User login
Troubling trend as both diabetes types rise among U.S. youth
The incidence of type 1 and type 2 diabetes continues to rise among children and adolescents in the United States, new data from the SEARCH for Diabetes in Youth study show.
The SEARCH data demonstrate an increase in the youth population aged 0-19 diagnosed with type 1 or type 2 diabetes in five representative U.S. centers. Between 2002 and 2018, the annual incidence rose by about 2% per year for type 1 diabetes and 5% per year for type 2 diabetes. The rates of increase for both types were greater among non-White than White youth.
These increases “will result in an expanding population of young adults at risk of developing early complications of diabetes whose health care needs will exceed those of their peers,” write Lynne E. Wagenknecht, DrPH, of Wake Forest University School of Medicine, Winston-Salem, N.C., and colleagues in their article, recently published in The Lancet Diabetes & Endocrinology.
In an accompanying editorial, Jonathan E. Shaw, MD, and Dianna J. Magliano, PhD, both at the Baker Heart and Diabetes Institute, Melbourne, write that one of the most “concerning findings” was a 7%-9% annual increase in the incidence of type 2 diabetes among Hispanic, Asian, and Pacific Islander populations.
“This is a health care crisis in the making. ...Youth and young-adult-onset type 2 diabetes are growing problems leading to poor outcomes and to widening social inequality, adversely affecting a population that might already be disadvantaged. Better information about its natural history, prevention, and management is urgently needed,” they write.
Upward trends in both diabetes types
Overall, 18,169 children and adolescents with type 1 diabetes and 5,293 with type 2 diabetes were identified over the 17-year study period in SEARCH. After adjustment for age, sex, and race/ethnicity, there was a significant increase in type 1 diabetes incidence from 19.5 cases/100,000 population in 2002-2003 to 22.2/100,000 in 2017-2018, a 2.02% annual increase.
The upward trend was even greater for type 2 diabetes, from 9.0/100,000 in 2002-2003 to 17.9/100,000 in 2017-2018, a 5.31% annual increase.
The annual rate of increase in type 1 diabetes was highest among Asian/Pacific Islander youth (4.84%), followed by Hispanic (4.14%) and Black youth (2.93%): All significantly rose over the 17 years.
For type 2 diabetes, significant annual rates of increase were also highest for Asian/Pacific Islanders (8.92%), followed by Hispanic (7.17%) and Black youth (5.99%).
Among youth aged 15-19 years, the overall incidence of type 2 diabetes exceeded that of type 1 diabetes (19.7 vs. 14.6/100,000).
The incidence of type 2 diabetes may be rising because of increased rates of obesity, as well as increased screening of at-risk youth, the authors say.
And, the editorialists note, obesity is also a risk factor for type 1 diabetes.
Peak incidence of type 1 diabetes occurred at age 10 years, while for type 2 diabetes, the peak was at 16 years. There were also seasonal peaks, occurring in January for type 1 diabetes and in August for type 2 diabetes. Those seasonal patterns have been previously reported; they are possibly because of increased viral infections and decreased sun exposure for the former, and increased physical exams in preparation for school in the latter, the authors speculate.
Dr. Shaw and Dr. Magliano note that the reduced incidence after age 16 years “might simply reflect a failure to diagnose,” suggesting that there will likely be an upturn in incidence in the subsequent decade.
The editorialists also point out: “Not only does the long duration of diabetes that youth-onset leads to cause a large burden of fatal and nonfatal complications, but it magnifies intergenerational effects.”
“When type 2 diabetes is already present before pregnancy, birth outcomes are worse, and the long-term metabolic health of the offspring is adversely affected. This does not bode well for the epidemic of diabetes and its complications.”
The study was funded by the Centers for Disease Control and Prevention and National Institutes of Health. The authors and Dr. Magliano have reported no relevant financial relationships. Dr. Shaw has reported receiving honoraria for lectures and for advisory boards and grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Eli Lilly, Sanofi, Roche, Mylan, and Zuellig Pharma.
A version of this article originally appeared on Medscape.com.
The incidence of type 1 and type 2 diabetes continues to rise among children and adolescents in the United States, new data from the SEARCH for Diabetes in Youth study show.
The SEARCH data demonstrate an increase in the youth population aged 0-19 diagnosed with type 1 or type 2 diabetes in five representative U.S. centers. Between 2002 and 2018, the annual incidence rose by about 2% per year for type 1 diabetes and 5% per year for type 2 diabetes. The rates of increase for both types were greater among non-White than White youth.
These increases “will result in an expanding population of young adults at risk of developing early complications of diabetes whose health care needs will exceed those of their peers,” write Lynne E. Wagenknecht, DrPH, of Wake Forest University School of Medicine, Winston-Salem, N.C., and colleagues in their article, recently published in The Lancet Diabetes & Endocrinology.
In an accompanying editorial, Jonathan E. Shaw, MD, and Dianna J. Magliano, PhD, both at the Baker Heart and Diabetes Institute, Melbourne, write that one of the most “concerning findings” was a 7%-9% annual increase in the incidence of type 2 diabetes among Hispanic, Asian, and Pacific Islander populations.
“This is a health care crisis in the making. ...Youth and young-adult-onset type 2 diabetes are growing problems leading to poor outcomes and to widening social inequality, adversely affecting a population that might already be disadvantaged. Better information about its natural history, prevention, and management is urgently needed,” they write.
Upward trends in both diabetes types
Overall, 18,169 children and adolescents with type 1 diabetes and 5,293 with type 2 diabetes were identified over the 17-year study period in SEARCH. After adjustment for age, sex, and race/ethnicity, there was a significant increase in type 1 diabetes incidence from 19.5 cases/100,000 population in 2002-2003 to 22.2/100,000 in 2017-2018, a 2.02% annual increase.
The upward trend was even greater for type 2 diabetes, from 9.0/100,000 in 2002-2003 to 17.9/100,000 in 2017-2018, a 5.31% annual increase.
The annual rate of increase in type 1 diabetes was highest among Asian/Pacific Islander youth (4.84%), followed by Hispanic (4.14%) and Black youth (2.93%): All significantly rose over the 17 years.
For type 2 diabetes, significant annual rates of increase were also highest for Asian/Pacific Islanders (8.92%), followed by Hispanic (7.17%) and Black youth (5.99%).
Among youth aged 15-19 years, the overall incidence of type 2 diabetes exceeded that of type 1 diabetes (19.7 vs. 14.6/100,000).
The incidence of type 2 diabetes may be rising because of increased rates of obesity, as well as increased screening of at-risk youth, the authors say.
And, the editorialists note, obesity is also a risk factor for type 1 diabetes.
Peak incidence of type 1 diabetes occurred at age 10 years, while for type 2 diabetes, the peak was at 16 years. There were also seasonal peaks, occurring in January for type 1 diabetes and in August for type 2 diabetes. Those seasonal patterns have been previously reported; they are possibly because of increased viral infections and decreased sun exposure for the former, and increased physical exams in preparation for school in the latter, the authors speculate.
Dr. Shaw and Dr. Magliano note that the reduced incidence after age 16 years “might simply reflect a failure to diagnose,” suggesting that there will likely be an upturn in incidence in the subsequent decade.
The editorialists also point out: “Not only does the long duration of diabetes that youth-onset leads to cause a large burden of fatal and nonfatal complications, but it magnifies intergenerational effects.”
“When type 2 diabetes is already present before pregnancy, birth outcomes are worse, and the long-term metabolic health of the offspring is adversely affected. This does not bode well for the epidemic of diabetes and its complications.”
The study was funded by the Centers for Disease Control and Prevention and National Institutes of Health. The authors and Dr. Magliano have reported no relevant financial relationships. Dr. Shaw has reported receiving honoraria for lectures and for advisory boards and grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Eli Lilly, Sanofi, Roche, Mylan, and Zuellig Pharma.
A version of this article originally appeared on Medscape.com.
The incidence of type 1 and type 2 diabetes continues to rise among children and adolescents in the United States, new data from the SEARCH for Diabetes in Youth study show.
The SEARCH data demonstrate an increase in the youth population aged 0-19 diagnosed with type 1 or type 2 diabetes in five representative U.S. centers. Between 2002 and 2018, the annual incidence rose by about 2% per year for type 1 diabetes and 5% per year for type 2 diabetes. The rates of increase for both types were greater among non-White than White youth.
These increases “will result in an expanding population of young adults at risk of developing early complications of diabetes whose health care needs will exceed those of their peers,” write Lynne E. Wagenknecht, DrPH, of Wake Forest University School of Medicine, Winston-Salem, N.C., and colleagues in their article, recently published in The Lancet Diabetes & Endocrinology.
In an accompanying editorial, Jonathan E. Shaw, MD, and Dianna J. Magliano, PhD, both at the Baker Heart and Diabetes Institute, Melbourne, write that one of the most “concerning findings” was a 7%-9% annual increase in the incidence of type 2 diabetes among Hispanic, Asian, and Pacific Islander populations.
“This is a health care crisis in the making. ...Youth and young-adult-onset type 2 diabetes are growing problems leading to poor outcomes and to widening social inequality, adversely affecting a population that might already be disadvantaged. Better information about its natural history, prevention, and management is urgently needed,” they write.
Upward trends in both diabetes types
Overall, 18,169 children and adolescents with type 1 diabetes and 5,293 with type 2 diabetes were identified over the 17-year study period in SEARCH. After adjustment for age, sex, and race/ethnicity, there was a significant increase in type 1 diabetes incidence from 19.5 cases/100,000 population in 2002-2003 to 22.2/100,000 in 2017-2018, a 2.02% annual increase.
The upward trend was even greater for type 2 diabetes, from 9.0/100,000 in 2002-2003 to 17.9/100,000 in 2017-2018, a 5.31% annual increase.
The annual rate of increase in type 1 diabetes was highest among Asian/Pacific Islander youth (4.84%), followed by Hispanic (4.14%) and Black youth (2.93%): All significantly rose over the 17 years.
For type 2 diabetes, significant annual rates of increase were also highest for Asian/Pacific Islanders (8.92%), followed by Hispanic (7.17%) and Black youth (5.99%).
Among youth aged 15-19 years, the overall incidence of type 2 diabetes exceeded that of type 1 diabetes (19.7 vs. 14.6/100,000).
The incidence of type 2 diabetes may be rising because of increased rates of obesity, as well as increased screening of at-risk youth, the authors say.
And, the editorialists note, obesity is also a risk factor for type 1 diabetes.
Peak incidence of type 1 diabetes occurred at age 10 years, while for type 2 diabetes, the peak was at 16 years. There were also seasonal peaks, occurring in January for type 1 diabetes and in August for type 2 diabetes. Those seasonal patterns have been previously reported; they are possibly because of increased viral infections and decreased sun exposure for the former, and increased physical exams in preparation for school in the latter, the authors speculate.
Dr. Shaw and Dr. Magliano note that the reduced incidence after age 16 years “might simply reflect a failure to diagnose,” suggesting that there will likely be an upturn in incidence in the subsequent decade.
The editorialists also point out: “Not only does the long duration of diabetes that youth-onset leads to cause a large burden of fatal and nonfatal complications, but it magnifies intergenerational effects.”
“When type 2 diabetes is already present before pregnancy, birth outcomes are worse, and the long-term metabolic health of the offspring is adversely affected. This does not bode well for the epidemic of diabetes and its complications.”
The study was funded by the Centers for Disease Control and Prevention and National Institutes of Health. The authors and Dr. Magliano have reported no relevant financial relationships. Dr. Shaw has reported receiving honoraria for lectures and for advisory boards and grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Eli Lilly, Sanofi, Roche, Mylan, and Zuellig Pharma.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
An earlier hep B biomarker for clinical outcomes?
Low serum levels of the hepatitis B core-related antigen (HBcrAg) could be an early biomarker of a functional cure of a hepatitis B infection, according to new findings from a retrospective study.
A drop in HBcrAg predicted the seroclearance of hepatitis B surface antigen (HBsAg), the widely accepted measure of optimal liver-related outcomes in patient care and clinical trials, long before HBsAg levels actually fell.
“In a large retrospective cohort study of chronic hepatitis B patients, we found lower levels of HBcrAg were associated with higher probability of clearing HBsAg,” wrote Tai-Chung Tseng and coauthors at National Taiwan University Hospital in Taipei. “Reduction of HBcrAg developed 10 years before decline of HBsAg in patients with high HBsAg levels at baseline.”
Nearly 300 million people worldwide are estimated to be positive for the HBsAg antigen, a marker of active hepatitis B virus (HBV) infection. Chronic HBV puts individuals at greater risk of cirrhosis, hepatocellular carcinoma (HCC), and other liver complications.
Seroclearance of HBsAg is generally regarded as signaling a functional cure, because it is associated with low viral activity and good clinical outcomes. Patients with low HBsAg levels may transition to complete clearance, while those with levels of 1,000 IU/mL or higher rarely achieve clearance either spontaneously or through treatment.
As with HBsAg, higher serum levels of HBcrAg have been linked to a raised risk of adverse events, including increased viral activity and heightened risk of developing hepatitis B e antigen-negative hepatitis, cirrhosis, and HCC. Lower HBcrAg levels are associated with a greater likelihood of HBsAg seroclearance in chronic hepatitis B patients who discontinued antiviral therapy.
In a study published in Gastroenterology, researchers conducted a retrospective Taiwanese cohort study of 2,614 untreated patients with hepatitis B who underwent long-term follow-up at National Taiwan University Hospital. The median age was 38.2 years, and 60.6% were men. At baseline, 14.8% had HBsAg levels of less than 100 IU/mL, and 47.7% had HBcrAg levels less than 10,000 IU/mL. Most (77.5%) were infected with HBV genotype B. From stored serum samples, the researchers quantified levels of HBV DNA, HBsAg, and HBcrAg and evaluated the relationships with spontaneous HBsAg seroclearance.
Over an average follow-up of about 12 years, 465 patients cleared HBsAg, an incidence of 1.43% per year. Researchers stratified patients by levels of viral markers. Compared to those with the highest HBcrAg levels (> 100,000 IU/mL), lower levels of HBcrAg were associated with greater likelihood of HBsAg clearance.
Specifically, intermediate levels (10,000-99,999 IU/mL) were associated with nearly double the chance of HBsAg clearance (hazard ratio [HR], 1.95; 95% confidence interval [CI], 1.44-2.65), and the lowest levels (< 10,000 IU/mL) were associated with just over triple the chance of clearance (HR, 3.15; 95% CI, 2.45-4.05). These associations held up with multivariable analyses, and HBV DNA levels were not significantly associated with HBsAg clearance.
“Not surprisingly, HBsAg levels still serve as a better predictor than the other two biomarkers,” the authors wrote. “Notably, the HBsAg levels are more like a short-term predictor” (within 5 years).
For patients with higher HBsAg levels (> 1,000 IU/mL), it took a median of 16 years to achieve HBsAg clearance. A subanalysis of the 1,539 patients with HbsAg levels > 1,000 IU/mL found that only HBcrAg levels below 10,000 IU/mL predicted HBsAg seroclearance versus 100,000 U/mL or higher (adjusted HR, 1.95; 95% CI, 1.16-3.27).
HBsAg levels began to decline later, often between 5 and 9 years before HBsAg seroclearance occurs. However, HBcrAg levels became undetectable 10-14 years before HBsAg seroclearance. Among patients achieving undetectable levels of HBcrAg, the annual HBsAg seroclearance rate was higher in the second decade of follow-up than in the first decade (3.75% versus 0.97%).
