User login
Patient satisfaction doesn’t equal better hospital care
What happens when you give children everything they ask for? They get spoiled, of course. Any parent can tell you that.
The problem is that you’re trying to raise children to (eventually) be responsible adults. Part of this is teaching them that you can’t always win, you should always share, and you can’t always get what you want.
Most kids don’t like it. (I know I didn’t.) They only see that the candy or toy they want is being refused and don’t grasp the long-term plan of growing up to be a decent person. Across a thousand human cultures, any parent would agree.
But the same principle doesn’t seem to apply in modern health care. What would you think is more important in a hospital: competent staff or having a beverage offered to you after being checked into the emergency department?
Sadly, things like the latter seem to be winning because of the recent emphasis on patient satisfaction scores. In today’s world, 30% of a hospital’s Medicare reimbursement is based on these scores. That’s a lot of money.
Unfortunately, quality of care doesn’t necessarily have the same meaning between doctors and patients. The former will say it means you left the hospital with a good outcome. The latter will agree but also will throw in things like whether they got enough pain meds or their call light answered fast enough. If you’re having chest pain or severe dyspnea, getting that call light answered quickly is pretty important. But if all you want is a soda or for someone to hand you the TV remote … not so much.
The problem is that the patient satisfaction surveys (and yes, speed of call-light response is on there) don’t take that key point into account. What might make some patients happy isn’t necessarily in their best interest. The post-CABG patient who wants a double cheeseburger won’t be thrilled if he gets a salad instead. Another patient in for detox won’t be pleased if she doesn’t get Dilaudid on demand. A third will be angry that he’s not allowed to smoke. Those refusals are an integral part of their successful treatment and recovery plan, but they may not see it that way. And they’ll be sure to mark it on the survey.
As a result, the hospital gets penalized in spite of the fact that they’re doing their best to provide quality care. And the business-minded CEOs, who generally have no medical background, only care about this part of it.
Measuring what counts is important. But the idea that hospital care should be held to the same standards as Burger King and Walmart is fundamentally flawed. The things that are done in hospitals – cut people open, draw blood, biopsy bone marrow, put in endotracheal and feeding tubes – aren’t intended as recreational experiences. We try to make them as painless as possible, but in health care “do no harm” often means doing some harm in order to prevent a catastrophe.
The side effects of chemotherapy are (hopefully) offset by the successful treatment of cancer. But that doesn’t mean hair loss, nausea, vomiting, diarrhea, and other toxic symptoms are part of “customer satisfaction.” One study even found that the most satisfied patients had the highest mortality.
We owe patients the very best care we can give them, but they also need to understand that “best care” doesn’t always mean what they want in the short term. We’re focused on a goal that’s beyond the immediate horizon.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What happens when you give children everything they ask for? They get spoiled, of course. Any parent can tell you that.
The problem is that you’re trying to raise children to (eventually) be responsible adults. Part of this is teaching them that you can’t always win, you should always share, and you can’t always get what you want.
Most kids don’t like it. (I know I didn’t.) They only see that the candy or toy they want is being refused and don’t grasp the long-term plan of growing up to be a decent person. Across a thousand human cultures, any parent would agree.
But the same principle doesn’t seem to apply in modern health care. What would you think is more important in a hospital: competent staff or having a beverage offered to you after being checked into the emergency department?
Sadly, things like the latter seem to be winning because of the recent emphasis on patient satisfaction scores. In today’s world, 30% of a hospital’s Medicare reimbursement is based on these scores. That’s a lot of money.
Unfortunately, quality of care doesn’t necessarily have the same meaning between doctors and patients. The former will say it means you left the hospital with a good outcome. The latter will agree but also will throw in things like whether they got enough pain meds or their call light answered fast enough. If you’re having chest pain or severe dyspnea, getting that call light answered quickly is pretty important. But if all you want is a soda or for someone to hand you the TV remote … not so much.
The problem is that the patient satisfaction surveys (and yes, speed of call-light response is on there) don’t take that key point into account. What might make some patients happy isn’t necessarily in their best interest. The post-CABG patient who wants a double cheeseburger won’t be thrilled if he gets a salad instead. Another patient in for detox won’t be pleased if she doesn’t get Dilaudid on demand. A third will be angry that he’s not allowed to smoke. Those refusals are an integral part of their successful treatment and recovery plan, but they may not see it that way. And they’ll be sure to mark it on the survey.
As a result, the hospital gets penalized in spite of the fact that they’re doing their best to provide quality care. And the business-minded CEOs, who generally have no medical background, only care about this part of it.
Measuring what counts is important. But the idea that hospital care should be held to the same standards as Burger King and Walmart is fundamentally flawed. The things that are done in hospitals – cut people open, draw blood, biopsy bone marrow, put in endotracheal and feeding tubes – aren’t intended as recreational experiences. We try to make them as painless as possible, but in health care “do no harm” often means doing some harm in order to prevent a catastrophe.
The side effects of chemotherapy are (hopefully) offset by the successful treatment of cancer. But that doesn’t mean hair loss, nausea, vomiting, diarrhea, and other toxic symptoms are part of “customer satisfaction.” One study even found that the most satisfied patients had the highest mortality.
We owe patients the very best care we can give them, but they also need to understand that “best care” doesn’t always mean what they want in the short term. We’re focused on a goal that’s beyond the immediate horizon.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What happens when you give children everything they ask for? They get spoiled, of course. Any parent can tell you that.
The problem is that you’re trying to raise children to (eventually) be responsible adults. Part of this is teaching them that you can’t always win, you should always share, and you can’t always get what you want.
Most kids don’t like it. (I know I didn’t.) They only see that the candy or toy they want is being refused and don’t grasp the long-term plan of growing up to be a decent person. Across a thousand human cultures, any parent would agree.
But the same principle doesn’t seem to apply in modern health care. What would you think is more important in a hospital: competent staff or having a beverage offered to you after being checked into the emergency department?
Sadly, things like the latter seem to be winning because of the recent emphasis on patient satisfaction scores. In today’s world, 30% of a hospital’s Medicare reimbursement is based on these scores. That’s a lot of money.
Unfortunately, quality of care doesn’t necessarily have the same meaning between doctors and patients. The former will say it means you left the hospital with a good outcome. The latter will agree but also will throw in things like whether they got enough pain meds or their call light answered fast enough. If you’re having chest pain or severe dyspnea, getting that call light answered quickly is pretty important. But if all you want is a soda or for someone to hand you the TV remote … not so much.
The problem is that the patient satisfaction surveys (and yes, speed of call-light response is on there) don’t take that key point into account. What might make some patients happy isn’t necessarily in their best interest. The post-CABG patient who wants a double cheeseburger won’t be thrilled if he gets a salad instead. Another patient in for detox won’t be pleased if she doesn’t get Dilaudid on demand. A third will be angry that he’s not allowed to smoke. Those refusals are an integral part of their successful treatment and recovery plan, but they may not see it that way. And they’ll be sure to mark it on the survey.
As a result, the hospital gets penalized in spite of the fact that they’re doing their best to provide quality care. And the business-minded CEOs, who generally have no medical background, only care about this part of it.
Measuring what counts is important. But the idea that hospital care should be held to the same standards as Burger King and Walmart is fundamentally flawed. The things that are done in hospitals – cut people open, draw blood, biopsy bone marrow, put in endotracheal and feeding tubes – aren’t intended as recreational experiences. We try to make them as painless as possible, but in health care “do no harm” often means doing some harm in order to prevent a catastrophe.
The side effects of chemotherapy are (hopefully) offset by the successful treatment of cancer. But that doesn’t mean hair loss, nausea, vomiting, diarrhea, and other toxic symptoms are part of “customer satisfaction.” One study even found that the most satisfied patients had the highest mortality.
We owe patients the very best care we can give them, but they also need to understand that “best care” doesn’t always mean what they want in the short term. We’re focused on a goal that’s beyond the immediate horizon.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Improving targeted therapy for leukemia, other diseases

