User login
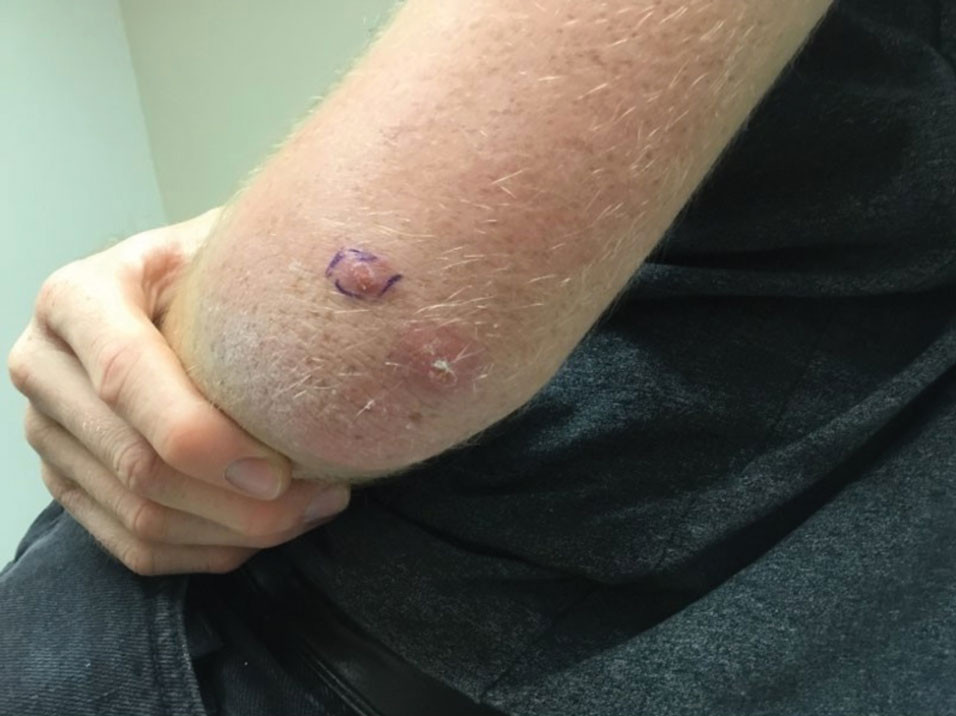
Painful Nodules With a Crawling Sensation
The Diagnosis: Cutaneous Furuncular Myiasis
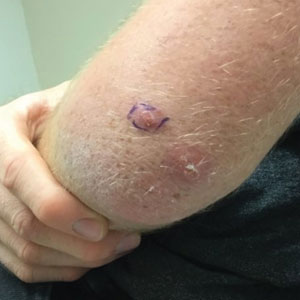
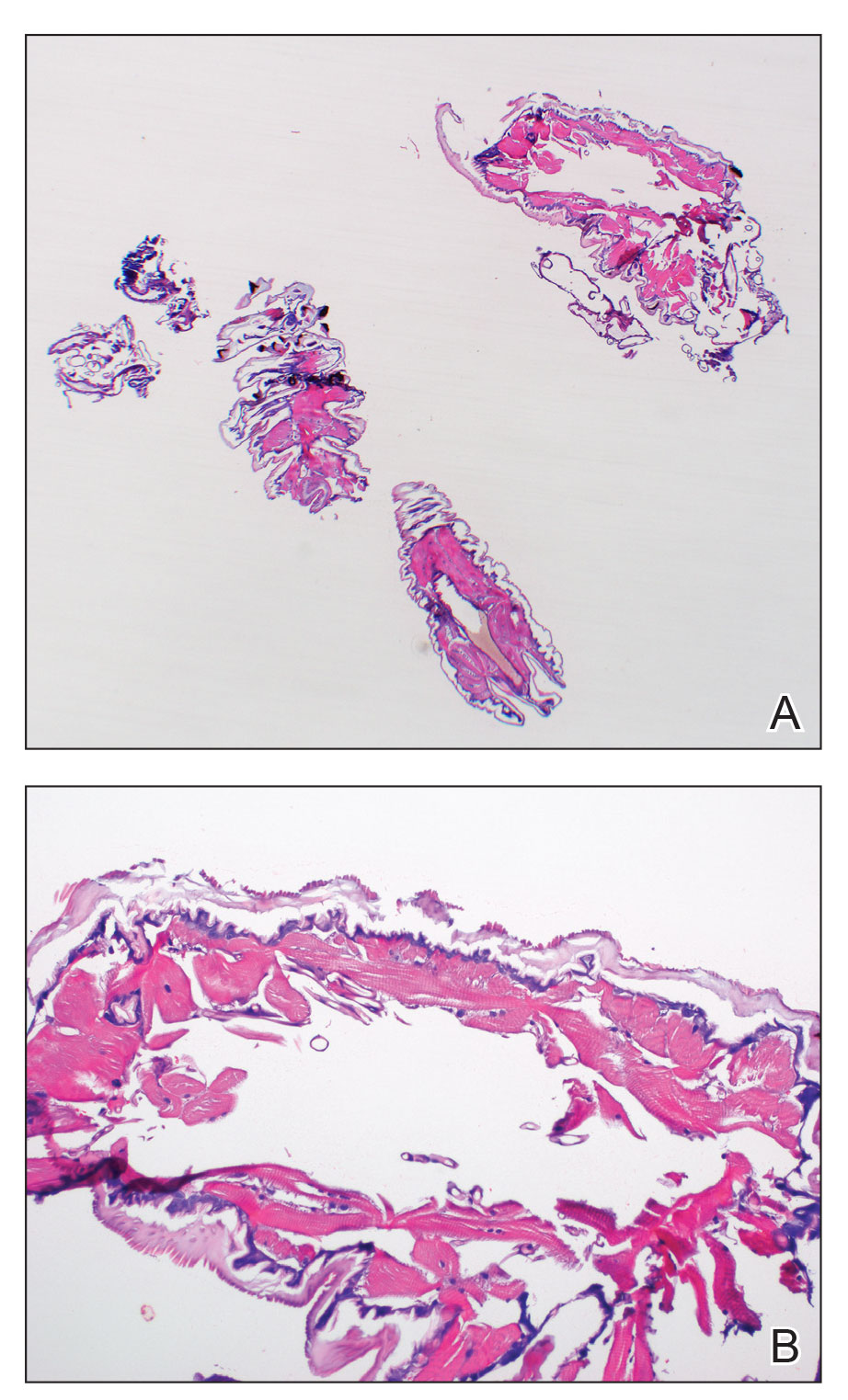
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).
Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4
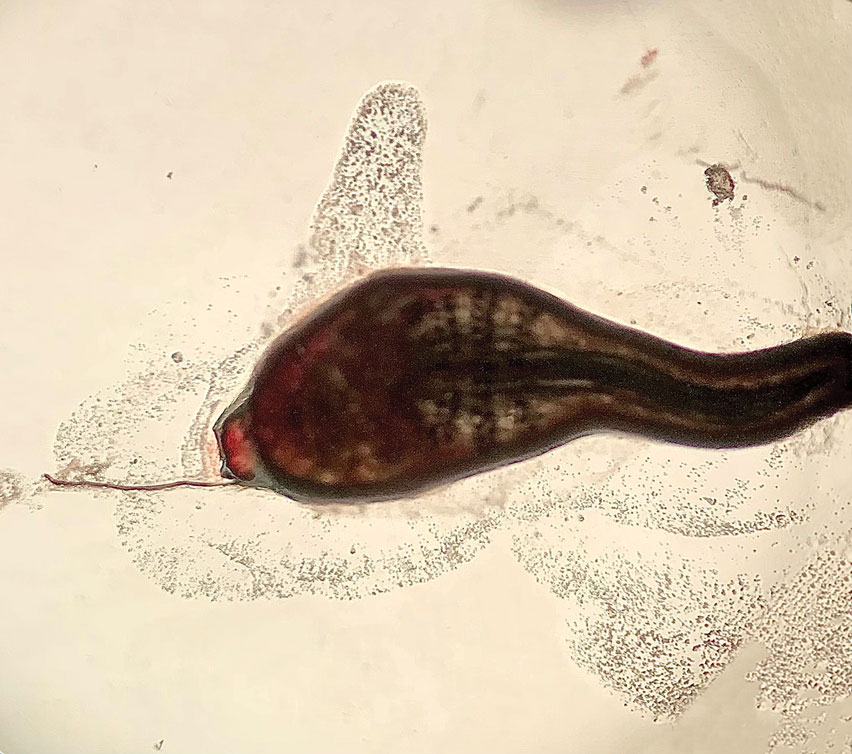
Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
The Diagnosis: Cutaneous Furuncular Myiasis
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).

Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4

Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
The Diagnosis: Cutaneous Furuncular Myiasis
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).

Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4

Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
A 20-year-old man presented with progressively enlarging, painful lesions on the arm with a crawling sensation of 3 weeks’ duration. The lesions appeared after a recent trip to Brazil where he was hiking in the Amazon. He noted that the pain occurred suddenly and there was some serous drainage from the lesions. He denied any trauma to the area and reported no history of similar eruptions, treatments, or systemic symptoms. Physical examination revealed 2 tender erythematous nodules, each measuring 0.6 cm in diameter, with associated crust and a reported crawling sensation on the posterior aspect of the left arm. No drainage was seen. A punch biopsy was performed.
Diagnosis by dog: Canines detect COVID in schoolchildren with no symptoms
Scent-detecting dogs have long been used to sniff out medical conditions ranging from low blood sugar and cancer to malaria, impending seizures, and migraines – not to mention explosives and narcotics.
Recently, the sensitivity of the canine nose has been tested as a strategy for screening for SARS-CoV-2 infection in schoolchildren showing no outward symptoms of the virus. A pilot study led by Carol A. Glaser, DVM, MD, of the California Department of Public Health in Richmond, found that trained dogs had an accuracy of more than 95% for detecting the odor of volatile organic compounds, or VOCs, produced by COVID-infected individuals.
The authors believe that odor-based diagnosis with dogs could eventually provide a rapid, inexpensive, and noninvasive way to screen large groups for COVID-19 without the need for antigen testing.
“This is a new program with research ongoing, so it would be premature to consider it from a consumer’s perspective,” Dr. Glaser said in an interview. “However, the data look promising and we are hopeful we can continue to pilot various programs in various settings to see where, and if, dogs can be used for biomedical detection.”
In the lab and in the field
In a study published online in JAMA Pediatrics, Dr. Glaser’s group found that after 2 months’ training on COVID-19 scent samples in the laboratory, the dogs detected the presence of the virus more than 95% of the time. Antigen tests were used as a comparative reference.
In medical terms, the dogs achieved a greater than 95% accuracy on two important measures of effectiveness: sensitivity – a test’s ability to correctly detect the positive presence of disease – and specificity – the ability of a test to accurately rule out the presence of disease and identify as negative an uninfected person.
Next, the researchers piloted field tests in 50 visits at 27 schools from April 1 to May 25, 2022, to compare dogs’ detection ability with that of standard laboratory antigen testing. Participants in the completely voluntary screening numbered 1,558 and ranged in age from 9 to 17 years. Of these, 56% were girls and 89% were students. Almost 70% were screened at least twice.
Overall, the field test compared 3,897 paired antigen-vs.-dog screenings. The dogs accurately signaled the presence of 85 infections and ruled out 3,411 infections, for an overall accuracy of 90%. In 383 cases, however, they inaccurately signaled the presence of infection (false positives) and missed 18 actual infections (false negatives). That translated to a sensitivity in the field of 83%, considerably lower than that of their lab performance.
Direct screening of individuals with dogs outside of the lab involved circumstantial factors that likely contributed to decreased sensitivity and specificity, the authors acknowledged. These included such distractions as noise and the presence of excitable young children as well environmental conditions such as wind and other odors. What about dog phobia and dog hair allergy? “Dog screening takes only a few seconds per student and the dogs do not generally touch the participant as they run a line and sniff at ankles,” Dr. Glaser explained.
As for allergies, the rapid, ankle-level screening occurred in outdoor settings. “The chance of allergies is very low. This would be similar to someone who is out walking on the sidewalk and walks by a dog,” Dr. Glaser said.
Last year, a British trial of almost 4,000 adults tested six dogs trained to detect differences in VOCs between COVID-infected and uninfected individuals. Given samples from both groups, the dogs were able to distinguish between infected and uninfected samples with a sensitivity for detecting the virus ranging from 82% to 94% and a specificity for ruling it out of 76% to 92%. And they were able to smell the VOCs even when the viral load was low. The study also tested organic sensors, which proved even more accurate than the canines.
According to lead author James G. Logan, PhD, a disease control expert at the London School of Hygiene & Tropical Medicine in London, “Odour-based diagnostics using dogs and/or sensors may prove a rapid and effective tool for screening large numbers of people. Mathematical modelling suggests that dog screening plus a confirmatory PCR test could detect up to 89% of SARS-CoV-2 infections, averting up to 2.2 times as much transmission compared to isolation of symptomatic individuals only.”
Funding was provided by the Centers for Disease Control and Prevention Foundation (CDCF) to Early Alert Canines for the purchase and care of the dogs and the support of the handlers and trainers. The CDCF had no other role in the study. Coauthor Carol A. Edwards of Early Alert Canines reported receiving grants from the CDCF.
Scent-detecting dogs have long been used to sniff out medical conditions ranging from low blood sugar and cancer to malaria, impending seizures, and migraines – not to mention explosives and narcotics.
Recently, the sensitivity of the canine nose has been tested as a strategy for screening for SARS-CoV-2 infection in schoolchildren showing no outward symptoms of the virus. A pilot study led by Carol A. Glaser, DVM, MD, of the California Department of Public Health in Richmond, found that trained dogs had an accuracy of more than 95% for detecting the odor of volatile organic compounds, or VOCs, produced by COVID-infected individuals.
The authors believe that odor-based diagnosis with dogs could eventually provide a rapid, inexpensive, and noninvasive way to screen large groups for COVID-19 without the need for antigen testing.
“This is a new program with research ongoing, so it would be premature to consider it from a consumer’s perspective,” Dr. Glaser said in an interview. “However, the data look promising and we are hopeful we can continue to pilot various programs in various settings to see where, and if, dogs can be used for biomedical detection.”
In the lab and in the field
In a study published online in JAMA Pediatrics, Dr. Glaser’s group found that after 2 months’ training on COVID-19 scent samples in the laboratory, the dogs detected the presence of the virus more than 95% of the time. Antigen tests were used as a comparative reference.
In medical terms, the dogs achieved a greater than 95% accuracy on two important measures of effectiveness: sensitivity – a test’s ability to correctly detect the positive presence of disease – and specificity – the ability of a test to accurately rule out the presence of disease and identify as negative an uninfected person.
Next, the researchers piloted field tests in 50 visits at 27 schools from April 1 to May 25, 2022, to compare dogs’ detection ability with that of standard laboratory antigen testing. Participants in the completely voluntary screening numbered 1,558 and ranged in age from 9 to 17 years. Of these, 56% were girls and 89% were students. Almost 70% were screened at least twice.
Overall, the field test compared 3,897 paired antigen-vs.-dog screenings. The dogs accurately signaled the presence of 85 infections and ruled out 3,411 infections, for an overall accuracy of 90%. In 383 cases, however, they inaccurately signaled the presence of infection (false positives) and missed 18 actual infections (false negatives). That translated to a sensitivity in the field of 83%, considerably lower than that of their lab performance.
Direct screening of individuals with dogs outside of the lab involved circumstantial factors that likely contributed to decreased sensitivity and specificity, the authors acknowledged. These included such distractions as noise and the presence of excitable young children as well environmental conditions such as wind and other odors. What about dog phobia and dog hair allergy? “Dog screening takes only a few seconds per student and the dogs do not generally touch the participant as they run a line and sniff at ankles,” Dr. Glaser explained.
As for allergies, the rapid, ankle-level screening occurred in outdoor settings. “The chance of allergies is very low. This would be similar to someone who is out walking on the sidewalk and walks by a dog,” Dr. Glaser said.
Last year, a British trial of almost 4,000 adults tested six dogs trained to detect differences in VOCs between COVID-infected and uninfected individuals. Given samples from both groups, the dogs were able to distinguish between infected and uninfected samples with a sensitivity for detecting the virus ranging from 82% to 94% and a specificity for ruling it out of 76% to 92%. And they were able to smell the VOCs even when the viral load was low. The study also tested organic sensors, which proved even more accurate than the canines.
According to lead author James G. Logan, PhD, a disease control expert at the London School of Hygiene & Tropical Medicine in London, “Odour-based diagnostics using dogs and/or sensors may prove a rapid and effective tool for screening large numbers of people. Mathematical modelling suggests that dog screening plus a confirmatory PCR test could detect up to 89% of SARS-CoV-2 infections, averting up to 2.2 times as much transmission compared to isolation of symptomatic individuals only.”
Funding was provided by the Centers for Disease Control and Prevention Foundation (CDCF) to Early Alert Canines for the purchase and care of the dogs and the support of the handlers and trainers. The CDCF had no other role in the study. Coauthor Carol A. Edwards of Early Alert Canines reported receiving grants from the CDCF.
Scent-detecting dogs have long been used to sniff out medical conditions ranging from low blood sugar and cancer to malaria, impending seizures, and migraines – not to mention explosives and narcotics.
Recently, the sensitivity of the canine nose has been tested as a strategy for screening for SARS-CoV-2 infection in schoolchildren showing no outward symptoms of the virus. A pilot study led by Carol A. Glaser, DVM, MD, of the California Department of Public Health in Richmond, found that trained dogs had an accuracy of more than 95% for detecting the odor of volatile organic compounds, or VOCs, produced by COVID-infected individuals.
The authors believe that odor-based diagnosis with dogs could eventually provide a rapid, inexpensive, and noninvasive way to screen large groups for COVID-19 without the need for antigen testing.
“This is a new program with research ongoing, so it would be premature to consider it from a consumer’s perspective,” Dr. Glaser said in an interview. “However, the data look promising and we are hopeful we can continue to pilot various programs in various settings to see where, and if, dogs can be used for biomedical detection.”
In the lab and in the field
In a study published online in JAMA Pediatrics, Dr. Glaser’s group found that after 2 months’ training on COVID-19 scent samples in the laboratory, the dogs detected the presence of the virus more than 95% of the time. Antigen tests were used as a comparative reference.
In medical terms, the dogs achieved a greater than 95% accuracy on two important measures of effectiveness: sensitivity – a test’s ability to correctly detect the positive presence of disease – and specificity – the ability of a test to accurately rule out the presence of disease and identify as negative an uninfected person.
