User login
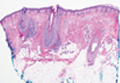
Lichen Planopilaris
Primary care-centric ACOs are working
Guess what? Physician-driven accountable care organizations with a strong primary care core are working – and, in a historic change, primary care physicians are the most highly compensated group.
The even better news? This trend is predictable and inevitable.
ACOs are working
As earlier posts to this column show, there are eight fairly straightforward elements required to create a successful and sustainable ACO:
• A change in financial incentives from those that reward volume, such as fee-for-service, to those that reward value, such as shared savings, if quality benchmarks are met.
• A primary care core.
• Physician cultural change.
• Patient engagement.
• Robust data collection.
• Clinical best practices.
• Administrative infrastructure.
• Enough scale.
A number of ACOs that do not have these elements will fail; but fortunately, more and more are being set up properly.
Recently, the Boston Consulting Group reported that ACO-like Medicare Advantage plans are reporting positive results. They are all distinguished by having "a selective network of providers, financial incentives that are aligned with clinical best practices, and active care management that emphasizes prevention in an effort to minimize expensive acute care."
Not only are emergency department and ambulatory surgery procedures down 20%-30% at these plans, but analysis of their data on 3 million Medicare patients showed that quality went up. These patients had lower single-year mortality rates, shorter average hospital stays, fewer readmissions, and better sustainability of health over time.1
Physician-led ACOs are better
If ACOs are good, physician-sponsored ones are better.
At a recent national meeting of health insurance companies, Paul Ginsburg, Ph.D., president of the Center for Studying Health System Change, told the insurers, "I think physician-led ACOs inherently make markets more competitive, because they have an opportunity to shift patients toward high-value hospitals."
Similarly, Charlie Baker, former secretary of health and human services for Massachusetts, told the group that nearly all of the Medicare Advantage risk contracts are with physician groups and not hospitals. Medicare Advantage participants are chosen by insurers, and he indicated that they know that contracting with physician ACOs is the best way to save money.2
As reported in an earlier column, this truth is becoming more evident, and there are now more physician-led ACOs than any other.
Primary care reaping rewards
Primary care is the only discipline mandated to be in ACOs participating in the Medicare Shared Savings Program. This is because ACO success stems from keeping people out of the hospital, avoiding expensive procedures, and reducing unnecessary tests and imaging. The "target-rich fields" for ACOs to accomplish this are primarily prevention and wellness, coordination of high-cost complex patients, reduced hospitalizations, and transition management across our fragmented system. These are all in primary care’s wheelhouse. It is no wonder that you are the darlings of the accountable care movement.
Successful and sustainable ACOs will tie shared savings distributions to relative contribution. A merit system thus likely will be primary care weighted.
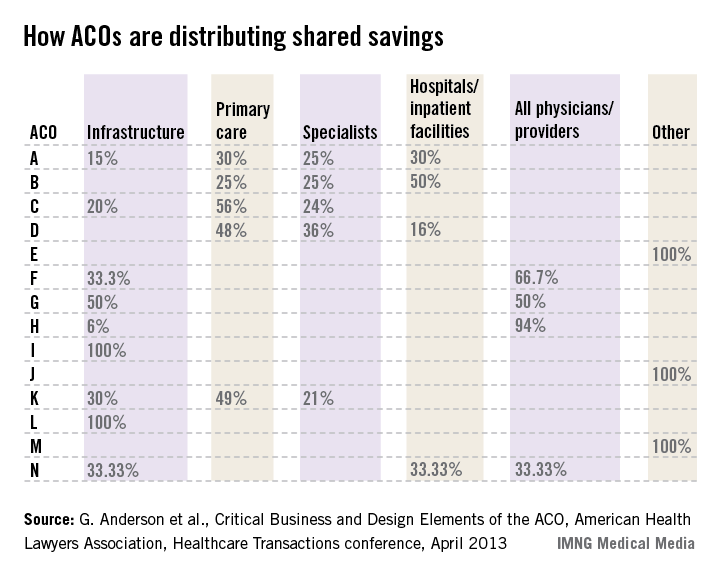
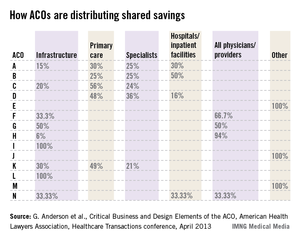
For example, one ACO posted this planned distribution of shared savings: 12% to infrastructure; of the remainder, 60% to primary care, 40% to specialists, and 0% to hospitals.
The following small sample survey shows widely varying models; but in all cases where distribution is broken out, primary care receives as much or more than specialists and, with one exception, hospitals.
There are some primary-care-only ACOs that are distributing 100% of savings to their primary care physicians under Medicare Advantage risk or Medicare Shared Savings Program contracts. One interviewed primary care physician ACO member stated that for his full-risk Medicare Advantage patient population, he was seeing half as many patients and making three times the income.
While income recognition for what you do is way overdue, keeping all the savings might be going too far. A fully evolved ACO should incentivize all providers and facilities along the entire continuum of care, but always in proportion to their value-adding contribution.
Primary care physicians tell me that while this economic reward is gratifying and validating, their surprise biggest reward has been empowerment to do health care right and regain control of the physician/patient relationship. They say that seeing happier, healthier patients, and being able to spend more time with them, has returned the fun to the practice of medicine.
References
1. Kaplan, J., et al., Alternative Payer Models Show Improved Health-Care Value, BCG Perspective, May 14, 2013.
2. Pittman, D., Doc-Led ACOs Better Model for Saving $$$, MedPage Today, May 15, 2013.
3. Anderson, G., et al., Critical Business and Design Elements of the ACO, American Health Lawyers Association, Healthcare Transactions conference (April 2013).
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, North Carolina. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Guess what? Physician-driven accountable care organizations with a strong primary care core are working – and, in a historic change, primary care physicians are the most highly compensated group.
The even better news? This trend is predictable and inevitable.

ACOs are working
As earlier posts to this column show, there are eight fairly straightforward elements required to create a successful and sustainable ACO:
• A change in financial incentives from those that reward volume, such as fee-for-service, to those that reward value, such as shared savings, if quality benchmarks are met.
• A primary care core.
• Physician cultural change.
• Patient engagement.
• Robust data collection.
• Clinical best practices.
• Administrative infrastructure.
• Enough scale.
A number of ACOs that do not have these elements will fail; but fortunately, more and more are being set up properly.
Recently, the Boston Consulting Group reported that ACO-like Medicare Advantage plans are reporting positive results. They are all distinguished by having "a selective network of providers, financial incentives that are aligned with clinical best practices, and active care management that emphasizes prevention in an effort to minimize expensive acute care."
Not only are emergency department and ambulatory surgery procedures down 20%-30% at these plans, but analysis of their data on 3 million Medicare patients showed that quality went up. These patients had lower single-year mortality rates, shorter average hospital stays, fewer readmissions, and better sustainability of health over time.1
Physician-led ACOs are better
If ACOs are good, physician-sponsored ones are better.
At a recent national meeting of health insurance companies, Paul Ginsburg, Ph.D., president of the Center for Studying Health System Change, told the insurers, "I think physician-led ACOs inherently make markets more competitive, because they have an opportunity to shift patients toward high-value hospitals."
Similarly, Charlie Baker, former secretary of health and human services for Massachusetts, told the group that nearly all of the Medicare Advantage risk contracts are with physician groups and not hospitals. Medicare Advantage participants are chosen by insurers, and he indicated that they know that contracting with physician ACOs is the best way to save money.2
As reported in an earlier column, this truth is becoming more evident, and there are now more physician-led ACOs than any other.
Primary care reaping rewards
Primary care is the only discipline mandated to be in ACOs participating in the Medicare Shared Savings Program. This is because ACO success stems from keeping people out of the hospital, avoiding expensive procedures, and reducing unnecessary tests and imaging. The "target-rich fields" for ACOs to accomplish this are primarily prevention and wellness, coordination of high-cost complex patients, reduced hospitalizations, and transition management across our fragmented system. These are all in primary care’s wheelhouse. It is no wonder that you are the darlings of the accountable care movement.
Successful and sustainable ACOs will tie shared savings distributions to relative contribution. A merit system thus likely will be primary care weighted.
For example, one ACO posted this planned distribution of shared savings: 12% to infrastructure; of the remainder, 60% to primary care, 40% to specialists, and 0% to hospitals.
The following small sample survey shows widely varying models; but in all cases where distribution is broken out, primary care receives as much or more than specialists and, with one exception, hospitals.
There are some primary-care-only ACOs that are distributing 100% of savings to their primary care physicians under Medicare Advantage risk or Medicare Shared Savings Program contracts. One interviewed primary care physician ACO member stated that for his full-risk Medicare Advantage patient population, he was seeing half as many patients and making three times the income.
While income recognition for what you do is way overdue, keeping all the savings might be going too far. A fully evolved ACO should incentivize all providers and facilities along the entire continuum of care, but always in proportion to their value-adding contribution.
Primary care physicians tell me that while this economic reward is gratifying and validating, their surprise biggest reward has been empowerment to do health care right and regain control of the physician/patient relationship. They say that seeing happier, healthier patients, and being able to spend more time with them, has returned the fun to the practice of medicine.
References
1. Kaplan, J., et al., Alternative Payer Models Show Improved Health-Care Value, BCG Perspective, May 14, 2013.
2. Pittman, D., Doc-Led ACOs Better Model for Saving $$$, MedPage Today, May 15, 2013.
3. Anderson, G., et al., Critical Business and Design Elements of the ACO, American Health Lawyers Association, Healthcare Transactions conference (April 2013).
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, North Carolina. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Guess what? Physician-driven accountable care organizations with a strong primary care core are working – and, in a historic change, primary care physicians are the most highly compensated group.
