User login
Racial morphing: A conundrum in cosmetic dermatology
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Cancer clinical trials: Can industry stack the deck?
A year before the COVID-19 pandemic began, a team of clinical statisticians at the University of Texas MD Anderson Cancer Center sat together in small office for a year, painstakingly hand coding data from the U.S. clinical trials database, www.clinicaltrials.gov.
“We found marked disparities across different disease sites. ... The patients that are enrolling on studies are markedly younger than the average patient seen in the population with those same conditions,” said team leader Ethan Ludmir, MD, assistant professor, Division of Radiation Oncology at the University of Texas.
And this age disparity was significantly greater in industry-funded trials.
Researchers have known for 20 years that cancer trial participants are not representative of the wider cancer population, and numerous government guidance documents have been issued on the matter. However, this Texas team’s findings were the first unambiguous evidence that pharmaceutical companies seem to be selecting younger patients to test their drugs.
“If we’re being generous then perhaps the answer is: They’re looking for some element of homogeneity, which is to say they don’t want competing risks to make the signal-to-noise ratio uninterpretable,” said Dr. Ludmir.
Dr. Laura Bothwell, PhD, assistant professor, Yale School of Public Health, recently coauthored a 259-page consensus report for the National Academies of Sciences, Engineering and Medicine on how to increase the research involvement of under-represented groups.
Dr. Bothwell said, “The problem with industry funded research is that ... it’s an inevitable conflict of interest that exists. They want the research to show that their products work. And older populations ... have a lot more complications, which leads to potentially less favorable results.”
The MD Anderson findings were published in JAMA Oncology. “That was the starting point in our journey,” said Dr. Ludmir. For the next 3 years, the researchers mined their painstakingly constructed database to understand what was preventing greater numbers of older patients from enrollment in cancer trials.
Meanwhile, answers were coming from elsewhere. In parallel with the work at MD Anderson, a team in California led by Mina Sedrak, MD, a medical oncologist at the City of Hope National Medical Center, had also started investigating age disparities in clinical trials.
Dr. Sedrak, who also serves as deputy director of Clinical Trials at the Center for Cancer and Aging, said he had become increasingly concerned that he did not have adequate information on new cancer therapies for his older patients.
“I was caring for a large number of people who were ... older adults,” said Dr. Sedrak, “But the data that was being used to get the standard-of-care treatment for cancer did not include older adults. And so there was this lack of applicability.”
He summed up the challenges in a 2021 review paper: “Most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients.”
By 2030, it is estimated that 70% of all new cancer diagnoses will be in patients 65 years old and older. By contrast, patients over age 65 still account for only 40% of patients in cancer trials registered with the FDA (2015 figures) and older adults make up only 44% of participants in practice-changing cancer trials, according to a 2022 study.
So what is going on? Are studies specifically designed to squeeze out older patients?
Surprisingly, patients are not being kept out of trials by formal age limits, according to Dr. Ludmir. His team found that only 10% of phase 3 trials over the past 30 years had an upper limit for age, and age restrictions have been dropping by 1% a year. (For example, 16% of trials that enrolled in 2002-2005 had an upper age limit, compared with just 8% of trials that started in 2010-2014.)
Dr. Sedrak’s team found that “clinician bias” may be a factor, a situation in which trial investigators – particularly academic oncologists – are subconsciously picking younger, healthier patients for trials and excluding older, sicker patients to protect them from drug toxicities.
Dr. Ludmir said this was understandable, especially in the case of industry-driven trials, which tend to have demanding endpoints and “an overall posture of more treatment aggressiveness.”
“These are typically not trials where they’re saying, `Hey, if we add acupuncture ... are we going to see improved patient reported outcomes?’” Dr. Ludmir explained. “You’re asking ... I’ve got this cocktail of two pretty rough chemos: I want to see what happens if I add an immunotherapy to that. If I’m the clinician in clinic, I might reasonably, subconsciously, say, is the 75-year-old really who I want on this?”
What about patient bias? Perhaps fewer older patients wish to join clinical trials?
Not so, at least not at community cancer centers, said Dr. Sedrak. His team’s analysis of the National Cancer Institute Community Oncology Research Program database for 2016-2019 revealed that older patients were just as keen as the younger patients to participate in trials (68% of patients aged 50-69 years and 65% of patients 70+; P = .28).
However, drug companies may be excluding older patients by more subtle means. One-fifth of patients over 65 have had a prior cancer. Dr. Ludmir and coauthor Roshal Patel, MD, used their hand-coded www.clinicaltrials.gov database to look at prior malignancy exclusion criteria (PMEC). The analysis found “pervasive utilization” of PMEC in phase 3 trials, cropping up in 41% of studies over the past 30 years.
PMEC was significantly associated with age disparities and was significantly more common in industry-funded trials.
When asked whether PMEC are “age restriction by stealth” on the part of drug companies, Dr. Ludmir was reluctant to assign blame, but stood by his data: “The wider you restrict people in terms of having a prior cancer, the wider the age disparities in the subsequent studies, which to me is about as strong, in terms of causal understanding of these phenomena, as you can reasonably get at this level.”
In March the FDA released a guidance document titled Inclusion of Older Adults in Cancer Clinical Trials. However, its recommendations are “nonbinding” and “do not have the force and effect of law.”
To fix the issues, said Dr. Sedrak, the FDA must be given teeth.
“Okay, you write guidelines,” he said. “But if you don’t actually hold people accountable to following the guidelines, how are we going to implement and make sure that we’re transforming policy into action?”
Dr. Bothwell of Yale’s School of Public Health agreed. “Accountability has been the weakest link for decades now.”
She concluded, “In medicine there’s a tendency to believe that a therapy, because it exists and it has been tested and it’s shown some efficacy, it’s useful. But we don’t know the answer to that question unless we have statistically valid research in the population that we’re using it in.”
Dr. Bothwell and Dr. Ludmir report no conflicts of interest. In his publications, Dr. Sedrak reports industry grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation.
A year before the COVID-19 pandemic began, a team of clinical statisticians at the University of Texas MD Anderson Cancer Center sat together in small office for a year, painstakingly hand coding data from the U.S. clinical trials database, www.clinicaltrials.gov.
“We found marked disparities across different disease sites. ... The patients that are enrolling on studies are markedly younger than the average patient seen in the population with those same conditions,” said team leader Ethan Ludmir, MD, assistant professor, Division of Radiation Oncology at the University of Texas.
And this age disparity was significantly greater in industry-funded trials.
Researchers have known for 20 years that cancer trial participants are not representative of the wider cancer population, and numerous government guidance documents have been issued on the matter. However, this Texas team’s findings were the first unambiguous evidence that pharmaceutical companies seem to be selecting younger patients to test their drugs.
