User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
What Skin Manifestations Are Associated With Pediatric IBD?
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Company Announces Regulatory Filing for Nemolizumab for Two Indications
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
On February 14, 2024, Galderma announced that .
A first-in-class investigational monoclonal antibody specifically designed to inhibit interleukin (IL) IL-31 signaling, nemolizumab has also been granted FDA Priority Review for prurigo nodularis, according to a press release from the company. The European Medicines Agency has also accepted Galderma’s Marketing Authorization Applications for nemolizumab for both prurigo nodularis and atopic dermatitis.
The regulatory developments follow data from the phase III OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in patients with prurigo nodularis (NCT04501679 and NCT04501666). According to the press release, in OLYMPIA 1 and 2, 58% and 56% of patients, respectively, achieved at least a least four-point reduction in itch intensity as measured by the peak-pruritus numerical rating scale (PP-NRS), compared with 17% and 21% in the placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the investigator’s global assessment (IGA) score, compared with 7% and 11% in the placebo groups (P < .0001).
FDA Approves Drug to Reduce Accidental Food Allergies
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved omalizumab (Xolair, Genentech) for reducing allergic reactions to foods in adults and most children. The drug is meant to be taken regularly by patients with food allergies to reduce the risk for reactions, including anaphylaxis, in case of accidental exposure to one or more allergens. The injection is not approved for emergency treatment of an allergic reaction.
Omalizumab first was approved for persistent allergic asthma in 2003. It also is approved for chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.
, the FDA said. Peanut-allergen powder (Palforzia) can reduce reactions to peanut, but its benefits are limited to that allergy.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs,” said Kelly Stone, MD, PhD, associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a news release.
The safety and efficacy of the monoclonal antibody in reducing allergic reactions was studied in a double-blind, placebo-controlled study of 168 children and adults who were allergic to peanut and at least two other foods, including milk, egg, wheat, cashew, hazelnut, or walnut. Patients received omalizumab or placebo for 16-20 weeks. At the end of the study, patients consumed peanut protein (equivalent to 2.5 peanuts). Of those who received the drug, 68% were able to consume peanut without moderate or severe allergic symptoms, versus 6% in the placebo group.
More patients who received the medication also avoided moderate or severe reactions to cashews (42% vs 3%), milk (66% vs 11%), and eggs (67% vs 0%).
The most common side effects of omalizumab included injection site reactions and fever. The drug’s label includes warnings and precautions about anaphylaxis, cancer, fever, joint pain, rash, parasitic (worm) infection, and abnormal laboratory tests. Omalizumab comes with a boxed warning for anaphylaxis and should be started only in a healthcare setting equipped to manage anaphylaxis, according to the FDA.
A version of this article appeared on Medscape.com.
An 8-Year-Old Male With Asymptomatic Brown Rough Plaques on the Dorsum of the Right Hand and Fingers, Accompanied by Widening of the Knuckles
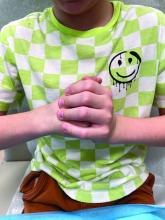
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
During examination, the patient was observed repetitively cracking his knuckles, making a fist with the right hand, placing the left hand on top, and rubbing the hand, a behavior he routinely did multiple times daily. The observed pattern of finger involvement on the dorsum of the right hand corresponded to areas subjected to significant pressure during the described activity. Consequently, a diagnosis of lichen simplex chronicus (LSC) secondary to mechanical rubbing, along with associated pachydermodactyly on the fingers of the right hand, was established.
Lichen simplex chronicus and pachydermodactyly are both attributed to microtrauma inflicted upon the skin. Lichen simplex chronicus often constitutes a diagnosis of exclusion and is characterized by repetitive trauma-induced keratinocyte proliferation and melanocyte activation, resulting in hyperpigmentation and skin thickening. Although typically observed in women between the fourth and fifth decades of life, LSC is rarely reported in children. In adults, LSC-related rubbing or scratching frequently arises from chronic pruritic dermatitis such as eczema or psoriasis, neurodermatitis from dysesthesia, or habitual movements, as exhibited by this young patient. While generally benign, LSC may become infected. In rare instances, malignant transformation may occur.
The association with pachydermodactyly implicates microtrauma, necessitating careful observation and questioning to elucidate the cause, as demonstrated in this case. Lesions are typically hyperpigmented, though cases of associated hypopigmentation or depigmentation have been documented. Affected areas typically fall within the patient’s hand and finger reach, with lesion improvement over several months achievable through trigger avoidance.
Pachydermodactyly, a rare but benign fibromatosis around the proximal interphalangeal joints, is often misdiagnosed as juvenile idiopathic arthritis, potentially leading to unnecessary treatments and patient anxiety. Microtrauma history due to digit manipulation is prevalent among affected individuals, with most also exhibiting neuropsychiatric disorders. Histological examination of pachydermodactyly reveals hypergranulosis and dermal thickening, accompanied by increased fibroblasts and collagen types I, III, and V, differing from the epidermal changes seen in LSC.
The differential diagnosis also included phytophotodermatitis, a phototoxic dermatologic reaction following exposure to ultraviolet light subsequent to contact with furocoumarin-containing plant chemicals. However, the persistence of the patient’s lesions for over a year precluded this diagnosis. Secondary hyperpigmentation was also contemplated but excluded due to the absence of preceding inflammatory dermatitis.
Treatment of LSC primarily involves identifying and addressing any underlying conditions, repairing the skin barrier, reducing inflammation, and modifying behaviors contributing to chronic microtrauma, as observed in this patient. Topical corticosteroids may aid in decreasing epidermal thickening and discoloration, though lesion resolution necessitates behavior cessation.
It’s important to identify these types of skin changes in children to avoid unnecessary medical treatments for these benign conditions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
Seier JA, Dissemond J. Lichen Simplex Chronicus Due to Mechanical Irritation. Dtsch Arztebl Int. 2022 Nov 18;119(46):802. doi: 10.3238/arztebl.m2022.0213.
Small S et al. A 12-Year-Old Boy Presenting With Unilateral Proximal Interphalangeal Joint Swelling. BMJ Case Rep. 2011 Apr 13:2011:bcr0120113719. doi: 10.1136/bcr.01.2011.3719.
Voicu C et al Lichen Simplex Chronicus as an Essential Part of the Dermatologic Masquerade. Open Access Maced J Med Sci. 2017 Jul 24;5(4):556-557. doi: 10.3889/oamjms.2017.133.
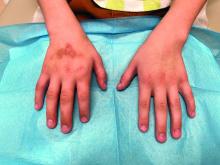
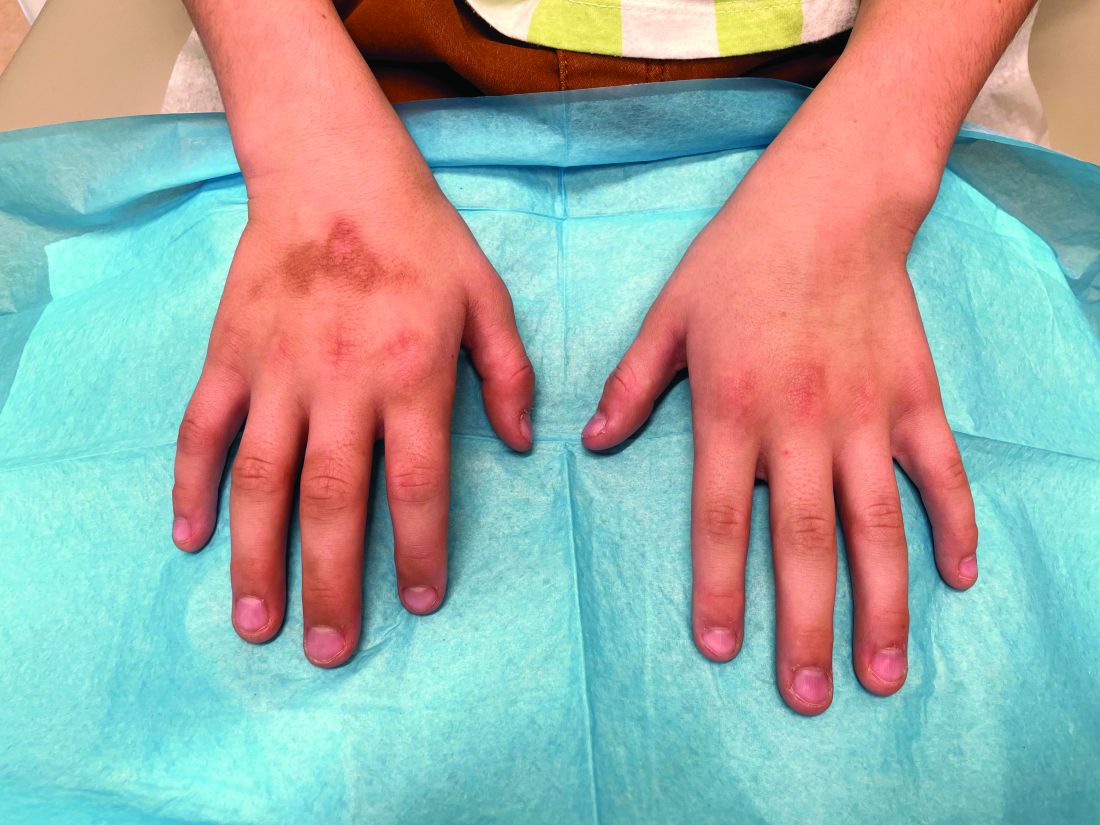
The patient was otherwise healthy, with no current medication intake, and he engaged in baseball and soccer activities. Upon physical examination, a hyperpigmented lichenified irregular plaque was observed on the dorsum of the right hand, along with irregular hyperpigmented macules and plaques on the fingers. Fusiform widening of the interphalangeal joints on the second, third, and fourth fingers of the right hand was noted, without associated pain, edema, or erythema.
Parent-Led Digital CBT Effective for Childhood Anxiety
while substantially reducing cost and therapist time, new research showed.
In a randomized controlled trial, children participating in the program Online Support and Intervention (OSI) for Child Anxiety showed similar reductions in anxiety and improvements in daily functioning as peers receiving standard CBT.
