User login
Azathioprine Hypersensitivity Presenting as Neutrophilic Dermatosis and Erythema Nodosum
To the Editor:
Azathioprine (AZA) hypersensitivity is an immunologically mediated reaction that presents within 1 to 4 weeks of drug initiation.1 Its cutaneous manifestations include Sweet syndrome, erythema nodosum (EN), and acute generalized exanthematous pustulosis, with 88% of cases presenting as neutrophilic dermatoses.2 Confirmation with cutaneous biopsy and cessation of medication is essential to prevent life-threatening anaphylactoid reactions.
A 58-year-old man with a history of Crohn disease was admitted with high fevers (>38.9°C); abdominal pain; diarrhea; and a nonpruritic “pimplelike” rash on the face, chest, and back with a tender nodule on the right leg of 5 days’ duration. Eight days prior to admission, he had started AZA for treatment of Crohn disease. In the hospital he received intravenous metronidazole for a presumed bowel infection; however, the lesions and symptoms did not resolve. Other medical history included psoriatic arthritis for which he was taking oral prednisone 50 mg daily; prednisone was continued during hospitalization.
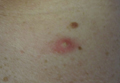
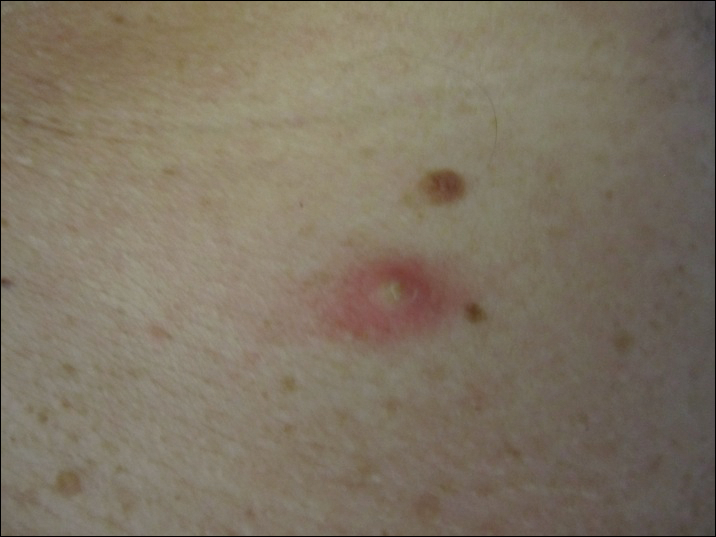
Physical examination showed that the patient was alert and well appearing. On the face, upper chest and back (Figure 1), shoulders, and knees were fewer than 20 sparsely distributed, nontender, 3- to 4-mm pustules. The patient’s scalp, lower back, abdomen, arms, and feet were spared. There also was a solitary 3.5-cm, tender, erythematous nodule on the right lower leg (Figure 2). Blood tests revealed leukocytosis (15,000/mm3 [reference range, 4300–10,300/mm3]) with neutrophilia (90%) and an elevated C-reactive protein level of 173 mg/L (reference range, <10 mg/L). Liver function tests were normal. Thiopurine methyltransferase (TPMT) was on the low end of the reference range. Tissue culture of a shoulder pustule grew only Staphylococcus non-aureus. Blood cultures were negative. A 4-mm punch biopsy specimen from the right leg nodule revealed septal panniculitis with neutrophilic and granulomatous infiltrate consistent with EN.
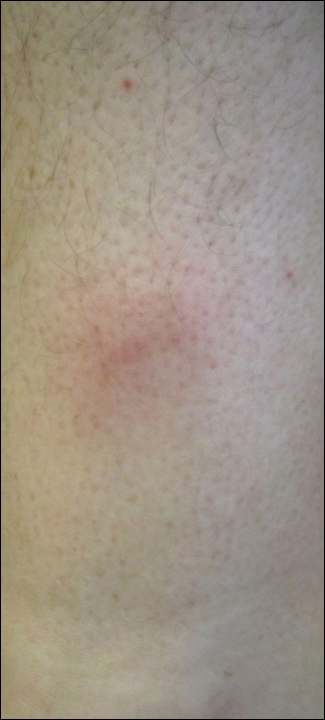
A clinical diagnosis of AZA hypersensitivity was made. Antibiotics and AZA were discontinued and the patient’s lesions resolved within 6 days. Medication rechallenge was not attempted and the patient is now managed with infliximab.
Azathioprine is a well-known and commonly used drug for inflammatory bowel diseases, rheumatoid arthritis, and prevention of transplant rejection. Hypersensitivity is a lesser-known complication of AZA therapy, with most reactions occurring within 4 weeks of treatment initiation. A PubMed search of articles indexed for MEDLINE using the search terms azathioprine and hypersensitivity found only 67 documented cases of AZA hypersensitivity between 1986 and 2009.2 Common findings include fever, malaise, arthralgia, nausea, vomiting, diarrhea, headache, and neutrophilic dermatoses.
Previously reported cases of AZA hypersensitivity with cutaneous manifestations include Sweet syndrome (17.9%), small vessel vasculitis (10.4%), EN (4.4%), acute generalized exanthematous pustulosis (4.4%), and nonspecific cutaneous findings (11.9%).2 One other case reported AZA hypersensitivity presenting as EN with a neutrophilic pustular dermatosis.3 Although Sweet syndrome–like lesions, EN, and acute generalized exanthematous pustulosis have been reported in the context of inflammatory bowel disease, in this case the appearance of these symptoms within 1 week of AZA initiation and resolution after AZA discontinuation is highly suggestive of AZA hypersensitivity. Also, several reports have documented rapid (within a few hours) recurrence of symptoms on rechallenge with AZA.4-6 Moreover, cases of cutaneous AZA hypersensitivity reactions in patients with no history of inflammatory bowel diseases have been reported.6-8
As in this case, cutaneous AZA hypersensitivity can occur even in the setting of normal TPMT levels, suggesting that this phenomenon is a dose-independent reaction.2 Abnormal metabolism of AZA does not appear to be related to previously reported neutrophilic pustular dermatosis3,4 or EN.4 Although the mechanism of hypersensitivity is unclear, there is a report of a patient who developed AZA hypersensitivity but was able to tolerate 6-mercaptopurine, a metabolite of AZA. The authors suggested that the imidazole component of AZA might be responsible for hypersensitivity reactions.9
The differential diagnosis of a patient with these findings includes infectious, rheumatologic, neurologic, or autoimmune diseases, as well as septic shock. Hence, negative cultures and a failure to respond to antibiotics make infection less likely. An appropriate time course of AZA initiation, the development of rash, and a cutaneous biopsy can lead to prompt diagnosis and cessation of AZA.
Once AZA hypersensitivity is suspected, the drug should be discontinued and the reaction should resolve within 2 to 3 days2 and the skin lesions within 5 to 6 days.2,10 Medication rechallenge is contraindicated because AZA rarely has been associated with shock syndrome and hypotension.11-19
Azathioprine hypersensitivity is a serious yet still underrecognized condition in the dermatologic community. In our case, symptoms appeared rapidly and resolved quickly after AZA was discontinued. Azathioprine-induced neutrophilic dermatosis presenting with EN should be recognized as a potential dermatologic manifestation of AZA hypersensitivity, which is a dose-dependent reaction even with normal TPMT levels. Rechallenge with AZA is not recommended due to the risk of a life-threatening anaphylactoid reaction.
- Meggitt SJ, Anstey AV, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711-734.
- Bidinger JJ, Sky K, Battafarano DF, et al. The cutaneous and systemic manifestations of azathioprine hypersensitivity syndrome. J Am Acad Dermatol. 2011;65:184-191.
- Hurtado-Garcia R, Escribano-Stablé JC, Pascual JC, et al. Neutrophilic dermatosis caused by azathioprine hypersensitivity. Int J Dermatol. 2012;51:1522-1525.
- De Fonclare AL, Khosrotehrani K, Aractingi S, et al. Erythema nodosum-like eruption as a manifestation of azathioprine hypersensitivity in patients with inflammatory bowel disease. Arch Dermatol. 2007;143:744-748.
- Jeurissen ME, Boerbooms AM, van de Putte LB, et al. Azathioprine induced fever, chills, rash, and hepatotoxicity in rheumatoid arthritis. Ann Rheum Dis. 1990;49:25-27.
- Goldenberg DL, Stor RA. Azathioprine hypersensitivity mimicking an acute exacerbation of dermatomyositis. J Rheumatol. 1975;2:346-349.
- Watts GF, Corston R. Hypersensitivity to azathioprine in myasthenia gravis. Postgrad Med J. 1984;60:362-363.
- El-Azhary RA, Brunner KL, Gibson LE. Sweet syndrome as a manifestation of azathioprine hypersensitivity. Mayo Clin Proc. 2008;83:1026-1030.
- Stetter M, Schmidl M, Krapf R. Azathioprine hypersensitivity mimicking Goodpasture’s syndrome. Am J Kidney Dis. 1994;23:874-877.
- Cyrus N, Stavert R, Mason AR, et al. Neutrophilic dermatosis after azathioprine exposure. JAMA Dermatol. 2013;149:592-597.
- Cunningham T, Barraclough D, Muirdin K. Azathioprine induced shock. Br Med J. 1981;283:823-824.
- Elston GE, Johnston GA, Mortimer NJ, et al. Acute generalized exanthematous pustulosis associated with azathioprine hypersensitivity. Clin Exp Dermatol. 2007;32:52-53.
- Fields CL, Robinson JW, Roy TM, et al. Hypersensitivity reaction to azathioprine. South Med J. 1998;91:471-474.
- Keystone E, Schabas R. Hypotension with oliguria: a side effect of azathioprine. Arthritis Rheum. 1981;24:1453-1454.
- Rosenthal E. Azathioprine shock. Postgrad Med J. 1986;62:677-678.
- Sofat N, Houghton J, McHale J, et al. Azathioprine hypersensitivity. Ann Rheum Dis. 2001;60:719-720.
- Knowles SR, Gupta AK, Shear NH, et al. Azathioprine hypersensitivity-like reactions—a case report and a review of the literature. Clin Exp Dermatol. 1995;20:353-356.
- Demirtaş-Ertan G, Rowshani AT, ten Berge IJ. Azathioprine-induced shock in a patient suffering from undifferentiated erosive oligoarthritis. Neth J Med. 2006;64:124-126.
- Zaltzman M, Kallenbach J, Shapiro T, et al. Life-threatening hypotension associated with azathioprine therapy. a case report. S Afr Med J. 1984;65:306.
To the Editor:
Azathioprine (AZA) hypersensitivity is an immunologically mediated reaction that presents within 1 to 4 weeks of drug initiation.1 Its cutaneous manifestations include Sweet syndrome, erythema nodosum (EN), and acute generalized exanthematous pustulosis, with 88% of cases presenting as neutrophilic dermatoses.2 Confirmation with cutaneous biopsy and cessation of medication is essential to prevent life-threatening anaphylactoid reactions.
A 58-year-old man with a history of Crohn disease was admitted with high fevers (>38.9°C); abdominal pain; diarrhea; and a nonpruritic “pimplelike” rash on the face, chest, and back with a tender nodule on the right leg of 5 days’ duration. Eight days prior to admission, he had started AZA for treatment of Crohn disease. In the hospital he received intravenous metronidazole for a presumed bowel infection; however, the lesions and symptoms did not resolve. Other medical history included psoriatic arthritis for which he was taking oral prednisone 50 mg daily; prednisone was continued during hospitalization.
Physical examination showed that the patient was alert and well appearing. On the face, upper chest and back (Figure 1), shoulders, and knees were fewer than 20 sparsely distributed, nontender, 3- to 4-mm pustules. The patient’s scalp, lower back, abdomen, arms, and feet were spared. There also was a solitary 3.5-cm, tender, erythematous nodule on the right lower leg (Figure 2). Blood tests revealed leukocytosis (15,000/mm3 [reference range, 4300–10,300/mm3]) with neutrophilia (90%) and an elevated C-reactive protein level of 173 mg/L (reference range, <10 mg/L). Liver function tests were normal. Thiopurine methyltransferase (TPMT) was on the low end of the reference range. Tissue culture of a shoulder pustule grew only Staphylococcus non-aureus. Blood cultures were negative. A 4-mm punch biopsy specimen from the right leg nodule revealed septal panniculitis with neutrophilic and granulomatous infiltrate consistent with EN.


A clinical diagnosis of AZA hypersensitivity was made. Antibiotics and AZA were discontinued and the patient’s lesions resolved within 6 days. Medication rechallenge was not attempted and the patient is now managed with infliximab.
Azathioprine is a well-known and commonly used drug for inflammatory bowel diseases, rheumatoid arthritis, and prevention of transplant rejection. Hypersensitivity is a lesser-known complication of AZA therapy, with most reactions occurring within 4 weeks of treatment initiation. A PubMed search of articles indexed for MEDLINE using the search terms azathioprine and hypersensitivity found only 67 documented cases of AZA hypersensitivity between 1986 and 2009.2 Common findings include fever, malaise, arthralgia, nausea, vomiting, diarrhea, headache, and neutrophilic dermatoses.
Previously reported cases of AZA hypersensitivity with cutaneous manifestations include Sweet syndrome (17.9%), small vessel vasculitis (10.4%), EN (4.4%), acute generalized exanthematous pustulosis (4.4%), and nonspecific cutaneous findings (11.9%).2 One other case reported AZA hypersensitivity presenting as EN with a neutrophilic pustular dermatosis.3 Although Sweet syndrome–like lesions, EN, and acute generalized exanthematous pustulosis have been reported in the context of inflammatory bowel disease, in this case the appearance of these symptoms within 1 week of AZA initiation and resolution after AZA discontinuation is highly suggestive of AZA hypersensitivity. Also, several reports have documented rapid (within a few hours) recurrence of symptoms on rechallenge with AZA.4-6 Moreover, cases of cutaneous AZA hypersensitivity reactions in patients with no history of inflammatory bowel diseases have been reported.6-8
As in this case, cutaneous AZA hypersensitivity can occur even in the setting of normal TPMT levels, suggesting that this phenomenon is a dose-independent reaction.2 Abnormal metabolism of AZA does not appear to be related to previously reported neutrophilic pustular dermatosis3,4 or EN.4 Although the mechanism of hypersensitivity is unclear, there is a report of a patient who developed AZA hypersensitivity but was able to tolerate 6-mercaptopurine, a metabolite of AZA. The authors suggested that the imidazole component of AZA might be responsible for hypersensitivity reactions.9
The differential diagnosis of a patient with these findings includes infectious, rheumatologic, neurologic, or autoimmune diseases, as well as septic shock. Hence, negative cultures and a failure to respond to antibiotics make infection less likely. An appropriate time course of AZA initiation, the development of rash, and a cutaneous biopsy can lead to prompt diagnosis and cessation of AZA.
Once AZA hypersensitivity is suspected, the drug should be discontinued and the reaction should resolve within 2 to 3 days2 and the skin lesions within 5 to 6 days.2,10 Medication rechallenge is contraindicated because AZA rarely has been associated with shock syndrome and hypotension.11-19
Azathioprine hypersensitivity is a serious yet still underrecognized condition in the dermatologic community. In our case, symptoms appeared rapidly and resolved quickly after AZA was discontinued. Azathioprine-induced neutrophilic dermatosis presenting with EN should be recognized as a potential dermatologic manifestation of AZA hypersensitivity, which is a dose-dependent reaction even with normal TPMT levels. Rechallenge with AZA is not recommended due to the risk of a life-threatening anaphylactoid reaction.
To the Editor:
Azathioprine (AZA) hypersensitivity is an immunologically mediated reaction that presents within 1 to 4 weeks of drug initiation.1 Its cutaneous manifestations include Sweet syndrome, erythema nodosum (EN), and acute generalized exanthematous pustulosis, with 88% of cases presenting as neutrophilic dermatoses.2 Confirmation with cutaneous biopsy and cessation of medication is essential to prevent life-threatening anaphylactoid reactions.
A 58-year-old man with a history of Crohn disease was admitted with high fevers (>38.9°C); abdominal pain; diarrhea; and a nonpruritic “pimplelike” rash on the face, chest, and back with a tender nodule on the right leg of 5 days’ duration. Eight days prior to admission, he had started AZA for treatment of Crohn disease. In the hospital he received intravenous metronidazole for a presumed bowel infection; however, the lesions and symptoms did not resolve. Other medical history included psoriatic arthritis for which he was taking oral prednisone 50 mg daily; prednisone was continued during hospitalization.
Physical examination showed that the patient was alert and well appearing. On the face, upper chest and back (Figure 1), shoulders, and knees were fewer than 20 sparsely distributed, nontender, 3- to 4-mm pustules. The patient’s scalp, lower back, abdomen, arms, and feet were spared. There also was a solitary 3.5-cm, tender, erythematous nodule on the right lower leg (Figure 2). Blood tests revealed leukocytosis (15,000/mm3 [reference range, 4300–10,300/mm3]) with neutrophilia (90%) and an elevated C-reactive protein level of 173 mg/L (reference range, <10 mg/L). Liver function tests were normal. Thiopurine methyltransferase (TPMT) was on the low end of the reference range. Tissue culture of a shoulder pustule grew only Staphylococcus non-aureus. Blood cultures were negative. A 4-mm punch biopsy specimen from the right leg nodule revealed septal panniculitis with neutrophilic and granulomatous infiltrate consistent with EN.