HBcrAg levels reflect the transcriptional activity of covalently closed circular DNA (cccDNA), the authors noted, while HBsAg can come from cccDNA and HBV-DNA integrated into the host genome. Several novel hepatitis B therapies in development target cccDNA transcription, but it isn’t known if the strategy will result in HBsAg clearance.
In the discussion section, the authors speculated about the possible pathology and treatment implications for several chronic hepatitis B scenarios. For example, the finding that HBcrAg clearance usually precedes HBsAg clearance suggests that reduction of cccDNA transcription is a requirement for curing hepatitis B, the authors speculate, but it also suggests that add-on treatment may need to target HBsAg transcribed from the integrated viral genome for a functional cure.
The researchers noted several study limitations, including that the cohort included only Asians largely with HBV genotypes B or C and that “further validation from Caucasian patients infected with genotypes types A or D is mandatory.”
Tai-Chung Tseng disclosed financial conflicts with Fujirebio, Bristol-Myers Squibb, and Gilead Sciences. The remaining authors had no conflicts of interest. The study received grant support from several institutions, including National Taiwan University Hospital.
Current hepatitis B virus (HBV) therapies do not eliminate the covalently closed circular DNA (cccDNA), and a single cccDNA can cause a infection. Hepatitis B core-related antigen (HBcrAg) has shown positive correlation with serum and hepatic HBV-DNA levels and cccDNA even in patients receiving antivirals for HBV. This is demonstrated by Tseng et al., where undetectable levels of HBcrAg predicted seroclearance of HBsAg by 10-14 years. This and past studies have shown HBcrAg to be a good predictor for cccDNA transcriptional activity, allowing health care providers to predict functional loss of HBsAg, flare-ups, treatment response, and when to conclude treatment.
Clinically, HBcrAg could be monitored in chronic HBV infection while patients are receiving treatment. A rise in HBcrAg has the ability to predict HBV flares, while a decrease in HBcrAg can forecast seroclearance of HBsAg. If there is undetectable level of HBsAg with detectable HBcrAg, it can mean the relapse of HBsAg+, and oral treatment could be continued. HBsAg and HBcrAg also can be used to determine when to stop treatment, especially with nucleos(t)ide analogs (NAs). The Mayo Clinic laboratories recently opened HBcrAg testing for patients with chronic HBV.
With emerging medications, HBV cure may be possible with multiple therapies. Hepatic cccDNA turnover may be halted by inhibiting capsid assembly and secretion, relaxed-circular DNA (rcDNA) nuclear delivery or conversion to cccDNA, and formation of viral RNAs. Since HBcrAg is a good indicator of cccDNA transcriptional activity, it should be used to determine the effectiveness of these new therapies in clinical trials.
Katerina Roma, DO, is with the department of internal medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas. Robert Gish, MD, is medical director of the Hepatitis B Foundation in Doylestown, Pa. They have no financial conflicts.
Current hepatitis B virus (HBV) therapies do not eliminate the covalently closed circular DNA (cccDNA), and a single cccDNA can cause a infection. Hepatitis B core-related antigen (HBcrAg) has shown positive correlation with serum and hepatic HBV-DNA levels and cccDNA even in patients receiving antivirals for HBV. This is demonstrated by Tseng et al., where undetectable levels of HBcrAg predicted seroclearance of HBsAg by 10-14 years. This and past studies have shown HBcrAg to be a good predictor for cccDNA transcriptional activity, allowing health care providers to predict functional loss of HBsAg, flare-ups, treatment response, and when to conclude treatment.
Clinically, HBcrAg could be monitored in chronic HBV infection while patients are receiving treatment. A rise in HBcrAg has the ability to predict HBV flares, while a decrease in HBcrAg can forecast seroclearance of HBsAg. If there is undetectable level of HBsAg with detectable HBcrAg, it can mean the relapse of HBsAg+, and oral treatment could be continued. HBsAg and HBcrAg also can be used to determine when to stop treatment, especially with nucleos(t)ide analogs (NAs). The Mayo Clinic laboratories recently opened HBcrAg testing for patients with chronic HBV.
With emerging medications, HBV cure may be possible with multiple therapies. Hepatic cccDNA turnover may be halted by inhibiting capsid assembly and secretion, relaxed-circular DNA (rcDNA) nuclear delivery or conversion to cccDNA, and formation of viral RNAs. Since HBcrAg is a good indicator of cccDNA transcriptional activity, it should be used to determine the effectiveness of these new therapies in clinical trials.
Katerina Roma, DO, is with the department of internal medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas. Robert Gish, MD, is medical director of the Hepatitis B Foundation in Doylestown, Pa. They have no financial conflicts.
Current hepatitis B virus (HBV) therapies do not eliminate the covalently closed circular DNA (cccDNA), and a single cccDNA can cause a infection. Hepatitis B core-related antigen (HBcrAg) has shown positive correlation with serum and hepatic HBV-DNA levels and cccDNA even in patients receiving antivirals for HBV. This is demonstrated by Tseng et al., where undetectable levels of HBcrAg predicted seroclearance of HBsAg by 10-14 years. This and past studies have shown HBcrAg to be a good predictor for cccDNA transcriptional activity, allowing health care providers to predict functional loss of HBsAg, flare-ups, treatment response, and when to conclude treatment.
Clinically, HBcrAg could be monitored in chronic HBV infection while patients are receiving treatment. A rise in HBcrAg has the ability to predict HBV flares, while a decrease in HBcrAg can forecast seroclearance of HBsAg. If there is undetectable level of HBsAg with detectable HBcrAg, it can mean the relapse of HBsAg+, and oral treatment could be continued. HBsAg and HBcrAg also can be used to determine when to stop treatment, especially with nucleos(t)ide analogs (NAs). The Mayo Clinic laboratories recently opened HBcrAg testing for patients with chronic HBV.
With emerging medications, HBV cure may be possible with multiple therapies. Hepatic cccDNA turnover may be halted by inhibiting capsid assembly and secretion, relaxed-circular DNA (rcDNA) nuclear delivery or conversion to cccDNA, and formation of viral RNAs. Since HBcrAg is a good indicator of cccDNA transcriptional activity, it should be used to determine the effectiveness of these new therapies in clinical trials.
Katerina Roma, DO, is with the department of internal medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas. Robert Gish, MD, is medical director of the Hepatitis B Foundation in Doylestown, Pa. They have no financial conflicts.
Low serum levels of the hepatitis B core-related antigen (HBcrAg) could be an early biomarker of a functional cure of a hepatitis B infection, according to new findings from a retrospective study.
A drop in HBcrAg predicted the seroclearance of hepatitis B surface antigen (HBsAg), the widely accepted measure of optimal liver-related outcomes in patient care and clinical trials, long before HBsAg levels actually fell.
“In a large retrospective cohort study of chronic hepatitis B patients, we found lower levels of HBcrAg were associated with higher probability of clearing HBsAg,” wrote Tai-Chung Tseng and coauthors at National Taiwan University Hospital in Taipei. “Reduction of HBcrAg developed 10 years before decline of HBsAg in patients with high HBsAg levels at baseline.”
Nearly 300 million people worldwide are estimated to be positive for the HBsAg antigen, a marker of active hepatitis B virus (HBV) infection. Chronic HBV puts individuals at greater risk of cirrhosis, hepatocellular carcinoma (HCC), and other liver complications.
Seroclearance of HBsAg is generally regarded as signaling a functional cure, because it is associated with low viral activity and good clinical outcomes. Patients with low HBsAg levels may transition to complete clearance, while those with levels of 1,000 IU/mL or higher rarely achieve clearance either spontaneously or through treatment.
As with HBsAg, higher serum levels of HBcrAg have been linked to a raised risk of adverse events, including increased viral activity and heightened risk of developing hepatitis B e antigen-negative hepatitis, cirrhosis, and HCC. Lower HBcrAg levels are associated with a greater likelihood of HBsAg seroclearance in chronic hepatitis B patients who discontinued antiviral therapy.
In a study published in Gastroenterology, researchers conducted a retrospective Taiwanese cohort study of 2,614 untreated patients with hepatitis B who underwent long-term follow-up at National Taiwan University Hospital. The median age was 38.2 years, and 60.6% were men. At baseline, 14.8% had HBsAg levels of less than 100 IU/mL, and 47.7% had HBcrAg levels less than 10,000 IU/mL. Most (77.5%) were infected with HBV genotype B. From stored serum samples, the researchers quantified levels of HBV DNA, HBsAg, and HBcrAg and evaluated the relationships with spontaneous HBsAg seroclearance.
Over an average follow-up of about 12 years, 465 patients cleared HBsAg, an incidence of 1.43% per year. Researchers stratified patients by levels of viral markers. Compared to those with the highest HBcrAg levels (> 100,000 IU/mL), lower levels of HBcrAg were associated with greater likelihood of HBsAg clearance.
Specifically, intermediate levels (10,000-99,999 IU/mL) were associated with nearly double the chance of HBsAg clearance (hazard ratio [HR], 1.95; 95% confidence interval [CI], 1.44-2.65), and the lowest levels (< 10,000 IU/mL) were associated with just over triple the chance of clearance (HR, 3.15; 95% CI, 2.45-4.05). These associations held up with multivariable analyses, and HBV DNA levels were not significantly associated with HBsAg clearance.
“Not surprisingly, HBsAg levels still serve as a better predictor than the other two biomarkers,” the authors wrote. “Notably, the HBsAg levels are more like a short-term predictor” (within 5 years).
For patients with higher HBsAg levels (> 1,000 IU/mL), it took a median of 16 years to achieve HBsAg clearance. A subanalysis of the 1,539 patients with HbsAg levels > 1,000 IU/mL found that only HBcrAg levels below 10,000 IU/mL predicted HBsAg seroclearance versus 100,000 U/mL or higher (adjusted HR, 1.95; 95% CI, 1.16-3.27).
HBsAg levels began to decline later, often between 5 and 9 years before HBsAg seroclearance occurs. However, HBcrAg levels became undetectable 10-14 years before HBsAg seroclearance. Among patients achieving undetectable levels of HBcrAg, the annual HBsAg seroclearance rate was higher in the second decade of follow-up than in the first decade (3.75% versus 0.97%).
HBcrAg levels reflect the transcriptional activity of covalently closed circular DNA (cccDNA), the authors noted, while HBsAg can come from cccDNA and HBV-DNA integrated into the host genome. Several novel hepatitis B therapies in development target cccDNA transcription, but it isn’t known if the strategy will result in HBsAg clearance.
In the discussion section, the authors speculated about the possible pathology and treatment implications for several chronic hepatitis B scenarios. For example, the finding that HBcrAg clearance usually precedes HBsAg clearance suggests that reduction of cccDNA transcription is a requirement for curing hepatitis B, the authors speculate, but it also suggests that add-on treatment may need to target HBsAg transcribed from the integrated viral genome for a functional cure.
The researchers noted several study limitations, including that the cohort included only Asians largely with HBV genotypes B or C and that “further validation from Caucasian patients infected with genotypes types A or D is mandatory.”
Tai-Chung Tseng disclosed financial conflicts with Fujirebio, Bristol-Myers Squibb, and Gilead Sciences. The remaining authors had no conflicts of interest. The study received grant support from several institutions, including National Taiwan University Hospital.
Low serum levels of the hepatitis B core-related antigen (HBcrAg) could be an early biomarker of a functional cure of a hepatitis B infection, according to new findings from a retrospective study.
A drop in HBcrAg predicted the seroclearance of hepatitis B surface antigen (HBsAg), the widely accepted measure of optimal liver-related outcomes in patient care and clinical trials, long before HBsAg levels actually fell.
“In a large retrospective cohort study of chronic hepatitis B patients, we found lower levels of HBcrAg were associated with higher probability of clearing HBsAg,” wrote Tai-Chung Tseng and coauthors at National Taiwan University Hospital in Taipei. “Reduction of HBcrAg developed 10 years before decline of HBsAg in patients with high HBsAg levels at baseline.”
Nearly 300 million people worldwide are estimated to be positive for the HBsAg antigen, a marker of active hepatitis B virus (HBV) infection. Chronic HBV puts individuals at greater risk of cirrhosis, hepatocellular carcinoma (HCC), and other liver complications.
Seroclearance of HBsAg is generally regarded as signaling a functional cure, because it is associated with low viral activity and good clinical outcomes. Patients with low HBsAg levels may transition to complete clearance, while those with levels of 1,000 IU/mL or higher rarely achieve clearance either spontaneously or through treatment.
As with HBsAg, higher serum levels of HBcrAg have been linked to a raised risk of adverse events, including increased viral activity and heightened risk of developing hepatitis B e antigen-negative hepatitis, cirrhosis, and HCC. Lower HBcrAg levels are associated with a greater likelihood of HBsAg seroclearance in chronic hepatitis B patients who discontinued antiviral therapy.
In a study published in Gastroenterology, researchers conducted a retrospective Taiwanese cohort study of 2,614 untreated patients with hepatitis B who underwent long-term follow-up at National Taiwan University Hospital. The median age was 38.2 years, and 60.6% were men. At baseline, 14.8% had HBsAg levels of less than 100 IU/mL, and 47.7% had HBcrAg levels less than 10,000 IU/mL. Most (77.5%) were infected with HBV genotype B. From stored serum samples, the researchers quantified levels of HBV DNA, HBsAg, and HBcrAg and evaluated the relationships with spontaneous HBsAg seroclearance.
Over an average follow-up of about 12 years, 465 patients cleared HBsAg, an incidence of 1.43% per year. Researchers stratified patients by levels of viral markers. Compared to those with the highest HBcrAg levels (> 100,000 IU/mL), lower levels of HBcrAg were associated with greater likelihood of HBsAg clearance.
Specifically, intermediate levels (10,000-99,999 IU/mL) were associated with nearly double the chance of HBsAg clearance (hazard ratio [HR], 1.95; 95% confidence interval [CI], 1.44-2.65), and the lowest levels (< 10,000 IU/mL) were associated with just over triple the chance of clearance (HR, 3.15; 95% CI, 2.45-4.05). These associations held up with multivariable analyses, and HBV DNA levels were not significantly associated with HBsAg clearance.
“Not surprisingly, HBsAg levels still serve as a better predictor than the other two biomarkers,” the authors wrote. “Notably, the HBsAg levels are more like a short-term predictor” (within 5 years).
For patients with higher HBsAg levels (> 1,000 IU/mL), it took a median of 16 years to achieve HBsAg clearance. A subanalysis of the 1,539 patients with HbsAg levels > 1,000 IU/mL found that only HBcrAg levels below 10,000 IU/mL predicted HBsAg seroclearance versus 100,000 U/mL or higher (adjusted HR, 1.95; 95% CI, 1.16-3.27).
HBsAg levels began to decline later, often between 5 and 9 years before HBsAg seroclearance occurs. However, HBcrAg levels became undetectable 10-14 years before HBsAg seroclearance. Among patients achieving undetectable levels of HBcrAg, the annual HBsAg seroclearance rate was higher in the second decade of follow-up than in the first decade (3.75% versus 0.97%).
HBcrAg levels reflect the transcriptional activity of covalently closed circular DNA (cccDNA), the authors noted, while HBsAg can come from cccDNA and HBV-DNA integrated into the host genome. Several novel hepatitis B therapies in development target cccDNA transcription, but it isn’t known if the strategy will result in HBsAg clearance.
In the discussion section, the authors speculated about the possible pathology and treatment implications for several chronic hepatitis B scenarios. For example, the finding that HBcrAg clearance usually precedes HBsAg clearance suggests that reduction of cccDNA transcription is a requirement for curing hepatitis B, the authors speculate, but it also suggests that add-on treatment may need to target HBsAg transcribed from the integrated viral genome for a functional cure.
The researchers noted several study limitations, including that the cohort included only Asians largely with HBV genotypes B or C and that “further validation from Caucasian patients infected with genotypes types A or D is mandatory.”
