User login
Patients Dissatisfied with Medicare Advantage Plans
NEW YORK - Medicare Advantage plans might not be meeting the needs of patients requiring the costliest and most complex levels of care, a new study suggests.
Between 2010 and 2011, such patients were more likely to switch from Medicare Advantage plans to traditional Medicare, rather than vice versa, researchers found.
The results suggest people should carefully consider all the benefits, payments, and quality measures before enrolling in Medicare Advantage plans, said lead author Dr. Momotazur Rahman of Brown University in Providence, R.I.
Unlike traditional Medicare, which is the U.S. health insurance program for the elderly and disabled, Medicare Advantage is offered by private insurance companies. While the plans cover all services provided under traditional Medicare, Advantage plans may also include added services like eye and dental coverage. They may also charge different out-of pocket costs and offer access to different sets of providers.
At the beginning of each month, the government pays Medicare Advantage companies a lump sum to cover enrollees' expenses - with higher sums for high-risk patients.
Rahman and his colleagues write in Health Affairs that lump sums encourage companies to keep healthcare costs low. But there's been some concern that companies were maximizing profits by enrolling healthier people, whereas traditional Medicare is obligated to enroll all comers.
According to the authors of the new study, legislation in 2003 aimed to address those concerns, and research suggests it helped close the gap in deaths and healthcare use and spending between people in the two types of plans.
Other studies, however, have suggested Advantage plans were still overpaid under the new system and switching between plans was limited to those needing the most care.
The researchers analyzed data on more than 36,000 Medicare beneficiaries, about a quarter of whom were enrolled in Medicare Advantage plans, to see how many switched from one type of plan to the other over the course of the year.
Overall, there was little difference, with 4 percent of traditional Medicare beneficiaries switching, compared to 5 percent of those in Medicare Advantage plans.
But there was a difference when the researchers looked at people requiring complex care - with more switching away from Medicare Advantage plans than from traditional Medicare.
For example, 17 percent of people in nursing homes for long stays switched from Medicare Advantage to traditional Medicare between 2010 and 2011, while only 3 percent moved in the opposite direction.
Also, 8 percent of people receiving home healthcare switched from Medicare Advantage during that time, compared to 3 percent switching from traditional Medicare.
The results were more exaggerated for people enrolled in both Medicare and Medicaid. Those people are allowed to switch anytime and usually use increasingly expensive care, Dr. Rahman said.
It's not clear why people needing higher levels of care are more likely to switch out of Medicare Advantage plans, said Dr. Gretchen Jacobson, associate director with the Kaiser Family Foundation's Program on Medicare Policy in Washington, D.C.
For example, it could be due to limited provider networks, unused extra benefits, or prescription drug needs, said Dr. Jacobson, who wasn't involved with the new study.
However, she said, it's important to point out that the vast majority of people remain in their chosen programs.
"Most people are not changing when they make an initial decision about their coverage, but this is an area that's ripe for more research," she said.
A representative of America's Health Insurance Plans (AHIP) also stressed that the study only looked at one point in time, and changes for Medicare Advantage plans were adopted since that period.
"More specifically, enrollment in Medicare Advantage has continued to increase year after year as program continues to offer coordinated care that leads to better outcomes for seniors and those with chronic conditions," said AHIP's Clare Krusing.
"If the type of disenrollment that was highlighted in this study was as pervasive as the authors suggest, there would be much greater evidence that beneficiaries were leaving the program in significant numbers," she said.
NEW YORK - Medicare Advantage plans might not be meeting the needs of patients requiring the costliest and most complex levels of care, a new study suggests.
Between 2010 and 2011, such patients were more likely to switch from Medicare Advantage plans to traditional Medicare, rather than vice versa, researchers found.
The results suggest people should carefully consider all the benefits, payments, and quality measures before enrolling in Medicare Advantage plans, said lead author Dr. Momotazur Rahman of Brown University in Providence, R.I.
Unlike traditional Medicare, which is the U.S. health insurance program for the elderly and disabled, Medicare Advantage is offered by private insurance companies. While the plans cover all services provided under traditional Medicare, Advantage plans may also include added services like eye and dental coverage. They may also charge different out-of pocket costs and offer access to different sets of providers.
At the beginning of each month, the government pays Medicare Advantage companies a lump sum to cover enrollees' expenses - with higher sums for high-risk patients.
Rahman and his colleagues write in Health Affairs that lump sums encourage companies to keep healthcare costs low. But there's been some concern that companies were maximizing profits by enrolling healthier people, whereas traditional Medicare is obligated to enroll all comers.
According to the authors of the new study, legislation in 2003 aimed to address those concerns, and research suggests it helped close the gap in deaths and healthcare use and spending between people in the two types of plans.
Other studies, however, have suggested Advantage plans were still overpaid under the new system and switching between plans was limited to those needing the most care.
The researchers analyzed data on more than 36,000 Medicare beneficiaries, about a quarter of whom were enrolled in Medicare Advantage plans, to see how many switched from one type of plan to the other over the course of the year.
Overall, there was little difference, with 4 percent of traditional Medicare beneficiaries switching, compared to 5 percent of those in Medicare Advantage plans.
But there was a difference when the researchers looked at people requiring complex care - with more switching away from Medicare Advantage plans than from traditional Medicare.
For example, 17 percent of people in nursing homes for long stays switched from Medicare Advantage to traditional Medicare between 2010 and 2011, while only 3 percent moved in the opposite direction.
Also, 8 percent of people receiving home healthcare switched from Medicare Advantage during that time, compared to 3 percent switching from traditional Medicare.
The results were more exaggerated for people enrolled in both Medicare and Medicaid. Those people are allowed to switch anytime and usually use increasingly expensive care, Dr. Rahman said.
It's not clear why people needing higher levels of care are more likely to switch out of Medicare Advantage plans, said Dr. Gretchen Jacobson, associate director with the Kaiser Family Foundation's Program on Medicare Policy in Washington, D.C.
For example, it could be due to limited provider networks, unused extra benefits, or prescription drug needs, said Dr. Jacobson, who wasn't involved with the new study.
However, she said, it's important to point out that the vast majority of people remain in their chosen programs.
"Most people are not changing when they make an initial decision about their coverage, but this is an area that's ripe for more research," she said.
A representative of America's Health Insurance Plans (AHIP) also stressed that the study only looked at one point in time, and changes for Medicare Advantage plans were adopted since that period.
"More specifically, enrollment in Medicare Advantage has continued to increase year after year as program continues to offer coordinated care that leads to better outcomes for seniors and those with chronic conditions," said AHIP's Clare Krusing.
"If the type of disenrollment that was highlighted in this study was as pervasive as the authors suggest, there would be much greater evidence that beneficiaries were leaving the program in significant numbers," she said.
NEW YORK - Medicare Advantage plans might not be meeting the needs of patients requiring the costliest and most complex levels of care, a new study suggests.
Between 2010 and 2011, such patients were more likely to switch from Medicare Advantage plans to traditional Medicare, rather than vice versa, researchers found.
The results suggest people should carefully consider all the benefits, payments, and quality measures before enrolling in Medicare Advantage plans, said lead author Dr. Momotazur Rahman of Brown University in Providence, R.I.
Unlike traditional Medicare, which is the U.S. health insurance program for the elderly and disabled, Medicare Advantage is offered by private insurance companies. While the plans cover all services provided under traditional Medicare, Advantage plans may also include added services like eye and dental coverage. They may also charge different out-of pocket costs and offer access to different sets of providers.
At the beginning of each month, the government pays Medicare Advantage companies a lump sum to cover enrollees' expenses - with higher sums for high-risk patients.
Rahman and his colleagues write in Health Affairs that lump sums encourage companies to keep healthcare costs low. But there's been some concern that companies were maximizing profits by enrolling healthier people, whereas traditional Medicare is obligated to enroll all comers.
According to the authors of the new study, legislation in 2003 aimed to address those concerns, and research suggests it helped close the gap in deaths and healthcare use and spending between people in the two types of plans.
Other studies, however, have suggested Advantage plans were still overpaid under the new system and switching between plans was limited to those needing the most care.
The researchers analyzed data on more than 36,000 Medicare beneficiaries, about a quarter of whom were enrolled in Medicare Advantage plans, to see how many switched from one type of plan to the other over the course of the year.
Overall, there was little difference, with 4 percent of traditional Medicare beneficiaries switching, compared to 5 percent of those in Medicare Advantage plans.
But there was a difference when the researchers looked at people requiring complex care - with more switching away from Medicare Advantage plans than from traditional Medicare.
For example, 17 percent of people in nursing homes for long stays switched from Medicare Advantage to traditional Medicare between 2010 and 2011, while only 3 percent moved in the opposite direction.
Also, 8 percent of people receiving home healthcare switched from Medicare Advantage during that time, compared to 3 percent switching from traditional Medicare.
The results were more exaggerated for people enrolled in both Medicare and Medicaid. Those people are allowed to switch anytime and usually use increasingly expensive care, Dr. Rahman said.
It's not clear why people needing higher levels of care are more likely to switch out of Medicare Advantage plans, said Dr. Gretchen Jacobson, associate director with the Kaiser Family Foundation's Program on Medicare Policy in Washington, D.C.
For example, it could be due to limited provider networks, unused extra benefits, or prescription drug needs, said Dr. Jacobson, who wasn't involved with the new study.
However, she said, it's important to point out that the vast majority of people remain in their chosen programs.
"Most people are not changing when they make an initial decision about their coverage, but this is an area that's ripe for more research," she said.
A representative of America's Health Insurance Plans (AHIP) also stressed that the study only looked at one point in time, and changes for Medicare Advantage plans were adopted since that period.
"More specifically, enrollment in Medicare Advantage has continued to increase year after year as program continues to offer coordinated care that leads to better outcomes for seniors and those with chronic conditions," said AHIP's Clare Krusing.
"If the type of disenrollment that was highlighted in this study was as pervasive as the authors suggest, there would be much greater evidence that beneficiaries were leaving the program in significant numbers," she said.
Practical tips help quell pseudofolliculitis barbae
LAS VEGAS – Stubble is okay, and not just because it’s trendy to sport a beard. This was a key message during a presentation on pseudofolliculitis barbae at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Changing up personal grooming habits is an important tactic for men who are plagued with pseudofolliculitis barbae, according to Dr. Andrew F. Alexis, chair of the department of dermatology and director of The Skin of Color Center, Mount Sinai St. Luke’s and Mount Sinai Roosevelt, New York.
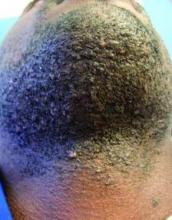
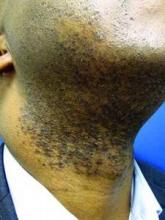
This very common skin condition, affecting 45%-83% of men of African ancestry, is best managed by avoiding close shaving and preventing a sharp hair shaft tip. For those who don’t want a full beard for personal or professional reasons, using single blade razors, electric clippers, and even depilatories can help, he said.
All of these techniques prevent curly beard hairs from repenetrating or recurving before emergence – the underpinning of the pathology of pseudofolliculitis barbae. The embedded hairs eventually form a papular or pustular lesion that mimics infectious folliculitis. The inflammatory process can also prompt keloid formation in susceptible individuals.
Providing treatment options is important because the condition can be disfiguring, with such long-term physical sequelae as scarring beard alopecia and postinflammatory hyperpigmentation – changes in appearance that can have a significant psychosocial impact on affected men, Dr. Alexis said.
Therapies are centered around avoiding close shaving and/or preventing a sharp hair shaft tip.
One primary treatment is to stop shaving. “Embedded hairs spontaneously release after about one centimeter of growth,” Dr. Alexis said. This process can take up to 2 months, he said, but military studies dating back to the 1970s showed that the vast majority of pseudofolliculitis barbae cases resolved when service members stopped close shaving practices.
However, many patients want a clean-shaven appearance. “We can work with them to modify their shaving practices. Historically, we have recommended single-blade razors over multiple blade razors” because they shave less closely, he said, pointing out that razor manufacturers have funded studies that challenge this finding.
“Electric clippers are a very good alternative” to razors, Dr. Alexis said. A blade setting that allows at least 0.5-1 mm stubble is desirable.
Chemical depilatories, which act by weakening keratin disulfide bonds, can be effective, since depilated hair does not have a sharp, beveled tip on regrowth and is therefore less likely to repuncture the skin. Patients should be aware, though, that these substances can cause irritant contact dermatitis, he pointed out. Newer formulations are less caustic, but also less efficacious, he said.
In terms of practical tips, shaving technique is important. “Don’t assume the patient knows. There are all sorts of varying techniques out there,” some of which can exacerbate pseudofolliculitis barbae, Dr. Alexis said.
Before shaving, men should wash with a mild cleanser, using a gentle circular technique to free any entrapped hairs, then a moisturizing shaving cream. Razors should be changed every five to seven shaves, and shaving should always be done in the direction of beard growth without pulling on the skin.
Post shave, topical benzoyl peroxide 5%/clindamycin 1% can significantly reduce papules and pustules. Topical retinoids are another effective option. A low-potency steroid can be helpful for inflammatory symptoms.
For cases that just don’t respond to conservative and medical management, laser hair removal is an option. A recent military-funded split-face study found further improvement when topical eflornithine was added to long-pulse Nd:Yag laser therapy, Dr. Alexis said.
Affected individuals may find it difficult to modify shaving practices when uniformed service regulations or office dress codes require men to be close shaven; a note from a physician can be helpful. Dr. Alexis provides patients with a form letter to show their employers, explaining that the patient has a skin disorder that is exacerbated by shaving, and that the patient should be permitted to maintain a well-groomed beard. “I end up writing a lot of these for New York police officers,” he said.
Dr. Alexis disclosed that he has received grants and research support from Allergan and Novartis, and speaker honoraria from Cipla. He has received consulting fees from Aclaris, Allergan, Amgen, Anacor, Bayer, Galderma, Johnson & Johnson, Leo, L’Oreal, Roche, Schick, Suneva, and Valeant.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Stubble is okay, and not just because it’s trendy to sport a beard. This was a key message during a presentation on pseudofolliculitis barbae at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Changing up personal grooming habits is an important tactic for men who are plagued with pseudofolliculitis barbae, according to Dr. Andrew F. Alexis, chair of the department of dermatology and director of The Skin of Color Center, Mount Sinai St. Luke’s and Mount Sinai Roosevelt, New York.
This very common skin condition, affecting 45%-83% of men of African ancestry, is best managed by avoiding close shaving and preventing a sharp hair shaft tip. For those who don’t want a full beard for personal or professional reasons, using single blade razors, electric clippers, and even depilatories can help, he said.
All of these techniques prevent curly beard hairs from repenetrating or recurving before emergence – the underpinning of the pathology of pseudofolliculitis barbae. The embedded hairs eventually form a papular or pustular lesion that mimics infectious folliculitis. The inflammatory process can also prompt keloid formation in susceptible individuals.
Providing treatment options is important because the condition can be disfiguring, with such long-term physical sequelae as scarring beard alopecia and postinflammatory hyperpigmentation – changes in appearance that can have a significant psychosocial impact on affected men, Dr. Alexis said.
Therapies are centered around avoiding close shaving and/or preventing a sharp hair shaft tip.
One primary treatment is to stop shaving. “Embedded hairs spontaneously release after about one centimeter of growth,” Dr. Alexis said. This process can take up to 2 months, he said, but military studies dating back to the 1970s showed that the vast majority of pseudofolliculitis barbae cases resolved when service members stopped close shaving practices.
However, many patients want a clean-shaven appearance. “We can work with them to modify their shaving practices. Historically, we have recommended single-blade razors over multiple blade razors” because they shave less closely, he said, pointing out that razor manufacturers have funded studies that challenge this finding.
“Electric clippers are a very good alternative” to razors, Dr. Alexis said. A blade setting that allows at least 0.5-1 mm stubble is desirable.
