User login
Most Common Dermatologic Conditions Encountered by Dermatologists and Nondermatologists
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
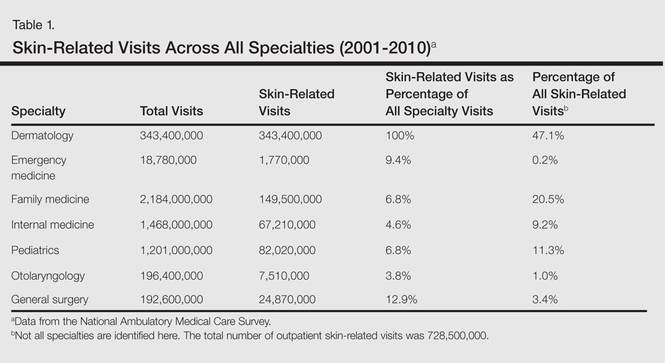
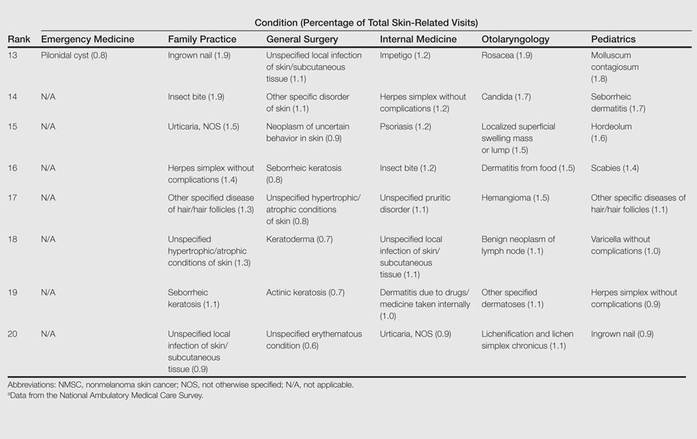
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).
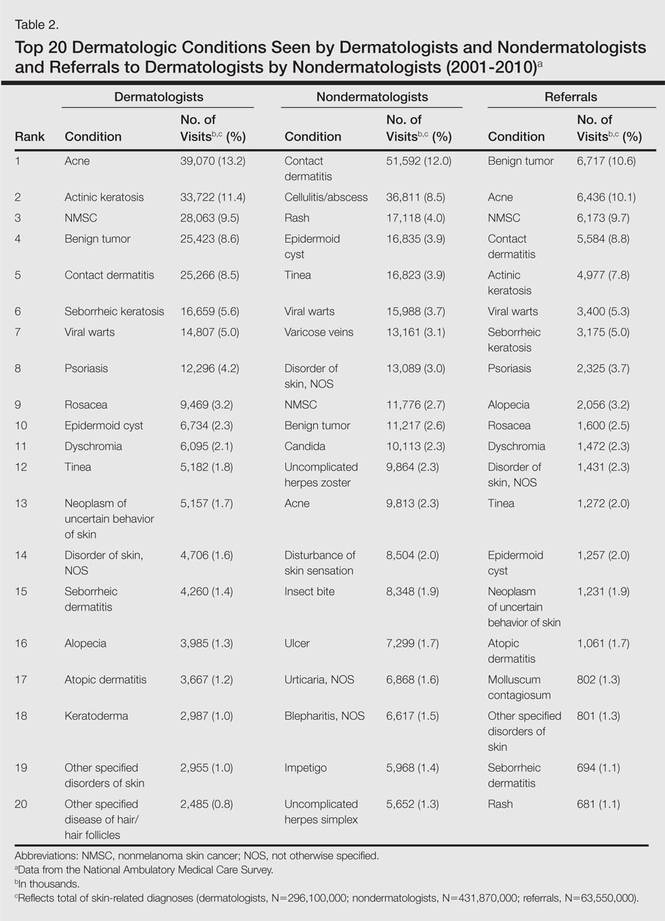
Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).
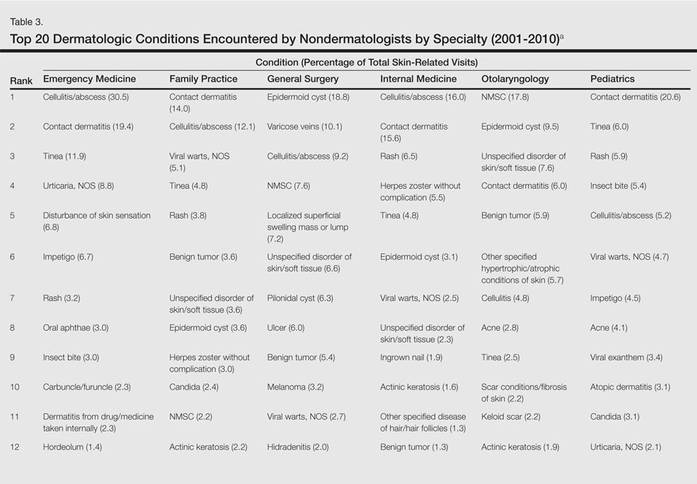
The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.
Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Practice Points
- Approximately half of skin-related visits are to nondermatologists, such as family medicine physicians, pediatricians, and internists.
- Skin conditions that most frequently present to nondermatologists are different from those seen by dermatologists.
- Education efforts in nondermatology specialties should be targeted toward the common skin diseases that present to these specialties to maximize the yield of medical education and improve diagnostic accuracy and patient outcomes.
Lower Transfusion Threshold for Sepsis Equals Fewer Transfusions, No Effect on Mortality
Clinical question
Does a lower transfusion threshold for critically ill patients with septic shock affect outcomes?
Bottom line
Using a lower threshold for transfusion for patients with septic shock in the intensive care unit (ICU) decreases the number of transfusions received without affecting mortality.
Reference
Study design
Randomized controlled trial (nonblinded); (LOE: 1b)
Setting
Inpatient (ICU only)
Synopsis
Using partial blinding and concealed allocation, these investigators randomized ICU patients with septic shock and a hemoglobin level of less than 9 g/dL to receive red blood cell transfusions at either a higher threshold (< 9 g/dL) or a lower threshold (< 7 g/dL). The intervention continued for the entire ICU stay, to a maximum of 90 days. The 2 groups were similar at baseline with an average age of 67 years and a median Sepsis-Related Organ Failure Assessment (SOFA) score of 10 out of 24. Analysis was by intention to treat. Not suprisingly, patients in the higher threshold group received twice as many transfusions as those in the lower threshold group (3088 transfusions vs 1545; P < .001). Notably, one third of the patients in the lower-threshold group required no transfusions at all compared with only 1% in the higher-threshold group (P < .001). For the primary outcome of death at 90 days, there was no significant difference detected between the 2 groups. The per-protocol analysis, which excluded patients with major protocol violations, also showed the same result. Secondary outcomes, including the use of life support and the number of ischemic events in the ICU (eg, acute myocardial or cerebral ischemia), were also similar in the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does a lower transfusion threshold for critically ill patients with septic shock affect outcomes?
Bottom line
Using a lower threshold for transfusion for patients with septic shock in the intensive care unit (ICU) decreases the number of transfusions received without affecting mortality.
Reference
Study design
Randomized controlled trial (nonblinded); (LOE: 1b)
Setting
Inpatient (ICU only)
Synopsis
Using partial blinding and concealed allocation, these investigators randomized ICU patients with septic shock and a hemoglobin level of less than 9 g/dL to receive red blood cell transfusions at either a higher threshold (< 9 g/dL) or a lower threshold (< 7 g/dL). The intervention continued for the entire ICU stay, to a maximum of 90 days. The 2 groups were similar at baseline with an average age of 67 years and a median Sepsis-Related Organ Failure Assessment (SOFA) score of 10 out of 24. Analysis was by intention to treat. Not suprisingly, patients in the higher threshold group received twice as many transfusions as those in the lower threshold group (3088 transfusions vs 1545; P < .001). Notably, one third of the patients in the lower-threshold group required no transfusions at all compared with only 1% in the higher-threshold group (P < .001). For the primary outcome of death at 90 days, there was no significant difference detected between the 2 groups. The per-protocol analysis, which excluded patients with major protocol violations, also showed the same result. Secondary outcomes, including the use of life support and the number of ischemic events in the ICU (eg, acute myocardial or cerebral ischemia), were also similar in the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does a lower transfusion threshold for critically ill patients with septic shock affect outcomes?
Bottom line
Using a lower threshold for transfusion for patients with septic shock in the intensive care unit (ICU) decreases the number of transfusions received without affecting mortality.
Reference
Study design
Randomized controlled trial (nonblinded); (LOE: 1b)
Setting
Inpatient (ICU only)
Synopsis
Using partial blinding and concealed allocation, these investigators randomized ICU patients with septic shock and a hemoglobin level of less than 9 g/dL to receive red blood cell transfusions at either a higher threshold (< 9 g/dL) or a lower threshold (< 7 g/dL). The intervention continued for the entire ICU stay, to a maximum of 90 days. The 2 groups were similar at baseline with an average age of 67 years and a median Sepsis-Related Organ Failure Assessment (SOFA) score of 10 out of 24. Analysis was by intention to treat. Not suprisingly, patients in the higher threshold group received twice as many transfusions as those in the lower threshold group (3088 transfusions vs 1545; P < .001). Notably, one third of the patients in the lower-threshold group required no transfusions at all compared with only 1% in the higher-threshold group (P < .001). For the primary outcome of death at 90 days, there was no significant difference detected between the 2 groups. The per-protocol analysis, which excluded patients with major protocol violations, also showed the same result. Secondary outcomes, including the use of life support and the number of ischemic events in the ICU (eg, acute myocardial or cerebral ischemia), were also similar in the 2 groups.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Targeted Therapy for Chronic Lymphocytic Leukemia
This presentation by Adrian Wiestner, MD, PhD, from the 2014 AVAHO Meeting in Portland, Oregon, provides an overview of new insights into the pathogenesis and treatment of CLL, how to interpret molecular targets during treatment, and the advantages and disadvantages of these treatment options for patients.
"The standard of care today is really chemo-immunotherapy," Wiestner said. "Ideally, we would like to have a more disease-directed therapy that is tolerable and active."
This presentation by Adrian Wiestner, MD, PhD, from the 2014 AVAHO Meeting in Portland, Oregon, provides an overview of new insights into the pathogenesis and treatment of CLL, how to interpret molecular targets during treatment, and the advantages and disadvantages of these treatment options for patients.
"The standard of care today is really chemo-immunotherapy," Wiestner said. "Ideally, we would like to have a more disease-directed therapy that is tolerable and active."
This presentation by Adrian Wiestner, MD, PhD, from the 2014 AVAHO Meeting in Portland, Oregon, provides an overview of new insights into the pathogenesis and treatment of CLL, how to interpret molecular targets during treatment, and the advantages and disadvantages of these treatment options for patients.
"The standard of care today is really chemo-immunotherapy," Wiestner said. "Ideally, we would like to have a more disease-directed therapy that is tolerable and active."
The Rural Surgeon: A new column
Surgeons who treat patients in rural areas are a unique group, distinct from other surgeons because of scope of practice, environment, and resources. These surgeons address a vast range of surgical problems, work in relative professional isolation, and have fewer resources available to get the job done. Nevertheless, these physicians play critical roles in their profession and are irreplaceable assets in their communities.
Although poorly defined, often misunderstood, arguably unfairly characterized, and inadequately documented, surgery has a long distinguished history and tradition in rural communities.
What are the main challenges faced by rural surgeons and what can be done to strengthen their practices, professional lives, and longevity in the field? Rural patients are more likely than urban patients to be elderly and poor and have chronic illnesses (J. Am Coll. Surg. 2014;219:814-8). The average age of rural surgeons suggests that many will retire in the coming decade. Who will replace them and care for 60 million citizens who reside in rural America? How will the gradual decline of residents choosing general surgery and rural practice affect patient care?
This new monthly column, Rural Surgeons Speak, focuses on these questions and searches for answers. Rural Surgeons Speak will introduce to all readers the voices, concerns, questions, and opinions of surgeons practicing in the small towns and rural regions of the United States. Although many of the challenges faced by rural surgeons are unique to this group, there is overlap with issues encountered by urban surgeons and those in academic settings. In spite of problems ranging from a shift from independent practice to hospital employment and to treating the poor with pronounced chronic illnesses, rural surgeons are dedicated and committed. Although these matters are common to all surgeons, rural surgeons’ experiences tend to be singular because of the high percentages of such patients and fewer institutional resources.
I have practiced surgery in small and large rural locations for 37 years. Rural surgery is not a specialty. My case log, filled with endoscopies, laparoscopies, laparotomies, and breast operations, may be even more varied than the logs of urban colleagues. In my early years of practice, I did orthopaedic surgery and gynecology. As a conscious choice, I practiced surgery where I wanted, and how I wanted. My choice was not by default. When I started, I sought a location that would allow me to “have a more complete life with less tension, excellent schools, opportunity for economical living,” and a fulfilling surgery practice. Unknown to me, Dr. Edwin F. Cave, ACS president, made those statements about rural surgery in the Daily Clinical Bulletin for the 37th Annual Clinical Congress decades before I started.
Like other rural surgeons, I wanted to embrace and be embraced by my community. I also saw opportunities for personal advancement (involvement with ACS), and for the development of my surgical talents (lasers, laparoscopies, endoscopies). I live just a mile from the hospital. I know my patients by their first names. They speak to me on the telephone. My number is in the book. When they meet me in church or at the grocery store, I am “Doc.” Occasionally a PEx is performed in such locations. It is personal. It is comfortable. There is a real sense of community and of belonging. I am involved. I provide an invaluable service “right at home,” where my patients want to be treated. In return, I am valued and supported.
After becoming a FACS in 1979, I involved myself in many activities of the ACS. Most recently, I participated in the rural surgery renaissance from its beginning. As part of my participation, I routinely contacted rural surgeons around the United States and gained intimate and detailed knowledge about their practices, successes, and concerns. I bring insights from my background to the writing of this column.
While glamour is at a minimum in rural America, personal satisfaction for many of these surgeons is at a maximum. Unfortunately, isolation is a given in most rural practices, and therein lies the problem. In spite of offering much to their patients and, in turn, to the profession, rural surgeons are easy targets of negative assessments, ill-conceived policy changes, and misunderstandings.
Despite their vital role in treating patients, rural surgeons often regard themselves as unrecognized and unappreciated by their peers. There is a subtle bias in the profession against a surgeon who would choose this life of relative isolation, alleged nonspecialized surgery, and overwork in communities with fewer cultural resources and fewer employment options for spouses. Yet their work is essential to the health of millions of people who live in rural areas. Thankfully, the profession as a whole has stopped ignoring rural surgery in recent years as the ACS leadership has stepped in and begun to increase support for rural surgeons.
In May 2011, during the 5th Annual Rural Surgery Symposium and Workshop, I presented a talk, “The ACS and the Rural Surgeon.” Past President Dr. LaMar McGinnis acknowledged the educational value of the presentation, which then went on to wider distribution. The Board of Regents received another talk about rural surgery in February 2012: “Us vs. Them.” Based on the talk and because of their keen insights, the Regents formed the Advisory Council on Rural Surgery – the first new advisory council in 50 years. Subsequently, a College-developed rural listserv became the most highly successful communications program in ACS history and led to the creation of the rural surgery community. In the following 2 years, rural surgeons exchanged 9 million emails on numerous, varied topics. An article in the July 2014 ACS Bulletin describes the program (http://bulletin.facs.org/2014/07/acs-rural-listserv-an-underdog-success-story/). Finally, in his inaugural address at the 2012 Clinical Congress, Dr. Brent Eastman identified rural surgery as one of the four fundamental components of surgery for the next 100 years (http://bulletin.facs.org/2012/12/presidential-address/). Recognition of rural surgery obviously has increased.
My experience of moderating the rural listserv with nearly 1,000 subscribers has revealed the amazing diversity, passion, commitment, and perseverance of rural surgeons who have united in a true community. Their perspectives on their professional lives and the lives of their patients are well worth closer acquaintance by surgeons from all sectors. They have a lot to teach us all. To learn about rural surgery will be to learn about all surgery. Understand rural surgery in 2014 and, consequently, understand all surgery. Recognize the benefits of rural surgery and then instill them in all practices. Supporting rural surgery today supports all surgery.
The column aims to offer commentary on diverse topics confronting rural surgeons today. Opinions, editorials, letters, economics, and clinical matters, all from the rural perspective, will be included. Over 60 individual “threads” appeared on the listserv this year and the subjects of many of these threads could be covered in future columns. Uncommon subjects such as YKYAARSI (“You know you are a rural surgeon if…”) should be entertaining and informative. Guest authors who are experts on varying subjects will contribute articles.
While its roots are in the rural surgery community, the column’s scope will touch all surgical practices. Rural surgery will be better defined, positively characterized, and documented. In partnership with the ACS to achieve the general goal of supporting rural surgery, Rural Surgeons Speak aims to enable all ACS Fellows to realize that they belong to a community of all surgeons, regardless of location or practice type.
Dr. Caropreso is a general surgeon at Keokuk (Iowa) Area Hospital and Clinical Professor of Surgery at the University of Iowa Carver College of Medicine. He has practiced surgery in the rural communities of Mason City, Iowa; Keokuk, Iowa; and Carthage, Ill., for 37 years.
Surgeons who treat patients in rural areas are a unique group, distinct from other surgeons because of scope of practice, environment, and resources. These surgeons address a vast range of surgical problems, work in relative professional isolation, and have fewer resources available to get the job done. Nevertheless, these physicians play critical roles in their profession and are irreplaceable assets in their communities.
Although poorly defined, often misunderstood, arguably unfairly characterized, and inadequately documented, surgery has a long distinguished history and tradition in rural communities.
What are the main challenges faced by rural surgeons and what can be done to strengthen their practices, professional lives, and longevity in the field? Rural patients are more likely than urban patients to be elderly and poor and have chronic illnesses (J. Am Coll. Surg. 2014;219:814-8). The average age of rural surgeons suggests that many will retire in the coming decade. Who will replace them and care for 60 million citizens who reside in rural America? How will the gradual decline of residents choosing general surgery and rural practice affect patient care?
This new monthly column, Rural Surgeons Speak, focuses on these questions and searches for answers. Rural Surgeons Speak will introduce to all readers the voices, concerns, questions, and opinions of surgeons practicing in the small towns and rural regions of the United States. Although many of the challenges faced by rural surgeons are unique to this group, there is overlap with issues encountered by urban surgeons and those in academic settings. In spite of problems ranging from a shift from independent practice to hospital employment and to treating the poor with pronounced chronic illnesses, rural surgeons are dedicated and committed. Although these matters are common to all surgeons, rural surgeons’ experiences tend to be singular because of the high percentages of such patients and fewer institutional resources.
I have practiced surgery in small and large rural locations for 37 years. Rural surgery is not a specialty. My case log, filled with endoscopies, laparoscopies, laparotomies, and breast operations, may be even more varied than the logs of urban colleagues. In my early years of practice, I did orthopaedic surgery and gynecology. As a conscious choice, I practiced surgery where I wanted, and how I wanted. My choice was not by default. When I started, I sought a location that would allow me to “have a more complete life with less tension, excellent schools, opportunity for economical living,” and a fulfilling surgery practice. Unknown to me, Dr. Edwin F. Cave, ACS president, made those statements about rural surgery in the Daily Clinical Bulletin for the 37th Annual Clinical Congress decades before I started.
Like other rural surgeons, I wanted to embrace and be embraced by my community. I also saw opportunities for personal advancement (involvement with ACS), and for the development of my surgical talents (lasers, laparoscopies, endoscopies). I live just a mile from the hospital. I know my patients by their first names. They speak to me on the telephone. My number is in the book. When they meet me in church or at the grocery store, I am “Doc.” Occasionally a PEx is performed in such locations. It is personal. It is comfortable. There is a real sense of community and of belonging. I am involved. I provide an invaluable service “right at home,” where my patients want to be treated. In return, I am valued and supported.
After becoming a FACS in 1979, I involved myself in many activities of the ACS. Most recently, I participated in the rural surgery renaissance from its beginning. As part of my participation, I routinely contacted rural surgeons around the United States and gained intimate and detailed knowledge about their practices, successes, and concerns. I bring insights from my background to the writing of this column.
While glamour is at a minimum in rural America, personal satisfaction for many of these surgeons is at a maximum. Unfortunately, isolation is a given in most rural practices, and therein lies the problem. In spite of offering much to their patients and, in turn, to the profession, rural surgeons are easy targets of negative assessments, ill-conceived policy changes, and misunderstandings.
Despite their vital role in treating patients, rural surgeons often regard themselves as unrecognized and unappreciated by their peers. There is a subtle bias in the profession against a surgeon who would choose this life of relative isolation, alleged nonspecialized surgery, and overwork in communities with fewer cultural resources and fewer employment options for spouses. Yet their work is essential to the health of millions of people who live in rural areas. Thankfully, the profession as a whole has stopped ignoring rural surgery in recent years as the ACS leadership has stepped in and begun to increase support for rural surgeons.
