User login
Improve Your Treatment of VTE
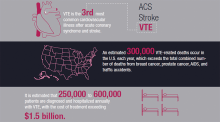
It is estimated that 250,000 to 600,000 patients are diagnosed and hospitalized annually with venous thromboembolism (VTE), the third most common cardiovascular illness after acute coronary syndrome and stroke. An estimated 300,000 VTE-related deaths occur in the U.S. each year, which exceeds the total number of deaths from breast cancer, prostate cancer, AIDS, and traffic accidents combined.
Communication among care providers is an essential part of medical care; it influences patients’ quality of life and effective disease treatment. Hospitalists educate both patients and providers regarding appropriate steps to take to improve care transitions and reduce any associated risks.
To ensure hospitalists have the latest information about diagnosis, treatment, and transition of inpatients with VTE, SHM is developing:
- An online tool kit, including a literature review; implementation guide; and other references, materials, and tools (e.g., discharge instructions and checklists)
- A webinar series with free CME
To be notified when SHM’s VTE resources become available, visit www.hospitalmedicine.org/VTEtreatment.
It is estimated that 250,000 to 600,000 patients are diagnosed and hospitalized annually with venous thromboembolism (VTE), the third most common cardiovascular illness after acute coronary syndrome and stroke. An estimated 300,000 VTE-related deaths occur in the U.S. each year, which exceeds the total number of deaths from breast cancer, prostate cancer, AIDS, and traffic accidents combined.
Communication among care providers is an essential part of medical care; it influences patients’ quality of life and effective disease treatment. Hospitalists educate both patients and providers regarding appropriate steps to take to improve care transitions and reduce any associated risks.
To ensure hospitalists have the latest information about diagnosis, treatment, and transition of inpatients with VTE, SHM is developing:
- An online tool kit, including a literature review; implementation guide; and other references, materials, and tools (e.g., discharge instructions and checklists)
- A webinar series with free CME
To be notified when SHM’s VTE resources become available, visit www.hospitalmedicine.org/VTEtreatment.
It is estimated that 250,000 to 600,000 patients are diagnosed and hospitalized annually with venous thromboembolism (VTE), the third most common cardiovascular illness after acute coronary syndrome and stroke. An estimated 300,000 VTE-related deaths occur in the U.S. each year, which exceeds the total number of deaths from breast cancer, prostate cancer, AIDS, and traffic accidents combined.
Communication among care providers is an essential part of medical care; it influences patients’ quality of life and effective disease treatment. Hospitalists educate both patients and providers regarding appropriate steps to take to improve care transitions and reduce any associated risks.
To ensure hospitalists have the latest information about diagnosis, treatment, and transition of inpatients with VTE, SHM is developing:
- An online tool kit, including a literature review; implementation guide; and other references, materials, and tools (e.g., discharge instructions and checklists)
- A webinar series with free CME
To be notified when SHM’s VTE resources become available, visit www.hospitalmedicine.org/VTEtreatment.
FDA grants drug accelerated approval for CLL

Photo courtesy of the CDC
The US Food and Drug Administration (FDA) has granted accelerated approval for venetoclax (Venclexta) to treat patients with chronic lymphocytic leukemia (CLL) who have 17p deletion and have received at least one prior therapy.
Venetoclax will be available in the US within about a week, according to the companies developing the drug, AbbVie and Genentech (a member of the
Roche Group).
The companies said they plan to offer patient assistance programs for qualifying patients who wish to receive venetoclax.
Venetoclax (formerly ABT-199) is the first FDA-approved treatment that targets the BCL-2 protein, which is overexpressed in many patients with CLL.
The drug is indicated for daily use after 17p deletion is confirmed via the FDA-approved companion diagnostic Vysis CLL FISH probe kit, which is manufactured by Abbott Molecular.
The FDA granted venetoclax accelerated approval rather than traditional approval because the drug has not yet shown a clinical benefit. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of venetoclax for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted venetoclax breakthrough therapy designation, priority review status, and orphan drug designation.
Phase 2 trial
Results from the pivotal phase 2 trial of venetoclax (M13-982, NCT01889186) were presented at the 2015 ASH Annual Meeting. According to those data, the trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
Eighty-five patients responded to treatment, for an overall response rate of 79.4%. Eight patients (7.5%) achieved a complete response or complete response with incomplete count recovery, 3 (2.8%) had a near partial response, and 74 (69.2%) had a partial response. Twenty-two patients (20.6%) did not respond.
As of the ASH presentation, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72.0%, and the overall survival estimate was 86.7%.
Treatment-emergent adverse events of any grade occurred in 96% of patients. The most frequent were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), fatigue (22%), pyrexia (20%), thrombocytopenia (19%), hyperphosphatemia (16%), vomiting (15%), and upper respiratory tract infection (15%). (About 22% of patients had neutropenia at baseline.)
The most frequent grade 3/4 adverse events were neutropenia (40%), anemia (18%), and thrombocytopenia (15%). Infections occurred in 72% of patients, with 20% of patients experiencing grade 3 or higher infections.
Serious adverse events occurred in 55% of patients, the most common being pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Laboratory tumor lysis syndrome (TLS) occurred in 5 patients during the ramp-up period only. Two patients required a dose interruption of 1 day each. There were no clinical TLS events.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS.
Venetoclax is currently being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL. ![]()

Photo courtesy of the CDC
The US Food and Drug Administration (FDA) has granted accelerated approval for venetoclax (Venclexta) to treat patients with chronic lymphocytic leukemia (CLL) who have 17p deletion and have received at least one prior therapy.
Venetoclax will be available in the US within about a week, according to the companies developing the drug, AbbVie and Genentech (a member of the
Roche Group).
The companies said they plan to offer patient assistance programs for qualifying patients who wish to receive venetoclax.
Venetoclax (formerly ABT-199) is the first FDA-approved treatment that targets the BCL-2 protein, which is overexpressed in many patients with CLL.
The drug is indicated for daily use after 17p deletion is confirmed via the FDA-approved companion diagnostic Vysis CLL FISH probe kit, which is manufactured by Abbott Molecular.
The FDA granted venetoclax accelerated approval rather than traditional approval because the drug has not yet shown a clinical benefit. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of venetoclax for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted venetoclax breakthrough therapy designation, priority review status, and orphan drug designation.
Phase 2 trial
Results from the pivotal phase 2 trial of venetoclax (M13-982, NCT01889186) were presented at the 2015 ASH Annual Meeting. According to those data, the trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
Eighty-five patients responded to treatment, for an overall response rate of 79.4%. Eight patients (7.5%) achieved a complete response or complete response with incomplete count recovery, 3 (2.8%) had a near partial response, and 74 (69.2%) had a partial response. Twenty-two patients (20.6%) did not respond.
As of the ASH presentation, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72.0%, and the overall survival estimate was 86.7%.
Treatment-emergent adverse events of any grade occurred in 96% of patients. The most frequent were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), fatigue (22%), pyrexia (20%), thrombocytopenia (19%), hyperphosphatemia (16%), vomiting (15%), and upper respiratory tract infection (15%). (About 22% of patients had neutropenia at baseline.)
The most frequent grade 3/4 adverse events were neutropenia (40%), anemia (18%), and thrombocytopenia (15%). Infections occurred in 72% of patients, with 20% of patients experiencing grade 3 or higher infections.
Serious adverse events occurred in 55% of patients, the most common being pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Laboratory tumor lysis syndrome (TLS) occurred in 5 patients during the ramp-up period only. Two patients required a dose interruption of 1 day each. There were no clinical TLS events.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS.
Venetoclax is currently being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL. ![]()

Photo courtesy of the CDC
The US Food and Drug Administration (FDA) has granted accelerated approval for venetoclax (Venclexta) to treat patients with chronic lymphocytic leukemia (CLL) who have 17p deletion and have received at least one prior therapy.
Venetoclax will be available in the US within about a week, according to the companies developing the drug, AbbVie and Genentech (a member of the
Roche Group).
The companies said they plan to offer patient assistance programs for qualifying patients who wish to receive venetoclax.
Venetoclax (formerly ABT-199) is the first FDA-approved treatment that targets the BCL-2 protein, which is overexpressed in many patients with CLL.
The drug is indicated for daily use after 17p deletion is confirmed via the FDA-approved companion diagnostic Vysis CLL FISH probe kit, which is manufactured by Abbott Molecular.
The FDA granted venetoclax accelerated approval rather than traditional approval because the drug has not yet shown a clinical benefit. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of venetoclax for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted venetoclax breakthrough therapy designation, priority review status, and orphan drug designation.
Phase 2 trial
Results from the pivotal phase 2 trial of venetoclax (M13-982, NCT01889186) were presented at the 2015 ASH Annual Meeting. According to those data, the trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
Eighty-five patients responded to treatment, for an overall response rate of 79.4%. Eight patients (7.5%) achieved a complete response or complete response with incomplete count recovery, 3 (2.8%) had a near partial response, and 74 (69.2%) had a partial response. Twenty-two patients (20.6%) did not respond.
As of the ASH presentation, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72.0%, and the overall survival estimate was 86.7%.
Treatment-emergent adverse events of any grade occurred in 96% of patients. The most frequent were neutropenia (43%), diarrhea (29%), nausea (29%), anemia (27%), fatigue (22%), pyrexia (20%), thrombocytopenia (19%), hyperphosphatemia (16%), vomiting (15%), and upper respiratory tract infection (15%). (About 22% of patients had neutropenia at baseline.)
The most frequent grade 3/4 adverse events were neutropenia (40%), anemia (18%), and thrombocytopenia (15%). Infections occurred in 72% of patients, with 20% of patients experiencing grade 3 or higher infections.
Serious adverse events occurred in 55% of patients, the most common being pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Laboratory tumor lysis syndrome (TLS) occurred in 5 patients during the ramp-up period only. Two patients required a dose interruption of 1 day each. There were no clinical TLS events.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS.
Venetoclax is currently being evaluated in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL. ![]()
Protein distribution impacts T cells’ fate
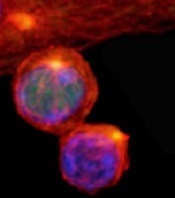
(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()

(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()

(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()
Team creates public repository of xenografts

Photo by Rhoda Baer
Researchers have established a public repository of leukemia and lymphoma xenografts, according to a report in Cancer Cell.
The repository, known as the Public Repository of Xenografts (PRoXe), contains material from bone marrow, peripheral blood, and lymph nodes of mice.
It also contains information on the patients from whom the cancer tissues were derived and details on the characteristics of the tumors themselves.
PRoXe is based at Dana-Farber Cancer Institute in Boston, Massachusetts, but it has a web portal that can be accessed by researchers around the world.
Those who register with PRoxE can access the repository and search for cells from patients with specific hematologic malignancies.
Researchers can then have frozen cells shipped to them and transplant the cells into mice to create patient-derived xenograft (PDX) models for testing drugs.
“About 90% of compounds that show anticancer activity in preclinical tests don’t work when given to patients,” said David Weinstock, MD, of the Dana-Farber Cancer Institute.
“By trying drugs in PDX models, we can ‘mimic’ large and expensive human clinical trials and get answers about efficacy more quickly, less expensively, and without the need for patients to get investigational drugs that won’t work.”
To demonstrate how PRoXe can be used, Dr Weinstock and his colleagues tested the MDM2 inhibitor CGM097 against B-cell acute lymphoblastic leukemia (B-ALL) in 2 groups of mice. One group had a mutation in the TP53 tumor suppressor gene, and the other did not.
The researchers observed superior survival in the mice with wild-type TP53. This result corresponds with the results of previous research, which showed that inhibitors that disrupt the MDM2-p53 interaction can be effective in tumor models and patient tumors with wild-type TP53.
Dr Weinstock and his colleagues said their results provide strong preclinical evidence for testing CGM097 in patients who have been extensively treated for B-ALL and have wild-type TP53.
Dr Weinstock also noted that the leaders of ProXe are negotiating with a number of academic centers in an attempt to incorporate their PDX models into the repository.
The Cancer Cell manuscript had 95 authors from 14 different centers that contributed samples, models, and/or effort to the project. ![]()

Photo by Rhoda Baer
Researchers have established a public repository of leukemia and lymphoma xenografts, according to a report in Cancer Cell.
The repository, known as the Public Repository of Xenografts (PRoXe), contains material from bone marrow, peripheral blood, and lymph nodes of mice.
It also contains information on the patients from whom the cancer tissues were derived and details on the characteristics of the tumors themselves.
PRoXe is based at Dana-Farber Cancer Institute in Boston, Massachusetts, but it has a web portal that can be accessed by researchers around the world.
Those who register with PRoxE can access the repository and search for cells from patients with specific hematologic malignancies.
Researchers can then have frozen cells shipped to them and transplant the cells into mice to create patient-derived xenograft (PDX) models for testing drugs.
“About 90% of compounds that show anticancer activity in preclinical tests don’t work when given to patients,” said David Weinstock, MD, of the Dana-Farber Cancer Institute.
“By trying drugs in PDX models, we can ‘mimic’ large and expensive human clinical trials and get answers about efficacy more quickly, less expensively, and without the need for patients to get investigational drugs that won’t work.”
To demonstrate how PRoXe can be used, Dr Weinstock and his colleagues tested the MDM2 inhibitor CGM097 against B-cell acute lymphoblastic leukemia (B-ALL) in 2 groups of mice. One group had a mutation in the TP53 tumor suppressor gene, and the other did not.
The researchers observed superior survival in the mice with wild-type TP53. This result corresponds with the results of previous research, which showed that inhibitors that disrupt the MDM2-p53 interaction can be effective in tumor models and patient tumors with wild-type TP53.
Dr Weinstock and his colleagues said their results provide strong preclinical evidence for testing CGM097 in patients who have been extensively treated for B-ALL and have wild-type TP53.
Dr Weinstock also noted that the leaders of ProXe are negotiating with a number of academic centers in an attempt to incorporate their PDX models into the repository.
The Cancer Cell manuscript had 95 authors from 14 different centers that contributed samples, models, and/or effort to the project. ![]()

Photo by Rhoda Baer
Researchers have established a public repository of leukemia and lymphoma xenografts, according to a report in Cancer Cell.
The repository, known as the Public Repository of Xenografts (PRoXe), contains material from bone marrow, peripheral blood, and lymph nodes of mice.
It also contains information on the patients from whom the cancer tissues were derived and details on the characteristics of the tumors themselves.
PRoXe is based at Dana-Farber Cancer Institute in Boston, Massachusetts, but it has a web portal that can be accessed by researchers around the world.
Those who register with PRoxE can access the repository and search for cells from patients with specific hematologic malignancies.
Researchers can then have frozen cells shipped to them and transplant the cells into mice to create patient-derived xenograft (PDX) models for testing drugs.
“About 90% of compounds that show anticancer activity in preclinical tests don’t work when given to patients,” said David Weinstock, MD, of the Dana-Farber Cancer Institute.
“By trying drugs in PDX models, we can ‘mimic’ large and expensive human clinical trials and get answers about efficacy more quickly, less expensively, and without the need for patients to get investigational drugs that won’t work.”
To demonstrate how PRoXe can be used, Dr Weinstock and his colleagues tested the MDM2 inhibitor CGM097 against B-cell acute lymphoblastic leukemia (B-ALL) in 2 groups of mice. One group had a mutation in the TP53 tumor suppressor gene, and the other did not.
The researchers observed superior survival in the mice with wild-type TP53. This result corresponds with the results of previous research, which showed that inhibitors that disrupt the MDM2-p53 interaction can be effective in tumor models and patient tumors with wild-type TP53.
Dr Weinstock and his colleagues said their results provide strong preclinical evidence for testing CGM097 in patients who have been extensively treated for B-ALL and have wild-type TP53.
Dr Weinstock also noted that the leaders of ProXe are negotiating with a number of academic centers in an attempt to incorporate their PDX models into the repository.
The Cancer Cell manuscript had 95 authors from 14 different centers that contributed samples, models, and/or effort to the project. ![]()
PET-guided chemo improves PFS in advanced HL

Image by Jens Langner
Using PET imaging to guide chemotherapy can improve outcomes for patients with advanced Hodgkin lymphoma (HL), according to research published in the Journal of Clinical Oncology.
The study indicated that PET-guided treatment can improve progression-free survival (PFS) among patients who do not achieve remission with 2 cycles of ABVD.
The results also suggested the approach can spare some patients unnecessary toxicity.
“The goal of cancer treatment is to cure as many people as possible with as little toxicity as possible,” said study author Oliver Press, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“We found a promising way to do that by tailoring treatment to Hodgkin patients, an approach which could lead to a new standard of care.”
Dr Press and his colleagues began this study with 358 HIV-negative patients with advanced HL, but only 336 of them were eligible and evaluable at baseline.
The patients’ median age was 32 (range, 18 to 60). Fifty-two percent had stage III disease, 48% had stage IV, 49% had an International Prognostic Score of 0 to 2, and 51% had a score of 3 to 7.
All of the patients received ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and underwent a PET scan to gauge their response after 2 cycles (PET2).
If the scan was negative, patients received a final 4 cycles of ABVD. If the scan was positive, patients received 6 cycles of eBEACOPP (escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone).
Central review of the PET2 scan was performed in 331 patients. Most (82%, n=271) had a negative scan.
Forty-nine of the 60 PET2-positive patients went on to receive eBEACOPP as planned, but 11 patients declined.
The median follow-up was 39.7 months. For the entire study cohort, the estimated 2-year overall survival was 98%, and the estimated 2-year PFS was 79%.
The 2-year estimated PFS was 82% for PET2-negative patients and 64% for PET2-positive patients. The researchers noted that, typically, if HL patients have a positive PET scan after 2 rounds of ABVD, their expected 2-year PFS ranges from about 15% to 30%.
The researchers also pointed out that eBEACOPP was significantly more toxic than ABVD. The incidence of grade 4/5 toxicities was 85.7% and 36.7%, respectively (P<0.001).
There were 3 treatment-related deaths—1 in the ABVD group and 2 in the eBEACOPP group. And 6 patients developed secondary malignancies—3 in each group—including 2 non-Hodgkin lymphomas, 2 kidney cancers, 1 melanoma, and 1 skin cancer.
“[O]nly 20% of the patients in our trial were exposed to eBEACOPP, which means [most patients] weren’t exposed to its bad effects,” said Jonathan Friedberg, MD, of the University of Rochester Medical Center in Rochester, New York.
“That’s important because many people diagnosed with Hodgkin lymphoma are in their 20s and 30s and want to have children. This response-adapted therapy would ensure that the people who need the more toxic drugs receive them and would spare others from infertility and serious toxicities.” ![]()

Image by Jens Langner
Using PET imaging to guide chemotherapy can improve outcomes for patients with advanced Hodgkin lymphoma (HL), according to research published in the Journal of Clinical Oncology.
The study indicated that PET-guided treatment can improve progression-free survival (PFS) among patients who do not achieve remission with 2 cycles of ABVD.
The results also suggested the approach can spare some patients unnecessary toxicity.
“The goal of cancer treatment is to cure as many people as possible with as little toxicity as possible,” said study author Oliver Press, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“We found a promising way to do that by tailoring treatment to Hodgkin patients, an approach which could lead to a new standard of care.”