“This study shows that by making the most of digital tools, we can deliver effective treatments more efficiently, helping services to better meet the growing demand for mental health services for children in ways that can also be more accessible for many families,” lead investigator Cathy Creswell, PhD, Departments of Experimental Psychology and Psychiatry, Oxford University, Oxford, England, told this news organization.
“I believe by incorporating this approach into standard care, we could address some of the major challenges faced by services and families,” Dr. Creswell added. “We are now moving the work out of the research environment into routine practice.”
The study was published online in The Lancet Psychiatry
Care Gaps for Common Problem
Anxiety is common in children, yet gaps exist between needed and available care, which investigators say could be filled by digitally augmented psychological treatments.
OSI, the digital platform used in the current study, was designed with therapists and families to aid parents in helping their children overcome problems with anxiety with remote therapist support.
The program provides parents with the core CBT content in accessible forms, including information in text, audio, and video and exercises supported by worksheets and quizzes.
There is also an optional child game app to help motivate the child to engage with the intervention. Parents are supported with weekly brief telephone or video call sessions with the therapist.
The two-arm randomized controlled non-inferiority trial included 444 families from 34 participating Child and Adolescent Mental Health Services (CAMHS) sites in England and Northern Ireland. Half received OSI plus therapist support and half CAMHS treatment as usual. The children were between 5 and 12 years old.
A total of 176 (79%) participants in the OSI plus therapist support group and 164 (74%) in the treatment as usual group completed the 26-week assessment.
‘Compelling’ Evidence
The primary clinical outcome was parent-reported interference caused by child anxiety at 26 weeks, using the Child Anxiety Impact Scale-Parent report.
On this measure, OSI plus therapist support was non-inferior to usual treatment, with a standardized mean difference of only 0.01 (95% CI, −0.15 to 0.17; P < .0001).
The intervention was also significantly non-inferior to usual treatment across all secondary outcomes, including total anxiety and depression scores, overall functioning, peer relationship problems, and prosocial behavior.
In addition, OSI plus therapist support was associated with nearly 60% less therapist time (182 min on average vs 307 min) and with lower costs than standard treatment. The OSI program was “likely to be cost-effective under several scenarios,” the researchers reported. Qualitative interviews showed “good” acceptability of the online program.
“This trial presents compelling clinical evidence and promising cost-effectiveness evidence that digitally augmented psychological therapies with therapist support can increase efficiencies in and access to child mental health services without compromising patient outcomes,” Dr. Creswell and colleagues concluded.
“Efforts are now needed to take full advantage of the opportunity that digitally augmented psychological treatments can bring to drive a step change in children’s mental health services, learning from successful examples of digital implementation elsewhere in health services,” they added.
‘Call to Action’
“We desperately need more trials” like this one, which showed the “clear value of a digitally augmented intervention over the usual face-to-face treatment” for child anxiety, wrote the authors of an accompanying editorial.
“Moreover, with the intervention delivered across 34 CAMHS settings in England and Northern Ireland, this study gives us confidence that the new intervention is effective in a range of clinical contexts at least in high-income countries and offers invaluable information about barriers and facilitators to future implementation,” wrote Sam Cartwright-Hatton, PhD, with the University of Sussex, Brighton and Hove, and Abby Dunn, PhD, with the University of Surrey, Guilford, England. “The potential benefits to overburdened services are clear.”
“That regular CAMHS clinicians, with minimal training and support from researchers, delivered the intervention within their standard caseload shows that it can be embedded within routine practice without a requirement for highly prepared and supervised clinicians,” they added.
However, more information is needed on the content and quality of the traditional CBT provided in the control group. It’s also important to determine if the program would be as effective with even less clinical support and in all types of childhood anxiety.
In addition, most clinicians in the OSI group only treated one patient with the new program and didn’t take advantage of the additional support offered by the research team, “which means we have not seen the true effectiveness of this intervention in the hands of well-practiced and well-trained staff,” Drs. Cartwright-Hatton and Dunn wrote.
Analyses included recruitment at the lower target amount, and one fifth of children were not offered treatment within the 12-week window recommended in the trial, they added.
“Although these issues place limits on the conclusions that can be drawn, they should also be seen as a call to action,” they wrote, adding that real-world clinical trials with greater clinician participation are needed. “All credit to this exceptional team for making this trial happen and for making it work as well as it did.”
The study was funded by the Department for Health and Social Care, UK Research and Innovation Research Grant, National Institute for Health and Care (NIHR) Research Policy Research Programme, Oxford and Thames Valley NIHR Applied Research Collaboration, and Oxford Health NIHR Biomedical Research Centre. Dr. Creswell is co-developer of the OSI platform and the author of a book for parents that is used in many of the participating clinical teams to augment treatment as usual for child anxiety problems and receives royalties from sales. Dr. Cartwright-Hatton and Dr. Dunn had no disclosures.
A version of this article appeared on Medscape.com.
while substantially reducing cost and therapist time, new research showed.
In a randomized controlled trial, children participating in the program Online Support and Intervention (OSI) for Child Anxiety showed similar reductions in anxiety and improvements in daily functioning as peers receiving standard CBT.
“This study shows that by making the most of digital tools, we can deliver effective treatments more efficiently, helping services to better meet the growing demand for mental health services for children in ways that can also be more accessible for many families,” lead investigator Cathy Creswell, PhD, Departments of Experimental Psychology and Psychiatry, Oxford University, Oxford, England, told this news organization.
“I believe by incorporating this approach into standard care, we could address some of the major challenges faced by services and families,” Dr. Creswell added. “We are now moving the work out of the research environment into routine practice.”
The study was published online in The Lancet Psychiatry
Care Gaps for Common Problem
Anxiety is common in children, yet gaps exist between needed and available care, which investigators say could be filled by digitally augmented psychological treatments.
OSI, the digital platform used in the current study, was designed with therapists and families to aid parents in helping their children overcome problems with anxiety with remote therapist support.
The program provides parents with the core CBT content in accessible forms, including information in text, audio, and video and exercises supported by worksheets and quizzes.
There is also an optional child game app to help motivate the child to engage with the intervention. Parents are supported with weekly brief telephone or video call sessions with the therapist.
The two-arm randomized controlled non-inferiority trial included 444 families from 34 participating Child and Adolescent Mental Health Services (CAMHS) sites in England and Northern Ireland. Half received OSI plus therapist support and half CAMHS treatment as usual. The children were between 5 and 12 years old.
A total of 176 (79%) participants in the OSI plus therapist support group and 164 (74%) in the treatment as usual group completed the 26-week assessment.
‘Compelling’ Evidence
The primary clinical outcome was parent-reported interference caused by child anxiety at 26 weeks, using the Child Anxiety Impact Scale-Parent report.
On this measure, OSI plus therapist support was non-inferior to usual treatment, with a standardized mean difference of only 0.01 (95% CI, −0.15 to 0.17; P < .0001).
The intervention was also significantly non-inferior to usual treatment across all secondary outcomes, including total anxiety and depression scores, overall functioning, peer relationship problems, and prosocial behavior.
In addition, OSI plus therapist support was associated with nearly 60% less therapist time (182 min on average vs 307 min) and with lower costs than standard treatment. The OSI program was “likely to be cost-effective under several scenarios,” the researchers reported. Qualitative interviews showed “good” acceptability of the online program.
“This trial presents compelling clinical evidence and promising cost-effectiveness evidence that digitally augmented psychological therapies with therapist support can increase efficiencies in and access to child mental health services without compromising patient outcomes,” Dr. Creswell and colleagues concluded.
“Efforts are now needed to take full advantage of the opportunity that digitally augmented psychological treatments can bring to drive a step change in children’s mental health services, learning from successful examples of digital implementation elsewhere in health services,” they added.
‘Call to Action’
“We desperately need more trials” like this one, which showed the “clear value of a digitally augmented intervention over the usual face-to-face treatment” for child anxiety, wrote the authors of an accompanying editorial.
“Moreover, with the intervention delivered across 34 CAMHS settings in England and Northern Ireland, this study gives us confidence that the new intervention is effective in a range of clinical contexts at least in high-income countries and offers invaluable information about barriers and facilitators to future implementation,” wrote Sam Cartwright-Hatton, PhD, with the University of Sussex, Brighton and Hove, and Abby Dunn, PhD, with the University of Surrey, Guilford, England. “The potential benefits to overburdened services are clear.”
“That regular CAMHS clinicians, with minimal training and support from researchers, delivered the intervention within their standard caseload shows that it can be embedded within routine practice without a requirement for highly prepared and supervised clinicians,” they added.
However, more information is needed on the content and quality of the traditional CBT provided in the control group. It’s also important to determine if the program would be as effective with even less clinical support and in all types of childhood anxiety.
In addition, most clinicians in the OSI group only treated one patient with the new program and didn’t take advantage of the additional support offered by the research team, “which means we have not seen the true effectiveness of this intervention in the hands of well-practiced and well-trained staff,” Drs. Cartwright-Hatton and Dunn wrote.
Analyses included recruitment at the lower target amount, and one fifth of children were not offered treatment within the 12-week window recommended in the trial, they added.
“Although these issues place limits on the conclusions that can be drawn, they should also be seen as a call to action,” they wrote, adding that real-world clinical trials with greater clinician participation are needed. “All credit to this exceptional team for making this trial happen and for making it work as well as it did.”
The study was funded by the Department for Health and Social Care, UK Research and Innovation Research Grant, National Institute for Health and Care (NIHR) Research Policy Research Programme, Oxford and Thames Valley NIHR Applied Research Collaboration, and Oxford Health NIHR Biomedical Research Centre. Dr. Creswell is co-developer of the OSI platform and the author of a book for parents that is used in many of the participating clinical teams to augment treatment as usual for child anxiety problems and receives royalties from sales. Dr. Cartwright-Hatton and Dr. Dunn had no disclosures.
A version of this article appeared on Medscape.com.
while substantially reducing cost and therapist time, new research showed.
In a randomized controlled trial, children participating in the program Online Support and Intervention (OSI) for Child Anxiety showed similar reductions in anxiety and improvements in daily functioning as peers receiving standard CBT.
