User login
Anticoagulation no benefit in presumed AFib detected by cardiac devices
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Cardiac arrest centers no benefit in OHCA without STEMI
Survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation who were transported to the nearest hospital emergency department had outcomes similar to those of patients transported to specialist cardiac arrest centers, in the ARREST trial.
Both groups had the same 30-day survival, the primary outcome, as well as 3-month survival and neurologic outcomes.
senior author Simon R. Redwood, MD, principal investigator of ARREST, from Guy’s and St. Thomas’ NHS Trust Hospitals and King’s College, London, said during a press briefing. “These results may allow better resource allocation elsewhere.”
Importantly, this study excluded patients who clearly had myocardial infarction (MI), he stressed. Cardiac arrest can result from cardiac causes or from other events, including trauma, overdose, drowning, or electrocution, he noted.
On the other hand, patients with MI “will benefit from going straight to a heart attack center and having an attempt at reopening the artery,” he emphasized.
Tiffany Patterson, PhD, clinical lead of ARREST, with the same affiliations as Dr. Redwood, presented the trial findings at the annual congress of the European Society of Cardiology in Amsterdam, on Aug. 27. The study was simultaneously published online in The Lancet.
Observational studies of registry data suggest that postarrest care for patients resuscitated after cardiac arrest, without ST-segment elevation, may be best delivered in a specialized center, she noted.
The International Liaison Committee on Resuscitation called for a randomized clinical trial of patients resuscitated after cardiac arrest without ST-segment elevation to clarify this.
In the ARREST trial, among 800 patients with return of spontaneous circulation following OHCA without ST-segment elevation who were randomly assigned to be transported to specialized centers or an emergency department, there was no survival benefit, she summarized.
ARREST was “not simply a negative trial, but a new evidence-based starting point,” according to the trial discussant Lia Crotti, MD, PhD, IRCCS Istituto Auxologico Italiano and University Milano-Bicocca, Italy.
She drew attention to two findings: First, among the 862 patients who were enrolled, whom paramedics judged as being without an obvious noncardiac cause of the cardiac arrest, “only 60% ended up having a cardiac cause for their cardiac arrest and only around one quarter of the total had coronary artery disease.”
The small number of patients who could have benefited from early access to a catheterization laboratory probably contributed to the negative result obtained in this trial, with the loss of statistical power, she said.
Second, London is a dense urban area with high-quality acute care hospitals, so the standard of care in the nearest emergency department may be not so different from that in cardiac arrest centers, she noted. Furthermore, four of the seven cardiac arrest centers have an emergency department, and some of the standard care patients may have been transported there.
“If the clinical trial would be extended to the entire country, including rural areas, maybe the result would be different,” she said.
The study authors acknowledge that the main limitation of this study was that “it was done across London with a dense population in a small geographic area,” and “the London Ambulance Service has rapid response times and short transit times and delivers high quality prehospital care, which could limit generalizability.”
Asked during the press conference here why the results were so different from the registry study findings, Dr. Redwood said, “We’ve seen time and time again that registry data think they are telling us the answer. They’re actually not.”
The session cochairs, Rudolf de Boer, MD, PhD, of Erasmus University Medical Centre, Rotterdam, the Netherlands, and Faiez Zannad, MD, PhD, from University of Lorraine–Vandoeuvre-lès-Nancy, France, each congratulated the researchers on a well-done study.
Dr. de Boer wanted to know whether, for example, 100% of these resuscitated OHCA patients without ST-segment elevation had a cardiac cause, “Would results differ? Or is this just real life?” he asked. Dr. Patterson replied that the paramedics excluded obvious noncardiac causes and the findings were based on current facilities.
“Does this trial provide a definitive answer?” Dr. de Boer asked. Dr. Patterson replied that for the moment, subgroup analysis did not identify any subgroup that might benefit from expedited transport to a cardiac arrest center.
Dr. Zannad wanted to know how informed consent was obtained. Dr. Patterson noted that they have an excellent ethical committee that allowed them to undertake this research in vulnerable patients. Written informed consent was obtained from the patient once the initial emergency had passed if they had regained capacity.
Rationale and trial findings
“It’s very well established that early bystander CPR [cardiopulmonary resuscitation], early defibrillation, and advanced in-hospital care improves survival,” Dr. Redwood noted. “Despite this, only 1 in 10 survive to leave the hospital.”
Therefore, “a cardiac arrest center has been proposed as a way of improving outcome.” These centers have a catheterization laboratory, open 24 hours a day, 7 days a week, advanced critical care including advanced ventilation, temperature management of the patient, hemodynamic support, and neuroprognostication and rehab “because often these patients will have brain injury.
“There’s quite overwhelming registry data to suggest that these cardiac arrest centers improve outcome,” he said, “but these are limited by bias.”
Between January 2018 and December 2022, London Ambulance paramedics randomly assigned 862 patients who were successfully resuscitated and without a confirmed MI to be transported the nearest hospital emergency department or the catheterization laboratory in a cardiac arrest center.
Data were available for 822 participants. They had a mean age of 63 years, and 68% were male.
The primary endpoint, 30-day mortality, occurred in 258 (63%) of 411 participants in the cardiac arrest center group and in 258 (63%) of 412 in the standard care group (unadjusted risk ratio for survival, 1.00; 95% confidence interval [CI], 0.90-1.11; P = 0.96).
Mortality at 3 months was similar in both groups: 64% in the standard care group and 65% in the cardiac arrest center group.
Neurologic outcomes at discharge and 3 months were similar in both groups.
Eight (2%) of 414 patients in the cardiac arrest center group and three (1%) of 413 in the standard care group had serious adverse events, none of which were deemed related to the trial intervention.
A cardiac cause of arrest was identified in roughly 60% of patients in each group, and of these, roughly 42% were coronary causes, 33% were arrhythmia, and 17% were cardiomyopathy.
The median time from cardiac arrest to hospital arrival was 84 minutes in the cardiac arrest center group and 77 minutes in the standard care group.
“Surprising and important RCT evidence”
In an accompanying editorial, Carolina Malta Hansen, MD, PhD, University of Copenhagen, and colleagues wrote that “this study provides randomized trial evidence that in urban settings such as London, there is no survival advantage of a strategy of transporting patients who have been resuscitated to centres with specialty expertise in care of cardiac arrest.
“This result is surprising and important, since this complex and critically ill population would be expected to benefit from centres with more expertise.”
However, “it would be a mistake to conclude that the trial results apply to regions where local hospitals provide a lower quality of care than those in this trial,” they cautioned.
“Where does this leave the medical community, researchers, and society in general?” they asked rhetorically. “Prioritising a minimum standard of care at local hospitals caring for this population is at least as important as ensuring high-quality care or advanced treatment at tertiary centres.
“This trial also calls for more focus on the basics, including efforts to increase bystander cardiopulmonary resuscitation and early defibrillation, aspects of care that are currently being assessed in two ongoing clinical trials (NCT04660526 and NCT03835403) and are most strongly associated with improved survival, when coupled with high-quality prehospital care with trained staff and short response times,” they concluded.
The study was fully funded by the British Heart Foundation. The authors reported that they have no relevant financial disclosures. The financial disclosures of the editorialists are listed with the editorial.
A version of this article first appeared on Medscape.com.
Survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation who were transported to the nearest hospital emergency department had outcomes similar to those of patients transported to specialist cardiac arrest centers, in the ARREST trial.
Both groups had the same 30-day survival, the primary outcome, as well as 3-month survival and neurologic outcomes.
senior author Simon R. Redwood, MD, principal investigator of ARREST, from Guy’s and St. Thomas’ NHS Trust Hospitals and King’s College, London, said during a press briefing. “These results may allow better resource allocation elsewhere.”
Importantly, this study excluded patients who clearly had myocardial infarction (MI), he stressed. Cardiac arrest can result from cardiac causes or from other events, including trauma, overdose, drowning, or electrocution, he noted.
On the other hand, patients with MI “will benefit from going straight to a heart attack center and having an attempt at reopening the artery,” he emphasized.
Tiffany Patterson, PhD, clinical lead of ARREST, with the same affiliations as Dr. Redwood, presented the trial findings at the annual congress of the European Society of Cardiology in Amsterdam, on Aug. 27. The study was simultaneously published online in The Lancet.
Observational studies of registry data suggest that postarrest care for patients resuscitated after cardiac arrest, without ST-segment elevation, may be best delivered in a specialized center, she noted.
The International Liaison Committee on Resuscitation called for a randomized clinical trial of patients resuscitated after cardiac arrest without ST-segment elevation to clarify this.
In the ARREST trial, among 800 patients with return of spontaneous circulation following OHCA without ST-segment elevation who were randomly assigned to be transported to specialized centers or an emergency department, there was no survival benefit, she summarized.
ARREST was “not simply a negative trial, but a new evidence-based starting point,” according to the trial discussant Lia Crotti, MD, PhD, IRCCS Istituto Auxologico Italiano and University Milano-Bicocca, Italy.
She drew attention to two findings: First, among the 862 patients who were enrolled, whom paramedics judged as being without an obvious noncardiac cause of the cardiac arrest, “only 60% ended up having a cardiac cause for their cardiac arrest and only around one quarter of the total had coronary artery disease.”
The small number of patients who could have benefited from early access to a catheterization laboratory probably contributed to the negative result obtained in this trial, with the loss of statistical power, she said.
Second, London is a dense urban area with high-quality acute care hospitals, so the standard of care in the nearest emergency department may be not so different from that in cardiac arrest centers, she noted. Furthermore, four of the seven cardiac arrest centers have an emergency department, and some of the standard care patients may have been transported there.
“If the clinical trial would be extended to the entire country, including rural areas, maybe the result would be different,” she said.
The study authors acknowledge that the main limitation of this study was that “it was done across London with a dense population in a small geographic area,” and “the London Ambulance Service has rapid response times and short transit times and delivers high quality prehospital care, which could limit generalizability.”
Asked during the press conference here why the results were so different from the registry study findings, Dr. Redwood said, “We’ve seen time and time again that registry data think they are telling us the answer. They’re actually not.”
The session cochairs, Rudolf de Boer, MD, PhD, of Erasmus University Medical Centre, Rotterdam, the Netherlands, and Faiez Zannad, MD, PhD, from University of Lorraine–Vandoeuvre-lès-Nancy, France, each congratulated the researchers on a well-done study.
Dr. de Boer wanted to know whether, for example, 100% of these resuscitated OHCA patients without ST-segment elevation had a cardiac cause, “Would results differ? Or is this just real life?” he asked. Dr. Patterson replied that the paramedics excluded obvious noncardiac causes and the findings were based on current facilities.
“Does this trial provide a definitive answer?” Dr. de Boer asked. Dr. Patterson replied that for the moment, subgroup analysis did not identify any subgroup that might benefit from expedited transport to a cardiac arrest center.
Dr. Zannad wanted to know how informed consent was obtained. Dr. Patterson noted that they have an excellent ethical committee that allowed them to undertake this research in vulnerable patients. Written informed consent was obtained from the patient once the initial emergency had passed if they had regained capacity.
Rationale and trial findings
“It’s very well established that early bystander CPR [cardiopulmonary resuscitation], early defibrillation, and advanced in-hospital care improves survival,” Dr. Redwood noted. “Despite this, only 1 in 10 survive to leave the hospital.”
Therefore, “a cardiac arrest center has been proposed as a way of improving outcome.” These centers have a catheterization laboratory, open 24 hours a day, 7 days a week, advanced critical care including advanced ventilation, temperature management of the patient, hemodynamic support, and neuroprognostication and rehab “because often these patients will have brain injury.
“There’s quite overwhelming registry data to suggest that these cardiac arrest centers improve outcome,” he said, “but these are limited by bias.”
Between January 2018 and December 2022, London Ambulance paramedics randomly assigned 862 patients who were successfully resuscitated and without a confirmed MI to be transported the nearest hospital emergency department or the catheterization laboratory in a cardiac arrest center.
Data were available for 822 participants. They had a mean age of 63 years, and 68% were male.
The primary endpoint, 30-day mortality, occurred in 258 (63%) of 411 participants in the cardiac arrest center group and in 258 (63%) of 412 in the standard care group (unadjusted risk ratio for survival, 1.00; 95% confidence interval [CI], 0.90-1.11; P = 0.96).
Mortality at 3 months was similar in both groups: 64% in the standard care group and 65% in the cardiac arrest center group.
Neurologic outcomes at discharge and 3 months were similar in both groups.
Eight (2%) of 414 patients in the cardiac arrest center group and three (1%) of 413 in the standard care group had serious adverse events, none of which were deemed related to the trial intervention.
A cardiac cause of arrest was identified in roughly 60% of patients in each group, and of these, roughly 42% were coronary causes, 33% were arrhythmia, and 17% were cardiomyopathy.
The median time from cardiac arrest to hospital arrival was 84 minutes in the cardiac arrest center group and 77 minutes in the standard care group.
“Surprising and important RCT evidence”
In an accompanying editorial, Carolina Malta Hansen, MD, PhD, University of Copenhagen, and colleagues wrote that “this study provides randomized trial evidence that in urban settings such as London, there is no survival advantage of a strategy of transporting patients who have been resuscitated to centres with specialty expertise in care of cardiac arrest.
“This result is surprising and important, since this complex and critically ill population would be expected to benefit from centres with more expertise.”
However, “it would be a mistake to conclude that the trial results apply to regions where local hospitals provide a lower quality of care than those in this trial,” they cautioned.
“Where does this leave the medical community, researchers, and society in general?” they asked rhetorically. “Prioritising a minimum standard of care at local hospitals caring for this population is at least as important as ensuring high-quality care or advanced treatment at tertiary centres.
“This trial also calls for more focus on the basics, including efforts to increase bystander cardiopulmonary resuscitation and early defibrillation, aspects of care that are currently being assessed in two ongoing clinical trials (NCT04660526 and NCT03835403) and are most strongly associated with improved survival, when coupled with high-quality prehospital care with trained staff and short response times,” they concluded.
The study was fully funded by the British Heart Foundation. The authors reported that they have no relevant financial disclosures. The financial disclosures of the editorialists are listed with the editorial.
A version of this article first appeared on Medscape.com.
Survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation who were transported to the nearest hospital emergency department had outcomes similar to those of patients transported to specialist cardiac arrest centers, in the ARREST trial.
