User login
Furuncular Myiasis in 2 American Travelers Returning From Senegal
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
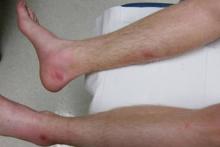
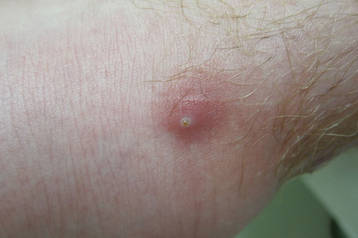
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
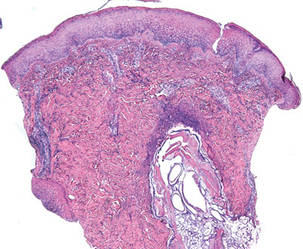
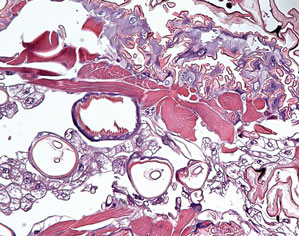
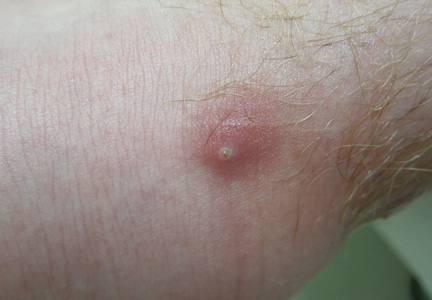
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.

|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.


|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.


|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Practice Points
- Cutaneous myiasis is caused by an infestation of fly larvae and can present as furuncles (furuncular myiasis), migratory inflammatory linear plaques (migratory myiasis), and worsening tissue destruction in existing wounds (wound myiasis).
- Furuncular myiasis should be included in the differential diagnosis in patients with furuncular skin lesions who have recently traveled to Central America, South America, or sub-Saharan Africa.
- Furuncular myiasis may be treated by both occlusive and extraction techniques.
Reduced resident duty hours haven’t changed patient outcomes
Patient mortality and morbidity outcomes have not changed since the most recent round of reforms to medical residents’ duty hours in 2011, according to two of the first nationwide studies to assess these “improvements,” which both were published online Dec. 9 in JAMA.
In addition, one of the studies found no difference between pre-reform and post-reform scores or on pass rates for oral or written national in-training and board certification examinations.
Thus, two separate studies involving millions of hospitalized patients across the country have both found that these reforms had no discernible effect on patient care. However, both groups of researchers cautioned that their studies were observational and therefore subject to potential biases and that they covered only the first 2 years that the duty-hours reforms have been in place.
The 2011 requirements expanded on those enacted in 2003 by further restricting residents’ duty hours, in the hope of reducing medical errors attributed to exhausted residents. The hours of continuous in-hospital duty were reduced from 30 to 16 for first-year residents and to 24 for upper-year residents, and the interval between shifts was increased to at least 8 hours off for first-year residents and at least 14 hours off for upper-year residents.
“Duty hour reform is arguably one of the largest efforts ever undertaken to improve the quality and safety of patient care in teaching hospitals,” said Dr. Mitesh S. Patel of the University of Pennsylvania and the Veterans Affairs Hospital Center for Health Equity Research and Promotion, both in Philadelphia, and his associates.
They assessed 30-day mortality and readmissions among 2,790,356 Medicare patients who were treated either for acute MI, stroke, gastrointestinal bleeding, or heart failure, or who underwent general, orthopedic, or vascular surgery, at 3,104 hospitals between 2009 and 2012. The investigators found no significant associations, either positive or negative, between the reforms to residents’ duty hours and any patient outcomes. Sensitivity analyses confirmed the results of the primary data analyses.
“Our findings suggest that ... the goals of improving the quality and safety of patient care ... were not being achieved. Conversely, concerns that outcomes might actually worsen because of decreased continuity of care have not been borne out,” Dr. Patel and his associates said (JAMA 2014 Dec. 9 [doi:10.1001/jama.2014.15273]).
The investigators noted that their study was limited in that it could not take into account hospitals’ adherence to the new requirements. Their study also did not assess other outcomes such as patient safety indicators or complication rates, which “may better elucidate the relative effects of decreased resident fatigue and increased patient hand offs.” And their study couldn’t address any possible confounding effects from other concurrent policy initiatives aimed at improving care for Medicare beneficiaries, such as the Hospital Readmissions Reduction Program.
In the other study, a separate group of researchers used data from the American College of Surgeons National Surgical Quality Improvement Program to assess outcomes for 535,499 patients who underwent general surgery at 131 hospitals during the 2 years before and the 2 years after the reforms to residents’ duty hours were implemented. This included 23 teaching hospitals in which residents were involved in at least 95% of general surgeries, said Dr. Ravi Rajaram of the division of research and optimal patient care, American College of Surgeons, and the Institute for Public Health and Medicine at Northwestern University, both in Chicago, and his associates.
The reforms were not associated with any change in rates of patient mortality or serious morbidity, either in the study population as a whole or in the subgroups of high-risk and low-risk patients. They also had no effect on secondary outcomes such as surgical-site infection or sepsis. These results remained consistent across several sensitivity analyses.
Neither mean scores for in-training, written board, and oral board examinations nor pass rates for those examinations showed any significant changes during the study period.
“Moreover, first-year trainees, who were most directly affected by the 2011 reforms, did not improve their ABSITE [American Board of Surgery In-Training Examination] scores, despite presumably more free time to prepare,” Dr. Rajaram and his associates said (JAMA 2014 Dec. 9 [doi:10.1001/JAMA.2014.15277]).
They cautioned that their study assessed only the first 2 years following duty-hour reform, and “there may be differences in patient care or resident examination performance that are evident only several years after implementation and adoption of new duty-hour requirements.” In addition, a retrospective observational study such as this one could not produce the high-level evidence needed to guide policy decisions. “To that end, a national multicenter cluster-randomized trial is being conducted (the Flexibility In duty hour Requirements for Surgical Trainees [FIRST] trial), comparing current duty-hour requirements with flexible duty hours to assess the effects of this intervention on patient outcomes and resident well-being. This trial may further inform the debate of how to optimally structure postgraduate training,” they said.
The results of these two large studies are aligned with those of most previous research into the effects of duty hour requirements on patient outcomes. There is a consistent theme: a lack of a major beneficial effect.
Complex problems often demand complex answers. The goal is for the medical profession to move forward with more comprehensive and nuanced approaches to help fulfill its responsibility to provide trainees with the necessary skills to manage fatigue and allow the safest environment for quality care.
Dr. James A. Arrigh is chair of the Accreditation Council for Graduate Medical Education (ACGME) residency review committee for internal medicine. Dr. James C. Hebert is chair of the ACGME Council of Review Committee Chairs. They made these remarks in an editorial accompanying the studies.
The results of these two large studies are aligned with those of most previous research into the effects of duty hour requirements on patient outcomes. There is a consistent theme: a lack of a major beneficial effect.
Complex problems often demand complex answers. The goal is for the medical profession to move forward with more comprehensive and nuanced approaches to help fulfill its responsibility to provide trainees with the necessary skills to manage fatigue and allow the safest environment for quality care.
Dr. James A. Arrigh is chair of the Accreditation Council for Graduate Medical Education (ACGME) residency review committee for internal medicine. Dr. James C. Hebert is chair of the ACGME Council of Review Committee Chairs. They made these remarks in an editorial accompanying the studies.
The results of these two large studies are aligned with those of most previous research into the effects of duty hour requirements on patient outcomes. There is a consistent theme: a lack of a major beneficial effect.
Complex problems often demand complex answers. The goal is for the medical profession to move forward with more comprehensive and nuanced approaches to help fulfill its responsibility to provide trainees with the necessary skills to manage fatigue and allow the safest environment for quality care.
Dr. James A. Arrigh is chair of the Accreditation Council for Graduate Medical Education (ACGME) residency review committee for internal medicine. Dr. James C. Hebert is chair of the ACGME Council of Review Committee Chairs. They made these remarks in an editorial accompanying the studies.
Patient mortality and morbidity outcomes have not changed since the most recent round of reforms to medical residents’ duty hours in 2011, according to two of the first nationwide studies to assess these “improvements,” which both were published online Dec. 9 in JAMA.
In addition, one of the studies found no difference between pre-reform and post-reform scores or on pass rates for oral or written national in-training and board certification examinations.
Thus, two separate studies involving millions of hospitalized patients across the country have both found that these reforms had no discernible effect on patient care. However, both groups of researchers cautioned that their studies were observational and therefore subject to potential biases and that they covered only the first 2 years that the duty-hours reforms have been in place.
The 2011 requirements expanded on those enacted in 2003 by further restricting residents’ duty hours, in the hope of reducing medical errors attributed to exhausted residents. The hours of continuous in-hospital duty were reduced from 30 to 16 for first-year residents and to 24 for upper-year residents, and the interval between shifts was increased to at least 8 hours off for first-year residents and at least 14 hours off for upper-year residents.
“Duty hour reform is arguably one of the largest efforts ever undertaken to improve the quality and safety of patient care in teaching hospitals,” said Dr. Mitesh S. Patel of the University of Pennsylvania and the Veterans Affairs Hospital Center for Health Equity Research and Promotion, both in Philadelphia, and his associates.
They assessed 30-day mortality and readmissions among 2,790,356 Medicare patients who were treated either for acute MI, stroke, gastrointestinal bleeding, or heart failure, or who underwent general, orthopedic, or vascular surgery, at 3,104 hospitals between 2009 and 2012. The investigators found no significant associations, either positive or negative, between the reforms to residents’ duty hours and any patient outcomes. Sensitivity analyses confirmed the results of the primary data analyses.
“Our findings suggest that ... the goals of improving the quality and safety of patient care ... were not being achieved. Conversely, concerns that outcomes might actually worsen because of decreased continuity of care have not been borne out,” Dr. Patel and his associates said (JAMA 2014 Dec. 9 [doi:10.1001/jama.2014.15273]).
The investigators noted that their study was limited in that it could not take into account hospitals’ adherence to the new requirements. Their study also did not assess other outcomes such as patient safety indicators or complication rates, which “may better elucidate the relative effects of decreased resident fatigue and increased patient hand offs.” And their study couldn’t address any possible confounding effects from other concurrent policy initiatives aimed at improving care for Medicare beneficiaries, such as the Hospital Readmissions Reduction Program.
In the other study, a separate group of researchers used data from the American College of Surgeons National Surgical Quality Improvement Program to assess outcomes for 535,499 patients who underwent general surgery at 131 hospitals during the 2 years before and the 2 years after the reforms to residents’ duty hours were implemented. This included 23 teaching hospitals in which residents were involved in at least 95% of general surgeries, said Dr. Ravi Rajaram of the division of research and optimal patient care, American College of Surgeons, and the Institute for Public Health and Medicine at Northwestern University, both in Chicago, and his associates.
The reforms were not associated with any change in rates of patient mortality or serious morbidity, either in the study population as a whole or in the subgroups of high-risk and low-risk patients. They also had no effect on secondary outcomes such as surgical-site infection or sepsis. These results remained consistent across several sensitivity analyses.
Neither mean scores for in-training, written board, and oral board examinations nor pass rates for those examinations showed any significant changes during the study period.
“Moreover, first-year trainees, who were most directly affected by the 2011 reforms, did not improve their ABSITE [American Board of Surgery In-Training Examination] scores, despite presumably more free time to prepare,” Dr. Rajaram and his associates said (JAMA 2014 Dec. 9 [doi:10.1001/JAMA.2014.15277]).
They cautioned that their study assessed only the first 2 years following duty-hour reform, and “there may be differences in patient care or resident examination performance that are evident only several years after implementation and adoption of new duty-hour requirements.” In addition, a retrospective observational study such as this one could not produce the high-level evidence needed to guide policy decisions. “To that end, a national multicenter cluster-randomized trial is being conducted (the Flexibility In duty hour Requirements for Surgical Trainees [FIRST] trial), comparing current duty-hour requirements with flexible duty hours to assess the effects of this intervention on patient outcomes and resident well-being. This trial may further inform the debate of how to optimally structure postgraduate training,” they said.
