted unchanged several months after the acute HZ episode. In the literature, there are reports of primary cutaneous dermal mucinosis associated with altered thyroid function34; autoimmune connective tissue diseases, mostly lupus erythematosus35; monoclonal gammopathy36; and human immunodeficiency virus infection,37 but these possibilities were ruled out in our patient by pertinent laboratory studies.
The pathogenesis of the postherpetic isotopic response remains unknown, but several mechanisms have been proposed. Some authors have suggested that postzoster dermatoses may represent isomorphic response of Köbner phenomenon.13,15 Although isomorphic and isotopic responses share some similarities, these terms describe 2 different phenomena: the first refers to the appearance of the same cutaneous disorder at a different site favored by trauma, while the second manifests a new and unrelated disease at the same location.38 Local anatomic changes such as altered microcirculation, collagen rearrangement, and an imperfect skin barrier may promote a prolonged local inflammatory response. Moreover, the destruction of nerve fibers by the varicella-zoster virus may indirectly influence the local immune system through the release of specific neuropeptides in the skin.39 It has been speculated that some secondary reactions may be the result of type III and type IV hypersensitivity reactions40 to viral antigens or to tissue antigens modified by the virus, inducing either immune hypersensitivity or local immune suppression.41 Some authors have documented the presence of varicella-zoster DNA within early postzoster lesions6,7 by using polymerase chain reaction in early lesions but not in late-stage and residual lesions.12,22 Nikkels et al42 studied early granulomatous lesions by immunohistochemistry and in situ hybridization techniques and concluded that major viral envelope glycoproteins (glycoproteins I and II) rather than complete viral particles could be responsible for delayed-type hypersensitivity reactions. All these findings suggest that secondary reactions presenting on HZ scars are mainly the result of atypical immune reactions to local antigenic stimuli.
The pathogenesis of our case is unknown. From a theoretical point of view, it is possible that varicella-zoster virus may induce fibroblastic proliferation and mucin production on HZ scars; however, if HZ is a frequent process and the virus may induce mucin production, then focal dermal mucinosis in an HZ scar should be a common finding. In our patient, there was no associated disease favoring the development of the cutaneous mucinosis. These localized variants of primary cutaneous mucinosis usually do not require therapy, and a wait-and-see approach is recommended. Topical applications of corticosteroids, pimecrolimus, or tacrolimus, as well as oral isotretinoin, may have some benefit,43 but spontaneous resolution may occur.44 In our patient, topical corticosteroids were applied 2 months following initial presentation without any benefit and the cutaneous lesions gradually improved without any therapy during the subsequent 6 months. Focal dermal mucinosis should be added to the list of cutaneous reactions that may develop in HZ scars.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
- Truhan AP, Roenigk HH Jr. The cutaneous mucinoses. J Am Acad Dermatol. 1986;14:1-18.
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. BMJ. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Ruocco E. Genital warts at the site of healed herpes progenitalis: the isotopic response. Int J Dermatol. 2000;39:705-706.
- Serfling U, Penneys NS, Zhu WY, et al. Varicella-zoster virus DNA in granulomatous skin lesions following herpes zoster. a study by the polymerase chain reaction. J Cutan Pathol. 1993;20:28-33.
- Gibney MD, Nahass GT, Leonardi CL. Cutaneous reactions following herpes zoster infections: report of three cases and a review of the literature. Br J Dermatol. 1996;134:504-509.
- Huang CW, Tu ME, Wu YH, et al. Isotopic response of fungal granuloma following facial herpes zoster infections-report of three cases. Int J Dermatol. 2007;46:1141-1145.
- Tüzün Y, Işçimen A, Göksügür N, et al. Wolf’s isotopic response: Trichophyton rubrum folliculitis appearing on a herpes zoster scar. Int J Dermatol. 2000;39:766-768.
- Allegue F, Fachal C, Romo M, et al. Psoriasis at the site of healed herpes zoster: Wolf’s isotopic response. Actas Dermosifiliogr. 2007;98:576-578.
- Forschner A, Metzler G, Rassner G, et al. Morphea with features of lichen sclerosus et atrophicus at the site of a herpes zoster scar: another case of an isotopic response. Int J Dermatol. 2005;44:524-525.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Niedt GW, Prioleau PG. Kaposi’s sarcoma occurring in a dermatome previously involved by herpes zoster. J Am Acad Dermatol. 1988;18:448-451.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Cecchi R, Giomi A. Scar sarcoidosis following herpes zoster. J Eur Acad Dermatol Venereol. 1999;12:280-282.
- Fernández-Redondo V, Amrouni B, Varela E, et al. Granulomatous folliculitis at sites of herpes zoster scars: Wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2002;16:628-630.
- Sanchez-Salas MP. Appearance of comedones at the site of healed herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2011;50:633-634.
- Ghorpade A. Wolf’s isotopic response—furuncles at the site of healed herpes zoster in an Indian male. Int J Dermatol. 2010;49:105-107.
- Lee HW, Lee DK, Rhee DY, et al. Erythema annulare centrifugum following herpes zoster infection: Wolf’s isotopic response? Br J Dermatol. 2005;153:1241-1243.
- Mitsuhashi Y, Kondo S. Post-zoster eosinophilic dermatosis. Br J Dermatol. 1997;136:465-466.
- Roo E, Villegas C, Lopez-Bran E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130:661-663.
- Langenberg A, Yen TS, LeBoit PE. Granulomatous vasculitis occurring after cutaneous herpes zoster despite absence of viral genome. J Am Acad Dermatol. 1991;24:429-433.
- Weidman F, Boston LN. Generalized xanthoma tuberosum with xantomathous changes in fresh scars of intercurrent zoster. Arch Intern Med. 1937;59:793-822.
- Olalquiaga J, Minaño R, Barrio J. Granuloma tuberculoide post-herpético en un paciente con leucemia linfocítica crónica. Med Cutan ILA. 1995;23:113-115.
- Stubbings JM, Goodfield MJ. An unusual distribution of an acneiform rash due to herpes zoster infection. Clin Exp Dermatol. 1993;18:92-93.
- Shemer A, Weiss G, Trau H. Wolf’s isotopic response: a case of zosteriform lichen planus on the site of healed herpes zoster. J Eur Acad Dermatol Venereol. 2001;15:445-447.
- Bang SW, Kim YK, Whang KU. Acquired reactive perforating collagenosis: unilateral umbilicated papules along the lesions of herpes zoster. J Am Acad Dermatol. 1997;36:778-779.
- Paydaş S, Sahin B, Yavuz S, et al. Lymphomatous skin infiltration at the site of previous varicella zoster virus infection in a patient with T cell lymphoma. Leuk Lymphoma. 2000;37:229-232.
- Cerroni L, Kerl H. Cutaneous localization of B-cell chronic lymphocytic leukemia at the site of varicella/herpes virus eruptions. J Am Acad Dermatol. 1997;37:1022.
- Hudson CP, Hanno R, Callen JP. Cutaneous angiosarcoma in a site of healed herpes zoster. Int J Dermatol. 1984;23:404-407.
- Wyburn-Mason R. Visceral lesions in herpes zoster. Br Med J. 1957;1:678-681.
- Caroti A. Metastasi cutanee di a adenocarcinoma papillifero ovarico in sede di herpes zoster. Chron Dermatol. 1987;18:769-773.
- Kim MB, Jwa SW, Ko HC, et al. A case of secondary cutaneous mucinosis following herpes zoster: Wolf’s isotopic response. Int J Dermatol. 2009;48:212-214.
- Burman KD, McKinley-Grant L. Dermatologic aspects of thyroid disease. Clin Dermatol. 2006;24:247-255.
- Shekari AM, Ghiasi M, Ghasemi E, et al. Papulonodular mucinosis indicating systemic lupus erythematosus. Clin Exp Dermatol. 2009;34:558-560.
- Dinneen AM, Dicken CH. Scleromyxedema. J Am Acad Dermatol. 1995;33:37-43.
- Rongioletti F, Ghigliotti G, De Marchi R, et al. Cutaneous mucinoses and HIV infection. Br J Dermatol. 1998;139:1077-1080.
- Krahl D, Hartschuh W, Tilgen W. Granuloma annulare perforans in herpes zoster scars. J Am Acad Dermatol. 1993;29:859-862.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- Fisher G, Jaworski R. Granuloma formation in herpes zoster scars. J Am Acad Dermatol. 1987;16:1261-1263.
- Ruocco V, Grimaldi Filioli F. La risposta isotopica post-erpetica: possibile sequela di un locus minoris resistentiae acquisito. G Ital Dermatol Venereol. 1999;134:547-552.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;5:530-532.
- Kwon OS, Moon SE, Kim JA, et al. Lichen myxodematosus with rapid spontaneous regression. Br J Dermatol. 1997;136:295-296.
Practice Points
- Focal mucinosis is a histopathologic finding that may be seen in different cutaneous disorders. It is an exceptional histopathologic finding that has rarely been described in herpes zoster scars.
- In most cases, focal mucinosis is just a histopathologic finding with no therapeutic consequences.