Both groups had the same 30-day survival, the primary outcome, as well as 3-month survival and neurologic outcomes.
senior author Simon R. Redwood, MD, principal investigator of ARREST, from Guy’s and St. Thomas’ NHS Trust Hospitals and King’s College, London, said during a press briefing. “These results may allow better resource allocation elsewhere.”
Importantly, this study excluded patients who clearly had myocardial infarction (MI), he stressed. Cardiac arrest can result from cardiac causes or from other events, including trauma, overdose, drowning, or electrocution, he noted.
On the other hand, patients with MI “will benefit from going straight to a heart attack center and having an attempt at reopening the artery,” he emphasized.
Tiffany Patterson, PhD, clinical lead of ARREST, with the same affiliations as Dr. Redwood, presented the trial findings at the annual congress of the European Society of Cardiology in Amsterdam, on Aug. 27. The study was simultaneously published online in The Lancet.
Observational studies of registry data suggest that postarrest care for patients resuscitated after cardiac arrest, without ST-segment elevation, may be best delivered in a specialized center, she noted.
The International Liaison Committee on Resuscitation called for a randomized clinical trial of patients resuscitated after cardiac arrest without ST-segment elevation to clarify this.
In the ARREST trial, among 800 patients with return of spontaneous circulation following OHCA without ST-segment elevation who were randomly assigned to be transported to specialized centers or an emergency department, there was no survival benefit, she summarized.
ARREST was “not simply a negative trial, but a new evidence-based starting point,” according to the trial discussant Lia Crotti, MD, PhD, IRCCS Istituto Auxologico Italiano and University Milano-Bicocca, Italy.
She drew attention to two findings: First, among the 862 patients who were enrolled, whom paramedics judged as being without an obvious noncardiac cause of the cardiac arrest, “only 60% ended up having a cardiac cause for their cardiac arrest and only around one quarter of the total had coronary artery disease.”
The small number of patients who could have benefited from early access to a catheterization laboratory probably contributed to the negative result obtained in this trial, with the loss of statistical power, she said.
Second, London is a dense urban area with high-quality acute care hospitals, so the standard of care in the nearest emergency department may be not so different from that in cardiac arrest centers, she noted. Furthermore, four of the seven cardiac arrest centers have an emergency department, and some of the standard care patients may have been transported there.
“If the clinical trial would be extended to the entire country, including rural areas, maybe the result would be different,” she said.
The study authors acknowledge that the main limitation of this study was that “it was done across London with a dense population in a small geographic area,” and “the London Ambulance Service has rapid response times and short transit times and delivers high quality prehospital care, which could limit generalizability.”
Asked during the press conference here why the results were so different from the registry study findings, Dr. Redwood said, “We’ve seen time and time again that registry data think they are telling us the answer. They’re actually not.”
The session cochairs, Rudolf de Boer, MD, PhD, of Erasmus University Medical Centre, Rotterdam, the Netherlands, and Faiez Zannad, MD, PhD, from University of Lorraine–Vandoeuvre-lès-Nancy, France, each congratulated the researchers on a well-done study.
Dr. de Boer wanted to know whether, for example, 100% of these resuscitated OHCA patients without ST-segment elevation had a cardiac cause, “Would results differ? Or is this just real life?” he asked. Dr. Patterson replied that the paramedics excluded obvious noncardiac causes and the findings were based on current facilities.
“Does this trial provide a definitive answer?” Dr. de Boer asked. Dr. Patterson replied that for the moment, subgroup analysis did not identify any subgroup that might benefit from expedited transport to a cardiac arrest center.
Dr. Zannad wanted to know how informed consent was obtained. Dr. Patterson noted that they have an excellent ethical committee that allowed them to undertake this research in vulnerable patients. Written informed consent was obtained from the patient once the initial emergency had passed if they had regained capacity.
Rationale and trial findings
“It’s very well established that early bystander CPR [cardiopulmonary resuscitation], early defibrillation, and advanced in-hospital care improves survival,” Dr. Redwood noted. “Despite this, only 1 in 10 survive to leave the hospital.”
Therefore, “a cardiac arrest center has been proposed as a way of improving outcome.” These centers have a catheterization laboratory, open 24 hours a day, 7 days a week, advanced critical care including advanced ventilation, temperature management of the patient, hemodynamic support, and neuroprognostication and rehab “because often these patients will have brain injury.
“There’s quite overwhelming registry data to suggest that these cardiac arrest centers improve outcome,” he said, “but these are limited by bias.”
Between January 2018 and December 2022, London Ambulance paramedics randomly assigned 862 patients who were successfully resuscitated and without a confirmed MI to be transported the nearest hospital emergency department or the catheterization laboratory in a cardiac arrest center.
Data were available for 822 participants. They had a mean age of 63 years, and 68% were male.
The primary endpoint, 30-day mortality, occurred in 258 (63%) of 411 participants in the cardiac arrest center group and in 258 (63%) of 412 in the standard care group (unadjusted risk ratio for survival, 1.00; 95% confidence interval [CI], 0.90-1.11; P = 0.96).
Mortality at 3 months was similar in both groups: 64% in the standard care group and 65% in the cardiac arrest center group.
Neurologic outcomes at discharge and 3 months were similar in both groups.
Eight (2%) of 414 patients in the cardiac arrest center group and three (1%) of 413 in the standard care group had serious adverse events, none of which were deemed related to the trial intervention.
A cardiac cause of arrest was identified in roughly 60% of patients in each group, and of these, roughly 42% were coronary causes, 33% were arrhythmia, and 17% were cardiomyopathy.
The median time from cardiac arrest to hospital arrival was 84 minutes in the cardiac arrest center group and 77 minutes in the standard care group.
“Surprising and important RCT evidence”
In an accompanying editorial, Carolina Malta Hansen, MD, PhD, University of Copenhagen, and colleagues wrote that “this study provides randomized trial evidence that in urban settings such as London, there is no survival advantage of a strategy of transporting patients who have been resuscitated to centres with specialty expertise in care of cardiac arrest.
“This result is surprising and important, since this complex and critically ill population would be expected to benefit from centres with more expertise.”
However, “it would be a mistake to conclude that the trial results apply to regions where local hospitals provide a lower quality of care than those in this trial,” they cautioned.
“Where does this leave the medical community, researchers, and society in general?” they asked rhetorically. “Prioritising a minimum standard of care at local hospitals caring for this population is at least as important as ensuring high-quality care or advanced treatment at tertiary centres.
“This trial also calls for more focus on the basics, including efforts to increase bystander cardiopulmonary resuscitation and early defibrillation, aspects of care that are currently being assessed in two ongoing clinical trials (NCT04660526 and NCT03835403) and are most strongly associated with improved survival, when coupled with high-quality prehospital care with trained staff and short response times,” they concluded.
The study was fully funded by the British Heart Foundation. The authors reported that they have no relevant financial disclosures. The financial disclosures of the editorialists are listed with the editorial.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
No reduction in AFib after noncardiac surgery with colchicine: COP-AF
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.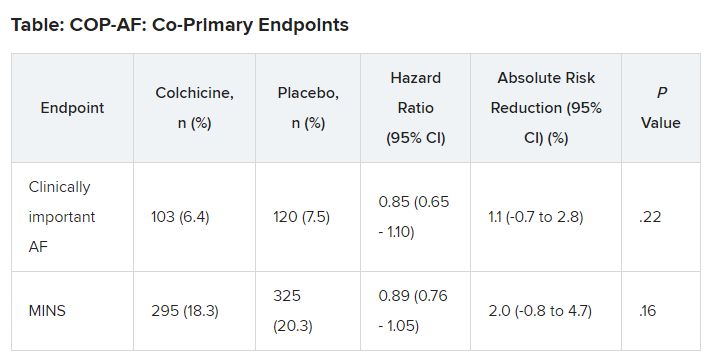
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
ESC issues new guidelines on infective endocarditis
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
FIRE a win for physiology-guided MI complete revascularization in older patients
(MVD) in a large, randomized trial.
In the study with more than 1,400 patients, CR was guided by assessments of the functional effect of coronary lesions other than the MI culprit, a process that selects or excludes the lesions, regardless of angiographic profile, as targets for percutaneous coronary intervention (PCI).
Such physiology-guided CR led to a significant 27% drop in risk for a composite primary endpoint over 1 year in the trial, called FIRE (Functional Assessment in Elderly MI Patients with Multivessel Disease), compared with the culprit-only approach. The endpoint included death, MI, stroke, or ischemia-driven revascularization.
Risk for cardiovascular (CV) death or MI fell by 36% in the trial, and all-cause mortality declined 30%. The differences were significant, although the study wasn’t powered for those secondary endpoints. Safety outcomes were similar for the two revascularization approaches.
FIRE was noteworthy for entering only patients with ST-segment elevation or non–ST-segment elevation MI (STEMI or NSTEMI) who were age 75 years or older, a higher-risk age group poorly represented in earlier CR trials. Such patients in practice are usually managed with the culprit lesion–only approach because of a lack of good evidence supporting CR, observed Simone Biscaglia, MD, the study’s principal investigator.
“This is the first trial actually showing a benefit” from physiology-guided CR in older patients with acute MI that is similar to what the strategy can offer younger patients, said Dr. Biscaglia, from Azienda Ospedaliera Universitaria S. Anna, Ferrara, Italy.
Biscaglia made the comments at a media briefing on FIRE held during the annual congress of the European Society of Cardiology, where he presented the study. He is also lead author on its publication in the New England Journal of Medicine.
“This is a remarkable trial that adds substantially to prior studies that examined the topic of complete versus culprit-only revascularization,” Deepak L. Bhatt, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
It shows “quite clearly” that physiology-guided CR is superior to the culprit-only approach in patients with acute MI, said Dr. Bhatt, who is also director of Mount Sinai Heart at Mount Sinai Hospital and not connected to FIRE.
The primary findings applied to a range of different patient subgroups, including those older than 80. That’s important, he said, because “it is sometimes incorrectly assumed that patients who are older may not benefit from complete revascularization in this setting.”
And the trial’s finding of reduced risk for CV death or MI in the CR group “really should make the complete revascularization approach the standard of care in MI patients without contraindications,” Dr. Bhatt said. And certainly, “age per se should no longer be considered a contraindication.”
“First and foremost, the FIRE trial confirms the benefit of complete revascularization that has been observed in previous trials and provides additional evidence for this approach in older patients,” wrote the author of an editorial accompanying the published report.
The mortality reduction with CR at 1 years “is particularly notable” and underscores that CR should be considered in all patients with acute MI, “regardless of age,” wrote Shamir R. Mehta, MD, McMaster University, Hamilton, Ont., and Hamilton Health Sciences.
Dr. Mehta was principal investigator for the 2019 COMPLETE trial, which made the case for CR, guided by standard angiography, in patients with MVD and STEMI; their age averaged about 62 years.
FIRE definitely ought to sway practice toward greater use of physiology-guided CR regardless of age, observed Vijay Kunadian, MBBS, MD, invited discussant for the Biscaglia presentation. “My oldest patient is 98,” she said, “and it is beneficial without a doubt.”
But Dr. Kunadian, from Newcastle (England) University, said that the trial results can’t be generalized to all older patients. That’s because their outcomes after CR could vary depending on, for example, their different frailties or comorbidities, cognition, or CV history. “So, there is an absolute need to individualize care.”
FIRE enrolled patients 75 years or older with MVD, about 64% male, who had been admitted with acute STEMI or NSTEMI at 34 sites in Italy, Spain, and Poland. All underwent successful culprit-lesion PCI using, as “strongly” recommended, the same model of sirolimus-eluting stent.
Patients were randomly assigned to physiology-guided CR of nonculprit lesions, at the same session or at least during the same hospitalization, or to no further revascularization: 720 and 725 patients, respectively.
The hazard ratio for the composite primary outcome, CR versus culprit-only PCI was 0.73 (95% confidence interval, 0.57-0.93; P = .01). The benefit was driven by reductions in three individual components of the primary endpoint: death, MI, and revascularization, but not stroke.
The HR for CV death or MI was 0.64 (95% CI, 0.47-0.88) and for death from any cause was 0.70 (95% CI, 0.51-0.96).
There was no significant difference in the primary safety outcome, a composite of contrast-related acute kidney injury, stroke, or Bleeding Academic Research Consortium grade 3 to 5 bleeding at 1 year. The rates were 22.5% in those assigned to CR and 20.4% in the culprit-only group.
The functional effect of individual lesions was assessed by either of two methods, crossing them with a standard “pressure wire” or by angiographic derivation of their quantitative flow ratio.
The choice was “left to operator discretion,” Dr. Biscaglia said in an interview, “because we wanted to mirror clinical practice at the participating centers.” Still, the CR primary benefit was independent of the physiology-guidance method.
FIRE’s sponsor – the nonprofit Consorzio Futuro in Ricerca, Italy – received grant support from Sahajanand Medical Technologies, Medis Medical Imaging systems, Eukon, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr. Biscaglia had no other disclosures. Dr. Mehta reported receiving grants from Abbott Vascular and personal fees from Amgen, Janssen, and Bristol Myers Squibb. Dr. Bhatt reported numerous disclosures with various companies and organizations. Dr. Kunadian had no disclosures.
A version of this article first appeared on Medscape.com.
(MVD) in a large, randomized trial.
In the study with more than 1,400 patients, CR was guided by assessments of the functional effect of coronary lesions other than the MI culprit, a process that selects or excludes the lesions, regardless of angiographic profile, as targets for percutaneous coronary intervention (PCI).
Such physiology-guided CR led to a significant 27% drop in risk for a composite primary endpoint over 1 year in the trial, called FIRE (Functional Assessment in Elderly MI Patients with Multivessel Disease), compared with the culprit-only approach. The endpoint included death, MI, stroke, or ischemia-driven revascularization.
Risk for cardiovascular (CV) death or MI fell by 36% in the trial, and all-cause mortality declined 30%. The differences were significant, although the study wasn’t powered for those secondary endpoints. Safety outcomes were similar for the two revascularization approaches.
FIRE was noteworthy for entering only patients with ST-segment elevation or non–ST-segment elevation MI (STEMI or NSTEMI) who were age 75 years or older, a higher-risk age group poorly represented in earlier CR trials. Such patients in practice are usually managed with the culprit lesion–only approach because of a lack of good evidence supporting CR, observed Simone Biscaglia, MD, the study’s principal investigator.
