User login
Management of Simultaneous Ipsilateral Dislocation of Hip, Knee, and Ankle
New Products/Product News
Epidermolytic Hyperkeratosis and Congenital Platelike Osteoma Cutis in a Child
Fungal Foes: Presentations of Chromoblastomycosis Post–Hurricane Ike
The perils of PSA screening
This issue includes a Priority Update from the Research Literature (PURL) that evaluates the results of 2 studies concerning PSA screening.1,2 No sooner had this PURL been completed than The New England Journal of Medicine (NEJM) published the results of a randomized controlled trial of radical prostatectomy vs watchful waiting in early prostate cancer,3- accompanied by an editorial titled, “Effective treatment for early-stage prostate cancer—possible, necessary, or both?”4 Meanwhile, we await the results of 2 trials being touted as definitive: the Prostate cancer Intervention Versus Observation Trial (PIVOT)5 and the Prostate testing for cancer and Treatment (ProtecT) trial.6
Keeping up with this area of practice is beginning to feel like a full-time job.
But I am going to go out on a limb here and suggest that, until we have fundamentally changed strategies for targeted case finding or early intervention (think genomic and proteomic markers), it is time to stop this screening nonsense. The facts speak for themselves: A trial of 182,000 patients finds in a post hoc analysis of a very narrow population that death can be averted in one of 723 individuals who are screened.2 What about the complications associated with diagnosis, work-up, and treatment?
It is time for urologists and primary care physicians to tell patients that PSA screening is unlikely to benefit them.
Some of you will suggest that we counsel patients about PSA testing to facilitate informed decision-making. But do we advise patients to play the lottery or try futile therapies?
The only men who stand to get even a small benefit from PSA screening are those in excellent health, and it is pretty darn hard to improve on that. I urge all of us to stop offering routine PSA testing and, when asked, to advise against this risky intervention.
1. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543.-
2. Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
3. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708-1717.
4. Smith MR. Effective treatment for early-stage prostate cancer—possible, necessary, or both? N Engl J Med. 2011;364:1770-1772.
5. Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Co-operative Studies Program#407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81-87.
6. Lane JA, Hamdy FC, Martin RM, et al. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer. 2010;46:3095-3101.
This issue includes a Priority Update from the Research Literature (PURL) that evaluates the results of 2 studies concerning PSA screening.1,2 No sooner had this PURL been completed than The New England Journal of Medicine (NEJM) published the results of a randomized controlled trial of radical prostatectomy vs watchful waiting in early prostate cancer,3- accompanied by an editorial titled, “Effective treatment for early-stage prostate cancer—possible, necessary, or both?”4 Meanwhile, we await the results of 2 trials being touted as definitive: the Prostate cancer Intervention Versus Observation Trial (PIVOT)5 and the Prostate testing for cancer and Treatment (ProtecT) trial.6
Keeping up with this area of practice is beginning to feel like a full-time job.
But I am going to go out on a limb here and suggest that, until we have fundamentally changed strategies for targeted case finding or early intervention (think genomic and proteomic markers), it is time to stop this screening nonsense. The facts speak for themselves: A trial of 182,000 patients finds in a post hoc analysis of a very narrow population that death can be averted in one of 723 individuals who are screened.2 What about the complications associated with diagnosis, work-up, and treatment?
It is time for urologists and primary care physicians to tell patients that PSA screening is unlikely to benefit them.
Some of you will suggest that we counsel patients about PSA testing to facilitate informed decision-making. But do we advise patients to play the lottery or try futile therapies?
The only men who stand to get even a small benefit from PSA screening are those in excellent health, and it is pretty darn hard to improve on that. I urge all of us to stop offering routine PSA testing and, when asked, to advise against this risky intervention.
This issue includes a Priority Update from the Research Literature (PURL) that evaluates the results of 2 studies concerning PSA screening.1,2 No sooner had this PURL been completed than The New England Journal of Medicine (NEJM) published the results of a randomized controlled trial of radical prostatectomy vs watchful waiting in early prostate cancer,3- accompanied by an editorial titled, “Effective treatment for early-stage prostate cancer—possible, necessary, or both?”4 Meanwhile, we await the results of 2 trials being touted as definitive: the Prostate cancer Intervention Versus Observation Trial (PIVOT)5 and the Prostate testing for cancer and Treatment (ProtecT) trial.6
Keeping up with this area of practice is beginning to feel like a full-time job.
But I am going to go out on a limb here and suggest that, until we have fundamentally changed strategies for targeted case finding or early intervention (think genomic and proteomic markers), it is time to stop this screening nonsense. The facts speak for themselves: A trial of 182,000 patients finds in a post hoc analysis of a very narrow population that death can be averted in one of 723 individuals who are screened.2 What about the complications associated with diagnosis, work-up, and treatment?
It is time for urologists and primary care physicians to tell patients that PSA screening is unlikely to benefit them.
Some of you will suggest that we counsel patients about PSA testing to facilitate informed decision-making. But do we advise patients to play the lottery or try futile therapies?
The only men who stand to get even a small benefit from PSA screening are those in excellent health, and it is pretty darn hard to improve on that. I urge all of us to stop offering routine PSA testing and, when asked, to advise against this risky intervention.
1. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543.-
2. Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
3. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708-1717.
4. Smith MR. Effective treatment for early-stage prostate cancer—possible, necessary, or both? N Engl J Med. 2011;364:1770-1772.
5. Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Co-operative Studies Program#407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81-87.
6. Lane JA, Hamdy FC, Martin RM, et al. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer. 2010;46:3095-3101.
1. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543.-
2. Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
3. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708-1717.
4. Smith MR. Effective treatment for early-stage prostate cancer—possible, necessary, or both? N Engl J Med. 2011;364:1770-1772.
5. Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Co-operative Studies Program#407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81-87.
6. Lane JA, Hamdy FC, Martin RM, et al. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer. 2010;46:3095-3101.
PSA testing: When it’s useful, when it’s not
Do not routinely screen all men over the age of 50 for prostate cancer with the prostate-specific antigen (PSA) test. Consider screening men younger than 75 with no cardiovascular or cancer risk factors—the only patient population for whom PSA testing appears to provide even a small benefit.1,2
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis of 6 randomized controlled trials (RCTs) with methodological limitations, and a post hoc analysis of a large RCT.
Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.
Crawford ED, Grubb R 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
ILLUSTRATIVE CASES
A 65-year-old obese man with high blood pressure comes in for a complete physical and asks if he should have the “blood test for cancer.” He had a normal prostate specific antigen (PSA) the last time he was tested, but that was 10 years ago. What should you tell him?
A 55-year-old man schedules a routine check-up and requests a PSA test. His last test, at age 50, was normal. The patient has no known medical problems and no family history of prostate cancer, and he exercises regularly and doesn’t smoke. How should you respond to his request for a PSA test?
Prostate cancer is the second leading cause of cancer deaths among men in the United States, after lung cancer. One in 6 American men will be diagnosed with prostate cancer; for about 3% of them, the cancer will be fatal.3,4
Widespread testing without evidence of efficacy
The PSA test was approved by the US Food and Drug Administration (FDA) in 1986.5 Its potential to detect early prostate cancer in the hope of decreasing morbidity and mortality led to widespread PSA screening in the 1990s, before data on the efficacy of routine screening existed.
By 2002, only one low-quality RCT that compared screening with no screening had been published. The investigators concluded that screening resulted in lower mortality rates, but a subsequent (and superior) intention-to-treat analysis showed no mortality benefit.6 Two large RCTs, both published in 2009, reported conflicting results.7,8
The European Randomized Study of Screening for Prostate Cancer (ERSPC) enrolled 182,000 men ages 50 to 74 years and randomized them to either PSA screening every 4 years or no screening. Prostate cancer-specific mortality was 20% lower for those in the screening group compared with the no-screening group; however, the absolute risk reduction was only 0.71 deaths per 1000 men.7
The US Prostate, Lung, Colorectal, Ovarian Cancer (PLCO) Screening Trial randomized 77,000 men ages 55 to 74 years to either annual PSA and digital rectal examination (DRE) screening or usual care. After 7 years of follow-up, no significant difference was found in prostate cancer deaths or all-cause mortality in the screening group vs the control group. It is important to note, however, that 52% of the men in the control group had ≥1 PSA screening during the study period, which decreased the researchers’ ability to fully assess the benefits of screening.8
PSA’s limitations and potential harmful effects
The PSA test’s significant limitations and potentially harmful effects counter the potential benefits of screening. About 75% of positive tests are false positives, which are associated with psychological harm in some men for up to a year after the test.6 In addition, diagnostic testing and treatment for what may be nonlife-threatening prostate cancer can cause harm, including erectile dysfunction (ED), urinary incontinence, bowel dysfunction, and death. Rates of ED and incontinence 18 months after radical prostatectomy are an estimated 59.9% and 8.4%, respectively.9
Do the benefits of PSA testing outweigh the harms—and for which men? The meta-analysis and post hoc analysis detailed in this PURL help clear up the controversy.
STUDY SUMMARY: Widespread screening doesn’t save lives
Djulbegovic et al examined 6 RCTs, including the ERSPC and PLCO studies described earlier, that compared screening for prostate cancer (PSA with or without DRE) with no screening or usual care.1 Together, the studies included nearly 390,000 men ages 45 to 80 years, and had 4 to 15 years of follow-up. The results showed that routine screening for prostate cancer had no statistically significant effect on all-cause mortality (relative risk [RR]=0.99; 95% confidence interval [CI], 0.97-1.01), death from prostate cancer (RR=0.88; 95% CI, 0.71-1.09), or diagnosis of stage III or IV prostate cancer (RR=0.94; 95% CI, 0.85-1.04). Routine screening did, however, increase the probability of being diagnosed with prostate cancer at any stage, especially at stage I. For every 1000 men screened, on average, 20 more cases of prostate cancer were diagnosed.
Healthy men may benefit from screening
Crawford et al conducted a post hoc analysis of the PLCO trial, which had found no benefit to annual PSA testing and serial DRE compared with usual care for the general population.2 Their analysis compared the mortality benefits (both prostate cancer–specific and overall) of annual PSA screening for healthy men with no or minimal comorbidities vs the mortality benefits for men with any risk factor for the 2 leading causes of death: cancer and cardiovascular disease.
Annual PSA testing yielded more diagnoses of prostate cancer in both healthy and at-risk men. Deaths from prostate cancer were infrequent in both groups, occurring in 0.22% (164/73,378) of all participants.
Men with ≥1 risk factor had similar prostate cancer–specific deaths with both yearly screening and usual care (62 vs 42 deaths, adjusted hazard ratio [AHR]=1.43; 95% CI, 0.96-2.11); their prostate cancer–specific mortality rate was 0.27% (95% CI, 0.21-0.34) and 0.19% (95% CI, 0.14-0.25), respectively.
However, healthy men younger than 75 years had fewer prostate cancer–specific deaths with annual PSA screenings (22 vs 38; AHR=0.56; 95% CI, 0.33-0.95; P=.03). Specifically, the prostate cancer mortality rate was 0.17% (95% CI, 0.11-0.25) in the group that received screening vs 0.31% (95% CI, 0.22-0.42) in the usual care group. Thus, the absolute risk reduction for prostate cancer-specific mortality in men without comorbidities who received yearly screening instead of usual care was 0.14% (0.31% vs 0.17%, P=.03), with a number needed to screen of 723 to prevent one death from prostate cancer. There was a non-significant reduction in all-cause mortality in the intervention group vs the control group (AHR=0.93; 95% CI, 0.86-1.02; P=.11).
WHAT’S NEW: At best, screening has a small benefit
These trials indicate that only a small group of men will potentially benefit from PSA screening. Prior to this meta-analysis, a Cochrane review published in 2006 had concluded that there was insufficient evidence to support or refute the routine use of mass screening for prostate can-cer.10 The meta-analysis by Djulbegovic et al, which included 4 additional trials, 2 of them large, found no benefit of PSA screening in reducing mortality from prostate cancer for the general population.1
Annual screening does appear to provide a small reduction in prostate cancer deaths but no significant reduction in all-cause mortality in men younger than age 75 who have no risk factors for cancer or cardiovascular disease.
CAVEATS: Study limitations, some unknowns
These studies did not address whether certain groups at higher risk of developing prostate cancer, such as African American men and those with a family history of prostate cancer, would benefit from PSA screening. In addition, both of the studies detailed in this PURL had substantive weaknesses.
Methodological limitations of the studies in the meta-analysis included the lack of intention-to-treat analysis and allocation concealment, which favors finding a benefit for the screening arm, and PSA screening in the nonscreening arm, which biases the results toward not finding a screening benefit that might exist. Despite these weaknesses, this meta-analysis brings together the best available evidence of the value of screening for prostate cancer.
In addition, there was no quantitative assessment of complication rates included in the meta-analysis. None of the 6 trials collected data on the effect of screening or treatment on participants’ quality of life.
In the post hoc study showing a benefit for screening healthy men, the decrease in prostate cancer deaths was small in magnitude, did not have an impact on all-cause mortality, and was of marginal statistical significance. Although the data came from the largest multicenter study to date of prostate cancer screening, the results of a post hoc analysis of a single trial should be interpreted with caution. The study was initially designed to test the effect of screening on a general population. Whenever a study deviates from the original hypothesis to evaluate a subset of the study population, the investigators increase the risk of finding a difference where none exists. Thus, it is possible that the findings of benefit for healthy men may not truly be present.
What’s more, the risk factors identified by the authors could be interpreted as arbitrary. They included diverticulosis, which is not known to increase the likelihood of cancer or heart disease, as a risk factor. By the same token, smoking—a known risk factor for both cancer and cardiovascular disease—was not addressed. Finally, potential harms associated with false-positive tests and prostate cancer treatment were not addressed in these studies.
CHALLENGES TO IMPLEMENTATION: Old habits die hard
Clinicians have recommended PSA screening for men >50 years, and men have requested such screening, for more than 2 decades. Physicians often opt to order a PSA test rather than to take the time to explain potential harms and benefits and listen to the patient’s thoughts and feelings about the value of screening. In addition, physicians who believe the lack of benefit from screening does not apply to their patients will continue to order the PSA test. (See “The perils of PSA screening”.)
Patients may opt to continue to be screened although they have developed a risk factor for cardiovascular disease. Also, a decision not to screen directly contradicts the recommendation of the American Urological Association, which calls for annual PSA testing for asymptomatic men with a life expectancy >10 years starting at 40 years of age.11
Shared decision-making
The US Preventive Services Task Force (USPSTF) provides a basis for shared decision-making between physicians and patients concerning prostate cancer screening. The USPSTF states that there is insufficient evidence to recommend for or against prostate cancer screening for the general male population younger than age 75 and recommends against screening men age 75 and older or those with a life expectancy of less than 10 years.12
Decisions regarding PSA screening should be shared and documented for all men between the ages of 50 and 75 years. Advise patients with risk factors that the evidence shows little value and possible harm from screening. Tell healthier men that PSA testing appears to offer a small benefit, at best.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources; the grant is a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.-
2. Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
3. American Cancer Society. Cancer facts & figures 2010. Atlanta, Ga: American Cancer Society; 2010. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Accessed April 13, 2011.
4. American Cancer Society. Prostate cancer. Last medical review November 22, 2010. Available at: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed April 13, 2011.
5. National Institutes of Health. Prostate cancer. Last updated February 14, 2011. Available at: http://report.nih.gov/NIHfactsheets/ViewFactSheet.aspx?csid=60. Accessed May 9, 2011.
