User login
Dermatitis in an intestinal transplant candidate
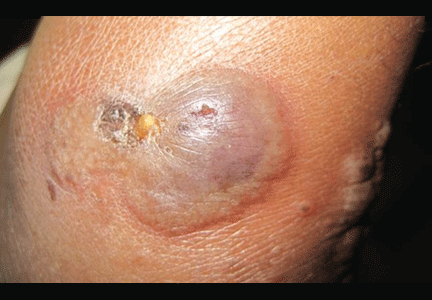
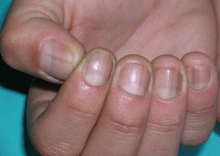
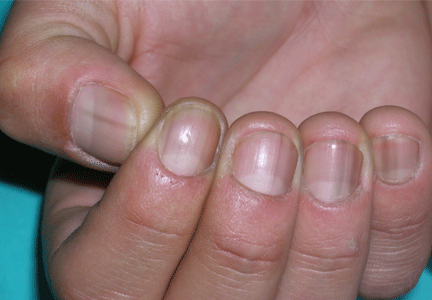
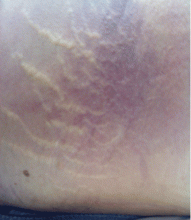
A 36-year-old woman on total parenteral nutrition because of short-bowel syndrome presented with a 2-week history of skin lesions on the face, arms, and legs, but no fever. Examination revealed prominent vesicular lesions on the left arm (Figure 1), face, palms, and soles. Cultures of biopsy specimens were negative for viral, bacterial, and fungal organisms.
Q: Which is the most likely diagnosis?
- Herpes simplex infection
- Varicella zoster infection
- Coxsackievirus infection
- Micronutrient deficiency
- Pemphigus vulgaris
A: Micronutrient deficiency is most likely the cause of her lesions—specifically, severe zinc deficiency, as she was found to have a serum zinc concentration of 12 μg/dL (reference range 55–150). Biopsy specimens showed characteristic intraepidermal blistering with necrosis and minimal inflammation. Serum levels of other micronutrients (iron, copper, selenium) were normal.
Her total parenteral nutrition regimen contained no zinc. Zinc supplementation was started, and a few days later the lesions began to resolve.
Herpes viral infections can cause similar blistering lesions, but this diagnosis was unlikely given the negative viral culture and direct fluorescence antibody test. Coxsackievirus infection is most often seen in children and typically causes fever and mouth sores, which this patient did not have. Lesions of pemphigus vulgaris typically exhibit the Nikolsky sign, ie, they are flaccid, they rupture easily, and the surrounding superficial skin separates from the deeper layers with rubbing or minor trauma. Our patient’s blisters were tense, with a negative Nikolsky sign, and skin biopsy was not consistent with pemphigus vulgaris.
Dermatitis can result from zinc deficiency, which can occur in conditions that cause severe malnutrition due to malabsorption or reduced dietary intake—eg, inflammatory bowel disease, anorexia nervosa, chronic alcoholism, and cystic fibrosis. The lesions can be complicated by secondary bacterial infection, which can cause significant morbidity. Zinc deficiency can also suppress cell-mediated and humoral immunity.
Zinc deficiency can be diagnosed on the basis of clinical findings, skin biopsy, and serum zinc levels. Other micronutrient deficiencies can coexist and should be ruled out. Perioral and acral skin lesions are typically more prominent. Zinc supplementation usually produces rapid resolution of the lesions.
Our patient’s presentation highlights the importance of monitoring micronutrient levels, including zinc, in patients on long-term total parenteral nutrition. Nutritional deficiencies should be considered as a possible cause of dermatitis in such patients.
- Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 2010; 22:107–112.
A 36-year-old woman on total parenteral nutrition because of short-bowel syndrome presented with a 2-week history of skin lesions on the face, arms, and legs, but no fever. Examination revealed prominent vesicular lesions on the left arm (Figure 1), face, palms, and soles. Cultures of biopsy specimens were negative for viral, bacterial, and fungal organisms.
Q: Which is the most likely diagnosis?
- Herpes simplex infection
- Varicella zoster infection
- Coxsackievirus infection
- Micronutrient deficiency
- Pemphigus vulgaris
A: Micronutrient deficiency is most likely the cause of her lesions—specifically, severe zinc deficiency, as she was found to have a serum zinc concentration of 12 μg/dL (reference range 55–150). Biopsy specimens showed characteristic intraepidermal blistering with necrosis and minimal inflammation. Serum levels of other micronutrients (iron, copper, selenium) were normal.
Her total parenteral nutrition regimen contained no zinc. Zinc supplementation was started, and a few days later the lesions began to resolve.
Herpes viral infections can cause similar blistering lesions, but this diagnosis was unlikely given the negative viral culture and direct fluorescence antibody test. Coxsackievirus infection is most often seen in children and typically causes fever and mouth sores, which this patient did not have. Lesions of pemphigus vulgaris typically exhibit the Nikolsky sign, ie, they are flaccid, they rupture easily, and the surrounding superficial skin separates from the deeper layers with rubbing or minor trauma. Our patient’s blisters were tense, with a negative Nikolsky sign, and skin biopsy was not consistent with pemphigus vulgaris.
Dermatitis can result from zinc deficiency, which can occur in conditions that cause severe malnutrition due to malabsorption or reduced dietary intake—eg, inflammatory bowel disease, anorexia nervosa, chronic alcoholism, and cystic fibrosis. The lesions can be complicated by secondary bacterial infection, which can cause significant morbidity. Zinc deficiency can also suppress cell-mediated and humoral immunity.
Zinc deficiency can be diagnosed on the basis of clinical findings, skin biopsy, and serum zinc levels. Other micronutrient deficiencies can coexist and should be ruled out. Perioral and acral skin lesions are typically more prominent. Zinc supplementation usually produces rapid resolution of the lesions.
Our patient’s presentation highlights the importance of monitoring micronutrient levels, including zinc, in patients on long-term total parenteral nutrition. Nutritional deficiencies should be considered as a possible cause of dermatitis in such patients.
A 36-year-old woman on total parenteral nutrition because of short-bowel syndrome presented with a 2-week history of skin lesions on the face, arms, and legs, but no fever. Examination revealed prominent vesicular lesions on the left arm (Figure 1), face, palms, and soles. Cultures of biopsy specimens were negative for viral, bacterial, and fungal organisms.
Q: Which is the most likely diagnosis?
- Herpes simplex infection
- Varicella zoster infection
- Coxsackievirus infection
- Micronutrient deficiency
- Pemphigus vulgaris
A: Micronutrient deficiency is most likely the cause of her lesions—specifically, severe zinc deficiency, as she was found to have a serum zinc concentration of 12 μg/dL (reference range 55–150). Biopsy specimens showed characteristic intraepidermal blistering with necrosis and minimal inflammation. Serum levels of other micronutrients (iron, copper, selenium) were normal.
Her total parenteral nutrition regimen contained no zinc. Zinc supplementation was started, and a few days later the lesions began to resolve.
Herpes viral infections can cause similar blistering lesions, but this diagnosis was unlikely given the negative viral culture and direct fluorescence antibody test. Coxsackievirus infection is most often seen in children and typically causes fever and mouth sores, which this patient did not have. Lesions of pemphigus vulgaris typically exhibit the Nikolsky sign, ie, they are flaccid, they rupture easily, and the surrounding superficial skin separates from the deeper layers with rubbing or minor trauma. Our patient’s blisters were tense, with a negative Nikolsky sign, and skin biopsy was not consistent with pemphigus vulgaris.
Dermatitis can result from zinc deficiency, which can occur in conditions that cause severe malnutrition due to malabsorption or reduced dietary intake—eg, inflammatory bowel disease, anorexia nervosa, chronic alcoholism, and cystic fibrosis. The lesions can be complicated by secondary bacterial infection, which can cause significant morbidity. Zinc deficiency can also suppress cell-mediated and humoral immunity.
Zinc deficiency can be diagnosed on the basis of clinical findings, skin biopsy, and serum zinc levels. Other micronutrient deficiencies can coexist and should be ruled out. Perioral and acral skin lesions are typically more prominent. Zinc supplementation usually produces rapid resolution of the lesions.
Our patient’s presentation highlights the importance of monitoring micronutrient levels, including zinc, in patients on long-term total parenteral nutrition. Nutritional deficiencies should be considered as a possible cause of dermatitis in such patients.
- Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 2010; 22:107–112.
- Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 2010; 22:107–112.
It’s all in the P wave
A 49-year-old man with rheumatic mitral valve stenosis, which had been diagnosed 3 years previously, presented to the outpatient department with worsening exertional dyspnea, fatigue, and cough.
At rest, he appeared comfortable; his pulse rate was 94 bpm and his blood pressure was 117/82 mm Hg. Cardiac auscultation revealed a loud first heart sound, a mid-diastolic murmur with presystolic accentuation at the cardiac apex, and a pansystolic murmur at the left lower sternal border that increased in intensity with inspiration. A prominent left parasternal heave was present.
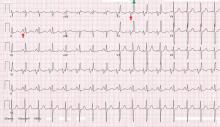
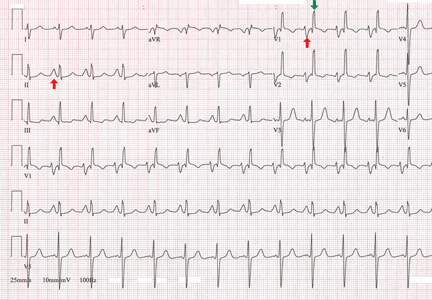
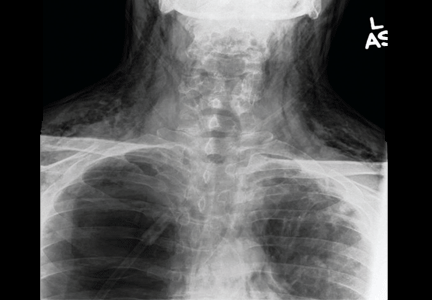
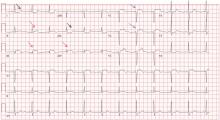
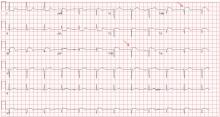
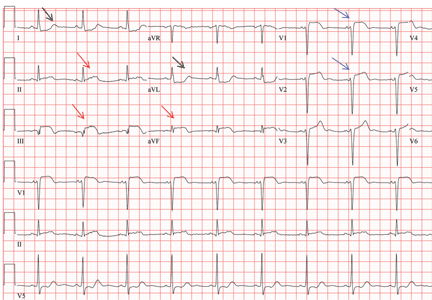
His 12-lead electrocardiogram is shown in Figure 1.