“This is the first trial actually showing a benefit” from physiology-guided CR in older patients with acute MI that is similar to what the strategy can offer younger patients, said Dr. Biscaglia, from Azienda Ospedaliera Universitaria S. Anna, Ferrara, Italy.
Biscaglia made the comments at a media briefing on FIRE held during the annual congress of the European Society of Cardiology, where he presented the study. He is also lead author on its publication in the New England Journal of Medicine.
“This is a remarkable trial that adds substantially to prior studies that examined the topic of complete versus culprit-only revascularization,” Deepak L. Bhatt, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
It shows “quite clearly” that physiology-guided CR is superior to the culprit-only approach in patients with acute MI, said Dr. Bhatt, who is also director of Mount Sinai Heart at Mount Sinai Hospital and not connected to FIRE.
The primary findings applied to a range of different patient subgroups, including those older than 80. That’s important, he said, because “it is sometimes incorrectly assumed that patients who are older may not benefit from complete revascularization in this setting.”
And the trial’s finding of reduced risk for CV death or MI in the CR group “really should make the complete revascularization approach the standard of care in MI patients without contraindications,” Dr. Bhatt said. And certainly, “age per se should no longer be considered a contraindication.”
“First and foremost, the FIRE trial confirms the benefit of complete revascularization that has been observed in previous trials and provides additional evidence for this approach in older patients,” wrote the author of an editorial accompanying the published report.
The mortality reduction with CR at 1 years “is particularly notable” and underscores that CR should be considered in all patients with acute MI, “regardless of age,” wrote Shamir R. Mehta, MD, McMaster University, Hamilton, Ont., and Hamilton Health Sciences.
Dr. Mehta was principal investigator for the 2019 COMPLETE trial, which made the case for CR, guided by standard angiography, in patients with MVD and STEMI; their age averaged about 62 years.
FIRE definitely ought to sway practice toward greater use of physiology-guided CR regardless of age, observed Vijay Kunadian, MBBS, MD, invited discussant for the Biscaglia presentation. “My oldest patient is 98,” she said, “and it is beneficial without a doubt.”
But Dr. Kunadian, from Newcastle (England) University, said that the trial results can’t be generalized to all older patients. That’s because their outcomes after CR could vary depending on, for example, their different frailties or comorbidities, cognition, or CV history. “So, there is an absolute need to individualize care.”
FIRE enrolled patients 75 years or older with MVD, about 64% male, who had been admitted with acute STEMI or NSTEMI at 34 sites in Italy, Spain, and Poland. All underwent successful culprit-lesion PCI using, as “strongly” recommended, the same model of sirolimus-eluting stent.
Patients were randomly assigned to physiology-guided CR of nonculprit lesions, at the same session or at least during the same hospitalization, or to no further revascularization: 720 and 725 patients, respectively.
The hazard ratio for the composite primary outcome, CR versus culprit-only PCI was 0.73 (95% confidence interval, 0.57-0.93; P = .01). The benefit was driven by reductions in three individual components of the primary endpoint: death, MI, and revascularization, but not stroke.
The HR for CV death or MI was 0.64 (95% CI, 0.47-0.88) and for death from any cause was 0.70 (95% CI, 0.51-0.96).
There was no significant difference in the primary safety outcome, a composite of contrast-related acute kidney injury, stroke, or Bleeding Academic Research Consortium grade 3 to 5 bleeding at 1 year. The rates were 22.5% in those assigned to CR and 20.4% in the culprit-only group.
The functional effect of individual lesions was assessed by either of two methods, crossing them with a standard “pressure wire” or by angiographic derivation of their quantitative flow ratio.
The choice was “left to operator discretion,” Dr. Biscaglia said in an interview, “because we wanted to mirror clinical practice at the participating centers.” Still, the CR primary benefit was independent of the physiology-guidance method.
FIRE’s sponsor – the nonprofit Consorzio Futuro in Ricerca, Italy – received grant support from Sahajanand Medical Technologies, Medis Medical Imaging systems, Eukon, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr. Biscaglia had no other disclosures. Dr. Mehta reported receiving grants from Abbott Vascular and personal fees from Amgen, Janssen, and Bristol Myers Squibb. Dr. Bhatt reported numerous disclosures with various companies and organizations. Dr. Kunadian had no disclosures.
A version of this article first appeared on Medscape.com.
(MVD) in a large, randomized trial.
In the study with more than 1,400 patients, CR was guided by assessments of the functional effect of coronary lesions other than the MI culprit, a process that selects or excludes the lesions, regardless of angiographic profile, as targets for percutaneous coronary intervention (PCI).
Such physiology-guided CR led to a significant 27% drop in risk for a composite primary endpoint over 1 year in the trial, called FIRE (Functional Assessment in Elderly MI Patients with Multivessel Disease), compared with the culprit-only approach. The endpoint included death, MI, stroke, or ischemia-driven revascularization.
Risk for cardiovascular (CV) death or MI fell by 36% in the trial, and all-cause mortality declined 30%. The differences were significant, although the study wasn’t powered for those secondary endpoints. Safety outcomes were similar for the two revascularization approaches.
FIRE was noteworthy for entering only patients with ST-segment elevation or non–ST-segment elevation MI (STEMI or NSTEMI) who were age 75 years or older, a higher-risk age group poorly represented in earlier CR trials. Such patients in practice are usually managed with the culprit lesion–only approach because of a lack of good evidence supporting CR, observed Simone Biscaglia, MD, the study’s principal investigator.
“This is the first trial actually showing a benefit” from physiology-guided CR in older patients with acute MI that is similar to what the strategy can offer younger patients, said Dr. Biscaglia, from Azienda Ospedaliera Universitaria S. Anna, Ferrara, Italy.
Biscaglia made the comments at a media briefing on FIRE held during the annual congress of the European Society of Cardiology, where he presented the study. He is also lead author on its publication in the New England Journal of Medicine.
“This is a remarkable trial that adds substantially to prior studies that examined the topic of complete versus culprit-only revascularization,” Deepak L. Bhatt, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
It shows “quite clearly” that physiology-guided CR is superior to the culprit-only approach in patients with acute MI, said Dr. Bhatt, who is also director of Mount Sinai Heart at Mount Sinai Hospital and not connected to FIRE.
The primary findings applied to a range of different patient subgroups, including those older than 80. That’s important, he said, because “it is sometimes incorrectly assumed that patients who are older may not benefit from complete revascularization in this setting.”
And the trial’s finding of reduced risk for CV death or MI in the CR group “really should make the complete revascularization approach the standard of care in MI patients without contraindications,” Dr. Bhatt said. And certainly, “age per se should no longer be considered a contraindication.”
“First and foremost, the FIRE trial confirms the benefit of complete revascularization that has been observed in previous trials and provides additional evidence for this approach in older patients,” wrote the author of an editorial accompanying the published report.
The mortality reduction with CR at 1 years “is particularly notable” and underscores that CR should be considered in all patients with acute MI, “regardless of age,” wrote Shamir R. Mehta, MD, McMaster University, Hamilton, Ont., and Hamilton Health Sciences.
Dr. Mehta was principal investigator for the 2019 COMPLETE trial, which made the case for CR, guided by standard angiography, in patients with MVD and STEMI; their age averaged about 62 years.
FIRE definitely ought to sway practice toward greater use of physiology-guided CR regardless of age, observed Vijay Kunadian, MBBS, MD, invited discussant for the Biscaglia presentation. “My oldest patient is 98,” she said, “and it is beneficial without a doubt.”
But Dr. Kunadian, from Newcastle (England) University, said that the trial results can’t be generalized to all older patients. That’s because their outcomes after CR could vary depending on, for example, their different frailties or comorbidities, cognition, or CV history. “So, there is an absolute need to individualize care.”
FIRE enrolled patients 75 years or older with MVD, about 64% male, who had been admitted with acute STEMI or NSTEMI at 34 sites in Italy, Spain, and Poland. All underwent successful culprit-lesion PCI using, as “strongly” recommended, the same model of sirolimus-eluting stent.
Patients were randomly assigned to physiology-guided CR of nonculprit lesions, at the same session or at least during the same hospitalization, or to no further revascularization: 720 and 725 patients, respectively.
The hazard ratio for the composite primary outcome, CR versus culprit-only PCI was 0.73 (95% confidence interval, 0.57-0.93; P = .01). The benefit was driven by reductions in three individual components of the primary endpoint: death, MI, and revascularization, but not stroke.
The HR for CV death or MI was 0.64 (95% CI, 0.47-0.88) and for death from any cause was 0.70 (95% CI, 0.51-0.96).
There was no significant difference in the primary safety outcome, a composite of contrast-related acute kidney injury, stroke, or Bleeding Academic Research Consortium grade 3 to 5 bleeding at 1 year. The rates were 22.5% in those assigned to CR and 20.4% in the culprit-only group.
The functional effect of individual lesions was assessed by either of two methods, crossing them with a standard “pressure wire” or by angiographic derivation of their quantitative flow ratio.
The choice was “left to operator discretion,” Dr. Biscaglia said in an interview, “because we wanted to mirror clinical practice at the participating centers.” Still, the CR primary benefit was independent of the physiology-guidance method.
FIRE’s sponsor – the nonprofit Consorzio Futuro in Ricerca, Italy – received grant support from Sahajanand Medical Technologies, Medis Medical Imaging systems, Eukon, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr. Biscaglia had no other disclosures. Dr. Mehta reported receiving grants from Abbott Vascular and personal fees from Amgen, Janssen, and Bristol Myers Squibb. Dr. Bhatt reported numerous disclosures with various companies and organizations. Dr. Kunadian had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
ESC backs SGLT2 inhibitor plus GLP-1 in diabetes with high CVD risk
AMSTERDAM – The era of guidelines that recommended treatment with either a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or a glucagonlike peptide-1 (GLP-1) receptor agonist in people with type 2 diabetes mellitus and established cardiovascular disease (CVD) ended with new recommendations from the European Society of Cardiology that call for starting both classes simultaneously.
said Darren K. McGuire, MD, at the annual congress of the European Society of Cardiology.
The society’s new guidelines for managing CVD in patients with diabetes, released on Aug. 25 and presented in several sessions at the Congress, also break with the past by calling for starting treatment with both an SGLT-2 inhibitor and a GLP-1 receptor agonist without regard to a person’s existing level of glucose control, including their current and target hemoglobin A1c levels, and regardless of background therapy, added Dr. McGuire, a cardiologist and professor at the UT Southwestern Medical Center in Dallas and a member of the ESC panel that wrote the new guidelines.
Instead, the new guidance calls for starting both drug classes promptly in people diagnosed with type 2 diabetes and established atherosclerotic CVD.
Both the previous ESC guidelines from 2019 as well as the current Standards of Care for 2023 document from the American Diabetes Association call for using one class or the other, but they hedge on combined treatment as discretionary.
Different mechanisms mean additive benefits
“With increasing numbers of patients with type 2 diabetes in trials for SGLT-2 inhibitors or GLP-1 receptor agonists who were also on the other drug class, we’ve done large, stratified analyses that suggest no treatment-effect modification” when people received agents from both drug classes, Dr. McGuire explained in an interview. “While we don’t understand the mechanisms of action of these drugs for CVD, we’ve become very confident that they use different mechanisms” that appear to have at least partially additive effects.
“Their benefits for CVD risk reduction are completely independent of their glucose effects. They are cardiology drugs,” Dr. McGuire added.
The new ESC guidelines highlight two other clinical settings where people with type 2 diabetes should receive an SGLT-2 inhibitor regardless of their existing level of glucose control and any other medical treatment: people with heart failure and people with chronic kidney disease (CKD) based on a depressed estimated glomerular filtration rate and an elevated urine albumin-to-creatinine ratio.
Nephropathy was considered by the ESC’s guideline panel to confer risk that is similar to that of established atherosclerotic CVD, Dr. McGuire said.
The guidelines also, for the first time for ESC recommendations, made treatment with finerenone (Kerendia, Bayer) a class 1 level A recommendation for people with type 2 diabetes and CKD.
SCORE2-Diabetes risk estimator
Another major change in the new ESC guideline revision is introduction of a CVD risk calculator intended to estimate the risk among people with type 2 diabetes but without established CVD, heart failure, or CKD.
Called the SCORE2-Diabetes risk estimator, it calculates a person’s 10-year risk for CVD and includes adjustment based on the European region where a person lives; it also tallies different risk levels for women and for men.
The researchers who developed the SCORE2-Diabetes calculator used data from nearly 230,000 people to devise the tool and then validated it with data from an additional 217,000 Europeans with type 2 diabetes.
Key features of the calculator include its use of routinely collected clinical values, such as age, sex, systolic blood pressure, smoking status, serum cholesterol levels, age at diabetes diagnosis, hemoglobin A1c level, and estimated glomerular filtration rate.
“For the first time we have a clear score to categorize risk” in people with type 2 diabetes and identify who needs more aggressive treatment to prevent CVD development,” said Emanuele Di Angelantonio, MD, PhD, deputy director of the cardiovascular epidemiology unit at the University of Cambridge (England).
The guidelines say that people who have a low (< 5%) or moderate (5%-9%) 10-year risk for CVD are possible candidates for metformin treatment. Those with high (10%-19%) or very high (≥ 20%) risk are possible candidates for treatment with metformin and/or an SGLT-2 inhibitor and/or a GLP-1 receptor agonist, said Dr. Di Angelantonio during his talk at the congress on the new risk score.
“The risk score is a good addition” because it estimates future CVD risk better and more systematically than usual practice, which generally relies on no systematic tool, said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow (Scotland) and also a member of the guideline-writing panel.
The new risk score “is a reasonable way” to identify people without CVD but at elevated risk who might benefit from treatment with a relatively expensive drug, such as an SGLT-2 inhibitor, Dr. Sattar said in an interview. “It doesn’t rely on any fancy biomarkers or imaging, and it takes about 30 seconds to calculate. It’s not perfect, but it gets the job done,” and it will increase the number of people with type 2 diabetes who will receive an SGLT-2 inhibitor, he predicted.
Dr. McGuire has been a consultant to Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Intercept, Lexion, Lilly, Merck, New Amsterdam, and Pfizer. Dr. Di Angelantonio had no disclosures. Dr. Sattar has been a consultant to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Roche Diagnostics.
A version of this article appeared on Medscape.com.