6. Lin K, Lipsitz R, Miller T, et al. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:192-199.
7. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
8. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310-1319.
9. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354-360.
10. Ilic D, O’Connor D, Greens, Wilt T. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;(3):CD004720.-
11. American Urological Association. Prostate-specific antigen best practice statement: 2009 update. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. Accessed March 16, 2011.
12. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185-191.
Do not routinely screen all men over the age of 50 for prostate cancer with the prostate-specific antigen (PSA) test. Consider screening men younger than 75 with no cardiovascular or cancer risk factors—the only patient population for whom PSA testing appears to provide even a small benefit.1,2
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis of 6 randomized controlled trials (RCTs) with methodological limitations, and a post hoc analysis of a large RCT.
Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.
Crawford ED, Grubb R 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
ILLUSTRATIVE CASES
A 65-year-old obese man with high blood pressure comes in for a complete physical and asks if he should have the “blood test for cancer.” He had a normal prostate specific antigen (PSA) the last time he was tested, but that was 10 years ago. What should you tell him?
A 55-year-old man schedules a routine check-up and requests a PSA test. His last test, at age 50, was normal. The patient has no known medical problems and no family history of prostate cancer, and he exercises regularly and doesn’t smoke. How should you respond to his request for a PSA test?
Prostate cancer is the second leading cause of cancer deaths among men in the United States, after lung cancer. One in 6 American men will be diagnosed with prostate cancer; for about 3% of them, the cancer will be fatal.3,4
Widespread testing without evidence of efficacy
The PSA test was approved by the US Food and Drug Administration (FDA) in 1986.5 Its potential to detect early prostate cancer in the hope of decreasing morbidity and mortality led to widespread PSA screening in the 1990s, before data on the efficacy of routine screening existed.
By 2002, only one low-quality RCT that compared screening with no screening had been published. The investigators concluded that screening resulted in lower mortality rates, but a subsequent (and superior) intention-to-treat analysis showed no mortality benefit.6 Two large RCTs, both published in 2009, reported conflicting results.7,8
The European Randomized Study of Screening for Prostate Cancer (ERSPC) enrolled 182,000 men ages 50 to 74 years and randomized them to either PSA screening every 4 years or no screening. Prostate cancer-specific mortality was 20% lower for those in the screening group compared with the no-screening group; however, the absolute risk reduction was only 0.71 deaths per 1000 men.7
The US Prostate, Lung, Colorectal, Ovarian Cancer (PLCO) Screening Trial randomized 77,000 men ages 55 to 74 years to either annual PSA and digital rectal examination (DRE) screening or usual care. After 7 years of follow-up, no significant difference was found in prostate cancer deaths or all-cause mortality in the screening group vs the control group. It is important to note, however, that 52% of the men in the control group had ≥1 PSA screening during the study period, which decreased the researchers’ ability to fully assess the benefits of screening.8
PSA’s limitations and potential harmful effects
The PSA test’s significant limitations and potentially harmful effects counter the potential benefits of screening. About 75% of positive tests are false positives, which are associated with psychological harm in some men for up to a year after the test.6 In addition, diagnostic testing and treatment for what may be nonlife-threatening prostate cancer can cause harm, including erectile dysfunction (ED), urinary incontinence, bowel dysfunction, and death. Rates of ED and incontinence 18 months after radical prostatectomy are an estimated 59.9% and 8.4%, respectively.9
Do the benefits of PSA testing outweigh the harms—and for which men? The meta-analysis and post hoc analysis detailed in this PURL help clear up the controversy.
STUDY SUMMARY: Widespread screening doesn’t save lives
Djulbegovic et al examined 6 RCTs, including the ERSPC and PLCO studies described earlier, that compared screening for prostate cancer (PSA with or without DRE) with no screening or usual care.1 Together, the studies included nearly 390,000 men ages 45 to 80 years, and had 4 to 15 years of follow-up. The results showed that routine screening for prostate cancer had no statistically significant effect on all-cause mortality (relative risk [RR]=0.99; 95% confidence interval [CI], 0.97-1.01), death from prostate cancer (RR=0.88; 95% CI, 0.71-1.09), or diagnosis of stage III or IV prostate cancer (RR=0.94; 95% CI, 0.85-1.04). Routine screening did, however, increase the probability of being diagnosed with prostate cancer at any stage, especially at stage I. For every 1000 men screened, on average, 20 more cases of prostate cancer were diagnosed.
Healthy men may benefit from screening
Crawford et al conducted a post hoc analysis of the PLCO trial, which had found no benefit to annual PSA testing and serial DRE compared with usual care for the general population.2 Their analysis compared the mortality benefits (both prostate cancer–specific and overall) of annual PSA screening for healthy men with no or minimal comorbidities vs the mortality benefits for men with any risk factor for the 2 leading causes of death: cancer and cardiovascular disease.
Annual PSA testing yielded more diagnoses of prostate cancer in both healthy and at-risk men. Deaths from prostate cancer were infrequent in both groups, occurring in 0.22% (164/73,378) of all participants.
Men with ≥1 risk factor had similar prostate cancer–specific deaths with both yearly screening and usual care (62 vs 42 deaths, adjusted hazard ratio [AHR]=1.43; 95% CI, 0.96-2.11); their prostate cancer–specific mortality rate was 0.27% (95% CI, 0.21-0.34) and 0.19% (95% CI, 0.14-0.25), respectively.
However, healthy men younger than 75 years had fewer prostate cancer–specific deaths with annual PSA screenings (22 vs 38; AHR=0.56; 95% CI, 0.33-0.95; P=.03). Specifically, the prostate cancer mortality rate was 0.17% (95% CI, 0.11-0.25) in the group that received screening vs 0.31% (95% CI, 0.22-0.42) in the usual care group. Thus, the absolute risk reduction for prostate cancer-specific mortality in men without comorbidities who received yearly screening instead of usual care was 0.14% (0.31% vs 0.17%, P=.03), with a number needed to screen of 723 to prevent one death from prostate cancer. There was a non-significant reduction in all-cause mortality in the intervention group vs the control group (AHR=0.93; 95% CI, 0.86-1.02; P=.11).
WHAT’S NEW: At best, screening has a small benefit
These trials indicate that only a small group of men will potentially benefit from PSA screening. Prior to this meta-analysis, a Cochrane review published in 2006 had concluded that there was insufficient evidence to support or refute the routine use of mass screening for prostate can-cer.10 The meta-analysis by Djulbegovic et al, which included 4 additional trials, 2 of them large, found no benefit of PSA screening in reducing mortality from prostate cancer for the general population.1
Annual screening does appear to provide a small reduction in prostate cancer deaths but no significant reduction in all-cause mortality in men younger than age 75 who have no risk factors for cancer or cardiovascular disease.
CAVEATS: Study limitations, some unknowns
These studies did not address whether certain groups at higher risk of developing prostate cancer, such as African American men and those with a family history of prostate cancer, would benefit from PSA screening. In addition, both of the studies detailed in this PURL had substantive weaknesses.
Methodological limitations of the studies in the meta-analysis included the lack of intention-to-treat analysis and allocation concealment, which favors finding a benefit for the screening arm, and PSA screening in the nonscreening arm, which biases the results toward not finding a screening benefit that might exist. Despite these weaknesses, this meta-analysis brings together the best available evidence of the value of screening for prostate cancer.
In addition, there was no quantitative assessment of complication rates included in the meta-analysis. None of the 6 trials collected data on the effect of screening or treatment on participants’ quality of life.
In the post hoc study showing a benefit for screening healthy men, the decrease in prostate cancer deaths was small in magnitude, did not have an impact on all-cause mortality, and was of marginal statistical significance. Although the data came from the largest multicenter study to date of prostate cancer screening, the results of a post hoc analysis of a single trial should be interpreted with caution. The study was initially designed to test the effect of screening on a general population. Whenever a study deviates from the original hypothesis to evaluate a subset of the study population, the investigators increase the risk of finding a difference where none exists. Thus, it is possible that the findings of benefit for healthy men may not truly be present.
What’s more, the risk factors identified by the authors could be interpreted as arbitrary. They included diverticulosis, which is not known to increase the likelihood of cancer or heart disease, as a risk factor. By the same token, smoking—a known risk factor for both cancer and cardiovascular disease—was not addressed. Finally, potential harms associated with false-positive tests and prostate cancer treatment were not addressed in these studies.
CHALLENGES TO IMPLEMENTATION: Old habits die hard
Clinicians have recommended PSA screening for men >50 years, and men have requested such screening, for more than 2 decades. Physicians often opt to order a PSA test rather than to take the time to explain potential harms and benefits and listen to the patient’s thoughts and feelings about the value of screening. In addition, physicians who believe the lack of benefit from screening does not apply to their patients will continue to order the PSA test. (See “The perils of PSA screening”.)
Patients may opt to continue to be screened although they have developed a risk factor for cardiovascular disease. Also, a decision not to screen directly contradicts the recommendation of the American Urological Association, which calls for annual PSA testing for asymptomatic men with a life expectancy >10 years starting at 40 years of age.11
Shared decision-making
The US Preventive Services Task Force (USPSTF) provides a basis for shared decision-making between physicians and patients concerning prostate cancer screening. The USPSTF states that there is insufficient evidence to recommend for or against prostate cancer screening for the general male population younger than age 75 and recommends against screening men age 75 and older or those with a life expectancy of less than 10 years.12
Decisions regarding PSA screening should be shared and documented for all men between the ages of 50 and 75 years. Advise patients with risk factors that the evidence shows little value and possible harm from screening. Tell healthier men that PSA testing appears to offer a small benefit, at best.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources; the grant is a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Do not routinely screen all men over the age of 50 for prostate cancer with the prostate-specific antigen (PSA) test. Consider screening men younger than 75 with no cardiovascular or cancer risk factors—the only patient population for whom PSA testing appears to provide even a small benefit.1,2
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis of 6 randomized controlled trials (RCTs) with methodological limitations, and a post hoc analysis of a large RCT.
Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.
Crawford ED, Grubb R 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
ILLUSTRATIVE CASES
A 65-year-old obese man with high blood pressure comes in for a complete physical and asks if he should have the “blood test for cancer.” He had a normal prostate specific antigen (PSA) the last time he was tested, but that was 10 years ago. What should you tell him?
A 55-year-old man schedules a routine check-up and requests a PSA test. His last test, at age 50, was normal. The patient has no known medical problems and no family history of prostate cancer, and he exercises regularly and doesn’t smoke. How should you respond to his request for a PSA test?
Prostate cancer is the second leading cause of cancer deaths among men in the United States, after lung cancer. One in 6 American men will be diagnosed with prostate cancer; for about 3% of them, the cancer will be fatal.3,4
Widespread testing without evidence of efficacy
The PSA test was approved by the US Food and Drug Administration (FDA) in 1986.5 Its potential to detect early prostate cancer in the hope of decreasing morbidity and mortality led to widespread PSA screening in the 1990s, before data on the efficacy of routine screening existed.
By 2002, only one low-quality RCT that compared screening with no screening had been published. The investigators concluded that screening resulted in lower mortality rates, but a subsequent (and superior) intention-to-treat analysis showed no mortality benefit.6 Two large RCTs, both published in 2009, reported conflicting results.7,8
The European Randomized Study of Screening for Prostate Cancer (ERSPC) enrolled 182,000 men ages 50 to 74 years and randomized them to either PSA screening every 4 years or no screening. Prostate cancer-specific mortality was 20% lower for those in the screening group compared with the no-screening group; however, the absolute risk reduction was only 0.71 deaths per 1000 men.7
The US Prostate, Lung, Colorectal, Ovarian Cancer (PLCO) Screening Trial randomized 77,000 men ages 55 to 74 years to either annual PSA and digital rectal examination (DRE) screening or usual care. After 7 years of follow-up, no significant difference was found in prostate cancer deaths or all-cause mortality in the screening group vs the control group. It is important to note, however, that 52% of the men in the control group had ≥1 PSA screening during the study period, which decreased the researchers’ ability to fully assess the benefits of screening.8
PSA’s limitations and potential harmful effects
The PSA test’s significant limitations and potentially harmful effects counter the potential benefits of screening. About 75% of positive tests are false positives, which are associated with psychological harm in some men for up to a year after the test.6 In addition, diagnostic testing and treatment for what may be nonlife-threatening prostate cancer can cause harm, including erectile dysfunction (ED), urinary incontinence, bowel dysfunction, and death. Rates of ED and incontinence 18 months after radical prostatectomy are an estimated 59.9% and 8.4%, respectively.9
Do the benefits of PSA testing outweigh the harms—and for which men? The meta-analysis and post hoc analysis detailed in this PURL help clear up the controversy.
STUDY SUMMARY: Widespread screening doesn’t save lives
Djulbegovic et al examined 6 RCTs, including the ERSPC and PLCO studies described earlier, that compared screening for prostate cancer (PSA with or without DRE) with no screening or usual care.1 Together, the studies included nearly 390,000 men ages 45 to 80 years, and had 4 to 15 years of follow-up. The results showed that routine screening for prostate cancer had no statistically significant effect on all-cause mortality (relative risk [RR]=0.99; 95% confidence interval [CI], 0.97-1.01), death from prostate cancer (RR=0.88; 95% CI, 0.71-1.09), or diagnosis of stage III or IV prostate cancer (RR=0.94; 95% CI, 0.85-1.04). Routine screening did, however, increase the probability of being diagnosed with prostate cancer at any stage, especially at stage I. For every 1000 men screened, on average, 20 more cases of prostate cancer were diagnosed.
Healthy men may benefit from screening
Crawford et al conducted a post hoc analysis of the PLCO trial, which had found no benefit to annual PSA testing and serial DRE compared with usual care for the general population.2 Their analysis compared the mortality benefits (both prostate cancer–specific and overall) of annual PSA screening for healthy men with no or minimal comorbidities vs the mortality benefits for men with any risk factor for the 2 leading causes of death: cancer and cardiovascular disease.
Annual PSA testing yielded more diagnoses of prostate cancer in both healthy and at-risk men. Deaths from prostate cancer were infrequent in both groups, occurring in 0.22% (164/73,378) of all participants.
Men with ≥1 risk factor had similar prostate cancer–specific deaths with both yearly screening and usual care (62 vs 42 deaths, adjusted hazard ratio [AHR]=1.43; 95% CI, 0.96-2.11); their prostate cancer–specific mortality rate was 0.27% (95% CI, 0.21-0.34) and 0.19% (95% CI, 0.14-0.25), respectively.
However, healthy men younger than 75 years had fewer prostate cancer–specific deaths with annual PSA screenings (22 vs 38; AHR=0.56; 95% CI, 0.33-0.95; P=.03). Specifically, the prostate cancer mortality rate was 0.17% (95% CI, 0.11-0.25) in the group that received screening vs 0.31% (95% CI, 0.22-0.42) in the usual care group. Thus, the absolute risk reduction for prostate cancer-specific mortality in men without comorbidities who received yearly screening instead of usual care was 0.14% (0.31% vs 0.17%, P=.03), with a number needed to screen of 723 to prevent one death from prostate cancer. There was a non-significant reduction in all-cause mortality in the intervention group vs the control group (AHR=0.93; 95% CI, 0.86-1.02; P=.11).
