User login
New insecticide targets malaria-carrying mosquito

Photo courtesy of the CDC
Recent progress in halting the spread of malaria has hinged, in part, on the use of insecticide-treated bed nets and spraying programs that target Anopheles gambiae mosquitoes.
Unfortunately, the mosquitoes are developing resistance to insecticides such as pyrethroid.
Wondering if they could defeat the mosquitoes by developing a new insecticide,a group of researchers set out to make blood meals toxic for Anopheles gambiae.
The team described their method in The Journal of Experimental Biology.
The researchers decided to target the mosquito glutamate gated chloride channel (AgGluCl), which is an essential component of the insect’s nervous system.
They generated antibodies that specifically targeted a portion of the protein exposed on the surface of nerves, a strategy they acknowledged was somewhat risky.
“Antibodies against a single mosquito antigen have never been shown to have mosquitocidal properties before, and the majority of previous research had focused on midgut antigens, while we were targeting a neuronal antigen expressed only in tissues found outside of the midgut,” said study author Jacob Meyers, a graduate student at Colorado State University in Fort Collins.
After injecting rabbits with a tiny portion of the surface of the AgGluCl protein channel, the researchers waited for the rabbits’ immune systems to begin producing antibodies tailored to the channel.
Then, the team collected the antibodies, mixed them with fresh blood, and fed the mixture to malaria-carrying mosquitoes (Anopheles gambiae), yellow fever-carrying mosquitoes (Aedes aegypti), and mosquitoes that carry the West Nile virus (Culex tarsalis).
Neither the yellow fever nor West Nile virus mosquitoes responded to the spiked blood. However, significant numbers of Anopheles gambiae mosquitoes died after consuming the blood/antibody cocktail. The highest antibody doses killed more than 90% of the insects within a day.
The researchers looked into why the yellow fever and West Nile virus mosquitoes had been immune to the antibodies and found the antibodies could not pass across the mosquitoes’ guts into the hemolymph. But the antibodies passed into the hemolymph of Anopheles gambiae mosquitoes with ease.
Intrigued by the antibodies’ mode of action, the researchers fed the insects a blood meal laced with the antibodies and a lethal dose of ivermectin, an insecticide that also targets the AgGluCl protein channel.
Then, the team monitored the mosquitoes’ survival to find out more about how the antibodies may destroy the insects. Mosquitoes that received ivermectin with the antibodies were more likely to survive than insects that received ivermectin alone.
“We believe that ivermectin is able to bind to AgGluCl,” Meyers said, “but the antibody keeps the channel from opening and becoming active.”
Having shown that antibodies targeted to the glutamate gated chloride channel in blood meals can be effective insecticides, the researchers are interested to find out if antibody-laced blood meals are equally deadly outside the lab.
First, though, the team plans to immunize cattle against the AgGluCl antigen and feed Anopheles gambiae on the immunized cattle in the lab. If the strategy proves successful, Meyers envisages a large-scale cattle immunization program as part of a combined attack on the malaria parasite. ![]()

Photo courtesy of the CDC
Recent progress in halting the spread of malaria has hinged, in part, on the use of insecticide-treated bed nets and spraying programs that target Anopheles gambiae mosquitoes.
Unfortunately, the mosquitoes are developing resistance to insecticides such as pyrethroid.
Wondering if they could defeat the mosquitoes by developing a new insecticide,a group of researchers set out to make blood meals toxic for Anopheles gambiae.
The team described their method in The Journal of Experimental Biology.
The researchers decided to target the mosquito glutamate gated chloride channel (AgGluCl), which is an essential component of the insect’s nervous system.
They generated antibodies that specifically targeted a portion of the protein exposed on the surface of nerves, a strategy they acknowledged was somewhat risky.
“Antibodies against a single mosquito antigen have never been shown to have mosquitocidal properties before, and the majority of previous research had focused on midgut antigens, while we were targeting a neuronal antigen expressed only in tissues found outside of the midgut,” said study author Jacob Meyers, a graduate student at Colorado State University in Fort Collins.
After injecting rabbits with a tiny portion of the surface of the AgGluCl protein channel, the researchers waited for the rabbits’ immune systems to begin producing antibodies tailored to the channel.
Then, the team collected the antibodies, mixed them with fresh blood, and fed the mixture to malaria-carrying mosquitoes (Anopheles gambiae), yellow fever-carrying mosquitoes (Aedes aegypti), and mosquitoes that carry the West Nile virus (Culex tarsalis).
Neither the yellow fever nor West Nile virus mosquitoes responded to the spiked blood. However, significant numbers of Anopheles gambiae mosquitoes died after consuming the blood/antibody cocktail. The highest antibody doses killed more than 90% of the insects within a day.
The researchers looked into why the yellow fever and West Nile virus mosquitoes had been immune to the antibodies and found the antibodies could not pass across the mosquitoes’ guts into the hemolymph. But the antibodies passed into the hemolymph of Anopheles gambiae mosquitoes with ease.
Intrigued by the antibodies’ mode of action, the researchers fed the insects a blood meal laced with the antibodies and a lethal dose of ivermectin, an insecticide that also targets the AgGluCl protein channel.
Then, the team monitored the mosquitoes’ survival to find out more about how the antibodies may destroy the insects. Mosquitoes that received ivermectin with the antibodies were more likely to survive than insects that received ivermectin alone.
“We believe that ivermectin is able to bind to AgGluCl,” Meyers said, “but the antibody keeps the channel from opening and becoming active.”
Having shown that antibodies targeted to the glutamate gated chloride channel in blood meals can be effective insecticides, the researchers are interested to find out if antibody-laced blood meals are equally deadly outside the lab.
First, though, the team plans to immunize cattle against the AgGluCl antigen and feed Anopheles gambiae on the immunized cattle in the lab. If the strategy proves successful, Meyers envisages a large-scale cattle immunization program as part of a combined attack on the malaria parasite. ![]()

Photo courtesy of the CDC
Recent progress in halting the spread of malaria has hinged, in part, on the use of insecticide-treated bed nets and spraying programs that target Anopheles gambiae mosquitoes.
Unfortunately, the mosquitoes are developing resistance to insecticides such as pyrethroid.
Wondering if they could defeat the mosquitoes by developing a new insecticide,a group of researchers set out to make blood meals toxic for Anopheles gambiae.
The team described their method in The Journal of Experimental Biology.
The researchers decided to target the mosquito glutamate gated chloride channel (AgGluCl), which is an essential component of the insect’s nervous system.
They generated antibodies that specifically targeted a portion of the protein exposed on the surface of nerves, a strategy they acknowledged was somewhat risky.
“Antibodies against a single mosquito antigen have never been shown to have mosquitocidal properties before, and the majority of previous research had focused on midgut antigens, while we were targeting a neuronal antigen expressed only in tissues found outside of the midgut,” said study author Jacob Meyers, a graduate student at Colorado State University in Fort Collins.
After injecting rabbits with a tiny portion of the surface of the AgGluCl protein channel, the researchers waited for the rabbits’ immune systems to begin producing antibodies tailored to the channel.
Then, the team collected the antibodies, mixed them with fresh blood, and fed the mixture to malaria-carrying mosquitoes (Anopheles gambiae), yellow fever-carrying mosquitoes (Aedes aegypti), and mosquitoes that carry the West Nile virus (Culex tarsalis).
Neither the yellow fever nor West Nile virus mosquitoes responded to the spiked blood. However, significant numbers of Anopheles gambiae mosquitoes died after consuming the blood/antibody cocktail. The highest antibody doses killed more than 90% of the insects within a day.
The researchers looked into why the yellow fever and West Nile virus mosquitoes had been immune to the antibodies and found the antibodies could not pass across the mosquitoes’ guts into the hemolymph. But the antibodies passed into the hemolymph of Anopheles gambiae mosquitoes with ease.