AMSTERDAM – The era of guidelines that recommended treatment with either a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or a glucagonlike peptide-1 (GLP-1) receptor agonist in people with type 2 diabetes mellitus and established cardiovascular disease (CVD) ended with new recommendations from the European Society of Cardiology that call for starting both classes simultaneously.
said Darren K. McGuire, MD, at the annual congress of the European Society of Cardiology.
The society’s new guidelines for managing CVD in patients with diabetes, released on Aug. 25 and presented in several sessions at the Congress, also break with the past by calling for starting treatment with both an SGLT-2 inhibitor and a GLP-1 receptor agonist without regard to a person’s existing level of glucose control, including their current and target hemoglobin A1c levels, and regardless of background therapy, added Dr. McGuire, a cardiologist and professor at the UT Southwestern Medical Center in Dallas and a member of the ESC panel that wrote the new guidelines.
Instead, the new guidance calls for starting both drug classes promptly in people diagnosed with type 2 diabetes and established atherosclerotic CVD.
Both the previous ESC guidelines from 2019 as well as the current Standards of Care for 2023 document from the American Diabetes Association call for using one class or the other, but they hedge on combined treatment as discretionary.
Different mechanisms mean additive benefits
“With increasing numbers of patients with type 2 diabetes in trials for SGLT-2 inhibitors or GLP-1 receptor agonists who were also on the other drug class, we’ve done large, stratified analyses that suggest no treatment-effect modification” when people received agents from both drug classes, Dr. McGuire explained in an interview. “While we don’t understand the mechanisms of action of these drugs for CVD, we’ve become very confident that they use different mechanisms” that appear to have at least partially additive effects.
“Their benefits for CVD risk reduction are completely independent of their glucose effects. They are cardiology drugs,” Dr. McGuire added.
The new ESC guidelines highlight two other clinical settings where people with type 2 diabetes should receive an SGLT-2 inhibitor regardless of their existing level of glucose control and any other medical treatment: people with heart failure and people with chronic kidney disease (CKD) based on a depressed estimated glomerular filtration rate and an elevated urine albumin-to-creatinine ratio.
Nephropathy was considered by the ESC’s guideline panel to confer risk that is similar to that of established atherosclerotic CVD, Dr. McGuire said.
The guidelines also, for the first time for ESC recommendations, made treatment with finerenone (Kerendia, Bayer) a class 1 level A recommendation for people with type 2 diabetes and CKD.
SCORE2-Diabetes risk estimator
Another major change in the new ESC guideline revision is introduction of a CVD risk calculator intended to estimate the risk among people with type 2 diabetes but without established CVD, heart failure, or CKD.
Called the SCORE2-Diabetes risk estimator, it calculates a person’s 10-year risk for CVD and includes adjustment based on the European region where a person lives; it also tallies different risk levels for women and for men.
The researchers who developed the SCORE2-Diabetes calculator used data from nearly 230,000 people to devise the tool and then validated it with data from an additional 217,000 Europeans with type 2 diabetes.
Key features of the calculator include its use of routinely collected clinical values, such as age, sex, systolic blood pressure, smoking status, serum cholesterol levels, age at diabetes diagnosis, hemoglobin A1c level, and estimated glomerular filtration rate.
“For the first time we have a clear score to categorize risk” in people with type 2 diabetes and identify who needs more aggressive treatment to prevent CVD development,” said Emanuele Di Angelantonio, MD, PhD, deputy director of the cardiovascular epidemiology unit at the University of Cambridge (England).
The guidelines say that people who have a low (< 5%) or moderate (5%-9%) 10-year risk for CVD are possible candidates for metformin treatment. Those with high (10%-19%) or very high (≥ 20%) risk are possible candidates for treatment with metformin and/or an SGLT-2 inhibitor and/or a GLP-1 receptor agonist, said Dr. Di Angelantonio during his talk at the congress on the new risk score.
“The risk score is a good addition” because it estimates future CVD risk better and more systematically than usual practice, which generally relies on no systematic tool, said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow (Scotland) and also a member of the guideline-writing panel.
The new risk score “is a reasonable way” to identify people without CVD but at elevated risk who might benefit from treatment with a relatively expensive drug, such as an SGLT-2 inhibitor, Dr. Sattar said in an interview. “It doesn’t rely on any fancy biomarkers or imaging, and it takes about 30 seconds to calculate. It’s not perfect, but it gets the job done,” and it will increase the number of people with type 2 diabetes who will receive an SGLT-2 inhibitor, he predicted.
Dr. McGuire has been a consultant to Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Intercept, Lexion, Lilly, Merck, New Amsterdam, and Pfizer. Dr. Di Angelantonio had no disclosures. Dr. Sattar has been a consultant to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Roche Diagnostics.
A version of this article appeared on Medscape.com.
AMSTERDAM – The era of guidelines that recommended treatment with either a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or a glucagonlike peptide-1 (GLP-1) receptor agonist in people with type 2 diabetes mellitus and established cardiovascular disease (CVD) ended with new recommendations from the European Society of Cardiology that call for starting both classes simultaneously.
said Darren K. McGuire, MD, at the annual congress of the European Society of Cardiology.
The society’s new guidelines for managing CVD in patients with diabetes, released on Aug. 25 and presented in several sessions at the Congress, also break with the past by calling for starting treatment with both an SGLT-2 inhibitor and a GLP-1 receptor agonist without regard to a person’s existing level of glucose control, including their current and target hemoglobin A1c levels, and regardless of background therapy, added Dr. McGuire, a cardiologist and professor at the UT Southwestern Medical Center in Dallas and a member of the ESC panel that wrote the new guidelines.
Instead, the new guidance calls for starting both drug classes promptly in people diagnosed with type 2 diabetes and established atherosclerotic CVD.
Both the previous ESC guidelines from 2019 as well as the current Standards of Care for 2023 document from the American Diabetes Association call for using one class or the other, but they hedge on combined treatment as discretionary.
Different mechanisms mean additive benefits
“With increasing numbers of patients with type 2 diabetes in trials for SGLT-2 inhibitors or GLP-1 receptor agonists who were also on the other drug class, we’ve done large, stratified analyses that suggest no treatment-effect modification” when people received agents from both drug classes, Dr. McGuire explained in an interview. “While we don’t understand the mechanisms of action of these drugs for CVD, we’ve become very confident that they use different mechanisms” that appear to have at least partially additive effects.
“Their benefits for CVD risk reduction are completely independent of their glucose effects. They are cardiology drugs,” Dr. McGuire added.
The new ESC guidelines highlight two other clinical settings where people with type 2 diabetes should receive an SGLT-2 inhibitor regardless of their existing level of glucose control and any other medical treatment: people with heart failure and people with chronic kidney disease (CKD) based on a depressed estimated glomerular filtration rate and an elevated urine albumin-to-creatinine ratio.
Nephropathy was considered by the ESC’s guideline panel to confer risk that is similar to that of established atherosclerotic CVD, Dr. McGuire said.
The guidelines also, for the first time for ESC recommendations, made treatment with finerenone (Kerendia, Bayer) a class 1 level A recommendation for people with type 2 diabetes and CKD.
SCORE2-Diabetes risk estimator
Another major change in the new ESC guideline revision is introduction of a CVD risk calculator intended to estimate the risk among people with type 2 diabetes but without established CVD, heart failure, or CKD.
Called the SCORE2-Diabetes risk estimator, it calculates a person’s 10-year risk for CVD and includes adjustment based on the European region where a person lives; it also tallies different risk levels for women and for men.
The researchers who developed the SCORE2-Diabetes calculator used data from nearly 230,000 people to devise the tool and then validated it with data from an additional 217,000 Europeans with type 2 diabetes.
Key features of the calculator include its use of routinely collected clinical values, such as age, sex, systolic blood pressure, smoking status, serum cholesterol levels, age at diabetes diagnosis, hemoglobin A1c level, and estimated glomerular filtration rate.
“For the first time we have a clear score to categorize risk” in people with type 2 diabetes and identify who needs more aggressive treatment to prevent CVD development,” said Emanuele Di Angelantonio, MD, PhD, deputy director of the cardiovascular epidemiology unit at the University of Cambridge (England).
The guidelines say that people who have a low (< 5%) or moderate (5%-9%) 10-year risk for CVD are possible candidates for metformin treatment. Those with high (10%-19%) or very high (≥ 20%) risk are possible candidates for treatment with metformin and/or an SGLT-2 inhibitor and/or a GLP-1 receptor agonist, said Dr. Di Angelantonio during his talk at the congress on the new risk score.
“The risk score is a good addition” because it estimates future CVD risk better and more systematically than usual practice, which generally relies on no systematic tool, said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow (Scotland) and also a member of the guideline-writing panel.
The new risk score “is a reasonable way” to identify people without CVD but at elevated risk who might benefit from treatment with a relatively expensive drug, such as an SGLT-2 inhibitor, Dr. Sattar said in an interview. “It doesn’t rely on any fancy biomarkers or imaging, and it takes about 30 seconds to calculate. It’s not perfect, but it gets the job done,” and it will increase the number of people with type 2 diabetes who will receive an SGLT-2 inhibitor, he predicted.
Dr. McGuire has been a consultant to Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Intercept, Lexion, Lilly, Merck, New Amsterdam, and Pfizer. Dr. Di Angelantonio had no disclosures. Dr. Sattar has been a consultant to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Roche Diagnostics.
A version of this article appeared on Medscape.com.
AT ESC CONGRESS 2023
Acoramidis shows encouraging results in ATTR cardiomyopathy
AMSTERDAM –
The drug, acoramidis (BridgeBio Pharma), showed a significant reduction, compared with placebo, in the primary endpoint, a hierarchical analysis of all-cause mortality, cumulative frequency of cardiovascular hospitalizations, and change from baseline in N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) and 6-minute walking distance, in the ATTRibute-CM trial.
The combination of all-cause mortality/cardiovascular hospitalization was also significantly reduced.
The trial was presented at the annual congress of the European Society of Cardiology by Julian Gillmore, MD, head of the University College London Centre for Amyloidosis.
“ATTRibute-CM was a robustly positive trial, showing benefits across the board for acoramidis, and suggest the tantalizing possibility of genuine clinical improvements,” Dr. Gillmore concluded.
ATTR-CM is a debilitating and progressive condition that increases mortality and reduces quality of life. Although this form of cardiomyopathy was considered to be very rare not long ago, improvements in imaging techniques and treatment developments have resulted in an upsurge in diagnosis throughout the world, and the disease is being diagnosed at an earlier stage, Dr. Gillmore noted.
ATTR-CM results from aggregation and deposition of transthyretin amyloid fibrils in the heart and various tissues. Acoramidis stabilizes the TTR tetramer and avoids the production of the fibrils.
Another similar drug, tafamidis (Vyndaqel, Vyndamax, Pfizer), was approved by the Food and Drug Administration in 2019 for ATTR-CM and is now available in several counties, including Japan and Europe.
BridgeBio Pharma is planning to file for FDA approval for acoramidis toward the end of 2023 and in other countries in 2024, Dr. Gillmore reported.
“It will be a huge benefit to patients to have another effective drug available,” he said.
Tafamidis also showed impressive results with its pivotal trial – ATTR-ACT – including a significant reduction in all-cause mortality, which was not seen in the ATTRibute-CM trial with acoramidis.
Asked about this, Dr. Gillmore replied: “It is difficult to comment on comparison with tafamidis as there isn’t a head-to-head trial. All I can say is that these results with acoramidis are fantastically encouraging, and I think we are going to have two effective drugs to treat this progressive and fatal condition.”
He elaborated that the difference in all-cause mortality results between the trials was “entirely consistent” with differences in the trial populations, with the ATTRibute-CM trial recruiting much lower-risk patients, in line with the earlier diagnosis of the condition that is now occurring.
“The survival in the placebo group in the ATTRibute study was greater than that in the treatment group in the ATTR-ACT study. So, it’s not all that surprising, given the reduced number of events, that mortality alone was not statistically significant in ATTRibute. What is important is that the trend in mortality was in the right direction, with an impressive risk reduction,” Dr. Gillmore noted.
“Incredibly, survival at 30 months and hospitalization rates among patients receiving acoramidis approached that of age-matched individuals who do not have ATTR,” he added.
Noting that more patients in the placebo group started taking tafamidis during the trial, Dr. Gillmore suggested that this would be expected to dilute the treatment effect of acoramidis.
“To have such a strongly positive study despite the change in the patient population and drop-in use of tafamidis is incredibly powerful,” he concluded.
ATTRibute trial
The randomized double-blind ATTRibute-CM trial included 632 patients with ATTR-CM and New York Heart Association class I-III heart failure.
They were randomly assigned 2:1 to acoramidis (800 mg twice daily) or placebo, with a follow-up of 30 months. After the first 12 months, tafamidis was permitted if available. This was more prevalent in the placebo arm (22% vs. 14%).
The trial met the primary endpoint – a hierarchical analysis of all-cause mortality, cumulative frequency of cardiovascular hospitalizations, and change from baseline in NT-proBNP and 6-minute walking distance – with a win ratio of 1.8, which was highly statistically significant (P < .0001).
Results were consistent across all components of the primary endpoint and across all subgroups, Dr. Gillmore reported.
“Importantly, 58% of the win ratio ties were broken by the first two components of the hierarchical analysis – all-cause mortality and cardiovascular hospitalizations – and a separate analysis of these two components alone was also statistically significant,” he noted.
A trend was seen toward a treatment effect on all-cause mortality favoring acoramidis, with an 81% survival rate in the treated group, representing an absolute risk reduction of 6.4 percentage points and a relative risk reduction of 25%.
Of the deaths reported in the study, 78% were cardiovascular in nature. Cardiovascular death also showed a trend favoring treatment with the study drug (14.9% in the acoramidis group vs. 21.3% in the placebo group), giving an absolute risk reduction of 6.4 percentage points and a relative risk reduction of 30%.
Acoramidis was also associated with 50% reduction in cardiovascular hospitalizations, which was highly significant (P < .0001).
A treatment effect was also seen in terms of functional status; at 30 months, the difference in 6-minute walk distance between the groups was 40 meters, a “highly statistically significant improvement and clinically important difference, Dr. Gillmore said. Improvement from baseline occurred in 40% of the acoramidis group versus 22% of the placebo group.