WHAT’S NEW: At best, screening has a small benefit
These trials indicate that only a small group of men will potentially benefit from PSA screening. Prior to this meta-analysis, a Cochrane review published in 2006 had concluded that there was insufficient evidence to support or refute the routine use of mass screening for prostate can-cer.10 The meta-analysis by Djulbegovic et al, which included 4 additional trials, 2 of them large, found no benefit of PSA screening in reducing mortality from prostate cancer for the general population.1
Annual screening does appear to provide a small reduction in prostate cancer deaths but no significant reduction in all-cause mortality in men younger than age 75 who have no risk factors for cancer or cardiovascular disease.
CAVEATS: Study limitations, some unknowns
These studies did not address whether certain groups at higher risk of developing prostate cancer, such as African American men and those with a family history of prostate cancer, would benefit from PSA screening. In addition, both of the studies detailed in this PURL had substantive weaknesses.
Methodological limitations of the studies in the meta-analysis included the lack of intention-to-treat analysis and allocation concealment, which favors finding a benefit for the screening arm, and PSA screening in the nonscreening arm, which biases the results toward not finding a screening benefit that might exist. Despite these weaknesses, this meta-analysis brings together the best available evidence of the value of screening for prostate cancer.
In addition, there was no quantitative assessment of complication rates included in the meta-analysis. None of the 6 trials collected data on the effect of screening or treatment on participants’ quality of life.
In the post hoc study showing a benefit for screening healthy men, the decrease in prostate cancer deaths was small in magnitude, did not have an impact on all-cause mortality, and was of marginal statistical significance. Although the data came from the largest multicenter study to date of prostate cancer screening, the results of a post hoc analysis of a single trial should be interpreted with caution. The study was initially designed to test the effect of screening on a general population. Whenever a study deviates from the original hypothesis to evaluate a subset of the study population, the investigators increase the risk of finding a difference where none exists. Thus, it is possible that the findings of benefit for healthy men may not truly be present.
What’s more, the risk factors identified by the authors could be interpreted as arbitrary. They included diverticulosis, which is not known to increase the likelihood of cancer or heart disease, as a risk factor. By the same token, smoking—a known risk factor for both cancer and cardiovascular disease—was not addressed. Finally, potential harms associated with false-positive tests and prostate cancer treatment were not addressed in these studies.
CHALLENGES TO IMPLEMENTATION: Old habits die hard
Clinicians have recommended PSA screening for men >50 years, and men have requested such screening, for more than 2 decades. Physicians often opt to order a PSA test rather than to take the time to explain potential harms and benefits and listen to the patient’s thoughts and feelings about the value of screening. In addition, physicians who believe the lack of benefit from screening does not apply to their patients will continue to order the PSA test. (See “The perils of PSA screening”.)
Patients may opt to continue to be screened although they have developed a risk factor for cardiovascular disease. Also, a decision not to screen directly contradicts the recommendation of the American Urological Association, which calls for annual PSA testing for asymptomatic men with a life expectancy >10 years starting at 40 years of age.11
Shared decision-making
The US Preventive Services Task Force (USPSTF) provides a basis for shared decision-making between physicians and patients concerning prostate cancer screening. The USPSTF states that there is insufficient evidence to recommend for or against prostate cancer screening for the general male population younger than age 75 and recommends against screening men age 75 and older or those with a life expectancy of less than 10 years.12
Decisions regarding PSA screening should be shared and documented for all men between the ages of 50 and 75 years. Advise patients with risk factors that the evidence shows little value and possible harm from screening. Tell healthier men that PSA testing appears to offer a small benefit, at best.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources; the grant is a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.-
2. Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
3. American Cancer Society. Cancer facts & figures 2010. Atlanta, Ga: American Cancer Society; 2010. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Accessed April 13, 2011.
4. American Cancer Society. Prostate cancer. Last medical review November 22, 2010. Available at: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed April 13, 2011.
5. National Institutes of Health. Prostate cancer. Last updated February 14, 2011. Available at: http://report.nih.gov/NIHfactsheets/ViewFactSheet.aspx?csid=60. Accessed May 9, 2011.
6. Lin K, Lipsitz R, Miller T, et al. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:192-199.
7. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
8. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310-1319.
9. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354-360.
10. Ilic D, O’Connor D, Greens, Wilt T. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;(3):CD004720.-
11. American Urological Association. Prostate-specific antigen best practice statement: 2009 update. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. Accessed March 16, 2011.
12. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185-191.
1. Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543.-
2. Crawford ED, Grubb R, 3rd, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361.
3. American Cancer Society. Cancer facts & figures 2010. Atlanta, Ga: American Cancer Society; 2010. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Accessed April 13, 2011.
4. American Cancer Society. Prostate cancer. Last medical review November 22, 2010. Available at: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Accessed April 13, 2011.
5. National Institutes of Health. Prostate cancer. Last updated February 14, 2011. Available at: http://report.nih.gov/NIHfactsheets/ViewFactSheet.aspx?csid=60. Accessed May 9, 2011.
6. Lin K, Lipsitz R, Miller T, et al. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:192-199.
7. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
8. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310-1319.
9. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354-360.
10. Ilic D, O’Connor D, Greens, Wilt T. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;(3):CD004720.-
11. American Urological Association. Prostate-specific antigen best practice statement: 2009 update. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf. Accessed March 16, 2011.
12. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185-191.
Copyright © 2011 The Family Physicians Inquiries Network.
All rights reserved.
Pregnant and moving involuntarily
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
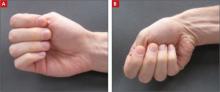
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
High uterosacral vaginal vault suspension to repair enterocele and apical prolapse
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).
FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).
FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).
When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.
FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.
FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).

FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).

FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).

When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.

FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.

FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).

FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).

FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).

When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.

FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.

FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
“Doctor, my thumb hurts”
• Do not treat de Quervain’s tenosynovitis with a corticosteroid injection plus a nonsteroidal anti-inflammatory drug; the combination is no more effective than the injection alone. B
• Resection arthroplasty of the carpometacarpal (CMC) joint is the gold standard for surgical treatment of thumb CMC osteoarthritis, but should be offered only if conservative measures fail. C
• Percutaneous release of trigger thumb combined with a corticosteroid injection provides greater symptom relief than the injection alone. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many possible causes of nontraumatic thumb pain are 3 conditions that primary care physicians are likely to encounter again and again: de Quervain’s tenosynovitis (dQT), first carpometacarpal osteoarthritis (CMC OA), and trigger thumb (TT). Common as they are, however, there are no consensus guidelines for the treatment of these conditions.
With that in mind, we did a literature search for studies of treatments for common causes of nontraumatic thumb pain. After reviewing the findings, we developed this evidence-based summary—and the “bottom line” treatment guide—as an aid to clinical decision making.
de Quervain’s tenosynovitis: An overuse injury
dQT is characterized by a gradual onset of pain in the first dorsal compartment of the wrist. The pain is reproduced on physical exam with clenched fist ulnar deviation of the wrist (Finkelstein test) (FIGURE). The suspected cause is overuse, leading to thickening of the tendons of the first dorsal compartment and subsequent resisted gliding of the tendons in their fibro-osseous canal.1
FIGURE
Finkelstein test for de Quervain’s tenosynovitis
With elbows flexed to 90°, the forearms parallel to each other and the floor, and the thumb clenched gently inside a fist (A), the patient drops the hand down (adduction) at the wrist (B). Pain over the first dorsal compartment is considered a positive test.
NSAIDs and injection: No better than injection alone
Conservative treatment of dQT consists of topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs), splinting, and corticosteroid injection.1 We identified 2 studies using such conservative modalities. The first was a randomized double-blind, placebo-controlled trial, which found that oral NSAIDs combined with corticosteroid injection provided no statistically significant benefit compared with corticosteroid injection alone (P=.69).2 The second study was a pooled qualitative analysis and showed that 83% (n=495) of patients were asymptomatic after corticosteroid injection alone.3 Treatment failure in the remaining 17% of patients was attributed to poor technique and anatomic variation within the first dorsal compartment.
Another arm of the study compared the combination of corticosteroid injection and splinting with splinting alone, which yielded 61% and 14% success rates, respectively. Some patients were treated with NSAIDs and rest alone, but this intervention had a 0% success rate.3
Surgery has a high “cure rate”
Symptoms of dQT of >9 months’ duration may not respond as well to conservative therapy.4 In such cases—and for patients for whom conservative measures bring only short-term relief—a surgical referral may be the best approach.
Surgery for dQT, a relatively simple procedure in which the sheaths surrounding the inflamed tendons at the base of the thumb are released to relieve the pain and swelling, has uniformly positive results. The “cure rate”—resolution of symptoms without complications—is reported to be >90%.1 One researcher found a positive correlation between a longer duration (>9 months) of preoperative symptoms and increased postoperative satisfaction (P<.4). 4
First carpometacarpal OA: Pain, deformity, functional impairment
In a study of patients with joint-specific arthritis of the hand, the prevalence of first CMC OA was reported at 21%.5 Symptoms include pain and deformity that may result in significant functional impairment of the thumb. Physical findings may include pain with palpation and swelling and warmth over the dorsal aspect of the CMC joint. The “grind test”—axial compression with internal and external rotation of the CMC joint—should reproduce the pain and may demonstrate crepitus.6 As with osteoarthritis in general, CMC OA radiographic findings do not directly correlate with the physical exam.
Splinting and physical therapy bring considerable relief
Conservative treatment options for CMC OA include NSAIDs, physical therapy, splinting, and corticosteroid injection. American College of Rheumatology guidelines support NSAIDs or acetaminophen as a first-line treatment for osteoarthritis pain of the knees and hips, but no guidelines specifically address CMC OA.7 Nor have there been any studies focused on NSAID therapy for CMC OA.
One retrospective study (n=130) evaluated splinting the thumb in abduction, and found that it reduced symptoms of CMC OA by an average of 54% to 61% at 6-month follow-up.8 The researchers studied the results of splinting in patients with stage 1 or 2 (mild to moderate) CMC OA vs those with stage 3 or 4 (moderate to severe) CMC OA, and found no significant difference in levels of improvement. In another study of patients with first CMC OA who were treated with splinting and physical therapy for 7 months, 70% of those who underwent treatment declined subsequent surgery, suggesting symptom improvement.9
Corticosteroid injections alone for CMC OA have had mixed results. One study compared corticosteroid injection with saline injection (n=40) and reported no difference at 24 weeks’ posttreatment.10 Another found short-term improvement from a corticosteroid injection (n=25), as measured on a visual analog scale at 1 month (P<.001), but no significant improvement in symptoms after 3 months.11
Consider surgery if conservative measures fail
As with most cases of osteoarthritis, surgery for CMC OA should be considered only after failure of conservative treatment. Surgical treatment options should be individualized, depending on the extent of disease.
Resection arthroplasty of the CMC joint is the gold standard for surgical treatment of thumb CMC OA.6 In one small study (n=24), researchers found that 90% of patients were satisfied with the outcome after 15 years.12 There are numerous surgical alternatives, however, and research addressing resurfacing, synthetic implants, and spacer materials is ongoing.6
Trigger thumb: Swelling, pain, limited motion
TT, also known as stenosing tenosynovitis, is characterized by swelling, limitation of thumb range of motion, and a “catching” sensation when the thumb is flexed. Pain is usually referred to the first dorsal compartment of the hand. The primary pathology is thickening of the A1 pulley, with resultant entrapment of the flexor tendon, thus forming a triggering mechanism.13
Early treatment leads to better response
Conservative treatment options for TT include splinting and corticosteroid injection; NSAIDs alone have not been found to provide any benefit.14 One study found that corticosteroid injection followed by splinting in 10° to 15° flexion for 3 to 12 weeks relieved symptoms for 66% of those with any trigger digit—but only 50% of patients with TT reported an improvement in symptoms.15
Overall, patients with TT symptoms for <4 months have been found to respond significantly better to any treatment (P=.01).16 This finding may be related to repeat injury to the tendon sheath, which leads to chronic inflammation and permanent sheath hypertrophy and scarring,16 and highlights the importance of early diagnosis and treatment.
Limited research has been done on the effect of corticosteroid injection alone on TT. Maneerit et al performed a prospective study (n=115) comparing steroid injection alone with percutaneous release combined with corticosteroid injection, and found that the injection alone was successful in improving symptoms in 47% of patients.17 (The combination of percutaneous release and steroid injection, discussed below, had a much higher success rate.)
A retrospective study of treatment for trigger digits demonstrated significant improvement with corticosteroid injection in patients who did not have diabetes; 52% had full resolution and 47% had improvement in symptoms (P=.04).18 In contrast, corticosteroid injection led to symptom resolution for only 32% of patients with diabetes.
Surgery for TT: Percutaneous or open release
Surgical treatment options for TT include percutaneous or open release. Complications of surgical intervention for trigger digits include infection, digital nerve injury, scarring, tenderness, and joint contractures. Nimigan et al reported a 99% improvement in symptoms and return to activity with open surgical release for patients with TT (n=72).18
In the study by Maneerit et al cited earlier, percutaneous release combined with corticosteroid injection had a success rate (indicated by decreased pain and triggering) of 91%, vs a 47% response rate for the group who received corticosteroid injection alone (P=.001).17 In another study, 25 patients with TT that had failed to respond to conservative treatment underwent percutaneous release. The result: An 84% success rate, as shown by a decrease in reported pain on a visual analog scale (P<.001), with no digital nerve damage reported.13
Digital nerve damage is more of a concern with percutaneous release than with open release, because of the proximity of the digital nerves to the A1 pulley.13 Success rates for percutaneous release vary from 38% to 100%, with improvement shown after appropriate physician training.13
de Quervain’s tenosynovitis. Initially, corticosteroid injection has been found to be the most appropriate first-line treatment for dQT;1,4,5 the addition of an oral nonsteroidal anti-inflammatory drug (NSAID) does not result in any additional benefit.5 What’s more, oral NSAIDs and thumb splinting are not effective.3 Overall, surgical repair has demonstrated the greatest success, but it is invasive and costly.1,4
First carpometacarpal osteoarthritis. There are few valid clinical trials for CMC OA. The available evidence, however, suggests starting with NSAIDs and progressing to splinting and physical therapy, as needed. Corticosteroid injections provide no long-term pain relief.10,11 As with osteoarthritis in general, surgery for CMC OA is usually reserved for patients who fail to respond to conservative treatments.
Trigger thumb. There are various methods and levels of success for trigger digit treatment, but few studies specifically examining treatment of TT. The evidence suggests starting with conservative treatment—corticosteroid injection and splinting—in patients who are opposed to surgery.15 Both open and percutaneous surgical release of TT have high success rates, however, and can be offered at any time.13
Acknowledgement
The authors thank Joshua Hodge, MD, for his constructive critique of this article.
CORRESPONDENCE
Christopher W. Bunt, MD, Major, USAF, MC, FAAFP, 2501 Capehart Road, Offutt AFB, NE 68113; [email protected]
1. Ilyas AM, Ast M, Schaffer AA, Thoder J. De Quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757-764.
2. Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, et al. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86A:2700-2706.
3. Richie A, Briner W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102-106.
4. Ta KT, Eidelman D, Thomson JG. Patient satisfaction and outcomes of surgery for De Quervain’s tenosynovitis. J Hand Surg Am. 1999;24:1071-1077.
5. Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953-957.
6. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140-151.
7. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
8. Swigart CR, Eaton RG, Glickel SZ, et al. Splinting in the treatment of arthritis of the first carpometacarpal joint. J Hand Surg Am. 1999;24:86-91.
