User login
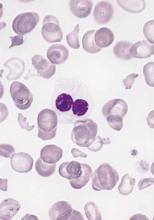
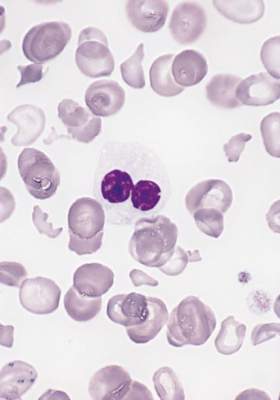
FDA approves venetoclax for CLL with 17p deletion
Venetoclax has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a 17p deletion and who have been treated with a least one prior therapy, the Food and Drug Administration has announced.
The drug will be marketed as Venclexta, and is indicated for daily use after detection of a 17p deletion is confirmed through the use of the FDA-approved companion diagnostic test, the Vysis CLL FISH probe kit. A 17p deletion occurs in about 10% of patients with untreated CLL and in about 20% of patients with relapsed CLL. Venetoclax targets the B-cell lymphoma 2 (BCL-2) protein, according to the FDA press release.
“Up to half of people whose CLL progressed have 17p deletion,” Dr. Sandra Horning, chief medical officer and head of Global Product Development for Genentech, said in a press release issued by the company. Venclexta will be marketed by AbbVie and Genentech USA. The Vysis CLL FISH probe kit is manufactured by Abbott Molecular.
“For certain patients with CLL who have not had favorable outcomes with other therapies, Venclexta may provide a new option,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release issued by the FDA.
The approval was based on a clinical trial of 106 patients who had CLL and 17p deletions and who had received at least one prior therapy. Trial participants took oral venetoclax daily, beginning with a 20 mg dose that was increased over a 5-week period to 400 mg. A complete or partial remission of CLL occurred in 80% of trial participants. Data on venetoclax also was presented at the annual meeting of the American Society of Hematology.
The most common side effects of venetoclax include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
The FDA granted the Venclexta application breakthrough therapy designation, priority review status, and accelerated approval for this indication. Venclexta also received orphan drug designation.
On Twitter @maryjodales
Venetoclax has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a 17p deletion and who have been treated with a least one prior therapy, the Food and Drug Administration has announced.
The drug will be marketed as Venclexta, and is indicated for daily use after detection of a 17p deletion is confirmed through the use of the FDA-approved companion diagnostic test, the Vysis CLL FISH probe kit. A 17p deletion occurs in about 10% of patients with untreated CLL and in about 20% of patients with relapsed CLL. Venetoclax targets the B-cell lymphoma 2 (BCL-2) protein, according to the FDA press release.
“Up to half of people whose CLL progressed have 17p deletion,” Dr. Sandra Horning, chief medical officer and head of Global Product Development for Genentech, said in a press release issued by the company. Venclexta will be marketed by AbbVie and Genentech USA. The Vysis CLL FISH probe kit is manufactured by Abbott Molecular.
“For certain patients with CLL who have not had favorable outcomes with other therapies, Venclexta may provide a new option,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release issued by the FDA.
The approval was based on a clinical trial of 106 patients who had CLL and 17p deletions and who had received at least one prior therapy. Trial participants took oral venetoclax daily, beginning with a 20 mg dose that was increased over a 5-week period to 400 mg. A complete or partial remission of CLL occurred in 80% of trial participants. Data on venetoclax also was presented at the annual meeting of the American Society of Hematology.
The most common side effects of venetoclax include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
The FDA granted the Venclexta application breakthrough therapy designation, priority review status, and accelerated approval for this indication. Venclexta also received orphan drug designation.
On Twitter @maryjodales
Venetoclax has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a 17p deletion and who have been treated with a least one prior therapy, the Food and Drug Administration has announced.
The drug will be marketed as Venclexta, and is indicated for daily use after detection of a 17p deletion is confirmed through the use of the FDA-approved companion diagnostic test, the Vysis CLL FISH probe kit. A 17p deletion occurs in about 10% of patients with untreated CLL and in about 20% of patients with relapsed CLL. Venetoclax targets the B-cell lymphoma 2 (BCL-2) protein, according to the FDA press release.
“Up to half of people whose CLL progressed have 17p deletion,” Dr. Sandra Horning, chief medical officer and head of Global Product Development for Genentech, said in a press release issued by the company. Venclexta will be marketed by AbbVie and Genentech USA. The Vysis CLL FISH probe kit is manufactured by Abbott Molecular.
“For certain patients with CLL who have not had favorable outcomes with other therapies, Venclexta may provide a new option,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release issued by the FDA.
The approval was based on a clinical trial of 106 patients who had CLL and 17p deletions and who had received at least one prior therapy. Trial participants took oral venetoclax daily, beginning with a 20 mg dose that was increased over a 5-week period to 400 mg. A complete or partial remission of CLL occurred in 80% of trial participants. Data on venetoclax also was presented at the annual meeting of the American Society of Hematology.
The most common side effects of venetoclax include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
The FDA granted the Venclexta application breakthrough therapy designation, priority review status, and accelerated approval for this indication. Venclexta also received orphan drug designation.
