User login
Risk score predicts rehospitalization after heart surgery
PHOENIX – A simple, five-element formula can help identify the patients undergoing heart surgery who face the greatest risk for a hospital readmission within 30 days following discharge from their index hospitalization.
The surgeons who developed this formula hope to use it in an investigational program that will target intensified management resources in postsurgical patients who face the highest readmission risk, to cut rehospitalizations and better improve their clinical status and quality of life.
The analysis that produced this formula also documented that the worst offender for triggering rehospitalizations following heart surgery is fluid overload, the proximate readmission cause for 23% of postsurgical patients, Dr. Arman Kilic said at the annual meeting of the Society of Thoracic Surgeons. The next most common cause was infection, which led to 20% of readmissions, followed by arrhythmias, responsible for 8% of readmissions, said Dr. Kilic, a thoracic surgeon at the University of Pennsylvania in Philadelphia.
Because fluid overload, often in the form of pleural effusion, is such an important driver of rehospitalizations, a more targeted management program would include better titration of diuretic treatment to patients following heart surgery, thoracentesis, and closer monitoring of clinical features that flag fluid overload such as weight.
“The volume overload issue is where the money is. If we can reduce that, it could really impact readmissions,” Dr. Kilic said in an interview.
An investigational program to target rehospitalization risk in heart surgery patients is planned at Johns Hopkins Hospital in Baltimore, where Dr. Kilic worked when he performed this analysis. Surgeons at Johns Hopkins are now in the process of getting funding for this pilot program, said Dr. John V. Conte Jr., professor of surgery and director of mechanical circulatory support at Johns Hopkins and a collaborator with Dr. Kilic on developing the risk formula.
“We’ll tailor postoperative follow-up. We’ll get high-risk patients back to the clinic sooner, and we’ll send nurse practitioners to see them to make sure they’re taking their medications and are getting weighed daily,” Dr. Conte said in an interview. “When a patient has heart surgery, they typically retain about 5-10 pounds of fluid. Patients with good renal function give up that fluid easily, but others are difficult to diurese. Many patients go home before they have been fully diuresed, and we need to follow these patients and transition them better to out-of-hospital care.”
He noted that other situations also come up that unnecessarily drive patients back to the hospital when an alternative and less expensive intervention might be equally effective. For example, some patients return to the hospital out of concern for how their chest wound is healing. Instead of being rehospitalized, such patients could be reassured by having them send a nurse a photo of their wound or by coming to an outpatient clinic.
“We need to engage more often with recently discharged patients,” Dr. Conte said in an interview. “Discharging them doesn’t mean separating them from the health care system; it should mean interacting with patients in a different way” that produces better outcomes and patient satisfaction for less money. Developing improved ways to manage recent heart surgery patients following discharge becomes even more critical later this year when, in July, the Centers for Medicare & Medicaid Services adds 30-day readmissions following coronary artery bypass grafting (CABG) to its list of procedures that can generate a penalty to hospitals if they exceed U.S. norms for readmission rates.
The risk model developed by Dr. Kilic, Dr. Conte, and their associates used data collected from 5,360 heart surgery patients treated at Johns Hopkins during 2008-2013. Nearly half the patients underwent isolated CABG, and 20% had isolated valve surgery. Overall, 585 patients (11%) had a hospital readmission within 30 days of their index discharge. One limitation of the analysis was it used data only on readmissions back to Johns Hopkins Hospital.
The researchers used data from three-quarters of the database to derive the risk formula, and from the remaining 25% of the database to validate the formula. A multivariate analysis of demographic and clinical characteristics that significantly linked with an elevated risk for readmissions identified five factors that independently made a significant contribution to readmission risk. The researchers assigned each of these five factors points depending on its relative contribution to readmission risk in the adjusted model: Severe chronic lung disease received 6 points; placement of a ventricular assist device received 5 points, while other types of heart surgery that was not CABG or valve surgery received 4 points (isolated CABG, isolated valve, or combined CABG and valve surgery received 0 points); development of acute renal failure postoperatively but before index discharge received 4 points; an index length of stay beyond 7 days received 4 points; and African American race received 3 points. The maximum number of points a patient could receive was 22.
Patients with a score of 0 had a 6% rate of a 30-day readmission; those with a score of 22 had a 63% readmission rate. For simplicity, Dr. Kilic suggested dividing patients into three categories based on their readmission risk score: Low-risk patients with a score of 0 had a readmission risk of 6%, medium-risk patients with a score of 1-10 had a readmission risk of 12%, and high-risk patients with a score of 11 or more had a readmission risk of 31%. The researchers found a 96% correlation when comparing these predicted readmission risk rates based on the derivation-subgroup analysis with the actual readmission rates seen in the validation subgroup of their database. The targeted risk-management program planned by Dr. Conte would primarily focus on high-risk patients.
Dr. Kilic and Dr. Conte said they had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
Dr. Kilic's data illustrates common factors resulting in rehospitalization after cardiac surgery. Fastidious fluid management in these patients and others is critical to reduce hospital readmissions. A further point to consider is that many pleural effusions, especially those on the left side, are due to retained hemothorax rather than fluid overload. In those instances, early surgical intervention with video-assisted thoracoscopic surgery, rather than prolonged diuresis, would be optimal.
Dr. Francis J. Podbielski, FCCP, serves on the editorial advisory board for CHEST Physician.
Dr. Kilic's data illustrates common factors resulting in rehospitalization after cardiac surgery. Fastidious fluid management in these patients and others is critical to reduce hospital readmissions. A further point to consider is that many pleural effusions, especially those on the left side, are due to retained hemothorax rather than fluid overload. In those instances, early surgical intervention with video-assisted thoracoscopic surgery, rather than prolonged diuresis, would be optimal.
Dr. Francis J. Podbielski, FCCP, serves on the editorial advisory board for CHEST Physician.
Dr. Kilic's data illustrates common factors resulting in rehospitalization after cardiac surgery. Fastidious fluid management in these patients and others is critical to reduce hospital readmissions. A further point to consider is that many pleural effusions, especially those on the left side, are due to retained hemothorax rather than fluid overload. In those instances, early surgical intervention with video-assisted thoracoscopic surgery, rather than prolonged diuresis, would be optimal.
Dr. Francis J. Podbielski, FCCP, serves on the editorial advisory board for CHEST Physician.
PHOENIX – A simple, five-element formula can help identify the patients undergoing heart surgery who face the greatest risk for a hospital readmission within 30 days following discharge from their index hospitalization.
The surgeons who developed this formula hope to use it in an investigational program that will target intensified management resources in postsurgical patients who face the highest readmission risk, to cut rehospitalizations and better improve their clinical status and quality of life.
The analysis that produced this formula also documented that the worst offender for triggering rehospitalizations following heart surgery is fluid overload, the proximate readmission cause for 23% of postsurgical patients, Dr. Arman Kilic said at the annual meeting of the Society of Thoracic Surgeons. The next most common cause was infection, which led to 20% of readmissions, followed by arrhythmias, responsible for 8% of readmissions, said Dr. Kilic, a thoracic surgeon at the University of Pennsylvania in Philadelphia.
Because fluid overload, often in the form of pleural effusion, is such an important driver of rehospitalizations, a more targeted management program would include better titration of diuretic treatment to patients following heart surgery, thoracentesis, and closer monitoring of clinical features that flag fluid overload such as weight.
“The volume overload issue is where the money is. If we can reduce that, it could really impact readmissions,” Dr. Kilic said in an interview.
An investigational program to target rehospitalization risk in heart surgery patients is planned at Johns Hopkins Hospital in Baltimore, where Dr. Kilic worked when he performed this analysis. Surgeons at Johns Hopkins are now in the process of getting funding for this pilot program, said Dr. John V. Conte Jr., professor of surgery and director of mechanical circulatory support at Johns Hopkins and a collaborator with Dr. Kilic on developing the risk formula.
“We’ll tailor postoperative follow-up. We’ll get high-risk patients back to the clinic sooner, and we’ll send nurse practitioners to see them to make sure they’re taking their medications and are getting weighed daily,” Dr. Conte said in an interview. “When a patient has heart surgery, they typically retain about 5-10 pounds of fluid. Patients with good renal function give up that fluid easily, but others are difficult to diurese. Many patients go home before they have been fully diuresed, and we need to follow these patients and transition them better to out-of-hospital care.”
He noted that other situations also come up that unnecessarily drive patients back to the hospital when an alternative and less expensive intervention might be equally effective. For example, some patients return to the hospital out of concern for how their chest wound is healing. Instead of being rehospitalized, such patients could be reassured by having them send a nurse a photo of their wound or by coming to an outpatient clinic.
“We need to engage more often with recently discharged patients,” Dr. Conte said in an interview. “Discharging them doesn’t mean separating them from the health care system; it should mean interacting with patients in a different way” that produces better outcomes and patient satisfaction for less money. Developing improved ways to manage recent heart surgery patients following discharge becomes even more critical later this year when, in July, the Centers for Medicare & Medicaid Services adds 30-day readmissions following coronary artery bypass grafting (CABG) to its list of procedures that can generate a penalty to hospitals if they exceed U.S. norms for readmission rates.
The risk model developed by Dr. Kilic, Dr. Conte, and their associates used data collected from 5,360 heart surgery patients treated at Johns Hopkins during 2008-2013. Nearly half the patients underwent isolated CABG, and 20% had isolated valve surgery. Overall, 585 patients (11%) had a hospital readmission within 30 days of their index discharge. One limitation of the analysis was it used data only on readmissions back to Johns Hopkins Hospital.