Transthoracic echocardiography confirmed severe mitral stenosis with an estimated mitral valve area of 0.7 cm2 without significant mitral regurgitation. In addition, right ventricular dilatation with moderately severe systolic dysfunction and 4+ (severe) tricuspid regurgitation were present. On the basis of the peak tricuspid regurgitant velocity, the right ventricular systolic pressure was calculated to be 80 mm Hg, consistent with severe pulmonary hypertension. The left ventricular end-diastolic volume was reduced and the ejection fraction was normal.
On right heart catheterization, the pulmonary artery pressure was 92/51 mm Hg.
Q: Electrocardiographic findings that support a diagnosis of pulmonary hypertension include which of the following?
- QRS complex axis of +110°
- R/S (QRS complex) ratio greater than 1 in lead V1
- Sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV
- All of the above
A: The correct answer is all of the above. Regardless of the cause, patients with long-standing pulmonary hypertension possess varying degrees of right ventricular hypertrophy that may be accompanied by right ventricular enlargement and systolic dysfunction. A QRS complex axis of 110° or more, an R/S (QRS complex) ratio greater than 1 in lead V1, and the sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV all support right ventricular hypertrophy.1
As noted in this electrocardiogram, T-wave inversion in leads V1 and V2 supports a right ventricular repolarization abnormality secondary to the hypertrophy.2
Q: Important electrocardiographic findings in this patient that support secondary pulmonary hypertension due to mitral stenosis include which of the following?
- Tall peaked P waves in lead II of at least 0.25 mV and positive P waves in V1 greater than 0.15 mV
- Prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms
- Right ventricular hypertrophy
- All of the above
A: The correct answer is prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms.
Abnormal surface electrocardiographic findings reflecting atrial enlargement or slowed atrial conduction are difficult to differentiate and are best characterized as “atrial abnormalities.” On surface electrocardiography, an atrial abnormality is represented by a P wave morphology that is best studied in leads II and V1. In lead II, a tall peaked P wave of at least 0.25 mV supports right atrial abnormality, and a prolonged P wave (≥ 120 ms) supports left atrial abnormality. In lead V1, right atrial abnormality is suggested by a positive P wave in V1 greater than 0.15 mV, and a terminally negative P wave greater than 40 ms in duration and greater than 0.1 mV deep supports left atrial abnormality.3
It is well recognized that the pathophysiology of pulmonary hypertension involves both the right ventricle and the right atrium.4,5 Therefore, irrespective of the cause of pulmonary hypertension, electrocardiography may additionally reveal right atrial abnormality.6
When the findings suggest pulmonary hypertension (ie, right ventricular hypertrophy with or without right atrial abnormality), it is also important to evaluate for concurrent left atrial abnormality. If present, concomitant left atrial abnormality is a valuable, more specific clue that may help characterize secondary pulmonary hypertension from left-sided heart disease, as illustrated in this example with long-standing severe mitral stenosis.2
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002.
- Goldberger AL. Atrial and ventricular enlargement. In: Clinical Electrocardiography: A Simplified Approach. 7th ed. Philadelphia, PA: Mosby Elsevier; 2006:59–71.
- Bayés-de-Luna A, Goldwasser D, Fiol M, Bayés-Genis A. Surface electrocardiography. In: Hurst’s The Heart. 13th ed. New York, NY: McGraw-Hill Medical; 2011.
- Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr 2007; 8:322–331.
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39:1214–1219.
- Al-Naamani K, Hijal T, Nguyen V, Andrew S, Nguyen T, Huynh T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127:214–218.
A 49-year-old man with rheumatic mitral valve stenosis, which had been diagnosed 3 years previously, presented to the outpatient department with worsening exertional dyspnea, fatigue, and cough.
At rest, he appeared comfortable; his pulse rate was 94 bpm and his blood pressure was 117/82 mm Hg. Cardiac auscultation revealed a loud first heart sound, a mid-diastolic murmur with presystolic accentuation at the cardiac apex, and a pansystolic murmur at the left lower sternal border that increased in intensity with inspiration. A prominent left parasternal heave was present.
His 12-lead electrocardiogram is shown in Figure 1.
Transthoracic echocardiography confirmed severe mitral stenosis with an estimated mitral valve area of 0.7 cm2 without significant mitral regurgitation. In addition, right ventricular dilatation with moderately severe systolic dysfunction and 4+ (severe) tricuspid regurgitation were present. On the basis of the peak tricuspid regurgitant velocity, the right ventricular systolic pressure was calculated to be 80 mm Hg, consistent with severe pulmonary hypertension. The left ventricular end-diastolic volume was reduced and the ejection fraction was normal.
On right heart catheterization, the pulmonary artery pressure was 92/51 mm Hg.
Q: Electrocardiographic findings that support a diagnosis of pulmonary hypertension include which of the following?
- QRS complex axis of +110°
- R/S (QRS complex) ratio greater than 1 in lead V1
- Sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV
- All of the above
A: The correct answer is all of the above. Regardless of the cause, patients with long-standing pulmonary hypertension possess varying degrees of right ventricular hypertrophy that may be accompanied by right ventricular enlargement and systolic dysfunction. A QRS complex axis of 110° or more, an R/S (QRS complex) ratio greater than 1 in lead V1, and the sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV all support right ventricular hypertrophy.1
As noted in this electrocardiogram, T-wave inversion in leads V1 and V2 supports a right ventricular repolarization abnormality secondary to the hypertrophy.2
Q: Important electrocardiographic findings in this patient that support secondary pulmonary hypertension due to mitral stenosis include which of the following?
- Tall peaked P waves in lead II of at least 0.25 mV and positive P waves in V1 greater than 0.15 mV
- Prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms
- Right ventricular hypertrophy
- All of the above
A: The correct answer is prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms.
Abnormal surface electrocardiographic findings reflecting atrial enlargement or slowed atrial conduction are difficult to differentiate and are best characterized as “atrial abnormalities.” On surface electrocardiography, an atrial abnormality is represented by a P wave morphology that is best studied in leads II and V1. In lead II, a tall peaked P wave of at least 0.25 mV supports right atrial abnormality, and a prolonged P wave (≥ 120 ms) supports left atrial abnormality. In lead V1, right atrial abnormality is suggested by a positive P wave in V1 greater than 0.15 mV, and a terminally negative P wave greater than 40 ms in duration and greater than 0.1 mV deep supports left atrial abnormality.3
It is well recognized that the pathophysiology of pulmonary hypertension involves both the right ventricle and the right atrium.4,5 Therefore, irrespective of the cause of pulmonary hypertension, electrocardiography may additionally reveal right atrial abnormality.6
When the findings suggest pulmonary hypertension (ie, right ventricular hypertrophy with or without right atrial abnormality), it is also important to evaluate for concurrent left atrial abnormality. If present, concomitant left atrial abnormality is a valuable, more specific clue that may help characterize secondary pulmonary hypertension from left-sided heart disease, as illustrated in this example with long-standing severe mitral stenosis.2
A 49-year-old man with rheumatic mitral valve stenosis, which had been diagnosed 3 years previously, presented to the outpatient department with worsening exertional dyspnea, fatigue, and cough.
At rest, he appeared comfortable; his pulse rate was 94 bpm and his blood pressure was 117/82 mm Hg. Cardiac auscultation revealed a loud first heart sound, a mid-diastolic murmur with presystolic accentuation at the cardiac apex, and a pansystolic murmur at the left lower sternal border that increased in intensity with inspiration. A prominent left parasternal heave was present.
His 12-lead electrocardiogram is shown in Figure 1.
Transthoracic echocardiography confirmed severe mitral stenosis with an estimated mitral valve area of 0.7 cm2 without significant mitral regurgitation. In addition, right ventricular dilatation with moderately severe systolic dysfunction and 4+ (severe) tricuspid regurgitation were present. On the basis of the peak tricuspid regurgitant velocity, the right ventricular systolic pressure was calculated to be 80 mm Hg, consistent with severe pulmonary hypertension. The left ventricular end-diastolic volume was reduced and the ejection fraction was normal.
On right heart catheterization, the pulmonary artery pressure was 92/51 mm Hg.
Q: Electrocardiographic findings that support a diagnosis of pulmonary hypertension include which of the following?
- QRS complex axis of +110°
- R/S (QRS complex) ratio greater than 1 in lead V1
- Sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV
- All of the above
A: The correct answer is all of the above. Regardless of the cause, patients with long-standing pulmonary hypertension possess varying degrees of right ventricular hypertrophy that may be accompanied by right ventricular enlargement and systolic dysfunction. A QRS complex axis of 110° or more, an R/S (QRS complex) ratio greater than 1 in lead V1, and the sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV all support right ventricular hypertrophy.1
As noted in this electrocardiogram, T-wave inversion in leads V1 and V2 supports a right ventricular repolarization abnormality secondary to the hypertrophy.2
Q: Important electrocardiographic findings in this patient that support secondary pulmonary hypertension due to mitral stenosis include which of the following?
- Tall peaked P waves in lead II of at least 0.25 mV and positive P waves in V1 greater than 0.15 mV
- Prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms
- Right ventricular hypertrophy
- All of the above
A: The correct answer is prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms.
Abnormal surface electrocardiographic findings reflecting atrial enlargement or slowed atrial conduction are difficult to differentiate and are best characterized as “atrial abnormalities.” On surface electrocardiography, an atrial abnormality is represented by a P wave morphology that is best studied in leads II and V1. In lead II, a tall peaked P wave of at least 0.25 mV supports right atrial abnormality, and a prolonged P wave (≥ 120 ms) supports left atrial abnormality. In lead V1, right atrial abnormality is suggested by a positive P wave in V1 greater than 0.15 mV, and a terminally negative P wave greater than 40 ms in duration and greater than 0.1 mV deep supports left atrial abnormality.3
It is well recognized that the pathophysiology of pulmonary hypertension involves both the right ventricle and the right atrium.4,5 Therefore, irrespective of the cause of pulmonary hypertension, electrocardiography may additionally reveal right atrial abnormality.6
When the findings suggest pulmonary hypertension (ie, right ventricular hypertrophy with or without right atrial abnormality), it is also important to evaluate for concurrent left atrial abnormality. If present, concomitant left atrial abnormality is a valuable, more specific clue that may help characterize secondary pulmonary hypertension from left-sided heart disease, as illustrated in this example with long-standing severe mitral stenosis.2
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002.
- Goldberger AL. Atrial and ventricular enlargement. In: Clinical Electrocardiography: A Simplified Approach. 7th ed. Philadelphia, PA: Mosby Elsevier; 2006:59–71.