Olanzapine helps prevent nausea in patients on chemo
Olanzapine is more effective than placebo, in combination with a 5-HT3-receptor antagonist and an NK1-receptor antagonist, in preventing nausea in patients undergoing chemotherapy, according to investigators.
“This large, randomized, double-blind, placebo-controlled, phase III trial showed that it is more effective to combine olanzapine than placebo with an NK1-receptor antagonist, a 5-HT3–receptor antagonist, and dexamethasone for the prevention of nausea and vomiting in patients who have not received previous chemotherapy but are currently receiving highly emetogenic chemotherapy,” reported Rudolph Navari, MD, PhD, of the World Health Organization, Geneva, and his associates (N Engl J Med. 2016;375:134-42).
Patients were randomized to receive olanzapine or the placebo, along with a 5-HT3-receptor antagonist (either palonosetron intravenously, granisetron intravenously or orally, or ondansetron intravenously or orally) and an NK1-receptor antagonist (fosaprepitant intravenously or aprepitant orally). The olanzapine (n = 192) and placebo (n = 188) groups were balanced with respect to age, race, sex, and chemotherapy administered.
Patients kept daily records of nausea and episodes of vomiting. The proportion of patients who reported no nausea or who experienced no clinically significant nausea was significantly greater in the olanzapine group than in the placebo group (37% vs. 22%, P = .002 and 67% vs. 49%, P = .001).
Patients receiving olanzapine had significantly increased sedation (severe in 5%) on day 2 compared with baseline, Dr. Navari and his associates reported. The sedation resolved on days 3, 4, and 5 even though patients continued to receive the drug on days 3 and 4. No patients discontinued the study because of sedation.
The National Cancer Institute funded the study. One investigator reported receiving financial support from Merck and Co. The other investigators reported having no disclosures.
On Twitter @jessnicolecraig
Olanzapine is more effective than placebo, in combination with a 5-HT3-receptor antagonist and an NK1-receptor antagonist, in preventing nausea in patients undergoing chemotherapy, according to investigators.
“This large, randomized, double-blind, placebo-controlled, phase III trial showed that it is more effective to combine olanzapine than placebo with an NK1-receptor antagonist, a 5-HT3–receptor antagonist, and dexamethasone for the prevention of nausea and vomiting in patients who have not received previous chemotherapy but are currently receiving highly emetogenic chemotherapy,” reported Rudolph Navari, MD, PhD, of the World Health Organization, Geneva, and his associates (N Engl J Med. 2016;375:134-42).
Patients were randomized to receive olanzapine or the placebo, along with a 5-HT3-receptor antagonist (either palonosetron intravenously, granisetron intravenously or orally, or ondansetron intravenously or orally) and an NK1-receptor antagonist (fosaprepitant intravenously or aprepitant orally). The olanzapine (n = 192) and placebo (n = 188) groups were balanced with respect to age, race, sex, and chemotherapy administered.
Patients kept daily records of nausea and episodes of vomiting. The proportion of patients who reported no nausea or who experienced no clinically significant nausea was significantly greater in the olanzapine group than in the placebo group (37% vs. 22%, P = .002 and 67% vs. 49%, P = .001).
Patients receiving olanzapine had significantly increased sedation (severe in 5%) on day 2 compared with baseline, Dr. Navari and his associates reported. The sedation resolved on days 3, 4, and 5 even though patients continued to receive the drug on days 3 and 4. No patients discontinued the study because of sedation.
The National Cancer Institute funded the study. One investigator reported receiving financial support from Merck and Co. The other investigators reported having no disclosures.
On Twitter @jessnicolecraig
Olanzapine is more effective than placebo, in combination with a 5-HT3-receptor antagonist and an NK1-receptor antagonist, in preventing nausea in patients undergoing chemotherapy, according to investigators.
“This large, randomized, double-blind, placebo-controlled, phase III trial showed that it is more effective to combine olanzapine than placebo with an NK1-receptor antagonist, a 5-HT3–receptor antagonist, and dexamethasone for the prevention of nausea and vomiting in patients who have not received previous chemotherapy but are currently receiving highly emetogenic chemotherapy,” reported Rudolph Navari, MD, PhD, of the World Health Organization, Geneva, and his associates (N Engl J Med. 2016;375:134-42).
Patients were randomized to receive olanzapine or the placebo, along with a 5-HT3-receptor antagonist (either palonosetron intravenously, granisetron intravenously or orally, or ondansetron intravenously or orally) and an NK1-receptor antagonist (fosaprepitant intravenously or aprepitant orally). The olanzapine (n = 192) and placebo (n = 188) groups were balanced with respect to age, race, sex, and chemotherapy administered.
Patients kept daily records of nausea and episodes of vomiting. The proportion of patients who reported no nausea or who experienced no clinically significant nausea was significantly greater in the olanzapine group than in the placebo group (37% vs. 22%, P = .002 and 67% vs. 49%, P = .001).
Patients receiving olanzapine had significantly increased sedation (severe in 5%) on day 2 compared with baseline, Dr. Navari and his associates reported. The sedation resolved on days 3, 4, and 5 even though patients continued to receive the drug on days 3 and 4. No patients discontinued the study because of sedation.
The National Cancer Institute funded the study. One investigator reported receiving financial support from Merck and Co. The other investigators reported having no disclosures.
On Twitter @jessnicolecraig
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Olanzapine significantly reduced episodes of nausea, compared with placebo.
Major finding: The proportion of patients who experienced no clinically significant nausea was significantly greater in the olanzapine group than in the placebo group (67% vs. 49%, P = .001).
Data source: A randomized, double-blind phase III trial of 380 patients receiving chemotherapy for malignant cancer.
Disclosures: The National Cancer Institute funded the study. One investigator reported receiving financial support from Merck and Co. The other investigators reported having no disclosures.
Ipilimumab may restore antitumor immunity after relapse from HSCT
Early data hint that immune checkpoint inhibitors may be able to restore antitumor activity in patients with hematologic malignancies that have relapsed after allogeneic transplant.
Among 22 patients with relapsed hematologic cancers following allogeneic hematopoietic stem cell transplantation (HSCT) in a phase I/Ib study, treatment with the anti-CTLA-4 antibody ipilimumab (Yervoy) at a dose of 10 mg/kg was associated with complete responses in five patients, partial responses in two, and decreased tumor burden in six, reported Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues.
“CTLA-4 blockade was a feasible approach for the treatment of patients with relapsed hematologic cancer after transplantation. Complete remissions with some durability were observed, even in patients with refractory myeloid cancers,” they wrote (N Engl J Med. 2016 Jul 14. doi: 10.1056/NEJMoa1601202).
More than one-third of patients who undergo HSCT for hematologic malignancies such as lymphoma, multiple myeloma, or leukemia will experience a relapse, and most will die within a year of relapse despite salvage therapies or retransplantation, the authors noted.
“Immune escape (i.e., tumor evasion of the donor immune system) contributes to relapse after allogeneic HSCT, and immune checkpoint inhibitory pathways probably play an important role,” they wrote.
Selective CTLA-4 blockade has been shown in mouse models to treat late relapse after transplantation by augmenting graft-versus-tumor response without apparent exacerbation of graft-versus-host disease (GVHD). To see whether the use of a CTLA-4 inhibitor could have the same effect in humans, the investigators instituted a single-group, open-label, dose-finding, safety and efficacy study of ipilimumab in 28 patients from six treatment sites.
The patients had all undergone allogeneic HSCT more than 3 months before the start of the study. The diagnoses included acute myeloid leukemia (AML) in 12 patients (including 3 with leukemia cutis and 1 with a myeloid sarcoma), Hodgkin lymphoma in 7, non-Hodgkin lymphoma in 4, myelodysplastic syndrome (MDS) in 2, and multiple myeloma, myeloproliferative neoplasm, and acute lymphoblastic leukemia in 1 patient each. Eight of the patients had previously had either grade I or II acute GVHD; 16 had had chronic GVHD.
Patients received induction therapy with ipilimumab at a dose of either 3 mg/kg (6 patients), or 10 mg/kg (22 patients) every 3 weeks for a total of 4 doses. Patients who experienced a clinical benefit from the drug could receive additional doses every 12 weeks for up to 60 weeks.
There were no clinical responses meeting study criteria in any of the patients who received the 3-mg/kg dose. Among the 22 who received the 10-mg/kg dose, however, the rate of complete responses was 23% (5 of 22), partial responses 9% (2 of 22), and decreased tumor burden 27% (6 of 22). The remaining nine patients experienced disease progression.
Four of the complete responses occurred in patients with extramedullary AML, and one occurred in a patient with MDS transforming into AML.
The safety analysis, which included all 28 patients evaluable for adverse events, showed four discontinuations due to dose-limiting chronic GVHD of the liver in the 3 patients, and acute GVHD of the gut in 1, and to severe immune-related events in one additional patient, leading to the patient’s death.
Other grade 3 or greater adverse events possibly related to ipilimumab included acute kidney injury (one patient) , corneal ulcer (one), thrombocytopenia (nine), neutropenia (three), anemia and pleural effusion (two).