A clinical diagnosis of AZA hypersensitivity was made. Antibiotics and AZA were discontinued and the patient’s lesions resolved within 6 days. Medication rechallenge was not attempted and the patient is now managed with infliximab.
Azathioprine is a well-known and commonly used drug for inflammatory bowel diseases, rheumatoid arthritis, and prevention of transplant rejection. Hypersensitivity is a lesser-known complication of AZA therapy, with most reactions occurring within 4 weeks of treatment initiation. A PubMed search of articles indexed for MEDLINE using the search terms azathioprine and hypersensitivity found only 67 documented cases of AZA hypersensitivity between 1986 and 2009.2 Common findings include fever, malaise, arthralgia, nausea, vomiting, diarrhea, headache, and neutrophilic dermatoses.
Previously reported cases of AZA hypersensitivity with cutaneous manifestations include Sweet syndrome (17.9%), small vessel vasculitis (10.4%), EN (4.4%), acute generalized exanthematous pustulosis (4.4%), and nonspecific cutaneous findings (11.9%).2 One other case reported AZA hypersensitivity presenting as EN with a neutrophilic pustular dermatosis.3 Although Sweet syndrome–like lesions, EN, and acute generalized exanthematous pustulosis have been reported in the context of inflammatory bowel disease, in this case the appearance of these symptoms within 1 week of AZA initiation and resolution after AZA discontinuation is highly suggestive of AZA hypersensitivity. Also, several reports have documented rapid (within a few hours) recurrence of symptoms on rechallenge with AZA.4-6 Moreover, cases of cutaneous AZA hypersensitivity reactions in patients with no history of inflammatory bowel diseases have been reported.6-8
As in this case, cutaneous AZA hypersensitivity can occur even in the setting of normal TPMT levels, suggesting that this phenomenon is a dose-independent reaction.2 Abnormal metabolism of AZA does not appear to be related to previously reported neutrophilic pustular dermatosis3,4 or EN.4 Although the mechanism of hypersensitivity is unclear, there is a report of a patient who developed AZA hypersensitivity but was able to tolerate 6-mercaptopurine, a metabolite of AZA. The authors suggested that the imidazole component of AZA might be responsible for hypersensitivity reactions.9
The differential diagnosis of a patient with these findings includes infectious, rheumatologic, neurologic, or autoimmune diseases, as well as septic shock. Hence, negative cultures and a failure to respond to antibiotics make infection less likely. An appropriate time course of AZA initiation, the development of rash, and a cutaneous biopsy can lead to prompt diagnosis and cessation of AZA.
Once AZA hypersensitivity is suspected, the drug should be discontinued and the reaction should resolve within 2 to 3 days2 and the skin lesions within 5 to 6 days.2,10 Medication rechallenge is contraindicated because AZA rarely has been associated with shock syndrome and hypotension.11-19
Azathioprine hypersensitivity is a serious yet still underrecognized condition in the dermatologic community. In our case, symptoms appeared rapidly and resolved quickly after AZA was discontinued. Azathioprine-induced neutrophilic dermatosis presenting with EN should be recognized as a potential dermatologic manifestation of AZA hypersensitivity, which is a dose-dependent reaction even with normal TPMT levels. Rechallenge with AZA is not recommended due to the risk of a life-threatening anaphylactoid reaction.
- Meggitt SJ, Anstey AV, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711-734.
- Bidinger JJ, Sky K, Battafarano DF, et al. The cutaneous and systemic manifestations of azathioprine hypersensitivity syndrome. J Am Acad Dermatol. 2011;65:184-191.
- Hurtado-Garcia R, Escribano-Stablé JC, Pascual JC, et al. Neutrophilic dermatosis caused by azathioprine hypersensitivity. Int J Dermatol. 2012;51:1522-1525.
- De Fonclare AL, Khosrotehrani K, Aractingi S, et al. Erythema nodosum-like eruption as a manifestation of azathioprine hypersensitivity in patients with inflammatory bowel disease. Arch Dermatol. 2007;143:744-748.
- Jeurissen ME, Boerbooms AM, van de Putte LB, et al. Azathioprine induced fever, chills, rash, and hepatotoxicity in rheumatoid arthritis. Ann Rheum Dis. 1990;49:25-27.
- Goldenberg DL, Stor RA. Azathioprine hypersensitivity mimicking an acute exacerbation of dermatomyositis. J Rheumatol. 1975;2:346-349.
- Watts GF, Corston R. Hypersensitivity to azathioprine in myasthenia gravis. Postgrad Med J. 1984;60:362-363.
- El-Azhary RA, Brunner KL, Gibson LE. Sweet syndrome as a manifestation of azathioprine hypersensitivity. Mayo Clin Proc. 2008;83:1026-1030.
- Stetter M, Schmidl M, Krapf R. Azathioprine hypersensitivity mimicking Goodpasture’s syndrome. Am J Kidney Dis. 1994;23:874-877.
- Cyrus N, Stavert R, Mason AR, et al. Neutrophilic dermatosis after azathioprine exposure. JAMA Dermatol. 2013;149:592-597.
- Cunningham T, Barraclough D, Muirdin K. Azathioprine induced shock. Br Med J. 1981;283:823-824.
- Elston GE, Johnston GA, Mortimer NJ, et al. Acute generalized exanthematous pustulosis associated with azathioprine hypersensitivity. Clin Exp Dermatol. 2007;32:52-53.
- Fields CL, Robinson JW, Roy TM, et al. Hypersensitivity reaction to azathioprine. South Med J. 1998;91:471-474.
- Keystone E, Schabas R. Hypotension with oliguria: a side effect of azathioprine. Arthritis Rheum. 1981;24:1453-1454.
- Rosenthal E. Azathioprine shock. Postgrad Med J. 1986;62:677-678.
- Sofat N, Houghton J, McHale J, et al. Azathioprine hypersensitivity. Ann Rheum Dis. 2001;60:719-720.
- Knowles SR, Gupta AK, Shear NH, et al. Azathioprine hypersensitivity-like reactions—a case report and a review of the literature. Clin Exp Dermatol. 1995;20:353-356.
- Demirtaş-Ertan G, Rowshani AT, ten Berge IJ. Azathioprine-induced shock in a patient suffering from undifferentiated erosive oligoarthritis. Neth J Med. 2006;64:124-126.
- Zaltzman M, Kallenbach J, Shapiro T, et al. Life-threatening hypotension associated with azathioprine therapy. a case report. S Afr Med J. 1984;65:306.
- Meggitt SJ, Anstey AV, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711-734.
- Bidinger JJ, Sky K, Battafarano DF, et al. The cutaneous and systemic manifestations of azathioprine hypersensitivity syndrome. J Am Acad Dermatol. 2011;65:184-191.
- Hurtado-Garcia R, Escribano-Stablé JC, Pascual JC, et al. Neutrophilic dermatosis caused by azathioprine hypersensitivity. Int J Dermatol. 2012;51:1522-1525.
- De Fonclare AL, Khosrotehrani K, Aractingi S, et al. Erythema nodosum-like eruption as a manifestation of azathioprine hypersensitivity in patients with inflammatory bowel disease. Arch Dermatol. 2007;143:744-748.
- Jeurissen ME, Boerbooms AM, van de Putte LB, et al. Azathioprine induced fever, chills, rash, and hepatotoxicity in rheumatoid arthritis. Ann Rheum Dis. 1990;49:25-27.
- Goldenberg DL, Stor RA. Azathioprine hypersensitivity mimicking an acute exacerbation of dermatomyositis. J Rheumatol. 1975;2:346-349.
- Watts GF, Corston R. Hypersensitivity to azathioprine in myasthenia gravis. Postgrad Med J. 1984;60:362-363.
- El-Azhary RA, Brunner KL, Gibson LE. Sweet syndrome as a manifestation of azathioprine hypersensitivity. Mayo Clin Proc. 2008;83:1026-1030.
- Stetter M, Schmidl M, Krapf R. Azathioprine hypersensitivity mimicking Goodpasture’s syndrome. Am J Kidney Dis. 1994;23:874-877.
- Cyrus N, Stavert R, Mason AR, et al. Neutrophilic dermatosis after azathioprine exposure. JAMA Dermatol. 2013;149:592-597.
- Cunningham T, Barraclough D, Muirdin K. Azathioprine induced shock. Br Med J. 1981;283:823-824.
- Elston GE, Johnston GA, Mortimer NJ, et al. Acute generalized exanthematous pustulosis associated with azathioprine hypersensitivity. Clin Exp Dermatol. 2007;32:52-53.
- Fields CL, Robinson JW, Roy TM, et al. Hypersensitivity reaction to azathioprine. South Med J. 1998;91:471-474.
- Keystone E, Schabas R. Hypotension with oliguria: a side effect of azathioprine. Arthritis Rheum. 1981;24:1453-1454.
- Rosenthal E. Azathioprine shock. Postgrad Med J. 1986;62:677-678.
- Sofat N, Houghton J, McHale J, et al. Azathioprine hypersensitivity. Ann Rheum Dis. 2001;60:719-720.
- Knowles SR, Gupta AK, Shear NH, et al. Azathioprine hypersensitivity-like reactions—a case report and a review of the literature. Clin Exp Dermatol. 1995;20:353-356.
- Demirtaş-Ertan G, Rowshani AT, ten Berge IJ. Azathioprine-induced shock in a patient suffering from undifferentiated erosive oligoarthritis. Neth J Med. 2006;64:124-126.
- Zaltzman M, Kallenbach J, Shapiro T, et al. Life-threatening hypotension associated with azathioprine therapy. a case report. S Afr Med J. 1984;65:306.
Practice Points
- Azathioprine is a well-known immunosuppressant for renal transplant recipients and inflammatory bowel disease with several off-label uses in dermatology including immunobullous dermatoses, neutrophilic dermatoses, and autoimmune connective tissue diseases.
- Azathioprine hypersensitivity is rare and can present with systemic symptoms of fever and a neutrophilic dermatosis, which is usually self-limited but can progress to an anaphylactoid reaction with multiorgan failure.
- If a more mild hypersensitivity reaction is appreciated, then a rechallenge is not recommended and should be avoided.
Resolution of Disseminated Granuloma Annulare With Removal of Surgical Hardware
To the Editor:
Disseminated granuloma annulare is a noninfectious granulomatous disease of unknown etiology. Reported precipitating factors include trauma, sun exposure, viral infection, vaccination, and malignancy.1 In contrast to a localized variant, disseminated granuloma annulare is associated with a later age of onset, longer duration, and recalcitrance to therapy.2 Although a variety of therapeutic approaches exist, there are limited efficacy data, which is complicated by the spontaneous, self-limited nature of the disease.3,4
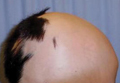
A 47-year-old man presented with an eruption of a thick red plaque on the dorsal aspect of the left hand (Figure). The eruption began 6 weeks following fixation of a Galeazzi fracture of the right radius with a stainless steel volar plate. Subsequent to the initial eruption, similar indurated plaques developed on the left thenar area, bilateral axillae, and bilateral legs. A punch biopsy was conducted to rule out necrobiosis lipoidica diabeticorum and sarcoidosis as well as to histopathologically confirm the clinical diagnosis of disseminated granuloma annulare. Following diagnosis, the patient received topical clobetasol for application to the advancing borders of the plaques. At 4-month follow-up, additional plaques continued to develop. The patient was not interested in pursuing alternative courses of therapy and felt that the implantation of surgical hardware was the cause. To the best of our knowledge, there have been no reports of precipitation of disseminated granuloma annulare in response to surgical hardware. Given the time course of onset of the eruption it was plausible that the hardware was the inciting event. The orthopedist thought that the fracture had healed sufficiently to remove the volar plate. The patient elected to have the hardware removed to potentially resolve or arrest the progression of the plaques. Resolution of the plaques was observed by the patient 2 weeks following surgical removal of the volar plate. At 4 months following hardware removal, the patient only had 2 slightly pink, hyperpigmented lesions on the left hand in the areas most severely affected, with complete resolution of all other plaques. The patient was given topical clobetasol for the residual lesions.
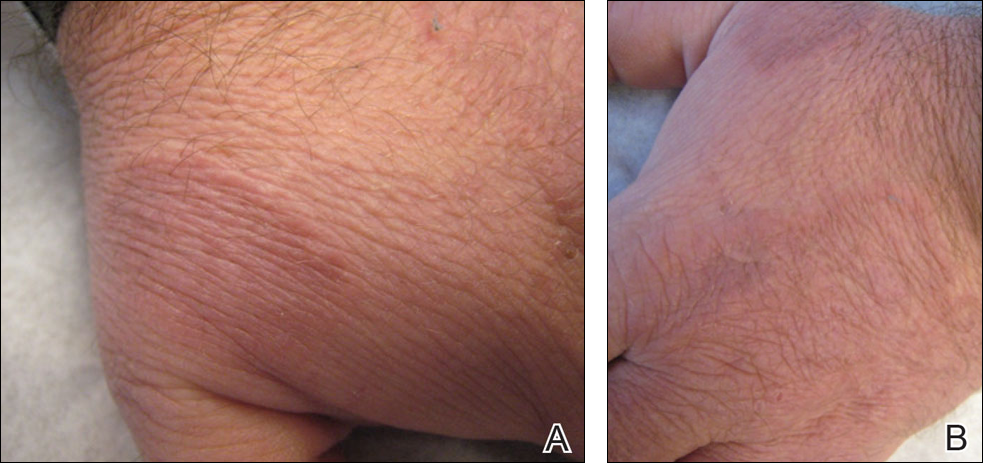
Precipitation and spontaneous resolution of disseminated granuloma annulare following the implantation and removal of surgical hardware is rare. Resolution following hardware removal is consistent with the theory that pathogenesis is due to a delayed-type hypersensitivity reaction to an inciting factor.5 Our case suggests that disseminated granuloma annulare may occur as a delayed-type hypersensitivity reaction to implanted surgical hardware, which should be considered in the etiology and potential therapeutic options for this disorder.
- Mills A, Chetty R. Auricular granuloma annulare. a consequence of trauma? Am J Dermatopathol. 1992;14:431-433.
- Dicken CH, Carrington SG, Winkelmann RK. Generalized granuloma annulare. Arch Dermatol. 1969;99:556-563.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea [published online May 31, 2009]. Ann Dermatol. 2009:21:113-119
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Buechner SA, Winkelmann RK, Banks PM. Identification of T-cell subpopulations in granuloma annulare. Arch Dermatol. 1983;119:125-128.
To the Editor:
Disseminated granuloma annulare is a noninfectious granulomatous disease of unknown etiology. Reported precipitating factors include trauma, sun exposure, viral infection, vaccination, and malignancy.1 In contrast to a localized variant, disseminated granuloma annulare is associated with a later age of onset, longer duration, and recalcitrance to therapy.2 Although a variety of therapeutic approaches exist, there are limited efficacy data, which is complicated by the spontaneous, self-limited nature of the disease.3,4
A 47-year-old man presented with an eruption of a thick red plaque on the dorsal aspect of the left hand (Figure). The eruption began 6 weeks following fixation of a Galeazzi fracture of the right radius with a stainless steel volar plate. Subsequent to the initial eruption, similar indurated plaques developed on the left thenar area, bilateral axillae, and bilateral legs. A punch biopsy was conducted to rule out necrobiosis lipoidica diabeticorum and sarcoidosis as well as to histopathologically confirm the clinical diagnosis of disseminated granuloma annulare. Following diagnosis, the patient received topical clobetasol for application to the advancing borders of the plaques. At 4-month follow-up, additional plaques continued to develop. The patient was not interested in pursuing alternative courses of therapy and felt that the implantation of surgical hardware was the cause. To the best of our knowledge, there have been no reports of precipitation of disseminated granuloma annulare in response to surgical hardware. Given the time course of onset of the eruption it was plausible that the hardware was the inciting event. The orthopedist thought that the fracture had healed sufficiently to remove the volar plate. The patient elected to have the hardware removed to potentially resolve or arrest the progression of the plaques. Resolution of the plaques was observed by the patient 2 weeks following surgical removal of the volar plate. At 4 months following hardware removal, the patient only had 2 slightly pink, hyperpigmented lesions on the left hand in the areas most severely affected, with complete resolution of all other plaques. The patient was given topical clobetasol for the residual lesions.

Precipitation and spontaneous resolution of disseminated granuloma annulare following the implantation and removal of surgical hardware is rare. Resolution following hardware removal is consistent with the theory that pathogenesis is due to a delayed-type hypersensitivity reaction to an inciting factor.5 Our case suggests that disseminated granuloma annulare may occur as a delayed-type hypersensitivity reaction to implanted surgical hardware, which should be considered in the etiology and potential therapeutic options for this disorder.
To the Editor:
Disseminated granuloma annulare is a noninfectious granulomatous disease of unknown etiology. Reported precipitating factors include trauma, sun exposure, viral infection, vaccination, and malignancy.1 In contrast to a localized variant, disseminated granuloma annulare is associated with a later age of onset, longer duration, and recalcitrance to therapy.2 Although a variety of therapeutic approaches exist, there are limited efficacy data, which is complicated by the spontaneous, self-limited nature of the disease.3,4
A 47-year-old man presented with an eruption of a thick red plaque on the dorsal aspect of the left hand (Figure). The eruption began 6 weeks following fixation of a Galeazzi fracture of the right radius with a stainless steel volar plate. Subsequent to the initial eruption, similar indurated plaques developed on the left thenar area, bilateral axillae, and bilateral legs. A punch biopsy was conducted to rule out necrobiosis lipoidica diabeticorum and sarcoidosis as well as to histopathologically confirm the clinical diagnosis of disseminated granuloma annulare. Following diagnosis, the patient received topical clobetasol for application to the advancing borders of the plaques. At 4-month follow-up, additional plaques continued to develop. The patient was not interested in pursuing alternative courses of therapy and felt that the implantation of surgical hardware was the cause. To the best of our knowledge, there have been no reports of precipitation of disseminated granuloma annulare in response to surgical hardware. Given the time course of onset of the eruption it was plausible that the hardware was the inciting event. The orthopedist thought that the fracture had healed sufficiently to remove the volar plate. The patient elected to have the hardware removed to potentially resolve or arrest the progression of the plaques. Resolution of the plaques was observed by the patient 2 weeks following surgical removal of the volar plate. At 4 months following hardware removal, the patient only had 2 slightly pink, hyperpigmented lesions on the left hand in the areas most severely affected, with complete resolution of all other plaques. The patient was given topical clobetasol for the residual lesions.