- Bayés-de-Luna A, Goldwasser D, Fiol M, Bayés-Genis A. Surface electrocardiography. In: Hurst’s The Heart. 13th ed. New York, NY: McGraw-Hill Medical; 2011.
- Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr 2007; 8:322–331.
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39:1214–1219.
- Al-Naamani K, Hijal T, Nguyen V, Andrew S, Nguyen T, Huynh T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127:214–218.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002.
- Goldberger AL. Atrial and ventricular enlargement. In: Clinical Electrocardiography: A Simplified Approach. 7th ed. Philadelphia, PA: Mosby Elsevier; 2006:59–71.
- Bayés-de-Luna A, Goldwasser D, Fiol M, Bayés-Genis A. Surface electrocardiography. In: Hurst’s The Heart. 13th ed. New York, NY: McGraw-Hill Medical; 2011.
- Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr 2007; 8:322–331.
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39:1214–1219.
- Al-Naamani K, Hijal T, Nguyen V, Andrew S, Nguyen T, Huynh T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127:214–218.
Odynophagia, peripheral facial nerve paralysis, mucocutaneous lesions
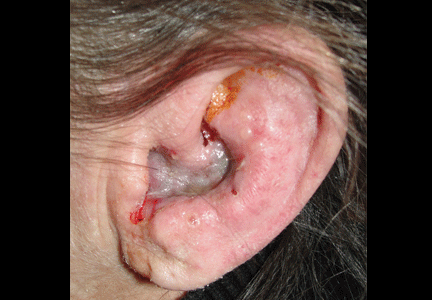
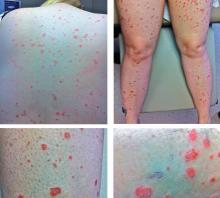
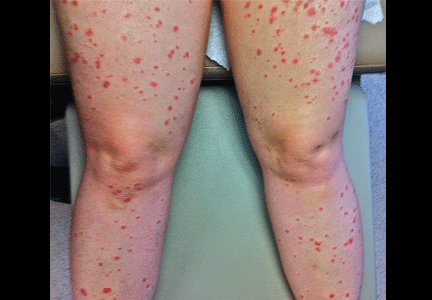
A 54-year-old woman presented with a 7-day history of odynophagia, pharyngeal swelling, and painful skin lesions on her left ear. She had been on antiretroviral therapy for human immunodeficiency virus infection but had not been fully compliant with the treatment.
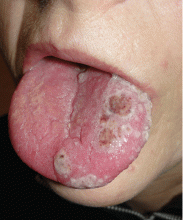
On examination, she had painful erythematous vesicles and pustules on the left auricle and in the external auditory canal (Figure 1), as well as small vesicles and circumscribed erosions on the left anterior twothirds of her tongue (Figure 2) and left palate. Facial sensory function was normal; however, she had lagophthalmos, a flattened nasolabial fold, ptosis of the oral commissure, and a loss of the forehead wrinkles on the left side of her face—all signs of peripheral facial nerve paralysis.
Q: Which is the most likely diagnosis?
- Ramsay Hunt syndrome
- Herpes simplex
- Contact dermatitis
- Malignant external otitis
- Erysipelas
A: This patient had Ramsay Hunt syndrome, also known as herpes zoster oticus. It is a rare complication of herpes zoster in which the reactivation of latent varicella-zoster virus infection in the geniculate ganglion causes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Taste perception, hearing (eg, tinnitus, hyperacusis), and lacrimation can be affected.1
Ramsay Hunt syndrome is generally considered a polycranial neuropathy of cranial nerves VII (facial) and VIII (acoustic). In some cases other cranial neuropathies may be present and may involve cranial nerves V (trigeminal), IX (glossopharyngeal), and X (vagus). Vestibular disturbances such as vertigo are also often reported. It is more severe in patients with immune deficiency. Because the classic symptoms are not always present at the onset, the syndrome can be misdiagnosed.
DIAGNOSIS
Once the vesicular rash caused by herpes zoster has appeared, the diagnosis is usually readily apparent. The other main disease to consider in the differential diagnosis is herpes simplex. Herpes zoster infection is characterized by a painful sensory prodrome, dermatomal distribution, and lack of a history of a similar rash. However, if the patient has had a similar vesicular rash in the same location, then recurrent zosteriform herpes simplex should be considered. A noninfectious cause to consider is contact dermatitis. However, contact dermatitis usually produces intense itch rather than pain.
If the clinical presentation is uncertain, then viral culture, direct immunofluorescence testing, and a polymerase chain reaction assay is indicated to confirm the diagnosis. Polymerase chain reaction testing is the most sensitive test.3
TREATMENT
The rapid start of antiviral therapy is particularly critical in immunocompromised patients,4 even if the vesicles have been present for 72 hours. Immunocompromised patients with Ramsay Hunt syndrome and other forms of complicated herpes zoster infection should be hospitalized for intravenous acyclovir therapy.
Corticosteroids and oral acyclovir (10 mg/kg three times daily for 7 days) are commonly used in Ramsay Hunt syndrome. In a recent review,5 combination therapy with a corticosteroid and intravenous acyclovir did not show a benefit over corticosteroids alone in promoting resolution of facial neuropathy after 6 months.5 However, randomized clinical trials are needed to evaluate both therapies.
Although antiviral therapy reduces pain associated with acute neuritis, pain syndromes associated with herpes zoster can still be severe. Nonsteroidal antiinflammatory drugs and acetaminophen are useful for mild pain, either alone or in combination with a weak opioid analgesic (eg, tramadol, codeine). For moderate to severe pain that disturbs sleep, a stronger opioid analgesic (eg, oxycodone, morphine) may be necessary.6
Vestibular suppressants may be helpful if vestibular symptoms are severe. Temporary relief of otalgia may be achieved by applying a local anesthetic to the trigger point, if in the external auditory canal. Carbamazepine may be helpful, especially in cases of idiopathic geniculate neuralgia.7
OTHER CONSIDERATIONS
Once drug therapy is started, the patient should be seen at 2 weeks, 6 weeks, and 3 months to monitor the evolution of nerve paralysis.8
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg 1990; 102:177–179.
- Adour KK. Otological complications of herpes zoster. Ann Neurol 1994; 35(suppl):S62–S64.
- Stránská R, Schuurman R, de Vos M, van Loon AM. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J Clin Virol 2004; 30:39–44.
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007; 7:741–747.
- Uscategui T, Dorée C, Chamberlain IJ, Burton MJ. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev 2008;(4):CD006851.
- Dworkin RH, Barbano RL, Tyring SK, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009; 142:209–217.
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011; 45:1483–1490.
- Ryu EW, Lee HY, Lee SY, Park MS, Yeo SG. Clinical manifestations and prognosis of patients with Ramsay Hunt syndrome. Am J Otolaryngol 2012; 33:313–318.
A 54-year-old woman presented with a 7-day history of odynophagia, pharyngeal swelling, and painful skin lesions on her left ear. She had been on antiretroviral therapy for human immunodeficiency virus infection but had not been fully compliant with the treatment.
On examination, she had painful erythematous vesicles and pustules on the left auricle and in the external auditory canal (Figure 1), as well as small vesicles and circumscribed erosions on the left anterior twothirds of her tongue (Figure 2) and left palate. Facial sensory function was normal; however, she had lagophthalmos, a flattened nasolabial fold, ptosis of the oral commissure, and a loss of the forehead wrinkles on the left side of her face—all signs of peripheral facial nerve paralysis.
Q: Which is the most likely diagnosis?
- Ramsay Hunt syndrome
- Herpes simplex
- Contact dermatitis
- Malignant external otitis
- Erysipelas
A: This patient had Ramsay Hunt syndrome, also known as herpes zoster oticus. It is a rare complication of herpes zoster in which the reactivation of latent varicella-zoster virus infection in the geniculate ganglion causes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Taste perception, hearing (eg, tinnitus, hyperacusis), and lacrimation can be affected.1
Ramsay Hunt syndrome is generally considered a polycranial neuropathy of cranial nerves VII (facial) and VIII (acoustic). In some cases other cranial neuropathies may be present and may involve cranial nerves V (trigeminal), IX (glossopharyngeal), and X (vagus). Vestibular disturbances such as vertigo are also often reported. It is more severe in patients with immune deficiency. Because the classic symptoms are not always present at the onset, the syndrome can be misdiagnosed.
DIAGNOSIS
Once the vesicular rash caused by herpes zoster has appeared, the diagnosis is usually readily apparent. The other main disease to consider in the differential diagnosis is herpes simplex. Herpes zoster infection is characterized by a painful sensory prodrome, dermatomal distribution, and lack of a history of a similar rash. However, if the patient has had a similar vesicular rash in the same location, then recurrent zosteriform herpes simplex should be considered. A noninfectious cause to consider is contact dermatitis. However, contact dermatitis usually produces intense itch rather than pain.
If the clinical presentation is uncertain, then viral culture, direct immunofluorescence testing, and a polymerase chain reaction assay is indicated to confirm the diagnosis. Polymerase chain reaction testing is the most sensitive test.3
TREATMENT
The rapid start of antiviral therapy is particularly critical in immunocompromised patients,4 even if the vesicles have been present for 72 hours. Immunocompromised patients with Ramsay Hunt syndrome and other forms of complicated herpes zoster infection should be hospitalized for intravenous acyclovir therapy.
Corticosteroids and oral acyclovir (10 mg/kg three times daily for 7 days) are commonly used in Ramsay Hunt syndrome. In a recent review,5 combination therapy with a corticosteroid and intravenous acyclovir did not show a benefit over corticosteroids alone in promoting resolution of facial neuropathy after 6 months.5 However, randomized clinical trials are needed to evaluate both therapies.
Although antiviral therapy reduces pain associated with acute neuritis, pain syndromes associated with herpes zoster can still be severe. Nonsteroidal antiinflammatory drugs and acetaminophen are useful for mild pain, either alone or in combination with a weak opioid analgesic (eg, tramadol, codeine). For moderate to severe pain that disturbs sleep, a stronger opioid analgesic (eg, oxycodone, morphine) may be necessary.6
Vestibular suppressants may be helpful if vestibular symptoms are severe. Temporary relief of otalgia may be achieved by applying a local anesthetic to the trigger point, if in the external auditory canal. Carbamazepine may be helpful, especially in cases of idiopathic geniculate neuralgia.7
OTHER CONSIDERATIONS
Once drug therapy is started, the patient should be seen at 2 weeks, 6 weeks, and 3 months to monitor the evolution of nerve paralysis.8
A 54-year-old woman presented with a 7-day history of odynophagia, pharyngeal swelling, and painful skin lesions on her left ear. She had been on antiretroviral therapy for human immunodeficiency virus infection but had not been fully compliant with the treatment.
