User login
FDA: Increased PAH Risk Seen With Dasatinib
Treatment with the leukemia drug dasatinib has been associated with an increased risk for pulmonary arterial hypertension, which can occur at any time after starting treatment, the Food and Drug Administration announced on Oct. 11.
None of the cases was fatal, and PAH "may be reversible" if treatment is discontinued, according to the statement, posted on the agency’s MedWatch site.
Dasatinib, a kinase inhibitor marketed as Sprycel by Bristol-Myers Squibb, is approved for treating certain adults with Philadelphia chromosome-positive chronic myeloid leukemia (CML) or acute lymphoblastic leukemia (ALL). It is an oral therapy, administered once daily.
Since dasatinib was approved in 2006, the BMS global pharmacovigilance database has identified cases of PAH in treated patients, the statement said. In 12 of these cases, right heart catheterization confirmed the diagnosis, and dasatinib was considered "the most likely cause," the FDA said. These patients had developed symptoms at various time intervals after starting treatment, including more than 12 months afterward, and they often were taking other medications or had comorbidities, so "there may be a combination of factors contributing to the development of PAH" in patients taking dasatinib, the FDA said.
Because dyspnea, fatigue, hypoxia, fluid retention, and other PAH symptoms overlap with those of other conditions, "a diagnosis of Sprycel-associated PAH should be considered" if other causes have been ruled out in symptomatic patients, the FDA advises. Health care professionals should also evaluate patients for signs and symptoms of underlying cardiopulmonary disease before starting treatment and during treatment. The drug should be permanently discontinued if a diagnosis of PAH is confirmed.
Improvements in hemodynamic and clinical parameters were observed following discontinuation in some patients, the FDA statement said.
This information has been added to the drug’s prescribing information. Serious adverse events associated with dasatinib should be reported to the FDA’s MedWatch program at 800-332-1088.
Treatment with the leukemia drug dasatinib has been associated with an increased risk for pulmonary arterial hypertension, which can occur at any time after starting treatment, the Food and Drug Administration announced on Oct. 11.
None of the cases was fatal, and PAH "may be reversible" if treatment is discontinued, according to the statement, posted on the agency’s MedWatch site.
Dasatinib, a kinase inhibitor marketed as Sprycel by Bristol-Myers Squibb, is approved for treating certain adults with Philadelphia chromosome-positive chronic myeloid leukemia (CML) or acute lymphoblastic leukemia (ALL). It is an oral therapy, administered once daily.
Since dasatinib was approved in 2006, the BMS global pharmacovigilance database has identified cases of PAH in treated patients, the statement said. In 12 of these cases, right heart catheterization confirmed the diagnosis, and dasatinib was considered "the most likely cause," the FDA said. These patients had developed symptoms at various time intervals after starting treatment, including more than 12 months afterward, and they often were taking other medications or had comorbidities, so "there may be a combination of factors contributing to the development of PAH" in patients taking dasatinib, the FDA said.
Because dyspnea, fatigue, hypoxia, fluid retention, and other PAH symptoms overlap with those of other conditions, "a diagnosis of Sprycel-associated PAH should be considered" if other causes have been ruled out in symptomatic patients, the FDA advises. Health care professionals should also evaluate patients for signs and symptoms of underlying cardiopulmonary disease before starting treatment and during treatment. The drug should be permanently discontinued if a diagnosis of PAH is confirmed.
Improvements in hemodynamic and clinical parameters were observed following discontinuation in some patients, the FDA statement said.
This information has been added to the drug’s prescribing information. Serious adverse events associated with dasatinib should be reported to the FDA’s MedWatch program at 800-332-1088.
Treatment with the leukemia drug dasatinib has been associated with an increased risk for pulmonary arterial hypertension, which can occur at any time after starting treatment, the Food and Drug Administration announced on Oct. 11.
None of the cases was fatal, and PAH "may be reversible" if treatment is discontinued, according to the statement, posted on the agency’s MedWatch site.
Dasatinib, a kinase inhibitor marketed as Sprycel by Bristol-Myers Squibb, is approved for treating certain adults with Philadelphia chromosome-positive chronic myeloid leukemia (CML) or acute lymphoblastic leukemia (ALL). It is an oral therapy, administered once daily.
Since dasatinib was approved in 2006, the BMS global pharmacovigilance database has identified cases of PAH in treated patients, the statement said. In 12 of these cases, right heart catheterization confirmed the diagnosis, and dasatinib was considered "the most likely cause," the FDA said. These patients had developed symptoms at various time intervals after starting treatment, including more than 12 months afterward, and they often were taking other medications or had comorbidities, so "there may be a combination of factors contributing to the development of PAH" in patients taking dasatinib, the FDA said.
Because dyspnea, fatigue, hypoxia, fluid retention, and other PAH symptoms overlap with those of other conditions, "a diagnosis of Sprycel-associated PAH should be considered" if other causes have been ruled out in symptomatic patients, the FDA advises. Health care professionals should also evaluate patients for signs and symptoms of underlying cardiopulmonary disease before starting treatment and during treatment. The drug should be permanently discontinued if a diagnosis of PAH is confirmed.
Improvements in hemodynamic and clinical parameters were observed following discontinuation in some patients, the FDA statement said.
This information has been added to the drug’s prescribing information. Serious adverse events associated with dasatinib should be reported to the FDA’s MedWatch program at 800-332-1088.
Age, Location of Bruises Flag Child Abuse
SAN FRANCISCO – The location of bruising and the age of a child can help hone clinical suspicion of child abuse, thanks to a study identifying these predictors.
Because of this study, clinicians now have a better way of raising the topic with parents, which is always a difficult scenario, Dr. Robert Sidbury said.
Instead of physicians having to say that they need to discuss the possibility the bruises might be from nonaccidental trauma, they can now can say, "I’ve got this paper that says bruises in this certain location in a child of this age make me have to check this out," he said at the Women’s and Pediatric Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF). "To me, that sounds different."
The study compared the characteristics of bruises on 95 infants aged 0-48 months seen in a pediatric ICU, 53 of whom had accidental trauma and 42 of whom were victims of abuse. Bruising on the torso, ear, or neck ("Think TEN," he suggested) in a child younger than 4 years of age increased the possibility of abuse (Pediatrics 2010;125:67-74).
"Does that mean a child can’t fall and bruise an ear? Of course not," said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital. "It is one thing to add to the list when we’re doing an assessment of the interaction with the parent, interaction with the child, [and] any other signs of trauma – all the things we go through" when considering the possibility of abuse.
"Remember, if they can’t cruise, they can’t bruise." Also, bruising anywhere on an infant younger than 4 months of age was suggestive of abuse. "Remember, if they can’t cruise, they can’t bruise," he said. "Is that evidence of abuse? It is not. Is it something we should pay attention to? I think it is."
Bruising on multiple sites was not in the study’s model, but also is suggestive of child abuse, Dr. Sidbury added.
He described seeing a 2-month-old patient with multiple linear, angulated bruises, some of them in the TEN locations. "The index of suspicion was high, and sadly, this was absolutely a case of abuse," he said.
The more data like this that can be gathered, the easier it will make the physician’s job when assessing a child that might be a victim of abuse.
"It is a wrenching issue," he said. "It is wrenching if it is abuse, and it is equally wrenching if you falsely raise the specter of abuse."
Dr. Sidbury said he had no relevant conflicts of interest.
SDEF and this news organization are owned by Elsevier.
SAN FRANCISCO – The location of bruising and the age of a child can help hone clinical suspicion of child abuse, thanks to a study identifying these predictors.
Because of this study, clinicians now have a better way of raising the topic with parents, which is always a difficult scenario, Dr. Robert Sidbury said.
Instead of physicians having to say that they need to discuss the possibility the bruises might be from nonaccidental trauma, they can now can say, "I’ve got this paper that says bruises in this certain location in a child of this age make me have to check this out," he said at the Women’s and Pediatric Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF). "To me, that sounds different."
The study compared the characteristics of bruises on 95 infants aged 0-48 months seen in a pediatric ICU, 53 of whom had accidental trauma and 42 of whom were victims of abuse. Bruising on the torso, ear, or neck ("Think TEN," he suggested) in a child younger than 4 years of age increased the possibility of abuse (Pediatrics 2010;125:67-74).
"Does that mean a child can’t fall and bruise an ear? Of course not," said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital. "It is one thing to add to the list when we’re doing an assessment of the interaction with the parent, interaction with the child, [and] any other signs of trauma – all the things we go through" when considering the possibility of abuse.
"Remember, if they can’t cruise, they can’t bruise." Also, bruising anywhere on an infant younger than 4 months of age was suggestive of abuse. "Remember, if they can’t cruise, they can’t bruise," he said. "Is that evidence of abuse? It is not. Is it something we should pay attention to? I think it is."
Bruising on multiple sites was not in the study’s model, but also is suggestive of child abuse, Dr. Sidbury added.
He described seeing a 2-month-old patient with multiple linear, angulated bruises, some of them in the TEN locations. "The index of suspicion was high, and sadly, this was absolutely a case of abuse," he said.
The more data like this that can be gathered, the easier it will make the physician’s job when assessing a child that might be a victim of abuse.
"It is a wrenching issue," he said. "It is wrenching if it is abuse, and it is equally wrenching if you falsely raise the specter of abuse."
Dr. Sidbury said he had no relevant conflicts of interest.
SDEF and this news organization are owned by Elsevier.
SAN FRANCISCO – The location of bruising and the age of a child can help hone clinical suspicion of child abuse, thanks to a study identifying these predictors.
Because of this study, clinicians now have a better way of raising the topic with parents, which is always a difficult scenario, Dr. Robert Sidbury said.
Instead of physicians having to say that they need to discuss the possibility the bruises might be from nonaccidental trauma, they can now can say, "I’ve got this paper that says bruises in this certain location in a child of this age make me have to check this out," he said at the Women’s and Pediatric Dermatology Seminar, sponsored by Skin Disease Education Foundation (SDEF). "To me, that sounds different."
The study compared the characteristics of bruises on 95 infants aged 0-48 months seen in a pediatric ICU, 53 of whom had accidental trauma and 42 of whom were victims of abuse. Bruising on the torso, ear, or neck ("Think TEN," he suggested) in a child younger than 4 years of age increased the possibility of abuse (Pediatrics 2010;125:67-74).
"Does that mean a child can’t fall and bruise an ear? Of course not," said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital. "It is one thing to add to the list when we’re doing an assessment of the interaction with the parent, interaction with the child, [and] any other signs of trauma – all the things we go through" when considering the possibility of abuse.
"Remember, if they can’t cruise, they can’t bruise." Also, bruising anywhere on an infant younger than 4 months of age was suggestive of abuse. "Remember, if they can’t cruise, they can’t bruise," he said. "Is that evidence of abuse? It is not. Is it something we should pay attention to? I think it is."
Bruising on multiple sites was not in the study’s model, but also is suggestive of child abuse, Dr. Sidbury added.
He described seeing a 2-month-old patient with multiple linear, angulated bruises, some of them in the TEN locations. "The index of suspicion was high, and sadly, this was absolutely a case of abuse," he said.
The more data like this that can be gathered, the easier it will make the physician’s job when assessing a child that might be a victim of abuse.
"It is a wrenching issue," he said. "It is wrenching if it is abuse, and it is equally wrenching if you falsely raise the specter of abuse."
Dr. Sidbury said he had no relevant conflicts of interest.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Cracking the Case
A 43‐year‐old woman presented to an outside hospital with painful plaques and patches on her bilateral lower extremities. Two weeks prior to presentation, she had noticed a single red lesion on her left ankle. Over the next two weeks, the lesion enlarged to involve the lower half of her posterior calf and subsequently turned purple and became exquisitely tender. Similar but smaller purple, tender lesions simultaneously appeared, first over her right shin and then on her bilateral thighs and hips. She also reported fatigue as well as diffuse joint pains in her hands and wrists bilaterally for the past month. She denied any swelling of these joints or functional impairment. She denied fevers, weight loss, headache, sinus symptoms, difficulty breathing, or abdominal pain.
Although we do not yet have a physical exam, the tempo, pattern of spread, and accompanying features allow some early hypotheses to be considered. Distal lower extremity lesions which darkened and spread could be erythema nodosum or erythema induratum. Malignancies rarely have such prominent skin manifestations, although leukemia cutis or an aggressive cutaneous T cell lymphoma might present with disseminated and darkened plaques, and Kaposi's sarcoma is characteristically purple and multifocal. Autoimmune disorders such as sarcoidosis, cutaneous lupus, and psoriasis may similarly present with widespread plaques. Most disseminated infections that start with patches evolve to pustules, ulcers, bullae, or other forms that reflect the invasive nature of the infection; syphilis warrants consideration for any widespread eruption of unknown etiology. Antecedent arthralgias with fatigue suggest an autoimmune condition, although infections such as hepatitis or parvovirus can do the same. Systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) would be favored initially on account of her demographics and the hand and wrist involvement, and each can be associated with vasculitis.
The significant pain as described is not compatible with most of the aforementioned diagnoses. Its presence, coupled with potential autoimmune symptoms, suggests a vasculitis such as polyarteritis nodosa (which can have prominent diffuse skin involvement), Henoch Schonlein purpura (with its predilection for the lower extremities, including extension to the hips and buttocks), cryoglobulinemia, or SLE‐ or RA‐associated vasculitis. Calciphylaxis is another ischemic vascular disorder that can cause diffuse dark painful lesions, but this only warrants consideration if advanced renal disease is present.
A skin biopsy ofher right hip was taken at the outside hospital. She was discharged on a two‐week course of prednisone for suspected vasculitis while biopsy results were pending. Over the next two weeks, none of the skin lesions improved, despite compliance with this treatment, and the skin over her left posterior calf and right shin lesions began to erode and bleed. In addition, small purple, tender lesions appeared over the pinnae of both ears. Three weeks after her initial evaluation, she presented to another emergency department for ulcerating skin lesions and worsening pain. At that point, the initial skin biopsy result was available and revealed vasculopathy of the small vessels with thrombi but no vasculitis.
The patient had no children,and denied a history of miscarriages. Her past medical history was unremarkable. She did not report any history of thrombotic events. She started a new job as a software engineer one month ago and was feeling stressed about her new responsibilities. She denied any high‐risk sexual behavior and any history of intravenous drug use. She had not traveled recently and did not own any pets. There was no family history of rheumatologic disorders, hypercoagulable states, or thrombotic events.
This picture of occluded but noninflamed vessels shifts the diagnosis away from vasculitis and focuses attention on hypercoagulable states with prominent dermal manifestations, including antiphospholipid antibody syndrome (APLS) and livedoid vasculopathy. In this young woman with arthralgias, consideration of SLE and APLS is warranted. Her recent increase in stress and widespread purpuric and ulcerative lesions could bring to mind a factitious disorder, but the histology results eliminate this possibility.
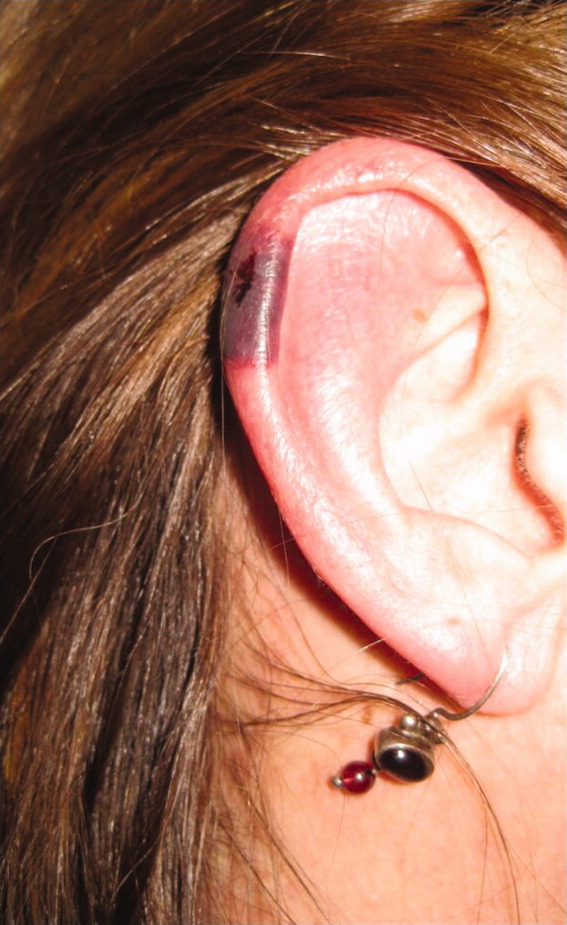
The patient's temperaturewas 36.5C, her blood pressure was 110/70 mmHg, respiratory rate was 16 breaths per minute, and her heart rate was 65 beats per minute. She was well‐appearing but in moderate pain. She did not have any oral lesions. Her cardiac, respiratory, and abdominal exams were normal. Skin exam revealed a 10‐cm by 4‐cm area of bloody granulation tissue draining serosanguinous fluid, surrounded by stellate palpable netlike purpura on her left posterior calf. There was a similar 4‐cm by 2‐cm ulcerated lesion on her right shin. Both lesions were exquisitely tender to palpation. On her bilateral thighs and hips, there were multiple stellate purpuric patches, all 4 cm in diameter or less, and only minimally tender to palpation. She also had 1‐cm purpuric bullae on the helices of both ears (Figure 1) which were slightly tender to palpation. Splinter hemorrhages were also noted on multiple nail beds bilaterally. Musculoskeletal exam did not reveal any synovitis.
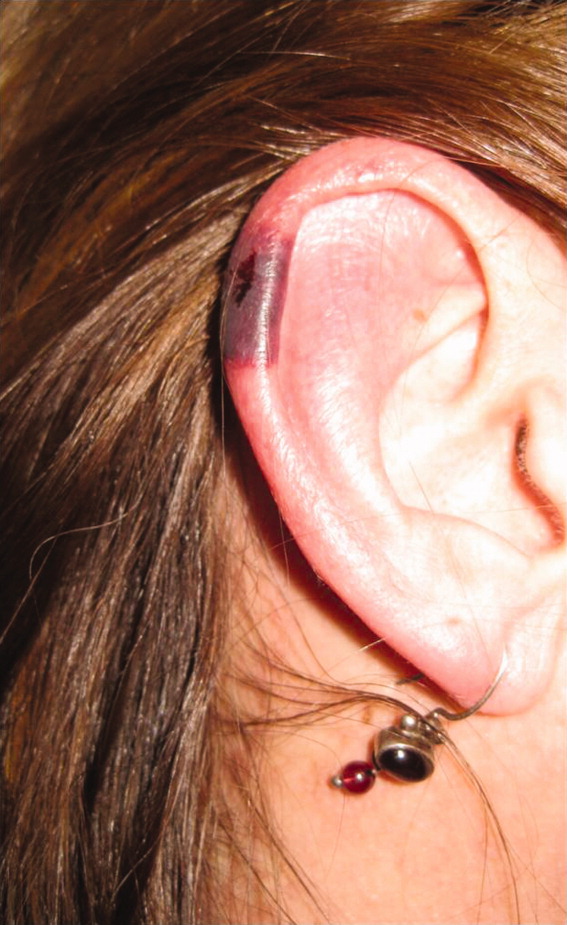
The original purpura on her calf and ear demonstrate a clear demarcation corresponding to cutaneous vascular insufficiency. The development of bullae (ear) and ulceration (calf) are compatible with ischemia. Despite the presence of multiple splinter hemorrhages, the distribution of lesions is very unusual for an embolic phenomenon (eg, endocarditis, cholesterol emboli, or atrial myxoma). The multifocal nature of the skin lesions with progression to well‐demarcated cutaneous necrosis is reminiscent of calciphylaxis or warfarin‐induced skin necrosis, although she lacks the relevant risk factors. A toxin such as cocaine or methamphetamine mediating multifocal vasoconstriction or hypercoagulability should be excluded.
The bilateral ear involvement remains decidedly unusual and makes me wonder if there is something about the ear, such as the nature of its circulation or its potentially lower temperature (as an acral organ) that might render it particularly susceptible, for instance, to cryoglobulinemia or cryofibrinogenemia‐mediated ischemia.
Laboratory studiesdemonstrated: white blood cell count of 1500/mm3 (37.3% neutrophils, 5.1% lymphocytes, 6.7% monocytes, and 1.3% eosinophils); hemoglobin 9.3 g/dl (mean corpuscular volume 91 fL); platelet count 212/mm3; erythrocyte sedimentation rate 62 mm/hr; C‐reactive protein 14.6 mg/L. Serum electrolytes, liver tests, coagulation studies, and urinalysis were normal. Fecal occult blood test was negative.
Her neutropenia and anemia suggest decreased production in the marrow by infection, malignancy, or toxin, or increased destruction, perhaps from an autoimmune process. The associated infections are usually viral, such as human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV), although their linkage with her cutaneous disease is tenuous. It is possible that malignancy could be present in the marrow with resultant dermal hypercoagulability and ischemia, but this seems unlikely. We do not know about any toxins that she has been exposed to, but these hematologic findings would mandate directed inquiry along those lines. In this young woman with cutaneous ulcers secondary to thrombotic vasculopathy, bicytopenia, antecedent arthralgias without synovitis, and elevated inflammatory markers, I favor an autoimmune process such as SLE, which I would evaluate with an antinuclear antibody (ANA) and antiphospholipid antibody studies.
She was admittedto the hospital and received hydromorphone for pain control. Corticosteroids were not administered. Peripheral blood morphology was normal. Antibodies against HIV1 and 2 were negative, as were antibodies against cytomegalovirus, EBV, parvovirus B19, mycoplasma pneumoniae, and hepatitis C virus. Bilateral lower extremity ultrasound was negative for deep vein thrombosis. Transthoracic echocardiogram was normal. Repeat skin biopsy confirmed small vessel vasculopathy without vasculitis (Figure 2). The results of the following investigations were also negative: ANA, rheumatoid factor, double‐stranded DNA (dsDNA), cyclic citrullinated peptide, ribonucleoprotein (RNP), and anti‐Smith antibodies. C3 and C4 complement levels were normal.
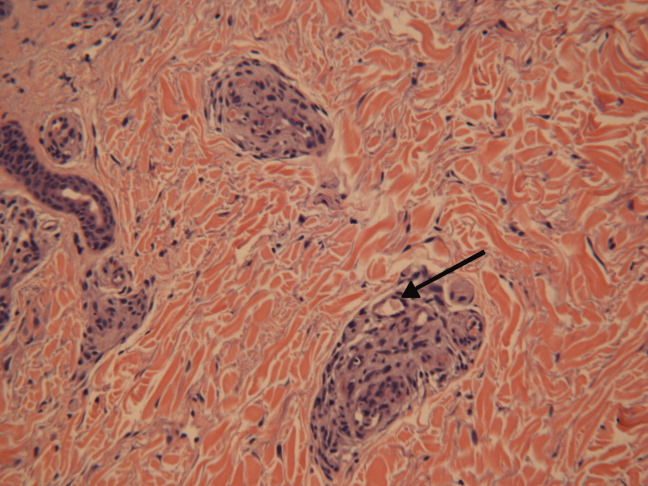
Given how much the histology is driving the clinical reasoning and focusing the differential diagnosis in this case, I agree with the decision to repeat the biopsy. In complex or undiagnosed cases, repeat histology samples allow for confirmation of the original interpretation (often with the perspective of new clinicians and pathologists) and sometimes reveal pathognomonic or additional findings that only appear after the disease has evolved over time. HIV seronegativity helps constrain the differential diagnosis, and parvovirus is another excellent consideration for arthralgias and cytopenias (with the predilection to involve cells lines other than RBCs particularly seen in HIV), although ulcers are not seen with this condition. Herpes simplex virus (HSV) is another viral infection that can cause painful skin ulcerations and cytopenias, although the duration and distribution are highly atypical. The negative ANA and dsDNA and normal complement levels make SLE unlikely. The negative lower extremity ultrasound helps frame the thromboses as a local cutaneous process rather than a systemic hypercoagulable state. Although the peripheral blood smear is normal, a bone marrow biopsy will be necessary to exclude a marrow invasive process, such as leukemia or lymphoma. A bone marrow biopsy would also provide another opportunity to examine tissue for mycobacteria or fungi which can cause ulcerations and cytopenias, although there is little reason to suspect she is susceptible to those pathogens. As this clinical picture fails to fit clearly with an infectious, autoimmune, or neoplastic disorder, I would revisit the possibility of toxinsprescription, complementary, over‐the‐counter, or illegal (eg, cocaine) at this time.
In further discussionwith the patient, she reported using cocaine intranasally for the past three months. Her urine toxicology was positive for cocaine. She was found to have positive perinuclear antineutrophil cytoplasmic antibodies (p‐ANCA), antimyeloperoxidase (MPO) antibodies, anticardiolipin (ACL) antibodies, and lupus anticoagulant (LAC). By hospital day 3, her lesions had significantly improved without any intervention, and her absolute neutrophil count increased to 1080/mm3.
The presence of widespread cutaneous ischemia (with bland thrombosis) and detectable ACL and LAC antibodies is compatible with APLS; the APLS could be deemed primary, because there is no clear associated rheumatologic or other systemic disease. However, neutropenia is not a characteristic of APLS, which has thrombocytopenia as its more frequently associated hematologic abnormality. Livedoid vasculopathy, a related disorder, is also supported by the ACL and LAC results, but also does not feature neutropenia. While the presence of diffuse thrombosis could be attributed to a widespread secondary effect of cocaine vasoconstriction, the appearance of ANCA (which can be drug‐induced, eg, propylthiouracil [PTU]) and the slowly resolving neutropenia during hospitalization without specific treatment is very suggestive of a toxin. The demographic, diffuse skin ulcers, and hematologic and serologic profile is compatible with the recently described toxidrome related to levamisole adulteration of cocaine.
A send‐out studyof a urine sample returned positive for levamisole. Based on purpuric skin lesions with a predilection for the ears, agranulocytosis, and skin biopsy revealing thrombotic vasculopathy, she was diagnosed with levamisole‐adulterated cocaine exposure. One week after discharge, her lower extremity pain and ulcerations were significantly improved. Her absolute neutrophil count increased to 2820/mm.3 Her urine toxicology screen was negative for cocaine.
DISCUSSION
Levamisole was initially developed in 1964 as an antihelminthic agent. Its incidentally discovered immunomodulatory effects led to trials for the treatment of chronic infections, inflammatory bowel disease, rheumatic diseases,1 and nephrotic syndrome in children.2 By 1990, 3 major studies supported levamisole as an adjunctive therapy in melanoma3 and colon cancer.4
Although levamisole appeared to be nontoxic at single or low doses, long‐term use in clinical trials demonstrated that 2.5%‐13% of patients developed life‐threatening agranulocytosis, and up to 10% of those instances resulted in death.5 A distinctive cutaneous pseudovasculitis was noted in children on therapeutic levamisole. They presented with purpura that had a predilection for the ears, cheeks, and thighs,6 and positive serologic markers for ANCA and antiphospholipid antibodies. Skin biopsies of the purpuric lesions revealed leukocytoclastic vasculitis, thrombotic vasculitis, and/or vascular occlusions.
Levamisole was withdrawn from the market in 2000 in the United States due to its side effects,7 but quickly found its way onto the black market. It was first detected in cocaine in 2002, and the percentage of cocaine containing levamisole has steadily been increasing since then. In July 2009, over 70% of cocaine seized by the Drug Enforcement Administration was found to contain levamisole.8 It is unclear exactly why this drug is used as an adulterant in cocaine. Theories include potentiation of the euphoric effects of cocaine, serving as a bulking agent, or functioning as a chemical signature to track distribution.9
The resurgence of levamisole has brought a new face to a problem seen over a decade ago. Current reports of levamisole toxicity describe adults presenting with purpura preferentially involving the ears, neutropenia, positive ANCA, and positive antiphospholipid antibodies.1012 Since 2002, there have been at least 20 confirmed cases of agranulocytosis and two deaths associated with levamisole‐adulterated cocaine.8, 13, 14 In September 2009, the Department of Health and Human Services issued a public health alert warning of an impending increase in levamisole‐related illness.
Levamisole is not detected on routine toxicology screens, but can be tested for using gas chromatography and mass spectrometry. Most laboratories do not offer testing for levamisole and send‐out testing is required. Given its half‐life of 5.6 hours, levamisole can only be detected in the blood within 24 hours, and in the urine within 48‐72 hours of exposure.15, 16 Urine samples are preferred over blood samples, since blood levels decline more rapidly and have lower sensitivity. Cocaine can also be sent out to local or state forensics laboratories to be tested for levamisole. The only definitive treatment for levamisole‐induced cutaneous pseudovasculitis and neutropenia is cessation of toxin exposure.
Although the discussant had familiarity with this toxidrome from local and published cases, he was only able to settle on levamisole toxicity after a series of competing hypotheses were ruled out on the basis of irreconcilable features (vasculitis and histology results; APLS and neutropenia; SLE and negative ANA with no visceral involvement) and by using analogical reasoning (eg, to infer the presence of a toxin on the basis of neutropenia [as seen with chemotherapy and other drugs] and ANCA induction [as seen with PTU]). It was a laborious process of hypothesis testing, but one that ultimately allowed him to crack the case.
Key Teaching Points
-
In patients presenting with neutropenia and purpuric skin lesionsparticularly with a predilection for the earsconsider levamisole‐adulterated cocaine exposure.
-
Tests supporting this diagnosis include positive serologies for ANCA and antiphospholipid antibodies, and skin biopsies that show leukocytoclastic vasculitis, thrombotic vasculitis, or vascular occlusion. Urine studies for levamisole are definitive if sent within 48 to 72 hours of exposure.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,.Levamisole, the story and the lessons.Int J Immunopharmocol.1992;14(3):481–486.
- British Association for Paediatric Nephrology.Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood.Lancet.1991;337:1555–1557.
- ,,,,.Improved survival in patients with poor‐prognosis malignant melanoma treated with adjuvant levamisole: a phase III study by the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.1991;9:729–735.
- ,,.Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.N Engl J Med.1990;322:352–358.
- ,,.Studies on levamisole‐induced agranulocytosis.Blood.1980;56(3):388–396.
- ,,.Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long‐term treatment with levamisole in children.Br J Dermatol.1999;140:948–951.
- . Janssen Discontinues Ergamisol. Available at: http://findarticles.com/p/articles/mi_m3374/is_18_22/ai_68536218/. Accessed July 25,2010.
- SAMHSA. Nationwide Public Health Alert Issued Concerning Life‐Threatening Risk Posed by Cocaine Laced with Veterinary Anti‐Parasite Drug. Available at: http://www.samhsa.gov/newsroom/advisories/090921vet5101.aspx. Accessed July 20,2010.
- .Unusual adulterants in cocaine seized on Italian clandestine market.Forensic Sci Int.2007;172(2–3):e1.
- ,,.Levamisole‐induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Jun 12.
- ,,,.Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole.Ann Intern Med.2010;1;152(11):758–759.
- ,,,,.Cocaine‐associated retiform purpura and neutropenia: is levamisole the culprit?J Am Acad Dermatol.2010;Mar 19.
- ,,,.A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Apr 1.
- Centers for Disease Control and Prevention.Agranulocytosis associated with cocaine use—four States, March 2008–November 2009.MMWR.2009;58(49):1381–1385.
- ,,.Levamisole as a contaminant of illicit cocaine.Journal of the Clandestine Laboratory Investigating Chemists Association.2006;16:6–11. Available at: http://www.tiaft2006.org/proceedings/pdf/PT‐p‐06.pdf. Accessed July 20, 2010.
- . Cocaine Cutting Agents—A Discussion. Laboratory Medicine and Pathology, University of Alberta. Available at: http://www.vandu.org/documents/Levamisole_Cocaine.pdf. Accessed July 20,2010.
A 43‐year‐old woman presented to an outside hospital with painful plaques and patches on her bilateral lower extremities. Two weeks prior to presentation, she had noticed a single red lesion on her left ankle. Over the next two weeks, the lesion enlarged to involve the lower half of her posterior calf and subsequently turned purple and became exquisitely tender. Similar but smaller purple, tender lesions simultaneously appeared, first over her right shin and then on her bilateral thighs and hips. She also reported fatigue as well as diffuse joint pains in her hands and wrists bilaterally for the past month. She denied any swelling of these joints or functional impairment. She denied fevers, weight loss, headache, sinus symptoms, difficulty breathing, or abdominal pain.
Although we do not yet have a physical exam, the tempo, pattern of spread, and accompanying features allow some early hypotheses to be considered. Distal lower extremity lesions which darkened and spread could be erythema nodosum or erythema induratum. Malignancies rarely have such prominent skin manifestations, although leukemia cutis or an aggressive cutaneous T cell lymphoma might present with disseminated and darkened plaques, and Kaposi's sarcoma is characteristically purple and multifocal. Autoimmune disorders such as sarcoidosis, cutaneous lupus, and psoriasis may similarly present with widespread plaques. Most disseminated infections that start with patches evolve to pustules, ulcers, bullae, or other forms that reflect the invasive nature of the infection; syphilis warrants consideration for any widespread eruption of unknown etiology. Antecedent arthralgias with fatigue suggest an autoimmune condition, although infections such as hepatitis or parvovirus can do the same. Systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) would be favored initially on account of her demographics and the hand and wrist involvement, and each can be associated with vasculitis.
The significant pain as described is not compatible with most of the aforementioned diagnoses. Its presence, coupled with potential autoimmune symptoms, suggests a vasculitis such as polyarteritis nodosa (which can have prominent diffuse skin involvement), Henoch Schonlein purpura (with its predilection for the lower extremities, including extension to the hips and buttocks), cryoglobulinemia, or SLE‐ or RA‐associated vasculitis. Calciphylaxis is another ischemic vascular disorder that can cause diffuse dark painful lesions, but this only warrants consideration if advanced renal disease is present.
A skin biopsy ofher right hip was taken at the outside hospital. She was discharged on a two‐week course of prednisone for suspected vasculitis while biopsy results were pending. Over the next two weeks, none of the skin lesions improved, despite compliance with this treatment, and the skin over her left posterior calf and right shin lesions began to erode and bleed. In addition, small purple, tender lesions appeared over the pinnae of both ears. Three weeks after her initial evaluation, she presented to another emergency department for ulcerating skin lesions and worsening pain. At that point, the initial skin biopsy result was available and revealed vasculopathy of the small vessels with thrombi but no vasculitis.
The patient had no children,and denied a history of miscarriages. Her past medical history was unremarkable. She did not report any history of thrombotic events. She started a new job as a software engineer one month ago and was feeling stressed about her new responsibilities. She denied any high‐risk sexual behavior and any history of intravenous drug use. She had not traveled recently and did not own any pets. There was no family history of rheumatologic disorders, hypercoagulable states, or thrombotic events.
This picture of occluded but noninflamed vessels shifts the diagnosis away from vasculitis and focuses attention on hypercoagulable states with prominent dermal manifestations, including antiphospholipid antibody syndrome (APLS) and livedoid vasculopathy. In this young woman with arthralgias, consideration of SLE and APLS is warranted. Her recent increase in stress and widespread purpuric and ulcerative lesions could bring to mind a factitious disorder, but the histology results eliminate this possibility.
The patient's temperaturewas 36.5C, her blood pressure was 110/70 mmHg, respiratory rate was 16 breaths per minute, and her heart rate was 65 beats per minute. She was well‐appearing but in moderate pain. She did not have any oral lesions. Her cardiac, respiratory, and abdominal exams were normal. Skin exam revealed a 10‐cm by 4‐cm area of bloody granulation tissue draining serosanguinous fluid, surrounded by stellate palpable netlike purpura on her left posterior calf. There was a similar 4‐cm by 2‐cm ulcerated lesion on her right shin. Both lesions were exquisitely tender to palpation. On her bilateral thighs and hips, there were multiple stellate purpuric patches, all 4 cm in diameter or less, and only minimally tender to palpation. She also had 1‐cm purpuric bullae on the helices of both ears (Figure 1) which were slightly tender to palpation. Splinter hemorrhages were also noted on multiple nail beds bilaterally. Musculoskeletal exam did not reveal any synovitis.

The original purpura on her calf and ear demonstrate a clear demarcation corresponding to cutaneous vascular insufficiency. The development of bullae (ear) and ulceration (calf) are compatible with ischemia. Despite the presence of multiple splinter hemorrhages, the distribution of lesions is very unusual for an embolic phenomenon (eg, endocarditis, cholesterol emboli, or atrial myxoma). The multifocal nature of the skin lesions with progression to well‐demarcated cutaneous necrosis is reminiscent of calciphylaxis or warfarin‐induced skin necrosis, although she lacks the relevant risk factors. A toxin such as cocaine or methamphetamine mediating multifocal vasoconstriction or hypercoagulability should be excluded.
The bilateral ear involvement remains decidedly unusual and makes me wonder if there is something about the ear, such as the nature of its circulation or its potentially lower temperature (as an acral organ) that might render it particularly susceptible, for instance, to cryoglobulinemia or cryofibrinogenemia‐mediated ischemia.
Laboratory studiesdemonstrated: white blood cell count of 1500/mm3 (37.3% neutrophils, 5.1% lymphocytes, 6.7% monocytes, and 1.3% eosinophils); hemoglobin 9.3 g/dl (mean corpuscular volume 91 fL); platelet count 212/mm3; erythrocyte sedimentation rate 62 mm/hr; C‐reactive protein 14.6 mg/L. Serum electrolytes, liver tests, coagulation studies, and urinalysis were normal. Fecal occult blood test was negative.
Her neutropenia and anemia suggest decreased production in the marrow by infection, malignancy, or toxin, or increased destruction, perhaps from an autoimmune process. The associated infections are usually viral, such as human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV), although their linkage with her cutaneous disease is tenuous. It is possible that malignancy could be present in the marrow with resultant dermal hypercoagulability and ischemia, but this seems unlikely. We do not know about any toxins that she has been exposed to, but these hematologic findings would mandate directed inquiry along those lines. In this young woman with cutaneous ulcers secondary to thrombotic vasculopathy, bicytopenia, antecedent arthralgias without synovitis, and elevated inflammatory markers, I favor an autoimmune process such as SLE, which I would evaluate with an antinuclear antibody (ANA) and antiphospholipid antibody studies.
She was admittedto the hospital and received hydromorphone for pain control. Corticosteroids were not administered. Peripheral blood morphology was normal. Antibodies against HIV1 and 2 were negative, as were antibodies against cytomegalovirus, EBV, parvovirus B19, mycoplasma pneumoniae, and hepatitis C virus. Bilateral lower extremity ultrasound was negative for deep vein thrombosis. Transthoracic echocardiogram was normal. Repeat skin biopsy confirmed small vessel vasculopathy without vasculitis (Figure 2). The results of the following investigations were also negative: ANA, rheumatoid factor, double‐stranded DNA (dsDNA), cyclic citrullinated peptide, ribonucleoprotein (RNP), and anti‐Smith antibodies. C3 and C4 complement levels were normal.

Given how much the histology is driving the clinical reasoning and focusing the differential diagnosis in this case, I agree with the decision to repeat the biopsy. In complex or undiagnosed cases, repeat histology samples allow for confirmation of the original interpretation (often with the perspective of new clinicians and pathologists) and sometimes reveal pathognomonic or additional findings that only appear after the disease has evolved over time. HIV seronegativity helps constrain the differential diagnosis, and parvovirus is another excellent consideration for arthralgias and cytopenias (with the predilection to involve cells lines other than RBCs particularly seen in HIV), although ulcers are not seen with this condition. Herpes simplex virus (HSV) is another viral infection that can cause painful skin ulcerations and cytopenias, although the duration and distribution are highly atypical. The negative ANA and dsDNA and normal complement levels make SLE unlikely. The negative lower extremity ultrasound helps frame the thromboses as a local cutaneous process rather than a systemic hypercoagulable state. Although the peripheral blood smear is normal, a bone marrow biopsy will be necessary to exclude a marrow invasive process, such as leukemia or lymphoma. A bone marrow biopsy would also provide another opportunity to examine tissue for mycobacteria or fungi which can cause ulcerations and cytopenias, although there is little reason to suspect she is susceptible to those pathogens. As this clinical picture fails to fit clearly with an infectious, autoimmune, or neoplastic disorder, I would revisit the possibility of toxinsprescription, complementary, over‐the‐counter, or illegal (eg, cocaine) at this time.
In further discussionwith the patient, she reported using cocaine intranasally for the past three months. Her urine toxicology was positive for cocaine. She was found to have positive perinuclear antineutrophil cytoplasmic antibodies (p‐ANCA), antimyeloperoxidase (MPO) antibodies, anticardiolipin (ACL) antibodies, and lupus anticoagulant (LAC). By hospital day 3, her lesions had significantly improved without any intervention, and her absolute neutrophil count increased to 1080/mm3.
The presence of widespread cutaneous ischemia (with bland thrombosis) and detectable ACL and LAC antibodies is compatible with APLS; the APLS could be deemed primary, because there is no clear associated rheumatologic or other systemic disease. However, neutropenia is not a characteristic of APLS, which has thrombocytopenia as its more frequently associated hematologic abnormality. Livedoid vasculopathy, a related disorder, is also supported by the ACL and LAC results, but also does not feature neutropenia. While the presence of diffuse thrombosis could be attributed to a widespread secondary effect of cocaine vasoconstriction, the appearance of ANCA (which can be drug‐induced, eg, propylthiouracil [PTU]) and the slowly resolving neutropenia during hospitalization without specific treatment is very suggestive of a toxin. The demographic, diffuse skin ulcers, and hematologic and serologic profile is compatible with the recently described toxidrome related to levamisole adulteration of cocaine.
A send‐out studyof a urine sample returned positive for levamisole. Based on purpuric skin lesions with a predilection for the ears, agranulocytosis, and skin biopsy revealing thrombotic vasculopathy, she was diagnosed with levamisole‐adulterated cocaine exposure. One week after discharge, her lower extremity pain and ulcerations were significantly improved. Her absolute neutrophil count increased to 2820/mm.3 Her urine toxicology screen was negative for cocaine.
DISCUSSION
Levamisole was initially developed in 1964 as an antihelminthic agent. Its incidentally discovered immunomodulatory effects led to trials for the treatment of chronic infections, inflammatory bowel disease, rheumatic diseases,1 and nephrotic syndrome in children.2 By 1990, 3 major studies supported levamisole as an adjunctive therapy in melanoma3 and colon cancer.4
Although levamisole appeared to be nontoxic at single or low doses, long‐term use in clinical trials demonstrated that 2.5%‐13% of patients developed life‐threatening agranulocytosis, and up to 10% of those instances resulted in death.5 A distinctive cutaneous pseudovasculitis was noted in children on therapeutic levamisole. They presented with purpura that had a predilection for the ears, cheeks, and thighs,6 and positive serologic markers for ANCA and antiphospholipid antibodies. Skin biopsies of the purpuric lesions revealed leukocytoclastic vasculitis, thrombotic vasculitis, and/or vascular occlusions.
Levamisole was withdrawn from the market in 2000 in the United States due to its side effects,7 but quickly found its way onto the black market. It was first detected in cocaine in 2002, and the percentage of cocaine containing levamisole has steadily been increasing since then. In July 2009, over 70% of cocaine seized by the Drug Enforcement Administration was found to contain levamisole.8 It is unclear exactly why this drug is used as an adulterant in cocaine. Theories include potentiation of the euphoric effects of cocaine, serving as a bulking agent, or functioning as a chemical signature to track distribution.9
The resurgence of levamisole has brought a new face to a problem seen over a decade ago. Current reports of levamisole toxicity describe adults presenting with purpura preferentially involving the ears, neutropenia, positive ANCA, and positive antiphospholipid antibodies.1012 Since 2002, there have been at least 20 confirmed cases of agranulocytosis and two deaths associated with levamisole‐adulterated cocaine.8, 13, 14 In September 2009, the Department of Health and Human Services issued a public health alert warning of an impending increase in levamisole‐related illness.
Levamisole is not detected on routine toxicology screens, but can be tested for using gas chromatography and mass spectrometry. Most laboratories do not offer testing for levamisole and send‐out testing is required. Given its half‐life of 5.6 hours, levamisole can only be detected in the blood within 24 hours, and in the urine within 48‐72 hours of exposure.15, 16 Urine samples are preferred over blood samples, since blood levels decline more rapidly and have lower sensitivity. Cocaine can also be sent out to local or state forensics laboratories to be tested for levamisole. The only definitive treatment for levamisole‐induced cutaneous pseudovasculitis and neutropenia is cessation of toxin exposure.
Although the discussant had familiarity with this toxidrome from local and published cases, he was only able to settle on levamisole toxicity after a series of competing hypotheses were ruled out on the basis of irreconcilable features (vasculitis and histology results; APLS and neutropenia; SLE and negative ANA with no visceral involvement) and by using analogical reasoning (eg, to infer the presence of a toxin on the basis of neutropenia [as seen with chemotherapy and other drugs] and ANCA induction [as seen with PTU]). It was a laborious process of hypothesis testing, but one that ultimately allowed him to crack the case.
Key Teaching Points
-
In patients presenting with neutropenia and purpuric skin lesionsparticularly with a predilection for the earsconsider levamisole‐adulterated cocaine exposure.
-
Tests supporting this diagnosis include positive serologies for ANCA and antiphospholipid antibodies, and skin biopsies that show leukocytoclastic vasculitis, thrombotic vasculitis, or vascular occlusion. Urine studies for levamisole are definitive if sent within 48 to 72 hours of exposure.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 43‐year‐old woman presented to an outside hospital with painful plaques and patches on her bilateral lower extremities. Two weeks prior to presentation, she had noticed a single red lesion on her left ankle. Over the next two weeks, the lesion enlarged to involve the lower half of her posterior calf and subsequently turned purple and became exquisitely tender. Similar but smaller purple, tender lesions simultaneously appeared, first over her right shin and then on her bilateral thighs and hips. She also reported fatigue as well as diffuse joint pains in her hands and wrists bilaterally for the past month. She denied any swelling of these joints or functional impairment. She denied fevers, weight loss, headache, sinus symptoms, difficulty breathing, or abdominal pain.
Although we do not yet have a physical exam, the tempo, pattern of spread, and accompanying features allow some early hypotheses to be considered. Distal lower extremity lesions which darkened and spread could be erythema nodosum or erythema induratum. Malignancies rarely have such prominent skin manifestations, although leukemia cutis or an aggressive cutaneous T cell lymphoma might present with disseminated and darkened plaques, and Kaposi's sarcoma is characteristically purple and multifocal. Autoimmune disorders such as sarcoidosis, cutaneous lupus, and psoriasis may similarly present with widespread plaques. Most disseminated infections that start with patches evolve to pustules, ulcers, bullae, or other forms that reflect the invasive nature of the infection; syphilis warrants consideration for any widespread eruption of unknown etiology. Antecedent arthralgias with fatigue suggest an autoimmune condition, although infections such as hepatitis or parvovirus can do the same. Systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) would be favored initially on account of her demographics and the hand and wrist involvement, and each can be associated with vasculitis.
The significant pain as described is not compatible with most of the aforementioned diagnoses. Its presence, coupled with potential autoimmune symptoms, suggests a vasculitis such as polyarteritis nodosa (which can have prominent diffuse skin involvement), Henoch Schonlein purpura (with its predilection for the lower extremities, including extension to the hips and buttocks), cryoglobulinemia, or SLE‐ or RA‐associated vasculitis. Calciphylaxis is another ischemic vascular disorder that can cause diffuse dark painful lesions, but this only warrants consideration if advanced renal disease is present.
A skin biopsy ofher right hip was taken at the outside hospital. She was discharged on a two‐week course of prednisone for suspected vasculitis while biopsy results were pending. Over the next two weeks, none of the skin lesions improved, despite compliance with this treatment, and the skin over her left posterior calf and right shin lesions began to erode and bleed. In addition, small purple, tender lesions appeared over the pinnae of both ears. Three weeks after her initial evaluation, she presented to another emergency department for ulcerating skin lesions and worsening pain. At that point, the initial skin biopsy result was available and revealed vasculopathy of the small vessels with thrombi but no vasculitis.
The patient had no children,and denied a history of miscarriages. Her past medical history was unremarkable. She did not report any history of thrombotic events. She started a new job as a software engineer one month ago and was feeling stressed about her new responsibilities. She denied any high‐risk sexual behavior and any history of intravenous drug use. She had not traveled recently and did not own any pets. There was no family history of rheumatologic disorders, hypercoagulable states, or thrombotic events.
This picture of occluded but noninflamed vessels shifts the diagnosis away from vasculitis and focuses attention on hypercoagulable states with prominent dermal manifestations, including antiphospholipid antibody syndrome (APLS) and livedoid vasculopathy. In this young woman with arthralgias, consideration of SLE and APLS is warranted. Her recent increase in stress and widespread purpuric and ulcerative lesions could bring to mind a factitious disorder, but the histology results eliminate this possibility.
The patient's temperaturewas 36.5C, her blood pressure was 110/70 mmHg, respiratory rate was 16 breaths per minute, and her heart rate was 65 beats per minute. She was well‐appearing but in moderate pain. She did not have any oral lesions. Her cardiac, respiratory, and abdominal exams were normal. Skin exam revealed a 10‐cm by 4‐cm area of bloody granulation tissue draining serosanguinous fluid, surrounded by stellate palpable netlike purpura on her left posterior calf. There was a similar 4‐cm by 2‐cm ulcerated lesion on her right shin. Both lesions were exquisitely tender to palpation. On her bilateral thighs and hips, there were multiple stellate purpuric patches, all 4 cm in diameter or less, and only minimally tender to palpation. She also had 1‐cm purpuric bullae on the helices of both ears (Figure 1) which were slightly tender to palpation. Splinter hemorrhages were also noted on multiple nail beds bilaterally. Musculoskeletal exam did not reveal any synovitis.

The original purpura on her calf and ear demonstrate a clear demarcation corresponding to cutaneous vascular insufficiency. The development of bullae (ear) and ulceration (calf) are compatible with ischemia. Despite the presence of multiple splinter hemorrhages, the distribution of lesions is very unusual for an embolic phenomenon (eg, endocarditis, cholesterol emboli, or atrial myxoma). The multifocal nature of the skin lesions with progression to well‐demarcated cutaneous necrosis is reminiscent of calciphylaxis or warfarin‐induced skin necrosis, although she lacks the relevant risk factors. A toxin such as cocaine or methamphetamine mediating multifocal vasoconstriction or hypercoagulability should be excluded.
The bilateral ear involvement remains decidedly unusual and makes me wonder if there is something about the ear, such as the nature of its circulation or its potentially lower temperature (as an acral organ) that might render it particularly susceptible, for instance, to cryoglobulinemia or cryofibrinogenemia‐mediated ischemia.
Laboratory studiesdemonstrated: white blood cell count of 1500/mm3 (37.3% neutrophils, 5.1% lymphocytes, 6.7% monocytes, and 1.3% eosinophils); hemoglobin 9.3 g/dl (mean corpuscular volume 91 fL); platelet count 212/mm3; erythrocyte sedimentation rate 62 mm/hr; C‐reactive protein 14.6 mg/L. Serum electrolytes, liver tests, coagulation studies, and urinalysis were normal. Fecal occult blood test was negative.
Her neutropenia and anemia suggest decreased production in the marrow by infection, malignancy, or toxin, or increased destruction, perhaps from an autoimmune process. The associated infections are usually viral, such as human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV), although their linkage with her cutaneous disease is tenuous. It is possible that malignancy could be present in the marrow with resultant dermal hypercoagulability and ischemia, but this seems unlikely. We do not know about any toxins that she has been exposed to, but these hematologic findings would mandate directed inquiry along those lines. In this young woman with cutaneous ulcers secondary to thrombotic vasculopathy, bicytopenia, antecedent arthralgias without synovitis, and elevated inflammatory markers, I favor an autoimmune process such as SLE, which I would evaluate with an antinuclear antibody (ANA) and antiphospholipid antibody studies.
She was admittedto the hospital and received hydromorphone for pain control. Corticosteroids were not administered. Peripheral blood morphology was normal. Antibodies against HIV1 and 2 were negative, as were antibodies against cytomegalovirus, EBV, parvovirus B19, mycoplasma pneumoniae, and hepatitis C virus. Bilateral lower extremity ultrasound was negative for deep vein thrombosis. Transthoracic echocardiogram was normal. Repeat skin biopsy confirmed small vessel vasculopathy without vasculitis (Figure 2). The results of the following investigations were also negative: ANA, rheumatoid factor, double‐stranded DNA (dsDNA), cyclic citrullinated peptide, ribonucleoprotein (RNP), and anti‐Smith antibodies. C3 and C4 complement levels were normal.

Given how much the histology is driving the clinical reasoning and focusing the differential diagnosis in this case, I agree with the decision to repeat the biopsy. In complex or undiagnosed cases, repeat histology samples allow for confirmation of the original interpretation (often with the perspective of new clinicians and pathologists) and sometimes reveal pathognomonic or additional findings that only appear after the disease has evolved over time. HIV seronegativity helps constrain the differential diagnosis, and parvovirus is another excellent consideration for arthralgias and cytopenias (with the predilection to involve cells lines other than RBCs particularly seen in HIV), although ulcers are not seen with this condition. Herpes simplex virus (HSV) is another viral infection that can cause painful skin ulcerations and cytopenias, although the duration and distribution are highly atypical. The negative ANA and dsDNA and normal complement levels make SLE unlikely. The negative lower extremity ultrasound helps frame the thromboses as a local cutaneous process rather than a systemic hypercoagulable state. Although the peripheral blood smear is normal, a bone marrow biopsy will be necessary to exclude a marrow invasive process, such as leukemia or lymphoma. A bone marrow biopsy would also provide another opportunity to examine tissue for mycobacteria or fungi which can cause ulcerations and cytopenias, although there is little reason to suspect she is susceptible to those pathogens. As this clinical picture fails to fit clearly with an infectious, autoimmune, or neoplastic disorder, I would revisit the possibility of toxinsprescription, complementary, over‐the‐counter, or illegal (eg, cocaine) at this time.
In further discussionwith the patient, she reported using cocaine intranasally for the past three months. Her urine toxicology was positive for cocaine. She was found to have positive perinuclear antineutrophil cytoplasmic antibodies (p‐ANCA), antimyeloperoxidase (MPO) antibodies, anticardiolipin (ACL) antibodies, and lupus anticoagulant (LAC). By hospital day 3, her lesions had significantly improved without any intervention, and her absolute neutrophil count increased to 1080/mm3.
The presence of widespread cutaneous ischemia (with bland thrombosis) and detectable ACL and LAC antibodies is compatible with APLS; the APLS could be deemed primary, because there is no clear associated rheumatologic or other systemic disease. However, neutropenia is not a characteristic of APLS, which has thrombocytopenia as its more frequently associated hematologic abnormality. Livedoid vasculopathy, a related disorder, is also supported by the ACL and LAC results, but also does not feature neutropenia. While the presence of diffuse thrombosis could be attributed to a widespread secondary effect of cocaine vasoconstriction, the appearance of ANCA (which can be drug‐induced, eg, propylthiouracil [PTU]) and the slowly resolving neutropenia during hospitalization without specific treatment is very suggestive of a toxin. The demographic, diffuse skin ulcers, and hematologic and serologic profile is compatible with the recently described toxidrome related to levamisole adulteration of cocaine.
A send‐out studyof a urine sample returned positive for levamisole. Based on purpuric skin lesions with a predilection for the ears, agranulocytosis, and skin biopsy revealing thrombotic vasculopathy, she was diagnosed with levamisole‐adulterated cocaine exposure. One week after discharge, her lower extremity pain and ulcerations were significantly improved. Her absolute neutrophil count increased to 2820/mm.3 Her urine toxicology screen was negative for cocaine.
DISCUSSION
Levamisole was initially developed in 1964 as an antihelminthic agent. Its incidentally discovered immunomodulatory effects led to trials for the treatment of chronic infections, inflammatory bowel disease, rheumatic diseases,1 and nephrotic syndrome in children.2 By 1990, 3 major studies supported levamisole as an adjunctive therapy in melanoma3 and colon cancer.4
Although levamisole appeared to be nontoxic at single or low doses, long‐term use in clinical trials demonstrated that 2.5%‐13% of patients developed life‐threatening agranulocytosis, and up to 10% of those instances resulted in death.5 A distinctive cutaneous pseudovasculitis was noted in children on therapeutic levamisole. They presented with purpura that had a predilection for the ears, cheeks, and thighs,6 and positive serologic markers for ANCA and antiphospholipid antibodies. Skin biopsies of the purpuric lesions revealed leukocytoclastic vasculitis, thrombotic vasculitis, and/or vascular occlusions.
Levamisole was withdrawn from the market in 2000 in the United States due to its side effects,7 but quickly found its way onto the black market. It was first detected in cocaine in 2002, and the percentage of cocaine containing levamisole has steadily been increasing since then. In July 2009, over 70% of cocaine seized by the Drug Enforcement Administration was found to contain levamisole.8 It is unclear exactly why this drug is used as an adulterant in cocaine. Theories include potentiation of the euphoric effects of cocaine, serving as a bulking agent, or functioning as a chemical signature to track distribution.9
The resurgence of levamisole has brought a new face to a problem seen over a decade ago. Current reports of levamisole toxicity describe adults presenting with purpura preferentially involving the ears, neutropenia, positive ANCA, and positive antiphospholipid antibodies.1012 Since 2002, there have been at least 20 confirmed cases of agranulocytosis and two deaths associated with levamisole‐adulterated cocaine.8, 13, 14 In September 2009, the Department of Health and Human Services issued a public health alert warning of an impending increase in levamisole‐related illness.
