User login
Avoiding Diabetes in Patients With Hepatitis C
Epidemiologic data suggest that patients with chronic hepatitis C virus (HCV) infection have an increased risk for insulin resistance—and for overt diabetes.1,2 Specifically, Serfaty and Capteau have reported evidence for “a triangular interaction” between steatosis, inflammatory processes, and insulin resistance.1
Averting these metabolic risks is essential for several reasons: Most importantly, their development is associated with increased liver inflammation and progression to fibrosis and cirrhosis, as well as impaired response to antiviral medications.3,4 Additionally, type 2 diabetes in patients with chronic HCV has been associated with a 1.7-fold increased risk for hepatocellular carcinoma (HCC) and other malignancies.5,6
Though never directly linked with the metabolic syndrome, HCV has been associated with impaired insulin signaling and insulin resistance, in addition to hypocholesterolemia and steatosis. Lonardo et al went so far as to mark this constellation of effects as “a distinct HCV-associated dysmetabolic syndrome.” 4
The dysmetabolic syndrome places affected patients at increased risk for cardiovascular disease, according to Rzouq et al. Statins, they report, are safe and effective in patients with chronic HCV and appear to confer anti-HCV proliferative benefits, making them a potentially “life-saving therapy.”7
Because insulin resistance in the hepatic and peripheral tissues is at the very least “an obvious and significantly detrimental pathophysiologic feature of HCV infection,” Kawaguchi and Mazuta suggest that patients with chronic HCV be encouraged to follow the same dietary and lifestyle recommendations made to those with diabetes, obesity, and metabolic syndrome.2 Maintaining a healthy body weight and following a reasonable regimen of diet and exercise help protect the liver in HCV-infected patients, whereas overweight and obesity, high cholesterol levels, and fatty liver are associated with accelerated liver damage.3
Continue for another component of diabetes risk reduction >>
Another component of diabetes risk reduction in patients with HCV is controlling hypertension.8 Of note, treatment with angiotensin-blocking agents has been associated with reduced liver fibrosis in HCV patients, compared with those receiving no antihypertensives or diuretics, vasodilators, or calcium channel antagonists.8,9
Patients with HCV who do develop diabetes are advised against using insulin or a sulfonylurea (ie, glipizide, glimepiride, glyburide).2 Metformin is considered a safer option, and its use has been linked to a reduced risk for HCC.2,10 Diabetic patients with HCV can also reduce their risk for HCC by maintaining an A1C level below 7.0%, according to Arase et al.5
REFERENCES
1. Serfaty L, Capteau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29(suppl 2):13-25.
2. Kawaguchi Y, Mazuta T. Interaction between hepatitis C virus and metabolic factors. World J Gasterentol. 2014;20(11):2888-2901.
3. US Department of Veterans Affairs. Viral hepatitis: diet and nutrition. http://www.hepatitis.va.gov/patient/daily/diet/single-page.asp. Accessed May 26, 2015.
4. Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med. 2008;3(2):99-108.
5. Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964-973.
6. Takahashi H, Mizuta T, Eguchi Y, et al. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46(6):790-798.
7. Rzouq F, Alahdab F, Olyaee M. Statins and hepatitis C virus infection: an old therapy with new scope. Am J Med Sci. 2014;348(5):426-430.
8. Arase Y, Suzuki F, Suzuki Y, et al. Losartan reduces the onset of type 2 diabetes in hypertensive Japanese patients with chronic hepatitis C. J Med Virol. 2009;81(9):1584-1590.
9. Corey KE, Shah N, Misdraji J, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int. 2009; 29(5):748-753.
10. Harris K, Smith L. Safety and efficacy of metformin in patients with type 2 diabetes mellitus and chronic hepatitis C. Ann Pharmacother. 2013;47(10):1348-1352.
Epidemiologic data suggest that patients with chronic hepatitis C virus (HCV) infection have an increased risk for insulin resistance—and for overt diabetes.1,2 Specifically, Serfaty and Capteau have reported evidence for “a triangular interaction” between steatosis, inflammatory processes, and insulin resistance.1
Averting these metabolic risks is essential for several reasons: Most importantly, their development is associated with increased liver inflammation and progression to fibrosis and cirrhosis, as well as impaired response to antiviral medications.3,4 Additionally, type 2 diabetes in patients with chronic HCV has been associated with a 1.7-fold increased risk for hepatocellular carcinoma (HCC) and other malignancies.5,6
Though never directly linked with the metabolic syndrome, HCV has been associated with impaired insulin signaling and insulin resistance, in addition to hypocholesterolemia and steatosis. Lonardo et al went so far as to mark this constellation of effects as “a distinct HCV-associated dysmetabolic syndrome.” 4
The dysmetabolic syndrome places affected patients at increased risk for cardiovascular disease, according to Rzouq et al. Statins, they report, are safe and effective in patients with chronic HCV and appear to confer anti-HCV proliferative benefits, making them a potentially “life-saving therapy.”7
Because insulin resistance in the hepatic and peripheral tissues is at the very least “an obvious and significantly detrimental pathophysiologic feature of HCV infection,” Kawaguchi and Mazuta suggest that patients with chronic HCV be encouraged to follow the same dietary and lifestyle recommendations made to those with diabetes, obesity, and metabolic syndrome.2 Maintaining a healthy body weight and following a reasonable regimen of diet and exercise help protect the liver in HCV-infected patients, whereas overweight and obesity, high cholesterol levels, and fatty liver are associated with accelerated liver damage.3
Continue for another component of diabetes risk reduction >>
Another component of diabetes risk reduction in patients with HCV is controlling hypertension.8 Of note, treatment with angiotensin-blocking agents has been associated with reduced liver fibrosis in HCV patients, compared with those receiving no antihypertensives or diuretics, vasodilators, or calcium channel antagonists.8,9
Patients with HCV who do develop diabetes are advised against using insulin or a sulfonylurea (ie, glipizide, glimepiride, glyburide).2 Metformin is considered a safer option, and its use has been linked to a reduced risk for HCC.2,10 Diabetic patients with HCV can also reduce their risk for HCC by maintaining an A1C level below 7.0%, according to Arase et al.5
REFERENCES
1. Serfaty L, Capteau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29(suppl 2):13-25.
2. Kawaguchi Y, Mazuta T. Interaction between hepatitis C virus and metabolic factors. World J Gasterentol. 2014;20(11):2888-2901.
3. US Department of Veterans Affairs. Viral hepatitis: diet and nutrition. http://www.hepatitis.va.gov/patient/daily/diet/single-page.asp. Accessed May 26, 2015.
4. Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med. 2008;3(2):99-108.
5. Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964-973.
6. Takahashi H, Mizuta T, Eguchi Y, et al. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46(6):790-798.
7. Rzouq F, Alahdab F, Olyaee M. Statins and hepatitis C virus infection: an old therapy with new scope. Am J Med Sci. 2014;348(5):426-430.
8. Arase Y, Suzuki F, Suzuki Y, et al. Losartan reduces the onset of type 2 diabetes in hypertensive Japanese patients with chronic hepatitis C. J Med Virol. 2009;81(9):1584-1590.
9. Corey KE, Shah N, Misdraji J, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int. 2009; 29(5):748-753.
10. Harris K, Smith L. Safety and efficacy of metformin in patients with type 2 diabetes mellitus and chronic hepatitis C. Ann Pharmacother. 2013;47(10):1348-1352.
Epidemiologic data suggest that patients with chronic hepatitis C virus (HCV) infection have an increased risk for insulin resistance—and for overt diabetes.1,2 Specifically, Serfaty and Capteau have reported evidence for “a triangular interaction” between steatosis, inflammatory processes, and insulin resistance.1
Averting these metabolic risks is essential for several reasons: Most importantly, their development is associated with increased liver inflammation and progression to fibrosis and cirrhosis, as well as impaired response to antiviral medications.3,4 Additionally, type 2 diabetes in patients with chronic HCV has been associated with a 1.7-fold increased risk for hepatocellular carcinoma (HCC) and other malignancies.5,6
Though never directly linked with the metabolic syndrome, HCV has been associated with impaired insulin signaling and insulin resistance, in addition to hypocholesterolemia and steatosis. Lonardo et al went so far as to mark this constellation of effects as “a distinct HCV-associated dysmetabolic syndrome.” 4
The dysmetabolic syndrome places affected patients at increased risk for cardiovascular disease, according to Rzouq et al. Statins, they report, are safe and effective in patients with chronic HCV and appear to confer anti-HCV proliferative benefits, making them a potentially “life-saving therapy.”7
Because insulin resistance in the hepatic and peripheral tissues is at the very least “an obvious and significantly detrimental pathophysiologic feature of HCV infection,” Kawaguchi and Mazuta suggest that patients with chronic HCV be encouraged to follow the same dietary and lifestyle recommendations made to those with diabetes, obesity, and metabolic syndrome.2 Maintaining a healthy body weight and following a reasonable regimen of diet and exercise help protect the liver in HCV-infected patients, whereas overweight and obesity, high cholesterol levels, and fatty liver are associated with accelerated liver damage.3
Continue for another component of diabetes risk reduction >>
Another component of diabetes risk reduction in patients with HCV is controlling hypertension.8 Of note, treatment with angiotensin-blocking agents has been associated with reduced liver fibrosis in HCV patients, compared with those receiving no antihypertensives or diuretics, vasodilators, or calcium channel antagonists.8,9
Patients with HCV who do develop diabetes are advised against using insulin or a sulfonylurea (ie, glipizide, glimepiride, glyburide).2 Metformin is considered a safer option, and its use has been linked to a reduced risk for HCC.2,10 Diabetic patients with HCV can also reduce their risk for HCC by maintaining an A1C level below 7.0%, according to Arase et al.5
REFERENCES
1. Serfaty L, Capteau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29(suppl 2):13-25.
2. Kawaguchi Y, Mazuta T. Interaction between hepatitis C virus and metabolic factors. World J Gasterentol. 2014;20(11):2888-2901.
3. US Department of Veterans Affairs. Viral hepatitis: diet and nutrition. http://www.hepatitis.va.gov/patient/daily/diet/single-page.asp. Accessed May 26, 2015.
4. Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med. 2008;3(2):99-108.
5. Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964-973.
6. Takahashi H, Mizuta T, Eguchi Y, et al. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46(6):790-798.
7. Rzouq F, Alahdab F, Olyaee M. Statins and hepatitis C virus infection: an old therapy with new scope. Am J Med Sci. 2014;348(5):426-430.
8. Arase Y, Suzuki F, Suzuki Y, et al. Losartan reduces the onset of type 2 diabetes in hypertensive Japanese patients with chronic hepatitis C. J Med Virol. 2009;81(9):1584-1590.
9. Corey KE, Shah N, Misdraji J, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int. 2009; 29(5):748-753.
10. Harris K, Smith L. Safety and efficacy of metformin in patients with type 2 diabetes mellitus and chronic hepatitis C. Ann Pharmacother. 2013;47(10):1348-1352.
Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
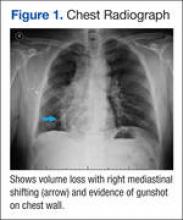
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
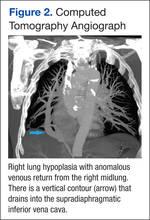
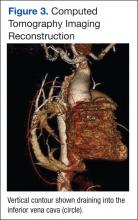
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
Treatment of Ampicillin-Resistant Enterococcus faecium Urinary Tract Infections
Enterococcus species account for about 110,000 urinary tract infections (UTIs) annually in the U.S.1 The most common species isolated are Enterococcus faecalis and Enterococcus faecium (E faecium). Amoxicillin is the drug of choice for the treatment of enterococcal UTIs. Second-line therapies include vancomycin and nitrofurantoin. Alternative therapies include daptomycin and linezolid; however, these newer agents ideally would be reserved for more serious infections to preserve activity.2
Increased E faecium resistance to ampicillin and vancomycin has limited the therapeutic options. The results of a study by Zhanel and colleagues assessed the prevalence of resistant enterococcal urine isolates in North America.3 Of the 658 E faecium urine isolates, about 96% were resistant to ampicillin and 94% were resistant to vancoymcin.3 Nitrofurantoin has much lower resistance rates; however, its use is contraindicated in patients with a creatinine clearance (CrCl) < 60 mL/min.4 Data supporting the contraindication are limited, but the results of a study by Oplinger and Andrews suggested that using nitrofurantoin in patients with a CrCl ≥ 40 mL/min may be safe and effective.5 A therapeutic dilemma may occur when resistant E faecium UTIs are encountered and viable treatment options are limited due to intolerances, administration difficulties, lack of susceptibility data, or cost.
Related: Antimicrobial Stewardship in an Outpatient Parenteral Antibiotic Therapy Program
Based on the current Clinical and Laboratory Standards Institute standard, Enterococcus species with a minimal inhibitory concentration (MIC) ≥ 16 μg/mL are considered ampicillin resistant. Microbiology laboratories use the same breakpoint regardless of the site of infection.6 Amoxicillin concentrates in the urine; therefore, urinary concentrations are much higher than serum concentrations. The mean serum peak concentration after a single dose of oral amoxicillin 500 mg is 7.6 μg/mL.7 After a single dose of oral amoxicillin 500 mg, the average concentration in pooled urine collected over 6 hours was 1,100 μg/mL.8
In 2002, Williamson and colleagues analyzed 30 ampicillin- resistant E faecium urine isolates. Reported MICs were 128 μg/mL (30%), 256 μg/mL (60%), and 512 μg/mL (10%).9 A more recent retrospective analysis analyzed 234 ampicillin-resistant E faecium urine isolates. The MIC ranged from 32 to 1,024 μg/mL, with a median MIC of 256 μg/mL. Only 5 isolates had an MIC value > 1,000 μg/mL, but each of these isolates was within 1 dilution of 512 μg/mL.10 Because penicillins exhibit time-dependent killing, an optimal response will occur as long as the urine concentration is above the MIC for at least 50% of the dosing interval.11 Therefore, therapeutic doses of amoxicillin are expected to produce urine concentrations that exceed the MIC of resistant E faecium urine isolates. The purpose of this study was to determine if amoxicillin was a viable treatment option for ampicillin-resistant E faecium UTIs based on this in vitro theory.
Methods
Veterans aged ≥ 18 years with a positive urine culture for ampicillin- resistant E faecium who received antibiotic therapy for cystitis at the Jesse Brown VA Medical Center (JBVAMC) from January 1, 2005, through June 22, 2010, were evaluated in this retrospective cohort study. Exclusion criteria were the presence of any other organisms in the initial urine culture, prostatic involvement, and the presence of E faecium in a blood culture. Subjects treated with multiple antibiotics concurrently and with sequential treatment of different antibiotics with no evaluation of efficacy between courses were also excluded.
Related: Urologist Workforce Variation Across the VHA
All included subjects were evaluated for resolution of symptoms; improvement in leukocyte esterase count and white blood cell (WBC) count from urine analysis (UA); and eradication of E faecium from a repeat urine culture. The response to treatment was classified as cure, presumed cure, or failure. The criteria for cure were based on the following: resolution of symptoms if present at baseline; repeat UA indicating improvement from the initial positive UA (if obtained); and eradication of E faecium in a repeat urine culture (if obtained).
At least 1 of the aforementioned criteria must have been met to be classified as cure. If more than 1 of the aforementioned criteria was present, then each one must have been met to be classified as cure. To be evaluated for presumed cure, the subject must have had symptoms at baseline. No documentation of ongoing symptoms in subjects who had an appropriate follow-up but did not have a repeat UA or urine culture indicated presumed cure. Persistence or worsening of pretreatment symptoms, a repeat UA without improvement from the initial positive UA, or a repeat urine culture demonstrating continued presence of E faecium indicated failure. The primary endpoint for the study was to determine whether amoxicillin was effective for the management of ampicillin-resistant E faecium UTIs. This study was conducted in compliance with the University of Illinois at Chicago Institutional Review Board and JBVAMC Human Subjects Research Committee requirements.
Results
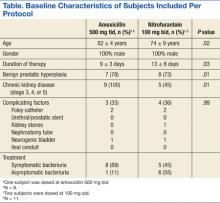
This study included 20 positive urine cultures for ampicillin-resistant E faecium in 19 subjects. Nine cases were treated with amoxicillin, and 11 cases were treated with nitrofurantoin. At baseline, the mean age was 75 years, mean duration of therapy was 14 days, and all the subjects were male. The baseline characteristics of the 2 groups were similar with the exception of an older population, shorter duration of therapy, and increased incidence of chronic kidney disease in the amoxicillin treatment group, P = .02, .03, and .01, respectively.
Symptoms were documented in 8 of 9 (89%) cases at the time of the positive culture in the amoxicillin treatment group and 5 of 11 (45%) cases in the nitrofurantoin treatment group (Table). The asymptomatic amoxicillin treatment group case and 5 of the 6 nitrofurantoin treatment group asymptomatic cases received treatment prior to a urologic procedure in accordance with the Infectious Diseases Society of America (IDSA) guidelines for the treatment of asymptomatic bacteriuria. The urologic procedures included transurethral resection of a bladder tumor, cystoscopy, urethral dilation, cystometrogram, and transurethral resection of the prostate. One asymptomatic subject in the nitrofurantoin group did not have any documentation to support an appropriate indication for treatment. All positive cultures were > 100,000 colonies/mL except for 1 culture in the nitrofurantoin treatment group, which was 45,000 colonies/mL, but because the subject was symptomatic, treatment was administered and a repeat urine culture was negative.
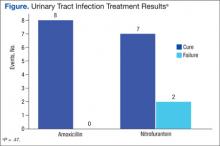
There were 8 cases classified as cure, 1 presumed cure, and no failures in the amoxicillin group. In the nitrofurantoin group, 7 cases were classified as cure, 1 presumed cure, and 3 failures. The presumed cures were excluded from the statistical analysis due to inability to ensure these cases were truly cured. Also excluded from the statistical analysis was one of the failures in the nitrofurantoin group, because the subject was asymptomatic with no known indication for treatment. This left 8 cases classified as cure and no failures in the amoxicillin group compared with 7 cases classified as cure and 2 failures in the nitrofurantoin group, P = .47 (Figure). Statistical analysis was performed using the Fisher exact test.
Discussion
There was no statistically significant difference between amoxicillin and nitrofurantoin for the treatment of ampicillin-resistant E faecium UTIs. There were no failures in the amoxicillin group despite all isolates displaying resistance based on current breakpoints, supporting the theory that higher urine concentrations of amoxicillin may overcome the MIC of resistant isolates.
Related: Novel Therapy for Treating Complicated UTIs
Of the 11 cases treated with nitrofurantoin, 3 were classified failures. The first failure in the nitrofurantoin group was an asymptomatic subject who did not have a repeat urine culture but had a repeat UA, which showed a persistent elevation in WBC and leukocyte esterase count. This subject was removed from the statistical analysis, as treatment was not indicated per IDSA guidelines. No reason could be identified for the second failure, as a repeat culture demonstrated continued presence of E faecium. Chronic kidney disease (CKD) contributed to the third failure in the nitrofurantoin treatment group; the subject’s CrCl was about 17 mL/min. After treatment, the subject had a repeat urine culture, which indicated the continued presence of E faecium. The subject was later successfully treated with amoxicillin. Both cultures in the same subject were included in the final analysis per protocol, as the subject had an adequate evaluation of efficacy between courses. Four additional cases with CKD were treated with nitrofurantoin; however, their CrCl ranged from 40 to 55 mL/min, and all were classified cure or presumed cure.
Limitations
There were several limitations to this study. Due to the strict inclusion and exclusion criteria, a limited number of subjects were evaluated. Given that this was a retrospective study, it is possible that symptoms were reported by a subject but not appropriately documented. Another significant limitation of this trial was that MICs were not determined due to the retrospective nature of the study. External validity was also limited due to a predominately elderly and male population. Safety data regarding different therapies were not collected, as this study evaluated only the efficacy of therapies.
Conclusion
Although this was a very small retrospective analysis, to the authors knowledge this is the first clinical study supporting the in vitro theory that amoxicillin (500 mg every 8 hours) may overcome the MIC of resistant isolates due to achievement of higher urinary concentrations. Because this was a small retrospective analysis, more prospective evidence is needed to confirm these results.
Acknowledgements
Heather Kim, biostatistician, University of Illinois at Chicago. CCTS Support: UL1RR029879.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4(2):239-249.
2. Heintz BH, Halilovic J, Christensen CL. Vancomycin -resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30(11):1136-1149.
3. Zhanel GG, Laing NM, Nichol KA, et al; NAVRESS Group. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J Antimicrob Chemother. 2003;52(3):382-388.
4. Macrobid [package insert]. Pine Brook, NJ: Almatica Pharma; 2013.
5. Oplinger M, Andrews CO. Nitrofurantoin contraindicated in patients with a creatinine clearance below 60 mL/min: looking for the evidence. Ann Pharmacother. 2013;47(1):106-111.
6. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
7. Gordon RC, Regamey C, Kirby WM. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob Agents Chemother. 1972;1(6):504-507.
8. Sutherland R, Croydon EA, Rolinson GN. Amoxycillin: a new semi-synthetic penicillin. Br Med J. 1972;3(5817):13-16.
9. Williamson JC, Craft DW, Butts JD, Raasch RH. In vitro assessment of urinary isolates of ampicillin-resistant enterococci. Ann Pharmacother. 2002;36(2):246-250.
10. Dumkow LE, Perri MB, Zervos M. Time to stop using alternatives to ampicillin for enterococcal UTIs? In-vitro susceptibility trends for enterococcus urinary isolates over a one-year period in Detroit. Poster presented at: 53rd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC); September 10-13, 2013; Denver, CO.
11. Quintiliani R. Using pharmacodynamics and pharmacokinetics concepts to optimize treatment of infectious diseases. Infect Med. 2004;21(5):219-232.
Enterococcus species account for about 110,000 urinary tract infections (UTIs) annually in the U.S.1 The most common species isolated are Enterococcus faecalis and Enterococcus faecium (E faecium). Amoxicillin is the drug of choice for the treatment of enterococcal UTIs. Second-line therapies include vancomycin and nitrofurantoin. Alternative therapies include daptomycin and linezolid; however, these newer agents ideally would be reserved for more serious infections to preserve activity.2
Increased E faecium resistance to ampicillin and vancomycin has limited the therapeutic options. The results of a study by Zhanel and colleagues assessed the prevalence of resistant enterococcal urine isolates in North America.3 Of the 658 E faecium urine isolates, about 96% were resistant to ampicillin and 94% were resistant to vancoymcin.3 Nitrofurantoin has much lower resistance rates; however, its use is contraindicated in patients with a creatinine clearance (CrCl) < 60 mL/min.4 Data supporting the contraindication are limited, but the results of a study by Oplinger and Andrews suggested that using nitrofurantoin in patients with a CrCl ≥ 40 mL/min may be safe and effective.5 A therapeutic dilemma may occur when resistant E faecium UTIs are encountered and viable treatment options are limited due to intolerances, administration difficulties, lack of susceptibility data, or cost.
Related: Antimicrobial Stewardship in an Outpatient Parenteral Antibiotic Therapy Program
Based on the current Clinical and Laboratory Standards Institute standard, Enterococcus species with a minimal inhibitory concentration (MIC) ≥ 16 μg/mL are considered ampicillin resistant. Microbiology laboratories use the same breakpoint regardless of the site of infection.6 Amoxicillin concentrates in the urine; therefore, urinary concentrations are much higher than serum concentrations. The mean serum peak concentration after a single dose of oral amoxicillin 500 mg is 7.6 μg/mL.7 After a single dose of oral amoxicillin 500 mg, the average concentration in pooled urine collected over 6 hours was 1,100 μg/mL.8
In 2002, Williamson and colleagues analyzed 30 ampicillin- resistant E faecium urine isolates. Reported MICs were 128 μg/mL (30%), 256 μg/mL (60%), and 512 μg/mL (10%).9 A more recent retrospective analysis analyzed 234 ampicillin-resistant E faecium urine isolates. The MIC ranged from 32 to 1,024 μg/mL, with a median MIC of 256 μg/mL. Only 5 isolates had an MIC value > 1,000 μg/mL, but each of these isolates was within 1 dilution of 512 μg/mL.10 Because penicillins exhibit time-dependent killing, an optimal response will occur as long as the urine concentration is above the MIC for at least 50% of the dosing interval.11 Therefore, therapeutic doses of amoxicillin are expected to produce urine concentrations that exceed the MIC of resistant E faecium urine isolates. The purpose of this study was to determine if amoxicillin was a viable treatment option for ampicillin-resistant E faecium UTIs based on this in vitro theory.
Methods
Veterans aged ≥ 18 years with a positive urine culture for ampicillin- resistant E faecium who received antibiotic therapy for cystitis at the Jesse Brown VA Medical Center (JBVAMC) from January 1, 2005, through June 22, 2010, were evaluated in this retrospective cohort study. Exclusion criteria were the presence of any other organisms in the initial urine culture, prostatic involvement, and the presence of E faecium in a blood culture. Subjects treated with multiple antibiotics concurrently and with sequential treatment of different antibiotics with no evaluation of efficacy between courses were also excluded.
Related: Urologist Workforce Variation Across the VHA
All included subjects were evaluated for resolution of symptoms; improvement in leukocyte esterase count and white blood cell (WBC) count from urine analysis (UA); and eradication of E faecium from a repeat urine culture. The response to treatment was classified as cure, presumed cure, or failure. The criteria for cure were based on the following: resolution of symptoms if present at baseline; repeat UA indicating improvement from the initial positive UA (if obtained); and eradication of E faecium in a repeat urine culture (if obtained).
At least 1 of the aforementioned criteria must have been met to be classified as cure. If more than 1 of the aforementioned criteria was present, then each one must have been met to be classified as cure. To be evaluated for presumed cure, the subject must have had symptoms at baseline. No documentation of ongoing symptoms in subjects who had an appropriate follow-up but did not have a repeat UA or urine culture indicated presumed cure. Persistence or worsening of pretreatment symptoms, a repeat UA without improvement from the initial positive UA, or a repeat urine culture demonstrating continued presence of E faecium indicated failure. The primary endpoint for the study was to determine whether amoxicillin was effective for the management of ampicillin-resistant E faecium UTIs. This study was conducted in compliance with the University of Illinois at Chicago Institutional Review Board and JBVAMC Human Subjects Research Committee requirements.
Results
This study included 20 positive urine cultures for ampicillin-resistant E faecium in 19 subjects. Nine cases were treated with amoxicillin, and 11 cases were treated with nitrofurantoin. At baseline, the mean age was 75 years, mean duration of therapy was 14 days, and all the subjects were male. The baseline characteristics of the 2 groups were similar with the exception of an older population, shorter duration of therapy, and increased incidence of chronic kidney disease in the amoxicillin treatment group, P = .02, .03, and .01, respectively.
Symptoms were documented in 8 of 9 (89%) cases at the time of the positive culture in the amoxicillin treatment group and 5 of 11 (45%) cases in the nitrofurantoin treatment group (Table). The asymptomatic amoxicillin treatment group case and 5 of the 6 nitrofurantoin treatment group asymptomatic cases received treatment prior to a urologic procedure in accordance with the Infectious Diseases Society of America (IDSA) guidelines for the treatment of asymptomatic bacteriuria. The urologic procedures included transurethral resection of a bladder tumor, cystoscopy, urethral dilation, cystometrogram, and transurethral resection of the prostate. One asymptomatic subject in the nitrofurantoin group did not have any documentation to support an appropriate indication for treatment. All positive cultures were > 100,000 colonies/mL except for 1 culture in the nitrofurantoin treatment group, which was 45,000 colonies/mL, but because the subject was symptomatic, treatment was administered and a repeat urine culture was negative.
There were 8 cases classified as cure, 1 presumed cure, and no failures in the amoxicillin group. In the nitrofurantoin group, 7 cases were classified as cure, 1 presumed cure, and 3 failures. The presumed cures were excluded from the statistical analysis due to inability to ensure these cases were truly cured. Also excluded from the statistical analysis was one of the failures in the nitrofurantoin group, because the subject was asymptomatic with no known indication for treatment. This left 8 cases classified as cure and no failures in the amoxicillin group compared with 7 cases classified as cure and 2 failures in the nitrofurantoin group, P = .47 (Figure). Statistical analysis was performed using the Fisher exact test.
Discussion
There was no statistically significant difference between amoxicillin and nitrofurantoin for the treatment of ampicillin-resistant E faecium UTIs. There were no failures in the amoxicillin group despite all isolates displaying resistance based on current breakpoints, supporting the theory that higher urine concentrations of amoxicillin may overcome the MIC of resistant isolates.
Related: Novel Therapy for Treating Complicated UTIs
Of the 11 cases treated with nitrofurantoin, 3 were classified failures. The first failure in the nitrofurantoin group was an asymptomatic subject who did not have a repeat urine culture but had a repeat UA, which showed a persistent elevation in WBC and leukocyte esterase count. This subject was removed from the statistical analysis, as treatment was not indicated per IDSA guidelines. No reason could be identified for the second failure, as a repeat culture demonstrated continued presence of E faecium. Chronic kidney disease (CKD) contributed to the third failure in the nitrofurantoin treatment group; the subject’s CrCl was about 17 mL/min. After treatment, the subject had a repeat urine culture, which indicated the continued presence of E faecium. The subject was later successfully treated with amoxicillin. Both cultures in the same subject were included in the final analysis per protocol, as the subject had an adequate evaluation of efficacy between courses. Four additional cases with CKD were treated with nitrofurantoin; however, their CrCl ranged from 40 to 55 mL/min, and all were classified cure or presumed cure.
Limitations
There were several limitations to this study. Due to the strict inclusion and exclusion criteria, a limited number of subjects were evaluated. Given that this was a retrospective study, it is possible that symptoms were reported by a subject but not appropriately documented. Another significant limitation of this trial was that MICs were not determined due to the retrospective nature of the study. External validity was also limited due to a predominately elderly and male population. Safety data regarding different therapies were not collected, as this study evaluated only the efficacy of therapies.
Conclusion
Although this was a very small retrospective analysis, to the authors knowledge this is the first clinical study supporting the in vitro theory that amoxicillin (500 mg every 8 hours) may overcome the MIC of resistant isolates due to achievement of higher urinary concentrations. Because this was a small retrospective analysis, more prospective evidence is needed to confirm these results.
Acknowledgements
Heather Kim, biostatistician, University of Illinois at Chicago. CCTS Support: UL1RR029879.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Enterococcus species account for about 110,000 urinary tract infections (UTIs) annually in the U.S.1 The most common species isolated are Enterococcus faecalis and Enterococcus faecium (E faecium). Amoxicillin is the drug of choice for the treatment of enterococcal UTIs. Second-line therapies include vancomycin and nitrofurantoin. Alternative therapies include daptomycin and linezolid; however, these newer agents ideally would be reserved for more serious infections to preserve activity.2
Increased E faecium resistance to ampicillin and vancomycin has limited the therapeutic options. The results of a study by Zhanel and colleagues assessed the prevalence of resistant enterococcal urine isolates in North America.3 Of the 658 E faecium urine isolates, about 96% were resistant to ampicillin and 94% were resistant to vancoymcin.3 Nitrofurantoin has much lower resistance rates; however, its use is contraindicated in patients with a creatinine clearance (CrCl) < 60 mL/min.4 Data supporting the contraindication are limited, but the results of a study by Oplinger and Andrews suggested that using nitrofurantoin in patients with a CrCl ≥ 40 mL/min may be safe and effective.5 A therapeutic dilemma may occur when resistant E faecium UTIs are encountered and viable treatment options are limited due to intolerances, administration difficulties, lack of susceptibility data, or cost.
Related: Antimicrobial Stewardship in an Outpatient Parenteral Antibiotic Therapy Program
Based on the current Clinical and Laboratory Standards Institute standard, Enterococcus species with a minimal inhibitory concentration (MIC) ≥ 16 μg/mL are considered ampicillin resistant. Microbiology laboratories use the same breakpoint regardless of the site of infection.6 Amoxicillin concentrates in the urine; therefore, urinary concentrations are much higher than serum concentrations. The mean serum peak concentration after a single dose of oral amoxicillin 500 mg is 7.6 μg/mL.7 After a single dose of oral amoxicillin 500 mg, the average concentration in pooled urine collected over 6 hours was 1,100 μg/mL.8
In 2002, Williamson and colleagues analyzed 30 ampicillin- resistant E faecium urine isolates. Reported MICs were 128 μg/mL (30%), 256 μg/mL (60%), and 512 μg/mL (10%).9 A more recent retrospective analysis analyzed 234 ampicillin-resistant E faecium urine isolates. The MIC ranged from 32 to 1,024 μg/mL, with a median MIC of 256 μg/mL. Only 5 isolates had an MIC value > 1,000 μg/mL, but each of these isolates was within 1 dilution of 512 μg/mL.10 Because penicillins exhibit time-dependent killing, an optimal response will occur as long as the urine concentration is above the MIC for at least 50% of the dosing interval.11 Therefore, therapeutic doses of amoxicillin are expected to produce urine concentrations that exceed the MIC of resistant E faecium urine isolates. The purpose of this study was to determine if amoxicillin was a viable treatment option for ampicillin-resistant E faecium UTIs based on this in vitro theory.
Methods
Veterans aged ≥ 18 years with a positive urine culture for ampicillin- resistant E faecium who received antibiotic therapy for cystitis at the Jesse Brown VA Medical Center (JBVAMC) from January 1, 2005, through June 22, 2010, were evaluated in this retrospective cohort study. Exclusion criteria were the presence of any other organisms in the initial urine culture, prostatic involvement, and the presence of E faecium in a blood culture. Subjects treated with multiple antibiotics concurrently and with sequential treatment of different antibiotics with no evaluation of efficacy between courses were also excluded.
Related: Urologist Workforce Variation Across the VHA
All included subjects were evaluated for resolution of symptoms; improvement in leukocyte esterase count and white blood cell (WBC) count from urine analysis (UA); and eradication of E faecium from a repeat urine culture. The response to treatment was classified as cure, presumed cure, or failure. The criteria for cure were based on the following: resolution of symptoms if present at baseline; repeat UA indicating improvement from the initial positive UA (if obtained); and eradication of E faecium in a repeat urine culture (if obtained).
At least 1 of the aforementioned criteria must have been met to be classified as cure. If more than 1 of the aforementioned criteria was present, then each one must have been met to be classified as cure. To be evaluated for presumed cure, the subject must have had symptoms at baseline. No documentation of ongoing symptoms in subjects who had an appropriate follow-up but did not have a repeat UA or urine culture indicated presumed cure. Persistence or worsening of pretreatment symptoms, a repeat UA without improvement from the initial positive UA, or a repeat urine culture demonstrating continued presence of E faecium indicated failure. The primary endpoint for the study was to determine whether amoxicillin was effective for the management of ampicillin-resistant E faecium UTIs. This study was conducted in compliance with the University of Illinois at Chicago Institutional Review Board and JBVAMC Human Subjects Research Committee requirements.
Results
This study included 20 positive urine cultures for ampicillin-resistant E faecium in 19 subjects. Nine cases were treated with amoxicillin, and 11 cases were treated with nitrofurantoin. At baseline, the mean age was 75 years, mean duration of therapy was 14 days, and all the subjects were male. The baseline characteristics of the 2 groups were similar with the exception of an older population, shorter duration of therapy, and increased incidence of chronic kidney disease in the amoxicillin treatment group, P = .02, .03, and .01, respectively.
Symptoms were documented in 8 of 9 (89%) cases at the time of the positive culture in the amoxicillin treatment group and 5 of 11 (45%) cases in the nitrofurantoin treatment group (Table). The asymptomatic amoxicillin treatment group case and 5 of the 6 nitrofurantoin treatment group asymptomatic cases received treatment prior to a urologic procedure in accordance with the Infectious Diseases Society of America (IDSA) guidelines for the treatment of asymptomatic bacteriuria. The urologic procedures included transurethral resection of a bladder tumor, cystoscopy, urethral dilation, cystometrogram, and transurethral resection of the prostate. One asymptomatic subject in the nitrofurantoin group did not have any documentation to support an appropriate indication for treatment. All positive cultures were > 100,000 colonies/mL except for 1 culture in the nitrofurantoin treatment group, which was 45,000 colonies/mL, but because the subject was symptomatic, treatment was administered and a repeat urine culture was negative.
There were 8 cases classified as cure, 1 presumed cure, and no failures in the amoxicillin group. In the nitrofurantoin group, 7 cases were classified as cure, 1 presumed cure, and 3 failures. The presumed cures were excluded from the statistical analysis due to inability to ensure these cases were truly cured. Also excluded from the statistical analysis was one of the failures in the nitrofurantoin group, because the subject was asymptomatic with no known indication for treatment. This left 8 cases classified as cure and no failures in the amoxicillin group compared with 7 cases classified as cure and 2 failures in the nitrofurantoin group, P = .47 (Figure). Statistical analysis was performed using the Fisher exact test.
Discussion
There was no statistically significant difference between amoxicillin and nitrofurantoin for the treatment of ampicillin-resistant E faecium UTIs. There were no failures in the amoxicillin group despite all isolates displaying resistance based on current breakpoints, supporting the theory that higher urine concentrations of amoxicillin may overcome the MIC of resistant isolates.