On examination, she had painful erythematous vesicles and pustules on the left auricle and in the external auditory canal (Figure 1), as well as small vesicles and circumscribed erosions on the left anterior twothirds of her tongue (Figure 2) and left palate. Facial sensory function was normal; however, she had lagophthalmos, a flattened nasolabial fold, ptosis of the oral commissure, and a loss of the forehead wrinkles on the left side of her face—all signs of peripheral facial nerve paralysis.
Q: Which is the most likely diagnosis?
- Ramsay Hunt syndrome
- Herpes simplex
- Contact dermatitis
- Malignant external otitis
- Erysipelas
A: This patient had Ramsay Hunt syndrome, also known as herpes zoster oticus. It is a rare complication of herpes zoster in which the reactivation of latent varicella-zoster virus infection in the geniculate ganglion causes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Taste perception, hearing (eg, tinnitus, hyperacusis), and lacrimation can be affected.1
Ramsay Hunt syndrome is generally considered a polycranial neuropathy of cranial nerves VII (facial) and VIII (acoustic). In some cases other cranial neuropathies may be present and may involve cranial nerves V (trigeminal), IX (glossopharyngeal), and X (vagus). Vestibular disturbances such as vertigo are also often reported. It is more severe in patients with immune deficiency. Because the classic symptoms are not always present at the onset, the syndrome can be misdiagnosed.
DIAGNOSIS
Once the vesicular rash caused by herpes zoster has appeared, the diagnosis is usually readily apparent. The other main disease to consider in the differential diagnosis is herpes simplex. Herpes zoster infection is characterized by a painful sensory prodrome, dermatomal distribution, and lack of a history of a similar rash. However, if the patient has had a similar vesicular rash in the same location, then recurrent zosteriform herpes simplex should be considered. A noninfectious cause to consider is contact dermatitis. However, contact dermatitis usually produces intense itch rather than pain.
If the clinical presentation is uncertain, then viral culture, direct immunofluorescence testing, and a polymerase chain reaction assay is indicated to confirm the diagnosis. Polymerase chain reaction testing is the most sensitive test.3
TREATMENT
The rapid start of antiviral therapy is particularly critical in immunocompromised patients,4 even if the vesicles have been present for 72 hours. Immunocompromised patients with Ramsay Hunt syndrome and other forms of complicated herpes zoster infection should be hospitalized for intravenous acyclovir therapy.
Corticosteroids and oral acyclovir (10 mg/kg three times daily for 7 days) are commonly used in Ramsay Hunt syndrome. In a recent review,5 combination therapy with a corticosteroid and intravenous acyclovir did not show a benefit over corticosteroids alone in promoting resolution of facial neuropathy after 6 months.5 However, randomized clinical trials are needed to evaluate both therapies.
Although antiviral therapy reduces pain associated with acute neuritis, pain syndromes associated with herpes zoster can still be severe. Nonsteroidal antiinflammatory drugs and acetaminophen are useful for mild pain, either alone or in combination with a weak opioid analgesic (eg, tramadol, codeine). For moderate to severe pain that disturbs sleep, a stronger opioid analgesic (eg, oxycodone, morphine) may be necessary.6
Vestibular suppressants may be helpful if vestibular symptoms are severe. Temporary relief of otalgia may be achieved by applying a local anesthetic to the trigger point, if in the external auditory canal. Carbamazepine may be helpful, especially in cases of idiopathic geniculate neuralgia.7
OTHER CONSIDERATIONS
Once drug therapy is started, the patient should be seen at 2 weeks, 6 weeks, and 3 months to monitor the evolution of nerve paralysis.8
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg 1990; 102:177–179.
- Adour KK. Otological complications of herpes zoster. Ann Neurol 1994; 35(suppl):S62–S64.
- Stránská R, Schuurman R, de Vos M, van Loon AM. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J Clin Virol 2004; 30:39–44.
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007; 7:741–747.
- Uscategui T, Dorée C, Chamberlain IJ, Burton MJ. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev 2008;(4):CD006851.
- Dworkin RH, Barbano RL, Tyring SK, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009; 142:209–217.
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011; 45:1483–1490.
- Ryu EW, Lee HY, Lee SY, Park MS, Yeo SG. Clinical manifestations and prognosis of patients with Ramsay Hunt syndrome. Am J Otolaryngol 2012; 33:313–318.
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg 1990; 102:177–179.
- Adour KK. Otological complications of herpes zoster. Ann Neurol 1994; 35(suppl):S62–S64.
- Stránská R, Schuurman R, de Vos M, van Loon AM. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J Clin Virol 2004; 30:39–44.
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007; 7:741–747.
- Uscategui T, Dorée C, Chamberlain IJ, Burton MJ. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev 2008;(4):CD006851.
- Dworkin RH, Barbano RL, Tyring SK, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009; 142:209–217.
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011; 45:1483–1490.
- Ryu EW, Lee HY, Lee SY, Park MS, Yeo SG. Clinical manifestations and prognosis of patients with Ramsay Hunt syndrome. Am J Otolaryngol 2012; 33:313–318.
Right upper-abdominal pain in a 97-year-old
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
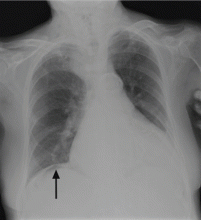
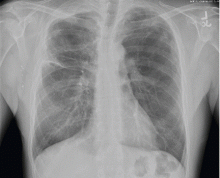
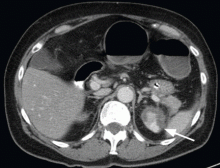
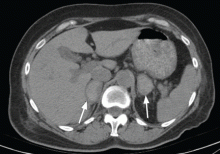
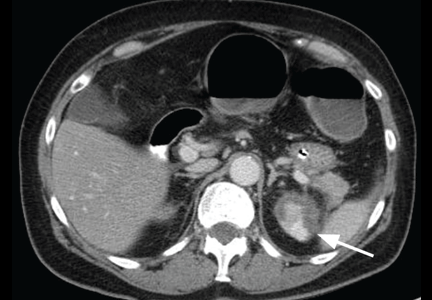
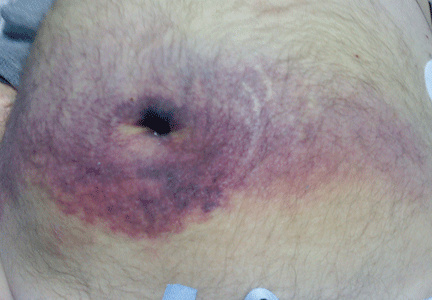
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
A 47-year-old man with chest and neck pain
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
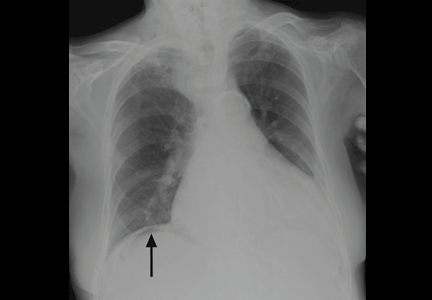
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
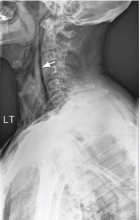
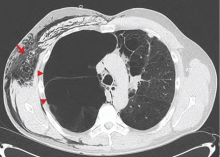
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
Bilateral adrenal masses
A 68-year-old woman presented to the emergency department with constipation and abdominal pain 13 days after left hip arthroplasty. Abdominal computed tomography (CT) revealed possible bowel obstruction, left renal infarction, and a thrombus in the abdominal aorta near the left renal artery (Figure 1).
Because of the aortic thrombus, anticoagulation with intravenous heparin and with warfarin (Coumadin) was started. Three days later, her platelet count decreased to 54 × 109/L (reference range 150–400), her serum creatinine rose to 2.38 mg/dL (0.70–1.40), sodium was stable at 131 mmol/L (132–148), and potassium was 4.1 mmol/L (3.5–5.0).
Physical examination revealed a temperature of 100.2°F (37.9°C), blood pressure 110/59 mm Hg, pulse 100 bpm, and no abdominal pain on palpation. Renal ultrasonography revealed a mass (2.4 × 2.1 × 3.6 cm) above the right kidney. Abdominal CT without contrast showed bilateral high-density adrenal masses (Figure 2).
Q: Which is the most likely diagnosis?
- Metastasis to the adrenal glands
- Adrenal adenoma
- Pheochromocytoma
- Adrenal cortical carcinoma
- Adrenal gland hemorrhage
A: The correct diagnosis is adrenal gland hemorrhage.
Acute or subacute hemorrhage typically results in an oval hyperdense mass with an attenuation of 50 to 90 Hounsfield units (H) on noncontrast CT (Figure 2),1,2 and this attenuation does not increase with the use of contrast.2
In contrast, adrenal cortical carcinoma typically appears as a large heterogeneous mass, with some lesions demonstrating central necrosis or calcification. Pheochromocytoma is well defined, with intense enhancement after contrast is given. Adenoma is usually homogenous, with well-defined margins. Many adenomas have increased intracytoplasmic lipid content and, therefore, will have an attenuation of less than 10 H or will demonstrate rapid washout of contrast. Metastasis to the adrenal gland may not have a characteristic radiographic appearance but typically has a slower contrast washout rate than adenoma.1
This patient’s initial abdominal image showed normal-appearing adrenal glands, thus making adenoma, adrenal metastasis, pheochromocytoma, and adrenal cortical carcinoma unlikely.
The patient’s baseline cortisol level, a random afternoon reading, was 0.4 μg/dL (reference range 3.4–26.9), and a 1-hour cortrosyn-stimulated cortisol was 0 μg/dL, which is diagnostic of primary adrenal insufficiency in the context of this clinical setting. She received hydrocortisone and fludrocortisone, and 8 am cortisol measurements 3 weeks and 5 months later, after the patient was off hydrocortisone for 24 hours, remained undetectable.