Intrigued by the antibodies’ mode of action, the researchers fed the insects a blood meal laced with the antibodies and a lethal dose of ivermectin, an insecticide that also targets the AgGluCl protein channel.
Then, the team monitored the mosquitoes’ survival to find out more about how the antibodies may destroy the insects. Mosquitoes that received ivermectin with the antibodies were more likely to survive than insects that received ivermectin alone.
“We believe that ivermectin is able to bind to AgGluCl,” Meyers said, “but the antibody keeps the channel from opening and becoming active.”
Having shown that antibodies targeted to the glutamate gated chloride channel in blood meals can be effective insecticides, the researchers are interested to find out if antibody-laced blood meals are equally deadly outside the lab.
First, though, the team plans to immunize cattle against the AgGluCl antigen and feed Anopheles gambiae on the immunized cattle in the lab. If the strategy proves successful, Meyers envisages a large-scale cattle immunization program as part of a combined attack on the malaria parasite. ![]()
System can identify life-threatening bleeding

Photo courtesy of UAB Hospital
A computerized system that analyzes vital signs can help healthcare professionals more accurately diagnose trauma patients with life-threatening bleeding, according to research published in Shock.
The system, known as APPRAISE, simultaneously analyzes blood pressure, heart rate, and breathing patterns during emergency transport.
And investigators found that APPRAISE accurately detected most cases of life-threatening bleeding.
“Providing faster care to patients who are bleeding to death saves lives,” said study author Andrew Reisner, MD, of Massachusetts General Hospital in Boston.
“While the clinical information that ambulance crews call in to trauma centers was sufficient to determine the presence of a life-threatening hemorrhage in about half the patients we studied, many other patients were in a ‘gray area’ and may or may not have been at risk of bleeding to death.”
“Our study demonstrated that automated analysis of patients’ vital signs during prehospital transport was significantly better at discriminating between patients who did and did not have life-threatening hemorrhage.”
The APPRAISE system incorporates software based on statistical techniques currently used in stock market trading and manufacturing to determine whether particular data points represent real problems and not random fluctuations.
The system uses an ultracompact personal computer to analyze data gathered by a standard patient monitor used in emergency transport vehicles.
For this study, the system was installed in 2 MedFlight helicopters and collected data on more than 200 trauma patients transported to participating Boston hospitals from February 2010 to December 2012. So that patients’ care was not affected by a still-unproven system, the APPRAISE system’s analysis was not provided to MedFlight crews.
The researchers also analyzed information from a 2005 study of vital sign data gathered manually by a Houston-based air ambulance system.
The team found the APPRAISE system could identify, with 76% sensitivity, patients who needed 9 or more units of packed red blood cells within 24 hours.
This was significantly more sensitive (P<0.05) than any prehospital Shock Index of 1.4 or higher (59%), initial systolic blood pressure less than 110 mmHg (50%), and any prehospital systolic blood pressure less than 90 mmHg (50%).
However, there was no signficant difference between the different measures with regard to specificity for identifying patients who did not need a blood transfusion within 24 hours.
Specificity was 87% for APPRAISE, 88% for any Shock Index of 1.4 or higher, 88% for initial systolic blood pressure less than 110 mmHg, and 90% for any prehospital systolic blood pressure less than 90 mmHg.
Notifications provided by APPRAISE would have been available within 10 minutes of initial monitoring and a median of 20 minutes before patients arrived at the trauma centers.
“The fact that decisions to proceed with surgery or to replenish lost blood often occur only after patients’ arrival means there are delays—sometimes brief but sometimes prolonged—in initiating such life-saving interventions,” Dr Reisner said.
“We are now working on a follow-up study to use this system in actual trauma care and will be measuring whether it truly leads to faster treatment of life-threatening hemorrhage and better patient outcomes. This approach could also be helpful for patients transported by ground ambulance and for hospitalized patients at risk of unexpected hemorrhage, such as during recovery from major surgery.” ![]()

Photo courtesy of UAB Hospital
A computerized system that analyzes vital signs can help healthcare professionals more accurately diagnose trauma patients with life-threatening bleeding, according to research published in Shock.
The system, known as APPRAISE, simultaneously analyzes blood pressure, heart rate, and breathing patterns during emergency transport.
And investigators found that APPRAISE accurately detected most cases of life-threatening bleeding.
“Providing faster care to patients who are bleeding to death saves lives,” said study author Andrew Reisner, MD, of Massachusetts General Hospital in Boston.
“While the clinical information that ambulance crews call in to trauma centers was sufficient to determine the presence of a life-threatening hemorrhage in about half the patients we studied, many other patients were in a ‘gray area’ and may or may not have been at risk of bleeding to death.”
“Our study demonstrated that automated analysis of patients’ vital signs during prehospital transport was significantly better at discriminating between patients who did and did not have life-threatening hemorrhage.”
The APPRAISE system incorporates software based on statistical techniques currently used in stock market trading and manufacturing to determine whether particular data points represent real problems and not random fluctuations.
The system uses an ultracompact personal computer to analyze data gathered by a standard patient monitor used in emergency transport vehicles.
For this study, the system was installed in 2 MedFlight helicopters and collected data on more than 200 trauma patients transported to participating Boston hospitals from February 2010 to December 2012. So that patients’ care was not affected by a still-unproven system, the APPRAISE system’s analysis was not provided to MedFlight crews.
The researchers also analyzed information from a 2005 study of vital sign data gathered manually by a Houston-based air ambulance system.
The team found the APPRAISE system could identify, with 76% sensitivity, patients who needed 9 or more units of packed red blood cells within 24 hours.
This was significantly more sensitive (P<0.05) than any prehospital Shock Index of 1.4 or higher (59%), initial systolic blood pressure less than 110 mmHg (50%), and any prehospital systolic blood pressure less than 90 mmHg (50%).
However, there was no signficant difference between the different measures with regard to specificity for identifying patients who did not need a blood transfusion within 24 hours.
Specificity was 87% for APPRAISE, 88% for any Shock Index of 1.4 or higher, 88% for initial systolic blood pressure less than 110 mmHg, and 90% for any prehospital systolic blood pressure less than 90 mmHg.
Notifications provided by APPRAISE would have been available within 10 minutes of initial monitoring and a median of 20 minutes before patients arrived at the trauma centers.
“The fact that decisions to proceed with surgery or to replenish lost blood often occur only after patients’ arrival means there are delays—sometimes brief but sometimes prolonged—in initiating such life-saving interventions,” Dr Reisner said.
“We are now working on a follow-up study to use this system in actual trauma care and will be measuring whether it truly leads to faster treatment of life-threatening hemorrhage and better patient outcomes. This approach could also be helpful for patients transported by ground ambulance and for hospitalized patients at risk of unexpected hemorrhage, such as during recovery from major surgery.” ![]()

Photo courtesy of UAB Hospital
A computerized system that analyzes vital signs can help healthcare professionals more accurately diagnose trauma patients with life-threatening bleeding, according to research published in Shock.
The system, known as APPRAISE, simultaneously analyzes blood pressure, heart rate, and breathing patterns during emergency transport.
And investigators found that APPRAISE accurately detected most cases of life-threatening bleeding.
“Providing faster care to patients who are bleeding to death saves lives,” said study author Andrew Reisner, MD, of Massachusetts General Hospital in Boston.
“While the clinical information that ambulance crews call in to trauma centers was sufficient to determine the presence of a life-threatening hemorrhage in about half the patients we studied, many other patients were in a ‘gray area’ and may or may not have been at risk of bleeding to death.”
“Our study demonstrated that automated analysis of patients’ vital signs during prehospital transport was significantly better at discriminating between patients who did and did not have life-threatening hemorrhage.”