Photo by Sam Ogden
A chemical strategy may allow researchers to target “undruggable” proteins and overcome resistance to current targeted therapies, according to a report published in Science.
The strategy uses tumor cells’ own protein-elimination system to break down and dispose of the proteins that drive cancer growth.
When tested in vitro and in vivo, the approach caused leukemia cells to die more quickly than they do with conventional targeted
therapies.
“One of the reasons [treatment] resistance occurs is that cancer-related proteins often have multiple functions within the cell, and conventional targeted therapies inhibit just one or a few of those functions,” said study author James Bradner, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Conventional drugs allow the targeted protein to adapt to the drug, and the cell finds alternate routes for its growth signals. We began designing approaches that cause the target protein to disintegrate, rather than merely be inhibited. It would be very powerful if we could chemically convert an inhibitor drug into a degrader drug.”
With this in mind, Dr Bradner’s team designed a chemical adapter that attaches to a targeted drug molecule. The adapter enables the drug to tow the cell’s protein-degradation machinery directly to the protein of interest. Once bound to the protein, the combination drug-and-protein-degrader essentially demolishes it.
The investigators tested the technology in leukemia cells. They built an adapter out of phthalimide, a chemical derivative of the drug thalidomide, and attached it to the BRD4 inhibitor JQ1. The phthalimide was designed to “hijack” the cereblon E3 ubiquitin ligase complex.
When the researchers treated the leukemia cells with a JQ1-phthalimide conjugate called dBET1, the BRD4 protein within the cells was degraded in less than an hour. The team said such rapid and extensive degradation suggests conjugates may be able to prevent or hinder cancer cells from developing resistance to targeted therapies.
“The potency, selectivity, and rapidity of this approach—namely, the ability to home in specifically on BRD4—are unprecedented in clinical approaches to protein degradation,” Dr Bradner said.
To determine how selective dBET1 actually is, the investigators measured the levels of all proteins in leukemia cells at 1 hour and 2 hours after treatment.
“We were stunned to find that only 3 proteins of more than 7000 in the entire cell were degraded: BRD2, 3, and 4, an exceptional degree of selectivity guided by the intended targets of JQ1,” Dr Bradner said. “It’s as though dBET1 is laser-guided to deliver protein-degrading machinery to targeted proteins.”
The researchers then tested dBET1 in mice bearing leukemia. As in the cell samples, there was a rapid degradation of BRD4 in the tumor cells and a potent anti-leukemic effect, with few noticeable side effects.
To see if compounds other than JQ1 can be used as a guidance system for a conjugate, the investigators created a set of molecules that lock the protein-degradation machinery onto a compound called SLF, which targets the protein FKBP12.
When they treated cancer cells with SLF, the team found it degraded the vast majority of FKBP12 in the cells within a few hours.
Buoyed by these results, the researchers are working to create a derivative of dBET1 that can be used as a drug in humans and to extend the conjugate strategy for the treatment of other diseases.
“The dBET1 and the dFKBP12 compounds are presently in a late stage of lead optimization for therapeutic development in both cancer and non-malignant diseases,” said Prem Das, PhD, chief research business development officer at Dana-Farber.
“Composition-of-matter and method-of-use patent applications have been filed on these and other additional targeted agents, as well as on the chemistry platform. They will be licensed for commercialization to an appropriate company according to standard Dana-Farber practice.” ![]()

Photo by Sam Ogden
A chemical strategy may allow researchers to target “undruggable” proteins and overcome resistance to current targeted therapies, according to a report published in Science.
The strategy uses tumor cells’ own protein-elimination system to break down and dispose of the proteins that drive cancer growth.
When tested in vitro and in vivo, the approach caused leukemia cells to die more quickly than they do with conventional targeted
therapies.
“One of the reasons [treatment] resistance occurs is that cancer-related proteins often have multiple functions within the cell, and conventional targeted therapies inhibit just one or a few of those functions,” said study author James Bradner, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Conventional drugs allow the targeted protein to adapt to the drug, and the cell finds alternate routes for its growth signals. We began designing approaches that cause the target protein to disintegrate, rather than merely be inhibited. It would be very powerful if we could chemically convert an inhibitor drug into a degrader drug.”
With this in mind, Dr Bradner’s team designed a chemical adapter that attaches to a targeted drug molecule. The adapter enables the drug to tow the cell’s protein-degradation machinery directly to the protein of interest. Once bound to the protein, the combination drug-and-protein-degrader essentially demolishes it.
The investigators tested the technology in leukemia cells. They built an adapter out of phthalimide, a chemical derivative of the drug thalidomide, and attached it to the BRD4 inhibitor JQ1. The phthalimide was designed to “hijack” the cereblon E3 ubiquitin ligase complex.
When the researchers treated the leukemia cells with a JQ1-phthalimide conjugate called dBET1, the BRD4 protein within the cells was degraded in less than an hour. The team said such rapid and extensive degradation suggests conjugates may be able to prevent or hinder cancer cells from developing resistance to targeted therapies.
“The potency, selectivity, and rapidity of this approach—namely, the ability to home in specifically on BRD4—are unprecedented in clinical approaches to protein degradation,” Dr Bradner said.
To determine how selective dBET1 actually is, the investigators measured the levels of all proteins in leukemia cells at 1 hour and 2 hours after treatment.
“We were stunned to find that only 3 proteins of more than 7000 in the entire cell were degraded: BRD2, 3, and 4, an exceptional degree of selectivity guided by the intended targets of JQ1,” Dr Bradner said. “It’s as though dBET1 is laser-guided to deliver protein-degrading machinery to targeted proteins.”
The researchers then tested dBET1 in mice bearing leukemia. As in the cell samples, there was a rapid degradation of BRD4 in the tumor cells and a potent anti-leukemic effect, with few noticeable side effects.
To see if compounds other than JQ1 can be used as a guidance system for a conjugate, the investigators created a set of molecules that lock the protein-degradation machinery onto a compound called SLF, which targets the protein FKBP12.
When they treated cancer cells with SLF, the team found it degraded the vast majority of FKBP12 in the cells within a few hours.
Buoyed by these results, the researchers are working to create a derivative of dBET1 that can be used as a drug in humans and to extend the conjugate strategy for the treatment of other diseases.
“The dBET1 and the dFKBP12 compounds are presently in a late stage of lead optimization for therapeutic development in both cancer and non-malignant diseases,” said Prem Das, PhD, chief research business development officer at Dana-Farber.
“Composition-of-matter and method-of-use patent applications have been filed on these and other additional targeted agents, as well as on the chemistry platform. They will be licensed for commercialization to an appropriate company according to standard Dana-Farber practice.” ![]()

