User login
Placenta-derived cells may improve recovery after HSCT

Cells derived from placenta can increase blood counts after hematopoietic stem cell transplant (HSCT), preclinical research suggests.
Investigators evaluated PLX-R18, a product consisting of mesenchymal-like adherent stromal cells derived from full-term human placentas, in mice undergoing HSCT.
Mice that received PLX-R18 in conjunction with HSCT had significantly faster hematopoietic recovery than mice that received placebo with their transplants.
Pluristem Therapeutics, Inc., the company developing PLX-R18, recently announced these results.
The study included 78 irradiated mice divided into 4 groups. One group received a transplant of 4 million HSCs plus an intra-muscular (IM) injection of 1 million PLX-R18 cells on days 1 and 10. A second group received 8 million HSCs plus an IM injection of 1 million PLX-R18 cells on days 1 and 10.
The first control group received 4 million HSCs plus an IM injection of placebo on days 1 and 10. And the second control group received 8 million HSCs plus an IM injection of placebo on days 1 and 10.
The investigators performed complete blood counts on day 9 after HSCT and the first dose of PLX-R18 or placebo, on day 16 after the second dose of PLX-R18 or placebo, and on day 23.
Nine days after transplantation with a low dose of HSCs (4 million) and concurrent administration of either PLX-R18 or placebo, mice treated with PLX-R18 had statistically significant increases in platelets and granulocytes when compared to controls (P=0.0059 and P=0.0267, respectively).
PLX-R18-treated mice also had more lymphocytes and total white blood cells, but these increases were not statistically significant.
Nine days after transplantation with a high dose of HSCs (8 million) and concurrent administration of either PLX-R18 or placebo, mice treated with PLX-R18 had statistically significant increases in platelet levels (P=0.0015).
One week later, at 16 days after a low-dose HSCT, mice treated with PLX-R18 had more platelets than controls, although the difference wasn’t significant.
Also on day 16, mice treated with PLX-R18 and a high dose of HSCs had statistically significant increases in platelets, granulocytes, and total white blood cells compared to controls (P=0.0053, P=0.0122, and P=0.0262 respectively).
On day 23, there were no significant differences in the number of cells between the treatment groups.
Taking these results together, the investigators concluded that PLX-R18 cells can significantly accelerate the recovery of several components of normal blood counts.
“A statistically significant increase in blood counts soon after bone marrow transplant is very meaningful,” said Reuven Or, MD, of Hadassah Medical Center in Haifa, Israel.
“We were particularly encouraged to see that the administration of PLX-R18 cells resulted in the greatest early improvement when using a lower dose of bone marrow cells. This means we could one day potentially achieve success with lower bone marrow transplant doses, thus addressing both treatment costs and donor availability.” ![]()

Cells derived from placenta can increase blood counts after hematopoietic stem cell transplant (HSCT), preclinical research suggests.
Investigators evaluated PLX-R18, a product consisting of mesenchymal-like adherent stromal cells derived from full-term human placentas, in mice undergoing HSCT.
Mice that received PLX-R18 in conjunction with HSCT had significantly faster hematopoietic recovery than mice that received placebo with their transplants.
Pluristem Therapeutics, Inc., the company developing PLX-R18, recently announced these results.
The study included 78 irradiated mice divided into 4 groups. One group received a transplant of 4 million HSCs plus an intra-muscular (IM) injection of 1 million PLX-R18 cells on days 1 and 10. A second group received 8 million HSCs plus an IM injection of 1 million PLX-R18 cells on days 1 and 10.
The first control group received 4 million HSCs plus an IM injection of placebo on days 1 and 10. And the second control group received 8 million HSCs plus an IM injection of placebo on days 1 and 10.
The investigators performed complete blood counts on day 9 after HSCT and the first dose of PLX-R18 or placebo, on day 16 after the second dose of PLX-R18 or placebo, and on day 23.
Nine days after transplantation with a low dose of HSCs (4 million) and concurrent administration of either PLX-R18 or placebo, mice treated with PLX-R18 had statistically significant increases in platelets and granulocytes when compared to controls (P=0.0059 and P=0.0267, respectively).
PLX-R18-treated mice also had more lymphocytes and total white blood cells, but these increases were not statistically significant.
Nine days after transplantation with a high dose of HSCs (8 million) and concurrent administration of either PLX-R18 or placebo, mice treated with PLX-R18 had statistically significant increases in platelet levels (P=0.0015).
One week later, at 16 days after a low-dose HSCT, mice treated with PLX-R18 had more platelets than controls, although the difference wasn’t significant.
Also on day 16, mice treated with PLX-R18 and a high dose of HSCs had statistically significant increases in platelets, granulocytes, and total white blood cells compared to controls (P=0.0053, P=0.0122, and P=0.0262 respectively).
On day 23, there were no significant differences in the number of cells between the treatment groups.
Taking these results together, the investigators concluded that PLX-R18 cells can significantly accelerate the recovery of several components of normal blood counts.
“A statistically significant increase in blood counts soon after bone marrow transplant is very meaningful,” said Reuven Or, MD, of Hadassah Medical Center in Haifa, Israel.
“We were particularly encouraged to see that the administration of PLX-R18 cells resulted in the greatest early improvement when using a lower dose of bone marrow cells. This means we could one day potentially achieve success with lower bone marrow transplant doses, thus addressing both treatment costs and donor availability.” ![]()

Cells derived from placenta can increase blood counts after hematopoietic stem cell transplant (HSCT), preclinical research suggests.
Investigators evaluated PLX-R18, a product consisting of mesenchymal-like adherent stromal cells derived from full-term human placentas, in mice undergoing HSCT.
Mice that received PLX-R18 in conjunction with HSCT had significantly faster hematopoietic recovery than mice that received placebo with their transplants.
Pluristem Therapeutics, Inc., the company developing PLX-R18, recently announced these results.
The study included 78 irradiated mice divided into 4 groups. One group received a transplant of 4 million HSCs plus an intra-muscular (IM) injection of 1 million PLX-R18 cells on days 1 and 10. A second group received 8 million HSCs plus an IM injection of 1 million PLX-R18 cells on days 1 and 10.
The first control group received 4 million HSCs plus an IM injection of placebo on days 1 and 10. And the second control group received 8 million HSCs plus an IM injection of placebo on days 1 and 10.
The investigators performed complete blood counts on day 9 after HSCT and the first dose of PLX-R18 or placebo, on day 16 after the second dose of PLX-R18 or placebo, and on day 23.
Nine days after transplantation with a low dose of HSCs (4 million) and concurrent administration of either PLX-R18 or placebo, mice treated with PLX-R18 had statistically significant increases in platelets and granulocytes when compared to controls (P=0.0059 and P=0.0267, respectively).
PLX-R18-treated mice also had more lymphocytes and total white blood cells, but these increases were not statistically significant.
Nine days after transplantation with a high dose of HSCs (8 million) and concurrent administration of either PLX-R18 or placebo, mice treated with PLX-R18 had statistically significant increases in platelet levels (P=0.0015).
One week later, at 16 days after a low-dose HSCT, mice treated with PLX-R18 had more platelets than controls, although the difference wasn’t significant.
Also on day 16, mice treated with PLX-R18 and a high dose of HSCs had statistically significant increases in platelets, granulocytes, and total white blood cells compared to controls (P=0.0053, P=0.0122, and P=0.0262 respectively).
On day 23, there were no significant differences in the number of cells between the treatment groups.
Taking these results together, the investigators concluded that PLX-R18 cells can significantly accelerate the recovery of several components of normal blood counts.
“A statistically significant increase in blood counts soon after bone marrow transplant is very meaningful,” said Reuven Or, MD, of Hadassah Medical Center in Haifa, Israel.
“We were particularly encouraged to see that the administration of PLX-R18 cells resulted in the greatest early improvement when using a lower dose of bone marrow cells. This means we could one day potentially achieve success with lower bone marrow transplant doses, thus addressing both treatment costs and donor availability.” ![]()
New radiation guidelines for pediatric HL
New guidelines on radiation therapy aim to help physicians more effectively treat pediatric Hodgkin lymphoma (HL) while reducing the radiation dose to normal tissue.
Previous guidelines for pediatric HL have focused on 2D imaging and bony landmarks to define dose volumes for radiation therapy, and they’ve recommended treating large volumes of normal tissue, in part, because of uncertainty about which lymph node areas were involved.
The new guidelines, published in Practical Radiation Oncology, describe how to use modern imaging and advances in radiation therapy planning technology to treat patients with pediatric HL while decreasing the risk of late side effects, including second cancers and heart disease.
The authors describe methods for identifying target volumes for radiation therapy and how to implement the concept of involved-site radiation to define radiation target volumes and limit the dose to normal organs at risk.
According to the guidelines, accurate assessment of the extent and location of disease requires both contrast-enhanced CT as well as FDG-PET.
The document describes how the evaluation of response to chemotherapy influences the targeting of the lymphoma and the volume of normal tissue treated, by fusing CT and FDG-PET images taken before and after chemotherapy to CT imaging taken for radiation therapy planning.
“The emergence of new imaging technologies, more accurate ways of delivering radiation therapy, and more detailed patient selection criteria have made a significant change in our ability to customize treatment for many cancer patients,” said lead guideline author David C. Hodgson, MD, of the University of Toronto in Ontario, Canada.
“This guideline has the potential to reduce the radiation therapy breast dose by about 80% and the heart dose by about 65% for an adolescent girl with Hodgkin lymphoma. This shift in more personalized treatment planning tailored to the individual patient’s disease will optimize risk-benefit considerations for our patients and reduce the likelihood that they will suffer late effects from radiation therapy.” ![]()

New guidelines on radiation therapy aim to help physicians more effectively treat pediatric Hodgkin lymphoma (HL) while reducing the radiation dose to normal tissue.
Previous guidelines for pediatric HL have focused on 2D imaging and bony landmarks to define dose volumes for radiation therapy, and they’ve recommended treating large volumes of normal tissue, in part, because of uncertainty about which lymph node areas were involved.
The new guidelines, published in Practical Radiation Oncology, describe how to use modern imaging and advances in radiation therapy planning technology to treat patients with pediatric HL while decreasing the risk of late side effects, including second cancers and heart disease.
The authors describe methods for identifying target volumes for radiation therapy and how to implement the concept of involved-site radiation to define radiation target volumes and limit the dose to normal organs at risk.
According to the guidelines, accurate assessment of the extent and location of disease requires both contrast-enhanced CT as well as FDG-PET.
The document describes how the evaluation of response to chemotherapy influences the targeting of the lymphoma and the volume of normal tissue treated, by fusing CT and FDG-PET images taken before and after chemotherapy to CT imaging taken for radiation therapy planning.
“The emergence of new imaging technologies, more accurate ways of delivering radiation therapy, and more detailed patient selection criteria have made a significant change in our ability to customize treatment for many cancer patients,” said lead guideline author David C. Hodgson, MD, of the University of Toronto in Ontario, Canada.
“This guideline has the potential to reduce the radiation therapy breast dose by about 80% and the heart dose by about 65% for an adolescent girl with Hodgkin lymphoma. This shift in more personalized treatment planning tailored to the individual patient’s disease will optimize risk-benefit considerations for our patients and reduce the likelihood that they will suffer late effects from radiation therapy.” ![]()

New guidelines on radiation therapy aim to help physicians more effectively treat pediatric Hodgkin lymphoma (HL) while reducing the radiation dose to normal tissue.
Previous guidelines for pediatric HL have focused on 2D imaging and bony landmarks to define dose volumes for radiation therapy, and they’ve recommended treating large volumes of normal tissue, in part, because of uncertainty about which lymph node areas were involved.
The new guidelines, published in Practical Radiation Oncology, describe how to use modern imaging and advances in radiation therapy planning technology to treat patients with pediatric HL while decreasing the risk of late side effects, including second cancers and heart disease.
The authors describe methods for identifying target volumes for radiation therapy and how to implement the concept of involved-site radiation to define radiation target volumes and limit the dose to normal organs at risk.
According to the guidelines, accurate assessment of the extent and location of disease requires both contrast-enhanced CT as well as FDG-PET.
The document describes how the evaluation of response to chemotherapy influences the targeting of the lymphoma and the volume of normal tissue treated, by fusing CT and FDG-PET images taken before and after chemotherapy to CT imaging taken for radiation therapy planning.
“The emergence of new imaging technologies, more accurate ways of delivering radiation therapy, and more detailed patient selection criteria have made a significant change in our ability to customize treatment for many cancer patients,” said lead guideline author David C. Hodgson, MD, of the University of Toronto in Ontario, Canada.
“This guideline has the potential to reduce the radiation therapy breast dose by about 80% and the heart dose by about 65% for an adolescent girl with Hodgkin lymphoma. This shift in more personalized treatment planning tailored to the individual patient’s disease will optimize risk-benefit considerations for our patients and reduce the likelihood that they will suffer late effects from radiation therapy.” ![]()
Parasite discovery could aid malaria treatment
Image by Ke Hu & John Murray
Researchers say they have gained new insight into how malaria-related parasites spread inside humans and other animals.
The team discovered how the malaria relative Toxoplasma gondii manages to replicate its chromosomes up to thousands of times before spinning off into daughter cells—all while avoiding cell death.
The findings, published in PLOS Biology, may have implications for malaria treatment, according to the researchers.
Once transmitted into an animal or human, malaria-related parasites can hide out in a single cell in many different tissues, replicating thousands of times before the host’s immune system can detect them.
Then, they burst forth as daughter cells, which are unleashed in massive quantities, quickly overwhelming the body’s immune response.
The researchers found that Toxoplasma parasites pull this off thanks to the centrosome, which imposes order on the replication chaos.
“Unlike the comparatively simple centrosome present in human cells, the parasite [centrosome] has 2 distinct operating machines,” said study author Michael White, PhD, of the University of South Florida in Tampa.
“One machine controls chromosome copying, while the other machine regulates when to form daughter cell bodies. Working together, but with independent responsibilities, parasite centrosome machines can dictate the scale and timing of pathogen replication.”
This discovery of the centrosome’s function leads to a critical conclusion, Dr White said. Disrupting the centrosome machines kills the parasite. Breaking any part of the highly efficient but highly fragile replication function shuts everything down.
With these findings and the new knowledge of the parasites’ vulnerabilities, Dr White and his fellow researchers are planning to explore drug development for malaria. Whether the team is able to find an already-approved drug or must develop one from scratch, they said the drug will need to be used in conjunction with other therapies.
Dr White noted that current drugs used to treat malaria target the pathogen’s metabolism. But the goal of the new drug will be to undermine the parasite’s foundation in enough of the spreading cells to allow the immune system to fight back and not become overwhelmed. ![]()

Image by Ke Hu & John Murray
Researchers say they have gained new insight into how malaria-related parasites spread inside humans and other animals.
The team discovered how the malaria relative Toxoplasma gondii manages to replicate its chromosomes up to thousands of times before spinning off into daughter cells—all while avoiding cell death.
The findings, published in PLOS Biology, may have implications for malaria treatment, according to the researchers.
Once transmitted into an animal or human, malaria-related parasites can hide out in a single cell in many different tissues, replicating thousands of times before the host’s immune system can detect them.
Then, they burst forth as daughter cells, which are unleashed in massive quantities, quickly overwhelming the body’s immune response.
The researchers found that Toxoplasma parasites pull this off thanks to the centrosome, which imposes order on the replication chaos.
“Unlike the comparatively simple centrosome present in human cells, the parasite [centrosome] has 2 distinct operating machines,” said study author Michael White, PhD, of the University of South Florida in Tampa.
“One machine controls chromosome copying, while the other machine regulates when to form daughter cell bodies. Working together, but with independent responsibilities, parasite centrosome machines can dictate the scale and timing of pathogen replication.”
This discovery of the centrosome’s function leads to a critical conclusion, Dr White said. Disrupting the centrosome machines kills the parasite. Breaking any part of the highly efficient but highly fragile replication function shuts everything down.
With these findings and the new knowledge of the parasites’ vulnerabilities, Dr White and his fellow researchers are planning to explore drug development for malaria. Whether the team is able to find an already-approved drug or must develop one from scratch, they said the drug will need to be used in conjunction with other therapies.
Dr White noted that current drugs used to treat malaria target the pathogen’s metabolism. But the goal of the new drug will be to undermine the parasite’s foundation in enough of the spreading cells to allow the immune system to fight back and not become overwhelmed. ![]()

Image by Ke Hu & John Murray
Researchers say they have gained new insight into how malaria-related parasites spread inside humans and other animals.
The team discovered how the malaria relative Toxoplasma gondii manages to replicate its chromosomes up to thousands of times before spinning off into daughter cells—all while avoiding cell death.
The findings, published in PLOS Biology, may have implications for malaria treatment, according to the researchers.
Once transmitted into an animal or human, malaria-related parasites can hide out in a single cell in many different tissues, replicating thousands of times before the host’s immune system can detect them.
Then, they burst forth as daughter cells, which are unleashed in massive quantities, quickly overwhelming the body’s immune response.
The researchers found that Toxoplasma parasites pull this off thanks to the centrosome, which imposes order on the replication chaos.
“Unlike the comparatively simple centrosome present in human cells, the parasite [centrosome] has 2 distinct operating machines,” said study author Michael White, PhD, of the University of South Florida in Tampa.
“One machine controls chromosome copying, while the other machine regulates when to form daughter cell bodies. Working together, but with independent responsibilities, parasite centrosome machines can dictate the scale and timing of pathogen replication.”
This discovery of the centrosome’s function leads to a critical conclusion, Dr White said. Disrupting the centrosome machines kills the parasite. Breaking any part of the highly efficient but highly fragile replication function shuts everything down.
With these findings and the new knowledge of the parasites’ vulnerabilities, Dr White and his fellow researchers are planning to explore drug development for malaria. Whether the team is able to find an already-approved drug or must develop one from scratch, they said the drug will need to be used in conjunction with other therapies.
Dr White noted that current drugs used to treat malaria target the pathogen’s metabolism. But the goal of the new drug will be to undermine the parasite’s foundation in enough of the spreading cells to allow the immune system to fight back and not become overwhelmed. ![]()
OUs and Patient Outcomes
Many pediatric hospitalizations are of short duration, and more than half of short‐stay hospitalizations are designated as observation status.[1, 2] Observation status is an administrative label assigned to patients who do not meet hospital or payer criteria for inpatient‐status care. Short‐stay observation‐status patients do not fit in traditional models of emergency department (ED) or inpatient care. EDs often focus on discharging or admitting patients within a matter of hours, whereas inpatient units tend to measure length of stay (LOS) in terms of days[3] and may not have systems in place to facilitate rapid discharge of short‐stay patients.[4] Observation units (OUs) have been established in some hospitals to address the unique care needs of short‐stay patients.[5, 6, 7]
Single‐site reports from children's hospitals with successful OUs have demonstrated shorter LOS and lower costs compared with inpatient settings.[6, 8, 9, 10, 11, 12, 13, 14] No prior study has examined hospital‐level effects of an OU on observation‐status patient outcomes. The Pediatric Health Information System (PHIS) database provides a unique opportunity to explore this question, because unlike other national hospital administrative databases,[15, 16] the PHIS dataset contains information about children under observation status. In addition, we know which PHIS hospitals had a dedicated OU in 2011.7
We hypothesized that overall observation‐status stays in hospitals with a dedicated OU would be of shorter duration with earlier discharges at lower cost than observation‐status stays in hospitals without a dedicated OU. We compared hospitals with and without a dedicated OU on secondary outcomes including rates of conversion to inpatient status and return care for any reason.
METHODS
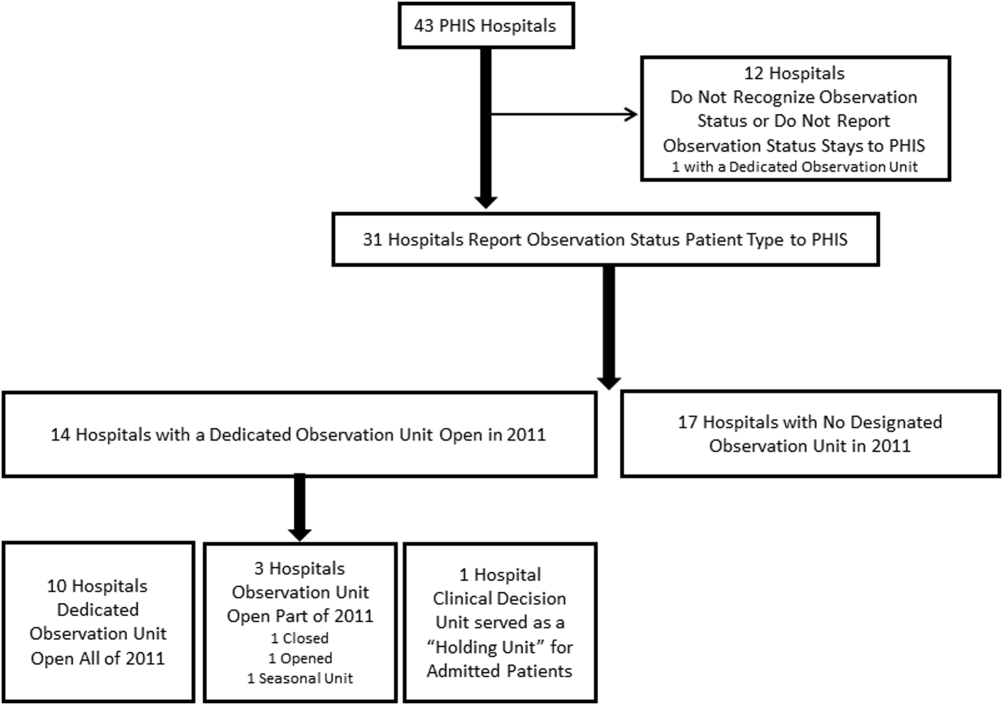
We conducted a cross‐sectional analysis of hospital administrative data using the 2011 PHIS databasea national administrative database that contains resource utilization data from 43 participating hospitals located in 26 states plus the District of Columbia. These hospitals account for approximately 20% of pediatric hospitalizations in the United States.
For each hospital encounter, PHIS includes patient demographics, up to 41 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses, up to 41 ICD‐9‐CM procedures, and hospital charges for services. Data are deidentified prior to inclusion, but unique identifiers allow for determination of return visits and readmissions following an index visit for an individual patient. Data quality and reliability are assured jointly by the Children's Hospital Association (formerly Child Health Corporation of America, Overland Park, KS), participating hospitals, and Truven Health Analytics (New York, NY). This study, using administrative data, was not considered human subjects research by the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board.
Hospital Selection and Hospital Characteristics
The study sample was drawn from the 31 hospitals that reported observation‐status patient data to PHIS in 2011. Analyses were conducted in 2013, at which time 2011 was the most recent year of data. We categorized 14 hospitals as having a dedicated OU during 2011 based on information collected in 2013.7 To summarize briefly, we interviewed by telephone representatives of hospitals responding to an email query as to the presence of a geographically distinct OU for the care of unscheduled patients from the ED. Three of the 14 representatives reported their hospital had 2 OUs, 1 of which was a separate surgical OU. Ten OUs cared for both ED patients and patients with scheduled procedures; 8 units received patients from non‐ED sources. Hospitalists provided staffing in more than half of the OUs.
We attempted to identify administrative data that would signal care delivered in a dedicated OU using hospital charge codes reported to PHIS, but learned this was not possible due to between‐hospital variation in the specificity of the charge codes. Therefore, we were unable to determine if patient care was delivered in a dedicated OU or another setting, such as a general inpatient unit or the ED. Other hospital characteristics available from the PHIS dataset included the number of inpatient beds, ED visits, inpatient admissions, observation‐status stays, and payer mix. We calculated the percentage of ED visits resulting in admission by dividing the number of ED visits with associated inpatient or observation status by the total number of ED visits and the percentage of admissions under observation status by dividing the number of observation‐status stays by the total number of admissions under observation or inpatient status.
Visit Selection and Patient Characteristics
All observation‐status stays regardless of the point of entry into the hospital were eligible for this study. We excluded stays that were birth‐related, included intensive care, or resulted in transfer or death. Patient demographic characteristics used to describe the cohort included age, gender, race/ethnicity, and primary payer. Stays that began in the ED were identified by an emergency room charge within PHIS. Eligible stays were categorized using All Patient Refined Diagnosis Related Groups (APR‐DRGs) version 24 using the ICD‐9‐CM code‐based proprietary 3M software (3M Health Information Systems, St. Paul, MN). We determined the 15 top‐ranking APR‐DRGs among observation‐status stays in hospitals with a dedicated OU and hospitals without. Procedural stays were identified based on procedural APR‐DRGs (eg, tonsil and adenoid procedures) or the presence of an ICD‐9‐CM procedure code (eg, 331 spinal tap).
Measured Outcomes
Outcomes of observation‐status stays were determined within 4 categories: (1) LOS, (2) standardized costs, (3) conversion to inpatient status, and (4) return visits and readmissions. LOS was calculated in terms of nights spent in hospital for all stays by subtracting the discharge date from the admission date and in terms of hours for stays in the 28 hospitals that report admission and discharge hour to the PHIS database. Discharge timing was examined in 4, 6‐hour blocks starting at midnight. Standardized costs were derived from a charge master index that was created by taking the median costs from all PHIS hospitals for each charged service.[17] Standardized costs represent the estimated cost of providing any particular clinical activity but are not the cost to patients, nor do they represent the actual cost to any given hospital. This approach allows for cost comparisons across hospitals, without biases arising from using charges or from deriving costs using hospitals' ratios of costs to charges.[18] Conversion from observation to inpatient status was calculated by dividing the number of inpatient‐status stays with observation codes by the number of observation‐statusonly stays plus the number of inpatient‐status stays with observation codes. All‐cause 3‐day ED return visits and 30‐day readmissions to the same hospital were assessed using patient‐specific identifiers that allowed for tracking of ED return visits and readmissions following the index observation stay.
