User login
Update on Management of Barrett’s Esophagus for Primary Care Providers
From the Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX.
Abstract
- Objective: To provide an update on management of Barrett’s esophagus.
- Methods: Review of the literature.
- Results: Management of Barrett’s esophagus depends on the degree of dysplasia. Surveillance by endoscopy every 3–5 years is recommended in patients with Barrett’s esophagus without dysplasia. Patients with Barrett’s esophagus and low-grade dysplasia should undergo surveillance by endoscopy in 3 months for confirmation of the diagnosis; if the diagnosis is confirmed then surveillance by endoscopy or eradication of Barrett’s epithelium by ablation or endoscopic resection are recommended. There is a sufficient evidence to recommend radiofrequency ablation of high-grade dysplasia within Barrett’s esophagus or to perform endoscopic mucosal resection of nodular Barrett’s esophagus with any degree of dysplasia. Early esophageal cancers that are limited to the mucosa can be treated by endoscopic resection, while cancer invading into the deep submucosa or muscularis propria may need esophagectomy with or without chemoradiation.
- Conclusion: The management of Barrett’s esophagus depends on the degree of dysplasia. Radiofrequency ablation and endoscopic mucosal resection are the most commonly used treatment for Barrett’s esophagus with dysplasia.
Keywords: Barrett’s esophagus; radiofrequency ablation; endoscopic submucosal dissection; endoscopic mucosal resection; early esophageal cancer.
Barrett’s esophagus is a common complication of chronic reflux disease [1]. Metaplastic changes that occur at the distal esophageal epithelium are usually asymptomatic [2,3] and occur as reparative adaptations to the insult of the gastric acid [4]. The management of Barrett’s esophagus after diagnosis is currently debated amongst experts without a clear consensus [5,6]. This review is generally consistent with the 2016 guidelines from the American College of Gastroenterology [1], a 2012 guideline from the American Society of Gastrointestinal Endoscopy [7], a 2011 guideline, and a 2016 expert review from the American Gastroenterological Association [8,9].
D efinition
Barrett’s esophagus is a metaplasia of the stratified squamous epithelium to a specialized columnar intestinal epithelium of mucus cells and goblet cells at the distal esophagus secondary to gastroesophageal reflux disease (GERD) [7,10]. Barrett’s esophagus is unstable tissue which can progress to esophageal adenocarcinoma. When unmanaged, the risk of cancer in dysplastic mucosa is at least thirty-fold greater than that for the general population [11–14], with recent studies suggesting a 0.4–0.7 occurrence rate per year [11,15]. With no dysplasia, the risk is low [16].
Epidemiology
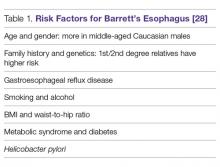
The prevalence of Barrett’s esophagus is ~10% in patients with GERD [11–13,17], an estimate tied to the prevalence of GERD. However, due to the lack of symptoms of Barrett’s esophagus, no solid data supports this assumption [18–20]. A European study estimated the prevalence of Barrett’s esophagus to be 1.6% among the general population [21,22]. Barrett’s esophagus is usually diagnosed during endoscopic examinations of middle-aged and older adults, with the mean age being 55 years of age. It is most commonly found in Caucasian males and associated with the use of smoking tobacco. The male-to-female ratio is approximately 2:1 [1] and it appears to be uncommon in African Americans [23,24]. Abdominal obesity as measured by an increased waist-to-hip ratio is associated with an increased risk of Barrett’s esophagus [25,26]. Germline mutations in the MSR1, ASCC1, and CTHRC1 genes have been associated with the presence of Barrett’s esophagus and esophageal adenocarcinoma [27]. Risk factors are listed in Table 1.
Clinical Symptoms
Columnar metaplasia itself does not cause any symptoms but is merely the adaptation of the cells to the repeated effect of the acid. The main clinical symptoms of the disease would initially be symptoms associated with GERD, such as heartburn, water brash, and dysphagia [1]. Severe presentations of GERD, such as esophageal ulceration, stricture, and hemorrhage, usually occur with long-segment Barrett’s esophagus [29,30]. However, 40% of patients presenting with adenocarcinoma had no history of GERD or symptoms of heartburn [13]. Furthermore, as few as 5% of those presenting with adenocarcinoma were known to have Barrett’s esophagus [31].
D iagnosis
Barrett’s esophagus generally requires an endoscopic examination with biopsy confirmation from the distal esophagus showing specialized intestinal columnar epithelium [32]. The biopsy specimen is acquired from the cellular lining proximal to gastroesophageal junction [5,33]. Barrett’s esophagus is classified into long- and short-segment based on the length of salmon-colored mucosa in the distal esophagus. A distance longer than 3 cm is
The Prague classification was presented by an international research group in 2006 and is regarded as the standard for measuring the length of Barrett’s esophagus. The lower measurement boundary is formed by the proximal cardial notch, and the 2 upper measurement boundaries are marked by the proximal limit of the circumferential Barrett’s segment and the longest tongue of Barrett’s [37].Confirmation of the diagnosis of dysplastic
Once the initial diagnosis of Barrett’s esophagus is made, we recommend referring the patient to a Barrett’s esophagus specialized center in order to offer the patient a second opinion from a team of experts in Barrett’s esophagus. This would avoid possible false-positive results, which can be as high as 40% [1,8]. It would also offer the patient a comprehensive multidisciplinary approach and adequate long-term management. It is further preferred if an advanced intervention is offered to the patient such as endoscopic mucosal resection or endoscopic submucosal dissection. Expert endoscopists
The availability of advanced endoscopic tools improves diagnostic yield. Adopting advanced techniques can reduce errors during biopsy sampling [40–42]. Some of the newer advanced imaging endoscopic tools include chromoendoscopy, optical coherence tomography, confocal microendoscopy, autofluorescence endoscopy, narrow band imaging (NBI), and Fujinon intelligent chromoendoscopy (FICE) [43,44]. In a meta-analysis examining whether advanced techniques improved diagnostic yield, it was found that advanced imaging increased the diagnostic yield by 34%. Advanced technology mentioned here is not mandated in current guidelines [5,45].
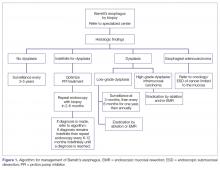
Histopathology categories and TNM staging system for Barrett’s esophagus are shown in Table 2 and Table 3. A management algorithm based on histologic findings is presented in Figure 1.
S creening
Logically, Barrett’s esophagus screening should be offered to every patient with GERD; however, this is against current recommendations because it is cost-prohibitive [46,47]. It is a given that the rationale behind the screening is to decrease morbidity and mortality from esophageal adenocarcinoma by offering early and definitive intervention [14,48,49]. The gold standard for screening is upper endoscopy, although nonendoscopic methods are being studied [1]. For example, a capsule attached to a string can be swallowed by the patient, the capsule is then deployed and pulled through the esophagus to obtains a brush sample of the cells. It is a promising technology due to high sensitivity and specificity [50]; however, it is not regularly utilized in practice.
Approaches to screening for Barrett’s esophagus has been addressed by multiple societies with several guidelines currently available [51]. None of these approaches have been proven to be superior in clinical studies. The American Gastroenterological Association (AGA) recommends screening patients with multiple risk factors associated with esophageal adenocarcinoma for Barrett’s esophagus. Risk factors listed by the AGA include white patients, male patients, patients above the age of 50 with a history of chronic GERD, hiatal hernia, elevated body mass index, and certain body fat distribution. The AGA recommends against screening the general population with GERD [9]. The American College of Gastroenterology (ACG) recommends upper endoscopy only in the presence of alarm symptoms (eg, dysphagia, weight loss, gastrointestinal bleeding) and for screening of patients at high risk for complications [52]. The American College of Physicians recommends upper endoscopy for screening for Barrett’s esophagus in men older than 50 years with GERD symptoms for more than 5 years with any risk factors like nocturnal reflux symptoms, hiatus hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat [1].
Overall, the sensitivity of endoscopy to diagnose Barrett’s esophagus, as seen in a Veterans Affairs (VA) cohort study for the detection of Barrett’s, is about 80%. The concluded 80% sensitivity rate was based on the performance of 2 endoscopies, 6 weeks apart for each patient, with subsequent labeling of the diagnosis if intestinal metaplasia was found in either of the 2 biopsy samples taken from the 2 procedures [53].
Promising molecular biomarkers have been associated with Barrett’s esophagus including p53 and cyclin D1 expression. However, additional studies are needed before they are incorporated as part of screening practices [5].
General Management
Medical Management of GERD
All patients with Barrett’s esophagus should be treated with a proton pump inhibitor (PPI) indefinitely based on multiple studies [54–56]. Effective control of GERD was associated with a decreased risk of dysplasia and adenocarcinoma (adjusted odds ratio 0.29, 95% CI 0.12–0.79) [57].
Effective control of GERD decreases chronic esophageal inflammation, which could progress to Barrett’s esophagus and has a risk of possible progression to adenocarcinoma. In one study, patients with proven Barrett’s esophagus showed partial regression of the intestinal metaplasia with aggressive PPI therapy [57–59]. Despite this, it is not clear whether the regression decreased risk of malignant progression [58,60,61]. In a study of 68 patients, aggressive acid reduction with omeprazole 40 mg twice a day lead to partial regression of Barrett’s esophagus when compared to mild suppression with ranitidine 150 mg twice a day. However, there was no reduction in the risk of cancer [59].
