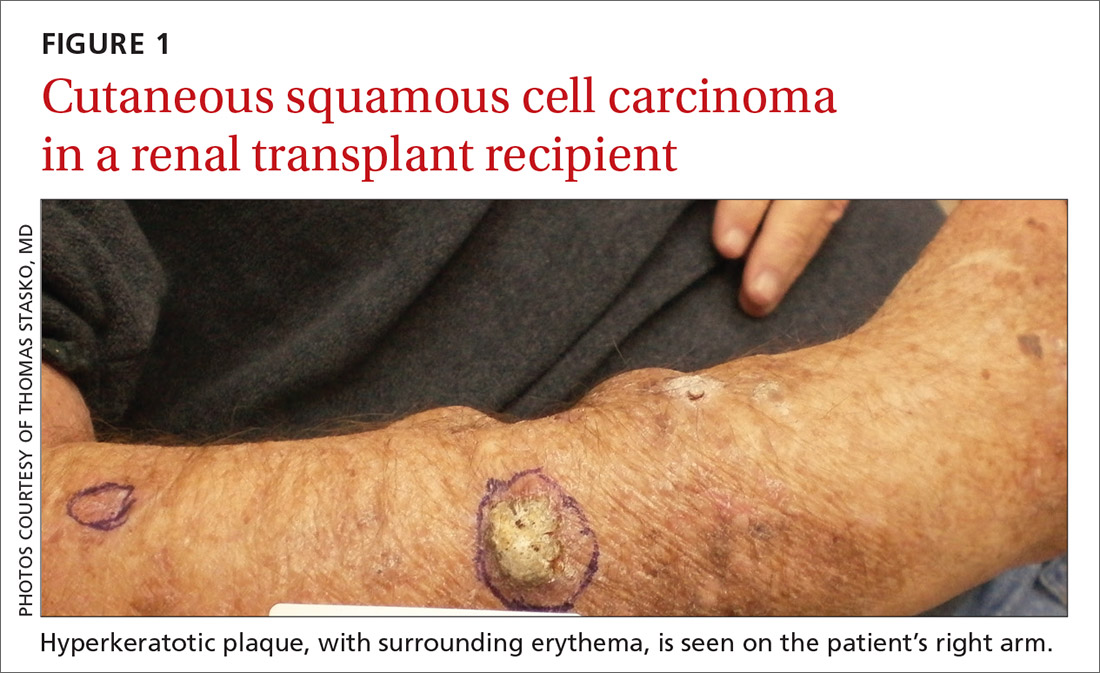
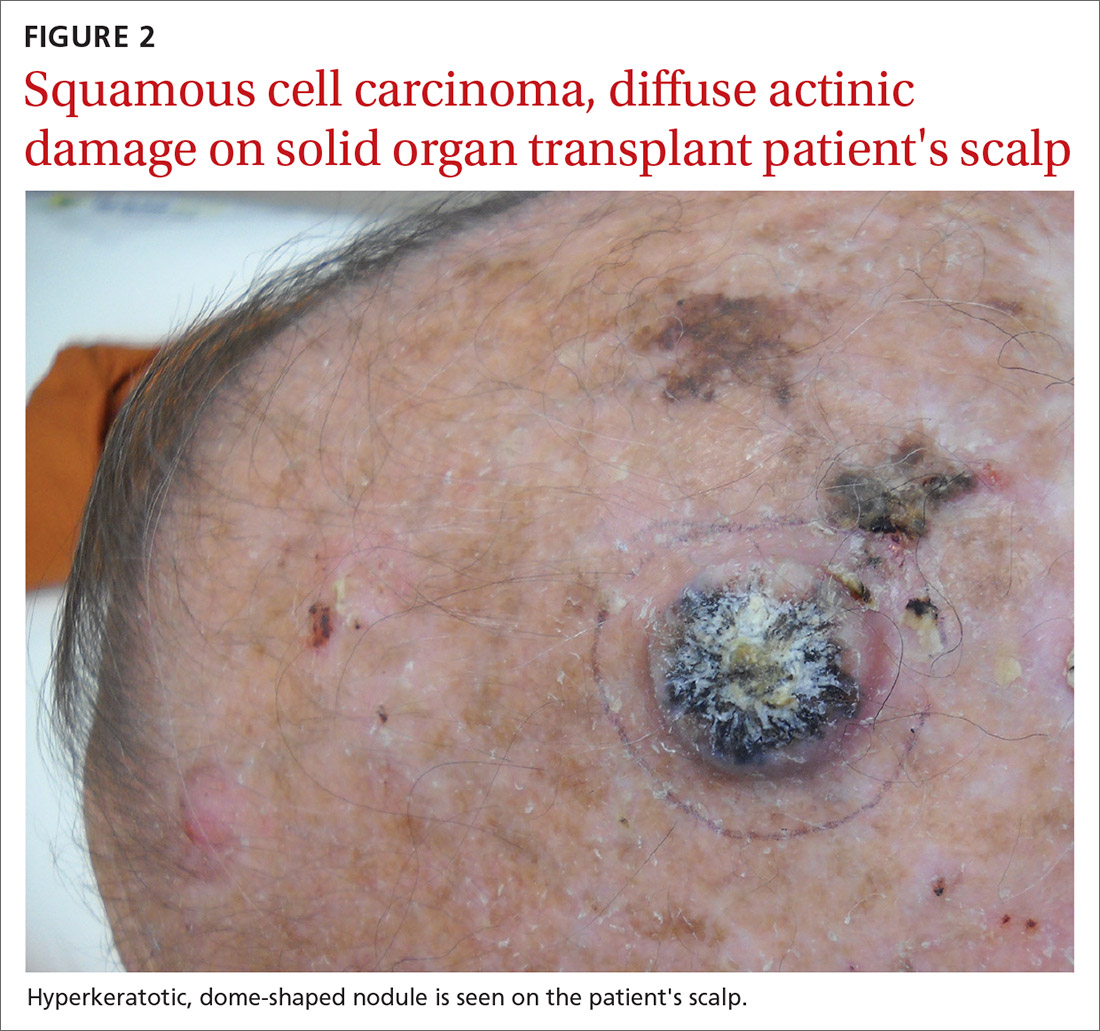
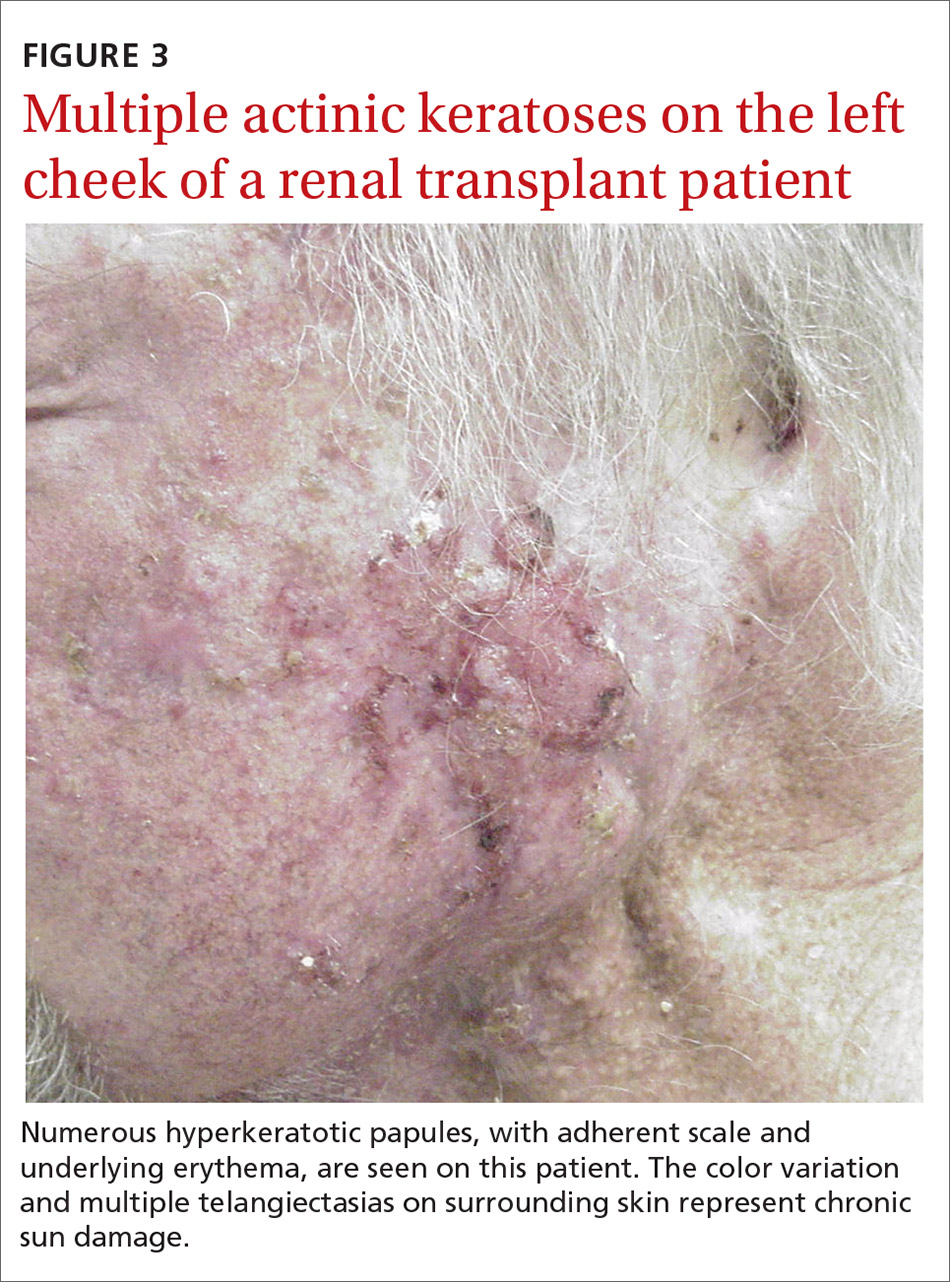
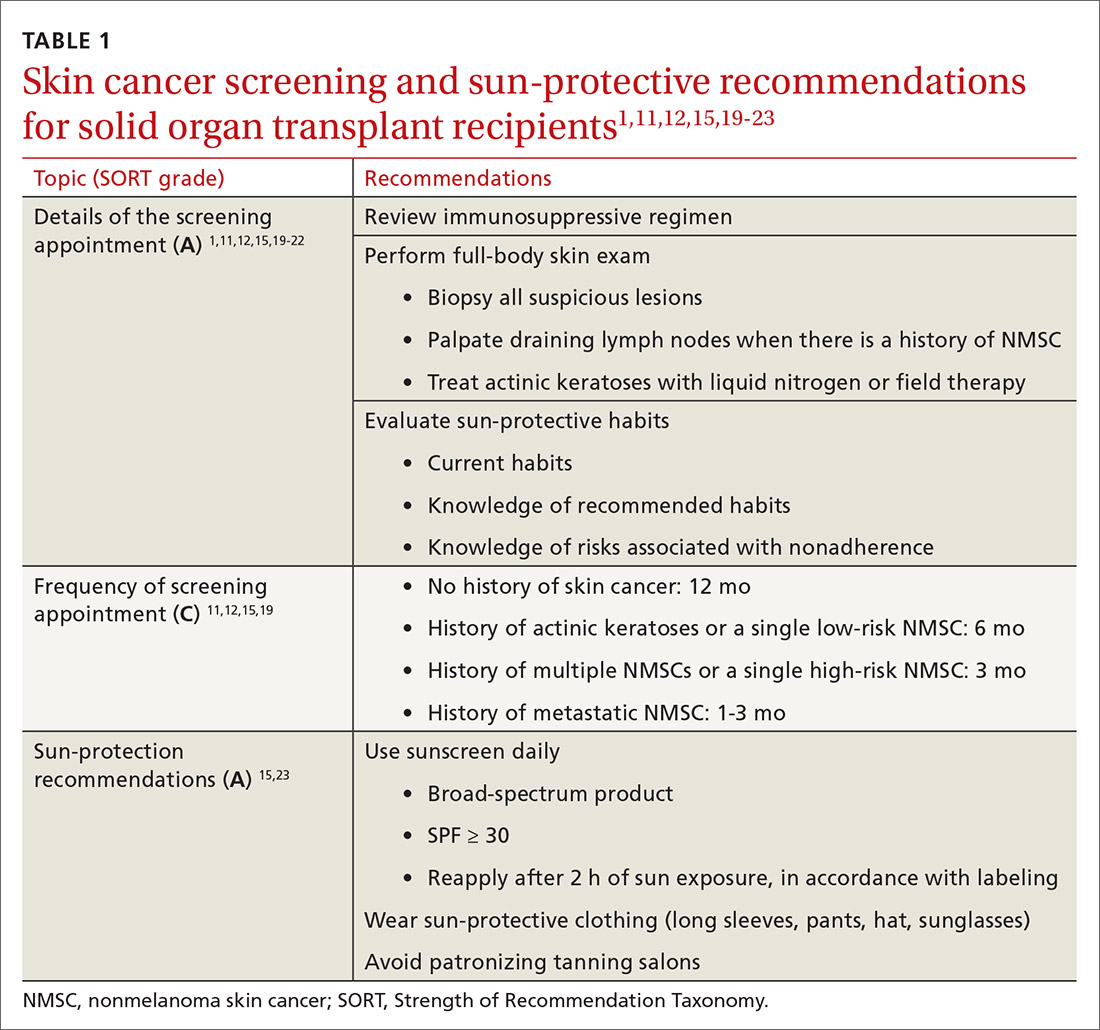
User login
How to help runners steer clear of injury
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.
This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
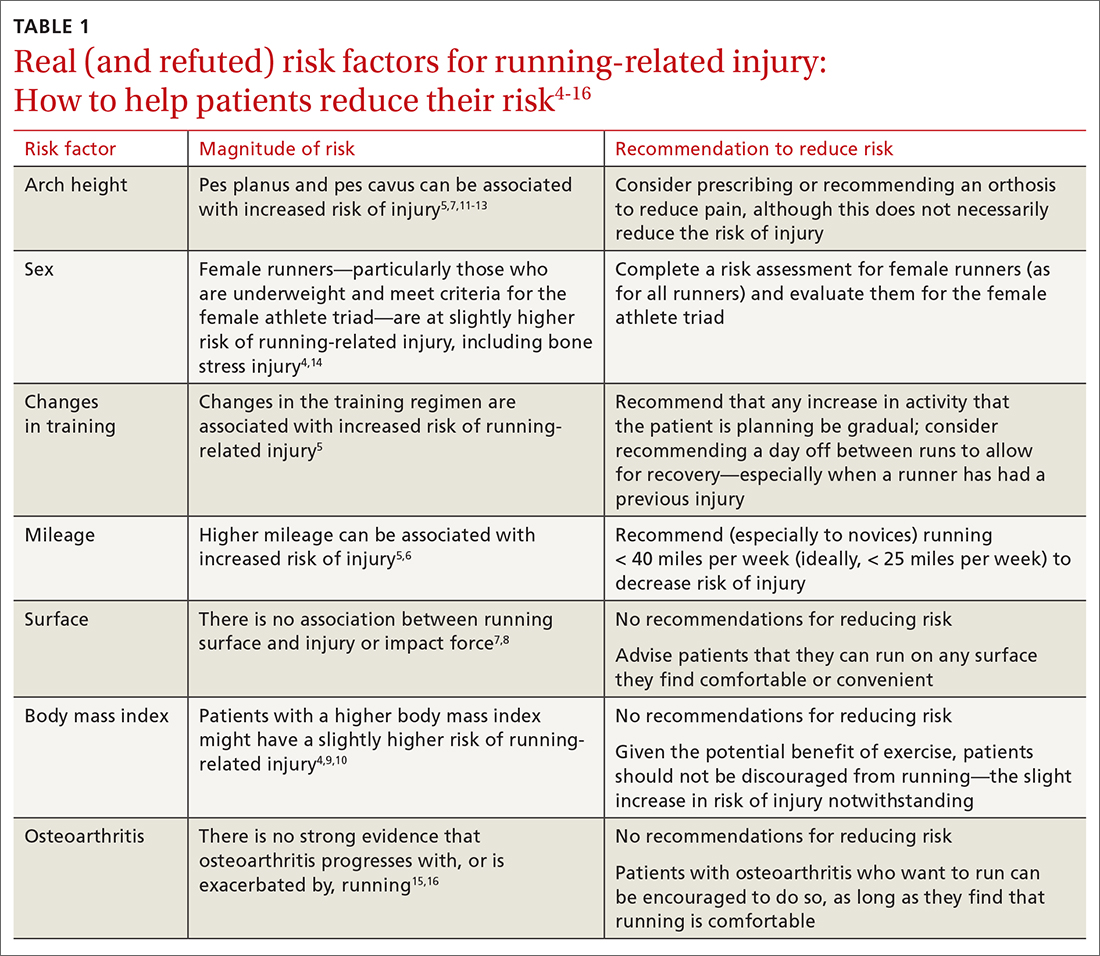
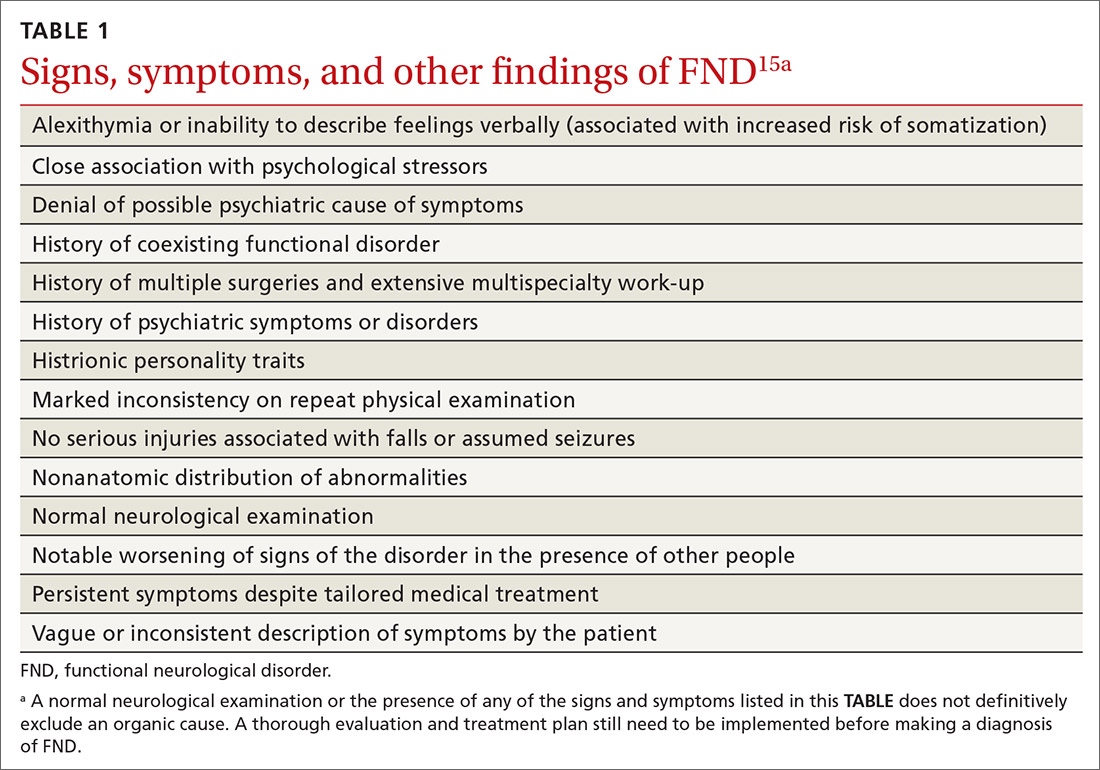
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
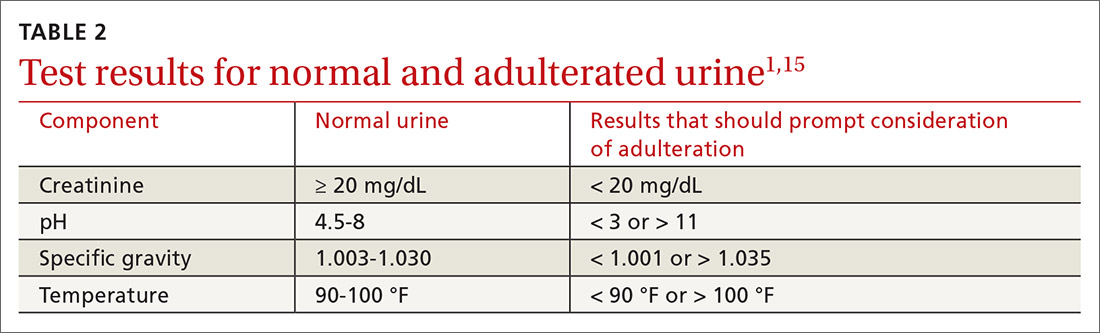
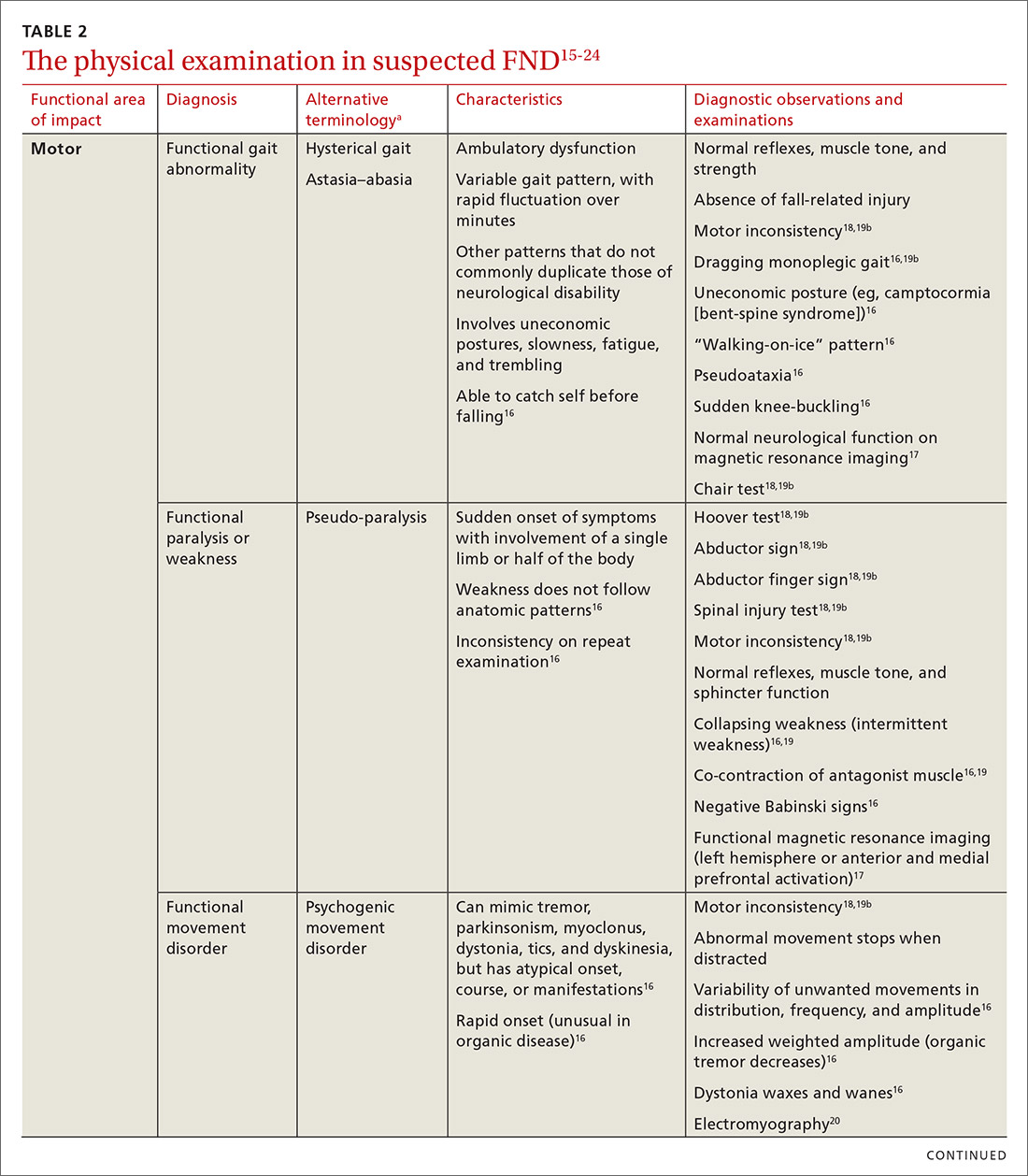
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.
Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; [email protected]
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.
This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.
Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; [email protected]
Approximately 60 million people in the United States run for exercise at least once a calendar year, with approximately 11 million of them running > 100 days a year.1,2 Running is an affordable, convenient, and efficient form of exercise, whose benefits include a decrease in the risk of all-cause early mortality, cancer, and diabetes; an improved lipid profile; and better mental health.3
However, running is also the cause of a significant percentage of exercise-associated injuries: More than 60% of runners report overuse injury annually.4 Given the high incidence of running-related injury, an important component of primary care is accurately diagnosing and managing such injuries and counseling patients about how to prevent them.
This article reviews risk factors for running-related injury and summarizes evidence-based recommendations for prevention.
