User login
Drive, chip, and putt your way to osteoarthritis relief
Taking a swing against arthritis
Osteoarthritis is a tough disease to manage. Exercise helps ease the stiffness and pain of the joints, but at the same time, the disease makes it difficult to do that beneficial exercise. Even a relatively simple activity like jogging can hurt more than it helps. If only there were a low-impact exercise that was incredibly popular among the generally older population who are likely to have arthritis.
We love a good golf study here at LOTME, and a group of Australian and U.K. researchers have provided. Osteoarthritis affects 2 million people in the land down under, making it the most common source of disability there. In that population, only 64% reported their physical health to be good, very good, or excellent. Among the 459 golfers with OA that the study authors surveyed, however, the percentage reporting good health rose to more than 90%.
A similar story emerged when they looked at mental health. Nearly a quarter of nongolfers with OA reported high or very high levels of psychological distress, compared with just 8% of golfers. This pattern of improved physical and mental health remained when the researchers looked at the general, non-OA population.
This isn’t the first time golf’s been connected with improved health, and previous studies have shown golf to reduce the risks of cardiovascular disease, diabetes, and obesity, among other things. Just walking one 18-hole round significantly exceeds the CDC’s recommended 150 minutes of physical activity per week. Go out multiple times a week – leaving the cart and beer at home, American golfers – and you’ll be fit for a lifetime.
The golfers on our staff, however, are still waiting for those mental health benefits to kick in. Because when we’re adding up our scorecard after that string of four double bogeys to end the round, we’re most definitely thinking: “Yes, this sport is reducing my psychological distress. I am having fun right now.”
Battle of the sexes’ intestines
There are, we’re sure you’ve noticed, some differences between males and females. Females, for one thing, have longer small intestines than males. Everybody knows that, right? You didn’t know? Really? … Really?
Well, then, we’re guessing you haven’t read “Hidden diversity: Comparative functional morphology of humans and other species” by Erin A. McKenney, PhD, of North Carolina State University, Raleigh, and associates, which just appeared in PeerJ. We couldn’t put it down, even in the shower – a real page-turner/scroller. (It’s a great way to clean a phone, for those who also like to scroll, text, or talk on the toilet.)
The researchers got out their rulers, calipers, and string and took many measurements of the digestive systems of 45 human cadavers (21 female and 24 male), which were compared with data from 10 rats, 10 pigs, and 10 bullfrogs, which had been collected (the measurements, not the animals) by undergraduate students enrolled in a comparative anatomy laboratory course at the university.
There was little intestinal-length variation among the four-legged subjects, but when it comes to humans, females have “consistently and significantly longer small intestines than males,” the investigators noted.
The women’s small intestines, almost 14 feet long on average, were about a foot longer than the men’s, which suggests that women are better able to extract nutrients from food and “supports the canalization hypothesis, which posits that women are better able to survive during periods of stress,” coauthor Amanda Hale said in a written statement from the school. The way to a man’s heart may be through his stomach, but the way to a woman’s heart is through her duodenum, it seems.
Fascinating stuff, to be sure, but the thing that really caught our eye in the PeerJ article was the authors’ suggestion “that organs behave independently of one another, both within and across species.” Organs behaving independently? A somewhat ominous concept, no doubt, but it does explain a lot of the sounds we hear coming from our guts, which can get pretty frightening, especially on chili night.
Dog walking is dangerous business
Yes, you did read that right. A lot of strange things can send you to the emergency department. Go ahead and add dog walking onto that list.
Investigators from Johns Hopkins University estimate that over 422,000 adults presented to U.S. emergency departments with leash-dependent dog walking-related injuries between 2001 and 2020.
With almost 53% of U.S. households owning at least one dog in 2021-2022 in the wake of the COVID pet boom, this kind of occurrence is becoming more common than you think. The annual number of dog-walking injuries more than quadrupled from 7,300 to 32,000 over the course of the study, and the researchers link that spike to the promotion of dog walking for fitness, along with the boost of ownership itself.
The most common injuries listed in the National Electronic Injury Surveillance System database were finger fracture, traumatic brain injury, and shoulder sprain or strain. These mostly involved falls from being pulled, tripped, or tangled up in the leash while walking. For those aged 65 years and older, traumatic brain injury and hip fracture were the most common.
Women were 50% more likely to sustain a fracture than were men, and dog owners aged 65 and older were three times as likely to fall, twice as likely to get a fracture, and 60% more likely to have brain injury than were younger people. Now, that’s not to say younger people don’t also get hurt. After all, dogs aren’t ageists. The researchers have that data but it’s coming out later.
Meanwhile, the pitfalls involved with just trying to get our daily steps in while letting Muffin do her business have us on the lookout for random squirrels.
Taking a swing against arthritis
Osteoarthritis is a tough disease to manage. Exercise helps ease the stiffness and pain of the joints, but at the same time, the disease makes it difficult to do that beneficial exercise. Even a relatively simple activity like jogging can hurt more than it helps. If only there were a low-impact exercise that was incredibly popular among the generally older population who are likely to have arthritis.
We love a good golf study here at LOTME, and a group of Australian and U.K. researchers have provided. Osteoarthritis affects 2 million people in the land down under, making it the most common source of disability there. In that population, only 64% reported their physical health to be good, very good, or excellent. Among the 459 golfers with OA that the study authors surveyed, however, the percentage reporting good health rose to more than 90%.
A similar story emerged when they looked at mental health. Nearly a quarter of nongolfers with OA reported high or very high levels of psychological distress, compared with just 8% of golfers. This pattern of improved physical and mental health remained when the researchers looked at the general, non-OA population.
This isn’t the first time golf’s been connected with improved health, and previous studies have shown golf to reduce the risks of cardiovascular disease, diabetes, and obesity, among other things. Just walking one 18-hole round significantly exceeds the CDC’s recommended 150 minutes of physical activity per week. Go out multiple times a week – leaving the cart and beer at home, American golfers – and you’ll be fit for a lifetime.
The golfers on our staff, however, are still waiting for those mental health benefits to kick in. Because when we’re adding up our scorecard after that string of four double bogeys to end the round, we’re most definitely thinking: “Yes, this sport is reducing my psychological distress. I am having fun right now.”
Battle of the sexes’ intestines
There are, we’re sure you’ve noticed, some differences between males and females. Females, for one thing, have longer small intestines than males. Everybody knows that, right? You didn’t know? Really? … Really?
Well, then, we’re guessing you haven’t read “Hidden diversity: Comparative functional morphology of humans and other species” by Erin A. McKenney, PhD, of North Carolina State University, Raleigh, and associates, which just appeared in PeerJ. We couldn’t put it down, even in the shower – a real page-turner/scroller. (It’s a great way to clean a phone, for those who also like to scroll, text, or talk on the toilet.)
The researchers got out their rulers, calipers, and string and took many measurements of the digestive systems of 45 human cadavers (21 female and 24 male), which were compared with data from 10 rats, 10 pigs, and 10 bullfrogs, which had been collected (the measurements, not the animals) by undergraduate students enrolled in a comparative anatomy laboratory course at the university.
There was little intestinal-length variation among the four-legged subjects, but when it comes to humans, females have “consistently and significantly longer small intestines than males,” the investigators noted.
The women’s small intestines, almost 14 feet long on average, were about a foot longer than the men’s, which suggests that women are better able to extract nutrients from food and “supports the canalization hypothesis, which posits that women are better able to survive during periods of stress,” coauthor Amanda Hale said in a written statement from the school. The way to a man’s heart may be through his stomach, but the way to a woman’s heart is through her duodenum, it seems.
Fascinating stuff, to be sure, but the thing that really caught our eye in the PeerJ article was the authors’ suggestion “that organs behave independently of one another, both within and across species.” Organs behaving independently? A somewhat ominous concept, no doubt, but it does explain a lot of the sounds we hear coming from our guts, which can get pretty frightening, especially on chili night.
Dog walking is dangerous business
Yes, you did read that right. A lot of strange things can send you to the emergency department. Go ahead and add dog walking onto that list.
Investigators from Johns Hopkins University estimate that over 422,000 adults presented to U.S. emergency departments with leash-dependent dog walking-related injuries between 2001 and 2020.
With almost 53% of U.S. households owning at least one dog in 2021-2022 in the wake of the COVID pet boom, this kind of occurrence is becoming more common than you think. The annual number of dog-walking injuries more than quadrupled from 7,300 to 32,000 over the course of the study, and the researchers link that spike to the promotion of dog walking for fitness, along with the boost of ownership itself.
The most common injuries listed in the National Electronic Injury Surveillance System database were finger fracture, traumatic brain injury, and shoulder sprain or strain. These mostly involved falls from being pulled, tripped, or tangled up in the leash while walking. For those aged 65 years and older, traumatic brain injury and hip fracture were the most common.
Women were 50% more likely to sustain a fracture than were men, and dog owners aged 65 and older were three times as likely to fall, twice as likely to get a fracture, and 60% more likely to have brain injury than were younger people. Now, that’s not to say younger people don’t also get hurt. After all, dogs aren’t ageists. The researchers have that data but it’s coming out later.
Meanwhile, the pitfalls involved with just trying to get our daily steps in while letting Muffin do her business have us on the lookout for random squirrels.
Taking a swing against arthritis
Osteoarthritis is a tough disease to manage. Exercise helps ease the stiffness and pain of the joints, but at the same time, the disease makes it difficult to do that beneficial exercise. Even a relatively simple activity like jogging can hurt more than it helps. If only there were a low-impact exercise that was incredibly popular among the generally older population who are likely to have arthritis.
We love a good golf study here at LOTME, and a group of Australian and U.K. researchers have provided. Osteoarthritis affects 2 million people in the land down under, making it the most common source of disability there. In that population, only 64% reported their physical health to be good, very good, or excellent. Among the 459 golfers with OA that the study authors surveyed, however, the percentage reporting good health rose to more than 90%.
A similar story emerged when they looked at mental health. Nearly a quarter of nongolfers with OA reported high or very high levels of psychological distress, compared with just 8% of golfers. This pattern of improved physical and mental health remained when the researchers looked at the general, non-OA population.