9. Berggren M, Joost-Davidsson A, Lindstrand J, et al. Reduction in the need for operation after conservative treatment of osteoarthritis of the first carpometacarpal joint: a seven year prospective study. Scand J Plast Reconstr Surg Hand Surg. 2001;35:415-417.
10. Meenagh GK, Patton J, Kynes C, et al. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63:1260-1263.
11. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32:1305-1306.
12. Freedman DM, Clickel SZ, Eaton RG. Long-term follow-up of volar ligament reconstruction of the thumb. J Hand Surg Am. 2000;25A:297-304.
13. Cebesoy O, Kose KC, Baltaci ET, et al. Percutaneous release of the trigger thumb: is it safe, cheap and effective? Int Orthop. 2007;31:345-349.
14. Akhtar S, Bradley MJ, Quinton DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30-33.
15. Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17:110>-113.
16. Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236-238.
17. Maneerit J, Sriworakun C, Budhraja N, et al. Trigger thumb: results of a prospective randomized study of percutaneous release with steroid injection versus steroid injection alone. J Hand Surg Br. 2003;28:586-589.
18. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
• Do not treat de Quervain’s tenosynovitis with a corticosteroid injection plus a nonsteroidal anti-inflammatory drug; the combination is no more effective than the injection alone. B
• Resection arthroplasty of the carpometacarpal (CMC) joint is the gold standard for surgical treatment of thumb CMC osteoarthritis, but should be offered only if conservative measures fail. C
• Percutaneous release of trigger thumb combined with a corticosteroid injection provides greater symptom relief than the injection alone. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many possible causes of nontraumatic thumb pain are 3 conditions that primary care physicians are likely to encounter again and again: de Quervain’s tenosynovitis (dQT), first carpometacarpal osteoarthritis (CMC OA), and trigger thumb (TT). Common as they are, however, there are no consensus guidelines for the treatment of these conditions.
With that in mind, we did a literature search for studies of treatments for common causes of nontraumatic thumb pain. After reviewing the findings, we developed this evidence-based summary—and the “bottom line” treatment guide—as an aid to clinical decision making.
de Quervain’s tenosynovitis: An overuse injury
dQT is characterized by a gradual onset of pain in the first dorsal compartment of the wrist. The pain is reproduced on physical exam with clenched fist ulnar deviation of the wrist (Finkelstein test) (FIGURE). The suspected cause is overuse, leading to thickening of the tendons of the first dorsal compartment and subsequent resisted gliding of the tendons in their fibro-osseous canal.1
FIGURE
Finkelstein test for de Quervain’s tenosynovitis
With elbows flexed to 90°, the forearms parallel to each other and the floor, and the thumb clenched gently inside a fist (A), the patient drops the hand down (adduction) at the wrist (B). Pain over the first dorsal compartment is considered a positive test.
NSAIDs and injection: No better than injection alone
Conservative treatment of dQT consists of topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs), splinting, and corticosteroid injection.1 We identified 2 studies using such conservative modalities. The first was a randomized double-blind, placebo-controlled trial, which found that oral NSAIDs combined with corticosteroid injection provided no statistically significant benefit compared with corticosteroid injection alone (P=.69).2 The second study was a pooled qualitative analysis and showed that 83% (n=495) of patients were asymptomatic after corticosteroid injection alone.3 Treatment failure in the remaining 17% of patients was attributed to poor technique and anatomic variation within the first dorsal compartment.
Another arm of the study compared the combination of corticosteroid injection and splinting with splinting alone, which yielded 61% and 14% success rates, respectively. Some patients were treated with NSAIDs and rest alone, but this intervention had a 0% success rate.3
Surgery has a high “cure rate”
Symptoms of dQT of >9 months’ duration may not respond as well to conservative therapy.4 In such cases—and for patients for whom conservative measures bring only short-term relief—a surgical referral may be the best approach.
Surgery for dQT, a relatively simple procedure in which the sheaths surrounding the inflamed tendons at the base of the thumb are released to relieve the pain and swelling, has uniformly positive results. The “cure rate”—resolution of symptoms without complications—is reported to be >90%.1 One researcher found a positive correlation between a longer duration (>9 months) of preoperative symptoms and increased postoperative satisfaction (P<.4). 4
First carpometacarpal OA: Pain, deformity, functional impairment
In a study of patients with joint-specific arthritis of the hand, the prevalence of first CMC OA was reported at 21%.5 Symptoms include pain and deformity that may result in significant functional impairment of the thumb. Physical findings may include pain with palpation and swelling and warmth over the dorsal aspect of the CMC joint. The “grind test”—axial compression with internal and external rotation of the CMC joint—should reproduce the pain and may demonstrate crepitus.6 As with osteoarthritis in general, CMC OA radiographic findings do not directly correlate with the physical exam.
Splinting and physical therapy bring considerable relief
Conservative treatment options for CMC OA include NSAIDs, physical therapy, splinting, and corticosteroid injection. American College of Rheumatology guidelines support NSAIDs or acetaminophen as a first-line treatment for osteoarthritis pain of the knees and hips, but no guidelines specifically address CMC OA.7 Nor have there been any studies focused on NSAID therapy for CMC OA.
One retrospective study (n=130) evaluated splinting the thumb in abduction, and found that it reduced symptoms of CMC OA by an average of 54% to 61% at 6-month follow-up.8 The researchers studied the results of splinting in patients with stage 1 or 2 (mild to moderate) CMC OA vs those with stage 3 or 4 (moderate to severe) CMC OA, and found no significant difference in levels of improvement. In another study of patients with first CMC OA who were treated with splinting and physical therapy for 7 months, 70% of those who underwent treatment declined subsequent surgery, suggesting symptom improvement.9
Corticosteroid injections alone for CMC OA have had mixed results. One study compared corticosteroid injection with saline injection (n=40) and reported no difference at 24 weeks’ posttreatment.10 Another found short-term improvement from a corticosteroid injection (n=25), as measured on a visual analog scale at 1 month (P<.001), but no significant improvement in symptoms after 3 months.11
Consider surgery if conservative measures fail
As with most cases of osteoarthritis, surgery for CMC OA should be considered only after failure of conservative treatment. Surgical treatment options should be individualized, depending on the extent of disease.
Resection arthroplasty of the CMC joint is the gold standard for surgical treatment of thumb CMC OA.6 In one small study (n=24), researchers found that 90% of patients were satisfied with the outcome after 15 years.12 There are numerous surgical alternatives, however, and research addressing resurfacing, synthetic implants, and spacer materials is ongoing.6
Trigger thumb: Swelling, pain, limited motion
TT, also known as stenosing tenosynovitis, is characterized by swelling, limitation of thumb range of motion, and a “catching” sensation when the thumb is flexed. Pain is usually referred to the first dorsal compartment of the hand. The primary pathology is thickening of the A1 pulley, with resultant entrapment of the flexor tendon, thus forming a triggering mechanism.13
Early treatment leads to better response
Conservative treatment options for TT include splinting and corticosteroid injection; NSAIDs alone have not been found to provide any benefit.14 One study found that corticosteroid injection followed by splinting in 10° to 15° flexion for 3 to 12 weeks relieved symptoms for 66% of those with any trigger digit—but only 50% of patients with TT reported an improvement in symptoms.15
Overall, patients with TT symptoms for <4 months have been found to respond significantly better to any treatment (P=.01).16 This finding may be related to repeat injury to the tendon sheath, which leads to chronic inflammation and permanent sheath hypertrophy and scarring,16 and highlights the importance of early diagnosis and treatment.
Limited research has been done on the effect of corticosteroid injection alone on TT. Maneerit et al performed a prospective study (n=115) comparing steroid injection alone with percutaneous release combined with corticosteroid injection, and found that the injection alone was successful in improving symptoms in 47% of patients.17 (The combination of percutaneous release and steroid injection, discussed below, had a much higher success rate.)
A retrospective study of treatment for trigger digits demonstrated significant improvement with corticosteroid injection in patients who did not have diabetes; 52% had full resolution and 47% had improvement in symptoms (P=.04).18 In contrast, corticosteroid injection led to symptom resolution for only 32% of patients with diabetes.
Surgery for TT: Percutaneous or open release
Surgical treatment options for TT include percutaneous or open release. Complications of surgical intervention for trigger digits include infection, digital nerve injury, scarring, tenderness, and joint contractures. Nimigan et al reported a 99% improvement in symptoms and return to activity with open surgical release for patients with TT (n=72).18
In the study by Maneerit et al cited earlier, percutaneous release combined with corticosteroid injection had a success rate (indicated by decreased pain and triggering) of 91%, vs a 47% response rate for the group who received corticosteroid injection alone (P=.001).17 In another study, 25 patients with TT that had failed to respond to conservative treatment underwent percutaneous release. The result: An 84% success rate, as shown by a decrease in reported pain on a visual analog scale (P<.001), with no digital nerve damage reported.13
Digital nerve damage is more of a concern with percutaneous release than with open release, because of the proximity of the digital nerves to the A1 pulley.13 Success rates for percutaneous release vary from 38% to 100%, with improvement shown after appropriate physician training.13
de Quervain’s tenosynovitis. Initially, corticosteroid injection has been found to be the most appropriate first-line treatment for dQT;1,4,5 the addition of an oral nonsteroidal anti-inflammatory drug (NSAID) does not result in any additional benefit.5 What’s more, oral NSAIDs and thumb splinting are not effective.3 Overall, surgical repair has demonstrated the greatest success, but it is invasive and costly.1,4
First carpometacarpal osteoarthritis. There are few valid clinical trials for CMC OA. The available evidence, however, suggests starting with NSAIDs and progressing to splinting and physical therapy, as needed. Corticosteroid injections provide no long-term pain relief.10,11 As with osteoarthritis in general, surgery for CMC OA is usually reserved for patients who fail to respond to conservative treatments.
Trigger thumb. There are various methods and levels of success for trigger digit treatment, but few studies specifically examining treatment of TT. The evidence suggests starting with conservative treatment—corticosteroid injection and splinting—in patients who are opposed to surgery.15 Both open and percutaneous surgical release of TT have high success rates, however, and can be offered at any time.13
Acknowledgement
The authors thank Joshua Hodge, MD, for his constructive critique of this article.
CORRESPONDENCE
Christopher W. Bunt, MD, Major, USAF, MC, FAAFP, 2501 Capehart Road, Offutt AFB, NE 68113; [email protected]
• Do not treat de Quervain’s tenosynovitis with a corticosteroid injection plus a nonsteroidal anti-inflammatory drug; the combination is no more effective than the injection alone. B
• Resection arthroplasty of the carpometacarpal (CMC) joint is the gold standard for surgical treatment of thumb CMC osteoarthritis, but should be offered only if conservative measures fail. C
• Percutaneous release of trigger thumb combined with a corticosteroid injection provides greater symptom relief than the injection alone. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Among the many possible causes of nontraumatic thumb pain are 3 conditions that primary care physicians are likely to encounter again and again: de Quervain’s tenosynovitis (dQT), first carpometacarpal osteoarthritis (CMC OA), and trigger thumb (TT). Common as they are, however, there are no consensus guidelines for the treatment of these conditions.
With that in mind, we did a literature search for studies of treatments for common causes of nontraumatic thumb pain. After reviewing the findings, we developed this evidence-based summary—and the “bottom line” treatment guide—as an aid to clinical decision making.
de Quervain’s tenosynovitis: An overuse injury
dQT is characterized by a gradual onset of pain in the first dorsal compartment of the wrist. The pain is reproduced on physical exam with clenched fist ulnar deviation of the wrist (Finkelstein test) (FIGURE). The suspected cause is overuse, leading to thickening of the tendons of the first dorsal compartment and subsequent resisted gliding of the tendons in their fibro-osseous canal.1
FIGURE
Finkelstein test for de Quervain’s tenosynovitis
With elbows flexed to 90°, the forearms parallel to each other and the floor, and the thumb clenched gently inside a fist (A), the patient drops the hand down (adduction) at the wrist (B). Pain over the first dorsal compartment is considered a positive test.
NSAIDs and injection: No better than injection alone
Conservative treatment of dQT consists of topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs), splinting, and corticosteroid injection.1 We identified 2 studies using such conservative modalities. The first was a randomized double-blind, placebo-controlled trial, which found that oral NSAIDs combined with corticosteroid injection provided no statistically significant benefit compared with corticosteroid injection alone (P=.69).2 The second study was a pooled qualitative analysis and showed that 83% (n=495) of patients were asymptomatic after corticosteroid injection alone.3 Treatment failure in the remaining 17% of patients was attributed to poor technique and anatomic variation within the first dorsal compartment.
Another arm of the study compared the combination of corticosteroid injection and splinting with splinting alone, which yielded 61% and 14% success rates, respectively. Some patients were treated with NSAIDs and rest alone, but this intervention had a 0% success rate.3
Surgery has a high “cure rate”
Symptoms of dQT of >9 months’ duration may not respond as well to conservative therapy.4 In such cases—and for patients for whom conservative measures bring only short-term relief—a surgical referral may be the best approach.
Surgery for dQT, a relatively simple procedure in which the sheaths surrounding the inflamed tendons at the base of the thumb are released to relieve the pain and swelling, has uniformly positive results. The “cure rate”—resolution of symptoms without complications—is reported to be >90%.1 One researcher found a positive correlation between a longer duration (>9 months) of preoperative symptoms and increased postoperative satisfaction (P<.4). 4
First carpometacarpal OA: Pain, deformity, functional impairment
In a study of patients with joint-specific arthritis of the hand, the prevalence of first CMC OA was reported at 21%.5 Symptoms include pain and deformity that may result in significant functional impairment of the thumb. Physical findings may include pain with palpation and swelling and warmth over the dorsal aspect of the CMC joint. The “grind test”—axial compression with internal and external rotation of the CMC joint—should reproduce the pain and may demonstrate crepitus.6 As with osteoarthritis in general, CMC OA radiographic findings do not directly correlate with the physical exam.
Splinting and physical therapy bring considerable relief
Conservative treatment options for CMC OA include NSAIDs, physical therapy, splinting, and corticosteroid injection. American College of Rheumatology guidelines support NSAIDs or acetaminophen as a first-line treatment for osteoarthritis pain of the knees and hips, but no guidelines specifically address CMC OA.7 Nor have there been any studies focused on NSAID therapy for CMC OA.
One retrospective study (n=130) evaluated splinting the thumb in abduction, and found that it reduced symptoms of CMC OA by an average of 54% to 61% at 6-month follow-up.8 The researchers studied the results of splinting in patients with stage 1 or 2 (mild to moderate) CMC OA vs those with stage 3 or 4 (moderate to severe) CMC OA, and found no significant difference in levels of improvement. In another study of patients with first CMC OA who were treated with splinting and physical therapy for 7 months, 70% of those who underwent treatment declined subsequent surgery, suggesting symptom improvement.9
Corticosteroid injections alone for CMC OA have had mixed results. One study compared corticosteroid injection with saline injection (n=40) and reported no difference at 24 weeks’ posttreatment.10 Another found short-term improvement from a corticosteroid injection (n=25), as measured on a visual analog scale at 1 month (P<.001), but no significant improvement in symptoms after 3 months.11
Consider surgery if conservative measures fail
As with most cases of osteoarthritis, surgery for CMC OA should be considered only after failure of conservative treatment. Surgical treatment options should be individualized, depending on the extent of disease.