Precipitation and spontaneous resolution of disseminated granuloma annulare following the implantation and removal of surgical hardware is rare. Resolution following hardware removal is consistent with the theory that pathogenesis is due to a delayed-type hypersensitivity reaction to an inciting factor.5 Our case suggests that disseminated granuloma annulare may occur as a delayed-type hypersensitivity reaction to implanted surgical hardware, which should be considered in the etiology and potential therapeutic options for this disorder.
- Mills A, Chetty R. Auricular granuloma annulare. a consequence of trauma? Am J Dermatopathol. 1992;14:431-433.
- Dicken CH, Carrington SG, Winkelmann RK. Generalized granuloma annulare. Arch Dermatol. 1969;99:556-563.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea [published online May 31, 2009]. Ann Dermatol. 2009:21:113-119
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Buechner SA, Winkelmann RK, Banks PM. Identification of T-cell subpopulations in granuloma annulare. Arch Dermatol. 1983;119:125-128.
- Mills A, Chetty R. Auricular granuloma annulare. a consequence of trauma? Am J Dermatopathol. 1992;14:431-433.
- Dicken CH, Carrington SG, Winkelmann RK. Generalized granuloma annulare. Arch Dermatol. 1969;99:556-563.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea [published online May 31, 2009]. Ann Dermatol. 2009:21:113-119
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Buechner SA, Winkelmann RK, Banks PM. Identification of T-cell subpopulations in granuloma annulare. Arch Dermatol. 1983;119:125-128.
Practice Points
- Disseminated granuloma annulare may occur as a delayed-type hypersensitivity reaction to implanted surgical hardware.
- Resolution may occur following removal of surgical hardware.
JAK-1 inhibitors heading for validation in phase III trials
LONDON – ABT-494 and filgotinib – two investigational and highly selective oral Janus kinase-1 inhibitors – are both showing promise in the treatment of patients with rheumatoid arthritis, according to the results of two separate phase II studies presented at the European Congress of Rheumatology.
In the BALANCE-2 study, 62%-80% patients who had an inadequate response to methotrexate alone achieved the primary endpoint of an ACR20 response after 12 weeks of combination treatment with methotrexate and ABT-494, depending on the dose used, versus 46% for placebo plus methotrexate. The secondary endpoint of ACR50 was reached by a respective 38%-50% vs. 18%, and ACR70 response was achieved by 16%-28% vs. 6%.
And in the DARWIN-1 study, 56%-79% of patients treated with different doses of filgotinib plus methotrexate achieved the trial’s primary endpoint, which was again ACR20 at 12 weeks, versus 44% for placebo plus methotrexate. ACR50 and ACR70 responses were also similarly high and maintained up to 24 weeks of follow-up.
Both drugs had safety and tolerability data that supported their further development, the respective study investigators said.
“I think the results are rather straightforward. There was significant improvement in signs and symptoms of RA with fast onset,” said René Westhovens, MD, PhD, of the University of Leuven (Belgium), who presented the data from the DARWIN-1 study. “These robust data support the future development of filgotinib in RA,” he said.
Mark Genovese, MD, of Stanford (Calif.) University, who presented the findings of the BALANCE-2 study, said: “ABT-494 has been shown to have significant improvements in symptoms and signs [of RA] based on our endpoints of ACR [response], DAS[28], and CDAI [clinical disease activity index].” Like ACR50 and ACR70, DAS28 and CDAI were secondary efficacy endpoints and showed significantly greater changes from baseline versus placebo, started from around 2 weeks.
In an interview, Peter Taylor, PhD, who chaired the session at the meeting where the findings were presented, said: “We’ve seen a lot of data about JAK inhibitors at various stages of development at EULAR 2016, with varying selectivity, and the clinical data unequivocally validates Janus kinases as a therapeutic target.”
Dr. Taylor, the Norman Collisson Professor of Musculoskeletal Sciences at the University of Oxford (England), added: “[JAK inhibitors] show very significant promise with favorable safety data overall, but there are subtle differences between the drugs which need further detailed analysis to understand what it means in a clinical context.”
BALANCE-2 was a double-blind, placebo-controlled, dose-ranging phase IIB study designed to look at the safety and efficacy of ABT-494 in adult patients with moderately to severely active rheumatoid arthritis who had an inadequate response to methotrexate. Five doses of ABT-494 were tested: four given twice-daily (3, 6, 12, and 18 mg) and one given once-daily (24 mg). A total of 300 patients were enrolled and 299 were randomized, 50 to placebo, 50 each to the once-daily doses, and 49 to the twice-daily dose group. The mean weekly methotrexate dose at baseline was 14-16 mg across the groups.
“In general, the safety and tolerability of ABT-494 was satisfactory at the doses tested, consistent with what would have been expected,” Dr. Genovese said.
There was a numerically higher rate of any adverse event in the groups treated with ABT-494, at 40%, 46%, 58%, and 50% for the twice-daily regimens of 3, 6, 12, and 18 mg, as well as 35% for the once-daily 24-mg dose. The rate was 26% for placebo plus methotrexate.
Infections occurred in a respective 20%, 14%, 24%, 22% across the twice-daily dosing groups, respectively, compared with 18% for the once-daily 24-mg dose and 14% for placebo plus methotrexate, he noted. While there were some grade 2-3 abnormalities in liver enzymes and dose-related decreases in hemoglobin seen at higher doses, these did not appear to have significant clinical impact. The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) was also affected slightly.
DARWIN-1 involved a total of 599 enrolled and 594 randomized and exposed patients with RA treated with placebo plus methotrexate or methotrexate plus one of six dosing regimens of filgotinib: 50, 100, or 200 mg once daily, or 25, 50, or 100 mg twice daily, for 24 weeks, with around 85 patients in each group. Each patient previously had an inadequate response to methotrexate alone. At the 12-week halfway point, patients taking placebo and the 50-mg dose could be reassigned to filgotinib 100 mg once daily or 50 mg twice daily if their tender or swollen joint counts had not improved. The mean weekly dose of methotrexate at baseline was 16.4-17.5 mg across the groups.
In addition to the improved ACR responses, significant improvements with filgotinib versus placebo were seen in the secondary endpoints of DAS28 (including DAS28 based on C-reactive protein), CDAI, and the Health Assessment Questionnaire-Disability Index.
There were infrequent serious adverse events, which included serious infections, and adverse events leading to discontinuations, Dr. Westhovens observed, and nothing that would not have been expected or different from placebo. There was a small decrease in neutrophil counts and increase in creatinine, but neither had any clinical consequences. Interestingly, there was a dose-dependent increase in hemoglobin but no reduction in lymphocyte counts, he said. HDL-C increased more than LDL-C.
Five phase III trials with ABT-494 are currently underway in patients with RA:
• SELECT-COMPARE will enroll an estimated 1,500 RA patients who have had an inadequate response to a stable dose of methotrexate and will compare additional treatment with ABT-494 against additional treatment with adalimumab (Humira) or placebo.
• SELECT-NEXT will enroll an estimated 600 RA patients who have had an inadequate response to stable doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and are then given ABT-494 or placebo on top.
• SELECT-BEYOND will enroll around 450 RA patients on stable csDMARDs who have an inadequate response or intolerance to biologic DMARDs and compare adding ABT-494 or placebo.
• SELECT-MONOTHERAPY will enroll 600 RA patients who have had an inadequate methotrexate response and compare ABT-494 monotherapy to methotrexate monotherapy.
• SELECT-EARLY will enroll 975 methotrexate-naive, moderately-to-severely active RA patients and compare giving ABT-494 monotherapy to methotrexate monotherapy.
Most of these trials should have primary endpoint data available for analysis by mid to late 2017 or 2018 and be finished by 2020 or 2021.
Filgotinib, formerly known as GLPG0634, is also about to enter phase III trials, but the details of these trials have not yet been revealed other than that they will begin mid-2016.
The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead.
The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb.
Dr. Taylor was not involved in either study but has consulted for Eli Lilly, Pfizer, and Galapagos.
LONDON – ABT-494 and filgotinib – two investigational and highly selective oral Janus kinase-1 inhibitors – are both showing promise in the treatment of patients with rheumatoid arthritis, according to the results of two separate phase II studies presented at the European Congress of Rheumatology.
In the BALANCE-2 study, 62%-80% patients who had an inadequate response to methotrexate alone achieved the primary endpoint of an ACR20 response after 12 weeks of combination treatment with methotrexate and ABT-494, depending on the dose used, versus 46% for placebo plus methotrexate. The secondary endpoint of ACR50 was reached by a respective 38%-50% vs. 18%, and ACR70 response was achieved by 16%-28% vs. 6%.
And in the DARWIN-1 study, 56%-79% of patients treated with different doses of filgotinib plus methotrexate achieved the trial’s primary endpoint, which was again ACR20 at 12 weeks, versus 44% for placebo plus methotrexate. ACR50 and ACR70 responses were also similarly high and maintained up to 24 weeks of follow-up.
Both drugs had safety and tolerability data that supported their further development, the respective study investigators said.
“I think the results are rather straightforward. There was significant improvement in signs and symptoms of RA with fast onset,” said René Westhovens, MD, PhD, of the University of Leuven (Belgium), who presented the data from the DARWIN-1 study. “These robust data support the future development of filgotinib in RA,” he said.
Mark Genovese, MD, of Stanford (Calif.) University, who presented the findings of the BALANCE-2 study, said: “ABT-494 has been shown to have significant improvements in symptoms and signs [of RA] based on our endpoints of ACR [response], DAS[28], and CDAI [clinical disease activity index].” Like ACR50 and ACR70, DAS28 and CDAI were secondary efficacy endpoints and showed significantly greater changes from baseline versus placebo, started from around 2 weeks.
In an interview, Peter Taylor, PhD, who chaired the session at the meeting where the findings were presented, said: “We’ve seen a lot of data about JAK inhibitors at various stages of development at EULAR 2016, with varying selectivity, and the clinical data unequivocally validates Janus kinases as a therapeutic target.”
Dr. Taylor, the Norman Collisson Professor of Musculoskeletal Sciences at the University of Oxford (England), added: “[JAK inhibitors] show very significant promise with favorable safety data overall, but there are subtle differences between the drugs which need further detailed analysis to understand what it means in a clinical context.”
BALANCE-2 was a double-blind, placebo-controlled, dose-ranging phase IIB study designed to look at the safety and efficacy of ABT-494 in adult patients with moderately to severely active rheumatoid arthritis who had an inadequate response to methotrexate. Five doses of ABT-494 were tested: four given twice-daily (3, 6, 12, and 18 mg) and one given once-daily (24 mg). A total of 300 patients were enrolled and 299 were randomized, 50 to placebo, 50 each to the once-daily doses, and 49 to the twice-daily dose group. The mean weekly methotrexate dose at baseline was 14-16 mg across the groups.
“In general, the safety and tolerability of ABT-494 was satisfactory at the doses tested, consistent with what would have been expected,” Dr. Genovese said.
There was a numerically higher rate of any adverse event in the groups treated with ABT-494, at 40%, 46%, 58%, and 50% for the twice-daily regimens of 3, 6, 12, and 18 mg, as well as 35% for the once-daily 24-mg dose. The rate was 26% for placebo plus methotrexate.
Infections occurred in a respective 20%, 14%, 24%, 22% across the twice-daily dosing groups, respectively, compared with 18% for the once-daily 24-mg dose and 14% for placebo plus methotrexate, he noted. While there were some grade 2-3 abnormalities in liver enzymes and dose-related decreases in hemoglobin seen at higher doses, these did not appear to have significant clinical impact. The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) was also affected slightly.
DARWIN-1 involved a total of 599 enrolled and 594 randomized and exposed patients with RA treated with placebo plus methotrexate or methotrexate plus one of six dosing regimens of filgotinib: 50, 100, or 200 mg once daily, or 25, 50, or 100 mg twice daily, for 24 weeks, with around 85 patients in each group. Each patient previously had an inadequate response to methotrexate alone. At the 12-week halfway point, patients taking placebo and the 50-mg dose could be reassigned to filgotinib 100 mg once daily or 50 mg twice daily if their tender or swollen joint counts had not improved. The mean weekly dose of methotrexate at baseline was 16.4-17.5 mg across the groups.
In addition to the improved ACR responses, significant improvements with filgotinib versus placebo were seen in the secondary endpoints of DAS28 (including DAS28 based on C-reactive protein), CDAI, and the Health Assessment Questionnaire-Disability Index.
There were infrequent serious adverse events, which included serious infections, and adverse events leading to discontinuations, Dr. Westhovens observed, and nothing that would not have been expected or different from placebo. There was a small decrease in neutrophil counts and increase in creatinine, but neither had any clinical consequences. Interestingly, there was a dose-dependent increase in hemoglobin but no reduction in lymphocyte counts, he said. HDL-C increased more than LDL-C.
Five phase III trials with ABT-494 are currently underway in patients with RA:
• SELECT-COMPARE will enroll an estimated 1,500 RA patients who have had an inadequate response to a stable dose of methotrexate and will compare additional treatment with ABT-494 against additional treatment with adalimumab (Humira) or placebo.
• SELECT-NEXT will enroll an estimated 600 RA patients who have had an inadequate response to stable doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and are then given ABT-494 or placebo on top.
• SELECT-BEYOND will enroll around 450 RA patients on stable csDMARDs who have an inadequate response or intolerance to biologic DMARDs and compare adding ABT-494 or placebo.
• SELECT-MONOTHERAPY will enroll 600 RA patients who have had an inadequate methotrexate response and compare ABT-494 monotherapy to methotrexate monotherapy.
• SELECT-EARLY will enroll 975 methotrexate-naive, moderately-to-severely active RA patients and compare giving ABT-494 monotherapy to methotrexate monotherapy.
Most of these trials should have primary endpoint data available for analysis by mid to late 2017 or 2018 and be finished by 2020 or 2021.
Filgotinib, formerly known as GLPG0634, is also about to enter phase III trials, but the details of these trials have not yet been revealed other than that they will begin mid-2016.
The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead.
The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb.
Dr. Taylor was not involved in either study but has consulted for Eli Lilly, Pfizer, and Galapagos.
LONDON – ABT-494 and filgotinib – two investigational and highly selective oral Janus kinase-1 inhibitors – are both showing promise in the treatment of patients with rheumatoid arthritis, according to the results of two separate phase II studies presented at the European Congress of Rheumatology.
In the BALANCE-2 study, 62%-80% patients who had an inadequate response to methotrexate alone achieved the primary endpoint of an ACR20 response after 12 weeks of combination treatment with methotrexate and ABT-494, depending on the dose used, versus 46% for placebo plus methotrexate. The secondary endpoint of ACR50 was reached by a respective 38%-50% vs. 18%, and ACR70 response was achieved by 16%-28% vs. 6%.
And in the DARWIN-1 study, 56%-79% of patients treated with different doses of filgotinib plus methotrexate achieved the trial’s primary endpoint, which was again ACR20 at 12 weeks, versus 44% for placebo plus methotrexate. ACR50 and ACR70 responses were also similarly high and maintained up to 24 weeks of follow-up.
Both drugs had safety and tolerability data that supported their further development, the respective study investigators said.
“I think the results are rather straightforward. There was significant improvement in signs and symptoms of RA with fast onset,” said René Westhovens, MD, PhD, of the University of Leuven (Belgium), who presented the data from the DARWIN-1 study. “These robust data support the future development of filgotinib in RA,” he said.
Mark Genovese, MD, of Stanford (Calif.) University, who presented the findings of the BALANCE-2 study, said: “ABT-494 has been shown to have significant improvements in symptoms and signs [of RA] based on our endpoints of ACR [response], DAS[28], and CDAI [clinical disease activity index].” Like ACR50 and ACR70, DAS28 and CDAI were secondary efficacy endpoints and showed significantly greater changes from baseline versus placebo, started from around 2 weeks.
In an interview, Peter Taylor, PhD, who chaired the session at the meeting where the findings were presented, said: “We’ve seen a lot of data about JAK inhibitors at various stages of development at EULAR 2016, with varying selectivity, and the clinical data unequivocally validates Janus kinases as a therapeutic target.”
Dr. Taylor, the Norman Collisson Professor of Musculoskeletal Sciences at the University of Oxford (England), added: “[JAK inhibitors] show very significant promise with favorable safety data overall, but there are subtle differences between the drugs which need further detailed analysis to understand what it means in a clinical context.”
BALANCE-2 was a double-blind, placebo-controlled, dose-ranging phase IIB study designed to look at the safety and efficacy of ABT-494 in adult patients with moderately to severely active rheumatoid arthritis who had an inadequate response to methotrexate. Five doses of ABT-494 were tested: four given twice-daily (3, 6, 12, and 18 mg) and one given once-daily (24 mg). A total of 300 patients were enrolled and 299 were randomized, 50 to placebo, 50 each to the once-daily doses, and 49 to the twice-daily dose group. The mean weekly methotrexate dose at baseline was 14-16 mg across the groups.
“In general, the safety and tolerability of ABT-494 was satisfactory at the doses tested, consistent with what would have been expected,” Dr. Genovese said.
There was a numerically higher rate of any adverse event in the groups treated with ABT-494, at 40%, 46%, 58%, and 50% for the twice-daily regimens of 3, 6, 12, and 18 mg, as well as 35% for the once-daily 24-mg dose. The rate was 26% for placebo plus methotrexate.
Infections occurred in a respective 20%, 14%, 24%, 22% across the twice-daily dosing groups, respectively, compared with 18% for the once-daily 24-mg dose and 14% for placebo plus methotrexate, he noted. While there were some grade 2-3 abnormalities in liver enzymes and dose-related decreases in hemoglobin seen at higher doses, these did not appear to have significant clinical impact. The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) was also affected slightly.
DARWIN-1 involved a total of 599 enrolled and 594 randomized and exposed patients with RA treated with placebo plus methotrexate or methotrexate plus one of six dosing regimens of filgotinib: 50, 100, or 200 mg once daily, or 25, 50, or 100 mg twice daily, for 24 weeks, with around 85 patients in each group. Each patient previously had an inadequate response to methotrexate alone. At the 12-week halfway point, patients taking placebo and the 50-mg dose could be reassigned to filgotinib 100 mg once daily or 50 mg twice daily if their tender or swollen joint counts had not improved. The mean weekly dose of methotrexate at baseline was 16.4-17.5 mg across the groups.
In addition to the improved ACR responses, significant improvements with filgotinib versus placebo were seen in the secondary endpoints of DAS28 (including DAS28 based on C-reactive protein), CDAI, and the Health Assessment Questionnaire-Disability Index.
There were infrequent serious adverse events, which included serious infections, and adverse events leading to discontinuations, Dr. Westhovens observed, and nothing that would not have been expected or different from placebo. There was a small decrease in neutrophil counts and increase in creatinine, but neither had any clinical consequences. Interestingly, there was a dose-dependent increase in hemoglobin but no reduction in lymphocyte counts, he said. HDL-C increased more than LDL-C.
Five phase III trials with ABT-494 are currently underway in patients with RA:
• SELECT-COMPARE will enroll an estimated 1,500 RA patients who have had an inadequate response to a stable dose of methotrexate and will compare additional treatment with ABT-494 against additional treatment with adalimumab (Humira) or placebo.
• SELECT-NEXT will enroll an estimated 600 RA patients who have had an inadequate response to stable doses of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and are then given ABT-494 or placebo on top.
• SELECT-BEYOND will enroll around 450 RA patients on stable csDMARDs who have an inadequate response or intolerance to biologic DMARDs and compare adding ABT-494 or placebo.
• SELECT-MONOTHERAPY will enroll 600 RA patients who have had an inadequate methotrexate response and compare ABT-494 monotherapy to methotrexate monotherapy.
• SELECT-EARLY will enroll 975 methotrexate-naive, moderately-to-severely active RA patients and compare giving ABT-494 monotherapy to methotrexate monotherapy.
Most of these trials should have primary endpoint data available for analysis by mid to late 2017 or 2018 and be finished by 2020 or 2021.
Filgotinib, formerly known as GLPG0634, is also about to enter phase III trials, but the details of these trials have not yet been revealed other than that they will begin mid-2016.
The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead.
The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb.
Dr. Taylor was not involved in either study but has consulted for Eli Lilly, Pfizer, and Galapagos.
Key clinical point: Two new oral Janus kinase inhibitors selective for JAK-1 have shown promising efficacy and safety in separate phase II trials.
Major finding: ACR20 responses were achieved by 62%-80% of patients treated with different dosing regimens of ABT-494 plus methotrexate (vs. 46% with placebo plus methotrexate) in one trial and by 56%-79% of patients treated with different dosing regimens of filgotinib plus methotrexate (vs. 44% with placebo and methotrexate) in the other.
Data source: Two phase II studies addressing the efficacy and safety of the selective JAK-1 inhibitors ABT-494 and filgotinib in patients with rheumatoid arthritis and an inadequate response to methotrexate.
Disclosures: The BALANCE-2 study was funded by AbbVie. Dr. Genovese is a consultant for, and has received grants from AbbVie, Eli Lilly, Astellas, Vertex, Pfizer, Galapagos, and Gilead. The DARWIN-1 study was funded by Galapagos. Dr. Westhovens is the principal investigator for the study. He also disclosed receiving research funding from Roche and speaker’s honoraria from Bristol-Myers Squibb. Dr. Taylor has consulted for Eli Lilly, Pfizer, and Galapagos.
Medical errors and the law
Question: A patient was admitted with heart failure, developed deep vein thrombosis, and was started on warfarin and Lovenox as “bridge” therapy. On day 4, the patient achieved anticoagulation with a prothrombin time of 29.8 and international normalized ratio (INR) of 2.86, but continued to receive both warfarin and Lovenox for a total of 13 days. Both medications were dispensed and administered for 2 days when the PT was greater than 50; the supratherapeutic coagulation profile result was overlooked. Medications held on day 14 (PT, 68; INR, 8.35). The patient developed a right subdural hematoma and was transferred to a tertiary care facility for neurosurgery consult.
Given these facts, which of the following statements is best?
A. The hospital is under a legal obligation to disclose the error.
B. The doctor should be sympathetic and apologize for the injury, but not admit fault.
C. All jurisdictions have so-called “apology statutes,” which encourage error disclosure in return for immunity.
D. This is a case of medication, not medical, error.
E. Silence is golden.
Answer: B. A recent publication concluded, “If medical error were a disease, it would rank as the third leading cause of death in the United States.”1 This is the latest follow through on the original landmark report from the Institute of Medicine in 2000, which drew the public’s attention to the fact that medical errors were responsible for between 44,000 and 98,000 annual fatalities in the United States.
A medical error denotes a preventable adverse event, which in turn can be described as an injury caused by medical mismanagement rather than the underlying condition of the patient. It is more formally defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.”2 The term is not synonymous with medical negligence, which is a legal term of art encompassing four separate elements: duty, breach, causation, and damages.