Data Analysis
Descriptive statistics were calculated for hospital and patient characteristics using medians and interquartile ranges (IQRs) for continuous factors and frequencies with percentages for categorical factors. Comparisons of these factors between hospitals with dedicated OUs and without were made using [2] and Wilcoxon rank sum tests as appropriate. Multivariable regression was performed using generalized linear mixed models treating hospital as a random effect and used patient age, the case‐mix index based on the APR‐DRG severity of illness, ED visit, and procedures associated with the index observation‐status stay. For continuous outcomes, we performed a log transformation on the outcome, confirmed the normality assumption, and back transformed the results. Sensitivity analyses were conducted to compare LOS, standardized costs, and conversation rates by hospital type for 10 of the 15 top‐ranking APR‐DRGs commonly cared for by pediatric hospitalists and to compare hospitals that reported the presence of an OU that was consistently open (24 hours per day, 7 days per week) and operating during the entire 2011 calendar year, and those without. Based on information gathered from the telephone interviews, hospitals with partially open OUs were similar to hospitals with continuously open OUs, such that they were included in our main analyses. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Hospital Characteristics
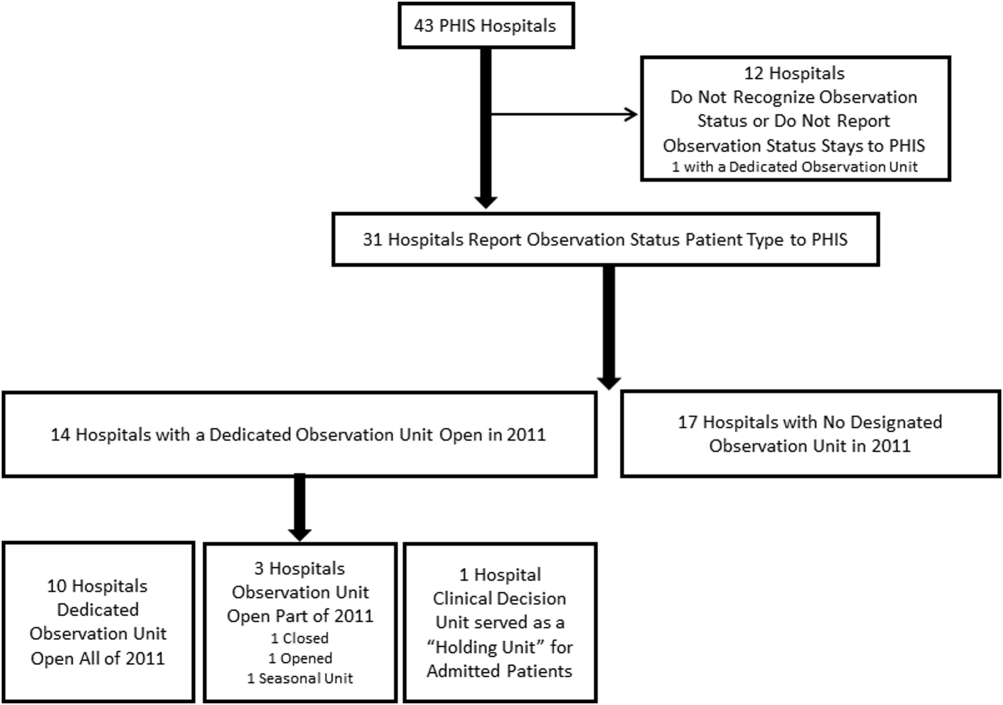
Dedicated OUs were present in 14 of the 31 hospitals that reported observation‐status patient data to PHIS (Figure 1). Three of these hospitals had OUs that were open for 5 months or less in 2011; 1 unit opened, 1 unit closed, and 1 hospital operated a seasonal unit. The remaining 17 hospitals reported no OU that admitted unscheduled patients from the ED during 2011. Hospitals with a dedicated OU had more inpatient beds and higher median number of inpatient admissions than those without (Table 1). Hospitals were statistically similar in terms of total volume of ED visits, percentage of ED visits resulting in admission, total number of observation‐status stays, percentage of admissions under observation status, and payer mix.
| Overall, Median (IQR) | Hospitals With a Dedicated Observation Unit, Median (IQR) | Hospitals Without a Dedicated Observation Unit, Median (IQR) | P Value | |
|---|---|---|---|---|
| ||||
| No. of hospitals | 31 | 14 | 17 | |
| Total no. of inpatient beds | 273 (213311) | 304 (269425) | 246 (175293) | 0.006 |
| Total no. ED visits | 62971 (47,50497,723) | 87,892 (55,102117,119) | 53,151 (4750470,882) | 0.21 |
| ED visits resulting in admission, % | 13.1 (9.715.0) | 13.8 (10.5, 19.1) | 12.5 (9.714.5) | 0.31 |
| Total no. of inpatient admissions | 11,537 (9,26814,568) | 13,206 (11,32517,869) | 10,207 (8,64013,363) | 0.04 |
| Admissions under observation status, % | 25.7 (19.733.8) | 25.5 (21.431.4) | 26.0 (16.935.1) | 0.98 |
| Total no. of observation stays | 3,820 (27935672) | 4,850 (3,309 6,196) | 3,141 (2,3654,616) | 0.07 |
| Government payer, % | 60.2 (53.371.2) | 62.1 (54.9, 65.9) | 59.2 (53.373.7) | 0.89 |
Observation‐Status Patients by Hospital Type
In 2011, there were a total of 136,239 observation‐status stays69,983 (51.4%) within the 14 hospitals with a dedicated OU and 66,256 (48.6%) within the 17 hospitals without. Patient care originated in the ED for 57.8% observation‐status stays in hospitals with an OU compared with 53.0% of observation‐status stays in hospitals without (P<0.001). Compared with hospitals with a dedicated OU, those without a dedicated OU had higher percentages of observation‐status patients older than 12 years and non‐Hispanic and a higher percentage of observation‐status patients with private payer type (Table 2). The 15 top‐ranking APR‐DRGs accounted for roughly half of all observation‐status stays and were relatively consistent between hospitals with and without a dedicated OU (Table 3). Procedural care was frequently associated with observation‐status stays.
| Overall, No. (%) | Hospitals With a Dedicated Observation Unit, No. (%)* | Hospitals Without a Dedicated Observation Unit, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Age | ||||
| <1 year | 23,845 (17.5) | 12,101 (17.3) | 11,744 (17.7) | <0.001 |
| 15 years | 53,405 (38.5) | 28,052 (40.1) | 24,353 (36.8) | |
| 612 years | 33,674 (24.7) | 17,215 (24.6) | 16,459 (24.8) | |
| 1318 years | 23,607 (17.3) | 11,472 (16.4) | 12,135 (18.3) | |
| >18 years | 2,708 (2) | 1,143 (1.6) | 1,565 (2.4) | |
| Gender | ||||
| Male | 76,142 (55.9) | 39,178 (56) | 36,964 (55.8) | 0.43 |
| Female | 60,025 (44.1) | 30,756 (44) | 29,269 (44.2) | |
| Race/ethnicity | ||||
| Non‐Hispanic white | 72,183 (53.0) | 30,653 (43.8) | 41,530 (62.7) | <0.001 |
| Non‐Hispanic black | 30,995 (22.8) | 16,314 (23.3) | 14,681 (22.2) | |
| Hispanic | 21,255 (15.6) | 16,583 (23.7) | 4,672 (7.1) | |
| Asian | 2,075 (1.5) | 1,313 (1.9) | 762 (1.2) | |
| Non‐Hispanic other | 9,731 (7.1) | 5,120 (7.3) | 4,611 (7.0) | |
| Payer | ||||
| Government | 68,725 (50.4) | 36,967 (52.8) | 31,758 (47.9) | <0.001 |
| Private | 48,416 (35.5) | 21,112 (30.2) | 27,304 (41.2) | |
| Other | 19,098 (14.0) | 11,904 (17) | 7,194 (10.9) | |
| Observation‐Status Patients in Hospitals With a Dedicated Observation Unit* | Observation‐Status Patients in Hospitals Without a Dedicated Observation Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank | APR‐DRG | No. | % of All Observation Status Stays | % Began in ED | Rank | APR‐DRG | No. | % of All Observation Status Stays | % Began in ED |
| |||||||||
| 1 | Tonsil and adenoid procedures | 4,621 | 6.6 | 1.3 | 1 | Tonsil and adenoid procedures | 3,806 | 5.7 | 1.6 |
| 2 | Asthma | 4,246 | 6.1 | 85.3 | 2 | Asthma | 3,756 | 5.7 | 79.0 |
| 3 | Seizure | 3,516 | 5.0 | 52.0 | 3 | Seizure | 2,846 | 4.3 | 54.9 |
| 4 | Nonbacterial gastroenteritis | 3,286 | 4.7 | 85.8 | 4 | Upper respiratory infections | 2,733 | 4.1 | 69.6 |
| 5 | Bronchiolitis, RSV pneumonia | 3,093 | 4.4 | 78.5 | 5 | Nonbacterial gastroenteritis | 2,682 | 4.0 | 74.5 |
| 6 | Upper respiratory infections | 2,923 | 4.2 | 80.0 | 6 | Other digestive system diagnoses | 2,545 | 3.8 | 66.3 |
| 7 | Other digestive system diagnoses | 2,064 | 2.9 | 74.0 | 7 | Bronchiolitis, RSV pneumonia | 2,544 | 3.8 | 69.2 |
| 8 | Respiratory signs, symptoms, diagnoses | 2,052 | 2.9 | 81.6 | 8 | Shoulder and arm procedures | 1,862 | 2.8 | 72.6 |
| 9 | Other ENT/cranial/facial diagnoses | 1,684 | 2.4 | 43.6 | 9 | Appendectomy | 1,785 | 2.7 | 79.2 |
| 10 | Shoulder and arm procedures | 1,624 | 2.3 | 79.1 | 10 | Other ENT/cranial/facial diagnoses | 1,624 | 2.5 | 29.9 |
| 11 | Abdominal pain | 1,612 | 2.3 | 86.2 | 11 | Abdominal pain | 1,461 | 2.2 | 82.3 |
| 12 | Fever | 1,494 | 2.1 | 85.1 | 12 | Other factors influencing health status | 1,461 | 2.2 | 66.3 |
| 13 | Appendectomy | 1,465 | 2.1 | 66.4 | 13 | Cellulitis/other bacterial skin infections | 1,383 | 2.1 | 84.2 |
| 14 | Cellulitis/other bacterial skin infections | 1,393 | 2.0 | 86.4 | 14 | Respiratory signs, symptoms, diagnoses | 1,308 | 2.0 | 39.1 |
| 15 | Pneumonia NEC | 1,356 | 1.9 | 79.1 | 15 | Pneumonia NEC | 1,245 | 1.9 | 73.1 |
| Total | 36,429 | 52.0 | 57.8 | Total | 33,041 | 49.87 | 53.0 | ||
Outcomes of Observation‐Status Stays
A greater percentage of observation‐status stays in hospitals with a dedicated OU experienced a same‐day discharge (Table 4). In addition, a higher percentage of discharges occurred between midnight and 11 am in hospitals with a dedicated OU. However, overall risk‐adjusted LOS in hours (12.8 vs 12.2 hours, P=0.90) and risk‐adjusted total standardized costs ($2551 vs $2433, P=0.75) were similar between hospital types. These findings were consistent within the 1 APR‐DRGs commonly cared for by pediatric hospitalists (see Supporting Information, Appendix 1, in the online version of this article). Overall, conversion from observation to inpatient status was significantly higher in hospitals with a dedicated OU compared with hospitals without; however, this pattern was not consistent across the 10 APR‐DRGs commonly cared for by pediatric hospitalists (see Supporting Information, Appendix 1, in the online version of this article). Adjusted odds of 3‐day ED return visits and 30‐day readmissions were comparable between hospital groups.
| Observation‐Status Patients in Hospitals With a Dedicated Observation Unit | Observation‐Status Patients in Hospitals Without a Dedicated Observation Unit | P Value | |
|---|---|---|---|
| |||
| No. of hospitals | 14 | 17 | |
| Length of stay, h, median (IQR) | 12.8 (6.923.7) | 12.2 (721.3) | 0.90 |
| 0 midnights, no. (%) | 16,678 (23.8) | 14,648 (22.1) | <.001 |
| 1 midnight, no. (%) | 46,144 (65.9) | 44,559 (67.3) | |
| 2 midnights or more, no. (%) | 7,161 (10.2) | 7,049 (10.6) | |
| Discharge timing, no. (%) | |||
| Midnight5 am | 1,223 (1.9) | 408 (0.7) | <0.001 |
| 6 am11 am | 18,916 (29.3) | 15,914 (27.1) | |
| Noon5 pm | 32,699 (50.7) | 31,619 (53.9) | |
| 6 pm11 pm | 11,718 (18.2) | 10,718 (18.3) | |
| Total standardized costs, $, median (IQR) | 2,551.3 (2,053.93,169.1) | 2,433.4 (1,998.42,963) | 0.75 |
| Conversion to inpatient status | 11.06% | 9.63% | <0.01 |
| Return care, AOR (95% CI) | |||
| 3‐day ED return visit | 0.93 (0.77‐1.12) | Referent | 0.46 |
| 30‐day readmission | 0.88 (0.67‐1.15) | Referent | 0.36 |
We found similar results in sensitivity analyses comparing observation‐status stays in hospitals with a continuously open OU (open 24 hours per day, 7 days per week, for all of 2011 [n=10 hospitals]) to those without(see Supporting Information, Appendix 2, in the online version of this article). However, there were, on average, more observation‐status stays in hospitals with a continuously open OU (median 5605, IQR 42077089) than hospitals without (median 3309, IQR 26784616) (P=0.04). In contrast to our main results, conversion to inpatient status was lower in hospitals with a continuously open OU compared with hospitals without (8.52% vs 11.57%, P<0.01).
DISCUSSION
Counter to our hypothesis, we did not find hospital‐level differences in length of stay or costs for observation‐status patients cared for in hospitals with and without a dedicated OU, though hospitals with dedicated OUs did have more same‐day discharges and more morning discharges. The lack of observed differences in LOS and costs may reflect the fact that many children under observation status are treated throughout the hospital, even in facilities with a dedicated OU. Access to a dedicated OU is limited by factors including small numbers of OU beds and specific low acuity/low complexity OU admission criteria.[7] The inclusion of all children admitted under observation status in our analyses may have diluted any effect of dedicated OUs at the hospital level, but was necessary due to the inability to identify location of care for children admitted under observation status. Location of care is an important variable that should be incorporated into administrative databases to allow for comparative effectiveness research designs. Until such data are available, chart review at individual hospitals would be necessary to determine which patients received care in an OU.
We did find that discharges for observation‐status patients occurred earlier in the day in hospitals with a dedicated OU when compared with observation‐status patients in hospitals without a dedicated OU. In addition, the percentage of same‐day discharges was higher among observation‐status patients treated in hospitals with a dedicated OU. These differences may stem from policies and procedures that encourage rapid discharge in dedicated OUs, and those practices may affect other care areas. For example, OUs may enforce policies requiring family presence at the bedside or utilize staffing models where doctors and nurses are in frequent communication, both of which would facilitate discharge as soon as a patient no longer required hospital‐based care.[7] A retrospective chart review study design could be used to identify discharge processes and other key characteristics of highly performing OUs.
We found conflicting results in our main and sensitivity analyses related to conversion to inpatient status. Lower percentages of observation‐status patients converting to inpatient status indicates greater success in the delivery of observation care based on established performance metrics.[19] Lower rates of conversion to inpatient status may be the result of stricter admission criteria for some diagnosis and in hospitals with a continuously open dedicate OU, more refined processes for utilization review that allow for patients to be placed into the correct status (observation vs inpatient) at the time of admission, or efforts to educate providers about the designation of observation status.[7] It is also possible that fewer observation‐status patients convert to inpatient status in hospitals with a continuously open dedicated OU because such a change would require movement of the patient to an inpatient bed.
These analyses were more comprehensive than our prior studies[2, 20] in that we included both patients who were treated first in the ED and those who were not. In addition to the APR‐DRGs representative of conditions that have been successfully treated in ED‐based pediatric OUs (eg, asthma, seizures, gastroenteritis, cellulitis),[8, 9, 21, 22] we found observation‐status was commonly associated with procedural care. This population of patients may be relevant to hospitalists who staff OUs that provide both unscheduled and postprocedural care. The colocation of medical and postprocedural patients has been described by others[8, 23] and was reported to occur in over half of the OUs included in this study.[7] The extent to which postprocedure observation care is provided in general OUs staffed by hospitalists represents another opportunity for further study.
Hospitals face many considerations when determining if and how they will provide observation services to patients expected to experience short stays.[7] Some hospitals may be unable to justify an OU for all or part of the year based on the volume of admissions or the costs to staff an OU.[24, 25] Other hospitals may open an OU to promote patient flow and reduce ED crowding.[26] Hospitals may also be influenced by reimbursement policies related to observation‐status stays. Although we did not observe differences in overall payer mix, we did find higher percentages of observation‐status patients in hospitals with dedicated OUs to have public insurance. Although hospital contracts with payers around observation status patients are complex and beyond the scope of this analysis, it is possible that hospitals have established OUs because of increasingly stringent rules or criteria to meet inpatient status or experiences with high volumes of observation‐status patients covered by a particular payer. Nevertheless, the brief nature of many pediatric hospitalizations and the scarcity of pediatric OU beds must be considered in policy changes that result from national discussions about the appropriateness of inpatient stays shorter than 2 nights in duration.[27]
Limitations
The primary limitation to our analyses is the lack of ability to identify patients who were treated in a dedicated OU because few hospitals provided data to PHIS that allowed for the identification of the unit or location of care. Second, it is possible that some hospitals were misclassified as not having a dedicated OU based on our survey, which initially inquired about OUs that provided care to patients first treated in the ED. Therefore, OUs that exclusively care for postoperative patients or patients with scheduled treatments may be present in hospitals that we have labeled as not having a dedicated OU. This potential misclassification would bias our results toward finding no differences. Third, in any study of administrative data there is potential that diagnosis codes are incomplete or inaccurately capture the underlying reason for the episode of care. Fourth, the experiences of the free‐standing children's hospitals that contribute data to PHIS may not be generalizable to other hospitals that provide observation care to children. Finally, return care may be underestimated, as children could receive treatment at another hospital following discharge from a PHIS hospital. Care outside of PHIS hospitals would not be captured, but we do not expect this to differ for hospitals with and without dedicated OUs. It is possible that health information exchanges will permit more comprehensive analyses of care across different hospitals in the future.
CONCLUSION
Observation status patients are similar in hospitals with and without dedicated observation units that admit children from the ED. The presence of a dedicated OU appears to have an influence on same‐day and morning discharges across all observation‐status stays without impacting other hospital‐level outcomes. Inclusion of location of care (eg, geographically distinct dedicated OU vs general inpatient unit vs ED) in hospital administrative datasets would allow for meaningful comparisons of different models of care for short‐stay observation‐status patients.
Acknowledgements
The authors thank John P. Harding, MBA, FACHE, Children's Hospital of the King's Daughters, Norfolk, Virginia for his input on the study design.
Disclosures: Dr. Hall had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Internal funds from the Children's Hospital Association supported the conduct of this work. The authors have no financial relationships or conflicts of interest to disclose.
Many pediatric hospitalizations are of short duration, and more than half of short‐stay hospitalizations are designated as observation status.[1, 2] Observation status is an administrative label assigned to patients who do not meet hospital or payer criteria for inpatient‐status care. Short‐stay observation‐status patients do not fit in traditional models of emergency department (ED) or inpatient care. EDs often focus on discharging or admitting patients within a matter of hours, whereas inpatient units tend to measure length of stay (LOS) in terms of days[3] and may not have systems in place to facilitate rapid discharge of short‐stay patients.[4] Observation units (OUs) have been established in some hospitals to address the unique care needs of short‐stay patients.[5, 6, 7]
Single‐site reports from children's hospitals with successful OUs have demonstrated shorter LOS and lower costs compared with inpatient settings.[6, 8, 9, 10, 11, 12, 13, 14] No prior study has examined hospital‐level effects of an OU on observation‐status patient outcomes. The Pediatric Health Information System (PHIS) database provides a unique opportunity to explore this question, because unlike other national hospital administrative databases,[15, 16] the PHIS dataset contains information about children under observation status. In addition, we know which PHIS hospitals had a dedicated OU in 2011.7
We hypothesized that overall observation‐status stays in hospitals with a dedicated OU would be of shorter duration with earlier discharges at lower cost than observation‐status stays in hospitals without a dedicated OU. We compared hospitals with and without a dedicated OU on secondary outcomes including rates of conversion to inpatient status and return care for any reason.
METHODS
We conducted a cross‐sectional analysis of hospital administrative data using the 2011 PHIS databasea national administrative database that contains resource utilization data from 43 participating hospitals located in 26 states plus the District of Columbia. These hospitals account for approximately 20% of pediatric hospitalizations in the United States.
For each hospital encounter, PHIS includes patient demographics, up to 41 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses, up to 41 ICD‐9‐CM procedures, and hospital charges for services. Data are deidentified prior to inclusion, but unique identifiers allow for determination of return visits and readmissions following an index visit for an individual patient. Data quality and reliability are assured jointly by the Children's Hospital Association (formerly Child Health Corporation of America, Overland Park, KS), participating hospitals, and Truven Health Analytics (New York, NY). This study, using administrative data, was not considered human subjects research by the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board.
Hospital Selection and Hospital Characteristics
The study sample was drawn from the 31 hospitals that reported observation‐status patient data to PHIS in 2011. Analyses were conducted in 2013, at which time 2011 was the most recent year of data. We categorized 14 hospitals as having a dedicated OU during 2011 based on information collected in 2013.7 To summarize briefly, we interviewed by telephone representatives of hospitals responding to an email query as to the presence of a geographically distinct OU for the care of unscheduled patients from the ED. Three of the 14 representatives reported their hospital had 2 OUs, 1 of which was a separate surgical OU. Ten OUs cared for both ED patients and patients with scheduled procedures; 8 units received patients from non‐ED sources. Hospitalists provided staffing in more than half of the OUs.
We attempted to identify administrative data that would signal care delivered in a dedicated OU using hospital charge codes reported to PHIS, but learned this was not possible due to between‐hospital variation in the specificity of the charge codes. Therefore, we were unable to determine if patient care was delivered in a dedicated OU or another setting, such as a general inpatient unit or the ED. Other hospital characteristics available from the PHIS dataset included the number of inpatient beds, ED visits, inpatient admissions, observation‐status stays, and payer mix. We calculated the percentage of ED visits resulting in admission by dividing the number of ED visits with associated inpatient or observation status by the total number of ED visits and the percentage of admissions under observation status by dividing the number of observation‐status stays by the total number of admissions under observation or inpatient status.
Visit Selection and Patient Characteristics
All observation‐status stays regardless of the point of entry into the hospital were eligible for this study. We excluded stays that were birth‐related, included intensive care, or resulted in transfer or death. Patient demographic characteristics used to describe the cohort included age, gender, race/ethnicity, and primary payer. Stays that began in the ED were identified by an emergency room charge within PHIS. Eligible stays were categorized using All Patient Refined Diagnosis Related Groups (APR‐DRGs) version 24 using the ICD‐9‐CM code‐based proprietary 3M software (3M Health Information Systems, St. Paul, MN). We determined the 15 top‐ranking APR‐DRGs among observation‐status stays in hospitals with a dedicated OU and hospitals without. Procedural stays were identified based on procedural APR‐DRGs (eg, tonsil and adenoid procedures) or the presence of an ICD‐9‐CM procedure code (eg, 331 spinal tap).
Measured Outcomes
Outcomes of observation‐status stays were determined within 4 categories: (1) LOS, (2) standardized costs, (3) conversion to inpatient status, and (4) return visits and readmissions. LOS was calculated in terms of nights spent in hospital for all stays by subtracting the discharge date from the admission date and in terms of hours for stays in the 28 hospitals that report admission and discharge hour to the PHIS database. Discharge timing was examined in 4, 6‐hour blocks starting at midnight. Standardized costs were derived from a charge master index that was created by taking the median costs from all PHIS hospitals for each charged service.[17] Standardized costs represent the estimated cost of providing any particular clinical activity but are not the cost to patients, nor do they represent the actual cost to any given hospital. This approach allows for cost comparisons across hospitals, without biases arising from using charges or from deriving costs using hospitals' ratios of costs to charges.[18] Conversion from observation to inpatient status was calculated by dividing the number of inpatient‐status stays with observation codes by the number of observation‐statusonly stays plus the number of inpatient‐status stays with observation codes. All‐cause 3‐day ED return visits and 30‐day readmissions to the same hospital were assessed using patient‐specific identifiers that allowed for tracking of ED return visits and readmissions following the index observation stay.
Data Analysis
Descriptive statistics were calculated for hospital and patient characteristics using medians and interquartile ranges (IQRs) for continuous factors and frequencies with percentages for categorical factors. Comparisons of these factors between hospitals with dedicated OUs and without were made using [2] and Wilcoxon rank sum tests as appropriate. Multivariable regression was performed using generalized linear mixed models treating hospital as a random effect and used patient age, the case‐mix index based on the APR‐DRG severity of illness, ED visit, and procedures associated with the index observation‐status stay. For continuous outcomes, we performed a log transformation on the outcome, confirmed the normality assumption, and back transformed the results. Sensitivity analyses were conducted to compare LOS, standardized costs, and conversation rates by hospital type for 10 of the 15 top‐ranking APR‐DRGs commonly cared for by pediatric hospitalists and to compare hospitals that reported the presence of an OU that was consistently open (24 hours per day, 7 days per week) and operating during the entire 2011 calendar year, and those without. Based on information gathered from the telephone interviews, hospitals with partially open OUs were similar to hospitals with continuously open OUs, such that they were included in our main analyses. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Hospital Characteristics
Dedicated OUs were present in 14 of the 31 hospitals that reported observation‐status patient data to PHIS (Figure 1). Three of these hospitals had OUs that were open for 5 months or less in 2011; 1 unit opened, 1 unit closed, and 1 hospital operated a seasonal unit. The remaining 17 hospitals reported no OU that admitted unscheduled patients from the ED during 2011. Hospitals with a dedicated OU had more inpatient beds and higher median number of inpatient admissions than those without (Table 1). Hospitals were statistically similar in terms of total volume of ED visits, percentage of ED visits resulting in admission, total number of observation‐status stays, percentage of admissions under observation status, and payer mix.

| Overall, Median (IQR) | Hospitals With a Dedicated Observation Unit, Median (IQR) | Hospitals Without a Dedicated Observation Unit, Median (IQR) | P Value | |
|---|---|---|---|---|
| ||||
| No. of hospitals | 31 | 14 | 17 | |
| Total no. of inpatient beds | 273 (213311) | 304 (269425) | 246 (175293) | 0.006 |
| Total no. ED visits | 62971 (47,50497,723) | 87,892 (55,102117,119) | 53,151 (4750470,882) | 0.21 |
| ED visits resulting in admission, % | 13.1 (9.715.0) | 13.8 (10.5, 19.1) | 12.5 (9.714.5) | 0.31 |
| Total no. of inpatient admissions | 11,537 (9,26814,568) | 13,206 (11,32517,869) | 10,207 (8,64013,363) | 0.04 |
| Admissions under observation status, % | 25.7 (19.733.8) | 25.5 (21.431.4) | 26.0 (16.935.1) | 0.98 |
| Total no. of observation stays | 3,820 (27935672) | 4,850 (3,309 6,196) | 3,141 (2,3654,616) | 0.07 |
| Government payer, % | 60.2 (53.371.2) | 62.1 (54.9, 65.9) | 59.2 (53.373.7) | 0.89 |
Observation‐Status Patients by Hospital Type
In 2011, there were a total of 136,239 observation‐status stays69,983 (51.4%) within the 14 hospitals with a dedicated OU and 66,256 (48.6%) within the 17 hospitals without. Patient care originated in the ED for 57.8% observation‐status stays in hospitals with an OU compared with 53.0% of observation‐status stays in hospitals without (P<0.001). Compared with hospitals with a dedicated OU, those without a dedicated OU had higher percentages of observation‐status patients older than 12 years and non‐Hispanic and a higher percentage of observation‐status patients with private payer type (Table 2). The 15 top‐ranking APR‐DRGs accounted for roughly half of all observation‐status stays and were relatively consistent between hospitals with and without a dedicated OU (Table 3). Procedural care was frequently associated with observation‐status stays.
| Overall, No. (%) | Hospitals With a Dedicated Observation Unit, No. (%)* | Hospitals Without a Dedicated Observation Unit, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Age | ||||
| <1 year | 23,845 (17.5) | 12,101 (17.3) | 11,744 (17.7) | <0.001 |
| 15 years | 53,405 (38.5) | 28,052 (40.1) | 24,353 (36.8) | |
| 612 years | 33,674 (24.7) | 17,215 (24.6) | 16,459 (24.8) | |
| 1318 years | 23,607 (17.3) | 11,472 (16.4) | 12,135 (18.3) | |
| >18 years | 2,708 (2) | 1,143 (1.6) | 1,565 (2.4) | |
| Gender | ||||
| Male | 76,142 (55.9) | 39,178 (56) | 36,964 (55.8) | 0.43 |
| Female | 60,025 (44.1) | 30,756 (44) | 29,269 (44.2) | |
| Race/ethnicity | ||||
| Non‐Hispanic white | 72,183 (53.0) | 30,653 (43.8) | 41,530 (62.7) | <0.001 |
| Non‐Hispanic black | 30,995 (22.8) | 16,314 (23.3) | 14,681 (22.2) | |
| Hispanic | 21,255 (15.6) | 16,583 (23.7) | 4,672 (7.1) | |
| Asian | 2,075 (1.5) | 1,313 (1.9) | 762 (1.2) | |
| Non‐Hispanic other | 9,731 (7.1) | 5,120 (7.3) | 4,611 (7.0) | |
| Payer | ||||
| Government | 68,725 (50.4) | 36,967 (52.8) | 31,758 (47.9) | <0.001 |
| Private | 48,416 (35.5) | 21,112 (30.2) | 27,304 (41.2) | |
| Other | 19,098 (14.0) | 11,904 (17) | 7,194 (10.9) | |
| Observation‐Status Patients in Hospitals With a Dedicated Observation Unit* | Observation‐Status Patients in Hospitals Without a Dedicated Observation Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank | APR‐DRG | No. | % of All Observation Status Stays | % Began in ED | Rank | APR‐DRG | No. | % of All Observation Status Stays | % Began in ED |
| |||||||||
| 1 | Tonsil and adenoid procedures | 4,621 | 6.6 | 1.3 | 1 | Tonsil and adenoid procedures | 3,806 | 5.7 | 1.6 |
| 2 | Asthma | 4,246 | 6.1 | 85.3 | 2 | Asthma | 3,756 | 5.7 | 79.0 |
| 3 | Seizure | 3,516 | 5.0 | 52.0 | 3 | Seizure | 2,846 | 4.3 | 54.9 |
| 4 | Nonbacterial gastroenteritis | 3,286 | 4.7 | 85.8 | 4 | Upper respiratory infections | 2,733 | 4.1 | 69.6 |
| 5 | Bronchiolitis, RSV pneumonia | 3,093 | 4.4 | 78.5 | 5 | Nonbacterial gastroenteritis | 2,682 | 4.0 | 74.5 |
| 6 | Upper respiratory infections | 2,923 | 4.2 | 80.0 | 6 | Other digestive system diagnoses | 2,545 | 3.8 | 66.3 |
| 7 | Other digestive system diagnoses | 2,064 | 2.9 | 74.0 | 7 | Bronchiolitis, RSV pneumonia | 2,544 | 3.8 | 69.2 |
| 8 | Respiratory signs, symptoms, diagnoses | 2,052 | 2.9 | 81.6 | 8 | Shoulder and arm procedures | 1,862 | 2.8 | 72.6 |
| 9 | Other ENT/cranial/facial diagnoses | 1,684 | 2.4 | 43.6 | 9 | Appendectomy | 1,785 | 2.7 | 79.2 |
| 10 | Shoulder and arm procedures | 1,624 | 2.3 | 79.1 | 10 | Other ENT/cranial/facial diagnoses | 1,624 | 2.5 | 29.9 |
| 11 | Abdominal pain | 1,612 | 2.3 | 86.2 | 11 | Abdominal pain | 1,461 | 2.2 | 82.3 |
| 12 | Fever | 1,494 | 2.1 | 85.1 | 12 | Other factors influencing health status | 1,461 | 2.2 | 66.3 |
| 13 | Appendectomy | 1,465 | 2.1 | 66.4 | 13 | Cellulitis/other bacterial skin infections | 1,383 | 2.1 | 84.2 |
| 14 | Cellulitis/other bacterial skin infections | 1,393 | 2.0 | 86.4 | 14 | Respiratory signs, symptoms, diagnoses | 1,308 | 2.0 | 39.1 |
| 15 | Pneumonia NEC | 1,356 | 1.9 | 79.1 | 15 | Pneumonia NEC | 1,245 | 1.9 | 73.1 |
| Total | 36,429 | 52.0 | 57.8 | Total | 33,041 | 49.87 | 53.0 | ||
Outcomes of Observation‐Status Stays
A greater percentage of observation‐status stays in hospitals with a dedicated OU experienced a same‐day discharge (Table 4). In addition, a higher percentage of discharges occurred between midnight and 11 am in hospitals with a dedicated OU. However, overall risk‐adjusted LOS in hours (12.8 vs 12.2 hours, P=0.90) and risk‐adjusted total standardized costs ($2551 vs $2433, P=0.75) were similar between hospital types. These findings were consistent within the 1 APR‐DRGs commonly cared for by pediatric hospitalists (see Supporting Information, Appendix 1, in the online version of this article). Overall, conversion from observation to inpatient status was significantly higher in hospitals with a dedicated OU compared with hospitals without; however, this pattern was not consistent across the 10 APR‐DRGs commonly cared for by pediatric hospitalists (see Supporting Information, Appendix 1, in the online version of this article). Adjusted odds of 3‐day ED return visits and 30‐day readmissions were comparable between hospital groups.
| Observation‐Status Patients in Hospitals With a Dedicated Observation Unit | Observation‐Status Patients in Hospitals Without a Dedicated Observation Unit | P Value | |
|---|---|---|---|
| |||
| No. of hospitals | 14 | 17 | |
| Length of stay, h, median (IQR) | 12.8 (6.923.7) | 12.2 (721.3) | 0.90 |
| 0 midnights, no. (%) | 16,678 (23.8) | 14,648 (22.1) | <.001 |
| 1 midnight, no. (%) | 46,144 (65.9) | 44,559 (67.3) | |
| 2 midnights or more, no. (%) | 7,161 (10.2) | 7,049 (10.6) | |
| Discharge timing, no. (%) | |||
| Midnight5 am | 1,223 (1.9) | 408 (0.7) | <0.001 |
| 6 am11 am | 18,916 (29.3) | 15,914 (27.1) | |
| Noon5 pm | 32,699 (50.7) | 31,619 (53.9) | |
| 6 pm11 pm | 11,718 (18.2) | 10,718 (18.3) | |
| Total standardized costs, $, median (IQR) | 2,551.3 (2,053.93,169.1) | 2,433.4 (1,998.42,963) | 0.75 |
| Conversion to inpatient status | 11.06% | 9.63% | <0.01 |
| Return care, AOR (95% CI) | |||
| 3‐day ED return visit | 0.93 (0.77‐1.12) | Referent | 0.46 |
| 30‐day readmission | 0.88 (0.67‐1.15) | Referent | 0.36 |
We found similar results in sensitivity analyses comparing observation‐status stays in hospitals with a continuously open OU (open 24 hours per day, 7 days per week, for all of 2011 [n=10 hospitals]) to those without(see Supporting Information, Appendix 2, in the online version of this article). However, there were, on average, more observation‐status stays in hospitals with a continuously open OU (median 5605, IQR 42077089) than hospitals without (median 3309, IQR 26784616) (P=0.04). In contrast to our main results, conversion to inpatient status was lower in hospitals with a continuously open OU compared with hospitals without (8.52% vs 11.57%, P<0.01).