Next, the researchers piloted field tests in 50 visits at 27 schools from April 1 to May 25, 2022, to compare dogs’ detection ability with that of standard laboratory antigen testing. Participants in the completely voluntary screening numbered 1,558 and ranged in age from 9 to 17 years. Of these, 56% were girls and 89% were students. Almost 70% were screened at least twice.
Overall, the field test compared 3,897 paired antigen-vs.-dog screenings. The dogs accurately signaled the presence of 85 infections and ruled out 3,411 infections, for an overall accuracy of 90%. In 383 cases, however, they inaccurately signaled the presence of infection (false positives) and missed 18 actual infections (false negatives). That translated to a sensitivity in the field of 83%, considerably lower than that of their lab performance.
Direct screening of individuals with dogs outside of the lab involved circumstantial factors that likely contributed to decreased sensitivity and specificity, the authors acknowledged. These included such distractions as noise and the presence of excitable young children as well environmental conditions such as wind and other odors. What about dog phobia and dog hair allergy? “Dog screening takes only a few seconds per student and the dogs do not generally touch the participant as they run a line and sniff at ankles,” Dr. Glaser explained.
As for allergies, the rapid, ankle-level screening occurred in outdoor settings. “The chance of allergies is very low. This would be similar to someone who is out walking on the sidewalk and walks by a dog,” Dr. Glaser said.
Last year, a British trial of almost 4,000 adults tested six dogs trained to detect differences in VOCs between COVID-infected and uninfected individuals. Given samples from both groups, the dogs were able to distinguish between infected and uninfected samples with a sensitivity for detecting the virus ranging from 82% to 94% and a specificity for ruling it out of 76% to 92%. And they were able to smell the VOCs even when the viral load was low. The study also tested organic sensors, which proved even more accurate than the canines.
According to lead author James G. Logan, PhD, a disease control expert at the London School of Hygiene & Tropical Medicine in London, “Odour-based diagnostics using dogs and/or sensors may prove a rapid and effective tool for screening large numbers of people. Mathematical modelling suggests that dog screening plus a confirmatory PCR test could detect up to 89% of SARS-CoV-2 infections, averting up to 2.2 times as much transmission compared to isolation of symptomatic individuals only.”
Funding was provided by the Centers for Disease Control and Prevention Foundation (CDCF) to Early Alert Canines for the purchase and care of the dogs and the support of the handlers and trainers. The CDCF had no other role in the study. Coauthor Carol A. Edwards of Early Alert Canines reported receiving grants from the CDCF.
FROM JAMA PEDIATRICS
Hybrid ablation superior for persistent AFib: CEASE-AF
BARCELONA – Staged hybrid ablation provided superior freedom from atrial arrhythmias compared with endocardial catheter ablation alone, including the need for repeat ablations in patients with advanced atrial fibrillation (AF), new data show.
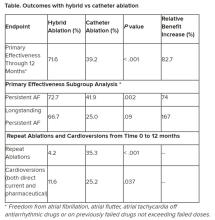
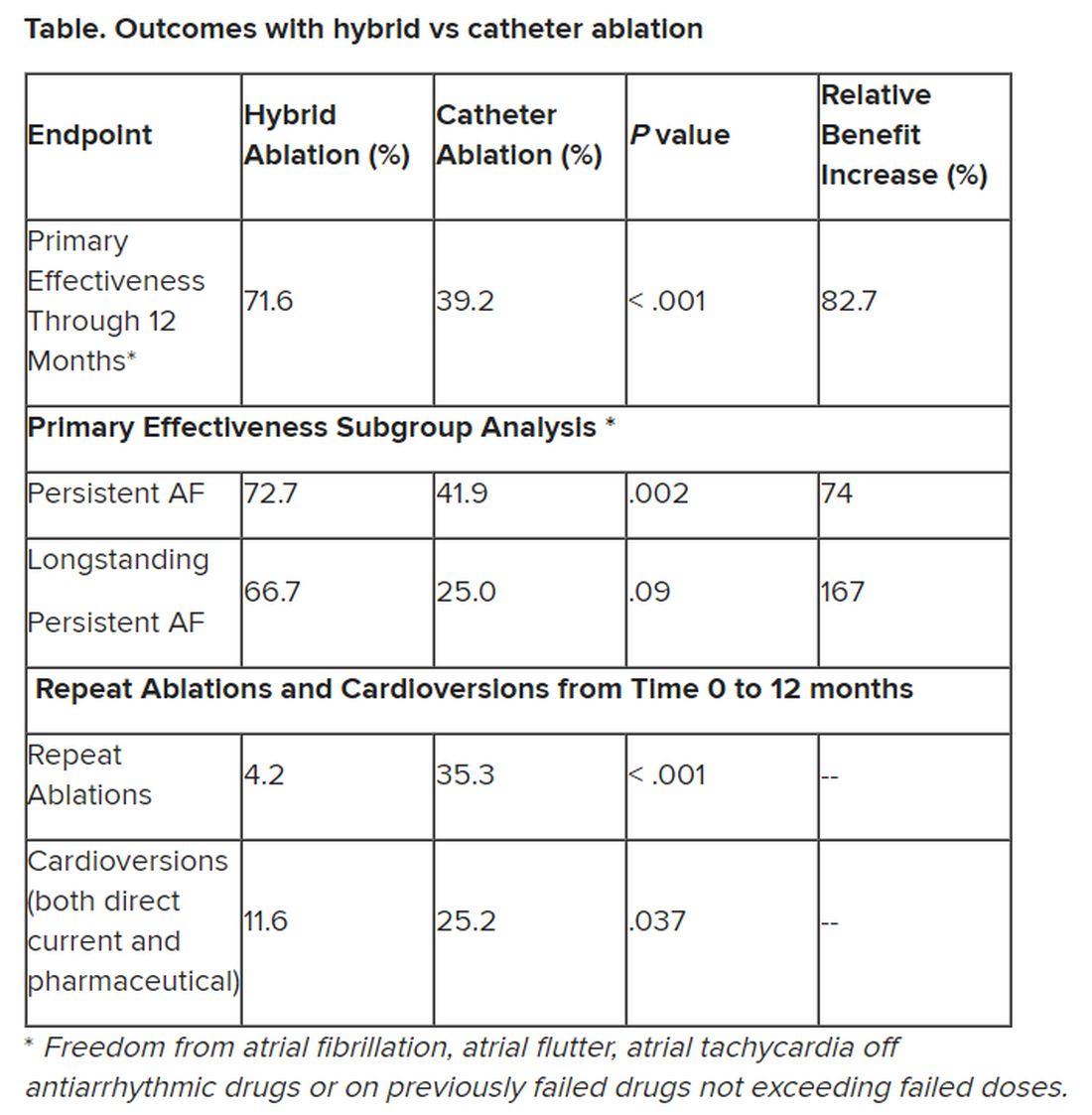
“We have seen that hybrid ablation resulted in 32.4% absolute benefit increase in effectiveness and 83% relative benefit increase, so this is a huge difference,” concluded cardiac surgeon Nicholas Doll, MD, PhD, Schüchtermann Clinic, Bad Rothenfelde, Germany.
Dr. Doll presented the 12-month follow up results of the Combined Endoscopic Epicardial and Percutaneous Endocardial Ablation Versus Repeated Catheter Ablation in Persistent and Longstanding Persistent Atrial Fibrillation (CEASE-AF) trial at the European Heart Rhythm Association 2023 Congress, held recently in Barcelona and virtually.
He said CEASE-AF is the largest multicenter randomized clinical trial comparing these two approaches for control of atrial arrhythmias.
Safety outcomes were numerically higher in the hybrid ablation (HA) group of the trial but not statistically different from the catheter ablation (CA) group.
Unstable wavefront
As background, Dr. Doll explained that in advanced AF, there is a high degree of endocardial-epicardial dissociation with unstable wavefront propagation transitioning between the endocardial and epicardial surfaces. Endocardial mapping and ablation alone may be insufficient to address the mechanism of AF.
“So, the hypothesis of the CEASE-AF study was a minimally invasive hybrid ablation approach which combines endocardial and epicardial ablation to achieve superior effectiveness when compared to endocardial catheter ablation alone,” he said.
This prospective clinical trial randomized patients 2:1 at nine sites in five countries to HA (n = 102) or CA (n = 52). All had left atrial diameter of 4 cm to 6 cm and persistent AF for up to 1 year or longstanding persistent AF for greater than 1 year up to 10 years.
Any patient with a previous ablation procedure, BMI greater than 35 kg/m2, or left ventricular ejection fraction less than 30% was excluded.
For HA, stage 1 consisted of epicardial lesions for pulmonary vein isolation (PVI) plus the posterior wall box plus left atrial appendage exclusion using the AtriClip (AtriCure Inc.) left atrial appendage exclusion device. Stage 2 involved endocardial mapping and catheter ablation to address gaps.
For CA, the index procedure involved catheter-mediated PVI plus repeat endocardial ablation as clinically indicated. For both HA and CA, additional ablation techniques and lesions were allowed for nonparoxysmal AF.
The HA timeline was the first stage, index procedure at time 0 (n = 102), a 90-day blanking period, and then the second stage, endocardial procedure at 90 to 180 days from the index procedure (n = 93).
For the CA arm of the trial, endocardial catheter ablation was performed on a minimal endocardial lesion set at time 0. Then after a 90-day blanking period, repeat catheter ablation was performed if clinically indicated (6/52).
Repeat ablations and electrical or pharmaceutical cardioversions were allowed during the 12-month follow-up period from time 0.
The primary efficacy endpoint was freedom from AF, atrial flutter, or atrial tachycardia of greater than 30 seconds through 12 months in the absence of class I/III antiarrhythmic drugs except ones that previously had failed, at doses not exceeding those previously failed doses. The safety endpoint was a composite rate of major complications over the course of the study.
Even with relatively modest cohort sizes, the HA and CA arms of the trial were well matched at baseline for age (approximately 60 years), gender (75.5% and 73.1% male, respectively), BMI (29.7 and 29.8 kg/m2), and persistent AF (79.4% and 82.7%).
The groups had persistent AF for 2.94 ± 3.29 years and 3.34 ± 3.52 years, respectively. The mean left atrial size was 4.7 ± 0.5 cm for the HA group and 4.7 ± 0.4 cm for the CA group.
Outcomes favored hybrid ablation over catheter ablation, the researchers reported. “We never would have expected these huge differences,” Dr. Doll told the congress. “We have seen that hybrid ablation resulted in 32.4% absolute benefit increase in effectiveness and 83% relative benefit increase.”
Subgroup analyses were consistent with the primary endpoint, but he said they would not be published because the trial was not powered for such comparisons.
Still, he noted that “there are only slightly reduced outcomes in the long-standing [persistent AF subgroup] in a really challenging patient arm, and we still have a success rate of 67%.” And the repeat ablations in about one-third of patients in the CA arm and need for cardioversions in about one quarter of them may have implications for reduced quality of life.
The total procedure duration was higher for the hybrid group at 336.4 ± 97 minutes, taking into account the index procedure plus the second stage procedure, vs. endocardial ablation at 251.9 ± 114 minutes, which includes the index procedure plus any repeat ablations (HA vs AF total duration, P < .001). Overall fluoroscopy time was approximately 8 minutes shorter for the HA arm.
Complications were assessed for 30 days post index procedure and 30 days post second stage procedure for the HA arm and for 30 days post index procedure and any repeat ablation for the CA arm.
The HA arm showed a complication rate of 7.8% vs. 5.8% for the CA arm (P = .751). Two patients in the former and three patients in the latter group had more than one major complication. There was one death in the HA group 93 days after the index procedure, and it was adjudicated as unrelated to the procedure.
“If you look back in the past, other studies showed a ... higher complication rate in the hybrid arm, so we feel very comfortable with these complication rates, which [are] very low and almost comparable,” Dr. Doll said.
Limitations of the study included symptom-driven electrocardiogram monitoring performed at unscheduled visits. Also, ablation beyond PVI in the CA arm and PVI/posterior box in the HA arm was not standardized and was performed according to standard practices in the participating countries.
“Success of epicardial-endocardial approach emphasizes the role of the collaborative heart team approach in the treatment of nonparoxysmal atrial fibrillation, and if I sum it up together, we can do it better” together, Dr. Doll advised.
‘Exceptional’ trial
After Dr. Doll’s presentation, appointed discussant Stylianos Tzeis, MD, PhD, head of the cardiology clinic and electrophysiology and pacing department at Mitera Hospital in Athens, congratulated the investigators and called CEASE-AF “an exceptional trial. It was really challenging to enroll patients in such a randomized controlled clinical trial.”
But Dr. Tzeis questioned whether pitting CA against HA was a fair comparison.
“Were the ablation targets similar between the two groups?” he asked. He noted that for the HA group, in the first stage the patients had PVI, posterior wall isolation, exclusion of the left atrial appendage, and additional lesions at the discretion of the operator. Ninety percent proceeded to the second stage, which was endocardial catheter ablation with verification of posterior wall isolation and PVI and additional lesions made if needed.
In the CA group, repeat catheter ablation could be performed after the 90-day blanking period if clinically indicated. “Please take note that only 10% were offered the second ablation. So at least in my perspective, this was a comparison of a two-stage approach versus a single-stage approach with a much more aggressive ablation protocol in the hybrid ablation group as compared to the endocardial group,” he said.
Seeing the higher success rate of the HA group in achieving the primary efficacy endpoint of freedom from all arrhythmias at 12 months, Dr. Tzeis asked, “Does this reflect the superiority of the epi-endo approach, or does it reflect the suboptimal performance of the catheter ablation approach?”
There was a 40% success rate in the CA patient population, a cohort that he deemed “not the most challenging persistent AF population in the world”: those with left atrial diameter of 47 millimeters and with 80% having an AF duration less than 12 months.
He also noted that “the average duration of the catheter ablation for the PVI in the vast majority of cases was 4 hours, which does not reflect what really happens in the everyday practice.”
All those critiques having been advanced, Dr. Tzeis said, “Definitely do not doubt my first comment that the authors should be congratulated, and I strongly believe that the main objective has been achieved to bring electrophysiologist and cardiac surgeons ... closer.”
The study sponsor was AtriCure Inc. with collaboration of Cardialysis BV. Doll has received consulting fees or royalties and/or has ownership or stockholder interest in AtriCure. Tzeis reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BARCELONA – Staged hybrid ablation provided superior freedom from atrial arrhythmias compared with endocardial catheter ablation alone, including the need for repeat ablations in patients with advanced atrial fibrillation (AF), new data show.
“We have seen that hybrid ablation resulted in 32.4% absolute benefit increase in effectiveness and 83% relative benefit increase, so this is a huge difference,” concluded cardiac surgeon Nicholas Doll, MD, PhD, Schüchtermann Clinic, Bad Rothenfelde, Germany.
Dr. Doll presented the 12-month follow up results of the Combined Endoscopic Epicardial and Percutaneous Endocardial Ablation Versus Repeated Catheter Ablation in Persistent and Longstanding Persistent Atrial Fibrillation (CEASE-AF) trial at the European Heart Rhythm Association 2023 Congress, held recently in Barcelona and virtually.
He said CEASE-AF is the largest multicenter randomized clinical trial comparing these two approaches for control of atrial arrhythmias.
Safety outcomes were numerically higher in the hybrid ablation (HA) group of the trial but not statistically different from the catheter ablation (CA) group.
Unstable wavefront
As background, Dr. Doll explained that in advanced AF, there is a high degree of endocardial-epicardial dissociation with unstable wavefront propagation transitioning between the endocardial and epicardial surfaces. Endocardial mapping and ablation alone may be insufficient to address the mechanism of AF.
“So, the hypothesis of the CEASE-AF study was a minimally invasive hybrid ablation approach which combines endocardial and epicardial ablation to achieve superior effectiveness when compared to endocardial catheter ablation alone,” he said.
This prospective clinical trial randomized patients 2:1 at nine sites in five countries to HA (n = 102) or CA (n = 52). All had left atrial diameter of 4 cm to 6 cm and persistent AF for up to 1 year or longstanding persistent AF for greater than 1 year up to 10 years.
Any patient with a previous ablation procedure, BMI greater than 35 kg/m2, or left ventricular ejection fraction less than 30% was excluded.
For HA, stage 1 consisted of epicardial lesions for pulmonary vein isolation (PVI) plus the posterior wall box plus left atrial appendage exclusion using the AtriClip (AtriCure Inc.) left atrial appendage exclusion device. Stage 2 involved endocardial mapping and catheter ablation to address gaps.
For CA, the index procedure involved catheter-mediated PVI plus repeat endocardial ablation as clinically indicated. For both HA and CA, additional ablation techniques and lesions were allowed for nonparoxysmal AF.
The HA timeline was the first stage, index procedure at time 0 (n = 102), a 90-day blanking period, and then the second stage, endocardial procedure at 90 to 180 days from the index procedure (n = 93).
For the CA arm of the trial, endocardial catheter ablation was performed on a minimal endocardial lesion set at time 0. Then after a 90-day blanking period, repeat catheter ablation was performed if clinically indicated (6/52).
Repeat ablations and electrical or pharmaceutical cardioversions were allowed during the 12-month follow-up period from time 0.
The primary efficacy endpoint was freedom from AF, atrial flutter, or atrial tachycardia of greater than 30 seconds through 12 months in the absence of class I/III antiarrhythmic drugs except ones that previously had failed, at doses not exceeding those previously failed doses. The safety endpoint was a composite rate of major complications over the course of the study.
Even with relatively modest cohort sizes, the HA and CA arms of the trial were well matched at baseline for age (approximately 60 years), gender (75.5% and 73.1% male, respectively), BMI (29.7 and 29.8 kg/m2), and persistent AF (79.4% and 82.7%).
The groups had persistent AF for 2.94 ± 3.29 years and 3.34 ± 3.52 years, respectively. The mean left atrial size was 4.7 ± 0.5 cm for the HA group and 4.7 ± 0.4 cm for the CA group.
Outcomes favored hybrid ablation over catheter ablation, the researchers reported. “We never would have expected these huge differences,” Dr. Doll told the congress. “We have seen that hybrid ablation resulted in 32.4% absolute benefit increase in effectiveness and 83% relative benefit increase.”
Subgroup analyses were consistent with the primary endpoint, but he said they would not be published because the trial was not powered for such comparisons.
Still, he noted that “there are only slightly reduced outcomes in the long-standing [persistent AF subgroup] in a really challenging patient arm, and we still have a success rate of 67%.” And the repeat ablations in about one-third of patients in the CA arm and need for cardioversions in about one quarter of them may have implications for reduced quality of life.