“If we’re being generous then perhaps the answer is: They’re looking for some element of homogeneity, which is to say they don’t want competing risks to make the signal-to-noise ratio uninterpretable,” said Dr. Ludmir.
Dr. Laura Bothwell, PhD, assistant professor, Yale School of Public Health, recently coauthored a 259-page consensus report for the National Academies of Sciences, Engineering and Medicine on how to increase the research involvement of under-represented groups.
Dr. Bothwell said, “The problem with industry funded research is that ... it’s an inevitable conflict of interest that exists. They want the research to show that their products work. And older populations ... have a lot more complications, which leads to potentially less favorable results.”
The MD Anderson findings were published in JAMA Oncology. “That was the starting point in our journey,” said Dr. Ludmir. For the next 3 years, the researchers mined their painstakingly constructed database to understand what was preventing greater numbers of older patients from enrollment in cancer trials.
Meanwhile, answers were coming from elsewhere. In parallel with the work at MD Anderson, a team in California led by Mina Sedrak, MD, a medical oncologist at the City of Hope National Medical Center, had also started investigating age disparities in clinical trials.
Dr. Sedrak, who also serves as deputy director of Clinical Trials at the Center for Cancer and Aging, said he had become increasingly concerned that he did not have adequate information on new cancer therapies for his older patients.
“I was caring for a large number of people who were ... older adults,” said Dr. Sedrak, “But the data that was being used to get the standard-of-care treatment for cancer did not include older adults. And so there was this lack of applicability.”
He summed up the challenges in a 2021 review paper: “Most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients.”
By 2030, it is estimated that 70% of all new cancer diagnoses will be in patients 65 years old and older. By contrast, patients over age 65 still account for only 40% of patients in cancer trials registered with the FDA (2015 figures) and older adults make up only 44% of participants in practice-changing cancer trials, according to a 2022 study.
So what is going on? Are studies specifically designed to squeeze out older patients?
Surprisingly, patients are not being kept out of trials by formal age limits, according to Dr. Ludmir. His team found that only 10% of phase 3 trials over the past 30 years had an upper limit for age, and age restrictions have been dropping by 1% a year. (For example, 16% of trials that enrolled in 2002-2005 had an upper age limit, compared with just 8% of trials that started in 2010-2014.)
Dr. Sedrak’s team found that “clinician bias” may be a factor, a situation in which trial investigators – particularly academic oncologists – are subconsciously picking younger, healthier patients for trials and excluding older, sicker patients to protect them from drug toxicities.
Dr. Ludmir said this was understandable, especially in the case of industry-driven trials, which tend to have demanding endpoints and “an overall posture of more treatment aggressiveness.”
“These are typically not trials where they’re saying, `Hey, if we add acupuncture ... are we going to see improved patient reported outcomes?’” Dr. Ludmir explained. “You’re asking ... I’ve got this cocktail of two pretty rough chemos: I want to see what happens if I add an immunotherapy to that. If I’m the clinician in clinic, I might reasonably, subconsciously, say, is the 75-year-old really who I want on this?”
What about patient bias? Perhaps fewer older patients wish to join clinical trials?
Not so, at least not at community cancer centers, said Dr. Sedrak. His team’s analysis of the National Cancer Institute Community Oncology Research Program database for 2016-2019 revealed that older patients were just as keen as the younger patients to participate in trials (68% of patients aged 50-69 years and 65% of patients 70+; P = .28).
However, drug companies may be excluding older patients by more subtle means. One-fifth of patients over 65 have had a prior cancer. Dr. Ludmir and coauthor Roshal Patel, MD, used their hand-coded www.clinicaltrials.gov database to look at prior malignancy exclusion criteria (PMEC). The analysis found “pervasive utilization” of PMEC in phase 3 trials, cropping up in 41% of studies over the past 30 years.
PMEC was significantly associated with age disparities and was significantly more common in industry-funded trials.
When asked whether PMEC are “age restriction by stealth” on the part of drug companies, Dr. Ludmir was reluctant to assign blame, but stood by his data: “The wider you restrict people in terms of having a prior cancer, the wider the age disparities in the subsequent studies, which to me is about as strong, in terms of causal understanding of these phenomena, as you can reasonably get at this level.”
In March the FDA released a guidance document titled Inclusion of Older Adults in Cancer Clinical Trials. However, its recommendations are “nonbinding” and “do not have the force and effect of law.”
To fix the issues, said Dr. Sedrak, the FDA must be given teeth.
“Okay, you write guidelines,” he said. “But if you don’t actually hold people accountable to following the guidelines, how are we going to implement and make sure that we’re transforming policy into action?”
Dr. Bothwell of Yale’s School of Public Health agreed. “Accountability has been the weakest link for decades now.”
She concluded, “In medicine there’s a tendency to believe that a therapy, because it exists and it has been tested and it’s shown some efficacy, it’s useful. But we don’t know the answer to that question unless we have statistically valid research in the population that we’re using it in.”
Dr. Bothwell and Dr. Ludmir report no conflicts of interest. In his publications, Dr. Sedrak reports industry grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation.
A year before the COVID-19 pandemic began, a team of clinical statisticians at the University of Texas MD Anderson Cancer Center sat together in small office for a year, painstakingly hand coding data from the U.S. clinical trials database, www.clinicaltrials.gov.
“We found marked disparities across different disease sites. ... The patients that are enrolling on studies are markedly younger than the average patient seen in the population with those same conditions,” said team leader Ethan Ludmir, MD, assistant professor, Division of Radiation Oncology at the University of Texas.
And this age disparity was significantly greater in industry-funded trials.
Researchers have known for 20 years that cancer trial participants are not representative of the wider cancer population, and numerous government guidance documents have been issued on the matter. However, this Texas team’s findings were the first unambiguous evidence that pharmaceutical companies seem to be selecting younger patients to test their drugs.
“If we’re being generous then perhaps the answer is: They’re looking for some element of homogeneity, which is to say they don’t want competing risks to make the signal-to-noise ratio uninterpretable,” said Dr. Ludmir.
Dr. Laura Bothwell, PhD, assistant professor, Yale School of Public Health, recently coauthored a 259-page consensus report for the National Academies of Sciences, Engineering and Medicine on how to increase the research involvement of under-represented groups.
Dr. Bothwell said, “The problem with industry funded research is that ... it’s an inevitable conflict of interest that exists. They want the research to show that their products work. And older populations ... have a lot more complications, which leads to potentially less favorable results.”
The MD Anderson findings were published in JAMA Oncology. “That was the starting point in our journey,” said Dr. Ludmir. For the next 3 years, the researchers mined their painstakingly constructed database to understand what was preventing greater numbers of older patients from enrollment in cancer trials.