Photo by Sam Ogden
A chemical strategy may allow researchers to target “undruggable” proteins and overcome resistance to current targeted therapies, according to a report published in Science.
The strategy uses tumor cells’ own protein-elimination system to break down and dispose of the proteins that drive cancer growth.
When tested in vitro and in vivo, the approach caused leukemia cells to die more quickly than they do with conventional targeted
therapies.
“One of the reasons [treatment] resistance occurs is that cancer-related proteins often have multiple functions within the cell, and conventional targeted therapies inhibit just one or a few of those functions,” said study author James Bradner, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“Conventional drugs allow the targeted protein to adapt to the drug, and the cell finds alternate routes for its growth signals. We began designing approaches that cause the target protein to disintegrate, rather than merely be inhibited. It would be very powerful if we could chemically convert an inhibitor drug into a degrader drug.”
With this in mind, Dr Bradner’s team designed a chemical adapter that attaches to a targeted drug molecule. The adapter enables the drug to tow the cell’s protein-degradation machinery directly to the protein of interest. Once bound to the protein, the combination drug-and-protein-degrader essentially demolishes it.
The investigators tested the technology in leukemia cells. They built an adapter out of phthalimide, a chemical derivative of the drug thalidomide, and attached it to the BRD4 inhibitor JQ1. The phthalimide was designed to “hijack” the cereblon E3 ubiquitin ligase complex.
When the researchers treated the leukemia cells with a JQ1-phthalimide conjugate called dBET1, the BRD4 protein within the cells was degraded in less than an hour. The team said such rapid and extensive degradation suggests conjugates may be able to prevent or hinder cancer cells from developing resistance to targeted therapies.
“The potency, selectivity, and rapidity of this approach—namely, the ability to home in specifically on BRD4—are unprecedented in clinical approaches to protein degradation,” Dr Bradner said.
To determine how selective dBET1 actually is, the investigators measured the levels of all proteins in leukemia cells at 1 hour and 2 hours after treatment.
“We were stunned to find that only 3 proteins of more than 7000 in the entire cell were degraded: BRD2, 3, and 4, an exceptional degree of selectivity guided by the intended targets of JQ1,” Dr Bradner said. “It’s as though dBET1 is laser-guided to deliver protein-degrading machinery to targeted proteins.”
The researchers then tested dBET1 in mice bearing leukemia. As in the cell samples, there was a rapid degradation of BRD4 in the tumor cells and a potent anti-leukemic effect, with few noticeable side effects.
To see if compounds other than JQ1 can be used as a guidance system for a conjugate, the investigators created a set of molecules that lock the protein-degradation machinery onto a compound called SLF, which targets the protein FKBP12.
When they treated cancer cells with SLF, the team found it degraded the vast majority of FKBP12 in the cells within a few hours.
Buoyed by these results, the researchers are working to create a derivative of dBET1 that can be used as a drug in humans and to extend the conjugate strategy for the treatment of other diseases.
“The dBET1 and the dFKBP12 compounds are presently in a late stage of lead optimization for therapeutic development in both cancer and non-malignant diseases,” said Prem Das, PhD, chief research business development officer at Dana-Farber.
“Composition-of-matter and method-of-use patent applications have been filed on these and other additional targeted agents, as well as on the chemistry platform. They will be licensed for commercialization to an appropriate company according to standard Dana-Farber practice.” ![]()
Anticoagulant type doesn’t affect stent thrombosis risk

PARIS—New research suggests that patients who have undergone primary percutaneous coronary intervention (PCI) have a low risk of stent thrombosis, regardless of the anticoagulant therapy they receive.
In a large, registry-based study, stent thrombosis occurred in less than 1% of patients, regardless of whether they received bivalirudin with or without heparin, heparin alone, or a GP IIb/IIIa inhibitor (GPI) with or without heparin.
The study also showed that patients who experienced stent thrombosis between days 2 and 30, regardless of drug regimen, were more likely to die within a year than patients who developed stent thrombosis within the first 24 hours of their procedure.
Per Grimfjard, of Vasteras Hospital/Uppsala University in Sweden, presented these findings at EuroPCR 2015.
A number of recent studies have raised concerns that bivalirudin may increase the risk of stent thrombosis compared with heparin. But rates of stent thrombosis have differed substantially between studies.
So Dr Grimfjard and his colleagues decided to review stent thrombosis rates by drug choice among more than 30,000 patients who were treated with primary PCI for ST-elevation myocardial infarction (STEMI) between January 2007 and July 2014 in the Swedish Coronary Angiography and Angioplasty Register (SCAAR).
The researchers divided patients into 3 treatment groups: bivalirudin, heparin, and GPI. However, 77% of patients in the bivalirudin group also received heparin, and 3.6% received a GPI prior to or during the PCI procedure. In the GPI group, 77% of patients also received heparin.
The rates of stent thrombosis were low in all 3 groups—0.84% in the bivalirudin group, 0.94% in the heparin group, and 0.83% in the GPI group.
For all 3 drugs, mortality at 1 year was numerically higher if the stent thrombosis occurred between 2 and 30 days, as compared with day 0 to 1 post-PCI.
“[A] possible explanation is that a stent thrombosis that happens once the patient has left the hospital is likely to cause a more substantial infarction, the reason being longer delay from symptoms to revascularization,” Dr Grimfjard said.
He added that a more substantial myocardial infarction typically leads to more heart failure and arrhythmia long-term. Unfortunately, the findings regarding the timing of stent thrombosis do not offer any guidance for choosing optimal antithrombotic treatment.
He and his colleagues are currently enrolling patients in a 6000-patient, registry-based, randomized clinical trial called SWEDEHART-Validate. The team will compare heparin alone to bivalirudin and optional low-dose heparin in STEMI and non-STEMI patients undergoing PCI.
“Hopefully, this large, randomized trial will bring clarity to the choice of antithrombotic treatment strategy in these patients,” Dr Grimfjard said. ![]()