The most common type of medical error is a medication error, which is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health professional, patient, or consumer.
Medication errors account for 6.3%-30% of all malpractice claims, and a 1999 Texas case is an example.3 A 43-year-old Hispanic man with chest pain was prescribed the anti-angina drug Isordil (isosorbide dinitrate) by his cardiologist, to be taken four times a day in doses of 20 mg. The pharmacist misread the order as Plendil (felodipine), a calcium channel blocker for treatment of hypertension. This exceeded the drug’s top dose, and the patient suffered a heart attack and died several days later.
The cardiologist’s illegible prescription was the sole reason for the error, and his overall quality of care was not at issue. The jury returned a verdict for the plaintiff, awarding $450,000 to his estate: $225,000 from the cardiologist, and $225,000 from the pharmacist.
Many, but not all, jurisdictions now require some form of reporting of medical errors occurring in a hospital setting. States such as California and Florida mandate disclosure to patients. Pennsylvania actually requires hospitals to issue a written disclosure within 7 days of a serious event.
Most states have enacted “apology statutes” to encourage open discussions with patients and their families about adverse results. The apologies may cover expressions of regret, sympathy, and compassion, and they are barred from being presented to the jury should a trial ensue. However, an acknowledgment of fault remains admissible into evidence.
Typical is California’s Evidence Code 1160(a), which provides that only “the portions of statements or benevolent gestures expressing sympathy” are inadmissible against a treating physician. On the other hand, some states have chosen to exclude all disclosures, including admissions of fault. An example is Colorado’s Apology Statute (Colo. Rev. Stat. Ann. 13-25-135), which provides that “any and all statements, affirmations, gestures, or conduct expressing apology, fault, sympathy, commiseration, condolence, compassion, or a general sense of benevolence ... shall be inadmissible as evidence of an admission of liability or as evidence of an admission against interest.”
Ohio is one of only a few states whose apology statutes fail to clearly distinguish between the admissibility of a physician’s statement of sympathy and one acknowledging fault. R.C. 2317.43, enacted by the Ohio General Assembly in 2004, renders inadmissible “statements, affirmations, gestures, or conduct expressing apology, sympathy, commiseration, condolence, compassion, or a general sense of benevolence.”
In Davis v. Wooster Orthopaedics & Sports Medicine,4 the plaintiff alleged that Dr. Michael Knapic, an orthopedic surgeon, negligently performed a lumbar microdiscectomy, severing the patient’s common iliac artery. The surgeon allegedly then said to the patient’s husband, “It’s my fault. I take full responsibility.”
In a wrongful-death action, the plaintiff argued that the statute did not prohibit the use of statements of fault, responsibility, or liability as compared to statements of sympathy or condolence.
Ohio’s Ninth Appellate District concluded that the intent behind the apology statute was to protect pure expressions of sympathy but not admissions of fault. The court held that Dr. Knapic’s statements constituted an admission of liability that could be admitted into evidence. The jury awarded damages of $3 million.
More recently, the Ohio Supreme Court ruled that Dr. Randall Smith’s alleged confession regarding accidentally sectioning his patient’s common bile duct was properly excluded from evidence, even though the incident took place before the Ohio law went into effect.5
The patient had to be readmitted within 3 weeks for obstructive jaundice. After the doctor informed her that she would have to undergo additional surgery, she became very emotional. He reportedly took her hand, saying, “I take full responsibility for this.”
The Ohio Supreme Court ruled, “The trial court had determined that Dr. Smith was faced with a distressed patient who was upset and made a statement that was designed to comfort his patient. This is precisely the type of evidence that R.C. 2317.43 was designed to exclude as evidence of liability in a medical-malpractice case.”
Do disclosures and apologies work?
Both claim frequency and severity have diminished following the adoption of a humanistic risk management policy at the Lexington Veterans Affairs Medical Center since 1987.6 The protocol includes early injury review, steadfast maintenance of the relationship between the hospital and the patient, proactive disclosure to patients who have been injured because of accidents or medical negligence, and fair compensation for injuries.
Other institutions such as the University of Michigan have adopted “disclosure and offer” in place of “denial and defend” policies, but these have yet to achieve widespread use.
Many health care providers continue to heed the traditional legal advice to say and admit nothing, believing that it is unsettled whether an apology will influence any decision to sue the doctor. They argue that the odds of a lawsuit are low to begin with.
In the oft-cited Harvard study, there was only one malpractice claim for every 7.6 adverse events caused by negligence among the 3.7% of hospitalized patients who suffered significant iatrogenic injuries, typically from errors or negligence.7
Notwithstanding the controversy, the AMA has properly taken the moral high ground: It asserts that error disclosure is the right thing to do.
References
2. Institute of Medicine: To Err is Human: Building a Safer Health System. National Academy Press, Washington, 2000.
3. Estate of Velasquez v. Albertsons, Inc. et al., Civ. No. A-103-042 (Ector Cnty, TX 1999).
4. Davis v. Wooster Orthopaedics & Sports Medicine, Inc., 193 Ohio App.3d 581 (2011).
5. Estate of Johnson v. Randall Smith, Inc., 131 Ohio St.3d 1543 (2013).
6. N Engl J Med. 2010 Apr 15;362(15):1353-6.
7. N Engl J Med. 1991 Jul 25;325(4):245-51.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical or legal advice. It is adapted from the author’s book, “Medical Malpractice: Understanding the Law, Managing the Risk” (2006). For additional information, readers may contact the author at [email protected].
Question: A patient was admitted with heart failure, developed deep vein thrombosis, and was started on warfarin and Lovenox as “bridge” therapy. On day 4, the patient achieved anticoagulation with a prothrombin time of 29.8 and international normalized ratio (INR) of 2.86, but continued to receive both warfarin and Lovenox for a total of 13 days. Both medications were dispensed and administered for 2 days when the PT was greater than 50; the supratherapeutic coagulation profile result was overlooked. Medications held on day 14 (PT, 68; INR, 8.35). The patient developed a right subdural hematoma and was transferred to a tertiary care facility for neurosurgery consult.
Given these facts, which of the following statements is best?
A. The hospital is under a legal obligation to disclose the error.
B. The doctor should be sympathetic and apologize for the injury, but not admit fault.
C. All jurisdictions have so-called “apology statutes,” which encourage error disclosure in return for immunity.
D. This is a case of medication, not medical, error.
E. Silence is golden.
Answer: B. A recent publication concluded, “If medical error were a disease, it would rank as the third leading cause of death in the United States.”1 This is the latest follow through on the original landmark report from the Institute of Medicine in 2000, which drew the public’s attention to the fact that medical errors were responsible for between 44,000 and 98,000 annual fatalities in the United States.
A medical error denotes a preventable adverse event, which in turn can be described as an injury caused by medical mismanagement rather than the underlying condition of the patient. It is more formally defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.”2 The term is not synonymous with medical negligence, which is a legal term of art encompassing four separate elements: duty, breach, causation, and damages.
The most common type of medical error is a medication error, which is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health professional, patient, or consumer.
Medication errors account for 6.3%-30% of all malpractice claims, and a 1999 Texas case is an example.3 A 43-year-old Hispanic man with chest pain was prescribed the anti-angina drug Isordil (isosorbide dinitrate) by his cardiologist, to be taken four times a day in doses of 20 mg. The pharmacist misread the order as Plendil (felodipine), a calcium channel blocker for treatment of hypertension. This exceeded the drug’s top dose, and the patient suffered a heart attack and died several days later.
The cardiologist’s illegible prescription was the sole reason for the error, and his overall quality of care was not at issue. The jury returned a verdict for the plaintiff, awarding $450,000 to his estate: $225,000 from the cardiologist, and $225,000 from the pharmacist.
Many, but not all, jurisdictions now require some form of reporting of medical errors occurring in a hospital setting. States such as California and Florida mandate disclosure to patients. Pennsylvania actually requires hospitals to issue a written disclosure within 7 days of a serious event.
Most states have enacted “apology statutes” to encourage open discussions with patients and their families about adverse results. The apologies may cover expressions of regret, sympathy, and compassion, and they are barred from being presented to the jury should a trial ensue. However, an acknowledgment of fault remains admissible into evidence.
Typical is California’s Evidence Code 1160(a), which provides that only “the portions of statements or benevolent gestures expressing sympathy” are inadmissible against a treating physician. On the other hand, some states have chosen to exclude all disclosures, including admissions of fault. An example is Colorado’s Apology Statute (Colo. Rev. Stat. Ann. 13-25-135), which provides that “any and all statements, affirmations, gestures, or conduct expressing apology, fault, sympathy, commiseration, condolence, compassion, or a general sense of benevolence ... shall be inadmissible as evidence of an admission of liability or as evidence of an admission against interest.”
Ohio is one of only a few states whose apology statutes fail to clearly distinguish between the admissibility of a physician’s statement of sympathy and one acknowledging fault. R.C. 2317.43, enacted by the Ohio General Assembly in 2004, renders inadmissible “statements, affirmations, gestures, or conduct expressing apology, sympathy, commiseration, condolence, compassion, or a general sense of benevolence.”
In Davis v. Wooster Orthopaedics & Sports Medicine,4 the plaintiff alleged that Dr. Michael Knapic, an orthopedic surgeon, negligently performed a lumbar microdiscectomy, severing the patient’s common iliac artery. The surgeon allegedly then said to the patient’s husband, “It’s my fault. I take full responsibility.”
In a wrongful-death action, the plaintiff argued that the statute did not prohibit the use of statements of fault, responsibility, or liability as compared to statements of sympathy or condolence.
Ohio’s Ninth Appellate District concluded that the intent behind the apology statute was to protect pure expressions of sympathy but not admissions of fault. The court held that Dr. Knapic’s statements constituted an admission of liability that could be admitted into evidence. The jury awarded damages of $3 million.
More recently, the Ohio Supreme Court ruled that Dr. Randall Smith’s alleged confession regarding accidentally sectioning his patient’s common bile duct was properly excluded from evidence, even though the incident took place before the Ohio law went into effect.5
The patient had to be readmitted within 3 weeks for obstructive jaundice. After the doctor informed her that she would have to undergo additional surgery, she became very emotional. He reportedly took her hand, saying, “I take full responsibility for this.”
The Ohio Supreme Court ruled, “The trial court had determined that Dr. Smith was faced with a distressed patient who was upset and made a statement that was designed to comfort his patient. This is precisely the type of evidence that R.C. 2317.43 was designed to exclude as evidence of liability in a medical-malpractice case.”
Do disclosures and apologies work?
Both claim frequency and severity have diminished following the adoption of a humanistic risk management policy at the Lexington Veterans Affairs Medical Center since 1987.6 The protocol includes early injury review, steadfast maintenance of the relationship between the hospital and the patient, proactive disclosure to patients who have been injured because of accidents or medical negligence, and fair compensation for injuries.
Other institutions such as the University of Michigan have adopted “disclosure and offer” in place of “denial and defend” policies, but these have yet to achieve widespread use.
Many health care providers continue to heed the traditional legal advice to say and admit nothing, believing that it is unsettled whether an apology will influence any decision to sue the doctor. They argue that the odds of a lawsuit are low to begin with.
In the oft-cited Harvard study, there was only one malpractice claim for every 7.6 adverse events caused by negligence among the 3.7% of hospitalized patients who suffered significant iatrogenic injuries, typically from errors or negligence.7
Notwithstanding the controversy, the AMA has properly taken the moral high ground: It asserts that error disclosure is the right thing to do.
References
2. Institute of Medicine: To Err is Human: Building a Safer Health System. National Academy Press, Washington, 2000.
3. Estate of Velasquez v. Albertsons, Inc. et al., Civ. No. A-103-042 (Ector Cnty, TX 1999).
4. Davis v. Wooster Orthopaedics & Sports Medicine, Inc., 193 Ohio App.3d 581 (2011).
5. Estate of Johnson v. Randall Smith, Inc., 131 Ohio St.3d 1543 (2013).
6. N Engl J Med. 2010 Apr 15;362(15):1353-6.
7. N Engl J Med. 1991 Jul 25;325(4):245-51.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical or legal advice. It is adapted from the author’s book, “Medical Malpractice: Understanding the Law, Managing the Risk” (2006). For additional information, readers may contact the author at [email protected].
Question: A patient was admitted with heart failure, developed deep vein thrombosis, and was started on warfarin and Lovenox as “bridge” therapy. On day 4, the patient achieved anticoagulation with a prothrombin time of 29.8 and international normalized ratio (INR) of 2.86, but continued to receive both warfarin and Lovenox for a total of 13 days. Both medications were dispensed and administered for 2 days when the PT was greater than 50; the supratherapeutic coagulation profile result was overlooked. Medications held on day 14 (PT, 68; INR, 8.35). The patient developed a right subdural hematoma and was transferred to a tertiary care facility for neurosurgery consult.
Given these facts, which of the following statements is best?
A. The hospital is under a legal obligation to disclose the error.
B. The doctor should be sympathetic and apologize for the injury, but not admit fault.
C. All jurisdictions have so-called “apology statutes,” which encourage error disclosure in return for immunity.
D. This is a case of medication, not medical, error.
E. Silence is golden.
Answer: B. A recent publication concluded, “If medical error were a disease, it would rank as the third leading cause of death in the United States.”1 This is the latest follow through on the original landmark report from the Institute of Medicine in 2000, which drew the public’s attention to the fact that medical errors were responsible for between 44,000 and 98,000 annual fatalities in the United States.
A medical error denotes a preventable adverse event, which in turn can be described as an injury caused by medical mismanagement rather than the underlying condition of the patient. It is more formally defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.”2 The term is not synonymous with medical negligence, which is a legal term of art encompassing four separate elements: duty, breach, causation, and damages.
The most common type of medical error is a medication error, which is any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health professional, patient, or consumer.
Medication errors account for 6.3%-30% of all malpractice claims, and a 1999 Texas case is an example.3 A 43-year-old Hispanic man with chest pain was prescribed the anti-angina drug Isordil (isosorbide dinitrate) by his cardiologist, to be taken four times a day in doses of 20 mg. The pharmacist misread the order as Plendil (felodipine), a calcium channel blocker for treatment of hypertension. This exceeded the drug’s top dose, and the patient suffered a heart attack and died several days later.
The cardiologist’s illegible prescription was the sole reason for the error, and his overall quality of care was not at issue. The jury returned a verdict for the plaintiff, awarding $450,000 to his estate: $225,000 from the cardiologist, and $225,000 from the pharmacist.
Many, but not all, jurisdictions now require some form of reporting of medical errors occurring in a hospital setting. States such as California and Florida mandate disclosure to patients. Pennsylvania actually requires hospitals to issue a written disclosure within 7 days of a serious event.
Most states have enacted “apology statutes” to encourage open discussions with patients and their families about adverse results. The apologies may cover expressions of regret, sympathy, and compassion, and they are barred from being presented to the jury should a trial ensue. However, an acknowledgment of fault remains admissible into evidence.
Typical is California’s Evidence Code 1160(a), which provides that only “the portions of statements or benevolent gestures expressing sympathy” are inadmissible against a treating physician. On the other hand, some states have chosen to exclude all disclosures, including admissions of fault. An example is Colorado’s Apology Statute (Colo. Rev. Stat. Ann. 13-25-135), which provides that “any and all statements, affirmations, gestures, or conduct expressing apology, fault, sympathy, commiseration, condolence, compassion, or a general sense of benevolence ... shall be inadmissible as evidence of an admission of liability or as evidence of an admission against interest.”
Ohio is one of only a few states whose apology statutes fail to clearly distinguish between the admissibility of a physician’s statement of sympathy and one acknowledging fault. R.C. 2317.43, enacted by the Ohio General Assembly in 2004, renders inadmissible “statements, affirmations, gestures, or conduct expressing apology, sympathy, commiseration, condolence, compassion, or a general sense of benevolence.”
In Davis v. Wooster Orthopaedics & Sports Medicine,4 the plaintiff alleged that Dr. Michael Knapic, an orthopedic surgeon, negligently performed a lumbar microdiscectomy, severing the patient’s common iliac artery. The surgeon allegedly then said to the patient’s husband, “It’s my fault. I take full responsibility.”
In a wrongful-death action, the plaintiff argued that the statute did not prohibit the use of statements of fault, responsibility, or liability as compared to statements of sympathy or condolence.
Ohio’s Ninth Appellate District concluded that the intent behind the apology statute was to protect pure expressions of sympathy but not admissions of fault. The court held that Dr. Knapic’s statements constituted an admission of liability that could be admitted into evidence. The jury awarded damages of $3 million.
More recently, the Ohio Supreme Court ruled that Dr. Randall Smith’s alleged confession regarding accidentally sectioning his patient’s common bile duct was properly excluded from evidence, even though the incident took place before the Ohio law went into effect.5
The patient had to be readmitted within 3 weeks for obstructive jaundice. After the doctor informed her that she would have to undergo additional surgery, she became very emotional. He reportedly took her hand, saying, “I take full responsibility for this.”
The Ohio Supreme Court ruled, “The trial court had determined that Dr. Smith was faced with a distressed patient who was upset and made a statement that was designed to comfort his patient. This is precisely the type of evidence that R.C. 2317.43 was designed to exclude as evidence of liability in a medical-malpractice case.”
Do disclosures and apologies work?
Both claim frequency and severity have diminished following the adoption of a humanistic risk management policy at the Lexington Veterans Affairs Medical Center since 1987.6 The protocol includes early injury review, steadfast maintenance of the relationship between the hospital and the patient, proactive disclosure to patients who have been injured because of accidents or medical negligence, and fair compensation for injuries.
Other institutions such as the University of Michigan have adopted “disclosure and offer” in place of “denial and defend” policies, but these have yet to achieve widespread use.
Many health care providers continue to heed the traditional legal advice to say and admit nothing, believing that it is unsettled whether an apology will influence any decision to sue the doctor. They argue that the odds of a lawsuit are low to begin with.
In the oft-cited Harvard study, there was only one malpractice claim for every 7.6 adverse events caused by negligence among the 3.7% of hospitalized patients who suffered significant iatrogenic injuries, typically from errors or negligence.7
Notwithstanding the controversy, the AMA has properly taken the moral high ground: It asserts that error disclosure is the right thing to do.
References
2. Institute of Medicine: To Err is Human: Building a Safer Health System. National Academy Press, Washington, 2000.
3. Estate of Velasquez v. Albertsons, Inc. et al., Civ. No. A-103-042 (Ector Cnty, TX 1999).
4. Davis v. Wooster Orthopaedics & Sports Medicine, Inc., 193 Ohio App.3d 581 (2011).
5. Estate of Johnson v. Randall Smith, Inc., 131 Ohio St.3d 1543 (2013).
6. N Engl J Med. 2010 Apr 15;362(15):1353-6.
7. N Engl J Med. 1991 Jul 25;325(4):245-51.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical or legal advice. It is adapted from the author’s book, “Medical Malpractice: Understanding the Law, Managing the Risk” (2006). For additional information, readers may contact the author at [email protected].
Clinical Characteristics and HLA Alleles of a Family With Simultaneously Occurring Alopecia Areata
Alopecia areata (AA) presents as sudden, nonscarring, recurrent hair loss characterized by well-circumscribed hairless patches. Although AA may be observed on any hair-bearing areas of the body, the most commonly affected sites are the scalp, beard area, eyebrows, and eyelashes.1 The incidence of AA is 1% to 2% in the general population and it is more common in males than females younger than 40 years.2 Although the majority of patients present with self-limited and well-circumscribed hairless patches that resolve within 2 years, 7% to 10% display a chronic and severe prognosis.3
The etiopathogenesis of AA is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.2 Alopecia areata is observed to occur sporadically in most patients. Family history has been found in 3% to 42% of cases, but simultaneous occurrence of AA in family members is rare.4 In this case series, we present 4 cases of active AA lesions occurring simultaneously in a family who also had associated psychologic disorders.
Case Series
Patient 1 (Proband)
An 11-year-old boy presented with a 6-year history of ongoing AA with recurrent improvement and relapses on the scalp, eyebrows, and eyelashes. Various topical and oral medications had been prescribed by several outside dermatologists; however, these treatments provided minimal benefit and resulted in the recurrence of AA. Dermatologic examination revealed hair loss on the entire frontal, parietal, and temporal regions of the scalp, as well as half of the occipital region and one-third of the lateral side of the eyebrows (Figure 1). Psychological evaluation revealed introvert personality characteristics, lack of self-confidence, and signs of depression and anxiety.
Patient 2 (Proband’s Father)
A 38-year-old man presented with a 16-year history of recurrent loss and regrowth of hair on the scalp and beard area and white spots on the penis and arms. He previously had not undergone any treatments. Dermatologic examination revealed well-circumscribed, 1- to 4-cm, hairless patches on the occipital region of the scalp and in the beard area (Figure 2A) and multiple, 2- to 10-mm, vitiliginous lesions on both forearms (Figure 2B) and the penis. The patient had been unemployed for 6 months. Psychological evaluation revealed obsessive-compulsive disorder and obsessive-compulsive personality disorder.
Patient 3 (Proband’s Mother)
A 32-year-old woman presented with a 3-year history of chronic AA. She previously had not undergone any treatments. Dermatologic examination revealed 2 well-circumscribed, 3- to 4-cm patches of hair loss on the occipital and left temporal regions of the scalp (Figure 3). Psychological evaluation revealed obsessive-compulsive personality disorder and depression. The patient did not have any autoimmune diseases.

