User login
CDC coronavirus testing decision likely to haunt nation for months to come
As the novel coronavirus snaked its way across the globe, the Centers for Disease Control and Prevention in early February distributed 200 test kits it had produced to more than 100 public health labs run by states and counties nationwide.
Each kit contained material to test a mere 300-400 patients. And labs, whether serving the population of New York or tiny towns in rural America, apparently received the same kits.
The kits were distributed roughly equally to locales in all 50 states. That decision presaged weeks of chaos, in which the availability of COVID-19 tests seemed oddly out of sync with where testing was needed.
A woman in South Dakota with mild symptoms and no fever readily got the test and the results. Meanwhile, political leaders and public officials in places like New York, Boston, Seattle, and the San Francisco Bay area – all in the throes of serious outbreaks – couldn’t get enough tests to screen ill patients or, thereby, the information they needed to protect the general public and stem the outbreak of the virus, whose symptoms mimic those of common respiratory illnesses.
Rapid testing is crucial in the early stages of an outbreak. It allows health workers and families to identify and focus on treating those infected and isolate them.
Yet health officials in New York and such states as New York, Washington, Pennsylvania, and Georgia confirmed that they each initially got one test kit, calling into question whether they would have even stood a chance to contain the outbreaks that would emerge. They would soon discover that the tests they did receive were flawed, lacking critical components, and delivering faulty results.
During those early weeks, the virus took off, infecting thousands of people and leading to nationwide social distancing and sheltering in place. Public health officials are just beginning to grapple with the fallout from that early bungling of testing, which is likely to haunt the country in the months to come.
Too little too late
The first shipment to Washington state arrived more than 2 weeks after officials there announced the first U.S. case of coronavirus, and at a moment when deadly outbreaks of the disease were already festering in places like the Life Care Center in Kirkland. Within weeks, three dozen people infected with COVID-19 would die at the nursing home in the suburbs of Seattle.
The spread of COVID-19 would not take long to overwhelm the state, which as of March 20, 2020, had more than 1,300 cases.
The Trump administration in recent days has attempted to speed testing for the virus after early missteps hampered the government’s response to contain the contagion, and officials have had to respond to a barrage of criticism from public health experts, state officials, and members of Congress.
Federal health officials have eased the process for university and commercial labs to perform their own tests, and they are ramping up their capacity. As of March 16, public and private labs in the United States had the ability to test more than 36,000 people a day, according to estimates compiled by the American Enterprise Institute, a conservative-leaning think tank in Washington, a figure expected to rapidly escalate in coming weeks. That figure, however, can vary considerably by state and does not indicate how many tests are actually given to patients.
“We are now beginning to see that they have spread out in a prioritized way. We asked them to prioritize the regions that were mostly affected,” Deborah Birx, the coronavirus response coordinator for the White House Coronavirus Task Force, said March 18 of private labs’ testing, without elaboration.
The scaling up of testing is set to take place after weeks of faltering and hundreds, if not thousands, of undiagnosed people spreading the virus. For example, New York’s state health department received a faulty CDC test kit on Feb. 8 for 800 patient specimens, an amount that’s consistent with other states, according to a spokesperson. It later began testing patients with a test that state officials developed based on the CDC protocol and has significantly increased testing – as of March 20, more than 7,200 people had tested positive statewide.
In New York City, the first batch was obtained on Feb. 7.
“The other state and local public health laboratories got test kits as they became available,” said Eric Blank, chief program officer of the Association of Public Health Laboratories.
Places in the middle of the country with no outbreaks had the luxury of time to plan. For example, Missouri officials have had about 800 tests to work with, leading to only 395 performed so far in the region by public health labs – 26 of which were positive. When private lab tests are accounted for, as of March 20 there were 47 confirmed cases.
Health care providers and public health staff in the state, however, benefited from the fact that there is less international travel to the region, according to infectious disease expert Steven Lawrence, MD, of Washington University, St. Louis.
“This is very similar to 1918 with the influenza pandemic – St. Louis had more time to prepare and was able to put measures in place to flatten the curve than, say, Philadelphia,” Dr. Lawrence said. “Seattle didn’t have an opportunity to prepare as much in advance.”
While commercial labs are coming online, strict restrictions are limiting testing capabilities, Dr. Lawrence said.
“The state has had their hands tied,” he added.
Waiting And wondering
Because of a widespread lag in testing, it is still a mystery for thousands of people to know whether they’ve come into contact with an infected person until well after it happens. As of March 20, the pandemic had killed more than 11,000 globally. More than 16,000 Americans were confirmed infected and at least 216 have died.
“CDC will distribute tests based on where they can do the most good. But without hospital-based testing and commercial testing, it will not be possible to meet the need,” said Tom Frieden, who led the CDC during President Obama’s administration and is a former commissioner of the New York City Health Department.
In California, public school teacher Claire Dugan, whose state was among the hardest hit in the initial wave of U.S. coronavirus cases, was told she didn’t qualify for testing because she had not traveled abroad to any country with an outbreak of the virus or been in contact with an infected person. Ms. Dugan, who lives in the San Francisco Bay area and is already medically fragile after a stray bullet nearly killed her while driving 4 years ago, sought a test from her doctor after registering a temperature of 100.7° F earlier this month.
“There are a lot of layers as to why this is so messed up,” said Ms. Dugan, who relies on a feeding tube and said she sought a test not only to protect herself but her students. “It’s community spreading right now, so it’s kind of silly we’re still insisting on [the early criteria for testing]. How would I know?”
Since the CDC’s initial distribution, states have been reordering more tests through the office’s International Reagent Resource – a long-standing tool that public health labs have relied on. They have also revised testing protocols to use only one sample per person, which boosts the number of people screened.
Yet problems still abound with tests or other materials needed to be able to detect the virus. California Gov. Gavin Newsom (D) said on March 12 that county public health labs can’t use all of the 8,000 test kits the state has because they are missing key components.
In Pennsylvania, state officials weren’t able to begin testing until March 2 because of problems with the CDC’s initial kit, according to Nate Wardle, a spokesperson at its department of health. New York received two newly manufactured CDC test kits on Feb. 29 and also began performing tests March 2, according to its health department.
“We are still limited on extraction kits,” Mandy Cohen, the Health & Human Services secretary in North Carolina, said in an interview in mid-March. Officials earlier this month could test only 300 patients because of shortages in the extraction materials needed to register whether the novel coronavirus is present.
In North Dakota, Loralyn Hegland wrote her physician’s practice an email on March 10 with the subject line “dry cough,” wondering if she should come in for testing after learning that was one symptom of COVID-19. The recommendation she got echoes those of countless others across the United States, saying her risk of being exposed was very low because she hadn’t traveled outside the United States and had not come into contact with a person who had been “definitely” diagnosed with the virus.
Ms. Hegland, who lives in Fargo, didn’t have a fever but decided to shelter herself, anyway, out of caution.
Would she push to get a test?
“What’s the point?” she said. “You can’t know what you don’t know. It’s just that simple. How else do you explain it to people when you’re not testing?”
KHN Midwest correspondent Lauren Weber in St. Louis contributed to this article.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
As the novel coronavirus snaked its way across the globe, the Centers for Disease Control and Prevention in early February distributed 200 test kits it had produced to more than 100 public health labs run by states and counties nationwide.
Each kit contained material to test a mere 300-400 patients. And labs, whether serving the population of New York or tiny towns in rural America, apparently received the same kits.
The kits were distributed roughly equally to locales in all 50 states. That decision presaged weeks of chaos, in which the availability of COVID-19 tests seemed oddly out of sync with where testing was needed.
A woman in South Dakota with mild symptoms and no fever readily got the test and the results. Meanwhile, political leaders and public officials in places like New York, Boston, Seattle, and the San Francisco Bay area – all in the throes of serious outbreaks – couldn’t get enough tests to screen ill patients or, thereby, the information they needed to protect the general public and stem the outbreak of the virus, whose symptoms mimic those of common respiratory illnesses.
Rapid testing is crucial in the early stages of an outbreak. It allows health workers and families to identify and focus on treating those infected and isolate them.
Yet health officials in New York and such states as New York, Washington, Pennsylvania, and Georgia confirmed that they each initially got one test kit, calling into question whether they would have even stood a chance to contain the outbreaks that would emerge. They would soon discover that the tests they did receive were flawed, lacking critical components, and delivering faulty results.
During those early weeks, the virus took off, infecting thousands of people and leading to nationwide social distancing and sheltering in place. Public health officials are just beginning to grapple with the fallout from that early bungling of testing, which is likely to haunt the country in the months to come.
Too little too late
The first shipment to Washington state arrived more than 2 weeks after officials there announced the first U.S. case of coronavirus, and at a moment when deadly outbreaks of the disease were already festering in places like the Life Care Center in Kirkland. Within weeks, three dozen people infected with COVID-19 would die at the nursing home in the suburbs of Seattle.
The spread of COVID-19 would not take long to overwhelm the state, which as of March 20, 2020, had more than 1,300 cases.
The Trump administration in recent days has attempted to speed testing for the virus after early missteps hampered the government’s response to contain the contagion, and officials have had to respond to a barrage of criticism from public health experts, state officials, and members of Congress.
Federal health officials have eased the process for university and commercial labs to perform their own tests, and they are ramping up their capacity. As of March 16, public and private labs in the United States had the ability to test more than 36,000 people a day, according to estimates compiled by the American Enterprise Institute, a conservative-leaning think tank in Washington, a figure expected to rapidly escalate in coming weeks. That figure, however, can vary considerably by state and does not indicate how many tests are actually given to patients.
“We are now beginning to see that they have spread out in a prioritized way. We asked them to prioritize the regions that were mostly affected,” Deborah Birx, the coronavirus response coordinator for the White House Coronavirus Task Force, said March 18 of private labs’ testing, without elaboration.
The scaling up of testing is set to take place after weeks of faltering and hundreds, if not thousands, of undiagnosed people spreading the virus. For example, New York’s state health department received a faulty CDC test kit on Feb. 8 for 800 patient specimens, an amount that’s consistent with other states, according to a spokesperson. It later began testing patients with a test that state officials developed based on the CDC protocol and has significantly increased testing – as of March 20, more than 7,200 people had tested positive statewide.
In New York City, the first batch was obtained on Feb. 7.
“The other state and local public health laboratories got test kits as they became available,” said Eric Blank, chief program officer of the Association of Public Health Laboratories.
Places in the middle of the country with no outbreaks had the luxury of time to plan. For example, Missouri officials have had about 800 tests to work with, leading to only 395 performed so far in the region by public health labs – 26 of which were positive. When private lab tests are accounted for, as of March 20 there were 47 confirmed cases.
Health care providers and public health staff in the state, however, benefited from the fact that there is less international travel to the region, according to infectious disease expert Steven Lawrence, MD, of Washington University, St. Louis.
“This is very similar to 1918 with the influenza pandemic – St. Louis had more time to prepare and was able to put measures in place to flatten the curve than, say, Philadelphia,” Dr. Lawrence said. “Seattle didn’t have an opportunity to prepare as much in advance.”
While commercial labs are coming online, strict restrictions are limiting testing capabilities, Dr. Lawrence said.
“The state has had their hands tied,” he added.
Waiting And wondering
Because of a widespread lag in testing, it is still a mystery for thousands of people to know whether they’ve come into contact with an infected person until well after it happens. As of March 20, the pandemic had killed more than 11,000 globally. More than 16,000 Americans were confirmed infected and at least 216 have died.
“CDC will distribute tests based on where they can do the most good. But without hospital-based testing and commercial testing, it will not be possible to meet the need,” said Tom Frieden, who led the CDC during President Obama’s administration and is a former commissioner of the New York City Health Department.
In California, public school teacher Claire Dugan, whose state was among the hardest hit in the initial wave of U.S. coronavirus cases, was told she didn’t qualify for testing because she had not traveled abroad to any country with an outbreak of the virus or been in contact with an infected person. Ms. Dugan, who lives in the San Francisco Bay area and is already medically fragile after a stray bullet nearly killed her while driving 4 years ago, sought a test from her doctor after registering a temperature of 100.7° F earlier this month.
“There are a lot of layers as to why this is so messed up,” said Ms. Dugan, who relies on a feeding tube and said she sought a test not only to protect herself but her students. “It’s community spreading right now, so it’s kind of silly we’re still insisting on [the early criteria for testing]. How would I know?”
Since the CDC’s initial distribution, states have been reordering more tests through the office’s International Reagent Resource – a long-standing tool that public health labs have relied on. They have also revised testing protocols to use only one sample per person, which boosts the number of people screened.
Yet problems still abound with tests or other materials needed to be able to detect the virus. California Gov. Gavin Newsom (D) said on March 12 that county public health labs can’t use all of the 8,000 test kits the state has because they are missing key components.
In Pennsylvania, state officials weren’t able to begin testing until March 2 because of problems with the CDC’s initial kit, according to Nate Wardle, a spokesperson at its department of health. New York received two newly manufactured CDC test kits on Feb. 29 and also began performing tests March 2, according to its health department.
“We are still limited on extraction kits,” Mandy Cohen, the Health & Human Services secretary in North Carolina, said in an interview in mid-March. Officials earlier this month could test only 300 patients because of shortages in the extraction materials needed to register whether the novel coronavirus is present.
In North Dakota, Loralyn Hegland wrote her physician’s practice an email on March 10 with the subject line “dry cough,” wondering if she should come in for testing after learning that was one symptom of COVID-19. The recommendation she got echoes those of countless others across the United States, saying her risk of being exposed was very low because she hadn’t traveled outside the United States and had not come into contact with a person who had been “definitely” diagnosed with the virus.
Ms. Hegland, who lives in Fargo, didn’t have a fever but decided to shelter herself, anyway, out of caution.
Would she push to get a test?
“What’s the point?” she said. “You can’t know what you don’t know. It’s just that simple. How else do you explain it to people when you’re not testing?”
KHN Midwest correspondent Lauren Weber in St. Louis contributed to this article.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
As the novel coronavirus snaked its way across the globe, the Centers for Disease Control and Prevention in early February distributed 200 test kits it had produced to more than 100 public health labs run by states and counties nationwide.
Each kit contained material to test a mere 300-400 patients. And labs, whether serving the population of New York or tiny towns in rural America, apparently received the same kits.
The kits were distributed roughly equally to locales in all 50 states. That decision presaged weeks of chaos, in which the availability of COVID-19 tests seemed oddly out of sync with where testing was needed.
A woman in South Dakota with mild symptoms and no fever readily got the test and the results. Meanwhile, political leaders and public officials in places like New York, Boston, Seattle, and the San Francisco Bay area – all in the throes of serious outbreaks – couldn’t get enough tests to screen ill patients or, thereby, the information they needed to protect the general public and stem the outbreak of the virus, whose symptoms mimic those of common respiratory illnesses.
Rapid testing is crucial in the early stages of an outbreak. It allows health workers and families to identify and focus on treating those infected and isolate them.
Yet health officials in New York and such states as New York, Washington, Pennsylvania, and Georgia confirmed that they each initially got one test kit, calling into question whether they would have even stood a chance to contain the outbreaks that would emerge. They would soon discover that the tests they did receive were flawed, lacking critical components, and delivering faulty results.
During those early weeks, the virus took off, infecting thousands of people and leading to nationwide social distancing and sheltering in place. Public health officials are just beginning to grapple with the fallout from that early bungling of testing, which is likely to haunt the country in the months to come.
Too little too late
The first shipment to Washington state arrived more than 2 weeks after officials there announced the first U.S. case of coronavirus, and at a moment when deadly outbreaks of the disease were already festering in places like the Life Care Center in Kirkland. Within weeks, three dozen people infected with COVID-19 would die at the nursing home in the suburbs of Seattle.
The spread of COVID-19 would not take long to overwhelm the state, which as of March 20, 2020, had more than 1,300 cases.
The Trump administration in recent days has attempted to speed testing for the virus after early missteps hampered the government’s response to contain the contagion, and officials have had to respond to a barrage of criticism from public health experts, state officials, and members of Congress.
Federal health officials have eased the process for university and commercial labs to perform their own tests, and they are ramping up their capacity. As of March 16, public and private labs in the United States had the ability to test more than 36,000 people a day, according to estimates compiled by the American Enterprise Institute, a conservative-leaning think tank in Washington, a figure expected to rapidly escalate in coming weeks. That figure, however, can vary considerably by state and does not indicate how many tests are actually given to patients.
“We are now beginning to see that they have spread out in a prioritized way. We asked them to prioritize the regions that were mostly affected,” Deborah Birx, the coronavirus response coordinator for the White House Coronavirus Task Force, said March 18 of private labs’ testing, without elaboration.
The scaling up of testing is set to take place after weeks of faltering and hundreds, if not thousands, of undiagnosed people spreading the virus. For example, New York’s state health department received a faulty CDC test kit on Feb. 8 for 800 patient specimens, an amount that’s consistent with other states, according to a spokesperson. It later began testing patients with a test that state officials developed based on the CDC protocol and has significantly increased testing – as of March 20, more than 7,200 people had tested positive statewide.
In New York City, the first batch was obtained on Feb. 7.
“The other state and local public health laboratories got test kits as they became available,” said Eric Blank, chief program officer of the Association of Public Health Laboratories.
Places in the middle of the country with no outbreaks had the luxury of time to plan. For example, Missouri officials have had about 800 tests to work with, leading to only 395 performed so far in the region by public health labs – 26 of which were positive. When private lab tests are accounted for, as of March 20 there were 47 confirmed cases.
Health care providers and public health staff in the state, however, benefited from the fact that there is less international travel to the region, according to infectious disease expert Steven Lawrence, MD, of Washington University, St. Louis.
“This is very similar to 1918 with the influenza pandemic – St. Louis had more time to prepare and was able to put measures in place to flatten the curve than, say, Philadelphia,” Dr. Lawrence said. “Seattle didn’t have an opportunity to prepare as much in advance.”
While commercial labs are coming online, strict restrictions are limiting testing capabilities, Dr. Lawrence said.
“The state has had their hands tied,” he added.
Waiting And wondering
Because of a widespread lag in testing, it is still a mystery for thousands of people to know whether they’ve come into contact with an infected person until well after it happens. As of March 20, the pandemic had killed more than 11,000 globally. More than 16,000 Americans were confirmed infected and at least 216 have died.
“CDC will distribute tests based on where they can do the most good. But without hospital-based testing and commercial testing, it will not be possible to meet the need,” said Tom Frieden, who led the CDC during President Obama’s administration and is a former commissioner of the New York City Health Department.
In California, public school teacher Claire Dugan, whose state was among the hardest hit in the initial wave of U.S. coronavirus cases, was told she didn’t qualify for testing because she had not traveled abroad to any country with an outbreak of the virus or been in contact with an infected person. Ms. Dugan, who lives in the San Francisco Bay area and is already medically fragile after a stray bullet nearly killed her while driving 4 years ago, sought a test from her doctor after registering a temperature of 100.7° F earlier this month.
“There are a lot of layers as to why this is so messed up,” said Ms. Dugan, who relies on a feeding tube and said she sought a test not only to protect herself but her students. “It’s community spreading right now, so it’s kind of silly we’re still insisting on [the early criteria for testing]. How would I know?”
Since the CDC’s initial distribution, states have been reordering more tests through the office’s International Reagent Resource – a long-standing tool that public health labs have relied on. They have also revised testing protocols to use only one sample per person, which boosts the number of people screened.
Yet problems still abound with tests or other materials needed to be able to detect the virus. California Gov. Gavin Newsom (D) said on March 12 that county public health labs can’t use all of the 8,000 test kits the state has because they are missing key components.
In Pennsylvania, state officials weren’t able to begin testing until March 2 because of problems with the CDC’s initial kit, according to Nate Wardle, a spokesperson at its department of health. New York received two newly manufactured CDC test kits on Feb. 29 and also began performing tests March 2, according to its health department.