Patient mortality and morbidity outcomes have not changed since the most recent round of reforms to medical residents’ duty hours in 2011, according to two of the first nationwide studies to assess these “improvements,” which both were published online Dec. 9 in JAMA.
In addition, one of the studies found no difference between pre-reform and post-reform scores or on pass rates for oral or written national in-training and board certification examinations.
Thus, two separate studies involving millions of hospitalized patients across the country have both found that these reforms had no discernible effect on patient care. However, both groups of researchers cautioned that their studies were observational and therefore subject to potential biases and that they covered only the first 2 years that the duty-hours reforms have been in place.
The 2011 requirements expanded on those enacted in 2003 by further restricting residents’ duty hours, in the hope of reducing medical errors attributed to exhausted residents. The hours of continuous in-hospital duty were reduced from 30 to 16 for first-year residents and to 24 for upper-year residents, and the interval between shifts was increased to at least 8 hours off for first-year residents and at least 14 hours off for upper-year residents.
“Duty hour reform is arguably one of the largest efforts ever undertaken to improve the quality and safety of patient care in teaching hospitals,” said Dr. Mitesh S. Patel of the University of Pennsylvania and the Veterans Affairs Hospital Center for Health Equity Research and Promotion, both in Philadelphia, and his associates.
They assessed 30-day mortality and readmissions among 2,790,356 Medicare patients who were treated either for acute MI, stroke, gastrointestinal bleeding, or heart failure, or who underwent general, orthopedic, or vascular surgery, at 3,104 hospitals between 2009 and 2012. The investigators found no significant associations, either positive or negative, between the reforms to residents’ duty hours and any patient outcomes. Sensitivity analyses confirmed the results of the primary data analyses.
“Our findings suggest that ... the goals of improving the quality and safety of patient care ... were not being achieved. Conversely, concerns that outcomes might actually worsen because of decreased continuity of care have not been borne out,” Dr. Patel and his associates said (JAMA 2014 Dec. 9 [doi:10.1001/jama.2014.15273]).
The investigators noted that their study was limited in that it could not take into account hospitals’ adherence to the new requirements. Their study also did not assess other outcomes such as patient safety indicators or complication rates, which “may better elucidate the relative effects of decreased resident fatigue and increased patient hand offs.” And their study couldn’t address any possible confounding effects from other concurrent policy initiatives aimed at improving care for Medicare beneficiaries, such as the Hospital Readmissions Reduction Program.
In the other study, a separate group of researchers used data from the American College of Surgeons National Surgical Quality Improvement Program to assess outcomes for 535,499 patients who underwent general surgery at 131 hospitals during the 2 years before and the 2 years after the reforms to residents’ duty hours were implemented. This included 23 teaching hospitals in which residents were involved in at least 95% of general surgeries, said Dr. Ravi Rajaram of the division of research and optimal patient care, American College of Surgeons, and the Institute for Public Health and Medicine at Northwestern University, both in Chicago, and his associates.
The reforms were not associated with any change in rates of patient mortality or serious morbidity, either in the study population as a whole or in the subgroups of high-risk and low-risk patients. They also had no effect on secondary outcomes such as surgical-site infection or sepsis. These results remained consistent across several sensitivity analyses.
Neither mean scores for in-training, written board, and oral board examinations nor pass rates for those examinations showed any significant changes during the study period.
“Moreover, first-year trainees, who were most directly affected by the 2011 reforms, did not improve their ABSITE [American Board of Surgery In-Training Examination] scores, despite presumably more free time to prepare,” Dr. Rajaram and his associates said (JAMA 2014 Dec. 9 [doi:10.1001/JAMA.2014.15277]).
They cautioned that their study assessed only the first 2 years following duty-hour reform, and “there may be differences in patient care or resident examination performance that are evident only several years after implementation and adoption of new duty-hour requirements.” In addition, a retrospective observational study such as this one could not produce the high-level evidence needed to guide policy decisions. “To that end, a national multicenter cluster-randomized trial is being conducted (the Flexibility In duty hour Requirements for Surgical Trainees [FIRST] trial), comparing current duty-hour requirements with flexible duty hours to assess the effects of this intervention on patient outcomes and resident well-being. This trial may further inform the debate of how to optimally structure postgraduate training,” they said.
Key clinical point: The newest (2011) reforms to resident duty hours haven’t changed patient mortality or morbidity outcomes.
Major finding: 30-day mortality and readmissions among almost 3 million Medicare patients at 3,104 hospitals did not change between 2009 and 2012.
Data source: Two observational cohort studies of millions of hospitalized adults across the country, comparing patient outcomes before with those after the 2011 reforms in duty hours for residents.
Disclosures: Dr. Patel’s study was funded in part by the National Heart, Lung, and Blood Institute, the Department of Veterans Affairs, and the Robert Wood Johnson Foundation. Dr. Rajaram’s study was supported by the Agency for Healthcare Research and Quality, the American College of Surgeons, and Merck. All of the investigators reported having no relevant financial conflicts of interest.
Investigational sotatercept improves heme parameters in MDS
SAN FRANCISCO – A first-in-class investigational agent called sotatercept appears to be safe and to improve hematologic parameters in patients with lower-risk myelodysplastic syndrome or nonproliferative chronic myelomonocytic leukemia and anemia requiring transfusion, a study showed.
In the open-label phase II dose-finding study of sotatercept in patients with myelodysplastic syndrome (MDS) or nonproliferative chronic myelomonocytic leukemia (CMML), hematologic improvement according to International Working Group (IWG) 2006 criteria was seen in 24 of 53 evaluable patients, said Dr. Rami Komrokji of the Moffitt Cancer Center,Tampa.
The patients were all refractory to, or were deemed to have a low chance of responding to, an erythropoiesis-stimulating agent (ESA), Dr. Komrokji said at the annual meeting of the American Society of Hematology.
“A medication like sotatercept would probably have a role in the management of anemia in lower-risk MDS patients. The treatment is administered every 3 weeks, which makes it also logistically easier for the patients to get the treatment. I don’t think we have seen any safety concern, at least at this point, about the chronic use of this medication,” he said in an interview.
Sotatercept (ACE-011) is an activin type IIA receptor fusion protein that acts on late-stage erythropoiesis to increase the release of mature erythrocytes into circulation. The mechanism of action is distinct from that of erythropoietins such as epoetin alfa (Procrit, Epogen) or darbapoietin alfa (Aranesp).
In clinical trials with healthy volunteers, sotatercept has been shown to increase hemoglobin levels, suggesting that it could help to reduce anemia and perhaps lessen dependence on transfusions among patients with lower-risk MDS, Dr. Komrokji said.
He and his colleagues at centers in the United States and France enrolled patients with low-risk or intermediate-1–risk MDS as defined by the International Prognostic Scoring System (IPSS), or nonproliferative CMML (fewer than 13,000 white blood cells per microliter). The patients had to have anemia requiring at least 2 red blood cell (RBC) transfusions in the 12 weeks before enrollment for hemoglobin levels below 9.0 g/dL, and no response, loss of response, or a low chance of response to an ESA. Those patients with serum erythropoietin levels greater than 500 mIU/mL were considered to have a low chance of responding to an ESA.
The patients received subcutaneous injections of sotatercept at doses of 0.1, 0.3, 0.5, or 1.0 mg/kg once every 3 weeks.
As noted, the rate of overall hematologic improvement by IWG 2006 criteria was 45%, occurring in 24 of 53 patients available for evaluation. Five of 44 patients with a high transfusion burden (4 or more RBC units required within 8 weeks) were able to be free of RBC transfusions for at least 8 weeks, as were 5 of 9 with a low transfusion burden (fewer than 4 RBC units over a period of 8 weeks).
Looking at the efficacy in patients with a high transfusion burden, the investigators found that 4 of 6 assigned to the 0.3-mg/kg dose group and 8 of 14 assigned to the 1-mg/kg dose group had a reduction in transfusion burden. The median duration of effect was 106 days, with the longest response lasting for 150 days.
There were no major adverse events in the study, and no apparent increase in risk for thrombosis, as had been seen in some studies of ESAs. Another theoretical risk with this type of agent is hypertension, but there was only one grade 3 case and no grade 4 cases of hypertension in the study, Dr. Komrokji said.
Sotatercept is currently in phase II trials for anemia related to hematologic malignancies and other diseases.
SAN FRANCISCO – A first-in-class investigational agent called sotatercept appears to be safe and to improve hematologic parameters in patients with lower-risk myelodysplastic syndrome or nonproliferative chronic myelomonocytic leukemia and anemia requiring transfusion, a study showed.
In the open-label phase II dose-finding study of sotatercept in patients with myelodysplastic syndrome (MDS) or nonproliferative chronic myelomonocytic leukemia (CMML), hematologic improvement according to International Working Group (IWG) 2006 criteria was seen in 24 of 53 evaluable patients, said Dr. Rami Komrokji of the Moffitt Cancer Center,Tampa.
The patients were all refractory to, or were deemed to have a low chance of responding to, an erythropoiesis-stimulating agent (ESA), Dr. Komrokji said at the annual meeting of the American Society of Hematology.
“A medication like sotatercept would probably have a role in the management of anemia in lower-risk MDS patients. The treatment is administered every 3 weeks, which makes it also logistically easier for the patients to get the treatment. I don’t think we have seen any safety concern, at least at this point, about the chronic use of this medication,” he said in an interview.
Sotatercept (ACE-011) is an activin type IIA receptor fusion protein that acts on late-stage erythropoiesis to increase the release of mature erythrocytes into circulation. The mechanism of action is distinct from that of erythropoietins such as epoetin alfa (Procrit, Epogen) or darbapoietin alfa (Aranesp).
In clinical trials with healthy volunteers, sotatercept has been shown to increase hemoglobin levels, suggesting that it could help to reduce anemia and perhaps lessen dependence on transfusions among patients with lower-risk MDS, Dr. Komrokji said.
He and his colleagues at centers in the United States and France enrolled patients with low-risk or intermediate-1–risk MDS as defined by the International Prognostic Scoring System (IPSS), or nonproliferative CMML (fewer than 13,000 white blood cells per microliter). The patients had to have anemia requiring at least 2 red blood cell (RBC) transfusions in the 12 weeks before enrollment for hemoglobin levels below 9.0 g/dL, and no response, loss of response, or a low chance of response to an ESA. Those patients with serum erythropoietin levels greater than 500 mIU/mL were considered to have a low chance of responding to an ESA.
The patients received subcutaneous injections of sotatercept at doses of 0.1, 0.3, 0.5, or 1.0 mg/kg once every 3 weeks.
As noted, the rate of overall hematologic improvement by IWG 2006 criteria was 45%, occurring in 24 of 53 patients available for evaluation. Five of 44 patients with a high transfusion burden (4 or more RBC units required within 8 weeks) were able to be free of RBC transfusions for at least 8 weeks, as were 5 of 9 with a low transfusion burden (fewer than 4 RBC units over a period of 8 weeks).
Looking at the efficacy in patients with a high transfusion burden, the investigators found that 4 of 6 assigned to the 0.3-mg/kg dose group and 8 of 14 assigned to the 1-mg/kg dose group had a reduction in transfusion burden. The median duration of effect was 106 days, with the longest response lasting for 150 days.
There were no major adverse events in the study, and no apparent increase in risk for thrombosis, as had been seen in some studies of ESAs. Another theoretical risk with this type of agent is hypertension, but there was only one grade 3 case and no grade 4 cases of hypertension in the study, Dr. Komrokji said.
Sotatercept is currently in phase II trials for anemia related to hematologic malignancies and other diseases.
SAN FRANCISCO – A first-in-class investigational agent called sotatercept appears to be safe and to improve hematologic parameters in patients with lower-risk myelodysplastic syndrome or nonproliferative chronic myelomonocytic leukemia and anemia requiring transfusion, a study showed.
In the open-label phase II dose-finding study of sotatercept in patients with myelodysplastic syndrome (MDS) or nonproliferative chronic myelomonocytic leukemia (CMML), hematologic improvement according to International Working Group (IWG) 2006 criteria was seen in 24 of 53 evaluable patients, said Dr. Rami Komrokji of the Moffitt Cancer Center,Tampa.