Resection arthroplasty of the CMC joint is the gold standard for surgical treatment of thumb CMC OA.6 In one small study (n=24), researchers found that 90% of patients were satisfied with the outcome after 15 years.12 There are numerous surgical alternatives, however, and research addressing resurfacing, synthetic implants, and spacer materials is ongoing.6
Trigger thumb: Swelling, pain, limited motion
TT, also known as stenosing tenosynovitis, is characterized by swelling, limitation of thumb range of motion, and a “catching” sensation when the thumb is flexed. Pain is usually referred to the first dorsal compartment of the hand. The primary pathology is thickening of the A1 pulley, with resultant entrapment of the flexor tendon, thus forming a triggering mechanism.13
Early treatment leads to better response
Conservative treatment options for TT include splinting and corticosteroid injection; NSAIDs alone have not been found to provide any benefit.14 One study found that corticosteroid injection followed by splinting in 10° to 15° flexion for 3 to 12 weeks relieved symptoms for 66% of those with any trigger digit—but only 50% of patients with TT reported an improvement in symptoms.15
Overall, patients with TT symptoms for <4 months have been found to respond significantly better to any treatment (P=.01).16 This finding may be related to repeat injury to the tendon sheath, which leads to chronic inflammation and permanent sheath hypertrophy and scarring,16 and highlights the importance of early diagnosis and treatment.
Limited research has been done on the effect of corticosteroid injection alone on TT. Maneerit et al performed a prospective study (n=115) comparing steroid injection alone with percutaneous release combined with corticosteroid injection, and found that the injection alone was successful in improving symptoms in 47% of patients.17 (The combination of percutaneous release and steroid injection, discussed below, had a much higher success rate.)
A retrospective study of treatment for trigger digits demonstrated significant improvement with corticosteroid injection in patients who did not have diabetes; 52% had full resolution and 47% had improvement in symptoms (P=.04).18 In contrast, corticosteroid injection led to symptom resolution for only 32% of patients with diabetes.
Surgery for TT: Percutaneous or open release
Surgical treatment options for TT include percutaneous or open release. Complications of surgical intervention for trigger digits include infection, digital nerve injury, scarring, tenderness, and joint contractures. Nimigan et al reported a 99% improvement in symptoms and return to activity with open surgical release for patients with TT (n=72).18
In the study by Maneerit et al cited earlier, percutaneous release combined with corticosteroid injection had a success rate (indicated by decreased pain and triggering) of 91%, vs a 47% response rate for the group who received corticosteroid injection alone (P=.001).17 In another study, 25 patients with TT that had failed to respond to conservative treatment underwent percutaneous release. The result: An 84% success rate, as shown by a decrease in reported pain on a visual analog scale (P<.001), with no digital nerve damage reported.13
Digital nerve damage is more of a concern with percutaneous release than with open release, because of the proximity of the digital nerves to the A1 pulley.13 Success rates for percutaneous release vary from 38% to 100%, with improvement shown after appropriate physician training.13
de Quervain’s tenosynovitis. Initially, corticosteroid injection has been found to be the most appropriate first-line treatment for dQT;1,4,5 the addition of an oral nonsteroidal anti-inflammatory drug (NSAID) does not result in any additional benefit.5 What’s more, oral NSAIDs and thumb splinting are not effective.3 Overall, surgical repair has demonstrated the greatest success, but it is invasive and costly.1,4
First carpometacarpal osteoarthritis. There are few valid clinical trials for CMC OA. The available evidence, however, suggests starting with NSAIDs and progressing to splinting and physical therapy, as needed. Corticosteroid injections provide no long-term pain relief.10,11 As with osteoarthritis in general, surgery for CMC OA is usually reserved for patients who fail to respond to conservative treatments.
Trigger thumb. There are various methods and levels of success for trigger digit treatment, but few studies specifically examining treatment of TT. The evidence suggests starting with conservative treatment—corticosteroid injection and splinting—in patients who are opposed to surgery.15 Both open and percutaneous surgical release of TT have high success rates, however, and can be offered at any time.13
Acknowledgement
The authors thank Joshua Hodge, MD, for his constructive critique of this article.
CORRESPONDENCE
Christopher W. Bunt, MD, Major, USAF, MC, FAAFP, 2501 Capehart Road, Offutt AFB, NE 68113; [email protected]
1. Ilyas AM, Ast M, Schaffer AA, Thoder J. De Quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757-764.
2. Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, et al. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86A:2700-2706.
3. Richie A, Briner W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102-106.
4. Ta KT, Eidelman D, Thomson JG. Patient satisfaction and outcomes of surgery for De Quervain’s tenosynovitis. J Hand Surg Am. 1999;24:1071-1077.
5. Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953-957.
6. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140-151.
7. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
8. Swigart CR, Eaton RG, Glickel SZ, et al. Splinting in the treatment of arthritis of the first carpometacarpal joint. J Hand Surg Am. 1999;24:86-91.
9. Berggren M, Joost-Davidsson A, Lindstrand J, et al. Reduction in the need for operation after conservative treatment of osteoarthritis of the first carpometacarpal joint: a seven year prospective study. Scand J Plast Reconstr Surg Hand Surg. 2001;35:415-417.
10. Meenagh GK, Patton J, Kynes C, et al. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63:1260-1263.
11. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32:1305-1306.
12. Freedman DM, Clickel SZ, Eaton RG. Long-term follow-up of volar ligament reconstruction of the thumb. J Hand Surg Am. 2000;25A:297-304.
13. Cebesoy O, Kose KC, Baltaci ET, et al. Percutaneous release of the trigger thumb: is it safe, cheap and effective? Int Orthop. 2007;31:345-349.
14. Akhtar S, Bradley MJ, Quinton DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30-33.
15. Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17:110>-113.
16. Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236-238.
17. Maneerit J, Sriworakun C, Budhraja N, et al. Trigger thumb: results of a prospective randomized study of percutaneous release with steroid injection versus steroid injection alone. J Hand Surg Br. 2003;28:586-589.
18. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
1. Ilyas AM, Ast M, Schaffer AA, Thoder J. De Quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757-764.
2. Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, et al. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2004;86A:2700-2706.
3. Richie A, Briner W. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102-106.
4. Ta KT, Eidelman D, Thomson JG. Patient satisfaction and outcomes of surgery for De Quervain’s tenosynovitis. J Hand Surg Am. 1999;24:1071-1077.
5. Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953-957.
6. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140-151.
7. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
8. Swigart CR, Eaton RG, Glickel SZ, et al. Splinting in the treatment of arthritis of the first carpometacarpal joint. J Hand Surg Am. 1999;24:86-91.
9. Berggren M, Joost-Davidsson A, Lindstrand J, et al. Reduction in the need for operation after conservative treatment of osteoarthritis of the first carpometacarpal joint: a seven year prospective study. Scand J Plast Reconstr Surg Hand Surg. 2001;35:415-417.
10. Meenagh GK, Patton J, Kynes C, et al. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63:1260-1263.
11. Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32:1305-1306.
12. Freedman DM, Clickel SZ, Eaton RG. Long-term follow-up of volar ligament reconstruction of the thumb. J Hand Surg Am. 2000;25A:297-304.
13. Cebesoy O, Kose KC, Baltaci ET, et al. Percutaneous release of the trigger thumb: is it safe, cheap and effective? Int Orthop. 2007;31:345-349.
14. Akhtar S, Bradley MJ, Quinton DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30-33.
15. Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992;17:110>-113.
16. Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984;190:236-238.
17. Maneerit J, Sriworakun C, Budhraja N, et al. Trigger thumb: results of a prospective randomized study of percutaneous release with steroid injection versus steroid injection alone. J Hand Surg Br. 2003;28:586-589.
18. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
You wrote the prescription, but will it get filled?
Purpose Despite numerous studies on adherence, there is little research on the first-fill rate of antihypertensive prescriptions. Our study took advantage of the recent increase in electronic prescribing (e-prescribing) and used data from e-prescribing physicians to determine the first-fill failure rate of antihypertensive prescriptions and to assess which factors predict first-fill failure.
Methods This retrospective study reviewed claims from a Mid-Atlantic managed care organization (MCO). We included adult members with continuous medical and pharmacy coverage who were prescribed an antihypertensive in 2008 by an e-prescribing physician. First-fill failure occurred when the patient did not obtain the antihypertensive medication due to either a denial by the MCO or reversal by the dispensing pharmacist. (Pharmacists reverse claims when a patient fails to pick up a medication.) Multivariate regression analysis determined the clinical and demographic factors associated with failure to fill.
Results The cohort consisted of 14,693 antihypertensive prescriptions, prescribed by 164 e-prescribing physicians for 7061 unique members. There were 2289 out of 14,693 prescriptions (15.6%) that went unfilled, affecting 24.3% of patients. Of the prescriptions not obtained, 1466 (64%) were denied by the MCO and 823 (36%) were reversed. Significant factors associated with first-fill failure were new diagnosis of hypertension, new antihypertensive agent, higher co-payment, and enrollment in a health maintenance organization or preferred provider organization.
Conclusions Patients newly diagnosed with hypertension and those prescribed a new antihypertensive were at particularly high risk for not obtaining their medication. Because nearly a quarter of patients did not obtain their initial fill of an antihypertensive prescription, future research should determine efficient and cost-effective systems to address first-fill failure in primary care.
Poor patient adherence to medical directives—the main cause of unsuccessful efforts to control hypertension1—is often difficult to assess in daily practice and in research. A common example of nonadherence is the failure to fill new prescriptions or to refill existing ones. In measuring adherence to first-fill and refilled prescriptions, investigators have often relied on patient self-report.2,3 However, this means of evaluation may be biased. One study found that patients markedly overstated their adherence to antihypertensive regimens, when compared with adherence measured by prescriptions actually filled.4
Objectively determining the rate of first-fill failure (not obtaining the initial fill of a prescription) has typically been cumbersome, requiring time-consuming chart reviews, which is unrealistic for studying large populations. A more efficient way to collect these data is through electronic prescribing (e-prescribing)—the electronic transmission of prescription or prescription-related information between a prescriber, a dispenser, and a pharmacy benefit manager or health plan, either directly or through an intermediary service.5
Our study sought to extend previous knowledge of adherence by determining the rate of first-fill failure for antihypertensive agents prescribed by electronic means, as well as identifying the clinical and demographic factors most closely associated with that failure. We believe e-prescribing may offer a way to improve antihypertensive medication adherence, especially for particular subgroups of patients, by providing information on the patient’s formulary and fill status notification.
Methods
This retrospective study used administrative, medical, and pharmacy data from a Mid-Atlantic managed care organization (MCO) serving 3.3 million medical and 1.2 million pharmacy members. To be eligible for inclusion, a member had to have an antihypertensive agent prescribed by a physician using e-prescribing. Our assumption in reviewing only prescriptions written by e-prescribing physicians was that each prescription would have a corresponding claim. To recruit a minimum of 100 electronic prescribers, we began by surveying physicians who had prescribed the highest volume of antihypertensive medication during the first half of 2008. We faxed a survey to these physicians, and if we received no response, we followed up by phone. Our final sample of physicians comprised those who were e-prescribing at least 75% of the time before January 1, 2008.6
A pharmacy claims query identified all antihypertensive prescriptions from January 1, 2008 through December 31, 2008 that were coded as new (a new prescription for either a new agent or the same agent the patient had been taking) and were prescribed by our group of electronic prescribers. We excluded members (and their prescription claims) who were younger than 18 years on the date of their first prescription and those who were not continuously enrolled in the same medical and pharmacy benefit plan from July 1, 2006 through December 31, 2008.
For each prescription, 3 claim options were possible: paid, denied, or reversed.
A paid claim meant that the prescription was approved by the MCO for coverage and that the member obtained the medication.
A denied claim occurred when coverage for the prescribed product was refused by the MCO.
A reversed claim meant that the prescription had been approved by the MCO for coverage, but the approved claim was later reversed by the pharmacist when a patient failed to pick up the prescription within 14 days.
A denied or reversed claim meant that the patient did not receive the medication, and this occurrence was labeled as failure to fill.
For members in the cohort, a medical claims query identified hypertension using specific International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes in the 401 category from July 1, 2006 through December 31, 2008. We deemed patients as newly diagnosed with hypertension if their first ICD-9-CM code for hypertension was within the preceding 6 months of the first antihypertensive prescription.
Outcome measures
The primary outcome measure was the percentage of new antihypertensive prescriptions that patients failed to obtain. To calculate this number, we designated the numerator as the number of denied or reversed claims for antihypertensive prescriptions, and the denominator as the overall number of antihypertensive prescriptions in the cohort.
The secondary outcome measure was the set of clinical and demographic factors related to failure to obtain the first fill. We evaluated the possible association of first-fill failure with age, sex, prior antihypertensive use, hypertension diagnosis, formulary status, co-payment, monotherapy vs combination product, pharmacologic category, type of health plan, and number of antihypertensive prescriptions.
All data collection conformed to patient privacy standards set by the Health Insurance Portability and Accountability Act (HIPAA), and the dataset was delivered to the researchers with de-identified patient information. The University of Maryland, Baltimore (UMB) Institutional Review Board (IRB) fully approved the research protocol.
Statistical analysis
Statistical analysis included descriptive statistics such as percentages for discrete variables (eg, sex) and calculations of means and standard deviations (SD) for continuous variables (eg, age). Univariate analyses examined the correlation between clinical/demographic characteristics and first-fill rates. We used binomial logistic regression to assess predictors of first-fill failure. We set statistical significance at an accepted alpha (P<.05).
Results
E-prescribing physicians
There were 1313 e-prescribing physicians ranked according to antihypertensive prescription volume. We contacted 457 physicians who prescribed the highest volume of medications and selected a final group of 164.
Patient/prescription cohort
The cohort consisted of 14,693 antihypertensive prescriptions prescribed by the 164 e-prescribing physicians. There were 7061 unique members with a mean age of 55.1±11.4 years (TABLE 1). Half were men (51.8%), and each member had a mean of 1.9 antihypertensive prescriptions. About two-thirds had a diagnosis of hypertension, as represented by ICD-9-CM codes.
Three-fourths of the prescriptions were for generic antihypertensives. ACE inhibitors were the most commonly prescribed class of drug, at 24.2%, followed by beta-blockers, angiotensin-receptor blockers, diuretics, and calcium channel blockers. Agents not fitting into the above classes were prescribed the least, at 2.8% of prescription volume. The mean co-payment per antihypertensive prescription was $17.00±$20.73.
Primary outcome
Patients failed to obtain the antihypertensive medication for 2289/14,693 (15.6%) prescriptions. Of the prescriptions not obtained, 1466 (64%) were denied and 823 (36%) were reversed. Failure to obtain the 2289 prescriptions affected 24.3% (1713/7061) of patients.
Secondary outcome
We compared clinical and demographic characteristics between the 2 outcome groups (TABLE 2). Univariate analyses revealed statistically significant differences for age, history of antihypertensive use and hypertension diagnosis, formulary status, medication characteristics, and type of health plan. Patients prescribed a new antihypertensive product or receiving a new diagnosis of hypertension were significantly less likely to fill their prescriptions (P<.001). Prescriptions for brand and combination products were, respectively, 2.2 percentage points (P=.025) and 3 percentage points (P=.002) higher in the failure-to-obtain category than in the obtained category. The difference in mean co-payment was $2.56 higher in the failure-to-obtain group (P<.001). There were differences between the obtained and failure-to-obtain groups based on the member’s type of health plan (P<.001).
