User login
Emergency Imaging: Severe Left Testicular Swelling
A 32-year-old man presented to the ED with acute onset of left testicular swelling and pain. He described the pain as severe, radiating to his lower back and lower abdomen. Regarding his medical history, the patient stated he had experienced similar episodes of significant testicular swelling in the past, for which he was treated with antibiotics.
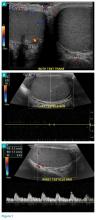
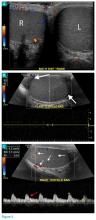
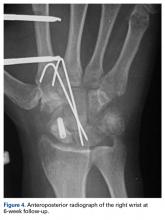
Physical examination revealed mild enlargement of the left testis with tenderness to palpation. The right testis was normal in appearance and nontender. An ultrasound study of the testicles was ordered; representative images are shown (Figures 1a-1c).
What is the diagnosis?
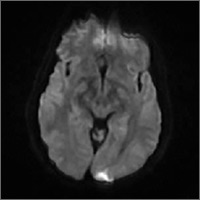
The transverse image of both testes demonstrated an enlarged left testicle compared to the right testicle (Figure 2a). On color-flow Doppler ultrasound, spots of color within the testicle were noted within the right testicle only. The lack of blood flow was confirmed on the sagittal image of the left testicle, which also revealed a small hydrocele (white arrows, Figure 2b). A sagittal color Doppler image of the normal right testicle showed color flow (white arrows, Figure 2c) and normal vascular waveforms (red arrow, Figure 2c) within the testis, but no hydrocele, confirming the diagnosis of left testicular torsion. The Doppler ultrasound of the right testicle (white arrows, Figure 2c) further confirmed a normal right testicle but no evidence of flow in the left testicle. These findings were further consistent with the presence of left testicular torsion.
Answer
Testicular Torsion
Testicular torsion is a urological emergency that results from a twisting of the spermatic cord, cutting off arterial flow to, and venous drainage from, the affected testis. There are two types of testicular torsion depending on which side of the tunica vaginalis (the serous membrane pouch covering the testes) the torsion occurs: extra vaginal, seen mainly in newborns; and intravaginal, which can occur at any age, but is more common in adolescents.
“Bell clapper deformity” is a predisposing congenital condition resulting from intravaginal torsion of the testis in which the tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate.1 Testicular torsion most commonly occurs in young males, with an estimated incidence of 4.5 cases per 100,000 patients between ages 1 and 25 years.2
Clinical Presentation
Patients with testicular torsion typically experience a sudden onset of severe unilateral pain often accompanied by nausea and vomiting, which can occur spontaneously or after vigorous physical activity or trauma. Associated complaints may include urinary symptoms and/or fever.3 The affected testis may lie transversely in the scrotum and be retracted, although physical examination is often nonspecific and unreliable. Since an absence of the cremasteric reflex is neither sensitive nor specific in determining the need for surgical intervention, further diagnostic testing is required.4
Doppler Ultrasound
Ultrasound utilizing color and spectral Doppler techniques is the imaging test of choice to evaluate for testicular torsion, and has a reported sensitivity of 82% to 89%, and a specificity of 98% to 100%.5,6 Ultrasound findings include enlargement and decreased echogenicity of the affected testicle due to edema. Scrotal wall thickening and a small hydrocele also may be seen. Doppler imaging also typically demonstrates absence of flow, though hyperemia and increased flow may be present early in the disease process.
It is important to note that torsion may be intermittent; therefore, imaging studies can appear normal during periods of intermittent perfusion. If there is incomplete torsion and some arterial flow persists in the affected testis, comparison of the two testes using transverse views is very useful in making the diagnosis.7
With respect to the differential diagnoses, ultrasound imaging studies are also useful in diagnosing other conditions associated with testicular pain, including torsion of the appendix testis, epididymitis, orchitis, trauma, varicocele, and tumors.
Treatment
Rapid diagnosis of testicular torsion is important, as delay in diagnosis may lead to irreversible damage and loss of the testicle. Infertility can result even with a normal contralateral testis.8 When surgical intervention is performed within 6 hours from onset of torsion, salvage of the testicle has been reported to be 90% to 100%, but only 50% and 10% at 12 and 24 hours, respectively.3 The patient in this case was taken immediately for emergent surgical detorsion, and the left testicle was salvaged.
1. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44 (1):114-116.
2. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167-1171. doi:10.1001/archpedi.159.12.1167.
3. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835-840.
4. Mellick LB. Torsion of the testicle: It is time to stop tossing the dice. Pediatr Emerg Care. 2012;28:80Y86. doi:10.1097/PEC.0b013e31823f5ed9.
5. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604-607.
6. Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821. doi:10.1148/radiology.175.3.2188301.
7. Aso C, Enríquez G, Fité M, et al. Gray-scale and color doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197-1214. doi:10.1148/rg.255045109.
8. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158-1160.
A 32-year-old man presented to the ED with acute onset of left testicular swelling and pain. He described the pain as severe, radiating to his lower back and lower abdomen. Regarding his medical history, the patient stated he had experienced similar episodes of significant testicular swelling in the past, for which he was treated with antibiotics.
Physical examination revealed mild enlargement of the left testis with tenderness to palpation. The right testis was normal in appearance and nontender. An ultrasound study of the testicles was ordered; representative images are shown (Figures 1a-1c).
What is the diagnosis?
The transverse image of both testes demonstrated an enlarged left testicle compared to the right testicle (Figure 2a). On color-flow Doppler ultrasound, spots of color within the testicle were noted within the right testicle only. The lack of blood flow was confirmed on the sagittal image of the left testicle, which also revealed a small hydrocele (white arrows, Figure 2b). A sagittal color Doppler image of the normal right testicle showed color flow (white arrows, Figure 2c) and normal vascular waveforms (red arrow, Figure 2c) within the testis, but no hydrocele, confirming the diagnosis of left testicular torsion. The Doppler ultrasound of the right testicle (white arrows, Figure 2c) further confirmed a normal right testicle but no evidence of flow in the left testicle. These findings were further consistent with the presence of left testicular torsion.
Answer
Testicular Torsion
Testicular torsion is a urological emergency that results from a twisting of the spermatic cord, cutting off arterial flow to, and venous drainage from, the affected testis. There are two types of testicular torsion depending on which side of the tunica vaginalis (the serous membrane pouch covering the testes) the torsion occurs: extra vaginal, seen mainly in newborns; and intravaginal, which can occur at any age, but is more common in adolescents.
“Bell clapper deformity” is a predisposing congenital condition resulting from intravaginal torsion of the testis in which the tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate.1 Testicular torsion most commonly occurs in young males, with an estimated incidence of 4.5 cases per 100,000 patients between ages 1 and 25 years.2
Clinical Presentation
Patients with testicular torsion typically experience a sudden onset of severe unilateral pain often accompanied by nausea and vomiting, which can occur spontaneously or after vigorous physical activity or trauma. Associated complaints may include urinary symptoms and/or fever.3 The affected testis may lie transversely in the scrotum and be retracted, although physical examination is often nonspecific and unreliable. Since an absence of the cremasteric reflex is neither sensitive nor specific in determining the need for surgical intervention, further diagnostic testing is required.4
Doppler Ultrasound
Ultrasound utilizing color and spectral Doppler techniques is the imaging test of choice to evaluate for testicular torsion, and has a reported sensitivity of 82% to 89%, and a specificity of 98% to 100%.5,6 Ultrasound findings include enlargement and decreased echogenicity of the affected testicle due to edema. Scrotal wall thickening and a small hydrocele also may be seen. Doppler imaging also typically demonstrates absence of flow, though hyperemia and increased flow may be present early in the disease process.
It is important to note that torsion may be intermittent; therefore, imaging studies can appear normal during periods of intermittent perfusion. If there is incomplete torsion and some arterial flow persists in the affected testis, comparison of the two testes using transverse views is very useful in making the diagnosis.7
With respect to the differential diagnoses, ultrasound imaging studies are also useful in diagnosing other conditions associated with testicular pain, including torsion of the appendix testis, epididymitis, orchitis, trauma, varicocele, and tumors.
Treatment
Rapid diagnosis of testicular torsion is important, as delay in diagnosis may lead to irreversible damage and loss of the testicle. Infertility can result even with a normal contralateral testis.8 When surgical intervention is performed within 6 hours from onset of torsion, salvage of the testicle has been reported to be 90% to 100%, but only 50% and 10% at 12 and 24 hours, respectively.3 The patient in this case was taken immediately for emergent surgical detorsion, and the left testicle was salvaged.
A 32-year-old man presented to the ED with acute onset of left testicular swelling and pain. He described the pain as severe, radiating to his lower back and lower abdomen. Regarding his medical history, the patient stated he had experienced similar episodes of significant testicular swelling in the past, for which he was treated with antibiotics.
Physical examination revealed mild enlargement of the left testis with tenderness to palpation. The right testis was normal in appearance and nontender. An ultrasound study of the testicles was ordered; representative images are shown (Figures 1a-1c).
What is the diagnosis?
The transverse image of both testes demonstrated an enlarged left testicle compared to the right testicle (Figure 2a). On color-flow Doppler ultrasound, spots of color within the testicle were noted within the right testicle only. The lack of blood flow was confirmed on the sagittal image of the left testicle, which also revealed a small hydrocele (white arrows, Figure 2b). A sagittal color Doppler image of the normal right testicle showed color flow (white arrows, Figure 2c) and normal vascular waveforms (red arrow, Figure 2c) within the testis, but no hydrocele, confirming the diagnosis of left testicular torsion. The Doppler ultrasound of the right testicle (white arrows, Figure 2c) further confirmed a normal right testicle but no evidence of flow in the left testicle. These findings were further consistent with the presence of left testicular torsion.
Answer
Testicular Torsion
Testicular torsion is a urological emergency that results from a twisting of the spermatic cord, cutting off arterial flow to, and venous drainage from, the affected testis. There are two types of testicular torsion depending on which side of the tunica vaginalis (the serous membrane pouch covering the testes) the torsion occurs: extra vaginal, seen mainly in newborns; and intravaginal, which can occur at any age, but is more common in adolescents.
“Bell clapper deformity” is a predisposing congenital condition resulting from intravaginal torsion of the testis in which the tunica vaginalis joins high on the spermatic cord, leaving the testis free to rotate.1 Testicular torsion most commonly occurs in young males, with an estimated incidence of 4.5 cases per 100,000 patients between ages 1 and 25 years.2
Clinical Presentation
Patients with testicular torsion typically experience a sudden onset of severe unilateral pain often accompanied by nausea and vomiting, which can occur spontaneously or after vigorous physical activity or trauma. Associated complaints may include urinary symptoms and/or fever.3 The affected testis may lie transversely in the scrotum and be retracted, although physical examination is often nonspecific and unreliable. Since an absence of the cremasteric reflex is neither sensitive nor specific in determining the need for surgical intervention, further diagnostic testing is required.4
Doppler Ultrasound
Ultrasound utilizing color and spectral Doppler techniques is the imaging test of choice to evaluate for testicular torsion, and has a reported sensitivity of 82% to 89%, and a specificity of 98% to 100%.5,6 Ultrasound findings include enlargement and decreased echogenicity of the affected testicle due to edema. Scrotal wall thickening and a small hydrocele also may be seen. Doppler imaging also typically demonstrates absence of flow, though hyperemia and increased flow may be present early in the disease process.
It is important to note that torsion may be intermittent; therefore, imaging studies can appear normal during periods of intermittent perfusion. If there is incomplete torsion and some arterial flow persists in the affected testis, comparison of the two testes using transverse views is very useful in making the diagnosis.7
With respect to the differential diagnoses, ultrasound imaging studies are also useful in diagnosing other conditions associated with testicular pain, including torsion of the appendix testis, epididymitis, orchitis, trauma, varicocele, and tumors.
Treatment
Rapid diagnosis of testicular torsion is important, as delay in diagnosis may lead to irreversible damage and loss of the testicle. Infertility can result even with a normal contralateral testis.8 When surgical intervention is performed within 6 hours from onset of torsion, salvage of the testicle has been reported to be 90% to 100%, but only 50% and 10% at 12 and 24 hours, respectively.3 The patient in this case was taken immediately for emergent surgical detorsion, and the left testicle was salvaged.
1. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44 (1):114-116.
2. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167-1171. doi:10.1001/archpedi.159.12.1167.
3. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835-840.
4. Mellick LB. Torsion of the testicle: It is time to stop tossing the dice. Pediatr Emerg Care. 2012;28:80Y86. doi:10.1097/PEC.0b013e31823f5ed9.
5. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604-607.
6. Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821. doi:10.1148/radiology.175.3.2188301.
7. Aso C, Enríquez G, Fité M, et al. Gray-scale and color doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197-1214. doi:10.1148/rg.255045109.
8. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158-1160.
1. Caesar RE, Kaplan GW. Incidence of the bell-clapper deformity in an autopsy series. Urology. 1994;44 (1):114-116.
2. Mansbach JM, Forbes P, Peters C. Testicular torsion and risk factors for orchiectomy. Arch Pediatr Adolesc Med. 2005;159(12):1167-1171. doi:10.1001/archpedi.159.12.1167.
3. Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88(12):835-840.
4. Mellick LB. Torsion of the testicle: It is time to stop tossing the dice. Pediatr Emerg Care. 2012;28:80Y86. doi:10.1097/PEC.0b013e31823f5ed9.
5. Baker LA, Sigman D, Mathews RI, Benson J, Docimo SG. An analysis of clinical outcomes using color doppler testicular ultrasound for testicular torsion. Pediatrics. 2000;105(3 Pt 1):604-607.
6. Burks DD, Markey BJ, Burkhard TK, Balsara ZN, Haluszka MM, Canning DA. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175(3):815-821. doi:10.1148/radiology.175.3.2188301.
7. Aso C, Enríquez G, Fité M, et al. Gray-scale and color doppler sonography of scrotal disorders in children: an update. Radiographics. 2005;25(5):1197-1214. doi:10.1148/rg.255045109.
8. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158-1160.
Parkinsonism and Vitamin C Deficiency
Vitamin C (ascorbic acid) deficiency is known to affect brain function and is associated with parkinsonism.1 In 1752, James Lind, MD, described emotional and behavioral changes that herald the onset of scurvy and precede hemorrhagic findings.2 The World Health Organization (WHO) today refers to this stage as latent scurvy.3 The 2 case studies that follow present examples of patients with vitamin C deficiencies whose parkinsonism responded robustly to vitamin C replacement. These cases suggest that vitamin C deficiency may be a treatable cause of parkinsonism.
Case 1
Mr. A, a 60-year-old white male, was admitted to the Medicine Service for alcohol detoxification. The patient had a history of alcohol dependence, alcohol withdrawal seizures, tobacco dependence, and hyperlipidemia. He took no medications as an outpatient. On admission Mr. A’s body mass index (BMI) was 27.2. An initial examination revealed a marked resting tremor of the patient’s right hand with cogwheeling, which had not been present in examinations conducted in the previous 3 years. Mr. A had no prior history of a tremor. He had no cerebellar findings and no evidence of asterixis or of tremulousness associated with high-output cardiac states, such as de Musset sign.
Mr. A reported he had experienced the tremor for a month and that it had been worsening. He also was having difficulty using his dominant right hand, for routine daily activities. Mr. A was oriented, and his short-term memory was intact. He was ill-appearing, irritable with psychomotor slowing, and did not wish to rise from his bed. He had no gingival or periungual bleeding and did not bruise easily. He had no corkscrew hairs. The patient was started on no medications known to cause extrapyramidal symptoms (EPS).
In the hospital, the tremor persisted unabated for 2 days. On the third day, Mr. A was started on 1,000 mg vitamin C IV twice daily. He received a total of 2,000 mg IV that day, but the IV fell out, and he refused its replacement. Several hours later, Mr. A stated that he felt much better, got out of bed, and asked to go outside to smoke. The author noted complete resolution of the right hand tremor and cogwheeling 20 hours after starting the vitamin C IV. Mr. A refused a repeat serum vitamin C assay.
Laboratory studies initially revealed that Mr. A had hyponatremia with a serum sodium of 121 mmol/L (normal range: 133 to 145 mmol/L) as well as hypokalemia with a serum potassium of 3.2 mmol/L (normal range: 3.5 to 5.0 mmol/L). He was hypoosmolar, with a serum osmolality of 276 mOsm/kg (normal range: 278 to 305 mOsm/kg). His vitamin C level was low at 0.2 mg/dL (normal range: 0.4 to 2.0 mg/dL). Mr. A also had a serum vitamin C level drawn 2 years prior that showed no symptoms of EPS, and at that time, the reading was 0.7 mg/dL. At admission to Medicine Services, Mr. A had a serum alcohol level of 211 mg/dL. Neuroimaging revealed diffuse cerebral and cerebellar volume loss.
Normal laboratory results included serum levels of vitamin B12, red cell folate, homocysteine, methylmalonic acid, free and total carnitine, alkaline phosphatase, manganese, and zinc. A urine drug screen was negative.
Case 2
Mr. B, a 69-year-old black male, was admitted to the hospital for depression complicated by alcohol dependence. He also had tobacco dependence, type 2 diabetes mellitus, hypertension, and gout. The patient’s BMI at admission was 16.1. Mr. B appeared ill, was worried about his health, and remained recumbent unless asked to move. He reported that his right hand had begun to shake at rest in the month prior to admission. The tremor made it difficult for him to drink. He pointed out stains on his hospital gurney from an attempt to drink orange juice prior to being assessed.
A physical examination revealed a distinct resting tremor with cogwheeling of the right hand; there was no other evidence of EPS, nor was there evidence of cognitive, cerebellar, or skin abnormalities, such as hemorrhages or corkscrew hairs. Asterixis was absent as was evidence of a high-output cardiac state that might produce a tremor, such as de Musset sign. A serum vitamin C level was obtained and returned at 0.0 mg/dL. A head computed tomography scan obtained the next day revealed mild cerebellar volume loss. A serum alkaline phosphatase level was elevated slightly at 136 U/L (normal range: 42 to 113 U/L). Normal serum values were returned for zinc, vitamins B12 and folate, rapid plasma reagin, sodium, and serum osmolality. A urine drug screen was negative, and serum alcohol level was < 5.0 mg/dL.
Mr. B took no medications expected to cause EPS. He received no micronutrient replacement until the day after admission when he began receiving oral vitamin C 1,000 mg twice a day. After receiving 3 doses, Mr. B’s resting tremor and cogwheeling completely resolved. He noticed he had stopped shaking and could now drink without spilling fluids. He also got out of bed and began interacting with others. Mr. B said he felt he was “doing well.” A repeat serum vitamin C level was 0.2 mg/dL on that day. The improvement was sustained over 3 days, and Mr. B was discharged to home.
Discussion
Both Mr. A and Mr. B presented with a typical picture of latent scurvy and the additional finding of parkinsonism. These cases are important for 2 reasons. First, the swift and full response of these patients’ parkinsonism to vitamin C replacement underscores the importance of considering a vitamin C deficiency when confronted with EPS. And second, both patients lacked signs of bleeding or of impaired collagen synthesis. This differs from the classic presentation of scurvy as a disorder primarily of collagen metabolism.4
Lind described the onset of scurvy as one in which striking emotional and behavior changes developed and later were followed by abnormal bleeding and even death.2 These early changes also were recognized by Shapter in 1847.5 Furthermore, the evidence that exists about the time-course of scurvy’s development suggests that neuropsychiatric findings precede the hemorrhagic.6 Indeed, classic skin findings, such as petechiae or corkscrew hairs, may develop years after the onset of neuropsychiatric changes.7,8
Despite WHO characterizing it as latent scurvy, the distinct syndromal presentation of hypovitaminosis C with parkinsonism along with the rapid response to vitamin C replacement argues for its recognition as a distinct clinical entity and not just a prelude to the hemorrhagic state. To assist in recognizing neuropsychiatric scurvy, the author suggests the operationalized approach described in Table 1.9
Pathophysiology
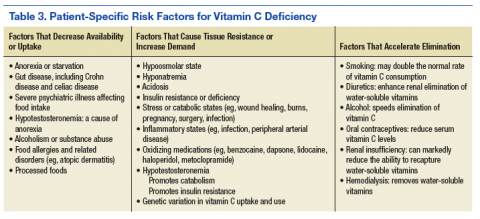
Vitamin C has an intimate role in the normal functioning of the basal ganglia. It is involved in the synthesis of catechecholamines, the regulation of the release and postsynaptic activities of various neurotransmitters, and managing the oxyradical toxicity of aerobic metabolism. Table 2 outlines some of the normal brain functions of vitamin C and the potential consequences of inadequate central vitamin C.9,10 Risk factors for vitamin C deficiency include those affecting the uptake, response to, and elimination of this vitamin (Table 3).11-14
The potential role of alcohol use by both patients also warrants mention. Current data suggest a nonlinear relationship between alcohol use and neurotoxicity. Epidemiologic data show that moderate alcohol consumption protects against the development of such neurodegenerative processes as Parkinson disease and Alzheimer disease.15,16 But the cases here reflect excessive use of alcohol. In this situation, a variety of progressive insults, such as those caused by oxyradical toxicity as well as malnutrition may foster the development of basal ganglia dysfunction.17
Measuring Deficiency
A deficiency of vitamin C may be determined in several ways. The most frequently used laboratory measure of vitamin C status is the serum vitamin C level. This level is included in the WHO’s recommendations for diagnosis.3 However, this assay is limited because when facing total body depletion, the kidneys may restrict the elimination of vitamin C and tend to maintain serum vitamin C levels even as target tissue levels fall. An interesting example of this is the 0.2 mg/dL value that each patient registered. In Mr. A’s case, this reflected a systemic deficit of vitamin C, while in Mr. B’s case it correlated with the onset of effective repletion of body’s stores.
A fall in urinary output of vitamin C is another marker of hypovitaminosis C. When available, this laboratory test can be used with the serum level to assess total body stores of vitamin C. Lymphocytes, neutrophils, and platelets also store vitamin C. These target tissues tend to saturate when the oral intake ranges between 100 mg to 200 mg a day. This is the same point at which serum vitamin C levels peak and level off in normal, healthy adults.18,19 Once again, the limited availability of target-tissue assays puts these studies out of reach for most clinicians.
No evaluation is complete without some assurance of what the disease is not. Deficiencies of biotin, zinc, folate, and B12 all may affect the function of the basal ganglia.20 The biotin deficiencies literature is particularly robust. Biotin deficiencies affecting basal ganglia function are best known as inherited disorders of metabolism.21 Manganese intoxication also may present as a movement disorder.22
Treatment
Treatment of neuropsychiatric scurvy has relied on IV administration of vitamin C. Although the bioavailability of oral vitamin C among healthy adult volunteers is nearly complete up to about 200 mg a day, a patient with neuropsychiatric scurvy may need substantially more than that amount to accommodate total body deficiencies and increased demands.23 The IV route allows serum vitamin C levels up to 100 times higher than by the oral route.24 Mr. B is, in fact, the first person reported in the literature with neuropsychiatric scurvy to respond to oral vitamin C replacement alone. Once repletion of vitamin C is complete, it is useful to consider a maintenance replacement dose based on a patient’s risk factors and needs.
A healthy adult should ingest about 120 mg of vitamin C daily. Smokers and pregnant women may require more, but this recommendation was intended to address their needs as well.25 Many commercial multivitamins use the old recommended daily allowance of 60 mg, so it may be safest to recommend specifically a vitamin C tablet with at least 120 mg when ordering vitamin C replacement.
Tight control of the serum vitamin C concentration means that little additional vitamin C will be taken up by the gut beyond 200 mg orally a day, which helps minimize any concerns about long-term toxicity. It takes several weeks to deplete vitamin C from the human body when vitamin C is removed from the diet, so a patient with a previously treated deficiency of vitamin C should wait a month before a repeat serum vitamin C level measurement.
The half life of vitamin C is normally ≤ 2 hours. When renal function is intact, vitamin C in excess of immediate need is lost through renal filtration. Toxicity is rare under these conditions.26 When vitamin C toxicity has been reported, it has occurred in the setting of prolonged supplementation, usually when a patient already experienced a renal injury. The main toxicities attributed to vitamin C are oxalate crystal formation with subsequent renal injury and exacerbation of glucose 6-phosphate dehydrogenase deficiency (G6PD).24
Oxalate formation due to vitamin C replacement is uncommon, but patients with preexisting calcium oxalate stones may be at risk for further stone formation when they receive additional vitamin C.27 This is most likely to occur when treatment with parenteral vitamin C is prolonged, which is not typical for patients with neuropsychiatric scurvy who tend to respond rapidly to vitamin C replenishment. Reports of acute hemolytic episodes among patient with G6PD deficiency receiving vitamin C exist, although these cases are rare.28 Furthermore, some authors advocate for the use of ascorbic acid to treat methemoglobinemia associated with G6PD deficiency, when methylene blue is not available.29 It may be reasonable to begin treatment with oral vitamin C for patients with NPS and G6PD deficiency. This is equivalent to a low-dose form of vitamin C replacement and may help avoid the theoretically pro-oxidant effects of larger, IV doses of vitamin C.30
Conclusion
The recent discovery of movement disorders in scurvy has enlarged the picture of vitamin C deficiency. The cases here demonstrate how hypovitaminosis C with central nervous system manifestations may be identified and treated. This relationship fits well within the established basic science and clinical framework for scurvy, and the clinical implications for scurvy remain in many ways unchanged. First, malnutrition must be considered even when a patient’s habitus suggests he is well fed. Also, it is more likely to see scurvy without all of the classic findings than an end-stage case of the disease.31 In the right clinical setting, it is reasonable to think of a vitamin C deficiency before the patient develops bleeding gums and corkscrew hairs. And as is typical of vitamin deficiencies, the treatment of a vitamin C deficiency usually results in swift improvement. Finally, for those who treat movement disorders or who prescribe agents such as antipsychotics that may cause movement disorders, it is important to recognize vitamin C deficiency as another potential explanation for EPS.
1. Ide K, Yamada H, Umegaki K, et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition. 2015;31(2):406-408.
2. Lind J. The diagnostics, or symptoms. A Treatise on the Scurvy, in Three Parts. 3rd ed. London: S. Crowder, D. Wilson and G. Nicholls, T. Cadell, T. Becket and Co., G. Pearch, and Woodfall; 1772:98-129.
3. World Health Organization. Scurvy and its prevention and control in major emergencies. http://whqlibdoc.who.int/hq/1999/WHO_NHD_99.11.pdf. Published 1999. Accessed July 6, 2017.
4. Sasseville D. Scurvy: curse and cure in New France. JAMA Dermatol. 2015;151(4):431.
5. Shapter T. On the recent occurrence of scurvy in Exeter and the neighbourhood. Prov Med Surg J. 1847;11(11):281-285.
6. Kinsman RA, Hood J. Some behavioral effects of ascorbic acid deficiency. Am J Clin Nutr. 1971;24(4):455-464.
7. DeSantis J. Scurvy and psychiatric symptoms. Perspect Psychiatr Care. 1993;29(1):18-22.
8. Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130(2):177-179.
9. Brown TM. Neuropsychiatric scurvy. Psychosomatics. 2015;56(1):12-20.
10. Feuerstein TJ, Weinheimer G, Lang G, Ginap T, Rossner R. Inhibition by ascorbic acid of NMDA-evoked acetylcholine release in rabbit caudate nucleus. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(5):549-551.
11. Kim J, Kwon J, Noh G, Lee SS. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7(6):488-494.
12. Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103(14):1863-1868.
13. Nappe TM, Pacelli AM, Katz K. An atypical case of methemoglobinemia due to self-administered benzocaine. Case Rep Emerg Med. 2015;2015:670979.
14. Wright AD, Stevens E, Ali M, Carroll DW, Brown TM. The neuropsychiatry of scurvy. Psychosomatics. 2014;55(2):179-185.
15. Bate C, Williams A. Ethanol protects cultured neurons against amyloid-β and α-synuclein-induced synapse damage. Neuropharmacology. 2011;61(8):1406-1412.
16. Vasanthi HR, Parameswari RP, DeLeiris J, Das DK. Health benefits of wine and alcohol from neuroprotection to heart health. Front Biosci (Elite Ed). 2012;4:1505-1512.
17. Vaglini F, Viaggi C, Piro V, et al. Acetaldehyde and parkinsonism: role of CYP450 2E1. Front Behav Neurosci. 2013;7:71.
18. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98(17):9842-9846.
19. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
20. Quiroga MJ, Carroll DW, Brown TM. Ascorbate- and zinc-responsive parkinsonism. Ann Pharmacother. 2014;48(11):1515-1520.
21. Tabarki B, Al-Shafi S, Al-Shahwan S, et al. Biotin-responsive basal ganglia disease revisited: clinical, radiologic, and genetic findings. Neurology. 2013;80(3):261-267.
22. Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277-312.
23. Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93(8):3704-3709.
24. Wilson MK, Baguley BC, Wall C, Jameson MB, Findlay MP. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol. 2014;10(1):22-37.
25. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086-1107.
26. Nielsen TK, Højgaard M, Andersen JT, Poulsen HE, Lykkesfeldt J, Mikines KJ. Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: a pharmacokinetic evaluation. Basic Clin Pharmacol Toxicol. 2015;116(4):343-348.
27. Baxmann AC, De O G Mendonça C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003;63(3):1066-1071.
28. Huang YC, Chang TK, Fu YC, Jan SL. C for colored urine: acute hemolysis induced by high-dose ascorbic acid. Clin Toxicol (Phila). 2014;52(9):984.
29. Rino PB, Scolnik D, Fustiñana A, Mitelpunkt A, Glatstein M. Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Am J Ther. 2014;21(4):240-243.
30. Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826(2):443-457.
31. Fouron JC, Chicoine L. Le scorbut: aspects particuliers de l’association rachitisme-scorbut. Can Med Assoc J. 1962;86(26):1191-1196.
Vitamin C (ascorbic acid) deficiency is known to affect brain function and is associated with parkinsonism.1 In 1752, James Lind, MD, described emotional and behavioral changes that herald the onset of scurvy and precede hemorrhagic findings.2 The World Health Organization (WHO) today refers to this stage as latent scurvy.3 The 2 case studies that follow present examples of patients with vitamin C deficiencies whose parkinsonism responded robustly to vitamin C replacement. These cases suggest that vitamin C deficiency may be a treatable cause of parkinsonism.
Case 1
Mr. A, a 60-year-old white male, was admitted to the Medicine Service for alcohol detoxification. The patient had a history of alcohol dependence, alcohol withdrawal seizures, tobacco dependence, and hyperlipidemia. He took no medications as an outpatient. On admission Mr. A’s body mass index (BMI) was 27.2. An initial examination revealed a marked resting tremor of the patient’s right hand with cogwheeling, which had not been present in examinations conducted in the previous 3 years. Mr. A had no prior history of a tremor. He had no cerebellar findings and no evidence of asterixis or of tremulousness associated with high-output cardiac states, such as de Musset sign.
Mr. A reported he had experienced the tremor for a month and that it had been worsening. He also was having difficulty using his dominant right hand, for routine daily activities. Mr. A was oriented, and his short-term memory was intact. He was ill-appearing, irritable with psychomotor slowing, and did not wish to rise from his bed. He had no gingival or periungual bleeding and did not bruise easily. He had no corkscrew hairs. The patient was started on no medications known to cause extrapyramidal symptoms (EPS).
