User login
‘Father of hematopoietic cytokines’ dies

Photo courtesy of The Walter
and Eliza Hall Institute
of Medical Research
Donald Metcalf, MD, an Australian researcher who has been called “the father of hematopoietic cytokines,” has died at the age of 85.
Dr Metcalf’s studies of blood production led to his speculation that there must be a biological mechanism—one or more hormones—that control white blood cell production.
These substances, which he termed colony-stimulating factors (CSFs), were the focus of more than 50 years of research.
Over this time, Dr Metcalf led researchers to characterize and purify 4 separate CSFs—granulocyte CSF (G-CSF), granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), and multi-CSF (now called interleukin-3).
Dr Metcalf recognized that CSFs had a potential role in clinical medicine, and his team was among the first in the world to discover the genes for CSFs.
Dr Metcalf was a central figure in the international clinical trials of CSFs in the 1980s, assessing whether CSFs could boost immune cell numbers in cancer patients whose immune system was weakened as a side effect of chemotherapy. On the basis of these studies, G-CSF (Neupogen) was approved for clinical use in 1991.
Now, an estimated 20 million people have been treated with CSFs. As well as boosting the immune system in patients who receive chemotherapy or have other immune deficiencies, CSFs are thought to have revolutionized hematopoietic stem cell transplantation.
A man with many achievements
Dr Metcalf was born in 1929 and started school at the age of 3, by which time he was already reading. He entered university at the age of 16, ultimately obtaining bachelor’s and medical degrees from the University of Sydney.
After an internship at the Royal Prince Alfred Hospital in Sydney, Dr Metcalf joined the staff of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne in 1954. He was supported by Cancer Council Victoria’s Carden Fellowship, an award he held until his retirement in 2014. (Dr Metcalf officially retired in 1996 but continued to do research until 2014.)
Dr Metcalf spent his early years at WEHI studying vaccinia virus. In 1965, he began studying blood cell formation and, by association, leukemia. In 1966, he became deputy director of WEHI and the head of its Cancer Research Unit.
Dr Metcalf took several sabbaticals from WEHI, serving as a visiting scientist at Harvard Medical School in Boston, Massachusetts; the Roswell Park Memorial Institute in Buffalo, New York; the Swiss Institute for Experimental Cancer Research in Lausanne, Switzerland; the Radiobiological Institute in Rijswijk, The Netherlands; and the University of Cambridge in the UK.
Among Dr Metcalf’s many honors and awards are the Companion of the Order of Australia (1993), the Albert Lasker Award for Clinical Medical Research (1993), the Gairdner Foundation International Award (1994), the Royal Medal of the Royal Society (1995), the Victoria Prize (2000), and the Prime Minister’s Prize for Science (2001).
Dr Metcalf is survived by his wife Jo; daughters Kate, Johanna, Penelope, and Mary-Ann; grandchildren James, Martin, Patrick, Elizabeth, Rose, and Robert; and their extended families. ![]()

Photo courtesy of The Walter
and Eliza Hall Institute
of Medical Research
Donald Metcalf, MD, an Australian researcher who has been called “the father of hematopoietic cytokines,” has died at the age of 85.
Dr Metcalf’s studies of blood production led to his speculation that there must be a biological mechanism—one or more hormones—that control white blood cell production.
These substances, which he termed colony-stimulating factors (CSFs), were the focus of more than 50 years of research.
Over this time, Dr Metcalf led researchers to characterize and purify 4 separate CSFs—granulocyte CSF (G-CSF), granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), and multi-CSF (now called interleukin-3).
Dr Metcalf recognized that CSFs had a potential role in clinical medicine, and his team was among the first in the world to discover the genes for CSFs.
Dr Metcalf was a central figure in the international clinical trials of CSFs in the 1980s, assessing whether CSFs could boost immune cell numbers in cancer patients whose immune system was weakened as a side effect of chemotherapy. On the basis of these studies, G-CSF (Neupogen) was approved for clinical use in 1991.
Now, an estimated 20 million people have been treated with CSFs. As well as boosting the immune system in patients who receive chemotherapy or have other immune deficiencies, CSFs are thought to have revolutionized hematopoietic stem cell transplantation.
A man with many achievements
Dr Metcalf was born in 1929 and started school at the age of 3, by which time he was already reading. He entered university at the age of 16, ultimately obtaining bachelor’s and medical degrees from the University of Sydney.
After an internship at the Royal Prince Alfred Hospital in Sydney, Dr Metcalf joined the staff of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne in 1954. He was supported by Cancer Council Victoria’s Carden Fellowship, an award he held until his retirement in 2014. (Dr Metcalf officially retired in 1996 but continued to do research until 2014.)
Dr Metcalf spent his early years at WEHI studying vaccinia virus. In 1965, he began studying blood cell formation and, by association, leukemia. In 1966, he became deputy director of WEHI and the head of its Cancer Research Unit.
Dr Metcalf took several sabbaticals from WEHI, serving as a visiting scientist at Harvard Medical School in Boston, Massachusetts; the Roswell Park Memorial Institute in Buffalo, New York; the Swiss Institute for Experimental Cancer Research in Lausanne, Switzerland; the Radiobiological Institute in Rijswijk, The Netherlands; and the University of Cambridge in the UK.
Among Dr Metcalf’s many honors and awards are the Companion of the Order of Australia (1993), the Albert Lasker Award for Clinical Medical Research (1993), the Gairdner Foundation International Award (1994), the Royal Medal of the Royal Society (1995), the Victoria Prize (2000), and the Prime Minister’s Prize for Science (2001).
Dr Metcalf is survived by his wife Jo; daughters Kate, Johanna, Penelope, and Mary-Ann; grandchildren James, Martin, Patrick, Elizabeth, Rose, and Robert; and their extended families. ![]()

Photo courtesy of The Walter
and Eliza Hall Institute
of Medical Research
Donald Metcalf, MD, an Australian researcher who has been called “the father of hematopoietic cytokines,” has died at the age of 85.
Dr Metcalf’s studies of blood production led to his speculation that there must be a biological mechanism—one or more hormones—that control white blood cell production.
These substances, which he termed colony-stimulating factors (CSFs), were the focus of more than 50 years of research.
Over this time, Dr Metcalf led researchers to characterize and purify 4 separate CSFs—granulocyte CSF (G-CSF), granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), and multi-CSF (now called interleukin-3).
Dr Metcalf recognized that CSFs had a potential role in clinical medicine, and his team was among the first in the world to discover the genes for CSFs.
Dr Metcalf was a central figure in the international clinical trials of CSFs in the 1980s, assessing whether CSFs could boost immune cell numbers in cancer patients whose immune system was weakened as a side effect of chemotherapy. On the basis of these studies, G-CSF (Neupogen) was approved for clinical use in 1991.
Now, an estimated 20 million people have been treated with CSFs. As well as boosting the immune system in patients who receive chemotherapy or have other immune deficiencies, CSFs are thought to have revolutionized hematopoietic stem cell transplantation.
A man with many achievements
Dr Metcalf was born in 1929 and started school at the age of 3, by which time he was already reading. He entered university at the age of 16, ultimately obtaining bachelor’s and medical degrees from the University of Sydney.
After an internship at the Royal Prince Alfred Hospital in Sydney, Dr Metcalf joined the staff of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne in 1954. He was supported by Cancer Council Victoria’s Carden Fellowship, an award he held until his retirement in 2014. (Dr Metcalf officially retired in 1996 but continued to do research until 2014.)
Dr Metcalf spent his early years at WEHI studying vaccinia virus. In 1965, he began studying blood cell formation and, by association, leukemia. In 1966, he became deputy director of WEHI and the head of its Cancer Research Unit.
Dr Metcalf took several sabbaticals from WEHI, serving as a visiting scientist at Harvard Medical School in Boston, Massachusetts; the Roswell Park Memorial Institute in Buffalo, New York; the Swiss Institute for Experimental Cancer Research in Lausanne, Switzerland; the Radiobiological Institute in Rijswijk, The Netherlands; and the University of Cambridge in the UK.
Among Dr Metcalf’s many honors and awards are the Companion of the Order of Australia (1993), the Albert Lasker Award for Clinical Medical Research (1993), the Gairdner Foundation International Award (1994), the Royal Medal of the Royal Society (1995), the Victoria Prize (2000), and the Prime Minister’s Prize for Science (2001).
Dr Metcalf is survived by his wife Jo; daughters Kate, Johanna, Penelope, and Mary-Ann; grandchildren James, Martin, Patrick, Elizabeth, Rose, and Robert; and their extended families. ![]()
Burns, fainting, eyes most common indoor tanning injuries
Skin burns, fainting, and eye injuries are the most common indoor tanning injuries requiring a trip to the local emergency department, according to a research letter published online Dec. 15 in JAMA Internal Medicine.
Indoor tanning exposes users to intense ultraviolet radiation, a known carcinogen, but little is known about the more immediate adverse events related to tanning, wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention, and coauthors.
After analyzing data from a nationally representative sample of hospital emergency departments from 2003 to 2012, the investigators estimated an average 3,234 indoor tanning–related injuries were treated each year in U.S. hospitals.
The most common injury was skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%).
Injuries occurred most commonly among younger adults and non-Hispanic white females, the populations with the highest rates of indoor tanning.
However, indoor tanning injuries were on the decline, decreasing from 6,487 in 2003 to 1,957 in 2012 (P < .001), a finding that was most likely due to a decline in the use of indoor sun beds, the researchers said.
Most patients did not require hospitalization, but burns severe enough to warrant a trip to the emergency department indicate an overexposure to ultraviolet radiation and an increased risk of skin cancer, the investigators added.
Although the Food and Drug Administration required tanning device manufacturers to install timers to limit exposure, several cases described patients falling asleep while tanning, raising concerns about timers malfunctioning or being intentionally overridden, the researchers noted.
The researchers said they had no relevant financial conflicts to disclose.
Skin burns, fainting, and eye injuries are the most common indoor tanning injuries requiring a trip to the local emergency department, according to a research letter published online Dec. 15 in JAMA Internal Medicine.
Indoor tanning exposes users to intense ultraviolet radiation, a known carcinogen, but little is known about the more immediate adverse events related to tanning, wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention, and coauthors.
After analyzing data from a nationally representative sample of hospital emergency departments from 2003 to 2012, the investigators estimated an average 3,234 indoor tanning–related injuries were treated each year in U.S. hospitals.
The most common injury was skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%).
Injuries occurred most commonly among younger adults and non-Hispanic white females, the populations with the highest rates of indoor tanning.
However, indoor tanning injuries were on the decline, decreasing from 6,487 in 2003 to 1,957 in 2012 (P < .001), a finding that was most likely due to a decline in the use of indoor sun beds, the researchers said.
Most patients did not require hospitalization, but burns severe enough to warrant a trip to the emergency department indicate an overexposure to ultraviolet radiation and an increased risk of skin cancer, the investigators added.
Although the Food and Drug Administration required tanning device manufacturers to install timers to limit exposure, several cases described patients falling asleep while tanning, raising concerns about timers malfunctioning or being intentionally overridden, the researchers noted.
The researchers said they had no relevant financial conflicts to disclose.
Skin burns, fainting, and eye injuries are the most common indoor tanning injuries requiring a trip to the local emergency department, according to a research letter published online Dec. 15 in JAMA Internal Medicine.
Indoor tanning exposes users to intense ultraviolet radiation, a known carcinogen, but little is known about the more immediate adverse events related to tanning, wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention, and coauthors.
After analyzing data from a nationally representative sample of hospital emergency departments from 2003 to 2012, the investigators estimated an average 3,234 indoor tanning–related injuries were treated each year in U.S. hospitals.
The most common injury was skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%).
Injuries occurred most commonly among younger adults and non-Hispanic white females, the populations with the highest rates of indoor tanning.
However, indoor tanning injuries were on the decline, decreasing from 6,487 in 2003 to 1,957 in 2012 (P < .001), a finding that was most likely due to a decline in the use of indoor sun beds, the researchers said.
Most patients did not require hospitalization, but burns severe enough to warrant a trip to the emergency department indicate an overexposure to ultraviolet radiation and an increased risk of skin cancer, the investigators added.
Although the Food and Drug Administration required tanning device manufacturers to install timers to limit exposure, several cases described patients falling asleep while tanning, raising concerns about timers malfunctioning or being intentionally overridden, the researchers noted.
The researchers said they had no relevant financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
Alpinia officinarum
Alpinia officinarum (and its close relative Alpinia galanga), a member of the Zingiberaceae family (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5), has long been used in Chinese medicinals (Bioorg. Med. Chem. 2009;17:6048-53). Specifically, the plant is used in traditional Chinese medicine as an aphrodisiac, abortifacient, carminative, antipyretic, anti-inflammatory, and emmenagogue as well as to treat disorders of the heart and kidneys, bronchitis, chronic enteritis, renal calculus, diabetes, and rheumatism (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53). Stomach ailments are the most typical application of the herb in traditional Chinese and Thai medicine; it is also used in Ayurveda and Sidda medicine. A. officinarum is widely cultivated throughout Asia, including China, Thailand, India, Sri Lanka, Malaysia, and Indonesia, as well as the Middle East and Northern Africa (Saudi Arabia and Egypt, respectively) (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5).
The flavonoid galangin (3,5,7-trihydroxyflavone) is the primary active constituent of A. officinarum (Phytother. Res. 2014;28:1533-8; J. Cell Biochem. 2013;114:152-61). In vitro, it has demonstrated a cytotoxic effect on multiple cancer cell lines (J. Cell Biochem. 2013;114[1]:152-61). Traditional Uighur medicine in China has incorporated galangin for the treatment of vitiligo (Phytother. Res. 2014;28:1533-8). Overall, A. officinarum rhizomes have been associated with antiemetic, antigenotoxic, antimutagenic, and antioxidant activity, as well as inhibitory effects on prostaglandin and leukotriene biosynthesis, and modulatory effects on cytochrome P450 enzymes (Bioorg. Med. Chem. 2009;17:6048-53; J. Cell Biochem. 2013;114:152-61). The rhizomes of A. officinarum have been used externally to treat skin infections, gum diseases, and skin cancer (J. Nat. Med. 2008;62:374-8).