Patient 4 (Proband’s Sister)
A 10-year-old girl presented with a 6-year history of recurrent, self-limited AA on various areas of scalp. She previously had not undergone any treatments. Dermatologic examination revealed a 3-cm hairless patch on the occipital region of the scalp (Figure 4). Psychiatric evaluation revealed narcissistic personality disorder, anxiety, and lack of self-confidence.
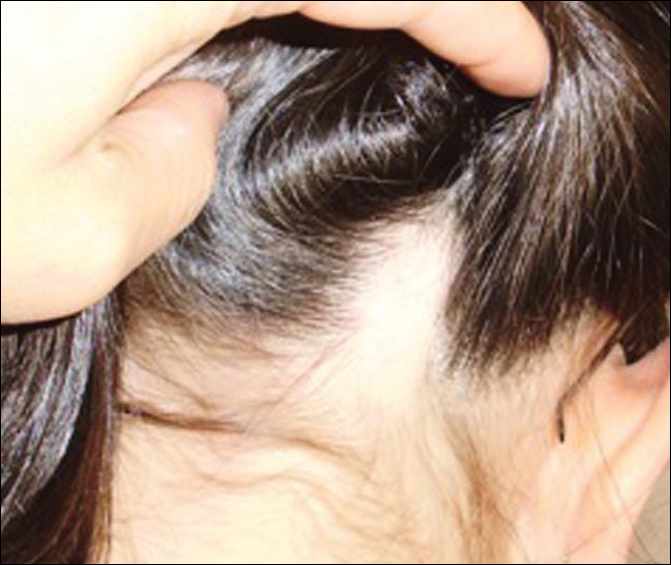
Laboratory Evaluation and HLA Antigen DNA Typing
Laboratory testing including complete blood cell count; liver, kidney, and thyroid function; and vitamin B12, zinc, folic acid, and fasting blood sugar levels were performed in all patients.
HLA antigen DNA typing was performed by polymerase chain reaction with sequence-specific primers in all patients after informed consent was obtained.
Clinical and laboratory examinations revealed no symptoms or findings of Epstein-Barr virus and cytomegalovirus infections, cicatricial alopecia, or connective tissue diseases in any of the patients. HLA antigen DNA typing revealed the following HLA alleles: B*35/40, C*04/15, DRB1*08/10, and DQB1*03/05 in patient 1; B*04/13, C*06/15, DRB1*07/10, and DQB1*02/05 in patient 2; B*33/37, C*04/06, DRB1*08/15, and DQ*06/06 in patient 3; B*13/37, C*06/06, DRB1*07/15, and DQB1*02/06 in patient 4.
Laboratory testing revealed vitamin B12 deficiency in patient 2 and iron deficiency anemia in patient 3; all other laboratory tests were within reference range. Antithyroglobulin and antithyroid peroxidase autoantibodies were all negative. Clinical features and laboratory analyses for all patients are summarized in the Table.
Treatment
All patients were recommended psychiatric therapy and started on dermatologic treatments. Topical corticosteroids, intralesional triamcinolone acetonide (8 mg/mL) injections into areas of hair loss, 8 total sessions of cryotherapy administered at 3-week intervals, and minoxidil solution 2% were administered respectively to all 4 patients. Alopecia areata in patients 3 and 4 completely regressed; however, no benefit was observed in patients 1 and 2 after 1 year of treatment. Because there was no response to the prior interventions, patient 1 was started on treatment with cyclosporine 2.5 mg/kg twice daily. However, therapy was discontinued after 1 month and treatment with narrowband UVB (3 times per week for 7 months [total of 57 sessions]) and topical corticosteroids were initiated (Table). The patient partially benefited from these regimens and recurrence was observed during the course of the treatment.
Although it was recommended that all 4 patients undergo psychiatric treatment and follow-up regularly with a psychiatrist, the patients declined. After approximately 1 year of dermatologic treatment, all 4 patients were lost to follow-up.
Comment
The etiopathogenesis of AA is unclear, but there is strong evidence suggesting that it is a T-cell–mediated autoimmune disease targeting the hair follicles. Common association of AA with autoimmune diseases such as vitiligo and thyroiditis support the immunological origin of the disease.3 In our case, patient 2 had AA along with vitiligo, but no associated autoimmune diseases (eg, vitiligo, diabetes mellitus, pernicious anemia, thyroid diseases) were noted in the other patients. Genetic and environmental factors are known to be influential as much as immune dysfunction in the etiology of AA.2
The presence of family history in 20% of patients supports the genetic predisposition of AA.4 In a genetic study by Martinez-Mir et al,5 susceptibility loci for AA were demonstrated on chromosomes 6, 10, 16, and 18. HLA antigen alleles, which provide predisposition to AA, have been investigated and associations with many different HLA antigens have been described for AA. In these studies, a relationship between AA and HLA class I antigens was not determined. Notable results mainly focused on HLA class II antigens.6-8 Colombe et al7 and Marques Da Costa et al8 demonstrated that long-lasting alopecia totalis or alopecia universalis (AT/AU) patients had a strong relationship with HLA-DRB1*1104; DRB1*04/05 was reported to be the most frequent HLA group among all patients with AA.6-10 In contrast, we did not detect these alleles in our patients. Colombe et al7,11 noted that HLA-DQB1*03 is a marker for both patch-type AA and AT/AU. Colombe et al10 showed that HLA-DQB1*03 was present in more than 80% of patients (N=286) with long-lasting AA. Barahmani et al9 confirmed a strong association between HLA-DQB1*0301, DRB1*1104, and AT/AU. In our patients, we detected HLA-DQB1*03/05 in patient 1 who had the earliest onset and most severe presentation of AA. In some studies, HLA-DRB1*03 was found to be less frequent in patients with AA, and this allele was suggested to be a protective factor.6,12 However, this allele was not detected in any of our patients.
The association of HLA alleles and AA has been investigated in Turkish patients with AA.13-15 Akar et al13 and Kavak et al14 detected that the frequency of HLA-DQB1*03 allele was remarkably higher in patients with AA than in healthy controls. These results were consistent with Colombe et al.10 On the other hand, Kavak et al14 reported that the frequency of HLA-DR16 was decreased in the patient group with AA. In another study, the frequency of HLA-B62 was increased in patients with AA compared to healthy controls.15 The HLA-DQB1*03 allele was found to be associated with AA in only patient 1 in our case series, and HLA alleles were not commonly shared among the 4 patients. Additionally, lack of consanguinity between patients 2 and 3 (the parents) also suggested that genetic factors were not involved in our familial cases.
Blaumeiser et al16 reported a lifetime risk of 7.4% in parents and 7.1% in siblings of 206 AA patients; however, because these studies investigated the presence of AA in any given life period of the family members, their results do not reflect frequency of simultaneous AA presence within one family. In a literature search using PubMed, Google Scholar, and other national databases for the terms alopecia areata as well as family, sibling, concurrently, concomitant, co-existent, and simultaneously, only 2 cases involving a husband and wife and 1 case of 2 siblings who concurrently had AA have been previously reported.17,18 Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.19 Among our patients, despite different age of onset and duration, AA was simultaneously present in the entire family.
Moreover, Rodriguez et al20 reported that the concordance rate of AA in identical twins was 42% and dizygotic twins was 10%. Environmental factors and infections also have been implicated in the etiology of AA. Infections caused by viruses such as cytomegalovirus and Epstein-Barr virus have been thought to be potential triggering factors; however, no evidence has been found.21,22 The clinical and laboratory examinations in our study did not reveal any presence and/or history of any known infectious disease, and there was no history of contact with water infected by acrylamide or a similar chemical.
Various life events and intense psychological stress may play an important role in triggering AA. Depression, hysteria, psychopathic deviance, psychasthenia, schizophrenia, anxiety, health concerns, bizarre thoughts, and family problems were found to be more frequent in patients with AA than healthy controls.23 The most common psychological disorders associated with AA are generalized anxiety disorder, major depressive disorder, adjustment disorders, and phobias.1,24 Ruiz-Doblado et al25 determined the presence of psychiatric comorbidities in 66% (21/32) of AA cases. Chu et al26 reported that the differences in ages of onset of AA revealed differences in psychiatric comorbidities. The risk for depression was higher in patients with AA younger than 20 years. An increased rate of anxiety was detected with patients with an onset of AA between the ages of 20 and 39 years. Obsessive-compulsive disorder and anxiety were more common in patients aged 40 to 59 years. Interestingly, the investigators also observed that approximately 50% of psychiatric disorders occurred prior to onset of AA.26 One study showed higher rates of stressful life events in children than in controls.27 Ghanizadeh24 reported at least 1 psychiatric disorder in 78% (11/14) of children and adolescents with AA. In the same study, obsessive-compulsive disorder was found to be the second common condition following major depression in AA.24
In our patients, psychiatric evaluations revealed obsessive-compulsive personality disorder in patients 2 and 3, depression in patient 3, and symptoms of anxiety with a lack of self-confidence in patients 1 and 4. Psychiatric disorders affecting the entire family may stem from unemployment of the father. Similar to the results noted in prior studies, depression, the most commonly associated psychiatric disorder of AA, was present in 2 of 4 patients. Obsessive-compulsive disorder, the second most common psychiatric disorder among AA patients, was present in patients 2 and 3. These results indicate that AA may be associated with shared stressful events and psychiatric disorders. Therefore, in addition to dermatologic treatment, it was recommended that all patients undergo psychiatric treatment and follow-up regularly with a psychiatrist; however, the patients declined. At the end of a 1-year treatment period and follow-up, resistance to therapy with minimal recovery followed by a rapid recurrence was determined in patients 1 and 2.
Conclusion
This report demonstrated that familial AA was strongly associated with psychological disorders that were detected in all patients. In our patients, HLA alleles did not seem to have a role in the development of familial AA. These results suggest that HLA was not associated with AA triggered by psychological stress. We believe that psychological disorders and stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
- García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, et al. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625-632.
- Bhat YJ, Manzoor S, Khan AR, et al. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29-31.
- Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364-370.
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213-218.
- Martinez-Mir A, Zlotogorski A, Gordon D, et al.Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316-328.
- Entz P, Blaumeiser B, Betz RC, et al. Investigation of the HLA-DRB1 locus in alopecia areata. Eur J Dermatol. 2006;16:363-367.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995;104(suppl 5):4-5.
- Marques Da Costa C, Dupont E, Van der Cruys M, et al. Earlier occurrence of severe alopecia areata in HLA-DRB1*11-positive patients. Dermatology. 2006;213:12-14.
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216-219.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5, pt 1):757-764.
- Broniarczyk-Dyła G, Prusińska-Bratoś M, Dubla-Berner M, et al. The protective role of the HLA-DR locus in patients with various clinical types of alopecia areata. Arch Immunol Ther Exp (Warsz). 2002;50:333-336.
- Akar A, Orkunuglu E, Sengul A, et al. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12:236-239.
- Kavak A, Baykal C, Ozarmagan G, et al. HLA in alopecia areata. Int J Dermatol. 2000;30:589-592.
- Aliagaoglu C, Pirim I, Atasoy M, et al. Association between alopecia areata and HLA class I and II in Turkey. J Dermatol. 2005;32:711-714.
- Blaumeiser B, Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627-632.
- Zalka AD, Byarlay JA, Goldsmith LA. Alopecia a deux: simultaneous occurrence of alopecia in a husband and wife. Arch Dermatol. 1994;130:390-392.
- Menon R, Kiran C. Concomitant presentation of alopecia areata in siblings: a rare occurrence. Int J Trichology. 2012;4:86-88.
- Valsecchi R, Vicari O, Frigeni A, et al. Familial alopecia areata-genetic susceptibility or coincidence? Acta Derm Venereol (Stockh). 1985;65:175-177.
- Rodriguez TA, Fernandes KE, Dresser KL, et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62:525-527.
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137-139.
- Offidani A, Amerio P, Bernardini ML, et al. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4:63-65.
- Alfani S, Antinone V, Mozzetta A, et al. Psychological status of patients with alopecia areata. Acta Derm Venereol. 2012;92:304-306.
- Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118-1120.
- Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434-437.
- Chu SY, Chen YJ, Tseng WC, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012;166:525-531.
- Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109.
Alopecia areata (AA) presents as sudden, nonscarring, recurrent hair loss characterized by well-circumscribed hairless patches. Although AA may be observed on any hair-bearing areas of the body, the most commonly affected sites are the scalp, beard area, eyebrows, and eyelashes.1 The incidence of AA is 1% to 2% in the general population and it is more common in males than females younger than 40 years.2 Although the majority of patients present with self-limited and well-circumscribed hairless patches that resolve within 2 years, 7% to 10% display a chronic and severe prognosis.3
The etiopathogenesis of AA is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.2 Alopecia areata is observed to occur sporadically in most patients. Family history has been found in 3% to 42% of cases, but simultaneous occurrence of AA in family members is rare.4 In this case series, we present 4 cases of active AA lesions occurring simultaneously in a family who also had associated psychologic disorders.
Case Series
Patient 1 (Proband)
An 11-year-old boy presented with a 6-year history of ongoing AA with recurrent improvement and relapses on the scalp, eyebrows, and eyelashes. Various topical and oral medications had been prescribed by several outside dermatologists; however, these treatments provided minimal benefit and resulted in the recurrence of AA. Dermatologic examination revealed hair loss on the entire frontal, parietal, and temporal regions of the scalp, as well as half of the occipital region and one-third of the lateral side of the eyebrows (Figure 1). Psychological evaluation revealed introvert personality characteristics, lack of self-confidence, and signs of depression and anxiety.