Tai-Chung Tseng disclosed financial conflicts with Fujirebio, Bristol-Myers Squibb, and Gilead Sciences. The remaining authors had no conflicts of interest. The study received grant support from several institutions, including National Taiwan University Hospital.
FROM GASTROENTEROLOGY
Physical activity is a growing priority for patients with MS
SAN DIEGO – As , researchers have developed a mobile app to encourage young patients with the disease to become more active. The smartphone-based app provides tailored physical activity information, coaching advice, and tools to increase social connectedness.
A pilot study examining whether the intervention changes activity, depression, and fatigue levels should be wrapped up later this year, but it looks as though the app is succeeding.
“The feedback we’ve gotten so far from our coaches is that the kids seem highly motivated,” said one of the creators, E. Ann Yeh, MD, professor in the faculty of medicine at the University of Toronto and director of the pediatric MS and neuroinflammatory disorders program at the Hospital for Sick Children.
Preliminary work showed that use of the app was associated with a 31% increase in physical activity.
They discussed this and other studies of the role of exercise in MS at the annual meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
Higher levels of depression and fatigue
Studies show that youths with MS who are less physically active are more likely to experience higher levels of fatigue and depression. Evidence suggests just 15-30 more minutes of moderate to vigorous physical activity (MVPA) makes a clinical difference in terms of improved depression and fatigue scores, said Dr. Yeh.
With moderate physical activity (for example, a brisk walk or raking the yard), the maximal heart rate (HRmax) reaches 64%-76%, while with vigorous physical activity (which includes jogging/running or participating in a strenuous fitness class), the HRmax reaches 77%-93%.
Dr. Yeh described vigorous physical activity as “the stuff that makes you sweat, makes your heart rate go up, and makes you not be able to talk when you’re moving.”
As it stands, kids get very little MVPA – 9.5 min/day, which is well below the recommended 60 min/day. Adults do a bit better – 18.7 min/day of MVPA – but this is still below the recommended 30 min/day.
Being physically active improves fatigue for adults as well as kids, said Dr. Yeh. She referred to a network meta-analysis of 27 studies involving 1,470 participants that evaluated 10 types of exercise interventions, including yoga, resistance training, dance, and aquatic activities. It found that exercise “does move the needle,” she said. “Regardless of the kind of activity that was studied, fatigue seemed to improve.”
The authors of that study ranked aquatic exercise as the most effective intervention. “It’s possible that aquatics worked better because people who can’t move well feel more comfortable in the water,” Dr. Yeh said.
But she cautioned that the one study in the meta-analysis that found a “quite strong” effect of aquatic exercise was “very small.”
With regard to depression, which affects about 30% of people with MS, Dr. Yeh told meeting attendees, “unfortunately, the data are less clear” when it comes to physical activity for adults. One meta-analysis of 15 randomized controlled trials involving 331 exercising participants and 260 control persons found that only a few studies showed positive effects of exercise on depressive symptoms.
However, Dr. Yeh noted that in this review, the baseline depressive symptoms of participants were “above the cutoff level,” which makes it more difficult to demonstrate change in depression levels.
Clear structural effects
Researchers have also described clear brain structural and functional effects from being physically active. For example, MVPA has been shown to affect brain volume, and it has been associated with better optical coherence tomography (OCT) metrics, which measures retinal thinning.
As for the impact of exercise on memory deficits, which is of interest, given the current focus on Alzheimer’s disease, “the jury is still out,” said Dr. Yeh. One 24-week randomized controlled trial found no difference in results on the Brief Repeatable Battery of Neuropsychological tests between participants who engaged in progressive aerobic exercise and control persons.
However, said Dr. Yeh, “the problem may not be with the intervention but with the outcome measures” and potentially with the populations studied.
It might be a different story for high-intensity exercise, though. A study by Danish researchers assessed the effects of a 24-week high-intensity intervention among 84 adult patients with mild-severe impairment.
The primary outcome of that study, which was the percentage of brain volume change, was not met, possibly because the study was too short. There were significant results for some secondary endpoints, including improved cardiorespiratory fitness and lower relapse rate.
“Even though on the face of it, it sounds like a negative study, there were important outcomes,” said Dr. Yeh.
Research into the possible mechanisms behind positive effects of physical activity is limited with regard to patients with MS, said Dr. Yeh. Some studies have implicated certain circulating factors, such as the cytokine irisin and brain-derived neurotrophic factor, but more work is needed, she said.
“There is need for further mechanistic knowledge related to exercise in MS, and this must be accomplished through prospective, randomized studies.”
While exercise likely makes some difference for MS patients, the problem is in getting them to be more active. “You can’t just write a prescription,” said Dr. Yeh.
“Patients should be doing whatever they can, but gradually, and should not go crazy at the beginning because they’ll just burn out,” she said.
She stressed that patients need to find what works for them personally. It’s also important for them to find ways to be active with a friend who can be “a motivator” to help sustain physical activity goals, said Dr. Yeh.
Patients can also look online for remote physical activity programs geared to people with MS, which popped up during the pandemic.
Improved mood, cognition, pain, sleep
In a comment, Marwa Kaisey, MD, assistant professor of neurology at Cedars-Sinai Medical Center, in Los Angeles, who cochaired the session highlighting the presentation, praised Dr. Yeh’s “excellent talk,” which highlighted the “strong benefit” of exercise for patients with MS.
“As a clinician, I often talk to my patients about the importance of physical exercise and have heard countless anecdotes of how their workout programs helped improve mood, cognition, pain, or sleep.”
However, she agreed there are several areas “where we need more data-driven solutions and a mechanistic understanding of the benefits of physical exercise.”
The pilot study was funded by the Consortium of Multiple Sclerosis Centers. The MS Society of Canada funded early work on the app, and the National MS Society is funding the trial of the app. Dr. Yeh receives support from the MS Society of Canada. Dr. Kaisey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – As , researchers have developed a mobile app to encourage young patients with the disease to become more active. The smartphone-based app provides tailored physical activity information, coaching advice, and tools to increase social connectedness.
A pilot study examining whether the intervention changes activity, depression, and fatigue levels should be wrapped up later this year, but it looks as though the app is succeeding.
“The feedback we’ve gotten so far from our coaches is that the kids seem highly motivated,” said one of the creators, E. Ann Yeh, MD, professor in the faculty of medicine at the University of Toronto and director of the pediatric MS and neuroinflammatory disorders program at the Hospital for Sick Children.
Preliminary work showed that use of the app was associated with a 31% increase in physical activity.
They discussed this and other studies of the role of exercise in MS at the annual meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
Higher levels of depression and fatigue
Studies show that youths with MS who are less physically active are more likely to experience higher levels of fatigue and depression. Evidence suggests just 15-30 more minutes of moderate to vigorous physical activity (MVPA) makes a clinical difference in terms of improved depression and fatigue scores, said Dr. Yeh.
With moderate physical activity (for example, a brisk walk or raking the yard), the maximal heart rate (HRmax) reaches 64%-76%, while with vigorous physical activity (which includes jogging/running or participating in a strenuous fitness class), the HRmax reaches 77%-93%.
Dr. Yeh described vigorous physical activity as “the stuff that makes you sweat, makes your heart rate go up, and makes you not be able to talk when you’re moving.”
As it stands, kids get very little MVPA – 9.5 min/day, which is well below the recommended 60 min/day. Adults do a bit better – 18.7 min/day of MVPA – but this is still below the recommended 30 min/day.
Being physically active improves fatigue for adults as well as kids, said Dr. Yeh. She referred to a network meta-analysis of 27 studies involving 1,470 participants that evaluated 10 types of exercise interventions, including yoga, resistance training, dance, and aquatic activities. It found that exercise “does move the needle,” she said. “Regardless of the kind of activity that was studied, fatigue seemed to improve.”
The authors of that study ranked aquatic exercise as the most effective intervention. “It’s possible that aquatics worked better because people who can’t move well feel more comfortable in the water,” Dr. Yeh said.
But she cautioned that the one study in the meta-analysis that found a “quite strong” effect of aquatic exercise was “very small.”
With regard to depression, which affects about 30% of people with MS, Dr. Yeh told meeting attendees, “unfortunately, the data are less clear” when it comes to physical activity for adults. One meta-analysis of 15 randomized controlled trials involving 331 exercising participants and 260 control persons found that only a few studies showed positive effects of exercise on depressive symptoms.
However, Dr. Yeh noted that in this review, the baseline depressive symptoms of participants were “above the cutoff level,” which makes it more difficult to demonstrate change in depression levels.
Clear structural effects
Researchers have also described clear brain structural and functional effects from being physically active. For example, MVPA has been shown to affect brain volume, and it has been associated with better optical coherence tomography (OCT) metrics, which measures retinal thinning.
As for the impact of exercise on memory deficits, which is of interest, given the current focus on Alzheimer’s disease, “the jury is still out,” said Dr. Yeh. One 24-week randomized controlled trial found no difference in results on the Brief Repeatable Battery of Neuropsychological tests between participants who engaged in progressive aerobic exercise and control persons.
However, said Dr. Yeh, “the problem may not be with the intervention but with the outcome measures” and potentially with the populations studied.
It might be a different story for high-intensity exercise, though. A study by Danish researchers assessed the effects of a 24-week high-intensity intervention among 84 adult patients with mild-severe impairment.
The primary outcome of that study, which was the percentage of brain volume change, was not met, possibly because the study was too short. There were significant results for some secondary endpoints, including improved cardiorespiratory fitness and lower relapse rate.
“Even though on the face of it, it sounds like a negative study, there were important outcomes,” said Dr. Yeh.
Research into the possible mechanisms behind positive effects of physical activity is limited with regard to patients with MS, said Dr. Yeh. Some studies have implicated certain circulating factors, such as the cytokine irisin and brain-derived neurotrophic factor, but more work is needed, she said.
“There is need for further mechanistic knowledge related to exercise in MS, and this must be accomplished through prospective, randomized studies.”
While exercise likely makes some difference for MS patients, the problem is in getting them to be more active. “You can’t just write a prescription,” said Dr. Yeh.
“Patients should be doing whatever they can, but gradually, and should not go crazy at the beginning because they’ll just burn out,” she said.
She stressed that patients need to find what works for them personally. It’s also important for them to find ways to be active with a friend who can be “a motivator” to help sustain physical activity goals, said Dr. Yeh.
Patients can also look online for remote physical activity programs geared to people with MS, which popped up during the pandemic.
Improved mood, cognition, pain, sleep
In a comment, Marwa Kaisey, MD, assistant professor of neurology at Cedars-Sinai Medical Center, in Los Angeles, who cochaired the session highlighting the presentation, praised Dr. Yeh’s “excellent talk,” which highlighted the “strong benefit” of exercise for patients with MS.
“As a clinician, I often talk to my patients about the importance of physical exercise and have heard countless anecdotes of how their workout programs helped improve mood, cognition, pain, or sleep.”
However, she agreed there are several areas “where we need more data-driven solutions and a mechanistic understanding of the benefits of physical exercise.”
The pilot study was funded by the Consortium of Multiple Sclerosis Centers. The MS Society of Canada funded early work on the app, and the National MS Society is funding the trial of the app. Dr. Yeh receives support from the MS Society of Canada. Dr. Kaisey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – As , researchers have developed a mobile app to encourage young patients with the disease to become more active. The smartphone-based app provides tailored physical activity information, coaching advice, and tools to increase social connectedness.
A pilot study examining whether the intervention changes activity, depression, and fatigue levels should be wrapped up later this year, but it looks as though the app is succeeding.
“The feedback we’ve gotten so far from our coaches is that the kids seem highly motivated,” said one of the creators, E. Ann Yeh, MD, professor in the faculty of medicine at the University of Toronto and director of the pediatric MS and neuroinflammatory disorders program at the Hospital for Sick Children.
Preliminary work showed that use of the app was associated with a 31% increase in physical activity.
They discussed this and other studies of the role of exercise in MS at the annual meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
Higher levels of depression and fatigue
Studies show that youths with MS who are less physically active are more likely to experience higher levels of fatigue and depression. Evidence suggests just 15-30 more minutes of moderate to vigorous physical activity (MVPA) makes a clinical difference in terms of improved depression and fatigue scores, said Dr. Yeh.
With moderate physical activity (for example, a brisk walk or raking the yard), the maximal heart rate (HRmax) reaches 64%-76%, while with vigorous physical activity (which includes jogging/running or participating in a strenuous fitness class), the HRmax reaches 77%-93%.
Dr. Yeh described vigorous physical activity as “the stuff that makes you sweat, makes your heart rate go up, and makes you not be able to talk when you’re moving.”
As it stands, kids get very little MVPA – 9.5 min/day, which is well below the recommended 60 min/day. Adults do a bit better – 18.7 min/day of MVPA – but this is still below the recommended 30 min/day.
Being physically active improves fatigue for adults as well as kids, said Dr. Yeh. She referred to a network meta-analysis of 27 studies involving 1,470 participants that evaluated 10 types of exercise interventions, including yoga, resistance training, dance, and aquatic activities. It found that exercise “does move the needle,” she said. “Regardless of the kind of activity that was studied, fatigue seemed to improve.”
The authors of that study ranked aquatic exercise as the most effective intervention. “It’s possible that aquatics worked better because people who can’t move well feel more comfortable in the water,” Dr. Yeh said.
But she cautioned that the one study in the meta-analysis that found a “quite strong” effect of aquatic exercise was “very small.”
With regard to depression, which affects about 30% of people with MS, Dr. Yeh told meeting attendees, “unfortunately, the data are less clear” when it comes to physical activity for adults. One meta-analysis of 15 randomized controlled trials involving 331 exercising participants and 260 control persons found that only a few studies showed positive effects of exercise on depressive symptoms.
However, Dr. Yeh noted that in this review, the baseline depressive symptoms of participants were “above the cutoff level,” which makes it more difficult to demonstrate change in depression levels.
Clear structural effects
Researchers have also described clear brain structural and functional effects from being physically active. For example, MVPA has been shown to affect brain volume, and it has been associated with better optical coherence tomography (OCT) metrics, which measures retinal thinning.
