User login
DANAMI 3-iPOST: No significant benefit with ischemic postconditioning after STEMI
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
CHICAGO – Ischemic postconditioning in patients with ST-segment elevation myocardial infarction failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
At a mean follow-up of 37.5 months, the primary composite endpoint of death from any cause and hospitalization for heart failure occurred in 69 of 617 patients with STEMI who received standard angioplasty and in 65 of 617 patients who received ischemic postconditioning in DANAMI 3-iPOST (the Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: iPOST conditioning during primary PCI). The 7% difference (hazard ratio, 0.93) was not statistically significant, Dr. Thomas Engstrøm reported at the annual meeting of the American College of Cardiology.
For the individual component of all-cause mortality, the reduction in the ischemic postconditioning group was 25%, occurring in 50 patients, compared with 38 in the conventionally treated group (hazard ratio, 0.75), but this difference also did not reach statistical significance, Dr. Engstrøm said.
Thirty patients in each group required hospitalization for heart failure.
However, an improvement in a secondary endpoint of left ventricular ejection fraction above 45% in patients with anterior infarcts was statistically significant, occurring in 72% of patients in the standard angioplasty group and 80% of those in the ischemic postconditioning group, said Dr. Engstrøm of Rigshospitalet University of Copenhagen.
This finding may translate into improved survival with longer follow-up, he noted.
Patients in the DANAMI-3 iPOST trial, who had a mean age of age 61 years, had acute STEMI (ST-segment elevation MI) symptoms of less than 12 hours’ duration at the time of randomization. They were followed for at least 2 years.
Ischemic postconditioning – a variation on angioplasty that involves using 30-second bursts of blood flow interspersed with 30-second pauses to restore blood flow to the heart – was shown in earlier studies to improve ST-segment resolution, reduce damage to heart muscle, and – in some patients – limit the extent of reperfusion injury.
Whether these factors would reduce hospitalizations or improve patient survival remained unclear, Dr. Engstrøm said.
Abrupt reperfusion by angioplasty may itself damage the heart muscle. In fact, up to 35% of patients may experience such injury during angioplasty.
“The thinking was that performing the reperfusion in a gentle, graded fashion would protect the heart against reperfusion injury,” Dr. Engstrøm explained.
The findings of DANAMI 3-iPOST – the first large clinical trial designed to evaluate clinical outcomes in STEMI patients (as opposed to surrogate endpoints such as ST-segment resolution) were disappointing, but larger trials may be required to definitively establish whether ischemic postconditioning improves clinical outcomes, Dr. Engstrøm said.
The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
AT ACC 16
Key clinical point: Ischemic postconditioning in patients with STEMI failed to significantly reduce death from any cause or hospitalization for heart failure in the randomized, controlled DANAMI 3-iPOST trial.
Major finding: No significant difference was seen in the primary composite endpoint of death from any cause and hospitalization for heart failure in standard angioplasty and ischemic postconditioning patients (HR, 0.93).
Data source: A randomized, controlled, open-label study of 1,234 patients from the DANAMI 3-iPOST trial.
Disclosures: The DANAMI 3-iPOST trial was funded by the Danish Agency for Science, Technology, and Innovation and the Danish Council for Strategic Research. Dr. Engstrøm reported having no relevant financial disclosures.
VIDEO: HOPE-3 bolsters primary prevention in intermediate-risk patients
CHICAGO – Results from the HOPE-3 trial confirm what guidelines have already recommended: patients with intermediate risk for cardiovascular disease should be treated for primary prevention of coronary event, Dr. Prakash Deedwania said in an interview at the annual meeting of the American College of Cardiology.
In the Heart Outcomes Prevention Evaluation (HOPE)-3 trial, nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease were randomized to either lipid lowering with rosuvastatin at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke, compared with placebo-treated controls, regardless of baseline LDL-cholesterol level. However, only subjects with a baseline pressure of greater than 143.5 mm Hg benefited from the dual-antihypertensive therapy.
In a video interview, Dr. Deedwania, professor of medicine at the University of California, San Francisco, Fresno, gave three takeaways from the HOPE-3 trial regarding primary prevention of cardiovascular events in patients at intermediate risk, how the results of the dual-antihypertensive treatment arm match up to guidelines, and whether there’s a future for the polypill.
Dr. Deedwania has received consultant fees and/or honoraria from Amgen, Pfizer, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results from the HOPE-3 trial confirm what guidelines have already recommended: patients with intermediate risk for cardiovascular disease should be treated for primary prevention of coronary event, Dr. Prakash Deedwania said in an interview at the annual meeting of the American College of Cardiology.
In the Heart Outcomes Prevention Evaluation (HOPE)-3 trial, nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease were randomized to either lipid lowering with rosuvastatin at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke, compared with placebo-treated controls, regardless of baseline LDL-cholesterol level. However, only subjects with a baseline pressure of greater than 143.5 mm Hg benefited from the dual-antihypertensive therapy.
In a video interview, Dr. Deedwania, professor of medicine at the University of California, San Francisco, Fresno, gave three takeaways from the HOPE-3 trial regarding primary prevention of cardiovascular events in patients at intermediate risk, how the results of the dual-antihypertensive treatment arm match up to guidelines, and whether there’s a future for the polypill.
Dr. Deedwania has received consultant fees and/or honoraria from Amgen, Pfizer, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results from the HOPE-3 trial confirm what guidelines have already recommended: patients with intermediate risk for cardiovascular disease should be treated for primary prevention of coronary event, Dr. Prakash Deedwania said in an interview at the annual meeting of the American College of Cardiology.
In the Heart Outcomes Prevention Evaluation (HOPE)-3 trial, nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease were randomized to either lipid lowering with rosuvastatin at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke, compared with placebo-treated controls, regardless of baseline LDL-cholesterol level. However, only subjects with a baseline pressure of greater than 143.5 mm Hg benefited from the dual-antihypertensive therapy.
In a video interview, Dr. Deedwania, professor of medicine at the University of California, San Francisco, Fresno, gave three takeaways from the HOPE-3 trial regarding primary prevention of cardiovascular events in patients at intermediate risk, how the results of the dual-antihypertensive treatment arm match up to guidelines, and whether there’s a future for the polypill.
Dr. Deedwania has received consultant fees and/or honoraria from Amgen, Pfizer, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Standard incubation can miss P. acnes in infective endocarditis
AMSTERDAM – Accounting for less than 1% of cases, Propionibacterium acnes has been considered an uncommon cause of infective endocarditis.
But data presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases suggest that the common anaerobe may be responsible for many more cases than is now believed. The bacteria are difficult to culture and grow very slowly, Dr. Jona Banzon said at the meeting. Incubating it for the standard 5 days may simply not be long enough.
“Due to this slow-growing nature, the standard incubation period may not be enough to detect it,” said Dr. Banzon, an infectious disease fellow at the Cleveland Clinic. “And since P. acnes is part of our commensal flora, it’s frequently a contaminate in culture, and we might be inappropriately dismissing it as such. In fact, we now wonder if this lack of extended incubation may be accounting for a significant proportion of negative blood cultures, and whether more prosthetic valve endocarditis than we think is actually being caused by P. acnes.”
Dr. Banzon presented a series of 23 cases included in the Cleveland Clinic Infective Endocarditis Registry from the period of 2007-2015. All had P. acnes confirmed as the causative organism. The group comprises 3.3% of the institution’s entire infective endocarditis registry, “making infective carditis with P. acnes already much more common than it is said to be in the literature,” she noted.
All of the cases were confirmed by any of the following standards:
• Two or more blood cultures positive for P. acnes.
• Two or more valve cultures positive for P. acnes.
• Two or more valve sequencing rests positive for P. acnes.
• At least two of the following: a positive blood culture, a positive valve culture or valve sequencing, or histopathologic demonstration of microorganism consistent with P. acnes.