“We are still limited on extraction kits,” Mandy Cohen, the Health & Human Services secretary in North Carolina, said in an interview in mid-March. Officials earlier this month could test only 300 patients because of shortages in the extraction materials needed to register whether the novel coronavirus is present.
In North Dakota, Loralyn Hegland wrote her physician’s practice an email on March 10 with the subject line “dry cough,” wondering if she should come in for testing after learning that was one symptom of COVID-19. The recommendation she got echoes those of countless others across the United States, saying her risk of being exposed was very low because she hadn’t traveled outside the United States and had not come into contact with a person who had been “definitely” diagnosed with the virus.
Ms. Hegland, who lives in Fargo, didn’t have a fever but decided to shelter herself, anyway, out of caution.
Would she push to get a test?
“What’s the point?” she said. “You can’t know what you don’t know. It’s just that simple. How else do you explain it to people when you’re not testing?”
KHN Midwest correspondent Lauren Weber in St. Louis contributed to this article.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
COVID-19: U.S. cardiology groups reaffirm continued use of RAAS-active drugs
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
Managing children’s fear, anxiety in the age of COVID-19
With coronavirus disease (COVID-19) reaching epidemic proportions, many US children are growing increasingly anxious about what this means for their own health and safety and that of their friends and family.
The constantly changing numbers of people affected by the virus and the evolving situation mean daily life for many children is affected in some way, with school trips, sports tournaments, and family vacations being postponed or canceled.
All children may have a heightened level of worry, and some who are normally anxious might be obsessing more about handwashing or getting sick.
Experts say there are ways to manage this fear to help children feel safe and appropriately informed.
Clinicians and other adults should provide children with honest and accurate information geared to their age and developmental level, said David Fassler, MD, clinical professor of psychiatry, University of Vermont Larner College of Medicine, Burlington, and member of the Consumer Issues Committee of the American Academy of Child and Adolescent Psychiatry.
That said, it’s also acceptable to let children know that some questions can’t be answered, said Fassler.
Be truthful, calm
“This is partly because the information keeps changing as we learn more about how the virus spreads, how to best protect communities, and how to treat people who get sick,” he added.
Clinicians and parents should remind children “that there are a lot of adults who are working very hard to keep them safe,” said Eli R. Lebowitz, PhD, associate professor in the Child Study Center, Yale School of Medicine, New Haven, Connecticut, who directs a program for anxiety.
It’s important for adults to pay attention not only to what they say to children but also how they say it, said Lebowitz. He highlighted the importance of talking about the virus “in a calm and matter-of-fact way” rather than in an anxious way.
“If you look scared or tense or your voice is conveying that you’re really scared, the child is going to absorb that and feel anxious as well,” he noted.
This advice also applies when adults are discussing the issue among themselves. They should be aware that “children are listening” and are picking up any anxiety or panic adults are expressing.
Children are soaking up information about this virus from the Internet, the media, friends, teachers, and elsewhere. Lebowitz suggests asking children what they have already heard, which provides an opportunity to correct rumors and inaccurate information.
“A child might have a very inflated sense of what the actual risk is. For example, they may think that anyone who gets the virus dies,” he said.
Myth busting
Adults should let children know that not everything they hear from friends or on the Internet “is necessarily correct,” he added.
Some children who have experienced serious illness or losses may be particularly vulnerable to experiencing intense reactions to graphic news reports or images of illness or death and may need extra support, said Fassler.
Adults could use the “framework of knowledge” that children already have, said Lebowitz. He noted that all children are aware of sickness.
“They know people get sick, and they themselves have probably been sick, so you can tell them that this is a sickness like a bad flu,” he said.
Children should be encouraged to approach adults they trust, such as their pediatrician, a parent, or a teacher, with their questions, said Lebowitz. “Those are the people who are able to give them the most accurate information.”
Fassler noted that accurate, up-to-date information is available via fact sheets developed by the Centers for Disease Control and Prevention and the World Health Organization.
Although it’s helpful and appropriate to be reassuring, Fassler advises not to make unrealistic promises.
“It’s fine to tell kids that you’ll deal with whatever happens, even if it means altering travel plans or work schedules, but you can’t promise that no one in your state or community will get sick,” he said.
Maintain healthy habits
Physicians and other adults can tell children “in an age-appropriate way” how the virus is transmitted and what the symptoms are, but it’s important to emphasize that most people who are sick don’t have COVID-19, said Lebowitz.
“I would emphasize that the people who are the sickest are the elderly who are already sick, rather than healthy younger people,” he said.
Lebowitz recommends continuing to follow guidelines on staying healthy, including coughing into a sleeve instead of your hand and regular handwashing.
It’s also important at this time for children to maintain healthy habits – getting enough physical activity and sleep, eating well, and being outside – because this regime will go a long way toward reducing anxiety, said Lebowitz. Deep breathing and muscle-relaxing exercises can also help, he said.
Lebowitz also suggests maintaining a supportive attitude and showing “some acceptance and validation of what children are feeling, as well as some confidence that they can cope and tolerate feeling uncomfortable sometimes, that they can handle some anxiety.”
While accepting that the child could be anxious, it’s important not to encourage excessive avoidance or unhealthy coping strategies. Fassler and Lebowitz agree that children who are overly anxious or preoccupied with concerns about the coronavirus should be evaluated by a trained, qualified mental health professional.
Signs that a child may need additional help include ongoing sleep difficulties, intrusive thoughts or worries, obsessive-compulsive behaviors, or reluctance or refusal to go to school, said Fassler.
The good news is that most children are resilient, said Fassler. “They’ll adjust, adapt, and go on with their lives.”
This article first appeared on Medscape.com.
With coronavirus disease (COVID-19) reaching epidemic proportions, many US children are growing increasingly anxious about what this means for their own health and safety and that of their friends and family.
The constantly changing numbers of people affected by the virus and the evolving situation mean daily life for many children is affected in some way, with school trips, sports tournaments, and family vacations being postponed or canceled.
All children may have a heightened level of worry, and some who are normally anxious might be obsessing more about handwashing or getting sick.
Experts say there are ways to manage this fear to help children feel safe and appropriately informed.
Clinicians and other adults should provide children with honest and accurate information geared to their age and developmental level, said David Fassler, MD, clinical professor of psychiatry, University of Vermont Larner College of Medicine, Burlington, and member of the Consumer Issues Committee of the American Academy of Child and Adolescent Psychiatry.
That said, it’s also acceptable to let children know that some questions can’t be answered, said Fassler.
Be truthful, calm
“This is partly because the information keeps changing as we learn more about how the virus spreads, how to best protect communities, and how to treat people who get sick,” he added.
Clinicians and parents should remind children “that there are a lot of adults who are working very hard to keep them safe,” said Eli R. Lebowitz, PhD, associate professor in the Child Study Center, Yale School of Medicine, New Haven, Connecticut, who directs a program for anxiety.
It’s important for adults to pay attention not only to what they say to children but also how they say it, said Lebowitz. He highlighted the importance of talking about the virus “in a calm and matter-of-fact way” rather than in an anxious way.
“If you look scared or tense or your voice is conveying that you’re really scared, the child is going to absorb that and feel anxious as well,” he noted.
This advice also applies when adults are discussing the issue among themselves. They should be aware that “children are listening” and are picking up any anxiety or panic adults are expressing.
Children are soaking up information about this virus from the Internet, the media, friends, teachers, and elsewhere. Lebowitz suggests asking children what they have already heard, which provides an opportunity to correct rumors and inaccurate information.
“A child might have a very inflated sense of what the actual risk is. For example, they may think that anyone who gets the virus dies,” he said.
Myth busting
Adults should let children know that not everything they hear from friends or on the Internet “is necessarily correct,” he added.
Some children who have experienced serious illness or losses may be particularly vulnerable to experiencing intense reactions to graphic news reports or images of illness or death and may need extra support, said Fassler.
Adults could use the “framework of knowledge” that children already have, said Lebowitz. He noted that all children are aware of sickness.
“They know people get sick, and they themselves have probably been sick, so you can tell them that this is a sickness like a bad flu,” he said.
Children should be encouraged to approach adults they trust, such as their pediatrician, a parent, or a teacher, with their questions, said Lebowitz. “Those are the people who are able to give them the most accurate information.”
Fassler noted that accurate, up-to-date information is available via fact sheets developed by the Centers for Disease Control and Prevention and the World Health Organization.
Although it’s helpful and appropriate to be reassuring, Fassler advises not to make unrealistic promises.
“It’s fine to tell kids that you’ll deal with whatever happens, even if it means altering travel plans or work schedules, but you can’t promise that no one in your state or community will get sick,” he said.
Maintain healthy habits
Physicians and other adults can tell children “in an age-appropriate way” how the virus is transmitted and what the symptoms are, but it’s important to emphasize that most people who are sick don’t have COVID-19, said Lebowitz.
“I would emphasize that the people who are the sickest are the elderly who are already sick, rather than healthy younger people,” he said.
Lebowitz recommends continuing to follow guidelines on staying healthy, including coughing into a sleeve instead of your hand and regular handwashing.
It’s also important at this time for children to maintain healthy habits – getting enough physical activity and sleep, eating well, and being outside – because this regime will go a long way toward reducing anxiety, said Lebowitz. Deep breathing and muscle-relaxing exercises can also help, he said.
Lebowitz also suggests maintaining a supportive attitude and showing “some acceptance and validation of what children are feeling, as well as some confidence that they can cope and tolerate feeling uncomfortable sometimes, that they can handle some anxiety.”
While accepting that the child could be anxious, it’s important not to encourage excessive avoidance or unhealthy coping strategies. Fassler and Lebowitz agree that children who are overly anxious or preoccupied with concerns about the coronavirus should be evaluated by a trained, qualified mental health professional.
Signs that a child may need additional help include ongoing sleep difficulties, intrusive thoughts or worries, obsessive-compulsive behaviors, or reluctance or refusal to go to school, said Fassler.
The good news is that most children are resilient, said Fassler. “They’ll adjust, adapt, and go on with their lives.”
This article first appeared on Medscape.com.
With coronavirus disease (COVID-19) reaching epidemic proportions, many US children are growing increasingly anxious about what this means for their own health and safety and that of their friends and family.
The constantly changing numbers of people affected by the virus and the evolving situation mean daily life for many children is affected in some way, with school trips, sports tournaments, and family vacations being postponed or canceled.
All children may have a heightened level of worry, and some who are normally anxious might be obsessing more about handwashing or getting sick.
Experts say there are ways to manage this fear to help children feel safe and appropriately informed.
Clinicians and other adults should provide children with honest and accurate information geared to their age and developmental level, said David Fassler, MD, clinical professor of psychiatry, University of Vermont Larner College of Medicine, Burlington, and member of the Consumer Issues Committee of the American Academy of Child and Adolescent Psychiatry.
That said, it’s also acceptable to let children know that some questions can’t be answered, said Fassler.
Be truthful, calm
“This is partly because the information keeps changing as we learn more about how the virus spreads, how to best protect communities, and how to treat people who get sick,” he added.
Clinicians and parents should remind children “that there are a lot of adults who are working very hard to keep them safe,” said Eli R. Lebowitz, PhD, associate professor in the Child Study Center, Yale School of Medicine, New Haven, Connecticut, who directs a program for anxiety.
It’s important for adults to pay attention not only to what they say to children but also how they say it, said Lebowitz. He highlighted the importance of talking about the virus “in a calm and matter-of-fact way” rather than in an anxious way.
“If you look scared or tense or your voice is conveying that you’re really scared, the child is going to absorb that and feel anxious as well,” he noted.
This advice also applies when adults are discussing the issue among themselves. They should be aware that “children are listening” and are picking up any anxiety or panic adults are expressing.
Children are soaking up information about this virus from the Internet, the media, friends, teachers, and elsewhere. Lebowitz suggests asking children what they have already heard, which provides an opportunity to correct rumors and inaccurate information.
“A child might have a very inflated sense of what the actual risk is. For example, they may think that anyone who gets the virus dies,” he said.
Myth busting
Adults should let children know that not everything they hear from friends or on the Internet “is necessarily correct,” he added.
Some children who have experienced serious illness or losses may be particularly vulnerable to experiencing intense reactions to graphic news reports or images of illness or death and may need extra support, said Fassler.
Adults could use the “framework of knowledge” that children already have, said Lebowitz. He noted that all children are aware of sickness.
“They know people get sick, and they themselves have probably been sick, so you can tell them that this is a sickness like a bad flu,” he said.
Children should be encouraged to approach adults they trust, such as their pediatrician, a parent, or a teacher, with their questions, said Lebowitz. “Those are the people who are able to give them the most accurate information.”
Fassler noted that accurate, up-to-date information is available via fact sheets developed by the Centers for Disease Control and Prevention and the World Health Organization.
Although it’s helpful and appropriate to be reassuring, Fassler advises not to make unrealistic promises.
“It’s fine to tell kids that you’ll deal with whatever happens, even if it means altering travel plans or work schedules, but you can’t promise that no one in your state or community will get sick,” he said.
Maintain healthy habits
Physicians and other adults can tell children “in an age-appropriate way” how the virus is transmitted and what the symptoms are, but it’s important to emphasize that most people who are sick don’t have COVID-19, said Lebowitz.
“I would emphasize that the people who are the sickest are the elderly who are already sick, rather than healthy younger people,” he said.
Lebowitz recommends continuing to follow guidelines on staying healthy, including coughing into a sleeve instead of your hand and regular handwashing.
It’s also important at this time for children to maintain healthy habits – getting enough physical activity and sleep, eating well, and being outside – because this regime will go a long way toward reducing anxiety, said Lebowitz. Deep breathing and muscle-relaxing exercises can also help, he said.
Lebowitz also suggests maintaining a supportive attitude and showing “some acceptance and validation of what children are feeling, as well as some confidence that they can cope and tolerate feeling uncomfortable sometimes, that they can handle some anxiety.”
While accepting that the child could be anxious, it’s important not to encourage excessive avoidance or unhealthy coping strategies. Fassler and Lebowitz agree that children who are overly anxious or preoccupied with concerns about the coronavirus should be evaluated by a trained, qualified mental health professional.
Signs that a child may need additional help include ongoing sleep difficulties, intrusive thoughts or worries, obsessive-compulsive behaviors, or reluctance or refusal to go to school, said Fassler.
The good news is that most children are resilient, said Fassler. “They’ll adjust, adapt, and go on with their lives.”
This article first appeared on Medscape.com.
An epidemic of fear and misinformation
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
Nontuberculous Mycobacterial Pulmonary Disease
Nontuberculous mycobacterial pulmonary disease is a broad term for a group of pulmonary disorders caused and characterized by exposure to environmental mycobacteria other than those belonging to the Mycobacterium tuberculosis complex and Mycobacterium leprae. Mycobacteria are aerobic, nonmotile organisms that appear positive with acid-fast alcohol stains. Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and have been recovered from domestic and natural water sources, soil, and food products, and from around livestock, cattle, and wildlife.1-3 To date, no evidence exists of human-to-human or animal-to-human transmission of NTM in the general population. Infections in humans are usually acquired from environmental exposures, although the specific source of infection cannot always be identified. Similarly, the mode of infection with NTM has not been established with certainty, but it is highly likely that the organism is implanted, ingested, aspirated, or inhaled. Aerosolization of droplets associated with use of bathroom showerheads and municipal water sources and soil contamination are some of the factors associated with the transmission of infection. Proven routes of transmission include showerheads and potting soil dust.2,3
NTM pulmonary disease occurs in individuals with or without comorbid conditions such as bronchiectasis, chronic obstructive pulmonary disease, pulmonary fibrosis, or structural lung diseases. Slender, middle-aged or elderly white females with marfanoid body habitus, with or without apparent immune or genetic disorders, showing impaired airway and mucus clearance present with this infection as a form of underlying bronchiectasis (Lady Windermere syndrome). It is unclear why NTM infections and escalation to clinical disease occur in certain individuals. Many risk factors, including inherited and acquired defects of host immune response (eg, cystic fibrosis trait and α1 antitrypsin deficiency), have been associated with increased susceptibility to NTM infections.4
NTM infection can lead to chronic symptoms, frequent exacerbations, progressive functional and structural lung destruction, and impaired quality of life, and is associated with an increased risk of hospitalization and higher 5-year all-cause mortality. As such, NTM disease is drawing increasing attention at the clinical, academic, and research levels.5 This case-based review outlines the clinical features of NTM infection, with a focus on the challenges in diagnosis, treatment, and management of NTM pulmonary disease. The cases use Mycobacterium avium complex (MAC), a slow-growing mycobacteria (SGM), and Mycobacterium abscessus, a rapidly growing mycobacteria (RGM), as prototypes in a non–cystic fibrosis, non-HIV clinical setting.
Epidemiology
Of the almost 200 isolated species of NTM, the most prevalent pathogens for respiratory disease in the United States are MAC, Mycobacterium kansasii, and M. abscessus. MAC accounts for more than 80% of cases of NTM respiratory disease in the United States.6 The prevalence of NTM disease is increasing at a rate of about 8% each year, with 75,000 to 105,000 patients diagnosed with NTM lung disease in the United States annually. NTM infections in the United States are increasing among patients aged 65 years and older, a population that is expected to nearly double by 2030.7,8
Isolation and prevalence of many NTM species are higher in certain geographic areas of the United States, especially in the southeast. The US coastal regions have a higher prevalence of NTM pulmonary disease, and account for 70% of NTM cases in the United States each year. Half of patients diagnosed with NTM lung disease reside in 7 states: Florida, New York, Texas, California, Pennsylvania, New Jersey, and Ohio, with 1 in 7 residing in Florida. Three parishes in Louisiana are among the top 10 counties with the highest prevalence in United States. The prevalence of NTM infection–associated hospitalizations is increasing worldwide as well. Co-infection with tuberculosis and multiple NTMs in individual patients has been observed clinically and documented in patients with and without HIV.9,10
It is not clear why the prevalence of NTM pulmonary disease is increasing, but there may be several contributing factors: (1) an increased awareness and identification of NTM infection sources in the environment; (2) an expanding cohort of immunocompromised individuals with exogenous or endogenous immune deficiencies; (3) availability of improved diagnostic techniques, such as use of high-performance liquid chromatography (HPLC), DNA probes, and gene sequencing; and (4) an increased awareness of the morbidity and mortality associated with NTM pulmonary disease. However, it is important to recognize that to best understand the clinical relevance of epidemiologic studies based on laboratory diagnosis and identification, the findings must be evaluated by correlating them with the microbiological and other clinical criteria established by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.11
Continue to: Mycobacterium avium Complex
Mycobacterium avium Complex
Case Patient 1
A 48-year-old woman who has never smoked and has no past medical problems, except seasonal allergic rhinitis and “colds and flu-like illness” once or twice a year, is evaluated for a chronic lingering cough with occasional sputum production. The patient denies any other chronic symptoms and is otherwise active. Physical examination reveals no specific findings except mild pectus excavatum and mild scoliosis. Body mass index is 22 kg/m2. Chest radiograph shows nonspecific increased markings in the lower zones. Computed tomography (CT) scan of the chest reveals minimal nodular and cylindrical bronchiectasis in both lungs (Figure 1). No previous radiographs are available for comparison. The patient is HIV-negative. Sputum tests reveal normal flora, and both fungus and acid-fast bacilli smear are negative. Culture for mycobacteria shows scanty growth of MAC in 1 specimen.
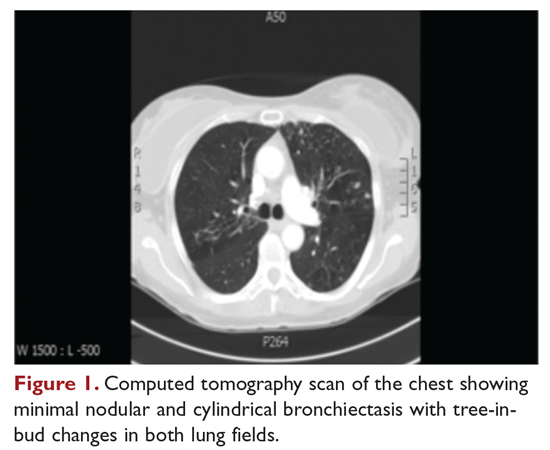
What is the clinical presentation of MAC pulmonary disease?
Among NTM, MAC is the most common cause of pulmonary disease worldwide.6 MAC primarily includes 2 species: M. avium and Mycobacterium intracellulare. M. avium is the more important pathogen in disseminated disease, whereas M. intracellulare is the more common respiratory pathogen.11 These organisms are genetically similar and generally not differentiated in the clinical microbiology laboratory, although there are isolated reports in the literature suggesting differences in prevalence, presentation, and prognosis in M. avium infection versus M. intracellulare infection.12
Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced HIV infection; and cervical lymphadenitis.13 Pulmonary disease caused by MAC may take on 1 of several clinically different forms, including asymptomatic “colonization” or persistent minimal infection without obvious clinical significance; endobronchial involvement; progressive pulmonary disease with radiographic and clinical deterioration and nodular bronchiectasis or cavitary lung disease; hypersensitivity pneumonitis; or persistent, overwhelming mycobacterial growth with symptomatic manifestations, often in a lung with underlying damage due to either chronic obstructive lung disease or pulmonary fibrosis (Table 1).14