As for the impact of exercise on memory deficits, which is of interest, given the current focus on Alzheimer’s disease, “the jury is still out,” said Dr. Yeh. One 24-week randomized controlled trial found no difference in results on the Brief Repeatable Battery of Neuropsychological tests between participants who engaged in progressive aerobic exercise and control persons.
However, said Dr. Yeh, “the problem may not be with the intervention but with the outcome measures” and potentially with the populations studied.
It might be a different story for high-intensity exercise, though. A study by Danish researchers assessed the effects of a 24-week high-intensity intervention among 84 adult patients with mild-severe impairment.
The primary outcome of that study, which was the percentage of brain volume change, was not met, possibly because the study was too short. There were significant results for some secondary endpoints, including improved cardiorespiratory fitness and lower relapse rate.
“Even though on the face of it, it sounds like a negative study, there were important outcomes,” said Dr. Yeh.
Research into the possible mechanisms behind positive effects of physical activity is limited with regard to patients with MS, said Dr. Yeh. Some studies have implicated certain circulating factors, such as the cytokine irisin and brain-derived neurotrophic factor, but more work is needed, she said.
“There is need for further mechanistic knowledge related to exercise in MS, and this must be accomplished through prospective, randomized studies.”
While exercise likely makes some difference for MS patients, the problem is in getting them to be more active. “You can’t just write a prescription,” said Dr. Yeh.
“Patients should be doing whatever they can, but gradually, and should not go crazy at the beginning because they’ll just burn out,” she said.
She stressed that patients need to find what works for them personally. It’s also important for them to find ways to be active with a friend who can be “a motivator” to help sustain physical activity goals, said Dr. Yeh.
Patients can also look online for remote physical activity programs geared to people with MS, which popped up during the pandemic.
Improved mood, cognition, pain, sleep
In a comment, Marwa Kaisey, MD, assistant professor of neurology at Cedars-Sinai Medical Center, in Los Angeles, who cochaired the session highlighting the presentation, praised Dr. Yeh’s “excellent talk,” which highlighted the “strong benefit” of exercise for patients with MS.
“As a clinician, I often talk to my patients about the importance of physical exercise and have heard countless anecdotes of how their workout programs helped improve mood, cognition, pain, or sleep.”
However, she agreed there are several areas “where we need more data-driven solutions and a mechanistic understanding of the benefits of physical exercise.”
The pilot study was funded by the Consortium of Multiple Sclerosis Centers. The MS Society of Canada funded early work on the app, and the National MS Society is funding the trial of the app. Dr. Yeh receives support from the MS Society of Canada. Dr. Kaisey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACTRIMS FORUM 2023
New insight into preventing antipsychotic-induced weight gain
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.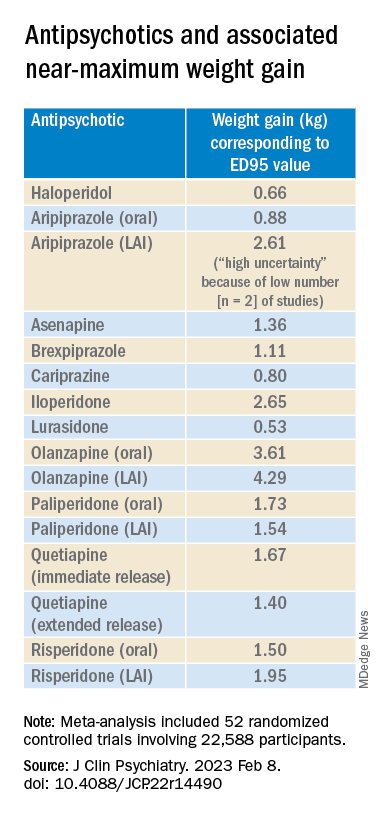
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Encouraging 3-year data for TAVR in low-risk patients: EVOLUT
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Protuberant, Pink, Irritated Growth on the Buttocks
The Diagnosis: Superficial Angiomyxoma
Superficial angiomyxoma is a rare, benign, cutaneous tumor of a myxoid matrix and blood vessels that was first described in association with Carney complex.1 Tumors may be solitary or multiple. A recent review of cases in the literature revealed a roughly equal distribution of superficial angiomyxomas in males and females occurring most frequently on the head and neck, extremities, and trunk or back. The peak incidence is between the fourth and fifth decades of life.2 Superficial angiomyxomas can occur sporadically or in association with Carney complex, an autosomal-dominant condition with germline inactivating mutations in protein kinase A, PRKAR1A. Interestingly, sporadic cases of superficial angiomyxoma also have shown loss of PRKAR1A expression on immunohistochemistry (IHC).3
Common histologic mimics of superficial angiomyxoma include aggressive angiomyxoma and angiomyofibroblastoma.4 It is thought that these 3 distinct tumor entities may arise from a common pluripotent cell of origin located near connective tissue vasculature, which may contribute to the similarities observed between them.5 For example, aggressive angiomyxomas and angiomyofibroblastomas also demonstrate a similar myxoid background and vascular proliferation that can closely mimic superficial angiomyxomas clinically. However, the vessels of superficial angiomyxomas tend to be long and thin walled, while aggressive angiomyxomas are characterized by large and thick-walled vessels and angiomyofibroblastomas by abundant smaller vessels. Additionally, unlike superficial angiomyxomas, both aggressive angiomyxomas and angiomyofibroblastomas typically occur in the genital tract of young to middle-aged women.6
Histopathologic examination is imperative for differentiating between superficial angiomyxoma and more aggressive histologic mimics. Superficial angiomyxomas typically consist of a rich myxoid stroma, thin-walled or arborizing blood vessels, and spindled to stellate fibroblastlike cells (quiz image 2).3 Although not prominent in our case, superficial angiomyxomas also frequently present with stromal neutrophils and epithelial components, including keratinous cysts, basaloid buds, and strands of squamous epithelium.7 Minimal cellular atypia, mitotic activity, and nuclear pleomorphism often are seen, with IHC negative for desmin, estrogen receptor, and progesterone receptor; positive for CD34 and smooth muscle actin; and variable for S-100 and muscle-specific actin. Although IHC has limited utility in the diagnosis of superficial angiomyxomas, it may be useful to rule out other differential diagnoses.2,3 Superficial angiomyxomas usually show fibroblastic stromal cells, proteoglycan matrix, and collagen fibers on electron microscopy.8 Importantly, histopathologic examination of aggressive angiomyxoma will comparatively present with more invasive, infiltrative, and less well-circumscribed tumors.9 Other differential diagnoses on histology may include neurofibroma, focal cutaneous mucinosis, spindle cell lipoma, and myxofibrosarcoma. Additional considerations include fibroepithelial polyp, nevus lipomatosis, angiomyxolipoma, and anetoderma.
An important differential diagnosis in the evaluation of superficial angiomyxoma is neurofibroma, a benign peripheral nerve sheath tumor that presents as a smooth, flesh-colored, and painless papule or nodule commonly associated with the buttonhole sign. Histopathology of neurofibroma features elongated spindle cells with comma-shaped or buckled wavy nuclei and variably sized collagen bundles described as “shredded carrots” (Figure 1).10 Occasional mast cells also can be seen. Immunohistochemistry targeting elements of peripheral nerve sheaths may assist in the diagnosis of neurofibromas, including positive S-100 and SOX10 in Schwann cells, epithelial membrane antigen in perineural cells, and fingerprint positivity for CD34 in fibroblasts.10
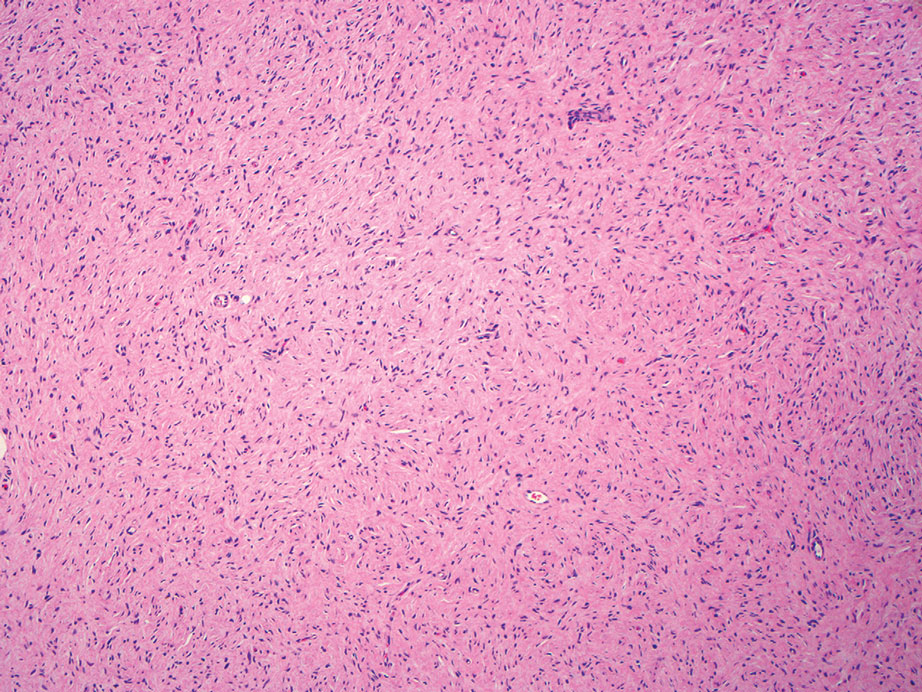
Cutaneous mucinoses encompass a diverse group of connective tissue disorders characterized by accumulation of mucin in the skin. Solitary focal cutaneous mucinoses (FCMs) are individual isolated lesions of mucin deposits that are unassociated with systemic conditions.11 Conversely, multiple FCMs presenting with multiple cutaneous lesions also have been described in association with systemic diseases such as scleroderma, systemic lupus erythematosus, and thyroid disease.12 Solitary FCM typically presents as an asymptomatic, flesh-colored papule or nodule on the extremities. It often arises in mid to late adulthood with a slightly increased frequency among males.12 Histopathology of solitary FCM commonly demonstrates a dome-shaped pool of basophilic mucin in the upper dermis sparing involvement of the underlying subcutaneous tissue (Figure 2).13 Notably, FCM often lacks the vascularity as well as stromal neutrophils and epithelial elements that are seen in superficial angiomyxomas. Although hematoxylin and eosin stains can be sufficient for diagnosis of solitary FCM, additional stains for mucin such as Alcian blue, colloidal iron, or toluidine blue also may be considered to support the diagnosis.12
Spindle cell lipomas (SCLs) are rare, benign, subcutaneous, adipocytic tumors that arise on the upper back, posterior neck, or shoulders of middle-aged or elderly adult males.14 The clinical presentation often is an asymptomatic, well-circumscribed, mobile subcutaneous mass that is firmer than a common lipoma. Histologically, SCLs are characterized by mature adipocytes, spindle cells, and wire or ropelike collagen fibers in a myxoid background (Figure 3). The spindle cells usually are bland with a notable bipolar shape and blunted ends. Infiltrative growth patterns or mitotic figures are uncommon. Diagnosis can be supported by IHC, as SCLs stain diffusely positive for CD34 with loss of the retinoblastoma protein.7
Another important differential diagnosis to consider is myxofibrosarcoma, a rare and malignant myxoid cutaneous tumor. Clinically, it presents asymptomatically as an indolent, slow-growing nodule on the limbs and limb girdles.7 Histopathologic features demonstrate a multilobular tumor composed of a mixture of hypocellular and hypercellular regions with incomplete fibrous septae (Figure 4). The presence of curvilinear vasculature is characteristic. Multinucleated giant cells and cellular atypia with nuclear pleomorphism also can be seen. Although IHC findings generally are not specific, they can be used to rule out other potential diagnoses. Myxofibrosarcomas stain positive for vimentin and occasionally smooth muscle actin, muscle-specific actin, and CD34.7
Superficial angiomyxomas are benign; however, excision is recommended to distinguish between mimics. Local recurrence after excision is common in 30% to 40% of patients.15 Mohs micrographic surgery has been considered, especially if the following are present: tumor characteristics (eg, poorly circumscribed), location (eg, head and neck or other cosmetically or functionally sensitive areas), and likelihood of recurrence (high for superficial angiomyxomas). 16 This case otherwise highlights a rare example of superficial angiomyxomas involving the buttocks.
- Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12:519-530. doi:10.1097 /00000478-198807000-00003
- Sharma A, Khaitan N, Ko JS, et al. A clinicopathologic analysis of 54 cases of cutaneous myxoma. Hum Pathol. 2021:S0046-8177(21) 00201-X. doi:10.1016/j.humpath.2021.12.003
- Hafeez F, Krakowski AC, Lian CG, et al. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression [published online March 17, 2022]. Histopathology. 2022;80:1001-1003. doi:10.1111/his.14568
- Mehrotra K, Bhandari M, Khullar G, et al. Large superficial angiomyxoma of the vulva: report of two cases with varied clinical presentation. Indian Dermatol Online J. 2021;12:605-607. doi:10.4103/idoj.IDOJ_489_20
- Alameda F, Munné A, Baró T, et al. Vulvar angiomyxoma, aggressive angiomyxoma, and angiomyofibroblastoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193-205. doi:10.1080/01913120500520911
- Haroon S, Irshad L, Zia S, et al. Aggressive angiomyxoma, angiomyofibroblastoma, and cellular angiofibroma of the lower female genital tract: related entities with different outcomes. Cureus. 2022;14:E29250. doi:10.7759/cureus.29250
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918. doi:10.1111/cup.12749
- Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4:99-123. doi:10.1016 /s1092-9134(00)90019-4
- Lee C-C, Chen Y-L, Liau J-Y, et al. Superficial angiomyxoma on the vulva of an adolescent. Taiwan J Obstet Gynecol. 2014;53:104-106. doi:10.1016/j.tjog.2013.08.001
- Magro G, Amico P, Vecchio GM, et al. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: a clinicopathologic study of 94 cases. Virchows Arch. 2010;456:71-76. doi:10.1007/s00428-009-0859-y
- Kuo KL, Lee LY, Kuo TT. Solitary cutaneous focal mucinosis: a clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J Dermatol. 2017;44:335-338. doi:10.1111/1346-8138.13523
- Gutierrez N, Erickson C, Calame A, et al. Solitary cutaneous focal mucinosis. Cureus. 2021;13:E18618. doi:10.7759/cureus.18618
- Biondo G, Sola S, Pastorino C, et al. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An Bras Dermatol. 2019;94:334-336. doi:10.1590/abd1806-4841.20198381
- Chen S, Huang H, He S, et al. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12:2613-2621.
- Bembem K, Jaiswal A, Singh M, et al. Cyto-histo correlation of a very rare tumor: superficial angiomyxoma. J Cytol. 2017;34:230-232. doi:10.4103/0970-9371.216119
- Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. 2016;42: 1014-1016. doi:10.1097/DSS.0000000000000782
The Diagnosis: Superficial Angiomyxoma
Superficial angiomyxoma is a rare, benign, cutaneous tumor of a myxoid matrix and blood vessels that was first described in association with Carney complex.1 Tumors may be solitary or multiple. A recent review of cases in the literature revealed a roughly equal distribution of superficial angiomyxomas in males and females occurring most frequently on the head and neck, extremities, and trunk or back. The peak incidence is between the fourth and fifth decades of life.2 Superficial angiomyxomas can occur sporadically or in association with Carney complex, an autosomal-dominant condition with germline inactivating mutations in protein kinase A, PRKAR1A. Interestingly, sporadic cases of superficial angiomyxoma also have shown loss of PRKAR1A expression on immunohistochemistry (IHC).3
Common histologic mimics of superficial angiomyxoma include aggressive angiomyxoma and angiomyofibroblastoma.4 It is thought that these 3 distinct tumor entities may arise from a common pluripotent cell of origin located near connective tissue vasculature, which may contribute to the similarities observed between them.5 For example, aggressive angiomyxomas and angiomyofibroblastomas also demonstrate a similar myxoid background and vascular proliferation that can closely mimic superficial angiomyxomas clinically. However, the vessels of superficial angiomyxomas tend to be long and thin walled, while aggressive angiomyxomas are characterized by large and thick-walled vessels and angiomyofibroblastomas by abundant smaller vessels. Additionally, unlike superficial angiomyxomas, both aggressive angiomyxomas and angiomyofibroblastomas typically occur in the genital tract of young to middle-aged women.6
Histopathologic examination is imperative for differentiating between superficial angiomyxoma and more aggressive histologic mimics. Superficial angiomyxomas typically consist of a rich myxoid stroma, thin-walled or arborizing blood vessels, and spindled to stellate fibroblastlike cells (quiz image 2).3 Although not prominent in our case, superficial angiomyxomas also frequently present with stromal neutrophils and epithelial components, including keratinous cysts, basaloid buds, and strands of squamous epithelium.7 Minimal cellular atypia, mitotic activity, and nuclear pleomorphism often are seen, with IHC negative for desmin, estrogen receptor, and progesterone receptor; positive for CD34 and smooth muscle actin; and variable for S-100 and muscle-specific actin. Although IHC has limited utility in the diagnosis of superficial angiomyxomas, it may be useful to rule out other differential diagnoses.2,3 Superficial angiomyxomas usually show fibroblastic stromal cells, proteoglycan matrix, and collagen fibers on electron microscopy.8 Importantly, histopathologic examination of aggressive angiomyxoma will comparatively present with more invasive, infiltrative, and less well-circumscribed tumors.9 Other differential diagnoses on histology may include neurofibroma, focal cutaneous mucinosis, spindle cell lipoma, and myxofibrosarcoma. Additional considerations include fibroepithelial polyp, nevus lipomatosis, angiomyxolipoma, and anetoderma.
An important differential diagnosis in the evaluation of superficial angiomyxoma is neurofibroma, a benign peripheral nerve sheath tumor that presents as a smooth, flesh-colored, and painless papule or nodule commonly associated with the buttonhole sign. Histopathology of neurofibroma features elongated spindle cells with comma-shaped or buckled wavy nuclei and variably sized collagen bundles described as “shredded carrots” (Figure 1).10 Occasional mast cells also can be seen. Immunohistochemistry targeting elements of peripheral nerve sheaths may assist in the diagnosis of neurofibromas, including positive S-100 and SOX10 in Schwann cells, epithelial membrane antigen in perineural cells, and fingerprint positivity for CD34 in fibroblasts.10