Dr Press and his colleagues began this study with 358 HIV-negative patients with advanced HL, but only 336 of them were eligible and evaluable at baseline.
The patients’ median age was 32 (range, 18 to 60). Fifty-two percent had stage III disease, 48% had stage IV, 49% had an International Prognostic Score of 0 to 2, and 51% had a score of 3 to 7.
All of the patients received ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and underwent a PET scan to gauge their response after 2 cycles (PET2).
If the scan was negative, patients received a final 4 cycles of ABVD. If the scan was positive, patients received 6 cycles of eBEACOPP (escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone).
Central review of the PET2 scan was performed in 331 patients. Most (82%, n=271) had a negative scan.
Forty-nine of the 60 PET2-positive patients went on to receive eBEACOPP as planned, but 11 patients declined.
The median follow-up was 39.7 months. For the entire study cohort, the estimated 2-year overall survival was 98%, and the estimated 2-year PFS was 79%.
The 2-year estimated PFS was 82% for PET2-negative patients and 64% for PET2-positive patients. The researchers noted that, typically, if HL patients have a positive PET scan after 2 rounds of ABVD, their expected 2-year PFS ranges from about 15% to 30%.
The researchers also pointed out that eBEACOPP was significantly more toxic than ABVD. The incidence of grade 4/5 toxicities was 85.7% and 36.7%, respectively (P<0.001).
There were 3 treatment-related deaths—1 in the ABVD group and 2 in the eBEACOPP group. And 6 patients developed secondary malignancies—3 in each group—including 2 non-Hodgkin lymphomas, 2 kidney cancers, 1 melanoma, and 1 skin cancer.
“[O]nly 20% of the patients in our trial were exposed to eBEACOPP, which means [most patients] weren’t exposed to its bad effects,” said Jonathan Friedberg, MD, of the University of Rochester Medical Center in Rochester, New York.
“That’s important because many people diagnosed with Hodgkin lymphoma are in their 20s and 30s and want to have children. This response-adapted therapy would ensure that the people who need the more toxic drugs receive them and would spare others from infertility and serious toxicities.” ![]()

Image by Jens Langner
Using PET imaging to guide chemotherapy can improve outcomes for patients with advanced Hodgkin lymphoma (HL), according to research published in the Journal of Clinical Oncology.
The study indicated that PET-guided treatment can improve progression-free survival (PFS) among patients who do not achieve remission with 2 cycles of ABVD.
The results also suggested the approach can spare some patients unnecessary toxicity.
“The goal of cancer treatment is to cure as many people as possible with as little toxicity as possible,” said study author Oliver Press, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“We found a promising way to do that by tailoring treatment to Hodgkin patients, an approach which could lead to a new standard of care.”
Dr Press and his colleagues began this study with 358 HIV-negative patients with advanced HL, but only 336 of them were eligible and evaluable at baseline.
The patients’ median age was 32 (range, 18 to 60). Fifty-two percent had stage III disease, 48% had stage IV, 49% had an International Prognostic Score of 0 to 2, and 51% had a score of 3 to 7.
All of the patients received ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and underwent a PET scan to gauge their response after 2 cycles (PET2).
If the scan was negative, patients received a final 4 cycles of ABVD. If the scan was positive, patients received 6 cycles of eBEACOPP (escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone).
Central review of the PET2 scan was performed in 331 patients. Most (82%, n=271) had a negative scan.
Forty-nine of the 60 PET2-positive patients went on to receive eBEACOPP as planned, but 11 patients declined.
The median follow-up was 39.7 months. For the entire study cohort, the estimated 2-year overall survival was 98%, and the estimated 2-year PFS was 79%.
The 2-year estimated PFS was 82% for PET2-negative patients and 64% for PET2-positive patients. The researchers noted that, typically, if HL patients have a positive PET scan after 2 rounds of ABVD, their expected 2-year PFS ranges from about 15% to 30%.
The researchers also pointed out that eBEACOPP was significantly more toxic than ABVD. The incidence of grade 4/5 toxicities was 85.7% and 36.7%, respectively (P<0.001).
There were 3 treatment-related deaths—1 in the ABVD group and 2 in the eBEACOPP group. And 6 patients developed secondary malignancies—3 in each group—including 2 non-Hodgkin lymphomas, 2 kidney cancers, 1 melanoma, and 1 skin cancer.
“[O]nly 20% of the patients in our trial were exposed to eBEACOPP, which means [most patients] weren’t exposed to its bad effects,” said Jonathan Friedberg, MD, of the University of Rochester Medical Center in Rochester, New York.
“That’s important because many people diagnosed with Hodgkin lymphoma are in their 20s and 30s and want to have children. This response-adapted therapy would ensure that the people who need the more toxic drugs receive them and would spare others from infertility and serious toxicities.” ![]()
USPSTF updates guideline for preventive aspirin therapy
Many patients aged 50-59 years should start low-dose aspirin for the primary prevention of cardiovascular disease and colorectal cancer, according to the U.S. Preventive Services Task Force’s updated clinical practice guideline on aspirin therapy, published online April 11 in Annals of Internal Medicine.
The evidence is clear that the benefits outweigh the potential harms of low-dose aspirin in this age group if patients have a 10% or greater 10-year cardiovascular disease (CVD) risk, are not at increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take the treatment for at least 10 years, said Dr. Albert L. Siu and his associates in the USPSTF.
The organization based this guideline on 2 systematic reviews of the literature, updating its 2009 review on aspirin therapy to prevent cardiovascular disease and updating its 2007 review on aspirin therapy to prevent colorectal cancer. The findings from these reviews of the current evidence were used to develop a decision-analysis model to weigh the benefits and harms of treatment in various patient groups defined by age, gender, and risk factors.
Recent studies of primary prevention of CVD, which included 118,445 participants, “consistently demonstrated effectiveness of aspirin in preventing nonfatal MI and stroke.” Pooled analyses showed that low-dose aspirin reduced nonfatal MI and coronary events 17% (risk ratio, 0.83) and that any aspirin dose reduced them 22%. Low-dose aspirin also reduced all-cause mortality risk (RR, 0.95) in pooled analyses.
Aspirin therapy also reduced the risk of colorectal cancer, but this benefit didn’t appear until after 5-10 years of treatment. Three trials reported a 40% reduction (RR, 0.60) after 10-20 years of daily low-dose aspirin.
On the other side of the equation, major GI bleeding increased by 65% among aspirin users when the data from 15 CVD prevention trials were pooled. Similarly, pooled analyses showed a 33% increase in hemorrhagic stroke among aspirin users, compared with nonusers.
The benefits of low-dose aspirin were highest and the harms were lowest in patients aged 50-59 years, hence the first recommendation in the new guideline. In patients aged 60-69 years, the benefit-to-harm balance isn’t as clear-cut, so the decision to initiate or continue aspirin therapy in this age group must be made on an individual basis. “Some adults [at this age] may decide that avoiding an MI or stroke is very important and that having a GI bleeding event is not as significant. They may decide to take aspirin at a lower CVD risk level than those who are more concerned about GI bleeding. Adults who have a high likelihood of benefit with little potential for harm should be encouraged to consider aspirin use.
“Conversely, adults who have little potential of benefit or high risk for GI bleeding should be discouraged” from taking aspirin therapy, the investigators said (Ann Intern Med. 2016 Apr 11. doi: 10.7326/M16-0577).
The task force found that current evidence is insufficient to assess the balance of benefits and harms regarding aspirin therapy for adults younger than age 50 or older than age 70. In the latter group in particular, the picture is complicated by the effects of age, use of other medications, and concomitant illness. However, since cardiovascular risks are increased after age 70 and the incidences of MI and stroke are relatively high, the benefits of preventive aspirin could be substantial in this age group, said Dr. Siu of Icahn School of Medicine Mount Sinai, New York, and the Veterans Affairs Medical Center, the Bronx, and his associates.
The USPSTF guideline generally accords with existing recommendations from the American Heart Association, the American Stroke Association, the American Diabetes Association, the American Academy of Family Physicians, and the American College of Chest Physicians. At present, the American Cancer Society doesn’t have recommendations for or against aspirin therapy; the American Gastroenterological Association and the National Comprehensive Care Network “limit their recommendations to patients who are at increased risk for colorectal cancer,” Dr. Siu and his associates added.
Copies of the guideline and of the supporting literature reviews and the decision-analysis tool are available at www.uspreventiveservicestaskforce.org.
Patients and providers must read beyond the headlines advocating expanded aspirin use. The USPSTF explicitly endorses low-dose aspirin only for those with a 10-year cardiovascular disease (CVD) risk of 10% or greater, who are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take the medication for at least that long. Balancing these competing risks is no small task. While Internet-based CVD risk calculators are readily available, they are not routinely used. GI risk is less easily quantified, with prior ulcer history or bleeding most important to consider, but concomitant medications (NSAIDs, anticoagulants, and SSRIs) must also be recognized. Proton pump inhibitors can reduce aspirin-induced upper GI bleeding and are cost effective in a primary prevention population with increased GI risk (Arch Intern Med. 2011;171:218-25). There is no intervention to reduce bleeding events in the small bowel and colon.
The USPSTF acknowledged that the benefits of aspirin in reducing colon cancer incidence and mortality were not established in the primary CVD prevention population. Its impact in other patient populations required 10 years or more – leading to their targeting the 50-70-year-old group. Aspirin therapy should not be considered a substitute for colonoscopy, and among those undergoing screening it remains of uncertain incremental value (Ann Intern Med. 2001;135:769-81). Gastroenterologists must play an active role to ensure the appropriate use of aspirin therapy in contributing to improved global patient outcomes.
Dr. James M. Scheiman is professor of internal medicine in the University of Michigan Health System, Ann Arbor. His is a consultant to Aralez, Pfizer, Stryker, Intec, and Teva.
Patients and providers must read beyond the headlines advocating expanded aspirin use. The USPSTF explicitly endorses low-dose aspirin only for those with a 10-year cardiovascular disease (CVD) risk of 10% or greater, who are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take the medication for at least that long. Balancing these competing risks is no small task. While Internet-based CVD risk calculators are readily available, they are not routinely used. GI risk is less easily quantified, with prior ulcer history or bleeding most important to consider, but concomitant medications (NSAIDs, anticoagulants, and SSRIs) must also be recognized. Proton pump inhibitors can reduce aspirin-induced upper GI bleeding and are cost effective in a primary prevention population with increased GI risk (Arch Intern Med. 2011;171:218-25). There is no intervention to reduce bleeding events in the small bowel and colon.
The USPSTF acknowledged that the benefits of aspirin in reducing colon cancer incidence and mortality were not established in the primary CVD prevention population. Its impact in other patient populations required 10 years or more – leading to their targeting the 50-70-year-old group. Aspirin therapy should not be considered a substitute for colonoscopy, and among those undergoing screening it remains of uncertain incremental value (Ann Intern Med. 2001;135:769-81). Gastroenterologists must play an active role to ensure the appropriate use of aspirin therapy in contributing to improved global patient outcomes.
Dr. James M. Scheiman is professor of internal medicine in the University of Michigan Health System, Ann Arbor. His is a consultant to Aralez, Pfizer, Stryker, Intec, and Teva.
Patients and providers must read beyond the headlines advocating expanded aspirin use. The USPSTF explicitly endorses low-dose aspirin only for those with a 10-year cardiovascular disease (CVD) risk of 10% or greater, who are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take the medication for at least that long. Balancing these competing risks is no small task. While Internet-based CVD risk calculators are readily available, they are not routinely used. GI risk is less easily quantified, with prior ulcer history or bleeding most important to consider, but concomitant medications (NSAIDs, anticoagulants, and SSRIs) must also be recognized. Proton pump inhibitors can reduce aspirin-induced upper GI bleeding and are cost effective in a primary prevention population with increased GI risk (Arch Intern Med. 2011;171:218-25). There is no intervention to reduce bleeding events in the small bowel and colon.
The USPSTF acknowledged that the benefits of aspirin in reducing colon cancer incidence and mortality were not established in the primary CVD prevention population. Its impact in other patient populations required 10 years or more – leading to their targeting the 50-70-year-old group. Aspirin therapy should not be considered a substitute for colonoscopy, and among those undergoing screening it remains of uncertain incremental value (Ann Intern Med. 2001;135:769-81). Gastroenterologists must play an active role to ensure the appropriate use of aspirin therapy in contributing to improved global patient outcomes.
Dr. James M. Scheiman is professor of internal medicine in the University of Michigan Health System, Ann Arbor. His is a consultant to Aralez, Pfizer, Stryker, Intec, and Teva.
Many patients aged 50-59 years should start low-dose aspirin for the primary prevention of cardiovascular disease and colorectal cancer, according to the U.S. Preventive Services Task Force’s updated clinical practice guideline on aspirin therapy, published online April 11 in Annals of Internal Medicine.
The evidence is clear that the benefits outweigh the potential harms of low-dose aspirin in this age group if patients have a 10% or greater 10-year cardiovascular disease (CVD) risk, are not at increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take the treatment for at least 10 years, said Dr. Albert L. Siu and his associates in the USPSTF.
The organization based this guideline on 2 systematic reviews of the literature, updating its 2009 review on aspirin therapy to prevent cardiovascular disease and updating its 2007 review on aspirin therapy to prevent colorectal cancer. The findings from these reviews of the current evidence were used to develop a decision-analysis model to weigh the benefits and harms of treatment in various patient groups defined by age, gender, and risk factors.
Recent studies of primary prevention of CVD, which included 118,445 participants, “consistently demonstrated effectiveness of aspirin in preventing nonfatal MI and stroke.” Pooled analyses showed that low-dose aspirin reduced nonfatal MI and coronary events 17% (risk ratio, 0.83) and that any aspirin dose reduced them 22%. Low-dose aspirin also reduced all-cause mortality risk (RR, 0.95) in pooled analyses.
Aspirin therapy also reduced the risk of colorectal cancer, but this benefit didn’t appear until after 5-10 years of treatment. Three trials reported a 40% reduction (RR, 0.60) after 10-20 years of daily low-dose aspirin.
On the other side of the equation, major GI bleeding increased by 65% among aspirin users when the data from 15 CVD prevention trials were pooled. Similarly, pooled analyses showed a 33% increase in hemorrhagic stroke among aspirin users, compared with nonusers.
The benefits of low-dose aspirin were highest and the harms were lowest in patients aged 50-59 years, hence the first recommendation in the new guideline. In patients aged 60-69 years, the benefit-to-harm balance isn’t as clear-cut, so the decision to initiate or continue aspirin therapy in this age group must be made on an individual basis. “Some adults [at this age] may decide that avoiding an MI or stroke is very important and that having a GI bleeding event is not as significant. They may decide to take aspirin at a lower CVD risk level than those who are more concerned about GI bleeding. Adults who have a high likelihood of benefit with little potential for harm should be encouraged to consider aspirin use.
“Conversely, adults who have little potential of benefit or high risk for GI bleeding should be discouraged” from taking aspirin therapy, the investigators said (Ann Intern Med. 2016 Apr 11. doi: 10.7326/M16-0577).
The task force found that current evidence is insufficient to assess the balance of benefits and harms regarding aspirin therapy for adults younger than age 50 or older than age 70. In the latter group in particular, the picture is complicated by the effects of age, use of other medications, and concomitant illness. However, since cardiovascular risks are increased after age 70 and the incidences of MI and stroke are relatively high, the benefits of preventive aspirin could be substantial in this age group, said Dr. Siu of Icahn School of Medicine Mount Sinai, New York, and the Veterans Affairs Medical Center, the Bronx, and his associates.
The USPSTF guideline generally accords with existing recommendations from the American Heart Association, the American Stroke Association, the American Diabetes Association, the American Academy of Family Physicians, and the American College of Chest Physicians. At present, the American Cancer Society doesn’t have recommendations for or against aspirin therapy; the American Gastroenterological Association and the National Comprehensive Care Network “limit their recommendations to patients who are at increased risk for colorectal cancer,” Dr. Siu and his associates added.
Copies of the guideline and of the supporting literature reviews and the decision-analysis tool are available at www.uspreventiveservicestaskforce.org.
Many patients aged 50-59 years should start low-dose aspirin for the primary prevention of cardiovascular disease and colorectal cancer, according to the U.S. Preventive Services Task Force’s updated clinical practice guideline on aspirin therapy, published online April 11 in Annals of Internal Medicine.
The evidence is clear that the benefits outweigh the potential harms of low-dose aspirin in this age group if patients have a 10% or greater 10-year cardiovascular disease (CVD) risk, are not at increased risk of bleeding, have a life expectancy of at least 10 years, and are willing to take the treatment for at least 10 years, said Dr. Albert L. Siu and his associates in the USPSTF.
The organization based this guideline on 2 systematic reviews of the literature, updating its 2009 review on aspirin therapy to prevent cardiovascular disease and updating its 2007 review on aspirin therapy to prevent colorectal cancer. The findings from these reviews of the current evidence were used to develop a decision-analysis model to weigh the benefits and harms of treatment in various patient groups defined by age, gender, and risk factors.
Recent studies of primary prevention of CVD, which included 118,445 participants, “consistently demonstrated effectiveness of aspirin in preventing nonfatal MI and stroke.” Pooled analyses showed that low-dose aspirin reduced nonfatal MI and coronary events 17% (risk ratio, 0.83) and that any aspirin dose reduced them 22%. Low-dose aspirin also reduced all-cause mortality risk (RR, 0.95) in pooled analyses.
Aspirin therapy also reduced the risk of colorectal cancer, but this benefit didn’t appear until after 5-10 years of treatment. Three trials reported a 40% reduction (RR, 0.60) after 10-20 years of daily low-dose aspirin.
On the other side of the equation, major GI bleeding increased by 65% among aspirin users when the data from 15 CVD prevention trials were pooled. Similarly, pooled analyses showed a 33% increase in hemorrhagic stroke among aspirin users, compared with nonusers.
The benefits of low-dose aspirin were highest and the harms were lowest in patients aged 50-59 years, hence the first recommendation in the new guideline. In patients aged 60-69 years, the benefit-to-harm balance isn’t as clear-cut, so the decision to initiate or continue aspirin therapy in this age group must be made on an individual basis. “Some adults [at this age] may decide that avoiding an MI or stroke is very important and that having a GI bleeding event is not as significant. They may decide to take aspirin at a lower CVD risk level than those who are more concerned about GI bleeding. Adults who have a high likelihood of benefit with little potential for harm should be encouraged to consider aspirin use.
“Conversely, adults who have little potential of benefit or high risk for GI bleeding should be discouraged” from taking aspirin therapy, the investigators said (Ann Intern Med. 2016 Apr 11. doi: 10.7326/M16-0577).
The task force found that current evidence is insufficient to assess the balance of benefits and harms regarding aspirin therapy for adults younger than age 50 or older than age 70. In the latter group in particular, the picture is complicated by the effects of age, use of other medications, and concomitant illness. However, since cardiovascular risks are increased after age 70 and the incidences of MI and stroke are relatively high, the benefits of preventive aspirin could be substantial in this age group, said Dr. Siu of Icahn School of Medicine Mount Sinai, New York, and the Veterans Affairs Medical Center, the Bronx, and his associates.