CASE
During a health maintenance examination, Clara K, a 47-year-old woman who is obese (body mass index [BMI], 34) and has bilateral knee osteoarthritis (OA), inquires about establishing a weight-loss strategy. Ms. K is interested in starting an exercise regimen involving running but is worried about provoking a flare of OA pain.
Risk factors for running injuries
Several risk factors—some modifiable, others nonmodifiable—are associated with running-related injury (TABLE 14-16). In addition, research suggests that other variables once thought to be risk factors, such as running surface and the Q-angle (described later), are not associated with running-related injury.
Modifiable risk factors
Changes in a training regimen or type of training. Many runners escalate training regimens as their fitness improves. Increasing mileage and changing the type of training (such as introducing hills or interval training) are independent risk factors for sustaining injury.5
The traditional recommendation has been for a runner to slowly increase or modify training with a 10% weekly increase in mileage or intensity.17 However, a randomized controlled trial failed to show a lower incidence of injury among amateur runners who adopted a graded exercise program.18 Regardless: It is still prudent to recommend a gradual increase in activity, such as taking ≥ 1 day off between running workouts or starting with a walking or jogging program, especially when there is a history of injury.19
Continue to: Excessive mileage
Excessive mileage. Many runners aspire to complete high-mileage runs. There is low-quality evidence demonstrating that high-mileage running, especially > 40 miles per week, is associated with increased risk of running-related injury.5 Injuries that occur with higher mileage are more often those of the hip and hamstring.5 A study noted that running ≤ 25 miles a week was protective against calf injury.6
Overall, there is little evidence to show that high-mileage running is associated with increased risk of running-related injury. However, this is still a risk factor that you should address with patients who have a running program—especially novices and those who ramp up mileage quickly.
Type of surface. Access to running surfaces—concrete, pavement, trails, treadmills, and athletic tracks—varies by time of day and season. Softer surfaces include treadmill, tracks, and trails; harder surfaces include asphalt and concrete.
There are limited data linking running surface with risk of injury.7 A study did not find an association between peak impact force based on running surface8; the authors hypothesized that runners compensate for a harder surface by making kinematic adjustments to minimize impact. With no strong evidence to link running-related injury to a particular running surface, patients should not be restricted to a softer running surface unless they notice a difference in comfort, because it is likely that they can compensate for a harder surface by adapting their gait.
Patients can therefore be counseled to run locally on sidewalks and neighborhood streets—if safe to do so—instead of obtaining a gym membership or driving to run on a trail. Such reassurance can increase a patient’s access to running and reduce barriers to exercise.