Levamisole is not detected on routine toxicology screens, but can be tested for using gas chromatography and mass spectrometry. Most laboratories do not offer testing for levamisole and send‐out testing is required. Given its half‐life of 5.6 hours, levamisole can only be detected in the blood within 24 hours, and in the urine within 48‐72 hours of exposure.15, 16 Urine samples are preferred over blood samples, since blood levels decline more rapidly and have lower sensitivity. Cocaine can also be sent out to local or state forensics laboratories to be tested for levamisole. The only definitive treatment for levamisole‐induced cutaneous pseudovasculitis and neutropenia is cessation of toxin exposure.
Although the discussant had familiarity with this toxidrome from local and published cases, he was only able to settle on levamisole toxicity after a series of competing hypotheses were ruled out on the basis of irreconcilable features (vasculitis and histology results; APLS and neutropenia; SLE and negative ANA with no visceral involvement) and by using analogical reasoning (eg, to infer the presence of a toxin on the basis of neutropenia [as seen with chemotherapy and other drugs] and ANCA induction [as seen with PTU]). It was a laborious process of hypothesis testing, but one that ultimately allowed him to crack the case.
Key Teaching Points
-
In patients presenting with neutropenia and purpuric skin lesionsparticularly with a predilection for the earsconsider levamisole‐adulterated cocaine exposure.
-
Tests supporting this diagnosis include positive serologies for ANCA and antiphospholipid antibodies, and skin biopsies that show leukocytoclastic vasculitis, thrombotic vasculitis, or vascular occlusion. Urine studies for levamisole are definitive if sent within 48 to 72 hours of exposure.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,.Levamisole, the story and the lessons.Int J Immunopharmocol.1992;14(3):481–486.
- British Association for Paediatric Nephrology.Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood.Lancet.1991;337:1555–1557.
- ,,,,.Improved survival in patients with poor‐prognosis malignant melanoma treated with adjuvant levamisole: a phase III study by the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.1991;9:729–735.
- ,,.Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.N Engl J Med.1990;322:352–358.
- ,,.Studies on levamisole‐induced agranulocytosis.Blood.1980;56(3):388–396.
- ,,.Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long‐term treatment with levamisole in children.Br J Dermatol.1999;140:948–951.
- . Janssen Discontinues Ergamisol. Available at: http://findarticles.com/p/articles/mi_m3374/is_18_22/ai_68536218/. Accessed July 25,2010.
- SAMHSA. Nationwide Public Health Alert Issued Concerning Life‐Threatening Risk Posed by Cocaine Laced with Veterinary Anti‐Parasite Drug. Available at: http://www.samhsa.gov/newsroom/advisories/090921vet5101.aspx. Accessed July 20,2010.
- .Unusual adulterants in cocaine seized on Italian clandestine market.Forensic Sci Int.2007;172(2–3):e1.
- ,,.Levamisole‐induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Jun 12.
- ,,,.Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole.Ann Intern Med.2010;1;152(11):758–759.
- ,,,,.Cocaine‐associated retiform purpura and neutropenia: is levamisole the culprit?J Am Acad Dermatol.2010;Mar 19.
- ,,,.A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Apr 1.
- Centers for Disease Control and Prevention.Agranulocytosis associated with cocaine use—four States, March 2008–November 2009.MMWR.2009;58(49):1381–1385.
- ,,.Levamisole as a contaminant of illicit cocaine.Journal of the Clandestine Laboratory Investigating Chemists Association.2006;16:6–11. Available at: http://www.tiaft2006.org/proceedings/pdf/PT‐p‐06.pdf. Accessed July 20, 2010.
- . Cocaine Cutting Agents—A Discussion. Laboratory Medicine and Pathology, University of Alberta. Available at: http://www.vandu.org/documents/Levamisole_Cocaine.pdf. Accessed July 20,2010.
- ,.Levamisole, the story and the lessons.Int J Immunopharmocol.1992;14(3):481–486.
- British Association for Paediatric Nephrology.Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood.Lancet.1991;337:1555–1557.
- ,,,,.Improved survival in patients with poor‐prognosis malignant melanoma treated with adjuvant levamisole: a phase III study by the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.1991;9:729–735.
- ,,.Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.N Engl J Med.1990;322:352–358.
- ,,.Studies on levamisole‐induced agranulocytosis.Blood.1980;56(3):388–396.
- ,,.Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long‐term treatment with levamisole in children.Br J Dermatol.1999;140:948–951.
- . Janssen Discontinues Ergamisol. Available at: http://findarticles.com/p/articles/mi_m3374/is_18_22/ai_68536218/. Accessed July 25,2010.
- SAMHSA. Nationwide Public Health Alert Issued Concerning Life‐Threatening Risk Posed by Cocaine Laced with Veterinary Anti‐Parasite Drug. Available at: http://www.samhsa.gov/newsroom/advisories/090921vet5101.aspx. Accessed July 20,2010.
- .Unusual adulterants in cocaine seized on Italian clandestine market.Forensic Sci Int.2007;172(2–3):e1.
- ,,.Levamisole‐induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Jun 12.
- ,,,.Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole.Ann Intern Med.2010;1;152(11):758–759.
- ,,,,.Cocaine‐associated retiform purpura and neutropenia: is levamisole the culprit?J Am Acad Dermatol.2010;Mar 19.
- ,,,.A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Apr 1.
- Centers for Disease Control and Prevention.Agranulocytosis associated with cocaine use—four States, March 2008–November 2009.MMWR.2009;58(49):1381–1385.
- ,,.Levamisole as a contaminant of illicit cocaine.Journal of the Clandestine Laboratory Investigating Chemists Association.2006;16:6–11. Available at: http://www.tiaft2006.org/proceedings/pdf/PT‐p‐06.pdf. Accessed July 20, 2010.
- . Cocaine Cutting Agents—A Discussion. Laboratory Medicine and Pathology, University of Alberta. Available at: http://www.vandu.org/documents/Levamisole_Cocaine.pdf. Accessed July 20,2010.
Treating AWS with Oral Baclofen
In the United States, unhealthy alcohol use affects medical care on several levels. The prevalence of alcohol problems is 7%20% or higher among outpatients,1 30%40% among emergency room patients, and 50% among patients with trauma.13 In 2006, approximately 430,000 hospital discharges in the United States were for persons with a principal (first‐listed) alcohol‐related diagnosis, and 1.7 million discharges listed at least one alcohol‐related diagnosis, representing 1.3% and 5.3% of all hospital discharges, respectively.4 Alcoholic psychosis (34.5%) and alcohol dependence syndrome (29.5%) together accounted for the majority of principal alcohol‐related diagnoses.4 Additionally, many patients hospitalized for other indications are susceptible to withdrawal symptoms due to physiological habituation to alcohol. Abrupt cessation of alcohol intake causes habituated drinkers to experience symptoms of alcohol withdrawal syndrome (AWS), which significantly increases intensity and cost of care. Trauma patients who develop AWS were found to have increased morbidity, more intensive care and ventilator days, and higher hospital costs than trauma patients without AWS.5 Unfortunately, attempts to develop predictive models to accurately forecast the likelihood of developing severe AWS in an individual case have been modestly successful at best.68
Regimens used to treat AWS have evolved over time, taking advantage of advances in the understanding of addiction neurophysiology. There is no specific ethanol receptor.9 Much of alcohol's acute effects on the central nervous system are mediated by its stimulation of the gamma‐aminobutyric acid (GABA) system, which is neuroinhibitory.10 Chronic alcohol use leads to habituation partly by inducing configuration changes of GABA‐A receptor subunits. This renders the GABA‐A receptor less sensitive to alcohol, barbiturates, and benzodiazepines.11 Although both GABA‐A and GABA‐B receptor activation cause increased GABA neuronal output, the GABA‐A receptor is rendered relatively less sensitive by chronic exposure to alcohol. Baclofen is a pure GABA‐B receptor agonist,12 and its GABA‐B stimulatory effect is maintained even in habituated alcoholics.13, 14 The absence of cross‐tolerance between baclofen and ethanol suggests that low doses of baclofen may be helpful in the management of AWS.
Currently, AWS is usually managed with benzodiazepines, using variable dosing depending on the severity of withdrawal symptoms. Such symptom‐triggered treatment is generally preferred over fixed‐dose regimens,15 in part because when using this method, many cases of AWS can be managed with less medication. Benzodiazepine regimens using high doses have been found to be associated with substantial morbidity and prolonged hospitalizations.16, 17
In a series of small studies, Addolorato's research team has reported decreases in AWS symptoms in association with the use of low doses of baclofen in an outpatient population,18 and has found baclofen to be associated with reduced alcohol craving in the long‐term management of alcohol dependence.11, 19, 20 Addolorato and colleagues' studies of baclofen in relieving AWS symptoms prompted our group to apply the use of baclofen in a larger group of inpatients with AWS.11, 18, 21 We conducted this study to improve understanding of the role of baclofen in the management of acute AWS in an inpatient population of subjects at risk for AWS, drawn from general hospital admissions.
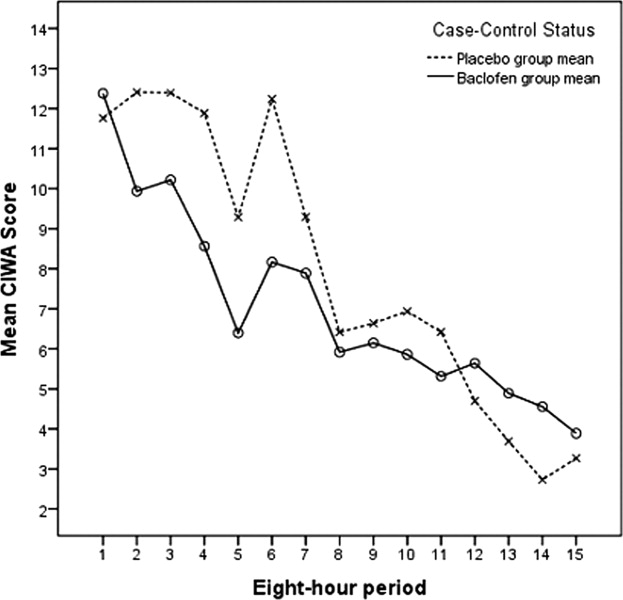
Our primary null hypothesis was that Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in acutely withdrawing alcoholic patients are equal in baclofen‐treated and placebo‐treated subject groups. Our secondary null hypothesis was that benzodiazepine doses used to treat acutely withdrawing alcoholic patients are equal in the baclofen‐treated and placebo‐treated groups.
METHODS
The protocol for this study was approved by the Essentia Health Institutional Review Board, Duluth, Minnesota.
This was a randomized, placebo‐controlled, double‐blind trial. Subjects were recruited from among patients who were admitted to 1 of 2 regional general hospitals in Duluth, Minnesota (St. Mary's Medical Center or Miller‐Dwan Medical Center) and who were identified by clinical staff as being at risk for AWS. Potential subjects were not required to have an alcohol‐related condition as their primary reason for admission, but were required to have a history of AWS or of alcohol use suggestive of significant risk for AWS, and to be able to provide informed consent, as described below.
Patients were not eligible for enrollment in the study if they had other active drug dependence in addition to alcohol; were using baclofen at the time of study enrollment; were using benzodiazepines chronically at the time of study enrollment; had a known baclofen or benzodiazepine sensitivity; were unable to take oral medications; were pregnant or breast‐feeding; had a serum creatinine level 2.0; had a history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; or were unable to complete the consenting procedures.
Consenting and Enrollment Procedures
Patients who were identified by clinical staff as being at high risk for AWS were approached for possible enrollment in the study. Potential subjects who met other inclusion criteria were screened to assess their mental status with the Mini‐Mental Status Exam (MMSE), using methods developed for subjects with cognitive impairment.22 Potential subjects who scored 24 of a possible score of 30 or higher on the exam were considered capable of providing informed consent. Potential subjects who scored between 20 and 23 were considered to be capable of providing consent if they were able to answer 4 questions about the study (why the study is being done, what will be required of them if they participate, how long they will be in the study, and how it will be determined if they will receive the investigational drug or the placebo).
Patients who met inclusion criteria were enrolled in the study. Subjects were randomized only if they developed signs of AWS sufficient to meet Diagnostic and Statistical Manual of Mental Disorders IV (DSMIV) criteria for AWS diagnosis, and reached at least a score of 11 (of a possible 67) on the CIWA‐Ar. All subjects received symptom‐triggered benzodiazepine treatment, and also were randomized to receive either baclofen (10 mg) or placebo every 8 hours (q8h) orally as inpatients for 72 hours or until discharge, whichever was shorter. Lorazepam was selected for the symptom‐triggered benzodiazepine treatment, as it has been used for managing AWS in the participating hospitals for several years. The initial research protocol called for 5 days of participation (15 doses of study drug), but we found that many subjects were discharged before the 5 days had elapsed after enrollment, and that compliance with follow‐up outpatient visits was poor. Accordingly, the protocol was amended to shorten the treatment period to 72 hours of participation (9 doses) or until discharge if prior to 72 hours, with the minimum observation period set to 72 hours.
Data Collection
Baseline data were collected at the time of enrollment, both from the patient and the medical record. Demographic data (age, gender, race) were obtained, as well as data on alcohol history, including approximate duration and intensity of alcohol use, and prior experience with AWS; data on comorbid conditions and medical history; and history of beta‐blocker use. During the period of observation following randomization, data were obtained on CIWA‐Ar scores; benzodiazepine doses administered; and adverse events, including use of sedatives in addition to benzodiazepines, use of restraints, use of intensive care, and clinical complications during the AWS course.
Study Procedures
The research pharmacy provided study medications (baclofen or placebo in identical form) for enrolled subjects. Subjects and study personnel were blinded to treatment group (baclofen vs placebo).
Nurses on inpatient units were provided with training in CIWA‐Ar assessment. All subjects were monitored for CIWA‐Ar scores at the time of study enrollment and for at least the next 72 hours. In monitoring the subjects, the nurses used the CIWA‐Ar protocol, in which subjects were assessed and potentially dosed with lorazepam hourly if their scores were 11 or higher. If the CIWA‐Ar score was less than 11, the subjects were assessed every 4 hours and at study discharge. CIWA‐AR results were reported as averaged over 8‐hour periods starting at study enrollment.
Data Analysis
Demographic and baseline variables with ordinal and continuous measurements, such as age, MMSE total score, and drinks per day were evaluated using group t test analysis, with two‐tailed significance estimates. Variables with prevalence reported were evaluated using the chi‐square test of significance. In accordance with the protocol, data from patients who had CIWA‐Ar assessments for at least 72 hours following randomization were included in the final study analyses. Repeated measures analysis of variance were conducted for the 2 treatment groups (baclofen and placebo), to evaluate mean CIWA‐Ar scores and mean lorazepam doses within each 8‐hour interval, as well as cumulative lorazepam dose. No covariates were included in the models. The last‐observation‐carried‐forward approach was used for those subjects who were missing CIWA‐Ar scores between baseline and their last CIWA‐Ar score. Doses for postdischarge patients without lorazepam prescriptions were set to 0 mg/8 hr. Cumulative lorazepam dose at 72 hours was also analyzed by defining the upper 25th percentile of existing doses (the upper 8 of 31) as high dose. This high‐dose lorazepam treatment level was determined to include all study participants receiving 20 mg or more of lorazepam during the first 72 hours. Fisher's exact test (two‐tailed) was then used to assess the difference in treatment group (baclofen vs placebo) for high‐dose lorazepam treatment.
RESULTS
Seventy‐nine subjects met study inclusion criteria, and provided informed consent for participation in the study. Of these, 44 subjects developed signs of AWS sufficient to meet DSMIV criteria for AWS diagnosis, and were randomized to receive either baclofen or placebo, in addition to benzodiazepine therapy. As summarized in Table 1, subjects who developed signs of AWS were similar to subjects who did not enter withdrawal, differing principally in that those who developed AWS reported more drinks per day, and more significant history of previous AWS.
| Withdrawal/Randomized | ||||
|---|---|---|---|---|
| No Withdrawal | All | Placebo | Baclofen | |
| Characteristic | (N = 35) | (N = 44) | (N = 19) | (N = 25) |
| ||||
| % Male | 82.9 | 84.1 | 94.7 | 76.0 |
| Age at admission (mean/SD) | 52.0/12.0 | 46.9/10.9 | 46.1/11.9 | 47.5/10.3 |
| MMSE total score (mean/SD) | 26.5/1.7 | 25.8/3.3 | 25.4/4.1 | 26.0/2.4 |
| Drinking history | ||||
| Age began drinking (mean/SD) | 16.7/4.2 | 16.2/4.3 | 15.5/4.5 | 16.7/4.2 |
| Years drinking (mean/SD) | 34.1/10.8 | 30.2/10.0 | 30.0/12.8 | 30.3/7.8 |
| Drinks per day (mean/SD)* | 11.2/8.8 | 16.3/9.7 | 14.4/7.8 | 18.0/11.0 |
| % Daily drinker | 65.7 | 64.1 | 57.9 | 70.0 |
| Days since last drink (mean/SD) | 1.5/0.9 | 1.3/1.3 | 1.0/0.8 | 1.6/1.5 |
| Medical history, % with history of | ||||
| Alcohol withdrawal syndrome | 60.6 | 87.5 | 87.5 | 87.5 |
| Seizures*, | 30.0 | 53.8 | 33.3 | 66.7 |
| DTs* | 48.3 | 74.3 | 80.0 | 70.0 |
| Medications, % at time of admission | ||||
| Alcohol treatment | 0.0 | 4.5 | 5.3 | 4.0 |
| Beta‐blocker | 31.4 | 25.0 | 26.3 | 24.0 |
| Sleep agent | 2.9 | 6.8 | 5.3 | 8.0 |
| Narcotic pain medication | 37.1 | 40.9 | 31.6 | 48.0 |
| Depakote | 0.0 | 4.5 | 0.0 | 8.0 |
| Benzodiazepine | 2.9 | 11.4 | 10.5 | 12.0 |
| Anti‐anxiety medication | 2.9 | 2.3 | 0.0 | 4.0 |
| Anti‐psychotic medication | 5.7 | 6.8 | 10.5 | 4.0 |
| Other psychiatric medication | 2.9 | 9.1 | 5.3 | 12.0 |
The 79 subjects who were enrolled were drawn from a population of 237 potential subjects who were screened for the study. The most common reasons that the 158 potential subjects were not enrolled were refusal (29.7%), low risk of withdrawal (19.0%), inability to provide consent (9.5%), and concurrent use of benzodiazepines (8.9%). The 15 patients who were unable to provide consent were those who scored 24 or lower on the MMSE, and did not have a surrogate decision‐maker available.
Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments, as summarized in Table 2. These assessments were completed either entirely as inpatients (24 subjects) or with inpatient assessments followed by outpatient assessments after discharge (7 subjects). Discharges prior to 72 hours occurred in 3 of the 18 subjects receiving baclofen, and in 4 of the 13 receiving placebo (odds ratio = 0.45, 95% CI = 0.082.49). Mean CIWA‐Ar scores for the 31 subjects who completed 72 hours of CIWA‐Ar assessments are presented in Figure 1.
| All | Placebo | Baclofen | |
|---|---|---|---|
| Characteristic | (N = 31) | (N = 13) | (N = 18) |
| |||
| % Male | 87.1 | 92.3 | 83.3 |
| Age at admission (mean/SD) | 47.5/10.2 | 45.7/9.3 | 48.7/10.9 |
| MMSE total score (mean/SD) | 26.3/2.2 | 26.3/1.8 | 26.3/2.6 |
| Charlson Comorbidity Score | 1.0/1.1 | 1.1/0.9 | 1.0/1.3 |
| Drinking history | |||
| Age began drinking (mean/SD) | 16.7/4.6 | 16.1/4.3 | 17.1/5.0 |
| Years drinking (mean/SD) | 29.8/8.6 | 29.5/10.0 | 30.0/7.9 |
| Drinks per day (mean/SD) | 16.0/9.9 | 12.9/8.4 | 18.6/10.5 |
| % Daily drinker | 58.1 | 53.8 | 78.6 |
| Days since last drink (mean/SD) | 1.2/0.9 | 1.1/0.8 | 1.3/1.1 |
| Current hospitalization, primary diagnosis | |||
| Alcohol withdrawal syndrome/alcoholism | 48.4 | 46.2 | 50.0 |
| Probably related to alcoholism* | 35.5 | 38.5 | 33.3 |
| Other | 16.1 | 15.4 | 16.7 |
| Medical history, % with history of | |||
| Alcohol withdrawal syndrome | 89.7 | 83.3 | 94.1 |
| Seizures | 60.7 | 45.5 | 70.6 |
| DTs | 79.2 | 80.0 | 78.6 |
| Medications, % at time of admission | |||
| Alcohol treatment | 6.5 | 7.7 | 5.6 |
| Beta‐blocker | 29.1 | 23.1 | 33.3 |
| Sleep agent | 6.5 | 7.7 | 5.6 |
| Narcotic pain medication | 45.2 | 30.8 | 55.6 |
| Depakote | 6.5 | 0.0 | 11.1 |
| Benzodiazepine | 9.7 | 15.4 | 5.6 |
| Anti‐anxiety medication | 3.2 | 0.0 | 5.6 |
| Anti‐psychotic medication | 9.7 | 15.4 | 5.6 |
| Other psychiatric medication | 6.5 | 7.7 | 5.6 |
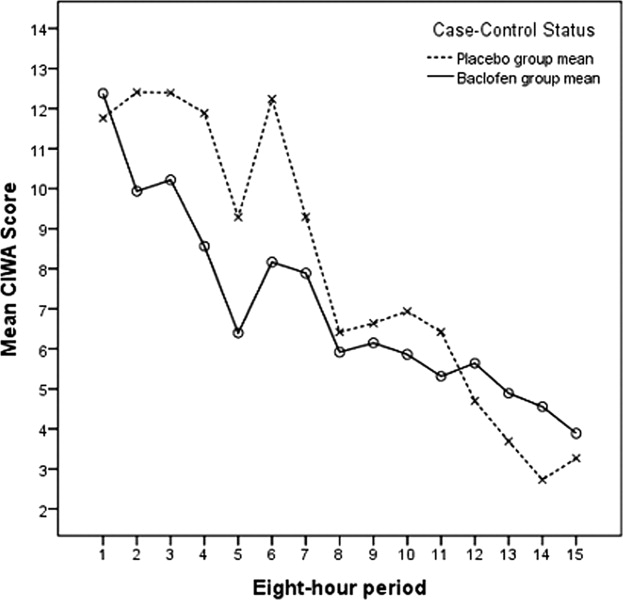
Figures 2 and 3 summarize the mean lorazepam doses in each 8‐hour period, and the cumulative lorazepam doses for the subjects in the 2 arms of the study, respectively. The cumulative dose of lorazepam administered to the 31 subjects ranged from 0 to 1035 mg in the 72 hours following randomization, with a range of 1 to 1035 mg in the placebo group and 0 to 39 mg in the baclofen group. The 8 subjects who received the highest doses of lorazepam (20 mg or more) included 1 of the 18 subjects who received baclofen and 7 of the 13 subjects who received placebo (P = 0.004). Only 4 subjects required >50 mg of lorazepam over the 72 hours; all 4 of these were patients in the placebo group (P = 0.023).
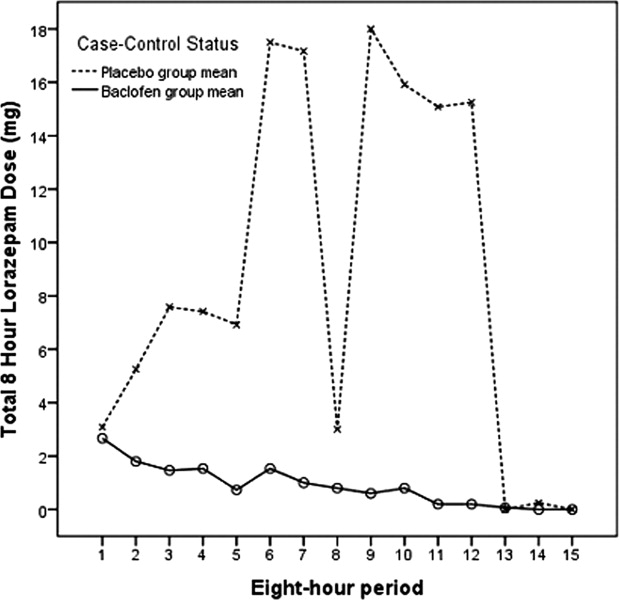
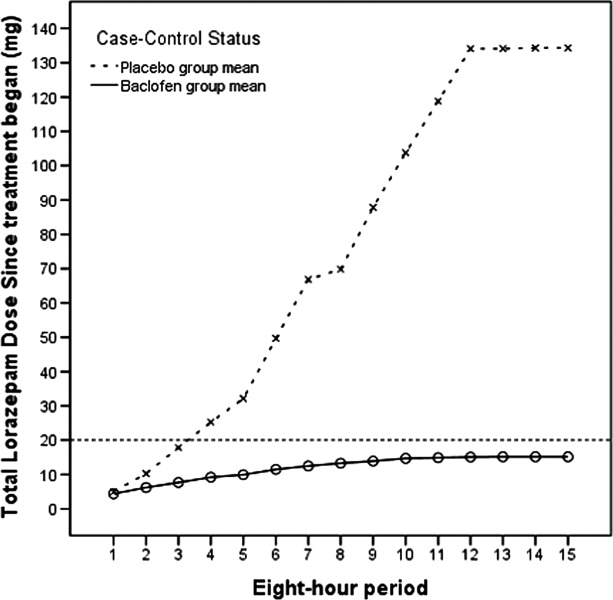
Subjects who received baclofen and subjects who received placebo did not differ significantly in regard to the use of sedatives other than benzodiazepines, the use of restraints, the use of intensive care, or other clinical complications. On the basis this analysis, the study's primary null hypothesis was not rejected (the CIWA‐Ar scores in the baclofen and placebo groups were not different), but the secondary null hypothesis was rejected (baclofen was associated with lower likelihood of the use of high doses of benzodiazepines).
DISCUSSION
Benzodiazepines are effective drugs in the treatment of alcohol withdrawal syndrome. They remain the gold standard of treatment. The most effective method of administering benzodiazepines to acutely withdrawing patients has been shown to be variable dosing, based on withdrawal symptoms.
Our small study of acutely withdrawing inpatients confirmed that benzodiazepines, administered at frequencies and doses dependent on AWS symptoms, work well to control CIWA‐Ar scores over a relatively short time span. Our study also demonstrated that the addition of the GABA‐B agonist baclofen orally, at a fixed dose of 30 mg daily, will allow the same level of AWS symptom control, while reducing the risk that high doses of benzodiazepines will be needed to achieve that control. Reducing the use of high‐dose benzodiazepines has the potential to improve patient safety. In addition, since the frequency of nursing assessments parallel CIWA‐AR scores and benzodiazepine dosing frequency, using oral baclofen in this setting has the potential to decrease the nursing time required to control withdrawal symptoms.
A larger study of AWS will be needed to assess the role of baclofen in managing the frequency and severity of the complications of AWS, such as prolonged sedation, intensive care admission, and ventilator days. The current study was not powered to assess differences in the frequency of relatively rare events, and we excluded patients who required intravenous benzodiazepines for AWS symptom management. However, in light of the well‐documented risk of sedation and respiratory depression from high‐dose benzodiazepines, our findings suggest that a larger study of the role of baclofen in AWS management is warranted.
Either baclofen or benzodiazepines may have severe adverse effects in high doses. In this study, we used a low, fixed dose of baclofen (10 mg every 8 hours), a level at which severe side effects such as respiratory depression are uncommon. Our principal finding was that the use of low‐dose baclofen is associated with reduced use of high‐dose benzodiazepines in some AWS patients.
Further Research
The use of baclofen and other adjunctive treatments in the management of AWS and other alcohol dependency syndromes warrants future study. If the benzodiazepine‐sparing effects of baclofen in AWS management are confirmed in additional studies, baclofen may become an important adjunct to benzodiazepines in AWS management, particularly in settings where the use of symptom‐triggered therapy is difficult.
It is difficult to predict which suddenly abstinent alcoholics will experience severe AWS. In the current study, 44 of the 79 patients judged by experienced clinicians to be at high risk for acute AWS reached the CIWA‐Ar threshold (a score of 11 or more) to be randomized. We found that those who experienced significant withdrawal were younger, drank more heavily, and had more prior experience with severe AWS, which is consistent with earlier studies.23, 24 Nevertheless, patient history is not a reliable predictor of risk for AWS; clinicians are often obliged to watch and wait until clinical signs of AWS develop. In light of the growing evidence that baclofen alleviates many symptoms of alcohol dependence, both in patients with AWS and in those in recovery,19, 20, 2529 future research should also examine the role of baclofen in preventing AWS in at‐risk patients.
Since ethanol has effects on several neurotransmitters and receptor systems, combinations of medications that modify GABA, glutamate, and adrenergic activity in low doses may be more effective and safer in managing AWS than using high doses of a single agent. Future research should seek to identify the most effective combination of low‐dose medications in managing AWS.
Study Limitations
This study was subject to several significant limitations. With a small study population (31 subjects), the experiences of individual subjects had a strong effect on the findings (such as the dip in mean lorazepam doses for placebo subjects in Figure 2, which was due to a high‐dose subject sleeping through the observation period). However, the overall finding of the lorazepam‐sparing effect of baclofen was consistent. We excluded patients who required intravenous benzodiazepines for AWS management, and so our study did not include the most severe AWS subjects. However, all of our subjects showed signs of mild‐to‐moderate alcohol withdrawal upon enrollment (CIWA‐Ar scores of 11 or more upon randomization), and all were at risk for more severe AWS; many went on to much higher CIWA‐AR scores during the course of their AWS. Of the 44 subjects who were randomized, 13 did not complete the study; the impact that these subjects might have had on the findings is unknown. However, the subjects who did not complete the study (baclofen 54%, placebo 46%) did not differ from the remaining subjects in regard to any of the variables used in the study. More baclofen‐treated than placebo‐treated subjects were taking narcotics and/or beta‐blockers at the time of enrollment, although these differences were not statistically significant, and their impact upon our findings are unknown. This study was conducted in northeast Minnesota; the study population reflected the limited diversity of the region. Caution should be used in generalizing the findings to other populations.
CONCLUSION
These findings suggest that baclofen may have potential as an adjunct in the management of acute alcohol withdrawal.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,, et al.Patients with alcohol problems in the emergency department, part 1: improving detection. SAEM Substance Abuse Task Force. Society for Academic Emergency Medicine.Acad Emerg Med.1998;5(12):1200–1209.
- ,,.Screening for alcohol problems in primary care: a systematic review.Arch Intern Med.2000;160(13):1977–1989.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Arlington, VA:National Institute on Alcohol Abuse and Alcoholism; August2008.
- ,,, et al.Alcohol withdrawal syndrome: turning minor injuries into a major problem.J Trauma.2006;61(6):1441–1446.
- ,,,,,.An assessment of the potential value of elevated homocysteine in predicting alcohol‐withdrawal seizures.Epilepsia.2006;47(5):934–938.
- ,.Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients.Alcohol Alcohol.2005;40(6):515–519.
- .A model for predicting alcohol withdrawal delirium.Psychiatr Serv.2001;52(6):820–823.
- .[GABA‐system and alcohol: does an “ethanol receptor” exist?] [review].Zh Nevrol Psikhiatr Im S S Korsakova.2007;Suppl 1:56–62.
- ,,, et al.Identification of molecular targets associated with ethanol toxicity and implications in drug development.Curr Pharm Des.2010;16(11):1313–1355.
- ,,,,.Baclofen: a new drug for the treatment of alcohol dependence.Int J Clin Pract.2006;60(8):1003–1008.
- ,.3H‐baclofen and 3H‐GABA bind to bicuculline‐insensitive GABA B sites in rat brain.Nature.1981;290(5802):149–152.
- ,.Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice.Psychopharmacology (Berl).1999;141(2):197–205.
- .Cerebral GABA receptors.Alcohol Alcohol Suppl.1994;2:181–186.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal.Arch Intern Med.2002;162(10):1117–1121.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacother.2001;21(9):1140–1144.
- ,,,,,.The use of continuous IV sedation is associated with prolongation of mechanical ventilation.Chest.1998;114:541–548.
- ,,, et al.Rapid suppression of alcohol withdrawal syndrome by baclofen.Am J Med.2002;112(3):226–229.
- ,,,,,.Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence.Alcohol Clin Exp Res.2000;24(1):67–71.
- ,,, et al.Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study.Alcohol Alcohol.2002;37(5):504–508.
- ,,, et al.Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam.Am J Med.2006;119:276.e213–276.e218.
- ,,,,.Relationship between Alzheimer's disease severity and patient participation in decisions about their care.J Geriatr Psychiatry Neurol.2002;15(2):68–72.
- ,,.Impact of age on the severity, course, and complications of alcohol withdrawal.Arch Intern Med.1997;157(19):2234–2241.
- ,,.Independent clinical correlates of severe alcohol withdrawal.Substance Abuse.2003;24(4):197–209.
- ,,, et al.Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind study.Lancet.2007;370:1915–1922.
- ,,, et al.Baclofen for alcohol dependence: a preliminary open‐label study.Alcohol Clin Exp Res.2004;28(10):1517–1523.
- ,,,.[Pharmacological treatment options for prevention of alcohol relapse].Fortschr Neurol Psychiatr.2008;76(7):421–428.
- ,.Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms.Pharmacol Ther.2006;111:855–876.
- ,.Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review.J Okla State Med Assoc.2007;100(9):354–360.
In the United States, unhealthy alcohol use affects medical care on several levels. The prevalence of alcohol problems is 7%20% or higher among outpatients,1 30%40% among emergency room patients, and 50% among patients with trauma.13 In 2006, approximately 430,000 hospital discharges in the United States were for persons with a principal (first‐listed) alcohol‐related diagnosis, and 1.7 million discharges listed at least one alcohol‐related diagnosis, representing 1.3% and 5.3% of all hospital discharges, respectively.4 Alcoholic psychosis (34.5%) and alcohol dependence syndrome (29.5%) together accounted for the majority of principal alcohol‐related diagnoses.4 Additionally, many patients hospitalized for other indications are susceptible to withdrawal symptoms due to physiological habituation to alcohol. Abrupt cessation of alcohol intake causes habituated drinkers to experience symptoms of alcohol withdrawal syndrome (AWS), which significantly increases intensity and cost of care. Trauma patients who develop AWS were found to have increased morbidity, more intensive care and ventilator days, and higher hospital costs than trauma patients without AWS.5 Unfortunately, attempts to develop predictive models to accurately forecast the likelihood of developing severe AWS in an individual case have been modestly successful at best.68
Regimens used to treat AWS have evolved over time, taking advantage of advances in the understanding of addiction neurophysiology. There is no specific ethanol receptor.9 Much of alcohol's acute effects on the central nervous system are mediated by its stimulation of the gamma‐aminobutyric acid (GABA) system, which is neuroinhibitory.10 Chronic alcohol use leads to habituation partly by inducing configuration changes of GABA‐A receptor subunits. This renders the GABA‐A receptor less sensitive to alcohol, barbiturates, and benzodiazepines.11 Although both GABA‐A and GABA‐B receptor activation cause increased GABA neuronal output, the GABA‐A receptor is rendered relatively less sensitive by chronic exposure to alcohol. Baclofen is a pure GABA‐B receptor agonist,12 and its GABA‐B stimulatory effect is maintained even in habituated alcoholics.13, 14 The absence of cross‐tolerance between baclofen and ethanol suggests that low doses of baclofen may be helpful in the management of AWS.
Currently, AWS is usually managed with benzodiazepines, using variable dosing depending on the severity of withdrawal symptoms. Such symptom‐triggered treatment is generally preferred over fixed‐dose regimens,15 in part because when using this method, many cases of AWS can be managed with less medication. Benzodiazepine regimens using high doses have been found to be associated with substantial morbidity and prolonged hospitalizations.16, 17
In a series of small studies, Addolorato's research team has reported decreases in AWS symptoms in association with the use of low doses of baclofen in an outpatient population,18 and has found baclofen to be associated with reduced alcohol craving in the long‐term management of alcohol dependence.11, 19, 20 Addolorato and colleagues' studies of baclofen in relieving AWS symptoms prompted our group to apply the use of baclofen in a larger group of inpatients with AWS.11, 18, 21 We conducted this study to improve understanding of the role of baclofen in the management of acute AWS in an inpatient population of subjects at risk for AWS, drawn from general hospital admissions.
Our primary null hypothesis was that Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in acutely withdrawing alcoholic patients are equal in baclofen‐treated and placebo‐treated subject groups. Our secondary null hypothesis was that benzodiazepine doses used to treat acutely withdrawing alcoholic patients are equal in the baclofen‐treated and placebo‐treated groups.
METHODS
The protocol for this study was approved by the Essentia Health Institutional Review Board, Duluth, Minnesota.
This was a randomized, placebo‐controlled, double‐blind trial. Subjects were recruited from among patients who were admitted to 1 of 2 regional general hospitals in Duluth, Minnesota (St. Mary's Medical Center or Miller‐Dwan Medical Center) and who were identified by clinical staff as being at risk for AWS. Potential subjects were not required to have an alcohol‐related condition as their primary reason for admission, but were required to have a history of AWS or of alcohol use suggestive of significant risk for AWS, and to be able to provide informed consent, as described below.
Patients were not eligible for enrollment in the study if they had other active drug dependence in addition to alcohol; were using baclofen at the time of study enrollment; were using benzodiazepines chronically at the time of study enrollment; had a known baclofen or benzodiazepine sensitivity; were unable to take oral medications; were pregnant or breast‐feeding; had a serum creatinine level 2.0; had a history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; or were unable to complete the consenting procedures.
Consenting and Enrollment Procedures
Patients who were identified by clinical staff as being at high risk for AWS were approached for possible enrollment in the study. Potential subjects who met other inclusion criteria were screened to assess their mental status with the Mini‐Mental Status Exam (MMSE), using methods developed for subjects with cognitive impairment.22 Potential subjects who scored 24 of a possible score of 30 or higher on the exam were considered capable of providing informed consent. Potential subjects who scored between 20 and 23 were considered to be capable of providing consent if they were able to answer 4 questions about the study (why the study is being done, what will be required of them if they participate, how long they will be in the study, and how it will be determined if they will receive the investigational drug or the placebo).
Patients who met inclusion criteria were enrolled in the study. Subjects were randomized only if they developed signs of AWS sufficient to meet Diagnostic and Statistical Manual of Mental Disorders IV (DSMIV) criteria for AWS diagnosis, and reached at least a score of 11 (of a possible 67) on the CIWA‐Ar. All subjects received symptom‐triggered benzodiazepine treatment, and also were randomized to receive either baclofen (10 mg) or placebo every 8 hours (q8h) orally as inpatients for 72 hours or until discharge, whichever was shorter. Lorazepam was selected for the symptom‐triggered benzodiazepine treatment, as it has been used for managing AWS in the participating hospitals for several years. The initial research protocol called for 5 days of participation (15 doses of study drug), but we found that many subjects were discharged before the 5 days had elapsed after enrollment, and that compliance with follow‐up outpatient visits was poor. Accordingly, the protocol was amended to shorten the treatment period to 72 hours of participation (9 doses) or until discharge if prior to 72 hours, with the minimum observation period set to 72 hours.
Data Collection
Baseline data were collected at the time of enrollment, both from the patient and the medical record. Demographic data (age, gender, race) were obtained, as well as data on alcohol history, including approximate duration and intensity of alcohol use, and prior experience with AWS; data on comorbid conditions and medical history; and history of beta‐blocker use. During the period of observation following randomization, data were obtained on CIWA‐Ar scores; benzodiazepine doses administered; and adverse events, including use of sedatives in addition to benzodiazepines, use of restraints, use of intensive care, and clinical complications during the AWS course.
Study Procedures
The research pharmacy provided study medications (baclofen or placebo in identical form) for enrolled subjects. Subjects and study personnel were blinded to treatment group (baclofen vs placebo).
Nurses on inpatient units were provided with training in CIWA‐Ar assessment. All subjects were monitored for CIWA‐Ar scores at the time of study enrollment and for at least the next 72 hours. In monitoring the subjects, the nurses used the CIWA‐Ar protocol, in which subjects were assessed and potentially dosed with lorazepam hourly if their scores were 11 or higher. If the CIWA‐Ar score was less than 11, the subjects were assessed every 4 hours and at study discharge. CIWA‐AR results were reported as averaged over 8‐hour periods starting at study enrollment.
Data Analysis
Demographic and baseline variables with ordinal and continuous measurements, such as age, MMSE total score, and drinks per day were evaluated using group t test analysis, with two‐tailed significance estimates. Variables with prevalence reported were evaluated using the chi‐square test of significance. In accordance with the protocol, data from patients who had CIWA‐Ar assessments for at least 72 hours following randomization were included in the final study analyses. Repeated measures analysis of variance were conducted for the 2 treatment groups (baclofen and placebo), to evaluate mean CIWA‐Ar scores and mean lorazepam doses within each 8‐hour interval, as well as cumulative lorazepam dose. No covariates were included in the models. The last‐observation‐carried‐forward approach was used for those subjects who were missing CIWA‐Ar scores between baseline and their last CIWA‐Ar score. Doses for postdischarge patients without lorazepam prescriptions were set to 0 mg/8 hr. Cumulative lorazepam dose at 72 hours was also analyzed by defining the upper 25th percentile of existing doses (the upper 8 of 31) as high dose. This high‐dose lorazepam treatment level was determined to include all study participants receiving 20 mg or more of lorazepam during the first 72 hours. Fisher's exact test (two‐tailed) was then used to assess the difference in treatment group (baclofen vs placebo) for high‐dose lorazepam treatment.
RESULTS
Seventy‐nine subjects met study inclusion criteria, and provided informed consent for participation in the study. Of these, 44 subjects developed signs of AWS sufficient to meet DSMIV criteria for AWS diagnosis, and were randomized to receive either baclofen or placebo, in addition to benzodiazepine therapy. As summarized in Table 1, subjects who developed signs of AWS were similar to subjects who did not enter withdrawal, differing principally in that those who developed AWS reported more drinks per day, and more significant history of previous AWS.
| Withdrawal/Randomized | ||||
|---|---|---|---|---|
| No Withdrawal | All | Placebo | Baclofen | |
| Characteristic | (N = 35) | (N = 44) | (N = 19) | (N = 25) |
| ||||
| % Male | 82.9 | 84.1 | 94.7 | 76.0 |
| Age at admission (mean/SD) | 52.0/12.0 | 46.9/10.9 | 46.1/11.9 | 47.5/10.3 |
| MMSE total score (mean/SD) | 26.5/1.7 | 25.8/3.3 | 25.4/4.1 | 26.0/2.4 |
| Drinking history | ||||
| Age began drinking (mean/SD) | 16.7/4.2 | 16.2/4.3 | 15.5/4.5 | 16.7/4.2 |
| Years drinking (mean/SD) | 34.1/10.8 | 30.2/10.0 | 30.0/12.8 | 30.3/7.8 |
| Drinks per day (mean/SD)* | 11.2/8.8 | 16.3/9.7 | 14.4/7.8 | 18.0/11.0 |
| % Daily drinker | 65.7 | 64.1 | 57.9 | 70.0 |
| Days since last drink (mean/SD) | 1.5/0.9 | 1.3/1.3 | 1.0/0.8 | 1.6/1.5 |
| Medical history, % with history of | ||||
| Alcohol withdrawal syndrome | 60.6 | 87.5 | 87.5 | 87.5 |
| Seizures*, | 30.0 | 53.8 | 33.3 | 66.7 |
| DTs* | 48.3 | 74.3 | 80.0 | 70.0 |
| Medications, % at time of admission | ||||
| Alcohol treatment | 0.0 | 4.5 | 5.3 | 4.0 |
| Beta‐blocker | 31.4 | 25.0 | 26.3 | 24.0 |
| Sleep agent | 2.9 | 6.8 | 5.3 | 8.0 |
| Narcotic pain medication | 37.1 | 40.9 | 31.6 | 48.0 |
| Depakote | 0.0 | 4.5 | 0.0 | 8.0 |
| Benzodiazepine | 2.9 | 11.4 | 10.5 | 12.0 |
| Anti‐anxiety medication | 2.9 | 2.3 | 0.0 | 4.0 |
| Anti‐psychotic medication | 5.7 | 6.8 | 10.5 | 4.0 |
| Other psychiatric medication | 2.9 | 9.1 | 5.3 | 12.0 |
The 79 subjects who were enrolled were drawn from a population of 237 potential subjects who were screened for the study. The most common reasons that the 158 potential subjects were not enrolled were refusal (29.7%), low risk of withdrawal (19.0%), inability to provide consent (9.5%), and concurrent use of benzodiazepines (8.9%). The 15 patients who were unable to provide consent were those who scored 24 or lower on the MMSE, and did not have a surrogate decision‐maker available.
Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments, as summarized in Table 2. These assessments were completed either entirely as inpatients (24 subjects) or with inpatient assessments followed by outpatient assessments after discharge (7 subjects). Discharges prior to 72 hours occurred in 3 of the 18 subjects receiving baclofen, and in 4 of the 13 receiving placebo (odds ratio = 0.45, 95% CI = 0.082.49). Mean CIWA‐Ar scores for the 31 subjects who completed 72 hours of CIWA‐Ar assessments are presented in Figure 1.
| All | Placebo | Baclofen | |
|---|---|---|---|
| Characteristic | (N = 31) | (N = 13) | (N = 18) |
| |||
| % Male | 87.1 | 92.3 | 83.3 |
| Age at admission (mean/SD) | 47.5/10.2 | 45.7/9.3 | 48.7/10.9 |
| MMSE total score (mean/SD) | 26.3/2.2 | 26.3/1.8 | 26.3/2.6 |
| Charlson Comorbidity Score | 1.0/1.1 | 1.1/0.9 | 1.0/1.3 |
| Drinking history | |||
| Age began drinking (mean/SD) | 16.7/4.6 | 16.1/4.3 | 17.1/5.0 |
| Years drinking (mean/SD) | 29.8/8.6 | 29.5/10.0 | 30.0/7.9 |
| Drinks per day (mean/SD) | 16.0/9.9 | 12.9/8.4 | 18.6/10.5 |
| % Daily drinker | 58.1 | 53.8 | 78.6 |
| Days since last drink (mean/SD) | 1.2/0.9 | 1.1/0.8 | 1.3/1.1 |
| Current hospitalization, primary diagnosis | |||
| Alcohol withdrawal syndrome/alcoholism | 48.4 | 46.2 | 50.0 |
| Probably related to alcoholism* | 35.5 | 38.5 | 33.3 |
| Other | 16.1 | 15.4 | 16.7 |
| Medical history, % with history of | |||
| Alcohol withdrawal syndrome | 89.7 | 83.3 | 94.1 |
| Seizures | 60.7 | 45.5 | 70.6 |
| DTs | 79.2 | 80.0 | 78.6 |
| Medications, % at time of admission | |||
| Alcohol treatment | 6.5 | 7.7 | 5.6 |
| Beta‐blocker | 29.1 | 23.1 | 33.3 |
| Sleep agent | 6.5 | 7.7 | 5.6 |
| Narcotic pain medication | 45.2 | 30.8 | 55.6 |
| Depakote | 6.5 | 0.0 | 11.1 |
| Benzodiazepine | 9.7 | 15.4 | 5.6 |
| Anti‐anxiety medication | 3.2 | 0.0 | 5.6 |
| Anti‐psychotic medication | 9.7 | 15.4 | 5.6 |
| Other psychiatric medication | 6.5 | 7.7 | 5.6 |

Figures 2 and 3 summarize the mean lorazepam doses in each 8‐hour period, and the cumulative lorazepam doses for the subjects in the 2 arms of the study, respectively. The cumulative dose of lorazepam administered to the 31 subjects ranged from 0 to 1035 mg in the 72 hours following randomization, with a range of 1 to 1035 mg in the placebo group and 0 to 39 mg in the baclofen group. The 8 subjects who received the highest doses of lorazepam (20 mg or more) included 1 of the 18 subjects who received baclofen and 7 of the 13 subjects who received placebo (P = 0.004). Only 4 subjects required >50 mg of lorazepam over the 72 hours; all 4 of these were patients in the placebo group (P = 0.023).


Subjects who received baclofen and subjects who received placebo did not differ significantly in regard to the use of sedatives other than benzodiazepines, the use of restraints, the use of intensive care, or other clinical complications. On the basis this analysis, the study's primary null hypothesis was not rejected (the CIWA‐Ar scores in the baclofen and placebo groups were not different), but the secondary null hypothesis was rejected (baclofen was associated with lower likelihood of the use of high doses of benzodiazepines).
DISCUSSION
Benzodiazepines are effective drugs in the treatment of alcohol withdrawal syndrome. They remain the gold standard of treatment. The most effective method of administering benzodiazepines to acutely withdrawing patients has been shown to be variable dosing, based on withdrawal symptoms.
Our small study of acutely withdrawing inpatients confirmed that benzodiazepines, administered at frequencies and doses dependent on AWS symptoms, work well to control CIWA‐Ar scores over a relatively short time span. Our study also demonstrated that the addition of the GABA‐B agonist baclofen orally, at a fixed dose of 30 mg daily, will allow the same level of AWS symptom control, while reducing the risk that high doses of benzodiazepines will be needed to achieve that control. Reducing the use of high‐dose benzodiazepines has the potential to improve patient safety. In addition, since the frequency of nursing assessments parallel CIWA‐AR scores and benzodiazepine dosing frequency, using oral baclofen in this setting has the potential to decrease the nursing time required to control withdrawal symptoms.
A larger study of AWS will be needed to assess the role of baclofen in managing the frequency and severity of the complications of AWS, such as prolonged sedation, intensive care admission, and ventilator days. The current study was not powered to assess differences in the frequency of relatively rare events, and we excluded patients who required intravenous benzodiazepines for AWS symptom management. However, in light of the well‐documented risk of sedation and respiratory depression from high‐dose benzodiazepines, our findings suggest that a larger study of the role of baclofen in AWS management is warranted.
Either baclofen or benzodiazepines may have severe adverse effects in high doses. In this study, we used a low, fixed dose of baclofen (10 mg every 8 hours), a level at which severe side effects such as respiratory depression are uncommon. Our principal finding was that the use of low‐dose baclofen is associated with reduced use of high‐dose benzodiazepines in some AWS patients.
Further Research
The use of baclofen and other adjunctive treatments in the management of AWS and other alcohol dependency syndromes warrants future study. If the benzodiazepine‐sparing effects of baclofen in AWS management are confirmed in additional studies, baclofen may become an important adjunct to benzodiazepines in AWS management, particularly in settings where the use of symptom‐triggered therapy is difficult.
It is difficult to predict which suddenly abstinent alcoholics will experience severe AWS. In the current study, 44 of the 79 patients judged by experienced clinicians to be at high risk for acute AWS reached the CIWA‐Ar threshold (a score of 11 or more) to be randomized. We found that those who experienced significant withdrawal were younger, drank more heavily, and had more prior experience with severe AWS, which is consistent with earlier studies.23, 24 Nevertheless, patient history is not a reliable predictor of risk for AWS; clinicians are often obliged to watch and wait until clinical signs of AWS develop. In light of the growing evidence that baclofen alleviates many symptoms of alcohol dependence, both in patients with AWS and in those in recovery,19, 20, 2529 future research should also examine the role of baclofen in preventing AWS in at‐risk patients.
Since ethanol has effects on several neurotransmitters and receptor systems, combinations of medications that modify GABA, glutamate, and adrenergic activity in low doses may be more effective and safer in managing AWS than using high doses of a single agent. Future research should seek to identify the most effective combination of low‐dose medications in managing AWS.
Study Limitations
This study was subject to several significant limitations. With a small study population (31 subjects), the experiences of individual subjects had a strong effect on the findings (such as the dip in mean lorazepam doses for placebo subjects in Figure 2, which was due to a high‐dose subject sleeping through the observation period). However, the overall finding of the lorazepam‐sparing effect of baclofen was consistent. We excluded patients who required intravenous benzodiazepines for AWS management, and so our study did not include the most severe AWS subjects. However, all of our subjects showed signs of mild‐to‐moderate alcohol withdrawal upon enrollment (CIWA‐Ar scores of 11 or more upon randomization), and all were at risk for more severe AWS; many went on to much higher CIWA‐AR scores during the course of their AWS. Of the 44 subjects who were randomized, 13 did not complete the study; the impact that these subjects might have had on the findings is unknown. However, the subjects who did not complete the study (baclofen 54%, placebo 46%) did not differ from the remaining subjects in regard to any of the variables used in the study. More baclofen‐treated than placebo‐treated subjects were taking narcotics and/or beta‐blockers at the time of enrollment, although these differences were not statistically significant, and their impact upon our findings are unknown. This study was conducted in northeast Minnesota; the study population reflected the limited diversity of the region. Caution should be used in generalizing the findings to other populations.
CONCLUSION
These findings suggest that baclofen may have potential as an adjunct in the management of acute alcohol withdrawal.
In the United States, unhealthy alcohol use affects medical care on several levels. The prevalence of alcohol problems is 7%20% or higher among outpatients,1 30%40% among emergency room patients, and 50% among patients with trauma.13 In 2006, approximately 430,000 hospital discharges in the United States were for persons with a principal (first‐listed) alcohol‐related diagnosis, and 1.7 million discharges listed at least one alcohol‐related diagnosis, representing 1.3% and 5.3% of all hospital discharges, respectively.4 Alcoholic psychosis (34.5%) and alcohol dependence syndrome (29.5%) together accounted for the majority of principal alcohol‐related diagnoses.4 Additionally, many patients hospitalized for other indications are susceptible to withdrawal symptoms due to physiological habituation to alcohol. Abrupt cessation of alcohol intake causes habituated drinkers to experience symptoms of alcohol withdrawal syndrome (AWS), which significantly increases intensity and cost of care. Trauma patients who develop AWS were found to have increased morbidity, more intensive care and ventilator days, and higher hospital costs than trauma patients without AWS.5 Unfortunately, attempts to develop predictive models to accurately forecast the likelihood of developing severe AWS in an individual case have been modestly successful at best.68
Regimens used to treat AWS have evolved over time, taking advantage of advances in the understanding of addiction neurophysiology. There is no specific ethanol receptor.9 Much of alcohol's acute effects on the central nervous system are mediated by its stimulation of the gamma‐aminobutyric acid (GABA) system, which is neuroinhibitory.10 Chronic alcohol use leads to habituation partly by inducing configuration changes of GABA‐A receptor subunits. This renders the GABA‐A receptor less sensitive to alcohol, barbiturates, and benzodiazepines.11 Although both GABA‐A and GABA‐B receptor activation cause increased GABA neuronal output, the GABA‐A receptor is rendered relatively less sensitive by chronic exposure to alcohol. Baclofen is a pure GABA‐B receptor agonist,12 and its GABA‐B stimulatory effect is maintained even in habituated alcoholics.13, 14 The absence of cross‐tolerance between baclofen and ethanol suggests that low doses of baclofen may be helpful in the management of AWS.
Currently, AWS is usually managed with benzodiazepines, using variable dosing depending on the severity of withdrawal symptoms. Such symptom‐triggered treatment is generally preferred over fixed‐dose regimens,15 in part because when using this method, many cases of AWS can be managed with less medication. Benzodiazepine regimens using high doses have been found to be associated with substantial morbidity and prolonged hospitalizations.16, 17
In a series of small studies, Addolorato's research team has reported decreases in AWS symptoms in association with the use of low doses of baclofen in an outpatient population,18 and has found baclofen to be associated with reduced alcohol craving in the long‐term management of alcohol dependence.11, 19, 20 Addolorato and colleagues' studies of baclofen in relieving AWS symptoms prompted our group to apply the use of baclofen in a larger group of inpatients with AWS.11, 18, 21 We conducted this study to improve understanding of the role of baclofen in the management of acute AWS in an inpatient population of subjects at risk for AWS, drawn from general hospital admissions.
Our primary null hypothesis was that Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in acutely withdrawing alcoholic patients are equal in baclofen‐treated and placebo‐treated subject groups. Our secondary null hypothesis was that benzodiazepine doses used to treat acutely withdrawing alcoholic patients are equal in the baclofen‐treated and placebo‐treated groups.
METHODS
The protocol for this study was approved by the Essentia Health Institutional Review Board, Duluth, Minnesota.