The researchers used data from three-quarters of the database to derive the risk formula, and from the remaining 25% of the database to validate the formula. A multivariate analysis of demographic and clinical characteristics that significantly linked with an elevated risk for readmissions identified five factors that independently made a significant contribution to readmission risk. The researchers assigned each of these five factors points depending on its relative contribution to readmission risk in the adjusted model: Severe chronic lung disease received 6 points; placement of a ventricular assist device received 5 points, while other types of heart surgery that was not CABG or valve surgery received 4 points (isolated CABG, isolated valve, or combined CABG and valve surgery received 0 points); development of acute renal failure postoperatively but before index discharge received 4 points; an index length of stay beyond 7 days received 4 points; and African American race received 3 points. The maximum number of points a patient could receive was 22.
Patients with a score of 0 had a 6% rate of a 30-day readmission; those with a score of 22 had a 63% readmission rate. For simplicity, Dr. Kilic suggested dividing patients into three categories based on their readmission risk score: Low-risk patients with a score of 0 had a readmission risk of 6%, medium-risk patients with a score of 1-10 had a readmission risk of 12%, and high-risk patients with a score of 11 or more had a readmission risk of 31%. The researchers found a 96% correlation when comparing these predicted readmission risk rates based on the derivation-subgroup analysis with the actual readmission rates seen in the validation subgroup of their database. The targeted risk-management program planned by Dr. Conte would primarily focus on high-risk patients.
Dr. Kilic and Dr. Conte said they had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
PHOENIX – A simple, five-element formula can help identify the patients undergoing heart surgery who face the greatest risk for a hospital readmission within 30 days following discharge from their index hospitalization.
The surgeons who developed this formula hope to use it in an investigational program that will target intensified management resources in postsurgical patients who face the highest readmission risk, to cut rehospitalizations and better improve their clinical status and quality of life.
The analysis that produced this formula also documented that the worst offender for triggering rehospitalizations following heart surgery is fluid overload, the proximate readmission cause for 23% of postsurgical patients, Dr. Arman Kilic said at the annual meeting of the Society of Thoracic Surgeons. The next most common cause was infection, which led to 20% of readmissions, followed by arrhythmias, responsible for 8% of readmissions, said Dr. Kilic, a thoracic surgeon at the University of Pennsylvania in Philadelphia.
Because fluid overload, often in the form of pleural effusion, is such an important driver of rehospitalizations, a more targeted management program would include better titration of diuretic treatment to patients following heart surgery, thoracentesis, and closer monitoring of clinical features that flag fluid overload such as weight.
“The volume overload issue is where the money is. If we can reduce that, it could really impact readmissions,” Dr. Kilic said in an interview.
An investigational program to target rehospitalization risk in heart surgery patients is planned at Johns Hopkins Hospital in Baltimore, where Dr. Kilic worked when he performed this analysis. Surgeons at Johns Hopkins are now in the process of getting funding for this pilot program, said Dr. John V. Conte Jr., professor of surgery and director of mechanical circulatory support at Johns Hopkins and a collaborator with Dr. Kilic on developing the risk formula.
“We’ll tailor postoperative follow-up. We’ll get high-risk patients back to the clinic sooner, and we’ll send nurse practitioners to see them to make sure they’re taking their medications and are getting weighed daily,” Dr. Conte said in an interview. “When a patient has heart surgery, they typically retain about 5-10 pounds of fluid. Patients with good renal function give up that fluid easily, but others are difficult to diurese. Many patients go home before they have been fully diuresed, and we need to follow these patients and transition them better to out-of-hospital care.”
He noted that other situations also come up that unnecessarily drive patients back to the hospital when an alternative and less expensive intervention might be equally effective. For example, some patients return to the hospital out of concern for how their chest wound is healing. Instead of being rehospitalized, such patients could be reassured by having them send a nurse a photo of their wound or by coming to an outpatient clinic.
“We need to engage more often with recently discharged patients,” Dr. Conte said in an interview. “Discharging them doesn’t mean separating them from the health care system; it should mean interacting with patients in a different way” that produces better outcomes and patient satisfaction for less money. Developing improved ways to manage recent heart surgery patients following discharge becomes even more critical later this year when, in July, the Centers for Medicare & Medicaid Services adds 30-day readmissions following coronary artery bypass grafting (CABG) to its list of procedures that can generate a penalty to hospitals if they exceed U.S. norms for readmission rates.
The risk model developed by Dr. Kilic, Dr. Conte, and their associates used data collected from 5,360 heart surgery patients treated at Johns Hopkins during 2008-2013. Nearly half the patients underwent isolated CABG, and 20% had isolated valve surgery. Overall, 585 patients (11%) had a hospital readmission within 30 days of their index discharge. One limitation of the analysis was it used data only on readmissions back to Johns Hopkins Hospital.
The researchers used data from three-quarters of the database to derive the risk formula, and from the remaining 25% of the database to validate the formula. A multivariate analysis of demographic and clinical characteristics that significantly linked with an elevated risk for readmissions identified five factors that independently made a significant contribution to readmission risk. The researchers assigned each of these five factors points depending on its relative contribution to readmission risk in the adjusted model: Severe chronic lung disease received 6 points; placement of a ventricular assist device received 5 points, while other types of heart surgery that was not CABG or valve surgery received 4 points (isolated CABG, isolated valve, or combined CABG and valve surgery received 0 points); development of acute renal failure postoperatively but before index discharge received 4 points; an index length of stay beyond 7 days received 4 points; and African American race received 3 points. The maximum number of points a patient could receive was 22.
Patients with a score of 0 had a 6% rate of a 30-day readmission; those with a score of 22 had a 63% readmission rate. For simplicity, Dr. Kilic suggested dividing patients into three categories based on their readmission risk score: Low-risk patients with a score of 0 had a readmission risk of 6%, medium-risk patients with a score of 1-10 had a readmission risk of 12%, and high-risk patients with a score of 11 or more had a readmission risk of 31%. The researchers found a 96% correlation when comparing these predicted readmission risk rates based on the derivation-subgroup analysis with the actual readmission rates seen in the validation subgroup of their database. The targeted risk-management program planned by Dr. Conte would primarily focus on high-risk patients.
Dr. Kilic and Dr. Conte said they had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
Key clinical point: A risk score predicted which heart surgery patients faced the greatest risk for hospital readmission within 30 days of their index discharge.
Major finding: Patients with a 0 score had a 6% 30-day readmission rate; a high score of 22 linked with a 63% rate.
Data source: A review of 5,360 heart surgery patients treated at one U.S. center.
Disclosures: Dr. Kilic and Dr. Conte said they had no relevant financial disclosures.
A Case of Nerves
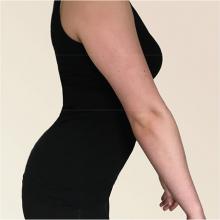
1. After several years of progressively worsening symptoms, starting with intermittent muscular rigidity and spasms in the lower back, this patient now has stiff leg muscles (more pronounced on the right side). On exam, she demonstrates a slow, stiff manner of walking and lordosis of the lower spine.
Diagnosis: Moersch-Woltman syndrome, or stiff person syndrome, is a rare acquired neurologic disorder, affecting both men and women of any age; most, however, are women. Thought to be an autoimmune disorder, this syndrome may localized to one area of the body or may be widespread, and rapidly progressive, including the body, brain stem, and spinal cord. If untreated, the patient may have difficulty walking and performing activities of daily living.
For more information, see “Stiff Person Syndrome.”
For the next photograph, proceed to the next page >>
2. This patient lost control of her eye and eyelid rather suddenly and then noticed difficulty swallowing and slurred speech. She is now experiencing limb weakness that worsens as the day progresses.
Diagnosis: Myasthenia gravis is a chronic autoimmune disease characterized by varying degrees of weakness of the skeletal muscles of the body. Muscles that control eye and eyelid movement, facial expression, chewing, talking, and swallowing are often, but not always, involved in the disorder. The muscles that control breathing and neck and limb movements may also be affected.
For more information, see “Test Accurately Diagnoses Myasthenia Gravis.” Neurology Reviews. 2016;24(3):36.
For the next photograph, proceed to the next page >>
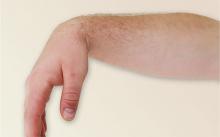
3. After a night out on the town, this patient is concerned that something is wrong, and it’s not the splitting headache: He is unable to extend his right wrist, thumb, or fingers in the pronated position. Furthermore, the radial and dorsal skin is numb. During the history, he reports that he fell asleep sitting upright with his arm over the back of a chair.
Diagnosis: Radial neuropathy, or Saturday night palsy, is typically produced by direct, prolonged pressure on the radial nerve. The nerve is especially susceptible as it spirals around the midportion of the humerus, in this case by allowing the hard edge of a chair back to compress it all night long. Although alcohol and drugs are usually implicated, radial palsy can also result from fracturing the humerus, leaning on crutches, or from long-term constriction of the wrist (tight watch or bracelet).
For more information, see “9.19 Radial Neuropathy (Saturday Night Palsy).”
For the next photograph, proceed to the next page >>
4. The patient reports spontaneous severe pain near his right shoulder, unrelated to any trauma. Two weeks later, the shoulder protrudes in a winglike position on the upper back, made more apparent when the same side arm pushes against resistance. He has limited ability to lift that arm above his head.