Constituents
The rhizomes of the plant, commonly referred to as galangal, contain several key active constituents, including essential oils, tannins, neolignans, phenol, glycosides, monoterpenes, diarylheptanoids, phenylpropanoids, carbohydrates, gallic acid glycoside, galangoisoflavonoid, beta-sitosterol, galangin, alpinin, zerumbone, and kampferide (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53; J. Nat. Med. 2008;62:374-8).
In 2009, Matsuda et al. reported that the 80% aqueous acetone extract of the rhizomes of A. officinarum suppressed melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. They found that several isolated constituents had significant IC50 values (10-48 mcm) for inhibiting melanogenesis, including four diarylheptanoids (5-hydroxy-1,7-diphenyl-3-heptanone, 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, 5-hydroxy-7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenyl-3-heptanone, and 3,5-dihydroxy-1,7-diphenylheptane) and two flavonol constituents (kaempferide and galangin). The mRNA expression of tyrosinase and tyrosinase-related proteins-1 and -2 was also hindered by 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, kaempferide, and galangin, as was the protein level of a microphthalmia-associated transcription factor, the authors noted (Bioorg. Med. Chem. 2009;17:6048-53).
Biologic activity
Penetration enhancement: In 2000, Shen et al. found that volatile oils from galangal, among other herbs, were effective in enhancing the skin permeation of 5-fluorouracil and notably more effective than azone (Zhong Yao Cai 2000;23:697-9).
Anti-inflammatory effects: In 2008, Yasukawa et al. examined the inhibitory effect of galangal in a two-stage in vivo carcinogenesis model in mice. They observed that the A. officinarum rhizomes displayed significant antitumor-promoting activity against 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)–promoted lesions. Seven diarylheptanoids isolated from the active fraction of the methanol extracts demonstrated significant anti-inflammatory effects against TPA-induced inflammation (J. Nat. Med. 2008;62:374-8).
Cancer prevention and pigmentary effects: Heo et al. reported in 2001 that in vitro and in vivo studies have demonstrated that the flavonoid galangin, found in high concentrations in A. officinarum, as well as the bee product propolis, exhibits significant antioxidant activity and can influence enzyme activities and inhibit genotoxicity without introducing a pro-oxidant effect. They concluded that galangin warrants consideration for its potential as a chemical cancer-preventing agent (Mutat. Res. 2001;488:135-50).
In 2007, Lu et al. investigated the whitening effects of the flavonoid components of A. officinarum on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition, and UV absorption. They found that galangin and the flavonoid mixture both decreased melanin content more than controls and also lowered melanin production, with galangin more effective than the flavonoid mixture. In addition, galangin and the flavonoid mixture exerted greater tyrosinase inhibition at lower concentrations. The A. officinarum constituents also displayed a broad absorption band in the UVB area (270 to 290 nm). The researchers concluded that galangin may be a viable whitening agent with the potential to prevent skin cancer (J. Enzyme Inhib. Med. Chem. 2007;22:433-8).
Six years later, Zhang et al. noted that various doses of galangin resulted in the inhibition of B16F10 melanoma cell proliferation. The investigators also showed that galangin achieved an antimetastatic effect in vivo in C57BL/6J mice, reducing focal adhesion kinase. They concluded that focal adhesion kinase is a viable target in melanoma therapy, with B16F10 melanoma metastasis apparently checked by galangin in mice and in cell cultures (J. Cell Biochem. 2013;114:152-61).
In 2014, Huo et al. tested galangin in a mouse model of vitiligo induced in C57BL/6 mice through the daily topical application of hydroquinone (2.5%) on shaved dorsal skin for 60 days. Thirty days after the final hydroquinone application, investigators began oral administration of galangin for 30 days. Hair grew back after treatment darker than the original color, with histologic analysis revealing that mice treated with galangin and the positive control 8-methoxypsoralen had an increased number of melanin-containing hair follicles, compared with untreated animals. In addition, galangin treatment was associated with significant increases in the number of cutaneous basal layer melanocytes and melanin-containing epidermal cells. Compared with controls, treatment with galangin and 8-methoxypsoralen led to increased serum levels of tyrosinase and decreased levels of malondialdehyde and lower cholinesterase activity. Galangin and 8-methoxypsoralen use also increased the expression of tyrosinase protein in treated skin. The investigators concluded that galangin improved hydroquinone-induced vitiligo in mice and warrants further study as a potential vitiligo treatment in humans (Phytother. Res. 2014 28:1533-8).
Conclusion
Alpinia officinarum is one of many botanical agents with a long history of applications in traditional folk medicine, particularly in Asia. There is a relative paucity of evidence regarding the dermatologic applications of this plant, but recent findings support continued research into its various potential cutaneous benefits, with particular focus on the main active ingredient galangin.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Alpinia officinarum (and its close relative Alpinia galanga), a member of the Zingiberaceae family (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5), has long been used in Chinese medicinals (Bioorg. Med. Chem. 2009;17:6048-53). Specifically, the plant is used in traditional Chinese medicine as an aphrodisiac, abortifacient, carminative, antipyretic, anti-inflammatory, and emmenagogue as well as to treat disorders of the heart and kidneys, bronchitis, chronic enteritis, renal calculus, diabetes, and rheumatism (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53). Stomach ailments are the most typical application of the herb in traditional Chinese and Thai medicine; it is also used in Ayurveda and Sidda medicine. A. officinarum is widely cultivated throughout Asia, including China, Thailand, India, Sri Lanka, Malaysia, and Indonesia, as well as the Middle East and Northern Africa (Saudi Arabia and Egypt, respectively) (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5).
The flavonoid galangin (3,5,7-trihydroxyflavone) is the primary active constituent of A. officinarum (Phytother. Res. 2014;28:1533-8; J. Cell Biochem. 2013;114:152-61). In vitro, it has demonstrated a cytotoxic effect on multiple cancer cell lines (J. Cell Biochem. 2013;114[1]:152-61). Traditional Uighur medicine in China has incorporated galangin for the treatment of vitiligo (Phytother. Res. 2014;28:1533-8). Overall, A. officinarum rhizomes have been associated with antiemetic, antigenotoxic, antimutagenic, and antioxidant activity, as well as inhibitory effects on prostaglandin and leukotriene biosynthesis, and modulatory effects on cytochrome P450 enzymes (Bioorg. Med. Chem. 2009;17:6048-53; J. Cell Biochem. 2013;114:152-61). The rhizomes of A. officinarum have been used externally to treat skin infections, gum diseases, and skin cancer (J. Nat. Med. 2008;62:374-8).
Constituents
The rhizomes of the plant, commonly referred to as galangal, contain several key active constituents, including essential oils, tannins, neolignans, phenol, glycosides, monoterpenes, diarylheptanoids, phenylpropanoids, carbohydrates, gallic acid glycoside, galangoisoflavonoid, beta-sitosterol, galangin, alpinin, zerumbone, and kampferide (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53; J. Nat. Med. 2008;62:374-8).
In 2009, Matsuda et al. reported that the 80% aqueous acetone extract of the rhizomes of A. officinarum suppressed melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. They found that several isolated constituents had significant IC50 values (10-48 mcm) for inhibiting melanogenesis, including four diarylheptanoids (5-hydroxy-1,7-diphenyl-3-heptanone, 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, 5-hydroxy-7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenyl-3-heptanone, and 3,5-dihydroxy-1,7-diphenylheptane) and two flavonol constituents (kaempferide and galangin). The mRNA expression of tyrosinase and tyrosinase-related proteins-1 and -2 was also hindered by 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, kaempferide, and galangin, as was the protein level of a microphthalmia-associated transcription factor, the authors noted (Bioorg. Med. Chem. 2009;17:6048-53).
Biologic activity
Penetration enhancement: In 2000, Shen et al. found that volatile oils from galangal, among other herbs, were effective in enhancing the skin permeation of 5-fluorouracil and notably more effective than azone (Zhong Yao Cai 2000;23:697-9).
Anti-inflammatory effects: In 2008, Yasukawa et al. examined the inhibitory effect of galangal in a two-stage in vivo carcinogenesis model in mice. They observed that the A. officinarum rhizomes displayed significant antitumor-promoting activity against 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)–promoted lesions. Seven diarylheptanoids isolated from the active fraction of the methanol extracts demonstrated significant anti-inflammatory effects against TPA-induced inflammation (J. Nat. Med. 2008;62:374-8).
Cancer prevention and pigmentary effects: Heo et al. reported in 2001 that in vitro and in vivo studies have demonstrated that the flavonoid galangin, found in high concentrations in A. officinarum, as well as the bee product propolis, exhibits significant antioxidant activity and can influence enzyme activities and inhibit genotoxicity without introducing a pro-oxidant effect. They concluded that galangin warrants consideration for its potential as a chemical cancer-preventing agent (Mutat. Res. 2001;488:135-50).
In 2007, Lu et al. investigated the whitening effects of the flavonoid components of A. officinarum on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition, and UV absorption. They found that galangin and the flavonoid mixture both decreased melanin content more than controls and also lowered melanin production, with galangin more effective than the flavonoid mixture. In addition, galangin and the flavonoid mixture exerted greater tyrosinase inhibition at lower concentrations. The A. officinarum constituents also displayed a broad absorption band in the UVB area (270 to 290 nm). The researchers concluded that galangin may be a viable whitening agent with the potential to prevent skin cancer (J. Enzyme Inhib. Med. Chem. 2007;22:433-8).
Six years later, Zhang et al. noted that various doses of galangin resulted in the inhibition of B16F10 melanoma cell proliferation. The investigators also showed that galangin achieved an antimetastatic effect in vivo in C57BL/6J mice, reducing focal adhesion kinase. They concluded that focal adhesion kinase is a viable target in melanoma therapy, with B16F10 melanoma metastasis apparently checked by galangin in mice and in cell cultures (J. Cell Biochem. 2013;114:152-61).
In 2014, Huo et al. tested galangin in a mouse model of vitiligo induced in C57BL/6 mice through the daily topical application of hydroquinone (2.5%) on shaved dorsal skin for 60 days. Thirty days after the final hydroquinone application, investigators began oral administration of galangin for 30 days. Hair grew back after treatment darker than the original color, with histologic analysis revealing that mice treated with galangin and the positive control 8-methoxypsoralen had an increased number of melanin-containing hair follicles, compared with untreated animals. In addition, galangin treatment was associated with significant increases in the number of cutaneous basal layer melanocytes and melanin-containing epidermal cells. Compared with controls, treatment with galangin and 8-methoxypsoralen led to increased serum levels of tyrosinase and decreased levels of malondialdehyde and lower cholinesterase activity. Galangin and 8-methoxypsoralen use also increased the expression of tyrosinase protein in treated skin. The investigators concluded that galangin improved hydroquinone-induced vitiligo in mice and warrants further study as a potential vitiligo treatment in humans (Phytother. Res. 2014 28:1533-8).
Conclusion
Alpinia officinarum is one of many botanical agents with a long history of applications in traditional folk medicine, particularly in Asia. There is a relative paucity of evidence regarding the dermatologic applications of this plant, but recent findings support continued research into its various potential cutaneous benefits, with particular focus on the main active ingredient galangin.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Alpinia officinarum (and its close relative Alpinia galanga), a member of the Zingiberaceae family (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5), has long been used in Chinese medicinals (Bioorg. Med. Chem. 2009;17:6048-53). Specifically, the plant is used in traditional Chinese medicine as an aphrodisiac, abortifacient, carminative, antipyretic, anti-inflammatory, and emmenagogue as well as to treat disorders of the heart and kidneys, bronchitis, chronic enteritis, renal calculus, diabetes, and rheumatism (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53). Stomach ailments are the most typical application of the herb in traditional Chinese and Thai medicine; it is also used in Ayurveda and Sidda medicine. A. officinarum is widely cultivated throughout Asia, including China, Thailand, India, Sri Lanka, Malaysia, and Indonesia, as well as the Middle East and Northern Africa (Saudi Arabia and Egypt, respectively) (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5).
The flavonoid galangin (3,5,7-trihydroxyflavone) is the primary active constituent of A. officinarum (Phytother. Res. 2014;28:1533-8; J. Cell Biochem. 2013;114:152-61). In vitro, it has demonstrated a cytotoxic effect on multiple cancer cell lines (J. Cell Biochem. 2013;114[1]:152-61). Traditional Uighur medicine in China has incorporated galangin for the treatment of vitiligo (Phytother. Res. 2014;28:1533-8). Overall, A. officinarum rhizomes have been associated with antiemetic, antigenotoxic, antimutagenic, and antioxidant activity, as well as inhibitory effects on prostaglandin and leukotriene biosynthesis, and modulatory effects on cytochrome P450 enzymes (Bioorg. Med. Chem. 2009;17:6048-53; J. Cell Biochem. 2013;114:152-61). The rhizomes of A. officinarum have been used externally to treat skin infections, gum diseases, and skin cancer (J. Nat. Med. 2008;62:374-8).
Constituents
The rhizomes of the plant, commonly referred to as galangal, contain several key active constituents, including essential oils, tannins, neolignans, phenol, glycosides, monoterpenes, diarylheptanoids, phenylpropanoids, carbohydrates, gallic acid glycoside, galangoisoflavonoid, beta-sitosterol, galangin, alpinin, zerumbone, and kampferide (Zhong Xi Yi Jie He Xue Bao 2011;9:1061-5; Bioorg. Med. Chem. 2009;17:6048-53; J. Nat. Med. 2008;62:374-8).
In 2009, Matsuda et al. reported that the 80% aqueous acetone extract of the rhizomes of A. officinarum suppressed melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. They found that several isolated constituents had significant IC50 values (10-48 mcm) for inhibiting melanogenesis, including four diarylheptanoids (5-hydroxy-1,7-diphenyl-3-heptanone, 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, 5-hydroxy-7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenyl-3-heptanone, and 3,5-dihydroxy-1,7-diphenylheptane) and two flavonol constituents (kaempferide and galangin). The mRNA expression of tyrosinase and tyrosinase-related proteins-1 and -2 was also hindered by 7-(4(‘’)-hydroxy-3(‘’)-methoxyphenyl)-1-phenylhept-4-en-3-one, kaempferide, and galangin, as was the protein level of a microphthalmia-associated transcription factor, the authors noted (Bioorg. Med. Chem. 2009;17:6048-53).
Biologic activity
Penetration enhancement: In 2000, Shen et al. found that volatile oils from galangal, among other herbs, were effective in enhancing the skin permeation of 5-fluorouracil and notably more effective than azone (Zhong Yao Cai 2000;23:697-9).