The total procedure duration was higher for the hybrid group at 336.4 ± 97 minutes, taking into account the index procedure plus the second stage procedure, vs. endocardial ablation at 251.9 ± 114 minutes, which includes the index procedure plus any repeat ablations (HA vs AF total duration, P < .001). Overall fluoroscopy time was approximately 8 minutes shorter for the HA arm.
Complications were assessed for 30 days post index procedure and 30 days post second stage procedure for the HA arm and for 30 days post index procedure and any repeat ablation for the CA arm.
The HA arm showed a complication rate of 7.8% vs. 5.8% for the CA arm (P = .751). Two patients in the former and three patients in the latter group had more than one major complication. There was one death in the HA group 93 days after the index procedure, and it was adjudicated as unrelated to the procedure.
“If you look back in the past, other studies showed a ... higher complication rate in the hybrid arm, so we feel very comfortable with these complication rates, which [are] very low and almost comparable,” Dr. Doll said.
Limitations of the study included symptom-driven electrocardiogram monitoring performed at unscheduled visits. Also, ablation beyond PVI in the CA arm and PVI/posterior box in the HA arm was not standardized and was performed according to standard practices in the participating countries.
“Success of epicardial-endocardial approach emphasizes the role of the collaborative heart team approach in the treatment of nonparoxysmal atrial fibrillation, and if I sum it up together, we can do it better” together, Dr. Doll advised.
‘Exceptional’ trial
After Dr. Doll’s presentation, appointed discussant Stylianos Tzeis, MD, PhD, head of the cardiology clinic and electrophysiology and pacing department at Mitera Hospital in Athens, congratulated the investigators and called CEASE-AF “an exceptional trial. It was really challenging to enroll patients in such a randomized controlled clinical trial.”
But Dr. Tzeis questioned whether pitting CA against HA was a fair comparison.
“Were the ablation targets similar between the two groups?” he asked. He noted that for the HA group, in the first stage the patients had PVI, posterior wall isolation, exclusion of the left atrial appendage, and additional lesions at the discretion of the operator. Ninety percent proceeded to the second stage, which was endocardial catheter ablation with verification of posterior wall isolation and PVI and additional lesions made if needed.
In the CA group, repeat catheter ablation could be performed after the 90-day blanking period if clinically indicated. “Please take note that only 10% were offered the second ablation. So at least in my perspective, this was a comparison of a two-stage approach versus a single-stage approach with a much more aggressive ablation protocol in the hybrid ablation group as compared to the endocardial group,” he said.
Seeing the higher success rate of the HA group in achieving the primary efficacy endpoint of freedom from all arrhythmias at 12 months, Dr. Tzeis asked, “Does this reflect the superiority of the epi-endo approach, or does it reflect the suboptimal performance of the catheter ablation approach?”
There was a 40% success rate in the CA patient population, a cohort that he deemed “not the most challenging persistent AF population in the world”: those with left atrial diameter of 47 millimeters and with 80% having an AF duration less than 12 months.
He also noted that “the average duration of the catheter ablation for the PVI in the vast majority of cases was 4 hours, which does not reflect what really happens in the everyday practice.”
All those critiques having been advanced, Dr. Tzeis said, “Definitely do not doubt my first comment that the authors should be congratulated, and I strongly believe that the main objective has been achieved to bring electrophysiologist and cardiac surgeons ... closer.”
The study sponsor was AtriCure Inc. with collaboration of Cardialysis BV. Doll has received consulting fees or royalties and/or has ownership or stockholder interest in AtriCure. Tzeis reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BARCELONA – Staged hybrid ablation provided superior freedom from atrial arrhythmias compared with endocardial catheter ablation alone, including the need for repeat ablations in patients with advanced atrial fibrillation (AF), new data show.
“We have seen that hybrid ablation resulted in 32.4% absolute benefit increase in effectiveness and 83% relative benefit increase, so this is a huge difference,” concluded cardiac surgeon Nicholas Doll, MD, PhD, Schüchtermann Clinic, Bad Rothenfelde, Germany.
Dr. Doll presented the 12-month follow up results of the Combined Endoscopic Epicardial and Percutaneous Endocardial Ablation Versus Repeated Catheter Ablation in Persistent and Longstanding Persistent Atrial Fibrillation (CEASE-AF) trial at the European Heart Rhythm Association 2023 Congress, held recently in Barcelona and virtually.
He said CEASE-AF is the largest multicenter randomized clinical trial comparing these two approaches for control of atrial arrhythmias.
Safety outcomes were numerically higher in the hybrid ablation (HA) group of the trial but not statistically different from the catheter ablation (CA) group.
Unstable wavefront
As background, Dr. Doll explained that in advanced AF, there is a high degree of endocardial-epicardial dissociation with unstable wavefront propagation transitioning between the endocardial and epicardial surfaces. Endocardial mapping and ablation alone may be insufficient to address the mechanism of AF.
“So, the hypothesis of the CEASE-AF study was a minimally invasive hybrid ablation approach which combines endocardial and epicardial ablation to achieve superior effectiveness when compared to endocardial catheter ablation alone,” he said.
This prospective clinical trial randomized patients 2:1 at nine sites in five countries to HA (n = 102) or CA (n = 52). All had left atrial diameter of 4 cm to 6 cm and persistent AF for up to 1 year or longstanding persistent AF for greater than 1 year up to 10 years.
Any patient with a previous ablation procedure, BMI greater than 35 kg/m2, or left ventricular ejection fraction less than 30% was excluded.
For HA, stage 1 consisted of epicardial lesions for pulmonary vein isolation (PVI) plus the posterior wall box plus left atrial appendage exclusion using the AtriClip (AtriCure Inc.) left atrial appendage exclusion device. Stage 2 involved endocardial mapping and catheter ablation to address gaps.
For CA, the index procedure involved catheter-mediated PVI plus repeat endocardial ablation as clinically indicated. For both HA and CA, additional ablation techniques and lesions were allowed for nonparoxysmal AF.
The HA timeline was the first stage, index procedure at time 0 (n = 102), a 90-day blanking period, and then the second stage, endocardial procedure at 90 to 180 days from the index procedure (n = 93).
For the CA arm of the trial, endocardial catheter ablation was performed on a minimal endocardial lesion set at time 0. Then after a 90-day blanking period, repeat catheter ablation was performed if clinically indicated (6/52).
Repeat ablations and electrical or pharmaceutical cardioversions were allowed during the 12-month follow-up period from time 0.
The primary efficacy endpoint was freedom from AF, atrial flutter, or atrial tachycardia of greater than 30 seconds through 12 months in the absence of class I/III antiarrhythmic drugs except ones that previously had failed, at doses not exceeding those previously failed doses. The safety endpoint was a composite rate of major complications over the course of the study.
Even with relatively modest cohort sizes, the HA and CA arms of the trial were well matched at baseline for age (approximately 60 years), gender (75.5% and 73.1% male, respectively), BMI (29.7 and 29.8 kg/m2), and persistent AF (79.4% and 82.7%).
The groups had persistent AF for 2.94 ± 3.29 years and 3.34 ± 3.52 years, respectively. The mean left atrial size was 4.7 ± 0.5 cm for the HA group and 4.7 ± 0.4 cm for the CA group.
Outcomes favored hybrid ablation over catheter ablation, the researchers reported. “We never would have expected these huge differences,” Dr. Doll told the congress. “We have seen that hybrid ablation resulted in 32.4% absolute benefit increase in effectiveness and 83% relative benefit increase.”
Subgroup analyses were consistent with the primary endpoint, but he said they would not be published because the trial was not powered for such comparisons.
Still, he noted that “there are only slightly reduced outcomes in the long-standing [persistent AF subgroup] in a really challenging patient arm, and we still have a success rate of 67%.” And the repeat ablations in about one-third of patients in the CA arm and need for cardioversions in about one quarter of them may have implications for reduced quality of life.
The total procedure duration was higher for the hybrid group at 336.4 ± 97 minutes, taking into account the index procedure plus the second stage procedure, vs. endocardial ablation at 251.9 ± 114 minutes, which includes the index procedure plus any repeat ablations (HA vs AF total duration, P < .001). Overall fluoroscopy time was approximately 8 minutes shorter for the HA arm.
Complications were assessed for 30 days post index procedure and 30 days post second stage procedure for the HA arm and for 30 days post index procedure and any repeat ablation for the CA arm.
The HA arm showed a complication rate of 7.8% vs. 5.8% for the CA arm (P = .751). Two patients in the former and three patients in the latter group had more than one major complication. There was one death in the HA group 93 days after the index procedure, and it was adjudicated as unrelated to the procedure.
“If you look back in the past, other studies showed a ... higher complication rate in the hybrid arm, so we feel very comfortable with these complication rates, which [are] very low and almost comparable,” Dr. Doll said.
Limitations of the study included symptom-driven electrocardiogram monitoring performed at unscheduled visits. Also, ablation beyond PVI in the CA arm and PVI/posterior box in the HA arm was not standardized and was performed according to standard practices in the participating countries.
“Success of epicardial-endocardial approach emphasizes the role of the collaborative heart team approach in the treatment of nonparoxysmal atrial fibrillation, and if I sum it up together, we can do it better” together, Dr. Doll advised.
‘Exceptional’ trial
After Dr. Doll’s presentation, appointed discussant Stylianos Tzeis, MD, PhD, head of the cardiology clinic and electrophysiology and pacing department at Mitera Hospital in Athens, congratulated the investigators and called CEASE-AF “an exceptional trial. It was really challenging to enroll patients in such a randomized controlled clinical trial.”
But Dr. Tzeis questioned whether pitting CA against HA was a fair comparison.
“Were the ablation targets similar between the two groups?” he asked. He noted that for the HA group, in the first stage the patients had PVI, posterior wall isolation, exclusion of the left atrial appendage, and additional lesions at the discretion of the operator. Ninety percent proceeded to the second stage, which was endocardial catheter ablation with verification of posterior wall isolation and PVI and additional lesions made if needed.
In the CA group, repeat catheter ablation could be performed after the 90-day blanking period if clinically indicated. “Please take note that only 10% were offered the second ablation. So at least in my perspective, this was a comparison of a two-stage approach versus a single-stage approach with a much more aggressive ablation protocol in the hybrid ablation group as compared to the endocardial group,” he said.
Seeing the higher success rate of the HA group in achieving the primary efficacy endpoint of freedom from all arrhythmias at 12 months, Dr. Tzeis asked, “Does this reflect the superiority of the epi-endo approach, or does it reflect the suboptimal performance of the catheter ablation approach?”
There was a 40% success rate in the CA patient population, a cohort that he deemed “not the most challenging persistent AF population in the world”: those with left atrial diameter of 47 millimeters and with 80% having an AF duration less than 12 months.
He also noted that “the average duration of the catheter ablation for the PVI in the vast majority of cases was 4 hours, which does not reflect what really happens in the everyday practice.”
All those critiques having been advanced, Dr. Tzeis said, “Definitely do not doubt my first comment that the authors should be congratulated, and I strongly believe that the main objective has been achieved to bring electrophysiologist and cardiac surgeons ... closer.”
The study sponsor was AtriCure Inc. with collaboration of Cardialysis BV. Doll has received consulting fees or royalties and/or has ownership or stockholder interest in AtriCure. Tzeis reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT EHRA 2023
Motixafortide may improve MM outcomes
Motixafortide, a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity , appears to increase the number of stem cells that can be harvested from transplant candidates, thereby increasing the likelihood of successful transplant, the authors reported.
An application for a new drug approval is currently under review by the Food and Drug Administration.
In the prospective, international, phase 3 GENESIS clinical trial , motixafortide plus granulocyte colony-stimulating factor (G-CSF) – the standard therapy for mobilizing stem cells – significantly increased the number stem cells harvested, when compared with standard therapy plus placebo. After one collection procedure, the combination approach allowed for harvesting of an optimal number of cells in 88% versus 9% of patients who received G-CSF plus placebo. After two collections, optimal collection occurred in 92% versus 26% of patients in the groups, respectively, first author Zachary D. Crees, MD, and colleagues found.
Motixafortide plus G-CSF was also associated with a tenfold increase in the number of primitive stem cells that could be collected. These stem cells are particularly effective for reconstituting red blood cells, white blood cells, and platelets, which all are important for patients’ recovery, they noted.
Stem cells mobilized by motixafortide were also associated with increased expression of genes and genetic pathways involved in self-renewal and regeneration, which are also of benefit for increasing the effectiveness of stem cell transplantation.
The findings were published in Nature Medicine.
“Stem cell transplantation is central to the treatment of multiple myeloma, but some patients don’t see as much benefit because standard therapies can’t harvest enough stem cells for the transplant to be effective, senior author John F. DiPersio, MD, PhD, stated in a news release . “This study suggests motixafortide works extremely well in combination with [G-CSF] in mobilizing stem cells in patients with multiple myeloma.
“The study also found that the combination worked rapidly and was generally well tolerated by patients,” added Dr. DiPersio, the Virginia E. & Sam J. Goldman Professor of Medicine at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University.
Dr. DiPersio is the lead author of another study investigating therapies beyond stem cell transplants. He and his colleagues recently reported the first comprehensive genomic and protein-based analysis of bone marrow samples from patients with multiple myeloma in an effort to identify targets for immunotherapies.
That study, published online in Cancer Research, identified 53 genes that could be targets, including 38 that are responsible for creating abnormal proteins on the surface of multiple myeloma cells; 11 of the 38 had not been previously identified as potential targets.
Dr. DiPersio and Dr. Crees, an assistant professor of medicine and the assistant clinical director of the Washington University Center for Gene and Cellular Immunotherapy, are also evaluating motixafortide’s potential for mobilizing stem cells to support “the genetic correction of the inherited disease sickle cell anemia.”
“This work is of particular importance because patients with sickle cell disease can’t be treated with G-CSF … due to dangerous side effects,” the news release stated. “The hope is that development of a novel, effective, and well-tolerated stem cell mobilizing regimen for a viral-based gene therapy approach using CRISPR-based gene editing will lead to improved outcomes for patients with sickle cell disease.”
The study published in Nature Medicine was supported by the National Institutes of Health and BioLineRx, which makes motixafortide. The study published in Cancer Research was supported by the Paula C. And Rodger O. Riney Blood Cancer Research Fund and the National Cancer Institute.
Dr. Crees reported research funding from BioLineRx. Dr. DiPersio reported relationships with Magenta Therapeutics, WUGEN, Incyte, RiverVest Venture Partners, Cellworks Group, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, and BioLineRx.
Correction, 4/26/23: The headline on an earlier version of this article mischaracterized the study findings.
Motixafortide, a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity , appears to increase the number of stem cells that can be harvested from transplant candidates, thereby increasing the likelihood of successful transplant, the authors reported.
An application for a new drug approval is currently under review by the Food and Drug Administration.
In the prospective, international, phase 3 GENESIS clinical trial , motixafortide plus granulocyte colony-stimulating factor (G-CSF) – the standard therapy for mobilizing stem cells – significantly increased the number stem cells harvested, when compared with standard therapy plus placebo. After one collection procedure, the combination approach allowed for harvesting of an optimal number of cells in 88% versus 9% of patients who received G-CSF plus placebo. After two collections, optimal collection occurred in 92% versus 26% of patients in the groups, respectively, first author Zachary D. Crees, MD, and colleagues found.
Motixafortide plus G-CSF was also associated with a tenfold increase in the number of primitive stem cells that could be collected. These stem cells are particularly effective for reconstituting red blood cells, white blood cells, and platelets, which all are important for patients’ recovery, they noted.
Stem cells mobilized by motixafortide were also associated with increased expression of genes and genetic pathways involved in self-renewal and regeneration, which are also of benefit for increasing the effectiveness of stem cell transplantation.
The findings were published in Nature Medicine.
“Stem cell transplantation is central to the treatment of multiple myeloma, but some patients don’t see as much benefit because standard therapies can’t harvest enough stem cells for the transplant to be effective, senior author John F. DiPersio, MD, PhD, stated in a news release . “This study suggests motixafortide works extremely well in combination with [G-CSF] in mobilizing stem cells in patients with multiple myeloma.
“The study also found that the combination worked rapidly and was generally well tolerated by patients,” added Dr. DiPersio, the Virginia E. & Sam J. Goldman Professor of Medicine at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University.
Dr. DiPersio is the lead author of another study investigating therapies beyond stem cell transplants. He and his colleagues recently reported the first comprehensive genomic and protein-based analysis of bone marrow samples from patients with multiple myeloma in an effort to identify targets for immunotherapies.
That study, published online in Cancer Research, identified 53 genes that could be targets, including 38 that are responsible for creating abnormal proteins on the surface of multiple myeloma cells; 11 of the 38 had not been previously identified as potential targets.
Dr. DiPersio and Dr. Crees, an assistant professor of medicine and the assistant clinical director of the Washington University Center for Gene and Cellular Immunotherapy, are also evaluating motixafortide’s potential for mobilizing stem cells to support “the genetic correction of the inherited disease sickle cell anemia.”
“This work is of particular importance because patients with sickle cell disease can’t be treated with G-CSF … due to dangerous side effects,” the news release stated. “The hope is that development of a novel, effective, and well-tolerated stem cell mobilizing regimen for a viral-based gene therapy approach using CRISPR-based gene editing will lead to improved outcomes for patients with sickle cell disease.”
The study published in Nature Medicine was supported by the National Institutes of Health and BioLineRx, which makes motixafortide. The study published in Cancer Research was supported by the Paula C. And Rodger O. Riney Blood Cancer Research Fund and the National Cancer Institute.
Dr. Crees reported research funding from BioLineRx. Dr. DiPersio reported relationships with Magenta Therapeutics, WUGEN, Incyte, RiverVest Venture Partners, Cellworks Group, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, and BioLineRx.
Correction, 4/26/23: The headline on an earlier version of this article mischaracterized the study findings.
Motixafortide, a novel cyclic-peptide CXCR4 inhibitor with extended in vivo activity , appears to increase the number of stem cells that can be harvested from transplant candidates, thereby increasing the likelihood of successful transplant, the authors reported.
An application for a new drug approval is currently under review by the Food and Drug Administration.
In the prospective, international, phase 3 GENESIS clinical trial , motixafortide plus granulocyte colony-stimulating factor (G-CSF) – the standard therapy for mobilizing stem cells – significantly increased the number stem cells harvested, when compared with standard therapy plus placebo. After one collection procedure, the combination approach allowed for harvesting of an optimal number of cells in 88% versus 9% of patients who received G-CSF plus placebo. After two collections, optimal collection occurred in 92% versus 26% of patients in the groups, respectively, first author Zachary D. Crees, MD, and colleagues found.