Surveillance
The reasoning behind surveillance is to detect dysplasia or adenocarcinoma in patients known to have Barrett’s esophagus early enough to provide early and efficient treatment to improve the outcome. Surveillance is done by endoscopy with biopsies in addition to sampling any irregularity [1,62,63]; however, the evidence to back up the benefit of surveillance is not clear [5,64]. It should be noted that surveillance carries risks, and morbidity associated with repeated procedures may affect patients psychologically and financially. Patients who have Barrett’s esophagus are more likely to die from other more common diseases, such as coronary heart disease, prior to developing adenocarcinoma [65]. In a recent meta-analysis, the mortality rate due to esophageal adenocarcinoma was 3.0 per 1000 person-years, whereas the mortality rate due to other causes was 37.1 per 1000 person-years [65]. An ongoing randomized, multicenter trial which assesses scheduled endoscopy every 2 years will shed more light on the overall survival after applying surveillance recommendations for each grade of dysplasia [66].
The following are ACG recommendations for surveillance of Barrett’s esophagus based on the histopathology report. The histopathology report delineates 1 of 3 types of columnar epithelium [68]: cardiac epithelium of mucus-secreting cells, atrophic gastric fundic type epithelium, or specialized columnar cells with goblet cells. The latter type is the most common with high potential for cancer [32]. Degrees of dysplasia and possible adenocarcinoma is usually described in the report as well. The degree of dysplasia if found helps the endoscopist plan the next step in management. It is worth mentioning that sampling errors can lead to missing a diagnosis. In a meta-analysis, 13% of patients diagnosed with high-grade dysplasia who underwent resection were found to have invasive cancer [69].
Surveillance for Patients with No Dysplasia
We suggest surveillance every 3 to 5 years since the rate of neoplasia is low [14]. For management of select patients with no dysplasia and with additional risk factors, radiofrequency ablation (RFA) may be an option, although it remains a controversial approach. For example, in a patient under 50 years of age, family history would be an argument for proceeding with RFA instead of prolonged surveillance. In a prospective cohort study of 139 patients with 10-year follow-up after ablation, recurrent Barrett’s occurred in less than 5% of the patients. [70]
Surveillance for Patients with Biopsy Showing “Indefinite for Dysplasia”
Aggressive treatment with PPI twice daily is recommended to avoid the misinterpretation of reactive esophageal changes secondary to reflux as dysplasia on the following endoscopy with biopsy. These patients will require a repeat endoscopy with biopsies after 3 months of aggressive treatment with PPI. Biopsies should be taken every 1 centimeter within Barrett’s epithelium [1].
If it remains indefinite, biopsies should be examined by a second pathologist with expertise in Barrett’s esophagus. If the second pathologist agrees on the indefinite diagnosis for dysplasia, then endoscopy every 12 months is recommended [1]. Treatment versus surveillance after repeat endoscopy and biopsy should be tailored to the new histopathology results on the most recent exam.
Surveillance for Patients with Biopsy Results showing Low-Grade Dysplasia (LGD), High-Grade Dysplasia (HGD), or Intramucosal Carcinoma
Surveillance recommendations are discussed under Management of Dysplasia or Intramucosal Carcinoma, below.
Efficacy of Surveillance
Asymptomatic adenocarcinoma could be discovered during surveillance, and neoplasia detected during surveillance is usually less advanced than those found after development of symptoms such as dysphagia, bleeding or weight loss [2,3,71–75]. These studies obviously had lead–time bias and did not document terminal cancer in patients adherent to surveillance protocol.
Management of Dysplasia or Intramucosal Carcinoma
Overview
Historically, dysplasia was managed with esophagectomy, which was associated with high morbidity and mortality. With advancement in the field of endoscopy, dysplasia is managed quite differently today, with endoscopic eradication therapy, which includes the use of endoscopic ablation techniques and endoscopic resection. The advantage of endoscopic resection is preservation of resected tissue for further examination, thus providing valuable information regarding the stage of the tumor (depth). Histological examination is not possible with photo or thermal ablation techniques as destroyed mucosa cannot be submitted for tissue analysis.
Low-Grade Dysplasia
If low-grade dysplasia is found, it is followed by a repeat endoscopy 8 weeks after aggressive PPI therapy. The repeat endoscopy should be performed with high definition/high-resolution endoscopy. The rationale of a second endoscopy is to ensure that the metaplastic mucosa was adequately inspected and biopsied prior to further intervention [1,9]. If the diagnosis is confirmed as low-grade dysplasia, and the patient prefers to go with the path of intervention instead of conservative management (endoscopic surveillance every 6 months for 1 year then annually with biopsies), then multiple options are available for the patient, including RFA or cryotherapy [8] [76]. In a randomized clinical trial in patients with Barrett’s esophagus and LGD, RFA was shown to reduce risk of neoplastic progression over a 3-year follow-up. The study included 136 patients randomized to receive ablation and 68 patients who underwent endoscopic surveillance. In the ablation group, the risk of progression to HGD or esophageal adenocarcinoma was reduced by 25% and the risk of progression to adenocarcinoma was reduced by 7.4%. In the ablation group, complete eradication of dysplasia and intestinal metaplasia occurred in 92.6% and 88.2% of patients respectively. In the endoscopic surveillance group, complete eradication of dysplasia and intestinal metaplasia was seen in 27.9% and 0.0% of patients respectively. [76]. Treatment-related adverse events occurred in 19.1% of patients receiving ablation (P < 0.001). The most common adverse event was stricture, occurring in 8 patients receiving ablation (11.8%), all resolved by endoscopic dilation [76,78].
Surveillance after ablation of LGD is still an ongoing debate, and further evidence is needed to establish guidelines [8]. Due to lack of evidence, we would lean towards surveillance for those patients with an annual esophagogastroduodenoscopy with biopsy examination (author’s opinion, no associated level of evidence).
High-Grade Dysplasia or Intramucosal Carcinoma
For patients with HGD or intramucosal carcinoma without submucosal invasion, eradication is the treatment of choice. Current guidelines advocate for endoscopic eradication therapy for most if not all patients with HGD or intramucosal carcinoma with a goal of removing all metaplastic and dysplastic tissue [1,5,62,64]. It should be noted that the biopsy specimen should be extensive to decrease the error margin. If the diagnosis were made on endoscopy without procuring extensive biopsies, then repeat endoscopy with extensive biopsies is needed prior to deciding the treatment path. The rationale behind extensive biopsies is to confirm the diagnosis and to determine the extent of dysplasia. Other factors potentially influencing the treatment path include the patient’s age, comorbid conditions, quality of life, and available access to an advanced endoscopist or specialized surgeon. Patient’s preferences and adherence to follow-up visits should also be a consideration.
The long-term benefits of endoscopic intervention versus surgical intervention are not well established. Esophagectomy is no longer a preferred method of treatment due to high morbidity and mortality associated with the procedure when compared to endoscopic interventions. However, it is still a preferred choice amongst a select group of patients unwilling to follow-up. A cost-effective analysis found that endoscopic ablation provided the longest quality adjusted life expectancy for Barrett’s esophagus with HGD [79,80].
Endoscopic Therapy in Barrett’s Esophagus
Endoscopic Ablative Therapies
With the advancement of endoscopic intervention, we now have multiple tools to ablate abnormal epithelium in Barrett’s esophagus. Examples of ablation techniques include thermal, photochemical, and mechanical techniques [81,82]. RFA is the treatment of choice for ablation [83]. However, non-contact ablative therapy, such as cryoablation, may be prefered if topography of the esophagus doesn’t allow contact ablation.
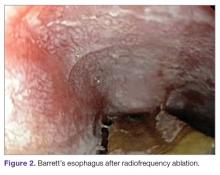
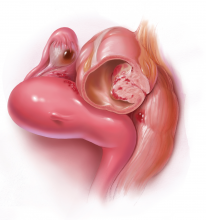
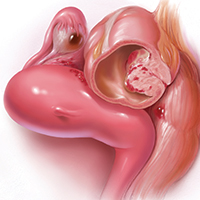
Radiofrequency Ablation (Figure 2). RFA is a procedure in which heat is generated from medium frequency alternating current and leads to thermal injury [84]. In Barrett’s esophagus, RFA uses radiofrequency energy delivered by a balloon that has a series of closely spaced electrodes in a
Most patients will require multiple sessions of RFA to achieve eradication. It is very rarely a one-time procedure. In a meta-analysis of 18 studies including 3082 patients, the most common adverse effects of RFA were stricture in 5% [76,98], bleeding in 1%, and pain in 3% of patients [99].
It is crucial for successful RFA to continue medical treatment for acid suppression, in order to allow healthy regeneration of the squamous cell lining. It is suggested to use PPI twice a day with sucralfate and ranitidine after the intervention [100,101]. Adhering to a liquid diet for 24 hours is needed, followed by a soft diet to allow faster regeneration of the epithelium.
The caveat with RFA is that new evidence shows a higher rate of recurrence than previously thought. In one study of 246 patients, recurrence of dysplasia occurred in 25% of patients at 48 months after eradication in 80% of the patients, and metaplasia occurred in 50% at 60 months [102]. The other risk is buried Barrett’s, a condition occurring after incomplete ablation, in which squamous cell epithelium covers patches of incompletely destroyed intestinal lining, leading to possible progression of the disease to adenocarcinoma under the surface [103].
It has been reported that patients who underwent RFA had remarkable improvement in quality of life even if RFA did not achieve eradication. Patients reported less depression, less stress and better quality of life [104].
Based on a survey of experts, follow-up at 3 months, 6 months, and then annually is recommended after ablation [1,105]. Biopsies should be taken distal to neosquamous epithelium and from suspicious areas [97,106].