PARIS—New research suggests that patients who have undergone primary percutaneous coronary intervention (PCI) have a low risk of stent thrombosis, regardless of the anticoagulant therapy they receive.
In a large, registry-based study, stent thrombosis occurred in less than 1% of patients, regardless of whether they received bivalirudin with or without heparin, heparin alone, or a GP IIb/IIIa inhibitor (GPI) with or without heparin.
The study also showed that patients who experienced stent thrombosis between days 2 and 30, regardless of drug regimen, were more likely to die within a year than patients who developed stent thrombosis within the first 24 hours of their procedure.
Per Grimfjard, of Vasteras Hospital/Uppsala University in Sweden, presented these findings at EuroPCR 2015.
A number of recent studies have raised concerns that bivalirudin may increase the risk of stent thrombosis compared with heparin. But rates of stent thrombosis have differed substantially between studies.
So Dr Grimfjard and his colleagues decided to review stent thrombosis rates by drug choice among more than 30,000 patients who were treated with primary PCI for ST-elevation myocardial infarction (STEMI) between January 2007 and July 2014 in the Swedish Coronary Angiography and Angioplasty Register (SCAAR).
The researchers divided patients into 3 treatment groups: bivalirudin, heparin, and GPI. However, 77% of patients in the bivalirudin group also received heparin, and 3.6% received a GPI prior to or during the PCI procedure. In the GPI group, 77% of patients also received heparin.
The rates of stent thrombosis were low in all 3 groups—0.84% in the bivalirudin group, 0.94% in the heparin group, and 0.83% in the GPI group.
For all 3 drugs, mortality at 1 year was numerically higher if the stent thrombosis occurred between 2 and 30 days, as compared with day 0 to 1 post-PCI.
“[A] possible explanation is that a stent thrombosis that happens once the patient has left the hospital is likely to cause a more substantial infarction, the reason being longer delay from symptoms to revascularization,” Dr Grimfjard said.
He added that a more substantial myocardial infarction typically leads to more heart failure and arrhythmia long-term. Unfortunately, the findings regarding the timing of stent thrombosis do not offer any guidance for choosing optimal antithrombotic treatment.
He and his colleagues are currently enrolling patients in a 6000-patient, registry-based, randomized clinical trial called SWEDEHART-Validate. The team will compare heparin alone to bivalirudin and optional low-dose heparin in STEMI and non-STEMI patients undergoing PCI.
“Hopefully, this large, randomized trial will bring clarity to the choice of antithrombotic treatment strategy in these patients,” Dr Grimfjard said. ![]()

PARIS—New research suggests that patients who have undergone primary percutaneous coronary intervention (PCI) have a low risk of stent thrombosis, regardless of the anticoagulant therapy they receive.
In a large, registry-based study, stent thrombosis occurred in less than 1% of patients, regardless of whether they received bivalirudin with or without heparin, heparin alone, or a GP IIb/IIIa inhibitor (GPI) with or without heparin.
The study also showed that patients who experienced stent thrombosis between days 2 and 30, regardless of drug regimen, were more likely to die within a year than patients who developed stent thrombosis within the first 24 hours of their procedure.
Per Grimfjard, of Vasteras Hospital/Uppsala University in Sweden, presented these findings at EuroPCR 2015.
A number of recent studies have raised concerns that bivalirudin may increase the risk of stent thrombosis compared with heparin. But rates of stent thrombosis have differed substantially between studies.
So Dr Grimfjard and his colleagues decided to review stent thrombosis rates by drug choice among more than 30,000 patients who were treated with primary PCI for ST-elevation myocardial infarction (STEMI) between January 2007 and July 2014 in the Swedish Coronary Angiography and Angioplasty Register (SCAAR).
The researchers divided patients into 3 treatment groups: bivalirudin, heparin, and GPI. However, 77% of patients in the bivalirudin group also received heparin, and 3.6% received a GPI prior to or during the PCI procedure. In the GPI group, 77% of patients also received heparin.
The rates of stent thrombosis were low in all 3 groups—0.84% in the bivalirudin group, 0.94% in the heparin group, and 0.83% in the GPI group.
For all 3 drugs, mortality at 1 year was numerically higher if the stent thrombosis occurred between 2 and 30 days, as compared with day 0 to 1 post-PCI.
“[A] possible explanation is that a stent thrombosis that happens once the patient has left the hospital is likely to cause a more substantial infarction, the reason being longer delay from symptoms to revascularization,” Dr Grimfjard said.
He added that a more substantial myocardial infarction typically leads to more heart failure and arrhythmia long-term. Unfortunately, the findings regarding the timing of stent thrombosis do not offer any guidance for choosing optimal antithrombotic treatment.
He and his colleagues are currently enrolling patients in a 6000-patient, registry-based, randomized clinical trial called SWEDEHART-Validate. The team will compare heparin alone to bivalirudin and optional low-dose heparin in STEMI and non-STEMI patients undergoing PCI.
“Hopefully, this large, randomized trial will bring clarity to the choice of antithrombotic treatment strategy in these patients,” Dr Grimfjard said. ![]()
Team reports new method to identify immune cells

Photo by Graham Colm
A new method for identifying immune cells could pave the way for rapid detection of hematologic malignancies from a small blood sample, according to researchers.
The team found they could use wavelength modulated Raman spectroscopy (WMRS) to identify subsets of T cells, natural killer cells, and dendritic cells.
Traditional methods of identifying these cells usually involve labeling them with fluorescent or magnetically labeled antibodies.
Using WMRS, the researchers were able to identify immune cells with no labeling at all, thus permitting rapid identification and further analysis to take place with no potential alteration to the cells.
Simon Powis, PhD, of the University of St Andrews in Fife, Scotland, and his colleagues described this work in PLOS ONE.
Raman scattering refers to light scattering from molecules in a sample where the light energy can be shifted up or down and recorded as a “molecular fingerprint” that can be used for identification. Normally, this process is very weak and further hampered by other background light (eg, fluorescence).
WMRS subtly changes the incident laser light that, in turn, results in a modulation of the Raman signal, allowing it to be extracted from any (stationary) interfering signal.
Using WMRS, Dr Powis and his colleagues found they could identify CD4+ T cells, CD8+ T cells, CD56+ natural killer cells, CD303+ lymphoid/plasmacytoid dendritic cells, and CD1c+ myeloid dendritic cells.
“Under a normal light microscope, these immune cells essentially all look identical,” Dr Powis said. “With this new method, we can identify key cell types without any labeling.”
“Our next goal is to make a full catalogue of all the normal cell types of the immune system that can be detected in the bloodstream. Once we have this completed, we can then collaborate with our clinical colleagues to start identifying when these immune cells are altered, in conditions such as leukemia and lymphoma, potentially providing a rapid detection system from just a small blood sample.” ![]()

Photo by Graham Colm
A new method for identifying immune cells could pave the way for rapid detection of hematologic malignancies from a small blood sample, according to researchers.
The team found they could use wavelength modulated Raman spectroscopy (WMRS) to identify subsets of T cells, natural killer cells, and dendritic cells.
Traditional methods of identifying these cells usually involve labeling them with fluorescent or magnetically labeled antibodies.
Using WMRS, the researchers were able to identify immune cells with no labeling at all, thus permitting rapid identification and further analysis to take place with no potential alteration to the cells.
Simon Powis, PhD, of the University of St Andrews in Fife, Scotland, and his colleagues described this work in PLOS ONE.
Raman scattering refers to light scattering from molecules in a sample where the light energy can be shifted up or down and recorded as a “molecular fingerprint” that can be used for identification. Normally, this process is very weak and further hampered by other background light (eg, fluorescence).
WMRS subtly changes the incident laser light that, in turn, results in a modulation of the Raman signal, allowing it to be extracted from any (stationary) interfering signal.
Using WMRS, Dr Powis and his colleagues found they could identify CD4+ T cells, CD8+ T cells, CD56+ natural killer cells, CD303+ lymphoid/plasmacytoid dendritic cells, and CD1c+ myeloid dendritic cells.
“Under a normal light microscope, these immune cells essentially all look identical,” Dr Powis said. “With this new method, we can identify key cell types without any labeling.”
“Our next goal is to make a full catalogue of all the normal cell types of the immune system that can be detected in the bloodstream. Once we have this completed, we can then collaborate with our clinical colleagues to start identifying when these immune cells are altered, in conditions such as leukemia and lymphoma, potentially providing a rapid detection system from just a small blood sample.” ![]()