Patient 2 (Proband’s Father)
A 38-year-old man presented with a 16-year history of recurrent loss and regrowth of hair on the scalp and beard area and white spots on the penis and arms. He previously had not undergone any treatments. Dermatologic examination revealed well-circumscribed, 1- to 4-cm, hairless patches on the occipital region of the scalp and in the beard area (Figure 2A) and multiple, 2- to 10-mm, vitiliginous lesions on both forearms (Figure 2B) and the penis. The patient had been unemployed for 6 months. Psychological evaluation revealed obsessive-compulsive disorder and obsessive-compulsive personality disorder.

Patient 3 (Proband’s Mother)
A 32-year-old woman presented with a 3-year history of chronic AA. She previously had not undergone any treatments. Dermatologic examination revealed 2 well-circumscribed, 3- to 4-cm patches of hair loss on the occipital and left temporal regions of the scalp (Figure 3). Psychological evaluation revealed obsessive-compulsive personality disorder and depression. The patient did not have any autoimmune diseases.

Patient 4 (Proband’s Sister)
A 10-year-old girl presented with a 6-year history of recurrent, self-limited AA on various areas of scalp. She previously had not undergone any treatments. Dermatologic examination revealed a 3-cm hairless patch on the occipital region of the scalp (Figure 4). Psychiatric evaluation revealed narcissistic personality disorder, anxiety, and lack of self-confidence.

Laboratory Evaluation and HLA Antigen DNA Typing
Laboratory testing including complete blood cell count; liver, kidney, and thyroid function; and vitamin B12, zinc, folic acid, and fasting blood sugar levels were performed in all patients.
HLA antigen DNA typing was performed by polymerase chain reaction with sequence-specific primers in all patients after informed consent was obtained.
Clinical and laboratory examinations revealed no symptoms or findings of Epstein-Barr virus and cytomegalovirus infections, cicatricial alopecia, or connective tissue diseases in any of the patients. HLA antigen DNA typing revealed the following HLA alleles: B*35/40, C*04/15, DRB1*08/10, and DQB1*03/05 in patient 1; B*04/13, C*06/15, DRB1*07/10, and DQB1*02/05 in patient 2; B*33/37, C*04/06, DRB1*08/15, and DQ*06/06 in patient 3; B*13/37, C*06/06, DRB1*07/15, and DQB1*02/06 in patient 4.
Laboratory testing revealed vitamin B12 deficiency in patient 2 and iron deficiency anemia in patient 3; all other laboratory tests were within reference range. Antithyroglobulin and antithyroid peroxidase autoantibodies were all negative. Clinical features and laboratory analyses for all patients are summarized in the Table.
Treatment
All patients were recommended psychiatric therapy and started on dermatologic treatments. Topical corticosteroids, intralesional triamcinolone acetonide (8 mg/mL) injections into areas of hair loss, 8 total sessions of cryotherapy administered at 3-week intervals, and minoxidil solution 2% were administered respectively to all 4 patients. Alopecia areata in patients 3 and 4 completely regressed; however, no benefit was observed in patients 1 and 2 after 1 year of treatment. Because there was no response to the prior interventions, patient 1 was started on treatment with cyclosporine 2.5 mg/kg twice daily. However, therapy was discontinued after 1 month and treatment with narrowband UVB (3 times per week for 7 months [total of 57 sessions]) and topical corticosteroids were initiated (Table). The patient partially benefited from these regimens and recurrence was observed during the course of the treatment.

Although it was recommended that all 4 patients undergo psychiatric treatment and follow-up regularly with a psychiatrist, the patients declined. After approximately 1 year of dermatologic treatment, all 4 patients were lost to follow-up.
Comment
The etiopathogenesis of AA is unclear, but there is strong evidence suggesting that it is a T-cell–mediated autoimmune disease targeting the hair follicles. Common association of AA with autoimmune diseases such as vitiligo and thyroiditis support the immunological origin of the disease.3 In our case, patient 2 had AA along with vitiligo, but no associated autoimmune diseases (eg, vitiligo, diabetes mellitus, pernicious anemia, thyroid diseases) were noted in the other patients. Genetic and environmental factors are known to be influential as much as immune dysfunction in the etiology of AA.2
The presence of family history in 20% of patients supports the genetic predisposition of AA.4 In a genetic study by Martinez-Mir et al,5 susceptibility loci for AA were demonstrated on chromosomes 6, 10, 16, and 18. HLA antigen alleles, which provide predisposition to AA, have been investigated and associations with many different HLA antigens have been described for AA. In these studies, a relationship between AA and HLA class I antigens was not determined. Notable results mainly focused on HLA class II antigens.6-8 Colombe et al7 and Marques Da Costa et al8 demonstrated that long-lasting alopecia totalis or alopecia universalis (AT/AU) patients had a strong relationship with HLA-DRB1*1104; DRB1*04/05 was reported to be the most frequent HLA group among all patients with AA.6-10 In contrast, we did not detect these alleles in our patients. Colombe et al7,11 noted that HLA-DQB1*03 is a marker for both patch-type AA and AT/AU. Colombe et al10 showed that HLA-DQB1*03 was present in more than 80% of patients (N=286) with long-lasting AA. Barahmani et al9 confirmed a strong association between HLA-DQB1*0301, DRB1*1104, and AT/AU. In our patients, we detected HLA-DQB1*03/05 in patient 1 who had the earliest onset and most severe presentation of AA. In some studies, HLA-DRB1*03 was found to be less frequent in patients with AA, and this allele was suggested to be a protective factor.6,12 However, this allele was not detected in any of our patients.
The association of HLA alleles and AA has been investigated in Turkish patients with AA.13-15 Akar et al13 and Kavak et al14 detected that the frequency of HLA-DQB1*03 allele was remarkably higher in patients with AA than in healthy controls. These results were consistent with Colombe et al.10 On the other hand, Kavak et al14 reported that the frequency of HLA-DR16 was decreased in the patient group with AA. In another study, the frequency of HLA-B62 was increased in patients with AA compared to healthy controls.15 The HLA-DQB1*03 allele was found to be associated with AA in only patient 1 in our case series, and HLA alleles were not commonly shared among the 4 patients. Additionally, lack of consanguinity between patients 2 and 3 (the parents) also suggested that genetic factors were not involved in our familial cases.
Blaumeiser et al16 reported a lifetime risk of 7.4% in parents and 7.1% in siblings of 206 AA patients; however, because these studies investigated the presence of AA in any given life period of the family members, their results do not reflect frequency of simultaneous AA presence within one family. In a literature search using PubMed, Google Scholar, and other national databases for the terms alopecia areata as well as family, sibling, concurrently, concomitant, co-existent, and simultaneously, only 2 cases involving a husband and wife and 1 case of 2 siblings who concurrently had AA have been previously reported.17,18 Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.19 Among our patients, despite different age of onset and duration, AA was simultaneously present in the entire family.
Moreover, Rodriguez et al20 reported that the concordance rate of AA in identical twins was 42% and dizygotic twins was 10%. Environmental factors and infections also have been implicated in the etiology of AA. Infections caused by viruses such as cytomegalovirus and Epstein-Barr virus have been thought to be potential triggering factors; however, no evidence has been found.21,22 The clinical and laboratory examinations in our study did not reveal any presence and/or history of any known infectious disease, and there was no history of contact with water infected by acrylamide or a similar chemical.
Various life events and intense psychological stress may play an important role in triggering AA. Depression, hysteria, psychopathic deviance, psychasthenia, schizophrenia, anxiety, health concerns, bizarre thoughts, and family problems were found to be more frequent in patients with AA than healthy controls.23 The most common psychological disorders associated with AA are generalized anxiety disorder, major depressive disorder, adjustment disorders, and phobias.1,24 Ruiz-Doblado et al25 determined the presence of psychiatric comorbidities in 66% (21/32) of AA cases. Chu et al26 reported that the differences in ages of onset of AA revealed differences in psychiatric comorbidities. The risk for depression was higher in patients with AA younger than 20 years. An increased rate of anxiety was detected with patients with an onset of AA between the ages of 20 and 39 years. Obsessive-compulsive disorder and anxiety were more common in patients aged 40 to 59 years. Interestingly, the investigators also observed that approximately 50% of psychiatric disorders occurred prior to onset of AA.26 One study showed higher rates of stressful life events in children than in controls.27 Ghanizadeh24 reported at least 1 psychiatric disorder in 78% (11/14) of children and adolescents with AA. In the same study, obsessive-compulsive disorder was found to be the second common condition following major depression in AA.24
In our patients, psychiatric evaluations revealed obsessive-compulsive personality disorder in patients 2 and 3, depression in patient 3, and symptoms of anxiety with a lack of self-confidence in patients 1 and 4. Psychiatric disorders affecting the entire family may stem from unemployment of the father. Similar to the results noted in prior studies, depression, the most commonly associated psychiatric disorder of AA, was present in 2 of 4 patients. Obsessive-compulsive disorder, the second most common psychiatric disorder among AA patients, was present in patients 2 and 3. These results indicate that AA may be associated with shared stressful events and psychiatric disorders. Therefore, in addition to dermatologic treatment, it was recommended that all patients undergo psychiatric treatment and follow-up regularly with a psychiatrist; however, the patients declined. At the end of a 1-year treatment period and follow-up, resistance to therapy with minimal recovery followed by a rapid recurrence was determined in patients 1 and 2.
Conclusion
This report demonstrated that familial AA was strongly associated with psychological disorders that were detected in all patients. In our patients, HLA alleles did not seem to have a role in the development of familial AA. These results suggest that HLA was not associated with AA triggered by psychological stress. We believe that psychological disorders and stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
Alopecia areata (AA) presents as sudden, nonscarring, recurrent hair loss characterized by well-circumscribed hairless patches. Although AA may be observed on any hair-bearing areas of the body, the most commonly affected sites are the scalp, beard area, eyebrows, and eyelashes.1 The incidence of AA is 1% to 2% in the general population and it is more common in males than females younger than 40 years.2 Although the majority of patients present with self-limited and well-circumscribed hairless patches that resolve within 2 years, 7% to 10% display a chronic and severe prognosis.3
The etiopathogenesis of AA is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.2 Alopecia areata is observed to occur sporadically in most patients. Family history has been found in 3% to 42% of cases, but simultaneous occurrence of AA in family members is rare.4 In this case series, we present 4 cases of active AA lesions occurring simultaneously in a family who also had associated psychologic disorders.
Case Series
Patient 1 (Proband)
An 11-year-old boy presented with a 6-year history of ongoing AA with recurrent improvement and relapses on the scalp, eyebrows, and eyelashes. Various topical and oral medications had been prescribed by several outside dermatologists; however, these treatments provided minimal benefit and resulted in the recurrence of AA. Dermatologic examination revealed hair loss on the entire frontal, parietal, and temporal regions of the scalp, as well as half of the occipital region and one-third of the lateral side of the eyebrows (Figure 1). Psychological evaluation revealed introvert personality characteristics, lack of self-confidence, and signs of depression and anxiety.

Patient 2 (Proband’s Father)
A 38-year-old man presented with a 16-year history of recurrent loss and regrowth of hair on the scalp and beard area and white spots on the penis and arms. He previously had not undergone any treatments. Dermatologic examination revealed well-circumscribed, 1- to 4-cm, hairless patches on the occipital region of the scalp and in the beard area (Figure 2A) and multiple, 2- to 10-mm, vitiliginous lesions on both forearms (Figure 2B) and the penis. The patient had been unemployed for 6 months. Psychological evaluation revealed obsessive-compulsive disorder and obsessive-compulsive personality disorder.

Patient 3 (Proband’s Mother)
A 32-year-old woman presented with a 3-year history of chronic AA. She previously had not undergone any treatments. Dermatologic examination revealed 2 well-circumscribed, 3- to 4-cm patches of hair loss on the occipital and left temporal regions of the scalp (Figure 3). Psychological evaluation revealed obsessive-compulsive personality disorder and depression. The patient did not have any autoimmune diseases.

Patient 4 (Proband’s Sister)
A 10-year-old girl presented with a 6-year history of recurrent, self-limited AA on various areas of scalp. She previously had not undergone any treatments. Dermatologic examination revealed a 3-cm hairless patch on the occipital region of the scalp (Figure 4). Psychiatric evaluation revealed narcissistic personality disorder, anxiety, and lack of self-confidence.

Laboratory Evaluation and HLA Antigen DNA Typing
Laboratory testing including complete blood cell count; liver, kidney, and thyroid function; and vitamin B12, zinc, folic acid, and fasting blood sugar levels were performed in all patients.
HLA antigen DNA typing was performed by polymerase chain reaction with sequence-specific primers in all patients after informed consent was obtained.
Clinical and laboratory examinations revealed no symptoms or findings of Epstein-Barr virus and cytomegalovirus infections, cicatricial alopecia, or connective tissue diseases in any of the patients. HLA antigen DNA typing revealed the following HLA alleles: B*35/40, C*04/15, DRB1*08/10, and DQB1*03/05 in patient 1; B*04/13, C*06/15, DRB1*07/10, and DQB1*02/05 in patient 2; B*33/37, C*04/06, DRB1*08/15, and DQ*06/06 in patient 3; B*13/37, C*06/06, DRB1*07/15, and DQB1*02/06 in patient 4.
Laboratory testing revealed vitamin B12 deficiency in patient 2 and iron deficiency anemia in patient 3; all other laboratory tests were within reference range. Antithyroglobulin and antithyroid peroxidase autoantibodies were all negative. Clinical features and laboratory analyses for all patients are summarized in the Table.
Treatment
All patients were recommended psychiatric therapy and started on dermatologic treatments. Topical corticosteroids, intralesional triamcinolone acetonide (8 mg/mL) injections into areas of hair loss, 8 total sessions of cryotherapy administered at 3-week intervals, and minoxidil solution 2% were administered respectively to all 4 patients. Alopecia areata in patients 3 and 4 completely regressed; however, no benefit was observed in patients 1 and 2 after 1 year of treatment. Because there was no response to the prior interventions, patient 1 was started on treatment with cyclosporine 2.5 mg/kg twice daily. However, therapy was discontinued after 1 month and treatment with narrowband UVB (3 times per week for 7 months [total of 57 sessions]) and topical corticosteroids were initiated (Table). The patient partially benefited from these regimens and recurrence was observed during the course of the treatment.