Meanwhile, answers were coming from elsewhere. In parallel with the work at MD Anderson, a team in California led by Mina Sedrak, MD, a medical oncologist at the City of Hope National Medical Center, had also started investigating age disparities in clinical trials.
Dr. Sedrak, who also serves as deputy director of Clinical Trials at the Center for Cancer and Aging, said he had become increasingly concerned that he did not have adequate information on new cancer therapies for his older patients.
“I was caring for a large number of people who were ... older adults,” said Dr. Sedrak, “But the data that was being used to get the standard-of-care treatment for cancer did not include older adults. And so there was this lack of applicability.”
He summed up the challenges in a 2021 review paper: “Most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients.”
By 2030, it is estimated that 70% of all new cancer diagnoses will be in patients 65 years old and older. By contrast, patients over age 65 still account for only 40% of patients in cancer trials registered with the FDA (2015 figures) and older adults make up only 44% of participants in practice-changing cancer trials, according to a 2022 study.
So what is going on? Are studies specifically designed to squeeze out older patients?
Surprisingly, patients are not being kept out of trials by formal age limits, according to Dr. Ludmir. His team found that only 10% of phase 3 trials over the past 30 years had an upper limit for age, and age restrictions have been dropping by 1% a year. (For example, 16% of trials that enrolled in 2002-2005 had an upper age limit, compared with just 8% of trials that started in 2010-2014.)
Dr. Sedrak’s team found that “clinician bias” may be a factor, a situation in which trial investigators – particularly academic oncologists – are subconsciously picking younger, healthier patients for trials and excluding older, sicker patients to protect them from drug toxicities.
Dr. Ludmir said this was understandable, especially in the case of industry-driven trials, which tend to have demanding endpoints and “an overall posture of more treatment aggressiveness.”
“These are typically not trials where they’re saying, `Hey, if we add acupuncture ... are we going to see improved patient reported outcomes?’” Dr. Ludmir explained. “You’re asking ... I’ve got this cocktail of two pretty rough chemos: I want to see what happens if I add an immunotherapy to that. If I’m the clinician in clinic, I might reasonably, subconsciously, say, is the 75-year-old really who I want on this?”
What about patient bias? Perhaps fewer older patients wish to join clinical trials?
Not so, at least not at community cancer centers, said Dr. Sedrak. His team’s analysis of the National Cancer Institute Community Oncology Research Program database for 2016-2019 revealed that older patients were just as keen as the younger patients to participate in trials (68% of patients aged 50-69 years and 65% of patients 70+; P = .28).
However, drug companies may be excluding older patients by more subtle means. One-fifth of patients over 65 have had a prior cancer. Dr. Ludmir and coauthor Roshal Patel, MD, used their hand-coded www.clinicaltrials.gov database to look at prior malignancy exclusion criteria (PMEC). The analysis found “pervasive utilization” of PMEC in phase 3 trials, cropping up in 41% of studies over the past 30 years.
PMEC was significantly associated with age disparities and was significantly more common in industry-funded trials.
When asked whether PMEC are “age restriction by stealth” on the part of drug companies, Dr. Ludmir was reluctant to assign blame, but stood by his data: “The wider you restrict people in terms of having a prior cancer, the wider the age disparities in the subsequent studies, which to me is about as strong, in terms of causal understanding of these phenomena, as you can reasonably get at this level.”
In March the FDA released a guidance document titled Inclusion of Older Adults in Cancer Clinical Trials. However, its recommendations are “nonbinding” and “do not have the force and effect of law.”
To fix the issues, said Dr. Sedrak, the FDA must be given teeth.
“Okay, you write guidelines,” he said. “But if you don’t actually hold people accountable to following the guidelines, how are we going to implement and make sure that we’re transforming policy into action?”
Dr. Bothwell of Yale’s School of Public Health agreed. “Accountability has been the weakest link for decades now.”
She concluded, “In medicine there’s a tendency to believe that a therapy, because it exists and it has been tested and it’s shown some efficacy, it’s useful. But we don’t know the answer to that question unless we have statistically valid research in the population that we’re using it in.”
Dr. Bothwell and Dr. Ludmir report no conflicts of interest. In his publications, Dr. Sedrak reports industry grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation.
Ob.gyns. reveal heavier suicide ideation burden than most specialists
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.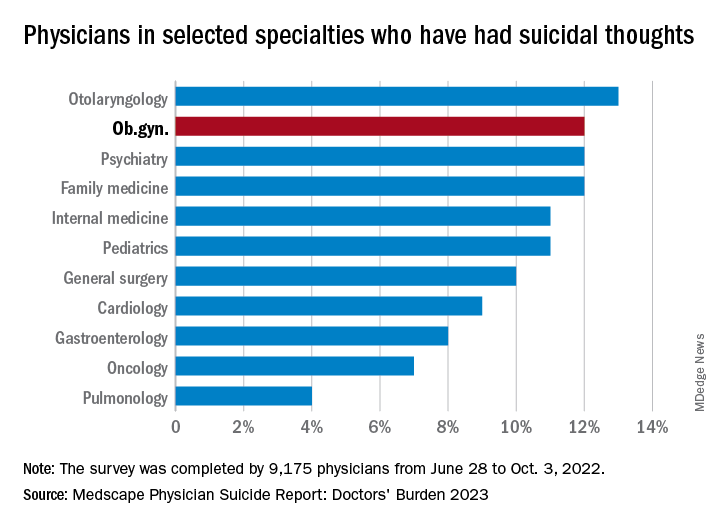
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.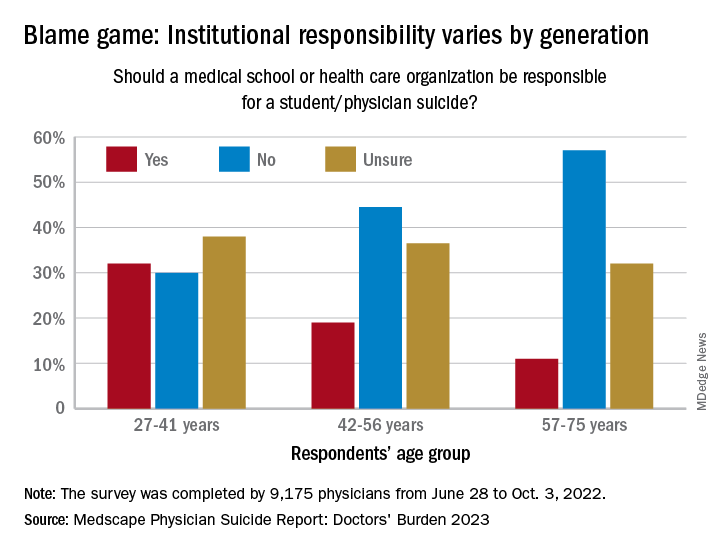
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Defensiveness may drive refusal for colon cancer screening
An Irish study attempting to get at the root of why men and women delay colon cancer screening found that, despite an uptick in colon cancer cases among younger adults, screening isn’t a priority for some adults while others are under the impression that a healthy diet with regular bowel movements negates the need for regular screening.