In the hospital, the tremor persisted unabated for 2 days. On the third day, Mr. A was started on 1,000 mg vitamin C IV twice daily. He received a total of 2,000 mg IV that day, but the IV fell out, and he refused its replacement. Several hours later, Mr. A stated that he felt much better, got out of bed, and asked to go outside to smoke. The author noted complete resolution of the right hand tremor and cogwheeling 20 hours after starting the vitamin C IV. Mr. A refused a repeat serum vitamin C assay.
Laboratory studies initially revealed that Mr. A had hyponatremia with a serum sodium of 121 mmol/L (normal range: 133 to 145 mmol/L) as well as hypokalemia with a serum potassium of 3.2 mmol/L (normal range: 3.5 to 5.0 mmol/L). He was hypoosmolar, with a serum osmolality of 276 mOsm/kg (normal range: 278 to 305 mOsm/kg). His vitamin C level was low at 0.2 mg/dL (normal range: 0.4 to 2.0 mg/dL). Mr. A also had a serum vitamin C level drawn 2 years prior that showed no symptoms of EPS, and at that time, the reading was 0.7 mg/dL. At admission to Medicine Services, Mr. A had a serum alcohol level of 211 mg/dL. Neuroimaging revealed diffuse cerebral and cerebellar volume loss.
Normal laboratory results included serum levels of vitamin B12, red cell folate, homocysteine, methylmalonic acid, free and total carnitine, alkaline phosphatase, manganese, and zinc. A urine drug screen was negative.
Case 2
Mr. B, a 69-year-old black male, was admitted to the hospital for depression complicated by alcohol dependence. He also had tobacco dependence, type 2 diabetes mellitus, hypertension, and gout. The patient’s BMI at admission was 16.1. Mr. B appeared ill, was worried about his health, and remained recumbent unless asked to move. He reported that his right hand had begun to shake at rest in the month prior to admission. The tremor made it difficult for him to drink. He pointed out stains on his hospital gurney from an attempt to drink orange juice prior to being assessed.
A physical examination revealed a distinct resting tremor with cogwheeling of the right hand; there was no other evidence of EPS, nor was there evidence of cognitive, cerebellar, or skin abnormalities, such as hemorrhages or corkscrew hairs. Asterixis was absent as was evidence of a high-output cardiac state that might produce a tremor, such as de Musset sign. A serum vitamin C level was obtained and returned at 0.0 mg/dL. A head computed tomography scan obtained the next day revealed mild cerebellar volume loss. A serum alkaline phosphatase level was elevated slightly at 136 U/L (normal range: 42 to 113 U/L). Normal serum values were returned for zinc, vitamins B12 and folate, rapid plasma reagin, sodium, and serum osmolality. A urine drug screen was negative, and serum alcohol level was < 5.0 mg/dL.
Mr. B took no medications expected to cause EPS. He received no micronutrient replacement until the day after admission when he began receiving oral vitamin C 1,000 mg twice a day. After receiving 3 doses, Mr. B’s resting tremor and cogwheeling completely resolved. He noticed he had stopped shaking and could now drink without spilling fluids. He also got out of bed and began interacting with others. Mr. B said he felt he was “doing well.” A repeat serum vitamin C level was 0.2 mg/dL on that day. The improvement was sustained over 3 days, and Mr. B was discharged to home.
Discussion
Both Mr. A and Mr. B presented with a typical picture of latent scurvy and the additional finding of parkinsonism. These cases are important for 2 reasons. First, the swift and full response of these patients’ parkinsonism to vitamin C replacement underscores the importance of considering a vitamin C deficiency when confronted with EPS. And second, both patients lacked signs of bleeding or of impaired collagen synthesis. This differs from the classic presentation of scurvy as a disorder primarily of collagen metabolism.4
Lind described the onset of scurvy as one in which striking emotional and behavior changes developed and later were followed by abnormal bleeding and even death.2 These early changes also were recognized by Shapter in 1847.5 Furthermore, the evidence that exists about the time-course of scurvy’s development suggests that neuropsychiatric findings precede the hemorrhagic.6 Indeed, classic skin findings, such as petechiae or corkscrew hairs, may develop years after the onset of neuropsychiatric changes.7,8
Despite WHO characterizing it as latent scurvy, the distinct syndromal presentation of hypovitaminosis C with parkinsonism along with the rapid response to vitamin C replacement argues for its recognition as a distinct clinical entity and not just a prelude to the hemorrhagic state. To assist in recognizing neuropsychiatric scurvy, the author suggests the operationalized approach described in Table 1.9
Pathophysiology
Vitamin C has an intimate role in the normal functioning of the basal ganglia. It is involved in the synthesis of catechecholamines, the regulation of the release and postsynaptic activities of various neurotransmitters, and managing the oxyradical toxicity of aerobic metabolism. Table 2 outlines some of the normal brain functions of vitamin C and the potential consequences of inadequate central vitamin C.9,10 Risk factors for vitamin C deficiency include those affecting the uptake, response to, and elimination of this vitamin (Table 3).11-14
The potential role of alcohol use by both patients also warrants mention. Current data suggest a nonlinear relationship between alcohol use and neurotoxicity. Epidemiologic data show that moderate alcohol consumption protects against the development of such neurodegenerative processes as Parkinson disease and Alzheimer disease.15,16 But the cases here reflect excessive use of alcohol. In this situation, a variety of progressive insults, such as those caused by oxyradical toxicity as well as malnutrition may foster the development of basal ganglia dysfunction.17
Measuring Deficiency
A deficiency of vitamin C may be determined in several ways. The most frequently used laboratory measure of vitamin C status is the serum vitamin C level. This level is included in the WHO’s recommendations for diagnosis.3 However, this assay is limited because when facing total body depletion, the kidneys may restrict the elimination of vitamin C and tend to maintain serum vitamin C levels even as target tissue levels fall. An interesting example of this is the 0.2 mg/dL value that each patient registered. In Mr. A’s case, this reflected a systemic deficit of vitamin C, while in Mr. B’s case it correlated with the onset of effective repletion of body’s stores.
A fall in urinary output of vitamin C is another marker of hypovitaminosis C. When available, this laboratory test can be used with the serum level to assess total body stores of vitamin C. Lymphocytes, neutrophils, and platelets also store vitamin C. These target tissues tend to saturate when the oral intake ranges between 100 mg to 200 mg a day. This is the same point at which serum vitamin C levels peak and level off in normal, healthy adults.18,19 Once again, the limited availability of target-tissue assays puts these studies out of reach for most clinicians.
No evaluation is complete without some assurance of what the disease is not. Deficiencies of biotin, zinc, folate, and B12 all may affect the function of the basal ganglia.20 The biotin deficiencies literature is particularly robust. Biotin deficiencies affecting basal ganglia function are best known as inherited disorders of metabolism.21 Manganese intoxication also may present as a movement disorder.22
Treatment
Treatment of neuropsychiatric scurvy has relied on IV administration of vitamin C. Although the bioavailability of oral vitamin C among healthy adult volunteers is nearly complete up to about 200 mg a day, a patient with neuropsychiatric scurvy may need substantially more than that amount to accommodate total body deficiencies and increased demands.23 The IV route allows serum vitamin C levels up to 100 times higher than by the oral route.24 Mr. B is, in fact, the first person reported in the literature with neuropsychiatric scurvy to respond to oral vitamin C replacement alone. Once repletion of vitamin C is complete, it is useful to consider a maintenance replacement dose based on a patient’s risk factors and needs.
A healthy adult should ingest about 120 mg of vitamin C daily. Smokers and pregnant women may require more, but this recommendation was intended to address their needs as well.25 Many commercial multivitamins use the old recommended daily allowance of 60 mg, so it may be safest to recommend specifically a vitamin C tablet with at least 120 mg when ordering vitamin C replacement.
Tight control of the serum vitamin C concentration means that little additional vitamin C will be taken up by the gut beyond 200 mg orally a day, which helps minimize any concerns about long-term toxicity. It takes several weeks to deplete vitamin C from the human body when vitamin C is removed from the diet, so a patient with a previously treated deficiency of vitamin C should wait a month before a repeat serum vitamin C level measurement.
The half life of vitamin C is normally ≤ 2 hours. When renal function is intact, vitamin C in excess of immediate need is lost through renal filtration. Toxicity is rare under these conditions.26 When vitamin C toxicity has been reported, it has occurred in the setting of prolonged supplementation, usually when a patient already experienced a renal injury. The main toxicities attributed to vitamin C are oxalate crystal formation with subsequent renal injury and exacerbation of glucose 6-phosphate dehydrogenase deficiency (G6PD).24
Oxalate formation due to vitamin C replacement is uncommon, but patients with preexisting calcium oxalate stones may be at risk for further stone formation when they receive additional vitamin C.27 This is most likely to occur when treatment with parenteral vitamin C is prolonged, which is not typical for patients with neuropsychiatric scurvy who tend to respond rapidly to vitamin C replenishment. Reports of acute hemolytic episodes among patient with G6PD deficiency receiving vitamin C exist, although these cases are rare.28 Furthermore, some authors advocate for the use of ascorbic acid to treat methemoglobinemia associated with G6PD deficiency, when methylene blue is not available.29 It may be reasonable to begin treatment with oral vitamin C for patients with NPS and G6PD deficiency. This is equivalent to a low-dose form of vitamin C replacement and may help avoid the theoretically pro-oxidant effects of larger, IV doses of vitamin C.30
Conclusion
The recent discovery of movement disorders in scurvy has enlarged the picture of vitamin C deficiency. The cases here demonstrate how hypovitaminosis C with central nervous system manifestations may be identified and treated. This relationship fits well within the established basic science and clinical framework for scurvy, and the clinical implications for scurvy remain in many ways unchanged. First, malnutrition must be considered even when a patient’s habitus suggests he is well fed. Also, it is more likely to see scurvy without all of the classic findings than an end-stage case of the disease.31 In the right clinical setting, it is reasonable to think of a vitamin C deficiency before the patient develops bleeding gums and corkscrew hairs. And as is typical of vitamin deficiencies, the treatment of a vitamin C deficiency usually results in swift improvement. Finally, for those who treat movement disorders or who prescribe agents such as antipsychotics that may cause movement disorders, it is important to recognize vitamin C deficiency as another potential explanation for EPS.
Vitamin C (ascorbic acid) deficiency is known to affect brain function and is associated with parkinsonism.1 In 1752, James Lind, MD, described emotional and behavioral changes that herald the onset of scurvy and precede hemorrhagic findings.2 The World Health Organization (WHO) today refers to this stage as latent scurvy.3 The 2 case studies that follow present examples of patients with vitamin C deficiencies whose parkinsonism responded robustly to vitamin C replacement. These cases suggest that vitamin C deficiency may be a treatable cause of parkinsonism.
Case 1
Mr. A, a 60-year-old white male, was admitted to the Medicine Service for alcohol detoxification. The patient had a history of alcohol dependence, alcohol withdrawal seizures, tobacco dependence, and hyperlipidemia. He took no medications as an outpatient. On admission Mr. A’s body mass index (BMI) was 27.2. An initial examination revealed a marked resting tremor of the patient’s right hand with cogwheeling, which had not been present in examinations conducted in the previous 3 years. Mr. A had no prior history of a tremor. He had no cerebellar findings and no evidence of asterixis or of tremulousness associated with high-output cardiac states, such as de Musset sign.
Mr. A reported he had experienced the tremor for a month and that it had been worsening. He also was having difficulty using his dominant right hand, for routine daily activities. Mr. A was oriented, and his short-term memory was intact. He was ill-appearing, irritable with psychomotor slowing, and did not wish to rise from his bed. He had no gingival or periungual bleeding and did not bruise easily. He had no corkscrew hairs. The patient was started on no medications known to cause extrapyramidal symptoms (EPS).
In the hospital, the tremor persisted unabated for 2 days. On the third day, Mr. A was started on 1,000 mg vitamin C IV twice daily. He received a total of 2,000 mg IV that day, but the IV fell out, and he refused its replacement. Several hours later, Mr. A stated that he felt much better, got out of bed, and asked to go outside to smoke. The author noted complete resolution of the right hand tremor and cogwheeling 20 hours after starting the vitamin C IV. Mr. A refused a repeat serum vitamin C assay.
Laboratory studies initially revealed that Mr. A had hyponatremia with a serum sodium of 121 mmol/L (normal range: 133 to 145 mmol/L) as well as hypokalemia with a serum potassium of 3.2 mmol/L (normal range: 3.5 to 5.0 mmol/L). He was hypoosmolar, with a serum osmolality of 276 mOsm/kg (normal range: 278 to 305 mOsm/kg). His vitamin C level was low at 0.2 mg/dL (normal range: 0.4 to 2.0 mg/dL). Mr. A also had a serum vitamin C level drawn 2 years prior that showed no symptoms of EPS, and at that time, the reading was 0.7 mg/dL. At admission to Medicine Services, Mr. A had a serum alcohol level of 211 mg/dL. Neuroimaging revealed diffuse cerebral and cerebellar volume loss.
Normal laboratory results included serum levels of vitamin B12, red cell folate, homocysteine, methylmalonic acid, free and total carnitine, alkaline phosphatase, manganese, and zinc. A urine drug screen was negative.
Case 2
Mr. B, a 69-year-old black male, was admitted to the hospital for depression complicated by alcohol dependence. He also had tobacco dependence, type 2 diabetes mellitus, hypertension, and gout. The patient’s BMI at admission was 16.1. Mr. B appeared ill, was worried about his health, and remained recumbent unless asked to move. He reported that his right hand had begun to shake at rest in the month prior to admission. The tremor made it difficult for him to drink. He pointed out stains on his hospital gurney from an attempt to drink orange juice prior to being assessed.
A physical examination revealed a distinct resting tremor with cogwheeling of the right hand; there was no other evidence of EPS, nor was there evidence of cognitive, cerebellar, or skin abnormalities, such as hemorrhages or corkscrew hairs. Asterixis was absent as was evidence of a high-output cardiac state that might produce a tremor, such as de Musset sign. A serum vitamin C level was obtained and returned at 0.0 mg/dL. A head computed tomography scan obtained the next day revealed mild cerebellar volume loss. A serum alkaline phosphatase level was elevated slightly at 136 U/L (normal range: 42 to 113 U/L). Normal serum values were returned for zinc, vitamins B12 and folate, rapid plasma reagin, sodium, and serum osmolality. A urine drug screen was negative, and serum alcohol level was < 5.0 mg/dL.
Mr. B took no medications expected to cause EPS. He received no micronutrient replacement until the day after admission when he began receiving oral vitamin C 1,000 mg twice a day. After receiving 3 doses, Mr. B’s resting tremor and cogwheeling completely resolved. He noticed he had stopped shaking and could now drink without spilling fluids. He also got out of bed and began interacting with others. Mr. B said he felt he was “doing well.” A repeat serum vitamin C level was 0.2 mg/dL on that day. The improvement was sustained over 3 days, and Mr. B was discharged to home.
Discussion
Both Mr. A and Mr. B presented with a typical picture of latent scurvy and the additional finding of parkinsonism. These cases are important for 2 reasons. First, the swift and full response of these patients’ parkinsonism to vitamin C replacement underscores the importance of considering a vitamin C deficiency when confronted with EPS. And second, both patients lacked signs of bleeding or of impaired collagen synthesis. This differs from the classic presentation of scurvy as a disorder primarily of collagen metabolism.4
Lind described the onset of scurvy as one in which striking emotional and behavior changes developed and later were followed by abnormal bleeding and even death.2 These early changes also were recognized by Shapter in 1847.5 Furthermore, the evidence that exists about the time-course of scurvy’s development suggests that neuropsychiatric findings precede the hemorrhagic.6 Indeed, classic skin findings, such as petechiae or corkscrew hairs, may develop years after the onset of neuropsychiatric changes.7,8
Despite WHO characterizing it as latent scurvy, the distinct syndromal presentation of hypovitaminosis C with parkinsonism along with the rapid response to vitamin C replacement argues for its recognition as a distinct clinical entity and not just a prelude to the hemorrhagic state. To assist in recognizing neuropsychiatric scurvy, the author suggests the operationalized approach described in Table 1.9
Pathophysiology
Vitamin C has an intimate role in the normal functioning of the basal ganglia. It is involved in the synthesis of catechecholamines, the regulation of the release and postsynaptic activities of various neurotransmitters, and managing the oxyradical toxicity of aerobic metabolism. Table 2 outlines some of the normal brain functions of vitamin C and the potential consequences of inadequate central vitamin C.9,10 Risk factors for vitamin C deficiency include those affecting the uptake, response to, and elimination of this vitamin (Table 3).11-14
The potential role of alcohol use by both patients also warrants mention. Current data suggest a nonlinear relationship between alcohol use and neurotoxicity. Epidemiologic data show that moderate alcohol consumption protects against the development of such neurodegenerative processes as Parkinson disease and Alzheimer disease.15,16 But the cases here reflect excessive use of alcohol. In this situation, a variety of progressive insults, such as those caused by oxyradical toxicity as well as malnutrition may foster the development of basal ganglia dysfunction.17
Measuring Deficiency
A deficiency of vitamin C may be determined in several ways. The most frequently used laboratory measure of vitamin C status is the serum vitamin C level. This level is included in the WHO’s recommendations for diagnosis.3 However, this assay is limited because when facing total body depletion, the kidneys may restrict the elimination of vitamin C and tend to maintain serum vitamin C levels even as target tissue levels fall. An interesting example of this is the 0.2 mg/dL value that each patient registered. In Mr. A’s case, this reflected a systemic deficit of vitamin C, while in Mr. B’s case it correlated with the onset of effective repletion of body’s stores.
A fall in urinary output of vitamin C is another marker of hypovitaminosis C. When available, this laboratory test can be used with the serum level to assess total body stores of vitamin C. Lymphocytes, neutrophils, and platelets also store vitamin C. These target tissues tend to saturate when the oral intake ranges between 100 mg to 200 mg a day. This is the same point at which serum vitamin C levels peak and level off in normal, healthy adults.18,19 Once again, the limited availability of target-tissue assays puts these studies out of reach for most clinicians.
No evaluation is complete without some assurance of what the disease is not. Deficiencies of biotin, zinc, folate, and B12 all may affect the function of the basal ganglia.20 The biotin deficiencies literature is particularly robust. Biotin deficiencies affecting basal ganglia function are best known as inherited disorders of metabolism.21 Manganese intoxication also may present as a movement disorder.22
Treatment
Treatment of neuropsychiatric scurvy has relied on IV administration of vitamin C. Although the bioavailability of oral vitamin C among healthy adult volunteers is nearly complete up to about 200 mg a day, a patient with neuropsychiatric scurvy may need substantially more than that amount to accommodate total body deficiencies and increased demands.23 The IV route allows serum vitamin C levels up to 100 times higher than by the oral route.24 Mr. B is, in fact, the first person reported in the literature with neuropsychiatric scurvy to respond to oral vitamin C replacement alone. Once repletion of vitamin C is complete, it is useful to consider a maintenance replacement dose based on a patient’s risk factors and needs.
A healthy adult should ingest about 120 mg of vitamin C daily. Smokers and pregnant women may require more, but this recommendation was intended to address their needs as well.25 Many commercial multivitamins use the old recommended daily allowance of 60 mg, so it may be safest to recommend specifically a vitamin C tablet with at least 120 mg when ordering vitamin C replacement.
Tight control of the serum vitamin C concentration means that little additional vitamin C will be taken up by the gut beyond 200 mg orally a day, which helps minimize any concerns about long-term toxicity. It takes several weeks to deplete vitamin C from the human body when vitamin C is removed from the diet, so a patient with a previously treated deficiency of vitamin C should wait a month before a repeat serum vitamin C level measurement.
The half life of vitamin C is normally ≤ 2 hours. When renal function is intact, vitamin C in excess of immediate need is lost through renal filtration. Toxicity is rare under these conditions.26 When vitamin C toxicity has been reported, it has occurred in the setting of prolonged supplementation, usually when a patient already experienced a renal injury. The main toxicities attributed to vitamin C are oxalate crystal formation with subsequent renal injury and exacerbation of glucose 6-phosphate dehydrogenase deficiency (G6PD).24
Oxalate formation due to vitamin C replacement is uncommon, but patients with preexisting calcium oxalate stones may be at risk for further stone formation when they receive additional vitamin C.27 This is most likely to occur when treatment with parenteral vitamin C is prolonged, which is not typical for patients with neuropsychiatric scurvy who tend to respond rapidly to vitamin C replenishment. Reports of acute hemolytic episodes among patient with G6PD deficiency receiving vitamin C exist, although these cases are rare.28 Furthermore, some authors advocate for the use of ascorbic acid to treat methemoglobinemia associated with G6PD deficiency, when methylene blue is not available.29 It may be reasonable to begin treatment with oral vitamin C for patients with NPS and G6PD deficiency. This is equivalent to a low-dose form of vitamin C replacement and may help avoid the theoretically pro-oxidant effects of larger, IV doses of vitamin C.30
Conclusion
The recent discovery of movement disorders in scurvy has enlarged the picture of vitamin C deficiency. The cases here demonstrate how hypovitaminosis C with central nervous system manifestations may be identified and treated. This relationship fits well within the established basic science and clinical framework for scurvy, and the clinical implications for scurvy remain in many ways unchanged. First, malnutrition must be considered even when a patient’s habitus suggests he is well fed. Also, it is more likely to see scurvy without all of the classic findings than an end-stage case of the disease.31 In the right clinical setting, it is reasonable to think of a vitamin C deficiency before the patient develops bleeding gums and corkscrew hairs. And as is typical of vitamin deficiencies, the treatment of a vitamin C deficiency usually results in swift improvement. Finally, for those who treat movement disorders or who prescribe agents such as antipsychotics that may cause movement disorders, it is important to recognize vitamin C deficiency as another potential explanation for EPS.
1. Ide K, Yamada H, Umegaki K, et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition. 2015;31(2):406-408.
2. Lind J. The diagnostics, or symptoms. A Treatise on the Scurvy, in Three Parts. 3rd ed. London: S. Crowder, D. Wilson and G. Nicholls, T. Cadell, T. Becket and Co., G. Pearch, and Woodfall; 1772:98-129.
3. World Health Organization. Scurvy and its prevention and control in major emergencies. http://whqlibdoc.who.int/hq/1999/WHO_NHD_99.11.pdf. Published 1999. Accessed July 6, 2017.
4. Sasseville D. Scurvy: curse and cure in New France. JAMA Dermatol. 2015;151(4):431.
5. Shapter T. On the recent occurrence of scurvy in Exeter and the neighbourhood. Prov Med Surg J. 1847;11(11):281-285.
6. Kinsman RA, Hood J. Some behavioral effects of ascorbic acid deficiency. Am J Clin Nutr. 1971;24(4):455-464.
7. DeSantis J. Scurvy and psychiatric symptoms. Perspect Psychiatr Care. 1993;29(1):18-22.
8. Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130(2):177-179.
9. Brown TM. Neuropsychiatric scurvy. Psychosomatics. 2015;56(1):12-20.
10. Feuerstein TJ, Weinheimer G, Lang G, Ginap T, Rossner R. Inhibition by ascorbic acid of NMDA-evoked acetylcholine release in rabbit caudate nucleus. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(5):549-551.
11. Kim J, Kwon J, Noh G, Lee SS. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7(6):488-494.
12. Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103(14):1863-1868.
13. Nappe TM, Pacelli AM, Katz K. An atypical case of methemoglobinemia due to self-administered benzocaine. Case Rep Emerg Med. 2015;2015:670979.
14. Wright AD, Stevens E, Ali M, Carroll DW, Brown TM. The neuropsychiatry of scurvy. Psychosomatics. 2014;55(2):179-185.
15. Bate C, Williams A. Ethanol protects cultured neurons against amyloid-β and α-synuclein-induced synapse damage. Neuropharmacology. 2011;61(8):1406-1412.
16. Vasanthi HR, Parameswari RP, DeLeiris J, Das DK. Health benefits of wine and alcohol from neuroprotection to heart health. Front Biosci (Elite Ed). 2012;4:1505-1512.
17. Vaglini F, Viaggi C, Piro V, et al. Acetaldehyde and parkinsonism: role of CYP450 2E1. Front Behav Neurosci. 2013;7:71.
18. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98(17):9842-9846.
19. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
20. Quiroga MJ, Carroll DW, Brown TM. Ascorbate- and zinc-responsive parkinsonism. Ann Pharmacother. 2014;48(11):1515-1520.
21. Tabarki B, Al-Shafi S, Al-Shahwan S, et al. Biotin-responsive basal ganglia disease revisited: clinical, radiologic, and genetic findings. Neurology. 2013;80(3):261-267.
22. Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277-312.
23. Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93(8):3704-3709.
24. Wilson MK, Baguley BC, Wall C, Jameson MB, Findlay MP. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol. 2014;10(1):22-37.
25. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086-1107.
26. Nielsen TK, Højgaard M, Andersen JT, Poulsen HE, Lykkesfeldt J, Mikines KJ. Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: a pharmacokinetic evaluation. Basic Clin Pharmacol Toxicol. 2015;116(4):343-348.
27. Baxmann AC, De O G Mendonça C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003;63(3):1066-1071.
28. Huang YC, Chang TK, Fu YC, Jan SL. C for colored urine: acute hemolysis induced by high-dose ascorbic acid. Clin Toxicol (Phila). 2014;52(9):984.
29. Rino PB, Scolnik D, Fustiñana A, Mitelpunkt A, Glatstein M. Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Am J Ther. 2014;21(4):240-243.
30. Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826(2):443-457.
31. Fouron JC, Chicoine L. Le scorbut: aspects particuliers de l’association rachitisme-scorbut. Can Med Assoc J. 1962;86(26):1191-1196.
1. Ide K, Yamada H, Umegaki K, et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition. 2015;31(2):406-408.
2. Lind J. The diagnostics, or symptoms. A Treatise on the Scurvy, in Three Parts. 3rd ed. London: S. Crowder, D. Wilson and G. Nicholls, T. Cadell, T. Becket and Co., G. Pearch, and Woodfall; 1772:98-129.
3. World Health Organization. Scurvy and its prevention and control in major emergencies. http://whqlibdoc.who.int/hq/1999/WHO_NHD_99.11.pdf. Published 1999. Accessed July 6, 2017.
4. Sasseville D. Scurvy: curse and cure in New France. JAMA Dermatol. 2015;151(4):431.
5. Shapter T. On the recent occurrence of scurvy in Exeter and the neighbourhood. Prov Med Surg J. 1847;11(11):281-285.
6. Kinsman RA, Hood J. Some behavioral effects of ascorbic acid deficiency. Am J Clin Nutr. 1971;24(4):455-464.
7. DeSantis J. Scurvy and psychiatric symptoms. Perspect Psychiatr Care. 1993;29(1):18-22.
8. Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130(2):177-179.
9. Brown TM. Neuropsychiatric scurvy. Psychosomatics. 2015;56(1):12-20.
10. Feuerstein TJ, Weinheimer G, Lang G, Ginap T, Rossner R. Inhibition by ascorbic acid of NMDA-evoked acetylcholine release in rabbit caudate nucleus. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(5):549-551.
11. Kim J, Kwon J, Noh G, Lee SS. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7(6):488-494.
12. Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103(14):1863-1868.
13. Nappe TM, Pacelli AM, Katz K. An atypical case of methemoglobinemia due to self-administered benzocaine. Case Rep Emerg Med. 2015;2015:670979.
14. Wright AD, Stevens E, Ali M, Carroll DW, Brown TM. The neuropsychiatry of scurvy. Psychosomatics. 2014;55(2):179-185.
15. Bate C, Williams A. Ethanol protects cultured neurons against amyloid-β and α-synuclein-induced synapse damage. Neuropharmacology. 2011;61(8):1406-1412.
16. Vasanthi HR, Parameswari RP, DeLeiris J, Das DK. Health benefits of wine and alcohol from neuroprotection to heart health. Front Biosci (Elite Ed). 2012;4:1505-1512.
17. Vaglini F, Viaggi C, Piro V, et al. Acetaldehyde and parkinsonism: role of CYP450 2E1. Front Behav Neurosci. 2013;7:71.
18. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98(17):9842-9846.
19. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
20. Quiroga MJ, Carroll DW, Brown TM. Ascorbate- and zinc-responsive parkinsonism. Ann Pharmacother. 2014;48(11):1515-1520.
21. Tabarki B, Al-Shafi S, Al-Shahwan S, et al. Biotin-responsive basal ganglia disease revisited: clinical, radiologic, and genetic findings. Neurology. 2013;80(3):261-267.
22. Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277-312.
23. Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93(8):3704-3709.
24. Wilson MK, Baguley BC, Wall C, Jameson MB, Findlay MP. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol. 2014;10(1):22-37.
25. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086-1107.
26. Nielsen TK, Højgaard M, Andersen JT, Poulsen HE, Lykkesfeldt J, Mikines KJ. Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: a pharmacokinetic evaluation. Basic Clin Pharmacol Toxicol. 2015;116(4):343-348.
27. Baxmann AC, De O G Mendonça C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003;63(3):1066-1071.
28. Huang YC, Chang TK, Fu YC, Jan SL. C for colored urine: acute hemolysis induced by high-dose ascorbic acid. Clin Toxicol (Phila). 2014;52(9):984.
29. Rino PB, Scolnik D, Fustiñana A, Mitelpunkt A, Glatstein M. Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Am J Ther. 2014;21(4):240-243.
30. Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826(2):443-457.
31. Fouron JC, Chicoine L. Le scorbut: aspects particuliers de l’association rachitisme-scorbut. Can Med Assoc J. 1962;86(26):1191-1196.
Hyaluronic Acid Gel Filler for Nipple Enhancement Following Breast Reconstruction
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
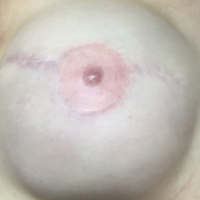
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
Practice Points
- The use of injectable hyaluronic acid (HA) gel to restore 3-dimensional contour of the nipple following nipple-areola complex (NAC) reconstruction is a noninvasive procedure that contributes to patient well-being.
- The use of HA gel for NAC augmentation can be performed in an office setting and may eliminate the need for secondary reconstructive surgeries.
Aggressive Merkel Cell Carcinoma in a Liver Transplant Recipient
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.
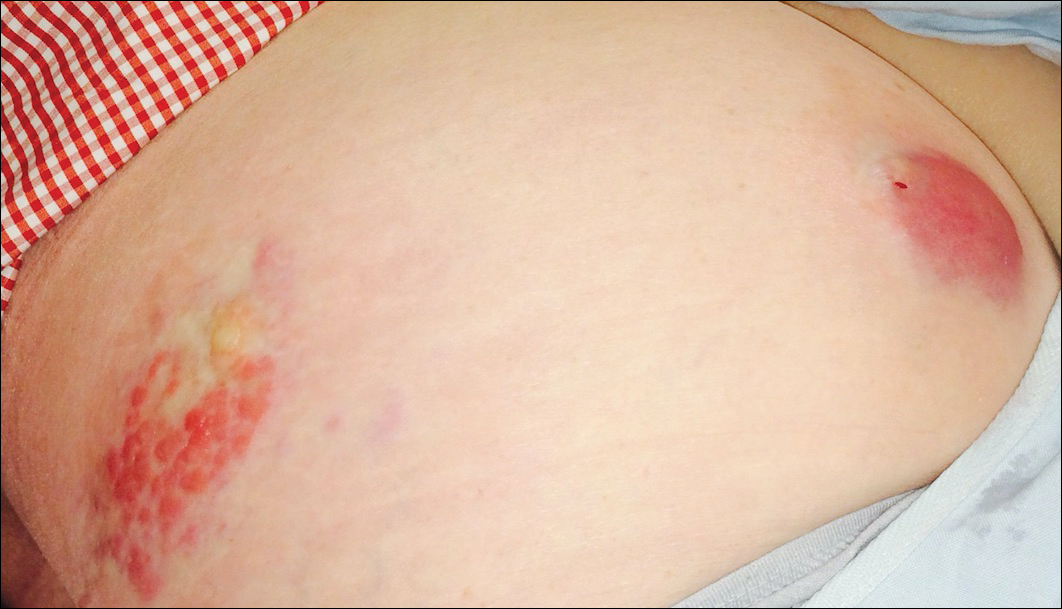
Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.
Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.
Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Practice Points
- Organ transplant recipients are at an increased risk for Merkel cell carcinoma (MCC).
- Early recognition and diagnosis of MCC is important to improve morbidity and mortality.
Annular Atrophic Lichen Planus Responds to Hydroxychloroquine and Acitretin
Annular atrophic lichen planus (AALP) is a rare variant of lichen planus that was first described by Friedman and Hashimoto1 in 1991. Clinically, it combines the configuration and morphological features of both annular and atrophic lichen planus. It is a rare entity. We report a case of AALP in a 69-year-old black man. The clinical and histopathological presentation depicted the defining features of this entity with a characteristic loss of elastic fibers corresponding to central atrophy of active lesions.
Case Report
A 69-year-old black man with a history of hepatitis C virus infection and hypothyroidism presented to the dermatology clinic with a pruritic rash on the trunk, extremities, groin, and scalp of 4 months' duration. He denied any new medications, recent illnesses, or sick contacts. Physical examination demonstrated well-demarcated violaceous papules and plaques on the trunk, extensor aspect of the forearms, and thighs involving 10% of the body surface area (Figure 1A). The lesions were annular with raised borders and central depigmented atrophic scarring (Figure 1B). The examination also revealed several large hypopigmented atrophic patches and plaques in the right inguinal region and on the dorsal aspect of the penile shaft and buttocks as well as a single atrophic plaque on the scalp. No oral lesions were seen. An initial punch biopsy was consistent with a nonspecific lichenoid dermatitis (Figure 2), and the patient was prescribed triamcinolone ointment 0.1% for the trunk and extremities and tacrolimus ointment 0.1% for the groin and genital region.