Although it was recommended that all 4 patients undergo psychiatric treatment and follow-up regularly with a psychiatrist, the patients declined. After approximately 1 year of dermatologic treatment, all 4 patients were lost to follow-up.
Comment
The etiopathogenesis of AA is unclear, but there is strong evidence suggesting that it is a T-cell–mediated autoimmune disease targeting the hair follicles. Common association of AA with autoimmune diseases such as vitiligo and thyroiditis support the immunological origin of the disease.3 In our case, patient 2 had AA along with vitiligo, but no associated autoimmune diseases (eg, vitiligo, diabetes mellitus, pernicious anemia, thyroid diseases) were noted in the other patients. Genetic and environmental factors are known to be influential as much as immune dysfunction in the etiology of AA.2
The presence of family history in 20% of patients supports the genetic predisposition of AA.4 In a genetic study by Martinez-Mir et al,5 susceptibility loci for AA were demonstrated on chromosomes 6, 10, 16, and 18. HLA antigen alleles, which provide predisposition to AA, have been investigated and associations with many different HLA antigens have been described for AA. In these studies, a relationship between AA and HLA class I antigens was not determined. Notable results mainly focused on HLA class II antigens.6-8 Colombe et al7 and Marques Da Costa et al8 demonstrated that long-lasting alopecia totalis or alopecia universalis (AT/AU) patients had a strong relationship with HLA-DRB1*1104; DRB1*04/05 was reported to be the most frequent HLA group among all patients with AA.6-10 In contrast, we did not detect these alleles in our patients. Colombe et al7,11 noted that HLA-DQB1*03 is a marker for both patch-type AA and AT/AU. Colombe et al10 showed that HLA-DQB1*03 was present in more than 80% of patients (N=286) with long-lasting AA. Barahmani et al9 confirmed a strong association between HLA-DQB1*0301, DRB1*1104, and AT/AU. In our patients, we detected HLA-DQB1*03/05 in patient 1 who had the earliest onset and most severe presentation of AA. In some studies, HLA-DRB1*03 was found to be less frequent in patients with AA, and this allele was suggested to be a protective factor.6,12 However, this allele was not detected in any of our patients.
The association of HLA alleles and AA has been investigated in Turkish patients with AA.13-15 Akar et al13 and Kavak et al14 detected that the frequency of HLA-DQB1*03 allele was remarkably higher in patients with AA than in healthy controls. These results were consistent with Colombe et al.10 On the other hand, Kavak et al14 reported that the frequency of HLA-DR16 was decreased in the patient group with AA. In another study, the frequency of HLA-B62 was increased in patients with AA compared to healthy controls.15 The HLA-DQB1*03 allele was found to be associated with AA in only patient 1 in our case series, and HLA alleles were not commonly shared among the 4 patients. Additionally, lack of consanguinity between patients 2 and 3 (the parents) also suggested that genetic factors were not involved in our familial cases.
Blaumeiser et al16 reported a lifetime risk of 7.4% in parents and 7.1% in siblings of 206 AA patients; however, because these studies investigated the presence of AA in any given life period of the family members, their results do not reflect frequency of simultaneous AA presence within one family. In a literature search using PubMed, Google Scholar, and other national databases for the terms alopecia areata as well as family, sibling, concurrently, concomitant, co-existent, and simultaneously, only 2 cases involving a husband and wife and 1 case of 2 siblings who concurrently had AA have been previously reported.17,18 Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.19 Among our patients, despite different age of onset and duration, AA was simultaneously present in the entire family.
Moreover, Rodriguez et al20 reported that the concordance rate of AA in identical twins was 42% and dizygotic twins was 10%. Environmental factors and infections also have been implicated in the etiology of AA. Infections caused by viruses such as cytomegalovirus and Epstein-Barr virus have been thought to be potential triggering factors; however, no evidence has been found.21,22 The clinical and laboratory examinations in our study did not reveal any presence and/or history of any known infectious disease, and there was no history of contact with water infected by acrylamide or a similar chemical.
Various life events and intense psychological stress may play an important role in triggering AA. Depression, hysteria, psychopathic deviance, psychasthenia, schizophrenia, anxiety, health concerns, bizarre thoughts, and family problems were found to be more frequent in patients with AA than healthy controls.23 The most common psychological disorders associated with AA are generalized anxiety disorder, major depressive disorder, adjustment disorders, and phobias.1,24 Ruiz-Doblado et al25 determined the presence of psychiatric comorbidities in 66% (21/32) of AA cases. Chu et al26 reported that the differences in ages of onset of AA revealed differences in psychiatric comorbidities. The risk for depression was higher in patients with AA younger than 20 years. An increased rate of anxiety was detected with patients with an onset of AA between the ages of 20 and 39 years. Obsessive-compulsive disorder and anxiety were more common in patients aged 40 to 59 years. Interestingly, the investigators also observed that approximately 50% of psychiatric disorders occurred prior to onset of AA.26 One study showed higher rates of stressful life events in children than in controls.27 Ghanizadeh24 reported at least 1 psychiatric disorder in 78% (11/14) of children and adolescents with AA. In the same study, obsessive-compulsive disorder was found to be the second common condition following major depression in AA.24
In our patients, psychiatric evaluations revealed obsessive-compulsive personality disorder in patients 2 and 3, depression in patient 3, and symptoms of anxiety with a lack of self-confidence in patients 1 and 4. Psychiatric disorders affecting the entire family may stem from unemployment of the father. Similar to the results noted in prior studies, depression, the most commonly associated psychiatric disorder of AA, was present in 2 of 4 patients. Obsessive-compulsive disorder, the second most common psychiatric disorder among AA patients, was present in patients 2 and 3. These results indicate that AA may be associated with shared stressful events and psychiatric disorders. Therefore, in addition to dermatologic treatment, it was recommended that all patients undergo psychiatric treatment and follow-up regularly with a psychiatrist; however, the patients declined. At the end of a 1-year treatment period and follow-up, resistance to therapy with minimal recovery followed by a rapid recurrence was determined in patients 1 and 2.
Conclusion
This report demonstrated that familial AA was strongly associated with psychological disorders that were detected in all patients. In our patients, HLA alleles did not seem to have a role in the development of familial AA. These results suggest that HLA was not associated with AA triggered by psychological stress. We believe that psychological disorders and stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
- García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, et al. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625-632.
- Bhat YJ, Manzoor S, Khan AR, et al. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29-31.
- Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364-370.
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213-218.
- Martinez-Mir A, Zlotogorski A, Gordon D, et al.Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316-328.
- Entz P, Blaumeiser B, Betz RC, et al. Investigation of the HLA-DRB1 locus in alopecia areata. Eur J Dermatol. 2006;16:363-367.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995;104(suppl 5):4-5.
- Marques Da Costa C, Dupont E, Van der Cruys M, et al. Earlier occurrence of severe alopecia areata in HLA-DRB1*11-positive patients. Dermatology. 2006;213:12-14.
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216-219.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5, pt 1):757-764.
- Broniarczyk-Dyła G, Prusińska-Bratoś M, Dubla-Berner M, et al. The protective role of the HLA-DR locus in patients with various clinical types of alopecia areata. Arch Immunol Ther Exp (Warsz). 2002;50:333-336.
- Akar A, Orkunuglu E, Sengul A, et al. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12:236-239.
- Kavak A, Baykal C, Ozarmagan G, et al. HLA in alopecia areata. Int J Dermatol. 2000;30:589-592.
- Aliagaoglu C, Pirim I, Atasoy M, et al. Association between alopecia areata and HLA class I and II in Turkey. J Dermatol. 2005;32:711-714.
- Blaumeiser B, Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627-632.
- Zalka AD, Byarlay JA, Goldsmith LA. Alopecia a deux: simultaneous occurrence of alopecia in a husband and wife. Arch Dermatol. 1994;130:390-392.
- Menon R, Kiran C. Concomitant presentation of alopecia areata in siblings: a rare occurrence. Int J Trichology. 2012;4:86-88.
- Valsecchi R, Vicari O, Frigeni A, et al. Familial alopecia areata-genetic susceptibility or coincidence? Acta Derm Venereol (Stockh). 1985;65:175-177.
- Rodriguez TA, Fernandes KE, Dresser KL, et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62:525-527.
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137-139.
- Offidani A, Amerio P, Bernardini ML, et al. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4:63-65.
- Alfani S, Antinone V, Mozzetta A, et al. Psychological status of patients with alopecia areata. Acta Derm Venereol. 2012;92:304-306.
- Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118-1120.
- Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434-437.
- Chu SY, Chen YJ, Tseng WC, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012;166:525-531.
- Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109.
- García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, et al. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol. 1999;26:625-632.
- Bhat YJ, Manzoor S, Khan AR, et al. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:29-31.
- Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364-370.
- Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213-218.
- Martinez-Mir A, Zlotogorski A, Gordon D, et al.Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80:316-328.
- Entz P, Blaumeiser B, Betz RC, et al. Investigation of the HLA-DRB1 locus in alopecia areata. Eur J Dermatol. 2006;16:363-367.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995;104(suppl 5):4-5.
- Marques Da Costa C, Dupont E, Van der Cruys M, et al. Earlier occurrence of severe alopecia areata in HLA-DRB1*11-positive patients. Dermatology. 2006;213:12-14.
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216-219.
- Colombe BW, Price VH, Khoury EL, et al. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33(5, pt 1):757-764.
- Broniarczyk-Dyła G, Prusińska-Bratoś M, Dubla-Berner M, et al. The protective role of the HLA-DR locus in patients with various clinical types of alopecia areata. Arch Immunol Ther Exp (Warsz). 2002;50:333-336.
- Akar A, Orkunuglu E, Sengul A, et al. HLA class II alleles in patients with alopecia areata. Eur J Dermatol. 2002;12:236-239.
- Kavak A, Baykal C, Ozarmagan G, et al. HLA in alopecia areata. Int J Dermatol. 2000;30:589-592.
- Aliagaoglu C, Pirim I, Atasoy M, et al. Association between alopecia areata and HLA class I and II in Turkey. J Dermatol. 2005;32:711-714.
- Blaumeiser B, Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54:627-632.
- Zalka AD, Byarlay JA, Goldsmith LA. Alopecia a deux: simultaneous occurrence of alopecia in a husband and wife. Arch Dermatol. 1994;130:390-392.
- Menon R, Kiran C. Concomitant presentation of alopecia areata in siblings: a rare occurrence. Int J Trichology. 2012;4:86-88.
- Valsecchi R, Vicari O, Frigeni A, et al. Familial alopecia areata-genetic susceptibility or coincidence? Acta Derm Venereol (Stockh). 1985;65:175-177.
- Rodriguez TA, Fernandes KE, Dresser KL, et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62:525-527.
- Rodriguez TA, Duvic M. Onset of alopecia areata after Epstein Barr virus infectious mononucleosis. J Am Acad Dermatol. 2008;59:137-139.
- Offidani A, Amerio P, Bernardini ML, et al. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4:63-65.
- Alfani S, Antinone V, Mozzetta A, et al. Psychological status of patients with alopecia areata. Acta Derm Venereol. 2012;92:304-306.
- Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118-1120.
- Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434-437.
- Chu SY, Chen YJ, Tseng WC, et al. Psychiatric comorbidities in patients with alopecia areata in Taiwan: a case-control study. Br J Dermatol. 2012;166:525-531.
- Manolache L, Petrescu-Seceleanu D, Benea V. Alopecia areata and stressful events in children. J Eur Acad Dermatol Venereol. 2009;23:107-109.
Practice Points
- The etiopathogenesis of alopecia areata (AA) is not clearly understood, but its occurrence and progression can involve immune dysfunction, genetic predisposition, infections, and physical and psychological trauma.
- Alopecia areata is observed to occur sporadically in most patients. Simultaneous presence of AA in more than 3 members of the same family is rare, and these cases have been observed in different generations and time periods.
- HLA antigen alleles, which provide predisposition to AA, have been investigated, and associations with many different HLA antigens have been described for AA. In previous studies, HLA-DQB1*03 allele was reported as the most common HLA allele in patients with AA.
- Psychological disorders and shared stressful life events may play an important role in the occurrence of AA and lead to the development of resistance against treatment in familial and resistant AA cases.
Dual immune checkpoint blockade found durable in melanoma
CHICAGO – Immune checkpoint blockade, especially with a combination of agents having complementary mechanisms of action, has durable efficacy when used as initial therapy for advanced melanoma, according to an update of the CheckMate 067 trial.
The trial randomized 945 treatment-naive patients with unresectable stage III or IV melanoma evenly to double-blind treatment with nivolumab, an antibody to the cell surface receptor programmed death 1 (PD-1); ipilimumab, an antibody to the T-cell receptor cytotoxic T-lymphocyte–associated antigen 4 (CTLA4); or the combination.
Initial results, after a median follow-up of about 12.4 months, showed that the risk of progression-free survival events was 58% lower with the combination and 43% lower with nivolumab alone as compared with ipilimumab alone (N Engl J Med. 2015;373[1]:23-34).
The update, now with a median follow-up of 20.7 months, showed that these results held up, with respective 58% and 45% reductions in the risk of events, researchers reported at the annual meeting of the American Society of Clinical Oncology. The combination was also superior to nivolumab alone, netting a 24% lower risk of events. Additionally, no cumulative or new toxicities were seen.
“Based on available evidence, the combination of nivolumab and ipilimumab represents a means to improve outcomes versus nivolumab alone,” said first author Jedd D. Wolchok, MD, PhD, chief of the Melanoma & Immunotherapeutics Service at the Memorial Sloan Kettering Cancer Center in New York. “Additional insights will be gained with the emergence of overall survival data.”
Neither tumor expression of PD-L1, a ligand of PD-1, nor presence of a BRAF mutation was very helpful in identifying patients who would benefit to a greater extent from these therapies.
The findings add to evidence establishing the efficacy of combination immunotherapy in melanoma, according to invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. At the same time, the trial left unanswered questions such as what strategy should be used after progression on either or both agents, and what are the appropriate doses and durations of therapy. Also unclear is which type of therapy to use first line in patients with BRAF mutations, he added. “Whether you start with immunotherapy or targeted therapy in BRAF-mutated patients is still in equipoise, and I would encourage everyone here to participate in the ECOG 6134 trial looking at the randomization of immune checkpoint therapy versus targeted therapy in BRAF-mutated patients,” he said. “The biomarker studies are still provocative, and we still need a lot more data to be able to preselect patients who might benefit from either of these therapies.”
“One has to recognize that these agents are costly,” Dr. Ernstoff maintained, with the acquisition cost of the checkpoint inhibitors ranging from roughly $140,000 to $290,000 per year depending on the agent(s) used. This issue will also have to be addressed going forward.
“The future is very bright. There are now 76 trials listed in PDQ [Physician Data Query] of combination PD-1 therapies in melanoma alone,” he concluded. “Immunotherapy continues to capture our imagination.”
The updated intent-to-treat analyses of CheckMate 067 – conducted after all patients had at least 18 months of follow-up – showed that median progression-free survival, one of the trial’s primary endpoints, was now 11.5 months with the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), 6.9 months with nivolumab alone, and 2.9 months with ipilimumab alone, Dr. Wolchok reported at the meeting.
The differences translated to significantly better outcomes with the combination (hazard ratio, 0.42) and with nivolumab (HR, 0.55) as compared with ipilimumab. Moreover, the combination was superior to nivolumab (HR, 0.76).
The overall response rate, the trial’s other primary endpoint, was 57.6% with the combination and 43.7% with nivolumab alone, both of which were superior to the 19.0% with ipilimumab alone.
“While the response rates have not changed, some partial responses have evolved into complete responses over time,” Dr. Wolchok noted.
Findings were similar when patients were stratified by BRAF mutational status. And in exploratory analyses, outcomes were numerically better with the combination than with nivolumab alone regardless of whether tumors had high or low PD-L1 expression.
Safety results were much the same as previously reported. The rate of grade 3 or 4 treatment-related adverse events was 56.5% with the combination, 19.8% with nivolumab monotherapy, and 27.0% with ipilimumab monotherapy. There were no treatment-related deaths with the combination and one with each of the monotherapies.
“There is no common signature adverse event with this combination,” Dr. Wolchok pointed out. “The majority of grade 3 or 4 adverse events resolved in all of the groups with the use of established algorithms. However, as observed in prior studies, most of the endocrine events did not resolve and required hormone replacement.”
About 40% of the combination therapy group stopped treatment because of adverse events. “Interestingly, 68% of these patients who discontinued due to treatment-related adverse events developed a response, and 50% of these responses occurred after treatment had ended,” he reported. “This is very important information for us as we talk to patients and their families about the difficulties of stopping treatment.”
Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti–PD-L1 immunohistochemistry assay.
CHICAGO – Immune checkpoint blockade, especially with a combination of agents having complementary mechanisms of action, has durable efficacy when used as initial therapy for advanced melanoma, according to an update of the CheckMate 067 trial.
The trial randomized 945 treatment-naive patients with unresectable stage III or IV melanoma evenly to double-blind treatment with nivolumab, an antibody to the cell surface receptor programmed death 1 (PD-1); ipilimumab, an antibody to the T-cell receptor cytotoxic T-lymphocyte–associated antigen 4 (CTLA4); or the combination.
Initial results, after a median follow-up of about 12.4 months, showed that the risk of progression-free survival events was 58% lower with the combination and 43% lower with nivolumab alone as compared with ipilimumab alone (N Engl J Med. 2015;373[1]:23-34).
The update, now with a median follow-up of 20.7 months, showed that these results held up, with respective 58% and 45% reductions in the risk of events, researchers reported at the annual meeting of the American Society of Clinical Oncology. The combination was also superior to nivolumab alone, netting a 24% lower risk of events. Additionally, no cumulative or new toxicities were seen.
“Based on available evidence, the combination of nivolumab and ipilimumab represents a means to improve outcomes versus nivolumab alone,” said first author Jedd D. Wolchok, MD, PhD, chief of the Melanoma & Immunotherapeutics Service at the Memorial Sloan Kettering Cancer Center in New York. “Additional insights will be gained with the emergence of overall survival data.”
Neither tumor expression of PD-L1, a ligand of PD-1, nor presence of a BRAF mutation was very helpful in identifying patients who would benefit to a greater extent from these therapies.
The findings add to evidence establishing the efficacy of combination immunotherapy in melanoma, according to invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. At the same time, the trial left unanswered questions such as what strategy should be used after progression on either or both agents, and what are the appropriate doses and durations of therapy. Also unclear is which type of therapy to use first line in patients with BRAF mutations, he added. “Whether you start with immunotherapy or targeted therapy in BRAF-mutated patients is still in equipoise, and I would encourage everyone here to participate in the ECOG 6134 trial looking at the randomization of immune checkpoint therapy versus targeted therapy in BRAF-mutated patients,” he said. “The biomarker studies are still provocative, and we still need a lot more data to be able to preselect patients who might benefit from either of these therapies.”
“One has to recognize that these agents are costly,” Dr. Ernstoff maintained, with the acquisition cost of the checkpoint inhibitors ranging from roughly $140,000 to $290,000 per year depending on the agent(s) used. This issue will also have to be addressed going forward.
“The future is very bright. There are now 76 trials listed in PDQ [Physician Data Query] of combination PD-1 therapies in melanoma alone,” he concluded. “Immunotherapy continues to capture our imagination.”
The updated intent-to-treat analyses of CheckMate 067 – conducted after all patients had at least 18 months of follow-up – showed that median progression-free survival, one of the trial’s primary endpoints, was now 11.5 months with the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), 6.9 months with nivolumab alone, and 2.9 months with ipilimumab alone, Dr. Wolchok reported at the meeting.
The differences translated to significantly better outcomes with the combination (hazard ratio, 0.42) and with nivolumab (HR, 0.55) as compared with ipilimumab. Moreover, the combination was superior to nivolumab (HR, 0.76).
The overall response rate, the trial’s other primary endpoint, was 57.6% with the combination and 43.7% with nivolumab alone, both of which were superior to the 19.0% with ipilimumab alone.
“While the response rates have not changed, some partial responses have evolved into complete responses over time,” Dr. Wolchok noted.
Findings were similar when patients were stratified by BRAF mutational status. And in exploratory analyses, outcomes were numerically better with the combination than with nivolumab alone regardless of whether tumors had high or low PD-L1 expression.
Safety results were much the same as previously reported. The rate of grade 3 or 4 treatment-related adverse events was 56.5% with the combination, 19.8% with nivolumab monotherapy, and 27.0% with ipilimumab monotherapy. There were no treatment-related deaths with the combination and one with each of the monotherapies.
“There is no common signature adverse event with this combination,” Dr. Wolchok pointed out. “The majority of grade 3 or 4 adverse events resolved in all of the groups with the use of established algorithms. However, as observed in prior studies, most of the endocrine events did not resolve and required hormone replacement.”
About 40% of the combination therapy group stopped treatment because of adverse events. “Interestingly, 68% of these patients who discontinued due to treatment-related adverse events developed a response, and 50% of these responses occurred after treatment had ended,” he reported. “This is very important information for us as we talk to patients and their families about the difficulties of stopping treatment.”
Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti–PD-L1 immunohistochemistry assay.
CHICAGO – Immune checkpoint blockade, especially with a combination of agents having complementary mechanisms of action, has durable efficacy when used as initial therapy for advanced melanoma, according to an update of the CheckMate 067 trial.
The trial randomized 945 treatment-naive patients with unresectable stage III or IV melanoma evenly to double-blind treatment with nivolumab, an antibody to the cell surface receptor programmed death 1 (PD-1); ipilimumab, an antibody to the T-cell receptor cytotoxic T-lymphocyte–associated antigen 4 (CTLA4); or the combination.
Initial results, after a median follow-up of about 12.4 months, showed that the risk of progression-free survival events was 58% lower with the combination and 43% lower with nivolumab alone as compared with ipilimumab alone (N Engl J Med. 2015;373[1]:23-34).
The update, now with a median follow-up of 20.7 months, showed that these results held up, with respective 58% and 45% reductions in the risk of events, researchers reported at the annual meeting of the American Society of Clinical Oncology. The combination was also superior to nivolumab alone, netting a 24% lower risk of events. Additionally, no cumulative or new toxicities were seen.
“Based on available evidence, the combination of nivolumab and ipilimumab represents a means to improve outcomes versus nivolumab alone,” said first author Jedd D. Wolchok, MD, PhD, chief of the Melanoma & Immunotherapeutics Service at the Memorial Sloan Kettering Cancer Center in New York. “Additional insights will be gained with the emergence of overall survival data.”
Neither tumor expression of PD-L1, a ligand of PD-1, nor presence of a BRAF mutation was very helpful in identifying patients who would benefit to a greater extent from these therapies.
The findings add to evidence establishing the efficacy of combination immunotherapy in melanoma, according to invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. At the same time, the trial left unanswered questions such as what strategy should be used after progression on either or both agents, and what are the appropriate doses and durations of therapy. Also unclear is which type of therapy to use first line in patients with BRAF mutations, he added. “Whether you start with immunotherapy or targeted therapy in BRAF-mutated patients is still in equipoise, and I would encourage everyone here to participate in the ECOG 6134 trial looking at the randomization of immune checkpoint therapy versus targeted therapy in BRAF-mutated patients,” he said. “The biomarker studies are still provocative, and we still need a lot more data to be able to preselect patients who might benefit from either of these therapies.”
“One has to recognize that these agents are costly,” Dr. Ernstoff maintained, with the acquisition cost of the checkpoint inhibitors ranging from roughly $140,000 to $290,000 per year depending on the agent(s) used. This issue will also have to be addressed going forward.
“The future is very bright. There are now 76 trials listed in PDQ [Physician Data Query] of combination PD-1 therapies in melanoma alone,” he concluded. “Immunotherapy continues to capture our imagination.”
The updated intent-to-treat analyses of CheckMate 067 – conducted after all patients had at least 18 months of follow-up – showed that median progression-free survival, one of the trial’s primary endpoints, was now 11.5 months with the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), 6.9 months with nivolumab alone, and 2.9 months with ipilimumab alone, Dr. Wolchok reported at the meeting.
The differences translated to significantly better outcomes with the combination (hazard ratio, 0.42) and with nivolumab (HR, 0.55) as compared with ipilimumab. Moreover, the combination was superior to nivolumab (HR, 0.76).
The overall response rate, the trial’s other primary endpoint, was 57.6% with the combination and 43.7% with nivolumab alone, both of which were superior to the 19.0% with ipilimumab alone.
“While the response rates have not changed, some partial responses have evolved into complete responses over time,” Dr. Wolchok noted.
Findings were similar when patients were stratified by BRAF mutational status. And in exploratory analyses, outcomes were numerically better with the combination than with nivolumab alone regardless of whether tumors had high or low PD-L1 expression.
Safety results were much the same as previously reported. The rate of grade 3 or 4 treatment-related adverse events was 56.5% with the combination, 19.8% with nivolumab monotherapy, and 27.0% with ipilimumab monotherapy. There were no treatment-related deaths with the combination and one with each of the monotherapies.
“There is no common signature adverse event with this combination,” Dr. Wolchok pointed out. “The majority of grade 3 or 4 adverse events resolved in all of the groups with the use of established algorithms. However, as observed in prior studies, most of the endocrine events did not resolve and required hormone replacement.”
About 40% of the combination therapy group stopped treatment because of adverse events. “Interestingly, 68% of these patients who discontinued due to treatment-related adverse events developed a response, and 50% of these responses occurred after treatment had ended,” he reported. “This is very important information for us as we talk to patients and their families about the difficulties of stopping treatment.”
Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti–PD-L1 immunohistochemistry assay.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Nivolumab-ipilimumab combination therapy and nivolumab monotherapy are more efficacious than ipilimumab monotherapy when used in the first line for advanced melanoma.
Major finding: The risk of progression-free survival events was lower with nivolumab plus ipilimumab (HR, 0.42) and with nivolumab alone (HR, 0.55) as compared with ipilimumab alone.
Data source: A phase III randomized trial among 945 treatment-naive patients with advanced melanoma (CheckMate 067).
Disclosures: Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti-PD-L1 immunohistochemistry assay.
2017 Fellows Application Process Now Open
SHM Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently 2,000 hospitalists who have earned the FHM/SFHM designation by demonstrating core values of leadership, teamwork, and quality improvement.
The application process is now open. Apply by Sept. 15 to receive an early decision on or before Oct. 28. The regular decision application will remain open through Nov. 30, with a decision notification on or before Dec. 31. Apply now and learn how you can join other hospitalists who have earned this exclusive designation and recognition at www.hospitalmedicine.org/fellows.
SHM Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently 2,000 hospitalists who have earned the FHM/SFHM designation by demonstrating core values of leadership, teamwork, and quality improvement.
The application process is now open. Apply by Sept. 15 to receive an early decision on or before Oct. 28. The regular decision application will remain open through Nov. 30, with a decision notification on or before Dec. 31. Apply now and learn how you can join other hospitalists who have earned this exclusive designation and recognition at www.hospitalmedicine.org/fellows.
SHM Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently 2,000 hospitalists who have earned the FHM/SFHM designation by demonstrating core values of leadership, teamwork, and quality improvement.
The application process is now open. Apply by Sept. 15 to receive an early decision on or before Oct. 28. The regular decision application will remain open through Nov. 30, with a decision notification on or before Dec. 31. Apply now and learn how you can join other hospitalists who have earned this exclusive designation and recognition at www.hospitalmedicine.org/fellows.
Academic Hospitalist Academy Has New Location
The eighth annual Academic Hospitalist Academy (AHA) will be held Sept. 12–15 at the Lakeway Resort and Spa in Austin, Texas. This is a can’t-miss event for academic hospitalists. At AHA, you will:
- Gain valuable tools for career development
- Establish a national network
- Take advantage of an effective learning environment with a 1:10
faculty-to-student ratio
- Develop scholarly work and increase scholarly output
- Earn CME credit
Seats are limited. Reserve your spot now at www.academichospitalist.org.
The eighth annual Academic Hospitalist Academy (AHA) will be held Sept. 12–15 at the Lakeway Resort and Spa in Austin, Texas. This is a can’t-miss event for academic hospitalists. At AHA, you will:
- Gain valuable tools for career development
- Establish a national network
- Take advantage of an effective learning environment with a 1:10
faculty-to-student ratio
- Develop scholarly work and increase scholarly output
- Earn CME credit
Seats are limited. Reserve your spot now at www.academichospitalist.org.
The eighth annual Academic Hospitalist Academy (AHA) will be held Sept. 12–15 at the Lakeway Resort and Spa in Austin, Texas. This is a can’t-miss event for academic hospitalists. At AHA, you will:
- Gain valuable tools for career development
- Establish a national network
- Take advantage of an effective learning environment with a 1:10
faculty-to-student ratio
- Develop scholarly work and increase scholarly output
- Earn CME credit
Seats are limited. Reserve your spot now at www.academichospitalist.org.
EC extends marketing authorization for brentuximab vedotin