The diagnosis of adrenal hemorrhage can be difficult because the symptoms can be nonspecific and attributable to other clinical factors. In a review of 141 patients with adrenal hemorrhage,3 only 19% of patients with bilateral adrenal hemorrhage developed hypotension with a systolic blood pressure less than 90 mm Hg, only 15% developed hyponatremia (sodium < 130 mmol/L), and only 24% developed hyperkalemia (potassium > 5 mmol/L).3
If unrecognized, adrenal insufficiency from adrenal hemorrhage is fatal. Abdominal CT or magnetic resonance imaging can diagnose adrenal hemorrhage. Adrenal function may recover (although it did not in this patient), and a morning cortisol level should be obtained to reevaluate adrenal function.3
Risk factors for adrenal hemorrhage include anticoagulation therapy, sepsis, surgery, hypotension, and coagulopathy as seen in heparininduced thrombocytopenia and disseminated intravascular coagulation. This patient had coagulopathy, as evidenced by her abdominal aortic thrombus, for which she was placed on anticoagulation. Patients without these risk factors for adrenal gland hemorrhage should be investigated for an underlying adrenal neoplasm or cyst.2
- Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol 2002; 179:559–568.
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999; 19:949–963.
- Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001; 76:161–168.
A 68-year-old woman presented to the emergency department with constipation and abdominal pain 13 days after left hip arthroplasty. Abdominal computed tomography (CT) revealed possible bowel obstruction, left renal infarction, and a thrombus in the abdominal aorta near the left renal artery (Figure 1).
Because of the aortic thrombus, anticoagulation with intravenous heparin and with warfarin (Coumadin) was started. Three days later, her platelet count decreased to 54 × 109/L (reference range 150–400), her serum creatinine rose to 2.38 mg/dL (0.70–1.40), sodium was stable at 131 mmol/L (132–148), and potassium was 4.1 mmol/L (3.5–5.0).
Physical examination revealed a temperature of 100.2°F (37.9°C), blood pressure 110/59 mm Hg, pulse 100 bpm, and no abdominal pain on palpation. Renal ultrasonography revealed a mass (2.4 × 2.1 × 3.6 cm) above the right kidney. Abdominal CT without contrast showed bilateral high-density adrenal masses (Figure 2).
Q: Which is the most likely diagnosis?
- Metastasis to the adrenal glands
- Adrenal adenoma
- Pheochromocytoma
- Adrenal cortical carcinoma
- Adrenal gland hemorrhage
A: The correct diagnosis is adrenal gland hemorrhage.
Acute or subacute hemorrhage typically results in an oval hyperdense mass with an attenuation of 50 to 90 Hounsfield units (H) on noncontrast CT (Figure 2),1,2 and this attenuation does not increase with the use of contrast.2
In contrast, adrenal cortical carcinoma typically appears as a large heterogeneous mass, with some lesions demonstrating central necrosis or calcification. Pheochromocytoma is well defined, with intense enhancement after contrast is given. Adenoma is usually homogenous, with well-defined margins. Many adenomas have increased intracytoplasmic lipid content and, therefore, will have an attenuation of less than 10 H or will demonstrate rapid washout of contrast. Metastasis to the adrenal gland may not have a characteristic radiographic appearance but typically has a slower contrast washout rate than adenoma.1
This patient’s initial abdominal image showed normal-appearing adrenal glands, thus making adenoma, adrenal metastasis, pheochromocytoma, and adrenal cortical carcinoma unlikely.
The patient’s baseline cortisol level, a random afternoon reading, was 0.4 μg/dL (reference range 3.4–26.9), and a 1-hour cortrosyn-stimulated cortisol was 0 μg/dL, which is diagnostic of primary adrenal insufficiency in the context of this clinical setting. She received hydrocortisone and fludrocortisone, and 8 am cortisol measurements 3 weeks and 5 months later, after the patient was off hydrocortisone for 24 hours, remained undetectable.
The diagnosis of adrenal hemorrhage can be difficult because the symptoms can be nonspecific and attributable to other clinical factors. In a review of 141 patients with adrenal hemorrhage,3 only 19% of patients with bilateral adrenal hemorrhage developed hypotension with a systolic blood pressure less than 90 mm Hg, only 15% developed hyponatremia (sodium < 130 mmol/L), and only 24% developed hyperkalemia (potassium > 5 mmol/L).3
If unrecognized, adrenal insufficiency from adrenal hemorrhage is fatal. Abdominal CT or magnetic resonance imaging can diagnose adrenal hemorrhage. Adrenal function may recover (although it did not in this patient), and a morning cortisol level should be obtained to reevaluate adrenal function.3
Risk factors for adrenal hemorrhage include anticoagulation therapy, sepsis, surgery, hypotension, and coagulopathy as seen in heparininduced thrombocytopenia and disseminated intravascular coagulation. This patient had coagulopathy, as evidenced by her abdominal aortic thrombus, for which she was placed on anticoagulation. Patients without these risk factors for adrenal gland hemorrhage should be investigated for an underlying adrenal neoplasm or cyst.2
A 68-year-old woman presented to the emergency department with constipation and abdominal pain 13 days after left hip arthroplasty. Abdominal computed tomography (CT) revealed possible bowel obstruction, left renal infarction, and a thrombus in the abdominal aorta near the left renal artery (Figure 1).
Because of the aortic thrombus, anticoagulation with intravenous heparin and with warfarin (Coumadin) was started. Three days later, her platelet count decreased to 54 × 109/L (reference range 150–400), her serum creatinine rose to 2.38 mg/dL (0.70–1.40), sodium was stable at 131 mmol/L (132–148), and potassium was 4.1 mmol/L (3.5–5.0).
Physical examination revealed a temperature of 100.2°F (37.9°C), blood pressure 110/59 mm Hg, pulse 100 bpm, and no abdominal pain on palpation. Renal ultrasonography revealed a mass (2.4 × 2.1 × 3.6 cm) above the right kidney. Abdominal CT without contrast showed bilateral high-density adrenal masses (Figure 2).
Q: Which is the most likely diagnosis?
- Metastasis to the adrenal glands
- Adrenal adenoma
- Pheochromocytoma
- Adrenal cortical carcinoma
- Adrenal gland hemorrhage
A: The correct diagnosis is adrenal gland hemorrhage.
Acute or subacute hemorrhage typically results in an oval hyperdense mass with an attenuation of 50 to 90 Hounsfield units (H) on noncontrast CT (Figure 2),1,2 and this attenuation does not increase with the use of contrast.2
In contrast, adrenal cortical carcinoma typically appears as a large heterogeneous mass, with some lesions demonstrating central necrosis or calcification. Pheochromocytoma is well defined, with intense enhancement after contrast is given. Adenoma is usually homogenous, with well-defined margins. Many adenomas have increased intracytoplasmic lipid content and, therefore, will have an attenuation of less than 10 H or will demonstrate rapid washout of contrast. Metastasis to the adrenal gland may not have a characteristic radiographic appearance but typically has a slower contrast washout rate than adenoma.1
This patient’s initial abdominal image showed normal-appearing adrenal glands, thus making adenoma, adrenal metastasis, pheochromocytoma, and adrenal cortical carcinoma unlikely.
The patient’s baseline cortisol level, a random afternoon reading, was 0.4 μg/dL (reference range 3.4–26.9), and a 1-hour cortrosyn-stimulated cortisol was 0 μg/dL, which is diagnostic of primary adrenal insufficiency in the context of this clinical setting. She received hydrocortisone and fludrocortisone, and 8 am cortisol measurements 3 weeks and 5 months later, after the patient was off hydrocortisone for 24 hours, remained undetectable.
The diagnosis of adrenal hemorrhage can be difficult because the symptoms can be nonspecific and attributable to other clinical factors. In a review of 141 patients with adrenal hemorrhage,3 only 19% of patients with bilateral adrenal hemorrhage developed hypotension with a systolic blood pressure less than 90 mm Hg, only 15% developed hyponatremia (sodium < 130 mmol/L), and only 24% developed hyperkalemia (potassium > 5 mmol/L).3
If unrecognized, adrenal insufficiency from adrenal hemorrhage is fatal. Abdominal CT or magnetic resonance imaging can diagnose adrenal hemorrhage. Adrenal function may recover (although it did not in this patient), and a morning cortisol level should be obtained to reevaluate adrenal function.3
Risk factors for adrenal hemorrhage include anticoagulation therapy, sepsis, surgery, hypotension, and coagulopathy as seen in heparininduced thrombocytopenia and disseminated intravascular coagulation. This patient had coagulopathy, as evidenced by her abdominal aortic thrombus, for which she was placed on anticoagulation. Patients without these risk factors for adrenal gland hemorrhage should be investigated for an underlying adrenal neoplasm or cyst.2
- Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol 2002; 179:559–568.
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999; 19:949–963.
- Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001; 76:161–168.
- Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol 2002; 179:559–568.
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999; 19:949–963.
- Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001; 76:161–168.
Nail pigmentation and fatigue
A 49-year-old man presented with pigmentation on the nails of both hands during the past 6 months. He had no history of significant medical conditions, but he complained of generalized asthenia, with no fever, during the previous 6 weeks. He said that he was a volcanologist and had recently returned from an expedition in the mountains of Peru that involved intense physical activity at high altitudes. He was a non-smoker and did not drink alcohol; he was not taking any medications or nutritional supplements. A biochemical profile including a white blood cell count 3 months ago was normal.
On physical examination, he had splenomegaly (about 2 cm below the costal margin) and low systolic blood pressure (90 mm Hg, with the diastolic pressure at 60 mm Hg). Heart and lung examinations, including chest radiography, were normal.
Q: What is the diagnosis?
- Addison disease
- Onychotillomania
- Longitudinal melanonychia in human immunodeficiency virus (HIV) infection
- Systemic lupus erythematosus
A: The patient was diagnosed with longitudinal melanonychia with underlying HIV infection.
In addition to the clinical findings noted above, laboratory testing now revealed a low white blood cell count (2.9 × 109/L, reference range 4.5–11.0) and a low CD4+ T-cell count (182 cells/mL). An enzyme-linked immunosorbent assay and a Western blot assay were positive for HIV, and his viral load was 120,000 copies/mL as determined by reverse transcription-polymerase chain reaction testing. On more detailed questioning, the patient admitted to sexual practices that put him at high risk for HIV infection.
Longitudinal melanonychia is characterized by a tan, brown, or black longitudinal streak, originating in the matrix and resulting from increased synthesis of melanin (by either normal or abnormal melanocytes) and its deposition within the nail plate.1
If the cause is not apparent (eg, trauma, application of chemical agents, concomitant onychomycosis or treatments), this pigmentation usually reflects the presence of a benign lesion within the matrix caused by a melanocytic nevus, simple lentigo, or increased activation of benign melanocytes. This finding has been reported in a US study2 to be more common in people with dark skin, occurring in 77% of blacks over age 20 and in almost 100% of those over age 50.
Melanoma and other nonmelanocytic tumors may present as longitudinal melanonychia, but these rarely involve more than one digit simultaneously, and they are usually detected with specific clinical clues, such as the Hutchinson sign, ie, periungual spread of pigmentation into the proximal and lateral nail folds.