Chemical depilatories, which act by weakening keratin disulfide bonds, can be effective, since depilated hair does not have a sharp, beveled tip on regrowth and is therefore less likely to repuncture the skin. Patients should be aware, though, that these substances can cause irritant contact dermatitis, he pointed out. Newer formulations are less caustic, but also less efficacious, he said.
In terms of practical tips, shaving technique is important. “Don’t assume the patient knows. There are all sorts of varying techniques out there,” some of which can exacerbate pseudofolliculitis barbae, Dr. Alexis said.
Before shaving, men should wash with a mild cleanser, using a gentle circular technique to free any entrapped hairs, then a moisturizing shaving cream. Razors should be changed every five to seven shaves, and shaving should always be done in the direction of beard growth without pulling on the skin.
Post shave, topical benzoyl peroxide 5%/clindamycin 1% can significantly reduce papules and pustules. Topical retinoids are another effective option. A low-potency steroid can be helpful for inflammatory symptoms.
For cases that just don’t respond to conservative and medical management, laser hair removal is an option. A recent military-funded split-face study found further improvement when topical eflornithine was added to long-pulse Nd:Yag laser therapy, Dr. Alexis said.
Affected individuals may find it difficult to modify shaving practices when uniformed service regulations or office dress codes require men to be close shaven; a note from a physician can be helpful. Dr. Alexis provides patients with a form letter to show their employers, explaining that the patient has a skin disorder that is exacerbated by shaving, and that the patient should be permitted to maintain a well-groomed beard. “I end up writing a lot of these for New York police officers,” he said.
Dr. Alexis disclosed that he has received grants and research support from Allergan and Novartis, and speaker honoraria from Cipla. He has received consulting fees from Aclaris, Allergan, Amgen, Anacor, Bayer, Galderma, Johnson & Johnson, Leo, L’Oreal, Roche, Schick, Suneva, and Valeant.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Stubble is okay, and not just because it’s trendy to sport a beard. This was a key message during a presentation on pseudofolliculitis barbae at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Changing up personal grooming habits is an important tactic for men who are plagued with pseudofolliculitis barbae, according to Dr. Andrew F. Alexis, chair of the department of dermatology and director of The Skin of Color Center, Mount Sinai St. Luke’s and Mount Sinai Roosevelt, New York.
This very common skin condition, affecting 45%-83% of men of African ancestry, is best managed by avoiding close shaving and preventing a sharp hair shaft tip. For those who don’t want a full beard for personal or professional reasons, using single blade razors, electric clippers, and even depilatories can help, he said.
All of these techniques prevent curly beard hairs from repenetrating or recurving before emergence – the underpinning of the pathology of pseudofolliculitis barbae. The embedded hairs eventually form a papular or pustular lesion that mimics infectious folliculitis. The inflammatory process can also prompt keloid formation in susceptible individuals.
Providing treatment options is important because the condition can be disfiguring, with such long-term physical sequelae as scarring beard alopecia and postinflammatory hyperpigmentation – changes in appearance that can have a significant psychosocial impact on affected men, Dr. Alexis said.
Therapies are centered around avoiding close shaving and/or preventing a sharp hair shaft tip.
One primary treatment is to stop shaving. “Embedded hairs spontaneously release after about one centimeter of growth,” Dr. Alexis said. This process can take up to 2 months, he said, but military studies dating back to the 1970s showed that the vast majority of pseudofolliculitis barbae cases resolved when service members stopped close shaving practices.
However, many patients want a clean-shaven appearance. “We can work with them to modify their shaving practices. Historically, we have recommended single-blade razors over multiple blade razors” because they shave less closely, he said, pointing out that razor manufacturers have funded studies that challenge this finding.
“Electric clippers are a very good alternative” to razors, Dr. Alexis said. A blade setting that allows at least 0.5-1 mm stubble is desirable.
Chemical depilatories, which act by weakening keratin disulfide bonds, can be effective, since depilated hair does not have a sharp, beveled tip on regrowth and is therefore less likely to repuncture the skin. Patients should be aware, though, that these substances can cause irritant contact dermatitis, he pointed out. Newer formulations are less caustic, but also less efficacious, he said.
In terms of practical tips, shaving technique is important. “Don’t assume the patient knows. There are all sorts of varying techniques out there,” some of which can exacerbate pseudofolliculitis barbae, Dr. Alexis said.
Before shaving, men should wash with a mild cleanser, using a gentle circular technique to free any entrapped hairs, then a moisturizing shaving cream. Razors should be changed every five to seven shaves, and shaving should always be done in the direction of beard growth without pulling on the skin.
Post shave, topical benzoyl peroxide 5%/clindamycin 1% can significantly reduce papules and pustules. Topical retinoids are another effective option. A low-potency steroid can be helpful for inflammatory symptoms.
For cases that just don’t respond to conservative and medical management, laser hair removal is an option. A recent military-funded split-face study found further improvement when topical eflornithine was added to long-pulse Nd:Yag laser therapy, Dr. Alexis said.
Affected individuals may find it difficult to modify shaving practices when uniformed service regulations or office dress codes require men to be close shaven; a note from a physician can be helpful. Dr. Alexis provides patients with a form letter to show their employers, explaining that the patient has a skin disorder that is exacerbated by shaving, and that the patient should be permitted to maintain a well-groomed beard. “I end up writing a lot of these for New York police officers,” he said.
Dr. Alexis disclosed that he has received grants and research support from Allergan and Novartis, and speaker honoraria from Cipla. He has received consulting fees from Aclaris, Allergan, Amgen, Anacor, Bayer, Galderma, Johnson & Johnson, Leo, L’Oreal, Roche, Schick, Suneva, and Valeant.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Long-term risk of hospitalization in cancer survivors

patient and her father
Photo by Rhoda Baer
Results of a large study suggest that adolescent and young adult cancer survivors have an increased risk of hospitalization up to 34 years after their diagnosis.
Cancer survivors with the highest risk of hospitalization were those who had been diagnosed with leukemia, brain cancer, or Hodgkin lymphoma.
Kathrine Rugbjerg, PhD, and Jørgen H. Olsen, MD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, reported these results in JAMA Oncology.
The pair examined the risk of hospitalization in 33,555 subjects who had cancer as adolescents or young adults and survived at least 5 years. The subjects were diagnosed from 1943 through 2004, when they were 15 to 39 years of age.
The researchers compared the cancer survivors to a cohort of 228,447 subjects from the general population who were matched to the cancer survivors by sex and year of birth.
All study subjects were followed up for hospitalizations in the Danish Patient Register through December 2010. The median follow-up was 14 years.
There were 53,032 hospitalizations among the cancer survivors, but only 38,423 were expected. So the standardized hospitalization rate ratio (RR) was 1.38.
The highest risks of hospitalization were for diseases of blood and blood-forming organs (RR=2.00), infectious and parasitic diseases (RR=1.69), and malignant neoplasms (RR=1.63).
The overall absolute excess risk of hospitalization for the cancer survivors was 2803 per 100,000 person-years. The highest absolute excess risks were for malignant neoplasms (18%), diseases of digestive organs (15%), and diseases of the circulatory system (14%).
The researchers said these results suggest that survivors of adolescent and young adult cancers face persistent risks for a broad range of somatic diseases that require hospitalization. And the morbidity pattern is highly dependent on the type of cancer being treated. ![]()

patient and her father
Photo by Rhoda Baer
Results of a large study suggest that adolescent and young adult cancer survivors have an increased risk of hospitalization up to 34 years after their diagnosis.
Cancer survivors with the highest risk of hospitalization were those who had been diagnosed with leukemia, brain cancer, or Hodgkin lymphoma.
Kathrine Rugbjerg, PhD, and Jørgen H. Olsen, MD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, reported these results in JAMA Oncology.
The pair examined the risk of hospitalization in 33,555 subjects who had cancer as adolescents or young adults and survived at least 5 years. The subjects were diagnosed from 1943 through 2004, when they were 15 to 39 years of age.
The researchers compared the cancer survivors to a cohort of 228,447 subjects from the general population who were matched to the cancer survivors by sex and year of birth.
All study subjects were followed up for hospitalizations in the Danish Patient Register through December 2010. The median follow-up was 14 years.
There were 53,032 hospitalizations among the cancer survivors, but only 38,423 were expected. So the standardized hospitalization rate ratio (RR) was 1.38.
The highest risks of hospitalization were for diseases of blood and blood-forming organs (RR=2.00), infectious and parasitic diseases (RR=1.69), and malignant neoplasms (RR=1.63).
The overall absolute excess risk of hospitalization for the cancer survivors was 2803 per 100,000 person-years. The highest absolute excess risks were for malignant neoplasms (18%), diseases of digestive organs (15%), and diseases of the circulatory system (14%).
The researchers said these results suggest that survivors of adolescent and young adult cancers face persistent risks for a broad range of somatic diseases that require hospitalization. And the morbidity pattern is highly dependent on the type of cancer being treated. ![]()

patient and her father
Photo by Rhoda Baer
Results of a large study suggest that adolescent and young adult cancer survivors have an increased risk of hospitalization up to 34 years after their diagnosis.
Cancer survivors with the highest risk of hospitalization were those who had been diagnosed with leukemia, brain cancer, or Hodgkin lymphoma.
Kathrine Rugbjerg, PhD, and Jørgen H. Olsen, MD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, reported these results in JAMA Oncology.
The pair examined the risk of hospitalization in 33,555 subjects who had cancer as adolescents or young adults and survived at least 5 years. The subjects were diagnosed from 1943 through 2004, when they were 15 to 39 years of age.
The researchers compared the cancer survivors to a cohort of 228,447 subjects from the general population who were matched to the cancer survivors by sex and year of birth.
All study subjects were followed up for hospitalizations in the Danish Patient Register through December 2010. The median follow-up was 14 years.
There were 53,032 hospitalizations among the cancer survivors, but only 38,423 were expected. So the standardized hospitalization rate ratio (RR) was 1.38.
The highest risks of hospitalization were for diseases of blood and blood-forming organs (RR=2.00), infectious and parasitic diseases (RR=1.69), and malignant neoplasms (RR=1.63).
The overall absolute excess risk of hospitalization for the cancer survivors was 2803 per 100,000 person-years. The highest absolute excess risks were for malignant neoplasms (18%), diseases of digestive organs (15%), and diseases of the circulatory system (14%).
The researchers said these results suggest that survivors of adolescent and young adult cancers face persistent risks for a broad range of somatic diseases that require hospitalization. And the morbidity pattern is highly dependent on the type of cancer being treated. ![]()
Unexpected findings in young cancer patients

Photo by Bill Branson
Many young cancer patients—not just those with a family history of cancer—may benefit from comprehensive genomic screening, according to a study published in NEJM.
The research revealed germline mutations in cancer-predisposing genes in 8.5% of the children and adolescents studied.
Information on family history was available for roughly 60% of these cancer patients, and, in this group, only 40% of patients had a family history of cancer.
Prior to this study, the presence of such germline mutations was thought to be extremely rare and restricted to children in families with strong histories of cancer.
“This paper marks an important turning point in our understanding of pediatric cancer risk and will likely change how patients are evaluated,” said James R. Downing, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“For many pediatric cancer patients, comprehensive next-generation DNA sequencing of both their tumor and normal tissue may provide valuable information that will not only influence their clinical management but also lead to genetic counseling and testing of their parents and siblings who may be at risk and would benefit from ongoing surveillance.”
To conduct this research, Dr Downing and his colleagues performed next-generation sequencing of both the tumor and normal tissues of 1120 cancer patients younger than 20 years of age.
The investigators sequenced the whole genome in 595 patients, the whole exome in 456 patients, and both in 69 patients.
The team analyzed the DNA sequences of 565 genes, including 60 that have been associated with autosomal dominant cancer-predisposition syndromes, for the presence of germline mutations.
A total of 95 patients, or 8.5%, had germline mutations in 21 of the 60 genes. In comparison, only 1.1% of individuals in a non-cancer cohort had alterations in the same genes.
Fifty-eight of the cancer patients who had a cancer-predisposing mutation also had available information on their family history. Forty percent (n=23) of these patients had a family history of cancer.
The frequency of germline mutations in cancer-predisposition genes varied by the type of cancer a patient had. The highest frequency, 16.7%, was in patients with non-central nervous system (CNS) solid tumors, followed by CNS tumors, at 9%, and leukemia, at 4.4%.
The most commonly mutated genes were TP53 (n=50), APC (n=6), BRCA2 (n=6), NF1 (n=4), PMS2 (n=4), RB1 (n=3), and RUNX1 (n=3).
The investigators were surprised to find mutations in the breast and ovarian cancer genes BRCA1 and BRCA2 in a number of the patients. These genes are not currently included in pediatric cancer genetic screening.
“Another surprising finding to emerge from this study was the prevalence of germline mutations in 6 patients with Ewing sarcoma,” said study author Kim Nichols, MD, also of St. Jude. “[This cancer] was not previously thought to be part of any cancer-predisposition syndrome.” ![]()

Photo by Bill Branson
Many young cancer patients—not just those with a family history of cancer—may benefit from comprehensive genomic screening, according to a study published in NEJM.
The research revealed germline mutations in cancer-predisposing genes in 8.5% of the children and adolescents studied.
Information on family history was available for roughly 60% of these cancer patients, and, in this group, only 40% of patients had a family history of cancer.
Prior to this study, the presence of such germline mutations was thought to be extremely rare and restricted to children in families with strong histories of cancer.
“This paper marks an important turning point in our understanding of pediatric cancer risk and will likely change how patients are evaluated,” said James R. Downing, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“For many pediatric cancer patients, comprehensive next-generation DNA sequencing of both their tumor and normal tissue may provide valuable information that will not only influence their clinical management but also lead to genetic counseling and testing of their parents and siblings who may be at risk and would benefit from ongoing surveillance.”
To conduct this research, Dr Downing and his colleagues performed next-generation sequencing of both the tumor and normal tissues of 1120 cancer patients younger than 20 years of age.
The investigators sequenced the whole genome in 595 patients, the whole exome in 456 patients, and both in 69 patients.
The team analyzed the DNA sequences of 565 genes, including 60 that have been associated with autosomal dominant cancer-predisposition syndromes, for the presence of germline mutations.
A total of 95 patients, or 8.5%, had germline mutations in 21 of the 60 genes. In comparison, only 1.1% of individuals in a non-cancer cohort had alterations in the same genes.
Fifty-eight of the cancer patients who had a cancer-predisposing mutation also had available information on their family history. Forty percent (n=23) of these patients had a family history of cancer.
The frequency of germline mutations in cancer-predisposition genes varied by the type of cancer a patient had. The highest frequency, 16.7%, was in patients with non-central nervous system (CNS) solid tumors, followed by CNS tumors, at 9%, and leukemia, at 4.4%.
The most commonly mutated genes were TP53 (n=50), APC (n=6), BRCA2 (n=6), NF1 (n=4), PMS2 (n=4), RB1 (n=3), and RUNX1 (n=3).
The investigators were surprised to find mutations in the breast and ovarian cancer genes BRCA1 and BRCA2 in a number of the patients. These genes are not currently included in pediatric cancer genetic screening.
“Another surprising finding to emerge from this study was the prevalence of germline mutations in 6 patients with Ewing sarcoma,” said study author Kim Nichols, MD, also of St. Jude. “[This cancer] was not previously thought to be part of any cancer-predisposition syndrome.” ![]()

Photo by Bill Branson
Many young cancer patients—not just those with a family history of cancer—may benefit from comprehensive genomic screening, according to a study published in NEJM.
The research revealed germline mutations in cancer-predisposing genes in 8.5% of the children and adolescents studied.
Information on family history was available for roughly 60% of these cancer patients, and, in this group, only 40% of patients had a family history of cancer.