Diagnosis: Winged scapula injury is due to dysfunction of the anterior serratus muscle, which is supplied by the injury-prone long thoracic nerve.
Damage can be caused by a contusion or blunt trauma of the shoulder, traction of the neck, and sometimes a viral illness. Paralysis of the serratus anterior muscle has been well documented among athletes and laborers. To identify an injury, the patient stands facing a wall from about two feet away and then pushes against the wall with flat palms at waist level. Conservative treatment is often recommended; if after 6-24 months if there is no recovery, patients become candidates for corrective surgery.
For more information, see “Scapular winging: anatomical review, diagnosis, and treatments.” Curr Rev Musculoskelet Med. 2008;1(1):1-11.
Related article
For more on peripheral neuropathies, see “An easy approach to evaluating peripheral neuropathy.” J Fam Pract. 2006 October;55(10):853-861.
Photographs courtesy of Ashley Owens and Alexander Orban. They are included for illustration only and do not represent actual cases.
1. After several years of progressively worsening symptoms, starting with intermittent muscular rigidity and spasms in the lower back, this patient now has stiff leg muscles (more pronounced on the right side). On exam, she demonstrates a slow, stiff manner of walking and lordosis of the lower spine.
Diagnosis: Moersch-Woltman syndrome, or stiff person syndrome, is a rare acquired neurologic disorder, affecting both men and women of any age; most, however, are women. Thought to be an autoimmune disorder, this syndrome may localized to one area of the body or may be widespread, and rapidly progressive, including the body, brain stem, and spinal cord. If untreated, the patient may have difficulty walking and performing activities of daily living.
For more information, see “Stiff Person Syndrome.”
For the next photograph, proceed to the next page >>
2. This patient lost control of her eye and eyelid rather suddenly and then noticed difficulty swallowing and slurred speech. She is now experiencing limb weakness that worsens as the day progresses.
Diagnosis: Myasthenia gravis is a chronic autoimmune disease characterized by varying degrees of weakness of the skeletal muscles of the body. Muscles that control eye and eyelid movement, facial expression, chewing, talking, and swallowing are often, but not always, involved in the disorder. The muscles that control breathing and neck and limb movements may also be affected.
For more information, see “Test Accurately Diagnoses Myasthenia Gravis.” Neurology Reviews. 2016;24(3):36.
For the next photograph, proceed to the next page >>
3. After a night out on the town, this patient is concerned that something is wrong, and it’s not the splitting headache: He is unable to extend his right wrist, thumb, or fingers in the pronated position. Furthermore, the radial and dorsal skin is numb. During the history, he reports that he fell asleep sitting upright with his arm over the back of a chair.
Diagnosis: Radial neuropathy, or Saturday night palsy, is typically produced by direct, prolonged pressure on the radial nerve. The nerve is especially susceptible as it spirals around the midportion of the humerus, in this case by allowing the hard edge of a chair back to compress it all night long. Although alcohol and drugs are usually implicated, radial palsy can also result from fracturing the humerus, leaning on crutches, or from long-term constriction of the wrist (tight watch or bracelet).
For more information, see “9.19 Radial Neuropathy (Saturday Night Palsy).”
For the next photograph, proceed to the next page >>
4. The patient reports spontaneous severe pain near his right shoulder, unrelated to any trauma. Two weeks later, the shoulder protrudes in a winglike position on the upper back, made more apparent when the same side arm pushes against resistance. He has limited ability to lift that arm above his head.
Diagnosis: Winged scapula injury is due to dysfunction of the anterior serratus muscle, which is supplied by the injury-prone long thoracic nerve.
Damage can be caused by a contusion or blunt trauma of the shoulder, traction of the neck, and sometimes a viral illness. Paralysis of the serratus anterior muscle has been well documented among athletes and laborers. To identify an injury, the patient stands facing a wall from about two feet away and then pushes against the wall with flat palms at waist level. Conservative treatment is often recommended; if after 6-24 months if there is no recovery, patients become candidates for corrective surgery.
For more information, see “Scapular winging: anatomical review, diagnosis, and treatments.” Curr Rev Musculoskelet Med. 2008;1(1):1-11.
Related article
For more on peripheral neuropathies, see “An easy approach to evaluating peripheral neuropathy.” J Fam Pract. 2006 October;55(10):853-861.
Photographs courtesy of Ashley Owens and Alexander Orban. They are included for illustration only and do not represent actual cases.
1. After several years of progressively worsening symptoms, starting with intermittent muscular rigidity and spasms in the lower back, this patient now has stiff leg muscles (more pronounced on the right side). On exam, she demonstrates a slow, stiff manner of walking and lordosis of the lower spine.
Diagnosis: Moersch-Woltman syndrome, or stiff person syndrome, is a rare acquired neurologic disorder, affecting both men and women of any age; most, however, are women. Thought to be an autoimmune disorder, this syndrome may localized to one area of the body or may be widespread, and rapidly progressive, including the body, brain stem, and spinal cord. If untreated, the patient may have difficulty walking and performing activities of daily living.
For more information, see “Stiff Person Syndrome.”
For the next photograph, proceed to the next page >>
2. This patient lost control of her eye and eyelid rather suddenly and then noticed difficulty swallowing and slurred speech. She is now experiencing limb weakness that worsens as the day progresses.
Diagnosis: Myasthenia gravis is a chronic autoimmune disease characterized by varying degrees of weakness of the skeletal muscles of the body. Muscles that control eye and eyelid movement, facial expression, chewing, talking, and swallowing are often, but not always, involved in the disorder. The muscles that control breathing and neck and limb movements may also be affected.
For more information, see “Test Accurately Diagnoses Myasthenia Gravis.” Neurology Reviews. 2016;24(3):36.
For the next photograph, proceed to the next page >>
3. After a night out on the town, this patient is concerned that something is wrong, and it’s not the splitting headache: He is unable to extend his right wrist, thumb, or fingers in the pronated position. Furthermore, the radial and dorsal skin is numb. During the history, he reports that he fell asleep sitting upright with his arm over the back of a chair.
Diagnosis: Radial neuropathy, or Saturday night palsy, is typically produced by direct, prolonged pressure on the radial nerve. The nerve is especially susceptible as it spirals around the midportion of the humerus, in this case by allowing the hard edge of a chair back to compress it all night long. Although alcohol and drugs are usually implicated, radial palsy can also result from fracturing the humerus, leaning on crutches, or from long-term constriction of the wrist (tight watch or bracelet).
For more information, see “9.19 Radial Neuropathy (Saturday Night Palsy).”
For the next photograph, proceed to the next page >>
4. The patient reports spontaneous severe pain near his right shoulder, unrelated to any trauma. Two weeks later, the shoulder protrudes in a winglike position on the upper back, made more apparent when the same side arm pushes against resistance. He has limited ability to lift that arm above his head.
Diagnosis: Winged scapula injury is due to dysfunction of the anterior serratus muscle, which is supplied by the injury-prone long thoracic nerve.
Damage can be caused by a contusion or blunt trauma of the shoulder, traction of the neck, and sometimes a viral illness. Paralysis of the serratus anterior muscle has been well documented among athletes and laborers. To identify an injury, the patient stands facing a wall from about two feet away and then pushes against the wall with flat palms at waist level. Conservative treatment is often recommended; if after 6-24 months if there is no recovery, patients become candidates for corrective surgery.
For more information, see “Scapular winging: anatomical review, diagnosis, and treatments.” Curr Rev Musculoskelet Med. 2008;1(1):1-11.
Related article
For more on peripheral neuropathies, see “An easy approach to evaluating peripheral neuropathy.” J Fam Pract. 2006 October;55(10):853-861.
Photographs courtesy of Ashley Owens and Alexander Orban. They are included for illustration only and do not represent actual cases.
Impact of trimodality treatment on patient quality of life and arm function for superior sulcus tumors
Background Trimodality treatment leads to improved survival for superior sulcus tumor (SST) patients. Not much is known about the impact of this treatment on arm function and patient quality of life.
Objective To analyze arm function and quality of life in SST patients undergoing trimodality treatment.
Methods This was a prospective cohort study of consecutive SST patients treated with trimodality treatment that was conducted between April 1, 2010 and October 31, 2012. We obtained informed consent for 20 of 22 eligible patients. The 36-item Short Form Health Survey (SF-36) and disabilities of the arm, shoulder, and hand (DASH) questionnaires were used to asses patient quality of life and subjective arm function at 0 (preoperative day), 3, and 12 months after trimodality treatment.
Results DASH scores were significantly lower at 3 and 12 months (P = .024 and P = .011) compared with preoperative scores. Significantly lower scores were reported for the SF-36 domains of physical functioning at 12 months (P = .020) and of physical role functioning at 3 months (P = .041), and significantly more pain was reported at 3 and 12 months (P = .006 and P = .019, respectively). Patients who underwent T1 nerve root resection had lower scores for the SF-36 domain health change at 3 months (P = .037) compared with those in whom the T1 root was spared. For all other domains no differences were found.
Limitations Small sample size; patient pre-chemoradiation function and quality of life unknown.
Conclusion Subjective arm function and patient quality of life is reduced following trimodality treatment. Resection of the T1 nerve root has no significant long-term effect on the subjective arm function and quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Background Trimodality treatment leads to improved survival for superior sulcus tumor (SST) patients. Not much is known about the impact of this treatment on arm function and patient quality of life.
Objective To analyze arm function and quality of life in SST patients undergoing trimodality treatment.