Photo from Business Wire
The European Commission (EC) has extended the current conditional marketing authorization of brentuximab vedotin (Adcetris) to include the treatment of adults with CD30+ Hodgkin lymphoma (HL) who are at an increased risk of relapse or progression following autologous stem cell transplant (ASCT).
Conditional marketing authorizations are valid for 1 year and are reviewed annually.
The company developing the drug is required to provide comprehensive data confirming the drug’s benefit-risk balance is positive. Once these data are available, the marketing authorization may be converted into a standard marketing authorization.
Drugs are eligible for conditional marketing authorization if they are designated as orphan medicines, intended for use in emergency situations, or designed to treat, prevent, or diagnose seriously debilitating or life-threatening diseases.
The EC previously granted brentuximab vedotin conditional marketing authorization for 2 indications:
- To treat adults with relapsed or refractory CD30+ HL after ASCT or following at least 2 prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- To treat adults with relapsed or refractory systemic anaplastic large-cell lymphoma (sALCL).
In January 2016, the EC approved a Type II variation to include data on the retreatment of adult patients with HL or sALCL who previously responded to brentuximab vedotin and later relapsed.
Brentuximab vedotin is under joint development by Seattle Genetics and Takeda Pharmaceutical Company Limited.
AETHERA trial
The EC’s decision to extend the conditional marketing authorization of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015 and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()

Photo from Business Wire
The European Commission (EC) has extended the current conditional marketing authorization of brentuximab vedotin (Adcetris) to include the treatment of adults with CD30+ Hodgkin lymphoma (HL) who are at an increased risk of relapse or progression following autologous stem cell transplant (ASCT).
Conditional marketing authorizations are valid for 1 year and are reviewed annually.
The company developing the drug is required to provide comprehensive data confirming the drug’s benefit-risk balance is positive. Once these data are available, the marketing authorization may be converted into a standard marketing authorization.
Drugs are eligible for conditional marketing authorization if they are designated as orphan medicines, intended for use in emergency situations, or designed to treat, prevent, or diagnose seriously debilitating or life-threatening diseases.
The EC previously granted brentuximab vedotin conditional marketing authorization for 2 indications:
- To treat adults with relapsed or refractory CD30+ HL after ASCT or following at least 2 prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- To treat adults with relapsed or refractory systemic anaplastic large-cell lymphoma (sALCL).
In January 2016, the EC approved a Type II variation to include data on the retreatment of adult patients with HL or sALCL who previously responded to brentuximab vedotin and later relapsed.
Brentuximab vedotin is under joint development by Seattle Genetics and Takeda Pharmaceutical Company Limited.
AETHERA trial
The EC’s decision to extend the conditional marketing authorization of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015 and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()

Photo from Business Wire
The European Commission (EC) has extended the current conditional marketing authorization of brentuximab vedotin (Adcetris) to include the treatment of adults with CD30+ Hodgkin lymphoma (HL) who are at an increased risk of relapse or progression following autologous stem cell transplant (ASCT).
Conditional marketing authorizations are valid for 1 year and are reviewed annually.
The company developing the drug is required to provide comprehensive data confirming the drug’s benefit-risk balance is positive. Once these data are available, the marketing authorization may be converted into a standard marketing authorization.
Drugs are eligible for conditional marketing authorization if they are designated as orphan medicines, intended for use in emergency situations, or designed to treat, prevent, or diagnose seriously debilitating or life-threatening diseases.
The EC previously granted brentuximab vedotin conditional marketing authorization for 2 indications:
- To treat adults with relapsed or refractory CD30+ HL after ASCT or following at least 2 prior therapies when ASCT or multi-agent chemotherapy is not a treatment option
- To treat adults with relapsed or refractory systemic anaplastic large-cell lymphoma (sALCL).
In January 2016, the EC approved a Type II variation to include data on the retreatment of adult patients with HL or sALCL who previously responded to brentuximab vedotin and later relapsed.
Brentuximab vedotin is under joint development by Seattle Genetics and Takeda Pharmaceutical Company Limited.
AETHERA trial
The EC’s decision to extend the conditional marketing authorization of brentuximab vedotin is based on results from the phase 3 AETHERA trial.
The trial was designed to compare brentuximab vedotin to placebo, both administered for up to 16 cycles (approximately 1 year) every 3 weeks following ASCT. Results from the trial were published in The Lancet in March 2015 and presented at the 2014 ASH Annual Meeting.
The study enrolled 329 HL patients at risk of relapse or progression, including 165 on the brentuximab vedotin arm and 164 on the placebo arm.
Patients were eligible for enrollment if they had a history of primary refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-ASCT relapse.
Brentuximab vedotin conferred a significant increase in progression-free survival over placebo, with a hazard ratio of 0.57 (P=0.001). The median progression-free survival was 43 months for patients who received brentuximab vedotin and 24 months for those who received placebo.
The most common adverse events (≥20%), of any grade and regardless of causality, in the brentuximab vedotin arm were neutropenia (78%), peripheral sensory neuropathy (56%), thrombocytopenia (41%), anemia (27%), upper respiratory tract infection (26%), fatigue (24%), peripheral motor neuropathy (23%), nausea (22%), cough (21%), and diarrhea (20%).
The most common adverse events (≥20%), of any grade and regardless of causality, in the placebo arm were neutropenia (34%), upper respiratory tract infection (23%), and thrombocytopenia (20%).
In all, 67% of patients on the brentuximab vedotin arm experienced peripheral neuropathy. Of those patients, 85% had resolution (59%) or partial improvement (26%) in symptoms at the time of their last evaluation, with a median time to improvement of 23 weeks (range, 0.1-138). ![]()
NHL patients may have higher risk of second cancer

Photo courtesy of NIH
Compared to patients with other common cancers, patients with non-Hodgkin lymphoma (NHL) have a higher risk of developing a second, unrelated malignancy, according to a new study.
Researchers looked at data on more than 2.1 million patients with 10 of the most common cancers and found that patients with NHL or bladder cancer had the highest risk of developing a second malignancy.
The researchers reported these findings in Cancer.
For this study, Karim Chamie, MD, of the University of California, Los Angeles, and his colleagues looked at data from Surveillance, Epidemiology, and End Results database.
The team identified patients age 18 and older who were diagnosed with one of the 10 most common cancers—NHL, melanoma, and prostate, breast, lung, colon, rectal, bladder, uterine, and kidney cancers—between 1992 and 2008.
Of the 2,116,163 patients identified, 170,865 (8.1%) developed a second primary malignancy.
In multivariable analysis, patients with NHL or bladder cancer had the highest risk of developing a second malignancy.
The hazard ratios for patients with NHL were 2.70 for men and 2.88 for women. The hazard ratios for bladder cancer were 1.88 for men and 1.66 for women.
Lung cancer was a common second malignancy for both NHL and bladder cancer patients. NHL patients also tended to develop prostate and breast cancer.
Among patients with 2 incident cancers, 13% died of their initial cancer, and 55% died of their second primary malignancy. Lung cancer was the cause of death in 12% of the patients.
“As clinicians, we can become so focused on surveilling our patients to see if a primary cancer recurs that we sometimes may not be aware that patients can be at risk of developing a second, unrelated cancer,” Dr Chamie said.
He and his colleagues believe this study makes a case for monitoring cancer patients for second malignancies. ![]()

Photo courtesy of NIH
Compared to patients with other common cancers, patients with non-Hodgkin lymphoma (NHL) have a higher risk of developing a second, unrelated malignancy, according to a new study.
Researchers looked at data on more than 2.1 million patients with 10 of the most common cancers and found that patients with NHL or bladder cancer had the highest risk of developing a second malignancy.
The researchers reported these findings in Cancer.
For this study, Karim Chamie, MD, of the University of California, Los Angeles, and his colleagues looked at data from Surveillance, Epidemiology, and End Results database.
The team identified patients age 18 and older who were diagnosed with one of the 10 most common cancers—NHL, melanoma, and prostate, breast, lung, colon, rectal, bladder, uterine, and kidney cancers—between 1992 and 2008.
Of the 2,116,163 patients identified, 170,865 (8.1%) developed a second primary malignancy.
In multivariable analysis, patients with NHL or bladder cancer had the highest risk of developing a second malignancy.
The hazard ratios for patients with NHL were 2.70 for men and 2.88 for women. The hazard ratios for bladder cancer were 1.88 for men and 1.66 for women.
Lung cancer was a common second malignancy for both NHL and bladder cancer patients. NHL patients also tended to develop prostate and breast cancer.
Among patients with 2 incident cancers, 13% died of their initial cancer, and 55% died of their second primary malignancy. Lung cancer was the cause of death in 12% of the patients.
“As clinicians, we can become so focused on surveilling our patients to see if a primary cancer recurs that we sometimes may not be aware that patients can be at risk of developing a second, unrelated cancer,” Dr Chamie said.
He and his colleagues believe this study makes a case for monitoring cancer patients for second malignancies. ![]()

Photo courtesy of NIH
Compared to patients with other common cancers, patients with non-Hodgkin lymphoma (NHL) have a higher risk of developing a second, unrelated malignancy, according to a new study.
Researchers looked at data on more than 2.1 million patients with 10 of the most common cancers and found that patients with NHL or bladder cancer had the highest risk of developing a second malignancy.
The researchers reported these findings in Cancer.
For this study, Karim Chamie, MD, of the University of California, Los Angeles, and his colleagues looked at data from Surveillance, Epidemiology, and End Results database.
The team identified patients age 18 and older who were diagnosed with one of the 10 most common cancers—NHL, melanoma, and prostate, breast, lung, colon, rectal, bladder, uterine, and kidney cancers—between 1992 and 2008.
Of the 2,116,163 patients identified, 170,865 (8.1%) developed a second primary malignancy.
In multivariable analysis, patients with NHL or bladder cancer had the highest risk of developing a second malignancy.
The hazard ratios for patients with NHL were 2.70 for men and 2.88 for women. The hazard ratios for bladder cancer were 1.88 for men and 1.66 for women.
Lung cancer was a common second malignancy for both NHL and bladder cancer patients. NHL patients also tended to develop prostate and breast cancer.
Among patients with 2 incident cancers, 13% died of their initial cancer, and 55% died of their second primary malignancy. Lung cancer was the cause of death in 12% of the patients.
“As clinicians, we can become so focused on surveilling our patients to see if a primary cancer recurs that we sometimes may not be aware that patients can be at risk of developing a second, unrelated cancer,” Dr Chamie said.
He and his colleagues believe this study makes a case for monitoring cancer patients for second malignancies. ![]()