Continue to: BMI
BMI. Elevated BMI increases joint contact forces, which might increase risk of pain and injury.20 Results of studies investigating the link between BMI and running injury are mixed; some report that, in regard to bone stress injury, overweight BMI (> 25) is a risk factor for male runners and underweight BMI (< 18.5) is a risk factor for female runners.4,6 An observational study concluded that, among half-marathon and marathon runners, there was no significant increase in race-related injury, based on BMI.9 However, another study showed a higher rate of running-related injury in novice runners who had a higher BMI.10 A prospective cohort study found that runners with a higher BMI reported increased knee stiffness, which can place a runner at higher risk of overuse injury.4
Although these results conflict, there is consistency in the finding that obese novice runners are likely at increased risk of running-related injury; it is reasonable, therefore, for you to discuss strategies to reduce the risk of other modifiable factors, especially among obese novice runners. Patients with a higher BMI should not be discouraged from running, because exercise in combination with healthy eating habits is essential to decrease the myriad adverse health outcomes associated with obesity.
Female runners with a lower BMI, especially in the presence of other components of the female athlete triad (inadequate nutrition, amenorrhea, and low bone density), should be counseled about their increased risk of bone stress injury.21 Notably, a study of female US Navy recruits randomized to receive a trial of dietary supplementation of vitamin D plus calcium, or placebo, showed a 21% lower incidence of bone stress injury in the active-treatment group.22 To mitigate risk of injury associated with low BMI and the female athlete triad, therefore, a multidisciplinary approach of nutrition intervention, dietary optimization of vitamin D and calcium, and, possibly, activity modification should be implemented when appropriate.
Running gait. A study using 2-dimensional gait analysis to visualize biomechanical running patterns in injured and noninjured runners found that, in regard to mechanical variables, running-related injury was most strongly associated with contralateral pelvic drop.23 Gait retraining can be employed to help decrease contralateral pelvic drop.24 In addition, pelvic drop is often a result of weak gluteal muscles, and can be improved by doing strengthening exercises at home or with physical therapy.
Longer stride is also associated with running-related injury.25 A study showed improvement in patellofemoral pain by having runners increase stride rate by 10%, which reduces stride length to a significant degree.25,26 These improvements were maintained at 1-month and 3-month follow-up, and required only 1 gait retraining session.
Continue to: Get analysis is not feasible...
Gait analysis is not feasible in most primary care clinics. Instead, patients who run and (1) in whom pain persists despite more traditional treatments and (2) who have had recurring injury should be referred to a gait lab for analysis, usually by a physical therapist.
Nonmodifiable risk factors
Arch height. A high arch (pes cavus) is associated with increased risk of running-related injury, including bone stress injury, Achilles tendinopathy, plantar fasciitis, and patellofemoral pain syndrome.5 The mechanism of injury is thought to be increased forefoot loading forces.1
A review article showed that patients with pes cavus have reduced pain when using an orthosis, although there is no associated decrease in the risk of injury.5 To the contrary, a prospective study concluded that arch height was unrelated to increased risk of running-related injury.7
Evidence regarding flat feet (pes planus) and risk of injury is also mixed. Some studies show that pes planus is not associated with increased risk of injury in athletes.12 A cross-sectional study in older patients showed those with pes planus morphology had a higher rate of knee pain and wearing away of medial compartment cartilage.13 Because this study comprised only older adults, it is not generalizable to runners—nor can conclusions be drawn about causation, given the cross-sectional nature of the study.
Although a foot orthosis can correct mechanical differences caused by pes planus morphology, there is not enough evidence to conclude that correction results in a lower rate of injury. In sum, data are mixed with regard to arch height as a risk factor for running-related injury.
Continue to: Patients with...
Patients with pes planus or pes cavus should not be discouraged from running, however. If they experience pain with running, they might benefit from a trial of arch support inserts; or consider referral to an orthotist for evaluation for a custom orthosis.
Sex. Based on a prospective cohort study, female runners have a slightly higher rate of running injury than male counterparts.4 Similarly, a study showed that female military members generally had a higher incidence of stress fractures than male military members—specifically, femoral shaft and neck stress fractures.14 Runners who fall in the spectrum of the female athlete triad, as described earlier, are particularly vulnerable to bone stress injury. It is reasonable, therefore, to review risk factors for injury with female runners (as it is with all runners), especially those who have sustained a prior running-related injury.
Increased Q-angle (an obsolete risk factor). The Q-angle is approximated by drawing a line from the anterior superior iliac spine to the patella and a second line from the patella to the tibial tubercle. In males, a normal Q-angle is 14°; in females, 17° (SD = 4.5°). The Q-angle can be obtained by goniometric or radiographic measurement.
An increased Q-angle had been considered an intrinsic risk factor for running injury but has not been shown to be associated with increased risk of running-related injury or patellofemoral pain syndrome.27,28 Because the Q-angle is not a clinically relevant tool in assessing risk of injury, do not routinely measure it or include it in risk-factor counseling.
OA. Based on a systematic review of observational studies, data are inconclusive with regard to whether running contributes to, or is protective against, knee OA.15 In a large cohort study, running (1) was protective against development of hip OA and (2) decreased the risk of requiring hip replacement.29 This finding was supported by animal-model research that concluded that it is inactivity that results in thinning of articular cartilage.29 In addition, a systematic review of randomized controlled trials concluded that knee joint-loading exercises are not harmful to articular cartilage (this is low-quality evidence, however).16
Continue to: Given that there...
Given that there are no high-quality studies suggesting that running contributes to or exacerbates OA, patients with OA can be counseled to start or continue running as tolerated because the health benefit of running likely outweighs risk. Patients with pre-existing moderate-to-severe OA might report knee and hip pain that is already exacerbated by certain activities; if a high-impact activity, such as running, makes that pain worse, exercise counseling that you provide can be tailored to include lower-impact alternatives, such as swimming, cycling, or an elliptical workout.
CASE
In response to Ms. K’s interest in beginning an exercise regimen that includes running, you perform a complete routine pre-participation evaluation and appropriate cardiac screening. You discuss risk factors for running injury, focusing on modifiable risk factors.
Ms. K is perimenopausal but reports a history of regular menstrual cycles. She eats a relatively well-balanced diet. You advise that her BMI should not restrict her from incorporating running into her fitness regimen. Also, you reassure her that she should not restrict running based on a diagnosis of OA; instead, you advise her to monitor her symptoms and reconsider her program if running makes her knee pain worse.
At this point, Ms. K is ready to run. She tells you that, based on your guidance, she feels more comfortable and safe starting a running program.
Preventing injury
After reviewing risk factors for running-related injury with patients, encourage other evidence-based methods of reducing that risk.
Continue to: Shoes
Shoes
The running shoe industry offers a variety of running shoes, from minimalist shoes to cushioned stability shoes that vary based on the amount of cushioning, level of motion control, and amount of heel-to-toe drop. With so many options, new runners might wonder which shoes can reduce their risk of injury and how they should select a pair.
Stability. A characteristic of running shoes promoted by the industry is their stability: ie, their motion control. Stability shoes are marketed to runners who overpronate and therefore limit motion to prevent overpronation. The benefit of stability shoes, or stability insoles, is unclear.30 A randomized controlled trial showed that, in runners who overpronate, motion-control shoes reduced their risk of injury.31 However, another study assessed whether shoes that had been “prescribed” based on foot morphology and stride reduced the risk of injury (compared to neutral, cushioned shoes) and found no change in the incidence of soft-tissue injury.32 Given no strong evidence to suggest otherwise, runners can be advised to buy shoes based on comfort rather than on foot morphology or running stride.
Heel-to-toe drop. Another component of shoe variability is heel-to-toe drop (the height difference between heel and forefoot). A study suggests that moderate-to-high (8-12 mm) heel-to-toe drop is associated with a reduced risk of running injury.33 Barefoot running shoes, which, typically, have no heel-to-toe drop, are associated with increased risk of injury—specifically, foot stress fracture (especially in runners who are even moderately overweight).34,35
Shoe age and shoe wear can be modified to reduce injury. There is evidence that running shoes lose approximately 50% of cushioning after 300 to 500 miles of use.36 Another study found that rotating running shoes—ideally, different types or brands—can lead to fewer running-related injuries.37
In general, patients can be counseled to use shoes that feel comfortable, as long as they replace them regularly (TABLE 2). Runners can also consider alternating pairs of different running shoes between runs. Overweight runners should avoid minimally cushioned and low heel-to-toe drop running shoes.
Continue to: Cross-training
Cross-training
Cross-training exercises for runners include cycling, an elliptical workout, swimming, and weightlifting. Incorporating cross-training can be protective against running injury because cross-training requires different movement patterns, prevents overuse, and equalizes muscle imbalances that occur with running.7 In addition, replacing running with a cross-training activity can decrease weekly running time and mileage, which can further reduce risk of running-related injury.7 Runners—especially higher-mileage runners—should be encouraged to incorporate cross-training into their workout regimen to decrease their risk of injury.
Stretching. The authors of a Cochrane review concluded that there is no significant reduction in injury associated with hamstring or gastrocnemius stretching.32 A small randomized, controlled, crossover study concluded that participants subjectively felt their performance was better when warm-ups included stretching.38 This perceived improvement in performance was similar between groups who completed dynamic or static stretching. However, no difference was noted in flexibility or objective performance between groups who stretched or did not stretch before activity.
Although there is no supporting evidence that stretching reduces the risk of injury, stretching is a low-risk intervention. Because stretching might provide subjective benefit to runners, you need not discourage patients from including this activity in their running program.
CORRESPONDENCE
Kartik Sidhar, MD, 15370 Huff Way, Brookfield, WI, 53005; [email protected]
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
1. Brown CR Jr. Common injuries from running. In: Imboden JB, Hellerman, DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. McGraw-Hill; 2013.