Methods This was a prospective cohort study of consecutive SST patients treated with trimodality treatment that was conducted between April 1, 2010 and October 31, 2012. We obtained informed consent for 20 of 22 eligible patients. The 36-item Short Form Health Survey (SF-36) and disabilities of the arm, shoulder, and hand (DASH) questionnaires were used to asses patient quality of life and subjective arm function at 0 (preoperative day), 3, and 12 months after trimodality treatment.
Results DASH scores were significantly lower at 3 and 12 months (P = .024 and P = .011) compared with preoperative scores. Significantly lower scores were reported for the SF-36 domains of physical functioning at 12 months (P = .020) and of physical role functioning at 3 months (P = .041), and significantly more pain was reported at 3 and 12 months (P = .006 and P = .019, respectively). Patients who underwent T1 nerve root resection had lower scores for the SF-36 domain health change at 3 months (P = .037) compared with those in whom the T1 root was spared. For all other domains no differences were found.
Limitations Small sample size; patient pre-chemoradiation function and quality of life unknown.
Conclusion Subjective arm function and patient quality of life is reduced following trimodality treatment. Resection of the T1 nerve root has no significant long-term effect on the subjective arm function and quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Background Trimodality treatment leads to improved survival for superior sulcus tumor (SST) patients. Not much is known about the impact of this treatment on arm function and patient quality of life.
Objective To analyze arm function and quality of life in SST patients undergoing trimodality treatment.
Methods This was a prospective cohort study of consecutive SST patients treated with trimodality treatment that was conducted between April 1, 2010 and October 31, 2012. We obtained informed consent for 20 of 22 eligible patients. The 36-item Short Form Health Survey (SF-36) and disabilities of the arm, shoulder, and hand (DASH) questionnaires were used to asses patient quality of life and subjective arm function at 0 (preoperative day), 3, and 12 months after trimodality treatment.
Results DASH scores were significantly lower at 3 and 12 months (P = .024 and P = .011) compared with preoperative scores. Significantly lower scores were reported for the SF-36 domains of physical functioning at 12 months (P = .020) and of physical role functioning at 3 months (P = .041), and significantly more pain was reported at 3 and 12 months (P = .006 and P = .019, respectively). Patients who underwent T1 nerve root resection had lower scores for the SF-36 domain health change at 3 months (P = .037) compared with those in whom the T1 root was spared. For all other domains no differences were found.
Limitations Small sample size; patient pre-chemoradiation function and quality of life unknown.
Conclusion Subjective arm function and patient quality of life is reduced following trimodality treatment. Resection of the T1 nerve root has no significant long-term effect on the subjective arm function and quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Testing, Risk Factors & Considerations of Treatment: Nilotinib & Dasatinib
Dr. Kalaycio: I'm starting with the less controversial questions to begin with. I think the next set have the potential for some more controversy. Before we leave the initial assessment of CML, do either of you have observations regarding referrals that you get about which you would like to either dispel myths or remind practitioners about best practices in patients newly diagnosed with CML?
I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. – Michael Deininger, MD, PhDDr. Mauro: I can mention one thing. I think when thinking about initial molecular diagnostic results, it is important to point out that testing should screen broadly for different fusions, namely P190 and P210, or variant (p230) transcripts. There are rare patients in chronic phase with non-p210 fusion who need to be followed with specific PCR. On this same topic, the measurement of transcripts at presentation (ie, before treatment) has become quite important and whereas formerly had not been emphasized, presently all patients should have ”baseline” transcript levels.
Dr. Deininger: I think one issue that comes up once in a while is that spleen measurements are done by ultrasound. Technically it's more accurate, but all the clinical risk scores and the prognostication is based on the old fashioned—but probably highly inaccurate—technology of palpation. This is what counts, this is the value to document. I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. Getting a good handle of those risk factors at diagnosis is also important.
Dr. Kalaycio: I think that's a very important point. That's where I was going to go next with this conversation. I was going to avoid the conversation about which TKI to choose as initial treatment. I think that's a debate unto itself.
I would like to ask you how you might assess cardiovascular risk before placing a patient on nilotinib. You got to that a little bit, Dr. Deininger. Could you expand on what else you might review as far as whether or not you feel a patient is a good candidate to start on nilotinib?
Dr. Deininger: Specifically, with regard to nilotinib, we would always get a baseline electrocardiogram. We would do a clinical exam. We would not do an echocardiogram just routinely in the absence of a cardiovascular history or any clinical evidence for heart failure or other cardiovascular issues.
We've adopted the practice of doing a lipid panel. Of course we would include fasting glucose as well. Some of these recommendations are probably somewhat on the soft side, because it's not yet clear what to do with the information.
On the other hand, I think for a patient who is being considered for nilotinib one wants to make sure that one really does the best to minimize the cardiovascular risk factors. Of course that would include smoking history and taking blood pressure and making sure that these risk factors are controlled.
If people have a presentation that is really out of whack in terms of their risk factor management, I would send them to an internist or even a cardiologist to help me optimize the cardiovascular prevention strategy.
Dr. Kalaycio: Great. Similarly, Dr. Mauro, how do you assess pulmonary risk before placing a patient on dasatinib?
Dr. Mauro: I think here we are focused on the less frequent and also less well understood potential toxicity of pulmonary hypertension, coupled with the more common risk of pleural and pericardial effusions.
I'm not sure how much we've learned in clinical studies looking at baseline chest X-rays or timing of X-rays during treatment. I think our best tool in the prevention and management of pleural and pericardial effusions is full discussion with patients about risk and what to look for, attention to any and all symptoms, and appropriate deployment of diagnostics as indicated.
It's interesting to consider whether baseline echocardiography for measurement of pulmonary pressures is warranted. I would say now we're on probably somewhat softer ground, first because on routine echocardiogram pulmonary pressure can't be measured readily unless there is some valvular regurgitation. As well, it is stated that pulmonary hypertension is only properly diagnosed by right heart catheterization. While I'm tempted to do routine echocardiogram studies, I think that such a recommendation still may be perhaps the realm of a clinical study. We need to explore that further. I think with dasatinib there may be certain patients at higher risk, although the data are somewhat limited. There seem to be certain conditions potentially associated with more pleural and pericardial toxicity, including cardiovascular disease and autoimmune disease. There may be circumstances during treatment—lymphocytosis, for example—that may be associated with greater risk. I think expectant management may still be the right approach and echocardiography and more aggressive diagnostics be reserved for patients in whom there might be much more clinical consequence.
Dr. Kalaycio: I'd like to pursue that a little bit further because sometimes the patients will come to us having already had an echocardiogram that may actually show some mild pulmonary hypertension and maybe they've got significant cardiovascular risk factors where you would otherwise be thinking about using dasatinib. Here's someone with pulmonary hypertension, at least by echocardiographic criteria, would that be enough to dissuade you from the use of dasatinib?
Dr. Mauro: I think it would certainly require significant consideration, understanding what the basis of the pulmonary hypertension is for that patient, and risk with adding dasatinib. I think the good news is the low incidence and the reversibility for the most part of dasatinib-associated pulmonary hypertension.
Again, I think the mechanism of action and the pathophysiology isn't completely understood, although there is the intriguing notion that imatinib has been reported to potentially mitigate pulmonary hypertension whereas dasatinib triggers it—a ”closed loop” if you will and an area ripe for research.
I would probably think that a patient with preexisting pulmonary hypertension in the new diagnosis setting might be the kind of patient for whom you really might weigh the pluses versus the minuses of a second generation TKI versus imatinib.
E-mail: [email protected]
Dr. Kalaycio: I'm starting with the less controversial questions to begin with. I think the next set have the potential for some more controversy. Before we leave the initial assessment of CML, do either of you have observations regarding referrals that you get about which you would like to either dispel myths or remind practitioners about best practices in patients newly diagnosed with CML?
I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. – Michael Deininger, MD, PhDDr. Mauro: I can mention one thing. I think when thinking about initial molecular diagnostic results, it is important to point out that testing should screen broadly for different fusions, namely P190 and P210, or variant (p230) transcripts. There are rare patients in chronic phase with non-p210 fusion who need to be followed with specific PCR. On this same topic, the measurement of transcripts at presentation (ie, before treatment) has become quite important and whereas formerly had not been emphasized, presently all patients should have ”baseline” transcript levels.
Dr. Deininger: I think one issue that comes up once in a while is that spleen measurements are done by ultrasound. Technically it's more accurate, but all the clinical risk scores and the prognostication is based on the old fashioned—but probably highly inaccurate—technology of palpation. This is what counts, this is the value to document. I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. Getting a good handle of those risk factors at diagnosis is also important.
Dr. Kalaycio: I think that's a very important point. That's where I was going to go next with this conversation. I was going to avoid the conversation about which TKI to choose as initial treatment. I think that's a debate unto itself.
I would like to ask you how you might assess cardiovascular risk before placing a patient on nilotinib. You got to that a little bit, Dr. Deininger. Could you expand on what else you might review as far as whether or not you feel a patient is a good candidate to start on nilotinib?
Dr. Deininger: Specifically, with regard to nilotinib, we would always get a baseline electrocardiogram. We would do a clinical exam. We would not do an echocardiogram just routinely in the absence of a cardiovascular history or any clinical evidence for heart failure or other cardiovascular issues.
We've adopted the practice of doing a lipid panel. Of course we would include fasting glucose as well. Some of these recommendations are probably somewhat on the soft side, because it's not yet clear what to do with the information.