Prior to this study, the presence of such germline mutations was thought to be extremely rare and restricted to children in families with strong histories of cancer.
“This paper marks an important turning point in our understanding of pediatric cancer risk and will likely change how patients are evaluated,” said James R. Downing, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“For many pediatric cancer patients, comprehensive next-generation DNA sequencing of both their tumor and normal tissue may provide valuable information that will not only influence their clinical management but also lead to genetic counseling and testing of their parents and siblings who may be at risk and would benefit from ongoing surveillance.”
To conduct this research, Dr Downing and his colleagues performed next-generation sequencing of both the tumor and normal tissues of 1120 cancer patients younger than 20 years of age.
The investigators sequenced the whole genome in 595 patients, the whole exome in 456 patients, and both in 69 patients.
The team analyzed the DNA sequences of 565 genes, including 60 that have been associated with autosomal dominant cancer-predisposition syndromes, for the presence of germline mutations.
A total of 95 patients, or 8.5%, had germline mutations in 21 of the 60 genes. In comparison, only 1.1% of individuals in a non-cancer cohort had alterations in the same genes.
Fifty-eight of the cancer patients who had a cancer-predisposing mutation also had available information on their family history. Forty percent (n=23) of these patients had a family history of cancer.
The frequency of germline mutations in cancer-predisposition genes varied by the type of cancer a patient had. The highest frequency, 16.7%, was in patients with non-central nervous system (CNS) solid tumors, followed by CNS tumors, at 9%, and leukemia, at 4.4%.
The most commonly mutated genes were TP53 (n=50), APC (n=6), BRCA2 (n=6), NF1 (n=4), PMS2 (n=4), RB1 (n=3), and RUNX1 (n=3).
The investigators were surprised to find mutations in the breast and ovarian cancer genes BRCA1 and BRCA2 in a number of the patients. These genes are not currently included in pediatric cancer genetic screening.
“Another surprising finding to emerge from this study was the prevalence of germline mutations in 6 patients with Ewing sarcoma,” said study author Kim Nichols, MD, also of St. Jude. “[This cancer] was not previously thought to be part of any cancer-predisposition syndrome.” ![]()
NICE issues guideline for blood transfusions

Photo courtesy of UAB Hospital
The National Institute for Health and Care Excellence (NICE) has issued its first guideline on blood transfusions.
The agency said that national audits on the use of blood in England have suggested that at least one-fifth of transfusions are unnecessary.
So NICE developed a guideline to provide recommendations on when a transfusion should be used and when alternatives should be considered.
“This guideline will ensure blood products are used safely and efficiently,” said Mark Baker, MD, director of clinical practice at NICE.
“Hundreds of thousands of people receive blood transfusions every year in England and Wales. We must do all we can to ensure that their experience is a safe one. We know that practice is improving, and, with this guideline, we want to drive things even further so we get closer to transfusions being completely risk-free and people are spared from avoidable harm.”
The guideline contains recommendations to help hospitals reduce the need for transfusions wherever possible. This includes encouraging clinicians to prescribe tranexamic acid, which can be given to patients undergoing surgery to stop them losing too much blood.
The guideline also calls for hospitals to consider using a system that electronically identifies patients to make the transfusion process safer and more efficient.
Electronic patient identification systems prompt staff to carry out key steps in the correct order and ensure that transfusions are given to the right patients through scanning of barcodes on patient wristbands and blood component containers.
“Electronic systems make it easy for staff to do the right thing every time and avoid errors,” said Mike Murphy, MD, a consultant hematologist at Oxford University Hospitals and chair of the committee that developed the guideline.
“They can also provide ‘decision support’ for doctors when they are ordering blood and promote the restrictive use of blood. The guideline also, importantly, highlights the benefits of the routine use of tranexamic acid in patients undergoing surgery. Routine implementation of these measures in the [National Health Service] will make best use of a valuable resource, be safer for patients, and save money for hospitals.”
The guideline is available on the NICE website. NICE has also published a guide for members of the public so they know what care they should expect to receive. ![]()

Photo courtesy of UAB Hospital
The National Institute for Health and Care Excellence (NICE) has issued its first guideline on blood transfusions.
The agency said that national audits on the use of blood in England have suggested that at least one-fifth of transfusions are unnecessary.
So NICE developed a guideline to provide recommendations on when a transfusion should be used and when alternatives should be considered.
“This guideline will ensure blood products are used safely and efficiently,” said Mark Baker, MD, director of clinical practice at NICE.
“Hundreds of thousands of people receive blood transfusions every year in England and Wales. We must do all we can to ensure that their experience is a safe one. We know that practice is improving, and, with this guideline, we want to drive things even further so we get closer to transfusions being completely risk-free and people are spared from avoidable harm.”
The guideline contains recommendations to help hospitals reduce the need for transfusions wherever possible. This includes encouraging clinicians to prescribe tranexamic acid, which can be given to patients undergoing surgery to stop them losing too much blood.
The guideline also calls for hospitals to consider using a system that electronically identifies patients to make the transfusion process safer and more efficient.
Electronic patient identification systems prompt staff to carry out key steps in the correct order and ensure that transfusions are given to the right patients through scanning of barcodes on patient wristbands and blood component containers.
“Electronic systems make it easy for staff to do the right thing every time and avoid errors,” said Mike Murphy, MD, a consultant hematologist at Oxford University Hospitals and chair of the committee that developed the guideline.
“They can also provide ‘decision support’ for doctors when they are ordering blood and promote the restrictive use of blood. The guideline also, importantly, highlights the benefits of the routine use of tranexamic acid in patients undergoing surgery. Routine implementation of these measures in the [National Health Service] will make best use of a valuable resource, be safer for patients, and save money for hospitals.”
The guideline is available on the NICE website. NICE has also published a guide for members of the public so they know what care they should expect to receive. ![]()

Photo courtesy of UAB Hospital
The National Institute for Health and Care Excellence (NICE) has issued its first guideline on blood transfusions.
The agency said that national audits on the use of blood in England have suggested that at least one-fifth of transfusions are unnecessary.
So NICE developed a guideline to provide recommendations on when a transfusion should be used and when alternatives should be considered.
“This guideline will ensure blood products are used safely and efficiently,” said Mark Baker, MD, director of clinical practice at NICE.
“Hundreds of thousands of people receive blood transfusions every year in England and Wales. We must do all we can to ensure that their experience is a safe one. We know that practice is improving, and, with this guideline, we want to drive things even further so we get closer to transfusions being completely risk-free and people are spared from avoidable harm.”
The guideline contains recommendations to help hospitals reduce the need for transfusions wherever possible. This includes encouraging clinicians to prescribe tranexamic acid, which can be given to patients undergoing surgery to stop them losing too much blood.
The guideline also calls for hospitals to consider using a system that electronically identifies patients to make the transfusion process safer and more efficient.
Electronic patient identification systems prompt staff to carry out key steps in the correct order and ensure that transfusions are given to the right patients through scanning of barcodes on patient wristbands and blood component containers.
“Electronic systems make it easy for staff to do the right thing every time and avoid errors,” said Mike Murphy, MD, a consultant hematologist at Oxford University Hospitals and chair of the committee that developed the guideline.
“They can also provide ‘decision support’ for doctors when they are ordering blood and promote the restrictive use of blood. The guideline also, importantly, highlights the benefits of the routine use of tranexamic acid in patients undergoing surgery. Routine implementation of these measures in the [National Health Service] will make best use of a valuable resource, be safer for patients, and save money for hospitals.”
The guideline is available on the NICE website. NICE has also published a guide for members of the public so they know what care they should expect to receive. ![]()
Model enables screening of MM drugs

showing multiple myeloma
Researchers have created a 3-dimensional model that can be used to screen drugs designed to treat multiple myeloma (MM).
The team developed 3D tissue-engineered bone marrow (3DTEBM) cultures that could, ideally, help physicians determine which drug or combination therapy might be most effective for a particular MM patient.
The cultures also provide guidance regarding dosage.
The researchers described this work in Biomaterials.
“Even before the patient completes all of the MRIs, CT scans, and other imaging procedures following diagnosis, we can have a recommendation for which drug and dosage to prescribe,” said study author Kareem Azab, PhD, of Washington University School of Medicine in St Louis, Missouri. “The test results come in 3 to 4 days.”
Dr Azab and his colleagues developed their 3DTEBM cultures using bone marrow samples from MM patients. The team took small samples of a patient’s cells and remodeled them in the lab.
This tumor microenvironment includes the cancer cells and neighboring blood vessels, immune cells, and other components whose interaction can help or inhibit the tumor cells’ growth. Drugs are tested on the remodeled patient cells to determine which treatment is likely to be most effective.
Dr Azab’s method gauges the sensitivity of a patient’s cells to different drugs at any time in the course of the disease.
Therefore, as a patient’s disease becomes more resistant to particular drugs, continued drug screening could suggest when to change therapies. This could save valuable time, Dr Azab said.
“Now, we have a drug test that closely replicates what’s going on with a patient at any given moment,” he noted. “We think this method has a better chance of working than existing options.”
The 3DTEBM cultures are currently being tested in a clinical trial of MM patients.
To further the technology, Dr Azab and his colleagues have launched a company, Cellatrix, in coordination with Washington University’s Office of Technology Management and BioGenerator, a nonprofit organization that helps launch bioscience companies.
Cellatrix is scheduled to begin testing potential therapies on behalf of pharmaceutical companies soon. Dr Azab’s team is also studying how well the screening method works for patients with leukemia or lymphoma. ![]()

showing multiple myeloma
Researchers have created a 3-dimensional model that can be used to screen drugs designed to treat multiple myeloma (MM).
The team developed 3D tissue-engineered bone marrow (3DTEBM) cultures that could, ideally, help physicians determine which drug or combination therapy might be most effective for a particular MM patient.
The cultures also provide guidance regarding dosage.
The researchers described this work in Biomaterials.
“Even before the patient completes all of the MRIs, CT scans, and other imaging procedures following diagnosis, we can have a recommendation for which drug and dosage to prescribe,” said study author Kareem Azab, PhD, of Washington University School of Medicine in St Louis, Missouri. “The test results come in 3 to 4 days.”
Dr Azab and his colleagues developed their 3DTEBM cultures using bone marrow samples from MM patients. The team took small samples of a patient’s cells and remodeled them in the lab.
This tumor microenvironment includes the cancer cells and neighboring blood vessels, immune cells, and other components whose interaction can help or inhibit the tumor cells’ growth. Drugs are tested on the remodeled patient cells to determine which treatment is likely to be most effective.
Dr Azab’s method gauges the sensitivity of a patient’s cells to different drugs at any time in the course of the disease.
Therefore, as a patient’s disease becomes more resistant to particular drugs, continued drug screening could suggest when to change therapies. This could save valuable time, Dr Azab said.
“Now, we have a drug test that closely replicates what’s going on with a patient at any given moment,” he noted. “We think this method has a better chance of working than existing options.”
The 3DTEBM cultures are currently being tested in a clinical trial of MM patients.
To further the technology, Dr Azab and his colleagues have launched a company, Cellatrix, in coordination with Washington University’s Office of Technology Management and BioGenerator, a nonprofit organization that helps launch bioscience companies.
Cellatrix is scheduled to begin testing potential therapies on behalf of pharmaceutical companies soon. Dr Azab’s team is also studying how well the screening method works for patients with leukemia or lymphoma. ![]()

showing multiple myeloma
Researchers have created a 3-dimensional model that can be used to screen drugs designed to treat multiple myeloma (MM).
The team developed 3D tissue-engineered bone marrow (3DTEBM) cultures that could, ideally, help physicians determine which drug or combination therapy might be most effective for a particular MM patient.
The cultures also provide guidance regarding dosage.
The researchers described this work in Biomaterials.
“Even before the patient completes all of the MRIs, CT scans, and other imaging procedures following diagnosis, we can have a recommendation for which drug and dosage to prescribe,” said study author Kareem Azab, PhD, of Washington University School of Medicine in St Louis, Missouri. “The test results come in 3 to 4 days.”
Dr Azab and his colleagues developed their 3DTEBM cultures using bone marrow samples from MM patients. The team took small samples of a patient’s cells and remodeled them in the lab.
This tumor microenvironment includes the cancer cells and neighboring blood vessels, immune cells, and other components whose interaction can help or inhibit the tumor cells’ growth. Drugs are tested on the remodeled patient cells to determine which treatment is likely to be most effective.
Dr Azab’s method gauges the sensitivity of a patient’s cells to different drugs at any time in the course of the disease.
Therefore, as a patient’s disease becomes more resistant to particular drugs, continued drug screening could suggest when to change therapies. This could save valuable time, Dr Azab said.
“Now, we have a drug test that closely replicates what’s going on with a patient at any given moment,” he noted. “We think this method has a better chance of working than existing options.”
The 3DTEBM cultures are currently being tested in a clinical trial of MM patients.
To further the technology, Dr Azab and his colleagues have launched a company, Cellatrix, in coordination with Washington University’s Office of Technology Management and BioGenerator, a nonprofit organization that helps launch bioscience companies.
Cellatrix is scheduled to begin testing potential therapies on behalf of pharmaceutical companies soon. Dr Azab’s team is also studying how well the screening method works for patients with leukemia or lymphoma. ![]()
Artificial pancreas improved glycemia after islet cell transplant
A closed-loop insulin pump with continuous glucose monitor produced significantly better blood glucose control in patients who have received islet cell transplants after pancreatectomy, compared with regular insulin injections, a pilot study has found.
Fourteen adults who received auto-islet transplants after pancreatectomy for chronic pancreatitis were randomized either to receive a closed-loop insulin pump system or the usual treatment of multiple insulin injections for 72 hours after transition from intravenous to subcutaneous insulin following surgery.
Researchers observed a significantly lower mean serum glucose in the insulin pump group, compared with the control group, with the highest average serum glucose level in individual patients in the pump group still being lower than the lowest average in the control group.
These improvements in glycemia were not associated with an increased risk of hypoglycemia in the closed-loop pump group and patients in the closed-loop pump group also required a significantly lower total daily insulin dose than did the control group, according to a paper published Nov. 20 in the American Journal of Transplantation.
“Success of islet engraftment is heavily dependent on maintenance of narrow-range euglycemia in the post-transplant period,” wrote Dr. Gregory P. Forlenza of the University of Minnesota Medical Center, Minneapolis, and his coauthors (Am J Transplant. 2015 Nov 20. doi: 10.1111/ajt.13539).
“This technology was shown in this study to provide some statistically and clinically significant improvements in glycemic parameters in adults after TP [total pancreatectomy] with IAT [intraportal islet autotransplantation] without producing associated increased episodes of hypoglycemia or adverse events.”
The study was funded by the Vikings Children’s Research Fund and the University of Minnesota. Medtronic Diabetes provided supplies as part of an investigator-initiated grant. No conflicts of interest were declared.
A closed-loop insulin pump with continuous glucose monitor produced significantly better blood glucose control in patients who have received islet cell transplants after pancreatectomy, compared with regular insulin injections, a pilot study has found.
Fourteen adults who received auto-islet transplants after pancreatectomy for chronic pancreatitis were randomized either to receive a closed-loop insulin pump system or the usual treatment of multiple insulin injections for 72 hours after transition from intravenous to subcutaneous insulin following surgery.
Researchers observed a significantly lower mean serum glucose in the insulin pump group, compared with the control group, with the highest average serum glucose level in individual patients in the pump group still being lower than the lowest average in the control group.
These improvements in glycemia were not associated with an increased risk of hypoglycemia in the closed-loop pump group and patients in the closed-loop pump group also required a significantly lower total daily insulin dose than did the control group, according to a paper published Nov. 20 in the American Journal of Transplantation.
“Success of islet engraftment is heavily dependent on maintenance of narrow-range euglycemia in the post-transplant period,” wrote Dr. Gregory P. Forlenza of the University of Minnesota Medical Center, Minneapolis, and his coauthors (Am J Transplant. 2015 Nov 20. doi: 10.1111/ajt.13539).