This was a randomized, placebo‐controlled, double‐blind trial. Subjects were recruited from among patients who were admitted to 1 of 2 regional general hospitals in Duluth, Minnesota (St. Mary's Medical Center or Miller‐Dwan Medical Center) and who were identified by clinical staff as being at risk for AWS. Potential subjects were not required to have an alcohol‐related condition as their primary reason for admission, but were required to have a history of AWS or of alcohol use suggestive of significant risk for AWS, and to be able to provide informed consent, as described below.
Patients were not eligible for enrollment in the study if they had other active drug dependence in addition to alcohol; were using baclofen at the time of study enrollment; were using benzodiazepines chronically at the time of study enrollment; had a known baclofen or benzodiazepine sensitivity; were unable to take oral medications; were pregnant or breast‐feeding; had a serum creatinine level 2.0; had a history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; or were unable to complete the consenting procedures.
Consenting and Enrollment Procedures
Patients who were identified by clinical staff as being at high risk for AWS were approached for possible enrollment in the study. Potential subjects who met other inclusion criteria were screened to assess their mental status with the Mini‐Mental Status Exam (MMSE), using methods developed for subjects with cognitive impairment.22 Potential subjects who scored 24 of a possible score of 30 or higher on the exam were considered capable of providing informed consent. Potential subjects who scored between 20 and 23 were considered to be capable of providing consent if they were able to answer 4 questions about the study (why the study is being done, what will be required of them if they participate, how long they will be in the study, and how it will be determined if they will receive the investigational drug or the placebo).
Patients who met inclusion criteria were enrolled in the study. Subjects were randomized only if they developed signs of AWS sufficient to meet Diagnostic and Statistical Manual of Mental Disorders IV (DSMIV) criteria for AWS diagnosis, and reached at least a score of 11 (of a possible 67) on the CIWA‐Ar. All subjects received symptom‐triggered benzodiazepine treatment, and also were randomized to receive either baclofen (10 mg) or placebo every 8 hours (q8h) orally as inpatients for 72 hours or until discharge, whichever was shorter. Lorazepam was selected for the symptom‐triggered benzodiazepine treatment, as it has been used for managing AWS in the participating hospitals for several years. The initial research protocol called for 5 days of participation (15 doses of study drug), but we found that many subjects were discharged before the 5 days had elapsed after enrollment, and that compliance with follow‐up outpatient visits was poor. Accordingly, the protocol was amended to shorten the treatment period to 72 hours of participation (9 doses) or until discharge if prior to 72 hours, with the minimum observation period set to 72 hours.
Data Collection
Baseline data were collected at the time of enrollment, both from the patient and the medical record. Demographic data (age, gender, race) were obtained, as well as data on alcohol history, including approximate duration and intensity of alcohol use, and prior experience with AWS; data on comorbid conditions and medical history; and history of beta‐blocker use. During the period of observation following randomization, data were obtained on CIWA‐Ar scores; benzodiazepine doses administered; and adverse events, including use of sedatives in addition to benzodiazepines, use of restraints, use of intensive care, and clinical complications during the AWS course.
Study Procedures
The research pharmacy provided study medications (baclofen or placebo in identical form) for enrolled subjects. Subjects and study personnel were blinded to treatment group (baclofen vs placebo).
Nurses on inpatient units were provided with training in CIWA‐Ar assessment. All subjects were monitored for CIWA‐Ar scores at the time of study enrollment and for at least the next 72 hours. In monitoring the subjects, the nurses used the CIWA‐Ar protocol, in which subjects were assessed and potentially dosed with lorazepam hourly if their scores were 11 or higher. If the CIWA‐Ar score was less than 11, the subjects were assessed every 4 hours and at study discharge. CIWA‐AR results were reported as averaged over 8‐hour periods starting at study enrollment.
Data Analysis
Demographic and baseline variables with ordinal and continuous measurements, such as age, MMSE total score, and drinks per day were evaluated using group t test analysis, with two‐tailed significance estimates. Variables with prevalence reported were evaluated using the chi‐square test of significance. In accordance with the protocol, data from patients who had CIWA‐Ar assessments for at least 72 hours following randomization were included in the final study analyses. Repeated measures analysis of variance were conducted for the 2 treatment groups (baclofen and placebo), to evaluate mean CIWA‐Ar scores and mean lorazepam doses within each 8‐hour interval, as well as cumulative lorazepam dose. No covariates were included in the models. The last‐observation‐carried‐forward approach was used for those subjects who were missing CIWA‐Ar scores between baseline and their last CIWA‐Ar score. Doses for postdischarge patients without lorazepam prescriptions were set to 0 mg/8 hr. Cumulative lorazepam dose at 72 hours was also analyzed by defining the upper 25th percentile of existing doses (the upper 8 of 31) as high dose. This high‐dose lorazepam treatment level was determined to include all study participants receiving 20 mg or more of lorazepam during the first 72 hours. Fisher's exact test (two‐tailed) was then used to assess the difference in treatment group (baclofen vs placebo) for high‐dose lorazepam treatment.
RESULTS
Seventy‐nine subjects met study inclusion criteria, and provided informed consent for participation in the study. Of these, 44 subjects developed signs of AWS sufficient to meet DSMIV criteria for AWS diagnosis, and were randomized to receive either baclofen or placebo, in addition to benzodiazepine therapy. As summarized in Table 1, subjects who developed signs of AWS were similar to subjects who did not enter withdrawal, differing principally in that those who developed AWS reported more drinks per day, and more significant history of previous AWS.
| Withdrawal/Randomized | ||||
|---|---|---|---|---|
| No Withdrawal | All | Placebo | Baclofen | |
| Characteristic | (N = 35) | (N = 44) | (N = 19) | (N = 25) |
| ||||
| % Male | 82.9 | 84.1 | 94.7 | 76.0 |
| Age at admission (mean/SD) | 52.0/12.0 | 46.9/10.9 | 46.1/11.9 | 47.5/10.3 |
| MMSE total score (mean/SD) | 26.5/1.7 | 25.8/3.3 | 25.4/4.1 | 26.0/2.4 |
| Drinking history | ||||
| Age began drinking (mean/SD) | 16.7/4.2 | 16.2/4.3 | 15.5/4.5 | 16.7/4.2 |
| Years drinking (mean/SD) | 34.1/10.8 | 30.2/10.0 | 30.0/12.8 | 30.3/7.8 |
| Drinks per day (mean/SD)* | 11.2/8.8 | 16.3/9.7 | 14.4/7.8 | 18.0/11.0 |
| % Daily drinker | 65.7 | 64.1 | 57.9 | 70.0 |
| Days since last drink (mean/SD) | 1.5/0.9 | 1.3/1.3 | 1.0/0.8 | 1.6/1.5 |
| Medical history, % with history of | ||||
| Alcohol withdrawal syndrome | 60.6 | 87.5 | 87.5 | 87.5 |
| Seizures*, | 30.0 | 53.8 | 33.3 | 66.7 |
| DTs* | 48.3 | 74.3 | 80.0 | 70.0 |
| Medications, % at time of admission | ||||
| Alcohol treatment | 0.0 | 4.5 | 5.3 | 4.0 |
| Beta‐blocker | 31.4 | 25.0 | 26.3 | 24.0 |
| Sleep agent | 2.9 | 6.8 | 5.3 | 8.0 |
| Narcotic pain medication | 37.1 | 40.9 | 31.6 | 48.0 |
| Depakote | 0.0 | 4.5 | 0.0 | 8.0 |
| Benzodiazepine | 2.9 | 11.4 | 10.5 | 12.0 |
| Anti‐anxiety medication | 2.9 | 2.3 | 0.0 | 4.0 |
| Anti‐psychotic medication | 5.7 | 6.8 | 10.5 | 4.0 |
| Other psychiatric medication | 2.9 | 9.1 | 5.3 | 12.0 |
The 79 subjects who were enrolled were drawn from a population of 237 potential subjects who were screened for the study. The most common reasons that the 158 potential subjects were not enrolled were refusal (29.7%), low risk of withdrawal (19.0%), inability to provide consent (9.5%), and concurrent use of benzodiazepines (8.9%). The 15 patients who were unable to provide consent were those who scored 24 or lower on the MMSE, and did not have a surrogate decision‐maker available.
Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments, as summarized in Table 2. These assessments were completed either entirely as inpatients (24 subjects) or with inpatient assessments followed by outpatient assessments after discharge (7 subjects). Discharges prior to 72 hours occurred in 3 of the 18 subjects receiving baclofen, and in 4 of the 13 receiving placebo (odds ratio = 0.45, 95% CI = 0.082.49). Mean CIWA‐Ar scores for the 31 subjects who completed 72 hours of CIWA‐Ar assessments are presented in Figure 1.
| All | Placebo | Baclofen | |
|---|---|---|---|
| Characteristic | (N = 31) | (N = 13) | (N = 18) |
| |||
| % Male | 87.1 | 92.3 | 83.3 |
| Age at admission (mean/SD) | 47.5/10.2 | 45.7/9.3 | 48.7/10.9 |
| MMSE total score (mean/SD) | 26.3/2.2 | 26.3/1.8 | 26.3/2.6 |
| Charlson Comorbidity Score | 1.0/1.1 | 1.1/0.9 | 1.0/1.3 |
| Drinking history | |||
| Age began drinking (mean/SD) | 16.7/4.6 | 16.1/4.3 | 17.1/5.0 |
| Years drinking (mean/SD) | 29.8/8.6 | 29.5/10.0 | 30.0/7.9 |
| Drinks per day (mean/SD) | 16.0/9.9 | 12.9/8.4 | 18.6/10.5 |
| % Daily drinker | 58.1 | 53.8 | 78.6 |
| Days since last drink (mean/SD) | 1.2/0.9 | 1.1/0.8 | 1.3/1.1 |
| Current hospitalization, primary diagnosis | |||
| Alcohol withdrawal syndrome/alcoholism | 48.4 | 46.2 | 50.0 |
| Probably related to alcoholism* | 35.5 | 38.5 | 33.3 |
| Other | 16.1 | 15.4 | 16.7 |
| Medical history, % with history of | |||
| Alcohol withdrawal syndrome | 89.7 | 83.3 | 94.1 |
| Seizures | 60.7 | 45.5 | 70.6 |
| DTs | 79.2 | 80.0 | 78.6 |
| Medications, % at time of admission | |||
| Alcohol treatment | 6.5 | 7.7 | 5.6 |
| Beta‐blocker | 29.1 | 23.1 | 33.3 |
| Sleep agent | 6.5 | 7.7 | 5.6 |
| Narcotic pain medication | 45.2 | 30.8 | 55.6 |
| Depakote | 6.5 | 0.0 | 11.1 |
| Benzodiazepine | 9.7 | 15.4 | 5.6 |
| Anti‐anxiety medication | 3.2 | 0.0 | 5.6 |
| Anti‐psychotic medication | 9.7 | 15.4 | 5.6 |
| Other psychiatric medication | 6.5 | 7.7 | 5.6 |

Figures 2 and 3 summarize the mean lorazepam doses in each 8‐hour period, and the cumulative lorazepam doses for the subjects in the 2 arms of the study, respectively. The cumulative dose of lorazepam administered to the 31 subjects ranged from 0 to 1035 mg in the 72 hours following randomization, with a range of 1 to 1035 mg in the placebo group and 0 to 39 mg in the baclofen group. The 8 subjects who received the highest doses of lorazepam (20 mg or more) included 1 of the 18 subjects who received baclofen and 7 of the 13 subjects who received placebo (P = 0.004). Only 4 subjects required >50 mg of lorazepam over the 72 hours; all 4 of these were patients in the placebo group (P = 0.023).


Subjects who received baclofen and subjects who received placebo did not differ significantly in regard to the use of sedatives other than benzodiazepines, the use of restraints, the use of intensive care, or other clinical complications. On the basis this analysis, the study's primary null hypothesis was not rejected (the CIWA‐Ar scores in the baclofen and placebo groups were not different), but the secondary null hypothesis was rejected (baclofen was associated with lower likelihood of the use of high doses of benzodiazepines).
DISCUSSION
Benzodiazepines are effective drugs in the treatment of alcohol withdrawal syndrome. They remain the gold standard of treatment. The most effective method of administering benzodiazepines to acutely withdrawing patients has been shown to be variable dosing, based on withdrawal symptoms.
Our small study of acutely withdrawing inpatients confirmed that benzodiazepines, administered at frequencies and doses dependent on AWS symptoms, work well to control CIWA‐Ar scores over a relatively short time span. Our study also demonstrated that the addition of the GABA‐B agonist baclofen orally, at a fixed dose of 30 mg daily, will allow the same level of AWS symptom control, while reducing the risk that high doses of benzodiazepines will be needed to achieve that control. Reducing the use of high‐dose benzodiazepines has the potential to improve patient safety. In addition, since the frequency of nursing assessments parallel CIWA‐AR scores and benzodiazepine dosing frequency, using oral baclofen in this setting has the potential to decrease the nursing time required to control withdrawal symptoms.
A larger study of AWS will be needed to assess the role of baclofen in managing the frequency and severity of the complications of AWS, such as prolonged sedation, intensive care admission, and ventilator days. The current study was not powered to assess differences in the frequency of relatively rare events, and we excluded patients who required intravenous benzodiazepines for AWS symptom management. However, in light of the well‐documented risk of sedation and respiratory depression from high‐dose benzodiazepines, our findings suggest that a larger study of the role of baclofen in AWS management is warranted.
Either baclofen or benzodiazepines may have severe adverse effects in high doses. In this study, we used a low, fixed dose of baclofen (10 mg every 8 hours), a level at which severe side effects such as respiratory depression are uncommon. Our principal finding was that the use of low‐dose baclofen is associated with reduced use of high‐dose benzodiazepines in some AWS patients.
Further Research
The use of baclofen and other adjunctive treatments in the management of AWS and other alcohol dependency syndromes warrants future study. If the benzodiazepine‐sparing effects of baclofen in AWS management are confirmed in additional studies, baclofen may become an important adjunct to benzodiazepines in AWS management, particularly in settings where the use of symptom‐triggered therapy is difficult.
It is difficult to predict which suddenly abstinent alcoholics will experience severe AWS. In the current study, 44 of the 79 patients judged by experienced clinicians to be at high risk for acute AWS reached the CIWA‐Ar threshold (a score of 11 or more) to be randomized. We found that those who experienced significant withdrawal were younger, drank more heavily, and had more prior experience with severe AWS, which is consistent with earlier studies.23, 24 Nevertheless, patient history is not a reliable predictor of risk for AWS; clinicians are often obliged to watch and wait until clinical signs of AWS develop. In light of the growing evidence that baclofen alleviates many symptoms of alcohol dependence, both in patients with AWS and in those in recovery,19, 20, 2529 future research should also examine the role of baclofen in preventing AWS in at‐risk patients.
Since ethanol has effects on several neurotransmitters and receptor systems, combinations of medications that modify GABA, glutamate, and adrenergic activity in low doses may be more effective and safer in managing AWS than using high doses of a single agent. Future research should seek to identify the most effective combination of low‐dose medications in managing AWS.
Study Limitations
This study was subject to several significant limitations. With a small study population (31 subjects), the experiences of individual subjects had a strong effect on the findings (such as the dip in mean lorazepam doses for placebo subjects in Figure 2, which was due to a high‐dose subject sleeping through the observation period). However, the overall finding of the lorazepam‐sparing effect of baclofen was consistent. We excluded patients who required intravenous benzodiazepines for AWS management, and so our study did not include the most severe AWS subjects. However, all of our subjects showed signs of mild‐to‐moderate alcohol withdrawal upon enrollment (CIWA‐Ar scores of 11 or more upon randomization), and all were at risk for more severe AWS; many went on to much higher CIWA‐AR scores during the course of their AWS. Of the 44 subjects who were randomized, 13 did not complete the study; the impact that these subjects might have had on the findings is unknown. However, the subjects who did not complete the study (baclofen 54%, placebo 46%) did not differ from the remaining subjects in regard to any of the variables used in the study. More baclofen‐treated than placebo‐treated subjects were taking narcotics and/or beta‐blockers at the time of enrollment, although these differences were not statistically significant, and their impact upon our findings are unknown. This study was conducted in northeast Minnesota; the study population reflected the limited diversity of the region. Caution should be used in generalizing the findings to other populations.
CONCLUSION
These findings suggest that baclofen may have potential as an adjunct in the management of acute alcohol withdrawal.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,, et al.Patients with alcohol problems in the emergency department, part 1: improving detection. SAEM Substance Abuse Task Force. Society for Academic Emergency Medicine.Acad Emerg Med.1998;5(12):1200–1209.
- ,,.Screening for alcohol problems in primary care: a systematic review.Arch Intern Med.2000;160(13):1977–1989.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Arlington, VA:National Institute on Alcohol Abuse and Alcoholism; August2008.
- ,,, et al.Alcohol withdrawal syndrome: turning minor injuries into a major problem.J Trauma.2006;61(6):1441–1446.
- ,,,,,.An assessment of the potential value of elevated homocysteine in predicting alcohol‐withdrawal seizures.Epilepsia.2006;47(5):934–938.
- ,.Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients.Alcohol Alcohol.2005;40(6):515–519.
- .A model for predicting alcohol withdrawal delirium.Psychiatr Serv.2001;52(6):820–823.
- .[GABA‐system and alcohol: does an “ethanol receptor” exist?] [review].Zh Nevrol Psikhiatr Im S S Korsakova.2007;Suppl 1:56–62.
- ,,, et al.Identification of molecular targets associated with ethanol toxicity and implications in drug development.Curr Pharm Des.2010;16(11):1313–1355.
- ,,,,.Baclofen: a new drug for the treatment of alcohol dependence.Int J Clin Pract.2006;60(8):1003–1008.
- ,.3H‐baclofen and 3H‐GABA bind to bicuculline‐insensitive GABA B sites in rat brain.Nature.1981;290(5802):149–152.
- ,.Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice.Psychopharmacology (Berl).1999;141(2):197–205.
- .Cerebral GABA receptors.Alcohol Alcohol Suppl.1994;2:181–186.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal.Arch Intern Med.2002;162(10):1117–1121.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacother.2001;21(9):1140–1144.
- ,,,,,.The use of continuous IV sedation is associated with prolongation of mechanical ventilation.Chest.1998;114:541–548.
- ,,, et al.Rapid suppression of alcohol withdrawal syndrome by baclofen.Am J Med.2002;112(3):226–229.
- ,,,,,.Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence.Alcohol Clin Exp Res.2000;24(1):67–71.
- ,,, et al.Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study.Alcohol Alcohol.2002;37(5):504–508.
- ,,, et al.Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam.Am J Med.2006;119:276.e213–276.e218.
- ,,,,.Relationship between Alzheimer's disease severity and patient participation in decisions about their care.J Geriatr Psychiatry Neurol.2002;15(2):68–72.
- ,,.Impact of age on the severity, course, and complications of alcohol withdrawal.Arch Intern Med.1997;157(19):2234–2241.
- ,,.Independent clinical correlates of severe alcohol withdrawal.Substance Abuse.2003;24(4):197–209.
- ,,, et al.Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind study.Lancet.2007;370:1915–1922.
- ,,, et al.Baclofen for alcohol dependence: a preliminary open‐label study.Alcohol Clin Exp Res.2004;28(10):1517–1523.
- ,,,.[Pharmacological treatment options for prevention of alcohol relapse].Fortschr Neurol Psychiatr.2008;76(7):421–428.
- ,.Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms.Pharmacol Ther.2006;111:855–876.
- ,.Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review.J Okla State Med Assoc.2007;100(9):354–360.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,, et al.Patients with alcohol problems in the emergency department, part 1: improving detection. SAEM Substance Abuse Task Force. Society for Academic Emergency Medicine.Acad Emerg Med.1998;5(12):1200–1209.
- ,,.Screening for alcohol problems in primary care: a systematic review.Arch Intern Med.2000;160(13):1977–1989.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Arlington, VA:National Institute on Alcohol Abuse and Alcoholism; August2008.
- ,,, et al.Alcohol withdrawal syndrome: turning minor injuries into a major problem.J Trauma.2006;61(6):1441–1446.
- ,,,,,.An assessment of the potential value of elevated homocysteine in predicting alcohol‐withdrawal seizures.Epilepsia.2006;47(5):934–938.
- ,.Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients.Alcohol Alcohol.2005;40(6):515–519.
- .A model for predicting alcohol withdrawal delirium.Psychiatr Serv.2001;52(6):820–823.
- .[GABA‐system and alcohol: does an “ethanol receptor” exist?] [review].Zh Nevrol Psikhiatr Im S S Korsakova.2007;Suppl 1:56–62.
- ,,, et al.Identification of molecular targets associated with ethanol toxicity and implications in drug development.Curr Pharm Des.2010;16(11):1313–1355.
- ,,,,.Baclofen: a new drug for the treatment of alcohol dependence.Int J Clin Pract.2006;60(8):1003–1008.
- ,.3H‐baclofen and 3H‐GABA bind to bicuculline‐insensitive GABA B sites in rat brain.Nature.1981;290(5802):149–152.
- ,.Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice.Psychopharmacology (Berl).1999;141(2):197–205.
- .Cerebral GABA receptors.Alcohol Alcohol Suppl.1994;2:181–186.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal.Arch Intern Med.2002;162(10):1117–1121.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacother.2001;21(9):1140–1144.
- ,,,,,.The use of continuous IV sedation is associated with prolongation of mechanical ventilation.Chest.1998;114:541–548.
- ,,, et al.Rapid suppression of alcohol withdrawal syndrome by baclofen.Am J Med.2002;112(3):226–229.
- ,,,,,.Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence.Alcohol Clin Exp Res.2000;24(1):67–71.
- ,,, et al.Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study.Alcohol Alcohol.2002;37(5):504–508.
- ,,, et al.Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam.Am J Med.2006;119:276.e213–276.e218.
- ,,,,.Relationship between Alzheimer's disease severity and patient participation in decisions about their care.J Geriatr Psychiatry Neurol.2002;15(2):68–72.
- ,,.Impact of age on the severity, course, and complications of alcohol withdrawal.Arch Intern Med.1997;157(19):2234–2241.
- ,,.Independent clinical correlates of severe alcohol withdrawal.Substance Abuse.2003;24(4):197–209.
- ,,, et al.Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind study.Lancet.2007;370:1915–1922.
- ,,, et al.Baclofen for alcohol dependence: a preliminary open‐label study.Alcohol Clin Exp Res.2004;28(10):1517–1523.
- ,,,.[Pharmacological treatment options for prevention of alcohol relapse].Fortschr Neurol Psychiatr.2008;76(7):421–428.
- ,.Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms.Pharmacol Ther.2006;111:855–876.
- ,.Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review.J Okla State Med Assoc.2007;100(9):354–360.
Copyright © 2011 Society of Hospital Medicine
Too Many Cooks in the Kitchen?
The tension between continuity of care and specialization is not new, but may have reached a tipping point when the hospitalist movement erupted onto the American medical scene in the late 1990s. By definition, when a hospitalist cares for an inpatient, there is some fragmentation of care, which is, at least in theory, avoidableif the primary care provider (PCP) can serve as attending physician in the hospital. Literature has since emerged suggesting that clinical and economic outcomes of care by hospitalists are at least as good as that provided by PCPs, and that patients are not, in general, opposed to hospitalist care.13
However, the degree of discontinuity is not just a feature of whether a hospitalist assumes care of the hospitalized patient. Discontinuity can be exacerbated by changing attendings throughout the hospital stay. And inpatient continuity is a potential issue for both the hospitalist model and traditional model of care (in which the PCP serves as inpatient attending physician). While one might assume that the hospitalist model fosters more inpatient discontinuity because most hospitalistswhether working a 7‐on7‐off schedule or another scheduledo not commit to caring for a patient throughout an entire hospitalization the way a PCP might, this question has not previously been examined. Even if the hospitalist model is a fait accompli in many hospitals, it is worth knowing how inpatient continuity differs between the 2 models.
In this issue of the Journal, Fletcher and colleagues4 used billing data to examine trends in inpatient continuity of care over a 10‐year period ending in 2006, and sought to determine: (1) whether inpatient care has become more fragmented over time (as defined by the number of generalists caring for a patient over the course of an average hospitalization), and (2) whether inpatient care provided by hospitalists tends to be more fragmented than care provided by PCPs. They found that continuity of inpatient care has indeed decreased over time. In 1996, just over 70% of patients received care from 1 generalist; this number declined to just under 60% a decade later, despite a decrease in length‐of‐stay during that period. However, and perhaps surprisingly, patients cared for exclusively by hospitalists saw fewer generalists in the hospital (ie, fewer different hospitalists) than those cared for exclusively by outpatient providers. The authors conclude that the doctorpatient continuity over the course of a hospital stay is not worse in the hospitalist model than in the traditional model. While reassuring, it is important to remember that the patient experience does not begin at admission or end at discharge, and a more patient‐centered analysis might take into account the outpatient providers too (ie, those seeing the patient before admission and after discharge), and would probably show that the hospitalist model indeed leads to more care fragmentation. After all, there are at least 2 providers involved in every patient's care when a hospitalist model is used, whereas a large subset of patients cared for by PCPs would have only 1 provider involved.
While not the primary focus of the analysis, Fletcher and colleagues4 identified additional predictors of inpatient continuity of care. Higher socioeconomic class and white race were associated with lower continuity. This suggests that care fragmentation is not a feature of inferior, or at least cheap, care. In keeping with this observation, there was also enormous geographic variation in inpatient care continuity, marked by greater fragmentation of care in the New England and the mid‐Atlantic regions than in other areas of the country, and more fragmentation in larger hospitals serving heavily populated metropolitan areas. This pattern is strikingly similar to the cost‐of‐care patterns observed by the Dartmouth Atlas researchers.5, 6 Densely populated areas tend to have more specialists per capita and also tend to deliver more expensive carewithout demonstrably higher quality. In parallel, it is easy to see how care fragmentation might increase length‐of‐stay7 and lead to excessive diagnostic testing and consultation. More cooks in the kitchen might make costlier stew.
How hospitalists tackle the issue of inpatient continuity is not only a matter of quality of care, but also a matter of job sustainability. The simple way to maximize continuityworking many consecutive dayscan lead to burnout if taken too far. But there are creative ways to assign admissions that maximize continuity for the average inpatient while allowing providers needed time off. The CICLE initiative (Creating Incentives and Continuity Leading to Efficiency in hospital medicine) at the Johns Hopkins Bayview Medical Center, for instance, assigns physicians to 4‐day cycles of clinical work; the first day of the cycle (a long‐call day) involves admitting a large number of patients during a busy shift, with no new patients admitted on the remaining days of the cycle. Thus, all patients whose length of stay is less than 5 days will have a single attending‐of‐record. Not only does this model increase continuity, it also incentivizes providers to augment throughput: more discharged patients on Tuesday means fewer patients to see on Wednesday, without any expectation to backfill. Other less aggressive but similar approaches are used elsewhere, such as exempting hospitalists from accepting new patients on the last 1 or 2 of the consecutive days they work. We eagerly await data on the impact of these programs on quality of care, patient satisfaction, and provider satisfaction.
The impact of other providers and staff cannot be ignored. While the most important handoff in many cases may indeed be between the PCP and attending hospitalist tasked with coordinating the overall care of the patient, for some patients, there may be a specialist who has known the patient for years who is driving the plan of care. For patients with severe chronic illnesses, such as end‐stage renal disease or asthma, a well‐structured specialty clinic may even serve as a patient‐centered medical home.8 And the current inpatient team includes night coverage physicians (whether moonlighters, house staff, or covering hospitalists), and an ever‐increasing number of non‐physicians who play a critical role in hospital care (non‐physician providers, nurses, social workers, pharmacists, case managers, physical therapists, and others). While it is tempting to focus on the attending physician as the main driver of healthcare quality, continuity, and the inpatient experience, this is an oversimplification.
If there is a take‐home message, it is probably that most hospitalized patients will be cared for by multiple providers and a team of non‐physicians. The Marcus Welby practice model may not be completely dead, but if Dr. Welby were still in practice, it would be a safe bet that he would be slower at computerized order entry than the average intern, that financial pressures would make it hard for him to attend to his hospitalized patients, and that he probably would have turned over much of his inpatient practice to the physicians and non‐physician caregivers who make the hospital their primary workplace.9 Going forward, research should examine ways to optimize care coordination under the hospitalist model,1013 rather than comparing it to the traditional model of inpatient care. The ingredients for success include coordinated care by a committee of caregivers, effective handoffs (throughout hospitalization and at discharge),12, 14 focused and deliberate multidisciplinary communication, and effective patient education,15 regardless of the attending‐du‐jour.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,,.How physicians perceive hospitalist services after implementation: anticipation vs reality.Arch Intern Med.2003;163:2330–2336.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,,,.Trends in inpatient continuity of care for a cohort of Medicare patients, 1996–2006.J Hosp Med.2011;6:438–444.
- ,,,,,.The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care.Ann Intern Med.2003;138:288–298.
- ,,,,,.The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care.Ann Intern Med.2003;138:273–287.
- ,,,,.The impact of fragmentation of hospitalist care on length of stay.J Hosp Med.2010;5:335–338.
- ,.Specialists/subspecialists and the patient‐centered medical home.Chest.2010;137:200–204.
- ,.U.S. trends in hospitalization and generalist physician workforce and the emergence of hospitalists.J Gen Intern Med.2010;25:453–459.
- ,,,.Understanding communication during hospitalist service changes: a mixed methods study.J Hosp Med.2009;4:535–540.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,,,.Hospitalist handoffs: a systematic review and task force recommendations.J Hosp Med.2009;4:433–440.
- .The hospitalist field turns 15: new opportunities and challenges.J Hosp Med.2011;6:E1–E4.
- ,,, et al.Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Gen Intern Med.2009;24:971–976.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150:178–187.
The tension between continuity of care and specialization is not new, but may have reached a tipping point when the hospitalist movement erupted onto the American medical scene in the late 1990s. By definition, when a hospitalist cares for an inpatient, there is some fragmentation of care, which is, at least in theory, avoidableif the primary care provider (PCP) can serve as attending physician in the hospital. Literature has since emerged suggesting that clinical and economic outcomes of care by hospitalists are at least as good as that provided by PCPs, and that patients are not, in general, opposed to hospitalist care.13
However, the degree of discontinuity is not just a feature of whether a hospitalist assumes care of the hospitalized patient. Discontinuity can be exacerbated by changing attendings throughout the hospital stay. And inpatient continuity is a potential issue for both the hospitalist model and traditional model of care (in which the PCP serves as inpatient attending physician). While one might assume that the hospitalist model fosters more inpatient discontinuity because most hospitalistswhether working a 7‐on7‐off schedule or another scheduledo not commit to caring for a patient throughout an entire hospitalization the way a PCP might, this question has not previously been examined. Even if the hospitalist model is a fait accompli in many hospitals, it is worth knowing how inpatient continuity differs between the 2 models.
In this issue of the Journal, Fletcher and colleagues4 used billing data to examine trends in inpatient continuity of care over a 10‐year period ending in 2006, and sought to determine: (1) whether inpatient care has become more fragmented over time (as defined by the number of generalists caring for a patient over the course of an average hospitalization), and (2) whether inpatient care provided by hospitalists tends to be more fragmented than care provided by PCPs. They found that continuity of inpatient care has indeed decreased over time. In 1996, just over 70% of patients received care from 1 generalist; this number declined to just under 60% a decade later, despite a decrease in length‐of‐stay during that period. However, and perhaps surprisingly, patients cared for exclusively by hospitalists saw fewer generalists in the hospital (ie, fewer different hospitalists) than those cared for exclusively by outpatient providers. The authors conclude that the doctorpatient continuity over the course of a hospital stay is not worse in the hospitalist model than in the traditional model. While reassuring, it is important to remember that the patient experience does not begin at admission or end at discharge, and a more patient‐centered analysis might take into account the outpatient providers too (ie, those seeing the patient before admission and after discharge), and would probably show that the hospitalist model indeed leads to more care fragmentation. After all, there are at least 2 providers involved in every patient's care when a hospitalist model is used, whereas a large subset of patients cared for by PCPs would have only 1 provider involved.
While not the primary focus of the analysis, Fletcher and colleagues4 identified additional predictors of inpatient continuity of care. Higher socioeconomic class and white race were associated with lower continuity. This suggests that care fragmentation is not a feature of inferior, or at least cheap, care. In keeping with this observation, there was also enormous geographic variation in inpatient care continuity, marked by greater fragmentation of care in the New England and the mid‐Atlantic regions than in other areas of the country, and more fragmentation in larger hospitals serving heavily populated metropolitan areas. This pattern is strikingly similar to the cost‐of‐care patterns observed by the Dartmouth Atlas researchers.5, 6 Densely populated areas tend to have more specialists per capita and also tend to deliver more expensive carewithout demonstrably higher quality. In parallel, it is easy to see how care fragmentation might increase length‐of‐stay7 and lead to excessive diagnostic testing and consultation. More cooks in the kitchen might make costlier stew.
How hospitalists tackle the issue of inpatient continuity is not only a matter of quality of care, but also a matter of job sustainability. The simple way to maximize continuityworking many consecutive dayscan lead to burnout if taken too far. But there are creative ways to assign admissions that maximize continuity for the average inpatient while allowing providers needed time off. The CICLE initiative (Creating Incentives and Continuity Leading to Efficiency in hospital medicine) at the Johns Hopkins Bayview Medical Center, for instance, assigns physicians to 4‐day cycles of clinical work; the first day of the cycle (a long‐call day) involves admitting a large number of patients during a busy shift, with no new patients admitted on the remaining days of the cycle. Thus, all patients whose length of stay is less than 5 days will have a single attending‐of‐record. Not only does this model increase continuity, it also incentivizes providers to augment throughput: more discharged patients on Tuesday means fewer patients to see on Wednesday, without any expectation to backfill. Other less aggressive but similar approaches are used elsewhere, such as exempting hospitalists from accepting new patients on the last 1 or 2 of the consecutive days they work. We eagerly await data on the impact of these programs on quality of care, patient satisfaction, and provider satisfaction.
The impact of other providers and staff cannot be ignored. While the most important handoff in many cases may indeed be between the PCP and attending hospitalist tasked with coordinating the overall care of the patient, for some patients, there may be a specialist who has known the patient for years who is driving the plan of care. For patients with severe chronic illnesses, such as end‐stage renal disease or asthma, a well‐structured specialty clinic may even serve as a patient‐centered medical home.8 And the current inpatient team includes night coverage physicians (whether moonlighters, house staff, or covering hospitalists), and an ever‐increasing number of non‐physicians who play a critical role in hospital care (non‐physician providers, nurses, social workers, pharmacists, case managers, physical therapists, and others). While it is tempting to focus on the attending physician as the main driver of healthcare quality, continuity, and the inpatient experience, this is an oversimplification.
If there is a take‐home message, it is probably that most hospitalized patients will be cared for by multiple providers and a team of non‐physicians. The Marcus Welby practice model may not be completely dead, but if Dr. Welby were still in practice, it would be a safe bet that he would be slower at computerized order entry than the average intern, that financial pressures would make it hard for him to attend to his hospitalized patients, and that he probably would have turned over much of his inpatient practice to the physicians and non‐physician caregivers who make the hospital their primary workplace.9 Going forward, research should examine ways to optimize care coordination under the hospitalist model,1013 rather than comparing it to the traditional model of inpatient care. The ingredients for success include coordinated care by a committee of caregivers, effective handoffs (throughout hospitalization and at discharge),12, 14 focused and deliberate multidisciplinary communication, and effective patient education,15 regardless of the attending‐du‐jour.
The tension between continuity of care and specialization is not new, but may have reached a tipping point when the hospitalist movement erupted onto the American medical scene in the late 1990s. By definition, when a hospitalist cares for an inpatient, there is some fragmentation of care, which is, at least in theory, avoidableif the primary care provider (PCP) can serve as attending physician in the hospital. Literature has since emerged suggesting that clinical and economic outcomes of care by hospitalists are at least as good as that provided by PCPs, and that patients are not, in general, opposed to hospitalist care.13
However, the degree of discontinuity is not just a feature of whether a hospitalist assumes care of the hospitalized patient. Discontinuity can be exacerbated by changing attendings throughout the hospital stay. And inpatient continuity is a potential issue for both the hospitalist model and traditional model of care (in which the PCP serves as inpatient attending physician). While one might assume that the hospitalist model fosters more inpatient discontinuity because most hospitalistswhether working a 7‐on7‐off schedule or another scheduledo not commit to caring for a patient throughout an entire hospitalization the way a PCP might, this question has not previously been examined. Even if the hospitalist model is a fait accompli in many hospitals, it is worth knowing how inpatient continuity differs between the 2 models.
In this issue of the Journal, Fletcher and colleagues4 used billing data to examine trends in inpatient continuity of care over a 10‐year period ending in 2006, and sought to determine: (1) whether inpatient care has become more fragmented over time (as defined by the number of generalists caring for a patient over the course of an average hospitalization), and (2) whether inpatient care provided by hospitalists tends to be more fragmented than care provided by PCPs. They found that continuity of inpatient care has indeed decreased over time. In 1996, just over 70% of patients received care from 1 generalist; this number declined to just under 60% a decade later, despite a decrease in length‐of‐stay during that period. However, and perhaps surprisingly, patients cared for exclusively by hospitalists saw fewer generalists in the hospital (ie, fewer different hospitalists) than those cared for exclusively by outpatient providers. The authors conclude that the doctorpatient continuity over the course of a hospital stay is not worse in the hospitalist model than in the traditional model. While reassuring, it is important to remember that the patient experience does not begin at admission or end at discharge, and a more patient‐centered analysis might take into account the outpatient providers too (ie, those seeing the patient before admission and after discharge), and would probably show that the hospitalist model indeed leads to more care fragmentation. After all, there are at least 2 providers involved in every patient's care when a hospitalist model is used, whereas a large subset of patients cared for by PCPs would have only 1 provider involved.
While not the primary focus of the analysis, Fletcher and colleagues4 identified additional predictors of inpatient continuity of care. Higher socioeconomic class and white race were associated with lower continuity. This suggests that care fragmentation is not a feature of inferior, or at least cheap, care. In keeping with this observation, there was also enormous geographic variation in inpatient care continuity, marked by greater fragmentation of care in the New England and the mid‐Atlantic regions than in other areas of the country, and more fragmentation in larger hospitals serving heavily populated metropolitan areas. This pattern is strikingly similar to the cost‐of‐care patterns observed by the Dartmouth Atlas researchers.5, 6 Densely populated areas tend to have more specialists per capita and also tend to deliver more expensive carewithout demonstrably higher quality. In parallel, it is easy to see how care fragmentation might increase length‐of‐stay7 and lead to excessive diagnostic testing and consultation. More cooks in the kitchen might make costlier stew.
How hospitalists tackle the issue of inpatient continuity is not only a matter of quality of care, but also a matter of job sustainability. The simple way to maximize continuityworking many consecutive dayscan lead to burnout if taken too far. But there are creative ways to assign admissions that maximize continuity for the average inpatient while allowing providers needed time off. The CICLE initiative (Creating Incentives and Continuity Leading to Efficiency in hospital medicine) at the Johns Hopkins Bayview Medical Center, for instance, assigns physicians to 4‐day cycles of clinical work; the first day of the cycle (a long‐call day) involves admitting a large number of patients during a busy shift, with no new patients admitted on the remaining days of the cycle. Thus, all patients whose length of stay is less than 5 days will have a single attending‐of‐record. Not only does this model increase continuity, it also incentivizes providers to augment throughput: more discharged patients on Tuesday means fewer patients to see on Wednesday, without any expectation to backfill. Other less aggressive but similar approaches are used elsewhere, such as exempting hospitalists from accepting new patients on the last 1 or 2 of the consecutive days they work. We eagerly await data on the impact of these programs on quality of care, patient satisfaction, and provider satisfaction.
The impact of other providers and staff cannot be ignored. While the most important handoff in many cases may indeed be between the PCP and attending hospitalist tasked with coordinating the overall care of the patient, for some patients, there may be a specialist who has known the patient for years who is driving the plan of care. For patients with severe chronic illnesses, such as end‐stage renal disease or asthma, a well‐structured specialty clinic may even serve as a patient‐centered medical home.8 And the current inpatient team includes night coverage physicians (whether moonlighters, house staff, or covering hospitalists), and an ever‐increasing number of non‐physicians who play a critical role in hospital care (non‐physician providers, nurses, social workers, pharmacists, case managers, physical therapists, and others). While it is tempting to focus on the attending physician as the main driver of healthcare quality, continuity, and the inpatient experience, this is an oversimplification.
If there is a take‐home message, it is probably that most hospitalized patients will be cared for by multiple providers and a team of non‐physicians. The Marcus Welby practice model may not be completely dead, but if Dr. Welby were still in practice, it would be a safe bet that he would be slower at computerized order entry than the average intern, that financial pressures would make it hard for him to attend to his hospitalized patients, and that he probably would have turned over much of his inpatient practice to the physicians and non‐physician caregivers who make the hospital their primary workplace.9 Going forward, research should examine ways to optimize care coordination under the hospitalist model,1013 rather than comparing it to the traditional model of inpatient care. The ingredients for success include coordinated care by a committee of caregivers, effective handoffs (throughout hospitalization and at discharge),12, 14 focused and deliberate multidisciplinary communication, and effective patient education,15 regardless of the attending‐du‐jour.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,,.How physicians perceive hospitalist services after implementation: anticipation vs reality.Arch Intern Med.2003;163:2330–2336.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,,,.Trends in inpatient continuity of care for a cohort of Medicare patients, 1996–2006.J Hosp Med.2011;6:438–444.
- ,,,,,.The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care.Ann Intern Med.2003;138:288–298.
- ,,,,,.The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care.Ann Intern Med.2003;138:273–287.
- ,,,,.The impact of fragmentation of hospitalist care on length of stay.J Hosp Med.2010;5:335–338.
- ,.Specialists/subspecialists and the patient‐centered medical home.Chest.2010;137:200–204.
- ,.U.S. trends in hospitalization and generalist physician workforce and the emergence of hospitalists.J Gen Intern Med.2010;25:453–459.
- ,,,.Understanding communication during hospitalist service changes: a mixed methods study.J Hosp Med.2009;4:535–540.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,,,.Hospitalist handoffs: a systematic review and task force recommendations.J Hosp Med.2009;4:433–440.
- .The hospitalist field turns 15: new opportunities and challenges.J Hosp Med.2011;6:E1–E4.
- ,,, et al.Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Gen Intern Med.2009;24:971–976.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150:178–187.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,,.How physicians perceive hospitalist services after implementation: anticipation vs reality.Arch Intern Med.2003;163:2330–2336.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,,,.Trends in inpatient continuity of care for a cohort of Medicare patients, 1996–2006.J Hosp Med.2011;6:438–444.
- ,,,,,.The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care.Ann Intern Med.2003;138:288–298.
- ,,,,,.The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care.Ann Intern Med.2003;138:273–287.
- ,,,,.The impact of fragmentation of hospitalist care on length of stay.J Hosp Med.2010;5:335–338.
- ,.Specialists/subspecialists and the patient‐centered medical home.Chest.2010;137:200–204.
- ,.U.S. trends in hospitalization and generalist physician workforce and the emergence of hospitalists.J Gen Intern Med.2010;25:453–459.
- ,,,.Understanding communication during hospitalist service changes: a mixed methods study.J Hosp Med.2009;4:535–540.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,,,,.Hospitalist handoffs: a systematic review and task force recommendations.J Hosp Med.2009;4:433–440.
- .The hospitalist field turns 15: new opportunities and challenges.J Hosp Med.2011;6:E1–E4.
- ,,, et al.Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine.J Gen Intern Med.2009;24:971–976.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150:178–187.
Rethinking Resident Supervision
Close supervision of residents potentially leads to fewer errors, lower patient mortality, and improved quality of care.19 An Institute of Medicine (IOM) report3 recommended improving supervision through more frequent consultations between residents and their supervisors. Although current Accreditation Council for Graduate Medical Education (ACGME) guidelines also recommend that attending physicians (attendings) supervise residents, detailed guidance about what constitutes adequate supervision and how it should be implemented is not well defined.10, 11 The ACGME stresses that supervision should promote resident autonomy in clinical care.10 However, when trainees act independently, it might lead to critical communication breakdowns and other patient safety concerns.5, 6, 1214 Although attendings can encourage (or discourage) residents from seeking advice,15, 16 residents also play important roles in asking for help (ie, initiating their own supervision).1719 Additional research is needed on how residents walk the fine line between exercising independence and seeking supervision.
Lack of resident supervision is especially problematic in high‐risk settings such as the medical intensive care unit (ICU), where medical errors are as frequent as 1.7 errors per patient per day,20, 21 and the adverse drug event rate is twice that of non‐ICU settings.22 Because medication errors are one of the most common errors residents make,23, 24 resident interactions with nursing and pharmacy staff may significantly influence medication safety in error‐prone ICUs.2529 Studies of traditional hierarchical supervision tend to overlook how interactions with other professionals influence resident training.12, 18, 30, 31
We define supervision as a process of providing trainees with monitoring, guidance, and feedback9(p828) as they care for patients.3 Whereas traditionally, supervisors are identified by their positions of formal authority in the medical chain of command; we conceptualize supervision as a process in which professionals engaged in supervisory activities need not have formal authority over their trainees.
To examine how residents seek supervision through both the traditional medical hierarchical chain of command (including attendings, fellows and senior residents) and interprofessional communication channels (including nursing and pharmacy staff), we conducted a qualitative study of residents working in ICUs in three tertiary care hospitals. Using semi‐structured interviews, we asked residents to describe how they experienced supervision as they provided medications to patients. Two broad research questions guided data analysis:
How do residents receive supervision from physicians in the traditional medical hierarchy?
How do residents receive supervision from other professionals (ie, nurses, staff pharmacists, and clinical pharmacists)?
METHODS
Study Design and Sample
We conducted a qualitative study using data from interviews with 17 residents working in the medical ICUs of three large tertiary care hospitals (henceforth referred to as South, West, and North hospitals). The interviews were conducted as part of a longitudinal research project that examined how hospitals learn from medication errors.32 The research project focused on hospitals where medication error prevention was salient because of a vulnerable patient population and/or extensive high‐hazard drug usage. For each ICU, the research design included interviews with 6 attendings, 6 fellows, and a purposeful random sample33 of 6 residents. The goal was to reduce bias from supervisors selecting study participants, and thus enhance the credibility of the small sample, rather than generalize from it.32 Surgical residents were excluded, because of the medication focus. The local Institutional Review Boards approved the research.
Drawing on preliminary analyses of research project data, we designed the current study to examine how residents experienced supervision.33 A qualitative research design was particularly appropriate, because this study is exploratory34 and examines the processes of how supervision is implemented.33 By gathering longitudinal data from 2001 to 2007 and from ICUs in different hospitals, we were able to search for persistent patterns (and systematic variations over time) in how residents experienced supervision that might not have been revealed by a cross‐sectional study in one hospital ICU.
Data Collection
The principal investigator ([PI] M.T.) interviewed residents to gather data about their experiences with medication safety and supervision when providing medication to ICU patients. A general interview guide33 addressed residents' personal experiences with ordering medications, receiving supervision, and their perceptions of institutional medication safety programs (see Supporting Table 1 in the online version of this article). The interviewer consistently prompted residents to provide examples of their supervision experiences. The PI conducted confidential interviews in a private location near the ICU. Using confidential open‐ended, in‐depth interviews33 enabled the participating residents to provide frank answers to potentially sensitive questions.
The current study focuses on interviews with 17 residents; 8 from South Hospital, 6 from West Hospital, and 3 from North Hospital ICUs. Residents were at different training stages (years 14), and none declined participation. Interviews were audio‐recorded, transcribed professionally, checked for accuracy of transcription, and de‐identified. On average, each interview lasted about an hour, resulted in a 30‐page transcript, and focused on how residents experienced supervision for over two‐thirds of the transcript. Interviewees frequently described specific examples in vivid detail, yielding rich information. These data are consistent with Patton's observation that the validity, meaningfulness, and insights generated from qualitative inquiry have more to do with the information richness of the cases selected than with sample size.33(p245) Field notes, document review, and observations of routine activities supplemented the interviews.
Data Analysis
We coded and analyzed interview transcripts by applying the constant comparative method, in which we systematically examined and refined variations in the concepts that emerged from the data.33 To focus on the residents' perceptions of their training experiences, we began the data analysis without preexisting codes. We refined and reconstructed the coding scheme in several iterative stages. Based on the initial review by two investigators (M.T., H.S.), the PI and the coding team (T.D.G., S.M.) developed a preliminary coding scheme by induction, considering the residents' description of their experiences in the context of organizational research.34 They applied the coding scheme to three interview transcripts, and reevaluated and revised it based on comments from other investigators (H.S., E.J.T.).
The PI and the coding team met regularly to review and refine the codes. The PI and the coding team finalized the coding scheme only after it was validated by two other investigators and reapplied to the first set of interview transcripts. Constructing a detailed coding guide, we defined specific codes and classified them under seven broad themes.
We engaged in an iterative coding process to ensure credibility33 and consistent data analysis.34 Both coding team members independently coded each interview and resolved differences through consensus. The PI reviewed each coded transcript and met with the team to resolve any remaining coding disagreements. We used ATLAS.ti 5.0 software (ATLAS.ti Scientific Software Development, Berlin, Germany) to manage data, assist in detecting patterns, and compile relevant quotations.
We observed patterns in the data; we inductively identified themes that emerged from the data as well as those related to organizational research. During the period that we conducted interviews, new rules limiting residents' working hours were implemented.10 We did not discern any pattern changes before and after the new rules. To enhance data analysis credibility,34 two investigators (H.S., E.J.T.), serving as peer debriefers,35 examined whether the themes accurately reflected the data and rigorously searched for counter‐examples that contradicted the proposed themes.
RESULTS
Residents described how they were supervised not only by other physicians within the traditional medical hierarchy, but also by other professionals, including nurses, staff pharmacists, and clinical pharmacists, ie, interprofessional supervision (Figure 1). After presenting these results, we examine how physicians and other professionals used communication strategies during interprofessional supervision. Here we use the term residents to include trainees at all levels, from interns to upper‐level residents, and male pronouns for de‐identification.
Initiating Supervision in the Traditional Medical Hierarchy
Residents described teaching rounds as the formal setting where the attending and other team members guided and gave feedback on their medication‐related decisions. After rounds, residents referred to the formal chain of command (from senior resident to fellow or attending) for their questions. However, residents also described enacting their own supervision by deciding when and how to ask for advice.
Residents developed different strategies for initiating supervision (Table 1). Some described a rule of thumb or personal decision‐making routine for determining when to approach a supervising physician with a question (eg, if the patient is in serious condition) (Table 1, columns 1 and 2). Others described how they decided when and how to ask an attending about their mistakes (Table 1, columns 3 and 4). As might be expected, residents' strategies usually reflected a desire for professional autonomy tempered with varying assessments of their own limitations (Table 1, columns 1 and 2, see Autonomy).
| Strategies for Asking Questions | Strategies for Seeking Feedback on Mistakes | ||
|---|---|---|---|
| When to Ask | When Not to Ask | When to Disclose a Mistake | How to Disclose a Mistake |
| Potential for adverse patient outcome: | Autonomy: | Potential for adverse patient outcome: | Direct: |
If you expect this is really bad, you try to cover yourselfand try to get the experience of somebody else, how to fix it .[And if it's less serious?] Yeah, then you can handle it. If I know it's a busy night, I let two or three admissions come in and then I call the fellow. But if the patient is really, really sick I call the fellow. | There's always a fellow to help us if we have questions. Being like almost a third year though, a lot of the things we kind of can handle on our own. Replacing the electrolytes and blood pressure medicines; we don't need hardly any oversight. | Well, I don't want to call a fellow. I think this medication, if it is wrong, is not going to kill a patient, is not going to adversely affect the outcome. | And I went straight up to the attending and I'll be like: Listen, this is what happened. Now I know. I know what happened, but how can I prevent this from happening again or what should I have done differently? |
| Medication choice: | Nights: | Medication choice and potential for adverse patient outcome: | Indirect: |
| If it's what type of medicine we give, then I usually contact my fellow. But most of the time I just make a decision on my own. | I never call Dr. [Attending] at night because you can get in touch with the fellow. The intern should talk to the attending, but the intern couldn't reach the attending. Sometimes it's like 2:00 or 3:00 in the morning. Then you can wait. If it's not an emergency, not in bad shape, you can wait. In the morning, when the attending physician is there, we'll talk about it. We can then ask. | If I know I have made a small mistake and I think it is inconsequential, I am not going to bother anybody. But if it is a different antibiotic that needed to be started, or what other medications might I have forgotten I would say [to the attending], I forgot to do this yesterday and I am sorry. | Instead of going up and saying, I made this mistake, you know, This is what I did and this is what happened, was it wrong? And I will let them tell me that this was a mistake, or not a mistake, and why. [If it's] really bad, you kind of talk with a fellow and say, This is what I've done. Is it okay? |
| Divergence from plan: | |||
If it's not something in the plan and we have to call someone, like an attending in a neurology service. Things that are discussed in advance, that may be potentially serious, I won't discuss, but basically anything that wasn't discussed in advance that I judge to be serious, then I will ask. | |||
We also identified patterns in how residents and their supervising physicians communicated when residents initiated supervision (Table 2, column 1). In general, residents considered attendings and fellows to be receptive to their questions. One resident explained: There is no one here who is unapproachableeven an attending. Nonetheless, residents reported using deferential language when initiating supervision (Table 2, column 1, row 2). Residents noted that attendings and fellows varied in their responses to questions and mistakes, as reflected in how they communicated with residents (Table 2, column 1, rows 1 and 3).
| Communication Strategies | Hierarchical Supervision: Resident Initiated Supervision | Interprofessional Supervision: Other Professional Initiated Supervision |
|---|---|---|
| ||
| Nonjudgmental language* | Fellow to resident: | Resident to nurse: |
| There's no dumb question. Ask. You can call me any time. | I'll say, It's not such a good idea for this reason. I feel they've [nurses] questioned you on it, so you deserve an appropriate answer. It's not okay to just be like, No, we're not gonna do that. | |
| Attending to resident: | ||
| Listen, [the mistake] could have happened to anybody . Now you know. Next time [you] do this, but [the patient is] gonna be okay. | ||
| Deferential language | Resident to difficult attending: | Pharmacist questions resident: |
| And when you call, you're polite and respectful: I'm sorry sir, I hate to bother you but I have a dumb question | The pharmacy called me up and said, Now listen, are you sure you want to give that dosage? | |
| Resident to fellow: | Nurse questions resident: | |
| Listen, in humbleness say, I don't know this, or am I doing this right? Can you help me out here? | [Nurses] might say like, Oh, you really? You sure you want to do this? | |
| Nurse guides resident: | ||
| Hey I know it's your decision, but this is what Dr. [Attending] would do. | ||
| Judgmental language | Attending response to a gross error: | Nurses questions resident: |
| What the hell were you thinking? We'll try to fix it, but God, what were you thinking? | At first [the nurses] were making fun of the resident who wrote [an unfamiliar medication order] . They just assume you're stupid until you prove them wrong, which is fine. But it gets annoying, too, because we did go to school for a long timewe actually know what the hell we're doing. | |
| Fellow response to resident question: | ||
| The cardiology fellow on call at 2 AM, when you call with a question will be like, Why would you even ask me that question? How could you not know that? | ||
Despite recognizing the importance of asking questions, several residents expressed conflicting beliefs; they raised concerns about the personal consequences of seeking assistance. For instance, one resident advocated: My point of view is I think it's wonderful when you ask questions. Cause that means you're conscientious enough to care about the patientsenough to do the right thing. However, we observed that when he interrupted the research interview to consult with a fellow, he prefaced his query with: Hey, I think this is a dumb question. Some residents expressed contradictory beliefs when they described their embarrassment over appearing stupid and fears of looking weak in front of supervising physicians, even those they perceived as being approachable. Indeed, for one resident, the attending's accessibility increased his anxiety: I don't want to lose respect by asking a stupid question.