In May 2011, during the 5th Annual Rural Surgery Symposium and Workshop, I presented a talk, “The ACS and the Rural Surgeon.” Past President Dr. LaMar McGinnis acknowledged the educational value of the presentation, which then went on to wider distribution. The Board of Regents received another talk about rural surgery in February 2012: “Us vs. Them.” Based on the talk and because of their keen insights, the Regents formed the Advisory Council on Rural Surgery – the first new advisory council in 50 years. Subsequently, a College-developed rural listserv became the most highly successful communications program in ACS history and led to the creation of the rural surgery community. In the following 2 years, rural surgeons exchanged 9 million emails on numerous, varied topics. An article in the July 2014 ACS Bulletin describes the program (http://bulletin.facs.org/2014/07/acs-rural-listserv-an-underdog-success-story/). Finally, in his inaugural address at the 2012 Clinical Congress, Dr. Brent Eastman identified rural surgery as one of the four fundamental components of surgery for the next 100 years (http://bulletin.facs.org/2012/12/presidential-address/). Recognition of rural surgery obviously has increased.
My experience of moderating the rural listserv with nearly 1,000 subscribers has revealed the amazing diversity, passion, commitment, and perseverance of rural surgeons who have united in a true community. Their perspectives on their professional lives and the lives of their patients are well worth closer acquaintance by surgeons from all sectors. They have a lot to teach us all. To learn about rural surgery will be to learn about all surgery. Understand rural surgery in 2014 and, consequently, understand all surgery. Recognize the benefits of rural surgery and then instill them in all practices. Supporting rural surgery today supports all surgery.
The column aims to offer commentary on diverse topics confronting rural surgeons today. Opinions, editorials, letters, economics, and clinical matters, all from the rural perspective, will be included. Over 60 individual “threads” appeared on the listserv this year and the subjects of many of these threads could be covered in future columns. Uncommon subjects such as YKYAARSI (“You know you are a rural surgeon if…”) should be entertaining and informative. Guest authors who are experts on varying subjects will contribute articles.
While its roots are in the rural surgery community, the column’s scope will touch all surgical practices. Rural surgery will be better defined, positively characterized, and documented. In partnership with the ACS to achieve the general goal of supporting rural surgery, Rural Surgeons Speak aims to enable all ACS Fellows to realize that they belong to a community of all surgeons, regardless of location or practice type.
Dr. Caropreso is a general surgeon at Keokuk (Iowa) Area Hospital and Clinical Professor of Surgery at the University of Iowa Carver College of Medicine. He has practiced surgery in the rural communities of Mason City, Iowa; Keokuk, Iowa; and Carthage, Ill., for 37 years.
Surgeons who treat patients in rural areas are a unique group, distinct from other surgeons because of scope of practice, environment, and resources. These surgeons address a vast range of surgical problems, work in relative professional isolation, and have fewer resources available to get the job done. Nevertheless, these physicians play critical roles in their profession and are irreplaceable assets in their communities.
Although poorly defined, often misunderstood, arguably unfairly characterized, and inadequately documented, surgery has a long distinguished history and tradition in rural communities.
What are the main challenges faced by rural surgeons and what can be done to strengthen their practices, professional lives, and longevity in the field? Rural patients are more likely than urban patients to be elderly and poor and have chronic illnesses (J. Am Coll. Surg. 2014;219:814-8). The average age of rural surgeons suggests that many will retire in the coming decade. Who will replace them and care for 60 million citizens who reside in rural America? How will the gradual decline of residents choosing general surgery and rural practice affect patient care?
This new monthly column, Rural Surgeons Speak, focuses on these questions and searches for answers. Rural Surgeons Speak will introduce to all readers the voices, concerns, questions, and opinions of surgeons practicing in the small towns and rural regions of the United States. Although many of the challenges faced by rural surgeons are unique to this group, there is overlap with issues encountered by urban surgeons and those in academic settings. In spite of problems ranging from a shift from independent practice to hospital employment and to treating the poor with pronounced chronic illnesses, rural surgeons are dedicated and committed. Although these matters are common to all surgeons, rural surgeons’ experiences tend to be singular because of the high percentages of such patients and fewer institutional resources.
I have practiced surgery in small and large rural locations for 37 years. Rural surgery is not a specialty. My case log, filled with endoscopies, laparoscopies, laparotomies, and breast operations, may be even more varied than the logs of urban colleagues. In my early years of practice, I did orthopaedic surgery and gynecology. As a conscious choice, I practiced surgery where I wanted, and how I wanted. My choice was not by default. When I started, I sought a location that would allow me to “have a more complete life with less tension, excellent schools, opportunity for economical living,” and a fulfilling surgery practice. Unknown to me, Dr. Edwin F. Cave, ACS president, made those statements about rural surgery in the Daily Clinical Bulletin for the 37th Annual Clinical Congress decades before I started.
Like other rural surgeons, I wanted to embrace and be embraced by my community. I also saw opportunities for personal advancement (involvement with ACS), and for the development of my surgical talents (lasers, laparoscopies, endoscopies). I live just a mile from the hospital. I know my patients by their first names. They speak to me on the telephone. My number is in the book. When they meet me in church or at the grocery store, I am “Doc.” Occasionally a PEx is performed in such locations. It is personal. It is comfortable. There is a real sense of community and of belonging. I am involved. I provide an invaluable service “right at home,” where my patients want to be treated. In return, I am valued and supported.
After becoming a FACS in 1979, I involved myself in many activities of the ACS. Most recently, I participated in the rural surgery renaissance from its beginning. As part of my participation, I routinely contacted rural surgeons around the United States and gained intimate and detailed knowledge about their practices, successes, and concerns. I bring insights from my background to the writing of this column.
While glamour is at a minimum in rural America, personal satisfaction for many of these surgeons is at a maximum. Unfortunately, isolation is a given in most rural practices, and therein lies the problem. In spite of offering much to their patients and, in turn, to the profession, rural surgeons are easy targets of negative assessments, ill-conceived policy changes, and misunderstandings.
Despite their vital role in treating patients, rural surgeons often regard themselves as unrecognized and unappreciated by their peers. There is a subtle bias in the profession against a surgeon who would choose this life of relative isolation, alleged nonspecialized surgery, and overwork in communities with fewer cultural resources and fewer employment options for spouses. Yet their work is essential to the health of millions of people who live in rural areas. Thankfully, the profession as a whole has stopped ignoring rural surgery in recent years as the ACS leadership has stepped in and begun to increase support for rural surgeons.
In May 2011, during the 5th Annual Rural Surgery Symposium and Workshop, I presented a talk, “The ACS and the Rural Surgeon.” Past President Dr. LaMar McGinnis acknowledged the educational value of the presentation, which then went on to wider distribution. The Board of Regents received another talk about rural surgery in February 2012: “Us vs. Them.” Based on the talk and because of their keen insights, the Regents formed the Advisory Council on Rural Surgery – the first new advisory council in 50 years. Subsequently, a College-developed rural listserv became the most highly successful communications program in ACS history and led to the creation of the rural surgery community. In the following 2 years, rural surgeons exchanged 9 million emails on numerous, varied topics. An article in the July 2014 ACS Bulletin describes the program (http://bulletin.facs.org/2014/07/acs-rural-listserv-an-underdog-success-story/). Finally, in his inaugural address at the 2012 Clinical Congress, Dr. Brent Eastman identified rural surgery as one of the four fundamental components of surgery for the next 100 years (http://bulletin.facs.org/2012/12/presidential-address/). Recognition of rural surgery obviously has increased.
My experience of moderating the rural listserv with nearly 1,000 subscribers has revealed the amazing diversity, passion, commitment, and perseverance of rural surgeons who have united in a true community. Their perspectives on their professional lives and the lives of their patients are well worth closer acquaintance by surgeons from all sectors. They have a lot to teach us all. To learn about rural surgery will be to learn about all surgery. Understand rural surgery in 2014 and, consequently, understand all surgery. Recognize the benefits of rural surgery and then instill them in all practices. Supporting rural surgery today supports all surgery.
The column aims to offer commentary on diverse topics confronting rural surgeons today. Opinions, editorials, letters, economics, and clinical matters, all from the rural perspective, will be included. Over 60 individual “threads” appeared on the listserv this year and the subjects of many of these threads could be covered in future columns. Uncommon subjects such as YKYAARSI (“You know you are a rural surgeon if…”) should be entertaining and informative. Guest authors who are experts on varying subjects will contribute articles.
While its roots are in the rural surgery community, the column’s scope will touch all surgical practices. Rural surgery will be better defined, positively characterized, and documented. In partnership with the ACS to achieve the general goal of supporting rural surgery, Rural Surgeons Speak aims to enable all ACS Fellows to realize that they belong to a community of all surgeons, regardless of location or practice type.
Dr. Caropreso is a general surgeon at Keokuk (Iowa) Area Hospital and Clinical Professor of Surgery at the University of Iowa Carver College of Medicine. He has practiced surgery in the rural communities of Mason City, Iowa; Keokuk, Iowa; and Carthage, Ill., for 37 years.
More isn’t always better with daunorubicin induction in AML

Photo courtesy of ASH
SAN FRANCISCO—Results regarding daunorubicin escalation in induction for patients with acute myeloid leukemia (AML) have varied among different studies.
And a 90 mg/m2 dose has been shown to be more effective than 45. Now, results of the UK NCRI AML17 trial have added yet another dimension to the discussion—the use of 60 mg/m2 compared to 90.
Alan K. Burnett, MD, of Cardiff University in the UK, presented the data as abstract 7 at the 2014 ASH Annual Meeting.
He explained that ECOG E1900 had demonstrated superior remission and overall survival for a 90-mg dose of daunorubicin compared to a 45-mg dose in adults younger than 60.
The HOVON trial showed superior remission but no difference in overall survival for the higher dose of daunorubicin in patients older than 60.
The Korean trial demonstrated superior remission and survival rates for the 90-mg dose in patients younger than 60.
And in the GOELAMS trial, investigators found no difference between a 90-mg and a 60-mg dose level.
So the UK AML Study Group undertook to clarify the issue by comparing 90 mg to 60 mg in induction.
The investigators randomized 1206 patients younger than 60 with de novo or secondary AML or high-risk myelodysplastic syndromes (MDS) to receive 90 or 60 mg of daunorubicin on days 1, 3, and 5 of their first induction course, followed by 50 mg/m2 on days 1, 3, and 5 in course 2.
All patients received ara-C during courses 1 and 2. Patients had to have LVEF of 45% or greater to be included in the trial.
The median follow-up was 29 months. Patient characteristics were comparable between the 2 groups.
The median age was 53 in both groups (range, 16-72 years), and slightly more than half of patients were male. Eighty-five percent and 84% had de novo AML in the 60-mg and 90-mg arms, respectively. Ten percent in each group had secondary AML. And 5% and 6%, respectively, had high-risk MDS.
Eleven percent in the 60-mg arm had favorable cytogenetics, compared with 9% in the 90-mg arm. Eighteen percent in each arm had mutant FLT3-ITD, and 40% in each arm were poor risk.
The investigators found no significant difference in response between the two arms—84% in the 60-mg arm and 81% in the 90-mg arm.
However, they observed a trend for increased 30-day mortality in the 90-mg arm and a significant difference in the 60-day mortality rate, which was 5% in the 60-mg arm and 10% in the 90-mg arm (P=0.001).
Twenty-nine people died by day 60 in the 60-mg arm compared with 58 in the 90-mg arm.
The main reasons for 60-day mortality in the 60-mg and 90-mg dose groups, respectively, included infection (11 vs 25 deaths), hemorrhage (3 vs 5 deaths), infection plus hemorrhage (3 vs 1 death), and resistant disease (2 vs 14 deaths), among other causes.
At 24 months, overall survival between the 2 groups was comparable, at 60% in the 60-mg arm and 59% in the 90-mg arm.
The cumulative incidence of relapse at 24 months from complete response was 41% in the 60-mg arm and 37% in the 90-mg arm.
One hundred ninety-seven patients in the 60-mg arm and 169 patients in the 90-mg arm went on to receive a stem cell transplant.
When survival was censored at transplant, there was also no difference between the arms, at 60% for the 90-mg group and 61% for the 60-mg group.
The investigators conducted subgroup analyses and found no significant benefit for 90 mg/m2 in any subgroup. Dr Burnett noted, however, that there could be a potential late benefit for FLT3-ITD mutated patients who receive a 90-mg dose.
FLT3 mutated patients had a non-significant survival benefit (HR 0.74 [0.47-1.17] P=0.2) with a 90-mg dose. However, survival was significantly worse for FLT3 wild type patients receiving 90 mg (HR 1.31 [1.03-1.67] P=0.03).
Otherwise, the group found that there is no evidence to suggest that 90 mg is superior to 60 mg. ![]()

Photo courtesy of ASH
SAN FRANCISCO—Results regarding daunorubicin escalation in induction for patients with acute myeloid leukemia (AML) have varied among different studies.
And a 90 mg/m2 dose has been shown to be more effective than 45. Now, results of the UK NCRI AML17 trial have added yet another dimension to the discussion—the use of 60 mg/m2 compared to 90.
Alan K. Burnett, MD, of Cardiff University in the UK, presented the data as abstract 7 at the 2014 ASH Annual Meeting.
He explained that ECOG E1900 had demonstrated superior remission and overall survival for a 90-mg dose of daunorubicin compared to a 45-mg dose in adults younger than 60.
The HOVON trial showed superior remission but no difference in overall survival for the higher dose of daunorubicin in patients older than 60.
The Korean trial demonstrated superior remission and survival rates for the 90-mg dose in patients younger than 60.
And in the GOELAMS trial, investigators found no difference between a 90-mg and a 60-mg dose level.
So the UK AML Study Group undertook to clarify the issue by comparing 90 mg to 60 mg in induction.
The investigators randomized 1206 patients younger than 60 with de novo or secondary AML or high-risk myelodysplastic syndromes (MDS) to receive 90 or 60 mg of daunorubicin on days 1, 3, and 5 of their first induction course, followed by 50 mg/m2 on days 1, 3, and 5 in course 2.
All patients received ara-C during courses 1 and 2. Patients had to have LVEF of 45% or greater to be included in the trial.
The median follow-up was 29 months. Patient characteristics were comparable between the 2 groups.
The median age was 53 in both groups (range, 16-72 years), and slightly more than half of patients were male. Eighty-five percent and 84% had de novo AML in the 60-mg and 90-mg arms, respectively. Ten percent in each group had secondary AML. And 5% and 6%, respectively, had high-risk MDS.
Eleven percent in the 60-mg arm had favorable cytogenetics, compared with 9% in the 90-mg arm. Eighteen percent in each arm had mutant FLT3-ITD, and 40% in each arm were poor risk.
The investigators found no significant difference in response between the two arms—84% in the 60-mg arm and 81% in the 90-mg arm.
However, they observed a trend for increased 30-day mortality in the 90-mg arm and a significant difference in the 60-day mortality rate, which was 5% in the 60-mg arm and 10% in the 90-mg arm (P=0.001).
Twenty-nine people died by day 60 in the 60-mg arm compared with 58 in the 90-mg arm.
The main reasons for 60-day mortality in the 60-mg and 90-mg dose groups, respectively, included infection (11 vs 25 deaths), hemorrhage (3 vs 5 deaths), infection plus hemorrhage (3 vs 1 death), and resistant disease (2 vs 14 deaths), among other causes.
At 24 months, overall survival between the 2 groups was comparable, at 60% in the 60-mg arm and 59% in the 90-mg arm.
The cumulative incidence of relapse at 24 months from complete response was 41% in the 60-mg arm and 37% in the 90-mg arm.
One hundred ninety-seven patients in the 60-mg arm and 169 patients in the 90-mg arm went on to receive a stem cell transplant.
When survival was censored at transplant, there was also no difference between the arms, at 60% for the 90-mg group and 61% for the 60-mg group.
The investigators conducted subgroup analyses and found no significant benefit for 90 mg/m2 in any subgroup. Dr Burnett noted, however, that there could be a potential late benefit for FLT3-ITD mutated patients who receive a 90-mg dose.
FLT3 mutated patients had a non-significant survival benefit (HR 0.74 [0.47-1.17] P=0.2) with a 90-mg dose. However, survival was significantly worse for FLT3 wild type patients receiving 90 mg (HR 1.31 [1.03-1.67] P=0.03).
Otherwise, the group found that there is no evidence to suggest that 90 mg is superior to 60 mg. ![]()

Photo courtesy of ASH
SAN FRANCISCO—Results regarding daunorubicin escalation in induction for patients with acute myeloid leukemia (AML) have varied among different studies.
And a 90 mg/m2 dose has been shown to be more effective than 45. Now, results of the UK NCRI AML17 trial have added yet another dimension to the discussion—the use of 60 mg/m2 compared to 90.
Alan K. Burnett, MD, of Cardiff University in the UK, presented the data as abstract 7 at the 2014 ASH Annual Meeting.
He explained that ECOG E1900 had demonstrated superior remission and overall survival for a 90-mg dose of daunorubicin compared to a 45-mg dose in adults younger than 60.
The HOVON trial showed superior remission but no difference in overall survival for the higher dose of daunorubicin in patients older than 60.
The Korean trial demonstrated superior remission and survival rates for the 90-mg dose in patients younger than 60.
And in the GOELAMS trial, investigators found no difference between a 90-mg and a 60-mg dose level.
So the UK AML Study Group undertook to clarify the issue by comparing 90 mg to 60 mg in induction.
The investigators randomized 1206 patients younger than 60 with de novo or secondary AML or high-risk myelodysplastic syndromes (MDS) to receive 90 or 60 mg of daunorubicin on days 1, 3, and 5 of their first induction course, followed by 50 mg/m2 on days 1, 3, and 5 in course 2.
All patients received ara-C during courses 1 and 2. Patients had to have LVEF of 45% or greater to be included in the trial.
The median follow-up was 29 months. Patient characteristics were comparable between the 2 groups.
The median age was 53 in both groups (range, 16-72 years), and slightly more than half of patients were male. Eighty-five percent and 84% had de novo AML in the 60-mg and 90-mg arms, respectively. Ten percent in each group had secondary AML. And 5% and 6%, respectively, had high-risk MDS.
Eleven percent in the 60-mg arm had favorable cytogenetics, compared with 9% in the 90-mg arm. Eighteen percent in each arm had mutant FLT3-ITD, and 40% in each arm were poor risk.
The investigators found no significant difference in response between the two arms—84% in the 60-mg arm and 81% in the 90-mg arm.
However, they observed a trend for increased 30-day mortality in the 90-mg arm and a significant difference in the 60-day mortality rate, which was 5% in the 60-mg arm and 10% in the 90-mg arm (P=0.001).
Twenty-nine people died by day 60 in the 60-mg arm compared with 58 in the 90-mg arm.
The main reasons for 60-day mortality in the 60-mg and 90-mg dose groups, respectively, included infection (11 vs 25 deaths), hemorrhage (3 vs 5 deaths), infection plus hemorrhage (3 vs 1 death), and resistant disease (2 vs 14 deaths), among other causes.
At 24 months, overall survival between the 2 groups was comparable, at 60% in the 60-mg arm and 59% in the 90-mg arm.
The cumulative incidence of relapse at 24 months from complete response was 41% in the 60-mg arm and 37% in the 90-mg arm.
One hundred ninety-seven patients in the 60-mg arm and 169 patients in the 90-mg arm went on to receive a stem cell transplant.
When survival was censored at transplant, there was also no difference between the arms, at 60% for the 90-mg group and 61% for the 60-mg group.
The investigators conducted subgroup analyses and found no significant benefit for 90 mg/m2 in any subgroup. Dr Burnett noted, however, that there could be a potential late benefit for FLT3-ITD mutated patients who receive a 90-mg dose.
FLT3 mutated patients had a non-significant survival benefit (HR 0.74 [0.47-1.17] P=0.2) with a 90-mg dose. However, survival was significantly worse for FLT3 wild type patients receiving 90 mg (HR 1.31 [1.03-1.67] P=0.03).
Otherwise, the group found that there is no evidence to suggest that 90 mg is superior to 60 mg. ![]()
Encapsulating doxorubicin can reduce heart damage

Credit: USDA
VIENNA—Encapsulating the anthracycline doxorubicin in a liposome can reduce the risk of developing heart damage, according to a study presented at EuroEcho-Imaging 2014.
Researchers administered doxorubicin encased in a liposome to a small group of pigs and compared cardiac outcomes to those in pigs that received unmanipulated doxorubicin or epirubicin.
Pigs that received encapsulated doxorubicin still developed cardiotoxicity, but at lower rates than pigs that received traditional doxorubicin.
Pigs that received epirubicin were excluded due to low survival rates.
“[M]any chemotherapies—in particular, anthracyclines—cause cardiac side effects that can lead to cardiomyopathy and severe heart failure,” said study investigator Jutta Bergler-Klein, MD, of the Medical University of Vienna in Austria. “Cardiotoxicity can occur acutely or up to 30 years after chemotherapy and is the second most common cause of death in cancer patients, after secondary malignancy in childhood cancer survivors.”
“Liposomal encapsulation is a new technique which wraps the chemotherapy drug in a fatty cover called a liposome. More of the drug reaches the cancer cells because there is less degradation, and there are fewer side effects on healthy cells because the fat cover acts as a barrier.”
“The drug stays in the bloodstream longer, allowing higher cumulative doses to be given. We tested whether non-pegylated liposome encapsulation of the anthracycline doxorubicin (called Myocet) could decrease its cardiotoxicity compared to conventional doxorubicin or epirubicin, another anthracycline.”
The study included 24 pigs that were randomized to receive the human dose-equivalent of Myocet, conventional doxorubicin, or epirubicin in 3 cycles. The epirubicin group was excluded from the final analyses because of low survival levels.