The even better news? This trend is predictable and inevitable.

ACOs are working
As earlier posts to this column show, there are eight fairly straightforward elements required to create a successful and sustainable ACO:
• A change in financial incentives from those that reward volume, such as fee-for-service, to those that reward value, such as shared savings, if quality benchmarks are met.
• A primary care core.
• Physician cultural change.
• Patient engagement.
• Robust data collection.
• Clinical best practices.
• Administrative infrastructure.
• Enough scale.
A number of ACOs that do not have these elements will fail; but fortunately, more and more are being set up properly.
Recently, the Boston Consulting Group reported that ACO-like Medicare Advantage plans are reporting positive results. They are all distinguished by having "a selective network of providers, financial incentives that are aligned with clinical best practices, and active care management that emphasizes prevention in an effort to minimize expensive acute care."
Not only are emergency department and ambulatory surgery procedures down 20%-30% at these plans, but analysis of their data on 3 million Medicare patients showed that quality went up. These patients had lower single-year mortality rates, shorter average hospital stays, fewer readmissions, and better sustainability of health over time.1
Physician-led ACOs are better
If ACOs are good, physician-sponsored ones are better.
At a recent national meeting of health insurance companies, Paul Ginsburg, Ph.D., president of the Center for Studying Health System Change, told the insurers, "I think physician-led ACOs inherently make markets more competitive, because they have an opportunity to shift patients toward high-value hospitals."
Similarly, Charlie Baker, former secretary of health and human services for Massachusetts, told the group that nearly all of the Medicare Advantage risk contracts are with physician groups and not hospitals. Medicare Advantage participants are chosen by insurers, and he indicated that they know that contracting with physician ACOs is the best way to save money.2
As reported in an earlier column, this truth is becoming more evident, and there are now more physician-led ACOs than any other.
Primary care reaping rewards
Primary care is the only discipline mandated to be in ACOs participating in the Medicare Shared Savings Program. This is because ACO success stems from keeping people out of the hospital, avoiding expensive procedures, and reducing unnecessary tests and imaging. The "target-rich fields" for ACOs to accomplish this are primarily prevention and wellness, coordination of high-cost complex patients, reduced hospitalizations, and transition management across our fragmented system. These are all in primary care’s wheelhouse. It is no wonder that you are the darlings of the accountable care movement.
Successful and sustainable ACOs will tie shared savings distributions to relative contribution. A merit system thus likely will be primary care weighted.
For example, one ACO posted this planned distribution of shared savings: 12% to infrastructure; of the remainder, 60% to primary care, 40% to specialists, and 0% to hospitals.
The following small sample survey shows widely varying models; but in all cases where distribution is broken out, primary care receives as much or more than specialists and, with one exception, hospitals.
There are some primary-care-only ACOs that are distributing 100% of savings to their primary care physicians under Medicare Advantage risk or Medicare Shared Savings Program contracts. One interviewed primary care physician ACO member stated that for his full-risk Medicare Advantage patient population, he was seeing half as many patients and making three times the income.
While income recognition for what you do is way overdue, keeping all the savings might be going too far. A fully evolved ACO should incentivize all providers and facilities along the entire continuum of care, but always in proportion to their value-adding contribution.
Primary care physicians tell me that while this economic reward is gratifying and validating, their surprise biggest reward has been empowerment to do health care right and regain control of the physician/patient relationship. They say that seeing happier, healthier patients, and being able to spend more time with them, has returned the fun to the practice of medicine.
References
1. Kaplan, J., et al., Alternative Payer Models Show Improved Health-Care Value, BCG Perspective, May 14, 2013.
2. Pittman, D., Doc-Led ACOs Better Model for Saving $$$, MedPage Today, May 15, 2013.
3. Anderson, G., et al., Critical Business and Design Elements of the ACO, American Health Lawyers Association, Healthcare Transactions conference (April 2013).
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, North Carolina. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Does myo-inositol supplementation reduce the rate of gestational diabetes in pregnant women with a family history of type 2 diabetes?
Inositol has generated increasing attention as a treatment for conditions related to pregnancy, including polycystic ovary syndrome1 and neural tube defects.2,3 In this study, pregnant women with a family history of type 2 diabetes were randomly allocated to:
- folic acid alone (400 µg daily)—placebo group
- folic acid plus myo-inositol (400 µg and 4 g daily, respectively)—treatment group.
The goal was to determine whether the addition of myo-inositol could prevent GDM and macrosomia. Because the study was conducted in Italy, GDM was diagnosed using recommendations from the International Association of Diabetes and Pregnancy Study Groups (IADPSG).4
Of the 197 women who completed the study, those given myo-inositol had a lower incidence of GDM than those given placebo: 6 of 99 women in the treatment group developed GDM, compared with 15 of 98 in the placebo group (P = .04). None of the women in the treatment group gave birth to a macrosomic infant (>4,000 g), compared with seven women in the placebo group (P = .007). However, other adverse outcomes, such as cesarean delivery, gestational hypertension, shoulder dystocia, preterm delivery, and fetal respiratory distress syndrome occurred at similar frequencies in the two groups.
RELATED ARTICLE Weight changes between pregnancies tied to risk of gestational diabetes mellitus (Web NEWS, June 2011)
How to improve outcomes in gestational diabetes—for mother and baby
E. Albert Reece, MD, PhD, MBA (March 2011)
Strengths and limitations of the study
Investigators conducted a prospective, randomized, placebo-controlled trial, one of the first moderately sized trials to examine the therapeutic effects of myo-inositol in pregnant women who had a family history of type 2 diabetes (in one or both parents). This study builds upon a previous report from the same investigators of the positive effects of myo-inositol in reducing insulin resistance in a smaller group of women (n = 69) with GDM.5
Another strength is that the investigators focused on a compound, inositol, readily found in foods, which suggests that women could derive benefits from a diet fortified with this supplement.
However, there are several concerns, also touched upon in a previous commentary about this study,6 which include:
- Only white women were included in the study. (As an ethnic group, white women have a lower risk of GDM.)
- All of the women enrolled in this trial had a prepregnancy body mass index within the normal range. Women who have a family history of type 2 diabetes who are also obese are a significant population to study.
- More work is needed to understand the mechanism of action of myo-inositol to help determine the optimal concentration to administer and the route of administration (ie, in pill form or as part of a diet plan).
Another important point: Investigators used IADPSG criteria to diagnose GDM; these criteria tend to identify significantly more cases of diabetes than the Carpenter and Coustan criteria, which are currently used in the United States.7,8 Indeed, a National Institutes of Health expert panel recommended continuing use of the Carpenter and Coustan criteria to diagnose GDM in the United States until more data are collected to show correlation between changing the diagnostic threshold and improved fetal and maternal outcomes.9
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Current standards for identifying GDM include appropriate screening. Methods for prevention of GDM including dietary counseling and advice on an exercise program to reduce fetal macrosomia, but no interventions, to date, have effectively controlled GDM.
The concept of using a dietary supplement to prevent GDM is intriguing, and the results of this trial, coupled with a second recent report on the beneficial effects of myo-inositol to prevent GDM,10 are promising. However, the many concerns raised here—especially the use of IADPSG criteria—make it difficult to apply these findings to traditional diagnostic criteria for US practitioners. Follow-up, large-scale trials are needed to determine how effective this supplement is in preventing GDM and whether its effects translate into benefits for women at increased risk of developing diabetes. The routine use of myo-inositol in patients with a family history of type 2 diabetes should await further studies, including the aforementioned trials.
E. Albert Reece, MD, PhD, MBA
- Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011;95(8):2642–2644.
- Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57(2):79–84.
- Cavalli P, Tonni G, Grosso E, Poggiani C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(11):962–965.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975.
- Coustan DR. Can a dietary supplement prevent gestational diabetes mellitus? Diabetes Care. 2013;36(4):777–779.
- Reece EA, Moore T. The diagnostic criteria for gestational diabetes: To change or not to change? Am J Obstet Gynecol. 2013;208(4):255–259.
- Langer O, Umans JG, Miodovnik M. Perspectives on the proposed gestational diabetes mellitus diagnostic criteria. Obstet Gynecol. 2013;121(1):177–182.
- National Institutes of Health Draft Consensus Development Panel. Draft Statement: National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellius; March 4–6, 2013; Bethesda, Maryland. http://prevention.nih.gov/cdp/conferences/2013/gdm/files/DraftStatement.pdf. Published March 6, 2013. Accessed May 17, 2013.
- Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial [published online ahead of print March 1, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.766691.
Inositol has generated increasing attention as a treatment for conditions related to pregnancy, including polycystic ovary syndrome1 and neural tube defects.2,3 In this study, pregnant women with a family history of type 2 diabetes were randomly allocated to:
- folic acid alone (400 µg daily)—placebo group
- folic acid plus myo-inositol (400 µg and 4 g daily, respectively)—treatment group.
The goal was to determine whether the addition of myo-inositol could prevent GDM and macrosomia. Because the study was conducted in Italy, GDM was diagnosed using recommendations from the International Association of Diabetes and Pregnancy Study Groups (IADPSG).4
Of the 197 women who completed the study, those given myo-inositol had a lower incidence of GDM than those given placebo: 6 of 99 women in the treatment group developed GDM, compared with 15 of 98 in the placebo group (P = .04). None of the women in the treatment group gave birth to a macrosomic infant (>4,000 g), compared with seven women in the placebo group (P = .007). However, other adverse outcomes, such as cesarean delivery, gestational hypertension, shoulder dystocia, preterm delivery, and fetal respiratory distress syndrome occurred at similar frequencies in the two groups.