The patients were all refractory to, or were deemed to have a low chance of responding to, an erythropoiesis-stimulating agent (ESA), Dr. Komrokji said at the annual meeting of the American Society of Hematology.
“A medication like sotatercept would probably have a role in the management of anemia in lower-risk MDS patients. The treatment is administered every 3 weeks, which makes it also logistically easier for the patients to get the treatment. I don’t think we have seen any safety concern, at least at this point, about the chronic use of this medication,” he said in an interview.
Sotatercept (ACE-011) is an activin type IIA receptor fusion protein that acts on late-stage erythropoiesis to increase the release of mature erythrocytes into circulation. The mechanism of action is distinct from that of erythropoietins such as epoetin alfa (Procrit, Epogen) or darbapoietin alfa (Aranesp).
In clinical trials with healthy volunteers, sotatercept has been shown to increase hemoglobin levels, suggesting that it could help to reduce anemia and perhaps lessen dependence on transfusions among patients with lower-risk MDS, Dr. Komrokji said.
He and his colleagues at centers in the United States and France enrolled patients with low-risk or intermediate-1–risk MDS as defined by the International Prognostic Scoring System (IPSS), or nonproliferative CMML (fewer than 13,000 white blood cells per microliter). The patients had to have anemia requiring at least 2 red blood cell (RBC) transfusions in the 12 weeks before enrollment for hemoglobin levels below 9.0 g/dL, and no response, loss of response, or a low chance of response to an ESA. Those patients with serum erythropoietin levels greater than 500 mIU/mL were considered to have a low chance of responding to an ESA.
The patients received subcutaneous injections of sotatercept at doses of 0.1, 0.3, 0.5, or 1.0 mg/kg once every 3 weeks.
As noted, the rate of overall hematologic improvement by IWG 2006 criteria was 45%, occurring in 24 of 53 patients available for evaluation. Five of 44 patients with a high transfusion burden (4 or more RBC units required within 8 weeks) were able to be free of RBC transfusions for at least 8 weeks, as were 5 of 9 with a low transfusion burden (fewer than 4 RBC units over a period of 8 weeks).
Looking at the efficacy in patients with a high transfusion burden, the investigators found that 4 of 6 assigned to the 0.3-mg/kg dose group and 8 of 14 assigned to the 1-mg/kg dose group had a reduction in transfusion burden. The median duration of effect was 106 days, with the longest response lasting for 150 days.
There were no major adverse events in the study, and no apparent increase in risk for thrombosis, as had been seen in some studies of ESAs. Another theoretical risk with this type of agent is hypertension, but there was only one grade 3 case and no grade 4 cases of hypertension in the study, Dr. Komrokji said.
Sotatercept is currently in phase II trials for anemia related to hematologic malignancies and other diseases.
Key clinical point: Sotatercept is a first-in-its-class agent that stimulates erythropoiesis through a mechanism different from that of erythropoietins.
Major finding: The rate of overall hematologic improvement by IWG 2006 criteria was 45%, occurring in 24 of 53 patients available for evaluation.
Data source: An ongoing phase II study with data available on 53 patients with MDS or nonproliferative CMML.
Disclosures: The study is sponsored by Celgene. Dr. Komrokji reported consulting for and receiving research funding from the company.
Nilotinib plus chemotherapy pays off for older patients with Ph+ALL
SAN FRANCISCO– The study was small but encouraging: Among 47 older patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome, 41 had a complete hematologic response to a combination of chemotherapy and the targeted agent nilotinib (Tasigna), report investigators from a European consortium.
“The data I have presented show that the combination of nilotinib with this age-adapted chemotherapy is highly effective. We do have quite a reasonable overall survival estimate at 2 years of just more than 70%,” said Dr. Oliver Ottmann of Goethe University in Frankfurt, on behalf of colleagues in the European Working Group for Adult ALL (EWALL).
The study also shows that although some centers are reluctant to offer allogeneic stem cell transplantation (SCT) to older patients, it is still a viable treatment option in this population, Dr. Ottmann said at a briefing at the annual meeting of the American Society of Hematology.
Although older patients with newly diagnosed Philadelphia-positive (Ph+) ALL have a high complete hematologic response rate (CHR) with imatinib (Gleevec), they generally have a poor prognosis because of a high rate of relapse.
Because nilotinib, a potent inhibitor of the ABL kinase, has good efficacy in the chronic and accelerated phase of Ph+ chronic myeloid leukemia, the EWALL investigators initiated a study to evaluate it in combination with chemotherapy in the front-line setting.
Adults aged 55 years and older with ALL positive for the Philadelphia chromosome and/or BCR-ABL1 fusion who were treatment naive or had not received therapy other than corticosteroids, single-dose vincristine, or three doses of cyclophosphamide were eligible.
Details of the combination regimen are available online.
Briefly, following a prephase with dexamethasone and optional cyclophosphamide, patients receive nilotinib 400 mg twice daily starting with induction and continuously thereafter. During induction, nilotinib is given with intravenous injections of vincristine and dexamethasone for 4 weeks, followed by consolidation with nilotinib, methotrexate, asparaginase and cytarabine. Maintenance consists of nilotinib, 6-mercaptopurine, and methotrexate once weekly for 1 month then every other month, and dexamethasone and vincristine in 2 month intervals up to 24 months.
The data Dr. Ottmann reported come from an interim analysis of the ongoing study. As of August 2014, data on 47 of 56 patients was available for an efficacy analysis, As noted before, the rate of CHR was 87%, occurring in 41 of 47 patients. The treatment evoked a partial response or no response in 2 patients, and there was one death during the induction phase. Additionally, three patients discontinued therapy early and were not included in the assessment, but at least one had a complete response later on, Dr. Ottmann noted.
The median time to a complete response (CR) was 41 days, but CRs occurred as early as 25 days and as late as 62 days after the start of therapy. The remissions at the time of data cutoff appeared to be durable, but follow-up is still early, he said.
Overall survival at a median follow-up for all patients of 8.6 months was 72.7% at 30 months for patients who did not undergo SCT (allowed under the protocol), and 67.1% at 30 months for patients who underwent SCT. This difference was not significant, but only nine patients at the time of data cutoff had undergone transplantation.
“It will be interesting to see how this will proceed if the transplant-free patients will do as well as the others,” Dr. Ottmann said.
An analysis of molecular response by minimal residual disease (MRD) time point showed a significant further increase with the consolidation chemotherapy and kinase inhibitor, emphasizing that “continuing the treatment in this form emphasizes the depth of response. If we then look at the rate of MRD negativity using high quality assays, then a quarter of the patients have undetectable polymerase chain reaction during the consolidation cycles, and approximately 80% achieves something that we call a major molecular response,” he said.
Dr. Ottmann did not provide updated safety data, but at the time of the data cutoff, there had been 34 serious adverse events reported, 11 of which occurred during induction, 16 during consolidation, 6 during maintenance, and 1 following discontinuation. The most-common adverse events were infections and neutropenic fevers. Single serious adverse events included metabolic, cardiovascular, neurologic, renal, and hepatic events.
The trial is expected to be completed in the next few months.
SAN FRANCISCO– The study was small but encouraging: Among 47 older patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome, 41 had a complete hematologic response to a combination of chemotherapy and the targeted agent nilotinib (Tasigna), report investigators from a European consortium.
“The data I have presented show that the combination of nilotinib with this age-adapted chemotherapy is highly effective. We do have quite a reasonable overall survival estimate at 2 years of just more than 70%,” said Dr. Oliver Ottmann of Goethe University in Frankfurt, on behalf of colleagues in the European Working Group for Adult ALL (EWALL).
The study also shows that although some centers are reluctant to offer allogeneic stem cell transplantation (SCT) to older patients, it is still a viable treatment option in this population, Dr. Ottmann said at a briefing at the annual meeting of the American Society of Hematology.
Although older patients with newly diagnosed Philadelphia-positive (Ph+) ALL have a high complete hematologic response rate (CHR) with imatinib (Gleevec), they generally have a poor prognosis because of a high rate of relapse.
Because nilotinib, a potent inhibitor of the ABL kinase, has good efficacy in the chronic and accelerated phase of Ph+ chronic myeloid leukemia, the EWALL investigators initiated a study to evaluate it in combination with chemotherapy in the front-line setting.
Adults aged 55 years and older with ALL positive for the Philadelphia chromosome and/or BCR-ABL1 fusion who were treatment naive or had not received therapy other than corticosteroids, single-dose vincristine, or three doses of cyclophosphamide were eligible.
Details of the combination regimen are available online.
Briefly, following a prephase with dexamethasone and optional cyclophosphamide, patients receive nilotinib 400 mg twice daily starting with induction and continuously thereafter. During induction, nilotinib is given with intravenous injections of vincristine and dexamethasone for 4 weeks, followed by consolidation with nilotinib, methotrexate, asparaginase and cytarabine. Maintenance consists of nilotinib, 6-mercaptopurine, and methotrexate once weekly for 1 month then every other month, and dexamethasone and vincristine in 2 month intervals up to 24 months.
The data Dr. Ottmann reported come from an interim analysis of the ongoing study. As of August 2014, data on 47 of 56 patients was available for an efficacy analysis, As noted before, the rate of CHR was 87%, occurring in 41 of 47 patients. The treatment evoked a partial response or no response in 2 patients, and there was one death during the induction phase. Additionally, three patients discontinued therapy early and were not included in the assessment, but at least one had a complete response later on, Dr. Ottmann noted.
The median time to a complete response (CR) was 41 days, but CRs occurred as early as 25 days and as late as 62 days after the start of therapy. The remissions at the time of data cutoff appeared to be durable, but follow-up is still early, he said.
Overall survival at a median follow-up for all patients of 8.6 months was 72.7% at 30 months for patients who did not undergo SCT (allowed under the protocol), and 67.1% at 30 months for patients who underwent SCT. This difference was not significant, but only nine patients at the time of data cutoff had undergone transplantation.
“It will be interesting to see how this will proceed if the transplant-free patients will do as well as the others,” Dr. Ottmann said.
An analysis of molecular response by minimal residual disease (MRD) time point showed a significant further increase with the consolidation chemotherapy and kinase inhibitor, emphasizing that “continuing the treatment in this form emphasizes the depth of response. If we then look at the rate of MRD negativity using high quality assays, then a quarter of the patients have undetectable polymerase chain reaction during the consolidation cycles, and approximately 80% achieves something that we call a major molecular response,” he said.
Dr. Ottmann did not provide updated safety data, but at the time of the data cutoff, there had been 34 serious adverse events reported, 11 of which occurred during induction, 16 during consolidation, 6 during maintenance, and 1 following discontinuation. The most-common adverse events were infections and neutropenic fevers. Single serious adverse events included metabolic, cardiovascular, neurologic, renal, and hepatic events.
The trial is expected to be completed in the next few months.
SAN FRANCISCO– The study was small but encouraging: Among 47 older patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome, 41 had a complete hematologic response to a combination of chemotherapy and the targeted agent nilotinib (Tasigna), report investigators from a European consortium.
“The data I have presented show that the combination of nilotinib with this age-adapted chemotherapy is highly effective. We do have quite a reasonable overall survival estimate at 2 years of just more than 70%,” said Dr. Oliver Ottmann of Goethe University in Frankfurt, on behalf of colleagues in the European Working Group for Adult ALL (EWALL).
The study also shows that although some centers are reluctant to offer allogeneic stem cell transplantation (SCT) to older patients, it is still a viable treatment option in this population, Dr. Ottmann said at a briefing at the annual meeting of the American Society of Hematology.
Although older patients with newly diagnosed Philadelphia-positive (Ph+) ALL have a high complete hematologic response rate (CHR) with imatinib (Gleevec), they generally have a poor prognosis because of a high rate of relapse.
Because nilotinib, a potent inhibitor of the ABL kinase, has good efficacy in the chronic and accelerated phase of Ph+ chronic myeloid leukemia, the EWALL investigators initiated a study to evaluate it in combination with chemotherapy in the front-line setting.