The researchers assessed cardiac function by echocardiography and MRI at baseline and follow-up (after about 3 months). Laboratory follow-up included hematology, renal function, and measurement of the cardiac enzymes troponin and BNP.
“The dose, imaging methodology, and blood parameters simulate the monitoring that patients on this treatment would receive and produces valuable translational data,” Dr Bergler-Klein said.
The researchers found that the group receiving Myocet had better diastolic and systolic function in the left and right ventricles, compared to conventional doxorubicin. The Myocet group also had less fibrosis in the myocardium, as shown by MRI and histology staining.
“Our study shows that doxorubicin encapsulated in a liposome had fewer cardiac side effects than doxorubicin given in the conventional way,” Dr Bergler-Klein said.
“We did find cardiac toxicity in the Myocet group as well, despite the fact that the pigs were young, healthy, and received anthracyclines for only a short period. This emphasizes how important it is for all cancer patients taking anthracyclines to receive cardiac monitoring using echocardiography and biomarkers, and MRI where indicated.”
“Many patients who recover after chemotherapy have asymptomatic heart damage, which can become symptomatic as they get older. When heart problems are picked up early, patients can be given preventive treatment, including ACE inhibitors, angiotensin receptor blockers, or beta-blockers, to prevent the progression to overt heart failure.”
The researchers are now conducting gene-expression profiling on the histology samples, hoping to explain the better outcome and cardiac function after Myocet therapy. They have found differences in the expression of genes that control energy use and the metabolic state, with better regulation in the Myocet group. ![]()

Credit: USDA
VIENNA—Encapsulating the anthracycline doxorubicin in a liposome can reduce the risk of developing heart damage, according to a study presented at EuroEcho-Imaging 2014.
Researchers administered doxorubicin encased in a liposome to a small group of pigs and compared cardiac outcomes to those in pigs that received unmanipulated doxorubicin or epirubicin.
Pigs that received encapsulated doxorubicin still developed cardiotoxicity, but at lower rates than pigs that received traditional doxorubicin.
Pigs that received epirubicin were excluded due to low survival rates.
“[M]any chemotherapies—in particular, anthracyclines—cause cardiac side effects that can lead to cardiomyopathy and severe heart failure,” said study investigator Jutta Bergler-Klein, MD, of the Medical University of Vienna in Austria. “Cardiotoxicity can occur acutely or up to 30 years after chemotherapy and is the second most common cause of death in cancer patients, after secondary malignancy in childhood cancer survivors.”
“Liposomal encapsulation is a new technique which wraps the chemotherapy drug in a fatty cover called a liposome. More of the drug reaches the cancer cells because there is less degradation, and there are fewer side effects on healthy cells because the fat cover acts as a barrier.”
“The drug stays in the bloodstream longer, allowing higher cumulative doses to be given. We tested whether non-pegylated liposome encapsulation of the anthracycline doxorubicin (called Myocet) could decrease its cardiotoxicity compared to conventional doxorubicin or epirubicin, another anthracycline.”
The study included 24 pigs that were randomized to receive the human dose-equivalent of Myocet, conventional doxorubicin, or epirubicin in 3 cycles. The epirubicin group was excluded from the final analyses because of low survival levels.
The researchers assessed cardiac function by echocardiography and MRI at baseline and follow-up (after about 3 months). Laboratory follow-up included hematology, renal function, and measurement of the cardiac enzymes troponin and BNP.
“The dose, imaging methodology, and blood parameters simulate the monitoring that patients on this treatment would receive and produces valuable translational data,” Dr Bergler-Klein said.
The researchers found that the group receiving Myocet had better diastolic and systolic function in the left and right ventricles, compared to conventional doxorubicin. The Myocet group also had less fibrosis in the myocardium, as shown by MRI and histology staining.
“Our study shows that doxorubicin encapsulated in a liposome had fewer cardiac side effects than doxorubicin given in the conventional way,” Dr Bergler-Klein said.
“We did find cardiac toxicity in the Myocet group as well, despite the fact that the pigs were young, healthy, and received anthracyclines for only a short period. This emphasizes how important it is for all cancer patients taking anthracyclines to receive cardiac monitoring using echocardiography and biomarkers, and MRI where indicated.”
“Many patients who recover after chemotherapy have asymptomatic heart damage, which can become symptomatic as they get older. When heart problems are picked up early, patients can be given preventive treatment, including ACE inhibitors, angiotensin receptor blockers, or beta-blockers, to prevent the progression to overt heart failure.”
The researchers are now conducting gene-expression profiling on the histology samples, hoping to explain the better outcome and cardiac function after Myocet therapy. They have found differences in the expression of genes that control energy use and the metabolic state, with better regulation in the Myocet group. ![]()

Credit: USDA
VIENNA—Encapsulating the anthracycline doxorubicin in a liposome can reduce the risk of developing heart damage, according to a study presented at EuroEcho-Imaging 2014.
Researchers administered doxorubicin encased in a liposome to a small group of pigs and compared cardiac outcomes to those in pigs that received unmanipulated doxorubicin or epirubicin.
Pigs that received encapsulated doxorubicin still developed cardiotoxicity, but at lower rates than pigs that received traditional doxorubicin.
Pigs that received epirubicin were excluded due to low survival rates.
“[M]any chemotherapies—in particular, anthracyclines—cause cardiac side effects that can lead to cardiomyopathy and severe heart failure,” said study investigator Jutta Bergler-Klein, MD, of the Medical University of Vienna in Austria. “Cardiotoxicity can occur acutely or up to 30 years after chemotherapy and is the second most common cause of death in cancer patients, after secondary malignancy in childhood cancer survivors.”
“Liposomal encapsulation is a new technique which wraps the chemotherapy drug in a fatty cover called a liposome. More of the drug reaches the cancer cells because there is less degradation, and there are fewer side effects on healthy cells because the fat cover acts as a barrier.”
“The drug stays in the bloodstream longer, allowing higher cumulative doses to be given. We tested whether non-pegylated liposome encapsulation of the anthracycline doxorubicin (called Myocet) could decrease its cardiotoxicity compared to conventional doxorubicin or epirubicin, another anthracycline.”
The study included 24 pigs that were randomized to receive the human dose-equivalent of Myocet, conventional doxorubicin, or epirubicin in 3 cycles. The epirubicin group was excluded from the final analyses because of low survival levels.
The researchers assessed cardiac function by echocardiography and MRI at baseline and follow-up (after about 3 months). Laboratory follow-up included hematology, renal function, and measurement of the cardiac enzymes troponin and BNP.
“The dose, imaging methodology, and blood parameters simulate the monitoring that patients on this treatment would receive and produces valuable translational data,” Dr Bergler-Klein said.
The researchers found that the group receiving Myocet had better diastolic and systolic function in the left and right ventricles, compared to conventional doxorubicin. The Myocet group also had less fibrosis in the myocardium, as shown by MRI and histology staining.
“Our study shows that doxorubicin encapsulated in a liposome had fewer cardiac side effects than doxorubicin given in the conventional way,” Dr Bergler-Klein said.
“We did find cardiac toxicity in the Myocet group as well, despite the fact that the pigs were young, healthy, and received anthracyclines for only a short period. This emphasizes how important it is for all cancer patients taking anthracyclines to receive cardiac monitoring using echocardiography and biomarkers, and MRI where indicated.”
“Many patients who recover after chemotherapy have asymptomatic heart damage, which can become symptomatic as they get older. When heart problems are picked up early, patients can be given preventive treatment, including ACE inhibitors, angiotensin receptor blockers, or beta-blockers, to prevent the progression to overt heart failure.”
The researchers are now conducting gene-expression profiling on the histology samples, hoping to explain the better outcome and cardiac function after Myocet therapy. They have found differences in the expression of genes that control energy use and the metabolic state, with better regulation in the Myocet group. ![]()
The Best Times to Try Abiraterone
Until recently, there have been few treatment options for advanced prostate cancer that is resistant to androgen-directed therapies. Newer treatments that target residual androgen production offer some hope of prolonging the interval before chemotherapy, with fewer adverse effects (AEs) and better efficacy. One of those is abiraterone, which blocks extragonadal, testicular, and tumor androgen biosynthesis.
An ongoing multinational phase 3 study is evaluating the clinical benefits of abiraterone plus prednisone vs prednisone alone in patients with progressive metastatic castration-resistant prostate cancer (mCRPC). Follow-up for the study has now exceeded 27 months, giving a good opportunity to evaluate safety and efficacy. Thus, after having reviewed outcomes so far, the independent data-monitoring committee recommended that the study be unblinded and patients be allowed to cross over from prednisone to abiraterone. The researchers reported the results of the third interim analysis, with updated analysis.
Patients were stratified by Eastern Cooperative Oncology Group performance status (ECOG-PS) and randomly assigned to receive abiraterone 1,000 mg plus prednisone 5 mg twice daily or placebo plus prednisone.
Patients who received abiraterone had, compared with those on prednisone, statistically significant improvement in radiographic progression-free survival (PFS), with a median time to disease progression or death of 16.5 months, vs 8.2 months (95% CI, 0.45-0.61).
Overall survival also lengthened, from a median of 35.3 months vs 30.1 months (95% CI, 0.66-0.95).
All secondary endpoints also favored abiraterone over prednisone. For instance, abiraterone treatment delayed the time to the need for opiates for cancer-related pain and the time to initiation of chemotherapy. Abiraterone also delayed the time to deterioration in ECOG-PS and prostate-specific antigen (PSA) progression. Abiraterone more than doubled the PSA response rate: 68% vs 29% with prednisone.
Patients reported more pain relief. Those receiving abiraterone had statistically significant improvement in pain interference (P = .005), although the improvement in mean pain intensity was not significant.
Adverse effects leading to dose modifications or interruption of treatment were reported in 21% of patients on abiraterone, compared with 12% of the prednisone group. Six patients (1%) in each group died of drug-related treatment-emergent AEs. The AEs of “special interest,” the researchers say, included events related to mineralocorticoid excess, such as hypertension, hypokalemia, and fluid retention—all unsurprising, given the known mechanism of action of abiraterone. Grade 3 or 4 AEs with increased alanine aminotransferase and aspartate aminotransferase were more common in the abiraterone group.
The most common subsequent therapy for patients who terminated the study was docetaxel. However, another recent study, from Johns Hopkins researchers in Baltimore, Maryland, indicates the transition warrants caution: The findings suggest a potential cross-resistance between docetaxel and abiraterone.
Their study compared outcomes in 24 men who received abiraterone before docetaxel with 95 who were abiraterone-naïve. Men who were on abiraterone were less likely to achieve a PSA response, and their cancer was more likely to progress.
The researchers concede that their study groups were small; they also say it is possible that differences in disease severity may have influenced the time to progression. However, they say the fact that PSA-PFS was significantly different between the 2 groups (P = .002) supports their initial hypothesis—that is, that abiraterone pretreatment reduces responsiveness to docetaxel.
In spite of its limitations, the researchers say their study represents the only comparative analysis of PSA-PFS and PFS after docetaxel treatment for patients who have or have not received prior abiraterone. Their report, they add, offers the “strongest available evidence to date” of a clinically meaningful cross-resistance between abiraterone and docetaxel. They conclude that their findings provide “valuable information” about which patients are likely to derive the most benefit from docetaxel.
Sources
Rathkopf DE, Smith MR, de Bono JS, et al. Eur Urol. 2014;66(5):815-825.
doi: 10.1016/j.eururo.2014.02.056.
Schewizer MT, Zhou XC, Wang H, et al. Eur Urol. 2014;66(4):646-652.
doi: 10.1016/j.eururo.2014.01.018.
Until recently, there have been few treatment options for advanced prostate cancer that is resistant to androgen-directed therapies. Newer treatments that target residual androgen production offer some hope of prolonging the interval before chemotherapy, with fewer adverse effects (AEs) and better efficacy. One of those is abiraterone, which blocks extragonadal, testicular, and tumor androgen biosynthesis.
An ongoing multinational phase 3 study is evaluating the clinical benefits of abiraterone plus prednisone vs prednisone alone in patients with progressive metastatic castration-resistant prostate cancer (mCRPC). Follow-up for the study has now exceeded 27 months, giving a good opportunity to evaluate safety and efficacy. Thus, after having reviewed outcomes so far, the independent data-monitoring committee recommended that the study be unblinded and patients be allowed to cross over from prednisone to abiraterone. The researchers reported the results of the third interim analysis, with updated analysis.
Patients were stratified by Eastern Cooperative Oncology Group performance status (ECOG-PS) and randomly assigned to receive abiraterone 1,000 mg plus prednisone 5 mg twice daily or placebo plus prednisone.
Patients who received abiraterone had, compared with those on prednisone, statistically significant improvement in radiographic progression-free survival (PFS), with a median time to disease progression or death of 16.5 months, vs 8.2 months (95% CI, 0.45-0.61).
Overall survival also lengthened, from a median of 35.3 months vs 30.1 months (95% CI, 0.66-0.95).
All secondary endpoints also favored abiraterone over prednisone. For instance, abiraterone treatment delayed the time to the need for opiates for cancer-related pain and the time to initiation of chemotherapy. Abiraterone also delayed the time to deterioration in ECOG-PS and prostate-specific antigen (PSA) progression. Abiraterone more than doubled the PSA response rate: 68% vs 29% with prednisone.
Patients reported more pain relief. Those receiving abiraterone had statistically significant improvement in pain interference (P = .005), although the improvement in mean pain intensity was not significant.
Adverse effects leading to dose modifications or interruption of treatment were reported in 21% of patients on abiraterone, compared with 12% of the prednisone group. Six patients (1%) in each group died of drug-related treatment-emergent AEs. The AEs of “special interest,” the researchers say, included events related to mineralocorticoid excess, such as hypertension, hypokalemia, and fluid retention—all unsurprising, given the known mechanism of action of abiraterone. Grade 3 or 4 AEs with increased alanine aminotransferase and aspartate aminotransferase were more common in the abiraterone group.
The most common subsequent therapy for patients who terminated the study was docetaxel. However, another recent study, from Johns Hopkins researchers in Baltimore, Maryland, indicates the transition warrants caution: The findings suggest a potential cross-resistance between docetaxel and abiraterone.
Their study compared outcomes in 24 men who received abiraterone before docetaxel with 95 who were abiraterone-naïve. Men who were on abiraterone were less likely to achieve a PSA response, and their cancer was more likely to progress.
The researchers concede that their study groups were small; they also say it is possible that differences in disease severity may have influenced the time to progression. However, they say the fact that PSA-PFS was significantly different between the 2 groups (P = .002) supports their initial hypothesis—that is, that abiraterone pretreatment reduces responsiveness to docetaxel.
In spite of its limitations, the researchers say their study represents the only comparative analysis of PSA-PFS and PFS after docetaxel treatment for patients who have or have not received prior abiraterone. Their report, they add, offers the “strongest available evidence to date” of a clinically meaningful cross-resistance between abiraterone and docetaxel. They conclude that their findings provide “valuable information” about which patients are likely to derive the most benefit from docetaxel.
Sources
Rathkopf DE, Smith MR, de Bono JS, et al. Eur Urol. 2014;66(5):815-825.
doi: 10.1016/j.eururo.2014.02.056.
Schewizer MT, Zhou XC, Wang H, et al. Eur Urol. 2014;66(4):646-652.
doi: 10.1016/j.eururo.2014.01.018.
Until recently, there have been few treatment options for advanced prostate cancer that is resistant to androgen-directed therapies. Newer treatments that target residual androgen production offer some hope of prolonging the interval before chemotherapy, with fewer adverse effects (AEs) and better efficacy. One of those is abiraterone, which blocks extragonadal, testicular, and tumor androgen biosynthesis.
An ongoing multinational phase 3 study is evaluating the clinical benefits of abiraterone plus prednisone vs prednisone alone in patients with progressive metastatic castration-resistant prostate cancer (mCRPC). Follow-up for the study has now exceeded 27 months, giving a good opportunity to evaluate safety and efficacy. Thus, after having reviewed outcomes so far, the independent data-monitoring committee recommended that the study be unblinded and patients be allowed to cross over from prednisone to abiraterone. The researchers reported the results of the third interim analysis, with updated analysis.
Patients were stratified by Eastern Cooperative Oncology Group performance status (ECOG-PS) and randomly assigned to receive abiraterone 1,000 mg plus prednisone 5 mg twice daily or placebo plus prednisone.
Patients who received abiraterone had, compared with those on prednisone, statistically significant improvement in radiographic progression-free survival (PFS), with a median time to disease progression or death of 16.5 months, vs 8.2 months (95% CI, 0.45-0.61).
Overall survival also lengthened, from a median of 35.3 months vs 30.1 months (95% CI, 0.66-0.95).
All secondary endpoints also favored abiraterone over prednisone. For instance, abiraterone treatment delayed the time to the need for opiates for cancer-related pain and the time to initiation of chemotherapy. Abiraterone also delayed the time to deterioration in ECOG-PS and prostate-specific antigen (PSA) progression. Abiraterone more than doubled the PSA response rate: 68% vs 29% with prednisone.
Patients reported more pain relief. Those receiving abiraterone had statistically significant improvement in pain interference (P = .005), although the improvement in mean pain intensity was not significant.
Adverse effects leading to dose modifications or interruption of treatment were reported in 21% of patients on abiraterone, compared with 12% of the prednisone group. Six patients (1%) in each group died of drug-related treatment-emergent AEs. The AEs of “special interest,” the researchers say, included events related to mineralocorticoid excess, such as hypertension, hypokalemia, and fluid retention—all unsurprising, given the known mechanism of action of abiraterone. Grade 3 or 4 AEs with increased alanine aminotransferase and aspartate aminotransferase were more common in the abiraterone group.
The most common subsequent therapy for patients who terminated the study was docetaxel. However, another recent study, from Johns Hopkins researchers in Baltimore, Maryland, indicates the transition warrants caution: The findings suggest a potential cross-resistance between docetaxel and abiraterone.
Their study compared outcomes in 24 men who received abiraterone before docetaxel with 95 who were abiraterone-naïve. Men who were on abiraterone were less likely to achieve a PSA response, and their cancer was more likely to progress.
The researchers concede that their study groups were small; they also say it is possible that differences in disease severity may have influenced the time to progression. However, they say the fact that PSA-PFS was significantly different between the 2 groups (P = .002) supports their initial hypothesis—that is, that abiraterone pretreatment reduces responsiveness to docetaxel.
In spite of its limitations, the researchers say their study represents the only comparative analysis of PSA-PFS and PFS after docetaxel treatment for patients who have or have not received prior abiraterone. Their report, they add, offers the “strongest available evidence to date” of a clinically meaningful cross-resistance between abiraterone and docetaxel. They conclude that their findings provide “valuable information” about which patients are likely to derive the most benefit from docetaxel.
Sources
Rathkopf DE, Smith MR, de Bono JS, et al. Eur Urol. 2014;66(5):815-825.
doi: 10.1016/j.eururo.2014.02.056.
Schewizer MT, Zhou XC, Wang H, et al. Eur Urol. 2014;66(4):646-652.
doi: 10.1016/j.eururo.2014.01.018.
Disordered methylation compromises CLL treatment

Credit: Christoph Bock
New research suggests disordered methylation is one of the defining characteristics of cancer and helps tumors adapt to changing circumstances.
The study, published in Cancer Cell, showed that disordered methylation has a direct bearing on the effectiveness of cancer therapy.
In patients with chronic lymphocytic leukemia (CLL), researchers found that treatment produced shorter remissions if the tumor tissue showed signs of highly disordered methylation.
The findings indicate that such disorganization can actually benefit tumors and render them less vulnerable to anticancer drugs.
“The behavior of a cancer cell is dictated not only by genetics . . . but also by epigenetics,” said study author Catherine Wu, MD, of the Dana-Farber Cancer Institute in Boston.
“We know that tumors are composed of many subgroups of cells, each with its own array of gene mutations. In this study, we wanted to see if that type of genetic diversity coincides with epigenetic diversity. In other words, does the range of methylation patterns mirror the genetic variety we find in tumors?”
To find out, the researchers used bisulfite sequencing, which allows scientists to track the presence or absence of methyl groups at specific rungs on the DNA ladder.
They also devised a simple measure called PDR—percent discordant reads—for quantifying the extent of irregular methylation within a tissue sample. The higher the PDR, the more variability in how the methyl groups are arranged.
They measured the PDR and the amount of genetic diversity in 104 CLL samples and 27 samples of normal B cells.
“We thought the epigenetic structure would map right onto the genetic structure,” said study author Alexander Meissner, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts.
“That is, the degree of genetic diversity in each sample would match the variation in methylation marks in an organized fashion.”
To the researchers’ surprise, the methylation patterns showed a tremendous degree of random disarray.
“We know that individual tumors are checkered with genetically distinct groups of cells,” Dr Meissner explained. “Bisulfite sequencing enabled us to see that the placement of methyl groups across tumor cell DNA also varies substantially among cells in the same tumor. In fact, disorderly methylation pervades the entire tumor.”
The results revealed that the diversity within individual tumors apparently proceeds along two independent, yet interrelated tracks: one resulting in a genetic hodgepodge of cell groups, the other resulting in haphazard methylation.
The methylation irregularities, technically known as “local methylation disorder,” were highly evident in CLL and other types of cancer.
Because methyl groups control the expression of genes, disorderly methylation might be expected to cause wildly inconsistent gene activity even within a single tumor. This, in fact, is what the researchers found.
The disruption of methylation machinery might seem hazardous to tumor survival, but the researchers theorize that tumors can turn the disorderliness to their own advantage.