Interprofessional Supervision
Residents described how other professionals used various methods of supervising their decision‐making (Table 3). Nurses and pharmacists intercepted medication orders and asked for clarifications, whereas clinical pharmacists also advised residents on ordering alternative medications (Table 3, row 1). Other professionals regularly double‐checked order implementation (Table 3, row 2). Nurses, in particular, routinely guided the future actions of residents by giving them cues and suggesting the next therapeutic tasks they should perform (Table 3, row 3). When assessing residents' clinical decisions, these professionals applied different guidelines (Table 4). Nurses compared residents' clinical decisions to their expectations for usual experience‐based practices (Table 4, column 1); pharmacists consulted and noticed deviations from national and hospital pharmacy standards (Table 4, column 2); and clinical pharmacists supplemented pharmacy standards with their professional judgment (Table 4, column 3).
| Provider Type | Example |
|---|---|
| Intercepting medication orders | |
| Nurses and pharmacists | Clarifying and correcting orders: |
The [pharmacist] said, How much do you really want to give? I was like, Okay. Let me take a look at it. And when I looked at it, I knew it wasn't calculated right. The nurse will call me and say, or the pharmacist will call me and say, Can you please change this? This is not the right dose. | |
| Clinical pharmacists | Suggesting alternative medications: |
| You know, this might be a better medication to use because the half life is | |
| Double‐checking order implementation | |
| Nurses | The nurses in [the unit] are wonderful about doing their own calculations, so if it's a rate, like if it's a drip, I've seen almost all the nurses go back over my drip and do the doses. |
| Clinical pharmacists | Cause even after rounds, he'll go back through and look at all, everything. And if he sees something that doesn't make sense or we could do different, he lets us know. |
| Guiding future actions | |
| Nurses | [The nurses] talk to you about everything. They see the labs before you. They see the labs in the morning and are like, His potassium is high, can you fix this? His blood pressure has been running up, do you want to give him something? They guide you towards making the right decision. |
| Clinical pharmacists | I wouldn't give these two [medications] together. There may be an interaction. |
| Nurses | Staff Pharmacists | Clinical Pharmacists |
|---|---|---|
| Experience on unit and with patients: | Standardized pharmacy guidelines for normal dosage ranges: | Standardized pharmacy guidelines for normal dosage ranges: |
| They're with the patients 12 hours a day. Some of them, they've been doing this for 30 years. | No, [the pharmacists] wouldn't have known on that one [error] because it was a normal it's within a normal range of dosing and it's not that it would cause any harm to the patient, but it was just that it needed to go to a higher dose. [I] did a very high dose, compared with the current dose. Then [the pharmacist] called me back and said, I think this is not the right dose. | [The clinical pharmacist is] the one who says, Oh, by the way, do you really want it IV or PO? Or It should be q 6 versus q 8. |
| Expectations for practice norms: | Patient‐specific dosage guidelines: | Clinical judgment based on specialized pharmacology expertise: |
| [The nurses] can pick up mistakes just as easily as anyone else because they are used to this environment and they are used to seeing all the orders that are written generally. | The [unit‐based] pharmacist came to me and said, This patient's almost in renal failure. Did you want to give them a smaller dose because of the renal failure? And I said, Oh, yeah. I didn't even think about that. | That's all [clinical pharmacists] know is medicine and research and studies, and so you know, there may be a paper that came out last week that none of us have even had a chance to read. But they would be up to date on it. So as far as all the drug trials and everything. |
| The usual practices in the unit: | ||
| An experienced nurse came to me and told me that in the unit, doctor, we used to do it 1 gram, not 0.5 gram. | ||
| The attending's preferences: | ||
| I know sometimes you'll want to start a certain pressor and the nurse will be like Well, Dr. [Attending] likes to use this pressor instead. | ||
| Formal standards: | ||
| A nurse would say, especially in the medications I wrote out to be canceled because of the antibiotic policy here . Doctor, the patient doesn't have any more doses of [antibiotic], what do you want me to start, or do you need to call the [antibiotic policy] team? |
Initiating Interprofessional Supervision
Residents, in turn, sought advice from other professionals. They actively engaged pharmacists in their supervision by asking questions ranging from basic clarifications to complex technical queries. You can just take [the clinical pharmacist] to the side and say, Hey listen. I forgot this medication. What am I supposed to give? It starts with an L, explained a resident. Other residents consulted clinical pharmacists for specialized expertise: The [clinical pharmacists] usually have a protocol that they like to follow that a lot of the residents and probably even a lot of the attendings aren't aware of. In one hospital, residents depended on the clinical pharmacists: They're always available and they really help out the team. In another hospital, unit‐based (on‐site) pharmacists served as an informal but extremely useful resource. Residents also relied on central pharmacy‐based staff, who provided essential backup, especially after‐hours: [The pharmacy is] always available, like if you have a questionthere's a medicine you've never given, but it's the middle of the night, nobody else around, you want to call the pharmacist. Residents uniformly noted that nurses monitored their decisions (Table 2, column 2; Table 4, column 1), and one specifically mentioned soliciting advice from nurses on organizing intravenous lines.
Communication Strategies for Managing Differences in Status and Expertise
Unlike the medical hierarchy that clearly differentiates among residents, fellows, and attendings, interdisciplinary differences were less clearly delineated. Residents were perceived as having higher status than other professionals, due in part to their medical education and responsibility for signing orders. Nurses and pharmacists, however, often had extensive experience and/or specialized training, and thus more expertise than residents. For instance, residents noticed their ambiguous status compared to nurses:
I don't know if some people might psychologically think it was better or worse, worse because it was coming from a nurse and maybe somebody would think that they wouldn't know as much or something like that. But other people would think of it as, they're a team member and they have the perfect right to know more. And maybe it's better because that way like maybe the fellow or attending wouldn't find out that you made a mistake [emphasis added].
To manage the ambiguous differences in their status, experience, and expertise, residents and other professionals used various communication strategies (Table 2, column 2). Residents consistently recounted that pharmacists and nurses used deferential language, for example, by asking questions, rather than directly stating their concerns (Table 2, column 2, row 2). One resident appreciated the unit‐nurses' indirect language: Over here they're really cool about it. They'll say, Is this right, are you sure about this? However, some residents also recalled that nurses used more direct language, such as I am not comfortable, especially when giving residents feedback on IV drug administration. In contrast, when asking pharmacists questions, residents consistently reported using nonjudgmental language, but not deferential language. However, some residents used judgmental language when they disagreed with a pharmacist's intervention.
Individual residents bitterly recalled their encounters with other professionals during previous rotations. One described nurses who were resident‐unfriendly and used judgmental language to mock a resident's choice of medications (Table 2, column 2, row 3). Another worked with clinical pharmacists who feel like they are teaching the residents and they are above the residents. These interactions illustrate how communication choices can create interprofessional tensions, especially when differences in status and expertise conflict or are unclear.
DISCUSSION
We analyzed interviews of residents working in medical ICUs to understand their supervision experiences related to medication safety. Although residents espoused beliefs in seeking assistance from supervising physicians and articulated strategies for doing so, many experienced difficulties in initiating supervision through the traditional medical hierarchy. Some residents were embarrassed by their mistaken decisions; others were concerned that their questions would reflect poorly on them.
Residents also received interprofessional supervision from nurses and pharmacists, who proactively monitored, intervened in, and guided residents' decisions. Other professionals evaluated residents' decisions by comparing them to distinctive professional guidelines and routinely used deferential language when conveying their concerns. Residents, in turn, asked other professionals for assistance.
We posit that interprofessional supervision clearly meets an accepted definition of supervision.3, 9 Residents received monitoring, guidance and feedback9(p828) from other professionals, who engaged in routine monitoring and in situation‐specific double‐checks of residents' clinical decisions, similar to those performed by supervising physicians.30 Moreover, other professionals demonstrated the ability to anticipate a doctor's strengths and weaknesses in particular clinical situations in order to maximize patient safety.9(p829)
Our study results have implications for graduate medical education (GME) reform. First, trainees experienced supervision as a two‐way interaction.36 Residents balanced the countervailing pressures to act independently or to seek a supervising physician's advice, in part, by developing strategies for deciding when to ask questions. Kennedy et al. identified similar rhetorical strategies.18 By asking questions about their clinical decisions, residents requested that supervising physicians guide their work; thus, they proactively initiated and thereby enacted their own supervision. Fostering the conditions for initiating supervision is essential, especially given the association between lack of effective supervision and adverse outcomes.5, 6, 1214
Second, residents expressed contradictory expectations about seeking advice from supervising physicians. Some residents were wary of approaching attending physicians for fear of appearing incompetent or being ridiculed.12, 16, 18, 31 However, we found that other residents remained reluctant to seek advice despite simultaneously appreciating that attendings encouraged them to ask for assistance. Whereas the perceived approachability of supervising physicians was important,18, 19 our exploratory findings suggest that it may be a necessary, but not a sufficient, condition for creating a learning environment. Creating a supportive learning environmentin which residents feel comfortable in revealing their perceived shortcomings to supervising physicians3begins with cultural changes, such as building medical teams,6 but such changes can be slow to develop.
Third, interprofessional supervision offers a strategy for improving supervision. The ubiquitous involvement of nursing and pharmacy staff in monitoring and intervening in residents' medication‐related decisions could result in overlooking their unique contributions to resident supervision. Mindful that supervising physicians evaluate them, residents selectively sought nonjudgmental advice from professionals outside the medical hierarchy. Therefore, improving supervision could entail offering residents ready access to other professionals who can advise them, especially during late night hours when supervising physicians might not be present.17, 27
The importance of interprofessional supervision has not been adequately recognized and emphasized in GME. Our study findings, if supported by future research, highlight how interpersonal communication techniques could influence both interprofessional supervision and hierarchical supervision among physicians. Medical team training programs3739 emphasize developing skills, such as mutual performance monitoring,40(p13) by training providers to raise and respond to potentially sensitive questions. Improving supervision by enhancing interpersonal communication skills may be important, not only when relative status differences are clear (ie, physician hierarchy), but also when status differences are ambiguous (ie, residents and other professionals). GME programs could consider incorporating these techniques into their formal curricula, as could programs for nursing and pharmacy staff.
Our study has several limitations. Because of the larger research project objectives, we focused on medication safety in medical ICU settings, where nurses and pharmacists may be especially vigilant and proactive in monitoring residents. Thus, our findings may be specific to medication issues and less relevant outside ICUs. We had a relatively small sample size and do not claim to generalize from it, although we believe it offers meaningful insights. We also did not continue enlarging our sample until reaching redundancy.35(p202) Nevertheless, the purposeful random sample of residents produced rich information. Indeed, some study results are consistent with previous resident education research,18 adding validity to our findings. Although the interview protocol was not designed specifically to investigate supervision, the resulting interviews yielded abundant data containing residents' detailed descriptions of how they experienced supervision. Whereas we were careful to note whether particular perceptions were unique to one resident, or shared by others, we recognize that the value of residents' observations is assessed by the quality of the insights they provide, not necessarily by the number of residents who described the same experience.
In conclusion, we found that residents experienced difficulties in initiating traditional hierarchical supervision related to medication safety in the ICU. However, they reported ubiquitous interprofessional supervision, albeit limited in scope, which they relied upon for nonjudgmental guidance in their therapeutic decision‐making, especially after‐hours. In our study, interprofessional supervision proved crucial to improving medication safety in the ICU.
- ,,.Resident supervision in the operating room: Does this impact on outcome?J Trauma.1993;35:556–560.
- ,.Supervision in the outpatient clinic: Effects on teaching and patient care.J Gen Intern Med.1993;8:378–380.
- Institute of Medicine (IOM).Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.Washington, DC:National Academic Press;2008.
- Joint Committee of the Group on Resident Affairs and Organization of Resident Representatives. Patient Safety and Graduate Medical Education. Washington, DC: Association of American Medical Colleges; 2003. Available at: https://services.aamc.org/publications/showfile.cfm?file=version13.pdf145:592–598.
- ,,,.Medical errors involving trainees: A study of closed malpractice claims from 5 insurers.Arch Intern Med.2007;167:2030–2036.
- .Resident duty hour reform and mortality in hospitalized patients.JAMA.2007;298:2865–2866.
- ,,,.Progressive independence in clinical training: A tradition worth defending?Acad Med.2005;80:S106–S111.
- ,.Effective supervision in clinical practice settings: A literature review.Med Educ.2000;34:827–840.
- Accreditation Council for Graduate Medical Education. ACGME Residency Review Committee Program Requirements in Critical Care Medicine. 2007. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/142pr707_ims.pdf Accessed August 14, 2009.
- ,.Resident supervision.Accreditation Council for Graduate Medical Education Bulletin.2005; September:15–17. Available at: http://www.acgme.org/acWebsite/bulletin/bulletin09_05. pdf. Accessed March 14,year="2009"2009.
- ,,,,.Resident uncertainty in clinical decision making and impact on patient care: A qualitative study.Qual Saf Health Care.2008;17:122–126.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204:533–540.
- ,,.Communication failures: An insidious contributor to medical mishaps.Acad Med.2004;79:186–194.
- ,,, et al.Attending doctors' perspectives on how residents learn.Med Educ.2007;41:1050–1058.
- ,,.Teaching but not learning: How medical residency programs handle errors.J Organiz Behav.2006;27:869–896.
- ,,,,.On‐call supervision and resident autonomy: From micromanager to absentee attending.Am J Med.2009;122:784–788.
- ,,,.Preserving professional credibility: Grounded theory study of medical trainees' requests for clinical support.BMJ.2009;338:b128.
- ,,,,,.Who wants feedback? An investigation of the variables influencing residents' feedback‐seeking behavior in relation to night shifts.Acad Med.2009;84:910–917.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23:294–300.
- ,,, et al.The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care.Crit Care Med.2005;33:1694–1700.
- ,,,,,.Preventable adverse drug events in hospitalized patients: A comparative study of intensive care and general care units.Crit Care Med.1997;25:1289–1297.
- ,,,,,.Residents report on adverse events and their causes.Arch Intern Med.2005;165:2607–2613.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- ,,,,.Unit‐based clinical pharmacists' prevention of serious medication errors in pediatric inpatients.Am J Health Syst Pharm.2008;65:1254–1260.
- ,,,,.Improving medication safety in the ICU: The pharmacist's role.Hospital Pharmacy.2007;42:337–344.
- ,,,,,.Collaboration between pharmacists, physicians and nurse practitioners: A qualitative investigation of working relationships in the inpatient medical setting.J Interprof Care.2009;23:169–184.
- ,,,.Role of registered nurses in error prevention, discovery and correction.Qual Saf Health Care.2008;17:117–121.
- ,,, et al.Recovery from medical errors: The critical care nursing safety net.Jt Comm J Qual Patient Saf.2006;32:63–72.
- ,,,,.Clinical oversight: Conceptualizing the relationship between supervision and safety.J Gen Intern Med.2007;22:1080–1085.
- .To call or not to call: A judgment of risk by pre‐registration house officers.Med Educ.2008;42:938–944.
- ,.Classifying and interpreting threats to patient safety in hospitals: Insights from aviation.J Organiz Behav.2006;27:919–940.
- .Qualitative Research and Evaluation Methods.3rd ed.Thousand Oaks, CA:Sage Publications;2002.
- ,.Qualitative Data Analysis.2nd. ed.Thousand Oaks, CA:Sage Publications;2006.
- ,.Naturalistic Inquiry.Beverly Hills, CA:Sage Publications;1985.
- ,,.Supervision: A 2‐way street.Arch Intern Med.2008;168:1117.
- ,,, et al.Effects of teamwork training on adverse outcomes and process of care in labor and delivery: A randomized controlled trial.Obstet Gynecol.2007;109:48–55.
- ,,,.Does crew resource management training work? An update, an extension, and some critical needs.Hum Factors.2006;48:392–412.
- ,,,,,.Team training in the neonatal resuscitation program for interns: Teamwork and quality of resuscitations.Pediatrics.2010;125:539–546.
- ,,,,.Medical Teamwork and Patient Safety: The Evidence‐Based Relation.Rockville, MD:Agency for Healthcare Research and Quality 2005. Publication No. 05–0053. Available at: http://www.ahrq.gov/qual/medteam. Accessed October 15,2010.
Close supervision of residents potentially leads to fewer errors, lower patient mortality, and improved quality of care.19 An Institute of Medicine (IOM) report3 recommended improving supervision through more frequent consultations between residents and their supervisors. Although current Accreditation Council for Graduate Medical Education (ACGME) guidelines also recommend that attending physicians (attendings) supervise residents, detailed guidance about what constitutes adequate supervision and how it should be implemented is not well defined.10, 11 The ACGME stresses that supervision should promote resident autonomy in clinical care.10 However, when trainees act independently, it might lead to critical communication breakdowns and other patient safety concerns.5, 6, 1214 Although attendings can encourage (or discourage) residents from seeking advice,15, 16 residents also play important roles in asking for help (ie, initiating their own supervision).1719 Additional research is needed on how residents walk the fine line between exercising independence and seeking supervision.
Lack of resident supervision is especially problematic in high‐risk settings such as the medical intensive care unit (ICU), where medical errors are as frequent as 1.7 errors per patient per day,20, 21 and the adverse drug event rate is twice that of non‐ICU settings.22 Because medication errors are one of the most common errors residents make,23, 24 resident interactions with nursing and pharmacy staff may significantly influence medication safety in error‐prone ICUs.2529 Studies of traditional hierarchical supervision tend to overlook how interactions with other professionals influence resident training.12, 18, 30, 31
We define supervision as a process of providing trainees with monitoring, guidance, and feedback9(p828) as they care for patients.3 Whereas traditionally, supervisors are identified by their positions of formal authority in the medical chain of command; we conceptualize supervision as a process in which professionals engaged in supervisory activities need not have formal authority over their trainees.
To examine how residents seek supervision through both the traditional medical hierarchical chain of command (including attendings, fellows and senior residents) and interprofessional communication channels (including nursing and pharmacy staff), we conducted a qualitative study of residents working in ICUs in three tertiary care hospitals. Using semi‐structured interviews, we asked residents to describe how they experienced supervision as they provided medications to patients. Two broad research questions guided data analysis:
How do residents receive supervision from physicians in the traditional medical hierarchy?
How do residents receive supervision from other professionals (ie, nurses, staff pharmacists, and clinical pharmacists)?
METHODS
Study Design and Sample
We conducted a qualitative study using data from interviews with 17 residents working in the medical ICUs of three large tertiary care hospitals (henceforth referred to as South, West, and North hospitals). The interviews were conducted as part of a longitudinal research project that examined how hospitals learn from medication errors.32 The research project focused on hospitals where medication error prevention was salient because of a vulnerable patient population and/or extensive high‐hazard drug usage. For each ICU, the research design included interviews with 6 attendings, 6 fellows, and a purposeful random sample33 of 6 residents. The goal was to reduce bias from supervisors selecting study participants, and thus enhance the credibility of the small sample, rather than generalize from it.32 Surgical residents were excluded, because of the medication focus. The local Institutional Review Boards approved the research.
Drawing on preliminary analyses of research project data, we designed the current study to examine how residents experienced supervision.33 A qualitative research design was particularly appropriate, because this study is exploratory34 and examines the processes of how supervision is implemented.33 By gathering longitudinal data from 2001 to 2007 and from ICUs in different hospitals, we were able to search for persistent patterns (and systematic variations over time) in how residents experienced supervision that might not have been revealed by a cross‐sectional study in one hospital ICU.
Data Collection
The principal investigator ([PI] M.T.) interviewed residents to gather data about their experiences with medication safety and supervision when providing medication to ICU patients. A general interview guide33 addressed residents' personal experiences with ordering medications, receiving supervision, and their perceptions of institutional medication safety programs (see Supporting Table 1 in the online version of this article). The interviewer consistently prompted residents to provide examples of their supervision experiences. The PI conducted confidential interviews in a private location near the ICU. Using confidential open‐ended, in‐depth interviews33 enabled the participating residents to provide frank answers to potentially sensitive questions.
The current study focuses on interviews with 17 residents; 8 from South Hospital, 6 from West Hospital, and 3 from North Hospital ICUs. Residents were at different training stages (years 14), and none declined participation. Interviews were audio‐recorded, transcribed professionally, checked for accuracy of transcription, and de‐identified. On average, each interview lasted about an hour, resulted in a 30‐page transcript, and focused on how residents experienced supervision for over two‐thirds of the transcript. Interviewees frequently described specific examples in vivid detail, yielding rich information. These data are consistent with Patton's observation that the validity, meaningfulness, and insights generated from qualitative inquiry have more to do with the information richness of the cases selected than with sample size.33(p245) Field notes, document review, and observations of routine activities supplemented the interviews.
Data Analysis
We coded and analyzed interview transcripts by applying the constant comparative method, in which we systematically examined and refined variations in the concepts that emerged from the data.33 To focus on the residents' perceptions of their training experiences, we began the data analysis without preexisting codes. We refined and reconstructed the coding scheme in several iterative stages. Based on the initial review by two investigators (M.T., H.S.), the PI and the coding team (T.D.G., S.M.) developed a preliminary coding scheme by induction, considering the residents' description of their experiences in the context of organizational research.34 They applied the coding scheme to three interview transcripts, and reevaluated and revised it based on comments from other investigators (H.S., E.J.T.).
The PI and the coding team met regularly to review and refine the codes. The PI and the coding team finalized the coding scheme only after it was validated by two other investigators and reapplied to the first set of interview transcripts. Constructing a detailed coding guide, we defined specific codes and classified them under seven broad themes.
We engaged in an iterative coding process to ensure credibility33 and consistent data analysis.34 Both coding team members independently coded each interview and resolved differences through consensus. The PI reviewed each coded transcript and met with the team to resolve any remaining coding disagreements. We used ATLAS.ti 5.0 software (ATLAS.ti Scientific Software Development, Berlin, Germany) to manage data, assist in detecting patterns, and compile relevant quotations.
We observed patterns in the data; we inductively identified themes that emerged from the data as well as those related to organizational research. During the period that we conducted interviews, new rules limiting residents' working hours were implemented.10 We did not discern any pattern changes before and after the new rules. To enhance data analysis credibility,34 two investigators (H.S., E.J.T.), serving as peer debriefers,35 examined whether the themes accurately reflected the data and rigorously searched for counter‐examples that contradicted the proposed themes.
RESULTS
Residents described how they were supervised not only by other physicians within the traditional medical hierarchy, but also by other professionals, including nurses, staff pharmacists, and clinical pharmacists, ie, interprofessional supervision (Figure 1). After presenting these results, we examine how physicians and other professionals used communication strategies during interprofessional supervision. Here we use the term residents to include trainees at all levels, from interns to upper‐level residents, and male pronouns for de‐identification.

Initiating Supervision in the Traditional Medical Hierarchy
Residents described teaching rounds as the formal setting where the attending and other team members guided and gave feedback on their medication‐related decisions. After rounds, residents referred to the formal chain of command (from senior resident to fellow or attending) for their questions. However, residents also described enacting their own supervision by deciding when and how to ask for advice.
Residents developed different strategies for initiating supervision (Table 1). Some described a rule of thumb or personal decision‐making routine for determining when to approach a supervising physician with a question (eg, if the patient is in serious condition) (Table 1, columns 1 and 2). Others described how they decided when and how to ask an attending about their mistakes (Table 1, columns 3 and 4). As might be expected, residents' strategies usually reflected a desire for professional autonomy tempered with varying assessments of their own limitations (Table 1, columns 1 and 2, see Autonomy).
| Strategies for Asking Questions | Strategies for Seeking Feedback on Mistakes | ||
|---|---|---|---|
| When to Ask | When Not to Ask | When to Disclose a Mistake | How to Disclose a Mistake |
| Potential for adverse patient outcome: | Autonomy: | Potential for adverse patient outcome: | Direct: |
If you expect this is really bad, you try to cover yourselfand try to get the experience of somebody else, how to fix it .[And if it's less serious?] Yeah, then you can handle it. If I know it's a busy night, I let two or three admissions come in and then I call the fellow. But if the patient is really, really sick I call the fellow. | There's always a fellow to help us if we have questions. Being like almost a third year though, a lot of the things we kind of can handle on our own. Replacing the electrolytes and blood pressure medicines; we don't need hardly any oversight. | Well, I don't want to call a fellow. I think this medication, if it is wrong, is not going to kill a patient, is not going to adversely affect the outcome. | And I went straight up to the attending and I'll be like: Listen, this is what happened. Now I know. I know what happened, but how can I prevent this from happening again or what should I have done differently? |
| Medication choice: | Nights: | Medication choice and potential for adverse patient outcome: | Indirect: |
| If it's what type of medicine we give, then I usually contact my fellow. But most of the time I just make a decision on my own. | I never call Dr. [Attending] at night because you can get in touch with the fellow. The intern should talk to the attending, but the intern couldn't reach the attending. Sometimes it's like 2:00 or 3:00 in the morning. Then you can wait. If it's not an emergency, not in bad shape, you can wait. In the morning, when the attending physician is there, we'll talk about it. We can then ask. | If I know I have made a small mistake and I think it is inconsequential, I am not going to bother anybody. But if it is a different antibiotic that needed to be started, or what other medications might I have forgotten I would say [to the attending], I forgot to do this yesterday and I am sorry. | Instead of going up and saying, I made this mistake, you know, This is what I did and this is what happened, was it wrong? And I will let them tell me that this was a mistake, or not a mistake, and why. [If it's] really bad, you kind of talk with a fellow and say, This is what I've done. Is it okay? |
| Divergence from plan: | |||
If it's not something in the plan and we have to call someone, like an attending in a neurology service. Things that are discussed in advance, that may be potentially serious, I won't discuss, but basically anything that wasn't discussed in advance that I judge to be serious, then I will ask. | |||
We also identified patterns in how residents and their supervising physicians communicated when residents initiated supervision (Table 2, column 1). In general, residents considered attendings and fellows to be receptive to their questions. One resident explained: There is no one here who is unapproachableeven an attending. Nonetheless, residents reported using deferential language when initiating supervision (Table 2, column 1, row 2). Residents noted that attendings and fellows varied in their responses to questions and mistakes, as reflected in how they communicated with residents (Table 2, column 1, rows 1 and 3).
| Communication Strategies | Hierarchical Supervision: Resident Initiated Supervision | Interprofessional Supervision: Other Professional Initiated Supervision |
|---|---|---|
| ||
| Nonjudgmental language* | Fellow to resident: | Resident to nurse: |
| There's no dumb question. Ask. You can call me any time. | I'll say, It's not such a good idea for this reason. I feel they've [nurses] questioned you on it, so you deserve an appropriate answer. It's not okay to just be like, No, we're not gonna do that. | |
| Attending to resident: | ||
| Listen, [the mistake] could have happened to anybody . Now you know. Next time [you] do this, but [the patient is] gonna be okay. | ||
| Deferential language | Resident to difficult attending: | Pharmacist questions resident: |
| And when you call, you're polite and respectful: I'm sorry sir, I hate to bother you but I have a dumb question | The pharmacy called me up and said, Now listen, are you sure you want to give that dosage? | |
| Resident to fellow: | Nurse questions resident: | |
| Listen, in humbleness say, I don't know this, or am I doing this right? Can you help me out here? | [Nurses] might say like, Oh, you really? You sure you want to do this? | |
| Nurse guides resident: | ||
| Hey I know it's your decision, but this is what Dr. [Attending] would do. | ||
| Judgmental language | Attending response to a gross error: | Nurses questions resident: |
| What the hell were you thinking? We'll try to fix it, but God, what were you thinking? | At first [the nurses] were making fun of the resident who wrote [an unfamiliar medication order] . They just assume you're stupid until you prove them wrong, which is fine. But it gets annoying, too, because we did go to school for a long timewe actually know what the hell we're doing. | |
| Fellow response to resident question: | ||
| The cardiology fellow on call at 2 AM, when you call with a question will be like, Why would you even ask me that question? How could you not know that? | ||
Despite recognizing the importance of asking questions, several residents expressed conflicting beliefs; they raised concerns about the personal consequences of seeking assistance. For instance, one resident advocated: My point of view is I think it's wonderful when you ask questions. Cause that means you're conscientious enough to care about the patientsenough to do the right thing. However, we observed that when he interrupted the research interview to consult with a fellow, he prefaced his query with: Hey, I think this is a dumb question. Some residents expressed contradictory beliefs when they described their embarrassment over appearing stupid and fears of looking weak in front of supervising physicians, even those they perceived as being approachable. Indeed, for one resident, the attending's accessibility increased his anxiety: I don't want to lose respect by asking a stupid question.
Interprofessional Supervision
Residents described how other professionals used various methods of supervising their decision‐making (Table 3). Nurses and pharmacists intercepted medication orders and asked for clarifications, whereas clinical pharmacists also advised residents on ordering alternative medications (Table 3, row 1). Other professionals regularly double‐checked order implementation (Table 3, row 2). Nurses, in particular, routinely guided the future actions of residents by giving them cues and suggesting the next therapeutic tasks they should perform (Table 3, row 3). When assessing residents' clinical decisions, these professionals applied different guidelines (Table 4). Nurses compared residents' clinical decisions to their expectations for usual experience‐based practices (Table 4, column 1); pharmacists consulted and noticed deviations from national and hospital pharmacy standards (Table 4, column 2); and clinical pharmacists supplemented pharmacy standards with their professional judgment (Table 4, column 3).
| Provider Type | Example |
|---|---|
| Intercepting medication orders | |
| Nurses and pharmacists | Clarifying and correcting orders: |
The [pharmacist] said, How much do you really want to give? I was like, Okay. Let me take a look at it. And when I looked at it, I knew it wasn't calculated right. The nurse will call me and say, or the pharmacist will call me and say, Can you please change this? This is not the right dose. | |
| Clinical pharmacists | Suggesting alternative medications: |
| You know, this might be a better medication to use because the half life is | |
| Double‐checking order implementation | |
| Nurses | The nurses in [the unit] are wonderful about doing their own calculations, so if it's a rate, like if it's a drip, I've seen almost all the nurses go back over my drip and do the doses. |
| Clinical pharmacists | Cause even after rounds, he'll go back through and look at all, everything. And if he sees something that doesn't make sense or we could do different, he lets us know. |
| Guiding future actions | |
| Nurses | [The nurses] talk to you about everything. They see the labs before you. They see the labs in the morning and are like, His potassium is high, can you fix this? His blood pressure has been running up, do you want to give him something? They guide you towards making the right decision. |
| Clinical pharmacists | I wouldn't give these two [medications] together. There may be an interaction. |
| Nurses | Staff Pharmacists | Clinical Pharmacists |
|---|---|---|
| Experience on unit and with patients: | Standardized pharmacy guidelines for normal dosage ranges: | Standardized pharmacy guidelines for normal dosage ranges: |
| They're with the patients 12 hours a day. Some of them, they've been doing this for 30 years. | No, [the pharmacists] wouldn't have known on that one [error] because it was a normal it's within a normal range of dosing and it's not that it would cause any harm to the patient, but it was just that it needed to go to a higher dose. [I] did a very high dose, compared with the current dose. Then [the pharmacist] called me back and said, I think this is not the right dose. | [The clinical pharmacist is] the one who says, Oh, by the way, do you really want it IV or PO? Or It should be q 6 versus q 8. |
| Expectations for practice norms: | Patient‐specific dosage guidelines: | Clinical judgment based on specialized pharmacology expertise: |
| [The nurses] can pick up mistakes just as easily as anyone else because they are used to this environment and they are used to seeing all the orders that are written generally. | The [unit‐based] pharmacist came to me and said, This patient's almost in renal failure. Did you want to give them a smaller dose because of the renal failure? And I said, Oh, yeah. I didn't even think about that. | That's all [clinical pharmacists] know is medicine and research and studies, and so you know, there may be a paper that came out last week that none of us have even had a chance to read. But they would be up to date on it. So as far as all the drug trials and everything. |
| The usual practices in the unit: | ||
| An experienced nurse came to me and told me that in the unit, doctor, we used to do it 1 gram, not 0.5 gram. | ||
| The attending's preferences: | ||
| I know sometimes you'll want to start a certain pressor and the nurse will be like Well, Dr. [Attending] likes to use this pressor instead. | ||
| Formal standards: | ||
| A nurse would say, especially in the medications I wrote out to be canceled because of the antibiotic policy here . Doctor, the patient doesn't have any more doses of [antibiotic], what do you want me to start, or do you need to call the [antibiotic policy] team? |
Initiating Interprofessional Supervision
Residents, in turn, sought advice from other professionals. They actively engaged pharmacists in their supervision by asking questions ranging from basic clarifications to complex technical queries. You can just take [the clinical pharmacist] to the side and say, Hey listen. I forgot this medication. What am I supposed to give? It starts with an L, explained a resident. Other residents consulted clinical pharmacists for specialized expertise: The [clinical pharmacists] usually have a protocol that they like to follow that a lot of the residents and probably even a lot of the attendings aren't aware of. In one hospital, residents depended on the clinical pharmacists: They're always available and they really help out the team. In another hospital, unit‐based (on‐site) pharmacists served as an informal but extremely useful resource. Residents also relied on central pharmacy‐based staff, who provided essential backup, especially after‐hours: [The pharmacy is] always available, like if you have a questionthere's a medicine you've never given, but it's the middle of the night, nobody else around, you want to call the pharmacist. Residents uniformly noted that nurses monitored their decisions (Table 2, column 2; Table 4, column 1), and one specifically mentioned soliciting advice from nurses on organizing intravenous lines.
Communication Strategies for Managing Differences in Status and Expertise
Unlike the medical hierarchy that clearly differentiates among residents, fellows, and attendings, interdisciplinary differences were less clearly delineated. Residents were perceived as having higher status than other professionals, due in part to their medical education and responsibility for signing orders. Nurses and pharmacists, however, often had extensive experience and/or specialized training, and thus more expertise than residents. For instance, residents noticed their ambiguous status compared to nurses:
I don't know if some people might psychologically think it was better or worse, worse because it was coming from a nurse and maybe somebody would think that they wouldn't know as much or something like that. But other people would think of it as, they're a team member and they have the perfect right to know more. And maybe it's better because that way like maybe the fellow or attending wouldn't find out that you made a mistake [emphasis added].
To manage the ambiguous differences in their status, experience, and expertise, residents and other professionals used various communication strategies (Table 2, column 2). Residents consistently recounted that pharmacists and nurses used deferential language, for example, by asking questions, rather than directly stating their concerns (Table 2, column 2, row 2). One resident appreciated the unit‐nurses' indirect language: Over here they're really cool about it. They'll say, Is this right, are you sure about this? However, some residents also recalled that nurses used more direct language, such as I am not comfortable, especially when giving residents feedback on IV drug administration. In contrast, when asking pharmacists questions, residents consistently reported using nonjudgmental language, but not deferential language. However, some residents used judgmental language when they disagreed with a pharmacist's intervention.
Individual residents bitterly recalled their encounters with other professionals during previous rotations. One described nurses who were resident‐unfriendly and used judgmental language to mock a resident's choice of medications (Table 2, column 2, row 3). Another worked with clinical pharmacists who feel like they are teaching the residents and they are above the residents. These interactions illustrate how communication choices can create interprofessional tensions, especially when differences in status and expertise conflict or are unclear.
DISCUSSION
We analyzed interviews of residents working in medical ICUs to understand their supervision experiences related to medication safety. Although residents espoused beliefs in seeking assistance from supervising physicians and articulated strategies for doing so, many experienced difficulties in initiating supervision through the traditional medical hierarchy. Some residents were embarrassed by their mistaken decisions; others were concerned that their questions would reflect poorly on them.
Residents also received interprofessional supervision from nurses and pharmacists, who proactively monitored, intervened in, and guided residents' decisions. Other professionals evaluated residents' decisions by comparing them to distinctive professional guidelines and routinely used deferential language when conveying their concerns. Residents, in turn, asked other professionals for assistance.
We posit that interprofessional supervision clearly meets an accepted definition of supervision.3, 9 Residents received monitoring, guidance and feedback9(p828) from other professionals, who engaged in routine monitoring and in situation‐specific double‐checks of residents' clinical decisions, similar to those performed by supervising physicians.30 Moreover, other professionals demonstrated the ability to anticipate a doctor's strengths and weaknesses in particular clinical situations in order to maximize patient safety.9(p829)
Our study results have implications for graduate medical education (GME) reform. First, trainees experienced supervision as a two‐way interaction.36 Residents balanced the countervailing pressures to act independently or to seek a supervising physician's advice, in part, by developing strategies for deciding when to ask questions. Kennedy et al. identified similar rhetorical strategies.18 By asking questions about their clinical decisions, residents requested that supervising physicians guide their work; thus, they proactively initiated and thereby enacted their own supervision. Fostering the conditions for initiating supervision is essential, especially given the association between lack of effective supervision and adverse outcomes.5, 6, 1214
Second, residents expressed contradictory expectations about seeking advice from supervising physicians. Some residents were wary of approaching attending physicians for fear of appearing incompetent or being ridiculed.12, 16, 18, 31 However, we found that other residents remained reluctant to seek advice despite simultaneously appreciating that attendings encouraged them to ask for assistance. Whereas the perceived approachability of supervising physicians was important,18, 19 our exploratory findings suggest that it may be a necessary, but not a sufficient, condition for creating a learning environment. Creating a supportive learning environmentin which residents feel comfortable in revealing their perceived shortcomings to supervising physicians3begins with cultural changes, such as building medical teams,6 but such changes can be slow to develop.
Third, interprofessional supervision offers a strategy for improving supervision. The ubiquitous involvement of nursing and pharmacy staff in monitoring and intervening in residents' medication‐related decisions could result in overlooking their unique contributions to resident supervision. Mindful that supervising physicians evaluate them, residents selectively sought nonjudgmental advice from professionals outside the medical hierarchy. Therefore, improving supervision could entail offering residents ready access to other professionals who can advise them, especially during late night hours when supervising physicians might not be present.17, 27
The importance of interprofessional supervision has not been adequately recognized and emphasized in GME. Our study findings, if supported by future research, highlight how interpersonal communication techniques could influence both interprofessional supervision and hierarchical supervision among physicians. Medical team training programs3739 emphasize developing skills, such as mutual performance monitoring,40(p13) by training providers to raise and respond to potentially sensitive questions. Improving supervision by enhancing interpersonal communication skills may be important, not only when relative status differences are clear (ie, physician hierarchy), but also when status differences are ambiguous (ie, residents and other professionals). GME programs could consider incorporating these techniques into their formal curricula, as could programs for nursing and pharmacy staff.
Our study has several limitations. Because of the larger research project objectives, we focused on medication safety in medical ICU settings, where nurses and pharmacists may be especially vigilant and proactive in monitoring residents. Thus, our findings may be specific to medication issues and less relevant outside ICUs. We had a relatively small sample size and do not claim to generalize from it, although we believe it offers meaningful insights. We also did not continue enlarging our sample until reaching redundancy.35(p202) Nevertheless, the purposeful random sample of residents produced rich information. Indeed, some study results are consistent with previous resident education research,18 adding validity to our findings. Although the interview protocol was not designed specifically to investigate supervision, the resulting interviews yielded abundant data containing residents' detailed descriptions of how they experienced supervision. Whereas we were careful to note whether particular perceptions were unique to one resident, or shared by others, we recognize that the value of residents' observations is assessed by the quality of the insights they provide, not necessarily by the number of residents who described the same experience.
In conclusion, we found that residents experienced difficulties in initiating traditional hierarchical supervision related to medication safety in the ICU. However, they reported ubiquitous interprofessional supervision, albeit limited in scope, which they relied upon for nonjudgmental guidance in their therapeutic decision‐making, especially after‐hours. In our study, interprofessional supervision proved crucial to improving medication safety in the ICU.
Close supervision of residents potentially leads to fewer errors, lower patient mortality, and improved quality of care.19 An Institute of Medicine (IOM) report3 recommended improving supervision through more frequent consultations between residents and their supervisors. Although current Accreditation Council for Graduate Medical Education (ACGME) guidelines also recommend that attending physicians (attendings) supervise residents, detailed guidance about what constitutes adequate supervision and how it should be implemented is not well defined.10, 11 The ACGME stresses that supervision should promote resident autonomy in clinical care.10 However, when trainees act independently, it might lead to critical communication breakdowns and other patient safety concerns.5, 6, 1214 Although attendings can encourage (or discourage) residents from seeking advice,15, 16 residents also play important roles in asking for help (ie, initiating their own supervision).1719 Additional research is needed on how residents walk the fine line between exercising independence and seeking supervision.
Lack of resident supervision is especially problematic in high‐risk settings such as the medical intensive care unit (ICU), where medical errors are as frequent as 1.7 errors per patient per day,20, 21 and the adverse drug event rate is twice that of non‐ICU settings.22 Because medication errors are one of the most common errors residents make,23, 24 resident interactions with nursing and pharmacy staff may significantly influence medication safety in error‐prone ICUs.2529 Studies of traditional hierarchical supervision tend to overlook how interactions with other professionals influence resident training.12, 18, 30, 31
We define supervision as a process of providing trainees with monitoring, guidance, and feedback9(p828) as they care for patients.3 Whereas traditionally, supervisors are identified by their positions of formal authority in the medical chain of command; we conceptualize supervision as a process in which professionals engaged in supervisory activities need not have formal authority over their trainees.
To examine how residents seek supervision through both the traditional medical hierarchical chain of command (including attendings, fellows and senior residents) and interprofessional communication channels (including nursing and pharmacy staff), we conducted a qualitative study of residents working in ICUs in three tertiary care hospitals. Using semi‐structured interviews, we asked residents to describe how they experienced supervision as they provided medications to patients. Two broad research questions guided data analysis:
How do residents receive supervision from physicians in the traditional medical hierarchy?
How do residents receive supervision from other professionals (ie, nurses, staff pharmacists, and clinical pharmacists)?
METHODS
Study Design and Sample
We conducted a qualitative study using data from interviews with 17 residents working in the medical ICUs of three large tertiary care hospitals (henceforth referred to as South, West, and North hospitals). The interviews were conducted as part of a longitudinal research project that examined how hospitals learn from medication errors.32 The research project focused on hospitals where medication error prevention was salient because of a vulnerable patient population and/or extensive high‐hazard drug usage. For each ICU, the research design included interviews with 6 attendings, 6 fellows, and a purposeful random sample33 of 6 residents. The goal was to reduce bias from supervisors selecting study participants, and thus enhance the credibility of the small sample, rather than generalize from it.32 Surgical residents were excluded, because of the medication focus. The local Institutional Review Boards approved the research.
Drawing on preliminary analyses of research project data, we designed the current study to examine how residents experienced supervision.33 A qualitative research design was particularly appropriate, because this study is exploratory34 and examines the processes of how supervision is implemented.33 By gathering longitudinal data from 2001 to 2007 and from ICUs in different hospitals, we were able to search for persistent patterns (and systematic variations over time) in how residents experienced supervision that might not have been revealed by a cross‐sectional study in one hospital ICU.
Data Collection
The principal investigator ([PI] M.T.) interviewed residents to gather data about their experiences with medication safety and supervision when providing medication to ICU patients. A general interview guide33 addressed residents' personal experiences with ordering medications, receiving supervision, and their perceptions of institutional medication safety programs (see Supporting Table 1 in the online version of this article). The interviewer consistently prompted residents to provide examples of their supervision experiences. The PI conducted confidential interviews in a private location near the ICU. Using confidential open‐ended, in‐depth interviews33 enabled the participating residents to provide frank answers to potentially sensitive questions.
The current study focuses on interviews with 17 residents; 8 from South Hospital, 6 from West Hospital, and 3 from North Hospital ICUs. Residents were at different training stages (years 14), and none declined participation. Interviews were audio‐recorded, transcribed professionally, checked for accuracy of transcription, and de‐identified. On average, each interview lasted about an hour, resulted in a 30‐page transcript, and focused on how residents experienced supervision for over two‐thirds of the transcript. Interviewees frequently described specific examples in vivid detail, yielding rich information. These data are consistent with Patton's observation that the validity, meaningfulness, and insights generated from qualitative inquiry have more to do with the information richness of the cases selected than with sample size.33(p245) Field notes, document review, and observations of routine activities supplemented the interviews.
Data Analysis
We coded and analyzed interview transcripts by applying the constant comparative method, in which we systematically examined and refined variations in the concepts that emerged from the data.33 To focus on the residents' perceptions of their training experiences, we began the data analysis without preexisting codes. We refined and reconstructed the coding scheme in several iterative stages. Based on the initial review by two investigators (M.T., H.S.), the PI and the coding team (T.D.G., S.M.) developed a preliminary coding scheme by induction, considering the residents' description of their experiences in the context of organizational research.34 They applied the coding scheme to three interview transcripts, and reevaluated and revised it based on comments from other investigators (H.S., E.J.T.).
The PI and the coding team met regularly to review and refine the codes. The PI and the coding team finalized the coding scheme only after it was validated by two other investigators and reapplied to the first set of interview transcripts. Constructing a detailed coding guide, we defined specific codes and classified them under seven broad themes.
We engaged in an iterative coding process to ensure credibility33 and consistent data analysis.34 Both coding team members independently coded each interview and resolved differences through consensus. The PI reviewed each coded transcript and met with the team to resolve any remaining coding disagreements. We used ATLAS.ti 5.0 software (ATLAS.ti Scientific Software Development, Berlin, Germany) to manage data, assist in detecting patterns, and compile relevant quotations.
We observed patterns in the data; we inductively identified themes that emerged from the data as well as those related to organizational research. During the period that we conducted interviews, new rules limiting residents' working hours were implemented.10 We did not discern any pattern changes before and after the new rules. To enhance data analysis credibility,34 two investigators (H.S., E.J.T.), serving as peer debriefers,35 examined whether the themes accurately reflected the data and rigorously searched for counter‐examples that contradicted the proposed themes.
RESULTS
Residents described how they were supervised not only by other physicians within the traditional medical hierarchy, but also by other professionals, including nurses, staff pharmacists, and clinical pharmacists, ie, interprofessional supervision (Figure 1). After presenting these results, we examine how physicians and other professionals used communication strategies during interprofessional supervision. Here we use the term residents to include trainees at all levels, from interns to upper‐level residents, and male pronouns for de‐identification.

Initiating Supervision in the Traditional Medical Hierarchy
Residents described teaching rounds as the formal setting where the attending and other team members guided and gave feedback on their medication‐related decisions. After rounds, residents referred to the formal chain of command (from senior resident to fellow or attending) for their questions. However, residents also described enacting their own supervision by deciding when and how to ask for advice.
Residents developed different strategies for initiating supervision (Table 1). Some described a rule of thumb or personal decision‐making routine for determining when to approach a supervising physician with a question (eg, if the patient is in serious condition) (Table 1, columns 1 and 2). Others described how they decided when and how to ask an attending about their mistakes (Table 1, columns 3 and 4). As might be expected, residents' strategies usually reflected a desire for professional autonomy tempered with varying assessments of their own limitations (Table 1, columns 1 and 2, see Autonomy).
| Strategies for Asking Questions | Strategies for Seeking Feedback on Mistakes | ||
|---|---|---|---|
| When to Ask | When Not to Ask | When to Disclose a Mistake | How to Disclose a Mistake |
| Potential for adverse patient outcome: | Autonomy: | Potential for adverse patient outcome: | Direct: |
If you expect this is really bad, you try to cover yourselfand try to get the experience of somebody else, how to fix it .[And if it's less serious?] Yeah, then you can handle it. If I know it's a busy night, I let two or three admissions come in and then I call the fellow. But if the patient is really, really sick I call the fellow. | There's always a fellow to help us if we have questions. Being like almost a third year though, a lot of the things we kind of can handle on our own. Replacing the electrolytes and blood pressure medicines; we don't need hardly any oversight. | Well, I don't want to call a fellow. I think this medication, if it is wrong, is not going to kill a patient, is not going to adversely affect the outcome. | And I went straight up to the attending and I'll be like: Listen, this is what happened. Now I know. I know what happened, but how can I prevent this from happening again or what should I have done differently? |
| Medication choice: | Nights: | Medication choice and potential for adverse patient outcome: | Indirect: |
| If it's what type of medicine we give, then I usually contact my fellow. But most of the time I just make a decision on my own. | I never call Dr. [Attending] at night because you can get in touch with the fellow. The intern should talk to the attending, but the intern couldn't reach the attending. Sometimes it's like 2:00 or 3:00 in the morning. Then you can wait. If it's not an emergency, not in bad shape, you can wait. In the morning, when the attending physician is there, we'll talk about it. We can then ask. | If I know I have made a small mistake and I think it is inconsequential, I am not going to bother anybody. But if it is a different antibiotic that needed to be started, or what other medications might I have forgotten I would say [to the attending], I forgot to do this yesterday and I am sorry. | Instead of going up and saying, I made this mistake, you know, This is what I did and this is what happened, was it wrong? And I will let them tell me that this was a mistake, or not a mistake, and why. [If it's] really bad, you kind of talk with a fellow and say, This is what I've done. Is it okay? |
| Divergence from plan: | |||
If it's not something in the plan and we have to call someone, like an attending in a neurology service. Things that are discussed in advance, that may be potentially serious, I won't discuss, but basically anything that wasn't discussed in advance that I judge to be serious, then I will ask. | |||
We also identified patterns in how residents and their supervising physicians communicated when residents initiated supervision (Table 2, column 1). In general, residents considered attendings and fellows to be receptive to their questions. One resident explained: There is no one here who is unapproachableeven an attending. Nonetheless, residents reported using deferential language when initiating supervision (Table 2, column 1, row 2). Residents noted that attendings and fellows varied in their responses to questions and mistakes, as reflected in how they communicated with residents (Table 2, column 1, rows 1 and 3).
| Communication Strategies | Hierarchical Supervision: Resident Initiated Supervision | Interprofessional Supervision: Other Professional Initiated Supervision |
|---|---|---|
| ||
| Nonjudgmental language* | Fellow to resident: | Resident to nurse: |
| There's no dumb question. Ask. You can call me any time. | I'll say, It's not such a good idea for this reason. I feel they've [nurses] questioned you on it, so you deserve an appropriate answer. It's not okay to just be like, No, we're not gonna do that. | |
| Attending to resident: | ||
| Listen, [the mistake] could have happened to anybody . Now you know. Next time [you] do this, but [the patient is] gonna be okay. | ||
| Deferential language | Resident to difficult attending: | Pharmacist questions resident: |
| And when you call, you're polite and respectful: I'm sorry sir, I hate to bother you but I have a dumb question | The pharmacy called me up and said, Now listen, are you sure you want to give that dosage? | |
| Resident to fellow: | Nurse questions resident: | |
| Listen, in humbleness say, I don't know this, or am I doing this right? Can you help me out here? | [Nurses] might say like, Oh, you really? You sure you want to do this? | |
| Nurse guides resident: | ||
| Hey I know it's your decision, but this is what Dr. [Attending] would do. | ||
| Judgmental language | Attending response to a gross error: | Nurses questions resident: |
| What the hell were you thinking? We'll try to fix it, but God, what were you thinking? | At first [the nurses] were making fun of the resident who wrote [an unfamiliar medication order] . They just assume you're stupid until you prove them wrong, which is fine. But it gets annoying, too, because we did go to school for a long timewe actually know what the hell we're doing. | |
| Fellow response to resident question: | ||
| The cardiology fellow on call at 2 AM, when you call with a question will be like, Why would you even ask me that question? How could you not know that? | ||
Despite recognizing the importance of asking questions, several residents expressed conflicting beliefs; they raised concerns about the personal consequences of seeking assistance. For instance, one resident advocated: My point of view is I think it's wonderful when you ask questions. Cause that means you're conscientious enough to care about the patientsenough to do the right thing. However, we observed that when he interrupted the research interview to consult with a fellow, he prefaced his query with: Hey, I think this is a dumb question. Some residents expressed contradictory beliefs when they described their embarrassment over appearing stupid and fears of looking weak in front of supervising physicians, even those they perceived as being approachable. Indeed, for one resident, the attending's accessibility increased his anxiety: I don't want to lose respect by asking a stupid question.
Interprofessional Supervision
Residents described how other professionals used various methods of supervising their decision‐making (Table 3). Nurses and pharmacists intercepted medication orders and asked for clarifications, whereas clinical pharmacists also advised residents on ordering alternative medications (Table 3, row 1). Other professionals regularly double‐checked order implementation (Table 3, row 2). Nurses, in particular, routinely guided the future actions of residents by giving them cues and suggesting the next therapeutic tasks they should perform (Table 3, row 3). When assessing residents' clinical decisions, these professionals applied different guidelines (Table 4). Nurses compared residents' clinical decisions to their expectations for usual experience‐based practices (Table 4, column 1); pharmacists consulted and noticed deviations from national and hospital pharmacy standards (Table 4, column 2); and clinical pharmacists supplemented pharmacy standards with their professional judgment (Table 4, column 3).
| Provider Type | Example |
|---|---|
| Intercepting medication orders | |
| Nurses and pharmacists | Clarifying and correcting orders: |
The [pharmacist] said, How much do you really want to give? I was like, Okay. Let me take a look at it. And when I looked at it, I knew it wasn't calculated right. The nurse will call me and say, or the pharmacist will call me and say, Can you please change this? This is not the right dose. | |
| Clinical pharmacists | Suggesting alternative medications: |
| You know, this might be a better medication to use because the half life is | |
| Double‐checking order implementation | |
| Nurses | The nurses in [the unit] are wonderful about doing their own calculations, so if it's a rate, like if it's a drip, I've seen almost all the nurses go back over my drip and do the doses. |
| Clinical pharmacists | Cause even after rounds, he'll go back through and look at all, everything. And if he sees something that doesn't make sense or we could do different, he lets us know. |
| Guiding future actions | |
| Nurses | [The nurses] talk to you about everything. They see the labs before you. They see the labs in the morning and are like, His potassium is high, can you fix this? His blood pressure has been running up, do you want to give him something? They guide you towards making the right decision. |
| Clinical pharmacists | I wouldn't give these two [medications] together. There may be an interaction. |
| Nurses | Staff Pharmacists | Clinical Pharmacists |
|---|---|---|
| Experience on unit and with patients: | Standardized pharmacy guidelines for normal dosage ranges: | Standardized pharmacy guidelines for normal dosage ranges: |
| They're with the patients 12 hours a day. Some of them, they've been doing this for 30 years. | No, [the pharmacists] wouldn't have known on that one [error] because it was a normal it's within a normal range of dosing and it's not that it would cause any harm to the patient, but it was just that it needed to go to a higher dose. [I] did a very high dose, compared with the current dose. Then [the pharmacist] called me back and said, I think this is not the right dose. | [The clinical pharmacist is] the one who says, Oh, by the way, do you really want it IV or PO? Or It should be q 6 versus q 8. |
| Expectations for practice norms: | Patient‐specific dosage guidelines: | Clinical judgment based on specialized pharmacology expertise: |
| [The nurses] can pick up mistakes just as easily as anyone else because they are used to this environment and they are used to seeing all the orders that are written generally. | The [unit‐based] pharmacist came to me and said, This patient's almost in renal failure. Did you want to give them a smaller dose because of the renal failure? And I said, Oh, yeah. I didn't even think about that. | That's all [clinical pharmacists] know is medicine and research and studies, and so you know, there may be a paper that came out last week that none of us have even had a chance to read. But they would be up to date on it. So as far as all the drug trials and everything. |
| The usual practices in the unit: | ||
| An experienced nurse came to me and told me that in the unit, doctor, we used to do it 1 gram, not 0.5 gram. | ||
| The attending's preferences: | ||
| I know sometimes you'll want to start a certain pressor and the nurse will be like Well, Dr. [Attending] likes to use this pressor instead. | ||
| Formal standards: | ||
| A nurse would say, especially in the medications I wrote out to be canceled because of the antibiotic policy here . Doctor, the patient doesn't have any more doses of [antibiotic], what do you want me to start, or do you need to call the [antibiotic policy] team? |
Initiating Interprofessional Supervision
Residents, in turn, sought advice from other professionals. They actively engaged pharmacists in their supervision by asking questions ranging from basic clarifications to complex technical queries. You can just take [the clinical pharmacist] to the side and say, Hey listen. I forgot this medication. What am I supposed to give? It starts with an L, explained a resident. Other residents consulted clinical pharmacists for specialized expertise: The [clinical pharmacists] usually have a protocol that they like to follow that a lot of the residents and probably even a lot of the attendings aren't aware of. In one hospital, residents depended on the clinical pharmacists: They're always available and they really help out the team. In another hospital, unit‐based (on‐site) pharmacists served as an informal but extremely useful resource. Residents also relied on central pharmacy‐based staff, who provided essential backup, especially after‐hours: [The pharmacy is] always available, like if you have a questionthere's a medicine you've never given, but it's the middle of the night, nobody else around, you want to call the pharmacist. Residents uniformly noted that nurses monitored their decisions (Table 2, column 2; Table 4, column 1), and one specifically mentioned soliciting advice from nurses on organizing intravenous lines.
Communication Strategies for Managing Differences in Status and Expertise
Unlike the medical hierarchy that clearly differentiates among residents, fellows, and attendings, interdisciplinary differences were less clearly delineated. Residents were perceived as having higher status than other professionals, due in part to their medical education and responsibility for signing orders. Nurses and pharmacists, however, often had extensive experience and/or specialized training, and thus more expertise than residents. For instance, residents noticed their ambiguous status compared to nurses:
I don't know if some people might psychologically think it was better or worse, worse because it was coming from a nurse and maybe somebody would think that they wouldn't know as much or something like that. But other people would think of it as, they're a team member and they have the perfect right to know more. And maybe it's better because that way like maybe the fellow or attending wouldn't find out that you made a mistake [emphasis added].
To manage the ambiguous differences in their status, experience, and expertise, residents and other professionals used various communication strategies (Table 2, column 2). Residents consistently recounted that pharmacists and nurses used deferential language, for example, by asking questions, rather than directly stating their concerns (Table 2, column 2, row 2). One resident appreciated the unit‐nurses' indirect language: Over here they're really cool about it. They'll say, Is this right, are you sure about this? However, some residents also recalled that nurses used more direct language, such as I am not comfortable, especially when giving residents feedback on IV drug administration. In contrast, when asking pharmacists questions, residents consistently reported using nonjudgmental language, but not deferential language. However, some residents used judgmental language when they disagreed with a pharmacist's intervention.