Adults aged 55 years and older with ALL positive for the Philadelphia chromosome and/or BCR-ABL1 fusion who were treatment naive or had not received therapy other than corticosteroids, single-dose vincristine, or three doses of cyclophosphamide were eligible.
Details of the combination regimen are available online.
Briefly, following a prephase with dexamethasone and optional cyclophosphamide, patients receive nilotinib 400 mg twice daily starting with induction and continuously thereafter. During induction, nilotinib is given with intravenous injections of vincristine and dexamethasone for 4 weeks, followed by consolidation with nilotinib, methotrexate, asparaginase and cytarabine. Maintenance consists of nilotinib, 6-mercaptopurine, and methotrexate once weekly for 1 month then every other month, and dexamethasone and vincristine in 2 month intervals up to 24 months.
The data Dr. Ottmann reported come from an interim analysis of the ongoing study. As of August 2014, data on 47 of 56 patients was available for an efficacy analysis, As noted before, the rate of CHR was 87%, occurring in 41 of 47 patients. The treatment evoked a partial response or no response in 2 patients, and there was one death during the induction phase. Additionally, three patients discontinued therapy early and were not included in the assessment, but at least one had a complete response later on, Dr. Ottmann noted.
The median time to a complete response (CR) was 41 days, but CRs occurred as early as 25 days and as late as 62 days after the start of therapy. The remissions at the time of data cutoff appeared to be durable, but follow-up is still early, he said.
Overall survival at a median follow-up for all patients of 8.6 months was 72.7% at 30 months for patients who did not undergo SCT (allowed under the protocol), and 67.1% at 30 months for patients who underwent SCT. This difference was not significant, but only nine patients at the time of data cutoff had undergone transplantation.
“It will be interesting to see how this will proceed if the transplant-free patients will do as well as the others,” Dr. Ottmann said.
An analysis of molecular response by minimal residual disease (MRD) time point showed a significant further increase with the consolidation chemotherapy and kinase inhibitor, emphasizing that “continuing the treatment in this form emphasizes the depth of response. If we then look at the rate of MRD negativity using high quality assays, then a quarter of the patients have undetectable polymerase chain reaction during the consolidation cycles, and approximately 80% achieves something that we call a major molecular response,” he said.
Dr. Ottmann did not provide updated safety data, but at the time of the data cutoff, there had been 34 serious adverse events reported, 11 of which occurred during induction, 16 during consolidation, 6 during maintenance, and 1 following discontinuation. The most-common adverse events were infections and neutropenic fevers. Single serious adverse events included metabolic, cardiovascular, neurologic, renal, and hepatic events.
The trial is expected to be completed in the next few months.
Key clinical point: Combining a kinase inhibitor with an intensive chemotherapy regimen produces a high complete response rate in patients age 55 and older who have Ph+All.
Major finding: The complete hematologic response rate for the combination of chemotherapy and nilotinib was 87%.
Data source: Investigator initiated study in 56 patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome or BCR/ABL fusion.
Disclosures: The study was sponsored by participating institution. Dr. Ottmann disclosed consultancy, honoraria, and research funding from Novartis, maker of nilotinib.
PFS improvement will translate to OS, speaker says
SAN FRANCISCO—Administering brentuximab vedotin immediately after autologous stem cell transplant can improve progression-free survival (PFS) in patients with Hodgkin lymphoma (HL), results of the phase 3 AETHERA trial suggest.
The overall survival (OS) data for this study are not yet mature, but the significant improvement in PFS will likely translate to improved OS in a few years’ time, according to Craig Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr Moskowitz presented results from the AETHERA trial at the 2014 ASH Annual Meeting as abstract 673. The trial was funded by Seattle Genetics, Inc., and Takeda Pharmaceutical Company Limited, the companies developing brentuximab.
The trial included HL patients with at least one risk factor for progression. Eligible patients must have had a history of refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-transplant relapse.
Researchers enrolled 329 patients, and they were randomized to receive brentuximab or placebo every 3 weeks for up to about a year. Baseline characteristics were similar between the 2 arms.
Dr Moskowitz pointed out that 43% of patients in the brentuximab arm and 48% in the placebo arm had required 2 or more prior salvage therapies, and 60% and 59%, respectively, had primary refractory HL.
Patients in both arms received a median of 15 treatment cycles, with an average of 12 cycles on the brentuximab arm and 11 cycles on the placebo arm.
“Patients who progressed in the placebo arm could be unblinded and subsequently receive brentuximab on a companion study,” Dr Moskowitz noted. “So technically, this was a cross-over design, making overall survival at 24 months quite unlikely.”
Efficacy/survival results
About half of patients in each arm completed treatment—47% in the brentuximab arm and 49% in the placebo arm. The reasons for discontinuation included disease progression (15% and 42%, respectively), adverse events (33% and 6%, respectively), and patient decision (5% and 2%, respectively).
Still, the trial achieved its primary endpoint, demonstrating a significant increase in PFS, according to an independent review facility (IRF).
The median PFS per the IRF was 43 months for patients in the brentuximab arm and 24 months in the placebo arm (hazard ratio=0.57, P=0.001). The 2-year PFS rates per the IRF were 63% and 51%, respectively.
The 2-year PFS rate according to investigators was 65% in the brentuximab arm and 45% in the placebo arm. The median PFS per investigators has not yet been reached for brentuximab but was 16 months for placebo.
The PFS benefit was consistent across all pre-specified subgroups, Dr Moskowitz noted, including primary refractory patients, patients who relapsed within 12 months of frontline therapy, and patients who relapsed after 12 months with extranodal disease.
Patients who experienced disease progression received a variety of subsequent therapies.
In the brentuximab arm, 16% of patients receiving subsequent therapy were treated with brentuximab after relapse. In the placebo arm, 85% of patients receiving subsequent therapy were treated with single-agent brentuximab.
Twenty-eight percent of patients in the placebo arm and 25% in the brentuximab arm received stem cell transplant as subsequent therapy, the majority of which were allogeneic transplants. Dr Moskowitz said a second transplant could have improved survival in these patients, but whether it actually did is unclear.
He noted that the OS data are immature, but there is currently no significant difference in OS between the treatment arms (hazard ratio=1.15; P=0.62).
“The median follow-up right now is 24 months,” he said. “So one will have to wait for a survival advantage or disadvantage, but from my point of view, a PFS of 65% at 2 years will translate to an overall survival difference. We’re just going to have to wait a few more years.”
Dr Moskowitz said another analysis of OS is planned in 2016.
Safety data
The most common adverse events in the brentuximab arm were peripheral sensory neuropathy (56%), neutropenia (35%), upper respiratory tract infection (26%), fatigue (24%), and peripheral motor neuropathy (23%).
The most common adverse events in the placebo arm were upper respiratory tract infection (23%), fatigue (18%), peripheral sensory neuropathy (16%), cough (16%), and neutropenia (12%).
Eighty-five percent of patients with peripheral neuropathy in the brentuximab arm had a resolution or improvement in symptoms, with a median time to improvement of 23.4 weeks.
Grade 3 or higher adverse events in the brentuximab arm included neutropenia, peripheral sensory neuropathy, peripheral motor neuropathy, nausea, fatigue, and diarrhea.
Grade 3 or higher adverse events in the placebo arm included neutropenia, fatigue, peripheral motor neuropathy, diarrhea, and peripheral sensory neuropathy. No Grade 4 peripheral neuropathy events occurred.
One death occurred within 30 days of brentuximab treatment. The patient died from treatment-related acute respiratory distress syndrome (ARDS) associated with pneumonitis.
Another death occurred on the brentuximab arm at day 40 from ARDS following an episode of treatment-related acute pancreatitis, which had resolved at the time of death.
Nevertheless, Dr Moskowitz characterized brentuximab consolidation as “very well-tolerated” in this patient population.
He concluded, “For patients with a remission duration of less than a year, patients with primary refractory Hodgkin lymphoma, and patients with Hodgkin lymphoma with extranodal involvement, I do believe this will become standard treatment.” ![]()
SAN FRANCISCO—Administering brentuximab vedotin immediately after autologous stem cell transplant can improve progression-free survival (PFS) in patients with Hodgkin lymphoma (HL), results of the phase 3 AETHERA trial suggest.
The overall survival (OS) data for this study are not yet mature, but the significant improvement in PFS will likely translate to improved OS in a few years’ time, according to Craig Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr Moskowitz presented results from the AETHERA trial at the 2014 ASH Annual Meeting as abstract 673. The trial was funded by Seattle Genetics, Inc., and Takeda Pharmaceutical Company Limited, the companies developing brentuximab.
The trial included HL patients with at least one risk factor for progression. Eligible patients must have had a history of refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-transplant relapse.
Researchers enrolled 329 patients, and they were randomized to receive brentuximab or placebo every 3 weeks for up to about a year. Baseline characteristics were similar between the 2 arms.
Dr Moskowitz pointed out that 43% of patients in the brentuximab arm and 48% in the placebo arm had required 2 or more prior salvage therapies, and 60% and 59%, respectively, had primary refractory HL.
Patients in both arms received a median of 15 treatment cycles, with an average of 12 cycles on the brentuximab arm and 11 cycles on the placebo arm.
“Patients who progressed in the placebo arm could be unblinded and subsequently receive brentuximab on a companion study,” Dr Moskowitz noted. “So technically, this was a cross-over design, making overall survival at 24 months quite unlikely.”
Efficacy/survival results
About half of patients in each arm completed treatment—47% in the brentuximab arm and 49% in the placebo arm. The reasons for discontinuation included disease progression (15% and 42%, respectively), adverse events (33% and 6%, respectively), and patient decision (5% and 2%, respectively).
Still, the trial achieved its primary endpoint, demonstrating a significant increase in PFS, according to an independent review facility (IRF).
The median PFS per the IRF was 43 months for patients in the brentuximab arm and 24 months in the placebo arm (hazard ratio=0.57, P=0.001). The 2-year PFS rates per the IRF were 63% and 51%, respectively.
The 2-year PFS rate according to investigators was 65% in the brentuximab arm and 45% in the placebo arm. The median PFS per investigators has not yet been reached for brentuximab but was 16 months for placebo.
The PFS benefit was consistent across all pre-specified subgroups, Dr Moskowitz noted, including primary refractory patients, patients who relapsed within 12 months of frontline therapy, and patients who relapsed after 12 months with extranodal disease.
Patients who experienced disease progression received a variety of subsequent therapies.
In the brentuximab arm, 16% of patients receiving subsequent therapy were treated with brentuximab after relapse. In the placebo arm, 85% of patients receiving subsequent therapy were treated with single-agent brentuximab.
Twenty-eight percent of patients in the placebo arm and 25% in the brentuximab arm received stem cell transplant as subsequent therapy, the majority of which were allogeneic transplants. Dr Moskowitz said a second transplant could have improved survival in these patients, but whether it actually did is unclear.
He noted that the OS data are immature, but there is currently no significant difference in OS between the treatment arms (hazard ratio=1.15; P=0.62).
“The median follow-up right now is 24 months,” he said. “So one will have to wait for a survival advantage or disadvantage, but from my point of view, a PFS of 65% at 2 years will translate to an overall survival difference. We’re just going to have to wait a few more years.”
Dr Moskowitz said another analysis of OS is planned in 2016.
Safety data
The most common adverse events in the brentuximab arm were peripheral sensory neuropathy (56%), neutropenia (35%), upper respiratory tract infection (26%), fatigue (24%), and peripheral motor neuropathy (23%).
The most common adverse events in the placebo arm were upper respiratory tract infection (23%), fatigue (18%), peripheral sensory neuropathy (16%), cough (16%), and neutropenia (12%).
Eighty-five percent of patients with peripheral neuropathy in the brentuximab arm had a resolution or improvement in symptoms, with a median time to improvement of 23.4 weeks.
Grade 3 or higher adverse events in the brentuximab arm included neutropenia, peripheral sensory neuropathy, peripheral motor neuropathy, nausea, fatigue, and diarrhea.
Grade 3 or higher adverse events in the placebo arm included neutropenia, fatigue, peripheral motor neuropathy, diarrhea, and peripheral sensory neuropathy. No Grade 4 peripheral neuropathy events occurred.