Related: Novel Therapy for Treating Complicated UTIs
Of the 11 cases treated with nitrofurantoin, 3 were classified failures. The first failure in the nitrofurantoin group was an asymptomatic subject who did not have a repeat urine culture but had a repeat UA, which showed a persistent elevation in WBC and leukocyte esterase count. This subject was removed from the statistical analysis, as treatment was not indicated per IDSA guidelines. No reason could be identified for the second failure, as a repeat culture demonstrated continued presence of E faecium. Chronic kidney disease (CKD) contributed to the third failure in the nitrofurantoin treatment group; the subject’s CrCl was about 17 mL/min. After treatment, the subject had a repeat urine culture, which indicated the continued presence of E faecium. The subject was later successfully treated with amoxicillin. Both cultures in the same subject were included in the final analysis per protocol, as the subject had an adequate evaluation of efficacy between courses. Four additional cases with CKD were treated with nitrofurantoin; however, their CrCl ranged from 40 to 55 mL/min, and all were classified cure or presumed cure.
Limitations
There were several limitations to this study. Due to the strict inclusion and exclusion criteria, a limited number of subjects were evaluated. Given that this was a retrospective study, it is possible that symptoms were reported by a subject but not appropriately documented. Another significant limitation of this trial was that MICs were not determined due to the retrospective nature of the study. External validity was also limited due to a predominately elderly and male population. Safety data regarding different therapies were not collected, as this study evaluated only the efficacy of therapies.
Conclusion
Although this was a very small retrospective analysis, to the authors knowledge this is the first clinical study supporting the in vitro theory that amoxicillin (500 mg every 8 hours) may overcome the MIC of resistant isolates due to achievement of higher urinary concentrations. Because this was a small retrospective analysis, more prospective evidence is needed to confirm these results.
Acknowledgements
Heather Kim, biostatistician, University of Illinois at Chicago. CCTS Support: UL1RR029879.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4(2):239-249.
2. Heintz BH, Halilovic J, Christensen CL. Vancomycin -resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30(11):1136-1149.
3. Zhanel GG, Laing NM, Nichol KA, et al; NAVRESS Group. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J Antimicrob Chemother. 2003;52(3):382-388.
4. Macrobid [package insert]. Pine Brook, NJ: Almatica Pharma; 2013.
5. Oplinger M, Andrews CO. Nitrofurantoin contraindicated in patients with a creatinine clearance below 60 mL/min: looking for the evidence. Ann Pharmacother. 2013;47(1):106-111.
6. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
7. Gordon RC, Regamey C, Kirby WM. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob Agents Chemother. 1972;1(6):504-507.
8. Sutherland R, Croydon EA, Rolinson GN. Amoxycillin: a new semi-synthetic penicillin. Br Med J. 1972;3(5817):13-16.
9. Williamson JC, Craft DW, Butts JD, Raasch RH. In vitro assessment of urinary isolates of ampicillin-resistant enterococci. Ann Pharmacother. 2002;36(2):246-250.
10. Dumkow LE, Perri MB, Zervos M. Time to stop using alternatives to ampicillin for enterococcal UTIs? In-vitro susceptibility trends for enterococcus urinary isolates over a one-year period in Detroit. Poster presented at: 53rd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC); September 10-13, 2013; Denver, CO.
11. Quintiliani R. Using pharmacodynamics and pharmacokinetics concepts to optimize treatment of infectious diseases. Infect Med. 2004;21(5):219-232.
1. Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4(2):239-249.
2. Heintz BH, Halilovic J, Christensen CL. Vancomycin -resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30(11):1136-1149.
3. Zhanel GG, Laing NM, Nichol KA, et al; NAVRESS Group. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J Antimicrob Chemother. 2003;52(3):382-388.
4. Macrobid [package insert]. Pine Brook, NJ: Almatica Pharma; 2013.
5. Oplinger M, Andrews CO. Nitrofurantoin contraindicated in patients with a creatinine clearance below 60 mL/min: looking for the evidence. Ann Pharmacother. 2013;47(1):106-111.
6. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
7. Gordon RC, Regamey C, Kirby WM. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob Agents Chemother. 1972;1(6):504-507.
8. Sutherland R, Croydon EA, Rolinson GN. Amoxycillin: a new semi-synthetic penicillin. Br Med J. 1972;3(5817):13-16.
9. Williamson JC, Craft DW, Butts JD, Raasch RH. In vitro assessment of urinary isolates of ampicillin-resistant enterococci. Ann Pharmacother. 2002;36(2):246-250.
10. Dumkow LE, Perri MB, Zervos M. Time to stop using alternatives to ampicillin for enterococcal UTIs? In-vitro susceptibility trends for enterococcus urinary isolates over a one-year period in Detroit. Poster presented at: 53rd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC); September 10-13, 2013; Denver, CO.
11. Quintiliani R. Using pharmacodynamics and pharmacokinetics concepts to optimize treatment of infectious diseases. Infect Med. 2004;21(5):219-232.
Testosterone Replacement Therapy: Playing Catch-up With Patients
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
Deployment-Related Lung Disorders
Military deployed from World War II through the Vietnam War have had enough time for respiratory disorders with both short and long latencies to manifest. More recent deployments over the past 13 years to Iraq, Kuwait, Afghanistan, and other regions in southwest Asia (SWA) have been associated with a unique spectrum of respiratory disorders. The long-term respiratory effects of SWA deployments are unknown. This review will discuss deployment-related lung cancer and then focus primarily on the emerging respiratory disorders related to SWA deployment and case examples of deployment-related lung disease.
As the number of recent veterans in the VA health care system increases, primary care providers (PCPs) and specialists are increasingly faced with questions about potential hazards of deployment, referring patients to the VA Airborne Hazards and Open Burn Pit Registry, and evaluating patients with new-onset respiratory symptoms following deployment. Previous reviews and white papers have offered recommendations for evaluation and management; however, little has been reported in the form of case examples of patients with deployment-related lung disorders and their clinical course.1,2
Deployment-Associated Lung Cancer
Lung cancer is the leading cause of cancer death in the U.S. and around the world.3 Lung cancer in the U.S. causes more deaths than does the combination of breast, prostate, colon, and rectal cancers. Lung cancer is the second most common cancer and causes more deaths than does any other cancer in the VHA.4 Most cancers with an environmental cause have a significant latent period of decades between the exposure and cancer incidence. Thus, although lung cancer risk is relatively low in active-duty military personnel, the rate of lung cancer in VA patients is nearly double that of the general population, suggesting causes associated with military service.5
Tobacco
The main cause of lung cancer is tobacco smoking, which accounts for 85% to 90% of lung cancer in the U.S. The latent period between initiation of tobacco smoking and lung cancer incidence is typically ≥ 30 years. Military service has long been associated with tobacco smoking, due to past practices that included the provision of free cigarettes, the availability of cigarettes at reduced cost, smoking breaks, perceived relief from both stress and boredom, and social factors.6 More recently, the adverse effects (AEs) of smoking on health and readiness have been appreciated, and many incentives encouraging tobacco smoking have been eliminated. In 2009, the Institute of Medicine called for a tobacco-free military, and both the Secretary of the Navy and Secretary of Defense have seriously considered this change.7
The additional effect of deployment on smoking has been reported.8 The longitudinal Millennium Cohort study compared several smoking measures between 55,021 deployers and nondeployers who completed both baseline (acquired July 2001-June 2003) and follow-up questionnaires (acquired June 2004-January 2006). Smoking initiation affected 2.3% of deployers and 1.3% of nondeployers; smoking resumption showed a similar pattern with an increase of 39.4% compared with 28.7%. The overall prevalence of smoking increased 44% among nondeployers and 57% among deployers. Those never smokers exposed to combat were 60% more likely to initiate smoking compared with noncombat deployers. Thus, it is clear that tobacco smoking should be considered a deployment-related exposure that contributes to lung cancer risk.
Asbestos
In 1955, Doll published an analysis associating asbestos exposure with risk for lung cancer.9 Many naval veterans and shipyard workers had asbestos exposure, resulting in a spectrum of asbestos-related diseases, including bronchogenic cancer.10
Depleted Uranium
Depleted uranium was used in munitions during the first Gulf War and more recently during military operations in SWA as a part of Operation New Dawn (OND), Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF). Because of concerns of military personnel having complex exposure to depleted uranium, including via inhalation, the VA established the Depleted Uranium Surveillance Program, which has followed a cohort of service members exposed to inhaled depleted uranium during friendly fire in 1991. No significant differences between individuals with high urinary uranium levels and low urinary uranium levels were found in self-reported respiratory symptoms and pulmonary function testing (PFT). Additionally, 20 years after exposure to depleted uranium, there was no statistically significant difference of low-dose chest computed tomography (CT) evidence of lung cancer in these 2 groups.11
Mustard Gas
Mustard gas is considered a definite lung carcinogen.12,13 Both long-term, low-dose and short-term, high- intensity exposures are known to cause human lung cancer.14 Mustard gas was first widely used in warfare in World War I. Mustard gas was used in training for World War II; training accidents resulted in acute toxicity even in lower exposures. It was later used as a chemical warfare agent in the Iran-Iraq conflict in the late 1980s and early 1990s. It is estimated that about 4,000 U.S. service members have been acutely exposed to high concentrations of mustard gas. Sulfur mustard may be incorporated into improvised explosive devices, and there is concern that troops in Iraq have been exposed to this agent in sites previously used for manufacturing and storage.15
Agent Orange
The herbicide Agent Orange is commonly contaminated with dioxin, which has been demonstrated to be a tumor promoter in animal studies. Agent Orange was used widely in the Vietnam War. The National Academy of Sciences issued a report in 2001 reviewing evidence for a link between Agent Orange and various neoplasms. Evidence was strongest for Hodgkin lymphoma and soft tissue sarcoma. The evidence of an association between Agent Orange exposure and lung cancer was deemed only suggestive.16
Respiratory Disease Associated With Southwest Asia Deployment
Over the past 14 years, > 2.5 million U.S. military personnel and civilian contractors have been deployed as part of 3 major military operations: OEF in Afghanistan (2001 to present), OIF in Iraq (2003 to 2010), and OND in Iraq (2010 to present).17,18 Deployed personnel encounter a wide variety of inhalational exposures that include desert dust particulate matter, burn pit combustion products, environmental tobacco smoke, vehicular diesel exhaust, debris from detonations and explosions, and other unique or specific job-related exposures (Table 1).19,20
A number of recent studies have helped identify and characterize an emerging spectrum of deployment-related lung disorders, including asthma, rhinosinusitis, emphysema, bronchiolitis, granulomatous pneumonitis, and less common conditions such as acute eosinophilic pneumonia and rapidly progressive pulmonary fibrosis (Table 2).20-30 Still, diagnosis of these conditions is often challenging, and traditional diagnostic tools such as PFT and chest radiography may be normal or mildly abnormal despite significant histopathologic abnormalities on surgical lung biopsy.24,30,31
Deployment-Related Exposures
As listed in Table 1, there are a number of other exposures that may be encountered during deployment. Environmental air sampling was conducted in several locations in Iraq, Afghanistan, and sites in SWA as part of the Enhanced Particulate Matter Survey. All sites were notable for air pollutant levels that exceeded 15 μg/m3, the military exposure guideline for fine particulate matter (PM2.5). The PM2.5 fraction comprised geologic dust, burn pit emissions, and the heavy metals aluminum, cadmium, and lead.32,33
Respiratory Disorders
Reports of deployers with respiratory symptoms during and after deployment surfaced as early as 2004.34 The Millennium Cohort study reported a 1.7-fold higher rate of new-onset respiratory symptoms that was independent of smoking status, such as cough and shortness of breath, in deployers compared with nondeployers. These increased symptom rates were associated with land-based deployment and longer deployment duration.35 A number of epidemiologic studies also demonstrated an association between respiratory symptoms and environmental exposures encountered during deployment.36-39
Respiratory diseases such as asthma, acute eosinophilic pneumonia, and constrictive bronchiolitis have been reported following deployment to SWA, but a review of the literature supports a more expansive list of deployment-related respiratory diseases (Table 2).20-30 The following case examples describe findings in veterans referred to the authors’ clinic for evaluation of chest symptoms associated with deployment.
OEF/OIF/OND Case Studies
Case Study 1
A 42-year-old male never smoker presented to his VA PCP for evaluation of nonproductive cough, dyspnea on exertion, chest tightness, and recurrent episodes of bronchitis since 2004 when he was deployed to Afghanistan. He had no history of asthma or other chronic respiratory disease in childhood or adolescence.
The patient served as a Civil Affairs officer in the U.S. Army and was deployed to Bosnia in 1997, Afghanistan in 2004, and Camp Arif-Jan in Kuwait as well as Mosul, Iraq, in 2005. He was exposed to depleted uranium while serving in Bosnia. He also had exposures to sandstorms, desert dust, and burn pit combustion products while deployed to Afghanistan and Iraq. He developed symptoms of chest tightness and dyspnea on exertion during his 2004 deployment, with these symptoms persisting after returning home from deployment. His symptoms occurred frequently while running and limited his ability to pass his military physical fitness test requirements and train for marathons as he had done previously. He also had symptoms of chest tightness and excessive coughing at rest, which were treated with antibiotics by his medical provider as recurrent acute infectious/viral bronchitis.
The patient was medically discharged from the U.S. Army in July 2005, primarily due to musculoskeletal injuries. His past medical history was notable for PTSD, recurrent allergic rhinosinusitis, and lumbosacral back pain. Given persistent respiratory symptoms of dyspnea after walking 1 block, the patient presented to his VA PCP in early 2006.
The patient’s vital signs and physical examination were normal. Spirometry showed a mixed restrictive and obstructive pattern, prompting referral for pulmonary consultation. Full PFT demonstrated an abnormally increased residual volume and mildly decreased diffusion capacity (Table 3). Laryngoscopy was negative for vocal cord dysfunction. A chest X-ray showed mild airway wall thickening bilaterally in the lower lung fields. Subsequent high-resolution CT of the chest demonstrated diffuse centrilobular nodularity (Figure 1). Serial spirometry measurements over 8 months showed severe and worsening airflow limitation despite treatment with inhaled bronchodilator and corticosteroid therapy. Seeking diagnostic clarity, the patient was referred for surgical lung biopsy via video-assisted thorascopic surgery (VATS) within 6 months of initial consultation.
The patient’s lung biopsy demonstrated constrictive changes in bronchioles, hyperinflation, and multiple chemodectomas in all 3 lobes of the right lung (Figures 2 and 3). Three pulmonary pathologists reviewed the biopsy and confirmed findings of constrictive bronchiolitis. Serologies for connective tissue disease were negative, indicating no autoimmune cause of bronchiolitis.
As no specific etiology was identified, the patient was referred for a second opinion with a pulmonologist with expertise in interstitial lung disease. Finding no evidence of post- infectious or autoimmune bronchiolitis, the patient’s diagnosis of constrictive bronchiolitis was deemed to be idiopathic. A number of years later, following publication of a case series of 38 OEF/OIF deployers with biopsy-proven constrictive bronchiolitis, the patient was referred for consultation to an occupational lung disease clinic.24 He subsequently was diagnosed with deployment-related lung disease, as his constrictive bronchiolitis was thought to be related to exposures encountered during his OEF/OIF deployments from 2003 to 2005.
The patient was monitored with spirometry over the next few months. After observing a 10% decline in forced expiratory volume in 1 second (FEV1) over 9 months despite stable lung volumes and diffusion capacity, the patient was started on macrolide therapy with erythromycin 500 mg daily. He was switched to azithromycin 250 mg daily due to gastrointestinal AEs of nausea and diarrhea while taking erythromycin. He continued use of an inhaled corticosteroid (ICS), as well as bronchodilator therapy with albuterol and formoterol and had stable dyspnea.
The patient was treated briefly with prednisone 40 mg, but he discontinued this medication after 5 days due to worsening anxiety and PTSD symptoms. Azithromycin therapy was discontinued after 4 years, because no significant improvement was noted in the patient’s lung function. Spirometry, lung volumes, and diffusion testing were unchanged for 2 years following discontinuation of azithromycin and continuing therapy with an ICS, long-acting beta-agonist, and albuterol. The patient has stable dyspnea on exertion but exercises regularly and recently was able to complete a marathon.
Case Study 2
A 43-year-old female ex-smoker presented to a VA chest clinic for evaluation of cough that started during a 2003 deployment to Iraq as well as dyspnea on exertion and chest tightness that had been present since her 2010 to 2011 deployment to Afghanistan. The patient had no history of asthma or other chronic respiratory disease during childhood.
She enlisted in the U.S. Navy in 1987 and later served as a medic while in the Navy Reserves. When she joined the U.S. Navy, she easily passed a 1.5-mile physical fitness readiness test run-time requirement with an 8.5-minute run time. She had no respiratory symptoms and ran in several marathons until her first SWA deployment in 2003.
In April 2003, she was deployed for 3 months to work as a combat medic near the Kuwait and Iraq border. She had frequent exposure to desert dust and recalled 5 sandstorms that appeared like a “wall of sand” coming toward the base. A few weeks into this deployment, the patient developed a nonproductive cough that persisted after returning to the U.S. She stopped smoking for a few months after returning home but continued to have a nonproductive cough. She did not seek further medical attention, because she had no exercise-limiting symptoms.
The patient joined the Army National Guard in 2006 and was activated in 2009 to deploy to Afghanistan from January 2010 through January 2011. She was stationed at Bagram Airbase for the entire deployment and worked as a military police officer in the prison. She had exposure to sandstorms and burn pit combustion products. The prison was about 2 miles downwind from a large burn pit.
In October 2010, she quit smoking again because of new-onset chest tightness and dyspnea on exertion. However, her symptoms did not abate, and she noted increased chest tightness and difficulty catching her breath when running near the burn pit. While she tried to avoid the burn pit, she participated in competitive races and a 10-mile run along paths that were near the burn pit.
After returning from deployment, the patient presented to her VA PCP for evaluation of persistent nonproductive cough, chest tightness, and dyspnea on exertion. She was not taking any respiratory or allergy medications at the time of evaluation. Initial chest X-ray and spirometry were normal, and she was referred to the chest clinic for consultation. At the time of pulmonary consultation, the patient had a total smoking history of 15 pack-years but had now abstained from smoking for about 2 years. She reported residential exposure to pet birds for > 20 years. High-resolution chest imaging and full PFT with lung volumes and diffusion capacity were performed to evaluate for hypersensitivity pneumonitis.
Her vital signs, physical examination pulmonary function testing with spirometry, lung volumes, and diffusion testing were all normal (Table 4). Bronchial challenge to methacholine demonstrated airways hyperresponsiveness at a PC[-20] FEV1 of 1.25 mg/mL. High-resolution chest CT did not demonstrate air trapping, centrilobular nodules, or other evidence of chronic interstitial lung disease. A cardiopulmonary maximum multistage exercise test with arterial line placement showed normal exercise tolerance with the patient achieving 109% of the maximum predicted workload and 90% of predicted VO2 max.
The patient was diagnosed with deployment-related asthma based on the finding of airways hyperresponsiveness after bronchial challenge testing. Her asthma was considered deployment-related based on the temporal onset of cough and later chest tightness and dyspnea on exertion that occurred during deployment. Ongoing smoking cessation was emphasized.
The patient was started on bronchodilator therapy with albuterol prior to exercise and as needed, but she continued to have symptoms of chest tightness while exercising. Eventually, a low-dose ICS was initiated in conjunction with albuterol as needed. Her symptoms did not resolve with this regimen, but she did experience improvement in exertional chest tightness. This patient was not referred for biopsy given clinical findings of asthma. She will continue pulmonary monitoring every 6 months. However, if her symptoms worsen, she will undergo full PFT, which includes lung volumes and diffusion testing and possible repeat chest imaging.
Conclusion
These 2 cases are representative of the spectrum of deployment-related lung disease. This assessment requires a detailed chronologic occupational and environmental history, establishing a temporal link between respiratory symptoms and deployment exposures and evidence of lung disease on noninvasive testing (or confirmation by surgical lung biopsy in select cases) in which noninvasive testing is nondiagnostic.
Referral for surgical lung biopsy was particularly helpful in the first case, because it ruled out other lung diseases that are more responsive to systemic therapy. However, referral for surgical lung biopsy is not recommended in all patients, and in-depth discussion of the risks and benefits associated with surgery is recommended. Although diagnostic clarity is a benefit of surgical lung biopsy, the authors also discuss with patients that there is no currently available therapy for deployment-related lung disease and thus management is unlikely to change after biopsy. The recommended approach to diagnostic evaluation is shown in Figure 4.
In the authors’ experience, treatment of deployment-related asthma with standard asthma treatment usually improves or stabilizes respiratory symptoms but often does not result in complete resolution of symptoms. Improvement in lung function with systemic pharmacotherapy in the management of deployment-related lung diseases, such as constrictive bronchiolitis, respiratory bronchiolitis, emphysema, or granulomatous pneumonitis has not been observed. Although little is currently known about prognosis, utilization of data collected from the VA Airborne Hazards and Open Burn Pit Registry may contribute to the understanding of deployment exposures and long-term respiratory health effects.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Rose C, Abraham J, Harkins D, et al. Overview and recommendations for medical screening and diagnostic evaluation for postdeployment lung disease in returning US warfighters. J Occup Environ Med. 2012;54(6):746-751.
2. Morris MJ, Lucero PF, Zanders TB, Zacher LL. Diagnosis and management of chronic lung disease in deployed military personnel. Ther Adv Respir Dis. 2013;7(4):235-245.
3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
4. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
5. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745.
6. Smith EA, Jahnke SA, Poston WS, et al. Is it time for a tobacco-free military? N Engl J Med. 2014;371(7):589-591.
7. Combating Tobacco in Military and Veteran Populations. In: Bondurant S, Wedge R, eds. Washington, DC: National Academies Press; 2009.
8. Smith B, Ryan MA, Wingard DL, Patterson TL, Slymen DJ, Macera CA; Millennium Cohort Study Team. Cigarette smoking and military deployment: a prospective evaluation. Am J Prev Med. 2008;35(6):539-546.
9. Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12(2):81-86.
10. Krstev S, Stewart P, Rusiecki J, Blair A. Mortality among shipyard Coast Guard workers: a retrospective cohort study. Occup Environ Med. 2007;64(10):651-658.
11. Hines SE, Gucer P, Kligerman S, et al. Pulmonary health effects in Gulf War I service members exposed to depleted uranium. J Occup Environ Med. 2013;55(8):937-944.
12. World Health Organization, International Agency for Research on Cancer. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: some aziridines, N-, S- & O-mustards and selenium. IARC Monogr Eval Carcinog Risk Chem Man. 1975;9:1-268.
13. Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33(4):681-703.
14. Ghanei M, Harandi AA. Lung carcinogenicity of sulfur mustard. Clin Lung Cancer. 2010;11(1):13-17.
15. Chivers CJ. The secret casualties of Iraq’s abandoned chemical weapons. New York Times. October 14, 2014. http://www.nytimes.com /interactive/2014/10/14/world/middleeast/us-casualties-of-iraq-chemical-weapons.html?_r=0. Accessed May 13, 2015.
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Third Biennial Update). Veterans and Agent Orange: Update 2000. Washington, DC: National Academies Press; 2001.
17. How to help military & veteran families before, during, and after deployment. Military Family Research Institute Web site. https://www.mfri.purdue.edu/resources/public/hth/HowToHelp _FamilyFriendNeighbor.pdf. Accessed November 6, 2014.
18. Torreon BS. U.S. periods of war and dates of current conflicts. Washington, DC: Congressional Research Service Report for Congress; December 28, 2012. http://fas.org/sgp/crs/natsec/RS21405.pdf. Accessed November 6, 2014.
19. Rose CS. Military service and lung disease. Clin Chest Med. 2012;33(4):705-714.
20. Szema AM. Occupational lung diseases among soldiers deployed to Iraq and Afghanistan. Occup Med Health Aff. 2013;1:10.4172/2329-6879.1000117.
21. Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med. 2014;190(1):77-84.
22. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004;292(24): 2997-3005.
23. Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172(12):1264-1269.
24. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan [published correction appears in N Engl J Med. 2011;365(18):1749]. N Engl J Med. 2011;365(3):222-230.
25. Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
26. Stecker T, Fortney J, Owen R, McGovern MP, Williams S. Co-occurring medical, psychiatric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51(6):503-507.
27. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71.
28. Scoville SL. Acute eosinophilic pneumonia (AEP) among U.S. military personnel in the U.S. Central Command Area of Responsibility (USCENTCOM AOR). USACHPPM Information Paper. http://www.pdhealth.mil/AEP_Info_paper_01oct09.pdf. Published October 1, 2009. Accessed January 27, 2015.
29. Zembrzuska H, Collen J, Roop S. Pulmonary fibrosis presenting at post-deployment health screening. Am J Respir Crit Care Med. 2011;183:A4780. Abstract.
30. Dhoma S, Gottschall B, Robinson M, et al. Lung disease in deployers returning from Afghanistan and Iraq. Am J Respir Crit Care Med. 2013;187:A3669. Abstract.
31. Dhoma S, Cox C, Chung JH, et al. Chest tomography may predict histopathologic abnormalities in symptomatic deployers returning from Iraq and Afghanistan. Am J Respir Crit Care Med. 2014;189:A5102. Abstract.
32. Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part I: ambient sampling. Inhal Toxicol. 2009;21(4):297-326.
33. Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part 2: grab samples and re-suspensions. Inhal Toxicol. 2009;21(4):327-336.
34. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480.
35. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD, Ryan MAK; for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442.
36. Abraham JH, DeBakey SF, Reid L, Zhou J, Baird CP. Does deployment to Iraq and Afghanistan affect respiratory health of US military personnel? J Occup Environ Med. 2012;54(6):740-745.
37. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669.
38. Quigley KS, McAndrew LM, Almeida L, et al. Prevalence of environmental and other military exposure concerns in Operation Enduring Freedom and Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):659-664.
39. Teichman R. Exposures of concern to veterans returning from Afghanistan and Iraq. J Occup Environ Med. 2012;54(6):677-681.
Military deployed from World War II through the Vietnam War have had enough time for respiratory disorders with both short and long latencies to manifest. More recent deployments over the past 13 years to Iraq, Kuwait, Afghanistan, and other regions in southwest Asia (SWA) have been associated with a unique spectrum of respiratory disorders. The long-term respiratory effects of SWA deployments are unknown. This review will discuss deployment-related lung cancer and then focus primarily on the emerging respiratory disorders related to SWA deployment and case examples of deployment-related lung disease.
As the number of recent veterans in the VA health care system increases, primary care providers (PCPs) and specialists are increasingly faced with questions about potential hazards of deployment, referring patients to the VA Airborne Hazards and Open Burn Pit Registry, and evaluating patients with new-onset respiratory symptoms following deployment. Previous reviews and white papers have offered recommendations for evaluation and management; however, little has been reported in the form of case examples of patients with deployment-related lung disorders and their clinical course.1,2
Deployment-Associated Lung Cancer
Lung cancer is the leading cause of cancer death in the U.S. and around the world.3 Lung cancer in the U.S. causes more deaths than does the combination of breast, prostate, colon, and rectal cancers. Lung cancer is the second most common cancer and causes more deaths than does any other cancer in the VHA.4 Most cancers with an environmental cause have a significant latent period of decades between the exposure and cancer incidence. Thus, although lung cancer risk is relatively low in active-duty military personnel, the rate of lung cancer in VA patients is nearly double that of the general population, suggesting causes associated with military service.5
Tobacco
The main cause of lung cancer is tobacco smoking, which accounts for 85% to 90% of lung cancer in the U.S. The latent period between initiation of tobacco smoking and lung cancer incidence is typically ≥ 30 years. Military service has long been associated with tobacco smoking, due to past practices that included the provision of free cigarettes, the availability of cigarettes at reduced cost, smoking breaks, perceived relief from both stress and boredom, and social factors.6 More recently, the adverse effects (AEs) of smoking on health and readiness have been appreciated, and many incentives encouraging tobacco smoking have been eliminated. In 2009, the Institute of Medicine called for a tobacco-free military, and both the Secretary of the Navy and Secretary of Defense have seriously considered this change.7
The additional effect of deployment on smoking has been reported.8 The longitudinal Millennium Cohort study compared several smoking measures between 55,021 deployers and nondeployers who completed both baseline (acquired July 2001-June 2003) and follow-up questionnaires (acquired June 2004-January 2006). Smoking initiation affected 2.3% of deployers and 1.3% of nondeployers; smoking resumption showed a similar pattern with an increase of 39.4% compared with 28.7%. The overall prevalence of smoking increased 44% among nondeployers and 57% among deployers. Those never smokers exposed to combat were 60% more likely to initiate smoking compared with noncombat deployers. Thus, it is clear that tobacco smoking should be considered a deployment-related exposure that contributes to lung cancer risk.
Asbestos
In 1955, Doll published an analysis associating asbestos exposure with risk for lung cancer.9 Many naval veterans and shipyard workers had asbestos exposure, resulting in a spectrum of asbestos-related diseases, including bronchogenic cancer.10
Depleted Uranium
Depleted uranium was used in munitions during the first Gulf War and more recently during military operations in SWA as a part of Operation New Dawn (OND), Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF). Because of concerns of military personnel having complex exposure to depleted uranium, including via inhalation, the VA established the Depleted Uranium Surveillance Program, which has followed a cohort of service members exposed to inhaled depleted uranium during friendly fire in 1991. No significant differences between individuals with high urinary uranium levels and low urinary uranium levels were found in self-reported respiratory symptoms and pulmonary function testing (PFT). Additionally, 20 years after exposure to depleted uranium, there was no statistically significant difference of low-dose chest computed tomography (CT) evidence of lung cancer in these 2 groups.11
Mustard Gas
Mustard gas is considered a definite lung carcinogen.12,13 Both long-term, low-dose and short-term, high- intensity exposures are known to cause human lung cancer.14 Mustard gas was first widely used in warfare in World War I. Mustard gas was used in training for World War II; training accidents resulted in acute toxicity even in lower exposures. It was later used as a chemical warfare agent in the Iran-Iraq conflict in the late 1980s and early 1990s. It is estimated that about 4,000 U.S. service members have been acutely exposed to high concentrations of mustard gas. Sulfur mustard may be incorporated into improvised explosive devices, and there is concern that troops in Iraq have been exposed to this agent in sites previously used for manufacturing and storage.15
Agent Orange
The herbicide Agent Orange is commonly contaminated with dioxin, which has been demonstrated to be a tumor promoter in animal studies. Agent Orange was used widely in the Vietnam War. The National Academy of Sciences issued a report in 2001 reviewing evidence for a link between Agent Orange and various neoplasms. Evidence was strongest for Hodgkin lymphoma and soft tissue sarcoma. The evidence of an association between Agent Orange exposure and lung cancer was deemed only suggestive.16
Respiratory Disease Associated With Southwest Asia Deployment
Over the past 14 years, > 2.5 million U.S. military personnel and civilian contractors have been deployed as part of 3 major military operations: OEF in Afghanistan (2001 to present), OIF in Iraq (2003 to 2010), and OND in Iraq (2010 to present).17,18 Deployed personnel encounter a wide variety of inhalational exposures that include desert dust particulate matter, burn pit combustion products, environmental tobacco smoke, vehicular diesel exhaust, debris from detonations and explosions, and other unique or specific job-related exposures (Table 1).19,20
A number of recent studies have helped identify and characterize an emerging spectrum of deployment-related lung disorders, including asthma, rhinosinusitis, emphysema, bronchiolitis, granulomatous pneumonitis, and less common conditions such as acute eosinophilic pneumonia and rapidly progressive pulmonary fibrosis (Table 2).20-30 Still, diagnosis of these conditions is often challenging, and traditional diagnostic tools such as PFT and chest radiography may be normal or mildly abnormal despite significant histopathologic abnormalities on surgical lung biopsy.24,30,31
Deployment-Related Exposures
As listed in Table 1, there are a number of other exposures that may be encountered during deployment. Environmental air sampling was conducted in several locations in Iraq, Afghanistan, and sites in SWA as part of the Enhanced Particulate Matter Survey. All sites were notable for air pollutant levels that exceeded 15 μg/m3, the military exposure guideline for fine particulate matter (PM2.5). The PM2.5 fraction comprised geologic dust, burn pit emissions, and the heavy metals aluminum, cadmium, and lead.32,33
Respiratory Disorders
Reports of deployers with respiratory symptoms during and after deployment surfaced as early as 2004.34 The Millennium Cohort study reported a 1.7-fold higher rate of new-onset respiratory symptoms that was independent of smoking status, such as cough and shortness of breath, in deployers compared with nondeployers. These increased symptom rates were associated with land-based deployment and longer deployment duration.35 A number of epidemiologic studies also demonstrated an association between respiratory symptoms and environmental exposures encountered during deployment.36-39
Respiratory diseases such as asthma, acute eosinophilic pneumonia, and constrictive bronchiolitis have been reported following deployment to SWA, but a review of the literature supports a more expansive list of deployment-related respiratory diseases (Table 2).20-30 The following case examples describe findings in veterans referred to the authors’ clinic for evaluation of chest symptoms associated with deployment.
OEF/OIF/OND Case Studies
Case Study 1
A 42-year-old male never smoker presented to his VA PCP for evaluation of nonproductive cough, dyspnea on exertion, chest tightness, and recurrent episodes of bronchitis since 2004 when he was deployed to Afghanistan. He had no history of asthma or other chronic respiratory disease in childhood or adolescence.
The patient served as a Civil Affairs officer in the U.S. Army and was deployed to Bosnia in 1997, Afghanistan in 2004, and Camp Arif-Jan in Kuwait as well as Mosul, Iraq, in 2005. He was exposed to depleted uranium while serving in Bosnia. He also had exposures to sandstorms, desert dust, and burn pit combustion products while deployed to Afghanistan and Iraq. He developed symptoms of chest tightness and dyspnea on exertion during his 2004 deployment, with these symptoms persisting after returning home from deployment. His symptoms occurred frequently while running and limited his ability to pass his military physical fitness test requirements and train for marathons as he had done previously. He also had symptoms of chest tightness and excessive coughing at rest, which were treated with antibiotics by his medical provider as recurrent acute infectious/viral bronchitis.
The patient was medically discharged from the U.S. Army in July 2005, primarily due to musculoskeletal injuries. His past medical history was notable for PTSD, recurrent allergic rhinosinusitis, and lumbosacral back pain. Given persistent respiratory symptoms of dyspnea after walking 1 block, the patient presented to his VA PCP in early 2006.
The patient’s vital signs and physical examination were normal. Spirometry showed a mixed restrictive and obstructive pattern, prompting referral for pulmonary consultation. Full PFT demonstrated an abnormally increased residual volume and mildly decreased diffusion capacity (Table 3). Laryngoscopy was negative for vocal cord dysfunction. A chest X-ray showed mild airway wall thickening bilaterally in the lower lung fields. Subsequent high-resolution CT of the chest demonstrated diffuse centrilobular nodularity (Figure 1). Serial spirometry measurements over 8 months showed severe and worsening airflow limitation despite treatment with inhaled bronchodilator and corticosteroid therapy. Seeking diagnostic clarity, the patient was referred for surgical lung biopsy via video-assisted thorascopic surgery (VATS) within 6 months of initial consultation.
The patient’s lung biopsy demonstrated constrictive changes in bronchioles, hyperinflation, and multiple chemodectomas in all 3 lobes of the right lung (Figures 2 and 3). Three pulmonary pathologists reviewed the biopsy and confirmed findings of constrictive bronchiolitis. Serologies for connective tissue disease were negative, indicating no autoimmune cause of bronchiolitis.
As no specific etiology was identified, the patient was referred for a second opinion with a pulmonologist with expertise in interstitial lung disease. Finding no evidence of post- infectious or autoimmune bronchiolitis, the patient’s diagnosis of constrictive bronchiolitis was deemed to be idiopathic. A number of years later, following publication of a case series of 38 OEF/OIF deployers with biopsy-proven constrictive bronchiolitis, the patient was referred for consultation to an occupational lung disease clinic.24 He subsequently was diagnosed with deployment-related lung disease, as his constrictive bronchiolitis was thought to be related to exposures encountered during his OEF/OIF deployments from 2003 to 2005.
The patient was monitored with spirometry over the next few months. After observing a 10% decline in forced expiratory volume in 1 second (FEV1) over 9 months despite stable lung volumes and diffusion capacity, the patient was started on macrolide therapy with erythromycin 500 mg daily. He was switched to azithromycin 250 mg daily due to gastrointestinal AEs of nausea and diarrhea while taking erythromycin. He continued use of an inhaled corticosteroid (ICS), as well as bronchodilator therapy with albuterol and formoterol and had stable dyspnea.
The patient was treated briefly with prednisone 40 mg, but he discontinued this medication after 5 days due to worsening anxiety and PTSD symptoms. Azithromycin therapy was discontinued after 4 years, because no significant improvement was noted in the patient’s lung function. Spirometry, lung volumes, and diffusion testing were unchanged for 2 years following discontinuation of azithromycin and continuing therapy with an ICS, long-acting beta-agonist, and albuterol. The patient has stable dyspnea on exertion but exercises regularly and recently was able to complete a marathon.
Case Study 2
A 43-year-old female ex-smoker presented to a VA chest clinic for evaluation of cough that started during a 2003 deployment to Iraq as well as dyspnea on exertion and chest tightness that had been present since her 2010 to 2011 deployment to Afghanistan. The patient had no history of asthma or other chronic respiratory disease during childhood.