This isn’t the first time golf’s been connected with improved health, and previous studies have shown golf to reduce the risks of cardiovascular disease, diabetes, and obesity, among other things. Just walking one 18-hole round significantly exceeds the CDC’s recommended 150 minutes of physical activity per week. Go out multiple times a week – leaving the cart and beer at home, American golfers – and you’ll be fit for a lifetime.
The golfers on our staff, however, are still waiting for those mental health benefits to kick in. Because when we’re adding up our scorecard after that string of four double bogeys to end the round, we’re most definitely thinking: “Yes, this sport is reducing my psychological distress. I am having fun right now.”
Battle of the sexes’ intestines
There are, we’re sure you’ve noticed, some differences between males and females. Females, for one thing, have longer small intestines than males. Everybody knows that, right? You didn’t know? Really? … Really?
Well, then, we’re guessing you haven’t read “Hidden diversity: Comparative functional morphology of humans and other species” by Erin A. McKenney, PhD, of North Carolina State University, Raleigh, and associates, which just appeared in PeerJ. We couldn’t put it down, even in the shower – a real page-turner/scroller. (It’s a great way to clean a phone, for those who also like to scroll, text, or talk on the toilet.)
The researchers got out their rulers, calipers, and string and took many measurements of the digestive systems of 45 human cadavers (21 female and 24 male), which were compared with data from 10 rats, 10 pigs, and 10 bullfrogs, which had been collected (the measurements, not the animals) by undergraduate students enrolled in a comparative anatomy laboratory course at the university.
There was little intestinal-length variation among the four-legged subjects, but when it comes to humans, females have “consistently and significantly longer small intestines than males,” the investigators noted.
The women’s small intestines, almost 14 feet long on average, were about a foot longer than the men’s, which suggests that women are better able to extract nutrients from food and “supports the canalization hypothesis, which posits that women are better able to survive during periods of stress,” coauthor Amanda Hale said in a written statement from the school. The way to a man’s heart may be through his stomach, but the way to a woman’s heart is through her duodenum, it seems.
Fascinating stuff, to be sure, but the thing that really caught our eye in the PeerJ article was the authors’ suggestion “that organs behave independently of one another, both within and across species.” Organs behaving independently? A somewhat ominous concept, no doubt, but it does explain a lot of the sounds we hear coming from our guts, which can get pretty frightening, especially on chili night.
Dog walking is dangerous business
Yes, you did read that right. A lot of strange things can send you to the emergency department. Go ahead and add dog walking onto that list.
Investigators from Johns Hopkins University estimate that over 422,000 adults presented to U.S. emergency departments with leash-dependent dog walking-related injuries between 2001 and 2020.
With almost 53% of U.S. households owning at least one dog in 2021-2022 in the wake of the COVID pet boom, this kind of occurrence is becoming more common than you think. The annual number of dog-walking injuries more than quadrupled from 7,300 to 32,000 over the course of the study, and the researchers link that spike to the promotion of dog walking for fitness, along with the boost of ownership itself.
The most common injuries listed in the National Electronic Injury Surveillance System database were finger fracture, traumatic brain injury, and shoulder sprain or strain. These mostly involved falls from being pulled, tripped, or tangled up in the leash while walking. For those aged 65 years and older, traumatic brain injury and hip fracture were the most common.
Women were 50% more likely to sustain a fracture than were men, and dog owners aged 65 and older were three times as likely to fall, twice as likely to get a fracture, and 60% more likely to have brain injury than were younger people. Now, that’s not to say younger people don’t also get hurt. After all, dogs aren’t ageists. The researchers have that data but it’s coming out later.
Meanwhile, the pitfalls involved with just trying to get our daily steps in while letting Muffin do her business have us on the lookout for random squirrels.
FDA gives fast-track approval to new ALS drug
the debilitating and deadly disease for which there is no cure.
Most people with ALS die within 3-5 years of when symptoms appear, usually of respiratory failure.
The newly approved drug, called Qalsody, is made by the Swiss company Biogen. The FDA fast-tracked the approval based on early trial results. The agency said in a news release that its decision was based on the demonstrated ability of the drug to reduce a protein in the blood that is a sign of degeneration of brain and nerve cells.
While the drug was shown to impact the chemical process in the body linked to degeneration, there was no significant change in people’s symptoms during the first 28 weeks that they took the drug, Biogen said in a news release. But the company noted that some patients did see improved functioning after starting treatment.
“I have observed the positive impact Qalsody has on slowing the progression of ALS in people with SOD1 mutations,” Timothy M. Miller, MD, PhD, researcher and codirector of the ALS Center at Washington University in St. Louis, said in a statement released by Biogen. “The FDA’s approval of Qalsody gives me hope that people living with this rare form of ALS could experience a reduction in decline in strength, clinical function, and respiratory function.”
Qalsody is given to people via a spinal injection, with an initial course of three injections every 2 weeks. People then get the injection once every 28 days.
The new treatment is approved only for people with a rare kind of ALS called SOD1-ALS, which is known for a genetic mutation. While ALS affects up to 32,000 people in the United States, just 2% of people with ALS have the SOD1 gene mutation. The FDA says the number of people in the United States who could use Qalsody is about 500.
In trials, 147 people received either Qalsody or a placebo, and the treatment significantly reduced the level of a protein in people’s blood that is associated with the loss of control of voluntary muscles.
Because Qalsody received a fast-track approval from the FDA, it must still provide more research data in the future, including from a trial examining how the drug affects people who carry the SOD1 gene but do not yet show symptoms of ALS.
A version of this article first appeared on Medscape.com.
the debilitating and deadly disease for which there is no cure.
Most people with ALS die within 3-5 years of when symptoms appear, usually of respiratory failure.
The newly approved drug, called Qalsody, is made by the Swiss company Biogen. The FDA fast-tracked the approval based on early trial results. The agency said in a news release that its decision was based on the demonstrated ability of the drug to reduce a protein in the blood that is a sign of degeneration of brain and nerve cells.
While the drug was shown to impact the chemical process in the body linked to degeneration, there was no significant change in people’s symptoms during the first 28 weeks that they took the drug, Biogen said in a news release. But the company noted that some patients did see improved functioning after starting treatment.
“I have observed the positive impact Qalsody has on slowing the progression of ALS in people with SOD1 mutations,” Timothy M. Miller, MD, PhD, researcher and codirector of the ALS Center at Washington University in St. Louis, said in a statement released by Biogen. “The FDA’s approval of Qalsody gives me hope that people living with this rare form of ALS could experience a reduction in decline in strength, clinical function, and respiratory function.”
Qalsody is given to people via a spinal injection, with an initial course of three injections every 2 weeks. People then get the injection once every 28 days.
The new treatment is approved only for people with a rare kind of ALS called SOD1-ALS, which is known for a genetic mutation. While ALS affects up to 32,000 people in the United States, just 2% of people with ALS have the SOD1 gene mutation. The FDA says the number of people in the United States who could use Qalsody is about 500.
In trials, 147 people received either Qalsody or a placebo, and the treatment significantly reduced the level of a protein in people’s blood that is associated with the loss of control of voluntary muscles.
Because Qalsody received a fast-track approval from the FDA, it must still provide more research data in the future, including from a trial examining how the drug affects people who carry the SOD1 gene but do not yet show symptoms of ALS.
A version of this article first appeared on Medscape.com.
the debilitating and deadly disease for which there is no cure.
Most people with ALS die within 3-5 years of when symptoms appear, usually of respiratory failure.
The newly approved drug, called Qalsody, is made by the Swiss company Biogen. The FDA fast-tracked the approval based on early trial results. The agency said in a news release that its decision was based on the demonstrated ability of the drug to reduce a protein in the blood that is a sign of degeneration of brain and nerve cells.
While the drug was shown to impact the chemical process in the body linked to degeneration, there was no significant change in people’s symptoms during the first 28 weeks that they took the drug, Biogen said in a news release. But the company noted that some patients did see improved functioning after starting treatment.
“I have observed the positive impact Qalsody has on slowing the progression of ALS in people with SOD1 mutations,” Timothy M. Miller, MD, PhD, researcher and codirector of the ALS Center at Washington University in St. Louis, said in a statement released by Biogen. “The FDA’s approval of Qalsody gives me hope that people living with this rare form of ALS could experience a reduction in decline in strength, clinical function, and respiratory function.”
Qalsody is given to people via a spinal injection, with an initial course of three injections every 2 weeks. People then get the injection once every 28 days.
The new treatment is approved only for people with a rare kind of ALS called SOD1-ALS, which is known for a genetic mutation. While ALS affects up to 32,000 people in the United States, just 2% of people with ALS have the SOD1 gene mutation. The FDA says the number of people in the United States who could use Qalsody is about 500.
In trials, 147 people received either Qalsody or a placebo, and the treatment significantly reduced the level of a protein in people’s blood that is associated with the loss of control of voluntary muscles.
Because Qalsody received a fast-track approval from the FDA, it must still provide more research data in the future, including from a trial examining how the drug affects people who carry the SOD1 gene but do not yet show symptoms of ALS.
A version of this article first appeared on Medscape.com.
Branding tattoo removal helps sex trafficking survivor close door on painful past
PHOENIX – When Kathy Givens walked onstage during a plenary session at the annual conference of the American Society for Laser Medicine and Surgery to reflect on her 9-month ordeal being sex trafficked in Texas more than 20 years ago, you could hear a pin drop.
“One of the scariest things about the life of sex trafficking is not knowing who’s going to be on the other side,” said Ms. Givens, who now lives in Houston. “There was some violence. There were some horrible things that happened. But you know what was really scary? When I got out. People may ask, ‘How’s that so? You escaped your trafficker. The past is behind you. Why were you afraid?’ I was afraid because I didn’t know that I had community. I didn’t know that community or that society would care about someone like me.”
She said that she found herself immobilized by fear of being shamed in society and labeled a sex trafficking victim, and wondered if she could overcome that fear and if anyone would view her as human again. Once free from her trafficker, she began a “healing journey,” which included getting married, raising four children, and re-enrolling in college with hopes of becoming a social worker. In 2020, she and her husband founded Twelve 11 Partners, an organization committed to supporting human trafficking survivors.