On the other hand, I think for a patient who is being considered for nilotinib one wants to make sure that one really does the best to minimize the cardiovascular risk factors. Of course that would include smoking history and taking blood pressure and making sure that these risk factors are controlled.
If people have a presentation that is really out of whack in terms of their risk factor management, I would send them to an internist or even a cardiologist to help me optimize the cardiovascular prevention strategy.
Dr. Kalaycio: Great. Similarly, Dr. Mauro, how do you assess pulmonary risk before placing a patient on dasatinib?
Dr. Mauro: I think here we are focused on the less frequent and also less well understood potential toxicity of pulmonary hypertension, coupled with the more common risk of pleural and pericardial effusions.
I'm not sure how much we've learned in clinical studies looking at baseline chest X-rays or timing of X-rays during treatment. I think our best tool in the prevention and management of pleural and pericardial effusions is full discussion with patients about risk and what to look for, attention to any and all symptoms, and appropriate deployment of diagnostics as indicated.
It's interesting to consider whether baseline echocardiography for measurement of pulmonary pressures is warranted. I would say now we're on probably somewhat softer ground, first because on routine echocardiogram pulmonary pressure can't be measured readily unless there is some valvular regurgitation. As well, it is stated that pulmonary hypertension is only properly diagnosed by right heart catheterization. While I'm tempted to do routine echocardiogram studies, I think that such a recommendation still may be perhaps the realm of a clinical study. We need to explore that further. I think with dasatinib there may be certain patients at higher risk, although the data are somewhat limited. There seem to be certain conditions potentially associated with more pleural and pericardial toxicity, including cardiovascular disease and autoimmune disease. There may be circumstances during treatment—lymphocytosis, for example—that may be associated with greater risk. I think expectant management may still be the right approach and echocardiography and more aggressive diagnostics be reserved for patients in whom there might be much more clinical consequence.
Dr. Kalaycio: I'd like to pursue that a little bit further because sometimes the patients will come to us having already had an echocardiogram that may actually show some mild pulmonary hypertension and maybe they've got significant cardiovascular risk factors where you would otherwise be thinking about using dasatinib. Here's someone with pulmonary hypertension, at least by echocardiographic criteria, would that be enough to dissuade you from the use of dasatinib?
Dr. Mauro: I think it would certainly require significant consideration, understanding what the basis of the pulmonary hypertension is for that patient, and risk with adding dasatinib. I think the good news is the low incidence and the reversibility for the most part of dasatinib-associated pulmonary hypertension.
Again, I think the mechanism of action and the pathophysiology isn't completely understood, although there is the intriguing notion that imatinib has been reported to potentially mitigate pulmonary hypertension whereas dasatinib triggers it—a ”closed loop” if you will and an area ripe for research.
I would probably think that a patient with preexisting pulmonary hypertension in the new diagnosis setting might be the kind of patient for whom you really might weigh the pluses versus the minuses of a second generation TKI versus imatinib.
E-mail: [email protected]
Dr. Kalaycio: I'm starting with the less controversial questions to begin with. I think the next set have the potential for some more controversy. Before we leave the initial assessment of CML, do either of you have observations regarding referrals that you get about which you would like to either dispel myths or remind practitioners about best practices in patients newly diagnosed with CML?
I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. – Michael Deininger, MD, PhDDr. Mauro: I can mention one thing. I think when thinking about initial molecular diagnostic results, it is important to point out that testing should screen broadly for different fusions, namely P190 and P210, or variant (p230) transcripts. There are rare patients in chronic phase with non-p210 fusion who need to be followed with specific PCR. On this same topic, the measurement of transcripts at presentation (ie, before treatment) has become quite important and whereas formerly had not been emphasized, presently all patients should have ”baseline” transcript levels.
Dr. Deininger: I think one issue that comes up once in a while is that spleen measurements are done by ultrasound. Technically it's more accurate, but all the clinical risk scores and the prognostication is based on the old fashioned—but probably highly inaccurate—technology of palpation. This is what counts, this is the value to document. I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. Getting a good handle of those risk factors at diagnosis is also important.
Dr. Kalaycio: I think that's a very important point. That's where I was going to go next with this conversation. I was going to avoid the conversation about which TKI to choose as initial treatment. I think that's a debate unto itself.
I would like to ask you how you might assess cardiovascular risk before placing a patient on nilotinib. You got to that a little bit, Dr. Deininger. Could you expand on what else you might review as far as whether or not you feel a patient is a good candidate to start on nilotinib?
Dr. Deininger: Specifically, with regard to nilotinib, we would always get a baseline electrocardiogram. We would do a clinical exam. We would not do an echocardiogram just routinely in the absence of a cardiovascular history or any clinical evidence for heart failure or other cardiovascular issues.
We've adopted the practice of doing a lipid panel. Of course we would include fasting glucose as well. Some of these recommendations are probably somewhat on the soft side, because it's not yet clear what to do with the information.
On the other hand, I think for a patient who is being considered for nilotinib one wants to make sure that one really does the best to minimize the cardiovascular risk factors. Of course that would include smoking history and taking blood pressure and making sure that these risk factors are controlled.
If people have a presentation that is really out of whack in terms of their risk factor management, I would send them to an internist or even a cardiologist to help me optimize the cardiovascular prevention strategy.
Dr. Kalaycio: Great. Similarly, Dr. Mauro, how do you assess pulmonary risk before placing a patient on dasatinib?
Dr. Mauro: I think here we are focused on the less frequent and also less well understood potential toxicity of pulmonary hypertension, coupled with the more common risk of pleural and pericardial effusions.
I'm not sure how much we've learned in clinical studies looking at baseline chest X-rays or timing of X-rays during treatment. I think our best tool in the prevention and management of pleural and pericardial effusions is full discussion with patients about risk and what to look for, attention to any and all symptoms, and appropriate deployment of diagnostics as indicated.
It's interesting to consider whether baseline echocardiography for measurement of pulmonary pressures is warranted. I would say now we're on probably somewhat softer ground, first because on routine echocardiogram pulmonary pressure can't be measured readily unless there is some valvular regurgitation. As well, it is stated that pulmonary hypertension is only properly diagnosed by right heart catheterization. While I'm tempted to do routine echocardiogram studies, I think that such a recommendation still may be perhaps the realm of a clinical study. We need to explore that further. I think with dasatinib there may be certain patients at higher risk, although the data are somewhat limited. There seem to be certain conditions potentially associated with more pleural and pericardial toxicity, including cardiovascular disease and autoimmune disease. There may be circumstances during treatment—lymphocytosis, for example—that may be associated with greater risk. I think expectant management may still be the right approach and echocardiography and more aggressive diagnostics be reserved for patients in whom there might be much more clinical consequence.
Dr. Kalaycio: I'd like to pursue that a little bit further because sometimes the patients will come to us having already had an echocardiogram that may actually show some mild pulmonary hypertension and maybe they've got significant cardiovascular risk factors where you would otherwise be thinking about using dasatinib. Here's someone with pulmonary hypertension, at least by echocardiographic criteria, would that be enough to dissuade you from the use of dasatinib?
Dr. Mauro: I think it would certainly require significant consideration, understanding what the basis of the pulmonary hypertension is for that patient, and risk with adding dasatinib. I think the good news is the low incidence and the reversibility for the most part of dasatinib-associated pulmonary hypertension.
Again, I think the mechanism of action and the pathophysiology isn't completely understood, although there is the intriguing notion that imatinib has been reported to potentially mitigate pulmonary hypertension whereas dasatinib triggers it—a ”closed loop” if you will and an area ripe for research.
I would probably think that a patient with preexisting pulmonary hypertension in the new diagnosis setting might be the kind of patient for whom you really might weigh the pluses versus the minuses of a second generation TKI versus imatinib.
E-mail: [email protected]
Financial toxicity in cancer care
The cost of cancer care is increasing, with important implications for the delivery of high-quality, patient-centered care. In the clinical setting, patients and physicians express a desire to discuss out-of-pocket costs. Nevertheless, both groups feel inadequately prepared to participate in these discussions, and perhaps not surprisingly, the integration of these discussions into clinical practice seems to be the exception rather than the rule.
Click on the PDF icon at the top of this introduction to read the full article.
The cost of cancer care is increasing, with important implications for the delivery of high-quality, patient-centered care. In the clinical setting, patients and physicians express a desire to discuss out-of-pocket costs. Nevertheless, both groups feel inadequately prepared to participate in these discussions, and perhaps not surprisingly, the integration of these discussions into clinical practice seems to be the exception rather than the rule.
Click on the PDF icon at the top of this introduction to read the full article.
The cost of cancer care is increasing, with important implications for the delivery of high-quality, patient-centered care. In the clinical setting, patients and physicians express a desire to discuss out-of-pocket costs. Nevertheless, both groups feel inadequately prepared to participate in these discussions, and perhaps not surprisingly, the integration of these discussions into clinical practice seems to be the exception rather than the rule.
Click on the PDF icon at the top of this introduction to read the full article.
Opioid risk assessment in palliative medicine
Pain management with opioids is an integral part of palliative medicine. As the doses and durations of opioid therapy increase, the inherent risks of opioid therapy rise. Although opioids are effective analgesics, they bring with them complex medical and psychological side effects. Aberrant behavior is dangerous and can be difficult to identify as it results in a splitting in the goals of treatment between the patient and providers. One effective strategy in preventing that situation is through the early identification of at-risk patients.