The investigators point out that therapy to stimulate a graft-versus-tumor effect has the potential to promote or exacerbate GVHD, as occurred in four patients in the study. The GVHD in these patients was effectively managed with glucocorticoids, however.
The National Institutes of Health, Leukemia and Lymphoma Society, Pasquarello Tissue Bank, and Dana-Farber Cancer Institute supported the study. Dr. Davids disclosed grants from ASCO, the Pasquarello Tissue Bank, NIH, NCI, and Leukemia and Lymphoma society, and personal fees from several companies outside the study. Several coauthors disclosed relationships with various pharmaceutical companies, including Bristol-Myers Squibb, maker of ipilimumab.
Early data hint that immune checkpoint inhibitors may be able to restore antitumor activity in patients with hematologic malignancies that have relapsed after allogeneic transplant.
Among 22 patients with relapsed hematologic cancers following allogeneic hematopoietic stem cell transplantation (HSCT) in a phase I/Ib study, treatment with the anti-CTLA-4 antibody ipilimumab (Yervoy) at a dose of 10 mg/kg was associated with complete responses in five patients, partial responses in two, and decreased tumor burden in six, reported Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues.
“CTLA-4 blockade was a feasible approach for the treatment of patients with relapsed hematologic cancer after transplantation. Complete remissions with some durability were observed, even in patients with refractory myeloid cancers,” they wrote (N Engl J Med. 2016 Jul 14. doi: 10.1056/NEJMoa1601202).
More than one-third of patients who undergo HSCT for hematologic malignancies such as lymphoma, multiple myeloma, or leukemia will experience a relapse, and most will die within a year of relapse despite salvage therapies or retransplantation, the authors noted.
“Immune escape (i.e., tumor evasion of the donor immune system) contributes to relapse after allogeneic HSCT, and immune checkpoint inhibitory pathways probably play an important role,” they wrote.
Selective CTLA-4 blockade has been shown in mouse models to treat late relapse after transplantation by augmenting graft-versus-tumor response without apparent exacerbation of graft-versus-host disease (GVHD). To see whether the use of a CTLA-4 inhibitor could have the same effect in humans, the investigators instituted a single-group, open-label, dose-finding, safety and efficacy study of ipilimumab in 28 patients from six treatment sites.
The patients had all undergone allogeneic HSCT more than 3 months before the start of the study. The diagnoses included acute myeloid leukemia (AML) in 12 patients (including 3 with leukemia cutis and 1 with a myeloid sarcoma), Hodgkin lymphoma in 7, non-Hodgkin lymphoma in 4, myelodysplastic syndrome (MDS) in 2, and multiple myeloma, myeloproliferative neoplasm, and acute lymphoblastic leukemia in 1 patient each. Eight of the patients had previously had either grade I or II acute GVHD; 16 had had chronic GVHD.
Patients received induction therapy with ipilimumab at a dose of either 3 mg/kg (6 patients), or 10 mg/kg (22 patients) every 3 weeks for a total of 4 doses. Patients who experienced a clinical benefit from the drug could receive additional doses every 12 weeks for up to 60 weeks.
There were no clinical responses meeting study criteria in any of the patients who received the 3-mg/kg dose. Among the 22 who received the 10-mg/kg dose, however, the rate of complete responses was 23% (5 of 22), partial responses 9% (2 of 22), and decreased tumor burden 27% (6 of 22). The remaining nine patients experienced disease progression.
Four of the complete responses occurred in patients with extramedullary AML, and one occurred in a patient with MDS transforming into AML.
The safety analysis, which included all 28 patients evaluable for adverse events, showed four discontinuations due to dose-limiting chronic GVHD of the liver in the 3 patients, and acute GVHD of the gut in 1, and to severe immune-related events in one additional patient, leading to the patient’s death.
Other grade 3 or greater adverse events possibly related to ipilimumab included acute kidney injury (one patient) , corneal ulcer (one), thrombocytopenia (nine), neutropenia (three), anemia and pleural effusion (two).
The investigators point out that therapy to stimulate a graft-versus-tumor effect has the potential to promote or exacerbate GVHD, as occurred in four patients in the study. The GVHD in these patients was effectively managed with glucocorticoids, however.
The National Institutes of Health, Leukemia and Lymphoma Society, Pasquarello Tissue Bank, and Dana-Farber Cancer Institute supported the study. Dr. Davids disclosed grants from ASCO, the Pasquarello Tissue Bank, NIH, NCI, and Leukemia and Lymphoma society, and personal fees from several companies outside the study. Several coauthors disclosed relationships with various pharmaceutical companies, including Bristol-Myers Squibb, maker of ipilimumab.
Early data hint that immune checkpoint inhibitors may be able to restore antitumor activity in patients with hematologic malignancies that have relapsed after allogeneic transplant.
Among 22 patients with relapsed hematologic cancers following allogeneic hematopoietic stem cell transplantation (HSCT) in a phase I/Ib study, treatment with the anti-CTLA-4 antibody ipilimumab (Yervoy) at a dose of 10 mg/kg was associated with complete responses in five patients, partial responses in two, and decreased tumor burden in six, reported Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues.
“CTLA-4 blockade was a feasible approach for the treatment of patients with relapsed hematologic cancer after transplantation. Complete remissions with some durability were observed, even in patients with refractory myeloid cancers,” they wrote (N Engl J Med. 2016 Jul 14. doi: 10.1056/NEJMoa1601202).
More than one-third of patients who undergo HSCT for hematologic malignancies such as lymphoma, multiple myeloma, or leukemia will experience a relapse, and most will die within a year of relapse despite salvage therapies or retransplantation, the authors noted.
“Immune escape (i.e., tumor evasion of the donor immune system) contributes to relapse after allogeneic HSCT, and immune checkpoint inhibitory pathways probably play an important role,” they wrote.
Selective CTLA-4 blockade has been shown in mouse models to treat late relapse after transplantation by augmenting graft-versus-tumor response without apparent exacerbation of graft-versus-host disease (GVHD). To see whether the use of a CTLA-4 inhibitor could have the same effect in humans, the investigators instituted a single-group, open-label, dose-finding, safety and efficacy study of ipilimumab in 28 patients from six treatment sites.
The patients had all undergone allogeneic HSCT more than 3 months before the start of the study. The diagnoses included acute myeloid leukemia (AML) in 12 patients (including 3 with leukemia cutis and 1 with a myeloid sarcoma), Hodgkin lymphoma in 7, non-Hodgkin lymphoma in 4, myelodysplastic syndrome (MDS) in 2, and multiple myeloma, myeloproliferative neoplasm, and acute lymphoblastic leukemia in 1 patient each. Eight of the patients had previously had either grade I or II acute GVHD; 16 had had chronic GVHD.
Patients received induction therapy with ipilimumab at a dose of either 3 mg/kg (6 patients), or 10 mg/kg (22 patients) every 3 weeks for a total of 4 doses. Patients who experienced a clinical benefit from the drug could receive additional doses every 12 weeks for up to 60 weeks.
There were no clinical responses meeting study criteria in any of the patients who received the 3-mg/kg dose. Among the 22 who received the 10-mg/kg dose, however, the rate of complete responses was 23% (5 of 22), partial responses 9% (2 of 22), and decreased tumor burden 27% (6 of 22). The remaining nine patients experienced disease progression.
Four of the complete responses occurred in patients with extramedullary AML, and one occurred in a patient with MDS transforming into AML.
The safety analysis, which included all 28 patients evaluable for adverse events, showed four discontinuations due to dose-limiting chronic GVHD of the liver in the 3 patients, and acute GVHD of the gut in 1, and to severe immune-related events in one additional patient, leading to the patient’s death.
Other grade 3 or greater adverse events possibly related to ipilimumab included acute kidney injury (one patient) , corneal ulcer (one), thrombocytopenia (nine), neutropenia (three), anemia and pleural effusion (two).
The investigators point out that therapy to stimulate a graft-versus-tumor effect has the potential to promote or exacerbate GVHD, as occurred in four patients in the study. The GVHD in these patients was effectively managed with glucocorticoids, however.
The National Institutes of Health, Leukemia and Lymphoma Society, Pasquarello Tissue Bank, and Dana-Farber Cancer Institute supported the study. Dr. Davids disclosed grants from ASCO, the Pasquarello Tissue Bank, NIH, NCI, and Leukemia and Lymphoma society, and personal fees from several companies outside the study. Several coauthors disclosed relationships with various pharmaceutical companies, including Bristol-Myers Squibb, maker of ipilimumab.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Anti-CTLA-4 therapy may restore graft-versus-tumor effect in patients with hematologic malignancies relapsed after allogeneic transplantation.
Major finding: Five of 22 patients on a 10-mg/kg dose of ipilimumab had a complete response.
Data source: Phase I/Ib investigator-initiated study of 28 patients with hematologic malignancies relapsed after allogeneic hematopoietic stem cell transplantation.