Of the cohort, 22 had prosthetic valve endocarditis. One patient had endocarditis on a native valve. This is an important point, Dr. Banzon said.
The organism is being increasingly recognized for causing infections of prosthetic material, including shoulder joint infections and shunts, but it rarely seems to affect native tissue. The patient who had native valve endocarditis had experienced an episode of Staphylococcus aureus endocarditis about 18 months earlier. He was not treated surgically, and ended up with a damaged valve. After the initial illness, he was readmitted several times with symptoms of endocarditis, but all of his blood cultures were negative. This was attributed to the receipt of antibiotics. “When he was finally operated on, there were three valve sequencing specimens and all were positive for P. acnes,” Dr. Banzon said.
The patients in the cohort were a mean of 74 years old; about 75% were men. All of the cases were left-sided endocarditis, with the majority (74%) involving the aortic valve. Other sites included the mitral valve (18%), aortic plus mitral (4%), and aortic plus tricuspid (4%).
The most common predisposing factor was having a prosthetic valve (96%). Other factors included having a cardiac implantable device (17%), and a prior episode of infective endocarditis (13%). None of the patients had indwelling vascular catheters or used injectable drugs.
The cases presented with severe disease, Dr. Banzon said. Almost half (48%) had a perivalvular abscess at presentation. A third (35%) had valve dehiscence, and 35% had severe valvular regurgitation. There was a vegetation of more than 1 cm in 9% of cases.
Emboli were not uncommon; 17% had emboli in the central nervous system, and 17% had peripheral emboli. Two of these patients had kidney and spleen infarcts and two had acute arterial thromboembolism that required thrombectomy.
All of the patients underwent blood cultures; overall, 30% of the cultures were positive for P. acnes. But there were “striking differences” when the tests were broken down by incubation time, Dr. Banzon said.
Most of the cultures (16) were incubated for the standard of 5 days or less. Among these, 12.5% were positive. However, cultures on seven patients were incubated for more than 5 days and in this group, 71.4% were positive for P. acnes. The median time to positivity overall was 7 days, with a range of 3-9 days.
Other diagnostic methods were important in closing this gap, she said. Valve culturing was positive in 57% of the cases, while valve sequencing was positive in 95%. The median time to positive for valve culture was somewhat shorter than for blood culture (5.5 days).
In nine cases, no organism would have been identified without valve sequencing, Dr. Banzon said.
Because they presented with severe disease, almost all of the patients (22) underwent surgery as their intimal treatment. At the time of surgery, everyone was taking an antibiotic that covered P. acnes. Single-agent therapy was the definitive treatment for most, with vancomycin being most commonly employed (59%), followed by ceftriaxone (25%). A few patients had a combination of both drugs or a combination of vancomycin and rifampin. One patient took penicillin.
The single patient who was medically treated received 6 weeks of intravenous ceftriaxone. After 1 month, he was readmitted with blood cultures positive for P. acnes. He underwent surgery and a valve sequencing confirmed P. acnes as the infective agent.
There were two in-hospital deaths, but the rest of the patients were discharged on antibiotic therapy and recovered with no additional deaths or relapses.
The extended time P. acnes required to show in culture was enough for the Cleveland Clinic to reconsider incubation guidelines for the microorganism, Dr. Banzon said.
“There are enough cases taking 9 or 10 days that we now always hold these cultures for at least 10 days when we’re looking for P. acnes.”
She had no financial disclosures.
AMSTERDAM – Accounting for less than 1% of cases, Propionibacterium acnes has been considered an uncommon cause of infective endocarditis.
But data presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases suggest that the common anaerobe may be responsible for many more cases than is now believed. The bacteria are difficult to culture and grow very slowly, Dr. Jona Banzon said at the meeting. Incubating it for the standard 5 days may simply not be long enough.
“Due to this slow-growing nature, the standard incubation period may not be enough to detect it,” said Dr. Banzon, an infectious disease fellow at the Cleveland Clinic. “And since P. acnes is part of our commensal flora, it’s frequently a contaminate in culture, and we might be inappropriately dismissing it as such. In fact, we now wonder if this lack of extended incubation may be accounting for a significant proportion of negative blood cultures, and whether more prosthetic valve endocarditis than we think is actually being caused by P. acnes.”
Dr. Banzon presented a series of 23 cases included in the Cleveland Clinic Infective Endocarditis Registry from the period of 2007-2015. All had P. acnes confirmed as the causative organism. The group comprises 3.3% of the institution’s entire infective endocarditis registry, “making infective carditis with P. acnes already much more common than it is said to be in the literature,” she noted.
All of the cases were confirmed by any of the following standards:
• Two or more blood cultures positive for P. acnes.
• Two or more valve cultures positive for P. acnes.
• Two or more valve sequencing rests positive for P. acnes.
• At least two of the following: a positive blood culture, a positive valve culture or valve sequencing, or histopathologic demonstration of microorganism consistent with P. acnes.
Of the cohort, 22 had prosthetic valve endocarditis. One patient had endocarditis on a native valve. This is an important point, Dr. Banzon said.
The organism is being increasingly recognized for causing infections of prosthetic material, including shoulder joint infections and shunts, but it rarely seems to affect native tissue. The patient who had native valve endocarditis had experienced an episode of Staphylococcus aureus endocarditis about 18 months earlier. He was not treated surgically, and ended up with a damaged valve. After the initial illness, he was readmitted several times with symptoms of endocarditis, but all of his blood cultures were negative. This was attributed to the receipt of antibiotics. “When he was finally operated on, there were three valve sequencing specimens and all were positive for P. acnes,” Dr. Banzon said.
The patients in the cohort were a mean of 74 years old; about 75% were men. All of the cases were left-sided endocarditis, with the majority (74%) involving the aortic valve. Other sites included the mitral valve (18%), aortic plus mitral (4%), and aortic plus tricuspid (4%).
The most common predisposing factor was having a prosthetic valve (96%). Other factors included having a cardiac implantable device (17%), and a prior episode of infective endocarditis (13%). None of the patients had indwelling vascular catheters or used injectable drugs.
The cases presented with severe disease, Dr. Banzon said. Almost half (48%) had a perivalvular abscess at presentation. A third (35%) had valve dehiscence, and 35% had severe valvular regurgitation. There was a vegetation of more than 1 cm in 9% of cases.
Emboli were not uncommon; 17% had emboli in the central nervous system, and 17% had peripheral emboli. Two of these patients had kidney and spleen infarcts and two had acute arterial thromboembolism that required thrombectomy.
All of the patients underwent blood cultures; overall, 30% of the cultures were positive for P. acnes. But there were “striking differences” when the tests were broken down by incubation time, Dr. Banzon said.
Most of the cultures (16) were incubated for the standard of 5 days or less. Among these, 12.5% were positive. However, cultures on seven patients were incubated for more than 5 days and in this group, 71.4% were positive for P. acnes. The median time to positivity overall was 7 days, with a range of 3-9 days.
Other diagnostic methods were important in closing this gap, she said. Valve culturing was positive in 57% of the cases, while valve sequencing was positive in 95%. The median time to positive for valve culture was somewhat shorter than for blood culture (5.5 days).
In nine cases, no organism would have been identified without valve sequencing, Dr. Banzon said.
Because they presented with severe disease, almost all of the patients (22) underwent surgery as their intimal treatment. At the time of surgery, everyone was taking an antibiotic that covered P. acnes. Single-agent therapy was the definitive treatment for most, with vancomycin being most commonly employed (59%), followed by ceftriaxone (25%). A few patients had a combination of both drugs or a combination of vancomycin and rifampin. One patient took penicillin.
The single patient who was medically treated received 6 weeks of intravenous ceftriaxone. After 1 month, he was readmitted with blood cultures positive for P. acnes. He underwent surgery and a valve sequencing confirmed P. acnes as the infective agent.
There were two in-hospital deaths, but the rest of the patients were discharged on antibiotic therapy and recovered with no additional deaths or relapses.