Acoramidis recipients showed a blunting of the progressive rise of NT-proBNP, which Dr. Gillmore noted has been shown to be strongly associated with outcomes, with 45% of the acoramidis treated patients showing an improvement in NT-proBNP levels, compared with 9% of placebo group.
There was also a relative preservation of quality of life in the acoramidis group consistent with the separation of NT-proBNP curves, he added.
“Consistent with the mechanism of action and preclinical data showing near-complete stabilization of TTR at therapeutic drug concentrations, serum TTR (an in vivo reflection of TTR stabilization) was promptly and persistently elevated in patients receiving acoramidis,” Dr. Gillmore said.
Safety data showed that treatment-related adverse events were equal between the two groups, and there were fewer treatment emergent serious adverse events in the acoramidis group. The drug was said to be “generally well tolerated, with no findings of potential clinical concern.”
Second primary endpoint not significant
Discussant of the study at the ESC Hotline session, Thibaud Damy, MD, Hospital Henri Mondor, Paris East Creteil University, pointed out that a second primary endpoint of the study, change from baseline to month 12 in the 6-minute walking test, did not significantly differ between acoramidis and placebo.
Dr. Damy also highlighted the significant all-cause mortality reduction seen with tafamidis in ATTR-ACT but not achieved with acoramidis in ATTRibute.
He agreed with Dr. Gillmore’s interpretation that this was probably stemmed from the ATTRibute trial recruiting lower-risk patients, pointing out that patients in this trial had lower levels of NT-proBNP and less severe heart failure.
“It is clear that there is a place for acoramidis in patients with ATTR-CM,” Dr. Damy concluded, adding that many other treatments are in development.
The ATTribute trial was supported by BridgeBio Pharma. Dr. Gillmore reported advisory/consultant roles with BridgeBio, Alnylam, Ionis, AstraZeneca, Intellia, Pfizer, ATTRalus, and Lycia.
A version of this article first appeared on Medscape.com.
AMSTERDAM –
The drug, acoramidis (BridgeBio Pharma), showed a significant reduction, compared with placebo, in the primary endpoint, a hierarchical analysis of all-cause mortality, cumulative frequency of cardiovascular hospitalizations, and change from baseline in N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) and 6-minute walking distance, in the ATTRibute-CM trial.
The combination of all-cause mortality/cardiovascular hospitalization was also significantly reduced.
The trial was presented at the annual congress of the European Society of Cardiology by Julian Gillmore, MD, head of the University College London Centre for Amyloidosis.
“ATTRibute-CM was a robustly positive trial, showing benefits across the board for acoramidis, and suggest the tantalizing possibility of genuine clinical improvements,” Dr. Gillmore concluded.
ATTR-CM is a debilitating and progressive condition that increases mortality and reduces quality of life. Although this form of cardiomyopathy was considered to be very rare not long ago, improvements in imaging techniques and treatment developments have resulted in an upsurge in diagnosis throughout the world, and the disease is being diagnosed at an earlier stage, Dr. Gillmore noted.
ATTR-CM results from aggregation and deposition of transthyretin amyloid fibrils in the heart and various tissues. Acoramidis stabilizes the TTR tetramer and avoids the production of the fibrils.
Another similar drug, tafamidis (Vyndaqel, Vyndamax, Pfizer), was approved by the Food and Drug Administration in 2019 for ATTR-CM and is now available in several counties, including Japan and Europe.
BridgeBio Pharma is planning to file for FDA approval for acoramidis toward the end of 2023 and in other countries in 2024, Dr. Gillmore reported.
“It will be a huge benefit to patients to have another effective drug available,” he said.
Tafamidis also showed impressive results with its pivotal trial – ATTR-ACT – including a significant reduction in all-cause mortality, which was not seen in the ATTRibute-CM trial with acoramidis.
Asked about this, Dr. Gillmore replied: “It is difficult to comment on comparison with tafamidis as there isn’t a head-to-head trial. All I can say is that these results with acoramidis are fantastically encouraging, and I think we are going to have two effective drugs to treat this progressive and fatal condition.”
He elaborated that the difference in all-cause mortality results between the trials was “entirely consistent” with differences in the trial populations, with the ATTRibute-CM trial recruiting much lower-risk patients, in line with the earlier diagnosis of the condition that is now occurring.
“The survival in the placebo group in the ATTRibute study was greater than that in the treatment group in the ATTR-ACT study. So, it’s not all that surprising, given the reduced number of events, that mortality alone was not statistically significant in ATTRibute. What is important is that the trend in mortality was in the right direction, with an impressive risk reduction,” Dr. Gillmore noted.
“Incredibly, survival at 30 months and hospitalization rates among patients receiving acoramidis approached that of age-matched individuals who do not have ATTR,” he added.
Noting that more patients in the placebo group started taking tafamidis during the trial, Dr. Gillmore suggested that this would be expected to dilute the treatment effect of acoramidis.
“To have such a strongly positive study despite the change in the patient population and drop-in use of tafamidis is incredibly powerful,” he concluded.
ATTRibute trial
The randomized double-blind ATTRibute-CM trial included 632 patients with ATTR-CM and New York Heart Association class I-III heart failure.
They were randomly assigned 2:1 to acoramidis (800 mg twice daily) or placebo, with a follow-up of 30 months. After the first 12 months, tafamidis was permitted if available. This was more prevalent in the placebo arm (22% vs. 14%).
The trial met the primary endpoint – a hierarchical analysis of all-cause mortality, cumulative frequency of cardiovascular hospitalizations, and change from baseline in NT-proBNP and 6-minute walking distance – with a win ratio of 1.8, which was highly statistically significant (P < .0001).
Results were consistent across all components of the primary endpoint and across all subgroups, Dr. Gillmore reported.
“Importantly, 58% of the win ratio ties were broken by the first two components of the hierarchical analysis – all-cause mortality and cardiovascular hospitalizations – and a separate analysis of these two components alone was also statistically significant,” he noted.
A trend was seen toward a treatment effect on all-cause mortality favoring acoramidis, with an 81% survival rate in the treated group, representing an absolute risk reduction of 6.4 percentage points and a relative risk reduction of 25%.
Of the deaths reported in the study, 78% were cardiovascular in nature. Cardiovascular death also showed a trend favoring treatment with the study drug (14.9% in the acoramidis group vs. 21.3% in the placebo group), giving an absolute risk reduction of 6.4 percentage points and a relative risk reduction of 30%.
Acoramidis was also associated with 50% reduction in cardiovascular hospitalizations, which was highly significant (P < .0001).
A treatment effect was also seen in terms of functional status; at 30 months, the difference in 6-minute walk distance between the groups was 40 meters, a “highly statistically significant improvement and clinically important difference, Dr. Gillmore said. Improvement from baseline occurred in 40% of the acoramidis group versus 22% of the placebo group.
Acoramidis recipients showed a blunting of the progressive rise of NT-proBNP, which Dr. Gillmore noted has been shown to be strongly associated with outcomes, with 45% of the acoramidis treated patients showing an improvement in NT-proBNP levels, compared with 9% of placebo group.
There was also a relative preservation of quality of life in the acoramidis group consistent with the separation of NT-proBNP curves, he added.
“Consistent with the mechanism of action and preclinical data showing near-complete stabilization of TTR at therapeutic drug concentrations, serum TTR (an in vivo reflection of TTR stabilization) was promptly and persistently elevated in patients receiving acoramidis,” Dr. Gillmore said.
Safety data showed that treatment-related adverse events were equal between the two groups, and there were fewer treatment emergent serious adverse events in the acoramidis group. The drug was said to be “generally well tolerated, with no findings of potential clinical concern.”
Second primary endpoint not significant
Discussant of the study at the ESC Hotline session, Thibaud Damy, MD, Hospital Henri Mondor, Paris East Creteil University, pointed out that a second primary endpoint of the study, change from baseline to month 12 in the 6-minute walking test, did not significantly differ between acoramidis and placebo.
Dr. Damy also highlighted the significant all-cause mortality reduction seen with tafamidis in ATTR-ACT but not achieved with acoramidis in ATTRibute.
He agreed with Dr. Gillmore’s interpretation that this was probably stemmed from the ATTRibute trial recruiting lower-risk patients, pointing out that patients in this trial had lower levels of NT-proBNP and less severe heart failure.
“It is clear that there is a place for acoramidis in patients with ATTR-CM,” Dr. Damy concluded, adding that many other treatments are in development.
The ATTribute trial was supported by BridgeBio Pharma. Dr. Gillmore reported advisory/consultant roles with BridgeBio, Alnylam, Ionis, AstraZeneca, Intellia, Pfizer, ATTRalus, and Lycia.
A version of this article first appeared on Medscape.com.
AMSTERDAM –
The drug, acoramidis (BridgeBio Pharma), showed a significant reduction, compared with placebo, in the primary endpoint, a hierarchical analysis of all-cause mortality, cumulative frequency of cardiovascular hospitalizations, and change from baseline in N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) and 6-minute walking distance, in the ATTRibute-CM trial.
The combination of all-cause mortality/cardiovascular hospitalization was also significantly reduced.
The trial was presented at the annual congress of the European Society of Cardiology by Julian Gillmore, MD, head of the University College London Centre for Amyloidosis.
“ATTRibute-CM was a robustly positive trial, showing benefits across the board for acoramidis, and suggest the tantalizing possibility of genuine clinical improvements,” Dr. Gillmore concluded.
ATTR-CM is a debilitating and progressive condition that increases mortality and reduces quality of life. Although this form of cardiomyopathy was considered to be very rare not long ago, improvements in imaging techniques and treatment developments have resulted in an upsurge in diagnosis throughout the world, and the disease is being diagnosed at an earlier stage, Dr. Gillmore noted.
ATTR-CM results from aggregation and deposition of transthyretin amyloid fibrils in the heart and various tissues. Acoramidis stabilizes the TTR tetramer and avoids the production of the fibrils.
Another similar drug, tafamidis (Vyndaqel, Vyndamax, Pfizer), was approved by the Food and Drug Administration in 2019 for ATTR-CM and is now available in several counties, including Japan and Europe.
BridgeBio Pharma is planning to file for FDA approval for acoramidis toward the end of 2023 and in other countries in 2024, Dr. Gillmore reported.
“It will be a huge benefit to patients to have another effective drug available,” he said.
Tafamidis also showed impressive results with its pivotal trial – ATTR-ACT – including a significant reduction in all-cause mortality, which was not seen in the ATTRibute-CM trial with acoramidis.
Asked about this, Dr. Gillmore replied: “It is difficult to comment on comparison with tafamidis as there isn’t a head-to-head trial. All I can say is that these results with acoramidis are fantastically encouraging, and I think we are going to have two effective drugs to treat this progressive and fatal condition.”
He elaborated that the difference in all-cause mortality results between the trials was “entirely consistent” with differences in the trial populations, with the ATTRibute-CM trial recruiting much lower-risk patients, in line with the earlier diagnosis of the condition that is now occurring.
“The survival in the placebo group in the ATTRibute study was greater than that in the treatment group in the ATTR-ACT study. So, it’s not all that surprising, given the reduced number of events, that mortality alone was not statistically significant in ATTRibute. What is important is that the trend in mortality was in the right direction, with an impressive risk reduction,” Dr. Gillmore noted.
“Incredibly, survival at 30 months and hospitalization rates among patients receiving acoramidis approached that of age-matched individuals who do not have ATTR,” he added.
Noting that more patients in the placebo group started taking tafamidis during the trial, Dr. Gillmore suggested that this would be expected to dilute the treatment effect of acoramidis.
“To have such a strongly positive study despite the change in the patient population and drop-in use of tafamidis is incredibly powerful,” he concluded.
ATTRibute trial
The randomized double-blind ATTRibute-CM trial included 632 patients with ATTR-CM and New York Heart Association class I-III heart failure.
They were randomly assigned 2:1 to acoramidis (800 mg twice daily) or placebo, with a follow-up of 30 months. After the first 12 months, tafamidis was permitted if available. This was more prevalent in the placebo arm (22% vs. 14%).
The trial met the primary endpoint – a hierarchical analysis of all-cause mortality, cumulative frequency of cardiovascular hospitalizations, and change from baseline in NT-proBNP and 6-minute walking distance – with a win ratio of 1.8, which was highly statistically significant (P < .0001).
Results were consistent across all components of the primary endpoint and across all subgroups, Dr. Gillmore reported.
“Importantly, 58% of the win ratio ties were broken by the first two components of the hierarchical analysis – all-cause mortality and cardiovascular hospitalizations – and a separate analysis of these two components alone was also statistically significant,” he noted.
A trend was seen toward a treatment effect on all-cause mortality favoring acoramidis, with an 81% survival rate in the treated group, representing an absolute risk reduction of 6.4 percentage points and a relative risk reduction of 25%.
Of the deaths reported in the study, 78% were cardiovascular in nature. Cardiovascular death also showed a trend favoring treatment with the study drug (14.9% in the acoramidis group vs. 21.3% in the placebo group), giving an absolute risk reduction of 6.4 percentage points and a relative risk reduction of 30%.
Acoramidis was also associated with 50% reduction in cardiovascular hospitalizations, which was highly significant (P < .0001).
A treatment effect was also seen in terms of functional status; at 30 months, the difference in 6-minute walk distance between the groups was 40 meters, a “highly statistically significant improvement and clinically important difference, Dr. Gillmore said. Improvement from baseline occurred in 40% of the acoramidis group versus 22% of the placebo group.
Acoramidis recipients showed a blunting of the progressive rise of NT-proBNP, which Dr. Gillmore noted has been shown to be strongly associated with outcomes, with 45% of the acoramidis treated patients showing an improvement in NT-proBNP levels, compared with 9% of placebo group.
There was also a relative preservation of quality of life in the acoramidis group consistent with the separation of NT-proBNP curves, he added.
“Consistent with the mechanism of action and preclinical data showing near-complete stabilization of TTR at therapeutic drug concentrations, serum TTR (an in vivo reflection of TTR stabilization) was promptly and persistently elevated in patients receiving acoramidis,” Dr. Gillmore said.
Safety data showed that treatment-related adverse events were equal between the two groups, and there were fewer treatment emergent serious adverse events in the acoramidis group. The drug was said to be “generally well tolerated, with no findings of potential clinical concern.”