“This study shows that by making the most of digital tools, we can deliver effective treatments more efficiently, helping services to better meet the growing demand for mental health services for children in ways that can also be more accessible for many families,” lead investigator Cathy Creswell, PhD, Departments of Experimental Psychology and Psychiatry, Oxford University, Oxford, England, told this news organization.
“I believe by incorporating this approach into standard care, we could address some of the major challenges faced by services and families,” Dr. Creswell added. “We are now moving the work out of the research environment into routine practice.”
The study was published online in The Lancet Psychiatry
Care Gaps for Common Problem
Anxiety is common in children, yet gaps exist between needed and available care, which investigators say could be filled by digitally augmented psychological treatments.
OSI, the digital platform used in the current study, was designed with therapists and families to aid parents in helping their children overcome problems with anxiety with remote therapist support.
The program provides parents with the core CBT content in accessible forms, including information in text, audio, and video and exercises supported by worksheets and quizzes.
There is also an optional child game app to help motivate the child to engage with the intervention. Parents are supported with weekly brief telephone or video call sessions with the therapist.
The two-arm randomized controlled non-inferiority trial included 444 families from 34 participating Child and Adolescent Mental Health Services (CAMHS) sites in England and Northern Ireland. Half received OSI plus therapist support and half CAMHS treatment as usual. The children were between 5 and 12 years old.
A total of 176 (79%) participants in the OSI plus therapist support group and 164 (74%) in the treatment as usual group completed the 26-week assessment.
‘Compelling’ Evidence
The primary clinical outcome was parent-reported interference caused by child anxiety at 26 weeks, using the Child Anxiety Impact Scale-Parent report.
On this measure, OSI plus therapist support was non-inferior to usual treatment, with a standardized mean difference of only 0.01 (95% CI, −0.15 to 0.17; P < .0001).
The intervention was also significantly non-inferior to usual treatment across all secondary outcomes, including total anxiety and depression scores, overall functioning, peer relationship problems, and prosocial behavior.
In addition, OSI plus therapist support was associated with nearly 60% less therapist time (182 min on average vs 307 min) and with lower costs than standard treatment. The OSI program was “likely to be cost-effective under several scenarios,” the researchers reported. Qualitative interviews showed “good” acceptability of the online program.
“This trial presents compelling clinical evidence and promising cost-effectiveness evidence that digitally augmented psychological therapies with therapist support can increase efficiencies in and access to child mental health services without compromising patient outcomes,” Dr. Creswell and colleagues concluded.
“Efforts are now needed to take full advantage of the opportunity that digitally augmented psychological treatments can bring to drive a step change in children’s mental health services, learning from successful examples of digital implementation elsewhere in health services,” they added.
‘Call to Action’
“We desperately need more trials” like this one, which showed the “clear value of a digitally augmented intervention over the usual face-to-face treatment” for child anxiety, wrote the authors of an accompanying editorial.
“Moreover, with the intervention delivered across 34 CAMHS settings in England and Northern Ireland, this study gives us confidence that the new intervention is effective in a range of clinical contexts at least in high-income countries and offers invaluable information about barriers and facilitators to future implementation,” wrote Sam Cartwright-Hatton, PhD, with the University of Sussex, Brighton and Hove, and Abby Dunn, PhD, with the University of Surrey, Guilford, England. “The potential benefits to overburdened services are clear.”
“That regular CAMHS clinicians, with minimal training and support from researchers, delivered the intervention within their standard caseload shows that it can be embedded within routine practice without a requirement for highly prepared and supervised clinicians,” they added.
However, more information is needed on the content and quality of the traditional CBT provided in the control group. It’s also important to determine if the program would be as effective with even less clinical support and in all types of childhood anxiety.
In addition, most clinicians in the OSI group only treated one patient with the new program and didn’t take advantage of the additional support offered by the research team, “which means we have not seen the true effectiveness of this intervention in the hands of well-practiced and well-trained staff,” Drs. Cartwright-Hatton and Dunn wrote.
Analyses included recruitment at the lower target amount, and one fifth of children were not offered treatment within the 12-week window recommended in the trial, they added.
“Although these issues place limits on the conclusions that can be drawn, they should also be seen as a call to action,” they wrote, adding that real-world clinical trials with greater clinician participation are needed. “All credit to this exceptional team for making this trial happen and for making it work as well as it did.”
The study was funded by the Department for Health and Social Care, UK Research and Innovation Research Grant, National Institute for Health and Care (NIHR) Research Policy Research Programme, Oxford and Thames Valley NIHR Applied Research Collaboration, and Oxford Health NIHR Biomedical Research Centre. Dr. Creswell is co-developer of the OSI platform and the author of a book for parents that is used in many of the participating clinical teams to augment treatment as usual for child anxiety problems and receives royalties from sales. Dr. Cartwright-Hatton and Dr. Dunn had no disclosures.
A version of this article appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
Urologist Sues Health System Over Noncompete Clause
The lawsuit brings renewed attention to the ongoing public discourse around restrictive covenants for physicians as more state and federal legislators signal plans to limit or ban the practice.
According to a civil suit filed on January 30 in the Court of Common Pleas, Scranton, Pennsylvania, Eric Rottenberg, MD, signed a 3-year employment agreement with Commonwealth Health Physician Network (CPN) in November 2022. He worked for the health system from May to November 2023, seeing patients at several of its locations, including Wilkes-Barre General Hospital and other facilities throughout northeast and central Pennsylvania.
Although Dr. Rottenberg previously practiced in Albany, New York, court records state he did not bring a significant referral or patient base to the new role, receive any specialized training, or have knowledge of CPN’s trade secrets during his tenure.
Instead, he was a “9-to-5 practitioner,” or a physician-employee like a “locum tenens whose replacement would not cost the employer more than his traditional compensation,” the complaint said. Dr. Rottenberg only treated patients assigned to him by CPN and its parent company, Commonwealth Health Systems, and did not take a patient base with him upon his departure from CPN.
Commonwealth Health spokesperson Annmarie Poslock declined to comment on pending litigation.
After becoming frustrated by “restrictions on his ability to practice medicine” at CPN, Dr. Rottenberg submitted the required 90-day written notice to terminate the employment agreement. He subsequently received a letter from Simon Ratliff, CPN’s chief executive officer, confirming that his last day of employment would be February 11, 2024. Ratliff also reiterated that the noncompete clause would be enforced, essentially banning Dr. Rottenberg from practicing within a 20-mile radius of his previous CPN locations for the next 2 years, court documents said.
Dr. Rottenberg was recruited by Lehigh Valley Physician Group (LVPG), part of Lehigh Valley Health Network, in December 2023 for a urology position at its Dickson City and Scranton locations — some of which are within 20 miles of CPN facilities, the complaint said.
Employers often include noncompete terms in physician contracts because they want to keep the departing physician’s patients from following them to a competitor. However, about a dozen states and the District of Columbia have passed legislation that allows physicians and other clinicians to more easily exit contracts and change jobs.
For example, an Indiana law took effect on July 1 that prohibits employers from entering a noncompete agreement with primary care physicians. Minnesota legislators also banned new noncompete agreements for all employees effective July 1.
“There’s actually been a long-standing push for bans on physician noncompetes going back to some of the first states to pass them, like Colorado, Delaware, and Massachusetts, in the late 1970s and early 1980s,” said Evan Starr, PhD, associate professor of management and organization at the Robert H. Smith School of Business at the University of Maryland.
Although New York Governor Kathy Hochul recently vetoed a bill that would have outlawed restrictive covenants, more states may consider passing laws that limit or ban noncompetes amid increasing patient equity and care access concerns. Dr. Starr told this news organization that one reason to eliminate restrictive covenants is because they can cause “third-party harm” to patients. “The patient doesn’t get the choice to sign a noncompete, but they’re going to be impacted by that agreement if the physician has to leave the area,” he said.
Interestingly, one profession — lawyers — is the only occupation in the US for which noncompete agreements are banned, says Dr. Starr. “Basically, the American Medical Association (AMA) and other physician governing bodies haven’t made the same policies to exempt themselves that the lawyers have.”
That may be changing. In June, the AMA’s House of Delegates adopted policies to support the prohibition of noncompete contracts for employed physicians. The change came several months after the Federal Trade Commission (FTC) proposed a new rule that could more broadly ban companies from enforcing noncompete clauses.
Despite Rottenberg’s attorney, Ryan Campbell, Esq, claiming that the noncompete is unenforceable without a protectable business interest, CPN would not release him from the agreement and opted to move forward with litigation, court records said. The suit cites several other cases where Pennsylvania judges have released physicians from similar restrictive covenants.
Mr. Campbell told this news organization that he and his client are “working diligently with CPN and its counsel to resolve the matter amicably and without further litigation.”
As employers await the FTC’s final rule, Dr. Starr says they could take steps to eliminate noncompete agreements altogether in favor of other stipulations. Contract terms prohibiting physicians from soliciting former patients could protect business interests and still allow patients to seek their preferred physician on their own accord.
A version of this article appeared on Medscape.com .
The lawsuit brings renewed attention to the ongoing public discourse around restrictive covenants for physicians as more state and federal legislators signal plans to limit or ban the practice.
According to a civil suit filed on January 30 in the Court of Common Pleas, Scranton, Pennsylvania, Eric Rottenberg, MD, signed a 3-year employment agreement with Commonwealth Health Physician Network (CPN) in November 2022. He worked for the health system from May to November 2023, seeing patients at several of its locations, including Wilkes-Barre General Hospital and other facilities throughout northeast and central Pennsylvania.
Although Dr. Rottenberg previously practiced in Albany, New York, court records state he did not bring a significant referral or patient base to the new role, receive any specialized training, or have knowledge of CPN’s trade secrets during his tenure.
Instead, he was a “9-to-5 practitioner,” or a physician-employee like a “locum tenens whose replacement would not cost the employer more than his traditional compensation,” the complaint said. Dr. Rottenberg only treated patients assigned to him by CPN and its parent company, Commonwealth Health Systems, and did not take a patient base with him upon his departure from CPN.
Commonwealth Health spokesperson Annmarie Poslock declined to comment on pending litigation.