The extended time P. acnes required to show in culture was enough for the Cleveland Clinic to reconsider incubation guidelines for the microorganism, Dr. Banzon said.
“There are enough cases taking 9 or 10 days that we now always hold these cultures for at least 10 days when we’re looking for P. acnes.”
She had no financial disclosures.
AMSTERDAM – Accounting for less than 1% of cases, Propionibacterium acnes has been considered an uncommon cause of infective endocarditis.
But data presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases suggest that the common anaerobe may be responsible for many more cases than is now believed. The bacteria are difficult to culture and grow very slowly, Dr. Jona Banzon said at the meeting. Incubating it for the standard 5 days may simply not be long enough.
“Due to this slow-growing nature, the standard incubation period may not be enough to detect it,” said Dr. Banzon, an infectious disease fellow at the Cleveland Clinic. “And since P. acnes is part of our commensal flora, it’s frequently a contaminate in culture, and we might be inappropriately dismissing it as such. In fact, we now wonder if this lack of extended incubation may be accounting for a significant proportion of negative blood cultures, and whether more prosthetic valve endocarditis than we think is actually being caused by P. acnes.”
Dr. Banzon presented a series of 23 cases included in the Cleveland Clinic Infective Endocarditis Registry from the period of 2007-2015. All had P. acnes confirmed as the causative organism. The group comprises 3.3% of the institution’s entire infective endocarditis registry, “making infective carditis with P. acnes already much more common than it is said to be in the literature,” she noted.
All of the cases were confirmed by any of the following standards:
• Two or more blood cultures positive for P. acnes.
• Two or more valve cultures positive for P. acnes.
• Two or more valve sequencing rests positive for P. acnes.
• At least two of the following: a positive blood culture, a positive valve culture or valve sequencing, or histopathologic demonstration of microorganism consistent with P. acnes.
Of the cohort, 22 had prosthetic valve endocarditis. One patient had endocarditis on a native valve. This is an important point, Dr. Banzon said.
The organism is being increasingly recognized for causing infections of prosthetic material, including shoulder joint infections and shunts, but it rarely seems to affect native tissue. The patient who had native valve endocarditis had experienced an episode of Staphylococcus aureus endocarditis about 18 months earlier. He was not treated surgically, and ended up with a damaged valve. After the initial illness, he was readmitted several times with symptoms of endocarditis, but all of his blood cultures were negative. This was attributed to the receipt of antibiotics. “When he was finally operated on, there were three valve sequencing specimens and all were positive for P. acnes,” Dr. Banzon said.
The patients in the cohort were a mean of 74 years old; about 75% were men. All of the cases were left-sided endocarditis, with the majority (74%) involving the aortic valve. Other sites included the mitral valve (18%), aortic plus mitral (4%), and aortic plus tricuspid (4%).
The most common predisposing factor was having a prosthetic valve (96%). Other factors included having a cardiac implantable device (17%), and a prior episode of infective endocarditis (13%). None of the patients had indwelling vascular catheters or used injectable drugs.
The cases presented with severe disease, Dr. Banzon said. Almost half (48%) had a perivalvular abscess at presentation. A third (35%) had valve dehiscence, and 35% had severe valvular regurgitation. There was a vegetation of more than 1 cm in 9% of cases.
Emboli were not uncommon; 17% had emboli in the central nervous system, and 17% had peripheral emboli. Two of these patients had kidney and spleen infarcts and two had acute arterial thromboembolism that required thrombectomy.
All of the patients underwent blood cultures; overall, 30% of the cultures were positive for P. acnes. But there were “striking differences” when the tests were broken down by incubation time, Dr. Banzon said.
Most of the cultures (16) were incubated for the standard of 5 days or less. Among these, 12.5% were positive. However, cultures on seven patients were incubated for more than 5 days and in this group, 71.4% were positive for P. acnes. The median time to positivity overall was 7 days, with a range of 3-9 days.
Other diagnostic methods were important in closing this gap, she said. Valve culturing was positive in 57% of the cases, while valve sequencing was positive in 95%. The median time to positive for valve culture was somewhat shorter than for blood culture (5.5 days).
In nine cases, no organism would have been identified without valve sequencing, Dr. Banzon said.
Because they presented with severe disease, almost all of the patients (22) underwent surgery as their intimal treatment. At the time of surgery, everyone was taking an antibiotic that covered P. acnes. Single-agent therapy was the definitive treatment for most, with vancomycin being most commonly employed (59%), followed by ceftriaxone (25%). A few patients had a combination of both drugs or a combination of vancomycin and rifampin. One patient took penicillin.
The single patient who was medically treated received 6 weeks of intravenous ceftriaxone. After 1 month, he was readmitted with blood cultures positive for P. acnes. He underwent surgery and a valve sequencing confirmed P. acnes as the infective agent.
There were two in-hospital deaths, but the rest of the patients were discharged on antibiotic therapy and recovered with no additional deaths or relapses.
The extended time P. acnes required to show in culture was enough for the Cleveland Clinic to reconsider incubation guidelines for the microorganism, Dr. Banzon said.
“There are enough cases taking 9 or 10 days that we now always hold these cultures for at least 10 days when we’re looking for P. acnes.”
She had no financial disclosures.
AT ECCMID 2016
Key clinical point: P. acnes’ slow growth may result in false negative blood cultures in infective endocarditis.
Major finding: Just 12% of blood cultures incubated for 5 days were positive, while 71% of those incubated for more than 5 days were positive.
Data source: The Cleveland Clinic Infective Endocarditis Registry.
Disclosures: Dr. Banzon had no financial disclosures.
Asthma, eczema in children unrelated to allergic sensitization
Atopy was not related to development of eczema or asthma in children under age 13 years, according to Ann-Marie Malby Schoos, Ph.D., and her associates at the University of Copenhagen.
Allergic sensitization increased with age in the 399 children tested, rising from 12% at 6 months to 54% at 13 years. The incidence of asthma was highest at age 4 years at 16%, but decreased afterward, falling to 12% at 13 years. The incidence of eczema peaked at 39% in children aged 1.5 years old, but decreased steadily to only 12% in 13-year-olds.
Asthma and allergic sensitization were related only in late childhood, with an odds ratio of 4.49 in 13-year-olds. This pattern was seen throughout allergic sensitization subgroups. There were strong associations between eczema and allergic sensitization at 6 months (OR, 6.02), 1.5 years (OR, 2.06), and 6 years (OR, 2.77), but no association at 13 years. The proportion of children with allergic sensitization who did not have asthma or eczema also increased with age.
“The tradition of using atopy as a particular endotype of asthma and eczema seems unfounded because it depends on the method of testing for sensitization, type of allergens, and age of the patient. This questions the relevance of the terms atopic asthma and atopic eczema as true endotypes,” the investigators concluded.
Find the full study in the Journal of Allergy and Clinical Immunology (doi: 10.1016/j.jaci.2015.10.004).
Atopy was not related to development of eczema or asthma in children under age 13 years, according to Ann-Marie Malby Schoos, Ph.D., and her associates at the University of Copenhagen.
Allergic sensitization increased with age in the 399 children tested, rising from 12% at 6 months to 54% at 13 years. The incidence of asthma was highest at age 4 years at 16%, but decreased afterward, falling to 12% at 13 years. The incidence of eczema peaked at 39% in children aged 1.5 years old, but decreased steadily to only 12% in 13-year-olds.
Asthma and allergic sensitization were related only in late childhood, with an odds ratio of 4.49 in 13-year-olds. This pattern was seen throughout allergic sensitization subgroups. There were strong associations between eczema and allergic sensitization at 6 months (OR, 6.02), 1.5 years (OR, 2.06), and 6 years (OR, 2.77), but no association at 13 years. The proportion of children with allergic sensitization who did not have asthma or eczema also increased with age.