Disclosures: The National Institutes of Health, Leukemia and Lymphoma Society, Pasquarello Tissue Bank, and Dana-Farber Cancer Institute supported the study. Dr. Davids disclosed grants from ASCO, the Pasquarello Tissue Bank, NIH, NCI, and Leukemia and Lymphoma society, and personal fees from several companies outside the study. Several coauthors disclosed relationships with various pharmaceutical companies, including Bristol-Myers Squibb, maker of ipilimumab.
CMS to Congress: We might delay MACRA start
Implementation of some MACRA provisions could be delayed, CMS Acting Administrator Andy Slavitt told committee members at a July 13 hearing of the Senate Finance Committee.
Officials at the Centers for Medicare & Medicaid Services are considering “alternative start dates, looking at whether shorter periods could be used, and finding other ways for physicians to get experience with the program before the impact of it really hits them,” Mr. Slavitt testified.
Many of the comments that were submitted by physicians and other stakeholders on the proposed MACRA rule called for delayed implementation, Mr. Slavitt said. Other key themes included ensuring that patients are the focus, simplifying the rules, creating more pathways to advanced payment models, and providing a greater consideration of the needs of small and solo practices, particularly rural ones.
“We need to launch this program so that it begins on the right foot. That means that every physician in the country needs to feel like they are set up for success,” Mr. Slavitt said. He added that CMS may release an interim final rule to allow for more comment on changes stemming from the feedback received on the initial proposal.
Many Senators on the committee asked Mr. Slavitt how the proposed regulations would impact solo and small practices, especially ones in rural areas.
“The focus on small independent practices and their ability to continue to practice independently is a very high priority for us,” Mr. Slavitt said. “I would add that it’s not just small practices, it’s all also any physician that practices in a rural location. They have a very different set of dynamics than other physicians do. ... We need every physician set up for success and the challenges in small practices are far greater.”
He noted that for many small or solo practices, even a small amount of additional paperwork could affect how much time the physician can spend with patients.
Another area of concern in the MACRA regulation comments was the delay in the development of virtual groups that would allow physicians to virtually pool reporting requirements to allow for greater participation in value-based programs. The proposed MACRA rule calls for these groups to be delayed for 1 year .
“I think this going to be a high priority for us, and I think it’s going to be something that is going to need a lot more input from physicians to make sure we get it right,” Mr. Slavitt said. Virtual groups are “a whole new way of reporting, and we need to make a number of decisions, and physicians would need to make a number of decisions and they are not yet used to practicing that way.”
He added that CMS is asking physicians how they want to go about it, and the feedback so far is promising that physicians want to participate, but there needs to be operational and technology infrastructure to make it happen.
“I don’t think this is something that can’t be solved with just a little bit more time, but it’s certainly not something that’s ready to be launched in months,” he said, adding that the agency aims to launch virtual group reporting in the second year of the MACRA implementation.
Implementation of some MACRA provisions could be delayed, CMS Acting Administrator Andy Slavitt told committee members at a July 13 hearing of the Senate Finance Committee.
Officials at the Centers for Medicare & Medicaid Services are considering “alternative start dates, looking at whether shorter periods could be used, and finding other ways for physicians to get experience with the program before the impact of it really hits them,” Mr. Slavitt testified.
Many of the comments that were submitted by physicians and other stakeholders on the proposed MACRA rule called for delayed implementation, Mr. Slavitt said. Other key themes included ensuring that patients are the focus, simplifying the rules, creating more pathways to advanced payment models, and providing a greater consideration of the needs of small and solo practices, particularly rural ones.
“We need to launch this program so that it begins on the right foot. That means that every physician in the country needs to feel like they are set up for success,” Mr. Slavitt said. He added that CMS may release an interim final rule to allow for more comment on changes stemming from the feedback received on the initial proposal.
Many Senators on the committee asked Mr. Slavitt how the proposed regulations would impact solo and small practices, especially ones in rural areas.
“The focus on small independent practices and their ability to continue to practice independently is a very high priority for us,” Mr. Slavitt said. “I would add that it’s not just small practices, it’s all also any physician that practices in a rural location. They have a very different set of dynamics than other physicians do. ... We need every physician set up for success and the challenges in small practices are far greater.”
He noted that for many small or solo practices, even a small amount of additional paperwork could affect how much time the physician can spend with patients.
Another area of concern in the MACRA regulation comments was the delay in the development of virtual groups that would allow physicians to virtually pool reporting requirements to allow for greater participation in value-based programs. The proposed MACRA rule calls for these groups to be delayed for 1 year .
“I think this going to be a high priority for us, and I think it’s going to be something that is going to need a lot more input from physicians to make sure we get it right,” Mr. Slavitt said. Virtual groups are “a whole new way of reporting, and we need to make a number of decisions, and physicians would need to make a number of decisions and they are not yet used to practicing that way.”
He added that CMS is asking physicians how they want to go about it, and the feedback so far is promising that physicians want to participate, but there needs to be operational and technology infrastructure to make it happen.
“I don’t think this is something that can’t be solved with just a little bit more time, but it’s certainly not something that’s ready to be launched in months,” he said, adding that the agency aims to launch virtual group reporting in the second year of the MACRA implementation.
Implementation of some MACRA provisions could be delayed, CMS Acting Administrator Andy Slavitt told committee members at a July 13 hearing of the Senate Finance Committee.
Officials at the Centers for Medicare & Medicaid Services are considering “alternative start dates, looking at whether shorter periods could be used, and finding other ways for physicians to get experience with the program before the impact of it really hits them,” Mr. Slavitt testified.
Many of the comments that were submitted by physicians and other stakeholders on the proposed MACRA rule called for delayed implementation, Mr. Slavitt said. Other key themes included ensuring that patients are the focus, simplifying the rules, creating more pathways to advanced payment models, and providing a greater consideration of the needs of small and solo practices, particularly rural ones.
“We need to launch this program so that it begins on the right foot. That means that every physician in the country needs to feel like they are set up for success,” Mr. Slavitt said. He added that CMS may release an interim final rule to allow for more comment on changes stemming from the feedback received on the initial proposal.
Many Senators on the committee asked Mr. Slavitt how the proposed regulations would impact solo and small practices, especially ones in rural areas.
“The focus on small independent practices and their ability to continue to practice independently is a very high priority for us,” Mr. Slavitt said. “I would add that it’s not just small practices, it’s all also any physician that practices in a rural location. They have a very different set of dynamics than other physicians do. ... We need every physician set up for success and the challenges in small practices are far greater.”
He noted that for many small or solo practices, even a small amount of additional paperwork could affect how much time the physician can spend with patients.
Another area of concern in the MACRA regulation comments was the delay in the development of virtual groups that would allow physicians to virtually pool reporting requirements to allow for greater participation in value-based programs. The proposed MACRA rule calls for these groups to be delayed for 1 year .
“I think this going to be a high priority for us, and I think it’s going to be something that is going to need a lot more input from physicians to make sure we get it right,” Mr. Slavitt said. Virtual groups are “a whole new way of reporting, and we need to make a number of decisions, and physicians would need to make a number of decisions and they are not yet used to practicing that way.”
He added that CMS is asking physicians how they want to go about it, and the feedback so far is promising that physicians want to participate, but there needs to be operational and technology infrastructure to make it happen.
“I don’t think this is something that can’t be solved with just a little bit more time, but it’s certainly not something that’s ready to be launched in months,” he said, adding that the agency aims to launch virtual group reporting in the second year of the MACRA implementation.
FROM A SENATE FINANCE COMMITTEE HEARING
The ‘guilty’ associates of silent thoracic aneurysm fingered
NEW YORK – Aortic aneurysm ranks as one of the top 20 causes of death in the United States. Most of these aneurysms are clinically silent until they rupture, but Yale cardiovascular surgeon John A. Elefteriades, MD, has developed a clinical paradigm that identifies eight markers that physicians can use to detect the disease before it strikes.
Dr. Elefteriades calls his paradigm “Guilt by Association.” It is based on an article he published online last year in the journal Open Heart (2015;2:e000169).
“What we need is for our colleagues in affiliated disciplines to recognize the importance of these offenders in indicating the presence of thoracic aortic aneurysm,” he said, reporting on the paradigm at the meeting sponsored by the American Association for Thoracic Surgery. He noted that studies from Japan of people who had died from out-of-hospital cardiac arrest found that 8% of them had a type A aortic dissection (Am J Cardiol 2016;117:1826-30).
He outlined eight “associates” of thoracic aortic aneurysm (TAA): intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; bicuspid aortic valve; family history; positive thumb-palm test; and temporal arteritis and other autoimmune disorders.
A patient with TAA has a 10% likelihood of harboring an intracranial aneurysm (Am J Cardiol 2010;105:417-20). “It’s even more common in the descending, compared to the ascending group in examples that we’ve identified,” Dr. Elefteriades said. Particularly vulnerable are patients over age 70 and those with an intracranial aneurysm larger than 4 mm: the former has a 9% chance of harboring a TAA, the latter a 6% chance, he said.
The bovine arch had been thought to be benign, but, Dr. Elefteriades said, “We don’t think it is.”