Endoscopic Spray Cryotherapy. This technique involves application of liquid nitrogen or carbon dioxide gas by endoscope on the tissue to freeze it off. Although it has been shown to eliminate HGD in over 95% of the cases and all dysplasia in over 85% of the cases, it was effective in eradicating intestinal metaplasia in only 55% of patients [103,108,109]. Thus, RFA as ablation therapy is still superior to cryotherapy and is still the first-line treatment for dysplastic Barrett’s esophagus. In comparison to cryotherapy, RFA efficacy has been studied extensively with well documented outcomes. However, there is a role for cryotherapy over RFA in certain clinical situations (such as severe chest pain from RFA or lack of efficacy in eradicating intestinal metaplasia or dysplasia by RFA).
Similar to RFA, on occasions of partial ablation, the remaining metaplastic tissue may get buried beneath a layer of squamous epithelium and can possibly progress to adenocarcinoma [110].
Photodynamic Therapy (PDT). This technique works by producing cytotoxicity at the cellular level by exposure to light at a specific wavelength in the presence of a chemical agent known as photosensitize [107]. Although superior to omeprazole, PDT has a significant rate of complications, mainly stricture, and a high occurrence of esophageal cancer during follow-up. For this reason, it is less favorable compared to RFA [107] and mentioned here as a historical therapy.
Endoscopic Resection Techniques: Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
Unlike flat mucosa in Barrett’s esophagus, which respond to ablative techniques such as RFA or cryotherapy, nodular Barrett’s esophagus is hard to treat and requires endoscopic resection prior to ablation. Endoscopic mucosal resection (EMR) is the most widely used technique and it is available in most tertiary referral centers. Another technique named endoscopic submucosal dissection (ESD) allows the removal of large nodular areas of Barrett’s esophagus in one piece to ensure complete removal of nodular dysplasia. ESD is technically challenging and it is only available in a handful of centers in the US. Endoscopic resection techniques are the preferred interventions for nodular dysplasia due to their ability to provide valuable information for staging the lesion [111–113]. Endoscopic resection techniques are safer and more effective with similar or better results when compared with other approaches [114].
EMR is completed by the excision of esophageal mucosa down to the submucosa and submitting a large tissue specimen to the pathologist. It additionally serves as a therapeutic measure in cases of no submucosal extension. Another advantage of EMR is the ability to predict lymph node metastasis. The rationale is based on the fact that the most important predictor of lymph node metastasis is the depth of the tumor; hence, invasive tumors would likely be associated with lymph node metastasis [115,116].
In a systematic review of 11 studies, complete EMR was as equally effective in the short-term treatment of dysplastic Barrett’s esophagus when compared to RFA, but adverse event rates were greater with complete EMR (mainly strictures). Strictures are more likely to occur in patients undergoing extensive EMR. In another meta-analysis of 22 studies comparing the efficacy of EMR to RFA, both techniques were effective in eradicating dysplasia (95% in EMR group and 92% in RFA group). However, extensive EMR was associated with higher complication rates suggesting that a combined endoscopic approach of focal EMR followed by RFA is preferred over extensive EMR alone [86].
It should be noted that EMR and ESD information were derived from highly specialized center and these results may not be duplicated in community settings [113,117].
Efficacy of Endoscopic Resection. Endoscopic resection has a success rate comparable to surgical esophagectomy with fewer complications [113,114,118–121] in patients with HGD and early stages of esophageal cancer [122]. Complete remission can be as high as 89%. Recurrence occurred in 6% to 30% of patients [114,118,119], which was attributed to incomplete removal, large lesions, failure to use adjunct therapy, or lack of follow-up [123]. Even when recurrence occurred, it was successfully managed by endoscopic intervention [124].
In a large cohort study of 1000 patients with early mucosal adenocarcinoma who were treated with endoscopic resection, long-term complete remission occurred in 94% of patients. There was no mortality and less than 2% of patients had major complications. Infrequent complications include bleeding, perforations, and strictures [123,125,126]. The rate of complications is lower in highly specialized centers [127–129].
Surgery was necessary in 12 patients (3.7%) after endoscopic therapy failed [123]. Post-resection care and follow-up is similar to the post-RFA care discussed above.
Management of Invasive Esophageal Adenocarcinoma
Patients diagnosed with an invasive adenocarcinoma need to be referred to an oncologist for staging and to discuss treatment options. A select number of patients may be referred by oncology for endoscopic resection, yet the need for a multidisciplinary approach in these situations is absolutely necessary [1].
Esophagectomy
Esophagectomy offers the complete removal of the HGD along with any adenocarcinoma in the regional lymph nodes. However, mortality rates are as high as 12% immediately after the procedure [130]. The multitude of short- and long-term morbidity has significant effects on quality of life. Short-term morbidity is as high as 30%. Patients may develop serious postoperative complications such as myocardial infarction, hospital associated pneumonia, or anatomic leak [131].
Examples of long-term morbidity include dysphagia, transection of vagal nerve, and dumping syndrome. Recent development in minimally invasive surgeries for esophagectomy has not reduced postoperative morbidity rates [132].
Advocates of esophagectomy illustrate the advantage of eradication of occult lymph node metastasis. The counter argument has been established by a systemic review in which occult lymph node metastasis occurred in less than 2% of patients with HGD and intramucosal carcinoma; whereas the mortality rate after esophagectomy is substantially higher with no guarantee of curing metastatic disease [133].
Prevention of Barrett’s Esophagus
Since Barrett’s esophagus precedes most of the cases of EAC if not all [1,134], methods that aim at decreasing the incidence of Barrett’s esophagus could help in prevention. The modifiable risk factors listed by the AGA include BMI, GERD, and hiatal hernia management. Along with diet and exercise, the advent of new therapies to help patients manage their weight could in return help in avoiding a plethora of medical conditions including Barrett’s esophagus. Hiatal hernia management could lower the risk of Barrett’s by restoring normal anatomy. Lastly, proper management of GERD would lower the risk of developing Barrett’s esophagus as discussed in this article [1,9].
It is worth noting that a large trial on the efficacy and safety of aspirin for prevention of adenocarcinoma progression in Barrett’s esophagus is ongoing in the UK (AspECT trial). The AspECT trial examines the efficacy of low dose vs. high dose PPI with or without aspirin for the chemoprevention of esophageal adenocarcinoma. The theory behind the study is the inhibition of COX 2 receptors in Barrett’s cells can decrease tissue progression to cancer. This chempreventive effect of nonsteroidal anti-inflammatory drugs was shown to be augmented when combined with statin intake [56,135–138].
Conclusion
Barrett’s esophagus is usually diagnosed during routine endoscopic examination. The initial symptoms are those associated with GERD, like heartburn, dyspepsia, and regurgitation. Specialized columnar epithelium is the hallmark of histopathological diagnosis. Recommendations of the ACG and AGA suggest treatment based on biopsy results. The intervention would vary on a wide spectrum starting from acid suppression, radiofrequency ablation, endoscopic resection therapy, and rarely, esophagectomy.
Corresponding author: Mohamed O. Othman, MD, Gastroenterology and Hepatology Section, Baylor College of Medicine, 7200 Cambridge St., Suite 8C, Houston, TX 77030, [email protected].
Financial disclosures: Dr. Othman has received grant support from Abbvie and has served as a consultant for Olympus.
1. Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51.
2. Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2.
3. Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8.
4. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836–45.
5. Spechler SJ, Sharma P, Souza RF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91.
6. Sharma P, McQuaid K, Dent J, et al; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127:310–30.
7. ASGE Standards of Practice Committee, Evans JA, Early DS; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94.
8. Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low–grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835.
9. American Gastroenterological, A., et al., American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology, 2011. 140(3): p. 1084–91. [Same as #5]
10. Van Eyken P. Definition of Barrett’s oesophagus. Acta Gastroenterol Belg 2000;63:10–2.
11. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 1999;116(2):277–85.
12. Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar–lined (Barrett’s) esophagus. Comparison of population–based clinical and autopsy findings. Gastroenterology 1990;99:918–22.
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7.
14. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989;30:14–8.
15. Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24.
16. Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220–7;quiz e26.
17. Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7.
18. Rex DK, Cummings OW, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7.
19. Rubenstein JH, Taylor JB. Meta–analysis: the association of oesophageal adenocarcinoma with symptoms of gastro–oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
20. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
21. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology, 2005;129:1825–31.
22. Sampliner RE. A population prevalence of Barrett’s esophagus––finally. Gastroenterology 2005; 129:2101–3.
23. Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30–4.
24. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community–based study, 1994–2006. Gut 2009;58:182–8.
25. Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta–analysis. Ann Thorac Surg 2009;87:655–62.
26. Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett’s oesophagus in women. Gut 2009;58:1460–6.
27. Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410–9.
28. Sharma N, Ho KY. Risk Factors for Barrett’s oesophagus. Gastrointest Tumors 2016;3:103–8.
29. Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis 1996;7:51–60.
30. Ronkainen J, Talley NJ, Storskrubb T. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community–based endoscopic follow–up study. Am J Gastroenterol 2011;106:1946–52.
31. Kim SL, Wo JM, Hunter JG. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53–5.
32. Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med 2002;346:836–42.
33. Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink––is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588–94.
34. Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus––the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033–6.
35. Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43.
36. Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612–20.
37. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.
38. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population–based study. J Natl Cancer Inst 2011;103:1049–57.
39. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577–80.
40. Canto MI, Setrakian S, Willis J, et al. Methylene blue–directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560–8.
41. Scotiniotis IA, Kochman ML, Lewis JD. Accuracy of EUS in the evaluation of Barrett’s esophagus and high–grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 2001;54:689–96.
42. Kobayashi K, Izatt JA, Kulkarni MD, et al. High–resolution cross–sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515–23.
43. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light–scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001. 120: 1620–9.
44. Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe–based confocal laser endomicroscopy. Gastrointest Endosc 2010;72:19–24.
45. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta–analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70.e1–2.–
46. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434–42.
47. Inadomi JM, Sampliner R, Lagergren J. Screening and surveillance for Barrett esophagus in high–risk groups: a cost–utility analysis. Ann Intern Med 2003;138:176–86.
48. Conio M, Blanchi S, Lapertosa G, et al. Long–term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931–9.
49. Rastogi A, Puli S, El–Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high–grade dysplasia: a meta–analysis. Gastrointest Endosc 2008;67:394–8.
50. Kadri SR, Lao–Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non–endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372.
51. Sharma, P., et al., A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology, 2004. 127(1): p. 310–30. [Same as #6]
52. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
53. Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945–9.
54. Kastelein F, Spaander MC, Steyerberg EW, et al; ProBar Study Group. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2013;11:382–8.
55. El–Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877–83.
56. Nguyen DM, El–Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
57. Singh S, Garg SK, Singh PP, et al. Acid–suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta–analysis. Gut 2014;63:1229–37.
58. Spechler SJ, Lee E, Ahnen D, et al. Long–term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow–up of a randomized controlled trial. JAMA 2001;285:2331–8.
59. Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489–94.
60. Ouatu–Lascar R, Triadafilopoulos G. –Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1998;93:711–6.
61. Maret–Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta–analysis. Ann Surg 2016;263:251–7.
62. Evans, J.A., et al., The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc, 2012. 76(6): p. 1087–94. [Same as #7]
63. ASGE Standards of Practice Committee, Evans JA, Early DS,et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
64. Bennett C, Moayyedi P, Corley DA, et al; BOB CAT Consortium. BOB CAT: A large–scale review and delphi consensus for ,anagement of Barrett’s esophagus with no dysplasia, indefinite for, or low–grade dysplasia. Am J Gastroenterol 2015;110:662–82; quiz 683.
65. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta–analysis. Clin Gastroenterol Hepatol 2010;8:235–44; quiz e32.
66. Old O, Moayyedi P, Love S, et al; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64.
67. Weston AP, Sharma P, Topalovski M, et al. Long–term follow–up of Barrett’s high–grade dysplasia. Am J Gastroenterol 2000;95:1888–93.
68. Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80.
69. Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high–grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64.
70. Allison H, Banchs MA, Bonis PA, Guelrud M. Long–term remission of nondysplastic Barrett’s esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow–up. Gastrointest Endosc 2011;73:651–8.
71. Corley DA, Levin TR, Habel LA, Weiss NS, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population–based study. Gastroenterology 2002;122:633–40.
72. Wong T, Tian J, Nagar AB. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med 2010;123:462–7.
73. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003.
74. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population–based cohort study. Am J Gastroenterol 2014;109:1215–22.
75. Kastelein F, van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 2016;65:548–54.
76. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low–grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17.
77. Wang WL, Chang IW, Chen CC, et al. Radiofrequency ablation versus endoscopic submucosal dissection in treating large early esophageal squamous cell neoplasia. Medicine (Baltimore) 2015;94:e2240.
78. Lim CH, Treanor D, Dixon MF, Axon AT. Low–grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy 2007;39:581–7.
79. Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut 2004;53:1736–44.
80. Vij R, Triadafilopoulos G, Owens DK, et al. Cost–effectiveness of photodynamic therapy for high–grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;60:739–56.
81. van den Boogert J, v an Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett’s esophagus with high–grade dysplasia: a review. Am J Gastroenterol 1999;94:1153–60.
82. Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc 2004;59:66–9.
83. Sharma VK, Wang KK, Overholt BF, et al. Balloon–based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1–year follow–up of 100 patients. Gastrointest Endosc 2007;65:185–95.
84. Hanlon CR. Textbook of surgery: The biological basis of modern surgical practice, 14th edition. Ann Surg 1992;216:94.
85. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007;246:1016–20.
86. Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718–31.e3.
87. Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370–9.
88. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9.
89. Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733–9.
90. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5–year follow–up. Gastrointest Endosc 2008;68:867–76.
91. Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310–7.
92. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high–grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc 2008;68:35–40.
93. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1.
94. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8.
95. Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1.
96. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5–year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9.
97. Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61:515–21.
98. Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8.
99. Orman ES, Li N, Shaheen NJ.Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta–analysis. Clin Gastroenterol Hepatol 2013;11:1245–55.
100. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–81.
101. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra–esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625–32.
102. Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high–grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc 2015;81:1158–66.e1–4.
103. Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high–grade dysplasia. Gastrointest Endosc 2010;71:680–5.
104. Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 2010;42:790–9.
105. Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow–up practices after successful endoscopic eradication therapy for Barrett’s esophagus with high–grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc 2013;78:696–701.
106. Sampliner RE, Camargo E, Prasad AR. Prasad, Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277–9.
107. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long–term results. Gastrointest Endosc 2003;58:183–8.
108. Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high–grade dysplasia: long–term results. Gastrointest Endosc 2013;78:260–5.
109. Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:582–91.
110. Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re–epithelialisation of Barrett’s oesophagus. Gut 2000;46:574–7.
111. Pech O, May A, Gossner L, et al. Management of pre–malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004;18:61–76.
112. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
113. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high–grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670–7.
114. Pech O, Behrens A, May A, et al. Long–term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high–grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6.
115. Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 2004;36:776–81.
116. Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high–grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10.
117. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high–grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 2000;52:328–32.
118. Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41–5.
119. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3–10.
120. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high–grade dysplasia and intramucosal carcinoma––an American single–center experience. Am J Gastroenterol 2009;104:2684–92.
121. Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high–volume centers. Ann Surg 2011;254:67–72.
122. Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta–analysis. Gastrointest Endosc 2014;79:233–241.e2.
123. Pech O, May A, Manner H, et al. Long–term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–660.e1.
124. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
125. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high–grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute–phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14(10):1085–91.
126. Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761–71.
127. Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753–60.
128. Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc 2005;62:860–5.
129. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853–8.
130. van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8.
131. Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635–43.
132. Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg 2000;70:1651–5.
133. Dunbar KB, Spechler SJ. The risk of lymph–node metastases in patients with high–grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol 2012;107:850–62; quiz 863.
134. Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 2002;360:1587–9.
135. Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012;10:722–7.
136. Abnet CC, Freedman ND, Kamangar F, et al. Non–steroidal anti–inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta–analysis. Br J Cancer 2009;100:551–7.
137. Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti–inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011;141:2000–8; quiz e13–4.
138. Zhang S, Zhang XQ, Ding XW, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta–analysis. Br J Cancer 2014;110: 2378–88.
From the Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX.
Abstract
- Objective: To provide an update on management of Barrett’s esophagus.
- Methods: Review of the literature.
- Results: Management of Barrett’s esophagus depends on the degree of dysplasia. Surveillance by endoscopy every 3–5 years is recommended in patients with Barrett’s esophagus without dysplasia. Patients with Barrett’s esophagus and low-grade dysplasia should undergo surveillance by endoscopy in 3 months for confirmation of the diagnosis; if the diagnosis is confirmed then surveillance by endoscopy or eradication of Barrett’s epithelium by ablation or endoscopic resection are recommended. There is a sufficient evidence to recommend radiofrequency ablation of high-grade dysplasia within Barrett’s esophagus or to perform endoscopic mucosal resection of nodular Barrett’s esophagus with any degree of dysplasia. Early esophageal cancers that are limited to the mucosa can be treated by endoscopic resection, while cancer invading into the deep submucosa or muscularis propria may need esophagectomy with or without chemoradiation.
- Conclusion: The management of Barrett’s esophagus depends on the degree of dysplasia. Radiofrequency ablation and endoscopic mucosal resection are the most commonly used treatment for Barrett’s esophagus with dysplasia.
Keywords: Barrett’s esophagus; radiofrequency ablation; endoscopic submucosal dissection; endoscopic mucosal resection; early esophageal cancer.
Barrett’s esophagus is a common complication of chronic reflux disease [1]. Metaplastic changes that occur at the distal esophageal epithelium are usually asymptomatic [2,3] and occur as reparative adaptations to the insult of the gastric acid [4]. The management of Barrett’s esophagus after diagnosis is currently debated amongst experts without a clear consensus [5,6]. This review is generally consistent with the 2016 guidelines from the American College of Gastroenterology [1], a 2012 guideline from the American Society of Gastrointestinal Endoscopy [7], a 2011 guideline, and a 2016 expert review from the American Gastroenterological Association [8,9].
D efinition
Barrett’s esophagus is a metaplasia of the stratified squamous epithelium to a specialized columnar intestinal epithelium of mucus cells and goblet cells at the distal esophagus secondary to gastroesophageal reflux disease (GERD) [7,10]. Barrett’s esophagus is unstable tissue which can progress to esophageal adenocarcinoma. When unmanaged, the risk of cancer in dysplastic mucosa is at least thirty-fold greater than that for the general population [11–14], with recent studies suggesting a 0.4–0.7 occurrence rate per year [11,15]. With no dysplasia, the risk is low [16].