On Twitter @maryjodales
FDA approves first leadless pacemaker
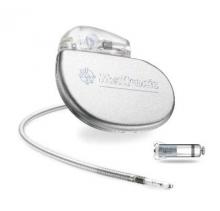
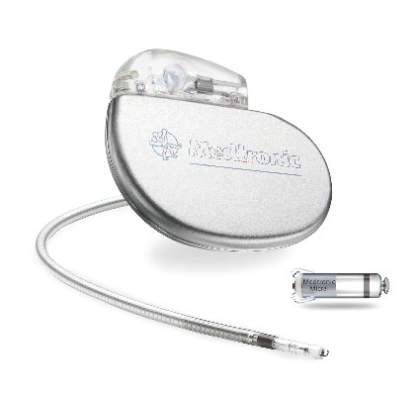
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
HM16 AUDIO: U.S. Surgeon General Vivek Murthy, MD, MBA, Discusses Hospital Medicine's Role in Public Health
U.S. Surgeon General Vivek Murthy, MD, MBA, talks about the role hospitalists can play in public health.
U.S. Surgeon General Vivek Murthy, MD, MBA, talks about the role hospitalists can play in public health.
U.S. Surgeon General Vivek Murthy, MD, MBA, talks about the role hospitalists can play in public health.
HM16 AUDIO: Alyssa Stephany, MD, Talks about the HM16 RIV Scientific Abstract Competition
Alyssa Stephany, MD, then assistant professor at Duke and now section chief of pediatric hospital medicine at Children’s Hospital of Wisconsin, talks about the evolution in training stemming from her experience in the HM16 RIV competition. This year, she oversaw a study for which resident
Jennifer Ladd, MD, won an award for pediatric clinical vignette.
Alyssa Stephany, MD, then assistant professor at Duke and now section chief of pediatric hospital medicine at Children’s Hospital of Wisconsin, talks about the evolution in training stemming from her experience in the HM16 RIV competition. This year, she oversaw a study for which resident
Jennifer Ladd, MD, won an award for pediatric clinical vignette.
Alyssa Stephany, MD, then assistant professor at Duke and now section chief of pediatric hospital medicine at Children’s Hospital of Wisconsin, talks about the evolution in training stemming from her experience in the HM16 RIV competition. This year, she oversaw a study for which resident
Jennifer Ladd, MD, won an award for pediatric clinical vignette.
HM16 AUDIO: Vineet Chopra, MD, MSc, Chats up His Research on Costs and Complications with PICC Line Usage
RIV winner Vineet Chopra, MD, MSc, assistant professor at the University of Michigan in Ann Arbor, talks about his research on the costs and complications associated with PICC line use.
RIV winner Vineet Chopra, MD, MSc, assistant professor at the University of Michigan in Ann Arbor, talks about his research on the costs and complications associated with PICC line use.
RIV winner Vineet Chopra, MD, MSc, assistant professor at the University of Michigan in Ann Arbor, talks about his research on the costs and complications associated with PICC line use.
HM16 AUDIO: Jordan Romano Discusses Getting Published, Hospitalist Burnout
Jordan Romano, DO, a hospitalist at Massachusetts General Hospital, talks about lessons gleaned at HM16 on the importance of taking small steps toward your goals of getting published and how burnout can be relative.
Jordan Romano, DO, a hospitalist at Massachusetts General Hospital, talks about lessons gleaned at HM16 on the importance of taking small steps toward your goals of getting published and how burnout can be relative.
Jordan Romano, DO, a hospitalist at Massachusetts General Hospital, talks about lessons gleaned at HM16 on the importance of taking small steps toward your goals of getting published and how burnout can be relative.
Sickle cell anemia: Stroke screening still underused
Children and adolescents with sickle cell anemia still are not being screened for stroke risk using transcranial Doppler, despite clinical guidelines that strongly recommend annual screening and despite these patients’ frequent health care encounters, according to a report published online April 11 in JAMA Pediatrics.
Approximately 10% of children and adolescents with sickle cell anemia experience stroke before the age of 20 years, unless those at high risk are identified and treated preemptively with blood transfusions, which reduces stroke risk by 92%. The National Heart, Lung, and Blood Institute clinical practice guideline on treating sickle cell disease calls for patients aged 2-16 years to undergo transcranial Doppler every year to detect any elevated velocity of cerebral blood flow, which indicates high stroke risk, said Sarah L. Reeves, Ph.D., of the Child Health Evaluation and Research Unit, University of Michigan, Ann Arbor, and her associates.
To assess screening rates, the investigators performed a retrospective cross-sectional analysis of administrative claims data for 4,775 affected children and adolescents treated during a 5-year period in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas. This yielded 10,787 person-years of data.
Overall, screening rates increased somewhat across all six states during the study period – from 22% to 44% – but “even the highest rates we report are suboptimal,” Dr. Reeves and her associates noted (JAMA Ped. 2016 Apr 11. doi: 10.1001/jamapediatrics.2015.4859).
This is especially true given that the average patient had 20.0 disease-related outpatient visits, 2.1 disease-related hospitalizations, 3.7 emergency department visits, and 1 well-child visit each year – numerous missed opportunities when they could have been referred for screening.
One way to improve screening rates would be to integrate transcranial Doppler exams into comprehensive sickle-cell healthcare, rather than requiring separate scheduled appointments at imaging facilities, they added.
This study was funded by the Agency for Healthcare Research and Quality and the Centers for Medicare & Medicaid Services. Dr. Reeves and her associates reported having no relevant financial disclosures.
Children and adolescents with sickle cell anemia still are not being screened for stroke risk using transcranial Doppler, despite clinical guidelines that strongly recommend annual screening and despite these patients’ frequent health care encounters, according to a report published online April 11 in JAMA Pediatrics.
Approximately 10% of children and adolescents with sickle cell anemia experience stroke before the age of 20 years, unless those at high risk are identified and treated preemptively with blood transfusions, which reduces stroke risk by 92%. The National Heart, Lung, and Blood Institute clinical practice guideline on treating sickle cell disease calls for patients aged 2-16 years to undergo transcranial Doppler every year to detect any elevated velocity of cerebral blood flow, which indicates high stroke risk, said Sarah L. Reeves, Ph.D., of the Child Health Evaluation and Research Unit, University of Michigan, Ann Arbor, and her associates.