She enlisted in the U.S. Navy in 1987 and later served as a medic while in the Navy Reserves. When she joined the U.S. Navy, she easily passed a 1.5-mile physical fitness readiness test run-time requirement with an 8.5-minute run time. She had no respiratory symptoms and ran in several marathons until her first SWA deployment in 2003.
In April 2003, she was deployed for 3 months to work as a combat medic near the Kuwait and Iraq border. She had frequent exposure to desert dust and recalled 5 sandstorms that appeared like a “wall of sand” coming toward the base. A few weeks into this deployment, the patient developed a nonproductive cough that persisted after returning to the U.S. She stopped smoking for a few months after returning home but continued to have a nonproductive cough. She did not seek further medical attention, because she had no exercise-limiting symptoms.
The patient joined the Army National Guard in 2006 and was activated in 2009 to deploy to Afghanistan from January 2010 through January 2011. She was stationed at Bagram Airbase for the entire deployment and worked as a military police officer in the prison. She had exposure to sandstorms and burn pit combustion products. The prison was about 2 miles downwind from a large burn pit.
In October 2010, she quit smoking again because of new-onset chest tightness and dyspnea on exertion. However, her symptoms did not abate, and she noted increased chest tightness and difficulty catching her breath when running near the burn pit. While she tried to avoid the burn pit, she participated in competitive races and a 10-mile run along paths that were near the burn pit.
After returning from deployment, the patient presented to her VA PCP for evaluation of persistent nonproductive cough, chest tightness, and dyspnea on exertion. She was not taking any respiratory or allergy medications at the time of evaluation. Initial chest X-ray and spirometry were normal, and she was referred to the chest clinic for consultation. At the time of pulmonary consultation, the patient had a total smoking history of 15 pack-years but had now abstained from smoking for about 2 years. She reported residential exposure to pet birds for > 20 years. High-resolution chest imaging and full PFT with lung volumes and diffusion capacity were performed to evaluate for hypersensitivity pneumonitis.
Her vital signs, physical examination pulmonary function testing with spirometry, lung volumes, and diffusion testing were all normal (Table 4). Bronchial challenge to methacholine demonstrated airways hyperresponsiveness at a PC[-20] FEV1 of 1.25 mg/mL. High-resolution chest CT did not demonstrate air trapping, centrilobular nodules, or other evidence of chronic interstitial lung disease. A cardiopulmonary maximum multistage exercise test with arterial line placement showed normal exercise tolerance with the patient achieving 109% of the maximum predicted workload and 90% of predicted VO2 max.
The patient was diagnosed with deployment-related asthma based on the finding of airways hyperresponsiveness after bronchial challenge testing. Her asthma was considered deployment-related based on the temporal onset of cough and later chest tightness and dyspnea on exertion that occurred during deployment. Ongoing smoking cessation was emphasized.
The patient was started on bronchodilator therapy with albuterol prior to exercise and as needed, but she continued to have symptoms of chest tightness while exercising. Eventually, a low-dose ICS was initiated in conjunction with albuterol as needed. Her symptoms did not resolve with this regimen, but she did experience improvement in exertional chest tightness. This patient was not referred for biopsy given clinical findings of asthma. She will continue pulmonary monitoring every 6 months. However, if her symptoms worsen, she will undergo full PFT, which includes lung volumes and diffusion testing and possible repeat chest imaging.
Conclusion
These 2 cases are representative of the spectrum of deployment-related lung disease. This assessment requires a detailed chronologic occupational and environmental history, establishing a temporal link between respiratory symptoms and deployment exposures and evidence of lung disease on noninvasive testing (or confirmation by surgical lung biopsy in select cases) in which noninvasive testing is nondiagnostic.
Referral for surgical lung biopsy was particularly helpful in the first case, because it ruled out other lung diseases that are more responsive to systemic therapy. However, referral for surgical lung biopsy is not recommended in all patients, and in-depth discussion of the risks and benefits associated with surgery is recommended. Although diagnostic clarity is a benefit of surgical lung biopsy, the authors also discuss with patients that there is no currently available therapy for deployment-related lung disease and thus management is unlikely to change after biopsy. The recommended approach to diagnostic evaluation is shown in Figure 4.
In the authors’ experience, treatment of deployment-related asthma with standard asthma treatment usually improves or stabilizes respiratory symptoms but often does not result in complete resolution of symptoms. Improvement in lung function with systemic pharmacotherapy in the management of deployment-related lung diseases, such as constrictive bronchiolitis, respiratory bronchiolitis, emphysema, or granulomatous pneumonitis has not been observed. Although little is currently known about prognosis, utilization of data collected from the VA Airborne Hazards and Open Burn Pit Registry may contribute to the understanding of deployment exposures and long-term respiratory health effects.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Military deployed from World War II through the Vietnam War have had enough time for respiratory disorders with both short and long latencies to manifest. More recent deployments over the past 13 years to Iraq, Kuwait, Afghanistan, and other regions in southwest Asia (SWA) have been associated with a unique spectrum of respiratory disorders. The long-term respiratory effects of SWA deployments are unknown. This review will discuss deployment-related lung cancer and then focus primarily on the emerging respiratory disorders related to SWA deployment and case examples of deployment-related lung disease.
As the number of recent veterans in the VA health care system increases, primary care providers (PCPs) and specialists are increasingly faced with questions about potential hazards of deployment, referring patients to the VA Airborne Hazards and Open Burn Pit Registry, and evaluating patients with new-onset respiratory symptoms following deployment. Previous reviews and white papers have offered recommendations for evaluation and management; however, little has been reported in the form of case examples of patients with deployment-related lung disorders and their clinical course.1,2
Deployment-Associated Lung Cancer
Lung cancer is the leading cause of cancer death in the U.S. and around the world.3 Lung cancer in the U.S. causes more deaths than does the combination of breast, prostate, colon, and rectal cancers. Lung cancer is the second most common cancer and causes more deaths than does any other cancer in the VHA.4 Most cancers with an environmental cause have a significant latent period of decades between the exposure and cancer incidence. Thus, although lung cancer risk is relatively low in active-duty military personnel, the rate of lung cancer in VA patients is nearly double that of the general population, suggesting causes associated with military service.5
Tobacco
The main cause of lung cancer is tobacco smoking, which accounts for 85% to 90% of lung cancer in the U.S. The latent period between initiation of tobacco smoking and lung cancer incidence is typically ≥ 30 years. Military service has long been associated with tobacco smoking, due to past practices that included the provision of free cigarettes, the availability of cigarettes at reduced cost, smoking breaks, perceived relief from both stress and boredom, and social factors.6 More recently, the adverse effects (AEs) of smoking on health and readiness have been appreciated, and many incentives encouraging tobacco smoking have been eliminated. In 2009, the Institute of Medicine called for a tobacco-free military, and both the Secretary of the Navy and Secretary of Defense have seriously considered this change.7
The additional effect of deployment on smoking has been reported.8 The longitudinal Millennium Cohort study compared several smoking measures between 55,021 deployers and nondeployers who completed both baseline (acquired July 2001-June 2003) and follow-up questionnaires (acquired June 2004-January 2006). Smoking initiation affected 2.3% of deployers and 1.3% of nondeployers; smoking resumption showed a similar pattern with an increase of 39.4% compared with 28.7%. The overall prevalence of smoking increased 44% among nondeployers and 57% among deployers. Those never smokers exposed to combat were 60% more likely to initiate smoking compared with noncombat deployers. Thus, it is clear that tobacco smoking should be considered a deployment-related exposure that contributes to lung cancer risk.
Asbestos
In 1955, Doll published an analysis associating asbestos exposure with risk for lung cancer.9 Many naval veterans and shipyard workers had asbestos exposure, resulting in a spectrum of asbestos-related diseases, including bronchogenic cancer.10
Depleted Uranium
Depleted uranium was used in munitions during the first Gulf War and more recently during military operations in SWA as a part of Operation New Dawn (OND), Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF). Because of concerns of military personnel having complex exposure to depleted uranium, including via inhalation, the VA established the Depleted Uranium Surveillance Program, which has followed a cohort of service members exposed to inhaled depleted uranium during friendly fire in 1991. No significant differences between individuals with high urinary uranium levels and low urinary uranium levels were found in self-reported respiratory symptoms and pulmonary function testing (PFT). Additionally, 20 years after exposure to depleted uranium, there was no statistically significant difference of low-dose chest computed tomography (CT) evidence of lung cancer in these 2 groups.11
Mustard Gas
Mustard gas is considered a definite lung carcinogen.12,13 Both long-term, low-dose and short-term, high- intensity exposures are known to cause human lung cancer.14 Mustard gas was first widely used in warfare in World War I. Mustard gas was used in training for World War II; training accidents resulted in acute toxicity even in lower exposures. It was later used as a chemical warfare agent in the Iran-Iraq conflict in the late 1980s and early 1990s. It is estimated that about 4,000 U.S. service members have been acutely exposed to high concentrations of mustard gas. Sulfur mustard may be incorporated into improvised explosive devices, and there is concern that troops in Iraq have been exposed to this agent in sites previously used for manufacturing and storage.15
Agent Orange
The herbicide Agent Orange is commonly contaminated with dioxin, which has been demonstrated to be a tumor promoter in animal studies. Agent Orange was used widely in the Vietnam War. The National Academy of Sciences issued a report in 2001 reviewing evidence for a link between Agent Orange and various neoplasms. Evidence was strongest for Hodgkin lymphoma and soft tissue sarcoma. The evidence of an association between Agent Orange exposure and lung cancer was deemed only suggestive.16
Respiratory Disease Associated With Southwest Asia Deployment
Over the past 14 years, > 2.5 million U.S. military personnel and civilian contractors have been deployed as part of 3 major military operations: OEF in Afghanistan (2001 to present), OIF in Iraq (2003 to 2010), and OND in Iraq (2010 to present).17,18 Deployed personnel encounter a wide variety of inhalational exposures that include desert dust particulate matter, burn pit combustion products, environmental tobacco smoke, vehicular diesel exhaust, debris from detonations and explosions, and other unique or specific job-related exposures (Table 1).19,20
A number of recent studies have helped identify and characterize an emerging spectrum of deployment-related lung disorders, including asthma, rhinosinusitis, emphysema, bronchiolitis, granulomatous pneumonitis, and less common conditions such as acute eosinophilic pneumonia and rapidly progressive pulmonary fibrosis (Table 2).20-30 Still, diagnosis of these conditions is often challenging, and traditional diagnostic tools such as PFT and chest radiography may be normal or mildly abnormal despite significant histopathologic abnormalities on surgical lung biopsy.24,30,31
Deployment-Related Exposures
As listed in Table 1, there are a number of other exposures that may be encountered during deployment. Environmental air sampling was conducted in several locations in Iraq, Afghanistan, and sites in SWA as part of the Enhanced Particulate Matter Survey. All sites were notable for air pollutant levels that exceeded 15 μg/m3, the military exposure guideline for fine particulate matter (PM2.5). The PM2.5 fraction comprised geologic dust, burn pit emissions, and the heavy metals aluminum, cadmium, and lead.32,33
Respiratory Disorders
Reports of deployers with respiratory symptoms during and after deployment surfaced as early as 2004.34 The Millennium Cohort study reported a 1.7-fold higher rate of new-onset respiratory symptoms that was independent of smoking status, such as cough and shortness of breath, in deployers compared with nondeployers. These increased symptom rates were associated with land-based deployment and longer deployment duration.35 A number of epidemiologic studies also demonstrated an association between respiratory symptoms and environmental exposures encountered during deployment.36-39
Respiratory diseases such as asthma, acute eosinophilic pneumonia, and constrictive bronchiolitis have been reported following deployment to SWA, but a review of the literature supports a more expansive list of deployment-related respiratory diseases (Table 2).20-30 The following case examples describe findings in veterans referred to the authors’ clinic for evaluation of chest symptoms associated with deployment.
OEF/OIF/OND Case Studies
Case Study 1
A 42-year-old male never smoker presented to his VA PCP for evaluation of nonproductive cough, dyspnea on exertion, chest tightness, and recurrent episodes of bronchitis since 2004 when he was deployed to Afghanistan. He had no history of asthma or other chronic respiratory disease in childhood or adolescence.
The patient served as a Civil Affairs officer in the U.S. Army and was deployed to Bosnia in 1997, Afghanistan in 2004, and Camp Arif-Jan in Kuwait as well as Mosul, Iraq, in 2005. He was exposed to depleted uranium while serving in Bosnia. He also had exposures to sandstorms, desert dust, and burn pit combustion products while deployed to Afghanistan and Iraq. He developed symptoms of chest tightness and dyspnea on exertion during his 2004 deployment, with these symptoms persisting after returning home from deployment. His symptoms occurred frequently while running and limited his ability to pass his military physical fitness test requirements and train for marathons as he had done previously. He also had symptoms of chest tightness and excessive coughing at rest, which were treated with antibiotics by his medical provider as recurrent acute infectious/viral bronchitis.
The patient was medically discharged from the U.S. Army in July 2005, primarily due to musculoskeletal injuries. His past medical history was notable for PTSD, recurrent allergic rhinosinusitis, and lumbosacral back pain. Given persistent respiratory symptoms of dyspnea after walking 1 block, the patient presented to his VA PCP in early 2006.
The patient’s vital signs and physical examination were normal. Spirometry showed a mixed restrictive and obstructive pattern, prompting referral for pulmonary consultation. Full PFT demonstrated an abnormally increased residual volume and mildly decreased diffusion capacity (Table 3). Laryngoscopy was negative for vocal cord dysfunction. A chest X-ray showed mild airway wall thickening bilaterally in the lower lung fields. Subsequent high-resolution CT of the chest demonstrated diffuse centrilobular nodularity (Figure 1). Serial spirometry measurements over 8 months showed severe and worsening airflow limitation despite treatment with inhaled bronchodilator and corticosteroid therapy. Seeking diagnostic clarity, the patient was referred for surgical lung biopsy via video-assisted thorascopic surgery (VATS) within 6 months of initial consultation.
The patient’s lung biopsy demonstrated constrictive changes in bronchioles, hyperinflation, and multiple chemodectomas in all 3 lobes of the right lung (Figures 2 and 3). Three pulmonary pathologists reviewed the biopsy and confirmed findings of constrictive bronchiolitis. Serologies for connective tissue disease were negative, indicating no autoimmune cause of bronchiolitis.
As no specific etiology was identified, the patient was referred for a second opinion with a pulmonologist with expertise in interstitial lung disease. Finding no evidence of post- infectious or autoimmune bronchiolitis, the patient’s diagnosis of constrictive bronchiolitis was deemed to be idiopathic. A number of years later, following publication of a case series of 38 OEF/OIF deployers with biopsy-proven constrictive bronchiolitis, the patient was referred for consultation to an occupational lung disease clinic.24 He subsequently was diagnosed with deployment-related lung disease, as his constrictive bronchiolitis was thought to be related to exposures encountered during his OEF/OIF deployments from 2003 to 2005.
The patient was monitored with spirometry over the next few months. After observing a 10% decline in forced expiratory volume in 1 second (FEV1) over 9 months despite stable lung volumes and diffusion capacity, the patient was started on macrolide therapy with erythromycin 500 mg daily. He was switched to azithromycin 250 mg daily due to gastrointestinal AEs of nausea and diarrhea while taking erythromycin. He continued use of an inhaled corticosteroid (ICS), as well as bronchodilator therapy with albuterol and formoterol and had stable dyspnea.
The patient was treated briefly with prednisone 40 mg, but he discontinued this medication after 5 days due to worsening anxiety and PTSD symptoms. Azithromycin therapy was discontinued after 4 years, because no significant improvement was noted in the patient’s lung function. Spirometry, lung volumes, and diffusion testing were unchanged for 2 years following discontinuation of azithromycin and continuing therapy with an ICS, long-acting beta-agonist, and albuterol. The patient has stable dyspnea on exertion but exercises regularly and recently was able to complete a marathon.
Case Study 2
A 43-year-old female ex-smoker presented to a VA chest clinic for evaluation of cough that started during a 2003 deployment to Iraq as well as dyspnea on exertion and chest tightness that had been present since her 2010 to 2011 deployment to Afghanistan. The patient had no history of asthma or other chronic respiratory disease during childhood.
She enlisted in the U.S. Navy in 1987 and later served as a medic while in the Navy Reserves. When she joined the U.S. Navy, she easily passed a 1.5-mile physical fitness readiness test run-time requirement with an 8.5-minute run time. She had no respiratory symptoms and ran in several marathons until her first SWA deployment in 2003.
In April 2003, she was deployed for 3 months to work as a combat medic near the Kuwait and Iraq border. She had frequent exposure to desert dust and recalled 5 sandstorms that appeared like a “wall of sand” coming toward the base. A few weeks into this deployment, the patient developed a nonproductive cough that persisted after returning to the U.S. She stopped smoking for a few months after returning home but continued to have a nonproductive cough. She did not seek further medical attention, because she had no exercise-limiting symptoms.
The patient joined the Army National Guard in 2006 and was activated in 2009 to deploy to Afghanistan from January 2010 through January 2011. She was stationed at Bagram Airbase for the entire deployment and worked as a military police officer in the prison. She had exposure to sandstorms and burn pit combustion products. The prison was about 2 miles downwind from a large burn pit.
In October 2010, she quit smoking again because of new-onset chest tightness and dyspnea on exertion. However, her symptoms did not abate, and she noted increased chest tightness and difficulty catching her breath when running near the burn pit. While she tried to avoid the burn pit, she participated in competitive races and a 10-mile run along paths that were near the burn pit.
After returning from deployment, the patient presented to her VA PCP for evaluation of persistent nonproductive cough, chest tightness, and dyspnea on exertion. She was not taking any respiratory or allergy medications at the time of evaluation. Initial chest X-ray and spirometry were normal, and she was referred to the chest clinic for consultation. At the time of pulmonary consultation, the patient had a total smoking history of 15 pack-years but had now abstained from smoking for about 2 years. She reported residential exposure to pet birds for > 20 years. High-resolution chest imaging and full PFT with lung volumes and diffusion capacity were performed to evaluate for hypersensitivity pneumonitis.
Her vital signs, physical examination pulmonary function testing with spirometry, lung volumes, and diffusion testing were all normal (Table 4). Bronchial challenge to methacholine demonstrated airways hyperresponsiveness at a PC[-20] FEV1 of 1.25 mg/mL. High-resolution chest CT did not demonstrate air trapping, centrilobular nodules, or other evidence of chronic interstitial lung disease. A cardiopulmonary maximum multistage exercise test with arterial line placement showed normal exercise tolerance with the patient achieving 109% of the maximum predicted workload and 90% of predicted VO2 max.
The patient was diagnosed with deployment-related asthma based on the finding of airways hyperresponsiveness after bronchial challenge testing. Her asthma was considered deployment-related based on the temporal onset of cough and later chest tightness and dyspnea on exertion that occurred during deployment. Ongoing smoking cessation was emphasized.
The patient was started on bronchodilator therapy with albuterol prior to exercise and as needed, but she continued to have symptoms of chest tightness while exercising. Eventually, a low-dose ICS was initiated in conjunction with albuterol as needed. Her symptoms did not resolve with this regimen, but she did experience improvement in exertional chest tightness. This patient was not referred for biopsy given clinical findings of asthma. She will continue pulmonary monitoring every 6 months. However, if her symptoms worsen, she will undergo full PFT, which includes lung volumes and diffusion testing and possible repeat chest imaging.
Conclusion
These 2 cases are representative of the spectrum of deployment-related lung disease. This assessment requires a detailed chronologic occupational and environmental history, establishing a temporal link between respiratory symptoms and deployment exposures and evidence of lung disease on noninvasive testing (or confirmation by surgical lung biopsy in select cases) in which noninvasive testing is nondiagnostic.
Referral for surgical lung biopsy was particularly helpful in the first case, because it ruled out other lung diseases that are more responsive to systemic therapy. However, referral for surgical lung biopsy is not recommended in all patients, and in-depth discussion of the risks and benefits associated with surgery is recommended. Although diagnostic clarity is a benefit of surgical lung biopsy, the authors also discuss with patients that there is no currently available therapy for deployment-related lung disease and thus management is unlikely to change after biopsy. The recommended approach to diagnostic evaluation is shown in Figure 4.
In the authors’ experience, treatment of deployment-related asthma with standard asthma treatment usually improves or stabilizes respiratory symptoms but often does not result in complete resolution of symptoms. Improvement in lung function with systemic pharmacotherapy in the management of deployment-related lung diseases, such as constrictive bronchiolitis, respiratory bronchiolitis, emphysema, or granulomatous pneumonitis has not been observed. Although little is currently known about prognosis, utilization of data collected from the VA Airborne Hazards and Open Burn Pit Registry may contribute to the understanding of deployment exposures and long-term respiratory health effects.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Rose C, Abraham J, Harkins D, et al. Overview and recommendations for medical screening and diagnostic evaluation for postdeployment lung disease in returning US warfighters. J Occup Environ Med. 2012;54(6):746-751.
2. Morris MJ, Lucero PF, Zanders TB, Zacher LL. Diagnosis and management of chronic lung disease in deployed military personnel. Ther Adv Respir Dis. 2013;7(4):235-245.
3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
4. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
5. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745.
6. Smith EA, Jahnke SA, Poston WS, et al. Is it time for a tobacco-free military? N Engl J Med. 2014;371(7):589-591.
7. Combating Tobacco in Military and Veteran Populations. In: Bondurant S, Wedge R, eds. Washington, DC: National Academies Press; 2009.
8. Smith B, Ryan MA, Wingard DL, Patterson TL, Slymen DJ, Macera CA; Millennium Cohort Study Team. Cigarette smoking and military deployment: a prospective evaluation. Am J Prev Med. 2008;35(6):539-546.
9. Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12(2):81-86.
10. Krstev S, Stewart P, Rusiecki J, Blair A. Mortality among shipyard Coast Guard workers: a retrospective cohort study. Occup Environ Med. 2007;64(10):651-658.
11. Hines SE, Gucer P, Kligerman S, et al. Pulmonary health effects in Gulf War I service members exposed to depleted uranium. J Occup Environ Med. 2013;55(8):937-944.
12. World Health Organization, International Agency for Research on Cancer. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: some aziridines, N-, S- & O-mustards and selenium. IARC Monogr Eval Carcinog Risk Chem Man. 1975;9:1-268.
13. Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33(4):681-703.
14. Ghanei M, Harandi AA. Lung carcinogenicity of sulfur mustard. Clin Lung Cancer. 2010;11(1):13-17.
15. Chivers CJ. The secret casualties of Iraq’s abandoned chemical weapons. New York Times. October 14, 2014. http://www.nytimes.com /interactive/2014/10/14/world/middleeast/us-casualties-of-iraq-chemical-weapons.html?_r=0. Accessed May 13, 2015.
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Third Biennial Update). Veterans and Agent Orange: Update 2000. Washington, DC: National Academies Press; 2001.
17. How to help military & veteran families before, during, and after deployment. Military Family Research Institute Web site. https://www.mfri.purdue.edu/resources/public/hth/HowToHelp _FamilyFriendNeighbor.pdf. Accessed November 6, 2014.
18. Torreon BS. U.S. periods of war and dates of current conflicts. Washington, DC: Congressional Research Service Report for Congress; December 28, 2012. http://fas.org/sgp/crs/natsec/RS21405.pdf. Accessed November 6, 2014.
19. Rose CS. Military service and lung disease. Clin Chest Med. 2012;33(4):705-714.
20. Szema AM. Occupational lung diseases among soldiers deployed to Iraq and Afghanistan. Occup Med Health Aff. 2013;1:10.4172/2329-6879.1000117.
21. Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med. 2014;190(1):77-84.
22. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004;292(24): 2997-3005.
23. Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172(12):1264-1269.
24. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan [published correction appears in N Engl J Med. 2011;365(18):1749]. N Engl J Med. 2011;365(3):222-230.
25. Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
26. Stecker T, Fortney J, Owen R, McGovern MP, Williams S. Co-occurring medical, psychiatric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51(6):503-507.
27. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71.
28. Scoville SL. Acute eosinophilic pneumonia (AEP) among U.S. military personnel in the U.S. Central Command Area of Responsibility (USCENTCOM AOR). USACHPPM Information Paper. http://www.pdhealth.mil/AEP_Info_paper_01oct09.pdf. Published October 1, 2009. Accessed January 27, 2015.
29. Zembrzuska H, Collen J, Roop S. Pulmonary fibrosis presenting at post-deployment health screening. Am J Respir Crit Care Med. 2011;183:A4780. Abstract.
30. Dhoma S, Gottschall B, Robinson M, et al. Lung disease in deployers returning from Afghanistan and Iraq. Am J Respir Crit Care Med. 2013;187:A3669. Abstract.
31. Dhoma S, Cox C, Chung JH, et al. Chest tomography may predict histopathologic abnormalities in symptomatic deployers returning from Iraq and Afghanistan. Am J Respir Crit Care Med. 2014;189:A5102. Abstract.
32. Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part I: ambient sampling. Inhal Toxicol. 2009;21(4):297-326.
33. Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part 2: grab samples and re-suspensions. Inhal Toxicol. 2009;21(4):327-336.
34. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480.
35. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD, Ryan MAK; for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442.
36. Abraham JH, DeBakey SF, Reid L, Zhou J, Baird CP. Does deployment to Iraq and Afghanistan affect respiratory health of US military personnel? J Occup Environ Med. 2012;54(6):740-745.
37. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669.
38. Quigley KS, McAndrew LM, Almeida L, et al. Prevalence of environmental and other military exposure concerns in Operation Enduring Freedom and Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):659-664.
39. Teichman R. Exposures of concern to veterans returning from Afghanistan and Iraq. J Occup Environ Med. 2012;54(6):677-681.
1. Rose C, Abraham J, Harkins D, et al. Overview and recommendations for medical screening and diagnostic evaluation for postdeployment lung disease in returning US warfighters. J Occup Environ Med. 2012;54(6):746-751.
2. Morris MJ, Lucero PF, Zanders TB, Zacher LL. Diagnosis and management of chronic lung disease in deployed military personnel. Ther Adv Respir Dis. 2013;7(4):235-245.
3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
4. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
5. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745.
6. Smith EA, Jahnke SA, Poston WS, et al. Is it time for a tobacco-free military? N Engl J Med. 2014;371(7):589-591.
7. Combating Tobacco in Military and Veteran Populations. In: Bondurant S, Wedge R, eds. Washington, DC: National Academies Press; 2009.
8. Smith B, Ryan MA, Wingard DL, Patterson TL, Slymen DJ, Macera CA; Millennium Cohort Study Team. Cigarette smoking and military deployment: a prospective evaluation. Am J Prev Med. 2008;35(6):539-546.
9. Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12(2):81-86.
10. Krstev S, Stewart P, Rusiecki J, Blair A. Mortality among shipyard Coast Guard workers: a retrospective cohort study. Occup Environ Med. 2007;64(10):651-658.
11. Hines SE, Gucer P, Kligerman S, et al. Pulmonary health effects in Gulf War I service members exposed to depleted uranium. J Occup Environ Med. 2013;55(8):937-944.
12. World Health Organization, International Agency for Research on Cancer. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: some aziridines, N-, S- & O-mustards and selenium. IARC Monogr Eval Carcinog Risk Chem Man. 1975;9:1-268.
13. Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33(4):681-703.
14. Ghanei M, Harandi AA. Lung carcinogenicity of sulfur mustard. Clin Lung Cancer. 2010;11(1):13-17.
15. Chivers CJ. The secret casualties of Iraq’s abandoned chemical weapons. New York Times. October 14, 2014. http://www.nytimes.com /interactive/2014/10/14/world/middleeast/us-casualties-of-iraq-chemical-weapons.html?_r=0. Accessed May 13, 2015.
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Third Biennial Update). Veterans and Agent Orange: Update 2000. Washington, DC: National Academies Press; 2001.
17. How to help military & veteran families before, during, and after deployment. Military Family Research Institute Web site. https://www.mfri.purdue.edu/resources/public/hth/HowToHelp _FamilyFriendNeighbor.pdf. Accessed November 6, 2014.
18. Torreon BS. U.S. periods of war and dates of current conflicts. Washington, DC: Congressional Research Service Report for Congress; December 28, 2012. http://fas.org/sgp/crs/natsec/RS21405.pdf. Accessed November 6, 2014.
19. Rose CS. Military service and lung disease. Clin Chest Med. 2012;33(4):705-714.
20. Szema AM. Occupational lung diseases among soldiers deployed to Iraq and Afghanistan. Occup Med Health Aff. 2013;1:10.4172/2329-6879.1000117.
21. Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med. 2014;190(1):77-84.
22. Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004;292(24): 2997-3005.
23. Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172(12):1264-1269.
24. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan [published correction appears in N Engl J Med. 2011;365(18):1749]. N Engl J Med. 2011;365(3):222-230.
25. Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
26. Stecker T, Fortney J, Owen R, McGovern MP, Williams S. Co-occurring medical, psychiatric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51(6):503-507.
27. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71.
28. Scoville SL. Acute eosinophilic pneumonia (AEP) among U.S. military personnel in the U.S. Central Command Area of Responsibility (USCENTCOM AOR). USACHPPM Information Paper. http://www.pdhealth.mil/AEP_Info_paper_01oct09.pdf. Published October 1, 2009. Accessed January 27, 2015.
29. Zembrzuska H, Collen J, Roop S. Pulmonary fibrosis presenting at post-deployment health screening. Am J Respir Crit Care Med. 2011;183:A4780. Abstract.
30. Dhoma S, Gottschall B, Robinson M, et al. Lung disease in deployers returning from Afghanistan and Iraq. Am J Respir Crit Care Med. 2013;187:A3669. Abstract.
31. Dhoma S, Cox C, Chung JH, et al. Chest tomography may predict histopathologic abnormalities in symptomatic deployers returning from Iraq and Afghanistan. Am J Respir Crit Care Med. 2014;189:A5102. Abstract.
32. Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part I: ambient sampling. Inhal Toxicol. 2009;21(4):297-326.
33. Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part 2: grab samples and re-suspensions. Inhal Toxicol. 2009;21(4):327-336.
34. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480.
35. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD, Ryan MAK; for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442.
36. Abraham JH, DeBakey SF, Reid L, Zhou J, Baird CP. Does deployment to Iraq and Afghanistan affect respiratory health of US military personnel? J Occup Environ Med. 2012;54(6):740-745.
37. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669.
38. Quigley KS, McAndrew LM, Almeida L, et al. Prevalence of environmental and other military exposure concerns in Operation Enduring Freedom and Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):659-664.
39. Teichman R. Exposures of concern to veterans returning from Afghanistan and Iraq. J Occup Environ Med. 2012;54(6):677-681.
Comparison of Carpal Tunnel Release Methods and Complications
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
Idiopathic Follicular Mucinosis or Mycosis Fungoides? Classification and Diagnostic Challenges
When follicular mucinosis (FM) is defined as an epithelial reaction pattern characterized by intrafollicular and perifollicular mucin accumulation, it cannot be considered a distinct disease entity, as this pattern is ubiquitously present in various inflammatory and neoplastic skin conditions.1,2 The distinction between idiopathic FM and lymphoma-associated follicular mucinosis (LAFM) was made several years ago by authors who evaluated the differences in the clinical presentation of these entities, including patient age at onset, number of lesions, pattern of distribution, and most importantly clinical progression.1 In this article, we discuss the importance of close clinical follow-up in patients with FM or patch-stage mycosis fungoides (MF) in whom histopathologic evaluation is ambiguous or nondiagnostic. We also highlight the value of ancillary testing, including T-cell receptor gene rearrangement, flow cytometry, and immunohistochemistry, as a component in the diagnostic process rather than the sole diagnostic moiety. A review of the pertinent literature also is performed.
History of FM and MF
Pinkus3 first described an entity he termed alopecia mucinosa in 1957. Pinkus noted 3 distinct patterns: an idiopathic form of alopecia mucinosa, lymphoblastoma with associated FM, and alopecia mucinosa that later transformed into lymphoblastoma.4 In 1983, however, Pinkus4 described uncertainty if alopecia mucinosa represented the first stage of MF or if patients with alopecia mucinosa were simply at an increased risk for developing lymphoma. He believed there were too many cases of lymphoma following a diagnosis of alopecia mucinosa for the relation to be coincidental, yet he noted that many of the cases resolved either spontaneously or following treatment with x-rays or topical steroids. He concluded his report with a sentiment that is echoed in many current studies regarding this entity: “Many questions surrounding this entity are as unanswerable today as they were 25 years ago.”4
Jablonska et al5 were the first to coin the term mucinosis follicularis, now known as FM, to replace alopecia mucinosa because they felt the description was more accurate, as lesions also arise on non–hair-bearing skin. Although there is general agreement that there is a form of MF that has associated FM, this is where the agreement ends with regard to the diagnosis of MF versus FM. Böer et al6 discussed the historic evolution of these terms, mostly to highlight the origins of the confusion. The investigators proposed that FM should only be used as a descriptive term and that all cases of alopecia mucinosa represent MF. They also concluded that many benign dermatoses associated with a risk for evolution to MF (eg, small and large plaque psoriasis [LPP]) should simply be diagnosed as MF.6 Subsequently, the proposal that idiopathic FM and LAFM are not 2 distinct entities but rather a clinicopathologic continuum and that idiopathic FM is simply a variant of MF along this spectrum has gained some approval.6,7 However, this belief is not shared among all authorities in the field, and attempts to define diagnostic criteria that distinguish between a benign clinical course and a course that is more progressive and fatal continue. Currently, it is agreed upon that when distinguishing between these 2 clinical courses, primary (idiopathic) follicular mucinosis refers to a benign course with no overt sign of malignancy, and lymphoma-associated follicular mucinosis refers to a diagnostic malignant condition. Lymphoma-associated follicular mucinosis refers to FM associated with cutaneous T-cell lymphoma, the most common form of which is FM. Many authors8-15 have sought ancillary methodologies in addition to clinical parameters to assist in the evaluation between both disease courses. Methodologies have included assessment of T-cell receptor gene rearrangements, flow cytometry, and immunohistochemical staining, mostly as an effort to establish monoclonality as a defining characteristic of LAFM; however, monoclonality in cutaneous T-cell infiltrates should be interpreted with caution and should not be considered as a confirmation of malignancy due to recent findings of monoclonality in benign inflammatory dermatoses such as lichen planus. The Table outlines several of the most common benign inflammatory dermatoses that demonstrate monoclonality, but this list should not be considered exhaustive, as there are many others in which monoclonality is sometimes seen.8-15 The lack of definitive criteria to distinguish between the 2 groups has led to confusion and consternation regarding the diagnosis of idiopathic FM versus LAFM and has led many in the field to consider the 2 conditions to be one and the same.