The APPRAISE system incorporates software based on statistical techniques currently used in stock market trading and manufacturing to determine whether particular data points represent real problems and not random fluctuations.
The system uses an ultracompact personal computer to analyze data gathered by a standard patient monitor used in emergency transport vehicles.
For this study, the system was installed in 2 MedFlight helicopters and collected data on more than 200 trauma patients transported to participating Boston hospitals from February 2010 to December 2012. So that patients’ care was not affected by a still-unproven system, the APPRAISE system’s analysis was not provided to MedFlight crews.
The researchers also analyzed information from a 2005 study of vital sign data gathered manually by a Houston-based air ambulance system.
The team found the APPRAISE system could identify, with 76% sensitivity, patients who needed 9 or more units of packed red blood cells within 24 hours.
This was significantly more sensitive (P<0.05) than any prehospital Shock Index of 1.4 or higher (59%), initial systolic blood pressure less than 110 mmHg (50%), and any prehospital systolic blood pressure less than 90 mmHg (50%).
However, there was no signficant difference between the different measures with regard to specificity for identifying patients who did not need a blood transfusion within 24 hours.
Specificity was 87% for APPRAISE, 88% for any Shock Index of 1.4 or higher, 88% for initial systolic blood pressure less than 110 mmHg, and 90% for any prehospital systolic blood pressure less than 90 mmHg.
Notifications provided by APPRAISE would have been available within 10 minutes of initial monitoring and a median of 20 minutes before patients arrived at the trauma centers.
“The fact that decisions to proceed with surgery or to replenish lost blood often occur only after patients’ arrival means there are delays—sometimes brief but sometimes prolonged—in initiating such life-saving interventions,” Dr Reisner said.
“We are now working on a follow-up study to use this system in actual trauma care and will be measuring whether it truly leads to faster treatment of life-threatening hemorrhage and better patient outcomes. This approach could also be helpful for patients transported by ground ambulance and for hospitalized patients at risk of unexpected hemorrhage, such as during recovery from major surgery.” ![]()
Modified T cells may enhance MM treatment
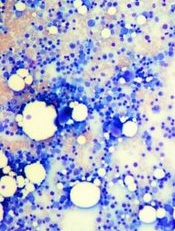
Image by Daniel E. Sabath
Modified T cells derived from patients’ bone marrow can help treat multiple myeloma (MM), according to researchers.
They modified marrow-infiltrating lymphocytes (MILs) to target MM cells and administered them to patients following chemotherapy and hematopoietic stem cell transplant (HSCT).
Seven of the 22 patients treated achieved at least a 90% reduction in disease burden, which translated to an improvement in progression-free survival.
The researchers reported these results in Science Translational Medicine.
“What we learned in this small trial is that large numbers of activated MILs can selectively target and kill myeloma cells,” said Ivan Borrello, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Borrello and his colleagues set out to test the MILs in 25 patients with newly diagnosed (n=14) or relapsed (n=11) MM. The patients’ median age was 56 years (range, 30-71), 64% were male, and 82% were white.
The median number of prior treatments was 2.13 (range, 1-6). For this trial, the patients received high-dose melphalan, autologous HSCT, and MIL therapy.
Patient outcomes
Prior to HSCT, 14 patients had a partial response (PR, 64%), 3 had a very good PR (14%), 2 had a minor response (8%), and 3 had stable disease (SD, 14%). After HSCT, 7 patients were in complete response (CR, 32%), 6 had a PR (27%), 6 had SD (27%), and 3 had progressive disease (PD, 14%).
Patients received MILs when they obtained the maximal response to the last therapy. The researchers retrieved MILs from each patient’s bone marrow and expanded them with anti-CD3/CD28 beads plus interleukin-2.
Twenty-two patients received a median of 9.5×108 MILs. Three patients relapsed before they could receive the MILs.
After MIL treatment, the overall response rate was 54%. Twenty-seven percent of patients achieved a CR, 27% had a PR, 23% had SD, and 14% had PD.
Patients who achieved at least a 90% reduction in disease burden (n=7) had superior progression-free survival compared to patients who did not respond as well to treatment (n=15).
The median progression-free survival was 25.1 months and 11.8 months, respectively (P=0.01). There was no significant difference between the groups with regard to overall survival.
The researchers said none of the patients experienced serious adverse events related to the MIL therapy.
Future directions
Dr Borrello said several US cancer centers have tested similar experimental treatments, but his team is believed to be the only one testing MILs. Other types of tumor-infiltrating cells can be used, but they are usually less plentiful in patients’ tumors and may not grow as well outside the body, he said.
“Typically, immune cells from solid tumors, called tumor-infiltrating lymphocytes, can be harvested and grown in only about 25% of patients who could potentially be eligible for the therapy,” said Kimberly Noonan, PhD, also of the Johns Hopkins University School of Medicine. “But in our clinical trial, we were able to harvest and grow MILs from all 22 patients.”
Dr Noonan said this small trial helped the researchers learn more about which patients may benefit from MIL therapy. For example, the team was able to determine how many of the MILs grown in the lab were specifically targeted to a patient’s tumor and whether they continued to target the tumor after being infused.
Additionally, the researchers found that patients with a high number of central memory cells before treatment had a better response to MIL therapy. Patients who began treatment with signs of an overactive immune response did not respond as well.
Dr Noonan said the team has used these data to guide 2 other ongoing trials of MILs. Those studies, she said, are trying to extend antitumor response and tumor specificity by combining the MILs with a cancer vaccine called GVAX and the drug lenalidomide, which stimulates T-cell responses.
All of the trials have also shed light on new ways to grow the MILs.
“In most of these trials, you see that the more cells you get, the better response you get in patients,” Dr Noonan said. “Learning how to improve cell growth may therefore improve the therapy.”
Researchers are also developing MILs to treat solid tumors such as lung, esophageal, and gastric cancers, as well as the pediatric cancers neuroblastoma and Ewing’s sarcoma. ![]()

Image by Daniel E. Sabath
Modified T cells derived from patients’ bone marrow can help treat multiple myeloma (MM), according to researchers.
They modified marrow-infiltrating lymphocytes (MILs) to target MM cells and administered them to patients following chemotherapy and hematopoietic stem cell transplant (HSCT).
Seven of the 22 patients treated achieved at least a 90% reduction in disease burden, which translated to an improvement in progression-free survival.
The researchers reported these results in Science Translational Medicine.
“What we learned in this small trial is that large numbers of activated MILs can selectively target and kill myeloma cells,” said Ivan Borrello, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Borrello and his colleagues set out to test the MILs in 25 patients with newly diagnosed (n=14) or relapsed (n=11) MM. The patients’ median age was 56 years (range, 30-71), 64% were male, and 82% were white.
The median number of prior treatments was 2.13 (range, 1-6). For this trial, the patients received high-dose melphalan, autologous HSCT, and MIL therapy.
Patient outcomes
Prior to HSCT, 14 patients had a partial response (PR, 64%), 3 had a very good PR (14%), 2 had a minor response (8%), and 3 had stable disease (SD, 14%). After HSCT, 7 patients were in complete response (CR, 32%), 6 had a PR (27%), 6 had SD (27%), and 3 had progressive disease (PD, 14%).
Patients received MILs when they obtained the maximal response to the last therapy. The researchers retrieved MILs from each patient’s bone marrow and expanded them with anti-CD3/CD28 beads plus interleukin-2.
Twenty-two patients received a median of 9.5×108 MILs. Three patients relapsed before they could receive the MILs.
After MIL treatment, the overall response rate was 54%. Twenty-seven percent of patients achieved a CR, 27% had a PR, 23% had SD, and 14% had PD.
Patients who achieved at least a 90% reduction in disease burden (n=7) had superior progression-free survival compared to patients who did not respond as well to treatment (n=15).