Anti-inflammatory effects: In 2008, Yasukawa et al. examined the inhibitory effect of galangal in a two-stage in vivo carcinogenesis model in mice. They observed that the A. officinarum rhizomes displayed significant antitumor-promoting activity against 7,12-dimethylbenz[a]anthracene (DMBA)-initiated and 12-O-tetradecanoylphorbol-13-acetate (TPA)–promoted lesions. Seven diarylheptanoids isolated from the active fraction of the methanol extracts demonstrated significant anti-inflammatory effects against TPA-induced inflammation (J. Nat. Med. 2008;62:374-8).
Cancer prevention and pigmentary effects: Heo et al. reported in 2001 that in vitro and in vivo studies have demonstrated that the flavonoid galangin, found in high concentrations in A. officinarum, as well as the bee product propolis, exhibits significant antioxidant activity and can influence enzyme activities and inhibit genotoxicity without introducing a pro-oxidant effect. They concluded that galangin warrants consideration for its potential as a chemical cancer-preventing agent (Mutat. Res. 2001;488:135-50).
In 2007, Lu et al. investigated the whitening effects of the flavonoid components of A. officinarum on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition, and UV absorption. They found that galangin and the flavonoid mixture both decreased melanin content more than controls and also lowered melanin production, with galangin more effective than the flavonoid mixture. In addition, galangin and the flavonoid mixture exerted greater tyrosinase inhibition at lower concentrations. The A. officinarum constituents also displayed a broad absorption band in the UVB area (270 to 290 nm). The researchers concluded that galangin may be a viable whitening agent with the potential to prevent skin cancer (J. Enzyme Inhib. Med. Chem. 2007;22:433-8).
Six years later, Zhang et al. noted that various doses of galangin resulted in the inhibition of B16F10 melanoma cell proliferation. The investigators also showed that galangin achieved an antimetastatic effect in vivo in C57BL/6J mice, reducing focal adhesion kinase. They concluded that focal adhesion kinase is a viable target in melanoma therapy, with B16F10 melanoma metastasis apparently checked by galangin in mice and in cell cultures (J. Cell Biochem. 2013;114:152-61).
In 2014, Huo et al. tested galangin in a mouse model of vitiligo induced in C57BL/6 mice through the daily topical application of hydroquinone (2.5%) on shaved dorsal skin for 60 days. Thirty days after the final hydroquinone application, investigators began oral administration of galangin for 30 days. Hair grew back after treatment darker than the original color, with histologic analysis revealing that mice treated with galangin and the positive control 8-methoxypsoralen had an increased number of melanin-containing hair follicles, compared with untreated animals. In addition, galangin treatment was associated with significant increases in the number of cutaneous basal layer melanocytes and melanin-containing epidermal cells. Compared with controls, treatment with galangin and 8-methoxypsoralen led to increased serum levels of tyrosinase and decreased levels of malondialdehyde and lower cholinesterase activity. Galangin and 8-methoxypsoralen use also increased the expression of tyrosinase protein in treated skin. The investigators concluded that galangin improved hydroquinone-induced vitiligo in mice and warrants further study as a potential vitiligo treatment in humans (Phytother. Res. 2014 28:1533-8).
Conclusion
Alpinia officinarum is one of many botanical agents with a long history of applications in traditional folk medicine, particularly in Asia. There is a relative paucity of evidence regarding the dermatologic applications of this plant, but recent findings support continued research into its various potential cutaneous benefits, with particular focus on the main active ingredient galangin.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
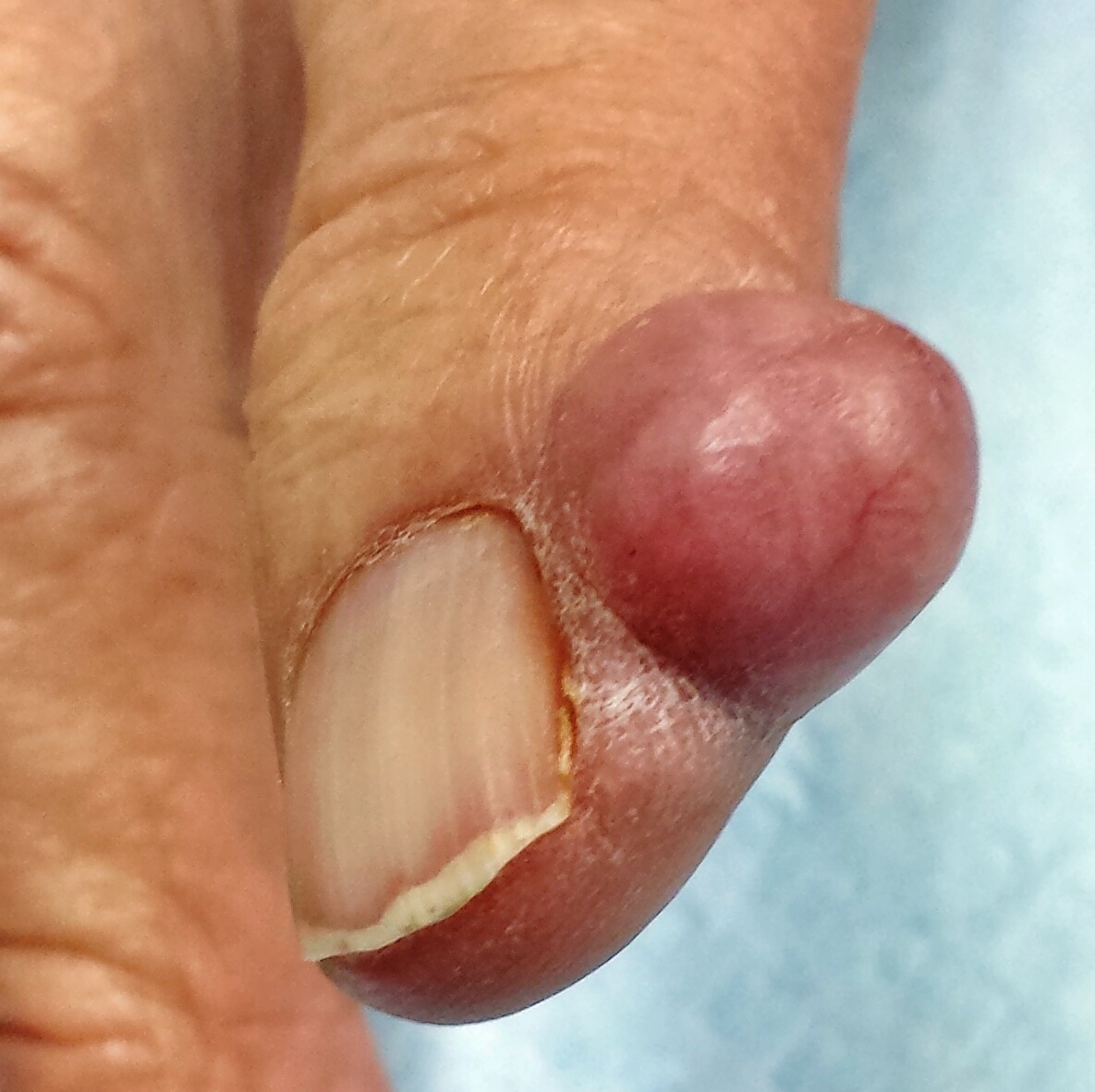
Finger Lesion Is Painful When Bumped
A 67-year-old man has had a small lesion on his index finger for several years. Recently, it has grown large enough to start interfering with his normal activities. The lesion is not painful unless the patient bumps it—but as it grows, avoiding such incidents is becoming more difficult.
The diagnosis has thus far been a mystery: What is it? The patient’s primary care provider didn’t know and, per the patient’s report, had no interest in trying to remove the lesion. Thus, the patient finds himself referred to dermatology.
The patient can recall no specific injury. He does, however, report that he worked for years as an upholsterer, shaping, sewing, and stapling heavy fabric onto chairs and sofas—often puncturing his fingers in the process.
His health is otherwise “decent.” He is taking medication for hypertension and an a-blocker due to “problems” with his prostate.
EXAMINATION
The finger lesion is a 2-cm, round, firm nodule with a smooth, telangiectatic surface. It is located on the perionychial area of patient’s index finger (lateral aspect). The lesion is attached to the finger by a broad base that rises seamlessly from the skin. It is not translucent or tender to touch. No similar lesions are seen on either hand, and no nodes are felt in the epitrochlear or axillary locations.
Within a few weeks, the patient returns to have the lesion excised. This is done with a combination of a digital block and local anesthesia, with a tourniquet to control bleeding. After primary closure, the lesion is submitted to pathology. The report indicates the lesion is a cyst, full of cheesy material.
What is the diagnosis?
DISCUSSION
This type of cyst is sometimes referred to as an implantation cyst and occurs when epidermal tissue, including sebaceous glands, is implanted into deeper tissue by a penetrating injury and continues to grow. Although this is an utterly common and benign diagnosis, the differential for a “fingeroma” includes digital mucous cyst, pyogenic granuloma, acquired digital fibrokeratoma, calcinosis cutis, tophaceous gout, wart, and giant cell tumor. Had the lesion been of bony origin, it could have represented connective tissue cancer (sarcoma) or even metastatic cancer.
Implantation cysts must be removed completely to prevent recurrence. The size and location of this particular cyst made it conducive to simple excision and primary closure. Larger lesions in more difficult locations often require the expertise of a hand surgeon for disposition.
TAKE-HOME LEARNING POINTS
• “Fingeromas” are quite common but have an extensive differential diagnosis—mostly benign, but occasionally malignant.
• Regarding malignancy, it is helpful to remember that any tissue in the body can undergo malignant transformation and that many types of primary cancers can metastasize to distant areas (including the hands).
• Removal, or at least biopsy, is the best way to differentiate between types of fingeroma lesions.
• Implantation cysts, as seen in this case, result from traumatic implantation of epidermal structures (including sebaceous glands) into deeper tissue, where they continue to grow.
• When in doubt, consider referring affected patients to dermatology or to a hand surgeon.
A 67-year-old man has had a small lesion on his index finger for several years. Recently, it has grown large enough to start interfering with his normal activities. The lesion is not painful unless the patient bumps it—but as it grows, avoiding such incidents is becoming more difficult.
The diagnosis has thus far been a mystery: What is it? The patient’s primary care provider didn’t know and, per the patient’s report, had no interest in trying to remove the lesion. Thus, the patient finds himself referred to dermatology.
The patient can recall no specific injury. He does, however, report that he worked for years as an upholsterer, shaping, sewing, and stapling heavy fabric onto chairs and sofas—often puncturing his fingers in the process.
His health is otherwise “decent.” He is taking medication for hypertension and an a-blocker due to “problems” with his prostate.
EXAMINATION
The finger lesion is a 2-cm, round, firm nodule with a smooth, telangiectatic surface. It is located on the perionychial area of patient’s index finger (lateral aspect). The lesion is attached to the finger by a broad base that rises seamlessly from the skin. It is not translucent or tender to touch. No similar lesions are seen on either hand, and no nodes are felt in the epitrochlear or axillary locations.
Within a few weeks, the patient returns to have the lesion excised. This is done with a combination of a digital block and local anesthesia, with a tourniquet to control bleeding. After primary closure, the lesion is submitted to pathology. The report indicates the lesion is a cyst, full of cheesy material.
What is the diagnosis?
DISCUSSION
This type of cyst is sometimes referred to as an implantation cyst and occurs when epidermal tissue, including sebaceous glands, is implanted into deeper tissue by a penetrating injury and continues to grow. Although this is an utterly common and benign diagnosis, the differential for a “fingeroma” includes digital mucous cyst, pyogenic granuloma, acquired digital fibrokeratoma, calcinosis cutis, tophaceous gout, wart, and giant cell tumor. Had the lesion been of bony origin, it could have represented connective tissue cancer (sarcoma) or even metastatic cancer.
Implantation cysts must be removed completely to prevent recurrence. The size and location of this particular cyst made it conducive to simple excision and primary closure. Larger lesions in more difficult locations often require the expertise of a hand surgeon for disposition.
TAKE-HOME LEARNING POINTS
• “Fingeromas” are quite common but have an extensive differential diagnosis—mostly benign, but occasionally malignant.
• Regarding malignancy, it is helpful to remember that any tissue in the body can undergo malignant transformation and that many types of primary cancers can metastasize to distant areas (including the hands).
• Removal, or at least biopsy, is the best way to differentiate between types of fingeroma lesions.
• Implantation cysts, as seen in this case, result from traumatic implantation of epidermal structures (including sebaceous glands) into deeper tissue, where they continue to grow.
• When in doubt, consider referring affected patients to dermatology or to a hand surgeon.
A 67-year-old man has had a small lesion on his index finger for several years. Recently, it has grown large enough to start interfering with his normal activities. The lesion is not painful unless the patient bumps it—but as it grows, avoiding such incidents is becoming more difficult.
The diagnosis has thus far been a mystery: What is it? The patient’s primary care provider didn’t know and, per the patient’s report, had no interest in trying to remove the lesion. Thus, the patient finds himself referred to dermatology.
The patient can recall no specific injury. He does, however, report that he worked for years as an upholsterer, shaping, sewing, and stapling heavy fabric onto chairs and sofas—often puncturing his fingers in the process.
His health is otherwise “decent.” He is taking medication for hypertension and an a-blocker due to “problems” with his prostate.
EXAMINATION
The finger lesion is a 2-cm, round, firm nodule with a smooth, telangiectatic surface. It is located on the perionychial area of patient’s index finger (lateral aspect). The lesion is attached to the finger by a broad base that rises seamlessly from the skin. It is not translucent or tender to touch. No similar lesions are seen on either hand, and no nodes are felt in the epitrochlear or axillary locations.
Within a few weeks, the patient returns to have the lesion excised. This is done with a combination of a digital block and local anesthesia, with a tourniquet to control bleeding. After primary closure, the lesion is submitted to pathology. The report indicates the lesion is a cyst, full of cheesy material.
What is the diagnosis?
DISCUSSION
This type of cyst is sometimes referred to as an implantation cyst and occurs when epidermal tissue, including sebaceous glands, is implanted into deeper tissue by a penetrating injury and continues to grow. Although this is an utterly common and benign diagnosis, the differential for a “fingeroma” includes digital mucous cyst, pyogenic granuloma, acquired digital fibrokeratoma, calcinosis cutis, tophaceous gout, wart, and giant cell tumor. Had the lesion been of bony origin, it could have represented connective tissue cancer (sarcoma) or even metastatic cancer.