DISCUSSION
Counter to our hypothesis, we did not find hospital‐level differences in length of stay or costs for observation‐status patients cared for in hospitals with and without a dedicated OU, though hospitals with dedicated OUs did have more same‐day discharges and more morning discharges. The lack of observed differences in LOS and costs may reflect the fact that many children under observation status are treated throughout the hospital, even in facilities with a dedicated OU. Access to a dedicated OU is limited by factors including small numbers of OU beds and specific low acuity/low complexity OU admission criteria.[7] The inclusion of all children admitted under observation status in our analyses may have diluted any effect of dedicated OUs at the hospital level, but was necessary due to the inability to identify location of care for children admitted under observation status. Location of care is an important variable that should be incorporated into administrative databases to allow for comparative effectiveness research designs. Until such data are available, chart review at individual hospitals would be necessary to determine which patients received care in an OU.
We did find that discharges for observation‐status patients occurred earlier in the day in hospitals with a dedicated OU when compared with observation‐status patients in hospitals without a dedicated OU. In addition, the percentage of same‐day discharges was higher among observation‐status patients treated in hospitals with a dedicated OU. These differences may stem from policies and procedures that encourage rapid discharge in dedicated OUs, and those practices may affect other care areas. For example, OUs may enforce policies requiring family presence at the bedside or utilize staffing models where doctors and nurses are in frequent communication, both of which would facilitate discharge as soon as a patient no longer required hospital‐based care.[7] A retrospective chart review study design could be used to identify discharge processes and other key characteristics of highly performing OUs.
We found conflicting results in our main and sensitivity analyses related to conversion to inpatient status. Lower percentages of observation‐status patients converting to inpatient status indicates greater success in the delivery of observation care based on established performance metrics.[19] Lower rates of conversion to inpatient status may be the result of stricter admission criteria for some diagnosis and in hospitals with a continuously open dedicate OU, more refined processes for utilization review that allow for patients to be placed into the correct status (observation vs inpatient) at the time of admission, or efforts to educate providers about the designation of observation status.[7] It is also possible that fewer observation‐status patients convert to inpatient status in hospitals with a continuously open dedicated OU because such a change would require movement of the patient to an inpatient bed.
These analyses were more comprehensive than our prior studies[2, 20] in that we included both patients who were treated first in the ED and those who were not. In addition to the APR‐DRGs representative of conditions that have been successfully treated in ED‐based pediatric OUs (eg, asthma, seizures, gastroenteritis, cellulitis),[8, 9, 21, 22] we found observation‐status was commonly associated with procedural care. This population of patients may be relevant to hospitalists who staff OUs that provide both unscheduled and postprocedural care. The colocation of medical and postprocedural patients has been described by others[8, 23] and was reported to occur in over half of the OUs included in this study.[7] The extent to which postprocedure observation care is provided in general OUs staffed by hospitalists represents another opportunity for further study.
Hospitals face many considerations when determining if and how they will provide observation services to patients expected to experience short stays.[7] Some hospitals may be unable to justify an OU for all or part of the year based on the volume of admissions or the costs to staff an OU.[24, 25] Other hospitals may open an OU to promote patient flow and reduce ED crowding.[26] Hospitals may also be influenced by reimbursement policies related to observation‐status stays. Although we did not observe differences in overall payer mix, we did find higher percentages of observation‐status patients in hospitals with dedicated OUs to have public insurance. Although hospital contracts with payers around observation status patients are complex and beyond the scope of this analysis, it is possible that hospitals have established OUs because of increasingly stringent rules or criteria to meet inpatient status or experiences with high volumes of observation‐status patients covered by a particular payer. Nevertheless, the brief nature of many pediatric hospitalizations and the scarcity of pediatric OU beds must be considered in policy changes that result from national discussions about the appropriateness of inpatient stays shorter than 2 nights in duration.[27]
Limitations
The primary limitation to our analyses is the lack of ability to identify patients who were treated in a dedicated OU because few hospitals provided data to PHIS that allowed for the identification of the unit or location of care. Second, it is possible that some hospitals were misclassified as not having a dedicated OU based on our survey, which initially inquired about OUs that provided care to patients first treated in the ED. Therefore, OUs that exclusively care for postoperative patients or patients with scheduled treatments may be present in hospitals that we have labeled as not having a dedicated OU. This potential misclassification would bias our results toward finding no differences. Third, in any study of administrative data there is potential that diagnosis codes are incomplete or inaccurately capture the underlying reason for the episode of care. Fourth, the experiences of the free‐standing children's hospitals that contribute data to PHIS may not be generalizable to other hospitals that provide observation care to children. Finally, return care may be underestimated, as children could receive treatment at another hospital following discharge from a PHIS hospital. Care outside of PHIS hospitals would not be captured, but we do not expect this to differ for hospitals with and without dedicated OUs. It is possible that health information exchanges will permit more comprehensive analyses of care across different hospitals in the future.
CONCLUSION
Observation status patients are similar in hospitals with and without dedicated observation units that admit children from the ED. The presence of a dedicated OU appears to have an influence on same‐day and morning discharges across all observation‐status stays without impacting other hospital‐level outcomes. Inclusion of location of care (eg, geographically distinct dedicated OU vs general inpatient unit vs ED) in hospital administrative datasets would allow for meaningful comparisons of different models of care for short‐stay observation‐status patients.
Acknowledgements
The authors thank John P. Harding, MBA, FACHE, Children's Hospital of the King's Daughters, Norfolk, Virginia for his input on the study design.
Disclosures: Dr. Hall had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Internal funds from the Children's Hospital Association supported the conduct of this work. The authors have no financial relationships or conflicts of interest to disclose.
Many pediatric hospitalizations are of short duration, and more than half of short‐stay hospitalizations are designated as observation status.[1, 2] Observation status is an administrative label assigned to patients who do not meet hospital or payer criteria for inpatient‐status care. Short‐stay observation‐status patients do not fit in traditional models of emergency department (ED) or inpatient care. EDs often focus on discharging or admitting patients within a matter of hours, whereas inpatient units tend to measure length of stay (LOS) in terms of days[3] and may not have systems in place to facilitate rapid discharge of short‐stay patients.[4] Observation units (OUs) have been established in some hospitals to address the unique care needs of short‐stay patients.[5, 6, 7]
Single‐site reports from children's hospitals with successful OUs have demonstrated shorter LOS and lower costs compared with inpatient settings.[6, 8, 9, 10, 11, 12, 13, 14] No prior study has examined hospital‐level effects of an OU on observation‐status patient outcomes. The Pediatric Health Information System (PHIS) database provides a unique opportunity to explore this question, because unlike other national hospital administrative databases,[15, 16] the PHIS dataset contains information about children under observation status. In addition, we know which PHIS hospitals had a dedicated OU in 2011.7
We hypothesized that overall observation‐status stays in hospitals with a dedicated OU would be of shorter duration with earlier discharges at lower cost than observation‐status stays in hospitals without a dedicated OU. We compared hospitals with and without a dedicated OU on secondary outcomes including rates of conversion to inpatient status and return care for any reason.
METHODS
We conducted a cross‐sectional analysis of hospital administrative data using the 2011 PHIS databasea national administrative database that contains resource utilization data from 43 participating hospitals located in 26 states plus the District of Columbia. These hospitals account for approximately 20% of pediatric hospitalizations in the United States.
For each hospital encounter, PHIS includes patient demographics, up to 41 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses, up to 41 ICD‐9‐CM procedures, and hospital charges for services. Data are deidentified prior to inclusion, but unique identifiers allow for determination of return visits and readmissions following an index visit for an individual patient. Data quality and reliability are assured jointly by the Children's Hospital Association (formerly Child Health Corporation of America, Overland Park, KS), participating hospitals, and Truven Health Analytics (New York, NY). This study, using administrative data, was not considered human subjects research by the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board.
Hospital Selection and Hospital Characteristics
The study sample was drawn from the 31 hospitals that reported observation‐status patient data to PHIS in 2011. Analyses were conducted in 2013, at which time 2011 was the most recent year of data. We categorized 14 hospitals as having a dedicated OU during 2011 based on information collected in 2013.7 To summarize briefly, we interviewed by telephone representatives of hospitals responding to an email query as to the presence of a geographically distinct OU for the care of unscheduled patients from the ED. Three of the 14 representatives reported their hospital had 2 OUs, 1 of which was a separate surgical OU. Ten OUs cared for both ED patients and patients with scheduled procedures; 8 units received patients from non‐ED sources. Hospitalists provided staffing in more than half of the OUs.
We attempted to identify administrative data that would signal care delivered in a dedicated OU using hospital charge codes reported to PHIS, but learned this was not possible due to between‐hospital variation in the specificity of the charge codes. Therefore, we were unable to determine if patient care was delivered in a dedicated OU or another setting, such as a general inpatient unit or the ED. Other hospital characteristics available from the PHIS dataset included the number of inpatient beds, ED visits, inpatient admissions, observation‐status stays, and payer mix. We calculated the percentage of ED visits resulting in admission by dividing the number of ED visits with associated inpatient or observation status by the total number of ED visits and the percentage of admissions under observation status by dividing the number of observation‐status stays by the total number of admissions under observation or inpatient status.
Visit Selection and Patient Characteristics
All observation‐status stays regardless of the point of entry into the hospital were eligible for this study. We excluded stays that were birth‐related, included intensive care, or resulted in transfer or death. Patient demographic characteristics used to describe the cohort included age, gender, race/ethnicity, and primary payer. Stays that began in the ED were identified by an emergency room charge within PHIS. Eligible stays were categorized using All Patient Refined Diagnosis Related Groups (APR‐DRGs) version 24 using the ICD‐9‐CM code‐based proprietary 3M software (3M Health Information Systems, St. Paul, MN). We determined the 15 top‐ranking APR‐DRGs among observation‐status stays in hospitals with a dedicated OU and hospitals without. Procedural stays were identified based on procedural APR‐DRGs (eg, tonsil and adenoid procedures) or the presence of an ICD‐9‐CM procedure code (eg, 331 spinal tap).
Measured Outcomes
Outcomes of observation‐status stays were determined within 4 categories: (1) LOS, (2) standardized costs, (3) conversion to inpatient status, and (4) return visits and readmissions. LOS was calculated in terms of nights spent in hospital for all stays by subtracting the discharge date from the admission date and in terms of hours for stays in the 28 hospitals that report admission and discharge hour to the PHIS database. Discharge timing was examined in 4, 6‐hour blocks starting at midnight. Standardized costs were derived from a charge master index that was created by taking the median costs from all PHIS hospitals for each charged service.[17] Standardized costs represent the estimated cost of providing any particular clinical activity but are not the cost to patients, nor do they represent the actual cost to any given hospital. This approach allows for cost comparisons across hospitals, without biases arising from using charges or from deriving costs using hospitals' ratios of costs to charges.[18] Conversion from observation to inpatient status was calculated by dividing the number of inpatient‐status stays with observation codes by the number of observation‐statusonly stays plus the number of inpatient‐status stays with observation codes. All‐cause 3‐day ED return visits and 30‐day readmissions to the same hospital were assessed using patient‐specific identifiers that allowed for tracking of ED return visits and readmissions following the index observation stay.
Data Analysis
Descriptive statistics were calculated for hospital and patient characteristics using medians and interquartile ranges (IQRs) for continuous factors and frequencies with percentages for categorical factors. Comparisons of these factors between hospitals with dedicated OUs and without were made using [2] and Wilcoxon rank sum tests as appropriate. Multivariable regression was performed using generalized linear mixed models treating hospital as a random effect and used patient age, the case‐mix index based on the APR‐DRG severity of illness, ED visit, and procedures associated with the index observation‐status stay. For continuous outcomes, we performed a log transformation on the outcome, confirmed the normality assumption, and back transformed the results. Sensitivity analyses were conducted to compare LOS, standardized costs, and conversation rates by hospital type for 10 of the 15 top‐ranking APR‐DRGs commonly cared for by pediatric hospitalists and to compare hospitals that reported the presence of an OU that was consistently open (24 hours per day, 7 days per week) and operating during the entire 2011 calendar year, and those without. Based on information gathered from the telephone interviews, hospitals with partially open OUs were similar to hospitals with continuously open OUs, such that they were included in our main analyses. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Hospital Characteristics
Dedicated OUs were present in 14 of the 31 hospitals that reported observation‐status patient data to PHIS (Figure 1). Three of these hospitals had OUs that were open for 5 months or less in 2011; 1 unit opened, 1 unit closed, and 1 hospital operated a seasonal unit. The remaining 17 hospitals reported no OU that admitted unscheduled patients from the ED during 2011. Hospitals with a dedicated OU had more inpatient beds and higher median number of inpatient admissions than those without (Table 1). Hospitals were statistically similar in terms of total volume of ED visits, percentage of ED visits resulting in admission, total number of observation‐status stays, percentage of admissions under observation status, and payer mix.

| Overall, Median (IQR) | Hospitals With a Dedicated Observation Unit, Median (IQR) | Hospitals Without a Dedicated Observation Unit, Median (IQR) | P Value | |
|---|---|---|---|---|
| ||||
| No. of hospitals | 31 | 14 | 17 | |
| Total no. of inpatient beds | 273 (213311) | 304 (269425) | 246 (175293) | 0.006 |
| Total no. ED visits | 62971 (47,50497,723) | 87,892 (55,102117,119) | 53,151 (4750470,882) | 0.21 |
| ED visits resulting in admission, % | 13.1 (9.715.0) | 13.8 (10.5, 19.1) | 12.5 (9.714.5) | 0.31 |
| Total no. of inpatient admissions | 11,537 (9,26814,568) | 13,206 (11,32517,869) | 10,207 (8,64013,363) | 0.04 |
| Admissions under observation status, % | 25.7 (19.733.8) | 25.5 (21.431.4) | 26.0 (16.935.1) | 0.98 |
| Total no. of observation stays | 3,820 (27935672) | 4,850 (3,309 6,196) | 3,141 (2,3654,616) | 0.07 |
| Government payer, % | 60.2 (53.371.2) | 62.1 (54.9, 65.9) | 59.2 (53.373.7) | 0.89 |
Observation‐Status Patients by Hospital Type
In 2011, there were a total of 136,239 observation‐status stays69,983 (51.4%) within the 14 hospitals with a dedicated OU and 66,256 (48.6%) within the 17 hospitals without. Patient care originated in the ED for 57.8% observation‐status stays in hospitals with an OU compared with 53.0% of observation‐status stays in hospitals without (P<0.001). Compared with hospitals with a dedicated OU, those without a dedicated OU had higher percentages of observation‐status patients older than 12 years and non‐Hispanic and a higher percentage of observation‐status patients with private payer type (Table 2). The 15 top‐ranking APR‐DRGs accounted for roughly half of all observation‐status stays and were relatively consistent between hospitals with and without a dedicated OU (Table 3). Procedural care was frequently associated with observation‐status stays.
| Overall, No. (%) | Hospitals With a Dedicated Observation Unit, No. (%)* | Hospitals Without a Dedicated Observation Unit, No. (%) | P Value | |
|---|---|---|---|---|
| ||||
| Age | ||||
| <1 year | 23,845 (17.5) | 12,101 (17.3) | 11,744 (17.7) | <0.001 |
| 15 years | 53,405 (38.5) | 28,052 (40.1) | 24,353 (36.8) | |
| 612 years | 33,674 (24.7) | 17,215 (24.6) | 16,459 (24.8) | |
| 1318 years | 23,607 (17.3) | 11,472 (16.4) | 12,135 (18.3) | |
| >18 years | 2,708 (2) | 1,143 (1.6) | 1,565 (2.4) | |
| Gender | ||||
| Male | 76,142 (55.9) | 39,178 (56) | 36,964 (55.8) | 0.43 |
| Female | 60,025 (44.1) | 30,756 (44) | 29,269 (44.2) | |
| Race/ethnicity | ||||
| Non‐Hispanic white | 72,183 (53.0) | 30,653 (43.8) | 41,530 (62.7) | <0.001 |
| Non‐Hispanic black | 30,995 (22.8) | 16,314 (23.3) | 14,681 (22.2) | |
| Hispanic | 21,255 (15.6) | 16,583 (23.7) | 4,672 (7.1) | |
| Asian | 2,075 (1.5) | 1,313 (1.9) | 762 (1.2) | |
| Non‐Hispanic other | 9,731 (7.1) | 5,120 (7.3) | 4,611 (7.0) | |
| Payer | ||||
| Government | 68,725 (50.4) | 36,967 (52.8) | 31,758 (47.9) | <0.001 |
| Private | 48,416 (35.5) | 21,112 (30.2) | 27,304 (41.2) | |
| Other | 19,098 (14.0) | 11,904 (17) | 7,194 (10.9) | |
| Observation‐Status Patients in Hospitals With a Dedicated Observation Unit* | Observation‐Status Patients in Hospitals Without a Dedicated Observation Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank | APR‐DRG | No. | % of All Observation Status Stays | % Began in ED | Rank | APR‐DRG | No. | % of All Observation Status Stays | % Began in ED |
| |||||||||
| 1 | Tonsil and adenoid procedures | 4,621 | 6.6 | 1.3 | 1 | Tonsil and adenoid procedures | 3,806 | 5.7 | 1.6 |
| 2 | Asthma | 4,246 | 6.1 | 85.3 | 2 | Asthma | 3,756 | 5.7 | 79.0 |
| 3 | Seizure | 3,516 | 5.0 | 52.0 | 3 | Seizure | 2,846 | 4.3 | 54.9 |
| 4 | Nonbacterial gastroenteritis | 3,286 | 4.7 | 85.8 | 4 | Upper respiratory infections | 2,733 | 4.1 | 69.6 |
| 5 | Bronchiolitis, RSV pneumonia | 3,093 | 4.4 | 78.5 | 5 | Nonbacterial gastroenteritis | 2,682 | 4.0 | 74.5 |
| 6 | Upper respiratory infections | 2,923 | 4.2 | 80.0 | 6 | Other digestive system diagnoses | 2,545 | 3.8 | 66.3 |
| 7 | Other digestive system diagnoses | 2,064 | 2.9 | 74.0 | 7 | Bronchiolitis, RSV pneumonia | 2,544 | 3.8 | 69.2 |
| 8 | Respiratory signs, symptoms, diagnoses | 2,052 | 2.9 | 81.6 | 8 | Shoulder and arm procedures | 1,862 | 2.8 | 72.6 |
| 9 | Other ENT/cranial/facial diagnoses | 1,684 | 2.4 | 43.6 | 9 | Appendectomy | 1,785 | 2.7 | 79.2 |
| 10 | Shoulder and arm procedures | 1,624 | 2.3 | 79.1 | 10 | Other ENT/cranial/facial diagnoses | 1,624 | 2.5 | 29.9 |
| 11 | Abdominal pain | 1,612 | 2.3 | 86.2 | 11 | Abdominal pain | 1,461 | 2.2 | 82.3 |
| 12 | Fever | 1,494 | 2.1 | 85.1 | 12 | Other factors influencing health status | 1,461 | 2.2 | 66.3 |
| 13 | Appendectomy | 1,465 | 2.1 | 66.4 | 13 | Cellulitis/other bacterial skin infections | 1,383 | 2.1 | 84.2 |
| 14 | Cellulitis/other bacterial skin infections | 1,393 | 2.0 | 86.4 | 14 | Respiratory signs, symptoms, diagnoses | 1,308 | 2.0 | 39.1 |
| 15 | Pneumonia NEC | 1,356 | 1.9 | 79.1 | 15 | Pneumonia NEC | 1,245 | 1.9 | 73.1 |
| Total | 36,429 | 52.0 | 57.8 | Total | 33,041 | 49.87 | 53.0 | ||
Outcomes of Observation‐Status Stays
A greater percentage of observation‐status stays in hospitals with a dedicated OU experienced a same‐day discharge (Table 4). In addition, a higher percentage of discharges occurred between midnight and 11 am in hospitals with a dedicated OU. However, overall risk‐adjusted LOS in hours (12.8 vs 12.2 hours, P=0.90) and risk‐adjusted total standardized costs ($2551 vs $2433, P=0.75) were similar between hospital types. These findings were consistent within the 1 APR‐DRGs commonly cared for by pediatric hospitalists (see Supporting Information, Appendix 1, in the online version of this article). Overall, conversion from observation to inpatient status was significantly higher in hospitals with a dedicated OU compared with hospitals without; however, this pattern was not consistent across the 10 APR‐DRGs commonly cared for by pediatric hospitalists (see Supporting Information, Appendix 1, in the online version of this article). Adjusted odds of 3‐day ED return visits and 30‐day readmissions were comparable between hospital groups.
| Observation‐Status Patients in Hospitals With a Dedicated Observation Unit | Observation‐Status Patients in Hospitals Without a Dedicated Observation Unit | P Value | |
|---|---|---|---|
| |||
| No. of hospitals | 14 | 17 | |
| Length of stay, h, median (IQR) | 12.8 (6.923.7) | 12.2 (721.3) | 0.90 |
| 0 midnights, no. (%) | 16,678 (23.8) | 14,648 (22.1) | <.001 |
| 1 midnight, no. (%) | 46,144 (65.9) | 44,559 (67.3) | |
| 2 midnights or more, no. (%) | 7,161 (10.2) | 7,049 (10.6) | |
| Discharge timing, no. (%) | |||
| Midnight5 am | 1,223 (1.9) | 408 (0.7) | <0.001 |
| 6 am11 am | 18,916 (29.3) | 15,914 (27.1) | |
| Noon5 pm | 32,699 (50.7) | 31,619 (53.9) | |
| 6 pm11 pm | 11,718 (18.2) | 10,718 (18.3) | |
| Total standardized costs, $, median (IQR) | 2,551.3 (2,053.93,169.1) | 2,433.4 (1,998.42,963) | 0.75 |
| Conversion to inpatient status | 11.06% | 9.63% | <0.01 |
| Return care, AOR (95% CI) | |||
| 3‐day ED return visit | 0.93 (0.77‐1.12) | Referent | 0.46 |
| 30‐day readmission | 0.88 (0.67‐1.15) | Referent | 0.36 |
We found similar results in sensitivity analyses comparing observation‐status stays in hospitals with a continuously open OU (open 24 hours per day, 7 days per week, for all of 2011 [n=10 hospitals]) to those without(see Supporting Information, Appendix 2, in the online version of this article). However, there were, on average, more observation‐status stays in hospitals with a continuously open OU (median 5605, IQR 42077089) than hospitals without (median 3309, IQR 26784616) (P=0.04). In contrast to our main results, conversion to inpatient status was lower in hospitals with a continuously open OU compared with hospitals without (8.52% vs 11.57%, P<0.01).
DISCUSSION
Counter to our hypothesis, we did not find hospital‐level differences in length of stay or costs for observation‐status patients cared for in hospitals with and without a dedicated OU, though hospitals with dedicated OUs did have more same‐day discharges and more morning discharges. The lack of observed differences in LOS and costs may reflect the fact that many children under observation status are treated throughout the hospital, even in facilities with a dedicated OU. Access to a dedicated OU is limited by factors including small numbers of OU beds and specific low acuity/low complexity OU admission criteria.[7] The inclusion of all children admitted under observation status in our analyses may have diluted any effect of dedicated OUs at the hospital level, but was necessary due to the inability to identify location of care for children admitted under observation status. Location of care is an important variable that should be incorporated into administrative databases to allow for comparative effectiveness research designs. Until such data are available, chart review at individual hospitals would be necessary to determine which patients received care in an OU.
We did find that discharges for observation‐status patients occurred earlier in the day in hospitals with a dedicated OU when compared with observation‐status patients in hospitals without a dedicated OU. In addition, the percentage of same‐day discharges was higher among observation‐status patients treated in hospitals with a dedicated OU. These differences may stem from policies and procedures that encourage rapid discharge in dedicated OUs, and those practices may affect other care areas. For example, OUs may enforce policies requiring family presence at the bedside or utilize staffing models where doctors and nurses are in frequent communication, both of which would facilitate discharge as soon as a patient no longer required hospital‐based care.[7] A retrospective chart review study design could be used to identify discharge processes and other key characteristics of highly performing OUs.
We found conflicting results in our main and sensitivity analyses related to conversion to inpatient status. Lower percentages of observation‐status patients converting to inpatient status indicates greater success in the delivery of observation care based on established performance metrics.[19] Lower rates of conversion to inpatient status may be the result of stricter admission criteria for some diagnosis and in hospitals with a continuously open dedicate OU, more refined processes for utilization review that allow for patients to be placed into the correct status (observation vs inpatient) at the time of admission, or efforts to educate providers about the designation of observation status.[7] It is also possible that fewer observation‐status patients convert to inpatient status in hospitals with a continuously open dedicated OU because such a change would require movement of the patient to an inpatient bed.