To assess screening rates, the investigators performed a retrospective cross-sectional analysis of administrative claims data for 4,775 affected children and adolescents treated during a 5-year period in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas. This yielded 10,787 person-years of data.
Overall, screening rates increased somewhat across all six states during the study period – from 22% to 44% – but “even the highest rates we report are suboptimal,” Dr. Reeves and her associates noted (JAMA Ped. 2016 Apr 11. doi: 10.1001/jamapediatrics.2015.4859).
This is especially true given that the average patient had 20.0 disease-related outpatient visits, 2.1 disease-related hospitalizations, 3.7 emergency department visits, and 1 well-child visit each year – numerous missed opportunities when they could have been referred for screening.
One way to improve screening rates would be to integrate transcranial Doppler exams into comprehensive sickle-cell healthcare, rather than requiring separate scheduled appointments at imaging facilities, they added.
This study was funded by the Agency for Healthcare Research and Quality and the Centers for Medicare & Medicaid Services. Dr. Reeves and her associates reported having no relevant financial disclosures.
Children and adolescents with sickle cell anemia still are not being screened for stroke risk using transcranial Doppler, despite clinical guidelines that strongly recommend annual screening and despite these patients’ frequent health care encounters, according to a report published online April 11 in JAMA Pediatrics.
Approximately 10% of children and adolescents with sickle cell anemia experience stroke before the age of 20 years, unless those at high risk are identified and treated preemptively with blood transfusions, which reduces stroke risk by 92%. The National Heart, Lung, and Blood Institute clinical practice guideline on treating sickle cell disease calls for patients aged 2-16 years to undergo transcranial Doppler every year to detect any elevated velocity of cerebral blood flow, which indicates high stroke risk, said Sarah L. Reeves, Ph.D., of the Child Health Evaluation and Research Unit, University of Michigan, Ann Arbor, and her associates.
To assess screening rates, the investigators performed a retrospective cross-sectional analysis of administrative claims data for 4,775 affected children and adolescents treated during a 5-year period in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas. This yielded 10,787 person-years of data.
Overall, screening rates increased somewhat across all six states during the study period – from 22% to 44% – but “even the highest rates we report are suboptimal,” Dr. Reeves and her associates noted (JAMA Ped. 2016 Apr 11. doi: 10.1001/jamapediatrics.2015.4859).
This is especially true given that the average patient had 20.0 disease-related outpatient visits, 2.1 disease-related hospitalizations, 3.7 emergency department visits, and 1 well-child visit each year – numerous missed opportunities when they could have been referred for screening.
One way to improve screening rates would be to integrate transcranial Doppler exams into comprehensive sickle-cell healthcare, rather than requiring separate scheduled appointments at imaging facilities, they added.
This study was funded by the Agency for Healthcare Research and Quality and the Centers for Medicare & Medicaid Services. Dr. Reeves and her associates reported having no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Transcranial Doppler screening for stroke risk is still underused among children and adolescents with sickle cell anemia, despite clinical guidelines that strongly recommend annual screening.
Major finding: Screening rates increased somewhat across all six states studied, from 22% to 44%, but even the highest rates were suboptimal.
Data source: A retrospective cross-sectional analysis of administrative claims data for 4,775 pediatric patients treated in a 5-year period.
Disclosures: This study was funded by the Agency for Healthcare Research and Quality and the Centers for Medicare & Medicaid Services. Dr. Reeves and her associates reported having no relevant financial disclosures.
In myelodysplastic syndrome, improved tool for predicting death after HCT
A new risk-stratification tool goes one better than the standard tools used to predict survival in those undergoing allogeneic hematopoietic cell transplantation (allo HCT) for myelodysplastic syndrome, based on a study published online April 4 in the Journal of Clinical Oncology.
The concordance index for the new risk-stratification tool was modestly better at 0.575, compared with 0.538 for the standard International Prognostic Scoring System (IPSS) and 0.554 for the revised IPSS (IPSS-R), according to Dr. Brian C. Shaffer of Memorial Sloan Kettering Cancer Center, New York, and his colleagues who participate in the Center for International Blood and Marrow Transplant Research (CIBMTR) network.
“The proposed system generally agrees with the IPSS-R in the very high–risk subcategory; however, a significant portion of patients in high- and very high–risk IPSS-R groups were represented in the low- and intermediate-risk proposed scoring subcategories. The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system,” the researchers wrote.
Further, the “scoring system uses readily available clinical data and can be calculated quickly, facilitating patient consultation with respect to allo HCT, and may also be used to identify high-risk populations where interventions such as post–allo HCT maintenance therapies may be of benefit,” they wrote (J Clin Oncol. 2016 April 4. doi: 10.1200/JCO.2015.65.0515).
The data were obtained from the CIBMTR, a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
The researchers applied the prognostic tool to 2,133 patients with MDS undergoing HLA-matched (n = 1,728) or -mismatched (n = 405) allo HCT. Factors prognostic of mortality were identified in a training subset (n = 1,151) of the HLA-matched cohort. A weighted score using these factors was then assigned to the validation cohort of 577 remaining patients undergoing HLA-matched allo HCT as well as to patients undergoing HLA-mismatched allo HCT. The training data set was used to develop a prognostic scoring system, and the validation data set was used to assess the prognostic ability of the scoring system, the researchers noted.