The findings are based on a survey of over 2,000 adults who participated in a population-based fecal immunochemical test (FIT) screening program. The authors found that denying the immediacy of the need to be tested and self-exempting from screening because of a belief in a healthy lifestyle were key drivers for opting out of FIT screening.
“What we found was that people who didn’t take part [in the survey] responded much more defensively to the invitation,” said Nicholas Clarke, PhD, a researcher at Dublin City University, who served as the lead author of the study published in the journal Cancer.
The domain of denying immediacy, which covers decisions like putting off a test because of more pressing life events, was associated with a 47% reduction in screening probability. “That’s quite a high percentage. They’re not saying I won’t do it. They’re saying: ‘I’ll wait to get tested for colon cancer until my other health concerns are under control, or until there’s a better test,’ ” he said.
The other suppression category of self-exempting was associated with a 20% reduction in the odds of participation. “They’re saying: ‘I don’t need to be tested because I have enough vegetables in my diet or because I have regular bowel movements,’ ” Dr. Clarke said.
Despite the proven efficacy of screening, many individuals still resist screening. In previous research, Dr. Clarke found that men in Ireland were less likely than women to undergo screening.
FIT works by identifying small amounts of blood in the stool that could suggest the presence of a tumor or precancerous polyps. The test also looks for methylation and DNA mutations that are indicative of precancer polyps or tumors. A positive test calls for a follow-up procedure such as a colonoscopy, where precancerous polyps can be removed to prevent them from developing into tumors.
FIT has similar sensitivity to colonoscopy in detecting cancers (93% vs. 95%), but is less effective with respect to polyps (42% vs. 75%-93%). For average risk adults between 50-75 years old, the U.S Preventive Services Task Force recommends colonoscopy every 10 years; flexible sigmoidoscopy or CT colonography every 5 years, or flexible sigmoidoscopy every 10 years plus fecal immunochemical test (FIT) every year; FIT DNA test every 3 years; guaiac-based fecal occult blood test or FIT test annually.
Findings from the new study
In the new study, researchers contacted both 2,299 responders and nonresponders to FIT tests that had been mailed out as part of a Dublin colorectal cancer screening program between 2008 and 2012. Researchers employed the McQueen defensive information processing (DIP) measure, which includes four domains of defensive attitudes that include information avoidance, mental disengagement or denial, suppression through belief that one is immune, and arguing against the evidence.
In this study, 7,476 men and women in Dublin were invited to participate in a population‐based FIT screening program. In follow-up questionnaires sent to those who did or did not complete FIT, 53% of those who completed FIT screening answered the questionnaire, while 8% of those who did not complete the screening returned the questionnaire. Those who didn’t complete the FIT test had higher DIP scores suggesting more rates of opting out of receiving health information, avoiding doctor visits, prevention avoidance, continually delaying screening, either claiming colon cancer is rare or normalizing cancer risk, and falsely aligning regular bowel movements with good health which was directly associated with less screening.
Increasing rates of early onset colorectal cancer
The research may shed light on reasons for increasing rates of early-onset colorectal cancer. “Often younger people feel invincible and as Beverly Green, MD, MPH, pointed out in an editorial on defensive information processing, invincibility is a good example of self-exemption DIP,” Dr. Clarke said.
“I think what’s underlying these two pieces is a lack of awareness of the trajectory of colorectal cancer, but it’s also the future consequences of not taking part [in screening]. A person can have their colorectal cancer for about 10 years before they begin to feel any symptoms from it, and usually at that point, the disease has gone to an advanced stage, so it’s much more difficult to treat, and the person will have much poorer outcomes. If it’s detected at stage 1, the outcomes are far better,” Dr. Clarke said.
Doctors should react calmly to defensiveness and listen to the patient’s concerns. “Informing them of the aim of screening, i.e. to detect it when its precancerous or at the earliest possible stage, is very important. Letting them know they are taking responsibility for their own health and giving them the best chance of a healthy old age may be a good way of counteracting defensiveness,” he said.
Dr. Green noted that nonresponders claiming lack of immediacy could be swayed with the right approach. She has conducted similar research and subjects themselves suggested the use of marketing techniques “like what happens on Amazon. People remind you frequently the same thing when they get a clue that you have an interest in that behavior. Or, they tell you it’s on sale, and you might lose out from that big bargain if you don’t buy it now. There’s a deadline. I think a lot of the things we might do to nudge people are similar to what’s already happening in marketing,” said Dr. Green, who is a family physician and a researcher at the Kaiser Permanente Washington Health Research Institute.
Dr. Clarke and Dr. Green have no relevant financial disclosures.
An Irish study attempting to get at the root of why men and women delay colon cancer screening found that, despite an uptick in colon cancer cases among younger adults, screening isn’t a priority for some adults while others are under the impression that a healthy diet with regular bowel movements negates the need for regular screening.
The findings are based on a survey of over 2,000 adults who participated in a population-based fecal immunochemical test (FIT) screening program. The authors found that denying the immediacy of the need to be tested and self-exempting from screening because of a belief in a healthy lifestyle were key drivers for opting out of FIT screening.
“What we found was that people who didn’t take part [in the survey] responded much more defensively to the invitation,” said Nicholas Clarke, PhD, a researcher at Dublin City University, who served as the lead author of the study published in the journal Cancer.
The domain of denying immediacy, which covers decisions like putting off a test because of more pressing life events, was associated with a 47% reduction in screening probability. “That’s quite a high percentage. They’re not saying I won’t do it. They’re saying: ‘I’ll wait to get tested for colon cancer until my other health concerns are under control, or until there’s a better test,’ ” he said.
The other suppression category of self-exempting was associated with a 20% reduction in the odds of participation. “They’re saying: ‘I don’t need to be tested because I have enough vegetables in my diet or because I have regular bowel movements,’ ” Dr. Clarke said.
Despite the proven efficacy of screening, many individuals still resist screening. In previous research, Dr. Clarke found that men in Ireland were less likely than women to undergo screening.
FIT works by identifying small amounts of blood in the stool that could suggest the presence of a tumor or precancerous polyps. The test also looks for methylation and DNA mutations that are indicative of precancer polyps or tumors. A positive test calls for a follow-up procedure such as a colonoscopy, where precancerous polyps can be removed to prevent them from developing into tumors.