The USPSTF guideline generally accords with existing recommendations from the American Heart Association, the American Stroke Association, the American Diabetes Association, the American Academy of Family Physicians, and the American College of Chest Physicians. At present, the American Cancer Society doesn’t have recommendations for or against aspirin therapy; the American Gastroenterological Association and the National Comprehensive Care Network “limit their recommendations to patients who are at increased risk for colorectal cancer,” Dr. Siu and his associates added.
Copies of the guideline and of the supporting literature reviews and the decision-analysis tool are available at www.uspreventiveservicestaskforce.org.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: The USPSTF recommends that many adults aged 50-59 years start low-dose aspirin for primary prevention of cardiovascular disease and colorectal cancer.
Major finding: Low-dose aspirin reduced nonfatal MI and coronary events 17% (RR, 0.83), and 10-20 years of daily aspirin reduced the risk of colorectal cancer 40%.
Data source: Three systematic reviews of the literature and a compilation of clinical practice guidelines for preventive aspirin therapy.
Disclosures: The USPSTF is an independent, voluntary group funded by the Agency for Healthcare Research and Quality by mandate of the U.S. Congress. Dr. Siu and his associates reported having no relevant financial disclosures.
On-site reporting from the Society of Gynecologic Surgeons 2016 annual meeting
4/13/16. DAY 4 AT SGS
A jam-packed day of sessions, posters, awards, and clinical updates
Our last educational day was kicked off by a fascinating lecture by Dr. Amy Park on the “Genetic Determinants of Pelvic Organ Prolapse in Women of European American Descent: The Women’s Health Initiative.” Dr. Park and her colleagues found that there is evidence of phenotypic and genotypic heterogeneity in patients with pelvic organ prolapse, and there were 4 genetic loci identified that correlated with prolapse. Any uterine prolapse was associated with a genome-wide significant intergenic variant on chromosome 13, cystocele was associated with LOXL2, and all prolapse was associated with BMP.
Surmounting surgical site infection
Dr. Sarah Andiman then gave a lecture on the effects of a perioperative bundle and offered timely feedback for surgical site infection (SSI) prevention in hysterectomy. We all know that wound infections are a major morbidity associated with surgery, and Dr. Andiman’s group at Yale found that, by implementing this prevention program, the surgical site infection decreased 52.8%. Another suggestion from the audience was to have all patients use chlorhexidine wipes the night before and morning of a surgery to decrease wound infection. Similarly, Dr. Ali Bazzi gave a lecture on “Chlorhexidine-Alcohol Compared with Povidone-Iodine for Surgical-Site Antisepsis after Abdominal Hysterectomy.” The chlorhexidine was associated with 30% lower odds of SSI compared with povidone-iodine, even though this group had several medical comorbidities and risk factors known for SSIs.
Oral poster presenters make several interesting points
Dr. Christopher Ripperda from UT Southwestern Medical Center in Dallas, Texas, found that medical comorbidities and the presence of detrusor overactivity and PVR are predictors of early postoperative voiding dysfunction following a midurethral sling placement. Dr. Nabila Noor then described a fabulous surgical alternative to the use of morcellation to remove the uterus after a supracervical hysterectomy. She described the technique for performing a posterior colpotomy and stated that patients who had a surgery performed at her institution using this technique did not experience increased postoperative pain or longer postoperative stay.
Dr. Jennifer Thompson then shed some light on a very important question related to the Sunshine Act: Are physicians including all of their disclosures when they submit articles to a conference? When the physicians who submitted an abstract to the Society of Gynecologic Surgeons (SGS) in 2015 were searched on the Centers for Medicare and Medicaid Services (CMS) website, 62% of them had incomplete disclosures, with a total of nondisclosed CMS transactions equaling $1.3 million. We can do better!
Status update: The FPRN and passing of the torch
Congratulations to Dr. Kristin Jacobs, the new Fellows’ Pelvic Research Network (FPRN) Chair! The morning session ended with an innovative video from Dr. Janet Li and colleagues on the “Use of Suprapubic Carter-Thomason Needle to Assist in Cystoscopic Excision of Intravesical Foreign Object.”
The winner of the Distinguished Surgeon Award was given to Dr. Javier Magrina, and the SGS gavel was passed on to Dr. Vivian Sung! Congratulations!
Dr. Stephanie Pickett kicked off the eighth scientific session with a study entitled, “Comparing Methods of NSAID Delivery for Postoperative Pain.” When she and her colleagues compared IV toradol to IV ibuprofen for postoperative pain control after urogynecologic surgery, they found that patients experienced similar rates of pain control and satisfaction regardless of the type of analgesia.
Why are patients being readmitted after gynecologic oncology surgery?
The answer to this question is important as readmission rates are being considered for physician and hospital reimbursement. Dr. MaryAnn Wilbur and colleagues looked at the rates of unplanned 30-day readmission in gyn oncology patients. The patients who were readmitted had the following characteristics: ovarian cancer, creation of ostomy, Charleston score >5, language barrier, and positive discharge screen. Gastrointestinal disturbance and SSI were the most common reasons for readmission, and the total readmission-related costs for these patients was about $4.5 million.
Considering tissue extraction, surgical complications, and cognitive impairment
We then had 3 fabulous oral poster presentations. Dr. Emily Von Bargen and colleagues presented a study entitled, “Prevalence of Occult Pre-malignant or Malignant Pathology at the time of Uterine Morcellation for Benign Disease.” They performed a multicenter retrospective cohort study and found that 1.2% of women had a premalignant or malignant uterine pathology after surgery, with a prevalence of 0.66% of occult malignancy. She was unable to identify risk factors for those patients who had a premalignant or malignant pathology. Overall she found a low prevalence of premalignant or malignant uterine pathology when uterine morcellation was performed for benign disease.
Dr. Alix Leader-Cramer from Northwestern University pointed out “risk factors for a 30-day perioperative complications for total vaginal hysterectomy,” including chronic steroid use, higher ASA classification, current smoking status, diabetes, and lower preoperative serum albumin and sodium levels. Adnexectomy and resident participation were also associated with increased perioperative complications. About 9.5% of patients had a perioperative complication in the study population studied.
Finally, Dr. Elisa Trowbridge pointed out in her talk, on “Cognitive Impairment among Elderly Urogynecologic Patients,” that cognitive impairment is very prevalent among such women aged older than 75 years, and the Mini-Cog is a feasible screening tool.
What a way to end an AMAZING conference!
The conference ended with a fabulous video presentation by Dr. Andrea Benton, entitled “Surgical Approaches to the Management of Bladder and Ureteral Endometriosis.” Overall, the themes of this year’s SGS conference were to:
- emphasize the importance of performing a vaginal hysterectomy when feasible
- continue to strive to balance innovation with experimentation
- ensure that patients are being operated on by surgeons who are competent and frequently performing the indicated procedure.
Thank you to everyone who shared their research to educate the attendees at the conference! I can’t wait until next year!
4/12/16. DAY 3 AT SGS
Vaginal hysterectomy, fecal incontinence, transgender surgery amid tackled topics in Palm Springs
I could get used to sipping my coffee with the sunny background here in Palm Springs! It was a beautiful morning to learn.
We started the day with breakfast and a fabulous lecture on the inferior gluteal neurovascular anatomy in female cadavers, and an insightful lecture by Dr. Bhumy Dave, who brought up concerns about tracking for obstetrics and gynecology residency programs. She specifically highlighted the need for more experience with vaginal hysterectomies for residents who are going to be in a subspecialty that performs vaginal surgery. As the number of hysterectomies, specifically vaginal hysterectomies, declines every year, we need to make sure we are adequately training the physicians who will be performing this procedure in the future. One solution is to have residents join a track their 4th year of residency, after their future career path has been established. Another possible solution would be to increase use of a simulation model for vaginal hysterectomy, as described by Dr. Douglas Miyazaki.
Indigo carmine replacement? A recent issue in gynecologic surgery resulted when there was a national shortage of indigo carmine, which is commonly used to identify UO efflux at the time of cystoscopy. Dr. Katie Propst provided a solution: In the setting of planned cystoscopy, she found that preoperative phenazopyridine is an acceptable alternative and its use led to similar UO identification as with indigo carmine without an increase in complications.
Who should have a vaginal hysterectomy? Dr. Jennifer Schmitt from the Mayo Clinic gave another wonderful lecture describing a decision-tree algorithm for determining the ideal route of hysterectomy. According to a retrospective review of patients at their institution, vaginal hysterectomy was associated with lower infection rates, operative times, and costs.
The highlight of the morning was a very insightful Presidential Address by Dr. Andrew Walter! Not only is a he a very entertaining speaker but he also shed some light on 2 challenges that we currently face in the field. He eloquently stated that, “every woman who is a candidate for a vaginal hysterectomy should be able to get one and have it performed competently, and every woman who needs surgery should have one performed by someone who performs enough surgeries every year to competently perform the surgery.” He frequently alluded to the wise Dr. Mayo, who stated, “The best interest of the patient is the only interest to be considered…” Let’s never forget this as physicians!
Do you find removing the adnexa challenging when performing vaginal surgery? Check out the amazing idea from the Cleveland Clinic shown in video presentation 05! They clearly demonstrate using a single-site gel port or surgical glove placed in the vaginal incision to perform laparoscopy through the vagina. What a novel idea to avoid any abdominal incisions when there is a difficult vaginal adnexal surgery!
After the morning break, we watched a master vaginal surgeon perform a sacral colpopexy through the vaginal route. For details on this method, please reference video presentation 06.
An increase in health care costs is a huge problem in this country. Dr. Mary Van Meter suggested that one area in which we can improve is in the operating room (OR), specifically through decreasing the number of instruments sterilized. She found that only approximately 20% of the instruments on the operating field were actually used by the OR physician. It costs about $3 per instrument on the tray at Vanderbilt University, she said. When you think of the few number of instruments actually used, if we work to limit the number of instruments on the trays, we could cut significant costs. As reimbursement is getting increasingly linked to cost reduction, we all should be thinking about innovative ways to cut costs.
The oral posters were all excellent!
Dr. Ann Peters showed that, at a high-volume tertiary-care center, multimodal preoperative testing failed to definitively identify leiomyosarcoma (LMS), and the factors associated with increased LMS risk included older age, menopause, and presence of fibroids with concurrent pelvic, uterine, or adnexal mass.
Dr. Kevin Kremer found that preoperative antibiotics were used in 23% of cases in which they were not indicated, and the increased use rate was associated with entrance into the abdomen during surgery, the individual surgeon, and time under anesthesia.
Dr. Jessica Heft then stated that the incontinence rate after midurethral sling revision for vaginal exposure or pain was significantly higher with a partial as opposed to complete mesh excision.
Fecal incontinence treatment options
Dr. Peter Rosenblatt tackled the issue of “Innovation or experimentation: Where do we draw the line?” in the TeLinde lecture. He started by describing a condition for which he feels we do not have an acceptable amount of treatment options for patients: fecal incontinence. He described how he observed that the puborectalis muscle, not the external anal sphincter, was critical for fecal continence. He then devised a surgery that created a sling that would act as a synthetic puborectalis muscle. He had amazing success until one patient had a serious complication. Should you abandon a successful, innovative surgery due to one very severe complication, was the question. Where do we draw the line? I don’t have an answer…but I do know that we all need to thank our puborectalis muscle for our daily fecal continence!
This is not a new issue in medicine, explained Dr. Rosenblatt. From the beginning of medicine, there has been a balance between innovation and experimentation. So, what is innovation? It is when someone improves upon a device or process. In medicine, is it innovation or just variation? We frequently use medications and devices “off label,” and these techniques often improve our surgeries and outcomes for our patients. Innovation comes about through careful planning, a necessity created by an emergency, advances in technology, and through evolution of a procedure. Who are the innovators? YOU are! We are the ones who understand the unmet needs and the potential opportunities for improving medicine. Let’s all work together to innovate NOT experiment and make medicine better so our patients have the best care possible!
“New Frontiers in Gynecologic Surgery: Transgender Surgery and Functional Clitoroplasty after Female Genital Mutilation”
The final talk for the day was by the groundbreaking Dr. Marci Bowers, whose lecture started by reminding everyone that there is a difference between gender identity and gender expression. Once a person knows his or her gender identity they use gender expression to express this to the world, she pointed out. Dr. Bowers is a true innovator and is performing gender reassignment surgeries as well as female genital mutilation corrective surgeries. She has perfected a one-stage vaginoplasty, and she showed a video of the procedure, which results in a 90% chance of orgasm. She also noted that she is able to restore clitoral function in 100% of the cases of genital mutilation, and she performs these procedures free of charge.
She reminded us that all ObGyns need to be trained in treating transgender patients, and given the high rate of suicide among adolescent transgender individuals, we all need to work together to provide a supportive medical environment to help these patients. What a fascinating end to the day!
Some relaxation, and entertainment, amid the sun and outdoors
We then had the afternoon to compete in a golf tournament, explore the area on mountain bikes, or lounge by the pool. Regardless of the daytime activities, we all came back together for the great lip-synch competition! All 4 teams gave an impressive performance, but the team from Brown University went home with the win! Such an entertaining event! (Thanks to incoming SGS President Vivian Sung @wih_sung for the pic!)
4/11/16. DAY 2 AT SGS
Experts talk surgical innovations, complementary therapies, value-based payment, and much more at SGS day 2
Wake up and learn! The second day of the 42nd Annual Scientific Meeting of the Society of Gynecologic Surgeons began with a review of research posters at the Poster Session as the sun rose over the Palm Springs mountains. We then moved indoors for the scientific sessions.
In the first lecture, "Reasons for Unplanned 30-Day Readmission After Hysterectomy for Benign Disease," Dr. Courtney Penn and colleagues reiterated that the postsurgical readmission rate is higher for patients undergoing abdominal hysterectomy compared with those who have a laparoscopic or vaginal hysterectomy. Similarly, Dr. Jennifer Schmitt and colleagues suggested that in a patient with a relative contraindication for vaginal hysterectomy, when this procedure is performed by an experienced surgeon there may not be increased complications. However, keep in mind that the study described—"A Comparison of Vaginal and Robotic Hysterectomy for Commonly Cited Relative Contraindications to Vaginal Hysterectomy"—was performed at the Mayo Clinic by expert, highly trained surgeons, and this fact may limit the generalizability of the study. As one audience member eloquently stated, it is important to remember that: "Just because it is feasible does not mean it is the correct procedure."
That yoga or barre class may be doing more good than you think! Lunge, bridge, and cat-into-cow movements may provide a greater degree of pelvic floor muscle unit recruitment than traditional Kegel exercises, according to a presentation by Dr. Bruce Crawford on Kegels versus specialized movement.
Perhaps those exercises should be recommended for surgeons as well. As Dr. Ruchira Singh pointed out, surgeons experience a high amount of musculoskeletal strain when performing vaginal procedures while sitting, regardless of the type of chair used during surgery. Dr. Singh and colleagues’ study, "Effect of Different Chairs on Work-Related Musculoskeletal Discomfort During Vaginal Surgery," found that while the round stool with a backrest and the Capisco chair were more comfortable, they did not eliminate the high risk for musculoskeletal strain, particularly in the head and neck.
Dr. Ann Peters and colleagues, from Magee-Womens Hospital of the University of Pittsburgh Medical Center, gave a fabulous video presentation on "Anatomic and Vascular Considerations in Laparoscopic Uterine Artery Ligation During Hysterectomy."
Need a novel treatment for interstitial cystitis/bladder pain syndrome? Consider mindfulness-based stress reduction. A randomized controlled study performed by Dr. Gregg Kanter and colleagues describes how this technique may help patients and could be considered a first-line therapy.
What is value-based payment and this new trend in reimbursement? And how does it apply to vaginal hysterectomy? Dr. Tina Groat addressed these issues in her keynote lecture. According to the American Congress of Obstetricians and Gynecologists, “Evidence demonstrates that, in general, vaginal hysterectomy is associated with better outcomes and fewer complications than laparoscopic or abdominal hysterectomy.” This is in opposition to what is actually being performed clinically. Dr. Groat explained that United Healthcare decided to incentivize physicians by requiring a prior authorization for all hysterectomies for benign disease. There is both a quality and cost benefit to performing a vaginal hysterectomy. Most insurance companies are moving away from a “fee for service” structure to performance-based payment. Change is always scary and, while I think the overall goal of moving toward the best care for our patients is a positive, this approach may create new challenges for the medical field. What do you think? Is performance-based payment beneficial? Or does it limit physicians and potentially force them to perform a procedure they do not feel as comfortable performing? Will this result in physicians rejecting certain patient populations? [Note from OBG MANAGEMENT: Let Dr. Collins know your thoughts through social media, or email OBG MANAGEMENT with a Letter to the Editor ([email protected]).]
The debate on the best route for hysterectomy continues: According to Dr. Carolyn Swenson and colleagues in their presentation, "Comparison of Robotic and Other Minimally Invasive Routes of Hysterectomy for Benign Indications," while there may be lower complications associated with robotic hysterectomy, the cost of performing a robotic hysterectomy is significantly higher than the cost of laparoscopic or vaginal hysterectomy, thus limiting its utility.
How can we teach a rare surgical procedure to learners? We channel our inner Martha Stewart and make a model out of a beef tongue and chicken. For about $8 a challenging and rare surgery can be taught to residents and medical students, according to the video presentation by Dr. Jana Illston and colleagues, titled "Modified Beef Tongue Model for Fourth-Degree Laceration Repair Simulation."
After the Day 2 lunch break, there was a rousing debate surrounding "Surgeons as Innovators—What Is the Patient Expecting?" Where do we draw the line between using an older more proven therapy as opposed to trying an innovative technology that may actually offer a potential benefit? Dr. Dennis Miller made a good point regarding innovation and pharmaceutical and device companies: If we ignore industry, we lose the ability to help with innovation and shape the future of medical treatments. Perhaps we should use the golden rule: If we would perform the surgery or use the device on ourselves, then we should perform it on our patient. Patients have a greater burden now, because there are more treatment options that they must choose among. Our job as physicians is to educate our patients and to guide them to innovative and evidence-based treatments.
Highlights from the afternoon oral poster session included a presentation by Dr. Caryn Russman that noted the high risk for recurrent urinary tract infection (UTI) after a mid-urethral sling procedure, which seems to be related to specific preoperative risk factors (such as a history of recurrent UTI). Dr. Tanya Hoke suggested that residents and attending physicians have inaccurate estimates of uterine weight, and an educational program may be necessary to improve these estimates. Finally, a study from Massachusetts General Hospital showed that a shorter stay in the hospital, ideally same-day surgery, resulted in a lower complication risk, lower number of emergency department visits, and a decreased readmission rate for patients undergoing urogynecology procedures.
The following recommendations were then suggested regarding vaginal hysterectomy:
- Preoperative prep with 4% chlorhexidine or povidine iodine
- Intracervical vasopressin injection to decrease blood loss
- Use of a pedicle-sealing device for pedicle ligation
- Vertical cuff closure is preferred to maximize vaginal length
Another important point made was that a prior cesarean delivery is not a contraindication to performing a vaginal hysterectomy.
Recommendations regarding recurrent UTI were also made, which include the recommendation for preoperative use of antibiotics to decrease the rate of UTI, with no benefit for a longer course of antibiotics.