One death occurred within 30 days of brentuximab treatment. The patient died from treatment-related acute respiratory distress syndrome (ARDS) associated with pneumonitis.
Another death occurred on the brentuximab arm at day 40 from ARDS following an episode of treatment-related acute pancreatitis, which had resolved at the time of death.
Nevertheless, Dr Moskowitz characterized brentuximab consolidation as “very well-tolerated” in this patient population.
He concluded, “For patients with a remission duration of less than a year, patients with primary refractory Hodgkin lymphoma, and patients with Hodgkin lymphoma with extranodal involvement, I do believe this will become standard treatment.” ![]()
SAN FRANCISCO—Administering brentuximab vedotin immediately after autologous stem cell transplant can improve progression-free survival (PFS) in patients with Hodgkin lymphoma (HL), results of the phase 3 AETHERA trial suggest.
The overall survival (OS) data for this study are not yet mature, but the significant improvement in PFS will likely translate to improved OS in a few years’ time, according to Craig Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr Moskowitz presented results from the AETHERA trial at the 2014 ASH Annual Meeting as abstract 673. The trial was funded by Seattle Genetics, Inc., and Takeda Pharmaceutical Company Limited, the companies developing brentuximab.
The trial included HL patients with at least one risk factor for progression. Eligible patients must have had a history of refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-transplant relapse.
Researchers enrolled 329 patients, and they were randomized to receive brentuximab or placebo every 3 weeks for up to about a year. Baseline characteristics were similar between the 2 arms.
Dr Moskowitz pointed out that 43% of patients in the brentuximab arm and 48% in the placebo arm had required 2 or more prior salvage therapies, and 60% and 59%, respectively, had primary refractory HL.
Patients in both arms received a median of 15 treatment cycles, with an average of 12 cycles on the brentuximab arm and 11 cycles on the placebo arm.
“Patients who progressed in the placebo arm could be unblinded and subsequently receive brentuximab on a companion study,” Dr Moskowitz noted. “So technically, this was a cross-over design, making overall survival at 24 months quite unlikely.”
Efficacy/survival results
About half of patients in each arm completed treatment—47% in the brentuximab arm and 49% in the placebo arm. The reasons for discontinuation included disease progression (15% and 42%, respectively), adverse events (33% and 6%, respectively), and patient decision (5% and 2%, respectively).
Still, the trial achieved its primary endpoint, demonstrating a significant increase in PFS, according to an independent review facility (IRF).
The median PFS per the IRF was 43 months for patients in the brentuximab arm and 24 months in the placebo arm (hazard ratio=0.57, P=0.001). The 2-year PFS rates per the IRF were 63% and 51%, respectively.
The 2-year PFS rate according to investigators was 65% in the brentuximab arm and 45% in the placebo arm. The median PFS per investigators has not yet been reached for brentuximab but was 16 months for placebo.
The PFS benefit was consistent across all pre-specified subgroups, Dr Moskowitz noted, including primary refractory patients, patients who relapsed within 12 months of frontline therapy, and patients who relapsed after 12 months with extranodal disease.
Patients who experienced disease progression received a variety of subsequent therapies.
In the brentuximab arm, 16% of patients receiving subsequent therapy were treated with brentuximab after relapse. In the placebo arm, 85% of patients receiving subsequent therapy were treated with single-agent brentuximab.
Twenty-eight percent of patients in the placebo arm and 25% in the brentuximab arm received stem cell transplant as subsequent therapy, the majority of which were allogeneic transplants. Dr Moskowitz said a second transplant could have improved survival in these patients, but whether it actually did is unclear.
He noted that the OS data are immature, but there is currently no significant difference in OS between the treatment arms (hazard ratio=1.15; P=0.62).
“The median follow-up right now is 24 months,” he said. “So one will have to wait for a survival advantage or disadvantage, but from my point of view, a PFS of 65% at 2 years will translate to an overall survival difference. We’re just going to have to wait a few more years.”
Dr Moskowitz said another analysis of OS is planned in 2016.
Safety data
The most common adverse events in the brentuximab arm were peripheral sensory neuropathy (56%), neutropenia (35%), upper respiratory tract infection (26%), fatigue (24%), and peripheral motor neuropathy (23%).
The most common adverse events in the placebo arm were upper respiratory tract infection (23%), fatigue (18%), peripheral sensory neuropathy (16%), cough (16%), and neutropenia (12%).
Eighty-five percent of patients with peripheral neuropathy in the brentuximab arm had a resolution or improvement in symptoms, with a median time to improvement of 23.4 weeks.
Grade 3 or higher adverse events in the brentuximab arm included neutropenia, peripheral sensory neuropathy, peripheral motor neuropathy, nausea, fatigue, and diarrhea.
Grade 3 or higher adverse events in the placebo arm included neutropenia, fatigue, peripheral motor neuropathy, diarrhea, and peripheral sensory neuropathy. No Grade 4 peripheral neuropathy events occurred.
One death occurred within 30 days of brentuximab treatment. The patient died from treatment-related acute respiratory distress syndrome (ARDS) associated with pneumonitis.
Another death occurred on the brentuximab arm at day 40 from ARDS following an episode of treatment-related acute pancreatitis, which had resolved at the time of death.
Nevertheless, Dr Moskowitz characterized brentuximab consolidation as “very well-tolerated” in this patient population.
He concluded, “For patients with a remission duration of less than a year, patients with primary refractory Hodgkin lymphoma, and patients with Hodgkin lymphoma with extranodal involvement, I do believe this will become standard treatment.” ![]()
Method may predict likelihood of GVHD

Credit: Darren Baker
Researchers say that computer modeling of next-generation DNA sequencing data can help us understand the variable outcomes of stem cell transplant and provide a theoretical framework to make transplant a possibility for more patients who don’t have a related donor.
The team analyzed data obtained from whole-exome sequencing of 9 donor-recipient pairs (DRPs) and found it’s possible to predict the risk of graft-vs-host disease (GVHD).
This finding could one day help physicians tailor immunosuppressive therapies to possibly improve transplant outcomes.
The investigators say their data provide evidence that the way a patient’s immune system rebuilds itself following transplant is representative of a dynamical system, a system in which the current state determines what future state will follow.
“The immune system seems chaotic, but that is because there are so many variables involved,” said Amir Toor, MD, of the Virginia Commonwealth University in Richmond.
“We have found evidence of an underlying order. Using next-generation DNA sequencing technology, it may be possible to account for many of the molecular variables that eventually determine how well a donor’s immune system will graft to a patient.”
Dr Toor and his colleagues describe this work in two articles in Frontiers in Immunology.
In the first paper, the researchers recount how they used whole-exome sequencing to examine variation in minor histocompatibility antigens (mHAs) of transplant DRPs.
Using advanced computer-based analysis, the investigators examined potential interactions between mHAs and HLAs and discovered a high level of mHA variation in HLA-matched DRPs that could potentially contribute to GVHD.
These findings may help explain why many HLA-matched recipients experience GVHD, but why some HLA-mismatched recipients do not develop GVHD remains a mystery.
The researchers offer an explanation for this seeming paradox in a companion article. In this paper, they suggest that by inhibiting peptide generation through immunosuppressive therapies in the earliest weeks following stem cell transplant, antigen presentation to donor T cells could be diminished, which reduces the risk of GVHD as the recipients reconstitute their T-cell repertoire.
In previous research, Dr Toor and his colleagues discovered a fractal pattern in the DNA of recipients’ T-cell repertoires. (Fractals are self-similar patterns that repeat themselves at every scale.)
Based on their data, the researchers believe that the presentation of mHAs following transplant helps shape the development of T-cell clonal families.
Thus, inhibiting this antigen presentation through immunosuppressive therapies in patients who have high mHA variation can potentially reduce the risk of GVHD by influencing the development of their T-cell repertoire. This is supported by data from clinical studies showing immune suppression soon after transplant improves outcomes in unrelated DRPs.
The investigators suggest that an equation such as the logistic model of growth, a mathematical formula used to explain population growth, could be employed to predict the evolution of T-cell clones and determine a patient’s future risk of GVHD.
“Currently, we rely on population-based outcomes derived from probabilistic studies to determine the best way to perform stem cell transplants,” Dr Toor said. “The development of accurate mathematical models that account for the key variables influencing transplant outcomes may allow us to treat patients using a systematic and personalized approach.”
“We plan to keep exploring this concept in hopes that we can tailor the transplantation process to each individual in order to improve outcomes and make transplantation an option for more patients.” ![]()

Credit: Darren Baker
Researchers say that computer modeling of next-generation DNA sequencing data can help us understand the variable outcomes of stem cell transplant and provide a theoretical framework to make transplant a possibility for more patients who don’t have a related donor.
The team analyzed data obtained from whole-exome sequencing of 9 donor-recipient pairs (DRPs) and found it’s possible to predict the risk of graft-vs-host disease (GVHD).
This finding could one day help physicians tailor immunosuppressive therapies to possibly improve transplant outcomes.
The investigators say their data provide evidence that the way a patient’s immune system rebuilds itself following transplant is representative of a dynamical system, a system in which the current state determines what future state will follow.
“The immune system seems chaotic, but that is because there are so many variables involved,” said Amir Toor, MD, of the Virginia Commonwealth University in Richmond.
“We have found evidence of an underlying order. Using next-generation DNA sequencing technology, it may be possible to account for many of the molecular variables that eventually determine how well a donor’s immune system will graft to a patient.”
Dr Toor and his colleagues describe this work in two articles in Frontiers in Immunology.
In the first paper, the researchers recount how they used whole-exome sequencing to examine variation in minor histocompatibility antigens (mHAs) of transplant DRPs.
Using advanced computer-based analysis, the investigators examined potential interactions between mHAs and HLAs and discovered a high level of mHA variation in HLA-matched DRPs that could potentially contribute to GVHD.
These findings may help explain why many HLA-matched recipients experience GVHD, but why some HLA-mismatched recipients do not develop GVHD remains a mystery.
The researchers offer an explanation for this seeming paradox in a companion article. In this paper, they suggest that by inhibiting peptide generation through immunosuppressive therapies in the earliest weeks following stem cell transplant, antigen presentation to donor T cells could be diminished, which reduces the risk of GVHD as the recipients reconstitute their T-cell repertoire.
In previous research, Dr Toor and his colleagues discovered a fractal pattern in the DNA of recipients’ T-cell repertoires. (Fractals are self-similar patterns that repeat themselves at every scale.)
Based on their data, the researchers believe that the presentation of mHAs following transplant helps shape the development of T-cell clonal families.
Thus, inhibiting this antigen presentation through immunosuppressive therapies in patients who have high mHA variation can potentially reduce the risk of GVHD by influencing the development of their T-cell repertoire. This is supported by data from clinical studies showing immune suppression soon after transplant improves outcomes in unrelated DRPs.
The investigators suggest that an equation such as the logistic model of growth, a mathematical formula used to explain population growth, could be employed to predict the evolution of T-cell clones and determine a patient’s future risk of GVHD.
“Currently, we rely on population-based outcomes derived from probabilistic studies to determine the best way to perform stem cell transplants,” Dr Toor said. “The development of accurate mathematical models that account for the key variables influencing transplant outcomes may allow us to treat patients using a systematic and personalized approach.”
“We plan to keep exploring this concept in hopes that we can tailor the transplantation process to each individual in order to improve outcomes and make transplantation an option for more patients.” ![]()

Credit: Darren Baker
Researchers say that computer modeling of next-generation DNA sequencing data can help us understand the variable outcomes of stem cell transplant and provide a theoretical framework to make transplant a possibility for more patients who don’t have a related donor.
The team analyzed data obtained from whole-exome sequencing of 9 donor-recipient pairs (DRPs) and found it’s possible to predict the risk of graft-vs-host disease (GVHD).
This finding could one day help physicians tailor immunosuppressive therapies to possibly improve transplant outcomes.
The investigators say their data provide evidence that the way a patient’s immune system rebuilds itself following transplant is representative of a dynamical system, a system in which the current state determines what future state will follow.
“The immune system seems chaotic, but that is because there are so many variables involved,” said Amir Toor, MD, of the Virginia Commonwealth University in Richmond.