The patient continued to develop new annular atrophic skin lesions over the next several months. Repeat punch biopsies of lesional and uninvolved perilesional skin from the trunk were obtained for histopathologic confirmation and special staining. Lichenoid dermatitis again was noted on the lesional biopsy, and no notable histopathologic changes were observed on the perilesional biopsy. Verhoeff-van Gieson staining for elastic fibers was performed on both biopsies, which revealed destruction of elastic fibers in the central papillary dermis and upper reticular dermis of the lesional biopsy (Figure 3A). The elastic fibers on the perilesional biopsy were preserved (Figure 3B).

The clinical presentation and histopathological findings confirmed a diagnosis of AALP. The patient was prescribed a short taper of oral prednisone, which halted further disease progression. The patient was then started on pentoxifylline and continued on tacrolimus ointment 0.1% with minimal improvement in existing lesions. These medications were discontinued after 3 months. Hydroxychloroquine 400 mg once daily was administered, which initially resulted in some thinning of the plaques on the trunk; however, further progression of the disease was noted after 3 months. Acitretin 25 mg once daily was added to his treatment regimen. Marked thinning of active lesions, hyperpigmentation, and residual scarring was noted after 2 months of combined therapy with acitretin and hydroxychloroquine (Figure 4), with continued improvement appreciable several months later.
Comment
Lichen planus is a common pruritic inflammatory disease of the skin, mucous membranes, hair follicles, and nails with a highly variable clinical pattern and disease course that typically affects the adult population.2 There are many clinical variants of lichen planus, which all demonstrate lichenoid dermatitis on histology. Annular lichen planus is an uncommon variant most commonly seen in men with asymptomatic lesions involving the axillae and groin.2 Atrophic lichen planus is another variant demonstrating atrophic papules and plaques on the trunk and extremities.3 Annular atrophic lichen planus is the rarest variant of lichen planus, incorporating features of both annular and atrophic lichen planus.
The first case of AALP involved a 56-year-old black man with a 25-year history of annular atrophic papules and plaques on the trunk and extremities.1 The second case reported by Requena et al4 in 1994 described a 65-year-old woman with characteristic lesions on the right elbow and left knee. Lipsker et al5 reported a third case in a 41-year-old man with a history of Sneddon syndrome who had lesions typical for AALP for 20 years. In all of these cases, histopathologic examination revealed a lichenoid infiltrate with thinning of the epidermis and loss of elastic fibers in the center of the active lesions.
In more recent cases of AALP, the characteristic findings primarily occurred on the trunk and extremities.6-10 Treatment with topical corticosteroids failed in most cases and some patients noted moderate improvement with tacrolimus ointment 0.1%. Sugashima and Yamamoto11 reported a unique case in 2012 of a 32-year-old woman with AALP on the lower lip. She had notable improvement with tacrolimus ointment 0.1% after 6 months.11
All of the known cases of AALP to date have occurred in adults, both male and female, presenting with a limited number of annular plaques with slightly elevated borders and depressed atrophic centers.1,3-11 Disease duration of AALP has ranged from 2 months to 25 years.11 Histopathologic findings characteristically demonstrate a lichenoid dermatitis of the raised lesional border with a flattened epidermis, loss of rete ridges, and fibrosis of dermal papillae in the lesion center.7 The elastic fibers are destroyed in the papillary dermis of the lesion center, presumably due to elastolytic activity of inflammatory cells.1 Macrophages present in the lichenoid infiltrate of acute lesions release elastases contributing to this destruction.7 Furthermore, elastic fibers appear fragmented on electron microscopy.1
The clinical course of AALP has proven to be chronic in most cases and frequently is resistant to treatment with topical corticosteroids, retinoids, phototherapy, and immunosuppressive agents.3 Treatment administered early in the disease course may provide a more favorable outcome.11 Lesions characteristically heal with scarring and hyperpigmentation. Our case displayed more extensive involvement than has previously been reported. Our patient showed minimal improvement with topical therapy; however, he demonstrated thinning and regression of active lesions after 2 months of combined treatment with hydroxychloroquine and acitretin. Our use of oral pentoxifylline, hydroxychloroquine, and acitretin has not been previously reported in the other cases of AALP we reviewed. Acitretin is the only systemic agent for lichen planus that has achieved level A evidence, as it previously was shown to be highly effective in a placebo-controlled, double-blind study of 65 patients.12
Conclusion
Annular atrophic lichen planus is a known variant of lichen planus characterized by a loss of elastic fibers in the papillary dermis in the center of active lesions. Treatment with topical corticosteroids and phototherapy frequently is ineffective. To our knowledge, there are no studies to date regarding the efficacy of systemic therapy in treatment of AALP. Hydroxychloroquine and acitretin may prove to be beneficial treatment options for resistant AALP. Additional alternative treatments continue to be explored. We encourage reporting additional cases of AALP to further characterize its clinical presentation and response to treatments.
- Friedman DB, Hashimoto K. Annular atrophic lichen planus. J Am Acad Dermatol. 1991;25:392-394.
- James WD, Berger TG, Elston DM. Lichen planus and related conditions. In: James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:213-215.
- Kim BS, Seo SH, Jang BS, et al. A case of annular atrophic lichen planus. J Eur Acad Dermatol Venereol. 2007;21:989-990.
- Requena L, Olivares M, Pique E, et al. Annular atrophic lichen planus. Dermatology. 1994;189:95-98.
- Lipsker D, Piette JC, Laporte JL, et al. Annular atrophic lichen planus and Sneddon's syndrome. Dermatology. 1997;105:402-403.
- Mseddi M, Bouassadi S, Marrakchi S, et al. Annular atrophic lichen planus. Dermatology. 2003;207:208-209.
- Morales-Callaghan A Jr, Martinez G, Aragoneses H, et al. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52:906-908.
- Ponce-Olivera RM, Tirado-Sánchez A, Montes-de-Oca-Sánchez G, et al. Annular atrophic lichen planus. Int J Dermatol. 2007;46:490-491.
- Kim JS, Kang MS, Sagong C, et al. Annular atrophic lichen planus associated with hypertrophic lichen planus. Clin Exp Dermatol. 2008;33:195-197.
- Li B, Li JH, Xiao T, et al. Annular atrophic lichen planus. Eur J Dermatol. 2010;20:842-843.
- Sugashima Y, Yamamoto T. Annular atrophic lichen planus of the lip. Dermatol Online J. 2012;18:14.
- Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-991.
Annular atrophic lichen planus (AALP) is a rare variant of lichen planus that was first described by Friedman and Hashimoto1 in 1991. Clinically, it combines the configuration and morphological features of both annular and atrophic lichen planus. It is a rare entity. We report a case of AALP in a 69-year-old black man. The clinical and histopathological presentation depicted the defining features of this entity with a characteristic loss of elastic fibers corresponding to central atrophy of active lesions.
Case Report
A 69-year-old black man with a history of hepatitis C virus infection and hypothyroidism presented to the dermatology clinic with a pruritic rash on the trunk, extremities, groin, and scalp of 4 months' duration. He denied any new medications, recent illnesses, or sick contacts. Physical examination demonstrated well-demarcated violaceous papules and plaques on the trunk, extensor aspect of the forearms, and thighs involving 10% of the body surface area (Figure 1A). The lesions were annular with raised borders and central depigmented atrophic scarring (Figure 1B). The examination also revealed several large hypopigmented atrophic patches and plaques in the right inguinal region and on the dorsal aspect of the penile shaft and buttocks as well as a single atrophic plaque on the scalp. No oral lesions were seen. An initial punch biopsy was consistent with a nonspecific lichenoid dermatitis (Figure 2), and the patient was prescribed triamcinolone ointment 0.1% for the trunk and extremities and tacrolimus ointment 0.1% for the groin and genital region.


The patient continued to develop new annular atrophic skin lesions over the next several months. Repeat punch biopsies of lesional and uninvolved perilesional skin from the trunk were obtained for histopathologic confirmation and special staining. Lichenoid dermatitis again was noted on the lesional biopsy, and no notable histopathologic changes were observed on the perilesional biopsy. Verhoeff-van Gieson staining for elastic fibers was performed on both biopsies, which revealed destruction of elastic fibers in the central papillary dermis and upper reticular dermis of the lesional biopsy (Figure 3A). The elastic fibers on the perilesional biopsy were preserved (Figure 3B).