A SIGN OF BENIGN AND MALIGNANT CONDITIONS
Longitudinal melanonychia can have benign causes, and these need to be investigated. It can occur with repeated trauma, friction, or pressure on the nails. The physician should question the patient about habits such as picking or chewing of the finger tips or pushing back the cuticles, as well as about athletic activities or manual work that could affect the nails. In addition, blood thinners can cause nail discoloration via subungual hemorrhage.
A number of pathologic conditions can cause longitudinal discoloration of the nails. Infection with certain dermatophytes can cause discoloration, but this pigmentation is usually wider and more distal at the hyponychium rather than proximal, with a black streak with several pointed extensions proximally.
Adrenocortical insufficiency (Addison disease), nutritional disorders (deficiency of folic acid or vitamin B12), and, more rarely, systemic lupus erythematosus and scleroderma should be ruled out, as they are typically associated with cutaneous and mucosal hyperpigmentation.
Chemotherapeutic agents such as doxorubicin (Adriamycin), hydroxyurea (Droxea), and cyclophosphamide and drugs such as antimalarials and tetracyclines can also cause longitudinal melanonychia.1,3
Nail lesions with a similar pattern are often seen in patients (especially black patients) taking zidovudine (Retrovir), and these lesions seem not to be related to drug dosage, HIV risk group, or severity of the infection. Nail discoloration in HIV patients not taking antiretroviral therapy has not been well documented.
The significance of melanonychia in the course of HIV remains unclear. A key role of overexpression of alpha-melanocyte-stimulating-hormone has been proposed.4 Although rare in fair-skinned people, longitudinal melanonychia represents an important clinical sign of disease progression. In reported cases, however, no significant improvement has been noted with treatment despite favorable virologic and immunologic responses.4,5
- Smith DF, Morgan MB, Bettencourt MS. Longitudinal melanonychia. Arch Dermatol 2003; 139:1209–1214.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989; 21:1165–1175.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001; 27:580–584.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002; 46:284–293.
- Ehnzadeh-Cheemeh P, Grimes RM, Rowan P, Huang YJ, Essien EJ, Lewis ST. Melanonychia in patients infected with human immunodeficiency virus original communication. Adv Infect Dis 2011; 1:15–19.
A 49-year-old man presented with pigmentation on the nails of both hands during the past 6 months. He had no history of significant medical conditions, but he complained of generalized asthenia, with no fever, during the previous 6 weeks. He said that he was a volcanologist and had recently returned from an expedition in the mountains of Peru that involved intense physical activity at high altitudes. He was a non-smoker and did not drink alcohol; he was not taking any medications or nutritional supplements. A biochemical profile including a white blood cell count 3 months ago was normal.
On physical examination, he had splenomegaly (about 2 cm below the costal margin) and low systolic blood pressure (90 mm Hg, with the diastolic pressure at 60 mm Hg). Heart and lung examinations, including chest radiography, were normal.
Q: What is the diagnosis?
- Addison disease
- Onychotillomania
- Longitudinal melanonychia in human immunodeficiency virus (HIV) infection
- Systemic lupus erythematosus
A: The patient was diagnosed with longitudinal melanonychia with underlying HIV infection.
In addition to the clinical findings noted above, laboratory testing now revealed a low white blood cell count (2.9 × 109/L, reference range 4.5–11.0) and a low CD4+ T-cell count (182 cells/mL). An enzyme-linked immunosorbent assay and a Western blot assay were positive for HIV, and his viral load was 120,000 copies/mL as determined by reverse transcription-polymerase chain reaction testing. On more detailed questioning, the patient admitted to sexual practices that put him at high risk for HIV infection.
Longitudinal melanonychia is characterized by a tan, brown, or black longitudinal streak, originating in the matrix and resulting from increased synthesis of melanin (by either normal or abnormal melanocytes) and its deposition within the nail plate.1
If the cause is not apparent (eg, trauma, application of chemical agents, concomitant onychomycosis or treatments), this pigmentation usually reflects the presence of a benign lesion within the matrix caused by a melanocytic nevus, simple lentigo, or increased activation of benign melanocytes. This finding has been reported in a US study2 to be more common in people with dark skin, occurring in 77% of blacks over age 20 and in almost 100% of those over age 50.
Melanoma and other nonmelanocytic tumors may present as longitudinal melanonychia, but these rarely involve more than one digit simultaneously, and they are usually detected with specific clinical clues, such as the Hutchinson sign, ie, periungual spread of pigmentation into the proximal and lateral nail folds.
A SIGN OF BENIGN AND MALIGNANT CONDITIONS
Longitudinal melanonychia can have benign causes, and these need to be investigated. It can occur with repeated trauma, friction, or pressure on the nails. The physician should question the patient about habits such as picking or chewing of the finger tips or pushing back the cuticles, as well as about athletic activities or manual work that could affect the nails. In addition, blood thinners can cause nail discoloration via subungual hemorrhage.
A number of pathologic conditions can cause longitudinal discoloration of the nails. Infection with certain dermatophytes can cause discoloration, but this pigmentation is usually wider and more distal at the hyponychium rather than proximal, with a black streak with several pointed extensions proximally.
Adrenocortical insufficiency (Addison disease), nutritional disorders (deficiency of folic acid or vitamin B12), and, more rarely, systemic lupus erythematosus and scleroderma should be ruled out, as they are typically associated with cutaneous and mucosal hyperpigmentation.
Chemotherapeutic agents such as doxorubicin (Adriamycin), hydroxyurea (Droxea), and cyclophosphamide and drugs such as antimalarials and tetracyclines can also cause longitudinal melanonychia.1,3
Nail lesions with a similar pattern are often seen in patients (especially black patients) taking zidovudine (Retrovir), and these lesions seem not to be related to drug dosage, HIV risk group, or severity of the infection. Nail discoloration in HIV patients not taking antiretroviral therapy has not been well documented.
The significance of melanonychia in the course of HIV remains unclear. A key role of overexpression of alpha-melanocyte-stimulating-hormone has been proposed.4 Although rare in fair-skinned people, longitudinal melanonychia represents an important clinical sign of disease progression. In reported cases, however, no significant improvement has been noted with treatment despite favorable virologic and immunologic responses.4,5
A 49-year-old man presented with pigmentation on the nails of both hands during the past 6 months. He had no history of significant medical conditions, but he complained of generalized asthenia, with no fever, during the previous 6 weeks. He said that he was a volcanologist and had recently returned from an expedition in the mountains of Peru that involved intense physical activity at high altitudes. He was a non-smoker and did not drink alcohol; he was not taking any medications or nutritional supplements. A biochemical profile including a white blood cell count 3 months ago was normal.
On physical examination, he had splenomegaly (about 2 cm below the costal margin) and low systolic blood pressure (90 mm Hg, with the diastolic pressure at 60 mm Hg). Heart and lung examinations, including chest radiography, were normal.
Q: What is the diagnosis?
- Addison disease
- Onychotillomania
- Longitudinal melanonychia in human immunodeficiency virus (HIV) infection
- Systemic lupus erythematosus
A: The patient was diagnosed with longitudinal melanonychia with underlying HIV infection.
In addition to the clinical findings noted above, laboratory testing now revealed a low white blood cell count (2.9 × 109/L, reference range 4.5–11.0) and a low CD4+ T-cell count (182 cells/mL). An enzyme-linked immunosorbent assay and a Western blot assay were positive for HIV, and his viral load was 120,000 copies/mL as determined by reverse transcription-polymerase chain reaction testing. On more detailed questioning, the patient admitted to sexual practices that put him at high risk for HIV infection.
Longitudinal melanonychia is characterized by a tan, brown, or black longitudinal streak, originating in the matrix and resulting from increased synthesis of melanin (by either normal or abnormal melanocytes) and its deposition within the nail plate.1
If the cause is not apparent (eg, trauma, application of chemical agents, concomitant onychomycosis or treatments), this pigmentation usually reflects the presence of a benign lesion within the matrix caused by a melanocytic nevus, simple lentigo, or increased activation of benign melanocytes. This finding has been reported in a US study2 to be more common in people with dark skin, occurring in 77% of blacks over age 20 and in almost 100% of those over age 50.
Melanoma and other nonmelanocytic tumors may present as longitudinal melanonychia, but these rarely involve more than one digit simultaneously, and they are usually detected with specific clinical clues, such as the Hutchinson sign, ie, periungual spread of pigmentation into the proximal and lateral nail folds.
A SIGN OF BENIGN AND MALIGNANT CONDITIONS
Longitudinal melanonychia can have benign causes, and these need to be investigated. It can occur with repeated trauma, friction, or pressure on the nails. The physician should question the patient about habits such as picking or chewing of the finger tips or pushing back the cuticles, as well as about athletic activities or manual work that could affect the nails. In addition, blood thinners can cause nail discoloration via subungual hemorrhage.
A number of pathologic conditions can cause longitudinal discoloration of the nails. Infection with certain dermatophytes can cause discoloration, but this pigmentation is usually wider and more distal at the hyponychium rather than proximal, with a black streak with several pointed extensions proximally.
Adrenocortical insufficiency (Addison disease), nutritional disorders (deficiency of folic acid or vitamin B12), and, more rarely, systemic lupus erythematosus and scleroderma should be ruled out, as they are typically associated with cutaneous and mucosal hyperpigmentation.
Chemotherapeutic agents such as doxorubicin (Adriamycin), hydroxyurea (Droxea), and cyclophosphamide and drugs such as antimalarials and tetracyclines can also cause longitudinal melanonychia.1,3
Nail lesions with a similar pattern are often seen in patients (especially black patients) taking zidovudine (Retrovir), and these lesions seem not to be related to drug dosage, HIV risk group, or severity of the infection. Nail discoloration in HIV patients not taking antiretroviral therapy has not been well documented.
The significance of melanonychia in the course of HIV remains unclear. A key role of overexpression of alpha-melanocyte-stimulating-hormone has been proposed.4 Although rare in fair-skinned people, longitudinal melanonychia represents an important clinical sign of disease progression. In reported cases, however, no significant improvement has been noted with treatment despite favorable virologic and immunologic responses.4,5
- Smith DF, Morgan MB, Bettencourt MS. Longitudinal melanonychia. Arch Dermatol 2003; 139:1209–1214.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989; 21:1165–1175.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001; 27:580–584.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002; 46:284–293.
- Ehnzadeh-Cheemeh P, Grimes RM, Rowan P, Huang YJ, Essien EJ, Lewis ST. Melanonychia in patients infected with human immunodeficiency virus original communication. Adv Infect Dis 2011; 1:15–19.