“We have found evidence of an underlying order. Using next-generation DNA sequencing technology, it may be possible to account for many of the molecular variables that eventually determine how well a donor’s immune system will graft to a patient.”
Dr Toor and his colleagues describe this work in two articles in Frontiers in Immunology.
In the first paper, the researchers recount how they used whole-exome sequencing to examine variation in minor histocompatibility antigens (mHAs) of transplant DRPs.
Using advanced computer-based analysis, the investigators examined potential interactions between mHAs and HLAs and discovered a high level of mHA variation in HLA-matched DRPs that could potentially contribute to GVHD.
These findings may help explain why many HLA-matched recipients experience GVHD, but why some HLA-mismatched recipients do not develop GVHD remains a mystery.
The researchers offer an explanation for this seeming paradox in a companion article. In this paper, they suggest that by inhibiting peptide generation through immunosuppressive therapies in the earliest weeks following stem cell transplant, antigen presentation to donor T cells could be diminished, which reduces the risk of GVHD as the recipients reconstitute their T-cell repertoire.
In previous research, Dr Toor and his colleagues discovered a fractal pattern in the DNA of recipients’ T-cell repertoires. (Fractals are self-similar patterns that repeat themselves at every scale.)
Based on their data, the researchers believe that the presentation of mHAs following transplant helps shape the development of T-cell clonal families.
Thus, inhibiting this antigen presentation through immunosuppressive therapies in patients who have high mHA variation can potentially reduce the risk of GVHD by influencing the development of their T-cell repertoire. This is supported by data from clinical studies showing immune suppression soon after transplant improves outcomes in unrelated DRPs.
The investigators suggest that an equation such as the logistic model of growth, a mathematical formula used to explain population growth, could be employed to predict the evolution of T-cell clones and determine a patient’s future risk of GVHD.
“Currently, we rely on population-based outcomes derived from probabilistic studies to determine the best way to perform stem cell transplants,” Dr Toor said. “The development of accurate mathematical models that account for the key variables influencing transplant outcomes may allow us to treat patients using a systematic and personalized approach.”
“We plan to keep exploring this concept in hopes that we can tailor the transplantation process to each individual in order to improve outcomes and make transplantation an option for more patients.” ![]()
Team identifies cells responsible for metastasis in MM

SAN FRANCISCO—Multiple myeloma (MM) is driven to spread by only a subset of the myeloma cells within a patient’s body, according to research presented at the 2014 ASH Annual Meeting.
Attacking those cells with targeted drugs may degrade MM’s ability to spread throughout the bone marrow, study investigators said.
The team had used a mouse model of MM to track which of 15 subclones of myeloma cells spread beyond their initial site in the animals’ hind legs.
By labeling the different subgroups with fluorescent dyes, the researchers determined that just one of the subclones was responsible for disease metastasis.
They then compared the pattern of gene abnormalities in the initial myeloma tissue and the metastatic tumors. And they found that 238 genes were significantly less active in the latter group, comprising a gene signature of metastatic myeloma.
“Out of all the genes that were differently expressed in the 2 groups, we found 11 that played a functional role in metastasis and therefore may be drivers of the disease,” said study investigator Irene Ghobrial, MD, of the Dana-Farber Cancer Institute in Boston.
If future studies confirm that role, the genes may become targets for therapies that inhibit MM metastasis, she added.
Dr Ghobrial and her colleagues presented this research in a poster session at ASH (abstract 3370). ![]()

SAN FRANCISCO—Multiple myeloma (MM) is driven to spread by only a subset of the myeloma cells within a patient’s body, according to research presented at the 2014 ASH Annual Meeting.
Attacking those cells with targeted drugs may degrade MM’s ability to spread throughout the bone marrow, study investigators said.
The team had used a mouse model of MM to track which of 15 subclones of myeloma cells spread beyond their initial site in the animals’ hind legs.
By labeling the different subgroups with fluorescent dyes, the researchers determined that just one of the subclones was responsible for disease metastasis.
They then compared the pattern of gene abnormalities in the initial myeloma tissue and the metastatic tumors. And they found that 238 genes were significantly less active in the latter group, comprising a gene signature of metastatic myeloma.
“Out of all the genes that were differently expressed in the 2 groups, we found 11 that played a functional role in metastasis and therefore may be drivers of the disease,” said study investigator Irene Ghobrial, MD, of the Dana-Farber Cancer Institute in Boston.
If future studies confirm that role, the genes may become targets for therapies that inhibit MM metastasis, she added.
Dr Ghobrial and her colleagues presented this research in a poster session at ASH (abstract 3370). ![]()

SAN FRANCISCO—Multiple myeloma (MM) is driven to spread by only a subset of the myeloma cells within a patient’s body, according to research presented at the 2014 ASH Annual Meeting.
Attacking those cells with targeted drugs may degrade MM’s ability to spread throughout the bone marrow, study investigators said.
The team had used a mouse model of MM to track which of 15 subclones of myeloma cells spread beyond their initial site in the animals’ hind legs.
By labeling the different subgroups with fluorescent dyes, the researchers determined that just one of the subclones was responsible for disease metastasis.
They then compared the pattern of gene abnormalities in the initial myeloma tissue and the metastatic tumors. And they found that 238 genes were significantly less active in the latter group, comprising a gene signature of metastatic myeloma.
“Out of all the genes that were differently expressed in the 2 groups, we found 11 that played a functional role in metastasis and therefore may be drivers of the disease,” said study investigator Irene Ghobrial, MD, of the Dana-Farber Cancer Institute in Boston.
If future studies confirm that role, the genes may become targets for therapies that inhibit MM metastasis, she added.
Dr Ghobrial and her colleagues presented this research in a poster session at ASH (abstract 3370). ![]()
Finding could help docs tailor treatment for myeloid leukemias

PHILADELPHIA—New research suggests that stiffness in the extracellular matrix (ECM) can predict how leukemias will respond to therapy.
Using a 3D model, investigators demonstrated that ECM stiffness can affect treatment response in both chronic and acute myeloid leukemia.
The researchers believe that correcting for the matrix effect could give hematologists a new tool for personalizing leukemia treatment.
The team presented this research at the 2014 ASCB/IFCB meeting (poster 429).
Jae-Won Shin, PhD, and David Mooney, PhD, both of Harvard University, knew that myeloid leukemia subtypes are defined by distinct genetic mutations and the activation of known signaling pathways.
But the investigators wanted to see if changes in matrix stiffness played a part in cancer cell proliferation and if myeloid leukemia subtypes could be sorted out by their responses.
The researchers built a 3D hydrogel system with tunable stiffness and attempted to evaluate how relative stiffness of the surrounding ECM affected the resistance of human myeloid leukemias to chemotherapeutic drugs.
They found, for example, that chronic myeloid leukemias (CMLs) grown in their viscous 3D gel system were more resistant to the tyrosine kinase inhibitor imatinib than those cultured in a rigid matrix.
Using this and other data from their variable ECM system, the team screened libraries of small-molecule drugs, identifying a subset of drugs they say are more likely to be effective against CML, regardless of the surrounding matrix.
By correcting for the matrix effect, Drs Shin and Mooney believe their novel approach to drug screening could more precisely tailor chemotherapy to a patient’s individual malignancy.
The investigators’ 3D hydrogel system allowed them to vary the stiffness of the matrix and uncover different growth patterns, which they used to profile different leukemia subtypes.
They also looked at a cellular signaling pathway, protein kinase B (AKT), known to be involved in mechanotransduction and therefore sensitive to stiffness in different leukemia subtypes.
They discovered that CML cells in the 3D hydrogel were resistant to an AKT inhibitor, while acute myeloid leukemia cells grown in the same conditions were responsive to the drug, supporting their idea that a tunable matrix system could be a way to sort out leukemia subtypes by drug resistance. ![]()

PHILADELPHIA—New research suggests that stiffness in the extracellular matrix (ECM) can predict how leukemias will respond to therapy.
Using a 3D model, investigators demonstrated that ECM stiffness can affect treatment response in both chronic and acute myeloid leukemia.
The researchers believe that correcting for the matrix effect could give hematologists a new tool for personalizing leukemia treatment.
The team presented this research at the 2014 ASCB/IFCB meeting (poster 429).
Jae-Won Shin, PhD, and David Mooney, PhD, both of Harvard University, knew that myeloid leukemia subtypes are defined by distinct genetic mutations and the activation of known signaling pathways.
But the investigators wanted to see if changes in matrix stiffness played a part in cancer cell proliferation and if myeloid leukemia subtypes could be sorted out by their responses.
The researchers built a 3D hydrogel system with tunable stiffness and attempted to evaluate how relative stiffness of the surrounding ECM affected the resistance of human myeloid leukemias to chemotherapeutic drugs.
They found, for example, that chronic myeloid leukemias (CMLs) grown in their viscous 3D gel system were more resistant to the tyrosine kinase inhibitor imatinib than those cultured in a rigid matrix.
Using this and other data from their variable ECM system, the team screened libraries of small-molecule drugs, identifying a subset of drugs they say are more likely to be effective against CML, regardless of the surrounding matrix.
By correcting for the matrix effect, Drs Shin and Mooney believe their novel approach to drug screening could more precisely tailor chemotherapy to a patient’s individual malignancy.
The investigators’ 3D hydrogel system allowed them to vary the stiffness of the matrix and uncover different growth patterns, which they used to profile different leukemia subtypes.
They also looked at a cellular signaling pathway, protein kinase B (AKT), known to be involved in mechanotransduction and therefore sensitive to stiffness in different leukemia subtypes.
They discovered that CML cells in the 3D hydrogel were resistant to an AKT inhibitor, while acute myeloid leukemia cells grown in the same conditions were responsive to the drug, supporting their idea that a tunable matrix system could be a way to sort out leukemia subtypes by drug resistance. ![]()

PHILADELPHIA—New research suggests that stiffness in the extracellular matrix (ECM) can predict how leukemias will respond to therapy.
Using a 3D model, investigators demonstrated that ECM stiffness can affect treatment response in both chronic and acute myeloid leukemia.
The researchers believe that correcting for the matrix effect could give hematologists a new tool for personalizing leukemia treatment.
The team presented this research at the 2014 ASCB/IFCB meeting (poster 429).
Jae-Won Shin, PhD, and David Mooney, PhD, both of Harvard University, knew that myeloid leukemia subtypes are defined by distinct genetic mutations and the activation of known signaling pathways.
But the investigators wanted to see if changes in matrix stiffness played a part in cancer cell proliferation and if myeloid leukemia subtypes could be sorted out by their responses.
The researchers built a 3D hydrogel system with tunable stiffness and attempted to evaluate how relative stiffness of the surrounding ECM affected the resistance of human myeloid leukemias to chemotherapeutic drugs.
They found, for example, that chronic myeloid leukemias (CMLs) grown in their viscous 3D gel system were more resistant to the tyrosine kinase inhibitor imatinib than those cultured in a rigid matrix.
Using this and other data from their variable ECM system, the team screened libraries of small-molecule drugs, identifying a subset of drugs they say are more likely to be effective against CML, regardless of the surrounding matrix.
By correcting for the matrix effect, Drs Shin and Mooney believe their novel approach to drug screening could more precisely tailor chemotherapy to a patient’s individual malignancy.
The investigators’ 3D hydrogel system allowed them to vary the stiffness of the matrix and uncover different growth patterns, which they used to profile different leukemia subtypes.
They also looked at a cellular signaling pathway, protein kinase B (AKT), known to be involved in mechanotransduction and therefore sensitive to stiffness in different leukemia subtypes.
They discovered that CML cells in the 3D hydrogel were resistant to an AKT inhibitor, while acute myeloid leukemia cells grown in the same conditions were responsive to the drug, supporting their idea that a tunable matrix system could be a way to sort out leukemia subtypes by drug resistance. ![]()
Vitamin D landscape marked by lack of consensus
If you’re stumped about what to tell patients who ask you if they should be adding supplemental vitamin D to their diet, you’re not alone.
Speaker after speaker at a public conference on vitamin D sponsored by the National Institutes of Health acknowledged that there is general disagreement among well-respected scientists and medical organizations not only about recommended intakes, but about whether supplementation of vitamin D (25-hydroxyvitamin D) has any impact on ailments ranging from depression and nonspecific pain to hypertension and fall prevention.