Statistically significant factors from univariate analyses were included in the logistic regression model (data not shown). Factors associated with failure to obtain an antihypertensive prescription fell into 3 categories: history of hypertension (new vs existing diagnosis), formulary status of antihypertensive agent, and type of health plan. Prescriptions for new antihypertensive products were 49.44 times more likely to go unfilled (P<.001). Prescriptions were 1.73 times more likely to remain unfilled for members with a new diagnosis for hypertension (P<.001). In addition, the formulary status of the prescription showed that prescriptions were less likely to be filled for brand products (P=.030) or for those requiring higher co-payments (P<.001). Compared with indemnity health plans, health maintenance organizations and preferred provider organizations were more likely to be associated with unfilled prescriptions (P<.001 and P=.044, respectively).
TABLE 2
Prescription characteristics associated with different claims outcomes (N=14,693)*
| Variable | Obtained antihyper-tensive (n=12,404) | Failed to obtain antihypertensive (n=2289) | P value |
|---|---|---|---|
| Age | |||
| Mean ±SD, y | 56.2 ±11.4 | 55.4 ±11.9 | .002 |
| Sex | |||
| Male | 6581 (53.1) | 1182 (51.6) | .212 |
| Female | 5823 (46.9) | 1107 (48.4) | |
| Prior antihypertensive prescriptions | |||
| New antihypertensive product (no pharmacy claims for this agent within prior 6 months) | 41 (0.3) | 383 (16.7) | <.001 |
| Hypertension diagnosis | |||
| New diagnosis (at least 1 medical claim for HTN <6 months prior and no medical claims for HTN >6 months prior to antihypertensive prescription) | 626 (5.0) | 367 (16.0) | <.001 |
| Monotherapy/combination product | |||
| Monotherapy | 9482 (76.4) | 1681 (73.4) | .002 |
| Combination | 2922 (23.6) | 608 (26.6) | |
| Brand or generic status of product | |||
| Generic | 9350 (75.4) | 1675 (73.2) | .025 |
| Brand | 3054 (24.6) | 614 (26.8) | |
| Tier status | |||
| Tier 1 | 9350 (75.4) | 1675 (73.2) | .074 |
| Tier 2 | 1282 (10.3) | 252 (11.0) | |
| Tier 3 | 1772 (14.3) | 362 (15.8) | |
| Co-payment | |||
| Mean ±SD | $16.60±$20.17 | $19.16±$23.43 | <.001 |
| Type of health plan | |||
| Health maintenance organization | 5574 (44.9) | 1188 (51.9) | <.001 |
| Preferred provider organization | 2372 (19.1) | 426 (18.6) | |
| Consumer directed | 1847 (14.9) | 278 (12.1) | |
| Indemnity | 2611 (21.0) | 397 (17.3) | |
| Number of antihypertensive prescriptions | |||
| Mean ±SD | 1.9 ±1.0 | 1.8 ±1.0 | .001 |
| Therapeutic class | |||
| Angiotensin-converting enzyme inhibitors | 3008 (24.3) | 548 (23.9) | .939 |
| Angiotensin-receptor blockers | 2296 (18.5) | 442 (19.3) | |
| Beta-blockers | 2461 (19.8) | 440 (19.2) | |
| Calcium channel blockers (including combination product with statin) | 2047 (16.5) | 382 (16.7) | |
| Diuretics | 2242 (18.1) | 410 (17.9) | |
| Other: Alpha-adrenergic blocking agents, central alpha-agonists, direct vasodilators, hypotensive agents, peripheral adrenergic inhibitors, renin inhibitors | 350 (2.8) | 67 (2.9) | |
| HTN, hypertension. *Data are presented as n(%) unless otherwise noted. | |||
Discussion
This study used e-prescribing to evaluate nonadherence to the first-fill of an antihypertensive prescription. Our findings that 24.3% of patients did not obtain the first-fill of a medication and that 15.6% of prescriptions remained unclaimed are comparable to those of other research using electronically obtained prescription data.7,8
In a cross-sectional study of 327 African American adults enrolled in a Medicaid managed care plan, the authors reported that 24.9% (433/1742) of antihypertensive prescriptions were unfilled.7 In a study of therapeutically naïve patients, the first-fill failure rate was 17%.8 These patients were less likely to fill their antihypertensive prescriptions if they were prescribed loop diuretics or had a higher prescription co-payment. The median co-payment was $2 higher for prescriptions not obtained, compared with those that were obtained (P<.001). This finding was similar to the $2.56 difference we found for mean co-payment.
Higher co-payment was a strong predictor of decreased adherence in other antihypertensive adherence studies.9,10 In a survey of Medicare patients, the most common reason cited for failing to fill any prescription was that “it would cost too much.”2 Prescribing a less costly agent based on an insurer’s formulary may reduce the first-fill failure rate.
Although educating patients about their disease, involving family, and increasing patient participation through self-monitoring of blood pressure all have a positive impact on adherence rates and blood pressure control, physicians are hard pressed for time during an office visit to address such interventions.11-19 E-prescribing potentially offers a more efficient way to improve antihypertensive medication adherence. A recent ruling by the Centers for Medicare & Medicaid Services (CMS) requires e-prescribing systems to have the capability of providing formulary and benefit transaction, medication history transaction, and fill status notification to prescribers.20 Prescribers can readily access patients’ insurance coverage information. Formulary decision support, as part of an e-prescribing system, has been shown to increase use of formulary products.21 Fill status notification allows two-way communication between the prescriber and pharmacy so that prescribers can be made aware if patients fail to fill prescriptions.
Unfortunately, e-prescribing is not yet widely used. Approximately 26% of office-based US physicians use e-prescribing, and only 30% of them take advantage of formulary information.22 E-prescribing authorities believe practices not using the fill status notification probably lack resources needed to manage patients who are nonadherent.22 While it may not be necessary to check whether all patients have obtained their prescription, it may be useful for subgroups of patients, such as those who have received a new diagnosis, whose disease is poorly controlled, who are prescribed new antihypertensive agents, or who are otherwise thought to be nonadherent.
Study limitations. First, claims data serve as a proxy for medication adherence. Even though a patient obtained an antihypertensive prescription according to claims data, this does not guarantee that the patient used the medication.
Second, not all patients had a diagnosis of hypertension designated by specific ICD-9-CM codes. Patients may have been prescribed antihypertensive medications for other indications, such as heart failure, migraine, anxiety, etc. Our results apply to all patients prescribed antihypertensive agents, and although most had hypertension, there may be differences in first-fill rates for those with and without hypertension.
Third, patients were required to fill their prescriptions through insurance. In community pharmacy settings, some prescriptions may be paid for with cash due to the availability of several inexpensive generic antihypertensive medications (eg, a 30-day supply for $4, or 90-day supply for $10).23 Patients taking advantage of these promotions would result in an overestimation of first-fill failure rate.
Fourth, patients may have received samples from their physician and subsequently failed to obtain their antihypertensive prescription at the pharmacy because the medication sample was ineffective or not tolerated.
Fifth, claims data from physicians who were e-prescribing were used to proxy electronic prescriptions. However, some physicians may have handed patients their prescriptions, which, if never taken to the pharmacy, would result in an underestimation of first-fill failure rate.
CORRESPONDENCE
Catherine E. Cooke, President, PosiHealth, Inc., 5106 Bonnie Branch Road, Ellicott City, MD 21043; [email protected]
1. Sabate E. ed. Adherence to long-term therapies: evidence for action. Geneva, Switzerland. World Health Organization, 20030. Available at: http://www.who.int/chp/knowledge/publications/adherence_report/en. Accessed May 20, 2011.
2. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of Medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553-560.
3. Esposito D, Schone E, Williams T, et al. Prevalence of unclaimed prescriptions at military pharmacies. J Manag Care Pharm. 2008;14:541-552.
4. Wang PS, Benner JS, Glynn RJ, et al. How well do patients report noncompliance with antihypertensive medications? a comparison of self-report versus filled prescriptions. Pharmacoepidemiol Drug Saf. 2004;13:11-19.
5. eHealth Initiative and The Center for Improving Medication Management. A clinician’s guide to electronic prescribing. White paper from the eHealth Initiative and The Center for Improving Medication Management, 2008. Available at: http://www.ehealthinitiative.org/sites/default/files/e-Prescribing_Clinicians_Guide_Final(1).pdf. Accessed March 21, 2010.
6. Solberg LI, Asche SE, Pawlson LG, et al. Practice systems are associated with high-quality care for diabetes. Am J Manag Care. 2008;14:85-92.
7. Lagu T, Weiner MG, Eachus S, et al. Effect of patient comorbidities on filling of antihypertensive prescriptions Am J Manag Care. 2009;15:24-30.
8. Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22:392-396.
9. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61-69.
10. Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff. 2004;23:227-236.
11. Bell RA, Kravitz RL. Physician counseling for hypertension: what do doctors really do? Patient Educ Couns. 2008;72:115-121.
12. Boulware LE, Daumit GL, Frick KD, et al. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21:221-232.
13. Feldman R, Bacher M, Campbell N, et al. Adherence to pharmacologic management of hypertension. Can J Public Health. 1998;89:I16-I18.
14. Fiscella K, Epstein RM. So much to do, so little time: care for the socially disadvantaged and the 15-minute visit. Arch Intern Med. 2008;168:1843-1852.
15. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028-3035.
16. Morisky DE, Levine DM, Green LW, et al. Five-year blood pressure control and mortality following health education for hypertensive patients. Am J Public Health. 1983;73:153-162.
17. Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657-665.
18. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care visits. Health Serv Res. 2007;42:1871-1894.
19. Yarnall KS, Pollak KI, Østbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
20. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; standards for e-prescribing under Medicare Part D and identification of backward compatible version of adopted standard for e-prescribing and the Medicare prescription drug program (version 8.1). Final rule. Fed Regist. 2008;73:18917-18942.
21. Fischer MA, Vogeli C, Stedman M, et al. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med. 2008;168:2433-2439.
22. SureScripts 2009 Progress Report on E-Prescribing. Available at: http://www.surescripts.com/downloads/NPR/national-progress-report.pdf. Accessed March 21, 2010.
23. Walmart. Retail prescription program drug list. Available at: http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed March 21, 2010.
Purpose Despite numerous studies on adherence, there is little research on the first-fill rate of antihypertensive prescriptions. Our study took advantage of the recent increase in electronic prescribing (e-prescribing) and used data from e-prescribing physicians to determine the first-fill failure rate of antihypertensive prescriptions and to assess which factors predict first-fill failure.
Methods This retrospective study reviewed claims from a Mid-Atlantic managed care organization (MCO). We included adult members with continuous medical and pharmacy coverage who were prescribed an antihypertensive in 2008 by an e-prescribing physician. First-fill failure occurred when the patient did not obtain the antihypertensive medication due to either a denial by the MCO or reversal by the dispensing pharmacist. (Pharmacists reverse claims when a patient fails to pick up a medication.) Multivariate regression analysis determined the clinical and demographic factors associated with failure to fill.
Results The cohort consisted of 14,693 antihypertensive prescriptions, prescribed by 164 e-prescribing physicians for 7061 unique members. There were 2289 out of 14,693 prescriptions (15.6%) that went unfilled, affecting 24.3% of patients. Of the prescriptions not obtained, 1466 (64%) were denied by the MCO and 823 (36%) were reversed. Significant factors associated with first-fill failure were new diagnosis of hypertension, new antihypertensive agent, higher co-payment, and enrollment in a health maintenance organization or preferred provider organization.
Conclusions Patients newly diagnosed with hypertension and those prescribed a new antihypertensive were at particularly high risk for not obtaining their medication. Because nearly a quarter of patients did not obtain their initial fill of an antihypertensive prescription, future research should determine efficient and cost-effective systems to address first-fill failure in primary care.
Poor patient adherence to medical directives—the main cause of unsuccessful efforts to control hypertension1—is often difficult to assess in daily practice and in research. A common example of nonadherence is the failure to fill new prescriptions or to refill existing ones. In measuring adherence to first-fill and refilled prescriptions, investigators have often relied on patient self-report.2,3 However, this means of evaluation may be biased. One study found that patients markedly overstated their adherence to antihypertensive regimens, when compared with adherence measured by prescriptions actually filled.4
Objectively determining the rate of first-fill failure (not obtaining the initial fill of a prescription) has typically been cumbersome, requiring time-consuming chart reviews, which is unrealistic for studying large populations. A more efficient way to collect these data is through electronic prescribing (e-prescribing)—the electronic transmission of prescription or prescription-related information between a prescriber, a dispenser, and a pharmacy benefit manager or health plan, either directly or through an intermediary service.5
Our study sought to extend previous knowledge of adherence by determining the rate of first-fill failure for antihypertensive agents prescribed by electronic means, as well as identifying the clinical and demographic factors most closely associated with that failure. We believe e-prescribing may offer a way to improve antihypertensive medication adherence, especially for particular subgroups of patients, by providing information on the patient’s formulary and fill status notification.
Methods
This retrospective study used administrative, medical, and pharmacy data from a Mid-Atlantic managed care organization (MCO) serving 3.3 million medical and 1.2 million pharmacy members. To be eligible for inclusion, a member had to have an antihypertensive agent prescribed by a physician using e-prescribing. Our assumption in reviewing only prescriptions written by e-prescribing physicians was that each prescription would have a corresponding claim. To recruit a minimum of 100 electronic prescribers, we began by surveying physicians who had prescribed the highest volume of antihypertensive medication during the first half of 2008. We faxed a survey to these physicians, and if we received no response, we followed up by phone. Our final sample of physicians comprised those who were e-prescribing at least 75% of the time before January 1, 2008.6
A pharmacy claims query identified all antihypertensive prescriptions from January 1, 2008 through December 31, 2008 that were coded as new (a new prescription for either a new agent or the same agent the patient had been taking) and were prescribed by our group of electronic prescribers. We excluded members (and their prescription claims) who were younger than 18 years on the date of their first prescription and those who were not continuously enrolled in the same medical and pharmacy benefit plan from July 1, 2006 through December 31, 2008.
For each prescription, 3 claim options were possible: paid, denied, or reversed.
A paid claim meant that the prescription was approved by the MCO for coverage and that the member obtained the medication.
A denied claim occurred when coverage for the prescribed product was refused by the MCO.
A reversed claim meant that the prescription had been approved by the MCO for coverage, but the approved claim was later reversed by the pharmacist when a patient failed to pick up the prescription within 14 days.
A denied or reversed claim meant that the patient did not receive the medication, and this occurrence was labeled as failure to fill.
For members in the cohort, a medical claims query identified hypertension using specific International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes in the 401 category from July 1, 2006 through December 31, 2008. We deemed patients as newly diagnosed with hypertension if their first ICD-9-CM code for hypertension was within the preceding 6 months of the first antihypertensive prescription.
Outcome measures
The primary outcome measure was the percentage of new antihypertensive prescriptions that patients failed to obtain. To calculate this number, we designated the numerator as the number of denied or reversed claims for antihypertensive prescriptions, and the denominator as the overall number of antihypertensive prescriptions in the cohort.
The secondary outcome measure was the set of clinical and demographic factors related to failure to obtain the first fill. We evaluated the possible association of first-fill failure with age, sex, prior antihypertensive use, hypertension diagnosis, formulary status, co-payment, monotherapy vs combination product, pharmacologic category, type of health plan, and number of antihypertensive prescriptions.