Click on the PDF icon at the top of this introduction to read the full article.
Pain management with opioids is an integral part of palliative medicine. As the doses and durations of opioid therapy increase, the inherent risks of opioid therapy rise. Although opioids are effective analgesics, they bring with them complex medical and psychological side effects. Aberrant behavior is dangerous and can be difficult to identify as it results in a splitting in the goals of treatment between the patient and providers. One effective strategy in preventing that situation is through the early identification of at-risk patients.
Click on the PDF icon at the top of this introduction to read the full article.
Pain management with opioids is an integral part of palliative medicine. As the doses and durations of opioid therapy increase, the inherent risks of opioid therapy rise. Although opioids are effective analgesics, they bring with them complex medical and psychological side effects. Aberrant behavior is dangerous and can be difficult to identify as it results in a splitting in the goals of treatment between the patient and providers. One effective strategy in preventing that situation is through the early identification of at-risk patients.
Click on the PDF icon at the top of this introduction to read the full article.
Lupus patients’ transition to adult care leaves gaps, delays in care
Patients with childhood-onset systemic lupus erythematosus (SLE) who transitioned to adult care without a formal transitioning process had long periods without care despite having moderate disease activity and also frequently reported anxiety and depression in a retrospective study of 50 patients during a 3-year period at Brigham and Women’s Hospital Lupus Center in Boston.
Dr. Mary Beth Son of Boston Children’s Hospital and her colleagues found that the patients went a mean of nearly 9 months from their last pediatric rheumatology visit to their first adult rheumatology visit, and 72% had at least one gap in care, defined as no appointments in a recommended time frame. A total of 32% had more than one missed appointment, although there was an average of only 9% of appointments missed per patient. The investigators determined that missed appointments were significantly associated with white race, education below the high school level, and medication nonadherence (Lupus. 2016 Mar 23. doi: 10.1177/0961203316640913).
“The finding of lower educational level leading to a suboptimal transition outcome may help to delineate an at-risk population that requires further support during the process of transition. ... Although missed appointments weren’t prominent, the majority of our patients experienced gaps in care whereby they didn’t schedule appointments within the recommended time frame per the treating physician. The lack of appropriate scheduling with its concomitant potentially serious consequences demonstrates the need to educate transition patients regarding the importance of scheduling their own appointments,” the investigators wrote.
Scores of the SLE Disease Activity Index remained stable from a mean of 5.7 at baseline to 4.7 at year 3, but Systemic Lupus International Collaborating Clinics/ACR Damage Index for Systemic Lupus Erythematosus scores rose significantly from 0.46 to 0.78. Depression and anxiety diagnoses or symptoms increased significantly from 10% to 26%, and the investigators noted that “more than one-quarter of them required support from social work for a variety of serious circumstances including homelessness, substance abuse, and incarceration.”
The patients were diagnosed at a mean age of 14.5 years, and most were white (42%), African American (22%), Hispanic (22%), or Asian (10%). They had a mean age of 19.5 years at their first Lupus Center visit and 21.9 years at their last.
The investigators noted that the patients’ relatively high socioeconomic status (zip code–based annual household income of $50,000-$100,000 in 60% and $100,000 or more in 32%) may limit the generalizability of the study.
The authors reported having no relevant conflicts of interest. Dr. Son was supported by a Boston Children’s Hospital Career Development Fellowship and another author’s grant from the National Institutes of Health helped to support the study.
Patients with childhood-onset systemic lupus erythematosus (SLE) who transitioned to adult care without a formal transitioning process had long periods without care despite having moderate disease activity and also frequently reported anxiety and depression in a retrospective study of 50 patients during a 3-year period at Brigham and Women’s Hospital Lupus Center in Boston.
Dr. Mary Beth Son of Boston Children’s Hospital and her colleagues found that the patients went a mean of nearly 9 months from their last pediatric rheumatology visit to their first adult rheumatology visit, and 72% had at least one gap in care, defined as no appointments in a recommended time frame. A total of 32% had more than one missed appointment, although there was an average of only 9% of appointments missed per patient. The investigators determined that missed appointments were significantly associated with white race, education below the high school level, and medication nonadherence (Lupus. 2016 Mar 23. doi: 10.1177/0961203316640913).
“The finding of lower educational level leading to a suboptimal transition outcome may help to delineate an at-risk population that requires further support during the process of transition. ... Although missed appointments weren’t prominent, the majority of our patients experienced gaps in care whereby they didn’t schedule appointments within the recommended time frame per the treating physician. The lack of appropriate scheduling with its concomitant potentially serious consequences demonstrates the need to educate transition patients regarding the importance of scheduling their own appointments,” the investigators wrote.
Scores of the SLE Disease Activity Index remained stable from a mean of 5.7 at baseline to 4.7 at year 3, but Systemic Lupus International Collaborating Clinics/ACR Damage Index for Systemic Lupus Erythematosus scores rose significantly from 0.46 to 0.78. Depression and anxiety diagnoses or symptoms increased significantly from 10% to 26%, and the investigators noted that “more than one-quarter of them required support from social work for a variety of serious circumstances including homelessness, substance abuse, and incarceration.”
The patients were diagnosed at a mean age of 14.5 years, and most were white (42%), African American (22%), Hispanic (22%), or Asian (10%). They had a mean age of 19.5 years at their first Lupus Center visit and 21.9 years at their last.
The investigators noted that the patients’ relatively high socioeconomic status (zip code–based annual household income of $50,000-$100,000 in 60% and $100,000 or more in 32%) may limit the generalizability of the study.
The authors reported having no relevant conflicts of interest. Dr. Son was supported by a Boston Children’s Hospital Career Development Fellowship and another author’s grant from the National Institutes of Health helped to support the study.
Patients with childhood-onset systemic lupus erythematosus (SLE) who transitioned to adult care without a formal transitioning process had long periods without care despite having moderate disease activity and also frequently reported anxiety and depression in a retrospective study of 50 patients during a 3-year period at Brigham and Women’s Hospital Lupus Center in Boston.
Dr. Mary Beth Son of Boston Children’s Hospital and her colleagues found that the patients went a mean of nearly 9 months from their last pediatric rheumatology visit to their first adult rheumatology visit, and 72% had at least one gap in care, defined as no appointments in a recommended time frame. A total of 32% had more than one missed appointment, although there was an average of only 9% of appointments missed per patient. The investigators determined that missed appointments were significantly associated with white race, education below the high school level, and medication nonadherence (Lupus. 2016 Mar 23. doi: 10.1177/0961203316640913).
“The finding of lower educational level leading to a suboptimal transition outcome may help to delineate an at-risk population that requires further support during the process of transition. ... Although missed appointments weren’t prominent, the majority of our patients experienced gaps in care whereby they didn’t schedule appointments within the recommended time frame per the treating physician. The lack of appropriate scheduling with its concomitant potentially serious consequences demonstrates the need to educate transition patients regarding the importance of scheduling their own appointments,” the investigators wrote.
Scores of the SLE Disease Activity Index remained stable from a mean of 5.7 at baseline to 4.7 at year 3, but Systemic Lupus International Collaborating Clinics/ACR Damage Index for Systemic Lupus Erythematosus scores rose significantly from 0.46 to 0.78. Depression and anxiety diagnoses or symptoms increased significantly from 10% to 26%, and the investigators noted that “more than one-quarter of them required support from social work for a variety of serious circumstances including homelessness, substance abuse, and incarceration.”
The patients were diagnosed at a mean age of 14.5 years, and most were white (42%), African American (22%), Hispanic (22%), or Asian (10%). They had a mean age of 19.5 years at their first Lupus Center visit and 21.9 years at their last.
The investigators noted that the patients’ relatively high socioeconomic status (zip code–based annual household income of $50,000-$100,000 in 60% and $100,000 or more in 32%) may limit the generalizability of the study.
The authors reported having no relevant conflicts of interest. Dr. Son was supported by a Boston Children’s Hospital Career Development Fellowship and another author’s grant from the National Institutes of Health helped to support the study.
FROM LUPUS
Key clinical point: Lupus patients undergoing transition need better education on the importance of prompt follow-up care with adult rheumatologists.
Major finding: Patients went a mean of nearly 9 months from their last pediatric rheumatology visit to their first adult rheumatology visit, and 72% had at least one gap in care.
Data source: A retrospective cohort study of 50 patients with childhood-onset SLE.
Disclosures: The authors reported having no relevant conflicts of interest. Dr. Son was supported by a Boston Children’s Hospital Career Development Fellowship and another author’s grant from the National Institutes of Health helped to support the study.
Lenalidomide prolongs PFS, not OS, in rel/ref MCL

Photo by Esther Dyson
Results of the phase 2 SPRINT trial suggest lenalidomide compares favorably to other single-agent therapies for patients with relapsed/refractory mantle cell lymphoma (MCL).
Patients who received lenalidomide had a significantly higher overall response rate and significantly longer progression-free survival (PFS) compared to patients who received single-agent therapy chosen by investigators.
However, there was no significant difference between the treatment arms with regard to overall survival (OS).
In addition, there were more treatment-related adverse events (AEs) in the lenalidomide arm than the investigator’s choice arm.
These results were published in The Lancet Oncology. The study was funded by Celgene Corporation, the company developing lenalidomide.
“The SPRINT study provided the first head-to-head, randomized, controlled study of single-agent lenalidomide compared with a range of single-agent comparators in previously treated MCL,” said study author Marek Trneny, MD, of Charles University Hospital in Prague, Czech Republic.