“Most people agree that at least in high-risk individuals with osteoporosis, vitamin D has an impact on bone and skeletal health, but maybe not in those who are asymptomatic and in healthy individuals as a preventive tool,” said Dr. Clifford J. Rosen, director of clinical and translational research and a senior scientist at Maine Medical Center Research Institute, Scarborough, Me. “There seems to be growing evidence that in high-risk individuals, or in those who repeatedly fall, vitamin D may have an impact, particularly in those with very-low levels of 25-D.”
Other relationships lack conclusive randomized control data, although there are strong observational data for vitamin D’s role in preventing type 2 diabetes. Dr. Rosen is one of the investigators in a National Institute of Diabetes and Digestive and Kidney Diseases–funded clinical trial known as D2D: a study of 4,000 IU of vitamin D vs. placebo in high-risk individuals with obesity and prediabetes. The primary outcome is time to onset of type 2 diabetes. “Currently, that [trial is] in its second year and is about 30% recruited,” said Dr. Rosen, who is also a member of the FDA Advisory Panel on Endocrinologic and Metabolic Drugs. “One of the biggest obstacles to recruitment has been the constant use of vitamin D by people being screened. [They say] ‘Why should I go into a clinical trial when I’m taking vitamin D, and my doctor tells me that it will prevent diabetes?’”
The potential benefit of vitamin D intake on reducing the risk for developing cardiovascular disease, cancer, and stroke is being investigated in the NIH-funded VITAL trial. Clinicians involved in this project have enrolled more than 28,000 men and women with no prior history of these illnesses, investigating the impact of taking vitamin D3 supplements (2,000 IU/day) or omega 3 fatty acids (1 G/day).
In the meantime, current vitamin D guidance and conclusions differ among leading medical organizations. For example, the American Geriatrics Society (AGS) recommends a daily dose of 4,000 IU for fall prevention in elderly individuals. This differs from the daily dose for adults recommended by the Endocrine Society (1,500-2,000 IU), Institute of Medicine (an average requirement of 400 IU and 600-800 IU meeting the greatest need), the United States Preventive Services Task Force (600-800 IU as a fall-prevention strategy), the Standing Committee of European Doctors (600-800 IU), and the National Osteoporosis Foundation (400-1,000 IU). “How do we reconcile vitamin D intake with vitamin D levels?” asked Dr. Rosen, who is also a professor of medicine at Tufts University. “This is one of the hallmarks of the questions or problems we have, or the lack of consistency of data. We know that intakes do not reflect serum 25-D levels to a great extent.”
In addition, the terminology for serum 25-D is not clear. “Is it a deficiency? Is it a disease? What does that mean?” he asked. “More importantly, we don’t really understand what vitamin D insufficiency is. Is it a disease? Not a disease? Is it inadequate intake?”
The definition of optimal 25-D is also a matter of debate, he continued. “What’s the upper level? What does pharmacological treatment mean with respect to long-term outcomes. What is the tolerable upper limit? What is the potentially toxic limit?”
A lack of consensus also exists regarding one’s risk of vitamin D deficiency. For example, the AGS puts this risk at less than 30 ng/mL, the Endocrine Society at less than 20 ng/mL, and the Institute of Medicine at less than 12 ng/mL. “We have a lot of inconsistency in the data,” Dr. Rosen concluded. “There’s not unanimity in recommendations, even among so-called experts.”
During the same session, Dr. Peter Millard presented findings from a national analysis of vitamin D level testing in adult patients conducted from January 2013 to September 2014. The sample, drawn from Athenahealth integrated electronic health records (EHRs), included more than 6,000 internists and family physicians and 2,000 nonphysician clinicians, translating into an estimated 900,000 patient encounters per month. During that time period 4%-5% of all adult patient encounters were associated with a vitamin D test ordered.
“Curiously, the Sunbelt states of Arizona and Nevada have a high testing rate, in the 8.5%-10.7% range,” said Dr. Millard, a family physician who practices at Seaport Community Health Center in Belfast, Me. “Perhaps it’s because of snowbirds coming down from Canada to get tested. I don’t know. There are certainly lots of retirees in those two states. There are also high levels of testing in Illinois, Maryland, Rhode Island, and Delaware. I don’t have a hypothesis as to why there are variations, but that’s about 10% of all encounters associated with a vitamin D test in those states, which seems like quite a high number.”
The greatest proportion of tests occurred in patients over the age of 65 (39%) years, and about 70% of all vitamin D tests were conducted in females.
Fewer than 0.1% of tests were associated with a diagnosis of osteoporosis. The most common diagnoses associated with ordering of a vitamin D test were depression and falls. “This was particularly true in the elderly group, where falls became much more important, and depression slightly less,” Dr. Millard said. He noted that the EHR findings “don’t answer many questions but clearly the cost for [vitamin D] testing itself is significant. The amount of time my colleagues are spending doing tests and interpreting tests for patients [and] deciding what to do about those results is very considerable. In the long run, the research to resolve these issues may not be anywhere near as expensive as continuing to do what we’re doing now.”
Dr. Rosen and Dr. Millard reporting having no financial disclosures.
On Twitter @dougbrunk
If you’re stumped about what to tell patients who ask you if they should be adding supplemental vitamin D to their diet, you’re not alone.
Speaker after speaker at a public conference on vitamin D sponsored by the National Institutes of Health acknowledged that there is general disagreement among well-respected scientists and medical organizations not only about recommended intakes, but about whether supplementation of vitamin D (25-hydroxyvitamin D) has any impact on ailments ranging from depression and nonspecific pain to hypertension and fall prevention.
“Most people agree that at least in high-risk individuals with osteoporosis, vitamin D has an impact on bone and skeletal health, but maybe not in those who are asymptomatic and in healthy individuals as a preventive tool,” said Dr. Clifford J. Rosen, director of clinical and translational research and a senior scientist at Maine Medical Center Research Institute, Scarborough, Me. “There seems to be growing evidence that in high-risk individuals, or in those who repeatedly fall, vitamin D may have an impact, particularly in those with very-low levels of 25-D.”
Other relationships lack conclusive randomized control data, although there are strong observational data for vitamin D’s role in preventing type 2 diabetes. Dr. Rosen is one of the investigators in a National Institute of Diabetes and Digestive and Kidney Diseases–funded clinical trial known as D2D: a study of 4,000 IU of vitamin D vs. placebo in high-risk individuals with obesity and prediabetes. The primary outcome is time to onset of type 2 diabetes. “Currently, that [trial is] in its second year and is about 30% recruited,” said Dr. Rosen, who is also a member of the FDA Advisory Panel on Endocrinologic and Metabolic Drugs. “One of the biggest obstacles to recruitment has been the constant use of vitamin D by people being screened. [They say] ‘Why should I go into a clinical trial when I’m taking vitamin D, and my doctor tells me that it will prevent diabetes?’”
The potential benefit of vitamin D intake on reducing the risk for developing cardiovascular disease, cancer, and stroke is being investigated in the NIH-funded VITAL trial. Clinicians involved in this project have enrolled more than 28,000 men and women with no prior history of these illnesses, investigating the impact of taking vitamin D3 supplements (2,000 IU/day) or omega 3 fatty acids (1 G/day).
In the meantime, current vitamin D guidance and conclusions differ among leading medical organizations. For example, the American Geriatrics Society (AGS) recommends a daily dose of 4,000 IU for fall prevention in elderly individuals. This differs from the daily dose for adults recommended by the Endocrine Society (1,500-2,000 IU), Institute of Medicine (an average requirement of 400 IU and 600-800 IU meeting the greatest need), the United States Preventive Services Task Force (600-800 IU as a fall-prevention strategy), the Standing Committee of European Doctors (600-800 IU), and the National Osteoporosis Foundation (400-1,000 IU). “How do we reconcile vitamin D intake with vitamin D levels?” asked Dr. Rosen, who is also a professor of medicine at Tufts University. “This is one of the hallmarks of the questions or problems we have, or the lack of consistency of data. We know that intakes do not reflect serum 25-D levels to a great extent.”
In addition, the terminology for serum 25-D is not clear. “Is it a deficiency? Is it a disease? What does that mean?” he asked. “More importantly, we don’t really understand what vitamin D insufficiency is. Is it a disease? Not a disease? Is it inadequate intake?”
The definition of optimal 25-D is also a matter of debate, he continued. “What’s the upper level? What does pharmacological treatment mean with respect to long-term outcomes. What is the tolerable upper limit? What is the potentially toxic limit?”
A lack of consensus also exists regarding one’s risk of vitamin D deficiency. For example, the AGS puts this risk at less than 30 ng/mL, the Endocrine Society at less than 20 ng/mL, and the Institute of Medicine at less than 12 ng/mL. “We have a lot of inconsistency in the data,” Dr. Rosen concluded. “There’s not unanimity in recommendations, even among so-called experts.”
During the same session, Dr. Peter Millard presented findings from a national analysis of vitamin D level testing in adult patients conducted from January 2013 to September 2014. The sample, drawn from Athenahealth integrated electronic health records (EHRs), included more than 6,000 internists and family physicians and 2,000 nonphysician clinicians, translating into an estimated 900,000 patient encounters per month. During that time period 4%-5% of all adult patient encounters were associated with a vitamin D test ordered.
“Curiously, the Sunbelt states of Arizona and Nevada have a high testing rate, in the 8.5%-10.7% range,” said Dr. Millard, a family physician who practices at Seaport Community Health Center in Belfast, Me. “Perhaps it’s because of snowbirds coming down from Canada to get tested. I don’t know. There are certainly lots of retirees in those two states. There are also high levels of testing in Illinois, Maryland, Rhode Island, and Delaware. I don’t have a hypothesis as to why there are variations, but that’s about 10% of all encounters associated with a vitamin D test in those states, which seems like quite a high number.”
The greatest proportion of tests occurred in patients over the age of 65 (39%) years, and about 70% of all vitamin D tests were conducted in females.
Fewer than 0.1% of tests were associated with a diagnosis of osteoporosis. The most common diagnoses associated with ordering of a vitamin D test were depression and falls. “This was particularly true in the elderly group, where falls became much more important, and depression slightly less,” Dr. Millard said. He noted that the EHR findings “don’t answer many questions but clearly the cost for [vitamin D] testing itself is significant. The amount of time my colleagues are spending doing tests and interpreting tests for patients [and] deciding what to do about those results is very considerable. In the long run, the research to resolve these issues may not be anywhere near as expensive as continuing to do what we’re doing now.”
Dr. Rosen and Dr. Millard reporting having no financial disclosures.
On Twitter @dougbrunk
If you’re stumped about what to tell patients who ask you if they should be adding supplemental vitamin D to their diet, you’re not alone.
Speaker after speaker at a public conference on vitamin D sponsored by the National Institutes of Health acknowledged that there is general disagreement among well-respected scientists and medical organizations not only about recommended intakes, but about whether supplementation of vitamin D (25-hydroxyvitamin D) has any impact on ailments ranging from depression and nonspecific pain to hypertension and fall prevention.
“Most people agree that at least in high-risk individuals with osteoporosis, vitamin D has an impact on bone and skeletal health, but maybe not in those who are asymptomatic and in healthy individuals as a preventive tool,” said Dr. Clifford J. Rosen, director of clinical and translational research and a senior scientist at Maine Medical Center Research Institute, Scarborough, Me. “There seems to be growing evidence that in high-risk individuals, or in those who repeatedly fall, vitamin D may have an impact, particularly in those with very-low levels of 25-D.”
Other relationships lack conclusive randomized control data, although there are strong observational data for vitamin D’s role in preventing type 2 diabetes. Dr. Rosen is one of the investigators in a National Institute of Diabetes and Digestive and Kidney Diseases–funded clinical trial known as D2D: a study of 4,000 IU of vitamin D vs. placebo in high-risk individuals with obesity and prediabetes. The primary outcome is time to onset of type 2 diabetes. “Currently, that [trial is] in its second year and is about 30% recruited,” said Dr. Rosen, who is also a member of the FDA Advisory Panel on Endocrinologic and Metabolic Drugs. “One of the biggest obstacles to recruitment has been the constant use of vitamin D by people being screened. [They say] ‘Why should I go into a clinical trial when I’m taking vitamin D, and my doctor tells me that it will prevent diabetes?’”