Implantation cysts must be removed completely to prevent recurrence. The size and location of this particular cyst made it conducive to simple excision and primary closure. Larger lesions in more difficult locations often require the expertise of a hand surgeon for disposition.
TAKE-HOME LEARNING POINTS
• “Fingeromas” are quite common but have an extensive differential diagnosis—mostly benign, but occasionally malignant.
• Regarding malignancy, it is helpful to remember that any tissue in the body can undergo malignant transformation and that many types of primary cancers can metastasize to distant areas (including the hands).
• Removal, or at least biopsy, is the best way to differentiate between types of fingeroma lesions.
• Implantation cysts, as seen in this case, result from traumatic implantation of epidermal structures (including sebaceous glands) into deeper tissue, where they continue to grow.
• When in doubt, consider referring affected patients to dermatology or to a hand surgeon.
Current Thinking in OTC Analgesia: Patient Considerations and Practical Insights
December 2014
A supplement to Family Practice News® and Internal Medicine News®. This supplement is sponsored by McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Topics
- Pain in the United States
- Critical Comorbid Illnesses
- Impact of Pain on Comorbidities and Patient Outcomes
- Pain Management for Patients Taking Concomitant Medications
- Benefits of OTC Analgesics
- Risks of OTC Analgesics
- A Nursing Perspective
- Not All Analgesics Are Appropriate for Every Patient
- Patients’ Safe Use of OTC Analgesics
- Conclusions
Faculty
Chairman
Charles P. Vega, Jr, MD, FAAFP
Clinical Professor, Department of Family Medicine
Director, Patient-Centered Advanced Clinical Education
Director, Program in Medical Education for the Latino Community
University of California, Irvine, School of Medicine
Irvine, California
Faculty
Christopher M. Chappel, MD
Medical Director
Chappel Health and Wellness
Kissimmee, Florida
Brett Badgley Snodgrass, MSN, APRN, FNP-BC
Family Nurse Practitioner
Saint Francis Hospital–Bartlett Interventional Pain Management Clinic
Chief Executive Officer and President
BBS Consultants, Inc.
Bartlett, Tennessee
Copyright © 2014 by Frontline Medical Communications Inc.
December 2014
A supplement to Family Practice News® and Internal Medicine News®. This supplement is sponsored by McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Topics
- Pain in the United States
- Critical Comorbid Illnesses
- Impact of Pain on Comorbidities and Patient Outcomes
- Pain Management for Patients Taking Concomitant Medications
- Benefits of OTC Analgesics
- Risks of OTC Analgesics
- A Nursing Perspective
- Not All Analgesics Are Appropriate for Every Patient
- Patients’ Safe Use of OTC Analgesics
- Conclusions
Faculty
Chairman
Charles P. Vega, Jr, MD, FAAFP
Clinical Professor, Department of Family Medicine
Director, Patient-Centered Advanced Clinical Education
Director, Program in Medical Education for the Latino Community
University of California, Irvine, School of Medicine
Irvine, California
Faculty
Christopher M. Chappel, MD
Medical Director
Chappel Health and Wellness
Kissimmee, Florida
Brett Badgley Snodgrass, MSN, APRN, FNP-BC
Family Nurse Practitioner
Saint Francis Hospital–Bartlett Interventional Pain Management Clinic
Chief Executive Officer and President
BBS Consultants, Inc.
Bartlett, Tennessee
Copyright © 2014 by Frontline Medical Communications Inc.
December 2014
A supplement to Family Practice News® and Internal Medicine News®. This supplement is sponsored by McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Topics
- Pain in the United States
- Critical Comorbid Illnesses
- Impact of Pain on Comorbidities and Patient Outcomes
- Pain Management for Patients Taking Concomitant Medications
- Benefits of OTC Analgesics
- Risks of OTC Analgesics
- A Nursing Perspective
- Not All Analgesics Are Appropriate for Every Patient
- Patients’ Safe Use of OTC Analgesics
- Conclusions
Faculty
Chairman
Charles P. Vega, Jr, MD, FAAFP
Clinical Professor, Department of Family Medicine
Director, Patient-Centered Advanced Clinical Education
Director, Program in Medical Education for the Latino Community
University of California, Irvine, School of Medicine
Irvine, California
Faculty
Christopher M. Chappel, MD
Medical Director
Chappel Health and Wellness
Kissimmee, Florida
Brett Badgley Snodgrass, MSN, APRN, FNP-BC
Family Nurse Practitioner
Saint Francis Hospital–Bartlett Interventional Pain Management Clinic
Chief Executive Officer and President
BBS Consultants, Inc.
Bartlett, Tennessee
Copyright © 2014 by Frontline Medical Communications Inc.
Current Thinking in OTC Analgesia: Patient Considerations and Practical Insights
December 2014
A supplement to Family Practice News® and Internal Medicine News®. This supplement is sponsored by McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Click here to open the supplement
Topics
- Pain in the United States
- Critical Comorbid Illnesses
- Impact of Pain on Comorbidities and Patient Outcomes
- Pain Management for Patients Taking Concomitant Medications
- Benefits of OTC Analgesics
- Risks of OTC Analgesics
- A Nursing Perspective
- Not All Analgesics Are Appropriate for Every Patient
- Patients’ Safe Use of OTC Analgesics
- Conclusions
Faculty
Chairman
Charles P. Vega, Jr, MD, FAAFP
Clinical Professor, Department of Family Medicine
Director, Patient-Centered Advanced Clinical Education
Director, Program in Medical Education for the Latino Community
University of California, Irvine, School of Medicine
Irvine, California
Faculty
Christopher M. Chappel, MD
Medical Director
Chappel Health and Wellness
Kissimmee, Florida
Brett Badgley Snodgrass, MSN, APRN, FNP-BC
Family Nurse Practitioner
Saint Francis Hospital–Bartlett Interventional Pain Management Clinic
Chief Executive Officer and President
BBS Consultants, Inc.
Bartlett, Tennessee
Copyright © 2014 by Frontline Medical Communications Inc.
December 2014
A supplement to Family Practice News® and Internal Medicine News®. This supplement is sponsored by McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Click here to open the supplement
Topics
- Pain in the United States
- Critical Comorbid Illnesses
- Impact of Pain on Comorbidities and Patient Outcomes
- Pain Management for Patients Taking Concomitant Medications
- Benefits of OTC Analgesics
- Risks of OTC Analgesics
- A Nursing Perspective
- Not All Analgesics Are Appropriate for Every Patient
- Patients’ Safe Use of OTC Analgesics
- Conclusions
Faculty
Chairman
Charles P. Vega, Jr, MD, FAAFP
Clinical Professor, Department of Family Medicine
Director, Patient-Centered Advanced Clinical Education
Director, Program in Medical Education for the Latino Community
University of California, Irvine, School of Medicine
Irvine, California
Faculty
Christopher M. Chappel, MD
Medical Director
Chappel Health and Wellness
Kissimmee, Florida
Brett Badgley Snodgrass, MSN, APRN, FNP-BC
Family Nurse Practitioner
Saint Francis Hospital–Bartlett Interventional Pain Management Clinic
Chief Executive Officer and President
BBS Consultants, Inc.
Bartlett, Tennessee
Copyright © 2014 by Frontline Medical Communications Inc.
December 2014
A supplement to Family Practice News® and Internal Medicine News®. This supplement is sponsored by McNeil Consumer Healthcare Division of McNEIL-PPC, Inc.
Click here to open the supplement
Topics
- Pain in the United States
- Critical Comorbid Illnesses
- Impact of Pain on Comorbidities and Patient Outcomes
- Pain Management for Patients Taking Concomitant Medications
- Benefits of OTC Analgesics
- Risks of OTC Analgesics
- A Nursing Perspective
- Not All Analgesics Are Appropriate for Every Patient
- Patients’ Safe Use of OTC Analgesics
- Conclusions
Faculty
Chairman
Charles P. Vega, Jr, MD, FAAFP
Clinical Professor, Department of Family Medicine
Director, Patient-Centered Advanced Clinical Education
Director, Program in Medical Education for the Latino Community
University of California, Irvine, School of Medicine
Irvine, California
Faculty
Christopher M. Chappel, MD
Medical Director
Chappel Health and Wellness
Kissimmee, Florida
Brett Badgley Snodgrass, MSN, APRN, FNP-BC
Family Nurse Practitioner
Saint Francis Hospital–Bartlett Interventional Pain Management Clinic
Chief Executive Officer and President
BBS Consultants, Inc.
Bartlett, Tennessee
Copyright © 2014 by Frontline Medical Communications Inc.
Product News: 12 2014
Heads Up! Education Program
The Skin Cancer Foundation is encouraging dermatologists to participate in Heads Up!, an education program that provides beauty professionals with tips on what to look for and how to speak to their clients if they spot a suspicious lesion. Dermatologists can host an educational event where hairstylists and aestheticians will learn about skin cancer and its warning signs. Because early detection is critical, the Heads Up! program ensures that this group of first responders will be prepared to give a “heads up” to their clients if they see something suspicious, encouraging the client to visit a dermatologist in a timely manner. For more information, visit www.skincancer.org/headsup.
Heliocare and Vitamin Angels
Ferndale Healthcare, Inc, announces support for Vitamin Angels and Walgreens with its Heliocare brand. Vitamin Angels helps at-risk populations in need, specifically pregnant women, new mothers, and children younger than 5 years, gain access to vitamins and minerals. Through 2017, a percentage of each Heliocare purchase at any Walgreens location will be donated to help Vitamin Angels. For more information, visit www.walgreens.com/vitaminangels.
Promiseb Topical Cream
Promius Pharma, LLC, introduces Promiseb Topical Cream in a 60-g box. Promiseb Topical Cream is a nonsteroidal cream for the management of seborrhea and seborrheic dermatitis that has demonstrated both anti-inflammatory and antifungal properties. The 60-g box provides an option for treatment of larger body surface areas, which may necessitate fewer refills. For more information, visit www.promiseb.com.
Total Relief Shampoo and Conditioner
Dr. Marder Skincare presents an over-the-counter scalp solution for itching, flaking, and scaling. This dermatologist-formulated hydrocortisone shampoo and conditioner can be used to relieve symptoms of psoriasis, seborrhea, and dandruff. Both products can be purchased online. For more information, visit www.drmarderskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected]
Heads Up! Education Program
The Skin Cancer Foundation is encouraging dermatologists to participate in Heads Up!, an education program that provides beauty professionals with tips on what to look for and how to speak to their clients if they spot a suspicious lesion. Dermatologists can host an educational event where hairstylists and aestheticians will learn about skin cancer and its warning signs. Because early detection is critical, the Heads Up! program ensures that this group of first responders will be prepared to give a “heads up” to their clients if they see something suspicious, encouraging the client to visit a dermatologist in a timely manner. For more information, visit www.skincancer.org/headsup.
Heliocare and Vitamin Angels
Ferndale Healthcare, Inc, announces support for Vitamin Angels and Walgreens with its Heliocare brand. Vitamin Angels helps at-risk populations in need, specifically pregnant women, new mothers, and children younger than 5 years, gain access to vitamins and minerals. Through 2017, a percentage of each Heliocare purchase at any Walgreens location will be donated to help Vitamin Angels. For more information, visit www.walgreens.com/vitaminangels.
Promiseb Topical Cream
Promius Pharma, LLC, introduces Promiseb Topical Cream in a 60-g box. Promiseb Topical Cream is a nonsteroidal cream for the management of seborrhea and seborrheic dermatitis that has demonstrated both anti-inflammatory and antifungal properties. The 60-g box provides an option for treatment of larger body surface areas, which may necessitate fewer refills. For more information, visit www.promiseb.com.
Total Relief Shampoo and Conditioner
Dr. Marder Skincare presents an over-the-counter scalp solution for itching, flaking, and scaling. This dermatologist-formulated hydrocortisone shampoo and conditioner can be used to relieve symptoms of psoriasis, seborrhea, and dandruff. Both products can be purchased online. For more information, visit www.drmarderskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected]
Heads Up! Education Program
The Skin Cancer Foundation is encouraging dermatologists to participate in Heads Up!, an education program that provides beauty professionals with tips on what to look for and how to speak to their clients if they spot a suspicious lesion. Dermatologists can host an educational event where hairstylists and aestheticians will learn about skin cancer and its warning signs. Because early detection is critical, the Heads Up! program ensures that this group of first responders will be prepared to give a “heads up” to their clients if they see something suspicious, encouraging the client to visit a dermatologist in a timely manner. For more information, visit www.skincancer.org/headsup.
Heliocare and Vitamin Angels
Ferndale Healthcare, Inc, announces support for Vitamin Angels and Walgreens with its Heliocare brand. Vitamin Angels helps at-risk populations in need, specifically pregnant women, new mothers, and children younger than 5 years, gain access to vitamins and minerals. Through 2017, a percentage of each Heliocare purchase at any Walgreens location will be donated to help Vitamin Angels. For more information, visit www.walgreens.com/vitaminangels.
Promiseb Topical Cream
Promius Pharma, LLC, introduces Promiseb Topical Cream in a 60-g box. Promiseb Topical Cream is a nonsteroidal cream for the management of seborrhea and seborrheic dermatitis that has demonstrated both anti-inflammatory and antifungal properties. The 60-g box provides an option for treatment of larger body surface areas, which may necessitate fewer refills. For more information, visit www.promiseb.com.
Total Relief Shampoo and Conditioner
Dr. Marder Skincare presents an over-the-counter scalp solution for itching, flaking, and scaling. This dermatologist-formulated hydrocortisone shampoo and conditioner can be used to relieve symptoms of psoriasis, seborrhea, and dandruff. Both products can be purchased online. For more information, visit www.drmarderskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected]
Magnesium disappoints in sickle cell disease

sickle cell disease
Credit: St. Jude Hospital
SAN FRANCISCO—Magnesium does not improve outcomes in children hospitalized for sickle cell pain crises, results of the MAGiC study suggest.
Researchers hypothesized that magnesium—a known vasodilator, anti-inflammatory, and pain reliever—could alter the pathophysiology of pain crises.
However, when compared to normal saline, intravenous (IV) magnesium did not shorten hospital stays, lessen opioid use, or improve patients’ quality of life.