RELATED ARTICLE Weight changes between pregnancies tied to risk of gestational diabetes mellitus (Web NEWS, June 2011)
How to improve outcomes in gestational diabetes—for mother and baby
E. Albert Reece, MD, PhD, MBA (March 2011)
Strengths and limitations of the study
Investigators conducted a prospective, randomized, placebo-controlled trial, one of the first moderately sized trials to examine the therapeutic effects of myo-inositol in pregnant women who had a family history of type 2 diabetes (in one or both parents). This study builds upon a previous report from the same investigators of the positive effects of myo-inositol in reducing insulin resistance in a smaller group of women (n = 69) with GDM.5
Another strength is that the investigators focused on a compound, inositol, readily found in foods, which suggests that women could derive benefits from a diet fortified with this supplement.
However, there are several concerns, also touched upon in a previous commentary about this study,6 which include:
- Only white women were included in the study. (As an ethnic group, white women have a lower risk of GDM.)
- All of the women enrolled in this trial had a prepregnancy body mass index within the normal range. Women who have a family history of type 2 diabetes who are also obese are a significant population to study.
- More work is needed to understand the mechanism of action of myo-inositol to help determine the optimal concentration to administer and the route of administration (ie, in pill form or as part of a diet plan).
Another important point: Investigators used IADPSG criteria to diagnose GDM; these criteria tend to identify significantly more cases of diabetes than the Carpenter and Coustan criteria, which are currently used in the United States.7,8 Indeed, a National Institutes of Health expert panel recommended continuing use of the Carpenter and Coustan criteria to diagnose GDM in the United States until more data are collected to show correlation between changing the diagnostic threshold and improved fetal and maternal outcomes.9
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Current standards for identifying GDM include appropriate screening. Methods for prevention of GDM including dietary counseling and advice on an exercise program to reduce fetal macrosomia, but no interventions, to date, have effectively controlled GDM.
The concept of using a dietary supplement to prevent GDM is intriguing, and the results of this trial, coupled with a second recent report on the beneficial effects of myo-inositol to prevent GDM,10 are promising. However, the many concerns raised here—especially the use of IADPSG criteria—make it difficult to apply these findings to traditional diagnostic criteria for US practitioners. Follow-up, large-scale trials are needed to determine how effective this supplement is in preventing GDM and whether its effects translate into benefits for women at increased risk of developing diabetes. The routine use of myo-inositol in patients with a family history of type 2 diabetes should await further studies, including the aforementioned trials.
E. Albert Reece, MD, PhD, MBA
Inositol has generated increasing attention as a treatment for conditions related to pregnancy, including polycystic ovary syndrome1 and neural tube defects.2,3 In this study, pregnant women with a family history of type 2 diabetes were randomly allocated to:
- folic acid alone (400 µg daily)—placebo group
- folic acid plus myo-inositol (400 µg and 4 g daily, respectively)—treatment group.
The goal was to determine whether the addition of myo-inositol could prevent GDM and macrosomia. Because the study was conducted in Italy, GDM was diagnosed using recommendations from the International Association of Diabetes and Pregnancy Study Groups (IADPSG).4
Of the 197 women who completed the study, those given myo-inositol had a lower incidence of GDM than those given placebo: 6 of 99 women in the treatment group developed GDM, compared with 15 of 98 in the placebo group (P = .04). None of the women in the treatment group gave birth to a macrosomic infant (>4,000 g), compared with seven women in the placebo group (P = .007). However, other adverse outcomes, such as cesarean delivery, gestational hypertension, shoulder dystocia, preterm delivery, and fetal respiratory distress syndrome occurred at similar frequencies in the two groups.
RELATED ARTICLE Weight changes between pregnancies tied to risk of gestational diabetes mellitus (Web NEWS, June 2011)
How to improve outcomes in gestational diabetes—for mother and baby
E. Albert Reece, MD, PhD, MBA (March 2011)
Strengths and limitations of the study
Investigators conducted a prospective, randomized, placebo-controlled trial, one of the first moderately sized trials to examine the therapeutic effects of myo-inositol in pregnant women who had a family history of type 2 diabetes (in one or both parents). This study builds upon a previous report from the same investigators of the positive effects of myo-inositol in reducing insulin resistance in a smaller group of women (n = 69) with GDM.5
Another strength is that the investigators focused on a compound, inositol, readily found in foods, which suggests that women could derive benefits from a diet fortified with this supplement.
However, there are several concerns, also touched upon in a previous commentary about this study,6 which include:
- Only white women were included in the study. (As an ethnic group, white women have a lower risk of GDM.)
- All of the women enrolled in this trial had a prepregnancy body mass index within the normal range. Women who have a family history of type 2 diabetes who are also obese are a significant population to study.
- More work is needed to understand the mechanism of action of myo-inositol to help determine the optimal concentration to administer and the route of administration (ie, in pill form or as part of a diet plan).
Another important point: Investigators used IADPSG criteria to diagnose GDM; these criteria tend to identify significantly more cases of diabetes than the Carpenter and Coustan criteria, which are currently used in the United States.7,8 Indeed, a National Institutes of Health expert panel recommended continuing use of the Carpenter and Coustan criteria to diagnose GDM in the United States until more data are collected to show correlation between changing the diagnostic threshold and improved fetal and maternal outcomes.9
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Current standards for identifying GDM include appropriate screening. Methods for prevention of GDM including dietary counseling and advice on an exercise program to reduce fetal macrosomia, but no interventions, to date, have effectively controlled GDM.
The concept of using a dietary supplement to prevent GDM is intriguing, and the results of this trial, coupled with a second recent report on the beneficial effects of myo-inositol to prevent GDM,10 are promising. However, the many concerns raised here—especially the use of IADPSG criteria—make it difficult to apply these findings to traditional diagnostic criteria for US practitioners. Follow-up, large-scale trials are needed to determine how effective this supplement is in preventing GDM and whether its effects translate into benefits for women at increased risk of developing diabetes. The routine use of myo-inositol in patients with a family history of type 2 diabetes should await further studies, including the aforementioned trials.
E. Albert Reece, MD, PhD, MBA
- Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011;95(8):2642–2644.
- Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57(2):79–84.
- Cavalli P, Tonni G, Grosso E, Poggiani C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(11):962–965.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975.
- Coustan DR. Can a dietary supplement prevent gestational diabetes mellitus? Diabetes Care. 2013;36(4):777–779.
- Reece EA, Moore T. The diagnostic criteria for gestational diabetes: To change or not to change? Am J Obstet Gynecol. 2013;208(4):255–259.
- Langer O, Umans JG, Miodovnik M. Perspectives on the proposed gestational diabetes mellitus diagnostic criteria. Obstet Gynecol. 2013;121(1):177–182.
- National Institutes of Health Draft Consensus Development Panel. Draft Statement: National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellius; March 4–6, 2013; Bethesda, Maryland. http://prevention.nih.gov/cdp/conferences/2013/gdm/files/DraftStatement.pdf. Published March 6, 2013. Accessed May 17, 2013.
- Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial [published online ahead of print March 1, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.766691.
- Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011;95(8):2642–2644.
- Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57(2):79–84.
- Cavalli P, Tonni G, Grosso E, Poggiani C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(11):962–965.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
- Corrado F, D’Anna R, Di Vieste G, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med. 2011;28(8):972–975.
- Coustan DR. Can a dietary supplement prevent gestational diabetes mellitus? Diabetes Care. 2013;36(4):777–779.
- Reece EA, Moore T. The diagnostic criteria for gestational diabetes: To change or not to change? Am J Obstet Gynecol. 2013;208(4):255–259.
- Langer O, Umans JG, Miodovnik M. Perspectives on the proposed gestational diabetes mellitus diagnostic criteria. Obstet Gynecol. 2013;121(1):177–182.
- National Institutes of Health Draft Consensus Development Panel. Draft Statement: National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellius; March 4–6, 2013; Bethesda, Maryland. http://prevention.nih.gov/cdp/conferences/2013/gdm/files/DraftStatement.pdf. Published March 6, 2013. Accessed May 17, 2013.
- Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial [published online ahead of print March 1, 2013]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2013.766691.
Practical considerations in the delivery of genetic counseling and testing services for inherited cancer predisposition
Many professional entities endorse the need to deliver cancer genetics risk assessment (CGRA) services through a multidisciplinary team that includes trained genetics professionals. However, market forces, a lack of regulation of genetic testing, patent laws, cost barriers, and a limited workforce in genetics have resulted in an increasing number of community practitioners who order and interpret genetic testing. In addition, varying state-level laws and licensure requirements for genetic counselors may contribute to the nonuniform delivery of CGRA services across the United States. Those who perform genetic testing without having adequate training or expertise may incur liability risks. Moreover, the patient might not enjoy the maximum benefit of testing at the hands of an inadequately trained individual. In the setting of a limited number of professional who are trained in CGRA and a dearth of education and training resources, it is a challenge to integrate genetic testing services into clinical care. With advances in genomics and the implementation of personalized medicine, the problem will only be magnified, and it is critical that there are more opportunities for high quality education and training in clinical cancer genetics free of commercial bias. Successful strategies for delivering comprehensive CGRA services include academic-community partnerships that focus on collaboration with nongenetics providers or the inclusion of a genetics professional in the community setting as part of multidisciplinary patient care. These approaches can leverage the expertise of genetics professionals while allowing patients to remain in their community and enjoy better access to resources for long-term follow-up care.
*Click on the link to the left for a PDF of the full article.