News from the Fellows’ Pelvic Research Network
So much exciting research currently is ongoing with the FPRN! New project ideas include comparison of trimethoprim with methenamie for treatment of recurrent UTI; comparison of laparoscopic/robotic sacrocolpopexy with vaginal USLS for management of apical prolapse; a survey study examining surgeon preferences for timing of midurethral sling placement when performed at the time of pelvic organ prolapse (POP) repair; an assessment of the effect of a midurethal sling on overactive bladder in surgical repair in POP; and a study evaluating female pelvic reconstructive surgery in the setting of human immunodeficiency virus infection. It is so great to see the fellows working together to provide groundbreaking research!
Fun with stats
Learning statistics at the end of the day is never easy, but Dr. Matthew Barber did a fabulous job explaining this often-confusing topic. He reminded attendees that the key to learning statistics is repetition. One new recommendation he offered to enhance understanding is to use common language instead of numbers for P values. For example, instead of saying P <.001, use “is superior,” and instead of saying P = .3, use “seems not superior” or “inconclusive.”
A night to remember
The night ended with a wonderful awards ceremony and the president’s reception. Overall, day 2 was a very educational—and fun—day!
4/10/16. DAY 1 AT SGS
Postgrad courses address pain management and social media education
Mastering pelvic pain
With the beautiful Palm Springs, California, backdrop of mountains and palm trees, the 2016 meeting of the Society of Gynecologic Surgeons kicked off with 4 postgraduate courses. At the “Mastering Pelvic Pain: Strategies and Techniques for a Multimodal Approach” course, directed by Dr. Cara King, Dr. Matt Siedhoff explained the key components of the history and physical examination: Keep in mind that this generally is a multifactorial issue and may require a multidisciplinary approach, he told attendees. It is also important to make sure that the patient is fully prepared to combat the chronic pelvic pain (CPP) symptoms by focusing on the fundamentals, he said, including smoking, diet, exercise, weight loss, sleep, and relationship stress.
How is your posture? It turns out that something as simple as altering your posture can significantly affect pelvic floor control. Carol Sobeck, PT, demonstrated this importance with a simple group exercise that proved how the ability to contract the pelvic floor changes significantly with posture. Get to know your pelvic floor physical therapist—they are critical in helping with the treatment of CPP.
But what to do when physical therapy fails? Dr. Jennifer Gunter shed some light on this very difficult medical situation. One suggestion, she said, is to consider the possibility of a local presentation of a systemic issue and screen for the following: fibromyalgia, Ehlers-Danlos syndrome, statin use, and diabetes mellitus. Other treatment ideas include trigger point injections and onabotulinumtoxin A (Botox) therapy. A trigger point is a hypercontracted focus of muscle. To treat a trigger point, a needle must mechanically disrupt the trigger point to reduce the pelvic pain, and injection of local anesthetic is only used to help with postprocedure discomfort. Botox in my pelvis, you ask? Yes! The goal is to block presynaptic release of acetylcholine, which is very effective at reducing muscle spasm.
Tackling endometriosis. Dr. King then described surgical techniques for endometriosis. She recommended that, for mild disease not in close proximity to vital structures, ablation is likely equivalent to excision. For deeply infiltrating endometriosis, or lesions in close proximity to vital structures, excision is more beneficial. Excision is always beneficial for pathologic diagnosis, she said. And she offered this tip for bladder endometriosis: place a stitch in the nodule to allow for counter traction. Here is another tip, for ovarian cystectomy: inject vasopressin (20 U in 60 mL) to help dissect the plane between the ovary and cyst wall, and consider presacral neurectomy for midline pain.
Dr. Frank Tu then explained that we should not think of it as the “terrible triad: endometriosis, irritable bowel syndrome, and interstitial cystsis,” but rather as a system out of balance. Both peripheral and central mechanisms are involved in the generation and maintenance of cross-organ sensitization, he pointed out, which may explain why patients receive multiple diagnoses that describe a myriad of complaints due to a lack of overall homeostasis.
While Dr. Alaa Abd-Elsayed described innovative nerve blocks for CPP, he emphasized that no one specific treatment will result in a complete resolution of symptoms.
Vaginal mesh placement and pain. Dr. Mario Castellanos gave a fabulous lecture describing the pain issues that surround vaginal mesh placement. Interestingly, he noted that many patients had pelvic pain prior to placement of vaginal mesh, and that pain likely only will worsen with mesh placement. Mesh may cause pain by causing inflammation, visceral injury, muscle injury, and nerve injury. While there are guidelines for where mesh should be placed for bladder slings (TVT and TOT) and for prolapse repair, studies show, he pointed out, that the mesh placement often disrupts several muscle groups and may directly injure a nerve.
Alternatives to the typical diagnoses for CPP. The morning ended with a lecture by Dr. Suzie As-Sanie, in which she reminded everyone to start with “gold standard” therapy but, if those fail, consider alternative diagnoses or a central pain disorder. She also suggested cognitive behavioral therapy for treatment of CPP. For patients who cannot afford cognitive behavioral therapy, there is a free online source at fibroguide.med.umich.edu. Other alternative treatment options include exercise, patient education, instruction on sleep hygiene, and neurostimulatory therapies.
Overall, all the speakers this morning agreed that CPP is rarely treated with one modality, and it is best treated with a multidisciplinary approach.
Let’s get social!
The afternoon was spent learning about ways to use social networking sites to educate our patients, in another postgraduate course on “Making Media Social,” presented by the SGS Social Media Committee. Given that a recent survey demonstrated that 74.1% of women have some type of social networking account, it can be a very useful source for medical information for patients. If you have any questions about what is appropriate on social media, check out ACOG committee opinion 622.
Here are some tips that I learned:
Facebook:
- Keep your private Facebook page private and create a separate professional Facebook page
- Adjust your login setting so only administrators can post on your professional page
- Adjust settings so your professional profile cannot be “tagged”
- If a patient contacts your personal page, direct them to your professional page
- Do not give medical advice since Facebook is not digitally encrypted
Twitter:
- “Short bursts of inconsequential information”
- Follow societies, medical centers, and medical journals
LinkedIn:
- It provides a great way to find jobs, people, and business opportunities that are recommended by someone in your contact group
- It is specifically there to help you grow your business and show people who you are and where you have come from
Doximity:
Before we get to tips, first, what is it? Well, Doximity is basically a “LinkedIn” for physicians. It is “a way to find relevant specialists for patients; a rolodex; an email and text service; and a virtual lounge.” It is currently transforming from social network into a ‘platform.’ Now more than 1,000 hospitals and health systems are part of Doximity. Tips:
- There is a secure message option, which is HIPAA compliant
- Provides a way for residents and fellows to understand possible future places of employment
- It is a great way to find someone to refer patients to in an unfamiliar location
Vimeo:
- A benefit over YouTube is the lack of advertisements
- Check out the SGS video archives!
- You also can set privacy settings and embedding stats
Fifty-nine percent of US adults have looked online for health information in the past year. Therefore, it is important for physicians to get good information out for people to see!
A relaxing day’s end
The night ended with a beautiful poolside reception! I can’t wait to see what day 2 will bring!
4/13/16. DAY 4 AT SGS
A jam-packed day of sessions, posters, awards, and clinical updates
Our last educational day was kicked off by a fascinating lecture by Dr. Amy Park on the “Genetic Determinants of Pelvic Organ Prolapse in Women of European American Descent: The Women’s Health Initiative.” Dr. Park and her colleagues found that there is evidence of phenotypic and genotypic heterogeneity in patients with pelvic organ prolapse, and there were 4 genetic loci identified that correlated with prolapse. Any uterine prolapse was associated with a genome-wide significant intergenic variant on chromosome 13, cystocele was associated with LOXL2, and all prolapse was associated with BMP.
Surmounting surgical site infection
Dr. Sarah Andiman then gave a lecture on the effects of a perioperative bundle and offered timely feedback for surgical site infection (SSI) prevention in hysterectomy. We all know that wound infections are a major morbidity associated with surgery, and Dr. Andiman’s group at Yale found that, by implementing this prevention program, the surgical site infection decreased 52.8%. Another suggestion from the audience was to have all patients use chlorhexidine wipes the night before and morning of a surgery to decrease wound infection. Similarly, Dr. Ali Bazzi gave a lecture on “Chlorhexidine-Alcohol Compared with Povidone-Iodine for Surgical-Site Antisepsis after Abdominal Hysterectomy.” The chlorhexidine was associated with 30% lower odds of SSI compared with povidone-iodine, even though this group had several medical comorbidities and risk factors known for SSIs.
Oral poster presenters make several interesting points
Dr. Christopher Ripperda from UT Southwestern Medical Center in Dallas, Texas, found that medical comorbidities and the presence of detrusor overactivity and PVR are predictors of early postoperative voiding dysfunction following a midurethral sling placement. Dr. Nabila Noor then described a fabulous surgical alternative to the use of morcellation to remove the uterus after a supracervical hysterectomy. She described the technique for performing a posterior colpotomy and stated that patients who had a surgery performed at her institution using this technique did not experience increased postoperative pain or longer postoperative stay.
Dr. Jennifer Thompson then shed some light on a very important question related to the Sunshine Act: Are physicians including all of their disclosures when they submit articles to a conference? When the physicians who submitted an abstract to the Society of Gynecologic Surgeons (SGS) in 2015 were searched on the Centers for Medicare and Medicaid Services (CMS) website, 62% of them had incomplete disclosures, with a total of nondisclosed CMS transactions equaling $1.3 million. We can do better!
Status update: The FPRN and passing of the torch
Congratulations to Dr. Kristin Jacobs, the new Fellows’ Pelvic Research Network (FPRN) Chair! The morning session ended with an innovative video from Dr. Janet Li and colleagues on the “Use of Suprapubic Carter-Thomason Needle to Assist in Cystoscopic Excision of Intravesical Foreign Object.”
The winner of the Distinguished Surgeon Award was given to Dr. Javier Magrina, and the SGS gavel was passed on to Dr. Vivian Sung! Congratulations!
Dr. Stephanie Pickett kicked off the eighth scientific session with a study entitled, “Comparing Methods of NSAID Delivery for Postoperative Pain.” When she and her colleagues compared IV toradol to IV ibuprofen for postoperative pain control after urogynecologic surgery, they found that patients experienced similar rates of pain control and satisfaction regardless of the type of analgesia.
Why are patients being readmitted after gynecologic oncology surgery?
The answer to this question is important as readmission rates are being considered for physician and hospital reimbursement. Dr. MaryAnn Wilbur and colleagues looked at the rates of unplanned 30-day readmission in gyn oncology patients. The patients who were readmitted had the following characteristics: ovarian cancer, creation of ostomy, Charleston score >5, language barrier, and positive discharge screen. Gastrointestinal disturbance and SSI were the most common reasons for readmission, and the total readmission-related costs for these patients was about $4.5 million.
Considering tissue extraction, surgical complications, and cognitive impairment
We then had 3 fabulous oral poster presentations. Dr. Emily Von Bargen and colleagues presented a study entitled, “Prevalence of Occult Pre-malignant or Malignant Pathology at the time of Uterine Morcellation for Benign Disease.” They performed a multicenter retrospective cohort study and found that 1.2% of women had a premalignant or malignant uterine pathology after surgery, with a prevalence of 0.66% of occult malignancy. She was unable to identify risk factors for those patients who had a premalignant or malignant pathology. Overall she found a low prevalence of premalignant or malignant uterine pathology when uterine morcellation was performed for benign disease.
Dr. Alix Leader-Cramer from Northwestern University pointed out “risk factors for a 30-day perioperative complications for total vaginal hysterectomy,” including chronic steroid use, higher ASA classification, current smoking status, diabetes, and lower preoperative serum albumin and sodium levels. Adnexectomy and resident participation were also associated with increased perioperative complications. About 9.5% of patients had a perioperative complication in the study population studied.
Finally, Dr. Elisa Trowbridge pointed out in her talk, on “Cognitive Impairment among Elderly Urogynecologic Patients,” that cognitive impairment is very prevalent among such women aged older than 75 years, and the Mini-Cog is a feasible screening tool.
What a way to end an AMAZING conference!
The conference ended with a fabulous video presentation by Dr. Andrea Benton, entitled “Surgical Approaches to the Management of Bladder and Ureteral Endometriosis.” Overall, the themes of this year’s SGS conference were to:
- emphasize the importance of performing a vaginal hysterectomy when feasible
- continue to strive to balance innovation with experimentation
- ensure that patients are being operated on by surgeons who are competent and frequently performing the indicated procedure.
Thank you to everyone who shared their research to educate the attendees at the conference! I can’t wait until next year!
4/12/16. DAY 3 AT SGS
Vaginal hysterectomy, fecal incontinence, transgender surgery amid tackled topics in Palm Springs
I could get used to sipping my coffee with the sunny background here in Palm Springs! It was a beautiful morning to learn.
We started the day with breakfast and a fabulous lecture on the inferior gluteal neurovascular anatomy in female cadavers, and an insightful lecture by Dr. Bhumy Dave, who brought up concerns about tracking for obstetrics and gynecology residency programs. She specifically highlighted the need for more experience with vaginal hysterectomies for residents who are going to be in a subspecialty that performs vaginal surgery. As the number of hysterectomies, specifically vaginal hysterectomies, declines every year, we need to make sure we are adequately training the physicians who will be performing this procedure in the future. One solution is to have residents join a track their 4th year of residency, after their future career path has been established. Another possible solution would be to increase use of a simulation model for vaginal hysterectomy, as described by Dr. Douglas Miyazaki.
Indigo carmine replacement? A recent issue in gynecologic surgery resulted when there was a national shortage of indigo carmine, which is commonly used to identify UO efflux at the time of cystoscopy. Dr. Katie Propst provided a solution: In the setting of planned cystoscopy, she found that preoperative phenazopyridine is an acceptable alternative and its use led to similar UO identification as with indigo carmine without an increase in complications.
Who should have a vaginal hysterectomy? Dr. Jennifer Schmitt from the Mayo Clinic gave another wonderful lecture describing a decision-tree algorithm for determining the ideal route of hysterectomy. According to a retrospective review of patients at their institution, vaginal hysterectomy was associated with lower infection rates, operative times, and costs.
The highlight of the morning was a very insightful Presidential Address by Dr. Andrew Walter! Not only is a he a very entertaining speaker but he also shed some light on 2 challenges that we currently face in the field. He eloquently stated that, “every woman who is a candidate for a vaginal hysterectomy should be able to get one and have it performed competently, and every woman who needs surgery should have one performed by someone who performs enough surgeries every year to competently perform the surgery.” He frequently alluded to the wise Dr. Mayo, who stated, “The best interest of the patient is the only interest to be considered…” Let’s never forget this as physicians!
Do you find removing the adnexa challenging when performing vaginal surgery? Check out the amazing idea from the Cleveland Clinic shown in video presentation 05! They clearly demonstrate using a single-site gel port or surgical glove placed in the vaginal incision to perform laparoscopy through the vagina. What a novel idea to avoid any abdominal incisions when there is a difficult vaginal adnexal surgery!
After the morning break, we watched a master vaginal surgeon perform a sacral colpopexy through the vaginal route. For details on this method, please reference video presentation 06.
An increase in health care costs is a huge problem in this country. Dr. Mary Van Meter suggested that one area in which we can improve is in the operating room (OR), specifically through decreasing the number of instruments sterilized. She found that only approximately 20% of the instruments on the operating field were actually used by the OR physician. It costs about $3 per instrument on the tray at Vanderbilt University, she said. When you think of the few number of instruments actually used, if we work to limit the number of instruments on the trays, we could cut significant costs. As reimbursement is getting increasingly linked to cost reduction, we all should be thinking about innovative ways to cut costs.
The oral posters were all excellent!
Dr. Ann Peters showed that, at a high-volume tertiary-care center, multimodal preoperative testing failed to definitively identify leiomyosarcoma (LMS), and the factors associated with increased LMS risk included older age, menopause, and presence of fibroids with concurrent pelvic, uterine, or adnexal mass.
Dr. Kevin Kremer found that preoperative antibiotics were used in 23% of cases in which they were not indicated, and the increased use rate was associated with entrance into the abdomen during surgery, the individual surgeon, and time under anesthesia.
Dr. Jessica Heft then stated that the incontinence rate after midurethral sling revision for vaginal exposure or pain was significantly higher with a partial as opposed to complete mesh excision.
Fecal incontinence treatment options
Dr. Peter Rosenblatt tackled the issue of “Innovation or experimentation: Where do we draw the line?” in the TeLinde lecture. He started by describing a condition for which he feels we do not have an acceptable amount of treatment options for patients: fecal incontinence. He described how he observed that the puborectalis muscle, not the external anal sphincter, was critical for fecal continence. He then devised a surgery that created a sling that would act as a synthetic puborectalis muscle. He had amazing success until one patient had a serious complication. Should you abandon a successful, innovative surgery due to one very severe complication, was the question. Where do we draw the line? I don’t have an answer…but I do know that we all need to thank our puborectalis muscle for our daily fecal continence!
This is not a new issue in medicine, explained Dr. Rosenblatt. From the beginning of medicine, there has been a balance between innovation and experimentation. So, what is innovation? It is when someone improves upon a device or process. In medicine, is it innovation or just variation? We frequently use medications and devices “off label,” and these techniques often improve our surgeries and outcomes for our patients. Innovation comes about through careful planning, a necessity created by an emergency, advances in technology, and through evolution of a procedure. Who are the innovators? YOU are! We are the ones who understand the unmet needs and the potential opportunities for improving medicine. Let’s all work together to innovate NOT experiment and make medicine better so our patients have the best care possible!
“New Frontiers in Gynecologic Surgery: Transgender Surgery and Functional Clitoroplasty after Female Genital Mutilation”
The final talk for the day was by the groundbreaking Dr. Marci Bowers, whose lecture started by reminding everyone that there is a difference between gender identity and gender expression. Once a person knows his or her gender identity they use gender expression to express this to the world, she pointed out. Dr. Bowers is a true innovator and is performing gender reassignment surgeries as well as female genital mutilation corrective surgeries. She has perfected a one-stage vaginoplasty, and she showed a video of the procedure, which results in a 90% chance of orgasm. She also noted that she is able to restore clitoral function in 100% of the cases of genital mutilation, and she performs these procedures free of charge.
She reminded us that all ObGyns need to be trained in treating transgender patients, and given the high rate of suicide among adolescent transgender individuals, we all need to work together to provide a supportive medical environment to help these patients. What a fascinating end to the day!
Some relaxation, and entertainment, amid the sun and outdoors
We then had the afternoon to compete in a golf tournament, explore the area on mountain bikes, or lounge by the pool. Regardless of the daytime activities, we all came back together for the great lip-synch competition! All 4 teams gave an impressive performance, but the team from Brown University went home with the win! Such an entertaining event! (Thanks to incoming SGS President Vivian Sung @wih_sung for the pic!)
4/11/16. DAY 2 AT SGS
Experts talk surgical innovations, complementary therapies, value-based payment, and much more at SGS day 2
Wake up and learn! The second day of the 42nd Annual Scientific Meeting of the Society of Gynecologic Surgeons began with a review of research posters at the Poster Session as the sun rose over the Palm Springs mountains. We then moved indoors for the scientific sessions.