Motixafortide plus G-CSF was also associated with a tenfold increase in the number of primitive stem cells that could be collected. These stem cells are particularly effective for reconstituting red blood cells, white blood cells, and platelets, which all are important for patients’ recovery, they noted.
Stem cells mobilized by motixafortide were also associated with increased expression of genes and genetic pathways involved in self-renewal and regeneration, which are also of benefit for increasing the effectiveness of stem cell transplantation.
The findings were published in Nature Medicine.
“Stem cell transplantation is central to the treatment of multiple myeloma, but some patients don’t see as much benefit because standard therapies can’t harvest enough stem cells for the transplant to be effective, senior author John F. DiPersio, MD, PhD, stated in a news release . “This study suggests motixafortide works extremely well in combination with [G-CSF] in mobilizing stem cells in patients with multiple myeloma.
“The study also found that the combination worked rapidly and was generally well tolerated by patients,” added Dr. DiPersio, the Virginia E. & Sam J. Goldman Professor of Medicine at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University.
Dr. DiPersio is the lead author of another study investigating therapies beyond stem cell transplants. He and his colleagues recently reported the first comprehensive genomic and protein-based analysis of bone marrow samples from patients with multiple myeloma in an effort to identify targets for immunotherapies.
That study, published online in Cancer Research, identified 53 genes that could be targets, including 38 that are responsible for creating abnormal proteins on the surface of multiple myeloma cells; 11 of the 38 had not been previously identified as potential targets.
Dr. DiPersio and Dr. Crees, an assistant professor of medicine and the assistant clinical director of the Washington University Center for Gene and Cellular Immunotherapy, are also evaluating motixafortide’s potential for mobilizing stem cells to support “the genetic correction of the inherited disease sickle cell anemia.”
“This work is of particular importance because patients with sickle cell disease can’t be treated with G-CSF … due to dangerous side effects,” the news release stated. “The hope is that development of a novel, effective, and well-tolerated stem cell mobilizing regimen for a viral-based gene therapy approach using CRISPR-based gene editing will lead to improved outcomes for patients with sickle cell disease.”
The study published in Nature Medicine was supported by the National Institutes of Health and BioLineRx, which makes motixafortide. The study published in Cancer Research was supported by the Paula C. And Rodger O. Riney Blood Cancer Research Fund and the National Cancer Institute.
Dr. Crees reported research funding from BioLineRx. Dr. DiPersio reported relationships with Magenta Therapeutics, WUGEN, Incyte, RiverVest Venture Partners, Cellworks Group, Amphivena Therapeutics, NeoImmune Tech, Macrogenics, and BioLineRx.
Correction, 4/26/23: The headline on an earlier version of this article mischaracterized the study findings.
FROM NATURE MEDICINE
Should antenatal testing be performed in patients with a pre-pregnancy BMI ≥ 35?
Possibly. Elevated body mass index (BMI) is associated with an increased risk for stillbirth (strength of recommendation (SOR), B; Cohort studies and meta-analysis of cohort studies). Three studies found an association between elevated BMI and stillbirth and one did not. However, no studies demonstrate that antenatal testing in pregnant people with higher BMIs decreases stillbirth rates, or that no harm is caused by unnecessary testing or resultant interventions.
Still, in 2021, the American College of Obstetricians and Gynecologists (ACOG) suggested weekly antenatal testing may be considered from 34 weeks' 0 days' gestation for pregnant people with a BMI ≥ 40.0 kg/m2 and from 37 weeks' 0 days' gestation for pregnant people with a BMI between 35.0 and 39.9 kg/m2 (SOR, C; consensus guideline). Thus, doing the antenatal testing recommended in the ACOG guideline in an attempt to prevent stillbirth is reasonable, given evidence that elevated BMI is associated with stillbirth.
Evidence summary
Association between higher maternal BMI and increased risk for stillbirth
The purpose of antenatal testing is to decrease the risk for stillbirth between visits. Because of the resources involved and the risk for false-positives when testing low-risk patients, antenatal testing is reserved for pregnant people with higher risk for stillbirth.
In a retrospective cohort study of more than 2.8 million singleton births including 9,030 stillbirths, pregnant people with an elevated BMI had an increased risk for stillbirth compared with those with a normal BMI. The adjusted hazard ratio was 1.71 (95% confidence interval (CI), 1.62-1.83) for those with a BMI of 30.0 to 34.9 kg/m2; 2.04 (95% CI, 1.8-2.21) for those with a BMI of 35.0 to 39.9 kg/m2; and 2.50 (95% CI, 2.28-2.74) for those with a BMI ≥ 40 kg/m2.1
A meta-analysis of 38 studies, which included data on 16,274 stillbirths, found that a 5-unit increase in BMI was associated with an increased risk for stillbirth (relative risk, 1.24; 95% CI, 1.18-1.30).2
Another meta-analysis included 6 cohort studies involving more than 1 million pregnancies and 3 case-control studies involving 2,530 stillbirths and 2,837 controls from 1980-2005. There was an association between increasing BMI and stillbirth: the odds ratio (OR) was 1.47 (95% CI, 1.08-1.94) for those with a BMI of 25.0 to 29.9 kg/m2 and 2.07 (95% CI, 1.59-2.74) for those with a BMI ≥ 30.0, compared with those with a normal BMI.3
However, a retrospective cohort study of 182,362 singleton births including 442 stillbirths found no association between stillbirth and increasing BMI. The OR was 1.10 (95% CI, 0.90-1.36) for those with a BMI of 25.0 to 29.9 and 1.09 (95% CI, 0.87-1.37) for those with a BMI ≥ 30.0 kg/m2, compared with those with a normal BMI.4 However, this cohort study may have been underpowered to detect an association between stillbirth and BMI.
Recommendations from others
In 2021, ACOG suggested that weekly antenatal testing may be considered from 34 weeks' and 0 days' gestation for pregnant people with a BMI ≥ 40.0 kg/m2 and from 37 weeks' and 0 days' gestation for pregnant people with a BMI between 35.0 and 39.9 kg/m2.5 The 2021 ACOG Practice Bulletin on obesity in pregnancy rates this recommendation as Level C—based primarily on consensus and expert opinion.6
A 2018 Royal College of Obstetricians and Gynecologists Green-top Guideline recognizes “definitive recommendations for fetal surveillance are hampered by the lack of randomized controlled trials demonstrating that antepartum fetal surveillance decreases perinatal morbidity or mortality in late-term and post-term gestations…. There are no definitive studies determining the optimal type or frequency of such testing and no evidence specific for women with obesity.”7
A 2019 Society of Obstetricians and Gynecologists of Canada practice guideline states “stillbirth is more common with maternal obesity” and recommends “increased fetal surveillance … in the third trimester if reduced fetal movements are reported.” The guideline notes “the role for non-stress tests … in surveillance of well-being in this population is uncertain.” Also, for pregnant people with a BMI > 30 kg/m2, “assessment of fetal well-being is … recommended weekly from 37 weeks until delivery.” Finally, increased fetal surveillance is recommended in the setting of increased BMI and an abnormal pulsatility index of the umbilical artery and/or maternal uterine artery.8
Editor’s takeaway
Evidence demonstrates that increased maternal BMI is associated with increased stillbirths. However, evidence has not shown that third-trimester antenatal testing decreases this morbidity and mortality. Expert opinion varies, with ACOG recommending weekly antenatal testing from 34 and 37 weeks’ gestation, respectively, for pregnant people with BMIs of ≥ 40 kg/m2 and of 35 to 39.9 kg/m2. ●
- Yao R, Ananth C, Park B, et al; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:e1-e9. doi: 10.1016/j. ajog. 2014.01.044
- Aune D, Saugstad O, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:15361546. doi: 10.1001/jama.2014.2269
- Chu S, Kim S, Lau J, et al. Maternal obesity and risk of stillbirth: a meta-analysis. Am J Obstet Gynecol. 2007;197:223-228. doi: 10.1016/j.ajog.2007.03.027
- Mahomed K, Chan G, Norton M. Obesity and the risk of stillbirth—a reappraisal—a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;255:25-28. doi: 10.1016/j. ejogrb. 2020.09.044
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197. doi: 10.1097/ AOG.0000000000004407
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;137:e128-e144. doi: 10.1097/ AOG.0000000000004395
- Denison F, Aedla N, Keag O, et al; Royal College of Obstetricians and Gynaecologists. Care of women with obesity in pregnancy: Green-top Guideline No. 72. BJOG. 2019;126:e62-e106. doi: 10.1111/1471-0528.15386
- Maxwell C, Gaudet L, Cassir G, et al. Guideline No. 391Pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41:1623-1640. doi: 10.1016/j.jogc. 2019.03.026
Possibly. Elevated body mass index (BMI) is associated with an increased risk for stillbirth (strength of recommendation (SOR), B; Cohort studies and meta-analysis of cohort studies). Three studies found an association between elevated BMI and stillbirth and one did not. However, no studies demonstrate that antenatal testing in pregnant people with higher BMIs decreases stillbirth rates, or that no harm is caused by unnecessary testing or resultant interventions.
Still, in 2021, the American College of Obstetricians and Gynecologists (ACOG) suggested weekly antenatal testing may be considered from 34 weeks' 0 days' gestation for pregnant people with a BMI ≥ 40.0 kg/m2 and from 37 weeks' 0 days' gestation for pregnant people with a BMI between 35.0 and 39.9 kg/m2 (SOR, C; consensus guideline). Thus, doing the antenatal testing recommended in the ACOG guideline in an attempt to prevent stillbirth is reasonable, given evidence that elevated BMI is associated with stillbirth.
Evidence summary
Association between higher maternal BMI and increased risk for stillbirth
The purpose of antenatal testing is to decrease the risk for stillbirth between visits. Because of the resources involved and the risk for false-positives when testing low-risk patients, antenatal testing is reserved for pregnant people with higher risk for stillbirth.
In a retrospective cohort study of more than 2.8 million singleton births including 9,030 stillbirths, pregnant people with an elevated BMI had an increased risk for stillbirth compared with those with a normal BMI. The adjusted hazard ratio was 1.71 (95% confidence interval (CI), 1.62-1.83) for those with a BMI of 30.0 to 34.9 kg/m2; 2.04 (95% CI, 1.8-2.21) for those with a BMI of 35.0 to 39.9 kg/m2; and 2.50 (95% CI, 2.28-2.74) for those with a BMI ≥ 40 kg/m2.1
A meta-analysis of 38 studies, which included data on 16,274 stillbirths, found that a 5-unit increase in BMI was associated with an increased risk for stillbirth (relative risk, 1.24; 95% CI, 1.18-1.30).2
Another meta-analysis included 6 cohort studies involving more than 1 million pregnancies and 3 case-control studies involving 2,530 stillbirths and 2,837 controls from 1980-2005. There was an association between increasing BMI and stillbirth: the odds ratio (OR) was 1.47 (95% CI, 1.08-1.94) for those with a BMI of 25.0 to 29.9 kg/m2 and 2.07 (95% CI, 1.59-2.74) for those with a BMI ≥ 30.0, compared with those with a normal BMI.3
However, a retrospective cohort study of 182,362 singleton births including 442 stillbirths found no association between stillbirth and increasing BMI. The OR was 1.10 (95% CI, 0.90-1.36) for those with a BMI of 25.0 to 29.9 and 1.09 (95% CI, 0.87-1.37) for those with a BMI ≥ 30.0 kg/m2, compared with those with a normal BMI.4 However, this cohort study may have been underpowered to detect an association between stillbirth and BMI.
Recommendations from others
In 2021, ACOG suggested that weekly antenatal testing may be considered from 34 weeks' and 0 days' gestation for pregnant people with a BMI ≥ 40.0 kg/m2 and from 37 weeks' and 0 days' gestation for pregnant people with a BMI between 35.0 and 39.9 kg/m2.5 The 2021 ACOG Practice Bulletin on obesity in pregnancy rates this recommendation as Level C—based primarily on consensus and expert opinion.6
A 2018 Royal College of Obstetricians and Gynecologists Green-top Guideline recognizes “definitive recommendations for fetal surveillance are hampered by the lack of randomized controlled trials demonstrating that antepartum fetal surveillance decreases perinatal morbidity or mortality in late-term and post-term gestations…. There are no definitive studies determining the optimal type or frequency of such testing and no evidence specific for women with obesity.”7
A 2019 Society of Obstetricians and Gynecologists of Canada practice guideline states “stillbirth is more common with maternal obesity” and recommends “increased fetal surveillance … in the third trimester if reduced fetal movements are reported.” The guideline notes “the role for non-stress tests … in surveillance of well-being in this population is uncertain.” Also, for pregnant people with a BMI > 30 kg/m2, “assessment of fetal well-being is … recommended weekly from 37 weeks until delivery.” Finally, increased fetal surveillance is recommended in the setting of increased BMI and an abnormal pulsatility index of the umbilical artery and/or maternal uterine artery.8
Editor’s takeaway
Evidence demonstrates that increased maternal BMI is associated with increased stillbirths. However, evidence has not shown that third-trimester antenatal testing decreases this morbidity and mortality. Expert opinion varies, with ACOG recommending weekly antenatal testing from 34 and 37 weeks’ gestation, respectively, for pregnant people with BMIs of ≥ 40 kg/m2 and of 35 to 39.9 kg/m2. ●
Possibly. Elevated body mass index (BMI) is associated with an increased risk for stillbirth (strength of recommendation (SOR), B; Cohort studies and meta-analysis of cohort studies). Three studies found an association between elevated BMI and stillbirth and one did not. However, no studies demonstrate that antenatal testing in pregnant people with higher BMIs decreases stillbirth rates, or that no harm is caused by unnecessary testing or resultant interventions.
Still, in 2021, the American College of Obstetricians and Gynecologists (ACOG) suggested weekly antenatal testing may be considered from 34 weeks' 0 days' gestation for pregnant people with a BMI ≥ 40.0 kg/m2 and from 37 weeks' 0 days' gestation for pregnant people with a BMI between 35.0 and 39.9 kg/m2 (SOR, C; consensus guideline). Thus, doing the antenatal testing recommended in the ACOG guideline in an attempt to prevent stillbirth is reasonable, given evidence that elevated BMI is associated with stillbirth.
Evidence summary
Association between higher maternal BMI and increased risk for stillbirth
The purpose of antenatal testing is to decrease the risk for stillbirth between visits. Because of the resources involved and the risk for false-positives when testing low-risk patients, antenatal testing is reserved for pregnant people with higher risk for stillbirth.
In a retrospective cohort study of more than 2.8 million singleton births including 9,030 stillbirths, pregnant people with an elevated BMI had an increased risk for stillbirth compared with those with a normal BMI. The adjusted hazard ratio was 1.71 (95% confidence interval (CI), 1.62-1.83) for those with a BMI of 30.0 to 34.9 kg/m2; 2.04 (95% CI, 1.8-2.21) for those with a BMI of 35.0 to 39.9 kg/m2; and 2.50 (95% CI, 2.28-2.74) for those with a BMI ≥ 40 kg/m2.1
A meta-analysis of 38 studies, which included data on 16,274 stillbirths, found that a 5-unit increase in BMI was associated with an increased risk for stillbirth (relative risk, 1.24; 95% CI, 1.18-1.30).2
Another meta-analysis included 6 cohort studies involving more than 1 million pregnancies and 3 case-control studies involving 2,530 stillbirths and 2,837 controls from 1980-2005. There was an association between increasing BMI and stillbirth: the odds ratio (OR) was 1.47 (95% CI, 1.08-1.94) for those with a BMI of 25.0 to 29.9 kg/m2 and 2.07 (95% CI, 1.59-2.74) for those with a BMI ≥ 30.0, compared with those with a normal BMI.3
However, a retrospective cohort study of 182,362 singleton births including 442 stillbirths found no association between stillbirth and increasing BMI. The OR was 1.10 (95% CI, 0.90-1.36) for those with a BMI of 25.0 to 29.9 and 1.09 (95% CI, 0.87-1.37) for those with a BMI ≥ 30.0 kg/m2, compared with those with a normal BMI.4 However, this cohort study may have been underpowered to detect an association between stillbirth and BMI.
Recommendations from others
In 2021, ACOG suggested that weekly antenatal testing may be considered from 34 weeks' and 0 days' gestation for pregnant people with a BMI ≥ 40.0 kg/m2 and from 37 weeks' and 0 days' gestation for pregnant people with a BMI between 35.0 and 39.9 kg/m2.5 The 2021 ACOG Practice Bulletin on obesity in pregnancy rates this recommendation as Level C—based primarily on consensus and expert opinion.6
A 2018 Royal College of Obstetricians and Gynecologists Green-top Guideline recognizes “definitive recommendations for fetal surveillance are hampered by the lack of randomized controlled trials demonstrating that antepartum fetal surveillance decreases perinatal morbidity or mortality in late-term and post-term gestations…. There are no definitive studies determining the optimal type or frequency of such testing and no evidence specific for women with obesity.”7
A 2019 Society of Obstetricians and Gynecologists of Canada practice guideline states “stillbirth is more common with maternal obesity” and recommends “increased fetal surveillance … in the third trimester if reduced fetal movements are reported.” The guideline notes “the role for non-stress tests … in surveillance of well-being in this population is uncertain.” Also, for pregnant people with a BMI > 30 kg/m2, “assessment of fetal well-being is … recommended weekly from 37 weeks until delivery.” Finally, increased fetal surveillance is recommended in the setting of increased BMI and an abnormal pulsatility index of the umbilical artery and/or maternal uterine artery.8
Editor’s takeaway
Evidence demonstrates that increased maternal BMI is associated with increased stillbirths. However, evidence has not shown that third-trimester antenatal testing decreases this morbidity and mortality. Expert opinion varies, with ACOG recommending weekly antenatal testing from 34 and 37 weeks’ gestation, respectively, for pregnant people with BMIs of ≥ 40 kg/m2 and of 35 to 39.9 kg/m2. ●
- Yao R, Ananth C, Park B, et al; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:e1-e9. doi: 10.1016/j. ajog. 2014.01.044
- Aune D, Saugstad O, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:15361546. doi: 10.1001/jama.2014.2269
- Chu S, Kim S, Lau J, et al. Maternal obesity and risk of stillbirth: a meta-analysis. Am J Obstet Gynecol. 2007;197:223-228. doi: 10.1016/j.ajog.2007.03.027
- Mahomed K, Chan G, Norton M. Obesity and the risk of stillbirth—a reappraisal—a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;255:25-28. doi: 10.1016/j. ejogrb. 2020.09.044
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197. doi: 10.1097/ AOG.0000000000004407
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;137:e128-e144. doi: 10.1097/ AOG.0000000000004395
- Denison F, Aedla N, Keag O, et al; Royal College of Obstetricians and Gynaecologists. Care of women with obesity in pregnancy: Green-top Guideline No. 72. BJOG. 2019;126:e62-e106. doi: 10.1111/1471-0528.15386
- Maxwell C, Gaudet L, Cassir G, et al. Guideline No. 391Pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41:1623-1640. doi: 10.1016/j.jogc. 2019.03.026
- Yao R, Ananth C, Park B, et al; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:e1-e9. doi: 10.1016/j. ajog. 2014.01.044
- Aune D, Saugstad O, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:15361546. doi: 10.1001/jama.2014.2269
- Chu S, Kim S, Lau J, et al. Maternal obesity and risk of stillbirth: a meta-analysis. Am J Obstet Gynecol. 2007;197:223-228. doi: 10.1016/j.ajog.2007.03.027
- Mahomed K, Chan G, Norton M. Obesity and the risk of stillbirth—a reappraisal—a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;255:25-28. doi: 10.1016/j. ejogrb. 2020.09.044
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197. doi: 10.1097/ AOG.0000000000004407
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;137:e128-e144. doi: 10.1097/ AOG.0000000000004395
- Denison F, Aedla N, Keag O, et al; Royal College of Obstetricians and Gynaecologists. Care of women with obesity in pregnancy: Green-top Guideline No. 72. BJOG. 2019;126:e62-e106. doi: 10.1111/1471-0528.15386
- Maxwell C, Gaudet L, Cassir G, et al. Guideline No. 391Pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41:1623-1640. doi: 10.1016/j.jogc. 2019.03.026
Ablation for atrial fibrillation may protect the aging brain
BOSTON – , new research suggests.