These analyses were more comprehensive than our prior studies[2, 20] in that we included both patients who were treated first in the ED and those who were not. In addition to the APR‐DRGs representative of conditions that have been successfully treated in ED‐based pediatric OUs (eg, asthma, seizures, gastroenteritis, cellulitis),[8, 9, 21, 22] we found observation‐status was commonly associated with procedural care. This population of patients may be relevant to hospitalists who staff OUs that provide both unscheduled and postprocedural care. The colocation of medical and postprocedural patients has been described by others[8, 23] and was reported to occur in over half of the OUs included in this study.[7] The extent to which postprocedure observation care is provided in general OUs staffed by hospitalists represents another opportunity for further study.
Hospitals face many considerations when determining if and how they will provide observation services to patients expected to experience short stays.[7] Some hospitals may be unable to justify an OU for all or part of the year based on the volume of admissions or the costs to staff an OU.[24, 25] Other hospitals may open an OU to promote patient flow and reduce ED crowding.[26] Hospitals may also be influenced by reimbursement policies related to observation‐status stays. Although we did not observe differences in overall payer mix, we did find higher percentages of observation‐status patients in hospitals with dedicated OUs to have public insurance. Although hospital contracts with payers around observation status patients are complex and beyond the scope of this analysis, it is possible that hospitals have established OUs because of increasingly stringent rules or criteria to meet inpatient status or experiences with high volumes of observation‐status patients covered by a particular payer. Nevertheless, the brief nature of many pediatric hospitalizations and the scarcity of pediatric OU beds must be considered in policy changes that result from national discussions about the appropriateness of inpatient stays shorter than 2 nights in duration.[27]
Limitations
The primary limitation to our analyses is the lack of ability to identify patients who were treated in a dedicated OU because few hospitals provided data to PHIS that allowed for the identification of the unit or location of care. Second, it is possible that some hospitals were misclassified as not having a dedicated OU based on our survey, which initially inquired about OUs that provided care to patients first treated in the ED. Therefore, OUs that exclusively care for postoperative patients or patients with scheduled treatments may be present in hospitals that we have labeled as not having a dedicated OU. This potential misclassification would bias our results toward finding no differences. Third, in any study of administrative data there is potential that diagnosis codes are incomplete or inaccurately capture the underlying reason for the episode of care. Fourth, the experiences of the free‐standing children's hospitals that contribute data to PHIS may not be generalizable to other hospitals that provide observation care to children. Finally, return care may be underestimated, as children could receive treatment at another hospital following discharge from a PHIS hospital. Care outside of PHIS hospitals would not be captured, but we do not expect this to differ for hospitals with and without dedicated OUs. It is possible that health information exchanges will permit more comprehensive analyses of care across different hospitals in the future.
CONCLUSION
Observation status patients are similar in hospitals with and without dedicated observation units that admit children from the ED. The presence of a dedicated OU appears to have an influence on same‐day and morning discharges across all observation‐status stays without impacting other hospital‐level outcomes. Inclusion of location of care (eg, geographically distinct dedicated OU vs general inpatient unit vs ED) in hospital administrative datasets would allow for meaningful comparisons of different models of care for short‐stay observation‐status patients.
Acknowledgements
The authors thank John P. Harding, MBA, FACHE, Children's Hospital of the King's Daughters, Norfolk, Virginia for his input on the study design.
Disclosures: Dr. Hall had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Internal funds from the Children's Hospital Association supported the conduct of this work. The authors have no financial relationships or conflicts of interest to disclose.
© 2015 Society of Hospital Medicine
Gender Disparities for Academic Hospitalists
Gender disparities still exist for women in academic medicine.[1, 2, 3, 4, 5, 6, 7, 8, 9] The most recent data from the Association of American Medical Colleges (AAMC) show that although gender disparities are decreasing, women are still under‐represented in the assistant, associate, and full‐professor ranks as well as in leadership positions.[1]
Some studies indicate that gender differences are less evident when examining younger cohorts.[1, 10, 11, 12, 13] Hospital medicine emerged around 1996, when the term hospitalist was first coined.[14] The gender distribution of academic hospitalists is likely nearly equal,[15, 16] and they are generally younger physicians.[15, 17, 18, 19, 20] Accordingly, we questioned whether gender disparities existed in academic hospital medicine (HM) and, if so, whether these disparities were greater than those that might exist in academic general internal medicine (GIM).
METHODS
This study consisted of both prospective and retrospective observation of data collected for academic adult hospitalists and general internists who practice in the United States. It was approved by the Colorado Multiple Institutional Review Board.
Gender distribution was assessed with respect to: (1) academic HM and GIM faculty, (2) leadership (ie, division or section heads), and (3) scholarly work (ie, speaking opportunities and publications). Data were collected between October 1, 2012 and August 31, 2014.
Gender Distribution of Faculty and Division/Section Heads
All US internal medicine residency programs were identified from the list of members or affiliates of the AAMC that were fully accredited by the Liaison Committee on Medical Education[21] using the Graduate Medical Education Directory.[22] We then determined the primary training hospital(s) affiliated with each program and selected those that were considered to be university hospitals and eliminated those that did not have divisions or sections of HM or GIM. We determined the gender of the respective division/section heads on the basis of the faculty member's first name (and often from accompanying photos) as well as from information obtained via Internet searches and, if necessary, contacted the individual institutions via email or phone call(s). We also determined the number and gender of all of the HM and GIM faculty members in a random sample of 25% of these hospitals from information on their respective websites.
Gender Distribution for Scholarly Productivity
We determined the gender and specialty of all speakers at the Society of Hospital Medicine and the Society of General Internal Medicine national conferences from 2006 to 2012. A list of speakers at each conference was obtained from conference pamphlets or agendas that were available via Internet searches or obtained directly from the organization. We also determined whether each presenter was a featured speaker (defined as one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), or if they spoke in a group format (also as indicated in the conference pamphlets). Because of the low number of featured and plenary speakers, these data were combined. Faculty labeled as additional faculty when presenting in a group format were excluded as were speakers at precourses, those presenting abstracts, and those participating in interest group sessions.
For authorship, a PubMed search was used to identify all articles published in the Journal of Hospital Medicine (JHM) and the Journal of General Internal Medicine (JGIM) from January 1, 2006 through December 31, 2012, and the gender and specialty of all the first and last authors were determined as described above. Specialty was determined from the division, section or department affiliation indicated for each author and by Internet searches. In some instances, it was necessary to contact the authors or their departments directly to verify their specialty. When articles had only 1 author, the author was considered a first author.
Duplicate records (eg, same author, same journal) and articles without an author were excluded, as were authors who did not have an MD, DO, or MBBS degree and those who were not affiliated with an institution in the United States. All manuscripts, with the exception of errata, were analyzed together as well as in 3 subgroups: original research, editorials, and others.
A second investigator corroborated data regarding gender and specialty for all speakers and authors to strengthen data integrity. On the rare occasion when discrepancies were found, a third investigator adjudicated the results.
Definitions
Physicians were defined as being hospitalists if they were listed as a member of a division or section of HM on their publications or if Internet searches indicated that they were a hospitalist or primarily worked on inpatient medical services. Physicians were considered to be general internists if they were listed as such on their publications and their specialty could be verified in Web‐based searches. If physicians appeared to have changing roles over time, we attempted to assign their specialty based upon their role at the time the article was published or the presentation was delivered. If necessary, phone calls and/or emails were also done to determine the physician's specialty.
Analysis
REDCap, a secure, Web‐based application for building and managing online surveys and databases, was used to collect and manage all study data.[23] All analyses were performed using SAS Enterprise Guide 4.3 (SAS Institute, Inc., Cary, NC). A [2] test was used to compare proportions of male versus female physicians, and data from hospitalists versus general internists. Because we performed multiple comparisons when analyzing presentations and publications, a Bonferroni adjustment was made such that a P<0.0125 for presentations and P<0.006 (within specialty) or P<0.0125 (between specialty) for the publication analyses were considered significant. P<0.05 was considered significant for all other comparisons.
RESULTS
Gender Distribution of Faculty
Eighteen HM and 20 GIM programs from university hospitals were randomly selected for review (see Supporting Figure 1 in the online version of this article). Seven of the HM programs and 1 of the GIM programs did not have a website, did not differentiate hospitalists from other faculty, or did not list their faculty on the website and were excluded from the analysis. In the remaining 11 HM programs and 19 GIM programs, women made up 277/568 (49%) and 555/1099 (51%) of the faculty, respectively (P=0.50).
Gender Distribution of Division/Section Heads
Eighty‐six of the programs were classified as university hospitals (see Supporting Figure 1 in the online version of this article), and in these, women led 11/69 (16%) of the HM divisions or sections and 28/80 (35%) of the GIM divisions (P=0.008).
Gender Distribution for Scholarly Productivity
Speaking Opportunities
A total of 1227 presentations were given at the 2 conferences from 2006 to 2012, with 1343 of the speakers meeting inclusion criteria (see Supporting Figure 2 in the online version of this article). Hospitalists accounted for 557 of the speakers, of which 146 (26%) were women. General internists accounted for 580 of the speakers, of which 291 (50%) were women (P<0.0001) (Table 1).
| Male, N (%) | Female, N (%) | |
|---|---|---|
| ||
| Hospitalists | ||
| All presentations | 411 (74) | 146 (26)* |
| Featured or plenary presentations | 49 (91) | 5 (9)* |
| General internists | ||
| All presentations | 289 (50) | 291 (50) |
| Featured or plenary presentations | 27 (55) | 22 (45) |
Of the 117 featured or plenary speakers, 54 were hospitalists and 5 (9%) of these were women. Of the 49 who were general internists, 22 (45%) were women (P<0.0001).
Authorship
The PubMed search identified a total of 3285 articles published in the JHM and the JGIM from 2006 to 2012, and 2172 first authors and 1869 last authors met inclusion criteria (see Supporting Figure 3 in the online version of this article). Hospitalists were listed as first or last authors on 464 and 305 articles, respectively, and of these, women were first authors on 153 (33%) and last authors on 63 (21%). General internists were listed as first or last authors on 895 and 769 articles, respectively, with women as first authors on 423 (47%) and last authors on 265 (34%). Compared with general internists, fewer women hospitalists were listed as either first or last authors (both P<0.0001) (Table 2).
| First Author | Last Author | |||
|---|---|---|---|---|
| Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | |
| ||||
| Hospitalists | ||||
| All publications | 311 (67) | 153 (33)* | 242 (79) | 63 (21)* |
| Original investigations/brief reports | 124 (61) | 79 (39)* | 96 (76) | 30 (24)* |
| Editorials | 34 (77) | 10 (23)* | 18 (86) | 3 (14)* |
| Other | 153 (71) | 64 (29)* | 128 (81) | 30 (19)* |
| General internists | ||||
| All publications | 472 (53) | 423 (47) | 504 (66) | 265 (34)* |
| Original investigations/brief reports | 218 (46) | 261 (54) | 310 (65) | 170 (35)* |
| Editorial | 98 (68) | 46 (32)* | 43 (73) | 16 (27)* |
| Other | 156 (57) | 116 (43) | 151 (66) | 79 (34)* |
Fewer women hospitalists were listed as first or last authors on all article types. For original research articles written by general internists, there was a trend for more women to be listed as first authors than men (261/479, 54%), but this difference was not statistically significant.
DISCUSSION
The important findings of this study are that, despite an equal gender distribution of academic HM and GIM faculty, fewer women were HM division/section chiefs, fewer women were speakers at the 2 selected national meetings, and fewer women were first or last authors of publications in 2 selected journals in comparison with general internists.
Previous studies have found that women lag behind their male counterparts with respect to academic productivity, leadership, and promotion.[1, 5, 7] Some studies suggest, however, that gender differences are reduced when younger cohorts are examined.[1, 10, 11, 12, 13] Surveys indicate that that the mean age of hospitalists is younger than most other specialties.[15, 19, 20, 24] The mean age of academic GIM physicians is unknown, but surveys of GIM (not differentiating academic from nonacademic) suggest that it is an older cohort than that of HM.[24] Despite hospitalists being a younger cohort, we found gender disparities in all areas investigated.
Our findings with respect to gender disparities in HM division or section leadership are consistent with the annual AAMC Women in US Academic Medicine and Science Benchmarking Report that found only 22% of all permanent division or section heads were women.[1]
Gender disparities with respect to authorship of medical publications have been previously noted,[3, 6, 15, 25] but to our knowledge, this is the first study to investigate the gender of authors who were hospitalists. Although we found a higher proportion of women hospitalists who were first or last authors than was observed by Jagsi and colleagues,[3] women hospitalists were still under‐represented with respect to this measure of academic productivity. Erren et al. reviewed 6 major journals from 2010 and 2011, and found that first authorship of original research by women ranged from 23.7% to 46.7%, and for last authorship from 18.3% to 28.8%.[25] Interestingly, we found no significant gender difference for first authors who were general internists, and there was a trend toward more women general internists being first authors than men for original research, reviews, and brief reports (data not shown).
Our study did not attempt to answer the question of why gender disparities persist, but many previous studies have explored this issue.[4, 8, 12, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42] Issues raised by others include the quantity of academic work (ie, publications and grants obtained), differences in hours worked and allocation of time, lack of mentorship, family responsibilities, discrimination, differences in career motivation, and levels of institutional support, to name a few.
The under‐representation of women hospitalists in leadership, authorship, and speaking opportunities may be consistent with gender‐related differences in research productivity. Fewer publications could lead to fewer national presentations, which could lead to fewer leadership opportunities. Our findings with respect to general internists are not consistent with this idea, however, as whereas women were under‐represented in GIM leadership positions, we found no disparities with respect to the gender of first authors or speakers at national meetings for general internists. The finding that hospitalists had gender disparities with respect to first authors and national speakers but general internists did not, argues against several hypotheses (ie, that women lack mentorship, have less career motivation, fewer career building opportunities).
One notable hypothesis, and perhaps one that is often discussed in the literature, is that women shoulder the majority of family responsibilities, and this may result in women having less time for their careers. Jolly and colleagues studied physician‐researchers and noted that women were more likely than men to have spouses or domestic partners who were fully employed, spent 8.5 more hours per week on domestic activities, and were more likely to take time off during disruptions of usual child care.[33] Carr and colleagues found that women with children (compared to men with children) had fewer publications, slower self‐perceived career progress, and lower career satisfaction, but having children had little effect on faculty aspirations and goals.[2] Kaplan et al., however, found that family responsibilities do not appear to account for sex differences in academic advancement.[4] Interestingly, in a study comparing physicians from Generation X to those of the Baby Boomer age, Generation X women reported working more than their male Generation X counterparts, and both had more of a focus on worklife balance than the older generation.[12]
The nature the of 2 specialties' work environment and job requirements could have also resulted in some of the differences seen. Primary care clinical work is typically conducted Monday through Friday, and hospitalist work frequently includes some weekend, evening, night, and holiday coverage. Although these are known differences, both specialties have also been noted to offer many advantages to women and men alike, including collaborative working environments and flexible work hours.[16]
Finally, finding disparity in leadership positions in both specialties supports the possibility that those responsible for hiring could have implicit gender biases. Under‐representation in entry‐level positions is also not a likely explanation for the differences we observed, because nearly an equal number of men and women graduate from medical school, pursue residency training in internal medicine, and become either academic hospitalists or general internists at university settings.[1, 15, 24] This hypothesis could, however, explain why disparities exist with respect to senior authorship and leadership positions, as typically, these individuals have been in practice longer and the current trends of improved gender equality have not always been the case.
Our study has a number of limitations. First, we only examined publications in 2 journals and presentations at 2 national conferences, although the journals and conferences selected are considered to be the major ones in the 2 specialties. Second, using Internet searches may have resulted in inaccurate gender and specialty assignment, but previous studies have used similar methodology.[3, 43] Additionally, we also attempted to contact individuals for direct confirmation when the information we obtained was not clear and had a second investigator independently verify the gender and specialty data. Third, we utilized division/department websites when available to determine the gender of HM divisions/sections. If not recently updated, these websites may not have reflected the most current leader of the unit, but this concern would seemingly pertain to both hospitalists and general internists. Fourth, we opted to only study faculty and division/section heads at university hospitals, as typically these institutions had GIM and hospitalist groups and also typically had websites. Because we only studied faculty and leadership at university hospitals, our data are not generalizable to all hospitalist and GIM groups. Finally, we excluded pediatric hospitalists, and thus, this study is representative of adult hospitalists only. Including pediatric hospitalists was out of the scope of this project.
Our study also had a number of strengths. To our knowledge, this is the first study to provide an estimate of the gender distribution in academic HM, of hospitalists as speakers at national meetings, as first and last authors, and of HM division or section heads, and is the first to compare these results with those observed for general internists. In addition, we examined 7 years of data from 2 of the major journals and national conferences for these specialties.
In summary, despite HM being a newer field with a younger cohort of physicians, we found that gender disparities exist for women with respect to authorship, national speaking opportunities, and division or section leadership. Identifying why these gender differences exist presents an important next step.
Disclosures: Nothing to report. Marisha Burden, MD and Maria G. Frank, MD are coprincipal authors.
- Association of American Medical Colleges. Women in U.S. academic medicine and science: Statistics and benchmarking report. 2012. Available at: https://members.aamc.org/eweb/upload/Women%20in%20U%20S%20%20Academic%20Medicine%20Statistics%20and%20Benchmarking%20Report%202011-20123.pdf. Accessed September 1, 2014.
- , , , et al. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Intern Med. 1998;129:532–538.
- , , , et al. The “gender gap” in authorship of academic medical literature—a 35‐year perspective. N Engl J Med. 2006;355:281–287.
- , , , , , . Sex differences in academic advancement. Results of a national study of pediatricians. N Engl J Med. 1996;335:1282–1289.
- . Women physicians in academic medicine: new insights from cohort studies. N Engl J Med. 2000;342:399–405.
- , , , , . Gender differences in academic productivity and leadership appointments of physicians throughout academic careers. Acad Med. 2011;86:43–47.
- , , , . Promotion of women physicians in academic medicine. Glass ceiling or sticky floor? JAMA. 1995;273:1022–1025.
- , , , . Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141:205–212.
- , , . Women physicians: choosing a career in academic medicine. Acad Med. 2012;87:105–114.
- , , , . The status of women at one academic medical center. Breaking through the glass ceiling. JAMA. 1990;264:1813–1817.
- , . Status of women in academic anesthesiology. Anesthesiology. 1986;64:496–500.
- , , . The generation and gender shifts in medicine: an exploratory survey of internal medicine physicians. BMC Health Serv Res. 2006;6:55.
- Pew Research Center. On pay gap, millenial women near parity—for now. December 2013. Available at: http://www.pewsocialtrends.org/files/2013/12/gender-and-work_final.pdf. Published December 11, 2013. Accessed February 5, 2015.
- , . The emerging role of "hospitalists" in the American health care system. N Engl J Med. 1996;335:514–517.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27:23–27.
- . The gender factor. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/the‐gender‐factor. Published March 1, 2006. Accessed September 1, 2014.
- Association of American Medical Colleges. Analysis in brief: Supplemental information for estimating the number and characteristics of hospitalist physicians in the United States and their possible workforce implications. Available at: https://www.aamc.org/download/300686/data/aibvol12_no3-supplemental.pdf. Published August 2012. Accessed September 1, 2014.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5–9.
- State of Hospital Medicine: 2011 Report Based on 2010 Data. Medical Group Management Association and Society of Hospital Medicine. www.mgma.com, www.hospitalmedicine.org.
- Today's Hospitalist Survey. Compensation and Career Survey Results. 2013. Available at: http://www.todayshospitalist.com/index.php?b=salary_survey_results. Accessed January 11, 2015.
- Association of American Medical Colleges. Women in U.S. Academic Medicine: Statistics and Benchmarking Report. 2009–2010. Available at: https://www.aamc.org/download/182674/data/gwims_stats_2009‐2010.pdf. Accessed September 1, 2014.
- American Medical Association. Graduate Medical Education Directory 2012–2013. Chicago, IL: American Medical Association; 2012:182–203.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- Association of American Medical Colleges. 2012 Physician Specialty Data Book. Center for Workforce Studies. Available at: https://www.aamc.org/download/313228/data/2012physicianspecialtydatabook.pdf. Published November 2012. Accessed September 1, 2014.
- , , , . Representation of women as authors, reviewers, editors in chief, and editorial board members at 6 general medical journals in 2010 and 2011. JAMA Intern Med. 2014;174:633–635.
- , , , et al. Relationships of gender and career motivation to medical faculty members' production of academic publications. Acad Med. 1998;73:180–186.
- , , , et al. Faculty perceptions of gender discrimination and sexual harassment in academic medicine. Ann Intern Med. 2000;132:889–896.
- , , , . Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey. Arch Intern Med. 2000;160:2625–2629.
- , , , , . A "ton of feathers": gender discrimination in academic medical careers and how to manage it. J Womens Health (Larchmt). 2003;12:1009–1018.
- , , . Perceived obstacles to career success for women in academic surgery. Arch Surg. 2000;135:972–977.
- , , , . Career satisfaction of US women physicians: results from the Women Physicians' Health Study. Society of General Internal Medicine Career Satisfaction Study Group. Arch Intern Med. 1999;159:1417–1426.
- . Doing the same and earning less: male and female physicians in a new medical specialty. Inquiry. 2004;41:301–315.
- , , , , , . Gender differences in time spent on parenting and domestic responsibilities by high‐achieving young physician‐researchers. Ann Intern Med. 2014;160:344–353.
- , , , , . Stories from early‐career women physicians who have left academic medicine: a qualitative study at a single institution. Acad Med. 2011;86:752–758.
- , , , . The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193–201.
- , , , , . Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28:201–207.
- . Gender pay gaps in hospital medicine. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/gender‐pay‐gaps‐in‐hospital‐medicine. Published February 29, 2012. Accessed September 1, 2014.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103–1115.
- . Inequality quantified: mind the gender gap. Nature. 2013;495:22–24.
- , , , et al. Gender differences in academic advancement: patterns, causes, and potential solutions in one US College of Medicine. Acad Med. 2003;78:500–508.
- , . Why aren't there more women leaders in academic medicine? The views of clinical department chairs. Acad Med. 2001;76:453–465.
- . Gender factors in reviewer recommendations for manuscript publication. J Appl Behav Anal. 1990;23:539–543.
- , , , . Scientific impact of women in academic surgery. J Surg Res. 2008;148:13–16.
Gender disparities still exist for women in academic medicine.[1, 2, 3, 4, 5, 6, 7, 8, 9] The most recent data from the Association of American Medical Colleges (AAMC) show that although gender disparities are decreasing, women are still under‐represented in the assistant, associate, and full‐professor ranks as well as in leadership positions.[1]
Some studies indicate that gender differences are less evident when examining younger cohorts.[1, 10, 11, 12, 13] Hospital medicine emerged around 1996, when the term hospitalist was first coined.[14] The gender distribution of academic hospitalists is likely nearly equal,[15, 16] and they are generally younger physicians.[15, 17, 18, 19, 20] Accordingly, we questioned whether gender disparities existed in academic hospital medicine (HM) and, if so, whether these disparities were greater than those that might exist in academic general internal medicine (GIM).
METHODS
This study consisted of both prospective and retrospective observation of data collected for academic adult hospitalists and general internists who practice in the United States. It was approved by the Colorado Multiple Institutional Review Board.
Gender distribution was assessed with respect to: (1) academic HM and GIM faculty, (2) leadership (ie, division or section heads), and (3) scholarly work (ie, speaking opportunities and publications). Data were collected between October 1, 2012 and August 31, 2014.
Gender Distribution of Faculty and Division/Section Heads
All US internal medicine residency programs were identified from the list of members or affiliates of the AAMC that were fully accredited by the Liaison Committee on Medical Education[21] using the Graduate Medical Education Directory.[22] We then determined the primary training hospital(s) affiliated with each program and selected those that were considered to be university hospitals and eliminated those that did not have divisions or sections of HM or GIM. We determined the gender of the respective division/section heads on the basis of the faculty member's first name (and often from accompanying photos) as well as from information obtained via Internet searches and, if necessary, contacted the individual institutions via email or phone call(s). We also determined the number and gender of all of the HM and GIM faculty members in a random sample of 25% of these hospitals from information on their respective websites.
Gender Distribution for Scholarly Productivity
We determined the gender and specialty of all speakers at the Society of Hospital Medicine and the Society of General Internal Medicine national conferences from 2006 to 2012. A list of speakers at each conference was obtained from conference pamphlets or agendas that were available via Internet searches or obtained directly from the organization. We also determined whether each presenter was a featured speaker (defined as one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), or if they spoke in a group format (also as indicated in the conference pamphlets). Because of the low number of featured and plenary speakers, these data were combined. Faculty labeled as additional faculty when presenting in a group format were excluded as were speakers at precourses, those presenting abstracts, and those participating in interest group sessions.
For authorship, a PubMed search was used to identify all articles published in the Journal of Hospital Medicine (JHM) and the Journal of General Internal Medicine (JGIM) from January 1, 2006 through December 31, 2012, and the gender and specialty of all the first and last authors were determined as described above. Specialty was determined from the division, section or department affiliation indicated for each author and by Internet searches. In some instances, it was necessary to contact the authors or their departments directly to verify their specialty. When articles had only 1 author, the author was considered a first author.
Duplicate records (eg, same author, same journal) and articles without an author were excluded, as were authors who did not have an MD, DO, or MBBS degree and those who were not affiliated with an institution in the United States. All manuscripts, with the exception of errata, were analyzed together as well as in 3 subgroups: original research, editorials, and others.
A second investigator corroborated data regarding gender and specialty for all speakers and authors to strengthen data integrity. On the rare occasion when discrepancies were found, a third investigator adjudicated the results.
Definitions
Physicians were defined as being hospitalists if they were listed as a member of a division or section of HM on their publications or if Internet searches indicated that they were a hospitalist or primarily worked on inpatient medical services. Physicians were considered to be general internists if they were listed as such on their publications and their specialty could be verified in Web‐based searches. If physicians appeared to have changing roles over time, we attempted to assign their specialty based upon their role at the time the article was published or the presentation was delivered. If necessary, phone calls and/or emails were also done to determine the physician's specialty.
Analysis
REDCap, a secure, Web‐based application for building and managing online surveys and databases, was used to collect and manage all study data.[23] All analyses were performed using SAS Enterprise Guide 4.3 (SAS Institute, Inc., Cary, NC). A [2] test was used to compare proportions of male versus female physicians, and data from hospitalists versus general internists. Because we performed multiple comparisons when analyzing presentations and publications, a Bonferroni adjustment was made such that a P<0.0125 for presentations and P<0.006 (within specialty) or P<0.0125 (between specialty) for the publication analyses were considered significant. P<0.05 was considered significant for all other comparisons.
RESULTS
Gender Distribution of Faculty
Eighteen HM and 20 GIM programs from university hospitals were randomly selected for review (see Supporting Figure 1 in the online version of this article). Seven of the HM programs and 1 of the GIM programs did not have a website, did not differentiate hospitalists from other faculty, or did not list their faculty on the website and were excluded from the analysis. In the remaining 11 HM programs and 19 GIM programs, women made up 277/568 (49%) and 555/1099 (51%) of the faculty, respectively (P=0.50).
Gender Distribution of Division/Section Heads
Eighty‐six of the programs were classified as university hospitals (see Supporting Figure 1 in the online version of this article), and in these, women led 11/69 (16%) of the HM divisions or sections and 28/80 (35%) of the GIM divisions (P=0.008).
Gender Distribution for Scholarly Productivity
Speaking Opportunities
A total of 1227 presentations were given at the 2 conferences from 2006 to 2012, with 1343 of the speakers meeting inclusion criteria (see Supporting Figure 2 in the online version of this article). Hospitalists accounted for 557 of the speakers, of which 146 (26%) were women. General internists accounted for 580 of the speakers, of which 291 (50%) were women (P<0.0001) (Table 1).
| Male, N (%) | Female, N (%) | |
|---|---|---|
| ||
| Hospitalists | ||
| All presentations | 411 (74) | 146 (26)* |
| Featured or plenary presentations | 49 (91) | 5 (9)* |
| General internists | ||
| All presentations | 289 (50) | 291 (50) |
| Featured or plenary presentations | 27 (55) | 22 (45) |
Of the 117 featured or plenary speakers, 54 were hospitalists and 5 (9%) of these were women. Of the 49 who were general internists, 22 (45%) were women (P<0.0001).