Diagnosis of FM and MF: Clinicopathologic Features
The World Health Organization (WHO) defined MF as an epidermotropic primary cutaneous T-cell lymphoma (CTCL) characterized by infiltrates of small- to medium-sized T lymphocytes with cerebriform nuclei. Further, the WHO stated that the term mycosis fungoides should be exclusively reserved for classical cases typified by the evolution of cutaneous patches, plaques, and tumors, or for variants that show a similar clinical course.16 Mycosis fungoides is divided into 3 stages—patch, plaque, and tumor—which are solely clinical descriptors.17 The WHO also described a clinical staging system with pathologic emphasis placed only on lymph node involvement and identification of Sézary cells.16 It lists folliculotropic MF as a variant, with only some cases presenting with mucinous degeneration of hair follicles. A lack of consensus among pathologists regarding criteria for diagnosis in patch-stage MF remains, but diagnosis of plaque-stage disease is not regularly debated due to its more reliably present, well-developed histologic features (eg, haloed lymphocytes, epidermotropism of lymphocytes, lymphocytes with convoluted nuceli, Pautrier microabscesses).18 Although there have been specific histologic findings reported to be associated with patch-stage MF, they have only been present in a few cases and are therefore of limited usefulness in practice.1,19 The categorization of patients with subtle histologic features common to both MF and inflammatory conditions such as parapsoriasis en plaques (the term plaque in this case is a misnomer because the word plaque means patch in French) continues to be elusive. A lack of agreement regarding LPP persists in the current literature in the same manner as FM. Some researchers have contended for many years that LPP is a type of MF, while others remain unconvinced, mainly due to the lack of evidence that lumping a benign condition (LPP) with an increased risk for malignant transformation and a known malignancy (MF) together is of any benefit to the patient. Assessment of clinicopathologic correlation, immunohistochemistry, clonality, and T-cell gene rearrangement have failed to positively identify patients who are at risk for disease progression, whether the diagnosis is called LPP or early patch-stage MF.20
Mycosis fungoides is more common in males and its incidence increases with age; however, diagnosis should not be ruled out based on age or gender. Typical presentation of early-stage disease includes erythematous patches or plaques, often with light scaling.19 Lesions routinely are of long-standing duration (months to years), are located in areas that are infrequently exposed to sunlight, and often are 5 cm in diameter or larger with irregular borders.21 Associated poikiloderma is relatively specific to MF but rarely is seen in other CTCLs, connective-tissue diseases, and some genodermatoses. Poikiloderma commonly is identified in LPP, which shows the same telangiectasia, mottled pigmentation, and epidermal atrophy as MF-associated poikiloderma, leading some to believe that there is no separation between the 2 conditions. In all stages of MF, lesions frequently are numerous and occur on multiple sites. Plaques and tumors can show spontaneous ulceration. When lesions are folliculotropic, they can cause localized alopecia, follicular-based papules, and fungating pseudotumors in more advanced stages.1 The clinical presentation of FM substantially overlaps with folliculotropic MF, and although FM lesions often are solitary and are located on the face or scalp, they also can present as multiple lesions located elsewhere on the body. It also has been proposed that folliculotropic MF should not be separated from FM-associated MF (or LAFM).22
The characteristic histologic picture of LAFM in patch or plaque stage shows mucin deposition within hair follicles, similar to idiopathic FM. On histology, both conditions demonstrate dense lymphoid infiltrates around and within hair follicles as well as in the dermis (Figure). Most cases of LAFM show epidermotropism of lymphocytes between follicles, but this finding is not present in every case and often disappears when the disease advances to the tumor stage.1,19 Although Pautrier microabscesses (collections of lymphocytes within the superficial epidermis) are considered to be somewhat specific to MF, they are only present in a minority of cases.20 In a study by the International Society for Cutaneous Lymphomas,21 the only histopathologic criteria that showed any appreciable sensitivity or specificity in the diagnosis of MF were the presence of lymphoid cells with variable nuclear and cytoplasmic features and/or strikingly irregular nuclear contours with the presence of lymphocytes larger than those usually seen in inflammatory dermatoses. Despite these criteria, the study reported a high misclassification rate. A complicated scoring system for diagnosis of MF in patch- or early plaque-stage disease was proposed by the International Society for Cutaneous Lymphomas,21 which integrates clinical, histopathologic, molecular, and immunophenotypic criteria. However, these criteria have been continually debated in the literature and are only discussed in this article in relation to the association between MF and FM. Diagnosis of tumor-stage MF is not addressed in this article, as it is readily identified as lymphoma and is not easily confused with idiopathic FM.
|
Clinical assessment of a patient’s medical history to identify persistent and progressive disease is paramount to the diagnosis of MF. Although MF lesions tend to increase in size and number over time, this presentation is not without exception.21 In early patch-stage disease, eliminating some of the patient’s current medications may be sufficient in clearing cutaneous patches that cannot be conclusively identified as either MF or a benign inflammatory lymphoid infiltrate, which further emphasizes the importance of clinical assessment of the patient’s medical history in the diagnosis of MF. The shape of the lesions also is helpful in distinguishing between MF and other skin disorders, such as digitate dermatosis or LPP; unlike the latter, the waxing and waning nature of MF lesions often produces irregularly shaped patches with little coalescence. Again, there are some investigators who believe that these lesions represent varying presentations of MF.6
In a study by Cerroni et al,1 44 patients with FM were divided into 2 groups: (1) a cohort of 16 patients with no history or clinical evidence of MF or Sézary syndrome (ie, LAFM), and (2) a cohort of 28 patients with clinicopathologic evidence of CTCL. Patients in both groups were followed for a maximum of 20 years. Results indicated that that the presence of perifollicular or intrafollicular mucin, epidermotropism of lymphocytes, monoclonality, and epidemiologic characteristics (eg, age, sex, race) cannot reliably distinguish the 2 disease forms. Furthermore, it was suggested that these conditions are not mutually exclusive entities and are actually variants of CTCL. The observation that the 2 diseases share prognostic overlap adds further credence to the already puzzling conundrum. Nineteen of 28 patients with MF were alive and well at follow-up, and all patients in the idiopathic FM group were alive, with only 9 of 16 patients showing residual disease and none with CTCL.1
Other clinical factors that may be helpful in the diagnosis of MF are the presentation of lesions in non–sun-exposed areas of the skin and multiple lesions, as unilesional MF is exceedingly uncommon.21 No histologic features have been proven to predict which early patch- or plaque-stage MFs will progress to full-blown CTCL versus benign idiopathic FM; thus, great caution should be taken in patients with early-stage disease to ensure they are not prematurely and/or incorrectly classified as CTCL. Such a diagnosis has medical, social, and economical ramifications that should not be overlooked.
If idiopathic FM and LAFM were considered distinct disease processes, the ambiguity in making a definitive diagnosis should give the physician pause, and a diagnosis of LAFM may only be appropriate when there is unequivocal clinicopathologic evidence. Otherwise, a lymphoma diagnosis is somewhat superfluous and potentially harmful. Definitive diagnosis of LAFM also is complicated by reports of other hematologic malignancies presenting with FM-like histopathologic findings, such as chronic myelogenous leukemia, leukemia-associated eosinophilic folliculitis, and acute myeloblastic leukemia.23,24 Although MF is the most common malignancy associated with FM, it is important to consider other less common malignancies that also may be present.
Diagnosis: Patient Consequences
Accurate diagnosis of idiopathic FM versus LAFM is critical, as the ramifications of a cancer diagnosis can have broad implications. For example, patients who receive cancer diagnoses often experience emotional trauma and social stigma, even when adequate patient education has been provided. The incidence of depression and anxiety also can increase following a cancer diagnosis and can be complicated by medical treatments (eg, systemic steroids, interferon),25 which are known to increase the frequency of these psychological disturbances. Health insurance premiums likely will be higher if a patient is diagnosed with cancer versus a benign inflammatory condition. Hesitation of the pathologist to assign a cancer diagnosis when unequivocal evidence is not present should not be regarded as trickery, malpractice, or deceit of the health care bylaws, as benign language with suggestion of close clinical follow-up in the setting of diagnostic uncertainty will “first, do no harm” and secondly, serve as a vehicle for patient advocacy.
If there is a definitive distinction between idiopathic FM and LAFM, it requires further research before it can be fully understood. Currently, the WHO does not recognize a diagnosis of FM-associated MF (or LAFM) and acknowledges that folliculotropic MF is not always associated with FM.16,26 Given uncertainty and repercussions associated with a cancer diagnosis, however indolent, it may be morally responsible and medically favorable for physicians to consider FM in the differential diagnosis when applicable rather than making a diagnosis of MF outright. Given the importance of both clinical and histologic factors, it may be beneficial for definitive diagnosis of FM versus MF to lie with the clinician, while the pathologist serves as an adjunct in the diagnostic process. Because the prognosis of idiopathic FM often is marred by possible transformation into MF or other CTCLs, therapeutic decisions should be dictated by close clinical follow-up. Additionally, stage of disease, patient age, treatment compliance, comorbidities, and possible side effects of medications should all be considered when evaluating potential therapeutic regimens.27
Conclusion
Research is underway to more accurately identify patients with FM who are at risk for progression to LAFM versus those with benign remitting FM. Once the required diagnostic criteria are established to accurately classify these patients, with an emphasis on prognosis and suggested treatments, it might be necessary to establish new, less debated terminology so pathologists and clinicians alike can improve patient care. Continued histopathologic scrutiny, use of sophisticated molecular techniques, and knowledge of other currently undiscovered modalities will shed light on this important disease process and aid in proper disease management, which may be advantageous to both patients and physicians.
1. Cerroni L, Fink-Puches R, Bäck B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2002;138:182-189.
2. Parker SR, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline [published online ahead of print July 25, 2009]. J Am Acad Dermatol. 2010;62:139-141.
3. Pinkus H. Alopecia mucinosa; inflammatory plaques with alopecia characterized by root-sheath mucinosis. AMA Arch Dermatol. 1957;76:419-424, 424-426.
4. Pinkus H. Alopecia mucinosa. additional data in 1983. Arch Dermatol. 1983;119:698-699.
5. Jablonska S, Chorzelski T, Lancucki J. Mucinosis follicularis [in German]. Hautarzt. 1959;10:27-33.
6. Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004;26:33-52.
7. Brown HA, Gibson LE, Pujol RM, et al. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and withoutclonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002;47:856-862.
8. Schiller PI, Flaig MJ, Puchta U, et al. Detection of clonal T cells in lichen planus. Arch Dermatol Res. 2000;292:568-569.
9. Cerroni L, Kerl H. Primary follicular mucinosis and association with mycosis fungoides and other cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:146-147.
10. Dereure O, Levi E, Kadin ME. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
11. Haeffner AC, Smoller BR, Zepter K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131:321-324.
12. Schultz JC, Granados S, Vonderheid EC, et al. T-cell clonality of peripheral blood lymphocytes in patients with lymphomatoid papulosis. J Am Acad Dermatol. 2005;53:152-155.
13. Pfaltz K, Kerl K, Palmedo G, et al. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662.
14. Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
15. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
16. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
17. Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides [published online ahead of print October 22, 2007]. Crit Rev Oncol Hematol. 2008;65:172-182.
18. Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
19. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957.
20. Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol. 2009;75:229-235.
21. Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
22. Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides. a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
23. Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009;15:16.
24. Wada T, Yoshinaga E, Oiso N, et al. Adult T-cell leukemia-lymphoma associated with follicular mucinosis. J Dermatol. 2009;36:638-642.
25. Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma [published online ahead of print December 16, 2008]. Br J Dermatol. 2009;160:815-822.
26. Boone SL, Guitart J, Gerami P. Follicular mycosis fungoides: a histopathologic, immunohistochemical, and genotypic review. G Ital Dermatol Venereol. 2008;143:409-414.
27. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome [published online ahead of print August 20, 2009]. Blood. 2009;114:4337-4353.
When follicular mucinosis (FM) is defined as an epithelial reaction pattern characterized by intrafollicular and perifollicular mucin accumulation, it cannot be considered a distinct disease entity, as this pattern is ubiquitously present in various inflammatory and neoplastic skin conditions.1,2 The distinction between idiopathic FM and lymphoma-associated follicular mucinosis (LAFM) was made several years ago by authors who evaluated the differences in the clinical presentation of these entities, including patient age at onset, number of lesions, pattern of distribution, and most importantly clinical progression.1 In this article, we discuss the importance of close clinical follow-up in patients with FM or patch-stage mycosis fungoides (MF) in whom histopathologic evaluation is ambiguous or nondiagnostic. We also highlight the value of ancillary testing, including T-cell receptor gene rearrangement, flow cytometry, and immunohistochemistry, as a component in the diagnostic process rather than the sole diagnostic moiety. A review of the pertinent literature also is performed.
History of FM and MF
Pinkus3 first described an entity he termed alopecia mucinosa in 1957. Pinkus noted 3 distinct patterns: an idiopathic form of alopecia mucinosa, lymphoblastoma with associated FM, and alopecia mucinosa that later transformed into lymphoblastoma.4 In 1983, however, Pinkus4 described uncertainty if alopecia mucinosa represented the first stage of MF or if patients with alopecia mucinosa were simply at an increased risk for developing lymphoma. He believed there were too many cases of lymphoma following a diagnosis of alopecia mucinosa for the relation to be coincidental, yet he noted that many of the cases resolved either spontaneously or following treatment with x-rays or topical steroids. He concluded his report with a sentiment that is echoed in many current studies regarding this entity: “Many questions surrounding this entity are as unanswerable today as they were 25 years ago.”4
Jablonska et al5 were the first to coin the term mucinosis follicularis, now known as FM, to replace alopecia mucinosa because they felt the description was more accurate, as lesions also arise on non–hair-bearing skin. Although there is general agreement that there is a form of MF that has associated FM, this is where the agreement ends with regard to the diagnosis of MF versus FM. Böer et al6 discussed the historic evolution of these terms, mostly to highlight the origins of the confusion. The investigators proposed that FM should only be used as a descriptive term and that all cases of alopecia mucinosa represent MF. They also concluded that many benign dermatoses associated with a risk for evolution to MF (eg, small and large plaque psoriasis [LPP]) should simply be diagnosed as MF.6 Subsequently, the proposal that idiopathic FM and LAFM are not 2 distinct entities but rather a clinicopathologic continuum and that idiopathic FM is simply a variant of MF along this spectrum has gained some approval.6,7 However, this belief is not shared among all authorities in the field, and attempts to define diagnostic criteria that distinguish between a benign clinical course and a course that is more progressive and fatal continue. Currently, it is agreed upon that when distinguishing between these 2 clinical courses, primary (idiopathic) follicular mucinosis refers to a benign course with no overt sign of malignancy, and lymphoma-associated follicular mucinosis refers to a diagnostic malignant condition. Lymphoma-associated follicular mucinosis refers to FM associated with cutaneous T-cell lymphoma, the most common form of which is FM. Many authors8-15 have sought ancillary methodologies in addition to clinical parameters to assist in the evaluation between both disease courses. Methodologies have included assessment of T-cell receptor gene rearrangements, flow cytometry, and immunohistochemical staining, mostly as an effort to establish monoclonality as a defining characteristic of LAFM; however, monoclonality in cutaneous T-cell infiltrates should be interpreted with caution and should not be considered as a confirmation of malignancy due to recent findings of monoclonality in benign inflammatory dermatoses such as lichen planus. The Table outlines several of the most common benign inflammatory dermatoses that demonstrate monoclonality, but this list should not be considered exhaustive, as there are many others in which monoclonality is sometimes seen.8-15 The lack of definitive criteria to distinguish between the 2 groups has led to confusion and consternation regarding the diagnosis of idiopathic FM versus LAFM and has led many in the field to consider the 2 conditions to be one and the same.
Diagnosis of FM and MF: Clinicopathologic Features
The World Health Organization (WHO) defined MF as an epidermotropic primary cutaneous T-cell lymphoma (CTCL) characterized by infiltrates of small- to medium-sized T lymphocytes with cerebriform nuclei. Further, the WHO stated that the term mycosis fungoides should be exclusively reserved for classical cases typified by the evolution of cutaneous patches, plaques, and tumors, or for variants that show a similar clinical course.16 Mycosis fungoides is divided into 3 stages—patch, plaque, and tumor—which are solely clinical descriptors.17 The WHO also described a clinical staging system with pathologic emphasis placed only on lymph node involvement and identification of Sézary cells.16 It lists folliculotropic MF as a variant, with only some cases presenting with mucinous degeneration of hair follicles. A lack of consensus among pathologists regarding criteria for diagnosis in patch-stage MF remains, but diagnosis of plaque-stage disease is not regularly debated due to its more reliably present, well-developed histologic features (eg, haloed lymphocytes, epidermotropism of lymphocytes, lymphocytes with convoluted nuceli, Pautrier microabscesses).18 Although there have been specific histologic findings reported to be associated with patch-stage MF, they have only been present in a few cases and are therefore of limited usefulness in practice.1,19 The categorization of patients with subtle histologic features common to both MF and inflammatory conditions such as parapsoriasis en plaques (the term plaque in this case is a misnomer because the word plaque means patch in French) continues to be elusive. A lack of agreement regarding LPP persists in the current literature in the same manner as FM. Some researchers have contended for many years that LPP is a type of MF, while others remain unconvinced, mainly due to the lack of evidence that lumping a benign condition (LPP) with an increased risk for malignant transformation and a known malignancy (MF) together is of any benefit to the patient. Assessment of clinicopathologic correlation, immunohistochemistry, clonality, and T-cell gene rearrangement have failed to positively identify patients who are at risk for disease progression, whether the diagnosis is called LPP or early patch-stage MF.20
Mycosis fungoides is more common in males and its incidence increases with age; however, diagnosis should not be ruled out based on age or gender. Typical presentation of early-stage disease includes erythematous patches or plaques, often with light scaling.19 Lesions routinely are of long-standing duration (months to years), are located in areas that are infrequently exposed to sunlight, and often are 5 cm in diameter or larger with irregular borders.21 Associated poikiloderma is relatively specific to MF but rarely is seen in other CTCLs, connective-tissue diseases, and some genodermatoses. Poikiloderma commonly is identified in LPP, which shows the same telangiectasia, mottled pigmentation, and epidermal atrophy as MF-associated poikiloderma, leading some to believe that there is no separation between the 2 conditions. In all stages of MF, lesions frequently are numerous and occur on multiple sites. Plaques and tumors can show spontaneous ulceration. When lesions are folliculotropic, they can cause localized alopecia, follicular-based papules, and fungating pseudotumors in more advanced stages.1 The clinical presentation of FM substantially overlaps with folliculotropic MF, and although FM lesions often are solitary and are located on the face or scalp, they also can present as multiple lesions located elsewhere on the body. It also has been proposed that folliculotropic MF should not be separated from FM-associated MF (or LAFM).22
The characteristic histologic picture of LAFM in patch or plaque stage shows mucin deposition within hair follicles, similar to idiopathic FM. On histology, both conditions demonstrate dense lymphoid infiltrates around and within hair follicles as well as in the dermis (Figure). Most cases of LAFM show epidermotropism of lymphocytes between follicles, but this finding is not present in every case and often disappears when the disease advances to the tumor stage.1,19 Although Pautrier microabscesses (collections of lymphocytes within the superficial epidermis) are considered to be somewhat specific to MF, they are only present in a minority of cases.20 In a study by the International Society for Cutaneous Lymphomas,21 the only histopathologic criteria that showed any appreciable sensitivity or specificity in the diagnosis of MF were the presence of lymphoid cells with variable nuclear and cytoplasmic features and/or strikingly irregular nuclear contours with the presence of lymphocytes larger than those usually seen in inflammatory dermatoses. Despite these criteria, the study reported a high misclassification rate. A complicated scoring system for diagnosis of MF in patch- or early plaque-stage disease was proposed by the International Society for Cutaneous Lymphomas,21 which integrates clinical, histopathologic, molecular, and immunophenotypic criteria. However, these criteria have been continually debated in the literature and are only discussed in this article in relation to the association between MF and FM. Diagnosis of tumor-stage MF is not addressed in this article, as it is readily identified as lymphoma and is not easily confused with idiopathic FM.
|
Clinical assessment of a patient’s medical history to identify persistent and progressive disease is paramount to the diagnosis of MF. Although MF lesions tend to increase in size and number over time, this presentation is not without exception.21 In early patch-stage disease, eliminating some of the patient’s current medications may be sufficient in clearing cutaneous patches that cannot be conclusively identified as either MF or a benign inflammatory lymphoid infiltrate, which further emphasizes the importance of clinical assessment of the patient’s medical history in the diagnosis of MF. The shape of the lesions also is helpful in distinguishing between MF and other skin disorders, such as digitate dermatosis or LPP; unlike the latter, the waxing and waning nature of MF lesions often produces irregularly shaped patches with little coalescence. Again, there are some investigators who believe that these lesions represent varying presentations of MF.6
In a study by Cerroni et al,1 44 patients with FM were divided into 2 groups: (1) a cohort of 16 patients with no history or clinical evidence of MF or Sézary syndrome (ie, LAFM), and (2) a cohort of 28 patients with clinicopathologic evidence of CTCL. Patients in both groups were followed for a maximum of 20 years. Results indicated that that the presence of perifollicular or intrafollicular mucin, epidermotropism of lymphocytes, monoclonality, and epidemiologic characteristics (eg, age, sex, race) cannot reliably distinguish the 2 disease forms. Furthermore, it was suggested that these conditions are not mutually exclusive entities and are actually variants of CTCL. The observation that the 2 diseases share prognostic overlap adds further credence to the already puzzling conundrum. Nineteen of 28 patients with MF were alive and well at follow-up, and all patients in the idiopathic FM group were alive, with only 9 of 16 patients showing residual disease and none with CTCL.1
Other clinical factors that may be helpful in the diagnosis of MF are the presentation of lesions in non–sun-exposed areas of the skin and multiple lesions, as unilesional MF is exceedingly uncommon.21 No histologic features have been proven to predict which early patch- or plaque-stage MFs will progress to full-blown CTCL versus benign idiopathic FM; thus, great caution should be taken in patients with early-stage disease to ensure they are not prematurely and/or incorrectly classified as CTCL. Such a diagnosis has medical, social, and economical ramifications that should not be overlooked.
If idiopathic FM and LAFM were considered distinct disease processes, the ambiguity in making a definitive diagnosis should give the physician pause, and a diagnosis of LAFM may only be appropriate when there is unequivocal clinicopathologic evidence. Otherwise, a lymphoma diagnosis is somewhat superfluous and potentially harmful. Definitive diagnosis of LAFM also is complicated by reports of other hematologic malignancies presenting with FM-like histopathologic findings, such as chronic myelogenous leukemia, leukemia-associated eosinophilic folliculitis, and acute myeloblastic leukemia.23,24 Although MF is the most common malignancy associated with FM, it is important to consider other less common malignancies that also may be present.
Diagnosis: Patient Consequences
Accurate diagnosis of idiopathic FM versus LAFM is critical, as the ramifications of a cancer diagnosis can have broad implications. For example, patients who receive cancer diagnoses often experience emotional trauma and social stigma, even when adequate patient education has been provided. The incidence of depression and anxiety also can increase following a cancer diagnosis and can be complicated by medical treatments (eg, systemic steroids, interferon),25 which are known to increase the frequency of these psychological disturbances. Health insurance premiums likely will be higher if a patient is diagnosed with cancer versus a benign inflammatory condition. Hesitation of the pathologist to assign a cancer diagnosis when unequivocal evidence is not present should not be regarded as trickery, malpractice, or deceit of the health care bylaws, as benign language with suggestion of close clinical follow-up in the setting of diagnostic uncertainty will “first, do no harm” and secondly, serve as a vehicle for patient advocacy.
If there is a definitive distinction between idiopathic FM and LAFM, it requires further research before it can be fully understood. Currently, the WHO does not recognize a diagnosis of FM-associated MF (or LAFM) and acknowledges that folliculotropic MF is not always associated with FM.16,26 Given uncertainty and repercussions associated with a cancer diagnosis, however indolent, it may be morally responsible and medically favorable for physicians to consider FM in the differential diagnosis when applicable rather than making a diagnosis of MF outright. Given the importance of both clinical and histologic factors, it may be beneficial for definitive diagnosis of FM versus MF to lie with the clinician, while the pathologist serves as an adjunct in the diagnostic process. Because the prognosis of idiopathic FM often is marred by possible transformation into MF or other CTCLs, therapeutic decisions should be dictated by close clinical follow-up. Additionally, stage of disease, patient age, treatment compliance, comorbidities, and possible side effects of medications should all be considered when evaluating potential therapeutic regimens.27
Conclusion
Research is underway to more accurately identify patients with FM who are at risk for progression to LAFM versus those with benign remitting FM. Once the required diagnostic criteria are established to accurately classify these patients, with an emphasis on prognosis and suggested treatments, it might be necessary to establish new, less debated terminology so pathologists and clinicians alike can improve patient care. Continued histopathologic scrutiny, use of sophisticated molecular techniques, and knowledge of other currently undiscovered modalities will shed light on this important disease process and aid in proper disease management, which may be advantageous to both patients and physicians.
When follicular mucinosis (FM) is defined as an epithelial reaction pattern characterized by intrafollicular and perifollicular mucin accumulation, it cannot be considered a distinct disease entity, as this pattern is ubiquitously present in various inflammatory and neoplastic skin conditions.1,2 The distinction between idiopathic FM and lymphoma-associated follicular mucinosis (LAFM) was made several years ago by authors who evaluated the differences in the clinical presentation of these entities, including patient age at onset, number of lesions, pattern of distribution, and most importantly clinical progression.1 In this article, we discuss the importance of close clinical follow-up in patients with FM or patch-stage mycosis fungoides (MF) in whom histopathologic evaluation is ambiguous or nondiagnostic. We also highlight the value of ancillary testing, including T-cell receptor gene rearrangement, flow cytometry, and immunohistochemistry, as a component in the diagnostic process rather than the sole diagnostic moiety. A review of the pertinent literature also is performed.
History of FM and MF
Pinkus3 first described an entity he termed alopecia mucinosa in 1957. Pinkus noted 3 distinct patterns: an idiopathic form of alopecia mucinosa, lymphoblastoma with associated FM, and alopecia mucinosa that later transformed into lymphoblastoma.4 In 1983, however, Pinkus4 described uncertainty if alopecia mucinosa represented the first stage of MF or if patients with alopecia mucinosa were simply at an increased risk for developing lymphoma. He believed there were too many cases of lymphoma following a diagnosis of alopecia mucinosa for the relation to be coincidental, yet he noted that many of the cases resolved either spontaneously or following treatment with x-rays or topical steroids. He concluded his report with a sentiment that is echoed in many current studies regarding this entity: “Many questions surrounding this entity are as unanswerable today as they were 25 years ago.”4
Jablonska et al5 were the first to coin the term mucinosis follicularis, now known as FM, to replace alopecia mucinosa because they felt the description was more accurate, as lesions also arise on non–hair-bearing skin. Although there is general agreement that there is a form of MF that has associated FM, this is where the agreement ends with regard to the diagnosis of MF versus FM. Böer et al6 discussed the historic evolution of these terms, mostly to highlight the origins of the confusion. The investigators proposed that FM should only be used as a descriptive term and that all cases of alopecia mucinosa represent MF. They also concluded that many benign dermatoses associated with a risk for evolution to MF (eg, small and large plaque psoriasis [LPP]) should simply be diagnosed as MF.6 Subsequently, the proposal that idiopathic FM and LAFM are not 2 distinct entities but rather a clinicopathologic continuum and that idiopathic FM is simply a variant of MF along this spectrum has gained some approval.6,7 However, this belief is not shared among all authorities in the field, and attempts to define diagnostic criteria that distinguish between a benign clinical course and a course that is more progressive and fatal continue. Currently, it is agreed upon that when distinguishing between these 2 clinical courses, primary (idiopathic) follicular mucinosis refers to a benign course with no overt sign of malignancy, and lymphoma-associated follicular mucinosis refers to a diagnostic malignant condition. Lymphoma-associated follicular mucinosis refers to FM associated with cutaneous T-cell lymphoma, the most common form of which is FM. Many authors8-15 have sought ancillary methodologies in addition to clinical parameters to assist in the evaluation between both disease courses. Methodologies have included assessment of T-cell receptor gene rearrangements, flow cytometry, and immunohistochemical staining, mostly as an effort to establish monoclonality as a defining characteristic of LAFM; however, monoclonality in cutaneous T-cell infiltrates should be interpreted with caution and should not be considered as a confirmation of malignancy due to recent findings of monoclonality in benign inflammatory dermatoses such as lichen planus. The Table outlines several of the most common benign inflammatory dermatoses that demonstrate monoclonality, but this list should not be considered exhaustive, as there are many others in which monoclonality is sometimes seen.8-15 The lack of definitive criteria to distinguish between the 2 groups has led to confusion and consternation regarding the diagnosis of idiopathic FM versus LAFM and has led many in the field to consider the 2 conditions to be one and the same.
Diagnosis of FM and MF: Clinicopathologic Features
The World Health Organization (WHO) defined MF as an epidermotropic primary cutaneous T-cell lymphoma (CTCL) characterized by infiltrates of small- to medium-sized T lymphocytes with cerebriform nuclei. Further, the WHO stated that the term mycosis fungoides should be exclusively reserved for classical cases typified by the evolution of cutaneous patches, plaques, and tumors, or for variants that show a similar clinical course.16 Mycosis fungoides is divided into 3 stages—patch, plaque, and tumor—which are solely clinical descriptors.17 The WHO also described a clinical staging system with pathologic emphasis placed only on lymph node involvement and identification of Sézary cells.16 It lists folliculotropic MF as a variant, with only some cases presenting with mucinous degeneration of hair follicles. A lack of consensus among pathologists regarding criteria for diagnosis in patch-stage MF remains, but diagnosis of plaque-stage disease is not regularly debated due to its more reliably present, well-developed histologic features (eg, haloed lymphocytes, epidermotropism of lymphocytes, lymphocytes with convoluted nuceli, Pautrier microabscesses).18 Although there have been specific histologic findings reported to be associated with patch-stage MF, they have only been present in a few cases and are therefore of limited usefulness in practice.1,19 The categorization of patients with subtle histologic features common to both MF and inflammatory conditions such as parapsoriasis en plaques (the term plaque in this case is a misnomer because the word plaque means patch in French) continues to be elusive. A lack of agreement regarding LPP persists in the current literature in the same manner as FM. Some researchers have contended for many years that LPP is a type of MF, while others remain unconvinced, mainly due to the lack of evidence that lumping a benign condition (LPP) with an increased risk for malignant transformation and a known malignancy (MF) together is of any benefit to the patient. Assessment of clinicopathologic correlation, immunohistochemistry, clonality, and T-cell gene rearrangement have failed to positively identify patients who are at risk for disease progression, whether the diagnosis is called LPP or early patch-stage MF.20
Mycosis fungoides is more common in males and its incidence increases with age; however, diagnosis should not be ruled out based on age or gender. Typical presentation of early-stage disease includes erythematous patches or plaques, often with light scaling.19 Lesions routinely are of long-standing duration (months to years), are located in areas that are infrequently exposed to sunlight, and often are 5 cm in diameter or larger with irregular borders.21 Associated poikiloderma is relatively specific to MF but rarely is seen in other CTCLs, connective-tissue diseases, and some genodermatoses. Poikiloderma commonly is identified in LPP, which shows the same telangiectasia, mottled pigmentation, and epidermal atrophy as MF-associated poikiloderma, leading some to believe that there is no separation between the 2 conditions. In all stages of MF, lesions frequently are numerous and occur on multiple sites. Plaques and tumors can show spontaneous ulceration. When lesions are folliculotropic, they can cause localized alopecia, follicular-based papules, and fungating pseudotumors in more advanced stages.1 The clinical presentation of FM substantially overlaps with folliculotropic MF, and although FM lesions often are solitary and are located on the face or scalp, they also can present as multiple lesions located elsewhere on the body. It also has been proposed that folliculotropic MF should not be separated from FM-associated MF (or LAFM).22
The characteristic histologic picture of LAFM in patch or plaque stage shows mucin deposition within hair follicles, similar to idiopathic FM. On histology, both conditions demonstrate dense lymphoid infiltrates around and within hair follicles as well as in the dermis (Figure). Most cases of LAFM show epidermotropism of lymphocytes between follicles, but this finding is not present in every case and often disappears when the disease advances to the tumor stage.1,19 Although Pautrier microabscesses (collections of lymphocytes within the superficial epidermis) are considered to be somewhat specific to MF, they are only present in a minority of cases.20 In a study by the International Society for Cutaneous Lymphomas,21 the only histopathologic criteria that showed any appreciable sensitivity or specificity in the diagnosis of MF were the presence of lymphoid cells with variable nuclear and cytoplasmic features and/or strikingly irregular nuclear contours with the presence of lymphocytes larger than those usually seen in inflammatory dermatoses. Despite these criteria, the study reported a high misclassification rate. A complicated scoring system for diagnosis of MF in patch- or early plaque-stage disease was proposed by the International Society for Cutaneous Lymphomas,21 which integrates clinical, histopathologic, molecular, and immunophenotypic criteria. However, these criteria have been continually debated in the literature and are only discussed in this article in relation to the association between MF and FM. Diagnosis of tumor-stage MF is not addressed in this article, as it is readily identified as lymphoma and is not easily confused with idiopathic FM.
|
Clinical assessment of a patient’s medical history to identify persistent and progressive disease is paramount to the diagnosis of MF. Although MF lesions tend to increase in size and number over time, this presentation is not without exception.21 In early patch-stage disease, eliminating some of the patient’s current medications may be sufficient in clearing cutaneous patches that cannot be conclusively identified as either MF or a benign inflammatory lymphoid infiltrate, which further emphasizes the importance of clinical assessment of the patient’s medical history in the diagnosis of MF. The shape of the lesions also is helpful in distinguishing between MF and other skin disorders, such as digitate dermatosis or LPP; unlike the latter, the waxing and waning nature of MF lesions often produces irregularly shaped patches with little coalescence. Again, there are some investigators who believe that these lesions represent varying presentations of MF.6
In a study by Cerroni et al,1 44 patients with FM were divided into 2 groups: (1) a cohort of 16 patients with no history or clinical evidence of MF or Sézary syndrome (ie, LAFM), and (2) a cohort of 28 patients with clinicopathologic evidence of CTCL. Patients in both groups were followed for a maximum of 20 years. Results indicated that that the presence of perifollicular or intrafollicular mucin, epidermotropism of lymphocytes, monoclonality, and epidemiologic characteristics (eg, age, sex, race) cannot reliably distinguish the 2 disease forms. Furthermore, it was suggested that these conditions are not mutually exclusive entities and are actually variants of CTCL. The observation that the 2 diseases share prognostic overlap adds further credence to the already puzzling conundrum. Nineteen of 28 patients with MF were alive and well at follow-up, and all patients in the idiopathic FM group were alive, with only 9 of 16 patients showing residual disease and none with CTCL.1
Other clinical factors that may be helpful in the diagnosis of MF are the presentation of lesions in non–sun-exposed areas of the skin and multiple lesions, as unilesional MF is exceedingly uncommon.21 No histologic features have been proven to predict which early patch- or plaque-stage MFs will progress to full-blown CTCL versus benign idiopathic FM; thus, great caution should be taken in patients with early-stage disease to ensure they are not prematurely and/or incorrectly classified as CTCL. Such a diagnosis has medical, social, and economical ramifications that should not be overlooked.
If idiopathic FM and LAFM were considered distinct disease processes, the ambiguity in making a definitive diagnosis should give the physician pause, and a diagnosis of LAFM may only be appropriate when there is unequivocal clinicopathologic evidence. Otherwise, a lymphoma diagnosis is somewhat superfluous and potentially harmful. Definitive diagnosis of LAFM also is complicated by reports of other hematologic malignancies presenting with FM-like histopathologic findings, such as chronic myelogenous leukemia, leukemia-associated eosinophilic folliculitis, and acute myeloblastic leukemia.23,24 Although MF is the most common malignancy associated with FM, it is important to consider other less common malignancies that also may be present.
Diagnosis: Patient Consequences
Accurate diagnosis of idiopathic FM versus LAFM is critical, as the ramifications of a cancer diagnosis can have broad implications. For example, patients who receive cancer diagnoses often experience emotional trauma and social stigma, even when adequate patient education has been provided. The incidence of depression and anxiety also can increase following a cancer diagnosis and can be complicated by medical treatments (eg, systemic steroids, interferon),25 which are known to increase the frequency of these psychological disturbances. Health insurance premiums likely will be higher if a patient is diagnosed with cancer versus a benign inflammatory condition. Hesitation of the pathologist to assign a cancer diagnosis when unequivocal evidence is not present should not be regarded as trickery, malpractice, or deceit of the health care bylaws, as benign language with suggestion of close clinical follow-up in the setting of diagnostic uncertainty will “first, do no harm” and secondly, serve as a vehicle for patient advocacy.
If there is a definitive distinction between idiopathic FM and LAFM, it requires further research before it can be fully understood. Currently, the WHO does not recognize a diagnosis of FM-associated MF (or LAFM) and acknowledges that folliculotropic MF is not always associated with FM.16,26 Given uncertainty and repercussions associated with a cancer diagnosis, however indolent, it may be morally responsible and medically favorable for physicians to consider FM in the differential diagnosis when applicable rather than making a diagnosis of MF outright. Given the importance of both clinical and histologic factors, it may be beneficial for definitive diagnosis of FM versus MF to lie with the clinician, while the pathologist serves as an adjunct in the diagnostic process. Because the prognosis of idiopathic FM often is marred by possible transformation into MF or other CTCLs, therapeutic decisions should be dictated by close clinical follow-up. Additionally, stage of disease, patient age, treatment compliance, comorbidities, and possible side effects of medications should all be considered when evaluating potential therapeutic regimens.27
Conclusion
Research is underway to more accurately identify patients with FM who are at risk for progression to LAFM versus those with benign remitting FM. Once the required diagnostic criteria are established to accurately classify these patients, with an emphasis on prognosis and suggested treatments, it might be necessary to establish new, less debated terminology so pathologists and clinicians alike can improve patient care. Continued histopathologic scrutiny, use of sophisticated molecular techniques, and knowledge of other currently undiscovered modalities will shed light on this important disease process and aid in proper disease management, which may be advantageous to both patients and physicians.
1. Cerroni L, Fink-Puches R, Bäck B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2002;138:182-189.
2. Parker SR, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline [published online ahead of print July 25, 2009]. J Am Acad Dermatol. 2010;62:139-141.
3. Pinkus H. Alopecia mucinosa; inflammatory plaques with alopecia characterized by root-sheath mucinosis. AMA Arch Dermatol. 1957;76:419-424, 424-426.
4. Pinkus H. Alopecia mucinosa. additional data in 1983. Arch Dermatol. 1983;119:698-699.
5. Jablonska S, Chorzelski T, Lancucki J. Mucinosis follicularis [in German]. Hautarzt. 1959;10:27-33.
6. Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004;26:33-52.
7. Brown HA, Gibson LE, Pujol RM, et al. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and withoutclonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002;47:856-862.
8. Schiller PI, Flaig MJ, Puchta U, et al. Detection of clonal T cells in lichen planus. Arch Dermatol Res. 2000;292:568-569.
9. Cerroni L, Kerl H. Primary follicular mucinosis and association with mycosis fungoides and other cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:146-147.
10. Dereure O, Levi E, Kadin ME. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
11. Haeffner AC, Smoller BR, Zepter K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131:321-324.
12. Schultz JC, Granados S, Vonderheid EC, et al. T-cell clonality of peripheral blood lymphocytes in patients with lymphomatoid papulosis. J Am Acad Dermatol. 2005;53:152-155.
13. Pfaltz K, Kerl K, Palmedo G, et al. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662.
14. Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
15. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
16. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
17. Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides [published online ahead of print October 22, 2007]. Crit Rev Oncol Hematol. 2008;65:172-182.
18. Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
19. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957.
20. Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol. 2009;75:229-235.
21. Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
22. Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides. a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
23. Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009;15:16.
24. Wada T, Yoshinaga E, Oiso N, et al. Adult T-cell leukemia-lymphoma associated with follicular mucinosis. J Dermatol. 2009;36:638-642.
25. Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma [published online ahead of print December 16, 2008]. Br J Dermatol. 2009;160:815-822.
26. Boone SL, Guitart J, Gerami P. Follicular mycosis fungoides: a histopathologic, immunohistochemical, and genotypic review. G Ital Dermatol Venereol. 2008;143:409-414.
27. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome [published online ahead of print August 20, 2009]. Blood. 2009;114:4337-4353.
1. Cerroni L, Fink-Puches R, Bäck B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2002;138:182-189.
2. Parker SR, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline [published online ahead of print July 25, 2009]. J Am Acad Dermatol. 2010;62:139-141.
3. Pinkus H. Alopecia mucinosa; inflammatory plaques with alopecia characterized by root-sheath mucinosis. AMA Arch Dermatol. 1957;76:419-424, 424-426.
4. Pinkus H. Alopecia mucinosa. additional data in 1983. Arch Dermatol. 1983;119:698-699.
5. Jablonska S, Chorzelski T, Lancucki J. Mucinosis follicularis [in German]. Hautarzt. 1959;10:27-33.
6. Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004;26:33-52.
7. Brown HA, Gibson LE, Pujol RM, et al. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and withoutclonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002;47:856-862.
8. Schiller PI, Flaig MJ, Puchta U, et al. Detection of clonal T cells in lichen planus. Arch Dermatol Res. 2000;292:568-569.
9. Cerroni L, Kerl H. Primary follicular mucinosis and association with mycosis fungoides and other cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:146-147.
10. Dereure O, Levi E, Kadin ME. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
11. Haeffner AC, Smoller BR, Zepter K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131:321-324.
12. Schultz JC, Granados S, Vonderheid EC, et al. T-cell clonality of peripheral blood lymphocytes in patients with lymphomatoid papulosis. J Am Acad Dermatol. 2005;53:152-155.
13. Pfaltz K, Kerl K, Palmedo G, et al. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662.
14. Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
15. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
16. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
17. Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides [published online ahead of print October 22, 2007]. Crit Rev Oncol Hematol. 2008;65:172-182.
18. Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
19. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957.
20. Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol. 2009;75:229-235.
21. Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
22. Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides. a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
23. Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009;15:16.
24. Wada T, Yoshinaga E, Oiso N, et al. Adult T-cell leukemia-lymphoma associated with follicular mucinosis. J Dermatol. 2009;36:638-642.
25. Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma [published online ahead of print December 16, 2008]. Br J Dermatol. 2009;160:815-822.
26. Boone SL, Guitart J, Gerami P. Follicular mycosis fungoides: a histopathologic, immunohistochemical, and genotypic review. G Ital Dermatol Venereol. 2008;143:409-414.
27. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome [published online ahead of print August 20, 2009]. Blood. 2009;114:4337-4353.
Practice Points
- An isolated patch in the head or neck area is much more likely to be follicular mucinosis (FM) than mycosis fungoides (MF).
- Monoclonality does not reliably distinguish FM from MF.
- Younger patients are more likely to have FM with spontaneous remission, and older patients are more likely to develop MF.
- None of the clinicopathologic features of FM or MF are without overlap.
Ergonomic Strain in Minimally Invasive Surgery: Addressing the Strain Epidemic
From the Division of Reproductive Endocrinology and Infertility, Department of Obstetrics, Gynecology and Reproductive Science, Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ (Dr. Fransasiak), and the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC (Dr. Gehrig).
Abstract
- Background: Minimally invasive surgery (MIS) has benefits to both patients and society and its use has increased markedly over the past 3 decades. With its introduction, new mental and physical challenges were presented to the surgeons, leading to concerns regarding operative ergonomics. Applied ergonomics has been used to study and improve operative techniques and technologies as they apply to MIS.
- Objective: To review the ergonomic challenges presented by both traditional MIS as well as robot-assisted MIS and discuss how ergonomic science has evolved to address these issues.
- Methods: Review of the literature involving MIS and applied ergonomics
- Results: Surgeon strain as it relates to MIS has historically been thought to occur in only approximately 15% of MIS surgeons. More recent data suggests this number is much higher. Rates of strain have been reported to be as high as 88% among traditional MIS surgeons and 45% among robotic-assisted MIS surgeons. Strain results from a number of factors, including instrument design and use, optics placement and resolution, patient and surgeon positioning, and the drive to implement surgical technologies which aim to further minimize the invasiveness of surgical procedures.
- Conclusion: Improvements in applied ergonomics in MIS have resulted in improved optics, more sophisticated and ergonomic instruments, and methods of optimizing positioning. However, despite these advancements, ergonomic strain rates amongst surgeons remain alarmingly high. With the ever-increasing demand for MIS, more research and development as well as MIS surgeon training are needed to improve the safety of surgeons and ensure the career longevity required to meet the patient and societal demand for MIS.
Since its introduction to North America in the 1980s, minimally invasive surgery (MIS) has become widely accepted and practiced across surgical disciplines including general surgery, gynecologic surgery, oncology, and thoracic surgery [1]. Procedures once done through large incisions, such as cholecystectomies, have been supplanted by those utilizing 2 or 3 small punctures as the gold standard.
The demand for MIS has been rising and is driven by both providers and patients. The minimally invasive approaches have been shown to decrease recovery time, result in less postoperative pain, and decrease blood loss and other surgical complications [2,3], allowing for patients and their supports to return to baseline function more quickly [4]. In this way, both individual patients and society as a whole derives benefits from MIS through decreased recovery times and return to productivity.
Despite the clear benefits to patients and society, there has been increasing evidence of an unanticipated side effect of MIS: surgeon ergonomic strain and injury [5].Although the same ultimate procedure is performed when open techniques are employed as when MIS is utilized, surgeons have reported increased physical stress and mental strain when utilizing minimally invasive technologies [6,7]. The phenomenon was first noted during the laparoscopic surgery boom of the early 1990s and has been revisited more recently in the setting of both traditional and robotic-assisted MIS techniques [5,8,9].
The source of the issues arises from the fact the surgeons are, by definition, operating with reduced access to the patient. This requires limiting the degrees of freedom in movements, employing specialized and often awkward or cumbersome instruments, and requiring use of an intracorporeal camera that projects the surgical field onto a screen, which causes increased mental strain due to perceptual challenges as well as visual strain [7,10–13].
The initial large survey studies characterizing surgeon strain during MIS revealed rates of strain and discomfort in the 12% to 18% range, with many reporting that strain was persistent and not simply limited to operative time [11,14]. In part, these early estimates focused on very experienced surgeons and thus may have underreported the rates of strain. Subsequently, other studies have quoted rates of strain in the 40% to 60% range [15,16]. These studies focused on a larger and more hetero-geneous group of MIS surgeons, which may explain the higher rates of strain. Most recently, in the setting of an ever growing demand for MIS, large survey studies have revealed rates of surgeon strain to be as high as 87% and 88% among traditional MIS surgeons and 45% among robotic-assisted MIS surgeons, with 26% reporting persistent strain beyond the robotic console time [5,9,17]. This prolonged strain can impact productivity, with 14% of surgeons limiting the number of surgical cases they do per day, and may impact quality of life, with 29% needing to seek treatment for strain related to MIS [9].
Here we review the 2 major forms of MIS, traditional and robotic-assisted surgery. The unique features of each type of MIS that predispose to surgeon strain are discussed along with the techniques and technologies that have been employed to improve the ergonomics of MIS and reduce surgeon strain.
Traditional MIS
Traditional MIS, developed in the 1980s, involves use of a surgeon-manipulated intracorporeal video camera to view the surgical field. Instruments are placed through fixed ports inserted through the body wall called trocars. Most MIS surgical suites involve 1 or more surgeons standing aside a patient holding the camera and surgical instruments and viewing the surgical field on monitors placed around the patient.
The field and technology have evolved greatly since its inception. However, there are a number of factors that persist in creating ergonomic strain during traditional MIS.
Instruments
MIS instruments are limited by several factors. They must have long, thin shafts that can be placed and removed through fixed trocars. The majority of trocars commonly used are 5 to 10 mm in diameter. Given this fixed point, the instrument motion is inverted in the operative cavity, which requires mental adjustment and scaling. Additionally, the range of motion of MIS instruments is limited to 5 degrees of freedom, which allows for less dexterity than is commonly enjoyed during open surgery through large incisions, which accommodate the surgeons hands and allows for more degrees of freedom and dexterity [18]. These limitations have historically yielded instruments that have not been ergonomically sound. Indeed, MIS instrument are identified as an ergonomic problem by over 80% of minimally invasive surgeons [19].
Additionally, given that the surgeon’s hands are often occupied with the camera and an operative tool, the activation of suction devices and electrocautery devices often requires use of instrument foot pedals. Requiring that the instrument and camera be optimally positioned and relatively stationary and the foot pedal be activated simultaneously can exacerbate poor posture and back strain as the surgeon balances on one foot. The addition of foot pedals around the operative table also further limits space for proper surgeon
positioning [19].
Ergonomic engineering has focused on instrument handles. To accommodate the varied sizes of surgeon hands, many companies have altered the size of the device handles allowing for a more comfortable grip. To address the issue of poor posture induced by the use of foot pedals, many instruments now have trigger finger or thumb-activated buttons on the handle of the device itself, which alleviates the need for positioning to activate a foot pedal. However, many of these may not be suitable to accommodate smaller hands.
Optics
A major limitation of MIS is the limited visual field. The video monitor is positioned outside of the sterile operative field, often requiring that the surgeon looks in one direction and operates in another direction, placing strain in both the axial or rotational and frontal or flexion/extension planes [20]. The surgeon does not have immediate visual access to the entire surgical field but rather must rely on movement of the camera, which can at times result in unnatural and uncomfortable positions in order to position the camera optimally [19]. Additionally, eye strain can result from constant visualization of the operative monitor throughout the surgery. Finally, until only recently, optic systems required operating in 2 dimensions, without the depth perception enjoyed during traditional open surgery.
To address neck and upper body strain as well as optic strain, operative monitor positioning has received significant emphasis. The original MIS video monitors were small, had poor resolution, and were fixed in their position. Over time, monitors have increased in size and resolution, allowing for easier viewing and decreased optic strain. Additionally, in the 1990s the MIS operative suite concept allowed for placement of monitors on swing arms, which allow for movement about the operating room with ease. Subsequently, use of systems that employed multiple monitors placed around the patient at different angles with independent height and inclination adjustment allowed for comfortable positioning for all members of the surgical team, particularly in cases where 2 or more surgeons are operating simultaneously [21]. The implementation of these monitor systems not only decrease ergonomic strain but have also been shown to improve intraoperative speed and surgical accuracy when performing standardized tasks [22,23].
The most recent advance in surgical optics has been the introduction of 3-dimensional (3D) imaging systems [21,24,25]. At present, most of these systems are cost prohibitive and have poorer resolution than the traditional 2-dimensional monitors which may in fact increase optic strain. The modern high-definition 2D monitor systems in current use have done much to decrease optic strain and further refinement of 3D technology may prove to mitigate this strain even further.
Operative Posture
MIS often involves assuming unnatural postures to manipulate instruments and visualize the operative monitors. When non-neutral posture is maintained, muscles require an increase in energy production in order to maintain the same contractile forces and the contractile forces required to stabilize joints is increased [20]. Maintaining these static positions for long periods of time results in rapid fatigue, muscle pain, and cramping, and strain that can persist after the operation is complete [19].
Attention to ideal posture is paramount during MIS. The surgeon should be upright next to the patient with the head slightly bent forward, ideally employing a shift in position of the neck from time to time throughout the surgery to avoid prolonged static positioning [11]. The arms should rest so that the elbow is at the side with a 90- to 120-degree bend to accommodate instrument manipulation. This angle can be tolerated for long period of time as opposed to angles that require the elbow be taken away from the side of the body [19]. The forearm should rest in the neutral rotating position between pronated and supinated whenever possible with the wrist slightly extended and the fingers slightly bent [26]. This neutral position allows for rapid and simple changes in grip.
Adjustment of table height or use of operative foot platforms is crucial to ensuring the arms remain in neutral position. Given that the patient is often positioned in steep Trendelenburg or reverse Trendelenburg for MIS, the standard operating beds may not be at a height that allows the surgeon to operate in a relaxed, neutral posture [27,28]. In these circumstances, rather than operating with arms and shoulders in an elevated position, a position that produces rapid upper extremity fatigue, surgeons should elevate themselves with the assistance of an operative platform or step.
Single Incision Laparoscopic Surgery
Most recently, single port laparoscopic surgery (SPLS), also called single incision laparoscopic surgery (SILS) has been introduced. This technique involves use of a slightly larger, single incision that allows for a single port, which accommodates several instruments and the operative camera. This enhances some of the challenges posed with traditional MIS, namely maintaining exposure of the operative field, sustaining pneumatic pressure in the operative space, avoiding instrument collision both intra- and extracorporeally, and avoiding instrument interference with optics [29].
A number of techniques have been employed to minimize these issues. For example, percutaneous sutures may be placed intraoperatively in order to assist with retraction and improve visualization. The most important technological advances have come in the form of coaxial, flexible, and articulating instruments to avoid collisions [29]. While there is a learning curve with these technologies in terms of instrument triangulation, they can be successfully employed to improve operative efficiency and ergonomics.
Robot-Assisted MIS
Robot-assisted MIS involves the use of intracorporeal instruments attached to robotic arms that have been docked to trocars. The surgeon controls these robotic arms with a computer console and a video monitor is available for the surgical assistants. Many robot-assisted surgeries involve the use of an assistant, who utilizes traditional MIS instruments and trocars. The same issues in ergonomics discussed above apply to the assistant surgeon. Here, we will focus on the ergonomic challenges unique to operating surgeon at a robotic console.
There have been several robotic systems developed for use during surgery. At present, only the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA) is in use in the United States. Several components of the robotic systems allow for potential advantages over traditional MIS in terms of ergonomics. First, as discussed, the primary surgeon is seated at a robotic console rather than standing next to the patient. The camera and instruments are held intracorporeally by the robotic system and controlled by the surgeon at the console. The mechanical engineering, which is associated with the instruments of the robotic system, allows for many for degrees of freedom in range of motion, which permits the surgeon to employ techniques which more closely mimic open surgical techniques.
The robotic surgical system has experienced rapid acceptance and growth over the past decade [30–33]. One small case comparison study and 1 large prospective analysis of over 200 procedures have suggested that robotic surgery is more ergonomically favorable and potentially less mentally stressful than conventional minimally invasive surgery [34,35]. However, although robotic surgery is often thought of as a tool to alleviate strain related to MIS [36,37], there are still high levels of strain, with some survey data indicating strain rates as high as 45%, involved with robotic surgery [8,9]. It is clear that, as with traditional MIS, effective interventions are needed to prevent and reduce strain to prevent work-related injury in robot-assisted MIS.
A primary cause of ergonomic strain during robot-assisted MIS is the lack of knowledge and training regard-ing proper ergonomic techniques at seated console work stations amongst surgeons, with as few as 16% of surgeons reporting any formal training [5,9,17,38]. Furthermore, when compared with traditional MIS, there is little available literature specific to robotic surgery ergonomics. Much of the data available has been extrapolated from recommendations from the U.S. Department of Labor’s Occupational Safety and Health Administration’s (OSHA’s) guidelines for working positions at workstations and on the available body of literature on the ergonomics of microscopy, which, due to somewhat similar positioning, have been adapted for robotic-assisted MIS [39].
The Robotic Console
When addressing applied ergonomics in robot-assisted MIS, the primary focuses in on the robotic console set-up and the surgeon positioning. The primary focus is on ensuring a comfortable headrest and adjustable ocular height, which relieves neck, shoulder, and upper back strain, and proper adjustment of armrests and finger controls aimed at minimizing arm and upper back strain due to static load forces [40].
The most common pitfalls during robotic-assisted MIS are when the arms are moved from the arm rest and the elbows flare out from the side of the operator. This departure from neutral position, common when attempting to reach for structures that are on the edge of the surgical field, causes significant tension and strain and is often not corrected for long period of time. Frequent use of the robotic clutch, which freezes the intracorporeal arms and allows for movement of the console arms freely, to bring the body back to neutral position is of paramount importance [17].
Conclusion
The field of applied ergonomics in surgery has never been more important. The fields of both traditional and robot-assisted MIS are growing rapidly and demand for these technologies will only increase as outcomes continue to improve. As the increasing workload of MIS is handled by a pool of surgeons that is not increasing rapidly enough to meet the demand, more volume will be handled by each surgeon.
With ergonomic strain now reported by nearly 90% of surgeons, much of it persistent strain beyond operative time, we will run into situations where surgeons may compensate for this persistent strain by decreasing operative volume and may decide to retire earlier than they otherwise might have. This could result in a health care supply problem as demand for MIS increases and the surgeon pool available to perform it stays constant or decreases.
To date, there has been relatively little research into this issue. The epidemiologic data on surgeon strain comes primarily from survey research done within various MIS subspecialties. There has been some data based on objective measures of strain utilizing validated strain indicators, but more work in this area is needed. Standardized methods of reporting strain will assist in clarifying both the epidemiology and standardize the response to interventions. Studies that aim to address the reported rates of strain are also needed. Much of the early work focused on operating room set-up and resulted in great improvements. More work is needed to assess optimal ergonomic positioning and formal surgeon training.
The solution will involve a combination of engineering advances in operating room set-up and equipment design along with a renewed focus on teaching ergonomic techniques and principles to MIS surgeons. While early data is promising and shows that training sessions in ergonomics are easy, acceptable by surgeons, and effective, more data is needed to develop optimal training session and modules when it comes to traditional and robot-assisted MIS ergonomics [17]. As ergonomic studies specifically designed to address this population accrue, more data driven guidelines can be developed and implemented.
Corresponding author: Jason M. Franasiak, MD, 140 Allen Rd., Basking Ridge, NJ 07920, [email protected].
Financial disclosures: None
1. Cuschieri A. Laparoscopic surgery: current status, issues and future developments. Surg J R Coll Surg Edinb Irel 2005;3:125–30, 132–3, 135–8.
2. Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol 2008;111:41–5.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 2009;27:5331–6.
4. Bell MC, Torgerson J, Seshadri-Kreaden U, et al. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol 2008;111:407–11.
5. Park A, Lee G, Seagull FJ, et al. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg 2010;210:306–13.
6. Kant IJ, de Jong LC, van Rijssen-Moll M, Borm PJ. A survey of static and dynamic work postures of operating room staff. Int Arch Occup Environ Health 1992;63:423–8.
7. Patkin M, Isabel L. Ergonomics, engineering and surgery of endosurgical dissection. J R Coll Surg Edinb 1995;40:120–32.
8. Craven R, Franasiak J, Mosaly P, Gehrig PA. Ergonomic deficits in robotic gynecologic oncology surgery: a need for intervention. J Minim Invasive Gynecol 2013;20:648–55.
9. Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol 2012;126:437–42.
10. Berguer R. Surgical technology and the ergonomics of laparoscopic instruments. Surg Endosc 1998;12:458–62.
11. Berguer R. Surgery and ergonomics. Arch Surg 1999;134:1011–6.
12. Berguer R, Forkey DL, Smith WD. The effect of laparoscopic instrument working angle on surgeons’ upper extremity workload. Surg Endosc 2001;15:1027–9.
13. Lawson EH, Curet MJ, Sanchez BR, et al. Postural ergonomics during robotic and laparoscopic gastric bypass surgery: a pilot project. J Robot Surg 2007;1:61–7.
14. Van Veelen MA, Meijer DW. Ergonomics and design of laparoscopic instruments: results of a survey among laparoscopic surgeons. J Laparoendosc Adv Surg Tech A 1999;9:481–9.
15. Van Veelen MA, Nederlof EA, Goossens RHM, et al. Ergonomic problems encountered by the medical team related to products used for minimally invasive surgery. Surg Endosc 2003;17:1077–81.
16. Lawther RE, Kirk GR, Regan MC. Laparoscopic procedures are associated with a significant risk of digital nerve injury for general surgeons. Ann R Coll Surg Engl 2002;84:443.
17. Franasiak J, Craven R, Mosaly P, Gehrig PA. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS 2014;18(4).
18. Ballantyne GH. The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 2002;12:1–5.
19. Matern U. Ergonomic deficiencies in the operating room: examples from minimally invasive surgery. Work 2009;33:165–8.
20. Van Det MJ, Meijerink WJHJ, Hoff C, et al. Optimal ergonomics for laparoscopic surgery in minimally invasive surgery suites: a review and guidelines. Surg Endosc 2009;23:1279–85.
21. Veelen MA, Jakimowicz JJ, Goossens RHM, et al. Evaluation of the usability of two types of image display systems, during laparoscopy. Surg Endosc 2002;16:674–8.
22. Haveran LA, Novitsky YW, Czerniach DR, et al. Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc 2007;21:980–4.
23. Hanna GB, Shimi SM, Cuschieri A. Task performance in endoscopic surgery is influenced by location of the image display. Ann Surg 1998;227:481–4.
24. Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc 2002;16:1389–402.
25. Boppart SA, Deutsch TF, Rattner DW. Optical imaging technology in minimally invasive surgery. Current status and future directions. Surg Endosc 1999;13:718–22.
26. Matern U, Waller P. Instruments for minimally invasive surgery: principles of ergonomic handles. Surg Endosc 1999;13:174–82.
27. Matern U, Waller P, Giebmeyer C, et al. Ergonomics: requirements for adjusting the height of laparoscopic operating tables. JSLS 2001;5:7–12.
28. Van Veelen MA, Kazemier G, Koopman J, et al. Assessment of the ergonomically optimal operating surface height for laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2002;12:47–52.
29. Tang B, Hou S, Cuschieri SA. Ergonomics of and technologies for single-port lapaxroscopic surgery. Minim Invasive Ther Allied Technol 2012;21:46–54.
30. Wexner SD, Bergamaschi R, Lacy A, et al. The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 2009;23:438–43.
31. Challacombe BJ, Khan MS, Murphy D, Dasgupta P. The history of robotics in urology. World J Urol 2006;24:120–7.
32. Ballantyne GH, Moll F. The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surg Clin North Am 2003;83:1293–304, vii.
33. Ruurda JP, van Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl 2002;84:223–6.
34. Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg 2005;140:779–86.
35. Talamini MA, Chapman S, Horgan S, Melvin WS; Academic Robotics Group. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 2003;17:1521–4.
36. Mucksavage P, Kerbl DC, Lee JY. The da Vinci Surgical System overcomes innate hand dominance. J Endourol 2011;25:1385–8.
37. Schreuder HW, Verheijen RH. Robotic surgery. BJOG 2009;116:198–213.
38. Stone R, McCloy R. Ergonomics in medicine and surgery. BMJ 2004;328:1115–8.
39. Lux MM, Marshall M, Erturk E, Joseph JV. Ergonomic evaluation and guidelines for use of the daVinci Robot system. J Endourol 2010;24:371–5.
40. Sillanpaa J, Nyberg M, Laippala P. A new table for work with a microscope, a solution to ergonomic problems. Appl Ergon 2003;34:621–8.
From the Division of Reproductive Endocrinology and Infertility, Department of Obstetrics, Gynecology and Reproductive Science, Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ (Dr. Fransasiak), and the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC (Dr. Gehrig).
Abstract
- Background: Minimally invasive surgery (MIS) has benefits to both patients and society and its use has increased markedly over the past 3 decades. With its introduction, new mental and physical challenges were presented to the surgeons, leading to concerns regarding operative ergonomics. Applied ergonomics has been used to study and improve operative techniques and technologies as they apply to MIS.
- Objective: To review the ergonomic challenges presented by both traditional MIS as well as robot-assisted MIS and discuss how ergonomic science has evolved to address these issues.
- Methods: Review of the literature involving MIS and applied ergonomics
- Results: Surgeon strain as it relates to MIS has historically been thought to occur in only approximately 15% of MIS surgeons. More recent data suggests this number is much higher. Rates of strain have been reported to be as high as 88% among traditional MIS surgeons and 45% among robotic-assisted MIS surgeons. Strain results from a number of factors, including instrument design and use, optics placement and resolution, patient and surgeon positioning, and the drive to implement surgical technologies which aim to further minimize the invasiveness of surgical procedures.
- Conclusion: Improvements in applied ergonomics in MIS have resulted in improved optics, more sophisticated and ergonomic instruments, and methods of optimizing positioning. However, despite these advancements, ergonomic strain rates amongst surgeons remain alarmingly high. With the ever-increasing demand for MIS, more research and development as well as MIS surgeon training are needed to improve the safety of surgeons and ensure the career longevity required to meet the patient and societal demand for MIS.
Since its introduction to North America in the 1980s, minimally invasive surgery (MIS) has become widely accepted and practiced across surgical disciplines including general surgery, gynecologic surgery, oncology, and thoracic surgery [1]. Procedures once done through large incisions, such as cholecystectomies, have been supplanted by those utilizing 2 or 3 small punctures as the gold standard.
The demand for MIS has been rising and is driven by both providers and patients. The minimally invasive approaches have been shown to decrease recovery time, result in less postoperative pain, and decrease blood loss and other surgical complications [2,3], allowing for patients and their supports to return to baseline function more quickly [4]. In this way, both individual patients and society as a whole derives benefits from MIS through decreased recovery times and return to productivity.
Despite the clear benefits to patients and society, there has been increasing evidence of an unanticipated side effect of MIS: surgeon ergonomic strain and injury [5].Although the same ultimate procedure is performed when open techniques are employed as when MIS is utilized, surgeons have reported increased physical stress and mental strain when utilizing minimally invasive technologies [6,7]. The phenomenon was first noted during the laparoscopic surgery boom of the early 1990s and has been revisited more recently in the setting of both traditional and robotic-assisted MIS techniques [5,8,9].
The source of the issues arises from the fact the surgeons are, by definition, operating with reduced access to the patient. This requires limiting the degrees of freedom in movements, employing specialized and often awkward or cumbersome instruments, and requiring use of an intracorporeal camera that projects the surgical field onto a screen, which causes increased mental strain due to perceptual challenges as well as visual strain [7,10–13].
The initial large survey studies characterizing surgeon strain during MIS revealed rates of strain and discomfort in the 12% to 18% range, with many reporting that strain was persistent and not simply limited to operative time [11,14]. In part, these early estimates focused on very experienced surgeons and thus may have underreported the rates of strain. Subsequently, other studies have quoted rates of strain in the 40% to 60% range [15,16]. These studies focused on a larger and more hetero-geneous group of MIS surgeons, which may explain the higher rates of strain. Most recently, in the setting of an ever growing demand for MIS, large survey studies have revealed rates of surgeon strain to be as high as 87% and 88% among traditional MIS surgeons and 45% among robotic-assisted MIS surgeons, with 26% reporting persistent strain beyond the robotic console time [5,9,17]. This prolonged strain can impact productivity, with 14% of surgeons limiting the number of surgical cases they do per day, and may impact quality of life, with 29% needing to seek treatment for strain related to MIS [9].
Here we review the 2 major forms of MIS, traditional and robotic-assisted surgery. The unique features of each type of MIS that predispose to surgeon strain are discussed along with the techniques and technologies that have been employed to improve the ergonomics of MIS and reduce surgeon strain.
Traditional MIS
Traditional MIS, developed in the 1980s, involves use of a surgeon-manipulated intracorporeal video camera to view the surgical field. Instruments are placed through fixed ports inserted through the body wall called trocars. Most MIS surgical suites involve 1 or more surgeons standing aside a patient holding the camera and surgical instruments and viewing the surgical field on monitors placed around the patient.
The field and technology have evolved greatly since its inception. However, there are a number of factors that persist in creating ergonomic strain during traditional MIS.
Instruments
MIS instruments are limited by several factors. They must have long, thin shafts that can be placed and removed through fixed trocars. The majority of trocars commonly used are 5 to 10 mm in diameter. Given this fixed point, the instrument motion is inverted in the operative cavity, which requires mental adjustment and scaling. Additionally, the range of motion of MIS instruments is limited to 5 degrees of freedom, which allows for less dexterity than is commonly enjoyed during open surgery through large incisions, which accommodate the surgeons hands and allows for more degrees of freedom and dexterity [18]. These limitations have historically yielded instruments that have not been ergonomically sound. Indeed, MIS instrument are identified as an ergonomic problem by over 80% of minimally invasive surgeons [19].
Additionally, given that the surgeon’s hands are often occupied with the camera and an operative tool, the activation of suction devices and electrocautery devices often requires use of instrument foot pedals. Requiring that the instrument and camera be optimally positioned and relatively stationary and the foot pedal be activated simultaneously can exacerbate poor posture and back strain as the surgeon balances on one foot. The addition of foot pedals around the operative table also further limits space for proper surgeon
positioning [19].
Ergonomic engineering has focused on instrument handles. To accommodate the varied sizes of surgeon hands, many companies have altered the size of the device handles allowing for a more comfortable grip. To address the issue of poor posture induced by the use of foot pedals, many instruments now have trigger finger or thumb-activated buttons on the handle of the device itself, which alleviates the need for positioning to activate a foot pedal. However, many of these may not be suitable to accommodate smaller hands.
Optics
A major limitation of MIS is the limited visual field. The video monitor is positioned outside of the sterile operative field, often requiring that the surgeon looks in one direction and operates in another direction, placing strain in both the axial or rotational and frontal or flexion/extension planes [20]. The surgeon does not have immediate visual access to the entire surgical field but rather must rely on movement of the camera, which can at times result in unnatural and uncomfortable positions in order to position the camera optimally [19]. Additionally, eye strain can result from constant visualization of the operative monitor throughout the surgery. Finally, until only recently, optic systems required operating in 2 dimensions, without the depth perception enjoyed during traditional open surgery.
To address neck and upper body strain as well as optic strain, operative monitor positioning has received significant emphasis. The original MIS video monitors were small, had poor resolution, and were fixed in their position. Over time, monitors have increased in size and resolution, allowing for easier viewing and decreased optic strain. Additionally, in the 1990s the MIS operative suite concept allowed for placement of monitors on swing arms, which allow for movement about the operating room with ease. Subsequently, use of systems that employed multiple monitors placed around the patient at different angles with independent height and inclination adjustment allowed for comfortable positioning for all members of the surgical team, particularly in cases where 2 or more surgeons are operating simultaneously [21]. The implementation of these monitor systems not only decrease ergonomic strain but have also been shown to improve intraoperative speed and surgical accuracy when performing standardized tasks [22,23].
The most recent advance in surgical optics has been the introduction of 3-dimensional (3D) imaging systems [21,24,25]. At present, most of these systems are cost prohibitive and have poorer resolution than the traditional 2-dimensional monitors which may in fact increase optic strain. The modern high-definition 2D monitor systems in current use have done much to decrease optic strain and further refinement of 3D technology may prove to mitigate this strain even further.
Operative Posture
MIS often involves assuming unnatural postures to manipulate instruments and visualize the operative monitors. When non-neutral posture is maintained, muscles require an increase in energy production in order to maintain the same contractile forces and the contractile forces required to stabilize joints is increased [20]. Maintaining these static positions for long periods of time results in rapid fatigue, muscle pain, and cramping, and strain that can persist after the operation is complete [19].
Attention to ideal posture is paramount during MIS. The surgeon should be upright next to the patient with the head slightly bent forward, ideally employing a shift in position of the neck from time to time throughout the surgery to avoid prolonged static positioning [11]. The arms should rest so that the elbow is at the side with a 90- to 120-degree bend to accommodate instrument manipulation. This angle can be tolerated for long period of time as opposed to angles that require the elbow be taken away from the side of the body [19]. The forearm should rest in the neutral rotating position between pronated and supinated whenever possible with the wrist slightly extended and the fingers slightly bent [26]. This neutral position allows for rapid and simple changes in grip.
Adjustment of table height or use of operative foot platforms is crucial to ensuring the arms remain in neutral position. Given that the patient is often positioned in steep Trendelenburg or reverse Trendelenburg for MIS, the standard operating beds may not be at a height that allows the surgeon to operate in a relaxed, neutral posture [27,28]. In these circumstances, rather than operating with arms and shoulders in an elevated position, a position that produces rapid upper extremity fatigue, surgeons should elevate themselves with the assistance of an operative platform or step.
Single Incision Laparoscopic Surgery
Most recently, single port laparoscopic surgery (SPLS), also called single incision laparoscopic surgery (SILS) has been introduced. This technique involves use of a slightly larger, single incision that allows for a single port, which accommodates several instruments and the operative camera. This enhances some of the challenges posed with traditional MIS, namely maintaining exposure of the operative field, sustaining pneumatic pressure in the operative space, avoiding instrument collision both intra- and extracorporeally, and avoiding instrument interference with optics [29].
A number of techniques have been employed to minimize these issues. For example, percutaneous sutures may be placed intraoperatively in order to assist with retraction and improve visualization. The most important technological advances have come in the form of coaxial, flexible, and articulating instruments to avoid collisions [29]. While there is a learning curve with these technologies in terms of instrument triangulation, they can be successfully employed to improve operative efficiency and ergonomics.
Robot-Assisted MIS
Robot-assisted MIS involves the use of intracorporeal instruments attached to robotic arms that have been docked to trocars. The surgeon controls these robotic arms with a computer console and a video monitor is available for the surgical assistants. Many robot-assisted surgeries involve the use of an assistant, who utilizes traditional MIS instruments and trocars. The same issues in ergonomics discussed above apply to the assistant surgeon. Here, we will focus on the ergonomic challenges unique to operating surgeon at a robotic console.
There have been several robotic systems developed for use during surgery. At present, only the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA) is in use in the United States. Several components of the robotic systems allow for potential advantages over traditional MIS in terms of ergonomics. First, as discussed, the primary surgeon is seated at a robotic console rather than standing next to the patient. The camera and instruments are held intracorporeally by the robotic system and controlled by the surgeon at the console. The mechanical engineering, which is associated with the instruments of the robotic system, allows for many for degrees of freedom in range of motion, which permits the surgeon to employ techniques which more closely mimic open surgical techniques.
The robotic surgical system has experienced rapid acceptance and growth over the past decade [30–33]. One small case comparison study and 1 large prospective analysis of over 200 procedures have suggested that robotic surgery is more ergonomically favorable and potentially less mentally stressful than conventional minimally invasive surgery [34,35]. However, although robotic surgery is often thought of as a tool to alleviate strain related to MIS [36,37], there are still high levels of strain, with some survey data indicating strain rates as high as 45%, involved with robotic surgery [8,9]. It is clear that, as with traditional MIS, effective interventions are needed to prevent and reduce strain to prevent work-related injury in robot-assisted MIS.
A primary cause of ergonomic strain during robot-assisted MIS is the lack of knowledge and training regard-ing proper ergonomic techniques at seated console work stations amongst surgeons, with as few as 16% of surgeons reporting any formal training [5,9,17,38]. Furthermore, when compared with traditional MIS, there is little available literature specific to robotic surgery ergonomics. Much of the data available has been extrapolated from recommendations from the U.S. Department of Labor’s Occupational Safety and Health Administration’s (OSHA’s) guidelines for working positions at workstations and on the available body of literature on the ergonomics of microscopy, which, due to somewhat similar positioning, have been adapted for robotic-assisted MIS [39].
The Robotic Console
When addressing applied ergonomics in robot-assisted MIS, the primary focuses in on the robotic console set-up and the surgeon positioning. The primary focus is on ensuring a comfortable headrest and adjustable ocular height, which relieves neck, shoulder, and upper back strain, and proper adjustment of armrests and finger controls aimed at minimizing arm and upper back strain due to static load forces [40].
The most common pitfalls during robotic-assisted MIS are when the arms are moved from the arm rest and the elbows flare out from the side of the operator. This departure from neutral position, common when attempting to reach for structures that are on the edge of the surgical field, causes significant tension and strain and is often not corrected for long period of time. Frequent use of the robotic clutch, which freezes the intracorporeal arms and allows for movement of the console arms freely, to bring the body back to neutral position is of paramount importance [17].
Conclusion
The field of applied ergonomics in surgery has never been more important. The fields of both traditional and robot-assisted MIS are growing rapidly and demand for these technologies will only increase as outcomes continue to improve. As the increasing workload of MIS is handled by a pool of surgeons that is not increasing rapidly enough to meet the demand, more volume will be handled by each surgeon.
With ergonomic strain now reported by nearly 90% of surgeons, much of it persistent strain beyond operative time, we will run into situations where surgeons may compensate for this persistent strain by decreasing operative volume and may decide to retire earlier than they otherwise might have. This could result in a health care supply problem as demand for MIS increases and the surgeon pool available to perform it stays constant or decreases.
To date, there has been relatively little research into this issue. The epidemiologic data on surgeon strain comes primarily from survey research done within various MIS subspecialties. There has been some data based on objective measures of strain utilizing validated strain indicators, but more work in this area is needed. Standardized methods of reporting strain will assist in clarifying both the epidemiology and standardize the response to interventions. Studies that aim to address the reported rates of strain are also needed. Much of the early work focused on operating room set-up and resulted in great improvements. More work is needed to assess optimal ergonomic positioning and formal surgeon training.