The clinical presentation and histopathological findings confirmed a diagnosis of AALP. The patient was prescribed a short taper of oral prednisone, which halted further disease progression. The patient was then started on pentoxifylline and continued on tacrolimus ointment 0.1% with minimal improvement in existing lesions. These medications were discontinued after 3 months. Hydroxychloroquine 400 mg once daily was administered, which initially resulted in some thinning of the plaques on the trunk; however, further progression of the disease was noted after 3 months. Acitretin 25 mg once daily was added to his treatment regimen. Marked thinning of active lesions, hyperpigmentation, and residual scarring was noted after 2 months of combined therapy with acitretin and hydroxychloroquine (Figure 4), with continued improvement appreciable several months later.

Comment
Lichen planus is a common pruritic inflammatory disease of the skin, mucous membranes, hair follicles, and nails with a highly variable clinical pattern and disease course that typically affects the adult population.2 There are many clinical variants of lichen planus, which all demonstrate lichenoid dermatitis on histology. Annular lichen planus is an uncommon variant most commonly seen in men with asymptomatic lesions involving the axillae and groin.2 Atrophic lichen planus is another variant demonstrating atrophic papules and plaques on the trunk and extremities.3 Annular atrophic lichen planus is the rarest variant of lichen planus, incorporating features of both annular and atrophic lichen planus.
The first case of AALP involved a 56-year-old black man with a 25-year history of annular atrophic papules and plaques on the trunk and extremities.1 The second case reported by Requena et al4 in 1994 described a 65-year-old woman with characteristic lesions on the right elbow and left knee. Lipsker et al5 reported a third case in a 41-year-old man with a history of Sneddon syndrome who had lesions typical for AALP for 20 years. In all of these cases, histopathologic examination revealed a lichenoid infiltrate with thinning of the epidermis and loss of elastic fibers in the center of the active lesions.
In more recent cases of AALP, the characteristic findings primarily occurred on the trunk and extremities.6-10 Treatment with topical corticosteroids failed in most cases and some patients noted moderate improvement with tacrolimus ointment 0.1%. Sugashima and Yamamoto11 reported a unique case in 2012 of a 32-year-old woman with AALP on the lower lip. She had notable improvement with tacrolimus ointment 0.1% after 6 months.11
All of the known cases of AALP to date have occurred in adults, both male and female, presenting with a limited number of annular plaques with slightly elevated borders and depressed atrophic centers.1,3-11 Disease duration of AALP has ranged from 2 months to 25 years.11 Histopathologic findings characteristically demonstrate a lichenoid dermatitis of the raised lesional border with a flattened epidermis, loss of rete ridges, and fibrosis of dermal papillae in the lesion center.7 The elastic fibers are destroyed in the papillary dermis of the lesion center, presumably due to elastolytic activity of inflammatory cells.1 Macrophages present in the lichenoid infiltrate of acute lesions release elastases contributing to this destruction.7 Furthermore, elastic fibers appear fragmented on electron microscopy.1
The clinical course of AALP has proven to be chronic in most cases and frequently is resistant to treatment with topical corticosteroids, retinoids, phototherapy, and immunosuppressive agents.3 Treatment administered early in the disease course may provide a more favorable outcome.11 Lesions characteristically heal with scarring and hyperpigmentation. Our case displayed more extensive involvement than has previously been reported. Our patient showed minimal improvement with topical therapy; however, he demonstrated thinning and regression of active lesions after 2 months of combined treatment with hydroxychloroquine and acitretin. Our use of oral pentoxifylline, hydroxychloroquine, and acitretin has not been previously reported in the other cases of AALP we reviewed. Acitretin is the only systemic agent for lichen planus that has achieved level A evidence, as it previously was shown to be highly effective in a placebo-controlled, double-blind study of 65 patients.12
Conclusion
Annular atrophic lichen planus is a known variant of lichen planus characterized by a loss of elastic fibers in the papillary dermis in the center of active lesions. Treatment with topical corticosteroids and phototherapy frequently is ineffective. To our knowledge, there are no studies to date regarding the efficacy of systemic therapy in treatment of AALP. Hydroxychloroquine and acitretin may prove to be beneficial treatment options for resistant AALP. Additional alternative treatments continue to be explored. We encourage reporting additional cases of AALP to further characterize its clinical presentation and response to treatments.
Annular atrophic lichen planus (AALP) is a rare variant of lichen planus that was first described by Friedman and Hashimoto1 in 1991. Clinically, it combines the configuration and morphological features of both annular and atrophic lichen planus. It is a rare entity. We report a case of AALP in a 69-year-old black man. The clinical and histopathological presentation depicted the defining features of this entity with a characteristic loss of elastic fibers corresponding to central atrophy of active lesions.
Case Report
A 69-year-old black man with a history of hepatitis C virus infection and hypothyroidism presented to the dermatology clinic with a pruritic rash on the trunk, extremities, groin, and scalp of 4 months' duration. He denied any new medications, recent illnesses, or sick contacts. Physical examination demonstrated well-demarcated violaceous papules and plaques on the trunk, extensor aspect of the forearms, and thighs involving 10% of the body surface area (Figure 1A). The lesions were annular with raised borders and central depigmented atrophic scarring (Figure 1B). The examination also revealed several large hypopigmented atrophic patches and plaques in the right inguinal region and on the dorsal aspect of the penile shaft and buttocks as well as a single atrophic plaque on the scalp. No oral lesions were seen. An initial punch biopsy was consistent with a nonspecific lichenoid dermatitis (Figure 2), and the patient was prescribed triamcinolone ointment 0.1% for the trunk and extremities and tacrolimus ointment 0.1% for the groin and genital region.


The patient continued to develop new annular atrophic skin lesions over the next several months. Repeat punch biopsies of lesional and uninvolved perilesional skin from the trunk were obtained for histopathologic confirmation and special staining. Lichenoid dermatitis again was noted on the lesional biopsy, and no notable histopathologic changes were observed on the perilesional biopsy. Verhoeff-van Gieson staining for elastic fibers was performed on both biopsies, which revealed destruction of elastic fibers in the central papillary dermis and upper reticular dermis of the lesional biopsy (Figure 3A). The elastic fibers on the perilesional biopsy were preserved (Figure 3B).

The clinical presentation and histopathological findings confirmed a diagnosis of AALP. The patient was prescribed a short taper of oral prednisone, which halted further disease progression. The patient was then started on pentoxifylline and continued on tacrolimus ointment 0.1% with minimal improvement in existing lesions. These medications were discontinued after 3 months. Hydroxychloroquine 400 mg once daily was administered, which initially resulted in some thinning of the plaques on the trunk; however, further progression of the disease was noted after 3 months. Acitretin 25 mg once daily was added to his treatment regimen. Marked thinning of active lesions, hyperpigmentation, and residual scarring was noted after 2 months of combined therapy with acitretin and hydroxychloroquine (Figure 4), with continued improvement appreciable several months later.

Comment
Lichen planus is a common pruritic inflammatory disease of the skin, mucous membranes, hair follicles, and nails with a highly variable clinical pattern and disease course that typically affects the adult population.2 There are many clinical variants of lichen planus, which all demonstrate lichenoid dermatitis on histology. Annular lichen planus is an uncommon variant most commonly seen in men with asymptomatic lesions involving the axillae and groin.2 Atrophic lichen planus is another variant demonstrating atrophic papules and plaques on the trunk and extremities.3 Annular atrophic lichen planus is the rarest variant of lichen planus, incorporating features of both annular and atrophic lichen planus.
The first case of AALP involved a 56-year-old black man with a 25-year history of annular atrophic papules and plaques on the trunk and extremities.1 The second case reported by Requena et al4 in 1994 described a 65-year-old woman with characteristic lesions on the right elbow and left knee. Lipsker et al5 reported a third case in a 41-year-old man with a history of Sneddon syndrome who had lesions typical for AALP for 20 years. In all of these cases, histopathologic examination revealed a lichenoid infiltrate with thinning of the epidermis and loss of elastic fibers in the center of the active lesions.
In more recent cases of AALP, the characteristic findings primarily occurred on the trunk and extremities.6-10 Treatment with topical corticosteroids failed in most cases and some patients noted moderate improvement with tacrolimus ointment 0.1%. Sugashima and Yamamoto11 reported a unique case in 2012 of a 32-year-old woman with AALP on the lower lip. She had notable improvement with tacrolimus ointment 0.1% after 6 months.11
All of the known cases of AALP to date have occurred in adults, both male and female, presenting with a limited number of annular plaques with slightly elevated borders and depressed atrophic centers.1,3-11 Disease duration of AALP has ranged from 2 months to 25 years.11 Histopathologic findings characteristically demonstrate a lichenoid dermatitis of the raised lesional border with a flattened epidermis, loss of rete ridges, and fibrosis of dermal papillae in the lesion center.7 The elastic fibers are destroyed in the papillary dermis of the lesion center, presumably due to elastolytic activity of inflammatory cells.1 Macrophages present in the lichenoid infiltrate of acute lesions release elastases contributing to this destruction.7 Furthermore, elastic fibers appear fragmented on electron microscopy.1
The clinical course of AALP has proven to be chronic in most cases and frequently is resistant to treatment with topical corticosteroids, retinoids, phototherapy, and immunosuppressive agents.3 Treatment administered early in the disease course may provide a more favorable outcome.11 Lesions characteristically heal with scarring and hyperpigmentation. Our case displayed more extensive involvement than has previously been reported. Our patient showed minimal improvement with topical therapy; however, he demonstrated thinning and regression of active lesions after 2 months of combined treatment with hydroxychloroquine and acitretin. Our use of oral pentoxifylline, hydroxychloroquine, and acitretin has not been previously reported in the other cases of AALP we reviewed. Acitretin is the only systemic agent for lichen planus that has achieved level A evidence, as it previously was shown to be highly effective in a placebo-controlled, double-blind study of 65 patients.12
Conclusion
Annular atrophic lichen planus is a known variant of lichen planus characterized by a loss of elastic fibers in the papillary dermis in the center of active lesions. Treatment with topical corticosteroids and phototherapy frequently is ineffective. To our knowledge, there are no studies to date regarding the efficacy of systemic therapy in treatment of AALP. Hydroxychloroquine and acitretin may prove to be beneficial treatment options for resistant AALP. Additional alternative treatments continue to be explored. We encourage reporting additional cases of AALP to further characterize its clinical presentation and response to treatments.
- Friedman DB, Hashimoto K. Annular atrophic lichen planus. J Am Acad Dermatol. 1991;25:392-394.
- James WD, Berger TG, Elston DM. Lichen planus and related conditions. In: James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:213-215.
- Kim BS, Seo SH, Jang BS, et al. A case of annular atrophic lichen planus. J Eur Acad Dermatol Venereol. 2007;21:989-990.
- Requena L, Olivares M, Pique E, et al. Annular atrophic lichen planus. Dermatology. 1994;189:95-98.
- Lipsker D, Piette JC, Laporte JL, et al. Annular atrophic lichen planus and Sneddon's syndrome. Dermatology. 1997;105:402-403.
- Mseddi M, Bouassadi S, Marrakchi S, et al. Annular atrophic lichen planus. Dermatology. 2003;207:208-209.
- Morales-Callaghan A Jr, Martinez G, Aragoneses H, et al. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52:906-908.
- Ponce-Olivera RM, Tirado-Sánchez A, Montes-de-Oca-Sánchez G, et al. Annular atrophic lichen planus. Int J Dermatol. 2007;46:490-491.
- Kim JS, Kang MS, Sagong C, et al. Annular atrophic lichen planus associated with hypertrophic lichen planus. Clin Exp Dermatol. 2008;33:195-197.
- Li B, Li JH, Xiao T, et al. Annular atrophic lichen planus. Eur J Dermatol. 2010;20:842-843.
- Sugashima Y, Yamamoto T. Annular atrophic lichen planus of the lip. Dermatol Online J. 2012;18:14.
- Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-991.
- Friedman DB, Hashimoto K. Annular atrophic lichen planus. J Am Acad Dermatol. 1991;25:392-394.
- James WD, Berger TG, Elston DM. Lichen planus and related conditions. In: James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:213-215.
- Kim BS, Seo SH, Jang BS, et al. A case of annular atrophic lichen planus. J Eur Acad Dermatol Venereol. 2007;21:989-990.
- Requena L, Olivares M, Pique E, et al. Annular atrophic lichen planus. Dermatology. 1994;189:95-98.
- Lipsker D, Piette JC, Laporte JL, et al. Annular atrophic lichen planus and Sneddon's syndrome. Dermatology. 1997;105:402-403.
- Mseddi M, Bouassadi S, Marrakchi S, et al. Annular atrophic lichen planus. Dermatology. 2003;207:208-209.
- Morales-Callaghan A Jr, Martinez G, Aragoneses H, et al. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52:906-908.
- Ponce-Olivera RM, Tirado-Sánchez A, Montes-de-Oca-Sánchez G, et al. Annular atrophic lichen planus. Int J Dermatol. 2007;46:490-491.
- Kim JS, Kang MS, Sagong C, et al. Annular atrophic lichen planus associated with hypertrophic lichen planus. Clin Exp Dermatol. 2008;33:195-197.
- Li B, Li JH, Xiao T, et al. Annular atrophic lichen planus. Eur J Dermatol. 2010;20:842-843.
- Sugashima Y, Yamamoto T. Annular atrophic lichen planus of the lip. Dermatol Online J. 2012;18:14.
- Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-991.
Asymptomatic Cutaneous Polyarteritis Nodosa: Treatment Options and Therapeutic Guidelines
In 1931, Lindberg1 described a cutaneous variant of polyarteritis nodosa, which lacked visceral involvement and possessed a more favorable prognosis.2 Cutaneous polyarteritis nodosa (CPAN) is a localized small- to medium-vessel vasculitis restricted to the skin. Both benign and chronic courses have been described, and systemic involvement does not occur.3 Diagnostic criteria proposed by Nakamura et al3 in 2009 included cutaneous nodules, livedo reticularis, purpura, or ulcers; histopathologic fibrinoid necrotizing vasculitis of small- to medium-sized vessels; and exclusion of systemic symptoms (eg, fever, hypertension, weight loss, renal failure, cerebral hemorrhage, neuropathy, myocardial infarction, ischemic heart disease, pericarditis, pleuritis, arthralgia/myalgia). Nodules occur in 30% to 50% of cases and can remain for years if left untreated. Ulcerations occur in up to 30% of patients. Myositis, arthritis, and weakness also have been reported with this condition.4 Cutaneous polyarteritis nodosa has been associated with abnormal antibody testing with elevations of antiphospholipid cofactor antibody, lupus anticoagulant, anticardiolipin antibody, and anti-β2-glycoprotein I–dependent cardiolipin antibody, as well as elevated anti–phosphatidylserine-prothrombin complex antibody.5 These antibodies suggest increased risk for thrombosis and systemic diseases such as lupus or other autoimmune connective tissue disease. The distinction of this entity from systemic polyartertitis nodosa is key when determining treatment options and monitoring parameters.
Case Report
A 66-year-old woman was referred to our facility by an outside dermatologist with a mildly pruritic, blanchable, reticulated erythema on the chest and bilateral arms and legs of 3 months’ duration consistent with livedo reticularis (Figure 1). Prior systemic therapy included prednisone 10 mg 3 times daily, fexofenadine, loratadine, and hydroxyzine. When the systemic steroid was tapered, the patient developed an asymptomatic flare of her eruption. On presentation, the lesions had waxed and waned, and the patient was taking only vitamin B12 and vitamin C. Her medical history was notable for an unknown-type lymphoma of the chest wall diagnosed at 46 years of age that was treated with an unknown chemotherapeutic agent, chronic pancreatitis that resulted in a duodenectomy at 61 years of age, chronic cholecystitis, and 1 first-trimester miscarriage. Outside laboratory tests, including a comprehensive metabolic panel, complete blood cell count, urinalysis, renal function, and liver function tests were within reference range, except for the finding of mild leukocytosis (11,000/µL)(reference range, 3800–10,800/µL), which resolved after steroids were discontinued, with otherwise normal results. Punch biopsy of a specimen from the right thigh revealed medium-vessel vasculitis consistent with polyarteritis nodosa (Figure 2). Laboratory workup by our facility including hepatitis panel, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, factor V Leiden, prothrombin time/international normalized ratio, anticardiolipin antibody, and proteins C and S were all within reference range. Abnormal values included a low positive but nondiagnostic antinuclear antibody screen with negative titers, and the lupus anticoagulant titer was mildly elevated at 44 IgG binding units (reference range, <40 IgG binding units). Serum protein electrophoresis (SPEP) and urine protein electrophoresis also were performed, and SPEP was low positive for elevated κ and γ light chains. The patient was referred to oncology, and further testing revealed no underlying malignancy. The patient was monitored and no treatment was initiated; her rash completely resolved within 3 months. Laboratory monitoring at 6 months including SPEP, urine protein electrophoresis, lupus anticoagulant, and clotting studies all were within reference range.
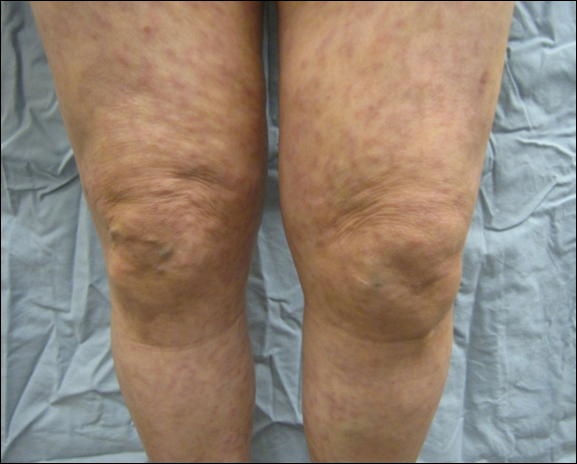
Comment
Although the treatment of systemic polyarteritis nodosa often is necessary and typically involves high-dose corticosteroids and cyclophosphamide, the treatment of CPAN initially is less aggressive. Of the options available for treatment of CPAN, each has associated risks and side effects. Chen6 classified CPAN into 3 groups: 1 (mild), 2 (severe with no systemic involvement), and 3 (severe with progression to systemic disease)(Table). The authors performed a review of all the published treatments and their respective side effects to evaluate if treatment should be instituted for asymptomatic (group 1) disease presenting with abnormal antibody findings as demonstrated in our case.
First-line treatment of CPAN includes nonsteroidal anti-inflammatory drugs (NSAIDS) and colchicine.7 Nonsteroidal anti-inflammatory drugs are preferred; however, they also have been associated with gastrointestinal tract upset and increased risk for peptic ulcer disease with long-term use. Although colchicine often is used in conjunction with NSAIDS8 for its anti-inflammatory activity, no studies have been performed on this drug as monotherapy, and the side effect of diarrhea often limits its use.
Other therapies include dapsone, which should be monitored carefully due to the risk for dapsone hypersensitivity syndrome.8,9 Topical corticosteroids have been proven effective for mild cases of confluent erythema with remission occurring as early as 4 weeks.4 Some reports emphasize the role of streptococcal infections in CPAN, especially in children.8,10-12 Consequently it is recommended that anti–streptolysin O titers should be included in the workup for CPAN. Long-term penicillin prophylaxis and tonsillectomy have been used to prevent disease flares with limited success.8,10-12
For more severe disease, especially with neuromuscular involvement, oral methylprednisolone up to 1 mg/kg daily has been used and has proven effective in the control of acute exacerbations.7,13 However, the many adverse effects of systemic steroids limit their use long-term, and taper will often result in flare of disease.4,7 Medications used in conjunction with steroids include hydroxychloroquine, dapsone, azathioprine, cyclophosphamide, methotrexate, sulfapyridine, pentoxifylline, infliximab, etanercept, and intravenous immunoglobulin.4,9,12-17
Low-dose methotrexate has shown some improvement in skin disease with CPAN, but other case reports suggest that complete remission is not achieved with this drug.15,18 More studies are needed to assess the use of methotrexate for CPAN.
Immunomodulators have been used in multiple case reports with varying levels of success. Rogalski and Sticherling4 reported 3 cases that cleared with methylprednisolone plus azathioprine ranging from 4 weeks to 6 months; nausea limited tolerance of azathioprine in 1 case. Mycophenolate mofetil also was successfully used in 2 cases with clearance at 17 weeks and 6 months. In this series of cases, cyclosporine was ineffective for CPAN.4 Two case reports documented cutaneous clearance with cyclophosphamide in conjunction with prednisolone.9,10 No prospective trials have been performed on these medications, and immunosuppressants should only be considered in steroid-resistant cases.
The use of intravenous immunoglobulin has been reported effective in prior cases that showed resistance to more conventional trials of steroids, azathioprine, and/or cyclophosphamide.12,14 Intravenous immunoglobulin may be regarded as a treatment option for severe resistant disease. Several case reports also have documented success using tumor necrosis factor α blockers, particularly infliximab, as an adjunct to steroids and etanercept as both a steroid adjunct and monotherapy.16,17,19 More studies are necessary to evaluate these treatments.
Additionally, single case reports have outlined the use of other therapeutic agents, including tamoxifen (10 mg twice daily increased to 20 mg twice daily during episodes of breakthrough lesions),20 hyperbaric oxygen therapy (100% oxygen for 90 minutes 5 times weekly at 1.5 atm absolute followed by 2 weeks of 2 atm absolute),21 and granulocyte-macrophage colony-stimulating factor (300 µg injection in small portion to ulcer edges twice monthly for 2 months).22 All of these treatments show promise, but data are limited.
Because thrombosis is postulated to be a potential mechanism leading to CPAN, agents such as pentoxifylline, clopidogrel, and warfarin have been examined as treatment options. Pentoxifylline in combination with mycophenolate mofetil has been successful in treating a case that was resistant to other immunosuppressants.23 Clopidogrel blocks the adenosine diphosphate pathway and impairs clot retraction. Clopidogrel was reported effective in an acute flare of CPAN for clearance of skin lesions and normalization of lupus anticoagulant.24 It also was used successfully in recurrent CPAN after steroid treatments in a patient with neuromuscular symptoms. There was no recurrence in either of the patients in this case report series. Warfarin therapy at an international normalized ratio of 3.0 also has demonstrated success in halting disease progression and in facilitating the resolution of skin changes and normalization of anti–phosphatidylserine-prothrombin complex antibodies.24 Our review of the literature did not reveal evidence of a standardized length of treatment following symptom resolution or if treatment is indicated in asymptomatic disease, or as in our case, with only mild elevations of antiphospholipid antibodies.
Conclusion
Multiple treatment options exist for CPAN, but the data on their efficacies is limited and based only on anecdotal evidence, not prospective analysis. We believe that it seems reasonable to initiate treatment only for symptomatic disease or cases in which the antibody titers suggest that the patient may be at high risk for thrombosis. Mild symptoms and mild cutaneous changes would suggest the likely choice of NSAIDs, colchicine, or dapsone as treatment options versus no treatment. In patients with antibody titers, pentoxifylline, clopidogrel, or warfarin may be considered first-line therapies. With severe ulcerative lesions and neuromuscular involvement, steroids, immunosuppressants, and other investigative agents should be contemplated. In our patient, the laboratory studies were repeated and normalized on complete resolution of her livedo eruption. She remained asymptomatic and clear for 8 months without any treatment. The incidence of this presentation of CPAN is unknown and is likely underreported, as we would not expect most patients to present to their physicians for the evaluation of otherwise asymptomatic livedo reticularis. In essence, our case report suggests that it may be prudent to simply monitor patients with asymptomatic CPAN.
- Lindberg K. Ein Beitrag zur Kenntnis der Periarteritis nodosa. Acta Med Scand. 1931;76:183-225.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa [published online August 26, 2005]. J Neurol. 2005;252:1155-1166.
- Nakamura T, Kanazawa N, Ikeda T, et al. Cutaneous polyarteritis nodosa: revisiting its definition and diagnostic criteria. Arch Dermatol Res. 2009;301:117-121.
- Rogalski C, Sticherling M. Panateritis cutanea benigna—an entity limited to the skin or cutaneous presentation of a systemic necrotizing vasculitis? report of seven cases and review of the literature. Int J Dermatol. 2007;46:817-821.
- Kawakami T, Yamazaki M, Mizoguchi M, et al. High titer of anti-phosphatidylserine-prothrombin complex antibodies in patients with cutaneous polyarteritis nodosa. Arthritis Rheum. 2007;57:1507-1513.
- Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;6:429-442.
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756.
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathologic analysis and review of the published work. J Dermatol. 2010;37:85-93.
- Flanagan N, Casey EB, Watson R, et al. Cutaneous polyartertitis nodosa with seronegative arthritis. Rheumatology (Oxford). 1999;38:1161-1162.
- Fathalla B, Miller L, Brady S, et al. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724-728.
- Misago N, Mochizuki Y, Sekiyama-Kodera H, et al. Cutaneous polyarteritis nodosa: therapy and clinical course in four cases. J Dermatol. 2001;28:719-727.
- Breda L, Franchini S, Marzetti V, et al. Intravenous immunoglobulins for cutaneous polyarteritis nodosa resistant to conventional treatment. Scand J Rheumatol. 2016;45:169-170.
- Maillard H, Szczesniak S, Martin L. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;26:125-129.
- Lobo I, Ferreira M, Silva E. Cutaneous polyarteritis nodosa treated with intravenous immunoglobulin. J Eur Acad Dermatol Venereol. 2007;22:880-882.
- Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000;136:167-169.
- Campanilho-Marques R, Ramos F, Canhão H, et al. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Joint Bone Spine. 2014;81:277-278.
- Inoue N, Shimizu M, Mizuta M, et al. Refractory cutaneous polyarteritis nodosa: successful treatment with etanercept. Pediatr Int. 2017;59:751-752.
- Schartz NE. Successful treatment in two cases of steroid dependent cutaneous polyarteritis nodosa with low-dose methotrexate. Dermatology. 2001;203:336-338.
- Valor L, Monteagudo I, de la Torre I, et al. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod Rheumatol. 2014;24:688-689.
- Cvancara JL, Meffert JJ, Elston DM. Estrogen sensitive cutaneous polyarteritis nodosa: response to tamoxifen. J Am Acad Dermatol. 1998;39:643-646.
- Mazokopakis E, Milkas A, Tsartsalis A, et al. Improvement of cutaneous polyarteritis nodosa with hyperbaric oxygen. Int J Dermatol. 2009;48:1017-1029.
- Tursen U, Api H, Kaya TI, et al. Rapid healing of chronic leg ulcers during perilesional injections of granulocyte-macrophage colony stimulating factor in a patient with cutaneous polyarteritis nodosa. J Eur Acad Dermatol Venereol. 2006;20:1341-1343.
- Kluger N, Guillot B, Bessis D. Ulcerative cutaneous polyarteritis nodosa treated with mycophenolate mofetil and pentoxifylline. J Dermatolog Treat. 2011;22:175-177.
- Kawakami T, Soma Y. Use of warfarin therapy at a target international normalized ratio of 3.0 for cutaneous polyarteritis nodosa. J Am Acad Dermatol. 2010;63:602-606.
In 1931, Lindberg1 described a cutaneous variant of polyarteritis nodosa, which lacked visceral involvement and possessed a more favorable prognosis.2 Cutaneous polyarteritis nodosa (CPAN) is a localized small- to medium-vessel vasculitis restricted to the skin. Both benign and chronic courses have been described, and systemic involvement does not occur.3 Diagnostic criteria proposed by Nakamura et al3 in 2009 included cutaneous nodules, livedo reticularis, purpura, or ulcers; histopathologic fibrinoid necrotizing vasculitis of small- to medium-sized vessels; and exclusion of systemic symptoms (eg, fever, hypertension, weight loss, renal failure, cerebral hemorrhage, neuropathy, myocardial infarction, ischemic heart disease, pericarditis, pleuritis, arthralgia/myalgia). Nodules occur in 30% to 50% of cases and can remain for years if left untreated. Ulcerations occur in up to 30% of patients. Myositis, arthritis, and weakness also have been reported with this condition.4 Cutaneous polyarteritis nodosa has been associated with abnormal antibody testing with elevations of antiphospholipid cofactor antibody, lupus anticoagulant, anticardiolipin antibody, and anti-β2-glycoprotein I–dependent cardiolipin antibody, as well as elevated anti–phosphatidylserine-prothrombin complex antibody.5 These antibodies suggest increased risk for thrombosis and systemic diseases such as lupus or other autoimmune connective tissue disease. The distinction of this entity from systemic polyartertitis nodosa is key when determining treatment options and monitoring parameters.
Case Report
A 66-year-old woman was referred to our facility by an outside dermatologist with a mildly pruritic, blanchable, reticulated erythema on the chest and bilateral arms and legs of 3 months’ duration consistent with livedo reticularis (Figure 1). Prior systemic therapy included prednisone 10 mg 3 times daily, fexofenadine, loratadine, and hydroxyzine. When the systemic steroid was tapered, the patient developed an asymptomatic flare of her eruption. On presentation, the lesions had waxed and waned, and the patient was taking only vitamin B12 and vitamin C. Her medical history was notable for an unknown-type lymphoma of the chest wall diagnosed at 46 years of age that was treated with an unknown chemotherapeutic agent, chronic pancreatitis that resulted in a duodenectomy at 61 years of age, chronic cholecystitis, and 1 first-trimester miscarriage. Outside laboratory tests, including a comprehensive metabolic panel, complete blood cell count, urinalysis, renal function, and liver function tests were within reference range, except for the finding of mild leukocytosis (11,000/µL)(reference range, 3800–10,800/µL), which resolved after steroids were discontinued, with otherwise normal results. Punch biopsy of a specimen from the right thigh revealed medium-vessel vasculitis consistent with polyarteritis nodosa (Figure 2). Laboratory workup by our facility including hepatitis panel, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, factor V Leiden, prothrombin time/international normalized ratio, anticardiolipin antibody, and proteins C and S were all within reference range. Abnormal values included a low positive but nondiagnostic antinuclear antibody screen with negative titers, and the lupus anticoagulant titer was mildly elevated at 44 IgG binding units (reference range, <40 IgG binding units). Serum protein electrophoresis (SPEP) and urine protein electrophoresis also were performed, and SPEP was low positive for elevated κ and γ light chains. The patient was referred to oncology, and further testing revealed no underlying malignancy. The patient was monitored and no treatment was initiated; her rash completely resolved within 3 months. Laboratory monitoring at 6 months including SPEP, urine protein electrophoresis, lupus anticoagulant, and clotting studies all were within reference range.