David C. Brousseau, MD, of the Medical College of Wisconsin and the Children’s Hospital of Wisconsin in Milwaukee, presented the results of this study at the 2014 ASH Annual Meeting (abstract 88).
Dr Brousseau noted that vasoocclusive crises are the most common acute complication of sickle cell disease and the most frequent cause of acute care or emergency department visits and hospitalizations. But recent changes in treatment have been minimal, with the judicious use of IV fluid and IV opioids being the mainstays of therapy.
“There have been few multicenter clinical trials evaluating new treatments, in part, due to a long history of difficulty with enrollment in interventional trials for sickle cell crises,” he continued. “These enrollment difficulties have been due to an inability to consent or to consent in a timely manner, leading to delayed initiation of study drug.”
With the MAGiC trial, Dr Brousseau and his colleagues sought to overcome this problem through a collaboration between pediatric emergency medicine physicians and pediatric hematologists.
In this randomized, double-blind trial, the researchers compared IV magnesium to normal saline. They enrolled children ages 4 to 21, with hemoglobin SS or hemoglobin SB° thalassemia, who were hospitalized after failing emergency department management for pain.
A total of 208 children were enrolled at 8 study sites over 3 years. Four children were excluded before receiving treatment, so 101 were randomized to receive magnesium and 103 to saline.
The children received 40 mg/kg of IV magnesium every 8 hours for a total of 6 doses or normal saline of an equivalent volume (1 mL/kg).
The treatment groups were well-balanced, with similar baseline age, sex, genotype, weight, history of acute chest syndrome or asthma, previous hospitalizations within the past 3 years, use of hydroxyurea, and days of pain prior to arrival.
The median time from the first emergency department opioid to the first study drug infusion was 7.3 hours in the magnesium group and 7.5 hours in the saline group.
For the study’s primary outcome, the researchers assessed patients’ length of stay from the first study drug infusion until 12 hours after the last IV opioid dose or the time of discharge, whichever came first.
“Approximately 50% of children [overall] met the study endpoint within 52 hours, and 25% met the study endpoint within 24 hours of the first drug infusion,” Dr Brousseau noted.
And there was no significant difference in the median length of stay between the treatment arms—56 hours in the magnesium arm and 47 hours in the placebo arm (P=0.264).
A secondary outcome was opioid use, recorded as morphine equivalents. There was no significant difference with this outcome, either. Patients in the magnesium arm received 1.46 mg/kg of morphine equivalents, compared to 1.28 mg/kg in the saline arm (P=0.12).
The researchers also assessed quality of life using the PedsQL sickle cell disease-specific module, fatigue module, and generic module. At 48 hours after the first infusion, there was no significant difference in quality of life scores between the treatment groups for any of the modules (P=0.17, 0.26, and 0.94, respectively). The same was true 1 week after discharge (P=0.55, 0.82, and 0.36, respectively).
As for safety, there was no significant difference between the treatment arms for most measures. However, patients in the magnesium arm were more likely to experience warmth upon infusion, at 26%, compared to 2% in the saline arm (P<0.01).
Acute chest syndrome occurred in 16% of patients in the magnesium arm and 14% in the saline arm (P=0.78). Hypotension occurred in 4% and 1%, respectively (P=0.39). And rehospitalization within 7 days occurred in 12% and 7%, respectively (P=0.11).
In closing, Dr Brousseau noted that, although the researchers did not prove their hypothesis correct, the MAGiC study was a success in one respect.
“Intravenous magnesium does not shorten length of stay, lessen opioid use, or improve quality of life in children hospitalized for sickle cell pain crises,” he said. “[However,] a collaboration between pediatric emergency department medicine physicians and pediatric hematologists allowed for successful enrollment in an acute intervention trial with a median time to first study drug of 7.5 hours.” ![]()

sickle cell disease
Credit: St. Jude Hospital
SAN FRANCISCO—Magnesium does not improve outcomes in children hospitalized for sickle cell pain crises, results of the MAGiC study suggest.
Researchers hypothesized that magnesium—a known vasodilator, anti-inflammatory, and pain reliever—could alter the pathophysiology of pain crises.
However, when compared to normal saline, intravenous (IV) magnesium did not shorten hospital stays, lessen opioid use, or improve patients’ quality of life.
David C. Brousseau, MD, of the Medical College of Wisconsin and the Children’s Hospital of Wisconsin in Milwaukee, presented the results of this study at the 2014 ASH Annual Meeting (abstract 88).
Dr Brousseau noted that vasoocclusive crises are the most common acute complication of sickle cell disease and the most frequent cause of acute care or emergency department visits and hospitalizations. But recent changes in treatment have been minimal, with the judicious use of IV fluid and IV opioids being the mainstays of therapy.
“There have been few multicenter clinical trials evaluating new treatments, in part, due to a long history of difficulty with enrollment in interventional trials for sickle cell crises,” he continued. “These enrollment difficulties have been due to an inability to consent or to consent in a timely manner, leading to delayed initiation of study drug.”
With the MAGiC trial, Dr Brousseau and his colleagues sought to overcome this problem through a collaboration between pediatric emergency medicine physicians and pediatric hematologists.
In this randomized, double-blind trial, the researchers compared IV magnesium to normal saline. They enrolled children ages 4 to 21, with hemoglobin SS or hemoglobin SB° thalassemia, who were hospitalized after failing emergency department management for pain.
A total of 208 children were enrolled at 8 study sites over 3 years. Four children were excluded before receiving treatment, so 101 were randomized to receive magnesium and 103 to saline.
The children received 40 mg/kg of IV magnesium every 8 hours for a total of 6 doses or normal saline of an equivalent volume (1 mL/kg).
The treatment groups were well-balanced, with similar baseline age, sex, genotype, weight, history of acute chest syndrome or asthma, previous hospitalizations within the past 3 years, use of hydroxyurea, and days of pain prior to arrival.
The median time from the first emergency department opioid to the first study drug infusion was 7.3 hours in the magnesium group and 7.5 hours in the saline group.
For the study’s primary outcome, the researchers assessed patients’ length of stay from the first study drug infusion until 12 hours after the last IV opioid dose or the time of discharge, whichever came first.
“Approximately 50% of children [overall] met the study endpoint within 52 hours, and 25% met the study endpoint within 24 hours of the first drug infusion,” Dr Brousseau noted.
And there was no significant difference in the median length of stay between the treatment arms—56 hours in the magnesium arm and 47 hours in the placebo arm (P=0.264).
A secondary outcome was opioid use, recorded as morphine equivalents. There was no significant difference with this outcome, either. Patients in the magnesium arm received 1.46 mg/kg of morphine equivalents, compared to 1.28 mg/kg in the saline arm (P=0.12).
The researchers also assessed quality of life using the PedsQL sickle cell disease-specific module, fatigue module, and generic module. At 48 hours after the first infusion, there was no significant difference in quality of life scores between the treatment groups for any of the modules (P=0.17, 0.26, and 0.94, respectively). The same was true 1 week after discharge (P=0.55, 0.82, and 0.36, respectively).
As for safety, there was no significant difference between the treatment arms for most measures. However, patients in the magnesium arm were more likely to experience warmth upon infusion, at 26%, compared to 2% in the saline arm (P<0.01).
Acute chest syndrome occurred in 16% of patients in the magnesium arm and 14% in the saline arm (P=0.78). Hypotension occurred in 4% and 1%, respectively (P=0.39). And rehospitalization within 7 days occurred in 12% and 7%, respectively (P=0.11).
In closing, Dr Brousseau noted that, although the researchers did not prove their hypothesis correct, the MAGiC study was a success in one respect.
“Intravenous magnesium does not shorten length of stay, lessen opioid use, or improve quality of life in children hospitalized for sickle cell pain crises,” he said. “[However,] a collaboration between pediatric emergency department medicine physicians and pediatric hematologists allowed for successful enrollment in an acute intervention trial with a median time to first study drug of 7.5 hours.” ![]()

sickle cell disease
Credit: St. Jude Hospital
SAN FRANCISCO—Magnesium does not improve outcomes in children hospitalized for sickle cell pain crises, results of the MAGiC study suggest.
Researchers hypothesized that magnesium—a known vasodilator, anti-inflammatory, and pain reliever—could alter the pathophysiology of pain crises.
However, when compared to normal saline, intravenous (IV) magnesium did not shorten hospital stays, lessen opioid use, or improve patients’ quality of life.
David C. Brousseau, MD, of the Medical College of Wisconsin and the Children’s Hospital of Wisconsin in Milwaukee, presented the results of this study at the 2014 ASH Annual Meeting (abstract 88).
Dr Brousseau noted that vasoocclusive crises are the most common acute complication of sickle cell disease and the most frequent cause of acute care or emergency department visits and hospitalizations. But recent changes in treatment have been minimal, with the judicious use of IV fluid and IV opioids being the mainstays of therapy.
“There have been few multicenter clinical trials evaluating new treatments, in part, due to a long history of difficulty with enrollment in interventional trials for sickle cell crises,” he continued. “These enrollment difficulties have been due to an inability to consent or to consent in a timely manner, leading to delayed initiation of study drug.”
With the MAGiC trial, Dr Brousseau and his colleagues sought to overcome this problem through a collaboration between pediatric emergency medicine physicians and pediatric hematologists.
In this randomized, double-blind trial, the researchers compared IV magnesium to normal saline. They enrolled children ages 4 to 21, with hemoglobin SS or hemoglobin SB° thalassemia, who were hospitalized after failing emergency department management for pain.
A total of 208 children were enrolled at 8 study sites over 3 years. Four children were excluded before receiving treatment, so 101 were randomized to receive magnesium and 103 to saline.
The children received 40 mg/kg of IV magnesium every 8 hours for a total of 6 doses or normal saline of an equivalent volume (1 mL/kg).
The treatment groups were well-balanced, with similar baseline age, sex, genotype, weight, history of acute chest syndrome or asthma, previous hospitalizations within the past 3 years, use of hydroxyurea, and days of pain prior to arrival.
The median time from the first emergency department opioid to the first study drug infusion was 7.3 hours in the magnesium group and 7.5 hours in the saline group.
For the study’s primary outcome, the researchers assessed patients’ length of stay from the first study drug infusion until 12 hours after the last IV opioid dose or the time of discharge, whichever came first.
“Approximately 50% of children [overall] met the study endpoint within 52 hours, and 25% met the study endpoint within 24 hours of the first drug infusion,” Dr Brousseau noted.
And there was no significant difference in the median length of stay between the treatment arms—56 hours in the magnesium arm and 47 hours in the placebo arm (P=0.264).
A secondary outcome was opioid use, recorded as morphine equivalents. There was no significant difference with this outcome, either. Patients in the magnesium arm received 1.46 mg/kg of morphine equivalents, compared to 1.28 mg/kg in the saline arm (P=0.12).
The researchers also assessed quality of life using the PedsQL sickle cell disease-specific module, fatigue module, and generic module. At 48 hours after the first infusion, there was no significant difference in quality of life scores between the treatment groups for any of the modules (P=0.17, 0.26, and 0.94, respectively). The same was true 1 week after discharge (P=0.55, 0.82, and 0.36, respectively).
As for safety, there was no significant difference between the treatment arms for most measures. However, patients in the magnesium arm were more likely to experience warmth upon infusion, at 26%, compared to 2% in the saline arm (P<0.01).
Acute chest syndrome occurred in 16% of patients in the magnesium arm and 14% in the saline arm (P=0.78). Hypotension occurred in 4% and 1%, respectively (P=0.39). And rehospitalization within 7 days occurred in 12% and 7%, respectively (P=0.11).
In closing, Dr Brousseau noted that, although the researchers did not prove their hypothesis correct, the MAGiC study was a success in one respect.
“Intravenous magnesium does not shorten length of stay, lessen opioid use, or improve quality of life in children hospitalized for sickle cell pain crises,” he said. “[However,] a collaboration between pediatric emergency department medicine physicians and pediatric hematologists allowed for successful enrollment in an acute intervention trial with a median time to first study drug of 7.5 hours.” ![]()
A Team Approach to Nonmelanotic Skin Cancer Procedures
For many decades, the treatment of choice for nonmetastatic but locally invasive nonmelanotic basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) has been complete surgical excision that ensures minimal tissue waste, yet retains adequate tumor-free resection margins. From early on, the primary challenge has been assessing the appropriateness of those margins at the time of the initial surgical procedure, rather than having to recall the patient later for an additional surgery to excise involved margins.
In 1953, Steven Mohs, MD, envisioned the use of a vital dye to distinguish benign from malignant skin tissue at the time of surgery.1-3 At that point intraoperative consultation with a pathologist and the process of examining frozen sections (FS) for diagnosis were not standards of care in oncologic surgery. This process allowed Mohs, with limited success, to excise tumors with negative margins. Mohs repeatedly revised and improved his procedure, including the utilization of intraoperative FS to examine the entire specimen margin, a process that is at the core of the Mohs micrographic surgery.1-3
Currently, the Mohs procedure is one of the most popular approaches to definitive skin cancer surgery, especially in the head and neck region where tissue preservation can be critical. It is usually performed as an outpatient or clinic procedure by a specially trained dermatologist who acts both as a surgeon and a pathologist, excising the lesion and processing it for FS diagnosis.4-6 In a hospital setting, other practitioners (surgeons and pathologists) often use the standard approach of limited sampling of resection margins for FS by serially sectioning a specimen that had already been inked or marked for the appropriate margins and freeze-sectioning representative portions of those margins. Reports published by experienced operators using these different approaches indicate variable cancer recurrence rates of 1% to 6%.7-9
At the VA it is a priority to deliver the same quality health care at a much lower price. In this setting it is prudent to periodically reexamine alternative approaches to patient care delivery that utilize existing resources or excess capacity to achieve comparable, if not superior, outcomes to the usually more costly private sector outsourcing contractual arrangements.
With that goal in mind, a few years ago Robley Rex VAMC (RRVAMC) embarked on a new team approach for resectable nonmelanotic skin cancer cases. The team consisted of a plastic surgeon and a pathologist with the appropriate technical and nursing support (histotechnicians, surgical nurse practitioners, and/or nurse anesthsesists) staff. None of the team members were exclusively dedicated to the procedure but were afforded adequate time and material resources to handle all such cases. In this report, the authors describe their experience and the impact of their approach on the affected patients.