“This technology was shown in this study to provide some statistically and clinically significant improvements in glycemic parameters in adults after TP [total pancreatectomy] with IAT [intraportal islet autotransplantation] without producing associated increased episodes of hypoglycemia or adverse events.”
The study was funded by the Vikings Children’s Research Fund and the University of Minnesota. Medtronic Diabetes provided supplies as part of an investigator-initiated grant. No conflicts of interest were declared.
A closed-loop insulin pump with continuous glucose monitor produced significantly better blood glucose control in patients who have received islet cell transplants after pancreatectomy, compared with regular insulin injections, a pilot study has found.
Fourteen adults who received auto-islet transplants after pancreatectomy for chronic pancreatitis were randomized either to receive a closed-loop insulin pump system or the usual treatment of multiple insulin injections for 72 hours after transition from intravenous to subcutaneous insulin following surgery.
Researchers observed a significantly lower mean serum glucose in the insulin pump group, compared with the control group, with the highest average serum glucose level in individual patients in the pump group still being lower than the lowest average in the control group.
These improvements in glycemia were not associated with an increased risk of hypoglycemia in the closed-loop pump group and patients in the closed-loop pump group also required a significantly lower total daily insulin dose than did the control group, according to a paper published Nov. 20 in the American Journal of Transplantation.
“Success of islet engraftment is heavily dependent on maintenance of narrow-range euglycemia in the post-transplant period,” wrote Dr. Gregory P. Forlenza of the University of Minnesota Medical Center, Minneapolis, and his coauthors (Am J Transplant. 2015 Nov 20. doi: 10.1111/ajt.13539).
“This technology was shown in this study to provide some statistically and clinically significant improvements in glycemic parameters in adults after TP [total pancreatectomy] with IAT [intraportal islet autotransplantation] without producing associated increased episodes of hypoglycemia or adverse events.”
The study was funded by the Vikings Children’s Research Fund and the University of Minnesota. Medtronic Diabetes provided supplies as part of an investigator-initiated grant. No conflicts of interest were declared.
FROM THE AMERICAN JOURNAL OF TRANSPLANTATION
Key clinical point: A closed-loop insulin pump with a continuous glucose monitor offers significantly better blood glucose control in patients who have received islet cell transplants after pancreatectomy.
Major finding: Closed-loop insulin pumps were associated with a significantly lower mean serum glucose, compared with multiple daily insulin injections.
Data source: Randomized, controlled pilot study in 14 patients receiving auto-islet transplants after pancreatectomy.
Disclosures: The study was funded by the Vikings Children’s Research Fund and the University of Minnesota. Medtronic Diabetes provided supplies as part of an investigator-initiated grant. No conflicts of interest were declared.
Interhospital Transfer Patients
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
© 2015 Society of Hospital Medicine
Hospitalists and Advanced Cancer Patients
Every year in the United States, approximately 4.7 million cancer‐related hospitalizations and 1.2 million hospital discharges with cancer as the principal diagnosis occur.[1] Limited evidence suggests that hospitalization of the cancer patient is associated with increased morbidity and mortality[2]; average length of survival of patients with advanced cancer after unplanned hospitalization is 3 to 5 months.[3] Furthermore, hospitalization of the cancer patient presents unique challenges in goals of care discussions and patient preferences. Given the high burden of cancer‐related hospitalization and limited survival in patients with advanced cancer, we must consider how hospitalists provide care for these patients. In this article, we describe the Hospital Medicine Service at Memorial Sloan Kettering Cancer Center (MSKCC) and use a hypothetical illustrative case (italicized) to provide a guide for inpatient care of the medical patient with advanced cancer while reviewing the current literature.
CLINICAL EXAMPLE
Mrs. A is a 70‐year‐old woman with recently diagnosed unresectable pancreatic adenocarcinoma, currently undergoing palliative chemotherapy with gemcitabine, who is admitted to the hospital with progressive early satiety, nausea, and increased abdominal girth. She attributes these symptoms to side effects of chemotherapy and presented to the emergency room when she developed intractable nausea and vomiting. How should her acute symptoms be evaluated and addressed? What is the hospitalist's role in her long‐term oncologic care? Is Mrs. A aware that her symptoms may be due to progression of disease rather than chemotherapy side effects? What is the best way to deliver information to Mrs. A? Who else should be involved in her care? What are her options upon discharge from the hospital?
HOSPITAL MEDICINE AT MSKCC
The Hospital Medicine Service at MSKCC consists of 7 full‐time academic hospitalists who attend on the gastrointestinal oncology, lymphoma, and general medicine inpatient services, as well as a larger number of nocturnists who work exclusively at night. In addition to being board certification in internal medicine, 1 member is board certified in medical oncology and 4 members are board certified in hospice and palliative medicine. In a recent article, we describe our experience with patients on our inpatient gastrointestinal oncology service; patients with pancreatic cancer accounted for a quarter of all inpatient admissions, and 90% of all patients had been diagnosed with metastatic disease.[4]
HOSPITALIZATION OF THE PATIENT WITH ADVANCED CANCER AND ROLE OF THE HOSPITALIST
Hospitalization of the patient with advanced cancer leads to an intense examination of health status in the face of terminal illness and an opportunity to explore patient preferences and define goals of care. It is a unique opportunity whereby hospitalists, serving as the primary inpatient physician for these patients, can encourage critical analysis of health and stimulate conversations about care. Hospitalization is a time of intense scrutiny and can reveal previously unknown medical, social, cultural, psychological, and spiritual concerns that often declare themselves in acute illness.[2, 3]
Care requires consideration not only of the malignancy and its complications, but also comorbidities that affect quality of life in terminal illness. Coordinating care in the hospitalized patient with advanced cancer is paramount; hospitalists are experts in hospital‐based care processes and can efficiently organize care between a patient's oncologist, consultants, nursing staff, social work, and case management. Coordination of care may possibly shorten length of stay, improve efficiency, and improve patient satisfaction. As hospitalists at a major cancer center, our experience has informed us of many issues involving care of these patients. Therefore, we offer the following guidelines.
PRACTICAL GUIDELINES FOR COORDINATING CARE IN HOSPITALIZED PATIENTS WITH ADVANCED CANCER
Diagnose and Treat Acute Illness and Put Into Context of Underlying Cancer
Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample on hospitalization in adults with cancer reported that the most frequent principal diagnoses were pneumonia, septicemia, maintenance chemotherapy or radiotherapy, congestive heart failure, chronic obstructive pulmonary disease, cardiac dysrhythmias, complications of surgical or medical care, osteoarthritis, complication of device, and fluid and electrolyte disorders.[1] A separate study of patients with gastrointestinal cancer found that the most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia.[5] Among our patients on the gastrointestinal oncology service, fever and pain were the 2 most common reasons for hospitalization.[4] The underlying natural disease course of cancer also deserves attention, and it is useful for patients and their families to understand this context. Patients may not realize that their acute symptoms are related to progression of cancer, and putting their symptoms into this context may be helpful. Acute illnesses that may be curable in isolation may not be so in the patient with advanced cancer, and trying to do so may cause more harm than good. Thus, placing the acute illness in the broader context of the cancer diagnosis is essential to the delivery of quality care.
In the case of Mrs. A, her symptoms were evaluated in the emergency room with a computed tomography (CT) scan of the abdomen and pelvis. Compared to her initial CT scan prior to beginning chemotherapy, there is now increased size of her primary pancreatic mass causing gastric outlet obstruction with a distended fluid‐filled stomach and new peritoneal carcinomatosis with a large amount of ascites.
Identify Decision Makers, Clarify Health Literacy, Manage Expectations, and Provide Anticipatory Guidance
Physicians should inquire about how medical decisions are made for each individual patient, as there is variability in the degree to which patients prefer to be involved in the process. If capacity is being threatened, a healthcare proxy should be designated for future decision making. If a patient is found to lack capacity in decision making, a surrogate should negotiate medical decisions.
Health literacy should be assessed so that patients are not misinformed in the decision‐making process. Begin by asking how much the patient would like to know and communicate with clear language. A probing question that we ask is: Some patients want to know everything about their medical care and others prefer that we communicate with family members. What is your preference? Explain the disease course of acute illness and provide anticipatory guidance on recovery.
It is essential for the hospitalist to understand what role the oncologist will play in the inpatient decision‐making team. In certain settings, the hospitalist is entirely responsible for inpatient care, and the oncologist plays an important but background role. In other settings, there may be a comanagement arrangement between the hospitalist and the oncologist. Understanding what role the oncologist will play and establishing clear communication at key decision points is necessary to ensure coordinated quality care. Reassuring the patient and family that the hospitalist maintains communication at key points with the oncologist is also important to building a trusting relationship.
We discuss the CT scan results with her oncologist over the phone and agree that further workup and interventions will focus on improving quality of life. No further chemotherapy is planned. Mrs. A is anxious to hear about her CT scan results, and though she has capacity for medical decision making tells us during rounds that she would like her husband and daughter to be present for the discussion.
Clarify Patient Understanding of Cancer and Goals of Care
The previous discussions will hopefully allow patients to have a full understanding of their acute illness and cancer. Further discussions may lead to shifting goals of care. To begin this process, physicians should clarify whether patients truly understand treatment intent. One study found that one‐third of patients with metastatic lung cancer thought they were receiving therapy with curative intent despite reports from their oncology team that they had been told prognosis and goals of care.[6] In 1 study of patients with head and neck cancer, 35% of patients believed palliative radiation to be curative.[7] Thus, it is critical to clarify the intent of treatment and manage expectations in regard to efficacy.
Patients may be hospitalized to undergo a procedure. It is critical to describe the rationale if these are palliative procedures. Among patients with gastrointestinal malignancies, we offer several procedures including drainage percutaneous gastrostomy for malignant small bowel obstruction, celiac plexus neurolysis for intractable pain, and stenting for symptomatic malignant biliary obstructions. In conversations describing these interventions and in the process of obtaining consent, it is crucial to explain their palliative intent.
Physicians should inquire about any advanced directives and ask hypothetical questions to assist in ascertaining goals of care. One study found that clearly documented advanced directives in patients with advanced cancer are completed approximately 25% of the time.[8] Goals‐of‐care discussions should include a discussion of palliative medicine and its role, beginning at diagnosis of advanced cancer, continuing throughout treatment, and providing end‐of‐life and follow‐up care. A landmark study by Temel et al. demonstrated that among patients with metastatic nonsmall‐cell lung cancer, early palliative care led to significant improvement in quality of life and mood, less aggressive care at end of life, and longer survival.[9]
Later that day, we return to the bedside after Mrs. A's family arrives. Our conversation reveals that they possess a good understanding of the palliative treatment intent of chemotherapy in her care. We review the CT scan findings and put these findings into the context that her cancer is progressing despite chemotherapy. They tell us that they want us to do whatever is going to help her feel better. We inform her of palliative interventions that we can offer to improve her symptoms and quality of life, namely a duodenal stent to relieve her gastric outlet obstruction to allow oral intake and Tenckhoff catheter for drainage of malignant ascites to relieve her abdominal distention and allow drainage of ascites at home. We discuss the role of hospice upon discharge from the hospital, and all agree that home hospice care is medically indicated and most consistent with her desire to be at home when her condition worsens. We address code status, and she tells us of her desire to have a natural death and we inform her a DNR order will be placed into her chart to which she agrees.
Make a Determination of Performance Status and Prognosis
The Eastern Cooperative Oncology Group (ECOG) score[10] is a simple measure of performance status in cancer patients that can be used to determine disease progression, prognosis, and resiliency to receive chemotherapy, and the physician should use this to ascertain baseline functional status. When combined with information about severity of current acute illness, the physician can estimate expected recovery.
In regard to prognostication, illness trajectories are conceptually and clinically useful. Three typical illness trajectories have been described in patients with progressive chronic illness: cancer, organ failure, and the frail elderly or dementia trajectory.[11, 12] These trajectories describe loss of function over time. The trajectory for cancer shows a period of clinical stability that is typically followed by a clear terminal phase with rapid reduction in performance status and impaired ability to care for self. The rapidity of this functional decline in advanced cancer can hinder the patient and family members' acceptance of the reality, and normalizing this pattern can be very helpful.
Using performance status, illness trajectories, generic prognosis based on cancer type, line of treatment, and input from the treating oncologist, physicians should estimate a prognosis. Prognosis can inform medical and nonmedical decision making. Prognostic uncertainty for patients can lead to uninformed decision making and hinder life planning. Wright and colleagues found that end‐of‐life discussions in patients with advanced cancer were associated with less aggressive medical care (eg, ventilation and resuscitation) near death and earlier hospice referrals. More aggressive care was found to be associated with worse patient quality of life and worse caregiver bereavement adjustment. Despite this, only 31% of dying cancer patients reported having direct discussions about death with their physicians.[13]
Often, physicians are concerned that hope is diminished when prognostic information is given. A study from Smith and colleagues showed that hope is maintained when patients with advanced cancer are given truthful prognostic and treatment information, even when the patient's chance of survival and being cured are zero.[14] Several studies identify the shortcoming of physicians when it comes to discussing end‐of‐life issues. In an exploratory analysis interviewing physicians and families of patients who died in the hospital, families reported that the attending physician never discussed the possibility of death 62% of the time, and that no one on the medical team discussed the possibility of death in 39% of cases.[15] A recent study by Rocque et al. surveyed admissions on an inpatient medical oncology service and found that despite a poor median survival of 4.7 months in the year 2000 and 3.4 months in 2010, hospice was recommended less than one‐quarter of the time, and 70% of patients were discharged home without additional services.[3]
During the conversation, Mrs. A's family inquires about prognosis. We assessed her performance status to be ECOG 3. We also note that the presence of malignant ascites and malignant bowel obstruction both portend a generic prognosis of less than 6 months. This information along with our knowledge of the illness trajectory for cancer allows us to estimate a prognosis of weeks to months. We communicate this prognosis to Mrs. A and her family and though saddened by the news, they are appreciative, as it will allow them to plan for her end‐of‐life care.
Assemble a Multidisciplinary Team
Patients with advanced cancer have complex needs that must be met within a short period of time, and it is essential for all clinical staff to be involved. If symptoms remain uncontrolled or end‐of‐life issues are looming, consultants in palliative medicine are experts in management of such issues. Case management is vital in establishing a discharge plan, as they possess information on prior discharge planning and readmissions, which may be more common in patients who do not have a clear understanding of their prognosis or when a discrepancy exists between physician‐communicated and patient‐perceived prognoses. Nursing and social work staffs are fundamental in exploring the role of the patient, family, and other caregivers who are involved in caring for the patient as well as the dynamics of interaction between them. Chaplaincy assists patients with spiritual needs and concerns. Throughout these interactions, it is important that communication remains clear, and any messages being conveyed by staff remain consistent. In line with this approach, we have found the importance of having all members on a single unit who are accustomed with particular cancer diagnoses and prognoses, as this familiarity and experience facilitates coordinated care. Acknowledging that such specialization of staff may be unrealistic in settings other than the comprehensive cancer center, the hospitalist's role as care coordinator is even more important.
Mrs. A undergoes duodenal stent placement and Tenckhoff catheter placement. She is now able to intake small amounts of food and liquids without nausea and vomiting. Her abdominal distention is relieved with ascites drainage, and she jokes she will be ready for swimsuit season soon. Our nurses and social worker work with Mrs. A and her family to assure she can adequately care for herself and has proper support at home. Our case manager identifies a nursing agency that provides home hospice care. She is discharged on hospital day 5 relieved of her symptoms.