In the scoring system, 1 point was assigned for the following factors: Blood blasts greater than 3%, platelet levels of 50 × 109/L or less at transplantation, Karnofsky performance status less than 90%, comprehensive cytogenetic risk score of poor or very poor, and age 30-49 years. Two points were assigned for monosomal karyotype and age 50 years or older.
Based on the scoring system, 3-year overall survival after transplantation was 71% in patients with scores of 1 point, 49% with scores of 2-3, 41% with scores of 4-5, and 25% with scores of 6 or more. Increasing score was predictive of increased relapse and treatment-related mortality in the HLA-matched set and of relapse in the HLA-mismatched cohort.
To develop the scoring system, the researchers used a model that weighed patient age; sex; and Karnofsky performance status; disease stage at transplantation; comprehensive cytogenetic risk status; bone marrow and peripheral blood blast percentages; hemoglobin, neutrophil, and platelet counts at diagnosis and pretransplantation; lactate dehydrogenase at transplantation; pretransplantation therapy (hypomethylating agents, chemotherapy, neither, or both); time from diagnosis to transplantation; year of transplantation; conditioning regimen and regimen intensity (myeloablative v reduced intensity); donor–recipient sex match or mismatch; graft-versus-host disease prophylaxis; graft type (bone marrow vs. peripheral blood); presence of secondary myelodysplastic syndrome; and unrelated donor vs. related donor.
There were no significant differences in overall survival at 1, 3, and 5 years or in the 3-year incidences of relapse and treatment-related mortality in the training subset and the validation cohort.
Data on somatic mutations have become relevant in myelodysplastic syndrome prognostication and were missing from this analysis, the researchers wrote. “The next generation of prognostic tools will need to account for this information.”
Dr. Shaffer had no relevant financial disclosures.
On Twitter @maryjodales
A new risk-stratification tool goes one better than the standard tools used to predict survival in those undergoing allogeneic hematopoietic cell transplantation (allo HCT) for myelodysplastic syndrome, based on a study published online April 4 in the Journal of Clinical Oncology.
The concordance index for the new risk-stratification tool was modestly better at 0.575, compared with 0.538 for the standard International Prognostic Scoring System (IPSS) and 0.554 for the revised IPSS (IPSS-R), according to Dr. Brian C. Shaffer of Memorial Sloan Kettering Cancer Center, New York, and his colleagues who participate in the Center for International Blood and Marrow Transplant Research (CIBMTR) network.
“The proposed system generally agrees with the IPSS-R in the very high–risk subcategory; however, a significant portion of patients in high- and very high–risk IPSS-R groups were represented in the low- and intermediate-risk proposed scoring subcategories. The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system,” the researchers wrote.
Further, the “scoring system uses readily available clinical data and can be calculated quickly, facilitating patient consultation with respect to allo HCT, and may also be used to identify high-risk populations where interventions such as post–allo HCT maintenance therapies may be of benefit,” they wrote (J Clin Oncol. 2016 April 4. doi: 10.1200/JCO.2015.65.0515).
The data were obtained from the CIBMTR, a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
The researchers applied the prognostic tool to 2,133 patients with MDS undergoing HLA-matched (n = 1,728) or -mismatched (n = 405) allo HCT. Factors prognostic of mortality were identified in a training subset (n = 1,151) of the HLA-matched cohort. A weighted score using these factors was then assigned to the validation cohort of 577 remaining patients undergoing HLA-matched allo HCT as well as to patients undergoing HLA-mismatched allo HCT. The training data set was used to develop a prognostic scoring system, and the validation data set was used to assess the prognostic ability of the scoring system, the researchers noted.
In the scoring system, 1 point was assigned for the following factors: Blood blasts greater than 3%, platelet levels of 50 × 109/L or less at transplantation, Karnofsky performance status less than 90%, comprehensive cytogenetic risk score of poor or very poor, and age 30-49 years. Two points were assigned for monosomal karyotype and age 50 years or older.
Based on the scoring system, 3-year overall survival after transplantation was 71% in patients with scores of 1 point, 49% with scores of 2-3, 41% with scores of 4-5, and 25% with scores of 6 or more. Increasing score was predictive of increased relapse and treatment-related mortality in the HLA-matched set and of relapse in the HLA-mismatched cohort.
To develop the scoring system, the researchers used a model that weighed patient age; sex; and Karnofsky performance status; disease stage at transplantation; comprehensive cytogenetic risk status; bone marrow and peripheral blood blast percentages; hemoglobin, neutrophil, and platelet counts at diagnosis and pretransplantation; lactate dehydrogenase at transplantation; pretransplantation therapy (hypomethylating agents, chemotherapy, neither, or both); time from diagnosis to transplantation; year of transplantation; conditioning regimen and regimen intensity (myeloablative v reduced intensity); donor–recipient sex match or mismatch; graft-versus-host disease prophylaxis; graft type (bone marrow vs. peripheral blood); presence of secondary myelodysplastic syndrome; and unrelated donor vs. related donor.
There were no significant differences in overall survival at 1, 3, and 5 years or in the 3-year incidences of relapse and treatment-related mortality in the training subset and the validation cohort.
Data on somatic mutations have become relevant in myelodysplastic syndrome prognostication and were missing from this analysis, the researchers wrote. “The next generation of prognostic tools will need to account for this information.”
Dr. Shaffer had no relevant financial disclosures.
On Twitter @maryjodales
A new risk-stratification tool goes one better than the standard tools used to predict survival in those undergoing allogeneic hematopoietic cell transplantation (allo HCT) for myelodysplastic syndrome, based on a study published online April 4 in the Journal of Clinical Oncology.