Methods
At RRVAMC, primary care providers were encouraged to refer patients suspected of nonmelanotic skin cancer directly to a hospital-based plastic surgeon, who schedules them for a FS-controlled surgical excision of the suspected lesion. The plastic surgeon also plans to cover the resulting wound, if too large for primary closure, with a micrograft during the same procedure. The procedure is usually performed under local anesthesia. A general surgeon or surgical fellow with basic training in plastic surgery may substitute for the plastic surgeon. When not performing this procedure, the surgeon carries on other routine surgical duties.
A dedicated FS room was set up next to an operating room (OR), which was designated for this specialized skin cancer surgery, among other surgeries. The pathologist could walk into the OR anytime to assess the lesion, its location, and the surgeon’s plan of resection, and both physicians could discuss the best strategy for the initial resection or any subsequent margin reexcision. Both could also discuss whether a permanent section would be more appropriate under the conditions.
A small window separated the FS room from the OR, allowing two-way communication and the delivery of specimens. If the specimen was more complex in terms of margin definition, the pathologist could personally take the specimen after its excision directly from the surgeon who could offer further explanation of the special attributes of the specimen. The specimen was usually placed on a topographic drawing of the body region with one or more permanent marks that denoted specific landmarks for orientation.
Once the specimen was in the FS room, the pathologist proceeded with standard gross description followed by color inking of the margins and sampling, according to the following rules:
- Small specimen (< 0.5 cm): Embed as is; FSs may be cut parallel to epidermal surface and examined until no more tumor is seen.
- Medium specimen (0.5-3.0 cm): Serially cross-section and embed all in ≥ 1 blocks; ≥ 6 FSs (cuts) examined from each block.
- Large specimen (> 3.0 cm): Peripheral margins shaved; few central sections taken through deep margin.
For the very small specimens excised from cosmetically or biologically critical areas, such as the head and neck region, the pathologist could use the classic Mohs sampling technique of freezing the entire specimen as is and sectioning parallel to the skin surface until free margins were reached or the entire specimen was exhausted. The pathologist could use serial cross-sectioning at 2 mm intervals in medium-sized excisions, or limited sampling of peripheral and deep margins in very large specimens. In these latter sampling approaches, at least 6 sections are cut from each slice (block), each 5 µm to 10 µm thick. The sections were mounted on glass slides, stained with hematoxylin-eosin (H&E), and examined thoroughly under a microscope before rendering a diagnosis (assessment of the resection margin).
The diagnosis was communicated directly to the surgeon by the pathologist who walked into the OR or while viewing the slides with the surgeon at a double-headed microscope located in the FS room. Remnants of any frozen or unprocessed tissue were submitted for permanent section, and the findings of both the FS and permanent diagnosis were compared the following day. Similar to the main laboratory procedures, 10% of cases were subjected to retroactive peer review for quality assurance.
Freeze section duty was handled by a pathologist and a histotechnician. Once the FS case was completed, the pathologist and histotechnician returned to the main laboratory to attend to other routine duties.
The patient’s state of comfort and satisfaction was assessed informally but routinely by the surgical team before discharge and at the follow-up visit. The patient was asked about the overall experience and invited to submit written comments to the RRVAMC patient representative. A generic mailback card was also available for feedback.
For the cost analysis, budgeting for the recurrent annual cost of labor and supplies was based on a presumed maximum workload of 300 cases/year (3-4 cases/day; 2 days/week or 0.4 full-time equivalent employee [FTEE] for each member of the team) and estimated additional OR and histology laboratory supplies of about $500/case. At the end of the fiscal year, the budgeted estimates were reconciled with the actual expenses or the added financial burden that was associated with the program to calculate the expense per case, which then was compared with the average CMS (Centers for Medicare and Medicaid Services) reimbursement rate for Mohs procedures as usually billed by private practitioners.
Results
From 2006 to 2007, 439 procedures were performed at the RRVAMC program. Patients were followed up for recurrence or other complications through the end of 2012. No serious complications were encountered during any of these procedures. Patients’ comments after each procedure indicated complete satisfaction with the process, and no negative feedback or complaint was received. More than 5 years of follow-up on the initial 439 procedures yielded a rate of cancer recurrence of about 0.5% (2 patients, a 30-year-old woman and a 77-year-old man, both with basal cell carcinoma [BCC] of the nose), which is comparable or slightly better than that reported in relevant literature for the various methods, including the classic Mohs.10,11
Table 1 shows the distribution of the cases by age, gender, specimen size, and type of cancer. Most patients were white men (98.5%), and almost all (99%) cancers were from the head and neck region. Basal cell carcinoma was the diagnosis in 80% of the cases; the remainder were squamous cell carcinomas (SCCs). Both types of cancer were prevalent in the older age groups (> 50 years). Basal cell carcinoma was more prevalent in the group aged 51 to 70 years, whereas SCC predominated in patients aged > 70 years. The patients ranged in age from 30 to 89 years. The majority of specimens were medium sized (86%); 11% were large and the remaining 3% were small specimens. These demographics of patient’s age, cancer location, and prevalent diagnosis, were comparable to those of most VAMCs.
All acrediatation standards of the Clinical Laboratory Improvement Amendments of 1988 (CLIA 88) and College of American Pathologists (CAP) were observed in the RRVAMC FS laboratory, including monitoring frozen vs permanent tissue diagnosis and 10% retroactive peer review. Those indicators were always well below established thresholds or reasonable pathology practice community standards. The RRVAMC laboratory overall error (major discrepancy) rate has been < 0.2%. The FS laboratory has also been in compliance with the technical quality CAP accreditation standards, such as those for equipment, reagents, personnel, and environment controls.
Cost analysis data are presented in Table 2. The data are based on realistic estimates in a hospital setting. The provided numbers for the FTEE salaries are average local estimates (based on VA-wide pay scale for employees according to their grades and within grade steps), though actual salary structure varied widely among institutions. Although budgeted estimates suggest an average expense of about $1,500 per case (including cases with multiple lesions that could be removed at the same session), the actual or realistic expense is far less, because some of the resources were preexisting or shared across the Surgical and Pathology Services, including FTEE time commitments. The RRVAMC planning strategy assumed 200 to 300 cases/year at $1,000 to $2,000/case.
Discussion
The RRVAMC approach of direct patient referral to the in-house plastic surgeon often spared the patient 2 additional clinical visits or procedures, which might otherwise have been required. Often, the primary care provider referred the patient to a dermatologist who would perform a shave or punch biopsy, awaiting a pathologist’s diagnosis before scheduling definitive (eg, Mohs) surgery with a separate provider. After that, the patient might be scheduled for reconstructive surgery, if necessary, by a plastic surgeon. With the RRVAMC approach, not only were the number of visits/procedures reduced, but the total time was shortened by several weeks, sparing the patient discomfort and uncertainty.
The RRVAMC cost analysis data show an average realistic cost at this setting (considering already available resources) of far less than $2,000 ($1,000-$1,500). This is substantially below the $2,000 to $10,000 cost per case (or lesion in patients with multiple lesions) that would have been required for a private sector referral, based on CMS reimbursement rates for Mohs procedures (CPT codes 17311-17315).
An important element in the cost-effectiveness, quality assurance, and time use in this approach is the flexibility of the key operators (surgery and pathology staff) and the sampling technique. For the latter, the pathologist can use the most efficient technique, depending on specimen source and size: The classic Mohs technique for very small (head and neck area) specimens, but serial cross-sectioning or limited sampling of peripheral and deep margins in other situations. All 3 sectioning approaches in the RRVAMC practice proved reliable in assessing the margins, as they were always verified either on permanent sections and/or through retroactive peer review. Furthermore, in a mostly elderly patient population, there is rarely a need for extremely conservative resection of the margins, as the skin often shows wrinkling or redundancy that allows for a more generous healthy rim around the lesion. In such cases, it may be indeed superfluous to apply the protracted and expensive Mohs procedural variant.
The quality assurance aspect of the RRVAMC approach is also important. Examining permanent sections as well as retroactive peer review can uncover diagnostic or processing errors even in the best of laboratories. That error rate in the surgical pathology community may reach more than 1% to 2%.12 In the RRVAMC practice, the major discrepancy rate is usually below 0.2%. There is a reason for concern in any FS laboratory where such monitoring is not done, considering that even BCC can be occasionally confused on FS with other small blue cell malignancies, such as lymphoma or Merkel cell carcinoma.
Conclusion
The authors offer the RRVAMC pathologist-plastic surgeon team approach to definitive skin cancer surgery as a reliable and less expensive in-house alternative to contractual outsourcing for those VA (or non-VA) medical centers that have a plastic surgeon (or trained equivalent) and a surgical pathologist on staff.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Robley Rex VAMC in Louisville, Kentucky.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Robins P, Albom MJ. Mohs’ surgery—fresh tissue technique. J Dermatol Surg. 1975;1(2):37-41.
2. Mohs FE. Mohs micrographic surgery. A historical perspective. Dermatol Clin. 1989;7(4):609-611.
3. Mohs FE. Origin and progress of Mohs micrographic surgery. In: Mikhail GR, ed. Mohs Micrographic Surgery. Philadelphia, PA: WB Saunders; 1991:1-10.
4. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990.
5. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13(6):617-620.
6. Smeets NW, Krekels GA, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: Randomised controlled trial. Lancet. 2004;364(9447):1766-1772.
7. Bentkover SH, Grande DM, Soto H, Kozlicak BA, Guillaume D, Girouard S. Excision of head and neck basal cell carcinoma with a rapid, cross-sectional, frozen-section technique. Arch Facial Plast Surg. 2002;4(2):114-119.
8. Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2004;53(3):469-474.
9. Dhingra N, Gajdasty A, Neal JW, Mukherjee AN, Lane CM. Confident complete excision of lid-margin BCCs using a marginal strip: An alternative to Mohs’ surgery. Brit J Ophthalmol. 2007;91(6):794-796.
10. Minton TJ. Contemporary Mohs surgery applications. Curr Opin Otolaryngol Head Neck Surg. 2008;16(4):376-380.
11. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9(12):1149-1156.
12. Weiss MA. Analytic variables; diagnostic accuracy. In: Nakhleh RE, Fitzgibbons PL, eds. Quality Management in Anatomic Pathology. Northfield, IL: College of American Pathologists; 2005:50-76.
For many decades, the treatment of choice for nonmetastatic but locally invasive nonmelanotic basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) has been complete surgical excision that ensures minimal tissue waste, yet retains adequate tumor-free resection margins. From early on, the primary challenge has been assessing the appropriateness of those margins at the time of the initial surgical procedure, rather than having to recall the patient later for an additional surgery to excise involved margins.
In 1953, Steven Mohs, MD, envisioned the use of a vital dye to distinguish benign from malignant skin tissue at the time of surgery.1-3 At that point intraoperative consultation with a pathologist and the process of examining frozen sections (FS) for diagnosis were not standards of care in oncologic surgery. This process allowed Mohs, with limited success, to excise tumors with negative margins. Mohs repeatedly revised and improved his procedure, including the utilization of intraoperative FS to examine the entire specimen margin, a process that is at the core of the Mohs micrographic surgery.1-3
Currently, the Mohs procedure is one of the most popular approaches to definitive skin cancer surgery, especially in the head and neck region where tissue preservation can be critical. It is usually performed as an outpatient or clinic procedure by a specially trained dermatologist who acts both as a surgeon and a pathologist, excising the lesion and processing it for FS diagnosis.4-6 In a hospital setting, other practitioners (surgeons and pathologists) often use the standard approach of limited sampling of resection margins for FS by serially sectioning a specimen that had already been inked or marked for the appropriate margins and freeze-sectioning representative portions of those margins. Reports published by experienced operators using these different approaches indicate variable cancer recurrence rates of 1% to 6%.7-9
At the VA it is a priority to deliver the same quality health care at a much lower price. In this setting it is prudent to periodically reexamine alternative approaches to patient care delivery that utilize existing resources or excess capacity to achieve comparable, if not superior, outcomes to the usually more costly private sector outsourcing contractual arrangements.
With that goal in mind, a few years ago Robley Rex VAMC (RRVAMC) embarked on a new team approach for resectable nonmelanotic skin cancer cases. The team consisted of a plastic surgeon and a pathologist with the appropriate technical and nursing support (histotechnicians, surgical nurse practitioners, and/or nurse anesthsesists) staff. None of the team members were exclusively dedicated to the procedure but were afforded adequate time and material resources to handle all such cases. In this report, the authors describe their experience and the impact of their approach on the affected patients.
Methods
At RRVAMC, primary care providers were encouraged to refer patients suspected of nonmelanotic skin cancer directly to a hospital-based plastic surgeon, who schedules them for a FS-controlled surgical excision of the suspected lesion. The plastic surgeon also plans to cover the resulting wound, if too large for primary closure, with a micrograft during the same procedure. The procedure is usually performed under local anesthesia. A general surgeon or surgical fellow with basic training in plastic surgery may substitute for the plastic surgeon. When not performing this procedure, the surgeon carries on other routine surgical duties.
A dedicated FS room was set up next to an operating room (OR), which was designated for this specialized skin cancer surgery, among other surgeries. The pathologist could walk into the OR anytime to assess the lesion, its location, and the surgeon’s plan of resection, and both physicians could discuss the best strategy for the initial resection or any subsequent margin reexcision. Both could also discuss whether a permanent section would be more appropriate under the conditions.
A small window separated the FS room from the OR, allowing two-way communication and the delivery of specimens. If the specimen was more complex in terms of margin definition, the pathologist could personally take the specimen after its excision directly from the surgeon who could offer further explanation of the special attributes of the specimen. The specimen was usually placed on a topographic drawing of the body region with one or more permanent marks that denoted specific landmarks for orientation.
Once the specimen was in the FS room, the pathologist proceeded with standard gross description followed by color inking of the margins and sampling, according to the following rules:
- Small specimen (< 0.5 cm): Embed as is; FSs may be cut parallel to epidermal surface and examined until no more tumor is seen.
- Medium specimen (0.5-3.0 cm): Serially cross-section and embed all in ≥ 1 blocks; ≥ 6 FSs (cuts) examined from each block.
- Large specimen (> 3.0 cm): Peripheral margins shaved; few central sections taken through deep margin.