“The tradition of using atopy as a particular endotype of asthma and eczema seems unfounded because it depends on the method of testing for sensitization, type of allergens, and age of the patient. This questions the relevance of the terms atopic asthma and atopic eczema as true endotypes,” the investigators concluded.
Find the full study in the Journal of Allergy and Clinical Immunology (doi: 10.1016/j.jaci.2015.10.004).
Atopy was not related to development of eczema or asthma in children under age 13 years, according to Ann-Marie Malby Schoos, Ph.D., and her associates at the University of Copenhagen.
Allergic sensitization increased with age in the 399 children tested, rising from 12% at 6 months to 54% at 13 years. The incidence of asthma was highest at age 4 years at 16%, but decreased afterward, falling to 12% at 13 years. The incidence of eczema peaked at 39% in children aged 1.5 years old, but decreased steadily to only 12% in 13-year-olds.
Asthma and allergic sensitization were related only in late childhood, with an odds ratio of 4.49 in 13-year-olds. This pattern was seen throughout allergic sensitization subgroups. There were strong associations between eczema and allergic sensitization at 6 months (OR, 6.02), 1.5 years (OR, 2.06), and 6 years (OR, 2.77), but no association at 13 years. The proportion of children with allergic sensitization who did not have asthma or eczema also increased with age.
“The tradition of using atopy as a particular endotype of asthma and eczema seems unfounded because it depends on the method of testing for sensitization, type of allergens, and age of the patient. This questions the relevance of the terms atopic asthma and atopic eczema as true endotypes,” the investigators concluded.
Find the full study in the Journal of Allergy and Clinical Immunology (doi: 10.1016/j.jaci.2015.10.004).
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Drug-eluting stent recipients can safely have surgery sooner
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
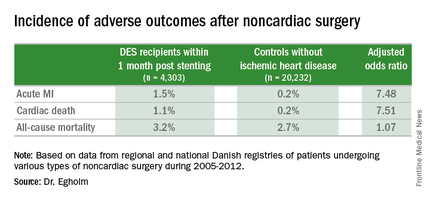
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.
Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
CHICAGO – Current U.S. and European guidelines recommending postponement of noncardiac surgery for 6-12 months after drug-eluting stent implantation appear to be excessive, Dr. Gro Egholm reported at the annual meeting of the American College of Cardiology.
She presented a large retrospective observational study of outcomes in patients undergoing various types of noncardiac surgery in western Denmark during 2005-2012. Among 4,303 patients who had noncardiac surgery within 12 months after receiving a drug-eluting stent (DES), only those whose operations took place during the first month post stenting had increased risks of acute MI and cardiac death within 30 days post surgery.
Risks of major adverse cardiac events among the DES recipients who had noncardiac surgery within that first month post–percutaneous coronary intervention were increased roughly 7.5-fold compared with controls, but for surgery performed after that the risks of MI and cardiac death dropped off abruptly and were no different from rates in 20,232 controls without ischemic heart disease or stents who were matched for age, gender, surgical procedure, and Charlson Comorbidity Index, according to Dr. Egholm of Aarhus (Denmark) University.
Moreover, even in DES recipients undergoing noncardiac surgery during the first month post stenting, all-cause mortality was no greater than in controls.
“Surgery could be performed much earlier than recommended,” she concluded.
Her study was carried out by linking data from comprehensive regional and national Danish health care registries. Most patients with DES remained on dual antiplatelet therapy periprocedurally. The exceptions were neurosurgical operations and others where it’s standard that dual antiplatelet therapy must be stopped.
“If you can continue only one antiplatelet agent, aspirin would be the most appealing,” she said.

Of the DES participants, 56% received their device as treatment for an acute coronary syndrome. The average time from stent placement to noncardiac surgery in this large series was 147 days.
Session co-chair Dr. Sunil V. Rao of Duke University in Durham, N.C., called this work “a very important study that’s relevant to daily practice.” However, he found the 23% incidence of noncardiac surgery within 12 months following DES implantation reported in Dr. Egholm’s study to be “shockingly high.” She agreed, noting that rates in some non-Danish registries she’s looked at are more in the 8%-15% range. But Denmark’s health care registries are known for rigorous accuracy and completeness.
Dr. Egholm reported having no financial conflicts regarding her study.
AT ACC 16
Key clinical point: The risk of noncardiac surgery is elevated only when the operation occurs during the first month after stenting.
Major finding: Danish drug-eluting stent recipients who underwent noncardiac surgery within 1 month after stent placement were at 7.5-fold increased risks of acute MI and cardiac death, but surgery performed 2-12 months post stenting carried no increased risks.
Data source: This retrospective observational study based upon large Danish patient registries compared outcomes of noncardiac surgery performed within 12 months after drug-eluting stent placement in 4,303 patients with 20,232 matched controls without ischemic heart disease who underwent the same operations.
Disclosures: The study was supported by Danish research funds. The presenter reported having no financial conflicts of interest.
FDG-PET guides need for eBEACOPP in advanced Hodgkin’s
Using FDG-PET (fluorodeoxyglucose positron emission tomography) imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to a more aggressive eBEACOPP regimen, according to the results of the Southwest Oncology Group (SWOG) S0816 study.
In this large U.S. trial of PET scanning to guide treatment approach in people with high-risk stage II or stage III-IV Hodgkin’s lymphoma, progression-free survival at 2 years for those with early interim positive PET scans was 64%, which is much higher than the expected progression-free survival of 15%-30%, according to Dr. Oliver Press, a SWOG member at Fred Hutchinson Cancer Research Center and the lead author of study, which was published ahead of print in the Journal of Clinical Oncology (2016 April 11. doi: 10.1200/JCO.2015.63.1119).
In addition, just 20% of patients in the trial were exposed to eBEACOPP, which usually results in infertility, can cause sustained heart or lung damage, and increases the risk of secondary cancers.
Researchers recruited 358 Hodgkin’s patients to the trial and were able to evaluate 331 of them. All trial volunteers were given two rounds of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, followed by a PET scan. If the scan was negative, patients received four more cycles of ABVD. If the scan was positive, with a Deauville score of 4-5, they were advised to switch to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), a seven-drug combination used in Europe. Of 60 patients with positive interim PET scans, 11 patients declined to switch, and 49 switched as planned to six cycles of eBEACOPP.
With a median follow-up of nearly 40 months, the Kaplan-Meier estimate for 2-year overall survival was 98%, and the 2-year estimate for progression-free survival was 79%. In the subset of patients who had positive PET scans after two cycles of ABVD, the 2-year estimate for progression-free survival was 64%, more than double the expected remission rate.
At least seven phase II and III cooperative group studies are underway testing this approach in advanced-stage Hodgkin’s lymphoma, the researchers wrote. “We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging, may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than is achievable with current technology.”
The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
On Twitter @maryjodales
Using FDG-PET (fluorodeoxyglucose positron emission tomography) imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to a more aggressive eBEACOPP regimen, according to the results of the Southwest Oncology Group (SWOG) S0816 study.
In this large U.S. trial of PET scanning to guide treatment approach in people with high-risk stage II or stage III-IV Hodgkin’s lymphoma, progression-free survival at 2 years for those with early interim positive PET scans was 64%, which is much higher than the expected progression-free survival of 15%-30%, according to Dr. Oliver Press, a SWOG member at Fred Hutchinson Cancer Research Center and the lead author of study, which was published ahead of print in the Journal of Clinical Oncology (2016 April 11. doi: 10.1200/JCO.2015.63.1119).
In addition, just 20% of patients in the trial were exposed to eBEACOPP, which usually results in infertility, can cause sustained heart or lung damage, and increases the risk of secondary cancers.
Researchers recruited 358 Hodgkin’s patients to the trial and were able to evaluate 331 of them. All trial volunteers were given two rounds of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, followed by a PET scan. If the scan was negative, patients received four more cycles of ABVD. If the scan was positive, with a Deauville score of 4-5, they were advised to switch to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), a seven-drug combination used in Europe. Of 60 patients with positive interim PET scans, 11 patients declined to switch, and 49 switched as planned to six cycles of eBEACOPP.