Photo by Graham Colm
A new method for identifying immune cells could pave the way for rapid detection of hematologic malignancies from a small blood sample, according to researchers.
The team found they could use wavelength modulated Raman spectroscopy (WMRS) to identify subsets of T cells, natural killer cells, and dendritic cells.
Traditional methods of identifying these cells usually involve labeling them with fluorescent or magnetically labeled antibodies.
Using WMRS, the researchers were able to identify immune cells with no labeling at all, thus permitting rapid identification and further analysis to take place with no potential alteration to the cells.
Simon Powis, PhD, of the University of St Andrews in Fife, Scotland, and his colleagues described this work in PLOS ONE.
Raman scattering refers to light scattering from molecules in a sample where the light energy can be shifted up or down and recorded as a “molecular fingerprint” that can be used for identification. Normally, this process is very weak and further hampered by other background light (eg, fluorescence).
WMRS subtly changes the incident laser light that, in turn, results in a modulation of the Raman signal, allowing it to be extracted from any (stationary) interfering signal.
Using WMRS, Dr Powis and his colleagues found they could identify CD4+ T cells, CD8+ T cells, CD56+ natural killer cells, CD303+ lymphoid/plasmacytoid dendritic cells, and CD1c+ myeloid dendritic cells.
“Under a normal light microscope, these immune cells essentially all look identical,” Dr Powis said. “With this new method, we can identify key cell types without any labeling.”
“Our next goal is to make a full catalogue of all the normal cell types of the immune system that can be detected in the bloodstream. Once we have this completed, we can then collaborate with our clinical colleagues to start identifying when these immune cells are altered, in conditions such as leukemia and lymphoma, potentially providing a rapid detection system from just a small blood sample.” ![]()
Co-infection may boost malaria mortality

Co-infection with malaria and a virus closely related to the Epstein-Barr virus (EBV) may make the malaria lethal, according to preclinical research published in PLOS Pathogens.
Children in sub-Saharan Africa become infected with EBV in infancy.
Within the same time period, they become susceptible to malaria parasite infection because protective antibodies from their mothers fade away.
“Where we think kids get into trouble is when both infections are happening at the same time, because case reports show EBV can produce a weeks-long suppression of the immune system,” said Tracey Lamb, PhD, of Emory University School of Medicine in Atlanta, Georgia.
Dr Lamb and her colleagues studied mice infected by the malaria parasite Plasmodium yoelii, which is usually non-lethal because the mice develop antibodies that control the parasites.
The researchers found that co-infection with murine gammaherpesvirus 68 (MHV68), a close relative of EBV that infects mice, made P yoelii lethal.
However, mice that had entered the chronic phase of MHV68 infection (several weeks to months after primary infection) were not affected.
The experiments indicated that MHV68 infection hinders the immune system in developing antibodies against P yoelii.
“These results are part of a pattern of evidence suggesting that clinicians treating severe malaria should check for acute EBV co-infection, and that ongoing malaria studies should include EBV as a potential risk factor for more severe forms of the disease,” said Caline Matar, a graduate student at Emory University School of Medicine.
“This phenomenon may not be unique to EBV,” added Sam Speck, PhD, also of Emory University School of Medicine.
“[I]nfections with other pathogens may also exacerbate malarial disease, since many pathogens have the capacity to suppress various components of the host immune response.” ![]()

Co-infection with malaria and a virus closely related to the Epstein-Barr virus (EBV) may make the malaria lethal, according to preclinical research published in PLOS Pathogens.
Children in sub-Saharan Africa become infected with EBV in infancy.
Within the same time period, they become susceptible to malaria parasite infection because protective antibodies from their mothers fade away.
“Where we think kids get into trouble is when both infections are happening at the same time, because case reports show EBV can produce a weeks-long suppression of the immune system,” said Tracey Lamb, PhD, of Emory University School of Medicine in Atlanta, Georgia.
Dr Lamb and her colleagues studied mice infected by the malaria parasite Plasmodium yoelii, which is usually non-lethal because the mice develop antibodies that control the parasites.
The researchers found that co-infection with murine gammaherpesvirus 68 (MHV68), a close relative of EBV that infects mice, made P yoelii lethal.
However, mice that had entered the chronic phase of MHV68 infection (several weeks to months after primary infection) were not affected.
The experiments indicated that MHV68 infection hinders the immune system in developing antibodies against P yoelii.
“These results are part of a pattern of evidence suggesting that clinicians treating severe malaria should check for acute EBV co-infection, and that ongoing malaria studies should include EBV as a potential risk factor for more severe forms of the disease,” said Caline Matar, a graduate student at Emory University School of Medicine.
“This phenomenon may not be unique to EBV,” added Sam Speck, PhD, also of Emory University School of Medicine.
“[I]nfections with other pathogens may also exacerbate malarial disease, since many pathogens have the capacity to suppress various components of the host immune response.” ![]()