- Smith DF, Morgan MB, Bettencourt MS. Longitudinal melanonychia. Arch Dermatol 2003; 139:1209–1214.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989; 21:1165–1175.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001; 27:580–584.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002; 46:284–293.
- Ehnzadeh-Cheemeh P, Grimes RM, Rowan P, Huang YJ, Essien EJ, Lewis ST. Melanonychia in patients infected with human immunodeficiency virus original communication. Adv Infect Dis 2011; 1:15–19.
A rash after streptococcal infection
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
A previously healthy 39-year-old woman presented to the emergency department with 7 days of a gradually worsening rash. One week before the onset of the rash, her primary care physician had diagnosed streptococcal pharyngitis, for which she was treated with oral amoxicillin. She had no history of skin or joint problems and was not currently taking any medications.
She was afebrile and her vital signs were normal. She had mild pharyngeal erythema but no palpable cervical lymph nodes. The skin examination showed well demarcated, erythmatous papules 1 cm in diameter, with overlying scales over the entire body, sparing the palms and the soles of the feet (Figure 1).
Q: Which is the most likely diagnosis?
- Impetigo
- Drug reaction
- Guttate psoriasis
- Nummular eczema
- Pityriasis rosea
A: The most likely diagnosis is guttate psoriasis.
Guttate psoriasis is a relatively uncommon condition that affects less than 2% of patients with psoriasis, primarily children and young adults. It is strongly associated with recent or concomitant beta-hemolytic streptococcal infection.1 The rash usually develops 1 to 2 weeks after the streptococcal pharyngitis or upper respiratory tract infection. Other organisms involved in guttate psoriasis are Staphylococcus aureus, Candida, and viruses such as human papillomavirus, human immunodeficiency virus, and human endogenous retrovirus. 2 Several commonly used drugs are also implicated in psoriasiform eruptions, including beta-blockers, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, lithium, metformin, and digoxin.
Acute onset of skin lesions caused by streptococcal infection can be either the first manifestation in a previously unaffected person or an acute exacerbation of long-standing psoriasis. Skin lesions are usually scaly, erythematous, and guttate (drop-shaped); they primarily involve the trunk but can spread to the rest of the body, sparing the palms and soles.
Throat culture should be done to confirm streptococcal infection. Titers of antistreptolysin O are elevated in more than half of patients with guttate psoriasis. Histopathologic examination can differentiate guttate psoriasis from other psoriasiform conditions, such as pityriasis rosea, secondary syphilis, and lichen simplex chronicus; however, the clinical appearance of the rash is so characteristic that biopsy is not usually needed to confirm the diagnosis.
Guttate psoriasis responds well to phototherapy with ultraviolet B radiation and medium-potency topical corticosteroids.3 And since streptococcal throat infection triggers the condition, it must also be treated for complete recovery.
CASE CONTINUED
Our patient was treated with topical steroid creams. Her rash improved slowly and had completely resolved in 6 weeks.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
- England RJ, Strachan DR, Knight LC. Streptococcal tonsillitis and its association with psoriasis: a review. Clin Otolaryngol Allied Sci 1997; 22:532–535.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007; 25:606–615.
- Thappa DM, Laxmisha C. Suit PUVA as an effective and safe modality of treatment in guttate psoriasis. J Eur Acad Dermatol Venereol 2006; 20:1146–1147.
A 60-year-old man with abdominal bruising
A 60-year-old man with hepatocellular carcinoma was admitted to the hospital with pulmonary emboli secondary to inferior vena caval thrombosis that extended to the right atrium.
He became hypotensive on the second day, with a heart rate of 124 per minute, respiratory rate 44 per minute, pulse oxygen saturation 79% on room air, and systolic blood pressure 70 mm Hg. Physical examination revealed abdominal ecchymoses resembling the Cullen sign and flank ecchymoses resembling the Grey Turner sign (Figures 1 and 2).
He was given a bolus of normal saline followed by infusion of fresh frozen plasma and packed red blood cells. His lactate level was 17.52 mg/dL (reference range 0.1–2.2). His hemoglobin and hematocrit decreased precipitously—the hemoglobin from 10.5 g/dL to 5.9 (reference range 14.0–17.5), and the hematocrit from 30.3% to 17.8% (reference range 41–50). He was transferred to the intensive care unit. A do-not-resuscitate order was instituted, and he died 12 hours later.
CONDITIONS RESULTING IN THE CULLEN AND GREY TURNER SIGNS
The Cullen sign, a bluish discoloration of the periumbilical skin, was originally described in 1918 by the gynecologist Thomas Cullen, MD, in a patient with a ruptured ectopic pregnancy.1 The Grey Turner sign, an ecchymotic discoloration of the lateral abdominal wall or flank, was first reported in 1920 by a surgeon, Dr. George Grey Turner, in a patient with acute pancreatitis.2
The signs occur in about 1% of patients with acute pancreatitis and predict a poor prognosis, with a reported death rate of 37%.3
The appearance of ecchymoses in the periumbilical area or flank has been taught as a hallmark of acute pancreatitis.4 However, the original patient described with the Cullen sign did not have pancreatitis,4,5 and it has been reported with many other conditions, including ruptured aortic aneurysm, splenic rupture, and rectus sheath hematoma, as a complication of anticoagulation or perforated duodenal ulcer, and, as in our patient, as a manifestation of liver disease.
How they occur
The common pathway leading to the occurrence of these subcutaneous ecchymoses is retroperitoneal bleeding followed by tracking of blood from the retroperitoneum through a defect in the transversalis fascia to the abdominal wall musculature and then to the periumbilical subcutaneous tissue. In the Cullen sign, blood diffuses from the retroperitoneum along the gastrohepatic and falciform ligaments to the umbilicus. In the Grey Turner sign, blood diffuses from the posterior pararenal space to the lateral edge of the quadratus lumborum muscle.6–8 Blood from a retroperitoneal hemorrhage may also diffuse and pool at the inguinal ligament (Fox sign) or at the scrotum (Bryant sign).5
The time to the appearance of the Cullen or the Grey Turner sign is thought to be at least 24 hours after the onset of retroperitoneal bleeding and averages about 3 days after the onset of pancreatitis.3
- Cullen TS. A new sign in ruptured extrauterine pregnancy. Am J Obstet Gynecol 1918; 78:457.
- Grey Turner G. Local discoloration of the abdominal wall as a sign of acute pancreatitis. Br J Surg 1910; 7:394–395.
- Dickson AP, Imrie CW. The incidence and prognosis of body wall ecchymosis in acute pancreatitis. Surg Gynecol Obstet 1984; 159:343–347.
- Harris S, Naina HV. Cullen’s sign revisited. Am J Med 2008; 121:682–683.
- Bosmann M, Schreiner O, Galle PR. Coexistence of Cullen’s and Grey Turner’s signs in acute pancreatitis. Am J Med 2009; 122:333–334.
- Bem J, Bradley EL. Subcutaneous manifestations of severe acute pancreatitis. Pancreas 1998; 16:551–555.
- Meyers MA, Feldberg MA, Oliphant M. Grey Turner’s sign and Cullen’s sign in acute pancreatitis. Gastrointest Radiol 1989; 14:31–37.
- Mabin TA, Gelfand M. Cullen’s sign, a feature in liver disease. Br Med J 1974; 1:493–494.
A 60-year-old man with hepatocellular carcinoma was admitted to the hospital with pulmonary emboli secondary to inferior vena caval thrombosis that extended to the right atrium.
He became hypotensive on the second day, with a heart rate of 124 per minute, respiratory rate 44 per minute, pulse oxygen saturation 79% on room air, and systolic blood pressure 70 mm Hg. Physical examination revealed abdominal ecchymoses resembling the Cullen sign and flank ecchymoses resembling the Grey Turner sign (Figures 1 and 2).
He was given a bolus of normal saline followed by infusion of fresh frozen plasma and packed red blood cells. His lactate level was 17.52 mg/dL (reference range 0.1–2.2). His hemoglobin and hematocrit decreased precipitously—the hemoglobin from 10.5 g/dL to 5.9 (reference range 14.0–17.5), and the hematocrit from 30.3% to 17.8% (reference range 41–50). He was transferred to the intensive care unit. A do-not-resuscitate order was instituted, and he died 12 hours later.
CONDITIONS RESULTING IN THE CULLEN AND GREY TURNER SIGNS
The Cullen sign, a bluish discoloration of the periumbilical skin, was originally described in 1918 by the gynecologist Thomas Cullen, MD, in a patient with a ruptured ectopic pregnancy.1 The Grey Turner sign, an ecchymotic discoloration of the lateral abdominal wall or flank, was first reported in 1920 by a surgeon, Dr. George Grey Turner, in a patient with acute pancreatitis.2
The signs occur in about 1% of patients with acute pancreatitis and predict a poor prognosis, with a reported death rate of 37%.3
The appearance of ecchymoses in the periumbilical area or flank has been taught as a hallmark of acute pancreatitis.4 However, the original patient described with the Cullen sign did not have pancreatitis,4,5 and it has been reported with many other conditions, including ruptured aortic aneurysm, splenic rupture, and rectus sheath hematoma, as a complication of anticoagulation or perforated duodenal ulcer, and, as in our patient, as a manifestation of liver disease.
How they occur
The common pathway leading to the occurrence of these subcutaneous ecchymoses is retroperitoneal bleeding followed by tracking of blood from the retroperitoneum through a defect in the transversalis fascia to the abdominal wall musculature and then to the periumbilical subcutaneous tissue. In the Cullen sign, blood diffuses from the retroperitoneum along the gastrohepatic and falciform ligaments to the umbilicus. In the Grey Turner sign, blood diffuses from the posterior pararenal space to the lateral edge of the quadratus lumborum muscle.6–8 Blood from a retroperitoneal hemorrhage may also diffuse and pool at the inguinal ligament (Fox sign) or at the scrotum (Bryant sign).5
The time to the appearance of the Cullen or the Grey Turner sign is thought to be at least 24 hours after the onset of retroperitoneal bleeding and averages about 3 days after the onset of pancreatitis.3
A 60-year-old man with hepatocellular carcinoma was admitted to the hospital with pulmonary emboli secondary to inferior vena caval thrombosis that extended to the right atrium.
He became hypotensive on the second day, with a heart rate of 124 per minute, respiratory rate 44 per minute, pulse oxygen saturation 79% on room air, and systolic blood pressure 70 mm Hg. Physical examination revealed abdominal ecchymoses resembling the Cullen sign and flank ecchymoses resembling the Grey Turner sign (Figures 1 and 2).