For the very small specimens excised from cosmetically or biologically critical areas, such as the head and neck region, the pathologist could use the classic Mohs sampling technique of freezing the entire specimen as is and sectioning parallel to the skin surface until free margins were reached or the entire specimen was exhausted. The pathologist could use serial cross-sectioning at 2 mm intervals in medium-sized excisions, or limited sampling of peripheral and deep margins in very large specimens. In these latter sampling approaches, at least 6 sections are cut from each slice (block), each 5 µm to 10 µm thick. The sections were mounted on glass slides, stained with hematoxylin-eosin (H&E), and examined thoroughly under a microscope before rendering a diagnosis (assessment of the resection margin).
The diagnosis was communicated directly to the surgeon by the pathologist who walked into the OR or while viewing the slides with the surgeon at a double-headed microscope located in the FS room. Remnants of any frozen or unprocessed tissue were submitted for permanent section, and the findings of both the FS and permanent diagnosis were compared the following day. Similar to the main laboratory procedures, 10% of cases were subjected to retroactive peer review for quality assurance.
Freeze section duty was handled by a pathologist and a histotechnician. Once the FS case was completed, the pathologist and histotechnician returned to the main laboratory to attend to other routine duties.
The patient’s state of comfort and satisfaction was assessed informally but routinely by the surgical team before discharge and at the follow-up visit. The patient was asked about the overall experience and invited to submit written comments to the RRVAMC patient representative. A generic mailback card was also available for feedback.
For the cost analysis, budgeting for the recurrent annual cost of labor and supplies was based on a presumed maximum workload of 300 cases/year (3-4 cases/day; 2 days/week or 0.4 full-time equivalent employee [FTEE] for each member of the team) and estimated additional OR and histology laboratory supplies of about $500/case. At the end of the fiscal year, the budgeted estimates were reconciled with the actual expenses or the added financial burden that was associated with the program to calculate the expense per case, which then was compared with the average CMS (Centers for Medicare and Medicaid Services) reimbursement rate for Mohs procedures as usually billed by private practitioners.
Results
From 2006 to 2007, 439 procedures were performed at the RRVAMC program. Patients were followed up for recurrence or other complications through the end of 2012. No serious complications were encountered during any of these procedures. Patients’ comments after each procedure indicated complete satisfaction with the process, and no negative feedback or complaint was received. More than 5 years of follow-up on the initial 439 procedures yielded a rate of cancer recurrence of about 0.5% (2 patients, a 30-year-old woman and a 77-year-old man, both with basal cell carcinoma [BCC] of the nose), which is comparable or slightly better than that reported in relevant literature for the various methods, including the classic Mohs.10,11
Table 1 shows the distribution of the cases by age, gender, specimen size, and type of cancer. Most patients were white men (98.5%), and almost all (99%) cancers were from the head and neck region. Basal cell carcinoma was the diagnosis in 80% of the cases; the remainder were squamous cell carcinomas (SCCs). Both types of cancer were prevalent in the older age groups (> 50 years). Basal cell carcinoma was more prevalent in the group aged 51 to 70 years, whereas SCC predominated in patients aged > 70 years. The patients ranged in age from 30 to 89 years. The majority of specimens were medium sized (86%); 11% were large and the remaining 3% were small specimens. These demographics of patient’s age, cancer location, and prevalent diagnosis, were comparable to those of most VAMCs.
All acrediatation standards of the Clinical Laboratory Improvement Amendments of 1988 (CLIA 88) and College of American Pathologists (CAP) were observed in the RRVAMC FS laboratory, including monitoring frozen vs permanent tissue diagnosis and 10% retroactive peer review. Those indicators were always well below established thresholds or reasonable pathology practice community standards. The RRVAMC laboratory overall error (major discrepancy) rate has been < 0.2%. The FS laboratory has also been in compliance with the technical quality CAP accreditation standards, such as those for equipment, reagents, personnel, and environment controls.
Cost analysis data are presented in Table 2. The data are based on realistic estimates in a hospital setting. The provided numbers for the FTEE salaries are average local estimates (based on VA-wide pay scale for employees according to their grades and within grade steps), though actual salary structure varied widely among institutions. Although budgeted estimates suggest an average expense of about $1,500 per case (including cases with multiple lesions that could be removed at the same session), the actual or realistic expense is far less, because some of the resources were preexisting or shared across the Surgical and Pathology Services, including FTEE time commitments. The RRVAMC planning strategy assumed 200 to 300 cases/year at $1,000 to $2,000/case.
Discussion
The RRVAMC approach of direct patient referral to the in-house plastic surgeon often spared the patient 2 additional clinical visits or procedures, which might otherwise have been required. Often, the primary care provider referred the patient to a dermatologist who would perform a shave or punch biopsy, awaiting a pathologist’s diagnosis before scheduling definitive (eg, Mohs) surgery with a separate provider. After that, the patient might be scheduled for reconstructive surgery, if necessary, by a plastic surgeon. With the RRVAMC approach, not only were the number of visits/procedures reduced, but the total time was shortened by several weeks, sparing the patient discomfort and uncertainty.
The RRVAMC cost analysis data show an average realistic cost at this setting (considering already available resources) of far less than $2,000 ($1,000-$1,500). This is substantially below the $2,000 to $10,000 cost per case (or lesion in patients with multiple lesions) that would have been required for a private sector referral, based on CMS reimbursement rates for Mohs procedures (CPT codes 17311-17315).
An important element in the cost-effectiveness, quality assurance, and time use in this approach is the flexibility of the key operators (surgery and pathology staff) and the sampling technique. For the latter, the pathologist can use the most efficient technique, depending on specimen source and size: The classic Mohs technique for very small (head and neck area) specimens, but serial cross-sectioning or limited sampling of peripheral and deep margins in other situations. All 3 sectioning approaches in the RRVAMC practice proved reliable in assessing the margins, as they were always verified either on permanent sections and/or through retroactive peer review. Furthermore, in a mostly elderly patient population, there is rarely a need for extremely conservative resection of the margins, as the skin often shows wrinkling or redundancy that allows for a more generous healthy rim around the lesion. In such cases, it may be indeed superfluous to apply the protracted and expensive Mohs procedural variant.
The quality assurance aspect of the RRVAMC approach is also important. Examining permanent sections as well as retroactive peer review can uncover diagnostic or processing errors even in the best of laboratories. That error rate in the surgical pathology community may reach more than 1% to 2%.12 In the RRVAMC practice, the major discrepancy rate is usually below 0.2%. There is a reason for concern in any FS laboratory where such monitoring is not done, considering that even BCC can be occasionally confused on FS with other small blue cell malignancies, such as lymphoma or Merkel cell carcinoma.
Conclusion
The authors offer the RRVAMC pathologist-plastic surgeon team approach to definitive skin cancer surgery as a reliable and less expensive in-house alternative to contractual outsourcing for those VA (or non-VA) medical centers that have a plastic surgeon (or trained equivalent) and a surgical pathologist on staff.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Robley Rex VAMC in Louisville, Kentucky.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
For many decades, the treatment of choice for nonmetastatic but locally invasive nonmelanotic basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) has been complete surgical excision that ensures minimal tissue waste, yet retains adequate tumor-free resection margins. From early on, the primary challenge has been assessing the appropriateness of those margins at the time of the initial surgical procedure, rather than having to recall the patient later for an additional surgery to excise involved margins.
In 1953, Steven Mohs, MD, envisioned the use of a vital dye to distinguish benign from malignant skin tissue at the time of surgery.1-3 At that point intraoperative consultation with a pathologist and the process of examining frozen sections (FS) for diagnosis were not standards of care in oncologic surgery. This process allowed Mohs, with limited success, to excise tumors with negative margins. Mohs repeatedly revised and improved his procedure, including the utilization of intraoperative FS to examine the entire specimen margin, a process that is at the core of the Mohs micrographic surgery.1-3
Currently, the Mohs procedure is one of the most popular approaches to definitive skin cancer surgery, especially in the head and neck region where tissue preservation can be critical. It is usually performed as an outpatient or clinic procedure by a specially trained dermatologist who acts both as a surgeon and a pathologist, excising the lesion and processing it for FS diagnosis.4-6 In a hospital setting, other practitioners (surgeons and pathologists) often use the standard approach of limited sampling of resection margins for FS by serially sectioning a specimen that had already been inked or marked for the appropriate margins and freeze-sectioning representative portions of those margins. Reports published by experienced operators using these different approaches indicate variable cancer recurrence rates of 1% to 6%.7-9
At the VA it is a priority to deliver the same quality health care at a much lower price. In this setting it is prudent to periodically reexamine alternative approaches to patient care delivery that utilize existing resources or excess capacity to achieve comparable, if not superior, outcomes to the usually more costly private sector outsourcing contractual arrangements.
With that goal in mind, a few years ago Robley Rex VAMC (RRVAMC) embarked on a new team approach for resectable nonmelanotic skin cancer cases. The team consisted of a plastic surgeon and a pathologist with the appropriate technical and nursing support (histotechnicians, surgical nurse practitioners, and/or nurse anesthsesists) staff. None of the team members were exclusively dedicated to the procedure but were afforded adequate time and material resources to handle all such cases. In this report, the authors describe their experience and the impact of their approach on the affected patients.
Methods
At RRVAMC, primary care providers were encouraged to refer patients suspected of nonmelanotic skin cancer directly to a hospital-based plastic surgeon, who schedules them for a FS-controlled surgical excision of the suspected lesion. The plastic surgeon also plans to cover the resulting wound, if too large for primary closure, with a micrograft during the same procedure. The procedure is usually performed under local anesthesia. A general surgeon or surgical fellow with basic training in plastic surgery may substitute for the plastic surgeon. When not performing this procedure, the surgeon carries on other routine surgical duties.
A dedicated FS room was set up next to an operating room (OR), which was designated for this specialized skin cancer surgery, among other surgeries. The pathologist could walk into the OR anytime to assess the lesion, its location, and the surgeon’s plan of resection, and both physicians could discuss the best strategy for the initial resection or any subsequent margin reexcision. Both could also discuss whether a permanent section would be more appropriate under the conditions.
A small window separated the FS room from the OR, allowing two-way communication and the delivery of specimens. If the specimen was more complex in terms of margin definition, the pathologist could personally take the specimen after its excision directly from the surgeon who could offer further explanation of the special attributes of the specimen. The specimen was usually placed on a topographic drawing of the body region with one or more permanent marks that denoted specific landmarks for orientation.
Once the specimen was in the FS room, the pathologist proceeded with standard gross description followed by color inking of the margins and sampling, according to the following rules:
- Small specimen (< 0.5 cm): Embed as is; FSs may be cut parallel to epidermal surface and examined until no more tumor is seen.
- Medium specimen (0.5-3.0 cm): Serially cross-section and embed all in ≥ 1 blocks; ≥ 6 FSs (cuts) examined from each block.
- Large specimen (> 3.0 cm): Peripheral margins shaved; few central sections taken through deep margin.
For the very small specimens excised from cosmetically or biologically critical areas, such as the head and neck region, the pathologist could use the classic Mohs sampling technique of freezing the entire specimen as is and sectioning parallel to the skin surface until free margins were reached or the entire specimen was exhausted. The pathologist could use serial cross-sectioning at 2 mm intervals in medium-sized excisions, or limited sampling of peripheral and deep margins in very large specimens. In these latter sampling approaches, at least 6 sections are cut from each slice (block), each 5 µm to 10 µm thick. The sections were mounted on glass slides, stained with hematoxylin-eosin (H&E), and examined thoroughly under a microscope before rendering a diagnosis (assessment of the resection margin).
The diagnosis was communicated directly to the surgeon by the pathologist who walked into the OR or while viewing the slides with the surgeon at a double-headed microscope located in the FS room. Remnants of any frozen or unprocessed tissue were submitted for permanent section, and the findings of both the FS and permanent diagnosis were compared the following day. Similar to the main laboratory procedures, 10% of cases were subjected to retroactive peer review for quality assurance.
Freeze section duty was handled by a pathologist and a histotechnician. Once the FS case was completed, the pathologist and histotechnician returned to the main laboratory to attend to other routine duties.
The patient’s state of comfort and satisfaction was assessed informally but routinely by the surgical team before discharge and at the follow-up visit. The patient was asked about the overall experience and invited to submit written comments to the RRVAMC patient representative. A generic mailback card was also available for feedback.
For the cost analysis, budgeting for the recurrent annual cost of labor and supplies was based on a presumed maximum workload of 300 cases/year (3-4 cases/day; 2 days/week or 0.4 full-time equivalent employee [FTEE] for each member of the team) and estimated additional OR and histology laboratory supplies of about $500/case. At the end of the fiscal year, the budgeted estimates were reconciled with the actual expenses or the added financial burden that was associated with the program to calculate the expense per case, which then was compared with the average CMS (Centers for Medicare and Medicaid Services) reimbursement rate for Mohs procedures as usually billed by private practitioners.
Results
From 2006 to 2007, 439 procedures were performed at the RRVAMC program. Patients were followed up for recurrence or other complications through the end of 2012. No serious complications were encountered during any of these procedures. Patients’ comments after each procedure indicated complete satisfaction with the process, and no negative feedback or complaint was received. More than 5 years of follow-up on the initial 439 procedures yielded a rate of cancer recurrence of about 0.5% (2 patients, a 30-year-old woman and a 77-year-old man, both with basal cell carcinoma [BCC] of the nose), which is comparable or slightly better than that reported in relevant literature for the various methods, including the classic Mohs.10,11
Table 1 shows the distribution of the cases by age, gender, specimen size, and type of cancer. Most patients were white men (98.5%), and almost all (99%) cancers were from the head and neck region. Basal cell carcinoma was the diagnosis in 80% of the cases; the remainder were squamous cell carcinomas (SCCs). Both types of cancer were prevalent in the older age groups (> 50 years). Basal cell carcinoma was more prevalent in the group aged 51 to 70 years, whereas SCC predominated in patients aged > 70 years. The patients ranged in age from 30 to 89 years. The majority of specimens were medium sized (86%); 11% were large and the remaining 3% were small specimens. These demographics of patient’s age, cancer location, and prevalent diagnosis, were comparable to those of most VAMCs.
All acrediatation standards of the Clinical Laboratory Improvement Amendments of 1988 (CLIA 88) and College of American Pathologists (CAP) were observed in the RRVAMC FS laboratory, including monitoring frozen vs permanent tissue diagnosis and 10% retroactive peer review. Those indicators were always well below established thresholds or reasonable pathology practice community standards. The RRVAMC laboratory overall error (major discrepancy) rate has been < 0.2%. The FS laboratory has also been in compliance with the technical quality CAP accreditation standards, such as those for equipment, reagents, personnel, and environment controls.