Many professional entities endorse the need to deliver cancer genetics risk assessment (CGRA) services through a multidisciplinary team that includes trained genetics professionals. However, market forces, a lack of regulation of genetic testing, patent laws, cost barriers, and a limited workforce in genetics have resulted in an increasing number of community practitioners who order and interpret genetic testing. In addition, varying state-level laws and licensure requirements for genetic counselors may contribute to the nonuniform delivery of CGRA services across the United States. Those who perform genetic testing without having adequate training or expertise may incur liability risks. Moreover, the patient might not enjoy the maximum benefit of testing at the hands of an inadequately trained individual. In the setting of a limited number of professional who are trained in CGRA and a dearth of education and training resources, it is a challenge to integrate genetic testing services into clinical care. With advances in genomics and the implementation of personalized medicine, the problem will only be magnified, and it is critical that there are more opportunities for high quality education and training in clinical cancer genetics free of commercial bias. Successful strategies for delivering comprehensive CGRA services include academic-community partnerships that focus on collaboration with nongenetics providers or the inclusion of a genetics professional in the community setting as part of multidisciplinary patient care. These approaches can leverage the expertise of genetics professionals while allowing patients to remain in their community and enjoy better access to resources for long-term follow-up care.
*Click on the link to the left for a PDF of the full article.
Many professional entities endorse the need to deliver cancer genetics risk assessment (CGRA) services through a multidisciplinary team that includes trained genetics professionals. However, market forces, a lack of regulation of genetic testing, patent laws, cost barriers, and a limited workforce in genetics have resulted in an increasing number of community practitioners who order and interpret genetic testing. In addition, varying state-level laws and licensure requirements for genetic counselors may contribute to the nonuniform delivery of CGRA services across the United States. Those who perform genetic testing without having adequate training or expertise may incur liability risks. Moreover, the patient might not enjoy the maximum benefit of testing at the hands of an inadequately trained individual. In the setting of a limited number of professional who are trained in CGRA and a dearth of education and training resources, it is a challenge to integrate genetic testing services into clinical care. With advances in genomics and the implementation of personalized medicine, the problem will only be magnified, and it is critical that there are more opportunities for high quality education and training in clinical cancer genetics free of commercial bias. Successful strategies for delivering comprehensive CGRA services include academic-community partnerships that focus on collaboration with nongenetics providers or the inclusion of a genetics professional in the community setting as part of multidisciplinary patient care. These approaches can leverage the expertise of genetics professionals while allowing patients to remain in their community and enjoy better access to resources for long-term follow-up care.
*Click on the link to the left for a PDF of the full article.
Good survival rates with temozolomide CRT for high-risk, low-grade gliomas
CHICAGO – Patients with high-risk, low-grade gliomas had 3-year survival rates significantly higher than those of historical controls when they were treated with a temozolomide-based chemoradiotherapy regimen, investigators reported at the annual meeting of the American Society of Clinical Oncology.
Preliminary results from the phase II RTOG 0424 trial showed a 73% overall survival rate for patients treated with temozolomide chemoradiotherapy, compared with 54% overall survival for historical controls (P less than .001), and exceeding the study’s hypothesized 65% overall survival, reported Dr. Barbara Fisher, a radiation oncologist at the University of Western Ontario in London.
At a median follow-up of 4 years, with a minimum potential follow-up of 3 years, median survival of patients has not been reached, and the median follow-up time for surviving patients is 5 years, Dr. Fisher noted.
Median progression-free survival was 4.5 years, and 3-year PFS was 59%. In contrast to historical controls, patients with four or five risk factors appeared to have an overall survival rate similar to that of patients with three risk factors, Dr. Fisher reported.
Although the trial was originally proposed in 2000 as a randomized controlled study, "there wasn’t much information about temozolomide and radiation at that point," Dr. Fisher said.
The control population comes from a 2002 study by Francesco Pignatti and his colleagues (J. Clin. Oncol. 2002;20:2076-84), which identified prognostic factors for survival in adults with low-grade glioma, based on data from two European Organization for Research and Treatment of Cancer (EORTC) trials conducted in the 1990s.
The risk factors are age 40 years and older; astrocytoma subtype; tumor crossing the midline; largest tumor diameter more than 6 cm preoperatively; and preoperative neurological function status (neurocognitive function greater than 1: moderate impairment).
A total of 136 patients were accrued, and 129 patients were eligible for the protocol, which consisted of temozolomide 75 mg/m2 per day for 6 weeks with concurrent conformal radiotherapy, consisting of 54 Gy divided into 30 fractions delivered 5 days each week for 6 weeks, followed by temozolomide 150-200 mg/m2 per day for days 1-5 of each 28-day cycle for a total of 12 cycles.
The patients had previously untreated, histologically proven supratentorial World Health Organization grade II astrocytoma (71 patients); oligodendroglioma (29) or oligoastrocytoma (29) confirmed by central pathology; and at least three of the aforementioned risk factors. The majority of patients (80%) were aged 40 years or older, 79% had tumors greater than 6 cm in the largest diameter, 53% had tumors that crossed the midline, 66% had a dominant astrocytoma subtype, and 50% had preoperative neurological function status scores greater than 1. In all, 89 patients had three risk factors, 32 had four, and 8 had five factors.
The investigators hypothesized that they would see a 43% relative increase in median survival, from 40.5 months in controls to 57.9 months, and a 20% improvement in 3-year survival, from 54% to 65%. As noted before, the survival rates exceeded their expectations.
In an analysis of survival by risk factors 5 years after trial registration, the authors found that 35 of the 89 patients with three risk factors (39%) had died, compared with 17 of the 40 patients with four or five risk factors (43%).
In all, 55 patients had grade 3 adverse events as their worst overall events, 13 had grade 4 toxicities, and 1 patient died of herpes simplex encephalitis, possibly related to treatment.
Grade 3 adverse events were seen in 43% of patients, and grade 4 toxicities in 10%. The most common toxicities were hematologic, constitutional, or gastrointestinal, including nausea and anorexia.
In her commentary, Dr. Helen Shih, the invited discussant, said that a randomized trial would have been preferable, but she acknowledged the difficulties the investigators had in designing and implementing the trial. Dr. Shih, of the radiation oncology department at Massachusetts General Hospital in Boston, also noted that the study used for control purposes "itself was conducted in a prior decade, when the management of radiation as well as surgery were slightly different, and perhaps those advancements also have affected overall survival of patients who are treated today."
In addition, the incidences of grade 3 and 4 toxicities in the study by Dr. Fisher and her colleagues were "not trivial, and should be kept in mind," Dr. Shih said.
The study was supported by the National Cancer Institute. Dr. Fisher and Dr. Shih reported having no relevant financial disclosures.
CHICAGO – Patients with high-risk, low-grade gliomas had 3-year survival rates significantly higher than those of historical controls when they were treated with a temozolomide-based chemoradiotherapy regimen, investigators reported at the annual meeting of the American Society of Clinical Oncology.
Preliminary results from the phase II RTOG 0424 trial showed a 73% overall survival rate for patients treated with temozolomide chemoradiotherapy, compared with 54% overall survival for historical controls (P less than .001), and exceeding the study’s hypothesized 65% overall survival, reported Dr. Barbara Fisher, a radiation oncologist at the University of Western Ontario in London.
At a median follow-up of 4 years, with a minimum potential follow-up of 3 years, median survival of patients has not been reached, and the median follow-up time for surviving patients is 5 years, Dr. Fisher noted.
Median progression-free survival was 4.5 years, and 3-year PFS was 59%. In contrast to historical controls, patients with four or five risk factors appeared to have an overall survival rate similar to that of patients with three risk factors, Dr. Fisher reported.
Although the trial was originally proposed in 2000 as a randomized controlled study, "there wasn’t much information about temozolomide and radiation at that point," Dr. Fisher said.
The control population comes from a 2002 study by Francesco Pignatti and his colleagues (J. Clin. Oncol. 2002;20:2076-84), which identified prognostic factors for survival in adults with low-grade glioma, based on data from two European Organization for Research and Treatment of Cancer (EORTC) trials conducted in the 1990s.
The risk factors are age 40 years and older; astrocytoma subtype; tumor crossing the midline; largest tumor diameter more than 6 cm preoperatively; and preoperative neurological function status (neurocognitive function greater than 1: moderate impairment).
A total of 136 patients were accrued, and 129 patients were eligible for the protocol, which consisted of temozolomide 75 mg/m2 per day for 6 weeks with concurrent conformal radiotherapy, consisting of 54 Gy divided into 30 fractions delivered 5 days each week for 6 weeks, followed by temozolomide 150-200 mg/m2 per day for days 1-5 of each 28-day cycle for a total of 12 cycles.
The patients had previously untreated, histologically proven supratentorial World Health Organization grade II astrocytoma (71 patients); oligodendroglioma (29) or oligoastrocytoma (29) confirmed by central pathology; and at least three of the aforementioned risk factors. The majority of patients (80%) were aged 40 years or older, 79% had tumors greater than 6 cm in the largest diameter, 53% had tumors that crossed the midline, 66% had a dominant astrocytoma subtype, and 50% had preoperative neurological function status scores greater than 1. In all, 89 patients had three risk factors, 32 had four, and 8 had five factors.
The investigators hypothesized that they would see a 43% relative increase in median survival, from 40.5 months in controls to 57.9 months, and a 20% improvement in 3-year survival, from 54% to 65%. As noted before, the survival rates exceeded their expectations.
In an analysis of survival by risk factors 5 years after trial registration, the authors found that 35 of the 89 patients with three risk factors (39%) had died, compared with 17 of the 40 patients with four or five risk factors (43%).
In all, 55 patients had grade 3 adverse events as their worst overall events, 13 had grade 4 toxicities, and 1 patient died of herpes simplex encephalitis, possibly related to treatment.
Grade 3 adverse events were seen in 43% of patients, and grade 4 toxicities in 10%. The most common toxicities were hematologic, constitutional, or gastrointestinal, including nausea and anorexia.
In her commentary, Dr. Helen Shih, the invited discussant, said that a randomized trial would have been preferable, but she acknowledged the difficulties the investigators had in designing and implementing the trial. Dr. Shih, of the radiation oncology department at Massachusetts General Hospital in Boston, also noted that the study used for control purposes "itself was conducted in a prior decade, when the management of radiation as well as surgery were slightly different, and perhaps those advancements also have affected overall survival of patients who are treated today."