Second primary endpoint not significant
Discussant of the study at the ESC Hotline session, Thibaud Damy, MD, Hospital Henri Mondor, Paris East Creteil University, pointed out that a second primary endpoint of the study, change from baseline to month 12 in the 6-minute walking test, did not significantly differ between acoramidis and placebo.
Dr. Damy also highlighted the significant all-cause mortality reduction seen with tafamidis in ATTR-ACT but not achieved with acoramidis in ATTRibute.
He agreed with Dr. Gillmore’s interpretation that this was probably stemmed from the ATTRibute trial recruiting lower-risk patients, pointing out that patients in this trial had lower levels of NT-proBNP and less severe heart failure.
“It is clear that there is a place for acoramidis in patients with ATTR-CM,” Dr. Damy concluded, adding that many other treatments are in development.
The ATTribute trial was supported by BridgeBio Pharma. Dr. Gillmore reported advisory/consultant roles with BridgeBio, Alnylam, Ionis, AstraZeneca, Intellia, Pfizer, ATTRalus, and Lycia.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Pulsed field ablation challenges conventional devices in AFib
in a head-to-head trial, an outcome that might favor PFA in the context of other considerations.
“The take-home message is that this is a new technology that has important safety benefits. Patients do not have to worry about the possibility – albeit rare – of esophageal fistulae and other problems. It is faster with at least the same efficacy,” reported Vivek Y. Reddy MD, director of cardiac arrhythmia services, Mount Sinai Hospital, New York.
As opposed to conventional catheter-based thermal ablation, which isolates pulmonary veins harboring AF triggers by heating or freezing the tissue, PFA uses microsecond high-voltage electrical fields to produce cellular necrosis. It is largely nonthermal, Dr. Reddy said.
New device might spare adjacent tissue
In experimental studies, PFA has demonstrated a high degree of ablative specificity, limiting effects on adjacent tissues, such as the esophagus and phrenic nerve, he explained.
Several previous clinical studies support the specificity of the PFA ablative effect, but the ADVENT trial, which Dr. Reddy presented Aug. 27 at the annual congress of the European Society of Cardiology, is the first trial in which patients have been randomly assigned to PFA or catheter-based ablation.
The study was published online in the New England Journal of Medicine simultaneously with the ESC presentation.
The primary efficacy endpoint was the absence of a composite of endpoints indicating incomplete ablation. These included an initial procedural failure, atrial tachyarrhythmias arising after a 3-month blanking period, subsequent use of antiarrhythmic drugs, cardioversion, or repeat ablation. The primary safety endpoint involved a composite of procedure-related adverse events.
The 607 patients enrolled in this trial had AF refractory to at least one antiarrhythmic drug class. They were randomly assigned in a 1:1 ratio to PFA with a catheter system (Farapulse–Boston Scientific) or to thermal ablation.
Of the thermal approaches, radiofrequency or cryoablation was permitted, but each center was required to use just one for the control arm. For the comparison to PFA, outcomes for the two thermal techniques, which were used in similar proportions of patients, were combined based on previous evidence that these approaches perform similarly.
At 1 year, 73.3% of patients in the PFA group and 71.3% of those in the control group met the primary outcome, meaning none of the events signaling ablation failure occurred. The numeric advantage of PFA confirmed noninferiority, although an evaluation of superiority for efficacy, which was triggered by the advantage of PFA, was not significant.
As predicted by previous studies, stratification of thermal ablation approaches showed that outcomes were similar, although the proportion of patients who remained free of events at 1 year was numerically higher in the cryoablation group relative to the radiofrequency group (73.6% vs. 69.2%).
An adverse safety event occurred in 2.1% of those who underwent PFA and in 1.5% of those who underwent thermal ablation. This 0.6–percentage point difference placed PFA well within the boundary of noninferiority for safety.
Of notable events, the only death in this study occurred in the PFA group, and the only stroke occurred in the control group. Phrenic nerve palsies occurred only in the control group (2 vs. 0) while pericarditis was seen only in the PFA group (2 vs. 0). One case of pulmonary edema occurred in each group.
“Catheter ablation is quite safe and effective,” said Dr. Reddy, explaining why this comparison was conducted on the basis of noninferiority.
Dr. Reddy emphasized that noninferiority for PFA was achieved by operators with little or no experience with this technology, whereas the catheter ablations were delivered by operators who typically had previously performed hundreds of interventions.
“With experience, one would expect even better rates of success. This is the floor,” Dr. Reddy said.
Procedure time faster with PFA
Despite working with a new technology, the mean procedure performance time with PFA was faster (105 vs. 123 minutes) even though mean fluoroscopy time was longer (21.1 vs 13.9 minutes). Dr. Reddy considers the difference in procedure time a meaningful demonstration of the efficiency of PFA.
“When you look at procedure performance, it is remarkable that the procedure times were statistically significantly shorter for a first-use technology in the hands of multiple operators,” Dr. Reddy said.
There was also a statistically significant advantage for PFA regarding change in the mean pulmonary vein cross-sectional area following the procedures (0.9% vs. 12%). Dr. Reddy acknowledged that small changes in pulmonary vein dimension are not clinically meaningful, but this result “gets at the question of whether we can achieve ablation without tissue proliferation that we see with conventional ablation.”
Overall, Dr. Reddy believes that the data from ADVENT provide several reasons “to get excited about PFA,” including the efficiency of this technique in the context of at least similar efficacy but a potential for fewer adverse events.
The ESC-invited discussant, Samuel Kiil Sørensen, MD, Gentofte University Hospital, Copenhagen, agreed that the ADVENT data support PFA as an alternative to thermal ablation. He suggested that the shorter procedure times are clinically meaningful given comparable safety and efficacy.
“Which property of PFA justifies noninferiority?” he asked. “Many of the complications of AF ablation are not specific to the energy modality. The devastating complications from damage to the esophagus, pulmonary veins, and phrenic nerve that the PFA technology may eliminate are rare, so they would not be expected to change the overall complication rate in a [randomized controlled trial] of realistic size.”
However, he suggested PFA might still prove to be an incremental advance for AF. He cited previous evidence that supports the specificity of its ablative activity and emphasized that ADVENT tested a first-generation device that might not capture the full advantages of the PFA technology.
The trial was supported by Farapulse–Boston Scientific. Dr. Reddy reports financial relationships with more than 30 pharmaceutical or device manufacturers, including Farapulse–Boston Scientific. Dr. Sørensen reports financial relationships with Medtronic and Biosense Webster.
A version of this article first appeared on Medscape.com.
in a head-to-head trial, an outcome that might favor PFA in the context of other considerations.
“The take-home message is that this is a new technology that has important safety benefits. Patients do not have to worry about the possibility – albeit rare – of esophageal fistulae and other problems. It is faster with at least the same efficacy,” reported Vivek Y. Reddy MD, director of cardiac arrhythmia services, Mount Sinai Hospital, New York.
As opposed to conventional catheter-based thermal ablation, which isolates pulmonary veins harboring AF triggers by heating or freezing the tissue, PFA uses microsecond high-voltage electrical fields to produce cellular necrosis. It is largely nonthermal, Dr. Reddy said.
New device might spare adjacent tissue
In experimental studies, PFA has demonstrated a high degree of ablative specificity, limiting effects on adjacent tissues, such as the esophagus and phrenic nerve, he explained.
Several previous clinical studies support the specificity of the PFA ablative effect, but the ADVENT trial, which Dr. Reddy presented Aug. 27 at the annual congress of the European Society of Cardiology, is the first trial in which patients have been randomly assigned to PFA or catheter-based ablation.
The study was published online in the New England Journal of Medicine simultaneously with the ESC presentation.
The primary efficacy endpoint was the absence of a composite of endpoints indicating incomplete ablation. These included an initial procedural failure, atrial tachyarrhythmias arising after a 3-month blanking period, subsequent use of antiarrhythmic drugs, cardioversion, or repeat ablation. The primary safety endpoint involved a composite of procedure-related adverse events.
The 607 patients enrolled in this trial had AF refractory to at least one antiarrhythmic drug class. They were randomly assigned in a 1:1 ratio to PFA with a catheter system (Farapulse–Boston Scientific) or to thermal ablation.
Of the thermal approaches, radiofrequency or cryoablation was permitted, but each center was required to use just one for the control arm. For the comparison to PFA, outcomes for the two thermal techniques, which were used in similar proportions of patients, were combined based on previous evidence that these approaches perform similarly.
At 1 year, 73.3% of patients in the PFA group and 71.3% of those in the control group met the primary outcome, meaning none of the events signaling ablation failure occurred. The numeric advantage of PFA confirmed noninferiority, although an evaluation of superiority for efficacy, which was triggered by the advantage of PFA, was not significant.
As predicted by previous studies, stratification of thermal ablation approaches showed that outcomes were similar, although the proportion of patients who remained free of events at 1 year was numerically higher in the cryoablation group relative to the radiofrequency group (73.6% vs. 69.2%).
An adverse safety event occurred in 2.1% of those who underwent PFA and in 1.5% of those who underwent thermal ablation. This 0.6–percentage point difference placed PFA well within the boundary of noninferiority for safety.
Of notable events, the only death in this study occurred in the PFA group, and the only stroke occurred in the control group. Phrenic nerve palsies occurred only in the control group (2 vs. 0) while pericarditis was seen only in the PFA group (2 vs. 0). One case of pulmonary edema occurred in each group.
“Catheter ablation is quite safe and effective,” said Dr. Reddy, explaining why this comparison was conducted on the basis of noninferiority.
Dr. Reddy emphasized that noninferiority for PFA was achieved by operators with little or no experience with this technology, whereas the catheter ablations were delivered by operators who typically had previously performed hundreds of interventions.
“With experience, one would expect even better rates of success. This is the floor,” Dr. Reddy said.
Procedure time faster with PFA
Despite working with a new technology, the mean procedure performance time with PFA was faster (105 vs. 123 minutes) even though mean fluoroscopy time was longer (21.1 vs 13.9 minutes). Dr. Reddy considers the difference in procedure time a meaningful demonstration of the efficiency of PFA.
“When you look at procedure performance, it is remarkable that the procedure times were statistically significantly shorter for a first-use technology in the hands of multiple operators,” Dr. Reddy said.
There was also a statistically significant advantage for PFA regarding change in the mean pulmonary vein cross-sectional area following the procedures (0.9% vs. 12%). Dr. Reddy acknowledged that small changes in pulmonary vein dimension are not clinically meaningful, but this result “gets at the question of whether we can achieve ablation without tissue proliferation that we see with conventional ablation.”
Overall, Dr. Reddy believes that the data from ADVENT provide several reasons “to get excited about PFA,” including the efficiency of this technique in the context of at least similar efficacy but a potential for fewer adverse events.
The ESC-invited discussant, Samuel Kiil Sørensen, MD, Gentofte University Hospital, Copenhagen, agreed that the ADVENT data support PFA as an alternative to thermal ablation. He suggested that the shorter procedure times are clinically meaningful given comparable safety and efficacy.
“Which property of PFA justifies noninferiority?” he asked. “Many of the complications of AF ablation are not specific to the energy modality. The devastating complications from damage to the esophagus, pulmonary veins, and phrenic nerve that the PFA technology may eliminate are rare, so they would not be expected to change the overall complication rate in a [randomized controlled trial] of realistic size.”
However, he suggested PFA might still prove to be an incremental advance for AF. He cited previous evidence that supports the specificity of its ablative activity and emphasized that ADVENT tested a first-generation device that might not capture the full advantages of the PFA technology.
The trial was supported by Farapulse–Boston Scientific. Dr. Reddy reports financial relationships with more than 30 pharmaceutical or device manufacturers, including Farapulse–Boston Scientific. Dr. Sørensen reports financial relationships with Medtronic and Biosense Webster.
A version of this article first appeared on Medscape.com.
in a head-to-head trial, an outcome that might favor PFA in the context of other considerations.
“The take-home message is that this is a new technology that has important safety benefits. Patients do not have to worry about the possibility – albeit rare – of esophageal fistulae and other problems. It is faster with at least the same efficacy,” reported Vivek Y. Reddy MD, director of cardiac arrhythmia services, Mount Sinai Hospital, New York.
As opposed to conventional catheter-based thermal ablation, which isolates pulmonary veins harboring AF triggers by heating or freezing the tissue, PFA uses microsecond high-voltage electrical fields to produce cellular necrosis. It is largely nonthermal, Dr. Reddy said.
New device might spare adjacent tissue
In experimental studies, PFA has demonstrated a high degree of ablative specificity, limiting effects on adjacent tissues, such as the esophagus and phrenic nerve, he explained.
Several previous clinical studies support the specificity of the PFA ablative effect, but the ADVENT trial, which Dr. Reddy presented Aug. 27 at the annual congress of the European Society of Cardiology, is the first trial in which patients have been randomly assigned to PFA or catheter-based ablation.
The study was published online in the New England Journal of Medicine simultaneously with the ESC presentation.
The primary efficacy endpoint was the absence of a composite of endpoints indicating incomplete ablation. These included an initial procedural failure, atrial tachyarrhythmias arising after a 3-month blanking period, subsequent use of antiarrhythmic drugs, cardioversion, or repeat ablation. The primary safety endpoint involved a composite of procedure-related adverse events.
The 607 patients enrolled in this trial had AF refractory to at least one antiarrhythmic drug class. They were randomly assigned in a 1:1 ratio to PFA with a catheter system (Farapulse–Boston Scientific) or to thermal ablation.
Of the thermal approaches, radiofrequency or cryoablation was permitted, but each center was required to use just one for the control arm. For the comparison to PFA, outcomes for the two thermal techniques, which were used in similar proportions of patients, were combined based on previous evidence that these approaches perform similarly.
At 1 year, 73.3% of patients in the PFA group and 71.3% of those in the control group met the primary outcome, meaning none of the events signaling ablation failure occurred. The numeric advantage of PFA confirmed noninferiority, although an evaluation of superiority for efficacy, which was triggered by the advantage of PFA, was not significant.
As predicted by previous studies, stratification of thermal ablation approaches showed that outcomes were similar, although the proportion of patients who remained free of events at 1 year was numerically higher in the cryoablation group relative to the radiofrequency group (73.6% vs. 69.2%).
An adverse safety event occurred in 2.1% of those who underwent PFA and in 1.5% of those who underwent thermal ablation. This 0.6–percentage point difference placed PFA well within the boundary of noninferiority for safety.