Cavitary Disease
The traditionally recognized presentation of MAC lung disease has been apical cavitary lung disease in men in their late 40s and early 50s who have a history of cigarette smoking, and frequently, excessive alcohol use. If left untreated, or in the case of erratic treatment or macrolide drug resistance, this form of disease is generally progressive within a relatively short time and can result in extensive cavitary lung destruction and progressive respiratory failure.15
Nodular Bronchiectasis
The more common presentation of MAC lung disease, which is outlined in the case described here, is interstitial nodular infiltrates, frequently involving the right middle lobe or lingula and predominantly occurring in postmenopausal, nonsmoking white women. This is sometimes labelled “Lady Windermere syndrome.” These patients with M. avium infection appear to have similar clinical characteristics and body types, including lean build, scoliosis, pectus excavatum, and mitral valve prolapse.16,17 The mechanism by which this body morphotype predisposes to pulmonary mycobacterial infection is not defined, but ineffective mucociliary clearance is a possible explanation. Evidence suggests that some patients may be predisposed to NTM lung disease because of preexisting bronchiectasis. Some potential etiologies of bronchiectasis in this population include chronic sinusitis, gastroesophageal reflux with chronic aspiration, α1 antitrypsin deficiency, and cystic fibrosis genetic traits and mutations.18 Risk factors for increased morbidity and mortality include the development of cavitary disease, age, weight loss, lower body mass index, and other comorbid conditions.
This form of disease, termed nodular bronchiectasis, tends to have a much slower progression than cavitary disease, such that long-term follow-up (months to years) may be necessary to demonstrate clinical or radiographic changes.11 The radiographic term “tree-in-bud” has been used to describe what may reflect inflammatory changes, including bronchiolitis. High-resolution CT scans of the chest are especially helpful for diagnosing this pattern of MAC lung disease, as bronchiectasis and small nodules may not be easily discernible on plain chest radiograph. The nodular/bronchiectasis radiographic pattern can also be seen with other NTM pathogens, including M. abscessus, Mycobacterium simiae, and M. kansasii. Solitary nodules and dense consolidation have also been described. Pleural effusions are uncommon, but reactive pleural thickening is frequently seen. Co-pathogens may be isolated from culture, including Pseudomonas aeruginosa, Staphylococcus aureus, and, occasionally, other NTM such as M. abscessus or Mycobacterium chelonae.19-21
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, initially described in patients who were exposed to hot tubs, mimics allergic hypersensitivity pneumonitis, with respiratory symptoms and culture/tissue identification of MAC or sometimes other NTM. It is unclear whether hypersensitivity pneumonitis is an inflammatory process, an infection, or both, and opinion regarding the need for specific antibiotic treatment is divided.11,22 However, avoidance of exposure is prudent and recommended.
Disseminated Disease
Disseminated NTM disease is associated with very low CD4+ lymphocyte counts and is seen in approximately 5% of patients with HIV infection.23-25 Although disseminated NTM disease is rarely seen in immunosuppressed patients without HIV infection, it has been reported in patients who have undergone renal or cardiac transplant, patients on long-term corticosteroid therapy, and those with leukemia or lymphoma. More than 90% of infections are caused by MAC; other potential pathogens include M. kansasii, M. chelonae, M. abscessus, and Mycobacterium haemophilum. Although seen less frequently since the advent of highly active antiretroviral therapy, disseminated infection can develop progressively from an apparently indolent or localized infection or a respiratory or gastrointestinal source. Signs and symptoms of disseminated infection (specifically MAC-associated disease) are nonspecific and include fever, night sweats, weight loss, and abdominal tenderness. Disseminated MAC disease occurs primarily in patients with more advanced HIV disease (CD4+ count typically < 50 cells/μL). Clinically, disseminated MAC manifests as intermittent or persistent fever, constitutional symptoms with organomegaly and organ-specific abnormalities (eg, anemia, neutropenia from bone marrow involvement, adenopathy, hepatosplenomegaly), and elevations of liver enzymes or lung infiltrates from pulmonary involvement.
Continue to: What are the criteria for diagnosing NTM pulmonary disease?
What are the criteria for diagnosing NTM pulmonary disease?
The diagnosis of NTM disease is based on clinical, radiologic, and mycobacterial correlation with good communication between the experts in this field. The ATS/IDSA criteria for diagnosing NTM lung disease are shown in Figure 2. These criteria best apply to MAC, M. kansasii, and M. abscessus, but are also clinically applied to other NTM respiratory pathogens. The diagnosis of MAC infection is most readily established by culture of blood, bone marrow, respiratory secretions/fluid, or tissue specimens from suspected sites of involvement. Due to erratic shedding of MAC into the respiratory secretions in patients with nodular bronchiectasis, as compared to those with the cavitary form of the disease, sputum may be intermittently positive, with variable colony counts and polyclonal infections.12 Prior to the advent of high-resolution CT, isolation of MAC organisms from the sputum of such patients was frequently dismissed as colonization.
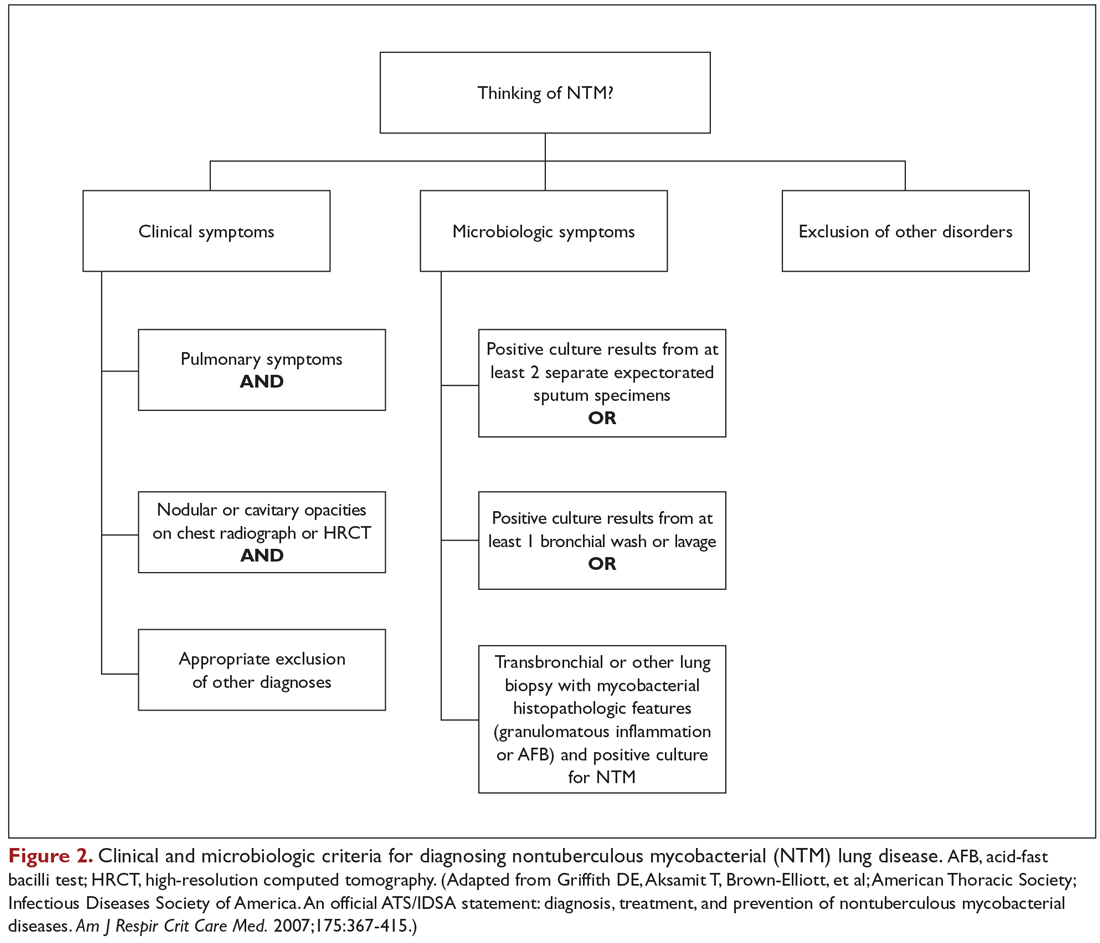
Mycobacterial Testing
Because of the nonspecific symptoms and lack of diagnostic specificity of chest imaging, the diagnosis of NTM lung disease requires microbiologic confirmation. Specimens sent to the laboratory for identification of NTM must be handled with care to prevent contamination and false-positive results. Transport media and preservatives should be avoided, and transportation of the specimens should be prompt. These measures will prevent bacterial overgrowth. Furthermore, the yield of NTM may be affected if the patient has used antibiotics, such as macrolides and fluoroquinolones, prior to obtaining the specimen.
NTM should be identified at the species and subspecies level, although this is not practical in community practice settings. The preferred staining procedure in the laboratory is the fluorochrome method. Some species require special growth conditions and/or lower incubation temperatures, and other identification methods may have to be employed, such as DNA probes, polymerase chain reaction genotyping, nucleic acid sequence determination, and high-performance liquid chromatography. As a gold standard, clinical specimens for mycobacterial cultures should be inoculated onto 1 or more solid media (eg, Middlebrook 7H11 media and/or Lowenstein-Jensen media, the former of which is the preferred medium for NTM) and into a liquid medium (eg, BACTEC 12B broth or Mycobacteria growth indicator tube broth). Growth of visible colonies on solid media typically requires 2 to 4 weeks, but liquid media (eg, the radiometric BACTEC system), used as a supplementary and not as an exclusive test, usually produce results within 10 to 14 days. Furthermore, even after initial growth, identification of specific isolates based on the growth characteristics on solid media requires additional time. Use of specific nucleic acid probes for MAC and M. kansasii and HPLC testing of mycolic acid patterns in acid-fast bacilli smear–positive specimens can reduce the turnaround time of specific identification of a primary culture–positive sample. However, HPLC is not sufficient for definitive identification of many NTM species, including the RGM. Other newer techniques, including 16S ribosomal DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism analysis, also allow NTM to be identified and speciated more reliably and rapidly from clinical specimens.
Cost and other practical considerations limit widespread adoption of these techniques. However, the recognition that M. abscessus can be separated into more than 1 subspecies, and that there are important prognostic implications of that separation, lends urgency to the broader adoption of newer molecular techniques in the mycobacteriology laboratory. Susceptibility testing is based on the broth microdilution method; RGM usually grow within 7 days of subculture, and the laboratory time to culture is a helpful hint, although not necessarily specific. Recognizing the morphology of mycobacterial colony growth may also be helpful in identification.
Are skin tests helpful in diagnosing NTM infection?
Tuberculin skin testing remains a nonspecific marker of mycobacterial infection and does not help in further elucidating NTM infection. However, epidemiologic and laboratory studies with well-characterized antigens have shown that dual skin testing with tuberculosis- versus NTM-derived tuberculin can discriminate between prior NTM and prior tuberculosis disease. Species-specific skin test antigens are not commercially available and are not helpful in the diagnosis of NTM disease because of cross-reactivity of M. tuberculosis and some NTM. However, increased prevalence of NTM sensitization based on purified protein derivative testing has been noted in a recent survey, which is consistent with an observed increase in the rates of NTM infections, specifically MAC, in the United States.26,27
Interferon-gamma release assays (IGRAs) are now being used as an alternative to tuberculin skin testing to diagnose M. tuberculosis infection. Certain NTM species also contain gene sequences that encode for ESAT-6 or CFP-10 antigens used in the IGRAs, and hence, yield a positive IGRA test. These include M. marinum, M. szulgai, and M. kansasii.28,29 However, MAC organisms do not produce positive results on assays that use these antigens.
Continue to: What is the approach to management of NTM pulmonary disease?
What is the approach to management of NTM pulmonary disease?
The correlation of symptoms with radiographic and microbiologic evidence is essential to categorize the disease and determine the need for therapy. Making the diagnosis of NTM lung disease does not necessitate the institution of therapy. The decision to treat should be weighed against potential risks and benefits to the individual patient based on symptomatic, radiographic, and microbiologic criteria, as well as underlying systemic or pulmonary immune status. In the absence of evidence of clinical, radiologic, or mycobacterial progression of disease, pursuing airway clearance therapy and clinical surveillance without initiating specific anti-MAC therapy is a reasonable option.11 Identifying the sustained presence of NTM infection, especially MAC, in a patient with underlying clinical and radiographic evidence of bronchiectasis is of value in determining comprehensive treatment and management strategies. Close observation is indicated if the decision not to treat is made. If treatment is initiated, comprehensive management includes long-term follow-up with periodic bacteriologic surveillance, watching for drug toxicity and drug-drug interactions, ensuring adherence and compliance to treatment, and managing comorbidity.
The Bronchiectasis Severity Index is a useful clinical predictive tool that identifies patients at risk of future mortality, hospitalization, and exacerbations and can be used to evaluate the need for specific treatment.30 The index is based on dyspnea score, lung function tests, colonization of pathogens, and extent of disease.
Case 1 Continued
After approximately 2 months of observation and symptomatic treatment, without specific antibiotic therapy, the patient’s symptoms continue. She now develops intermittent hemoptysis. Repeat sputum studies reveal moderate growth of M. avium. A follow-up CT scan shows progression of disease, with an increase in the tree-in-bud pattern (Figure 3).
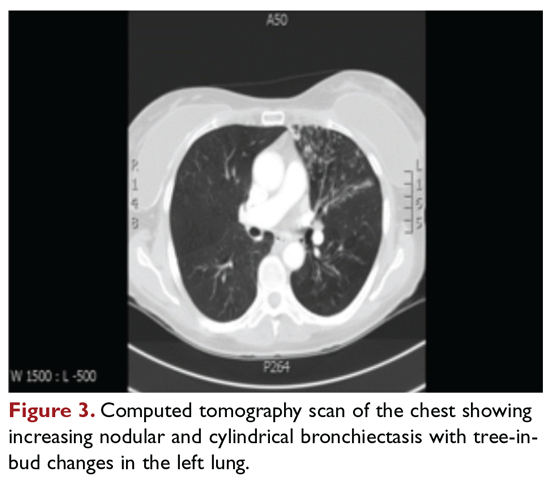
What treatment protocols are recommended for MAC pulmonary disease?
As per the ATS/IDSA statement, macrolides are the mainstay of treatment for pulmonary MAC disease.11 Macrolides achieve an increased concentration in the lung, and when used for treatment of pulmonary MAC disease, there is a strong correlation between in vitro susceptibility, in vivo (clinical) response, and the immunomodulating effects of macrolides.31,32 Macrolide-containing regimens have demonstrated efficacy in patients with MAC pulmonary disease33,34; however, macrolide monotherapy should be avoided to prevent the development of resistance.
At the outset, it is critical to establish the objective criteria for determining response and to ensure that the patient understands the goals of the treatment and expectations of the treatment plan. Moreover, experts suggest that due to the possibility of drug intolerance, side effects, and the need for prolonged therapy, a “step ladder” ramping up approach to treatment could be adopted, with gradual introduction of therapy within a short time period; this approach may improve compliance and adherence to treatment.11 If this approach is used, the doses may have to be divided. Patients who are unable to tolerate daily medications, even with dosage adjustment, should be tried on an intermittent treatment regimen. Older female patients frequently require gradual introduction of medications (ie, 1 medication added to the regimen every 1 to 2 weeks) to evaluate tolerance to each medication and medication dose.11 Commonly encountered adverse effects of NTM treatment include intolerance to clarithromycin due to gastrointestinal problems, low body mass index, or age older than 70 years.
After determining that the patient requires therapy, the standard recommended treatment for MAC pulmonary disease includes the following: for most patients with nodular/bronchiectasis disease, a thrice-weekly regimen of clarithromycin (1000 mg) or azithromycin (500 mg), rifampin (600 mg), and ethambutol (25 mg/kg) is recommended. For patients with cavitary MAC pulmonary disease or severe nodular/bronchiectasis disease, the guidelines recommend a daily regimen of clarithromycin (500-1000 mg) or azithromycin (250 mg), rifampin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg), with consideration of intravenous (IV) amikacin 3 times/week early in therapy (Table 2).11
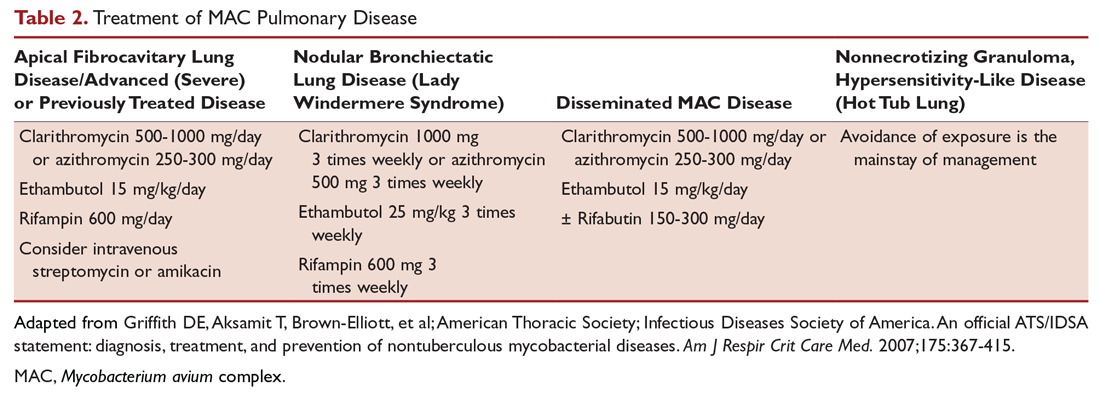
The treatment of MAC hypersensitivity-like disease speaks to the controversy of whether this is an inflammatory process, infectious process, or a combination of inflammation and infection. Avoidance of exposure is the mainstay of management. In some cases, steroids are used with or without a short course of anti-MAC therapy (ie, clarithromycin or azithromycin with rifampin and ethambutol).
Prophylaxis for disseminated MAC disease should be given to adults with HIV infection who have a CD4+ count less than 50 cells/μL. Azithromycin 1200 mg/week or clarithromycin 1000 mg/day has proven efficacy, and rifabutin 300 mg/day is also effective but less well tolerated. Rifabutin is more active in vitro against MAC than rifampin, and is used in HIV-positive patients because of drug-drug interaction between antiretroviral drugs and rifampin.
Continue to: Case 1 Continued
Case 1 Continued
The patient is treated with clarithromycin, rifampin, and ethambutol for 1 year, with sputum conversion after 9 months. In the latter part of her treatment, she experiences decreased visual acuity. Treatment is discontinued prematurely after 1 year due to drug toxicity and continued intolerance to drug therapy. The patient remains asymptomatic for 8 months, and then begins to experience mild to moderate hemoptysis, with increasing cough and sputum production associated with postural changes during exercise. Physical examination overall remains unchanged. Three sputum results reveal heavy growth of MAC, and a CT scan of the chest shows a cavitary lesion in the left upper lobe along with the nodular bronchiectasis (Figure 4).
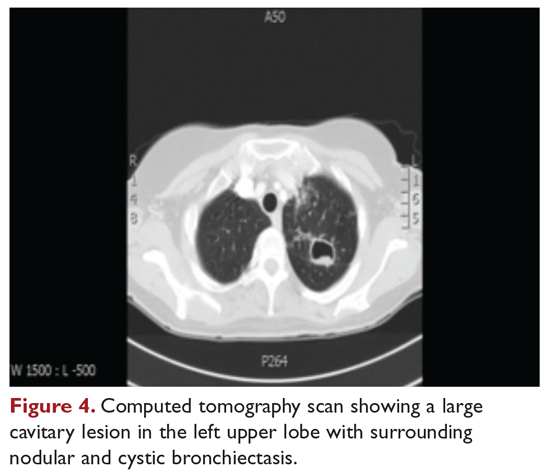
What are the management options at this stage?
Based on this patient’s continued symptoms, progression of radiologic abnormalities, and current culture growth, she requires re-treatment. With the adverse effects associated with ethambutol during the first round of therapy, the drug regimen needs to be modified. Several considerations are relevant at this stage. Relapse rates range from 20% to 30% after treatment with a macrolide-based therapy.11,34 Obtaining a culture-sensitivity profile is imperative in these cases. Of note, treatment should not be discontinued altogether, but instead the toxic agent should be removed from the treatment regimen. Continuing treatment with a 2-drug regimen of clarithromycin and rifampin may be considered in this patient. Re-infection with multiple genotypes may also occur after successful drug therapy, but this is primarily seen in MAC patients with nodular bronchiectasis.34,35 Patients in whom previous therapy has failed, even those with macrolide-susceptible MAC isolates, are less likely to respond to subsequent therapy. Data suggest that intermittent medication dosing is not effective for patients with severe or cavitary disease or in those in whom previous therapy has failed.36 In this case, treatment should include a daily 3-drug therapy, with an injectable thrice-weekly aminoglycoside. Other agents such as linezolid and clofazimine may have to be tried. Cycloserine, ethionamide, and other agents are sometimes used, but their efficacy is unproven and doubtful. Pyrazinamide and isoniazid have no activity against MAC.
Treatment Failure and Drug Resistance
Treatment failure is considered to have occurred if patients have not had a response (microbiologic, clinical, or radiographic) after 6 months of appropriate therapy or had not achieved conversion of sputum to culture-negative after 12 months of appropriate therapy.11 This occurs in about 40% of patients. Multiple factors can interfere with the successful treatment of MAC pulmonary disease, including medication nonadherence, medication side effects or intolerance, lack of response to a medication regimen, or the emergence of a macrolide-resistant or multidrug-resistant strain. Inducible macrolide resistance remains a potential factor.34-36 A number of characteristics of NTM contribute to the poor response to currently used antibiotics: the organisms have a lipid outer membrane and prefer to adhere to surfaces and form biofilms, which makes them relatively impermeable to antibiotics.37 Also, NTM replicate in phagocytic cells, allowing them to subvert normal cellular defense mechanisms. Furthermore, NTM can display colony variants, whereby single colony isolates switch between antibiotic-susceptible and -resistant variants. These factors have also impeded in development of new antibiotics for NTM infection.37
Recent limited approval of amikacin liposomal inhalation suspension (ALIS) for treatment failure and refractory MAC infection in combination with guideline-based antimicrobial therapy (GBT) is a promising addition to the available treatment armamentarium. In a multinational trial, the addition of ALIS to GBT for treatment-refractory MAC lung disease achieved significantly greater culture conversion rates by month 6 than GBT alone, with comparable rates of serious adverse events.38
Is therapeutic drug monitoring recommended during treatment of MAC pulmonary disease?
Treatment failure may also be drug-related, including poor drug penetration into the damaged lung tissue or drug-drug interactions leading to suboptimal drug levels. Peak serum concentrations have been found to be below target ranges in approximately 50% of patients using a macrolide and ethambutol. Concurrent use of rifampin decreases the peak serum concentration of macrolides and quinolones, with acceptable target levels seen in only 18% to 57% of cases. Whether this alters patient outcomes is not clear.39-42 Factors identified as contributing to the poor response to therapy include poor compliance, cavitary disease, previous treatment for MAC pulmonary disease, and a history of chronic obstructive lung disease. Studies by Koh and colleagues40 and van Ingen and colleagues41 with pharmacokinetic and pharmacodynamics data showed that, in patients on MAC treatment with both clarithromycin and rifampicin, plasma levels of clarithromycin were lower than the recommended minimal inhibitory concentrations (MIC) against MAC for that drug. The studies also showed that rifampicin lowered clarithromycin concentrations more than did rifabutin, with the AUC/MIC ratio being suboptimal in nearly half the cases. However, low plasma clarithromycin concentrations did not have any correlation with treatment outcomes, as the peak plasma drug concentrations and the peak plasma drug concentration/MIC ratios did not differ between patients with unfavorable treatment outcomes and those with favorable outcomes. This is further compounded by the fact that macrolides achieve higher levels in lung tissue than in plasma, and hence the significance of low plasma levels is unclear; however, it is postulated that achieving higher drug levels could, in fact, lead to better clinical outcomes. Pending specific well-designed, prospective randomized controlled trials, routine therapeutic drug monitoring is not currently recommended, although some referral centers do this as their practice pattern.
Is surgery an option in this case?
The overall 5-year mortality for MAC pulmonary disease was approximately 28% in a retrospective analysis, with patients with cavitary disease at increased risk for death at 5 years.42 As such, surgery is an option in selected cases as part of adjunctive therapy along with anti-MAC therapy based on mycobacterial sensitivity. Surgery is used as either a curative approach or a “debulking” measure.11 When present, clearly localized disease, especially in the upper lobe, lends itself best to surgical intervention. Surgical resection of a solitary pulmonary nodule due to MAC, in addition to concomitant medical treatment, is recommended. Surgical intervention should be considered early in the course of the disease because it may provide a cure without prolonged treatment and its associated problems, and this approach may lead to early sputum conversion. Surgery should also be considered in patients with macrolide-resistant or multidrug-resistant MAC infection or in those who cannot tolerate the side effects of therapy, provided that the disease is focal and limited. Patients with poor preoperative lung function have poorer outcomes than those with good lung function, and postoperative complications arising from treatment, especially with a right-sided pneumonectomy, tend to occur more frequently in these patients. Thoracic surgery for NTM pulmonary disease must be considered cautiously, as this is associated with significant morbidity and mortality and is best performed at specialized centers that have expertise and experience in this field.43
Continue to: Mycobacterium abscessus Complex
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).
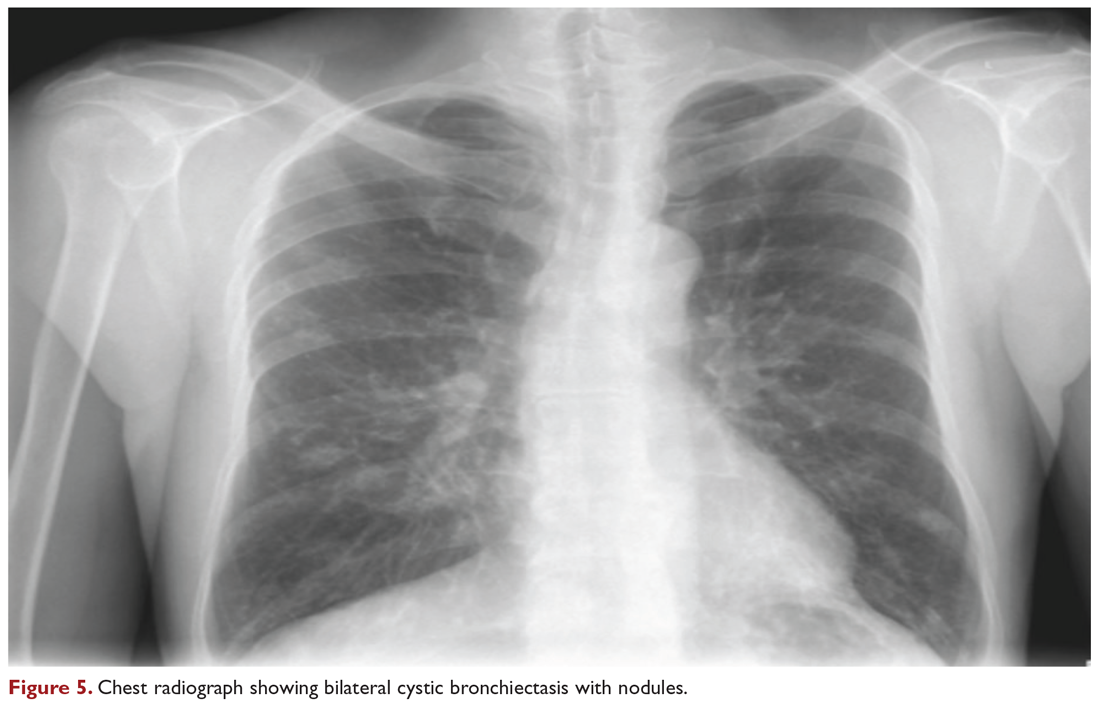
The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)
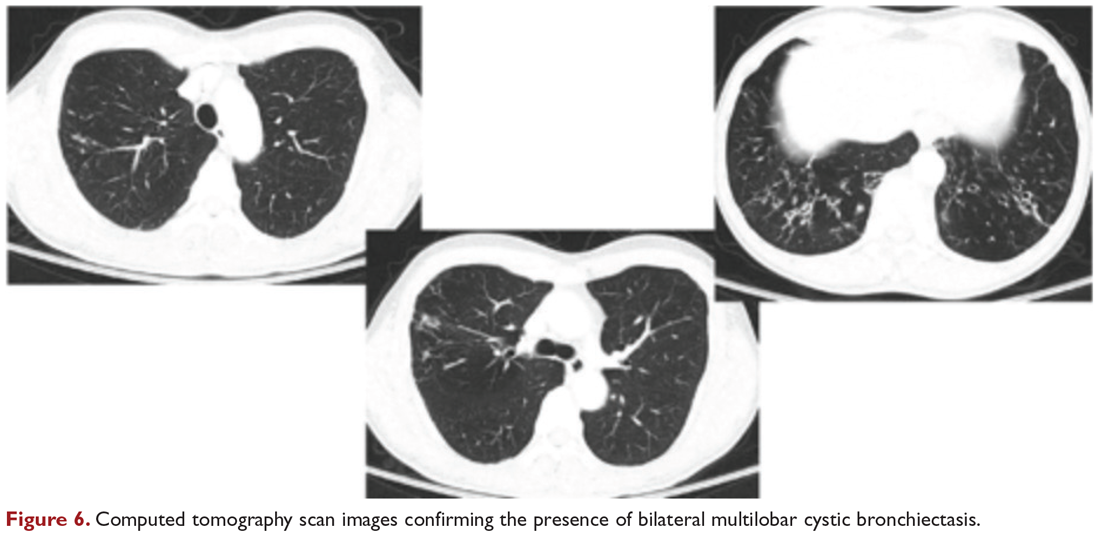
What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.
1. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210-220.
2. Falkinham JO III. Environmental sources of NTM. Clin Chest Med. 2015;36:35-41.
3. Falkinham JO III, Current epidemiological trends in NTM. Curr Environ Health Rep. 2016;3:161-167.
4. Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1-11.
5. Marras TK, Mirsaeidi M, Chou E, et al. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. Manag Care Spec Pharm. 2018;24:964-974.
6. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182:970-976.
7. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-982.
8. Adjemian, Olivier KN, Seitz AE, J et al. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185;881-886.
9. Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalizations associated with pulmonary nontuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
10. Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170.
11. Griffith DE, Aksamit T, Brown-Elliott, et al; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-415.
12. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235-1244.
13. Gordin FM, Horsburgh CR Jr. Mycobacterium avium complex. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier; 2015.
14. Chitty S, Ali J. Mycobacterium avium complex pulmonary disease in immune competent patients. South Med J. 2005;98:646-52.
15. Ramirez J, Mason C, Ali J, Lopez FA. MAC pulmonary disease: management options in HIV-negative patients. J La State Med Soc. 2008;160:248-254.
16. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914-916.
17. Kim RD, Greenburg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066-1074.
18. Ziedalski TM, Kao PN, Henig NR, et al. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995-1002.
19. Koh WJ, Lee KS, Kwon OJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282-288.
20. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994;105:49-52.
21. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033-1040.
22. Cappelluti E, Fraire AE, Schaefer OP. A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med. 2003;163:845-848.
23. Nightingale SD, Byrd LT, Southern PM, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082-1085.
24. Horsburgh CR Jr, Selik RM. The epidemiology of disseminated tuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989;139:4-7.
25. Chin DP, Hopewell PC, Yajko DM, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289-295.
26. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306-313.
27. Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990;12:760-767.
28. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
29. Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797-1807.
30. James D, Chalmers JD, Goeminne P, et al. The Bronchiectasis Severity Index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576-585.
31. Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988;32:1131-1136.
32. Horsburgh CR Jr, Mason UG 3rd, Heifits LB, et al. Response to therapy of pulmonary Mycobacterium avium intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135:418-421.
33. Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S-78S.
34. Wallace RJ Jr, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766-1772.
35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928-934.
36. Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283-1289.
37. Falkinham J III. Challenges of NTM drug development. Front Microbiol. 2018;9:1613.
38. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-1569.
39. Schluger NW. Treatment of pulmonary Mycobacterium avium complex infections: do drug levels matter? Am J Respir Crit Care Med. 2012;186:710-711.
40. Van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559-565.
41. Koh WJ, Jeong BH, Jeon K, et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:797-802.
42. Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary MAC disease. Int J Tuberc Lung Dis. 2012;16:408-414.
43. Yuji S, Yutsuki N, Keiichiso T, et al. Surgery for Mycobacterium avium lung disease in the clarithromycin era. Eur J Cardiothor Surg. 2002;21:314-318.
44. Tortoli E, Kohl TA, Brown-Elliott BA, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. Int J Syst Evol Microbiol. 2016; 66:4471-4479.
45. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-1278.
46. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309-316.
Nontuberculous mycobacterial pulmonary disease is a broad term for a group of pulmonary disorders caused and characterized by exposure to environmental mycobacteria other than those belonging to the Mycobacterium tuberculosis complex and Mycobacterium leprae. Mycobacteria are aerobic, nonmotile organisms that appear positive with acid-fast alcohol stains. Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and have been recovered from domestic and natural water sources, soil, and food products, and from around livestock, cattle, and wildlife.1-3 To date, no evidence exists of human-to-human or animal-to-human transmission of NTM in the general population. Infections in humans are usually acquired from environmental exposures, although the specific source of infection cannot always be identified. Similarly, the mode of infection with NTM has not been established with certainty, but it is highly likely that the organism is implanted, ingested, aspirated, or inhaled. Aerosolization of droplets associated with use of bathroom showerheads and municipal water sources and soil contamination are some of the factors associated with the transmission of infection. Proven routes of transmission include showerheads and potting soil dust.2,3
NTM pulmonary disease occurs in individuals with or without comorbid conditions such as bronchiectasis, chronic obstructive pulmonary disease, pulmonary fibrosis, or structural lung diseases. Slender, middle-aged or elderly white females with marfanoid body habitus, with or without apparent immune or genetic disorders, showing impaired airway and mucus clearance present with this infection as a form of underlying bronchiectasis (Lady Windermere syndrome). It is unclear why NTM infections and escalation to clinical disease occur in certain individuals. Many risk factors, including inherited and acquired defects of host immune response (eg, cystic fibrosis trait and α1 antitrypsin deficiency), have been associated with increased susceptibility to NTM infections.4
NTM infection can lead to chronic symptoms, frequent exacerbations, progressive functional and structural lung destruction, and impaired quality of life, and is associated with an increased risk of hospitalization and higher 5-year all-cause mortality. As such, NTM disease is drawing increasing attention at the clinical, academic, and research levels.5 This case-based review outlines the clinical features of NTM infection, with a focus on the challenges in diagnosis, treatment, and management of NTM pulmonary disease. The cases use Mycobacterium avium complex (MAC), a slow-growing mycobacteria (SGM), and Mycobacterium abscessus, a rapidly growing mycobacteria (RGM), as prototypes in a non–cystic fibrosis, non-HIV clinical setting.
Epidemiology
Of the almost 200 isolated species of NTM, the most prevalent pathogens for respiratory disease in the United States are MAC, Mycobacterium kansasii, and M. abscessus. MAC accounts for more than 80% of cases of NTM respiratory disease in the United States.6 The prevalence of NTM disease is increasing at a rate of about 8% each year, with 75,000 to 105,000 patients diagnosed with NTM lung disease in the United States annually. NTM infections in the United States are increasing among patients aged 65 years and older, a population that is expected to nearly double by 2030.7,8
Isolation and prevalence of many NTM species are higher in certain geographic areas of the United States, especially in the southeast. The US coastal regions have a higher prevalence of NTM pulmonary disease, and account for 70% of NTM cases in the United States each year. Half of patients diagnosed with NTM lung disease reside in 7 states: Florida, New York, Texas, California, Pennsylvania, New Jersey, and Ohio, with 1 in 7 residing in Florida. Three parishes in Louisiana are among the top 10 counties with the highest prevalence in United States. The prevalence of NTM infection–associated hospitalizations is increasing worldwide as well. Co-infection with tuberculosis and multiple NTMs in individual patients has been observed clinically and documented in patients with and without HIV.9,10
It is not clear why the prevalence of NTM pulmonary disease is increasing, but there may be several contributing factors: (1) an increased awareness and identification of NTM infection sources in the environment; (2) an expanding cohort of immunocompromised individuals with exogenous or endogenous immune deficiencies; (3) availability of improved diagnostic techniques, such as use of high-performance liquid chromatography (HPLC), DNA probes, and gene sequencing; and (4) an increased awareness of the morbidity and mortality associated with NTM pulmonary disease. However, it is important to recognize that to best understand the clinical relevance of epidemiologic studies based on laboratory diagnosis and identification, the findings must be evaluated by correlating them with the microbiological and other clinical criteria established by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.11
Continue to: Mycobacterium avium Complex
Mycobacterium avium Complex
Case Patient 1
A 48-year-old woman who has never smoked and has no past medical problems, except seasonal allergic rhinitis and “colds and flu-like illness” once or twice a year, is evaluated for a chronic lingering cough with occasional sputum production. The patient denies any other chronic symptoms and is otherwise active. Physical examination reveals no specific findings except mild pectus excavatum and mild scoliosis. Body mass index is 22 kg/m2. Chest radiograph shows nonspecific increased markings in the lower zones. Computed tomography (CT) scan of the chest reveals minimal nodular and cylindrical bronchiectasis in both lungs (Figure 1). No previous radiographs are available for comparison. The patient is HIV-negative. Sputum tests reveal normal flora, and both fungus and acid-fast bacilli smear are negative. Culture for mycobacteria shows scanty growth of MAC in 1 specimen.