“Just as in the case of genetic heterogeneity within tumors, increased random variation of the epigenetic profile may augment the diversity of malignant cells,” said study author Dan Landau, MD, PhD, of Dana-Farber and the Broad Institute.
“The ability of cancers to maintain high levels of diversity is an effective hedging strategy, enabling them to better adapt to therapy, as well as enhancing the ‘trial and error’ process in search of better evolutionary trajectories.”
“Cancer survives through some wildly inventive ways,” Dr Wu added. “Methylation disorder is one of the ways it creates the conditions that enable it to adapt.” ![]()

Credit: Christoph Bock
New research suggests disordered methylation is one of the defining characteristics of cancer and helps tumors adapt to changing circumstances.
The study, published in Cancer Cell, showed that disordered methylation has a direct bearing on the effectiveness of cancer therapy.
In patients with chronic lymphocytic leukemia (CLL), researchers found that treatment produced shorter remissions if the tumor tissue showed signs of highly disordered methylation.
The findings indicate that such disorganization can actually benefit tumors and render them less vulnerable to anticancer drugs.
“The behavior of a cancer cell is dictated not only by genetics . . . but also by epigenetics,” said study author Catherine Wu, MD, of the Dana-Farber Cancer Institute in Boston.
“We know that tumors are composed of many subgroups of cells, each with its own array of gene mutations. In this study, we wanted to see if that type of genetic diversity coincides with epigenetic diversity. In other words, does the range of methylation patterns mirror the genetic variety we find in tumors?”
To find out, the researchers used bisulfite sequencing, which allows scientists to track the presence or absence of methyl groups at specific rungs on the DNA ladder.
They also devised a simple measure called PDR—percent discordant reads—for quantifying the extent of irregular methylation within a tissue sample. The higher the PDR, the more variability in how the methyl groups are arranged.
They measured the PDR and the amount of genetic diversity in 104 CLL samples and 27 samples of normal B cells.
“We thought the epigenetic structure would map right onto the genetic structure,” said study author Alexander Meissner, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts.
“That is, the degree of genetic diversity in each sample would match the variation in methylation marks in an organized fashion.”
To the researchers’ surprise, the methylation patterns showed a tremendous degree of random disarray.
“We know that individual tumors are checkered with genetically distinct groups of cells,” Dr Meissner explained. “Bisulfite sequencing enabled us to see that the placement of methyl groups across tumor cell DNA also varies substantially among cells in the same tumor. In fact, disorderly methylation pervades the entire tumor.”
The results revealed that the diversity within individual tumors apparently proceeds along two independent, yet interrelated tracks: one resulting in a genetic hodgepodge of cell groups, the other resulting in haphazard methylation.
The methylation irregularities, technically known as “local methylation disorder,” were highly evident in CLL and other types of cancer.
Because methyl groups control the expression of genes, disorderly methylation might be expected to cause wildly inconsistent gene activity even within a single tumor. This, in fact, is what the researchers found.
The disruption of methylation machinery might seem hazardous to tumor survival, but the researchers theorize that tumors can turn the disorderliness to their own advantage.
“Just as in the case of genetic heterogeneity within tumors, increased random variation of the epigenetic profile may augment the diversity of malignant cells,” said study author Dan Landau, MD, PhD, of Dana-Farber and the Broad Institute.
“The ability of cancers to maintain high levels of diversity is an effective hedging strategy, enabling them to better adapt to therapy, as well as enhancing the ‘trial and error’ process in search of better evolutionary trajectories.”
“Cancer survives through some wildly inventive ways,” Dr Wu added. “Methylation disorder is one of the ways it creates the conditions that enable it to adapt.” ![]()

Credit: Christoph Bock
New research suggests disordered methylation is one of the defining characteristics of cancer and helps tumors adapt to changing circumstances.
The study, published in Cancer Cell, showed that disordered methylation has a direct bearing on the effectiveness of cancer therapy.
In patients with chronic lymphocytic leukemia (CLL), researchers found that treatment produced shorter remissions if the tumor tissue showed signs of highly disordered methylation.
The findings indicate that such disorganization can actually benefit tumors and render them less vulnerable to anticancer drugs.
“The behavior of a cancer cell is dictated not only by genetics . . . but also by epigenetics,” said study author Catherine Wu, MD, of the Dana-Farber Cancer Institute in Boston.
“We know that tumors are composed of many subgroups of cells, each with its own array of gene mutations. In this study, we wanted to see if that type of genetic diversity coincides with epigenetic diversity. In other words, does the range of methylation patterns mirror the genetic variety we find in tumors?”
To find out, the researchers used bisulfite sequencing, which allows scientists to track the presence or absence of methyl groups at specific rungs on the DNA ladder.
They also devised a simple measure called PDR—percent discordant reads—for quantifying the extent of irregular methylation within a tissue sample. The higher the PDR, the more variability in how the methyl groups are arranged.
They measured the PDR and the amount of genetic diversity in 104 CLL samples and 27 samples of normal B cells.
“We thought the epigenetic structure would map right onto the genetic structure,” said study author Alexander Meissner, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts.
“That is, the degree of genetic diversity in each sample would match the variation in methylation marks in an organized fashion.”
To the researchers’ surprise, the methylation patterns showed a tremendous degree of random disarray.
“We know that individual tumors are checkered with genetically distinct groups of cells,” Dr Meissner explained. “Bisulfite sequencing enabled us to see that the placement of methyl groups across tumor cell DNA also varies substantially among cells in the same tumor. In fact, disorderly methylation pervades the entire tumor.”
The results revealed that the diversity within individual tumors apparently proceeds along two independent, yet interrelated tracks: one resulting in a genetic hodgepodge of cell groups, the other resulting in haphazard methylation.
The methylation irregularities, technically known as “local methylation disorder,” were highly evident in CLL and other types of cancer.
Because methyl groups control the expression of genes, disorderly methylation might be expected to cause wildly inconsistent gene activity even within a single tumor. This, in fact, is what the researchers found.
The disruption of methylation machinery might seem hazardous to tumor survival, but the researchers theorize that tumors can turn the disorderliness to their own advantage.
“Just as in the case of genetic heterogeneity within tumors, increased random variation of the epigenetic profile may augment the diversity of malignant cells,” said study author Dan Landau, MD, PhD, of Dana-Farber and the Broad Institute.
“The ability of cancers to maintain high levels of diversity is an effective hedging strategy, enabling them to better adapt to therapy, as well as enhancing the ‘trial and error’ process in search of better evolutionary trajectories.”
“Cancer survives through some wildly inventive ways,” Dr Wu added. “Methylation disorder is one of the ways it creates the conditions that enable it to adapt.” ![]()
CLL drug can fight AML too, study suggests

Credit: FDA
SAN FRANCISCO—A BCL2 inhibitor that previously proved active against chronic lymphocytic leukemia has shown activity in certain patients with acute myelogenous leukemia (AML) as well.
This phase 2 trial was the first use of the inhibitor, ABT-199 (or venetoclax), in patients with relapsed or refractory AML.
Five of 32 patients treated with ABT-199 achieved a complete response (CR) or CR with incomplete blood count recovery (CRi), and several more had stable disease.
The drug appeared to be particularly active in patients with IDH mutations.
Marina Konopleva, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, presented these results at the 2014 ASH Annual Meeting (abstract 118). The research was funded by AbbVie, Inc., the company developing ABT-199.
The trial was launched on the basis of preclinical studies showing that ABT-199 could kill AML cell lines, patients’ AML cells, and patient-derived AML cells implanted in mice.
The researchers enrolled 32 AML patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a CR and 4 patients achieving a CRi. The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The team said these results suggest single-agent ABT-199 can have considerable clinical activity in patients with relapsed or refractory AML, and patients with mutations in IDH genes may be particularly sensitive to the drug.
The researchers also found the median bone marrow blast count in evaluable patients decreased 36% after treatment with ABT-199. And 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting. Grade 3 and 4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia.
No patients died as a result of treatment-related adverse events.
Furthermore, the maximum-tolerated dose was not reached, leaving open the possibility of higher doses in further trials. The next step is to carry out trials combining ABT-199 with other agents. These trials are currently opening at several sites.
AbbVie said ABT-199 will be studied in combination with common AML treatments, and the company is developing ABT-199 for, and evaluating the drug in, several hematologic malignancies. ![]()

Credit: FDA
SAN FRANCISCO—A BCL2 inhibitor that previously proved active against chronic lymphocytic leukemia has shown activity in certain patients with acute myelogenous leukemia (AML) as well.
This phase 2 trial was the first use of the inhibitor, ABT-199 (or venetoclax), in patients with relapsed or refractory AML.
Five of 32 patients treated with ABT-199 achieved a complete response (CR) or CR with incomplete blood count recovery (CRi), and several more had stable disease.
The drug appeared to be particularly active in patients with IDH mutations.
Marina Konopleva, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, presented these results at the 2014 ASH Annual Meeting (abstract 118). The research was funded by AbbVie, Inc., the company developing ABT-199.
The trial was launched on the basis of preclinical studies showing that ABT-199 could kill AML cell lines, patients’ AML cells, and patient-derived AML cells implanted in mice.
The researchers enrolled 32 AML patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a CR and 4 patients achieving a CRi. The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The team said these results suggest single-agent ABT-199 can have considerable clinical activity in patients with relapsed or refractory AML, and patients with mutations in IDH genes may be particularly sensitive to the drug.
The researchers also found the median bone marrow blast count in evaluable patients decreased 36% after treatment with ABT-199. And 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting. Grade 3 and 4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia.
No patients died as a result of treatment-related adverse events.
Furthermore, the maximum-tolerated dose was not reached, leaving open the possibility of higher doses in further trials. The next step is to carry out trials combining ABT-199 with other agents. These trials are currently opening at several sites.
AbbVie said ABT-199 will be studied in combination with common AML treatments, and the company is developing ABT-199 for, and evaluating the drug in, several hematologic malignancies. ![]()

Credit: FDA
SAN FRANCISCO—A BCL2 inhibitor that previously proved active against chronic lymphocytic leukemia has shown activity in certain patients with acute myelogenous leukemia (AML) as well.
This phase 2 trial was the first use of the inhibitor, ABT-199 (or venetoclax), in patients with relapsed or refractory AML.
Five of 32 patients treated with ABT-199 achieved a complete response (CR) or CR with incomplete blood count recovery (CRi), and several more had stable disease.
The drug appeared to be particularly active in patients with IDH mutations.
Marina Konopleva, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, presented these results at the 2014 ASH Annual Meeting (abstract 118). The research was funded by AbbVie, Inc., the company developing ABT-199.
The trial was launched on the basis of preclinical studies showing that ABT-199 could kill AML cell lines, patients’ AML cells, and patient-derived AML cells implanted in mice.
The researchers enrolled 32 AML patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a CR and 4 patients achieving a CRi. The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The team said these results suggest single-agent ABT-199 can have considerable clinical activity in patients with relapsed or refractory AML, and patients with mutations in IDH genes may be particularly sensitive to the drug.
The researchers also found the median bone marrow blast count in evaluable patients decreased 36% after treatment with ABT-199. And 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting. Grade 3 and 4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia.
No patients died as a result of treatment-related adverse events.
Furthermore, the maximum-tolerated dose was not reached, leaving open the possibility of higher doses in further trials. The next step is to carry out trials combining ABT-199 with other agents. These trials are currently opening at several sites.
AbbVie said ABT-199 will be studied in combination with common AML treatments, and the company is developing ABT-199 for, and evaluating the drug in, several hematologic malignancies. ![]()
Crisis Mode and Information Exchange
Using electronic health records to improve the continuity of care between hospital units does not replace the need for interpersonal communication to improve transitions of care. Hospital personnel play a critical role in accurately exchanging patient information during patient transfers, a process requiring accurate communication between hospital units to prevent system failures.[1] Because poor communication contributes to preventable adverse events,[2] and effective communication during handoffs decreases medical errors and readmissions,[3] hospitals need to ensure their work environments are conducive to effective communication.
Individuals working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information,[4] partially due to limited ability to accurately process and communicate information under stressful circumstances. Furthermore, because time‐constrained decision makers tend to use less information and less rigorous decision strategies,[5] work climates characterized by staff members doing too many things too quickly could cause patient health information to be lost during transitions of care across hospital units.
Current studies illustrate scenarios in which demanding or time‐constrained work environments caused information exchange errors. One study found that the increased rate of prescribing errors was partially attributed to a high‐demand work environment characterized by working after hours and multitasking.[6] Other studies found that clinicians' limited time to relay and respond to information and ask clarifying questions during patient handoffs was partially attributed to the fast‐faced and chaotic environment of the emergency room.[7, 8] These studies are consistent with another study that found patient handoffs between emergency departments and inpatient wards were inadequate, partially due to less interactive and more rushed communication.[9] The fact that communication breakdowns are widely cited as barriers to patient handoffs[7, 8, 10] and facilitators of medical errors,[7, 8] further underscores the detrimental effect that crisis mode work climates could have on exchanging patient information during transitions of care.
The objective of this analysis was to evaluate the extent to which a crisis mode work climate impacts the occurrence of patient information exchange problems. Estimating associations between hospital staff members' perceptions of crisis mode work climates and perceptions of information exchange problems provide insights as to whether high‐demand and time‐constrained work climates negatively impact the exchange of patient information. Because hospital staff members working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information, we hypothesized that higher levels of a perceived crisis mode work climate would be associated with higher levels of perceived problems with information exchange across hospital units.
METHODS
Data Source
Data originated from the Agency of Healthcare Research and Quality 2010 Hospital Survey on Patient Safety Culture. This validated survey, designed to assess the safety climate within acute‐care settings, remains an important annual survey deployed each year to track changes and factors impacting patient safety.[11] We included only those respondents who self‐reported their position as a nurse, physician, pharmacist, dietician, therapist, technician, patient care assistant, or hospital unit secretary, all of whom are likely responsible for exchanging patient information across hospital units. For this reason, we excluded respondents who self‐reported their position as administrative or miscellaneous. Applying these exclusion criteria resulted in 247,104 respondents across 884 hospitals.
Conceptual Framework
The relationship between perceived crisis mode work climates and patient information exchange problems is likely influenced by staff skill levels, work climate, and infrastructure (Figure 1). With respect to skill levels, hospital staff members with many years of experience compared to those with fewer years may be relatively desensitized to chaotic work environments and consequently have higher thresholds for perceiving crisis modes. Number of hours worked per week likely impacts perceived crisis mode as illustrated in 1 study finding that full‐time nurses reported a significantly lower work pace compared to part‐time nurses.[12] Years of experience likely impacts perception of information exchange problems, particularly if staff members with many years of experience are familiar enough with hospital systems or protocols to easily detect exchange errors or mishaps.
With respect to work climates, workers' perception of cooperation, coordination and patient safety, and specific hospital unit likely impact perceptions of crisis work mode and information exchange problems. For example, hospital staff members reporting high levels of cooperation, coordination, and patient safety likely perceive fewer crisis modes and information exchange problems compared to those in less‐cooperative hospital units. Furthermore, the heterogeneity of work cultures across departments within a hospital results in department‐specific perceptions of crisis mode climates and information exchange problems. Infrastructure factors, such as hospital size, teaching and ownership status, and census region, likely impact the amount of resources available for staffing and infrastructure, which in turn could impact work pace and information exchange accuracy.
Variable Definitions
We defined our predictor as the perceived presence of a crisis mode work climate as captured from the survey questionnaire item: We work in crisis mode trying to do too much, too quickly. This question item had a Likert response scale comprised of the following 5 answer choices: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. We created a 3‐level response variable by aggregating the agree and disagree responses, respectively, as the first 2 levels, and retaining the neutral response as the third level. Consequently, those responding strongly disagree or disagree were classified as working in lowcrisis mode work climates and those responding strongly agree or agree were classified as working in high crisis mode work climates. We defined our outcome measure as the presence of patient information exchange problems as captured from the survey questionnaire item: Problems often occur in the exchange of information across hospital units. Because this question item had a Likert response scale similar to the crisis mode question predictor, we also created a 3‐level categorical variable in the same fashion. Consequently, those responding strongly disagree or disagree were classified as perceiving no problems exchanging patient information, and those responding strongly agree or agree were classified as perceiving problems exchanging patient information. For the fewer than 10% of the respondents with missing data on either the predictor our outcome variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
We also included questionnaire items that captured staff skill levels, work climate, and infrastructure as covariates to account for potential confounders (Figure 1). The staff skill levels domain included years of experience working in the hospital, specialty, and unit; current staff position; and extent of patient contact. The work climate domain included respondent perceptions of coordination and cooperation, patient safety, and primary work area or unit in which the provider reported working. The hospital infrastructure domain included bed size, census region, teaching status, and government ownership status. For the fewer than 10% of the respondents with missing data on any of the categorical variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
Analytic Approach
We used multivariable ordinal regressions to estimate the likelihood of perceived problems in patient information exchange conditional upon perceptions of a crisis mode work climate, controlling for staff skill levels, work climate, and hospital infrastructure. Our estimates therefore reflect the likelihood of hospital staff responding strongly agree or agree to the question Problems often occur in the exchange of information across hospital units conditional upon responding strongly agree or agree to the question We work in crisis mode trying to do too much, too quickly. In addition to controlling for hospital‐specific response rates, we also adjusted our standard errors to account for the clustering of respondents within hospitals. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The hospital sample averaged 279 respondents per hospital with a 56% response rate. Most hospitals were located in the Central region of the United States, and 32% and 19% were teaching and government‐owned hospitals, respectively. Forty‐three percent and 44% of the hospitals in the sample were designated as small and medium hospitals, respectively (Table 1).
| Characteristics | % |
|---|---|
| |
| Hospital characteristics, N=884 | |
| Bed size | |
| Small, 199 | 43.5 |
| Medium, 100399 | 43.8 |
| Large, 400 plus | 12.7 |
| Teaching status | |
| Yes | 32.2 |
| No | 67.8 |
| Government ownership | |
| Yes | 19.5 |
| No | 80.5 |
| Census region | |
| Mid‐Atlantic and New England | 8.7 |
| South Atlantic | 14.8 |
| Central | 57.2 |
| Mountain | 7.7 |
| Pacific | 11.5 |
| Response rate, mean (SD) | 0.56 (0.28) |
| Respondents per hospital, mean (SD) | 279 (358) |
| Respondent characteristics, N=274,140 | |
| How long have you worked in your current specialty or profession? | |
| <1 year | 5.8 |
| 15 years | 32.8 |
| 610 years | 16.2 |
| 1115 years | 12.0 |
| 1620 years | 10.6 |
| 21 years | 22.7 |
| How long have you worked in this hospital? | |
| <1 year | 9.8 |
| 15 years | 42.8 |
| 610 years | 17.8 |
| 1115 years | 9.0 |
| 1620 years | 8.2 |
| 21 years | 12.4 |
| How long have you worked in your current hospital work area/unit? | |
| <1 year | 13.1 |
| 15 years | 48.0 |
| 610 years | 18.1 |
| 1115 years | 8.1 |
| 1620 years | 6.0 |
| 21 years | 6.7 |
| Typically, how many hours per week do you work in this hospital? | |
| <20 hours | 4.8 |
| 2039 hours | 39.9 |
| 4059 hours | 48.8 |
| 6079 hours | 4.2 |
| 8099 hours | 2.1 |
| 100 hours | 0.11 |
| What is your staff position in this hospital? | |
| Registered nurse | 51.2 |
| Technician (eg, ECG, lab, radiology) | 14.1 |
| Unit assistant/clerk/secretary | 8.5 |
| Patient care assistant/hospital aide/care partner | 7.4 |
| Physical, occupational, or speech therapist | 3.7 |
| Attending/staff physician | 3.5 |
| LVN/LPN | 3.0 |
| Respiratory therapist | 2.9 |
| Pharmacist | 2.2 |
| Physician assistant/nurse practitioner | 1.4 |
| Resident physician/physician in training | 1.2 |
| Dietician | 0.83 |
| In your staff position, do you typically have direct interaction or contact with patients? | |
| Yes | 86.6 |
| No | 13.4 |
| What is your primary work area or unit in this hospital? | |
| Other | 27.7 |
| Medicine (nonsurgical) | 11.1 |
| Surgery | 10.0 |
| Intensive care unit (any type) | 8.6 |
| Many different hospital units/no specific unit | 6.8 |
| Radiology | 6.2 |
| Emergency department | 5.8 |
| Obstetrics | 4.9 |
| Laboratory | 4.9 |
| Rehabilitation | 4.2 |
| Pediatrics | 3.8 |
| Pharmacy | 3.2 |
| Psychiatry/mental health | 2.1 |
| Anesthesiology | 0.55 |
Thirty‐seven percent of the respondents have worked in their current specialty or profession for 5 years or less (Table 1). Over half of the respondents have worked in their current hospital for 5 years or less, whereas 61% have worked in their current unit within the hospital for 5 years or less. Forty‐nine percent work at least 40 hours per week. Registered nurses and technicians represented the 2 largest subgroups of staff positions, comprising 51% and 14% of the sample, respectively. Dieticians and resident physicians, on the other hand, represented the 2 smallest subgroups of staff positions, comprising 0.83% and 1.2% of the sample, respectively. Eighty‐seven percent of the respondents have direct interaction or contact with patients. Apart from those responding other as their hospital unit, nonsurgical medicine and surgery represented the largest subgroup primary work areas, comprising 11% and 10% of the sample, respectively. In contrast, psychiatry and anesthesiology represented the 2 smallest subgroups of primary work areas, comprising 2.1% and 0.55% of the sample, respectively (Table 1).