The concordance index for the new risk-stratification tool was modestly better at 0.575, compared with 0.538 for the standard International Prognostic Scoring System (IPSS) and 0.554 for the revised IPSS (IPSS-R), according to Dr. Brian C. Shaffer of Memorial Sloan Kettering Cancer Center, New York, and his colleagues who participate in the Center for International Blood and Marrow Transplant Research (CIBMTR) network.
“The proposed system generally agrees with the IPSS-R in the very high–risk subcategory; however, a significant portion of patients in high- and very high–risk IPSS-R groups were represented in the low- and intermediate-risk proposed scoring subcategories. The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system,” the researchers wrote.
Further, the “scoring system uses readily available clinical data and can be calculated quickly, facilitating patient consultation with respect to allo HCT, and may also be used to identify high-risk populations where interventions such as post–allo HCT maintenance therapies may be of benefit,” they wrote (J Clin Oncol. 2016 April 4. doi: 10.1200/JCO.2015.65.0515).
The data were obtained from the CIBMTR, a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
The researchers applied the prognostic tool to 2,133 patients with MDS undergoing HLA-matched (n = 1,728) or -mismatched (n = 405) allo HCT. Factors prognostic of mortality were identified in a training subset (n = 1,151) of the HLA-matched cohort. A weighted score using these factors was then assigned to the validation cohort of 577 remaining patients undergoing HLA-matched allo HCT as well as to patients undergoing HLA-mismatched allo HCT. The training data set was used to develop a prognostic scoring system, and the validation data set was used to assess the prognostic ability of the scoring system, the researchers noted.
In the scoring system, 1 point was assigned for the following factors: Blood blasts greater than 3%, platelet levels of 50 × 109/L or less at transplantation, Karnofsky performance status less than 90%, comprehensive cytogenetic risk score of poor or very poor, and age 30-49 years. Two points were assigned for monosomal karyotype and age 50 years or older.
Based on the scoring system, 3-year overall survival after transplantation was 71% in patients with scores of 1 point, 49% with scores of 2-3, 41% with scores of 4-5, and 25% with scores of 6 or more. Increasing score was predictive of increased relapse and treatment-related mortality in the HLA-matched set and of relapse in the HLA-mismatched cohort.
To develop the scoring system, the researchers used a model that weighed patient age; sex; and Karnofsky performance status; disease stage at transplantation; comprehensive cytogenetic risk status; bone marrow and peripheral blood blast percentages; hemoglobin, neutrophil, and platelet counts at diagnosis and pretransplantation; lactate dehydrogenase at transplantation; pretransplantation therapy (hypomethylating agents, chemotherapy, neither, or both); time from diagnosis to transplantation; year of transplantation; conditioning regimen and regimen intensity (myeloablative v reduced intensity); donor–recipient sex match or mismatch; graft-versus-host disease prophylaxis; graft type (bone marrow vs. peripheral blood); presence of secondary myelodysplastic syndrome; and unrelated donor vs. related donor.
There were no significant differences in overall survival at 1, 3, and 5 years or in the 3-year incidences of relapse and treatment-related mortality in the training subset and the validation cohort.
Data on somatic mutations have become relevant in myelodysplastic syndrome prognostication and were missing from this analysis, the researchers wrote. “The next generation of prognostic tools will need to account for this information.”
Dr. Shaffer had no relevant financial disclosures.
On Twitter @maryjodales
FROM JCO
Key clinical point: A portion of patients with myelodysplastic syndrome in high- and very high–risk groups of the revised International Prognostic Scoring System (IPSS-R) were represented in the low- and intermediate-risk groups of the proposed scoring subcategories.
Major finding: The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system.
Data source: The Center for International Blood and Marrow Transplant Research (CIBMTR), a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
Disclosures: Dr. Shaffer had no relevant financial disclosures.
Proactive endocrine screening urged for pediatric brain tumor survivors
BOSTON – More than a third of 419 children treated for brain tumors at Cincinnati Children’s Hospital Medical Center later developed endocrine problems, according to a review presented at the Endocrine Society annual meeting.
Over 60% of the 96 suprasellar tumor patients developed endocrine dysfunction, which isn’t surprising considering the location of the tumor, but wide-ranging endocrine problems were also common in the 145 posterior fossa, 158 supratentorial, and 20 spinal cord cases, ranging from 14% in the spinal cord group to 42% in the posterior fossa group, after some combination of radiation, chemotherapy, and surgery based on tumor location and other factors.
“Even with tumors that aren’t supposed to be high risk, there was a high risk of endocrinopathies. We need yearly screening of these patients” for about 6 years, after which symptom-based screening may be sufficient. The clock should be restarted if there’s a recurrence. “Not everyone does this” at Cincinnati Children’s and probably most other institutions, said investigator and endocrinology fellow Dr. Vincent Horne.
The findings are “changing how our oncology department is thinking about [screening]; there’s a concentrated effort to increase proactive screening and follow these patients long term,” he said.
“Even within our specialized, multidisciplinary center,” endocrinopathy screening referrals were low, about 61% overall and only 80% in the suprasellar group. “Patients at highest risk” – those with craniopharyngioma – “are being seen early by us,” but others aren’t being referred. It’s possible that the extent of endocrine problems after pediatric brain tumor treatment is simply unrecognized, he said.
Endocrine abnormalities were found in 114 (45%) of the 254 patients evaluated, which translated to problems in more than a third of all patients.
More than half of the children had more than one problem, and most of the issues occurred within 6 years of treatment. Central hypothyroidism was found in 53% of the children, probably because Cincinnati Children’s already has thyroid screening in place.