In addition, the incidences of grade 3 and 4 toxicities in the study by Dr. Fisher and her colleagues were "not trivial, and should be kept in mind," Dr. Shih said.
The study was supported by the National Cancer Institute. Dr. Fisher and Dr. Shih reported having no relevant financial disclosures.
CHICAGO – Patients with high-risk, low-grade gliomas had 3-year survival rates significantly higher than those of historical controls when they were treated with a temozolomide-based chemoradiotherapy regimen, investigators reported at the annual meeting of the American Society of Clinical Oncology.
Preliminary results from the phase II RTOG 0424 trial showed a 73% overall survival rate for patients treated with temozolomide chemoradiotherapy, compared with 54% overall survival for historical controls (P less than .001), and exceeding the study’s hypothesized 65% overall survival, reported Dr. Barbara Fisher, a radiation oncologist at the University of Western Ontario in London.
At a median follow-up of 4 years, with a minimum potential follow-up of 3 years, median survival of patients has not been reached, and the median follow-up time for surviving patients is 5 years, Dr. Fisher noted.
Median progression-free survival was 4.5 years, and 3-year PFS was 59%. In contrast to historical controls, patients with four or five risk factors appeared to have an overall survival rate similar to that of patients with three risk factors, Dr. Fisher reported.
Although the trial was originally proposed in 2000 as a randomized controlled study, "there wasn’t much information about temozolomide and radiation at that point," Dr. Fisher said.
The control population comes from a 2002 study by Francesco Pignatti and his colleagues (J. Clin. Oncol. 2002;20:2076-84), which identified prognostic factors for survival in adults with low-grade glioma, based on data from two European Organization for Research and Treatment of Cancer (EORTC) trials conducted in the 1990s.
The risk factors are age 40 years and older; astrocytoma subtype; tumor crossing the midline; largest tumor diameter more than 6 cm preoperatively; and preoperative neurological function status (neurocognitive function greater than 1: moderate impairment).
A total of 136 patients were accrued, and 129 patients were eligible for the protocol, which consisted of temozolomide 75 mg/m2 per day for 6 weeks with concurrent conformal radiotherapy, consisting of 54 Gy divided into 30 fractions delivered 5 days each week for 6 weeks, followed by temozolomide 150-200 mg/m2 per day for days 1-5 of each 28-day cycle for a total of 12 cycles.
The patients had previously untreated, histologically proven supratentorial World Health Organization grade II astrocytoma (71 patients); oligodendroglioma (29) or oligoastrocytoma (29) confirmed by central pathology; and at least three of the aforementioned risk factors. The majority of patients (80%) were aged 40 years or older, 79% had tumors greater than 6 cm in the largest diameter, 53% had tumors that crossed the midline, 66% had a dominant astrocytoma subtype, and 50% had preoperative neurological function status scores greater than 1. In all, 89 patients had three risk factors, 32 had four, and 8 had five factors.
The investigators hypothesized that they would see a 43% relative increase in median survival, from 40.5 months in controls to 57.9 months, and a 20% improvement in 3-year survival, from 54% to 65%. As noted before, the survival rates exceeded their expectations.
In an analysis of survival by risk factors 5 years after trial registration, the authors found that 35 of the 89 patients with three risk factors (39%) had died, compared with 17 of the 40 patients with four or five risk factors (43%).
In all, 55 patients had grade 3 adverse events as their worst overall events, 13 had grade 4 toxicities, and 1 patient died of herpes simplex encephalitis, possibly related to treatment.
Grade 3 adverse events were seen in 43% of patients, and grade 4 toxicities in 10%. The most common toxicities were hematologic, constitutional, or gastrointestinal, including nausea and anorexia.
In her commentary, Dr. Helen Shih, the invited discussant, said that a randomized trial would have been preferable, but she acknowledged the difficulties the investigators had in designing and implementing the trial. Dr. Shih, of the radiation oncology department at Massachusetts General Hospital in Boston, also noted that the study used for control purposes "itself was conducted in a prior decade, when the management of radiation as well as surgery were slightly different, and perhaps those advancements also have affected overall survival of patients who are treated today."
In addition, the incidences of grade 3 and 4 toxicities in the study by Dr. Fisher and her colleagues were "not trivial, and should be kept in mind," Dr. Shih said.
The study was supported by the National Cancer Institute. Dr. Fisher and Dr. Shih reported having no relevant financial disclosures.
AT THE ASCO ANNUAL MEETING 2013
Major finding: With temozolomide chemoradiotherapy, 3-year overall survival was 73% for patients with low-grade gliomas and three or more risk factors for worse prognosis, compared with 54% for historical controls (P less than .001).
Data source: Single-arm, prospective phase II study in 129 patients compared with historical controls.
Disclosures: The study was supported by the National Cancer Institute. Dr. Fisher and Dr. Shih reported having no relevant financial disclosures.
Fontan reoperation mortality unexpectedly high in multicenter analysis
MINNEAPOLIS – Early mortality is substantially higher than initially thought for Fontan revision or conversion, the most common reoperation in adults with congenital heart disease.
Discharge mortality was 10.1% among adults undergoing a Fontan redo from 2007 to 2011 in an analysis of the STS-CHSD (Society of Thoracic Surgeons Congenital Heart Surgery Database), encompassing more than 90% of heart surgeries in the United States.
When Fontan conversion was first described in a single-center series, however, discharge mortality was less than 1% (Ann. Thorac. Surg. 2007;84:1457-65), observed Dr. Jeffrey P. Jacobs, chair of the STS-CHSD, and a cardiovascular surgeon with All Children’s Hospital, Johns Hopkins Medicine, St. Petersburg, Fla.
"This point really exemplifies the power of multi-institution data and exemplifies that the published literature reflecting an excellence experience at one center, may not reflect the reality of what is going on across the country or across the world," he said at the annual meeting of the American Association for Thoracic Surgery.
The STS-CHSD includes 108 congenital heart surgery hospitals in North America, 105 in the United States and 3 in Canada, or 84% of programs in the United States.
The investigators identified 92,603 index cardiac operations in the database from 2007-2011, after excluding those with missing data and patients weighing 2,500 g or less undergoing patent ductus arteriosus ligation as their primary procedure.
In all, 30,673 (33%) had one or more prior cardiopulmonary bypass cardiothoracic operation, which was used as a surrogate for reoperation.
Discharge mortality was 3.98% with no prior bypass cardiothoracic operations, 2.38% with one, 1.67% with two, 2.41% with three, 3.31% with four, 4.08% with five, and 5.07% with six or more reoperations, Dr. Jacobs said.
Mean length of stay was 14.8 days for the index procedure and increased in a stepwise manner from 10.8 days with one prior surgery to 14.07 with six or more surgeries.
Fontan (total cavopulmonary connection, external conduit) was the most common reoperation performed among all patients and had the highest discharge mortality whether it was fenestrated (1.8% among 1,870 patients) or nonfenestrated (1.6% among 1,403 patients).
Discharge mortality was lower among all patients for bidirectional cavopulmonary anastomosis (1.3%), pulmonic valve replacement (0.4%), conduit reoperation (0.8%), pacemaker procedure (0.3%), and permanent pacemaker implantation (1.4%).
The number of neonates dying before discharge was alarmingly high for various operations including total anomalous pulmonary venous connection repair (55.6%), pulmonary artery banding (36.4%), and the Norwood procedure (21.6%), but Dr. Jacobs cautioned that these reoperations are very rare events occurring in 18, 11, and 37 cases, respectively.
In contrast, discharge mortality among infants reached a high of 2.6% for a hemi-Fontan reoperation (8 deaths/311 procedures) and was half that for bidirectional cavopulmonary anastomosis, the most common reoperation among infants (29 deaths/2,271 procedures).
Discharge mortality for reoperations among children, aged 1-18 years, in the United States also remained below 2%, he said. The highest rate was 1.7% for a Fontan redo (TCPC, external conduit, fenestrated), the most common operation, performed in 1,852 children.
Finally, there were no double-digit discharge mortality rates among adults, save for the bloated 10.1% reported for Fontan revision or conversion. Rates were low for arrhythmia surgery–atrial surgical ablation (3.4%), right ventricle to pulmonary artery conduit placement (3.3%), permanent pacemaker implantation (2%), conduit reoperation (1.6%), pulmonic valve replacement (0.3%), and pacemaker procedure (0.2%).
Science tells us what we can do, guidelines what we should do, and registries what we are actually doing, Dr. Jacobs concluded.
Dr. Jacobs is chair of the STS Congenital Heart Surgery Database Taskforce; STS Task Force on Longitudinal Follow-Up and Linked Registries; and the STS Public Reporting Task Force.
MINNEAPOLIS – Early mortality is substantially higher than initially thought for Fontan revision or conversion, the most common reoperation in adults with congenital heart disease.
Discharge mortality was 10.1% among adults undergoing a Fontan redo from 2007 to 2011 in an analysis of the STS-CHSD (Society of Thoracic Surgeons Congenital Heart Surgery Database), encompassing more than 90% of heart surgeries in the United States.
When Fontan conversion was first described in a single-center series, however, discharge mortality was less than 1% (Ann. Thorac. Surg. 2007;84:1457-65), observed Dr. Jeffrey P. Jacobs, chair of the STS-CHSD, and a cardiovascular surgeon with All Children’s Hospital, Johns Hopkins Medicine, St. Petersburg, Fla.
"This point really exemplifies the power of multi-institution data and exemplifies that the published literature reflecting an excellence experience at one center, may not reflect the reality of what is going on across the country or across the world," he said at the annual meeting of the American Association for Thoracic Surgery.