The median progression-free survival was 25.1 months and 11.8 months, respectively (P=0.01). There was no significant difference between the groups with regard to overall survival.
The researchers said none of the patients experienced serious adverse events related to the MIL therapy.
Future directions
Dr Borrello said several US cancer centers have tested similar experimental treatments, but his team is believed to be the only one testing MILs. Other types of tumor-infiltrating cells can be used, but they are usually less plentiful in patients’ tumors and may not grow as well outside the body, he said.
“Typically, immune cells from solid tumors, called tumor-infiltrating lymphocytes, can be harvested and grown in only about 25% of patients who could potentially be eligible for the therapy,” said Kimberly Noonan, PhD, also of the Johns Hopkins University School of Medicine. “But in our clinical trial, we were able to harvest and grow MILs from all 22 patients.”
Dr Noonan said this small trial helped the researchers learn more about which patients may benefit from MIL therapy. For example, the team was able to determine how many of the MILs grown in the lab were specifically targeted to a patient’s tumor and whether they continued to target the tumor after being infused.
Additionally, the researchers found that patients with a high number of central memory cells before treatment had a better response to MIL therapy. Patients who began treatment with signs of an overactive immune response did not respond as well.
Dr Noonan said the team has used these data to guide 2 other ongoing trials of MILs. Those studies, she said, are trying to extend antitumor response and tumor specificity by combining the MILs with a cancer vaccine called GVAX and the drug lenalidomide, which stimulates T-cell responses.
All of the trials have also shed light on new ways to grow the MILs.
“In most of these trials, you see that the more cells you get, the better response you get in patients,” Dr Noonan said. “Learning how to improve cell growth may therefore improve the therapy.”
Researchers are also developing MILs to treat solid tumors such as lung, esophageal, and gastric cancers, as well as the pediatric cancers neuroblastoma and Ewing’s sarcoma. ![]()

Image by Daniel E. Sabath
Modified T cells derived from patients’ bone marrow can help treat multiple myeloma (MM), according to researchers.
They modified marrow-infiltrating lymphocytes (MILs) to target MM cells and administered them to patients following chemotherapy and hematopoietic stem cell transplant (HSCT).
Seven of the 22 patients treated achieved at least a 90% reduction in disease burden, which translated to an improvement in progression-free survival.
The researchers reported these results in Science Translational Medicine.
“What we learned in this small trial is that large numbers of activated MILs can selectively target and kill myeloma cells,” said Ivan Borrello, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Borrello and his colleagues set out to test the MILs in 25 patients with newly diagnosed (n=14) or relapsed (n=11) MM. The patients’ median age was 56 years (range, 30-71), 64% were male, and 82% were white.
The median number of prior treatments was 2.13 (range, 1-6). For this trial, the patients received high-dose melphalan, autologous HSCT, and MIL therapy.
Patient outcomes
Prior to HSCT, 14 patients had a partial response (PR, 64%), 3 had a very good PR (14%), 2 had a minor response (8%), and 3 had stable disease (SD, 14%). After HSCT, 7 patients were in complete response (CR, 32%), 6 had a PR (27%), 6 had SD (27%), and 3 had progressive disease (PD, 14%).
Patients received MILs when they obtained the maximal response to the last therapy. The researchers retrieved MILs from each patient’s bone marrow and expanded them with anti-CD3/CD28 beads plus interleukin-2.
Twenty-two patients received a median of 9.5×108 MILs. Three patients relapsed before they could receive the MILs.
After MIL treatment, the overall response rate was 54%. Twenty-seven percent of patients achieved a CR, 27% had a PR, 23% had SD, and 14% had PD.
Patients who achieved at least a 90% reduction in disease burden (n=7) had superior progression-free survival compared to patients who did not respond as well to treatment (n=15).
The median progression-free survival was 25.1 months and 11.8 months, respectively (P=0.01). There was no significant difference between the groups with regard to overall survival.
The researchers said none of the patients experienced serious adverse events related to the MIL therapy.
Future directions
Dr Borrello said several US cancer centers have tested similar experimental treatments, but his team is believed to be the only one testing MILs. Other types of tumor-infiltrating cells can be used, but they are usually less plentiful in patients’ tumors and may not grow as well outside the body, he said.
“Typically, immune cells from solid tumors, called tumor-infiltrating lymphocytes, can be harvested and grown in only about 25% of patients who could potentially be eligible for the therapy,” said Kimberly Noonan, PhD, also of the Johns Hopkins University School of Medicine. “But in our clinical trial, we were able to harvest and grow MILs from all 22 patients.”
Dr Noonan said this small trial helped the researchers learn more about which patients may benefit from MIL therapy. For example, the team was able to determine how many of the MILs grown in the lab were specifically targeted to a patient’s tumor and whether they continued to target the tumor after being infused.
Additionally, the researchers found that patients with a high number of central memory cells before treatment had a better response to MIL therapy. Patients who began treatment with signs of an overactive immune response did not respond as well.
Dr Noonan said the team has used these data to guide 2 other ongoing trials of MILs. Those studies, she said, are trying to extend antitumor response and tumor specificity by combining the MILs with a cancer vaccine called GVAX and the drug lenalidomide, which stimulates T-cell responses.
All of the trials have also shed light on new ways to grow the MILs.
“In most of these trials, you see that the more cells you get, the better response you get in patients,” Dr Noonan said. “Learning how to improve cell growth may therefore improve the therapy.”
Researchers are also developing MILs to treat solid tumors such as lung, esophageal, and gastric cancers, as well as the pediatric cancers neuroblastoma and Ewing’s sarcoma. ![]()
Rural surgery: A view from the front lines
Welcome to a new column about rural surgery! This quarterly feature will be a foray into the world of the rural surgeon by someone who has lived and worked in the rural milieu for many years. I want to bring to you a view of rural surgery from the “front lines.”
“Rural surgery: A view from the front lines” will be the second series about this timely topic published by ACS Surgery News. The first, “The Rural Surgeon” appeared in 2015 and was coordinated by Philip Caropreso, MD, FACS, a prominent and eloquent advocate for rural surgeons. The series was a great success. I was prevailed upon to begin another series. The goal for the new series is to delve into not only the important issues that rural surgeons face, but also to convey to readers who practice far from rural America what goes on in a rural practice.
I’ve been fortunate to be active in the leadership of the Michigan Chapter of the ACS and, through this association, I have learned how surgeons can impact care at the state level. I recently completed a 6-year term as an ACS Governor. As a governor, I’ve had the wonderful opportunity to serve as a member of the ACS Advisory Council for Rural Surgery, where I have learned about the many complex and unique challenges that U.S. rural surgeons confront. So I have my own lived experience as a rural surgeon and some exposure to what is happening in rural practices across the country, as well.
One thing I have learned is that not all rural surgery practices are the same. The work can depend on the size of the institution and the community. Surgical literature describes surgical practices in communities with a population of 10,000 or fewer as small rural surgical practices. In this setting, the hospital and staff level will be quite small. There are not likely to be cardiologists, pulmonologists, or many other medical specialists. Anesthesia may be provided by certified registered nurse anesthetists rather than anesthesiologists. A surgeon in a small rural surgical practice will do “bread and butter” elective cases such as hernia repairs, cholecystectomies, breast cancer surgery, and colectomies. Endoscopy is a large part of a rural surgeon’s practice since there would likely not be a gastroenterologist on staff. There may no urologist, ob.gyn., or orthopedist on staff, so a rural surgeon in this setting may do some urologic and orthopedic surgery and may provide C-section coverage.
A rural surgeon here may likely be the only general surgeon or may have one partner. These surgeons have a very high on-call burden. Because of all of these factors, surgeons in this setting will tend to be somewhat selective in which patients they choose to operate on. Elderly patients or those with multiple comorbidities may more likely be referred to a larger center.