Individual residents bitterly recalled their encounters with other professionals during previous rotations. One described nurses who were resident‐unfriendly and used judgmental language to mock a resident's choice of medications (Table 2, column 2, row 3). Another worked with clinical pharmacists who feel like they are teaching the residents and they are above the residents. These interactions illustrate how communication choices can create interprofessional tensions, especially when differences in status and expertise conflict or are unclear.
DISCUSSION
We analyzed interviews of residents working in medical ICUs to understand their supervision experiences related to medication safety. Although residents espoused beliefs in seeking assistance from supervising physicians and articulated strategies for doing so, many experienced difficulties in initiating supervision through the traditional medical hierarchy. Some residents were embarrassed by their mistaken decisions; others were concerned that their questions would reflect poorly on them.
Residents also received interprofessional supervision from nurses and pharmacists, who proactively monitored, intervened in, and guided residents' decisions. Other professionals evaluated residents' decisions by comparing them to distinctive professional guidelines and routinely used deferential language when conveying their concerns. Residents, in turn, asked other professionals for assistance.
We posit that interprofessional supervision clearly meets an accepted definition of supervision.3, 9 Residents received monitoring, guidance and feedback9(p828) from other professionals, who engaged in routine monitoring and in situation‐specific double‐checks of residents' clinical decisions, similar to those performed by supervising physicians.30 Moreover, other professionals demonstrated the ability to anticipate a doctor's strengths and weaknesses in particular clinical situations in order to maximize patient safety.9(p829)
Our study results have implications for graduate medical education (GME) reform. First, trainees experienced supervision as a two‐way interaction.36 Residents balanced the countervailing pressures to act independently or to seek a supervising physician's advice, in part, by developing strategies for deciding when to ask questions. Kennedy et al. identified similar rhetorical strategies.18 By asking questions about their clinical decisions, residents requested that supervising physicians guide their work; thus, they proactively initiated and thereby enacted their own supervision. Fostering the conditions for initiating supervision is essential, especially given the association between lack of effective supervision and adverse outcomes.5, 6, 1214
Second, residents expressed contradictory expectations about seeking advice from supervising physicians. Some residents were wary of approaching attending physicians for fear of appearing incompetent or being ridiculed.12, 16, 18, 31 However, we found that other residents remained reluctant to seek advice despite simultaneously appreciating that attendings encouraged them to ask for assistance. Whereas the perceived approachability of supervising physicians was important,18, 19 our exploratory findings suggest that it may be a necessary, but not a sufficient, condition for creating a learning environment. Creating a supportive learning environmentin which residents feel comfortable in revealing their perceived shortcomings to supervising physicians3begins with cultural changes, such as building medical teams,6 but such changes can be slow to develop.
Third, interprofessional supervision offers a strategy for improving supervision. The ubiquitous involvement of nursing and pharmacy staff in monitoring and intervening in residents' medication‐related decisions could result in overlooking their unique contributions to resident supervision. Mindful that supervising physicians evaluate them, residents selectively sought nonjudgmental advice from professionals outside the medical hierarchy. Therefore, improving supervision could entail offering residents ready access to other professionals who can advise them, especially during late night hours when supervising physicians might not be present.17, 27
The importance of interprofessional supervision has not been adequately recognized and emphasized in GME. Our study findings, if supported by future research, highlight how interpersonal communication techniques could influence both interprofessional supervision and hierarchical supervision among physicians. Medical team training programs3739 emphasize developing skills, such as mutual performance monitoring,40(p13) by training providers to raise and respond to potentially sensitive questions. Improving supervision by enhancing interpersonal communication skills may be important, not only when relative status differences are clear (ie, physician hierarchy), but also when status differences are ambiguous (ie, residents and other professionals). GME programs could consider incorporating these techniques into their formal curricula, as could programs for nursing and pharmacy staff.
Our study has several limitations. Because of the larger research project objectives, we focused on medication safety in medical ICU settings, where nurses and pharmacists may be especially vigilant and proactive in monitoring residents. Thus, our findings may be specific to medication issues and less relevant outside ICUs. We had a relatively small sample size and do not claim to generalize from it, although we believe it offers meaningful insights. We also did not continue enlarging our sample until reaching redundancy.35(p202) Nevertheless, the purposeful random sample of residents produced rich information. Indeed, some study results are consistent with previous resident education research,18 adding validity to our findings. Although the interview protocol was not designed specifically to investigate supervision, the resulting interviews yielded abundant data containing residents' detailed descriptions of how they experienced supervision. Whereas we were careful to note whether particular perceptions were unique to one resident, or shared by others, we recognize that the value of residents' observations is assessed by the quality of the insights they provide, not necessarily by the number of residents who described the same experience.
In conclusion, we found that residents experienced difficulties in initiating traditional hierarchical supervision related to medication safety in the ICU. However, they reported ubiquitous interprofessional supervision, albeit limited in scope, which they relied upon for nonjudgmental guidance in their therapeutic decision‐making, especially after‐hours. In our study, interprofessional supervision proved crucial to improving medication safety in the ICU.
- ,,.Resident supervision in the operating room: Does this impact on outcome?J Trauma.1993;35:556–560.
- ,.Supervision in the outpatient clinic: Effects on teaching and patient care.J Gen Intern Med.1993;8:378–380.
- Institute of Medicine (IOM).Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.Washington, DC:National Academic Press;2008.
- Joint Committee of the Group on Resident Affairs and Organization of Resident Representatives. Patient Safety and Graduate Medical Education. Washington, DC: Association of American Medical Colleges; 2003. Available at: https://services.aamc.org/publications/showfile.cfm?file=version13.pdf145:592–598.
- ,,,.Medical errors involving trainees: A study of closed malpractice claims from 5 insurers.Arch Intern Med.2007;167:2030–2036.
- .Resident duty hour reform and mortality in hospitalized patients.JAMA.2007;298:2865–2866.
- ,,,.Progressive independence in clinical training: A tradition worth defending?Acad Med.2005;80:S106–S111.
- ,.Effective supervision in clinical practice settings: A literature review.Med Educ.2000;34:827–840.
- Accreditation Council for Graduate Medical Education. ACGME Residency Review Committee Program Requirements in Critical Care Medicine. 2007. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/142pr707_ims.pdf Accessed August 14, 2009.
- ,.Resident supervision.Accreditation Council for Graduate Medical Education Bulletin.2005; September:15–17. Available at: http://www.acgme.org/acWebsite/bulletin/bulletin09_05. pdf. Accessed March 14,year="2009"2009.
- ,,,,.Resident uncertainty in clinical decision making and impact on patient care: A qualitative study.Qual Saf Health Care.2008;17:122–126.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204:533–540.
- ,,.Communication failures: An insidious contributor to medical mishaps.Acad Med.2004;79:186–194.
- ,,, et al.Attending doctors' perspectives on how residents learn.Med Educ.2007;41:1050–1058.
- ,,.Teaching but not learning: How medical residency programs handle errors.J Organiz Behav.2006;27:869–896.
- ,,,,.On‐call supervision and resident autonomy: From micromanager to absentee attending.Am J Med.2009;122:784–788.
- ,,,.Preserving professional credibility: Grounded theory study of medical trainees' requests for clinical support.BMJ.2009;338:b128.
- ,,,,,.Who wants feedback? An investigation of the variables influencing residents' feedback‐seeking behavior in relation to night shifts.Acad Med.2009;84:910–917.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23:294–300.
- ,,, et al.The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care.Crit Care Med.2005;33:1694–1700.
- ,,,,,.Preventable adverse drug events in hospitalized patients: A comparative study of intensive care and general care units.Crit Care Med.1997;25:1289–1297.
- ,,,,,.Residents report on adverse events and their causes.Arch Intern Med.2005;165:2607–2613.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- ,,,,.Unit‐based clinical pharmacists' prevention of serious medication errors in pediatric inpatients.Am J Health Syst Pharm.2008;65:1254–1260.
- ,,,,.Improving medication safety in the ICU: The pharmacist's role.Hospital Pharmacy.2007;42:337–344.
- ,,,,,.Collaboration between pharmacists, physicians and nurse practitioners: A qualitative investigation of working relationships in the inpatient medical setting.J Interprof Care.2009;23:169–184.
- ,,,.Role of registered nurses in error prevention, discovery and correction.Qual Saf Health Care.2008;17:117–121.
- ,,, et al.Recovery from medical errors: The critical care nursing safety net.Jt Comm J Qual Patient Saf.2006;32:63–72.
- ,,,,.Clinical oversight: Conceptualizing the relationship between supervision and safety.J Gen Intern Med.2007;22:1080–1085.
- .To call or not to call: A judgment of risk by pre‐registration house officers.Med Educ.2008;42:938–944.
- ,.Classifying and interpreting threats to patient safety in hospitals: Insights from aviation.J Organiz Behav.2006;27:919–940.
- .Qualitative Research and Evaluation Methods.3rd ed.Thousand Oaks, CA:Sage Publications;2002.
- ,.Qualitative Data Analysis.2nd. ed.Thousand Oaks, CA:Sage Publications;2006.
- ,.Naturalistic Inquiry.Beverly Hills, CA:Sage Publications;1985.
- ,,.Supervision: A 2‐way street.Arch Intern Med.2008;168:1117.
- ,,, et al.Effects of teamwork training on adverse outcomes and process of care in labor and delivery: A randomized controlled trial.Obstet Gynecol.2007;109:48–55.
- ,,,.Does crew resource management training work? An update, an extension, and some critical needs.Hum Factors.2006;48:392–412.
- ,,,,,.Team training in the neonatal resuscitation program for interns: Teamwork and quality of resuscitations.Pediatrics.2010;125:539–546.
- ,,,,.Medical Teamwork and Patient Safety: The Evidence‐Based Relation.Rockville, MD:Agency for Healthcare Research and Quality 2005. Publication No. 05–0053. Available at: http://www.ahrq.gov/qual/medteam. Accessed October 15,2010.
- ,,.Resident supervision in the operating room: Does this impact on outcome?J Trauma.1993;35:556–560.
- ,.Supervision in the outpatient clinic: Effects on teaching and patient care.J Gen Intern Med.1993;8:378–380.
- Institute of Medicine (IOM).Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.Washington, DC:National Academic Press;2008.
- Joint Committee of the Group on Resident Affairs and Organization of Resident Representatives. Patient Safety and Graduate Medical Education. Washington, DC: Association of American Medical Colleges; 2003. Available at: https://services.aamc.org/publications/showfile.cfm?file=version13.pdf145:592–598.
- ,,,.Medical errors involving trainees: A study of closed malpractice claims from 5 insurers.Arch Intern Med.2007;167:2030–2036.
- .Resident duty hour reform and mortality in hospitalized patients.JAMA.2007;298:2865–2866.
- ,,,.Progressive independence in clinical training: A tradition worth defending?Acad Med.2005;80:S106–S111.
- ,.Effective supervision in clinical practice settings: A literature review.Med Educ.2000;34:827–840.
- Accreditation Council for Graduate Medical Education. ACGME Residency Review Committee Program Requirements in Critical Care Medicine. 2007. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/142pr707_ims.pdf Accessed August 14, 2009.
- ,.Resident supervision.Accreditation Council for Graduate Medical Education Bulletin.2005; September:15–17. Available at: http://www.acgme.org/acWebsite/bulletin/bulletin09_05. pdf. Accessed March 14,year="2009"2009.
- ,,,,.Resident uncertainty in clinical decision making and impact on patient care: A qualitative study.Qual Saf Health Care.2008;17:122–126.
- ,,, et al.Patterns of communication breakdowns resulting in injury to surgical patients.J Am Coll Surg.2007;204:533–540.
- ,,.Communication failures: An insidious contributor to medical mishaps.Acad Med.2004;79:186–194.
- ,,, et al.Attending doctors' perspectives on how residents learn.Med Educ.2007;41:1050–1058.
- ,,.Teaching but not learning: How medical residency programs handle errors.J Organiz Behav.2006;27:869–896.
- ,,,,.On‐call supervision and resident autonomy: From micromanager to absentee attending.Am J Med.2009;122:784–788.
- ,,,.Preserving professional credibility: Grounded theory study of medical trainees' requests for clinical support.BMJ.2009;338:b128.
- ,,,,,.Who wants feedback? An investigation of the variables influencing residents' feedback‐seeking behavior in relation to night shifts.Acad Med.2009;84:910–917.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23:294–300.
- ,,, et al.The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care.Crit Care Med.2005;33:1694–1700.
- ,,,,,.Preventable adverse drug events in hospitalized patients: A comparative study of intensive care and general care units.Crit Care Med.1997;25:1289–1297.
- ,,,,,.Residents report on adverse events and their causes.Arch Intern Med.2005;165:2607–2613.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- ,,,,.Unit‐based clinical pharmacists' prevention of serious medication errors in pediatric inpatients.Am J Health Syst Pharm.2008;65:1254–1260.
- ,,,,.Improving medication safety in the ICU: The pharmacist's role.Hospital Pharmacy.2007;42:337–344.
- ,,,,,.Collaboration between pharmacists, physicians and nurse practitioners: A qualitative investigation of working relationships in the inpatient medical setting.J Interprof Care.2009;23:169–184.
- ,,,.Role of registered nurses in error prevention, discovery and correction.Qual Saf Health Care.2008;17:117–121.
- ,,, et al.Recovery from medical errors: The critical care nursing safety net.Jt Comm J Qual Patient Saf.2006;32:63–72.
- ,,,,.Clinical oversight: Conceptualizing the relationship between supervision and safety.J Gen Intern Med.2007;22:1080–1085.
- .To call or not to call: A judgment of risk by pre‐registration house officers.Med Educ.2008;42:938–944.
- ,.Classifying and interpreting threats to patient safety in hospitals: Insights from aviation.J Organiz Behav.2006;27:919–940.
- .Qualitative Research and Evaluation Methods.3rd ed.Thousand Oaks, CA:Sage Publications;2002.
- ,.Qualitative Data Analysis.2nd. ed.Thousand Oaks, CA:Sage Publications;2006.
- ,.Naturalistic Inquiry.Beverly Hills, CA:Sage Publications;1985.
- ,,.Supervision: A 2‐way street.Arch Intern Med.2008;168:1117.
- ,,, et al.Effects of teamwork training on adverse outcomes and process of care in labor and delivery: A randomized controlled trial.Obstet Gynecol.2007;109:48–55.
- ,,,.Does crew resource management training work? An update, an extension, and some critical needs.Hum Factors.2006;48:392–412.
- ,,,,,.Team training in the neonatal resuscitation program for interns: Teamwork and quality of resuscitations.Pediatrics.2010;125:539–546.
- ,,,,.Medical Teamwork and Patient Safety: The Evidence‐Based Relation.Rockville, MD:Agency for Healthcare Research and Quality 2005. Publication No. 05–0053. Available at: http://www.ahrq.gov/qual/medteam. Accessed October 15,2010.
Copyright © 2011 Society of Hospital Medicine
Hospital Performance Trends
The Joint Commission (TJC) currently accredits approximately 4546 acute care, critical access, and specialty hospitals,1 accounting for approximately 82% of U.S. hospitals (representing 92% of hospital beds). Hospitals seeking to earn and maintain accreditation undergo unannounced on‐site visits by a team of Joint Commission surveyors at least once every 3 years. These surveys address a variety of domains, including the environment of care, infection prevention and control, information management, adherence to a series of national patient safety goals, and leadership.1
The survey process has changed markedly in recent years. Since 2002, accredited hospitals have been required to continuously collect and submit selected performance measure data to The Joint Commission throughout the three‐year accreditation cycle. The tracer methodology, an evaluation method in which surveyors select a patient to follow through the organization in order to assess compliance with selected standards, was instituted in 2004. Soon thereafter, on‐site surveys went from announced to unannounced in 2006.
Despite the 50+ year history of hospital accreditation in the United States, there has been surprisingly little research on the link between accreditation status and measures of hospital quality (both processes and outcomes). It is only recently that a growing number of studies have attempted to examine this relationship. Empirical support for the relationship between accreditation and other quality measures is emerging. Accredited hospitals have been shown to provide better emergency response planning2 and training3 compared to non‐accredited hospitals. Accreditation has been observed to be a key predictor of patient safety system implementation4 and the primary driver of hospitals' patient‐safety initiatives.5 Accredited trauma centers have been associated with significant reductions in patient mortality,6 and accreditation has been linked to better compliance with evidence‐based methadone and substance abuse treatment.7, 8 Accredited hospitals have been shown to perform better on measures of hospital quality in acute myocardial infarction (AMI), heart failure, and pneumonia care.9, 10 Similarly, accreditation has been associated with lower risk‐adjusted in‐hospital mortality rates for congestive heart failure (CHF), stroke, and pneumonia.11, 12 The results of such research, however, have not always been consistent. Several studies have been unable to demonstrate a relationship between accreditation and quality measures. A study of financial and cost‐related outcome measures found no relationship to accreditation,13 and a study comparing medication error rates across different types of organizations found no relationship to accreditation status.14 Similarly, a comparison of accredited versus non‐accredited ambulatory surgical organizations found that patients were less likely to be hospitalized when treated at an accredited facility for colonoscopy procedures, but no such relationship was observed for the other 4 procedures studied.15
While the research to date has been generally supportive of the link between accreditation and other measures of health care quality, the studies were typically limited to only a few measures and/or involved relatively small samples of accredited and non‐accredited organizations. Over the last decade, however, changes in the performance measurement landscape have created previously unavailable opportunities to more robustly examine the relationship between accreditation and other indicators of hospital quality.
At about the same time that The Joint Commission's accreditation process was becoming more vigorous, the Centers for Medicare and Medicaid Services (CMS) began a program of publicly reporting quality data (
By using a population of hospitals and a range of standardized quality measures greater than those used in previous studies, we seek to address the following questions: Is Joint Commission accreditation status truly associated with higher quality care? And does accreditation status help identify hospitals that are more likely to improve their quality and safety over time?
METHODS
Performance Measures
Since July 2002, U.S. hospitals have been collecting data on standardized measures of quality developed by The Joint Commission and CMS. These measures have been endorsed by the National Quality Forum16 and adopted by the Hospital Quality Alliance.17 The first peer‐reviewed reports using The Joint Commission/CMS measure data confirmed that the measures could successfully monitor and track hospital improvement and identify disparities in performance,18, 19 as called for by the Institute of Medicine's (IOM) landmark 2001 report, Crossing the Quality Chasm.20
In order to promote transparency in health care, both CMSthrough the efforts of the Hospital Quality Allianceand The Joint Commission began publicly reporting measure rates in 2004 using identical measure and data element specifications. It is important to note that during the five‐year span covered by this study, both The Joint Commission and CMS emphasized the reporting of performance measure data. While performance improvement has been the clear objective of these efforts, neither organization established targets for measure rates or set benchmarks for performance improvement. Similarly, while Joint Commission‐accredited hospitals were required to submit performance measure data as a condition of accreditation, their actual performance on the measure rates did not factor into the accreditation decision. In the absence of such direct leverage, it is interesting to note that several studies have demonstrated the positive impact of public reporting on hospital performance,21 and on providing useful information to the general public and health care professionals regarding hospital quality.22
The 16 measures used in this study address hospital compliance with evidence‐based processes of care recommended by the clinical treatment guidelines of respected professional societies.23 Process of care measures are particularly well suited for quality improvement purposes, as they can identify deficiencies which can be immediately addressed by hospitals and do not require risk‐adjustment, as opposed to outcome measures, which do not necessarily directly identify obvious performance improvement opportunities.2426 The measures were also implemented in sets in order to provide hospitals with a more complete portrayal of quality than might be provided using unrelated individual measures. Research has demonstrated that greater collective performance on these process measures is associated with improved one‐year survival after heart failure hospitalization27 and inpatient mortality for those Medicare patients discharged with acute myocardial infarction, heart failure, and pneumonia,28 while other research has shown little association with short‐term outcomes.29
Using the Specifications Manual for National Hospital Inpatient Quality Measures,16 hospitals identify the initial measure populations through International Classification of Diseases (ICD‐CM‐9) codes and patient age obtained through administrative data. Trained abstractors then collect the data for measure‐specific data elements through medical record review on the identified measure population or a sample of this population. Measure algorithms then identify patients in the numerator and denominator of each measure.
Process measure rates reflect the number of times a hospital treated a patient in a manner consistent with specific evidence‐based clinical practice guidelines (numerator cases), divided by the number of patients who were eligible to receive such care (denominator cases). Because precise measure specifications permit the exclusion of patients contraindicated to receive the specific process of care for the measure, ideal performance should be characterized by measure rates that approach 100% (although rare or unpredictable situations, and the reality that no measure is perfect in its design, make consistent performance at 100% improbable). Accuracy of the measure data, as measured by data element agreement rates on reabstraction, has been reported to exceed 90%.30
In addition to the individual performance measures, hospital performance was assessed using 3 condition‐specific summary scores, one for each of the 3 clinical areas: acute myocardial infarction, heart failure, and pneumonia. The summary scores are a weighted average of the individual measure rates in the clinical area, where the weights are the sample sizes for each of the measures.31 A summary score was also calculated based on all 16 measures as a summary measure of overall compliance with recommended care.
One way of studying performance measurement in a way that relates to standards is to evaluate whether a hospital achieves a high rate of performance, where high is defined as a performance rate of 90% or more. In this context, measures were created from each of the 2004 and 2008 hospital performance rates by dichotomizing them as being either less than 90%, or greater than or equal to 90%.32
Data Sources
The data for the measures included in the study are available on the CMS Hospital Compare public databases or The Joint Commission for discharges in 2004 and 2008.33 These 16 measures, active for all 5 years of the study period, include: 7 measures related to acute myocardial infarction care; 4 measures related to heart failure care; and 5 measures related to pneumonia care. The majority of the performance data for the study were obtained from the yearly CMS Hospital Compare public download databases (
Hospital Characteristics
We then linked the CMS performance data, augmented by The Joint Commission performance data when necessary, to hospital characteristics data in the American Hospital Association (AHA) Annual Survey with respect to profit status, number of beds (<100 beds, 100299 beds, 300+ beds), rural status, geographic region, and whether or not the hospital was a critical access hospital. (Teaching status, although available in the AHA database, was not used in the analysis, as almost all teaching hospitals are Joint Commission accredited.) These characteristics were chosen since previous research has identified them as being associated with hospital quality.9, 19, 3437 Data on accreditation status were obtained from The Joint Commission's hospital accreditation database. Hospitals were grouped into 3 hospital accreditation strata based on longitudinal hospital accreditation status between 2004 and 2008: 1) hospitals not accredited in the study period; 2) hospitals accredited between one to four years; and 3) hospitals accredited for the entire study period. Analyses of this middle group (those hospitals accredited for part of the study period; n = 212, 5.4% of the whole sample) led to no significant change in our findings (their performance tended to be midway between always accredited and never‐accredited hospitals) and are thus omitted from our results. Instead, we present only hospitals who were never accredited (n = 762) and those who were accredited through the entire study period (n = 2917).
Statistical Analysis
We assessed the relationship between hospital characteristics and 2004 performance of Joint Commission‐accredited hospitals with hospitals that were not Joint Commission accredited using 2 tests for categorical variables and t tests for continuous variables. Linear regression was used to estimate the five‐year change in performance at each hospital as a function of accreditation group, controlling for hospital characteristics. Baseline hospital performance was also included in the regression models to control for ceiling effects for those hospitals with high baseline performance. To summarize the results, we used the regression models to calculate adjusted change in performance for each accreditation group, and calculated a 95% confidence interval and P value for the difference between the adjusted change scores, using bootstrap methods.38
Next we analyzed the association between accreditation and the likelihood of high 2008 hospital performance by dichotomizing the hospital rates, using a 90% cut point, and using logistic regression to estimate the probability of high performance as a function of accreditation group, controlling for hospital characteristics and baseline hospital performance. The logistic models were then used to calculate adjusted rates of high performance for each accreditation group in presenting the results.
We used two‐sided tests for significance; P < 0.05 was considered statistically significant. This study had no external funding source.
RESULTS
For the 16 individual measures used in this study, a total of 4798 hospitals participated in Hospital Compare or reported data to The Joint Commission in 2004 or 2008. Of these, 907 were excluded because the performance data were not available for either 2004 (576 hospitals) or 2008 (331 hospitals) resulting in a missing value for the change in performance score. Therefore, 3891 hospitals (81%) were included in the final analyses. The 907 excluded hospitals were more likely to be rural (50.8% vs 17.5%), be critical access hospitals (53.9% vs 13.9%), have less than 100 beds (77.4% vs 37.6%), be government owned (34.6% vs 22.1%), be for profit (61.4% vs 49.5%), or be unaccredited (79.8% vs 45.8% in 2004; 75.6% vs 12.8% in 2008), compared with the included hospitals (P < 0.001 for all comparisons).
Hospital Performance at Baseline
Joint Commission‐accredited hospitals were more likely to be large, for profit, or urban, and less likely to be government owned, from the Midwest, or critical access (Table 1). Non‐accredited hospitals performed more poorly than accredited hospitals on most of the publicly reported measures in 2004; the only exception is the timing of initial antibiotic therapy measure for pneumonia (Table 2).
| Characteristic | Non‐Accredited (n = 786) | Accredited (n = 3105) | P Value* |
|---|---|---|---|
| |||
| Profit status, No. (%) | <0.001 | ||
| For profit | 60 (7.6) | 586 (18.9) | |
| Government | 289 (36.8) | 569 (18.3) | |
| Not for profit | 437 (55.6) | 1,950 (62.8) | |
| Census region, No. (%) | <0.001 | ||
| Northeast | 72 (9.2) | 497 (16.0) | |
| Midwest | 345 (43.9) | 716 (23.1) | |
| South | 248 (31.6) | 1,291 (41.6) | |
| West | 121 (15.4) | 601 (19.4) | |
| Rural setting, No. (%) | <0.001 | ||
| Rural | 495 (63.0) | 833 (26.8) | |
| Urban | 291 (37.0) | 2,272 (73.2) | |
| Bed size | <0.001 | ||
| <100 beds | 603 (76.7) | 861 (27.7) | |
| 100299 beds | 158 (20.1) | 1,444 (46.5) | |
| 300+ beds | 25 (3.2) | 800 (25.8) | |
| Critical access hospital status, No. (%) | <0.001 | ||
| Critical access hospital | 376 (47.8) | 164 (5.3) | |
| Acute care hospital | 410 (52.2) | 2,941 (94.7) | |
| Quality Measure, Mean (SD)* | 2004 | 2008 | ||||
|---|---|---|---|---|---|---|
| Non‐Accredited | Accredited | P Value | Non‐Accredited | Accredited | P Value | |
| (n = 786) | (n = 3105) | (n = 950) | (n = 2,941) | |||
| ||||||
| AMI | ||||||
| Aspirin at admission | 87.1 (20.0) | 92.6 (9.4) | <0.001 | 88.6 (22.1) | 96.0 (8.6) | <0.001 |
| Aspirin at discharge | 81.2 (26.1) | 88.5 (14.9) | <0.001 | 87.8 (22.7) | 94.8 (10.1) | <0.001 |
| ACE inhibitor for LV dysfunction | 72.1 (33.4) | 76.7 (22.9) | 0.010 | 83.2 (30.5) | 92.1 (14.8) | <0.001 |
| Beta blocker at discharge | 78.2 (27.9) | 87.0 (16.2) | <0.001 | 87.4 (23.4) | 95.5 (9.9) | <0.001 |
| Smoking cessation advice | 59.6 (40.8) | 74.5 (29.9) | <0.001 | 87.2 (29.5) | 97.2 (11.3) | <0.001 |
| PCI received within 90 min | 60.3 (26.2) | 60.6 (23.8) | 0.946 | 70.1 (24.8) | 77.7 (19.2) | 0.006 |
| Thrombolytic agent within 30 min | 27.9 (35.5) | 32.1 (32.8) | 0.152 | 31.4 (40.7) | 43.7 (40.2) | 0.008 |
| Composite AMI score | 80.6 (20.3) | 87.7 (10.4) | <0.001 | 85.8 (20.0) | 94.6 (8.1) | <0.001 |
| Heart failure | ||||||
| Discharge instructions | 36.8 (32.3) | 49.7 (28.2) | <0.001 | 67.4 (29.6) | 82.3 (16.4) | <0.001 |
| Assessment of LV function | 63.3 (27.6) | 83.6 (14.9) | <0.001 | 79.6 (24.4) | 95.6 (8.1) | <0.001 |
| ACE inhibitor for LV dysfunction | 70.8 (27.6) | 75.7 (16.3) | <0.001 | 82.5 (22.7) | 91.5 (9.7) | <0.001 |
| Smoking cessation advice | 57.1 (36.4) | 68.6 (26.2) | <0.001 | 81.5 (29.9) | 96.1 (10.7) | <0.001 |
| Composite heart failure score | 56.3 (24.1) | 71.2 (15.6) | <0.001 | 75.4 (22.3) | 90.4 (9.4) | <0.001 |
| Pneumonia | ||||||
| Oxygenation assessment | 97.4 (7.3) | 98.4 (4.0) | <0.001 | 99.0 (3.2) | 99.7 (1.2) | <0.001 |
| Pneumococcal vaccination | 45.5 (29.0) | 48.7 (26.2) | 0.007 | 79.9 (21.3) | 87.9 (12.9) | <0.001 |
| Timing of initial antibiotic therapy | 80.6 (13.1) | 70.9 (14.0) | <0.001 | 93.4 (9.2) | 93.6 (6.1) | 0.525 |
| Smoking cessation advice | 56.6 (33.1) | 65.7 (24.8) | <0.001 | 81.6 (25.1) | 94.4 (11.4) | <0.001 |
| Initial antibiotic selection | 73.6 (19.6) | 74.1 (13.4) | 0.508 | 86.1 (13.8) | 88.6 (8.7) | <0.001 |
| Composite pneumonia score | 77.2 (10.2) | 76.6 (8.2) | 0.119 | 90.0 (9.6) | 93.6 (4.9) | <0.001 |
| Overall composite | 73.7 (10.6) | 78.0 (8.7) | <0.001 | 86.8 (11.1) | 93.3 (5.0) | <0.001 |
Five‐Year Changes in Hospital Performance
Between 2004 and 2008, Joint Commission‐accredited hospitals improved their performance more than did non‐accredited hospitals (Table 3). After adjustment for baseline characteristics previously shown to be associated with performance, the overall relative (absolute) difference in improvement was 26% (4.2%) (AMI score difference 67% [3.9%], CHF 48% [10.1%], and pneumonia 21% [3.7%]). Accredited hospitals improved their performance significantly more than non‐accredited for 13 of the 16 individual performance measures.
| Characteristic | Change in Performance* | Absolute Difference, Always vs Never (95% CI) | Relative Difference, % Always vs Never | P Value | |
|---|---|---|---|---|---|
| Never Accredited (n = 762) | Always Accredited (n = 2,917) | ||||
| |||||
| AMI | |||||
| Aspirin at admission | 1.1 | 2.0 | 3.2 (1.25.2) | 160 | 0.001 |
| Aspirin at discharge | 4.7 | 8.0 | 3.2 (1.45.1) | 40 | 0.008 |
| ACE inhibitor for LV dysfunction | 8.5 | 15.9 | 7.4 (3.711.5) | 47 | <0.001 |
| Beta blocker at discharge | 4.4 | 8.4 | 4.0 (2.06.0) | 48 | <0.001 |
| Smoking cessation advice | 18.6 | 22.4 | 3.7 (1.16.9) | 17 | 0.012 |
| PCI received within 90 min | 6.3 | 13.0 | 6.7 (0.314.2) | 52 | 0.070 |
| Thrombolytic agent within 30 min | 0.6 | 5.4 | 6.1 (9.520.4) | 113 | 0.421 |
| Composite AMI score | 2.0 | 5.8 | 3.9 (2.25.5) | 67 | <0.001 |
| Heart failure | |||||
| Discharge instructions | 24.2 | 35.6 | 11.4 (8.714.0) | 32 | <0.001 |
| Assessment of LV function | 4.6 | 12.8 | 8.3 (6.610.0) | 65 | <0.001 |
| ACE inhibitor for LV dysfunction | 10.1 | 15.2 | 5.1 (3.56.8) | 34 | <0.001 |
| Smoking cessation advice | 20.5 | 26.4 | 6.0 (3.38.7) | 23 | <0.001 |
| Composite heart failure score | 10.8 | 20.9 | 10.1 (8.312.0) | 48 | <0.001 |
| Pneumonia | |||||
| Oxygenation assessment | 0.9 | 1.4 | 0.6 (0.30.9) | 43 | <0.001 |
| Pneumococcal vaccination | 33.4 | 40.9 | 7.5 (5.69.4) | 18 | <0.001 |
| Timing of initial antibiotic therapy | 19.2 | 21.1 | 1.9 (1.12.7) | 9 | <0.001 |
| Smoking cessation advice | 21.8 | 27.9 | 6.0 (3.88.3) | 22 | <0.001 |
| Initial antibiotic selection | 13.6 | 14.3 | 0.7 (0.51.9) | 5 | 0.293 |
| Composite pneumonia score | 13.7 | 17.5 | 3.7 (2.84.6) | 21 | <0.001 |
| Overall composite | 12.0 | 16.1 | 4.2 (3.25.1) | 26 | <0.001 |
High Performing Hospitals in 2008
The likelihood that a hospital was a high performer in 2008 was significantly associated with Joint Commission accreditation status, with a higher proportion of accredited hospitals reaching the 90% threshold compared to never‐accredited hospitals (Table 4). Accredited hospitals attained the 90% threshold significantly more often for 13 of the 16 performance measures and all four summary scores, compared to non‐accredited hospitals. In 2008, 82% of Joint Commission‐accredited hospitals demonstrated greater than 90% on the overall summary score, compared to 48% of never‐accredited hospitals. Even after adjusting for differences among hospitals, including performance at baseline, Joint Commission‐accredited hospitals were more likely than never‐accredited hospitals to exceed 90% performance in 2008 (84% vs 69%).
| Characteristic | Percent of Hospitals with Performance Over 90% Adjusted (Actual) | Odds Ratio, Always vs Never (95% CI) | P Value | |
|---|---|---|---|---|
| Never Accredited (n = 762) | Always Accredited (n = 2,917) | |||
| ||||
| AMI | ||||
| Aspirin at admission | 91.8 (71.8) | 93.9 (90.7) | 1.38 (1.001.89) | 0.049 |
| Aspirin at discharge | 83.7 (69.2) | 88.2 (85.1) | 1.45 (1.081.94) | 0.013 |
| ACE inhibitor for LV dysfunction | 65.1 (65.8) | 77.2 (76.5) | 1.81 (1.322.50) | <0.001 |
| Beta blocker at discharge | 84.7 (69.4) | 90.9 (88.4) | 1.80 (1.332.44) | <0.001 |
| Smoking cessation advice | 91.1 (81.3) | 95.9 (94.1) | 2.29 (1.314.01) | 0.004 |
| PCI received within 90 min | 21.5 (16.2) | 29.9 (29.8) | 1.56 (0.713.40) | 0.265 |
| Thrombolytic agent within 30 min | 21.4 (21.3) | 22.7 (23.6) | 1.08 (0.422.74) | 0.879 |
| Composite AMI score | 80.5 (56.6) | 88.2 (85.9) | 1.82 (1.372.41) | <0.001 |
| Heart failure | ||||
| Discharge instructions | 27.0 (26.3) | 38.9 (39.3) | 1.72 (1.302.27) | <0.001 |
| Assessment of LV function | 76.2 (45.0) | 89.1 (88.8) | 2.54 (1.953.31) | <0.001 |
| ACE inhibitor for LV dysfunction | 58.0 (51.4) | 67.8 (68.5) | 1.52 (1.211.92) | <0.001 |
| Smoking cessation advice | 84.2 (62.3) | 90.3 (89.2) | 1.76 (1.282.43) | <0.001 |
| Composite heart failure score | 38.2 (27.6) | 61.5 (64.6) | 2.57 (2.033.26) | <0.001 |
| Pneumonia | ||||
| Oxygenation assessment | 100 (98.2) | 100 (99.8) | 4.38 (1.201.32) | 0.025 |
| Pneumococcal vaccination | 44.1 (40.3) | 57.3 (58.2) | 1.70 (1.362.12) | <0.001 |
| Timing of initial antibiotic therapy | 74.3 (79.1) | 84.2 (82.7) | 1.85 (1.402.46) | <0.001 |
| Smoking cessation advice | 76.2 (54.6) | 85.8 (84.2) | 1.89 (1.422.51) | <0.001 |
| Initial antibiotic selection | 51.8 (47.4) | 51.0 (51.8) | 0.97 (0.761.25) | 0.826 |
| Composite pneumonia score | 69.3 (59.4) | 85.3 (83.9) | 2.58 (2.013.31) | <0.001 |
| Overall composite | 69.0 (47.5) | 83.8 (82.0) | 2.32 (1.763.06) | <0.001 |
DISCUSSION
While accreditation has face validity and is desired by key stakeholders, it is expensive and time consuming. Stakeholders thus are justified in seeking evidence that accreditation is associated with better quality and safety. Ideally, not only would it be associated with better performance at a single point in time, it would also be associated with the pace of improvement over time.
Our study is the first, to our knowledge, to show the association of accreditation status with improvement in the trajectory of performance over a five‐year period. Taking advantage of the fact that the accreditation process changed substantially at about the same time that TJC and CMS began requiring public reporting of evidence‐based quality measures, we found that hospitals accredited by The Joint Commission had had larger improvements in hospital performance from 2004 to 2008 than non‐accredited hospitals, even though the former started with higher baseline performance levels. This accelerated improvement was broad‐based: Accredited hospitals were more likely to achieve superior performance (greater than 90% adherence to quality measures) in 2008 on 13 of 16 nationally standardized quality‐of‐care measures, three clinical area summary scores, and an overall score compared to hospitals that were not accredited. These results are consistent with other studies that have looked at both process and outcome measures and accreditation.912
It is important to note that the observed accreditation effect reflects a difference between hospitals that have elected to seek one particular self‐regulatory alternative to the more restrictive and extensive public regulatory or licensure requirements with those that have not.39 The non‐accredited hospitals that were included in this study are not considered to be sub‐standard hospitals. In fact, hospitals not accredited by The Joint Commission have also met the standards set by Medicare in the Conditions of Participation, and our study demonstrates that these hospitals achieved reasonably strong performance on publicly reported quality measures (86.8% adherence on the composite measure in 2008) and considerable improvement over the 5 years of public reporting (average improvement on composite measure from 2004 to 2008 of 11.8%). Moreover, there are many paths to improvement, and some non‐accredited hospitals achieve stellar performance on quality measures, perhaps by embracing other methods to catalyze improvement.
That said, our data demonstrate that, on average, accredited hospitals achieve superior performance on these evidence‐based quality measures, and their performance improved more strikingly over time. In interpreting these results, it is important to recognize that, while Joint Commission‐accredited hospitals must report quality data, performance on these measures is not directly factored into the accreditation decision; if this were not so, one could argue that this association is a statistical tautology. As it is, we believe that the 2 measures (accreditation and publicly reported quality measures) are two independent assessments of the quality of an organization, and, while the performance measures may not be a gold standard, a measure of their association does provide useful information about the degree to which accreditation is linked to organizational quality.
There are several potential limitations of the current study. First, while we adjusted for most of the known hospital demographic and organizational factors associated with performance, there may be unidentified factors that are associated with both accreditation and performance. This may not be relevant to a patient or payer choosing a hospital based on accreditation status (who may not care whether accreditation is simply associated with higher quality or actually helps produce such quality), but it is relevant to policy‐makers, who may weigh the value of embracing accreditation versus other maneuvers (such as pay for performance or new educational requirements) as a vehicle to promote high‐quality care.
A second limitation is that the specification of the measures can change over time due to the acquisition of new clinical knowledge, which makes longitudinal comparison and tracking of results over time difficult. There were two measures that had definitional changes that had noticeable impact on longitudinal trends: the AMI measure Primary Percutaneous Coronary Intervention (PCI) Received within 90 Minutes of Hospital Arrival (which in 2004 and 2005 used 120 minutes as the threshold), and the pneumonia measure Antibiotic Within 4 Hours of Arrival (which in 2007 changed the threshold to six hours). Other changes included adding angiotensin‐receptor blocker therapy (ARB) as an alternative to angiotensin‐converting enzyme inhibitor (ACEI) therapy in 2005 to the AMI and heart failure measures ACEI or ARB for left ventricular dysfunction. Other less significant changes have been made to the data collection methods for other measures, which could impact the interpretation of changes in performance over time. That said, these changes influenced both accredited and non‐accredited hospitals equally, and we cannot think of reasons that they would have created differential impacts.
Another limitation is that the 16 process measures provide a limited picture of hospital performance. Although the three conditions in the study account for over 15% of Medicare admissions,19 it is possible that non‐accredited hospitals performed as well as accredited hospitals on other measures of quality that were not captured by the 16 measures. As more standardized measures are added to The Joint Commission and CMS databases, it will be possible to use the same study methodology to incorporate these additional domains.
From the original cohort of 4798 hospitals reporting in 2004 or 2008, 19% were not included in the study due to missing data in either 2004 or 2008. Almost two‐thirds of the hospitals excluded from the study were missing 2004 data and, of these, 77% were critical access hospitals. The majority of these critical access hospitals (97%) were non‐accredited. This is in contrast to the hospitals missing 2008 data, of which only 13% were critical access. Since reporting of data to Hospital Compare was voluntary in 2004, it appears that critical access hospitals chose to wait later to report data to Hospital Compare, compared to acute care hospitals. Since critical access hospitals tended to have lower rates, smaller sample sizes, and be non‐accredited, the results of the study would be expected to slightly underestimate the difference between accredited and non‐accredited hospitals.
Finally, while we have argued that the publicly reported quality measures and TJC accreditation decisions provide different lenses into the quality of a given hospital, we cannot entirely exclude the possibility that there are subtle relationships between these two methods that might be partly responsible for our findings. For example, while performance measure rates do not factor directly into the accreditation decision, it is possible that Joint Commission surveyors may be influenced by their knowledge of these rates and biased in their scoring of unrelated standards during the survey process. While we cannot rule out such biases, we are aware of no research on the subject, and have no reason to believe that such biases may have confounded the analysis.
In summary, we found that Joint Commission‐accredited hospitals outperformed non‐accredited hospitals on nationally standardized quality measures of AMI, heart failure, and pneumonia. The performance gap between Joint Commission‐accredited and non‐accredited hospitals increased over the five years of the study. Future studies should incorporate more robust and varied measures of quality as outcomes, and seek to examine the nature of the observed relationship (ie, whether accreditation is simply a marker of higher quality and more rapid improvement, or the accreditation process actually helps create these salutary outcomes).
Acknowledgements
The authors thank Barbara Braun, PhD and Nicole Wineman, MPH, MBA for their literature review on the impact of accreditation, and Barbara Braun, PhD for her thoughtful review of the manuscript.
- The Joint Commission. Facts About Hospital Accreditation. Available at: http://www.jointcommission.org/assets/1/18/Hospital_Accreditation_1_31_11.pdf. Accessed on Feb 16, 2011.
- ,.Emergency Response Planning in Hospitals, United States: 2003–2004. Advance Data from Vital and Health Statistics; No. 391.Hattsville, MD:National Center for Health Statistics;2007.
- ,.Training for Terrorism‐Related Conditions in Hospitals, United States: 2003–2004. Advance Data from Vital and Health Statistics; No. 380.Hattsville, MD:National Center for Health Statistics;2006.
- ,,,.Hospital patient safety: characteristics of best‐performing hospitals.J Healthcare Manag.2007;52 (3):188–205.
- ,,.What is driving hospitals' patient‐safety efforts?Health Aff.2004;23(2):103–115.
- ,.The impact of trauma centre accreditation on patient outcome.Injury.2006;37(12):1166–1171.
- ,.Factors that influence staffing of outpatient substance abuse treatment programs.Psychiatr Serv.2005;56(8)934–939.
- ,.Changes in methadone treatment practices. Results from a national panel study, 1988–2000.JAMA.2002;288:850–856.
- ,,, et al.Quality of care for the treatment of acute medical conditions in US hospitals.Arch Intern Med.2006;166:2511–2517.
- ,,,.JCAHO accreditation and quality of care for acute myocardial infarction.Health Aff.2003;22(2):243–254.
- ,,, et al.Is JCAHO Accreditation Associated with Better Patient Outcomes in Rural Hospitals? Academy Health Annual Meeting; Boston, MA; June2005.
- .Hospital quality of care: the link between accreditation and mortality.J Clin Outcomes Manag.2003;10(9):473–480.
- , , . Structural versus outcome measures in hospitals: A comparison of Joint Commission and medicare outcome scores in hospitals. Qual Manage Health Care. 2002;10(2): 29–38.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,,,.Quality of care in accredited and non‐accredited ambulatory surgical centers.Jt Comm J Qual Patient Saf.2008;34(9):546–551.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures 2009. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed May 21,2009.
- Hospital Quality Alliance Homepage. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/index.html. Accessed May 6,2010
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004.N Engl J Med.2005;353(3):255–264.
- ,,,.Care in U.S. hospitals—the Hospital Quality Alliance Program.N Engl J Med.2005;353:265–274.
- Institute of Medicine, Committee on Quality Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:The National Academy Press;2001.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff.2003;22(2):84–94.
- ,,,.Performance of top ranked heart care hospitals on evidence‐based process measures.Circulation.2006;114:558–564.
- The Joint Commission Performance Measure Initiatives Homepage. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/default.htm. Accessed on July 27,2010.
- .Using health outcomes data to compare plans, networks and providers.Int J Qual Health Care.1998;10(6):477–483.
- .Process versus outcome indicators in the assessment of quality of health care.Int J Qual Health Care.2001;13:475–480.
- .Does paying for performance improve the quality of health care?Med Care Res Rev.2006;63(1):122S–125S.
- ,,, et al.Incremental survival benefit with adherence to standardized health failure core measures: a performance evaluation study of 2958 patients.J Card Fail.2008;14(2):95–102.
- ,,,.The inverse relationship between mortality rates and performance in the hospital quality alliance measures.Health Aff.2007;26(4):1104–1110.
- ,,, et al.Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality.JAMA.2006:296(1):72–78.
- ,,,,.Assessing the reliability of standardized performance measures.Int J Qual Health Care.2006;18:246–255.
- Centers for Medicare and Medicaid Services (CMS). CMS HQI Demonstration Project‐Composite Quality Score Methodology Overview. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalCompositeQualityScoreMethodologyOverview.pdf. Accessed March 8,2010.
- ,,,.Assessing the accuracy of hospital performance measures.Med Decis Making.2007;27:9–20.
- Quality Check Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed May 21,2009.
- ,,.Hospital characteristics and mortality rates.N Engl J Med.1989;321(25):1720–1725.
- ,.United States rural hospital quality in the Hospital Compare Database—accounting for hospital characteristics.Health Policy.2008;87:112–127.
- ,,,,,.Characteristics of hospitals demonstrating superior performance in patient experience and clinical process measures of care.Med Care Res Rev.2010;67(1):38–55.
- ,,.Comparison of change in quality of care between safety‐net and non‐safety‐net hospitals.JAMA.2008;299(18):2180–2187.
- ,.Bootstrap Methods and Their Application.New York:Cambridge;1997:chap 6.
- ,,,.The role of accreditation in an era of market‐driven accountability.Am J Manag Care.2005;11(5):290–293.
The Joint Commission (TJC) currently accredits approximately 4546 acute care, critical access, and specialty hospitals,1 accounting for approximately 82% of U.S. hospitals (representing 92% of hospital beds). Hospitals seeking to earn and maintain accreditation undergo unannounced on‐site visits by a team of Joint Commission surveyors at least once every 3 years. These surveys address a variety of domains, including the environment of care, infection prevention and control, information management, adherence to a series of national patient safety goals, and leadership.1
The survey process has changed markedly in recent years. Since 2002, accredited hospitals have been required to continuously collect and submit selected performance measure data to The Joint Commission throughout the three‐year accreditation cycle. The tracer methodology, an evaluation method in which surveyors select a patient to follow through the organization in order to assess compliance with selected standards, was instituted in 2004. Soon thereafter, on‐site surveys went from announced to unannounced in 2006.
Despite the 50+ year history of hospital accreditation in the United States, there has been surprisingly little research on the link between accreditation status and measures of hospital quality (both processes and outcomes). It is only recently that a growing number of studies have attempted to examine this relationship. Empirical support for the relationship between accreditation and other quality measures is emerging. Accredited hospitals have been shown to provide better emergency response planning2 and training3 compared to non‐accredited hospitals. Accreditation has been observed to be a key predictor of patient safety system implementation4 and the primary driver of hospitals' patient‐safety initiatives.5 Accredited trauma centers have been associated with significant reductions in patient mortality,6 and accreditation has been linked to better compliance with evidence‐based methadone and substance abuse treatment.7, 8 Accredited hospitals have been shown to perform better on measures of hospital quality in acute myocardial infarction (AMI), heart failure, and pneumonia care.9, 10 Similarly, accreditation has been associated with lower risk‐adjusted in‐hospital mortality rates for congestive heart failure (CHF), stroke, and pneumonia.11, 12 The results of such research, however, have not always been consistent. Several studies have been unable to demonstrate a relationship between accreditation and quality measures. A study of financial and cost‐related outcome measures found no relationship to accreditation,13 and a study comparing medication error rates across different types of organizations found no relationship to accreditation status.14 Similarly, a comparison of accredited versus non‐accredited ambulatory surgical organizations found that patients were less likely to be hospitalized when treated at an accredited facility for colonoscopy procedures, but no such relationship was observed for the other 4 procedures studied.15
While the research to date has been generally supportive of the link between accreditation and other measures of health care quality, the studies were typically limited to only a few measures and/or involved relatively small samples of accredited and non‐accredited organizations. Over the last decade, however, changes in the performance measurement landscape have created previously unavailable opportunities to more robustly examine the relationship between accreditation and other indicators of hospital quality.
At about the same time that The Joint Commission's accreditation process was becoming more vigorous, the Centers for Medicare and Medicaid Services (CMS) began a program of publicly reporting quality data (
By using a population of hospitals and a range of standardized quality measures greater than those used in previous studies, we seek to address the following questions: Is Joint Commission accreditation status truly associated with higher quality care? And does accreditation status help identify hospitals that are more likely to improve their quality and safety over time?
METHODS
Performance Measures
Since July 2002, U.S. hospitals have been collecting data on standardized measures of quality developed by The Joint Commission and CMS. These measures have been endorsed by the National Quality Forum16 and adopted by the Hospital Quality Alliance.17 The first peer‐reviewed reports using The Joint Commission/CMS measure data confirmed that the measures could successfully monitor and track hospital improvement and identify disparities in performance,18, 19 as called for by the Institute of Medicine's (IOM) landmark 2001 report, Crossing the Quality Chasm.20
In order to promote transparency in health care, both CMSthrough the efforts of the Hospital Quality Allianceand The Joint Commission began publicly reporting measure rates in 2004 using identical measure and data element specifications. It is important to note that during the five‐year span covered by this study, both The Joint Commission and CMS emphasized the reporting of performance measure data. While performance improvement has been the clear objective of these efforts, neither organization established targets for measure rates or set benchmarks for performance improvement. Similarly, while Joint Commission‐accredited hospitals were required to submit performance measure data as a condition of accreditation, their actual performance on the measure rates did not factor into the accreditation decision. In the absence of such direct leverage, it is interesting to note that several studies have demonstrated the positive impact of public reporting on hospital performance,21 and on providing useful information to the general public and health care professionals regarding hospital quality.22
The 16 measures used in this study address hospital compliance with evidence‐based processes of care recommended by the clinical treatment guidelines of respected professional societies.23 Process of care measures are particularly well suited for quality improvement purposes, as they can identify deficiencies which can be immediately addressed by hospitals and do not require risk‐adjustment, as opposed to outcome measures, which do not necessarily directly identify obvious performance improvement opportunities.2426 The measures were also implemented in sets in order to provide hospitals with a more complete portrayal of quality than might be provided using unrelated individual measures. Research has demonstrated that greater collective performance on these process measures is associated with improved one‐year survival after heart failure hospitalization27 and inpatient mortality for those Medicare patients discharged with acute myocardial infarction, heart failure, and pneumonia,28 while other research has shown little association with short‐term outcomes.29
Using the Specifications Manual for National Hospital Inpatient Quality Measures,16 hospitals identify the initial measure populations through International Classification of Diseases (ICD‐CM‐9) codes and patient age obtained through administrative data. Trained abstractors then collect the data for measure‐specific data elements through medical record review on the identified measure population or a sample of this population. Measure algorithms then identify patients in the numerator and denominator of each measure.
Process measure rates reflect the number of times a hospital treated a patient in a manner consistent with specific evidence‐based clinical practice guidelines (numerator cases), divided by the number of patients who were eligible to receive such care (denominator cases). Because precise measure specifications permit the exclusion of patients contraindicated to receive the specific process of care for the measure, ideal performance should be characterized by measure rates that approach 100% (although rare or unpredictable situations, and the reality that no measure is perfect in its design, make consistent performance at 100% improbable). Accuracy of the measure data, as measured by data element agreement rates on reabstraction, has been reported to exceed 90%.30
In addition to the individual performance measures, hospital performance was assessed using 3 condition‐specific summary scores, one for each of the 3 clinical areas: acute myocardial infarction, heart failure, and pneumonia. The summary scores are a weighted average of the individual measure rates in the clinical area, where the weights are the sample sizes for each of the measures.31 A summary score was also calculated based on all 16 measures as a summary measure of overall compliance with recommended care.
One way of studying performance measurement in a way that relates to standards is to evaluate whether a hospital achieves a high rate of performance, where high is defined as a performance rate of 90% or more. In this context, measures were created from each of the 2004 and 2008 hospital performance rates by dichotomizing them as being either less than 90%, or greater than or equal to 90%.32
Data Sources
The data for the measures included in the study are available on the CMS Hospital Compare public databases or The Joint Commission for discharges in 2004 and 2008.33 These 16 measures, active for all 5 years of the study period, include: 7 measures related to acute myocardial infarction care; 4 measures related to heart failure care; and 5 measures related to pneumonia care. The majority of the performance data for the study were obtained from the yearly CMS Hospital Compare public download databases (
Hospital Characteristics
We then linked the CMS performance data, augmented by The Joint Commission performance data when necessary, to hospital characteristics data in the American Hospital Association (AHA) Annual Survey with respect to profit status, number of beds (<100 beds, 100299 beds, 300+ beds), rural status, geographic region, and whether or not the hospital was a critical access hospital. (Teaching status, although available in the AHA database, was not used in the analysis, as almost all teaching hospitals are Joint Commission accredited.) These characteristics were chosen since previous research has identified them as being associated with hospital quality.9, 19, 3437 Data on accreditation status were obtained from The Joint Commission's hospital accreditation database. Hospitals were grouped into 3 hospital accreditation strata based on longitudinal hospital accreditation status between 2004 and 2008: 1) hospitals not accredited in the study period; 2) hospitals accredited between one to four years; and 3) hospitals accredited for the entire study period. Analyses of this middle group (those hospitals accredited for part of the study period; n = 212, 5.4% of the whole sample) led to no significant change in our findings (their performance tended to be midway between always accredited and never‐accredited hospitals) and are thus omitted from our results. Instead, we present only hospitals who were never accredited (n = 762) and those who were accredited through the entire study period (n = 2917).
Statistical Analysis
We assessed the relationship between hospital characteristics and 2004 performance of Joint Commission‐accredited hospitals with hospitals that were not Joint Commission accredited using 2 tests for categorical variables and t tests for continuous variables. Linear regression was used to estimate the five‐year change in performance at each hospital as a function of accreditation group, controlling for hospital characteristics. Baseline hospital performance was also included in the regression models to control for ceiling effects for those hospitals with high baseline performance. To summarize the results, we used the regression models to calculate adjusted change in performance for each accreditation group, and calculated a 95% confidence interval and P value for the difference between the adjusted change scores, using bootstrap methods.38
Next we analyzed the association between accreditation and the likelihood of high 2008 hospital performance by dichotomizing the hospital rates, using a 90% cut point, and using logistic regression to estimate the probability of high performance as a function of accreditation group, controlling for hospital characteristics and baseline hospital performance. The logistic models were then used to calculate adjusted rates of high performance for each accreditation group in presenting the results.
We used two‐sided tests for significance; P < 0.05 was considered statistically significant. This study had no external funding source.
RESULTS
For the 16 individual measures used in this study, a total of 4798 hospitals participated in Hospital Compare or reported data to The Joint Commission in 2004 or 2008. Of these, 907 were excluded because the performance data were not available for either 2004 (576 hospitals) or 2008 (331 hospitals) resulting in a missing value for the change in performance score. Therefore, 3891 hospitals (81%) were included in the final analyses. The 907 excluded hospitals were more likely to be rural (50.8% vs 17.5%), be critical access hospitals (53.9% vs 13.9%), have less than 100 beds (77.4% vs 37.6%), be government owned (34.6% vs 22.1%), be for profit (61.4% vs 49.5%), or be unaccredited (79.8% vs 45.8% in 2004; 75.6% vs 12.8% in 2008), compared with the included hospitals (P < 0.001 for all comparisons).