Bovine arch refers to a group of congenital aortic arch vessels with an aberrant origin of the left common carotid artery. “We recently looked at this as a marker for thoracic aortic disease, and please note that 20% of our TAA patients have a bovine arch,” he said. “This is much higher than in the general population.”
Abdominal aortic aneurysm has long been associated with TAA, he said. “When these aneurysms are identified by ultrasound, it’s important that the thoracic aorta be checked as well,” he said.
“This is a message for internists and our vascular colleagues.”
Simple renal cysts have been found in patients with TAA at a “much higher” rate than the general population, as high as 57% of those with descending aortic aneurysms vs. 11%-13.7% of the general population, Dr. Elefteriades said (J. Am. Heart Assoc. 2016;5:e002248). Simple renal cysts are detected by abdominal CT scan. “It’s just a matter of a few more slices with the CT scan to get the entirety of the thoracic aorta evaluated,” he said.
“We encourage our radiology colleagues to do this when a renal cyst is detected.”
Bicuspid aortic valve mandates “support from our cardiac colleagues when they find one of these to let the patient know he has to be monitored lifelong for later development of this aneurysm,” Dr. Elefteriades said.
Family history of TAA has been known as a strong predictor, but genetic studies have provided clarity on the association (Arch Surg. 1999;134:361‐7). “If the proband has a thoracic aortic aneurysm, there’s 21% likelihood there’s a family member who is affected with an aneurysm somewhere in the body,” he said.
Location of aneurysms in family members is also important, Dr. Elefteriades said. “If the proband has a ascending aortic aneurysm, the kindred also have an ascending aortic aneurysm; but if the proband has a descending aneurysm, the likelihood is that the kindred will have an abdominal aortic aneurysm,” he said. “To identify silent disease, it’s very important we check siblings and children, and now, of course, we’re using whole-exome sequencing.” So far, Dr. Elefteriades has obtained whole-exome sequencing in 200 patients.
The thumb-palm test involves the patient touching the thumb to the palm; the thumb crossing the edge of the flat palm is an indicator of connective tissue disease. “It doesn’t cost anything,” Dr. Elefteriades said. “It is a very simple thing for internists to do to identify those connective tissue diseases.”
Temporal arteritis has become increasingly common in elderly women. “They have a markedly increased likelihood of having a thoracic aortic aneurysm – about 8% in some studies,’ Dr. Elefteriades said. “So we want our neurology colleagues to be aware of this and to look for thoracic aortic aneurysm.”
Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtronic.
NEW YORK – Aortic aneurysm ranks as one of the top 20 causes of death in the United States. Most of these aneurysms are clinically silent until they rupture, but Yale cardiovascular surgeon John A. Elefteriades, MD, has developed a clinical paradigm that identifies eight markers that physicians can use to detect the disease before it strikes.
Dr. Elefteriades calls his paradigm “Guilt by Association.” It is based on an article he published online last year in the journal Open Heart (2015;2:e000169).
“What we need is for our colleagues in affiliated disciplines to recognize the importance of these offenders in indicating the presence of thoracic aortic aneurysm,” he said, reporting on the paradigm at the meeting sponsored by the American Association for Thoracic Surgery. He noted that studies from Japan of people who had died from out-of-hospital cardiac arrest found that 8% of them had a type A aortic dissection (Am J Cardiol 2016;117:1826-30).
He outlined eight “associates” of thoracic aortic aneurysm (TAA): intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; bicuspid aortic valve; family history; positive thumb-palm test; and temporal arteritis and other autoimmune disorders.
A patient with TAA has a 10% likelihood of harboring an intracranial aneurysm (Am J Cardiol 2010;105:417-20). “It’s even more common in the descending, compared to the ascending group in examples that we’ve identified,” Dr. Elefteriades said. Particularly vulnerable are patients over age 70 and those with an intracranial aneurysm larger than 4 mm: the former has a 9% chance of harboring a TAA, the latter a 6% chance, he said.
The bovine arch had been thought to be benign, but, Dr. Elefteriades said, “We don’t think it is.”
Bovine arch refers to a group of congenital aortic arch vessels with an aberrant origin of the left common carotid artery. “We recently looked at this as a marker for thoracic aortic disease, and please note that 20% of our TAA patients have a bovine arch,” he said. “This is much higher than in the general population.”
Abdominal aortic aneurysm has long been associated with TAA, he said. “When these aneurysms are identified by ultrasound, it’s important that the thoracic aorta be checked as well,” he said.
“This is a message for internists and our vascular colleagues.”
Simple renal cysts have been found in patients with TAA at a “much higher” rate than the general population, as high as 57% of those with descending aortic aneurysms vs. 11%-13.7% of the general population, Dr. Elefteriades said (J. Am. Heart Assoc. 2016;5:e002248). Simple renal cysts are detected by abdominal CT scan. “It’s just a matter of a few more slices with the CT scan to get the entirety of the thoracic aorta evaluated,” he said.
“We encourage our radiology colleagues to do this when a renal cyst is detected.”
Bicuspid aortic valve mandates “support from our cardiac colleagues when they find one of these to let the patient know he has to be monitored lifelong for later development of this aneurysm,” Dr. Elefteriades said.
Family history of TAA has been known as a strong predictor, but genetic studies have provided clarity on the association (Arch Surg. 1999;134:361‐7). “If the proband has a thoracic aortic aneurysm, there’s 21% likelihood there’s a family member who is affected with an aneurysm somewhere in the body,” he said.
Location of aneurysms in family members is also important, Dr. Elefteriades said. “If the proband has a ascending aortic aneurysm, the kindred also have an ascending aortic aneurysm; but if the proband has a descending aneurysm, the likelihood is that the kindred will have an abdominal aortic aneurysm,” he said. “To identify silent disease, it’s very important we check siblings and children, and now, of course, we’re using whole-exome sequencing.” So far, Dr. Elefteriades has obtained whole-exome sequencing in 200 patients.
The thumb-palm test involves the patient touching the thumb to the palm; the thumb crossing the edge of the flat palm is an indicator of connective tissue disease. “It doesn’t cost anything,” Dr. Elefteriades said. “It is a very simple thing for internists to do to identify those connective tissue diseases.”
Temporal arteritis has become increasingly common in elderly women. “They have a markedly increased likelihood of having a thoracic aortic aneurysm – about 8% in some studies,’ Dr. Elefteriades said. “So we want our neurology colleagues to be aware of this and to look for thoracic aortic aneurysm.”
Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtronic.
NEW YORK – Aortic aneurysm ranks as one of the top 20 causes of death in the United States. Most of these aneurysms are clinically silent until they rupture, but Yale cardiovascular surgeon John A. Elefteriades, MD, has developed a clinical paradigm that identifies eight markers that physicians can use to detect the disease before it strikes.
Dr. Elefteriades calls his paradigm “Guilt by Association.” It is based on an article he published online last year in the journal Open Heart (2015;2:e000169).
“What we need is for our colleagues in affiliated disciplines to recognize the importance of these offenders in indicating the presence of thoracic aortic aneurysm,” he said, reporting on the paradigm at the meeting sponsored by the American Association for Thoracic Surgery. He noted that studies from Japan of people who had died from out-of-hospital cardiac arrest found that 8% of them had a type A aortic dissection (Am J Cardiol 2016;117:1826-30).
He outlined eight “associates” of thoracic aortic aneurysm (TAA): intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; bicuspid aortic valve; family history; positive thumb-palm test; and temporal arteritis and other autoimmune disorders.
A patient with TAA has a 10% likelihood of harboring an intracranial aneurysm (Am J Cardiol 2010;105:417-20). “It’s even more common in the descending, compared to the ascending group in examples that we’ve identified,” Dr. Elefteriades said. Particularly vulnerable are patients over age 70 and those with an intracranial aneurysm larger than 4 mm: the former has a 9% chance of harboring a TAA, the latter a 6% chance, he said.
The bovine arch had been thought to be benign, but, Dr. Elefteriades said, “We don’t think it is.”
Bovine arch refers to a group of congenital aortic arch vessels with an aberrant origin of the left common carotid artery. “We recently looked at this as a marker for thoracic aortic disease, and please note that 20% of our TAA patients have a bovine arch,” he said. “This is much higher than in the general population.”
Abdominal aortic aneurysm has long been associated with TAA, he said. “When these aneurysms are identified by ultrasound, it’s important that the thoracic aorta be checked as well,” he said.
“This is a message for internists and our vascular colleagues.”
Simple renal cysts have been found in patients with TAA at a “much higher” rate than the general population, as high as 57% of those with descending aortic aneurysms vs. 11%-13.7% of the general population, Dr. Elefteriades said (J. Am. Heart Assoc. 2016;5:e002248). Simple renal cysts are detected by abdominal CT scan. “It’s just a matter of a few more slices with the CT scan to get the entirety of the thoracic aorta evaluated,” he said.
“We encourage our radiology colleagues to do this when a renal cyst is detected.”
Bicuspid aortic valve mandates “support from our cardiac colleagues when they find one of these to let the patient know he has to be monitored lifelong for later development of this aneurysm,” Dr. Elefteriades said.