Surgical practices in communities of 10,000-50,000 population, such as where I practice, are described as large rural surgical practices. The hospital in these communities will be larger and will offer more services. Our hospital has a cancer center, wound care clinic, and provides dialysis services. The medical staff will have more medical specialists. Our hospital has a pulmonologist, medical oncologist, radiation oncologist, neurologist, and a pathologist. Other specialties include cardiology, radiology, and pediatrics.
Hospitals in a community of this size will have more surgical specialists and will usually have anesthesiologists on staff. At our hospital, we have an orthopedic service, urology, ophthalmology, podiatry, ob.gyn. physicians, and MD anesthesia. There are no gastroenterologists, ICU specialists, neurosurgeons, infectious disease specialists, ENT surgeons, vascular surgeons, thoracic surgeons, or other surgical subspecialists at our hospital.
With a larger medical staff and a more developed hospital infrastructure, rural surgeons in a large rural practice setting can take care of more varied and complex patients. In my practice, we do “bread and butter” elective cases as mentioned above. We also do thyroid surgery, carotid endarterectomies, carpal tunnel release, melanoma surgery, amputations, and we place dialysis catheters and subcutaneous ports, and cover the wound care clinic. In other large rural practice settings, surgeons may do tendon repairs, endoscopic retrograde cholangiopancreatography, salivary gland surgery, pediatric hernia repair, and non–cardiac thoracic surgery. Many medical communities of this size will not have a gastroenterologist on staff, so endoscopy is a large part of a rural surgeon’s practice in this setting also. In my practice, endoscopy accounts for 60% of our billing. There will be more general surgeons on staff in a large rural setting, so the call burden is not as pronounced. I have three partners, so I am on call one in four.
Rural surgeons in both small and large rural practices frequently will see urgent and emergent surgical problems and, in my mind, that’s where rural surgeons can make the most difference. In these situations, the rural surgeon will take care of whatever he/she and their hospital have the capability to do. Rural areas have no lack of trauma and sometimes, the care a rural surgeon provides can be lifesaving. Common urgent or emergency surgical cases include small- or large-bowel obstructions, diverticulitis, perforated ulcers, incarcerated hernia, and necrotizing fasciitis. Rural surgeons also play a vital role in stabilizing critically ill or injured patients prior to arranging for transfer to a larger center.
Rural surgeons have a very broad scope of practice, but that’s not because they think they’re good enough to “do it all.” They aren’t being “cowboy surgeons.” A good rural surgeon simply tries to utilize as many of the skills learned during residency to properly care for as many patients of the community as possible.
By working on the front lines, rural surgeons can make a very real difference for the patients of their communities. More to come in future columns!
Dr. Puls is a general surgeon in Alpena, Mich. He serves as vice chair of the ACS Advisory Council for Rural Surgery.
Welcome to a new column about rural surgery! This quarterly feature will be a foray into the world of the rural surgeon by someone who has lived and worked in the rural milieu for many years. I want to bring to you a view of rural surgery from the “front lines.”
“Rural surgery: A view from the front lines” will be the second series about this timely topic published by ACS Surgery News. The first, “The Rural Surgeon” appeared in 2015 and was coordinated by Philip Caropreso, MD, FACS, a prominent and eloquent advocate for rural surgeons. The series was a great success. I was prevailed upon to begin another series. The goal for the new series is to delve into not only the important issues that rural surgeons face, but also to convey to readers who practice far from rural America what goes on in a rural practice.
I’ve been fortunate to be active in the leadership of the Michigan Chapter of the ACS and, through this association, I have learned how surgeons can impact care at the state level. I recently completed a 6-year term as an ACS Governor. As a governor, I’ve had the wonderful opportunity to serve as a member of the ACS Advisory Council for Rural Surgery, where I have learned about the many complex and unique challenges that U.S. rural surgeons confront. So I have my own lived experience as a rural surgeon and some exposure to what is happening in rural practices across the country, as well.
One thing I have learned is that not all rural surgery practices are the same. The work can depend on the size of the institution and the community. Surgical literature describes surgical practices in communities with a population of 10,000 or fewer as small rural surgical practices. In this setting, the hospital and staff level will be quite small. There are not likely to be cardiologists, pulmonologists, or many other medical specialists. Anesthesia may be provided by certified registered nurse anesthetists rather than anesthesiologists. A surgeon in a small rural surgical practice will do “bread and butter” elective cases such as hernia repairs, cholecystectomies, breast cancer surgery, and colectomies. Endoscopy is a large part of a rural surgeon’s practice since there would likely not be a gastroenterologist on staff. There may no urologist, ob.gyn., or orthopedist on staff, so a rural surgeon in this setting may do some urologic and orthopedic surgery and may provide C-section coverage.
A rural surgeon here may likely be the only general surgeon or may have one partner. These surgeons have a very high on-call burden. Because of all of these factors, surgeons in this setting will tend to be somewhat selective in which patients they choose to operate on. Elderly patients or those with multiple comorbidities may more likely be referred to a larger center.
Surgical practices in communities of 10,000-50,000 population, such as where I practice, are described as large rural surgical practices. The hospital in these communities will be larger and will offer more services. Our hospital has a cancer center, wound care clinic, and provides dialysis services. The medical staff will have more medical specialists. Our hospital has a pulmonologist, medical oncologist, radiation oncologist, neurologist, and a pathologist. Other specialties include cardiology, radiology, and pediatrics.
Hospitals in a community of this size will have more surgical specialists and will usually have anesthesiologists on staff. At our hospital, we have an orthopedic service, urology, ophthalmology, podiatry, ob.gyn. physicians, and MD anesthesia. There are no gastroenterologists, ICU specialists, neurosurgeons, infectious disease specialists, ENT surgeons, vascular surgeons, thoracic surgeons, or other surgical subspecialists at our hospital.
With a larger medical staff and a more developed hospital infrastructure, rural surgeons in a large rural practice setting can take care of more varied and complex patients. In my practice, we do “bread and butter” elective cases as mentioned above. We also do thyroid surgery, carotid endarterectomies, carpal tunnel release, melanoma surgery, amputations, and we place dialysis catheters and subcutaneous ports, and cover the wound care clinic. In other large rural practice settings, surgeons may do tendon repairs, endoscopic retrograde cholangiopancreatography, salivary gland surgery, pediatric hernia repair, and non–cardiac thoracic surgery. Many medical communities of this size will not have a gastroenterologist on staff, so endoscopy is a large part of a rural surgeon’s practice in this setting also. In my practice, endoscopy accounts for 60% of our billing. There will be more general surgeons on staff in a large rural setting, so the call burden is not as pronounced. I have three partners, so I am on call one in four.
Rural surgeons in both small and large rural practices frequently will see urgent and emergent surgical problems and, in my mind, that’s where rural surgeons can make the most difference. In these situations, the rural surgeon will take care of whatever he/she and their hospital have the capability to do. Rural areas have no lack of trauma and sometimes, the care a rural surgeon provides can be lifesaving. Common urgent or emergency surgical cases include small- or large-bowel obstructions, diverticulitis, perforated ulcers, incarcerated hernia, and necrotizing fasciitis. Rural surgeons also play a vital role in stabilizing critically ill or injured patients prior to arranging for transfer to a larger center.
Rural surgeons have a very broad scope of practice, but that’s not because they think they’re good enough to “do it all.” They aren’t being “cowboy surgeons.” A good rural surgeon simply tries to utilize as many of the skills learned during residency to properly care for as many patients of the community as possible.
By working on the front lines, rural surgeons can make a very real difference for the patients of their communities. More to come in future columns!
Dr. Puls is a general surgeon in Alpena, Mich. He serves as vice chair of the ACS Advisory Council for Rural Surgery.