The solution will involve a combination of engineering advances in operating room set-up and equipment design along with a renewed focus on teaching ergonomic techniques and principles to MIS surgeons. While early data is promising and shows that training sessions in ergonomics are easy, acceptable by surgeons, and effective, more data is needed to develop optimal training session and modules when it comes to traditional and robot-assisted MIS ergonomics [17]. As ergonomic studies specifically designed to address this population accrue, more data driven guidelines can be developed and implemented.
Corresponding author: Jason M. Franasiak, MD, 140 Allen Rd., Basking Ridge, NJ 07920, [email protected].
Financial disclosures: None
From the Division of Reproductive Endocrinology and Infertility, Department of Obstetrics, Gynecology and Reproductive Science, Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ (Dr. Fransasiak), and the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC (Dr. Gehrig).
Abstract
- Background: Minimally invasive surgery (MIS) has benefits to both patients and society and its use has increased markedly over the past 3 decades. With its introduction, new mental and physical challenges were presented to the surgeons, leading to concerns regarding operative ergonomics. Applied ergonomics has been used to study and improve operative techniques and technologies as they apply to MIS.
- Objective: To review the ergonomic challenges presented by both traditional MIS as well as robot-assisted MIS and discuss how ergonomic science has evolved to address these issues.
- Methods: Review of the literature involving MIS and applied ergonomics
- Results: Surgeon strain as it relates to MIS has historically been thought to occur in only approximately 15% of MIS surgeons. More recent data suggests this number is much higher. Rates of strain have been reported to be as high as 88% among traditional MIS surgeons and 45% among robotic-assisted MIS surgeons. Strain results from a number of factors, including instrument design and use, optics placement and resolution, patient and surgeon positioning, and the drive to implement surgical technologies which aim to further minimize the invasiveness of surgical procedures.
- Conclusion: Improvements in applied ergonomics in MIS have resulted in improved optics, more sophisticated and ergonomic instruments, and methods of optimizing positioning. However, despite these advancements, ergonomic strain rates amongst surgeons remain alarmingly high. With the ever-increasing demand for MIS, more research and development as well as MIS surgeon training are needed to improve the safety of surgeons and ensure the career longevity required to meet the patient and societal demand for MIS.
Since its introduction to North America in the 1980s, minimally invasive surgery (MIS) has become widely accepted and practiced across surgical disciplines including general surgery, gynecologic surgery, oncology, and thoracic surgery [1]. Procedures once done through large incisions, such as cholecystectomies, have been supplanted by those utilizing 2 or 3 small punctures as the gold standard.
The demand for MIS has been rising and is driven by both providers and patients. The minimally invasive approaches have been shown to decrease recovery time, result in less postoperative pain, and decrease blood loss and other surgical complications [2,3], allowing for patients and their supports to return to baseline function more quickly [4]. In this way, both individual patients and society as a whole derives benefits from MIS through decreased recovery times and return to productivity.
Despite the clear benefits to patients and society, there has been increasing evidence of an unanticipated side effect of MIS: surgeon ergonomic strain and injury [5].Although the same ultimate procedure is performed when open techniques are employed as when MIS is utilized, surgeons have reported increased physical stress and mental strain when utilizing minimally invasive technologies [6,7]. The phenomenon was first noted during the laparoscopic surgery boom of the early 1990s and has been revisited more recently in the setting of both traditional and robotic-assisted MIS techniques [5,8,9].
The source of the issues arises from the fact the surgeons are, by definition, operating with reduced access to the patient. This requires limiting the degrees of freedom in movements, employing specialized and often awkward or cumbersome instruments, and requiring use of an intracorporeal camera that projects the surgical field onto a screen, which causes increased mental strain due to perceptual challenges as well as visual strain [7,10–13].
The initial large survey studies characterizing surgeon strain during MIS revealed rates of strain and discomfort in the 12% to 18% range, with many reporting that strain was persistent and not simply limited to operative time [11,14]. In part, these early estimates focused on very experienced surgeons and thus may have underreported the rates of strain. Subsequently, other studies have quoted rates of strain in the 40% to 60% range [15,16]. These studies focused on a larger and more hetero-geneous group of MIS surgeons, which may explain the higher rates of strain. Most recently, in the setting of an ever growing demand for MIS, large survey studies have revealed rates of surgeon strain to be as high as 87% and 88% among traditional MIS surgeons and 45% among robotic-assisted MIS surgeons, with 26% reporting persistent strain beyond the robotic console time [5,9,17]. This prolonged strain can impact productivity, with 14% of surgeons limiting the number of surgical cases they do per day, and may impact quality of life, with 29% needing to seek treatment for strain related to MIS [9].
Here we review the 2 major forms of MIS, traditional and robotic-assisted surgery. The unique features of each type of MIS that predispose to surgeon strain are discussed along with the techniques and technologies that have been employed to improve the ergonomics of MIS and reduce surgeon strain.
Traditional MIS
Traditional MIS, developed in the 1980s, involves use of a surgeon-manipulated intracorporeal video camera to view the surgical field. Instruments are placed through fixed ports inserted through the body wall called trocars. Most MIS surgical suites involve 1 or more surgeons standing aside a patient holding the camera and surgical instruments and viewing the surgical field on monitors placed around the patient.
The field and technology have evolved greatly since its inception. However, there are a number of factors that persist in creating ergonomic strain during traditional MIS.
Instruments
MIS instruments are limited by several factors. They must have long, thin shafts that can be placed and removed through fixed trocars. The majority of trocars commonly used are 5 to 10 mm in diameter. Given this fixed point, the instrument motion is inverted in the operative cavity, which requires mental adjustment and scaling. Additionally, the range of motion of MIS instruments is limited to 5 degrees of freedom, which allows for less dexterity than is commonly enjoyed during open surgery through large incisions, which accommodate the surgeons hands and allows for more degrees of freedom and dexterity [18]. These limitations have historically yielded instruments that have not been ergonomically sound. Indeed, MIS instrument are identified as an ergonomic problem by over 80% of minimally invasive surgeons [19].
Additionally, given that the surgeon’s hands are often occupied with the camera and an operative tool, the activation of suction devices and electrocautery devices often requires use of instrument foot pedals. Requiring that the instrument and camera be optimally positioned and relatively stationary and the foot pedal be activated simultaneously can exacerbate poor posture and back strain as the surgeon balances on one foot. The addition of foot pedals around the operative table also further limits space for proper surgeon
positioning [19].
Ergonomic engineering has focused on instrument handles. To accommodate the varied sizes of surgeon hands, many companies have altered the size of the device handles allowing for a more comfortable grip. To address the issue of poor posture induced by the use of foot pedals, many instruments now have trigger finger or thumb-activated buttons on the handle of the device itself, which alleviates the need for positioning to activate a foot pedal. However, many of these may not be suitable to accommodate smaller hands.
Optics
A major limitation of MIS is the limited visual field. The video monitor is positioned outside of the sterile operative field, often requiring that the surgeon looks in one direction and operates in another direction, placing strain in both the axial or rotational and frontal or flexion/extension planes [20]. The surgeon does not have immediate visual access to the entire surgical field but rather must rely on movement of the camera, which can at times result in unnatural and uncomfortable positions in order to position the camera optimally [19]. Additionally, eye strain can result from constant visualization of the operative monitor throughout the surgery. Finally, until only recently, optic systems required operating in 2 dimensions, without the depth perception enjoyed during traditional open surgery.
To address neck and upper body strain as well as optic strain, operative monitor positioning has received significant emphasis. The original MIS video monitors were small, had poor resolution, and were fixed in their position. Over time, monitors have increased in size and resolution, allowing for easier viewing and decreased optic strain. Additionally, in the 1990s the MIS operative suite concept allowed for placement of monitors on swing arms, which allow for movement about the operating room with ease. Subsequently, use of systems that employed multiple monitors placed around the patient at different angles with independent height and inclination adjustment allowed for comfortable positioning for all members of the surgical team, particularly in cases where 2 or more surgeons are operating simultaneously [21]. The implementation of these monitor systems not only decrease ergonomic strain but have also been shown to improve intraoperative speed and surgical accuracy when performing standardized tasks [22,23].
The most recent advance in surgical optics has been the introduction of 3-dimensional (3D) imaging systems [21,24,25]. At present, most of these systems are cost prohibitive and have poorer resolution than the traditional 2-dimensional monitors which may in fact increase optic strain. The modern high-definition 2D monitor systems in current use have done much to decrease optic strain and further refinement of 3D technology may prove to mitigate this strain even further.
Operative Posture
MIS often involves assuming unnatural postures to manipulate instruments and visualize the operative monitors. When non-neutral posture is maintained, muscles require an increase in energy production in order to maintain the same contractile forces and the contractile forces required to stabilize joints is increased [20]. Maintaining these static positions for long periods of time results in rapid fatigue, muscle pain, and cramping, and strain that can persist after the operation is complete [19].
Attention to ideal posture is paramount during MIS. The surgeon should be upright next to the patient with the head slightly bent forward, ideally employing a shift in position of the neck from time to time throughout the surgery to avoid prolonged static positioning [11]. The arms should rest so that the elbow is at the side with a 90- to 120-degree bend to accommodate instrument manipulation. This angle can be tolerated for long period of time as opposed to angles that require the elbow be taken away from the side of the body [19]. The forearm should rest in the neutral rotating position between pronated and supinated whenever possible with the wrist slightly extended and the fingers slightly bent [26]. This neutral position allows for rapid and simple changes in grip.
Adjustment of table height or use of operative foot platforms is crucial to ensuring the arms remain in neutral position. Given that the patient is often positioned in steep Trendelenburg or reverse Trendelenburg for MIS, the standard operating beds may not be at a height that allows the surgeon to operate in a relaxed, neutral posture [27,28]. In these circumstances, rather than operating with arms and shoulders in an elevated position, a position that produces rapid upper extremity fatigue, surgeons should elevate themselves with the assistance of an operative platform or step.
Single Incision Laparoscopic Surgery
Most recently, single port laparoscopic surgery (SPLS), also called single incision laparoscopic surgery (SILS) has been introduced. This technique involves use of a slightly larger, single incision that allows for a single port, which accommodates several instruments and the operative camera. This enhances some of the challenges posed with traditional MIS, namely maintaining exposure of the operative field, sustaining pneumatic pressure in the operative space, avoiding instrument collision both intra- and extracorporeally, and avoiding instrument interference with optics [29].
A number of techniques have been employed to minimize these issues. For example, percutaneous sutures may be placed intraoperatively in order to assist with retraction and improve visualization. The most important technological advances have come in the form of coaxial, flexible, and articulating instruments to avoid collisions [29]. While there is a learning curve with these technologies in terms of instrument triangulation, they can be successfully employed to improve operative efficiency and ergonomics.
Robot-Assisted MIS
Robot-assisted MIS involves the use of intracorporeal instruments attached to robotic arms that have been docked to trocars. The surgeon controls these robotic arms with a computer console and a video monitor is available for the surgical assistants. Many robot-assisted surgeries involve the use of an assistant, who utilizes traditional MIS instruments and trocars. The same issues in ergonomics discussed above apply to the assistant surgeon. Here, we will focus on the ergonomic challenges unique to operating surgeon at a robotic console.
There have been several robotic systems developed for use during surgery. At present, only the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA) is in use in the United States. Several components of the robotic systems allow for potential advantages over traditional MIS in terms of ergonomics. First, as discussed, the primary surgeon is seated at a robotic console rather than standing next to the patient. The camera and instruments are held intracorporeally by the robotic system and controlled by the surgeon at the console. The mechanical engineering, which is associated with the instruments of the robotic system, allows for many for degrees of freedom in range of motion, which permits the surgeon to employ techniques which more closely mimic open surgical techniques.
The robotic surgical system has experienced rapid acceptance and growth over the past decade [30–33]. One small case comparison study and 1 large prospective analysis of over 200 procedures have suggested that robotic surgery is more ergonomically favorable and potentially less mentally stressful than conventional minimally invasive surgery [34,35]. However, although robotic surgery is often thought of as a tool to alleviate strain related to MIS [36,37], there are still high levels of strain, with some survey data indicating strain rates as high as 45%, involved with robotic surgery [8,9]. It is clear that, as with traditional MIS, effective interventions are needed to prevent and reduce strain to prevent work-related injury in robot-assisted MIS.
A primary cause of ergonomic strain during robot-assisted MIS is the lack of knowledge and training regard-ing proper ergonomic techniques at seated console work stations amongst surgeons, with as few as 16% of surgeons reporting any formal training [5,9,17,38]. Furthermore, when compared with traditional MIS, there is little available literature specific to robotic surgery ergonomics. Much of the data available has been extrapolated from recommendations from the U.S. Department of Labor’s Occupational Safety and Health Administration’s (OSHA’s) guidelines for working positions at workstations and on the available body of literature on the ergonomics of microscopy, which, due to somewhat similar positioning, have been adapted for robotic-assisted MIS [39].
The Robotic Console
When addressing applied ergonomics in robot-assisted MIS, the primary focuses in on the robotic console set-up and the surgeon positioning. The primary focus is on ensuring a comfortable headrest and adjustable ocular height, which relieves neck, shoulder, and upper back strain, and proper adjustment of armrests and finger controls aimed at minimizing arm and upper back strain due to static load forces [40].
The most common pitfalls during robotic-assisted MIS are when the arms are moved from the arm rest and the elbows flare out from the side of the operator. This departure from neutral position, common when attempting to reach for structures that are on the edge of the surgical field, causes significant tension and strain and is often not corrected for long period of time. Frequent use of the robotic clutch, which freezes the intracorporeal arms and allows for movement of the console arms freely, to bring the body back to neutral position is of paramount importance [17].
Conclusion
The field of applied ergonomics in surgery has never been more important. The fields of both traditional and robot-assisted MIS are growing rapidly and demand for these technologies will only increase as outcomes continue to improve. As the increasing workload of MIS is handled by a pool of surgeons that is not increasing rapidly enough to meet the demand, more volume will be handled by each surgeon.
With ergonomic strain now reported by nearly 90% of surgeons, much of it persistent strain beyond operative time, we will run into situations where surgeons may compensate for this persistent strain by decreasing operative volume and may decide to retire earlier than they otherwise might have. This could result in a health care supply problem as demand for MIS increases and the surgeon pool available to perform it stays constant or decreases.
To date, there has been relatively little research into this issue. The epidemiologic data on surgeon strain comes primarily from survey research done within various MIS subspecialties. There has been some data based on objective measures of strain utilizing validated strain indicators, but more work in this area is needed. Standardized methods of reporting strain will assist in clarifying both the epidemiology and standardize the response to interventions. Studies that aim to address the reported rates of strain are also needed. Much of the early work focused on operating room set-up and resulted in great improvements. More work is needed to assess optimal ergonomic positioning and formal surgeon training.
The solution will involve a combination of engineering advances in operating room set-up and equipment design along with a renewed focus on teaching ergonomic techniques and principles to MIS surgeons. While early data is promising and shows that training sessions in ergonomics are easy, acceptable by surgeons, and effective, more data is needed to develop optimal training session and modules when it comes to traditional and robot-assisted MIS ergonomics [17]. As ergonomic studies specifically designed to address this population accrue, more data driven guidelines can be developed and implemented.
Corresponding author: Jason M. Franasiak, MD, 140 Allen Rd., Basking Ridge, NJ 07920, [email protected].
Financial disclosures: None
1. Cuschieri A. Laparoscopic surgery: current status, issues and future developments. Surg J R Coll Surg Edinb Irel 2005;3:125–30, 132–3, 135–8.
2. Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol 2008;111:41–5.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 2009;27:5331–6.
4. Bell MC, Torgerson J, Seshadri-Kreaden U, et al. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol 2008;111:407–11.
5. Park A, Lee G, Seagull FJ, et al. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg 2010;210:306–13.
6. Kant IJ, de Jong LC, van Rijssen-Moll M, Borm PJ. A survey of static and dynamic work postures of operating room staff. Int Arch Occup Environ Health 1992;63:423–8.
7. Patkin M, Isabel L. Ergonomics, engineering and surgery of endosurgical dissection. J R Coll Surg Edinb 1995;40:120–32.
8. Craven R, Franasiak J, Mosaly P, Gehrig PA. Ergonomic deficits in robotic gynecologic oncology surgery: a need for intervention. J Minim Invasive Gynecol 2013;20:648–55.
9. Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol 2012;126:437–42.
10. Berguer R. Surgical technology and the ergonomics of laparoscopic instruments. Surg Endosc 1998;12:458–62.
11. Berguer R. Surgery and ergonomics. Arch Surg 1999;134:1011–6.
12. Berguer R, Forkey DL, Smith WD. The effect of laparoscopic instrument working angle on surgeons’ upper extremity workload. Surg Endosc 2001;15:1027–9.
13. Lawson EH, Curet MJ, Sanchez BR, et al. Postural ergonomics during robotic and laparoscopic gastric bypass surgery: a pilot project. J Robot Surg 2007;1:61–7.
14. Van Veelen MA, Meijer DW. Ergonomics and design of laparoscopic instruments: results of a survey among laparoscopic surgeons. J Laparoendosc Adv Surg Tech A 1999;9:481–9.
15. Van Veelen MA, Nederlof EA, Goossens RHM, et al. Ergonomic problems encountered by the medical team related to products used for minimally invasive surgery. Surg Endosc 2003;17:1077–81.
16. Lawther RE, Kirk GR, Regan MC. Laparoscopic procedures are associated with a significant risk of digital nerve injury for general surgeons. Ann R Coll Surg Engl 2002;84:443.
17. Franasiak J, Craven R, Mosaly P, Gehrig PA. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS 2014;18(4).
18. Ballantyne GH. The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 2002;12:1–5.
19. Matern U. Ergonomic deficiencies in the operating room: examples from minimally invasive surgery. Work 2009;33:165–8.
20. Van Det MJ, Meijerink WJHJ, Hoff C, et al. Optimal ergonomics for laparoscopic surgery in minimally invasive surgery suites: a review and guidelines. Surg Endosc 2009;23:1279–85.
21. Veelen MA, Jakimowicz JJ, Goossens RHM, et al. Evaluation of the usability of two types of image display systems, during laparoscopy. Surg Endosc 2002;16:674–8.
22. Haveran LA, Novitsky YW, Czerniach DR, et al. Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc 2007;21:980–4.
23. Hanna GB, Shimi SM, Cuschieri A. Task performance in endoscopic surgery is influenced by location of the image display. Ann Surg 1998;227:481–4.
24. Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc 2002;16:1389–402.
25. Boppart SA, Deutsch TF, Rattner DW. Optical imaging technology in minimally invasive surgery. Current status and future directions. Surg Endosc 1999;13:718–22.
26. Matern U, Waller P. Instruments for minimally invasive surgery: principles of ergonomic handles. Surg Endosc 1999;13:174–82.
27. Matern U, Waller P, Giebmeyer C, et al. Ergonomics: requirements for adjusting the height of laparoscopic operating tables. JSLS 2001;5:7–12.
28. Van Veelen MA, Kazemier G, Koopman J, et al. Assessment of the ergonomically optimal operating surface height for laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2002;12:47–52.
29. Tang B, Hou S, Cuschieri SA. Ergonomics of and technologies for single-port lapaxroscopic surgery. Minim Invasive Ther Allied Technol 2012;21:46–54.
30. Wexner SD, Bergamaschi R, Lacy A, et al. The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 2009;23:438–43.
31. Challacombe BJ, Khan MS, Murphy D, Dasgupta P. The history of robotics in urology. World J Urol 2006;24:120–7.
32. Ballantyne GH, Moll F. The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surg Clin North Am 2003;83:1293–304, vii.
33. Ruurda JP, van Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl 2002;84:223–6.
34. Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg 2005;140:779–86.
35. Talamini MA, Chapman S, Horgan S, Melvin WS; Academic Robotics Group. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 2003;17:1521–4.
36. Mucksavage P, Kerbl DC, Lee JY. The da Vinci Surgical System overcomes innate hand dominance. J Endourol 2011;25:1385–8.
37. Schreuder HW, Verheijen RH. Robotic surgery. BJOG 2009;116:198–213.
38. Stone R, McCloy R. Ergonomics in medicine and surgery. BMJ 2004;328:1115–8.
39. Lux MM, Marshall M, Erturk E, Joseph JV. Ergonomic evaluation and guidelines for use of the daVinci Robot system. J Endourol 2010;24:371–5.
40. Sillanpaa J, Nyberg M, Laippala P. A new table for work with a microscope, a solution to ergonomic problems. Appl Ergon 2003;34:621–8.
1. Cuschieri A. Laparoscopic surgery: current status, issues and future developments. Surg J R Coll Surg Edinb Irel 2005;3:125–30, 132–3, 135–8.
2. Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol 2008;111:41–5.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 2009;27:5331–6.
4. Bell MC, Torgerson J, Seshadri-Kreaden U, et al. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol 2008;111:407–11.
5. Park A, Lee G, Seagull FJ, et al. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg 2010;210:306–13.
6. Kant IJ, de Jong LC, van Rijssen-Moll M, Borm PJ. A survey of static and dynamic work postures of operating room staff. Int Arch Occup Environ Health 1992;63:423–8.
7. Patkin M, Isabel L. Ergonomics, engineering and surgery of endosurgical dissection. J R Coll Surg Edinb 1995;40:120–32.
8. Craven R, Franasiak J, Mosaly P, Gehrig PA. Ergonomic deficits in robotic gynecologic oncology surgery: a need for intervention. J Minim Invasive Gynecol 2013;20:648–55.
9. Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol 2012;126:437–42.
10. Berguer R. Surgical technology and the ergonomics of laparoscopic instruments. Surg Endosc 1998;12:458–62.
11. Berguer R. Surgery and ergonomics. Arch Surg 1999;134:1011–6.
12. Berguer R, Forkey DL, Smith WD. The effect of laparoscopic instrument working angle on surgeons’ upper extremity workload. Surg Endosc 2001;15:1027–9.
13. Lawson EH, Curet MJ, Sanchez BR, et al. Postural ergonomics during robotic and laparoscopic gastric bypass surgery: a pilot project. J Robot Surg 2007;1:61–7.
14. Van Veelen MA, Meijer DW. Ergonomics and design of laparoscopic instruments: results of a survey among laparoscopic surgeons. J Laparoendosc Adv Surg Tech A 1999;9:481–9.
15. Van Veelen MA, Nederlof EA, Goossens RHM, et al. Ergonomic problems encountered by the medical team related to products used for minimally invasive surgery. Surg Endosc 2003;17:1077–81.
16. Lawther RE, Kirk GR, Regan MC. Laparoscopic procedures are associated with a significant risk of digital nerve injury for general surgeons. Ann R Coll Surg Engl 2002;84:443.
17. Franasiak J, Craven R, Mosaly P, Gehrig PA. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS 2014;18(4).
18. Ballantyne GH. The pitfalls of laparoscopic surgery: challenges for robotics and telerobotic surgery. Surg Laparosc Endosc Percutan Tech 2002;12:1–5.
19. Matern U. Ergonomic deficiencies in the operating room: examples from minimally invasive surgery. Work 2009;33:165–8.
20. Van Det MJ, Meijerink WJHJ, Hoff C, et al. Optimal ergonomics for laparoscopic surgery in minimally invasive surgery suites: a review and guidelines. Surg Endosc 2009;23:1279–85.
21. Veelen MA, Jakimowicz JJ, Goossens RHM, et al. Evaluation of the usability of two types of image display systems, during laparoscopy. Surg Endosc 2002;16:674–8.
22. Haveran LA, Novitsky YW, Czerniach DR, et al. Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc 2007;21:980–4.
23. Hanna GB, Shimi SM, Cuschieri A. Task performance in endoscopic surgery is influenced by location of the image display. Ann Surg 1998;227:481–4.
24. Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc 2002;16:1389–402.
25. Boppart SA, Deutsch TF, Rattner DW. Optical imaging technology in minimally invasive surgery. Current status and future directions. Surg Endosc 1999;13:718–22.
26. Matern U, Waller P. Instruments for minimally invasive surgery: principles of ergonomic handles. Surg Endosc 1999;13:174–82.
27. Matern U, Waller P, Giebmeyer C, et al. Ergonomics: requirements for adjusting the height of laparoscopic operating tables. JSLS 2001;5:7–12.
28. Van Veelen MA, Kazemier G, Koopman J, et al. Assessment of the ergonomically optimal operating surface height for laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2002;12:47–52.
29. Tang B, Hou S, Cuschieri SA. Ergonomics of and technologies for single-port lapaxroscopic surgery. Minim Invasive Ther Allied Technol 2012;21:46–54.
30. Wexner SD, Bergamaschi R, Lacy A, et al. The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 2009;23:438–43.
31. Challacombe BJ, Khan MS, Murphy D, Dasgupta P. The history of robotics in urology. World J Urol 2006;24:120–7.
32. Ballantyne GH, Moll F. The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surg Clin North Am 2003;83:1293–304, vii.
33. Ruurda JP, van Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl 2002;84:223–6.
34. Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg 2005;140:779–86.
35. Talamini MA, Chapman S, Horgan S, Melvin WS; Academic Robotics Group. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 2003;17:1521–4.
36. Mucksavage P, Kerbl DC, Lee JY. The da Vinci Surgical System overcomes innate hand dominance. J Endourol 2011;25:1385–8.
37. Schreuder HW, Verheijen RH. Robotic surgery. BJOG 2009;116:198–213.
38. Stone R, McCloy R. Ergonomics in medicine and surgery. BMJ 2004;328:1115–8.
39. Lux MM, Marshall M, Erturk E, Joseph JV. Ergonomic evaluation and guidelines for use of the daVinci Robot system. J Endourol 2010;24:371–5.
40. Sillanpaa J, Nyberg M, Laippala P. A new table for work with a microscope, a solution to ergonomic problems. Appl Ergon 2003;34:621–8.
Early Parkinsonism: Distinguishing Idiopathic Parkinson’s Disease from Other Syndromes
From the VA Medical Center (Dr. Lehosit) and the Parkinson’s and Movement Disorders Center, Virginia Commonwealth University (Dr. Cloud), Richmond, VA.
Abstract
- Objective: To provide an overview of the importance and challenges of accurate diagnosis of early idiopathic Parkinson’s disease and practical guidelines for clinicians.
- Methods: Review of the relevant literature.
- Results: Idiopathic Parkinson’s disease is a common neurodegenerative disorder causing a wide spectrum of motor and nonmotor symptoms. The cardinal motor features include resting tremors, bradykinesia, rigidity, and postural instability. The diagnosis is clinical, and ancillary laboratory or radiology tests are unnecessary in typical cases. Despite the use of validated diagnostic criteria, misdiagnosis is common, especially early in the disease process. This is largely due to the phenotypic heterogeneity in the idiopathic Parkinson’s disease population as well phenotypic overlapping with other diseases. The diseases most commonly confused with idiopathic Parkinson’s disease are the Parkinson-plus syndromes (dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration), vascular parkinsonism, drug-induced parkinsonism, dopa responsive dystonia, normal pressure hydrocephalus, and essential tremor. Since the diagnosis of these other diseases is also clinical, familiarity with their typical presentations and most current diagnostic criteria is helpful. Brain MRI can be helpful in diagnosing some of the diseases, though brain imaging is most commonly unremarkable in idiopathic Parkinson’s disease. DaTscan has an FDA indication to assist in the evaluation of adults with parkinsonian syndromes. It should not be used in typical cases but can be a useful adjunct to other diagnostic evaluations in atypical cases.
- Conclusion: Despite the challenges involved, accurate and early diagnosis of idiopathic Parkinson’s disease is essential for optimal patient education, counseling, and treatment.
Idiopathic Parkinson’s disease (IPD) is a common neurodenerative disease, affecting 1% of the population over the age of 65 [1]. A definitive diagnosis requires the postmortem findings of degeneration of the substantia nigra pars compacta and the presence of Lewy bodies (insoluble cytoplasmic inclusions composed of aggregated alpha-synuclein). In the later stages of the disease, a correct clinical diagnosis is made in more than 90% of patients [2]. Early on, however, clinical diagnosis is less reliable. For clinicians, distinguishing early IPD from other parkinsonian syndromes can be extraordinarily challenging because these conditions, especially in the earliest stages, present with highly variable yet overlapping phenotypes [3]. Furthermore, most of the diseases in the differential diagnosis, including IPD itself, are clinical diagnoses made on the basis of history and examination without the benefit of laboratory or radiology data. A high level of clinical acumen is therefore required for early and accurate diagnosis. Recent clinical trials in which subspecialists performed stringent diagnostic assessments to identify subjects with clinically diagnosed IPD later found that some subjects had normal functional dopamine imaging, suggesting that they probably did not have IPD [4,5]. These trials served to highlight the possibility of misdiagnosis, even in the hands of highly trained subspecialists. Early and accurate diagnosis is of paramount importance for many reasons. First, treatment approaches differ significantly across many of these diseases. Second, as neuroprotective interventions that are currently under investigation become available, long-term outcomes may significantly improve with earlier diagnosis and intervention. Third, some of these diseases are prognostically very different from one another, so accurate diagnosis enables better counseling and setting realistic expectations for progression.
This review will discuss the most common presenting signs and symptoms of early IPD, present the most widely used diagnostic criteria, and introduce the ancillary laboratory and imaging tests that may be helpful in distinguishing it from its mimics. The diseases most commonly confused with early IPD will also be discussed with an emphasis on the ways they most commonly differ from IPD. We will begin our discussion with the presenting signs and symptoms of IPD.
Idiopathic Parkinson’s Disease
IPD typically has a subtle and insidious onset with characteristic features developing over months to years. IPD most often presents in patients after age 60, and age is the most consistent risk factor for developing IPD; however, approximately 5% of IPD cases begin before age 40 years. These young-onset cases are likely to be caused by genetic mutations [6]. The widely recognized cardinal motor features of IPD include asymmetric resting tremor, rigidity, bradykinesia and postural instability [7]. Asymmetry is a key feature, as symptoms typically start on one side and remain more prominent on that side as the disease progresses. In fact, lack of asymmetry suggests an alternative diagnosis. Of the cardinal motor features, tremor is most often reported by patients as the first symptom [8]. However, IPD can alternately present with various other motor or even nonmotor complaints that will be discussed later.
Motor Features
Resting tremor is the most common presenting sign/symptom of early IPD, found in approximately 70% of patients [8]. The tremor typically is asymmetric and intermittent at onset, often starting in one hand. It is sometimes, though not necessarily, described as a “pill-rolling” rhythmic movement of the thumb and first finger while the hand is at rest. Patients will usually report a worsening of tremor with stress, anxiety, and increased fatigue. The tremor does not persist during sleep and diminishes with voluntary activity of the affected limb(s). By having the patient perform mentally challenging tasks (such as counting backwards) or motor movements of other body parts (such as finger tapping with the other hand or walking), the examiner may notice an increase in tremor amplitude [11]. There may also be a resting tremor of the lip or lower jaw, but true head tremor suggests an alternate diagnosis such as essential tremor [12]. Postural tremor can co-exist with resting tremor in IPD, which often leads to diagnostic confusion, especially when the postural tremor is more prominent than the resting tremor. In this scenario, the distinction between IPD and essential tremor (discussed later) can become more difficult.
Rigidity is characterized as the presence of increased resistance to passive stretch throughout the range of motion [13]. “Lead pipe” rigidity remains sustained throughout the motion of the joint, while “cogwheel” rigidity is intermittent through the movement. The examiner must take care to distinguish between true rigidity and other forms of increased tone such as spasticity (a velocity dependent increase in tone) and paratonia (a resistance to passive motion created by the patient). Subtle rigidity can be enhanced in a limb by having the patient perform a voluntary movement of the contralateral limb [14]. Rigidity in early IPD is also asymmetric and most commonly found in the upper extremities, but it can be seen in the neck and lower extremities as well. Patients may initially complain of shoulder pain and stiffness that is diagnosed as rotator cuff disease or arthritis, when this pain is actually due to rigidity from Parkinson’s disease [15]. Severe axial rigidity out of proportion to appendicular rigidity, however, should suggest an alternate diagnosis in the early stages of the disease (such as progressive supranuclear palsy which is further discussed below).
Bradykinesia refers to decreased amplitude and speed of voluntary motor movements. This sign can be found throughout the body in the form of hypometric saccades, decreased blink rate, decreased facial expressions (“masked facies”) and softening of speech (hypophonia) [16]. Patients may initially report a general slowing down of movements as well as difficulty with handwriting due to their writing becoming smaller (micrographia) [17]. Bradykinesia is evaluated by testing the speed, amplitude, and rhythmicity of voluntary movements such as repetitive tapping of the thumb and first finger together, alternation of supination and pronation of the forearm and hand, opening and closing the hand and tapping the foot rhythmically on the floor. The examiner should also evaluate for generalized bradykinesia by viewing the patient rise from a seated to standing position as well as observing the patient’s normal speed of ambulation and speed and symmetry of arm swing.
Gait disturbance and postural instability can sometimes be found in early IPD; however, significant impairment of postural reflexes, gait impairment and early falls may point to a diagnosis other than IPD. Early IPD postural changes include mild flexion of the neck or trunk that may be accompanied by a slight leaning to one side. On examination of natural gait, the patient may exhibit asymmetrically reduced arm swing, slowing of gait and turning, shortened stride length and intermittent shuffling of the feet. With disease progression, all of these become more severe and there may be festination of gait (“hurried” gate with increased cadence and difficulty stopping). This can lead to instability and falls as the patient’s center of balance is displaced forward. Freezing of gait can also develop, but is rarely found in early IPD [18]. Postural stability is evaluated by the “pull test” where the patient is asked to stand in a comfortable stance with eyes open and feet apart and instructed to resist falling backwards when pulled by the examiner. The patient is allowed to take one step backwards with either foot if necessary to prevent falling. This test is usually normal in early IPD, but it often becomes abnormal with disease progression.
Because of dramatic heterogeneity in the expression of these cardinal motor features in IPD, patients are often subcategorized based upon the most prominent features of their motor exam. Well-recognized motor subtypes include tremor-predominant, akinetic-rigid, postural instability gait disorder PD (PIGD), and mixed [19]. Tremor-predominant patients are those with significant tremors that overshadow the other motor features of the disease, while akinetic-rigid patients have prominent bradykinesia and rigidity with little to no tremor. PIGD patients have prominent postural and gait abnormalities, while mixed patients have roughly equal amounts of all of the cardinal motor features. Recent research has suggested that these motor subtypes differ with regard to the frequency of comorbid nonmotor features, disease prognosis, and response to certain treatments [20–22]. For example, tremor-predominant patients generally have a good prognosis with slow disease progression while PIGD patients have a poor prognosis with rapid progression, dementia, and depression [19].
Nonmotor Symptoms
Along with the classic motor features of IPD, patients often suffer from a variety of nonmotor symptoms that can sometimes precede the onset of motor symptoms by several years [23]. When nonmotor symptoms are the presenting symptoms, diagnosis is often delayed at 1.6 years versus 1.0 year for individuals with motor presentations [2]. Recognition of a nonmotor prodrome of PD has instigated a debate about whether new diagnostic criteria for early-stage and prodromal PD should be created [24]; for now, however, a diagnosis of PD still requires the motor syndrome. The spectrum of nonmotor symptoms in IPD can include olfactory dysfunction, urinary dysfunction, constipation, depression, anxiety, apathy, cognitive decline, sleep disorders such as REM (rapid eye movement) sleep behavior disorder and restless legs syndrome, fatigue and orthostatic hypotension. While many of these nonmotor symptoms are common in the general population and are certainly not specific to IPD, their presence in conjunction with early parkinsonism can help further support an IPD diagnosis.
Patients with IPD should exhibit a robust and sustained response to levodopa therapy. Over time, as the degenerative disease progresses, doses need to be increased and complications of therapy are likely to emerge, most commonly levodopa-induced dyskinesia, motor and nonmotor fluctuations [25]. The various forms of parkinsonism (discussed later) may have an initial response to levodopa therapy; however, this response is generally transient and wanes quickly despite increases in dose. Many will have no response at all.
Differential Diagnosis
The differential diagnosis for IPD most commonly includes the Parkinson-plus syndromes (dementia with Lewy bodies, multiple system atrophy, progressive supra-nuclear palsy, and corticobasal degeneration), vascular parkinsonism, drug-induced parkinsonism, dopa responsive dystonia, normal pressure hydrocephalus, and essential tremor. Each of these conditions will be discussed in further detail below.
Parkinson-Plus Syndromes
Dementia with Lewy bodies (DLB) may initially resemble IPD as it can present with parkinsonian motor signs, but the distinguishing feature of this disease is the presence of a progressive dementia with deficits in attention and executive function that occurs before or within 1 year of the development of parkinsonian motor signs [26]. This is in contrast to the dementia that can develop in IPD, which usually occurs many years into the disease course. Patients with DLB often have well-formed, visual hallucinations with this disorder. Motor parkinsonian symptoms do not improve with dopaminergic therapy and caution should be used with these patients as psychiatric symptoms may be exacerbated by even small doses of these medications [27]. Diagnostic criteria for probable DLB require the presence of dementia plus at least 2 of the following 3 core features: fluctuating attention and concentration, recurrent well-formed visual hallucinations, and spontaneous parkinsonian motor signs. Suggestive clinical features include REM behavior disorder, severe neuroleptic sensitivity, and low dopamine transporter uptake in the basal ganglia on SPECT or PET imaging. In the absence of 2 core features, the diagnosis of probable DLB can also be made if dementia plus at least 1 suggestive feature is present with just 1 core feature. Possible DLB can be diagnosed with the presence of dementia plus 1 core or suggestive feature. These criteria are 83% sensitive and 95% specific for the presence of neocortical Lewy bodies at autopsy [27]. Other supportive clinical features include repeated falls, syncope, transient loss of consciousness, severe autonomic dysfunction, depression, and systematized delusions or hallucinations in other sensory and perceptual modalities [27]. Definitive diagnosis requires pathological confirmation.