Investigators found adults who had previously undergone catheter ablation were significantly less likely to be cognitively impaired during the 2-year study period, compared with those who receive medical management alone.
“Catheter ablation is intended to stop atrial fibrillation and restore the normal rhythm of the heart. By doing so, there is an improved cerebral hemodynamic profile,” said Bahadar S. Srichawla, DO, department of neurology, University of Massachusetts, Worcester.
“Thus, long-term cognitive outcomes may be improved due to improved blood flow to the brain by restoring the normal rhythm of the heart,” he added.
This research was presented at the 2023 annual meeting of the American Academy of Neurology.
Heart-brain connection
The study involved 887 older adults (mean age 75; 49% women) with atrial fibrillation participating in the SAGE-AF (Systematic Assessment of Geriatric Elements) study. A total of 193 (22%) participants underwent catheter ablation prior to enrollment. These individuals more frequently had an implantable cardiac device (46% vs. 28%, P < .001) and persistent atrial fibrillation (31% vs. 23%, P < .05).
Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) tool at baseline and 1 and 2 years, with cognitive impairment defined as a MoCA score of 23 or below. Individuals who had catheter ablation had an average MoCA score of 25, compared with an average score of 23 in those who didn’t have catheter ablation.
After adjusting for potential confounding factors such as heart disease, renal disease, sleep apnea, and atrial fibrillation risk score, those who underwent catheter ablation were 36% less likely to develop cognitive impairment over 2 years than those who were treated only with medication (adjusted odds ratio, 0.64; 95% CI, 0.46-0.88).
During his presentation, Dr. Srichawla noted there is a hypothesis that individuals who are anticoagulated with warfarin may be prone to cerebral microbleeds and may be more cognitively impaired over time.
However, in a subgroup analysis, “cognitive function was similar at 2-year follow-up in those anticoagulated with warfarin, compared with all other anticoagulants. However, it should be noted that in this study, a direct head-to-head comparison was not done,” Dr. Srichawla told attendees.
“In patients with atrial fibrillation, catheter ablation should be discussed as a potential treatment strategy, particularly in patients who have or are at risk for cognitive decline and dementia,” Dr. Srichawla said.
Intriguing findings
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said the study is “intriguing and adds to what we know from previous research connecting cardiovascular and cognitive health.”
“However, there are limitations to this study,” Dr. Griffin said, “including its predominantly White cohort and the use of only neuropsychiatric testing to diagnose dementia. More research is needed to fully understand the impact of atrial fibrillation on cognitive outcomes in all people.”
“It’s well known that the heart and the brain are intimately connected. Individuals experiencing any cardiovascular issues should speak to their doctor,” Dr. Griffin added.
Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, agreed. “If you ever get up too quickly and feel woozy, that is your brain not getting enough blood flow and you are getting all the warning signs to correct that – or else! Similarly, with atrial fibrillation, the heart is contracting, but not effectively pumping blood to the brain,” he said.
“This line of research shows that correcting the abnormal heart rhythm by zapping the faulty circuit with a catheter is actually better for your brain health than just taking medications alone,” added Dr. Lakhan, who was not involved with the study.
The study had no commercial funding. Dr. Srichawla, Dr. Griffin, and Dr. Lakhan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – , new research suggests.
Investigators found adults who had previously undergone catheter ablation were significantly less likely to be cognitively impaired during the 2-year study period, compared with those who receive medical management alone.
“Catheter ablation is intended to stop atrial fibrillation and restore the normal rhythm of the heart. By doing so, there is an improved cerebral hemodynamic profile,” said Bahadar S. Srichawla, DO, department of neurology, University of Massachusetts, Worcester.
“Thus, long-term cognitive outcomes may be improved due to improved blood flow to the brain by restoring the normal rhythm of the heart,” he added.
This research was presented at the 2023 annual meeting of the American Academy of Neurology.
Heart-brain connection
The study involved 887 older adults (mean age 75; 49% women) with atrial fibrillation participating in the SAGE-AF (Systematic Assessment of Geriatric Elements) study. A total of 193 (22%) participants underwent catheter ablation prior to enrollment. These individuals more frequently had an implantable cardiac device (46% vs. 28%, P < .001) and persistent atrial fibrillation (31% vs. 23%, P < .05).
Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) tool at baseline and 1 and 2 years, with cognitive impairment defined as a MoCA score of 23 or below. Individuals who had catheter ablation had an average MoCA score of 25, compared with an average score of 23 in those who didn’t have catheter ablation.
After adjusting for potential confounding factors such as heart disease, renal disease, sleep apnea, and atrial fibrillation risk score, those who underwent catheter ablation were 36% less likely to develop cognitive impairment over 2 years than those who were treated only with medication (adjusted odds ratio, 0.64; 95% CI, 0.46-0.88).
During his presentation, Dr. Srichawla noted there is a hypothesis that individuals who are anticoagulated with warfarin may be prone to cerebral microbleeds and may be more cognitively impaired over time.
However, in a subgroup analysis, “cognitive function was similar at 2-year follow-up in those anticoagulated with warfarin, compared with all other anticoagulants. However, it should be noted that in this study, a direct head-to-head comparison was not done,” Dr. Srichawla told attendees.
“In patients with atrial fibrillation, catheter ablation should be discussed as a potential treatment strategy, particularly in patients who have or are at risk for cognitive decline and dementia,” Dr. Srichawla said.
Intriguing findings
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said the study is “intriguing and adds to what we know from previous research connecting cardiovascular and cognitive health.”
“However, there are limitations to this study,” Dr. Griffin said, “including its predominantly White cohort and the use of only neuropsychiatric testing to diagnose dementia. More research is needed to fully understand the impact of atrial fibrillation on cognitive outcomes in all people.”
“It’s well known that the heart and the brain are intimately connected. Individuals experiencing any cardiovascular issues should speak to their doctor,” Dr. Griffin added.
Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, agreed. “If you ever get up too quickly and feel woozy, that is your brain not getting enough blood flow and you are getting all the warning signs to correct that – or else! Similarly, with atrial fibrillation, the heart is contracting, but not effectively pumping blood to the brain,” he said.
“This line of research shows that correcting the abnormal heart rhythm by zapping the faulty circuit with a catheter is actually better for your brain health than just taking medications alone,” added Dr. Lakhan, who was not involved with the study.
The study had no commercial funding. Dr. Srichawla, Dr. Griffin, and Dr. Lakhan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – , new research suggests.
Investigators found adults who had previously undergone catheter ablation were significantly less likely to be cognitively impaired during the 2-year study period, compared with those who receive medical management alone.
“Catheter ablation is intended to stop atrial fibrillation and restore the normal rhythm of the heart. By doing so, there is an improved cerebral hemodynamic profile,” said Bahadar S. Srichawla, DO, department of neurology, University of Massachusetts, Worcester.
“Thus, long-term cognitive outcomes may be improved due to improved blood flow to the brain by restoring the normal rhythm of the heart,” he added.
This research was presented at the 2023 annual meeting of the American Academy of Neurology.
Heart-brain connection
The study involved 887 older adults (mean age 75; 49% women) with atrial fibrillation participating in the SAGE-AF (Systematic Assessment of Geriatric Elements) study. A total of 193 (22%) participants underwent catheter ablation prior to enrollment. These individuals more frequently had an implantable cardiac device (46% vs. 28%, P < .001) and persistent atrial fibrillation (31% vs. 23%, P < .05).
Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) tool at baseline and 1 and 2 years, with cognitive impairment defined as a MoCA score of 23 or below. Individuals who had catheter ablation had an average MoCA score of 25, compared with an average score of 23 in those who didn’t have catheter ablation.
After adjusting for potential confounding factors such as heart disease, renal disease, sleep apnea, and atrial fibrillation risk score, those who underwent catheter ablation were 36% less likely to develop cognitive impairment over 2 years than those who were treated only with medication (adjusted odds ratio, 0.64; 95% CI, 0.46-0.88).
During his presentation, Dr. Srichawla noted there is a hypothesis that individuals who are anticoagulated with warfarin may be prone to cerebral microbleeds and may be more cognitively impaired over time.
However, in a subgroup analysis, “cognitive function was similar at 2-year follow-up in those anticoagulated with warfarin, compared with all other anticoagulants. However, it should be noted that in this study, a direct head-to-head comparison was not done,” Dr. Srichawla told attendees.
“In patients with atrial fibrillation, catheter ablation should be discussed as a potential treatment strategy, particularly in patients who have or are at risk for cognitive decline and dementia,” Dr. Srichawla said.
Intriguing findings
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said the study is “intriguing and adds to what we know from previous research connecting cardiovascular and cognitive health.”
“However, there are limitations to this study,” Dr. Griffin said, “including its predominantly White cohort and the use of only neuropsychiatric testing to diagnose dementia. More research is needed to fully understand the impact of atrial fibrillation on cognitive outcomes in all people.”
“It’s well known that the heart and the brain are intimately connected. Individuals experiencing any cardiovascular issues should speak to their doctor,” Dr. Griffin added.
Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, agreed. “If you ever get up too quickly and feel woozy, that is your brain not getting enough blood flow and you are getting all the warning signs to correct that – or else! Similarly, with atrial fibrillation, the heart is contracting, but not effectively pumping blood to the brain,” he said.
“This line of research shows that correcting the abnormal heart rhythm by zapping the faulty circuit with a catheter is actually better for your brain health than just taking medications alone,” added Dr. Lakhan, who was not involved with the study.
The study had no commercial funding. Dr. Srichawla, Dr. Griffin, and Dr. Lakhan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2023
Psilocybin promising for body dysmorphic disorder
WASHINGTON – Psilocybin is safe and effective in patients with body dysmorphic disorder (BDD), preliminary findings of a small pilot study show.
“The results suggest that psilocybin appears to be relatively safe and potentially helpful for people with BDD, and that it has a broader scope than just depression,” study investigator Franklin Schneier, MD, codirector of the Anxiety Disorders Clinic, New York State Psychiatric Institute, and special lecturer in psychiatry at Columbia University Medical Center in New York City, told this news organization.
So far, psilocybin has mostly been examined in clinical trials among patients with major depression. Dr. Schneier said he is aware of only a single case in the literature of its use in BDD: a patient who self-treated with psilocybin and reported symptom improvement.
The current study was presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Few treatment options
Patients with BDD are preoccupied with a body part they perceive as ugly or defective, “and not just mildly so,” said Dr. Schneier. “It bothers them to the extreme such that they may obsess about it on and off all day long.”
Such patients may engage in compulsive behaviors like constantly checking themselves in the mirror, and going to great lengths to conceal the body part they feel is defective. “They often seek out cosmetic procedures that objectively aren’t warranted,” said Dr. Schneier.
BDD patients often have comorbid depression, and many attempt suicide. As with other anxiety and depressive disorders, BDD is twice as prevalent in women vs. men, said Dr. Schneier.
Selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (CBT) are the only approved therapies for BDD.
The investigators thought there may be a good chance BDD patients could benefit from psilocybin. Psilocybin alters bodily self-awareness, which “might shake up people’s beliefs about their abnormal body perceptions,” said Dr. Schneier.
There’s also some suggestion that psilocybin relaxes inflexible thinking, he added. “People with BDD have very rigid beliefs about their body distortions that aren’t easily swayed by logic.”
The study included 12 adults (8 women, 4 men) with a mean age of 34 years and moderate to severe BDD who failed at least one SSRI course and had had BDD for an average of 21 years.
Participants had preliminary sessions with a therapist familiar with psilocybin who prepared them psychologically and discussed what to expect from the experience. On the day of the intervention, subjects took a single 25 mg oral dose of synthetic psilocybin in a comfortable setting.
Therapists were present for the next 8 hours to answer questions and support subjects through the experience.
High response rate
The primary efficacy outcome was change in the BDD Yale-Brown Obsessive Compulsive Disorder Scale Modified (BDD-YBOCS) total score.
The mean baseline BDD-YBOCS score was 29.17. Researchers regularly assessed this score in the following weeks.
At 12 weeks, BDD-YBOCS scores decreased significantly from baseline (P < .001) with a large effect size (partial eta squared = .54).
However, said Dr. Schneier, what really stood out was the proportion of responders. At week 12, seven (58%) of the 12 participants were responders, as defined by a 30% or greater decrease in the BDD-YBOCS score. Of these, three were “almost symptom-free,” he added.
A number of secondary outcomes, including conviction of belief, disability, and negative affect, also significantly improved.
It’s too early to determine if additional treatment is required. The investigators plan to follow-up with the cohort at 1 year.
Although exciting, these early results warrant caution, said Dr. Schneier. “On the one hand, this is a sample of people who have struggled for a long time and have failed previous therapies, so that’s good. But on the other hand, it’s an open trial with no placebo group, and everyone has high expectations, so we don’t know how much of a placebo effect there was.”
Most adverse events, including headaches and fatigue, were mild and resolved within the first week after dosing, and there were no serious adverse events.
Based on these findings, Dr. Schneier said controlled trials of psilocybin in BDD are warranted.
Need for scientific rigor
Commenting on the research, Charles B. Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, University of Texas at Austin, said while promising, psilocybin is “not for everyone” and patients need to be closely screened.
“We want to know their medical history and if they have a family history of schizophrenia or bipolar disorder. We don’t know whether these [psychedelic] medicines might trigger an episode.”
Dr. Nemeroff also noted there’s a risk of “troubling” side effects from the drug.
“My view is psilocybin clearly has therapeutic effects and we need to apply scientific rigor as we would any medicine in order to determine the risk/benefit ratio,” said Dr. Nemeroff, who was not associated with this psilocybin trial.
In addition, psilocybin is being tested in conditions other than BDD and major depression, including anorexia nervosa, postpartum depression, and alcohol use disorder, he added.
The study received funding from COMPASS Pathways PLC.
Dr. Nemeroff reports he has received research support from the NIH and Stanley Medical Research Institute; served as a consultant for Bracket (Clintara), Fortress Biotech, Intra-Cellular Therapies, Janssen Research and Development, Magstim, Navitor Pharmaceuticals, Sunovion Pharmaceuticals, Taisho Pharmaceuticals, Takeda, TC MSO, and Xhale; served on scientific advisory boards for the American Foundation for Suicide Prevention, the Anxiety and Depression Association of America, Bracket (Clintara), Brain and Behavior Research Foundation, Laureate Institute for Brain Research, Skyland Trail, and Xhale; is a stockholder in AbbVie, Antares, BI Gen Holdings, Celgene, OPKO Health, Seattle Genetics, and Xhale; serves on the board of directors for the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, and Gratitude America; has received income or equity of $10,000 or more from American Psychiatric Publishing, Bracket (Clintara), Magstim, CME Outfitters, and Intra-Cellular Therapies; and holds patents on a method and devices for transdermal delivery of lithium and a method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay.
A version of this article first appeared on Medscape.com.
WASHINGTON – Psilocybin is safe and effective in patients with body dysmorphic disorder (BDD), preliminary findings of a small pilot study show.
“The results suggest that psilocybin appears to be relatively safe and potentially helpful for people with BDD, and that it has a broader scope than just depression,” study investigator Franklin Schneier, MD, codirector of the Anxiety Disorders Clinic, New York State Psychiatric Institute, and special lecturer in psychiatry at Columbia University Medical Center in New York City, told this news organization.
So far, psilocybin has mostly been examined in clinical trials among patients with major depression. Dr. Schneier said he is aware of only a single case in the literature of its use in BDD: a patient who self-treated with psilocybin and reported symptom improvement.
The current study was presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Few treatment options
Patients with BDD are preoccupied with a body part they perceive as ugly or defective, “and not just mildly so,” said Dr. Schneier. “It bothers them to the extreme such that they may obsess about it on and off all day long.”
Such patients may engage in compulsive behaviors like constantly checking themselves in the mirror, and going to great lengths to conceal the body part they feel is defective. “They often seek out cosmetic procedures that objectively aren’t warranted,” said Dr. Schneier.
BDD patients often have comorbid depression, and many attempt suicide. As with other anxiety and depressive disorders, BDD is twice as prevalent in women vs. men, said Dr. Schneier.
Selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (CBT) are the only approved therapies for BDD.
The investigators thought there may be a good chance BDD patients could benefit from psilocybin. Psilocybin alters bodily self-awareness, which “might shake up people’s beliefs about their abnormal body perceptions,” said Dr. Schneier.
There’s also some suggestion that psilocybin relaxes inflexible thinking, he added. “People with BDD have very rigid beliefs about their body distortions that aren’t easily swayed by logic.”
The study included 12 adults (8 women, 4 men) with a mean age of 34 years and moderate to severe BDD who failed at least one SSRI course and had had BDD for an average of 21 years.
Participants had preliminary sessions with a therapist familiar with psilocybin who prepared them psychologically and discussed what to expect from the experience. On the day of the intervention, subjects took a single 25 mg oral dose of synthetic psilocybin in a comfortable setting.
Therapists were present for the next 8 hours to answer questions and support subjects through the experience.