Epidemiology
The prevalence of Barrett’s esophagus is ~10% in patients with GERD [11–13,17], an estimate tied to the prevalence of GERD. However, due to the lack of symptoms of Barrett’s esophagus, no solid data supports this assumption [18–20]. A European study estimated the prevalence of Barrett’s esophagus to be 1.6% among the general population [21,22]. Barrett’s esophagus is usually diagnosed during endoscopic examinations of middle-aged and older adults, with the mean age being 55 years of age. It is most commonly found in Caucasian males and associated with the use of smoking tobacco. The male-to-female ratio is approximately 2:1 [1] and it appears to be uncommon in African Americans [23,24]. Abdominal obesity as measured by an increased waist-to-hip ratio is associated with an increased risk of Barrett’s esophagus [25,26]. Germline mutations in the MSR1, ASCC1, and CTHRC1 genes have been associated with the presence of Barrett’s esophagus and esophageal adenocarcinoma [27]. Risk factors are listed in Table 1.
Clinical Symptoms
Columnar metaplasia itself does not cause any symptoms but is merely the adaptation of the cells to the repeated effect of the acid. The main clinical symptoms of the disease would initially be symptoms associated with GERD, such as heartburn, water brash, and dysphagia [1]. Severe presentations of GERD, such as esophageal ulceration, stricture, and hemorrhage, usually occur with long-segment Barrett’s esophagus [29,30]. However, 40% of patients presenting with adenocarcinoma had no history of GERD or symptoms of heartburn [13]. Furthermore, as few as 5% of those presenting with adenocarcinoma were known to have Barrett’s esophagus [31].
D iagnosis
Barrett’s esophagus generally requires an endoscopic examination with biopsy confirmation from the distal esophagus showing specialized intestinal columnar epithelium [32]. The biopsy specimen is acquired from the cellular lining proximal to gastroesophageal junction [5,33]. Barrett’s esophagus is classified into long- and short-segment based on the length of salmon-colored mucosa in the distal esophagus. A distance longer than 3 cm is
The Prague classification was presented by an international research group in 2006 and is regarded as the standard for measuring the length of Barrett’s esophagus. The lower measurement boundary is formed by the proximal cardial notch, and the 2 upper measurement boundaries are marked by the proximal limit of the circumferential Barrett’s segment and the longest tongue of Barrett’s [37].Confirmation of the diagnosis of dysplastic
Once the initial diagnosis of Barrett’s esophagus is made, we recommend referring the patient to a Barrett’s esophagus specialized center in order to offer the patient a second opinion from a team of experts in Barrett’s esophagus. This would avoid possible false-positive results, which can be as high as 40% [1,8]. It would also offer the patient a comprehensive multidisciplinary approach and adequate long-term management. It is further preferred if an advanced intervention is offered to the patient such as endoscopic mucosal resection or endoscopic submucosal dissection. Expert endoscopists
The availability of advanced endoscopic tools improves diagnostic yield. Adopting advanced techniques can reduce errors during biopsy sampling [40–42]. Some of the newer advanced imaging endoscopic tools include chromoendoscopy, optical coherence tomography, confocal microendoscopy, autofluorescence endoscopy, narrow band imaging (NBI), and Fujinon intelligent chromoendoscopy (FICE) [43,44]. In a meta-analysis examining whether advanced techniques improved diagnostic yield, it was found that advanced imaging increased the diagnostic yield by 34%. Advanced technology mentioned here is not mandated in current guidelines [5,45].
Histopathology categories and TNM staging system for Barrett’s esophagus are shown in Table 2 and Table 3. A management algorithm based on histologic findings is presented in Figure 1.
S creening
Logically, Barrett’s esophagus screening should be offered to every patient with GERD; however, this is against current recommendations because it is cost-prohibitive [46,47]. It is a given that the rationale behind the screening is to decrease morbidity and mortality from esophageal adenocarcinoma by offering early and definitive intervention [14,48,49]. The gold standard for screening is upper endoscopy, although nonendoscopic methods are being studied [1]. For example, a capsule attached to a string can be swallowed by the patient, the capsule is then deployed and pulled through the esophagus to obtains a brush sample of the cells. It is a promising technology due to high sensitivity and specificity [50]; however, it is not regularly utilized in practice.
Approaches to screening for Barrett’s esophagus has been addressed by multiple societies with several guidelines currently available [51]. None of these approaches have been proven to be superior in clinical studies. The American Gastroenterological Association (AGA) recommends screening patients with multiple risk factors associated with esophageal adenocarcinoma for Barrett’s esophagus. Risk factors listed by the AGA include white patients, male patients, patients above the age of 50 with a history of chronic GERD, hiatal hernia, elevated body mass index, and certain body fat distribution. The AGA recommends against screening the general population with GERD [9]. The American College of Gastroenterology (ACG) recommends upper endoscopy only in the presence of alarm symptoms (eg, dysphagia, weight loss, gastrointestinal bleeding) and for screening of patients at high risk for complications [52]. The American College of Physicians recommends upper endoscopy for screening for Barrett’s esophagus in men older than 50 years with GERD symptoms for more than 5 years with any risk factors like nocturnal reflux symptoms, hiatus hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat [1].
Overall, the sensitivity of endoscopy to diagnose Barrett’s esophagus, as seen in a Veterans Affairs (VA) cohort study for the detection of Barrett’s, is about 80%. The concluded 80% sensitivity rate was based on the performance of 2 endoscopies, 6 weeks apart for each patient, with subsequent labeling of the diagnosis if intestinal metaplasia was found in either of the 2 biopsy samples taken from the 2 procedures [53].
Promising molecular biomarkers have been associated with Barrett’s esophagus including p53 and cyclin D1 expression. However, additional studies are needed before they are incorporated as part of screening practices [5].
General Management
Medical Management of GERD
All patients with Barrett’s esophagus should be treated with a proton pump inhibitor (PPI) indefinitely based on multiple studies [54–56]. Effective control of GERD was associated with a decreased risk of dysplasia and adenocarcinoma (adjusted odds ratio 0.29, 95% CI 0.12–0.79) [57].
Effective control of GERD decreases chronic esophageal inflammation, which could progress to Barrett’s esophagus and has a risk of possible progression to adenocarcinoma. In one study, patients with proven Barrett’s esophagus showed partial regression of the intestinal metaplasia with aggressive PPI therapy [57–59]. Despite this, it is not clear whether the regression decreased risk of malignant progression [58,60,61]. In a study of 68 patients, aggressive acid reduction with omeprazole 40 mg twice a day lead to partial regression of Barrett’s esophagus when compared to mild suppression with ranitidine 150 mg twice a day. However, there was no reduction in the risk of cancer [59].
Surveillance
The reasoning behind surveillance is to detect dysplasia or adenocarcinoma in patients known to have Barrett’s esophagus early enough to provide early and efficient treatment to improve the outcome. Surveillance is done by endoscopy with biopsies in addition to sampling any irregularity [1,62,63]; however, the evidence to back up the benefit of surveillance is not clear [5,64]. It should be noted that surveillance carries risks, and morbidity associated with repeated procedures may affect patients psychologically and financially. Patients who have Barrett’s esophagus are more likely to die from other more common diseases, such as coronary heart disease, prior to developing adenocarcinoma [65]. In a recent meta-analysis, the mortality rate due to esophageal adenocarcinoma was 3.0 per 1000 person-years, whereas the mortality rate due to other causes was 37.1 per 1000 person-years [65]. An ongoing randomized, multicenter trial which assesses scheduled endoscopy every 2 years will shed more light on the overall survival after applying surveillance recommendations for each grade of dysplasia [66].
The following are ACG recommendations for surveillance of Barrett’s esophagus based on the histopathology report. The histopathology report delineates 1 of 3 types of columnar epithelium [68]: cardiac epithelium of mucus-secreting cells, atrophic gastric fundic type epithelium, or specialized columnar cells with goblet cells. The latter type is the most common with high potential for cancer [32]. Degrees of dysplasia and possible adenocarcinoma is usually described in the report as well. The degree of dysplasia if found helps the endoscopist plan the next step in management. It is worth mentioning that sampling errors can lead to missing a diagnosis. In a meta-analysis, 13% of patients diagnosed with high-grade dysplasia who underwent resection were found to have invasive cancer [69].
Surveillance for Patients with No Dysplasia
We suggest surveillance every 3 to 5 years since the rate of neoplasia is low [14]. For management of select patients with no dysplasia and with additional risk factors, radiofrequency ablation (RFA) may be an option, although it remains a controversial approach. For example, in a patient under 50 years of age, family history would be an argument for proceeding with RFA instead of prolonged surveillance. In a prospective cohort study of 139 patients with 10-year follow-up after ablation, recurrent Barrett’s occurred in less than 5% of the patients. [70]
Surveillance for Patients with Biopsy Showing “Indefinite for Dysplasia”
Aggressive treatment with PPI twice daily is recommended to avoid the misinterpretation of reactive esophageal changes secondary to reflux as dysplasia on the following endoscopy with biopsy. These patients will require a repeat endoscopy with biopsies after 3 months of aggressive treatment with PPI. Biopsies should be taken every 1 centimeter within Barrett’s epithelium [1].
If it remains indefinite, biopsies should be examined by a second pathologist with expertise in Barrett’s esophagus. If the second pathologist agrees on the indefinite diagnosis for dysplasia, then endoscopy every 12 months is recommended [1]. Treatment versus surveillance after repeat endoscopy and biopsy should be tailored to the new histopathology results on the most recent exam.
Surveillance for Patients with Biopsy Results showing Low-Grade Dysplasia (LGD), High-Grade Dysplasia (HGD), or Intramucosal Carcinoma
Surveillance recommendations are discussed under Management of Dysplasia or Intramucosal Carcinoma, below.
Efficacy of Surveillance
Asymptomatic adenocarcinoma could be discovered during surveillance, and neoplasia detected during surveillance is usually less advanced than those found after development of symptoms such as dysphagia, bleeding or weight loss [2,3,71–75]. These studies obviously had lead–time bias and did not document terminal cancer in patients adherent to surveillance protocol.