Cutaneous mucinoses encompass a diverse group of connective tissue disorders characterized by accumulation of mucin in the skin. Solitary focal cutaneous mucinoses (FCMs) are individual isolated lesions of mucin deposits that are unassociated with systemic conditions.11 Conversely, multiple FCMs presenting with multiple cutaneous lesions also have been described in association with systemic diseases such as scleroderma, systemic lupus erythematosus, and thyroid disease.12 Solitary FCM typically presents as an asymptomatic, flesh-colored papule or nodule on the extremities. It often arises in mid to late adulthood with a slightly increased frequency among males.12 Histopathology of solitary FCM commonly demonstrates a dome-shaped pool of basophilic mucin in the upper dermis sparing involvement of the underlying subcutaneous tissue (Figure 2).13 Notably, FCM often lacks the vascularity as well as stromal neutrophils and epithelial elements that are seen in superficial angiomyxomas. Although hematoxylin and eosin stains can be sufficient for diagnosis of solitary FCM, additional stains for mucin such as Alcian blue, colloidal iron, or toluidine blue also may be considered to support the diagnosis.12

Spindle cell lipomas (SCLs) are rare, benign, subcutaneous, adipocytic tumors that arise on the upper back, posterior neck, or shoulders of middle-aged or elderly adult males.14 The clinical presentation often is an asymptomatic, well-circumscribed, mobile subcutaneous mass that is firmer than a common lipoma. Histologically, SCLs are characterized by mature adipocytes, spindle cells, and wire or ropelike collagen fibers in a myxoid background (Figure 3). The spindle cells usually are bland with a notable bipolar shape and blunted ends. Infiltrative growth patterns or mitotic figures are uncommon. Diagnosis can be supported by IHC, as SCLs stain diffusely positive for CD34 with loss of the retinoblastoma protein.7