Of notable events, the only death in this study occurred in the PFA group, and the only stroke occurred in the control group. Phrenic nerve palsies occurred only in the control group (2 vs. 0) while pericarditis was seen only in the PFA group (2 vs. 0). One case of pulmonary edema occurred in each group.
“Catheter ablation is quite safe and effective,” said Dr. Reddy, explaining why this comparison was conducted on the basis of noninferiority.
Dr. Reddy emphasized that noninferiority for PFA was achieved by operators with little or no experience with this technology, whereas the catheter ablations were delivered by operators who typically had previously performed hundreds of interventions.
“With experience, one would expect even better rates of success. This is the floor,” Dr. Reddy said.
Procedure time faster with PFA
Despite working with a new technology, the mean procedure performance time with PFA was faster (105 vs. 123 minutes) even though mean fluoroscopy time was longer (21.1 vs 13.9 minutes). Dr. Reddy considers the difference in procedure time a meaningful demonstration of the efficiency of PFA.
“When you look at procedure performance, it is remarkable that the procedure times were statistically significantly shorter for a first-use technology in the hands of multiple operators,” Dr. Reddy said.
There was also a statistically significant advantage for PFA regarding change in the mean pulmonary vein cross-sectional area following the procedures (0.9% vs. 12%). Dr. Reddy acknowledged that small changes in pulmonary vein dimension are not clinically meaningful, but this result “gets at the question of whether we can achieve ablation without tissue proliferation that we see with conventional ablation.”
Overall, Dr. Reddy believes that the data from ADVENT provide several reasons “to get excited about PFA,” including the efficiency of this technique in the context of at least similar efficacy but a potential for fewer adverse events.
The ESC-invited discussant, Samuel Kiil Sørensen, MD, Gentofte University Hospital, Copenhagen, agreed that the ADVENT data support PFA as an alternative to thermal ablation. He suggested that the shorter procedure times are clinically meaningful given comparable safety and efficacy.
“Which property of PFA justifies noninferiority?” he asked. “Many of the complications of AF ablation are not specific to the energy modality. The devastating complications from damage to the esophagus, pulmonary veins, and phrenic nerve that the PFA technology may eliminate are rare, so they would not be expected to change the overall complication rate in a [randomized controlled trial] of realistic size.”
However, he suggested PFA might still prove to be an incremental advance for AF. He cited previous evidence that supports the specificity of its ablative activity and emphasized that ADVENT tested a first-generation device that might not capture the full advantages of the PFA technology.
The trial was supported by Farapulse–Boston Scientific. Dr. Reddy reports financial relationships with more than 30 pharmaceutical or device manufacturers, including Farapulse–Boston Scientific. Dr. Sørensen reports financial relationships with Medtronic and Biosense Webster.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
ECMO for shock in acute MI won’t help, may harm: ECLS-SHOCK
Patients with acute myocardial infarction (MI) and shock are often put on extracorporeal membrane oxygenation (ECMO) support before heading to the catheterization laboratory. But the practice, done routinely, doesn’t have much backing from randomized trials. Now it’s being challenged by one of the largest such studies to explore the issue.
ECMO-managed patients, moreover, had sharply increased risks for moderate and severe bleeding and vascular complications.
A challenge to common practice
The results undercut guidelines that promote mechanical circulatory support in MI-related cardiogenic shock primarily based on observational data, and they argue against what’s become common practice, said Holger Thiele, MD, Heart Center Leipzig, University of Leipzig, Germany.
Such use of ECMO could well offer some type of advantage in MI-related shock, but the data so far don’t show it, Dr. Thiele said at a press conference on the new study, called ECLS-SHOCK, at the annual congress of the European Society of Cardiology in Amsterdam. He formally presented the trial at the meeting and is lead author on its simultaneous publication in The New England Journal of Medicine.
Almost half of the trial’s patients died, whether or not they had been put on ECMO. All-cause mortality at 30 days, the primary endpoint, was about the same, at 47.8% and 49.0% for the ECMO and usual-care groups, respectively.
Meanwhile, Dr. Thiele reported, risks for moderate or severe bleeding more than doubled and serious peripheral vascular complications almost tripled with addition of ECMO support.
The findings, he noted, are consistent with a new meta-analysis of trials testing ECMO in MI-related shock that also showed increases in bleeding with survival gains using the devices. Dr. Thiele is senior author on that report, published in The Lancet to coincide with his ECLS-SHOCK presentation.
Would any subgroups benefit?
Importantly, he said in an interview, ECMO’s failure to improve 30-day survival in the trial probably applies across the spectrum of patients with MI-related shock. Subgroup analyses in both ECLS-SHOCK and the meta-analysis didn’t identify any groups that benefit, Dr. Thiele observed.
For example, there were no significant differences for the primary outcome by age, sex, whether the MI was ST-segment elevation MI or non–ST-segment elevation MI or anterior or nonanterior, or whether the patient had diabetes.
If there is a subgroup in MI-related shock that is likely to benefit from the intervention with lower mortality, he said, “it’s less than 1%, if you ask me.”
An accompanying editorial essentially agreed, arguing that ECLS-SHOCK contests the intervention’s broad application in MI-related shock without shedding light on any selective benefits.
“Will the results of the ECLS-SHOCK trial change current clinical practice? If the goal of [ECMO] is to improve 30-day mortality, these data should steer interventional and critical care cardiologists away from its early routine implementation for all or even most patients with myocardial infarction and cardiogenic shock,” the editorialists say.
“There will be some patients in this population for whom [ECMO] is necessary and lifesaving, but the results of the ECLS-SHOCK trial do not tell us which ones,” write Jane A. Leopold, MD, Brigham and Women’s Hospital, Boston, and Darren B. Taichman, MD, PhD, Penn Presbyterian Medical Center, Philadelphia.
“For now, the best course may be to reserve the early initiation of [ECMO] for those patients with infarct-related cardiogenic shock in whom the likely benefits more clearly outweigh the potential harms. We need further studies to tell us who they are,” write Dr. Leopold and Dr. Taichman, who are deputy editors with The New England Journal of Medicine.
ECLS-SHOCK randomly assigned 420 patients with acute MI complicated by shock and slated for coronary revascularization to receive standard care with or without early ECMO at 44 centers in Germany and Slovenia. Their median age was 63 years, and about 81% were men.
The relative risk for death from any cause, ECMO vs. usual care, was flatly nonsignificant at 0.98 (95% confidence interval, 0.80-1.19; P = .81).
ECMO came at the cost of significantly more cases of the primary safety endpoint, moderate or severe bleeding by Bleeding Academic Research Consortium criteria. That endpoint was met by 23.4% of ECMO patients and 9.6% of the control group, for an RR of 2.44 (95% CI, 1.50-3.95).
Rates of stroke or systemic embolization were nonsignificantly different at 3.8% and 2.9%, respectively (RR, 1.33; 95% CI, 0.47-3.76).
Speaking with this news organization, Sripal Bangalore, MD, MHA, pointed out that only 5.8% of the ECMO group but about 32% of those managed with usual care received some form of left ventricular (LV) unloading therapy.
Such measures can include atrial septostomy or the addition of an intra-aortic balloon pump or percutaneous LV-assist pump.
Given that ECMO increases afterload, “which is physiologically detrimental in patients with an ongoing MI, one is left to wonder if the results would have been different with greater use of LV unloading,” said Dr. Bangalore, of NYU Langone Health, New York, who isn’t associated with ECLS-SHOCK.
Also, he pointed out, about 78% of the trial’s patients had experienced some degree of cardiopulmonary resuscitation despite exclusion of anyone who had undergone it for more than 45 minutes. That may make the study more generalizable but also harder to show a benefit from ECMO. “The overall prognosis of that subset of patients despite heroic efforts is bleak at best.”
Dr. Thiele had no disclosures; statements for the other authors can be found at nejm.org. Dr. Bangalore has previously disclosed financial relationships with Abbott Vascular, Amgen, Biotronik, Inari, Pfizer, Reata, and Truvic. Dr. Leopold reports grants from Astellas and personal fees from United Therapeutics, Abbott Vascular, and North America Thrombosis Forum. Dr. Leopold and Dr. Taichman both report employment by The New England Journal of Medicine.
A version of this article appeared on Medscape.com.
Patients with acute myocardial infarction (MI) and shock are often put on extracorporeal membrane oxygenation (ECMO) support before heading to the catheterization laboratory. But the practice, done routinely, doesn’t have much backing from randomized trials. Now it’s being challenged by one of the largest such studies to explore the issue.
ECMO-managed patients, moreover, had sharply increased risks for moderate and severe bleeding and vascular complications.
A challenge to common practice
The results undercut guidelines that promote mechanical circulatory support in MI-related cardiogenic shock primarily based on observational data, and they argue against what’s become common practice, said Holger Thiele, MD, Heart Center Leipzig, University of Leipzig, Germany.
Such use of ECMO could well offer some type of advantage in MI-related shock, but the data so far don’t show it, Dr. Thiele said at a press conference on the new study, called ECLS-SHOCK, at the annual congress of the European Society of Cardiology in Amsterdam. He formally presented the trial at the meeting and is lead author on its simultaneous publication in The New England Journal of Medicine.
Almost half of the trial’s patients died, whether or not they had been put on ECMO. All-cause mortality at 30 days, the primary endpoint, was about the same, at 47.8% and 49.0% for the ECMO and usual-care groups, respectively.
Meanwhile, Dr. Thiele reported, risks for moderate or severe bleeding more than doubled and serious peripheral vascular complications almost tripled with addition of ECMO support.
The findings, he noted, are consistent with a new meta-analysis of trials testing ECMO in MI-related shock that also showed increases in bleeding with survival gains using the devices. Dr. Thiele is senior author on that report, published in The Lancet to coincide with his ECLS-SHOCK presentation.
Would any subgroups benefit?
Importantly, he said in an interview, ECMO’s failure to improve 30-day survival in the trial probably applies across the spectrum of patients with MI-related shock. Subgroup analyses in both ECLS-SHOCK and the meta-analysis didn’t identify any groups that benefit, Dr. Thiele observed.
For example, there were no significant differences for the primary outcome by age, sex, whether the MI was ST-segment elevation MI or non–ST-segment elevation MI or anterior or nonanterior, or whether the patient had diabetes.
If there is a subgroup in MI-related shock that is likely to benefit from the intervention with lower mortality, he said, “it’s less than 1%, if you ask me.”
An accompanying editorial essentially agreed, arguing that ECLS-SHOCK contests the intervention’s broad application in MI-related shock without shedding light on any selective benefits.
“Will the results of the ECLS-SHOCK trial change current clinical practice? If the goal of [ECMO] is to improve 30-day mortality, these data should steer interventional and critical care cardiologists away from its early routine implementation for all or even most patients with myocardial infarction and cardiogenic shock,” the editorialists say.
“There will be some patients in this population for whom [ECMO] is necessary and lifesaving, but the results of the ECLS-SHOCK trial do not tell us which ones,” write Jane A. Leopold, MD, Brigham and Women’s Hospital, Boston, and Darren B. Taichman, MD, PhD, Penn Presbyterian Medical Center, Philadelphia.
“For now, the best course may be to reserve the early initiation of [ECMO] for those patients with infarct-related cardiogenic shock in whom the likely benefits more clearly outweigh the potential harms. We need further studies to tell us who they are,” write Dr. Leopold and Dr. Taichman, who are deputy editors with The New England Journal of Medicine.
ECLS-SHOCK randomly assigned 420 patients with acute MI complicated by shock and slated for coronary revascularization to receive standard care with or without early ECMO at 44 centers in Germany and Slovenia. Their median age was 63 years, and about 81% were men.
The relative risk for death from any cause, ECMO vs. usual care, was flatly nonsignificant at 0.98 (95% confidence interval, 0.80-1.19; P = .81).
ECMO came at the cost of significantly more cases of the primary safety endpoint, moderate or severe bleeding by Bleeding Academic Research Consortium criteria. That endpoint was met by 23.4% of ECMO patients and 9.6% of the control group, for an RR of 2.44 (95% CI, 1.50-3.95).
Rates of stroke or systemic embolization were nonsignificantly different at 3.8% and 2.9%, respectively (RR, 1.33; 95% CI, 0.47-3.76).
Speaking with this news organization, Sripal Bangalore, MD, MHA, pointed out that only 5.8% of the ECMO group but about 32% of those managed with usual care received some form of left ventricular (LV) unloading therapy.
Such measures can include atrial septostomy or the addition of an intra-aortic balloon pump or percutaneous LV-assist pump.
Given that ECMO increases afterload, “which is physiologically detrimental in patients with an ongoing MI, one is left to wonder if the results would have been different with greater use of LV unloading,” said Dr. Bangalore, of NYU Langone Health, New York, who isn’t associated with ECLS-SHOCK.
Also, he pointed out, about 78% of the trial’s patients had experienced some degree of cardiopulmonary resuscitation despite exclusion of anyone who had undergone it for more than 45 minutes. That may make the study more generalizable but also harder to show a benefit from ECMO. “The overall prognosis of that subset of patients despite heroic efforts is bleak at best.”
Dr. Thiele had no disclosures; statements for the other authors can be found at nejm.org. Dr. Bangalore has previously disclosed financial relationships with Abbott Vascular, Amgen, Biotronik, Inari, Pfizer, Reata, and Truvic. Dr. Leopold reports grants from Astellas and personal fees from United Therapeutics, Abbott Vascular, and North America Thrombosis Forum. Dr. Leopold and Dr. Taichman both report employment by The New England Journal of Medicine.
A version of this article appeared on Medscape.com.
Patients with acute myocardial infarction (MI) and shock are often put on extracorporeal membrane oxygenation (ECMO) support before heading to the catheterization laboratory. But the practice, done routinely, doesn’t have much backing from randomized trials. Now it’s being challenged by one of the largest such studies to explore the issue.
ECMO-managed patients, moreover, had sharply increased risks for moderate and severe bleeding and vascular complications.
A challenge to common practice
The results undercut guidelines that promote mechanical circulatory support in MI-related cardiogenic shock primarily based on observational data, and they argue against what’s become common practice, said Holger Thiele, MD, Heart Center Leipzig, University of Leipzig, Germany.
Such use of ECMO could well offer some type of advantage in MI-related shock, but the data so far don’t show it, Dr. Thiele said at a press conference on the new study, called ECLS-SHOCK, at the annual congress of the European Society of Cardiology in Amsterdam. He formally presented the trial at the meeting and is lead author on its simultaneous publication in The New England Journal of Medicine.