About 40% were growth hormone deficient, and almost a third had precocious puberty. About 30% were gonadotropin-releasing hormone deficient, over 20% had primary hypothyroidism, and about the same had diabetes insipidus. Just over 6% were hyperprolactinemic.
Of the 151 patients who completed adrenocorticotropic hormone (ACTH) testing, 14.6% were deficient. ACTH deficient children were about evenly split between the suprasellar and supratentorial groups, with the remaining in the posterior fossa cohort.
“We are probably not thinking about” the risk of radiation “to locations like the posterior fossa. That group actually had the highest risk of primary hypothyroidism [20%] because of the spinal radiation. The supratentorial group is also receiving radiation; even though we think we are missing the hypothalamus, obviously that’s not necessarily the case,” Dr. Horne said.
His team looked into endocrine screening because previous studies “were limited and done years ago.” People are living longer now after treatment, “so we need to think about how to screen for endocrine disease. This is an attempt to clarify how we should do it,” he said.
Children were a median of 8 years old at diagnosis, and the median radiation dose was 54 Gy.
There was no industry funding for the work, and the investigators had no disclosures.
BOSTON – More than a third of 419 children treated for brain tumors at Cincinnati Children’s Hospital Medical Center later developed endocrine problems, according to a review presented at the Endocrine Society annual meeting.
Over 60% of the 96 suprasellar tumor patients developed endocrine dysfunction, which isn’t surprising considering the location of the tumor, but wide-ranging endocrine problems were also common in the 145 posterior fossa, 158 supratentorial, and 20 spinal cord cases, ranging from 14% in the spinal cord group to 42% in the posterior fossa group, after some combination of radiation, chemotherapy, and surgery based on tumor location and other factors.
“Even with tumors that aren’t supposed to be high risk, there was a high risk of endocrinopathies. We need yearly screening of these patients” for about 6 years, after which symptom-based screening may be sufficient. The clock should be restarted if there’s a recurrence. “Not everyone does this” at Cincinnati Children’s and probably most other institutions, said investigator and endocrinology fellow Dr. Vincent Horne.
The findings are “changing how our oncology department is thinking about [screening]; there’s a concentrated effort to increase proactive screening and follow these patients long term,” he said.
“Even within our specialized, multidisciplinary center,” endocrinopathy screening referrals were low, about 61% overall and only 80% in the suprasellar group. “Patients at highest risk” – those with craniopharyngioma – “are being seen early by us,” but others aren’t being referred. It’s possible that the extent of endocrine problems after pediatric brain tumor treatment is simply unrecognized, he said.
Endocrine abnormalities were found in 114 (45%) of the 254 patients evaluated, which translated to problems in more than a third of all patients.
More than half of the children had more than one problem, and most of the issues occurred within 6 years of treatment. Central hypothyroidism was found in 53% of the children, probably because Cincinnati Children’s already has thyroid screening in place.
About 40% were growth hormone deficient, and almost a third had precocious puberty. About 30% were gonadotropin-releasing hormone deficient, over 20% had primary hypothyroidism, and about the same had diabetes insipidus. Just over 6% were hyperprolactinemic.
Of the 151 patients who completed adrenocorticotropic hormone (ACTH) testing, 14.6% were deficient. ACTH deficient children were about evenly split between the suprasellar and supratentorial groups, with the remaining in the posterior fossa cohort.
“We are probably not thinking about” the risk of radiation “to locations like the posterior fossa. That group actually had the highest risk of primary hypothyroidism [20%] because of the spinal radiation. The supratentorial group is also receiving radiation; even though we think we are missing the hypothalamus, obviously that’s not necessarily the case,” Dr. Horne said.
His team looked into endocrine screening because previous studies “were limited and done years ago.” People are living longer now after treatment, “so we need to think about how to screen for endocrine disease. This is an attempt to clarify how we should do it,” he said.
Children were a median of 8 years old at diagnosis, and the median radiation dose was 54 Gy.
There was no industry funding for the work, and the investigators had no disclosures.
BOSTON – More than a third of 419 children treated for brain tumors at Cincinnati Children’s Hospital Medical Center later developed endocrine problems, according to a review presented at the Endocrine Society annual meeting.
Over 60% of the 96 suprasellar tumor patients developed endocrine dysfunction, which isn’t surprising considering the location of the tumor, but wide-ranging endocrine problems were also common in the 145 posterior fossa, 158 supratentorial, and 20 spinal cord cases, ranging from 14% in the spinal cord group to 42% in the posterior fossa group, after some combination of radiation, chemotherapy, and surgery based on tumor location and other factors.
“Even with tumors that aren’t supposed to be high risk, there was a high risk of endocrinopathies. We need yearly screening of these patients” for about 6 years, after which symptom-based screening may be sufficient. The clock should be restarted if there’s a recurrence. “Not everyone does this” at Cincinnati Children’s and probably most other institutions, said investigator and endocrinology fellow Dr. Vincent Horne.
The findings are “changing how our oncology department is thinking about [screening]; there’s a concentrated effort to increase proactive screening and follow these patients long term,” he said.
“Even within our specialized, multidisciplinary center,” endocrinopathy screening referrals were low, about 61% overall and only 80% in the suprasellar group. “Patients at highest risk” – those with craniopharyngioma – “are being seen early by us,” but others aren’t being referred. It’s possible that the extent of endocrine problems after pediatric brain tumor treatment is simply unrecognized, he said.
Endocrine abnormalities were found in 114 (45%) of the 254 patients evaluated, which translated to problems in more than a third of all patients.
More than half of the children had more than one problem, and most of the issues occurred within 6 years of treatment. Central hypothyroidism was found in 53% of the children, probably because Cincinnati Children’s already has thyroid screening in place.