Management of Dysplasia or Intramucosal Carcinoma
Overview
Historically, dysplasia was managed with esophagectomy, which was associated with high morbidity and mortality. With advancement in the field of endoscopy, dysplasia is managed quite differently today, with endoscopic eradication therapy, which includes the use of endoscopic ablation techniques and endoscopic resection. The advantage of endoscopic resection is preservation of resected tissue for further examination, thus providing valuable information regarding the stage of the tumor (depth). Histological examination is not possible with photo or thermal ablation techniques as destroyed mucosa cannot be submitted for tissue analysis.
Low-Grade Dysplasia
If low-grade dysplasia is found, it is followed by a repeat endoscopy 8 weeks after aggressive PPI therapy. The repeat endoscopy should be performed with high definition/high-resolution endoscopy. The rationale of a second endoscopy is to ensure that the metaplastic mucosa was adequately inspected and biopsied prior to further intervention [1,9]. If the diagnosis is confirmed as low-grade dysplasia, and the patient prefers to go with the path of intervention instead of conservative management (endoscopic surveillance every 6 months for 1 year then annually with biopsies), then multiple options are available for the patient, including RFA or cryotherapy [8] [76]. In a randomized clinical trial in patients with Barrett’s esophagus and LGD, RFA was shown to reduce risk of neoplastic progression over a 3-year follow-up. The study included 136 patients randomized to receive ablation and 68 patients who underwent endoscopic surveillance. In the ablation group, the risk of progression to HGD or esophageal adenocarcinoma was reduced by 25% and the risk of progression to adenocarcinoma was reduced by 7.4%. In the ablation group, complete eradication of dysplasia and intestinal metaplasia occurred in 92.6% and 88.2% of patients respectively. In the endoscopic surveillance group, complete eradication of dysplasia and intestinal metaplasia was seen in 27.9% and 0.0% of patients respectively. [76]. Treatment-related adverse events occurred in 19.1% of patients receiving ablation (P < 0.001). The most common adverse event was stricture, occurring in 8 patients receiving ablation (11.8%), all resolved by endoscopic dilation [76,78].
Surveillance after ablation of LGD is still an ongoing debate, and further evidence is needed to establish guidelines [8]. Due to lack of evidence, we would lean towards surveillance for those patients with an annual esophagogastroduodenoscopy with biopsy examination (author’s opinion, no associated level of evidence).
High-Grade Dysplasia or Intramucosal Carcinoma
For patients with HGD or intramucosal carcinoma without submucosal invasion, eradication is the treatment of choice. Current guidelines advocate for endoscopic eradication therapy for most if not all patients with HGD or intramucosal carcinoma with a goal of removing all metaplastic and dysplastic tissue [1,5,62,64]. It should be noted that the biopsy specimen should be extensive to decrease the error margin. If the diagnosis were made on endoscopy without procuring extensive biopsies, then repeat endoscopy with extensive biopsies is needed prior to deciding the treatment path. The rationale behind extensive biopsies is to confirm the diagnosis and to determine the extent of dysplasia. Other factors potentially influencing the treatment path include the patient’s age, comorbid conditions, quality of life, and available access to an advanced endoscopist or specialized surgeon. Patient’s preferences and adherence to follow-up visits should also be a consideration.
The long-term benefits of endoscopic intervention versus surgical intervention are not well established. Esophagectomy is no longer a preferred method of treatment due to high morbidity and mortality associated with the procedure when compared to endoscopic interventions. However, it is still a preferred choice amongst a select group of patients unwilling to follow-up. A cost-effective analysis found that endoscopic ablation provided the longest quality adjusted life expectancy for Barrett’s esophagus with HGD [79,80].
Endoscopic Therapy in Barrett’s Esophagus
Endoscopic Ablative Therapies
With the advancement of endoscopic intervention, we now have multiple tools to ablate abnormal epithelium in Barrett’s esophagus. Examples of ablation techniques include thermal, photochemical, and mechanical techniques [81,82]. RFA is the treatment of choice for ablation [83]. However, non-contact ablative therapy, such as cryoablation, may be prefered if topography of the esophagus doesn’t allow contact ablation.
Radiofrequency Ablation (Figure 2). RFA is a procedure in which heat is generated from medium frequency alternating current and leads to thermal injury [84]. In Barrett’s esophagus, RFA uses radiofrequency energy delivered by a balloon that has a series of closely spaced electrodes in a
Most patients will require multiple sessions of RFA to achieve eradication. It is very rarely a one-time procedure. In a meta-analysis of 18 studies including 3082 patients, the most common adverse effects of RFA were stricture in 5% [76,98], bleeding in 1%, and pain in 3% of patients [99].
It is crucial for successful RFA to continue medical treatment for acid suppression, in order to allow healthy regeneration of the squamous cell lining. It is suggested to use PPI twice a day with sucralfate and ranitidine after the intervention [100,101]. Adhering to a liquid diet for 24 hours is needed, followed by a soft diet to allow faster regeneration of the epithelium.
The caveat with RFA is that new evidence shows a higher rate of recurrence than previously thought. In one study of 246 patients, recurrence of dysplasia occurred in 25% of patients at 48 months after eradication in 80% of the patients, and metaplasia occurred in 50% at 60 months [102]. The other risk is buried Barrett’s, a condition occurring after incomplete ablation, in which squamous cell epithelium covers patches of incompletely destroyed intestinal lining, leading to possible progression of the disease to adenocarcinoma under the surface [103].
It has been reported that patients who underwent RFA had remarkable improvement in quality of life even if RFA did not achieve eradication. Patients reported less depression, less stress and better quality of life [104].
Based on a survey of experts, follow-up at 3 months, 6 months, and then annually is recommended after ablation [1,105]. Biopsies should be taken distal to neosquamous epithelium and from suspicious areas [97,106].
Endoscopic Spray Cryotherapy. This technique involves application of liquid nitrogen or carbon dioxide gas by endoscope on the tissue to freeze it off. Although it has been shown to eliminate HGD in over 95% of the cases and all dysplasia in over 85% of the cases, it was effective in eradicating intestinal metaplasia in only 55% of patients [103,108,109]. Thus, RFA as ablation therapy is still superior to cryotherapy and is still the first-line treatment for dysplastic Barrett’s esophagus. In comparison to cryotherapy, RFA efficacy has been studied extensively with well documented outcomes. However, there is a role for cryotherapy over RFA in certain clinical situations (such as severe chest pain from RFA or lack of efficacy in eradicating intestinal metaplasia or dysplasia by RFA).
Similar to RFA, on occasions of partial ablation, the remaining metaplastic tissue may get buried beneath a layer of squamous epithelium and can possibly progress to adenocarcinoma [110].
Photodynamic Therapy (PDT). This technique works by producing cytotoxicity at the cellular level by exposure to light at a specific wavelength in the presence of a chemical agent known as photosensitize [107]. Although superior to omeprazole, PDT has a significant rate of complications, mainly stricture, and a high occurrence of esophageal cancer during follow-up. For this reason, it is less favorable compared to RFA [107] and mentioned here as a historical therapy.
Endoscopic Resection Techniques: Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
Unlike flat mucosa in Barrett’s esophagus, which respond to ablative techniques such as RFA or cryotherapy, nodular Barrett’s esophagus is hard to treat and requires endoscopic resection prior to ablation. Endoscopic mucosal resection (EMR) is the most widely used technique and it is available in most tertiary referral centers. Another technique named endoscopic submucosal dissection (ESD) allows the removal of large nodular areas of Barrett’s esophagus in one piece to ensure complete removal of nodular dysplasia. ESD is technically challenging and it is only available in a handful of centers in the US. Endoscopic resection techniques are the preferred interventions for nodular dysplasia due to their ability to provide valuable information for staging the lesion [111–113]. Endoscopic resection techniques are safer and more effective with similar or better results when compared with other approaches [114].
EMR is completed by the excision of esophageal mucosa down to the submucosa and submitting a large tissue specimen to the pathologist. It additionally serves as a therapeutic measure in cases of no submucosal extension. Another advantage of EMR is the ability to predict lymph node metastasis. The rationale is based on the fact that the most important predictor of lymph node metastasis is the depth of the tumor; hence, invasive tumors would likely be associated with lymph node metastasis [115,116].
In a systematic review of 11 studies, complete EMR was as equally effective in the short-term treatment of dysplastic Barrett’s esophagus when compared to RFA, but adverse event rates were greater with complete EMR (mainly strictures). Strictures are more likely to occur in patients undergoing extensive EMR. In another meta-analysis of 22 studies comparing the efficacy of EMR to RFA, both techniques were effective in eradicating dysplasia (95% in EMR group and 92% in RFA group). However, extensive EMR was associated with higher complication rates suggesting that a combined endoscopic approach of focal EMR followed by RFA is preferred over extensive EMR alone [86].
It should be noted that EMR and ESD information were derived from highly specialized center and these results may not be duplicated in community settings [113,117].
Efficacy of Endoscopic Resection. Endoscopic resection has a success rate comparable to surgical esophagectomy with fewer complications [113,114,118–121] in patients with HGD and early stages of esophageal cancer [122]. Complete remission can be as high as 89%. Recurrence occurred in 6% to 30% of patients [114,118,119], which was attributed to incomplete removal, large lesions, failure to use adjunct therapy, or lack of follow-up [123]. Even when recurrence occurred, it was successfully managed by endoscopic intervention [124].
In a large cohort study of 1000 patients with early mucosal adenocarcinoma who were treated with endoscopic resection, long-term complete remission occurred in 94% of patients. There was no mortality and less than 2% of patients had major complications. Infrequent complications include bleeding, perforations, and strictures [123,125,126]. The rate of complications is lower in highly specialized centers [127–129].