After becoming frustrated by “restrictions on his ability to practice medicine” at CPN, Dr. Rottenberg submitted the required 90-day written notice to terminate the employment agreement. He subsequently received a letter from Simon Ratliff, CPN’s chief executive officer, confirming that his last day of employment would be February 11, 2024. Ratliff also reiterated that the noncompete clause would be enforced, essentially banning Dr. Rottenberg from practicing within a 20-mile radius of his previous CPN locations for the next 2 years, court documents said.
Dr. Rottenberg was recruited by Lehigh Valley Physician Group (LVPG), part of Lehigh Valley Health Network, in December 2023 for a urology position at its Dickson City and Scranton locations — some of which are within 20 miles of CPN facilities, the complaint said.
Employers often include noncompete terms in physician contracts because they want to keep the departing physician’s patients from following them to a competitor. However, about a dozen states and the District of Columbia have passed legislation that allows physicians and other clinicians to more easily exit contracts and change jobs.
For example, an Indiana law took effect on July 1 that prohibits employers from entering a noncompete agreement with primary care physicians. Minnesota legislators also banned new noncompete agreements for all employees effective July 1.
“There’s actually been a long-standing push for bans on physician noncompetes going back to some of the first states to pass them, like Colorado, Delaware, and Massachusetts, in the late 1970s and early 1980s,” said Evan Starr, PhD, associate professor of management and organization at the Robert H. Smith School of Business at the University of Maryland.
Although New York Governor Kathy Hochul recently vetoed a bill that would have outlawed restrictive covenants, more states may consider passing laws that limit or ban noncompetes amid increasing patient equity and care access concerns. Dr. Starr told this news organization that one reason to eliminate restrictive covenants is because they can cause “third-party harm” to patients. “The patient doesn’t get the choice to sign a noncompete, but they’re going to be impacted by that agreement if the physician has to leave the area,” he said.
Interestingly, one profession — lawyers — is the only occupation in the US for which noncompete agreements are banned, says Dr. Starr. “Basically, the American Medical Association (AMA) and other physician governing bodies haven’t made the same policies to exempt themselves that the lawyers have.”
That may be changing. In June, the AMA’s House of Delegates adopted policies to support the prohibition of noncompete contracts for employed physicians. The change came several months after the Federal Trade Commission (FTC) proposed a new rule that could more broadly ban companies from enforcing noncompete clauses.
Despite Rottenberg’s attorney, Ryan Campbell, Esq, claiming that the noncompete is unenforceable without a protectable business interest, CPN would not release him from the agreement and opted to move forward with litigation, court records said. The suit cites several other cases where Pennsylvania judges have released physicians from similar restrictive covenants.
Mr. Campbell told this news organization that he and his client are “working diligently with CPN and its counsel to resolve the matter amicably and without further litigation.”
As employers await the FTC’s final rule, Dr. Starr says they could take steps to eliminate noncompete agreements altogether in favor of other stipulations. Contract terms prohibiting physicians from soliciting former patients could protect business interests and still allow patients to seek their preferred physician on their own accord.
A version of this article appeared on Medscape.com .
The lawsuit brings renewed attention to the ongoing public discourse around restrictive covenants for physicians as more state and federal legislators signal plans to limit or ban the practice.
According to a civil suit filed on January 30 in the Court of Common Pleas, Scranton, Pennsylvania, Eric Rottenberg, MD, signed a 3-year employment agreement with Commonwealth Health Physician Network (CPN) in November 2022. He worked for the health system from May to November 2023, seeing patients at several of its locations, including Wilkes-Barre General Hospital and other facilities throughout northeast and central Pennsylvania.
Although Dr. Rottenberg previously practiced in Albany, New York, court records state he did not bring a significant referral or patient base to the new role, receive any specialized training, or have knowledge of CPN’s trade secrets during his tenure.
Instead, he was a “9-to-5 practitioner,” or a physician-employee like a “locum tenens whose replacement would not cost the employer more than his traditional compensation,” the complaint said. Dr. Rottenberg only treated patients assigned to him by CPN and its parent company, Commonwealth Health Systems, and did not take a patient base with him upon his departure from CPN.
Commonwealth Health spokesperson Annmarie Poslock declined to comment on pending litigation.
After becoming frustrated by “restrictions on his ability to practice medicine” at CPN, Dr. Rottenberg submitted the required 90-day written notice to terminate the employment agreement. He subsequently received a letter from Simon Ratliff, CPN’s chief executive officer, confirming that his last day of employment would be February 11, 2024. Ratliff also reiterated that the noncompete clause would be enforced, essentially banning Dr. Rottenberg from practicing within a 20-mile radius of his previous CPN locations for the next 2 years, court documents said.
Dr. Rottenberg was recruited by Lehigh Valley Physician Group (LVPG), part of Lehigh Valley Health Network, in December 2023 for a urology position at its Dickson City and Scranton locations — some of which are within 20 miles of CPN facilities, the complaint said.
Employers often include noncompete terms in physician contracts because they want to keep the departing physician’s patients from following them to a competitor. However, about a dozen states and the District of Columbia have passed legislation that allows physicians and other clinicians to more easily exit contracts and change jobs.
For example, an Indiana law took effect on July 1 that prohibits employers from entering a noncompete agreement with primary care physicians. Minnesota legislators also banned new noncompete agreements for all employees effective July 1.
“There’s actually been a long-standing push for bans on physician noncompetes going back to some of the first states to pass them, like Colorado, Delaware, and Massachusetts, in the late 1970s and early 1980s,” said Evan Starr, PhD, associate professor of management and organization at the Robert H. Smith School of Business at the University of Maryland.
Although New York Governor Kathy Hochul recently vetoed a bill that would have outlawed restrictive covenants, more states may consider passing laws that limit or ban noncompetes amid increasing patient equity and care access concerns. Dr. Starr told this news organization that one reason to eliminate restrictive covenants is because they can cause “third-party harm” to patients. “The patient doesn’t get the choice to sign a noncompete, but they’re going to be impacted by that agreement if the physician has to leave the area,” he said.
Interestingly, one profession — lawyers — is the only occupation in the US for which noncompete agreements are banned, says Dr. Starr. “Basically, the American Medical Association (AMA) and other physician governing bodies haven’t made the same policies to exempt themselves that the lawyers have.”
That may be changing. In June, the AMA’s House of Delegates adopted policies to support the prohibition of noncompete contracts for employed physicians. The change came several months after the Federal Trade Commission (FTC) proposed a new rule that could more broadly ban companies from enforcing noncompete clauses.
Despite Rottenberg’s attorney, Ryan Campbell, Esq, claiming that the noncompete is unenforceable without a protectable business interest, CPN would not release him from the agreement and opted to move forward with litigation, court records said. The suit cites several other cases where Pennsylvania judges have released physicians from similar restrictive covenants.
Mr. Campbell told this news organization that he and his client are “working diligently with CPN and its counsel to resolve the matter amicably and without further litigation.”
As employers await the FTC’s final rule, Dr. Starr says they could take steps to eliminate noncompete agreements altogether in favor of other stipulations. Contract terms prohibiting physicians from soliciting former patients could protect business interests and still allow patients to seek their preferred physician on their own accord.
A version of this article appeared on Medscape.com .
Nepali IMG Sues NBME for Invalidating USMLE Scores in Cheating Scandal
in response to a widespread cheating scandal.
Latika Giri, MBBS, of Kathmandu, claims the board violated its own procedures by invalidating exam scores before giving examinees a chance to argue and appeal, according to documents filed on February 12 in the US District Court for the District of Columbia. Dr. Giri alleges that the NBME’s actions were discriminatory against Nepali doctors and run afoul of the Civil Rights Act.
Dr. Giri is requesting that the court block NBME from invalidating her scores while the lawsuit continues and restore her original results. The complaint was filed as a class action suit on behalf of Dr. Giri and other as yet unnamed plaintiffs affected by the board’s action.
The lawsuit stems from a January 31 statement from the United States Medical Licensing Examination (USMLE) program that it was voiding scores attained by some examinees after an investigation revealed a pattern of anomalous exam performance associated with test-takers from Nepal.
The announcement came just before the report about the selling and buying of USMLE questions online, and concerns that cheating on the exam had become “rampant” in recent years. The article was cited in Dr. Giri’s lawsuit.
A spokesman for the NBME said the board does not comment on pending litigation.
Kritika Tara Deb, a Washington, DC–based attorney representing Dr. Giri, declined to answer specific questions about the case but expressed confidence in the outcome of the suit.
“A policy that explicitly denies employment to an entire nationality or ethnicity is counter to US law and the USMLE’s non-discrimination principles,” Deb told this news organization in an email. “Such a blatantly discriminatory policy severely punishes honest doctors while unfairly maligning an entire nationality, and we’re confident it will not stand.”
Doctor Says She Didn’t Cheat
Dr. Giri is one of 22,000 foreign medical school graduates who complete the USMLE each year, in addition to the 24,000 US medical school graduates who take the exam.
A 2022 graduate of the Kathmandu University School of Medical Sciences, Dr. Giri completed her board exams in 2023. According to her lawsuit, she studied hard and did not cheat, passing Step 1 and scoring a 252 on Step 2 and a 229 on Step 3. Dr. Giri took Step 1 in Nepal, Step 2 in India, and Step 3 in Connecticut, according to court documents. In January 2024, Dr. Giri was preparing to enter the residency match pool and hoping to start her training in the summer when she received an email from NBME saying her USMLE scores had been invalidated. She was accused of “extremely improbable answer similarity with other examinees testing on the same form at similar times, unusually high performance, and abnormal question response times,” according to the complaint.
Dr. Giri and other examinees affected by the invalidations were given until February 16 to choose from three options. They could request that NBME reconsider its decision, which could take up to 10 weeks; agree to retake the test; or do nothing, in which case their scores would remain invalid and their access to USMLE would be suspended for 3 years.
If examinees chose options 1 or 2, they would be required to waive their right to sue NBME, according to Dr. Giri’s lawsuit.
“Because of the schedule of medical-residency matching, all three options result in graduates being unable to practice medicine for at least a year,” attorneys for Dr. Giri wrote in the complaint. “All three options force many people to abruptly leave the country within 30 days and cause every affected person to lose their jobs or the opportunity to seek a job.”