The STS-CHSD includes 108 congenital heart surgery hospitals in North America, 105 in the United States and 3 in Canada, or 84% of programs in the United States.
The investigators identified 92,603 index cardiac operations in the database from 2007-2011, after excluding those with missing data and patients weighing 2,500 g or less undergoing patent ductus arteriosus ligation as their primary procedure.
In all, 30,673 (33%) had one or more prior cardiopulmonary bypass cardiothoracic operation, which was used as a surrogate for reoperation.
Discharge mortality was 3.98% with no prior bypass cardiothoracic operations, 2.38% with one, 1.67% with two, 2.41% with three, 3.31% with four, 4.08% with five, and 5.07% with six or more reoperations, Dr. Jacobs said.
Mean length of stay was 14.8 days for the index procedure and increased in a stepwise manner from 10.8 days with one prior surgery to 14.07 with six or more surgeries.
Fontan (total cavopulmonary connection, external conduit) was the most common reoperation performed among all patients and had the highest discharge mortality whether it was fenestrated (1.8% among 1,870 patients) or nonfenestrated (1.6% among 1,403 patients).
Discharge mortality was lower among all patients for bidirectional cavopulmonary anastomosis (1.3%), pulmonic valve replacement (0.4%), conduit reoperation (0.8%), pacemaker procedure (0.3%), and permanent pacemaker implantation (1.4%).
The number of neonates dying before discharge was alarmingly high for various operations including total anomalous pulmonary venous connection repair (55.6%), pulmonary artery banding (36.4%), and the Norwood procedure (21.6%), but Dr. Jacobs cautioned that these reoperations are very rare events occurring in 18, 11, and 37 cases, respectively.
In contrast, discharge mortality among infants reached a high of 2.6% for a hemi-Fontan reoperation (8 deaths/311 procedures) and was half that for bidirectional cavopulmonary anastomosis, the most common reoperation among infants (29 deaths/2,271 procedures).
Discharge mortality for reoperations among children, aged 1-18 years, in the United States also remained below 2%, he said. The highest rate was 1.7% for a Fontan redo (TCPC, external conduit, fenestrated), the most common operation, performed in 1,852 children.
Finally, there were no double-digit discharge mortality rates among adults, save for the bloated 10.1% reported for Fontan revision or conversion. Rates were low for arrhythmia surgery–atrial surgical ablation (3.4%), right ventricle to pulmonary artery conduit placement (3.3%), permanent pacemaker implantation (2%), conduit reoperation (1.6%), pulmonic valve replacement (0.3%), and pacemaker procedure (0.2%).
Science tells us what we can do, guidelines what we should do, and registries what we are actually doing, Dr. Jacobs concluded.
Dr. Jacobs is chair of the STS Congenital Heart Surgery Database Taskforce; STS Task Force on Longitudinal Follow-Up and Linked Registries; and the STS Public Reporting Task Force.
MINNEAPOLIS – Early mortality is substantially higher than initially thought for Fontan revision or conversion, the most common reoperation in adults with congenital heart disease.
Discharge mortality was 10.1% among adults undergoing a Fontan redo from 2007 to 2011 in an analysis of the STS-CHSD (Society of Thoracic Surgeons Congenital Heart Surgery Database), encompassing more than 90% of heart surgeries in the United States.
When Fontan conversion was first described in a single-center series, however, discharge mortality was less than 1% (Ann. Thorac. Surg. 2007;84:1457-65), observed Dr. Jeffrey P. Jacobs, chair of the STS-CHSD, and a cardiovascular surgeon with All Children’s Hospital, Johns Hopkins Medicine, St. Petersburg, Fla.
"This point really exemplifies the power of multi-institution data and exemplifies that the published literature reflecting an excellence experience at one center, may not reflect the reality of what is going on across the country or across the world," he said at the annual meeting of the American Association for Thoracic Surgery.
The STS-CHSD includes 108 congenital heart surgery hospitals in North America, 105 in the United States and 3 in Canada, or 84% of programs in the United States.
The investigators identified 92,603 index cardiac operations in the database from 2007-2011, after excluding those with missing data and patients weighing 2,500 g or less undergoing patent ductus arteriosus ligation as their primary procedure.
In all, 30,673 (33%) had one or more prior cardiopulmonary bypass cardiothoracic operation, which was used as a surrogate for reoperation.
Discharge mortality was 3.98% with no prior bypass cardiothoracic operations, 2.38% with one, 1.67% with two, 2.41% with three, 3.31% with four, 4.08% with five, and 5.07% with six or more reoperations, Dr. Jacobs said.
Mean length of stay was 14.8 days for the index procedure and increased in a stepwise manner from 10.8 days with one prior surgery to 14.07 with six or more surgeries.
Fontan (total cavopulmonary connection, external conduit) was the most common reoperation performed among all patients and had the highest discharge mortality whether it was fenestrated (1.8% among 1,870 patients) or nonfenestrated (1.6% among 1,403 patients).
Discharge mortality was lower among all patients for bidirectional cavopulmonary anastomosis (1.3%), pulmonic valve replacement (0.4%), conduit reoperation (0.8%), pacemaker procedure (0.3%), and permanent pacemaker implantation (1.4%).
The number of neonates dying before discharge was alarmingly high for various operations including total anomalous pulmonary venous connection repair (55.6%), pulmonary artery banding (36.4%), and the Norwood procedure (21.6%), but Dr. Jacobs cautioned that these reoperations are very rare events occurring in 18, 11, and 37 cases, respectively.
In contrast, discharge mortality among infants reached a high of 2.6% for a hemi-Fontan reoperation (8 deaths/311 procedures) and was half that for bidirectional cavopulmonary anastomosis, the most common reoperation among infants (29 deaths/2,271 procedures).
Discharge mortality for reoperations among children, aged 1-18 years, in the United States also remained below 2%, he said. The highest rate was 1.7% for a Fontan redo (TCPC, external conduit, fenestrated), the most common operation, performed in 1,852 children.
Finally, there were no double-digit discharge mortality rates among adults, save for the bloated 10.1% reported for Fontan revision or conversion. Rates were low for arrhythmia surgery–atrial surgical ablation (3.4%), right ventricle to pulmonary artery conduit placement (3.3%), permanent pacemaker implantation (2%), conduit reoperation (1.6%), pulmonic valve replacement (0.3%), and pacemaker procedure (0.2%).
Science tells us what we can do, guidelines what we should do, and registries what we are actually doing, Dr. Jacobs concluded.
Dr. Jacobs is chair of the STS Congenital Heart Surgery Database Taskforce; STS Task Force on Longitudinal Follow-Up and Linked Registries; and the STS Public Reporting Task Force.
Ensuring optimal adherence to BCR-ABL1 tyrosine kinase inhibitor therapy for chronic myeloid leukemia
The advent of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for the treatment of chronic myeloid leukemia (CML) has dramatically changed the management of patients with CML. With continuous long-term TKI therapy, CML can be managed like a chronic condition, and most patients can expect to have a normal life expectancy. Given the prospect of lifelong therapy, however, issues related to adherence become particularly important and warrant greater attention since attainment of favorable long-term survival depends in large part on consistent, appropriate treatment administration over years, if not decades. As the multidisciplinary care team approach to cancer care has gained traction at academic centers and community practices, midlevel providers, including nurse practitioners and physician assistants, have taken on greater patient-related responsibilities. Midlevel providers have the potential to foster and maintain meaningful provider-patient relationships that may span years, and are well positioned to recognize and manage problems that patients may have with adherence. Here we discuss the importance of achieving and maintaining responses to TKI therapy, describe the clinical consequences of poor adherence to TKI therapy in CML, and outline factors behind poor adherence. We also share strategies that we use at our center to improve adherence to long-term TKI therapy for CML.
*Click on the link to the left for a PDF of the full article.
The advent of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for the treatment of chronic myeloid leukemia (CML) has dramatically changed the management of patients with CML. With continuous long-term TKI therapy, CML can be managed like a chronic condition, and most patients can expect to have a normal life expectancy. Given the prospect of lifelong therapy, however, issues related to adherence become particularly important and warrant greater attention since attainment of favorable long-term survival depends in large part on consistent, appropriate treatment administration over years, if not decades. As the multidisciplinary care team approach to cancer care has gained traction at academic centers and community practices, midlevel providers, including nurse practitioners and physician assistants, have taken on greater patient-related responsibilities. Midlevel providers have the potential to foster and maintain meaningful provider-patient relationships that may span years, and are well positioned to recognize and manage problems that patients may have with adherence. Here we discuss the importance of achieving and maintaining responses to TKI therapy, describe the clinical consequences of poor adherence to TKI therapy in CML, and outline factors behind poor adherence. We also share strategies that we use at our center to improve adherence to long-term TKI therapy for CML.
*Click on the link to the left for a PDF of the full article.
The advent of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for the treatment of chronic myeloid leukemia (CML) has dramatically changed the management of patients with CML. With continuous long-term TKI therapy, CML can be managed like a chronic condition, and most patients can expect to have a normal life expectancy. Given the prospect of lifelong therapy, however, issues related to adherence become particularly important and warrant greater attention since attainment of favorable long-term survival depends in large part on consistent, appropriate treatment administration over years, if not decades. As the multidisciplinary care team approach to cancer care has gained traction at academic centers and community practices, midlevel providers, including nurse practitioners and physician assistants, have taken on greater patient-related responsibilities. Midlevel providers have the potential to foster and maintain meaningful provider-patient relationships that may span years, and are well positioned to recognize and manage problems that patients may have with adherence. Here we discuss the importance of achieving and maintaining responses to TKI therapy, describe the clinical consequences of poor adherence to TKI therapy in CML, and outline factors behind poor adherence. We also share strategies that we use at our center to improve adherence to long-term TKI therapy for CML.