About 40% were growth hormone deficient, and almost a third had precocious puberty. About 30% were gonadotropin-releasing hormone deficient, over 20% had primary hypothyroidism, and about the same had diabetes insipidus. Just over 6% were hyperprolactinemic.
Of the 151 patients who completed adrenocorticotropic hormone (ACTH) testing, 14.6% were deficient. ACTH deficient children were about evenly split between the suprasellar and supratentorial groups, with the remaining in the posterior fossa cohort.
“We are probably not thinking about” the risk of radiation “to locations like the posterior fossa. That group actually had the highest risk of primary hypothyroidism [20%] because of the spinal radiation. The supratentorial group is also receiving radiation; even though we think we are missing the hypothalamus, obviously that’s not necessarily the case,” Dr. Horne said.
His team looked into endocrine screening because previous studies “were limited and done years ago.” People are living longer now after treatment, “so we need to think about how to screen for endocrine disease. This is an attempt to clarify how we should do it,” he said.
Children were a median of 8 years old at diagnosis, and the median radiation dose was 54 Gy.
There was no industry funding for the work, and the investigators had no disclosures.
AT ENDO 2016
Key clinical point: Screen for endocrine dysfunction for 6 years after pediatric brain tumor treatment.
Major finding: Endocrine abnormalities were found in 114 of 254 patients (45%) evaluated, which translated to problems in more than a third of all patients.
Data source: Single-center review of 419 pediatric brain tumor cases.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
Surgeons commonly off the mark in estimating blood loss
MONTREAL – Surgeons, nurses, and anesthesia providers were all pretty bad at estimating surgical blood loss in a small study. And more experience doesn’t improve accuracy, though experienced providers were more confident in their estimates.
These were the findings from a study that simulated operating room scenarios and asked providers to estimate blood loss. “Estimation of blood loss is inaccurate and unreliable,” Dr. Luke Rothermel said at the Central Surgical Association’s annual meeting.
Dr. Rothermel, a resident at Case Western Reserve University, Cleveland, noted that although the Joint Commission requires operative notes to contain estimated blood loss, “no study in the United States has compared the characteristics of operating room personnel or conditions associated with improved accuracy or reliability of blood loss estimation.”
Beyond the required reporting, estimating blood loss (EBL) also provides important guidance in perioperative care. Still, said Dr. Rothermel, previous studies have shown that EBL is typically inaccurate.
To assess providers’ ability to be accurate and reliable in estimating blood loss, Dr. Rothermel and his collaborator, Dr. Jeremy Lipman, assistant residency director at MetroHealth, Cleveland, designed a study to simulate three different operating room scenarios, involving high, medium, and low blood loss volumes. The materials used, such as blood-soaked sponges and suction canisters, were identical to what’s actually used in the operating room (porcine blood was used in the simulations).
Before the study, Dr. Rothermel said that he and Dr. Lipman hypothesized that those providers who had more experience and those who were working at the operating field would be more accurate in estimating blood loss. They also hypothesized that estimations in procedures with lower volumes of blood loss would be more accurate.
The study recruited providers from the surgery, anesthesia, and nursing services at an urban level 1 trauma center. Each scenario included a written description of the procedure performed and the course of surgery, and participants could handle study materials for each scenario under the supervision of study staff.
A total of 60 participants (22 from surgery, 17 from anesthesia, and 21 from nursing) participated; they had an average of 12.8 years of experience. The surgical participants included surgical scrub techs, trainees, and attending physicians. Anesthesia participants included anesthesia assistants, CRNAs, trainees, and attending physicians. Nursing participants were all RNs.
The findings? All over the board: “There was no association between specialty, years of experience, or confidence in ability with the consistency or accuracy of estimated blood loss,” said Dr. Rothermel.
Most participants were far shy of the mark, with just 5% of study participants overall able to come within 25% accuracy in judging EBL in all scenarios. Just over a quarter were consistent in over- or underestimating blood loss.
These findings held true across scenarios, across disciplines, and regardless of the number of years of experience. “Increased years of experience trended toward increased error,” said Dr. Rothermel, though the difference was not statistically significant. However, those with more years of experience tended to be more confident of their judgments.
Dr. Rothermel noted the small study size and single institution studied as limitations. Also, “this model was not a high fidelity representation of the OR experience, “ he said, explaining that during surgery, caregivers continually assess intraoperative blood loss and may form an estimate in a different – and potentially more accurate – manner than occurs when presented with the contrived presentation of a scenario.
The study calls into question the validity of using EBL as a quality indicator in assessing physician performance and patient outcomes, said Dr. Rothermel, who had no financial disclosures.
On Twitter @karioakes
MONTREAL – Surgeons, nurses, and anesthesia providers were all pretty bad at estimating surgical blood loss in a small study. And more experience doesn’t improve accuracy, though experienced providers were more confident in their estimates.
These were the findings from a study that simulated operating room scenarios and asked providers to estimate blood loss. “Estimation of blood loss is inaccurate and unreliable,” Dr. Luke Rothermel said at the Central Surgical Association’s annual meeting.
Dr. Rothermel, a resident at Case Western Reserve University, Cleveland, noted that although the Joint Commission requires operative notes to contain estimated blood loss, “no study in the United States has compared the characteristics of operating room personnel or conditions associated with improved accuracy or reliability of blood loss estimation.”
Beyond the required reporting, estimating blood loss (EBL) also provides important guidance in perioperative care. Still, said Dr. Rothermel, previous studies have shown that EBL is typically inaccurate.