2. Lange D. Running & jogging - statistic and facts. Statista Web site. November 16, 2020. Accessed March 28, 2021. www.statista.com/topics/1743/running-and-jogging/
3. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541-556. doi:10.1097/HCO.0000000000000437
4. Messier SP, Martin DF, Mihalko SL, et al. A 2-year prospective cohort study of overuse running injuries: The Runners and Injury Longitudinal Study (TRAILS). Am J Sports Med. 2018;46:2211-2221. doi:10.1177/0363546518773755
5. Fields KB, Sykes JC, Walker KM, et al. Prevention of running injuries. Curr Sports Med Rep. 2010;9:176-182. doi:10.1249/JSR.0b013e3181de7ec5
6. van der Worp MP, ten Haaf DSM, van Cingel R. Injuries in runners; a systematic review on risk factors and sex differences. PLoS One. 2015;10:1-18. doi:10.1371/journal.pone.0114937
7. Taunton JE, Ryan MB, Clement DB, et al. A prospective study of running injuries: the Vancouver Sun Run “In Training” clinics. Br J Sports Med. 2003;37:239-244. doi:10.1136/bjsm.37.3.239
8. Dixon SJ, Collop AC, Batt ME. Surface effects on ground reaction forces and lower extremity kinematics in running. Med Sci Sports Exerc. 2000;32:1919-1926. doi:10.1097/00005768-200011000-00016
9. Vadeboncoeur TF, Silvers SM, Taylor WC, et al. Impact of a high body mass index on lower extremity injury in marathon/half-marathon participants. J Phys Act Health. 2012;9:96-103. doi:10.1123/jpah.9.1.96
10. Buist I, Bredeweg SW. Higher risk of injury in overweight novice runners. Br J Sports Med. 2011;45:338. http://dx.doi.org/10.1136/bjsm.2011.084038.79
11. Cowan DN, Jones BH, Robinson JR. Foot morphologic characteristics and risk of Exercise-related injury. Arch Fam Med. 1993;2:773-777. doi:10.1001/archfami.2.7.773
12. Michelson JD, Durant DM, McFarland E. The injury risk associated with pes planus in athletes. Foot Ankle Int. 2002;23:629-633. doi: 10.1177/107110070202300708
13. Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res (Hoboken). 2011;63:937-944. doi:10.1002/acr.20431
14. Waterman BR, Gun B, Bader JO, et al. Epidemiology of lower extremity stress fractures in the United States military. Mil Med. 2016;181:1308-1313. doi:10.7205/MILMED-D-15-00571
15. Timmins KA, Leech RD, Batt ME, et al. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447-1457. doi:10.1177/0363546516657531
16. Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2019;53:940-947. doi:10.1136/bjsports-2017-098661
17. Johnston CAM, Taunton JE, Lloyd-Smith DR, et al. Preventing running injuries. Practical approach for family doctors. Can Fam Physician. 2003;49:1101-1109.
18. Buist I, Bredeweg SW, van Mechelen W, et al. No effect of a graded training program on the number of running-related injuries in novice runners: a randomized controlled trial. Am J Sports Med. 2008;36:33-39. doi:10.1177/0363546507307505
19. Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44:749-765. doi:10.2519/jospt.2014.5334
20. Kim N, Browning RC, Lerner ZF. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Posture. 2019;73:209-214. doi:10.1016/j.gaitpost.2019.07.307
21. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139-149. doi:10.1016/j.pmr.2015.08.008
22. Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. doi:10.1359/jbmr.080102