Another important differential diagnosis to consider is myxofibrosarcoma, a rare and malignant myxoid cutaneous tumor. Clinically, it presents asymptomatically as an indolent, slow-growing nodule on the limbs and limb girdles.7 Histopathologic features demonstrate a multilobular tumor composed of a mixture of hypocellular and hypercellular regions with incomplete fibrous septae (Figure 4). The presence of curvilinear vasculature is characteristic. Multinucleated giant cells and cellular atypia with nuclear pleomorphism also can be seen. Although IHC findings generally are not specific, they can be used to rule out other potential diagnoses. Myxofibrosarcomas stain positive for vimentin and occasionally smooth muscle actin, muscle-specific actin, and CD34.7

Superficial angiomyxomas are benign; however, excision is recommended to distinguish between mimics. Local recurrence after excision is common in 30% to 40% of patients.15 Mohs micrographic surgery has been considered, especially if the following are present: tumor characteristics (eg, poorly circumscribed), location (eg, head and neck or other cosmetically or functionally sensitive areas), and likelihood of recurrence (high for superficial angiomyxomas). 16 This case otherwise highlights a rare example of superficial angiomyxomas involving the buttocks.
The Diagnosis: Superficial Angiomyxoma
Superficial angiomyxoma is a rare, benign, cutaneous tumor of a myxoid matrix and blood vessels that was first described in association with Carney complex.1 Tumors may be solitary or multiple. A recent review of cases in the literature revealed a roughly equal distribution of superficial angiomyxomas in males and females occurring most frequently on the head and neck, extremities, and trunk or back. The peak incidence is between the fourth and fifth decades of life.2 Superficial angiomyxomas can occur sporadically or in association with Carney complex, an autosomal-dominant condition with germline inactivating mutations in protein kinase A, PRKAR1A. Interestingly, sporadic cases of superficial angiomyxoma also have shown loss of PRKAR1A expression on immunohistochemistry (IHC).3
Common histologic mimics of superficial angiomyxoma include aggressive angiomyxoma and angiomyofibroblastoma.4 It is thought that these 3 distinct tumor entities may arise from a common pluripotent cell of origin located near connective tissue vasculature, which may contribute to the similarities observed between them.5 For example, aggressive angiomyxomas and angiomyofibroblastomas also demonstrate a similar myxoid background and vascular proliferation that can closely mimic superficial angiomyxomas clinically. However, the vessels of superficial angiomyxomas tend to be long and thin walled, while aggressive angiomyxomas are characterized by large and thick-walled vessels and angiomyofibroblastomas by abundant smaller vessels. Additionally, unlike superficial angiomyxomas, both aggressive angiomyxomas and angiomyofibroblastomas typically occur in the genital tract of young to middle-aged women.6
Histopathologic examination is imperative for differentiating between superficial angiomyxoma and more aggressive histologic mimics. Superficial angiomyxomas typically consist of a rich myxoid stroma, thin-walled or arborizing blood vessels, and spindled to stellate fibroblastlike cells (quiz image 2).3 Although not prominent in our case, superficial angiomyxomas also frequently present with stromal neutrophils and epithelial components, including keratinous cysts, basaloid buds, and strands of squamous epithelium.7 Minimal cellular atypia, mitotic activity, and nuclear pleomorphism often are seen, with IHC negative for desmin, estrogen receptor, and progesterone receptor; positive for CD34 and smooth muscle actin; and variable for S-100 and muscle-specific actin. Although IHC has limited utility in the diagnosis of superficial angiomyxomas, it may be useful to rule out other differential diagnoses.2,3 Superficial angiomyxomas usually show fibroblastic stromal cells, proteoglycan matrix, and collagen fibers on electron microscopy.8 Importantly, histopathologic examination of aggressive angiomyxoma will comparatively present with more invasive, infiltrative, and less well-circumscribed tumors.9 Other differential diagnoses on histology may include neurofibroma, focal cutaneous mucinosis, spindle cell lipoma, and myxofibrosarcoma. Additional considerations include fibroepithelial polyp, nevus lipomatosis, angiomyxolipoma, and anetoderma.
An important differential diagnosis in the evaluation of superficial angiomyxoma is neurofibroma, a benign peripheral nerve sheath tumor that presents as a smooth, flesh-colored, and painless papule or nodule commonly associated with the buttonhole sign. Histopathology of neurofibroma features elongated spindle cells with comma-shaped or buckled wavy nuclei and variably sized collagen bundles described as “shredded carrots” (Figure 1).10 Occasional mast cells also can be seen. Immunohistochemistry targeting elements of peripheral nerve sheaths may assist in the diagnosis of neurofibromas, including positive S-100 and SOX10 in Schwann cells, epithelial membrane antigen in perineural cells, and fingerprint positivity for CD34 in fibroblasts.10