“I was working in the anti-trafficking field helping other survivors ... who have experienced this horrific crime,” she said. “I thought I was on my way.” But one “stain” from her sex trafficking past remained: The name of her trafficker was tattooed on her skin, “a reminder of what I’d gone through.”
Ms. Givens was eventually introduced to Paul M. Friedman, MD, the current ASLMS president and one of the that was formed in 2022. According to a survey that Dr. Friedman and colleagues presented at the 2022 annual ASLMS conference, an estimated 1 in 2 sex trafficking survivors have branding tattoos, and at least 1,000 survivors a year could benefit from removal of those tattoos.
“To date, 87 physicians in the U.S. and one in Canada have stepped forward to volunteer their services to be part of this program,” Dr. Friedman, who directs the Dermatology and Laser Surgery Center in Houston, said at this year’s meeting. “My goal is to double this number by the next annual conference,” he added, noting that trauma-informed training is part of the program, “to support the survivor experience during the treatment process.”
ASLMS is also working on this issue in partnership with the American Academy of Dermatology (AAD) Ad Hoc Task Force on Dermatological Resources for the Intervention and Prevention of Human Trafficking, which is headed by Boston dermatologist Shadi Kourosh, MD.
“Dermatologists are uniquely positioned to aid in efforts to assist those experiences in trafficking with our training to recognize and diagnose relevant signs on the skin and to assist patients with certain aspects of care and recovery including the treatment of the disease of scars and tattoos,” Dr. Friedman said. “Ultimately, we hope to create a database together to improve recognition of branding tattoos to aid in identifying sex trafficking victims.”
Ms. Givens, who sits on the U.S. Advisory Council on Human Trafficking, said that she was able to truly close the door on her sex trafficking past thanks to the tattoo removal Dr. Friedman performed as part of New Beginnings. “It means the world to me to know that I can now be an advocate for other individuals who have experienced human trafficking,” she told meeting attendees.
“Again, one of the scariest things is not knowing that you have community. I was scared of losing hope, but I’m standing here today. I have all the hope that I need. You have the power to change lives.”
PHOENIX – When Kathy Givens walked onstage during a plenary session at the annual conference of the American Society for Laser Medicine and Surgery to reflect on her 9-month ordeal being sex trafficked in Texas more than 20 years ago, you could hear a pin drop.
“One of the scariest things about the life of sex trafficking is not knowing who’s going to be on the other side,” said Ms. Givens, who now lives in Houston. “There was some violence. There were some horrible things that happened. But you know what was really scary? When I got out. People may ask, ‘How’s that so? You escaped your trafficker. The past is behind you. Why were you afraid?’ I was afraid because I didn’t know that I had community. I didn’t know that community or that society would care about someone like me.”
She said that she found herself immobilized by fear of being shamed in society and labeled a sex trafficking victim, and wondered if she could overcome that fear and if anyone would view her as human again. Once free from her trafficker, she began a “healing journey,” which included getting married, raising four children, and re-enrolling in college with hopes of becoming a social worker. In 2020, she and her husband founded Twelve 11 Partners, an organization committed to supporting human trafficking survivors.
“I was working in the anti-trafficking field helping other survivors ... who have experienced this horrific crime,” she said. “I thought I was on my way.” But one “stain” from her sex trafficking past remained: The name of her trafficker was tattooed on her skin, “a reminder of what I’d gone through.”
Ms. Givens was eventually introduced to Paul M. Friedman, MD, the current ASLMS president and one of the that was formed in 2022. According to a survey that Dr. Friedman and colleagues presented at the 2022 annual ASLMS conference, an estimated 1 in 2 sex trafficking survivors have branding tattoos, and at least 1,000 survivors a year could benefit from removal of those tattoos.
“To date, 87 physicians in the U.S. and one in Canada have stepped forward to volunteer their services to be part of this program,” Dr. Friedman, who directs the Dermatology and Laser Surgery Center in Houston, said at this year’s meeting. “My goal is to double this number by the next annual conference,” he added, noting that trauma-informed training is part of the program, “to support the survivor experience during the treatment process.”
ASLMS is also working on this issue in partnership with the American Academy of Dermatology (AAD) Ad Hoc Task Force on Dermatological Resources for the Intervention and Prevention of Human Trafficking, which is headed by Boston dermatologist Shadi Kourosh, MD.
“Dermatologists are uniquely positioned to aid in efforts to assist those experiences in trafficking with our training to recognize and diagnose relevant signs on the skin and to assist patients with certain aspects of care and recovery including the treatment of the disease of scars and tattoos,” Dr. Friedman said. “Ultimately, we hope to create a database together to improve recognition of branding tattoos to aid in identifying sex trafficking victims.”
Ms. Givens, who sits on the U.S. Advisory Council on Human Trafficking, said that she was able to truly close the door on her sex trafficking past thanks to the tattoo removal Dr. Friedman performed as part of New Beginnings. “It means the world to me to know that I can now be an advocate for other individuals who have experienced human trafficking,” she told meeting attendees.
“Again, one of the scariest things is not knowing that you have community. I was scared of losing hope, but I’m standing here today. I have all the hope that I need. You have the power to change lives.”
PHOENIX – When Kathy Givens walked onstage during a plenary session at the annual conference of the American Society for Laser Medicine and Surgery to reflect on her 9-month ordeal being sex trafficked in Texas more than 20 years ago, you could hear a pin drop.
“One of the scariest things about the life of sex trafficking is not knowing who’s going to be on the other side,” said Ms. Givens, who now lives in Houston. “There was some violence. There were some horrible things that happened. But you know what was really scary? When I got out. People may ask, ‘How’s that so? You escaped your trafficker. The past is behind you. Why were you afraid?’ I was afraid because I didn’t know that I had community. I didn’t know that community or that society would care about someone like me.”
She said that she found herself immobilized by fear of being shamed in society and labeled a sex trafficking victim, and wondered if she could overcome that fear and if anyone would view her as human again. Once free from her trafficker, she began a “healing journey,” which included getting married, raising four children, and re-enrolling in college with hopes of becoming a social worker. In 2020, she and her husband founded Twelve 11 Partners, an organization committed to supporting human trafficking survivors.
“I was working in the anti-trafficking field helping other survivors ... who have experienced this horrific crime,” she said. “I thought I was on my way.” But one “stain” from her sex trafficking past remained: The name of her trafficker was tattooed on her skin, “a reminder of what I’d gone through.”
Ms. Givens was eventually introduced to Paul M. Friedman, MD, the current ASLMS president and one of the that was formed in 2022. According to a survey that Dr. Friedman and colleagues presented at the 2022 annual ASLMS conference, an estimated 1 in 2 sex trafficking survivors have branding tattoos, and at least 1,000 survivors a year could benefit from removal of those tattoos.
“To date, 87 physicians in the U.S. and one in Canada have stepped forward to volunteer their services to be part of this program,” Dr. Friedman, who directs the Dermatology and Laser Surgery Center in Houston, said at this year’s meeting. “My goal is to double this number by the next annual conference,” he added, noting that trauma-informed training is part of the program, “to support the survivor experience during the treatment process.”
ASLMS is also working on this issue in partnership with the American Academy of Dermatology (AAD) Ad Hoc Task Force on Dermatological Resources for the Intervention and Prevention of Human Trafficking, which is headed by Boston dermatologist Shadi Kourosh, MD.
“Dermatologists are uniquely positioned to aid in efforts to assist those experiences in trafficking with our training to recognize and diagnose relevant signs on the skin and to assist patients with certain aspects of care and recovery including the treatment of the disease of scars and tattoos,” Dr. Friedman said. “Ultimately, we hope to create a database together to improve recognition of branding tattoos to aid in identifying sex trafficking victims.”
Ms. Givens, who sits on the U.S. Advisory Council on Human Trafficking, said that she was able to truly close the door on her sex trafficking past thanks to the tattoo removal Dr. Friedman performed as part of New Beginnings. “It means the world to me to know that I can now be an advocate for other individuals who have experienced human trafficking,” she told meeting attendees.
“Again, one of the scariest things is not knowing that you have community. I was scared of losing hope, but I’m standing here today. I have all the hope that I need. You have the power to change lives.”
AT ASLMS 2023
Repeated CTs in childhood linked with increased cancer risk
In a population-based case-control study that included more than 85,000 participants, researchers found a ninefold increased risk of intracranial tumors among children who received four or more CT scans.
The results “indicate that judicious CT usage and radiation-reducing techniques should be advocated,” Yu-Hsuan Joni Shao, PhD, professor of biomedical informatics at Taipei (Taiwan) Medical University, and colleagues wrote.
The study was published in the Canadian Medical Association Journal.
Dose-response relationship
The investigators used the National Health Insurance Research Database in Taiwan to identify 7,807 patients under age 25 years with intracranial tumors (grades I-IV), leukemia, non-Hodgkin lymphomas, or Hodgkin lymphomas that had been diagnosed in a 14-year span between the years 2000 and 2013. They matched each case with 10 control participants without cancer by sex, date of birth, and date of entry into the cohort.
Radiation exposure was calculated for each patient according to number and type of CT scans received and an estimated organ-specific cumulative dose based on previously published models. The investigators excluded patients from the analysis if they had a diagnosis of any malignant disease before the study period or if they had any cancer-predisposing conditions, such as Down syndrome (which entails an increased risk of leukemia) or immunodeficiency (which may require multiple CT scans).
Compared with no exposure, exposure to a single pediatric CT scan was not associated with increased cancer risk. Exposure to two to three CT scans, however, was associated with an increased risk for intracranial tumour (adjusted odds ratio, 2.36), but not for leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma. Exposure to four or more CT scans was associated with increased risk for intracranial tumor (aOR, 9.01), leukemia (aOR, 4.80), and non-Hodgkin lymphoma (aOR, 6.76), but not for Hodgkin lymphoma.
The researchers also found a dose-response relationship. Participants in the top quintile of cumulative brain radiation dose had a significantly higher risk for intracranial tumor, compared with nonexposed participants (aOR, 3.61), although this relationship was not seen with the other cancers.
Age at exposure was also a significant factor. Children exposed to four or more CT scans at or before age 6 years had the highest risk for cancer (aOR, 22.95), followed by the same number of scans in those aged 7-12 years (aOR, 5.69) and those aged 13-18 years (aOR, 3.20).
The authors noted that, although these cancers are uncommon in children, “our work reinforces the importance of radiation protection strategies, addressed by the International Atomic Energy Agency. Unnecessary CT scans should be avoided, and special attention should be paid to patients who require repeated CT scans. Parents and pediatric patients should be well informed on risks and benefits before radiological procedures and encouraged to participate in decision-making around imaging.”
True risks underestimated?