Surgery was necessary in 12 patients (3.7%) after endoscopic therapy failed [123]. Post-resection care and follow-up is similar to the post-RFA care discussed above.
Management of Invasive Esophageal Adenocarcinoma
Patients diagnosed with an invasive adenocarcinoma need to be referred to an oncologist for staging and to discuss treatment options. A select number of patients may be referred by oncology for endoscopic resection, yet the need for a multidisciplinary approach in these situations is absolutely necessary [1].
Esophagectomy
Esophagectomy offers the complete removal of the HGD along with any adenocarcinoma in the regional lymph nodes. However, mortality rates are as high as 12% immediately after the procedure [130]. The multitude of short- and long-term morbidity has significant effects on quality of life. Short-term morbidity is as high as 30%. Patients may develop serious postoperative complications such as myocardial infarction, hospital associated pneumonia, or anatomic leak [131].
Examples of long-term morbidity include dysphagia, transection of vagal nerve, and dumping syndrome. Recent development in minimally invasive surgeries for esophagectomy has not reduced postoperative morbidity rates [132].
Advocates of esophagectomy illustrate the advantage of eradication of occult lymph node metastasis. The counter argument has been established by a systemic review in which occult lymph node metastasis occurred in less than 2% of patients with HGD and intramucosal carcinoma; whereas the mortality rate after esophagectomy is substantially higher with no guarantee of curing metastatic disease [133].
Prevention of Barrett’s Esophagus
Since Barrett’s esophagus precedes most of the cases of EAC if not all [1,134], methods that aim at decreasing the incidence of Barrett’s esophagus could help in prevention. The modifiable risk factors listed by the AGA include BMI, GERD, and hiatal hernia management. Along with diet and exercise, the advent of new therapies to help patients manage their weight could in return help in avoiding a plethora of medical conditions including Barrett’s esophagus. Hiatal hernia management could lower the risk of Barrett’s by restoring normal anatomy. Lastly, proper management of GERD would lower the risk of developing Barrett’s esophagus as discussed in this article [1,9].
It is worth noting that a large trial on the efficacy and safety of aspirin for prevention of adenocarcinoma progression in Barrett’s esophagus is ongoing in the UK (AspECT trial). The AspECT trial examines the efficacy of low dose vs. high dose PPI with or without aspirin for the chemoprevention of esophageal adenocarcinoma. The theory behind the study is the inhibition of COX 2 receptors in Barrett’s cells can decrease tissue progression to cancer. This chempreventive effect of nonsteroidal anti-inflammatory drugs was shown to be augmented when combined with statin intake [56,135–138].
Conclusion
Barrett’s esophagus is usually diagnosed during routine endoscopic examination. The initial symptoms are those associated with GERD, like heartburn, dyspepsia, and regurgitation. Specialized columnar epithelium is the hallmark of histopathological diagnosis. Recommendations of the ACG and AGA suggest treatment based on biopsy results. The intervention would vary on a wide spectrum starting from acid suppression, radiofrequency ablation, endoscopic resection therapy, and rarely, esophagectomy.
Corresponding author: Mohamed O. Othman, MD, Gastroenterology and Hepatology Section, Baylor College of Medicine, 7200 Cambridge St., Suite 8C, Houston, TX 77030, [email protected].
Financial disclosures: Dr. Othman has received grant support from Abbvie and has served as a consultant for Olympus.
From the Gastroenterology and Hepatology Section, Baylor College of Medicine, Houston, TX.
Abstract
- Objective: To provide an update on management of Barrett’s esophagus.
- Methods: Review of the literature.
- Results: Management of Barrett’s esophagus depends on the degree of dysplasia. Surveillance by endoscopy every 3–5 years is recommended in patients with Barrett’s esophagus without dysplasia. Patients with Barrett’s esophagus and low-grade dysplasia should undergo surveillance by endoscopy in 3 months for confirmation of the diagnosis; if the diagnosis is confirmed then surveillance by endoscopy or eradication of Barrett’s epithelium by ablation or endoscopic resection are recommended. There is a sufficient evidence to recommend radiofrequency ablation of high-grade dysplasia within Barrett’s esophagus or to perform endoscopic mucosal resection of nodular Barrett’s esophagus with any degree of dysplasia. Early esophageal cancers that are limited to the mucosa can be treated by endoscopic resection, while cancer invading into the deep submucosa or muscularis propria may need esophagectomy with or without chemoradiation.
- Conclusion: The management of Barrett’s esophagus depends on the degree of dysplasia. Radiofrequency ablation and endoscopic mucosal resection are the most commonly used treatment for Barrett’s esophagus with dysplasia.
Keywords: Barrett’s esophagus; radiofrequency ablation; endoscopic submucosal dissection; endoscopic mucosal resection; early esophageal cancer.
Barrett’s esophagus is a common complication of chronic reflux disease [1]. Metaplastic changes that occur at the distal esophageal epithelium are usually asymptomatic [2,3] and occur as reparative adaptations to the insult of the gastric acid [4]. The management of Barrett’s esophagus after diagnosis is currently debated amongst experts without a clear consensus [5,6]. This review is generally consistent with the 2016 guidelines from the American College of Gastroenterology [1], a 2012 guideline from the American Society of Gastrointestinal Endoscopy [7], a 2011 guideline, and a 2016 expert review from the American Gastroenterological Association [8,9].
D efinition
Barrett’s esophagus is a metaplasia of the stratified squamous epithelium to a specialized columnar intestinal epithelium of mucus cells and goblet cells at the distal esophagus secondary to gastroesophageal reflux disease (GERD) [7,10]. Barrett’s esophagus is unstable tissue which can progress to esophageal adenocarcinoma. When unmanaged, the risk of cancer in dysplastic mucosa is at least thirty-fold greater than that for the general population [11–14], with recent studies suggesting a 0.4–0.7 occurrence rate per year [11,15]. With no dysplasia, the risk is low [16].
Epidemiology
The prevalence of Barrett’s esophagus is ~10% in patients with GERD [11–13,17], an estimate tied to the prevalence of GERD. However, due to the lack of symptoms of Barrett’s esophagus, no solid data supports this assumption [18–20]. A European study estimated the prevalence of Barrett’s esophagus to be 1.6% among the general population [21,22]. Barrett’s esophagus is usually diagnosed during endoscopic examinations of middle-aged and older adults, with the mean age being 55 years of age. It is most commonly found in Caucasian males and associated with the use of smoking tobacco. The male-to-female ratio is approximately 2:1 [1] and it appears to be uncommon in African Americans [23,24]. Abdominal obesity as measured by an increased waist-to-hip ratio is associated with an increased risk of Barrett’s esophagus [25,26]. Germline mutations in the MSR1, ASCC1, and CTHRC1 genes have been associated with the presence of Barrett’s esophagus and esophageal adenocarcinoma [27]. Risk factors are listed in Table 1.
Clinical Symptoms
Columnar metaplasia itself does not cause any symptoms but is merely the adaptation of the cells to the repeated effect of the acid. The main clinical symptoms of the disease would initially be symptoms associated with GERD, such as heartburn, water brash, and dysphagia [1]. Severe presentations of GERD, such as esophageal ulceration, stricture, and hemorrhage, usually occur with long-segment Barrett’s esophagus [29,30]. However, 40% of patients presenting with adenocarcinoma had no history of GERD or symptoms of heartburn [13]. Furthermore, as few as 5% of those presenting with adenocarcinoma were known to have Barrett’s esophagus [31].
D iagnosis
Barrett’s esophagus generally requires an endoscopic examination with biopsy confirmation from the distal esophagus showing specialized intestinal columnar epithelium [32]. The biopsy specimen is acquired from the cellular lining proximal to gastroesophageal junction [5,33]. Barrett’s esophagus is classified into long- and short-segment based on the length of salmon-colored mucosa in the distal esophagus. A distance longer than 3 cm is
The Prague classification was presented by an international research group in 2006 and is regarded as the standard for measuring the length of Barrett’s esophagus. The lower measurement boundary is formed by the proximal cardial notch, and the 2 upper measurement boundaries are marked by the proximal limit of the circumferential Barrett’s segment and the longest tongue of Barrett’s [37].Confirmation of the diagnosis of dysplastic
Once the initial diagnosis of Barrett’s esophagus is made, we recommend referring the patient to a Barrett’s esophagus specialized center in order to offer the patient a second opinion from a team of experts in Barrett’s esophagus. This would avoid possible false-positive results, which can be as high as 40% [1,8]. It would also offer the patient a comprehensive multidisciplinary approach and adequate long-term management. It is further preferred if an advanced intervention is offered to the patient such as endoscopic mucosal resection or endoscopic submucosal dissection. Expert endoscopists
The availability of advanced endoscopic tools improves diagnostic yield. Adopting advanced techniques can reduce errors during biopsy sampling [40–42]. Some of the newer advanced imaging endoscopic tools include chromoendoscopy, optical coherence tomography, confocal microendoscopy, autofluorescence endoscopy, narrow band imaging (NBI), and Fujinon intelligent chromoendoscopy (FICE) [43,44]. In a meta-analysis examining whether advanced techniques improved diagnostic yield, it was found that advanced imaging increased the diagnostic yield by 34%. Advanced technology mentioned here is not mandated in current guidelines [5,45].
Histopathology categories and TNM staging system for Barrett’s esophagus are shown in Table 2 and Table 3. A management algorithm based on histologic findings is presented in Figure 1.