High response rate
The primary efficacy outcome was change in the BDD Yale-Brown Obsessive Compulsive Disorder Scale Modified (BDD-YBOCS) total score.
The mean baseline BDD-YBOCS score was 29.17. Researchers regularly assessed this score in the following weeks.
At 12 weeks, BDD-YBOCS scores decreased significantly from baseline (P < .001) with a large effect size (partial eta squared = .54).
However, said Dr. Schneier, what really stood out was the proportion of responders. At week 12, seven (58%) of the 12 participants were responders, as defined by a 30% or greater decrease in the BDD-YBOCS score. Of these, three were “almost symptom-free,” he added.
A number of secondary outcomes, including conviction of belief, disability, and negative affect, also significantly improved.
It’s too early to determine if additional treatment is required. The investigators plan to follow-up with the cohort at 1 year.
Although exciting, these early results warrant caution, said Dr. Schneier. “On the one hand, this is a sample of people who have struggled for a long time and have failed previous therapies, so that’s good. But on the other hand, it’s an open trial with no placebo group, and everyone has high expectations, so we don’t know how much of a placebo effect there was.”
Most adverse events, including headaches and fatigue, were mild and resolved within the first week after dosing, and there were no serious adverse events.
Based on these findings, Dr. Schneier said controlled trials of psilocybin in BDD are warranted.
Need for scientific rigor
Commenting on the research, Charles B. Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, University of Texas at Austin, said while promising, psilocybin is “not for everyone” and patients need to be closely screened.
“We want to know their medical history and if they have a family history of schizophrenia or bipolar disorder. We don’t know whether these [psychedelic] medicines might trigger an episode.”
Dr. Nemeroff also noted there’s a risk of “troubling” side effects from the drug.
“My view is psilocybin clearly has therapeutic effects and we need to apply scientific rigor as we would any medicine in order to determine the risk/benefit ratio,” said Dr. Nemeroff, who was not associated with this psilocybin trial.
In addition, psilocybin is being tested in conditions other than BDD and major depression, including anorexia nervosa, postpartum depression, and alcohol use disorder, he added.
The study received funding from COMPASS Pathways PLC.
Dr. Nemeroff reports he has received research support from the NIH and Stanley Medical Research Institute; served as a consultant for Bracket (Clintara), Fortress Biotech, Intra-Cellular Therapies, Janssen Research and Development, Magstim, Navitor Pharmaceuticals, Sunovion Pharmaceuticals, Taisho Pharmaceuticals, Takeda, TC MSO, and Xhale; served on scientific advisory boards for the American Foundation for Suicide Prevention, the Anxiety and Depression Association of America, Bracket (Clintara), Brain and Behavior Research Foundation, Laureate Institute for Brain Research, Skyland Trail, and Xhale; is a stockholder in AbbVie, Antares, BI Gen Holdings, Celgene, OPKO Health, Seattle Genetics, and Xhale; serves on the board of directors for the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, and Gratitude America; has received income or equity of $10,000 or more from American Psychiatric Publishing, Bracket (Clintara), Magstim, CME Outfitters, and Intra-Cellular Therapies; and holds patents on a method and devices for transdermal delivery of lithium and a method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay.
A version of this article first appeared on Medscape.com.
WASHINGTON – Psilocybin is safe and effective in patients with body dysmorphic disorder (BDD), preliminary findings of a small pilot study show.
“The results suggest that psilocybin appears to be relatively safe and potentially helpful for people with BDD, and that it has a broader scope than just depression,” study investigator Franklin Schneier, MD, codirector of the Anxiety Disorders Clinic, New York State Psychiatric Institute, and special lecturer in psychiatry at Columbia University Medical Center in New York City, told this news organization.
So far, psilocybin has mostly been examined in clinical trials among patients with major depression. Dr. Schneier said he is aware of only a single case in the literature of its use in BDD: a patient who self-treated with psilocybin and reported symptom improvement.
The current study was presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Few treatment options
Patients with BDD are preoccupied with a body part they perceive as ugly or defective, “and not just mildly so,” said Dr. Schneier. “It bothers them to the extreme such that they may obsess about it on and off all day long.”
Such patients may engage in compulsive behaviors like constantly checking themselves in the mirror, and going to great lengths to conceal the body part they feel is defective. “They often seek out cosmetic procedures that objectively aren’t warranted,” said Dr. Schneier.
BDD patients often have comorbid depression, and many attempt suicide. As with other anxiety and depressive disorders, BDD is twice as prevalent in women vs. men, said Dr. Schneier.
Selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (CBT) are the only approved therapies for BDD.
The investigators thought there may be a good chance BDD patients could benefit from psilocybin. Psilocybin alters bodily self-awareness, which “might shake up people’s beliefs about their abnormal body perceptions,” said Dr. Schneier.
There’s also some suggestion that psilocybin relaxes inflexible thinking, he added. “People with BDD have very rigid beliefs about their body distortions that aren’t easily swayed by logic.”
The study included 12 adults (8 women, 4 men) with a mean age of 34 years and moderate to severe BDD who failed at least one SSRI course and had had BDD for an average of 21 years.
Participants had preliminary sessions with a therapist familiar with psilocybin who prepared them psychologically and discussed what to expect from the experience. On the day of the intervention, subjects took a single 25 mg oral dose of synthetic psilocybin in a comfortable setting.
Therapists were present for the next 8 hours to answer questions and support subjects through the experience.
High response rate
The primary efficacy outcome was change in the BDD Yale-Brown Obsessive Compulsive Disorder Scale Modified (BDD-YBOCS) total score.
The mean baseline BDD-YBOCS score was 29.17. Researchers regularly assessed this score in the following weeks.
At 12 weeks, BDD-YBOCS scores decreased significantly from baseline (P < .001) with a large effect size (partial eta squared = .54).
However, said Dr. Schneier, what really stood out was the proportion of responders. At week 12, seven (58%) of the 12 participants were responders, as defined by a 30% or greater decrease in the BDD-YBOCS score. Of these, three were “almost symptom-free,” he added.
A number of secondary outcomes, including conviction of belief, disability, and negative affect, also significantly improved.
It’s too early to determine if additional treatment is required. The investigators plan to follow-up with the cohort at 1 year.
Although exciting, these early results warrant caution, said Dr. Schneier. “On the one hand, this is a sample of people who have struggled for a long time and have failed previous therapies, so that’s good. But on the other hand, it’s an open trial with no placebo group, and everyone has high expectations, so we don’t know how much of a placebo effect there was.”
Most adverse events, including headaches and fatigue, were mild and resolved within the first week after dosing, and there were no serious adverse events.
Based on these findings, Dr. Schneier said controlled trials of psilocybin in BDD are warranted.
Need for scientific rigor
Commenting on the research, Charles B. Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, University of Texas at Austin, said while promising, psilocybin is “not for everyone” and patients need to be closely screened.
“We want to know their medical history and if they have a family history of schizophrenia or bipolar disorder. We don’t know whether these [psychedelic] medicines might trigger an episode.”
Dr. Nemeroff also noted there’s a risk of “troubling” side effects from the drug.
“My view is psilocybin clearly has therapeutic effects and we need to apply scientific rigor as we would any medicine in order to determine the risk/benefit ratio,” said Dr. Nemeroff, who was not associated with this psilocybin trial.
In addition, psilocybin is being tested in conditions other than BDD and major depression, including anorexia nervosa, postpartum depression, and alcohol use disorder, he added.
The study received funding from COMPASS Pathways PLC.
Dr. Nemeroff reports he has received research support from the NIH and Stanley Medical Research Institute; served as a consultant for Bracket (Clintara), Fortress Biotech, Intra-Cellular Therapies, Janssen Research and Development, Magstim, Navitor Pharmaceuticals, Sunovion Pharmaceuticals, Taisho Pharmaceuticals, Takeda, TC MSO, and Xhale; served on scientific advisory boards for the American Foundation for Suicide Prevention, the Anxiety and Depression Association of America, Bracket (Clintara), Brain and Behavior Research Foundation, Laureate Institute for Brain Research, Skyland Trail, and Xhale; is a stockholder in AbbVie, Antares, BI Gen Holdings, Celgene, OPKO Health, Seattle Genetics, and Xhale; serves on the board of directors for the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, and Gratitude America; has received income or equity of $10,000 or more from American Psychiatric Publishing, Bracket (Clintara), Magstim, CME Outfitters, and Intra-Cellular Therapies; and holds patents on a method and devices for transdermal delivery of lithium and a method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
Phase 3 study of new levodopa/carbidopa delivery system meets all efficacy endpoints
BOSTON – presented at the 2023 annual meeting of the American Academy of Neurology.
When compared with optimized oral immediate-release medication, the delivery system, called ND0612 (NeuroDerm, Rehovot, Israel), improved ON time without troublesome dyskinesias while improving symptoms according to ratings from both patients and clinicians, according to Alberto J. Espay, MD, professor of neurology and director of the Gardner Family Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati.
The new delivery system addresses the challenge of reducing the variability in levodopa plasma concentrations, a major factor in motor fluctuations and diminishing benefit from orally administered drug, according to Dr. Espay. He said that continuous infusion strategies have long been sought as a method to preserve levodopa efficacy.
BouNDless findings
There were two phases to this multinational trial, called BouNDless. In the first, an open-label run-in phase, 381 patients with Parkinson’s disease were dose titrated for optimization of oral immediate-release levodopa and carbidopa. They were then optimized for the same drugs delivered with ND0612. The study was conducted over 12 weeks; 122 patients left the study after this phase due to adverse events, lack of efficacy, or withdrawal of consent.
In the second phase, the 259 remaining patients were randomized to the continuous infusion arm or to immediate release oral therapy. In this double-blind, double-dummy phase, those randomized to the ND0612 infusion also received oral placebos. Those randomized to oral therapy received a placebo infusion. Efficacy and safety were assessed at the end of 12 weeks.
At the end of phase 1, the ON time increased by about 3 hours when levodopa-carbidopa dosing was optimized on either delivery method. Dr. Espay attributed the improvement to the value of optimized dosing even in patients with relatively advanced disease.
However, for the purposes of the double-blind comparison, this improvement in ON time provided a new baseline for comparison of the two delivery methods. This is important for interpreting the primary result, which was a 1.72-hour difference in ON time at the end of the study. The difference was created when ON time was maintained with ND0612 continuous drug delivery but eroded in the group randomized to oral immediate-release treatment.
Several secondary endpoints supported the greater efficacy of continuous subcutaneous delivery. These included lower OFF time (0.50 vs. 1.90 hours), less accumulation of disability on the United Parkinson’s Disease Rating Scale part II-M-EDL (-0.30 vs. +2.75 points), and greater improvement on the Patient Global Impression of Change (+0.31 vs. +0.70 points), and the Clinical Global Impression of change (+0.31 vs. +0.77 points). The differences were highly statistically significant (all P < .0001).
The patients participating in the double-blind phase of the study were similar with a mean age of 63.5 years in both groups and time since Parkinson’s disease diagnosis (> 9 years). The median ON time without troublesome dyskinesias was about 12 hours at baseline in both groups and the median OFF time was about 3.5 hours.
The higher rate of treatment-related adverse events in the ND0612 group (67.2% vs. 52.7%) was largely explained by the greater rate of infusion site reactions (57.0% vs. 42.7%). The rates of severe reactions in the two groups were the same (0.8%), but both mild (43.8% vs. 36.6%) and moderate (12.5% vs. 5.3%) reactions occurred more commonly in the group receiving active therapy.
“Infusion reactions are the Achilles heel of all subcutaneous therapies,” acknowledged Dr. Espay, who expects other infusion systems in development to share this risk. He suggested that the clinical impact can be attenuated to some degree by rotating infusion sites.
BeyoND extension study
Data from an open-label extension (OLE) of the phase 2b BeyoND trial were also presented at the AAN meeting and generated generally similar results. Largely a safety study, there was no active control in the initial BeyoND or the BeyoND OLE. In BeyoND, the improvement in ON time from baseline was even greater than that seen in BouNDless, but, again, the optimization of dosing in the BouNDless run-in established a greater baseline of disease control.
In the OLE of BeyoND, presented by Aaron Ellenbogen, DO, a neurologist in Farmington, Mich., one of the notable findings was the retention of patients. After 2 years of follow-up, 82% completed at least 2 years of follow-up and 66.7% have now remained on treatment for at least 3 years. Dr. Ellenbogen maintains that this retention rate provides compelling evidence of a favorable benefit-to-risk ratio.
Fulfilling an unmet need
The favorable efficacy data from this trial represent “a big advance,” according to Ihtsham Ul Haq, MD, chief, movement disorders division, University of Miami, who was reached for comment. He noted that continuous infusion delivery has been anticipated for some time, and he expects these types of systems to fulfill an unmet need.
“This will be a useful option in a carefully selected group of patients,” said Dr. Haq, who considers the types of improvement in ON time to be highly clinically meaningful.
However, he cautioned that the nodules created by injection site reactions might limit the utility of this treatment option in at least some patients. Wearing the external device might also be a limiting factor for some patients.
In complex Parkinson’s disease, a stage that can be reached fairly rapidly in some patients but might take 15 years or more in others, all of the options involve a careful benefit-to-risk calculation, according to Dr. Haq. Deep brain stimulation is among the most effective options, but continuous infusion might appeal to some patients for delaying this procedure or as an alternative.
“We need multiple options for these types of patients, and it appears that continuous infusion will be one of them,” Dr. Haq said.
Dr. Espay has financial relationships with Acadia, Acorda, Amneal, AskBio, Bexion, Kyowa Kirin, Neuroderm, Neurocrine, and Sunovion. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva. Dr. Haq reports no potential conflicts of interest.
BOSTON – presented at the 2023 annual meeting of the American Academy of Neurology.
When compared with optimized oral immediate-release medication, the delivery system, called ND0612 (NeuroDerm, Rehovot, Israel), improved ON time without troublesome dyskinesias while improving symptoms according to ratings from both patients and clinicians, according to Alberto J. Espay, MD, professor of neurology and director of the Gardner Family Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati.
The new delivery system addresses the challenge of reducing the variability in levodopa plasma concentrations, a major factor in motor fluctuations and diminishing benefit from orally administered drug, according to Dr. Espay. He said that continuous infusion strategies have long been sought as a method to preserve levodopa efficacy.
BouNDless findings
There were two phases to this multinational trial, called BouNDless. In the first, an open-label run-in phase, 381 patients with Parkinson’s disease were dose titrated for optimization of oral immediate-release levodopa and carbidopa. They were then optimized for the same drugs delivered with ND0612. The study was conducted over 12 weeks; 122 patients left the study after this phase due to adverse events, lack of efficacy, or withdrawal of consent.
In the second phase, the 259 remaining patients were randomized to the continuous infusion arm or to immediate release oral therapy. In this double-blind, double-dummy phase, those randomized to the ND0612 infusion also received oral placebos. Those randomized to oral therapy received a placebo infusion. Efficacy and safety were assessed at the end of 12 weeks.
At the end of phase 1, the ON time increased by about 3 hours when levodopa-carbidopa dosing was optimized on either delivery method. Dr. Espay attributed the improvement to the value of optimized dosing even in patients with relatively advanced disease.
However, for the purposes of the double-blind comparison, this improvement in ON time provided a new baseline for comparison of the two delivery methods. This is important for interpreting the primary result, which was a 1.72-hour difference in ON time at the end of the study. The difference was created when ON time was maintained with ND0612 continuous drug delivery but eroded in the group randomized to oral immediate-release treatment.
Several secondary endpoints supported the greater efficacy of continuous subcutaneous delivery. These included lower OFF time (0.50 vs. 1.90 hours), less accumulation of disability on the United Parkinson’s Disease Rating Scale part II-M-EDL (-0.30 vs. +2.75 points), and greater improvement on the Patient Global Impression of Change (+0.31 vs. +0.70 points), and the Clinical Global Impression of change (+0.31 vs. +0.77 points). The differences were highly statistically significant (all P < .0001).
The patients participating in the double-blind phase of the study were similar with a mean age of 63.5 years in both groups and time since Parkinson’s disease diagnosis (> 9 years). The median ON time without troublesome dyskinesias was about 12 hours at baseline in both groups and the median OFF time was about 3.5 hours.
The higher rate of treatment-related adverse events in the ND0612 group (67.2% vs. 52.7%) was largely explained by the greater rate of infusion site reactions (57.0% vs. 42.7%). The rates of severe reactions in the two groups were the same (0.8%), but both mild (43.8% vs. 36.6%) and moderate (12.5% vs. 5.3%) reactions occurred more commonly in the group receiving active therapy.
“Infusion reactions are the Achilles heel of all subcutaneous therapies,” acknowledged Dr. Espay, who expects other infusion systems in development to share this risk. He suggested that the clinical impact can be attenuated to some degree by rotating infusion sites.
BeyoND extension study
Data from an open-label extension (OLE) of the phase 2b BeyoND trial were also presented at the AAN meeting and generated generally similar results. Largely a safety study, there was no active control in the initial BeyoND or the BeyoND OLE. In BeyoND, the improvement in ON time from baseline was even greater than that seen in BouNDless, but, again, the optimization of dosing in the BouNDless run-in established a greater baseline of disease control.
In the OLE of BeyoND, presented by Aaron Ellenbogen, DO, a neurologist in Farmington, Mich., one of the notable findings was the retention of patients. After 2 years of follow-up, 82% completed at least 2 years of follow-up and 66.7% have now remained on treatment for at least 3 years. Dr. Ellenbogen maintains that this retention rate provides compelling evidence of a favorable benefit-to-risk ratio.
Fulfilling an unmet need
The favorable efficacy data from this trial represent “a big advance,” according to Ihtsham Ul Haq, MD, chief, movement disorders division, University of Miami, who was reached for comment. He noted that continuous infusion delivery has been anticipated for some time, and he expects these types of systems to fulfill an unmet need.
“This will be a useful option in a carefully selected group of patients,” said Dr. Haq, who considers the types of improvement in ON time to be highly clinically meaningful.
However, he cautioned that the nodules created by injection site reactions might limit the utility of this treatment option in at least some patients. Wearing the external device might also be a limiting factor for some patients.
In complex Parkinson’s disease, a stage that can be reached fairly rapidly in some patients but might take 15 years or more in others, all of the options involve a careful benefit-to-risk calculation, according to Dr. Haq. Deep brain stimulation is among the most effective options, but continuous infusion might appeal to some patients for delaying this procedure or as an alternative.
“We need multiple options for these types of patients, and it appears that continuous infusion will be one of them,” Dr. Haq said.