Respondents scored relatively high with regard to teamwork and helping each other out under hurried or busy circumstances. For example, 85% agreed or strongly agreed that their unit worked together as a team to get work done when a lot of work needed to be completed quickly, and 68% agreed or strongly agreed that individuals within their unit helped out when an area in their unit became busy (Table 1). Despite this cooperation, 31% agreed or strongly agreed that hospital units did not coordinate well together. Paradoxically, 57% agreed or strongly agreed that there was good cooperation among hospital units that needed to work together. Seventy‐five percent of the respondents reported excellent or very good patient safety levels within their unit, although 53% agreed or strongly agreed that staff worked longer hours than was best for patient care (Table 1).
With regard to perceived crisis mode work climate, 32% and 47% reported agreeing and disagreeing, respectively, that their work unit worked in crisis mode trying to do too much too quickly (Table 2). With regard to perceived problems with patient information exchange, 27% and 36% reported agreeing and disagreeing, respectively, that information exchange problems occurred across hospital units (Table 2).
| Perceptions | % |
|---|---|
| We work in crisis mode trying to do too much, too quickly | |
| Strongly disagree | 8.1 |
| Disagree | 39.2 |
| Neutral | 21.0 |
| Agree | 24.3 |
| Strongly agree | 7.5 |
| Problems often occur in the exchange of information across hospital units | |
| Strongly disagree | 4.6 |
| Disagree | 31.3 |
| Neutral | 37.3 |
| Agree | 24.0 |
| Strongly agree | 2.7 |
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | |
| Strongly disagree | 1.5 |
| Disagree | 6.1 |
| Neutral | 7.5 |
| Agree | 53.6 |
| Strongly agree | 31.2 |
| When one area in this unit gets really busy, others help out. | |
| Strongly disagree | 3.9 |
| Disagree | 13.9 |
| Neutral | 13.7 |
| Agree | 52.6 |
| Strongly agree | 15.8 |
| Hospital units do not coordinate well with each other. | |
| Strongly disagree | 5.6 |
| Disagree | 38.8 |
| Neutral | 23.7 |
| Agree | 25.3 |
| Strongly agree | 6.6 |
| There is good cooperation among hospital units that need to work together. | |
| Strongly disagree | 2.7 |
| Disagree | 15.1 |
| Neutral | 24.7 |
| Agree | 51.1 |
| Strongly agree | 6.3 |
| Please give your work area/unit in this hospital an overall grade on patient safety. | |
| Excellent | 23.0 |
| Very good | 49.8 |
| Acceptable | 21.8 |
| Poor | 4.6 |
| Failing | 0.76 |
| Staff in this unit work longer hours than is best for patient care. | |
| Strongly disagree | 11.5 |
| Disagree | 42.2 |
| Neutral | 23.6 |
| Agree | 18.4 |
| Strongly agree | 6.3 |
In the unadjusted analyses, crisis mode perceptions and information exchange problem perceptions were significantly associated. Among those who agreed that their work unit worked in crisis mode, a larger proportion of respondents agreed (41%) versus disagreed (24%) that problems often occurred in exchanging patient information across units (Table 3). In contrast, among those who disagreed that their work unit worked in crisis mode, a larger proportion of respondents disagreed (47%) versus agreed (19%) that problems often occurred in exchanging patient information across units (Table 3).
| Problems Often Occur in Exchange of Information Across Hospital Units | |||
|---|---|---|---|
| Agree (N=66,115), Row %* | Neutral (N=92,228), Row % | Disagree (N=88,797), Row % | |
| |||
| Crisis Mode Work Climate | |||
| Agree (N=78,253) | 40.8 | 35.4 | 23.8 |
| Neutral (N=51,836)‖ | 22.9 | 48.9 | 28.2 |
| Disagree (N=116,781) | 19.0 | 33.5 | 47.5 |
In the multivariable ordinal regression, compared to those who disagreed that their unit worked in crisis mode, those who agreed were 1.6 times more likely to report that problems often occurred in exchanging patient information across units (odds ratio [OR]: 1.6, 95% confidence interval [CI]: 1.58‐1.65) (Table 4). Additionally, some key covariates were independently associated with perceptions of information exchange problems. Two of these covariates measured workplace coordination. Those who reported that hospital units did not cooperate well together were more likely to report problematic information exchange compared to those who reported that hospital units did cooperate well (OR: 4.7, 95% CI: 4.35.0). Relatedly, those who reported that hospital units did coordinate well were less likely to report problematic information exchange compared to those who reported that hospital units did not coordinate well (OR: 0.10, 95% CI: 0.10‐0.11). Two other covariates measured patient contact and perceptions about long working hours. Those who reported having direct interaction or contact with patients were less likely to report problematic information exchange compared to those who reported not having direct interaction or contact with patients (OR: 0.85, 95% CI: 0.83‐0.87). Those who reported that staff did not work longer hours than was better for patient care were less likely to report problematic information exchange compared to those who did report working longer hours than was better for patient care (OR: 0.76, 95% CI: 0.73 0.79). One covariate measured hospital size. Those who reported working in smaller hospitals were less likely to report problematic information exchange compared to those reporting working in large hospitals (OR: 0.66, 95% CI 0.59‐0.75) (Table 4).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| ||
| Primary predictor of interest | ||
| Crisis mode work climate | ||
| Agree | 3.0 (2.9‐3.1) | 1.6 (1.5‐1.6) |
| Neutral | 1.8 (1.7‐1.8) | 1.3 (1.2‐1.3) |
| Disagree | Reference | Reference |
| Hospital characteristics | ||
| Bed Size | ||
| Small, 624 | 0.51 (0.44‐0.59) | 0.66 (0.59‐0.75) |
| Small, 249 | 0.59 (0.53‐0.66) | 0.77 (0.70‐0.84) |
| Small, 5099 | 0.65 (0.58‐0.73) | 0.78 (0.71‐0.84) |
| Medium, 100199 | 0.85 (0.77‐0.95) | 0.92 (0.86‐1.0) |
| Medium, 200299 | 1.0 (0.98‐1.1) | 0.97 (0.90‐1.0) |
| Medium, 300399 | 0.96 (0.85‐1.1) | 1.0 (0.92‐1.1) |
| Large, 400499 | 0.99 (0.86‐1.1) | 0.96 (0.87‐1.0) |
| Large, 500 plus | Reference | Reference |
| Teaching status | ||
| No | 0.81 (0.76‐0.87) | 1.0 (0.95‐1.0) |
| Yes | Reference | Reference |
| Government ownership | ||
| No | 1.1 (1.01.2) | 1.0 (0.98‐1.1) |
| Yes | Reference | Reference |
| Census region | ||
| Mid‐Atlantic and New England | 1.0 (0.88‐1.1) | 0.91 (0.84‐0.99) |
| South Atlantic | 0.95 (0.85‐1.1) | 1.0 (0.95‐1.1) |
| Central 1 | 0.95 (0.85‐1.0) | 0.95 (0.89‐1.0) |
| Central 2 | 0.71 (0.62‐0.81) | 0.91 (0.83‐0.99) |
| Central 3 | 0.80 (0.71‐0.91) | 0.97 (0.90‐1.0) |
| Central 4 | 0.76 (0.68‐0.86) | 0.93 (0.85‐1.0) |
| Mountain | 0.84 (0.73‐0.96) | 0.98 (0.90‐1.1) |
| Pacific | Reference | Reference |
| Average survey response rate within hospital | 0.65 (0.58‐0.72) | 0.93 (0.82‐1.0) |
| Respondent characteristics | ||
| How long have you worked in your current specialty or profession? | ||
| <1 year | 0.75 (0.73‐0.78) | 1.03 (0.99‐1.1) |
| 15 years | 0.99 (0.97‐1.0) | 1.1 (1.1‐1.1) |
| 610 years | 1.0 (1.01.1) | 0.99 (0.96‐1.0) |
| 1115 years | 1.0 (1.01.1) | 1.0 (0.97‐1.0) |
| 1620 years | 1.0 (0.98‐1.0) | 0.97 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in this hospital? | ||
| <1 year | 0.75 (0.73‐0.77) | 0.90 (0.85‐0.90) |
| 15 years | 1.03 (1.001.05) | 0.99 (0.95‐1.0) |
| 610 years | 1.1 (1.1‐1.1) | 0.99 (0.95‐1.0) |
| 1115 years | 1.1 (1. 01.1) | 1.0 (0.96‐1.0) |
| 1620 years | 1.1 (1.01.1) | 0.98 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in your current hospital work area/unit? | ||
| <1 year | 0.79 (0.76‐0.82) | 0.98 (0.93‐1.0) |
| 15 years | 1.0 (1.01.1) | 1.0 (0.99‐1.1) |
| 610 years | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| 1115 years | 1.1 (1.01.1) | 1.0 (0.99‐1.1) |
| 1620 years | 1.1 (1.01.1) | 1.1 (1.01.1) |
| 21 years | Reference | Reference |
| Typically, how many hours per week do you work in this hospital? | ||
| <20 | 0.63 (0.50‐0.79) | 0.91 (0.72‐1.2) |
| 2039 | 0.75 (0.59‐0.94) | 0.90 (0.71‐1.1) |
| 4059 | 0.87 (0.69‐1.1) | 1.1 (0.85‐1.4) |
| 6079 | 0.95 (0.75‐1.2) | 1.0 (0.82‐1.3) |
| 8099 | 0.99 (0.78‐1.2) | 1.1 (0.86‐1.4) |
| 100 | Reference | Reference |
| What is your staff position in this hospital? | ||
| Registered nurse | 0.92 (0.90‐0.94) | 1.1 (0.98‐1.0) |
| Technician (eg, ECG, lab, radiology) | Reference | Reference |
| Unit assistant/clerk/secretary | 0.79 (0.76‐0.81) | 0.94 (0.80‐0.96) |
| Patient care assistant/hospital aide/care partner | 0.78 (0.75‐0.81) | 0.96 (0.90‐0.98) |
| Physical, occupational, or speech therapist | 0.88 (0.84‐0.92) | 1.2 (1.1‐1.2) |
| Attending/staff physician | 1.0 (0.97‐1.1) | 1.3 (1.2‐1.3) |
| LVN/LPN | 0.89 (0.85‐0.94) | 1.0 (0.92‐1.0) |
| Respiratory therapist | 0.84 (0.80‐0.88) | 0.97 (0.89‐1.0) |
| Pharmacist | 1.5 (1.4‐1.6) | 1.3 (1.1‐1.3) |
| Physician assistant/nurse practitioner | 0.93 (0.87‐1.0) | 1.2 (1.1‐1.2) |
| Resident physician/physician in training | 0.96 (0.89‐1.0) | 1.3 (1.2‐1.4) |
| Dietician | 0.86 (0.79‐0.94) | 1.2 (1.1‐1.3) |
| In your staff position, do you typically have direct interaction or contact with patients? | ||
| Yes | 0.83 (0.82‐0.85) | 0.85 (0.83‐0.87) |
| No | Reference | Reference |
| What is your primary work area or unit in this hospital? | ||
| Other | Reference | Reference |
| Medicine (nonsurgical) | 1.1 (1.01.1) | 0.84 (0.82‐0.89) |
| Surgery | 1.1 (1.1‐1.2) | 0.88 (0.86‐0.91) |
| Intensive care unit (any type) | 0.93 (0.90‐0.96) | 0.78 (0.76‐0.81) |
| Many different hospital units/no specific unit | 1.2 (1.1‐1.2) | 1.0 (0.98‐ 1.0) |
| Radiology | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| Emergency department | 1.0 (0.97‐1.0) | 0.57 (0.55‐0.60) |
| Obstetrics | 0.76 (0.73‐0.79) | 0.66 (0.63‐0.69) |
| Laboratory | 1.2 (1.2‐1.3) | 1.0 (1.01.1) |
| Rehabilitation | 1.0 (0.97‐1.0) | 1.0 (0.98‐1.1) |
| Pediatrics | 0.90 (0.86‐0.94) | 0.83 (0.80‐0.87) |
| Pharmacy | 1.6 (1.5‐1.7) | 1.1 (1.01.2) |
| Psychiatry/mental health | 1.2 (1.1‐1.2) | 0.96 (0.90‐1.0) |
| Anesthesiology | 1.1 (1.01.3) | 0.93 (0.83‐1.0) |
| Respondent perceptions | ||
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | ||
| Strongly disagree | 3.2 (3.03.4) | 1.0 (0.98‐1.1) |
| Disagree | 3.2 (3.13.3) | 1.0 (1.01.1) |
| Neutral | 2.3 (2.2‐2.4) | 0.98 (0.94‐1.0) |
| Agree | 2.3 (2.2‐2.4) | 1.0 (1.002‐1.04) |
| Strongly agree | Reference | Reference |
| Staff in this unit work longer hours than is best for patient care. | ||
| Strongly disagree | 0.51 (0.48‐0.53) | 0.76 (0.73‐0.79) |
| Disagree | 0.68 (0.67‐0.70) | 0.81 (0.78‐0.84) |
| Neutral | 0.94 (0.91‐0.97) | 0.93 (0.90‐0.97) |
| Agree | 1.0 (0.99‐1.1) | 0.94 (0.91‐0.98) |
| Strongly agree | Reference | Reference |
| When 1 area in this unit gets really busy, others help out. | ||
| Strongly disagree | 3.8 (3.7 ‐ 4.0) | 1.0 (0.96‐1.1) |
| Disagree | 3.0 (2.9‐3.1) | 1.0 (0.99‐1.1) |
| Neutral | 2.2 (2.12.3) | 1.0 (0.97‐1.0) |
| Agree | 1.5 (1.5‐1.6) | 0.99 (0.96‐1.0) |
| Strongly agree | Reference | Reference |
| Hospital units do not coordinate well with each other. | ||
| Strongly disagree | 0.03 (0.03‐0.04) | 0.10 (0.10‐0.11) |
| Disagree | 0.08 (0.08‐0.08) | 0.18 (0.17‐0.19) |
| Neutral | 0.21 (0.20‐0.22) | 0.32 (0.30‐0.33) |
| Agree | 0.50 (0.48‐0.52) | 0.61 (0.58‐0.63) |
| Strongly agree | Reference | Reference |
| There is good cooperation among hospital units that need to work together. | ||
| Strongly disagree | 20.1 (18.921.5) | 4.7 (4.35.0) |
| Disagree | 14.2 (13.614.9) | 4.2 (4.14.5) |
| Neutral | 6.7 (6.47.0) | 2.7 (2.6‐2.8) |
| Agree | 2.4 (2.3‐2.5) | 1.6 (1.6‐1.7) |
| Strongly agree | Reference | Reference |
| Please give your work area/unit in this hospital an overall grade on patient safety | ||
| Excellent | 0.13 (0.12‐0.14) | 0.47 (0.42‐0.52) |
| Very good | 0.24 (0.21‐0.26) | 0.63 (0.57‐0.70) |
| Acceptable | 0.49 (0.45‐0.54) | 0.79 (0.72‐0.88) |
| Poor | 0.83 (0.75‐0.92) | 0.92 (0.83‐1.03) |
| Failing | Reference | Reference |
DISCUSSION
Our results illustrate that when hospital staff agree that their hospital works in crisis mode, they are more likely to agree that their hospital unit had frequent problems exchanging patient information across units. Because hospital staff working under time constraints and heavy workloads could potentially be at risk of misinterpreting or delivering inaccurate information, these results imply that crisis mode work climates increase the risk of problematic health information exchange. An equally plausible interpretation could be that problematic patient health information exchange increases the risk of hospital staff perceiving crisis mode work climates. Given that information gaps are associated with patient handoff errors,[14] and that patient handoff errors are associated with adverse events,[2, 3, 6, 8] an urgent need exists to implement information exchange systems that prevent information gaps from harming patients. Consequently, hospitals need to implement workflow strategies that prevent information gaps from undermining patient safety during transitions of care.
Other factors affect information exchange apart from crisis mode work climate, as illustrated by the significant associations of key covariates in the multivariate model. The effect found between perceived coordination and information exchange implies that improving information exchange requires good cooperation and coordination. The effect found between patient contact and information exchange implies that working directly with patients improves either the accuracy or the perception of information exchange. Finally, the effect found between hospital size and information exchange suggests that small hospitals are less likely than large hospitals to have information exchange problem. The geographical dispersion and the complexity of larger institutions could result in information exchange problems due to more confusion and less in‐person communication.
Because problematic patient information exchanges are associated with hospital size, coordination, and patient contact, in addition to crisis mode work climate, multifaceted solutions are necessary to resolve the problem. For example, hospital interventions designed to improve coordination could in turn attenuate perceived crisis modes. Furthermore, tailoring these interventions to hospitals that belong to complex geographically dispersed provider networks would likely decrease errors during transitions of care. Because multiple factors cause information exchange problems, implementing interventions that improve both coordination and crisis mode work climates would likely result in a greater net improvement compared to interventions focused solely on decreasing crisis mode work climates.
Some limitations of our paper are worth noting. First, we did not have information on the volume of data exchanged or the functionality levels of the electronic health record systems, both of which likely impact the accuracy of patient information exchange. For example, hospitals with smaller versus larger amounts of data exchanged could be less prone to error. On the other hand, this risk of error could be reduced even further by implementing robust health information technology (IT) systems that improve the accuracy of information transfer. This is consistent with studies showing that hospitals without computerized provider order entry (CPOE) systems have been shown to have higher medication error rates compared to those hospitals with CPOE systems.[15] Therefore, omitting data volume and health IT capabilities from the multivariate model could introduce unobserved heterogeneity, resulting in biased associations between perceived crisis mode work climate and perceived information exchange problems. Second, the cross‐sectional design limits our ability to infer causality because we are not certain whether the perceived crisis mode occurred before, after, or simultaneously to perceived information exchange problems. Third, the self‐reported nature of the questionnaire items does not provide information on observed levels of crisis mode and exchange problems, which could be inconsistent with perceived levels. Fourth, the relatively low within‐hospital response rate decreases the external validity of our findings. For example, if responders' perceptions of crisis mode or information exchange problems significantly differed from nonresponders, our results would not be generalizable to the larger population of acute‐care hospitals across the United States. Therefore, conclusions should be viewed with caution if applying these results to hospitals with respondents significantly differing from those contained within our sample.
Despite these limitations, the large sample size in conjunction with the use of data from a survey having acceptable psychometric properties[16] strengthens the external and internal validity of our findings. Although questionnaire items measuring perceptions are relatively subjective in nature compared to using metrics that capture observed problems or crisis modes, we argue that staff perception data are equally informative, as they guide organization leaders on how to improve workplace performance. Because a core concept of high reliability organizations (HROs) is to preserve constant awareness by key leaders and staff of the state of the systems and processes that affect patient care,[17] HROs could benefit from knowing the extent to which staff perceptions impact patient care. From a methods perspective, the multivariable ordinal regressions enabled us to control for potential confounders that if omitted could have resulted in biased estimates. Furthermore, low levels of multicollinearity as illustrated by low variation inflation factors enabled us to isolate the independent effect of crisis mode perceptions. Including hospital size and hospital work unit as covariates was an additional methodological strength helping account for the unobserved heterogeneity caused by excluding volume of data exchanged or health IT system capability. For example, because larger compared to smaller hospitals usually have more sophisticated health IT systems,[15] including bed size in the model theoretically captures some of the variation that would have been captured if we were able to include a covariate measuring health IT capability. Last, using ordinal regression facilitates interpretation of the findings because the questionnaire items for the predictor and outcome were originally captured on a Likert scale.
Our findings underscore the significant impact that work climate has on accurate information exchange, and ultimately patient safety. Improving patient safety is imperative for hospitals, especially within the context of recent regulations stemming from the Affordable Care Act that incentivize hospitals to reduce readmissions[18] and improve transitions of care.[19] Because accurate health information exchange is a critical component of patient care, resolving barriers that decrease the accuracy of this exchange is essential. Therefore, future studies need to continue examining these associations within the context of study designs that incorporate longitudinal data and datasets that include objective measures capturing crisis mode work climates and information exchange problems. Because effective communication during handoffs is associated with decreases in medical errors and readmissions, hospitals need to continually ensure that work environments are conducive to effective patient information exchange.
Disclosures
Nothing to report
- , , . Safety and complexity: inter‐departmental relationships as a threat to patient safety in the operating department. J Health Organ Manag. 2006;20(2–3):227–242.
- , , , , . Communication loads on clinical staff in the emergency department. Med J Aust. 2002;176(9):415–418.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , . The Handbook of Group Communication Theory and Research. Thousand Oaks, CA: Sage Publications; 1999.
- , , . Judgment and decision making under time pressure. In: Svenson O, Maule AJ, eds. Time Pressure and Stress in Human Judgment and Decision Making. New York, NY: Plenum Press; 1993:27–40.
- , , , , . Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- , . Emergency physician intershift handovers: an analysis of our transitional care. Pediatr Emerg Care. 2006;22(10):751–754.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- , , , et al. Hospital Survey on Patient Safety Culture: 2010 user comparative database report. (Prepared by Westat, Rockville, MD, under Contract No. HHSA 290200710024C). Rockville, MD: Agency for Healthcare Research and Quality; February 2010. AHRQ Publication No. 10‐0026.
- , , , , . Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag. 2011;19(1):57–68.