Co-infection with malaria and a virus closely related to the Epstein-Barr virus (EBV) may make the malaria lethal, according to preclinical research published in PLOS Pathogens.
Children in sub-Saharan Africa become infected with EBV in infancy.
Within the same time period, they become susceptible to malaria parasite infection because protective antibodies from their mothers fade away.
“Where we think kids get into trouble is when both infections are happening at the same time, because case reports show EBV can produce a weeks-long suppression of the immune system,” said Tracey Lamb, PhD, of Emory University School of Medicine in Atlanta, Georgia.
Dr Lamb and her colleagues studied mice infected by the malaria parasite Plasmodium yoelii, which is usually non-lethal because the mice develop antibodies that control the parasites.
The researchers found that co-infection with murine gammaherpesvirus 68 (MHV68), a close relative of EBV that infects mice, made P yoelii lethal.
However, mice that had entered the chronic phase of MHV68 infection (several weeks to months after primary infection) were not affected.
The experiments indicated that MHV68 infection hinders the immune system in developing antibodies against P yoelii.
“These results are part of a pattern of evidence suggesting that clinicians treating severe malaria should check for acute EBV co-infection, and that ongoing malaria studies should include EBV as a potential risk factor for more severe forms of the disease,” said Caline Matar, a graduate student at Emory University School of Medicine.
“This phenomenon may not be unique to EBV,” added Sam Speck, PhD, also of Emory University School of Medicine.
“[I]nfections with other pathogens may also exacerbate malarial disease, since many pathogens have the capacity to suppress various components of the host immune response.” ![]()
Putting isthmocele into perspective
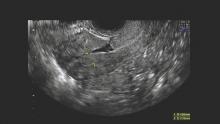
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
With the increase in cesarean sections worldwide, it is imperative that physicians properly inform their patients as to potential procedure risks. One potential postcesarean section problem that is receiving increasing attention is the isthmocele or niche.
Defined as an anechoic area in the cesarean section scar, it has been noted to occur in 24%-69% of women undergoing transvaginal sonography, and 56%-78% of women evaluated with transvaginal saline infused sonogram. While most cesarean section defects are asymptomatic, the isthmocele has been noted to be associated with abnormal uterine bleeding, including prolonged menstruation or postmenopausal spotting, and fertility concerns (BJOG. 2014;121:145-56).
Interestingly, it has been 40 years since Stewart, et al. first reported the relationship of abnormal uterine bleeding and cesarean section (Br. J. Gynaecol. 1975;82:682-6). Bloody fluid can be generated at the isthmocele site, which travels up the endometrial canal, thus impacting implantation. The niche can also be the site of ectopic pregnancy implantation.
In this edition of Master Class in gynecologic surgery, I have asked my newest partner, Dr. Kirsten Sasaki, to share our views on this increasingly important subject. Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston, where she was awarded the Outstanding Chief Resident Clinician Award. Dr. Sasaki then went on to become our second fellow in the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and SRS at Advocate Lutheran General Hospital, Park Ridge, Ill. Once again, Dr. Sasaki was singled out for her excellent teaching and research capabilities. Ultimately however, it was her tremendous surgical skills and surgical sense that led Dr. Aarathi Cholkeri-Singh and I to invite her into our practice.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and on the speakers bureau for Ethicon. He is also a consultant, on the speakers bureau, and has received grant and research support from Intuitive Surgical.
Diagnosis and treatment of uterine isthmocele
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
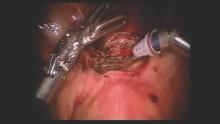
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
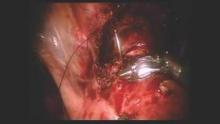
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
DXA screening: You’re doing it wrong
Dual-energy x-ray absorptiometry to screen for osteoporosis is both underused in women who meet the U.S. Preventive Services Task Force criteria for screening, and overused in low-risk younger women, new data suggest.
A retrospective, longitudinal cohort study of 50,995 women attending 13 primary care clinics found that among previously unscreened women who met the criteria for screening, the 7-year cumulative incidence of DXA screening ranged from 58.8% in women aged 60-64 years with at least one risk factor, to 42.7% in women aged 75 years and older.
But among women who didn’t meet screening criteria, the 7-year cumulative incidence was 45.5% in women aged 50-59 years and 58.6% in women aged 60-64 years without risk factors, according to a paper published May 19 in the Journal of General Internal Medicine (doi:10.1007/s11606-015-3349-8).
The U.S. Preventive Services Task Force currently recommends screening for osteoporosis with DXA for women aged 65 and older, as well as young women at increased risk for fracture. The American Academy of Family Physicians also advised against DXA screening in women younger than age 65 without osteoporosis risk factors as part of the “Choosing Wisely” campaign.
“Additional research is needed to elucidate patient, physician, and health system barriers to evidence-based screening so that interventions can maximize the value of population screening for osteoporosis,” wrote Dr. Anna Lee D. Amarnath of the University of California, Davis Health System, and her coauthors.
The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
Dual-energy x-ray absorptiometry to screen for osteoporosis is both underused in women who meet the U.S. Preventive Services Task Force criteria for screening, and overused in low-risk younger women, new data suggest.
A retrospective, longitudinal cohort study of 50,995 women attending 13 primary care clinics found that among previously unscreened women who met the criteria for screening, the 7-year cumulative incidence of DXA screening ranged from 58.8% in women aged 60-64 years with at least one risk factor, to 42.7% in women aged 75 years and older.
But among women who didn’t meet screening criteria, the 7-year cumulative incidence was 45.5% in women aged 50-59 years and 58.6% in women aged 60-64 years without risk factors, according to a paper published May 19 in the Journal of General Internal Medicine (doi:10.1007/s11606-015-3349-8).
The U.S. Preventive Services Task Force currently recommends screening for osteoporosis with DXA for women aged 65 and older, as well as young women at increased risk for fracture. The American Academy of Family Physicians also advised against DXA screening in women younger than age 65 without osteoporosis risk factors as part of the “Choosing Wisely” campaign.
“Additional research is needed to elucidate patient, physician, and health system barriers to evidence-based screening so that interventions can maximize the value of population screening for osteoporosis,” wrote Dr. Anna Lee D. Amarnath of the University of California, Davis Health System, and her coauthors.
The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
Dual-energy x-ray absorptiometry to screen for osteoporosis is both underused in women who meet the U.S. Preventive Services Task Force criteria for screening, and overused in low-risk younger women, new data suggest.
A retrospective, longitudinal cohort study of 50,995 women attending 13 primary care clinics found that among previously unscreened women who met the criteria for screening, the 7-year cumulative incidence of DXA screening ranged from 58.8% in women aged 60-64 years with at least one risk factor, to 42.7% in women aged 75 years and older.
But among women who didn’t meet screening criteria, the 7-year cumulative incidence was 45.5% in women aged 50-59 years and 58.6% in women aged 60-64 years without risk factors, according to a paper published May 19 in the Journal of General Internal Medicine (doi:10.1007/s11606-015-3349-8).
The U.S. Preventive Services Task Force currently recommends screening for osteoporosis with DXA for women aged 65 and older, as well as young women at increased risk for fracture. The American Academy of Family Physicians also advised against DXA screening in women younger than age 65 without osteoporosis risk factors as part of the “Choosing Wisely” campaign.
“Additional research is needed to elucidate patient, physician, and health system barriers to evidence-based screening so that interventions can maximize the value of population screening for osteoporosis,” wrote Dr. Anna Lee D. Amarnath of the University of California, Davis Health System, and her coauthors.
The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
FROM THE JOURNAL OF GENERAL INTERNAL MEDICINE
Key clinical point: DXA is underused in women who meet the U.S. Preventive Services Task Force criteria for osteoporosis screening and overused in women who do not.
Major finding: The 7-year cumulative incidence of DXA screening was virtually the same for women aged 60-64 years whether they met screening criteria (58.8%) or did not (58.6%).
Data source: A retrospective longitudinal cohort study of 50,995 women attending 13 primary care clinics.
Disclosures: The study was supported by the National Institutes of Health and the Agency for Healthcare Research and Quality. The researchers reported having no financial disclosures.
If It Didn’t Itch, She Wouldn’t Scratch!
After more than 20 years, this 60-year-old woman has had enough of her persistent leg condition, finally taking a friend’s advice to consult dermatology. When asked if she has ever scratched or rubbed the area, her response is, “Of course! It itches something awful! If it didn’t itch, I would leave it alone.”
She goes on to describe how she spends her work day fantasizing about getting home and scratching the spot. Sometimes she uses a hairbrush, sometimes a rag; but she always obtains exquisite, if temporary, pleasure from attacking the problem.
Some of her innumerable treatment attempts—with products topical and oral, OTC and prescription—have offered brief relief but no true resolution. Among the methods tried are moisturizers, antifungal creams and pills, and topical steroids.
The patient has long since forgotten precisely when or how the problem started. However, she denies having any other problems with her skin.
EXAMINATION
The condition is confined to the right anterior tibial area. The skin on the upper one-third of the leg is darkened and rough, with a leathery texture. No erythema is seen in or around the lesion, and no abnormalities are noted elsewhere.
What is the diagnosis?
DISCUSSION
This is an archetypical case of lichen simplex chronicus (LSC), previously known as neurodermatitis. LSC is, by definition, a secondary condition, starting with a bit of eczema, dry skin, or even a bug bite that draws the patient’s attention to the area.
For some, once the itching and scratching start, it becomes hard to stop, although the original insult is long gone. Scratched or rubbed long enough, the affected skin reacts by becoming thick and leathery. In patients with darker skin, postinflammatory hyperpigmentation will also be seen.
But the worst effect is that, in response to chronic scratching, the nerves in the affected area become more numerous and even more sensitive. It’s the classic “itch-scratch-itch” cycle that takes on a life of its own until broken. Even then, many patients resume scratching at some point, purely out of habit.
Effective treatment has three components: First, the patient has to apply a relatively strong topical steroid, usually twice a day, to reduce the itching. This paves the way for the second phase of treatment: cessation of scratching. Within a fairly short period of time, the skin will begin to lose its rough feel and hyperpigmentation and look more normal. Then the third part of successful treatment: The patient must stop looking for the cause and begin to understand his/her role in the perpetuation of the problem.
The differential for LSC includes psoriasis, eczema, lichen planus, and xerosis (dry skin). But the key features of LSC, illustrated nicely by this case, are: easily accessible area, chronicity (sometimes extreme), and lichenification and postinflammatory hyperpigmentation. This patient’s history of chronic excoriation of the site was an extremely helpful diagnostic clue.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is always secondary to another trigger, such as dry skin, eczema, bug bite, or minor skin injury that itches as it heals.
• LSC is characterized by localized thickening of the skin in an accessible area.
• Legs (especially the anterior tibial area) are common sites, but so is the nuchal scalp—particularly in women.
• LSC is treated with a potent topical steroid cream (eg, clobetasol 0.05%) and education of the patient as to his/her role in the creation and perpetuation of the problem.
After more than 20 years, this 60-year-old woman has had enough of her persistent leg condition, finally taking a friend’s advice to consult dermatology. When asked if she has ever scratched or rubbed the area, her response is, “Of course! It itches something awful! If it didn’t itch, I would leave it alone.”
She goes on to describe how she spends her work day fantasizing about getting home and scratching the spot. Sometimes she uses a hairbrush, sometimes a rag; but she always obtains exquisite, if temporary, pleasure from attacking the problem.
Some of her innumerable treatment attempts—with products topical and oral, OTC and prescription—have offered brief relief but no true resolution. Among the methods tried are moisturizers, antifungal creams and pills, and topical steroids.
The patient has long since forgotten precisely when or how the problem started. However, she denies having any other problems with her skin.
EXAMINATION
The condition is confined to the right anterior tibial area. The skin on the upper one-third of the leg is darkened and rough, with a leathery texture. No erythema is seen in or around the lesion, and no abnormalities are noted elsewhere.
What is the diagnosis?
DISCUSSION
This is an archetypical case of lichen simplex chronicus (LSC), previously known as neurodermatitis. LSC is, by definition, a secondary condition, starting with a bit of eczema, dry skin, or even a bug bite that draws the patient’s attention to the area.
For some, once the itching and scratching start, it becomes hard to stop, although the original insult is long gone. Scratched or rubbed long enough, the affected skin reacts by becoming thick and leathery. In patients with darker skin, postinflammatory hyperpigmentation will also be seen.
But the worst effect is that, in response to chronic scratching, the nerves in the affected area become more numerous and even more sensitive. It’s the classic “itch-scratch-itch” cycle that takes on a life of its own until broken. Even then, many patients resume scratching at some point, purely out of habit.
Effective treatment has three components: First, the patient has to apply a relatively strong topical steroid, usually twice a day, to reduce the itching. This paves the way for the second phase of treatment: cessation of scratching. Within a fairly short period of time, the skin will begin to lose its rough feel and hyperpigmentation and look more normal. Then the third part of successful treatment: The patient must stop looking for the cause and begin to understand his/her role in the perpetuation of the problem.
The differential for LSC includes psoriasis, eczema, lichen planus, and xerosis (dry skin). But the key features of LSC, illustrated nicely by this case, are: easily accessible area, chronicity (sometimes extreme), and lichenification and postinflammatory hyperpigmentation. This patient’s history of chronic excoriation of the site was an extremely helpful diagnostic clue.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is always secondary to another trigger, such as dry skin, eczema, bug bite, or minor skin injury that itches as it heals.
• LSC is characterized by localized thickening of the skin in an accessible area.
• Legs (especially the anterior tibial area) are common sites, but so is the nuchal scalp—particularly in women.
• LSC is treated with a potent topical steroid cream (eg, clobetasol 0.05%) and education of the patient as to his/her role in the creation and perpetuation of the problem.
After more than 20 years, this 60-year-old woman has had enough of her persistent leg condition, finally taking a friend’s advice to consult dermatology. When asked if she has ever scratched or rubbed the area, her response is, “Of course! It itches something awful! If it didn’t itch, I would leave it alone.”
She goes on to describe how she spends her work day fantasizing about getting home and scratching the spot. Sometimes she uses a hairbrush, sometimes a rag; but she always obtains exquisite, if temporary, pleasure from attacking the problem.
Some of her innumerable treatment attempts—with products topical and oral, OTC and prescription—have offered brief relief but no true resolution. Among the methods tried are moisturizers, antifungal creams and pills, and topical steroids.
The patient has long since forgotten precisely when or how the problem started. However, she denies having any other problems with her skin.
EXAMINATION
The condition is confined to the right anterior tibial area. The skin on the upper one-third of the leg is darkened and rough, with a leathery texture. No erythema is seen in or around the lesion, and no abnormalities are noted elsewhere.
What is the diagnosis?
DISCUSSION
This is an archetypical case of lichen simplex chronicus (LSC), previously known as neurodermatitis. LSC is, by definition, a secondary condition, starting with a bit of eczema, dry skin, or even a bug bite that draws the patient’s attention to the area.
For some, once the itching and scratching start, it becomes hard to stop, although the original insult is long gone. Scratched or rubbed long enough, the affected skin reacts by becoming thick and leathery. In patients with darker skin, postinflammatory hyperpigmentation will also be seen.
But the worst effect is that, in response to chronic scratching, the nerves in the affected area become more numerous and even more sensitive. It’s the classic “itch-scratch-itch” cycle that takes on a life of its own until broken. Even then, many patients resume scratching at some point, purely out of habit.
Effective treatment has three components: First, the patient has to apply a relatively strong topical steroid, usually twice a day, to reduce the itching. This paves the way for the second phase of treatment: cessation of scratching. Within a fairly short period of time, the skin will begin to lose its rough feel and hyperpigmentation and look more normal. Then the third part of successful treatment: The patient must stop looking for the cause and begin to understand his/her role in the perpetuation of the problem.
The differential for LSC includes psoriasis, eczema, lichen planus, and xerosis (dry skin). But the key features of LSC, illustrated nicely by this case, are: easily accessible area, chronicity (sometimes extreme), and lichenification and postinflammatory hyperpigmentation. This patient’s history of chronic excoriation of the site was an extremely helpful diagnostic clue.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is always secondary to another trigger, such as dry skin, eczema, bug bite, or minor skin injury that itches as it heals.
• LSC is characterized by localized thickening of the skin in an accessible area.
• Legs (especially the anterior tibial area) are common sites, but so is the nuchal scalp—particularly in women.
• LSC is treated with a potent topical steroid cream (eg, clobetasol 0.05%) and education of the patient as to his/her role in the creation and perpetuation of the problem.
IL-2 variant proves active against ATL, GVHD