Dr. Espay has financial relationships with Acadia, Acorda, Amneal, AskBio, Bexion, Kyowa Kirin, Neuroderm, Neurocrine, and Sunovion. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva. Dr. Haq reports no potential conflicts of interest.
BOSTON – presented at the 2023 annual meeting of the American Academy of Neurology.
When compared with optimized oral immediate-release medication, the delivery system, called ND0612 (NeuroDerm, Rehovot, Israel), improved ON time without troublesome dyskinesias while improving symptoms according to ratings from both patients and clinicians, according to Alberto J. Espay, MD, professor of neurology and director of the Gardner Family Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati.
The new delivery system addresses the challenge of reducing the variability in levodopa plasma concentrations, a major factor in motor fluctuations and diminishing benefit from orally administered drug, according to Dr. Espay. He said that continuous infusion strategies have long been sought as a method to preserve levodopa efficacy.
BouNDless findings
There were two phases to this multinational trial, called BouNDless. In the first, an open-label run-in phase, 381 patients with Parkinson’s disease were dose titrated for optimization of oral immediate-release levodopa and carbidopa. They were then optimized for the same drugs delivered with ND0612. The study was conducted over 12 weeks; 122 patients left the study after this phase due to adverse events, lack of efficacy, or withdrawal of consent.
In the second phase, the 259 remaining patients were randomized to the continuous infusion arm or to immediate release oral therapy. In this double-blind, double-dummy phase, those randomized to the ND0612 infusion also received oral placebos. Those randomized to oral therapy received a placebo infusion. Efficacy and safety were assessed at the end of 12 weeks.
At the end of phase 1, the ON time increased by about 3 hours when levodopa-carbidopa dosing was optimized on either delivery method. Dr. Espay attributed the improvement to the value of optimized dosing even in patients with relatively advanced disease.
However, for the purposes of the double-blind comparison, this improvement in ON time provided a new baseline for comparison of the two delivery methods. This is important for interpreting the primary result, which was a 1.72-hour difference in ON time at the end of the study. The difference was created when ON time was maintained with ND0612 continuous drug delivery but eroded in the group randomized to oral immediate-release treatment.
Several secondary endpoints supported the greater efficacy of continuous subcutaneous delivery. These included lower OFF time (0.50 vs. 1.90 hours), less accumulation of disability on the United Parkinson’s Disease Rating Scale part II-M-EDL (-0.30 vs. +2.75 points), and greater improvement on the Patient Global Impression of Change (+0.31 vs. +0.70 points), and the Clinical Global Impression of change (+0.31 vs. +0.77 points). The differences were highly statistically significant (all P < .0001).
The patients participating in the double-blind phase of the study were similar with a mean age of 63.5 years in both groups and time since Parkinson’s disease diagnosis (> 9 years). The median ON time without troublesome dyskinesias was about 12 hours at baseline in both groups and the median OFF time was about 3.5 hours.
The higher rate of treatment-related adverse events in the ND0612 group (67.2% vs. 52.7%) was largely explained by the greater rate of infusion site reactions (57.0% vs. 42.7%). The rates of severe reactions in the two groups were the same (0.8%), but both mild (43.8% vs. 36.6%) and moderate (12.5% vs. 5.3%) reactions occurred more commonly in the group receiving active therapy.
“Infusion reactions are the Achilles heel of all subcutaneous therapies,” acknowledged Dr. Espay, who expects other infusion systems in development to share this risk. He suggested that the clinical impact can be attenuated to some degree by rotating infusion sites.
BeyoND extension study
Data from an open-label extension (OLE) of the phase 2b BeyoND trial were also presented at the AAN meeting and generated generally similar results. Largely a safety study, there was no active control in the initial BeyoND or the BeyoND OLE. In BeyoND, the improvement in ON time from baseline was even greater than that seen in BouNDless, but, again, the optimization of dosing in the BouNDless run-in established a greater baseline of disease control.
In the OLE of BeyoND, presented by Aaron Ellenbogen, DO, a neurologist in Farmington, Mich., one of the notable findings was the retention of patients. After 2 years of follow-up, 82% completed at least 2 years of follow-up and 66.7% have now remained on treatment for at least 3 years. Dr. Ellenbogen maintains that this retention rate provides compelling evidence of a favorable benefit-to-risk ratio.
Fulfilling an unmet need
The favorable efficacy data from this trial represent “a big advance,” according to Ihtsham Ul Haq, MD, chief, movement disorders division, University of Miami, who was reached for comment. He noted that continuous infusion delivery has been anticipated for some time, and he expects these types of systems to fulfill an unmet need.
“This will be a useful option in a carefully selected group of patients,” said Dr. Haq, who considers the types of improvement in ON time to be highly clinically meaningful.
However, he cautioned that the nodules created by injection site reactions might limit the utility of this treatment option in at least some patients. Wearing the external device might also be a limiting factor for some patients.
In complex Parkinson’s disease, a stage that can be reached fairly rapidly in some patients but might take 15 years or more in others, all of the options involve a careful benefit-to-risk calculation, according to Dr. Haq. Deep brain stimulation is among the most effective options, but continuous infusion might appeal to some patients for delaying this procedure or as an alternative.
“We need multiple options for these types of patients, and it appears that continuous infusion will be one of them,” Dr. Haq said.
Dr. Espay has financial relationships with Acadia, Acorda, Amneal, AskBio, Bexion, Kyowa Kirin, Neuroderm, Neurocrine, and Sunovion. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva. Dr. Haq reports no potential conflicts of interest.
FROM AAN 2023
Cycle timing may reduce hormonal dosage for contraception
Progesterone and estrogen are often used for contraception by preventing ovulation, but the adverse effects associated with large doses of these hormones remain a concern, wrote Brenda Lyn A. Gavina, a PhD candidate at the University of the Philippines Diliman, Quezon City, and colleagues.
In a study published in PLoS Computational Biology, the researchers examined how the timing of hormone administration during a cycle might impact the amount of hormones needed for contraception. Previous research shown that combining hormones can reduce the dosage needed, but the impact of timing on further dose reduction has not been well studied, they said.
The researchers applied optimal control theory in a mathematical model to show the contraceptive effect of estrogen and/or progesterone at different times in the menstrual cycle. The model was based on a normal menstrual cycle with pituitary and ovarian phases. The model assumed that synthesis of luteinizing hormone and follicle-stimulating hormone occurs in the pituitary, that LH and FSH are held in reserve before release into the bloodstream, and that the follicular/luteal mass goes through nine ovarian stages of development. The model also included the activity of ovarian hormones estradiol (E2), progesterone (P4), and inhibin (Inh), in a normal cycle. In the model, LH, FSH, and E2 peaked in the late follicular phase, and P4 and Inh peaked in the luteal phase.
The pituitary model predicted the synthesis, release, and clearance of LH and FSH, and the response of the pituitary to E2, P4, and Inh. The ovarian model predicted the response of E2, P4, and Inh as functions of LH and FSH.
The researchers simulated a constant dose of exogenous progesterone monotherapy and combined exogenous estrogen/progesterone. They determined that a P4 peak of 4.99 ng/mL was taken as the optimum constant dosage for progesterone monotherapy, and for combination estrogen/progesterone.
The researchers then assessed the impact of time on dosage. They found that estrogen administration starting on the first day of a normal cycle preventing FHS from reaching maximum value, and that the low level of FHS in the follicular phase and additional P4 inhibition slowed follicular growth, and use of combination estrogen/progesterone caused similar inhibition at a later follicular stage.
“The combination therapy suggests that time-varying doses of estrogen and progesterone given simultaneously from the start to the end of the 28-day period, only requires a surge in estrogen dose around the 12th day of the cycle (a delayed administration compared to the estrogen monotherapy),” they noted.
With attention to timing, the maximum progesterone levels throughout a menstrual cycle were 4.43 ng/mL, 4.66 ng/mL, and 4.31 ng/mL for estrogen monotherapy, progesterone monotherapy, and combination therapy, respectively. Total doses of the optimal exogenous hormone were 77.76 pg/mL and 48.84 ng/mL for estrogen and progesterone monotherapy, respectively, and 35.58 pg/mL and 21.67 ng/mL for estrogen and progesterone in combination.
The findings were limited by the use of a standard model that does not account for variations in cycle length, the researchers noted. However, the results reflect other studies of hormonal activity, and the model can be used in future studies of the effect of hormones on cycle length, they said.
Overall, the researchers determined that timing dosage with estrogen monotherapy based on their model could provide effective contraception with about 92% of the minimum total constant dosage, while progesterone monotherapy would be effective with approximately 43% of the total constant dose.
Although more work is needed, the current study results may guide clinicians in experimenting with the optimal treatment regimen for anovulation, the researchers said.
“The results presented here give insights on construction of timed devices that give contraception at certain parts of the menstrual cycle,” they concluded.
Model aims to improve women’s control of contraception
“Aside from wanting to contribute to controlling population growth, we aim to empower women more by giving them more control on when to conceive and start motherhood,” and be in control of contraception in a safer way, said lead author Ms. Gavina, in an interview. In addition, studies are showing the noncontraceptive benefits of suppressing ovulation for managing premenstrual syndromes such as breast tenderness and irritability, and for managing diseases such as endometriosis, she said. “Anovulation also lowers the risk of ACL injuries in female athletes,” she added.
Ms. Gavina said that she was surprised primarily by the optimal control result for estrogen monotherapy. “It was surprising that, theoretically, our mathematical model, with the simplifying assumptions, showed that as low as 10% of the total dose in constant administration could achieve contraception as long as the administration of this dosage is perfectly timed, and the timing was also shown in our optimization result,” she said.
“Our model does not capture all factors in contraception, since the reproductive function in women is a very complex multiscale dynamical system highly dependent on both endogenous and exogenous hormones,” Ms. Gavina told this news organization. However, “with the emergence of more data, it can be refined to address other contraception issues. Further, although the results of this study are not directly translatable to the clinical setting, we hope that these results may aid clinicians in identifying the minimum dose and treatment schedule for contraception,” she said.
Future research directions include examining within and between women’s variabilities and adding a pharmacokinetics model to account for the effects of specific drugs, she said. “We also hope to expand or modify the current model to investigate reproductive health concerns in women, such as [polycystic ovary syndrome] and ovarian cysts,” she added.
Ms. Gavina disclosed support from the University of the Philippines Office of International Linkages, a Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement grant, and a Commission on Higher Education Faculty Development Program-II scholarship.
Progesterone and estrogen are often used for contraception by preventing ovulation, but the adverse effects associated with large doses of these hormones remain a concern, wrote Brenda Lyn A. Gavina, a PhD candidate at the University of the Philippines Diliman, Quezon City, and colleagues.
In a study published in PLoS Computational Biology, the researchers examined how the timing of hormone administration during a cycle might impact the amount of hormones needed for contraception. Previous research shown that combining hormones can reduce the dosage needed, but the impact of timing on further dose reduction has not been well studied, they said.
The researchers applied optimal control theory in a mathematical model to show the contraceptive effect of estrogen and/or progesterone at different times in the menstrual cycle. The model was based on a normal menstrual cycle with pituitary and ovarian phases. The model assumed that synthesis of luteinizing hormone and follicle-stimulating hormone occurs in the pituitary, that LH and FSH are held in reserve before release into the bloodstream, and that the follicular/luteal mass goes through nine ovarian stages of development. The model also included the activity of ovarian hormones estradiol (E2), progesterone (P4), and inhibin (Inh), in a normal cycle. In the model, LH, FSH, and E2 peaked in the late follicular phase, and P4 and Inh peaked in the luteal phase.
The pituitary model predicted the synthesis, release, and clearance of LH and FSH, and the response of the pituitary to E2, P4, and Inh. The ovarian model predicted the response of E2, P4, and Inh as functions of LH and FSH.
The researchers simulated a constant dose of exogenous progesterone monotherapy and combined exogenous estrogen/progesterone. They determined that a P4 peak of 4.99 ng/mL was taken as the optimum constant dosage for progesterone monotherapy, and for combination estrogen/progesterone.
The researchers then assessed the impact of time on dosage. They found that estrogen administration starting on the first day of a normal cycle preventing FHS from reaching maximum value, and that the low level of FHS in the follicular phase and additional P4 inhibition slowed follicular growth, and use of combination estrogen/progesterone caused similar inhibition at a later follicular stage.
“The combination therapy suggests that time-varying doses of estrogen and progesterone given simultaneously from the start to the end of the 28-day period, only requires a surge in estrogen dose around the 12th day of the cycle (a delayed administration compared to the estrogen monotherapy),” they noted.
With attention to timing, the maximum progesterone levels throughout a menstrual cycle were 4.43 ng/mL, 4.66 ng/mL, and 4.31 ng/mL for estrogen monotherapy, progesterone monotherapy, and combination therapy, respectively. Total doses of the optimal exogenous hormone were 77.76 pg/mL and 48.84 ng/mL for estrogen and progesterone monotherapy, respectively, and 35.58 pg/mL and 21.67 ng/mL for estrogen and progesterone in combination.
The findings were limited by the use of a standard model that does not account for variations in cycle length, the researchers noted. However, the results reflect other studies of hormonal activity, and the model can be used in future studies of the effect of hormones on cycle length, they said.
Overall, the researchers determined that timing dosage with estrogen monotherapy based on their model could provide effective contraception with about 92% of the minimum total constant dosage, while progesterone monotherapy would be effective with approximately 43% of the total constant dose.
Although more work is needed, the current study results may guide clinicians in experimenting with the optimal treatment regimen for anovulation, the researchers said.
“The results presented here give insights on construction of timed devices that give contraception at certain parts of the menstrual cycle,” they concluded.
Model aims to improve women’s control of contraception
“Aside from wanting to contribute to controlling population growth, we aim to empower women more by giving them more control on when to conceive and start motherhood,” and be in control of contraception in a safer way, said lead author Ms. Gavina, in an interview. In addition, studies are showing the noncontraceptive benefits of suppressing ovulation for managing premenstrual syndromes such as breast tenderness and irritability, and for managing diseases such as endometriosis, she said. “Anovulation also lowers the risk of ACL injuries in female athletes,” she added.
Ms. Gavina said that she was surprised primarily by the optimal control result for estrogen monotherapy. “It was surprising that, theoretically, our mathematical model, with the simplifying assumptions, showed that as low as 10% of the total dose in constant administration could achieve contraception as long as the administration of this dosage is perfectly timed, and the timing was also shown in our optimization result,” she said.
“Our model does not capture all factors in contraception, since the reproductive function in women is a very complex multiscale dynamical system highly dependent on both endogenous and exogenous hormones,” Ms. Gavina told this news organization. However, “with the emergence of more data, it can be refined to address other contraception issues. Further, although the results of this study are not directly translatable to the clinical setting, we hope that these results may aid clinicians in identifying the minimum dose and treatment schedule for contraception,” she said.
Future research directions include examining within and between women’s variabilities and adding a pharmacokinetics model to account for the effects of specific drugs, she said. “We also hope to expand or modify the current model to investigate reproductive health concerns in women, such as [polycystic ovary syndrome] and ovarian cysts,” she added.
Ms. Gavina disclosed support from the University of the Philippines Office of International Linkages, a Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement grant, and a Commission on Higher Education Faculty Development Program-II scholarship.
Progesterone and estrogen are often used for contraception by preventing ovulation, but the adverse effects associated with large doses of these hormones remain a concern, wrote Brenda Lyn A. Gavina, a PhD candidate at the University of the Philippines Diliman, Quezon City, and colleagues.
In a study published in PLoS Computational Biology, the researchers examined how the timing of hormone administration during a cycle might impact the amount of hormones needed for contraception. Previous research shown that combining hormones can reduce the dosage needed, but the impact of timing on further dose reduction has not been well studied, they said.
The researchers applied optimal control theory in a mathematical model to show the contraceptive effect of estrogen and/or progesterone at different times in the menstrual cycle. The model was based on a normal menstrual cycle with pituitary and ovarian phases. The model assumed that synthesis of luteinizing hormone and follicle-stimulating hormone occurs in the pituitary, that LH and FSH are held in reserve before release into the bloodstream, and that the follicular/luteal mass goes through nine ovarian stages of development. The model also included the activity of ovarian hormones estradiol (E2), progesterone (P4), and inhibin (Inh), in a normal cycle. In the model, LH, FSH, and E2 peaked in the late follicular phase, and P4 and Inh peaked in the luteal phase.
The pituitary model predicted the synthesis, release, and clearance of LH and FSH, and the response of the pituitary to E2, P4, and Inh. The ovarian model predicted the response of E2, P4, and Inh as functions of LH and FSH.
The researchers simulated a constant dose of exogenous progesterone monotherapy and combined exogenous estrogen/progesterone. They determined that a P4 peak of 4.99 ng/mL was taken as the optimum constant dosage for progesterone monotherapy, and for combination estrogen/progesterone.
The researchers then assessed the impact of time on dosage. They found that estrogen administration starting on the first day of a normal cycle preventing FHS from reaching maximum value, and that the low level of FHS in the follicular phase and additional P4 inhibition slowed follicular growth, and use of combination estrogen/progesterone caused similar inhibition at a later follicular stage.
“The combination therapy suggests that time-varying doses of estrogen and progesterone given simultaneously from the start to the end of the 28-day period, only requires a surge in estrogen dose around the 12th day of the cycle (a delayed administration compared to the estrogen monotherapy),” they noted.
With attention to timing, the maximum progesterone levels throughout a menstrual cycle were 4.43 ng/mL, 4.66 ng/mL, and 4.31 ng/mL for estrogen monotherapy, progesterone monotherapy, and combination therapy, respectively. Total doses of the optimal exogenous hormone were 77.76 pg/mL and 48.84 ng/mL for estrogen and progesterone monotherapy, respectively, and 35.58 pg/mL and 21.67 ng/mL for estrogen and progesterone in combination.
The findings were limited by the use of a standard model that does not account for variations in cycle length, the researchers noted. However, the results reflect other studies of hormonal activity, and the model can be used in future studies of the effect of hormones on cycle length, they said.
Overall, the researchers determined that timing dosage with estrogen monotherapy based on their model could provide effective contraception with about 92% of the minimum total constant dosage, while progesterone monotherapy would be effective with approximately 43% of the total constant dose.
Although more work is needed, the current study results may guide clinicians in experimenting with the optimal treatment regimen for anovulation, the researchers said.
“The results presented here give insights on construction of timed devices that give contraception at certain parts of the menstrual cycle,” they concluded.