Authorship
The PubMed search identified a total of 3285 articles published in the JHM and the JGIM from 2006 to 2012, and 2172 first authors and 1869 last authors met inclusion criteria (see Supporting Figure 3 in the online version of this article). Hospitalists were listed as first or last authors on 464 and 305 articles, respectively, and of these, women were first authors on 153 (33%) and last authors on 63 (21%). General internists were listed as first or last authors on 895 and 769 articles, respectively, with women as first authors on 423 (47%) and last authors on 265 (34%). Compared with general internists, fewer women hospitalists were listed as either first or last authors (both P<0.0001) (Table 2).
| First Author | Last Author | |||
|---|---|---|---|---|
| Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | |
| ||||
| Hospitalists | ||||
| All publications | 311 (67) | 153 (33)* | 242 (79) | 63 (21)* |
| Original investigations/brief reports | 124 (61) | 79 (39)* | 96 (76) | 30 (24)* |
| Editorials | 34 (77) | 10 (23)* | 18 (86) | 3 (14)* |
| Other | 153 (71) | 64 (29)* | 128 (81) | 30 (19)* |
| General internists | ||||
| All publications | 472 (53) | 423 (47) | 504 (66) | 265 (34)* |
| Original investigations/brief reports | 218 (46) | 261 (54) | 310 (65) | 170 (35)* |
| Editorial | 98 (68) | 46 (32)* | 43 (73) | 16 (27)* |
| Other | 156 (57) | 116 (43) | 151 (66) | 79 (34)* |
Fewer women hospitalists were listed as first or last authors on all article types. For original research articles written by general internists, there was a trend for more women to be listed as first authors than men (261/479, 54%), but this difference was not statistically significant.
DISCUSSION
The important findings of this study are that, despite an equal gender distribution of academic HM and GIM faculty, fewer women were HM division/section chiefs, fewer women were speakers at the 2 selected national meetings, and fewer women were first or last authors of publications in 2 selected journals in comparison with general internists.
Previous studies have found that women lag behind their male counterparts with respect to academic productivity, leadership, and promotion.[1, 5, 7] Some studies suggest, however, that gender differences are reduced when younger cohorts are examined.[1, 10, 11, 12, 13] Surveys indicate that that the mean age of hospitalists is younger than most other specialties.[15, 19, 20, 24] The mean age of academic GIM physicians is unknown, but surveys of GIM (not differentiating academic from nonacademic) suggest that it is an older cohort than that of HM.[24] Despite hospitalists being a younger cohort, we found gender disparities in all areas investigated.
Our findings with respect to gender disparities in HM division or section leadership are consistent with the annual AAMC Women in US Academic Medicine and Science Benchmarking Report that found only 22% of all permanent division or section heads were women.[1]
Gender disparities with respect to authorship of medical publications have been previously noted,[3, 6, 15, 25] but to our knowledge, this is the first study to investigate the gender of authors who were hospitalists. Although we found a higher proportion of women hospitalists who were first or last authors than was observed by Jagsi and colleagues,[3] women hospitalists were still under‐represented with respect to this measure of academic productivity. Erren et al. reviewed 6 major journals from 2010 and 2011, and found that first authorship of original research by women ranged from 23.7% to 46.7%, and for last authorship from 18.3% to 28.8%.[25] Interestingly, we found no significant gender difference for first authors who were general internists, and there was a trend toward more women general internists being first authors than men for original research, reviews, and brief reports (data not shown).
Our study did not attempt to answer the question of why gender disparities persist, but many previous studies have explored this issue.[4, 8, 12, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42] Issues raised by others include the quantity of academic work (ie, publications and grants obtained), differences in hours worked and allocation of time, lack of mentorship, family responsibilities, discrimination, differences in career motivation, and levels of institutional support, to name a few.
The under‐representation of women hospitalists in leadership, authorship, and speaking opportunities may be consistent with gender‐related differences in research productivity. Fewer publications could lead to fewer national presentations, which could lead to fewer leadership opportunities. Our findings with respect to general internists are not consistent with this idea, however, as whereas women were under‐represented in GIM leadership positions, we found no disparities with respect to the gender of first authors or speakers at national meetings for general internists. The finding that hospitalists had gender disparities with respect to first authors and national speakers but general internists did not, argues against several hypotheses (ie, that women lack mentorship, have less career motivation, fewer career building opportunities).
One notable hypothesis, and perhaps one that is often discussed in the literature, is that women shoulder the majority of family responsibilities, and this may result in women having less time for their careers. Jolly and colleagues studied physician‐researchers and noted that women were more likely than men to have spouses or domestic partners who were fully employed, spent 8.5 more hours per week on domestic activities, and were more likely to take time off during disruptions of usual child care.[33] Carr and colleagues found that women with children (compared to men with children) had fewer publications, slower self‐perceived career progress, and lower career satisfaction, but having children had little effect on faculty aspirations and goals.[2] Kaplan et al., however, found that family responsibilities do not appear to account for sex differences in academic advancement.[4] Interestingly, in a study comparing physicians from Generation X to those of the Baby Boomer age, Generation X women reported working more than their male Generation X counterparts, and both had more of a focus on worklife balance than the older generation.[12]
The nature the of 2 specialties' work environment and job requirements could have also resulted in some of the differences seen. Primary care clinical work is typically conducted Monday through Friday, and hospitalist work frequently includes some weekend, evening, night, and holiday coverage. Although these are known differences, both specialties have also been noted to offer many advantages to women and men alike, including collaborative working environments and flexible work hours.[16]
Finally, finding disparity in leadership positions in both specialties supports the possibility that those responsible for hiring could have implicit gender biases. Under‐representation in entry‐level positions is also not a likely explanation for the differences we observed, because nearly an equal number of men and women graduate from medical school, pursue residency training in internal medicine, and become either academic hospitalists or general internists at university settings.[1, 15, 24] This hypothesis could, however, explain why disparities exist with respect to senior authorship and leadership positions, as typically, these individuals have been in practice longer and the current trends of improved gender equality have not always been the case.
Our study has a number of limitations. First, we only examined publications in 2 journals and presentations at 2 national conferences, although the journals and conferences selected are considered to be the major ones in the 2 specialties. Second, using Internet searches may have resulted in inaccurate gender and specialty assignment, but previous studies have used similar methodology.[3, 43] Additionally, we also attempted to contact individuals for direct confirmation when the information we obtained was not clear and had a second investigator independently verify the gender and specialty data. Third, we utilized division/department websites when available to determine the gender of HM divisions/sections. If not recently updated, these websites may not have reflected the most current leader of the unit, but this concern would seemingly pertain to both hospitalists and general internists. Fourth, we opted to only study faculty and division/section heads at university hospitals, as typically these institutions had GIM and hospitalist groups and also typically had websites. Because we only studied faculty and leadership at university hospitals, our data are not generalizable to all hospitalist and GIM groups. Finally, we excluded pediatric hospitalists, and thus, this study is representative of adult hospitalists only. Including pediatric hospitalists was out of the scope of this project.
Our study also had a number of strengths. To our knowledge, this is the first study to provide an estimate of the gender distribution in academic HM, of hospitalists as speakers at national meetings, as first and last authors, and of HM division or section heads, and is the first to compare these results with those observed for general internists. In addition, we examined 7 years of data from 2 of the major journals and national conferences for these specialties.
In summary, despite HM being a newer field with a younger cohort of physicians, we found that gender disparities exist for women with respect to authorship, national speaking opportunities, and division or section leadership. Identifying why these gender differences exist presents an important next step.
Disclosures: Nothing to report. Marisha Burden, MD and Maria G. Frank, MD are coprincipal authors.
Gender disparities still exist for women in academic medicine.[1, 2, 3, 4, 5, 6, 7, 8, 9] The most recent data from the Association of American Medical Colleges (AAMC) show that although gender disparities are decreasing, women are still under‐represented in the assistant, associate, and full‐professor ranks as well as in leadership positions.[1]
Some studies indicate that gender differences are less evident when examining younger cohorts.[1, 10, 11, 12, 13] Hospital medicine emerged around 1996, when the term hospitalist was first coined.[14] The gender distribution of academic hospitalists is likely nearly equal,[15, 16] and they are generally younger physicians.[15, 17, 18, 19, 20] Accordingly, we questioned whether gender disparities existed in academic hospital medicine (HM) and, if so, whether these disparities were greater than those that might exist in academic general internal medicine (GIM).
METHODS
This study consisted of both prospective and retrospective observation of data collected for academic adult hospitalists and general internists who practice in the United States. It was approved by the Colorado Multiple Institutional Review Board.
Gender distribution was assessed with respect to: (1) academic HM and GIM faculty, (2) leadership (ie, division or section heads), and (3) scholarly work (ie, speaking opportunities and publications). Data were collected between October 1, 2012 and August 31, 2014.
Gender Distribution of Faculty and Division/Section Heads
All US internal medicine residency programs were identified from the list of members or affiliates of the AAMC that were fully accredited by the Liaison Committee on Medical Education[21] using the Graduate Medical Education Directory.[22] We then determined the primary training hospital(s) affiliated with each program and selected those that were considered to be university hospitals and eliminated those that did not have divisions or sections of HM or GIM. We determined the gender of the respective division/section heads on the basis of the faculty member's first name (and often from accompanying photos) as well as from information obtained via Internet searches and, if necessary, contacted the individual institutions via email or phone call(s). We also determined the number and gender of all of the HM and GIM faculty members in a random sample of 25% of these hospitals from information on their respective websites.
Gender Distribution for Scholarly Productivity
We determined the gender and specialty of all speakers at the Society of Hospital Medicine and the Society of General Internal Medicine national conferences from 2006 to 2012. A list of speakers at each conference was obtained from conference pamphlets or agendas that were available via Internet searches or obtained directly from the organization. We also determined whether each presenter was a featured speaker (defined as one whose talk was unopposed by other sessions), plenary speaker (defined as such in the conference pamphlets), or if they spoke in a group format (also as indicated in the conference pamphlets). Because of the low number of featured and plenary speakers, these data were combined. Faculty labeled as additional faculty when presenting in a group format were excluded as were speakers at precourses, those presenting abstracts, and those participating in interest group sessions.
For authorship, a PubMed search was used to identify all articles published in the Journal of Hospital Medicine (JHM) and the Journal of General Internal Medicine (JGIM) from January 1, 2006 through December 31, 2012, and the gender and specialty of all the first and last authors were determined as described above. Specialty was determined from the division, section or department affiliation indicated for each author and by Internet searches. In some instances, it was necessary to contact the authors or their departments directly to verify their specialty. When articles had only 1 author, the author was considered a first author.
Duplicate records (eg, same author, same journal) and articles without an author were excluded, as were authors who did not have an MD, DO, or MBBS degree and those who were not affiliated with an institution in the United States. All manuscripts, with the exception of errata, were analyzed together as well as in 3 subgroups: original research, editorials, and others.
A second investigator corroborated data regarding gender and specialty for all speakers and authors to strengthen data integrity. On the rare occasion when discrepancies were found, a third investigator adjudicated the results.
Definitions
Physicians were defined as being hospitalists if they were listed as a member of a division or section of HM on their publications or if Internet searches indicated that they were a hospitalist or primarily worked on inpatient medical services. Physicians were considered to be general internists if they were listed as such on their publications and their specialty could be verified in Web‐based searches. If physicians appeared to have changing roles over time, we attempted to assign their specialty based upon their role at the time the article was published or the presentation was delivered. If necessary, phone calls and/or emails were also done to determine the physician's specialty.
Analysis
REDCap, a secure, Web‐based application for building and managing online surveys and databases, was used to collect and manage all study data.[23] All analyses were performed using SAS Enterprise Guide 4.3 (SAS Institute, Inc., Cary, NC). A [2] test was used to compare proportions of male versus female physicians, and data from hospitalists versus general internists. Because we performed multiple comparisons when analyzing presentations and publications, a Bonferroni adjustment was made such that a P<0.0125 for presentations and P<0.006 (within specialty) or P<0.0125 (between specialty) for the publication analyses were considered significant. P<0.05 was considered significant for all other comparisons.
RESULTS
Gender Distribution of Faculty
Eighteen HM and 20 GIM programs from university hospitals were randomly selected for review (see Supporting Figure 1 in the online version of this article). Seven of the HM programs and 1 of the GIM programs did not have a website, did not differentiate hospitalists from other faculty, or did not list their faculty on the website and were excluded from the analysis. In the remaining 11 HM programs and 19 GIM programs, women made up 277/568 (49%) and 555/1099 (51%) of the faculty, respectively (P=0.50).
Gender Distribution of Division/Section Heads
Eighty‐six of the programs were classified as university hospitals (see Supporting Figure 1 in the online version of this article), and in these, women led 11/69 (16%) of the HM divisions or sections and 28/80 (35%) of the GIM divisions (P=0.008).
Gender Distribution for Scholarly Productivity
Speaking Opportunities
A total of 1227 presentations were given at the 2 conferences from 2006 to 2012, with 1343 of the speakers meeting inclusion criteria (see Supporting Figure 2 in the online version of this article). Hospitalists accounted for 557 of the speakers, of which 146 (26%) were women. General internists accounted for 580 of the speakers, of which 291 (50%) were women (P<0.0001) (Table 1).
| Male, N (%) | Female, N (%) | |
|---|---|---|
| ||
| Hospitalists | ||
| All presentations | 411 (74) | 146 (26)* |
| Featured or plenary presentations | 49 (91) | 5 (9)* |
| General internists | ||
| All presentations | 289 (50) | 291 (50) |
| Featured or plenary presentations | 27 (55) | 22 (45) |
Of the 117 featured or plenary speakers, 54 were hospitalists and 5 (9%) of these were women. Of the 49 who were general internists, 22 (45%) were women (P<0.0001).
Authorship
The PubMed search identified a total of 3285 articles published in the JHM and the JGIM from 2006 to 2012, and 2172 first authors and 1869 last authors met inclusion criteria (see Supporting Figure 3 in the online version of this article). Hospitalists were listed as first or last authors on 464 and 305 articles, respectively, and of these, women were first authors on 153 (33%) and last authors on 63 (21%). General internists were listed as first or last authors on 895 and 769 articles, respectively, with women as first authors on 423 (47%) and last authors on 265 (34%). Compared with general internists, fewer women hospitalists were listed as either first or last authors (both P<0.0001) (Table 2).
| First Author | Last Author | |||
|---|---|---|---|---|
| Male, N (%) | Female, N (%) | Male, N (%) | Female, N (%) | |
| ||||
| Hospitalists | ||||
| All publications | 311 (67) | 153 (33)* | 242 (79) | 63 (21)* |
| Original investigations/brief reports | 124 (61) | 79 (39)* | 96 (76) | 30 (24)* |
| Editorials | 34 (77) | 10 (23)* | 18 (86) | 3 (14)* |
| Other | 153 (71) | 64 (29)* | 128 (81) | 30 (19)* |
| General internists | ||||
| All publications | 472 (53) | 423 (47) | 504 (66) | 265 (34)* |
| Original investigations/brief reports | 218 (46) | 261 (54) | 310 (65) | 170 (35)* |
| Editorial | 98 (68) | 46 (32)* | 43 (73) | 16 (27)* |
| Other | 156 (57) | 116 (43) | 151 (66) | 79 (34)* |
Fewer women hospitalists were listed as first or last authors on all article types. For original research articles written by general internists, there was a trend for more women to be listed as first authors than men (261/479, 54%), but this difference was not statistically significant.
DISCUSSION
The important findings of this study are that, despite an equal gender distribution of academic HM and GIM faculty, fewer women were HM division/section chiefs, fewer women were speakers at the 2 selected national meetings, and fewer women were first or last authors of publications in 2 selected journals in comparison with general internists.
Previous studies have found that women lag behind their male counterparts with respect to academic productivity, leadership, and promotion.[1, 5, 7] Some studies suggest, however, that gender differences are reduced when younger cohorts are examined.[1, 10, 11, 12, 13] Surveys indicate that that the mean age of hospitalists is younger than most other specialties.[15, 19, 20, 24] The mean age of academic GIM physicians is unknown, but surveys of GIM (not differentiating academic from nonacademic) suggest that it is an older cohort than that of HM.[24] Despite hospitalists being a younger cohort, we found gender disparities in all areas investigated.
Our findings with respect to gender disparities in HM division or section leadership are consistent with the annual AAMC Women in US Academic Medicine and Science Benchmarking Report that found only 22% of all permanent division or section heads were women.[1]
Gender disparities with respect to authorship of medical publications have been previously noted,[3, 6, 15, 25] but to our knowledge, this is the first study to investigate the gender of authors who were hospitalists. Although we found a higher proportion of women hospitalists who were first or last authors than was observed by Jagsi and colleagues,[3] women hospitalists were still under‐represented with respect to this measure of academic productivity. Erren et al. reviewed 6 major journals from 2010 and 2011, and found that first authorship of original research by women ranged from 23.7% to 46.7%, and for last authorship from 18.3% to 28.8%.[25] Interestingly, we found no significant gender difference for first authors who were general internists, and there was a trend toward more women general internists being first authors than men for original research, reviews, and brief reports (data not shown).
Our study did not attempt to answer the question of why gender disparities persist, but many previous studies have explored this issue.[4, 8, 12, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42] Issues raised by others include the quantity of academic work (ie, publications and grants obtained), differences in hours worked and allocation of time, lack of mentorship, family responsibilities, discrimination, differences in career motivation, and levels of institutional support, to name a few.
The under‐representation of women hospitalists in leadership, authorship, and speaking opportunities may be consistent with gender‐related differences in research productivity. Fewer publications could lead to fewer national presentations, which could lead to fewer leadership opportunities. Our findings with respect to general internists are not consistent with this idea, however, as whereas women were under‐represented in GIM leadership positions, we found no disparities with respect to the gender of first authors or speakers at national meetings for general internists. The finding that hospitalists had gender disparities with respect to first authors and national speakers but general internists did not, argues against several hypotheses (ie, that women lack mentorship, have less career motivation, fewer career building opportunities).
One notable hypothesis, and perhaps one that is often discussed in the literature, is that women shoulder the majority of family responsibilities, and this may result in women having less time for their careers. Jolly and colleagues studied physician‐researchers and noted that women were more likely than men to have spouses or domestic partners who were fully employed, spent 8.5 more hours per week on domestic activities, and were more likely to take time off during disruptions of usual child care.[33] Carr and colleagues found that women with children (compared to men with children) had fewer publications, slower self‐perceived career progress, and lower career satisfaction, but having children had little effect on faculty aspirations and goals.[2] Kaplan et al., however, found that family responsibilities do not appear to account for sex differences in academic advancement.[4] Interestingly, in a study comparing physicians from Generation X to those of the Baby Boomer age, Generation X women reported working more than their male Generation X counterparts, and both had more of a focus on worklife balance than the older generation.[12]
The nature the of 2 specialties' work environment and job requirements could have also resulted in some of the differences seen. Primary care clinical work is typically conducted Monday through Friday, and hospitalist work frequently includes some weekend, evening, night, and holiday coverage. Although these are known differences, both specialties have also been noted to offer many advantages to women and men alike, including collaborative working environments and flexible work hours.[16]
Finally, finding disparity in leadership positions in both specialties supports the possibility that those responsible for hiring could have implicit gender biases. Under‐representation in entry‐level positions is also not a likely explanation for the differences we observed, because nearly an equal number of men and women graduate from medical school, pursue residency training in internal medicine, and become either academic hospitalists or general internists at university settings.[1, 15, 24] This hypothesis could, however, explain why disparities exist with respect to senior authorship and leadership positions, as typically, these individuals have been in practice longer and the current trends of improved gender equality have not always been the case.
Our study has a number of limitations. First, we only examined publications in 2 journals and presentations at 2 national conferences, although the journals and conferences selected are considered to be the major ones in the 2 specialties. Second, using Internet searches may have resulted in inaccurate gender and specialty assignment, but previous studies have used similar methodology.[3, 43] Additionally, we also attempted to contact individuals for direct confirmation when the information we obtained was not clear and had a second investigator independently verify the gender and specialty data. Third, we utilized division/department websites when available to determine the gender of HM divisions/sections. If not recently updated, these websites may not have reflected the most current leader of the unit, but this concern would seemingly pertain to both hospitalists and general internists. Fourth, we opted to only study faculty and division/section heads at university hospitals, as typically these institutions had GIM and hospitalist groups and also typically had websites. Because we only studied faculty and leadership at university hospitals, our data are not generalizable to all hospitalist and GIM groups. Finally, we excluded pediatric hospitalists, and thus, this study is representative of adult hospitalists only. Including pediatric hospitalists was out of the scope of this project.
Our study also had a number of strengths. To our knowledge, this is the first study to provide an estimate of the gender distribution in academic HM, of hospitalists as speakers at national meetings, as first and last authors, and of HM division or section heads, and is the first to compare these results with those observed for general internists. In addition, we examined 7 years of data from 2 of the major journals and national conferences for these specialties.
In summary, despite HM being a newer field with a younger cohort of physicians, we found that gender disparities exist for women with respect to authorship, national speaking opportunities, and division or section leadership. Identifying why these gender differences exist presents an important next step.
Disclosures: Nothing to report. Marisha Burden, MD and Maria G. Frank, MD are coprincipal authors.
- Association of American Medical Colleges. Women in U.S. academic medicine and science: Statistics and benchmarking report. 2012. Available at: https://members.aamc.org/eweb/upload/Women%20in%20U%20S%20%20Academic%20Medicine%20Statistics%20and%20Benchmarking%20Report%202011-20123.pdf. Accessed September 1, 2014.
- , , , et al. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Intern Med. 1998;129:532–538.
- , , , et al. The “gender gap” in authorship of academic medical literature—a 35‐year perspective. N Engl J Med. 2006;355:281–287.
- , , , , , . Sex differences in academic advancement. Results of a national study of pediatricians. N Engl J Med. 1996;335:1282–1289.
- . Women physicians in academic medicine: new insights from cohort studies. N Engl J Med. 2000;342:399–405.
- , , , , . Gender differences in academic productivity and leadership appointments of physicians throughout academic careers. Acad Med. 2011;86:43–47.
- , , , . Promotion of women physicians in academic medicine. Glass ceiling or sticky floor? JAMA. 1995;273:1022–1025.
- , , , . Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141:205–212.
- , , . Women physicians: choosing a career in academic medicine. Acad Med. 2012;87:105–114.
- , , , . The status of women at one academic medical center. Breaking through the glass ceiling. JAMA. 1990;264:1813–1817.
- , . Status of women in academic anesthesiology. Anesthesiology. 1986;64:496–500.
- , , . The generation and gender shifts in medicine: an exploratory survey of internal medicine physicians. BMC Health Serv Res. 2006;6:55.
- Pew Research Center. On pay gap, millenial women near parity—for now. December 2013. Available at: http://www.pewsocialtrends.org/files/2013/12/gender-and-work_final.pdf. Published December 11, 2013. Accessed February 5, 2015.
- , . The emerging role of "hospitalists" in the American health care system. N Engl J Med. 1996;335:514–517.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27:23–27.
- . The gender factor. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/the‐gender‐factor. Published March 1, 2006. Accessed September 1, 2014.
- Association of American Medical Colleges. Analysis in brief: Supplemental information for estimating the number and characteristics of hospitalist physicians in the United States and their possible workforce implications. Available at: https://www.aamc.org/download/300686/data/aibvol12_no3-supplemental.pdf. Published August 2012. Accessed September 1, 2014.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5–9.
- State of Hospital Medicine: 2011 Report Based on 2010 Data. Medical Group Management Association and Society of Hospital Medicine. www.mgma.com, www.hospitalmedicine.org.
- Today's Hospitalist Survey. Compensation and Career Survey Results. 2013. Available at: http://www.todayshospitalist.com/index.php?b=salary_survey_results. Accessed January 11, 2015.
- Association of American Medical Colleges. Women in U.S. Academic Medicine: Statistics and Benchmarking Report. 2009–2010. Available at: https://www.aamc.org/download/182674/data/gwims_stats_2009‐2010.pdf. Accessed September 1, 2014.
- American Medical Association. Graduate Medical Education Directory 2012–2013. Chicago, IL: American Medical Association; 2012:182–203.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- Association of American Medical Colleges. 2012 Physician Specialty Data Book. Center for Workforce Studies. Available at: https://www.aamc.org/download/313228/data/2012physicianspecialtydatabook.pdf. Published November 2012. Accessed September 1, 2014.
- , , , . Representation of women as authors, reviewers, editors in chief, and editorial board members at 6 general medical journals in 2010 and 2011. JAMA Intern Med. 2014;174:633–635.
- , , , et al. Relationships of gender and career motivation to medical faculty members' production of academic publications. Acad Med. 1998;73:180–186.
- , , , et al. Faculty perceptions of gender discrimination and sexual harassment in academic medicine. Ann Intern Med. 2000;132:889–896.
- , , , . Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey. Arch Intern Med. 2000;160:2625–2629.
- , , , , . A "ton of feathers": gender discrimination in academic medical careers and how to manage it. J Womens Health (Larchmt). 2003;12:1009–1018.
- , , . Perceived obstacles to career success for women in academic surgery. Arch Surg. 2000;135:972–977.
- , , , . Career satisfaction of US women physicians: results from the Women Physicians' Health Study. Society of General Internal Medicine Career Satisfaction Study Group. Arch Intern Med. 1999;159:1417–1426.
- . Doing the same and earning less: male and female physicians in a new medical specialty. Inquiry. 2004;41:301–315.
- , , , , , . Gender differences in time spent on parenting and domestic responsibilities by high‐achieving young physician‐researchers. Ann Intern Med. 2014;160:344–353.
- , , , , . Stories from early‐career women physicians who have left academic medicine: a qualitative study at a single institution. Acad Med. 2011;86:752–758.
- , , , . The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193–201.
- , , , , . Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28:201–207.
- . Gender pay gaps in hospital medicine. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/gender‐pay‐gaps‐in‐hospital‐medicine. Published February 29, 2012. Accessed September 1, 2014.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103–1115.
- . Inequality quantified: mind the gender gap. Nature. 2013;495:22–24.
- , , , et al. Gender differences in academic advancement: patterns, causes, and potential solutions in one US College of Medicine. Acad Med. 2003;78:500–508.
- , . Why aren't there more women leaders in academic medicine? The views of clinical department chairs. Acad Med. 2001;76:453–465.
- . Gender factors in reviewer recommendations for manuscript publication. J Appl Behav Anal. 1990;23:539–543.
- , , , . Scientific impact of women in academic surgery. J Surg Res. 2008;148:13–16.
- Association of American Medical Colleges. Women in U.S. academic medicine and science: Statistics and benchmarking report. 2012. Available at: https://members.aamc.org/eweb/upload/Women%20in%20U%20S%20%20Academic%20Medicine%20Statistics%20and%20Benchmarking%20Report%202011-20123.pdf. Accessed September 1, 2014.
- , , , et al. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Intern Med. 1998;129:532–538.
- , , , et al. The “gender gap” in authorship of academic medical literature—a 35‐year perspective. N Engl J Med. 2006;355:281–287.
- , , , , , . Sex differences in academic advancement. Results of a national study of pediatricians. N Engl J Med. 1996;335:1282–1289.
- . Women physicians in academic medicine: new insights from cohort studies. N Engl J Med. 2000;342:399–405.
- , , , , . Gender differences in academic productivity and leadership appointments of physicians throughout academic careers. Acad Med. 2011;86:43–47.
- , , , . Promotion of women physicians in academic medicine. Glass ceiling or sticky floor? JAMA. 1995;273:1022–1025.
- , , , . Compensation and advancement of women in academic medicine: is there equity? Ann Intern Med. 2004;141:205–212.
- , , . Women physicians: choosing a career in academic medicine. Acad Med. 2012;87:105–114.
- , , , . The status of women at one academic medical center. Breaking through the glass ceiling. JAMA. 1990;264:1813–1817.
- , . Status of women in academic anesthesiology. Anesthesiology. 1986;64:496–500.
- , , . The generation and gender shifts in medicine: an exploratory survey of internal medicine physicians. BMC Health Serv Res. 2006;6:55.
- Pew Research Center. On pay gap, millenial women near parity—for now. December 2013. Available at: http://www.pewsocialtrends.org/files/2013/12/gender-and-work_final.pdf. Published December 11, 2013. Accessed February 5, 2015.
- , . The emerging role of "hospitalists" in the American health care system. N Engl J Med. 1996;335:514–517.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27:23–27.
- . The gender factor. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/the‐gender‐factor. Published March 1, 2006. Accessed September 1, 2014.
- Association of American Medical Colleges. Analysis in brief: Supplemental information for estimating the number and characteristics of hospitalist physicians in the United States and their possible workforce implications. Available at: https://www.aamc.org/download/300686/data/aibvol12_no3-supplemental.pdf. Published August 2012. Accessed September 1, 2014.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5–9.
- State of Hospital Medicine: 2011 Report Based on 2010 Data. Medical Group Management Association and Society of Hospital Medicine. www.mgma.com, www.hospitalmedicine.org.
- Today's Hospitalist Survey. Compensation and Career Survey Results. 2013. Available at: http://www.todayshospitalist.com/index.php?b=salary_survey_results. Accessed January 11, 2015.
- Association of American Medical Colleges. Women in U.S. Academic Medicine: Statistics and Benchmarking Report. 2009–2010. Available at: https://www.aamc.org/download/182674/data/gwims_stats_2009‐2010.pdf. Accessed September 1, 2014.
- American Medical Association. Graduate Medical Education Directory 2012–2013. Chicago, IL: American Medical Association; 2012:182–203.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- Association of American Medical Colleges. 2012 Physician Specialty Data Book. Center for Workforce Studies. Available at: https://www.aamc.org/download/313228/data/2012physicianspecialtydatabook.pdf. Published November 2012. Accessed September 1, 2014.