With a median follow-up of nearly 40 months, the Kaplan-Meier estimate for 2-year overall survival was 98%, and the 2-year estimate for progression-free survival was 79%. In the subset of patients who had positive PET scans after two cycles of ABVD, the 2-year estimate for progression-free survival was 64%, more than double the expected remission rate.
At least seven phase II and III cooperative group studies are underway testing this approach in advanced-stage Hodgkin’s lymphoma, the researchers wrote. “We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging, may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than is achievable with current technology.”
The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
On Twitter @maryjodales
Using FDG-PET (fluorodeoxyglucose positron emission tomography) imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to a more aggressive eBEACOPP regimen, according to the results of the Southwest Oncology Group (SWOG) S0816 study.
In this large U.S. trial of PET scanning to guide treatment approach in people with high-risk stage II or stage III-IV Hodgkin’s lymphoma, progression-free survival at 2 years for those with early interim positive PET scans was 64%, which is much higher than the expected progression-free survival of 15%-30%, according to Dr. Oliver Press, a SWOG member at Fred Hutchinson Cancer Research Center and the lead author of study, which was published ahead of print in the Journal of Clinical Oncology (2016 April 11. doi: 10.1200/JCO.2015.63.1119).
In addition, just 20% of patients in the trial were exposed to eBEACOPP, which usually results in infertility, can cause sustained heart or lung damage, and increases the risk of secondary cancers.
Researchers recruited 358 Hodgkin’s patients to the trial and were able to evaluate 331 of them. All trial volunteers were given two rounds of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, followed by a PET scan. If the scan was negative, patients received four more cycles of ABVD. If the scan was positive, with a Deauville score of 4-5, they were advised to switch to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), a seven-drug combination used in Europe. Of 60 patients with positive interim PET scans, 11 patients declined to switch, and 49 switched as planned to six cycles of eBEACOPP.
With a median follow-up of nearly 40 months, the Kaplan-Meier estimate for 2-year overall survival was 98%, and the 2-year estimate for progression-free survival was 79%. In the subset of patients who had positive PET scans after two cycles of ABVD, the 2-year estimate for progression-free survival was 64%, more than double the expected remission rate.
At least seven phase II and III cooperative group studies are underway testing this approach in advanced-stage Hodgkin’s lymphoma, the researchers wrote. “We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging, may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than is achievable with current technology.”
The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
On Twitter @maryjodales
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Using FDG-PET imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to the eBEACOPP regimen.
Major finding: Progression-free survival at 2 years for those with early interim positive PET scans was 64%; the historical progression-free survival for this group is 15%-30%.
Data source: Evaluations of 331 patients in the Southwest Oncology Group S0816 study.
Disclosures: The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
CUDC-907 enters phase II for relapsed or refractory lymphoma and multiple myeloma
Another oral, small-molecule therapy called CUDC-907 is emerging from phase I testing as a treatment option for patients with relapsed or refractory lymphoma and multiple myeloma.
The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule, according to Dr. Anas Younes of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. A dose-expansion trial of this dose is ongoing, and the drug appears to be useful in particular for patients with refractory and relapsed diffuse large B-cell lymphoma.
The researchers tested CUDC-907, which is designed to inhibit histone deacetylase and PI3K enzyme pathways, for overall safety and response in 44 patients at four cancer centers. All participants had lymphoma or multiple myeloma and were refractory to treatment or had relapsed after two or more previous regimens.
The 44 participants were sequentially assigned to 21-day cycles of CUDC-907: 10 to once daily, 12 to twice weekly, 15 to three times weekly, and 7 to daily for 5 days followed by a 2-day break. The maximum tolerated doses were 60 mg for the once-daily schedule, 150 mg for the twice-weekly schedule, 150 mg for the three-times-weekly schedule, and 60 mg for the 5-on/2-off schedule. At data cutoff, 37 of the 44 patients had discontinued CUDC-907 because of disease progression, Dr. Younes and his associates reported in a study published online (Lancet Oncol. 2016 Mar 31. doi: 10.1016/S1470-2045(15)00584-7).
Four dose-limiting toxicities occurred in 3 of 40 evaluable patients. Grade 3 or worse adverse events occurred in 19 of 44 patients: 9 had thrombocytopenia, 3 had neutropenia, 3 had hyperglycemia. Adverse events led to dose reductions in six patients and treatment discontinuation in seven.
Of 37 response-evaluable patients, two had complete responses and three had partial responses. All five were seen in the subgroup of nine patients with diffuse large B-cell lymphoma, and three occurred in the five patients with transformed follicular disease. The 21 patients with stable disease included those with diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma. This ongoing trial is registered at ClinicalTrials.gov as NCT01742988.
The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
On Twitter @maryjodales
Another oral, small-molecule therapy called CUDC-907 is emerging from phase I testing as a treatment option for patients with relapsed or refractory lymphoma and multiple myeloma.
The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule, according to Dr. Anas Younes of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. A dose-expansion trial of this dose is ongoing, and the drug appears to be useful in particular for patients with refractory and relapsed diffuse large B-cell lymphoma.
The researchers tested CUDC-907, which is designed to inhibit histone deacetylase and PI3K enzyme pathways, for overall safety and response in 44 patients at four cancer centers. All participants had lymphoma or multiple myeloma and were refractory to treatment or had relapsed after two or more previous regimens.
The 44 participants were sequentially assigned to 21-day cycles of CUDC-907: 10 to once daily, 12 to twice weekly, 15 to three times weekly, and 7 to daily for 5 days followed by a 2-day break. The maximum tolerated doses were 60 mg for the once-daily schedule, 150 mg for the twice-weekly schedule, 150 mg for the three-times-weekly schedule, and 60 mg for the 5-on/2-off schedule. At data cutoff, 37 of the 44 patients had discontinued CUDC-907 because of disease progression, Dr. Younes and his associates reported in a study published online (Lancet Oncol. 2016 Mar 31. doi: 10.1016/S1470-2045(15)00584-7).
Four dose-limiting toxicities occurred in 3 of 40 evaluable patients. Grade 3 or worse adverse events occurred in 19 of 44 patients: 9 had thrombocytopenia, 3 had neutropenia, 3 had hyperglycemia. Adverse events led to dose reductions in six patients and treatment discontinuation in seven.
Of 37 response-evaluable patients, two had complete responses and three had partial responses. All five were seen in the subgroup of nine patients with diffuse large B-cell lymphoma, and three occurred in the five patients with transformed follicular disease. The 21 patients with stable disease included those with diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma. This ongoing trial is registered at ClinicalTrials.gov as NCT01742988.
The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
On Twitter @maryjodales
Another oral, small-molecule therapy called CUDC-907 is emerging from phase I testing as a treatment option for patients with relapsed or refractory lymphoma and multiple myeloma.
The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule, according to Dr. Anas Younes of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. A dose-expansion trial of this dose is ongoing, and the drug appears to be useful in particular for patients with refractory and relapsed diffuse large B-cell lymphoma.
The researchers tested CUDC-907, which is designed to inhibit histone deacetylase and PI3K enzyme pathways, for overall safety and response in 44 patients at four cancer centers. All participants had lymphoma or multiple myeloma and were refractory to treatment or had relapsed after two or more previous regimens.
The 44 participants were sequentially assigned to 21-day cycles of CUDC-907: 10 to once daily, 12 to twice weekly, 15 to three times weekly, and 7 to daily for 5 days followed by a 2-day break. The maximum tolerated doses were 60 mg for the once-daily schedule, 150 mg for the twice-weekly schedule, 150 mg for the three-times-weekly schedule, and 60 mg for the 5-on/2-off schedule. At data cutoff, 37 of the 44 patients had discontinued CUDC-907 because of disease progression, Dr. Younes and his associates reported in a study published online (Lancet Oncol. 2016 Mar 31. doi: 10.1016/S1470-2045(15)00584-7).