Welcome to a new column about rural surgery! This quarterly feature will be a foray into the world of the rural surgeon by someone who has lived and worked in the rural milieu for many years. I want to bring to you a view of rural surgery from the “front lines.”
“Rural surgery: A view from the front lines” will be the second series about this timely topic published by ACS Surgery News. The first, “The Rural Surgeon” appeared in 2015 and was coordinated by Philip Caropreso, MD, FACS, a prominent and eloquent advocate for rural surgeons. The series was a great success. I was prevailed upon to begin another series. The goal for the new series is to delve into not only the important issues that rural surgeons face, but also to convey to readers who practice far from rural America what goes on in a rural practice.
I’ve been fortunate to be active in the leadership of the Michigan Chapter of the ACS and, through this association, I have learned how surgeons can impact care at the state level. I recently completed a 6-year term as an ACS Governor. As a governor, I’ve had the wonderful opportunity to serve as a member of the ACS Advisory Council for Rural Surgery, where I have learned about the many complex and unique challenges that U.S. rural surgeons confront. So I have my own lived experience as a rural surgeon and some exposure to what is happening in rural practices across the country, as well.
One thing I have learned is that not all rural surgery practices are the same. The work can depend on the size of the institution and the community. Surgical literature describes surgical practices in communities with a population of 10,000 or fewer as small rural surgical practices. In this setting, the hospital and staff level will be quite small. There are not likely to be cardiologists, pulmonologists, or many other medical specialists. Anesthesia may be provided by certified registered nurse anesthetists rather than anesthesiologists. A surgeon in a small rural surgical practice will do “bread and butter” elective cases such as hernia repairs, cholecystectomies, breast cancer surgery, and colectomies. Endoscopy is a large part of a rural surgeon’s practice since there would likely not be a gastroenterologist on staff. There may no urologist, ob.gyn., or orthopedist on staff, so a rural surgeon in this setting may do some urologic and orthopedic surgery and may provide C-section coverage.
A rural surgeon here may likely be the only general surgeon or may have one partner. These surgeons have a very high on-call burden. Because of all of these factors, surgeons in this setting will tend to be somewhat selective in which patients they choose to operate on. Elderly patients or those with multiple comorbidities may more likely be referred to a larger center.
Surgical practices in communities of 10,000-50,000 population, such as where I practice, are described as large rural surgical practices. The hospital in these communities will be larger and will offer more services. Our hospital has a cancer center, wound care clinic, and provides dialysis services. The medical staff will have more medical specialists. Our hospital has a pulmonologist, medical oncologist, radiation oncologist, neurologist, and a pathologist. Other specialties include cardiology, radiology, and pediatrics.
Hospitals in a community of this size will have more surgical specialists and will usually have anesthesiologists on staff. At our hospital, we have an orthopedic service, urology, ophthalmology, podiatry, ob.gyn. physicians, and MD anesthesia. There are no gastroenterologists, ICU specialists, neurosurgeons, infectious disease specialists, ENT surgeons, vascular surgeons, thoracic surgeons, or other surgical subspecialists at our hospital.
With a larger medical staff and a more developed hospital infrastructure, rural surgeons in a large rural practice setting can take care of more varied and complex patients. In my practice, we do “bread and butter” elective cases as mentioned above. We also do thyroid surgery, carotid endarterectomies, carpal tunnel release, melanoma surgery, amputations, and we place dialysis catheters and subcutaneous ports, and cover the wound care clinic. In other large rural practice settings, surgeons may do tendon repairs, endoscopic retrograde cholangiopancreatography, salivary gland surgery, pediatric hernia repair, and non–cardiac thoracic surgery. Many medical communities of this size will not have a gastroenterologist on staff, so endoscopy is a large part of a rural surgeon’s practice in this setting also. In my practice, endoscopy accounts for 60% of our billing. There will be more general surgeons on staff in a large rural setting, so the call burden is not as pronounced. I have three partners, so I am on call one in four.
Rural surgeons in both small and large rural practices frequently will see urgent and emergent surgical problems and, in my mind, that’s where rural surgeons can make the most difference. In these situations, the rural surgeon will take care of whatever he/she and their hospital have the capability to do. Rural areas have no lack of trauma and sometimes, the care a rural surgeon provides can be lifesaving. Common urgent or emergency surgical cases include small- or large-bowel obstructions, diverticulitis, perforated ulcers, incarcerated hernia, and necrotizing fasciitis. Rural surgeons also play a vital role in stabilizing critically ill or injured patients prior to arranging for transfer to a larger center.
Rural surgeons have a very broad scope of practice, but that’s not because they think they’re good enough to “do it all.” They aren’t being “cowboy surgeons.” A good rural surgeon simply tries to utilize as many of the skills learned during residency to properly care for as many patients of the community as possible.
By working on the front lines, rural surgeons can make a very real difference for the patients of their communities. More to come in future columns!
Dr. Puls is a general surgeon in Alpena, Mich. He serves as vice chair of the ACS Advisory Council for Rural Surgery.
VIDEO: Is it IBS? Blood test may offer conclusive answer
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2015
Autar scale helps identify DVT risk, prevent DVT
The Autar scale is a successful tool for identifying orthopedic surgery patients at risk for deep vein thrombosis (DVT) – and can prevent DVT when patients follow prophylactic measures corresponding with their DVT risk level, according to researchers.
The study comprised 216 patients who were undergoing orthopedic surgery to the lower extremities in Henan, China. They were divided into a control group and an intervention group, consisting of 106 and 110 patients, respectively. Researchers used the Autar scale to assess the risk of a DVT occurring in all of the patients.
The scale is used mainly for evaluating the probability of DVT in a hospitalized patient undergoing surgery. It includes seven risk categories and 41 items, and is used to assign scores to patients indicating whether they are at no, low, moderate, or high risk of DVT.
Specific preventive measures were implemented among the intervention group’s members based on the Autar scale scores of each of these patients. The scores of patients in the control group were not used to implement DVT prophylaxis. Such patients, however, did receive routine nursing and mechanical and pharmacological prophylactic measures, if clinical experience and basic information caused their health care providers to identify them as being at high risk for DVT.
The Autar scale’s efficacy was confirmed by the fact that “the number of patients with DVT was in line with the number of high-risk patients in both groups,” according to Hui-Zhen Yin and Professor Ci-Ming Shan of Zhengzhou (China) University.
The numbers of DVTs that occurred in each group were significantly different from each other; 1.82% of patients in the intervention group got DVTs, compared to 9.43% of patients in the control group. Therefore, the study showed that the Autar scale is useful not only for predicting DVT, but also for preventing its incidence when patients receive the appropriate prophylactic and nursing interventions, the researchers noted.
They recommend wide use of the scale, because they believe it is “a comprehensive and valid instrument that improves the consistency of nursing assessment and creates a reference for preventing DVT in nursing practice.”
Read the full study in International Journal of Nursing Sciences (doi:10.1016/j.ijnss.2015.04.003).
The Autar scale is a successful tool for identifying orthopedic surgery patients at risk for deep vein thrombosis (DVT) – and can prevent DVT when patients follow prophylactic measures corresponding with their DVT risk level, according to researchers.
The study comprised 216 patients who were undergoing orthopedic surgery to the lower extremities in Henan, China. They were divided into a control group and an intervention group, consisting of 106 and 110 patients, respectively. Researchers used the Autar scale to assess the risk of a DVT occurring in all of the patients.
The scale is used mainly for evaluating the probability of DVT in a hospitalized patient undergoing surgery. It includes seven risk categories and 41 items, and is used to assign scores to patients indicating whether they are at no, low, moderate, or high risk of DVT.
Specific preventive measures were implemented among the intervention group’s members based on the Autar scale scores of each of these patients. The scores of patients in the control group were not used to implement DVT prophylaxis. Such patients, however, did receive routine nursing and mechanical and pharmacological prophylactic measures, if clinical experience and basic information caused their health care providers to identify them as being at high risk for DVT.