Comment
Although the treatment of systemic polyarteritis nodosa often is necessary and typically involves high-dose corticosteroids and cyclophosphamide, the treatment of CPAN initially is less aggressive. Of the options available for treatment of CPAN, each has associated risks and side effects. Chen6 classified CPAN into 3 groups: 1 (mild), 2 (severe with no systemic involvement), and 3 (severe with progression to systemic disease)(Table). The authors performed a review of all the published treatments and their respective side effects to evaluate if treatment should be instituted for asymptomatic (group 1) disease presenting with abnormal antibody findings as demonstrated in our case.

First-line treatment of CPAN includes nonsteroidal anti-inflammatory drugs (NSAIDS) and colchicine.7 Nonsteroidal anti-inflammatory drugs are preferred; however, they also have been associated with gastrointestinal tract upset and increased risk for peptic ulcer disease with long-term use. Although colchicine often is used in conjunction with NSAIDS8 for its anti-inflammatory activity, no studies have been performed on this drug as monotherapy, and the side effect of diarrhea often limits its use.
Other therapies include dapsone, which should be monitored carefully due to the risk for dapsone hypersensitivity syndrome.8,9 Topical corticosteroids have been proven effective for mild cases of confluent erythema with remission occurring as early as 4 weeks.4 Some reports emphasize the role of streptococcal infections in CPAN, especially in children.8,10-12 Consequently it is recommended that anti–streptolysin O titers should be included in the workup for CPAN. Long-term penicillin prophylaxis and tonsillectomy have been used to prevent disease flares with limited success.8,10-12
For more severe disease, especially with neuromuscular involvement, oral methylprednisolone up to 1 mg/kg daily has been used and has proven effective in the control of acute exacerbations.7,13 However, the many adverse effects of systemic steroids limit their use long-term, and taper will often result in flare of disease.4,7 Medications used in conjunction with steroids include hydroxychloroquine, dapsone, azathioprine, cyclophosphamide, methotrexate, sulfapyridine, pentoxifylline, infliximab, etanercept, and intravenous immunoglobulin.4,9,12-17
Low-dose methotrexate has shown some improvement in skin disease with CPAN, but other case reports suggest that complete remission is not achieved with this drug.15,18 More studies are needed to assess the use of methotrexate for CPAN.
Immunomodulators have been used in multiple case reports with varying levels of success. Rogalski and Sticherling4 reported 3 cases that cleared with methylprednisolone plus azathioprine ranging from 4 weeks to 6 months; nausea limited tolerance of azathioprine in 1 case. Mycophenolate mofetil also was successfully used in 2 cases with clearance at 17 weeks and 6 months. In this series of cases, cyclosporine was ineffective for CPAN.4 Two case reports documented cutaneous clearance with cyclophosphamide in conjunction with prednisolone.9,10 No prospective trials have been performed on these medications, and immunosuppressants should only be considered in steroid-resistant cases.
The use of intravenous immunoglobulin has been reported effective in prior cases that showed resistance to more conventional trials of steroids, azathioprine, and/or cyclophosphamide.12,14 Intravenous immunoglobulin may be regarded as a treatment option for severe resistant disease. Several case reports also have documented success using tumor necrosis factor α blockers, particularly infliximab, as an adjunct to steroids and etanercept as both a steroid adjunct and monotherapy.16,17,19 More studies are necessary to evaluate these treatments.
Additionally, single case reports have outlined the use of other therapeutic agents, including tamoxifen (10 mg twice daily increased to 20 mg twice daily during episodes of breakthrough lesions),20 hyperbaric oxygen therapy (100% oxygen for 90 minutes 5 times weekly at 1.5 atm absolute followed by 2 weeks of 2 atm absolute),21 and granulocyte-macrophage colony-stimulating factor (300 µg injection in small portion to ulcer edges twice monthly for 2 months).22 All of these treatments show promise, but data are limited.
Because thrombosis is postulated to be a potential mechanism leading to CPAN, agents such as pentoxifylline, clopidogrel, and warfarin have been examined as treatment options. Pentoxifylline in combination with mycophenolate mofetil has been successful in treating a case that was resistant to other immunosuppressants.23 Clopidogrel blocks the adenosine diphosphate pathway and impairs clot retraction. Clopidogrel was reported effective in an acute flare of CPAN for clearance of skin lesions and normalization of lupus anticoagulant.24 It also was used successfully in recurrent CPAN after steroid treatments in a patient with neuromuscular symptoms. There was no recurrence in either of the patients in this case report series. Warfarin therapy at an international normalized ratio of 3.0 also has demonstrated success in halting disease progression and in facilitating the resolution of skin changes and normalization of anti–phosphatidylserine-prothrombin complex antibodies.24 Our review of the literature did not reveal evidence of a standardized length of treatment following symptom resolution or if treatment is indicated in asymptomatic disease, or as in our case, with only mild elevations of antiphospholipid antibodies.
Conclusion
Multiple treatment options exist for CPAN, but the data on their efficacies is limited and based only on anecdotal evidence, not prospective analysis. We believe that it seems reasonable to initiate treatment only for symptomatic disease or cases in which the antibody titers suggest that the patient may be at high risk for thrombosis. Mild symptoms and mild cutaneous changes would suggest the likely choice of NSAIDs, colchicine, or dapsone as treatment options versus no treatment. In patients with antibody titers, pentoxifylline, clopidogrel, or warfarin may be considered first-line therapies. With severe ulcerative lesions and neuromuscular involvement, steroids, immunosuppressants, and other investigative agents should be contemplated. In our patient, the laboratory studies were repeated and normalized on complete resolution of her livedo eruption. She remained asymptomatic and clear for 8 months without any treatment. The incidence of this presentation of CPAN is unknown and is likely underreported, as we would not expect most patients to present to their physicians for the evaluation of otherwise asymptomatic livedo reticularis. In essence, our case report suggests that it may be prudent to simply monitor patients with asymptomatic CPAN.
In 1931, Lindberg1 described a cutaneous variant of polyarteritis nodosa, which lacked visceral involvement and possessed a more favorable prognosis.2 Cutaneous polyarteritis nodosa (CPAN) is a localized small- to medium-vessel vasculitis restricted to the skin. Both benign and chronic courses have been described, and systemic involvement does not occur.3 Diagnostic criteria proposed by Nakamura et al3 in 2009 included cutaneous nodules, livedo reticularis, purpura, or ulcers; histopathologic fibrinoid necrotizing vasculitis of small- to medium-sized vessels; and exclusion of systemic symptoms (eg, fever, hypertension, weight loss, renal failure, cerebral hemorrhage, neuropathy, myocardial infarction, ischemic heart disease, pericarditis, pleuritis, arthralgia/myalgia). Nodules occur in 30% to 50% of cases and can remain for years if left untreated. Ulcerations occur in up to 30% of patients. Myositis, arthritis, and weakness also have been reported with this condition.4 Cutaneous polyarteritis nodosa has been associated with abnormal antibody testing with elevations of antiphospholipid cofactor antibody, lupus anticoagulant, anticardiolipin antibody, and anti-β2-glycoprotein I–dependent cardiolipin antibody, as well as elevated anti–phosphatidylserine-prothrombin complex antibody.5 These antibodies suggest increased risk for thrombosis and systemic diseases such as lupus or other autoimmune connective tissue disease. The distinction of this entity from systemic polyartertitis nodosa is key when determining treatment options and monitoring parameters.
Case Report
A 66-year-old woman was referred to our facility by an outside dermatologist with a mildly pruritic, blanchable, reticulated erythema on the chest and bilateral arms and legs of 3 months’ duration consistent with livedo reticularis (Figure 1). Prior systemic therapy included prednisone 10 mg 3 times daily, fexofenadine, loratadine, and hydroxyzine. When the systemic steroid was tapered, the patient developed an asymptomatic flare of her eruption. On presentation, the lesions had waxed and waned, and the patient was taking only vitamin B12 and vitamin C. Her medical history was notable for an unknown-type lymphoma of the chest wall diagnosed at 46 years of age that was treated with an unknown chemotherapeutic agent, chronic pancreatitis that resulted in a duodenectomy at 61 years of age, chronic cholecystitis, and 1 first-trimester miscarriage. Outside laboratory tests, including a comprehensive metabolic panel, complete blood cell count, urinalysis, renal function, and liver function tests were within reference range, except for the finding of mild leukocytosis (11,000/µL)(reference range, 3800–10,800/µL), which resolved after steroids were discontinued, with otherwise normal results. Punch biopsy of a specimen from the right thigh revealed medium-vessel vasculitis consistent with polyarteritis nodosa (Figure 2). Laboratory workup by our facility including hepatitis panel, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, factor V Leiden, prothrombin time/international normalized ratio, anticardiolipin antibody, and proteins C and S were all within reference range. Abnormal values included a low positive but nondiagnostic antinuclear antibody screen with negative titers, and the lupus anticoagulant titer was mildly elevated at 44 IgG binding units (reference range, <40 IgG binding units). Serum protein electrophoresis (SPEP) and urine protein electrophoresis also were performed, and SPEP was low positive for elevated κ and γ light chains. The patient was referred to oncology, and further testing revealed no underlying malignancy. The patient was monitored and no treatment was initiated; her rash completely resolved within 3 months. Laboratory monitoring at 6 months including SPEP, urine protein electrophoresis, lupus anticoagulant, and clotting studies all were within reference range.


Comment
Although the treatment of systemic polyarteritis nodosa often is necessary and typically involves high-dose corticosteroids and cyclophosphamide, the treatment of CPAN initially is less aggressive. Of the options available for treatment of CPAN, each has associated risks and side effects. Chen6 classified CPAN into 3 groups: 1 (mild), 2 (severe with no systemic involvement), and 3 (severe with progression to systemic disease)(Table). The authors performed a review of all the published treatments and their respective side effects to evaluate if treatment should be instituted for asymptomatic (group 1) disease presenting with abnormal antibody findings as demonstrated in our case.