He was given a bolus of normal saline followed by infusion of fresh frozen plasma and packed red blood cells. His lactate level was 17.52 mg/dL (reference range 0.1–2.2). His hemoglobin and hematocrit decreased precipitously—the hemoglobin from 10.5 g/dL to 5.9 (reference range 14.0–17.5), and the hematocrit from 30.3% to 17.8% (reference range 41–50). He was transferred to the intensive care unit. A do-not-resuscitate order was instituted, and he died 12 hours later.
CONDITIONS RESULTING IN THE CULLEN AND GREY TURNER SIGNS
The Cullen sign, a bluish discoloration of the periumbilical skin, was originally described in 1918 by the gynecologist Thomas Cullen, MD, in a patient with a ruptured ectopic pregnancy.1 The Grey Turner sign, an ecchymotic discoloration of the lateral abdominal wall or flank, was first reported in 1920 by a surgeon, Dr. George Grey Turner, in a patient with acute pancreatitis.2
The signs occur in about 1% of patients with acute pancreatitis and predict a poor prognosis, with a reported death rate of 37%.3
The appearance of ecchymoses in the periumbilical area or flank has been taught as a hallmark of acute pancreatitis.4 However, the original patient described with the Cullen sign did not have pancreatitis,4,5 and it has been reported with many other conditions, including ruptured aortic aneurysm, splenic rupture, and rectus sheath hematoma, as a complication of anticoagulation or perforated duodenal ulcer, and, as in our patient, as a manifestation of liver disease.
How they occur
The common pathway leading to the occurrence of these subcutaneous ecchymoses is retroperitoneal bleeding followed by tracking of blood from the retroperitoneum through a defect in the transversalis fascia to the abdominal wall musculature and then to the periumbilical subcutaneous tissue. In the Cullen sign, blood diffuses from the retroperitoneum along the gastrohepatic and falciform ligaments to the umbilicus. In the Grey Turner sign, blood diffuses from the posterior pararenal space to the lateral edge of the quadratus lumborum muscle.6–8 Blood from a retroperitoneal hemorrhage may also diffuse and pool at the inguinal ligament (Fox sign) or at the scrotum (Bryant sign).5
The time to the appearance of the Cullen or the Grey Turner sign is thought to be at least 24 hours after the onset of retroperitoneal bleeding and averages about 3 days after the onset of pancreatitis.3
- Cullen TS. A new sign in ruptured extrauterine pregnancy. Am J Obstet Gynecol 1918; 78:457.
- Grey Turner G. Local discoloration of the abdominal wall as a sign of acute pancreatitis. Br J Surg 1910; 7:394–395.
- Dickson AP, Imrie CW. The incidence and prognosis of body wall ecchymosis in acute pancreatitis. Surg Gynecol Obstet 1984; 159:343–347.
- Harris S, Naina HV. Cullen’s sign revisited. Am J Med 2008; 121:682–683.
- Bosmann M, Schreiner O, Galle PR. Coexistence of Cullen’s and Grey Turner’s signs in acute pancreatitis. Am J Med 2009; 122:333–334.
- Bem J, Bradley EL. Subcutaneous manifestations of severe acute pancreatitis. Pancreas 1998; 16:551–555.
- Meyers MA, Feldberg MA, Oliphant M. Grey Turner’s sign and Cullen’s sign in acute pancreatitis. Gastrointest Radiol 1989; 14:31–37.
- Mabin TA, Gelfand M. Cullen’s sign, a feature in liver disease. Br Med J 1974; 1:493–494.
- Cullen TS. A new sign in ruptured extrauterine pregnancy. Am J Obstet Gynecol 1918; 78:457.
- Grey Turner G. Local discoloration of the abdominal wall as a sign of acute pancreatitis. Br J Surg 1910; 7:394–395.
- Dickson AP, Imrie CW. The incidence and prognosis of body wall ecchymosis in acute pancreatitis. Surg Gynecol Obstet 1984; 159:343–347.
- Harris S, Naina HV. Cullen’s sign revisited. Am J Med 2008; 121:682–683.
- Bosmann M, Schreiner O, Galle PR. Coexistence of Cullen’s and Grey Turner’s signs in acute pancreatitis. Am J Med 2009; 122:333–334.
- Bem J, Bradley EL. Subcutaneous manifestations of severe acute pancreatitis. Pancreas 1998; 16:551–555.
- Meyers MA, Feldberg MA, Oliphant M. Grey Turner’s sign and Cullen’s sign in acute pancreatitis. Gastrointest Radiol 1989; 14:31–37.
- Mabin TA, Gelfand M. Cullen’s sign, a feature in liver disease. Br Med J 1974; 1:493–494.
V1: The most important lead in inferior STEMI
Q: Which would be the most appropriate diagnosis?
- Pericarditis
- Acute inferior and right ventricular myocardial infarction
- Anterior and inferior myocardial infarction
- None of the above
A: The correct answer is acute inferior and right ventricular myocardial infarction.
Her electrocardiogram showed sinus rhythm and inferior ST-segment elevation myocardial infarction (STEMI) evidenced by ST-segment elevation in leads II, III, and aVF. Hemodynamic instability or ST-segment elevation of more than 1 mm in lead V1 raises the suspicion of right ventricular myocardial infarction. In such patients, the American Heart Association guidelines recommend electrocardiography with right-sided precordial leads.1
A 1-mm ST-segment elevation in the right precordial lead V4R is one of the most predictive electrocardiographic findings in right ventricular infarction.2 The electrocardiographic changes in this type of myocardial infarction may be transient and resolve within 10 hours in up to 48% of cases.3
Echocardiography can also be used to confirm the possibility of right ventricular infarction.
Q: Which clinical condition can occur as a complication of right ventricular myocardial infarction?
- Profound hypotension after nitrate administration
- High-degree heart block
- Atrial fibrillation
- All of the above
A: All of the conditions can occur.
Right ventricular involvement is very common, noted in up to 50% of patients with acute inferior STEMI in postmortem studies.4 However, hemodynamically significant right ventricular dysfunction is much less common.
Intravenous volume loading with normal saline is one of the first steps in the management of hypotension associated with right ventricular infarction. Patients with significant bradycardia or a high degree of atrioventricular block may require pacing. Early reperfusion should be achieved, if possible. Heightened suspicion is critical to the early diagnosis of this condition, since the prognosis is much worse than for isolated inferior STEMI.4
Our patient was found to have right coronary artery disease requiring percutaneous coronary intervention.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118:138–144.
- Braat SH, Brugada P, de Zwaan C, Coenegracht JM, Wellens HJ. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49:368–372.
- Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981–988.
Q: Which would be the most appropriate diagnosis?
- Pericarditis
- Acute inferior and right ventricular myocardial infarction
- Anterior and inferior myocardial infarction
- None of the above
A: The correct answer is acute inferior and right ventricular myocardial infarction.
Her electrocardiogram showed sinus rhythm and inferior ST-segment elevation myocardial infarction (STEMI) evidenced by ST-segment elevation in leads II, III, and aVF. Hemodynamic instability or ST-segment elevation of more than 1 mm in lead V1 raises the suspicion of right ventricular myocardial infarction. In such patients, the American Heart Association guidelines recommend electrocardiography with right-sided precordial leads.1
A 1-mm ST-segment elevation in the right precordial lead V4R is one of the most predictive electrocardiographic findings in right ventricular infarction.2 The electrocardiographic changes in this type of myocardial infarction may be transient and resolve within 10 hours in up to 48% of cases.3
Echocardiography can also be used to confirm the possibility of right ventricular infarction.
Q: Which clinical condition can occur as a complication of right ventricular myocardial infarction?
- Profound hypotension after nitrate administration
- High-degree heart block
- Atrial fibrillation
- All of the above
A: All of the conditions can occur.
Right ventricular involvement is very common, noted in up to 50% of patients with acute inferior STEMI in postmortem studies.4 However, hemodynamically significant right ventricular dysfunction is much less common.
Intravenous volume loading with normal saline is one of the first steps in the management of hypotension associated with right ventricular infarction. Patients with significant bradycardia or a high degree of atrioventricular block may require pacing. Early reperfusion should be achieved, if possible. Heightened suspicion is critical to the early diagnosis of this condition, since the prognosis is much worse than for isolated inferior STEMI.4
Our patient was found to have right coronary artery disease requiring percutaneous coronary intervention.
Q: Which would be the most appropriate diagnosis?
- Pericarditis
- Acute inferior and right ventricular myocardial infarction
- Anterior and inferior myocardial infarction
- None of the above
A: The correct answer is acute inferior and right ventricular myocardial infarction.
Her electrocardiogram showed sinus rhythm and inferior ST-segment elevation myocardial infarction (STEMI) evidenced by ST-segment elevation in leads II, III, and aVF. Hemodynamic instability or ST-segment elevation of more than 1 mm in lead V1 raises the suspicion of right ventricular myocardial infarction. In such patients, the American Heart Association guidelines recommend electrocardiography with right-sided precordial leads.1
A 1-mm ST-segment elevation in the right precordial lead V4R is one of the most predictive electrocardiographic findings in right ventricular infarction.2 The electrocardiographic changes in this type of myocardial infarction may be transient and resolve within 10 hours in up to 48% of cases.3
Echocardiography can also be used to confirm the possibility of right ventricular infarction.
Q: Which clinical condition can occur as a complication of right ventricular myocardial infarction?
- Profound hypotension after nitrate administration
- High-degree heart block
- Atrial fibrillation
- All of the above
A: All of the conditions can occur.
Right ventricular involvement is very common, noted in up to 50% of patients with acute inferior STEMI in postmortem studies.4 However, hemodynamically significant right ventricular dysfunction is much less common.
Intravenous volume loading with normal saline is one of the first steps in the management of hypotension associated with right ventricular infarction. Patients with significant bradycardia or a high degree of atrioventricular block may require pacing. Early reperfusion should be achieved, if possible. Heightened suspicion is critical to the early diagnosis of this condition, since the prognosis is much worse than for isolated inferior STEMI.4
Our patient was found to have right coronary artery disease requiring percutaneous coronary intervention.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118:138–144.
- Braat SH, Brugada P, de Zwaan C, Coenegracht JM, Wellens HJ. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49:368–372.
- Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981–988.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Robalino BD, Whitlow PL, Underwood DA, Salcedo EE. Electrocardiographic manifestations of right ventricular infarction. Am Heart J 1989; 118:138–144.
- Braat SH, Brugada P, de Zwaan C, Coenegracht JM, Wellens HJ. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br Heart J 1983; 49:368–372.
- Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981–988.