Lawsuit: Board Did Not Follow Published Practices
Dr. Giri contends that NBME’s handling of the suspected cheating violates its own published procedures and treats the subset of Nepali examinees differently from other medical graduates. Examinees suspected of cheating are typically first advised of the matter, given an opportunity to share relevant information, and provided the right to appeal, according to the suit. During the process, the test-taker’s score is treated as valid.
Dr. Giri and others were not provided this same treatment and had their scores invalidated on “the explicit basis that they were associated with Nepal,” the suit claims. The actions are in direct violation of the Civil Rights Act of 1964, which forbids discrimination against “any individual with respect to his terms, conditions, or privileges of employment, because of such individual’s race, color, religion, sex, or national origin,” according to the complaint.
About 800 people are in the subset of Nepali test-takers targeted by the NBME, according to the suit.
Dr. Giri said the score invalidations will cause plaintiffs “irreparable harm” if the NBME’s actions are not promptly halted.
“As of January 31, 2024, plaintiffs who are applying to medical residencies are all ineligible for the Match, the deadline for which is February 28, 2024,” attorneys for Dr. Giri wrote. “All plaintiffs will thus miss this year’s Match no matter what. And NBME has offered no explanation for why it waited until the day before the Match opened to abruptly suspended plaintiffs’ scores: Dr. Giri and many others took some of the invalidated exams more than a year ago.”
Dr. Giri is requesting a decision by the court by February 21. The NBME meanwhile, plans to issue a legal response by February 19, according to court documents.
Meanwhile, a petition started on change.org by a US emergency physician born calls for the USMLE program to degeneralize the wording of its January 31 statement. The USMLE statement “casts a shadow over the achievement of a supermajority of physicians from Nepal who succeeded through perseverance, honesty, and intelligence,” according to the petition. Petitioners want the USMLE program to change and clarify that it does “not mean to malign physicians from the entire country of Nepal.” More than 2700 people have signed the petition.
A version of this article appeared on Medscape.com.
in response to a widespread cheating scandal.
Latika Giri, MBBS, of Kathmandu, claims the board violated its own procedures by invalidating exam scores before giving examinees a chance to argue and appeal, according to documents filed on February 12 in the US District Court for the District of Columbia. Dr. Giri alleges that the NBME’s actions were discriminatory against Nepali doctors and run afoul of the Civil Rights Act.
Dr. Giri is requesting that the court block NBME from invalidating her scores while the lawsuit continues and restore her original results. The complaint was filed as a class action suit on behalf of Dr. Giri and other as yet unnamed plaintiffs affected by the board’s action.
The lawsuit stems from a January 31 statement from the United States Medical Licensing Examination (USMLE) program that it was voiding scores attained by some examinees after an investigation revealed a pattern of anomalous exam performance associated with test-takers from Nepal.
The announcement came just before the report about the selling and buying of USMLE questions online, and concerns that cheating on the exam had become “rampant” in recent years. The article was cited in Dr. Giri’s lawsuit.
A spokesman for the NBME said the board does not comment on pending litigation.
Kritika Tara Deb, a Washington, DC–based attorney representing Dr. Giri, declined to answer specific questions about the case but expressed confidence in the outcome of the suit.
“A policy that explicitly denies employment to an entire nationality or ethnicity is counter to US law and the USMLE’s non-discrimination principles,” Deb told this news organization in an email. “Such a blatantly discriminatory policy severely punishes honest doctors while unfairly maligning an entire nationality, and we’re confident it will not stand.”
Doctor Says She Didn’t Cheat
Dr. Giri is one of 22,000 foreign medical school graduates who complete the USMLE each year, in addition to the 24,000 US medical school graduates who take the exam.
A 2022 graduate of the Kathmandu University School of Medical Sciences, Dr. Giri completed her board exams in 2023. According to her lawsuit, she studied hard and did not cheat, passing Step 1 and scoring a 252 on Step 2 and a 229 on Step 3. Dr. Giri took Step 1 in Nepal, Step 2 in India, and Step 3 in Connecticut, according to court documents. In January 2024, Dr. Giri was preparing to enter the residency match pool and hoping to start her training in the summer when she received an email from NBME saying her USMLE scores had been invalidated. She was accused of “extremely improbable answer similarity with other examinees testing on the same form at similar times, unusually high performance, and abnormal question response times,” according to the complaint.
Dr. Giri and other examinees affected by the invalidations were given until February 16 to choose from three options. They could request that NBME reconsider its decision, which could take up to 10 weeks; agree to retake the test; or do nothing, in which case their scores would remain invalid and their access to USMLE would be suspended for 3 years.
If examinees chose options 1 or 2, they would be required to waive their right to sue NBME, according to Dr. Giri’s lawsuit.
“Because of the schedule of medical-residency matching, all three options result in graduates being unable to practice medicine for at least a year,” attorneys for Dr. Giri wrote in the complaint. “All three options force many people to abruptly leave the country within 30 days and cause every affected person to lose their jobs or the opportunity to seek a job.”
Lawsuit: Board Did Not Follow Published Practices
Dr. Giri contends that NBME’s handling of the suspected cheating violates its own published procedures and treats the subset of Nepali examinees differently from other medical graduates. Examinees suspected of cheating are typically first advised of the matter, given an opportunity to share relevant information, and provided the right to appeal, according to the suit. During the process, the test-taker’s score is treated as valid.
Dr. Giri and others were not provided this same treatment and had their scores invalidated on “the explicit basis that they were associated with Nepal,” the suit claims. The actions are in direct violation of the Civil Rights Act of 1964, which forbids discrimination against “any individual with respect to his terms, conditions, or privileges of employment, because of such individual’s race, color, religion, sex, or national origin,” according to the complaint.
About 800 people are in the subset of Nepali test-takers targeted by the NBME, according to the suit.
Dr. Giri said the score invalidations will cause plaintiffs “irreparable harm” if the NBME’s actions are not promptly halted.
“As of January 31, 2024, plaintiffs who are applying to medical residencies are all ineligible for the Match, the deadline for which is February 28, 2024,” attorneys for Dr. Giri wrote. “All plaintiffs will thus miss this year’s Match no matter what. And NBME has offered no explanation for why it waited until the day before the Match opened to abruptly suspended plaintiffs’ scores: Dr. Giri and many others took some of the invalidated exams more than a year ago.”
Dr. Giri is requesting a decision by the court by February 21. The NBME meanwhile, plans to issue a legal response by February 19, according to court documents.
Meanwhile, a petition started on change.org by a US emergency physician born calls for the USMLE program to degeneralize the wording of its January 31 statement. The USMLE statement “casts a shadow over the achievement of a supermajority of physicians from Nepal who succeeded through perseverance, honesty, and intelligence,” according to the petition. Petitioners want the USMLE program to change and clarify that it does “not mean to malign physicians from the entire country of Nepal.” More than 2700 people have signed the petition.
A version of this article appeared on Medscape.com.
in response to a widespread cheating scandal.
Latika Giri, MBBS, of Kathmandu, claims the board violated its own procedures by invalidating exam scores before giving examinees a chance to argue and appeal, according to documents filed on February 12 in the US District Court for the District of Columbia. Dr. Giri alleges that the NBME’s actions were discriminatory against Nepali doctors and run afoul of the Civil Rights Act.
Dr. Giri is requesting that the court block NBME from invalidating her scores while the lawsuit continues and restore her original results. The complaint was filed as a class action suit on behalf of Dr. Giri and other as yet unnamed plaintiffs affected by the board’s action.
The lawsuit stems from a January 31 statement from the United States Medical Licensing Examination (USMLE) program that it was voiding scores attained by some examinees after an investigation revealed a pattern of anomalous exam performance associated with test-takers from Nepal.
The announcement came just before the report about the selling and buying of USMLE questions online, and concerns that cheating on the exam had become “rampant” in recent years. The article was cited in Dr. Giri’s lawsuit.
A spokesman for the NBME said the board does not comment on pending litigation.
Kritika Tara Deb, a Washington, DC–based attorney representing Dr. Giri, declined to answer specific questions about the case but expressed confidence in the outcome of the suit.
“A policy that explicitly denies employment to an entire nationality or ethnicity is counter to US law and the USMLE’s non-discrimination principles,” Deb told this news organization in an email. “Such a blatantly discriminatory policy severely punishes honest doctors while unfairly maligning an entire nationality, and we’re confident it will not stand.”
Doctor Says She Didn’t Cheat
Dr. Giri is one of 22,000 foreign medical school graduates who complete the USMLE each year, in addition to the 24,000 US medical school graduates who take the exam.
A 2022 graduate of the Kathmandu University School of Medical Sciences, Dr. Giri completed her board exams in 2023. According to her lawsuit, she studied hard and did not cheat, passing Step 1 and scoring a 252 on Step 2 and a 229 on Step 3. Dr. Giri took Step 1 in Nepal, Step 2 in India, and Step 3 in Connecticut, according to court documents. In January 2024, Dr. Giri was preparing to enter the residency match pool and hoping to start her training in the summer when she received an email from NBME saying her USMLE scores had been invalidated. She was accused of “extremely improbable answer similarity with other examinees testing on the same form at similar times, unusually high performance, and abnormal question response times,” according to the complaint.
Dr. Giri and other examinees affected by the invalidations were given until February 16 to choose from three options. They could request that NBME reconsider its decision, which could take up to 10 weeks; agree to retake the test; or do nothing, in which case their scores would remain invalid and their access to USMLE would be suspended for 3 years.
If examinees chose options 1 or 2, they would be required to waive their right to sue NBME, according to Dr. Giri’s lawsuit.
“Because of the schedule of medical-residency matching, all three options result in graduates being unable to practice medicine for at least a year,” attorneys for Dr. Giri wrote in the complaint. “All three options force many people to abruptly leave the country within 30 days and cause every affected person to lose their jobs or the opportunity to seek a job.”
Lawsuit: Board Did Not Follow Published Practices
Dr. Giri contends that NBME’s handling of the suspected cheating violates its own published procedures and treats the subset of Nepali examinees differently from other medical graduates. Examinees suspected of cheating are typically first advised of the matter, given an opportunity to share relevant information, and provided the right to appeal, according to the suit. During the process, the test-taker’s score is treated as valid.