All data collection conformed to patient privacy standards set by the Health Insurance Portability and Accountability Act (HIPAA), and the dataset was delivered to the researchers with de-identified patient information. The University of Maryland, Baltimore (UMB) Institutional Review Board (IRB) fully approved the research protocol.
Statistical analysis
Statistical analysis included descriptive statistics such as percentages for discrete variables (eg, sex) and calculations of means and standard deviations (SD) for continuous variables (eg, age). Univariate analyses examined the correlation between clinical/demographic characteristics and first-fill rates. We used binomial logistic regression to assess predictors of first-fill failure. We set statistical significance at an accepted alpha (P<.05).
Results
E-prescribing physicians
There were 1313 e-prescribing physicians ranked according to antihypertensive prescription volume. We contacted 457 physicians who prescribed the highest volume of medications and selected a final group of 164.
Patient/prescription cohort
The cohort consisted of 14,693 antihypertensive prescriptions prescribed by the 164 e-prescribing physicians. There were 7061 unique members with a mean age of 55.1±11.4 years (TABLE 1). Half were men (51.8%), and each member had a mean of 1.9 antihypertensive prescriptions. About two-thirds had a diagnosis of hypertension, as represented by ICD-9-CM codes.
Three-fourths of the prescriptions were for generic antihypertensives. ACE inhibitors were the most commonly prescribed class of drug, at 24.2%, followed by beta-blockers, angiotensin-receptor blockers, diuretics, and calcium channel blockers. Agents not fitting into the above classes were prescribed the least, at 2.8% of prescription volume. The mean co-payment per antihypertensive prescription was $17.00±$20.73.
Primary outcome
Patients failed to obtain the antihypertensive medication for 2289/14,693 (15.6%) prescriptions. Of the prescriptions not obtained, 1466 (64%) were denied and 823 (36%) were reversed. Failure to obtain the 2289 prescriptions affected 24.3% (1713/7061) of patients.
Secondary outcome
We compared clinical and demographic characteristics between the 2 outcome groups (TABLE 2). Univariate analyses revealed statistically significant differences for age, history of antihypertensive use and hypertension diagnosis, formulary status, medication characteristics, and type of health plan. Patients prescribed a new antihypertensive product or receiving a new diagnosis of hypertension were significantly less likely to fill their prescriptions (P<.001). Prescriptions for brand and combination products were, respectively, 2.2 percentage points (P=.025) and 3 percentage points (P=.002) higher in the failure-to-obtain category than in the obtained category. The difference in mean co-payment was $2.56 higher in the failure-to-obtain group (P<.001). There were differences between the obtained and failure-to-obtain groups based on the member’s type of health plan (P<.001).
Statistically significant factors from univariate analyses were included in the logistic regression model (data not shown). Factors associated with failure to obtain an antihypertensive prescription fell into 3 categories: history of hypertension (new vs existing diagnosis), formulary status of antihypertensive agent, and type of health plan. Prescriptions for new antihypertensive products were 49.44 times more likely to go unfilled (P<.001). Prescriptions were 1.73 times more likely to remain unfilled for members with a new diagnosis for hypertension (P<.001). In addition, the formulary status of the prescription showed that prescriptions were less likely to be filled for brand products (P=.030) or for those requiring higher co-payments (P<.001). Compared with indemnity health plans, health maintenance organizations and preferred provider organizations were more likely to be associated with unfilled prescriptions (P<.001 and P=.044, respectively).
TABLE 2
Prescription characteristics associated with different claims outcomes (N=14,693)*
| Variable | Obtained antihyper-tensive (n=12,404) | Failed to obtain antihypertensive (n=2289) | P value |
|---|---|---|---|
| Age | |||
| Mean ±SD, y | 56.2 ±11.4 | 55.4 ±11.9 | .002 |
| Sex | |||
| Male | 6581 (53.1) | 1182 (51.6) | .212 |
| Female | 5823 (46.9) | 1107 (48.4) | |
| Prior antihypertensive prescriptions | |||
| New antihypertensive product (no pharmacy claims for this agent within prior 6 months) | 41 (0.3) | 383 (16.7) | <.001 |
| Hypertension diagnosis | |||
| New diagnosis (at least 1 medical claim for HTN <6 months prior and no medical claims for HTN >6 months prior to antihypertensive prescription) | 626 (5.0) | 367 (16.0) | <.001 |
| Monotherapy/combination product | |||
| Monotherapy | 9482 (76.4) | 1681 (73.4) | .002 |
| Combination | 2922 (23.6) | 608 (26.6) | |
| Brand or generic status of product | |||
| Generic | 9350 (75.4) | 1675 (73.2) | .025 |
| Brand | 3054 (24.6) | 614 (26.8) | |
| Tier status | |||
| Tier 1 | 9350 (75.4) | 1675 (73.2) | .074 |
| Tier 2 | 1282 (10.3) | 252 (11.0) | |
| Tier 3 | 1772 (14.3) | 362 (15.8) | |
| Co-payment | |||
| Mean ±SD | $16.60±$20.17 | $19.16±$23.43 | <.001 |
| Type of health plan | |||
| Health maintenance organization | 5574 (44.9) | 1188 (51.9) | <.001 |
| Preferred provider organization | 2372 (19.1) | 426 (18.6) | |
| Consumer directed | 1847 (14.9) | 278 (12.1) | |
| Indemnity | 2611 (21.0) | 397 (17.3) | |
| Number of antihypertensive prescriptions | |||
| Mean ±SD | 1.9 ±1.0 | 1.8 ±1.0 | .001 |
| Therapeutic class | |||
| Angiotensin-converting enzyme inhibitors | 3008 (24.3) | 548 (23.9) | .939 |
| Angiotensin-receptor blockers | 2296 (18.5) | 442 (19.3) | |
| Beta-blockers | 2461 (19.8) | 440 (19.2) | |
| Calcium channel blockers (including combination product with statin) | 2047 (16.5) | 382 (16.7) | |
| Diuretics | 2242 (18.1) | 410 (17.9) | |
| Other: Alpha-adrenergic blocking agents, central alpha-agonists, direct vasodilators, hypotensive agents, peripheral adrenergic inhibitors, renin inhibitors | 350 (2.8) | 67 (2.9) | |
| HTN, hypertension. *Data are presented as n(%) unless otherwise noted. | |||
Discussion
This study used e-prescribing to evaluate nonadherence to the first-fill of an antihypertensive prescription. Our findings that 24.3% of patients did not obtain the first-fill of a medication and that 15.6% of prescriptions remained unclaimed are comparable to those of other research using electronically obtained prescription data.7,8
In a cross-sectional study of 327 African American adults enrolled in a Medicaid managed care plan, the authors reported that 24.9% (433/1742) of antihypertensive prescriptions were unfilled.7 In a study of therapeutically naïve patients, the first-fill failure rate was 17%.8 These patients were less likely to fill their antihypertensive prescriptions if they were prescribed loop diuretics or had a higher prescription co-payment. The median co-payment was $2 higher for prescriptions not obtained, compared with those that were obtained (P<.001). This finding was similar to the $2.56 difference we found for mean co-payment.
Higher co-payment was a strong predictor of decreased adherence in other antihypertensive adherence studies.9,10 In a survey of Medicare patients, the most common reason cited for failing to fill any prescription was that “it would cost too much.”2 Prescribing a less costly agent based on an insurer’s formulary may reduce the first-fill failure rate.
Although educating patients about their disease, involving family, and increasing patient participation through self-monitoring of blood pressure all have a positive impact on adherence rates and blood pressure control, physicians are hard pressed for time during an office visit to address such interventions.11-19 E-prescribing potentially offers a more efficient way to improve antihypertensive medication adherence. A recent ruling by the Centers for Medicare & Medicaid Services (CMS) requires e-prescribing systems to have the capability of providing formulary and benefit transaction, medication history transaction, and fill status notification to prescribers.20 Prescribers can readily access patients’ insurance coverage information. Formulary decision support, as part of an e-prescribing system, has been shown to increase use of formulary products.21 Fill status notification allows two-way communication between the prescriber and pharmacy so that prescribers can be made aware if patients fail to fill prescriptions.
Unfortunately, e-prescribing is not yet widely used. Approximately 26% of office-based US physicians use e-prescribing, and only 30% of them take advantage of formulary information.22 E-prescribing authorities believe practices not using the fill status notification probably lack resources needed to manage patients who are nonadherent.22 While it may not be necessary to check whether all patients have obtained their prescription, it may be useful for subgroups of patients, such as those who have received a new diagnosis, whose disease is poorly controlled, who are prescribed new antihypertensive agents, or who are otherwise thought to be nonadherent.
Study limitations. First, claims data serve as a proxy for medication adherence. Even though a patient obtained an antihypertensive prescription according to claims data, this does not guarantee that the patient used the medication.
Second, not all patients had a diagnosis of hypertension designated by specific ICD-9-CM codes. Patients may have been prescribed antihypertensive medications for other indications, such as heart failure, migraine, anxiety, etc. Our results apply to all patients prescribed antihypertensive agents, and although most had hypertension, there may be differences in first-fill rates for those with and without hypertension.
Third, patients were required to fill their prescriptions through insurance. In community pharmacy settings, some prescriptions may be paid for with cash due to the availability of several inexpensive generic antihypertensive medications (eg, a 30-day supply for $4, or 90-day supply for $10).23 Patients taking advantage of these promotions would result in an overestimation of first-fill failure rate.
Fourth, patients may have received samples from their physician and subsequently failed to obtain their antihypertensive prescription at the pharmacy because the medication sample was ineffective or not tolerated.
Fifth, claims data from physicians who were e-prescribing were used to proxy electronic prescriptions. However, some physicians may have handed patients their prescriptions, which, if never taken to the pharmacy, would result in an underestimation of first-fill failure rate.
CORRESPONDENCE
Catherine E. Cooke, President, PosiHealth, Inc., 5106 Bonnie Branch Road, Ellicott City, MD 21043; [email protected]
Purpose Despite numerous studies on adherence, there is little research on the first-fill rate of antihypertensive prescriptions. Our study took advantage of the recent increase in electronic prescribing (e-prescribing) and used data from e-prescribing physicians to determine the first-fill failure rate of antihypertensive prescriptions and to assess which factors predict first-fill failure.
Methods This retrospective study reviewed claims from a Mid-Atlantic managed care organization (MCO). We included adult members with continuous medical and pharmacy coverage who were prescribed an antihypertensive in 2008 by an e-prescribing physician. First-fill failure occurred when the patient did not obtain the antihypertensive medication due to either a denial by the MCO or reversal by the dispensing pharmacist. (Pharmacists reverse claims when a patient fails to pick up a medication.) Multivariate regression analysis determined the clinical and demographic factors associated with failure to fill.
Results The cohort consisted of 14,693 antihypertensive prescriptions, prescribed by 164 e-prescribing physicians for 7061 unique members. There were 2289 out of 14,693 prescriptions (15.6%) that went unfilled, affecting 24.3% of patients. Of the prescriptions not obtained, 1466 (64%) were denied by the MCO and 823 (36%) were reversed. Significant factors associated with first-fill failure were new diagnosis of hypertension, new antihypertensive agent, higher co-payment, and enrollment in a health maintenance organization or preferred provider organization.
Conclusions Patients newly diagnosed with hypertension and those prescribed a new antihypertensive were at particularly high risk for not obtaining their medication. Because nearly a quarter of patients did not obtain their initial fill of an antihypertensive prescription, future research should determine efficient and cost-effective systems to address first-fill failure in primary care.
Poor patient adherence to medical directives—the main cause of unsuccessful efforts to control hypertension1—is often difficult to assess in daily practice and in research. A common example of nonadherence is the failure to fill new prescriptions or to refill existing ones. In measuring adherence to first-fill and refilled prescriptions, investigators have often relied on patient self-report.2,3 However, this means of evaluation may be biased. One study found that patients markedly overstated their adherence to antihypertensive regimens, when compared with adherence measured by prescriptions actually filled.4
Objectively determining the rate of first-fill failure (not obtaining the initial fill of a prescription) has typically been cumbersome, requiring time-consuming chart reviews, which is unrealistic for studying large populations. A more efficient way to collect these data is through electronic prescribing (e-prescribing)—the electronic transmission of prescription or prescription-related information between a prescriber, a dispenser, and a pharmacy benefit manager or health plan, either directly or through an intermediary service.5
Our study sought to extend previous knowledge of adherence by determining the rate of first-fill failure for antihypertensive agents prescribed by electronic means, as well as identifying the clinical and demographic factors most closely associated with that failure. We believe e-prescribing may offer a way to improve antihypertensive medication adherence, especially for particular subgroups of patients, by providing information on the patient’s formulary and fill status notification.
Methods
This retrospective study used administrative, medical, and pharmacy data from a Mid-Atlantic managed care organization (MCO) serving 3.3 million medical and 1.2 million pharmacy members. To be eligible for inclusion, a member had to have an antihypertensive agent prescribed by a physician using e-prescribing. Our assumption in reviewing only prescriptions written by e-prescribing physicians was that each prescription would have a corresponding claim. To recruit a minimum of 100 electronic prescribers, we began by surveying physicians who had prescribed the highest volume of antihypertensive medication during the first half of 2008. We faxed a survey to these physicians, and if we received no response, we followed up by phone. Our final sample of physicians comprised those who were e-prescribing at least 75% of the time before January 1, 2008.6
A pharmacy claims query identified all antihypertensive prescriptions from January 1, 2008 through December 31, 2008 that were coded as new (a new prescription for either a new agent or the same agent the patient had been taking) and were prescribed by our group of electronic prescribers. We excluded members (and their prescription claims) who were younger than 18 years on the date of their first prescription and those who were not continuously enrolled in the same medical and pharmacy benefit plan from July 1, 2006 through December 31, 2008.
For each prescription, 3 claim options were possible: paid, denied, or reversed.
A paid claim meant that the prescription was approved by the MCO for coverage and that the member obtained the medication.
A denied claim occurred when coverage for the prescribed product was refused by the MCO.
A reversed claim meant that the prescription had been approved by the MCO for coverage, but the approved claim was later reversed by the pharmacist when a patient failed to pick up the prescription within 14 days.
A denied or reversed claim meant that the patient did not receive the medication, and this occurrence was labeled as failure to fill.
For members in the cohort, a medical claims query identified hypertension using specific International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes in the 401 category from July 1, 2006 through December 31, 2008. We deemed patients as newly diagnosed with hypertension if their first ICD-9-CM code for hypertension was within the preceding 6 months of the first antihypertensive prescription.
Outcome measures
The primary outcome measure was the percentage of new antihypertensive prescriptions that patients failed to obtain. To calculate this number, we designated the numerator as the number of denied or reversed claims for antihypertensive prescriptions, and the denominator as the overall number of antihypertensive prescriptions in the cohort.
The secondary outcome measure was the set of clinical and demographic factors related to failure to obtain the first fill. We evaluated the possible association of first-fill failure with age, sex, prior antihypertensive use, hypertension diagnosis, formulary status, co-payment, monotherapy vs combination product, pharmacologic category, type of health plan, and number of antihypertensive prescriptions.
All data collection conformed to patient privacy standards set by the Health Insurance Portability and Accountability Act (HIPAA), and the dataset was delivered to the researchers with de-identified patient information. The University of Maryland, Baltimore (UMB) Institutional Review Board (IRB) fully approved the research protocol.