FIT has similar sensitivity to colonoscopy in detecting cancers (93% vs. 95%), but is less effective with respect to polyps (42% vs. 75%-93%). For average risk adults between 50-75 years old, the U.S Preventive Services Task Force recommends colonoscopy every 10 years; flexible sigmoidoscopy or CT colonography every 5 years, or flexible sigmoidoscopy every 10 years plus fecal immunochemical test (FIT) every year; FIT DNA test every 3 years; guaiac-based fecal occult blood test or FIT test annually.
Findings from the new study
In the new study, researchers contacted both 2,299 responders and nonresponders to FIT tests that had been mailed out as part of a Dublin colorectal cancer screening program between 2008 and 2012. Researchers employed the McQueen defensive information processing (DIP) measure, which includes four domains of defensive attitudes that include information avoidance, mental disengagement or denial, suppression through belief that one is immune, and arguing against the evidence.
In this study, 7,476 men and women in Dublin were invited to participate in a population‐based FIT screening program. In follow-up questionnaires sent to those who did or did not complete FIT, 53% of those who completed FIT screening answered the questionnaire, while 8% of those who did not complete the screening returned the questionnaire. Those who didn’t complete the FIT test had higher DIP scores suggesting more rates of opting out of receiving health information, avoiding doctor visits, prevention avoidance, continually delaying screening, either claiming colon cancer is rare or normalizing cancer risk, and falsely aligning regular bowel movements with good health which was directly associated with less screening.
Increasing rates of early onset colorectal cancer
The research may shed light on reasons for increasing rates of early-onset colorectal cancer. “Often younger people feel invincible and as Beverly Green, MD, MPH, pointed out in an editorial on defensive information processing, invincibility is a good example of self-exemption DIP,” Dr. Clarke said.
“I think what’s underlying these two pieces is a lack of awareness of the trajectory of colorectal cancer, but it’s also the future consequences of not taking part [in screening]. A person can have their colorectal cancer for about 10 years before they begin to feel any symptoms from it, and usually at that point, the disease has gone to an advanced stage, so it’s much more difficult to treat, and the person will have much poorer outcomes. If it’s detected at stage 1, the outcomes are far better,” Dr. Clarke said.
Doctors should react calmly to defensiveness and listen to the patient’s concerns. “Informing them of the aim of screening, i.e. to detect it when its precancerous or at the earliest possible stage, is very important. Letting them know they are taking responsibility for their own health and giving them the best chance of a healthy old age may be a good way of counteracting defensiveness,” he said.
Dr. Green noted that nonresponders claiming lack of immediacy could be swayed with the right approach. She has conducted similar research and subjects themselves suggested the use of marketing techniques “like what happens on Amazon. People remind you frequently the same thing when they get a clue that you have an interest in that behavior. Or, they tell you it’s on sale, and you might lose out from that big bargain if you don’t buy it now. There’s a deadline. I think a lot of the things we might do to nudge people are similar to what’s already happening in marketing,” said Dr. Green, who is a family physician and a researcher at the Kaiser Permanente Washington Health Research Institute.
Dr. Clarke and Dr. Green have no relevant financial disclosures.
An Irish study attempting to get at the root of why men and women delay colon cancer screening found that, despite an uptick in colon cancer cases among younger adults, screening isn’t a priority for some adults while others are under the impression that a healthy diet with regular bowel movements negates the need for regular screening.
The findings are based on a survey of over 2,000 adults who participated in a population-based fecal immunochemical test (FIT) screening program. The authors found that denying the immediacy of the need to be tested and self-exempting from screening because of a belief in a healthy lifestyle were key drivers for opting out of FIT screening.
“What we found was that people who didn’t take part [in the survey] responded much more defensively to the invitation,” said Nicholas Clarke, PhD, a researcher at Dublin City University, who served as the lead author of the study published in the journal Cancer.
The domain of denying immediacy, which covers decisions like putting off a test because of more pressing life events, was associated with a 47% reduction in screening probability. “That’s quite a high percentage. They’re not saying I won’t do it. They’re saying: ‘I’ll wait to get tested for colon cancer until my other health concerns are under control, or until there’s a better test,’ ” he said.
The other suppression category of self-exempting was associated with a 20% reduction in the odds of participation. “They’re saying: ‘I don’t need to be tested because I have enough vegetables in my diet or because I have regular bowel movements,’ ” Dr. Clarke said.
Despite the proven efficacy of screening, many individuals still resist screening. In previous research, Dr. Clarke found that men in Ireland were less likely than women to undergo screening.
FIT works by identifying small amounts of blood in the stool that could suggest the presence of a tumor or precancerous polyps. The test also looks for methylation and DNA mutations that are indicative of precancer polyps or tumors. A positive test calls for a follow-up procedure such as a colonoscopy, where precancerous polyps can be removed to prevent them from developing into tumors.
FIT has similar sensitivity to colonoscopy in detecting cancers (93% vs. 95%), but is less effective with respect to polyps (42% vs. 75%-93%). For average risk adults between 50-75 years old, the U.S Preventive Services Task Force recommends colonoscopy every 10 years; flexible sigmoidoscopy or CT colonography every 5 years, or flexible sigmoidoscopy every 10 years plus fecal immunochemical test (FIT) every year; FIT DNA test every 3 years; guaiac-based fecal occult blood test or FIT test annually.
Findings from the new study
In the new study, researchers contacted both 2,299 responders and nonresponders to FIT tests that had been mailed out as part of a Dublin colorectal cancer screening program between 2008 and 2012. Researchers employed the McQueen defensive information processing (DIP) measure, which includes four domains of defensive attitudes that include information avoidance, mental disengagement or denial, suppression through belief that one is immune, and arguing against the evidence.
In this study, 7,476 men and women in Dublin were invited to participate in a population‐based FIT screening program. In follow-up questionnaires sent to those who did or did not complete FIT, 53% of those who completed FIT screening answered the questionnaire, while 8% of those who did not complete the screening returned the questionnaire. Those who didn’t complete the FIT test had higher DIP scores suggesting more rates of opting out of receiving health information, avoiding doctor visits, prevention avoidance, continually delaying screening, either claiming colon cancer is rare or normalizing cancer risk, and falsely aligning regular bowel movements with good health which was directly associated with less screening.
Increasing rates of early onset colorectal cancer
The research may shed light on reasons for increasing rates of early-onset colorectal cancer. “Often younger people feel invincible and as Beverly Green, MD, MPH, pointed out in an editorial on defensive information processing, invincibility is a good example of self-exemption DIP,” Dr. Clarke said.