In the first lecture, "Reasons for Unplanned 30-Day Readmission After Hysterectomy for Benign Disease," Dr. Courtney Penn and colleagues reiterated that the postsurgical readmission rate is higher for patients undergoing abdominal hysterectomy compared with those who have a laparoscopic or vaginal hysterectomy. Similarly, Dr. Jennifer Schmitt and colleagues suggested that in a patient with a relative contraindication for vaginal hysterectomy, when this procedure is performed by an experienced surgeon there may not be increased complications. However, keep in mind that the study described—"A Comparison of Vaginal and Robotic Hysterectomy for Commonly Cited Relative Contraindications to Vaginal Hysterectomy"—was performed at the Mayo Clinic by expert, highly trained surgeons, and this fact may limit the generalizability of the study. As one audience member eloquently stated, it is important to remember that: "Just because it is feasible does not mean it is the correct procedure."
That yoga or barre class may be doing more good than you think! Lunge, bridge, and cat-into-cow movements may provide a greater degree of pelvic floor muscle unit recruitment than traditional Kegel exercises, according to a presentation by Dr. Bruce Crawford on Kegels versus specialized movement.
Perhaps those exercises should be recommended for surgeons as well. As Dr. Ruchira Singh pointed out, surgeons experience a high amount of musculoskeletal strain when performing vaginal procedures while sitting, regardless of the type of chair used during surgery. Dr. Singh and colleagues’ study, "Effect of Different Chairs on Work-Related Musculoskeletal Discomfort During Vaginal Surgery," found that while the round stool with a backrest and the Capisco chair were more comfortable, they did not eliminate the high risk for musculoskeletal strain, particularly in the head and neck.
Dr. Ann Peters and colleagues, from Magee-Womens Hospital of the University of Pittsburgh Medical Center, gave a fabulous video presentation on "Anatomic and Vascular Considerations in Laparoscopic Uterine Artery Ligation During Hysterectomy."
Need a novel treatment for interstitial cystitis/bladder pain syndrome? Consider mindfulness-based stress reduction. A randomized controlled study performed by Dr. Gregg Kanter and colleagues describes how this technique may help patients and could be considered a first-line therapy.
What is value-based payment and this new trend in reimbursement? And how does it apply to vaginal hysterectomy? Dr. Tina Groat addressed these issues in her keynote lecture. According to the American Congress of Obstetricians and Gynecologists, “Evidence demonstrates that, in general, vaginal hysterectomy is associated with better outcomes and fewer complications than laparoscopic or abdominal hysterectomy.” This is in opposition to what is actually being performed clinically. Dr. Groat explained that United Healthcare decided to incentivize physicians by requiring a prior authorization for all hysterectomies for benign disease. There is both a quality and cost benefit to performing a vaginal hysterectomy. Most insurance companies are moving away from a “fee for service” structure to performance-based payment. Change is always scary and, while I think the overall goal of moving toward the best care for our patients is a positive, this approach may create new challenges for the medical field. What do you think? Is performance-based payment beneficial? Or does it limit physicians and potentially force them to perform a procedure they do not feel as comfortable performing? Will this result in physicians rejecting certain patient populations? [Note from OBG MANAGEMENT: Let Dr. Collins know your thoughts through social media, or email OBG MANAGEMENT with a Letter to the Editor ([email protected]).]
The debate on the best route for hysterectomy continues: According to Dr. Carolyn Swenson and colleagues in their presentation, "Comparison of Robotic and Other Minimally Invasive Routes of Hysterectomy for Benign Indications," while there may be lower complications associated with robotic hysterectomy, the cost of performing a robotic hysterectomy is significantly higher than the cost of laparoscopic or vaginal hysterectomy, thus limiting its utility.
How can we teach a rare surgical procedure to learners? We channel our inner Martha Stewart and make a model out of a beef tongue and chicken. For about $8 a challenging and rare surgery can be taught to residents and medical students, according to the video presentation by Dr. Jana Illston and colleagues, titled "Modified Beef Tongue Model for Fourth-Degree Laceration Repair Simulation."
After the Day 2 lunch break, there was a rousing debate surrounding "Surgeons as Innovators—What Is the Patient Expecting?" Where do we draw the line between using an older more proven therapy as opposed to trying an innovative technology that may actually offer a potential benefit? Dr. Dennis Miller made a good point regarding innovation and pharmaceutical and device companies: If we ignore industry, we lose the ability to help with innovation and shape the future of medical treatments. Perhaps we should use the golden rule: If we would perform the surgery or use the device on ourselves, then we should perform it on our patient. Patients have a greater burden now, because there are more treatment options that they must choose among. Our job as physicians is to educate our patients and to guide them to innovative and evidence-based treatments.
Highlights from the afternoon oral poster session included a presentation by Dr. Caryn Russman that noted the high risk for recurrent urinary tract infection (UTI) after a mid-urethral sling procedure, which seems to be related to specific preoperative risk factors (such as a history of recurrent UTI). Dr. Tanya Hoke suggested that residents and attending physicians have inaccurate estimates of uterine weight, and an educational program may be necessary to improve these estimates. Finally, a study from Massachusetts General Hospital showed that a shorter stay in the hospital, ideally same-day surgery, resulted in a lower complication risk, lower number of emergency department visits, and a decreased readmission rate for patients undergoing urogynecology procedures.
The following recommendations were then suggested regarding vaginal hysterectomy:
- Preoperative prep with 4% chlorhexidine or povidine iodine
- Intracervical vasopressin injection to decrease blood loss
- Use of a pedicle-sealing device for pedicle ligation
- Vertical cuff closure is preferred to maximize vaginal length
Another important point made was that a prior cesarean delivery is not a contraindication to performing a vaginal hysterectomy.
Recommendations regarding recurrent UTI were also made, which include the recommendation for preoperative use of antibiotics to decrease the rate of UTI, with no benefit for a longer course of antibiotics.
News from the Fellows’ Pelvic Research Network
So much exciting research currently is ongoing with the FPRN! New project ideas include comparison of trimethoprim with methenamie for treatment of recurrent UTI; comparison of laparoscopic/robotic sacrocolpopexy with vaginal USLS for management of apical prolapse; a survey study examining surgeon preferences for timing of midurethral sling placement when performed at the time of pelvic organ prolapse (POP) repair; an assessment of the effect of a midurethal sling on overactive bladder in surgical repair in POP; and a study evaluating female pelvic reconstructive surgery in the setting of human immunodeficiency virus infection. It is so great to see the fellows working together to provide groundbreaking research!
Fun with stats
Learning statistics at the end of the day is never easy, but Dr. Matthew Barber did a fabulous job explaining this often-confusing topic. He reminded attendees that the key to learning statistics is repetition. One new recommendation he offered to enhance understanding is to use common language instead of numbers for P values. For example, instead of saying P <.001, use “is superior,” and instead of saying P = .3, use “seems not superior” or “inconclusive.”
A night to remember
The night ended with a wonderful awards ceremony and the president’s reception. Overall, day 2 was a very educational—and fun—day!
4/10/16. DAY 1 AT SGS
Postgrad courses address pain management and social media education
Mastering pelvic pain
With the beautiful Palm Springs, California, backdrop of mountains and palm trees, the 2016 meeting of the Society of Gynecologic Surgeons kicked off with 4 postgraduate courses. At the “Mastering Pelvic Pain: Strategies and Techniques for a Multimodal Approach” course, directed by Dr. Cara King, Dr. Matt Siedhoff explained the key components of the history and physical examination: Keep in mind that this generally is a multifactorial issue and may require a multidisciplinary approach, he told attendees. It is also important to make sure that the patient is fully prepared to combat the chronic pelvic pain (CPP) symptoms by focusing on the fundamentals, he said, including smoking, diet, exercise, weight loss, sleep, and relationship stress.
How is your posture? It turns out that something as simple as altering your posture can significantly affect pelvic floor control. Carol Sobeck, PT, demonstrated this importance with a simple group exercise that proved how the ability to contract the pelvic floor changes significantly with posture. Get to know your pelvic floor physical therapist—they are critical in helping with the treatment of CPP.
But what to do when physical therapy fails? Dr. Jennifer Gunter shed some light on this very difficult medical situation. One suggestion, she said, is to consider the possibility of a local presentation of a systemic issue and screen for the following: fibromyalgia, Ehlers-Danlos syndrome, statin use, and diabetes mellitus. Other treatment ideas include trigger point injections and onabotulinumtoxin A (Botox) therapy. A trigger point is a hypercontracted focus of muscle. To treat a trigger point, a needle must mechanically disrupt the trigger point to reduce the pelvic pain, and injection of local anesthetic is only used to help with postprocedure discomfort. Botox in my pelvis, you ask? Yes! The goal is to block presynaptic release of acetylcholine, which is very effective at reducing muscle spasm.
Tackling endometriosis. Dr. King then described surgical techniques for endometriosis. She recommended that, for mild disease not in close proximity to vital structures, ablation is likely equivalent to excision. For deeply infiltrating endometriosis, or lesions in close proximity to vital structures, excision is more beneficial. Excision is always beneficial for pathologic diagnosis, she said. And she offered this tip for bladder endometriosis: place a stitch in the nodule to allow for counter traction. Here is another tip, for ovarian cystectomy: inject vasopressin (20 U in 60 mL) to help dissect the plane between the ovary and cyst wall, and consider presacral neurectomy for midline pain.
Dr. Frank Tu then explained that we should not think of it as the “terrible triad: endometriosis, irritable bowel syndrome, and interstitial cystsis,” but rather as a system out of balance. Both peripheral and central mechanisms are involved in the generation and maintenance of cross-organ sensitization, he pointed out, which may explain why patients receive multiple diagnoses that describe a myriad of complaints due to a lack of overall homeostasis.
While Dr. Alaa Abd-Elsayed described innovative nerve blocks for CPP, he emphasized that no one specific treatment will result in a complete resolution of symptoms.
Vaginal mesh placement and pain. Dr. Mario Castellanos gave a fabulous lecture describing the pain issues that surround vaginal mesh placement. Interestingly, he noted that many patients had pelvic pain prior to placement of vaginal mesh, and that pain likely only will worsen with mesh placement. Mesh may cause pain by causing inflammation, visceral injury, muscle injury, and nerve injury. While there are guidelines for where mesh should be placed for bladder slings (TVT and TOT) and for prolapse repair, studies show, he pointed out, that the mesh placement often disrupts several muscle groups and may directly injure a nerve.
Alternatives to the typical diagnoses for CPP. The morning ended with a lecture by Dr. Suzie As-Sanie, in which she reminded everyone to start with “gold standard” therapy but, if those fail, consider alternative diagnoses or a central pain disorder. She also suggested cognitive behavioral therapy for treatment of CPP. For patients who cannot afford cognitive behavioral therapy, there is a free online source at fibroguide.med.umich.edu. Other alternative treatment options include exercise, patient education, instruction on sleep hygiene, and neurostimulatory therapies.
Overall, all the speakers this morning agreed that CPP is rarely treated with one modality, and it is best treated with a multidisciplinary approach.
Let’s get social!
The afternoon was spent learning about ways to use social networking sites to educate our patients, in another postgraduate course on “Making Media Social,” presented by the SGS Social Media Committee. Given that a recent survey demonstrated that 74.1% of women have some type of social networking account, it can be a very useful source for medical information for patients. If you have any questions about what is appropriate on social media, check out ACOG committee opinion 622.
Here are some tips that I learned:
Facebook:
- Keep your private Facebook page private and create a separate professional Facebook page
- Adjust your login setting so only administrators can post on your professional page
- Adjust settings so your professional profile cannot be “tagged”
- If a patient contacts your personal page, direct them to your professional page
- Do not give medical advice since Facebook is not digitally encrypted
Twitter:
- “Short bursts of inconsequential information”
- Follow societies, medical centers, and medical journals
LinkedIn:
- It provides a great way to find jobs, people, and business opportunities that are recommended by someone in your contact group
- It is specifically there to help you grow your business and show people who you are and where you have come from
Doximity:
Before we get to tips, first, what is it? Well, Doximity is basically a “LinkedIn” for physicians. It is “a way to find relevant specialists for patients; a rolodex; an email and text service; and a virtual lounge.” It is currently transforming from social network into a ‘platform.’ Now more than 1,000 hospitals and health systems are part of Doximity. Tips:
- There is a secure message option, which is HIPAA compliant
- Provides a way for residents and fellows to understand possible future places of employment
- It is a great way to find someone to refer patients to in an unfamiliar location
Vimeo:
- A benefit over YouTube is the lack of advertisements
- Check out the SGS video archives!
- You also can set privacy settings and embedding stats
Fifty-nine percent of US adults have looked online for health information in the past year. Therefore, it is important for physicians to get good information out for people to see!
A relaxing day’s end
The night ended with a beautiful poolside reception! I can’t wait to see what day 2 will bring!
4/13/16. DAY 4 AT SGS
A jam-packed day of sessions, posters, awards, and clinical updates
Our last educational day was kicked off by a fascinating lecture by Dr. Amy Park on the “Genetic Determinants of Pelvic Organ Prolapse in Women of European American Descent: The Women’s Health Initiative.” Dr. Park and her colleagues found that there is evidence of phenotypic and genotypic heterogeneity in patients with pelvic organ prolapse, and there were 4 genetic loci identified that correlated with prolapse. Any uterine prolapse was associated with a genome-wide significant intergenic variant on chromosome 13, cystocele was associated with LOXL2, and all prolapse was associated with BMP.
Surmounting surgical site infection
Dr. Sarah Andiman then gave a lecture on the effects of a perioperative bundle and offered timely feedback for surgical site infection (SSI) prevention in hysterectomy. We all know that wound infections are a major morbidity associated with surgery, and Dr. Andiman’s group at Yale found that, by implementing this prevention program, the surgical site infection decreased 52.8%. Another suggestion from the audience was to have all patients use chlorhexidine wipes the night before and morning of a surgery to decrease wound infection. Similarly, Dr. Ali Bazzi gave a lecture on “Chlorhexidine-Alcohol Compared with Povidone-Iodine for Surgical-Site Antisepsis after Abdominal Hysterectomy.” The chlorhexidine was associated with 30% lower odds of SSI compared with povidone-iodine, even though this group had several medical comorbidities and risk factors known for SSIs.
Oral poster presenters make several interesting points
Dr. Christopher Ripperda from UT Southwestern Medical Center in Dallas, Texas, found that medical comorbidities and the presence of detrusor overactivity and PVR are predictors of early postoperative voiding dysfunction following a midurethral sling placement. Dr. Nabila Noor then described a fabulous surgical alternative to the use of morcellation to remove the uterus after a supracervical hysterectomy. She described the technique for performing a posterior colpotomy and stated that patients who had a surgery performed at her institution using this technique did not experience increased postoperative pain or longer postoperative stay.
Dr. Jennifer Thompson then shed some light on a very important question related to the Sunshine Act: Are physicians including all of their disclosures when they submit articles to a conference? When the physicians who submitted an abstract to the Society of Gynecologic Surgeons (SGS) in 2015 were searched on the Centers for Medicare and Medicaid Services (CMS) website, 62% of them had incomplete disclosures, with a total of nondisclosed CMS transactions equaling $1.3 million. We can do better!
Status update: The FPRN and passing of the torch
Congratulations to Dr. Kristin Jacobs, the new Fellows’ Pelvic Research Network (FPRN) Chair! The morning session ended with an innovative video from Dr. Janet Li and colleagues on the “Use of Suprapubic Carter-Thomason Needle to Assist in Cystoscopic Excision of Intravesical Foreign Object.”
The winner of the Distinguished Surgeon Award was given to Dr. Javier Magrina, and the SGS gavel was passed on to Dr. Vivian Sung! Congratulations!
Dr. Stephanie Pickett kicked off the eighth scientific session with a study entitled, “Comparing Methods of NSAID Delivery for Postoperative Pain.” When she and her colleagues compared IV toradol to IV ibuprofen for postoperative pain control after urogynecologic surgery, they found that patients experienced similar rates of pain control and satisfaction regardless of the type of analgesia.
Why are patients being readmitted after gynecologic oncology surgery?
The answer to this question is important as readmission rates are being considered for physician and hospital reimbursement. Dr. MaryAnn Wilbur and colleagues looked at the rates of unplanned 30-day readmission in gyn oncology patients. The patients who were readmitted had the following characteristics: ovarian cancer, creation of ostomy, Charleston score >5, language barrier, and positive discharge screen. Gastrointestinal disturbance and SSI were the most common reasons for readmission, and the total readmission-related costs for these patients was about $4.5 million.
Considering tissue extraction, surgical complications, and cognitive impairment
We then had 3 fabulous oral poster presentations. Dr. Emily Von Bargen and colleagues presented a study entitled, “Prevalence of Occult Pre-malignant or Malignant Pathology at the time of Uterine Morcellation for Benign Disease.” They performed a multicenter retrospective cohort study and found that 1.2% of women had a premalignant or malignant uterine pathology after surgery, with a prevalence of 0.66% of occult malignancy. She was unable to identify risk factors for those patients who had a premalignant or malignant pathology. Overall she found a low prevalence of premalignant or malignant uterine pathology when uterine morcellation was performed for benign disease.
Dr. Alix Leader-Cramer from Northwestern University pointed out “risk factors for a 30-day perioperative complications for total vaginal hysterectomy,” including chronic steroid use, higher ASA classification, current smoking status, diabetes, and lower preoperative serum albumin and sodium levels. Adnexectomy and resident participation were also associated with increased perioperative complications. About 9.5% of patients had a perioperative complication in the study population studied.
Finally, Dr. Elisa Trowbridge pointed out in her talk, on “Cognitive Impairment among Elderly Urogynecologic Patients,” that cognitive impairment is very prevalent among such women aged older than 75 years, and the Mini-Cog is a feasible screening tool.
What a way to end an AMAZING conference!
The conference ended with a fabulous video presentation by Dr. Andrea Benton, entitled “Surgical Approaches to the Management of Bladder and Ureteral Endometriosis.” Overall, the themes of this year’s SGS conference were to:
- emphasize the importance of performing a vaginal hysterectomy when feasible
- continue to strive to balance innovation with experimentation
- ensure that patients are being operated on by surgeons who are competent and frequently performing the indicated procedure.
Thank you to everyone who shared their research to educate the attendees at the conference! I can’t wait until next year!
4/12/16. DAY 3 AT SGS
Vaginal hysterectomy, fecal incontinence, transgender surgery amid tackled topics in Palm Springs
I could get used to sipping my coffee with the sunny background here in Palm Springs! It was a beautiful morning to learn.
We started the day with breakfast and a fabulous lecture on the inferior gluteal neurovascular anatomy in female cadavers, and an insightful lecture by Dr. Bhumy Dave, who brought up concerns about tracking for obstetrics and gynecology residency programs. She specifically highlighted the need for more experience with vaginal hysterectomies for residents who are going to be in a subspecialty that performs vaginal surgery. As the number of hysterectomies, specifically vaginal hysterectomies, declines every year, we need to make sure we are adequately training the physicians who will be performing this procedure in the future. One solution is to have residents join a track their 4th year of residency, after their future career path has been established. Another possible solution would be to increase use of a simulation model for vaginal hysterectomy, as described by Dr. Douglas Miyazaki.