To assess providers’ ability to be accurate and reliable in estimating blood loss, Dr. Rothermel and his collaborator, Dr. Jeremy Lipman, assistant residency director at MetroHealth, Cleveland, designed a study to simulate three different operating room scenarios, involving high, medium, and low blood loss volumes. The materials used, such as blood-soaked sponges and suction canisters, were identical to what’s actually used in the operating room (porcine blood was used in the simulations).
Before the study, Dr. Rothermel said that he and Dr. Lipman hypothesized that those providers who had more experience and those who were working at the operating field would be more accurate in estimating blood loss. They also hypothesized that estimations in procedures with lower volumes of blood loss would be more accurate.
The study recruited providers from the surgery, anesthesia, and nursing services at an urban level 1 trauma center. Each scenario included a written description of the procedure performed and the course of surgery, and participants could handle study materials for each scenario under the supervision of study staff.
A total of 60 participants (22 from surgery, 17 from anesthesia, and 21 from nursing) participated; they had an average of 12.8 years of experience. The surgical participants included surgical scrub techs, trainees, and attending physicians. Anesthesia participants included anesthesia assistants, CRNAs, trainees, and attending physicians. Nursing participants were all RNs.
The findings? All over the board: “There was no association between specialty, years of experience, or confidence in ability with the consistency or accuracy of estimated blood loss,” said Dr. Rothermel.
Most participants were far shy of the mark, with just 5% of study participants overall able to come within 25% accuracy in judging EBL in all scenarios. Just over a quarter were consistent in over- or underestimating blood loss.
These findings held true across scenarios, across disciplines, and regardless of the number of years of experience. “Increased years of experience trended toward increased error,” said Dr. Rothermel, though the difference was not statistically significant. However, those with more years of experience tended to be more confident of their judgments.
Dr. Rothermel noted the small study size and single institution studied as limitations. Also, “this model was not a high fidelity representation of the OR experience, “ he said, explaining that during surgery, caregivers continually assess intraoperative blood loss and may form an estimate in a different – and potentially more accurate – manner than occurs when presented with the contrived presentation of a scenario.
The study calls into question the validity of using EBL as a quality indicator in assessing physician performance and patient outcomes, said Dr. Rothermel, who had no financial disclosures.
On Twitter @karioakes
MONTREAL – Surgeons, nurses, and anesthesia providers were all pretty bad at estimating surgical blood loss in a small study. And more experience doesn’t improve accuracy, though experienced providers were more confident in their estimates.
These were the findings from a study that simulated operating room scenarios and asked providers to estimate blood loss. “Estimation of blood loss is inaccurate and unreliable,” Dr. Luke Rothermel said at the Central Surgical Association’s annual meeting.
Dr. Rothermel, a resident at Case Western Reserve University, Cleveland, noted that although the Joint Commission requires operative notes to contain estimated blood loss, “no study in the United States has compared the characteristics of operating room personnel or conditions associated with improved accuracy or reliability of blood loss estimation.”
Beyond the required reporting, estimating blood loss (EBL) also provides important guidance in perioperative care. Still, said Dr. Rothermel, previous studies have shown that EBL is typically inaccurate.
To assess providers’ ability to be accurate and reliable in estimating blood loss, Dr. Rothermel and his collaborator, Dr. Jeremy Lipman, assistant residency director at MetroHealth, Cleveland, designed a study to simulate three different operating room scenarios, involving high, medium, and low blood loss volumes. The materials used, such as blood-soaked sponges and suction canisters, were identical to what’s actually used in the operating room (porcine blood was used in the simulations).
Before the study, Dr. Rothermel said that he and Dr. Lipman hypothesized that those providers who had more experience and those who were working at the operating field would be more accurate in estimating blood loss. They also hypothesized that estimations in procedures with lower volumes of blood loss would be more accurate.
The study recruited providers from the surgery, anesthesia, and nursing services at an urban level 1 trauma center. Each scenario included a written description of the procedure performed and the course of surgery, and participants could handle study materials for each scenario under the supervision of study staff.
A total of 60 participants (22 from surgery, 17 from anesthesia, and 21 from nursing) participated; they had an average of 12.8 years of experience. The surgical participants included surgical scrub techs, trainees, and attending physicians. Anesthesia participants included anesthesia assistants, CRNAs, trainees, and attending physicians. Nursing participants were all RNs.
The findings? All over the board: “There was no association between specialty, years of experience, or confidence in ability with the consistency or accuracy of estimated blood loss,” said Dr. Rothermel.
Most participants were far shy of the mark, with just 5% of study participants overall able to come within 25% accuracy in judging EBL in all scenarios. Just over a quarter were consistent in over- or underestimating blood loss.
These findings held true across scenarios, across disciplines, and regardless of the number of years of experience. “Increased years of experience trended toward increased error,” said Dr. Rothermel, though the difference was not statistically significant. However, those with more years of experience tended to be more confident of their judgments.
Dr. Rothermel noted the small study size and single institution studied as limitations. Also, “this model was not a high fidelity representation of the OR experience, “ he said, explaining that during surgery, caregivers continually assess intraoperative blood loss and may form an estimate in a different – and potentially more accurate – manner than occurs when presented with the contrived presentation of a scenario.
The study calls into question the validity of using EBL as a quality indicator in assessing physician performance and patient outcomes, said Dr. Rothermel, who had no financial disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Surgery, anesthesia, and nursing providers were inaccurate and unreliable in estimating surgical blood loss.
Major finding: Only 5% of providers could come within 25% accuracy of simulated surgical blood loss.
Data source: Simulations of surgical scenarios depicting varying amounts of blood loss using porcine blood, presented to 60 providers.
Disclosures: The study authors reported no relevant disclosures.