“I think what’s underlying these two pieces is a lack of awareness of the trajectory of colorectal cancer, but it’s also the future consequences of not taking part [in screening]. A person can have their colorectal cancer for about 10 years before they begin to feel any symptoms from it, and usually at that point, the disease has gone to an advanced stage, so it’s much more difficult to treat, and the person will have much poorer outcomes. If it’s detected at stage 1, the outcomes are far better,” Dr. Clarke said.
Doctors should react calmly to defensiveness and listen to the patient’s concerns. “Informing them of the aim of screening, i.e. to detect it when its precancerous or at the earliest possible stage, is very important. Letting them know they are taking responsibility for their own health and giving them the best chance of a healthy old age may be a good way of counteracting defensiveness,” he said.
Dr. Green noted that nonresponders claiming lack of immediacy could be swayed with the right approach. She has conducted similar research and subjects themselves suggested the use of marketing techniques “like what happens on Amazon. People remind you frequently the same thing when they get a clue that you have an interest in that behavior. Or, they tell you it’s on sale, and you might lose out from that big bargain if you don’t buy it now. There’s a deadline. I think a lot of the things we might do to nudge people are similar to what’s already happening in marketing,” said Dr. Green, who is a family physician and a researcher at the Kaiser Permanente Washington Health Research Institute.
Dr. Clarke and Dr. Green have no relevant financial disclosures.
FROM CANCER
In utero exposure to asthma medication not tied to risks of neurodevelopmental disorders
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Phase 3 results: Ponatinib bests imatinib for Ph+ALL
The agents were evaluated in the randomized, open-label, phase 3 PhALLCON study, the first head-to-head comparison of ponatinib and imatinib in combination with reduced-intensity chemotherapy in the Ph+ALL population.
Overall, patients in the ponatinib arm experienced a significantly higher minimal residual disease (MRD)–negative complete response rate as well as deeper and more durable responses compared with those in the imatinib arm, the investigators reported.
The findings were presented during an American Society of Clinical Oncology virtual plenary session.
In adults with ALL, Ph+ disease is the most frequent genetic subtype, accounting for about one third of cases. The current standard of care for newly diagnosed Ph+ALL, also known as BCR-ABL-1–positive ALL, is BCR-ABL1 TKIs in combination with chemotherapy or steroids. However, when treated with first- or second-generation TKIs, patients eventually progress due to the emergence of treatment resistance.
Before the advent of TKI therapies, Ph+ALL had a very poor prognosis, but the development of imatinib in 2001 was transformative, said Marlise R. Luskin, MD, a senior physician at Dana-Farber Cancer Institute, Boston, in the ASCO plenary session, exploring the state of the science.
Added to “backbone” chemotherapy regimens, imatinib improved complete response rates, increased eligibility for stem cell transplantation, and improved overall survival. Second-generation TKIs, including dasatinib and nilotinib further improved outcomes, said Dr. Luskin, also assistant professor at Harvard Medical School, Boston.
More recently, ponatinib has emerged as a promising treatment given its unique action against the ABLA1 T315I KD mutation present in about 75% of cases that relapse as well as the findings of improved MRD-negative complete response rates and event-free survival in retrospective studies, Dr. Luskin said.
The PhALLCON study was designed to further investigate promising results seen in retrospective studies of ponatinib.
To assess ponatinib versus imatinib, patients were enrolled and randomized two to one to receive either a 30-mg once-daily starting dose of ponatinib or a once-daily 600 mg dose of imatinib plus reduced-intensity chemotherapy. After cycle 20, patients received single agent ponatinib or imatinib until disease progression or unacceptable toxicity.
Of the 245 enrolled, 78 remained on treatment at the August 2022 data cutoff, including 42% of those in the ponatinib arm and 12% in the imatinib arm. The most common reasons for discontinuation included hematopoietic stem cell transplantation (31% for ponatinib and 37% for imatinib), adverse events (12% in both arms), and lack of efficacy (7% and 26%, respectively).
At median follow-up of 20 months among 164 patients in the ponatinib arm and 18 months among 81 patients in the imatinib arm, the MRD-negative complete response rates were 34.4% and 16.7%, respectively, said first author Elias J. Jabbour, MD, a professor of medicine at the University of Texas MD Anderson Cancer Center, Houston.
A trend toward improved event-free survival was also observed in the ponatinib arm, but the data were not mature at the time of the analysis, Dr. Jabbour noted.
The two treatments showed comparable safety. Treatment-emergent adverse event rates of any grade and of grade 3 or higher were similar in the two study arms. Arterial occlusive events were infrequent and were also similar between the arms.
“Taken together, for this patient population, the efficacy and safety results demonstrate a favorable risk-benefit assessment for ponatinib, which should be considered a standard of care for frontline therapy in patents with newly diagnosed Ph+ALL,” Dr. Jabbour said.
Although the PhALLCON findings are encouraging, invited discussant Anjali S. Advani, MD, of the Cleveland Clinic, noted some study “pitfalls and caveats,” including the generally younger age and low incidence of cardiovascular risk factors in the study population, which raises questions about the ability to extrapolate the findings to “the larger population, which may be older and have more comorbidities.”
Dr. Advani also said that the ponatinib versus imatinib comparison is a reasonable one, but that most clinicians are now using dasatinib, so “it would have been nice to have this comparison.”
Additionally, “the landscape is now changing with the use of blinatumomab plus TKIs – either dasatinib or ponatinib – in the up-front setting.”
“There is data now from various groups ... showing excellent results, although longer follow-up is needed on all of these,” she said.
One such study is the GIMEMA ALL2820 trial looking at ponatinib plus blinatumomab versus imatinib plus chemotherapy, said Nicolas Boissel, MD, PhD, of Hôpital Saint-Louis in Paris, an invited discussant who addressed the European perspective on the PhALLCON results.
“It is expected that access to ponatinib will be delayed in Europe, compared with the U.S., so meanwhile, clinical trials remain a good option to give access to ponatinib frontline,” he said.
Going forward, Dr. Boissel said it will be important to determine the role of second-generation TKIs in patients who are ineligible to receive ponatinib, the treatment duration needed to reduce long-term risk of relapse, and the potential for eliminating the need for postremission chemotherapy and stem cell transplantation in certain patients.
Dr. Advani added that when evaluating and comparing treatments, it will be important to look at genomic alterations and BCR-ABL mutation status, age and comorbidities, and patterns of disease relapse, including relapse sites and genomics. Longer follow-up results for event-free survival and overall survival are also needed.
“I think, particularly in younger patients with relatively few or no cardiovascular comorbidities, [ponatinib plus reduced-intensity chemotherapy] represents a really exciting option,” Dr. Advani said. “What’s difficult is that the landscape is changing quickly in this field, and so is the standard of care. I think what we struggle with is whether we should be using antibody-based therapies plus TKIs or look at an approach such as this, and further studies are going to be needed to answer that question.”