Indigo carmine replacement? A recent issue in gynecologic surgery resulted when there was a national shortage of indigo carmine, which is commonly used to identify UO efflux at the time of cystoscopy. Dr. Katie Propst provided a solution: In the setting of planned cystoscopy, she found that preoperative phenazopyridine is an acceptable alternative and its use led to similar UO identification as with indigo carmine without an increase in complications.
Who should have a vaginal hysterectomy? Dr. Jennifer Schmitt from the Mayo Clinic gave another wonderful lecture describing a decision-tree algorithm for determining the ideal route of hysterectomy. According to a retrospective review of patients at their institution, vaginal hysterectomy was associated with lower infection rates, operative times, and costs.
The highlight of the morning was a very insightful Presidential Address by Dr. Andrew Walter! Not only is a he a very entertaining speaker but he also shed some light on 2 challenges that we currently face in the field. He eloquently stated that, “every woman who is a candidate for a vaginal hysterectomy should be able to get one and have it performed competently, and every woman who needs surgery should have one performed by someone who performs enough surgeries every year to competently perform the surgery.” He frequently alluded to the wise Dr. Mayo, who stated, “The best interest of the patient is the only interest to be considered…” Let’s never forget this as physicians!
Do you find removing the adnexa challenging when performing vaginal surgery? Check out the amazing idea from the Cleveland Clinic shown in video presentation 05! They clearly demonstrate using a single-site gel port or surgical glove placed in the vaginal incision to perform laparoscopy through the vagina. What a novel idea to avoid any abdominal incisions when there is a difficult vaginal adnexal surgery!
After the morning break, we watched a master vaginal surgeon perform a sacral colpopexy through the vaginal route. For details on this method, please reference video presentation 06.
An increase in health care costs is a huge problem in this country. Dr. Mary Van Meter suggested that one area in which we can improve is in the operating room (OR), specifically through decreasing the number of instruments sterilized. She found that only approximately 20% of the instruments on the operating field were actually used by the OR physician. It costs about $3 per instrument on the tray at Vanderbilt University, she said. When you think of the few number of instruments actually used, if we work to limit the number of instruments on the trays, we could cut significant costs. As reimbursement is getting increasingly linked to cost reduction, we all should be thinking about innovative ways to cut costs.
The oral posters were all excellent!
Dr. Ann Peters showed that, at a high-volume tertiary-care center, multimodal preoperative testing failed to definitively identify leiomyosarcoma (LMS), and the factors associated with increased LMS risk included older age, menopause, and presence of fibroids with concurrent pelvic, uterine, or adnexal mass.
Dr. Kevin Kremer found that preoperative antibiotics were used in 23% of cases in which they were not indicated, and the increased use rate was associated with entrance into the abdomen during surgery, the individual surgeon, and time under anesthesia.
Dr. Jessica Heft then stated that the incontinence rate after midurethral sling revision for vaginal exposure or pain was significantly higher with a partial as opposed to complete mesh excision.
Fecal incontinence treatment options
Dr. Peter Rosenblatt tackled the issue of “Innovation or experimentation: Where do we draw the line?” in the TeLinde lecture. He started by describing a condition for which he feels we do not have an acceptable amount of treatment options for patients: fecal incontinence. He described how he observed that the puborectalis muscle, not the external anal sphincter, was critical for fecal continence. He then devised a surgery that created a sling that would act as a synthetic puborectalis muscle. He had amazing success until one patient had a serious complication. Should you abandon a successful, innovative surgery due to one very severe complication, was the question. Where do we draw the line? I don’t have an answer…but I do know that we all need to thank our puborectalis muscle for our daily fecal continence!
This is not a new issue in medicine, explained Dr. Rosenblatt. From the beginning of medicine, there has been a balance between innovation and experimentation. So, what is innovation? It is when someone improves upon a device or process. In medicine, is it innovation or just variation? We frequently use medications and devices “off label,” and these techniques often improve our surgeries and outcomes for our patients. Innovation comes about through careful planning, a necessity created by an emergency, advances in technology, and through evolution of a procedure. Who are the innovators? YOU are! We are the ones who understand the unmet needs and the potential opportunities for improving medicine. Let’s all work together to innovate NOT experiment and make medicine better so our patients have the best care possible!
“New Frontiers in Gynecologic Surgery: Transgender Surgery and Functional Clitoroplasty after Female Genital Mutilation”
The final talk for the day was by the groundbreaking Dr. Marci Bowers, whose lecture started by reminding everyone that there is a difference between gender identity and gender expression. Once a person knows his or her gender identity they use gender expression to express this to the world, she pointed out. Dr. Bowers is a true innovator and is performing gender reassignment surgeries as well as female genital mutilation corrective surgeries. She has perfected a one-stage vaginoplasty, and she showed a video of the procedure, which results in a 90% chance of orgasm. She also noted that she is able to restore clitoral function in 100% of the cases of genital mutilation, and she performs these procedures free of charge.
She reminded us that all ObGyns need to be trained in treating transgender patients, and given the high rate of suicide among adolescent transgender individuals, we all need to work together to provide a supportive medical environment to help these patients. What a fascinating end to the day!
Some relaxation, and entertainment, amid the sun and outdoors
We then had the afternoon to compete in a golf tournament, explore the area on mountain bikes, or lounge by the pool. Regardless of the daytime activities, we all came back together for the great lip-synch competition! All 4 teams gave an impressive performance, but the team from Brown University went home with the win! Such an entertaining event! (Thanks to incoming SGS President Vivian Sung @wih_sung for the pic!)
4/11/16. DAY 2 AT SGS
Experts talk surgical innovations, complementary therapies, value-based payment, and much more at SGS day 2
Wake up and learn! The second day of the 42nd Annual Scientific Meeting of the Society of Gynecologic Surgeons began with a review of research posters at the Poster Session as the sun rose over the Palm Springs mountains. We then moved indoors for the scientific sessions.
In the first lecture, "Reasons for Unplanned 30-Day Readmission After Hysterectomy for Benign Disease," Dr. Courtney Penn and colleagues reiterated that the postsurgical readmission rate is higher for patients undergoing abdominal hysterectomy compared with those who have a laparoscopic or vaginal hysterectomy. Similarly, Dr. Jennifer Schmitt and colleagues suggested that in a patient with a relative contraindication for vaginal hysterectomy, when this procedure is performed by an experienced surgeon there may not be increased complications. However, keep in mind that the study described—"A Comparison of Vaginal and Robotic Hysterectomy for Commonly Cited Relative Contraindications to Vaginal Hysterectomy"—was performed at the Mayo Clinic by expert, highly trained surgeons, and this fact may limit the generalizability of the study. As one audience member eloquently stated, it is important to remember that: "Just because it is feasible does not mean it is the correct procedure."
That yoga or barre class may be doing more good than you think! Lunge, bridge, and cat-into-cow movements may provide a greater degree of pelvic floor muscle unit recruitment than traditional Kegel exercises, according to a presentation by Dr. Bruce Crawford on Kegels versus specialized movement.
Perhaps those exercises should be recommended for surgeons as well. As Dr. Ruchira Singh pointed out, surgeons experience a high amount of musculoskeletal strain when performing vaginal procedures while sitting, regardless of the type of chair used during surgery. Dr. Singh and colleagues’ study, "Effect of Different Chairs on Work-Related Musculoskeletal Discomfort During Vaginal Surgery," found that while the round stool with a backrest and the Capisco chair were more comfortable, they did not eliminate the high risk for musculoskeletal strain, particularly in the head and neck.
Dr. Ann Peters and colleagues, from Magee-Womens Hospital of the University of Pittsburgh Medical Center, gave a fabulous video presentation on "Anatomic and Vascular Considerations in Laparoscopic Uterine Artery Ligation During Hysterectomy."
Need a novel treatment for interstitial cystitis/bladder pain syndrome? Consider mindfulness-based stress reduction. A randomized controlled study performed by Dr. Gregg Kanter and colleagues describes how this technique may help patients and could be considered a first-line therapy.
What is value-based payment and this new trend in reimbursement? And how does it apply to vaginal hysterectomy? Dr. Tina Groat addressed these issues in her keynote lecture. According to the American Congress of Obstetricians and Gynecologists, “Evidence demonstrates that, in general, vaginal hysterectomy is associated with better outcomes and fewer complications than laparoscopic or abdominal hysterectomy.” This is in opposition to what is actually being performed clinically. Dr. Groat explained that United Healthcare decided to incentivize physicians by requiring a prior authorization for all hysterectomies for benign disease. There is both a quality and cost benefit to performing a vaginal hysterectomy. Most insurance companies are moving away from a “fee for service” structure to performance-based payment. Change is always scary and, while I think the overall goal of moving toward the best care for our patients is a positive, this approach may create new challenges for the medical field. What do you think? Is performance-based payment beneficial? Or does it limit physicians and potentially force them to perform a procedure they do not feel as comfortable performing? Will this result in physicians rejecting certain patient populations? [Note from OBG MANAGEMENT: Let Dr. Collins know your thoughts through social media, or email OBG MANAGEMENT with a Letter to the Editor ([email protected]).]
The debate on the best route for hysterectomy continues: According to Dr. Carolyn Swenson and colleagues in their presentation, "Comparison of Robotic and Other Minimally Invasive Routes of Hysterectomy for Benign Indications," while there may be lower complications associated with robotic hysterectomy, the cost of performing a robotic hysterectomy is significantly higher than the cost of laparoscopic or vaginal hysterectomy, thus limiting its utility.
How can we teach a rare surgical procedure to learners? We channel our inner Martha Stewart and make a model out of a beef tongue and chicken. For about $8 a challenging and rare surgery can be taught to residents and medical students, according to the video presentation by Dr. Jana Illston and colleagues, titled "Modified Beef Tongue Model for Fourth-Degree Laceration Repair Simulation."
After the Day 2 lunch break, there was a rousing debate surrounding "Surgeons as Innovators—What Is the Patient Expecting?" Where do we draw the line between using an older more proven therapy as opposed to trying an innovative technology that may actually offer a potential benefit? Dr. Dennis Miller made a good point regarding innovation and pharmaceutical and device companies: If we ignore industry, we lose the ability to help with innovation and shape the future of medical treatments. Perhaps we should use the golden rule: If we would perform the surgery or use the device on ourselves, then we should perform it on our patient. Patients have a greater burden now, because there are more treatment options that they must choose among. Our job as physicians is to educate our patients and to guide them to innovative and evidence-based treatments.
Highlights from the afternoon oral poster session included a presentation by Dr. Caryn Russman that noted the high risk for recurrent urinary tract infection (UTI) after a mid-urethral sling procedure, which seems to be related to specific preoperative risk factors (such as a history of recurrent UTI). Dr. Tanya Hoke suggested that residents and attending physicians have inaccurate estimates of uterine weight, and an educational program may be necessary to improve these estimates. Finally, a study from Massachusetts General Hospital showed that a shorter stay in the hospital, ideally same-day surgery, resulted in a lower complication risk, lower number of emergency department visits, and a decreased readmission rate for patients undergoing urogynecology procedures.
The following recommendations were then suggested regarding vaginal hysterectomy:
- Preoperative prep with 4% chlorhexidine or povidine iodine
- Intracervical vasopressin injection to decrease blood loss
- Use of a pedicle-sealing device for pedicle ligation
- Vertical cuff closure is preferred to maximize vaginal length
Another important point made was that a prior cesarean delivery is not a contraindication to performing a vaginal hysterectomy.
Recommendations regarding recurrent UTI were also made, which include the recommendation for preoperative use of antibiotics to decrease the rate of UTI, with no benefit for a longer course of antibiotics.
News from the Fellows’ Pelvic Research Network
So much exciting research currently is ongoing with the FPRN! New project ideas include comparison of trimethoprim with methenamie for treatment of recurrent UTI; comparison of laparoscopic/robotic sacrocolpopexy with vaginal USLS for management of apical prolapse; a survey study examining surgeon preferences for timing of midurethral sling placement when performed at the time of pelvic organ prolapse (POP) repair; an assessment of the effect of a midurethal sling on overactive bladder in surgical repair in POP; and a study evaluating female pelvic reconstructive surgery in the setting of human immunodeficiency virus infection. It is so great to see the fellows working together to provide groundbreaking research!
Fun with stats
Learning statistics at the end of the day is never easy, but Dr. Matthew Barber did a fabulous job explaining this often-confusing topic. He reminded attendees that the key to learning statistics is repetition. One new recommendation he offered to enhance understanding is to use common language instead of numbers for P values. For example, instead of saying P <.001, use “is superior,” and instead of saying P = .3, use “seems not superior” or “inconclusive.”
A night to remember
The night ended with a wonderful awards ceremony and the president’s reception. Overall, day 2 was a very educational—and fun—day!
4/10/16. DAY 1 AT SGS
Postgrad courses address pain management and social media education
Mastering pelvic pain
With the beautiful Palm Springs, California, backdrop of mountains and palm trees, the 2016 meeting of the Society of Gynecologic Surgeons kicked off with 4 postgraduate courses. At the “Mastering Pelvic Pain: Strategies and Techniques for a Multimodal Approach” course, directed by Dr. Cara King, Dr. Matt Siedhoff explained the key components of the history and physical examination: Keep in mind that this generally is a multifactorial issue and may require a multidisciplinary approach, he told attendees. It is also important to make sure that the patient is fully prepared to combat the chronic pelvic pain (CPP) symptoms by focusing on the fundamentals, he said, including smoking, diet, exercise, weight loss, sleep, and relationship stress.
How is your posture? It turns out that something as simple as altering your posture can significantly affect pelvic floor control. Carol Sobeck, PT, demonstrated this importance with a simple group exercise that proved how the ability to contract the pelvic floor changes significantly with posture. Get to know your pelvic floor physical therapist—they are critical in helping with the treatment of CPP.
But what to do when physical therapy fails? Dr. Jennifer Gunter shed some light on this very difficult medical situation. One suggestion, she said, is to consider the possibility of a local presentation of a systemic issue and screen for the following: fibromyalgia, Ehlers-Danlos syndrome, statin use, and diabetes mellitus. Other treatment ideas include trigger point injections and onabotulinumtoxin A (Botox) therapy. A trigger point is a hypercontracted focus of muscle. To treat a trigger point, a needle must mechanically disrupt the trigger point to reduce the pelvic pain, and injection of local anesthetic is only used to help with postprocedure discomfort. Botox in my pelvis, you ask? Yes! The goal is to block presynaptic release of acetylcholine, which is very effective at reducing muscle spasm.
Tackling endometriosis. Dr. King then described surgical techniques for endometriosis. She recommended that, for mild disease not in close proximity to vital structures, ablation is likely equivalent to excision. For deeply infiltrating endometriosis, or lesions in close proximity to vital structures, excision is more beneficial. Excision is always beneficial for pathologic diagnosis, she said. And she offered this tip for bladder endometriosis: place a stitch in the nodule to allow for counter traction. Here is another tip, for ovarian cystectomy: inject vasopressin (20 U in 60 mL) to help dissect the plane between the ovary and cyst wall, and consider presacral neurectomy for midline pain.
Dr. Frank Tu then explained that we should not think of it as the “terrible triad: endometriosis, irritable bowel syndrome, and interstitial cystsis,” but rather as a system out of balance. Both peripheral and central mechanisms are involved in the generation and maintenance of cross-organ sensitization, he pointed out, which may explain why patients receive multiple diagnoses that describe a myriad of complaints due to a lack of overall homeostasis.
While Dr. Alaa Abd-Elsayed described innovative nerve blocks for CPP, he emphasized that no one specific treatment will result in a complete resolution of symptoms.
Vaginal mesh placement and pain. Dr. Mario Castellanos gave a fabulous lecture describing the pain issues that surround vaginal mesh placement. Interestingly, he noted that many patients had pelvic pain prior to placement of vaginal mesh, and that pain likely only will worsen with mesh placement. Mesh may cause pain by causing inflammation, visceral injury, muscle injury, and nerve injury. While there are guidelines for where mesh should be placed for bladder slings (TVT and TOT) and for prolapse repair, studies show, he pointed out, that the mesh placement often disrupts several muscle groups and may directly injure a nerve.
Alternatives to the typical diagnoses for CPP. The morning ended with a lecture by Dr. Suzie As-Sanie, in which she reminded everyone to start with “gold standard” therapy but, if those fail, consider alternative diagnoses or a central pain disorder. She also suggested cognitive behavioral therapy for treatment of CPP. For patients who cannot afford cognitive behavioral therapy, there is a free online source at fibroguide.med.umich.edu. Other alternative treatment options include exercise, patient education, instruction on sleep hygiene, and neurostimulatory therapies.
Overall, all the speakers this morning agreed that CPP is rarely treated with one modality, and it is best treated with a multidisciplinary approach.
Let’s get social!
The afternoon was spent learning about ways to use social networking sites to educate our patients, in another postgraduate course on “Making Media Social,” presented by the SGS Social Media Committee. Given that a recent survey demonstrated that 74.1% of women have some type of social networking account, it can be a very useful source for medical information for patients. If you have any questions about what is appropriate on social media, check out ACOG committee opinion 622.
Here are some tips that I learned:
Facebook:
- Keep your private Facebook page private and create a separate professional Facebook page
- Adjust your login setting so only administrators can post on your professional page
- Adjust settings so your professional profile cannot be “tagged”
- If a patient contacts your personal page, direct them to your professional page
- Do not give medical advice since Facebook is not digitally encrypted
Twitter:
- “Short bursts of inconsequential information”
- Follow societies, medical centers, and medical journals
LinkedIn:
- It provides a great way to find jobs, people, and business opportunities that are recommended by someone in your contact group
- It is specifically there to help you grow your business and show people who you are and where you have come from
Doximity:
Before we get to tips, first, what is it? Well, Doximity is basically a “LinkedIn” for physicians. It is “a way to find relevant specialists for patients; a rolodex; an email and text service; and a virtual lounge.” It is currently transforming from social network into a ‘platform.’ Now more than 1,000 hospitals and health systems are part of Doximity. Tips:
- There is a secure message option, which is HIPAA compliant
- Provides a way for residents and fellows to understand possible future places of employment
- It is a great way to find someone to refer patients to in an unfamiliar location
Vimeo:
- A benefit over YouTube is the lack of advertisements
- Check out the SGS video archives!
- You also can set privacy settings and embedding stats
Fifty-nine percent of US adults have looked online for health information in the past year. Therefore, it is important for physicians to get good information out for people to see!
A relaxing day’s end
The night ended with a beautiful poolside reception! I can’t wait to see what day 2 will bring!
Don’t assume that psychiatric patients lack capacity to make decisions about care
Some practitioners of medicine—including psychiatrists—might equate “psychosis” with incapacity, but that isn’t necessarily true. Even patients who, by most measures, are deemed psychotic might be capable of making wise and thoughtful decisions about their life. The case I describe in this article demonstrates that fact.
While rotating on a busy consultation service, I was asked to evaluate the capacity of a woman who had a diagnosis of schizophrenia and was being seen for worsening auditory hallucinations and progressive weight loss. She had a complicated medical course that eventually led to multiple requests to the consult team for a capacity evaluation.
The question of capacity in this patient, and in the psychiatric population generally, motivated me to review the literature, because the assumption by many on the medical teams involved in this patient’s care was that psychiatric patients do not have the capacity to participate in their own care. My goal here is to clarify the misconceptions in regard to this situation.