What is the clinical presentation of MAC pulmonary disease?
Among NTM, MAC is the most common cause of pulmonary disease worldwide.6 MAC primarily includes 2 species: M. avium and Mycobacterium intracellulare. M. avium is the more important pathogen in disseminated disease, whereas M. intracellulare is the more common respiratory pathogen.11 These organisms are genetically similar and generally not differentiated in the clinical microbiology laboratory, although there are isolated reports in the literature suggesting differences in prevalence, presentation, and prognosis in M. avium infection versus M. intracellulare infection.12
Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced HIV infection; and cervical lymphadenitis.13 Pulmonary disease caused by MAC may take on 1 of several clinically different forms, including asymptomatic “colonization” or persistent minimal infection without obvious clinical significance; endobronchial involvement; progressive pulmonary disease with radiographic and clinical deterioration and nodular bronchiectasis or cavitary lung disease; hypersensitivity pneumonitis; or persistent, overwhelming mycobacterial growth with symptomatic manifestations, often in a lung with underlying damage due to either chronic obstructive lung disease or pulmonary fibrosis (Table 1).14

Cavitary Disease
The traditionally recognized presentation of MAC lung disease has been apical cavitary lung disease in men in their late 40s and early 50s who have a history of cigarette smoking, and frequently, excessive alcohol use. If left untreated, or in the case of erratic treatment or macrolide drug resistance, this form of disease is generally progressive within a relatively short time and can result in extensive cavitary lung destruction and progressive respiratory failure.15
Nodular Bronchiectasis
The more common presentation of MAC lung disease, which is outlined in the case described here, is interstitial nodular infiltrates, frequently involving the right middle lobe or lingula and predominantly occurring in postmenopausal, nonsmoking white women. This is sometimes labelled “Lady Windermere syndrome.” These patients with M. avium infection appear to have similar clinical characteristics and body types, including lean build, scoliosis, pectus excavatum, and mitral valve prolapse.16,17 The mechanism by which this body morphotype predisposes to pulmonary mycobacterial infection is not defined, but ineffective mucociliary clearance is a possible explanation. Evidence suggests that some patients may be predisposed to NTM lung disease because of preexisting bronchiectasis. Some potential etiologies of bronchiectasis in this population include chronic sinusitis, gastroesophageal reflux with chronic aspiration, α1 antitrypsin deficiency, and cystic fibrosis genetic traits and mutations.18 Risk factors for increased morbidity and mortality include the development of cavitary disease, age, weight loss, lower body mass index, and other comorbid conditions.
This form of disease, termed nodular bronchiectasis, tends to have a much slower progression than cavitary disease, such that long-term follow-up (months to years) may be necessary to demonstrate clinical or radiographic changes.11 The radiographic term “tree-in-bud” has been used to describe what may reflect inflammatory changes, including bronchiolitis. High-resolution CT scans of the chest are especially helpful for diagnosing this pattern of MAC lung disease, as bronchiectasis and small nodules may not be easily discernible on plain chest radiograph. The nodular/bronchiectasis radiographic pattern can also be seen with other NTM pathogens, including M. abscessus, Mycobacterium simiae, and M. kansasii. Solitary nodules and dense consolidation have also been described. Pleural effusions are uncommon, but reactive pleural thickening is frequently seen. Co-pathogens may be isolated from culture, including Pseudomonas aeruginosa, Staphylococcus aureus, and, occasionally, other NTM such as M. abscessus or Mycobacterium chelonae.19-21
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, initially described in patients who were exposed to hot tubs, mimics allergic hypersensitivity pneumonitis, with respiratory symptoms and culture/tissue identification of MAC or sometimes other NTM. It is unclear whether hypersensitivity pneumonitis is an inflammatory process, an infection, or both, and opinion regarding the need for specific antibiotic treatment is divided.11,22 However, avoidance of exposure is prudent and recommended.
Disseminated Disease
Disseminated NTM disease is associated with very low CD4+ lymphocyte counts and is seen in approximately 5% of patients with HIV infection.23-25 Although disseminated NTM disease is rarely seen in immunosuppressed patients without HIV infection, it has been reported in patients who have undergone renal or cardiac transplant, patients on long-term corticosteroid therapy, and those with leukemia or lymphoma. More than 90% of infections are caused by MAC; other potential pathogens include M. kansasii, M. chelonae, M. abscessus, and Mycobacterium haemophilum. Although seen less frequently since the advent of highly active antiretroviral therapy, disseminated infection can develop progressively from an apparently indolent or localized infection or a respiratory or gastrointestinal source. Signs and symptoms of disseminated infection (specifically MAC-associated disease) are nonspecific and include fever, night sweats, weight loss, and abdominal tenderness. Disseminated MAC disease occurs primarily in patients with more advanced HIV disease (CD4+ count typically < 50 cells/μL). Clinically, disseminated MAC manifests as intermittent or persistent fever, constitutional symptoms with organomegaly and organ-specific abnormalities (eg, anemia, neutropenia from bone marrow involvement, adenopathy, hepatosplenomegaly), and elevations of liver enzymes or lung infiltrates from pulmonary involvement.
Continue to: What are the criteria for diagnosing NTM pulmonary disease?
What are the criteria for diagnosing NTM pulmonary disease?
The diagnosis of NTM disease is based on clinical, radiologic, and mycobacterial correlation with good communication between the experts in this field. The ATS/IDSA criteria for diagnosing NTM lung disease are shown in Figure 2. These criteria best apply to MAC, M. kansasii, and M. abscessus, but are also clinically applied to other NTM respiratory pathogens. The diagnosis of MAC infection is most readily established by culture of blood, bone marrow, respiratory secretions/fluid, or tissue specimens from suspected sites of involvement. Due to erratic shedding of MAC into the respiratory secretions in patients with nodular bronchiectasis, as compared to those with the cavitary form of the disease, sputum may be intermittently positive, with variable colony counts and polyclonal infections.12 Prior to the advent of high-resolution CT, isolation of MAC organisms from the sputum of such patients was frequently dismissed as colonization.