Photo by Rhoda Baer
Researchers say they have created interleukin-2 (IL-2) variants that function as IL-2-receptor signaling “clamps” and allow for “fine tuning” of the signaling amplitude.
One variant, known as H9-RETR, was able to inhibit the actions of endogenous IL-2 and IL-15, prolong survival in a mouse model of graft-vs-host disease (GVHD), and inhibit the proliferation of cells derived from a patient with smoldering adult T-cell
leukemia (ATL).
The researchers reported these results in Immunity.
Warren J. Leonard, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Maryland, and his colleagues developed IL-2 variants in which activity can be tuned to either boost or block immune responses, depending on the desired therapeutic application.
The researchers said these variants had high affinity for IL-2Rβ and inhibited binding of endogenous IL-2, but their interaction with γc was weakened, thereby weakening IL-2Rβ-γc heterodimerization.
The team found that IL-2 signaling strength was inversely correlated with the degree of mutation at the γc interface. And differential effects on cell proliferation were dependent upon the cells’ state of activation.
One of the IL-2 variants, H9-RETR, inhibited IL-2- and IL-15-mediated proliferation and cytotoxicity. H9-RETR inhibited cytokine signaling and natural killer cell activity as well or better than blocking antibodies to IL-2Rα and IL-2Rβ.
In experiments with cells isolated from a patient with smoldering ATL, H9-RETR blocked IL-2 signaling and inhibited the spontaneous proliferation of ATL cells. In this regard, H9-RETR was at least as effective as the anti-IL-2Rα antibody daclizumab and much more effective than the anti-IL-2Rβ antibody Mikβ1.
In a mouse model of GVHD, animals that received a stabilized, Fc-fusion version of H9-RETR (H9-RETR-Fc4) had significantly longer survival than control mice (which received only Fc4 protein).
All of the control mice had died by 40 days post-injection, but it took 60 days for all of the H9-RETR-Fc4-treated mice to die (P=0.0001).
The researchers believe their receptor-clamping approach could potentially be used to engineer other immune-system cytokines with therapeutic potential. ![]()