Hospital Performance at Baseline
Joint Commission‐accredited hospitals were more likely to be large, for profit, or urban, and less likely to be government owned, from the Midwest, or critical access (Table 1). Non‐accredited hospitals performed more poorly than accredited hospitals on most of the publicly reported measures in 2004; the only exception is the timing of initial antibiotic therapy measure for pneumonia (Table 2).
| Characteristic | Non‐Accredited (n = 786) | Accredited (n = 3105) | P Value* |
|---|---|---|---|
| |||
| Profit status, No. (%) | <0.001 | ||
| For profit | 60 (7.6) | 586 (18.9) | |
| Government | 289 (36.8) | 569 (18.3) | |
| Not for profit | 437 (55.6) | 1,950 (62.8) | |
| Census region, No. (%) | <0.001 | ||
| Northeast | 72 (9.2) | 497 (16.0) | |
| Midwest | 345 (43.9) | 716 (23.1) | |
| South | 248 (31.6) | 1,291 (41.6) | |
| West | 121 (15.4) | 601 (19.4) | |
| Rural setting, No. (%) | <0.001 | ||
| Rural | 495 (63.0) | 833 (26.8) | |
| Urban | 291 (37.0) | 2,272 (73.2) | |
| Bed size | <0.001 | ||
| <100 beds | 603 (76.7) | 861 (27.7) | |
| 100299 beds | 158 (20.1) | 1,444 (46.5) | |
| 300+ beds | 25 (3.2) | 800 (25.8) | |
| Critical access hospital status, No. (%) | <0.001 | ||
| Critical access hospital | 376 (47.8) | 164 (5.3) | |
| Acute care hospital | 410 (52.2) | 2,941 (94.7) | |
| Quality Measure, Mean (SD)* | 2004 | 2008 | ||||
|---|---|---|---|---|---|---|
| Non‐Accredited | Accredited | P Value | Non‐Accredited | Accredited | P Value | |
| (n = 786) | (n = 3105) | (n = 950) | (n = 2,941) | |||
| ||||||
| AMI | ||||||
| Aspirin at admission | 87.1 (20.0) | 92.6 (9.4) | <0.001 | 88.6 (22.1) | 96.0 (8.6) | <0.001 |
| Aspirin at discharge | 81.2 (26.1) | 88.5 (14.9) | <0.001 | 87.8 (22.7) | 94.8 (10.1) | <0.001 |
| ACE inhibitor for LV dysfunction | 72.1 (33.4) | 76.7 (22.9) | 0.010 | 83.2 (30.5) | 92.1 (14.8) | <0.001 |
| Beta blocker at discharge | 78.2 (27.9) | 87.0 (16.2) | <0.001 | 87.4 (23.4) | 95.5 (9.9) | <0.001 |
| Smoking cessation advice | 59.6 (40.8) | 74.5 (29.9) | <0.001 | 87.2 (29.5) | 97.2 (11.3) | <0.001 |
| PCI received within 90 min | 60.3 (26.2) | 60.6 (23.8) | 0.946 | 70.1 (24.8) | 77.7 (19.2) | 0.006 |
| Thrombolytic agent within 30 min | 27.9 (35.5) | 32.1 (32.8) | 0.152 | 31.4 (40.7) | 43.7 (40.2) | 0.008 |
| Composite AMI score | 80.6 (20.3) | 87.7 (10.4) | <0.001 | 85.8 (20.0) | 94.6 (8.1) | <0.001 |
| Heart failure | ||||||
| Discharge instructions | 36.8 (32.3) | 49.7 (28.2) | <0.001 | 67.4 (29.6) | 82.3 (16.4) | <0.001 |
| Assessment of LV function | 63.3 (27.6) | 83.6 (14.9) | <0.001 | 79.6 (24.4) | 95.6 (8.1) | <0.001 |
| ACE inhibitor for LV dysfunction | 70.8 (27.6) | 75.7 (16.3) | <0.001 | 82.5 (22.7) | 91.5 (9.7) | <0.001 |
| Smoking cessation advice | 57.1 (36.4) | 68.6 (26.2) | <0.001 | 81.5 (29.9) | 96.1 (10.7) | <0.001 |
| Composite heart failure score | 56.3 (24.1) | 71.2 (15.6) | <0.001 | 75.4 (22.3) | 90.4 (9.4) | <0.001 |
| Pneumonia | ||||||
| Oxygenation assessment | 97.4 (7.3) | 98.4 (4.0) | <0.001 | 99.0 (3.2) | 99.7 (1.2) | <0.001 |
| Pneumococcal vaccination | 45.5 (29.0) | 48.7 (26.2) | 0.007 | 79.9 (21.3) | 87.9 (12.9) | <0.001 |
| Timing of initial antibiotic therapy | 80.6 (13.1) | 70.9 (14.0) | <0.001 | 93.4 (9.2) | 93.6 (6.1) | 0.525 |
| Smoking cessation advice | 56.6 (33.1) | 65.7 (24.8) | <0.001 | 81.6 (25.1) | 94.4 (11.4) | <0.001 |
| Initial antibiotic selection | 73.6 (19.6) | 74.1 (13.4) | 0.508 | 86.1 (13.8) | 88.6 (8.7) | <0.001 |
| Composite pneumonia score | 77.2 (10.2) | 76.6 (8.2) | 0.119 | 90.0 (9.6) | 93.6 (4.9) | <0.001 |
| Overall composite | 73.7 (10.6) | 78.0 (8.7) | <0.001 | 86.8 (11.1) | 93.3 (5.0) | <0.001 |
Five‐Year Changes in Hospital Performance
Between 2004 and 2008, Joint Commission‐accredited hospitals improved their performance more than did non‐accredited hospitals (Table 3). After adjustment for baseline characteristics previously shown to be associated with performance, the overall relative (absolute) difference in improvement was 26% (4.2%) (AMI score difference 67% [3.9%], CHF 48% [10.1%], and pneumonia 21% [3.7%]). Accredited hospitals improved their performance significantly more than non‐accredited for 13 of the 16 individual performance measures.
| Characteristic | Change in Performance* | Absolute Difference, Always vs Never (95% CI) | Relative Difference, % Always vs Never | P Value | |
|---|---|---|---|---|---|
| Never Accredited (n = 762) | Always Accredited (n = 2,917) | ||||
| |||||
| AMI | |||||
| Aspirin at admission | 1.1 | 2.0 | 3.2 (1.25.2) | 160 | 0.001 |
| Aspirin at discharge | 4.7 | 8.0 | 3.2 (1.45.1) | 40 | 0.008 |
| ACE inhibitor for LV dysfunction | 8.5 | 15.9 | 7.4 (3.711.5) | 47 | <0.001 |
| Beta blocker at discharge | 4.4 | 8.4 | 4.0 (2.06.0) | 48 | <0.001 |
| Smoking cessation advice | 18.6 | 22.4 | 3.7 (1.16.9) | 17 | 0.012 |
| PCI received within 90 min | 6.3 | 13.0 | 6.7 (0.314.2) | 52 | 0.070 |
| Thrombolytic agent within 30 min | 0.6 | 5.4 | 6.1 (9.520.4) | 113 | 0.421 |
| Composite AMI score | 2.0 | 5.8 | 3.9 (2.25.5) | 67 | <0.001 |
| Heart failure | |||||
| Discharge instructions | 24.2 | 35.6 | 11.4 (8.714.0) | 32 | <0.001 |
| Assessment of LV function | 4.6 | 12.8 | 8.3 (6.610.0) | 65 | <0.001 |
| ACE inhibitor for LV dysfunction | 10.1 | 15.2 | 5.1 (3.56.8) | 34 | <0.001 |
| Smoking cessation advice | 20.5 | 26.4 | 6.0 (3.38.7) | 23 | <0.001 |
| Composite heart failure score | 10.8 | 20.9 | 10.1 (8.312.0) | 48 | <0.001 |
| Pneumonia | |||||
| Oxygenation assessment | 0.9 | 1.4 | 0.6 (0.30.9) | 43 | <0.001 |
| Pneumococcal vaccination | 33.4 | 40.9 | 7.5 (5.69.4) | 18 | <0.001 |
| Timing of initial antibiotic therapy | 19.2 | 21.1 | 1.9 (1.12.7) | 9 | <0.001 |
| Smoking cessation advice | 21.8 | 27.9 | 6.0 (3.88.3) | 22 | <0.001 |
| Initial antibiotic selection | 13.6 | 14.3 | 0.7 (0.51.9) | 5 | 0.293 |
| Composite pneumonia score | 13.7 | 17.5 | 3.7 (2.84.6) | 21 | <0.001 |
| Overall composite | 12.0 | 16.1 | 4.2 (3.25.1) | 26 | <0.001 |
High Performing Hospitals in 2008
The likelihood that a hospital was a high performer in 2008 was significantly associated with Joint Commission accreditation status, with a higher proportion of accredited hospitals reaching the 90% threshold compared to never‐accredited hospitals (Table 4). Accredited hospitals attained the 90% threshold significantly more often for 13 of the 16 performance measures and all four summary scores, compared to non‐accredited hospitals. In 2008, 82% of Joint Commission‐accredited hospitals demonstrated greater than 90% on the overall summary score, compared to 48% of never‐accredited hospitals. Even after adjusting for differences among hospitals, including performance at baseline, Joint Commission‐accredited hospitals were more likely than never‐accredited hospitals to exceed 90% performance in 2008 (84% vs 69%).
| Characteristic | Percent of Hospitals with Performance Over 90% Adjusted (Actual) | Odds Ratio, Always vs Never (95% CI) | P Value | |
|---|---|---|---|---|
| Never Accredited (n = 762) | Always Accredited (n = 2,917) | |||
| ||||
| AMI | ||||
| Aspirin at admission | 91.8 (71.8) | 93.9 (90.7) | 1.38 (1.001.89) | 0.049 |
| Aspirin at discharge | 83.7 (69.2) | 88.2 (85.1) | 1.45 (1.081.94) | 0.013 |
| ACE inhibitor for LV dysfunction | 65.1 (65.8) | 77.2 (76.5) | 1.81 (1.322.50) | <0.001 |
| Beta blocker at discharge | 84.7 (69.4) | 90.9 (88.4) | 1.80 (1.332.44) | <0.001 |
| Smoking cessation advice | 91.1 (81.3) | 95.9 (94.1) | 2.29 (1.314.01) | 0.004 |
| PCI received within 90 min | 21.5 (16.2) | 29.9 (29.8) | 1.56 (0.713.40) | 0.265 |
| Thrombolytic agent within 30 min | 21.4 (21.3) | 22.7 (23.6) | 1.08 (0.422.74) | 0.879 |
| Composite AMI score | 80.5 (56.6) | 88.2 (85.9) | 1.82 (1.372.41) | <0.001 |
| Heart failure | ||||
| Discharge instructions | 27.0 (26.3) | 38.9 (39.3) | 1.72 (1.302.27) | <0.001 |
| Assessment of LV function | 76.2 (45.0) | 89.1 (88.8) | 2.54 (1.953.31) | <0.001 |
| ACE inhibitor for LV dysfunction | 58.0 (51.4) | 67.8 (68.5) | 1.52 (1.211.92) | <0.001 |
| Smoking cessation advice | 84.2 (62.3) | 90.3 (89.2) | 1.76 (1.282.43) | <0.001 |
| Composite heart failure score | 38.2 (27.6) | 61.5 (64.6) | 2.57 (2.033.26) | <0.001 |
| Pneumonia | ||||
| Oxygenation assessment | 100 (98.2) | 100 (99.8) | 4.38 (1.201.32) | 0.025 |
| Pneumococcal vaccination | 44.1 (40.3) | 57.3 (58.2) | 1.70 (1.362.12) | <0.001 |
| Timing of initial antibiotic therapy | 74.3 (79.1) | 84.2 (82.7) | 1.85 (1.402.46) | <0.001 |
| Smoking cessation advice | 76.2 (54.6) | 85.8 (84.2) | 1.89 (1.422.51) | <0.001 |
| Initial antibiotic selection | 51.8 (47.4) | 51.0 (51.8) | 0.97 (0.761.25) | 0.826 |
| Composite pneumonia score | 69.3 (59.4) | 85.3 (83.9) | 2.58 (2.013.31) | <0.001 |
| Overall composite | 69.0 (47.5) | 83.8 (82.0) | 2.32 (1.763.06) | <0.001 |
DISCUSSION
While accreditation has face validity and is desired by key stakeholders, it is expensive and time consuming. Stakeholders thus are justified in seeking evidence that accreditation is associated with better quality and safety. Ideally, not only would it be associated with better performance at a single point in time, it would also be associated with the pace of improvement over time.
Our study is the first, to our knowledge, to show the association of accreditation status with improvement in the trajectory of performance over a five‐year period. Taking advantage of the fact that the accreditation process changed substantially at about the same time that TJC and CMS began requiring public reporting of evidence‐based quality measures, we found that hospitals accredited by The Joint Commission had had larger improvements in hospital performance from 2004 to 2008 than non‐accredited hospitals, even though the former started with higher baseline performance levels. This accelerated improvement was broad‐based: Accredited hospitals were more likely to achieve superior performance (greater than 90% adherence to quality measures) in 2008 on 13 of 16 nationally standardized quality‐of‐care measures, three clinical area summary scores, and an overall score compared to hospitals that were not accredited. These results are consistent with other studies that have looked at both process and outcome measures and accreditation.912
It is important to note that the observed accreditation effect reflects a difference between hospitals that have elected to seek one particular self‐regulatory alternative to the more restrictive and extensive public regulatory or licensure requirements with those that have not.39 The non‐accredited hospitals that were included in this study are not considered to be sub‐standard hospitals. In fact, hospitals not accredited by The Joint Commission have also met the standards set by Medicare in the Conditions of Participation, and our study demonstrates that these hospitals achieved reasonably strong performance on publicly reported quality measures (86.8% adherence on the composite measure in 2008) and considerable improvement over the 5 years of public reporting (average improvement on composite measure from 2004 to 2008 of 11.8%). Moreover, there are many paths to improvement, and some non‐accredited hospitals achieve stellar performance on quality measures, perhaps by embracing other methods to catalyze improvement.
That said, our data demonstrate that, on average, accredited hospitals achieve superior performance on these evidence‐based quality measures, and their performance improved more strikingly over time. In interpreting these results, it is important to recognize that, while Joint Commission‐accredited hospitals must report quality data, performance on these measures is not directly factored into the accreditation decision; if this were not so, one could argue that this association is a statistical tautology. As it is, we believe that the 2 measures (accreditation and publicly reported quality measures) are two independent assessments of the quality of an organization, and, while the performance measures may not be a gold standard, a measure of their association does provide useful information about the degree to which accreditation is linked to organizational quality.
There are several potential limitations of the current study. First, while we adjusted for most of the known hospital demographic and organizational factors associated with performance, there may be unidentified factors that are associated with both accreditation and performance. This may not be relevant to a patient or payer choosing a hospital based on accreditation status (who may not care whether accreditation is simply associated with higher quality or actually helps produce such quality), but it is relevant to policy‐makers, who may weigh the value of embracing accreditation versus other maneuvers (such as pay for performance or new educational requirements) as a vehicle to promote high‐quality care.
A second limitation is that the specification of the measures can change over time due to the acquisition of new clinical knowledge, which makes longitudinal comparison and tracking of results over time difficult. There were two measures that had definitional changes that had noticeable impact on longitudinal trends: the AMI measure Primary Percutaneous Coronary Intervention (PCI) Received within 90 Minutes of Hospital Arrival (which in 2004 and 2005 used 120 minutes as the threshold), and the pneumonia measure Antibiotic Within 4 Hours of Arrival (which in 2007 changed the threshold to six hours). Other changes included adding angiotensin‐receptor blocker therapy (ARB) as an alternative to angiotensin‐converting enzyme inhibitor (ACEI) therapy in 2005 to the AMI and heart failure measures ACEI or ARB for left ventricular dysfunction. Other less significant changes have been made to the data collection methods for other measures, which could impact the interpretation of changes in performance over time. That said, these changes influenced both accredited and non‐accredited hospitals equally, and we cannot think of reasons that they would have created differential impacts.
Another limitation is that the 16 process measures provide a limited picture of hospital performance. Although the three conditions in the study account for over 15% of Medicare admissions,19 it is possible that non‐accredited hospitals performed as well as accredited hospitals on other measures of quality that were not captured by the 16 measures. As more standardized measures are added to The Joint Commission and CMS databases, it will be possible to use the same study methodology to incorporate these additional domains.
From the original cohort of 4798 hospitals reporting in 2004 or 2008, 19% were not included in the study due to missing data in either 2004 or 2008. Almost two‐thirds of the hospitals excluded from the study were missing 2004 data and, of these, 77% were critical access hospitals. The majority of these critical access hospitals (97%) were non‐accredited. This is in contrast to the hospitals missing 2008 data, of which only 13% were critical access. Since reporting of data to Hospital Compare was voluntary in 2004, it appears that critical access hospitals chose to wait later to report data to Hospital Compare, compared to acute care hospitals. Since critical access hospitals tended to have lower rates, smaller sample sizes, and be non‐accredited, the results of the study would be expected to slightly underestimate the difference between accredited and non‐accredited hospitals.
Finally, while we have argued that the publicly reported quality measures and TJC accreditation decisions provide different lenses into the quality of a given hospital, we cannot entirely exclude the possibility that there are subtle relationships between these two methods that might be partly responsible for our findings. For example, while performance measure rates do not factor directly into the accreditation decision, it is possible that Joint Commission surveyors may be influenced by their knowledge of these rates and biased in their scoring of unrelated standards during the survey process. While we cannot rule out such biases, we are aware of no research on the subject, and have no reason to believe that such biases may have confounded the analysis.
In summary, we found that Joint Commission‐accredited hospitals outperformed non‐accredited hospitals on nationally standardized quality measures of AMI, heart failure, and pneumonia. The performance gap between Joint Commission‐accredited and non‐accredited hospitals increased over the five years of the study. Future studies should incorporate more robust and varied measures of quality as outcomes, and seek to examine the nature of the observed relationship (ie, whether accreditation is simply a marker of higher quality and more rapid improvement, or the accreditation process actually helps create these salutary outcomes).
Acknowledgements
The authors thank Barbara Braun, PhD and Nicole Wineman, MPH, MBA for their literature review on the impact of accreditation, and Barbara Braun, PhD for her thoughtful review of the manuscript.
The Joint Commission (TJC) currently accredits approximately 4546 acute care, critical access, and specialty hospitals,1 accounting for approximately 82% of U.S. hospitals (representing 92% of hospital beds). Hospitals seeking to earn and maintain accreditation undergo unannounced on‐site visits by a team of Joint Commission surveyors at least once every 3 years. These surveys address a variety of domains, including the environment of care, infection prevention and control, information management, adherence to a series of national patient safety goals, and leadership.1
The survey process has changed markedly in recent years. Since 2002, accredited hospitals have been required to continuously collect and submit selected performance measure data to The Joint Commission throughout the three‐year accreditation cycle. The tracer methodology, an evaluation method in which surveyors select a patient to follow through the organization in order to assess compliance with selected standards, was instituted in 2004. Soon thereafter, on‐site surveys went from announced to unannounced in 2006.
Despite the 50+ year history of hospital accreditation in the United States, there has been surprisingly little research on the link between accreditation status and measures of hospital quality (both processes and outcomes). It is only recently that a growing number of studies have attempted to examine this relationship. Empirical support for the relationship between accreditation and other quality measures is emerging. Accredited hospitals have been shown to provide better emergency response planning2 and training3 compared to non‐accredited hospitals. Accreditation has been observed to be a key predictor of patient safety system implementation4 and the primary driver of hospitals' patient‐safety initiatives.5 Accredited trauma centers have been associated with significant reductions in patient mortality,6 and accreditation has been linked to better compliance with evidence‐based methadone and substance abuse treatment.7, 8 Accredited hospitals have been shown to perform better on measures of hospital quality in acute myocardial infarction (AMI), heart failure, and pneumonia care.9, 10 Similarly, accreditation has been associated with lower risk‐adjusted in‐hospital mortality rates for congestive heart failure (CHF), stroke, and pneumonia.11, 12 The results of such research, however, have not always been consistent. Several studies have been unable to demonstrate a relationship between accreditation and quality measures. A study of financial and cost‐related outcome measures found no relationship to accreditation,13 and a study comparing medication error rates across different types of organizations found no relationship to accreditation status.14 Similarly, a comparison of accredited versus non‐accredited ambulatory surgical organizations found that patients were less likely to be hospitalized when treated at an accredited facility for colonoscopy procedures, but no such relationship was observed for the other 4 procedures studied.15
While the research to date has been generally supportive of the link between accreditation and other measures of health care quality, the studies were typically limited to only a few measures and/or involved relatively small samples of accredited and non‐accredited organizations. Over the last decade, however, changes in the performance measurement landscape have created previously unavailable opportunities to more robustly examine the relationship between accreditation and other indicators of hospital quality.
At about the same time that The Joint Commission's accreditation process was becoming more vigorous, the Centers for Medicare and Medicaid Services (CMS) began a program of publicly reporting quality data (
By using a population of hospitals and a range of standardized quality measures greater than those used in previous studies, we seek to address the following questions: Is Joint Commission accreditation status truly associated with higher quality care? And does accreditation status help identify hospitals that are more likely to improve their quality and safety over time?
METHODS
Performance Measures
Since July 2002, U.S. hospitals have been collecting data on standardized measures of quality developed by The Joint Commission and CMS. These measures have been endorsed by the National Quality Forum16 and adopted by the Hospital Quality Alliance.17 The first peer‐reviewed reports using The Joint Commission/CMS measure data confirmed that the measures could successfully monitor and track hospital improvement and identify disparities in performance,18, 19 as called for by the Institute of Medicine's (IOM) landmark 2001 report, Crossing the Quality Chasm.20
In order to promote transparency in health care, both CMSthrough the efforts of the Hospital Quality Allianceand The Joint Commission began publicly reporting measure rates in 2004 using identical measure and data element specifications. It is important to note that during the five‐year span covered by this study, both The Joint Commission and CMS emphasized the reporting of performance measure data. While performance improvement has been the clear objective of these efforts, neither organization established targets for measure rates or set benchmarks for performance improvement. Similarly, while Joint Commission‐accredited hospitals were required to submit performance measure data as a condition of accreditation, their actual performance on the measure rates did not factor into the accreditation decision. In the absence of such direct leverage, it is interesting to note that several studies have demonstrated the positive impact of public reporting on hospital performance,21 and on providing useful information to the general public and health care professionals regarding hospital quality.22
The 16 measures used in this study address hospital compliance with evidence‐based processes of care recommended by the clinical treatment guidelines of respected professional societies.23 Process of care measures are particularly well suited for quality improvement purposes, as they can identify deficiencies which can be immediately addressed by hospitals and do not require risk‐adjustment, as opposed to outcome measures, which do not necessarily directly identify obvious performance improvement opportunities.2426 The measures were also implemented in sets in order to provide hospitals with a more complete portrayal of quality than might be provided using unrelated individual measures. Research has demonstrated that greater collective performance on these process measures is associated with improved one‐year survival after heart failure hospitalization27 and inpatient mortality for those Medicare patients discharged with acute myocardial infarction, heart failure, and pneumonia,28 while other research has shown little association with short‐term outcomes.29
Using the Specifications Manual for National Hospital Inpatient Quality Measures,16 hospitals identify the initial measure populations through International Classification of Diseases (ICD‐CM‐9) codes and patient age obtained through administrative data. Trained abstractors then collect the data for measure‐specific data elements through medical record review on the identified measure population or a sample of this population. Measure algorithms then identify patients in the numerator and denominator of each measure.
Process measure rates reflect the number of times a hospital treated a patient in a manner consistent with specific evidence‐based clinical practice guidelines (numerator cases), divided by the number of patients who were eligible to receive such care (denominator cases). Because precise measure specifications permit the exclusion of patients contraindicated to receive the specific process of care for the measure, ideal performance should be characterized by measure rates that approach 100% (although rare or unpredictable situations, and the reality that no measure is perfect in its design, make consistent performance at 100% improbable). Accuracy of the measure data, as measured by data element agreement rates on reabstraction, has been reported to exceed 90%.30
In addition to the individual performance measures, hospital performance was assessed using 3 condition‐specific summary scores, one for each of the 3 clinical areas: acute myocardial infarction, heart failure, and pneumonia. The summary scores are a weighted average of the individual measure rates in the clinical area, where the weights are the sample sizes for each of the measures.31 A summary score was also calculated based on all 16 measures as a summary measure of overall compliance with recommended care.
One way of studying performance measurement in a way that relates to standards is to evaluate whether a hospital achieves a high rate of performance, where high is defined as a performance rate of 90% or more. In this context, measures were created from each of the 2004 and 2008 hospital performance rates by dichotomizing them as being either less than 90%, or greater than or equal to 90%.32
Data Sources
The data for the measures included in the study are available on the CMS Hospital Compare public databases or The Joint Commission for discharges in 2004 and 2008.33 These 16 measures, active for all 5 years of the study period, include: 7 measures related to acute myocardial infarction care; 4 measures related to heart failure care; and 5 measures related to pneumonia care. The majority of the performance data for the study were obtained from the yearly CMS Hospital Compare public download databases (
Hospital Characteristics
We then linked the CMS performance data, augmented by The Joint Commission performance data when necessary, to hospital characteristics data in the American Hospital Association (AHA) Annual Survey with respect to profit status, number of beds (<100 beds, 100299 beds, 300+ beds), rural status, geographic region, and whether or not the hospital was a critical access hospital. (Teaching status, although available in the AHA database, was not used in the analysis, as almost all teaching hospitals are Joint Commission accredited.) These characteristics were chosen since previous research has identified them as being associated with hospital quality.9, 19, 3437 Data on accreditation status were obtained from The Joint Commission's hospital accreditation database. Hospitals were grouped into 3 hospital accreditation strata based on longitudinal hospital accreditation status between 2004 and 2008: 1) hospitals not accredited in the study period; 2) hospitals accredited between one to four years; and 3) hospitals accredited for the entire study period. Analyses of this middle group (those hospitals accredited for part of the study period; n = 212, 5.4% of the whole sample) led to no significant change in our findings (their performance tended to be midway between always accredited and never‐accredited hospitals) and are thus omitted from our results. Instead, we present only hospitals who were never accredited (n = 762) and those who were accredited through the entire study period (n = 2917).
Statistical Analysis
We assessed the relationship between hospital characteristics and 2004 performance of Joint Commission‐accredited hospitals with hospitals that were not Joint Commission accredited using 2 tests for categorical variables and t tests for continuous variables. Linear regression was used to estimate the five‐year change in performance at each hospital as a function of accreditation group, controlling for hospital characteristics. Baseline hospital performance was also included in the regression models to control for ceiling effects for those hospitals with high baseline performance. To summarize the results, we used the regression models to calculate adjusted change in performance for each accreditation group, and calculated a 95% confidence interval and P value for the difference between the adjusted change scores, using bootstrap methods.38
Next we analyzed the association between accreditation and the likelihood of high 2008 hospital performance by dichotomizing the hospital rates, using a 90% cut point, and using logistic regression to estimate the probability of high performance as a function of accreditation group, controlling for hospital characteristics and baseline hospital performance. The logistic models were then used to calculate adjusted rates of high performance for each accreditation group in presenting the results.
We used two‐sided tests for significance; P < 0.05 was considered statistically significant. This study had no external funding source.
RESULTS
For the 16 individual measures used in this study, a total of 4798 hospitals participated in Hospital Compare or reported data to The Joint Commission in 2004 or 2008. Of these, 907 were excluded because the performance data were not available for either 2004 (576 hospitals) or 2008 (331 hospitals) resulting in a missing value for the change in performance score. Therefore, 3891 hospitals (81%) were included in the final analyses. The 907 excluded hospitals were more likely to be rural (50.8% vs 17.5%), be critical access hospitals (53.9% vs 13.9%), have less than 100 beds (77.4% vs 37.6%), be government owned (34.6% vs 22.1%), be for profit (61.4% vs 49.5%), or be unaccredited (79.8% vs 45.8% in 2004; 75.6% vs 12.8% in 2008), compared with the included hospitals (P < 0.001 for all comparisons).
Hospital Performance at Baseline
Joint Commission‐accredited hospitals were more likely to be large, for profit, or urban, and less likely to be government owned, from the Midwest, or critical access (Table 1). Non‐accredited hospitals performed more poorly than accredited hospitals on most of the publicly reported measures in 2004; the only exception is the timing of initial antibiotic therapy measure for pneumonia (Table 2).
| Characteristic | Non‐Accredited (n = 786) | Accredited (n = 3105) | P Value* |
|---|---|---|---|
| |||
| Profit status, No. (%) | <0.001 | ||
| For profit | 60 (7.6) | 586 (18.9) | |
| Government | 289 (36.8) | 569 (18.3) | |
| Not for profit | 437 (55.6) | 1,950 (62.8) | |
| Census region, No. (%) | <0.001 | ||
| Northeast | 72 (9.2) | 497 (16.0) | |
| Midwest | 345 (43.9) | 716 (23.1) | |
| South | 248 (31.6) | 1,291 (41.6) | |
| West | 121 (15.4) | 601 (19.4) | |
| Rural setting, No. (%) | <0.001 | ||
| Rural | 495 (63.0) | 833 (26.8) | |
| Urban | 291 (37.0) | 2,272 (73.2) | |
| Bed size | <0.001 | ||
| <100 beds | 603 (76.7) | 861 (27.7) | |
| 100299 beds | 158 (20.1) | 1,444 (46.5) | |
| 300+ beds | 25 (3.2) | 800 (25.8) | |
| Critical access hospital status, No. (%) | <0.001 | ||
| Critical access hospital | 376 (47.8) | 164 (5.3) | |
| Acute care hospital | 410 (52.2) | 2,941 (94.7) | |
| Quality Measure, Mean (SD)* | 2004 | 2008 | ||||
|---|---|---|---|---|---|---|
| Non‐Accredited | Accredited | P Value | Non‐Accredited | Accredited | P Value | |
| (n = 786) | (n = 3105) | (n = 950) | (n = 2,941) | |||
| ||||||
| AMI | ||||||
| Aspirin at admission | 87.1 (20.0) | 92.6 (9.4) | <0.001 | 88.6 (22.1) | 96.0 (8.6) | <0.001 |
| Aspirin at discharge | 81.2 (26.1) | 88.5 (14.9) | <0.001 | 87.8 (22.7) | 94.8 (10.1) | <0.001 |
| ACE inhibitor for LV dysfunction | 72.1 (33.4) | 76.7 (22.9) | 0.010 | 83.2 (30.5) | 92.1 (14.8) | <0.001 |
| Beta blocker at discharge | 78.2 (27.9) | 87.0 (16.2) | <0.001 | 87.4 (23.4) | 95.5 (9.9) | <0.001 |
| Smoking cessation advice | 59.6 (40.8) | 74.5 (29.9) | <0.001 | 87.2 (29.5) | 97.2 (11.3) | <0.001 |
| PCI received within 90 min | 60.3 (26.2) | 60.6 (23.8) | 0.946 | 70.1 (24.8) | 77.7 (19.2) | 0.006 |
| Thrombolytic agent within 30 min | 27.9 (35.5) | 32.1 (32.8) | 0.152 | 31.4 (40.7) | 43.7 (40.2) | 0.008 |
| Composite AMI score | 80.6 (20.3) | 87.7 (10.4) | <0.001 | 85.8 (20.0) | 94.6 (8.1) | <0.001 |
| Heart failure | ||||||
| Discharge instructions | 36.8 (32.3) | 49.7 (28.2) | <0.001 | 67.4 (29.6) | 82.3 (16.4) | <0.001 |
| Assessment of LV function | 63.3 (27.6) | 83.6 (14.9) | <0.001 | 79.6 (24.4) | 95.6 (8.1) | <0.001 |
| ACE inhibitor for LV dysfunction | 70.8 (27.6) | 75.7 (16.3) | <0.001 | 82.5 (22.7) | 91.5 (9.7) | <0.001 |
| Smoking cessation advice | 57.1 (36.4) | 68.6 (26.2) | <0.001 | 81.5 (29.9) | 96.1 (10.7) | <0.001 |
| Composite heart failure score | 56.3 (24.1) | 71.2 (15.6) | <0.001 | 75.4 (22.3) | 90.4 (9.4) | <0.001 |
| Pneumonia | ||||||
| Oxygenation assessment | 97.4 (7.3) | 98.4 (4.0) | <0.001 | 99.0 (3.2) | 99.7 (1.2) | <0.001 |
| Pneumococcal vaccination | 45.5 (29.0) | 48.7 (26.2) | 0.007 | 79.9 (21.3) | 87.9 (12.9) | <0.001 |
| Timing of initial antibiotic therapy | 80.6 (13.1) | 70.9 (14.0) | <0.001 | 93.4 (9.2) | 93.6 (6.1) | 0.525 |
| Smoking cessation advice | 56.6 (33.1) | 65.7 (24.8) | <0.001 | 81.6 (25.1) | 94.4 (11.4) | <0.001 |
| Initial antibiotic selection | 73.6 (19.6) | 74.1 (13.4) | 0.508 | 86.1 (13.8) | 88.6 (8.7) | <0.001 |
| Composite pneumonia score | 77.2 (10.2) | 76.6 (8.2) | 0.119 | 90.0 (9.6) | 93.6 (4.9) | <0.001 |
| Overall composite | 73.7 (10.6) | 78.0 (8.7) | <0.001 | 86.8 (11.1) | 93.3 (5.0) | <0.001 |
Five‐Year Changes in Hospital Performance
Between 2004 and 2008, Joint Commission‐accredited hospitals improved their performance more than did non‐accredited hospitals (Table 3). After adjustment for baseline characteristics previously shown to be associated with performance, the overall relative (absolute) difference in improvement was 26% (4.2%) (AMI score difference 67% [3.9%], CHF 48% [10.1%], and pneumonia 21% [3.7%]). Accredited hospitals improved their performance significantly more than non‐accredited for 13 of the 16 individual performance measures.
| Characteristic | Change in Performance* | Absolute Difference, Always vs Never (95% CI) | Relative Difference, % Always vs Never | P Value | |
|---|---|---|---|---|---|
| Never Accredited (n = 762) | Always Accredited (n = 2,917) | ||||
| |||||
| AMI | |||||
| Aspirin at admission | 1.1 | 2.0 | 3.2 (1.25.2) | 160 | 0.001 |
| Aspirin at discharge | 4.7 | 8.0 | 3.2 (1.45.1) | 40 | 0.008 |
| ACE inhibitor for LV dysfunction | 8.5 | 15.9 | 7.4 (3.711.5) | 47 | <0.001 |
| Beta blocker at discharge | 4.4 | 8.4 | 4.0 (2.06.0) | 48 | <0.001 |
| Smoking cessation advice | 18.6 | 22.4 | 3.7 (1.16.9) | 17 | 0.012 |
| PCI received within 90 min | 6.3 | 13.0 | 6.7 (0.314.2) | 52 | 0.070 |
| Thrombolytic agent within 30 min | 0.6 | 5.4 | 6.1 (9.520.4) | 113 | 0.421 |
| Composite AMI score | 2.0 | 5.8 | 3.9 (2.25.5) | 67 | <0.001 |
| Heart failure | |||||
| Discharge instructions | 24.2 | 35.6 | 11.4 (8.714.0) | 32 | <0.001 |
| Assessment of LV function | 4.6 | 12.8 | 8.3 (6.610.0) | 65 | <0.001 |
| ACE inhibitor for LV dysfunction | 10.1 | 15.2 | 5.1 (3.56.8) | 34 | <0.001 |
| Smoking cessation advice | 20.5 | 26.4 | 6.0 (3.38.7) | 23 | <0.001 |
| Composite heart failure score | 10.8 | 20.9 | 10.1 (8.312.0) | 48 | <0.001 |
| Pneumonia | |||||
| Oxygenation assessment | 0.9 | 1.4 | 0.6 (0.30.9) | 43 | <0.001 |
| Pneumococcal vaccination | 33.4 | 40.9 | 7.5 (5.69.4) | 18 | <0.001 |
| Timing of initial antibiotic therapy | 19.2 | 21.1 | 1.9 (1.12.7) | 9 | <0.001 |
| Smoking cessation advice | 21.8 | 27.9 | 6.0 (3.88.3) | 22 | <0.001 |
| Initial antibiotic selection | 13.6 | 14.3 | 0.7 (0.51.9) | 5 | 0.293 |
| Composite pneumonia score | 13.7 | 17.5 | 3.7 (2.84.6) | 21 | <0.001 |
| Overall composite | 12.0 | 16.1 | 4.2 (3.25.1) | 26 | <0.001 |
High Performing Hospitals in 2008
The likelihood that a hospital was a high performer in 2008 was significantly associated with Joint Commission accreditation status, with a higher proportion of accredited hospitals reaching the 90% threshold compared to never‐accredited hospitals (Table 4). Accredited hospitals attained the 90% threshold significantly more often for 13 of the 16 performance measures and all four summary scores, compared to non‐accredited hospitals. In 2008, 82% of Joint Commission‐accredited hospitals demonstrated greater than 90% on the overall summary score, compared to 48% of never‐accredited hospitals. Even after adjusting for differences among hospitals, including performance at baseline, Joint Commission‐accredited hospitals were more likely than never‐accredited hospitals to exceed 90% performance in 2008 (84% vs 69%).
| Characteristic | Percent of Hospitals with Performance Over 90% Adjusted (Actual) | Odds Ratio, Always vs Never (95% CI) | P Value | |
|---|---|---|---|---|
| Never Accredited (n = 762) | Always Accredited (n = 2,917) | |||
| ||||
| AMI | ||||
| Aspirin at admission | 91.8 (71.8) | 93.9 (90.7) | 1.38 (1.001.89) | 0.049 |
| Aspirin at discharge | 83.7 (69.2) | 88.2 (85.1) | 1.45 (1.081.94) | 0.013 |
| ACE inhibitor for LV dysfunction | 65.1 (65.8) | 77.2 (76.5) | 1.81 (1.322.50) | <0.001 |
| Beta blocker at discharge | 84.7 (69.4) | 90.9 (88.4) | 1.80 (1.332.44) | <0.001 |
| Smoking cessation advice | 91.1 (81.3) | 95.9 (94.1) | 2.29 (1.314.01) | 0.004 |
| PCI received within 90 min | 21.5 (16.2) | 29.9 (29.8) | 1.56 (0.713.40) | 0.265 |
| Thrombolytic agent within 30 min | 21.4 (21.3) | 22.7 (23.6) | 1.08 (0.422.74) | 0.879 |
| Composite AMI score | 80.5 (56.6) | 88.2 (85.9) | 1.82 (1.372.41) | <0.001 |
| Heart failure | ||||
| Discharge instructions | 27.0 (26.3) | 38.9 (39.3) | 1.72 (1.302.27) | <0.001 |
| Assessment of LV function | 76.2 (45.0) | 89.1 (88.8) | 2.54 (1.953.31) | <0.001 |
| ACE inhibitor for LV dysfunction | 58.0 (51.4) | 67.8 (68.5) | 1.52 (1.211.92) | <0.001 |
| Smoking cessation advice | 84.2 (62.3) | 90.3 (89.2) | 1.76 (1.282.43) | <0.001 |
| Composite heart failure score | 38.2 (27.6) | 61.5 (64.6) | 2.57 (2.033.26) | <0.001 |
| Pneumonia | ||||
| Oxygenation assessment | 100 (98.2) | 100 (99.8) | 4.38 (1.201.32) | 0.025 |
| Pneumococcal vaccination | 44.1 (40.3) | 57.3 (58.2) | 1.70 (1.362.12) | <0.001 |
| Timing of initial antibiotic therapy | 74.3 (79.1) | 84.2 (82.7) | 1.85 (1.402.46) | <0.001 |
| Smoking cessation advice | 76.2 (54.6) | 85.8 (84.2) | 1.89 (1.422.51) | <0.001 |
| Initial antibiotic selection | 51.8 (47.4) | 51.0 (51.8) | 0.97 (0.761.25) | 0.826 |
| Composite pneumonia score | 69.3 (59.4) | 85.3 (83.9) | 2.58 (2.013.31) | <0.001 |
| Overall composite | 69.0 (47.5) | 83.8 (82.0) | 2.32 (1.763.06) | <0.001 |
DISCUSSION
While accreditation has face validity and is desired by key stakeholders, it is expensive and time consuming. Stakeholders thus are justified in seeking evidence that accreditation is associated with better quality and safety. Ideally, not only would it be associated with better performance at a single point in time, it would also be associated with the pace of improvement over time.
Our study is the first, to our knowledge, to show the association of accreditation status with improvement in the trajectory of performance over a five‐year period. Taking advantage of the fact that the accreditation process changed substantially at about the same time that TJC and CMS began requiring public reporting of evidence‐based quality measures, we found that hospitals accredited by The Joint Commission had had larger improvements in hospital performance from 2004 to 2008 than non‐accredited hospitals, even though the former started with higher baseline performance levels. This accelerated improvement was broad‐based: Accredited hospitals were more likely to achieve superior performance (greater than 90% adherence to quality measures) in 2008 on 13 of 16 nationally standardized quality‐of‐care measures, three clinical area summary scores, and an overall score compared to hospitals that were not accredited. These results are consistent with other studies that have looked at both process and outcome measures and accreditation.912
It is important to note that the observed accreditation effect reflects a difference between hospitals that have elected to seek one particular self‐regulatory alternative to the more restrictive and extensive public regulatory or licensure requirements with those that have not.39 The non‐accredited hospitals that were included in this study are not considered to be sub‐standard hospitals. In fact, hospitals not accredited by The Joint Commission have also met the standards set by Medicare in the Conditions of Participation, and our study demonstrates that these hospitals achieved reasonably strong performance on publicly reported quality measures (86.8% adherence on the composite measure in 2008) and considerable improvement over the 5 years of public reporting (average improvement on composite measure from 2004 to 2008 of 11.8%). Moreover, there are many paths to improvement, and some non‐accredited hospitals achieve stellar performance on quality measures, perhaps by embracing other methods to catalyze improvement.
That said, our data demonstrate that, on average, accredited hospitals achieve superior performance on these evidence‐based quality measures, and their performance improved more strikingly over time. In interpreting these results, it is important to recognize that, while Joint Commission‐accredited hospitals must report quality data, performance on these measures is not directly factored into the accreditation decision; if this were not so, one could argue that this association is a statistical tautology. As it is, we believe that the 2 measures (accreditation and publicly reported quality measures) are two independent assessments of the quality of an organization, and, while the performance measures may not be a gold standard, a measure of their association does provide useful information about the degree to which accreditation is linked to organizational quality.
There are several potential limitations of the current study. First, while we adjusted for most of the known hospital demographic and organizational factors associated with performance, there may be unidentified factors that are associated with both accreditation and performance. This may not be relevant to a patient or payer choosing a hospital based on accreditation status (who may not care whether accreditation is simply associated with higher quality or actually helps produce such quality), but it is relevant to policy‐makers, who may weigh the value of embracing accreditation versus other maneuvers (such as pay for performance or new educational requirements) as a vehicle to promote high‐quality care.
A second limitation is that the specification of the measures can change over time due to the acquisition of new clinical knowledge, which makes longitudinal comparison and tracking of results over time difficult. There were two measures that had definitional changes that had noticeable impact on longitudinal trends: the AMI measure Primary Percutaneous Coronary Intervention (PCI) Received within 90 Minutes of Hospital Arrival (which in 2004 and 2005 used 120 minutes as the threshold), and the pneumonia measure Antibiotic Within 4 Hours of Arrival (which in 2007 changed the threshold to six hours). Other changes included adding angiotensin‐receptor blocker therapy (ARB) as an alternative to angiotensin‐converting enzyme inhibitor (ACEI) therapy in 2005 to the AMI and heart failure measures ACEI or ARB for left ventricular dysfunction. Other less significant changes have been made to the data collection methods for other measures, which could impact the interpretation of changes in performance over time. That said, these changes influenced both accredited and non‐accredited hospitals equally, and we cannot think of reasons that they would have created differential impacts.
Another limitation is that the 16 process measures provide a limited picture of hospital performance. Although the three conditions in the study account for over 15% of Medicare admissions,19 it is possible that non‐accredited hospitals performed as well as accredited hospitals on other measures of quality that were not captured by the 16 measures. As more standardized measures are added to The Joint Commission and CMS databases, it will be possible to use the same study methodology to incorporate these additional domains.
From the original cohort of 4798 hospitals reporting in 2004 or 2008, 19% were not included in the study due to missing data in either 2004 or 2008. Almost two‐thirds of the hospitals excluded from the study were missing 2004 data and, of these, 77% were critical access hospitals. The majority of these critical access hospitals (97%) were non‐accredited. This is in contrast to the hospitals missing 2008 data, of which only 13% were critical access. Since reporting of data to Hospital Compare was voluntary in 2004, it appears that critical access hospitals chose to wait later to report data to Hospital Compare, compared to acute care hospitals. Since critical access hospitals tended to have lower rates, smaller sample sizes, and be non‐accredited, the results of the study would be expected to slightly underestimate the difference between accredited and non‐accredited hospitals.
Finally, while we have argued that the publicly reported quality measures and TJC accreditation decisions provide different lenses into the quality of a given hospital, we cannot entirely exclude the possibility that there are subtle relationships between these two methods that might be partly responsible for our findings. For example, while performance measure rates do not factor directly into the accreditation decision, it is possible that Joint Commission surveyors may be influenced by their knowledge of these rates and biased in their scoring of unrelated standards during the survey process. While we cannot rule out such biases, we are aware of no research on the subject, and have no reason to believe that such biases may have confounded the analysis.
In summary, we found that Joint Commission‐accredited hospitals outperformed non‐accredited hospitals on nationally standardized quality measures of AMI, heart failure, and pneumonia. The performance gap between Joint Commission‐accredited and non‐accredited hospitals increased over the five years of the study. Future studies should incorporate more robust and varied measures of quality as outcomes, and seek to examine the nature of the observed relationship (ie, whether accreditation is simply a marker of higher quality and more rapid improvement, or the accreditation process actually helps create these salutary outcomes).
Acknowledgements
The authors thank Barbara Braun, PhD and Nicole Wineman, MPH, MBA for their literature review on the impact of accreditation, and Barbara Braun, PhD for her thoughtful review of the manuscript.
- The Joint Commission. Facts About Hospital Accreditation. Available at: http://www.jointcommission.org/assets/1/18/Hospital_Accreditation_1_31_11.pdf. Accessed on Feb 16, 2011.
- ,.Emergency Response Planning in Hospitals, United States: 2003–2004. Advance Data from Vital and Health Statistics; No. 391.Hattsville, MD:National Center for Health Statistics;2007.
- ,.Training for Terrorism‐Related Conditions in Hospitals, United States: 2003–2004. Advance Data from Vital and Health Statistics; No. 380.Hattsville, MD:National Center for Health Statistics;2006.
- ,,,.Hospital patient safety: characteristics of best‐performing hospitals.J Healthcare Manag.2007;52 (3):188–205.
- ,,.What is driving hospitals' patient‐safety efforts?Health Aff.2004;23(2):103–115.
- ,.The impact of trauma centre accreditation on patient outcome.Injury.2006;37(12):1166–1171.
- ,.Factors that influence staffing of outpatient substance abuse treatment programs.Psychiatr Serv.2005;56(8)934–939.
- ,.Changes in methadone treatment practices. Results from a national panel study, 1988–2000.JAMA.2002;288:850–856.
- ,,, et al.Quality of care for the treatment of acute medical conditions in US hospitals.Arch Intern Med.2006;166:2511–2517.
- ,,,.JCAHO accreditation and quality of care for acute myocardial infarction.Health Aff.2003;22(2):243–254.
- ,,, et al.Is JCAHO Accreditation Associated with Better Patient Outcomes in Rural Hospitals? Academy Health Annual Meeting; Boston, MA; June2005.
- .Hospital quality of care: the link between accreditation and mortality.J Clin Outcomes Manag.2003;10(9):473–480.
- , , . Structural versus outcome measures in hospitals: A comparison of Joint Commission and medicare outcome scores in hospitals. Qual Manage Health Care. 2002;10(2): 29–38.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,,,.Quality of care in accredited and non‐accredited ambulatory surgical centers.Jt Comm J Qual Patient Saf.2008;34(9):546–551.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures 2009. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed May 21,2009.
- Hospital Quality Alliance Homepage. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/index.html. Accessed May 6,2010
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004.N Engl J Med.2005;353(3):255–264.
- ,,,.Care in U.S. hospitals—the Hospital Quality Alliance Program.N Engl J Med.2005;353:265–274.
- Institute of Medicine, Committee on Quality Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:The National Academy Press;2001.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff.2003;22(2):84–94.
- ,,,.Performance of top ranked heart care hospitals on evidence‐based process measures.Circulation.2006;114:558–564.
- The Joint Commission Performance Measure Initiatives Homepage. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/default.htm. Accessed on July 27,2010.
- .Using health outcomes data to compare plans, networks and providers.Int J Qual Health Care.1998;10(6):477–483.
- .Process versus outcome indicators in the assessment of quality of health care.Int J Qual Health Care.2001;13:475–480.
- .Does paying for performance improve the quality of health care?Med Care Res Rev.2006;63(1):122S–125S.
- ,,, et al.Incremental survival benefit with adherence to standardized health failure core measures: a performance evaluation study of 2958 patients.J Card Fail.2008;14(2):95–102.
- ,,,.The inverse relationship between mortality rates and performance in the hospital quality alliance measures.Health Aff.2007;26(4):1104–1110.
- ,,, et al.Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality.JAMA.2006:296(1):72–78.
- ,,,,.Assessing the reliability of standardized performance measures.Int J Qual Health Care.2006;18:246–255.
- Centers for Medicare and Medicaid Services (CMS). CMS HQI Demonstration Project‐Composite Quality Score Methodology Overview. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalCompositeQualityScoreMethodologyOverview.pdf. Accessed March 8,2010.
- ,,,.Assessing the accuracy of hospital performance measures.Med Decis Making.2007;27:9–20.
- Quality Check Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed May 21,2009.
- ,,.Hospital characteristics and mortality rates.N Engl J Med.1989;321(25):1720–1725.
- ,.United States rural hospital quality in the Hospital Compare Database—accounting for hospital characteristics.Health Policy.2008;87:112–127.
- ,,,,,.Characteristics of hospitals demonstrating superior performance in patient experience and clinical process measures of care.Med Care Res Rev.2010;67(1):38–55.
- ,,.Comparison of change in quality of care between safety‐net and non‐safety‐net hospitals.JAMA.2008;299(18):2180–2187.
- ,.Bootstrap Methods and Their Application.New York:Cambridge;1997:chap 6.
- ,,,.The role of accreditation in an era of market‐driven accountability.Am J Manag Care.2005;11(5):290–293.
- The Joint Commission. Facts About Hospital Accreditation. Available at: http://www.jointcommission.org/assets/1/18/Hospital_Accreditation_1_31_11.pdf. Accessed on Feb 16, 2011.
- ,.Emergency Response Planning in Hospitals, United States: 2003–2004. Advance Data from Vital and Health Statistics; No. 391.Hattsville, MD:National Center for Health Statistics;2007.
- ,.Training for Terrorism‐Related Conditions in Hospitals, United States: 2003–2004. Advance Data from Vital and Health Statistics; No. 380.Hattsville, MD:National Center for Health Statistics;2006.
- ,,,.Hospital patient safety: characteristics of best‐performing hospitals.J Healthcare Manag.2007;52 (3):188–205.
- ,,.What is driving hospitals' patient‐safety efforts?Health Aff.2004;23(2):103–115.
- ,.The impact of trauma centre accreditation on patient outcome.Injury.2006;37(12):1166–1171.
- ,.Factors that influence staffing of outpatient substance abuse treatment programs.Psychiatr Serv.2005;56(8)934–939.
- ,.Changes in methadone treatment practices. Results from a national panel study, 1988–2000.JAMA.2002;288:850–856.
- ,,, et al.Quality of care for the treatment of acute medical conditions in US hospitals.Arch Intern Med.2006;166:2511–2517.
- ,,,.JCAHO accreditation and quality of care for acute myocardial infarction.Health Aff.2003;22(2):243–254.
- ,,, et al.Is JCAHO Accreditation Associated with Better Patient Outcomes in Rural Hospitals? Academy Health Annual Meeting; Boston, MA; June2005.
- .Hospital quality of care: the link between accreditation and mortality.J Clin Outcomes Manag.2003;10(9):473–480.
- , , . Structural versus outcome measures in hospitals: A comparison of Joint Commission and medicare outcome scores in hospitals. Qual Manage Health Care. 2002;10(2): 29–38.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,,,.Quality of care in accredited and non‐accredited ambulatory surgical centers.Jt Comm J Qual Patient Saf.2008;34(9):546–551.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures 2009. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed May 21,2009.
- Hospital Quality Alliance Homepage. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/index.html. Accessed May 6,2010
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002–2004.N Engl J Med.2005;353(3):255–264.
- ,,,.Care in U.S. hospitals—the Hospital Quality Alliance Program.N Engl J Med.2005;353:265–274.
- Institute of Medicine, Committee on Quality Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:The National Academy Press;2001.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff.2003;22(2):84–94.
- ,,,.Performance of top ranked heart care hospitals on evidence‐based process measures.Circulation.2006;114:558–564.
- The Joint Commission Performance Measure Initiatives Homepage. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/default.htm. Accessed on July 27,2010.
- .Using health outcomes data to compare plans, networks and providers.Int J Qual Health Care.1998;10(6):477–483.
- .Process versus outcome indicators in the assessment of quality of health care.Int J Qual Health Care.2001;13:475–480.
- .Does paying for performance improve the quality of health care?Med Care Res Rev.2006;63(1):122S–125S.
- ,,, et al.Incremental survival benefit with adherence to standardized health failure core measures: a performance evaluation study of 2958 patients.J Card Fail.2008;14(2):95–102.
- ,,,.The inverse relationship between mortality rates and performance in the hospital quality alliance measures.Health Aff.2007;26(4):1104–1110.
- ,,, et al.Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality.JAMA.2006:296(1):72–78.
- ,,,,.Assessing the reliability of standardized performance measures.Int J Qual Health Care.2006;18:246–255.
- Centers for Medicare and Medicaid Services (CMS). CMS HQI Demonstration Project‐Composite Quality Score Methodology Overview. Available at: http://www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalCompositeQualityScoreMethodologyOverview.pdf. Accessed March 8,2010.
- ,,,.Assessing the accuracy of hospital performance measures.Med Decis Making.2007;27:9–20.
- Quality Check Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed May 21,2009.
- ,,.Hospital characteristics and mortality rates.N Engl J Med.1989;321(25):1720–1725.
- ,.United States rural hospital quality in the Hospital Compare Database—accounting for hospital characteristics.Health Policy.2008;87:112–127.
- ,,,,,.Characteristics of hospitals demonstrating superior performance in patient experience and clinical process measures of care.Med Care Res Rev.2010;67(1):38–55.
- ,,.Comparison of change in quality of care between safety‐net and non‐safety‐net hospitals.JAMA.2008;299(18):2180–2187.
- ,.Bootstrap Methods and Their Application.New York:Cambridge;1997:chap 6.
- ,,,.The role of accreditation in an era of market‐driven accountability.Am J Manag Care.2005;11(5):290–293.
Copyright © 2011 Society of Hospital Medicine
Hospitalists and Alcohol Withdrawal
With 17 million Americans reporting heavy drinking (5 or more drinks on 5 different occasions in the last month) and 1.7 million hospital discharges in 2006 containing at least 1 alcohol‐related diagnosis, it would be hard to imagine a hospitalist who does not encounter patients with alcohol abuse.1, 2 Estimates from studies looking at the number of risky drinkers among medical inpatients vary widely2% to 60%with more detailed studies suggesting 17% to 25% prevalence.36 Yet despite the large numbers and great costs to the healthcare system, the inpatient treatment of alcohol withdrawal syndrome remains the ugly stepsister to more exciting topics, such as acute myocardial infarction, pulmonary embolism and procedures.7, 8 We hospitalists typically leave the clinical studies, research, and interest on substance abuse to addiction specialists and psychiatrists, perhaps due to our discomfort with these patients, negative attitudes, or belief that there is nothing new in the treatment of alcohol withdrawal syndrome since Dr Leo Henryk Sternbach discovered benzodiazepines in 1957.7, 9 Many of us just admit the alcoholic patient, check the alcohol‐pathway in our order entry system, and stop thinking about it.
But in this day of evidence‐based medicine and practice, what is the evidence behind the treatment of alcohol withdrawal, especially in relation to inpatient medicine? Shouldn't we hospitalists be thinking about this question? Hospitalists tend to see 2 types of inpatients with alcohol withdrawal: those solely admitted for withdrawal, and those admitted with active medical issues who then experience alcohol withdrawal. Is there a difference?