Four dose-limiting toxicities occurred in 3 of 40 evaluable patients. Grade 3 or worse adverse events occurred in 19 of 44 patients: 9 had thrombocytopenia, 3 had neutropenia, 3 had hyperglycemia. Adverse events led to dose reductions in six patients and treatment discontinuation in seven.
Of 37 response-evaluable patients, two had complete responses and three had partial responses. All five were seen in the subgroup of nine patients with diffuse large B-cell lymphoma, and three occurred in the five patients with transformed follicular disease. The 21 patients with stable disease included those with diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma. This ongoing trial is registered at ClinicalTrials.gov as NCT01742988.
The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
On Twitter @maryjodales
FROM THE LANCET ONCOLOGY
Key clinical point: The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule.
Major finding: Two complete responses and three partial responses were seen in the subgroup of nine patients with diffuse large B-cell lymphoma; three occurred in the five patients with transformed follicular disease.
Data source: A dose-escalation study involving 44 patients at four cancer centers.
Disclosures: The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
Modifying our behavior
“Just say no to overprescribing!” It has such a straightforward Nancy Reagan-ish sound to it. But when it comes to drugs, whether it is crack cocaine or a prescription antibiotic, simple slogans don’t alter behavior.
While most physicians aren’t drug addicts, we do share something in common with other substance abusers. We are all human, and we are all influenced by the social contexts that we inhabit. The global health problems rippling out from the overuse of antibiotics are significant, unmistakable, and well documented. Certainly, we physicians must share some of the blame with the food industry for this unfortunate situation. There is some glimmer of hope that pressure from consumers has begun to convince a few food producers to be more judicious in their use of antibiotics.
However, there seems to be little or no pressure from patients on physicians to curtail our antibiotic prescribing habits. If physicians feel any pressure from patients, it is in the form of stated or more often unstated requests for antibiotics to treat conditions for which we know they are inappropriate. There is some question as to how often this perception of patient pressure actually occurs. It may be that the pressure physicians are feeling could be better described as fear – fear that the patient will die because of an undiscovered and untreated infection. Regardless of what motivates physicians to overprescribe antibiotics, the fact is that this kind of clinical misbehavior is difficult to change.
I recently read an article in which three medical school professors describe several behavior modification strategies that they have found to be effective in discouraging overprescribing (“How to Stop Overprescribing Antibiotics,” by Craig R. Fox, Jeffrey A. Linder, and Jason N. Doctor, New York Times, March 25, 2016). In one study, the researchers found that physicians who posted a pledge to follow antibiotic guidelines reduced inappropriate prescribing by 20%. In another study the investigators found that when physicians were presented with a list of medications in a format that presented the “more aggressive” drugs in a group, as opposed to singly in a vertical column, the physicians were 12% less likely to prescribe those medications.
Better results were achieved when physicians were provided with monthly reports of their prescribing habits in comparison with those of their peers. The physicians whose prescribing patterns followed accepted guidelines most closely were complimented as being “top performers.” Those physicians who did less well were told, “You are not a top performer.” This strategy nearly eliminated inappropriate prescribing. Similar improvement occurred when physicians who clicked their mouse on an antibiotic in a clinical scenario where it was not appropriate were given a screen prompt asking them to type in a short “antibiotic justification note.”
What all of these strategies have in common is that none of them uses financial gain as a motivator. Previous studies have shown that if financial rewards work, it is only for short periods of time. Instead, these strategies leverage our inherent competitive nature and take advantage of the fact that most of us want to do the right thing. We just need a little nudge every now and then. It is also encouraging to learn that none of these strategies incorporates a punishment.
I suspect that further studies will show that a screen prompt in the medical record requiring the overprescribing physician to justify his or her prescription will be the most effective in the long run. In my experience, physicians will do anything to shorten the amount of time they spend at their office computers.
At least two of these strategies hold the promise of being very powerful behavior modifiers. Those wielding these powerful tools must exercise that power carefully and be sure that evidence supporting their target behaviors is solid and continually updated. More importantly, those of us whose behavior is being modified should have a voice in the choice of which behaviors are to be modified.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
“Just say no to overprescribing!” It has such a straightforward Nancy Reagan-ish sound to it. But when it comes to drugs, whether it is crack cocaine or a prescription antibiotic, simple slogans don’t alter behavior.
While most physicians aren’t drug addicts, we do share something in common with other substance abusers. We are all human, and we are all influenced by the social contexts that we inhabit. The global health problems rippling out from the overuse of antibiotics are significant, unmistakable, and well documented. Certainly, we physicians must share some of the blame with the food industry for this unfortunate situation. There is some glimmer of hope that pressure from consumers has begun to convince a few food producers to be more judicious in their use of antibiotics.
However, there seems to be little or no pressure from patients on physicians to curtail our antibiotic prescribing habits. If physicians feel any pressure from patients, it is in the form of stated or more often unstated requests for antibiotics to treat conditions for which we know they are inappropriate. There is some question as to how often this perception of patient pressure actually occurs. It may be that the pressure physicians are feeling could be better described as fear – fear that the patient will die because of an undiscovered and untreated infection. Regardless of what motivates physicians to overprescribe antibiotics, the fact is that this kind of clinical misbehavior is difficult to change.
I recently read an article in which three medical school professors describe several behavior modification strategies that they have found to be effective in discouraging overprescribing (“How to Stop Overprescribing Antibiotics,” by Craig R. Fox, Jeffrey A. Linder, and Jason N. Doctor, New York Times, March 25, 2016). In one study, the researchers found that physicians who posted a pledge to follow antibiotic guidelines reduced inappropriate prescribing by 20%. In another study the investigators found that when physicians were presented with a list of medications in a format that presented the “more aggressive” drugs in a group, as opposed to singly in a vertical column, the physicians were 12% less likely to prescribe those medications.
Better results were achieved when physicians were provided with monthly reports of their prescribing habits in comparison with those of their peers. The physicians whose prescribing patterns followed accepted guidelines most closely were complimented as being “top performers.” Those physicians who did less well were told, “You are not a top performer.” This strategy nearly eliminated inappropriate prescribing. Similar improvement occurred when physicians who clicked their mouse on an antibiotic in a clinical scenario where it was not appropriate were given a screen prompt asking them to type in a short “antibiotic justification note.”
What all of these strategies have in common is that none of them uses financial gain as a motivator. Previous studies have shown that if financial rewards work, it is only for short periods of time. Instead, these strategies leverage our inherent competitive nature and take advantage of the fact that most of us want to do the right thing. We just need a little nudge every now and then. It is also encouraging to learn that none of these strategies incorporates a punishment.
I suspect that further studies will show that a screen prompt in the medical record requiring the overprescribing physician to justify his or her prescription will be the most effective in the long run. In my experience, physicians will do anything to shorten the amount of time they spend at their office computers.
At least two of these strategies hold the promise of being very powerful behavior modifiers. Those wielding these powerful tools must exercise that power carefully and be sure that evidence supporting their target behaviors is solid and continually updated. More importantly, those of us whose behavior is being modified should have a voice in the choice of which behaviors are to be modified.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
“Just say no to overprescribing!” It has such a straightforward Nancy Reagan-ish sound to it. But when it comes to drugs, whether it is crack cocaine or a prescription antibiotic, simple slogans don’t alter behavior.
While most physicians aren’t drug addicts, we do share something in common with other substance abusers. We are all human, and we are all influenced by the social contexts that we inhabit. The global health problems rippling out from the overuse of antibiotics are significant, unmistakable, and well documented. Certainly, we physicians must share some of the blame with the food industry for this unfortunate situation. There is some glimmer of hope that pressure from consumers has begun to convince a few food producers to be more judicious in their use of antibiotics.