- , , , . Representation of women as authors, reviewers, editors in chief, and editorial board members at 6 general medical journals in 2010 and 2011. JAMA Intern Med. 2014;174:633–635.
- , , , et al. Relationships of gender and career motivation to medical faculty members' production of academic publications. Acad Med. 1998;73:180–186.
- , , , et al. Faculty perceptions of gender discrimination and sexual harassment in academic medicine. Ann Intern Med. 2000;132:889–896.
- , , , . Attitudes of clinical faculty about career progress, career success and recognition, and commitment to academic medicine. Results of a survey. Arch Intern Med. 2000;160:2625–2629.
- , , , , . A "ton of feathers": gender discrimination in academic medical careers and how to manage it. J Womens Health (Larchmt). 2003;12:1009–1018.
- , , . Perceived obstacles to career success for women in academic surgery. Arch Surg. 2000;135:972–977.
- , , , . Career satisfaction of US women physicians: results from the Women Physicians' Health Study. Society of General Internal Medicine Career Satisfaction Study Group. Arch Intern Med. 1999;159:1417–1426.
- . Doing the same and earning less: male and female physicians in a new medical specialty. Inquiry. 2004;41:301–315.
- , , , , , . Gender differences in time spent on parenting and domestic responsibilities by high‐achieving young physician‐researchers. Ann Intern Med. 2014;160:344–353.
- , , , , . Stories from early‐career women physicians who have left academic medicine: a qualitative study at a single institution. Acad Med. 2011;86:752–758.
- , , , . The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193–201.
- , , , , . Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28:201–207.
- . Gender pay gaps in hospital medicine. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/gender‐pay‐gaps‐in‐hospital‐medicine. Published February 29, 2012. Accessed September 1, 2014.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103–1115.
- . Inequality quantified: mind the gender gap. Nature. 2013;495:22–24.
- , , , et al. Gender differences in academic advancement: patterns, causes, and potential solutions in one US College of Medicine. Acad Med. 2003;78:500–508.
- , . Why aren't there more women leaders in academic medicine? The views of clinical department chairs. Acad Med. 2001;76:453–465.
- . Gender factors in reviewer recommendations for manuscript publication. J Appl Behav Anal. 1990;23:539–543.
- , , , . Scientific impact of women in academic surgery. J Surg Res. 2008;148:13–16.
© 2015 Society of Hospital Medicine
In‐hospital SIF
People who use illicit drugs (PWUDs) experience a wide range of health‐related harms and consequently often rely on acute and emergency services for care.[1, 2] Specifically, the poor health status of many PWUDs is often attributable to infectious diseases such as human immunodeficiency virus (HIV) and hepatitis C virus.[3, 4] Soft‐tissue infections associated with injection drug use are also common, and have increasingly accounted for the majority of hospitalizations among this population.[5] Many of these adverse health outcomes may require lengthy in‐patient hospital admissions and constitute a substantial financial burden for the healthcare system.[6]
PWUDs frequently experience barriers to conventional healthcare services. For example, negative experiences with healthcare providers and the healthcare system have often deterred PWUDs from accessing these services.[7, 8] Given that most hospitals operate under an abstinence‐based policy, PWUDs have minimal access to drug‐using paraphernalia while hospitalized, making it difficult for these individuals to safely manage their active drug use. As a result, many PWUDs may resort to high‐risk drug‐using practices (eg, syringe sharing, injecting alone) in the hospital that may lead to further adverse health outcomes, such as infectious disease transmission and overdose, respectively.[9] Past studies have also shown that many PWUDs do not complete hospital‐based treatments.[10, 11] Specifically, many PWUDs leave the hospital against medical advice (AMA) possibly because they are unable to continue their drug use practices in this setting,[10, 12] thus contributing to an increase in readmission rates and mortality among this population.[13] Past studies have indicated that approximately 30% of patients who inject drugs left the hospital AMA,[12, 14] and these individuals have shown to be as high as 4 times more likely to leave the hospital AMA compared to their nondrug‐using counterparts.[11]
Supervised injection facilities (SIFs) are sanctioned environments where PWUDs can inject preobtained illicit drugs under the supervision of healthcare staff. Internationally, SIFs have been shown to improve public health and public order within surrounding communities.[15, 16, 17] For example, a dramatic decline in fatal overdoses in Vancouver's Downtown Eastside neighborhood was attributed to the implementation of a SIF in the area.[15] Changes in risk‐injecting behavior have also been observed among individuals who access SIFs.[18] Although a large body of evidence supports SIFs as an effective approach for minimizing the drug‐ and health‐related harms within street‐based drug scenes,[17, 19] little is known about whether there is a role for SIFs within hospital settings. Currently in Vancouver, Canada, harm reduction services are generally not being provided within hospital settings. Therefore, we sought to conduct a needs assessment to identify the prevalence and correlates of willingness to access an in‐hospital SIF among PWUDs in Vancouver. These data may be crucial for planning appropriate programs and services to reduce health‐related harms and leaving the hospital AMA among PWUDs in hospital settings.
METHODS
The Vancouver Injection Drug Users Study (VIDUS) and the AIDS Care Cohort to Evaluate Exposure to Survival Services (ACCESS) are 2 prospective cohort studies of PWUDs who have been recruited through self‐referral and street outreach since May 1996. These cohorts have been described in detail previously.[20, 21] In brief, persons were eligible to enter the VIDUS study if they had injected illicit drugs at least once in the previous month and resided in the Greater Vancouver region at enrollment. Persons were eligible to enter the ACCESS study if they were HIV infected and used illicit drugs other than cannabinoids in the previous month. Individuals who seroconvert following recruitment are transferred from the VIDUS study into the ACCESS study. All eligible participants provided written informed consent. At baseline and semiannually, study participants complete a harmonized interviewer‐administered questionnaire (ie, participants in the VIDUS and ACCESS studies completed an identical questionnaire) and provide blood samples for HIV and hepatitis C virus testing, and HIV disease monitoring. At the conclusion of each visit, study participants receive Can$20 for their time. The study has received ethical approval from Providence Health Care/University of British Columbia's research ethics board.
The primary outcome of interest for this analysis was willingness to access an in‐hospital SIF (yes vs no or unsure), ascertained by asking participants the following hypothetical question: If you were admitted into a hospital, and if a supervised safe injection site was available in that hospital, would you use it? Given the existence of 2 SIFs in the local environment, PWUDs in Vancouver are familiar with the design and operation of such programs. We compared PWUDs who were and were not willing to access an in‐hospital SIF using bivariable and multivariable logistic regression analyses. Given that the variable measure was based on a hypothetical scenario, participants who completed the survey between June 2013 and November 2013 were eligible for inclusion regardless of their current injection drug use behavior. A complete case approach was used to analyze the data given that the extent of missingness was not significant (<5%). Variables considered included: age (per year increase); gender (male vs female); HIV serostatus (positive vs negative); heroin injection ( daily vs
To identify factors independently associated with willingness to access an in‐hospital SIF, a multivariable logistic regression model was constructed using an a prioridefined statistical protocol based on examination of the Akaike information criterion (AIC) and P values. First, we constructed a full model that included all variables significant at P<0.10 in bivariable analyses. After noting the AIC of the model, we removed the variable with the largest P value and built a reduced model. We continued this iterative process until no variables remained. We selected the multivariable model with the lowest AIC score. All P values were 2 sided. As a subanalysis, we asked participants who would be willing to access an in‐hospital SIF to indicate reasons why they would be willing to access such a facility.
RESULTS
Of the total 769 participants who were eligible for inclusion in the study, 732 PWUDs provided complete data and participated in the study; 37 (4.8%) were excluded due to missing data. In our study sample, 250 (34.2%) were female, the median age was 48 years (interquartile range: 4153 years), and 307 (41.5%) were HIV‐positive. Among our study sample, 499 (68.2%) participants would be willing to access an in‐hospital SIF if it were available. Bivariable analyses of factors associated with willingness to access an in‐hospital SIF are presented in Table 1.
| Characteristic | Willingness to Access an In‐hospital SIF | Odds Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Yes, n (%), n=499 | No, n (%), n=233 | ||||
| |||||
| Age | |||||
| Median | 48 | 48 | 0.98 (0.97‐1.00) | 0.085 | |
| IQR | (4153) | (4254) | |||
| Gender | |||||
| Male | 331 (66.3) | 151 (64.8) | 1.07 (0.77‐1.48) | 0.685 | |
| Female | 168 (33.7) | 82 (35.2) | |||
| HIV serostatus | |||||
| Positive | 203 (40.7) | 104 (44.6) | 0.85 (0.62‐1.16) | 0.313 | |
| Negative | 296 (59.3) | 129 (55.4) | |||
| Heroin injection* | |||||
| Daily | 106 (21.2) | 26 (11.2) | 2.15 (1.35‐3.40) | <0.001 | |
| < Daily | 393 (78.8) | 207 (88.8) | |||
| Cocaine injection* | |||||
| Daily | 46 (9.2) | 19 (8.2) | 1.14 (0.65‐2.00) | 0.637 | |
| < Daily | 453 (90.8) | 214 (91.8) | |||
| Crystal methamphetamine injection* | |||||
| Daily | 46 (9.2) | 16 (6.9) | 1.38 (0.76‐2.49) | 0.287 | |
| < Daily | 453 (90.8) | 217 (93.1) | |||
| Prescription opioid injection* | |||||
| Daily | 34 (6.8) | 9 (3.9) | 1.82 (0.86‐3.86) | 0.114 | |
| < Daily | 465 (93.2) | 224 (96.1) | |||
| Binge drug use* | |||||
| Yes | 141 (28.3) | 61 (26.2) | 1.11 (0.78‐1.58) | 0.558 | |
| No | 358 (71.7) | 172 (73.8) | |||
| Ever left hospital AMA | |||||
| Yes | 21 (4.2) | 2 (0.9) | 5.07 (1.1821.83) | 0.012 | |
| No | 478 (95.8) | 231 (99.1) | |||
| Ever used illicit drugs in hospital | |||||
| Yes | 238 (47.7) | 83 (35.6) | 1.65 (1.20‐2.27) | 0.002 | |
| No | 261 (52.3) | 150 (64.4) | |||
| Ever had negative experiences with healthcare providers | |||||
| Yes | 131 (26.3) | 64 (27.5) | 0.94 (0.66‐1.33) | 0.729 | |
| No | 368 (73.7) | 169 (72.5) | |||
| Ever had negative experiences with police | |||||
| Yes | 383 (76.8) | 169 (72.5) | 1.25 (0.88‐1.78) | 0.217 | |
| No | 116 (23.2) | 64 (27.5) | |||
| Used an SIF* | |||||
| Yes | 228 (45.7) | 77 (33.0) | 1.70 (1.23‐2.36) | 0.001 | |
| No | 271 (54.3) | 156 (67.0) | |||
As indicated in Table 2, in multivariable analyses, factors that remained significantly and positively associated with willingness to access an in‐hospital SIF included: daily heroin injection (adjusted odds ratio [AOR]=1.90; 95% confidence interval [CI]: 1.20‐3.11), ever used illicit drugs in the hospital (AOR=1.63; 95% CI: 1.18‐2.26), and previously used an SIF (AOR=1.53; 95% CI: 1.10‐2.15).
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Heroin injection* ( daily vs | 1.90 | 1.20‐3.11 | 0.008 |
| Ever left hospital AMA (yes vs no) | 3.74 | 1.0623.72 | 0.079 |
| Ever used illicit drugs in hospital (yes vs no) | 1.63 | 1.18‐2.26 | 0.003 |
| Used an SIF* (yes vs no) | 1.53 | 1.10‐2.15 | 0.013 |
Among participants who would be willing to access an in‐hospital SIF, the most common reasons included: to be able to stay in the hospital (229/499=45.9%), to reduce their drug‐related risks (189/499=37.9%), and to reduce stress associated with being kicked out of the hospital because they were using drugs (97/499=19.4%).
DISCUSSION
We found that over two‐thirds of PWUDs participating in our study would be willing to access an in‐hospital SIF if such a service was available. This finding is encouraging given that a large proportion of PWUDs are hospitalized annually for acute and chronic diseases.[5, 6] Previous studies have documented the positive impact of incorporating a harm reduction model within hospital settings, resulting in more comprehensive care for PWUDs.[22, 23] For example, the Dr. Peter Centre Day Health Program provides a SIF for HIV‐positive PWUDs to safely use illicit drugs under the supervision of trained nurses and was once located at St. Paul's Hospital.[24] Although the Dr. Peter Centre currently operates outside of St. Paul's Hospital, it may be advantageous to model an in‐hospital SIF after the Dr. Peter Centre's harm‐reduction room given their success in facilitating access and delivery of comprehensive care for PWUDs.[23] Specifically, nurses at the Dr. Peter Centre directly observe injections of preobtained illicit drugs for the purposes of preventing illness and promoting health. Our findings support recent calls to implement harm‐reduction services within hospital settings in an effort to minimize the harms associated with illicit drug use.[25, 26]
Previous studies have identified various high‐risk locations where PWUDs use illicit drugs to maintain their established drug‐use habits, including in locked washrooms in hospitals.[9] We found a positive association among PWUDs who had used illicit drugs in the hospital and a willingness to use an in‐hospital SIF. Our finding is reassuring given that studies have shown that these individuals are at a higher risk of negative health consequences (eg, fatal overdose) from using drugs in the hospital.[9] Harm reduction services within the hospital settings can play an important role in reducing this drug‐ and health‐related harm among PWUDs.
Our study also found that high‐frequency heroin injection was associated with a willingness to access an in‐hospital SIF. This relationship may be a result of the complex nature of treating opioid‐dependent patients for pain. For instance, some opioid‐dependent PWUDs may have already established a high tolerance for opioids due to the concomitant use of opioid substitution therapies and ongoing drug use, making it difficult to appropriately prescribe pain medication to these individuals.[27] High‐frequency heroin users may also face severe withdrawal given the unavailability of illicit opioids in hospital settings, resulting in their increased willingness to access an in‐hospital SIF. Furthermore, inadequate pain management may contribute to the continued need to use opioids, as some healthcare providers may be reluctant to provide pain medication out of fear that they would contribute to an existing addiction or relapse.[28, 29] Further, requests for pain mediation may be misinterpreted as drug‐seeking behavior.[30, 31] Given the complexities arising from high‐intensity heroin use, pain management, and healthcare professionals' perceptions regarding PWUDs, further research should seek to untangle the causal relationships underlying these associations.
We found an association between recent use of an SIF and willingness to access an in‐hospital SIF. As mentioned previously, earlier research has shown improvements in various health outcomes and reductions in related harm in surrounding communities where SIFs were implemented.[15, 17] It is unfortunate that although progress in reducing the harm of injection drug use has been seen in community settings globally, the same cannot be said about hospitals. Given that many PWUDs often present to emergency care late in the course of illness and require admission to a hospital bed,[2] it is important to ensure that harm reduction services that are available in the community are also made available in hospitals. However, given the lack of knowledge on in‐hospital SIFs, future research should seek to understand the benefits and consequences of implementing such a facility in a hospital from different perspectives. For example, it may be of interest to assess the attitudes and perceptions of healthcare providers toward an in‐hospital SIF.
A large body of evidence has documented the health harms associated with leaving the hospital AMA, including readmission for a worsened illness and mortality.[13, 32] However, when faced with the abstinence‐based policies that exist in most hospital settings, it is not uncommon for PWUDs to leave the hospital to maintain their active addiction or to address their drug withdrawal.[9] Although we failed to find a statistically significant association between being discharged AMA and willingness to access an in‐hospital SIF, it is noteworthy that in our subanalysis we found that PWUD who were more likely to access an in‐hospital SIF reported doing so because they wanted to stay in the hospital and reduce their drug‐related risks. Given that we observed low counts of reported AMA discharge events, further exploration of this topic is warranted.
Our study suggests that in‐hospital SIFs have the potential to minimize health harms among patients who use illicit drugs in hospitals; however, there are some legal issues that warrant consideration. Specifically, for the successful operation of SIFs, there is a need for changes to regulatory frameworks, including drug laws, to allow for the possession of illicit drugs by individuals accessing an SIF. Such frameworks have been developed in a range of settings and in a manner that is consistent with international drug control treaties. In hospitals, additional regulatory changes may be needed to address issues unique to these settings, such as the use of opioids among PWUDs being treated for pain.
There are several limitations to this study. First, the cross‐sectional design of the study limited our ability to determine a temporal or causal relationship between the explanatory and outcome variables. Second, it is noteworthy that the chosen mode of interviewer‐based questionnaire administration may have influenced our results by relying on self‐reported data that are susceptible to reporting biases, including socially desirable reporting and recall bias. However, we believe we have minimized response bias and maximized reliability in our data by placing sensitive questions toward the end of the interview to allow rapport to be established between the interviewer and participant. Last, given that the participants in the present study were not randomly selected, the interpretation of these results may not be representative or generalizable to other PWUD populations outside of Vancouver. However, it is noteworthy that over the past few decades, community‐based SIFs have been successfully operating in international settings such as Europe and Australia[33, 34]; thus, the concept of an in‐hospital SIF may not be far from actual inpatient practice in these settings. It is also important to acknowledge the progress made toward the implementation of community‐based SIFs in other settings, including the United States. For example, feasibility studies have been conducted in San Francisco and New York and have shown increasing support for the implementation of SIFs in these areas.[35, 36]
We found that a substantial proportion of PWUDs in our sample would be willing to access an in‐hospital SIF if this service was available. Those PWUDs who expressed a willingness to use an in‐hospital SIF were more likely to be high‐intensity heroin users, to have previously used illicit drugs in the hospital, and were more likely to have previously used an SIF. Our findings highlight the potential of in‐hospital SIFs to complement existing harm‐reduction programs that serve people who inject drugs.
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
Disclosures: The VIDUS study was supported by the US National Institutes of Health (R01DA011591). The ACCESS study was supported by the US National Institutes of Health (R01DA021525). The evaluation of the supervised injecting facility was supported by Vancouver Coastal Health and Canadian Institutes of Health Research (MOP‐111039). L.T. is supported by a Canadian Institutes of Health Research Doctoral Research Award. This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine, which supports E.W. The authors report no conflicts of interest.
- , , , et al. Emergency department utilization among a cohort of HIV‐positive injecting drug users in a Canadian setting. J Emerg Med. 2012;43(2):236–243.
- , , , et al. High rates of primary care and emergency department use among injection drug users in Vancouver. J Public Health (Oxf). 2005;27(1):62–66.
- , . Epidemiology of HIV among injecting and non‐injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep. 2010;7(2):99–106.
- , , , et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–284.
- , , , et al. Determinants of hospitalization for a cutaneous injection‐related infection among injection drug users: a cohort study. BMC Public Health. 2010;10:327.
- , , , et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165(4):415–420.
- , , , , . The association of stigma with self‐reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–1108.
- , , , . Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1–2):23–35.
- , , , . Hospitals as a “risk environment”: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105C:59–66.
- . “I'm going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255–260.
- , , , , , . Leaving hospital against medical advice among HIV‐positive patients. CMAJ. 2002;167(6):633–637.
- , , , et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56–59.
- , , . Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594–602.
- , . Welfare checks, drug consumption, and health: evidence from Vancouver injection drug users. J Hum Resour. 2006;41(1):138–161.
- , , , , . Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: a retrospective population‐based study. Lancet. 2011;377(9775):1429–1437.
- , , , , . The impact of a supervised injecting facility on ambulance call‐outs in Sydney, Australia. Addiction. 2010;105(4):676–683.
- , , , et al. Changes in public order after the opening of a medically supervised safer injecting facility for illicit injection drug users. CMAJ. 2004;171(7):731–734.
- , , , et al. Changes in injecting practices associated with the use of a medically supervised safer injection facility. J Public Health. 2007;29(1):35–39.
- , , , , , . Police and public health partnerships: Evidence from the evaluation of Vancouver's supervised injection facility. Subst Abuse Treat Prev Policy. 2008;3:11.
- , , , et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549.
- , , , et al. Unsafe injection practices in a cohort of injection drug users in Vancouver: could safer injecting rooms help? CMAJ. 2001;165(4):405–410.
- , , , . Expanding harm reduction services through a wound and abscess clinic. Am J Public Health. 2002;92(12):1915–1917.
- , , , . An integrated supervised injecting program within a care facility for HIV‐positive individuals: a qualitative evaluation. AIDS Care. 2009;21(5):638–644.
- Dr. Peter AIDS Foundation. Available at: http://www.drpeter.org. Accessed June 19, 2014.
- , . Drug use in hospitals: Is there a role for harm reduction? Hospital News. Available at: http://hospitalnews.com/drug‐use‐in‐hospitals‐is‐there‐a‐role‐for‐harm‐reduction. Published October 17, 2013. Accessed March 11, 2014.
- , , , . Harm reduction in hospitals: is it time? Harm Reduct J. 2009;6(1):19.
- , , . Pain intolerance in opioid‐maintained former opiate addicts: effect of long‐acting maintenance agent. Drug Alcohol Depend. 2001;63(2):139–146.
- , , , . Guilty until proven innocent: a qualitative study of the management of chronic non‐cancer pain among patients with a history of substance abuse. Addict Behav. 2010;35(3):270–272.
- , , , . Providers' experiences treating chronic pain among opioid‐dependent drug users. J Gen Intern Med. 2009;24(4):482–488.
- , , , . Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284–1293.
- , , , et al. Routines and rituals: a grounded theory of the pain management of drug users in acute care settings. J Clin Nurs. 2010;19(19–20):2730–2740.
- , , . Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926–929.
- , , , , . Supervised injection services: What has been demonstrated? A systematic literature review. Drug Alcohol Depend. 2014;145:48–68.
- , , , , , . Drug consumption facilities in Europe and the establishment of supervised injecting centres in Australia. Drug Alcohol Rev. 2000;19(3):337–346.
- , , , , , . Acceptability of a safer injection facility among injection drug users in San Francisco. Drug Alcohol Depend. 2010;110(1–2):160–163.
- , , , , , . Safer injection sites in New York City: a utilization survey of injection drug users. J Drug Issues. 2003;33(3):733–750.
People who use illicit drugs (PWUDs) experience a wide range of health‐related harms and consequently often rely on acute and emergency services for care.[1, 2] Specifically, the poor health status of many PWUDs is often attributable to infectious diseases such as human immunodeficiency virus (HIV) and hepatitis C virus.[3, 4] Soft‐tissue infections associated with injection drug use are also common, and have increasingly accounted for the majority of hospitalizations among this population.[5] Many of these adverse health outcomes may require lengthy in‐patient hospital admissions and constitute a substantial financial burden for the healthcare system.[6]
PWUDs frequently experience barriers to conventional healthcare services. For example, negative experiences with healthcare providers and the healthcare system have often deterred PWUDs from accessing these services.[7, 8] Given that most hospitals operate under an abstinence‐based policy, PWUDs have minimal access to drug‐using paraphernalia while hospitalized, making it difficult for these individuals to safely manage their active drug use. As a result, many PWUDs may resort to high‐risk drug‐using practices (eg, syringe sharing, injecting alone) in the hospital that may lead to further adverse health outcomes, such as infectious disease transmission and overdose, respectively.[9] Past studies have also shown that many PWUDs do not complete hospital‐based treatments.[10, 11] Specifically, many PWUDs leave the hospital against medical advice (AMA) possibly because they are unable to continue their drug use practices in this setting,[10, 12] thus contributing to an increase in readmission rates and mortality among this population.[13] Past studies have indicated that approximately 30% of patients who inject drugs left the hospital AMA,[12, 14] and these individuals have shown to be as high as 4 times more likely to leave the hospital AMA compared to their nondrug‐using counterparts.[11]
Supervised injection facilities (SIFs) are sanctioned environments where PWUDs can inject preobtained illicit drugs under the supervision of healthcare staff. Internationally, SIFs have been shown to improve public health and public order within surrounding communities.[15, 16, 17] For example, a dramatic decline in fatal overdoses in Vancouver's Downtown Eastside neighborhood was attributed to the implementation of a SIF in the area.[15] Changes in risk‐injecting behavior have also been observed among individuals who access SIFs.[18] Although a large body of evidence supports SIFs as an effective approach for minimizing the drug‐ and health‐related harms within street‐based drug scenes,[17, 19] little is known about whether there is a role for SIFs within hospital settings. Currently in Vancouver, Canada, harm reduction services are generally not being provided within hospital settings. Therefore, we sought to conduct a needs assessment to identify the prevalence and correlates of willingness to access an in‐hospital SIF among PWUDs in Vancouver. These data may be crucial for planning appropriate programs and services to reduce health‐related harms and leaving the hospital AMA among PWUDs in hospital settings.
METHODS
The Vancouver Injection Drug Users Study (VIDUS) and the AIDS Care Cohort to Evaluate Exposure to Survival Services (ACCESS) are 2 prospective cohort studies of PWUDs who have been recruited through self‐referral and street outreach since May 1996. These cohorts have been described in detail previously.[20, 21] In brief, persons were eligible to enter the VIDUS study if they had injected illicit drugs at least once in the previous month and resided in the Greater Vancouver region at enrollment. Persons were eligible to enter the ACCESS study if they were HIV infected and used illicit drugs other than cannabinoids in the previous month. Individuals who seroconvert following recruitment are transferred from the VIDUS study into the ACCESS study. All eligible participants provided written informed consent. At baseline and semiannually, study participants complete a harmonized interviewer‐administered questionnaire (ie, participants in the VIDUS and ACCESS studies completed an identical questionnaire) and provide blood samples for HIV and hepatitis C virus testing, and HIV disease monitoring. At the conclusion of each visit, study participants receive Can$20 for their time. The study has received ethical approval from Providence Health Care/University of British Columbia's research ethics board.
The primary outcome of interest for this analysis was willingness to access an in‐hospital SIF (yes vs no or unsure), ascertained by asking participants the following hypothetical question: If you were admitted into a hospital, and if a supervised safe injection site was available in that hospital, would you use it? Given the existence of 2 SIFs in the local environment, PWUDs in Vancouver are familiar with the design and operation of such programs. We compared PWUDs who were and were not willing to access an in‐hospital SIF using bivariable and multivariable logistic regression analyses. Given that the variable measure was based on a hypothetical scenario, participants who completed the survey between June 2013 and November 2013 were eligible for inclusion regardless of their current injection drug use behavior. A complete case approach was used to analyze the data given that the extent of missingness was not significant (<5%). Variables considered included: age (per year increase); gender (male vs female); HIV serostatus (positive vs negative); heroin injection ( daily vs
To identify factors independently associated with willingness to access an in‐hospital SIF, a multivariable logistic regression model was constructed using an a prioridefined statistical protocol based on examination of the Akaike information criterion (AIC) and P values. First, we constructed a full model that included all variables significant at P<0.10 in bivariable analyses. After noting the AIC of the model, we removed the variable with the largest P value and built a reduced model. We continued this iterative process until no variables remained. We selected the multivariable model with the lowest AIC score. All P values were 2 sided. As a subanalysis, we asked participants who would be willing to access an in‐hospital SIF to indicate reasons why they would be willing to access such a facility.