23. Bramah C, Preece SJ, Gill N, et al. Is there a pathological gait associated with common soft tissue running injuries? Am J Sports Med. 2018;46:3023-3031. doi:10.1177/0363546518793657
24. Willy RW, Scholz PT, Davis IS. Mirror gait retraining for the treatment of patellofemoral pain in female runners. Clin Biomech (Bristol Avon). 2012;27:1045-1051. doi:10.1016/j.clinbiomech.2012.07.011
25. Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014;6:210-217. doi:10.1177/1941738113508544
26. Bramah C, Preece SJ, Gill N et al. A 10% increase in step rate improves running kinematics and clinical outcomes in runners with patellofemoral pain at 4 weeks and 3 months. Am J Sports Med. 2019;47:3406-3413. doi: 10.1177/0363546519879693
27. Ramskov D, Jensen ML, Obling K, et al. No association between q-angle and foot posture with running-related injuries: a 10 week prospective follow-up study. Int J Sports Phys Ther. 2013;8:407-415.
28. Almeida GPL, Silva AP, França FJR, et al. Q-angle in patellofemoral pain: relationship with dynamic knee valgus, hip abductor torque, pain and function. Rev Bras Ortop. 2016;51:181-186. doi:10.1016/j.rboe.2016.01.010
29. Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45:1292-1297. doi:10.1249/MSS.0b013e3182885f26
30. Nigg BM, Baltich J, Hoerzer S, et al. Running shoes and running injuries: mythbusting and a proposal for two new paradigms: ‘Preferred movement path’ and ‘comfort filter.’ Br J Sports Med. 2015;49:1290-1294. doi:10.1136/bjsports-2015-095054
31. Malisoux L, Chambon N, Delattre N, et al. Injury risk in runners using standard or motion control shoes: a randomised controlled trial with participant and assessor blinding. Br J Sports Med. 2016;50:481-487. doi:10.1136/bjsports-2015-095031
32. Yeung SS, Yeung EW, Gillespie LD. Interventions for preventing lower limb soft-tissue running injuries. Cochrane Database Syst Rev. 2011(7):CD001256. doi:10.1002/14651858.cd001256.pub2
33. Malisoux L, Chambon N, Urhausen A, et al. Influence of the heel-to-toe drop of standard cushioned running shoes on injury risk in leisure-time runners: a randomized controlled trial with 6-month follow-up. Am J Sports Med. 2016;44:2933-2940. doi:10.1177/0363546516654690
34. Ryan M, Elashi M, Newsham-West R, et al. Examining injury risk and pain perception in runners using minimalist footwear. Br J Sports Med. 2014;48:1257-1262. doi:10.1136/bjsports-2012-092061
35. Fuller JT, Thewlis D, Buckley JD, et al.. Body mass and weekly training distance influence the pain and injuries experienced by runners using minimalist shoes: a randomized controlled trial. Am J Sports Med. 2017;45:1162-1170. doi:10.1177/0363546516682497
36. Cook SD, Kester MA, Brunet ME. Shock absorption characteristics of running shoes. Am J Sports Med. 1985;13:248-253. doi.org/10.1177/036354658501300406
37. Malisoux L, Ramesh J, Mann R, et al. Can parallel use of different running shoes decrease running-related injury risk? Scand J Med Sci Sport. 2015;25:110-115. doi:10.1111/sms.12154
38. Blazevich AJ, Gill ND, Kvorning T, et al. No effect of muscle stretching within a full, dynamic warm-up on athletic performance. Med Sci Sports Exerc. 2018;50:1258-1266. doi:10.1249/MSS.0000000000001539
PRACTICE RECOMMENDATIONS
› Counsel runners to cross-train, replace shoes regularly, and use shoes with moderate-to-high (8-12 mm) heel-to-toe drop. C