Model aims to improve women’s control of contraception
“Aside from wanting to contribute to controlling population growth, we aim to empower women more by giving them more control on when to conceive and start motherhood,” and be in control of contraception in a safer way, said lead author Ms. Gavina, in an interview. In addition, studies are showing the noncontraceptive benefits of suppressing ovulation for managing premenstrual syndromes such as breast tenderness and irritability, and for managing diseases such as endometriosis, she said. “Anovulation also lowers the risk of ACL injuries in female athletes,” she added.
Ms. Gavina said that she was surprised primarily by the optimal control result for estrogen monotherapy. “It was surprising that, theoretically, our mathematical model, with the simplifying assumptions, showed that as low as 10% of the total dose in constant administration could achieve contraception as long as the administration of this dosage is perfectly timed, and the timing was also shown in our optimization result,” she said.
“Our model does not capture all factors in contraception, since the reproductive function in women is a very complex multiscale dynamical system highly dependent on both endogenous and exogenous hormones,” Ms. Gavina told this news organization. However, “with the emergence of more data, it can be refined to address other contraception issues. Further, although the results of this study are not directly translatable to the clinical setting, we hope that these results may aid clinicians in identifying the minimum dose and treatment schedule for contraception,” she said.
Future research directions include examining within and between women’s variabilities and adding a pharmacokinetics model to account for the effects of specific drugs, she said. “We also hope to expand or modify the current model to investigate reproductive health concerns in women, such as [polycystic ovary syndrome] and ovarian cysts,” she added.
Ms. Gavina disclosed support from the University of the Philippines Office of International Linkages, a Continuous Operational and Outcomes-based Partnership for Excellence in Research and Academic Training Enhancement grant, and a Commission on Higher Education Faculty Development Program-II scholarship.
FROM PLOS COMPUTATIONAL BIOLOGY
Child’s health improves by applying new obesity guidelines
At age 15 years, Maya was referred by her primary care provider to our pediatric obesity center. She weighed 151 kg and had a body mass index (BMI) over 48 kg/m2. One year earlier, she had been diagnosed with hypertension and prediabetes.
A review of her growth charts showed that she had been in the 95th percentile at age 8 years. Her weight had steadily risen, with an exponential increase of 55 lb between 2020 and 2022, during the COVID-19 pandemic. Her primary care provider monitored her from age 8 to 12 years, providing nutrition and physical activity counseling.
In February, the American Academy of Pediatrics released new clinical practice guidelines for managing childhood obesity. A better understanding of the pathophysiology has challenged the old-worn concept of lack of will power and personal responsibility as the cause of obesity, which has been the basis for weight-related bias and stigma. The updated guidelines have also been influenced by lifestyle intervention studies, the US Food and Drug Administration approval of new anti-obesity medications, and the 2013 designation of obesity as a disease by the American Medical Association.
We used these updated guidelines in our approach to treating Maya.
Starting with the assessment
In the new AAP guidelines, assessing the genetic, environmental, and social-determinant risks for obesity form the basis for evaluation and intervention. Following this approach, we conducted a complete medical evaluation of Maya, including a review of her symptoms and her family history along with a physical examination to assess for comorbidities and other cause of obesity (for example, genetic, hypothyroidism).
We also collected information regarding her diet and behaviors (for example, drinking sweet beverages, fruit and vegetable intake, parent feeding style, portion sizes, emotional eating, hyperphagia), physical activity behaviors (for example, physical education, organized sports), screen time, social drivers of health (for example, food insecurity, neighborhood, school environment), family and household factors (for example, family composition, support, number of caregivers, parenting style) and mental and physical health (autism, attention-deficit/hyperactivity disorder, history of being bullied, developmental and physical disabilities). Because Maya had a BMI of 48, she met the criterion for severe obesity, which is having a BMI at least 120% of the 95th percentile.
The guidelines use BMI as a criterion for screening for obesity because it is inexpensive and easy to obtain in the clinic setting. The Centers for Disease Control and Prevention growth chart uses BMI as well. Recently, there has been controversy about solely using BMI to define obesity, which is a point that the guidelines address by emphasizing evaluation of the whole child along with BMI to make a diagnosis of obesity.
The child’s age and the severity of their obesity drive the evaluation for comorbidities and treatment. In children aged 10 years or older, pediatricians and other primary care providers should evaluate for lipid abnormalities, abnormal glucose metabolism, and abnormal liver function in children and adolescents with obesity (BMI ≥ 95th percentile).
Maya presented with snoring, early-morning headaches, daytime sleepiness, and abdominal pain. A sleep study revealed an apnea-hypopnea index of 15, indicating obstructive sleep apnea, and she was placed on a continuous positive airway pressure machine.
Her laboratory studies showed elevated triglycerides of 169 mg/dL and abnormal ALT (123 IU/L). Potential causes of elevated liver function test results (such as abnormal ceruloplasmin levels or infectious or autoimmune hepatitis) were excluded, and a liver ultrasound with elastography indicated steatohepatitis. Maya was referred to gastroenterology for nonalcoholic fatty liver disease.
Maya experienced depressive symptoms, including difficulty with peer relationships and declining academic performance. Her Patient Health Questionnaire–9 score was 21, with a moderate impact on her daily functioning. Prior attempts at counseling had been sporadic and not helpful. She was diagnosed with intermittent moderate clinical depression, started on a selective serotonin reuptake inhibitor, and resumed counseling with a new therapist.
Considering treatment options
Based on shared decision-making, our team began a more intensive lifestyle behavior treatment as recommended in the updated guidelines. Maya chose to decrease sugar-sweetened beverages as her initial nutrition goal, a change that can lead to a reduction of liver function test results and triglycerides, even in the absence of weight loss.
As emphasized in the guidelines, we stressed the importance of managing obesity and comorbidities concurrently to the family. In addition to lifestyle behavior intervention, once her mental health stabilized, Maya and her mother opted for bariatric surgery. Sleeve gastrectomy was elected because she met the criteria.
If the child already has obesity, the guidelines discourage watchful waiting (that is, the expectation that the child will grow into their weight) as Maya’s primary care provider had done when she was younger. The staged treatment approach where progressively more intensive interventions are adopted (a hallmark of the 2007 guidelines) is no longer recommended. Rather, the primary care provider should offer treatment options guided by age, severity of obesity, and comorbidities.
Maya completed a bariatric preoperative program, extensive mental health evaluation, and tolerated the sleeve gastrectomy well with no complications. At her 6-month postoperative visit, she had lost 99 lb (45 kg) since the surgery, with an 18% decline in BMI. She is taking daily multivitamins as well as calcium and vitamin D. She continues to incorporate healthy eating into her life, with a focus on adequate protein intake and is exercising three to four times per week in the apartment complex gym. She reports better physical and mental health, her school performance has improved, and she still receives regular counseling.
Maya’s story outlines the benefits of early and intensive intervention as recommended by the new AAP guidelines. The shift from some of the earlier recommendations is partly driven by the persistence of childhood obesity into adulthood, especially for older children with serious psychosocial and physical comorbidities. Hopefully by implementing the new guidelines, the physician can provide empathetic, bias-free, and effective care that recognizes the needs and environment of the whole child.
Dr. Salhah is a pediatric endocrinology fellow at Nationwide Children’s Hospital, Columbus, Ohio. Dr. Eneli is director of the Center for Healthy Weight and Nutrition at Nationwide Children’s Hospital. Dr. Salhah reported no conflicts of interest. Dr. Eneli reported receiving research grants and income from the National Institutes of Health, the AAP, and the National Academy of Medicine.
A version of this article first appeared on Medscape.com.
At age 15 years, Maya was referred by her primary care provider to our pediatric obesity center. She weighed 151 kg and had a body mass index (BMI) over 48 kg/m2. One year earlier, she had been diagnosed with hypertension and prediabetes.
A review of her growth charts showed that she had been in the 95th percentile at age 8 years. Her weight had steadily risen, with an exponential increase of 55 lb between 2020 and 2022, during the COVID-19 pandemic. Her primary care provider monitored her from age 8 to 12 years, providing nutrition and physical activity counseling.
In February, the American Academy of Pediatrics released new clinical practice guidelines for managing childhood obesity. A better understanding of the pathophysiology has challenged the old-worn concept of lack of will power and personal responsibility as the cause of obesity, which has been the basis for weight-related bias and stigma. The updated guidelines have also been influenced by lifestyle intervention studies, the US Food and Drug Administration approval of new anti-obesity medications, and the 2013 designation of obesity as a disease by the American Medical Association.
We used these updated guidelines in our approach to treating Maya.
Starting with the assessment
In the new AAP guidelines, assessing the genetic, environmental, and social-determinant risks for obesity form the basis for evaluation and intervention. Following this approach, we conducted a complete medical evaluation of Maya, including a review of her symptoms and her family history along with a physical examination to assess for comorbidities and other cause of obesity (for example, genetic, hypothyroidism).
We also collected information regarding her diet and behaviors (for example, drinking sweet beverages, fruit and vegetable intake, parent feeding style, portion sizes, emotional eating, hyperphagia), physical activity behaviors (for example, physical education, organized sports), screen time, social drivers of health (for example, food insecurity, neighborhood, school environment), family and household factors (for example, family composition, support, number of caregivers, parenting style) and mental and physical health (autism, attention-deficit/hyperactivity disorder, history of being bullied, developmental and physical disabilities). Because Maya had a BMI of 48, she met the criterion for severe obesity, which is having a BMI at least 120% of the 95th percentile.
The guidelines use BMI as a criterion for screening for obesity because it is inexpensive and easy to obtain in the clinic setting. The Centers for Disease Control and Prevention growth chart uses BMI as well. Recently, there has been controversy about solely using BMI to define obesity, which is a point that the guidelines address by emphasizing evaluation of the whole child along with BMI to make a diagnosis of obesity.
The child’s age and the severity of their obesity drive the evaluation for comorbidities and treatment. In children aged 10 years or older, pediatricians and other primary care providers should evaluate for lipid abnormalities, abnormal glucose metabolism, and abnormal liver function in children and adolescents with obesity (BMI ≥ 95th percentile).
Maya presented with snoring, early-morning headaches, daytime sleepiness, and abdominal pain. A sleep study revealed an apnea-hypopnea index of 15, indicating obstructive sleep apnea, and she was placed on a continuous positive airway pressure machine.
Her laboratory studies showed elevated triglycerides of 169 mg/dL and abnormal ALT (123 IU/L). Potential causes of elevated liver function test results (such as abnormal ceruloplasmin levels or infectious or autoimmune hepatitis) were excluded, and a liver ultrasound with elastography indicated steatohepatitis. Maya was referred to gastroenterology for nonalcoholic fatty liver disease.
Maya experienced depressive symptoms, including difficulty with peer relationships and declining academic performance. Her Patient Health Questionnaire–9 score was 21, with a moderate impact on her daily functioning. Prior attempts at counseling had been sporadic and not helpful. She was diagnosed with intermittent moderate clinical depression, started on a selective serotonin reuptake inhibitor, and resumed counseling with a new therapist.
Considering treatment options
Based on shared decision-making, our team began a more intensive lifestyle behavior treatment as recommended in the updated guidelines. Maya chose to decrease sugar-sweetened beverages as her initial nutrition goal, a change that can lead to a reduction of liver function test results and triglycerides, even in the absence of weight loss.
As emphasized in the guidelines, we stressed the importance of managing obesity and comorbidities concurrently to the family. In addition to lifestyle behavior intervention, once her mental health stabilized, Maya and her mother opted for bariatric surgery. Sleeve gastrectomy was elected because she met the criteria.
If the child already has obesity, the guidelines discourage watchful waiting (that is, the expectation that the child will grow into their weight) as Maya’s primary care provider had done when she was younger. The staged treatment approach where progressively more intensive interventions are adopted (a hallmark of the 2007 guidelines) is no longer recommended. Rather, the primary care provider should offer treatment options guided by age, severity of obesity, and comorbidities.
Maya completed a bariatric preoperative program, extensive mental health evaluation, and tolerated the sleeve gastrectomy well with no complications. At her 6-month postoperative visit, she had lost 99 lb (45 kg) since the surgery, with an 18% decline in BMI. She is taking daily multivitamins as well as calcium and vitamin D. She continues to incorporate healthy eating into her life, with a focus on adequate protein intake and is exercising three to four times per week in the apartment complex gym. She reports better physical and mental health, her school performance has improved, and she still receives regular counseling.
Maya’s story outlines the benefits of early and intensive intervention as recommended by the new AAP guidelines. The shift from some of the earlier recommendations is partly driven by the persistence of childhood obesity into adulthood, especially for older children with serious psychosocial and physical comorbidities. Hopefully by implementing the new guidelines, the physician can provide empathetic, bias-free, and effective care that recognizes the needs and environment of the whole child.
Dr. Salhah is a pediatric endocrinology fellow at Nationwide Children’s Hospital, Columbus, Ohio. Dr. Eneli is director of the Center for Healthy Weight and Nutrition at Nationwide Children’s Hospital. Dr. Salhah reported no conflicts of interest. Dr. Eneli reported receiving research grants and income from the National Institutes of Health, the AAP, and the National Academy of Medicine.
A version of this article first appeared on Medscape.com.
At age 15 years, Maya was referred by her primary care provider to our pediatric obesity center. She weighed 151 kg and had a body mass index (BMI) over 48 kg/m2. One year earlier, she had been diagnosed with hypertension and prediabetes.
A review of her growth charts showed that she had been in the 95th percentile at age 8 years. Her weight had steadily risen, with an exponential increase of 55 lb between 2020 and 2022, during the COVID-19 pandemic. Her primary care provider monitored her from age 8 to 12 years, providing nutrition and physical activity counseling.
In February, the American Academy of Pediatrics released new clinical practice guidelines for managing childhood obesity. A better understanding of the pathophysiology has challenged the old-worn concept of lack of will power and personal responsibility as the cause of obesity, which has been the basis for weight-related bias and stigma. The updated guidelines have also been influenced by lifestyle intervention studies, the US Food and Drug Administration approval of new anti-obesity medications, and the 2013 designation of obesity as a disease by the American Medical Association.
We used these updated guidelines in our approach to treating Maya.
Starting with the assessment
In the new AAP guidelines, assessing the genetic, environmental, and social-determinant risks for obesity form the basis for evaluation and intervention. Following this approach, we conducted a complete medical evaluation of Maya, including a review of her symptoms and her family history along with a physical examination to assess for comorbidities and other cause of obesity (for example, genetic, hypothyroidism).
We also collected information regarding her diet and behaviors (for example, drinking sweet beverages, fruit and vegetable intake, parent feeding style, portion sizes, emotional eating, hyperphagia), physical activity behaviors (for example, physical education, organized sports), screen time, social drivers of health (for example, food insecurity, neighborhood, school environment), family and household factors (for example, family composition, support, number of caregivers, parenting style) and mental and physical health (autism, attention-deficit/hyperactivity disorder, history of being bullied, developmental and physical disabilities). Because Maya had a BMI of 48, she met the criterion for severe obesity, which is having a BMI at least 120% of the 95th percentile.
The guidelines use BMI as a criterion for screening for obesity because it is inexpensive and easy to obtain in the clinic setting. The Centers for Disease Control and Prevention growth chart uses BMI as well. Recently, there has been controversy about solely using BMI to define obesity, which is a point that the guidelines address by emphasizing evaluation of the whole child along with BMI to make a diagnosis of obesity.
The child’s age and the severity of their obesity drive the evaluation for comorbidities and treatment. In children aged 10 years or older, pediatricians and other primary care providers should evaluate for lipid abnormalities, abnormal glucose metabolism, and abnormal liver function in children and adolescents with obesity (BMI ≥ 95th percentile).
Maya presented with snoring, early-morning headaches, daytime sleepiness, and abdominal pain. A sleep study revealed an apnea-hypopnea index of 15, indicating obstructive sleep apnea, and she was placed on a continuous positive airway pressure machine.
Her laboratory studies showed elevated triglycerides of 169 mg/dL and abnormal ALT (123 IU/L). Potential causes of elevated liver function test results (such as abnormal ceruloplasmin levels or infectious or autoimmune hepatitis) were excluded, and a liver ultrasound with elastography indicated steatohepatitis. Maya was referred to gastroenterology for nonalcoholic fatty liver disease.
Maya experienced depressive symptoms, including difficulty with peer relationships and declining academic performance. Her Patient Health Questionnaire–9 score was 21, with a moderate impact on her daily functioning. Prior attempts at counseling had been sporadic and not helpful. She was diagnosed with intermittent moderate clinical depression, started on a selective serotonin reuptake inhibitor, and resumed counseling with a new therapist.
Considering treatment options
Based on shared decision-making, our team began a more intensive lifestyle behavior treatment as recommended in the updated guidelines. Maya chose to decrease sugar-sweetened beverages as her initial nutrition goal, a change that can lead to a reduction of liver function test results and triglycerides, even in the absence of weight loss.
As emphasized in the guidelines, we stressed the importance of managing obesity and comorbidities concurrently to the family. In addition to lifestyle behavior intervention, once her mental health stabilized, Maya and her mother opted for bariatric surgery. Sleeve gastrectomy was elected because she met the criteria.
If the child already has obesity, the guidelines discourage watchful waiting (that is, the expectation that the child will grow into their weight) as Maya’s primary care provider had done when she was younger. The staged treatment approach where progressively more intensive interventions are adopted (a hallmark of the 2007 guidelines) is no longer recommended. Rather, the primary care provider should offer treatment options guided by age, severity of obesity, and comorbidities.
Maya completed a bariatric preoperative program, extensive mental health evaluation, and tolerated the sleeve gastrectomy well with no complications. At her 6-month postoperative visit, she had lost 99 lb (45 kg) since the surgery, with an 18% decline in BMI. She is taking daily multivitamins as well as calcium and vitamin D. She continues to incorporate healthy eating into her life, with a focus on adequate protein intake and is exercising three to four times per week in the apartment complex gym. She reports better physical and mental health, her school performance has improved, and she still receives regular counseling.
Maya’s story outlines the benefits of early and intensive intervention as recommended by the new AAP guidelines. The shift from some of the earlier recommendations is partly driven by the persistence of childhood obesity into adulthood, especially for older children with serious psychosocial and physical comorbidities. Hopefully by implementing the new guidelines, the physician can provide empathetic, bias-free, and effective care that recognizes the needs and environment of the whole child.
Dr. Salhah is a pediatric endocrinology fellow at Nationwide Children’s Hospital, Columbus, Ohio. Dr. Eneli is director of the Center for Healthy Weight and Nutrition at Nationwide Children’s Hospital. Dr. Salhah reported no conflicts of interest. Dr. Eneli reported receiving research grants and income from the National Institutes of Health, the AAP, and the National Academy of Medicine.
A version of this article first appeared on Medscape.com.