Corticobasal degeneration (CBD) is more rare than the previously described Parkinson-plus syndromes. CBD typically presents with a markedly unilateral/asymmetric motor features and can mimic early IPD, but other defining features include cortical signs of progressive unilateral apraxia, limb dystonia and visual-tactile neglect (“alien limb” sign) that can lead to loss of voluntary control of the extremity. This sign has been reported in approximately half of all patients with CBD [34]. As the disease progresses, cognitive decline, dementia, dysarthria, postural instability and gait dysfunction can all occur [35]. Patients with CBD typically do not show any response to dopaminergic therapy. CBD brain MRI findings include asymmetric cortical atrophy (most commonly in the superior parietal region), bilateral basal ganglia atrophy, corpus callosum atrophy and T2 hyperintensities of the subcortical white matter and posterolateral putamen [36]. In recently published consensus criteria, Armstrong et al broadened the clinical phenotype associated with CBD to acknowledge the spectrum and overlapping phenotypes of tau-related neurodegenerative diseases [37]. The criteria for probable corticobasal syndrome require asymmetric presentation of 2 of: (a) limb rigidity or akinesia, (b) limb dystonia, (c) limb myoclonus plus 2 of: (d) orobuccal or limb apraxia, (e) cortical sensory deficit, (f) alien limb phenomena (more than simple levitation). Possible corticobasal syndrome may be symmetric and requires 1 of: (a) limb rigidity or akinesia, (b) limb dystonia, (c) limb myoclonus plus 1 of: (d) orobuccal or limb apraxia, (e) cortical sensory deficit, (f) alien limb phenomena (more than simple levitation). Unfortunately, these new criteria have not improved the specificity of diagnosis compared to previous criteria as shown by a recent longitudinal clinical and neuropathological study that found that all of their patients with a cortiocobasal syndrome but without corticobasal pathology had all met the new diagnostic criteria for possible or probable CBD [38]. The reader should be aware that Armstrong et al acknowledged that memory dysfunction is common in CBD, although this was not incorporated into the diagnostic criteria.
Other Causes of Parkinsonism
Vascular parkinsonism results from the accumulation of multiple infarcts in the basal ganglia and/or subcortical white matter [39]. It may account for up to 12% of all cases of parkinsonism [40]. There are not any specific clinical diagnostic criteria for vascular parkinsonism; however, the clinical presentation is somewhat distinctive. Vascular parkinsonism initially presents with gait problems, and the upper extremities are less affected than the lower extremities. Vascular parkinsonism has been referred to as “lower body parkinsonism” due to this distribution of symptoms. Patients often present with a characteristic shuffling gait, but may also exhibit significant freezing of gait, even early in the course of the disease (in contrast to IPD). Tremor is reported less consistently and other pyramidal tract signs, urinary symptoms, dementia and pseudobulbar affect resulting from various ischemic lesions often co-exist [41]. Patients tend to have a history of cerebrovascular risk factors. Response to dopaminergic therapy is present in one-third to one-half of patients and is typically short-lived [42]. Brain MRI findings in vascular parkinsonism include diffuse subcortical white or gray matter lesions, particularly involving the globus pallidus, thalamus, substantia nigra and frontal lobes. One study reported a “cutoff” point to help differentiate between vascular parkinsonism and the normal vascular changes associated with aging at 0.6% lesioned volume of brain tissue [43]. It is important to remember that microvascular lesions are commonly seen on MRI scans of older patients and therefore the presence of these lesions on imaging does not necessarily convey a diagnosis of vascular parkinsonism.
Evaluation of any parkinsonian patient should involve careful scrutiny of the medication list (current and past) to exclude the possibility of drug-induced parkinsonism (DIP). DIP is typically, though not always, symmetric in onset. Drugs causing DIP include all of the typical and atypical antipsychotics, dopamine depleters such as reserpine and tetrabenazine, gastrointestinal drugs with dopamine receptor blocking activity such as antiemetics and metoclopramide, calcium channel blockers, valproic acid, selective serotonin reuptake inhibiters and lithium [44]. Traditionally this syndrome was thought to be reversible with discontinuation of the offending drug; however, resolution can require many months and at least 10% of patients with DIP develop persistent and progressive parkinsonism despite discontinuation of the drug [45].
Dopa-responsive dystonia (DRD) most typically presents in childhood with initial onset of lower limb dystonia with parkinsonism developing over time. Symptoms respond robustly to low doses of levodopa, hence the name DRD. Occasionally, however, DRD can present in adulthood. In adult-onset cases of DRD, parkinsonism usually develops before dystonia. Because it presents with parkinsonism and is levodopa responsive, adult-onset DRD can easily be confused with young-onset IPD [46]. Clues to the presence of DRD include diurnal fluctuation, stability of symptoms over time, and a normal DaTscan (discussed later) [46].
Other rare causes of parkinsonism include exposure to toxins (MPTP, manganese, carbon monoxide, methanol), metabolic disorders (hypoparathyroidism, hypothyroidism, acquired hepatocerebral degeneration), early-onset and genetic disorders (Wilson’s disease, juvenile Huntington’s disease, spinocerebellar ataxia types 2 and 3, and neurodegeneration with brain iron accumulation), infectious diseases, trauma, space-occupying brain lesions, autoimmune diseases (Sjogren’s syndrome) and paraneoplastic disorders [47–51]. Further discussion of these more rare causes parkinsonism is beyond the scope of this review; however, clinicians should always carefully consider the past medical, family, and social history, along with the review of systems, as these aspects of the patient history may point to one of these causes of parkinsonism.
Normal pressure hydrocephalus (NPH) refers to chronic communicating hydrocephalus with adult onset. The classic clinical triad of NPH includes cognitive impairment, urinary incontinence, and gait disturbance in the absence of signs of increased intracranial pressure such as papilledema. NPH can present with motor signs similar to those found in vascular parkinsonism, possibly due to the close proximity of basal ganglia structures to the ventricular system [52]. The gait of NPH typically shows a decrease in step height and foot clearance as well as a decrease in walking speed. This is often referred to as a “magnetic gait.” In contrast to Parkinson’s disease patients, the gait disturbance in NPH does not improve with visual cues or dopaminergic therapy [53]. Dementia also occurs early on in the course of NPH and is mostly characterized by apathy, forgetfulness, and impaired recall. Urinary incontinence and urgency is a later finding of the disease in contrast to IPD in which urinary dysfunction is often an early nonmotor symptom. MRI and CT scans of the brain reveal enlarged ventricles (out of proportion to surrounding cerebral atrophy if present) and should be followed by a diagnostic high volume lumbar puncture. Clinical improvement following lumbar puncture is supportive of the diagnosis of NPH and helps to identify patients who may benefit from ventriculoperitoneal shunting [54].
Essential tremor (ET) is characterized by postural and action tremors, rather than resting tremors, though some ET patients can have co-existing resting tremors. Though it is usually bilateral, it is often asymmetric, adding to the potential for diagnostic confusion with IPD. It typically has a higher frequency than the tremor of IPD. The absence of rigidity, bradykinesia, postural and gait disturbances and no response to dopaminergic therapy help distinguish it further from IPD [55]. There is phenotypic overlap between these two conditions and some patients with IPD have more postural tremor than rest tremor (or even postural tremor with no rest tremor), while some with long-standing essential tremor may go on to develop parkinsonism [56].
The Role of DaTscan in Diagnosing Early Parkinsonism
Final Thoughts
Despite the challenges involved, accurate and early diagnosis of IPD is essential for optimal patient education, counseling, and treatment. Careful attention to the initial presentation and examination may be all that is required for diagnosis in typical cases. In atypical cases, brain MRI to evaluate for other diseases or DaTscan may be helpful adjunctive tests. As research advances over the coming years, it is likely that additional imaging or fluid biomarkers will become available to assist us with the diagnosis of IPD (and related disorders) in the early stages. Until then, clinicians must remain highly vigilant in their efforts to make these often challenging clinical diagnoses.
Corresponding author: Leslie J. Cloud, MD, MSc, 6605 West Broad St., Ste. C, Richmond, VA 23230, [email protected].
Financial disclosures: None.
1. Wirdefeldt K, Adami HO, Cole P, et al. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 2011;26 Suppl 1:S1–58.
2. O’Sullivan SS, Williams DR, Gallagher DA, et al. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord 2008;23:101–6.
3. Ali K, Morris HR. Parkinson’s disease: chameleons and mimics. Pract Neurol 2015;15:14–25.
4. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61:1044–53.
5. Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 2003;54:93–101.
6. Wickremaratchi MM, Ben-Shlomo Y, Morris HR. The effect of onset age on the clinical features of Parkinson’s disease. Eur J Neurol 2009;16:450–6.
7. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–9.
8. Rajput AH, Rozdilsky B, Ang L. Occurrence of resting tremor in Parkinson’s disease. Neurology 1991;41:1298–9.
9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4.
10. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–7.
11. Raethjen J, Austermann K, Witt K, et al. Provocation of Parkinsonian tremor. Mov Disord 2008;23:1019–23.
12. Roze E, Coêlho-Braga MC, Gayraud D, et al. Head tremor in Parkinson’s disease. Mov Disord 2006;21:1245–8.
13. Hallett M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol 2003;91:19–28.
14. Broussolle E, Krack P, Thobois S, et al. Contribution of Jules Froment to the study of parkinsonian rigidity. Mov Disord 2007;22:909–14.
15. Riley D, Lang AE, Blair RD, et al. Frozen shoulder and other shoulder disturbances in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1989;52:63–6.
16. Rottach KG, Riley DE, DiScenna AO, et al. Dynamic properties of horizontal and vertical eye movements in parkinsonian syndromes. Ann Neurol 1996;39:368–77.
17. Cooper JA, Sagar HJ, Tidswell P, Jordan N. Slowed central processing in simple and go/no-go reaction time tasks in Parkinson’s disease. Brain 1994;117(Pt 3):517–29.
18. Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010;81:513–8.
19. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurology 2014;71:499–504.
20. Burn DJ, Rowan EN, Allan LM, et al. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–9.
21. Burn DJ, Landau S, Hindle JV, et al; PROMS-PD Study Group. Parkinson’s disease motor subtypes and mood. Mov Disord 2012;27:379–86.
22. Katz M, Luciano MS, Carlson K, et al; CSP 468 study group. Differential effects of deep brain stimulation target on motor subtypes in Parkinson’s disease. Ann Neurol 2015;77:710–9.
23. Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol 2010;67:798–801.
24. Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 2014;29:454–62.
25. Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord 2015;30:80–9.
26. Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord 2005;20 Suppl 12:S11–20.
27. Karantzoulis S, Galvin JE. Update on dementia with Lewy bodies. Curr Transl Geriatr Exp Gerontol Rep 2013;2:196–204.
28. Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–8.
29. Kim HJ, Jeon BS, Jellinger KA. Diagnosis and differential diagnosis of MSA: boundary issues. J Neurol 2015 Feb 7. [Epub ahead of print]
30. Lee EA, Cho HI, Kim SS, Lee WY. Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat Disord 2004;10:363–8.
31. Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology 1988;38:1031–4.
32. Maher ER, Lees AJ. The clinical features and natural history of the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1986;36:1005–8.
33. Gröschel K, Kastrup A, Litvan I, Schulz JB. Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology 2006;66:949–50.
34. Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain 1994117(Pt 5):1183–96.
35. Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999;53:1969–74.
36. Tokumaru AM, O’uchi T, Kuru Y, et al. Corticobasal degeneration: MR with histopathologic comparison. AJNR Am J Neuroradiol 1996;17:1849–52.
37. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503.
38. Alexander SK, Rittman T, Xuereb JH, et al. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 2014;85:925–9.
39. Sibon I, Fenelon G, Quinn NP, Tison F. Vascular parkinsonism. J Neurol 2004;251:513–24.
40. Thanvi B, Lo N, Robinson T. Vascular parkinsonism--an important cause of parkinsonism in older people. Age Ageing 2005;34:114–9.
41. Kalra S, Grosset DG, Benamer HT. Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: a systematic review. Mov Disord 2010;25:149–56.
42. Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol 2013; 12:597–608.
43. Josephs KA. Frontotemporal lobar degeneration. Neurol Clin 2007;25:683–96, vi.
44. Lopez-Sendon J, Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf 2013;12:487–96.
45. Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf 2006;5:759–71.
46. Brajkovic LD, Svetel MV, Kostic VS, et al. Dopamine transporter imaging (123)I-FP-CIT (DaTSCAN) SPET in differential diagnosis of dopa-responsive dystonia and young-onset Parkinson’s disease. Hell J Nucl Med 2012;15:134–8.
47. Krusz JC, Koller WC, Ziegler DK. Historical review: abnormal movements associated with epidemic encephalitis lethargica. Mov Disord 1987;2:137–41.
48. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983;219:979–80.
49. Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology 2005;64:2021–8.
50. Jankovic J, Kirkpatrick JB, Blomquist KA, et al. Late-onset Hallervorden-Spatz disease presenting as familial parkinsonism. Neurology 1985;35:227–34.
51. Cloud L, Jankovic J. Systemic disease and movement disorders. In: Burn DJ, editor. Oxford textbook of clinical neurology on movement disorders. Oxford University Press; 2013.
52. Bugalho P, Guimaraes J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord 2007;13:434–7.
53. Jankovic J, Newmark M, Peter P. Parkinsonism and acquired hydrocephalus. Mov Disord 1986;1:59–64.
54. Bergsneider M, Black PM, Klinge P, et al. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 Suppl): S29-39; discussion ii-v.
55. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology 2000; 54(11 Suppl 4): S7.
56. Jankovic J. Essential tremor and Parkinson’s disease. Ann Neurol 1989;25:211–2.
57. Catafau AM, Tolosa E; DaTSCAN Clinically Uncertain Parkinsonian Syndromes Study Group. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord 2004;19:1175–82.
58. Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 2013;84:1288–95.
59. Kagi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 2010;81:5–12.
60. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9.
From the VA Medical Center (Dr. Lehosit) and the Parkinson’s and Movement Disorders Center, Virginia Commonwealth University (Dr. Cloud), Richmond, VA.
Abstract
- Objective: To provide an overview of the importance and challenges of accurate diagnosis of early idiopathic Parkinson’s disease and practical guidelines for clinicians.
- Methods: Review of the relevant literature.
- Results: Idiopathic Parkinson’s disease is a common neurodegenerative disorder causing a wide spectrum of motor and nonmotor symptoms. The cardinal motor features include resting tremors, bradykinesia, rigidity, and postural instability. The diagnosis is clinical, and ancillary laboratory or radiology tests are unnecessary in typical cases. Despite the use of validated diagnostic criteria, misdiagnosis is common, especially early in the disease process. This is largely due to the phenotypic heterogeneity in the idiopathic Parkinson’s disease population as well phenotypic overlapping with other diseases. The diseases most commonly confused with idiopathic Parkinson’s disease are the Parkinson-plus syndromes (dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration), vascular parkinsonism, drug-induced parkinsonism, dopa responsive dystonia, normal pressure hydrocephalus, and essential tremor. Since the diagnosis of these other diseases is also clinical, familiarity with their typical presentations and most current diagnostic criteria is helpful. Brain MRI can be helpful in diagnosing some of the diseases, though brain imaging is most commonly unremarkable in idiopathic Parkinson’s disease. DaTscan has an FDA indication to assist in the evaluation of adults with parkinsonian syndromes. It should not be used in typical cases but can be a useful adjunct to other diagnostic evaluations in atypical cases.
- Conclusion: Despite the challenges involved, accurate and early diagnosis of idiopathic Parkinson’s disease is essential for optimal patient education, counseling, and treatment.
Idiopathic Parkinson’s disease (IPD) is a common neurodenerative disease, affecting 1% of the population over the age of 65 [1]. A definitive diagnosis requires the postmortem findings of degeneration of the substantia nigra pars compacta and the presence of Lewy bodies (insoluble cytoplasmic inclusions composed of aggregated alpha-synuclein). In the later stages of the disease, a correct clinical diagnosis is made in more than 90% of patients [2]. Early on, however, clinical diagnosis is less reliable. For clinicians, distinguishing early IPD from other parkinsonian syndromes can be extraordinarily challenging because these conditions, especially in the earliest stages, present with highly variable yet overlapping phenotypes [3]. Furthermore, most of the diseases in the differential diagnosis, including IPD itself, are clinical diagnoses made on the basis of history and examination without the benefit of laboratory or radiology data. A high level of clinical acumen is therefore required for early and accurate diagnosis. Recent clinical trials in which subspecialists performed stringent diagnostic assessments to identify subjects with clinically diagnosed IPD later found that some subjects had normal functional dopamine imaging, suggesting that they probably did not have IPD [4,5]. These trials served to highlight the possibility of misdiagnosis, even in the hands of highly trained subspecialists. Early and accurate diagnosis is of paramount importance for many reasons. First, treatment approaches differ significantly across many of these diseases. Second, as neuroprotective interventions that are currently under investigation become available, long-term outcomes may significantly improve with earlier diagnosis and intervention. Third, some of these diseases are prognostically very different from one another, so accurate diagnosis enables better counseling and setting realistic expectations for progression.
This review will discuss the most common presenting signs and symptoms of early IPD, present the most widely used diagnostic criteria, and introduce the ancillary laboratory and imaging tests that may be helpful in distinguishing it from its mimics. The diseases most commonly confused with early IPD will also be discussed with an emphasis on the ways they most commonly differ from IPD. We will begin our discussion with the presenting signs and symptoms of IPD.
Idiopathic Parkinson’s Disease
IPD typically has a subtle and insidious onset with characteristic features developing over months to years. IPD most often presents in patients after age 60, and age is the most consistent risk factor for developing IPD; however, approximately 5% of IPD cases begin before age 40 years. These young-onset cases are likely to be caused by genetic mutations [6]. The widely recognized cardinal motor features of IPD include asymmetric resting tremor, rigidity, bradykinesia and postural instability [7]. Asymmetry is a key feature, as symptoms typically start on one side and remain more prominent on that side as the disease progresses. In fact, lack of asymmetry suggests an alternative diagnosis. Of the cardinal motor features, tremor is most often reported by patients as the first symptom [8]. However, IPD can alternately present with various other motor or even nonmotor complaints that will be discussed later.
Motor Features
Resting tremor is the most common presenting sign/symptom of early IPD, found in approximately 70% of patients [8]. The tremor typically is asymmetric and intermittent at onset, often starting in one hand. It is sometimes, though not necessarily, described as a “pill-rolling” rhythmic movement of the thumb and first finger while the hand is at rest. Patients will usually report a worsening of tremor with stress, anxiety, and increased fatigue. The tremor does not persist during sleep and diminishes with voluntary activity of the affected limb(s). By having the patient perform mentally challenging tasks (such as counting backwards) or motor movements of other body parts (such as finger tapping with the other hand or walking), the examiner may notice an increase in tremor amplitude [11]. There may also be a resting tremor of the lip or lower jaw, but true head tremor suggests an alternate diagnosis such as essential tremor [12]. Postural tremor can co-exist with resting tremor in IPD, which often leads to diagnostic confusion, especially when the postural tremor is more prominent than the resting tremor. In this scenario, the distinction between IPD and essential tremor (discussed later) can become more difficult.
Rigidity is characterized as the presence of increased resistance to passive stretch throughout the range of motion [13]. “Lead pipe” rigidity remains sustained throughout the motion of the joint, while “cogwheel” rigidity is intermittent through the movement. The examiner must take care to distinguish between true rigidity and other forms of increased tone such as spasticity (a velocity dependent increase in tone) and paratonia (a resistance to passive motion created by the patient). Subtle rigidity can be enhanced in a limb by having the patient perform a voluntary movement of the contralateral limb [14]. Rigidity in early IPD is also asymmetric and most commonly found in the upper extremities, but it can be seen in the neck and lower extremities as well. Patients may initially complain of shoulder pain and stiffness that is diagnosed as rotator cuff disease or arthritis, when this pain is actually due to rigidity from Parkinson’s disease [15]. Severe axial rigidity out of proportion to appendicular rigidity, however, should suggest an alternate diagnosis in the early stages of the disease (such as progressive supranuclear palsy which is further discussed below).
Bradykinesia refers to decreased amplitude and speed of voluntary motor movements. This sign can be found throughout the body in the form of hypometric saccades, decreased blink rate, decreased facial expressions (“masked facies”) and softening of speech (hypophonia) [16]. Patients may initially report a general slowing down of movements as well as difficulty with handwriting due to their writing becoming smaller (micrographia) [17]. Bradykinesia is evaluated by testing the speed, amplitude, and rhythmicity of voluntary movements such as repetitive tapping of the thumb and first finger together, alternation of supination and pronation of the forearm and hand, opening and closing the hand and tapping the foot rhythmically on the floor. The examiner should also evaluate for generalized bradykinesia by viewing the patient rise from a seated to standing position as well as observing the patient’s normal speed of ambulation and speed and symmetry of arm swing.
Gait disturbance and postural instability can sometimes be found in early IPD; however, significant impairment of postural reflexes, gait impairment and early falls may point to a diagnosis other than IPD. Early IPD postural changes include mild flexion of the neck or trunk that may be accompanied by a slight leaning to one side. On examination of natural gait, the patient may exhibit asymmetrically reduced arm swing, slowing of gait and turning, shortened stride length and intermittent shuffling of the feet. With disease progression, all of these become more severe and there may be festination of gait (“hurried” gate with increased cadence and difficulty stopping). This can lead to instability and falls as the patient’s center of balance is displaced forward. Freezing of gait can also develop, but is rarely found in early IPD [18]. Postural stability is evaluated by the “pull test” where the patient is asked to stand in a comfortable stance with eyes open and feet apart and instructed to resist falling backwards when pulled by the examiner. The patient is allowed to take one step backwards with either foot if necessary to prevent falling. This test is usually normal in early IPD, but it often becomes abnormal with disease progression.
Because of dramatic heterogeneity in the expression of these cardinal motor features in IPD, patients are often subcategorized based upon the most prominent features of their motor exam. Well-recognized motor subtypes include tremor-predominant, akinetic-rigid, postural instability gait disorder PD (PIGD), and mixed [19]. Tremor-predominant patients are those with significant tremors that overshadow the other motor features of the disease, while akinetic-rigid patients have prominent bradykinesia and rigidity with little to no tremor. PIGD patients have prominent postural and gait abnormalities, while mixed patients have roughly equal amounts of all of the cardinal motor features. Recent research has suggested that these motor subtypes differ with regard to the frequency of comorbid nonmotor features, disease prognosis, and response to certain treatments [20–22]. For example, tremor-predominant patients generally have a good prognosis with slow disease progression while PIGD patients have a poor prognosis with rapid progression, dementia, and depression [19].
Nonmotor Symptoms
Along with the classic motor features of IPD, patients often suffer from a variety of nonmotor symptoms that can sometimes precede the onset of motor symptoms by several years [23]. When nonmotor symptoms are the presenting symptoms, diagnosis is often delayed at 1.6 years versus 1.0 year for individuals with motor presentations [2]. Recognition of a nonmotor prodrome of PD has instigated a debate about whether new diagnostic criteria for early-stage and prodromal PD should be created [24]; for now, however, a diagnosis of PD still requires the motor syndrome. The spectrum of nonmotor symptoms in IPD can include olfactory dysfunction, urinary dysfunction, constipation, depression, anxiety, apathy, cognitive decline, sleep disorders such as REM (rapid eye movement) sleep behavior disorder and restless legs syndrome, fatigue and orthostatic hypotension. While many of these nonmotor symptoms are common in the general population and are certainly not specific to IPD, their presence in conjunction with early parkinsonism can help further support an IPD diagnosis.
Patients with IPD should exhibit a robust and sustained response to levodopa therapy. Over time, as the degenerative disease progresses, doses need to be increased and complications of therapy are likely to emerge, most commonly levodopa-induced dyskinesia, motor and nonmotor fluctuations [25]. The various forms of parkinsonism (discussed later) may have an initial response to levodopa therapy; however, this response is generally transient and wanes quickly despite increases in dose. Many will have no response at all.
Differential Diagnosis
The differential diagnosis for IPD most commonly includes the Parkinson-plus syndromes (dementia with Lewy bodies, multiple system atrophy, progressive supra-nuclear palsy, and corticobasal degeneration), vascular parkinsonism, drug-induced parkinsonism, dopa responsive dystonia, normal pressure hydrocephalus, and essential tremor. Each of these conditions will be discussed in further detail below.
Parkinson-Plus Syndromes
Dementia with Lewy bodies (DLB) may initially resemble IPD as it can present with parkinsonian motor signs, but the distinguishing feature of this disease is the presence of a progressive dementia with deficits in attention and executive function that occurs before or within 1 year of the development of parkinsonian motor signs [26]. This is in contrast to the dementia that can develop in IPD, which usually occurs many years into the disease course. Patients with DLB often have well-formed, visual hallucinations with this disorder. Motor parkinsonian symptoms do not improve with dopaminergic therapy and caution should be used with these patients as psychiatric symptoms may be exacerbated by even small doses of these medications [27]. Diagnostic criteria for probable DLB require the presence of dementia plus at least 2 of the following 3 core features: fluctuating attention and concentration, recurrent well-formed visual hallucinations, and spontaneous parkinsonian motor signs. Suggestive clinical features include REM behavior disorder, severe neuroleptic sensitivity, and low dopamine transporter uptake in the basal ganglia on SPECT or PET imaging. In the absence of 2 core features, the diagnosis of probable DLB can also be made if dementia plus at least 1 suggestive feature is present with just 1 core feature. Possible DLB can be diagnosed with the presence of dementia plus 1 core or suggestive feature. These criteria are 83% sensitive and 95% specific for the presence of neocortical Lewy bodies at autopsy [27]. Other supportive clinical features include repeated falls, syncope, transient loss of consciousness, severe autonomic dysfunction, depression, and systematized delusions or hallucinations in other sensory and perceptual modalities [27]. Definitive diagnosis requires pathological confirmation.
Corticobasal degeneration (CBD) is more rare than the previously described Parkinson-plus syndromes. CBD typically presents with a markedly unilateral/asymmetric motor features and can mimic early IPD, but other defining features include cortical signs of progressive unilateral apraxia, limb dystonia and visual-tactile neglect (“alien limb” sign) that can lead to loss of voluntary control of the extremity. This sign has been reported in approximately half of all patients with CBD [34]. As the disease progresses, cognitive decline, dementia, dysarthria, postural instability and gait dysfunction can all occur [35]. Patients with CBD typically do not show any response to dopaminergic therapy. CBD brain MRI findings include asymmetric cortical atrophy (most commonly in the superior parietal region), bilateral basal ganglia atrophy, corpus callosum atrophy and T2 hyperintensities of the subcortical white matter and posterolateral putamen [36]. In recently published consensus criteria, Armstrong et al broadened the clinical phenotype associated with CBD to acknowledge the spectrum and overlapping phenotypes of tau-related neurodegenerative diseases [37]. The criteria for probable corticobasal syndrome require asymmetric presentation of 2 of: (a) limb rigidity or akinesia, (b) limb dystonia, (c) limb myoclonus plus 2 of: (d) orobuccal or limb apraxia, (e) cortical sensory deficit, (f) alien limb phenomena (more than simple levitation). Possible corticobasal syndrome may be symmetric and requires 1 of: (a) limb rigidity or akinesia, (b) limb dystonia, (c) limb myoclonus plus 1 of: (d) orobuccal or limb apraxia, (e) cortical sensory deficit, (f) alien limb phenomena (more than simple levitation). Unfortunately, these new criteria have not improved the specificity of diagnosis compared to previous criteria as shown by a recent longitudinal clinical and neuropathological study that found that all of their patients with a cortiocobasal syndrome but without corticobasal pathology had all met the new diagnostic criteria for possible or probable CBD [38]. The reader should be aware that Armstrong et al acknowledged that memory dysfunction is common in CBD, although this was not incorporated into the diagnostic criteria.
Other Causes of Parkinsonism
Vascular parkinsonism results from the accumulation of multiple infarcts in the basal ganglia and/or subcortical white matter [39]. It may account for up to 12% of all cases of parkinsonism [40]. There are not any specific clinical diagnostic criteria for vascular parkinsonism; however, the clinical presentation is somewhat distinctive. Vascular parkinsonism initially presents with gait problems, and the upper extremities are less affected than the lower extremities. Vascular parkinsonism has been referred to as “lower body parkinsonism” due to this distribution of symptoms. Patients often present with a characteristic shuffling gait, but may also exhibit significant freezing of gait, even early in the course of the disease (in contrast to IPD). Tremor is reported less consistently and other pyramidal tract signs, urinary symptoms, dementia and pseudobulbar affect resulting from various ischemic lesions often co-exist [41]. Patients tend to have a history of cerebrovascular risk factors. Response to dopaminergic therapy is present in one-third to one-half of patients and is typically short-lived [42]. Brain MRI findings in vascular parkinsonism include diffuse subcortical white or gray matter lesions, particularly involving the globus pallidus, thalamus, substantia nigra and frontal lobes. One study reported a “cutoff” point to help differentiate between vascular parkinsonism and the normal vascular changes associated with aging at 0.6% lesioned volume of brain tissue [43]. It is important to remember that microvascular lesions are commonly seen on MRI scans of older patients and therefore the presence of these lesions on imaging does not necessarily convey a diagnosis of vascular parkinsonism.
Evaluation of any parkinsonian patient should involve careful scrutiny of the medication list (current and past) to exclude the possibility of drug-induced parkinsonism (DIP). DIP is typically, though not always, symmetric in onset. Drugs causing DIP include all of the typical and atypical antipsychotics, dopamine depleters such as reserpine and tetrabenazine, gastrointestinal drugs with dopamine receptor blocking activity such as antiemetics and metoclopramide, calcium channel blockers, valproic acid, selective serotonin reuptake inhibiters and lithium [44]. Traditionally this syndrome was thought to be reversible with discontinuation of the offending drug; however, resolution can require many months and at least 10% of patients with DIP develop persistent and progressive parkinsonism despite discontinuation of the drug [45].
Dopa-responsive dystonia (DRD) most typically presents in childhood with initial onset of lower limb dystonia with parkinsonism developing over time. Symptoms respond robustly to low doses of levodopa, hence the name DRD. Occasionally, however, DRD can present in adulthood. In adult-onset cases of DRD, parkinsonism usually develops before dystonia. Because it presents with parkinsonism and is levodopa responsive, adult-onset DRD can easily be confused with young-onset IPD [46]. Clues to the presence of DRD include diurnal fluctuation, stability of symptoms over time, and a normal DaTscan (discussed later) [46].
Other rare causes of parkinsonism include exposure to toxins (MPTP, manganese, carbon monoxide, methanol), metabolic disorders (hypoparathyroidism, hypothyroidism, acquired hepatocerebral degeneration), early-onset and genetic disorders (Wilson’s disease, juvenile Huntington’s disease, spinocerebellar ataxia types 2 and 3, and neurodegeneration with brain iron accumulation), infectious diseases, trauma, space-occupying brain lesions, autoimmune diseases (Sjogren’s syndrome) and paraneoplastic disorders [47–51]. Further discussion of these more rare causes parkinsonism is beyond the scope of this review; however, clinicians should always carefully consider the past medical, family, and social history, along with the review of systems, as these aspects of the patient history may point to one of these causes of parkinsonism.
Normal pressure hydrocephalus (NPH) refers to chronic communicating hydrocephalus with adult onset. The classic clinical triad of NPH includes cognitive impairment, urinary incontinence, and gait disturbance in the absence of signs of increased intracranial pressure such as papilledema. NPH can present with motor signs similar to those found in vascular parkinsonism, possibly due to the close proximity of basal ganglia structures to the ventricular system [52]. The gait of NPH typically shows a decrease in step height and foot clearance as well as a decrease in walking speed. This is often referred to as a “magnetic gait.” In contrast to Parkinson’s disease patients, the gait disturbance in NPH does not improve with visual cues or dopaminergic therapy [53]. Dementia also occurs early on in the course of NPH and is mostly characterized by apathy, forgetfulness, and impaired recall. Urinary incontinence and urgency is a later finding of the disease in contrast to IPD in which urinary dysfunction is often an early nonmotor symptom. MRI and CT scans of the brain reveal enlarged ventricles (out of proportion to surrounding cerebral atrophy if present) and should be followed by a diagnostic high volume lumbar puncture. Clinical improvement following lumbar puncture is supportive of the diagnosis of NPH and helps to identify patients who may benefit from ventriculoperitoneal shunting [54].
Essential tremor (ET) is characterized by postural and action tremors, rather than resting tremors, though some ET patients can have co-existing resting tremors. Though it is usually bilateral, it is often asymmetric, adding to the potential for diagnostic confusion with IPD. It typically has a higher frequency than the tremor of IPD. The absence of rigidity, bradykinesia, postural and gait disturbances and no response to dopaminergic therapy help distinguish it further from IPD [55]. There is phenotypic overlap between these two conditions and some patients with IPD have more postural tremor than rest tremor (or even postural tremor with no rest tremor), while some with long-standing essential tremor may go on to develop parkinsonism [56].
The Role of DaTscan in Diagnosing Early Parkinsonism
Final Thoughts
Despite the challenges involved, accurate and early diagnosis of IPD is essential for optimal patient education, counseling, and treatment. Careful attention to the initial presentation and examination may be all that is required for diagnosis in typical cases. In atypical cases, brain MRI to evaluate for other diseases or DaTscan may be helpful adjunctive tests. As research advances over the coming years, it is likely that additional imaging or fluid biomarkers will become available to assist us with the diagnosis of IPD (and related disorders) in the early stages. Until then, clinicians must remain highly vigilant in their efforts to make these often challenging clinical diagnoses.
Corresponding author: Leslie J. Cloud, MD, MSc, 6605 West Broad St., Ste. C, Richmond, VA 23230, [email protected].
Financial disclosures: None.
From the VA Medical Center (Dr. Lehosit) and the Parkinson’s and Movement Disorders Center, Virginia Commonwealth University (Dr. Cloud), Richmond, VA.
Abstract
- Objective: To provide an overview of the importance and challenges of accurate diagnosis of early idiopathic Parkinson’s disease and practical guidelines for clinicians.
- Methods: Review of the relevant literature.
- Results: Idiopathic Parkinson’s disease is a common neurodegenerative disorder causing a wide spectrum of motor and nonmotor symptoms. The cardinal motor features include resting tremors, bradykinesia, rigidity, and postural instability. The diagnosis is clinical, and ancillary laboratory or radiology tests are unnecessary in typical cases. Despite the use of validated diagnostic criteria, misdiagnosis is common, especially early in the disease process. This is largely due to the phenotypic heterogeneity in the idiopathic Parkinson’s disease population as well phenotypic overlapping with other diseases. The diseases most commonly confused with idiopathic Parkinson’s disease are the Parkinson-plus syndromes (dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration), vascular parkinsonism, drug-induced parkinsonism, dopa responsive dystonia, normal pressure hydrocephalus, and essential tremor. Since the diagnosis of these other diseases is also clinical, familiarity with their typical presentations and most current diagnostic criteria is helpful. Brain MRI can be helpful in diagnosing some of the diseases, though brain imaging is most commonly unremarkable in idiopathic Parkinson’s disease. DaTscan has an FDA indication to assist in the evaluation of adults with parkinsonian syndromes. It should not be used in typical cases but can be a useful adjunct to other diagnostic evaluations in atypical cases.
- Conclusion: Despite the challenges involved, accurate and early diagnosis of idiopathic Parkinson’s disease is essential for optimal patient education, counseling, and treatment.
Idiopathic Parkinson’s disease (IPD) is a common neurodenerative disease, affecting 1% of the population over the age of 65 [1]. A definitive diagnosis requires the postmortem findings of degeneration of the substantia nigra pars compacta and the presence of Lewy bodies (insoluble cytoplasmic inclusions composed of aggregated alpha-synuclein). In the later stages of the disease, a correct clinical diagnosis is made in more than 90% of patients [2]. Early on, however, clinical diagnosis is less reliable. For clinicians, distinguishing early IPD from other parkinsonian syndromes can be extraordinarily challenging because these conditions, especially in the earliest stages, present with highly variable yet overlapping phenotypes [3]. Furthermore, most of the diseases in the differential diagnosis, including IPD itself, are clinical diagnoses made on the basis of history and examination without the benefit of laboratory or radiology data. A high level of clinical acumen is therefore required for early and accurate diagnosis. Recent clinical trials in which subspecialists performed stringent diagnostic assessments to identify subjects with clinically diagnosed IPD later found that some subjects had normal functional dopamine imaging, suggesting that they probably did not have IPD [4,5]. These trials served to highlight the possibility of misdiagnosis, even in the hands of highly trained subspecialists. Early and accurate diagnosis is of paramount importance for many reasons. First, treatment approaches differ significantly across many of these diseases. Second, as neuroprotective interventions that are currently under investigation become available, long-term outcomes may significantly improve with earlier diagnosis and intervention. Third, some of these diseases are prognostically very different from one another, so accurate diagnosis enables better counseling and setting realistic expectations for progression.