However, there seems to be little or no pressure from patients on physicians to curtail our antibiotic prescribing habits. If physicians feel any pressure from patients, it is in the form of stated or more often unstated requests for antibiotics to treat conditions for which we know they are inappropriate. There is some question as to how often this perception of patient pressure actually occurs. It may be that the pressure physicians are feeling could be better described as fear – fear that the patient will die because of an undiscovered and untreated infection. Regardless of what motivates physicians to overprescribe antibiotics, the fact is that this kind of clinical misbehavior is difficult to change.
I recently read an article in which three medical school professors describe several behavior modification strategies that they have found to be effective in discouraging overprescribing (“How to Stop Overprescribing Antibiotics,” by Craig R. Fox, Jeffrey A. Linder, and Jason N. Doctor, New York Times, March 25, 2016). In one study, the researchers found that physicians who posted a pledge to follow antibiotic guidelines reduced inappropriate prescribing by 20%. In another study the investigators found that when physicians were presented with a list of medications in a format that presented the “more aggressive” drugs in a group, as opposed to singly in a vertical column, the physicians were 12% less likely to prescribe those medications.
Better results were achieved when physicians were provided with monthly reports of their prescribing habits in comparison with those of their peers. The physicians whose prescribing patterns followed accepted guidelines most closely were complimented as being “top performers.” Those physicians who did less well were told, “You are not a top performer.” This strategy nearly eliminated inappropriate prescribing. Similar improvement occurred when physicians who clicked their mouse on an antibiotic in a clinical scenario where it was not appropriate were given a screen prompt asking them to type in a short “antibiotic justification note.”
What all of these strategies have in common is that none of them uses financial gain as a motivator. Previous studies have shown that if financial rewards work, it is only for short periods of time. Instead, these strategies leverage our inherent competitive nature and take advantage of the fact that most of us want to do the right thing. We just need a little nudge every now and then. It is also encouraging to learn that none of these strategies incorporates a punishment.
I suspect that further studies will show that a screen prompt in the medical record requiring the overprescribing physician to justify his or her prescription will be the most effective in the long run. In my experience, physicians will do anything to shorten the amount of time they spend at their office computers.
At least two of these strategies hold the promise of being very powerful behavior modifiers. Those wielding these powerful tools must exercise that power carefully and be sure that evidence supporting their target behaviors is solid and continually updated. More importantly, those of us whose behavior is being modified should have a voice in the choice of which behaviors are to be modified.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
Failure of Promising Treatments for Controlling Cholesterol Leads to More Studies
(Reuters) - New ways of controlling cholesterol, including possibly directly injecting "good" HDL cholesterol into patients, need to be studied following the failure of promising treatments from Eli Lilly, Pfizer Inc and Roche Holding AG, according to top heart researchers.
Lilly in October halted a 12,000-patient study of its experimental drug evacetrapib, an oral medication that in smaller earlier studies slashed "bad" LDL cholesterol and doubled levels of HDL.
But improved cholesterol levels did not prevent heart attacks and strokes, diminishing hopes for the approach to treating heart disease - by raising HDL through blockage of a protein called CETP.
Roche in 2012 scrapped its own CETP inhibitor after it also failed to help patients. Pfizer's similar drug was discontinued in 2006 after being linked to deaths in trials.
Although Merck & Co continues to develop its own CETP inhibitor in a 30,000-patient study expected to be completed next year, researchers on Sunday said the failures of the Lilly, Roche and Pfizer drugs bode poorly for it.
"Merck's drug is the fourth shot on goal for CETP inhibitors, but with disappointment or lack of success for the other agents you have to be increasingly pessimistic" about the class of drugs, said Dr. Stephen Nicholls, deputy director of the South Australian Health and Medical Research Institute in Adelaide, Australia. He was a lead investigator for the failed trial of Lilly's drug.
Nicholls and Dr. Steve Nissen, the head of cardiology for the Cleveland Clinic, who co-lead the evacetrapib study, on Sunday reviewed the baffling evacetrapib data in a presentation at the annual scientific sessions of the American College of Cardiology in Chicago.
"This drug lowered LDL by 37 percent and raised HDL by 130 percent and had absolutely no effect" on preventing deaths and heart attacks, Nissen said in an interview.
Although other ways of raising HDL cholesterol might eventually prove protective, Nissen said all attempts so far have been fruitless.
Nicholls said he remains hopeful of future HDL therapies and is testing whether artificial HDL can be made in the laboratory and injected directly into high-risk heart patients. "There is enthusiasm it may be able to shrink plaque" in heart arteries, he said.
He said he is studying variations of that approach with French drugmaker Cerenis Therapeutics and the Medicines Company. Nicholls said another possible approach would be to instruct the liver to make more HDL.
(Reuters) - New ways of controlling cholesterol, including possibly directly injecting "good" HDL cholesterol into patients, need to be studied following the failure of promising treatments from Eli Lilly, Pfizer Inc and Roche Holding AG, according to top heart researchers.
Lilly in October halted a 12,000-patient study of its experimental drug evacetrapib, an oral medication that in smaller earlier studies slashed "bad" LDL cholesterol and doubled levels of HDL.
But improved cholesterol levels did not prevent heart attacks and strokes, diminishing hopes for the approach to treating heart disease - by raising HDL through blockage of a protein called CETP.
Roche in 2012 scrapped its own CETP inhibitor after it also failed to help patients. Pfizer's similar drug was discontinued in 2006 after being linked to deaths in trials.
Although Merck & Co continues to develop its own CETP inhibitor in a 30,000-patient study expected to be completed next year, researchers on Sunday said the failures of the Lilly, Roche and Pfizer drugs bode poorly for it.
"Merck's drug is the fourth shot on goal for CETP inhibitors, but with disappointment or lack of success for the other agents you have to be increasingly pessimistic" about the class of drugs, said Dr. Stephen Nicholls, deputy director of the South Australian Health and Medical Research Institute in Adelaide, Australia. He was a lead investigator for the failed trial of Lilly's drug.
Nicholls and Dr. Steve Nissen, the head of cardiology for the Cleveland Clinic, who co-lead the evacetrapib study, on Sunday reviewed the baffling evacetrapib data in a presentation at the annual scientific sessions of the American College of Cardiology in Chicago.
"This drug lowered LDL by 37 percent and raised HDL by 130 percent and had absolutely no effect" on preventing deaths and heart attacks, Nissen said in an interview.
Although other ways of raising HDL cholesterol might eventually prove protective, Nissen said all attempts so far have been fruitless.
Nicholls said he remains hopeful of future HDL therapies and is testing whether artificial HDL can be made in the laboratory and injected directly into high-risk heart patients. "There is enthusiasm it may be able to shrink plaque" in heart arteries, he said.
He said he is studying variations of that approach with French drugmaker Cerenis Therapeutics and the Medicines Company. Nicholls said another possible approach would be to instruct the liver to make more HDL.
(Reuters) - New ways of controlling cholesterol, including possibly directly injecting "good" HDL cholesterol into patients, need to be studied following the failure of promising treatments from Eli Lilly, Pfizer Inc and Roche Holding AG, according to top heart researchers.
Lilly in October halted a 12,000-patient study of its experimental drug evacetrapib, an oral medication that in smaller earlier studies slashed "bad" LDL cholesterol and doubled levels of HDL.
But improved cholesterol levels did not prevent heart attacks and strokes, diminishing hopes for the approach to treating heart disease - by raising HDL through blockage of a protein called CETP.
Roche in 2012 scrapped its own CETP inhibitor after it also failed to help patients. Pfizer's similar drug was discontinued in 2006 after being linked to deaths in trials.
Although Merck & Co continues to develop its own CETP inhibitor in a 30,000-patient study expected to be completed next year, researchers on Sunday said the failures of the Lilly, Roche and Pfizer drugs bode poorly for it.
"Merck's drug is the fourth shot on goal for CETP inhibitors, but with disappointment or lack of success for the other agents you have to be increasingly pessimistic" about the class of drugs, said Dr. Stephen Nicholls, deputy director of the South Australian Health and Medical Research Institute in Adelaide, Australia. He was a lead investigator for the failed trial of Lilly's drug.