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) defines early alcohol withdrawal as the first 48 hours where there is central nervous system (CNS) stimulation, adrenergic hyperactivity, and the risk of seizures. Late withdrawal, after 48 hours, includes delirium tremens (DTs) and Wernicke's encephalopathy.10 This is based on studies done in the 1950s, where researchers observed patients as they withdrew from alcohol and took notes.11, 12
The goal in treatment of alcohol withdrawal is to minimize symptoms and prevent seizures and DTs which, prior to benzodiazepines, had a mortality rate of 5% to 20%. Before the US Food and Drug Administration (FDA) approval of the first benzodiazepine in 1960 (chlordiazepoxide), physicians treated alcohol withdrawal with ethanol, antipsychotics, or paraldehyde.12 (That is why there is a P in the mnemonic MUDPILES for anion gap acidosis.) The first study to show a real benefit from benzodiazepine was published in 1969, when 537 men in a veterans detoxification unit were randomized to chlordiazepoxide (Librium), chlorpromazine (Thorazine), antihistamine, thiamine, or placebo.12 The primary outcome of DTs and seizures occurred in 10% to 16% of the patients, except for the chlordiazepoxide group where only 2% developed seizures and DTs (there was no P value calculated). Further studies published in the 1970s and early 1980s were too small to demonstrate a benefit. A 1997 meta‐analysis of all these studies, including the 1969 article,12 confirmed benzodiazepines statistically reduced seizures and DTs.13 Which benzodiazepine to use, however, is less clear. Long‐acting benzodiazepines with liver clearance (eg, chlordiazepoxide or diazepam) versus short‐acting with renal clearance (eg, oxazepam or lorazepam) is debated. While there are many strong opinions among clinicians, the same meta‐analysis did not find any difference between them, and a small 2009 study found no difference between a short‐acting and long‐acting benzodiazepine.13, 14
How much benzodiazepine to give and how frequently to dose it was looked at in 2 classic studies.15, 16 Both studies demonstrated that symptom‐triggered dosing of benzodiazepines, based on the Clinical Institute Withdrawal Assessment (CIWA) scale, performed equally well in terms of clinical outcomes, with less medication required as compared with fixed‐dose regimens. Based on these articles, many hospitals created alcohol pathways using solely symptom‐triggered dosing.
The CIWA scale is one of multiple rating scales in the assessment of alcohol withdrawal.17, 18 The CIWA‐Ar is a modified scale that was designed and validated for clinical use in inpatient detoxification centers, and excluded any active medical illness. It has gained popularity, though initial time for staff training and time for administration are limitations to its usefulness. Interestingly, vital signs, which many institutions use in their alcohol withdrawal pathways, were not strongly predictive in the CIWA study of severe withdrawal, seizures, or DTs.17
Finally, what about treatment when the patient does develop seizures or DTs? The evidence on how best to treat alcohol withdrawal seizures comes from a 1999 article which demonstrated a benefit of using lorazepam for recurrent seizures.19, 20 Unfortunately, the treatment for DTs is less clear. A 2004 meta‐analysis on the treatment of delirium tremens found benzodiazepines better than chlorpromazine (Thorazine), but benzodiazepines versus, or in addition to, newer antipsychotics have not been tested. The amount of benzodiazepine to give in DTs is only a Grade C (ie, expert opinion) recommendation: dose for light somnolence.21
All of these studies, however, come back to the basic question: Do they apply to the inpatients that hospitalists care for? A key factor to consider: All of the above‐mentioned studies, including the derivation and validation of the CIWA scale, were done in outpatient centers or inpatient detoxification centers. Patients with active medical illness or comorbidities were excluded. This data may be relevant for the patients admitted solely for alcohol withdrawal, but what about the 60 year old with diabetes, coronary artery disease, and chronic obstructive lung disease admitted for pneumonia who starts to withdraw; or the 72‐year‐old woman who breaks her hip and begins to withdraw on post‐op day 2?
There are 6 relatively recent studies that evaluate PRN (as needed) dosing of benzodiazepines on general medical inpatients.2227 While ideally these articles should apply to a hospitalist's patients, 2 of the studies excluded anyone with acute medical illness.24, 27 From the remaining 4, what do we learn? Weaver and colleagues did a randomized study on general medical patients and found less lorazepam was given with PRN versus fixed dosing.26 Unfortunately, the study was not blinded and there were statistically significant protocol errors. Comorbidity data was not given, leaving us to wonder to which inpatients this applies. Repper‐DeLisi et al. did a retrospective chart review, after implementing an alcohol pathway (not based on the CIWA scale), and did not find a statistical difference in dosing, length of stay, or delirium.25 Foy et al. looked at both medical and surgical patients, and dosed benzodiazepines based on an 18‐item CIWA scale which included vital signs.22 They found that the higher score did correlate with risk of developing severe alcohol withdrawal. However, the scale had limitations. Many patients with illness were at higher risk for severe alcohol withdrawal than their score indicated, and some high scores were believed, in part, due to illness. Jeager et al. did a pre‐comparison and post‐comparison of the implementation of a PRN CIWA protocol by chart review.23 They found a reduction in delirium in patients treated with PRN dosing, but no different in total benzodiazepine given. Because it was chart review, the authors acknowledge that defining delirium tremens was less reliable, and controlling for comorbidities was difficult. The difficult part of delirium in inpatients with alcohol abuse is that the delirium is not always just from DTs.
Two recent studies raised alarm about using a PRN CIWA pathway on patients.28, 29 A 2008 study found that 52% of patients were inappropriately put on a CIWA sliding scale when they either could not communicate or had not been recently drinking, or both.29 (The CIWA scale requires the person be able to answer symptom questions and is not applicable to non‐drinkers.) In 2005, during the implementation of an alcohol pathway at San Francisco General Hospital, an increase in mortality was noted with a PRN CIWA scale on inpatients.28
One of the conundrums for physicians is that whereas alcohol withdrawal has morbidity and mortality risks, benzodiazepine treatment itself has its own risks. Over sedation, respiratory depression, aspiration pneumonia, deconditioning from prolonged sedation, paradoxical agitation and disinhibition are the consequences of the dosing difficulties in alcohol withdrawal. Case reports on astronomical doses required to treat withdrawal (eg, 1600 mg of lorazepam in a day) raise questions of benzodiazepine resistance.30 Hence, multiple studies have been done to find alternatives for benzodiazepines. Our European counterparts lead the way in looking at: carbemazepine, gabapentin, gamma‐hydroxybuterate, corticotropin‐releasing hormone, baclofen, pregabalin, and phenobarbital. Again, the key issue for hospitalists: Are these benzodiazepine alternatives or additives applicable to our patients? These studies are done on outpatients with no concurrent medical illnesses. Yet, logic would suggest that it is the vulnerable hospitalized patients who might benefit the most from reducing the benzodiazepine amount using other agents.
In this issue of the Journal of Hospital Medicine, Lyon et al. provide a glimpse into possible ways to reduce the total benzodiazepine dose for general medical inpatients.31 They randomized inpatients withdrawing from alcohol to baclofen or placebo. Both groups still received PRN lorazepam based on their hospital's CIWA protocol. Prior outpatient studies have shown baclofen benefits patients undergoing alcohol withdrawal and the pathophysiology makes sense; baclofen acts on GABA b receptors. Lyon and collegaues' study results show significant reduction in the amount of benzodiazepine needed with no difference in CIWA scores.31
Is this a practice changer? Well, not yet. The numbers in the study are small and this is only 1 institution. These patients had only moderate alcohol withdrawal and the study was not powered to detect outcomes related to prevention of seizures and delirium tremens. However, the authors should be applauded for looking at alcohol withdrawal in medical inpatients.31 Trying to reduce the harm we cause with our benzodiazepine treatment regimens is a laudable goal. Inpatient alcohol withdrawal, especially for patients with medical comorbidities, is an area ripe for study and certainly deserves to have a spotlight shown on it.
Who better to do this than hospitalists? The Society of Hospital Medicine (SHM) core competency on Alcohol and Drug Withdrawal states, Hospitalists can lead their institutions in evidence based treatment protocols that improve care, reduce costs‐ and length of stay, and facilitate better overall outcomes in patients with substance related withdrawal syndromes.32 Hopefully, Lyon and collegaues' work will lead to the formation of multicenter hospitalist‐initiated studies to provide us with the best evidence for the treatment of inpatient alcohol withdrawal on our patients with comorbidities.31 Given the prevalence and potential severity of alcohol withdrawal in complex inpatients, isn't it time we really knew how to treat them?
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Bethesda, MD:National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research;2008.
- Substance Abuse and Mental Health Services Administration (SAMHSA).Results From the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H‐32, DHHS Publication No SMA‐0704293).Rockville, MD:US Department of Health and Human Services;2007.
- ,.Alcohol‐related disease in hospital patients.Med J Aust.1986;144(10):515–517, 519.
- ,,,,.The effect of patient gender on the prevalence and recognition of alcoholism on a general medicine inpatient service.J Gen Intern Med.1992;7(1):38–45.
- ,,,,.The severity of unhealthy alcohol use in hospitalized medical patients. The spectrum is narrow.J Gen Intern Med.2006;21(4):381–385.
- ,,,,,.Prevalence, detection, and treatment of alcoholism in hospitalized patients.JAMA.1989;261(3):403–407.
- ,,,.Internal medicine residency training for unhealthy alcohol and other drug use: recommendations for curriculum design.BMC Med Educ.2010;10:22.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,,,.Good Chemistry: The Life and Legacy of Valium Inventor Leo Sternbach.New York, NY:McGraw Hill;2004.
- ,,,,.Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment.J Gen Intern Med.1989;4(5):432–444.
- ,,,,.An experimental study of the etiology of rum fits and delirium tremens.Q J Stud Alcohol.1955;16(1):1–33.
- ,,.Treatment of the acute alcohol withdrawal state: a comparison of four drugs.Am J Psychiatry.1969;125(12):1640–1646.
- .Pharmacological management of alcohol withdrawal. A meta‐analysis and evidence‐based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal.JAMA.1997;278(2):144–151.
- ,,.A randomized, double‐blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal.J Stud Alcohol Drugs.2009;70(3):467–474.
- ,,,,,.Individualized treatment for alcohol withdrawal. A randomized double‐blind controlled trial.JAMA.1994;272(7):519–523.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial.Arch Intern Med.2002;162(10):1117–1121.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar).Br J Addict.1989;84(11):1353–1357.
- ,,.A comparison of rating scales for the alcohol‐withdrawal syndrome.Alcohol Alcohol.2001;36(2):104–108.
- ,,,,.Lorazepam for the prevention of recurrent seizures related to alcohol.N Engl J Med.1999;340(12):915–919.
- ,,,.Anticonvulsants for alcohol withdrawal.Cochrane Database Syst Rev.2010(3):CD005064.
- ,,, et al.Management of alcohol withdrawal delirium. An evidence‐based practice guideline.Arch Intern Med.2004;164(13):1405–1412.
- ,,.Use of an objective clinical scale in the assessment and management of alcohol withdrawal in a large general hospital.Alcohol Clin Exp Res.1988;12(3):360–364.
- ,,.Symptom‐triggered therapy for alcohol withdrawal syndrome in medical inpatients.Mayo Clin Proc.2001;76(7):695–701.
- ,.Routine hospital alcohol detoxification practice compared to symptom triggered management with an objective withdrawal scale (CIWA‐Ar).Am J Addict.2000;9(2):135–144.
- ,,, et al.Successful implementation of an alcohol‐withdrawal pathway in a general hospital.Psychosomatics.2008;49(4):292–299.
- ,,,.Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity.J Addict Dis.2006;25(2):17–24.
- ,,.Benzodiazepine requirements during alcohol withdrawal syndrome: clinical implications of using a standardized withdrawal scale.J Clin Psychopharmacol.1991;11(5):291–295.
- ,,, et al.Unintended consequences of a quality improvement program designed to improve treatment of alcohol withdrawal in hospitalized patients.Jt Comm J Qual Patient Saf.2005;31(3):148–157.
- ,,,.Inappropriate use of symptom‐triggered therapy for alcohol withdrawal in the general hospital.Mayo Clin Proc.2008;83(3):274–279.
- ,,.A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours.CNS Spectr.2009;14(7):385–389.
- et al.J Hosp Med.2011;6:471–476.
- The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
With 17 million Americans reporting heavy drinking (5 or more drinks on 5 different occasions in the last month) and 1.7 million hospital discharges in 2006 containing at least 1 alcohol‐related diagnosis, it would be hard to imagine a hospitalist who does not encounter patients with alcohol abuse.1, 2 Estimates from studies looking at the number of risky drinkers among medical inpatients vary widely2% to 60%with more detailed studies suggesting 17% to 25% prevalence.36 Yet despite the large numbers and great costs to the healthcare system, the inpatient treatment of alcohol withdrawal syndrome remains the ugly stepsister to more exciting topics, such as acute myocardial infarction, pulmonary embolism and procedures.7, 8 We hospitalists typically leave the clinical studies, research, and interest on substance abuse to addiction specialists and psychiatrists, perhaps due to our discomfort with these patients, negative attitudes, or belief that there is nothing new in the treatment of alcohol withdrawal syndrome since Dr Leo Henryk Sternbach discovered benzodiazepines in 1957.7, 9 Many of us just admit the alcoholic patient, check the alcohol‐pathway in our order entry system, and stop thinking about it.
But in this day of evidence‐based medicine and practice, what is the evidence behind the treatment of alcohol withdrawal, especially in relation to inpatient medicine? Shouldn't we hospitalists be thinking about this question? Hospitalists tend to see 2 types of inpatients with alcohol withdrawal: those solely admitted for withdrawal, and those admitted with active medical issues who then experience alcohol withdrawal. Is there a difference?
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) defines early alcohol withdrawal as the first 48 hours where there is central nervous system (CNS) stimulation, adrenergic hyperactivity, and the risk of seizures. Late withdrawal, after 48 hours, includes delirium tremens (DTs) and Wernicke's encephalopathy.10 This is based on studies done in the 1950s, where researchers observed patients as they withdrew from alcohol and took notes.11, 12
The goal in treatment of alcohol withdrawal is to minimize symptoms and prevent seizures and DTs which, prior to benzodiazepines, had a mortality rate of 5% to 20%. Before the US Food and Drug Administration (FDA) approval of the first benzodiazepine in 1960 (chlordiazepoxide), physicians treated alcohol withdrawal with ethanol, antipsychotics, or paraldehyde.12 (That is why there is a P in the mnemonic MUDPILES for anion gap acidosis.) The first study to show a real benefit from benzodiazepine was published in 1969, when 537 men in a veterans detoxification unit were randomized to chlordiazepoxide (Librium), chlorpromazine (Thorazine), antihistamine, thiamine, or placebo.12 The primary outcome of DTs and seizures occurred in 10% to 16% of the patients, except for the chlordiazepoxide group where only 2% developed seizures and DTs (there was no P value calculated). Further studies published in the 1970s and early 1980s were too small to demonstrate a benefit. A 1997 meta‐analysis of all these studies, including the 1969 article,12 confirmed benzodiazepines statistically reduced seizures and DTs.13 Which benzodiazepine to use, however, is less clear. Long‐acting benzodiazepines with liver clearance (eg, chlordiazepoxide or diazepam) versus short‐acting with renal clearance (eg, oxazepam or lorazepam) is debated. While there are many strong opinions among clinicians, the same meta‐analysis did not find any difference between them, and a small 2009 study found no difference between a short‐acting and long‐acting benzodiazepine.13, 14
How much benzodiazepine to give and how frequently to dose it was looked at in 2 classic studies.15, 16 Both studies demonstrated that symptom‐triggered dosing of benzodiazepines, based on the Clinical Institute Withdrawal Assessment (CIWA) scale, performed equally well in terms of clinical outcomes, with less medication required as compared with fixed‐dose regimens. Based on these articles, many hospitals created alcohol pathways using solely symptom‐triggered dosing.
The CIWA scale is one of multiple rating scales in the assessment of alcohol withdrawal.17, 18 The CIWA‐Ar is a modified scale that was designed and validated for clinical use in inpatient detoxification centers, and excluded any active medical illness. It has gained popularity, though initial time for staff training and time for administration are limitations to its usefulness. Interestingly, vital signs, which many institutions use in their alcohol withdrawal pathways, were not strongly predictive in the CIWA study of severe withdrawal, seizures, or DTs.17
Finally, what about treatment when the patient does develop seizures or DTs? The evidence on how best to treat alcohol withdrawal seizures comes from a 1999 article which demonstrated a benefit of using lorazepam for recurrent seizures.19, 20 Unfortunately, the treatment for DTs is less clear. A 2004 meta‐analysis on the treatment of delirium tremens found benzodiazepines better than chlorpromazine (Thorazine), but benzodiazepines versus, or in addition to, newer antipsychotics have not been tested. The amount of benzodiazepine to give in DTs is only a Grade C (ie, expert opinion) recommendation: dose for light somnolence.21
All of these studies, however, come back to the basic question: Do they apply to the inpatients that hospitalists care for? A key factor to consider: All of the above‐mentioned studies, including the derivation and validation of the CIWA scale, were done in outpatient centers or inpatient detoxification centers. Patients with active medical illness or comorbidities were excluded. This data may be relevant for the patients admitted solely for alcohol withdrawal, but what about the 60 year old with diabetes, coronary artery disease, and chronic obstructive lung disease admitted for pneumonia who starts to withdraw; or the 72‐year‐old woman who breaks her hip and begins to withdraw on post‐op day 2?
There are 6 relatively recent studies that evaluate PRN (as needed) dosing of benzodiazepines on general medical inpatients.2227 While ideally these articles should apply to a hospitalist's patients, 2 of the studies excluded anyone with acute medical illness.24, 27 From the remaining 4, what do we learn? Weaver and colleagues did a randomized study on general medical patients and found less lorazepam was given with PRN versus fixed dosing.26 Unfortunately, the study was not blinded and there were statistically significant protocol errors. Comorbidity data was not given, leaving us to wonder to which inpatients this applies. Repper‐DeLisi et al. did a retrospective chart review, after implementing an alcohol pathway (not based on the CIWA scale), and did not find a statistical difference in dosing, length of stay, or delirium.25 Foy et al. looked at both medical and surgical patients, and dosed benzodiazepines based on an 18‐item CIWA scale which included vital signs.22 They found that the higher score did correlate with risk of developing severe alcohol withdrawal. However, the scale had limitations. Many patients with illness were at higher risk for severe alcohol withdrawal than their score indicated, and some high scores were believed, in part, due to illness. Jeager et al. did a pre‐comparison and post‐comparison of the implementation of a PRN CIWA protocol by chart review.23 They found a reduction in delirium in patients treated with PRN dosing, but no different in total benzodiazepine given. Because it was chart review, the authors acknowledge that defining delirium tremens was less reliable, and controlling for comorbidities was difficult. The difficult part of delirium in inpatients with alcohol abuse is that the delirium is not always just from DTs.
Two recent studies raised alarm about using a PRN CIWA pathway on patients.28, 29 A 2008 study found that 52% of patients were inappropriately put on a CIWA sliding scale when they either could not communicate or had not been recently drinking, or both.29 (The CIWA scale requires the person be able to answer symptom questions and is not applicable to non‐drinkers.) In 2005, during the implementation of an alcohol pathway at San Francisco General Hospital, an increase in mortality was noted with a PRN CIWA scale on inpatients.28
One of the conundrums for physicians is that whereas alcohol withdrawal has morbidity and mortality risks, benzodiazepine treatment itself has its own risks. Over sedation, respiratory depression, aspiration pneumonia, deconditioning from prolonged sedation, paradoxical agitation and disinhibition are the consequences of the dosing difficulties in alcohol withdrawal. Case reports on astronomical doses required to treat withdrawal (eg, 1600 mg of lorazepam in a day) raise questions of benzodiazepine resistance.30 Hence, multiple studies have been done to find alternatives for benzodiazepines. Our European counterparts lead the way in looking at: carbemazepine, gabapentin, gamma‐hydroxybuterate, corticotropin‐releasing hormone, baclofen, pregabalin, and phenobarbital. Again, the key issue for hospitalists: Are these benzodiazepine alternatives or additives applicable to our patients? These studies are done on outpatients with no concurrent medical illnesses. Yet, logic would suggest that it is the vulnerable hospitalized patients who might benefit the most from reducing the benzodiazepine amount using other agents.
In this issue of the Journal of Hospital Medicine, Lyon et al. provide a glimpse into possible ways to reduce the total benzodiazepine dose for general medical inpatients.31 They randomized inpatients withdrawing from alcohol to baclofen or placebo. Both groups still received PRN lorazepam based on their hospital's CIWA protocol. Prior outpatient studies have shown baclofen benefits patients undergoing alcohol withdrawal and the pathophysiology makes sense; baclofen acts on GABA b receptors. Lyon and collegaues' study results show significant reduction in the amount of benzodiazepine needed with no difference in CIWA scores.31
Is this a practice changer? Well, not yet. The numbers in the study are small and this is only 1 institution. These patients had only moderate alcohol withdrawal and the study was not powered to detect outcomes related to prevention of seizures and delirium tremens. However, the authors should be applauded for looking at alcohol withdrawal in medical inpatients.31 Trying to reduce the harm we cause with our benzodiazepine treatment regimens is a laudable goal. Inpatient alcohol withdrawal, especially for patients with medical comorbidities, is an area ripe for study and certainly deserves to have a spotlight shown on it.
Who better to do this than hospitalists? The Society of Hospital Medicine (SHM) core competency on Alcohol and Drug Withdrawal states, Hospitalists can lead their institutions in evidence based treatment protocols that improve care, reduce costs‐ and length of stay, and facilitate better overall outcomes in patients with substance related withdrawal syndromes.32 Hopefully, Lyon and collegaues' work will lead to the formation of multicenter hospitalist‐initiated studies to provide us with the best evidence for the treatment of inpatient alcohol withdrawal on our patients with comorbidities.31 Given the prevalence and potential severity of alcohol withdrawal in complex inpatients, isn't it time we really knew how to treat them?
With 17 million Americans reporting heavy drinking (5 or more drinks on 5 different occasions in the last month) and 1.7 million hospital discharges in 2006 containing at least 1 alcohol‐related diagnosis, it would be hard to imagine a hospitalist who does not encounter patients with alcohol abuse.1, 2 Estimates from studies looking at the number of risky drinkers among medical inpatients vary widely2% to 60%with more detailed studies suggesting 17% to 25% prevalence.36 Yet despite the large numbers and great costs to the healthcare system, the inpatient treatment of alcohol withdrawal syndrome remains the ugly stepsister to more exciting topics, such as acute myocardial infarction, pulmonary embolism and procedures.7, 8 We hospitalists typically leave the clinical studies, research, and interest on substance abuse to addiction specialists and psychiatrists, perhaps due to our discomfort with these patients, negative attitudes, or belief that there is nothing new in the treatment of alcohol withdrawal syndrome since Dr Leo Henryk Sternbach discovered benzodiazepines in 1957.7, 9 Many of us just admit the alcoholic patient, check the alcohol‐pathway in our order entry system, and stop thinking about it.
But in this day of evidence‐based medicine and practice, what is the evidence behind the treatment of alcohol withdrawal, especially in relation to inpatient medicine? Shouldn't we hospitalists be thinking about this question? Hospitalists tend to see 2 types of inpatients with alcohol withdrawal: those solely admitted for withdrawal, and those admitted with active medical issues who then experience alcohol withdrawal. Is there a difference?
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) defines early alcohol withdrawal as the first 48 hours where there is central nervous system (CNS) stimulation, adrenergic hyperactivity, and the risk of seizures. Late withdrawal, after 48 hours, includes delirium tremens (DTs) and Wernicke's encephalopathy.10 This is based on studies done in the 1950s, where researchers observed patients as they withdrew from alcohol and took notes.11, 12
The goal in treatment of alcohol withdrawal is to minimize symptoms and prevent seizures and DTs which, prior to benzodiazepines, had a mortality rate of 5% to 20%. Before the US Food and Drug Administration (FDA) approval of the first benzodiazepine in 1960 (chlordiazepoxide), physicians treated alcohol withdrawal with ethanol, antipsychotics, or paraldehyde.12 (That is why there is a P in the mnemonic MUDPILES for anion gap acidosis.) The first study to show a real benefit from benzodiazepine was published in 1969, when 537 men in a veterans detoxification unit were randomized to chlordiazepoxide (Librium), chlorpromazine (Thorazine), antihistamine, thiamine, or placebo.12 The primary outcome of DTs and seizures occurred in 10% to 16% of the patients, except for the chlordiazepoxide group where only 2% developed seizures and DTs (there was no P value calculated). Further studies published in the 1970s and early 1980s were too small to demonstrate a benefit. A 1997 meta‐analysis of all these studies, including the 1969 article,12 confirmed benzodiazepines statistically reduced seizures and DTs.13 Which benzodiazepine to use, however, is less clear. Long‐acting benzodiazepines with liver clearance (eg, chlordiazepoxide or diazepam) versus short‐acting with renal clearance (eg, oxazepam or lorazepam) is debated. While there are many strong opinions among clinicians, the same meta‐analysis did not find any difference between them, and a small 2009 study found no difference between a short‐acting and long‐acting benzodiazepine.13, 14
How much benzodiazepine to give and how frequently to dose it was looked at in 2 classic studies.15, 16 Both studies demonstrated that symptom‐triggered dosing of benzodiazepines, based on the Clinical Institute Withdrawal Assessment (CIWA) scale, performed equally well in terms of clinical outcomes, with less medication required as compared with fixed‐dose regimens. Based on these articles, many hospitals created alcohol pathways using solely symptom‐triggered dosing.
The CIWA scale is one of multiple rating scales in the assessment of alcohol withdrawal.17, 18 The CIWA‐Ar is a modified scale that was designed and validated for clinical use in inpatient detoxification centers, and excluded any active medical illness. It has gained popularity, though initial time for staff training and time for administration are limitations to its usefulness. Interestingly, vital signs, which many institutions use in their alcohol withdrawal pathways, were not strongly predictive in the CIWA study of severe withdrawal, seizures, or DTs.17
Finally, what about treatment when the patient does develop seizures or DTs? The evidence on how best to treat alcohol withdrawal seizures comes from a 1999 article which demonstrated a benefit of using lorazepam for recurrent seizures.19, 20 Unfortunately, the treatment for DTs is less clear. A 2004 meta‐analysis on the treatment of delirium tremens found benzodiazepines better than chlorpromazine (Thorazine), but benzodiazepines versus, or in addition to, newer antipsychotics have not been tested. The amount of benzodiazepine to give in DTs is only a Grade C (ie, expert opinion) recommendation: dose for light somnolence.21
All of these studies, however, come back to the basic question: Do they apply to the inpatients that hospitalists care for? A key factor to consider: All of the above‐mentioned studies, including the derivation and validation of the CIWA scale, were done in outpatient centers or inpatient detoxification centers. Patients with active medical illness or comorbidities were excluded. This data may be relevant for the patients admitted solely for alcohol withdrawal, but what about the 60 year old with diabetes, coronary artery disease, and chronic obstructive lung disease admitted for pneumonia who starts to withdraw; or the 72‐year‐old woman who breaks her hip and begins to withdraw on post‐op day 2?
There are 6 relatively recent studies that evaluate PRN (as needed) dosing of benzodiazepines on general medical inpatients.2227 While ideally these articles should apply to a hospitalist's patients, 2 of the studies excluded anyone with acute medical illness.24, 27 From the remaining 4, what do we learn? Weaver and colleagues did a randomized study on general medical patients and found less lorazepam was given with PRN versus fixed dosing.26 Unfortunately, the study was not blinded and there were statistically significant protocol errors. Comorbidity data was not given, leaving us to wonder to which inpatients this applies. Repper‐DeLisi et al. did a retrospective chart review, after implementing an alcohol pathway (not based on the CIWA scale), and did not find a statistical difference in dosing, length of stay, or delirium.25 Foy et al. looked at both medical and surgical patients, and dosed benzodiazepines based on an 18‐item CIWA scale which included vital signs.22 They found that the higher score did correlate with risk of developing severe alcohol withdrawal. However, the scale had limitations. Many patients with illness were at higher risk for severe alcohol withdrawal than their score indicated, and some high scores were believed, in part, due to illness. Jeager et al. did a pre‐comparison and post‐comparison of the implementation of a PRN CIWA protocol by chart review.23 They found a reduction in delirium in patients treated with PRN dosing, but no different in total benzodiazepine given. Because it was chart review, the authors acknowledge that defining delirium tremens was less reliable, and controlling for comorbidities was difficult. The difficult part of delirium in inpatients with alcohol abuse is that the delirium is not always just from DTs.
Two recent studies raised alarm about using a PRN CIWA pathway on patients.28, 29 A 2008 study found that 52% of patients were inappropriately put on a CIWA sliding scale when they either could not communicate or had not been recently drinking, or both.29 (The CIWA scale requires the person be able to answer symptom questions and is not applicable to non‐drinkers.) In 2005, during the implementation of an alcohol pathway at San Francisco General Hospital, an increase in mortality was noted with a PRN CIWA scale on inpatients.28
One of the conundrums for physicians is that whereas alcohol withdrawal has morbidity and mortality risks, benzodiazepine treatment itself has its own risks. Over sedation, respiratory depression, aspiration pneumonia, deconditioning from prolonged sedation, paradoxical agitation and disinhibition are the consequences of the dosing difficulties in alcohol withdrawal. Case reports on astronomical doses required to treat withdrawal (eg, 1600 mg of lorazepam in a day) raise questions of benzodiazepine resistance.30 Hence, multiple studies have been done to find alternatives for benzodiazepines. Our European counterparts lead the way in looking at: carbemazepine, gabapentin, gamma‐hydroxybuterate, corticotropin‐releasing hormone, baclofen, pregabalin, and phenobarbital. Again, the key issue for hospitalists: Are these benzodiazepine alternatives or additives applicable to our patients? These studies are done on outpatients with no concurrent medical illnesses. Yet, logic would suggest that it is the vulnerable hospitalized patients who might benefit the most from reducing the benzodiazepine amount using other agents.
In this issue of the Journal of Hospital Medicine, Lyon et al. provide a glimpse into possible ways to reduce the total benzodiazepine dose for general medical inpatients.31 They randomized inpatients withdrawing from alcohol to baclofen or placebo. Both groups still received PRN lorazepam based on their hospital's CIWA protocol. Prior outpatient studies have shown baclofen benefits patients undergoing alcohol withdrawal and the pathophysiology makes sense; baclofen acts on GABA b receptors. Lyon and collegaues' study results show significant reduction in the amount of benzodiazepine needed with no difference in CIWA scores.31
Is this a practice changer? Well, not yet. The numbers in the study are small and this is only 1 institution. These patients had only moderate alcohol withdrawal and the study was not powered to detect outcomes related to prevention of seizures and delirium tremens. However, the authors should be applauded for looking at alcohol withdrawal in medical inpatients.31 Trying to reduce the harm we cause with our benzodiazepine treatment regimens is a laudable goal. Inpatient alcohol withdrawal, especially for patients with medical comorbidities, is an area ripe for study and certainly deserves to have a spotlight shown on it.
Who better to do this than hospitalists? The Society of Hospital Medicine (SHM) core competency on Alcohol and Drug Withdrawal states, Hospitalists can lead their institutions in evidence based treatment protocols that improve care, reduce costs‐ and length of stay, and facilitate better overall outcomes in patients with substance related withdrawal syndromes.32 Hopefully, Lyon and collegaues' work will lead to the formation of multicenter hospitalist‐initiated studies to provide us with the best evidence for the treatment of inpatient alcohol withdrawal on our patients with comorbidities.31 Given the prevalence and potential severity of alcohol withdrawal in complex inpatients, isn't it time we really knew how to treat them?
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Bethesda, MD:National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research;2008.
- Substance Abuse and Mental Health Services Administration (SAMHSA).Results From the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H‐32, DHHS Publication No SMA‐0704293).Rockville, MD:US Department of Health and Human Services;2007.
- ,.Alcohol‐related disease in hospital patients.Med J Aust.1986;144(10):515–517, 519.
- ,,,,.The effect of patient gender on the prevalence and recognition of alcoholism on a general medicine inpatient service.J Gen Intern Med.1992;7(1):38–45.
- ,,,,.The severity of unhealthy alcohol use in hospitalized medical patients. The spectrum is narrow.J Gen Intern Med.2006;21(4):381–385.
- ,,,,,.Prevalence, detection, and treatment of alcoholism in hospitalized patients.JAMA.1989;261(3):403–407.
- ,,,.Internal medicine residency training for unhealthy alcohol and other drug use: recommendations for curriculum design.BMC Med Educ.2010;10:22.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,,,.Good Chemistry: The Life and Legacy of Valium Inventor Leo Sternbach.New York, NY:McGraw Hill;2004.
- ,,,,.Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment.J Gen Intern Med.1989;4(5):432–444.
- ,,,,.An experimental study of the etiology of rum fits and delirium tremens.Q J Stud Alcohol.1955;16(1):1–33.
- ,,.Treatment of the acute alcohol withdrawal state: a comparison of four drugs.Am J Psychiatry.1969;125(12):1640–1646.
- .Pharmacological management of alcohol withdrawal. A meta‐analysis and evidence‐based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal.JAMA.1997;278(2):144–151.
- ,,.A randomized, double‐blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal.J Stud Alcohol Drugs.2009;70(3):467–474.
- ,,,,,.Individualized treatment for alcohol withdrawal. A randomized double‐blind controlled trial.JAMA.1994;272(7):519–523.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial.Arch Intern Med.2002;162(10):1117–1121.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar).Br J Addict.1989;84(11):1353–1357.
- ,,.A comparison of rating scales for the alcohol‐withdrawal syndrome.Alcohol Alcohol.2001;36(2):104–108.
- ,,,,.Lorazepam for the prevention of recurrent seizures related to alcohol.N Engl J Med.1999;340(12):915–919.
- ,,,.Anticonvulsants for alcohol withdrawal.Cochrane Database Syst Rev.2010(3):CD005064.
- ,,, et al.Management of alcohol withdrawal delirium. An evidence‐based practice guideline.Arch Intern Med.2004;164(13):1405–1412.
- ,,.Use of an objective clinical scale in the assessment and management of alcohol withdrawal in a large general hospital.Alcohol Clin Exp Res.1988;12(3):360–364.
- ,,.Symptom‐triggered therapy for alcohol withdrawal syndrome in medical inpatients.Mayo Clin Proc.2001;76(7):695–701.
- ,.Routine hospital alcohol detoxification practice compared to symptom triggered management with an objective withdrawal scale (CIWA‐Ar).Am J Addict.2000;9(2):135–144.
- ,,, et al.Successful implementation of an alcohol‐withdrawal pathway in a general hospital.Psychosomatics.2008;49(4):292–299.
- ,,,.Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity.J Addict Dis.2006;25(2):17–24.
- ,,.Benzodiazepine requirements during alcohol withdrawal syndrome: clinical implications of using a standardized withdrawal scale.J Clin Psychopharmacol.1991;11(5):291–295.
- ,,, et al.Unintended consequences of a quality improvement program designed to improve treatment of alcohol withdrawal in hospitalized patients.Jt Comm J Qual Patient Saf.2005;31(3):148–157.
- ,,,.Inappropriate use of symptom‐triggered therapy for alcohol withdrawal in the general hospital.Mayo Clin Proc.2008;83(3):274–279.
- ,,.A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours.CNS Spectr.2009;14(7):385–389.
- et al.J Hosp Med.2011;6:471–476.
- The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Bethesda, MD:National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research;2008.
- Substance Abuse and Mental Health Services Administration (SAMHSA).Results From the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H‐32, DHHS Publication No SMA‐0704293).Rockville, MD:US Department of Health and Human Services;2007.
- ,.Alcohol‐related disease in hospital patients.Med J Aust.1986;144(10):515–517, 519.
- ,,,,.The effect of patient gender on the prevalence and recognition of alcoholism on a general medicine inpatient service.J Gen Intern Med.1992;7(1):38–45.
- ,,,,.The severity of unhealthy alcohol use in hospitalized medical patients. The spectrum is narrow.J Gen Intern Med.2006;21(4):381–385.
- ,,,,,.Prevalence, detection, and treatment of alcoholism in hospitalized patients.JAMA.1989;261(3):403–407.
- ,,,.Internal medicine residency training for unhealthy alcohol and other drug use: recommendations for curriculum design.BMC Med Educ.2010;10:22.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,,,.Good Chemistry: The Life and Legacy of Valium Inventor Leo Sternbach.New York, NY:McGraw Hill;2004.
- ,,,,.Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment.J Gen Intern Med.1989;4(5):432–444.
- ,,,,.An experimental study of the etiology of rum fits and delirium tremens.Q J Stud Alcohol.1955;16(1):1–33.
- ,,.Treatment of the acute alcohol withdrawal state: a comparison of four drugs.Am J Psychiatry.1969;125(12):1640–1646.
- .Pharmacological management of alcohol withdrawal. A meta‐analysis and evidence‐based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal.JAMA.1997;278(2):144–151.
- ,,.A randomized, double‐blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal.J Stud Alcohol Drugs.2009;70(3):467–474.
- ,,,,,.Individualized treatment for alcohol withdrawal. A randomized double‐blind controlled trial.JAMA.1994;272(7):519–523.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial.Arch Intern Med.2002;162(10):1117–1121.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar).Br J Addict.1989;84(11):1353–1357.
- ,,.A comparison of rating scales for the alcohol‐withdrawal syndrome.Alcohol Alcohol.2001;36(2):104–108.
- ,,,,.Lorazepam for the prevention of recurrent seizures related to alcohol.N Engl J Med.1999;340(12):915–919.
- ,,,.Anticonvulsants for alcohol withdrawal.Cochrane Database Syst Rev.2010(3):CD005064.
- ,,, et al.Management of alcohol withdrawal delirium. An evidence‐based practice guideline.Arch Intern Med.2004;164(13):1405–1412.
- ,,.Use of an objective clinical scale in the assessment and management of alcohol withdrawal in a large general hospital.Alcohol Clin Exp Res.1988;12(3):360–364.
- ,,.Symptom‐triggered therapy for alcohol withdrawal syndrome in medical inpatients.Mayo Clin Proc.2001;76(7):695–701.
- ,.Routine hospital alcohol detoxification practice compared to symptom triggered management with an objective withdrawal scale (CIWA‐Ar).Am J Addict.2000;9(2):135–144.
- ,,, et al.Successful implementation of an alcohol‐withdrawal pathway in a general hospital.Psychosomatics.2008;49(4):292–299.
- ,,,.Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity.J Addict Dis.2006;25(2):17–24.
- ,,.Benzodiazepine requirements during alcohol withdrawal syndrome: clinical implications of using a standardized withdrawal scale.J Clin Psychopharmacol.1991;11(5):291–295.
- ,,, et al.Unintended consequences of a quality improvement program designed to improve treatment of alcohol withdrawal in hospitalized patients.Jt Comm J Qual Patient Saf.2005;31(3):148–157.
- ,,,.Inappropriate use of symptom‐triggered therapy for alcohol withdrawal in the general hospital.Mayo Clin Proc.2008;83(3):274–279.
- ,,.A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours.CNS Spectr.2009;14(7):385–389.
- et al.J Hosp Med.2011;6:471–476.
- The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
Trends in Inpatient Continuity of Care
Continuity of care is considered by many physicians to be of critical importance in providing high‐quality patient care. Most of the research to date has focused on continuity in outpatient primary care. Research on outpatient continuity of care has been facilitated by the fact that a number of measurement tools for outpatient continuity exist.1 Outpatient continuity of care has been linked to better quality of life scores,2 lower costs,3 and less emergency room use.4 As hospital medicine has taken on more and more of the responsibility of inpatient care, primary care doctors have voiced concerns about the impact of hospitalists on overall continuity of care5 and the quality of the doctorpatient relationship.6
Recently, continuity of care in the hospital setting has also received attention. When the Accreditation Council for Graduate Medical Education (ACGME) first proposed restrictions to resident duty hours, the importance of continuity of inpatient care began to be debated in earnest in large part because of the increase in hand‐offs which accompanies discontinuity.7, 8 A recent study of hospitalist communication documented that as many as 13% of hand‐offs at the time of service changes are judged as incomplete by the receiving physician. These incomplete hand‐offs were more likely to be associated with uncertainty regarding the plan of care, as well as perceived near misses or adverse events.9 In addition, several case reports and studies suggest that systems with less continuity may have poorer outcomes.7, 1015
Continuity in the hospital setting is likely to be important for several reasons. First, the acuity of a patient's problem during a hospitalization is likely greater than during an outpatient visit. Thus the complexity of information to be transferred between physicians during a hospital stay is correspondingly greater. Second, the diagnostic uncertainty surrounding many admissions leads to complex thought processes that may be difficult to recreate when handing off patient care to another physician. Finally, knowledge of a patient's hospital course and the likely trajectory of care is facilitated by firsthand knowledge of where the patient has been. All this information can be difficult to distill into a brief sign‐out to another physician who assumes care of the patient.
In the current study, we sought to examine the trends over time in continuity of inpatient care. We chose patients likely to be cared for by general internists: those hospitalized for chronic obstructive pulmonary disease (COPD), pneumonia, and congestive heart failure (CHF). The general internists caring for patients in the hospital could be the patient's primary care physician (PCP), a physician covering for the patient's PCP, a physician assigned at admission by the hospital, or a hospitalist. Our goals were to describe the current level of continuity of care in the hospital setting, to examine whether continuity has changed over time, and to determine factors affecting continuity of care.
Methods
We used a 5% national sample of claims data from Medicare beneficiaries for the years 19962006.16 This included Medicare enrollment files, Medicare Provider Analysis and Review (MEDPAR) files, Medicare Carrier files, and Provider of Services (POS) files.17, 18
Establishment of the Study Cohort
Hospital admissions for COPD (Diagnosis Related Group [DRG] 088), pneumonia (DRG 089, 090), and CHF (DRG 127) from 1996 to 2006 for patients older than 66 years in MEDPAR were selected (n = 781,348). We excluded admissions for patients enrolled in health maintenance organizations (HMOs) or who did not have Medicare Parts A and B for the entire year prior to admission (n = 57,558). Admissions with a length of stay >18 days (n = 10,688) were considered outliers (exceeding the 99th percentile) and were excluded. Only admissions cared for by a general internist, family physician, general practitioner, or geriatrician were included (n = 528,453).
Measures
We categorized patients by age, gender, and ethnicity using Medicare enrollment files. We used the Medicaid indicator in the Medicare file as a proxy of low socioeconomic status. We used MEDPAR files to determine the origin of the admission (via the emergency department vs other), weekend versus weekday admission, and DRG. A comorbidity score was generated using the Elixhauser comorbidity scale using inpatient and outpatient billing data.19 In analyses, we listed the total number of comorbidities identified. The specialty of each physician was determined from the codes in the Medicare Carrier files. The 2004 POS files provided hospital‐level information such as zip code, metropolitan size, state, total number of beds, type of hospital, and medical school affiliation. We divided metropolitan size and total number of hospital beds into quartiles. We categorized hospitals as nonprofit, for profit, or public; medical school affiliation was categorized as non, minor, or major.
Determination of Primary Care Physician (PCP)
We identified outpatient visits using American Medical AssociationCommon Procedure Terminology (CPT) evaluation and management codes 99201 to 99205 (new patient) and 99221 to 99215 (established patient encounters). Individual providers were differentiated by using their Unique Provider Identification Number (UPIN). We defined a PCP as a general practitioner, family physician, internist, or geriatrician. Patients had to make at least 3 visits on different days to the same PCP within a year prior to the hospitalization to be categorized as having a PCP.20
Identification of Hospitalists Versus Other Generalist Physicians
As previously described, we defined hospitalists as general internal medicine physicians who derive at least 90% of their Medicare claims for Evaluation and Management services from care provided to hospitalized patients.21 Non‐hospitalist generalist physicians were those generalists who met the criteria for generalists but did not derive at least 90% of their Medicare claims from inpatient medicine.
Definition of Inpatient Continuity of Care
We measured inpatient continuity of care by number of generalist physicians (including hospitalists) who provided care during a hospitalization, through all inpatient claims made during that hospitalization. We considered patients to have had inpatient continuity of care if all billing by generalist physicians was done by one physician during the entire hospitalization.
Statistical Analyses
We calculated the percentage of admissions that received care from 1, 2, or 3 or more generalist physicians during the hospitalization, and stratified by selected patient and hospital characteristics. These proportions were also stratified by whether the patients were cared for by their outpatient PCP or not, and whether they were cared for by hospitalists or not. Based on who cared for the patient during the hospitalization, all admissions were classified as receiving care from: 1) non‐hospitalist generalist physicians, 2) a combination of generalist physicians and hospitalists, and 3) hospitalists only. The effect of patient and hospital characteristics on whether a patient experienced inpatient continuity was evaluated using a hierarchical generalized linear model (HGLM) with a logistic link, adjusting for clustering of admissions within hospitals and all covariates. We repeated our analyses using HGLM with an ordinal logit link to explore the factors associated with number of generalists seen in the hospital. All analyses were performed with SAS version 9.1 (SAS Inc, Cary, NC). The SAS GLIMMIX procedure was used to conduct multilevel analyses.
Results
Between 1996 and 2006, 528,453 patients hospitalized for COPD, pneumonia, and CHF received care by a generalist physician during their hospital stay. Of these, 64.3% were seen by one generalist physician, 26.9% by two generalist physicians, and 8.8% by three or more generalist physicians during hospitalization.
Figure 1 shows the percentage of all patients seen by 1, 2, and 3 or more generalist physicians between 1996 and 2006. The percentage of patients receiving care from one generalist physician declined from 70.7% in 1996 to 59.4% in 2006 (P < 0.001). During the same period, the percentage of patients receiving care from 3 or more generalist physicians increased from 6.5% to 10.7% (P < 0.001). Similar trends were seen for each of the 3 conditions. There was a decrease in overall length of stay during this period, from a mean of 5.7 to 4.9 days (P < 0.001). The increase in the number of generalist physicians providing care during the hospital stay did not correspond to an increase in total number of visits during the hospitalization. The average number of daily visits from a generalist physician was 0.94 (0.30) in 1996 and 0.96 (0.35) in 2006.
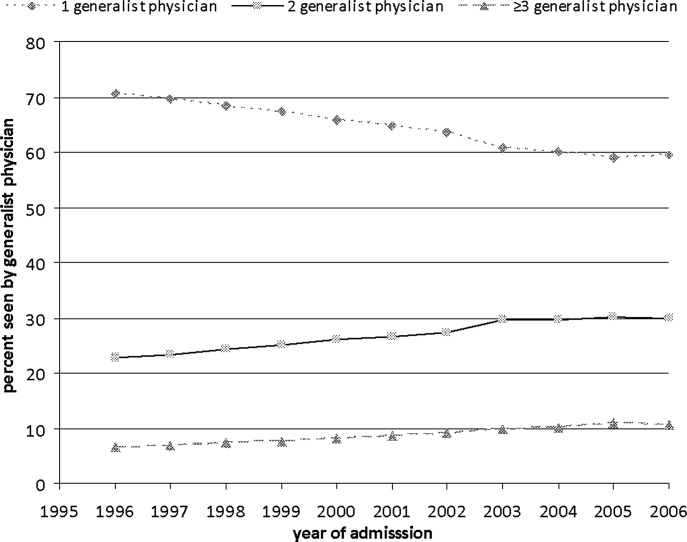
Table 1 presents the percentage of patients receiving care from 1, 2, and 3 or more generalist physicians during hospitalization stratified by patient and hospital characteristics. Older adults, females, non‐Hispanic whites, those with higher socioeconomic status, and those with more comorbidities were more likely to receive care by multiple generalist physicians. There was also large variation by geographic region, metropolitan area size, and hospital characteristics. All of these differences were significant at the P < 0.0001 level.
| No. of Generalist Physicians Seen During Hospitalization | ||||
|---|---|---|---|---|
| Characteristic | N | 1 | 2 | 3 (Percentage of Patients) |
| ||||
| Age at admission | ||||
| 6674 | 152,488 | 66.4 | 25.6 | 8.0 |
| 7584 | 226,802 | 63.8 | 27.3 | 8.9 |
| 85+ | 149,163 | 63.0 | 27.7 | 9.3 |
| Gender | ||||
| Male | 216,602 | 65.3 | 26.4 | 8.3 |
| Female | 311,851 | 63.6 | 27.3 | 9.1 |
| Ethnicity | ||||
| White | 461,543 | 63.7 | 27.4 | 9.0 |
| Black | 46,960 | 68.6 | 23.8 | 7.6 |
| Other | 19,950 | 67.9 | 24.5 | 7.6 |
| Low socioeconomic status | ||||
| No | 366,392 | 63.4 | 27.5 | 9.1 |
| Yes | 162,061 | 66.3 | 25.7 | 8.0 |
| Emergency admission | ||||
| No | 188,354 | 66.8 | 25.6 | 7.6 |
| Yes | 340,099 | 62.9 | 27.7 | 9.4 |
| Weekend admission | ||||
| No | 392,150 | 65.7 | 25.8 | 8.5 |
| Yes | 136,303 | 60.1 | 30.3 | 9.6 |
| Diagnosis‐related groups | ||||
| CHF | 213,914 | 65.0 | 26.3 | 8.7 |
| Pneumonia | 195,430 | 62.5 | 28.0 | 9.5 |
| COPD | 119,109 | 66.1 | 26.2 | 7.7 |
| Had a PCP | ||||
| No | 201,016 | 66.5 | 25.4 | 8.0 |
| Yes | 327,437 | 62.9 | 27.9 | 9.2 |
| Seen hospitalist | ||||
| No | 431,784 | 67.8 | 25.1 | 7.0 |
| Yes | 96,669 | 48.5 | 34.9 | 16.6 |
| Charlson comorbidity score | ||||
| 0 | 127,385 | 64.0 | 27.2 | 8.8 |
| 1 | 131,402 | 65.1 | 26.8 | 8.1 |
| 2 | 105,831 | 64.9 | 26.6 | 8.5 |
| 3 | 163,835 | 63.4 | 27.1 | 9.5 |
| ICU use | ||||
| No | 431,462 | 65.3 | 26.5 | 8.2 |
| Yes | 96,991 | 60.1 | 28.7 | 11.2 |
| Length of stay (in days) | ||||
| Mean (SD) | 4.7 (2.9) | 5.8 (3.1) | 8.1 (3.7) | |
| Geographic region | ||||
| New England | 23,572 | 55.7 | 30.8 | 13.5 |
| Middle Atlantic | 78,181 | 60.8 | 27.8 | 11.4 |
| East North Central | 98,072 | 65.7 | 26.3 | 8.0 |
| West North Central | 44,785 | 59.6 | 30.5 | 9.9 |
| South Atlantic | 104,894 | 63.8 | 27.0 | 9.2 |
| East South Central | 51,450 | 67.8 | 24.6 | 7.6 |
| West South Central | 63,493 | 69.2 | 24.8 | 6.0 |
| Mountain | 20,310 | 61.9 | 29.4 | 8.7 |
| Pacific | 36,484 | 66.7 | 26.3 | 7.0 |
| Size of metropolitan area* | ||||
| 1,000,000 | 229,145 | 63.7 | 26.5 | 9.8 |
| 250,000999,999 | 114,448 | 61.0 | 29.2 | 9.8 |
| 100,000249,999 | 11,448 | 61.3 | 30.4 | 8.3 |
| <100,000 | 171,585 | 67.4 | 25.8 | 6.8 |
| Medical school affiliation* | ||||
| Major | 77,605 | 62.9 | 26.8 | 10.3 |
| Minor | 107,144 | 61.5 | 28.4 | 10.1 |
| Non | 341,874 | 65.5 | 26.5 | 8.0 |
| Type of hospital* | ||||
| Nonprofit | 375,888 | 62.7 | 27.8 | 9.5 |
| For profit | 63,898 | 67.5 | 25.5 | 7.0 |
| Public | 86,837 | 68.9 | 24.2 | 6.9 |
| Hospital size* | . | . | . | |
| <200 beds | 232,869 | 67.2 | 25.7 | 7.1 |
| 200349 beds | 135,954 | 62.6 | 27.9 | 9.5 |
| 350499 beds | 77,080 | 61.1 | 28.3 | 10.6 |
| 500 beds | 80,723 | 61.7 | 27.6 | 10.7 |
| Discharge location | ||||
| Home | 361,893 | 66.6 | 26.0 | 7.4 |
| SNF | 94,723 | 57.6 | 30.1 | 12.3 |
| Rehab | 3,030 | 45.7 | 34.2 | 20.1 |
| Death | 22,133 | 63.1 | 25.4 | 11.5 |
| Other | 46,674 | 61.8 | 28.1 | 10.1 |
Table 2 presents the results of a multivariable analysis of factors independently associated with experiencing continuity of care. In this analysis, continuity of care was defined as receiving inpatient care from one generalist physician (vs two or more). In the unadjusted models, the odds of experiencing continuity of care decreased by 5.5% per year from 1996 through 2006, and this decrease did not substantially change after adjusting for all other variables (4.8% yearly decrease). Younger patients, females, black patients, and those with low socioeconomic status were slightly more likely to experience continuity of care. As expected, patients admitted on weekends, emergency admissions, and those with intensive care unit (ICU) stays were less likely to experience continuity. There were marked geographic variations in continuity, with continuity approximately half as likely in New England as in the South. Continuity was greatest in smaller metropolitan areas versus rural and large metropolitan areas. Hospital size and teaching status produced only minor variation.
| Characteristic | Odds Ratio (95% CI) |
|---|---|
| |
| Admission year (increase by year) | 0.952 (0.9500.954) |
| Length of stay (increase by day) | 0.822 (0.8200.823) |
| Had a PCP | |
| No | 1.0 |
| Yes | 0.762 (0.7520.773) |
| Seen by a hospitalist | |
| No | 1.0 |
| Yes | 0.391 (0.3840.398) |
| Age | |
| 6674 | 1.0 |
| 7584 | 0.959 (0.9440.973) |
| 85+ | 0.946 (0.9300.962) |
| Gender | |
| Male | 1.0 |
| Female | 1.047 (1.0331.060) |
| Ethnicity | |
| White | 1.0 |
| Black | 1.126 (1.0971.155) |
| Other | 1.062 (1.0231.103) |
| Low socioeconomic status | |
| No | 1.0 |
| Yes | 1.036 (1.0201.051) |
| Emergency admission | |
| No | 1.0 |
| Yes | 0.864 (0.8510.878) |
| Weekend admission | |
| No | 1.0 |
| Yes | 0.778 (0.7680.789) |
| Diagnosis‐related group | |
| CHF | 1.0 |
| Pneumonia | 0.964 (0.9500.978) |
| COPD | 1.002 (0.9851.019) |
| Charlson comorbidity score | |
| 0 | 1.0 |
| 1 | 1.053 (1.0351.072) |
| 2 | 1.062 (1.0421.083) |
| 3 | 1.040 (1.0221.058) |
| ICU use | |
| No | 1.0 |
| Yes | 0.918 (0.9020.935) |
| Geographic region | |
| Middle Atlantic | 1.0 |
| New England | 0.714 (0.6210.822) |
| East North Central | 1.015 (0.9221.119) |
| West North Central | 0.791 (0.7110.879) |
| South Atlantic | 1.074 (0.9711.186) |
| East South Central | 1.250 (1.1131.403) |
| West South Central | 1.377 (1.2401.530) |
| Mountain | 0.839 (0.7400.951) |
| Pacific | 0.985 (0.8841.097) |
| Size of metropolitan area | |
| 1,000,000 | 1.0 |
| 250,000999,999 | 0.743 (0.6910.798) |
| 100,000249,999 | 0.651 (0.5380.789) |
| <100,000 | 1.062 (0.9911.138) |
| Medical school affiliation | |
| None | 1.0 |
| Minor | 0.889 (0.8270.956) |
| Major | 1.048 (0.9521.154) |
| Type of hospital | |
| Nonprofit | 1.0 |
| For profit | 1.194 (1.1061.289) |
| Public | 1.394 (1.3091.484) |
| Size of hospital | |
| <200 beds | 1.0 |
| 200349 beds | 0.918 (0.8550.986) |
| 350499 beds | 0.962 (0.8721.061) |
| 500 beds | 1.000 (0.8931.119) |
In Table 2 we also show that patients with an established PCP and those who received care from a hospitalist in the hospital were substantially less likely to experience continuity of care. There are several possible interpretations for that finding. For example, it might be that patients admitted to a hospitalist service were likely to see multiple hospitalists. Alternatively, the decreased continuity associated with hospitalists could reflect the fact that some patients cared for predominantly by non‐hospitalists may have seen a hospitalist on call for a sudden change in health status. To further explore these possible explanatory pathways, we constructed three new cohorts: 1) patients receiving all their care from non‐hospitalists, 2) patients receiving all their care from hospitalists, and 3) patients seen by both. As shown in Table 3, in patients seen by non‐hospitalists only, the mean number of generalist physicians seen during hospitalization was slightly greater than in patients cared for only by hospitalists.
| Received Care During Entire Hospitalization | No. of Admissions | Mean (SD) No. of Generalist Physicians Seen During Hospitalization |
|---|---|---|
| ||
| Non‐hospitalist physician | 431,784 | 1.41 (0.68)* |
| Hospitalist physician | 64,662 | 1.34 (0.62)* |
| Both | 32,007 | 2.55 (0.83)* |
We also tested for interactions in Table 2 between admission year and other factors. There was a significant interaction between admission year and having an identifiable PCP in the year prior to admission (Table 2). The odds of experiencing continuity of care decreased more rapidly for patients who did not have a PCP (5.5% per year; 95% CI: 5.2%5.8%) than for those who had one (4.3% per year; 95% CI: 4.1%4.6%).