Address System‐Level Challenges
A study examining family perspectives on end‐of‐life care found that many people dying in institutions have unmet needs for symptom relief, physician communication, emotional support, and being treated with respect. Family members of decedents who received home hospice services were more likely to report a favorable dying experience.[16] Despite the appropriateness of hospice care for patients with advanced cancer, there are often challenges in making hospice a functioning reality. The delivery of hospice's promises depends on individual hospice nurses and agencies. Patients may want to retain their oncologist as their hospice physician (versus the medical director of the hospice agency) when enrolled in hospice. Although this is beneficial for continuity, it may be detrimental in cases where the oncologist is unfamiliar with particular hospice practices or has not received training in end‐of‐life care. Hospice services also greatly differ by region in terms of services offered, level and frequency of involvement, and availability of inpatient hospice services if necessary. Few acute care hospitals offer hospice care, and for many patients who have undergone intensive treatment at 1 institution, it may feel like abandonment if patients are then asked to transition care to a hospice organization. Therefore, although hospice is beneficial to the patient with advanced cancer, the physician should become familiar with the local system‐level challenges and barriers for this option and try to overcome them whenever possible.
Although we believe we have developed a strong model at our center for hospitalists to primarily care for patients with cancer, we recognize institutional challenges that may exist. Patients may expect their oncologist to primarily provide inpatient care, and issues of trust may emerge that require expectation management and reassurance. Hospitalists may feel uncomfortable and uncertain diagnosing and treating complications of advanced cancer, which may require education and experience. Due to the severity of illness and intensity of services required for patients with advanced cancer, hospitalists may face challenges related to increased length of stay, more frequent readmissions, and increased resource utilization and cost of hospitalization that may prompt questions about the quality of care being delivered, even if those concerns are unfounded. Hospital administration may be tentative about patients with cancer being cared for primarily by hospitalists, which may be ameliorated by recognition that a majority of medical issues faced by the hospitalized patient with cancer is within the realm of a hospitalist's capabilities and scope of practice. We have faced these challenges at our own institution and are optimistic that they can be overcome at other institutions.
CONCLUSIONS
Although this article provides a guide based on our experience and review of the literature, there are several potential areas of further investigation for hospitalists caring for patients with advanced cancer. Research areas including examining the impact of hospitalist versus oncologist inpatient care on length of stay, readmissions, resource utilization, patient satisfaction, and outcomes for patients with a broad array of cancer diagnosis remains to be delineated. Issues involving patient‐physician communication are also of interest to assess patients' preferences in the communication of bad news by hospitalists versus primary oncologists. The role of hospitalists as providers of primary palliative care in the inpatient setting and the impact on outcomes also warrants further investigation. Finally, the effects of formal use of guides such as the one proposed deserve further attention.
The care of the hospitalized patient with advanced cancer can be extremely gratifying, although the challenges are significant. An organized approach to maximizing opportunities, improving quality, and enhancing patient well‐being has been outlined in this article. Because patients with advanced cancer have complicated medical, surgical, nursing, spiritual, and social needs, the hospitalist‐led multidisciplinary team is very well suited for this population.
Disclosure
Nothing to report.
- , , . Cancer hospitalizations for adults, 2009. Agency for Healthcare Quality and Research. HCUP statistical brief #125. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb125.pdf. Published February 2012. Accessed May 15, 2015.
- , , , et al. Risk factors for in‐hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4:310–318.
- , , , et al. Inpatient hospitalization of oncology patients: are we missing an opportunity for end‐of‐life care? J Oncol Pract. 2013;9:51–54.
- , , , et al. Hospitalists on an inpatient tertiary care oncology teaching service. J Oncol Pract. 2015;11:e114–e119.
- , , , , . Patterns and predictors of unplanned hospitalization in a population‐based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533.
- , , , . Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–358.
- , , , et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001;13:204–208.
- , , . Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12:373–383.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- , . Living well at the end of life: adapting health care to serious chronic illness in old age. RAND Corporation, WP‐137, 2003. Available at: http://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed May 15, 2015.
- , . Predicting survival in patients with advanced disease. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. Oxford, United Kingdom: Oxford University Press; 2004.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673.
- , , , et al. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24:521–525.
- , , , , , . Diagnosing and discussing imminent death in the hospital: a secondary analysis of physician interviews. J Palliat Med. 2007;10:882–893.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291:88–93.
Every year in the United States, approximately 4.7 million cancer‐related hospitalizations and 1.2 million hospital discharges with cancer as the principal diagnosis occur.[1] Limited evidence suggests that hospitalization of the cancer patient is associated with increased morbidity and mortality[2]; average length of survival of patients with advanced cancer after unplanned hospitalization is 3 to 5 months.[3] Furthermore, hospitalization of the cancer patient presents unique challenges in goals of care discussions and patient preferences. Given the high burden of cancer‐related hospitalization and limited survival in patients with advanced cancer, we must consider how hospitalists provide care for these patients. In this article, we describe the Hospital Medicine Service at Memorial Sloan Kettering Cancer Center (MSKCC) and use a hypothetical illustrative case (italicized) to provide a guide for inpatient care of the medical patient with advanced cancer while reviewing the current literature.
CLINICAL EXAMPLE
Mrs. A is a 70‐year‐old woman with recently diagnosed unresectable pancreatic adenocarcinoma, currently undergoing palliative chemotherapy with gemcitabine, who is admitted to the hospital with progressive early satiety, nausea, and increased abdominal girth. She attributes these symptoms to side effects of chemotherapy and presented to the emergency room when she developed intractable nausea and vomiting. How should her acute symptoms be evaluated and addressed? What is the hospitalist's role in her long‐term oncologic care? Is Mrs. A aware that her symptoms may be due to progression of disease rather than chemotherapy side effects? What is the best way to deliver information to Mrs. A? Who else should be involved in her care? What are her options upon discharge from the hospital?
HOSPITAL MEDICINE AT MSKCC
The Hospital Medicine Service at MSKCC consists of 7 full‐time academic hospitalists who attend on the gastrointestinal oncology, lymphoma, and general medicine inpatient services, as well as a larger number of nocturnists who work exclusively at night. In addition to being board certification in internal medicine, 1 member is board certified in medical oncology and 4 members are board certified in hospice and palliative medicine. In a recent article, we describe our experience with patients on our inpatient gastrointestinal oncology service; patients with pancreatic cancer accounted for a quarter of all inpatient admissions, and 90% of all patients had been diagnosed with metastatic disease.[4]
HOSPITALIZATION OF THE PATIENT WITH ADVANCED CANCER AND ROLE OF THE HOSPITALIST
Hospitalization of the patient with advanced cancer leads to an intense examination of health status in the face of terminal illness and an opportunity to explore patient preferences and define goals of care. It is a unique opportunity whereby hospitalists, serving as the primary inpatient physician for these patients, can encourage critical analysis of health and stimulate conversations about care. Hospitalization is a time of intense scrutiny and can reveal previously unknown medical, social, cultural, psychological, and spiritual concerns that often declare themselves in acute illness.[2, 3]
Care requires consideration not only of the malignancy and its complications, but also comorbidities that affect quality of life in terminal illness. Coordinating care in the hospitalized patient with advanced cancer is paramount; hospitalists are experts in hospital‐based care processes and can efficiently organize care between a patient's oncologist, consultants, nursing staff, social work, and case management. Coordination of care may possibly shorten length of stay, improve efficiency, and improve patient satisfaction. As hospitalists at a major cancer center, our experience has informed us of many issues involving care of these patients. Therefore, we offer the following guidelines.
PRACTICAL GUIDELINES FOR COORDINATING CARE IN HOSPITALIZED PATIENTS WITH ADVANCED CANCER
Diagnose and Treat Acute Illness and Put Into Context of Underlying Cancer
Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample on hospitalization in adults with cancer reported that the most frequent principal diagnoses were pneumonia, septicemia, maintenance chemotherapy or radiotherapy, congestive heart failure, chronic obstructive pulmonary disease, cardiac dysrhythmias, complications of surgical or medical care, osteoarthritis, complication of device, and fluid and electrolyte disorders.[1] A separate study of patients with gastrointestinal cancer found that the most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia.[5] Among our patients on the gastrointestinal oncology service, fever and pain were the 2 most common reasons for hospitalization.[4] The underlying natural disease course of cancer also deserves attention, and it is useful for patients and their families to understand this context. Patients may not realize that their acute symptoms are related to progression of cancer, and putting their symptoms into this context may be helpful. Acute illnesses that may be curable in isolation may not be so in the patient with advanced cancer, and trying to do so may cause more harm than good. Thus, placing the acute illness in the broader context of the cancer diagnosis is essential to the delivery of quality care.
In the case of Mrs. A, her symptoms were evaluated in the emergency room with a computed tomography (CT) scan of the abdomen and pelvis. Compared to her initial CT scan prior to beginning chemotherapy, there is now increased size of her primary pancreatic mass causing gastric outlet obstruction with a distended fluid‐filled stomach and new peritoneal carcinomatosis with a large amount of ascites.
Identify Decision Makers, Clarify Health Literacy, Manage Expectations, and Provide Anticipatory Guidance
Physicians should inquire about how medical decisions are made for each individual patient, as there is variability in the degree to which patients prefer to be involved in the process. If capacity is being threatened, a healthcare proxy should be designated for future decision making. If a patient is found to lack capacity in decision making, a surrogate should negotiate medical decisions.
Health literacy should be assessed so that patients are not misinformed in the decision‐making process. Begin by asking how much the patient would like to know and communicate with clear language. A probing question that we ask is: Some patients want to know everything about their medical care and others prefer that we communicate with family members. What is your preference? Explain the disease course of acute illness and provide anticipatory guidance on recovery.
It is essential for the hospitalist to understand what role the oncologist will play in the inpatient decision‐making team. In certain settings, the hospitalist is entirely responsible for inpatient care, and the oncologist plays an important but background role. In other settings, there may be a comanagement arrangement between the hospitalist and the oncologist. Understanding what role the oncologist will play and establishing clear communication at key decision points is necessary to ensure coordinated quality care. Reassuring the patient and family that the hospitalist maintains communication at key points with the oncologist is also important to building a trusting relationship.
We discuss the CT scan results with her oncologist over the phone and agree that further workup and interventions will focus on improving quality of life. No further chemotherapy is planned. Mrs. A is anxious to hear about her CT scan results, and though she has capacity for medical decision making tells us during rounds that she would like her husband and daughter to be present for the discussion.
Clarify Patient Understanding of Cancer and Goals of Care
The previous discussions will hopefully allow patients to have a full understanding of their acute illness and cancer. Further discussions may lead to shifting goals of care. To begin this process, physicians should clarify whether patients truly understand treatment intent. One study found that one‐third of patients with metastatic lung cancer thought they were receiving therapy with curative intent despite reports from their oncology team that they had been told prognosis and goals of care.[6] In 1 study of patients with head and neck cancer, 35% of patients believed palliative radiation to be curative.[7] Thus, it is critical to clarify the intent of treatment and manage expectations in regard to efficacy.
Patients may be hospitalized to undergo a procedure. It is critical to describe the rationale if these are palliative procedures. Among patients with gastrointestinal malignancies, we offer several procedures including drainage percutaneous gastrostomy for malignant small bowel obstruction, celiac plexus neurolysis for intractable pain, and stenting for symptomatic malignant biliary obstructions. In conversations describing these interventions and in the process of obtaining consent, it is crucial to explain their palliative intent.
Physicians should inquire about any advanced directives and ask hypothetical questions to assist in ascertaining goals of care. One study found that clearly documented advanced directives in patients with advanced cancer are completed approximately 25% of the time.[8] Goals‐of‐care discussions should include a discussion of palliative medicine and its role, beginning at diagnosis of advanced cancer, continuing throughout treatment, and providing end‐of‐life and follow‐up care. A landmark study by Temel et al. demonstrated that among patients with metastatic nonsmall‐cell lung cancer, early palliative care led to significant improvement in quality of life and mood, less aggressive care at end of life, and longer survival.[9]
Later that day, we return to the bedside after Mrs. A's family arrives. Our conversation reveals that they possess a good understanding of the palliative treatment intent of chemotherapy in her care. We review the CT scan findings and put these findings into the context that her cancer is progressing despite chemotherapy. They tell us that they want us to do whatever is going to help her feel better. We inform her of palliative interventions that we can offer to improve her symptoms and quality of life, namely a duodenal stent to relieve her gastric outlet obstruction to allow oral intake and Tenckhoff catheter for drainage of malignant ascites to relieve her abdominal distention and allow drainage of ascites at home. We discuss the role of hospice upon discharge from the hospital, and all agree that home hospice care is medically indicated and most consistent with her desire to be at home when her condition worsens. We address code status, and she tells us of her desire to have a natural death and we inform her a DNR order will be placed into her chart to which she agrees.
Make a Determination of Performance Status and Prognosis
The Eastern Cooperative Oncology Group (ECOG) score[10] is a simple measure of performance status in cancer patients that can be used to determine disease progression, prognosis, and resiliency to receive chemotherapy, and the physician should use this to ascertain baseline functional status. When combined with information about severity of current acute illness, the physician can estimate expected recovery.
In regard to prognostication, illness trajectories are conceptually and clinically useful. Three typical illness trajectories have been described in patients with progressive chronic illness: cancer, organ failure, and the frail elderly or dementia trajectory.[11, 12] These trajectories describe loss of function over time. The trajectory for cancer shows a period of clinical stability that is typically followed by a clear terminal phase with rapid reduction in performance status and impaired ability to care for self. The rapidity of this functional decline in advanced cancer can hinder the patient and family members' acceptance of the reality, and normalizing this pattern can be very helpful.
Using performance status, illness trajectories, generic prognosis based on cancer type, line of treatment, and input from the treating oncologist, physicians should estimate a prognosis. Prognosis can inform medical and nonmedical decision making. Prognostic uncertainty for patients can lead to uninformed decision making and hinder life planning. Wright and colleagues found that end‐of‐life discussions in patients with advanced cancer were associated with less aggressive medical care (eg, ventilation and resuscitation) near death and earlier hospice referrals. More aggressive care was found to be associated with worse patient quality of life and worse caregiver bereavement adjustment. Despite this, only 31% of dying cancer patients reported having direct discussions about death with their physicians.[13]
Often, physicians are concerned that hope is diminished when prognostic information is given. A study from Smith and colleagues showed that hope is maintained when patients with advanced cancer are given truthful prognostic and treatment information, even when the patient's chance of survival and being cured are zero.[14] Several studies identify the shortcoming of physicians when it comes to discussing end‐of‐life issues. In an exploratory analysis interviewing physicians and families of patients who died in the hospital, families reported that the attending physician never discussed the possibility of death 62% of the time, and that no one on the medical team discussed the possibility of death in 39% of cases.[15] A recent study by Rocque et al. surveyed admissions on an inpatient medical oncology service and found that despite a poor median survival of 4.7 months in the year 2000 and 3.4 months in 2010, hospice was recommended less than one‐quarter of the time, and 70% of patients were discharged home without additional services.[3]
During the conversation, Mrs. A's family inquires about prognosis. We assessed her performance status to be ECOG 3. We also note that the presence of malignant ascites and malignant bowel obstruction both portend a generic prognosis of less than 6 months. This information along with our knowledge of the illness trajectory for cancer allows us to estimate a prognosis of weeks to months. We communicate this prognosis to Mrs. A and her family and though saddened by the news, they are appreciative, as it will allow them to plan for her end‐of‐life care.
Assemble a Multidisciplinary Team
Patients with advanced cancer have complex needs that must be met within a short period of time, and it is essential for all clinical staff to be involved. If symptoms remain uncontrolled or end‐of‐life issues are looming, consultants in palliative medicine are experts in management of such issues. Case management is vital in establishing a discharge plan, as they possess information on prior discharge planning and readmissions, which may be more common in patients who do not have a clear understanding of their prognosis or when a discrepancy exists between physician‐communicated and patient‐perceived prognoses. Nursing and social work staffs are fundamental in exploring the role of the patient, family, and other caregivers who are involved in caring for the patient as well as the dynamics of interaction between them. Chaplaincy assists patients with spiritual needs and concerns. Throughout these interactions, it is important that communication remains clear, and any messages being conveyed by staff remain consistent. In line with this approach, we have found the importance of having all members on a single unit who are accustomed with particular cancer diagnoses and prognoses, as this familiarity and experience facilitates coordinated care. Acknowledging that such specialization of staff may be unrealistic in settings other than the comprehensive cancer center, the hospitalist's role as care coordinator is even more important.