Statistical analysis
Statistical analysis included descriptive statistics such as percentages for discrete variables (eg, sex) and calculations of means and standard deviations (SD) for continuous variables (eg, age). Univariate analyses examined the correlation between clinical/demographic characteristics and first-fill rates. We used binomial logistic regression to assess predictors of first-fill failure. We set statistical significance at an accepted alpha (P<.05).
Results
E-prescribing physicians
There were 1313 e-prescribing physicians ranked according to antihypertensive prescription volume. We contacted 457 physicians who prescribed the highest volume of medications and selected a final group of 164.
Patient/prescription cohort
The cohort consisted of 14,693 antihypertensive prescriptions prescribed by the 164 e-prescribing physicians. There were 7061 unique members with a mean age of 55.1±11.4 years (TABLE 1). Half were men (51.8%), and each member had a mean of 1.9 antihypertensive prescriptions. About two-thirds had a diagnosis of hypertension, as represented by ICD-9-CM codes.
Three-fourths of the prescriptions were for generic antihypertensives. ACE inhibitors were the most commonly prescribed class of drug, at 24.2%, followed by beta-blockers, angiotensin-receptor blockers, diuretics, and calcium channel blockers. Agents not fitting into the above classes were prescribed the least, at 2.8% of prescription volume. The mean co-payment per antihypertensive prescription was $17.00±$20.73.
Primary outcome
Patients failed to obtain the antihypertensive medication for 2289/14,693 (15.6%) prescriptions. Of the prescriptions not obtained, 1466 (64%) were denied and 823 (36%) were reversed. Failure to obtain the 2289 prescriptions affected 24.3% (1713/7061) of patients.
Secondary outcome
We compared clinical and demographic characteristics between the 2 outcome groups (TABLE 2). Univariate analyses revealed statistically significant differences for age, history of antihypertensive use and hypertension diagnosis, formulary status, medication characteristics, and type of health plan. Patients prescribed a new antihypertensive product or receiving a new diagnosis of hypertension were significantly less likely to fill their prescriptions (P<.001). Prescriptions for brand and combination products were, respectively, 2.2 percentage points (P=.025) and 3 percentage points (P=.002) higher in the failure-to-obtain category than in the obtained category. The difference in mean co-payment was $2.56 higher in the failure-to-obtain group (P<.001). There were differences between the obtained and failure-to-obtain groups based on the member’s type of health plan (P<.001).
Statistically significant factors from univariate analyses were included in the logistic regression model (data not shown). Factors associated with failure to obtain an antihypertensive prescription fell into 3 categories: history of hypertension (new vs existing diagnosis), formulary status of antihypertensive agent, and type of health plan. Prescriptions for new antihypertensive products were 49.44 times more likely to go unfilled (P<.001). Prescriptions were 1.73 times more likely to remain unfilled for members with a new diagnosis for hypertension (P<.001). In addition, the formulary status of the prescription showed that prescriptions were less likely to be filled for brand products (P=.030) or for those requiring higher co-payments (P<.001). Compared with indemnity health plans, health maintenance organizations and preferred provider organizations were more likely to be associated with unfilled prescriptions (P<.001 and P=.044, respectively).
TABLE 2
Prescription characteristics associated with different claims outcomes (N=14,693)*
| Variable | Obtained antihyper-tensive (n=12,404) | Failed to obtain antihypertensive (n=2289) | P value |
|---|---|---|---|
| Age | |||
| Mean ±SD, y | 56.2 ±11.4 | 55.4 ±11.9 | .002 |
| Sex | |||
| Male | 6581 (53.1) | 1182 (51.6) | .212 |
| Female | 5823 (46.9) | 1107 (48.4) | |
| Prior antihypertensive prescriptions | |||
| New antihypertensive product (no pharmacy claims for this agent within prior 6 months) | 41 (0.3) | 383 (16.7) | <.001 |
| Hypertension diagnosis | |||
| New diagnosis (at least 1 medical claim for HTN <6 months prior and no medical claims for HTN >6 months prior to antihypertensive prescription) | 626 (5.0) | 367 (16.0) | <.001 |
| Monotherapy/combination product | |||
| Monotherapy | 9482 (76.4) | 1681 (73.4) | .002 |
| Combination | 2922 (23.6) | 608 (26.6) | |
| Brand or generic status of product | |||
| Generic | 9350 (75.4) | 1675 (73.2) | .025 |
| Brand | 3054 (24.6) | 614 (26.8) | |
| Tier status | |||
| Tier 1 | 9350 (75.4) | 1675 (73.2) | .074 |
| Tier 2 | 1282 (10.3) | 252 (11.0) | |
| Tier 3 | 1772 (14.3) | 362 (15.8) | |
| Co-payment | |||
| Mean ±SD | $16.60±$20.17 | $19.16±$23.43 | <.001 |
| Type of health plan | |||
| Health maintenance organization | 5574 (44.9) | 1188 (51.9) | <.001 |
| Preferred provider organization | 2372 (19.1) | 426 (18.6) | |
| Consumer directed | 1847 (14.9) | 278 (12.1) | |
| Indemnity | 2611 (21.0) | 397 (17.3) | |
| Number of antihypertensive prescriptions | |||
| Mean ±SD | 1.9 ±1.0 | 1.8 ±1.0 | .001 |
| Therapeutic class | |||
| Angiotensin-converting enzyme inhibitors | 3008 (24.3) | 548 (23.9) | .939 |
| Angiotensin-receptor blockers | 2296 (18.5) | 442 (19.3) | |
| Beta-blockers | 2461 (19.8) | 440 (19.2) | |
| Calcium channel blockers (including combination product with statin) | 2047 (16.5) | 382 (16.7) | |
| Diuretics | 2242 (18.1) | 410 (17.9) | |
| Other: Alpha-adrenergic blocking agents, central alpha-agonists, direct vasodilators, hypotensive agents, peripheral adrenergic inhibitors, renin inhibitors | 350 (2.8) | 67 (2.9) | |
| HTN, hypertension. *Data are presented as n(%) unless otherwise noted. | |||
Discussion
This study used e-prescribing to evaluate nonadherence to the first-fill of an antihypertensive prescription. Our findings that 24.3% of patients did not obtain the first-fill of a medication and that 15.6% of prescriptions remained unclaimed are comparable to those of other research using electronically obtained prescription data.7,8
In a cross-sectional study of 327 African American adults enrolled in a Medicaid managed care plan, the authors reported that 24.9% (433/1742) of antihypertensive prescriptions were unfilled.7 In a study of therapeutically naïve patients, the first-fill failure rate was 17%.8 These patients were less likely to fill their antihypertensive prescriptions if they were prescribed loop diuretics or had a higher prescription co-payment. The median co-payment was $2 higher for prescriptions not obtained, compared with those that were obtained (P<.001). This finding was similar to the $2.56 difference we found for mean co-payment.
Higher co-payment was a strong predictor of decreased adherence in other antihypertensive adherence studies.9,10 In a survey of Medicare patients, the most common reason cited for failing to fill any prescription was that “it would cost too much.”2 Prescribing a less costly agent based on an insurer’s formulary may reduce the first-fill failure rate.
Although educating patients about their disease, involving family, and increasing patient participation through self-monitoring of blood pressure all have a positive impact on adherence rates and blood pressure control, physicians are hard pressed for time during an office visit to address such interventions.11-19 E-prescribing potentially offers a more efficient way to improve antihypertensive medication adherence. A recent ruling by the Centers for Medicare & Medicaid Services (CMS) requires e-prescribing systems to have the capability of providing formulary and benefit transaction, medication history transaction, and fill status notification to prescribers.20 Prescribers can readily access patients’ insurance coverage information. Formulary decision support, as part of an e-prescribing system, has been shown to increase use of formulary products.21 Fill status notification allows two-way communication between the prescriber and pharmacy so that prescribers can be made aware if patients fail to fill prescriptions.
Unfortunately, e-prescribing is not yet widely used. Approximately 26% of office-based US physicians use e-prescribing, and only 30% of them take advantage of formulary information.22 E-prescribing authorities believe practices not using the fill status notification probably lack resources needed to manage patients who are nonadherent.22 While it may not be necessary to check whether all patients have obtained their prescription, it may be useful for subgroups of patients, such as those who have received a new diagnosis, whose disease is poorly controlled, who are prescribed new antihypertensive agents, or who are otherwise thought to be nonadherent.
Study limitations. First, claims data serve as a proxy for medication adherence. Even though a patient obtained an antihypertensive prescription according to claims data, this does not guarantee that the patient used the medication.
Second, not all patients had a diagnosis of hypertension designated by specific ICD-9-CM codes. Patients may have been prescribed antihypertensive medications for other indications, such as heart failure, migraine, anxiety, etc. Our results apply to all patients prescribed antihypertensive agents, and although most had hypertension, there may be differences in first-fill rates for those with and without hypertension.
Third, patients were required to fill their prescriptions through insurance. In community pharmacy settings, some prescriptions may be paid for with cash due to the availability of several inexpensive generic antihypertensive medications (eg, a 30-day supply for $4, or 90-day supply for $10).23 Patients taking advantage of these promotions would result in an overestimation of first-fill failure rate.
Fourth, patients may have received samples from their physician and subsequently failed to obtain their antihypertensive prescription at the pharmacy because the medication sample was ineffective or not tolerated.
Fifth, claims data from physicians who were e-prescribing were used to proxy electronic prescriptions. However, some physicians may have handed patients their prescriptions, which, if never taken to the pharmacy, would result in an underestimation of first-fill failure rate.
CORRESPONDENCE
Catherine E. Cooke, President, PosiHealth, Inc., 5106 Bonnie Branch Road, Ellicott City, MD 21043; [email protected]
1. Sabate E. ed. Adherence to long-term therapies: evidence for action. Geneva, Switzerland. World Health Organization, 20030. Available at: http://www.who.int/chp/knowledge/publications/adherence_report/en. Accessed May 20, 2011.
2. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of Medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553-560.
3. Esposito D, Schone E, Williams T, et al. Prevalence of unclaimed prescriptions at military pharmacies. J Manag Care Pharm. 2008;14:541-552.
4. Wang PS, Benner JS, Glynn RJ, et al. How well do patients report noncompliance with antihypertensive medications? a comparison of self-report versus filled prescriptions. Pharmacoepidemiol Drug Saf. 2004;13:11-19.
5. eHealth Initiative and The Center for Improving Medication Management. A clinician’s guide to electronic prescribing. White paper from the eHealth Initiative and The Center for Improving Medication Management, 2008. Available at: http://www.ehealthinitiative.org/sites/default/files/e-Prescribing_Clinicians_Guide_Final(1).pdf. Accessed March 21, 2010.
6. Solberg LI, Asche SE, Pawlson LG, et al. Practice systems are associated with high-quality care for diabetes. Am J Manag Care. 2008;14:85-92.
7. Lagu T, Weiner MG, Eachus S, et al. Effect of patient comorbidities on filling of antihypertensive prescriptions Am J Manag Care. 2009;15:24-30.
8. Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22:392-396.
9. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61-69.
10. Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff. 2004;23:227-236.
11. Bell RA, Kravitz RL. Physician counseling for hypertension: what do doctors really do? Patient Educ Couns. 2008;72:115-121.
12. Boulware LE, Daumit GL, Frick KD, et al. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21:221-232.
13. Feldman R, Bacher M, Campbell N, et al. Adherence to pharmacologic management of hypertension. Can J Public Health. 1998;89:I16-I18.
14. Fiscella K, Epstein RM. So much to do, so little time: care for the socially disadvantaged and the 15-minute visit. Arch Intern Med. 2008;168:1843-1852.
15. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028-3035.
16. Morisky DE, Levine DM, Green LW, et al. Five-year blood pressure control and mortality following health education for hypertensive patients. Am J Public Health. 1983;73:153-162.
17. Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657-665.
18. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care visits. Health Serv Res. 2007;42:1871-1894.
19. Yarnall KS, Pollak KI, Østbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
20. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; standards for e-prescribing under Medicare Part D and identification of backward compatible version of adopted standard for e-prescribing and the Medicare prescription drug program (version 8.1). Final rule. Fed Regist. 2008;73:18917-18942.
21. Fischer MA, Vogeli C, Stedman M, et al. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med. 2008;168:2433-2439.
22. SureScripts 2009 Progress Report on E-Prescribing. Available at: http://www.surescripts.com/downloads/NPR/national-progress-report.pdf. Accessed March 21, 2010.
23. Walmart. Retail prescription program drug list. Available at: http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed March 21, 2010.
1. Sabate E. ed. Adherence to long-term therapies: evidence for action. Geneva, Switzerland. World Health Organization, 20030. Available at: http://www.who.int/chp/knowledge/publications/adherence_report/en. Accessed May 20, 2011.
2. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of Medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553-560.
3. Esposito D, Schone E, Williams T, et al. Prevalence of unclaimed prescriptions at military pharmacies. J Manag Care Pharm. 2008;14:541-552.
4. Wang PS, Benner JS, Glynn RJ, et al. How well do patients report noncompliance with antihypertensive medications? a comparison of self-report versus filled prescriptions. Pharmacoepidemiol Drug Saf. 2004;13:11-19.
5. eHealth Initiative and The Center for Improving Medication Management. A clinician’s guide to electronic prescribing. White paper from the eHealth Initiative and The Center for Improving Medication Management, 2008. Available at: http://www.ehealthinitiative.org/sites/default/files/e-Prescribing_Clinicians_Guide_Final(1).pdf. Accessed March 21, 2010.
6. Solberg LI, Asche SE, Pawlson LG, et al. Practice systems are associated with high-quality care for diabetes. Am J Manag Care. 2008;14:85-92.
7. Lagu T, Weiner MG, Eachus S, et al. Effect of patient comorbidities on filling of antihypertensive prescriptions Am J Manag Care. 2009;15:24-30.
8. Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22:392-396.
9. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61-69.
10. Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff. 2004;23:227-236.
11. Bell RA, Kravitz RL. Physician counseling for hypertension: what do doctors really do? Patient Educ Couns. 2008;72:115-121.
12. Boulware LE, Daumit GL, Frick KD, et al. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21:221-232.
13. Feldman R, Bacher M, Campbell N, et al. Adherence to pharmacologic management of hypertension. Can J Public Health. 1998;89:I16-I18.
14. Fiscella K, Epstein RM. So much to do, so little time: care for the socially disadvantaged and the 15-minute visit. Arch Intern Med. 2008;168:1843-1852.
15. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028-3035.
16. Morisky DE, Levine DM, Green LW, et al. Five-year blood pressure control and mortality following health education for hypertensive patients. Am J Public Health. 1983;73:153-162.
17. Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657-665.
18. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care visits. Health Serv Res. 2007;42:1871-1894.
19. Yarnall KS, Pollak KI, Østbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
20. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; standards for e-prescribing under Medicare Part D and identification of backward compatible version of adopted standard for e-prescribing and the Medicare prescription drug program (version 8.1). Final rule. Fed Regist. 2008;73:18917-18942.
21. Fischer MA, Vogeli C, Stedman M, et al. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med. 2008;168:2433-2439.
22. SureScripts 2009 Progress Report on E-Prescribing. Available at: http://www.surescripts.com/downloads/NPR/national-progress-report.pdf. Accessed March 21, 2010.
23. Walmart. Retail prescription program drug list. Available at: http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed March 21, 2010.