Cost analysis data are presented in Table 2. The data are based on realistic estimates in a hospital setting. The provided numbers for the FTEE salaries are average local estimates (based on VA-wide pay scale for employees according to their grades and within grade steps), though actual salary structure varied widely among institutions. Although budgeted estimates suggest an average expense of about $1,500 per case (including cases with multiple lesions that could be removed at the same session), the actual or realistic expense is far less, because some of the resources were preexisting or shared across the Surgical and Pathology Services, including FTEE time commitments. The RRVAMC planning strategy assumed 200 to 300 cases/year at $1,000 to $2,000/case.
Discussion
The RRVAMC approach of direct patient referral to the in-house plastic surgeon often spared the patient 2 additional clinical visits or procedures, which might otherwise have been required. Often, the primary care provider referred the patient to a dermatologist who would perform a shave or punch biopsy, awaiting a pathologist’s diagnosis before scheduling definitive (eg, Mohs) surgery with a separate provider. After that, the patient might be scheduled for reconstructive surgery, if necessary, by a plastic surgeon. With the RRVAMC approach, not only were the number of visits/procedures reduced, but the total time was shortened by several weeks, sparing the patient discomfort and uncertainty.
The RRVAMC cost analysis data show an average realistic cost at this setting (considering already available resources) of far less than $2,000 ($1,000-$1,500). This is substantially below the $2,000 to $10,000 cost per case (or lesion in patients with multiple lesions) that would have been required for a private sector referral, based on CMS reimbursement rates for Mohs procedures (CPT codes 17311-17315).
An important element in the cost-effectiveness, quality assurance, and time use in this approach is the flexibility of the key operators (surgery and pathology staff) and the sampling technique. For the latter, the pathologist can use the most efficient technique, depending on specimen source and size: The classic Mohs technique for very small (head and neck area) specimens, but serial cross-sectioning or limited sampling of peripheral and deep margins in other situations. All 3 sectioning approaches in the RRVAMC practice proved reliable in assessing the margins, as they were always verified either on permanent sections and/or through retroactive peer review. Furthermore, in a mostly elderly patient population, there is rarely a need for extremely conservative resection of the margins, as the skin often shows wrinkling or redundancy that allows for a more generous healthy rim around the lesion. In such cases, it may be indeed superfluous to apply the protracted and expensive Mohs procedural variant.
The quality assurance aspect of the RRVAMC approach is also important. Examining permanent sections as well as retroactive peer review can uncover diagnostic or processing errors even in the best of laboratories. That error rate in the surgical pathology community may reach more than 1% to 2%.12 In the RRVAMC practice, the major discrepancy rate is usually below 0.2%. There is a reason for concern in any FS laboratory where such monitoring is not done, considering that even BCC can be occasionally confused on FS with other small blue cell malignancies, such as lymphoma or Merkel cell carcinoma.
Conclusion
The authors offer the RRVAMC pathologist-plastic surgeon team approach to definitive skin cancer surgery as a reliable and less expensive in-house alternative to contractual outsourcing for those VA (or non-VA) medical centers that have a plastic surgeon (or trained equivalent) and a surgical pathologist on staff.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Robley Rex VAMC in Louisville, Kentucky.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Robins P, Albom MJ. Mohs’ surgery—fresh tissue technique. J Dermatol Surg. 1975;1(2):37-41.
2. Mohs FE. Mohs micrographic surgery. A historical perspective. Dermatol Clin. 1989;7(4):609-611.
3. Mohs FE. Origin and progress of Mohs micrographic surgery. In: Mikhail GR, ed. Mohs Micrographic Surgery. Philadelphia, PA: WB Saunders; 1991:1-10.
4. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990.
5. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13(6):617-620.
6. Smeets NW, Krekels GA, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: Randomised controlled trial. Lancet. 2004;364(9447):1766-1772.
7. Bentkover SH, Grande DM, Soto H, Kozlicak BA, Guillaume D, Girouard S. Excision of head and neck basal cell carcinoma with a rapid, cross-sectional, frozen-section technique. Arch Facial Plast Surg. 2002;4(2):114-119.
8. Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2004;53(3):469-474.
9. Dhingra N, Gajdasty A, Neal JW, Mukherjee AN, Lane CM. Confident complete excision of lid-margin BCCs using a marginal strip: An alternative to Mohs’ surgery. Brit J Ophthalmol. 2007;91(6):794-796.
10. Minton TJ. Contemporary Mohs surgery applications. Curr Opin Otolaryngol Head Neck Surg. 2008;16(4):376-380.
11. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9(12):1149-1156.
12. Weiss MA. Analytic variables; diagnostic accuracy. In: Nakhleh RE, Fitzgibbons PL, eds. Quality Management in Anatomic Pathology. Northfield, IL: College of American Pathologists; 2005:50-76.
1. Robins P, Albom MJ. Mohs’ surgery—fresh tissue technique. J Dermatol Surg. 1975;1(2):37-41.
2. Mohs FE. Mohs micrographic surgery. A historical perspective. Dermatol Clin. 1989;7(4):609-611.
3. Mohs FE. Origin and progress of Mohs micrographic surgery. In: Mikhail GR, ed. Mohs Micrographic Surgery. Philadelphia, PA: WB Saunders; 1991:1-10.
4. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990.
5. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13(6):617-620.
6. Smeets NW, Krekels GA, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: Randomised controlled trial. Lancet. 2004;364(9447):1766-1772.
7. Bentkover SH, Grande DM, Soto H, Kozlicak BA, Guillaume D, Girouard S. Excision of head and neck basal cell carcinoma with a rapid, cross-sectional, frozen-section technique. Arch Facial Plast Surg. 2002;4(2):114-119.
8. Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2004;53(3):469-474.
9. Dhingra N, Gajdasty A, Neal JW, Mukherjee AN, Lane CM. Confident complete excision of lid-margin BCCs using a marginal strip: An alternative to Mohs’ surgery. Brit J Ophthalmol. 2007;91(6):794-796.
10. Minton TJ. Contemporary Mohs surgery applications. Curr Opin Otolaryngol Head Neck Surg. 2008;16(4):376-380.
11. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9(12):1149-1156.
12. Weiss MA. Analytic variables; diagnostic accuracy. In: Nakhleh RE, Fitzgibbons PL, eds. Quality Management in Anatomic Pathology. Northfield, IL: College of American Pathologists; 2005:50-76.
Reducing chemo drug’s cardiac side effects

Investigators have identified compounds that appear to prevent the cardiac damage caused by the chemotherapy drug doxorubicin.
The compounds target MDH2, an enzyme key to the generation of cellular energy in mitochondria.
And preclinical experiments showed that inhibiting MDH2 could prevent doxorubicin-induced damage to cardiac cells without reducing the drug’s antitumor effects.
The investigators detailed these experiments in Science Translational Medicine.
“Doxorubicin-induced cardiomyopathy limits the amount of the drug a patient can receive—which limits the ability to treat cancer—and even low, safer doses can lead to heart failure in up to 8% of patients,” explained study author Randall Peterson, PhD, of Massachusetts General Hospital in Charlestown.
“Finding an effective cardioprotective drug—essentially separating the good and bad effects of this form of chemotherapy—could increase the beneficial effects of doxorubicin against cancer while reducing the rate of heart failure in treated patients.”
To conduct a broad search for potential protective compounds, Dr Peterson and his colleagues developed a zebrafish model of doxorubicin-induced heart failure. They used this model to screen 3000 molecules from 2 chemical libraries for the ability to prevent the kind of cardiac damage caused by the drug.
Eight of the tested chemicals reduced damage to the hearts of zebrafish embryos, and two compounds—visnagin and diphenylurea—were the most potent in preventing both structural and functional damage.
Further in vitro and in vivo experiments revealed that either compound almost completely prevented the death of cardiac cells caused by doxorubicin. In mouse models of both high- and low-dose doxorubicin treatment, visnagin—a natural compound synthesized by the toothpick weed—was able to maintain cardiac function.
Investigation into the possible mechanism behind visnagin’s protective ability showed that the compound binds to and inhibits the action of MDH2, an enzyme essential to the generation of cellular energy by mitochondria.
Other agents that block MDH2 activity also protected zebrafish against doxorubicin-induced cardiac damage. And tests in both cellular and animal models of several types of cancer showed that neither visnagin nor diphenylurea reduced the antitumor action of doxorubicin.
“We are still trying to determine exactly how inhibition of MDH2 protects the heart, but one intriguing idea is that doxorubicin may kill cardiac and tumor cells in different ways,” Dr Peterson said. “Given the intense energy requirements of the beating heart, we speculate that cardiac cells may be especially susceptible to metabolic disturbance caused by doxorubicin and that inhibiting MDH2 may correct the metabolic imbalance and prevent the cells from dying.”
“It remains to be seen if visnagin’s protective effects are restricted to doxorubicin or if it can protect the heart from other kinds of damage. We are pursuing this question by testing its ability to protect heart muscle from oxygen deprivation during heart attacks and from the effects of other heart-damaging chemotherapy drugs.” ![]()

Investigators have identified compounds that appear to prevent the cardiac damage caused by the chemotherapy drug doxorubicin.
The compounds target MDH2, an enzyme key to the generation of cellular energy in mitochondria.
And preclinical experiments showed that inhibiting MDH2 could prevent doxorubicin-induced damage to cardiac cells without reducing the drug’s antitumor effects.
The investigators detailed these experiments in Science Translational Medicine.
“Doxorubicin-induced cardiomyopathy limits the amount of the drug a patient can receive—which limits the ability to treat cancer—and even low, safer doses can lead to heart failure in up to 8% of patients,” explained study author Randall Peterson, PhD, of Massachusetts General Hospital in Charlestown.
“Finding an effective cardioprotective drug—essentially separating the good and bad effects of this form of chemotherapy—could increase the beneficial effects of doxorubicin against cancer while reducing the rate of heart failure in treated patients.”
To conduct a broad search for potential protective compounds, Dr Peterson and his colleagues developed a zebrafish model of doxorubicin-induced heart failure. They used this model to screen 3000 molecules from 2 chemical libraries for the ability to prevent the kind of cardiac damage caused by the drug.
Eight of the tested chemicals reduced damage to the hearts of zebrafish embryos, and two compounds—visnagin and diphenylurea—were the most potent in preventing both structural and functional damage.
Further in vitro and in vivo experiments revealed that either compound almost completely prevented the death of cardiac cells caused by doxorubicin. In mouse models of both high- and low-dose doxorubicin treatment, visnagin—a natural compound synthesized by the toothpick weed—was able to maintain cardiac function.
Investigation into the possible mechanism behind visnagin’s protective ability showed that the compound binds to and inhibits the action of MDH2, an enzyme essential to the generation of cellular energy by mitochondria.
Other agents that block MDH2 activity also protected zebrafish against doxorubicin-induced cardiac damage. And tests in both cellular and animal models of several types of cancer showed that neither visnagin nor diphenylurea reduced the antitumor action of doxorubicin.
“We are still trying to determine exactly how inhibition of MDH2 protects the heart, but one intriguing idea is that doxorubicin may kill cardiac and tumor cells in different ways,” Dr Peterson said. “Given the intense energy requirements of the beating heart, we speculate that cardiac cells may be especially susceptible to metabolic disturbance caused by doxorubicin and that inhibiting MDH2 may correct the metabolic imbalance and prevent the cells from dying.”
“It remains to be seen if visnagin’s protective effects are restricted to doxorubicin or if it can protect the heart from other kinds of damage. We are pursuing this question by testing its ability to protect heart muscle from oxygen deprivation during heart attacks and from the effects of other heart-damaging chemotherapy drugs.” ![]()

Investigators have identified compounds that appear to prevent the cardiac damage caused by the chemotherapy drug doxorubicin.
The compounds target MDH2, an enzyme key to the generation of cellular energy in mitochondria.
And preclinical experiments showed that inhibiting MDH2 could prevent doxorubicin-induced damage to cardiac cells without reducing the drug’s antitumor effects.
The investigators detailed these experiments in Science Translational Medicine.
“Doxorubicin-induced cardiomyopathy limits the amount of the drug a patient can receive—which limits the ability to treat cancer—and even low, safer doses can lead to heart failure in up to 8% of patients,” explained study author Randall Peterson, PhD, of Massachusetts General Hospital in Charlestown.
“Finding an effective cardioprotective drug—essentially separating the good and bad effects of this form of chemotherapy—could increase the beneficial effects of doxorubicin against cancer while reducing the rate of heart failure in treated patients.”
To conduct a broad search for potential protective compounds, Dr Peterson and his colleagues developed a zebrafish model of doxorubicin-induced heart failure. They used this model to screen 3000 molecules from 2 chemical libraries for the ability to prevent the kind of cardiac damage caused by the drug.
Eight of the tested chemicals reduced damage to the hearts of zebrafish embryos, and two compounds—visnagin and diphenylurea—were the most potent in preventing both structural and functional damage.
Further in vitro and in vivo experiments revealed that either compound almost completely prevented the death of cardiac cells caused by doxorubicin. In mouse models of both high- and low-dose doxorubicin treatment, visnagin—a natural compound synthesized by the toothpick weed—was able to maintain cardiac function.
Investigation into the possible mechanism behind visnagin’s protective ability showed that the compound binds to and inhibits the action of MDH2, an enzyme essential to the generation of cellular energy by mitochondria.
Other agents that block MDH2 activity also protected zebrafish against doxorubicin-induced cardiac damage. And tests in both cellular and animal models of several types of cancer showed that neither visnagin nor diphenylurea reduced the antitumor action of doxorubicin.
“We are still trying to determine exactly how inhibition of MDH2 protects the heart, but one intriguing idea is that doxorubicin may kill cardiac and tumor cells in different ways,” Dr Peterson said. “Given the intense energy requirements of the beating heart, we speculate that cardiac cells may be especially susceptible to metabolic disturbance caused by doxorubicin and that inhibiting MDH2 may correct the metabolic imbalance and prevent the cells from dying.”
“It remains to be seen if visnagin’s protective effects are restricted to doxorubicin or if it can protect the heart from other kinds of damage. We are pursuing this question by testing its ability to protect heart muscle from oxygen deprivation during heart attacks and from the effects of other heart-damaging chemotherapy drugs.” ![]()