RESULTS
Of the total 769 participants who were eligible for inclusion in the study, 732 PWUDs provided complete data and participated in the study; 37 (4.8%) were excluded due to missing data. In our study sample, 250 (34.2%) were female, the median age was 48 years (interquartile range: 4153 years), and 307 (41.5%) were HIV‐positive. Among our study sample, 499 (68.2%) participants would be willing to access an in‐hospital SIF if it were available. Bivariable analyses of factors associated with willingness to access an in‐hospital SIF are presented in Table 1.
| Characteristic | Willingness to Access an In‐hospital SIF | Odds Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Yes, n (%), n=499 | No, n (%), n=233 | ||||
| |||||
| Age | |||||
| Median | 48 | 48 | 0.98 (0.97‐1.00) | 0.085 | |
| IQR | (4153) | (4254) | |||
| Gender | |||||
| Male | 331 (66.3) | 151 (64.8) | 1.07 (0.77‐1.48) | 0.685 | |
| Female | 168 (33.7) | 82 (35.2) | |||
| HIV serostatus | |||||
| Positive | 203 (40.7) | 104 (44.6) | 0.85 (0.62‐1.16) | 0.313 | |
| Negative | 296 (59.3) | 129 (55.4) | |||
| Heroin injection* | |||||
| Daily | 106 (21.2) | 26 (11.2) | 2.15 (1.35‐3.40) | <0.001 | |
| < Daily | 393 (78.8) | 207 (88.8) | |||
| Cocaine injection* | |||||
| Daily | 46 (9.2) | 19 (8.2) | 1.14 (0.65‐2.00) | 0.637 | |
| < Daily | 453 (90.8) | 214 (91.8) | |||
| Crystal methamphetamine injection* | |||||
| Daily | 46 (9.2) | 16 (6.9) | 1.38 (0.76‐2.49) | 0.287 | |
| < Daily | 453 (90.8) | 217 (93.1) | |||
| Prescription opioid injection* | |||||
| Daily | 34 (6.8) | 9 (3.9) | 1.82 (0.86‐3.86) | 0.114 | |
| < Daily | 465 (93.2) | 224 (96.1) | |||
| Binge drug use* | |||||
| Yes | 141 (28.3) | 61 (26.2) | 1.11 (0.78‐1.58) | 0.558 | |
| No | 358 (71.7) | 172 (73.8) | |||
| Ever left hospital AMA | |||||
| Yes | 21 (4.2) | 2 (0.9) | 5.07 (1.1821.83) | 0.012 | |
| No | 478 (95.8) | 231 (99.1) | |||
| Ever used illicit drugs in hospital | |||||
| Yes | 238 (47.7) | 83 (35.6) | 1.65 (1.20‐2.27) | 0.002 | |
| No | 261 (52.3) | 150 (64.4) | |||
| Ever had negative experiences with healthcare providers | |||||
| Yes | 131 (26.3) | 64 (27.5) | 0.94 (0.66‐1.33) | 0.729 | |
| No | 368 (73.7) | 169 (72.5) | |||
| Ever had negative experiences with police | |||||
| Yes | 383 (76.8) | 169 (72.5) | 1.25 (0.88‐1.78) | 0.217 | |
| No | 116 (23.2) | 64 (27.5) | |||
| Used an SIF* | |||||
| Yes | 228 (45.7) | 77 (33.0) | 1.70 (1.23‐2.36) | 0.001 | |
| No | 271 (54.3) | 156 (67.0) | |||
As indicated in Table 2, in multivariable analyses, factors that remained significantly and positively associated with willingness to access an in‐hospital SIF included: daily heroin injection (adjusted odds ratio [AOR]=1.90; 95% confidence interval [CI]: 1.20‐3.11), ever used illicit drugs in the hospital (AOR=1.63; 95% CI: 1.18‐2.26), and previously used an SIF (AOR=1.53; 95% CI: 1.10‐2.15).
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Heroin injection* ( daily vs | 1.90 | 1.20‐3.11 | 0.008 |
| Ever left hospital AMA (yes vs no) | 3.74 | 1.0623.72 | 0.079 |
| Ever used illicit drugs in hospital (yes vs no) | 1.63 | 1.18‐2.26 | 0.003 |
| Used an SIF* (yes vs no) | 1.53 | 1.10‐2.15 | 0.013 |
Among participants who would be willing to access an in‐hospital SIF, the most common reasons included: to be able to stay in the hospital (229/499=45.9%), to reduce their drug‐related risks (189/499=37.9%), and to reduce stress associated with being kicked out of the hospital because they were using drugs (97/499=19.4%).
DISCUSSION
We found that over two‐thirds of PWUDs participating in our study would be willing to access an in‐hospital SIF if such a service was available. This finding is encouraging given that a large proportion of PWUDs are hospitalized annually for acute and chronic diseases.[5, 6] Previous studies have documented the positive impact of incorporating a harm reduction model within hospital settings, resulting in more comprehensive care for PWUDs.[22, 23] For example, the Dr. Peter Centre Day Health Program provides a SIF for HIV‐positive PWUDs to safely use illicit drugs under the supervision of trained nurses and was once located at St. Paul's Hospital.[24] Although the Dr. Peter Centre currently operates outside of St. Paul's Hospital, it may be advantageous to model an in‐hospital SIF after the Dr. Peter Centre's harm‐reduction room given their success in facilitating access and delivery of comprehensive care for PWUDs.[23] Specifically, nurses at the Dr. Peter Centre directly observe injections of preobtained illicit drugs for the purposes of preventing illness and promoting health. Our findings support recent calls to implement harm‐reduction services within hospital settings in an effort to minimize the harms associated with illicit drug use.[25, 26]
Previous studies have identified various high‐risk locations where PWUDs use illicit drugs to maintain their established drug‐use habits, including in locked washrooms in hospitals.[9] We found a positive association among PWUDs who had used illicit drugs in the hospital and a willingness to use an in‐hospital SIF. Our finding is reassuring given that studies have shown that these individuals are at a higher risk of negative health consequences (eg, fatal overdose) from using drugs in the hospital.[9] Harm reduction services within the hospital settings can play an important role in reducing this drug‐ and health‐related harm among PWUDs.
Our study also found that high‐frequency heroin injection was associated with a willingness to access an in‐hospital SIF. This relationship may be a result of the complex nature of treating opioid‐dependent patients for pain. For instance, some opioid‐dependent PWUDs may have already established a high tolerance for opioids due to the concomitant use of opioid substitution therapies and ongoing drug use, making it difficult to appropriately prescribe pain medication to these individuals.[27] High‐frequency heroin users may also face severe withdrawal given the unavailability of illicit opioids in hospital settings, resulting in their increased willingness to access an in‐hospital SIF. Furthermore, inadequate pain management may contribute to the continued need to use opioids, as some healthcare providers may be reluctant to provide pain medication out of fear that they would contribute to an existing addiction or relapse.[28, 29] Further, requests for pain mediation may be misinterpreted as drug‐seeking behavior.[30, 31] Given the complexities arising from high‐intensity heroin use, pain management, and healthcare professionals' perceptions regarding PWUDs, further research should seek to untangle the causal relationships underlying these associations.
We found an association between recent use of an SIF and willingness to access an in‐hospital SIF. As mentioned previously, earlier research has shown improvements in various health outcomes and reductions in related harm in surrounding communities where SIFs were implemented.[15, 17] It is unfortunate that although progress in reducing the harm of injection drug use has been seen in community settings globally, the same cannot be said about hospitals. Given that many PWUDs often present to emergency care late in the course of illness and require admission to a hospital bed,[2] it is important to ensure that harm reduction services that are available in the community are also made available in hospitals. However, given the lack of knowledge on in‐hospital SIFs, future research should seek to understand the benefits and consequences of implementing such a facility in a hospital from different perspectives. For example, it may be of interest to assess the attitudes and perceptions of healthcare providers toward an in‐hospital SIF.
A large body of evidence has documented the health harms associated with leaving the hospital AMA, including readmission for a worsened illness and mortality.[13, 32] However, when faced with the abstinence‐based policies that exist in most hospital settings, it is not uncommon for PWUDs to leave the hospital to maintain their active addiction or to address their drug withdrawal.[9] Although we failed to find a statistically significant association between being discharged AMA and willingness to access an in‐hospital SIF, it is noteworthy that in our subanalysis we found that PWUD who were more likely to access an in‐hospital SIF reported doing so because they wanted to stay in the hospital and reduce their drug‐related risks. Given that we observed low counts of reported AMA discharge events, further exploration of this topic is warranted.
Our study suggests that in‐hospital SIFs have the potential to minimize health harms among patients who use illicit drugs in hospitals; however, there are some legal issues that warrant consideration. Specifically, for the successful operation of SIFs, there is a need for changes to regulatory frameworks, including drug laws, to allow for the possession of illicit drugs by individuals accessing an SIF. Such frameworks have been developed in a range of settings and in a manner that is consistent with international drug control treaties. In hospitals, additional regulatory changes may be needed to address issues unique to these settings, such as the use of opioids among PWUDs being treated for pain.
There are several limitations to this study. First, the cross‐sectional design of the study limited our ability to determine a temporal or causal relationship between the explanatory and outcome variables. Second, it is noteworthy that the chosen mode of interviewer‐based questionnaire administration may have influenced our results by relying on self‐reported data that are susceptible to reporting biases, including socially desirable reporting and recall bias. However, we believe we have minimized response bias and maximized reliability in our data by placing sensitive questions toward the end of the interview to allow rapport to be established between the interviewer and participant. Last, given that the participants in the present study were not randomly selected, the interpretation of these results may not be representative or generalizable to other PWUD populations outside of Vancouver. However, it is noteworthy that over the past few decades, community‐based SIFs have been successfully operating in international settings such as Europe and Australia[33, 34]; thus, the concept of an in‐hospital SIF may not be far from actual inpatient practice in these settings. It is also important to acknowledge the progress made toward the implementation of community‐based SIFs in other settings, including the United States. For example, feasibility studies have been conducted in San Francisco and New York and have shown increasing support for the implementation of SIFs in these areas.[35, 36]
We found that a substantial proportion of PWUDs in our sample would be willing to access an in‐hospital SIF if this service was available. Those PWUDs who expressed a willingness to use an in‐hospital SIF were more likely to be high‐intensity heroin users, to have previously used illicit drugs in the hospital, and were more likely to have previously used an SIF. Our findings highlight the potential of in‐hospital SIFs to complement existing harm‐reduction programs that serve people who inject drugs.
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
Disclosures: The VIDUS study was supported by the US National Institutes of Health (R01DA011591). The ACCESS study was supported by the US National Institutes of Health (R01DA021525). The evaluation of the supervised injecting facility was supported by Vancouver Coastal Health and Canadian Institutes of Health Research (MOP‐111039). L.T. is supported by a Canadian Institutes of Health Research Doctoral Research Award. This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine, which supports E.W. The authors report no conflicts of interest.
People who use illicit drugs (PWUDs) experience a wide range of health‐related harms and consequently often rely on acute and emergency services for care.[1, 2] Specifically, the poor health status of many PWUDs is often attributable to infectious diseases such as human immunodeficiency virus (HIV) and hepatitis C virus.[3, 4] Soft‐tissue infections associated with injection drug use are also common, and have increasingly accounted for the majority of hospitalizations among this population.[5] Many of these adverse health outcomes may require lengthy in‐patient hospital admissions and constitute a substantial financial burden for the healthcare system.[6]
PWUDs frequently experience barriers to conventional healthcare services. For example, negative experiences with healthcare providers and the healthcare system have often deterred PWUDs from accessing these services.[7, 8] Given that most hospitals operate under an abstinence‐based policy, PWUDs have minimal access to drug‐using paraphernalia while hospitalized, making it difficult for these individuals to safely manage their active drug use. As a result, many PWUDs may resort to high‐risk drug‐using practices (eg, syringe sharing, injecting alone) in the hospital that may lead to further adverse health outcomes, such as infectious disease transmission and overdose, respectively.[9] Past studies have also shown that many PWUDs do not complete hospital‐based treatments.[10, 11] Specifically, many PWUDs leave the hospital against medical advice (AMA) possibly because they are unable to continue their drug use practices in this setting,[10, 12] thus contributing to an increase in readmission rates and mortality among this population.[13] Past studies have indicated that approximately 30% of patients who inject drugs left the hospital AMA,[12, 14] and these individuals have shown to be as high as 4 times more likely to leave the hospital AMA compared to their nondrug‐using counterparts.[11]
Supervised injection facilities (SIFs) are sanctioned environments where PWUDs can inject preobtained illicit drugs under the supervision of healthcare staff. Internationally, SIFs have been shown to improve public health and public order within surrounding communities.[15, 16, 17] For example, a dramatic decline in fatal overdoses in Vancouver's Downtown Eastside neighborhood was attributed to the implementation of a SIF in the area.[15] Changes in risk‐injecting behavior have also been observed among individuals who access SIFs.[18] Although a large body of evidence supports SIFs as an effective approach for minimizing the drug‐ and health‐related harms within street‐based drug scenes,[17, 19] little is known about whether there is a role for SIFs within hospital settings. Currently in Vancouver, Canada, harm reduction services are generally not being provided within hospital settings. Therefore, we sought to conduct a needs assessment to identify the prevalence and correlates of willingness to access an in‐hospital SIF among PWUDs in Vancouver. These data may be crucial for planning appropriate programs and services to reduce health‐related harms and leaving the hospital AMA among PWUDs in hospital settings.
METHODS
The Vancouver Injection Drug Users Study (VIDUS) and the AIDS Care Cohort to Evaluate Exposure to Survival Services (ACCESS) are 2 prospective cohort studies of PWUDs who have been recruited through self‐referral and street outreach since May 1996. These cohorts have been described in detail previously.[20, 21] In brief, persons were eligible to enter the VIDUS study if they had injected illicit drugs at least once in the previous month and resided in the Greater Vancouver region at enrollment. Persons were eligible to enter the ACCESS study if they were HIV infected and used illicit drugs other than cannabinoids in the previous month. Individuals who seroconvert following recruitment are transferred from the VIDUS study into the ACCESS study. All eligible participants provided written informed consent. At baseline and semiannually, study participants complete a harmonized interviewer‐administered questionnaire (ie, participants in the VIDUS and ACCESS studies completed an identical questionnaire) and provide blood samples for HIV and hepatitis C virus testing, and HIV disease monitoring. At the conclusion of each visit, study participants receive Can$20 for their time. The study has received ethical approval from Providence Health Care/University of British Columbia's research ethics board.
The primary outcome of interest for this analysis was willingness to access an in‐hospital SIF (yes vs no or unsure), ascertained by asking participants the following hypothetical question: If you were admitted into a hospital, and if a supervised safe injection site was available in that hospital, would you use it? Given the existence of 2 SIFs in the local environment, PWUDs in Vancouver are familiar with the design and operation of such programs. We compared PWUDs who were and were not willing to access an in‐hospital SIF using bivariable and multivariable logistic regression analyses. Given that the variable measure was based on a hypothetical scenario, participants who completed the survey between June 2013 and November 2013 were eligible for inclusion regardless of their current injection drug use behavior. A complete case approach was used to analyze the data given that the extent of missingness was not significant (<5%). Variables considered included: age (per year increase); gender (male vs female); HIV serostatus (positive vs negative); heroin injection ( daily vs
To identify factors independently associated with willingness to access an in‐hospital SIF, a multivariable logistic regression model was constructed using an a prioridefined statistical protocol based on examination of the Akaike information criterion (AIC) and P values. First, we constructed a full model that included all variables significant at P<0.10 in bivariable analyses. After noting the AIC of the model, we removed the variable with the largest P value and built a reduced model. We continued this iterative process until no variables remained. We selected the multivariable model with the lowest AIC score. All P values were 2 sided. As a subanalysis, we asked participants who would be willing to access an in‐hospital SIF to indicate reasons why they would be willing to access such a facility.
RESULTS
Of the total 769 participants who were eligible for inclusion in the study, 732 PWUDs provided complete data and participated in the study; 37 (4.8%) were excluded due to missing data. In our study sample, 250 (34.2%) were female, the median age was 48 years (interquartile range: 4153 years), and 307 (41.5%) were HIV‐positive. Among our study sample, 499 (68.2%) participants would be willing to access an in‐hospital SIF if it were available. Bivariable analyses of factors associated with willingness to access an in‐hospital SIF are presented in Table 1.
| Characteristic | Willingness to Access an In‐hospital SIF | Odds Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Yes, n (%), n=499 | No, n (%), n=233 | ||||
| |||||
| Age | |||||
| Median | 48 | 48 | 0.98 (0.97‐1.00) | 0.085 | |
| IQR | (4153) | (4254) | |||
| Gender | |||||
| Male | 331 (66.3) | 151 (64.8) | 1.07 (0.77‐1.48) | 0.685 | |
| Female | 168 (33.7) | 82 (35.2) | |||
| HIV serostatus | |||||
| Positive | 203 (40.7) | 104 (44.6) | 0.85 (0.62‐1.16) | 0.313 | |
| Negative | 296 (59.3) | 129 (55.4) | |||
| Heroin injection* | |||||
| Daily | 106 (21.2) | 26 (11.2) | 2.15 (1.35‐3.40) | <0.001 | |
| < Daily | 393 (78.8) | 207 (88.8) | |||
| Cocaine injection* | |||||
| Daily | 46 (9.2) | 19 (8.2) | 1.14 (0.65‐2.00) | 0.637 | |
| < Daily | 453 (90.8) | 214 (91.8) | |||
| Crystal methamphetamine injection* | |||||
| Daily | 46 (9.2) | 16 (6.9) | 1.38 (0.76‐2.49) | 0.287 | |
| < Daily | 453 (90.8) | 217 (93.1) | |||
| Prescription opioid injection* | |||||
| Daily | 34 (6.8) | 9 (3.9) | 1.82 (0.86‐3.86) | 0.114 | |
| < Daily | 465 (93.2) | 224 (96.1) | |||
| Binge drug use* | |||||
| Yes | 141 (28.3) | 61 (26.2) | 1.11 (0.78‐1.58) | 0.558 | |
| No | 358 (71.7) | 172 (73.8) | |||
| Ever left hospital AMA | |||||
| Yes | 21 (4.2) | 2 (0.9) | 5.07 (1.1821.83) | 0.012 | |
| No | 478 (95.8) | 231 (99.1) | |||
| Ever used illicit drugs in hospital | |||||
| Yes | 238 (47.7) | 83 (35.6) | 1.65 (1.20‐2.27) | 0.002 | |
| No | 261 (52.3) | 150 (64.4) | |||
| Ever had negative experiences with healthcare providers | |||||
| Yes | 131 (26.3) | 64 (27.5) | 0.94 (0.66‐1.33) | 0.729 | |
| No | 368 (73.7) | 169 (72.5) | |||
| Ever had negative experiences with police | |||||
| Yes | 383 (76.8) | 169 (72.5) | 1.25 (0.88‐1.78) | 0.217 | |
| No | 116 (23.2) | 64 (27.5) | |||
| Used an SIF* | |||||
| Yes | 228 (45.7) | 77 (33.0) | 1.70 (1.23‐2.36) | 0.001 | |
| No | 271 (54.3) | 156 (67.0) | |||
As indicated in Table 2, in multivariable analyses, factors that remained significantly and positively associated with willingness to access an in‐hospital SIF included: daily heroin injection (adjusted odds ratio [AOR]=1.90; 95% confidence interval [CI]: 1.20‐3.11), ever used illicit drugs in the hospital (AOR=1.63; 95% CI: 1.18‐2.26), and previously used an SIF (AOR=1.53; 95% CI: 1.10‐2.15).
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Heroin injection* ( daily vs | 1.90 | 1.20‐3.11 | 0.008 |
| Ever left hospital AMA (yes vs no) | 3.74 | 1.0623.72 | 0.079 |
| Ever used illicit drugs in hospital (yes vs no) | 1.63 | 1.18‐2.26 | 0.003 |
| Used an SIF* (yes vs no) | 1.53 | 1.10‐2.15 | 0.013 |
Among participants who would be willing to access an in‐hospital SIF, the most common reasons included: to be able to stay in the hospital (229/499=45.9%), to reduce their drug‐related risks (189/499=37.9%), and to reduce stress associated with being kicked out of the hospital because they were using drugs (97/499=19.4%).
DISCUSSION
We found that over two‐thirds of PWUDs participating in our study would be willing to access an in‐hospital SIF if such a service was available. This finding is encouraging given that a large proportion of PWUDs are hospitalized annually for acute and chronic diseases.[5, 6] Previous studies have documented the positive impact of incorporating a harm reduction model within hospital settings, resulting in more comprehensive care for PWUDs.[22, 23] For example, the Dr. Peter Centre Day Health Program provides a SIF for HIV‐positive PWUDs to safely use illicit drugs under the supervision of trained nurses and was once located at St. Paul's Hospital.[24] Although the Dr. Peter Centre currently operates outside of St. Paul's Hospital, it may be advantageous to model an in‐hospital SIF after the Dr. Peter Centre's harm‐reduction room given their success in facilitating access and delivery of comprehensive care for PWUDs.[23] Specifically, nurses at the Dr. Peter Centre directly observe injections of preobtained illicit drugs for the purposes of preventing illness and promoting health. Our findings support recent calls to implement harm‐reduction services within hospital settings in an effort to minimize the harms associated with illicit drug use.[25, 26]
Previous studies have identified various high‐risk locations where PWUDs use illicit drugs to maintain their established drug‐use habits, including in locked washrooms in hospitals.[9] We found a positive association among PWUDs who had used illicit drugs in the hospital and a willingness to use an in‐hospital SIF. Our finding is reassuring given that studies have shown that these individuals are at a higher risk of negative health consequences (eg, fatal overdose) from using drugs in the hospital.[9] Harm reduction services within the hospital settings can play an important role in reducing this drug‐ and health‐related harm among PWUDs.
Our study also found that high‐frequency heroin injection was associated with a willingness to access an in‐hospital SIF. This relationship may be a result of the complex nature of treating opioid‐dependent patients for pain. For instance, some opioid‐dependent PWUDs may have already established a high tolerance for opioids due to the concomitant use of opioid substitution therapies and ongoing drug use, making it difficult to appropriately prescribe pain medication to these individuals.[27] High‐frequency heroin users may also face severe withdrawal given the unavailability of illicit opioids in hospital settings, resulting in their increased willingness to access an in‐hospital SIF. Furthermore, inadequate pain management may contribute to the continued need to use opioids, as some healthcare providers may be reluctant to provide pain medication out of fear that they would contribute to an existing addiction or relapse.[28, 29] Further, requests for pain mediation may be misinterpreted as drug‐seeking behavior.[30, 31] Given the complexities arising from high‐intensity heroin use, pain management, and healthcare professionals' perceptions regarding PWUDs, further research should seek to untangle the causal relationships underlying these associations.
We found an association between recent use of an SIF and willingness to access an in‐hospital SIF. As mentioned previously, earlier research has shown improvements in various health outcomes and reductions in related harm in surrounding communities where SIFs were implemented.[15, 17] It is unfortunate that although progress in reducing the harm of injection drug use has been seen in community settings globally, the same cannot be said about hospitals. Given that many PWUDs often present to emergency care late in the course of illness and require admission to a hospital bed,[2] it is important to ensure that harm reduction services that are available in the community are also made available in hospitals. However, given the lack of knowledge on in‐hospital SIFs, future research should seek to understand the benefits and consequences of implementing such a facility in a hospital from different perspectives. For example, it may be of interest to assess the attitudes and perceptions of healthcare providers toward an in‐hospital SIF.
A large body of evidence has documented the health harms associated with leaving the hospital AMA, including readmission for a worsened illness and mortality.[13, 32] However, when faced with the abstinence‐based policies that exist in most hospital settings, it is not uncommon for PWUDs to leave the hospital to maintain their active addiction or to address their drug withdrawal.[9] Although we failed to find a statistically significant association between being discharged AMA and willingness to access an in‐hospital SIF, it is noteworthy that in our subanalysis we found that PWUD who were more likely to access an in‐hospital SIF reported doing so because they wanted to stay in the hospital and reduce their drug‐related risks. Given that we observed low counts of reported AMA discharge events, further exploration of this topic is warranted.
Our study suggests that in‐hospital SIFs have the potential to minimize health harms among patients who use illicit drugs in hospitals; however, there are some legal issues that warrant consideration. Specifically, for the successful operation of SIFs, there is a need for changes to regulatory frameworks, including drug laws, to allow for the possession of illicit drugs by individuals accessing an SIF. Such frameworks have been developed in a range of settings and in a manner that is consistent with international drug control treaties. In hospitals, additional regulatory changes may be needed to address issues unique to these settings, such as the use of opioids among PWUDs being treated for pain.
There are several limitations to this study. First, the cross‐sectional design of the study limited our ability to determine a temporal or causal relationship between the explanatory and outcome variables. Second, it is noteworthy that the chosen mode of interviewer‐based questionnaire administration may have influenced our results by relying on self‐reported data that are susceptible to reporting biases, including socially desirable reporting and recall bias. However, we believe we have minimized response bias and maximized reliability in our data by placing sensitive questions toward the end of the interview to allow rapport to be established between the interviewer and participant. Last, given that the participants in the present study were not randomly selected, the interpretation of these results may not be representative or generalizable to other PWUD populations outside of Vancouver. However, it is noteworthy that over the past few decades, community‐based SIFs have been successfully operating in international settings such as Europe and Australia[33, 34]; thus, the concept of an in‐hospital SIF may not be far from actual inpatient practice in these settings. It is also important to acknowledge the progress made toward the implementation of community‐based SIFs in other settings, including the United States. For example, feasibility studies have been conducted in San Francisco and New York and have shown increasing support for the implementation of SIFs in these areas.[35, 36]
We found that a substantial proportion of PWUDs in our sample would be willing to access an in‐hospital SIF if this service was available. Those PWUDs who expressed a willingness to use an in‐hospital SIF were more likely to be high‐intensity heroin users, to have previously used illicit drugs in the hospital, and were more likely to have previously used an SIF. Our findings highlight the potential of in‐hospital SIFs to complement existing harm‐reduction programs that serve people who inject drugs.
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
Disclosures: The VIDUS study was supported by the US National Institutes of Health (R01DA011591). The ACCESS study was supported by the US National Institutes of Health (R01DA021525). The evaluation of the supervised injecting facility was supported by Vancouver Coastal Health and Canadian Institutes of Health Research (MOP‐111039). L.T. is supported by a Canadian Institutes of Health Research Doctoral Research Award. This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine, which supports E.W. The authors report no conflicts of interest.
- , , , et al. Emergency department utilization among a cohort of HIV‐positive injecting drug users in a Canadian setting. J Emerg Med. 2012;43(2):236–243.
- , , , et al. High rates of primary care and emergency department use among injection drug users in Vancouver. J Public Health (Oxf). 2005;27(1):62–66.
- , . Epidemiology of HIV among injecting and non‐injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep. 2010;7(2):99–106.
- , , , et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–284.
- , , , et al. Determinants of hospitalization for a cutaneous injection‐related infection among injection drug users: a cohort study. BMC Public Health. 2010;10:327.
- , , , et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165(4):415–420.
- , , , , . The association of stigma with self‐reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–1108.
- , , , . Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1–2):23–35.
- , , , . Hospitals as a “risk environment”: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105C:59–66.
- . “I'm going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255–260.
- , , , , , . Leaving hospital against medical advice among HIV‐positive patients. CMAJ. 2002;167(6):633–637.
- , , , et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56–59.
- , , . Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594–602.
- , . Welfare checks, drug consumption, and health: evidence from Vancouver injection drug users. J Hum Resour. 2006;41(1):138–161.
- , , , , . Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: a retrospective population‐based study. Lancet. 2011;377(9775):1429–1437.
- , , , , . The impact of a supervised injecting facility on ambulance call‐outs in Sydney, Australia. Addiction. 2010;105(4):676–683.
- , , , et al. Changes in public order after the opening of a medically supervised safer injecting facility for illicit injection drug users. CMAJ. 2004;171(7):731–734.
- , , , et al. Changes in injecting practices associated with the use of a medically supervised safer injection facility. J Public Health. 2007;29(1):35–39.
- , , , , , . Police and public health partnerships: Evidence from the evaluation of Vancouver's supervised injection facility. Subst Abuse Treat Prev Policy. 2008;3:11.
- , , , et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549.
- , , , et al. Unsafe injection practices in a cohort of injection drug users in Vancouver: could safer injecting rooms help? CMAJ. 2001;165(4):405–410.
- , , , . Expanding harm reduction services through a wound and abscess clinic. Am J Public Health. 2002;92(12):1915–1917.
- , , , . An integrated supervised injecting program within a care facility for HIV‐positive individuals: a qualitative evaluation. AIDS Care. 2009;21(5):638–644.
- Dr. Peter AIDS Foundation. Available at: http://www.drpeter.org. Accessed June 19, 2014.
- , . Drug use in hospitals: Is there a role for harm reduction? Hospital News. Available at: http://hospitalnews.com/drug‐use‐in‐hospitals‐is‐there‐a‐role‐for‐harm‐reduction. Published October 17, 2013. Accessed March 11, 2014.
- , , , . Harm reduction in hospitals: is it time? Harm Reduct J. 2009;6(1):19.
- , , . Pain intolerance in opioid‐maintained former opiate addicts: effect of long‐acting maintenance agent. Drug Alcohol Depend. 2001;63(2):139–146.
- , , , . Guilty until proven innocent: a qualitative study of the management of chronic non‐cancer pain among patients with a history of substance abuse. Addict Behav. 2010;35(3):270–272.
- , , , . Providers' experiences treating chronic pain among opioid‐dependent drug users. J Gen Intern Med. 2009;24(4):482–488.
- , , , . Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284–1293.
- , , , et al. Routines and rituals: a grounded theory of the pain management of drug users in acute care settings. J Clin Nurs. 2010;19(19–20):2730–2740.
- , , . Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926–929.
- , , , , . Supervised injection services: What has been demonstrated? A systematic literature review. Drug Alcohol Depend. 2014;145:48–68.
- , , , , , . Drug consumption facilities in Europe and the establishment of supervised injecting centres in Australia. Drug Alcohol Rev. 2000;19(3):337–346.
- , , , , , . Acceptability of a safer injection facility among injection drug users in San Francisco. Drug Alcohol Depend. 2010;110(1–2):160–163.
- , , , , , . Safer injection sites in New York City: a utilization survey of injection drug users. J Drug Issues. 2003;33(3):733–750.
- , , , et al. Emergency department utilization among a cohort of HIV‐positive injecting drug users in a Canadian setting. J Emerg Med. 2012;43(2):236–243.
- , , , et al. High rates of primary care and emergency department use among injection drug users in Vancouver. J Public Health (Oxf). 2005;27(1):62–66.