Commenting on the findings, Rebecca Smith-Bindman, MD, a radiologist at the University of California, San Francisco, and an expert on the impact of CT scans on patient outcomes, said that she trusts the authors’ overall findings. But “because of the direction of their biases,” the study design “doesn’t let me accept their conclusion that one CT does not elevate the risk.
“It’s an interesting study that found the risk of brain cancer is more than doubled in children who undergo two or more CT scans, but in many ways, their assumptions will underestimate the true risk,” said Dr. Smith-Bindman, who is a professor of epidemiology and biostatistics at UCSF. She said reasons for this include the fact that the investigators used estimated, rather than actual radiation doses; that their estimates “reflect doses far lower than we have found actually occur in clinical practice”; that they do not differentiate between a low-dose or a high-dose CT; and that that they include a long, 3-year lag during which leukemia can develop after a CT scan.
“They did a lot of really well-done adjustments to ensure that they were not overestimating risk,” said Dr. Smith-Bindman. “They made sure to delete children who had cancer susceptibility syndrome, they included a lag of 3 years, assuming that there could be hidden cancers for up to 3 years after the first imaging study when they might have had a preexisting cancer. These are decisions that ensure that any cancer risk they find is real, but it also means that the risks that are estimated are almost certainly an underestimate of the true risks.”
The study was conducted without external funding. The authors declared no relevant financial relationships. Dr. Smith-Bindman is a cofounder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
A version of this article first appeared on Medscape.com.
In a population-based case-control study that included more than 85,000 participants, researchers found a ninefold increased risk of intracranial tumors among children who received four or more CT scans.
The results “indicate that judicious CT usage and radiation-reducing techniques should be advocated,” Yu-Hsuan Joni Shao, PhD, professor of biomedical informatics at Taipei (Taiwan) Medical University, and colleagues wrote.
The study was published in the Canadian Medical Association Journal.
Dose-response relationship
The investigators used the National Health Insurance Research Database in Taiwan to identify 7,807 patients under age 25 years with intracranial tumors (grades I-IV), leukemia, non-Hodgkin lymphomas, or Hodgkin lymphomas that had been diagnosed in a 14-year span between the years 2000 and 2013. They matched each case with 10 control participants without cancer by sex, date of birth, and date of entry into the cohort.
Radiation exposure was calculated for each patient according to number and type of CT scans received and an estimated organ-specific cumulative dose based on previously published models. The investigators excluded patients from the analysis if they had a diagnosis of any malignant disease before the study period or if they had any cancer-predisposing conditions, such as Down syndrome (which entails an increased risk of leukemia) or immunodeficiency (which may require multiple CT scans).
Compared with no exposure, exposure to a single pediatric CT scan was not associated with increased cancer risk. Exposure to two to three CT scans, however, was associated with an increased risk for intracranial tumour (adjusted odds ratio, 2.36), but not for leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma. Exposure to four or more CT scans was associated with increased risk for intracranial tumor (aOR, 9.01), leukemia (aOR, 4.80), and non-Hodgkin lymphoma (aOR, 6.76), but not for Hodgkin lymphoma.
The researchers also found a dose-response relationship. Participants in the top quintile of cumulative brain radiation dose had a significantly higher risk for intracranial tumor, compared with nonexposed participants (aOR, 3.61), although this relationship was not seen with the other cancers.
Age at exposure was also a significant factor. Children exposed to four or more CT scans at or before age 6 years had the highest risk for cancer (aOR, 22.95), followed by the same number of scans in those aged 7-12 years (aOR, 5.69) and those aged 13-18 years (aOR, 3.20).
The authors noted that, although these cancers are uncommon in children, “our work reinforces the importance of radiation protection strategies, addressed by the International Atomic Energy Agency. Unnecessary CT scans should be avoided, and special attention should be paid to patients who require repeated CT scans. Parents and pediatric patients should be well informed on risks and benefits before radiological procedures and encouraged to participate in decision-making around imaging.”
True risks underestimated?
Commenting on the findings, Rebecca Smith-Bindman, MD, a radiologist at the University of California, San Francisco, and an expert on the impact of CT scans on patient outcomes, said that she trusts the authors’ overall findings. But “because of the direction of their biases,” the study design “doesn’t let me accept their conclusion that one CT does not elevate the risk.
“It’s an interesting study that found the risk of brain cancer is more than doubled in children who undergo two or more CT scans, but in many ways, their assumptions will underestimate the true risk,” said Dr. Smith-Bindman, who is a professor of epidemiology and biostatistics at UCSF. She said reasons for this include the fact that the investigators used estimated, rather than actual radiation doses; that their estimates “reflect doses far lower than we have found actually occur in clinical practice”; that they do not differentiate between a low-dose or a high-dose CT; and that that they include a long, 3-year lag during which leukemia can develop after a CT scan.
“They did a lot of really well-done adjustments to ensure that they were not overestimating risk,” said Dr. Smith-Bindman. “They made sure to delete children who had cancer susceptibility syndrome, they included a lag of 3 years, assuming that there could be hidden cancers for up to 3 years after the first imaging study when they might have had a preexisting cancer. These are decisions that ensure that any cancer risk they find is real, but it also means that the risks that are estimated are almost certainly an underestimate of the true risks.”
The study was conducted without external funding. The authors declared no relevant financial relationships. Dr. Smith-Bindman is a cofounder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
A version of this article first appeared on Medscape.com.
In a population-based case-control study that included more than 85,000 participants, researchers found a ninefold increased risk of intracranial tumors among children who received four or more CT scans.
The results “indicate that judicious CT usage and radiation-reducing techniques should be advocated,” Yu-Hsuan Joni Shao, PhD, professor of biomedical informatics at Taipei (Taiwan) Medical University, and colleagues wrote.
The study was published in the Canadian Medical Association Journal.
Dose-response relationship
The investigators used the National Health Insurance Research Database in Taiwan to identify 7,807 patients under age 25 years with intracranial tumors (grades I-IV), leukemia, non-Hodgkin lymphomas, or Hodgkin lymphomas that had been diagnosed in a 14-year span between the years 2000 and 2013. They matched each case with 10 control participants without cancer by sex, date of birth, and date of entry into the cohort.
Radiation exposure was calculated for each patient according to number and type of CT scans received and an estimated organ-specific cumulative dose based on previously published models. The investigators excluded patients from the analysis if they had a diagnosis of any malignant disease before the study period or if they had any cancer-predisposing conditions, such as Down syndrome (which entails an increased risk of leukemia) or immunodeficiency (which may require multiple CT scans).
Compared with no exposure, exposure to a single pediatric CT scan was not associated with increased cancer risk. Exposure to two to three CT scans, however, was associated with an increased risk for intracranial tumour (adjusted odds ratio, 2.36), but not for leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma. Exposure to four or more CT scans was associated with increased risk for intracranial tumor (aOR, 9.01), leukemia (aOR, 4.80), and non-Hodgkin lymphoma (aOR, 6.76), but not for Hodgkin lymphoma.
The researchers also found a dose-response relationship. Participants in the top quintile of cumulative brain radiation dose had a significantly higher risk for intracranial tumor, compared with nonexposed participants (aOR, 3.61), although this relationship was not seen with the other cancers.
Age at exposure was also a significant factor. Children exposed to four or more CT scans at or before age 6 years had the highest risk for cancer (aOR, 22.95), followed by the same number of scans in those aged 7-12 years (aOR, 5.69) and those aged 13-18 years (aOR, 3.20).
The authors noted that, although these cancers are uncommon in children, “our work reinforces the importance of radiation protection strategies, addressed by the International Atomic Energy Agency. Unnecessary CT scans should be avoided, and special attention should be paid to patients who require repeated CT scans. Parents and pediatric patients should be well informed on risks and benefits before radiological procedures and encouraged to participate in decision-making around imaging.”
True risks underestimated?
Commenting on the findings, Rebecca Smith-Bindman, MD, a radiologist at the University of California, San Francisco, and an expert on the impact of CT scans on patient outcomes, said that she trusts the authors’ overall findings. But “because of the direction of their biases,” the study design “doesn’t let me accept their conclusion that one CT does not elevate the risk.
“It’s an interesting study that found the risk of brain cancer is more than doubled in children who undergo two or more CT scans, but in many ways, their assumptions will underestimate the true risk,” said Dr. Smith-Bindman, who is a professor of epidemiology and biostatistics at UCSF. She said reasons for this include the fact that the investigators used estimated, rather than actual radiation doses; that their estimates “reflect doses far lower than we have found actually occur in clinical practice”; that they do not differentiate between a low-dose or a high-dose CT; and that that they include a long, 3-year lag during which leukemia can develop after a CT scan.
“They did a lot of really well-done adjustments to ensure that they were not overestimating risk,” said Dr. Smith-Bindman. “They made sure to delete children who had cancer susceptibility syndrome, they included a lag of 3 years, assuming that there could be hidden cancers for up to 3 years after the first imaging study when they might have had a preexisting cancer. These are decisions that ensure that any cancer risk they find is real, but it also means that the risks that are estimated are almost certainly an underestimate of the true risks.”
The study was conducted without external funding. The authors declared no relevant financial relationships. Dr. Smith-Bindman is a cofounder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
‘Shocking’ data on what’s really in melatonin gummies
New data may explain the recent massive jump in pediatric hospitalizations.
Thenvestigators found that consuming some products as directed could expose consumers, including children, to doses that are 40-130 times greater than what’s recommended.
“The results were quite shocking,” lead researcher Pieter Cohen, MD, with Harvard Medical School, Boston, and Cambridge Health Alliance, Somerville, Mass., said in an interview.
“Melatonin gummies contained up to 347% more melatonin than what was listed on the label, and some products also contained cannabidiol; in one brand of melatonin gummies, there was zero melatonin, just CBD,” Dr. Cohen said.
The study was published online in JAMA.
530% jump in pediatric hospitalizations
Melatonin products are not approved by the Food and Drug Administration but are sold over the counter or online.
Previous research from JAMA has shown the use of melatonin has increased over the past 2 decades among people of all ages.
With increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related ED visits for children.
Federal data show the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021. More than 4,000 of the reported ingestions led to a hospital stay; 287 children required intensive care, and two children died.