CASE REPORT
Schizophrenia, weight loss, back pain
Ms. V, age 67, a resident of a group home for the past 6 years, was brought to the emergency department (ED) because of weight loss and auditory hallucinations that had developed during the past few months. She had a history of paranoid schizophrenia that included several psychiatric hospitalizations but no known medical history.
The patient appeared cachectic and dehydrated. When approached, she was pleasant and reported hearing voices of the “devil.”
“They are not scary,” she confided. “They talk to me about art and literature.”
Over the past 6 months, Ms. V had lost 60 lb; she was now bedridden because of back pain. Collateral information obtained from staff members at the group home indicated that she had refused to get out of bed, and only intermittently took her medications or ate meals during the past few months. In general, however, she had been relatively stable over the course of her psychiatric illness, was adherent to psychiatric treatment, and had had no psychiatric hospitalizations in the past 3 decades.
Ominous development. Although the ED staff was convinced that Ms. V needed psychiatric admission, we (the consult team) first requested a detailed medical workup, including imaging studies. A CT scan showed multiple metastatic foci throughout her spine. She was admitted to the medical service.
Respiratory distress developed; her condition deteriorated. Numerous capacity consults were requested because she refused a medical workup or to sign do-not-resuscitate and do-not-intubate orders. Each time an evaluation was performed, Ms. V was deemed by various clinicians on the consult service to have decision-making capacity.
The patient grew unhappy with the staff’s insistence that she undergo more tests regardless of her stated wishes. The palliative care service determined that further workup would not benefit her medically: Ms. V’s condition would be grave and her prognosis poor regardless of what treatment she received.
The medical team continued to believe that, because that this patient had a mental illness and was actively hallucinating, she did not have the capacity to refuse any proposed treatments and tests.
What is capacity?
Capacity is an assessment of a person’s ability to make rational decisions. This includes the ability to understand, appreciate, and manipulate information in reaching such decisions. Determining whether a patient has the capacity to accept or refuse treatment is a medical decision that any physician can make; however, consultation−liaison psychiatrists are the experts who often are involved in this activity, particularly in patients who have a psychiatric comorbidity.
Capacity is evaluated by assessing 4 standards; that is, whether a patient can:
- communicate choice about proposed treatment
- understand her (his) medical situation
- appreciate the situation and its consequences
- manipulate information to reach a rational decision.1-3
- manipulate information to reach a rational decision.
CASE REPORT continued
Although Ms. V’s health was deteriorating and her auditory hallucinations were becoming worse, she appeared insightful about her medical problems, understood her prognosis, and wanted comfort care. She understood that having multiple metastases meant a poor prognosis, and that a biopsy might yield a medical diagnosis. She stated, “If it were caught earlier and I was better able to tolerate treatment, it would make sense to know for sure, but now it doesn’t make sense. I just want to have no pain in my end.”
Misconceptions
In a study by Ganzini et al,4,5 395 consultation−liaison psychiatrists, geriatricians, and geriatric psychologists responded to a survey in which they rated types of misunderstandings by clinicians who refer patients for assessment of decision-making capacity. Seventy percent reported that it is common that, when a patient has a mental illness such as schizophrenia, practitioners think that the patient lacks capacity to make medical decisions. However, results of a meta-analysis by Jeste et al,6 in which the magnitude of impairment of decisional capacity in patients with schizophrenia was assessed in comparison to that of normal subjects, suggest that the presence of schizophrenia does not necessarily mean the patient has impaired capacity.
Voluntary participation in research. Many patients with schizophrenia volunteer to participate in clinical trials even when they are acutely psychotic and admitted to a psychiatric hospital. Given the importance placed on participants’ voluntary informed consent as a prerequisite for ethical conduct of research, the cognitive and emotional impairments associated with schizophrenia raise questions about patients’ capacity to consent.
As is true in other areas of functional capacity, the ability of patients with schizophrenia to make competent decisions relates more to their overall cognitive functioning than to the presence or absence of specific symptoms of the disorder.7 Documentation of longitudinal consent-related abilities among research participants with schizophrenia in the long-term Clinical Antipsychotic Trials of Intervention Effectiveness study indicated that most participants had stable or improved consent-related abilities. Although almost 25% of participants experienced substantial worsening, only 4% fell below the study’s capacity threshold for enrollment.8
What I learned from Ms. V
A diagnosis of schizophrenia does not automatically render a person unable to make decisions about medical care. Even patients who have severe mental illness might have significant intact areas of reality testing. Ethically, it is important to at least consider that the chronically mentally ill can understand treatment options and express consistent choices.
Healthcare providers might tend to exclude psychiatric patients from end-of-life decisions because they (1) are worried about the emotional fragility of such patients and (2) assume that patients lack capacity to participate in making such important decisions. The case presented here is an example of a patient with a severe psychiatric diagnosis being able to participate in her care despite her mental state.
1. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
2. Leo RJ. Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131-141.
3. White MM, Lofwall MR. Challenges of the capacity evaluation for the consultation-liaison psychiatrist. J Psychiatr Pract. 2015;21(2):160-170.
4. Ganzini L, Volicer L, Nelson W, et al. Pitfalls in assessment of decision-making capacity. Psychosomatics. 2003;44(3):237-243.
5. Ganzini L, Volicer L, Nelson WA, et al. Ten myths about decision-making capacity. J Am Med Dir Assoc. 2005;6(3):S100-S104.
6. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005;32(1):121-128.
7. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2005;32(1):22-25.
8. Stroup TS, Appelbaum PS, Gu H, et al. Longitudinal consent-related abilities among research participants with schizophrenia: results from the CATIE study. Schizophr Res. 2011;130(1-3):47-52.
Some practitioners of medicine—including psychiatrists—might equate “psychosis” with incapacity, but that isn’t necessarily true. Even patients who, by most measures, are deemed psychotic might be capable of making wise and thoughtful decisions about their life. The case I describe in this article demonstrates that fact.
While rotating on a busy consultation service, I was asked to evaluate the capacity of a woman who had a diagnosis of schizophrenia and was being seen for worsening auditory hallucinations and progressive weight loss. She had a complicated medical course that eventually led to multiple requests to the consult team for a capacity evaluation.
The question of capacity in this patient, and in the psychiatric population generally, motivated me to review the literature, because the assumption by many on the medical teams involved in this patient’s care was that psychiatric patients do not have the capacity to participate in their own care. My goal here is to clarify the misconceptions in regard to this situation.
CASE REPORT
Schizophrenia, weight loss, back pain
Ms. V, age 67, a resident of a group home for the past 6 years, was brought to the emergency department (ED) because of weight loss and auditory hallucinations that had developed during the past few months. She had a history of paranoid schizophrenia that included several psychiatric hospitalizations but no known medical history.
The patient appeared cachectic and dehydrated. When approached, she was pleasant and reported hearing voices of the “devil.”
“They are not scary,” she confided. “They talk to me about art and literature.”
Over the past 6 months, Ms. V had lost 60 lb; she was now bedridden because of back pain. Collateral information obtained from staff members at the group home indicated that she had refused to get out of bed, and only intermittently took her medications or ate meals during the past few months. In general, however, she had been relatively stable over the course of her psychiatric illness, was adherent to psychiatric treatment, and had had no psychiatric hospitalizations in the past 3 decades.
Ominous development. Although the ED staff was convinced that Ms. V needed psychiatric admission, we (the consult team) first requested a detailed medical workup, including imaging studies. A CT scan showed multiple metastatic foci throughout her spine. She was admitted to the medical service.
Respiratory distress developed; her condition deteriorated. Numerous capacity consults were requested because she refused a medical workup or to sign do-not-resuscitate and do-not-intubate orders. Each time an evaluation was performed, Ms. V was deemed by various clinicians on the consult service to have decision-making capacity.
The patient grew unhappy with the staff’s insistence that she undergo more tests regardless of her stated wishes. The palliative care service determined that further workup would not benefit her medically: Ms. V’s condition would be grave and her prognosis poor regardless of what treatment she received.
The medical team continued to believe that, because that this patient had a mental illness and was actively hallucinating, she did not have the capacity to refuse any proposed treatments and tests.
What is capacity?
Capacity is an assessment of a person’s ability to make rational decisions. This includes the ability to understand, appreciate, and manipulate information in reaching such decisions. Determining whether a patient has the capacity to accept or refuse treatment is a medical decision that any physician can make; however, consultation−liaison psychiatrists are the experts who often are involved in this activity, particularly in patients who have a psychiatric comorbidity.
Capacity is evaluated by assessing 4 standards; that is, whether a patient can:
- communicate choice about proposed treatment
- understand her (his) medical situation
- appreciate the situation and its consequences
- manipulate information to reach a rational decision.1-3
- manipulate information to reach a rational decision.
CASE REPORT continued
Although Ms. V’s health was deteriorating and her auditory hallucinations were becoming worse, she appeared insightful about her medical problems, understood her prognosis, and wanted comfort care. She understood that having multiple metastases meant a poor prognosis, and that a biopsy might yield a medical diagnosis. She stated, “If it were caught earlier and I was better able to tolerate treatment, it would make sense to know for sure, but now it doesn’t make sense. I just want to have no pain in my end.”
Misconceptions
In a study by Ganzini et al,4,5 395 consultation−liaison psychiatrists, geriatricians, and geriatric psychologists responded to a survey in which they rated types of misunderstandings by clinicians who refer patients for assessment of decision-making capacity. Seventy percent reported that it is common that, when a patient has a mental illness such as schizophrenia, practitioners think that the patient lacks capacity to make medical decisions. However, results of a meta-analysis by Jeste et al,6 in which the magnitude of impairment of decisional capacity in patients with schizophrenia was assessed in comparison to that of normal subjects, suggest that the presence of schizophrenia does not necessarily mean the patient has impaired capacity.
Voluntary participation in research. Many patients with schizophrenia volunteer to participate in clinical trials even when they are acutely psychotic and admitted to a psychiatric hospital. Given the importance placed on participants’ voluntary informed consent as a prerequisite for ethical conduct of research, the cognitive and emotional impairments associated with schizophrenia raise questions about patients’ capacity to consent.
As is true in other areas of functional capacity, the ability of patients with schizophrenia to make competent decisions relates more to their overall cognitive functioning than to the presence or absence of specific symptoms of the disorder.7 Documentation of longitudinal consent-related abilities among research participants with schizophrenia in the long-term Clinical Antipsychotic Trials of Intervention Effectiveness study indicated that most participants had stable or improved consent-related abilities. Although almost 25% of participants experienced substantial worsening, only 4% fell below the study’s capacity threshold for enrollment.8
What I learned from Ms. V
A diagnosis of schizophrenia does not automatically render a person unable to make decisions about medical care. Even patients who have severe mental illness might have significant intact areas of reality testing. Ethically, it is important to at least consider that the chronically mentally ill can understand treatment options and express consistent choices.
Healthcare providers might tend to exclude psychiatric patients from end-of-life decisions because they (1) are worried about the emotional fragility of such patients and (2) assume that patients lack capacity to participate in making such important decisions. The case presented here is an example of a patient with a severe psychiatric diagnosis being able to participate in her care despite her mental state.
Some practitioners of medicine—including psychiatrists—might equate “psychosis” with incapacity, but that isn’t necessarily true. Even patients who, by most measures, are deemed psychotic might be capable of making wise and thoughtful decisions about their life. The case I describe in this article demonstrates that fact.
While rotating on a busy consultation service, I was asked to evaluate the capacity of a woman who had a diagnosis of schizophrenia and was being seen for worsening auditory hallucinations and progressive weight loss. She had a complicated medical course that eventually led to multiple requests to the consult team for a capacity evaluation.
The question of capacity in this patient, and in the psychiatric population generally, motivated me to review the literature, because the assumption by many on the medical teams involved in this patient’s care was that psychiatric patients do not have the capacity to participate in their own care. My goal here is to clarify the misconceptions in regard to this situation.
CASE REPORT
Schizophrenia, weight loss, back pain
Ms. V, age 67, a resident of a group home for the past 6 years, was brought to the emergency department (ED) because of weight loss and auditory hallucinations that had developed during the past few months. She had a history of paranoid schizophrenia that included several psychiatric hospitalizations but no known medical history.
The patient appeared cachectic and dehydrated. When approached, she was pleasant and reported hearing voices of the “devil.”
“They are not scary,” she confided. “They talk to me about art and literature.”
Over the past 6 months, Ms. V had lost 60 lb; she was now bedridden because of back pain. Collateral information obtained from staff members at the group home indicated that she had refused to get out of bed, and only intermittently took her medications or ate meals during the past few months. In general, however, she had been relatively stable over the course of her psychiatric illness, was adherent to psychiatric treatment, and had had no psychiatric hospitalizations in the past 3 decades.
Ominous development. Although the ED staff was convinced that Ms. V needed psychiatric admission, we (the consult team) first requested a detailed medical workup, including imaging studies. A CT scan showed multiple metastatic foci throughout her spine. She was admitted to the medical service.
Respiratory distress developed; her condition deteriorated. Numerous capacity consults were requested because she refused a medical workup or to sign do-not-resuscitate and do-not-intubate orders. Each time an evaluation was performed, Ms. V was deemed by various clinicians on the consult service to have decision-making capacity.
The patient grew unhappy with the staff’s insistence that she undergo more tests regardless of her stated wishes. The palliative care service determined that further workup would not benefit her medically: Ms. V’s condition would be grave and her prognosis poor regardless of what treatment she received.
The medical team continued to believe that, because that this patient had a mental illness and was actively hallucinating, she did not have the capacity to refuse any proposed treatments and tests.
What is capacity?
Capacity is an assessment of a person’s ability to make rational decisions. This includes the ability to understand, appreciate, and manipulate information in reaching such decisions. Determining whether a patient has the capacity to accept or refuse treatment is a medical decision that any physician can make; however, consultation−liaison psychiatrists are the experts who often are involved in this activity, particularly in patients who have a psychiatric comorbidity.
Capacity is evaluated by assessing 4 standards; that is, whether a patient can:
- communicate choice about proposed treatment
- understand her (his) medical situation
- appreciate the situation and its consequences
- manipulate information to reach a rational decision.1-3
- manipulate information to reach a rational decision.
CASE REPORT continued
Although Ms. V’s health was deteriorating and her auditory hallucinations were becoming worse, she appeared insightful about her medical problems, understood her prognosis, and wanted comfort care. She understood that having multiple metastases meant a poor prognosis, and that a biopsy might yield a medical diagnosis. She stated, “If it were caught earlier and I was better able to tolerate treatment, it would make sense to know for sure, but now it doesn’t make sense. I just want to have no pain in my end.”
Misconceptions
In a study by Ganzini et al,4,5 395 consultation−liaison psychiatrists, geriatricians, and geriatric psychologists responded to a survey in which they rated types of misunderstandings by clinicians who refer patients for assessment of decision-making capacity. Seventy percent reported that it is common that, when a patient has a mental illness such as schizophrenia, practitioners think that the patient lacks capacity to make medical decisions. However, results of a meta-analysis by Jeste et al,6 in which the magnitude of impairment of decisional capacity in patients with schizophrenia was assessed in comparison to that of normal subjects, suggest that the presence of schizophrenia does not necessarily mean the patient has impaired capacity.
Voluntary participation in research. Many patients with schizophrenia volunteer to participate in clinical trials even when they are acutely psychotic and admitted to a psychiatric hospital. Given the importance placed on participants’ voluntary informed consent as a prerequisite for ethical conduct of research, the cognitive and emotional impairments associated with schizophrenia raise questions about patients’ capacity to consent.
As is true in other areas of functional capacity, the ability of patients with schizophrenia to make competent decisions relates more to their overall cognitive functioning than to the presence or absence of specific symptoms of the disorder.7 Documentation of longitudinal consent-related abilities among research participants with schizophrenia in the long-term Clinical Antipsychotic Trials of Intervention Effectiveness study indicated that most participants had stable or improved consent-related abilities. Although almost 25% of participants experienced substantial worsening, only 4% fell below the study’s capacity threshold for enrollment.8
What I learned from Ms. V
A diagnosis of schizophrenia does not automatically render a person unable to make decisions about medical care. Even patients who have severe mental illness might have significant intact areas of reality testing. Ethically, it is important to at least consider that the chronically mentally ill can understand treatment options and express consistent choices.
Healthcare providers might tend to exclude psychiatric patients from end-of-life decisions because they (1) are worried about the emotional fragility of such patients and (2) assume that patients lack capacity to participate in making such important decisions. The case presented here is an example of a patient with a severe psychiatric diagnosis being able to participate in her care despite her mental state.
1. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
2. Leo RJ. Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131-141.
3. White MM, Lofwall MR. Challenges of the capacity evaluation for the consultation-liaison psychiatrist. J Psychiatr Pract. 2015;21(2):160-170.
4. Ganzini L, Volicer L, Nelson W, et al. Pitfalls in assessment of decision-making capacity. Psychosomatics. 2003;44(3):237-243.
5. Ganzini L, Volicer L, Nelson WA, et al. Ten myths about decision-making capacity. J Am Med Dir Assoc. 2005;6(3):S100-S104.
6. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005;32(1):121-128.
7. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2005;32(1):22-25.
8. Stroup TS, Appelbaum PS, Gu H, et al. Longitudinal consent-related abilities among research participants with schizophrenia: results from the CATIE study. Schizophr Res. 2011;130(1-3):47-52.
1. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
2. Leo RJ. Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131-141.
3. White MM, Lofwall MR. Challenges of the capacity evaluation for the consultation-liaison psychiatrist. J Psychiatr Pract. 2015;21(2):160-170.
4. Ganzini L, Volicer L, Nelson W, et al. Pitfalls in assessment of decision-making capacity. Psychosomatics. 2003;44(3):237-243.
5. Ganzini L, Volicer L, Nelson WA, et al. Ten myths about decision-making capacity. J Am Med Dir Assoc. 2005;6(3):S100-S104.
6. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005;32(1):121-128.
7. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schizophr Bull. 2005;32(1):22-25.
8. Stroup TS, Appelbaum PS, Gu H, et al. Longitudinal consent-related abilities among research participants with schizophrenia: results from the CATIE study. Schizophr Res. 2011;130(1-3):47-52.
Androgen deprivation therapy linked to depression
Men on androgen deprivation therapy for prostate cancer are at significantly increased risk for depression, a risk that increases with duration of therapy, investigators report.
A review of Surveillance, Epidemiology, and End Results (SEER) Medicare data on nearly 79,000 men older than 65 years with a diagnosis of prostate cancer showed that those who received androgen deprivation therapy (ADT) had a 23% increased risk for depression, compared with men who were not on ADT, reported Kathryn T. Dinh of Harvard Medical School, Boston, and her colleagues.
“We observed a significantly increased risk of depression and inpatient psychiatric treatment in men treated with ADT for prostate cancer, as well as a duration-response effect such that more ADT was linked to an increasing risk of depression and inpatient and outpatient psychiatric treatment. The possible psychiatric effects of ADT should be recognized by physicians and discussed with patients before initiating treatment,” they wrote (J Clin Oncol. 2016 Apr 11. doi: 10.1200/JCO.2015.64.1969).