Mycobacterial Testing
Because of the nonspecific symptoms and lack of diagnostic specificity of chest imaging, the diagnosis of NTM lung disease requires microbiologic confirmation. Specimens sent to the laboratory for identification of NTM must be handled with care to prevent contamination and false-positive results. Transport media and preservatives should be avoided, and transportation of the specimens should be prompt. These measures will prevent bacterial overgrowth. Furthermore, the yield of NTM may be affected if the patient has used antibiotics, such as macrolides and fluoroquinolones, prior to obtaining the specimen.
NTM should be identified at the species and subspecies level, although this is not practical in community practice settings. The preferred staining procedure in the laboratory is the fluorochrome method. Some species require special growth conditions and/or lower incubation temperatures, and other identification methods may have to be employed, such as DNA probes, polymerase chain reaction genotyping, nucleic acid sequence determination, and high-performance liquid chromatography. As a gold standard, clinical specimens for mycobacterial cultures should be inoculated onto 1 or more solid media (eg, Middlebrook 7H11 media and/or Lowenstein-Jensen media, the former of which is the preferred medium for NTM) and into a liquid medium (eg, BACTEC 12B broth or Mycobacteria growth indicator tube broth). Growth of visible colonies on solid media typically requires 2 to 4 weeks, but liquid media (eg, the radiometric BACTEC system), used as a supplementary and not as an exclusive test, usually produce results within 10 to 14 days. Furthermore, even after initial growth, identification of specific isolates based on the growth characteristics on solid media requires additional time. Use of specific nucleic acid probes for MAC and M. kansasii and HPLC testing of mycolic acid patterns in acid-fast bacilli smear–positive specimens can reduce the turnaround time of specific identification of a primary culture–positive sample. However, HPLC is not sufficient for definitive identification of many NTM species, including the RGM. Other newer techniques, including 16S ribosomal DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism analysis, also allow NTM to be identified and speciated more reliably and rapidly from clinical specimens.
Cost and other practical considerations limit widespread adoption of these techniques. However, the recognition that M. abscessus can be separated into more than 1 subspecies, and that there are important prognostic implications of that separation, lends urgency to the broader adoption of newer molecular techniques in the mycobacteriology laboratory. Susceptibility testing is based on the broth microdilution method; RGM usually grow within 7 days of subculture, and the laboratory time to culture is a helpful hint, although not necessarily specific. Recognizing the morphology of mycobacterial colony growth may also be helpful in identification.
Are skin tests helpful in diagnosing NTM infection?
Tuberculin skin testing remains a nonspecific marker of mycobacterial infection and does not help in further elucidating NTM infection. However, epidemiologic and laboratory studies with well-characterized antigens have shown that dual skin testing with tuberculosis- versus NTM-derived tuberculin can discriminate between prior NTM and prior tuberculosis disease. Species-specific skin test antigens are not commercially available and are not helpful in the diagnosis of NTM disease because of cross-reactivity of M. tuberculosis and some NTM. However, increased prevalence of NTM sensitization based on purified protein derivative testing has been noted in a recent survey, which is consistent with an observed increase in the rates of NTM infections, specifically MAC, in the United States.26,27
Interferon-gamma release assays (IGRAs) are now being used as an alternative to tuberculin skin testing to diagnose M. tuberculosis infection. Certain NTM species also contain gene sequences that encode for ESAT-6 or CFP-10 antigens used in the IGRAs, and hence, yield a positive IGRA test. These include M. marinum, M. szulgai, and M. kansasii.28,29 However, MAC organisms do not produce positive results on assays that use these antigens.
Continue to: What is the approach to management of NTM pulmonary disease?
What is the approach to management of NTM pulmonary disease?
The correlation of symptoms with radiographic and microbiologic evidence is essential to categorize the disease and determine the need for therapy. Making the diagnosis of NTM lung disease does not necessitate the institution of therapy. The decision to treat should be weighed against potential risks and benefits to the individual patient based on symptomatic, radiographic, and microbiologic criteria, as well as underlying systemic or pulmonary immune status. In the absence of evidence of clinical, radiologic, or mycobacterial progression of disease, pursuing airway clearance therapy and clinical surveillance without initiating specific anti-MAC therapy is a reasonable option.11 Identifying the sustained presence of NTM infection, especially MAC, in a patient with underlying clinical and radiographic evidence of bronchiectasis is of value in determining comprehensive treatment and management strategies. Close observation is indicated if the decision not to treat is made. If treatment is initiated, comprehensive management includes long-term follow-up with periodic bacteriologic surveillance, watching for drug toxicity and drug-drug interactions, ensuring adherence and compliance to treatment, and managing comorbidity.
The Bronchiectasis Severity Index is a useful clinical predictive tool that identifies patients at risk of future mortality, hospitalization, and exacerbations and can be used to evaluate the need for specific treatment.30 The index is based on dyspnea score, lung function tests, colonization of pathogens, and extent of disease.
Case 1 Continued
After approximately 2 months of observation and symptomatic treatment, without specific antibiotic therapy, the patient’s symptoms continue. She now develops intermittent hemoptysis. Repeat sputum studies reveal moderate growth of M. avium. A follow-up CT scan shows progression of disease, with an increase in the tree-in-bud pattern (Figure 3).

What treatment protocols are recommended for MAC pulmonary disease?
As per the ATS/IDSA statement, macrolides are the mainstay of treatment for pulmonary MAC disease.11 Macrolides achieve an increased concentration in the lung, and when used for treatment of pulmonary MAC disease, there is a strong correlation between in vitro susceptibility, in vivo (clinical) response, and the immunomodulating effects of macrolides.31,32 Macrolide-containing regimens have demonstrated efficacy in patients with MAC pulmonary disease33,34; however, macrolide monotherapy should be avoided to prevent the development of resistance.
At the outset, it is critical to establish the objective criteria for determining response and to ensure that the patient understands the goals of the treatment and expectations of the treatment plan. Moreover, experts suggest that due to the possibility of drug intolerance, side effects, and the need for prolonged therapy, a “step ladder” ramping up approach to treatment could be adopted, with gradual introduction of therapy within a short time period; this approach may improve compliance and adherence to treatment.11 If this approach is used, the doses may have to be divided. Patients who are unable to tolerate daily medications, even with dosage adjustment, should be tried on an intermittent treatment regimen. Older female patients frequently require gradual introduction of medications (ie, 1 medication added to the regimen every 1 to 2 weeks) to evaluate tolerance to each medication and medication dose.11 Commonly encountered adverse effects of NTM treatment include intolerance to clarithromycin due to gastrointestinal problems, low body mass index, or age older than 70 years.
After determining that the patient requires therapy, the standard recommended treatment for MAC pulmonary disease includes the following: for most patients with nodular/bronchiectasis disease, a thrice-weekly regimen of clarithromycin (1000 mg) or azithromycin (500 mg), rifampin (600 mg), and ethambutol (25 mg/kg) is recommended. For patients with cavitary MAC pulmonary disease or severe nodular/bronchiectasis disease, the guidelines recommend a daily regimen of clarithromycin (500-1000 mg) or azithromycin (250 mg), rifampin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg), with consideration of intravenous (IV) amikacin 3 times/week early in therapy (Table 2).11

The treatment of MAC hypersensitivity-like disease speaks to the controversy of whether this is an inflammatory process, infectious process, or a combination of inflammation and infection. Avoidance of exposure is the mainstay of management. In some cases, steroids are used with or without a short course of anti-MAC therapy (ie, clarithromycin or azithromycin with rifampin and ethambutol).
Prophylaxis for disseminated MAC disease should be given to adults with HIV infection who have a CD4+ count less than 50 cells/μL. Azithromycin 1200 mg/week or clarithromycin 1000 mg/day has proven efficacy, and rifabutin 300 mg/day is also effective but less well tolerated. Rifabutin is more active in vitro against MAC than rifampin, and is used in HIV-positive patients because of drug-drug interaction between antiretroviral drugs and rifampin.
Continue to: Case 1 Continued
Case 1 Continued
The patient is treated with clarithromycin, rifampin, and ethambutol for 1 year, with sputum conversion after 9 months. In the latter part of her treatment, she experiences decreased visual acuity. Treatment is discontinued prematurely after 1 year due to drug toxicity and continued intolerance to drug therapy. The patient remains asymptomatic for 8 months, and then begins to experience mild to moderate hemoptysis, with increasing cough and sputum production associated with postural changes during exercise. Physical examination overall remains unchanged. Three sputum results reveal heavy growth of MAC, and a CT scan of the chest shows a cavitary lesion in the left upper lobe along with the nodular bronchiectasis (Figure 4).

What are the management options at this stage?
Based on this patient’s continued symptoms, progression of radiologic abnormalities, and current culture growth, she requires re-treatment. With the adverse effects associated with ethambutol during the first round of therapy, the drug regimen needs to be modified. Several considerations are relevant at this stage. Relapse rates range from 20% to 30% after treatment with a macrolide-based therapy.11,34 Obtaining a culture-sensitivity profile is imperative in these cases. Of note, treatment should not be discontinued altogether, but instead the toxic agent should be removed from the treatment regimen. Continuing treatment with a 2-drug regimen of clarithromycin and rifampin may be considered in this patient. Re-infection with multiple genotypes may also occur after successful drug therapy, but this is primarily seen in MAC patients with nodular bronchiectasis.34,35 Patients in whom previous therapy has failed, even those with macrolide-susceptible MAC isolates, are less likely to respond to subsequent therapy. Data suggest that intermittent medication dosing is not effective for patients with severe or cavitary disease or in those in whom previous therapy has failed.36 In this case, treatment should include a daily 3-drug therapy, with an injectable thrice-weekly aminoglycoside. Other agents such as linezolid and clofazimine may have to be tried. Cycloserine, ethionamide, and other agents are sometimes used, but their efficacy is unproven and doubtful. Pyrazinamide and isoniazid have no activity against MAC.
Treatment Failure and Drug Resistance
Treatment failure is considered to have occurred if patients have not had a response (microbiologic, clinical, or radiographic) after 6 months of appropriate therapy or had not achieved conversion of sputum to culture-negative after 12 months of appropriate therapy.11 This occurs in about 40% of patients. Multiple factors can interfere with the successful treatment of MAC pulmonary disease, including medication nonadherence, medication side effects or intolerance, lack of response to a medication regimen, or the emergence of a macrolide-resistant or multidrug-resistant strain. Inducible macrolide resistance remains a potential factor.34-36 A number of characteristics of NTM contribute to the poor response to currently used antibiotics: the organisms have a lipid outer membrane and prefer to adhere to surfaces and form biofilms, which makes them relatively impermeable to antibiotics.37 Also, NTM replicate in phagocytic cells, allowing them to subvert normal cellular defense mechanisms. Furthermore, NTM can display colony variants, whereby single colony isolates switch between antibiotic-susceptible and -resistant variants. These factors have also impeded in development of new antibiotics for NTM infection.37
Recent limited approval of amikacin liposomal inhalation suspension (ALIS) for treatment failure and refractory MAC infection in combination with guideline-based antimicrobial therapy (GBT) is a promising addition to the available treatment armamentarium. In a multinational trial, the addition of ALIS to GBT for treatment-refractory MAC lung disease achieved significantly greater culture conversion rates by month 6 than GBT alone, with comparable rates of serious adverse events.38
Is therapeutic drug monitoring recommended during treatment of MAC pulmonary disease?
Treatment failure may also be drug-related, including poor drug penetration into the damaged lung tissue or drug-drug interactions leading to suboptimal drug levels. Peak serum concentrations have been found to be below target ranges in approximately 50% of patients using a macrolide and ethambutol. Concurrent use of rifampin decreases the peak serum concentration of macrolides and quinolones, with acceptable target levels seen in only 18% to 57% of cases. Whether this alters patient outcomes is not clear.39-42 Factors identified as contributing to the poor response to therapy include poor compliance, cavitary disease, previous treatment for MAC pulmonary disease, and a history of chronic obstructive lung disease. Studies by Koh and colleagues40 and van Ingen and colleagues41 with pharmacokinetic and pharmacodynamics data showed that, in patients on MAC treatment with both clarithromycin and rifampicin, plasma levels of clarithromycin were lower than the recommended minimal inhibitory concentrations (MIC) against MAC for that drug. The studies also showed that rifampicin lowered clarithromycin concentrations more than did rifabutin, with the AUC/MIC ratio being suboptimal in nearly half the cases. However, low plasma clarithromycin concentrations did not have any correlation with treatment outcomes, as the peak plasma drug concentrations and the peak plasma drug concentration/MIC ratios did not differ between patients with unfavorable treatment outcomes and those with favorable outcomes. This is further compounded by the fact that macrolides achieve higher levels in lung tissue than in plasma, and hence the significance of low plasma levels is unclear; however, it is postulated that achieving higher drug levels could, in fact, lead to better clinical outcomes. Pending specific well-designed, prospective randomized controlled trials, routine therapeutic drug monitoring is not currently recommended, although some referral centers do this as their practice pattern.
Is surgery an option in this case?
The overall 5-year mortality for MAC pulmonary disease was approximately 28% in a retrospective analysis, with patients with cavitary disease at increased risk for death at 5 years.42 As such, surgery is an option in selected cases as part of adjunctive therapy along with anti-MAC therapy based on mycobacterial sensitivity. Surgery is used as either a curative approach or a “debulking” measure.11 When present, clearly localized disease, especially in the upper lobe, lends itself best to surgical intervention. Surgical resection of a solitary pulmonary nodule due to MAC, in addition to concomitant medical treatment, is recommended. Surgical intervention should be considered early in the course of the disease because it may provide a cure without prolonged treatment and its associated problems, and this approach may lead to early sputum conversion. Surgery should also be considered in patients with macrolide-resistant or multidrug-resistant MAC infection or in those who cannot tolerate the side effects of therapy, provided that the disease is focal and limited. Patients with poor preoperative lung function have poorer outcomes than those with good lung function, and postoperative complications arising from treatment, especially with a right-sided pneumonectomy, tend to occur more frequently in these patients. Thoracic surgery for NTM pulmonary disease must be considered cautiously, as this is associated with significant morbidity and mortality and is best performed at specialized centers that have expertise and experience in this field.43
Continue to: Mycobacterium abscessus Complex
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).

The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)

What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.
Nontuberculous mycobacterial pulmonary disease is a broad term for a group of pulmonary disorders caused and characterized by exposure to environmental mycobacteria other than those belonging to the Mycobacterium tuberculosis complex and Mycobacterium leprae. Mycobacteria are aerobic, nonmotile organisms that appear positive with acid-fast alcohol stains. Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and have been recovered from domestic and natural water sources, soil, and food products, and from around livestock, cattle, and wildlife.1-3 To date, no evidence exists of human-to-human or animal-to-human transmission of NTM in the general population. Infections in humans are usually acquired from environmental exposures, although the specific source of infection cannot always be identified. Similarly, the mode of infection with NTM has not been established with certainty, but it is highly likely that the organism is implanted, ingested, aspirated, or inhaled. Aerosolization of droplets associated with use of bathroom showerheads and municipal water sources and soil contamination are some of the factors associated with the transmission of infection. Proven routes of transmission include showerheads and potting soil dust.2,3
NTM pulmonary disease occurs in individuals with or without comorbid conditions such as bronchiectasis, chronic obstructive pulmonary disease, pulmonary fibrosis, or structural lung diseases. Slender, middle-aged or elderly white females with marfanoid body habitus, with or without apparent immune or genetic disorders, showing impaired airway and mucus clearance present with this infection as a form of underlying bronchiectasis (Lady Windermere syndrome). It is unclear why NTM infections and escalation to clinical disease occur in certain individuals. Many risk factors, including inherited and acquired defects of host immune response (eg, cystic fibrosis trait and α1 antitrypsin deficiency), have been associated with increased susceptibility to NTM infections.4
NTM infection can lead to chronic symptoms, frequent exacerbations, progressive functional and structural lung destruction, and impaired quality of life, and is associated with an increased risk of hospitalization and higher 5-year all-cause mortality. As such, NTM disease is drawing increasing attention at the clinical, academic, and research levels.5 This case-based review outlines the clinical features of NTM infection, with a focus on the challenges in diagnosis, treatment, and management of NTM pulmonary disease. The cases use Mycobacterium avium complex (MAC), a slow-growing mycobacteria (SGM), and Mycobacterium abscessus, a rapidly growing mycobacteria (RGM), as prototypes in a non–cystic fibrosis, non-HIV clinical setting.
Epidemiology
Of the almost 200 isolated species of NTM, the most prevalent pathogens for respiratory disease in the United States are MAC, Mycobacterium kansasii, and M. abscessus. MAC accounts for more than 80% of cases of NTM respiratory disease in the United States.6 The prevalence of NTM disease is increasing at a rate of about 8% each year, with 75,000 to 105,000 patients diagnosed with NTM lung disease in the United States annually. NTM infections in the United States are increasing among patients aged 65 years and older, a population that is expected to nearly double by 2030.7,8
Isolation and prevalence of many NTM species are higher in certain geographic areas of the United States, especially in the southeast. The US coastal regions have a higher prevalence of NTM pulmonary disease, and account for 70% of NTM cases in the United States each year. Half of patients diagnosed with NTM lung disease reside in 7 states: Florida, New York, Texas, California, Pennsylvania, New Jersey, and Ohio, with 1 in 7 residing in Florida. Three parishes in Louisiana are among the top 10 counties with the highest prevalence in United States. The prevalence of NTM infection–associated hospitalizations is increasing worldwide as well. Co-infection with tuberculosis and multiple NTMs in individual patients has been observed clinically and documented in patients with and without HIV.9,10
It is not clear why the prevalence of NTM pulmonary disease is increasing, but there may be several contributing factors: (1) an increased awareness and identification of NTM infection sources in the environment; (2) an expanding cohort of immunocompromised individuals with exogenous or endogenous immune deficiencies; (3) availability of improved diagnostic techniques, such as use of high-performance liquid chromatography (HPLC), DNA probes, and gene sequencing; and (4) an increased awareness of the morbidity and mortality associated with NTM pulmonary disease. However, it is important to recognize that to best understand the clinical relevance of epidemiologic studies based on laboratory diagnosis and identification, the findings must be evaluated by correlating them with the microbiological and other clinical criteria established by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.11
Continue to: Mycobacterium avium Complex
Mycobacterium avium Complex
Case Patient 1
A 48-year-old woman who has never smoked and has no past medical problems, except seasonal allergic rhinitis and “colds and flu-like illness” once or twice a year, is evaluated for a chronic lingering cough with occasional sputum production. The patient denies any other chronic symptoms and is otherwise active. Physical examination reveals no specific findings except mild pectus excavatum and mild scoliosis. Body mass index is 22 kg/m2. Chest radiograph shows nonspecific increased markings in the lower zones. Computed tomography (CT) scan of the chest reveals minimal nodular and cylindrical bronchiectasis in both lungs (Figure 1). No previous radiographs are available for comparison. The patient is HIV-negative. Sputum tests reveal normal flora, and both fungus and acid-fast bacilli smear are negative. Culture for mycobacteria shows scanty growth of MAC in 1 specimen.