It was unclear why melatonin supplements were causing these harms, which led Dr. Cohen’s team to analyze 25 unique brands of “melatonin” gummies purchased online.
One product didn’t contain any melatonin but did contain 31.3 mg of CBD.
In the remaining products, the quantity of melatonin ranged from 1.3 mg to 13.1 mg per serving. The actual quantity of melatonin ranged from 74% to 347% of the labeled quantity, the researchers found.
They note that for a young adult who takes as little as 0.1-0.3 mg of melatonin, plasma concentrations can increase into the normal night-time range.
Of the 25 products (88%) analyzed, 22 were inaccurately labeled, and only 3 (12%) contained a quantity of melatonin that was within 10% (plus or minus) of the declared quantity.
Five products listed CBD as an ingredient. The listed quantity ranged from 10.6 mg to 31.3 mg per serving, although the actual quantity of CBD ranged from 104% to 118% of the labeled quantity.
Inquire about use in kids
A limitation of the study is that only one sample of each brand was analyzed, and only gummies were analyzed. It is not known whether the results are generalizable to melatonin products sold as tablets and capsules in the United States or whether the quantity of melatonin within an individual brand may vary from batch to batch.
A recent study from Canada showed similar results. In an analysis of 16 Canadian melatonin brands, the actual dose of melatonin ranged from 17% to 478% of the declared quantity.
It’s estimated that more than 1% of all U.S. children use melatonin supplements, most commonly for sleep, stress, and relaxation.
“Given new research as to the excessive quantities of melatonin in gummies, caution should be used if considering their use,” said Dr. Cohen.
“It’s important to inquire about melatonin use when caring for children, particularly when parents express concerns about their child’s sleep,” he added.
The American Academy of Sleep Medicine recently issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
Children don’t need melatonin
Commenting on the study, Michael Breus, PhD, clinical psychologist and founder of TheSleepDoctor.com, agreed that analyzing only one sample of each brand is a key limitation “because supplements are made in batches, and gummies in particular are difficult to distribute the active ingredient evenly.
“But even with that being said, 88% of them were labeled incorrectly, so even if there were a few single-sample issues, I kind of doubt its all of them,” Dr. Breus said.
“Kids as a general rule do not need melatonin. Their brains make almost four times the necessary amount already. If you start giving kids pills to help them sleep, then they start to have a pill problem, causing another issue,” Dr. Breus added.
“Most children’s falling asleep and staying sleep issues can be treated with behavioral measures like cognitive-behavioral therapy for insomnia,” he said.
The study had no specific funding. Dr. Cohen has received research support from Consumers Union and PEW Charitable Trusts and royalties from UptoDate. Dr. Breus disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data may explain the recent massive jump in pediatric hospitalizations.
Thenvestigators found that consuming some products as directed could expose consumers, including children, to doses that are 40-130 times greater than what’s recommended.
“The results were quite shocking,” lead researcher Pieter Cohen, MD, with Harvard Medical School, Boston, and Cambridge Health Alliance, Somerville, Mass., said in an interview.
“Melatonin gummies contained up to 347% more melatonin than what was listed on the label, and some products also contained cannabidiol; in one brand of melatonin gummies, there was zero melatonin, just CBD,” Dr. Cohen said.
The study was published online in JAMA.
530% jump in pediatric hospitalizations
Melatonin products are not approved by the Food and Drug Administration but are sold over the counter or online.
Previous research from JAMA has shown the use of melatonin has increased over the past 2 decades among people of all ages.
With increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related ED visits for children.
Federal data show the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021. More than 4,000 of the reported ingestions led to a hospital stay; 287 children required intensive care, and two children died.
It was unclear why melatonin supplements were causing these harms, which led Dr. Cohen’s team to analyze 25 unique brands of “melatonin” gummies purchased online.
One product didn’t contain any melatonin but did contain 31.3 mg of CBD.
In the remaining products, the quantity of melatonin ranged from 1.3 mg to 13.1 mg per serving. The actual quantity of melatonin ranged from 74% to 347% of the labeled quantity, the researchers found.
They note that for a young adult who takes as little as 0.1-0.3 mg of melatonin, plasma concentrations can increase into the normal night-time range.
Of the 25 products (88%) analyzed, 22 were inaccurately labeled, and only 3 (12%) contained a quantity of melatonin that was within 10% (plus or minus) of the declared quantity.
Five products listed CBD as an ingredient. The listed quantity ranged from 10.6 mg to 31.3 mg per serving, although the actual quantity of CBD ranged from 104% to 118% of the labeled quantity.
Inquire about use in kids
A limitation of the study is that only one sample of each brand was analyzed, and only gummies were analyzed. It is not known whether the results are generalizable to melatonin products sold as tablets and capsules in the United States or whether the quantity of melatonin within an individual brand may vary from batch to batch.
A recent study from Canada showed similar results. In an analysis of 16 Canadian melatonin brands, the actual dose of melatonin ranged from 17% to 478% of the declared quantity.
It’s estimated that more than 1% of all U.S. children use melatonin supplements, most commonly for sleep, stress, and relaxation.
“Given new research as to the excessive quantities of melatonin in gummies, caution should be used if considering their use,” said Dr. Cohen.
“It’s important to inquire about melatonin use when caring for children, particularly when parents express concerns about their child’s sleep,” he added.
The American Academy of Sleep Medicine recently issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
Children don’t need melatonin
Commenting on the study, Michael Breus, PhD, clinical psychologist and founder of TheSleepDoctor.com, agreed that analyzing only one sample of each brand is a key limitation “because supplements are made in batches, and gummies in particular are difficult to distribute the active ingredient evenly.
“But even with that being said, 88% of them were labeled incorrectly, so even if there were a few single-sample issues, I kind of doubt its all of them,” Dr. Breus said.
“Kids as a general rule do not need melatonin. Their brains make almost four times the necessary amount already. If you start giving kids pills to help them sleep, then they start to have a pill problem, causing another issue,” Dr. Breus added.
“Most children’s falling asleep and staying sleep issues can be treated with behavioral measures like cognitive-behavioral therapy for insomnia,” he said.
The study had no specific funding. Dr. Cohen has received research support from Consumers Union and PEW Charitable Trusts and royalties from UptoDate. Dr. Breus disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data may explain the recent massive jump in pediatric hospitalizations.
Thenvestigators found that consuming some products as directed could expose consumers, including children, to doses that are 40-130 times greater than what’s recommended.
“The results were quite shocking,” lead researcher Pieter Cohen, MD, with Harvard Medical School, Boston, and Cambridge Health Alliance, Somerville, Mass., said in an interview.
“Melatonin gummies contained up to 347% more melatonin than what was listed on the label, and some products also contained cannabidiol; in one brand of melatonin gummies, there was zero melatonin, just CBD,” Dr. Cohen said.
The study was published online in JAMA.
530% jump in pediatric hospitalizations
Melatonin products are not approved by the Food and Drug Administration but are sold over the counter or online.
Previous research from JAMA has shown the use of melatonin has increased over the past 2 decades among people of all ages.
With increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related ED visits for children.
Federal data show the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021. More than 4,000 of the reported ingestions led to a hospital stay; 287 children required intensive care, and two children died.
It was unclear why melatonin supplements were causing these harms, which led Dr. Cohen’s team to analyze 25 unique brands of “melatonin” gummies purchased online.
One product didn’t contain any melatonin but did contain 31.3 mg of CBD.
In the remaining products, the quantity of melatonin ranged from 1.3 mg to 13.1 mg per serving. The actual quantity of melatonin ranged from 74% to 347% of the labeled quantity, the researchers found.
They note that for a young adult who takes as little as 0.1-0.3 mg of melatonin, plasma concentrations can increase into the normal night-time range.
Of the 25 products (88%) analyzed, 22 were inaccurately labeled, and only 3 (12%) contained a quantity of melatonin that was within 10% (plus or minus) of the declared quantity.
Five products listed CBD as an ingredient. The listed quantity ranged from 10.6 mg to 31.3 mg per serving, although the actual quantity of CBD ranged from 104% to 118% of the labeled quantity.
Inquire about use in kids
A limitation of the study is that only one sample of each brand was analyzed, and only gummies were analyzed. It is not known whether the results are generalizable to melatonin products sold as tablets and capsules in the United States or whether the quantity of melatonin within an individual brand may vary from batch to batch.
A recent study from Canada showed similar results. In an analysis of 16 Canadian melatonin brands, the actual dose of melatonin ranged from 17% to 478% of the declared quantity.
It’s estimated that more than 1% of all U.S. children use melatonin supplements, most commonly for sleep, stress, and relaxation.
“Given new research as to the excessive quantities of melatonin in gummies, caution should be used if considering their use,” said Dr. Cohen.
“It’s important to inquire about melatonin use when caring for children, particularly when parents express concerns about their child’s sleep,” he added.
The American Academy of Sleep Medicine recently issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
Children don’t need melatonin
Commenting on the study, Michael Breus, PhD, clinical psychologist and founder of TheSleepDoctor.com, agreed that analyzing only one sample of each brand is a key limitation “because supplements are made in batches, and gummies in particular are difficult to distribute the active ingredient evenly.
“But even with that being said, 88% of them were labeled incorrectly, so even if there were a few single-sample issues, I kind of doubt its all of them,” Dr. Breus said.
“Kids as a general rule do not need melatonin. Their brains make almost four times the necessary amount already. If you start giving kids pills to help them sleep, then they start to have a pill problem, causing another issue,” Dr. Breus added.
“Most children’s falling asleep and staying sleep issues can be treated with behavioral measures like cognitive-behavioral therapy for insomnia,” he said.
The study had no specific funding. Dr. Cohen has received research support from Consumers Union and PEW Charitable Trusts and royalties from UptoDate. Dr. Breus disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Skin Diseases Associated With COVID-19: A Narrative Review
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
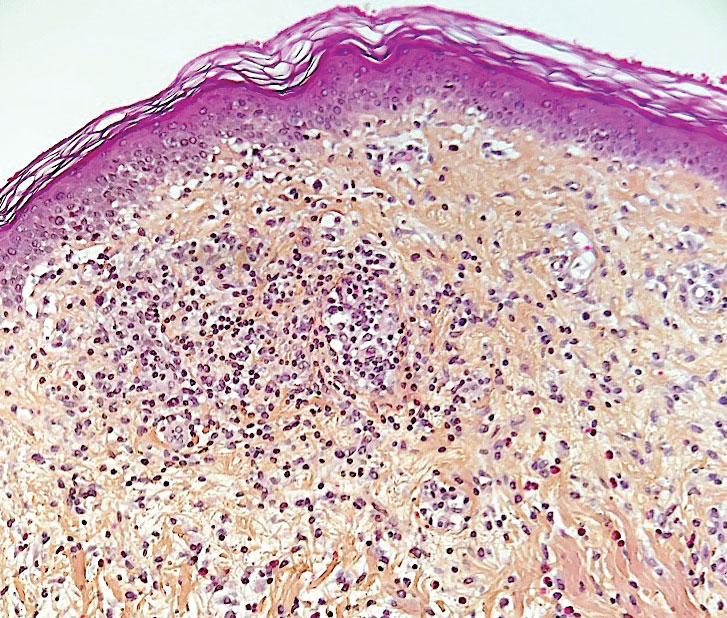
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.
Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
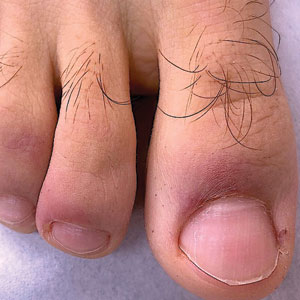
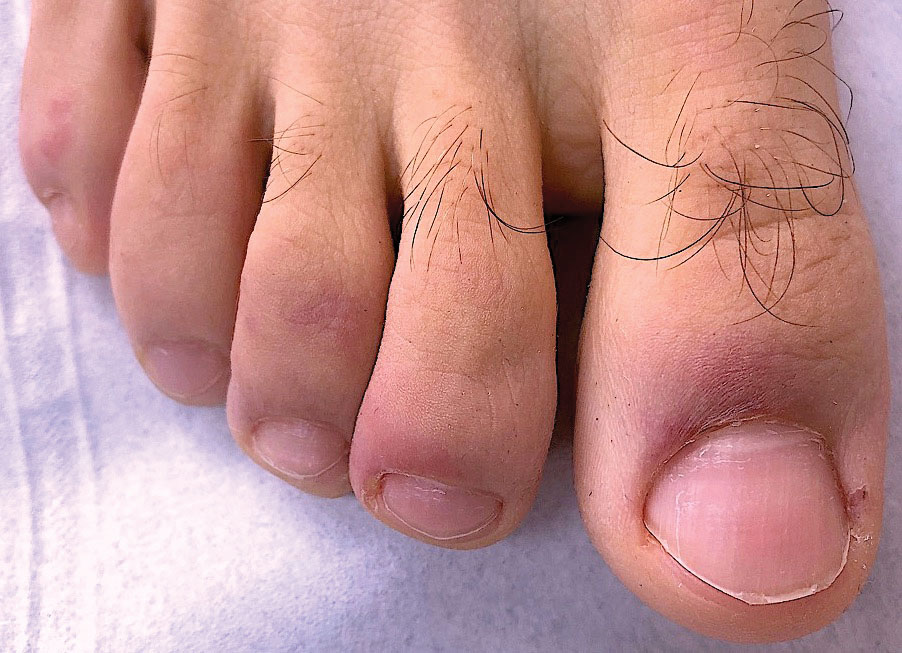
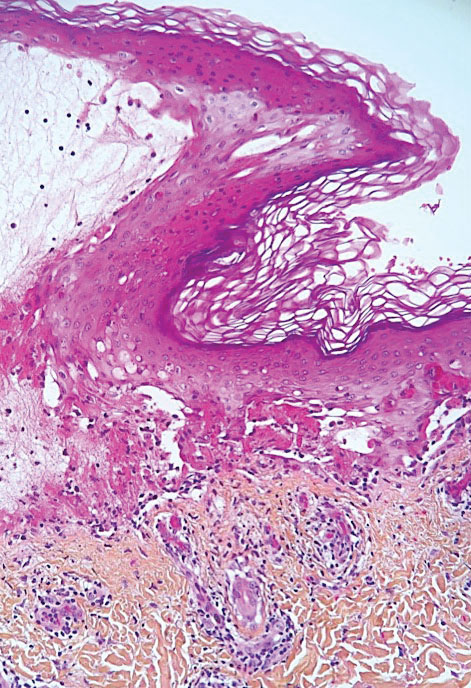
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30
Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).
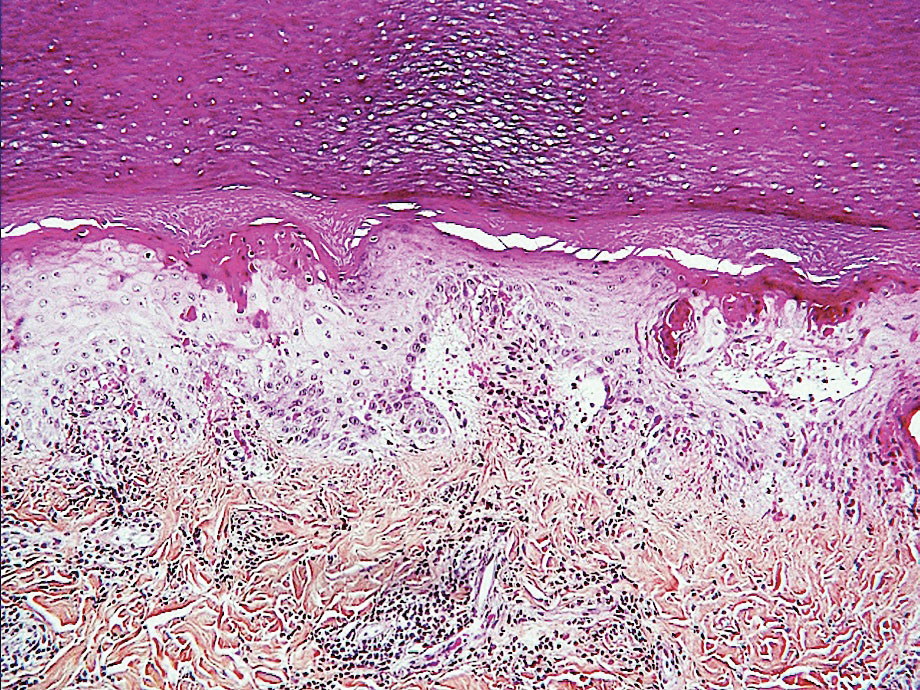
Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).
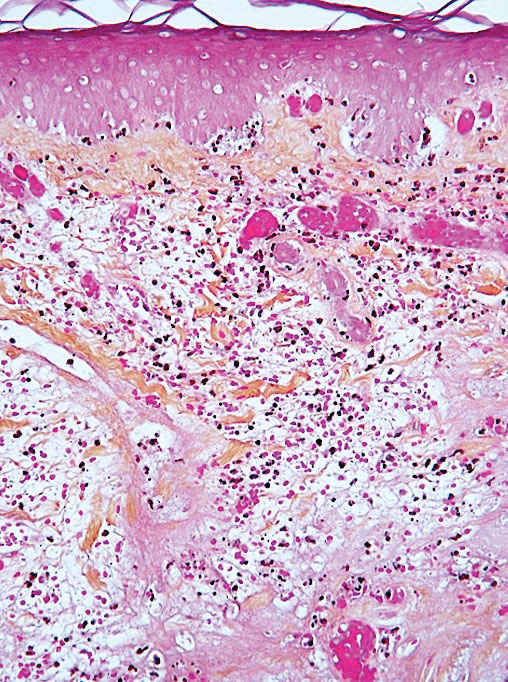
Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.
The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
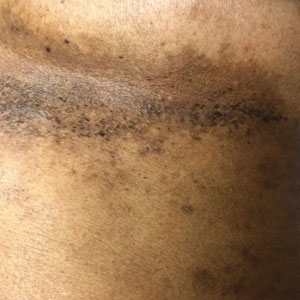
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54
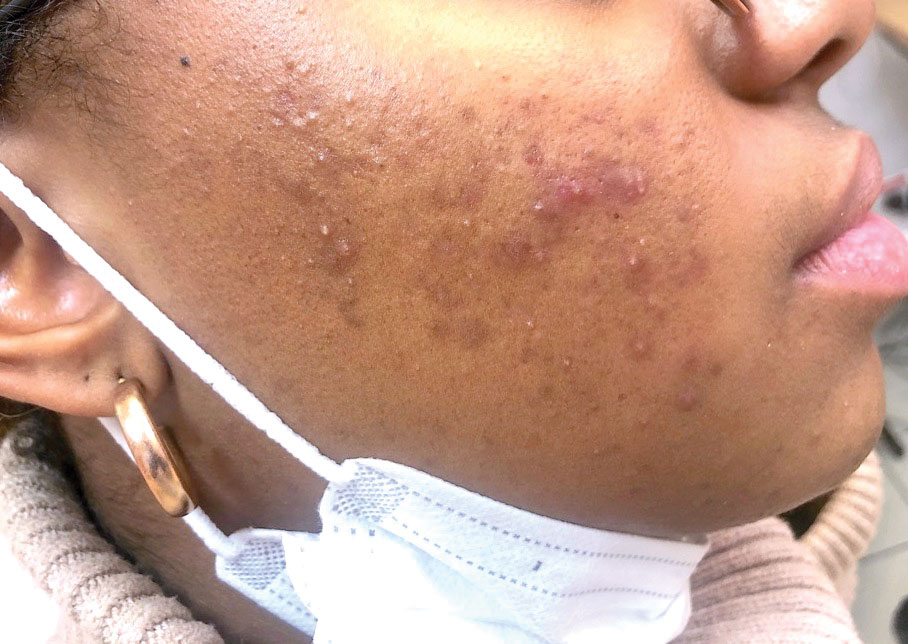
DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.

Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30

Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).

Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).

Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.

The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54

DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.

Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30

Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).

Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).

Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.

The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54

DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
Practice Points
- During the COVID-19 pandemic, several skin diseases were reported in association with this new infectious disease and were classified mainly according to their morphologic aspect. However, the pathogenetic mechanisms often are unclear and the causal link of the culprit virus (SARS-CoV-2) not always well established.
- Currently, most skin manifestations related to COVID-19 are reported after vaccination against COVID-19; remarkably, many of them are similar to those attributed to the natural infection.
Botulinum Toxin and Glycopyrrolate Combination Therapy for Hailey-Hailey Disease
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.
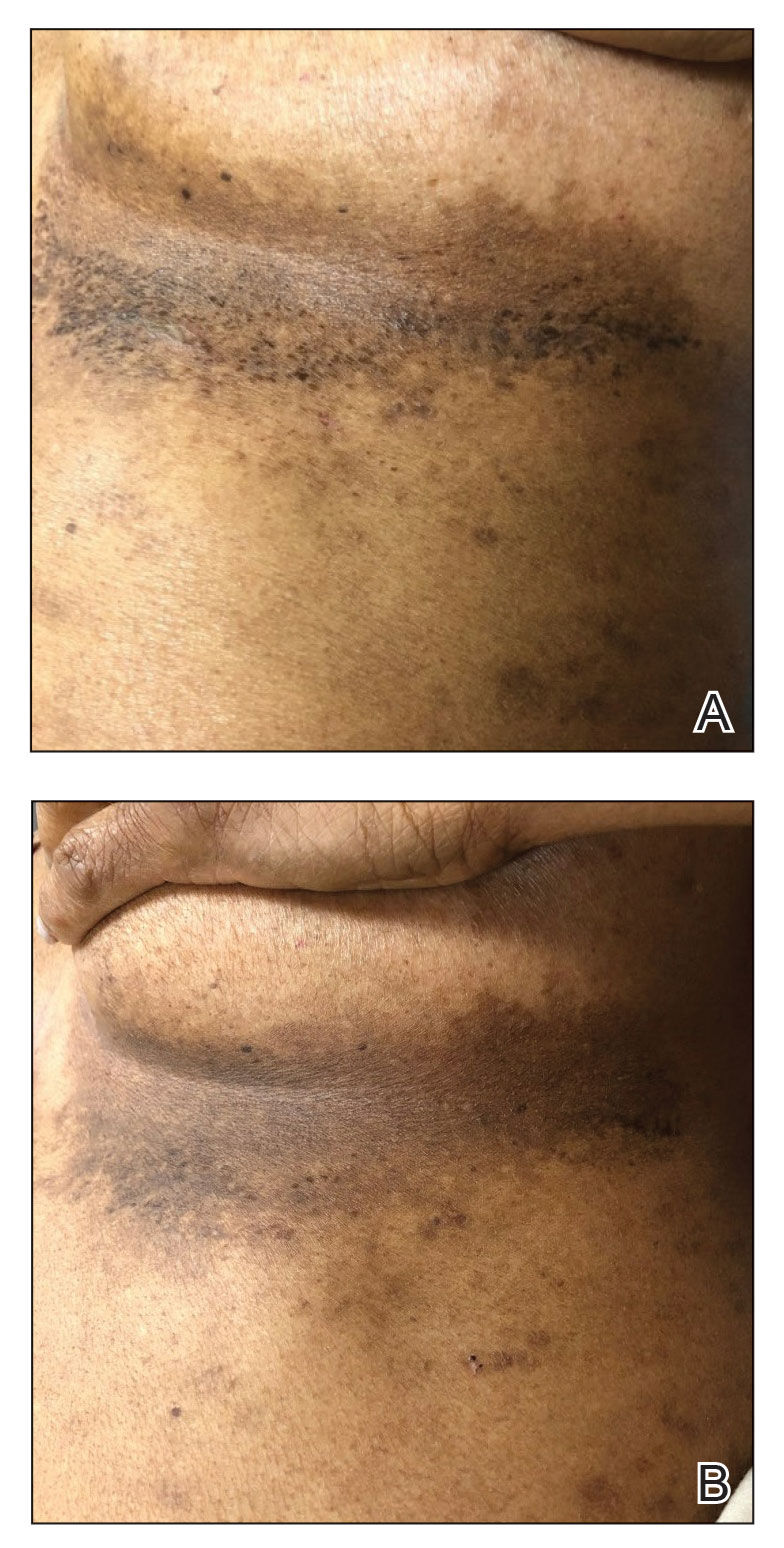
A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.
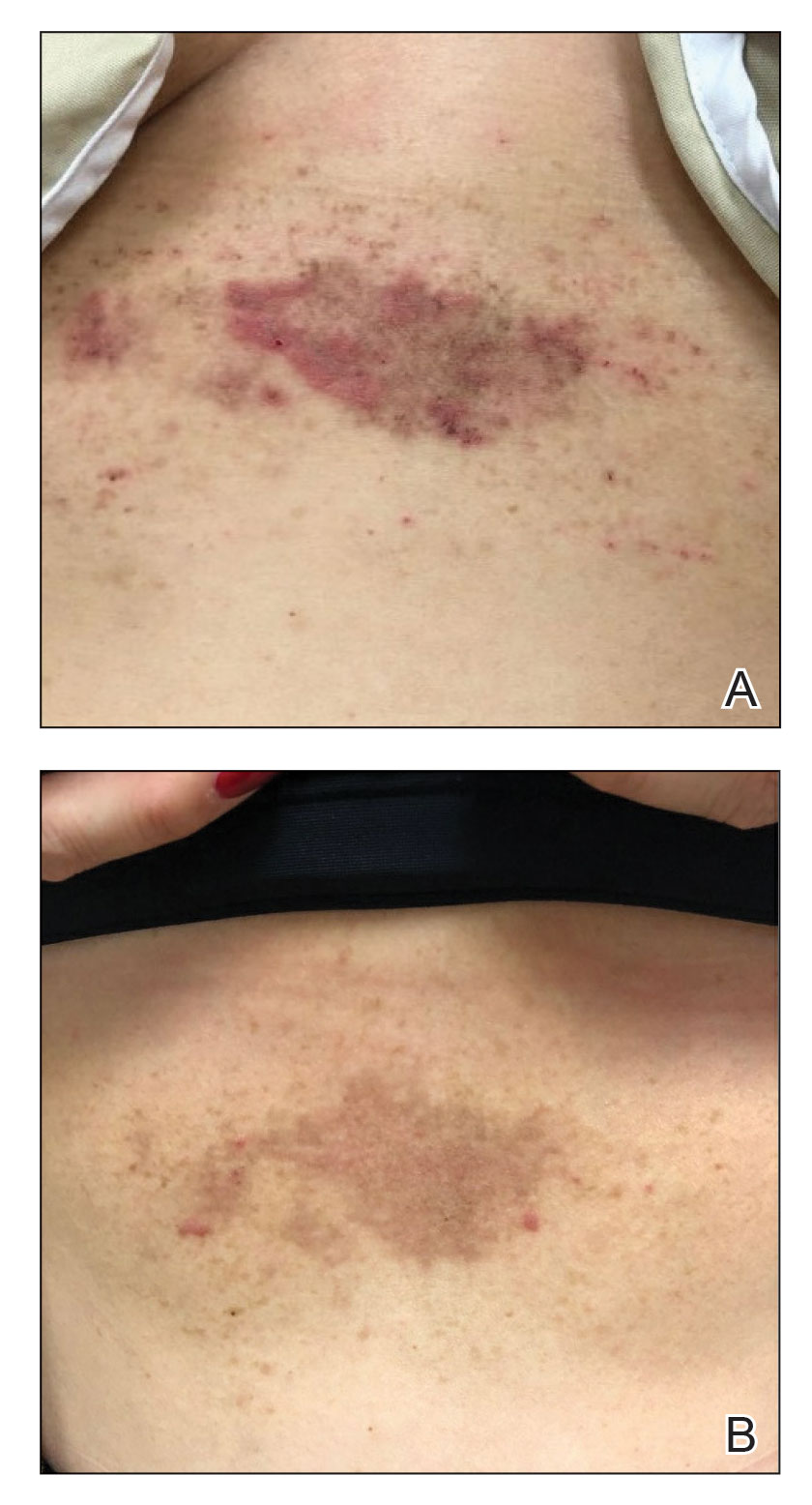
Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.

A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.

Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.

A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.

Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
Practice Points
- Hailey-Hailey disease is associated with decreased quality of life for patients, and current treatment options are limited.
- A combination of local neuromodulator injections and systemic oral anticholinergic therapy may provide sustained disease remission compared to neuromodulator therapy alone.
The newest form of mommy shaming: The 'narcissistic mother'
Narcissists appear to be everywhere. A few minutes on the Internet shows the dangers of narcissistic romantic partners, friends, and employers. Identifying and limiting the reach of their manipulative and self-centered endeavors is cast as both urgent and necessary. The destructive powers of the narcissistic mother are viewed as especially in need of remedy, and any bookstore can reveal the risks they pose: “Will I Ever Be Good Enough? Healing the Daughters of Narcissistic Mothers;” “You’re Not Crazy – It’s Your Mother: Freedom for Daughters of Narcissistic Mothers;” “Healing for Daughters of Narcissistic Mothers: A Practical Guide on How to Recover from the Childhood Trauma of Toxic Relationship with Your Mother and How You Can Handle Her Abuse Now As An Adult” – to name just a few (there are more).
As a psychologist specializing in parental estrangement, I (Dr. Coleman) regularly see letters from adult children explaining their discovery-through-therapy that their mother is a narcissist. The proclamation often comes when the therapist has never met the mother. Typically, the discovery is presented as a justification for ending the relationship with the parent. While these mothers could rightly be accused of being anxious, over-involved, depressed, or hurt by the lack of gratitude or reciprocity, the vast majority are not narcissists.
Which begs the question, why are so many being labeled in this way? Are therapists only now discovering the power of narcissistic mothers? Have they always existed, casting their spells upon unwary children? Are those now-grown children only today able to disentangle themselves from the longstanding, pervasive, and harmful influence of these parents, with the help of therapy? Or is this the newest form of mommy shaming as it engages head-on with our Diagnostic and Statistical Manuals?
We believe it is the latter.
Blaming mothers has a long reach. Mothers have been blamed for causing schizophrenia, autism, homosexuality, and effeminacy in men. While we used to call people selfish and “controlling,” narcissism is a more consequential label as it confers diagnostic validity from the mental health profession. Worse, it suggests an individual beyond reach, where the only answer is distance, containment, or estrangement.
The rise of the narcissistic mother comes during a time when, for the past 4 decades, the average working mother spends more time with her children than stay-at-home moms did in the supposed halcyon days of the 1960s’ middle class, before “parenting” was a common term. A variety of economists and sociologists observed that an increase in parental effort became necessary to launch children into adulthood given the retreat of governmental and corporate support for parents that began in the 1980s.
“The financial and emotional burden on families has grown in ways that were almost unimaginable just a half-century ago,” writes the University of Pennsylvania sociologist Frank Furstenberg in “On a New Schedule: Transitions to Adulthood and Family Change.” In addition, a view of children as vulnerable and in need of intense parental investment gained momentum over the course of the 20th century and has continued unabated into the present. As a result, an environment of intense maternal preoccupation, worry, guilt, and involvement with children’s grades, safety, health, and emotional states – referred to as “helicopter” and “tiger” mothering – grew into the norm across the classes.
While prior generations of parents could, by today’s standards, be viewed as being insufficiently involved, today’s parents have become “over-involved” – aided by the ability of parents to be in constant contact with their adult children through technology. While this shift to a more hands-on, more conscientious parenting has been a boon to parent–adult child relationships in the main, the downside has meant, for some, too much of a good thing. From that perspective, pathologizing a mother’s involvement or her expressions of hurt for that child’s lack of availability provides a shield against the child’s feelings of guilt or obligation.
Diagnoses can serve a social purpose: They can allow individuals to use the authority of our profession to decide who to be close to and who to let go. They can provide insulation against feelings of obligation or guilt. They create a way to label behavior as dysfunctional that in other eras or cultures would be considered normal, even valued. To that extent, diagnoses don’t occur in a cultural void. They are inextricably tied to larger ideals, be they individualistic – as exists in the United States – or collectivist, as exists in many other parts of the world.
While we have decided what parents owe our children, it is unclear what parents might ask in return. To that end, mothers who want more interest, availability, or gratitude today are vulnerable to being cast as selfish, uncaring, needy, and controlling. They can now be viewed as failing in their task of selfless devotion. Their desires for closeness or repair can be regarded as incompatible with the quest for the adult child’s self-fulfillment and identity; her identification with her children too great a barrier to their individuation.
There may well be good reasons to estrange family members for their intolerable behaviors, especially ones who have threatened personal safety. Yet, while there are plenty of problematic parents, few meet the diagnostic criteria of narcissistic personality disorder. More important, such labels can discourage a discussion of boundaries that both the parents and the adult children might find acceptable – which sometimes means asking family members to tolerate behavior or individuals not to their liking.
Diagnoses carry enormous social weight and can facilitate estrangements or negativity to mothers that are far more workable than our patients’ characterization of them might lead them or us to believe. ; it devalues their years of love and dedication, however flawed; and it weakens the fabric of connection that could otherwise exist. Rather than provide a path toward compassion or understanding, “narcissistic mother” just becomes the latest form of mommy shaming.
Dr. Coleman is a clinical psychologist and author of “Rules of Estrangement: Why Adult Children Cut Ties and How to Heal the Conflict” (New York: Penguin Random House, 2021). Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
Narcissists appear to be everywhere. A few minutes on the Internet shows the dangers of narcissistic romantic partners, friends, and employers. Identifying and limiting the reach of their manipulative and self-centered endeavors is cast as both urgent and necessary. The destructive powers of the narcissistic mother are viewed as especially in need of remedy, and any bookstore can reveal the risks they pose: “Will I Ever Be Good Enough? Healing the Daughters of Narcissistic Mothers;” “You’re Not Crazy – It’s Your Mother: Freedom for Daughters of Narcissistic Mothers;” “Healing for Daughters of Narcissistic Mothers: A Practical Guide on How to Recover from the Childhood Trauma of Toxic Relationship with Your Mother and How You Can Handle Her Abuse Now As An Adult” – to name just a few (there are more).
As a psychologist specializing in parental estrangement, I (Dr. Coleman) regularly see letters from adult children explaining their discovery-through-therapy that their mother is a narcissist. The proclamation often comes when the therapist has never met the mother. Typically, the discovery is presented as a justification for ending the relationship with the parent. While these mothers could rightly be accused of being anxious, over-involved, depressed, or hurt by the lack of gratitude or reciprocity, the vast majority are not narcissists.
Which begs the question, why are so many being labeled in this way? Are therapists only now discovering the power of narcissistic mothers? Have they always existed, casting their spells upon unwary children? Are those now-grown children only today able to disentangle themselves from the longstanding, pervasive, and harmful influence of these parents, with the help of therapy? Or is this the newest form of mommy shaming as it engages head-on with our Diagnostic and Statistical Manuals?
We believe it is the latter.
Blaming mothers has a long reach. Mothers have been blamed for causing schizophrenia, autism, homosexuality, and effeminacy in men. While we used to call people selfish and “controlling,” narcissism is a more consequential label as it confers diagnostic validity from the mental health profession. Worse, it suggests an individual beyond reach, where the only answer is distance, containment, or estrangement.
The rise of the narcissistic mother comes during a time when, for the past 4 decades, the average working mother spends more time with her children than stay-at-home moms did in the supposed halcyon days of the 1960s’ middle class, before “parenting” was a common term. A variety of economists and sociologists observed that an increase in parental effort became necessary to launch children into adulthood given the retreat of governmental and corporate support for parents that began in the 1980s.
“The financial and emotional burden on families has grown in ways that were almost unimaginable just a half-century ago,” writes the University of Pennsylvania sociologist Frank Furstenberg in “On a New Schedule: Transitions to Adulthood and Family Change.” In addition, a view of children as vulnerable and in need of intense parental investment gained momentum over the course of the 20th century and has continued unabated into the present. As a result, an environment of intense maternal preoccupation, worry, guilt, and involvement with children’s grades, safety, health, and emotional states – referred to as “helicopter” and “tiger” mothering – grew into the norm across the classes.
While prior generations of parents could, by today’s standards, be viewed as being insufficiently involved, today’s parents have become “over-involved” – aided by the ability of parents to be in constant contact with their adult children through technology. While this shift to a more hands-on, more conscientious parenting has been a boon to parent–adult child relationships in the main, the downside has meant, for some, too much of a good thing. From that perspective, pathologizing a mother’s involvement or her expressions of hurt for that child’s lack of availability provides a shield against the child’s feelings of guilt or obligation.
Diagnoses can serve a social purpose: They can allow individuals to use the authority of our profession to decide who to be close to and who to let go. They can provide insulation against feelings of obligation or guilt. They create a way to label behavior as dysfunctional that in other eras or cultures would be considered normal, even valued. To that extent, diagnoses don’t occur in a cultural void. They are inextricably tied to larger ideals, be they individualistic – as exists in the United States – or collectivist, as exists in many other parts of the world.
While we have decided what parents owe our children, it is unclear what parents might ask in return. To that end, mothers who want more interest, availability, or gratitude today are vulnerable to being cast as selfish, uncaring, needy, and controlling. They can now be viewed as failing in their task of selfless devotion. Their desires for closeness or repair can be regarded as incompatible with the quest for the adult child’s self-fulfillment and identity; her identification with her children too great a barrier to their individuation.
There may well be good reasons to estrange family members for their intolerable behaviors, especially ones who have threatened personal safety. Yet, while there are plenty of problematic parents, few meet the diagnostic criteria of narcissistic personality disorder. More important, such labels can discourage a discussion of boundaries that both the parents and the adult children might find acceptable – which sometimes means asking family members to tolerate behavior or individuals not to their liking.
Diagnoses carry enormous social weight and can facilitate estrangements or negativity to mothers that are far more workable than our patients’ characterization of them might lead them or us to believe. ; it devalues their years of love and dedication, however flawed; and it weakens the fabric of connection that could otherwise exist. Rather than provide a path toward compassion or understanding, “narcissistic mother” just becomes the latest form of mommy shaming.
Dr. Coleman is a clinical psychologist and author of “Rules of Estrangement: Why Adult Children Cut Ties and How to Heal the Conflict” (New York: Penguin Random House, 2021). Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
Narcissists appear to be everywhere. A few minutes on the Internet shows the dangers of narcissistic romantic partners, friends, and employers. Identifying and limiting the reach of their manipulative and self-centered endeavors is cast as both urgent and necessary. The destructive powers of the narcissistic mother are viewed as especially in need of remedy, and any bookstore can reveal the risks they pose: “Will I Ever Be Good Enough? Healing the Daughters of Narcissistic Mothers;” “You’re Not Crazy – It’s Your Mother: Freedom for Daughters of Narcissistic Mothers;” “Healing for Daughters of Narcissistic Mothers: A Practical Guide on How to Recover from the Childhood Trauma of Toxic Relationship with Your Mother and How You Can Handle Her Abuse Now As An Adult” – to name just a few (there are more).
As a psychologist specializing in parental estrangement, I (Dr. Coleman) regularly see letters from adult children explaining their discovery-through-therapy that their mother is a narcissist. The proclamation often comes when the therapist has never met the mother. Typically, the discovery is presented as a justification for ending the relationship with the parent. While these mothers could rightly be accused of being anxious, over-involved, depressed, or hurt by the lack of gratitude or reciprocity, the vast majority are not narcissists.
Which begs the question, why are so many being labeled in this way? Are therapists only now discovering the power of narcissistic mothers? Have they always existed, casting their spells upon unwary children? Are those now-grown children only today able to disentangle themselves from the longstanding, pervasive, and harmful influence of these parents, with the help of therapy? Or is this the newest form of mommy shaming as it engages head-on with our Diagnostic and Statistical Manuals?
We believe it is the latter.
Blaming mothers has a long reach. Mothers have been blamed for causing schizophrenia, autism, homosexuality, and effeminacy in men. While we used to call people selfish and “controlling,” narcissism is a more consequential label as it confers diagnostic validity from the mental health profession. Worse, it suggests an individual beyond reach, where the only answer is distance, containment, or estrangement.
The rise of the narcissistic mother comes during a time when, for the past 4 decades, the average working mother spends more time with her children than stay-at-home moms did in the supposed halcyon days of the 1960s’ middle class, before “parenting” was a common term. A variety of economists and sociologists observed that an increase in parental effort became necessary to launch children into adulthood given the retreat of governmental and corporate support for parents that began in the 1980s.
“The financial and emotional burden on families has grown in ways that were almost unimaginable just a half-century ago,” writes the University of Pennsylvania sociologist Frank Furstenberg in “On a New Schedule: Transitions to Adulthood and Family Change.” In addition, a view of children as vulnerable and in need of intense parental investment gained momentum over the course of the 20th century and has continued unabated into the present. As a result, an environment of intense maternal preoccupation, worry, guilt, and involvement with children’s grades, safety, health, and emotional states – referred to as “helicopter” and “tiger” mothering – grew into the norm across the classes.
While prior generations of parents could, by today’s standards, be viewed as being insufficiently involved, today’s parents have become “over-involved” – aided by the ability of parents to be in constant contact with their adult children through technology. While this shift to a more hands-on, more conscientious parenting has been a boon to parent–adult child relationships in the main, the downside has meant, for some, too much of a good thing. From that perspective, pathologizing a mother’s involvement or her expressions of hurt for that child’s lack of availability provides a shield against the child’s feelings of guilt or obligation.
Diagnoses can serve a social purpose: They can allow individuals to use the authority of our profession to decide who to be close to and who to let go. They can provide insulation against feelings of obligation or guilt. They create a way to label behavior as dysfunctional that in other eras or cultures would be considered normal, even valued. To that extent, diagnoses don’t occur in a cultural void. They are inextricably tied to larger ideals, be they individualistic – as exists in the United States – or collectivist, as exists in many other parts of the world.
While we have decided what parents owe our children, it is unclear what parents might ask in return. To that end, mothers who want more interest, availability, or gratitude today are vulnerable to being cast as selfish, uncaring, needy, and controlling. They can now be viewed as failing in their task of selfless devotion. Their desires for closeness or repair can be regarded as incompatible with the quest for the adult child’s self-fulfillment and identity; her identification with her children too great a barrier to their individuation.
There may well be good reasons to estrange family members for their intolerable behaviors, especially ones who have threatened personal safety. Yet, while there are plenty of problematic parents, few meet the diagnostic criteria of narcissistic personality disorder. More important, such labels can discourage a discussion of boundaries that both the parents and the adult children might find acceptable – which sometimes means asking family members to tolerate behavior or individuals not to their liking.
Diagnoses carry enormous social weight and can facilitate estrangements or negativity to mothers that are far more workable than our patients’ characterization of them might lead them or us to believe. ; it devalues their years of love and dedication, however flawed; and it weakens the fabric of connection that could otherwise exist. Rather than provide a path toward compassion or understanding, “narcissistic mother” just becomes the latest form of mommy shaming.
Dr. Coleman is a clinical psychologist and author of “Rules of Estrangement: Why Adult Children Cut Ties and How to Heal the Conflict” (New York: Penguin Random House, 2021). Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
Strong need for eating disorder screening in patients with PTSD
WASHINGTON –
“Eating-related and body-image concerns may be more prevalent than we think, and if not considered, these concerns can make psychotherapy treatment less effective,” study author Nick Powers, a doctoral student in clinical psychology, La Salle University, Philadelphia, told this news organization.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Common bedfellows
Although many patients with PTSD also have an eating disorder, they are not always properly assessed for eating pathology and related functional impairment.
Some therapists don’t feel adequately equipped to target eating-related concerns in these patients and so may refer them to other providers. This, said Mr. Powers, can prolong symptoms and further distress patients.
Mr. Powers noted childhood physical or sexual abuse may affect eating patterns in patients with PTSD. “The evidence suggests these types of trauma exposure can be risk factors for the development of an eating disorder.”
Undiagnosed eating pathology may exacerbate functional impairment from PTSD and weaken the impact of evidence-based treatment.