Mrs. A undergoes duodenal stent placement and Tenckhoff catheter placement. She is now able to intake small amounts of food and liquids without nausea and vomiting. Her abdominal distention is relieved with ascites drainage, and she jokes she will be ready for swimsuit season soon. Our nurses and social worker work with Mrs. A and her family to assure she can adequately care for herself and has proper support at home. Our case manager identifies a nursing agency that provides home hospice care. She is discharged on hospital day 5 relieved of her symptoms.
Address System‐Level Challenges
A study examining family perspectives on end‐of‐life care found that many people dying in institutions have unmet needs for symptom relief, physician communication, emotional support, and being treated with respect. Family members of decedents who received home hospice services were more likely to report a favorable dying experience.[16] Despite the appropriateness of hospice care for patients with advanced cancer, there are often challenges in making hospice a functioning reality. The delivery of hospice's promises depends on individual hospice nurses and agencies. Patients may want to retain their oncologist as their hospice physician (versus the medical director of the hospice agency) when enrolled in hospice. Although this is beneficial for continuity, it may be detrimental in cases where the oncologist is unfamiliar with particular hospice practices or has not received training in end‐of‐life care. Hospice services also greatly differ by region in terms of services offered, level and frequency of involvement, and availability of inpatient hospice services if necessary. Few acute care hospitals offer hospice care, and for many patients who have undergone intensive treatment at 1 institution, it may feel like abandonment if patients are then asked to transition care to a hospice organization. Therefore, although hospice is beneficial to the patient with advanced cancer, the physician should become familiar with the local system‐level challenges and barriers for this option and try to overcome them whenever possible.
Although we believe we have developed a strong model at our center for hospitalists to primarily care for patients with cancer, we recognize institutional challenges that may exist. Patients may expect their oncologist to primarily provide inpatient care, and issues of trust may emerge that require expectation management and reassurance. Hospitalists may feel uncomfortable and uncertain diagnosing and treating complications of advanced cancer, which may require education and experience. Due to the severity of illness and intensity of services required for patients with advanced cancer, hospitalists may face challenges related to increased length of stay, more frequent readmissions, and increased resource utilization and cost of hospitalization that may prompt questions about the quality of care being delivered, even if those concerns are unfounded. Hospital administration may be tentative about patients with cancer being cared for primarily by hospitalists, which may be ameliorated by recognition that a majority of medical issues faced by the hospitalized patient with cancer is within the realm of a hospitalist's capabilities and scope of practice. We have faced these challenges at our own institution and are optimistic that they can be overcome at other institutions.
CONCLUSIONS
Although this article provides a guide based on our experience and review of the literature, there are several potential areas of further investigation for hospitalists caring for patients with advanced cancer. Research areas including examining the impact of hospitalist versus oncologist inpatient care on length of stay, readmissions, resource utilization, patient satisfaction, and outcomes for patients with a broad array of cancer diagnosis remains to be delineated. Issues involving patient‐physician communication are also of interest to assess patients' preferences in the communication of bad news by hospitalists versus primary oncologists. The role of hospitalists as providers of primary palliative care in the inpatient setting and the impact on outcomes also warrants further investigation. Finally, the effects of formal use of guides such as the one proposed deserve further attention.
The care of the hospitalized patient with advanced cancer can be extremely gratifying, although the challenges are significant. An organized approach to maximizing opportunities, improving quality, and enhancing patient well‐being has been outlined in this article. Because patients with advanced cancer have complicated medical, surgical, nursing, spiritual, and social needs, the hospitalist‐led multidisciplinary team is very well suited for this population.
Disclosure
Nothing to report.
Every year in the United States, approximately 4.7 million cancer‐related hospitalizations and 1.2 million hospital discharges with cancer as the principal diagnosis occur.[1] Limited evidence suggests that hospitalization of the cancer patient is associated with increased morbidity and mortality[2]; average length of survival of patients with advanced cancer after unplanned hospitalization is 3 to 5 months.[3] Furthermore, hospitalization of the cancer patient presents unique challenges in goals of care discussions and patient preferences. Given the high burden of cancer‐related hospitalization and limited survival in patients with advanced cancer, we must consider how hospitalists provide care for these patients. In this article, we describe the Hospital Medicine Service at Memorial Sloan Kettering Cancer Center (MSKCC) and use a hypothetical illustrative case (italicized) to provide a guide for inpatient care of the medical patient with advanced cancer while reviewing the current literature.
CLINICAL EXAMPLE
Mrs. A is a 70‐year‐old woman with recently diagnosed unresectable pancreatic adenocarcinoma, currently undergoing palliative chemotherapy with gemcitabine, who is admitted to the hospital with progressive early satiety, nausea, and increased abdominal girth. She attributes these symptoms to side effects of chemotherapy and presented to the emergency room when she developed intractable nausea and vomiting. How should her acute symptoms be evaluated and addressed? What is the hospitalist's role in her long‐term oncologic care? Is Mrs. A aware that her symptoms may be due to progression of disease rather than chemotherapy side effects? What is the best way to deliver information to Mrs. A? Who else should be involved in her care? What are her options upon discharge from the hospital?
HOSPITAL MEDICINE AT MSKCC
The Hospital Medicine Service at MSKCC consists of 7 full‐time academic hospitalists who attend on the gastrointestinal oncology, lymphoma, and general medicine inpatient services, as well as a larger number of nocturnists who work exclusively at night. In addition to being board certification in internal medicine, 1 member is board certified in medical oncology and 4 members are board certified in hospice and palliative medicine. In a recent article, we describe our experience with patients on our inpatient gastrointestinal oncology service; patients with pancreatic cancer accounted for a quarter of all inpatient admissions, and 90% of all patients had been diagnosed with metastatic disease.[4]
HOSPITALIZATION OF THE PATIENT WITH ADVANCED CANCER AND ROLE OF THE HOSPITALIST
Hospitalization of the patient with advanced cancer leads to an intense examination of health status in the face of terminal illness and an opportunity to explore patient preferences and define goals of care. It is a unique opportunity whereby hospitalists, serving as the primary inpatient physician for these patients, can encourage critical analysis of health and stimulate conversations about care. Hospitalization is a time of intense scrutiny and can reveal previously unknown medical, social, cultural, psychological, and spiritual concerns that often declare themselves in acute illness.[2, 3]
Care requires consideration not only of the malignancy and its complications, but also comorbidities that affect quality of life in terminal illness. Coordinating care in the hospitalized patient with advanced cancer is paramount; hospitalists are experts in hospital‐based care processes and can efficiently organize care between a patient's oncologist, consultants, nursing staff, social work, and case management. Coordination of care may possibly shorten length of stay, improve efficiency, and improve patient satisfaction. As hospitalists at a major cancer center, our experience has informed us of many issues involving care of these patients. Therefore, we offer the following guidelines.
PRACTICAL GUIDELINES FOR COORDINATING CARE IN HOSPITALIZED PATIENTS WITH ADVANCED CANCER
Diagnose and Treat Acute Illness and Put Into Context of Underlying Cancer
Data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample on hospitalization in adults with cancer reported that the most frequent principal diagnoses were pneumonia, septicemia, maintenance chemotherapy or radiotherapy, congestive heart failure, chronic obstructive pulmonary disease, cardiac dysrhythmias, complications of surgical or medical care, osteoarthritis, complication of device, and fluid and electrolyte disorders.[1] A separate study of patients with gastrointestinal cancer found that the most common reasons for unplanned hospitalization were fluid and electrolyte disorders, intestinal obstruction, and pneumonia.[5] Among our patients on the gastrointestinal oncology service, fever and pain were the 2 most common reasons for hospitalization.[4] The underlying natural disease course of cancer also deserves attention, and it is useful for patients and their families to understand this context. Patients may not realize that their acute symptoms are related to progression of cancer, and putting their symptoms into this context may be helpful. Acute illnesses that may be curable in isolation may not be so in the patient with advanced cancer, and trying to do so may cause more harm than good. Thus, placing the acute illness in the broader context of the cancer diagnosis is essential to the delivery of quality care.
In the case of Mrs. A, her symptoms were evaluated in the emergency room with a computed tomography (CT) scan of the abdomen and pelvis. Compared to her initial CT scan prior to beginning chemotherapy, there is now increased size of her primary pancreatic mass causing gastric outlet obstruction with a distended fluid‐filled stomach and new peritoneal carcinomatosis with a large amount of ascites.
Identify Decision Makers, Clarify Health Literacy, Manage Expectations, and Provide Anticipatory Guidance
Physicians should inquire about how medical decisions are made for each individual patient, as there is variability in the degree to which patients prefer to be involved in the process. If capacity is being threatened, a healthcare proxy should be designated for future decision making. If a patient is found to lack capacity in decision making, a surrogate should negotiate medical decisions.
Health literacy should be assessed so that patients are not misinformed in the decision‐making process. Begin by asking how much the patient would like to know and communicate with clear language. A probing question that we ask is: Some patients want to know everything about their medical care and others prefer that we communicate with family members. What is your preference? Explain the disease course of acute illness and provide anticipatory guidance on recovery.
It is essential for the hospitalist to understand what role the oncologist will play in the inpatient decision‐making team. In certain settings, the hospitalist is entirely responsible for inpatient care, and the oncologist plays an important but background role. In other settings, there may be a comanagement arrangement between the hospitalist and the oncologist. Understanding what role the oncologist will play and establishing clear communication at key decision points is necessary to ensure coordinated quality care. Reassuring the patient and family that the hospitalist maintains communication at key points with the oncologist is also important to building a trusting relationship.
We discuss the CT scan results with her oncologist over the phone and agree that further workup and interventions will focus on improving quality of life. No further chemotherapy is planned. Mrs. A is anxious to hear about her CT scan results, and though she has capacity for medical decision making tells us during rounds that she would like her husband and daughter to be present for the discussion.
Clarify Patient Understanding of Cancer and Goals of Care
The previous discussions will hopefully allow patients to have a full understanding of their acute illness and cancer. Further discussions may lead to shifting goals of care. To begin this process, physicians should clarify whether patients truly understand treatment intent. One study found that one‐third of patients with metastatic lung cancer thought they were receiving therapy with curative intent despite reports from their oncology team that they had been told prognosis and goals of care.[6] In 1 study of patients with head and neck cancer, 35% of patients believed palliative radiation to be curative.[7] Thus, it is critical to clarify the intent of treatment and manage expectations in regard to efficacy.
Patients may be hospitalized to undergo a procedure. It is critical to describe the rationale if these are palliative procedures. Among patients with gastrointestinal malignancies, we offer several procedures including drainage percutaneous gastrostomy for malignant small bowel obstruction, celiac plexus neurolysis for intractable pain, and stenting for symptomatic malignant biliary obstructions. In conversations describing these interventions and in the process of obtaining consent, it is crucial to explain their palliative intent.
Physicians should inquire about any advanced directives and ask hypothetical questions to assist in ascertaining goals of care. One study found that clearly documented advanced directives in patients with advanced cancer are completed approximately 25% of the time.[8] Goals‐of‐care discussions should include a discussion of palliative medicine and its role, beginning at diagnosis of advanced cancer, continuing throughout treatment, and providing end‐of‐life and follow‐up care. A landmark study by Temel et al. demonstrated that among patients with metastatic nonsmall‐cell lung cancer, early palliative care led to significant improvement in quality of life and mood, less aggressive care at end of life, and longer survival.[9]
Later that day, we return to the bedside after Mrs. A's family arrives. Our conversation reveals that they possess a good understanding of the palliative treatment intent of chemotherapy in her care. We review the CT scan findings and put these findings into the context that her cancer is progressing despite chemotherapy. They tell us that they want us to do whatever is going to help her feel better. We inform her of palliative interventions that we can offer to improve her symptoms and quality of life, namely a duodenal stent to relieve her gastric outlet obstruction to allow oral intake and Tenckhoff catheter for drainage of malignant ascites to relieve her abdominal distention and allow drainage of ascites at home. We discuss the role of hospice upon discharge from the hospital, and all agree that home hospice care is medically indicated and most consistent with her desire to be at home when her condition worsens. We address code status, and she tells us of her desire to have a natural death and we inform her a DNR order will be placed into her chart to which she agrees.
Make a Determination of Performance Status and Prognosis
The Eastern Cooperative Oncology Group (ECOG) score[10] is a simple measure of performance status in cancer patients that can be used to determine disease progression, prognosis, and resiliency to receive chemotherapy, and the physician should use this to ascertain baseline functional status. When combined with information about severity of current acute illness, the physician can estimate expected recovery.
In regard to prognostication, illness trajectories are conceptually and clinically useful. Three typical illness trajectories have been described in patients with progressive chronic illness: cancer, organ failure, and the frail elderly or dementia trajectory.[11, 12] These trajectories describe loss of function over time. The trajectory for cancer shows a period of clinical stability that is typically followed by a clear terminal phase with rapid reduction in performance status and impaired ability to care for self. The rapidity of this functional decline in advanced cancer can hinder the patient and family members' acceptance of the reality, and normalizing this pattern can be very helpful.
Using performance status, illness trajectories, generic prognosis based on cancer type, line of treatment, and input from the treating oncologist, physicians should estimate a prognosis. Prognosis can inform medical and nonmedical decision making. Prognostic uncertainty for patients can lead to uninformed decision making and hinder life planning. Wright and colleagues found that end‐of‐life discussions in patients with advanced cancer were associated with less aggressive medical care (eg, ventilation and resuscitation) near death and earlier hospice referrals. More aggressive care was found to be associated with worse patient quality of life and worse caregiver bereavement adjustment. Despite this, only 31% of dying cancer patients reported having direct discussions about death with their physicians.[13]
Often, physicians are concerned that hope is diminished when prognostic information is given. A study from Smith and colleagues showed that hope is maintained when patients with advanced cancer are given truthful prognostic and treatment information, even when the patient's chance of survival and being cured are zero.[14] Several studies identify the shortcoming of physicians when it comes to discussing end‐of‐life issues. In an exploratory analysis interviewing physicians and families of patients who died in the hospital, families reported that the attending physician never discussed the possibility of death 62% of the time, and that no one on the medical team discussed the possibility of death in 39% of cases.[15] A recent study by Rocque et al. surveyed admissions on an inpatient medical oncology service and found that despite a poor median survival of 4.7 months in the year 2000 and 3.4 months in 2010, hospice was recommended less than one‐quarter of the time, and 70% of patients were discharged home without additional services.[3]
During the conversation, Mrs. A's family inquires about prognosis. We assessed her performance status to be ECOG 3. We also note that the presence of malignant ascites and malignant bowel obstruction both portend a generic prognosis of less than 6 months. This information along with our knowledge of the illness trajectory for cancer allows us to estimate a prognosis of weeks to months. We communicate this prognosis to Mrs. A and her family and though saddened by the news, they are appreciative, as it will allow them to plan for her end‐of‐life care.