First-line treatment of CPAN includes nonsteroidal anti-inflammatory drugs (NSAIDS) and colchicine.7 Nonsteroidal anti-inflammatory drugs are preferred; however, they also have been associated with gastrointestinal tract upset and increased risk for peptic ulcer disease with long-term use. Although colchicine often is used in conjunction with NSAIDS8 for its anti-inflammatory activity, no studies have been performed on this drug as monotherapy, and the side effect of diarrhea often limits its use.
Other therapies include dapsone, which should be monitored carefully due to the risk for dapsone hypersensitivity syndrome.8,9 Topical corticosteroids have been proven effective for mild cases of confluent erythema with remission occurring as early as 4 weeks.4 Some reports emphasize the role of streptococcal infections in CPAN, especially in children.8,10-12 Consequently it is recommended that anti–streptolysin O titers should be included in the workup for CPAN. Long-term penicillin prophylaxis and tonsillectomy have been used to prevent disease flares with limited success.8,10-12
For more severe disease, especially with neuromuscular involvement, oral methylprednisolone up to 1 mg/kg daily has been used and has proven effective in the control of acute exacerbations.7,13 However, the many adverse effects of systemic steroids limit their use long-term, and taper will often result in flare of disease.4,7 Medications used in conjunction with steroids include hydroxychloroquine, dapsone, azathioprine, cyclophosphamide, methotrexate, sulfapyridine, pentoxifylline, infliximab, etanercept, and intravenous immunoglobulin.4,9,12-17
Low-dose methotrexate has shown some improvement in skin disease with CPAN, but other case reports suggest that complete remission is not achieved with this drug.15,18 More studies are needed to assess the use of methotrexate for CPAN.
Immunomodulators have been used in multiple case reports with varying levels of success. Rogalski and Sticherling4 reported 3 cases that cleared with methylprednisolone plus azathioprine ranging from 4 weeks to 6 months; nausea limited tolerance of azathioprine in 1 case. Mycophenolate mofetil also was successfully used in 2 cases with clearance at 17 weeks and 6 months. In this series of cases, cyclosporine was ineffective for CPAN.4 Two case reports documented cutaneous clearance with cyclophosphamide in conjunction with prednisolone.9,10 No prospective trials have been performed on these medications, and immunosuppressants should only be considered in steroid-resistant cases.
The use of intravenous immunoglobulin has been reported effective in prior cases that showed resistance to more conventional trials of steroids, azathioprine, and/or cyclophosphamide.12,14 Intravenous immunoglobulin may be regarded as a treatment option for severe resistant disease. Several case reports also have documented success using tumor necrosis factor α blockers, particularly infliximab, as an adjunct to steroids and etanercept as both a steroid adjunct and monotherapy.16,17,19 More studies are necessary to evaluate these treatments.
Additionally, single case reports have outlined the use of other therapeutic agents, including tamoxifen (10 mg twice daily increased to 20 mg twice daily during episodes of breakthrough lesions),20 hyperbaric oxygen therapy (100% oxygen for 90 minutes 5 times weekly at 1.5 atm absolute followed by 2 weeks of 2 atm absolute),21 and granulocyte-macrophage colony-stimulating factor (300 µg injection in small portion to ulcer edges twice monthly for 2 months).22 All of these treatments show promise, but data are limited.
Because thrombosis is postulated to be a potential mechanism leading to CPAN, agents such as pentoxifylline, clopidogrel, and warfarin have been examined as treatment options. Pentoxifylline in combination with mycophenolate mofetil has been successful in treating a case that was resistant to other immunosuppressants.23 Clopidogrel blocks the adenosine diphosphate pathway and impairs clot retraction. Clopidogrel was reported effective in an acute flare of CPAN for clearance of skin lesions and normalization of lupus anticoagulant.24 It also was used successfully in recurrent CPAN after steroid treatments in a patient with neuromuscular symptoms. There was no recurrence in either of the patients in this case report series. Warfarin therapy at an international normalized ratio of 3.0 also has demonstrated success in halting disease progression and in facilitating the resolution of skin changes and normalization of anti–phosphatidylserine-prothrombin complex antibodies.24 Our review of the literature did not reveal evidence of a standardized length of treatment following symptom resolution or if treatment is indicated in asymptomatic disease, or as in our case, with only mild elevations of antiphospholipid antibodies.
Conclusion
Multiple treatment options exist for CPAN, but the data on their efficacies is limited and based only on anecdotal evidence, not prospective analysis. We believe that it seems reasonable to initiate treatment only for symptomatic disease or cases in which the antibody titers suggest that the patient may be at high risk for thrombosis. Mild symptoms and mild cutaneous changes would suggest the likely choice of NSAIDs, colchicine, or dapsone as treatment options versus no treatment. In patients with antibody titers, pentoxifylline, clopidogrel, or warfarin may be considered first-line therapies. With severe ulcerative lesions and neuromuscular involvement, steroids, immunosuppressants, and other investigative agents should be contemplated. In our patient, the laboratory studies were repeated and normalized on complete resolution of her livedo eruption. She remained asymptomatic and clear for 8 months without any treatment. The incidence of this presentation of CPAN is unknown and is likely underreported, as we would not expect most patients to present to their physicians for the evaluation of otherwise asymptomatic livedo reticularis. In essence, our case report suggests that it may be prudent to simply monitor patients with asymptomatic CPAN.
- Lindberg K. Ein Beitrag zur Kenntnis der Periarteritis nodosa. Acta Med Scand. 1931;76:183-225.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa [published online August 26, 2005]. J Neurol. 2005;252:1155-1166.
- Nakamura T, Kanazawa N, Ikeda T, et al. Cutaneous polyarteritis nodosa: revisiting its definition and diagnostic criteria. Arch Dermatol Res. 2009;301:117-121.
- Rogalski C, Sticherling M. Panateritis cutanea benigna—an entity limited to the skin or cutaneous presentation of a systemic necrotizing vasculitis? report of seven cases and review of the literature. Int J Dermatol. 2007;46:817-821.
- Kawakami T, Yamazaki M, Mizoguchi M, et al. High titer of anti-phosphatidylserine-prothrombin complex antibodies in patients with cutaneous polyarteritis nodosa. Arthritis Rheum. 2007;57:1507-1513.
- Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;6:429-442.
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756.
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathologic analysis and review of the published work. J Dermatol. 2010;37:85-93.
- Flanagan N, Casey EB, Watson R, et al. Cutaneous polyartertitis nodosa with seronegative arthritis. Rheumatology (Oxford). 1999;38:1161-1162.
- Fathalla B, Miller L, Brady S, et al. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724-728.
- Misago N, Mochizuki Y, Sekiyama-Kodera H, et al. Cutaneous polyarteritis nodosa: therapy and clinical course in four cases. J Dermatol. 2001;28:719-727.
- Breda L, Franchini S, Marzetti V, et al. Intravenous immunoglobulins for cutaneous polyarteritis nodosa resistant to conventional treatment. Scand J Rheumatol. 2016;45:169-170.
- Maillard H, Szczesniak S, Martin L. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;26:125-129.
- Lobo I, Ferreira M, Silva E. Cutaneous polyarteritis nodosa treated with intravenous immunoglobulin. J Eur Acad Dermatol Venereol. 2007;22:880-882.
- Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000;136:167-169.
- Campanilho-Marques R, Ramos F, Canhão H, et al. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Joint Bone Spine. 2014;81:277-278.
- Inoue N, Shimizu M, Mizuta M, et al. Refractory cutaneous polyarteritis nodosa: successful treatment with etanercept. Pediatr Int. 2017;59:751-752.
- Schartz NE. Successful treatment in two cases of steroid dependent cutaneous polyarteritis nodosa with low-dose methotrexate. Dermatology. 2001;203:336-338.
- Valor L, Monteagudo I, de la Torre I, et al. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod Rheumatol. 2014;24:688-689.
- Cvancara JL, Meffert JJ, Elston DM. Estrogen sensitive cutaneous polyarteritis nodosa: response to tamoxifen. J Am Acad Dermatol. 1998;39:643-646.
- Mazokopakis E, Milkas A, Tsartsalis A, et al. Improvement of cutaneous polyarteritis nodosa with hyperbaric oxygen. Int J Dermatol. 2009;48:1017-1029.
- Tursen U, Api H, Kaya TI, et al. Rapid healing of chronic leg ulcers during perilesional injections of granulocyte-macrophage colony stimulating factor in a patient with cutaneous polyarteritis nodosa. J Eur Acad Dermatol Venereol. 2006;20:1341-1343.
- Kluger N, Guillot B, Bessis D. Ulcerative cutaneous polyarteritis nodosa treated with mycophenolate mofetil and pentoxifylline. J Dermatolog Treat. 2011;22:175-177.
- Kawakami T, Soma Y. Use of warfarin therapy at a target international normalized ratio of 3.0 for cutaneous polyarteritis nodosa. J Am Acad Dermatol. 2010;63:602-606.
- Lindberg K. Ein Beitrag zur Kenntnis der Periarteritis nodosa. Acta Med Scand. 1931;76:183-225.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa [published online August 26, 2005]. J Neurol. 2005;252:1155-1166.
- Nakamura T, Kanazawa N, Ikeda T, et al. Cutaneous polyarteritis nodosa: revisiting its definition and diagnostic criteria. Arch Dermatol Res. 2009;301:117-121.
- Rogalski C, Sticherling M. Panateritis cutanea benigna—an entity limited to the skin or cutaneous presentation of a systemic necrotizing vasculitis? report of seven cases and review of the literature. Int J Dermatol. 2007;46:817-821.
- Kawakami T, Yamazaki M, Mizoguchi M, et al. High titer of anti-phosphatidylserine-prothrombin complex antibodies in patients with cutaneous polyarteritis nodosa. Arthritis Rheum. 2007;57:1507-1513.
- Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;6:429-442.
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756.
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathologic analysis and review of the published work. J Dermatol. 2010;37:85-93.
- Flanagan N, Casey EB, Watson R, et al. Cutaneous polyartertitis nodosa with seronegative arthritis. Rheumatology (Oxford). 1999;38:1161-1162.
- Fathalla B, Miller L, Brady S, et al. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724-728.
- Misago N, Mochizuki Y, Sekiyama-Kodera H, et al. Cutaneous polyarteritis nodosa: therapy and clinical course in four cases. J Dermatol. 2001;28:719-727.
- Breda L, Franchini S, Marzetti V, et al. Intravenous immunoglobulins for cutaneous polyarteritis nodosa resistant to conventional treatment. Scand J Rheumatol. 2016;45:169-170.
- Maillard H, Szczesniak S, Martin L. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;26:125-129.
- Lobo I, Ferreira M, Silva E. Cutaneous polyarteritis nodosa treated with intravenous immunoglobulin. J Eur Acad Dermatol Venereol. 2007;22:880-882.
- Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000;136:167-169.
- Campanilho-Marques R, Ramos F, Canhão H, et al. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Joint Bone Spine. 2014;81:277-278.
- Inoue N, Shimizu M, Mizuta M, et al. Refractory cutaneous polyarteritis nodosa: successful treatment with etanercept. Pediatr Int. 2017;59:751-752.
- Schartz NE. Successful treatment in two cases of steroid dependent cutaneous polyarteritis nodosa with low-dose methotrexate. Dermatology. 2001;203:336-338.
- Valor L, Monteagudo I, de la Torre I, et al. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod Rheumatol. 2014;24:688-689.
- Cvancara JL, Meffert JJ, Elston DM. Estrogen sensitive cutaneous polyarteritis nodosa: response to tamoxifen. J Am Acad Dermatol. 1998;39:643-646.
- Mazokopakis E, Milkas A, Tsartsalis A, et al. Improvement of cutaneous polyarteritis nodosa with hyperbaric oxygen. Int J Dermatol. 2009;48:1017-1029.
- Tursen U, Api H, Kaya TI, et al. Rapid healing of chronic leg ulcers during perilesional injections of granulocyte-macrophage colony stimulating factor in a patient with cutaneous polyarteritis nodosa. J Eur Acad Dermatol Venereol. 2006;20:1341-1343.
- Kluger N, Guillot B, Bessis D. Ulcerative cutaneous polyarteritis nodosa treated with mycophenolate mofetil and pentoxifylline. J Dermatolog Treat. 2011;22:175-177.
- Kawakami T, Soma Y. Use of warfarin therapy at a target international normalized ratio of 3.0 for cutaneous polyarteritis nodosa. J Am Acad Dermatol. 2010;63:602-606.
Practice Points
- Cutaneous polyarteritis nodosa should be in the differential of new-onset livedo reticularis.
- Workup with biopsy and specific blood work is important.
- Treatment options at this time are limited.
Low-grade fever, erythematous rash in pregnant woman • Dx?
THE CASE
A 31-year-old woman presented to her obstetrician’s office at 16 weeks’ gestation with a 2-day history of low-grade fever and an erythematous rash measuring 1 x 4 cm on her right groin. She had a medical history of a penicillin allergy (urticaria) and her outdoor activities included gardening and picnicking.
We suspected that she was experiencing an allergic reaction and recommended an antihistamine (diphenhydramine). The patient returned 4 days later with new symptoms including headache, photophobia, neck pain, unilateral large joint pain, and periorbital cellulitis, as well as expansion of her rash. She was afebrile and an examination revealed that the 1 x 4 cm rash on her groin had grown; it was now a demarcated erythematous rash with faint central clearing measuring 5 x 8 cm. Right periorbital erythema and nuchal rigidity were also noted.
Because of her expanding rash and nuchal rigidity, we suspected Lyme meningitis and we referred her to the emergency department. Within 24 hours, the rash had spread to her abdomen, thigh, and wrist, and was consistent with erythema migrans.
Laboratory evaluation revealed an increased number of white bloods cells (13.5 million cells/mcL; normal range 4.5-11.0 million cells/mcL), and an increased number of neutrophils (10.8 million cells/mcL; normal range 1.8-8 million cells/mcL), indicating leukocytosis with a left shift. Lab tests also revealed a low hemoglobin level (10.6 g/dL; normal range 12-16 g/dL) and a mean corpuscular volume of 85.6 fL/red cell (normal range 80-100 fL/red cell), indicating microcytic anemia. A lumbar puncture was negative for disseminated Lyme disease by Gram stain, culture, and polymerase chain reaction.
THE DIAGNOSIS
A diagnosis of Lyme disease was confirmed with a positive Lyme titer serology via an enzyme-linked immunosorbent assay. The rash and other symptoms responded promptly to intravenous ceftriaxone 2 g, and the patient was discharged home on oral cefuroxime 500 mg bid for 14 days.
DISCUSSION
Lyme disease is the most common vector-borne illness in the United States, concentrated heavily in the Northeast and upper Midwest.1 The most recent information released by the Centers for Disease Control and Prevention (CDC) lists Vermont, Maine, Pennsylvania, Rhode Island, Connecticut, New Jersey, Massachusetts, Delaware, New Hampshire, and Minnesota as the states with the highest incidence of Lyme disease.2
The number of reported cases in the United States has increased over the past 2 decades, from approximately 11,000 in 1995 to about 28,000 in 2015.3 Over the past year, we have seen several cases of Lyme disease in the obstetric population of our own practice.
Prompt treatment is crucial. Pregnant women who are acutely infected with Borrelia burgdorferi (the primary cause of Lyme disease) and do not receive treatment have experienced multiple adverse pregnancy outcomes, including preterm delivery, infants born with rash, and stillbirth.4 Additional concern exists for fetal cardiac anomalies, with data showing that there are twice as many cardiac defects in children born to mothers residing in endemic regions.5
What animal studies have taught us about Lyme disease
The potential causal relationship between Lyme disease and fetal demise was first studied in 2007. This case report involved the stillbirth of a full-term baby from an acutely infected woman who did not receive treatment. She experienced erythema multiforme 6 weeks prior to delivery.6
The vast majority of research on Lyme disease in pregnancy comes from work with mice and dogs. These studies confirmed that acute infection with Lyme disease is associated with an increased risk of adverse fetal outcomes, specifically fetal death.7
Silver et al further researched the association using murine models in the 1980s. They found that fetal death occurred in 12% of acutely infected mice, compared with none of the mice that were chronically infected.7
In 2010, Lakos and Solymosi examined the effects of Lyme disease on pregnancy outcomes in acutely infected women. Seven out of 95 pregnant women acutely infected with B burgdorferi experienced fetal demise, further supporting the association seen in animal experiments.8
Treating pregnant patients
Doxycycline and tetracycline, which are routinely used to treat Lyme disease, are not appropriate in the obstetric population. The CDC recommends up to a 3-week course of antibiotics; the standard regimen is amoxicillin 500 mg by mouth tid. For women who are allergic to penicillin, as was the case with our patient, cefuroxime 500 mg by mouth bid is the treatment of choice.9
Our patient underwent a detailed ultrasound at 21 weeks, which revealed normal fetal anatomy and no evidence of cardiac malformations. The remainder of her pregnancy was uncomplicated, and she gave birth vaginally at 41 weeks to a baby boy weighing 3700 g.
THE TAKEAWAY
There is a need to increase awareness of Lyme disease in pregnancy on a national level. It is the responsibility of every practitioner caring for obstetric patients in endemic regions to address new-onset rash promptly. There have been cases of women who experienced erythema migrans and arthralgias after exposure to a tick bite, later delivering infants with cardiac anomalies such as atrial and ventricular septal defects.10 In obstetric patients acutely infected during the first trimester, a fetal echocardiogram is reasonable, given the demonstrated high potential for fetal cardiac anomalies.
Preventing adverse fetal outcomes requires early treatment with antibiotics. The CDC maintains that there have been no life-threatening adverse fetal effects from Lyme disease seen in women who are appropriately treated, as well as no transmission of Lyme disease in the breast milk of lactating mothers.9
1. Centers for Disease Control and Prevention. Lyme disease. Data and statistics. Available at: https://www.cdc.gov/lyme/stats/. Accessed July 5, 2017.
2. Centers for Disease Control and Prevention. Lyme disease data tables. Reported cases of Lyme disease by state or locality, 2005-2015. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html. Accessed July 5, 2017.
3. Centers for Disease Control and Prevention. Lyme disease graphs. Reported cases of Lyme disease by year, United States, 1995-2015. Available at: https://www.cdc.gov/lyme/stats/graphs.html. Accessed July 5, 2017.
4. Maraspin V, Cimperman J, Lotric-Furlan S, et al. Erythema migrans in pregnancy. Wien Klin Wochenschr. 1999;111:933-940.
5. Strobino BA, Williams CL, Abid S, et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367-374.
6. Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27-2007. A 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918-925.
7. Silver RM, Yang L, Daynes RA, et al. Fetal outcome in murine Lyme disease. Infect Immun. 1995;63:66-72.
8. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcomes. Int J Infect Dis. 2010;14:e494-e498.
9. Centers for Disease Control and Prevention. Ticks and Lyme disease. Pregnancy and Lyme disease. Available at: https://www.cdc.gov/lyme/resources/toolkit/factsheets/10_508_Lyme%20disease_PregnantWoman_FACTSheet.pdf. Accessed July 5, 2017.
10. O’Brien JM, Martens MG. Lyme disease in pregnancy: a New Jersey medical advisory. MD Advis. 2014;7:24-27.
THE CASE
A 31-year-old woman presented to her obstetrician’s office at 16 weeks’ gestation with a 2-day history of low-grade fever and an erythematous rash measuring 1 x 4 cm on her right groin. She had a medical history of a penicillin allergy (urticaria) and her outdoor activities included gardening and picnicking.
We suspected that she was experiencing an allergic reaction and recommended an antihistamine (diphenhydramine). The patient returned 4 days later with new symptoms including headache, photophobia, neck pain, unilateral large joint pain, and periorbital cellulitis, as well as expansion of her rash. She was afebrile and an examination revealed that the 1 x 4 cm rash on her groin had grown; it was now a demarcated erythematous rash with faint central clearing measuring 5 x 8 cm. Right periorbital erythema and nuchal rigidity were also noted.
Because of her expanding rash and nuchal rigidity, we suspected Lyme meningitis and we referred her to the emergency department. Within 24 hours, the rash had spread to her abdomen, thigh, and wrist, and was consistent with erythema migrans.
Laboratory evaluation revealed an increased number of white bloods cells (13.5 million cells/mcL; normal range 4.5-11.0 million cells/mcL), and an increased number of neutrophils (10.8 million cells/mcL; normal range 1.8-8 million cells/mcL), indicating leukocytosis with a left shift. Lab tests also revealed a low hemoglobin level (10.6 g/dL; normal range 12-16 g/dL) and a mean corpuscular volume of 85.6 fL/red cell (normal range 80-100 fL/red cell), indicating microcytic anemia. A lumbar puncture was negative for disseminated Lyme disease by Gram stain, culture, and polymerase chain reaction.
THE DIAGNOSIS
A diagnosis of Lyme disease was confirmed with a positive Lyme titer serology via an enzyme-linked immunosorbent assay. The rash and other symptoms responded promptly to intravenous ceftriaxone 2 g, and the patient was discharged home on oral cefuroxime 500 mg bid for 14 days.
DISCUSSION
Lyme disease is the most common vector-borne illness in the United States, concentrated heavily in the Northeast and upper Midwest.1 The most recent information released by the Centers for Disease Control and Prevention (CDC) lists Vermont, Maine, Pennsylvania, Rhode Island, Connecticut, New Jersey, Massachusetts, Delaware, New Hampshire, and Minnesota as the states with the highest incidence of Lyme disease.2
The number of reported cases in the United States has increased over the past 2 decades, from approximately 11,000 in 1995 to about 28,000 in 2015.3 Over the past year, we have seen several cases of Lyme disease in the obstetric population of our own practice.
Prompt treatment is crucial. Pregnant women who are acutely infected with Borrelia burgdorferi (the primary cause of Lyme disease) and do not receive treatment have experienced multiple adverse pregnancy outcomes, including preterm delivery, infants born with rash, and stillbirth.4 Additional concern exists for fetal cardiac anomalies, with data showing that there are twice as many cardiac defects in children born to mothers residing in endemic regions.5
What animal studies have taught us about Lyme disease
The potential causal relationship between Lyme disease and fetal demise was first studied in 2007. This case report involved the stillbirth of a full-term baby from an acutely infected woman who did not receive treatment. She experienced erythema multiforme 6 weeks prior to delivery.6
The vast majority of research on Lyme disease in pregnancy comes from work with mice and dogs. These studies confirmed that acute infection with Lyme disease is associated with an increased risk of adverse fetal outcomes, specifically fetal death.7
Silver et al further researched the association using murine models in the 1980s. They found that fetal death occurred in 12% of acutely infected mice, compared with none of the mice that were chronically infected.7
In 2010, Lakos and Solymosi examined the effects of Lyme disease on pregnancy outcomes in acutely infected women. Seven out of 95 pregnant women acutely infected with B burgdorferi experienced fetal demise, further supporting the association seen in animal experiments.8
Treating pregnant patients
Doxycycline and tetracycline, which are routinely used to treat Lyme disease, are not appropriate in the obstetric population. The CDC recommends up to a 3-week course of antibiotics; the standard regimen is amoxicillin 500 mg by mouth tid. For women who are allergic to penicillin, as was the case with our patient, cefuroxime 500 mg by mouth bid is the treatment of choice.9
Our patient underwent a detailed ultrasound at 21 weeks, which revealed normal fetal anatomy and no evidence of cardiac malformations. The remainder of her pregnancy was uncomplicated, and she gave birth vaginally at 41 weeks to a baby boy weighing 3700 g.
THE TAKEAWAY
There is a need to increase awareness of Lyme disease in pregnancy on a national level. It is the responsibility of every practitioner caring for obstetric patients in endemic regions to address new-onset rash promptly. There have been cases of women who experienced erythema migrans and arthralgias after exposure to a tick bite, later delivering infants with cardiac anomalies such as atrial and ventricular septal defects.10 In obstetric patients acutely infected during the first trimester, a fetal echocardiogram is reasonable, given the demonstrated high potential for fetal cardiac anomalies.
Preventing adverse fetal outcomes requires early treatment with antibiotics. The CDC maintains that there have been no life-threatening adverse fetal effects from Lyme disease seen in women who are appropriately treated, as well as no transmission of Lyme disease in the breast milk of lactating mothers.9
THE CASE
A 31-year-old woman presented to her obstetrician’s office at 16 weeks’ gestation with a 2-day history of low-grade fever and an erythematous rash measuring 1 x 4 cm on her right groin. She had a medical history of a penicillin allergy (urticaria) and her outdoor activities included gardening and picnicking.
We suspected that she was experiencing an allergic reaction and recommended an antihistamine (diphenhydramine). The patient returned 4 days later with new symptoms including headache, photophobia, neck pain, unilateral large joint pain, and periorbital cellulitis, as well as expansion of her rash. She was afebrile and an examination revealed that the 1 x 4 cm rash on her groin had grown; it was now a demarcated erythematous rash with faint central clearing measuring 5 x 8 cm. Right periorbital erythema and nuchal rigidity were also noted.
Because of her expanding rash and nuchal rigidity, we suspected Lyme meningitis and we referred her to the emergency department. Within 24 hours, the rash had spread to her abdomen, thigh, and wrist, and was consistent with erythema migrans.
Laboratory evaluation revealed an increased number of white bloods cells (13.5 million cells/mcL; normal range 4.5-11.0 million cells/mcL), and an increased number of neutrophils (10.8 million cells/mcL; normal range 1.8-8 million cells/mcL), indicating leukocytosis with a left shift. Lab tests also revealed a low hemoglobin level (10.6 g/dL; normal range 12-16 g/dL) and a mean corpuscular volume of 85.6 fL/red cell (normal range 80-100 fL/red cell), indicating microcytic anemia. A lumbar puncture was negative for disseminated Lyme disease by Gram stain, culture, and polymerase chain reaction.
THE DIAGNOSIS
A diagnosis of Lyme disease was confirmed with a positive Lyme titer serology via an enzyme-linked immunosorbent assay. The rash and other symptoms responded promptly to intravenous ceftriaxone 2 g, and the patient was discharged home on oral cefuroxime 500 mg bid for 14 days.
DISCUSSION
Lyme disease is the most common vector-borne illness in the United States, concentrated heavily in the Northeast and upper Midwest.1 The most recent information released by the Centers for Disease Control and Prevention (CDC) lists Vermont, Maine, Pennsylvania, Rhode Island, Connecticut, New Jersey, Massachusetts, Delaware, New Hampshire, and Minnesota as the states with the highest incidence of Lyme disease.2
The number of reported cases in the United States has increased over the past 2 decades, from approximately 11,000 in 1995 to about 28,000 in 2015.3 Over the past year, we have seen several cases of Lyme disease in the obstetric population of our own practice.
Prompt treatment is crucial. Pregnant women who are acutely infected with Borrelia burgdorferi (the primary cause of Lyme disease) and do not receive treatment have experienced multiple adverse pregnancy outcomes, including preterm delivery, infants born with rash, and stillbirth.4 Additional concern exists for fetal cardiac anomalies, with data showing that there are twice as many cardiac defects in children born to mothers residing in endemic regions.5
What animal studies have taught us about Lyme disease
The potential causal relationship between Lyme disease and fetal demise was first studied in 2007. This case report involved the stillbirth of a full-term baby from an acutely infected woman who did not receive treatment. She experienced erythema multiforme 6 weeks prior to delivery.6
The vast majority of research on Lyme disease in pregnancy comes from work with mice and dogs. These studies confirmed that acute infection with Lyme disease is associated with an increased risk of adverse fetal outcomes, specifically fetal death.7
Silver et al further researched the association using murine models in the 1980s. They found that fetal death occurred in 12% of acutely infected mice, compared with none of the mice that were chronically infected.7
In 2010, Lakos and Solymosi examined the effects of Lyme disease on pregnancy outcomes in acutely infected women. Seven out of 95 pregnant women acutely infected with B burgdorferi experienced fetal demise, further supporting the association seen in animal experiments.8
Treating pregnant patients
Doxycycline and tetracycline, which are routinely used to treat Lyme disease, are not appropriate in the obstetric population. The CDC recommends up to a 3-week course of antibiotics; the standard regimen is amoxicillin 500 mg by mouth tid. For women who are allergic to penicillin, as was the case with our patient, cefuroxime 500 mg by mouth bid is the treatment of choice.9
Our patient underwent a detailed ultrasound at 21 weeks, which revealed normal fetal anatomy and no evidence of cardiac malformations. The remainder of her pregnancy was uncomplicated, and she gave birth vaginally at 41 weeks to a baby boy weighing 3700 g.
THE TAKEAWAY
There is a need to increase awareness of Lyme disease in pregnancy on a national level. It is the responsibility of every practitioner caring for obstetric patients in endemic regions to address new-onset rash promptly. There have been cases of women who experienced erythema migrans and arthralgias after exposure to a tick bite, later delivering infants with cardiac anomalies such as atrial and ventricular septal defects.10 In obstetric patients acutely infected during the first trimester, a fetal echocardiogram is reasonable, given the demonstrated high potential for fetal cardiac anomalies.
Preventing adverse fetal outcomes requires early treatment with antibiotics. The CDC maintains that there have been no life-threatening adverse fetal effects from Lyme disease seen in women who are appropriately treated, as well as no transmission of Lyme disease in the breast milk of lactating mothers.9
1. Centers for Disease Control and Prevention. Lyme disease. Data and statistics. Available at: https://www.cdc.gov/lyme/stats/. Accessed July 5, 2017.
2. Centers for Disease Control and Prevention. Lyme disease data tables. Reported cases of Lyme disease by state or locality, 2005-2015. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html. Accessed July 5, 2017.
3. Centers for Disease Control and Prevention. Lyme disease graphs. Reported cases of Lyme disease by year, United States, 1995-2015. Available at: https://www.cdc.gov/lyme/stats/graphs.html. Accessed July 5, 2017.
4. Maraspin V, Cimperman J, Lotric-Furlan S, et al. Erythema migrans in pregnancy. Wien Klin Wochenschr. 1999;111:933-940.
5. Strobino BA, Williams CL, Abid S, et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367-374.
6. Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27-2007. A 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918-925.
7. Silver RM, Yang L, Daynes RA, et al. Fetal outcome in murine Lyme disease. Infect Immun. 1995;63:66-72.
8. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcomes. Int J Infect Dis. 2010;14:e494-e498.
9. Centers for Disease Control and Prevention. Ticks and Lyme disease. Pregnancy and Lyme disease. Available at: https://www.cdc.gov/lyme/resources/toolkit/factsheets/10_508_Lyme%20disease_PregnantWoman_FACTSheet.pdf. Accessed July 5, 2017.
10. O’Brien JM, Martens MG. Lyme disease in pregnancy: a New Jersey medical advisory. MD Advis. 2014;7:24-27.
1. Centers for Disease Control and Prevention. Lyme disease. Data and statistics. Available at: https://www.cdc.gov/lyme/stats/. Accessed July 5, 2017.
2. Centers for Disease Control and Prevention. Lyme disease data tables. Reported cases of Lyme disease by state or locality, 2005-2015. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html. Accessed July 5, 2017.
3. Centers for Disease Control and Prevention. Lyme disease graphs. Reported cases of Lyme disease by year, United States, 1995-2015. Available at: https://www.cdc.gov/lyme/stats/graphs.html. Accessed July 5, 2017.
4. Maraspin V, Cimperman J, Lotric-Furlan S, et al. Erythema migrans in pregnancy. Wien Klin Wochenschr. 1999;111:933-940.
5. Strobino BA, Williams CL, Abid S, et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367-374.
6. Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27-2007. A 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918-925.
7. Silver RM, Yang L, Daynes RA, et al. Fetal outcome in murine Lyme disease. Infect Immun. 1995;63:66-72.
8. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcomes. Int J Infect Dis. 2010;14:e494-e498.
9. Centers for Disease Control and Prevention. Ticks and Lyme disease. Pregnancy and Lyme disease. Available at: https://www.cdc.gov/lyme/resources/toolkit/factsheets/10_508_Lyme%20disease_PregnantWoman_FACTSheet.pdf. Accessed July 5, 2017.
10. O’Brien JM, Martens MG. Lyme disease in pregnancy: a New Jersey medical advisory. MD Advis. 2014;7:24-27.
Active 46-year-old man with right-sided visual loss and no family history of stroke • Dx?
THE CASE
A 46-year-old man presented to the emergency department (ED) with sudden-onset right-sided visual loss. He had a history of asthma, but no family history of hypercoagulability, deep vein thrombosis (DVT), or stroke. The patient had an active lifestyle that included scuba diving, mountain biking, and hockey (coaching and playing). The physical examination revealed a right homonymous upper quadrantanopia. The neurologic examination was within normal limits, except for the visual deficit and unequal pupil size. A computerized tomography scan of the patient’s head did not reveal any lesions.
Based on the patient’s clinical picture, the ED physician prescribed alteplase, a tissue plasminogen activator (tPA), and admitted him to the intensive care unit for monitoring.
Subsequent magnetic resonance imaging (MRI) of the brain showed multiple small areas of acute infarct in the posterior circulation territory bilaterally, with involvement of small portions of the bilateral cerebellar hemispheres and parts of the left occipital lobe (FIGURE 1A and 1B).
An electrocardiogram showed no evidence of atrial fibrillation, and hypercoagulability studies were within normal limits. There was no evidence of May-Thurner anatomy, and an ultrasound of the lower extremities showed no DVT.
THE DIAGNOSIS
An echocardiogram with bubble study confirmed a diagnosis of patent foramen ovale (PFO) with bidirectional flow, a normal ejection fraction, and no evidence of left ventricular or left atrial thrombus. We started the patient on the anticoagulant enoxaparin 70 mg bid bridged with warfarin 5 mg/d.
Taking the patient’s active lifestyle into consideration, he was approved for PFO closure by the PFO committee and underwent closure. Following treatment, the patient was left with a residual 2-mm blind spot in the right visual field. At a 2-year follow-up visit, he showed no new focal deficits or recurrent symptoms.
DISCUSSION
Since 1988 when Lechat et al reported increased incidence of PFO in young stroke patients,1 many studies have supported the association between PFO and cryptogenic stroke (CS) in young adults.2 Because it remained controversial as to whether PFO is a risk factor for stroke or transient ischemic attack recurrence,3 researchers investigated PFO closure as a preventive measure to decrease stroke recurrence in patients with both CS and PFO.
A 2012 meta-analysis showed possible benefits of closure compared with medical management using antiplatelet or anticoagulation therapies.4 However, these results were not supported by results of other studies. These include the CLOSURE I trial,5 which compared device closure of PFO with medical therapy, and the RESPECT6 and PC trials,7 which did not show a significant difference in the primary end point of recurrent stroke between patients who received medical therapy and those who had PFO closure.
American Heart Association/American Stroke Association’s 2011 guidelines recommend only antiplatelet therapy for patients with CS and PFO.8 While there is consensus that surgical closure is not better than a medical approach to patients with CS and PFO, cases should be individualized, as a patient’s clinical or social factors may dictate otherwise.
Lifestyle may warrant PFO closure
No previous studies have considered occupation or hobbies as an indication for PFO closure in patients with CS. Our patient’s active lifestyle, particularly his scuba diving and participation in contact sports, made him a poor candidate for anticoagulation. Scuba diving is associated with decompression sickness and air emboli, which can be a mechanism of cerebral ischemia, especially in patients with a right-to-left shunt, such as with PFO.9
We did not observe a strong temporal relationship between diving and stroke in our patient. MRI findings suggested that he had multiple minor embolic events over time, which is consistent with a prior case report.9 This suggested air emboli as a possible source of stroke, in which case, our patient might not benefit from antiplatelet or anticoagulation therapy.
THE TAKEAWAY
This case illustrates the importance of a thorough social history and knowledge of the patient’s hobbies, occupation, and preferences in evaluating and treating individuals with CS associated with PFO. The current literature does not provide complete answers to the cause, diagnosis, and management of CS; additional research is needed.
The work-up involved in defining the etiology of stroke includes, but is not limited to, head and brain imaging, an echocardiogram, hypercoagulability tests, and vascular imaging. The work of Sanna et al showed that approximately 12% of patients with CS have atrial fibrillation when monitored over a one-year period, suggesting atrial fibrillation as a possible cause in some cases.10
As the case described here demonstrates, further research is warranted regarding how a patient’s occupation and lifestyle factor into decision-making for patients with PFO.
1. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152.
2. Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085-1096.
3. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol. 2010;257:1777-1787.
4. Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431.
5. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276.
9. Menkin M, Schwartzman RJ. Cerebral air embolism. Report of five cases and review of the literature. Arch Neurol. 1977;34:168-170.
10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
THE CASE
A 46-year-old man presented to the emergency department (ED) with sudden-onset right-sided visual loss. He had a history of asthma, but no family history of hypercoagulability, deep vein thrombosis (DVT), or stroke. The patient had an active lifestyle that included scuba diving, mountain biking, and hockey (coaching and playing). The physical examination revealed a right homonymous upper quadrantanopia. The neurologic examination was within normal limits, except for the visual deficit and unequal pupil size. A computerized tomography scan of the patient’s head did not reveal any lesions.
Based on the patient’s clinical picture, the ED physician prescribed alteplase, a tissue plasminogen activator (tPA), and admitted him to the intensive care unit for monitoring.
Subsequent magnetic resonance imaging (MRI) of the brain showed multiple small areas of acute infarct in the posterior circulation territory bilaterally, with involvement of small portions of the bilateral cerebellar hemispheres and parts of the left occipital lobe (FIGURE 1A and 1B).