“The study demonstrated a statistically significant reduction in the risk of disease progression or death for lenalidomide over investigator’s choice in patients with relapsed/refractory MCL.”
The study enrolled 254 patients with relapsed/refractory MCL who were ineligible for intensive chemotherapy or hematopoietic stem cell transplant. The patients’ median age was 68.5 (range, 44-88), and they had received a median of 2 previous treatment regimens (range, 1-≥4).
Treatment
The patients were randomized (2:1) to receive lenalidomide orally at 25 mg on days 1-21 every 28 days until progressive disease or intolerability (n=170) or single-agent investigator’s choice (n=84), which included rituximab, gemcitabine, fludarabine, chlorambucil, and cytarabine. Patients who progressed on investigator’s choice could cross over to the lenalidomide arm.
In all, 167 patients in the lenalidomide arm and 83 patients in the investigator’s choice arm received at least 1 dose of treatment. In the investigator’s choice arm, 33% of patients received rituximab, 24% got gemcitabine, 22% fludarabine, 13% chlorambucil, and 8% cytarabine.
After disease progression on investigator’s choice, 46% of patients crossed over to the lenalidomide arm.
Before cycle 6, half of the patients in the lenalidomide arm and two-thirds of those in the investigator’s choice arm had discontinued treatment. After discontinuation, 46% of patients in the lenalidomide arm and 50% in the investigator’s choice arm received 1 or more new antilymphoma therapies (excluding crossover patients).
The investigators said the proportion of patients who responded to subsequent therapies or had progressive disease did not substantially differ whether they had previously been randomized to lenalidomide or investigator’s choice.
Efficacy
As of the data cutoff point (March 7, 2014), the median follow-up was 15.9 months (range, 7.6 to 31.7).
The overall response rate was 40% in the lenalidomide arm and 11% in the investigator’s choice arm (P<0.001). The complete response rates were 5% and 0%, respectively (P=0.04). The median duration of response was 16 months and 10.4 months, respectively.
Lenalidomide significantly prolonged PFS. The median PFS was 8.7 months in the lenalidomide arm and 5.2 months in the investigator’s choice arm. The hazard ratio was 0.61 (P=0.004).
There was no significant difference in OS between the treatment arms. The median OS was 27.8 months in the lenalidomide arm and 21.2 months in the investigator’s choice arm. The hazard ratio was 0.89 (P=0.45)
A total of 128 patients (50%) died. Most deaths occurred during follow-up, and the most frequent cause of death was underlying lymphoma.
Safety
The incidence of treatment-related AEs was 84% in the lenalidomide arm and 60% in the investigator’s choice arm.
The most common grade 3 or higher AEs—in the lenalidomide and investigator’s choice arms, respectively—were neutropenia (44% vs 34%) without increased risk of infection, thrombocytopenia (18% vs 28%), leukopenia (8% vs 11%), and anemia (8% vs 7%).
The incidence of any-grade nasopharyngitis was 15% in the lenalidomide arm and 6% in the investigator’s choice arm. The incidence of upper respiratory tract infection was 12% and 6%, respectively. Febrile neutropenia was reported in 6% and 2% of patients, respectively.
Growth factors were used more often in the lenalidomide arm than the investigator’s choice arm—30% and 23%, respectively. But platelet transfusions were used less often in the lenalidomide arm—4% and 11%, respectively. ![]()

Photo by Esther Dyson
Results of the phase 2 SPRINT trial suggest lenalidomide compares favorably to other single-agent therapies for patients with relapsed/refractory mantle cell lymphoma (MCL).
Patients who received lenalidomide had a significantly higher overall response rate and significantly longer progression-free survival (PFS) compared to patients who received single-agent therapy chosen by investigators.
However, there was no significant difference between the treatment arms with regard to overall survival (OS).
In addition, there were more treatment-related adverse events (AEs) in the lenalidomide arm than the investigator’s choice arm.
These results were published in The Lancet Oncology. The study was funded by Celgene Corporation, the company developing lenalidomide.
“The SPRINT study provided the first head-to-head, randomized, controlled study of single-agent lenalidomide compared with a range of single-agent comparators in previously treated MCL,” said study author Marek Trneny, MD, of Charles University Hospital in Prague, Czech Republic.
“The study demonstrated a statistically significant reduction in the risk of disease progression or death for lenalidomide over investigator’s choice in patients with relapsed/refractory MCL.”
The study enrolled 254 patients with relapsed/refractory MCL who were ineligible for intensive chemotherapy or hematopoietic stem cell transplant. The patients’ median age was 68.5 (range, 44-88), and they had received a median of 2 previous treatment regimens (range, 1-≥4).
Treatment
The patients were randomized (2:1) to receive lenalidomide orally at 25 mg on days 1-21 every 28 days until progressive disease or intolerability (n=170) or single-agent investigator’s choice (n=84), which included rituximab, gemcitabine, fludarabine, chlorambucil, and cytarabine. Patients who progressed on investigator’s choice could cross over to the lenalidomide arm.
In all, 167 patients in the lenalidomide arm and 83 patients in the investigator’s choice arm received at least 1 dose of treatment. In the investigator’s choice arm, 33% of patients received rituximab, 24% got gemcitabine, 22% fludarabine, 13% chlorambucil, and 8% cytarabine.
After disease progression on investigator’s choice, 46% of patients crossed over to the lenalidomide arm.
Before cycle 6, half of the patients in the lenalidomide arm and two-thirds of those in the investigator’s choice arm had discontinued treatment. After discontinuation, 46% of patients in the lenalidomide arm and 50% in the investigator’s choice arm received 1 or more new antilymphoma therapies (excluding crossover patients).
The investigators said the proportion of patients who responded to subsequent therapies or had progressive disease did not substantially differ whether they had previously been randomized to lenalidomide or investigator’s choice.
Efficacy
As of the data cutoff point (March 7, 2014), the median follow-up was 15.9 months (range, 7.6 to 31.7).
The overall response rate was 40% in the lenalidomide arm and 11% in the investigator’s choice arm (P<0.001). The complete response rates were 5% and 0%, respectively (P=0.04). The median duration of response was 16 months and 10.4 months, respectively.
Lenalidomide significantly prolonged PFS. The median PFS was 8.7 months in the lenalidomide arm and 5.2 months in the investigator’s choice arm. The hazard ratio was 0.61 (P=0.004).
There was no significant difference in OS between the treatment arms. The median OS was 27.8 months in the lenalidomide arm and 21.2 months in the investigator’s choice arm. The hazard ratio was 0.89 (P=0.45)
A total of 128 patients (50%) died. Most deaths occurred during follow-up, and the most frequent cause of death was underlying lymphoma.
Safety
The incidence of treatment-related AEs was 84% in the lenalidomide arm and 60% in the investigator’s choice arm.
The most common grade 3 or higher AEs—in the lenalidomide and investigator’s choice arms, respectively—were neutropenia (44% vs 34%) without increased risk of infection, thrombocytopenia (18% vs 28%), leukopenia (8% vs 11%), and anemia (8% vs 7%).
The incidence of any-grade nasopharyngitis was 15% in the lenalidomide arm and 6% in the investigator’s choice arm. The incidence of upper respiratory tract infection was 12% and 6%, respectively. Febrile neutropenia was reported in 6% and 2% of patients, respectively.
Growth factors were used more often in the lenalidomide arm than the investigator’s choice arm—30% and 23%, respectively. But platelet transfusions were used less often in the lenalidomide arm—4% and 11%, respectively. ![]()

Photo by Esther Dyson
Results of the phase 2 SPRINT trial suggest lenalidomide compares favorably to other single-agent therapies for patients with relapsed/refractory mantle cell lymphoma (MCL).
Patients who received lenalidomide had a significantly higher overall response rate and significantly longer progression-free survival (PFS) compared to patients who received single-agent therapy chosen by investigators.
However, there was no significant difference between the treatment arms with regard to overall survival (OS).
In addition, there were more treatment-related adverse events (AEs) in the lenalidomide arm than the investigator’s choice arm.
These results were published in The Lancet Oncology. The study was funded by Celgene Corporation, the company developing lenalidomide.
“The SPRINT study provided the first head-to-head, randomized, controlled study of single-agent lenalidomide compared with a range of single-agent comparators in previously treated MCL,” said study author Marek Trneny, MD, of Charles University Hospital in Prague, Czech Republic.
“The study demonstrated a statistically significant reduction in the risk of disease progression or death for lenalidomide over investigator’s choice in patients with relapsed/refractory MCL.”
The study enrolled 254 patients with relapsed/refractory MCL who were ineligible for intensive chemotherapy or hematopoietic stem cell transplant. The patients’ median age was 68.5 (range, 44-88), and they had received a median of 2 previous treatment regimens (range, 1-≥4).
Treatment
The patients were randomized (2:1) to receive lenalidomide orally at 25 mg on days 1-21 every 28 days until progressive disease or intolerability (n=170) or single-agent investigator’s choice (n=84), which included rituximab, gemcitabine, fludarabine, chlorambucil, and cytarabine. Patients who progressed on investigator’s choice could cross over to the lenalidomide arm.
In all, 167 patients in the lenalidomide arm and 83 patients in the investigator’s choice arm received at least 1 dose of treatment. In the investigator’s choice arm, 33% of patients received rituximab, 24% got gemcitabine, 22% fludarabine, 13% chlorambucil, and 8% cytarabine.