Family history of TAA has been known as a strong predictor, but genetic studies have provided clarity on the association (Arch Surg. 1999;134:361‐7). “If the proband has a thoracic aortic aneurysm, there’s 21% likelihood there’s a family member who is affected with an aneurysm somewhere in the body,” he said.
Location of aneurysms in family members is also important, Dr. Elefteriades said. “If the proband has a ascending aortic aneurysm, the kindred also have an ascending aortic aneurysm; but if the proband has a descending aneurysm, the likelihood is that the kindred will have an abdominal aortic aneurysm,” he said. “To identify silent disease, it’s very important we check siblings and children, and now, of course, we’re using whole-exome sequencing.” So far, Dr. Elefteriades has obtained whole-exome sequencing in 200 patients.
The thumb-palm test involves the patient touching the thumb to the palm; the thumb crossing the edge of the flat palm is an indicator of connective tissue disease. “It doesn’t cost anything,” Dr. Elefteriades said. “It is a very simple thing for internists to do to identify those connective tissue diseases.”
Temporal arteritis has become increasingly common in elderly women. “They have a markedly increased likelihood of having a thoracic aortic aneurysm – about 8% in some studies,’ Dr. Elefteriades said. “So we want our neurology colleagues to be aware of this and to look for thoracic aortic aneurysm.”
Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtronic.
EXPERT ANALYSIS FROM AATS AORTIC SYMPOSIUM 2016
Key clinical point: Eight markers may detect silent thoracic aneurysms before rupture.
Major finding: The eight “associates” of TAA include intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; and bicuspid aortic valve.
Data source: The “Guilt by Association” paradigm was based upon a review of the literature by Dr. Elefteriades and his own published reports.
Disclosures: Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtroni
Metabolic Health Declining Among the Obese, Despite Improvements in BP and Lipids
Despite achieving significant improvements in blood pressure and cholesterol levels, obese Americans continue to grow fatter, with worsening blood glucose and an increasing incidence of diabetes.
From 1998 to 2014, national health data showed that mean diastolic and systolic blood pressures decreased in obese men and women in all racial and ethnic groups. Mean lipid measurements improved as well, including a “marked” 21-mg/dL decrease in total cholesterol and a significant increase in HDL cholesterol.
But markers of blood glucose health continued to decline over the same period, contributing to an overall worsening of metabolic health and a increase from 11% to 19% in the rate of diabetes, Fangjian Guo, MD, and W. Timothy Garvey, MD, reported in July 13 issue of the Journal of the American Heart Association (J Am Heart Assoc. 2016 Jul 13. 5:e003619 doi: 10.1161/JAHA.116.003619).
The rate of obese adults free of these three cardiovascular disease risk factors – diabetes, elevated cholesterol, and blood pressure – remained stable over the study period at about 15%. But the rate of obese adults with all three risk factors increased by 37% over the same period. By 2014, 22% reported having all three of those risk factors.
“The deteriorated blood glucose health among obese adults in the United States calls for lifestyle interventions (diet and exercise) on a national scale,” wrote Dr. Garvey, chair of nutrition science at the University of Alabama, Birmingham. “Community-based public health intervention programs may help increase physical activity and diet quality to alleviate the problem.”
The investigators examined trends in cardiometabolic health among 18,626 obese adults who participated in National Health and Nutrition Examination Surveys from 1988 to 2014. Over this period, mean body mass index increased significantly, from 34.7 to 36 kg/m2. Waist circumference increased as well, from 110 to 114.8 cm.
The picture was much better for blood pressure. Mean systolic pressures decreased about 2 points – from 126.1 to 124.4 mm Hg – in all age, racial and ethnic groups, and in both sexes. Mean diastolic blood pressure also decreased, dropping from 76.6 to 72.5 mm Hg. By 2014, 44% of the men and 51% of the women were below the blood pressure risk threshold.
Lipids also improved over the years, the investigators noted, with significant decreases in mean total cholesterol, from 214.5 to 193.7 mg/dL, and increases in HDL cholesterol, from 45.4 to 47.4 mg/dL.
Blood glucose worsened significantly, however. The mean hemoglobin A1c increased from 5.7% to 5.9%. The measurement rose in all ages, both sexes, and in all racial and ethnic groups except for non-Hispanic blacks.
Perhaps not surprisingly, the incidence of diabetes (a self-reported HbA1c of 6.5% or more) increased from 11% to 19% from 1988 to 2014. The increase occurred in all age groups and both sexes except for young adults aged 20-39 years. No racial or ethnic group was exempt from the increase.
The number of people having all three risk factors (hypertension, hypercholesterolemia, and hyperglycemia) increased from 16% in 1988 to 22% in 2014.
“The increase occurred in parallel with a decline in the prevalence of healthy blood glucose, which is the predominant explanation accounting for the rise in the prevalence of presence of all three risk factors,” the investigators said.
Only 15% of the study population was free from all of these risk factors – a percentage that remained unchanged during 1988-2014.
The findings should be a wake-up call for physicians and their patients, and a national call for action to improve cardiovascular health among obese adults, the team wrote.
“The increasing trend of obese people with all three cardiovascular risk factors, commensurate with a decline in those with one or two risk factors, suggests an overall deterioration in health among people with obesity. ... These patterns of worsening metabolic health constitute an increase in risk of type 2 diabetes mellitus and underlie increasing prevalence rates for diabetes mellitus,” the investigators wrote.
Aggressive treatment will be necessary to reverse these trends. This might include treatment with weight-loss medications in conjunction with lifestyle interventions, which should be especially targeted at obese individuals who are already metabolically unhealthy and in those who have complications or are at risk for developing them.
“In the context of the current data, those obese adults who are metabolically unhealthy or perhaps those with suboptimal metabolic health represent patients who will benefit most from intensive obesity management … coordinated efforts aligning cardiovascular disease prevention and control activities across the public and private sectors in the United States are needed reduce the burden of cardiovascular disease among the obese population,” Dr. Garvey concluded.
The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center.
Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.
Despite achieving significant improvements in blood pressure and cholesterol levels, obese Americans continue to grow fatter, with worsening blood glucose and an increasing incidence of diabetes.
From 1998 to 2014, national health data showed that mean diastolic and systolic blood pressures decreased in obese men and women in all racial and ethnic groups. Mean lipid measurements improved as well, including a “marked” 21-mg/dL decrease in total cholesterol and a significant increase in HDL cholesterol.
But markers of blood glucose health continued to decline over the same period, contributing to an overall worsening of metabolic health and a increase from 11% to 19% in the rate of diabetes, Fangjian Guo, MD, and W. Timothy Garvey, MD, reported in July 13 issue of the Journal of the American Heart Association (J Am Heart Assoc. 2016 Jul 13. 5:e003619 doi: 10.1161/JAHA.116.003619).
The rate of obese adults free of these three cardiovascular disease risk factors – diabetes, elevated cholesterol, and blood pressure – remained stable over the study period at about 15%. But the rate of obese adults with all three risk factors increased by 37% over the same period. By 2014, 22% reported having all three of those risk factors.
“The deteriorated blood glucose health among obese adults in the United States calls for lifestyle interventions (diet and exercise) on a national scale,” wrote Dr. Garvey, chair of nutrition science at the University of Alabama, Birmingham. “Community-based public health intervention programs may help increase physical activity and diet quality to alleviate the problem.”
The investigators examined trends in cardiometabolic health among 18,626 obese adults who participated in National Health and Nutrition Examination Surveys from 1988 to 2014. Over this period, mean body mass index increased significantly, from 34.7 to 36 kg/m2. Waist circumference increased as well, from 110 to 114.8 cm.
The picture was much better for blood pressure. Mean systolic pressures decreased about 2 points – from 126.1 to 124.4 mm Hg – in all age, racial and ethnic groups, and in both sexes. Mean diastolic blood pressure also decreased, dropping from 76.6 to 72.5 mm Hg. By 2014, 44% of the men and 51% of the women were below the blood pressure risk threshold.
Lipids also improved over the years, the investigators noted, with significant decreases in mean total cholesterol, from 214.5 to 193.7 mg/dL, and increases in HDL cholesterol, from 45.4 to 47.4 mg/dL.
Blood glucose worsened significantly, however. The mean hemoglobin A1c increased from 5.7% to 5.9%. The measurement rose in all ages, both sexes, and in all racial and ethnic groups except for non-Hispanic blacks.
Perhaps not surprisingly, the incidence of diabetes (a self-reported HbA1c of 6.5% or more) increased from 11% to 19% from 1988 to 2014. The increase occurred in all age groups and both sexes except for young adults aged 20-39 years. No racial or ethnic group was exempt from the increase.
The number of people having all three risk factors (hypertension, hypercholesterolemia, and hyperglycemia) increased from 16% in 1988 to 22% in 2014.
“The increase occurred in parallel with a decline in the prevalence of healthy blood glucose, which is the predominant explanation accounting for the rise in the prevalence of presence of all three risk factors,” the investigators said.
Only 15% of the study population was free from all of these risk factors – a percentage that remained unchanged during 1988-2014.
The findings should be a wake-up call for physicians and their patients, and a national call for action to improve cardiovascular health among obese adults, the team wrote.