Photo by Rhoda Baer
Researchers say they have created interleukin-2 (IL-2) variants that function as IL-2-receptor signaling “clamps” and allow for “fine tuning” of the signaling amplitude.
One variant, known as H9-RETR, was able to inhibit the actions of endogenous IL-2 and IL-15, prolong survival in a mouse model of graft-vs-host disease (GVHD), and inhibit the proliferation of cells derived from a patient with smoldering adult T-cell
leukemia (ATL).
The researchers reported these results in Immunity.
Warren J. Leonard, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Maryland, and his colleagues developed IL-2 variants in which activity can be tuned to either boost or block immune responses, depending on the desired therapeutic application.
The researchers said these variants had high affinity for IL-2Rβ and inhibited binding of endogenous IL-2, but their interaction with γc was weakened, thereby weakening IL-2Rβ-γc heterodimerization.
The team found that IL-2 signaling strength was inversely correlated with the degree of mutation at the γc interface. And differential effects on cell proliferation were dependent upon the cells’ state of activation.
One of the IL-2 variants, H9-RETR, inhibited IL-2- and IL-15-mediated proliferation and cytotoxicity. H9-RETR inhibited cytokine signaling and natural killer cell activity as well or better than blocking antibodies to IL-2Rα and IL-2Rβ.
In experiments with cells isolated from a patient with smoldering ATL, H9-RETR blocked IL-2 signaling and inhibited the spontaneous proliferation of ATL cells. In this regard, H9-RETR was at least as effective as the anti-IL-2Rα antibody daclizumab and much more effective than the anti-IL-2Rβ antibody Mikβ1.
In a mouse model of GVHD, animals that received a stabilized, Fc-fusion version of H9-RETR (H9-RETR-Fc4) had significantly longer survival than control mice (which received only Fc4 protein).
All of the control mice had died by 40 days post-injection, but it took 60 days for all of the H9-RETR-Fc4-treated mice to die (P=0.0001).
The researchers believe their receptor-clamping approach could potentially be used to engineer other immune-system cytokines with therapeutic potential. ![]()

Photo by Rhoda Baer
Researchers say they have created interleukin-2 (IL-2) variants that function as IL-2-receptor signaling “clamps” and allow for “fine tuning” of the signaling amplitude.
One variant, known as H9-RETR, was able to inhibit the actions of endogenous IL-2 and IL-15, prolong survival in a mouse model of graft-vs-host disease (GVHD), and inhibit the proliferation of cells derived from a patient with smoldering adult T-cell
leukemia (ATL).
The researchers reported these results in Immunity.
Warren J. Leonard, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Maryland, and his colleagues developed IL-2 variants in which activity can be tuned to either boost or block immune responses, depending on the desired therapeutic application.
The researchers said these variants had high affinity for IL-2Rβ and inhibited binding of endogenous IL-2, but their interaction with γc was weakened, thereby weakening IL-2Rβ-γc heterodimerization.
The team found that IL-2 signaling strength was inversely correlated with the degree of mutation at the γc interface. And differential effects on cell proliferation were dependent upon the cells’ state of activation.
One of the IL-2 variants, H9-RETR, inhibited IL-2- and IL-15-mediated proliferation and cytotoxicity. H9-RETR inhibited cytokine signaling and natural killer cell activity as well or better than blocking antibodies to IL-2Rα and IL-2Rβ.
In experiments with cells isolated from a patient with smoldering ATL, H9-RETR blocked IL-2 signaling and inhibited the spontaneous proliferation of ATL cells. In this regard, H9-RETR was at least as effective as the anti-IL-2Rα antibody daclizumab and much more effective than the anti-IL-2Rβ antibody Mikβ1.
In a mouse model of GVHD, animals that received a stabilized, Fc-fusion version of H9-RETR (H9-RETR-Fc4) had significantly longer survival than control mice (which received only Fc4 protein).
All of the control mice had died by 40 days post-injection, but it took 60 days for all of the H9-RETR-Fc4-treated mice to die (P=0.0001).
The researchers believe their receptor-clamping approach could potentially be used to engineer other immune-system cytokines with therapeutic potential. ![]()