This review will discuss the most common presenting signs and symptoms of early IPD, present the most widely used diagnostic criteria, and introduce the ancillary laboratory and imaging tests that may be helpful in distinguishing it from its mimics. The diseases most commonly confused with early IPD will also be discussed with an emphasis on the ways they most commonly differ from IPD. We will begin our discussion with the presenting signs and symptoms of IPD.
Idiopathic Parkinson’s Disease
IPD typically has a subtle and insidious onset with characteristic features developing over months to years. IPD most often presents in patients after age 60, and age is the most consistent risk factor for developing IPD; however, approximately 5% of IPD cases begin before age 40 years. These young-onset cases are likely to be caused by genetic mutations [6]. The widely recognized cardinal motor features of IPD include asymmetric resting tremor, rigidity, bradykinesia and postural instability [7]. Asymmetry is a key feature, as symptoms typically start on one side and remain more prominent on that side as the disease progresses. In fact, lack of asymmetry suggests an alternative diagnosis. Of the cardinal motor features, tremor is most often reported by patients as the first symptom [8]. However, IPD can alternately present with various other motor or even nonmotor complaints that will be discussed later.
Motor Features
Resting tremor is the most common presenting sign/symptom of early IPD, found in approximately 70% of patients [8]. The tremor typically is asymmetric and intermittent at onset, often starting in one hand. It is sometimes, though not necessarily, described as a “pill-rolling” rhythmic movement of the thumb and first finger while the hand is at rest. Patients will usually report a worsening of tremor with stress, anxiety, and increased fatigue. The tremor does not persist during sleep and diminishes with voluntary activity of the affected limb(s). By having the patient perform mentally challenging tasks (such as counting backwards) or motor movements of other body parts (such as finger tapping with the other hand or walking), the examiner may notice an increase in tremor amplitude [11]. There may also be a resting tremor of the lip or lower jaw, but true head tremor suggests an alternate diagnosis such as essential tremor [12]. Postural tremor can co-exist with resting tremor in IPD, which often leads to diagnostic confusion, especially when the postural tremor is more prominent than the resting tremor. In this scenario, the distinction between IPD and essential tremor (discussed later) can become more difficult.
Rigidity is characterized as the presence of increased resistance to passive stretch throughout the range of motion [13]. “Lead pipe” rigidity remains sustained throughout the motion of the joint, while “cogwheel” rigidity is intermittent through the movement. The examiner must take care to distinguish between true rigidity and other forms of increased tone such as spasticity (a velocity dependent increase in tone) and paratonia (a resistance to passive motion created by the patient). Subtle rigidity can be enhanced in a limb by having the patient perform a voluntary movement of the contralateral limb [14]. Rigidity in early IPD is also asymmetric and most commonly found in the upper extremities, but it can be seen in the neck and lower extremities as well. Patients may initially complain of shoulder pain and stiffness that is diagnosed as rotator cuff disease or arthritis, when this pain is actually due to rigidity from Parkinson’s disease [15]. Severe axial rigidity out of proportion to appendicular rigidity, however, should suggest an alternate diagnosis in the early stages of the disease (such as progressive supranuclear palsy which is further discussed below).
Bradykinesia refers to decreased amplitude and speed of voluntary motor movements. This sign can be found throughout the body in the form of hypometric saccades, decreased blink rate, decreased facial expressions (“masked facies”) and softening of speech (hypophonia) [16]. Patients may initially report a general slowing down of movements as well as difficulty with handwriting due to their writing becoming smaller (micrographia) [17]. Bradykinesia is evaluated by testing the speed, amplitude, and rhythmicity of voluntary movements such as repetitive tapping of the thumb and first finger together, alternation of supination and pronation of the forearm and hand, opening and closing the hand and tapping the foot rhythmically on the floor. The examiner should also evaluate for generalized bradykinesia by viewing the patient rise from a seated to standing position as well as observing the patient’s normal speed of ambulation and speed and symmetry of arm swing.
Gait disturbance and postural instability can sometimes be found in early IPD; however, significant impairment of postural reflexes, gait impairment and early falls may point to a diagnosis other than IPD. Early IPD postural changes include mild flexion of the neck or trunk that may be accompanied by a slight leaning to one side. On examination of natural gait, the patient may exhibit asymmetrically reduced arm swing, slowing of gait and turning, shortened stride length and intermittent shuffling of the feet. With disease progression, all of these become more severe and there may be festination of gait (“hurried” gate with increased cadence and difficulty stopping). This can lead to instability and falls as the patient’s center of balance is displaced forward. Freezing of gait can also develop, but is rarely found in early IPD [18]. Postural stability is evaluated by the “pull test” where the patient is asked to stand in a comfortable stance with eyes open and feet apart and instructed to resist falling backwards when pulled by the examiner. The patient is allowed to take one step backwards with either foot if necessary to prevent falling. This test is usually normal in early IPD, but it often becomes abnormal with disease progression.
Because of dramatic heterogeneity in the expression of these cardinal motor features in IPD, patients are often subcategorized based upon the most prominent features of their motor exam. Well-recognized motor subtypes include tremor-predominant, akinetic-rigid, postural instability gait disorder PD (PIGD), and mixed [19]. Tremor-predominant patients are those with significant tremors that overshadow the other motor features of the disease, while akinetic-rigid patients have prominent bradykinesia and rigidity with little to no tremor. PIGD patients have prominent postural and gait abnormalities, while mixed patients have roughly equal amounts of all of the cardinal motor features. Recent research has suggested that these motor subtypes differ with regard to the frequency of comorbid nonmotor features, disease prognosis, and response to certain treatments [20–22]. For example, tremor-predominant patients generally have a good prognosis with slow disease progression while PIGD patients have a poor prognosis with rapid progression, dementia, and depression [19].
Nonmotor Symptoms
Along with the classic motor features of IPD, patients often suffer from a variety of nonmotor symptoms that can sometimes precede the onset of motor symptoms by several years [23]. When nonmotor symptoms are the presenting symptoms, diagnosis is often delayed at 1.6 years versus 1.0 year for individuals with motor presentations [2]. Recognition of a nonmotor prodrome of PD has instigated a debate about whether new diagnostic criteria for early-stage and prodromal PD should be created [24]; for now, however, a diagnosis of PD still requires the motor syndrome. The spectrum of nonmotor symptoms in IPD can include olfactory dysfunction, urinary dysfunction, constipation, depression, anxiety, apathy, cognitive decline, sleep disorders such as REM (rapid eye movement) sleep behavior disorder and restless legs syndrome, fatigue and orthostatic hypotension. While many of these nonmotor symptoms are common in the general population and are certainly not specific to IPD, their presence in conjunction with early parkinsonism can help further support an IPD diagnosis.
Patients with IPD should exhibit a robust and sustained response to levodopa therapy. Over time, as the degenerative disease progresses, doses need to be increased and complications of therapy are likely to emerge, most commonly levodopa-induced dyskinesia, motor and nonmotor fluctuations [25]. The various forms of parkinsonism (discussed later) may have an initial response to levodopa therapy; however, this response is generally transient and wanes quickly despite increases in dose. Many will have no response at all.
Differential Diagnosis
The differential diagnosis for IPD most commonly includes the Parkinson-plus syndromes (dementia with Lewy bodies, multiple system atrophy, progressive supra-nuclear palsy, and corticobasal degeneration), vascular parkinsonism, drug-induced parkinsonism, dopa responsive dystonia, normal pressure hydrocephalus, and essential tremor. Each of these conditions will be discussed in further detail below.
Parkinson-Plus Syndromes
Dementia with Lewy bodies (DLB) may initially resemble IPD as it can present with parkinsonian motor signs, but the distinguishing feature of this disease is the presence of a progressive dementia with deficits in attention and executive function that occurs before or within 1 year of the development of parkinsonian motor signs [26]. This is in contrast to the dementia that can develop in IPD, which usually occurs many years into the disease course. Patients with DLB often have well-formed, visual hallucinations with this disorder. Motor parkinsonian symptoms do not improve with dopaminergic therapy and caution should be used with these patients as psychiatric symptoms may be exacerbated by even small doses of these medications [27]. Diagnostic criteria for probable DLB require the presence of dementia plus at least 2 of the following 3 core features: fluctuating attention and concentration, recurrent well-formed visual hallucinations, and spontaneous parkinsonian motor signs. Suggestive clinical features include REM behavior disorder, severe neuroleptic sensitivity, and low dopamine transporter uptake in the basal ganglia on SPECT or PET imaging. In the absence of 2 core features, the diagnosis of probable DLB can also be made if dementia plus at least 1 suggestive feature is present with just 1 core feature. Possible DLB can be diagnosed with the presence of dementia plus 1 core or suggestive feature. These criteria are 83% sensitive and 95% specific for the presence of neocortical Lewy bodies at autopsy [27]. Other supportive clinical features include repeated falls, syncope, transient loss of consciousness, severe autonomic dysfunction, depression, and systematized delusions or hallucinations in other sensory and perceptual modalities [27]. Definitive diagnosis requires pathological confirmation.
Corticobasal degeneration (CBD) is more rare than the previously described Parkinson-plus syndromes. CBD typically presents with a markedly unilateral/asymmetric motor features and can mimic early IPD, but other defining features include cortical signs of progressive unilateral apraxia, limb dystonia and visual-tactile neglect (“alien limb” sign) that can lead to loss of voluntary control of the extremity. This sign has been reported in approximately half of all patients with CBD [34]. As the disease progresses, cognitive decline, dementia, dysarthria, postural instability and gait dysfunction can all occur [35]. Patients with CBD typically do not show any response to dopaminergic therapy. CBD brain MRI findings include asymmetric cortical atrophy (most commonly in the superior parietal region), bilateral basal ganglia atrophy, corpus callosum atrophy and T2 hyperintensities of the subcortical white matter and posterolateral putamen [36]. In recently published consensus criteria, Armstrong et al broadened the clinical phenotype associated with CBD to acknowledge the spectrum and overlapping phenotypes of tau-related neurodegenerative diseases [37]. The criteria for probable corticobasal syndrome require asymmetric presentation of 2 of: (a) limb rigidity or akinesia, (b) limb dystonia, (c) limb myoclonus plus 2 of: (d) orobuccal or limb apraxia, (e) cortical sensory deficit, (f) alien limb phenomena (more than simple levitation). Possible corticobasal syndrome may be symmetric and requires 1 of: (a) limb rigidity or akinesia, (b) limb dystonia, (c) limb myoclonus plus 1 of: (d) orobuccal or limb apraxia, (e) cortical sensory deficit, (f) alien limb phenomena (more than simple levitation). Unfortunately, these new criteria have not improved the specificity of diagnosis compared to previous criteria as shown by a recent longitudinal clinical and neuropathological study that found that all of their patients with a cortiocobasal syndrome but without corticobasal pathology had all met the new diagnostic criteria for possible or probable CBD [38]. The reader should be aware that Armstrong et al acknowledged that memory dysfunction is common in CBD, although this was not incorporated into the diagnostic criteria.
Other Causes of Parkinsonism
Vascular parkinsonism results from the accumulation of multiple infarcts in the basal ganglia and/or subcortical white matter [39]. It may account for up to 12% of all cases of parkinsonism [40]. There are not any specific clinical diagnostic criteria for vascular parkinsonism; however, the clinical presentation is somewhat distinctive. Vascular parkinsonism initially presents with gait problems, and the upper extremities are less affected than the lower extremities. Vascular parkinsonism has been referred to as “lower body parkinsonism” due to this distribution of symptoms. Patients often present with a characteristic shuffling gait, but may also exhibit significant freezing of gait, even early in the course of the disease (in contrast to IPD). Tremor is reported less consistently and other pyramidal tract signs, urinary symptoms, dementia and pseudobulbar affect resulting from various ischemic lesions often co-exist [41]. Patients tend to have a history of cerebrovascular risk factors. Response to dopaminergic therapy is present in one-third to one-half of patients and is typically short-lived [42]. Brain MRI findings in vascular parkinsonism include diffuse subcortical white or gray matter lesions, particularly involving the globus pallidus, thalamus, substantia nigra and frontal lobes. One study reported a “cutoff” point to help differentiate between vascular parkinsonism and the normal vascular changes associated with aging at 0.6% lesioned volume of brain tissue [43]. It is important to remember that microvascular lesions are commonly seen on MRI scans of older patients and therefore the presence of these lesions on imaging does not necessarily convey a diagnosis of vascular parkinsonism.
Evaluation of any parkinsonian patient should involve careful scrutiny of the medication list (current and past) to exclude the possibility of drug-induced parkinsonism (DIP). DIP is typically, though not always, symmetric in onset. Drugs causing DIP include all of the typical and atypical antipsychotics, dopamine depleters such as reserpine and tetrabenazine, gastrointestinal drugs with dopamine receptor blocking activity such as antiemetics and metoclopramide, calcium channel blockers, valproic acid, selective serotonin reuptake inhibiters and lithium [44]. Traditionally this syndrome was thought to be reversible with discontinuation of the offending drug; however, resolution can require many months and at least 10% of patients with DIP develop persistent and progressive parkinsonism despite discontinuation of the drug [45].
Dopa-responsive dystonia (DRD) most typically presents in childhood with initial onset of lower limb dystonia with parkinsonism developing over time. Symptoms respond robustly to low doses of levodopa, hence the name DRD. Occasionally, however, DRD can present in adulthood. In adult-onset cases of DRD, parkinsonism usually develops before dystonia. Because it presents with parkinsonism and is levodopa responsive, adult-onset DRD can easily be confused with young-onset IPD [46]. Clues to the presence of DRD include diurnal fluctuation, stability of symptoms over time, and a normal DaTscan (discussed later) [46].
Other rare causes of parkinsonism include exposure to toxins (MPTP, manganese, carbon monoxide, methanol), metabolic disorders (hypoparathyroidism, hypothyroidism, acquired hepatocerebral degeneration), early-onset and genetic disorders (Wilson’s disease, juvenile Huntington’s disease, spinocerebellar ataxia types 2 and 3, and neurodegeneration with brain iron accumulation), infectious diseases, trauma, space-occupying brain lesions, autoimmune diseases (Sjogren’s syndrome) and paraneoplastic disorders [47–51]. Further discussion of these more rare causes parkinsonism is beyond the scope of this review; however, clinicians should always carefully consider the past medical, family, and social history, along with the review of systems, as these aspects of the patient history may point to one of these causes of parkinsonism.
Normal pressure hydrocephalus (NPH) refers to chronic communicating hydrocephalus with adult onset. The classic clinical triad of NPH includes cognitive impairment, urinary incontinence, and gait disturbance in the absence of signs of increased intracranial pressure such as papilledema. NPH can present with motor signs similar to those found in vascular parkinsonism, possibly due to the close proximity of basal ganglia structures to the ventricular system [52]. The gait of NPH typically shows a decrease in step height and foot clearance as well as a decrease in walking speed. This is often referred to as a “magnetic gait.” In contrast to Parkinson’s disease patients, the gait disturbance in NPH does not improve with visual cues or dopaminergic therapy [53]. Dementia also occurs early on in the course of NPH and is mostly characterized by apathy, forgetfulness, and impaired recall. Urinary incontinence and urgency is a later finding of the disease in contrast to IPD in which urinary dysfunction is often an early nonmotor symptom. MRI and CT scans of the brain reveal enlarged ventricles (out of proportion to surrounding cerebral atrophy if present) and should be followed by a diagnostic high volume lumbar puncture. Clinical improvement following lumbar puncture is supportive of the diagnosis of NPH and helps to identify patients who may benefit from ventriculoperitoneal shunting [54].
Essential tremor (ET) is characterized by postural and action tremors, rather than resting tremors, though some ET patients can have co-existing resting tremors. Though it is usually bilateral, it is often asymmetric, adding to the potential for diagnostic confusion with IPD. It typically has a higher frequency than the tremor of IPD. The absence of rigidity, bradykinesia, postural and gait disturbances and no response to dopaminergic therapy help distinguish it further from IPD [55]. There is phenotypic overlap between these two conditions and some patients with IPD have more postural tremor than rest tremor (or even postural tremor with no rest tremor), while some with long-standing essential tremor may go on to develop parkinsonism [56].
The Role of DaTscan in Diagnosing Early Parkinsonism
Final Thoughts
Despite the challenges involved, accurate and early diagnosis of IPD is essential for optimal patient education, counseling, and treatment. Careful attention to the initial presentation and examination may be all that is required for diagnosis in typical cases. In atypical cases, brain MRI to evaluate for other diseases or DaTscan may be helpful adjunctive tests. As research advances over the coming years, it is likely that additional imaging or fluid biomarkers will become available to assist us with the diagnosis of IPD (and related disorders) in the early stages. Until then, clinicians must remain highly vigilant in their efforts to make these often challenging clinical diagnoses.
Corresponding author: Leslie J. Cloud, MD, MSc, 6605 West Broad St., Ste. C, Richmond, VA 23230, [email protected].
Financial disclosures: None.
1. Wirdefeldt K, Adami HO, Cole P, et al. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 2011;26 Suppl 1:S1–58.
2. O’Sullivan SS, Williams DR, Gallagher DA, et al. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord 2008;23:101–6.
3. Ali K, Morris HR. Parkinson’s disease: chameleons and mimics. Pract Neurol 2015;15:14–25.
4. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61:1044–53.
5. Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 2003;54:93–101.
6. Wickremaratchi MM, Ben-Shlomo Y, Morris HR. The effect of onset age on the clinical features of Parkinson’s disease. Eur J Neurol 2009;16:450–6.
7. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–9.
8. Rajput AH, Rozdilsky B, Ang L. Occurrence of resting tremor in Parkinson’s disease. Neurology 1991;41:1298–9.
9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4.
10. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–7.
11. Raethjen J, Austermann K, Witt K, et al. Provocation of Parkinsonian tremor. Mov Disord 2008;23:1019–23.
12. Roze E, Coêlho-Braga MC, Gayraud D, et al. Head tremor in Parkinson’s disease. Mov Disord 2006;21:1245–8.
13. Hallett M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol 2003;91:19–28.
14. Broussolle E, Krack P, Thobois S, et al. Contribution of Jules Froment to the study of parkinsonian rigidity. Mov Disord 2007;22:909–14.
15. Riley D, Lang AE, Blair RD, et al. Frozen shoulder and other shoulder disturbances in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1989;52:63–6.
16. Rottach KG, Riley DE, DiScenna AO, et al. Dynamic properties of horizontal and vertical eye movements in parkinsonian syndromes. Ann Neurol 1996;39:368–77.
17. Cooper JA, Sagar HJ, Tidswell P, Jordan N. Slowed central processing in simple and go/no-go reaction time tasks in Parkinson’s disease. Brain 1994;117(Pt 3):517–29.
18. Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010;81:513–8.
19. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurology 2014;71:499–504.
20. Burn DJ, Rowan EN, Allan LM, et al. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–9.
21. Burn DJ, Landau S, Hindle JV, et al; PROMS-PD Study Group. Parkinson’s disease motor subtypes and mood. Mov Disord 2012;27:379–86.
22. Katz M, Luciano MS, Carlson K, et al; CSP 468 study group. Differential effects of deep brain stimulation target on motor subtypes in Parkinson’s disease. Ann Neurol 2015;77:710–9.
23. Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol 2010;67:798–801.
24. Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 2014;29:454–62.
25. Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord 2015;30:80–9.
26. Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord 2005;20 Suppl 12:S11–20.
27. Karantzoulis S, Galvin JE. Update on dementia with Lewy bodies. Curr Transl Geriatr Exp Gerontol Rep 2013;2:196–204.
28. Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–8.
29. Kim HJ, Jeon BS, Jellinger KA. Diagnosis and differential diagnosis of MSA: boundary issues. J Neurol 2015 Feb 7. [Epub ahead of print]
30. Lee EA, Cho HI, Kim SS, Lee WY. Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat Disord 2004;10:363–8.
31. Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology 1988;38:1031–4.
32. Maher ER, Lees AJ. The clinical features and natural history of the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1986;36:1005–8.
33. Gröschel K, Kastrup A, Litvan I, Schulz JB. Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology 2006;66:949–50.
34. Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain 1994117(Pt 5):1183–96.
35. Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999;53:1969–74.
36. Tokumaru AM, O’uchi T, Kuru Y, et al. Corticobasal degeneration: MR with histopathologic comparison. AJNR Am J Neuroradiol 1996;17:1849–52.
37. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503.
38. Alexander SK, Rittman T, Xuereb JH, et al. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 2014;85:925–9.
39. Sibon I, Fenelon G, Quinn NP, Tison F. Vascular parkinsonism. J Neurol 2004;251:513–24.
40. Thanvi B, Lo N, Robinson T. Vascular parkinsonism--an important cause of parkinsonism in older people. Age Ageing 2005;34:114–9.
41. Kalra S, Grosset DG, Benamer HT. Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: a systematic review. Mov Disord 2010;25:149–56.
42. Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol 2013; 12:597–608.
43. Josephs KA. Frontotemporal lobar degeneration. Neurol Clin 2007;25:683–96, vi.
44. Lopez-Sendon J, Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf 2013;12:487–96.
45. Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf 2006;5:759–71.
46. Brajkovic LD, Svetel MV, Kostic VS, et al. Dopamine transporter imaging (123)I-FP-CIT (DaTSCAN) SPET in differential diagnosis of dopa-responsive dystonia and young-onset Parkinson’s disease. Hell J Nucl Med 2012;15:134–8.
47. Krusz JC, Koller WC, Ziegler DK. Historical review: abnormal movements associated with epidemic encephalitis lethargica. Mov Disord 1987;2:137–41.
48. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983;219:979–80.
49. Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology 2005;64:2021–8.
50. Jankovic J, Kirkpatrick JB, Blomquist KA, et al. Late-onset Hallervorden-Spatz disease presenting as familial parkinsonism. Neurology 1985;35:227–34.
51. Cloud L, Jankovic J. Systemic disease and movement disorders. In: Burn DJ, editor. Oxford textbook of clinical neurology on movement disorders. Oxford University Press; 2013.
52. Bugalho P, Guimaraes J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord 2007;13:434–7.
53. Jankovic J, Newmark M, Peter P. Parkinsonism and acquired hydrocephalus. Mov Disord 1986;1:59–64.
54. Bergsneider M, Black PM, Klinge P, et al. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 Suppl): S29-39; discussion ii-v.
55. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology 2000; 54(11 Suppl 4): S7.
56. Jankovic J. Essential tremor and Parkinson’s disease. Ann Neurol 1989;25:211–2.
57. Catafau AM, Tolosa E; DaTSCAN Clinically Uncertain Parkinsonian Syndromes Study Group. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord 2004;19:1175–82.
58. Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 2013;84:1288–95.
59. Kagi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 2010;81:5–12.
60. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9.
1. Wirdefeldt K, Adami HO, Cole P, et al. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 2011;26 Suppl 1:S1–58.
2. O’Sullivan SS, Williams DR, Gallagher DA, et al. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord 2008;23:101–6.
3. Ali K, Morris HR. Parkinson’s disease: chameleons and mimics. Pract Neurol 2015;15:14–25.
4. Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004;61:1044–53.
5. Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol 2003;54:93–101.
6. Wickremaratchi MM, Ben-Shlomo Y, Morris HR. The effect of onset age on the clinical features of Parkinson’s disease. Eur J Neurol 2009;16:450–6.
7. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–9.
8. Rajput AH, Rozdilsky B, Ang L. Occurrence of resting tremor in Parkinson’s disease. Neurology 1991;41:1298–9.
9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4.
10. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–7.
11. Raethjen J, Austermann K, Witt K, et al. Provocation of Parkinsonian tremor. Mov Disord 2008;23:1019–23.
12. Roze E, Coêlho-Braga MC, Gayraud D, et al. Head tremor in Parkinson’s disease. Mov Disord 2006;21:1245–8.
13. Hallett M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol 2003;91:19–28.
14. Broussolle E, Krack P, Thobois S, et al. Contribution of Jules Froment to the study of parkinsonian rigidity. Mov Disord 2007;22:909–14.
15. Riley D, Lang AE, Blair RD, et al. Frozen shoulder and other shoulder disturbances in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1989;52:63–6.
16. Rottach KG, Riley DE, DiScenna AO, et al. Dynamic properties of horizontal and vertical eye movements in parkinsonian syndromes. Ann Neurol 1996;39:368–77.
17. Cooper JA, Sagar HJ, Tidswell P, Jordan N. Slowed central processing in simple and go/no-go reaction time tasks in Parkinson’s disease. Brain 1994;117(Pt 3):517–29.
18. Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010;81:513–8.
19. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurology 2014;71:499–504.
20. Burn DJ, Rowan EN, Allan LM, et al. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585–9.
21. Burn DJ, Landau S, Hindle JV, et al; PROMS-PD Study Group. Parkinson’s disease motor subtypes and mood. Mov Disord 2012;27:379–86.
22. Katz M, Luciano MS, Carlson K, et al; CSP 468 study group. Differential effects of deep brain stimulation target on motor subtypes in Parkinson’s disease. Ann Neurol 2015;77:710–9.
23. Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol 2010;67:798–801.
24. Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 2014;29:454–62.
25. Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord 2015;30:80–9.
26. Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord 2005;20 Suppl 12:S11–20.
27. Karantzoulis S, Galvin JE. Update on dementia with Lewy bodies. Curr Transl Geriatr Exp Gerontol Rep 2013;2:196–204.
28. Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–8.
29. Kim HJ, Jeon BS, Jellinger KA. Diagnosis and differential diagnosis of MSA: boundary issues. J Neurol 2015 Feb 7. [Epub ahead of print]
30. Lee EA, Cho HI, Kim SS, Lee WY. Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat Disord 2004;10:363–8.
31. Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology 1988;38:1031–4.
32. Maher ER, Lees AJ. The clinical features and natural history of the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1986;36:1005–8.
33. Gröschel K, Kastrup A, Litvan I, Schulz JB. Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology 2006;66:949–50.
34. Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain 1994117(Pt 5):1183–96.
35. Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999;53:1969–74.
36. Tokumaru AM, O’uchi T, Kuru Y, et al. Corticobasal degeneration: MR with histopathologic comparison. AJNR Am J Neuroradiol 1996;17:1849–52.
37. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503.
38. Alexander SK, Rittman T, Xuereb JH, et al. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 2014;85:925–9.
39. Sibon I, Fenelon G, Quinn NP, Tison F. Vascular parkinsonism. J Neurol 2004;251:513–24.
40. Thanvi B, Lo N, Robinson T. Vascular parkinsonism--an important cause of parkinsonism in older people. Age Ageing 2005;34:114–9.
41. Kalra S, Grosset DG, Benamer HT. Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: a systematic review. Mov Disord 2010;25:149–56.
42. Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol 2013; 12:597–608.
43. Josephs KA. Frontotemporal lobar degeneration. Neurol Clin 2007;25:683–96, vi.
44. Lopez-Sendon J, Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf 2013;12:487–96.
45. Mena MA, de Yebenes JG. Drug-induced parkinsonism. Expert Opin Drug Saf 2006;5:759–71.
46. Brajkovic LD, Svetel MV, Kostic VS, et al. Dopamine transporter imaging (123)I-FP-CIT (DaTSCAN) SPET in differential diagnosis of dopa-responsive dystonia and young-onset Parkinson’s disease. Hell J Nucl Med 2012;15:134–8.
47. Krusz JC, Koller WC, Ziegler DK. Historical review: abnormal movements associated with epidemic encephalitis lethargica. Mov Disord 1987;2:137–41.
48. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983;219:979–80.
49. Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology 2005;64:2021–8.
50. Jankovic J, Kirkpatrick JB, Blomquist KA, et al. Late-onset Hallervorden-Spatz disease presenting as familial parkinsonism. Neurology 1985;35:227–34.
51. Cloud L, Jankovic J. Systemic disease and movement disorders. In: Burn DJ, editor. Oxford textbook of clinical neurology on movement disorders. Oxford University Press; 2013.
52. Bugalho P, Guimaraes J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat Disord 2007;13:434–7.
53. Jankovic J, Newmark M, Peter P. Parkinsonism and acquired hydrocephalus. Mov Disord 1986;1:59–64.
54. Bergsneider M, Black PM, Klinge P, et al. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57(3 Suppl): S29-39; discussion ii-v.
55. Bain P, Brin M, Deuschl G, et al. Criteria for the diagnosis of essential tremor. Neurology 2000; 54(11 Suppl 4): S7.
56. Jankovic J. Essential tremor and Parkinson’s disease. Ann Neurol 1989;25:211–2.
57. Catafau AM, Tolosa E; DaTSCAN Clinically Uncertain Parkinsonian Syndromes Study Group. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord 2004;19:1175–82.
58. Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 2013;84:1288–95.
59. Kagi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 2010;81:5–12.
60. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9.
Exercise & Diet: The Latest
SEDENTARY TIME INCREASES HEALTH RISKS
Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123-132. doi: 10.7326/M14-1651.
The more time a person spends sedentary, regardless of physical activity, the greater the risk for deleterious health outcomes, a systematic review and meta-analysis of more than 1.5 million patients found.
Researchers reviewed 47 studies on the association between sedentary time and hospitalizations, all-cause mortality, cardiovascular disease, diabetes, and cancer in adults, and found that sedentary time was independently associated with the following negative health outcomes:
• All-cause mortality (hazard ratio [HR], 1.24)
• Cardiovascular disease mortality (HR, 1.18)
• Cardiovascular disease incidence (HR, 1.14)
• Cancer mortality (HR, 1.17)
• Cancer incidence (HR, 1.13)
• Type 2 diabetes incidence (HR, 1.91)
Sedentary time was assessed as either daily overall sedentary time, sitting time, television or screen time, or leisure time spent sitting. High sedentary time was defined as a range from five or more hours per day watching television to 11 hours a day total sedentary time, depending on the study.
DOES EXERCISE INTENSITY IMPACT ABDOMINAL OBESITY?
Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med. 2015;162(5):325-334. doi: 10.7326/M14-1189.
Exercise, regardless of the amount or intensity, produces similar reductions in abdominal obesity, but only high-amount, high-intensity exercise showed improvements in two-hour glucose readings, according to a 24-week trial of 300 abdominally obese adults.
Subjects were split into a control group and three groups told to exercise five times per week at varying amounts and intensity based on maximum oxygen capacity (VO2 peak)—low-amount, low-intensity (LALI); high-amount, low-intensity (HALI); and high-amount, high-intensity (HAHI)—with the following results (see Table).
CHOOSE FRUITS AND VEGETABLES FOR LONGEVITY
Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490.
Eating at least five servings of fruits and vegetables daily is associated with a reduced risk for mortality from all causes and cardiovascular disease, but not cancer, a meta-analysis of studies published in BMJ reports.
Researchers analyzed 16 prospective cohort studies from the United States, Europe, and Asia that reviewed the effects of fruit and vegetable consumption on mortality from all causes, cardiovascular disease, and cancer. The studies involved a combined total of 833,234 subjects and a follow-up period ranging from 4.6 to 26 years and showed
• Risk for all-cause mortality decreased by 5% per serving per day, up to five servings a day.
• Risk for cardiovascular mortality decreased by 4% per serving, per day, up to five servings a day.
• Risk for cancer mortality was not appreciably associated with increased fruit or vegetable consumption.
• Fruit provided the greatest reduction in risk for all-cause mortality at 6% per serving and cardiovascular mortality at 5% per serving. Vegetables reduced risks by 5% and 4%, respectively.
“This meta-analysis provides further evidence that higher consumption of fruits and vegetables is associated with a lower risk for mortality from all causes, particularly from cardiovascular diseases,” the authors conclude. “The results support current recommendations to increase consumption to promote health and overall longevity.”
SEDENTARY TIME INCREASES HEALTH RISKS
Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123-132. doi: 10.7326/M14-1651.
The more time a person spends sedentary, regardless of physical activity, the greater the risk for deleterious health outcomes, a systematic review and meta-analysis of more than 1.5 million patients found.
Researchers reviewed 47 studies on the association between sedentary time and hospitalizations, all-cause mortality, cardiovascular disease, diabetes, and cancer in adults, and found that sedentary time was independently associated with the following negative health outcomes:
• All-cause mortality (hazard ratio [HR], 1.24)
• Cardiovascular disease mortality (HR, 1.18)
• Cardiovascular disease incidence (HR, 1.14)
• Cancer mortality (HR, 1.17)
• Cancer incidence (HR, 1.13)
• Type 2 diabetes incidence (HR, 1.91)
Sedentary time was assessed as either daily overall sedentary time, sitting time, television or screen time, or leisure time spent sitting. High sedentary time was defined as a range from five or more hours per day watching television to 11 hours a day total sedentary time, depending on the study.
DOES EXERCISE INTENSITY IMPACT ABDOMINAL OBESITY?
Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med. 2015;162(5):325-334. doi: 10.7326/M14-1189.
Exercise, regardless of the amount or intensity, produces similar reductions in abdominal obesity, but only high-amount, high-intensity exercise showed improvements in two-hour glucose readings, according to a 24-week trial of 300 abdominally obese adults.
Subjects were split into a control group and three groups told to exercise five times per week at varying amounts and intensity based on maximum oxygen capacity (VO2 peak)—low-amount, low-intensity (LALI); high-amount, low-intensity (HALI); and high-amount, high-intensity (HAHI)—with the following results (see Table).
CHOOSE FRUITS AND VEGETABLES FOR LONGEVITY
Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490.
Eating at least five servings of fruits and vegetables daily is associated with a reduced risk for mortality from all causes and cardiovascular disease, but not cancer, a meta-analysis of studies published in BMJ reports.
Researchers analyzed 16 prospective cohort studies from the United States, Europe, and Asia that reviewed the effects of fruit and vegetable consumption on mortality from all causes, cardiovascular disease, and cancer. The studies involved a combined total of 833,234 subjects and a follow-up period ranging from 4.6 to 26 years and showed
• Risk for all-cause mortality decreased by 5% per serving per day, up to five servings a day.
• Risk for cardiovascular mortality decreased by 4% per serving, per day, up to five servings a day.
• Risk for cancer mortality was not appreciably associated with increased fruit or vegetable consumption.
• Fruit provided the greatest reduction in risk for all-cause mortality at 6% per serving and cardiovascular mortality at 5% per serving. Vegetables reduced risks by 5% and 4%, respectively.
“This meta-analysis provides further evidence that higher consumption of fruits and vegetables is associated with a lower risk for mortality from all causes, particularly from cardiovascular diseases,” the authors conclude. “The results support current recommendations to increase consumption to promote health and overall longevity.”
SEDENTARY TIME INCREASES HEALTH RISKS
Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123-132. doi: 10.7326/M14-1651.
The more time a person spends sedentary, regardless of physical activity, the greater the risk for deleterious health outcomes, a systematic review and meta-analysis of more than 1.5 million patients found.
Researchers reviewed 47 studies on the association between sedentary time and hospitalizations, all-cause mortality, cardiovascular disease, diabetes, and cancer in adults, and found that sedentary time was independently associated with the following negative health outcomes:
• All-cause mortality (hazard ratio [HR], 1.24)
• Cardiovascular disease mortality (HR, 1.18)
• Cardiovascular disease incidence (HR, 1.14)
• Cancer mortality (HR, 1.17)
• Cancer incidence (HR, 1.13)
• Type 2 diabetes incidence (HR, 1.91)
Sedentary time was assessed as either daily overall sedentary time, sitting time, television or screen time, or leisure time spent sitting. High sedentary time was defined as a range from five or more hours per day watching television to 11 hours a day total sedentary time, depending on the study.
DOES EXERCISE INTENSITY IMPACT ABDOMINAL OBESITY?
Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med. 2015;162(5):325-334. doi: 10.7326/M14-1189.
Exercise, regardless of the amount or intensity, produces similar reductions in abdominal obesity, but only high-amount, high-intensity exercise showed improvements in two-hour glucose readings, according to a 24-week trial of 300 abdominally obese adults.
Subjects were split into a control group and three groups told to exercise five times per week at varying amounts and intensity based on maximum oxygen capacity (VO2 peak)—low-amount, low-intensity (LALI); high-amount, low-intensity (HALI); and high-amount, high-intensity (HAHI)—with the following results (see Table).
CHOOSE FRUITS AND VEGETABLES FOR LONGEVITY
Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490.
Eating at least five servings of fruits and vegetables daily is associated with a reduced risk for mortality from all causes and cardiovascular disease, but not cancer, a meta-analysis of studies published in BMJ reports.
Researchers analyzed 16 prospective cohort studies from the United States, Europe, and Asia that reviewed the effects of fruit and vegetable consumption on mortality from all causes, cardiovascular disease, and cancer. The studies involved a combined total of 833,234 subjects and a follow-up period ranging from 4.6 to 26 years and showed
• Risk for all-cause mortality decreased by 5% per serving per day, up to five servings a day.
• Risk for cardiovascular mortality decreased by 4% per serving, per day, up to five servings a day.
• Risk for cancer mortality was not appreciably associated with increased fruit or vegetable consumption.
• Fruit provided the greatest reduction in risk for all-cause mortality at 6% per serving and cardiovascular mortality at 5% per serving. Vegetables reduced risks by 5% and 4%, respectively.
“This meta-analysis provides further evidence that higher consumption of fruits and vegetables is associated with a lower risk for mortality from all causes, particularly from cardiovascular diseases,” the authors conclude. “The results support current recommendations to increase consumption to promote health and overall longevity.”