Such patients are challenging to treat as they may not have the requisite skills to fully engage in exposure therapy, an evidence-based approach to treat PTSD, said Mr. Powers.
To determine whether PTSD would be significantly linked to greater eating disorder impairment (EDI) compared with other anxiety-related diagnoses and whether this would impair treatment, investigators studied 748 patients with an anxiety disorder who were attending a cognitive behavioral therapy (CBT) clinic. Anxiety disorders included PTSD, obsessive-compulsive disorder (OCD), social anxiety, and panic disorder.
Participants completed the 16-item Clinical Impairment Assessment (CIA) questionnaire, which includes questions about eating habits and feelings about food, body shape, and weight over the previous 4 weeks. Participants also reported anxiety symptom severity at the beginning, during, and end of treatment.
Need for better screening
Results showed that compared with those who had other anxiety disorders, patients with PTSD were three times more likely to have disordered eating (odds ratio [OR], 3.06; 95% confidence interval [CI], 1.47-6.37; P = .003).
In addition, higher baseline CIA scores predicted poorer PTSD treatment outcome (beta = –1.4; 95% CI, –1.67 to –1.10; P < .01).
“Having higher baseline CIA scores meant that patients’ PTSD symptoms did not remit as strongly compared to those with lower scores,” said Mr. Powers.
Patients with both PTSD and an eating disorder may have difficulty with regulating emotions and tolerating distress, he said.
“They may use binge eating, purging, or food restriction as strategies to regulate emotions. These behaviors may allow patients to become numb to or avoid heightened emotions that come from having PTSD and an eating disorder.”
Prior research linked perfectionism tendencies to poorer response to PTSD treatment. Those with an eating disorder may share similar tendencies, said Mr. Powers.
“If someone is consistently thinking negatively about their eating or body to the point where it interrupts their functioning, they may not be as likely to fully engage with PTSD treatment,” he said.
Ideally, clinicians would screen all patients with PTSD for an eating disorder, said Mr. Powers. “If screening instruments aren’t feasible or available, even just inquiring about body image or history of maladaptive eating behaviors can be helpful.”
He added this could open up a conversation about a traumatic event in the patient’s past.
Confirmatory research
Commenting on the study, Karen S. Mitchell, PhD, clinical research psychologist, National Center for PTSD, VA Boston Healthcare System, and associate professor in psychiatry, Boston University, said she was “excited” to see this research.
“Very few studies have examined the impact of baseline eating disorder symptoms on PTSD treatment outcomes or vice versa,” she said.
The study findings “add to the small but growing body of evidence suggesting that comorbid PTSD and eating disorder symptoms can impact recovery from each disorder,” she said.
She noted the importance of assessing comorbidity in patients presenting for treatment and of addressing comorbidity in both eating disorders and PTSD treatment. “But we need more research on how best to do this.”
Mr. Powers and Dr. Mitchell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
“Eating-related and body-image concerns may be more prevalent than we think, and if not considered, these concerns can make psychotherapy treatment less effective,” study author Nick Powers, a doctoral student in clinical psychology, La Salle University, Philadelphia, told this news organization.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Common bedfellows
Although many patients with PTSD also have an eating disorder, they are not always properly assessed for eating pathology and related functional impairment.
Some therapists don’t feel adequately equipped to target eating-related concerns in these patients and so may refer them to other providers. This, said Mr. Powers, can prolong symptoms and further distress patients.
Mr. Powers noted childhood physical or sexual abuse may affect eating patterns in patients with PTSD. “The evidence suggests these types of trauma exposure can be risk factors for the development of an eating disorder.”
Undiagnosed eating pathology may exacerbate functional impairment from PTSD and weaken the impact of evidence-based treatment.
Such patients are challenging to treat as they may not have the requisite skills to fully engage in exposure therapy, an evidence-based approach to treat PTSD, said Mr. Powers.
To determine whether PTSD would be significantly linked to greater eating disorder impairment (EDI) compared with other anxiety-related diagnoses and whether this would impair treatment, investigators studied 748 patients with an anxiety disorder who were attending a cognitive behavioral therapy (CBT) clinic. Anxiety disorders included PTSD, obsessive-compulsive disorder (OCD), social anxiety, and panic disorder.
Participants completed the 16-item Clinical Impairment Assessment (CIA) questionnaire, which includes questions about eating habits and feelings about food, body shape, and weight over the previous 4 weeks. Participants also reported anxiety symptom severity at the beginning, during, and end of treatment.
Need for better screening
Results showed that compared with those who had other anxiety disorders, patients with PTSD were three times more likely to have disordered eating (odds ratio [OR], 3.06; 95% confidence interval [CI], 1.47-6.37; P = .003).
In addition, higher baseline CIA scores predicted poorer PTSD treatment outcome (beta = –1.4; 95% CI, –1.67 to –1.10; P < .01).
“Having higher baseline CIA scores meant that patients’ PTSD symptoms did not remit as strongly compared to those with lower scores,” said Mr. Powers.
Patients with both PTSD and an eating disorder may have difficulty with regulating emotions and tolerating distress, he said.
“They may use binge eating, purging, or food restriction as strategies to regulate emotions. These behaviors may allow patients to become numb to or avoid heightened emotions that come from having PTSD and an eating disorder.”
Prior research linked perfectionism tendencies to poorer response to PTSD treatment. Those with an eating disorder may share similar tendencies, said Mr. Powers.
“If someone is consistently thinking negatively about their eating or body to the point where it interrupts their functioning, they may not be as likely to fully engage with PTSD treatment,” he said.
Ideally, clinicians would screen all patients with PTSD for an eating disorder, said Mr. Powers. “If screening instruments aren’t feasible or available, even just inquiring about body image or history of maladaptive eating behaviors can be helpful.”
He added this could open up a conversation about a traumatic event in the patient’s past.
Confirmatory research
Commenting on the study, Karen S. Mitchell, PhD, clinical research psychologist, National Center for PTSD, VA Boston Healthcare System, and associate professor in psychiatry, Boston University, said she was “excited” to see this research.
“Very few studies have examined the impact of baseline eating disorder symptoms on PTSD treatment outcomes or vice versa,” she said.
The study findings “add to the small but growing body of evidence suggesting that comorbid PTSD and eating disorder symptoms can impact recovery from each disorder,” she said.
She noted the importance of assessing comorbidity in patients presenting for treatment and of addressing comorbidity in both eating disorders and PTSD treatment. “But we need more research on how best to do this.”
Mr. Powers and Dr. Mitchell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
“Eating-related and body-image concerns may be more prevalent than we think, and if not considered, these concerns can make psychotherapy treatment less effective,” study author Nick Powers, a doctoral student in clinical psychology, La Salle University, Philadelphia, told this news organization.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Common bedfellows
Although many patients with PTSD also have an eating disorder, they are not always properly assessed for eating pathology and related functional impairment.
Some therapists don’t feel adequately equipped to target eating-related concerns in these patients and so may refer them to other providers. This, said Mr. Powers, can prolong symptoms and further distress patients.
Mr. Powers noted childhood physical or sexual abuse may affect eating patterns in patients with PTSD. “The evidence suggests these types of trauma exposure can be risk factors for the development of an eating disorder.”
Undiagnosed eating pathology may exacerbate functional impairment from PTSD and weaken the impact of evidence-based treatment.
Such patients are challenging to treat as they may not have the requisite skills to fully engage in exposure therapy, an evidence-based approach to treat PTSD, said Mr. Powers.
To determine whether PTSD would be significantly linked to greater eating disorder impairment (EDI) compared with other anxiety-related diagnoses and whether this would impair treatment, investigators studied 748 patients with an anxiety disorder who were attending a cognitive behavioral therapy (CBT) clinic. Anxiety disorders included PTSD, obsessive-compulsive disorder (OCD), social anxiety, and panic disorder.
Participants completed the 16-item Clinical Impairment Assessment (CIA) questionnaire, which includes questions about eating habits and feelings about food, body shape, and weight over the previous 4 weeks. Participants also reported anxiety symptom severity at the beginning, during, and end of treatment.
Need for better screening
Results showed that compared with those who had other anxiety disorders, patients with PTSD were three times more likely to have disordered eating (odds ratio [OR], 3.06; 95% confidence interval [CI], 1.47-6.37; P = .003).
In addition, higher baseline CIA scores predicted poorer PTSD treatment outcome (beta = –1.4; 95% CI, –1.67 to –1.10; P < .01).
“Having higher baseline CIA scores meant that patients’ PTSD symptoms did not remit as strongly compared to those with lower scores,” said Mr. Powers.
Patients with both PTSD and an eating disorder may have difficulty with regulating emotions and tolerating distress, he said.
“They may use binge eating, purging, or food restriction as strategies to regulate emotions. These behaviors may allow patients to become numb to or avoid heightened emotions that come from having PTSD and an eating disorder.”
Prior research linked perfectionism tendencies to poorer response to PTSD treatment. Those with an eating disorder may share similar tendencies, said Mr. Powers.
“If someone is consistently thinking negatively about their eating or body to the point where it interrupts their functioning, they may not be as likely to fully engage with PTSD treatment,” he said.
Ideally, clinicians would screen all patients with PTSD for an eating disorder, said Mr. Powers. “If screening instruments aren’t feasible or available, even just inquiring about body image or history of maladaptive eating behaviors can be helpful.”
He added this could open up a conversation about a traumatic event in the patient’s past.
Confirmatory research
Commenting on the study, Karen S. Mitchell, PhD, clinical research psychologist, National Center for PTSD, VA Boston Healthcare System, and associate professor in psychiatry, Boston University, said she was “excited” to see this research.
“Very few studies have examined the impact of baseline eating disorder symptoms on PTSD treatment outcomes or vice versa,” she said.
The study findings “add to the small but growing body of evidence suggesting that comorbid PTSD and eating disorder symptoms can impact recovery from each disorder,” she said.
She noted the importance of assessing comorbidity in patients presenting for treatment and of addressing comorbidity in both eating disorders and PTSD treatment. “But we need more research on how best to do this.”
Mr. Powers and Dr. Mitchell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
Commentary: PsA development risks, and a new index, May 2023
The differences between patients who have PsA with axial involvement (AxPsA) and patients who have axial spondyloarthritis with psoriasis (AxSpA+PsO) continue to remain a strong area of interest. Regierer and colleagues recently compared 359 patients with AxPsA vs 181 patients with AxSpA+PsO. These patients were enrolled into the RABBIT-SpA prospective longitudinal cohort study. Given the lack of definition of AxPsA, two definitions were used: 1) clinical judgment by the rheumatologist and 2) imaging (x-ray or MRI) findings. Regardless of clinical or imaging definition used, compared with patients who have AxSpA+PsO those with AxPsA were significantly more often women, were older, were less often HLA-B27 positive, and had more frequent peripheral manifestations but less frequent uveitis. The two diseases thus have significant differences; these should be carefully considered while making treatment decisions.
Another major research focus is on the influence of sex on PsA treatment response. Eder and colleagues conducted a post hoc analysis of pooled data from phase 3 randomized controlled trials that included 816 patients with PsA who received tofacitinib, adalimumab, or placebo. They demonstrate that at 3 months, tofacitinib was more efficacious than placebo, irrespective of sex. However, a higher proportion of men vs women receiving tofacitinib achieved minimal disease activity. This might be due to baseline differences in disease activity. The American College of Rheumatology 20/50/70 response rates were comparable. The incidence of treatment-emergent adverse events was similar in men and women receiving tofacitinib. Thus, sex significantly influences achieving low disease state. Understanding the mechanisms underlying sex differences will help improve treatment response rates in women with PsA.
Atherosclerotic vascular disease (ASVD) is an important comorbidity of PsA. Predicting ASVD remains difficult. The triglyceride-glucose (TyG) index — calculated as ln[fasting triglycerides (in mg/dL) × fasting glucose (in mg/dL)/2] — was recently identified as a marker of insulin resistance and ASVD. Xie and colleagues conducted a cross-sectional study in 165 patients with PsA who underwent carotid ultrasound and had data available for the TyG index. In a model that was adjusted for age, sex, comorbidities, smoking, BMI, low-density lipoprotein cholesterol, psoriasis area and severity index, and disease activity index for PsA, the TyG index was significantly associated with the presence of carotid atherosclerosis (adjusted odds ratio [aOR] 2.69; 95% CI 1.02-7.11) as well as carotid artery plaque (aOR 3.61; 95% CI 1.15-11.38). Thus, this easily calculated marker is associated with ASVD independent of demographic, traditional risk factors, and disease activity and needs further evaluation in prospective studies.
The differences between patients who have PsA with axial involvement (AxPsA) and patients who have axial spondyloarthritis with psoriasis (AxSpA+PsO) continue to remain a strong area of interest. Regierer and colleagues recently compared 359 patients with AxPsA vs 181 patients with AxSpA+PsO. These patients were enrolled into the RABBIT-SpA prospective longitudinal cohort study. Given the lack of definition of AxPsA, two definitions were used: 1) clinical judgment by the rheumatologist and 2) imaging (x-ray or MRI) findings. Regardless of clinical or imaging definition used, compared with patients who have AxSpA+PsO those with AxPsA were significantly more often women, were older, were less often HLA-B27 positive, and had more frequent peripheral manifestations but less frequent uveitis. The two diseases thus have significant differences; these should be carefully considered while making treatment decisions.
Another major research focus is on the influence of sex on PsA treatment response. Eder and colleagues conducted a post hoc analysis of pooled data from phase 3 randomized controlled trials that included 816 patients with PsA who received tofacitinib, adalimumab, or placebo. They demonstrate that at 3 months, tofacitinib was more efficacious than placebo, irrespective of sex. However, a higher proportion of men vs women receiving tofacitinib achieved minimal disease activity. This might be due to baseline differences in disease activity. The American College of Rheumatology 20/50/70 response rates were comparable. The incidence of treatment-emergent adverse events was similar in men and women receiving tofacitinib. Thus, sex significantly influences achieving low disease state. Understanding the mechanisms underlying sex differences will help improve treatment response rates in women with PsA.
Atherosclerotic vascular disease (ASVD) is an important comorbidity of PsA. Predicting ASVD remains difficult. The triglyceride-glucose (TyG) index — calculated as ln[fasting triglycerides (in mg/dL) × fasting glucose (in mg/dL)/2] — was recently identified as a marker of insulin resistance and ASVD. Xie and colleagues conducted a cross-sectional study in 165 patients with PsA who underwent carotid ultrasound and had data available for the TyG index. In a model that was adjusted for age, sex, comorbidities, smoking, BMI, low-density lipoprotein cholesterol, psoriasis area and severity index, and disease activity index for PsA, the TyG index was significantly associated with the presence of carotid atherosclerosis (adjusted odds ratio [aOR] 2.69; 95% CI 1.02-7.11) as well as carotid artery plaque (aOR 3.61; 95% CI 1.15-11.38). Thus, this easily calculated marker is associated with ASVD independent of demographic, traditional risk factors, and disease activity and needs further evaluation in prospective studies.
The differences between patients who have PsA with axial involvement (AxPsA) and patients who have axial spondyloarthritis with psoriasis (AxSpA+PsO) continue to remain a strong area of interest. Regierer and colleagues recently compared 359 patients with AxPsA vs 181 patients with AxSpA+PsO. These patients were enrolled into the RABBIT-SpA prospective longitudinal cohort study. Given the lack of definition of AxPsA, two definitions were used: 1) clinical judgment by the rheumatologist and 2) imaging (x-ray or MRI) findings. Regardless of clinical or imaging definition used, compared with patients who have AxSpA+PsO those with AxPsA were significantly more often women, were older, were less often HLA-B27 positive, and had more frequent peripheral manifestations but less frequent uveitis. The two diseases thus have significant differences; these should be carefully considered while making treatment decisions.
Another major research focus is on the influence of sex on PsA treatment response. Eder and colleagues conducted a post hoc analysis of pooled data from phase 3 randomized controlled trials that included 816 patients with PsA who received tofacitinib, adalimumab, or placebo. They demonstrate that at 3 months, tofacitinib was more efficacious than placebo, irrespective of sex. However, a higher proportion of men vs women receiving tofacitinib achieved minimal disease activity. This might be due to baseline differences in disease activity. The American College of Rheumatology 20/50/70 response rates were comparable. The incidence of treatment-emergent adverse events was similar in men and women receiving tofacitinib. Thus, sex significantly influences achieving low disease state. Understanding the mechanisms underlying sex differences will help improve treatment response rates in women with PsA.
Atherosclerotic vascular disease (ASVD) is an important comorbidity of PsA. Predicting ASVD remains difficult. The triglyceride-glucose (TyG) index — calculated as ln[fasting triglycerides (in mg/dL) × fasting glucose (in mg/dL)/2] — was recently identified as a marker of insulin resistance and ASVD. Xie and colleagues conducted a cross-sectional study in 165 patients with PsA who underwent carotid ultrasound and had data available for the TyG index. In a model that was adjusted for age, sex, comorbidities, smoking, BMI, low-density lipoprotein cholesterol, psoriasis area and severity index, and disease activity index for PsA, the TyG index was significantly associated with the presence of carotid atherosclerosis (adjusted odds ratio [aOR] 2.69; 95% CI 1.02-7.11) as well as carotid artery plaque (aOR 3.61; 95% CI 1.15-11.38). Thus, this easily calculated marker is associated with ASVD independent of demographic, traditional risk factors, and disease activity and needs further evaluation in prospective studies.