After disease progression on investigator’s choice, 46% of patients crossed over to the lenalidomide arm.
Before cycle 6, half of the patients in the lenalidomide arm and two-thirds of those in the investigator’s choice arm had discontinued treatment. After discontinuation, 46% of patients in the lenalidomide arm and 50% in the investigator’s choice arm received 1 or more new antilymphoma therapies (excluding crossover patients).
The investigators said the proportion of patients who responded to subsequent therapies or had progressive disease did not substantially differ whether they had previously been randomized to lenalidomide or investigator’s choice.
Efficacy
As of the data cutoff point (March 7, 2014), the median follow-up was 15.9 months (range, 7.6 to 31.7).
The overall response rate was 40% in the lenalidomide arm and 11% in the investigator’s choice arm (P<0.001). The complete response rates were 5% and 0%, respectively (P=0.04). The median duration of response was 16 months and 10.4 months, respectively.
Lenalidomide significantly prolonged PFS. The median PFS was 8.7 months in the lenalidomide arm and 5.2 months in the investigator’s choice arm. The hazard ratio was 0.61 (P=0.004).
There was no significant difference in OS between the treatment arms. The median OS was 27.8 months in the lenalidomide arm and 21.2 months in the investigator’s choice arm. The hazard ratio was 0.89 (P=0.45)
A total of 128 patients (50%) died. Most deaths occurred during follow-up, and the most frequent cause of death was underlying lymphoma.
Safety
The incidence of treatment-related AEs was 84% in the lenalidomide arm and 60% in the investigator’s choice arm.
The most common grade 3 or higher AEs—in the lenalidomide and investigator’s choice arms, respectively—were neutropenia (44% vs 34%) without increased risk of infection, thrombocytopenia (18% vs 28%), leukopenia (8% vs 11%), and anemia (8% vs 7%).
The incidence of any-grade nasopharyngitis was 15% in the lenalidomide arm and 6% in the investigator’s choice arm. The incidence of upper respiratory tract infection was 12% and 6%, respectively. Febrile neutropenia was reported in 6% and 2% of patients, respectively.
Growth factors were used more often in the lenalidomide arm than the investigator’s choice arm—30% and 23%, respectively. But platelet transfusions were used less often in the lenalidomide arm—4% and 11%, respectively. ![]()
Discontinuing Inhaled Corticosteroids in COPD Reduces Risk of Pneumonia
Clinical question: Is discontinuation of inhaled corticosteroids (ICSs) in patients with COPD associated with a decreased risk of pneumonia?
Background: ICSs are used in up to 85% of patients treated for COPD but may be associated with adverse systemic side effects including pneumonia. Trials looking at weaning patients off ICSs and replacing with long-acting bronchodilators have found few adverse outcomes; however, the benefits of discontinuation on adverse events, including pneumonia, have been unclear.
Study design: Case-control study.
Setting: Quebec health systems.
Synopsis: Using the Quebec health insurance databases, a study cohort of 103,386 patients with COPD on ICSs was created. Patients were followed for a mean of 4.9 years; 14,020 patients who were hospitalized for pneumonia or died from pneumonia outside the hospital were matched to control subjects. Discontinuation of ICSs was associated with a 37% decrease in serious pneumonia (relative risk [RR] 0.63; 95% CI, 0.60–0.66). The risk reduction occurred as early as one month after discontinuation of ICSs. Risk reduction was greater with fluticasone (RR 0.58; 95% CI, 0.54–0.61) than with budesonide (RR 0.87; 95% CI, 0.7–0.97).
Population size and follow-up may contribute to why risk reduction in pneumonia was seen in this study but not in other recent randomized trials on discontinuation of ICSs. A limitation of this study was its observational design; however, its results suggest that use of ICSs in COPD patients should be highly selective, as indiscriminate use can subject patients to elevated risk of hospitalization or death from pneumonia.
Bottom line: Discontinuation of ICSs in patients with COPD is associated with a decreased risk of contracting serious pneumonia. This reduction appears greatest with fluticasone.
Citation: Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177-1183.
Short Take
Increase in Rates of Prescription Drug Use and Polypharmacy Seen
The percentage of Americans who reported taking prescription medications increased substantially from 1999 to 2012 (51% to 59%), as did the percentage who reported taking at least five prescription medications.
Citation: Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1830.
Clinical question: Is discontinuation of inhaled corticosteroids (ICSs) in patients with COPD associated with a decreased risk of pneumonia?
Background: ICSs are used in up to 85% of patients treated for COPD but may be associated with adverse systemic side effects including pneumonia. Trials looking at weaning patients off ICSs and replacing with long-acting bronchodilators have found few adverse outcomes; however, the benefits of discontinuation on adverse events, including pneumonia, have been unclear.
Study design: Case-control study.
Setting: Quebec health systems.
Synopsis: Using the Quebec health insurance databases, a study cohort of 103,386 patients with COPD on ICSs was created. Patients were followed for a mean of 4.9 years; 14,020 patients who were hospitalized for pneumonia or died from pneumonia outside the hospital were matched to control subjects. Discontinuation of ICSs was associated with a 37% decrease in serious pneumonia (relative risk [RR] 0.63; 95% CI, 0.60–0.66). The risk reduction occurred as early as one month after discontinuation of ICSs. Risk reduction was greater with fluticasone (RR 0.58; 95% CI, 0.54–0.61) than with budesonide (RR 0.87; 95% CI, 0.7–0.97).
Population size and follow-up may contribute to why risk reduction in pneumonia was seen in this study but not in other recent randomized trials on discontinuation of ICSs. A limitation of this study was its observational design; however, its results suggest that use of ICSs in COPD patients should be highly selective, as indiscriminate use can subject patients to elevated risk of hospitalization or death from pneumonia.
Bottom line: Discontinuation of ICSs in patients with COPD is associated with a decreased risk of contracting serious pneumonia. This reduction appears greatest with fluticasone.
Citation: Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177-1183.
Short Take
Increase in Rates of Prescription Drug Use and Polypharmacy Seen
The percentage of Americans who reported taking prescription medications increased substantially from 1999 to 2012 (51% to 59%), as did the percentage who reported taking at least five prescription medications.
Citation: Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1830.
Clinical question: Is discontinuation of inhaled corticosteroids (ICSs) in patients with COPD associated with a decreased risk of pneumonia?
Background: ICSs are used in up to 85% of patients treated for COPD but may be associated with adverse systemic side effects including pneumonia. Trials looking at weaning patients off ICSs and replacing with long-acting bronchodilators have found few adverse outcomes; however, the benefits of discontinuation on adverse events, including pneumonia, have been unclear.
Study design: Case-control study.
Setting: Quebec health systems.
Synopsis: Using the Quebec health insurance databases, a study cohort of 103,386 patients with COPD on ICSs was created. Patients were followed for a mean of 4.9 years; 14,020 patients who were hospitalized for pneumonia or died from pneumonia outside the hospital were matched to control subjects. Discontinuation of ICSs was associated with a 37% decrease in serious pneumonia (relative risk [RR] 0.63; 95% CI, 0.60–0.66). The risk reduction occurred as early as one month after discontinuation of ICSs. Risk reduction was greater with fluticasone (RR 0.58; 95% CI, 0.54–0.61) than with budesonide (RR 0.87; 95% CI, 0.7–0.97).
Population size and follow-up may contribute to why risk reduction in pneumonia was seen in this study but not in other recent randomized trials on discontinuation of ICSs. A limitation of this study was its observational design; however, its results suggest that use of ICSs in COPD patients should be highly selective, as indiscriminate use can subject patients to elevated risk of hospitalization or death from pneumonia.
Bottom line: Discontinuation of ICSs in patients with COPD is associated with a decreased risk of contracting serious pneumonia. This reduction appears greatest with fluticasone.
Citation: Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177-1183.
Short Take
Increase in Rates of Prescription Drug Use and Polypharmacy Seen
The percentage of Americans who reported taking prescription medications increased substantially from 1999 to 2012 (51% to 59%), as did the percentage who reported taking at least five prescription medications.
Citation: Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1830.
MEDS Score for Sepsis Might Best Predict ED Mortality
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.
Clinical question: Which illness severity score best predicts outcomes in emergency department (ED) patients presenting with infection?
Background: Several scoring models have been developed to predict illness severity and mortality in patients with infection. Some scores were developed specifically for patients with sepsis and others for patients in a general critical care setting. These different scoring models have not been specifically compared and validated in the ED setting in patients with infection of various severities.
Study design: Prospective, observational study.
Setting: Adult ED in a metropolitan tertiary, university-affiliated hospital.
Synopsis: Investigators prospectively identified 8,871 adult inpatients with infection from a single-center ED. Data to calculate five prediction models were collected. The models were:
- Mortality in Emergency Department Sepsis (MEDS) score;
- Acute Physiology and Chronic Health Evaluation II (APACHE II);
- Simplified Acute Physiology Score II (SAPS II);
- Sequential Organ Failure Assessment (SOFA); and
- Severe Sepsis Score (SSS).
Severity score performance was assessed for the overall cohort and for subgroups, including infection without systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock. The MEDS score best predicted mortality in the cohort, with an area under the receiver operating characteristics curve of 0.92. However, older scoring models such as the APACHE II and SAPS II still discriminated well, especially in patients who were admitted to the ICU. All scores tended to overestimate mortality.
Bottom line: The MEDS score may best predict illness severity in septic patients presenting to the ED, but other scoring models may be better-suited for specific patient populations.
Citation: Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539-547.