Almost half of the trial’s patients died, whether or not they had been put on ECMO. All-cause mortality at 30 days, the primary endpoint, was about the same, at 47.8% and 49.0% for the ECMO and usual-care groups, respectively.
Meanwhile, Dr. Thiele reported, risks for moderate or severe bleeding more than doubled and serious peripheral vascular complications almost tripled with addition of ECMO support.
The findings, he noted, are consistent with a new meta-analysis of trials testing ECMO in MI-related shock that also showed increases in bleeding with survival gains using the devices. Dr. Thiele is senior author on that report, published in The Lancet to coincide with his ECLS-SHOCK presentation.
Would any subgroups benefit?
Importantly, he said in an interview, ECMO’s failure to improve 30-day survival in the trial probably applies across the spectrum of patients with MI-related shock. Subgroup analyses in both ECLS-SHOCK and the meta-analysis didn’t identify any groups that benefit, Dr. Thiele observed.
For example, there were no significant differences for the primary outcome by age, sex, whether the MI was ST-segment elevation MI or non–ST-segment elevation MI or anterior or nonanterior, or whether the patient had diabetes.
If there is a subgroup in MI-related shock that is likely to benefit from the intervention with lower mortality, he said, “it’s less than 1%, if you ask me.”
An accompanying editorial essentially agreed, arguing that ECLS-SHOCK contests the intervention’s broad application in MI-related shock without shedding light on any selective benefits.
“Will the results of the ECLS-SHOCK trial change current clinical practice? If the goal of [ECMO] is to improve 30-day mortality, these data should steer interventional and critical care cardiologists away from its early routine implementation for all or even most patients with myocardial infarction and cardiogenic shock,” the editorialists say.
“There will be some patients in this population for whom [ECMO] is necessary and lifesaving, but the results of the ECLS-SHOCK trial do not tell us which ones,” write Jane A. Leopold, MD, Brigham and Women’s Hospital, Boston, and Darren B. Taichman, MD, PhD, Penn Presbyterian Medical Center, Philadelphia.
“For now, the best course may be to reserve the early initiation of [ECMO] for those patients with infarct-related cardiogenic shock in whom the likely benefits more clearly outweigh the potential harms. We need further studies to tell us who they are,” write Dr. Leopold and Dr. Taichman, who are deputy editors with The New England Journal of Medicine.
ECLS-SHOCK randomly assigned 420 patients with acute MI complicated by shock and slated for coronary revascularization to receive standard care with or without early ECMO at 44 centers in Germany and Slovenia. Their median age was 63 years, and about 81% were men.
The relative risk for death from any cause, ECMO vs. usual care, was flatly nonsignificant at 0.98 (95% confidence interval, 0.80-1.19; P = .81).
ECMO came at the cost of significantly more cases of the primary safety endpoint, moderate or severe bleeding by Bleeding Academic Research Consortium criteria. That endpoint was met by 23.4% of ECMO patients and 9.6% of the control group, for an RR of 2.44 (95% CI, 1.50-3.95).
Rates of stroke or systemic embolization were nonsignificantly different at 3.8% and 2.9%, respectively (RR, 1.33; 95% CI, 0.47-3.76).
Speaking with this news organization, Sripal Bangalore, MD, MHA, pointed out that only 5.8% of the ECMO group but about 32% of those managed with usual care received some form of left ventricular (LV) unloading therapy.
Such measures can include atrial septostomy or the addition of an intra-aortic balloon pump or percutaneous LV-assist pump.
Given that ECMO increases afterload, “which is physiologically detrimental in patients with an ongoing MI, one is left to wonder if the results would have been different with greater use of LV unloading,” said Dr. Bangalore, of NYU Langone Health, New York, who isn’t associated with ECLS-SHOCK.
Also, he pointed out, about 78% of the trial’s patients had experienced some degree of cardiopulmonary resuscitation despite exclusion of anyone who had undergone it for more than 45 minutes. That may make the study more generalizable but also harder to show a benefit from ECMO. “The overall prognosis of that subset of patients despite heroic efforts is bleak at best.”
Dr. Thiele had no disclosures; statements for the other authors can be found at nejm.org. Dr. Bangalore has previously disclosed financial relationships with Abbott Vascular, Amgen, Biotronik, Inari, Pfizer, Reata, and Truvic. Dr. Leopold reports grants from Astellas and personal fees from United Therapeutics, Abbott Vascular, and North America Thrombosis Forum. Dr. Leopold and Dr. Taichman both report employment by The New England Journal of Medicine.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
Number of people with long COVID could be vastly underestimated
It’s been estimated that up to one-third of people who survive acute SARS-CoV-2 infection will suffer a post-viral syndrome with lingering neurologic and other symptoms – now known as long COVID or neurological postacute sequelae of SARS-CoV-2 infection (Neuro-PASC).
However, Researchers found a significant proportion of patients in their small study who had never tested positive for COVID-19 but who were having symptoms of long COVID nevertheless showed evidence of immune responses consistent with previous exposure.
“We estimate that millions of people got COVID in the U.S. during the first year of the pandemic and then developed long COVID, yet they did not get a positive COVID diagnosis because of testing limitations,” Igor J. Koralnik, MD, of Northwestern Medicine Comprehensive COVID-19 Center in Chicago, said in an interview.
He noted that many post-COVID-19 clinics in the United States don’t accept people with long COVID symptoms who do not have a positive test result.
Patients with long COVID symptoms but without laboratory evidence of prior infection, “who have often been rejected and stigmatized, should feel vindicated by the results of our study,” Dr. Koralnik said.
“We think that those patients deserve the same clinical care as those with a positive test, as well as inclusion in research studies. This is what we are doing at Northwestern Medicine’s Comprehensive COVID[-19] Center,” Dr. Koralnik added.
The study was published online in the journal Neurology: Neuroimmunology & Neuroinflammation.
Delayed care
The researchers measured SARS-CoV-2-specific humoral and cell-mediated immune responses against nucleocapsid protein and spike proteins, which indicate a prior COVID-19 infection, in 29 patients with post-viral syndrome after suspected COVID-19, including neurologic symptoms such as cognitive impairment, headache, and fatigue, but who did not have a confirmed positive COVID-19 test.
They did the same in 32 age- and sex-matched COVID long haulers with confirmed Neuro-PASC and 18 healthy controls with none of the symptoms of long COVID and no known exposure to SARS-CoV-2 or positive test result.
They found that 12 of the 29 patients (41%) with post-viral syndrome (but no positive COVID-19 test) had detectable humoral and cellular immune responses consistent with prior exposure to SARS-CoV-2. Three-quarters harbored antinucleocapsid and 50% harbored antispike responses.
“Our data suggest that at least 4 million people with post-viral syndrome similar to long COVID may indeed have detectable immune responses to support a COVID diagnosis,” Dr. Koralnik said in a news release.
The 12 patients with post-viral syndrome but without a confirmed COVID-19 test had neurologic symptoms similar to those of patients with confirmed Neuro-PASC.
However, lack of a confirmed COVID-19 diagnosis likely contributed to the 5-month delay in the median time from symptom onset to clinic visit, the researchers said. They were evaluated at a median of 10.7 months vs. 5.4 months for Neuro-PASC patients.
Dr. Koralnik said in an interview that the “most important take-home message” of the study is that patients with post-viral syndrome often present with clinical manifestations similar to those of confirmed patients with Neuro-PASC, suggesting that a positive result by commercially available SARS-CoV-2 diagnostic test should not be a prerequisite for accessing care.
Patients with post-viral syndrome may benefit from the same clinical care as confirmed patients with Neuro-PASC, and the absence of a positive SARS-CoV-2 test should not preclude or delay treatment, he added.
A version of this article first appeared on Medscape.com .
This article was updated 8/28/23.
It’s been estimated that up to one-third of people who survive acute SARS-CoV-2 infection will suffer a post-viral syndrome with lingering neurologic and other symptoms – now known as long COVID or neurological postacute sequelae of SARS-CoV-2 infection (Neuro-PASC).
However, Researchers found a significant proportion of patients in their small study who had never tested positive for COVID-19 but who were having symptoms of long COVID nevertheless showed evidence of immune responses consistent with previous exposure.
“We estimate that millions of people got COVID in the U.S. during the first year of the pandemic and then developed long COVID, yet they did not get a positive COVID diagnosis because of testing limitations,” Igor J. Koralnik, MD, of Northwestern Medicine Comprehensive COVID-19 Center in Chicago, said in an interview.
He noted that many post-COVID-19 clinics in the United States don’t accept people with long COVID symptoms who do not have a positive test result.
Patients with long COVID symptoms but without laboratory evidence of prior infection, “who have often been rejected and stigmatized, should feel vindicated by the results of our study,” Dr. Koralnik said.
“We think that those patients deserve the same clinical care as those with a positive test, as well as inclusion in research studies. This is what we are doing at Northwestern Medicine’s Comprehensive COVID[-19] Center,” Dr. Koralnik added.
The study was published online in the journal Neurology: Neuroimmunology & Neuroinflammation.
Delayed care
The researchers measured SARS-CoV-2-specific humoral and cell-mediated immune responses against nucleocapsid protein and spike proteins, which indicate a prior COVID-19 infection, in 29 patients with post-viral syndrome after suspected COVID-19, including neurologic symptoms such as cognitive impairment, headache, and fatigue, but who did not have a confirmed positive COVID-19 test.
They did the same in 32 age- and sex-matched COVID long haulers with confirmed Neuro-PASC and 18 healthy controls with none of the symptoms of long COVID and no known exposure to SARS-CoV-2 or positive test result.
They found that 12 of the 29 patients (41%) with post-viral syndrome (but no positive COVID-19 test) had detectable humoral and cellular immune responses consistent with prior exposure to SARS-CoV-2. Three-quarters harbored antinucleocapsid and 50% harbored antispike responses.
“Our data suggest that at least 4 million people with post-viral syndrome similar to long COVID may indeed have detectable immune responses to support a COVID diagnosis,” Dr. Koralnik said in a news release.
The 12 patients with post-viral syndrome but without a confirmed COVID-19 test had neurologic symptoms similar to those of patients with confirmed Neuro-PASC.
However, lack of a confirmed COVID-19 diagnosis likely contributed to the 5-month delay in the median time from symptom onset to clinic visit, the researchers said. They were evaluated at a median of 10.7 months vs. 5.4 months for Neuro-PASC patients.
Dr. Koralnik said in an interview that the “most important take-home message” of the study is that patients with post-viral syndrome often present with clinical manifestations similar to those of confirmed patients with Neuro-PASC, suggesting that a positive result by commercially available SARS-CoV-2 diagnostic test should not be a prerequisite for accessing care.
Patients with post-viral syndrome may benefit from the same clinical care as confirmed patients with Neuro-PASC, and the absence of a positive SARS-CoV-2 test should not preclude or delay treatment, he added.
A version of this article first appeared on Medscape.com .
This article was updated 8/28/23.
It’s been estimated that up to one-third of people who survive acute SARS-CoV-2 infection will suffer a post-viral syndrome with lingering neurologic and other symptoms – now known as long COVID or neurological postacute sequelae of SARS-CoV-2 infection (Neuro-PASC).
However, Researchers found a significant proportion of patients in their small study who had never tested positive for COVID-19 but who were having symptoms of long COVID nevertheless showed evidence of immune responses consistent with previous exposure.
“We estimate that millions of people got COVID in the U.S. during the first year of the pandemic and then developed long COVID, yet they did not get a positive COVID diagnosis because of testing limitations,” Igor J. Koralnik, MD, of Northwestern Medicine Comprehensive COVID-19 Center in Chicago, said in an interview.
He noted that many post-COVID-19 clinics in the United States don’t accept people with long COVID symptoms who do not have a positive test result.
Patients with long COVID symptoms but without laboratory evidence of prior infection, “who have often been rejected and stigmatized, should feel vindicated by the results of our study,” Dr. Koralnik said.
“We think that those patients deserve the same clinical care as those with a positive test, as well as inclusion in research studies. This is what we are doing at Northwestern Medicine’s Comprehensive COVID[-19] Center,” Dr. Koralnik added.
The study was published online in the journal Neurology: Neuroimmunology & Neuroinflammation.
Delayed care
The researchers measured SARS-CoV-2-specific humoral and cell-mediated immune responses against nucleocapsid protein and spike proteins, which indicate a prior COVID-19 infection, in 29 patients with post-viral syndrome after suspected COVID-19, including neurologic symptoms such as cognitive impairment, headache, and fatigue, but who did not have a confirmed positive COVID-19 test.
They did the same in 32 age- and sex-matched COVID long haulers with confirmed Neuro-PASC and 18 healthy controls with none of the symptoms of long COVID and no known exposure to SARS-CoV-2 or positive test result.
They found that 12 of the 29 patients (41%) with post-viral syndrome (but no positive COVID-19 test) had detectable humoral and cellular immune responses consistent with prior exposure to SARS-CoV-2. Three-quarters harbored antinucleocapsid and 50% harbored antispike responses.
“Our data suggest that at least 4 million people with post-viral syndrome similar to long COVID may indeed have detectable immune responses to support a COVID diagnosis,” Dr. Koralnik said in a news release.
The 12 patients with post-viral syndrome but without a confirmed COVID-19 test had neurologic symptoms similar to those of patients with confirmed Neuro-PASC.
However, lack of a confirmed COVID-19 diagnosis likely contributed to the 5-month delay in the median time from symptom onset to clinic visit, the researchers said. They were evaluated at a median of 10.7 months vs. 5.4 months for Neuro-PASC patients.
Dr. Koralnik said in an interview that the “most important take-home message” of the study is that patients with post-viral syndrome often present with clinical manifestations similar to those of confirmed patients with Neuro-PASC, suggesting that a positive result by commercially available SARS-CoV-2 diagnostic test should not be a prerequisite for accessing care.
Patients with post-viral syndrome may benefit from the same clinical care as confirmed patients with Neuro-PASC, and the absence of a positive SARS-CoV-2 test should not preclude or delay treatment, he added.
A version of this article first appeared on Medscape.com .
This article was updated 8/28/23.
FROM NEUROLOGY, NEUROIMMUNOLOGY & NEUROINFLAMMATION