Dr. Giri and others were not provided this same treatment and had their scores invalidated on “the explicit basis that they were associated with Nepal,” the suit claims. The actions are in direct violation of the Civil Rights Act of 1964, which forbids discrimination against “any individual with respect to his terms, conditions, or privileges of employment, because of such individual’s race, color, religion, sex, or national origin,” according to the complaint.
About 800 people are in the subset of Nepali test-takers targeted by the NBME, according to the suit.
Dr. Giri said the score invalidations will cause plaintiffs “irreparable harm” if the NBME’s actions are not promptly halted.
“As of January 31, 2024, plaintiffs who are applying to medical residencies are all ineligible for the Match, the deadline for which is February 28, 2024,” attorneys for Dr. Giri wrote. “All plaintiffs will thus miss this year’s Match no matter what. And NBME has offered no explanation for why it waited until the day before the Match opened to abruptly suspended plaintiffs’ scores: Dr. Giri and many others took some of the invalidated exams more than a year ago.”
Dr. Giri is requesting a decision by the court by February 21. The NBME meanwhile, plans to issue a legal response by February 19, according to court documents.
Meanwhile, a petition started on change.org by a US emergency physician born calls for the USMLE program to degeneralize the wording of its January 31 statement. The USMLE statement “casts a shadow over the achievement of a supermajority of physicians from Nepal who succeeded through perseverance, honesty, and intelligence,” according to the petition. Petitioners want the USMLE program to change and clarify that it does “not mean to malign physicians from the entire country of Nepal.” More than 2700 people have signed the petition.
A version of this article appeared on Medscape.com.
Stimulant Medications for ADHD — the Good, the Bad, and the Ugly
Children with attention-deficit/hyperactivity disorder (ADHD) are mainly cared for in primary care settings by us. Management of this chronic neurodevelopmental condition that affects 5+% of children worldwide should include proper diagnosis, assessment for contributing and comorbid conditions, behavioral intervention (the primary treatment for preschoolers), ensuring good sleep and nutrition, and usually medication.
Because stimulants are very effective for reducing ADHD symptoms, we may readily begin these first-line medications even on the initial visit when the diagnosis is determined. But are we really thoughtful about knowing and explaining the potential short- and long-term side effects of these medications that may then be used for many years? Considerable discussion with the child and parents may be needed to address their concerns, balanced with benefits, and to make a plan for their access and use of stimulants (and other medications for ADHD not the topic here).
Consider the Side Effects
In children older than 6 years, some form of either a methylphenidate (MPH) or a dextroamphetamine (DA) class of stimulant have been shown to be equally effective in reducing symptoms of ADHD in about 77% of cases, but side effects are common, mostly mild, and mostly in the first months of use. These include reduced appetite, abdominal pain, headache, weight loss, tics, jitteriness, and delays in falling asleep. About half of all children treated will have one of these adverse effects over 5 years, with reduced appetite the most common. There is no difference in effectiveness or side effects by presentation type, i.e. hyperactive, inattentive, or combined, but the DA forms are associated with more side effects than MPH (10% vs. 6%). Medicated preschoolers have more and different side effects which, in addition to those above, may include listlessness, social withdrawal, and repetitive movements. Fortunately, we can reassure families that side effects can usually be readily managed by slower ramp up of dose, spacing to ensure appetite for meals, extra snacks, attention to bowel patterns and bedtime routines, or change in medication class.
Rates of tics while on stimulants are low irrespective of whether DA or MPH is used, and are usually transient, but difficult cases may occur, sometimes as part of Tourette’s, although not a contraindication. Additional side effects of concern are anxiety, irritability, sadness, and overfocusing that may require a change in class of stimulant or to a nonstimulant. Keep in mind that these symptoms may represent comorbid conditions to ADHD, warranting counseling intervention rather than being a medication side effect. Both initial assessment for ADHD and monitoring should look for comorbidities whether medication is used or not.
Measuring height, weight, pulse, and blood pressure should be part of ADHD care. How concerned should you and the family be about variations? Growth rate declines are more common in preschool children; in the PATS study height varied by 20.3%, and weight by 55.2%, more in heavier children. Growth can be protected by providing favored food for school, encouraging eating when hungry, and an evening fourth meal. You can reassure families that, even with continual use of stimulant medicines for years and initial deficits of 2 cm and 2.7 kg compared to expected, no significant differences remain in adulthood.
This longitudinal growth data was collected when short-acting stimulants were the usual, rather than the now common long-acting stimulants given 7 days per week, however. Children on transdermal MPH with 12-hour release over 3 years showed a small but significant delay in growth with the mean deficit rates 1.3 kg/year mainly in the first year, and 0.68 cm/year in height in the second year. If we see growth not recovering as it is expected to after the first year of treatment, we can advise shorter-acting forms, and medication “holidays” on weekends or vacations, that reduce but do not end the deficits. When concerned, a nonstimulant can be selected.
Blood pressure and pulse rate are predictably slightly increased on stimulants (about 2-4 mm Hg and about 3-6 bpm) but not clinically significantly. Although ECGs are not routinely recommended, careful consideration and consultation is warranted before starting stimulants for any patient with structural cardiac abnormalities, unexplained syncope, exertional chest pain, or a family history of sudden death in children or young adults. Neither current nor former users of stimulants for ADHD were found to have greater rates of cardiac events than the general population, however.
Misuse and abuse
Misuse and diversion of stimulants is common (e.g. 26% diverted MPH in the past month; 14% of 12th graders divert DA), often undetected, and potentially dangerous. And the problem is not limited to just the kids. Sixteen percent of parents reported diversion of stimulant medication to another household member, mainly to themselves. Stimulant overdose can occur, especially taken parenterally, and presents with dilated pupils, tremor, agitation, hyperreflexia, combative behavior, confusion, hallucinations, delirium, anxiety, paranoia, movement disorders, and/or seizures. Fortunately, overdose of prescribed stimulants is rarely fatal if medically managed, but recent “fake” Adderall (not from pharmacies) has been circulating. These fake drugs may contain lethal amounts of fentanyl or methamphetamine. Point out to families that a peer-provided stimulant not prescribed for them may have underlying medical or psychiatric issues that increase adverse events. Selling stimulants can have serious legal implications, with punishments ranging from fines to incarceration. A record of arrest during adolescence increases the likelihood of high school dropout, lack of 4-year college education, and later employment barriers. Besides these serious outcomes, it is useful to remind patients that if they deviate from your recommended dosing that you, and others, will not prescribe for them in the future the medication that has been supporting their successful functioning.
You can be fooled about being able to tell if your patients are misusing or diverting the stimulants you prescribe. Most (59%) physicians suspect that more than one of their patients with ADHD has diverted or feigned symptoms (66%) to get a prescription. Women were less likely to suspect their patients than are men, though, so be vigilant! Child psychiatrists had the highest suspicion with their greater proportion of patients with ADHD plus conduct or substance use disorder, who account for 83% of misusers/diverters. We can use education about misuse, pill counts, contracts on dosing, or switching to long-acting or nonstimulants to curb this.
Additional concerns
With more ADHD diagnosis and stimulants used for many years should we worry about longer-term issues? There have been reports in rodent models and a few children of chromosomal changes with stimulant exposure, but reviewers do not interpret these as an individual cancer risk. Record review of patients who received stimulants showed lower numbers of cancer than expected. Nor is there evidence of reproductive effects of stimulants, although use during pregnancy is not cleared.
Stimulants carry a boxed warning as having high potential for abuse and psychological or physical dependence, which is unsurprising given their effects on brain reward pathways. However, neither past nor present use of stimulants for ADHD has been associated with greater substance use long term.
To top off these issues, recent shortages of stimulants complicate ADHD management. Most states require electronic prescribing, US rules only allowing one transfer of such e-prescriptions. With many pharmacies refusing to tell families about availability, we must make multiple calls to locate a source. Pharmacists could help us by looking up patient names of abusers on the registry and identifying sites with adequate supplies.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Children with attention-deficit/hyperactivity disorder (ADHD) are mainly cared for in primary care settings by us. Management of this chronic neurodevelopmental condition that affects 5+% of children worldwide should include proper diagnosis, assessment for contributing and comorbid conditions, behavioral intervention (the primary treatment for preschoolers), ensuring good sleep and nutrition, and usually medication.
Because stimulants are very effective for reducing ADHD symptoms, we may readily begin these first-line medications even on the initial visit when the diagnosis is determined. But are we really thoughtful about knowing and explaining the potential short- and long-term side effects of these medications that may then be used for many years? Considerable discussion with the child and parents may be needed to address their concerns, balanced with benefits, and to make a plan for their access and use of stimulants (and other medications for ADHD not the topic here).
Consider the Side Effects
In children older than 6 years, some form of either a methylphenidate (MPH) or a dextroamphetamine (DA) class of stimulant have been shown to be equally effective in reducing symptoms of ADHD in about 77% of cases, but side effects are common, mostly mild, and mostly in the first months of use. These include reduced appetite, abdominal pain, headache, weight loss, tics, jitteriness, and delays in falling asleep. About half of all children treated will have one of these adverse effects over 5 years, with reduced appetite the most common. There is no difference in effectiveness or side effects by presentation type, i.e. hyperactive, inattentive, or combined, but the DA forms are associated with more side effects than MPH (10% vs. 6%). Medicated preschoolers have more and different side effects which, in addition to those above, may include listlessness, social withdrawal, and repetitive movements. Fortunately, we can reassure families that side effects can usually be readily managed by slower ramp up of dose, spacing to ensure appetite for meals, extra snacks, attention to bowel patterns and bedtime routines, or change in medication class.
Rates of tics while on stimulants are low irrespective of whether DA or MPH is used, and are usually transient, but difficult cases may occur, sometimes as part of Tourette’s, although not a contraindication. Additional side effects of concern are anxiety, irritability, sadness, and overfocusing that may require a change in class of stimulant or to a nonstimulant. Keep in mind that these symptoms may represent comorbid conditions to ADHD, warranting counseling intervention rather than being a medication side effect. Both initial assessment for ADHD and monitoring should look for comorbidities whether medication is used or not.