› Don’t discourage running for exercise, as long as it is tolerated, in patients who have osteoarthritis. C
› Encourage moderation in running distance and intensity, especially in novice runners. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
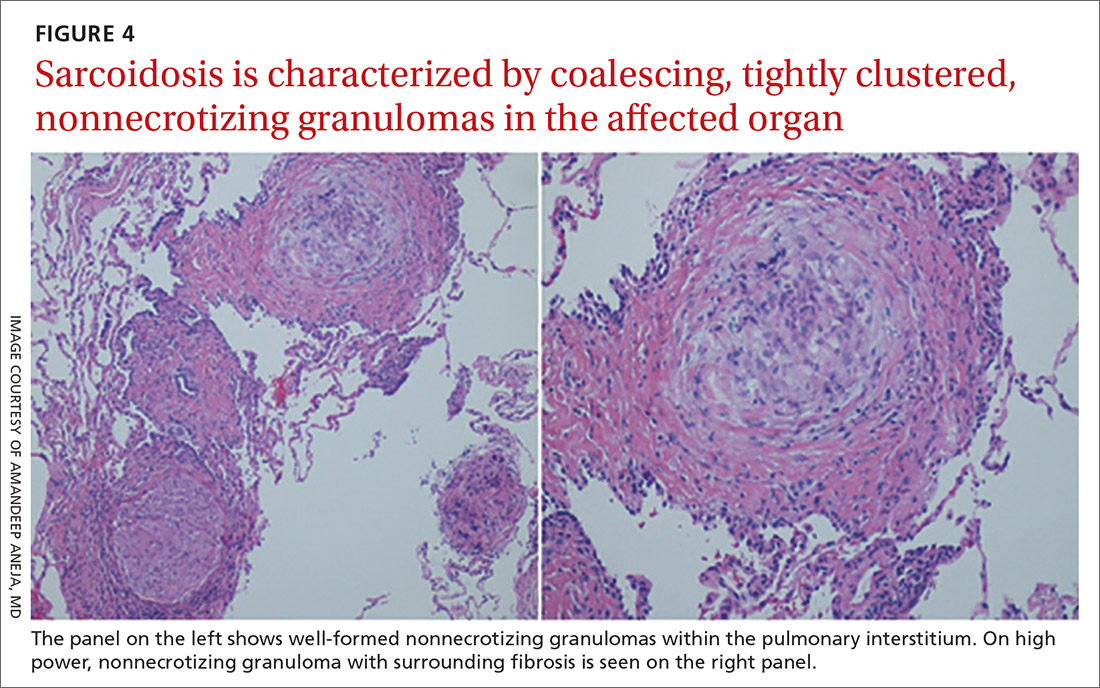
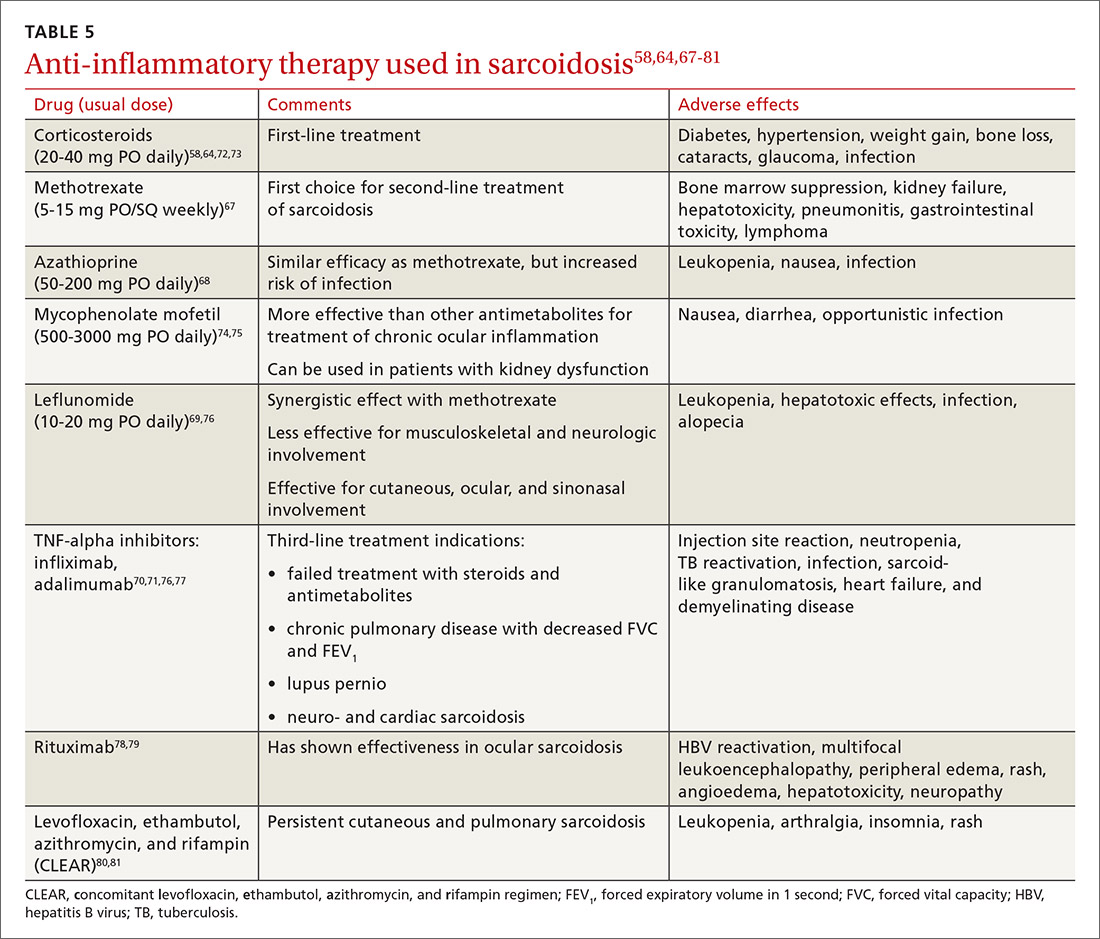
Sarcoidosis: An FP’s primer on an enigmatic disease
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
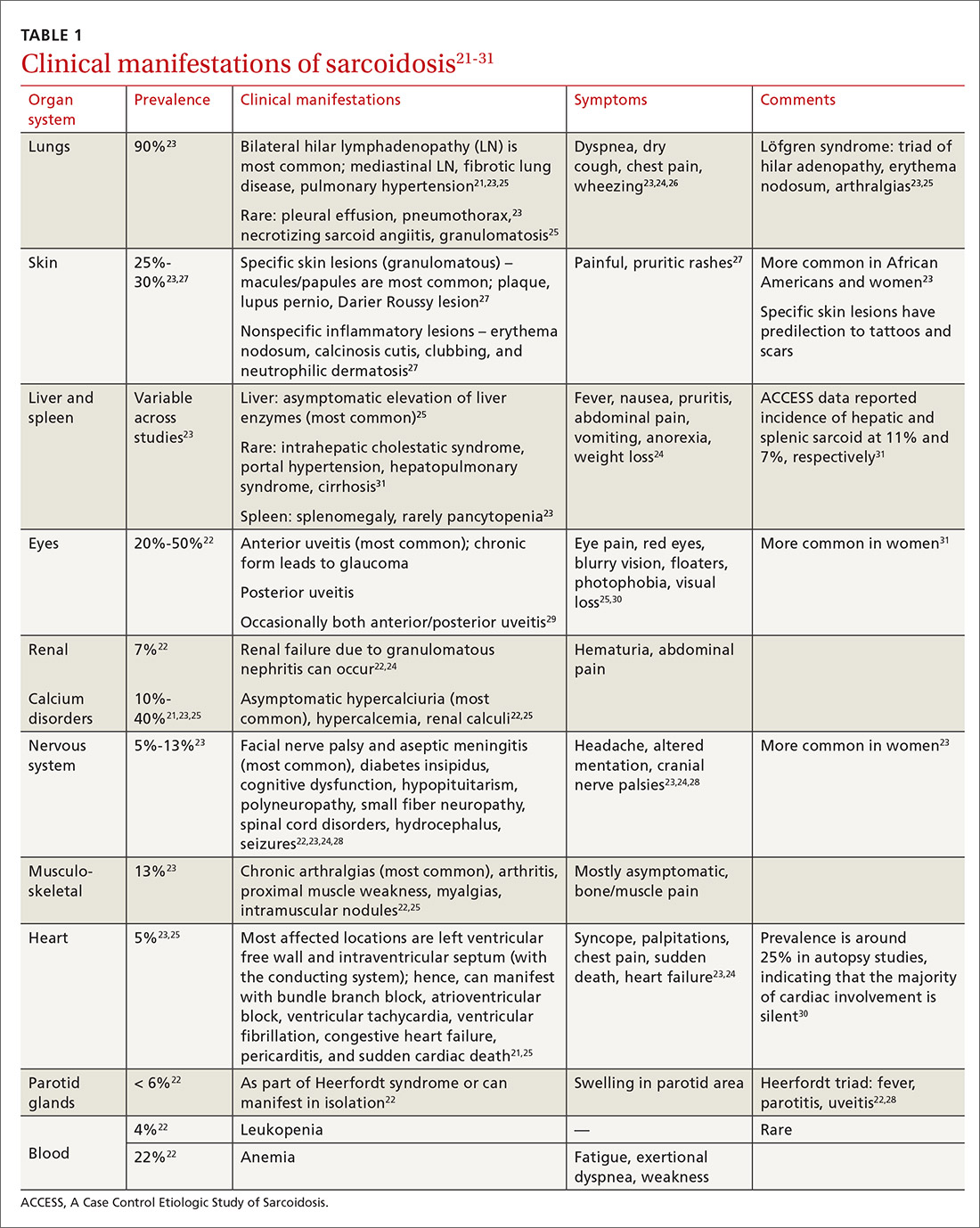
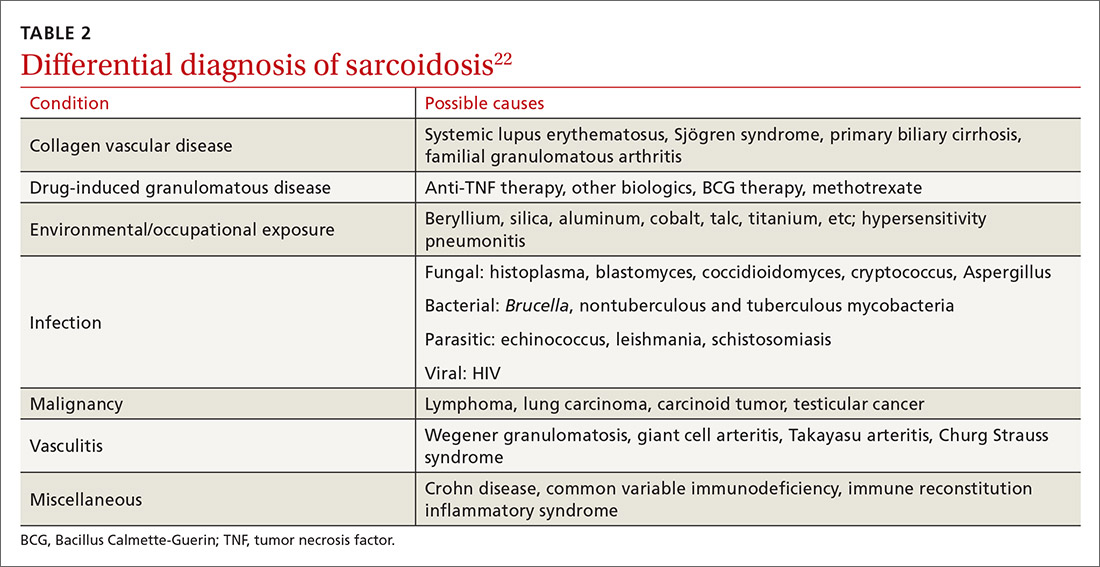
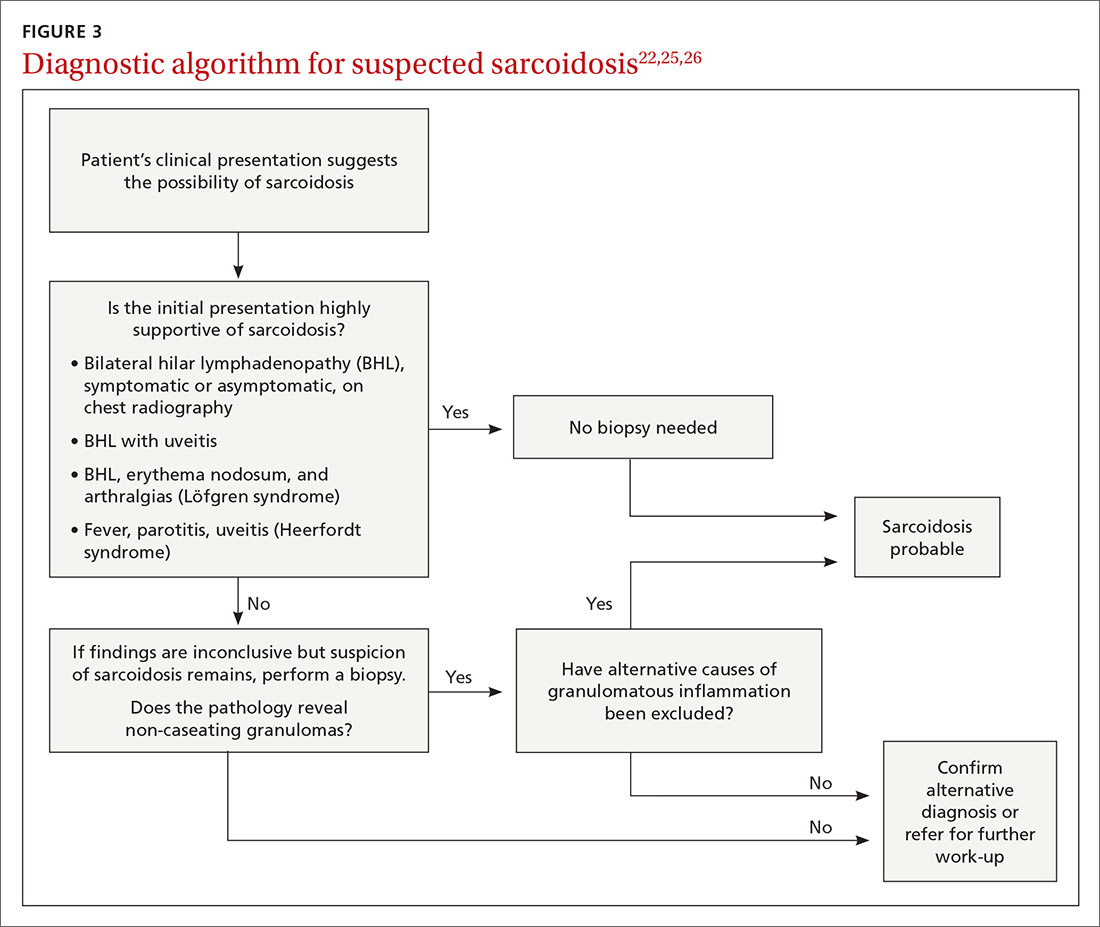
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
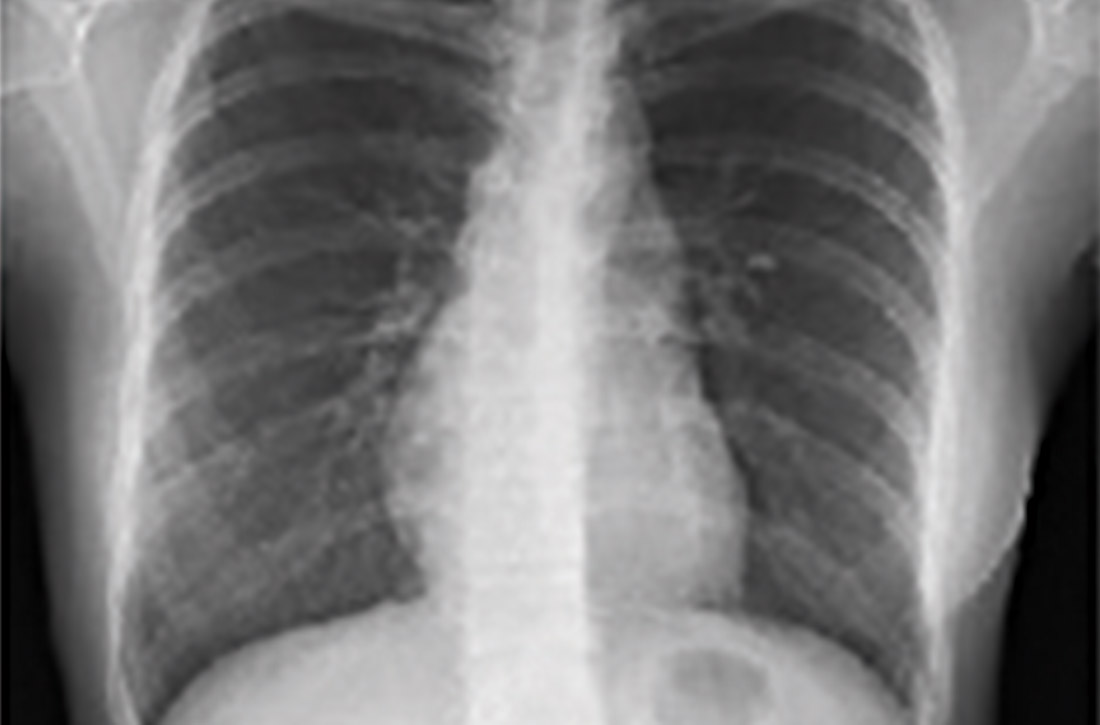
Radiologic studies
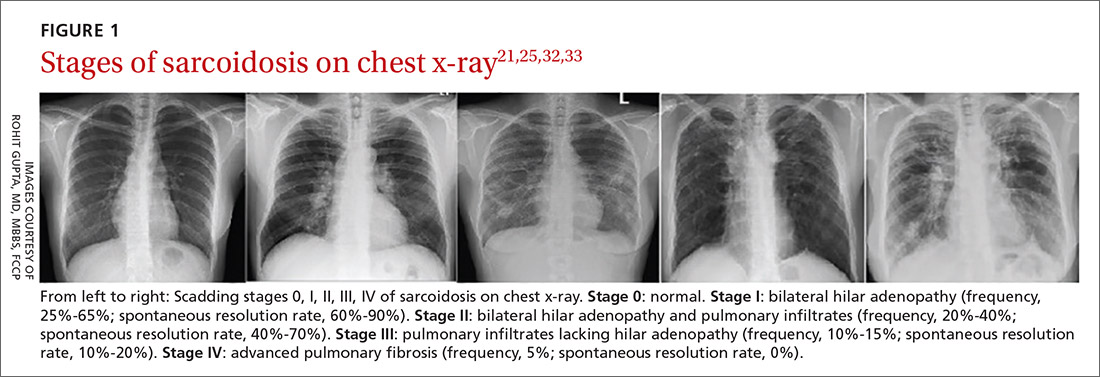
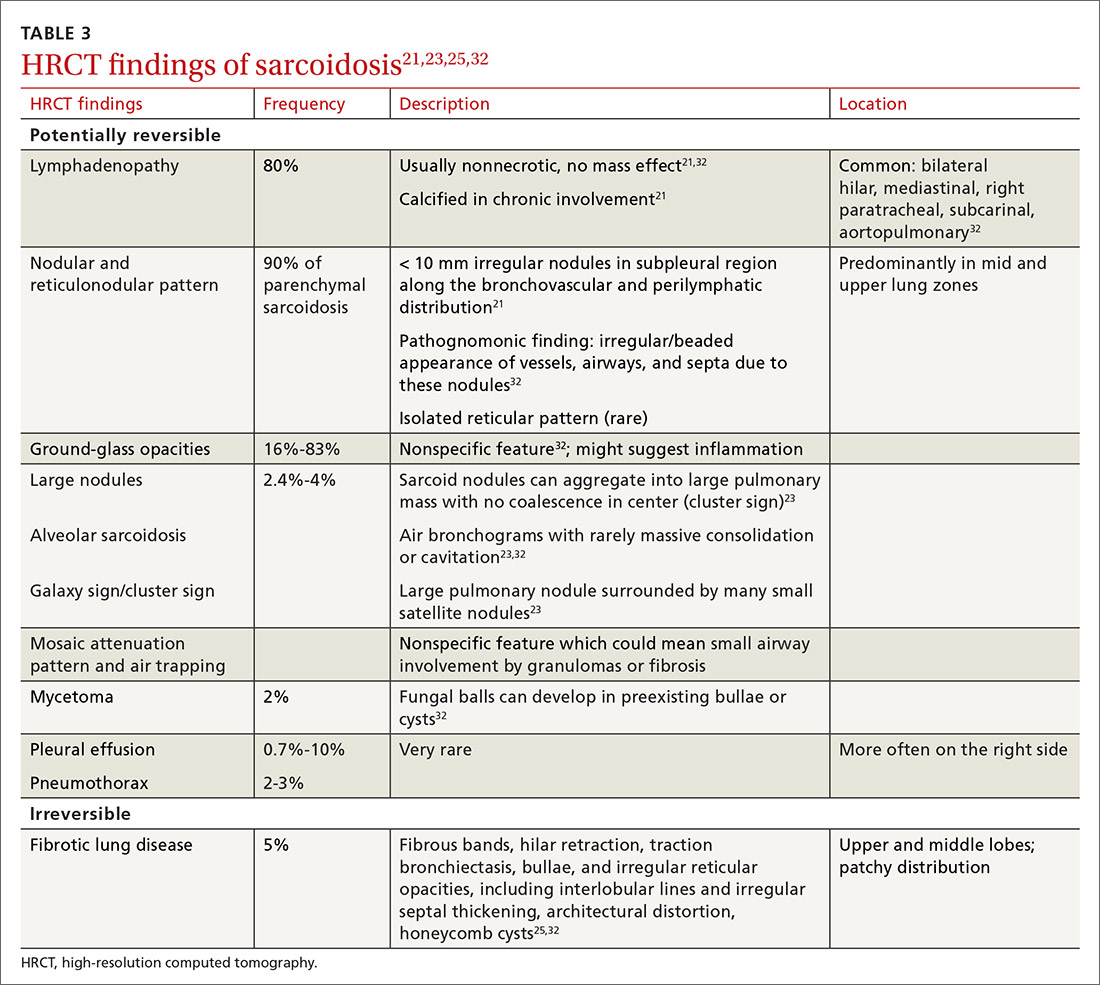
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
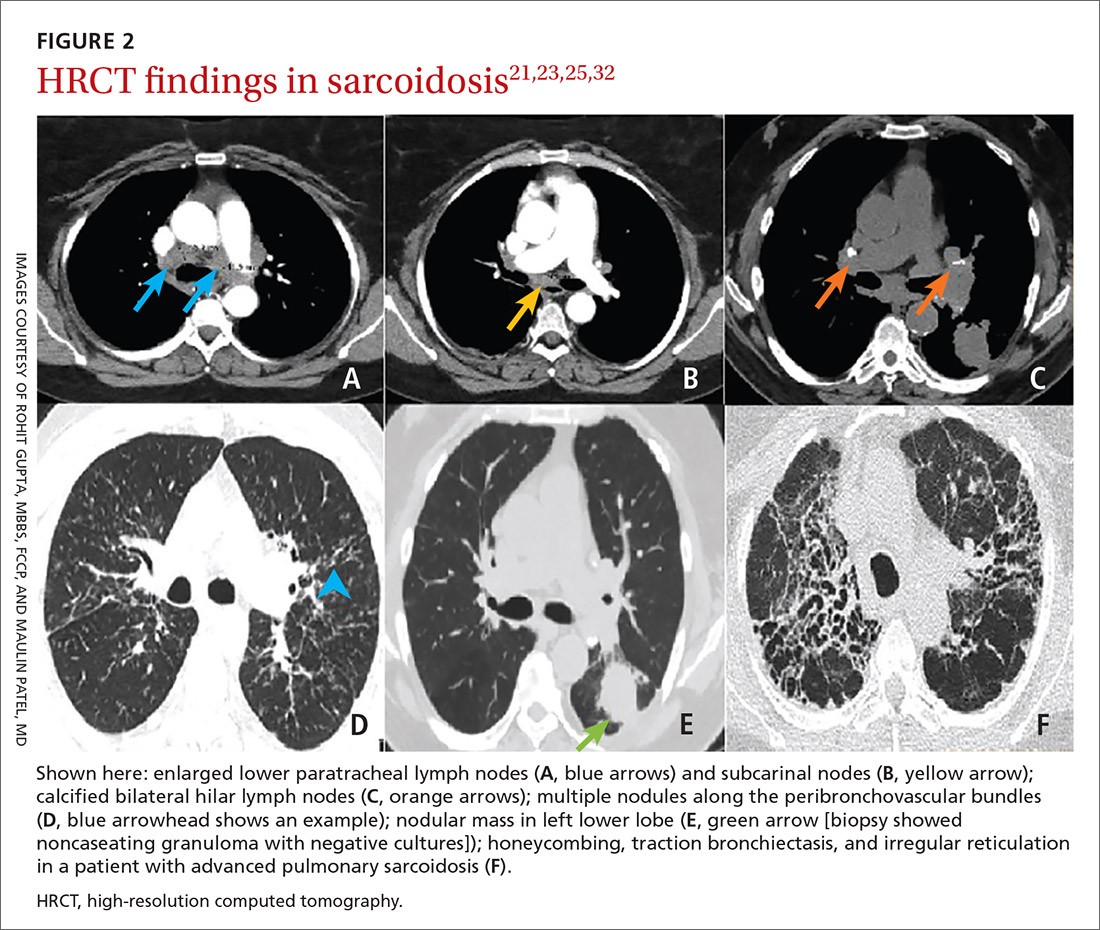
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
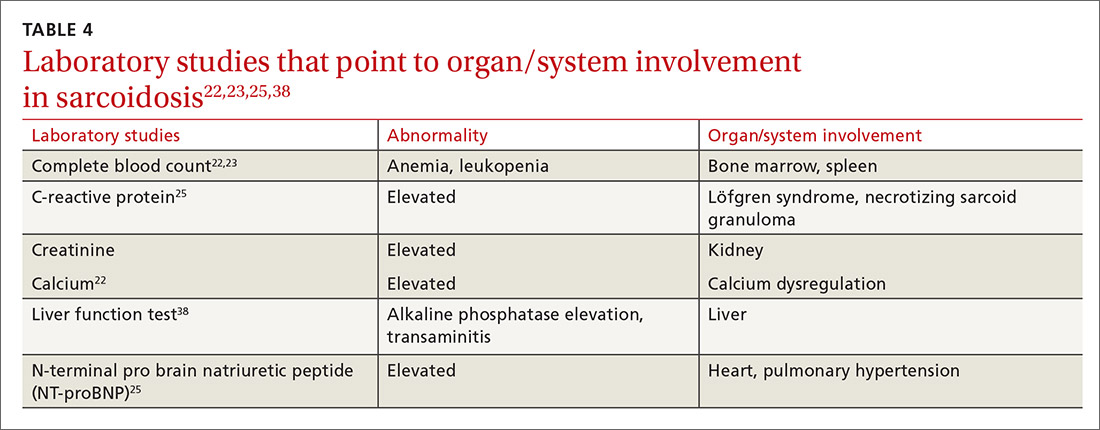
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
PRACTICE RECOMMENDATIONS
› Consider biopsy to aid in diagnosing sarcoidosis; it may be avoided with a high clinical suspicion for sarcoidosis (eg, Löfgren syndrome, lupus pernio, or Heerfordt syndrome). C
› Rule out alternative diagnoses such as infection, malignancy, collagen vascular disease, and vasculitis. C
› Identify extra-pulmonary organ involvement, as clinically indicated, by screening with a baseline eye examination; complete blood count; creatinine, alkaline phosphatase, and calcium levels; electrocardiogram, and other organ-specific studies. C
› Make a patient-centered decision whether to begin antiinflammatory treatment based on symptomatology and risk of organ failure or death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Guarding against nonmelanoma skin cancer in solid organ transplant recipients
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
PRACTICE RECOMMENDATIONS
› Conduct a full-body skin examination at least once annually for solid organ transplant recipients. C
› Encourage daily use of broad-spectrum SPF ≥ 30 sunscreen and sun-protective clothing (long sleeves, pants, wide-brimmed hats) for these patients. A
› Consider chemoprophylactic agents for patients at especially high risk of nonmelanoma skin cancer. A
› Treat nonmelanoma skin cancer in a solid organ transplant recipient aggressively because of their increased risk of recurrence, local invasion, and metastasis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Urine drug screening: A guide to monitoring Tx with controlled substances
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4
A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15
Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19
Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20
Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).
Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4
A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15
Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19
Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20
Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).
Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4
A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15
Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19
Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20
Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).
Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
PRACTICE RECOMMENDATIONS
› Consider developing a risk-based urine drug testing protocol for all patients who are on chronic opioid therapy. C
› Consider urine drug testing to augment a thorough history when identifying and offering treatment to patients with a substance use disorder. A
› Do not change your management plan based on results of a single screening urine test. Revisit unexpected positive or negative results with a thorough history or confirmatory testing. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Anticipating the care adolescents will need
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.
A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.
Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48
Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.
A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.
Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48
Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.
A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.
Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48
Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; [email protected].
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
PRACTICE RECOMMENDATIONS
› Consider using a 2-question screening tool for adolescents that asks about personal use of alcohol and use of alcohol by friends; this resource offers a risk assessment with recommendations. C
› Consider using the American Academy of Pediatrics Family Media Plan to provide age-specific guidelines to help parents or caregivers establish rules for online activities. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Conservative or surgical management for that shoulder dislocation?
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
PRACTICE RECOMMENDATIONS
› Start with conservative management of shoulder dislocation in patients older than 30 years and those with uncomplicated injuries. B
› Discourage strict immobilization; its utility is debated and it may not change outcomes. B
› Recommend a progressive rehabilitative program after the initial acute shoulder injury. B
› Consider surgical management for patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability or for the highly physically active individual. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Functional neurological disorder: A practical guide to an elusive Dx
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.
Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.
Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.
Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
PRACTICE RECOMMENDATIONS
› Avoid using stigmatizing terminology (eg, adding the prefix “pseudo” or the adjective “hysterical”) to characterize a suspected functional neurological disorder (FND) or a medically unexplained disorder. C
› Refrain from ordering functional magnetic resonance imaging as part of the routine evaluation of suspected FND. C
› Validate the patient‘s concerns with an appropriate diagnostic label; use layman’s terms to discuss the diagnostic parameters of FND and the cause of symptoms; and emphasize treatment possibilities and plans. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series