Although ADT has been identified in some studies as a risk factor for clinical depression, evidence for such a relationship has been spotty, the investigators said, prompting them to conduct a population-based retrospective study to get a better handle on the issue.
They reviewed SEER Medicare data on 78,552 men older than 65 years with a diagnosis of stage I-III prostate cancer treated with ADT from 1992 through 2006, excluding from the sample those patients who had a psychiatric diagnosis within the past 12 months.
Ms. Dinh and her associates found that the 33,882 patients (43%) who received ADT had a significantly higher 3-year cumulative incidence of depression than patients who did not have ADT (7.1% vs. 5.2%, P less than .001), and a significantly higher proportion had either inpatient psychiatric treatment (2.8% vs. 1.9%, P less than .001) or outpatient psychiatric therapy (3.4% vs. 2.5%, P less than .001).
In proportional hazard models controlling for demographic and clinical factors, receipt of ADT was associated with adjusted hazard ratios of 1.23 for depression and 1.29 (P less than .001 for both) for inpatient psychiatric treatment. There was no significant increase in risk for outpatient psychiatric treatment in this analysis, however.
In addition, the longer patients that were on ADT, the greater the risk for depression. The risk of depression was 12% for patients treated for 6 months or less, 26% for those on ADT for 7-11 months, and 37% for those on ADT for at least 1 year.
“The impact of ADT on depression may plausibly occur via deregulation of neurochemicals, such as serotonin, in addition to the well-described physical effects,” Ms. Dinh and her associates wrote.
Side effects of ADT that can impair quality of life also may contribute to clinical depression, they noted.
The study was supported by charitable grants and internal institutional sources. One investigator reported consulting or advisory roles with Medivation, GenomeDx, and Ferring. Three of the other ten coauthors also reported financial disclosures.
Men on androgen deprivation therapy for prostate cancer are at significantly increased risk for depression, a risk that increases with duration of therapy, investigators report.
A review of Surveillance, Epidemiology, and End Results (SEER) Medicare data on nearly 79,000 men older than 65 years with a diagnosis of prostate cancer showed that those who received androgen deprivation therapy (ADT) had a 23% increased risk for depression, compared with men who were not on ADT, reported Kathryn T. Dinh of Harvard Medical School, Boston, and her colleagues.
“We observed a significantly increased risk of depression and inpatient psychiatric treatment in men treated with ADT for prostate cancer, as well as a duration-response effect such that more ADT was linked to an increasing risk of depression and inpatient and outpatient psychiatric treatment. The possible psychiatric effects of ADT should be recognized by physicians and discussed with patients before initiating treatment,” they wrote (J Clin Oncol. 2016 Apr 11. doi: 10.1200/JCO.2015.64.1969).
Although ADT has been identified in some studies as a risk factor for clinical depression, evidence for such a relationship has been spotty, the investigators said, prompting them to conduct a population-based retrospective study to get a better handle on the issue.
They reviewed SEER Medicare data on 78,552 men older than 65 years with a diagnosis of stage I-III prostate cancer treated with ADT from 1992 through 2006, excluding from the sample those patients who had a psychiatric diagnosis within the past 12 months.
Ms. Dinh and her associates found that the 33,882 patients (43%) who received ADT had a significantly higher 3-year cumulative incidence of depression than patients who did not have ADT (7.1% vs. 5.2%, P less than .001), and a significantly higher proportion had either inpatient psychiatric treatment (2.8% vs. 1.9%, P less than .001) or outpatient psychiatric therapy (3.4% vs. 2.5%, P less than .001).
In proportional hazard models controlling for demographic and clinical factors, receipt of ADT was associated with adjusted hazard ratios of 1.23 for depression and 1.29 (P less than .001 for both) for inpatient psychiatric treatment. There was no significant increase in risk for outpatient psychiatric treatment in this analysis, however.
In addition, the longer patients that were on ADT, the greater the risk for depression. The risk of depression was 12% for patients treated for 6 months or less, 26% for those on ADT for 7-11 months, and 37% for those on ADT for at least 1 year.
“The impact of ADT on depression may plausibly occur via deregulation of neurochemicals, such as serotonin, in addition to the well-described physical effects,” Ms. Dinh and her associates wrote.
Side effects of ADT that can impair quality of life also may contribute to clinical depression, they noted.
The study was supported by charitable grants and internal institutional sources. One investigator reported consulting or advisory roles with Medivation, GenomeDx, and Ferring. Three of the other ten coauthors also reported financial disclosures.
Men on androgen deprivation therapy for prostate cancer are at significantly increased risk for depression, a risk that increases with duration of therapy, investigators report.
A review of Surveillance, Epidemiology, and End Results (SEER) Medicare data on nearly 79,000 men older than 65 years with a diagnosis of prostate cancer showed that those who received androgen deprivation therapy (ADT) had a 23% increased risk for depression, compared with men who were not on ADT, reported Kathryn T. Dinh of Harvard Medical School, Boston, and her colleagues.
“We observed a significantly increased risk of depression and inpatient psychiatric treatment in men treated with ADT for prostate cancer, as well as a duration-response effect such that more ADT was linked to an increasing risk of depression and inpatient and outpatient psychiatric treatment. The possible psychiatric effects of ADT should be recognized by physicians and discussed with patients before initiating treatment,” they wrote (J Clin Oncol. 2016 Apr 11. doi: 10.1200/JCO.2015.64.1969).
Although ADT has been identified in some studies as a risk factor for clinical depression, evidence for such a relationship has been spotty, the investigators said, prompting them to conduct a population-based retrospective study to get a better handle on the issue.
They reviewed SEER Medicare data on 78,552 men older than 65 years with a diagnosis of stage I-III prostate cancer treated with ADT from 1992 through 2006, excluding from the sample those patients who had a psychiatric diagnosis within the past 12 months.
Ms. Dinh and her associates found that the 33,882 patients (43%) who received ADT had a significantly higher 3-year cumulative incidence of depression than patients who did not have ADT (7.1% vs. 5.2%, P less than .001), and a significantly higher proportion had either inpatient psychiatric treatment (2.8% vs. 1.9%, P less than .001) or outpatient psychiatric therapy (3.4% vs. 2.5%, P less than .001).
In proportional hazard models controlling for demographic and clinical factors, receipt of ADT was associated with adjusted hazard ratios of 1.23 for depression and 1.29 (P less than .001 for both) for inpatient psychiatric treatment. There was no significant increase in risk for outpatient psychiatric treatment in this analysis, however.
In addition, the longer patients that were on ADT, the greater the risk for depression. The risk of depression was 12% for patients treated for 6 months or less, 26% for those on ADT for 7-11 months, and 37% for those on ADT for at least 1 year.
“The impact of ADT on depression may plausibly occur via deregulation of neurochemicals, such as serotonin, in addition to the well-described physical effects,” Ms. Dinh and her associates wrote.
Side effects of ADT that can impair quality of life also may contribute to clinical depression, they noted.
The study was supported by charitable grants and internal institutional sources. One investigator reported consulting or advisory roles with Medivation, GenomeDx, and Ferring. Three of the other ten coauthors also reported financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Androgen deprivation therapy for prostate cancer is associated with increased risk for clinical depression.
Major finding: Men who received androgen deprivation therapy (ADT) had a 23% increased risk for depression compared with men who were not on ADT.
Data source: Review of SEER Medicare data on 78,552 men with stage I-III prostate cancer.
Disclosures: The study was supported by charitable grants and internal institutional sources. One investigator reported consulting or advisory roles with Medivation, GenomeDx, and Ferring. Three of the other ten coauthors also reported financial disclosures.
Fecal transplant cures most with C. difficile, but one dies
AMSTERDAM – Fecal transplants effected a clinical cure in 97% of patients with recurrent Clostridium difficile infection, a small prospective study has determined.
However, the transplants, which were administered via duodenal intubation, were not without serious adverse events, Dr. Yvette van Beurden said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Five patients regurgitated or vomited fecal material, and one of these patients died, presumably from aspiration pneumonia related to the event, said Dr. van Beurden of the VU University Medical Center, Amsterdam.
The study was relatively small – 39 patients – but provided up to 2 years of follow-up on them. All were treated at Academic Medical Center, Amsterdam, from 2010 to 1016.
They were a mean of 73 years old, but the age range was wide (14-97 years). All had experienced recurrent C. difficile infections. The mean recurrence rate was four, but again this varied widely, from one recurrence to 10.
Thus, they had also experienced a mean of four courses of antibiotic treatment, with a range similar to the recurrence range. At the time of transplant, they were a mean of 6 months past their last recurrence.
The transplant protocol called for a minimum of 4 days of vancomycin treatment before transplant, and a full bowel prep 1 day before. The transplant itself consisted of 500 mL of fresh donor feces in solution; it was obtained from a household contact or healthy volunteer and administered by duodenal tube. Patients were discharged on the same day of infusion.
The mean follow-up was 21 months, also with a wide range (3-68 months).
A clinical cure – not microbiologically confirmed – occurred in 82% of the patients. There were seven recurrences (18%), which all happened within the first 3 months. Of these, two were thought to be related to antibiotic use within the first month of the procedure; the cause of the other recurrences was unknown.
Four of the patients with recurrent infections received antibiotics without a repeat transplant; three received fidaxomicin and one, metronidazole. Two underwent a successful repeat transplant. One patient had multiple treatments, including a course of fidaxomicin. This patient experienced another recurrence that was successfully treated with a second transplant.
Six of these seven patients experienced a clinical cure, bringing the secondary cure rate of the entire cohort to 97%.
There were nine serious adverse events (23%), most of which occurred during or shortly after the transplant procedure. This included the single death; four hospitalizations (one related to the transplant); and four transplant-related events.
The patient who died had an uncomplicated transplant, but within an hour started to feel nauseated and regurgitated the fecal material. “This didn’t appear to be severe,” Dr. van Beurden said. “But within a week, pneumonia developed and the patient died despite antibiotic treatment.”
She added that this patient was “medically fragile,” with a swallowing disorder that required a percutaneous endoscopic gastrostomy feeding tube.
Of the other four patients with transplant complications:
• One, following an uncomplicated transplant, was discharged and ate a large meal, then shortly after vomited food and donor feces.
• One experienced abdominal cramping during the procedure, which was immediately stopped. When the cramping subsided, the procedure was completed. However, within a few hours the cramping recurred, along with diarrhea, nausea, and vomiting of fecal material.
• One patient was “very stressed and anxious” during the procedure and regurgitated a mix of gastric juices and donor feces. The infusion tube was immediately removed. The patient was discharged after being symptom-free for 3 hours, but vomited fecal material on the way home.
• One patient experienced nausea during the transplant, which was immediately stopped with tube removal. Upon removal, the patient regurgitated donor material. Nausea shortly resolved.
During the discussion period, Dr. van Beurden fielded a question about duodenal administration rather than delivering the donor feces colonoscopically. She said that decision was made because the duodenal tube doesn’t require anesthesia, and because many of the patients had severely inflamed colons. However, the hospital’s experience with complications did help refine its transplant protocol, she said.
• Colonoscopic administration is mandatory for any patient with a swallowing disorder.
• A smaller volume of feces is now infused.
• Donor material is infused very slowly and immediately discontinued if there is any nausea, cramping, or regurgitation.
• There is no eating or drinking for at least 1 hour after the transplant.
• To minimize the risk of recurrent C. difficile, patients should have no nonessential antibiotic treatment within the first month after transplant.
She had no financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – Fecal transplants effected a clinical cure in 97% of patients with recurrent Clostridium difficile infection, a small prospective study has determined.
However, the transplants, which were administered via duodenal intubation, were not without serious adverse events, Dr. Yvette van Beurden said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Five patients regurgitated or vomited fecal material, and one of these patients died, presumably from aspiration pneumonia related to the event, said Dr. van Beurden of the VU University Medical Center, Amsterdam.
The study was relatively small – 39 patients – but provided up to 2 years of follow-up on them. All were treated at Academic Medical Center, Amsterdam, from 2010 to 1016.
They were a mean of 73 years old, but the age range was wide (14-97 years). All had experienced recurrent C. difficile infections. The mean recurrence rate was four, but again this varied widely, from one recurrence to 10.
Thus, they had also experienced a mean of four courses of antibiotic treatment, with a range similar to the recurrence range. At the time of transplant, they were a mean of 6 months past their last recurrence.
The transplant protocol called for a minimum of 4 days of vancomycin treatment before transplant, and a full bowel prep 1 day before. The transplant itself consisted of 500 mL of fresh donor feces in solution; it was obtained from a household contact or healthy volunteer and administered by duodenal tube. Patients were discharged on the same day of infusion.
The mean follow-up was 21 months, also with a wide range (3-68 months).
A clinical cure – not microbiologically confirmed – occurred in 82% of the patients. There were seven recurrences (18%), which all happened within the first 3 months. Of these, two were thought to be related to antibiotic use within the first month of the procedure; the cause of the other recurrences was unknown.
Four of the patients with recurrent infections received antibiotics without a repeat transplant; three received fidaxomicin and one, metronidazole. Two underwent a successful repeat transplant. One patient had multiple treatments, including a course of fidaxomicin. This patient experienced another recurrence that was successfully treated with a second transplant.
Six of these seven patients experienced a clinical cure, bringing the secondary cure rate of the entire cohort to 97%.
There were nine serious adverse events (23%), most of which occurred during or shortly after the transplant procedure. This included the single death; four hospitalizations (one related to the transplant); and four transplant-related events.
The patient who died had an uncomplicated transplant, but within an hour started to feel nauseated and regurgitated the fecal material. “This didn’t appear to be severe,” Dr. van Beurden said. “But within a week, pneumonia developed and the patient died despite antibiotic treatment.”
She added that this patient was “medically fragile,” with a swallowing disorder that required a percutaneous endoscopic gastrostomy feeding tube.
Of the other four patients with transplant complications:
• One, following an uncomplicated transplant, was discharged and ate a large meal, then shortly after vomited food and donor feces.
• One experienced abdominal cramping during the procedure, which was immediately stopped. When the cramping subsided, the procedure was completed. However, within a few hours the cramping recurred, along with diarrhea, nausea, and vomiting of fecal material.
• One patient was “very stressed and anxious” during the procedure and regurgitated a mix of gastric juices and donor feces. The infusion tube was immediately removed. The patient was discharged after being symptom-free for 3 hours, but vomited fecal material on the way home.
• One patient experienced nausea during the transplant, which was immediately stopped with tube removal. Upon removal, the patient regurgitated donor material. Nausea shortly resolved.
During the discussion period, Dr. van Beurden fielded a question about duodenal administration rather than delivering the donor feces colonoscopically. She said that decision was made because the duodenal tube doesn’t require anesthesia, and because many of the patients had severely inflamed colons. However, the hospital’s experience with complications did help refine its transplant protocol, she said.
• Colonoscopic administration is mandatory for any patient with a swallowing disorder.
• A smaller volume of feces is now infused.
• Donor material is infused very slowly and immediately discontinued if there is any nausea, cramping, or regurgitation.
• There is no eating or drinking for at least 1 hour after the transplant.
• To minimize the risk of recurrent C. difficile, patients should have no nonessential antibiotic treatment within the first month after transplant.
She had no financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – Fecal transplants effected a clinical cure in 97% of patients with recurrent Clostridium difficile infection, a small prospective study has determined.
However, the transplants, which were administered via duodenal intubation, were not without serious adverse events, Dr. Yvette van Beurden said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Five patients regurgitated or vomited fecal material, and one of these patients died, presumably from aspiration pneumonia related to the event, said Dr. van Beurden of the VU University Medical Center, Amsterdam.
The study was relatively small – 39 patients – but provided up to 2 years of follow-up on them. All were treated at Academic Medical Center, Amsterdam, from 2010 to 1016.
They were a mean of 73 years old, but the age range was wide (14-97 years). All had experienced recurrent C. difficile infections. The mean recurrence rate was four, but again this varied widely, from one recurrence to 10.
Thus, they had also experienced a mean of four courses of antibiotic treatment, with a range similar to the recurrence range. At the time of transplant, they were a mean of 6 months past their last recurrence.
The transplant protocol called for a minimum of 4 days of vancomycin treatment before transplant, and a full bowel prep 1 day before. The transplant itself consisted of 500 mL of fresh donor feces in solution; it was obtained from a household contact or healthy volunteer and administered by duodenal tube. Patients were discharged on the same day of infusion.
The mean follow-up was 21 months, also with a wide range (3-68 months).
A clinical cure – not microbiologically confirmed – occurred in 82% of the patients. There were seven recurrences (18%), which all happened within the first 3 months. Of these, two were thought to be related to antibiotic use within the first month of the procedure; the cause of the other recurrences was unknown.
Four of the patients with recurrent infections received antibiotics without a repeat transplant; three received fidaxomicin and one, metronidazole. Two underwent a successful repeat transplant. One patient had multiple treatments, including a course of fidaxomicin. This patient experienced another recurrence that was successfully treated with a second transplant.
Six of these seven patients experienced a clinical cure, bringing the secondary cure rate of the entire cohort to 97%.
There were nine serious adverse events (23%), most of which occurred during or shortly after the transplant procedure. This included the single death; four hospitalizations (one related to the transplant); and four transplant-related events.
The patient who died had an uncomplicated transplant, but within an hour started to feel nauseated and regurgitated the fecal material. “This didn’t appear to be severe,” Dr. van Beurden said. “But within a week, pneumonia developed and the patient died despite antibiotic treatment.”
She added that this patient was “medically fragile,” with a swallowing disorder that required a percutaneous endoscopic gastrostomy feeding tube.
Of the other four patients with transplant complications:
• One, following an uncomplicated transplant, was discharged and ate a large meal, then shortly after vomited food and donor feces.
• One experienced abdominal cramping during the procedure, which was immediately stopped. When the cramping subsided, the procedure was completed. However, within a few hours the cramping recurred, along with diarrhea, nausea, and vomiting of fecal material.
• One patient was “very stressed and anxious” during the procedure and regurgitated a mix of gastric juices and donor feces. The infusion tube was immediately removed. The patient was discharged after being symptom-free for 3 hours, but vomited fecal material on the way home.
• One patient experienced nausea during the transplant, which was immediately stopped with tube removal. Upon removal, the patient regurgitated donor material. Nausea shortly resolved.
During the discussion period, Dr. van Beurden fielded a question about duodenal administration rather than delivering the donor feces colonoscopically. She said that decision was made because the duodenal tube doesn’t require anesthesia, and because many of the patients had severely inflamed colons. However, the hospital’s experience with complications did help refine its transplant protocol, she said.
• Colonoscopic administration is mandatory for any patient with a swallowing disorder.
• A smaller volume of feces is now infused.
• Donor material is infused very slowly and immediately discontinued if there is any nausea, cramping, or regurgitation.
• There is no eating or drinking for at least 1 hour after the transplant.
• To minimize the risk of recurrent C. difficile, patients should have no nonessential antibiotic treatment within the first month after transplant.
She had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
Key clinical point: Fecal transplants cured most recurrent C. difficile infections, but could be dangerous as well.
Major finding: A duodenal administered fecal transplant cured 97% of patients with recurrent C. difficile, but one patient died after vomiting the fecal material.
Data source: The prospective study comprised 39 patients.
Disclosures: Dr. van Beurden had no financial disclosures.