The potential benefit of vitamin D intake on reducing the risk for developing cardiovascular disease, cancer, and stroke is being investigated in the NIH-funded VITAL trial. Clinicians involved in this project have enrolled more than 28,000 men and women with no prior history of these illnesses, investigating the impact of taking vitamin D3 supplements (2,000 IU/day) or omega 3 fatty acids (1 G/day).
In the meantime, current vitamin D guidance and conclusions differ among leading medical organizations. For example, the American Geriatrics Society (AGS) recommends a daily dose of 4,000 IU for fall prevention in elderly individuals. This differs from the daily dose for adults recommended by the Endocrine Society (1,500-2,000 IU), Institute of Medicine (an average requirement of 400 IU and 600-800 IU meeting the greatest need), the United States Preventive Services Task Force (600-800 IU as a fall-prevention strategy), the Standing Committee of European Doctors (600-800 IU), and the National Osteoporosis Foundation (400-1,000 IU). “How do we reconcile vitamin D intake with vitamin D levels?” asked Dr. Rosen, who is also a professor of medicine at Tufts University. “This is one of the hallmarks of the questions or problems we have, or the lack of consistency of data. We know that intakes do not reflect serum 25-D levels to a great extent.”
In addition, the terminology for serum 25-D is not clear. “Is it a deficiency? Is it a disease? What does that mean?” he asked. “More importantly, we don’t really understand what vitamin D insufficiency is. Is it a disease? Not a disease? Is it inadequate intake?”
The definition of optimal 25-D is also a matter of debate, he continued. “What’s the upper level? What does pharmacological treatment mean with respect to long-term outcomes. What is the tolerable upper limit? What is the potentially toxic limit?”
A lack of consensus also exists regarding one’s risk of vitamin D deficiency. For example, the AGS puts this risk at less than 30 ng/mL, the Endocrine Society at less than 20 ng/mL, and the Institute of Medicine at less than 12 ng/mL. “We have a lot of inconsistency in the data,” Dr. Rosen concluded. “There’s not unanimity in recommendations, even among so-called experts.”
During the same session, Dr. Peter Millard presented findings from a national analysis of vitamin D level testing in adult patients conducted from January 2013 to September 2014. The sample, drawn from Athenahealth integrated electronic health records (EHRs), included more than 6,000 internists and family physicians and 2,000 nonphysician clinicians, translating into an estimated 900,000 patient encounters per month. During that time period 4%-5% of all adult patient encounters were associated with a vitamin D test ordered.
“Curiously, the Sunbelt states of Arizona and Nevada have a high testing rate, in the 8.5%-10.7% range,” said Dr. Millard, a family physician who practices at Seaport Community Health Center in Belfast, Me. “Perhaps it’s because of snowbirds coming down from Canada to get tested. I don’t know. There are certainly lots of retirees in those two states. There are also high levels of testing in Illinois, Maryland, Rhode Island, and Delaware. I don’t have a hypothesis as to why there are variations, but that’s about 10% of all encounters associated with a vitamin D test in those states, which seems like quite a high number.”
The greatest proportion of tests occurred in patients over the age of 65 (39%) years, and about 70% of all vitamin D tests were conducted in females.
Fewer than 0.1% of tests were associated with a diagnosis of osteoporosis. The most common diagnoses associated with ordering of a vitamin D test were depression and falls. “This was particularly true in the elderly group, where falls became much more important, and depression slightly less,” Dr. Millard said. He noted that the EHR findings “don’t answer many questions but clearly the cost for [vitamin D] testing itself is significant. The amount of time my colleagues are spending doing tests and interpreting tests for patients [and] deciding what to do about those results is very considerable. In the long run, the research to resolve these issues may not be anywhere near as expensive as continuing to do what we’re doing now.”
Dr. Rosen and Dr. Millard reporting having no financial disclosures.
On Twitter @dougbrunk
Sorafenib boosts event-free survival of adult AML
SAN FRANCISCO – Adding a kinase inhibitor to a standard regimen for acute myeloid leukemia can prolong event-free and relapse-free survival in young adult patients, but the effect on overall survival is still unclear, investigators reported.
In a randomized controlled trial, 3-year event-free survival (EFS), the primary endpoint, was 40% among patients treated with chemotherapy and sorafenib (Nexavar), compared with 22% for patients treated with chemotherapy and a placebo (P = .013). The median EFS was 21 months and 9 months, respectively, reported Dr. Christoph Röllig of University Hospital in Dresden, Germany.
Relapse-free survival (RFS) at 3 years was 56% in the sorafenib-treated group, compared with 38% in the placebo group (P = .017). The median RFS was not reached in the sorafenib group, vs. 23 months in the placebo group, Dr. Röllig reported at the annual meeting of the American Society of Hematology.
“These data constitute the first randomized evidence that actually kinase inhibitors work in AML. What we judge is that, according to evidence-based medicine principles, a comparatory trial would be desirable in order to establish sorafenib in AML treatment,” he said at a briefing prior to his presentation of the data in a plenary session.
Dr. Röllig and colleagues in 25 centers enrolled patients from 18 to 60 years with newly diagnosed AML. Of the 276 enrolled, 267 went on to receive study treatment: 134 were assigned to sorafenib and 133 to placebo. The study drug was given along a chemotherapy regimen consisting of two cycles of induction with daunorubicin and cytarabine followed by three cycles of high-dose cytarabine consolidation. Patients who did not have a response after the first cycle of daunorubicin induction underwent a second induction attempt with cytarabine and mitoxantrone.
The assigned study medication was given on days 10-19 of induction cycles one and two, from day 8 of each consolidation cycle until 3 days before the start of the next consolidation cycle, and as maintenance for 12 months after the end of consolidation.
As noted before, EFS in an intention-to-treat analysis censored for stem-cell therapy favored the sorafenib-treated patients, as did RFS. In addition, there was evidence to suggest a benefit trend toward prolonged RFS and overall survival with sorafenib among patients positive for the FLT3-ITD mutation, which has been shown to be sensitive to kinase inhibitors.
Patients in the sorafenib arm had significantly more episodes of fever, bleeding events, and the hand-foot syndrome, but there were no significant differences in other adverse events, Dr. Röllig said.
In an interview, he said that the investigators chose sorafenib because of its good track record and its efficacy against multiple kinases. His center is also involved in clinical trials exploring whether a different kinase inhibitor, quizartinib, has similar or better efficacy against AML.
The study was supported by Bayer. Dr. Röllig reported having no relevant disclosures.
SAN FRANCISCO – Adding a kinase inhibitor to a standard regimen for acute myeloid leukemia can prolong event-free and relapse-free survival in young adult patients, but the effect on overall survival is still unclear, investigators reported.
In a randomized controlled trial, 3-year event-free survival (EFS), the primary endpoint, was 40% among patients treated with chemotherapy and sorafenib (Nexavar), compared with 22% for patients treated with chemotherapy and a placebo (P = .013). The median EFS was 21 months and 9 months, respectively, reported Dr. Christoph Röllig of University Hospital in Dresden, Germany.
Relapse-free survival (RFS) at 3 years was 56% in the sorafenib-treated group, compared with 38% in the placebo group (P = .017). The median RFS was not reached in the sorafenib group, vs. 23 months in the placebo group, Dr. Röllig reported at the annual meeting of the American Society of Hematology.
“These data constitute the first randomized evidence that actually kinase inhibitors work in AML. What we judge is that, according to evidence-based medicine principles, a comparatory trial would be desirable in order to establish sorafenib in AML treatment,” he said at a briefing prior to his presentation of the data in a plenary session.
Dr. Röllig and colleagues in 25 centers enrolled patients from 18 to 60 years with newly diagnosed AML. Of the 276 enrolled, 267 went on to receive study treatment: 134 were assigned to sorafenib and 133 to placebo. The study drug was given along a chemotherapy regimen consisting of two cycles of induction with daunorubicin and cytarabine followed by three cycles of high-dose cytarabine consolidation. Patients who did not have a response after the first cycle of daunorubicin induction underwent a second induction attempt with cytarabine and mitoxantrone.
The assigned study medication was given on days 10-19 of induction cycles one and two, from day 8 of each consolidation cycle until 3 days before the start of the next consolidation cycle, and as maintenance for 12 months after the end of consolidation.
As noted before, EFS in an intention-to-treat analysis censored for stem-cell therapy favored the sorafenib-treated patients, as did RFS. In addition, there was evidence to suggest a benefit trend toward prolonged RFS and overall survival with sorafenib among patients positive for the FLT3-ITD mutation, which has been shown to be sensitive to kinase inhibitors.
Patients in the sorafenib arm had significantly more episodes of fever, bleeding events, and the hand-foot syndrome, but there were no significant differences in other adverse events, Dr. Röllig said.
In an interview, he said that the investigators chose sorafenib because of its good track record and its efficacy against multiple kinases. His center is also involved in clinical trials exploring whether a different kinase inhibitor, quizartinib, has similar or better efficacy against AML.
The study was supported by Bayer. Dr. Röllig reported having no relevant disclosures.
SAN FRANCISCO – Adding a kinase inhibitor to a standard regimen for acute myeloid leukemia can prolong event-free and relapse-free survival in young adult patients, but the effect on overall survival is still unclear, investigators reported.
In a randomized controlled trial, 3-year event-free survival (EFS), the primary endpoint, was 40% among patients treated with chemotherapy and sorafenib (Nexavar), compared with 22% for patients treated with chemotherapy and a placebo (P = .013). The median EFS was 21 months and 9 months, respectively, reported Dr. Christoph Röllig of University Hospital in Dresden, Germany.
Relapse-free survival (RFS) at 3 years was 56% in the sorafenib-treated group, compared with 38% in the placebo group (P = .017). The median RFS was not reached in the sorafenib group, vs. 23 months in the placebo group, Dr. Röllig reported at the annual meeting of the American Society of Hematology.
“These data constitute the first randomized evidence that actually kinase inhibitors work in AML. What we judge is that, according to evidence-based medicine principles, a comparatory trial would be desirable in order to establish sorafenib in AML treatment,” he said at a briefing prior to his presentation of the data in a plenary session.
Dr. Röllig and colleagues in 25 centers enrolled patients from 18 to 60 years with newly diagnosed AML. Of the 276 enrolled, 267 went on to receive study treatment: 134 were assigned to sorafenib and 133 to placebo. The study drug was given along a chemotherapy regimen consisting of two cycles of induction with daunorubicin and cytarabine followed by three cycles of high-dose cytarabine consolidation. Patients who did not have a response after the first cycle of daunorubicin induction underwent a second induction attempt with cytarabine and mitoxantrone.
The assigned study medication was given on days 10-19 of induction cycles one and two, from day 8 of each consolidation cycle until 3 days before the start of the next consolidation cycle, and as maintenance for 12 months after the end of consolidation.
As noted before, EFS in an intention-to-treat analysis censored for stem-cell therapy favored the sorafenib-treated patients, as did RFS. In addition, there was evidence to suggest a benefit trend toward prolonged RFS and overall survival with sorafenib among patients positive for the FLT3-ITD mutation, which has been shown to be sensitive to kinase inhibitors.
Patients in the sorafenib arm had significantly more episodes of fever, bleeding events, and the hand-foot syndrome, but there were no significant differences in other adverse events, Dr. Röllig said.
In an interview, he said that the investigators chose sorafenib because of its good track record and its efficacy against multiple kinases. His center is also involved in clinical trials exploring whether a different kinase inhibitor, quizartinib, has similar or better efficacy against AML.
The study was supported by Bayer. Dr. Röllig reported having no relevant disclosures.
Key clinical point: This study is the first randomized trial to show efficacy of kinase inhibition in AML.
Major finding: 3-year event-free survival was 40% among patients treated with chemotherapy and sorafenib, compared with 22% for patients treated with chemotherapy and a placebo.
Data source: Randomized controlled trial involving 267 adults.
Disclosures: The study was supported by Bayer. Dr. Röllig reported having no relevant disclosures.