The Autar scale’s efficacy was confirmed by the fact that “the number of patients with DVT was in line with the number of high-risk patients in both groups,” according to Hui-Zhen Yin and Professor Ci-Ming Shan of Zhengzhou (China) University.
The numbers of DVTs that occurred in each group were significantly different from each other; 1.82% of patients in the intervention group got DVTs, compared to 9.43% of patients in the control group. Therefore, the study showed that the Autar scale is useful not only for predicting DVT, but also for preventing its incidence when patients receive the appropriate prophylactic and nursing interventions, the researchers noted.
They recommend wide use of the scale, because they believe it is “a comprehensive and valid instrument that improves the consistency of nursing assessment and creates a reference for preventing DVT in nursing practice.”
Read the full study in International Journal of Nursing Sciences (doi:10.1016/j.ijnss.2015.04.003).
The Autar scale is a successful tool for identifying orthopedic surgery patients at risk for deep vein thrombosis (DVT) – and can prevent DVT when patients follow prophylactic measures corresponding with their DVT risk level, according to researchers.
The study comprised 216 patients who were undergoing orthopedic surgery to the lower extremities in Henan, China. They were divided into a control group and an intervention group, consisting of 106 and 110 patients, respectively. Researchers used the Autar scale to assess the risk of a DVT occurring in all of the patients.
The scale is used mainly for evaluating the probability of DVT in a hospitalized patient undergoing surgery. It includes seven risk categories and 41 items, and is used to assign scores to patients indicating whether they are at no, low, moderate, or high risk of DVT.
Specific preventive measures were implemented among the intervention group’s members based on the Autar scale scores of each of these patients. The scores of patients in the control group were not used to implement DVT prophylaxis. Such patients, however, did receive routine nursing and mechanical and pharmacological prophylactic measures, if clinical experience and basic information caused their health care providers to identify them as being at high risk for DVT.
The Autar scale’s efficacy was confirmed by the fact that “the number of patients with DVT was in line with the number of high-risk patients in both groups,” according to Hui-Zhen Yin and Professor Ci-Ming Shan of Zhengzhou (China) University.
The numbers of DVTs that occurred in each group were significantly different from each other; 1.82% of patients in the intervention group got DVTs, compared to 9.43% of patients in the control group. Therefore, the study showed that the Autar scale is useful not only for predicting DVT, but also for preventing its incidence when patients receive the appropriate prophylactic and nursing interventions, the researchers noted.
They recommend wide use of the scale, because they believe it is “a comprehensive and valid instrument that improves the consistency of nursing assessment and creates a reference for preventing DVT in nursing practice.”
Read the full study in International Journal of Nursing Sciences (doi:10.1016/j.ijnss.2015.04.003).
Stricter DVT prophylaxis guidelines needed for cardiac and vascular surgery
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
FROM ANNALS OF VASCULAR SURGERY
Key clinical point: Intraoperative anticoagulation alone does not prevent DVT in patients undergoing vascular and cardiac surgery.
Major finding: The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery.
Data source: Retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database.
Disclosures: The authors did not report any financial disclosures.
Larger-gauge PICCs, VTE history increase PICC-DVT risk
Risk factors of upper-extremity deep vein thrombosis associated with peripherally inserted central catheters include a history of venous thromboembolism and a larger-gauge catheter, according to Dr. Vineet Chopra and his associates at the University of Michigan Health System, Ann Arbor.
Compared to 4-Fr gauge PICCs, 5-Fr and 6-Fr PICCs significantly increased PICC-DVT risk, with odds ratios of 2.7 and 7.4, respectively. The OR for patients with a prior history of VTE was 1.7. While any surgery with a PICC in place increased risk, the OR was higher in surgery lasting less than 2 hours (2.75 vs. 2.17). PICCs inserted into the brachial or cephalic vein also increased PICC-DVT risk, compared with the basilic vein, with ORs of 6.8 and 5.8, respectively.
Patients who received both aspirin and statins were at a significantly lower risk of PICC-DVT than those who received only one drug or neither, with an OR of 0.31 for those who got both, compared with 0.77 for aspirin and 0.61 for statins. Pharmacologic VTE prophylaxis also benefited patients with an OR of DVT at 0.72.
“Given the existence of other supportive data regarding the influence of VTE prophylaxis on PICC-DVT and a potentially protective role of statins on thrombosis, further controlled studies of VTE prophylaxis, antiplatelet treatment, and statins to prevent PICC-DVT appear warranted,” the investigators recommended.
Find the full study in Thrombosis Research (doi:10.1016/j.thromres.2015.02.0120).
Risk factors of upper-extremity deep vein thrombosis associated with peripherally inserted central catheters include a history of venous thromboembolism and a larger-gauge catheter, according to Dr. Vineet Chopra and his associates at the University of Michigan Health System, Ann Arbor.
Compared to 4-Fr gauge PICCs, 5-Fr and 6-Fr PICCs significantly increased PICC-DVT risk, with odds ratios of 2.7 and 7.4, respectively. The OR for patients with a prior history of VTE was 1.7. While any surgery with a PICC in place increased risk, the OR was higher in surgery lasting less than 2 hours (2.75 vs. 2.17). PICCs inserted into the brachial or cephalic vein also increased PICC-DVT risk, compared with the basilic vein, with ORs of 6.8 and 5.8, respectively.
Patients who received both aspirin and statins were at a significantly lower risk of PICC-DVT than those who received only one drug or neither, with an OR of 0.31 for those who got both, compared with 0.77 for aspirin and 0.61 for statins. Pharmacologic VTE prophylaxis also benefited patients with an OR of DVT at 0.72.
“Given the existence of other supportive data regarding the influence of VTE prophylaxis on PICC-DVT and a potentially protective role of statins on thrombosis, further controlled studies of VTE prophylaxis, antiplatelet treatment, and statins to prevent PICC-DVT appear warranted,” the investigators recommended.
Find the full study in Thrombosis Research (doi:10.1016/j.thromres.2015.02.0120).
Risk factors of upper-extremity deep vein thrombosis associated with peripherally inserted central catheters include a history of venous thromboembolism and a larger-gauge catheter, according to Dr. Vineet Chopra and his associates at the University of Michigan Health System, Ann Arbor.
Compared to 4-Fr gauge PICCs, 5-Fr and 6-Fr PICCs significantly increased PICC-DVT risk, with odds ratios of 2.7 and 7.4, respectively. The OR for patients with a prior history of VTE was 1.7. While any surgery with a PICC in place increased risk, the OR was higher in surgery lasting less than 2 hours (2.75 vs. 2.17). PICCs inserted into the brachial or cephalic vein also increased PICC-DVT risk, compared with the basilic vein, with ORs of 6.8 and 5.8, respectively.
Patients who received both aspirin and statins were at a significantly lower risk of PICC-DVT than those who received only one drug or neither, with an OR of 0.31 for those who got both, compared with 0.77 for aspirin and 0.61 for statins. Pharmacologic VTE prophylaxis also benefited patients with an OR of DVT at 0.72.
“Given the existence of other supportive data regarding the influence of VTE prophylaxis on PICC-DVT and a potentially protective role of statins on thrombosis, further controlled studies of VTE prophylaxis, antiplatelet treatment, and statins to prevent PICC-DVT appear warranted,” the investigators recommended.
Find the full study in Thrombosis Research (doi:10.1016/j.thromres.2015.02.0120).