Discussion
We conducted this study to better understand the degree to which hospitalized patients experience discontinuity of care within a hospital stay and to determine which patients are most likely to experience discontinuity. In our study, we specifically chose admission conditions that would likely be followed primarily by generalist physicians. We found that, over the past decade, discontinuity of care for hospitalized patients has increased substantially, as indicated by the proportion of patients taken care of by more than one generalist physician during a single hospital stay. This occurred even though overall length of stay was decreasing in this same period.
It is perhaps not surprising that inpatient continuity of care has been decreasing in the past 10 years. Outpatient practices are becoming busier, and more doctors are practicing in large group practices, which could lead to several different physicians in the same practice rounding on a hospitalized patient. We have previously demonstrated that hospitalists are caring for an increasing number of patients over this same time period,21 so another possibility is that hospitalist services are being used more often because of this heavy outpatient workload. Our analyses allowed us to test the hypothesis that having hospitalists involved in patient care increases discontinuity.
At first glance, it appears that being cared for by hospitalists may result in worse continuity of care. However, closer scrutiny of the data reveals that the discontinuity ascribed to the hospitalists in the multivariable model appears to be an artifact of defining the hospitalist variable as having been seen by any hospitalist during the hospital stay. This would include patients who saw a hospitalist in addition to their PCP or another non‐hospitalist generalist. When we compared hospitalist‐only care to other generalist care, we could not detect a difference in discontinuity. We know that generalist visits per day to patients has not substantially increased over time, so this discontinuity trend is not explained by having visits by both a hospitalist and the PCP. Therefore, this combination of findings suggests that the increased discontinuity associated with having a hospitalist involved in patient care is likely the result of system issues rather than hospitalist care per se. In fact, patients seem to experience slightly better continuity when they see only hospitalists as opposed to only non‐hospitalists.
What types of systems issues might lead to this finding? Generalists in most settings could choose to involve a hospitalist at any point in the patient's hospital stay. This could occur because of a change in patient acuity requiring the involvement of hospitalists who are present in the hospital more. It is also possible that hospitalists' schedules are created to maximize inpatient continuity of care with individual hospitalists. Even though hospitalists clearly work shifts, the 7 on, 7 off model22 likely results in patients seeing the same physician each day until the switch day. This is in contrast to outpatient primary care doctors whose concentration may be on maintaining continuity within their practice.
As the field of hospital medicine was emerging, many internal medicine physicians from various specialties were concerned about the impact of hospitalists on patient care. In one study, 73% of internal medicine physicians who were not hospitalists thought that hospitalists would worsen continuity of care.23 Primary care and subspecialist internal medicine physicians also expressed the concern that hospitalists could hurt their own relationships with patients,6 presumably because of lost continuity between the inpatient and outpatient settings. However, this fear seems to diminish once hospitalist programs are implemented and primary care doctors have experience with them.23 Our study suggests that the decrease in continuity that has occurred since these studies were published is not likely due to the emergence of hospital medicine, but rather due to other factors that influence who cares for hospitalized patients.
This study had some limitations. Length of stay is an obvious mediator of number of generalist physicians seen. Therefore, the sickest patients are likely to have both a long length of stay and low continuity. We adjusted for this in the multivariable modeling. In addition, given that this study used a large database, certain details are not discernable. For example, we chose to operationalize discontinuity as visits from multiple generalists during a single hospital stay. That is not a perfect definition, but it does represent multiple physicians directing the care of a patient. Importantly, this does not appear to represent continuity with one physician with extra visits from another, as the total number of generalist visits per day did not change over time. It is also possible that patients in the non‐hospitalist group saw physicians only from a single practice, but those details are not included in the database. Finally, we cannot tell what type of hand‐offs were occurring for individual patients during each hospital stay. Despite these disadvantages, using a large database like this one allows for detection of fairly small differences that could still be clinically important.
In summary, hospitalized patients appear to experience less continuity now than 10 years ago. However, the hospitalist model does not appear to play a role in this discontinuity. It is worth exploring in more detail why patients would see both hospitalists and other generalists. This pattern is not surprising, but may have some repercussions in terms of increasing the number of hand‐offs experienced by patients. These could lead to problems with patient safety and quality of care. Future work should explore the reasons for this discontinuity and look at the relationship between inpatient discontinuity outcomes such as quality of care and the doctorpatient relationship.
Acknowledgements
The authors thank Sarah Toombs Smith, PhD, for help in preparation of the manuscript.
- .Defining and measuring interpersonal continuity of care.Ann Fam Med.2003;1(3):134–143.
- ,,.Good continuity of care may improve quality of life in Type 2 diabetes.Diabetes Res Clin Pract.2001;51(1):21–27.
- ,,,.Provider continuity in family medicine: Does it make a difference for total health care costs?Ann Fam Med.2003;1(3):144–148.
- ,,.The effect of continuity of care on emergency department use.Arch Fam Med.2000;9(4):333–338.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: Results of a national survey.Am J Med.2000;109(8):648–653.
- ,,.Physician views on caring for hospitalized patients and the hospitalist model of inpatient care.J Gen Intern Med.2001;16(2):116–119.
- ,,,,,.Systematic review: Effects of resident work hours on patient safety.Ann Intern Med.2004;141(11):851–857.
- ,,.Balancing continuity of care with residents' limited work hours: Defining the implications.Acad Med.2005;80(1):39–43.
- ,,.Understanding communication during hospitalist sevice changes: A mixed methods study.J Hosp Med.2009;4:535–540.
- ,,,Center for Safety in Emergency C. Profiles in patient safety: Emergency care transitions.Acad Emerg Med.2003;10(4):364–367.
- .Fumbled handoffs: One dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- Agency for Healthcare Research and Quality. Fumbled handoff.2004. Available at: http://www.webmm.ahrq.gov/printview.aspx?caseID=55. Accessed December 27, 2005.
- ,,.Graduate medical education and patient safety: A busy—and occasionally hazardous—intersection.Ann Intern Med.2006;145(8):592–598.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269(3):374–378.
- Centers for Medicare and Medicaid Services. Standard analytical files. Available at: http://www.cms.hhs.gov/IdentifiableDataFiles/02_Standard AnalyticalFiles.asp. Accessed March 1,2009.
- Centers for Medicare and Medicaid Services. Nonidentifiable data files: Provider of services files. Available at: http://www.cms.hhs.gov/NonIdentifiableDataFiles/04_ProviderofSerrvicesFile.asp. Accessed March 1,2009.
- Research Data Assistance Center. Medicare data file description. Available at: http://www.resdac.umn.edu/Medicare/file_descriptions.asp. Accessed March 1,2009.
- ,,,.Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance.Am J Transplant.2009;9:506–516.
- ,,,,,.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301:1671–1680.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med. 2009;360:1102–1112.
- HCPro Inc.Medical Staff Leader blog.2010. Available at: http://blogs. hcpro.com/medicalstaff/2010/01/free‐form‐example‐seven‐day‐on‐seven‐day‐off‐hospitalist‐schedule/. Accessed November 20, 2010.
- ,,,.How physicians perceive hospitalist services after implementation: Anticipation vs reality.Arch Intern Med.2003;163(19):2330–2336.
Continuity of care is considered by many physicians to be of critical importance in providing high‐quality patient care. Most of the research to date has focused on continuity in outpatient primary care. Research on outpatient continuity of care has been facilitated by the fact that a number of measurement tools for outpatient continuity exist.1 Outpatient continuity of care has been linked to better quality of life scores,2 lower costs,3 and less emergency room use.4 As hospital medicine has taken on more and more of the responsibility of inpatient care, primary care doctors have voiced concerns about the impact of hospitalists on overall continuity of care5 and the quality of the doctorpatient relationship.6
Recently, continuity of care in the hospital setting has also received attention. When the Accreditation Council for Graduate Medical Education (ACGME) first proposed restrictions to resident duty hours, the importance of continuity of inpatient care began to be debated in earnest in large part because of the increase in hand‐offs which accompanies discontinuity.7, 8 A recent study of hospitalist communication documented that as many as 13% of hand‐offs at the time of service changes are judged as incomplete by the receiving physician. These incomplete hand‐offs were more likely to be associated with uncertainty regarding the plan of care, as well as perceived near misses or adverse events.9 In addition, several case reports and studies suggest that systems with less continuity may have poorer outcomes.7, 1015
Continuity in the hospital setting is likely to be important for several reasons. First, the acuity of a patient's problem during a hospitalization is likely greater than during an outpatient visit. Thus the complexity of information to be transferred between physicians during a hospital stay is correspondingly greater. Second, the diagnostic uncertainty surrounding many admissions leads to complex thought processes that may be difficult to recreate when handing off patient care to another physician. Finally, knowledge of a patient's hospital course and the likely trajectory of care is facilitated by firsthand knowledge of where the patient has been. All this information can be difficult to distill into a brief sign‐out to another physician who assumes care of the patient.
In the current study, we sought to examine the trends over time in continuity of inpatient care. We chose patients likely to be cared for by general internists: those hospitalized for chronic obstructive pulmonary disease (COPD), pneumonia, and congestive heart failure (CHF). The general internists caring for patients in the hospital could be the patient's primary care physician (PCP), a physician covering for the patient's PCP, a physician assigned at admission by the hospital, or a hospitalist. Our goals were to describe the current level of continuity of care in the hospital setting, to examine whether continuity has changed over time, and to determine factors affecting continuity of care.
Methods
We used a 5% national sample of claims data from Medicare beneficiaries for the years 19962006.16 This included Medicare enrollment files, Medicare Provider Analysis and Review (MEDPAR) files, Medicare Carrier files, and Provider of Services (POS) files.17, 18
Establishment of the Study Cohort
Hospital admissions for COPD (Diagnosis Related Group [DRG] 088), pneumonia (DRG 089, 090), and CHF (DRG 127) from 1996 to 2006 for patients older than 66 years in MEDPAR were selected (n = 781,348). We excluded admissions for patients enrolled in health maintenance organizations (HMOs) or who did not have Medicare Parts A and B for the entire year prior to admission (n = 57,558). Admissions with a length of stay >18 days (n = 10,688) were considered outliers (exceeding the 99th percentile) and were excluded. Only admissions cared for by a general internist, family physician, general practitioner, or geriatrician were included (n = 528,453).
Measures
We categorized patients by age, gender, and ethnicity using Medicare enrollment files. We used the Medicaid indicator in the Medicare file as a proxy of low socioeconomic status. We used MEDPAR files to determine the origin of the admission (via the emergency department vs other), weekend versus weekday admission, and DRG. A comorbidity score was generated using the Elixhauser comorbidity scale using inpatient and outpatient billing data.19 In analyses, we listed the total number of comorbidities identified. The specialty of each physician was determined from the codes in the Medicare Carrier files. The 2004 POS files provided hospital‐level information such as zip code, metropolitan size, state, total number of beds, type of hospital, and medical school affiliation. We divided metropolitan size and total number of hospital beds into quartiles. We categorized hospitals as nonprofit, for profit, or public; medical school affiliation was categorized as non, minor, or major.
Determination of Primary Care Physician (PCP)
We identified outpatient visits using American Medical AssociationCommon Procedure Terminology (CPT) evaluation and management codes 99201 to 99205 (new patient) and 99221 to 99215 (established patient encounters). Individual providers were differentiated by using their Unique Provider Identification Number (UPIN). We defined a PCP as a general practitioner, family physician, internist, or geriatrician. Patients had to make at least 3 visits on different days to the same PCP within a year prior to the hospitalization to be categorized as having a PCP.20
Identification of Hospitalists Versus Other Generalist Physicians
As previously described, we defined hospitalists as general internal medicine physicians who derive at least 90% of their Medicare claims for Evaluation and Management services from care provided to hospitalized patients.21 Non‐hospitalist generalist physicians were those generalists who met the criteria for generalists but did not derive at least 90% of their Medicare claims from inpatient medicine.
Definition of Inpatient Continuity of Care
We measured inpatient continuity of care by number of generalist physicians (including hospitalists) who provided care during a hospitalization, through all inpatient claims made during that hospitalization. We considered patients to have had inpatient continuity of care if all billing by generalist physicians was done by one physician during the entire hospitalization.
Statistical Analyses
We calculated the percentage of admissions that received care from 1, 2, or 3 or more generalist physicians during the hospitalization, and stratified by selected patient and hospital characteristics. These proportions were also stratified by whether the patients were cared for by their outpatient PCP or not, and whether they were cared for by hospitalists or not. Based on who cared for the patient during the hospitalization, all admissions were classified as receiving care from: 1) non‐hospitalist generalist physicians, 2) a combination of generalist physicians and hospitalists, and 3) hospitalists only. The effect of patient and hospital characteristics on whether a patient experienced inpatient continuity was evaluated using a hierarchical generalized linear model (HGLM) with a logistic link, adjusting for clustering of admissions within hospitals and all covariates. We repeated our analyses using HGLM with an ordinal logit link to explore the factors associated with number of generalists seen in the hospital. All analyses were performed with SAS version 9.1 (SAS Inc, Cary, NC). The SAS GLIMMIX procedure was used to conduct multilevel analyses.
Results
Between 1996 and 2006, 528,453 patients hospitalized for COPD, pneumonia, and CHF received care by a generalist physician during their hospital stay. Of these, 64.3% were seen by one generalist physician, 26.9% by two generalist physicians, and 8.8% by three or more generalist physicians during hospitalization.
Figure 1 shows the percentage of all patients seen by 1, 2, and 3 or more generalist physicians between 1996 and 2006. The percentage of patients receiving care from one generalist physician declined from 70.7% in 1996 to 59.4% in 2006 (P < 0.001). During the same period, the percentage of patients receiving care from 3 or more generalist physicians increased from 6.5% to 10.7% (P < 0.001). Similar trends were seen for each of the 3 conditions. There was a decrease in overall length of stay during this period, from a mean of 5.7 to 4.9 days (P < 0.001). The increase in the number of generalist physicians providing care during the hospital stay did not correspond to an increase in total number of visits during the hospitalization. The average number of daily visits from a generalist physician was 0.94 (0.30) in 1996 and 0.96 (0.35) in 2006.

Table 1 presents the percentage of patients receiving care from 1, 2, and 3 or more generalist physicians during hospitalization stratified by patient and hospital characteristics. Older adults, females, non‐Hispanic whites, those with higher socioeconomic status, and those with more comorbidities were more likely to receive care by multiple generalist physicians. There was also large variation by geographic region, metropolitan area size, and hospital characteristics. All of these differences were significant at the P < 0.0001 level.
| No. of Generalist Physicians Seen During Hospitalization | ||||
|---|---|---|---|---|
| Characteristic | N | 1 | 2 | 3 (Percentage of Patients) |
| ||||
| Age at admission | ||||
| 6674 | 152,488 | 66.4 | 25.6 | 8.0 |
| 7584 | 226,802 | 63.8 | 27.3 | 8.9 |
| 85+ | 149,163 | 63.0 | 27.7 | 9.3 |
| Gender | ||||
| Male | 216,602 | 65.3 | 26.4 | 8.3 |
| Female | 311,851 | 63.6 | 27.3 | 9.1 |
| Ethnicity | ||||
| White | 461,543 | 63.7 | 27.4 | 9.0 |
| Black | 46,960 | 68.6 | 23.8 | 7.6 |
| Other | 19,950 | 67.9 | 24.5 | 7.6 |
| Low socioeconomic status | ||||
| No | 366,392 | 63.4 | 27.5 | 9.1 |
| Yes | 162,061 | 66.3 | 25.7 | 8.0 |
| Emergency admission | ||||
| No | 188,354 | 66.8 | 25.6 | 7.6 |
| Yes | 340,099 | 62.9 | 27.7 | 9.4 |
| Weekend admission | ||||
| No | 392,150 | 65.7 | 25.8 | 8.5 |
| Yes | 136,303 | 60.1 | 30.3 | 9.6 |
| Diagnosis‐related groups | ||||
| CHF | 213,914 | 65.0 | 26.3 | 8.7 |
| Pneumonia | 195,430 | 62.5 | 28.0 | 9.5 |
| COPD | 119,109 | 66.1 | 26.2 | 7.7 |
| Had a PCP | ||||
| No | 201,016 | 66.5 | 25.4 | 8.0 |
| Yes | 327,437 | 62.9 | 27.9 | 9.2 |
| Seen hospitalist | ||||
| No | 431,784 | 67.8 | 25.1 | 7.0 |
| Yes | 96,669 | 48.5 | 34.9 | 16.6 |
| Charlson comorbidity score | ||||
| 0 | 127,385 | 64.0 | 27.2 | 8.8 |
| 1 | 131,402 | 65.1 | 26.8 | 8.1 |
| 2 | 105,831 | 64.9 | 26.6 | 8.5 |
| 3 | 163,835 | 63.4 | 27.1 | 9.5 |
| ICU use | ||||
| No | 431,462 | 65.3 | 26.5 | 8.2 |
| Yes | 96,991 | 60.1 | 28.7 | 11.2 |
| Length of stay (in days) | ||||
| Mean (SD) | 4.7 (2.9) | 5.8 (3.1) | 8.1 (3.7) | |
| Geographic region | ||||
| New England | 23,572 | 55.7 | 30.8 | 13.5 |
| Middle Atlantic | 78,181 | 60.8 | 27.8 | 11.4 |
| East North Central | 98,072 | 65.7 | 26.3 | 8.0 |
| West North Central | 44,785 | 59.6 | 30.5 | 9.9 |
| South Atlantic | 104,894 | 63.8 | 27.0 | 9.2 |
| East South Central | 51,450 | 67.8 | 24.6 | 7.6 |
| West South Central | 63,493 | 69.2 | 24.8 | 6.0 |
| Mountain | 20,310 | 61.9 | 29.4 | 8.7 |
| Pacific | 36,484 | 66.7 | 26.3 | 7.0 |
| Size of metropolitan area* | ||||
| 1,000,000 | 229,145 | 63.7 | 26.5 | 9.8 |
| 250,000999,999 | 114,448 | 61.0 | 29.2 | 9.8 |
| 100,000249,999 | 11,448 | 61.3 | 30.4 | 8.3 |
| <100,000 | 171,585 | 67.4 | 25.8 | 6.8 |
| Medical school affiliation* | ||||
| Major | 77,605 | 62.9 | 26.8 | 10.3 |
| Minor | 107,144 | 61.5 | 28.4 | 10.1 |
| Non | 341,874 | 65.5 | 26.5 | 8.0 |
| Type of hospital* | ||||
| Nonprofit | 375,888 | 62.7 | 27.8 | 9.5 |
| For profit | 63,898 | 67.5 | 25.5 | 7.0 |
| Public | 86,837 | 68.9 | 24.2 | 6.9 |
| Hospital size* | . | . | . | |
| <200 beds | 232,869 | 67.2 | 25.7 | 7.1 |
| 200349 beds | 135,954 | 62.6 | 27.9 | 9.5 |
| 350499 beds | 77,080 | 61.1 | 28.3 | 10.6 |
| 500 beds | 80,723 | 61.7 | 27.6 | 10.7 |
| Discharge location | ||||
| Home | 361,893 | 66.6 | 26.0 | 7.4 |
| SNF | 94,723 | 57.6 | 30.1 | 12.3 |
| Rehab | 3,030 | 45.7 | 34.2 | 20.1 |
| Death | 22,133 | 63.1 | 25.4 | 11.5 |
| Other | 46,674 | 61.8 | 28.1 | 10.1 |
Table 2 presents the results of a multivariable analysis of factors independently associated with experiencing continuity of care. In this analysis, continuity of care was defined as receiving inpatient care from one generalist physician (vs two or more). In the unadjusted models, the odds of experiencing continuity of care decreased by 5.5% per year from 1996 through 2006, and this decrease did not substantially change after adjusting for all other variables (4.8% yearly decrease). Younger patients, females, black patients, and those with low socioeconomic status were slightly more likely to experience continuity of care. As expected, patients admitted on weekends, emergency admissions, and those with intensive care unit (ICU) stays were less likely to experience continuity. There were marked geographic variations in continuity, with continuity approximately half as likely in New England as in the South. Continuity was greatest in smaller metropolitan areas versus rural and large metropolitan areas. Hospital size and teaching status produced only minor variation.
| Characteristic | Odds Ratio (95% CI) |
|---|---|
| |
| Admission year (increase by year) | 0.952 (0.9500.954) |
| Length of stay (increase by day) | 0.822 (0.8200.823) |
| Had a PCP | |
| No | 1.0 |
| Yes | 0.762 (0.7520.773) |
| Seen by a hospitalist | |
| No | 1.0 |
| Yes | 0.391 (0.3840.398) |
| Age | |
| 6674 | 1.0 |
| 7584 | 0.959 (0.9440.973) |
| 85+ | 0.946 (0.9300.962) |
| Gender | |
| Male | 1.0 |
| Female | 1.047 (1.0331.060) |
| Ethnicity | |
| White | 1.0 |
| Black | 1.126 (1.0971.155) |
| Other | 1.062 (1.0231.103) |
| Low socioeconomic status | |
| No | 1.0 |
| Yes | 1.036 (1.0201.051) |
| Emergency admission | |
| No | 1.0 |
| Yes | 0.864 (0.8510.878) |
| Weekend admission | |
| No | 1.0 |
| Yes | 0.778 (0.7680.789) |
| Diagnosis‐related group | |
| CHF | 1.0 |
| Pneumonia | 0.964 (0.9500.978) |
| COPD | 1.002 (0.9851.019) |
| Charlson comorbidity score | |
| 0 | 1.0 |
| 1 | 1.053 (1.0351.072) |
| 2 | 1.062 (1.0421.083) |
| 3 | 1.040 (1.0221.058) |
| ICU use | |
| No | 1.0 |
| Yes | 0.918 (0.9020.935) |
| Geographic region | |
| Middle Atlantic | 1.0 |
| New England | 0.714 (0.6210.822) |
| East North Central | 1.015 (0.9221.119) |
| West North Central | 0.791 (0.7110.879) |
| South Atlantic | 1.074 (0.9711.186) |
| East South Central | 1.250 (1.1131.403) |
| West South Central | 1.377 (1.2401.530) |
| Mountain | 0.839 (0.7400.951) |
| Pacific | 0.985 (0.8841.097) |
| Size of metropolitan area | |
| 1,000,000 | 1.0 |
| 250,000999,999 | 0.743 (0.6910.798) |
| 100,000249,999 | 0.651 (0.5380.789) |
| <100,000 | 1.062 (0.9911.138) |
| Medical school affiliation | |
| None | 1.0 |
| Minor | 0.889 (0.8270.956) |
| Major | 1.048 (0.9521.154) |
| Type of hospital | |
| Nonprofit | 1.0 |
| For profit | 1.194 (1.1061.289) |
| Public | 1.394 (1.3091.484) |
| Size of hospital | |
| <200 beds | 1.0 |
| 200349 beds | 0.918 (0.8550.986) |
| 350499 beds | 0.962 (0.8721.061) |
| 500 beds | 1.000 (0.8931.119) |
In Table 2 we also show that patients with an established PCP and those who received care from a hospitalist in the hospital were substantially less likely to experience continuity of care. There are several possible interpretations for that finding. For example, it might be that patients admitted to a hospitalist service were likely to see multiple hospitalists. Alternatively, the decreased continuity associated with hospitalists could reflect the fact that some patients cared for predominantly by non‐hospitalists may have seen a hospitalist on call for a sudden change in health status. To further explore these possible explanatory pathways, we constructed three new cohorts: 1) patients receiving all their care from non‐hospitalists, 2) patients receiving all their care from hospitalists, and 3) patients seen by both. As shown in Table 3, in patients seen by non‐hospitalists only, the mean number of generalist physicians seen during hospitalization was slightly greater than in patients cared for only by hospitalists.
| Received Care During Entire Hospitalization | No. of Admissions | Mean (SD) No. of Generalist Physicians Seen During Hospitalization |
|---|---|---|
| ||
| Non‐hospitalist physician | 431,784 | 1.41 (0.68)* |
| Hospitalist physician | 64,662 | 1.34 (0.62)* |
| Both | 32,007 | 2.55 (0.83)* |
We also tested for interactions in Table 2 between admission year and other factors. There was a significant interaction between admission year and having an identifiable PCP in the year prior to admission (Table 2). The odds of experiencing continuity of care decreased more rapidly for patients who did not have a PCP (5.5% per year; 95% CI: 5.2%5.8%) than for those who had one (4.3% per year; 95% CI: 4.1%4.6%).
Discussion
We conducted this study to better understand the degree to which hospitalized patients experience discontinuity of care within a hospital stay and to determine which patients are most likely to experience discontinuity. In our study, we specifically chose admission conditions that would likely be followed primarily by generalist physicians. We found that, over the past decade, discontinuity of care for hospitalized patients has increased substantially, as indicated by the proportion of patients taken care of by more than one generalist physician during a single hospital stay. This occurred even though overall length of stay was decreasing in this same period.
It is perhaps not surprising that inpatient continuity of care has been decreasing in the past 10 years. Outpatient practices are becoming busier, and more doctors are practicing in large group practices, which could lead to several different physicians in the same practice rounding on a hospitalized patient. We have previously demonstrated that hospitalists are caring for an increasing number of patients over this same time period,21 so another possibility is that hospitalist services are being used more often because of this heavy outpatient workload. Our analyses allowed us to test the hypothesis that having hospitalists involved in patient care increases discontinuity.
At first glance, it appears that being cared for by hospitalists may result in worse continuity of care. However, closer scrutiny of the data reveals that the discontinuity ascribed to the hospitalists in the multivariable model appears to be an artifact of defining the hospitalist variable as having been seen by any hospitalist during the hospital stay. This would include patients who saw a hospitalist in addition to their PCP or another non‐hospitalist generalist. When we compared hospitalist‐only care to other generalist care, we could not detect a difference in discontinuity. We know that generalist visits per day to patients has not substantially increased over time, so this discontinuity trend is not explained by having visits by both a hospitalist and the PCP. Therefore, this combination of findings suggests that the increased discontinuity associated with having a hospitalist involved in patient care is likely the result of system issues rather than hospitalist care per se. In fact, patients seem to experience slightly better continuity when they see only hospitalists as opposed to only non‐hospitalists.
What types of systems issues might lead to this finding? Generalists in most settings could choose to involve a hospitalist at any point in the patient's hospital stay. This could occur because of a change in patient acuity requiring the involvement of hospitalists who are present in the hospital more. It is also possible that hospitalists' schedules are created to maximize inpatient continuity of care with individual hospitalists. Even though hospitalists clearly work shifts, the 7 on, 7 off model22 likely results in patients seeing the same physician each day until the switch day. This is in contrast to outpatient primary care doctors whose concentration may be on maintaining continuity within their practice.
As the field of hospital medicine was emerging, many internal medicine physicians from various specialties were concerned about the impact of hospitalists on patient care. In one study, 73% of internal medicine physicians who were not hospitalists thought that hospitalists would worsen continuity of care.23 Primary care and subspecialist internal medicine physicians also expressed the concern that hospitalists could hurt their own relationships with patients,6 presumably because of lost continuity between the inpatient and outpatient settings. However, this fear seems to diminish once hospitalist programs are implemented and primary care doctors have experience with them.23 Our study suggests that the decrease in continuity that has occurred since these studies were published is not likely due to the emergence of hospital medicine, but rather due to other factors that influence who cares for hospitalized patients.
This study had some limitations. Length of stay is an obvious mediator of number of generalist physicians seen. Therefore, the sickest patients are likely to have both a long length of stay and low continuity. We adjusted for this in the multivariable modeling. In addition, given that this study used a large database, certain details are not discernable. For example, we chose to operationalize discontinuity as visits from multiple generalists during a single hospital stay. That is not a perfect definition, but it does represent multiple physicians directing the care of a patient. Importantly, this does not appear to represent continuity with one physician with extra visits from another, as the total number of generalist visits per day did not change over time. It is also possible that patients in the non‐hospitalist group saw physicians only from a single practice, but those details are not included in the database. Finally, we cannot tell what type of hand‐offs were occurring for individual patients during each hospital stay. Despite these disadvantages, using a large database like this one allows for detection of fairly small differences that could still be clinically important.
In summary, hospitalized patients appear to experience less continuity now than 10 years ago. However, the hospitalist model does not appear to play a role in this discontinuity. It is worth exploring in more detail why patients would see both hospitalists and other generalists. This pattern is not surprising, but may have some repercussions in terms of increasing the number of hand‐offs experienced by patients. These could lead to problems with patient safety and quality of care. Future work should explore the reasons for this discontinuity and look at the relationship between inpatient discontinuity outcomes such as quality of care and the doctorpatient relationship.
Acknowledgements
The authors thank Sarah Toombs Smith, PhD, for help in preparation of the manuscript.
Continuity of care is considered by many physicians to be of critical importance in providing high‐quality patient care. Most of the research to date has focused on continuity in outpatient primary care. Research on outpatient continuity of care has been facilitated by the fact that a number of measurement tools for outpatient continuity exist.1 Outpatient continuity of care has been linked to better quality of life scores,2 lower costs,3 and less emergency room use.4 As hospital medicine has taken on more and more of the responsibility of inpatient care, primary care doctors have voiced concerns about the impact of hospitalists on overall continuity of care5 and the quality of the doctorpatient relationship.6
Recently, continuity of care in the hospital setting has also received attention. When the Accreditation Council for Graduate Medical Education (ACGME) first proposed restrictions to resident duty hours, the importance of continuity of inpatient care began to be debated in earnest in large part because of the increase in hand‐offs which accompanies discontinuity.7, 8 A recent study of hospitalist communication documented that as many as 13% of hand‐offs at the time of service changes are judged as incomplete by the receiving physician. These incomplete hand‐offs were more likely to be associated with uncertainty regarding the plan of care, as well as perceived near misses or adverse events.9 In addition, several case reports and studies suggest that systems with less continuity may have poorer outcomes.7, 1015
Continuity in the hospital setting is likely to be important for several reasons. First, the acuity of a patient's problem during a hospitalization is likely greater than during an outpatient visit. Thus the complexity of information to be transferred between physicians during a hospital stay is correspondingly greater. Second, the diagnostic uncertainty surrounding many admissions leads to complex thought processes that may be difficult to recreate when handing off patient care to another physician. Finally, knowledge of a patient's hospital course and the likely trajectory of care is facilitated by firsthand knowledge of where the patient has been. All this information can be difficult to distill into a brief sign‐out to another physician who assumes care of the patient.
In the current study, we sought to examine the trends over time in continuity of inpatient care. We chose patients likely to be cared for by general internists: those hospitalized for chronic obstructive pulmonary disease (COPD), pneumonia, and congestive heart failure (CHF). The general internists caring for patients in the hospital could be the patient's primary care physician (PCP), a physician covering for the patient's PCP, a physician assigned at admission by the hospital, or a hospitalist. Our goals were to describe the current level of continuity of care in the hospital setting, to examine whether continuity has changed over time, and to determine factors affecting continuity of care.
Methods
We used a 5% national sample of claims data from Medicare beneficiaries for the years 19962006.16 This included Medicare enrollment files, Medicare Provider Analysis and Review (MEDPAR) files, Medicare Carrier files, and Provider of Services (POS) files.17, 18
Establishment of the Study Cohort
Hospital admissions for COPD (Diagnosis Related Group [DRG] 088), pneumonia (DRG 089, 090), and CHF (DRG 127) from 1996 to 2006 for patients older than 66 years in MEDPAR were selected (n = 781,348). We excluded admissions for patients enrolled in health maintenance organizations (HMOs) or who did not have Medicare Parts A and B for the entire year prior to admission (n = 57,558). Admissions with a length of stay >18 days (n = 10,688) were considered outliers (exceeding the 99th percentile) and were excluded. Only admissions cared for by a general internist, family physician, general practitioner, or geriatrician were included (n = 528,453).
Measures
We categorized patients by age, gender, and ethnicity using Medicare enrollment files. We used the Medicaid indicator in the Medicare file as a proxy of low socioeconomic status. We used MEDPAR files to determine the origin of the admission (via the emergency department vs other), weekend versus weekday admission, and DRG. A comorbidity score was generated using the Elixhauser comorbidity scale using inpatient and outpatient billing data.19 In analyses, we listed the total number of comorbidities identified. The specialty of each physician was determined from the codes in the Medicare Carrier files. The 2004 POS files provided hospital‐level information such as zip code, metropolitan size, state, total number of beds, type of hospital, and medical school affiliation. We divided metropolitan size and total number of hospital beds into quartiles. We categorized hospitals as nonprofit, for profit, or public; medical school affiliation was categorized as non, minor, or major.
Determination of Primary Care Physician (PCP)
We identified outpatient visits using American Medical AssociationCommon Procedure Terminology (CPT) evaluation and management codes 99201 to 99205 (new patient) and 99221 to 99215 (established patient encounters). Individual providers were differentiated by using their Unique Provider Identification Number (UPIN). We defined a PCP as a general practitioner, family physician, internist, or geriatrician. Patients had to make at least 3 visits on different days to the same PCP within a year prior to the hospitalization to be categorized as having a PCP.20
Identification of Hospitalists Versus Other Generalist Physicians
As previously described, we defined hospitalists as general internal medicine physicians who derive at least 90% of their Medicare claims for Evaluation and Management services from care provided to hospitalized patients.21 Non‐hospitalist generalist physicians were those generalists who met the criteria for generalists but did not derive at least 90% of their Medicare claims from inpatient medicine.
Definition of Inpatient Continuity of Care
We measured inpatient continuity of care by number of generalist physicians (including hospitalists) who provided care during a hospitalization, through all inpatient claims made during that hospitalization. We considered patients to have had inpatient continuity of care if all billing by generalist physicians was done by one physician during the entire hospitalization.
Statistical Analyses
We calculated the percentage of admissions that received care from 1, 2, or 3 or more generalist physicians during the hospitalization, and stratified by selected patient and hospital characteristics. These proportions were also stratified by whether the patients were cared for by their outpatient PCP or not, and whether they were cared for by hospitalists or not. Based on who cared for the patient during the hospitalization, all admissions were classified as receiving care from: 1) non‐hospitalist generalist physicians, 2) a combination of generalist physicians and hospitalists, and 3) hospitalists only. The effect of patient and hospital characteristics on whether a patient experienced inpatient continuity was evaluated using a hierarchical generalized linear model (HGLM) with a logistic link, adjusting for clustering of admissions within hospitals and all covariates. We repeated our analyses using HGLM with an ordinal logit link to explore the factors associated with number of generalists seen in the hospital. All analyses were performed with SAS version 9.1 (SAS Inc, Cary, NC). The SAS GLIMMIX procedure was used to conduct multilevel analyses.
Results
Between 1996 and 2006, 528,453 patients hospitalized for COPD, pneumonia, and CHF received care by a generalist physician during their hospital stay. Of these, 64.3% were seen by one generalist physician, 26.9% by two generalist physicians, and 8.8% by three or more generalist physicians during hospitalization.
Figure 1 shows the percentage of all patients seen by 1, 2, and 3 or more generalist physicians between 1996 and 2006. The percentage of patients receiving care from one generalist physician declined from 70.7% in 1996 to 59.4% in 2006 (P < 0.001). During the same period, the percentage of patients receiving care from 3 or more generalist physicians increased from 6.5% to 10.7% (P < 0.001). Similar trends were seen for each of the 3 conditions. There was a decrease in overall length of stay during this period, from a mean of 5.7 to 4.9 days (P < 0.001). The increase in the number of generalist physicians providing care during the hospital stay did not correspond to an increase in total number of visits during the hospitalization. The average number of daily visits from a generalist physician was 0.94 (0.30) in 1996 and 0.96 (0.35) in 2006.

Table 1 presents the percentage of patients receiving care from 1, 2, and 3 or more generalist physicians during hospitalization stratified by patient and hospital characteristics. Older adults, females, non‐Hispanic whites, those with higher socioeconomic status, and those with more comorbidities were more likely to receive care by multiple generalist physicians. There was also large variation by geographic region, metropolitan area size, and hospital characteristics. All of these differences were significant at the P < 0.0001 level.
| No. of Generalist Physicians Seen During Hospitalization | ||||
|---|---|---|---|---|
| Characteristic | N | 1 | 2 | 3 (Percentage of Patients) |
| ||||
| Age at admission | ||||
| 6674 | 152,488 | 66.4 | 25.6 | 8.0 |
| 7584 | 226,802 | 63.8 | 27.3 | 8.9 |
| 85+ | 149,163 | 63.0 | 27.7 | 9.3 |
| Gender | ||||
| Male | 216,602 | 65.3 | 26.4 | 8.3 |
| Female | 311,851 | 63.6 | 27.3 | 9.1 |
| Ethnicity | ||||
| White | 461,543 | 63.7 | 27.4 | 9.0 |
| Black | 46,960 | 68.6 | 23.8 | 7.6 |
| Other | 19,950 | 67.9 | 24.5 | 7.6 |
| Low socioeconomic status | ||||
| No | 366,392 | 63.4 | 27.5 | 9.1 |
| Yes | 162,061 | 66.3 | 25.7 | 8.0 |
| Emergency admission | ||||
| No | 188,354 | 66.8 | 25.6 | 7.6 |
| Yes | 340,099 | 62.9 | 27.7 | 9.4 |
| Weekend admission | ||||
| No | 392,150 | 65.7 | 25.8 | 8.5 |
| Yes | 136,303 | 60.1 | 30.3 | 9.6 |
| Diagnosis‐related groups | ||||
| CHF | 213,914 | 65.0 | 26.3 | 8.7 |
| Pneumonia | 195,430 | 62.5 | 28.0 | 9.5 |
| COPD | 119,109 | 66.1 | 26.2 | 7.7 |
| Had a PCP | ||||
| No | 201,016 | 66.5 | 25.4 | 8.0 |
| Yes | 327,437 | 62.9 | 27.9 | 9.2 |
| Seen hospitalist | ||||
| No | 431,784 | 67.8 | 25.1 | 7.0 |
| Yes | 96,669 | 48.5 | 34.9 | 16.6 |
| Charlson comorbidity score | ||||
| 0 | 127,385 | 64.0 | 27.2 | 8.8 |
| 1 | 131,402 | 65.1 | 26.8 | 8.1 |
| 2 | 105,831 | 64.9 | 26.6 | 8.5 |
| 3 | 163,835 | 63.4 | 27.1 | 9.5 |
| ICU use | ||||
| No | 431,462 | 65.3 | 26.5 | 8.2 |
| Yes | 96,991 | 60.1 | 28.7 | 11.2 |
| Length of stay (in days) | ||||
| Mean (SD) | 4.7 (2.9) | 5.8 (3.1) | 8.1 (3.7) | |
| Geographic region | ||||
| New England | 23,572 | 55.7 | 30.8 | 13.5 |
| Middle Atlantic | 78,181 | 60.8 | 27.8 | 11.4 |
| East North Central | 98,072 | 65.7 | 26.3 | 8.0 |
| West North Central | 44,785 | 59.6 | 30.5 | 9.9 |
| South Atlantic | 104,894 | 63.8 | 27.0 | 9.2 |
| East South Central | 51,450 | 67.8 | 24.6 | 7.6 |
| West South Central | 63,493 | 69.2 | 24.8 | 6.0 |
| Mountain | 20,310 | 61.9 | 29.4 | 8.7 |
| Pacific | 36,484 | 66.7 | 26.3 | 7.0 |
| Size of metropolitan area* | ||||
| 1,000,000 | 229,145 | 63.7 | 26.5 | 9.8 |
| 250,000999,999 | 114,448 | 61.0 | 29.2 | 9.8 |
| 100,000249,999 | 11,448 | 61.3 | 30.4 | 8.3 |
| <100,000 | 171,585 | 67.4 | 25.8 | 6.8 |
| Medical school affiliation* | ||||
| Major | 77,605 | 62.9 | 26.8 | 10.3 |
| Minor | 107,144 | 61.5 | 28.4 | 10.1 |
| Non | 341,874 | 65.5 | 26.5 | 8.0 |
| Type of hospital* | ||||
| Nonprofit | 375,888 | 62.7 | 27.8 | 9.5 |
| For profit | 63,898 | 67.5 | 25.5 | 7.0 |
| Public | 86,837 | 68.9 | 24.2 | 6.9 |
| Hospital size* | . | . | . | |
| <200 beds | 232,869 | 67.2 | 25.7 | 7.1 |
| 200349 beds | 135,954 | 62.6 | 27.9 | 9.5 |
| 350499 beds | 77,080 | 61.1 | 28.3 | 10.6 |
| 500 beds | 80,723 | 61.7 | 27.6 | 10.7 |
| Discharge location | ||||
| Home | 361,893 | 66.6 | 26.0 | 7.4 |
| SNF | 94,723 | 57.6 | 30.1 | 12.3 |
| Rehab | 3,030 | 45.7 | 34.2 | 20.1 |
| Death | 22,133 | 63.1 | 25.4 | 11.5 |
| Other | 46,674 | 61.8 | 28.1 | 10.1 |
Table 2 presents the results of a multivariable analysis of factors independently associated with experiencing continuity of care. In this analysis, continuity of care was defined as receiving inpatient care from one generalist physician (vs two or more). In the unadjusted models, the odds of experiencing continuity of care decreased by 5.5% per year from 1996 through 2006, and this decrease did not substantially change after adjusting for all other variables (4.8% yearly decrease). Younger patients, females, black patients, and those with low socioeconomic status were slightly more likely to experience continuity of care. As expected, patients admitted on weekends, emergency admissions, and those with intensive care unit (ICU) stays were less likely to experience continuity. There were marked geographic variations in continuity, with continuity approximately half as likely in New England as in the South. Continuity was greatest in smaller metropolitan areas versus rural and large metropolitan areas. Hospital size and teaching status produced only minor variation.
| Characteristic | Odds Ratio (95% CI) |
|---|---|
| |
| Admission year (increase by year) | 0.952 (0.9500.954) |
| Length of stay (increase by day) | 0.822 (0.8200.823) |
| Had a PCP | |
| No | 1.0 |
| Yes | 0.762 (0.7520.773) |
| Seen by a hospitalist | |
| No | 1.0 |
| Yes | 0.391 (0.3840.398) |
| Age | |
| 6674 | 1.0 |
| 7584 | 0.959 (0.9440.973) |
| 85+ | 0.946 (0.9300.962) |
| Gender | |
| Male | 1.0 |
| Female | 1.047 (1.0331.060) |
| Ethnicity | |
| White | 1.0 |
| Black | 1.126 (1.0971.155) |
| Other | 1.062 (1.0231.103) |
| Low socioeconomic status | |
| No | 1.0 |
| Yes | 1.036 (1.0201.051) |
| Emergency admission | |
| No | 1.0 |
| Yes | 0.864 (0.8510.878) |
| Weekend admission | |
| No | 1.0 |
| Yes | 0.778 (0.7680.789) |
| Diagnosis‐related group | |
| CHF | 1.0 |
| Pneumonia | 0.964 (0.9500.978) |
| COPD | 1.002 (0.9851.019) |
| Charlson comorbidity score | |
| 0 | 1.0 |
| 1 | 1.053 (1.0351.072) |
| 2 | 1.062 (1.0421.083) |
| 3 | 1.040 (1.0221.058) |
| ICU use | |
| No | 1.0 |
| Yes | 0.918 (0.9020.935) |
| Geographic region | |
| Middle Atlantic | 1.0 |
| New England | 0.714 (0.6210.822) |
| East North Central | 1.015 (0.9221.119) |
| West North Central | 0.791 (0.7110.879) |
| South Atlantic | 1.074 (0.9711.186) |
| East South Central | 1.250 (1.1131.403) |
| West South Central | 1.377 (1.2401.530) |
| Mountain | 0.839 (0.7400.951) |
| Pacific | 0.985 (0.8841.097) |
| Size of metropolitan area | |
| 1,000,000 | 1.0 |
| 250,000999,999 | 0.743 (0.6910.798) |
| 100,000249,999 | 0.651 (0.5380.789) |
| <100,000 | 1.062 (0.9911.138) |
| Medical school affiliation | |
| None | 1.0 |
| Minor | 0.889 (0.8270.956) |
| Major | 1.048 (0.9521.154) |
| Type of hospital | |
| Nonprofit | 1.0 |
| For profit | 1.194 (1.1061.289) |
| Public | 1.394 (1.3091.484) |
| Size of hospital | |
| <200 beds | 1.0 |
| 200349 beds | 0.918 (0.8550.986) |
| 350499 beds | 0.962 (0.8721.061) |
| 500 beds | 1.000 (0.8931.119) |
In Table 2 we also show that patients with an established PCP and those who received care from a hospitalist in the hospital were substantially less likely to experience continuity of care. There are several possible interpretations for that finding. For example, it might be that patients admitted to a hospitalist service were likely to see multiple hospitalists. Alternatively, the decreased continuity associated with hospitalists could reflect the fact that some patients cared for predominantly by non‐hospitalists may have seen a hospitalist on call for a sudden change in health status. To further explore these possible explanatory pathways, we constructed three new cohorts: 1) patients receiving all their care from non‐hospitalists, 2) patients receiving all their care from hospitalists, and 3) patients seen by both. As shown in Table 3, in patients seen by non‐hospitalists only, the mean number of generalist physicians seen during hospitalization was slightly greater than in patients cared for only by hospitalists.
| Received Care During Entire Hospitalization | No. of Admissions | Mean (SD) No. of Generalist Physicians Seen During Hospitalization |
|---|---|---|
| ||
| Non‐hospitalist physician | 431,784 | 1.41 (0.68)* |
| Hospitalist physician | 64,662 | 1.34 (0.62)* |
| Both | 32,007 | 2.55 (0.83)* |
We also tested for interactions in Table 2 between admission year and other factors. There was a significant interaction between admission year and having an identifiable PCP in the year prior to admission (Table 2). The odds of experiencing continuity of care decreased more rapidly for patients who did not have a PCP (5.5% per year; 95% CI: 5.2%5.8%) than for those who had one (4.3% per year; 95% CI: 4.1%4.6%).
Discussion
We conducted this study to better understand the degree to which hospitalized patients experience discontinuity of care within a hospital stay and to determine which patients are most likely to experience discontinuity. In our study, we specifically chose admission conditions that would likely be followed primarily by generalist physicians. We found that, over the past decade, discontinuity of care for hospitalized patients has increased substantially, as indicated by the proportion of patients taken care of by more than one generalist physician during a single hospital stay. This occurred even though overall length of stay was decreasing in this same period.
It is perhaps not surprising that inpatient continuity of care has been decreasing in the past 10 years. Outpatient practices are becoming busier, and more doctors are practicing in large group practices, which could lead to several different physicians in the same practice rounding on a hospitalized patient. We have previously demonstrated that hospitalists are caring for an increasing number of patients over this same time period,21 so another possibility is that hospitalist services are being used more often because of this heavy outpatient workload. Our analyses allowed us to test the hypothesis that having hospitalists involved in patient care increases discontinuity.
At first glance, it appears that being cared for by hospitalists may result in worse continuity of care. However, closer scrutiny of the data reveals that the discontinuity ascribed to the hospitalists in the multivariable model appears to be an artifact of defining the hospitalist variable as having been seen by any hospitalist during the hospital stay. This would include patients who saw a hospitalist in addition to their PCP or another non‐hospitalist generalist. When we compared hospitalist‐only care to other generalist care, we could not detect a difference in discontinuity. We know that generalist visits per day to patients has not substantially increased over time, so this discontinuity trend is not explained by having visits by both a hospitalist and the PCP. Therefore, this combination of findings suggests that the increased discontinuity associated with having a hospitalist involved in patient care is likely the result of system issues rather than hospitalist care per se. In fact, patients seem to experience slightly better continuity when they see only hospitalists as opposed to only non‐hospitalists.
What types of systems issues might lead to this finding? Generalists in most settings could choose to involve a hospitalist at any point in the patient's hospital stay. This could occur because of a change in patient acuity requiring the involvement of hospitalists who are present in the hospital more. It is also possible that hospitalists' schedules are created to maximize inpatient continuity of care with individual hospitalists. Even though hospitalists clearly work shifts, the 7 on, 7 off model22 likely results in patients seeing the same physician each day until the switch day. This is in contrast to outpatient primary care doctors whose concentration may be on maintaining continuity within their practice.
As the field of hospital medicine was emerging, many internal medicine physicians from various specialties were concerned about the impact of hospitalists on patient care. In one study, 73% of internal medicine physicians who were not hospitalists thought that hospitalists would worsen continuity of care.23 Primary care and subspecialist internal medicine physicians also expressed the concern that hospitalists could hurt their own relationships with patients,6 presumably because of lost continuity between the inpatient and outpatient settings. However, this fear seems to diminish once hospitalist programs are implemented and primary care doctors have experience with them.23 Our study suggests that the decrease in continuity that has occurred since these studies were published is not likely due to the emergence of hospital medicine, but rather due to other factors that influence who cares for hospitalized patients.
This study had some limitations. Length of stay is an obvious mediator of number of generalist physicians seen. Therefore, the sickest patients are likely to have both a long length of stay and low continuity. We adjusted for this in the multivariable modeling. In addition, given that this study used a large database, certain details are not discernable. For example, we chose to operationalize discontinuity as visits from multiple generalists during a single hospital stay. That is not a perfect definition, but it does represent multiple physicians directing the care of a patient. Importantly, this does not appear to represent continuity with one physician with extra visits from another, as the total number of generalist visits per day did not change over time. It is also possible that patients in the non‐hospitalist group saw physicians only from a single practice, but those details are not included in the database. Finally, we cannot tell what type of hand‐offs were occurring for individual patients during each hospital stay. Despite these disadvantages, using a large database like this one allows for detection of fairly small differences that could still be clinically important.
In summary, hospitalized patients appear to experience less continuity now than 10 years ago. However, the hospitalist model does not appear to play a role in this discontinuity. It is worth exploring in more detail why patients would see both hospitalists and other generalists. This pattern is not surprising, but may have some repercussions in terms of increasing the number of hand‐offs experienced by patients. These could lead to problems with patient safety and quality of care. Future work should explore the reasons for this discontinuity and look at the relationship between inpatient discontinuity outcomes such as quality of care and the doctorpatient relationship.
Acknowledgements
The authors thank Sarah Toombs Smith, PhD, for help in preparation of the manuscript.
- .Defining and measuring interpersonal continuity of care.Ann Fam Med.2003;1(3):134–143.
- ,,.Good continuity of care may improve quality of life in Type 2 diabetes.Diabetes Res Clin Pract.2001;51(1):21–27.
- ,,,.Provider continuity in family medicine: Does it make a difference for total health care costs?Ann Fam Med.2003;1(3):144–148.
- ,,.The effect of continuity of care on emergency department use.Arch Fam Med.2000;9(4):333–338.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: Results of a national survey.Am J Med.2000;109(8):648–653.
- ,,.Physician views on caring for hospitalized patients and the hospitalist model of inpatient care.J Gen Intern Med.2001;16(2):116–119.
- ,,,,,.Systematic review: Effects of resident work hours on patient safety.Ann Intern Med.2004;141(11):851–857.
- ,,.Balancing continuity of care with residents' limited work hours: Defining the implications.Acad Med.2005;80(1):39–43.
- ,,.Understanding communication during hospitalist sevice changes: A mixed methods study.J Hosp Med.2009;4:535–540.
- ,,,Center for Safety in Emergency C. Profiles in patient safety: Emergency care transitions.Acad Emerg Med.2003;10(4):364–367.
- .Fumbled handoffs: One dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- Agency for Healthcare Research and Quality. Fumbled handoff.2004. Available at: http://www.webmm.ahrq.gov/printview.aspx?caseID=55. Accessed December 27, 2005.
- ,,.Graduate medical education and patient safety: A busy—and occasionally hazardous—intersection.Ann Intern Med.2006;145(8):592–598.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269(3):374–378.
- Centers for Medicare and Medicaid Services. Standard analytical files. Available at: http://www.cms.hhs.gov/IdentifiableDataFiles/02_Standard AnalyticalFiles.asp. Accessed March 1,2009.
- Centers for Medicare and Medicaid Services. Nonidentifiable data files: Provider of services files. Available at: http://www.cms.hhs.gov/NonIdentifiableDataFiles/04_ProviderofSerrvicesFile.asp. Accessed March 1,2009.
- Research Data Assistance Center. Medicare data file description. Available at: http://www.resdac.umn.edu/Medicare/file_descriptions.asp. Accessed March 1,2009.
- ,,,.Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance.Am J Transplant.2009;9:506–516.
- ,,,,,.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301:1671–1680.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med. 2009;360:1102–1112.
- HCPro Inc.Medical Staff Leader blog.2010. Available at: http://blogs. hcpro.com/medicalstaff/2010/01/free‐form‐example‐seven‐day‐on‐seven‐day‐off‐hospitalist‐schedule/. Accessed November 20, 2010.
- ,,,.How physicians perceive hospitalist services after implementation: Anticipation vs reality.Arch Intern Med.2003;163(19):2330–2336.
- .Defining and measuring interpersonal continuity of care.Ann Fam Med.2003;1(3):134–143.
- ,,.Good continuity of care may improve quality of life in Type 2 diabetes.Diabetes Res Clin Pract.2001;51(1):21–27.
- ,,,.Provider continuity in family medicine: Does it make a difference for total health care costs?Ann Fam Med.2003;1(3):144–148.
- ,,.The effect of continuity of care on emergency department use.Arch Fam Med.2000;9(4):333–338.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: Results of a national survey.Am J Med.2000;109(8):648–653.
- ,,.Physician views on caring for hospitalized patients and the hospitalist model of inpatient care.J Gen Intern Med.2001;16(2):116–119.
- ,,,,,.Systematic review: Effects of resident work hours on patient safety.Ann Intern Med.2004;141(11):851–857.
- ,,.Balancing continuity of care with residents' limited work hours: Defining the implications.Acad Med.2005;80(1):39–43.
- ,,.Understanding communication during hospitalist sevice changes: A mixed methods study.J Hosp Med.2009;4:535–540.
- ,,,Center for Safety in Emergency C. Profiles in patient safety: Emergency care transitions.Acad Emerg Med.2003;10(4):364–367.
- .Fumbled handoffs: One dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- Agency for Healthcare Research and Quality. Fumbled handoff.2004. Available at: http://www.webmm.ahrq.gov/printview.aspx?caseID=55. Accessed December 27, 2005.
- ,,.Graduate medical education and patient safety: A busy—and occasionally hazardous—intersection.Ann Intern Med.2006;145(8):592–598.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121(11):866–872.
- ,,,.The impact of a regulation restricting medical house staff working hours on the quality of patient care.JAMA.1993;269(3):374–378.
- Centers for Medicare and Medicaid Services. Standard analytical files. Available at: http://www.cms.hhs.gov/IdentifiableDataFiles/02_Standard AnalyticalFiles.asp. Accessed March 1,2009.
- Centers for Medicare and Medicaid Services. Nonidentifiable data files: Provider of services files. Available at: http://www.cms.hhs.gov/NonIdentifiableDataFiles/04_ProviderofSerrvicesFile.asp. Accessed March 1,2009.
- Research Data Assistance Center. Medicare data file description. Available at: http://www.resdac.umn.edu/Medicare/file_descriptions.asp. Accessed March 1,2009.
- ,,,.Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance.Am J Transplant.2009;9:506–516.
- ,,,,,.Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301:1671–1680.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med. 2009;360:1102–1112.
- HCPro Inc.Medical Staff Leader blog.2010. Available at: http://blogs. hcpro.com/medicalstaff/2010/01/free‐form‐example‐seven‐day‐on‐seven‐day‐off‐hospitalist‐schedule/. Accessed November 20, 2010.
- ,,,.How physicians perceive hospitalist services after implementation: Anticipation vs reality.Arch Intern Med.2003;163(19):2330–2336.
Copyright © 2011 Society of Hospital Medicine
Continuing Medical Education Program in
If you wish to receive credit for this activity, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
The objectives need to be changed. Please remove the existing ones, and include these two:
-
Identify recent changes to the Joint Commission accreditation process.
-
Interpret the association between accreditation status and hospital performance in three common clinical conditions.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to
www.wileyblackwellcme.com -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
The objectives need to be changed. Please remove the existing ones, and include these two:
-
Identify recent changes to the Joint Commission accreditation process.
-
Interpret the association between accreditation status and hospital performance in three common clinical conditions.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to
www.wileyblackwellcme.com -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
The objectives need to be changed. Please remove the existing ones, and include these two:
-
Identify recent changes to the Joint Commission accreditation process.
-
Interpret the association between accreditation status and hospital performance in three common clinical conditions.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to
www.wileyblackwellcme.com -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.