What is the clinical presentation of MAC pulmonary disease?
Among NTM, MAC is the most common cause of pulmonary disease worldwide.6 MAC primarily includes 2 species: M. avium and Mycobacterium intracellulare. M. avium is the more important pathogen in disseminated disease, whereas M. intracellulare is the more common respiratory pathogen.11 These organisms are genetically similar and generally not differentiated in the clinical microbiology laboratory, although there are isolated reports in the literature suggesting differences in prevalence, presentation, and prognosis in M. avium infection versus M. intracellulare infection.12
Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced HIV infection; and cervical lymphadenitis.13 Pulmonary disease caused by MAC may take on 1 of several clinically different forms, including asymptomatic “colonization” or persistent minimal infection without obvious clinical significance; endobronchial involvement; progressive pulmonary disease with radiographic and clinical deterioration and nodular bronchiectasis or cavitary lung disease; hypersensitivity pneumonitis; or persistent, overwhelming mycobacterial growth with symptomatic manifestations, often in a lung with underlying damage due to either chronic obstructive lung disease or pulmonary fibrosis (Table 1).14

Cavitary Disease
The traditionally recognized presentation of MAC lung disease has been apical cavitary lung disease in men in their late 40s and early 50s who have a history of cigarette smoking, and frequently, excessive alcohol use. If left untreated, or in the case of erratic treatment or macrolide drug resistance, this form of disease is generally progressive within a relatively short time and can result in extensive cavitary lung destruction and progressive respiratory failure.15
Nodular Bronchiectasis
The more common presentation of MAC lung disease, which is outlined in the case described here, is interstitial nodular infiltrates, frequently involving the right middle lobe or lingula and predominantly occurring in postmenopausal, nonsmoking white women. This is sometimes labelled “Lady Windermere syndrome.” These patients with M. avium infection appear to have similar clinical characteristics and body types, including lean build, scoliosis, pectus excavatum, and mitral valve prolapse.16,17 The mechanism by which this body morphotype predisposes to pulmonary mycobacterial infection is not defined, but ineffective mucociliary clearance is a possible explanation. Evidence suggests that some patients may be predisposed to NTM lung disease because of preexisting bronchiectasis. Some potential etiologies of bronchiectasis in this population include chronic sinusitis, gastroesophageal reflux with chronic aspiration, α1 antitrypsin deficiency, and cystic fibrosis genetic traits and mutations.18 Risk factors for increased morbidity and mortality include the development of cavitary disease, age, weight loss, lower body mass index, and other comorbid conditions.
This form of disease, termed nodular bronchiectasis, tends to have a much slower progression than cavitary disease, such that long-term follow-up (months to years) may be necessary to demonstrate clinical or radiographic changes.11 The radiographic term “tree-in-bud” has been used to describe what may reflect inflammatory changes, including bronchiolitis. High-resolution CT scans of the chest are especially helpful for diagnosing this pattern of MAC lung disease, as bronchiectasis and small nodules may not be easily discernible on plain chest radiograph. The nodular/bronchiectasis radiographic pattern can also be seen with other NTM pathogens, including M. abscessus, Mycobacterium simiae, and M. kansasii. Solitary nodules and dense consolidation have also been described. Pleural effusions are uncommon, but reactive pleural thickening is frequently seen. Co-pathogens may be isolated from culture, including Pseudomonas aeruginosa, Staphylococcus aureus, and, occasionally, other NTM such as M. abscessus or Mycobacterium chelonae.19-21
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, initially described in patients who were exposed to hot tubs, mimics allergic hypersensitivity pneumonitis, with respiratory symptoms and culture/tissue identification of MAC or sometimes other NTM. It is unclear whether hypersensitivity pneumonitis is an inflammatory process, an infection, or both, and opinion regarding the need for specific antibiotic treatment is divided.11,22 However, avoidance of exposure is prudent and recommended.
Disseminated Disease
Disseminated NTM disease is associated with very low CD4+ lymphocyte counts and is seen in approximately 5% of patients with HIV infection.23-25 Although disseminated NTM disease is rarely seen in immunosuppressed patients without HIV infection, it has been reported in patients who have undergone renal or cardiac transplant, patients on long-term corticosteroid therapy, and those with leukemia or lymphoma. More than 90% of infections are caused by MAC; other potential pathogens include M. kansasii, M. chelonae, M. abscessus, and Mycobacterium haemophilum. Although seen less frequently since the advent of highly active antiretroviral therapy, disseminated infection can develop progressively from an apparently indolent or localized infection or a respiratory or gastrointestinal source. Signs and symptoms of disseminated infection (specifically MAC-associated disease) are nonspecific and include fever, night sweats, weight loss, and abdominal tenderness. Disseminated MAC disease occurs primarily in patients with more advanced HIV disease (CD4+ count typically < 50 cells/μL). Clinically, disseminated MAC manifests as intermittent or persistent fever, constitutional symptoms with organomegaly and organ-specific abnormalities (eg, anemia, neutropenia from bone marrow involvement, adenopathy, hepatosplenomegaly), and elevations of liver enzymes or lung infiltrates from pulmonary involvement.
Continue to: What are the criteria for diagnosing NTM pulmonary disease?
What are the criteria for diagnosing NTM pulmonary disease?
The diagnosis of NTM disease is based on clinical, radiologic, and mycobacterial correlation with good communication between the experts in this field. The ATS/IDSA criteria for diagnosing NTM lung disease are shown in Figure 2. These criteria best apply to MAC, M. kansasii, and M. abscessus, but are also clinically applied to other NTM respiratory pathogens. The diagnosis of MAC infection is most readily established by culture of blood, bone marrow, respiratory secretions/fluid, or tissue specimens from suspected sites of involvement. Due to erratic shedding of MAC into the respiratory secretions in patients with nodular bronchiectasis, as compared to those with the cavitary form of the disease, sputum may be intermittently positive, with variable colony counts and polyclonal infections.12 Prior to the advent of high-resolution CT, isolation of MAC organisms from the sputum of such patients was frequently dismissed as colonization.

Mycobacterial Testing
Because of the nonspecific symptoms and lack of diagnostic specificity of chest imaging, the diagnosis of NTM lung disease requires microbiologic confirmation. Specimens sent to the laboratory for identification of NTM must be handled with care to prevent contamination and false-positive results. Transport media and preservatives should be avoided, and transportation of the specimens should be prompt. These measures will prevent bacterial overgrowth. Furthermore, the yield of NTM may be affected if the patient has used antibiotics, such as macrolides and fluoroquinolones, prior to obtaining the specimen.
NTM should be identified at the species and subspecies level, although this is not practical in community practice settings. The preferred staining procedure in the laboratory is the fluorochrome method. Some species require special growth conditions and/or lower incubation temperatures, and other identification methods may have to be employed, such as DNA probes, polymerase chain reaction genotyping, nucleic acid sequence determination, and high-performance liquid chromatography. As a gold standard, clinical specimens for mycobacterial cultures should be inoculated onto 1 or more solid media (eg, Middlebrook 7H11 media and/or Lowenstein-Jensen media, the former of which is the preferred medium for NTM) and into a liquid medium (eg, BACTEC 12B broth or Mycobacteria growth indicator tube broth). Growth of visible colonies on solid media typically requires 2 to 4 weeks, but liquid media (eg, the radiometric BACTEC system), used as a supplementary and not as an exclusive test, usually produce results within 10 to 14 days. Furthermore, even after initial growth, identification of specific isolates based on the growth characteristics on solid media requires additional time. Use of specific nucleic acid probes for MAC and M. kansasii and HPLC testing of mycolic acid patterns in acid-fast bacilli smear–positive specimens can reduce the turnaround time of specific identification of a primary culture–positive sample. However, HPLC is not sufficient for definitive identification of many NTM species, including the RGM. Other newer techniques, including 16S ribosomal DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism analysis, also allow NTM to be identified and speciated more reliably and rapidly from clinical specimens.
Cost and other practical considerations limit widespread adoption of these techniques. However, the recognition that M. abscessus can be separated into more than 1 subspecies, and that there are important prognostic implications of that separation, lends urgency to the broader adoption of newer molecular techniques in the mycobacteriology laboratory. Susceptibility testing is based on the broth microdilution method; RGM usually grow within 7 days of subculture, and the laboratory time to culture is a helpful hint, although not necessarily specific. Recognizing the morphology of mycobacterial colony growth may also be helpful in identification.
Are skin tests helpful in diagnosing NTM infection?
Tuberculin skin testing remains a nonspecific marker of mycobacterial infection and does not help in further elucidating NTM infection. However, epidemiologic and laboratory studies with well-characterized antigens have shown that dual skin testing with tuberculosis- versus NTM-derived tuberculin can discriminate between prior NTM and prior tuberculosis disease. Species-specific skin test antigens are not commercially available and are not helpful in the diagnosis of NTM disease because of cross-reactivity of M. tuberculosis and some NTM. However, increased prevalence of NTM sensitization based on purified protein derivative testing has been noted in a recent survey, which is consistent with an observed increase in the rates of NTM infections, specifically MAC, in the United States.26,27
Interferon-gamma release assays (IGRAs) are now being used as an alternative to tuberculin skin testing to diagnose M. tuberculosis infection. Certain NTM species also contain gene sequences that encode for ESAT-6 or CFP-10 antigens used in the IGRAs, and hence, yield a positive IGRA test. These include M. marinum, M. szulgai, and M. kansasii.28,29 However, MAC organisms do not produce positive results on assays that use these antigens.
Continue to: What is the approach to management of NTM pulmonary disease?
What is the approach to management of NTM pulmonary disease?
The correlation of symptoms with radiographic and microbiologic evidence is essential to categorize the disease and determine the need for therapy. Making the diagnosis of NTM lung disease does not necessitate the institution of therapy. The decision to treat should be weighed against potential risks and benefits to the individual patient based on symptomatic, radiographic, and microbiologic criteria, as well as underlying systemic or pulmonary immune status. In the absence of evidence of clinical, radiologic, or mycobacterial progression of disease, pursuing airway clearance therapy and clinical surveillance without initiating specific anti-MAC therapy is a reasonable option.11 Identifying the sustained presence of NTM infection, especially MAC, in a patient with underlying clinical and radiographic evidence of bronchiectasis is of value in determining comprehensive treatment and management strategies. Close observation is indicated if the decision not to treat is made. If treatment is initiated, comprehensive management includes long-term follow-up with periodic bacteriologic surveillance, watching for drug toxicity and drug-drug interactions, ensuring adherence and compliance to treatment, and managing comorbidity.
The Bronchiectasis Severity Index is a useful clinical predictive tool that identifies patients at risk of future mortality, hospitalization, and exacerbations and can be used to evaluate the need for specific treatment.30 The index is based on dyspnea score, lung function tests, colonization of pathogens, and extent of disease.
Case 1 Continued
After approximately 2 months of observation and symptomatic treatment, without specific antibiotic therapy, the patient’s symptoms continue. She now develops intermittent hemoptysis. Repeat sputum studies reveal moderate growth of M. avium. A follow-up CT scan shows progression of disease, with an increase in the tree-in-bud pattern (Figure 3).

What treatment protocols are recommended for MAC pulmonary disease?
As per the ATS/IDSA statement, macrolides are the mainstay of treatment for pulmonary MAC disease.11 Macrolides achieve an increased concentration in the lung, and when used for treatment of pulmonary MAC disease, there is a strong correlation between in vitro susceptibility, in vivo (clinical) response, and the immunomodulating effects of macrolides.31,32 Macrolide-containing regimens have demonstrated efficacy in patients with MAC pulmonary disease33,34; however, macrolide monotherapy should be avoided to prevent the development of resistance.
At the outset, it is critical to establish the objective criteria for determining response and to ensure that the patient understands the goals of the treatment and expectations of the treatment plan. Moreover, experts suggest that due to the possibility of drug intolerance, side effects, and the need for prolonged therapy, a “step ladder” ramping up approach to treatment could be adopted, with gradual introduction of therapy within a short time period; this approach may improve compliance and adherence to treatment.11 If this approach is used, the doses may have to be divided. Patients who are unable to tolerate daily medications, even with dosage adjustment, should be tried on an intermittent treatment regimen. Older female patients frequently require gradual introduction of medications (ie, 1 medication added to the regimen every 1 to 2 weeks) to evaluate tolerance to each medication and medication dose.11 Commonly encountered adverse effects of NTM treatment include intolerance to clarithromycin due to gastrointestinal problems, low body mass index, or age older than 70 years.
After determining that the patient requires therapy, the standard recommended treatment for MAC pulmonary disease includes the following: for most patients with nodular/bronchiectasis disease, a thrice-weekly regimen of clarithromycin (1000 mg) or azithromycin (500 mg), rifampin (600 mg), and ethambutol (25 mg/kg) is recommended. For patients with cavitary MAC pulmonary disease or severe nodular/bronchiectasis disease, the guidelines recommend a daily regimen of clarithromycin (500-1000 mg) or azithromycin (250 mg), rifampin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg), with consideration of intravenous (IV) amikacin 3 times/week early in therapy (Table 2).11

The treatment of MAC hypersensitivity-like disease speaks to the controversy of whether this is an inflammatory process, infectious process, or a combination of inflammation and infection. Avoidance of exposure is the mainstay of management. In some cases, steroids are used with or without a short course of anti-MAC therapy (ie, clarithromycin or azithromycin with rifampin and ethambutol).
Prophylaxis for disseminated MAC disease should be given to adults with HIV infection who have a CD4+ count less than 50 cells/μL. Azithromycin 1200 mg/week or clarithromycin 1000 mg/day has proven efficacy, and rifabutin 300 mg/day is also effective but less well tolerated. Rifabutin is more active in vitro against MAC than rifampin, and is used in HIV-positive patients because of drug-drug interaction between antiretroviral drugs and rifampin.
Continue to: Case 1 Continued
Case 1 Continued
The patient is treated with clarithromycin, rifampin, and ethambutol for 1 year, with sputum conversion after 9 months. In the latter part of her treatment, she experiences decreased visual acuity. Treatment is discontinued prematurely after 1 year due to drug toxicity and continued intolerance to drug therapy. The patient remains asymptomatic for 8 months, and then begins to experience mild to moderate hemoptysis, with increasing cough and sputum production associated with postural changes during exercise. Physical examination overall remains unchanged. Three sputum results reveal heavy growth of MAC, and a CT scan of the chest shows a cavitary lesion in the left upper lobe along with the nodular bronchiectasis (Figure 4).

What are the management options at this stage?
Based on this patient’s continued symptoms, progression of radiologic abnormalities, and current culture growth, she requires re-treatment. With the adverse effects associated with ethambutol during the first round of therapy, the drug regimen needs to be modified. Several considerations are relevant at this stage. Relapse rates range from 20% to 30% after treatment with a macrolide-based therapy.11,34 Obtaining a culture-sensitivity profile is imperative in these cases. Of note, treatment should not be discontinued altogether, but instead the toxic agent should be removed from the treatment regimen. Continuing treatment with a 2-drug regimen of clarithromycin and rifampin may be considered in this patient. Re-infection with multiple genotypes may also occur after successful drug therapy, but this is primarily seen in MAC patients with nodular bronchiectasis.34,35 Patients in whom previous therapy has failed, even those with macrolide-susceptible MAC isolates, are less likely to respond to subsequent therapy. Data suggest that intermittent medication dosing is not effective for patients with severe or cavitary disease or in those in whom previous therapy has failed.36 In this case, treatment should include a daily 3-drug therapy, with an injectable thrice-weekly aminoglycoside. Other agents such as linezolid and clofazimine may have to be tried. Cycloserine, ethionamide, and other agents are sometimes used, but their efficacy is unproven and doubtful. Pyrazinamide and isoniazid have no activity against MAC.
Treatment Failure and Drug Resistance
Treatment failure is considered to have occurred if patients have not had a response (microbiologic, clinical, or radiographic) after 6 months of appropriate therapy or had not achieved conversion of sputum to culture-negative after 12 months of appropriate therapy.11 This occurs in about 40% of patients. Multiple factors can interfere with the successful treatment of MAC pulmonary disease, including medication nonadherence, medication side effects or intolerance, lack of response to a medication regimen, or the emergence of a macrolide-resistant or multidrug-resistant strain. Inducible macrolide resistance remains a potential factor.34-36 A number of characteristics of NTM contribute to the poor response to currently used antibiotics: the organisms have a lipid outer membrane and prefer to adhere to surfaces and form biofilms, which makes them relatively impermeable to antibiotics.37 Also, NTM replicate in phagocytic cells, allowing them to subvert normal cellular defense mechanisms. Furthermore, NTM can display colony variants, whereby single colony isolates switch between antibiotic-susceptible and -resistant variants. These factors have also impeded in development of new antibiotics for NTM infection.37
Recent limited approval of amikacin liposomal inhalation suspension (ALIS) for treatment failure and refractory MAC infection in combination with guideline-based antimicrobial therapy (GBT) is a promising addition to the available treatment armamentarium. In a multinational trial, the addition of ALIS to GBT for treatment-refractory MAC lung disease achieved significantly greater culture conversion rates by month 6 than GBT alone, with comparable rates of serious adverse events.38
Is therapeutic drug monitoring recommended during treatment of MAC pulmonary disease?
Treatment failure may also be drug-related, including poor drug penetration into the damaged lung tissue or drug-drug interactions leading to suboptimal drug levels. Peak serum concentrations have been found to be below target ranges in approximately 50% of patients using a macrolide and ethambutol. Concurrent use of rifampin decreases the peak serum concentration of macrolides and quinolones, with acceptable target levels seen in only 18% to 57% of cases. Whether this alters patient outcomes is not clear.39-42 Factors identified as contributing to the poor response to therapy include poor compliance, cavitary disease, previous treatment for MAC pulmonary disease, and a history of chronic obstructive lung disease. Studies by Koh and colleagues40 and van Ingen and colleagues41 with pharmacokinetic and pharmacodynamics data showed that, in patients on MAC treatment with both clarithromycin and rifampicin, plasma levels of clarithromycin were lower than the recommended minimal inhibitory concentrations (MIC) against MAC for that drug. The studies also showed that rifampicin lowered clarithromycin concentrations more than did rifabutin, with the AUC/MIC ratio being suboptimal in nearly half the cases. However, low plasma clarithromycin concentrations did not have any correlation with treatment outcomes, as the peak plasma drug concentrations and the peak plasma drug concentration/MIC ratios did not differ between patients with unfavorable treatment outcomes and those with favorable outcomes. This is further compounded by the fact that macrolides achieve higher levels in lung tissue than in plasma, and hence the significance of low plasma levels is unclear; however, it is postulated that achieving higher drug levels could, in fact, lead to better clinical outcomes. Pending specific well-designed, prospective randomized controlled trials, routine therapeutic drug monitoring is not currently recommended, although some referral centers do this as their practice pattern.
Is surgery an option in this case?
The overall 5-year mortality for MAC pulmonary disease was approximately 28% in a retrospective analysis, with patients with cavitary disease at increased risk for death at 5 years.42 As such, surgery is an option in selected cases as part of adjunctive therapy along with anti-MAC therapy based on mycobacterial sensitivity. Surgery is used as either a curative approach or a “debulking” measure.11 When present, clearly localized disease, especially in the upper lobe, lends itself best to surgical intervention. Surgical resection of a solitary pulmonary nodule due to MAC, in addition to concomitant medical treatment, is recommended. Surgical intervention should be considered early in the course of the disease because it may provide a cure without prolonged treatment and its associated problems, and this approach may lead to early sputum conversion. Surgery should also be considered in patients with macrolide-resistant or multidrug-resistant MAC infection or in those who cannot tolerate the side effects of therapy, provided that the disease is focal and limited. Patients with poor preoperative lung function have poorer outcomes than those with good lung function, and postoperative complications arising from treatment, especially with a right-sided pneumonectomy, tend to occur more frequently in these patients. Thoracic surgery for NTM pulmonary disease must be considered cautiously, as this is associated with significant morbidity and mortality and is best performed at specialized centers that have expertise and experience in this field.43
Continue to: Mycobacterium abscessus Complex
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).