Measuring height, weight, pulse, and blood pressure should be part of ADHD care. How concerned should you and the family be about variations? Growth rate declines are more common in preschool children; in the PATS study height varied by 20.3%, and weight by 55.2%, more in heavier children. Growth can be protected by providing favored food for school, encouraging eating when hungry, and an evening fourth meal. You can reassure families that, even with continual use of stimulant medicines for years and initial deficits of 2 cm and 2.7 kg compared to expected, no significant differences remain in adulthood.
This longitudinal growth data was collected when short-acting stimulants were the usual, rather than the now common long-acting stimulants given 7 days per week, however. Children on transdermal MPH with 12-hour release over 3 years showed a small but significant delay in growth with the mean deficit rates 1.3 kg/year mainly in the first year, and 0.68 cm/year in height in the second year. If we see growth not recovering as it is expected to after the first year of treatment, we can advise shorter-acting forms, and medication “holidays” on weekends or vacations, that reduce but do not end the deficits. When concerned, a nonstimulant can be selected.
Blood pressure and pulse rate are predictably slightly increased on stimulants (about 2-4 mm Hg and about 3-6 bpm) but not clinically significantly. Although ECGs are not routinely recommended, careful consideration and consultation is warranted before starting stimulants for any patient with structural cardiac abnormalities, unexplained syncope, exertional chest pain, or a family history of sudden death in children or young adults. Neither current nor former users of stimulants for ADHD were found to have greater rates of cardiac events than the general population, however.
Misuse and abuse
Misuse and diversion of stimulants is common (e.g. 26% diverted MPH in the past month; 14% of 12th graders divert DA), often undetected, and potentially dangerous. And the problem is not limited to just the kids. Sixteen percent of parents reported diversion of stimulant medication to another household member, mainly to themselves. Stimulant overdose can occur, especially taken parenterally, and presents with dilated pupils, tremor, agitation, hyperreflexia, combative behavior, confusion, hallucinations, delirium, anxiety, paranoia, movement disorders, and/or seizures. Fortunately, overdose of prescribed stimulants is rarely fatal if medically managed, but recent “fake” Adderall (not from pharmacies) has been circulating. These fake drugs may contain lethal amounts of fentanyl or methamphetamine. Point out to families that a peer-provided stimulant not prescribed for them may have underlying medical or psychiatric issues that increase adverse events. Selling stimulants can have serious legal implications, with punishments ranging from fines to incarceration. A record of arrest during adolescence increases the likelihood of high school dropout, lack of 4-year college education, and later employment barriers. Besides these serious outcomes, it is useful to remind patients that if they deviate from your recommended dosing that you, and others, will not prescribe for them in the future the medication that has been supporting their successful functioning.
You can be fooled about being able to tell if your patients are misusing or diverting the stimulants you prescribe. Most (59%) physicians suspect that more than one of their patients with ADHD has diverted or feigned symptoms (66%) to get a prescription. Women were less likely to suspect their patients than are men, though, so be vigilant! Child psychiatrists had the highest suspicion with their greater proportion of patients with ADHD plus conduct or substance use disorder, who account for 83% of misusers/diverters. We can use education about misuse, pill counts, contracts on dosing, or switching to long-acting or nonstimulants to curb this.
Additional concerns
With more ADHD diagnosis and stimulants used for many years should we worry about longer-term issues? There have been reports in rodent models and a few children of chromosomal changes with stimulant exposure, but reviewers do not interpret these as an individual cancer risk. Record review of patients who received stimulants showed lower numbers of cancer than expected. Nor is there evidence of reproductive effects of stimulants, although use during pregnancy is not cleared.
Stimulants carry a boxed warning as having high potential for abuse and psychological or physical dependence, which is unsurprising given their effects on brain reward pathways. However, neither past nor present use of stimulants for ADHD has been associated with greater substance use long term.
To top off these issues, recent shortages of stimulants complicate ADHD management. Most states require electronic prescribing, US rules only allowing one transfer of such e-prescriptions. With many pharmacies refusing to tell families about availability, we must make multiple calls to locate a source. Pharmacists could help us by looking up patient names of abusers on the registry and identifying sites with adequate supplies.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Children with attention-deficit/hyperactivity disorder (ADHD) are mainly cared for in primary care settings by us. Management of this chronic neurodevelopmental condition that affects 5+% of children worldwide should include proper diagnosis, assessment for contributing and comorbid conditions, behavioral intervention (the primary treatment for preschoolers), ensuring good sleep and nutrition, and usually medication.
Because stimulants are very effective for reducing ADHD symptoms, we may readily begin these first-line medications even on the initial visit when the diagnosis is determined. But are we really thoughtful about knowing and explaining the potential short- and long-term side effects of these medications that may then be used for many years? Considerable discussion with the child and parents may be needed to address their concerns, balanced with benefits, and to make a plan for their access and use of stimulants (and other medications for ADHD not the topic here).
Consider the Side Effects
In children older than 6 years, some form of either a methylphenidate (MPH) or a dextroamphetamine (DA) class of stimulant have been shown to be equally effective in reducing symptoms of ADHD in about 77% of cases, but side effects are common, mostly mild, and mostly in the first months of use. These include reduced appetite, abdominal pain, headache, weight loss, tics, jitteriness, and delays in falling asleep. About half of all children treated will have one of these adverse effects over 5 years, with reduced appetite the most common. There is no difference in effectiveness or side effects by presentation type, i.e. hyperactive, inattentive, or combined, but the DA forms are associated with more side effects than MPH (10% vs. 6%). Medicated preschoolers have more and different side effects which, in addition to those above, may include listlessness, social withdrawal, and repetitive movements. Fortunately, we can reassure families that side effects can usually be readily managed by slower ramp up of dose, spacing to ensure appetite for meals, extra snacks, attention to bowel patterns and bedtime routines, or change in medication class.
Rates of tics while on stimulants are low irrespective of whether DA or MPH is used, and are usually transient, but difficult cases may occur, sometimes as part of Tourette’s, although not a contraindication. Additional side effects of concern are anxiety, irritability, sadness, and overfocusing that may require a change in class of stimulant or to a nonstimulant. Keep in mind that these symptoms may represent comorbid conditions to ADHD, warranting counseling intervention rather than being a medication side effect. Both initial assessment for ADHD and monitoring should look for comorbidities whether medication is used or not.
Measuring height, weight, pulse, and blood pressure should be part of ADHD care. How concerned should you and the family be about variations? Growth rate declines are more common in preschool children; in the PATS study height varied by 20.3%, and weight by 55.2%, more in heavier children. Growth can be protected by providing favored food for school, encouraging eating when hungry, and an evening fourth meal. You can reassure families that, even with continual use of stimulant medicines for years and initial deficits of 2 cm and 2.7 kg compared to expected, no significant differences remain in adulthood.
This longitudinal growth data was collected when short-acting stimulants were the usual, rather than the now common long-acting stimulants given 7 days per week, however. Children on transdermal MPH with 12-hour release over 3 years showed a small but significant delay in growth with the mean deficit rates 1.3 kg/year mainly in the first year, and 0.68 cm/year in height in the second year. If we see growth not recovering as it is expected to after the first year of treatment, we can advise shorter-acting forms, and medication “holidays” on weekends or vacations, that reduce but do not end the deficits. When concerned, a nonstimulant can be selected.
Blood pressure and pulse rate are predictably slightly increased on stimulants (about 2-4 mm Hg and about 3-6 bpm) but not clinically significantly. Although ECGs are not routinely recommended, careful consideration and consultation is warranted before starting stimulants for any patient with structural cardiac abnormalities, unexplained syncope, exertional chest pain, or a family history of sudden death in children or young adults. Neither current nor former users of stimulants for ADHD were found to have greater rates of cardiac events than the general population, however.
Misuse and abuse
Misuse and diversion of stimulants is common (e.g. 26% diverted MPH in the past month; 14% of 12th graders divert DA), often undetected, and potentially dangerous. And the problem is not limited to just the kids. Sixteen percent of parents reported diversion of stimulant medication to another household member, mainly to themselves. Stimulant overdose can occur, especially taken parenterally, and presents with dilated pupils, tremor, agitation, hyperreflexia, combative behavior, confusion, hallucinations, delirium, anxiety, paranoia, movement disorders, and/or seizures. Fortunately, overdose of prescribed stimulants is rarely fatal if medically managed, but recent “fake” Adderall (not from pharmacies) has been circulating. These fake drugs may contain lethal amounts of fentanyl or methamphetamine. Point out to families that a peer-provided stimulant not prescribed for them may have underlying medical or psychiatric issues that increase adverse events. Selling stimulants can have serious legal implications, with punishments ranging from fines to incarceration. A record of arrest during adolescence increases the likelihood of high school dropout, lack of 4-year college education, and later employment barriers. Besides these serious outcomes, it is useful to remind patients that if they deviate from your recommended dosing that you, and others, will not prescribe for them in the future the medication that has been supporting their successful functioning.
You can be fooled about being able to tell if your patients are misusing or diverting the stimulants you prescribe. Most (59%) physicians suspect that more than one of their patients with ADHD has diverted or feigned symptoms (66%) to get a prescription. Women were less likely to suspect their patients than are men, though, so be vigilant! Child psychiatrists had the highest suspicion with their greater proportion of patients with ADHD plus conduct or substance use disorder, who account for 83% of misusers/diverters. We can use education about misuse, pill counts, contracts on dosing, or switching to long-acting or nonstimulants to curb this.
Additional concerns
With more ADHD diagnosis and stimulants used for many years should we worry about longer-term issues? There have been reports in rodent models and a few children of chromosomal changes with stimulant exposure, but reviewers do not interpret these as an individual cancer risk. Record review of patients who received stimulants showed lower numbers of cancer than expected. Nor is there evidence of reproductive effects of stimulants, although use during pregnancy is not cleared.
Stimulants carry a boxed warning as having high potential for abuse and psychological or physical dependence, which is unsurprising given their effects on brain reward pathways. However, neither past nor present use of stimulants for ADHD has been associated with greater substance use long term.