“The increasing trend of obese people with all three cardiovascular risk factors, commensurate with a decline in those with one or two risk factors, suggests an overall deterioration in health among people with obesity. ... These patterns of worsening metabolic health constitute an increase in risk of type 2 diabetes mellitus and underlie increasing prevalence rates for diabetes mellitus,” the investigators wrote.
Aggressive treatment will be necessary to reverse these trends. This might include treatment with weight-loss medications in conjunction with lifestyle interventions, which should be especially targeted at obese individuals who are already metabolically unhealthy and in those who have complications or are at risk for developing them.
“In the context of the current data, those obese adults who are metabolically unhealthy or perhaps those with suboptimal metabolic health represent patients who will benefit most from intensive obesity management … coordinated efforts aligning cardiovascular disease prevention and control activities across the public and private sectors in the United States are needed reduce the burden of cardiovascular disease among the obese population,” Dr. Garvey concluded.
The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center.
Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.
Despite achieving significant improvements in blood pressure and cholesterol levels, obese Americans continue to grow fatter, with worsening blood glucose and an increasing incidence of diabetes.
From 1998 to 2014, national health data showed that mean diastolic and systolic blood pressures decreased in obese men and women in all racial and ethnic groups. Mean lipid measurements improved as well, including a “marked” 21-mg/dL decrease in total cholesterol and a significant increase in HDL cholesterol.
But markers of blood glucose health continued to decline over the same period, contributing to an overall worsening of metabolic health and a increase from 11% to 19% in the rate of diabetes, Fangjian Guo, MD, and W. Timothy Garvey, MD, reported in July 13 issue of the Journal of the American Heart Association (J Am Heart Assoc. 2016 Jul 13. 5:e003619 doi: 10.1161/JAHA.116.003619).
The rate of obese adults free of these three cardiovascular disease risk factors – diabetes, elevated cholesterol, and blood pressure – remained stable over the study period at about 15%. But the rate of obese adults with all three risk factors increased by 37% over the same period. By 2014, 22% reported having all three of those risk factors.
“The deteriorated blood glucose health among obese adults in the United States calls for lifestyle interventions (diet and exercise) on a national scale,” wrote Dr. Garvey, chair of nutrition science at the University of Alabama, Birmingham. “Community-based public health intervention programs may help increase physical activity and diet quality to alleviate the problem.”
The investigators examined trends in cardiometabolic health among 18,626 obese adults who participated in National Health and Nutrition Examination Surveys from 1988 to 2014. Over this period, mean body mass index increased significantly, from 34.7 to 36 kg/m2. Waist circumference increased as well, from 110 to 114.8 cm.
The picture was much better for blood pressure. Mean systolic pressures decreased about 2 points – from 126.1 to 124.4 mm Hg – in all age, racial and ethnic groups, and in both sexes. Mean diastolic blood pressure also decreased, dropping from 76.6 to 72.5 mm Hg. By 2014, 44% of the men and 51% of the women were below the blood pressure risk threshold.
Lipids also improved over the years, the investigators noted, with significant decreases in mean total cholesterol, from 214.5 to 193.7 mg/dL, and increases in HDL cholesterol, from 45.4 to 47.4 mg/dL.
Blood glucose worsened significantly, however. The mean hemoglobin A1c increased from 5.7% to 5.9%. The measurement rose in all ages, both sexes, and in all racial and ethnic groups except for non-Hispanic blacks.
Perhaps not surprisingly, the incidence of diabetes (a self-reported HbA1c of 6.5% or more) increased from 11% to 19% from 1988 to 2014. The increase occurred in all age groups and both sexes except for young adults aged 20-39 years. No racial or ethnic group was exempt from the increase.
The number of people having all three risk factors (hypertension, hypercholesterolemia, and hyperglycemia) increased from 16% in 1988 to 22% in 2014.
“The increase occurred in parallel with a decline in the prevalence of healthy blood glucose, which is the predominant explanation accounting for the rise in the prevalence of presence of all three risk factors,” the investigators said.
Only 15% of the study population was free from all of these risk factors – a percentage that remained unchanged during 1988-2014.
The findings should be a wake-up call for physicians and their patients, and a national call for action to improve cardiovascular health among obese adults, the team wrote.
“The increasing trend of obese people with all three cardiovascular risk factors, commensurate with a decline in those with one or two risk factors, suggests an overall deterioration in health among people with obesity. ... These patterns of worsening metabolic health constitute an increase in risk of type 2 diabetes mellitus and underlie increasing prevalence rates for diabetes mellitus,” the investigators wrote.
Aggressive treatment will be necessary to reverse these trends. This might include treatment with weight-loss medications in conjunction with lifestyle interventions, which should be especially targeted at obese individuals who are already metabolically unhealthy and in those who have complications or are at risk for developing them.
“In the context of the current data, those obese adults who are metabolically unhealthy or perhaps those with suboptimal metabolic health represent patients who will benefit most from intensive obesity management … coordinated efforts aligning cardiovascular disease prevention and control activities across the public and private sectors in the United States are needed reduce the burden of cardiovascular disease among the obese population,” Dr. Garvey concluded.
The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center.
Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Metabolic health declining among the obese, despite improvements in BP and lipids
Despite achieving significant improvements in blood pressure and cholesterol levels, obese Americans continue to grow fatter, with worsening blood glucose and an increasing incidence of diabetes.
From 1998 to 2014, national health data showed that mean diastolic and systolic blood pressures decreased in obese men and women in all racial and ethnic groups. Mean lipid measurements improved as well, including a “marked” 21-mg/dL decrease in total cholesterol and a significant increase in HDL cholesterol.
But markers of blood glucose health continued to decline over the same period, contributing to an overall worsening of metabolic health and a increase from 11% to 19% in the rate of diabetes, Fangjian Guo, MD, and W. Timothy Garvey, MD, reported in July 13 issue of the Journal of the American Heart Association (J Am Heart Assoc. 2016 Jul 13. 5:e003619 doi: 10.1161/JAHA.116.003619).
The rate of obese adults free of these three cardiovascular disease risk factors – diabetes, elevated cholesterol, and blood pressure – remained stable over the study period at about 15%. But the rate of obese adults with all three risk factors increased by 37% over the same period. By 2014, 22% reported having all three of those risk factors.
“The deteriorated blood glucose health among obese adults in the United States calls for lifestyle interventions (diet and exercise) on a national scale,” wrote Dr. Garvey, chair of nutrition science at the University of Alabama, Birmingham. “Community-based public health intervention programs may help increase physical activity and diet quality to alleviate the problem.”
The investigators examined trends in cardiometabolic health among 18,626 obese adults who participated in National Health and Nutrition Examination Surveys from 1988 to 2014. Over this period, mean body mass index increased significantly, from 34.7 to 36 kg/m2. Waist circumference increased as well, from 110 to 114.8 cm.
The picture was much better for blood pressure. Mean systolic pressures decreased about 2 points – from 126.1 to 124.4 mm Hg – in all age, racial and ethnic groups, and in both sexes. Mean diastolic blood pressure also decreased, dropping from 76.6 to 72.5 mm Hg. By 2014, 44% of the men and 51% of the women were below the blood pressure risk threshold.
Lipids also improved over the years, the investigators noted, with significant decreases in mean total cholesterol, from 214.5 to 193.7 mg/dL, and increases in HDL cholesterol, from 45.4 to 47.4 mg/dL.
Blood glucose worsened significantly, however. The mean hemoglobin A1c increased from 5.7% to 5.9%. The measurement rose in all ages, both sexes, and in all racial and ethnic groups except for non-Hispanic blacks.
Perhaps not surprisingly, the incidence of diabetes (a self-reported HbA1c of 6.5% or more) increased from 11% to 19% from 1988 to 2014. The increase occurred in all age groups and both sexes except for young adults aged 20-39 years. No racial or ethnic group was exempt from the increase.
The number of people having all three risk factors (hypertension, hypercholesterolemia, and hyperglycemia) increased from 16% in 1988 to 22% in 2014.
“The increase occurred in parallel with a decline in the prevalence of healthy blood glucose, which is the predominant explanation accounting for the rise in the prevalence of presence of all three risk factors,” the investigators said.
Only 15% of the study population was free from all of these risk factors – a percentage that remained unchanged during 1988-2014.
The findings should be a wake-up call for physicians and their patients, and a national call for action to improve cardiovascular health among obese adults, the team wrote.
“The increasing trend of obese people with all three cardiovascular risk factors, commensurate with a decline in those with one or two risk factors, suggests an overall deterioration in health among people with obesity. ... These patterns of worsening metabolic health constitute an increase in risk of type 2 diabetes mellitus and underlie increasing prevalence rates for diabetes mellitus,” the investigators wrote.
Aggressive treatment will be necessary to reverse these trends. This might include treatment with weight-loss medications in conjunction with lifestyle interventions, which should be especially targeted at obese individuals who are already metabolically unhealthy and in those who have complications or are at risk for developing them.