Assemble a Multidisciplinary Team
Patients with advanced cancer have complex needs that must be met within a short period of time, and it is essential for all clinical staff to be involved. If symptoms remain uncontrolled or end‐of‐life issues are looming, consultants in palliative medicine are experts in management of such issues. Case management is vital in establishing a discharge plan, as they possess information on prior discharge planning and readmissions, which may be more common in patients who do not have a clear understanding of their prognosis or when a discrepancy exists between physician‐communicated and patient‐perceived prognoses. Nursing and social work staffs are fundamental in exploring the role of the patient, family, and other caregivers who are involved in caring for the patient as well as the dynamics of interaction between them. Chaplaincy assists patients with spiritual needs and concerns. Throughout these interactions, it is important that communication remains clear, and any messages being conveyed by staff remain consistent. In line with this approach, we have found the importance of having all members on a single unit who are accustomed with particular cancer diagnoses and prognoses, as this familiarity and experience facilitates coordinated care. Acknowledging that such specialization of staff may be unrealistic in settings other than the comprehensive cancer center, the hospitalist's role as care coordinator is even more important.
Mrs. A undergoes duodenal stent placement and Tenckhoff catheter placement. She is now able to intake small amounts of food and liquids without nausea and vomiting. Her abdominal distention is relieved with ascites drainage, and she jokes she will be ready for swimsuit season soon. Our nurses and social worker work with Mrs. A and her family to assure she can adequately care for herself and has proper support at home. Our case manager identifies a nursing agency that provides home hospice care. She is discharged on hospital day 5 relieved of her symptoms.
Address System‐Level Challenges
A study examining family perspectives on end‐of‐life care found that many people dying in institutions have unmet needs for symptom relief, physician communication, emotional support, and being treated with respect. Family members of decedents who received home hospice services were more likely to report a favorable dying experience.[16] Despite the appropriateness of hospice care for patients with advanced cancer, there are often challenges in making hospice a functioning reality. The delivery of hospice's promises depends on individual hospice nurses and agencies. Patients may want to retain their oncologist as their hospice physician (versus the medical director of the hospice agency) when enrolled in hospice. Although this is beneficial for continuity, it may be detrimental in cases where the oncologist is unfamiliar with particular hospice practices or has not received training in end‐of‐life care. Hospice services also greatly differ by region in terms of services offered, level and frequency of involvement, and availability of inpatient hospice services if necessary. Few acute care hospitals offer hospice care, and for many patients who have undergone intensive treatment at 1 institution, it may feel like abandonment if patients are then asked to transition care to a hospice organization. Therefore, although hospice is beneficial to the patient with advanced cancer, the physician should become familiar with the local system‐level challenges and barriers for this option and try to overcome them whenever possible.
Although we believe we have developed a strong model at our center for hospitalists to primarily care for patients with cancer, we recognize institutional challenges that may exist. Patients may expect their oncologist to primarily provide inpatient care, and issues of trust may emerge that require expectation management and reassurance. Hospitalists may feel uncomfortable and uncertain diagnosing and treating complications of advanced cancer, which may require education and experience. Due to the severity of illness and intensity of services required for patients with advanced cancer, hospitalists may face challenges related to increased length of stay, more frequent readmissions, and increased resource utilization and cost of hospitalization that may prompt questions about the quality of care being delivered, even if those concerns are unfounded. Hospital administration may be tentative about patients with cancer being cared for primarily by hospitalists, which may be ameliorated by recognition that a majority of medical issues faced by the hospitalized patient with cancer is within the realm of a hospitalist's capabilities and scope of practice. We have faced these challenges at our own institution and are optimistic that they can be overcome at other institutions.
CONCLUSIONS
Although this article provides a guide based on our experience and review of the literature, there are several potential areas of further investigation for hospitalists caring for patients with advanced cancer. Research areas including examining the impact of hospitalist versus oncologist inpatient care on length of stay, readmissions, resource utilization, patient satisfaction, and outcomes for patients with a broad array of cancer diagnosis remains to be delineated. Issues involving patient‐physician communication are also of interest to assess patients' preferences in the communication of bad news by hospitalists versus primary oncologists. The role of hospitalists as providers of primary palliative care in the inpatient setting and the impact on outcomes also warrants further investigation. Finally, the effects of formal use of guides such as the one proposed deserve further attention.
The care of the hospitalized patient with advanced cancer can be extremely gratifying, although the challenges are significant. An organized approach to maximizing opportunities, improving quality, and enhancing patient well‐being has been outlined in this article. Because patients with advanced cancer have complicated medical, surgical, nursing, spiritual, and social needs, the hospitalist‐led multidisciplinary team is very well suited for this population.
Disclosure
Nothing to report.
- , , . Cancer hospitalizations for adults, 2009. Agency for Healthcare Quality and Research. HCUP statistical brief #125. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb125.pdf. Published February 2012. Accessed May 15, 2015.
- , , , et al. Risk factors for in‐hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4:310–318.
- , , , et al. Inpatient hospitalization of oncology patients: are we missing an opportunity for end‐of‐life care? J Oncol Pract. 2013;9:51–54.
- , , , et al. Hospitalists on an inpatient tertiary care oncology teaching service. J Oncol Pract. 2015;11:e114–e119.
- , , , , . Patterns and predictors of unplanned hospitalization in a population‐based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533.
- , , , . Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–358.
- , , , et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001;13:204–208.
- , , . Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12:373–383.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- , . Living well at the end of life: adapting health care to serious chronic illness in old age. RAND Corporation, WP‐137, 2003. Available at: http://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed May 15, 2015.
- , . Predicting survival in patients with advanced disease. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. Oxford, United Kingdom: Oxford University Press; 2004.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673.
- , , , et al. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24:521–525.
- , , , , , . Diagnosing and discussing imminent death in the hospital: a secondary analysis of physician interviews. J Palliat Med. 2007;10:882–893.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291:88–93.
- , , . Cancer hospitalizations for adults, 2009. Agency for Healthcare Quality and Research. HCUP statistical brief #125. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb125.pdf. Published February 2012. Accessed May 15, 2015.
- , , , et al. Risk factors for in‐hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4:310–318.
- , , , et al. Inpatient hospitalization of oncology patients: are we missing an opportunity for end‐of‐life care? J Oncol Pract. 2013;9:51–54.
- , , , et al. Hospitalists on an inpatient tertiary care oncology teaching service. J Oncol Pract. 2015;11:e114–e119.
- , , , , . Patterns and predictors of unplanned hospitalization in a population‐based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533.
- , , , . Cancer patients' perceptions of their disease and its treatment. Br J Cancer. 1988;58:355–358.
- , , , et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol). 2001;13:204–208.
- , , . Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12:373–383.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655.
- , . Living well at the end of life: adapting health care to serious chronic illness in old age. RAND Corporation, WP‐137, 2003. Available at: http://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed May 15, 2015.
- , . Predicting survival in patients with advanced disease. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. Oxford, United Kingdom: Oxford University Press; 2004.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673.
- , , , et al. Giving honest information to patients with advanced cancer maintains hope. Oncology. 2010;24:521–525.
- , , , , , . Diagnosing and discussing imminent death in the hospital: a secondary analysis of physician interviews. J Palliat Med. 2007;10:882–893.
- , , , et al. Family perspectives on end‐of‐life care at the last place of care. JAMA. 2004;291:88–93.
Direct Admissions to the Hospital
Increasing use of emergency departments (EDs) throughout the United States has become a focus of national healthcare policy and reform efforts. ED growth continues to outpace population growth, with the Institute of Medicine describing our ED systems as fragmented, overburdened, and at the breaking point.[1] Associations between ED crowding and patient dissatisfaction, delays in treatment, medical errors, and patient mortality speak to the urgency of systems improvements.[2] One major factor contributing to ED volumes is the growing number of hospital admissions that begin in EDs. From 1993 to 2006, the proportion of hospitalizations originating in EDs increased from 33.5% to 43.8%, with more than 17 million hospital admissions originating in EDs annually.[3, 4] Despite these challenges, discussions about alternative approaches to hospital admission remain at the periphery of healthcare policy conversations.
Direct admission to the hospital, defined as hospitalization without first receiving care in the hospital's ED, is an alternative approach to hospital admission, and may be a vehicle to both observation and inpatient hospital stays. Direct admissions account for 25% of all nonelective pediatric hospitalizations and 15% of nonelective adult hospitalizations in the United States.[5, 6] This admission approach was considerably more common in the past, facilitated by primary care providers (PCPs) or specialists who provided both outpatient and hospital‐based care for their patients.[4] However, as the number of hospitalists in the United States has grown over the last 30 years, the number of direct admissions has decreased concurrently. In fact, from 2003 to 2009, the number of direct admissions from clinics and physicians' offices decreased by a total of 1.6 million.[4] Although this decline is undoubtedly multifactorial, hospitalists may have contributed, both deliberately and inadvertently, to the shifting epidemiology of hospital admissions. Although many factors influence the source of hospital admissions and admission processes, direct admission has 2 important prerequisites: patients require timely access to outpatient providers for acute care, and hospitals, in partnership with outpatient‐based clinics and practices, require systems to safely and efficiently facilitate admissions without ED involvement. However, we know little about hospital admission systems, developed in the era of hospital medicine, to facilitate admissions independent of the ED.
Direct admission offers a number of potential benefits for both patients and healthcare delivery systems including reductions in the number of sites and providers of care, improved communication and coordination between outpatient and hospital‐based healthcare providers, greater patient and referring physician satisfaction, and reduced ED volumes and subsequent costs.[7] However, there are also risks and potential harms associated with direct admission, including potential delays in initial evaluation and management, inconsistent admission processes, and difficulties determining direct admission appropriateness, all of which could adversely impact patient safety and quality of care.[7, 8, 9] One study of adults with sepsis found that direct admission was associated with increased mortality compared to ED admission, which the authors speculated to be related to less timely care.[9] Similarly, a study of unscheduled adult hospitalizations found that patients admitted directly had higher mortality for time‐sensitive conditions such as acute myocardial infarction and sepsis than patients admitted through EDs, differences not observed among adults admitted with pneumonia, asthma, cellulitis, and several other common, yet frequently less emergent, reasons for hospitalization.[8] Among children with pneumonia, the most common reason for pediatric hospitalization, direct admission has been associated with significantly lower costs than admissions originating in the ED, with no significant differences in rates of transfer to the intensive care unit or hospital readmission.[10]
There is significant variation across both diagnoses and hospitals in rates of direct admission, raising questions about the contextual factors unique to hospital medicine programs that perform a substantial proportion of direct admissions.[5] This variation also highlights opportunities to identify the populations, conditions, and systems that facilitate safe and effective direct admissions. Certainly, direct admission is unlikely to be appropriate for all populations or conditions. Patients requiring emergent care or rapid diagnostic imaging are likely to receive more timely care in the ED; sepsis, acute myocardial infarction, and trauma are but a few examples of conditions for which rapid ED care decreases morbidity and mortality. Similarly, patients for whom the need for hospitalization is uncertainfor example, dehydration, asthmamay be more appropriate for initial ED management followed by re‐evaluation to inform the need for hospitalization. Finally, patients for whom the admitting diagnosis is uncertain and who require consultation for several subspecialists may be more efficiently evaluated in EDs. In our national survey of pediatric direct admission guidelines, less than one‐third of hospitals reported having formal criteria to assess the appropriateness of direct admissions, and respondents' perspectives regarding populations and diagnoses appropriate for this admission approach varied considerably.[7] These results point to the need for further research and quality‐improvement initiatives to inform the development of direct admission guidelines and protocols.
During the last decade, hospitals' discharge processes have been the focus of tremendous research, policy, and quality improvement efforts. The phrase transition of care is now widely understood to describe the changes in patient care that begin with discharge planning and conclude when patients' have established care at home or another healthcare facility. Transitions of care have been a focus of the Journal of Hospital Medicine since its inception, including publication of the Transitions of Care Consensus Policy Statement in 2009, as well as numerous other studies highlighting both risks associated with transitions of care as well as methods to address these.[11, 12, 13, 14, 15, 16] Similar to hospital discharge, hospital admission is an inherent feature of every hospitalization, and admission and discharge processes share many commonalities. Both involve transitions in sites of care, and handoffs between healthcare providers. Most involve changes in medical therapies, including both the addition of new medications and changes to existing treatments. Moreover, both are associated with significant stress to patients and their families. As a result, hospital admissions expose patients to many of same risks that have been the focus of hospital discharge reform: unstructured patient handoffs, poor communication between healthcare providers, and costly, inefficient care. The Society of Hospital Medicine has been a leader in articulating the importance of patient‐centered, clinically relevant medication reconciliation across the healthcare continuum.[17] However, with the exception of this important work, research and policy focused on understanding and improving transitions of care into the hospital have received disproportionately little attention.
To facilitate research and quality improvement efforts focused on hospital admission, we suggest that the transitions of care framework, typically discussed in the context of hospital discharge, be expanded to reflect the different origins of hospitalizations and multiple transitions that can be experienced by patients as they enter the hospital. A broadening of the transitions of care framework to incorporate hospital admissions brings numerous questions previously addressed in hospital‐to‐home transitions to the forefront. How do transitions into the hospital impact patients and healthcare systems? When is direct admission safe and effective, and how does this vary across conditions and hospital settings? What protocols and tools might optimize the associated transitions and reduce the risks of error and harm? There are numerous stakeholders who will undoubtedly bring diverse perspectives to these questionspatients and their families, hospital‐based healthcare providers, PCPs and specialists, ED physicians, and payors.
Increasing ED volumes, long wait times, and rising ED costs speak to the importance of better understanding hospital admission alternatives and the associated risks and benefits. Encouraging more direct admissions may be a solution, but evidence to guide best practices must precede this. The growing presence of round‐the‐clock pediatric and adult hospitalists across the country creates unique opportunities to transform hospital admission systems for the vast number of patients who do not require emergent care. The Affordable Care Act's expansion of insurance coverage and incentivized coordinated care within patient‐centered medical homes creates a unique opportunity for this broadened view of transitions of care. This suggests that the time is ripe for pursuing strategies that will both improve patients' transitions from outpatient to inpatient care and reduce stress on our overburdened emergency departments.
Disclosure: Dr. Lagu was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. She has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services (CMS), for her work on a project to help health systems achieve disability competence, and from the Island Peer Review Organization, under contract to CMS, for her work on development of episodes of care for CMS payment purposes (both unrelated to the current work). Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors report no conflicts of interest.
- Institute of Medicine. Hospital‐based emergency care: At the breaking point. Washington, DC: National Academies Press; 2006. Available at: http://www.nap.edu/openbook.php?record_id=11621. Accessed September 13, 2015.
- , , , . National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann Emerg Med. 2012;60(6):679–686.e3.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393.
- , , , et al. The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corp.; 2013:1–79.
- , , , , . Direct admission to hospitals among children in the United States. JAMA Pediatr. 2015;169(5):500–502.
- Healthcare Cost and Utilization Project. National Inpatient Sample. 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed October 11, 2014.
- , , , , . Direct admission to hospital: a mixed methods survey of pediatric practices, benefits, and challenges [published online August 17, 2015]. Acad Pediatr.
- , , . Changes in the source of unscheduled hospitalizations in the United States. Med Care. 2013;51(8):689–698.
- , , , . Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med. 2012;30(3):432–439.
- , , , , . Variation and outcomes associated with direct admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . Safety in numbers: physicians joining forces to seal the cracks during transitions. J Hosp Med. 2009;4(6):329–330.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , . The successes and challenges of hospital to home transitions. J Hosp Med. 2014;9(4):271–273.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
Increasing use of emergency departments (EDs) throughout the United States has become a focus of national healthcare policy and reform efforts. ED growth continues to outpace population growth, with the Institute of Medicine describing our ED systems as fragmented, overburdened, and at the breaking point.[1] Associations between ED crowding and patient dissatisfaction, delays in treatment, medical errors, and patient mortality speak to the urgency of systems improvements.[2] One major factor contributing to ED volumes is the growing number of hospital admissions that begin in EDs. From 1993 to 2006, the proportion of hospitalizations originating in EDs increased from 33.5% to 43.8%, with more than 17 million hospital admissions originating in EDs annually.[3, 4] Despite these challenges, discussions about alternative approaches to hospital admission remain at the periphery of healthcare policy conversations.