To top off these issues, recent shortages of stimulants complicate ADHD management. Most states require electronic prescribing, US rules only allowing one transfer of such e-prescriptions. With many pharmacies refusing to tell families about availability, we must make multiple calls to locate a source. Pharmacists could help us by looking up patient names of abusers on the registry and identifying sites with adequate supplies.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Beware the Letter of Intent
I recently received an email from a distraught physician. Several months previously, He could continue running his office any way he wished, set his own hours and fees, and keep his employees. All his overhead expenses would disappear. His income would remain the same, maybe even increase. He signed it eagerly.
When he received the actual sale and employment contracts, none of the details promised in the LOI were included; but he figured that since they were spelled out in the LOI, which both he and the buyer had signed, he was covered. His attorney — a family friend with no experience in medical practice transactions — approved the documents.
The deal seemed too good to be true, and it was. The day after the sale closed, all his employees received termination notices. The group offered to rehire some of them, but at lower salaries and reduced benefits. (Most declined.) The new staffers he received were inadequately trained and unfamiliar with his standard office procedures. Patients complained that fees had increased substantially. His own compensation was contingent on meeting strict billing and performance goals. Malpractice premiums remained his responsibility. His office hours were lengthened to include evenings and Saturday mornings.
When he complained to the group that none of the things promised in the LOI had been delivered, he was informed that the LOI was not legally binding. In fact, the sale and employment contracts both clearly specified that they “replaced any previous written or oral agreements between the parties.”
There are some valuable lessons to be learned here. First, whether you are a young physician seeking a new job with a hospital or large practice, or an older one looking to sell an established practice, retain an attorney experienced in medical transactions early, before you sign anything, binding or not. Second, recognize that any promises made in an LOI must be spelled out in the employment and/or sale contract as well.
You might ask, if the terms in an LOI are not binding, why bother with one at all? For one thing, you want to make sure that you and your potential employer or buyer are on the same page with respect to major terms before you get down to details in the employment agreement and/or the medical practice sale agreement. For another, in most states certain LOI provisions are legally binding. For example, the document will most likely provide that each party is responsible for its own attorneys’ fees and for maintaining confidentiality during the negotiations, and that you will not negotiate with any other parties for some specified period of time. In most cases, such provisions are binding whether you go on to sign a formal contract or not.
When you receive an LOI, go through it carefully and identify areas of concern. The offering party will likely be in a rush to sign you up; but once you sign, you won’t be able to negotiate with anyone else for a while, which weakens your negotiating position. Regardless of what is said about time being “of the essence,” proceed slowly and with caution.
Bear in mind that employers and buyers never begin with their best offer. Unless you have been through this before, it is unlikely that you will know your value as an employee or the value of your practice, or what exactly you are entitled to ask for. Rather than signing something you don’t completely understand, explain to the offering party that you need time to consider and evaluate their offer.
This is the time to hire a competent medical attorney to do some due diligence on the offering party and review their offer, and to educate yourself about practice value and compensation benchmarks in your area. You and your counsel should assemble a list of things that you want changed in the LOI, then present them to the other side. They should be amenable to negotiation. If they are not (as was the case in the example presented earlier), you should reconsider whether you really want to be associated with that particular buyer or employer.
Once you have signed the LOI, experts say speed then works to your advantage. “Time kills all deals,” as one lawyer put it. “The longer it takes to close the transaction, the more that can go wrong.” The prospective employer or buyer could uncover information about you or your practice that decreases their perception of value, or economic conditions might change.
While speed is now important, and most of the core issues should already have been resolved in the LOI, don’t be afraid to ask for everything you want, whether it’s a better sale price, higher compensation, a favorable call schedule, more vacation time, or anything else. You won’t know what you can get if you don’t ask for it.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I recently received an email from a distraught physician. Several months previously, He could continue running his office any way he wished, set his own hours and fees, and keep his employees. All his overhead expenses would disappear. His income would remain the same, maybe even increase. He signed it eagerly.
When he received the actual sale and employment contracts, none of the details promised in the LOI were included; but he figured that since they were spelled out in the LOI, which both he and the buyer had signed, he was covered. His attorney — a family friend with no experience in medical practice transactions — approved the documents.
The deal seemed too good to be true, and it was. The day after the sale closed, all his employees received termination notices. The group offered to rehire some of them, but at lower salaries and reduced benefits. (Most declined.) The new staffers he received were inadequately trained and unfamiliar with his standard office procedures. Patients complained that fees had increased substantially. His own compensation was contingent on meeting strict billing and performance goals. Malpractice premiums remained his responsibility. His office hours were lengthened to include evenings and Saturday mornings.
When he complained to the group that none of the things promised in the LOI had been delivered, he was informed that the LOI was not legally binding. In fact, the sale and employment contracts both clearly specified that they “replaced any previous written or oral agreements between the parties.”
There are some valuable lessons to be learned here. First, whether you are a young physician seeking a new job with a hospital or large practice, or an older one looking to sell an established practice, retain an attorney experienced in medical transactions early, before you sign anything, binding or not. Second, recognize that any promises made in an LOI must be spelled out in the employment and/or sale contract as well.
You might ask, if the terms in an LOI are not binding, why bother with one at all? For one thing, you want to make sure that you and your potential employer or buyer are on the same page with respect to major terms before you get down to details in the employment agreement and/or the medical practice sale agreement. For another, in most states certain LOI provisions are legally binding. For example, the document will most likely provide that each party is responsible for its own attorneys’ fees and for maintaining confidentiality during the negotiations, and that you will not negotiate with any other parties for some specified period of time. In most cases, such provisions are binding whether you go on to sign a formal contract or not.
When you receive an LOI, go through it carefully and identify areas of concern. The offering party will likely be in a rush to sign you up; but once you sign, you won’t be able to negotiate with anyone else for a while, which weakens your negotiating position. Regardless of what is said about time being “of the essence,” proceed slowly and with caution.
Bear in mind that employers and buyers never begin with their best offer. Unless you have been through this before, it is unlikely that you will know your value as an employee or the value of your practice, or what exactly you are entitled to ask for. Rather than signing something you don’t completely understand, explain to the offering party that you need time to consider and evaluate their offer.
This is the time to hire a competent medical attorney to do some due diligence on the offering party and review their offer, and to educate yourself about practice value and compensation benchmarks in your area. You and your counsel should assemble a list of things that you want changed in the LOI, then present them to the other side. They should be amenable to negotiation. If they are not (as was the case in the example presented earlier), you should reconsider whether you really want to be associated with that particular buyer or employer.
Once you have signed the LOI, experts say speed then works to your advantage. “Time kills all deals,” as one lawyer put it. “The longer it takes to close the transaction, the more that can go wrong.” The prospective employer or buyer could uncover information about you or your practice that decreases their perception of value, or economic conditions might change.
While speed is now important, and most of the core issues should already have been resolved in the LOI, don’t be afraid to ask for everything you want, whether it’s a better sale price, higher compensation, a favorable call schedule, more vacation time, or anything else. You won’t know what you can get if you don’t ask for it.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I recently received an email from a distraught physician. Several months previously, He could continue running his office any way he wished, set his own hours and fees, and keep his employees. All his overhead expenses would disappear. His income would remain the same, maybe even increase. He signed it eagerly.
When he received the actual sale and employment contracts, none of the details promised in the LOI were included; but he figured that since they were spelled out in the LOI, which both he and the buyer had signed, he was covered. His attorney — a family friend with no experience in medical practice transactions — approved the documents.
The deal seemed too good to be true, and it was. The day after the sale closed, all his employees received termination notices. The group offered to rehire some of them, but at lower salaries and reduced benefits. (Most declined.) The new staffers he received were inadequately trained and unfamiliar with his standard office procedures. Patients complained that fees had increased substantially. His own compensation was contingent on meeting strict billing and performance goals. Malpractice premiums remained his responsibility. His office hours were lengthened to include evenings and Saturday mornings.
When he complained to the group that none of the things promised in the LOI had been delivered, he was informed that the LOI was not legally binding. In fact, the sale and employment contracts both clearly specified that they “replaced any previous written or oral agreements between the parties.”
There are some valuable lessons to be learned here. First, whether you are a young physician seeking a new job with a hospital or large practice, or an older one looking to sell an established practice, retain an attorney experienced in medical transactions early, before you sign anything, binding or not. Second, recognize that any promises made in an LOI must be spelled out in the employment and/or sale contract as well.
You might ask, if the terms in an LOI are not binding, why bother with one at all? For one thing, you want to make sure that you and your potential employer or buyer are on the same page with respect to major terms before you get down to details in the employment agreement and/or the medical practice sale agreement. For another, in most states certain LOI provisions are legally binding. For example, the document will most likely provide that each party is responsible for its own attorneys’ fees and for maintaining confidentiality during the negotiations, and that you will not negotiate with any other parties for some specified period of time. In most cases, such provisions are binding whether you go on to sign a formal contract or not.
When you receive an LOI, go through it carefully and identify areas of concern. The offering party will likely be in a rush to sign you up; but once you sign, you won’t be able to negotiate with anyone else for a while, which weakens your negotiating position. Regardless of what is said about time being “of the essence,” proceed slowly and with caution.
Bear in mind that employers and buyers never begin with their best offer. Unless you have been through this before, it is unlikely that you will know your value as an employee or the value of your practice, or what exactly you are entitled to ask for. Rather than signing something you don’t completely understand, explain to the offering party that you need time to consider and evaluate their offer.
This is the time to hire a competent medical attorney to do some due diligence on the offering party and review their offer, and to educate yourself about practice value and compensation benchmarks in your area. You and your counsel should assemble a list of things that you want changed in the LOI, then present them to the other side. They should be amenable to negotiation. If they are not (as was the case in the example presented earlier), you should reconsider whether you really want to be associated with that particular buyer or employer.
Once you have signed the LOI, experts say speed then works to your advantage. “Time kills all deals,” as one lawyer put it. “The longer it takes to close the transaction, the more that can go wrong.” The prospective employer or buyer could uncover information about you or your practice that decreases their perception of value, or economic conditions might change.
While speed is now important, and most of the core issues should already have been resolved in the LOI, don’t be afraid to ask for everything you want, whether it’s a better sale price, higher compensation, a favorable call schedule, more vacation time, or anything else. You won’t know what you can get if you don’t ask for it.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Long-Term Follow-Up Emphasizes HPV Vaccination Importance
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.