“In the context of the current data, those obese adults who are metabolically unhealthy or perhaps those with suboptimal metabolic health represent patients who will benefit most from intensive obesity management … coordinated efforts aligning cardiovascular disease prevention and control activities across the public and private sectors in the United States are needed reduce the burden of cardiovascular disease among the obese population,” Dr. Garvey concluded.
The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center.
Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.
Despite achieving significant improvements in blood pressure and cholesterol levels, obese Americans continue to grow fatter, with worsening blood glucose and an increasing incidence of diabetes.
From 1998 to 2014, national health data showed that mean diastolic and systolic blood pressures decreased in obese men and women in all racial and ethnic groups. Mean lipid measurements improved as well, including a “marked” 21-mg/dL decrease in total cholesterol and a significant increase in HDL cholesterol.
But markers of blood glucose health continued to decline over the same period, contributing to an overall worsening of metabolic health and a increase from 11% to 19% in the rate of diabetes, Fangjian Guo, MD, and W. Timothy Garvey, MD, reported in July 13 issue of the Journal of the American Heart Association (J Am Heart Assoc. 2016 Jul 13. 5:e003619 doi: 10.1161/JAHA.116.003619).
The rate of obese adults free of these three cardiovascular disease risk factors – diabetes, elevated cholesterol, and blood pressure – remained stable over the study period at about 15%. But the rate of obese adults with all three risk factors increased by 37% over the same period. By 2014, 22% reported having all three of those risk factors.
“The deteriorated blood glucose health among obese adults in the United States calls for lifestyle interventions (diet and exercise) on a national scale,” wrote Dr. Garvey, chair of nutrition science at the University of Alabama, Birmingham. “Community-based public health intervention programs may help increase physical activity and diet quality to alleviate the problem.”
The investigators examined trends in cardiometabolic health among 18,626 obese adults who participated in National Health and Nutrition Examination Surveys from 1988 to 2014. Over this period, mean body mass index increased significantly, from 34.7 to 36 kg/m2. Waist circumference increased as well, from 110 to 114.8 cm.
The picture was much better for blood pressure. Mean systolic pressures decreased about 2 points – from 126.1 to 124.4 mm Hg – in all age, racial and ethnic groups, and in both sexes. Mean diastolic blood pressure also decreased, dropping from 76.6 to 72.5 mm Hg. By 2014, 44% of the men and 51% of the women were below the blood pressure risk threshold.
Lipids also improved over the years, the investigators noted, with significant decreases in mean total cholesterol, from 214.5 to 193.7 mg/dL, and increases in HDL cholesterol, from 45.4 to 47.4 mg/dL.
Blood glucose worsened significantly, however. The mean hemoglobin A1c increased from 5.7% to 5.9%. The measurement rose in all ages, both sexes, and in all racial and ethnic groups except for non-Hispanic blacks.
Perhaps not surprisingly, the incidence of diabetes (a self-reported HbA1c of 6.5% or more) increased from 11% to 19% from 1988 to 2014. The increase occurred in all age groups and both sexes except for young adults aged 20-39 years. No racial or ethnic group was exempt from the increase.
The number of people having all three risk factors (hypertension, hypercholesterolemia, and hyperglycemia) increased from 16% in 1988 to 22% in 2014.
“The increase occurred in parallel with a decline in the prevalence of healthy blood glucose, which is the predominant explanation accounting for the rise in the prevalence of presence of all three risk factors,” the investigators said.
Only 15% of the study population was free from all of these risk factors – a percentage that remained unchanged during 1988-2014.
The findings should be a wake-up call for physicians and their patients, and a national call for action to improve cardiovascular health among obese adults, the team wrote.
“The increasing trend of obese people with all three cardiovascular risk factors, commensurate with a decline in those with one or two risk factors, suggests an overall deterioration in health among people with obesity. ... These patterns of worsening metabolic health constitute an increase in risk of type 2 diabetes mellitus and underlie increasing prevalence rates for diabetes mellitus,” the investigators wrote.
Aggressive treatment will be necessary to reverse these trends. This might include treatment with weight-loss medications in conjunction with lifestyle interventions, which should be especially targeted at obese individuals who are already metabolically unhealthy and in those who have complications or are at risk for developing them.
“In the context of the current data, those obese adults who are metabolically unhealthy or perhaps those with suboptimal metabolic health represent patients who will benefit most from intensive obesity management … coordinated efforts aligning cardiovascular disease prevention and control activities across the public and private sectors in the United States are needed reduce the burden of cardiovascular disease among the obese population,” Dr. Garvey concluded.
The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center.
Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.
Despite achieving significant improvements in blood pressure and cholesterol levels, obese Americans continue to grow fatter, with worsening blood glucose and an increasing incidence of diabetes.
From 1998 to 2014, national health data showed that mean diastolic and systolic blood pressures decreased in obese men and women in all racial and ethnic groups. Mean lipid measurements improved as well, including a “marked” 21-mg/dL decrease in total cholesterol and a significant increase in HDL cholesterol.
But markers of blood glucose health continued to decline over the same period, contributing to an overall worsening of metabolic health and a increase from 11% to 19% in the rate of diabetes, Fangjian Guo, MD, and W. Timothy Garvey, MD, reported in July 13 issue of the Journal of the American Heart Association (J Am Heart Assoc. 2016 Jul 13. 5:e003619 doi: 10.1161/JAHA.116.003619).
The rate of obese adults free of these three cardiovascular disease risk factors – diabetes, elevated cholesterol, and blood pressure – remained stable over the study period at about 15%. But the rate of obese adults with all three risk factors increased by 37% over the same period. By 2014, 22% reported having all three of those risk factors.
“The deteriorated blood glucose health among obese adults in the United States calls for lifestyle interventions (diet and exercise) on a national scale,” wrote Dr. Garvey, chair of nutrition science at the University of Alabama, Birmingham. “Community-based public health intervention programs may help increase physical activity and diet quality to alleviate the problem.”
The investigators examined trends in cardiometabolic health among 18,626 obese adults who participated in National Health and Nutrition Examination Surveys from 1988 to 2014. Over this period, mean body mass index increased significantly, from 34.7 to 36 kg/m2. Waist circumference increased as well, from 110 to 114.8 cm.
The picture was much better for blood pressure. Mean systolic pressures decreased about 2 points – from 126.1 to 124.4 mm Hg – in all age, racial and ethnic groups, and in both sexes. Mean diastolic blood pressure also decreased, dropping from 76.6 to 72.5 mm Hg. By 2014, 44% of the men and 51% of the women were below the blood pressure risk threshold.
Lipids also improved over the years, the investigators noted, with significant decreases in mean total cholesterol, from 214.5 to 193.7 mg/dL, and increases in HDL cholesterol, from 45.4 to 47.4 mg/dL.
Blood glucose worsened significantly, however. The mean hemoglobin A1c increased from 5.7% to 5.9%. The measurement rose in all ages, both sexes, and in all racial and ethnic groups except for non-Hispanic blacks.
Perhaps not surprisingly, the incidence of diabetes (a self-reported HbA1c of 6.5% or more) increased from 11% to 19% from 1988 to 2014. The increase occurred in all age groups and both sexes except for young adults aged 20-39 years. No racial or ethnic group was exempt from the increase.
The number of people having all three risk factors (hypertension, hypercholesterolemia, and hyperglycemia) increased from 16% in 1988 to 22% in 2014.
“The increase occurred in parallel with a decline in the prevalence of healthy blood glucose, which is the predominant explanation accounting for the rise in the prevalence of presence of all three risk factors,” the investigators said.
Only 15% of the study population was free from all of these risk factors – a percentage that remained unchanged during 1988-2014.
The findings should be a wake-up call for physicians and their patients, and a national call for action to improve cardiovascular health among obese adults, the team wrote.
“The increasing trend of obese people with all three cardiovascular risk factors, commensurate with a decline in those with one or two risk factors, suggests an overall deterioration in health among people with obesity. ... These patterns of worsening metabolic health constitute an increase in risk of type 2 diabetes mellitus and underlie increasing prevalence rates for diabetes mellitus,” the investigators wrote.
Aggressive treatment will be necessary to reverse these trends. This might include treatment with weight-loss medications in conjunction with lifestyle interventions, which should be especially targeted at obese individuals who are already metabolically unhealthy and in those who have complications or are at risk for developing them.
“In the context of the current data, those obese adults who are metabolically unhealthy or perhaps those with suboptimal metabolic health represent patients who will benefit most from intensive obesity management … coordinated efforts aligning cardiovascular disease prevention and control activities across the public and private sectors in the United States are needed reduce the burden of cardiovascular disease among the obese population,” Dr. Garvey concluded.
The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center.
Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: Blood glucose and diabetes are on the rise among America’s obese.
Major finding: Twenty-two percent of obese Americans now have three serious cardiovascular risk factors: hypertension, hypercholesterolemia, and hyperglycemia.
Data source: The 26-year observational study comprised more than 18,600 people.
Disclosures: The study was supported by the Department of Veterans Affairs, the National Institutes of Health, and the University of Alabama Diabetes Research Center. Dr. Guo had no financial disclosures. Dr. Garvey disclosed relationships with multiple pharmaceutical companies.