Cutaneous mucinoses encompass a diverse group of connective tissue disorders characterized by accumulation of mucin in the skin. Solitary focal cutaneous mucinoses (FCMs) are individual isolated lesions of mucin deposits that are unassociated with systemic conditions.11 Conversely, multiple FCMs presenting with multiple cutaneous lesions also have been described in association with systemic diseases such as scleroderma, systemic lupus erythematosus, and thyroid disease.12 Solitary FCM typically presents as an asymptomatic, flesh-colored papule or nodule on the extremities. It often arises in mid to late adulthood with a slightly increased frequency among males.12 Histopathology of solitary FCM commonly demonstrates a dome-shaped pool of basophilic mucin in the upper dermis sparing involvement of the underlying subcutaneous tissue (Figure 2).13 Notably, FCM often lacks the vascularity as well as stromal neutrophils and epithelial elements that are seen in superficial angiomyxomas. Although hematoxylin and eosin stains can be sufficient for diagnosis of solitary FCM, additional stains for mucin such as Alcian blue, colloidal iron, or toluidine blue also may be considered to support the diagnosis.12

Spindle cell lipomas (SCLs) are rare, benign, subcutaneous, adipocytic tumors that arise on the upper back, posterior neck, or shoulders of middle-aged or elderly adult males.14 The clinical presentation often is an asymptomatic, well-circumscribed, mobile subcutaneous mass that is firmer than a common lipoma. Histologically, SCLs are characterized by mature adipocytes, spindle cells, and wire or ropelike collagen fibers in a myxoid background (Figure 3). The spindle cells usually are bland with a notable bipolar shape and blunted ends. Infiltrative growth patterns or mitotic figures are uncommon. Diagnosis can be supported by IHC, as SCLs stain diffusely positive for CD34 with loss of the retinoblastoma protein.7

Another important differential diagnosis to consider is myxofibrosarcoma, a rare and malignant myxoid cutaneous tumor. Clinically, it presents asymptomatically as an indolent, slow-growing nodule on the limbs and limb girdles.7 Histopathologic features demonstrate a multilobular tumor composed of a mixture of hypocellular and hypercellular regions with incomplete fibrous septae (Figure 4). The presence of curvilinear vasculature is characteristic. Multinucleated giant cells and cellular atypia with nuclear pleomorphism also can be seen. Although IHC findings generally are not specific, they can be used to rule out other potential diagnoses. Myxofibrosarcomas stain positive for vimentin and occasionally smooth muscle actin, muscle-specific actin, and CD34.7

Superficial angiomyxomas are benign; however, excision is recommended to distinguish between mimics. Local recurrence after excision is common in 30% to 40% of patients.15 Mohs micrographic surgery has been considered, especially if the following are present: tumor characteristics (eg, poorly circumscribed), location (eg, head and neck or other cosmetically or functionally sensitive areas), and likelihood of recurrence (high for superficial angiomyxomas). 16 This case otherwise highlights a rare example of superficial angiomyxomas involving the buttocks.
- Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12:519-530. doi:10.1097 /00000478-198807000-00003
- Sharma A, Khaitan N, Ko JS, et al. A clinicopathologic analysis of 54 cases of cutaneous myxoma. Hum Pathol. 2021:S0046-8177(21) 00201-X. doi:10.1016/j.humpath.2021.12.003
- Hafeez F, Krakowski AC, Lian CG, et al. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression [published online March 17, 2022]. Histopathology. 2022;80:1001-1003. doi:10.1111/his.14568
- Mehrotra K, Bhandari M, Khullar G, et al. Large superficial angiomyxoma of the vulva: report of two cases with varied clinical presentation. Indian Dermatol Online J. 2021;12:605-607. doi:10.4103/idoj.IDOJ_489_20
- Alameda F, Munné A, Baró T, et al. Vulvar angiomyxoma, aggressive angiomyxoma, and angiomyofibroblastoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193-205. doi:10.1080/01913120500520911
- Haroon S, Irshad L, Zia S, et al. Aggressive angiomyxoma, angiomyofibroblastoma, and cellular angiofibroma of the lower female genital tract: related entities with different outcomes. Cureus. 2022;14:E29250. doi:10.7759/cureus.29250
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918. doi:10.1111/cup.12749
- Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4:99-123. doi:10.1016 /s1092-9134(00)90019-4
- Lee C-C, Chen Y-L, Liau J-Y, et al. Superficial angiomyxoma on the vulva of an adolescent. Taiwan J Obstet Gynecol. 2014;53:104-106. doi:10.1016/j.tjog.2013.08.001
- Magro G, Amico P, Vecchio GM, et al. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: a clinicopathologic study of 94 cases. Virchows Arch. 2010;456:71-76. doi:10.1007/s00428-009-0859-y
- Kuo KL, Lee LY, Kuo TT. Solitary cutaneous focal mucinosis: a clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J Dermatol. 2017;44:335-338. doi:10.1111/1346-8138.13523
- Gutierrez N, Erickson C, Calame A, et al. Solitary cutaneous focal mucinosis. Cureus. 2021;13:E18618. doi:10.7759/cureus.18618
- Biondo G, Sola S, Pastorino C, et al. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An Bras Dermatol. 2019;94:334-336. doi:10.1590/abd1806-4841.20198381
- Chen S, Huang H, He S, et al. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12:2613-2621.
- Bembem K, Jaiswal A, Singh M, et al. Cyto-histo correlation of a very rare tumor: superficial angiomyxoma. J Cytol. 2017;34:230-232. doi:10.4103/0970-9371.216119
- Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. 2016;42: 1014-1016. doi:10.1097/DSS.0000000000000782
- Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12:519-530. doi:10.1097 /00000478-198807000-00003
- Sharma A, Khaitan N, Ko JS, et al. A clinicopathologic analysis of 54 cases of cutaneous myxoma. Hum Pathol. 2021:S0046-8177(21) 00201-X. doi:10.1016/j.humpath.2021.12.003
- Hafeez F, Krakowski AC, Lian CG, et al. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression [published online March 17, 2022]. Histopathology. 2022;80:1001-1003. doi:10.1111/his.14568
- Mehrotra K, Bhandari M, Khullar G, et al. Large superficial angiomyxoma of the vulva: report of two cases with varied clinical presentation. Indian Dermatol Online J. 2021;12:605-607. doi:10.4103/idoj.IDOJ_489_20
- Alameda F, Munné A, Baró T, et al. Vulvar angiomyxoma, aggressive angiomyxoma, and angiomyofibroblastoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193-205. doi:10.1080/01913120500520911
- Haroon S, Irshad L, Zia S, et al. Aggressive angiomyxoma, angiomyofibroblastoma, and cellular angiofibroma of the lower female genital tract: related entities with different outcomes. Cureus. 2022;14:E29250. doi:10.7759/cureus.29250
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918. doi:10.1111/cup.12749
- Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4:99-123. doi:10.1016 /s1092-9134(00)90019-4
- Lee C-C, Chen Y-L, Liau J-Y, et al. Superficial angiomyxoma on the vulva of an adolescent. Taiwan J Obstet Gynecol. 2014;53:104-106. doi:10.1016/j.tjog.2013.08.001
- Magro G, Amico P, Vecchio GM, et al. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: a clinicopathologic study of 94 cases. Virchows Arch. 2010;456:71-76. doi:10.1007/s00428-009-0859-y
- Kuo KL, Lee LY, Kuo TT. Solitary cutaneous focal mucinosis: a clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J Dermatol. 2017;44:335-338. doi:10.1111/1346-8138.13523
- Gutierrez N, Erickson C, Calame A, et al. Solitary cutaneous focal mucinosis. Cureus. 2021;13:E18618. doi:10.7759/cureus.18618
- Biondo G, Sola S, Pastorino C, et al. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An Bras Dermatol. 2019;94:334-336. doi:10.1590/abd1806-4841.20198381
- Chen S, Huang H, He S, et al. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12:2613-2621.
- Bembem K, Jaiswal A, Singh M, et al. Cyto-histo correlation of a very rare tumor: superficial angiomyxoma. J Cytol. 2017;34:230-232. doi:10.4103/0970-9371.216119
- Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. 2016;42: 1014-1016. doi:10.1097/DSS.0000000000000782
A 25-year-old woman presented with an irritated growth on the left buttock of 6 months’ duration. The lesion had grown slowly over time and became irritated because of the constant rubbing on her clothing due to its location. Physical examination revealed a 1-cm, pink, protuberant, soft, dome-shaped nodule on the left upper medial buttock (inset). A biopsy was performed for diagnostic purposes.
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Severe Esophageal Lichen Planus Treated With Tofacitinib
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.
Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.
Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.
The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.
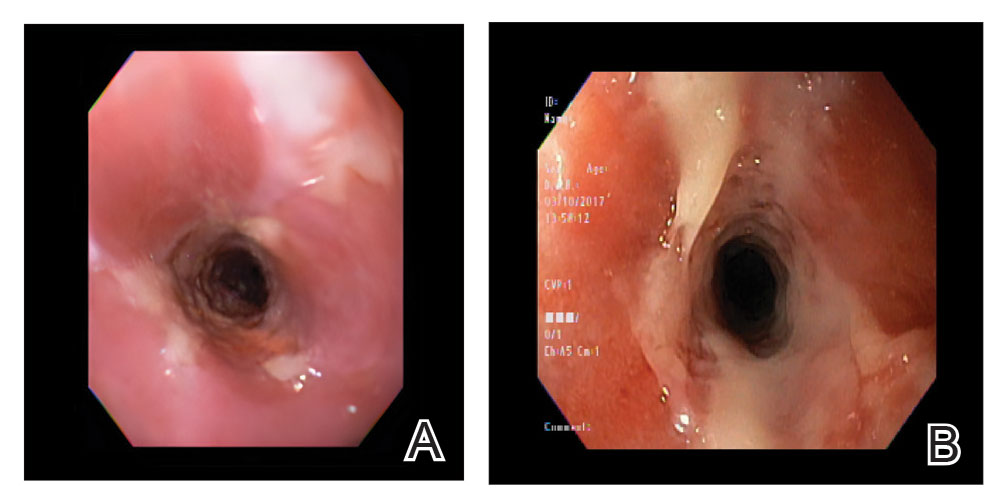
The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.
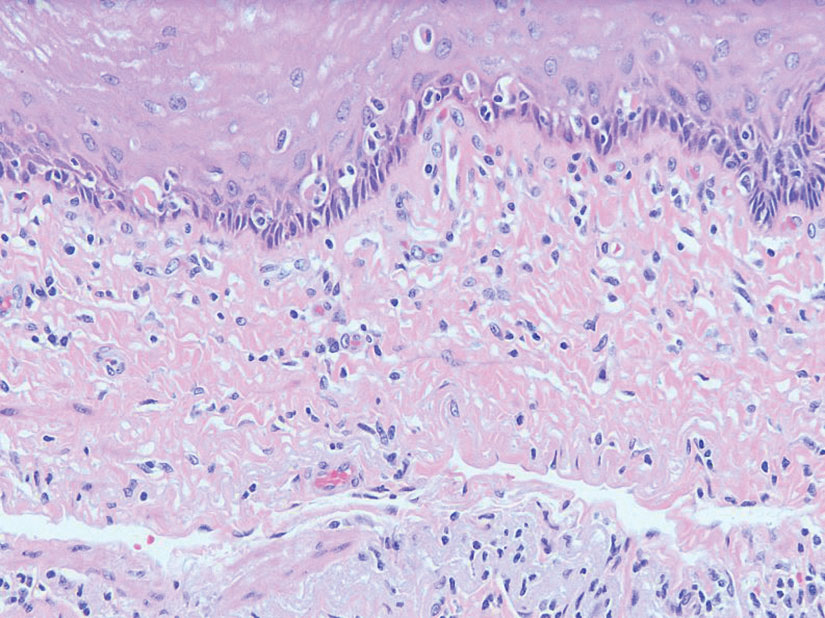
Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.
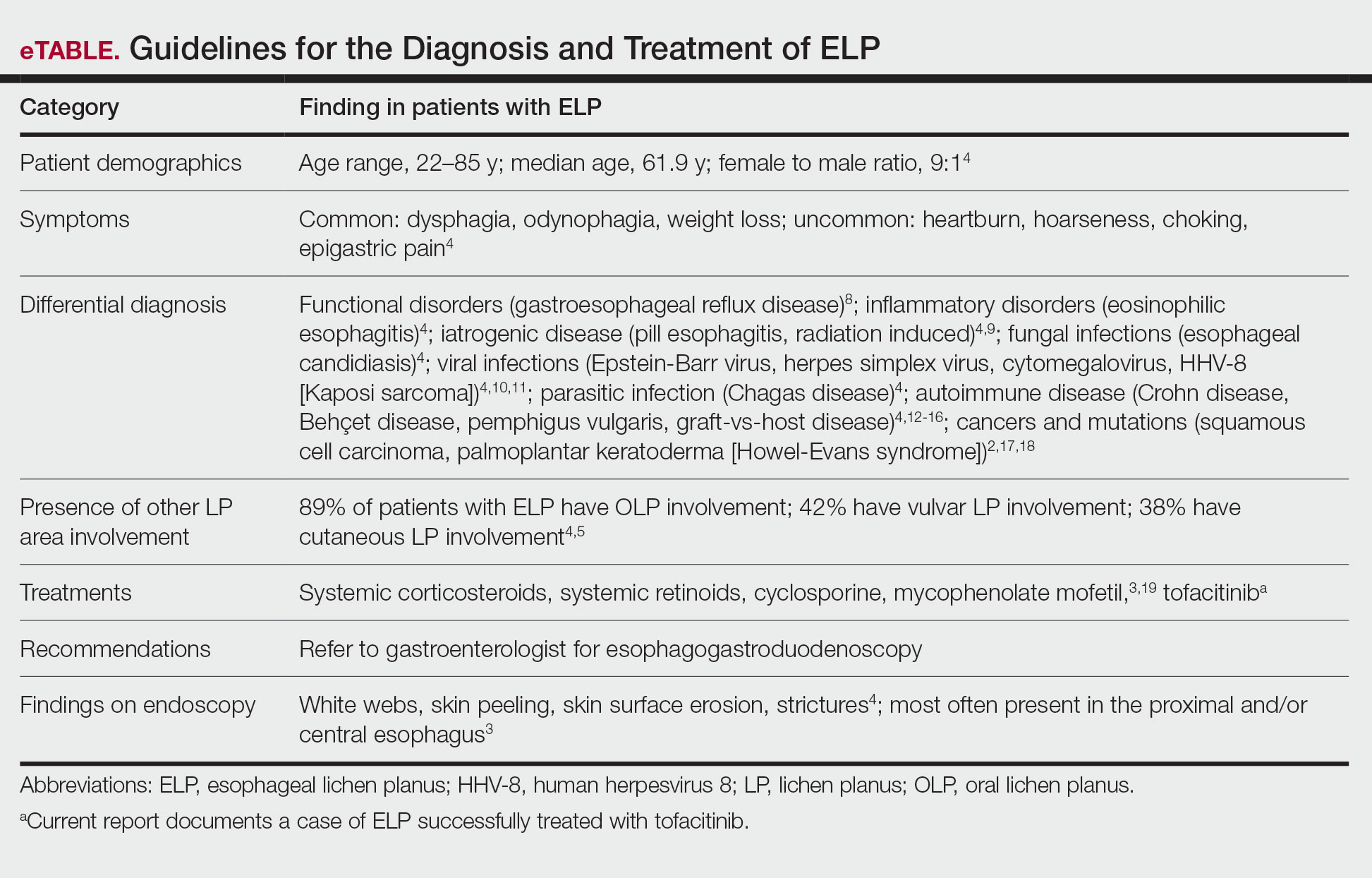
Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.

Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.

Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.

The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.

The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.

Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.

Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.

Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.

Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.

The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.

The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.

Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.

Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
Practice Points
- Patients diagnosed with lichen planus should be informed about the signs of esophageal lichen planus (ELP).
- Twenty-five percent to 50% of patients with oral lichen planus (OLP) have been shown to have concomitant ELP.
- Esophageal lichen planus may be asymptomatic and often is misdiagnosed.
- Tofacitinib should be considered for the treatment of ELP, OLP, and cutaneous lichen planus.
Characterization of Blood-borne Pathogen Exposures During Dermatologic Procedures: The Mayo Clinic Experience
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).
Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.
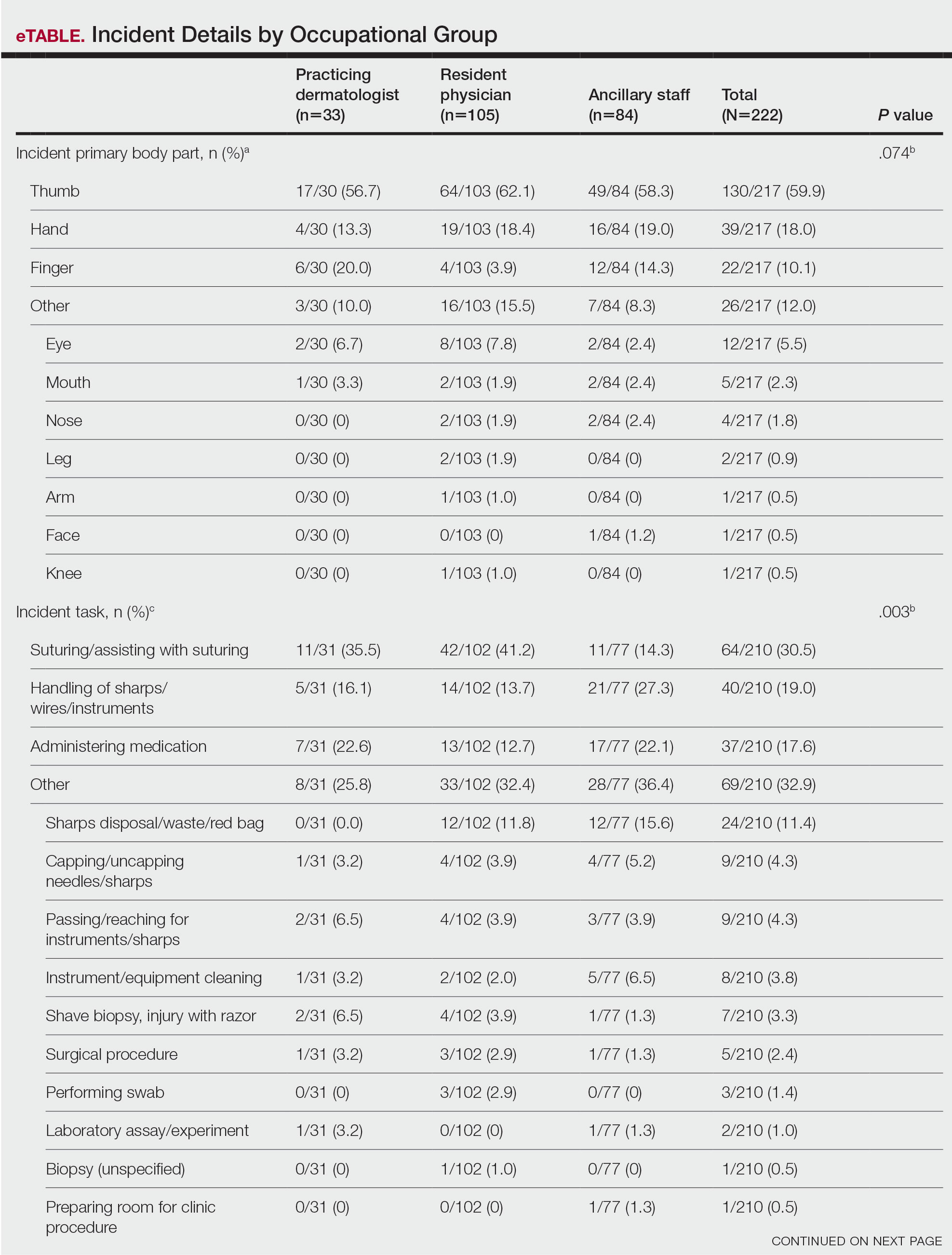
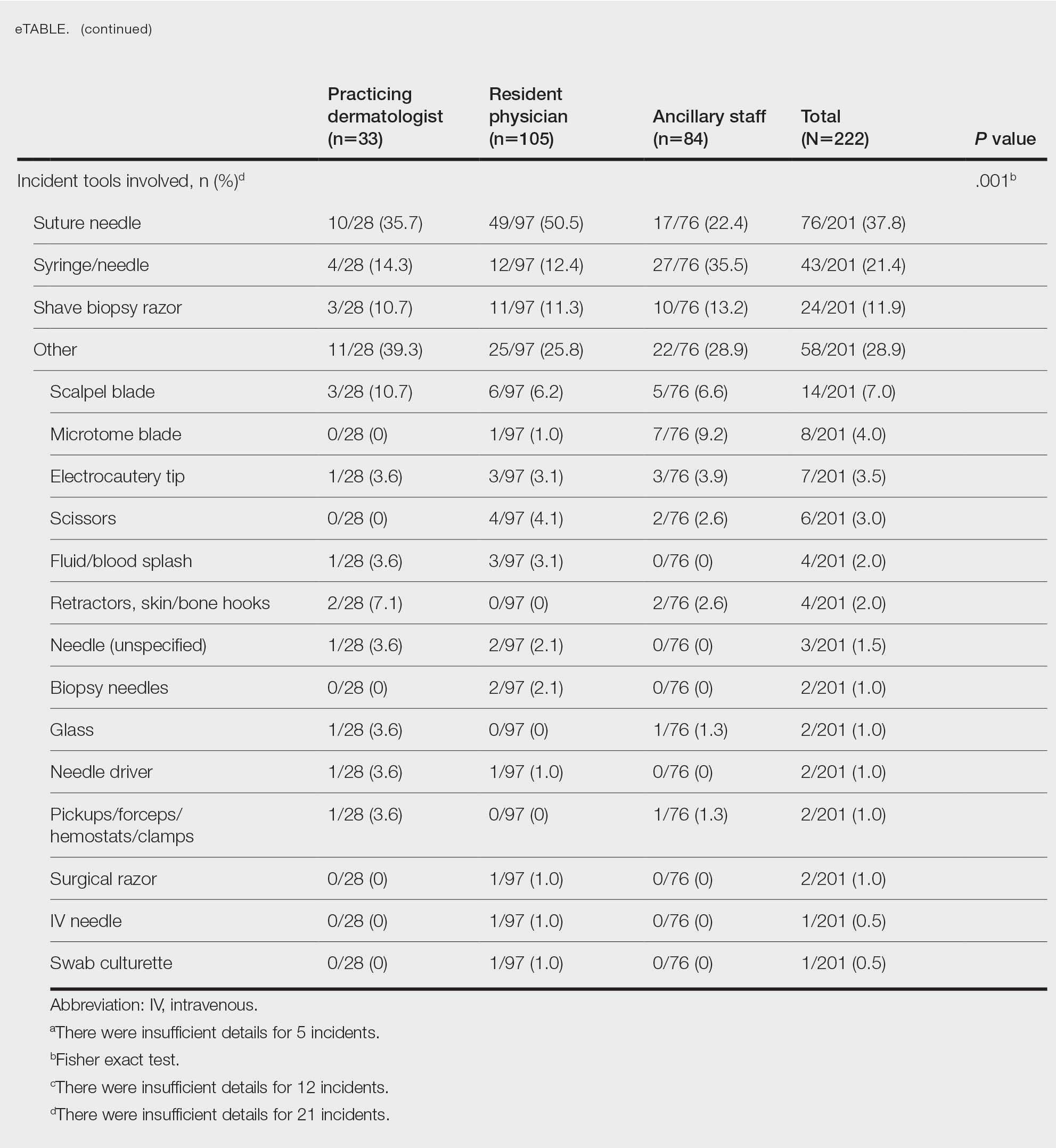
Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Practice Points
- Most blood-borne pathogen (BBP) exposures in dermatologic staff occur due to medical sharps as opposed to splash incidents.
- The most common implicated task in resident physicians and practicing dermatologists is suturing or assisting with suturing, and the most commonly associated instrument is the suture needle. In contrast, ancillary staff experience most BBP exposures during handling of sharps, wires, or instruments, and the injection syringe/needle is the most common instrument of injury.
- Quality improvement measures are needed in prevention of BBP exposures and should focus on identified risk factors among occupational groups in the workplace.
Two FDA clearances add diabetes technology options
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.