The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)

What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.
1. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210-220.
2. Falkinham JO III. Environmental sources of NTM. Clin Chest Med. 2015;36:35-41.
3. Falkinham JO III, Current epidemiological trends in NTM. Curr Environ Health Rep. 2016;3:161-167.
4. Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1-11.
5. Marras TK, Mirsaeidi M, Chou E, et al. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. Manag Care Spec Pharm. 2018;24:964-974.
6. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182:970-976.
7. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-982.
8. Adjemian, Olivier KN, Seitz AE, J et al. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185;881-886.
9. Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalizations associated with pulmonary nontuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
10. Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170.
11. Griffith DE, Aksamit T, Brown-Elliott, et al; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-415.
12. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235-1244.
13. Gordin FM, Horsburgh CR Jr. Mycobacterium avium complex. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier; 2015.
14. Chitty S, Ali J. Mycobacterium avium complex pulmonary disease in immune competent patients. South Med J. 2005;98:646-52.
15. Ramirez J, Mason C, Ali J, Lopez FA. MAC pulmonary disease: management options in HIV-negative patients. J La State Med Soc. 2008;160:248-254.
16. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914-916.
17. Kim RD, Greenburg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066-1074.
18. Ziedalski TM, Kao PN, Henig NR, et al. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995-1002.
19. Koh WJ, Lee KS, Kwon OJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282-288.
20. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994;105:49-52.
21. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033-1040.
22. Cappelluti E, Fraire AE, Schaefer OP. A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med. 2003;163:845-848.
23. Nightingale SD, Byrd LT, Southern PM, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082-1085.
24. Horsburgh CR Jr, Selik RM. The epidemiology of disseminated tuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989;139:4-7.
25. Chin DP, Hopewell PC, Yajko DM, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289-295.
26. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306-313.
27. Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990;12:760-767.
28. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
29. Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797-1807.
30. James D, Chalmers JD, Goeminne P, et al. The Bronchiectasis Severity Index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576-585.
31. Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988;32:1131-1136.
32. Horsburgh CR Jr, Mason UG 3rd, Heifits LB, et al. Response to therapy of pulmonary Mycobacterium avium intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135:418-421.
33. Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S-78S.
34. Wallace RJ Jr, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766-1772.
35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928-934.
36. Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283-1289.
37. Falkinham J III. Challenges of NTM drug development. Front Microbiol. 2018;9:1613.
38. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-1569.
39. Schluger NW. Treatment of pulmonary Mycobacterium avium complex infections: do drug levels matter? Am J Respir Crit Care Med. 2012;186:710-711.
40. Van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559-565.
41. Koh WJ, Jeong BH, Jeon K, et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:797-802.
42. Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary MAC disease. Int J Tuberc Lung Dis. 2012;16:408-414.
43. Yuji S, Yutsuki N, Keiichiso T, et al. Surgery for Mycobacterium avium lung disease in the clarithromycin era. Eur J Cardiothor Surg. 2002;21:314-318.
44. Tortoli E, Kohl TA, Brown-Elliott BA, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. Int J Syst Evol Microbiol. 2016; 66:4471-4479.
45. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-1278.
46. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309-316.
1. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210-220.
2. Falkinham JO III. Environmental sources of NTM. Clin Chest Med. 2015;36:35-41.
3. Falkinham JO III, Current epidemiological trends in NTM. Curr Environ Health Rep. 2016;3:161-167.
4. Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1-11.
5. Marras TK, Mirsaeidi M, Chou E, et al. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. Manag Care Spec Pharm. 2018;24:964-974.
6. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182:970-976.
7. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-982.
8. Adjemian, Olivier KN, Seitz AE, J et al. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185;881-886.
9. Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalizations associated with pulmonary nontuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
10. Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170.
11. Griffith DE, Aksamit T, Brown-Elliott, et al; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-415.
12. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235-1244.
13. Gordin FM, Horsburgh CR Jr. Mycobacterium avium complex. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier; 2015.
14. Chitty S, Ali J. Mycobacterium avium complex pulmonary disease in immune competent patients. South Med J. 2005;98:646-52.
15. Ramirez J, Mason C, Ali J, Lopez FA. MAC pulmonary disease: management options in HIV-negative patients. J La State Med Soc. 2008;160:248-254.
16. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914-916.
17. Kim RD, Greenburg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066-1074.
18. Ziedalski TM, Kao PN, Henig NR, et al. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995-1002.
19. Koh WJ, Lee KS, Kwon OJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282-288.
20. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994;105:49-52.
21. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033-1040.
22. Cappelluti E, Fraire AE, Schaefer OP. A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med. 2003;163:845-848.
23. Nightingale SD, Byrd LT, Southern PM, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082-1085.
24. Horsburgh CR Jr, Selik RM. The epidemiology of disseminated tuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989;139:4-7.
25. Chin DP, Hopewell PC, Yajko DM, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289-295.
26. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306-313.
27. Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990;12:760-767.
28. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
29. Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797-1807.
30. James D, Chalmers JD, Goeminne P, et al. The Bronchiectasis Severity Index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576-585.
31. Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988;32:1131-1136.
32. Horsburgh CR Jr, Mason UG 3rd, Heifits LB, et al. Response to therapy of pulmonary Mycobacterium avium intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135:418-421.
33. Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S-78S.
34. Wallace RJ Jr, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766-1772.
35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928-934.
36. Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283-1289.
37. Falkinham J III. Challenges of NTM drug development. Front Microbiol. 2018;9:1613.
38. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-1569.
39. Schluger NW. Treatment of pulmonary Mycobacterium avium complex infections: do drug levels matter? Am J Respir Crit Care Med. 2012;186:710-711.
40. Van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559-565.
41. Koh WJ, Jeong BH, Jeon K, et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:797-802.
42. Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary MAC disease. Int J Tuberc Lung Dis. 2012;16:408-414.
43. Yuji S, Yutsuki N, Keiichiso T, et al. Surgery for Mycobacterium avium lung disease in the clarithromycin era. Eur J Cardiothor Surg. 2002;21:314-318.
44. Tortoli E, Kohl TA, Brown-Elliott BA, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. Int J Syst Evol Microbiol. 2016; 66:4471-4479.
45. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-1278.
46. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309-316.
FDA approves novel pandemic influenza vaccine
The Food and Drug Administration has approved the first and only adjuvanted, cell-based pandemic vaccine to provide active immunization against the influenza A virus H5N1 strain.
Influenza A (H5N1) monovalent vaccine, adjuvanted (Audenz, Seqirus) is for use in individuals aged 6 months and older. It’s designed to be rapidly deployed to help protect the U.S. population and can be stockpiled for first responders in the event of a pandemic.
The vaccine and formulated prefilled syringes used in the vaccine are produced in a state-of-the-art production facility built and supported through a multiyear public-private partnership between Seqirus and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health & Human Services.
“Pandemic influenza viruses can be deadly and spread rapidly, making production of safe, effective vaccines essential in saving lives,” BARDA Director Rick Bright, PhD, said in a company news release.
“With this licensure – the latest FDA-approved vaccine to prevent H5N1 influenza — we celebrate a decade-long partnership to achieve health security goals set by the National Strategy for Pandemic Influenza and the 2019 Executive Order to speed the availability of influenza vaccine. Ultimately, this latest licensure means we can protect more people in an influenza pandemic,” said Bright.
“The approval of Audenz represents a key advance in influenza prevention and pandemic preparedness, combining leading-edge, cell-based manufacturing and adjuvant technologies,” Russell Basser, MD, chief scientist and senior vice president of research and development at Seqirus, said in the news release. “This pandemic influenza vaccine exemplifies our commitment to developing innovative technologies that can help provide rapid response during a pandemic emergency.”
Audenz had FDA fast track designation, a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first and only adjuvanted, cell-based pandemic vaccine to provide active immunization against the influenza A virus H5N1 strain.
Influenza A (H5N1) monovalent vaccine, adjuvanted (Audenz, Seqirus) is for use in individuals aged 6 months and older. It’s designed to be rapidly deployed to help protect the U.S. population and can be stockpiled for first responders in the event of a pandemic.
The vaccine and formulated prefilled syringes used in the vaccine are produced in a state-of-the-art production facility built and supported through a multiyear public-private partnership between Seqirus and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health & Human Services.
“Pandemic influenza viruses can be deadly and spread rapidly, making production of safe, effective vaccines essential in saving lives,” BARDA Director Rick Bright, PhD, said in a company news release.
“With this licensure – the latest FDA-approved vaccine to prevent H5N1 influenza — we celebrate a decade-long partnership to achieve health security goals set by the National Strategy for Pandemic Influenza and the 2019 Executive Order to speed the availability of influenza vaccine. Ultimately, this latest licensure means we can protect more people in an influenza pandemic,” said Bright.
“The approval of Audenz represents a key advance in influenza prevention and pandemic preparedness, combining leading-edge, cell-based manufacturing and adjuvant technologies,” Russell Basser, MD, chief scientist and senior vice president of research and development at Seqirus, said in the news release. “This pandemic influenza vaccine exemplifies our commitment to developing innovative technologies that can help provide rapid response during a pandemic emergency.”
Audenz had FDA fast track designation, a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first and only adjuvanted, cell-based pandemic vaccine to provide active immunization against the influenza A virus H5N1 strain.
Influenza A (H5N1) monovalent vaccine, adjuvanted (Audenz, Seqirus) is for use in individuals aged 6 months and older. It’s designed to be rapidly deployed to help protect the U.S. population and can be stockpiled for first responders in the event of a pandemic.
The vaccine and formulated prefilled syringes used in the vaccine are produced in a state-of-the-art production facility built and supported through a multiyear public-private partnership between Seqirus and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health & Human Services.
“Pandemic influenza viruses can be deadly and spread rapidly, making production of safe, effective vaccines essential in saving lives,” BARDA Director Rick Bright, PhD, said in a company news release.
“With this licensure – the latest FDA-approved vaccine to prevent H5N1 influenza — we celebrate a decade-long partnership to achieve health security goals set by the National Strategy for Pandemic Influenza and the 2019 Executive Order to speed the availability of influenza vaccine. Ultimately, this latest licensure means we can protect more people in an influenza pandemic,” said Bright.
“The approval of Audenz represents a key advance in influenza prevention and pandemic preparedness, combining leading-edge, cell-based manufacturing and adjuvant technologies,” Russell Basser, MD, chief scientist and senior vice president of research and development at Seqirus, said in the news release. “This pandemic influenza vaccine exemplifies our commitment to developing innovative technologies that can help provide rapid response during a pandemic emergency.”
Audenz had FDA fast track designation, a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
This article first appeared on Medscape.com.
A better approach to preventing active TB?
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 27-year-old daycare worker was tested for tuberculosis (TB) as part of a recent work physical. She presents to your office for follow-up for her positive purified protein derivative (PPD) skin test. You confirm the result with a quantiferon gold test and ensure she does not have active TB. What medication should you prescribe to treat her latent TB infection (LTBI)?
In 2017, there were 9093 cases of new active TB in the United States.2 It’s estimated that one-fourth of the world’s population has latent TB.3 Identifying and treating latent TB infection is vital to achieving TB’s elimination.4,5
Primary care clinicians are at the forefront of screening high-risk populations for TB. Once identified, treating LTBI can be challenging for providers and patients. Treatment guidelines recommend 4 to 9 months of daily isoniazid.5-8 Shorter treatment regimens were recommended previously; they tended to be rigorous, to involve multiple drugs, and to require high adherence rates. As such, they included directly observed therapy, which prevented widespread adoption.
Consequently, the mainstay for treating LTBI has been 9 months of daily isoniazid. However, isoniazid use is limited by hepatoxicity and by suboptimal treatment completion rates. A 2018 retrospective analysis of patients treated for LTBI reported a completion rate of only 49% for 9 months of isoniazid.9 Additionally, a Cochrane review last updated in 2013 suggests that shorter courses of rifampin are similar in efficacy to isoniazid (although with a wide confidence interval [CI]), and likely have higher adherence rates.10
STUDY SUMMARY
Rifampin is as effective as isoniazid with fewer adverse effects
The study by Menzies et al1 was a multisite, 9-country, open-label, randomized controlled trial (RCT) that compared 4 months of daily rifampin to 9 months of daily isoniazid for the treatment of LTBI in adults. Participants were eligible if they had a positive tuberculin skin test or interferon-gamma-release assay, were ≥ 18 years of age, had an increased risk for reactivation of active TB, and if their health care provider had recommended treatment with isoniazid. Exclusion criteria included current pregnancy or plans to become pregnant, exposure to a patient with TB whose isolates were resistant to either trial drug, an allergy to either of the trial drugs, use of a medication with serious potential interactions with the trial drugs, or current active TB.
Method, outcomes, patient characteristics. Patients received either isoniazid 5 mg/kg body weight (maximum dose 300 mg) daily for 9 months or rifampin 10 mg/kg (maximum dose 600 mg) daily for 4 months and were followed for 28 months. Patients in the isoniazid group also received pyridoxine (vitamin B6) if they were at risk for neuropathy. The primary outcome was the rate of active TB. Secondary outcomes included adverse events, medication regimen completion rate, and drug resistance, among others.
A total of 2989 patients were treated with isoniazid; 3023 patients were treated with rifampin. The mean age of the participants was 38.4 years, 41% of the population was male, and 71% of the groups had confirmed active TB in close contacts.
Continue to: Results
Results. Overall, rates of active TB were low with 9 cases in the isoniazid group and 8 in the rifampin group. In the intention-to-treat analysis, the rate difference for confirmed active TB was < 0.01 cases per 100 person-years (95% CI; −0.14 to 0.16). This met the prespecified noninferiority endpoint, but did not show superiority. A total of 79% of patients treated with rifampin vs 63% treated with isoniazid completed their respective medication courses (difference of 15.1 percentage points; 95% CI, 12.7-17.4; P < .001). Compared with patients in the isoniazid group, those taking rifampin had fewer adverse events, leading to discontinuation (5.6% vs 2.8%).
WHAT’S NEW?
First high-quality study to show that less is more
This is the first large, high-quality study to show that a shorter (4 month) rifampin-based regimen is not inferior to a longer (9 months) isoniazid-based regimen for the treatment of LTBI, and that rifampin is associated with improved adherence and fewer adverse events.
CAVEATS
Low rate of active TB infection and potential bias
The current study had lower-than-anticipated rates of active TB infection, which made the study’s conclusions less compelling. This may have been because of a small number of patients with human immunodeficiency virus enrolled in the study and/or that even participants who discontinued treatment received a median of 3 months of partial treatment.
In addition, the study was an open-label RCT, subjecting it to potential bias. However, the diagnosis of active TB and attribution of adverse events were made by an independent, blinded review panel.
CHALLENGES TO IMPLEMENTATION
No challenges to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
1. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
2. Stewart RJ, Tsang CA, Pratt RH, et al. Tuberculosis — United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:317-323.
3. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLoS Med. 2016;13:e1002152.
4. Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928-952.
5. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385:1799-1801.
6. Centers for Disease Control and Prevention. Treatment regimens for latent TB infection (LTBI). Last reviewed April 5, 2016. https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed January 15, 2020.
7. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management. 2018. Publication no. WHO/CDS/TB/2018.4. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed January 15, 2020.
8. Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67:723-726.
9. Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuber Lung Dis. 2018;22:1344-1349. 10. Sharma SK, Sharma A, Kadhiravan T, et al. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev. 2013;(7):CD007545.
PRACTICE CHANGER
Use 4 months of rifampin instead of 9 months of isoniazid to treat adults with latent tuberculosis; rifampin is associated with fewer adverse events and higher completion rates.
STRENGTH OF RECOMMENDATION
A: Based on a randomized controlled trial and a previous Cochrane review.
Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440-453.
New cystic fibrosis therapy raises hopes among specialists and patients
A newly approved triple-combination modulator to treat cystic fibrosis (CF) has raised expectations of a treatment turning point among patients and specialists. If the early results are sustained, elexacaftor/ivacaftor/tezacaftor (Trikafta) could prove to be the rare case of a much-touted new medicine that meets high expectations.
“CF even in infants causes inflammation, so we know that lung damage can start early and progress,” said Susan Millard, MD, FCCP, of Helen DeVos Children’s Hospital in Grand Rapids, Mich., and the local clinical research director for the pediatric pulmonary and sleep medicine section. “This oral drug therapy is actually treating the underlying problem, as opposed to many of the therapies we have that take hours to nebulize and only work locally in the airways.”
Dr. Millard is the recent past pediatric editor for Chest Physician and has been a local principal investigator at Helen DeVos Children’s Hospital for many Vertex-sponsored clinical studies.
The pivotal studies
The Food and Drug Administration approval of Trikafta rested on two pivotal phase 3, placebo-controlled studies, one in patients with two copies of the most common CF mutations, F508del, and the second in patients with one copy of F508del and a second mutation that was called a “minimal-function” mutation. The findings have ignited the hopes of many people with CF and their physicians. The drug was approved in October 2019 for patients aged 12 years and older who have at least one F508del mutation of the cystic fibrosis transmembrane conductance regulator gene. About 90% of patients in the United States have at least one copy of F508del. In the study looking at patients with one copy of F508del, the mean predicted forced expiratory volume in 1 second increased 13.8% in patients taking the drug versus placebo (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMoa1908639). The number of pulmonary exacerbations decreased by 63% in the Trikafta group, compared with placebo. Pulmonary exacerbations were described as a change in specific symptoms that required treatment with a new oral, intravenous, or inhaled antibiotic. Serious adverse drug reactions that occurred more frequently in patients receiving Trikafta, compared with placebo, were rash and influenza events.