- , . The treatment of missing values and its effect in the classifier accuracy. In: Banks D, House L, McMorris FR, Arabie P, Gaul W, eds. Classification, Clustering and Data Mining Applications. Berlin, Germany: Springer‐Verlag; 2004.
- , . A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274–284.
- , , , et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10:199.
- , , , et al. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. Prepared by the Lewin Group under contract no. 290‐04‐0011. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Health policy brief: Medicare hospital readmissions reduction program. Health Affairs. November 12, 2013. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102. Accessed August 11, 2014.
- , , , , . The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754.
Using electronic health records to improve the continuity of care between hospital units does not replace the need for interpersonal communication to improve transitions of care. Hospital personnel play a critical role in accurately exchanging patient information during patient transfers, a process requiring accurate communication between hospital units to prevent system failures.[1] Because poor communication contributes to preventable adverse events,[2] and effective communication during handoffs decreases medical errors and readmissions,[3] hospitals need to ensure their work environments are conducive to effective communication.
Individuals working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information,[4] partially due to limited ability to accurately process and communicate information under stressful circumstances. Furthermore, because time‐constrained decision makers tend to use less information and less rigorous decision strategies,[5] work climates characterized by staff members doing too many things too quickly could cause patient health information to be lost during transitions of care across hospital units.
Current studies illustrate scenarios in which demanding or time‐constrained work environments caused information exchange errors. One study found that the increased rate of prescribing errors was partially attributed to a high‐demand work environment characterized by working after hours and multitasking.[6] Other studies found that clinicians' limited time to relay and respond to information and ask clarifying questions during patient handoffs was partially attributed to the fast‐faced and chaotic environment of the emergency room.[7, 8] These studies are consistent with another study that found patient handoffs between emergency departments and inpatient wards were inadequate, partially due to less interactive and more rushed communication.[9] The fact that communication breakdowns are widely cited as barriers to patient handoffs[7, 8, 10] and facilitators of medical errors,[7, 8] further underscores the detrimental effect that crisis mode work climates could have on exchanging patient information during transitions of care.
The objective of this analysis was to evaluate the extent to which a crisis mode work climate impacts the occurrence of patient information exchange problems. Estimating associations between hospital staff members' perceptions of crisis mode work climates and perceptions of information exchange problems provide insights as to whether high‐demand and time‐constrained work climates negatively impact the exchange of patient information. Because hospital staff members working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information, we hypothesized that higher levels of a perceived crisis mode work climate would be associated with higher levels of perceived problems with information exchange across hospital units.
METHODS
Data Source
Data originated from the Agency of Healthcare Research and Quality 2010 Hospital Survey on Patient Safety Culture. This validated survey, designed to assess the safety climate within acute‐care settings, remains an important annual survey deployed each year to track changes and factors impacting patient safety.[11] We included only those respondents who self‐reported their position as a nurse, physician, pharmacist, dietician, therapist, technician, patient care assistant, or hospital unit secretary, all of whom are likely responsible for exchanging patient information across hospital units. For this reason, we excluded respondents who self‐reported their position as administrative or miscellaneous. Applying these exclusion criteria resulted in 247,104 respondents across 884 hospitals.
Conceptual Framework
The relationship between perceived crisis mode work climates and patient information exchange problems is likely influenced by staff skill levels, work climate, and infrastructure (Figure 1). With respect to skill levels, hospital staff members with many years of experience compared to those with fewer years may be relatively desensitized to chaotic work environments and consequently have higher thresholds for perceiving crisis modes. Number of hours worked per week likely impacts perceived crisis mode as illustrated in 1 study finding that full‐time nurses reported a significantly lower work pace compared to part‐time nurses.[12] Years of experience likely impacts perception of information exchange problems, particularly if staff members with many years of experience are familiar enough with hospital systems or protocols to easily detect exchange errors or mishaps.

With respect to work climates, workers' perception of cooperation, coordination and patient safety, and specific hospital unit likely impact perceptions of crisis work mode and information exchange problems. For example, hospital staff members reporting high levels of cooperation, coordination, and patient safety likely perceive fewer crisis modes and information exchange problems compared to those in less‐cooperative hospital units. Furthermore, the heterogeneity of work cultures across departments within a hospital results in department‐specific perceptions of crisis mode climates and information exchange problems. Infrastructure factors, such as hospital size, teaching and ownership status, and census region, likely impact the amount of resources available for staffing and infrastructure, which in turn could impact work pace and information exchange accuracy.
Variable Definitions
We defined our predictor as the perceived presence of a crisis mode work climate as captured from the survey questionnaire item: We work in crisis mode trying to do too much, too quickly. This question item had a Likert response scale comprised of the following 5 answer choices: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. We created a 3‐level response variable by aggregating the agree and disagree responses, respectively, as the first 2 levels, and retaining the neutral response as the third level. Consequently, those responding strongly disagree or disagree were classified as working in lowcrisis mode work climates and those responding strongly agree or agree were classified as working in high crisis mode work climates. We defined our outcome measure as the presence of patient information exchange problems as captured from the survey questionnaire item: Problems often occur in the exchange of information across hospital units. Because this question item had a Likert response scale similar to the crisis mode question predictor, we also created a 3‐level categorical variable in the same fashion. Consequently, those responding strongly disagree or disagree were classified as perceiving no problems exchanging patient information, and those responding strongly agree or agree were classified as perceiving problems exchanging patient information. For the fewer than 10% of the respondents with missing data on either the predictor our outcome variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
We also included questionnaire items that captured staff skill levels, work climate, and infrastructure as covariates to account for potential confounders (Figure 1). The staff skill levels domain included years of experience working in the hospital, specialty, and unit; current staff position; and extent of patient contact. The work climate domain included respondent perceptions of coordination and cooperation, patient safety, and primary work area or unit in which the provider reported working. The hospital infrastructure domain included bed size, census region, teaching status, and government ownership status. For the fewer than 10% of the respondents with missing data on any of the categorical variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
Analytic Approach
We used multivariable ordinal regressions to estimate the likelihood of perceived problems in patient information exchange conditional upon perceptions of a crisis mode work climate, controlling for staff skill levels, work climate, and hospital infrastructure. Our estimates therefore reflect the likelihood of hospital staff responding strongly agree or agree to the question Problems often occur in the exchange of information across hospital units conditional upon responding strongly agree or agree to the question We work in crisis mode trying to do too much, too quickly. In addition to controlling for hospital‐specific response rates, we also adjusted our standard errors to account for the clustering of respondents within hospitals. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The hospital sample averaged 279 respondents per hospital with a 56% response rate. Most hospitals were located in the Central region of the United States, and 32% and 19% were teaching and government‐owned hospitals, respectively. Forty‐three percent and 44% of the hospitals in the sample were designated as small and medium hospitals, respectively (Table 1).
| Characteristics | % |
|---|---|
| |
| Hospital characteristics, N=884 | |
| Bed size | |
| Small, 199 | 43.5 |
| Medium, 100399 | 43.8 |
| Large, 400 plus | 12.7 |
| Teaching status | |
| Yes | 32.2 |
| No | 67.8 |
| Government ownership | |
| Yes | 19.5 |
| No | 80.5 |
| Census region | |
| Mid‐Atlantic and New England | 8.7 |
| South Atlantic | 14.8 |
| Central | 57.2 |
| Mountain | 7.7 |
| Pacific | 11.5 |
| Response rate, mean (SD) | 0.56 (0.28) |
| Respondents per hospital, mean (SD) | 279 (358) |
| Respondent characteristics, N=274,140 | |
| How long have you worked in your current specialty or profession? | |
| <1 year | 5.8 |
| 15 years | 32.8 |
| 610 years | 16.2 |
| 1115 years | 12.0 |
| 1620 years | 10.6 |
| 21 years | 22.7 |
| How long have you worked in this hospital? | |
| <1 year | 9.8 |
| 15 years | 42.8 |
| 610 years | 17.8 |
| 1115 years | 9.0 |
| 1620 years | 8.2 |
| 21 years | 12.4 |
| How long have you worked in your current hospital work area/unit? | |
| <1 year | 13.1 |
| 15 years | 48.0 |
| 610 years | 18.1 |
| 1115 years | 8.1 |
| 1620 years | 6.0 |
| 21 years | 6.7 |
| Typically, how many hours per week do you work in this hospital? | |
| <20 hours | 4.8 |
| 2039 hours | 39.9 |
| 4059 hours | 48.8 |
| 6079 hours | 4.2 |
| 8099 hours | 2.1 |
| 100 hours | 0.11 |
| What is your staff position in this hospital? | |
| Registered nurse | 51.2 |
| Technician (eg, ECG, lab, radiology) | 14.1 |
| Unit assistant/clerk/secretary | 8.5 |
| Patient care assistant/hospital aide/care partner | 7.4 |
| Physical, occupational, or speech therapist | 3.7 |
| Attending/staff physician | 3.5 |
| LVN/LPN | 3.0 |
| Respiratory therapist | 2.9 |
| Pharmacist | 2.2 |
| Physician assistant/nurse practitioner | 1.4 |
| Resident physician/physician in training | 1.2 |
| Dietician | 0.83 |
| In your staff position, do you typically have direct interaction or contact with patients? | |
| Yes | 86.6 |
| No | 13.4 |
| What is your primary work area or unit in this hospital? | |
| Other | 27.7 |
| Medicine (nonsurgical) | 11.1 |
| Surgery | 10.0 |
| Intensive care unit (any type) | 8.6 |
| Many different hospital units/no specific unit | 6.8 |
| Radiology | 6.2 |
| Emergency department | 5.8 |
| Obstetrics | 4.9 |
| Laboratory | 4.9 |
| Rehabilitation | 4.2 |
| Pediatrics | 3.8 |
| Pharmacy | 3.2 |
| Psychiatry/mental health | 2.1 |
| Anesthesiology | 0.55 |
Thirty‐seven percent of the respondents have worked in their current specialty or profession for 5 years or less (Table 1). Over half of the respondents have worked in their current hospital for 5 years or less, whereas 61% have worked in their current unit within the hospital for 5 years or less. Forty‐nine percent work at least 40 hours per week. Registered nurses and technicians represented the 2 largest subgroups of staff positions, comprising 51% and 14% of the sample, respectively. Dieticians and resident physicians, on the other hand, represented the 2 smallest subgroups of staff positions, comprising 0.83% and 1.2% of the sample, respectively. Eighty‐seven percent of the respondents have direct interaction or contact with patients. Apart from those responding other as their hospital unit, nonsurgical medicine and surgery represented the largest subgroup primary work areas, comprising 11% and 10% of the sample, respectively. In contrast, psychiatry and anesthesiology represented the 2 smallest subgroups of primary work areas, comprising 2.1% and 0.55% of the sample, respectively (Table 1).
Respondents scored relatively high with regard to teamwork and helping each other out under hurried or busy circumstances. For example, 85% agreed or strongly agreed that their unit worked together as a team to get work done when a lot of work needed to be completed quickly, and 68% agreed or strongly agreed that individuals within their unit helped out when an area in their unit became busy (Table 1). Despite this cooperation, 31% agreed or strongly agreed that hospital units did not coordinate well together. Paradoxically, 57% agreed or strongly agreed that there was good cooperation among hospital units that needed to work together. Seventy‐five percent of the respondents reported excellent or very good patient safety levels within their unit, although 53% agreed or strongly agreed that staff worked longer hours than was best for patient care (Table 1).
With regard to perceived crisis mode work climate, 32% and 47% reported agreeing and disagreeing, respectively, that their work unit worked in crisis mode trying to do too much too quickly (Table 2). With regard to perceived problems with patient information exchange, 27% and 36% reported agreeing and disagreeing, respectively, that information exchange problems occurred across hospital units (Table 2).
| Perceptions | % |
|---|---|
| We work in crisis mode trying to do too much, too quickly | |
| Strongly disagree | 8.1 |
| Disagree | 39.2 |
| Neutral | 21.0 |
| Agree | 24.3 |
| Strongly agree | 7.5 |
| Problems often occur in the exchange of information across hospital units | |
| Strongly disagree | 4.6 |
| Disagree | 31.3 |
| Neutral | 37.3 |
| Agree | 24.0 |
| Strongly agree | 2.7 |
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | |
| Strongly disagree | 1.5 |
| Disagree | 6.1 |
| Neutral | 7.5 |
| Agree | 53.6 |
| Strongly agree | 31.2 |
| When one area in this unit gets really busy, others help out. | |
| Strongly disagree | 3.9 |
| Disagree | 13.9 |
| Neutral | 13.7 |
| Agree | 52.6 |
| Strongly agree | 15.8 |
| Hospital units do not coordinate well with each other. | |
| Strongly disagree | 5.6 |
| Disagree | 38.8 |
| Neutral | 23.7 |
| Agree | 25.3 |
| Strongly agree | 6.6 |
| There is good cooperation among hospital units that need to work together. | |
| Strongly disagree | 2.7 |
| Disagree | 15.1 |
| Neutral | 24.7 |
| Agree | 51.1 |
| Strongly agree | 6.3 |
| Please give your work area/unit in this hospital an overall grade on patient safety. | |
| Excellent | 23.0 |
| Very good | 49.8 |
| Acceptable | 21.8 |
| Poor | 4.6 |
| Failing | 0.76 |
| Staff in this unit work longer hours than is best for patient care. | |
| Strongly disagree | 11.5 |
| Disagree | 42.2 |
| Neutral | 23.6 |
| Agree | 18.4 |
| Strongly agree | 6.3 |
In the unadjusted analyses, crisis mode perceptions and information exchange problem perceptions were significantly associated. Among those who agreed that their work unit worked in crisis mode, a larger proportion of respondents agreed (41%) versus disagreed (24%) that problems often occurred in exchanging patient information across units (Table 3). In contrast, among those who disagreed that their work unit worked in crisis mode, a larger proportion of respondents disagreed (47%) versus agreed (19%) that problems often occurred in exchanging patient information across units (Table 3).
| Problems Often Occur in Exchange of Information Across Hospital Units | |||
|---|---|---|---|
| Agree (N=66,115), Row %* | Neutral (N=92,228), Row % | Disagree (N=88,797), Row % | |
| |||
| Crisis Mode Work Climate | |||
| Agree (N=78,253) | 40.8 | 35.4 | 23.8 |
| Neutral (N=51,836)‖ | 22.9 | 48.9 | 28.2 |
| Disagree (N=116,781) | 19.0 | 33.5 | 47.5 |
In the multivariable ordinal regression, compared to those who disagreed that their unit worked in crisis mode, those who agreed were 1.6 times more likely to report that problems often occurred in exchanging patient information across units (odds ratio [OR]: 1.6, 95% confidence interval [CI]: 1.58‐1.65) (Table 4). Additionally, some key covariates were independently associated with perceptions of information exchange problems. Two of these covariates measured workplace coordination. Those who reported that hospital units did not cooperate well together were more likely to report problematic information exchange compared to those who reported that hospital units did cooperate well (OR: 4.7, 95% CI: 4.35.0). Relatedly, those who reported that hospital units did coordinate well were less likely to report problematic information exchange compared to those who reported that hospital units did not coordinate well (OR: 0.10, 95% CI: 0.10‐0.11). Two other covariates measured patient contact and perceptions about long working hours. Those who reported having direct interaction or contact with patients were less likely to report problematic information exchange compared to those who reported not having direct interaction or contact with patients (OR: 0.85, 95% CI: 0.83‐0.87). Those who reported that staff did not work longer hours than was better for patient care were less likely to report problematic information exchange compared to those who did report working longer hours than was better for patient care (OR: 0.76, 95% CI: 0.73 0.79). One covariate measured hospital size. Those who reported working in smaller hospitals were less likely to report problematic information exchange compared to those reporting working in large hospitals (OR: 0.66, 95% CI 0.59‐0.75) (Table 4).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| ||
| Primary predictor of interest | ||
| Crisis mode work climate | ||
| Agree | 3.0 (2.9‐3.1) | 1.6 (1.5‐1.6) |
| Neutral | 1.8 (1.7‐1.8) | 1.3 (1.2‐1.3) |
| Disagree | Reference | Reference |
| Hospital characteristics | ||
| Bed Size | ||
| Small, 624 | 0.51 (0.44‐0.59) | 0.66 (0.59‐0.75) |
| Small, 249 | 0.59 (0.53‐0.66) | 0.77 (0.70‐0.84) |
| Small, 5099 | 0.65 (0.58‐0.73) | 0.78 (0.71‐0.84) |
| Medium, 100199 | 0.85 (0.77‐0.95) | 0.92 (0.86‐1.0) |
| Medium, 200299 | 1.0 (0.98‐1.1) | 0.97 (0.90‐1.0) |
| Medium, 300399 | 0.96 (0.85‐1.1) | 1.0 (0.92‐1.1) |
| Large, 400499 | 0.99 (0.86‐1.1) | 0.96 (0.87‐1.0) |
| Large, 500 plus | Reference | Reference |
| Teaching status | ||
| No | 0.81 (0.76‐0.87) | 1.0 (0.95‐1.0) |
| Yes | Reference | Reference |
| Government ownership | ||
| No | 1.1 (1.01.2) | 1.0 (0.98‐1.1) |
| Yes | Reference | Reference |
| Census region | ||
| Mid‐Atlantic and New England | 1.0 (0.88‐1.1) | 0.91 (0.84‐0.99) |
| South Atlantic | 0.95 (0.85‐1.1) | 1.0 (0.95‐1.1) |
| Central 1 | 0.95 (0.85‐1.0) | 0.95 (0.89‐1.0) |
| Central 2 | 0.71 (0.62‐0.81) | 0.91 (0.83‐0.99) |
| Central 3 | 0.80 (0.71‐0.91) | 0.97 (0.90‐1.0) |
| Central 4 | 0.76 (0.68‐0.86) | 0.93 (0.85‐1.0) |
| Mountain | 0.84 (0.73‐0.96) | 0.98 (0.90‐1.1) |
| Pacific | Reference | Reference |
| Average survey response rate within hospital | 0.65 (0.58‐0.72) | 0.93 (0.82‐1.0) |
| Respondent characteristics | ||
| How long have you worked in your current specialty or profession? | ||
| <1 year | 0.75 (0.73‐0.78) | 1.03 (0.99‐1.1) |
| 15 years | 0.99 (0.97‐1.0) | 1.1 (1.1‐1.1) |
| 610 years | 1.0 (1.01.1) | 0.99 (0.96‐1.0) |
| 1115 years | 1.0 (1.01.1) | 1.0 (0.97‐1.0) |
| 1620 years | 1.0 (0.98‐1.0) | 0.97 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in this hospital? | ||
| <1 year | 0.75 (0.73‐0.77) | 0.90 (0.85‐0.90) |
| 15 years | 1.03 (1.001.05) | 0.99 (0.95‐1.0) |
| 610 years | 1.1 (1.1‐1.1) | 0.99 (0.95‐1.0) |
| 1115 years | 1.1 (1. 01.1) | 1.0 (0.96‐1.0) |
| 1620 years | 1.1 (1.01.1) | 0.98 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in your current hospital work area/unit? | ||
| <1 year | 0.79 (0.76‐0.82) | 0.98 (0.93‐1.0) |
| 15 years | 1.0 (1.01.1) | 1.0 (0.99‐1.1) |
| 610 years | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| 1115 years | 1.1 (1.01.1) | 1.0 (0.99‐1.1) |
| 1620 years | 1.1 (1.01.1) | 1.1 (1.01.1) |
| 21 years | Reference | Reference |
| Typically, how many hours per week do you work in this hospital? | ||
| <20 | 0.63 (0.50‐0.79) | 0.91 (0.72‐1.2) |
| 2039 | 0.75 (0.59‐0.94) | 0.90 (0.71‐1.1) |
| 4059 | 0.87 (0.69‐1.1) | 1.1 (0.85‐1.4) |
| 6079 | 0.95 (0.75‐1.2) | 1.0 (0.82‐1.3) |
| 8099 | 0.99 (0.78‐1.2) | 1.1 (0.86‐1.4) |
| 100 | Reference | Reference |
| What is your staff position in this hospital? | ||
| Registered nurse | 0.92 (0.90‐0.94) | 1.1 (0.98‐1.0) |
| Technician (eg, ECG, lab, radiology) | Reference | Reference |
| Unit assistant/clerk/secretary | 0.79 (0.76‐0.81) | 0.94 (0.80‐0.96) |
| Patient care assistant/hospital aide/care partner | 0.78 (0.75‐0.81) | 0.96 (0.90‐0.98) |
| Physical, occupational, or speech therapist | 0.88 (0.84‐0.92) | 1.2 (1.1‐1.2) |
| Attending/staff physician | 1.0 (0.97‐1.1) | 1.3 (1.2‐1.3) |
| LVN/LPN | 0.89 (0.85‐0.94) | 1.0 (0.92‐1.0) |
| Respiratory therapist | 0.84 (0.80‐0.88) | 0.97 (0.89‐1.0) |
| Pharmacist | 1.5 (1.4‐1.6) | 1.3 (1.1‐1.3) |
| Physician assistant/nurse practitioner | 0.93 (0.87‐1.0) | 1.2 (1.1‐1.2) |
| Resident physician/physician in training | 0.96 (0.89‐1.0) | 1.3 (1.2‐1.4) |
| Dietician | 0.86 (0.79‐0.94) | 1.2 (1.1‐1.3) |
| In your staff position, do you typically have direct interaction or contact with patients? | ||
| Yes | 0.83 (0.82‐0.85) | 0.85 (0.83‐0.87) |
| No | Reference | Reference |
| What is your primary work area or unit in this hospital? | ||
| Other | Reference | Reference |
| Medicine (nonsurgical) | 1.1 (1.01.1) | 0.84 (0.82‐0.89) |
| Surgery | 1.1 (1.1‐1.2) | 0.88 (0.86‐0.91) |
| Intensive care unit (any type) | 0.93 (0.90‐0.96) | 0.78 (0.76‐0.81) |
| Many different hospital units/no specific unit | 1.2 (1.1‐1.2) | 1.0 (0.98‐ 1.0) |
| Radiology | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| Emergency department | 1.0 (0.97‐1.0) | 0.57 (0.55‐0.60) |
| Obstetrics | 0.76 (0.73‐0.79) | 0.66 (0.63‐0.69) |
| Laboratory | 1.2 (1.2‐1.3) | 1.0 (1.01.1) |
| Rehabilitation | 1.0 (0.97‐1.0) | 1.0 (0.98‐1.1) |
| Pediatrics | 0.90 (0.86‐0.94) | 0.83 (0.80‐0.87) |
| Pharmacy | 1.6 (1.5‐1.7) | 1.1 (1.01.2) |
| Psychiatry/mental health | 1.2 (1.1‐1.2) | 0.96 (0.90‐1.0) |
| Anesthesiology | 1.1 (1.01.3) | 0.93 (0.83‐1.0) |
| Respondent perceptions | ||
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | ||
| Strongly disagree | 3.2 (3.03.4) | 1.0 (0.98‐1.1) |
| Disagree | 3.2 (3.13.3) | 1.0 (1.01.1) |
| Neutral | 2.3 (2.2‐2.4) | 0.98 (0.94‐1.0) |
| Agree | 2.3 (2.2‐2.4) | 1.0 (1.002‐1.04) |
| Strongly agree | Reference | Reference |
| Staff in this unit work longer hours than is best for patient care. | ||
| Strongly disagree | 0.51 (0.48‐0.53) | 0.76 (0.73‐0.79) |
| Disagree | 0.68 (0.67‐0.70) | 0.81 (0.78‐0.84) |
| Neutral | 0.94 (0.91‐0.97) | 0.93 (0.90‐0.97) |
| Agree | 1.0 (0.99‐1.1) | 0.94 (0.91‐0.98) |
| Strongly agree | Reference | Reference |
| When 1 area in this unit gets really busy, others help out. | ||
| Strongly disagree | 3.8 (3.7 ‐ 4.0) | 1.0 (0.96‐1.1) |
| Disagree | 3.0 (2.9‐3.1) | 1.0 (0.99‐1.1) |
| Neutral | 2.2 (2.12.3) | 1.0 (0.97‐1.0) |
| Agree | 1.5 (1.5‐1.6) | 0.99 (0.96‐1.0) |
| Strongly agree | Reference | Reference |
| Hospital units do not coordinate well with each other. | ||
| Strongly disagree | 0.03 (0.03‐0.04) | 0.10 (0.10‐0.11) |
| Disagree | 0.08 (0.08‐0.08) | 0.18 (0.17‐0.19) |
| Neutral | 0.21 (0.20‐0.22) | 0.32 (0.30‐0.33) |
| Agree | 0.50 (0.48‐0.52) | 0.61 (0.58‐0.63) |
| Strongly agree | Reference | Reference |
| There is good cooperation among hospital units that need to work together. | ||
| Strongly disagree | 20.1 (18.921.5) | 4.7 (4.35.0) |
| Disagree | 14.2 (13.614.9) | 4.2 (4.14.5) |
| Neutral | 6.7 (6.47.0) | 2.7 (2.6‐2.8) |
| Agree | 2.4 (2.3‐2.5) | 1.6 (1.6‐1.7) |
| Strongly agree | Reference | Reference |
| Please give your work area/unit in this hospital an overall grade on patient safety | ||
| Excellent | 0.13 (0.12‐0.14) | 0.47 (0.42‐0.52) |
| Very good | 0.24 (0.21‐0.26) | 0.63 (0.57‐0.70) |
| Acceptable | 0.49 (0.45‐0.54) | 0.79 (0.72‐0.88) |
| Poor | 0.83 (0.75‐0.92) | 0.92 (0.83‐1.03) |
| Failing | Reference | Reference |
DISCUSSION
Our results illustrate that when hospital staff agree that their hospital works in crisis mode, they are more likely to agree that their hospital unit had frequent problems exchanging patient information across units. Because hospital staff working under time constraints and heavy workloads could potentially be at risk of misinterpreting or delivering inaccurate information, these results imply that crisis mode work climates increase the risk of problematic health information exchange. An equally plausible interpretation could be that problematic patient health information exchange increases the risk of hospital staff perceiving crisis mode work climates. Given that information gaps are associated with patient handoff errors,[14] and that patient handoff errors are associated with adverse events,[2, 3, 6, 8] an urgent need exists to implement information exchange systems that prevent information gaps from harming patients. Consequently, hospitals need to implement workflow strategies that prevent information gaps from undermining patient safety during transitions of care.