*Click on the link to the left for a PDF of the full article.
Biennial vs annual mammography: How I manage my patients
Controversy surrounds the issue of mammographic screening intervals for older women, with conflicting recommendations from professional organizations and governmental bodies. For example, the US Preventive Services Task Force recommends biennial screening for women aged 50 to 74 years,1 whereas the American College of Obstetricians and Gynecologists2 and the American Cancer Society3 both recommend annual screening for women aged 40 years and older, with no upper age limit. It also has been unclear how patient comorbidities affect screening.
Recently, Braithwaite and colleagues addressed both issues in a prospective trial of 3,000 women with breast cancer and 138,000 women without breast cancer—all of them aged 66 to 89 years.4
What did they find?
Details of the trial. This study was conducted between January 1999 and December 2006. Using logistic-regression analyses, the study authors calculated the odds of advanced tumors and the 10-year cumulative probability of false-positive findings by the frequency of screening (1 vs 2 years), age, and comorbidity score, as determined using the Klabunde approximation of the Charlson score.1
All women underwent mammography at a facility that participated in data linkage between the Breast Cancer Surveillance Consortium and Medicare claims.
Screening interval had no effect on odds of advanced tumors. The study authors found no difference in the rate of advanced breast cancer (adverse characteristics included stage IIb or higher, tumor size greater than 20 mm, or positive lymph nodes) when screening was biennial versus annual, and no effect of comorbidities on this percentage.4
Annual screening led to more false-positives. In fact, Braithwaite and colleagues found that 48% of women aged 66 to 74 years had at least one false-positive screen in the annual-screening group (95% confidence interval [CI], 46.1–49.9), compared with 29% of biennial screeners (95% CI, 28.1–29.9).1
Balance of data seems to tilt toward biennial screening
In this study, Braithwaite and colleagues observe that their findings are consistent with those of earlier studies indicating that biennial screening retains the benefits of annual assessment and reduces the false-positive rate.
RELATED ARTICLE: Update on Breast Health
Guiding my patients. Although I expect most of my patients aged 50 and older to continue to seek annual mammograms for the foreseeable future, I plan to be flexible about mammography intervals, given these findings. Therefore, if a patient aged 50 years or older is receptive to being screened less often than annually, I would encourage her to be screened every 2 years, provided she is not at elevated risk of breast cancer by virtue of family or personal history, genetic testing, or earlier findings.
1. US Preventive Services Task Force Screening for Breast Cancer. http://www.uspreventiveservicestask force.org/uspstf/uspsbrca.htm. Accessed May 17, 2013.
2. American College of Obstetricians-Gynecologists. Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-82.
3. Breast cancer: early detection. American Cancer Society Web site. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Accessed May 17, 2013.
4. Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: Does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013;105(5):334–341.
Controversy surrounds the issue of mammographic screening intervals for older women, with conflicting recommendations from professional organizations and governmental bodies. For example, the US Preventive Services Task Force recommends biennial screening for women aged 50 to 74 years,1 whereas the American College of Obstetricians and Gynecologists2 and the American Cancer Society3 both recommend annual screening for women aged 40 years and older, with no upper age limit. It also has been unclear how patient comorbidities affect screening.
Recently, Braithwaite and colleagues addressed both issues in a prospective trial of 3,000 women with breast cancer and 138,000 women without breast cancer—all of them aged 66 to 89 years.4
What did they find?
Details of the trial. This study was conducted between January 1999 and December 2006. Using logistic-regression analyses, the study authors calculated the odds of advanced tumors and the 10-year cumulative probability of false-positive findings by the frequency of screening (1 vs 2 years), age, and comorbidity score, as determined using the Klabunde approximation of the Charlson score.1
All women underwent mammography at a facility that participated in data linkage between the Breast Cancer Surveillance Consortium and Medicare claims.
Screening interval had no effect on odds of advanced tumors. The study authors found no difference in the rate of advanced breast cancer (adverse characteristics included stage IIb or higher, tumor size greater than 20 mm, or positive lymph nodes) when screening was biennial versus annual, and no effect of comorbidities on this percentage.4
Annual screening led to more false-positives. In fact, Braithwaite and colleagues found that 48% of women aged 66 to 74 years had at least one false-positive screen in the annual-screening group (95% confidence interval [CI], 46.1–49.9), compared with 29% of biennial screeners (95% CI, 28.1–29.9).1
Balance of data seems to tilt toward biennial screening
In this study, Braithwaite and colleagues observe that their findings are consistent with those of earlier studies indicating that biennial screening retains the benefits of annual assessment and reduces the false-positive rate.
RELATED ARTICLE: Update on Breast Health
Guiding my patients. Although I expect most of my patients aged 50 and older to continue to seek annual mammograms for the foreseeable future, I plan to be flexible about mammography intervals, given these findings. Therefore, if a patient aged 50 years or older is receptive to being screened less often than annually, I would encourage her to be screened every 2 years, provided she is not at elevated risk of breast cancer by virtue of family or personal history, genetic testing, or earlier findings.
Controversy surrounds the issue of mammographic screening intervals for older women, with conflicting recommendations from professional organizations and governmental bodies. For example, the US Preventive Services Task Force recommends biennial screening for women aged 50 to 74 years,1 whereas the American College of Obstetricians and Gynecologists2 and the American Cancer Society3 both recommend annual screening for women aged 40 years and older, with no upper age limit. It also has been unclear how patient comorbidities affect screening.
Recently, Braithwaite and colleagues addressed both issues in a prospective trial of 3,000 women with breast cancer and 138,000 women without breast cancer—all of them aged 66 to 89 years.4
What did they find?
Details of the trial. This study was conducted between January 1999 and December 2006. Using logistic-regression analyses, the study authors calculated the odds of advanced tumors and the 10-year cumulative probability of false-positive findings by the frequency of screening (1 vs 2 years), age, and comorbidity score, as determined using the Klabunde approximation of the Charlson score.1
All women underwent mammography at a facility that participated in data linkage between the Breast Cancer Surveillance Consortium and Medicare claims.
Screening interval had no effect on odds of advanced tumors. The study authors found no difference in the rate of advanced breast cancer (adverse characteristics included stage IIb or higher, tumor size greater than 20 mm, or positive lymph nodes) when screening was biennial versus annual, and no effect of comorbidities on this percentage.4
Annual screening led to more false-positives. In fact, Braithwaite and colleagues found that 48% of women aged 66 to 74 years had at least one false-positive screen in the annual-screening group (95% confidence interval [CI], 46.1–49.9), compared with 29% of biennial screeners (95% CI, 28.1–29.9).1
Balance of data seems to tilt toward biennial screening
In this study, Braithwaite and colleagues observe that their findings are consistent with those of earlier studies indicating that biennial screening retains the benefits of annual assessment and reduces the false-positive rate.
RELATED ARTICLE: Update on Breast Health
Guiding my patients. Although I expect most of my patients aged 50 and older to continue to seek annual mammograms for the foreseeable future, I plan to be flexible about mammography intervals, given these findings. Therefore, if a patient aged 50 years or older is receptive to being screened less often than annually, I would encourage her to be screened every 2 years, provided she is not at elevated risk of breast cancer by virtue of family or personal history, genetic testing, or earlier findings.
1. US Preventive Services Task Force Screening for Breast Cancer. http://www.uspreventiveservicestask force.org/uspstf/uspsbrca.htm. Accessed May 17, 2013.
2. American College of Obstetricians-Gynecologists. Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-82.
3. Breast cancer: early detection. American Cancer Society Web site. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Accessed May 17, 2013.
4. Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: Does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013;105(5):334–341.
1. US Preventive Services Task Force Screening for Breast Cancer. http://www.uspreventiveservicestask force.org/uspstf/uspsbrca.htm. Accessed May 17, 2013.
2. American College of Obstetricians-Gynecologists. Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-82.
3. Breast cancer: early detection. American Cancer Society Web site. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Accessed May 17, 2013.
4. Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: Does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013;105(5):334–341.
Proton-Pump Inhibitors Associated with Increased Mortality Risk
Clinical question: Is the use of proton-pump inhibitors (PPIs) associated with risk of mortality or combined risk of death or rehospitalization in older patients discharged from acute-care hospitals?
Background: Previous studies have shown that the use of PPIs could be associated with increased mortality in institutionalized older people and in patients discharged from acute-care hospitals. Older patients could be more vulnerable to adverse effects of PPIs, such as drug-drug interactions and absorption of nutrients, because of the higher incidence of polypharmacy and malnutrition in the elderly.
Study design: Prospective cohort.
Setting: Eleven acute-care medical wards participating in the Italian study Pharmacosurveillance in the Elderly Care.
Synopsis: All patients aged 65 years or older consecutively admitted to participating wards from April to June 2007 underwent screening. Excluding patients who refused, died during hospitalization, or were admitted to long-term care or rehabilitation units, a total of 491 patients were analyzed. The study team administered questionnaires during admission and conducted follow-up visits every three months for one year after discharge. Outcomes included one-year survival of patients discharged from acute-care medical wards and the combined endpoint of death or rehospitalization.
Overall, 174 patients (35.4%) had PPI exposure. After adjusting for age, cognitive impairment, disability, comorbidities, nutritional status, and number of drugs prescribed, patients exposed to PPIs had a significantly increased risk of death (adjusted HR 1.51, 95% CI 1.03-2.77). This association was strongest among patients receiving high-dose PPIs. No such association was found when considering the combined endpoint (HR 1.49, 95% CI 0.98-2.17). Limitations of the study include observational design, small size, potential for confounding by indication for PPI, and indeterminate PPI use prior to index hospitalization. Finally, the finding of an association between PPIs and increased mortality does not equate to a causative relationship between the two variables.
Bottom line: Proton-pump inhibitor use in older patients discharged from acute-care hospitals is associated with increased all-cause mortality at one year.