- , . Epidemiology of HIV among injecting and non‐injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep. 2010;7(2):99–106.
- , , , et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–284.
- , , , et al. Determinants of hospitalization for a cutaneous injection‐related infection among injection drug users: a cohort study. BMC Public Health. 2010;10:327.
- , , , et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165(4):415–420.
- , , , , . The association of stigma with self‐reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–1108.
- , , , . Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1–2):23–35.
- , , , . Hospitals as a “risk environment”: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105C:59–66.
- . “I'm going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255–260.
- , , , , , . Leaving hospital against medical advice among HIV‐positive patients. CMAJ. 2002;167(6):633–637.
- , , , et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56–59.
- , , . Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594–602.
- , . Welfare checks, drug consumption, and health: evidence from Vancouver injection drug users. J Hum Resour. 2006;41(1):138–161.
- , , , , . Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: a retrospective population‐based study. Lancet. 2011;377(9775):1429–1437.
- , , , , . The impact of a supervised injecting facility on ambulance call‐outs in Sydney, Australia. Addiction. 2010;105(4):676–683.
- , , , et al. Changes in public order after the opening of a medically supervised safer injecting facility for illicit injection drug users. CMAJ. 2004;171(7):731–734.
- , , , et al. Changes in injecting practices associated with the use of a medically supervised safer injection facility. J Public Health. 2007;29(1):35–39.
- , , , , , . Police and public health partnerships: Evidence from the evaluation of Vancouver's supervised injection facility. Subst Abuse Treat Prev Policy. 2008;3:11.
- , , , et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549.
- , , , et al. Unsafe injection practices in a cohort of injection drug users in Vancouver: could safer injecting rooms help? CMAJ. 2001;165(4):405–410.
- , , , . Expanding harm reduction services through a wound and abscess clinic. Am J Public Health. 2002;92(12):1915–1917.
- , , , . An integrated supervised injecting program within a care facility for HIV‐positive individuals: a qualitative evaluation. AIDS Care. 2009;21(5):638–644.
- Dr. Peter AIDS Foundation. Available at: http://www.drpeter.org. Accessed June 19, 2014.
- , . Drug use in hospitals: Is there a role for harm reduction? Hospital News. Available at: http://hospitalnews.com/drug‐use‐in‐hospitals‐is‐there‐a‐role‐for‐harm‐reduction. Published October 17, 2013. Accessed March 11, 2014.
- , , , . Harm reduction in hospitals: is it time? Harm Reduct J. 2009;6(1):19.
- , , . Pain intolerance in opioid‐maintained former opiate addicts: effect of long‐acting maintenance agent. Drug Alcohol Depend. 2001;63(2):139–146.
- , , , . Guilty until proven innocent: a qualitative study of the management of chronic non‐cancer pain among patients with a history of substance abuse. Addict Behav. 2010;35(3):270–272.
- , , , . Providers' experiences treating chronic pain among opioid‐dependent drug users. J Gen Intern Med. 2009;24(4):482–488.
- , , , . Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284–1293.
- , , , et al. Routines and rituals: a grounded theory of the pain management of drug users in acute care settings. J Clin Nurs. 2010;19(19–20):2730–2740.
- , , . Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med. 2010;25(9):926–929.
- , , , , . Supervised injection services: What has been demonstrated? A systematic literature review. Drug Alcohol Depend. 2014;145:48–68.
- , , , , , . Drug consumption facilities in Europe and the establishment of supervised injecting centres in Australia. Drug Alcohol Rev. 2000;19(3):337–346.
- , , , , , . Acceptability of a safer injection facility among injection drug users in San Francisco. Drug Alcohol Depend. 2010;110(1–2):160–163.
- , , , , , . Safer injection sites in New York City: a utilization survey of injection drug users. J Drug Issues. 2003;33(3):733–750.
© 2015 Society of Hospital Medicine
Dimensional aspects of DSM-5 personality disorder criteria discussed
HUNTINGTON BEACH, CALIF. – In the opinion of Dr. John M. Oldham, clinicians who deem the alternative personality disorder model of the DSM-5 as too confusing are misguided.
“If you’re going to compare DSM-5 alternative personality disorder model with the DSM-IV model, you have to do a fair comparison,” Dr. Oldham told attendees at the annual meeting of the American College of Psychiatrists. ”In fact, we reduced the number of items that you have to measure by 43%.”
So when people describe the DSM-5’s personality disorders criteria as more complicated, he continued, “what they really mean is, ‘it’s more complicated than what I do,’ not that it’s more complicated than [the] DSM-IV.”
Along with Dr. Andrew E. Skodol, Dr. Oldham cochaired a work group of experts convened by the American Psychiatric Association to update diagnostic criteria related to personality and personality disorders for the DSM-5. “We took our work and our charge seriously,” recalled Dr. Oldham, senior vice president and chief of staff at the Menninger Clinic, Houston. “It was not easy. We had many challenges. A great deal of research has been done in the factor analytic research psychology world around things like the five-factor model of personality. Such terms are not always terribly familiar in clinical medicine, so there was a problem with the lack of familiarity. Then there were vested interests different groups had that were influential in some ways.”
Ultimately, the alternative personality disorder model was placed in section III of the DSM-5. The model enables clinicians “to individually portray the dimensions of the patient’s pathology in a thorough and broad way,” Dr. Oldham explained. “We emphasize impairment in functioning. That’s an important new requirement. So you have to determine, by using the level of functioning scale, whether the person does or doesn’t have moderate or greater impairment. If you have a patient with mild impairment, you can describe what you’re concerned about, but you’re not putting that patient into a diagnostic box of pathology. There is a dimensional scope that enables you to capture many types of patients.”
An empirical study of 337 clinicians demonstrated that in 14 of 18 comparisons, respondents deemed the DSM-5 pathological personality traits as more clinical useful, compared with the DSM-IV, with respect to ease of use, communication of clinical information to other professionals, communication of clinical information to patients, comprehensiveness in describing pathology, and treatment planning (J. Abnorm. Psychol. 2013;122:836-41). “In fact, this was a preference to the new model, which was unfamiliar, compared to the model that these clinicians had been using for 20 years,” Dr. Oldham said.
The study also found that the new DSM-5 personality disorder model was more strongly related to clinical decision making in areas of global functioning, risk assessment, recommended treatment type and intensity, and prognosis.
According to unpublished data from the DSM-5 field trials conducted in the United States and Canada, more than 80% of clinicians in academic and routine clinical practice fields found the new personality disorder criteria “moderately” to “extremely” useful, compared with the DSM-IV. In fact, the respondents rated the new criteria as more useful than other changes to the DSM-5, including those related to bipolar and related disorders, schizophrenia spectrum and other psychotic disorders, and other conditions.
In addition, a test-retest reliability study conducted at 11 academic medical centers found that the new model for borderline personality disorder had a good test-retest reliability (.054), in the same ballpark as that for bipolar I disorder (0.56) and schizophrenia (.50) (Am. J. Psychiatry 2013;170:43-58). “This surprised a lot of people,” Dr. Oldham said.
About 1 year after the DSM-5’s release, Medscape Psychiatry surveyed almost 3,000 clinicians about their impressions of the new guidelines. Of the 2,828 respondents, nearly one-third (28%) were psychiatrists, 22% were psychologists, 13% were family medicine clinicians, and the rest were from other medical fields. The researchers found that 39% of survey respondents were considering the dimensional approaches offered in the new personality disorder criteria of the DSM-5.
“That’s not bad,” Dr. Oldham said.
He reported having no relevant financial conflicts.
On Twitter @dougbrunk
HUNTINGTON BEACH, CALIF. – In the opinion of Dr. John M. Oldham, clinicians who deem the alternative personality disorder model of the DSM-5 as too confusing are misguided.
“If you’re going to compare DSM-5 alternative personality disorder model with the DSM-IV model, you have to do a fair comparison,” Dr. Oldham told attendees at the annual meeting of the American College of Psychiatrists. ”In fact, we reduced the number of items that you have to measure by 43%.”
So when people describe the DSM-5’s personality disorders criteria as more complicated, he continued, “what they really mean is, ‘it’s more complicated than what I do,’ not that it’s more complicated than [the] DSM-IV.”
Along with Dr. Andrew E. Skodol, Dr. Oldham cochaired a work group of experts convened by the American Psychiatric Association to update diagnostic criteria related to personality and personality disorders for the DSM-5. “We took our work and our charge seriously,” recalled Dr. Oldham, senior vice president and chief of staff at the Menninger Clinic, Houston. “It was not easy. We had many challenges. A great deal of research has been done in the factor analytic research psychology world around things like the five-factor model of personality. Such terms are not always terribly familiar in clinical medicine, so there was a problem with the lack of familiarity. Then there were vested interests different groups had that were influential in some ways.”
Ultimately, the alternative personality disorder model was placed in section III of the DSM-5. The model enables clinicians “to individually portray the dimensions of the patient’s pathology in a thorough and broad way,” Dr. Oldham explained. “We emphasize impairment in functioning. That’s an important new requirement. So you have to determine, by using the level of functioning scale, whether the person does or doesn’t have moderate or greater impairment. If you have a patient with mild impairment, you can describe what you’re concerned about, but you’re not putting that patient into a diagnostic box of pathology. There is a dimensional scope that enables you to capture many types of patients.”
An empirical study of 337 clinicians demonstrated that in 14 of 18 comparisons, respondents deemed the DSM-5 pathological personality traits as more clinical useful, compared with the DSM-IV, with respect to ease of use, communication of clinical information to other professionals, communication of clinical information to patients, comprehensiveness in describing pathology, and treatment planning (J. Abnorm. Psychol. 2013;122:836-41). “In fact, this was a preference to the new model, which was unfamiliar, compared to the model that these clinicians had been using for 20 years,” Dr. Oldham said.
The study also found that the new DSM-5 personality disorder model was more strongly related to clinical decision making in areas of global functioning, risk assessment, recommended treatment type and intensity, and prognosis.
According to unpublished data from the DSM-5 field trials conducted in the United States and Canada, more than 80% of clinicians in academic and routine clinical practice fields found the new personality disorder criteria “moderately” to “extremely” useful, compared with the DSM-IV. In fact, the respondents rated the new criteria as more useful than other changes to the DSM-5, including those related to bipolar and related disorders, schizophrenia spectrum and other psychotic disorders, and other conditions.
In addition, a test-retest reliability study conducted at 11 academic medical centers found that the new model for borderline personality disorder had a good test-retest reliability (.054), in the same ballpark as that for bipolar I disorder (0.56) and schizophrenia (.50) (Am. J. Psychiatry 2013;170:43-58). “This surprised a lot of people,” Dr. Oldham said.
About 1 year after the DSM-5’s release, Medscape Psychiatry surveyed almost 3,000 clinicians about their impressions of the new guidelines. Of the 2,828 respondents, nearly one-third (28%) were psychiatrists, 22% were psychologists, 13% were family medicine clinicians, and the rest were from other medical fields. The researchers found that 39% of survey respondents were considering the dimensional approaches offered in the new personality disorder criteria of the DSM-5.
“That’s not bad,” Dr. Oldham said.
He reported having no relevant financial conflicts.
On Twitter @dougbrunk
HUNTINGTON BEACH, CALIF. – In the opinion of Dr. John M. Oldham, clinicians who deem the alternative personality disorder model of the DSM-5 as too confusing are misguided.
“If you’re going to compare DSM-5 alternative personality disorder model with the DSM-IV model, you have to do a fair comparison,” Dr. Oldham told attendees at the annual meeting of the American College of Psychiatrists. ”In fact, we reduced the number of items that you have to measure by 43%.”
So when people describe the DSM-5’s personality disorders criteria as more complicated, he continued, “what they really mean is, ‘it’s more complicated than what I do,’ not that it’s more complicated than [the] DSM-IV.”
Along with Dr. Andrew E. Skodol, Dr. Oldham cochaired a work group of experts convened by the American Psychiatric Association to update diagnostic criteria related to personality and personality disorders for the DSM-5. “We took our work and our charge seriously,” recalled Dr. Oldham, senior vice president and chief of staff at the Menninger Clinic, Houston. “It was not easy. We had many challenges. A great deal of research has been done in the factor analytic research psychology world around things like the five-factor model of personality. Such terms are not always terribly familiar in clinical medicine, so there was a problem with the lack of familiarity. Then there were vested interests different groups had that were influential in some ways.”
Ultimately, the alternative personality disorder model was placed in section III of the DSM-5. The model enables clinicians “to individually portray the dimensions of the patient’s pathology in a thorough and broad way,” Dr. Oldham explained. “We emphasize impairment in functioning. That’s an important new requirement. So you have to determine, by using the level of functioning scale, whether the person does or doesn’t have moderate or greater impairment. If you have a patient with mild impairment, you can describe what you’re concerned about, but you’re not putting that patient into a diagnostic box of pathology. There is a dimensional scope that enables you to capture many types of patients.”
An empirical study of 337 clinicians demonstrated that in 14 of 18 comparisons, respondents deemed the DSM-5 pathological personality traits as more clinical useful, compared with the DSM-IV, with respect to ease of use, communication of clinical information to other professionals, communication of clinical information to patients, comprehensiveness in describing pathology, and treatment planning (J. Abnorm. Psychol. 2013;122:836-41). “In fact, this was a preference to the new model, which was unfamiliar, compared to the model that these clinicians had been using for 20 years,” Dr. Oldham said.
The study also found that the new DSM-5 personality disorder model was more strongly related to clinical decision making in areas of global functioning, risk assessment, recommended treatment type and intensity, and prognosis.
According to unpublished data from the DSM-5 field trials conducted in the United States and Canada, more than 80% of clinicians in academic and routine clinical practice fields found the new personality disorder criteria “moderately” to “extremely” useful, compared with the DSM-IV. In fact, the respondents rated the new criteria as more useful than other changes to the DSM-5, including those related to bipolar and related disorders, schizophrenia spectrum and other psychotic disorders, and other conditions.
In addition, a test-retest reliability study conducted at 11 academic medical centers found that the new model for borderline personality disorder had a good test-retest reliability (.054), in the same ballpark as that for bipolar I disorder (0.56) and schizophrenia (.50) (Am. J. Psychiatry 2013;170:43-58). “This surprised a lot of people,” Dr. Oldham said.
About 1 year after the DSM-5’s release, Medscape Psychiatry surveyed almost 3,000 clinicians about their impressions of the new guidelines. Of the 2,828 respondents, nearly one-third (28%) were psychiatrists, 22% were psychologists, 13% were family medicine clinicians, and the rest were from other medical fields. The researchers found that 39% of survey respondents were considering the dimensional approaches offered in the new personality disorder criteria of the DSM-5.
“That’s not bad,” Dr. Oldham said.
He reported having no relevant financial conflicts.
On Twitter @dougbrunk
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF PSYCHIATRISTS
Gout may lower Alzheimer’s risk
Patients with a history of gout may be at a lower risk for developing Alzheimer’s disease, reported Na Lu of Boston University and coauthors.
The large cohort study used electronic medical records from general practices in the United Kingdom’s The Health Improvement Network (THIN) to find 309 new cases of Alzheimer’s in 55,224 patients with gout and 1,942 cases in a comparison group of 238,805 patients during a median follow-up of 5 years. Gout patients had a multivariate-adjusted 24% lower risk of developing Alzheimer’s, the investigators found (hazard ratio, 0.76; 95% confidence interval, 0.66-0.87).
This difference may be due to the potential neuroprotective effects of uric acid, Ms. Lu and colleagues said in the report. “Overall, these findings support the proposed hypothesis that supplementary use of the metabolic precursor to uric acid, like inosine or hypoxanthine, could prevent and attenuate the progression of AD,” they wrote.
Read the full paper in Annals of the Rheumatic Diseases (2015 March 4 [doi:10.1136/annrheumdis-2014-206917]).
Patients with a history of gout may be at a lower risk for developing Alzheimer’s disease, reported Na Lu of Boston University and coauthors.
The large cohort study used electronic medical records from general practices in the United Kingdom’s The Health Improvement Network (THIN) to find 309 new cases of Alzheimer’s in 55,224 patients with gout and 1,942 cases in a comparison group of 238,805 patients during a median follow-up of 5 years. Gout patients had a multivariate-adjusted 24% lower risk of developing Alzheimer’s, the investigators found (hazard ratio, 0.76; 95% confidence interval, 0.66-0.87).
This difference may be due to the potential neuroprotective effects of uric acid, Ms. Lu and colleagues said in the report. “Overall, these findings support the proposed hypothesis that supplementary use of the metabolic precursor to uric acid, like inosine or hypoxanthine, could prevent and attenuate the progression of AD,” they wrote.
Read the full paper in Annals of the Rheumatic Diseases (2015 March 4 [doi:10.1136/annrheumdis-2014-206917]).
Patients with a history of gout may be at a lower risk for developing Alzheimer’s disease, reported Na Lu of Boston University and coauthors.
The large cohort study used electronic medical records from general practices in the United Kingdom’s The Health Improvement Network (THIN) to find 309 new cases of Alzheimer’s in 55,224 patients with gout and 1,942 cases in a comparison group of 238,805 patients during a median follow-up of 5 years. Gout patients had a multivariate-adjusted 24% lower risk of developing Alzheimer’s, the investigators found (hazard ratio, 0.76; 95% confidence interval, 0.66-0.87).
This difference may be due to the potential neuroprotective effects of uric acid, Ms. Lu and colleagues said in the report. “Overall, these findings support the proposed hypothesis that supplementary use of the metabolic precursor to uric acid, like inosine or hypoxanthine, could prevent and attenuate the progression of AD,” they wrote.
Read the full paper in Annals of the Rheumatic Diseases (2015 March 4 [doi:10.1136/annrheumdis-2014-206917]).
Environmental factors could increase U.S. anthrax cases
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
AT THE ASM BIODEFENSE MEETING
Careful planning, technique, and counseling can minimize pediatric scarring
It’s a myth that children don’t scar like adults, according to Dr. Jon A. Dyer.
In fact, children often scar worse than adults, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Children have more aggressive healing and inflammatory responses, a highly elastic dermis and connective tissues, and a high activity level that increases the risk of stressing the wound, and they may pay less attention to it, he explained.
Additionally, children can be more difficult to treat for scars because they are often more fearful and anxious, they have a short attention span, and they move around – a lot, he said. This leads to greater anxiety on the part of both the dermatologist and the family, which can lead to rapidly completing a procedure, said Dr. Dyer of the University of Missouri, Columbia.
Successful treatment of a wound or lesion – that is, getting the best initial scar possible – requires good planning, proactive application of surgical principles, and careful patient and family counseling and follow-up, he said.
Among Dr. Dyer’s tips for minimizing scarring when treating lesions in children:
• Do the simplest possible procedure.
• Make the scar as small as possible, keeping in mind that pediatric skin is more elastic.
• Remember that fusiform closures in children do not always require a 3:1 ratio. Tips settle, and divots fill in in young children, he said.
• Operate before puberty when the lesion is located in a cosmetically important area.
Also, consider performing a staged excision if possible; this allows a reduction in final scar length, provides tissue expansion, and allows assessment of individual wound healing. Take as much of the lesion as possible the first time, and remember that central excision is preferable to lateral excision, Dr. Dyer said.
The ideal time to wait between stage 1 and 2 is 4-6 weeks. Longer intervals will lead to more spread and more hypertrophy, and will negate the benefits of staging, he said.
Keep in mind that purse-string sutures can be particularly effective for suturing small spaces and for closing dead spaces, he said, adding that use of a purse-string closure for a round defect can reduce the final scar length by at least 50%.
Scar prevention or minimization requires strict adherence to good surgical principles, Dr. Dyer said, explaining that attention must be paid to perfecting the excision through proper use of skin tension lines, clean wound edges, sharp corners with no or minimal boating, undermining, and removing bulk if necessary.
Track marks are more likely on nonfacial skin and can be avoided by using running subcuticular sutures; in some cases of excellent approximation with no tension, the wound surface can be secured with Dermabond, he said.
Dr. Dyer also outlined the best closure choices for specific areas. For the scalp, use running cuticular sutures; for the extremities, trunk, axilla, or groin, use running subcuticular sutures; for the face, feet, or hands, use interrupted sutures; and for a punch biopsy, consider not using any closure, he advised.
If cuticular stitches are used, minimize scarring by pulling them within 5 days on the face, and within 7-10 days on the body. If dehiscence is a concern, pull the cuticulars and follow with either Dermabond or SteriStrips, he said, but strongly consider using running subcuticular sutures.
Once a wound is closed, secure it with Dermabond or with Steri-Strips and Mastisol and provide protective dressing.
“The bulkier and more colorful the dressing, the happier and more compliant the child,” he said.
Minimize postoperative wound tension in high-risk scars or sites and restrict movement and trauma for as long as possible; 6 weeks is ideal, he added, noting that movement restriction is critical for ensuring a good outcome.
To reduce the trauma of suture removal, “cut ’em long and leave ’em long,” and be sure to use proper scissors, he said.
Running subcuticular suture removal doesn’t hurt; enlist parents’ help in convincing the child of this, he suggested.
Finally, be specific when counseling patients about restrictions, discuss possible complications, provide a written summary including contact numbers, address the scar, and give the follow-up appointment time in writing, he said.
If dehiscence occurs – and it’s not uncommon in children – let it heal and restart the process, he said, noting that prevention is best and can be achieved in many cases with aggressive pre- and postop education, restriction of movement, and proper dressing.
Dr. Dyer stressed that good surgical principles for use in children can also be utilized to improve results in adult patients.
Dr. Dyer reported having no disclosures. SDEF and this new organization are owned by the same parent company.
It’s a myth that children don’t scar like adults, according to Dr. Jon A. Dyer.
In fact, children often scar worse than adults, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Children have more aggressive healing and inflammatory responses, a highly elastic dermis and connective tissues, and a high activity level that increases the risk of stressing the wound, and they may pay less attention to it, he explained.
Additionally, children can be more difficult to treat for scars because they are often more fearful and anxious, they have a short attention span, and they move around – a lot, he said. This leads to greater anxiety on the part of both the dermatologist and the family, which can lead to rapidly completing a procedure, said Dr. Dyer of the University of Missouri, Columbia.
Successful treatment of a wound or lesion – that is, getting the best initial scar possible – requires good planning, proactive application of surgical principles, and careful patient and family counseling and follow-up, he said.
Among Dr. Dyer’s tips for minimizing scarring when treating lesions in children:
• Do the simplest possible procedure.
• Make the scar as small as possible, keeping in mind that pediatric skin is more elastic.
• Remember that fusiform closures in children do not always require a 3:1 ratio. Tips settle, and divots fill in in young children, he said.
• Operate before puberty when the lesion is located in a cosmetically important area.
Also, consider performing a staged excision if possible; this allows a reduction in final scar length, provides tissue expansion, and allows assessment of individual wound healing. Take as much of the lesion as possible the first time, and remember that central excision is preferable to lateral excision, Dr. Dyer said.
The ideal time to wait between stage 1 and 2 is 4-6 weeks. Longer intervals will lead to more spread and more hypertrophy, and will negate the benefits of staging, he said.
Keep in mind that purse-string sutures can be particularly effective for suturing small spaces and for closing dead spaces, he said, adding that use of a purse-string closure for a round defect can reduce the final scar length by at least 50%.
Scar prevention or minimization requires strict adherence to good surgical principles, Dr. Dyer said, explaining that attention must be paid to perfecting the excision through proper use of skin tension lines, clean wound edges, sharp corners with no or minimal boating, undermining, and removing bulk if necessary.
Track marks are more likely on nonfacial skin and can be avoided by using running subcuticular sutures; in some cases of excellent approximation with no tension, the wound surface can be secured with Dermabond, he said.
Dr. Dyer also outlined the best closure choices for specific areas. For the scalp, use running cuticular sutures; for the extremities, trunk, axilla, or groin, use running subcuticular sutures; for the face, feet, or hands, use interrupted sutures; and for a punch biopsy, consider not using any closure, he advised.
If cuticular stitches are used, minimize scarring by pulling them within 5 days on the face, and within 7-10 days on the body. If dehiscence is a concern, pull the cuticulars and follow with either Dermabond or SteriStrips, he said, but strongly consider using running subcuticular sutures.
Once a wound is closed, secure it with Dermabond or with Steri-Strips and Mastisol and provide protective dressing.
“The bulkier and more colorful the dressing, the happier and more compliant the child,” he said.
Minimize postoperative wound tension in high-risk scars or sites and restrict movement and trauma for as long as possible; 6 weeks is ideal, he added, noting that movement restriction is critical for ensuring a good outcome.
To reduce the trauma of suture removal, “cut ’em long and leave ’em long,” and be sure to use proper scissors, he said.
Running subcuticular suture removal doesn’t hurt; enlist parents’ help in convincing the child of this, he suggested.
Finally, be specific when counseling patients about restrictions, discuss possible complications, provide a written summary including contact numbers, address the scar, and give the follow-up appointment time in writing, he said.
If dehiscence occurs – and it’s not uncommon in children – let it heal and restart the process, he said, noting that prevention is best and can be achieved in many cases with aggressive pre- and postop education, restriction of movement, and proper dressing.
Dr. Dyer stressed that good surgical principles for use in children can also be utilized to improve results in adult patients.
Dr. Dyer reported having no disclosures. SDEF and this new organization are owned by the same parent company.
It’s a myth that children don’t scar like adults, according to Dr. Jon A. Dyer.
In fact, children often scar worse than adults, he said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Children have more aggressive healing and inflammatory responses, a highly elastic dermis and connective tissues, and a high activity level that increases the risk of stressing the wound, and they may pay less attention to it, he explained.
Additionally, children can be more difficult to treat for scars because they are often more fearful and anxious, they have a short attention span, and they move around – a lot, he said. This leads to greater anxiety on the part of both the dermatologist and the family, which can lead to rapidly completing a procedure, said Dr. Dyer of the University of Missouri, Columbia.
Successful treatment of a wound or lesion – that is, getting the best initial scar possible – requires good planning, proactive application of surgical principles, and careful patient and family counseling and follow-up, he said.
Among Dr. Dyer’s tips for minimizing scarring when treating lesions in children:
• Do the simplest possible procedure.
• Make the scar as small as possible, keeping in mind that pediatric skin is more elastic.
• Remember that fusiform closures in children do not always require a 3:1 ratio. Tips settle, and divots fill in in young children, he said.
• Operate before puberty when the lesion is located in a cosmetically important area.
Also, consider performing a staged excision if possible; this allows a reduction in final scar length, provides tissue expansion, and allows assessment of individual wound healing. Take as much of the lesion as possible the first time, and remember that central excision is preferable to lateral excision, Dr. Dyer said.
The ideal time to wait between stage 1 and 2 is 4-6 weeks. Longer intervals will lead to more spread and more hypertrophy, and will negate the benefits of staging, he said.
Keep in mind that purse-string sutures can be particularly effective for suturing small spaces and for closing dead spaces, he said, adding that use of a purse-string closure for a round defect can reduce the final scar length by at least 50%.
Scar prevention or minimization requires strict adherence to good surgical principles, Dr. Dyer said, explaining that attention must be paid to perfecting the excision through proper use of skin tension lines, clean wound edges, sharp corners with no or minimal boating, undermining, and removing bulk if necessary.
Track marks are more likely on nonfacial skin and can be avoided by using running subcuticular sutures; in some cases of excellent approximation with no tension, the wound surface can be secured with Dermabond, he said.
Dr. Dyer also outlined the best closure choices for specific areas. For the scalp, use running cuticular sutures; for the extremities, trunk, axilla, or groin, use running subcuticular sutures; for the face, feet, or hands, use interrupted sutures; and for a punch biopsy, consider not using any closure, he advised.
If cuticular stitches are used, minimize scarring by pulling them within 5 days on the face, and within 7-10 days on the body. If dehiscence is a concern, pull the cuticulars and follow with either Dermabond or SteriStrips, he said, but strongly consider using running subcuticular sutures.
Once a wound is closed, secure it with Dermabond or with Steri-Strips and Mastisol and provide protective dressing.
“The bulkier and more colorful the dressing, the happier and more compliant the child,” he said.
Minimize postoperative wound tension in high-risk scars or sites and restrict movement and trauma for as long as possible; 6 weeks is ideal, he added, noting that movement restriction is critical for ensuring a good outcome.
To reduce the trauma of suture removal, “cut ’em long and leave ’em long,” and be sure to use proper scissors, he said.
Running subcuticular suture removal doesn’t hurt; enlist parents’ help in convincing the child of this, he suggested.
Finally, be specific when counseling patients about restrictions, discuss possible complications, provide a written summary including contact numbers, address the scar, and give the follow-up appointment time in writing, he said.
If dehiscence occurs – and it’s not uncommon in children – let it heal and restart the process, he said, noting that prevention is best and can be achieved in many cases with aggressive pre- and postop education, restriction of movement, and proper dressing.
Dr. Dyer stressed that good surgical principles for use in children can also be utilized to improve results in adult patients.
Dr. Dyer reported having no disclosures. SDEF and this new organization are owned by the same parent company.