Other factors affect information exchange apart from crisis mode work climate, as illustrated by the significant associations of key covariates in the multivariate model. The effect found between perceived coordination and information exchange implies that improving information exchange requires good cooperation and coordination. The effect found between patient contact and information exchange implies that working directly with patients improves either the accuracy or the perception of information exchange. Finally, the effect found between hospital size and information exchange suggests that small hospitals are less likely than large hospitals to have information exchange problem. The geographical dispersion and the complexity of larger institutions could result in information exchange problems due to more confusion and less in‐person communication.
Because problematic patient information exchanges are associated with hospital size, coordination, and patient contact, in addition to crisis mode work climate, multifaceted solutions are necessary to resolve the problem. For example, hospital interventions designed to improve coordination could in turn attenuate perceived crisis modes. Furthermore, tailoring these interventions to hospitals that belong to complex geographically dispersed provider networks would likely decrease errors during transitions of care. Because multiple factors cause information exchange problems, implementing interventions that improve both coordination and crisis mode work climates would likely result in a greater net improvement compared to interventions focused solely on decreasing crisis mode work climates.
Some limitations of our paper are worth noting. First, we did not have information on the volume of data exchanged or the functionality levels of the electronic health record systems, both of which likely impact the accuracy of patient information exchange. For example, hospitals with smaller versus larger amounts of data exchanged could be less prone to error. On the other hand, this risk of error could be reduced even further by implementing robust health information technology (IT) systems that improve the accuracy of information transfer. This is consistent with studies showing that hospitals without computerized provider order entry (CPOE) systems have been shown to have higher medication error rates compared to those hospitals with CPOE systems.[15] Therefore, omitting data volume and health IT capabilities from the multivariate model could introduce unobserved heterogeneity, resulting in biased associations between perceived crisis mode work climate and perceived information exchange problems. Second, the cross‐sectional design limits our ability to infer causality because we are not certain whether the perceived crisis mode occurred before, after, or simultaneously to perceived information exchange problems. Third, the self‐reported nature of the questionnaire items does not provide information on observed levels of crisis mode and exchange problems, which could be inconsistent with perceived levels. Fourth, the relatively low within‐hospital response rate decreases the external validity of our findings. For example, if responders' perceptions of crisis mode or information exchange problems significantly differed from nonresponders, our results would not be generalizable to the larger population of acute‐care hospitals across the United States. Therefore, conclusions should be viewed with caution if applying these results to hospitals with respondents significantly differing from those contained within our sample.
Despite these limitations, the large sample size in conjunction with the use of data from a survey having acceptable psychometric properties[16] strengthens the external and internal validity of our findings. Although questionnaire items measuring perceptions are relatively subjective in nature compared to using metrics that capture observed problems or crisis modes, we argue that staff perception data are equally informative, as they guide organization leaders on how to improve workplace performance. Because a core concept of high reliability organizations (HROs) is to preserve constant awareness by key leaders and staff of the state of the systems and processes that affect patient care,[17] HROs could benefit from knowing the extent to which staff perceptions impact patient care. From a methods perspective, the multivariable ordinal regressions enabled us to control for potential confounders that if omitted could have resulted in biased estimates. Furthermore, low levels of multicollinearity as illustrated by low variation inflation factors enabled us to isolate the independent effect of crisis mode perceptions. Including hospital size and hospital work unit as covariates was an additional methodological strength helping account for the unobserved heterogeneity caused by excluding volume of data exchanged or health IT system capability. For example, because larger compared to smaller hospitals usually have more sophisticated health IT systems,[15] including bed size in the model theoretically captures some of the variation that would have been captured if we were able to include a covariate measuring health IT capability. Last, using ordinal regression facilitates interpretation of the findings because the questionnaire items for the predictor and outcome were originally captured on a Likert scale.
Our findings underscore the significant impact that work climate has on accurate information exchange, and ultimately patient safety. Improving patient safety is imperative for hospitals, especially within the context of recent regulations stemming from the Affordable Care Act that incentivize hospitals to reduce readmissions[18] and improve transitions of care.[19] Because accurate health information exchange is a critical component of patient care, resolving barriers that decrease the accuracy of this exchange is essential. Therefore, future studies need to continue examining these associations within the context of study designs that incorporate longitudinal data and datasets that include objective measures capturing crisis mode work climates and information exchange problems. Because effective communication during handoffs is associated with decreases in medical errors and readmissions, hospitals need to continually ensure that work environments are conducive to effective patient information exchange.
Disclosures
Nothing to report
Using electronic health records to improve the continuity of care between hospital units does not replace the need for interpersonal communication to improve transitions of care. Hospital personnel play a critical role in accurately exchanging patient information during patient transfers, a process requiring accurate communication between hospital units to prevent system failures.[1] Because poor communication contributes to preventable adverse events,[2] and effective communication during handoffs decreases medical errors and readmissions,[3] hospitals need to ensure their work environments are conducive to effective communication.
Individuals working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information,[4] partially due to limited ability to accurately process and communicate information under stressful circumstances. Furthermore, because time‐constrained decision makers tend to use less information and less rigorous decision strategies,[5] work climates characterized by staff members doing too many things too quickly could cause patient health information to be lost during transitions of care across hospital units.
Current studies illustrate scenarios in which demanding or time‐constrained work environments caused information exchange errors. One study found that the increased rate of prescribing errors was partially attributed to a high‐demand work environment characterized by working after hours and multitasking.[6] Other studies found that clinicians' limited time to relay and respond to information and ask clarifying questions during patient handoffs was partially attributed to the fast‐faced and chaotic environment of the emergency room.[7, 8] These studies are consistent with another study that found patient handoffs between emergency departments and inpatient wards were inadequate, partially due to less interactive and more rushed communication.[9] The fact that communication breakdowns are widely cited as barriers to patient handoffs[7, 8, 10] and facilitators of medical errors,[7, 8] further underscores the detrimental effect that crisis mode work climates could have on exchanging patient information during transitions of care.
The objective of this analysis was to evaluate the extent to which a crisis mode work climate impacts the occurrence of patient information exchange problems. Estimating associations between hospital staff members' perceptions of crisis mode work climates and perceptions of information exchange problems provide insights as to whether high‐demand and time‐constrained work climates negatively impact the exchange of patient information. Because hospital staff members working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information, we hypothesized that higher levels of a perceived crisis mode work climate would be associated with higher levels of perceived problems with information exchange across hospital units.
METHODS
Data Source
Data originated from the Agency of Healthcare Research and Quality 2010 Hospital Survey on Patient Safety Culture. This validated survey, designed to assess the safety climate within acute‐care settings, remains an important annual survey deployed each year to track changes and factors impacting patient safety.[11] We included only those respondents who self‐reported their position as a nurse, physician, pharmacist, dietician, therapist, technician, patient care assistant, or hospital unit secretary, all of whom are likely responsible for exchanging patient information across hospital units. For this reason, we excluded respondents who self‐reported their position as administrative or miscellaneous. Applying these exclusion criteria resulted in 247,104 respondents across 884 hospitals.
Conceptual Framework
The relationship between perceived crisis mode work climates and patient information exchange problems is likely influenced by staff skill levels, work climate, and infrastructure (Figure 1). With respect to skill levels, hospital staff members with many years of experience compared to those with fewer years may be relatively desensitized to chaotic work environments and consequently have higher thresholds for perceiving crisis modes. Number of hours worked per week likely impacts perceived crisis mode as illustrated in 1 study finding that full‐time nurses reported a significantly lower work pace compared to part‐time nurses.[12] Years of experience likely impacts perception of information exchange problems, particularly if staff members with many years of experience are familiar enough with hospital systems or protocols to easily detect exchange errors or mishaps.

With respect to work climates, workers' perception of cooperation, coordination and patient safety, and specific hospital unit likely impact perceptions of crisis work mode and information exchange problems. For example, hospital staff members reporting high levels of cooperation, coordination, and patient safety likely perceive fewer crisis modes and information exchange problems compared to those in less‐cooperative hospital units. Furthermore, the heterogeneity of work cultures across departments within a hospital results in department‐specific perceptions of crisis mode climates and information exchange problems. Infrastructure factors, such as hospital size, teaching and ownership status, and census region, likely impact the amount of resources available for staffing and infrastructure, which in turn could impact work pace and information exchange accuracy.
Variable Definitions
We defined our predictor as the perceived presence of a crisis mode work climate as captured from the survey questionnaire item: We work in crisis mode trying to do too much, too quickly. This question item had a Likert response scale comprised of the following 5 answer choices: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. We created a 3‐level response variable by aggregating the agree and disagree responses, respectively, as the first 2 levels, and retaining the neutral response as the third level. Consequently, those responding strongly disagree or disagree were classified as working in lowcrisis mode work climates and those responding strongly agree or agree were classified as working in high crisis mode work climates. We defined our outcome measure as the presence of patient information exchange problems as captured from the survey questionnaire item: Problems often occur in the exchange of information across hospital units. Because this question item had a Likert response scale similar to the crisis mode question predictor, we also created a 3‐level categorical variable in the same fashion. Consequently, those responding strongly disagree or disagree were classified as perceiving no problems exchanging patient information, and those responding strongly agree or agree were classified as perceiving problems exchanging patient information. For the fewer than 10% of the respondents with missing data on either the predictor our outcome variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
We also included questionnaire items that captured staff skill levels, work climate, and infrastructure as covariates to account for potential confounders (Figure 1). The staff skill levels domain included years of experience working in the hospital, specialty, and unit; current staff position; and extent of patient contact. The work climate domain included respondent perceptions of coordination and cooperation, patient safety, and primary work area or unit in which the provider reported working. The hospital infrastructure domain included bed size, census region, teaching status, and government ownership status. For the fewer than 10% of the respondents with missing data on any of the categorical variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
Analytic Approach
We used multivariable ordinal regressions to estimate the likelihood of perceived problems in patient information exchange conditional upon perceptions of a crisis mode work climate, controlling for staff skill levels, work climate, and hospital infrastructure. Our estimates therefore reflect the likelihood of hospital staff responding strongly agree or agree to the question Problems often occur in the exchange of information across hospital units conditional upon responding strongly agree or agree to the question We work in crisis mode trying to do too much, too quickly. In addition to controlling for hospital‐specific response rates, we also adjusted our standard errors to account for the clustering of respondents within hospitals. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The hospital sample averaged 279 respondents per hospital with a 56% response rate. Most hospitals were located in the Central region of the United States, and 32% and 19% were teaching and government‐owned hospitals, respectively. Forty‐three percent and 44% of the hospitals in the sample were designated as small and medium hospitals, respectively (Table 1).
| Characteristics | % |
|---|---|
| |
| Hospital characteristics, N=884 | |
| Bed size | |
| Small, 199 | 43.5 |
| Medium, 100399 | 43.8 |
| Large, 400 plus | 12.7 |
| Teaching status | |
| Yes | 32.2 |
| No | 67.8 |
| Government ownership | |
| Yes | 19.5 |
| No | 80.5 |
| Census region | |
| Mid‐Atlantic and New England | 8.7 |
| South Atlantic | 14.8 |
| Central | 57.2 |
| Mountain | 7.7 |
| Pacific | 11.5 |
| Response rate, mean (SD) | 0.56 (0.28) |
| Respondents per hospital, mean (SD) | 279 (358) |
| Respondent characteristics, N=274,140 | |
| How long have you worked in your current specialty or profession? | |
| <1 year | 5.8 |
| 15 years | 32.8 |
| 610 years | 16.2 |
| 1115 years | 12.0 |
| 1620 years | 10.6 |
| 21 years | 22.7 |
| How long have you worked in this hospital? | |
| <1 year | 9.8 |
| 15 years | 42.8 |
| 610 years | 17.8 |
| 1115 years | 9.0 |
| 1620 years | 8.2 |
| 21 years | 12.4 |
| How long have you worked in your current hospital work area/unit? | |
| <1 year | 13.1 |
| 15 years | 48.0 |
| 610 years | 18.1 |
| 1115 years | 8.1 |
| 1620 years | 6.0 |
| 21 years | 6.7 |
| Typically, how many hours per week do you work in this hospital? | |
| <20 hours | 4.8 |
| 2039 hours | 39.9 |
| 4059 hours | 48.8 |
| 6079 hours | 4.2 |
| 8099 hours | 2.1 |
| 100 hours | 0.11 |
| What is your staff position in this hospital? | |
| Registered nurse | 51.2 |
| Technician (eg, ECG, lab, radiology) | 14.1 |
| Unit assistant/clerk/secretary | 8.5 |
| Patient care assistant/hospital aide/care partner | 7.4 |
| Physical, occupational, or speech therapist | 3.7 |
| Attending/staff physician | 3.5 |
| LVN/LPN | 3.0 |
| Respiratory therapist | 2.9 |
| Pharmacist | 2.2 |
| Physician assistant/nurse practitioner | 1.4 |
| Resident physician/physician in training | 1.2 |
| Dietician | 0.83 |
| In your staff position, do you typically have direct interaction or contact with patients? | |
| Yes | 86.6 |
| No | 13.4 |
| What is your primary work area or unit in this hospital? | |
| Other | 27.7 |
| Medicine (nonsurgical) | 11.1 |
| Surgery | 10.0 |
| Intensive care unit (any type) | 8.6 |
| Many different hospital units/no specific unit | 6.8 |
| Radiology | 6.2 |
| Emergency department | 5.8 |
| Obstetrics | 4.9 |
| Laboratory | 4.9 |
| Rehabilitation | 4.2 |
| Pediatrics | 3.8 |
| Pharmacy | 3.2 |
| Psychiatry/mental health | 2.1 |
| Anesthesiology | 0.55 |
Thirty‐seven percent of the respondents have worked in their current specialty or profession for 5 years or less (Table 1). Over half of the respondents have worked in their current hospital for 5 years or less, whereas 61% have worked in their current unit within the hospital for 5 years or less. Forty‐nine percent work at least 40 hours per week. Registered nurses and technicians represented the 2 largest subgroups of staff positions, comprising 51% and 14% of the sample, respectively. Dieticians and resident physicians, on the other hand, represented the 2 smallest subgroups of staff positions, comprising 0.83% and 1.2% of the sample, respectively. Eighty‐seven percent of the respondents have direct interaction or contact with patients. Apart from those responding other as their hospital unit, nonsurgical medicine and surgery represented the largest subgroup primary work areas, comprising 11% and 10% of the sample, respectively. In contrast, psychiatry and anesthesiology represented the 2 smallest subgroups of primary work areas, comprising 2.1% and 0.55% of the sample, respectively (Table 1).
Respondents scored relatively high with regard to teamwork and helping each other out under hurried or busy circumstances. For example, 85% agreed or strongly agreed that their unit worked together as a team to get work done when a lot of work needed to be completed quickly, and 68% agreed or strongly agreed that individuals within their unit helped out when an area in their unit became busy (Table 1). Despite this cooperation, 31% agreed or strongly agreed that hospital units did not coordinate well together. Paradoxically, 57% agreed or strongly agreed that there was good cooperation among hospital units that needed to work together. Seventy‐five percent of the respondents reported excellent or very good patient safety levels within their unit, although 53% agreed or strongly agreed that staff worked longer hours than was best for patient care (Table 1).