Dr. Jabbour disclosed ties with Pfizer, Takeda, Amgen, AbbVie, Bristol-Myers Squibb, Incyte, Adaptive Biotechnologies, Astellas Pharma, Genentech, and Ascentage Pharma. Dr. Luskin reported relationships with Pfizer, Novartis, and Abbvie. Dr. Advani disclosed ties with Novartis, Glycomimetics, Kite Pharma, Seattle Genetics, Amgen, Beam Therapeutics, Mkarta, Taiho Oncology, Jazz Pharmaceuticals, Pfizer, and Kura Oncology. Dr. Boissel reported relationships with Amgen, ARIAD/Incyte, Novartis, SERVIER, and Astellas Pharma.
A version of this article originally appeared on Medscape.com.
The agents were evaluated in the randomized, open-label, phase 3 PhALLCON study, the first head-to-head comparison of ponatinib and imatinib in combination with reduced-intensity chemotherapy in the Ph+ALL population.
Overall, patients in the ponatinib arm experienced a significantly higher minimal residual disease (MRD)–negative complete response rate as well as deeper and more durable responses compared with those in the imatinib arm, the investigators reported.
The findings were presented during an American Society of Clinical Oncology virtual plenary session.
In adults with ALL, Ph+ disease is the most frequent genetic subtype, accounting for about one third of cases. The current standard of care for newly diagnosed Ph+ALL, also known as BCR-ABL-1–positive ALL, is BCR-ABL1 TKIs in combination with chemotherapy or steroids. However, when treated with first- or second-generation TKIs, patients eventually progress due to the emergence of treatment resistance.
Before the advent of TKI therapies, Ph+ALL had a very poor prognosis, but the development of imatinib in 2001 was transformative, said Marlise R. Luskin, MD, a senior physician at Dana-Farber Cancer Institute, Boston, in the ASCO plenary session, exploring the state of the science.
Added to “backbone” chemotherapy regimens, imatinib improved complete response rates, increased eligibility for stem cell transplantation, and improved overall survival. Second-generation TKIs, including dasatinib and nilotinib further improved outcomes, said Dr. Luskin, also assistant professor at Harvard Medical School, Boston.
More recently, ponatinib has emerged as a promising treatment given its unique action against the ABLA1 T315I KD mutation present in about 75% of cases that relapse as well as the findings of improved MRD-negative complete response rates and event-free survival in retrospective studies, Dr. Luskin said.
The PhALLCON study was designed to further investigate promising results seen in retrospective studies of ponatinib.
To assess ponatinib versus imatinib, patients were enrolled and randomized two to one to receive either a 30-mg once-daily starting dose of ponatinib or a once-daily 600 mg dose of imatinib plus reduced-intensity chemotherapy. After cycle 20, patients received single agent ponatinib or imatinib until disease progression or unacceptable toxicity.
Of the 245 enrolled, 78 remained on treatment at the August 2022 data cutoff, including 42% of those in the ponatinib arm and 12% in the imatinib arm. The most common reasons for discontinuation included hematopoietic stem cell transplantation (31% for ponatinib and 37% for imatinib), adverse events (12% in both arms), and lack of efficacy (7% and 26%, respectively).
At median follow-up of 20 months among 164 patients in the ponatinib arm and 18 months among 81 patients in the imatinib arm, the MRD-negative complete response rates were 34.4% and 16.7%, respectively, said first author Elias J. Jabbour, MD, a professor of medicine at the University of Texas MD Anderson Cancer Center, Houston.
A trend toward improved event-free survival was also observed in the ponatinib arm, but the data were not mature at the time of the analysis, Dr. Jabbour noted.
The two treatments showed comparable safety. Treatment-emergent adverse event rates of any grade and of grade 3 or higher were similar in the two study arms. Arterial occlusive events were infrequent and were also similar between the arms.
“Taken together, for this patient population, the efficacy and safety results demonstrate a favorable risk-benefit assessment for ponatinib, which should be considered a standard of care for frontline therapy in patents with newly diagnosed Ph+ALL,” Dr. Jabbour said.
Although the PhALLCON findings are encouraging, invited discussant Anjali S. Advani, MD, of the Cleveland Clinic, noted some study “pitfalls and caveats,” including the generally younger age and low incidence of cardiovascular risk factors in the study population, which raises questions about the ability to extrapolate the findings to “the larger population, which may be older and have more comorbidities.”
Dr. Advani also said that the ponatinib versus imatinib comparison is a reasonable one, but that most clinicians are now using dasatinib, so “it would have been nice to have this comparison.”
Additionally, “the landscape is now changing with the use of blinatumomab plus TKIs – either dasatinib or ponatinib – in the up-front setting.”
“There is data now from various groups ... showing excellent results, although longer follow-up is needed on all of these,” she said.
One such study is the GIMEMA ALL2820 trial looking at ponatinib plus blinatumomab versus imatinib plus chemotherapy, said Nicolas Boissel, MD, PhD, of Hôpital Saint-Louis in Paris, an invited discussant who addressed the European perspective on the PhALLCON results.
“It is expected that access to ponatinib will be delayed in Europe, compared with the U.S., so meanwhile, clinical trials remain a good option to give access to ponatinib frontline,” he said.
Going forward, Dr. Boissel said it will be important to determine the role of second-generation TKIs in patients who are ineligible to receive ponatinib, the treatment duration needed to reduce long-term risk of relapse, and the potential for eliminating the need for postremission chemotherapy and stem cell transplantation in certain patients.
Dr. Advani added that when evaluating and comparing treatments, it will be important to look at genomic alterations and BCR-ABL mutation status, age and comorbidities, and patterns of disease relapse, including relapse sites and genomics. Longer follow-up results for event-free survival and overall survival are also needed.
“I think, particularly in younger patients with relatively few or no cardiovascular comorbidities, [ponatinib plus reduced-intensity chemotherapy] represents a really exciting option,” Dr. Advani said. “What’s difficult is that the landscape is changing quickly in this field, and so is the standard of care. I think what we struggle with is whether we should be using antibody-based therapies plus TKIs or look at an approach such as this, and further studies are going to be needed to answer that question.”
Dr. Jabbour disclosed ties with Pfizer, Takeda, Amgen, AbbVie, Bristol-Myers Squibb, Incyte, Adaptive Biotechnologies, Astellas Pharma, Genentech, and Ascentage Pharma. Dr. Luskin reported relationships with Pfizer, Novartis, and Abbvie. Dr. Advani disclosed ties with Novartis, Glycomimetics, Kite Pharma, Seattle Genetics, Amgen, Beam Therapeutics, Mkarta, Taiho Oncology, Jazz Pharmaceuticals, Pfizer, and Kura Oncology. Dr. Boissel reported relationships with Amgen, ARIAD/Incyte, Novartis, SERVIER, and Astellas Pharma.