June 2015 Quiz 2
Q2: ANSWER: A
Critique
This pregnant patient has features compatible with Listeria monocytogenes infection. In any pregnant patient presenting with fever, Listeria needs to be considered after ruling out common conditions such as a urinary tract infection given the high morbidity associated with this condition. Blood cultures are used to make the diagnosis of Listeria, while stool and vaginal cultures are not helpful. While CSF cultures can be used in cases of Listeria meningitis, the yield of blood cultures is higher. Urine cultures are unlikely to add additional information in this patient’s case given the negative urine dipstick.
Reference
1. Jackson, K.A., Iwamoto, M., Swerdlow, D. Pregnancy-associated listeriosis. Epidemiol. Infect. 2010;138:1503-9.
Q2: ANSWER: A
Critique
This pregnant patient has features compatible with Listeria monocytogenes infection. In any pregnant patient presenting with fever, Listeria needs to be considered after ruling out common conditions such as a urinary tract infection given the high morbidity associated with this condition. Blood cultures are used to make the diagnosis of Listeria, while stool and vaginal cultures are not helpful. While CSF cultures can be used in cases of Listeria meningitis, the yield of blood cultures is higher. Urine cultures are unlikely to add additional information in this patient’s case given the negative urine dipstick.
Reference
1. Jackson, K.A., Iwamoto, M., Swerdlow, D. Pregnancy-associated listeriosis. Epidemiol. Infect. 2010;138:1503-9.
Q2: ANSWER: A
Critique
This pregnant patient has features compatible with Listeria monocytogenes infection. In any pregnant patient presenting with fever, Listeria needs to be considered after ruling out common conditions such as a urinary tract infection given the high morbidity associated with this condition. Blood cultures are used to make the diagnosis of Listeria, while stool and vaginal cultures are not helpful. While CSF cultures can be used in cases of Listeria meningitis, the yield of blood cultures is higher. Urine cultures are unlikely to add additional information in this patient’s case given the negative urine dipstick.
Reference
1. Jackson, K.A., Iwamoto, M., Swerdlow, D. Pregnancy-associated listeriosis. Epidemiol. Infect. 2010;138:1503-9.
June 2015 Quiz 1
Q1: ANSWER: D
Critique
The patient has had complicated recurrent Crohn’s disease and two resections. If this were his first resection of a short-segment ileal stricture, then choice (a) would be a reasonable alternative to starting medication at this time. This is based on the endoscopic scoring system of Rutgeerts in which endoscopic findings in the neoterminal ileum at 6-12 months postoperatively are somewhat predictive of clinical recurrence over the next 5 years. Short-term antibiotic therapy with ciprofloxacin has not been shown to be a good long-term solution to prevention of postoperative recurrence. Both azathioprine and 6-mercaptopurine have a modest effect on the prevention of postoperative recurrence of Crohn’s disease. Azathioprine at 1 mg/kg per day would be less than an optimal dose for this purpose. The D’Haens study used 1.5-2 mg/kg of azathioprine in combination with 3 months of metronidazole. At 1 year the endoscopic recurrence rate (i2-i4 lesions) was greater in the placebo group at 69% compared with 44% in the azathioprine-treated group. Infliximab has been shown in a small randomized trial to be very effective in preventing postoperative recurrence of Crohn’s disease. In a 24-subject randomized trial, 91% of the infliximab treated patients were free of endoscopic recurrence, compared to 9% of patients receiving placebo. In this patient, who is at a high risk for recurrence with recurrent inflammation, multiple surgeries, and continued smoking, anti-TNF therapy should be initiated within 4 weeks after surgery. Mesalamine has minimal postoperative preventative effects and would not be appropriate monotherapy to use in this high-risk patient.
References
1. D’Haens, G.R., Vermeire, S., Van Assche G., et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology 2008;135:1123-9.
2. Regueiro, M., Schraut, W., Baidoo, L., et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441-50.e1;quiz 716. Epub 2008 Oct 31.
3. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956-63.
4. Schwartz M, Regueiro M. Prevention and treatment of postoperative Crohn’s disease recurrence: an update for a new decade. Curr. Gastroenterol. Rep. 2011;13:95-100.
Q1: ANSWER: D
Critique
The patient has had complicated recurrent Crohn’s disease and two resections. If this were his first resection of a short-segment ileal stricture, then choice (a) would be a reasonable alternative to starting medication at this time. This is based on the endoscopic scoring system of Rutgeerts in which endoscopic findings in the neoterminal ileum at 6-12 months postoperatively are somewhat predictive of clinical recurrence over the next 5 years. Short-term antibiotic therapy with ciprofloxacin has not been shown to be a good long-term solution to prevention of postoperative recurrence. Both azathioprine and 6-mercaptopurine have a modest effect on the prevention of postoperative recurrence of Crohn’s disease. Azathioprine at 1 mg/kg per day would be less than an optimal dose for this purpose. The D’Haens study used 1.5-2 mg/kg of azathioprine in combination with 3 months of metronidazole. At 1 year the endoscopic recurrence rate (i2-i4 lesions) was greater in the placebo group at 69% compared with 44% in the azathioprine-treated group. Infliximab has been shown in a small randomized trial to be very effective in preventing postoperative recurrence of Crohn’s disease. In a 24-subject randomized trial, 91% of the infliximab treated patients were free of endoscopic recurrence, compared to 9% of patients receiving placebo. In this patient, who is at a high risk for recurrence with recurrent inflammation, multiple surgeries, and continued smoking, anti-TNF therapy should be initiated within 4 weeks after surgery. Mesalamine has minimal postoperative preventative effects and would not be appropriate monotherapy to use in this high-risk patient.
References
1. D’Haens, G.R., Vermeire, S., Van Assche G., et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology 2008;135:1123-9.
2. Regueiro, M., Schraut, W., Baidoo, L., et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441-50.e1;quiz 716. Epub 2008 Oct 31.
3. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956-63.
4. Schwartz M, Regueiro M. Prevention and treatment of postoperative Crohn’s disease recurrence: an update for a new decade. Curr. Gastroenterol. Rep. 2011;13:95-100.
Q1: ANSWER: D
Critique
The patient has had complicated recurrent Crohn’s disease and two resections. If this were his first resection of a short-segment ileal stricture, then choice (a) would be a reasonable alternative to starting medication at this time. This is based on the endoscopic scoring system of Rutgeerts in which endoscopic findings in the neoterminal ileum at 6-12 months postoperatively are somewhat predictive of clinical recurrence over the next 5 years. Short-term antibiotic therapy with ciprofloxacin has not been shown to be a good long-term solution to prevention of postoperative recurrence. Both azathioprine and 6-mercaptopurine have a modest effect on the prevention of postoperative recurrence of Crohn’s disease. Azathioprine at 1 mg/kg per day would be less than an optimal dose for this purpose. The D’Haens study used 1.5-2 mg/kg of azathioprine in combination with 3 months of metronidazole. At 1 year the endoscopic recurrence rate (i2-i4 lesions) was greater in the placebo group at 69% compared with 44% in the azathioprine-treated group. Infliximab has been shown in a small randomized trial to be very effective in preventing postoperative recurrence of Crohn’s disease. In a 24-subject randomized trial, 91% of the infliximab treated patients were free of endoscopic recurrence, compared to 9% of patients receiving placebo. In this patient, who is at a high risk for recurrence with recurrent inflammation, multiple surgeries, and continued smoking, anti-TNF therapy should be initiated within 4 weeks after surgery. Mesalamine has minimal postoperative preventative effects and would not be appropriate monotherapy to use in this high-risk patient.
References
1. D’Haens, G.R., Vermeire, S., Van Assche G., et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology 2008;135:1123-9.
2. Regueiro, M., Schraut, W., Baidoo, L., et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441-50.e1;quiz 716. Epub 2008 Oct 31.
3. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956-63.
4. Schwartz M, Regueiro M. Prevention and treatment of postoperative Crohn’s disease recurrence: an update for a new decade. Curr. Gastroenterol. Rep. 2011;13:95-100.