Citation: Maggio M, Corsonello A, Ceda GP, et al. Proton-pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
Clinical question: Is the use of proton-pump inhibitors (PPIs) associated with risk of mortality or combined risk of death or rehospitalization in older patients discharged from acute-care hospitals?
Background: Previous studies have shown that the use of PPIs could be associated with increased mortality in institutionalized older people and in patients discharged from acute-care hospitals. Older patients could be more vulnerable to adverse effects of PPIs, such as drug-drug interactions and absorption of nutrients, because of the higher incidence of polypharmacy and malnutrition in the elderly.
Study design: Prospective cohort.
Setting: Eleven acute-care medical wards participating in the Italian study Pharmacosurveillance in the Elderly Care.
Synopsis: All patients aged 65 years or older consecutively admitted to participating wards from April to June 2007 underwent screening. Excluding patients who refused, died during hospitalization, or were admitted to long-term care or rehabilitation units, a total of 491 patients were analyzed. The study team administered questionnaires during admission and conducted follow-up visits every three months for one year after discharge. Outcomes included one-year survival of patients discharged from acute-care medical wards and the combined endpoint of death or rehospitalization.
Overall, 174 patients (35.4%) had PPI exposure. After adjusting for age, cognitive impairment, disability, comorbidities, nutritional status, and number of drugs prescribed, patients exposed to PPIs had a significantly increased risk of death (adjusted HR 1.51, 95% CI 1.03-2.77). This association was strongest among patients receiving high-dose PPIs. No such association was found when considering the combined endpoint (HR 1.49, 95% CI 0.98-2.17). Limitations of the study include observational design, small size, potential for confounding by indication for PPI, and indeterminate PPI use prior to index hospitalization. Finally, the finding of an association between PPIs and increased mortality does not equate to a causative relationship between the two variables.
Bottom line: Proton-pump inhibitor use in older patients discharged from acute-care hospitals is associated with increased all-cause mortality at one year.
Citation: Maggio M, Corsonello A, Ceda GP, et al. Proton-pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
Clinical question: Is the use of proton-pump inhibitors (PPIs) associated with risk of mortality or combined risk of death or rehospitalization in older patients discharged from acute-care hospitals?
Background: Previous studies have shown that the use of PPIs could be associated with increased mortality in institutionalized older people and in patients discharged from acute-care hospitals. Older patients could be more vulnerable to adverse effects of PPIs, such as drug-drug interactions and absorption of nutrients, because of the higher incidence of polypharmacy and malnutrition in the elderly.
Study design: Prospective cohort.
Setting: Eleven acute-care medical wards participating in the Italian study Pharmacosurveillance in the Elderly Care.
Synopsis: All patients aged 65 years or older consecutively admitted to participating wards from April to June 2007 underwent screening. Excluding patients who refused, died during hospitalization, or were admitted to long-term care or rehabilitation units, a total of 491 patients were analyzed. The study team administered questionnaires during admission and conducted follow-up visits every three months for one year after discharge. Outcomes included one-year survival of patients discharged from acute-care medical wards and the combined endpoint of death or rehospitalization.
Overall, 174 patients (35.4%) had PPI exposure. After adjusting for age, cognitive impairment, disability, comorbidities, nutritional status, and number of drugs prescribed, patients exposed to PPIs had a significantly increased risk of death (adjusted HR 1.51, 95% CI 1.03-2.77). This association was strongest among patients receiving high-dose PPIs. No such association was found when considering the combined endpoint (HR 1.49, 95% CI 0.98-2.17). Limitations of the study include observational design, small size, potential for confounding by indication for PPI, and indeterminate PPI use prior to index hospitalization. Finally, the finding of an association between PPIs and increased mortality does not equate to a causative relationship between the two variables.
Bottom line: Proton-pump inhibitor use in older patients discharged from acute-care hospitals is associated with increased all-cause mortality at one year.
Citation: Maggio M, Corsonello A, Ceda GP, et al. Proton-pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
Diabetes Mellitus Does Not Increase Risk of Surgical Complications after Elective Total Knee Replacement Surgery
Clinical question: Does uncontrolled diabetes mellitus increase risk for post-operative complications after elective joint replacement surgery?
Background: Several previous studies suggested that patients with uncontrolled diabetes could be at higher risk of postoperative complications and have worse functional outcomes after joint replacement surgery than patients without diabetes. Preoperative glycemic control is a potentially modifiable risk factor in patients undergoing elective joint replacement surgery. Demand for elective joint replacement is expected to increase over time, and reducing the risk of postoperative complications is essential in order to optimize functional outcomes and reduce healthcare costs.
Study design: Retrospective cohort.
Setting: Five regions of the Kaiser Permanente healthcare system.
Synopsis: The study included 40,491 patients aged 18 years and older who underwent primary knee replacement between January 2001 and December 2009 in five regions of the Kaiser Permanente system. Patients were identified using the Kaiser Permanente Total Joint Replacement Registry. Clinical information on each patient was collected from two years before the procedure to one year after the procedure using Kaiser Permanente electronic health records. Subjects were classified as nondiabetic (81.3%), diabetic with good glycemic control (12.5%), or diabetic with poor glycemic control (6.2%). Glycemic control status was assessed using the latest hemoglobin A1c (HbA1c) value measured prior to the date of the index surgery, with HbA1c <7.0% defined as good glycemic control. Outcomes included revision arthroplasty, deep infection, DVT or PE, incident myocardial infarction, and rehospitalization.
There was no significant association identified between uncontrolled diabetes and any of the five outcomes.
Limitations of the study include retrospective design, rarity of all outcomes except all-cause rehospitalization, and the small number of patients with uncontrolled diabetes in the cohort. In addition, functional outcomes were not assessed in this study.
Bottom line: The effect of uncontrolled diabetes on the risk of adverse surgical outcomes following elective joint replacement remains unclear based on currently published data; more studies are needed.
Citation: Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001-2009. J Bone Joint Surg Am. 2013;95:481-487.
Clinical question: Does uncontrolled diabetes mellitus increase risk for post-operative complications after elective joint replacement surgery?
Background: Several previous studies suggested that patients with uncontrolled diabetes could be at higher risk of postoperative complications and have worse functional outcomes after joint replacement surgery than patients without diabetes. Preoperative glycemic control is a potentially modifiable risk factor in patients undergoing elective joint replacement surgery. Demand for elective joint replacement is expected to increase over time, and reducing the risk of postoperative complications is essential in order to optimize functional outcomes and reduce healthcare costs.
Study design: Retrospective cohort.
Setting: Five regions of the Kaiser Permanente healthcare system.
Synopsis: The study included 40,491 patients aged 18 years and older who underwent primary knee replacement between January 2001 and December 2009 in five regions of the Kaiser Permanente system. Patients were identified using the Kaiser Permanente Total Joint Replacement Registry. Clinical information on each patient was collected from two years before the procedure to one year after the procedure using Kaiser Permanente electronic health records. Subjects were classified as nondiabetic (81.3%), diabetic with good glycemic control (12.5%), or diabetic with poor glycemic control (6.2%). Glycemic control status was assessed using the latest hemoglobin A1c (HbA1c) value measured prior to the date of the index surgery, with HbA1c <7.0% defined as good glycemic control. Outcomes included revision arthroplasty, deep infection, DVT or PE, incident myocardial infarction, and rehospitalization.
There was no significant association identified between uncontrolled diabetes and any of the five outcomes.
Limitations of the study include retrospective design, rarity of all outcomes except all-cause rehospitalization, and the small number of patients with uncontrolled diabetes in the cohort. In addition, functional outcomes were not assessed in this study.
Bottom line: The effect of uncontrolled diabetes on the risk of adverse surgical outcomes following elective joint replacement remains unclear based on currently published data; more studies are needed.
Citation: Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001-2009. J Bone Joint Surg Am. 2013;95:481-487.
Clinical question: Does uncontrolled diabetes mellitus increase risk for post-operative complications after elective joint replacement surgery?
Background: Several previous studies suggested that patients with uncontrolled diabetes could be at higher risk of postoperative complications and have worse functional outcomes after joint replacement surgery than patients without diabetes. Preoperative glycemic control is a potentially modifiable risk factor in patients undergoing elective joint replacement surgery. Demand for elective joint replacement is expected to increase over time, and reducing the risk of postoperative complications is essential in order to optimize functional outcomes and reduce healthcare costs.
Study design: Retrospective cohort.
Setting: Five regions of the Kaiser Permanente healthcare system.
Synopsis: The study included 40,491 patients aged 18 years and older who underwent primary knee replacement between January 2001 and December 2009 in five regions of the Kaiser Permanente system. Patients were identified using the Kaiser Permanente Total Joint Replacement Registry. Clinical information on each patient was collected from two years before the procedure to one year after the procedure using Kaiser Permanente electronic health records. Subjects were classified as nondiabetic (81.3%), diabetic with good glycemic control (12.5%), or diabetic with poor glycemic control (6.2%). Glycemic control status was assessed using the latest hemoglobin A1c (HbA1c) value measured prior to the date of the index surgery, with HbA1c <7.0% defined as good glycemic control. Outcomes included revision arthroplasty, deep infection, DVT or PE, incident myocardial infarction, and rehospitalization.
There was no significant association identified between uncontrolled diabetes and any of the five outcomes.
Limitations of the study include retrospective design, rarity of all outcomes except all-cause rehospitalization, and the small number of patients with uncontrolled diabetes in the cohort. In addition, functional outcomes were not assessed in this study.
Bottom line: The effect of uncontrolled diabetes on the risk of adverse surgical outcomes following elective joint replacement remains unclear based on currently published data; more studies are needed.
Citation: Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001-2009. J Bone Joint Surg Am. 2013;95:481-487.