An electrocardiogram showed no evidence of atrial fibrillation, and hypercoagulability studies were within normal limits. There was no evidence of May-Thurner anatomy, and an ultrasound of the lower extremities showed no DVT.
THE DIAGNOSIS
An echocardiogram with bubble study confirmed a diagnosis of patent foramen ovale (PFO) with bidirectional flow, a normal ejection fraction, and no evidence of left ventricular or left atrial thrombus. We started the patient on the anticoagulant enoxaparin 70 mg bid bridged with warfarin 5 mg/d.
Taking the patient’s active lifestyle into consideration, he was approved for PFO closure by the PFO committee and underwent closure. Following treatment, the patient was left with a residual 2-mm blind spot in the right visual field. At a 2-year follow-up visit, he showed no new focal deficits or recurrent symptoms.
DISCUSSION
Since 1988 when Lechat et al reported increased incidence of PFO in young stroke patients,1 many studies have supported the association between PFO and cryptogenic stroke (CS) in young adults.2 Because it remained controversial as to whether PFO is a risk factor for stroke or transient ischemic attack recurrence,3 researchers investigated PFO closure as a preventive measure to decrease stroke recurrence in patients with both CS and PFO.
A 2012 meta-analysis showed possible benefits of closure compared with medical management using antiplatelet or anticoagulation therapies.4 However, these results were not supported by results of other studies. These include the CLOSURE I trial,5 which compared device closure of PFO with medical therapy, and the RESPECT6 and PC trials,7 which did not show a significant difference in the primary end point of recurrent stroke between patients who received medical therapy and those who had PFO closure.
American Heart Association/American Stroke Association’s 2011 guidelines recommend only antiplatelet therapy for patients with CS and PFO.8 While there is consensus that surgical closure is not better than a medical approach to patients with CS and PFO, cases should be individualized, as a patient’s clinical or social factors may dictate otherwise.
Lifestyle may warrant PFO closure
No previous studies have considered occupation or hobbies as an indication for PFO closure in patients with CS. Our patient’s active lifestyle, particularly his scuba diving and participation in contact sports, made him a poor candidate for anticoagulation. Scuba diving is associated with decompression sickness and air emboli, which can be a mechanism of cerebral ischemia, especially in patients with a right-to-left shunt, such as with PFO.9
We did not observe a strong temporal relationship between diving and stroke in our patient. MRI findings suggested that he had multiple minor embolic events over time, which is consistent with a prior case report.9 This suggested air emboli as a possible source of stroke, in which case, our patient might not benefit from antiplatelet or anticoagulation therapy.
THE TAKEAWAY
This case illustrates the importance of a thorough social history and knowledge of the patient’s hobbies, occupation, and preferences in evaluating and treating individuals with CS associated with PFO. The current literature does not provide complete answers to the cause, diagnosis, and management of CS; additional research is needed.
The work-up involved in defining the etiology of stroke includes, but is not limited to, head and brain imaging, an echocardiogram, hypercoagulability tests, and vascular imaging. The work of Sanna et al showed that approximately 12% of patients with CS have atrial fibrillation when monitored over a one-year period, suggesting atrial fibrillation as a possible cause in some cases.10
As the case described here demonstrates, further research is warranted regarding how a patient’s occupation and lifestyle factor into decision-making for patients with PFO.
THE CASE
A 46-year-old man presented to the emergency department (ED) with sudden-onset right-sided visual loss. He had a history of asthma, but no family history of hypercoagulability, deep vein thrombosis (DVT), or stroke. The patient had an active lifestyle that included scuba diving, mountain biking, and hockey (coaching and playing). The physical examination revealed a right homonymous upper quadrantanopia. The neurologic examination was within normal limits, except for the visual deficit and unequal pupil size. A computerized tomography scan of the patient’s head did not reveal any lesions.
Based on the patient’s clinical picture, the ED physician prescribed alteplase, a tissue plasminogen activator (tPA), and admitted him to the intensive care unit for monitoring.
Subsequent magnetic resonance imaging (MRI) of the brain showed multiple small areas of acute infarct in the posterior circulation territory bilaterally, with involvement of small portions of the bilateral cerebellar hemispheres and parts of the left occipital lobe (FIGURE 1A and 1B).

An electrocardiogram showed no evidence of atrial fibrillation, and hypercoagulability studies were within normal limits. There was no evidence of May-Thurner anatomy, and an ultrasound of the lower extremities showed no DVT.
THE DIAGNOSIS
An echocardiogram with bubble study confirmed a diagnosis of patent foramen ovale (PFO) with bidirectional flow, a normal ejection fraction, and no evidence of left ventricular or left atrial thrombus. We started the patient on the anticoagulant enoxaparin 70 mg bid bridged with warfarin 5 mg/d.
Taking the patient’s active lifestyle into consideration, he was approved for PFO closure by the PFO committee and underwent closure. Following treatment, the patient was left with a residual 2-mm blind spot in the right visual field. At a 2-year follow-up visit, he showed no new focal deficits or recurrent symptoms.
DISCUSSION
Since 1988 when Lechat et al reported increased incidence of PFO in young stroke patients,1 many studies have supported the association between PFO and cryptogenic stroke (CS) in young adults.2 Because it remained controversial as to whether PFO is a risk factor for stroke or transient ischemic attack recurrence,3 researchers investigated PFO closure as a preventive measure to decrease stroke recurrence in patients with both CS and PFO.
A 2012 meta-analysis showed possible benefits of closure compared with medical management using antiplatelet or anticoagulation therapies.4 However, these results were not supported by results of other studies. These include the CLOSURE I trial,5 which compared device closure of PFO with medical therapy, and the RESPECT6 and PC trials,7 which did not show a significant difference in the primary end point of recurrent stroke between patients who received medical therapy and those who had PFO closure.
American Heart Association/American Stroke Association’s 2011 guidelines recommend only antiplatelet therapy for patients with CS and PFO.8 While there is consensus that surgical closure is not better than a medical approach to patients with CS and PFO, cases should be individualized, as a patient’s clinical or social factors may dictate otherwise.
Lifestyle may warrant PFO closure
No previous studies have considered occupation or hobbies as an indication for PFO closure in patients with CS. Our patient’s active lifestyle, particularly his scuba diving and participation in contact sports, made him a poor candidate for anticoagulation. Scuba diving is associated with decompression sickness and air emboli, which can be a mechanism of cerebral ischemia, especially in patients with a right-to-left shunt, such as with PFO.9
We did not observe a strong temporal relationship between diving and stroke in our patient. MRI findings suggested that he had multiple minor embolic events over time, which is consistent with a prior case report.9 This suggested air emboli as a possible source of stroke, in which case, our patient might not benefit from antiplatelet or anticoagulation therapy.
THE TAKEAWAY
This case illustrates the importance of a thorough social history and knowledge of the patient’s hobbies, occupation, and preferences in evaluating and treating individuals with CS associated with PFO. The current literature does not provide complete answers to the cause, diagnosis, and management of CS; additional research is needed.
The work-up involved in defining the etiology of stroke includes, but is not limited to, head and brain imaging, an echocardiogram, hypercoagulability tests, and vascular imaging. The work of Sanna et al showed that approximately 12% of patients with CS have atrial fibrillation when monitored over a one-year period, suggesting atrial fibrillation as a possible cause in some cases.10
As the case described here demonstrates, further research is warranted regarding how a patient’s occupation and lifestyle factor into decision-making for patients with PFO.
1. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152.
2. Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085-1096.
3. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol. 2010;257:1777-1787.
4. Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431.
5. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276.
9. Menkin M, Schwartzman RJ. Cerebral air embolism. Report of five cases and review of the literature. Arch Neurol. 1977;34:168-170.
10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
1. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152.
2. Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085-1096.
3. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol. 2010;257:1777-1787.
4. Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431.
5. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276.
9. Menkin M, Schwartzman RJ. Cerebral air embolism. Report of five cases and review of the literature. Arch Neurol. 1977;34:168-170.
10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
Trans-Scaphoid Transcapitate Perilunate Fracture-Dislocation
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Co-occurrence of Steatocystoma Multiplex, Eruptive Vellus Hair Cysts, and Trichofolliculomas
An association between steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) has been recognized. They are related conditions representing nevoid malformations of the pilosebaceous junctions1-10 that have similar clinical features but distinctive histologic features. Both conditions most commonly involve the anterior aspect of the chest. Six cases of a rare facial variant of SCM have been reported,11-16 3 involving lesions limited to the forehead.13-15 Two patients with a rare facial variant of EVHC also have been reported.17 The development of separate lesions of SCM and EVHC on the trunk can uncommonly occur.5,6,10 One case of SCM and EVHC on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. The simultaneous occurrence of multiple trichoblastomas, trichoepitheliomas, and SCM on the face and trunk has been reported in 1 case.18 Milia, SCM, and EVHC on the face and trunk have been reported in 1 family.4 A report of facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus also has occurred in 1 patient.19 Here, we report the simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead.
Case Report
A 37-year-old man had an increasing number of flesh-colored to yellow papules on the forehead that had been present since puberty. Although the lesions were asymptomatic, some had recently become tender, which led him to seek medical care. There was no history of trauma, burns, irradiation, or application of topical agents to the area or use of eyeglasses or goggles. The patient’s father had similar lesions limited to the forehead, which developed during adolescence.
On evaluation at our clinic, skin examination revealed 16 discrete, 0.3- to 1-cm, flesh-colored, yellow to blue, mobile, smooth papules, as well as flesh-colored papules with a central black punctum, on the forehead (Figure 1). Similar lesions were not present on the rest of the face; around the ears; or on the scalp, neck, chest, back, abdomen, genitalia, buttocks, palms, soles, axillae, arms, or legs. There were no nail abnormalities.
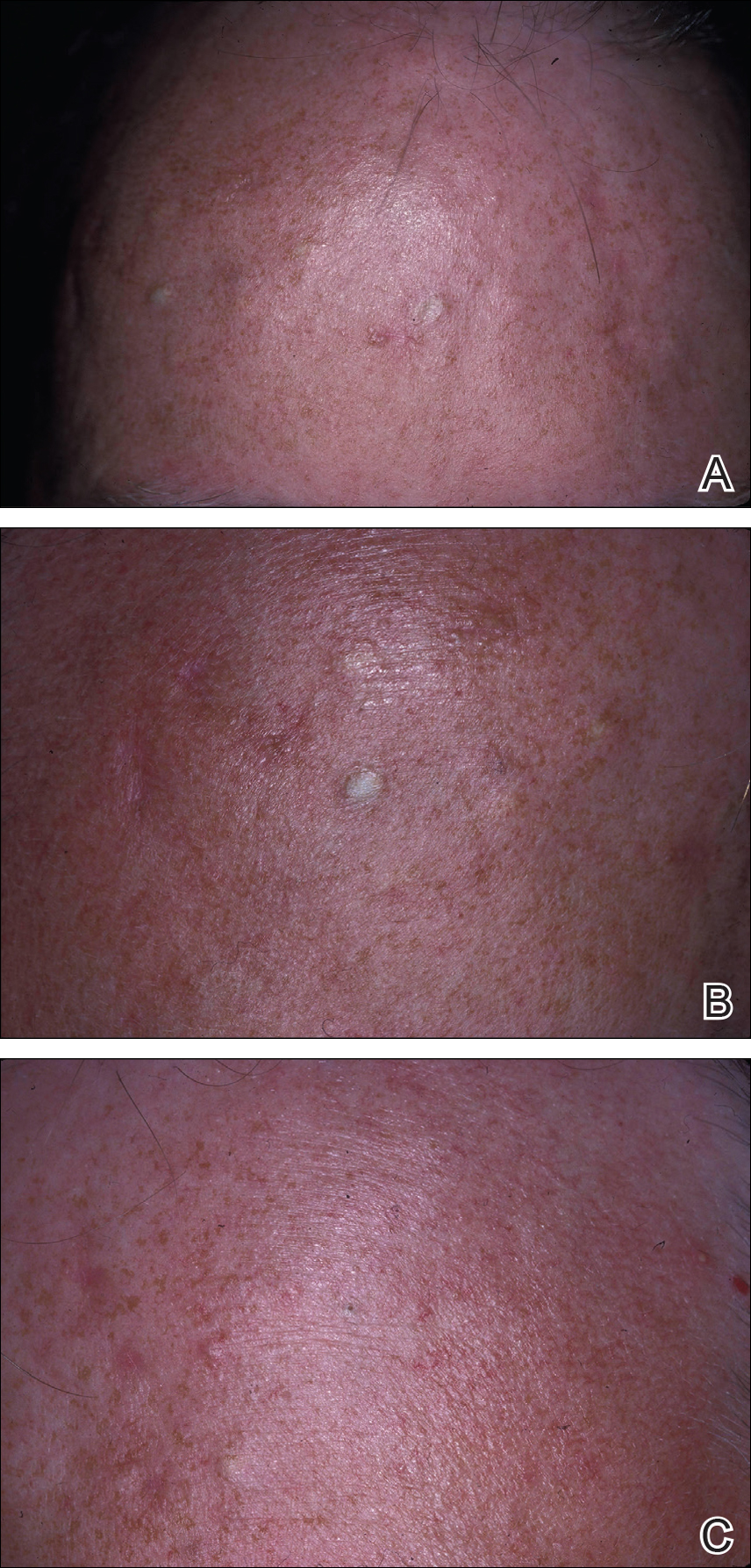
Multiple 3-, 4-, and 6-mm punch and excisional biopsies were performed to remove all 16 lesions on the forehead. Histologic examination revealed a collapsed cystic structure in the mid dermis in 10 lesions. The cysts were lined with a squamous epithelium without a granular layer but with an eosinophilic corrugated lining, and the cyst cavity contained scant homogeneous eosinophilic secretion. Mature sebaceous glands were adjacent to the outer portion of the cyst wall. These histologic findings were consistent with SCM (Figure 2).
In 3 lesions, histologic examination revealed a cystic structure lined by a few layers of stratified squamous epithelium in the mid dermis. The cyst cavity contained numerous small vellus hairs and laminated keratin. These histologic findings were consistent with EVHC (Figure 3).
In the other 3 lesions, histologic examination revealed a dilated central cystic cavity filled with laminated keratin in the mid dermis. Multiple small follicles arose from the cysts and showed differentiation toward germinative epithelium. The surrounding stroma was fibrotic and contained a patchy lymphocytic infiltrate. These histologic findings were consistent with trichofolliculomas (Figure 4).
Comment
Characteristics of SCM
Steatocystoma multiplex is an uncommon condition characterized by the formation of asymptomatic, 0.2- to 2-cm, yellow to flesh-colored, soft, mobile papules or nodules on the trunk, extremities, axillae, genitalia, and/or chest. The lesions contain a clear or opaque, oily, milky or yellow, odorless fluid and most commonly are located on the anterior aspect of the chest. The face is not a commonly involved site in this condition. Six cases of a rare facial variant of SCM have been reported,11-16 with lesions limited to the forehead in 3 cases.13-15
In 1937, Mount20 credited Bozellini for describing the first case, though 3 cases reported in the late 1800s probably were SCM.21 In 1899, Pringle22 coined the term steatocystoma multiplex for this condition. It can be sporadic or have an autosomal-dominant inheritance pattern. Steatocystoma multiplex can occur at any age, though lesions develop most frequently in adolescence or young adulthood. There is no sex predilection.
Steatocystoma multiplex with pachyonychia congenita has been reported in a familial case.23 Other findings reported in patients with SCM include ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf and hypertrophic lichen planus, hidradenitis suppurativa, hypotrichosis, multiple keratoacanthomas, and rheumatoid arthritis.12,24-26
Steatocystoma multiplex is a cyst lined by stratified squamous epithelium without a granular layer but with a thick eosinophilic cuticle. Mature sebaceous lobules are closely associated with the cyst wall. Steatocystoma multiplex arises from the sebaceous duct because the lining of the lumen is composed of undulating eosinophilic cuticle.
Characteristics of EVHCs
Eruptive vellus hair cysts, which were first described by Esterly et al,27 can occur at any age but develop most frequently in adolescents or young adults. Sometimes the lesions are congenital or appear in childhood. There is no sex predilection. They can be sporadic or have an autosomal-dominant inheritance pattern.
Eruptive vellus hair cysts are asymptomatic, 1- to 2-mm, smooth, crusted, or umbilicated papules on the chest or arms and legs. Eruptive vellus hair cysts most commonly involve the anterior aspect of the chest. The lesions are flesh-colored to yellow, though they have a slate gray color in darker-skinned individuals. A rare facial variant has been reported in 2 patients of Asian descent.17
Eruptive vellus hair cysts are small cystic structures lined by a stratified squamous epithelium with a granular layer. The cyst cavity contains numerous small vellus hair shafts and laminated keratin. Eruptive vellus hair cysts originate from the infundibulum or less frequently the isthmus or infundibular-isthmic junction of the hair follicle.
Characteristics of Trichofolliculomas
Trichofolliculomas are solitary, 3- to 5-mm, flesh-colored papules that occur on the face. They are highly differentiated, benign, neoplastic proliferations of an actively trichogenic epithelium, with structural components reflecting all portions of the pilosebaceous unit. Trichofolliculomas consist of a central dilated primary follicle contiguous with the surface epidermis embedded in a fibrous stroma. Multiple small secondary follicles with varying degrees of follicular differentiation arise from the primary follicle.
Co-occurrence of Lesions
An association between SCM and EVHC has been recognized.5-10 Steatocystoma multiplex and EVHC have similar clinical features but distinctive histologic features. They also have a similar age of onset, location/appearance of lesions, and mode of inheritance. Steatocystoma multiplex and EVHC can be distinguished by immunohistochemical techniques: SCM shows expression of keratin 10 and keratin 17, whereas EVHCs express only keratin 17.28
Steatocystoma multiplex and EVHC have only rarely been reported to occur together on the trunk. One case of SCM and EVHC occurring on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. Milia, SCM, and EVHC on the face and trunk have been reported in 1 family,4 and facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus was reported in 1 patient.19 Although trichofolliculomas have not been reported to occur with SCM or EVHC, 2 related follicular neoplasms—trichoepitheliomas and trichoblastomas—have been reported to occur in association with SCM on the face and chest and around the ears in 1 case.18
Differential Diagnosis
The clinical differential diagnosis includes multiple epidermoid cysts, dermoid cysts, Gardner syndrome, sebaceous adenomas, Muir-Torre syndrome, syringomas, milia, leiomyomas, lipomas, acneiform folliculitis, multiple familial and nonfamilial trichoepitheliomas, cylindromas, and angiofibromas.3,29
Conclusion
Our patient represents a rare case of simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead. The patient had multiple neoplasms involving differentiation toward various regions of the pilosebaceous unit. This case gives further support to the hypothesis that these benign follicular neoplasms are closely related but are distinct conditions within the spectrum of the same disease process. They represent nevoid malformations of the pilosebaceous unit that can be sporadic or inherited in an autosomal-dominant pattern. Pure types of these lesions may represent one end of the spectrum, but in some patients, there are overlapping features or hybrids of each condition. Several biopsies from patients with multiple lesions should be performed to establish an accurate diagnosis.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19:570-571.
- Sanchez Yus E, Requena L. Eruptive vellus hair cyst and steatocystoma multiplex. Am J Dermatopathol. 1990;12:536-537.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Kiene P, Hauschild A, Christophers E. Eruptive vellus hair cysts and steatocystoma multiplex: variants of one entity? Br J Dermatol. 1996;134:365-367.
- Hurlimann AF, Panizzon RG, Burg G. Eruptive vellus hair cyst and steatocystoma multiplex: hybrid cysts. Dermatology. 1996;192:64-66.
- Sexton M, Murdock DK. Eruptive vellus hair cysts: a follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1989;11:364-368.
- Sanchez-Yus E, Aguilar-Martinez A, Cristobal-Gil MC, et al. Eruptive vellus hair cyst and steatocystoma multiplex: two related conditions? J Cutan Pathol. 1988;15:40-42.
- Ahn SK, Chung J, Lee WS, et al. Hybrid cysts showing alternate combination of eruptive vellus hair cyst, steatocystoma multiplex, and epidermoid cyst, and an association among the three conditions. Am J Dermatopathol. 1996;18:645-649.
- Ahn SK, Hwang SM, Lee SH, et al. Steatocystoma multiplex localized only in the face. Int J Dermatol. 1997;36:372-373.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Hansen KK, Troy JL, Fairley JA. Multiple papules of the scalp and forehead. steatocystoma multiplex (facial papular variant). Arch Dermatol. 1995;131:835-838.
- Nishimura M, Kohda H, Urabe A. Steatocystoma multiplex: a facial popular variant. Arch Dermatol. 1986;122:205-207.
- Requena L, Martin L, Renedo G, et al. A facial variant of steatocystoma multiplex. Cutis. 1993;51:449-452.
- Holmes R, Black MM. Steatocystoma multiplex with unusually prominent cysts on the face. Br J Dermatol. 1980;102:711-713.
- Kumakiri M, Takashima I, Iju M, et al. Eruptive vellus hair cysts: a facial variant. J Am Acad Dermatol. 1982;7:461-467.
- Gianotti R, Cavicchini S, Alessi E. Simultaneous occurrence of multiple trichoblastomas and steatocystoma multiplex. Am J Dermatopathol. 1997;19:294-298.
- Sardana K, Sharma RC, Jain A, et al. Facial steatocystoma multiplex associated with pilar cyst and bilateral preauricular sinus. J Dermatol. 2002;29:157-159.
- Mount LB. Steatocystoma multiplex. Arch Dermatol Syphilol. 1937;36:31-39.
- Dubreuilh W, Auche B. Kystes grassieux sudoripares. Arch Clin de Bordeaux. 1896;5:387-391.
- Pringle JJ. A case of peculiar multiple sebaceous cysts (steatocystoma multiplex). Br J Dermatol. 1899;11:381-88.
- Vineyard WR, Scott RA. Steatocystoma multiplex with pachyonychia congenital: eight cases in four generations. Arch Dermatol. 1961;84:824-827.
- Contreras MA, Costello MJ. Steatocystoma multiplex with embryonal hair formation: case presentation and consideration of pathogenesis. AMA Arch Derm. 1957;76:720-725.
- Sohn D, Chin TC, Fellner MJ. Multiple keratoacanthomas associated with steatocystoma multiplex and rheumatoid arthritis: a case report. Arch Dermatol. 1980;116:913-915.
- Verbov J. Acrokeratosis verruciformis of Hopf with steatocystoma multiplex and hypertrophic lichen planus. Br J Dermatol. 1972;86:91-94.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Tomkova H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Feinstein A, Trau H, Movshovitz M, et al. Steatocystoma multiplex. Cutis. 1983;31:425-427.
An association between steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) has been recognized. They are related conditions representing nevoid malformations of the pilosebaceous junctions1-10 that have similar clinical features but distinctive histologic features. Both conditions most commonly involve the anterior aspect of the chest. Six cases of a rare facial variant of SCM have been reported,11-16 3 involving lesions limited to the forehead.13-15 Two patients with a rare facial variant of EVHC also have been reported.17 The development of separate lesions of SCM and EVHC on the trunk can uncommonly occur.5,6,10 One case of SCM and EVHC on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. The simultaneous occurrence of multiple trichoblastomas, trichoepitheliomas, and SCM on the face and trunk has been reported in 1 case.18 Milia, SCM, and EVHC on the face and trunk have been reported in 1 family.4 A report of facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus also has occurred in 1 patient.19 Here, we report the simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead.
Case Report
A 37-year-old man had an increasing number of flesh-colored to yellow papules on the forehead that had been present since puberty. Although the lesions were asymptomatic, some had recently become tender, which led him to seek medical care. There was no history of trauma, burns, irradiation, or application of topical agents to the area or use of eyeglasses or goggles. The patient’s father had similar lesions limited to the forehead, which developed during adolescence.
On evaluation at our clinic, skin examination revealed 16 discrete, 0.3- to 1-cm, flesh-colored, yellow to blue, mobile, smooth papules, as well as flesh-colored papules with a central black punctum, on the forehead (Figure 1). Similar lesions were not present on the rest of the face; around the ears; or on the scalp, neck, chest, back, abdomen, genitalia, buttocks, palms, soles, axillae, arms, or legs. There were no nail abnormalities.