Nicholls and Dr. Steve Nissen, the head of cardiology for the Cleveland Clinic, who co-lead the evacetrapib study, on Sunday reviewed the baffling evacetrapib data in a presentation at the annual scientific sessions of the American College of Cardiology in Chicago.
"This drug lowered LDL by 37 percent and raised HDL by 130 percent and had absolutely no effect" on preventing deaths and heart attacks, Nissen said in an interview.
Although other ways of raising HDL cholesterol might eventually prove protective, Nissen said all attempts so far have been fruitless.
Nicholls said he remains hopeful of future HDL therapies and is testing whether artificial HDL can be made in the laboratory and injected directly into high-risk heart patients. "There is enthusiasm it may be able to shrink plaque" in heart arteries, he said.
He said he is studying variations of that approach with French drugmaker Cerenis Therapeutics and the Medicines Company. Nicholls said another possible approach would be to instruct the liver to make more HDL.
HM16 Q&A: How Do You try to Make a Positive Impact on Public Health in Your Daily Work?
U.S. Surgeon General Vivek Murthy, MD, MBA, formerly a hospitalist in Boston, said in a keynote speech that hospitalists have more power than they think to improve public health. The Hospitalist asked HM16 attendees: As a hospitalist, how empowered do you feel in having a positive impact on public health, and how do you try to make such an impact in your daily work?
L. Scott Sussman, MD, associate director of the hospitalist service, Yale-New Haven Hospital, Conn.
“We see patients in a time of crisis, so they’re coming in, they’re scared, they’re sick, and it’s a really teachable moment. Because once we start treating them, they start feeling better … [and] what happens is we start to gain their confidence. We use that as a point where we can say, ‘Let’s talk about your other health issues.’”
Nazima Allaudeen, MD, hospitalist, director of quality improvement, Veterans Affairs Palo Alto Healthcare System, Calif.
“I don’t feel like I do [have an impact] very much, but I think his talk kind of made me feel like we probably can and should do a lot more. … You know how sometimes you’ll get an email that has a standard thing that you can send to your congressperson about something like that? I feel like we were all like, ‘Yes, we should,’ and it would have been a great opportunity. … I feel like we kind of need it spelled out for us, you know? But it’s a good reminder.”
Gilbert Wergowske, MD, TeamHealth hospitalist, Vallejo, Calif.
“We try to arrange the best follow-up you can. That’s a difficult problem. The area that I work in is not an area that really has a lot of options … as far as giving care is concerned. So it’s really important to try to get in touch with the primary care provider directly. If that doesn’t happen, most of our patients will end up using the ED as their primary care provider. It’s important mainly for two reasons: Disjointed care like that is suboptimal; the patients do not get the best benefit from the care. And also it’s very wasteful because tests and procedures tend to be repeated over and over again.”
Cory Ritter, MD, hospitalist, soon to be working at the Veterans Affairs Medical Center, Houston
“After Dr. Murthy’s talk, I feel much more empowered to call up my local government representatives and express what I feel should be done for public health in our area. Before today, I didn’t think there was much we could do except for supporting the Society of Hospital Medicine because I know they’ve been very engaged in Obamacare and CMS. But otherwise, before today, it wasn’t much on my radar. … On a one-on-one level, you have some impact, but I’ve never really thought much about a larger public health–scale type of impact.”
U.S. Surgeon General Vivek Murthy, MD, MBA, formerly a hospitalist in Boston, said in a keynote speech that hospitalists have more power than they think to improve public health. The Hospitalist asked HM16 attendees: As a hospitalist, how empowered do you feel in having a positive impact on public health, and how do you try to make such an impact in your daily work?
L. Scott Sussman, MD, associate director of the hospitalist service, Yale-New Haven Hospital, Conn.
“We see patients in a time of crisis, so they’re coming in, they’re scared, they’re sick, and it’s a really teachable moment. Because once we start treating them, they start feeling better … [and] what happens is we start to gain their confidence. We use that as a point where we can say, ‘Let’s talk about your other health issues.’”
Nazima Allaudeen, MD, hospitalist, director of quality improvement, Veterans Affairs Palo Alto Healthcare System, Calif.
“I don’t feel like I do [have an impact] very much, but I think his talk kind of made me feel like we probably can and should do a lot more. … You know how sometimes you’ll get an email that has a standard thing that you can send to your congressperson about something like that? I feel like we were all like, ‘Yes, we should,’ and it would have been a great opportunity. … I feel like we kind of need it spelled out for us, you know? But it’s a good reminder.”
Gilbert Wergowske, MD, TeamHealth hospitalist, Vallejo, Calif.
“We try to arrange the best follow-up you can. That’s a difficult problem. The area that I work in is not an area that really has a lot of options … as far as giving care is concerned. So it’s really important to try to get in touch with the primary care provider directly. If that doesn’t happen, most of our patients will end up using the ED as their primary care provider. It’s important mainly for two reasons: Disjointed care like that is suboptimal; the patients do not get the best benefit from the care. And also it’s very wasteful because tests and procedures tend to be repeated over and over again.”
Cory Ritter, MD, hospitalist, soon to be working at the Veterans Affairs Medical Center, Houston
“After Dr. Murthy’s talk, I feel much more empowered to call up my local government representatives and express what I feel should be done for public health in our area. Before today, I didn’t think there was much we could do except for supporting the Society of Hospital Medicine because I know they’ve been very engaged in Obamacare and CMS. But otherwise, before today, it wasn’t much on my radar. … On a one-on-one level, you have some impact, but I’ve never really thought much about a larger public health–scale type of impact.”
U.S. Surgeon General Vivek Murthy, MD, MBA, formerly a hospitalist in Boston, said in a keynote speech that hospitalists have more power than they think to improve public health. The Hospitalist asked HM16 attendees: As a hospitalist, how empowered do you feel in having a positive impact on public health, and how do you try to make such an impact in your daily work?
L. Scott Sussman, MD, associate director of the hospitalist service, Yale-New Haven Hospital, Conn.
“We see patients in a time of crisis, so they’re coming in, they’re scared, they’re sick, and it’s a really teachable moment. Because once we start treating them, they start feeling better … [and] what happens is we start to gain their confidence. We use that as a point where we can say, ‘Let’s talk about your other health issues.’”
Nazima Allaudeen, MD, hospitalist, director of quality improvement, Veterans Affairs Palo Alto Healthcare System, Calif.
“I don’t feel like I do [have an impact] very much, but I think his talk kind of made me feel like we probably can and should do a lot more. … You know how sometimes you’ll get an email that has a standard thing that you can send to your congressperson about something like that? I feel like we were all like, ‘Yes, we should,’ and it would have been a great opportunity. … I feel like we kind of need it spelled out for us, you know? But it’s a good reminder.”
Gilbert Wergowske, MD, TeamHealth hospitalist, Vallejo, Calif.
“We try to arrange the best follow-up you can. That’s a difficult problem. The area that I work in is not an area that really has a lot of options … as far as giving care is concerned. So it’s really important to try to get in touch with the primary care provider directly. If that doesn’t happen, most of our patients will end up using the ED as their primary care provider. It’s important mainly for two reasons: Disjointed care like that is suboptimal; the patients do not get the best benefit from the care. And also it’s very wasteful because tests and procedures tend to be repeated over and over again.”
Cory Ritter, MD, hospitalist, soon to be working at the Veterans Affairs Medical Center, Houston
“After Dr. Murthy’s talk, I feel much more empowered to call up my local government representatives and express what I feel should be done for public health in our area. Before today, I didn’t think there was much we could do except for supporting the Society of Hospital Medicine because I know they’ve been very engaged in Obamacare and CMS. But otherwise, before today, it wasn’t much on my radar. … On a one-on-one level, you have some impact, but I’ve never really thought much about a larger public health–scale type of impact.”