Direct admission to the hospital, defined as hospitalization without first receiving care in the hospital's ED, is an alternative approach to hospital admission, and may be a vehicle to both observation and inpatient hospital stays. Direct admissions account for 25% of all nonelective pediatric hospitalizations and 15% of nonelective adult hospitalizations in the United States.[5, 6] This admission approach was considerably more common in the past, facilitated by primary care providers (PCPs) or specialists who provided both outpatient and hospital‐based care for their patients.[4] However, as the number of hospitalists in the United States has grown over the last 30 years, the number of direct admissions has decreased concurrently. In fact, from 2003 to 2009, the number of direct admissions from clinics and physicians' offices decreased by a total of 1.6 million.[4] Although this decline is undoubtedly multifactorial, hospitalists may have contributed, both deliberately and inadvertently, to the shifting epidemiology of hospital admissions. Although many factors influence the source of hospital admissions and admission processes, direct admission has 2 important prerequisites: patients require timely access to outpatient providers for acute care, and hospitals, in partnership with outpatient‐based clinics and practices, require systems to safely and efficiently facilitate admissions without ED involvement. However, we know little about hospital admission systems, developed in the era of hospital medicine, to facilitate admissions independent of the ED.
Direct admission offers a number of potential benefits for both patients and healthcare delivery systems including reductions in the number of sites and providers of care, improved communication and coordination between outpatient and hospital‐based healthcare providers, greater patient and referring physician satisfaction, and reduced ED volumes and subsequent costs.[7] However, there are also risks and potential harms associated with direct admission, including potential delays in initial evaluation and management, inconsistent admission processes, and difficulties determining direct admission appropriateness, all of which could adversely impact patient safety and quality of care.[7, 8, 9] One study of adults with sepsis found that direct admission was associated with increased mortality compared to ED admission, which the authors speculated to be related to less timely care.[9] Similarly, a study of unscheduled adult hospitalizations found that patients admitted directly had higher mortality for time‐sensitive conditions such as acute myocardial infarction and sepsis than patients admitted through EDs, differences not observed among adults admitted with pneumonia, asthma, cellulitis, and several other common, yet frequently less emergent, reasons for hospitalization.[8] Among children with pneumonia, the most common reason for pediatric hospitalization, direct admission has been associated with significantly lower costs than admissions originating in the ED, with no significant differences in rates of transfer to the intensive care unit or hospital readmission.[10]
There is significant variation across both diagnoses and hospitals in rates of direct admission, raising questions about the contextual factors unique to hospital medicine programs that perform a substantial proportion of direct admissions.[5] This variation also highlights opportunities to identify the populations, conditions, and systems that facilitate safe and effective direct admissions. Certainly, direct admission is unlikely to be appropriate for all populations or conditions. Patients requiring emergent care or rapid diagnostic imaging are likely to receive more timely care in the ED; sepsis, acute myocardial infarction, and trauma are but a few examples of conditions for which rapid ED care decreases morbidity and mortality. Similarly, patients for whom the need for hospitalization is uncertainfor example, dehydration, asthmamay be more appropriate for initial ED management followed by re‐evaluation to inform the need for hospitalization. Finally, patients for whom the admitting diagnosis is uncertain and who require consultation for several subspecialists may be more efficiently evaluated in EDs. In our national survey of pediatric direct admission guidelines, less than one‐third of hospitals reported having formal criteria to assess the appropriateness of direct admissions, and respondents' perspectives regarding populations and diagnoses appropriate for this admission approach varied considerably.[7] These results point to the need for further research and quality‐improvement initiatives to inform the development of direct admission guidelines and protocols.
During the last decade, hospitals' discharge processes have been the focus of tremendous research, policy, and quality improvement efforts. The phrase transition of care is now widely understood to describe the changes in patient care that begin with discharge planning and conclude when patients' have established care at home or another healthcare facility. Transitions of care have been a focus of the Journal of Hospital Medicine since its inception, including publication of the Transitions of Care Consensus Policy Statement in 2009, as well as numerous other studies highlighting both risks associated with transitions of care as well as methods to address these.[11, 12, 13, 14, 15, 16] Similar to hospital discharge, hospital admission is an inherent feature of every hospitalization, and admission and discharge processes share many commonalities. Both involve transitions in sites of care, and handoffs between healthcare providers. Most involve changes in medical therapies, including both the addition of new medications and changes to existing treatments. Moreover, both are associated with significant stress to patients and their families. As a result, hospital admissions expose patients to many of same risks that have been the focus of hospital discharge reform: unstructured patient handoffs, poor communication between healthcare providers, and costly, inefficient care. The Society of Hospital Medicine has been a leader in articulating the importance of patient‐centered, clinically relevant medication reconciliation across the healthcare continuum.[17] However, with the exception of this important work, research and policy focused on understanding and improving transitions of care into the hospital have received disproportionately little attention.
To facilitate research and quality improvement efforts focused on hospital admission, we suggest that the transitions of care framework, typically discussed in the context of hospital discharge, be expanded to reflect the different origins of hospitalizations and multiple transitions that can be experienced by patients as they enter the hospital. A broadening of the transitions of care framework to incorporate hospital admissions brings numerous questions previously addressed in hospital‐to‐home transitions to the forefront. How do transitions into the hospital impact patients and healthcare systems? When is direct admission safe and effective, and how does this vary across conditions and hospital settings? What protocols and tools might optimize the associated transitions and reduce the risks of error and harm? There are numerous stakeholders who will undoubtedly bring diverse perspectives to these questionspatients and their families, hospital‐based healthcare providers, PCPs and specialists, ED physicians, and payors.
Increasing ED volumes, long wait times, and rising ED costs speak to the importance of better understanding hospital admission alternatives and the associated risks and benefits. Encouraging more direct admissions may be a solution, but evidence to guide best practices must precede this. The growing presence of round‐the‐clock pediatric and adult hospitalists across the country creates unique opportunities to transform hospital admission systems for the vast number of patients who do not require emergent care. The Affordable Care Act's expansion of insurance coverage and incentivized coordinated care within patient‐centered medical homes creates a unique opportunity for this broadened view of transitions of care. This suggests that the time is ripe for pursuing strategies that will both improve patients' transitions from outpatient to inpatient care and reduce stress on our overburdened emergency departments.
Disclosure: Dr. Lagu was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. She has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services (CMS), for her work on a project to help health systems achieve disability competence, and from the Island Peer Review Organization, under contract to CMS, for her work on development of episodes of care for CMS payment purposes (both unrelated to the current work). Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors report no conflicts of interest.
Increasing use of emergency departments (EDs) throughout the United States has become a focus of national healthcare policy and reform efforts. ED growth continues to outpace population growth, with the Institute of Medicine describing our ED systems as fragmented, overburdened, and at the breaking point.[1] Associations between ED crowding and patient dissatisfaction, delays in treatment, medical errors, and patient mortality speak to the urgency of systems improvements.[2] One major factor contributing to ED volumes is the growing number of hospital admissions that begin in EDs. From 1993 to 2006, the proportion of hospitalizations originating in EDs increased from 33.5% to 43.8%, with more than 17 million hospital admissions originating in EDs annually.[3, 4] Despite these challenges, discussions about alternative approaches to hospital admission remain at the periphery of healthcare policy conversations.
Direct admission to the hospital, defined as hospitalization without first receiving care in the hospital's ED, is an alternative approach to hospital admission, and may be a vehicle to both observation and inpatient hospital stays. Direct admissions account for 25% of all nonelective pediatric hospitalizations and 15% of nonelective adult hospitalizations in the United States.[5, 6] This admission approach was considerably more common in the past, facilitated by primary care providers (PCPs) or specialists who provided both outpatient and hospital‐based care for their patients.[4] However, as the number of hospitalists in the United States has grown over the last 30 years, the number of direct admissions has decreased concurrently. In fact, from 2003 to 2009, the number of direct admissions from clinics and physicians' offices decreased by a total of 1.6 million.[4] Although this decline is undoubtedly multifactorial, hospitalists may have contributed, both deliberately and inadvertently, to the shifting epidemiology of hospital admissions. Although many factors influence the source of hospital admissions and admission processes, direct admission has 2 important prerequisites: patients require timely access to outpatient providers for acute care, and hospitals, in partnership with outpatient‐based clinics and practices, require systems to safely and efficiently facilitate admissions without ED involvement. However, we know little about hospital admission systems, developed in the era of hospital medicine, to facilitate admissions independent of the ED.
Direct admission offers a number of potential benefits for both patients and healthcare delivery systems including reductions in the number of sites and providers of care, improved communication and coordination between outpatient and hospital‐based healthcare providers, greater patient and referring physician satisfaction, and reduced ED volumes and subsequent costs.[7] However, there are also risks and potential harms associated with direct admission, including potential delays in initial evaluation and management, inconsistent admission processes, and difficulties determining direct admission appropriateness, all of which could adversely impact patient safety and quality of care.[7, 8, 9] One study of adults with sepsis found that direct admission was associated with increased mortality compared to ED admission, which the authors speculated to be related to less timely care.[9] Similarly, a study of unscheduled adult hospitalizations found that patients admitted directly had higher mortality for time‐sensitive conditions such as acute myocardial infarction and sepsis than patients admitted through EDs, differences not observed among adults admitted with pneumonia, asthma, cellulitis, and several other common, yet frequently less emergent, reasons for hospitalization.[8] Among children with pneumonia, the most common reason for pediatric hospitalization, direct admission has been associated with significantly lower costs than admissions originating in the ED, with no significant differences in rates of transfer to the intensive care unit or hospital readmission.[10]
There is significant variation across both diagnoses and hospitals in rates of direct admission, raising questions about the contextual factors unique to hospital medicine programs that perform a substantial proportion of direct admissions.[5] This variation also highlights opportunities to identify the populations, conditions, and systems that facilitate safe and effective direct admissions. Certainly, direct admission is unlikely to be appropriate for all populations or conditions. Patients requiring emergent care or rapid diagnostic imaging are likely to receive more timely care in the ED; sepsis, acute myocardial infarction, and trauma are but a few examples of conditions for which rapid ED care decreases morbidity and mortality. Similarly, patients for whom the need for hospitalization is uncertainfor example, dehydration, asthmamay be more appropriate for initial ED management followed by re‐evaluation to inform the need for hospitalization. Finally, patients for whom the admitting diagnosis is uncertain and who require consultation for several subspecialists may be more efficiently evaluated in EDs. In our national survey of pediatric direct admission guidelines, less than one‐third of hospitals reported having formal criteria to assess the appropriateness of direct admissions, and respondents' perspectives regarding populations and diagnoses appropriate for this admission approach varied considerably.[7] These results point to the need for further research and quality‐improvement initiatives to inform the development of direct admission guidelines and protocols.
During the last decade, hospitals' discharge processes have been the focus of tremendous research, policy, and quality improvement efforts. The phrase transition of care is now widely understood to describe the changes in patient care that begin with discharge planning and conclude when patients' have established care at home or another healthcare facility. Transitions of care have been a focus of the Journal of Hospital Medicine since its inception, including publication of the Transitions of Care Consensus Policy Statement in 2009, as well as numerous other studies highlighting both risks associated with transitions of care as well as methods to address these.[11, 12, 13, 14, 15, 16] Similar to hospital discharge, hospital admission is an inherent feature of every hospitalization, and admission and discharge processes share many commonalities. Both involve transitions in sites of care, and handoffs between healthcare providers. Most involve changes in medical therapies, including both the addition of new medications and changes to existing treatments. Moreover, both are associated with significant stress to patients and their families. As a result, hospital admissions expose patients to many of same risks that have been the focus of hospital discharge reform: unstructured patient handoffs, poor communication between healthcare providers, and costly, inefficient care. The Society of Hospital Medicine has been a leader in articulating the importance of patient‐centered, clinically relevant medication reconciliation across the healthcare continuum.[17] However, with the exception of this important work, research and policy focused on understanding and improving transitions of care into the hospital have received disproportionately little attention.
To facilitate research and quality improvement efforts focused on hospital admission, we suggest that the transitions of care framework, typically discussed in the context of hospital discharge, be expanded to reflect the different origins of hospitalizations and multiple transitions that can be experienced by patients as they enter the hospital. A broadening of the transitions of care framework to incorporate hospital admissions brings numerous questions previously addressed in hospital‐to‐home transitions to the forefront. How do transitions into the hospital impact patients and healthcare systems? When is direct admission safe and effective, and how does this vary across conditions and hospital settings? What protocols and tools might optimize the associated transitions and reduce the risks of error and harm? There are numerous stakeholders who will undoubtedly bring diverse perspectives to these questionspatients and their families, hospital‐based healthcare providers, PCPs and specialists, ED physicians, and payors.
Increasing ED volumes, long wait times, and rising ED costs speak to the importance of better understanding hospital admission alternatives and the associated risks and benefits. Encouraging more direct admissions may be a solution, but evidence to guide best practices must precede this. The growing presence of round‐the‐clock pediatric and adult hospitalists across the country creates unique opportunities to transform hospital admission systems for the vast number of patients who do not require emergent care. The Affordable Care Act's expansion of insurance coverage and incentivized coordinated care within patient‐centered medical homes creates a unique opportunity for this broadened view of transitions of care. This suggests that the time is ripe for pursuing strategies that will both improve patients' transitions from outpatient to inpatient care and reduce stress on our overburdened emergency departments.
Disclosure: Dr. Lagu was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. She has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services (CMS), for her work on a project to help health systems achieve disability competence, and from the Island Peer Review Organization, under contract to CMS, for her work on development of episodes of care for CMS payment purposes (both unrelated to the current work). Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors report no conflicts of interest.
- Institute of Medicine. Hospital‐based emergency care: At the breaking point. Washington, DC: National Academies Press; 2006. Available at: http://www.nap.edu/openbook.php?record_id=11621. Accessed September 13, 2015.
- , , , . National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann Emerg Med. 2012;60(6):679–686.e3.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393.
- , , , et al. The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corp.; 2013:1–79.
- , , , , . Direct admission to hospitals among children in the United States. JAMA Pediatr. 2015;169(5):500–502.
- Healthcare Cost and Utilization Project. National Inpatient Sample. 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed October 11, 2014.
- , , , , . Direct admission to hospital: a mixed methods survey of pediatric practices, benefits, and challenges [published online August 17, 2015]. Acad Pediatr.
- , , . Changes in the source of unscheduled hospitalizations in the United States. Med Care. 2013;51(8):689–698.
- , , , . Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med. 2012;30(3):432–439.
- , , , , . Variation and outcomes associated with direct admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . Safety in numbers: physicians joining forces to seal the cracks during transitions. J Hosp Med. 2009;4(6):329–330.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , . The successes and challenges of hospital to home transitions. J Hosp Med. 2014;9(4):271–273.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- Institute of Medicine. Hospital‐based emergency care: At the breaking point. Washington, DC: National Academies Press; 2006. Available at: http://www.nap.edu/openbook.php?record_id=11621. Accessed September 13, 2015.
- , , , . National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann Emerg Med. 2012;60(6):679–686.e3.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393.
- , , , et al. The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corp.; 2013:1–79.
- , , , , . Direct admission to hospitals among children in the United States. JAMA Pediatr. 2015;169(5):500–502.
- Healthcare Cost and Utilization Project. National Inpatient Sample. 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed October 11, 2014.
- , , , , . Direct admission to hospital: a mixed methods survey of pediatric practices, benefits, and challenges [published online August 17, 2015]. Acad Pediatr.
- , , . Changes in the source of unscheduled hospitalizations in the United States. Med Care. 2013;51(8):689–698.
- , , , . Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med. 2012;30(3):432–439.
- , , , , . Variation and outcomes associated with direct admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . Safety in numbers: physicians joining forces to seal the cracks during transitions. J Hosp Med. 2009;4(6):329–330.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , . The successes and challenges of hospital to home transitions. J Hosp Med. 2014;9(4):271–273.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.