With regard to perceived crisis mode work climate, 32% and 47% reported agreeing and disagreeing, respectively, that their work unit worked in crisis mode trying to do too much too quickly (Table 2). With regard to perceived problems with patient information exchange, 27% and 36% reported agreeing and disagreeing, respectively, that information exchange problems occurred across hospital units (Table 2).
| Perceptions | % |
|---|---|
| We work in crisis mode trying to do too much, too quickly | |
| Strongly disagree | 8.1 |
| Disagree | 39.2 |
| Neutral | 21.0 |
| Agree | 24.3 |
| Strongly agree | 7.5 |
| Problems often occur in the exchange of information across hospital units | |
| Strongly disagree | 4.6 |
| Disagree | 31.3 |
| Neutral | 37.3 |
| Agree | 24.0 |
| Strongly agree | 2.7 |
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | |
| Strongly disagree | 1.5 |
| Disagree | 6.1 |
| Neutral | 7.5 |
| Agree | 53.6 |
| Strongly agree | 31.2 |
| When one area in this unit gets really busy, others help out. | |
| Strongly disagree | 3.9 |
| Disagree | 13.9 |
| Neutral | 13.7 |
| Agree | 52.6 |
| Strongly agree | 15.8 |
| Hospital units do not coordinate well with each other. | |
| Strongly disagree | 5.6 |
| Disagree | 38.8 |
| Neutral | 23.7 |
| Agree | 25.3 |
| Strongly agree | 6.6 |
| There is good cooperation among hospital units that need to work together. | |
| Strongly disagree | 2.7 |
| Disagree | 15.1 |
| Neutral | 24.7 |
| Agree | 51.1 |
| Strongly agree | 6.3 |
| Please give your work area/unit in this hospital an overall grade on patient safety. | |
| Excellent | 23.0 |
| Very good | 49.8 |
| Acceptable | 21.8 |
| Poor | 4.6 |
| Failing | 0.76 |
| Staff in this unit work longer hours than is best for patient care. | |
| Strongly disagree | 11.5 |
| Disagree | 42.2 |
| Neutral | 23.6 |
| Agree | 18.4 |
| Strongly agree | 6.3 |
In the unadjusted analyses, crisis mode perceptions and information exchange problem perceptions were significantly associated. Among those who agreed that their work unit worked in crisis mode, a larger proportion of respondents agreed (41%) versus disagreed (24%) that problems often occurred in exchanging patient information across units (Table 3). In contrast, among those who disagreed that their work unit worked in crisis mode, a larger proportion of respondents disagreed (47%) versus agreed (19%) that problems often occurred in exchanging patient information across units (Table 3).
| Problems Often Occur in Exchange of Information Across Hospital Units | |||
|---|---|---|---|
| Agree (N=66,115), Row %* | Neutral (N=92,228), Row % | Disagree (N=88,797), Row % | |
| |||
| Crisis Mode Work Climate | |||
| Agree (N=78,253) | 40.8 | 35.4 | 23.8 |
| Neutral (N=51,836)‖ | 22.9 | 48.9 | 28.2 |
| Disagree (N=116,781) | 19.0 | 33.5 | 47.5 |
In the multivariable ordinal regression, compared to those who disagreed that their unit worked in crisis mode, those who agreed were 1.6 times more likely to report that problems often occurred in exchanging patient information across units (odds ratio [OR]: 1.6, 95% confidence interval [CI]: 1.58‐1.65) (Table 4). Additionally, some key covariates were independently associated with perceptions of information exchange problems. Two of these covariates measured workplace coordination. Those who reported that hospital units did not cooperate well together were more likely to report problematic information exchange compared to those who reported that hospital units did cooperate well (OR: 4.7, 95% CI: 4.35.0). Relatedly, those who reported that hospital units did coordinate well were less likely to report problematic information exchange compared to those who reported that hospital units did not coordinate well (OR: 0.10, 95% CI: 0.10‐0.11). Two other covariates measured patient contact and perceptions about long working hours. Those who reported having direct interaction or contact with patients were less likely to report problematic information exchange compared to those who reported not having direct interaction or contact with patients (OR: 0.85, 95% CI: 0.83‐0.87). Those who reported that staff did not work longer hours than was better for patient care were less likely to report problematic information exchange compared to those who did report working longer hours than was better for patient care (OR: 0.76, 95% CI: 0.73 0.79). One covariate measured hospital size. Those who reported working in smaller hospitals were less likely to report problematic information exchange compared to those reporting working in large hospitals (OR: 0.66, 95% CI 0.59‐0.75) (Table 4).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| ||
| Primary predictor of interest | ||
| Crisis mode work climate | ||
| Agree | 3.0 (2.9‐3.1) | 1.6 (1.5‐1.6) |
| Neutral | 1.8 (1.7‐1.8) | 1.3 (1.2‐1.3) |
| Disagree | Reference | Reference |
| Hospital characteristics | ||
| Bed Size | ||
| Small, 624 | 0.51 (0.44‐0.59) | 0.66 (0.59‐0.75) |
| Small, 249 | 0.59 (0.53‐0.66) | 0.77 (0.70‐0.84) |
| Small, 5099 | 0.65 (0.58‐0.73) | 0.78 (0.71‐0.84) |
| Medium, 100199 | 0.85 (0.77‐0.95) | 0.92 (0.86‐1.0) |
| Medium, 200299 | 1.0 (0.98‐1.1) | 0.97 (0.90‐1.0) |
| Medium, 300399 | 0.96 (0.85‐1.1) | 1.0 (0.92‐1.1) |
| Large, 400499 | 0.99 (0.86‐1.1) | 0.96 (0.87‐1.0) |
| Large, 500 plus | Reference | Reference |
| Teaching status | ||
| No | 0.81 (0.76‐0.87) | 1.0 (0.95‐1.0) |
| Yes | Reference | Reference |
| Government ownership | ||
| No | 1.1 (1.01.2) | 1.0 (0.98‐1.1) |
| Yes | Reference | Reference |
| Census region | ||
| Mid‐Atlantic and New England | 1.0 (0.88‐1.1) | 0.91 (0.84‐0.99) |
| South Atlantic | 0.95 (0.85‐1.1) | 1.0 (0.95‐1.1) |
| Central 1 | 0.95 (0.85‐1.0) | 0.95 (0.89‐1.0) |
| Central 2 | 0.71 (0.62‐0.81) | 0.91 (0.83‐0.99) |
| Central 3 | 0.80 (0.71‐0.91) | 0.97 (0.90‐1.0) |
| Central 4 | 0.76 (0.68‐0.86) | 0.93 (0.85‐1.0) |
| Mountain | 0.84 (0.73‐0.96) | 0.98 (0.90‐1.1) |
| Pacific | Reference | Reference |
| Average survey response rate within hospital | 0.65 (0.58‐0.72) | 0.93 (0.82‐1.0) |
| Respondent characteristics | ||
| How long have you worked in your current specialty or profession? | ||
| <1 year | 0.75 (0.73‐0.78) | 1.03 (0.99‐1.1) |
| 15 years | 0.99 (0.97‐1.0) | 1.1 (1.1‐1.1) |
| 610 years | 1.0 (1.01.1) | 0.99 (0.96‐1.0) |
| 1115 years | 1.0 (1.01.1) | 1.0 (0.97‐1.0) |
| 1620 years | 1.0 (0.98‐1.0) | 0.97 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in this hospital? | ||
| <1 year | 0.75 (0.73‐0.77) | 0.90 (0.85‐0.90) |
| 15 years | 1.03 (1.001.05) | 0.99 (0.95‐1.0) |
| 610 years | 1.1 (1.1‐1.1) | 0.99 (0.95‐1.0) |
| 1115 years | 1.1 (1. 01.1) | 1.0 (0.96‐1.0) |
| 1620 years | 1.1 (1.01.1) | 0.98 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in your current hospital work area/unit? | ||
| <1 year | 0.79 (0.76‐0.82) | 0.98 (0.93‐1.0) |
| 15 years | 1.0 (1.01.1) | 1.0 (0.99‐1.1) |
| 610 years | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| 1115 years | 1.1 (1.01.1) | 1.0 (0.99‐1.1) |
| 1620 years | 1.1 (1.01.1) | 1.1 (1.01.1) |
| 21 years | Reference | Reference |
| Typically, how many hours per week do you work in this hospital? | ||
| <20 | 0.63 (0.50‐0.79) | 0.91 (0.72‐1.2) |
| 2039 | 0.75 (0.59‐0.94) | 0.90 (0.71‐1.1) |
| 4059 | 0.87 (0.69‐1.1) | 1.1 (0.85‐1.4) |
| 6079 | 0.95 (0.75‐1.2) | 1.0 (0.82‐1.3) |
| 8099 | 0.99 (0.78‐1.2) | 1.1 (0.86‐1.4) |
| 100 | Reference | Reference |
| What is your staff position in this hospital? | ||
| Registered nurse | 0.92 (0.90‐0.94) | 1.1 (0.98‐1.0) |
| Technician (eg, ECG, lab, radiology) | Reference | Reference |
| Unit assistant/clerk/secretary | 0.79 (0.76‐0.81) | 0.94 (0.80‐0.96) |
| Patient care assistant/hospital aide/care partner | 0.78 (0.75‐0.81) | 0.96 (0.90‐0.98) |
| Physical, occupational, or speech therapist | 0.88 (0.84‐0.92) | 1.2 (1.1‐1.2) |
| Attending/staff physician | 1.0 (0.97‐1.1) | 1.3 (1.2‐1.3) |
| LVN/LPN | 0.89 (0.85‐0.94) | 1.0 (0.92‐1.0) |
| Respiratory therapist | 0.84 (0.80‐0.88) | 0.97 (0.89‐1.0) |
| Pharmacist | 1.5 (1.4‐1.6) | 1.3 (1.1‐1.3) |
| Physician assistant/nurse practitioner | 0.93 (0.87‐1.0) | 1.2 (1.1‐1.2) |
| Resident physician/physician in training | 0.96 (0.89‐1.0) | 1.3 (1.2‐1.4) |
| Dietician | 0.86 (0.79‐0.94) | 1.2 (1.1‐1.3) |
| In your staff position, do you typically have direct interaction or contact with patients? | ||
| Yes | 0.83 (0.82‐0.85) | 0.85 (0.83‐0.87) |
| No | Reference | Reference |
| What is your primary work area or unit in this hospital? | ||
| Other | Reference | Reference |
| Medicine (nonsurgical) | 1.1 (1.01.1) | 0.84 (0.82‐0.89) |
| Surgery | 1.1 (1.1‐1.2) | 0.88 (0.86‐0.91) |
| Intensive care unit (any type) | 0.93 (0.90‐0.96) | 0.78 (0.76‐0.81) |
| Many different hospital units/no specific unit | 1.2 (1.1‐1.2) | 1.0 (0.98‐ 1.0) |
| Radiology | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| Emergency department | 1.0 (0.97‐1.0) | 0.57 (0.55‐0.60) |
| Obstetrics | 0.76 (0.73‐0.79) | 0.66 (0.63‐0.69) |
| Laboratory | 1.2 (1.2‐1.3) | 1.0 (1.01.1) |
| Rehabilitation | 1.0 (0.97‐1.0) | 1.0 (0.98‐1.1) |
| Pediatrics | 0.90 (0.86‐0.94) | 0.83 (0.80‐0.87) |
| Pharmacy | 1.6 (1.5‐1.7) | 1.1 (1.01.2) |
| Psychiatry/mental health | 1.2 (1.1‐1.2) | 0.96 (0.90‐1.0) |
| Anesthesiology | 1.1 (1.01.3) | 0.93 (0.83‐1.0) |
| Respondent perceptions | ||
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | ||
| Strongly disagree | 3.2 (3.03.4) | 1.0 (0.98‐1.1) |
| Disagree | 3.2 (3.13.3) | 1.0 (1.01.1) |
| Neutral | 2.3 (2.2‐2.4) | 0.98 (0.94‐1.0) |
| Agree | 2.3 (2.2‐2.4) | 1.0 (1.002‐1.04) |
| Strongly agree | Reference | Reference |
| Staff in this unit work longer hours than is best for patient care. | ||
| Strongly disagree | 0.51 (0.48‐0.53) | 0.76 (0.73‐0.79) |
| Disagree | 0.68 (0.67‐0.70) | 0.81 (0.78‐0.84) |
| Neutral | 0.94 (0.91‐0.97) | 0.93 (0.90‐0.97) |
| Agree | 1.0 (0.99‐1.1) | 0.94 (0.91‐0.98) |
| Strongly agree | Reference | Reference |
| When 1 area in this unit gets really busy, others help out. | ||
| Strongly disagree | 3.8 (3.7 ‐ 4.0) | 1.0 (0.96‐1.1) |
| Disagree | 3.0 (2.9‐3.1) | 1.0 (0.99‐1.1) |
| Neutral | 2.2 (2.12.3) | 1.0 (0.97‐1.0) |
| Agree | 1.5 (1.5‐1.6) | 0.99 (0.96‐1.0) |
| Strongly agree | Reference | Reference |
| Hospital units do not coordinate well with each other. | ||
| Strongly disagree | 0.03 (0.03‐0.04) | 0.10 (0.10‐0.11) |
| Disagree | 0.08 (0.08‐0.08) | 0.18 (0.17‐0.19) |
| Neutral | 0.21 (0.20‐0.22) | 0.32 (0.30‐0.33) |
| Agree | 0.50 (0.48‐0.52) | 0.61 (0.58‐0.63) |
| Strongly agree | Reference | Reference |
| There is good cooperation among hospital units that need to work together. | ||
| Strongly disagree | 20.1 (18.921.5) | 4.7 (4.35.0) |
| Disagree | 14.2 (13.614.9) | 4.2 (4.14.5) |
| Neutral | 6.7 (6.47.0) | 2.7 (2.6‐2.8) |
| Agree | 2.4 (2.3‐2.5) | 1.6 (1.6‐1.7) |
| Strongly agree | Reference | Reference |
| Please give your work area/unit in this hospital an overall grade on patient safety | ||
| Excellent | 0.13 (0.12‐0.14) | 0.47 (0.42‐0.52) |
| Very good | 0.24 (0.21‐0.26) | 0.63 (0.57‐0.70) |
| Acceptable | 0.49 (0.45‐0.54) | 0.79 (0.72‐0.88) |
| Poor | 0.83 (0.75‐0.92) | 0.92 (0.83‐1.03) |
| Failing | Reference | Reference |
DISCUSSION
Our results illustrate that when hospital staff agree that their hospital works in crisis mode, they are more likely to agree that their hospital unit had frequent problems exchanging patient information across units. Because hospital staff working under time constraints and heavy workloads could potentially be at risk of misinterpreting or delivering inaccurate information, these results imply that crisis mode work climates increase the risk of problematic health information exchange. An equally plausible interpretation could be that problematic patient health information exchange increases the risk of hospital staff perceiving crisis mode work climates. Given that information gaps are associated with patient handoff errors,[14] and that patient handoff errors are associated with adverse events,[2, 3, 6, 8] an urgent need exists to implement information exchange systems that prevent information gaps from harming patients. Consequently, hospitals need to implement workflow strategies that prevent information gaps from undermining patient safety during transitions of care.
Other factors affect information exchange apart from crisis mode work climate, as illustrated by the significant associations of key covariates in the multivariate model. The effect found between perceived coordination and information exchange implies that improving information exchange requires good cooperation and coordination. The effect found between patient contact and information exchange implies that working directly with patients improves either the accuracy or the perception of information exchange. Finally, the effect found between hospital size and information exchange suggests that small hospitals are less likely than large hospitals to have information exchange problem. The geographical dispersion and the complexity of larger institutions could result in information exchange problems due to more confusion and less in‐person communication.
Because problematic patient information exchanges are associated with hospital size, coordination, and patient contact, in addition to crisis mode work climate, multifaceted solutions are necessary to resolve the problem. For example, hospital interventions designed to improve coordination could in turn attenuate perceived crisis modes. Furthermore, tailoring these interventions to hospitals that belong to complex geographically dispersed provider networks would likely decrease errors during transitions of care. Because multiple factors cause information exchange problems, implementing interventions that improve both coordination and crisis mode work climates would likely result in a greater net improvement compared to interventions focused solely on decreasing crisis mode work climates.
Some limitations of our paper are worth noting. First, we did not have information on the volume of data exchanged or the functionality levels of the electronic health record systems, both of which likely impact the accuracy of patient information exchange. For example, hospitals with smaller versus larger amounts of data exchanged could be less prone to error. On the other hand, this risk of error could be reduced even further by implementing robust health information technology (IT) systems that improve the accuracy of information transfer. This is consistent with studies showing that hospitals without computerized provider order entry (CPOE) systems have been shown to have higher medication error rates compared to those hospitals with CPOE systems.[15] Therefore, omitting data volume and health IT capabilities from the multivariate model could introduce unobserved heterogeneity, resulting in biased associations between perceived crisis mode work climate and perceived information exchange problems. Second, the cross‐sectional design limits our ability to infer causality because we are not certain whether the perceived crisis mode occurred before, after, or simultaneously to perceived information exchange problems. Third, the self‐reported nature of the questionnaire items does not provide information on observed levels of crisis mode and exchange problems, which could be inconsistent with perceived levels. Fourth, the relatively low within‐hospital response rate decreases the external validity of our findings. For example, if responders' perceptions of crisis mode or information exchange problems significantly differed from nonresponders, our results would not be generalizable to the larger population of acute‐care hospitals across the United States. Therefore, conclusions should be viewed with caution if applying these results to hospitals with respondents significantly differing from those contained within our sample.
Despite these limitations, the large sample size in conjunction with the use of data from a survey having acceptable psychometric properties[16] strengthens the external and internal validity of our findings. Although questionnaire items measuring perceptions are relatively subjective in nature compared to using metrics that capture observed problems or crisis modes, we argue that staff perception data are equally informative, as they guide organization leaders on how to improve workplace performance. Because a core concept of high reliability organizations (HROs) is to preserve constant awareness by key leaders and staff of the state of the systems and processes that affect patient care,[17] HROs could benefit from knowing the extent to which staff perceptions impact patient care. From a methods perspective, the multivariable ordinal regressions enabled us to control for potential confounders that if omitted could have resulted in biased estimates. Furthermore, low levels of multicollinearity as illustrated by low variation inflation factors enabled us to isolate the independent effect of crisis mode perceptions. Including hospital size and hospital work unit as covariates was an additional methodological strength helping account for the unobserved heterogeneity caused by excluding volume of data exchanged or health IT system capability. For example, because larger compared to smaller hospitals usually have more sophisticated health IT systems,[15] including bed size in the model theoretically captures some of the variation that would have been captured if we were able to include a covariate measuring health IT capability. Last, using ordinal regression facilitates interpretation of the findings because the questionnaire items for the predictor and outcome were originally captured on a Likert scale.
Our findings underscore the significant impact that work climate has on accurate information exchange, and ultimately patient safety. Improving patient safety is imperative for hospitals, especially within the context of recent regulations stemming from the Affordable Care Act that incentivize hospitals to reduce readmissions[18] and improve transitions of care.[19] Because accurate health information exchange is a critical component of patient care, resolving barriers that decrease the accuracy of this exchange is essential. Therefore, future studies need to continue examining these associations within the context of study designs that incorporate longitudinal data and datasets that include objective measures capturing crisis mode work climates and information exchange problems. Because effective communication during handoffs is associated with decreases in medical errors and readmissions, hospitals need to continually ensure that work environments are conducive to effective patient information exchange.
Disclosures
Nothing to report
- , , . Safety and complexity: inter‐departmental relationships as a threat to patient safety in the operating department. J Health Organ Manag. 2006;20(2–3):227–242.
- , , , , . Communication loads on clinical staff in the emergency department. Med J Aust. 2002;176(9):415–418.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , . The Handbook of Group Communication Theory and Research. Thousand Oaks, CA: Sage Publications; 1999.
- , , . Judgment and decision making under time pressure. In: Svenson O, Maule AJ, eds. Time Pressure and Stress in Human Judgment and Decision Making. New York, NY: Plenum Press; 1993:27–40.
- , , , , . Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- , . Emergency physician intershift handovers: an analysis of our transitional care. Pediatr Emerg Care. 2006;22(10):751–754.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- , , , et al. Hospital Survey on Patient Safety Culture: 2010 user comparative database report. (Prepared by Westat, Rockville, MD, under Contract No. HHSA 290200710024C). Rockville, MD: Agency for Healthcare Research and Quality; February 2010. AHRQ Publication No. 10‐0026.
- , , , , . Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag. 2011;19(1):57–68.
- , . The treatment of missing values and its effect in the classifier accuracy. In: Banks D, House L, McMorris FR, Arabie P, Gaul W, eds. Classification, Clustering and Data Mining Applications. Berlin, Germany: Springer‐Verlag; 2004.
- , . A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274–284.
- , , , et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10:199.
- , , , et al. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. Prepared by the Lewin Group under contract no. 290‐04‐0011. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Health policy brief: Medicare hospital readmissions reduction program. Health Affairs. November 12, 2013. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102. Accessed August 11, 2014.
- , , , , . The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754.
- , , . Safety and complexity: inter‐departmental relationships as a threat to patient safety in the operating department. J Health Organ Manag. 2006;20(2–3):227–242.
- , , , , . Communication loads on clinical staff in the emergency department. Med J Aust. 2002;176(9):415–418.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , . The Handbook of Group Communication Theory and Research. Thousand Oaks, CA: Sage Publications; 1999.
- , , . Judgment and decision making under time pressure. In: Svenson O, Maule AJ, eds. Time Pressure and Stress in Human Judgment and Decision Making. New York, NY: Plenum Press; 1993:27–40.
- , , , , . Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- , . Emergency physician intershift handovers: an analysis of our transitional care. Pediatr Emerg Care. 2006;22(10):751–754.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- , , , et al. Hospital Survey on Patient Safety Culture: 2010 user comparative database report. (Prepared by Westat, Rockville, MD, under Contract No. HHSA 290200710024C). Rockville, MD: Agency for Healthcare Research and Quality; February 2010. AHRQ Publication No. 10‐0026.
- , , , , . Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag. 2011;19(1):57–68.
- , . The treatment of missing values and its effect in the classifier accuracy. In: Banks D, House L, McMorris FR, Arabie P, Gaul W, eds. Classification, Clustering and Data Mining Applications. Berlin, Germany: Springer‐Verlag; 2004.
- , . A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274–284.
- , , , et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10:199.
- , , , et al. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. Prepared by the Lewin Group under contract no. 290‐04‐0011. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Health policy brief: Medicare hospital readmissions reduction program. Health Affairs. November 12, 2013. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102. Accessed August 11, 2014.
- , , , , . The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754.
© 2014 Society of Hospital Medicine