A version of this article originally appeared on Medscape.com.
The agents were evaluated in the randomized, open-label, phase 3 PhALLCON study, the first head-to-head comparison of ponatinib and imatinib in combination with reduced-intensity chemotherapy in the Ph+ALL population.
Overall, patients in the ponatinib arm experienced a significantly higher minimal residual disease (MRD)–negative complete response rate as well as deeper and more durable responses compared with those in the imatinib arm, the investigators reported.
The findings were presented during an American Society of Clinical Oncology virtual plenary session.
In adults with ALL, Ph+ disease is the most frequent genetic subtype, accounting for about one third of cases. The current standard of care for newly diagnosed Ph+ALL, also known as BCR-ABL-1–positive ALL, is BCR-ABL1 TKIs in combination with chemotherapy or steroids. However, when treated with first- or second-generation TKIs, patients eventually progress due to the emergence of treatment resistance.
Before the advent of TKI therapies, Ph+ALL had a very poor prognosis, but the development of imatinib in 2001 was transformative, said Marlise R. Luskin, MD, a senior physician at Dana-Farber Cancer Institute, Boston, in the ASCO plenary session, exploring the state of the science.
Added to “backbone” chemotherapy regimens, imatinib improved complete response rates, increased eligibility for stem cell transplantation, and improved overall survival. Second-generation TKIs, including dasatinib and nilotinib further improved outcomes, said Dr. Luskin, also assistant professor at Harvard Medical School, Boston.
More recently, ponatinib has emerged as a promising treatment given its unique action against the ABLA1 T315I KD mutation present in about 75% of cases that relapse as well as the findings of improved MRD-negative complete response rates and event-free survival in retrospective studies, Dr. Luskin said.
The PhALLCON study was designed to further investigate promising results seen in retrospective studies of ponatinib.
To assess ponatinib versus imatinib, patients were enrolled and randomized two to one to receive either a 30-mg once-daily starting dose of ponatinib or a once-daily 600 mg dose of imatinib plus reduced-intensity chemotherapy. After cycle 20, patients received single agent ponatinib or imatinib until disease progression or unacceptable toxicity.
Of the 245 enrolled, 78 remained on treatment at the August 2022 data cutoff, including 42% of those in the ponatinib arm and 12% in the imatinib arm. The most common reasons for discontinuation included hematopoietic stem cell transplantation (31% for ponatinib and 37% for imatinib), adverse events (12% in both arms), and lack of efficacy (7% and 26%, respectively).
At median follow-up of 20 months among 164 patients in the ponatinib arm and 18 months among 81 patients in the imatinib arm, the MRD-negative complete response rates were 34.4% and 16.7%, respectively, said first author Elias J. Jabbour, MD, a professor of medicine at the University of Texas MD Anderson Cancer Center, Houston.
A trend toward improved event-free survival was also observed in the ponatinib arm, but the data were not mature at the time of the analysis, Dr. Jabbour noted.
The two treatments showed comparable safety. Treatment-emergent adverse event rates of any grade and of grade 3 or higher were similar in the two study arms. Arterial occlusive events were infrequent and were also similar between the arms.
“Taken together, for this patient population, the efficacy and safety results demonstrate a favorable risk-benefit assessment for ponatinib, which should be considered a standard of care for frontline therapy in patents with newly diagnosed Ph+ALL,” Dr. Jabbour said.
Although the PhALLCON findings are encouraging, invited discussant Anjali S. Advani, MD, of the Cleveland Clinic, noted some study “pitfalls and caveats,” including the generally younger age and low incidence of cardiovascular risk factors in the study population, which raises questions about the ability to extrapolate the findings to “the larger population, which may be older and have more comorbidities.”
Dr. Advani also said that the ponatinib versus imatinib comparison is a reasonable one, but that most clinicians are now using dasatinib, so “it would have been nice to have this comparison.”
Additionally, “the landscape is now changing with the use of blinatumomab plus TKIs – either dasatinib or ponatinib – in the up-front setting.”
“There is data now from various groups ... showing excellent results, although longer follow-up is needed on all of these,” she said.
One such study is the GIMEMA ALL2820 trial looking at ponatinib plus blinatumomab versus imatinib plus chemotherapy, said Nicolas Boissel, MD, PhD, of Hôpital Saint-Louis in Paris, an invited discussant who addressed the European perspective on the PhALLCON results.
“It is expected that access to ponatinib will be delayed in Europe, compared with the U.S., so meanwhile, clinical trials remain a good option to give access to ponatinib frontline,” he said.
Going forward, Dr. Boissel said it will be important to determine the role of second-generation TKIs in patients who are ineligible to receive ponatinib, the treatment duration needed to reduce long-term risk of relapse, and the potential for eliminating the need for postremission chemotherapy and stem cell transplantation in certain patients.
Dr. Advani added that when evaluating and comparing treatments, it will be important to look at genomic alterations and BCR-ABL mutation status, age and comorbidities, and patterns of disease relapse, including relapse sites and genomics. Longer follow-up results for event-free survival and overall survival are also needed.
“I think, particularly in younger patients with relatively few or no cardiovascular comorbidities, [ponatinib plus reduced-intensity chemotherapy] represents a really exciting option,” Dr. Advani said. “What’s difficult is that the landscape is changing quickly in this field, and so is the standard of care. I think what we struggle with is whether we should be using antibody-based therapies plus TKIs or look at an approach such as this, and further studies are going to be needed to answer that question.”
Dr. Jabbour disclosed ties with Pfizer, Takeda, Amgen, AbbVie, Bristol-Myers Squibb, Incyte, Adaptive Biotechnologies, Astellas Pharma, Genentech, and Ascentage Pharma. Dr. Luskin reported relationships with Pfizer, Novartis, and Abbvie. Dr. Advani disclosed ties with Novartis, Glycomimetics, Kite Pharma, Seattle Genetics, Amgen, Beam Therapeutics, Mkarta, Taiho Oncology, Jazz Pharmaceuticals, Pfizer, and Kura Oncology. Dr. Boissel reported relationships with Amgen, ARIAD/Incyte, Novartis, SERVIER, and Astellas Pharma.
A version of this article originally appeared on Medscape.com.
Cognitive remediation training reduces aggression in schizophrenia
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
Fixed-dose combo pill for PAH promises accelerated benefit: A DUE
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
AT ACC 2023
White male presents with pruritic, scaly, erythematous patches on his feet and left hand
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Ob.gyn. loses PhD after committee finds he made up research
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.