Multiple 3-, 4-, and 6-mm punch and excisional biopsies were performed to remove all 16 lesions on the forehead. Histologic examination revealed a collapsed cystic structure in the mid dermis in 10 lesions. The cysts were lined with a squamous epithelium without a granular layer but with an eosinophilic corrugated lining, and the cyst cavity contained scant homogeneous eosinophilic secretion. Mature sebaceous glands were adjacent to the outer portion of the cyst wall. These histologic findings were consistent with SCM (Figure 2).

In 3 lesions, histologic examination revealed a cystic structure lined by a few layers of stratified squamous epithelium in the mid dermis. The cyst cavity contained numerous small vellus hairs and laminated keratin. These histologic findings were consistent with EVHC (Figure 3).

In the other 3 lesions, histologic examination revealed a dilated central cystic cavity filled with laminated keratin in the mid dermis. Multiple small follicles arose from the cysts and showed differentiation toward germinative epithelium. The surrounding stroma was fibrotic and contained a patchy lymphocytic infiltrate. These histologic findings were consistent with trichofolliculomas (Figure 4).

Comment
Characteristics of SCM
Steatocystoma multiplex is an uncommon condition characterized by the formation of asymptomatic, 0.2- to 2-cm, yellow to flesh-colored, soft, mobile papules or nodules on the trunk, extremities, axillae, genitalia, and/or chest. The lesions contain a clear or opaque, oily, milky or yellow, odorless fluid and most commonly are located on the anterior aspect of the chest. The face is not a commonly involved site in this condition. Six cases of a rare facial variant of SCM have been reported,11-16 with lesions limited to the forehead in 3 cases.13-15
In 1937, Mount20 credited Bozellini for describing the first case, though 3 cases reported in the late 1800s probably were SCM.21 In 1899, Pringle22 coined the term steatocystoma multiplex for this condition. It can be sporadic or have an autosomal-dominant inheritance pattern. Steatocystoma multiplex can occur at any age, though lesions develop most frequently in adolescence or young adulthood. There is no sex predilection.
Steatocystoma multiplex with pachyonychia congenita has been reported in a familial case.23 Other findings reported in patients with SCM include ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf and hypertrophic lichen planus, hidradenitis suppurativa, hypotrichosis, multiple keratoacanthomas, and rheumatoid arthritis.12,24-26
Steatocystoma multiplex is a cyst lined by stratified squamous epithelium without a granular layer but with a thick eosinophilic cuticle. Mature sebaceous lobules are closely associated with the cyst wall. Steatocystoma multiplex arises from the sebaceous duct because the lining of the lumen is composed of undulating eosinophilic cuticle.
Characteristics of EVHCs
Eruptive vellus hair cysts, which were first described by Esterly et al,27 can occur at any age but develop most frequently in adolescents or young adults. Sometimes the lesions are congenital or appear in childhood. There is no sex predilection. They can be sporadic or have an autosomal-dominant inheritance pattern.
Eruptive vellus hair cysts are asymptomatic, 1- to 2-mm, smooth, crusted, or umbilicated papules on the chest or arms and legs. Eruptive vellus hair cysts most commonly involve the anterior aspect of the chest. The lesions are flesh-colored to yellow, though they have a slate gray color in darker-skinned individuals. A rare facial variant has been reported in 2 patients of Asian descent.17
Eruptive vellus hair cysts are small cystic structures lined by a stratified squamous epithelium with a granular layer. The cyst cavity contains numerous small vellus hair shafts and laminated keratin. Eruptive vellus hair cysts originate from the infundibulum or less frequently the isthmus or infundibular-isthmic junction of the hair follicle.
Characteristics of Trichofolliculomas
Trichofolliculomas are solitary, 3- to 5-mm, flesh-colored papules that occur on the face. They are highly differentiated, benign, neoplastic proliferations of an actively trichogenic epithelium, with structural components reflecting all portions of the pilosebaceous unit. Trichofolliculomas consist of a central dilated primary follicle contiguous with the surface epidermis embedded in a fibrous stroma. Multiple small secondary follicles with varying degrees of follicular differentiation arise from the primary follicle.
Co-occurrence of Lesions
An association between SCM and EVHC has been recognized.5-10 Steatocystoma multiplex and EVHC have similar clinical features but distinctive histologic features. They also have a similar age of onset, location/appearance of lesions, and mode of inheritance. Steatocystoma multiplex and EVHC can be distinguished by immunohistochemical techniques: SCM shows expression of keratin 10 and keratin 17, whereas EVHCs express only keratin 17.28
Steatocystoma multiplex and EVHC have only rarely been reported to occur together on the trunk. One case of SCM and EVHC occurring on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. Milia, SCM, and EVHC on the face and trunk have been reported in 1 family,4 and facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus was reported in 1 patient.19 Although trichofolliculomas have not been reported to occur with SCM or EVHC, 2 related follicular neoplasms—trichoepitheliomas and trichoblastomas—have been reported to occur in association with SCM on the face and chest and around the ears in 1 case.18
Differential Diagnosis
The clinical differential diagnosis includes multiple epidermoid cysts, dermoid cysts, Gardner syndrome, sebaceous adenomas, Muir-Torre syndrome, syringomas, milia, leiomyomas, lipomas, acneiform folliculitis, multiple familial and nonfamilial trichoepitheliomas, cylindromas, and angiofibromas.3,29
Conclusion
Our patient represents a rare case of simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead. The patient had multiple neoplasms involving differentiation toward various regions of the pilosebaceous unit. This case gives further support to the hypothesis that these benign follicular neoplasms are closely related but are distinct conditions within the spectrum of the same disease process. They represent nevoid malformations of the pilosebaceous unit that can be sporadic or inherited in an autosomal-dominant pattern. Pure types of these lesions may represent one end of the spectrum, but in some patients, there are overlapping features or hybrids of each condition. Several biopsies from patients with multiple lesions should be performed to establish an accurate diagnosis.
An association between steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) has been recognized. They are related conditions representing nevoid malformations of the pilosebaceous junctions1-10 that have similar clinical features but distinctive histologic features. Both conditions most commonly involve the anterior aspect of the chest. Six cases of a rare facial variant of SCM have been reported,11-16 3 involving lesions limited to the forehead.13-15 Two patients with a rare facial variant of EVHC also have been reported.17 The development of separate lesions of SCM and EVHC on the trunk can uncommonly occur.5,6,10 One case of SCM and EVHC on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. The simultaneous occurrence of multiple trichoblastomas, trichoepitheliomas, and SCM on the face and trunk has been reported in 1 case.18 Milia, SCM, and EVHC on the face and trunk have been reported in 1 family.4 A report of facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus also has occurred in 1 patient.19 Here, we report the simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead.
Case Report
A 37-year-old man had an increasing number of flesh-colored to yellow papules on the forehead that had been present since puberty. Although the lesions were asymptomatic, some had recently become tender, which led him to seek medical care. There was no history of trauma, burns, irradiation, or application of topical agents to the area or use of eyeglasses or goggles. The patient’s father had similar lesions limited to the forehead, which developed during adolescence.
On evaluation at our clinic, skin examination revealed 16 discrete, 0.3- to 1-cm, flesh-colored, yellow to blue, mobile, smooth papules, as well as flesh-colored papules with a central black punctum, on the forehead (Figure 1). Similar lesions were not present on the rest of the face; around the ears; or on the scalp, neck, chest, back, abdomen, genitalia, buttocks, palms, soles, axillae, arms, or legs. There were no nail abnormalities.

Multiple 3-, 4-, and 6-mm punch and excisional biopsies were performed to remove all 16 lesions on the forehead. Histologic examination revealed a collapsed cystic structure in the mid dermis in 10 lesions. The cysts were lined with a squamous epithelium without a granular layer but with an eosinophilic corrugated lining, and the cyst cavity contained scant homogeneous eosinophilic secretion. Mature sebaceous glands were adjacent to the outer portion of the cyst wall. These histologic findings were consistent with SCM (Figure 2).

In 3 lesions, histologic examination revealed a cystic structure lined by a few layers of stratified squamous epithelium in the mid dermis. The cyst cavity contained numerous small vellus hairs and laminated keratin. These histologic findings were consistent with EVHC (Figure 3).

In the other 3 lesions, histologic examination revealed a dilated central cystic cavity filled with laminated keratin in the mid dermis. Multiple small follicles arose from the cysts and showed differentiation toward germinative epithelium. The surrounding stroma was fibrotic and contained a patchy lymphocytic infiltrate. These histologic findings were consistent with trichofolliculomas (Figure 4).

Comment
Characteristics of SCM
Steatocystoma multiplex is an uncommon condition characterized by the formation of asymptomatic, 0.2- to 2-cm, yellow to flesh-colored, soft, mobile papules or nodules on the trunk, extremities, axillae, genitalia, and/or chest. The lesions contain a clear or opaque, oily, milky or yellow, odorless fluid and most commonly are located on the anterior aspect of the chest. The face is not a commonly involved site in this condition. Six cases of a rare facial variant of SCM have been reported,11-16 with lesions limited to the forehead in 3 cases.13-15
In 1937, Mount20 credited Bozellini for describing the first case, though 3 cases reported in the late 1800s probably were SCM.21 In 1899, Pringle22 coined the term steatocystoma multiplex for this condition. It can be sporadic or have an autosomal-dominant inheritance pattern. Steatocystoma multiplex can occur at any age, though lesions develop most frequently in adolescence or young adulthood. There is no sex predilection.
Steatocystoma multiplex with pachyonychia congenita has been reported in a familial case.23 Other findings reported in patients with SCM include ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf and hypertrophic lichen planus, hidradenitis suppurativa, hypotrichosis, multiple keratoacanthomas, and rheumatoid arthritis.12,24-26
Steatocystoma multiplex is a cyst lined by stratified squamous epithelium without a granular layer but with a thick eosinophilic cuticle. Mature sebaceous lobules are closely associated with the cyst wall. Steatocystoma multiplex arises from the sebaceous duct because the lining of the lumen is composed of undulating eosinophilic cuticle.
Characteristics of EVHCs
Eruptive vellus hair cysts, which were first described by Esterly et al,27 can occur at any age but develop most frequently in adolescents or young adults. Sometimes the lesions are congenital or appear in childhood. There is no sex predilection. They can be sporadic or have an autosomal-dominant inheritance pattern.
Eruptive vellus hair cysts are asymptomatic, 1- to 2-mm, smooth, crusted, or umbilicated papules on the chest or arms and legs. Eruptive vellus hair cysts most commonly involve the anterior aspect of the chest. The lesions are flesh-colored to yellow, though they have a slate gray color in darker-skinned individuals. A rare facial variant has been reported in 2 patients of Asian descent.17
Eruptive vellus hair cysts are small cystic structures lined by a stratified squamous epithelium with a granular layer. The cyst cavity contains numerous small vellus hair shafts and laminated keratin. Eruptive vellus hair cysts originate from the infundibulum or less frequently the isthmus or infundibular-isthmic junction of the hair follicle.
Characteristics of Trichofolliculomas
Trichofolliculomas are solitary, 3- to 5-mm, flesh-colored papules that occur on the face. They are highly differentiated, benign, neoplastic proliferations of an actively trichogenic epithelium, with structural components reflecting all portions of the pilosebaceous unit. Trichofolliculomas consist of a central dilated primary follicle contiguous with the surface epidermis embedded in a fibrous stroma. Multiple small secondary follicles with varying degrees of follicular differentiation arise from the primary follicle.
Co-occurrence of Lesions
An association between SCM and EVHC has been recognized.5-10 Steatocystoma multiplex and EVHC have similar clinical features but distinctive histologic features. They also have a similar age of onset, location/appearance of lesions, and mode of inheritance. Steatocystoma multiplex and EVHC can be distinguished by immunohistochemical techniques: SCM shows expression of keratin 10 and keratin 17, whereas EVHCs express only keratin 17.28
Steatocystoma multiplex and EVHC have only rarely been reported to occur together on the trunk. One case of SCM and EVHC occurring on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. Milia, SCM, and EVHC on the face and trunk have been reported in 1 family,4 and facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus was reported in 1 patient.19 Although trichofolliculomas have not been reported to occur with SCM or EVHC, 2 related follicular neoplasms—trichoepitheliomas and trichoblastomas—have been reported to occur in association with SCM on the face and chest and around the ears in 1 case.18
Differential Diagnosis
The clinical differential diagnosis includes multiple epidermoid cysts, dermoid cysts, Gardner syndrome, sebaceous adenomas, Muir-Torre syndrome, syringomas, milia, leiomyomas, lipomas, acneiform folliculitis, multiple familial and nonfamilial trichoepitheliomas, cylindromas, and angiofibromas.3,29
Conclusion
Our patient represents a rare case of simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead. The patient had multiple neoplasms involving differentiation toward various regions of the pilosebaceous unit. This case gives further support to the hypothesis that these benign follicular neoplasms are closely related but are distinct conditions within the spectrum of the same disease process. They represent nevoid malformations of the pilosebaceous unit that can be sporadic or inherited in an autosomal-dominant pattern. Pure types of these lesions may represent one end of the spectrum, but in some patients, there are overlapping features or hybrids of each condition. Several biopsies from patients with multiple lesions should be performed to establish an accurate diagnosis.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19:570-571.
- Sanchez Yus E, Requena L. Eruptive vellus hair cyst and steatocystoma multiplex. Am J Dermatopathol. 1990;12:536-537.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Kiene P, Hauschild A, Christophers E. Eruptive vellus hair cysts and steatocystoma multiplex: variants of one entity? Br J Dermatol. 1996;134:365-367.
- Hurlimann AF, Panizzon RG, Burg G. Eruptive vellus hair cyst and steatocystoma multiplex: hybrid cysts. Dermatology. 1996;192:64-66.
- Sexton M, Murdock DK. Eruptive vellus hair cysts: a follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1989;11:364-368.
- Sanchez-Yus E, Aguilar-Martinez A, Cristobal-Gil MC, et al. Eruptive vellus hair cyst and steatocystoma multiplex: two related conditions? J Cutan Pathol. 1988;15:40-42.
- Ahn SK, Chung J, Lee WS, et al. Hybrid cysts showing alternate combination of eruptive vellus hair cyst, steatocystoma multiplex, and epidermoid cyst, and an association among the three conditions. Am J Dermatopathol. 1996;18:645-649.
- Ahn SK, Hwang SM, Lee SH, et al. Steatocystoma multiplex localized only in the face. Int J Dermatol. 1997;36:372-373.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Hansen KK, Troy JL, Fairley JA. Multiple papules of the scalp and forehead. steatocystoma multiplex (facial papular variant). Arch Dermatol. 1995;131:835-838.
- Nishimura M, Kohda H, Urabe A. Steatocystoma multiplex: a facial popular variant. Arch Dermatol. 1986;122:205-207.
- Requena L, Martin L, Renedo G, et al. A facial variant of steatocystoma multiplex. Cutis. 1993;51:449-452.
- Holmes R, Black MM. Steatocystoma multiplex with unusually prominent cysts on the face. Br J Dermatol. 1980;102:711-713.
- Kumakiri M, Takashima I, Iju M, et al. Eruptive vellus hair cysts: a facial variant. J Am Acad Dermatol. 1982;7:461-467.
- Gianotti R, Cavicchini S, Alessi E. Simultaneous occurrence of multiple trichoblastomas and steatocystoma multiplex. Am J Dermatopathol. 1997;19:294-298.
- Sardana K, Sharma RC, Jain A, et al. Facial steatocystoma multiplex associated with pilar cyst and bilateral preauricular sinus. J Dermatol. 2002;29:157-159.
- Mount LB. Steatocystoma multiplex. Arch Dermatol Syphilol. 1937;36:31-39.
- Dubreuilh W, Auche B. Kystes grassieux sudoripares. Arch Clin de Bordeaux. 1896;5:387-391.
- Pringle JJ. A case of peculiar multiple sebaceous cysts (steatocystoma multiplex). Br J Dermatol. 1899;11:381-88.
- Vineyard WR, Scott RA. Steatocystoma multiplex with pachyonychia congenital: eight cases in four generations. Arch Dermatol. 1961;84:824-827.
- Contreras MA, Costello MJ. Steatocystoma multiplex with embryonal hair formation: case presentation and consideration of pathogenesis. AMA Arch Derm. 1957;76:720-725.
- Sohn D, Chin TC, Fellner MJ. Multiple keratoacanthomas associated with steatocystoma multiplex and rheumatoid arthritis: a case report. Arch Dermatol. 1980;116:913-915.
- Verbov J. Acrokeratosis verruciformis of Hopf with steatocystoma multiplex and hypertrophic lichen planus. Br J Dermatol. 1972;86:91-94.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Tomkova H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Feinstein A, Trau H, Movshovitz M, et al. Steatocystoma multiplex. Cutis. 1983;31:425-427.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19:570-571.
- Sanchez Yus E, Requena L. Eruptive vellus hair cyst and steatocystoma multiplex. Am J Dermatopathol. 1990;12:536-537.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Kiene P, Hauschild A, Christophers E. Eruptive vellus hair cysts and steatocystoma multiplex: variants of one entity? Br J Dermatol. 1996;134:365-367.
- Hurlimann AF, Panizzon RG, Burg G. Eruptive vellus hair cyst and steatocystoma multiplex: hybrid cysts. Dermatology. 1996;192:64-66.
- Sexton M, Murdock DK. Eruptive vellus hair cysts: a follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1989;11:364-368.
- Sanchez-Yus E, Aguilar-Martinez A, Cristobal-Gil MC, et al. Eruptive vellus hair cyst and steatocystoma multiplex: two related conditions? J Cutan Pathol. 1988;15:40-42.
- Ahn SK, Chung J, Lee WS, et al. Hybrid cysts showing alternate combination of eruptive vellus hair cyst, steatocystoma multiplex, and epidermoid cyst, and an association among the three conditions. Am J Dermatopathol. 1996;18:645-649.
- Ahn SK, Hwang SM, Lee SH, et al. Steatocystoma multiplex localized only in the face. Int J Dermatol. 1997;36:372-373.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Hansen KK, Troy JL, Fairley JA. Multiple papules of the scalp and forehead. steatocystoma multiplex (facial papular variant). Arch Dermatol. 1995;131:835-838.
- Nishimura M, Kohda H, Urabe A. Steatocystoma multiplex: a facial popular variant. Arch Dermatol. 1986;122:205-207.
- Requena L, Martin L, Renedo G, et al. A facial variant of steatocystoma multiplex. Cutis. 1993;51:449-452.
- Holmes R, Black MM. Steatocystoma multiplex with unusually prominent cysts on the face. Br J Dermatol. 1980;102:711-713.
- Kumakiri M, Takashima I, Iju M, et al. Eruptive vellus hair cysts: a facial variant. J Am Acad Dermatol. 1982;7:461-467.
- Gianotti R, Cavicchini S, Alessi E. Simultaneous occurrence of multiple trichoblastomas and steatocystoma multiplex. Am J Dermatopathol. 1997;19:294-298.
- Sardana K, Sharma RC, Jain A, et al. Facial steatocystoma multiplex associated with pilar cyst and bilateral preauricular sinus. J Dermatol. 2002;29:157-159.
- Mount LB. Steatocystoma multiplex. Arch Dermatol Syphilol. 1937;36:31-39.
- Dubreuilh W, Auche B. Kystes grassieux sudoripares. Arch Clin de Bordeaux. 1896;5:387-391.
- Pringle JJ. A case of peculiar multiple sebaceous cysts (steatocystoma multiplex). Br J Dermatol. 1899;11:381-88.
- Vineyard WR, Scott RA. Steatocystoma multiplex with pachyonychia congenital: eight cases in four generations. Arch Dermatol. 1961;84:824-827.
- Contreras MA, Costello MJ. Steatocystoma multiplex with embryonal hair formation: case presentation and consideration of pathogenesis. AMA Arch Derm. 1957;76:720-725.
- Sohn D, Chin TC, Fellner MJ. Multiple keratoacanthomas associated with steatocystoma multiplex and rheumatoid arthritis: a case report. Arch Dermatol. 1980;116:913-915.
- Verbov J. Acrokeratosis verruciformis of Hopf with steatocystoma multiplex and hypertrophic lichen planus. Br J Dermatol. 1972;86:91-94.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Tomkova H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Feinstein A, Trau H, Movshovitz M, et al. Steatocystoma multiplex. Cutis. 1983;31:425-427.
Practice Points
- Steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) have similar clinical features but distinctive histologic features.
- Milia, pilar cyst, trichoepitheliomas, and trichoblastomas simultaneously developing in association with SCM or EVHC on the face are rare.
- This case supports the hypothesis that these benign follicular neoplasms are related but distinct nevoid malformations of the pilosebaceous unit within the same disease spectrum.