In the study that included patients with two copies of F508del, on average, the lung function increased 10% versus patients on ivacaftor/tezacaftor at 4 weeks. In addition, there was a 45.1 mmol/L on average decrease in the sweat chloride level in the Trikafta group, compared with ivacaftor/tezacaftor.
A hopeful start
Robert Giusti, MD, a pediatric pulmonologist at New York University Langone Health, is also hopeful. “This could be the kind of treatment that will make a revolution in terms of [cystic fibrosis] care if it can be started very early in life shortly after diagnosis. We anticipate that patients will be disease free for a longer period of time.”
The Cystic Fibrosis Foundation’s (CFF) “venture philanthropy” initiative played an important role in the development of the drug by Vertex Pharmaceuticals. The CFF has invested many millions of dollars in research by drug companies since the 1980s and was an early backer of Vertex. According to a statement on the CFF website, the Foundation sold its royalty rights for treatments developed by Vertex for $3.3 billion in 2014. The drug has a list price of about $311,000 a year. Payment issues may arise in the future, but for now, Vertex has stated that insurers and some Medicaid programs have begun paying claims for Trikafta
Specialists who treat CF now are watching to see how well patients tolerate this highly anticipated drug – and how well it meets expectations. The Therapeutic Development Network, the clinical research division of the CFF, is enrolling patients taking Trikafta in an observational study to follow for long-term follow-up.
Meeting expectations
“[Long-term efficacy is] something that we’re always concerned about. When the drug comes to market, is it going to be as effective as we thought it might be?” said Ryan Thomas, MD, director of the Cystic Fibrosis Center at Michigan State University, East Lansing. The MSU Cystic Fibrosis Center receives funding from the Cystic Fibrosis Foundation.
Almost one in five patients could not tolerate treatment with Orkambi, most often because of adverse breathing events, according to a French study published in the American Journal of Respiratory and Critical Care Medicine. The investigators wrote: “Among the 845 patients (292 adolescents, 553 adults) who initiated lumacaftor/ivacaftor, 18.2% (154 patients) discontinued treatment, often due to respiratory (48.1%, 74 patients) or nonrespiratory (27.9%, 43 patients) adverse events” and that the discontinuation rate was considerably higher than previously reported in clinical trials.
“We thought [Orkambi] was going to be something that could have a big effect,” Dr. Thomas said. “It turned out that it was harder for people to tolerate than we thought and the improvements weren’t as sustained as we thought they might be. I really don’t think this will end up being the case with Trikafta.”
Longer-term data are starting to emerge, which may ease some of the concerns inherent in working with a newer medicine. “These [data] suggest that this is going to be a game changer,” Dr. Thomas said. “If Trikafta is this efficacious, well, we’re talking about having people with CF who will live full lifespans without a lung transplant, and that is so rare.”
The decrease in hospitalizations, improved CT scans, and lower rates of lung function decline suggest it could be “the Holy Grail,” Dr. Thomas said.
A different disease
Trikafta is the latest in a series of improvements of CF treatment in recent decades, recalled Dr. Giusti, who has been in this field for about 3 decades. “It used to be that I attended many funerals for children with CF. Now with patients living longer and healthier lives I am invited to attend their weddings and even their children’s baptisms and bris ceremonies. It is a very different disease than it used to be.”
The promise of Trikafta leaves behind the minority of patients for whom the drug won’t work. This is for the 10% of patients that have rare mutations. That can lead to difficult conversations with parents about why this new option is not a choice for their child, Dr. Millard said. “It just crushes you, but the Cystic Fibrosis Foundation is committing a lot of new research in that direction. Their mantra is ‘until it is done.’ ”
Realistic expectations
William (Randy) Hunt, MD, FAAP, FACP, assistant professor of medicine in the Division of Pulmonary, Allergy, Critical Care and Sleep, Emory University School of Medicine, Atlanta, agrees that Trikafta is an exciting development in CF treatment. He noted, “Starting this medication early in life may very well significantly attenuate the disease, but it is not a cure. For individuals who already have significant disease, we may not see the same level of improvements in lung function as what we saw in the studies. The studies generally excluded individuals with ppFEV1 < 40%. Nevertheless, I remain optimistic and have been prescribing it to nearly everyone that qualifies after a discussion.”
Dr. Hunt added, “Patients are asking if they can stop their current chronic CF therapies once they start Trikafta. The answer is “no, at least not right now.” While all the relatively short-term data around Trikafta are very promising, we do not yet know how sustained the long-term benefits will be. Still, safely removing therapeutic burden from our patient population is a real interest. There are plans underway by the CFF and other institutions to systematically research whether discontinuing chronic CF therapies is safe in the setting of Trikafta.”
He concluded that 10% of individuals with CF mutations still do not respond to the modulators currently available. “We will not leave that population behind, but treating these remaining mutations is going to take continued efforts and likely modulators that are therapeutically differently from the mechanism of actions of those that are currently available,” he said.
Therese Borden contributed to this article.
1/2/2020 - This story was updated.
A newly approved triple-combination modulator to treat cystic fibrosis (CF) has raised expectations of a treatment turning point among patients and specialists. If the early results are sustained, elexacaftor/ivacaftor/tezacaftor (Trikafta) could prove to be the rare case of a much-touted new medicine that meets high expectations.
“CF even in infants causes inflammation, so we know that lung damage can start early and progress,” said Susan Millard, MD, FCCP, of Helen DeVos Children’s Hospital in Grand Rapids, Mich., and the local clinical research director for the pediatric pulmonary and sleep medicine section. “This oral drug therapy is actually treating the underlying problem, as opposed to many of the therapies we have that take hours to nebulize and only work locally in the airways.”
Dr. Millard is the recent past pediatric editor for Chest Physician and has been a local principal investigator at Helen DeVos Children’s Hospital for many Vertex-sponsored clinical studies.
The pivotal studies
The Food and Drug Administration approval of Trikafta rested on two pivotal phase 3, placebo-controlled studies, one in patients with two copies of the most common CF mutations, F508del, and the second in patients with one copy of F508del and a second mutation that was called a “minimal-function” mutation. The findings have ignited the hopes of many people with CF and their physicians. The drug was approved in October 2019 for patients aged 12 years and older who have at least one F508del mutation of the cystic fibrosis transmembrane conductance regulator gene. About 90% of patients in the United States have at least one copy of F508del. In the study looking at patients with one copy of F508del, the mean predicted forced expiratory volume in 1 second increased 13.8% in patients taking the drug versus placebo (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMoa1908639). The number of pulmonary exacerbations decreased by 63% in the Trikafta group, compared with placebo. Pulmonary exacerbations were described as a change in specific symptoms that required treatment with a new oral, intravenous, or inhaled antibiotic. Serious adverse drug reactions that occurred more frequently in patients receiving Trikafta, compared with placebo, were rash and influenza events.
In the study that included patients with two copies of F508del, on average, the lung function increased 10% versus patients on ivacaftor/tezacaftor at 4 weeks. In addition, there was a 45.1 mmol/L on average decrease in the sweat chloride level in the Trikafta group, compared with ivacaftor/tezacaftor.
A hopeful start
Robert Giusti, MD, a pediatric pulmonologist at New York University Langone Health, is also hopeful. “This could be the kind of treatment that will make a revolution in terms of [cystic fibrosis] care if it can be started very early in life shortly after diagnosis. We anticipate that patients will be disease free for a longer period of time.”
The Cystic Fibrosis Foundation’s (CFF) “venture philanthropy” initiative played an important role in the development of the drug by Vertex Pharmaceuticals. The CFF has invested many millions of dollars in research by drug companies since the 1980s and was an early backer of Vertex. According to a statement on the CFF website, the Foundation sold its royalty rights for treatments developed by Vertex for $3.3 billion in 2014. The drug has a list price of about $311,000 a year. Payment issues may arise in the future, but for now, Vertex has stated that insurers and some Medicaid programs have begun paying claims for Trikafta
Specialists who treat CF now are watching to see how well patients tolerate this highly anticipated drug – and how well it meets expectations. The Therapeutic Development Network, the clinical research division of the CFF, is enrolling patients taking Trikafta in an observational study to follow for long-term follow-up.
Meeting expectations
“[Long-term efficacy is] something that we’re always concerned about. When the drug comes to market, is it going to be as effective as we thought it might be?” said Ryan Thomas, MD, director of the Cystic Fibrosis Center at Michigan State University, East Lansing. The MSU Cystic Fibrosis Center receives funding from the Cystic Fibrosis Foundation.
Almost one in five patients could not tolerate treatment with Orkambi, most often because of adverse breathing events, according to a French study published in the American Journal of Respiratory and Critical Care Medicine. The investigators wrote: “Among the 845 patients (292 adolescents, 553 adults) who initiated lumacaftor/ivacaftor, 18.2% (154 patients) discontinued treatment, often due to respiratory (48.1%, 74 patients) or nonrespiratory (27.9%, 43 patients) adverse events” and that the discontinuation rate was considerably higher than previously reported in clinical trials.
“We thought [Orkambi] was going to be something that could have a big effect,” Dr. Thomas said. “It turned out that it was harder for people to tolerate than we thought and the improvements weren’t as sustained as we thought they might be. I really don’t think this will end up being the case with Trikafta.”
Longer-term data are starting to emerge, which may ease some of the concerns inherent in working with a newer medicine. “These [data] suggest that this is going to be a game changer,” Dr. Thomas said. “If Trikafta is this efficacious, well, we’re talking about having people with CF who will live full lifespans without a lung transplant, and that is so rare.”
The decrease in hospitalizations, improved CT scans, and lower rates of lung function decline suggest it could be “the Holy Grail,” Dr. Thomas said.
A different disease
Trikafta is the latest in a series of improvements of CF treatment in recent decades, recalled Dr. Giusti, who has been in this field for about 3 decades. “It used to be that I attended many funerals for children with CF. Now with patients living longer and healthier lives I am invited to attend their weddings and even their children’s baptisms and bris ceremonies. It is a very different disease than it used to be.”
The promise of Trikafta leaves behind the minority of patients for whom the drug won’t work. This is for the 10% of patients that have rare mutations. That can lead to difficult conversations with parents about why this new option is not a choice for their child, Dr. Millard said. “It just crushes you, but the Cystic Fibrosis Foundation is committing a lot of new research in that direction. Their mantra is ‘until it is done.’ ”
Realistic expectations
William (Randy) Hunt, MD, FAAP, FACP, assistant professor of medicine in the Division of Pulmonary, Allergy, Critical Care and Sleep, Emory University School of Medicine, Atlanta, agrees that Trikafta is an exciting development in CF treatment. He noted, “Starting this medication early in life may very well significantly attenuate the disease, but it is not a cure. For individuals who already have significant disease, we may not see the same level of improvements in lung function as what we saw in the studies. The studies generally excluded individuals with ppFEV1 < 40%. Nevertheless, I remain optimistic and have been prescribing it to nearly everyone that qualifies after a discussion.”
Dr. Hunt added, “Patients are asking if they can stop their current chronic CF therapies once they start Trikafta. The answer is “no, at least not right now.” While all the relatively short-term data around Trikafta are very promising, we do not yet know how sustained the long-term benefits will be. Still, safely removing therapeutic burden from our patient population is a real interest. There are plans underway by the CFF and other institutions to systematically research whether discontinuing chronic CF therapies is safe in the setting of Trikafta.”
He concluded that 10% of individuals with CF mutations still do not respond to the modulators currently available. “We will not leave that population behind, but treating these remaining mutations is going to take continued efforts and likely modulators that are therapeutically differently from the mechanism of actions of those that are currently available,” he said.
Therese Borden contributed to this article.
1/2/2020 - This story was updated.
A newly approved triple-combination modulator to treat cystic fibrosis (CF) has raised expectations of a treatment turning point among patients and specialists. If the early results are sustained, elexacaftor/ivacaftor/tezacaftor (Trikafta) could prove to be the rare case of a much-touted new medicine that meets high expectations.
“CF even in infants causes inflammation, so we know that lung damage can start early and progress,” said Susan Millard, MD, FCCP, of Helen DeVos Children’s Hospital in Grand Rapids, Mich., and the local clinical research director for the pediatric pulmonary and sleep medicine section. “This oral drug therapy is actually treating the underlying problem, as opposed to many of the therapies we have that take hours to nebulize and only work locally in the airways.”
Dr. Millard is the recent past pediatric editor for Chest Physician and has been a local principal investigator at Helen DeVos Children’s Hospital for many Vertex-sponsored clinical studies.
The pivotal studies
The Food and Drug Administration approval of Trikafta rested on two pivotal phase 3, placebo-controlled studies, one in patients with two copies of the most common CF mutations, F508del, and the second in patients with one copy of F508del and a second mutation that was called a “minimal-function” mutation. The findings have ignited the hopes of many people with CF and their physicians. The drug was approved in October 2019 for patients aged 12 years and older who have at least one F508del mutation of the cystic fibrosis transmembrane conductance regulator gene. About 90% of patients in the United States have at least one copy of F508del. In the study looking at patients with one copy of F508del, the mean predicted forced expiratory volume in 1 second increased 13.8% in patients taking the drug versus placebo (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMoa1908639). The number of pulmonary exacerbations decreased by 63% in the Trikafta group, compared with placebo. Pulmonary exacerbations were described as a change in specific symptoms that required treatment with a new oral, intravenous, or inhaled antibiotic. Serious adverse drug reactions that occurred more frequently in patients receiving Trikafta, compared with placebo, were rash and influenza events.
In the study that included patients with two copies of F508del, on average, the lung function increased 10% versus patients on ivacaftor/tezacaftor at 4 weeks. In addition, there was a 45.1 mmol/L on average decrease in the sweat chloride level in the Trikafta group, compared with ivacaftor/tezacaftor.
A hopeful start
Robert Giusti, MD, a pediatric pulmonologist at New York University Langone Health, is also hopeful. “This could be the kind of treatment that will make a revolution in terms of [cystic fibrosis] care if it can be started very early in life shortly after diagnosis. We anticipate that patients will be disease free for a longer period of time.”
The Cystic Fibrosis Foundation’s (CFF) “venture philanthropy” initiative played an important role in the development of the drug by Vertex Pharmaceuticals. The CFF has invested many millions of dollars in research by drug companies since the 1980s and was an early backer of Vertex. According to a statement on the CFF website, the Foundation sold its royalty rights for treatments developed by Vertex for $3.3 billion in 2014. The drug has a list price of about $311,000 a year. Payment issues may arise in the future, but for now, Vertex has stated that insurers and some Medicaid programs have begun paying claims for Trikafta
Specialists who treat CF now are watching to see how well patients tolerate this highly anticipated drug – and how well it meets expectations. The Therapeutic Development Network, the clinical research division of the CFF, is enrolling patients taking Trikafta in an observational study to follow for long-term follow-up.
Meeting expectations
“[Long-term efficacy is] something that we’re always concerned about. When the drug comes to market, is it going to be as effective as we thought it might be?” said Ryan Thomas, MD, director of the Cystic Fibrosis Center at Michigan State University, East Lansing. The MSU Cystic Fibrosis Center receives funding from the Cystic Fibrosis Foundation.
Almost one in five patients could not tolerate treatment with Orkambi, most often because of adverse breathing events, according to a French study published in the American Journal of Respiratory and Critical Care Medicine. The investigators wrote: “Among the 845 patients (292 adolescents, 553 adults) who initiated lumacaftor/ivacaftor, 18.2% (154 patients) discontinued treatment, often due to respiratory (48.1%, 74 patients) or nonrespiratory (27.9%, 43 patients) adverse events” and that the discontinuation rate was considerably higher than previously reported in clinical trials.
“We thought [Orkambi] was going to be something that could have a big effect,” Dr. Thomas said. “It turned out that it was harder for people to tolerate than we thought and the improvements weren’t as sustained as we thought they might be. I really don’t think this will end up being the case with Trikafta.”
Longer-term data are starting to emerge, which may ease some of the concerns inherent in working with a newer medicine. “These [data] suggest that this is going to be a game changer,” Dr. Thomas said. “If Trikafta is this efficacious, well, we’re talking about having people with CF who will live full lifespans without a lung transplant, and that is so rare.”
The decrease in hospitalizations, improved CT scans, and lower rates of lung function decline suggest it could be “the Holy Grail,” Dr. Thomas said.
A different disease
Trikafta is the latest in a series of improvements of CF treatment in recent decades, recalled Dr. Giusti, who has been in this field for about 3 decades. “It used to be that I attended many funerals for children with CF. Now with patients living longer and healthier lives I am invited to attend their weddings and even their children’s baptisms and bris ceremonies. It is a very different disease than it used to be.”
The promise of Trikafta leaves behind the minority of patients for whom the drug won’t work. This is for the 10% of patients that have rare mutations. That can lead to difficult conversations with parents about why this new option is not a choice for their child, Dr. Millard said. “It just crushes you, but the Cystic Fibrosis Foundation is committing a lot of new research in that direction. Their mantra is ‘until it is done.’ ”
Realistic expectations
William (Randy) Hunt, MD, FAAP, FACP, assistant professor of medicine in the Division of Pulmonary, Allergy, Critical Care and Sleep, Emory University School of Medicine, Atlanta, agrees that Trikafta is an exciting development in CF treatment. He noted, “Starting this medication early in life may very well significantly attenuate the disease, but it is not a cure. For individuals who already have significant disease, we may not see the same level of improvements in lung function as what we saw in the studies. The studies generally excluded individuals with ppFEV1 < 40%. Nevertheless, I remain optimistic and have been prescribing it to nearly everyone that qualifies after a discussion.”
Dr. Hunt added, “Patients are asking if they can stop their current chronic CF therapies once they start Trikafta. The answer is “no, at least not right now.” While all the relatively short-term data around Trikafta are very promising, we do not yet know how sustained the long-term benefits will be. Still, safely removing therapeutic burden from our patient population is a real interest. There are plans underway by the CFF and other institutions to systematically research whether discontinuing chronic CF therapies is safe in the setting of Trikafta.”
He concluded that 10% of individuals with CF mutations still do not respond to the modulators currently available. “We will not leave that population behind, but treating these remaining mutations is going to take continued efforts and likely modulators that are therapeutically differently from the mechanism of actions of those that are currently available,” he said.
Therese Borden contributed to this article.
1/2/2020 - This story was updated.
When guideline treatment of asthma fails, consider a macrolide antibiotic
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
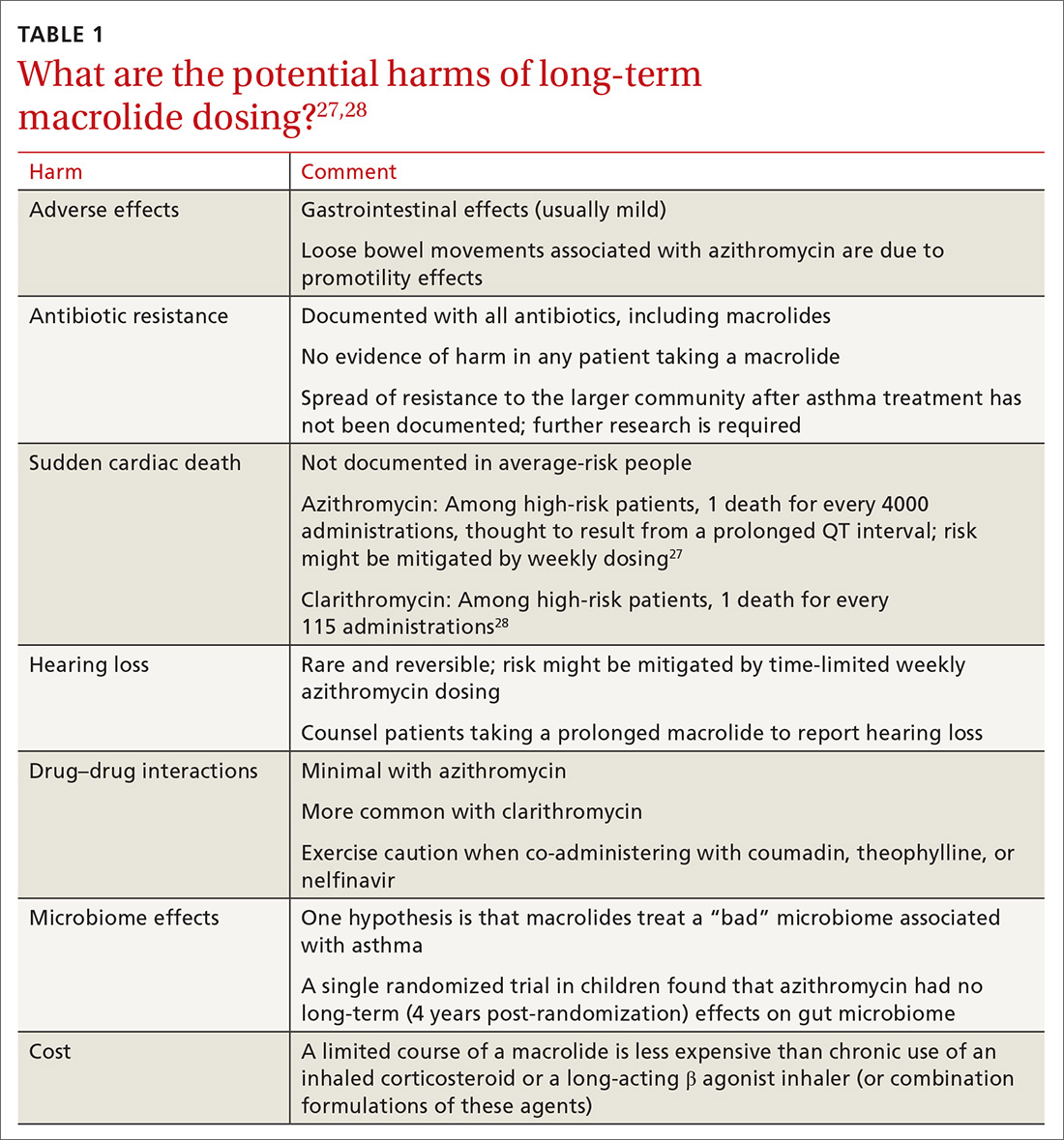
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
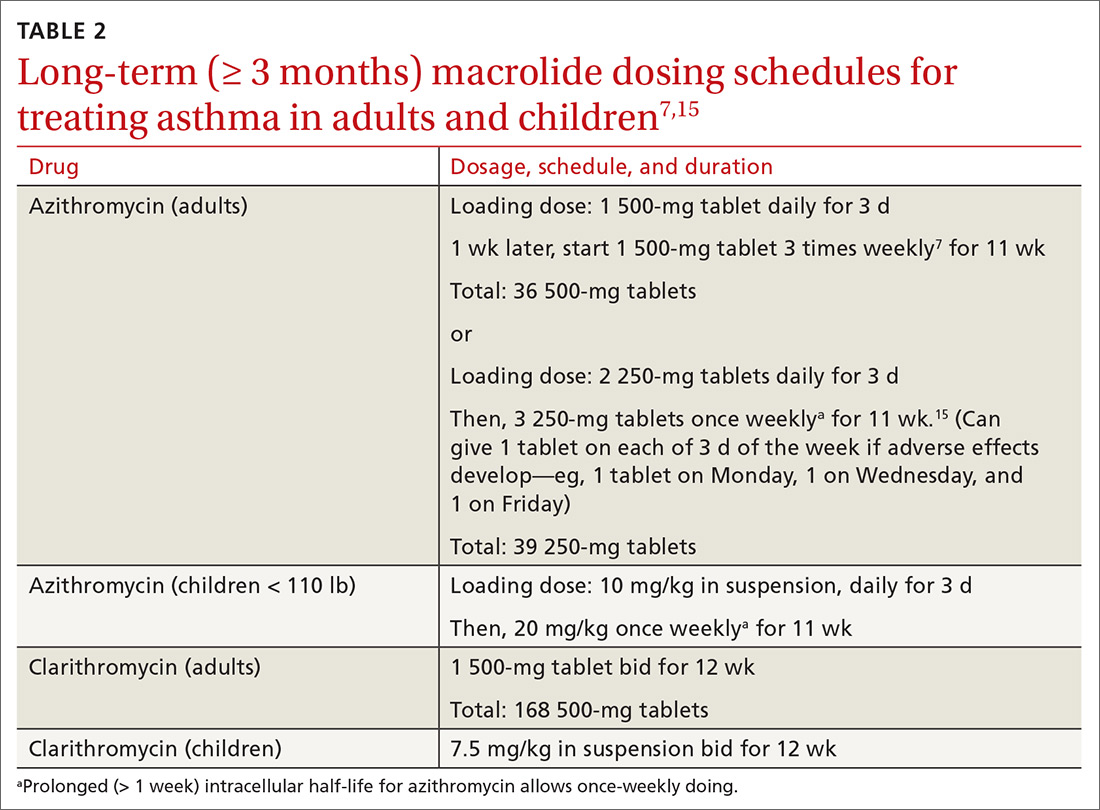
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
PRACTICE RECOMMENDATIONS
› Consider a trial of azithromycin for patients who have poorly controlled persistent asthma and are not responding to guideline treatment with the combination of an inhaled corticosteroid and either a long-acting bronchodilator or long-acting muscarinic antagonist. B
› Consider a trial of azithromycin in addition to first-line guideline therapy for patients who have new-onset asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series