S creening
Logically, Barrett’s esophagus screening should be offered to every patient with GERD; however, this is against current recommendations because it is cost-prohibitive [46,47]. It is a given that the rationale behind the screening is to decrease morbidity and mortality from esophageal adenocarcinoma by offering early and definitive intervention [14,48,49]. The gold standard for screening is upper endoscopy, although nonendoscopic methods are being studied [1]. For example, a capsule attached to a string can be swallowed by the patient, the capsule is then deployed and pulled through the esophagus to obtains a brush sample of the cells. It is a promising technology due to high sensitivity and specificity [50]; however, it is not regularly utilized in practice.
Approaches to screening for Barrett’s esophagus has been addressed by multiple societies with several guidelines currently available [51]. None of these approaches have been proven to be superior in clinical studies. The American Gastroenterological Association (AGA) recommends screening patients with multiple risk factors associated with esophageal adenocarcinoma for Barrett’s esophagus. Risk factors listed by the AGA include white patients, male patients, patients above the age of 50 with a history of chronic GERD, hiatal hernia, elevated body mass index, and certain body fat distribution. The AGA recommends against screening the general population with GERD [9]. The American College of Gastroenterology (ACG) recommends upper endoscopy only in the presence of alarm symptoms (eg, dysphagia, weight loss, gastrointestinal bleeding) and for screening of patients at high risk for complications [52]. The American College of Physicians recommends upper endoscopy for screening for Barrett’s esophagus in men older than 50 years with GERD symptoms for more than 5 years with any risk factors like nocturnal reflux symptoms, hiatus hernia, elevated body mass index, tobacco use, and intra-abdominal distribution of fat [1].
Overall, the sensitivity of endoscopy to diagnose Barrett’s esophagus, as seen in a Veterans Affairs (VA) cohort study for the detection of Barrett’s, is about 80%. The concluded 80% sensitivity rate was based on the performance of 2 endoscopies, 6 weeks apart for each patient, with subsequent labeling of the diagnosis if intestinal metaplasia was found in either of the 2 biopsy samples taken from the 2 procedures [53].
Promising molecular biomarkers have been associated with Barrett’s esophagus including p53 and cyclin D1 expression. However, additional studies are needed before they are incorporated as part of screening practices [5].
General Management
Medical Management of GERD
All patients with Barrett’s esophagus should be treated with a proton pump inhibitor (PPI) indefinitely based on multiple studies [54–56]. Effective control of GERD was associated with a decreased risk of dysplasia and adenocarcinoma (adjusted odds ratio 0.29, 95% CI 0.12–0.79) [57].
Effective control of GERD decreases chronic esophageal inflammation, which could progress to Barrett’s esophagus and has a risk of possible progression to adenocarcinoma. In one study, patients with proven Barrett’s esophagus showed partial regression of the intestinal metaplasia with aggressive PPI therapy [57–59]. Despite this, it is not clear whether the regression decreased risk of malignant progression [58,60,61]. In a study of 68 patients, aggressive acid reduction with omeprazole 40 mg twice a day lead to partial regression of Barrett’s esophagus when compared to mild suppression with ranitidine 150 mg twice a day. However, there was no reduction in the risk of cancer [59].
Surveillance
The reasoning behind surveillance is to detect dysplasia or adenocarcinoma in patients known to have Barrett’s esophagus early enough to provide early and efficient treatment to improve the outcome. Surveillance is done by endoscopy with biopsies in addition to sampling any irregularity [1,62,63]; however, the evidence to back up the benefit of surveillance is not clear [5,64]. It should be noted that surveillance carries risks, and morbidity associated with repeated procedures may affect patients psychologically and financially. Patients who have Barrett’s esophagus are more likely to die from other more common diseases, such as coronary heart disease, prior to developing adenocarcinoma [65]. In a recent meta-analysis, the mortality rate due to esophageal adenocarcinoma was 3.0 per 1000 person-years, whereas the mortality rate due to other causes was 37.1 per 1000 person-years [65]. An ongoing randomized, multicenter trial which assesses scheduled endoscopy every 2 years will shed more light on the overall survival after applying surveillance recommendations for each grade of dysplasia [66].
The following are ACG recommendations for surveillance of Barrett’s esophagus based on the histopathology report. The histopathology report delineates 1 of 3 types of columnar epithelium [68]: cardiac epithelium of mucus-secreting cells, atrophic gastric fundic type epithelium, or specialized columnar cells with goblet cells. The latter type is the most common with high potential for cancer [32]. Degrees of dysplasia and possible adenocarcinoma is usually described in the report as well. The degree of dysplasia if found helps the endoscopist plan the next step in management. It is worth mentioning that sampling errors can lead to missing a diagnosis. In a meta-analysis, 13% of patients diagnosed with high-grade dysplasia who underwent resection were found to have invasive cancer [69].
Surveillance for Patients with No Dysplasia
We suggest surveillance every 3 to 5 years since the rate of neoplasia is low [14]. For management of select patients with no dysplasia and with additional risk factors, radiofrequency ablation (RFA) may be an option, although it remains a controversial approach. For example, in a patient under 50 years of age, family history would be an argument for proceeding with RFA instead of prolonged surveillance. In a prospective cohort study of 139 patients with 10-year follow-up after ablation, recurrent Barrett’s occurred in less than 5% of the patients. [70]
Surveillance for Patients with Biopsy Showing “Indefinite for Dysplasia”
Aggressive treatment with PPI twice daily is recommended to avoid the misinterpretation of reactive esophageal changes secondary to reflux as dysplasia on the following endoscopy with biopsy. These patients will require a repeat endoscopy with biopsies after 3 months of aggressive treatment with PPI. Biopsies should be taken every 1 centimeter within Barrett’s epithelium [1].
If it remains indefinite, biopsies should be examined by a second pathologist with expertise in Barrett’s esophagus. If the second pathologist agrees on the indefinite diagnosis for dysplasia, then endoscopy every 12 months is recommended [1]. Treatment versus surveillance after repeat endoscopy and biopsy should be tailored to the new histopathology results on the most recent exam.
Surveillance for Patients with Biopsy Results showing Low-Grade Dysplasia (LGD), High-Grade Dysplasia (HGD), or Intramucosal Carcinoma
Surveillance recommendations are discussed under Management of Dysplasia or Intramucosal Carcinoma, below.
Efficacy of Surveillance
Asymptomatic adenocarcinoma could be discovered during surveillance, and neoplasia detected during surveillance is usually less advanced than those found after development of symptoms such as dysphagia, bleeding or weight loss [2,3,71–75]. These studies obviously had lead–time bias and did not document terminal cancer in patients adherent to surveillance protocol.
Management of Dysplasia or Intramucosal Carcinoma
Overview
Historically, dysplasia was managed with esophagectomy, which was associated with high morbidity and mortality. With advancement in the field of endoscopy, dysplasia is managed quite differently today, with endoscopic eradication therapy, which includes the use of endoscopic ablation techniques and endoscopic resection. The advantage of endoscopic resection is preservation of resected tissue for further examination, thus providing valuable information regarding the stage of the tumor (depth). Histological examination is not possible with photo or thermal ablation techniques as destroyed mucosa cannot be submitted for tissue analysis.
Low-Grade Dysplasia
If low-grade dysplasia is found, it is followed by a repeat endoscopy 8 weeks after aggressive PPI therapy. The repeat endoscopy should be performed with high definition/high-resolution endoscopy. The rationale of a second endoscopy is to ensure that the metaplastic mucosa was adequately inspected and biopsied prior to further intervention [1,9]. If the diagnosis is confirmed as low-grade dysplasia, and the patient prefers to go with the path of intervention instead of conservative management (endoscopic surveillance every 6 months for 1 year then annually with biopsies), then multiple options are available for the patient, including RFA or cryotherapy [8] [76]. In a randomized clinical trial in patients with Barrett’s esophagus and LGD, RFA was shown to reduce risk of neoplastic progression over a 3-year follow-up. The study included 136 patients randomized to receive ablation and 68 patients who underwent endoscopic surveillance. In the ablation group, the risk of progression to HGD or esophageal adenocarcinoma was reduced by 25% and the risk of progression to adenocarcinoma was reduced by 7.4%. In the ablation group, complete eradication of dysplasia and intestinal metaplasia occurred in 92.6% and 88.2% of patients respectively. In the endoscopic surveillance group, complete eradication of dysplasia and intestinal metaplasia was seen in 27.9% and 0.0% of patients respectively. [76]. Treatment-related adverse events occurred in 19.1% of patients receiving ablation (P < 0.001). The most common adverse event was stricture, occurring in 8 patients receiving ablation (11.8%), all resolved by endoscopic dilation [76,78].
Surveillance after ablation of LGD is still an ongoing debate, and further evidence is needed to establish guidelines [8]. Due to lack of evidence, we would lean towards surveillance for those patients with an annual esophagogastroduodenoscopy with biopsy examination (author’s opinion, no associated level of evidence).
High-Grade Dysplasia or Intramucosal Carcinoma
For patients with HGD or intramucosal carcinoma without submucosal invasion, eradication is the treatment of choice. Current guidelines advocate for endoscopic eradication therapy for most if not all patients with HGD or intramucosal carcinoma with a goal of removing all metaplastic and dysplastic tissue [1,5,62,64]. It should be noted that the biopsy specimen should be extensive to decrease the error margin. If the diagnosis were made on endoscopy without procuring extensive biopsies, then repeat endoscopy with extensive biopsies is needed prior to deciding the treatment path. The rationale behind extensive biopsies is to confirm the diagnosis and to determine the extent of dysplasia. Other factors potentially influencing the treatment path include the patient’s age, comorbid conditions, quality of life, and available access to an advanced endoscopist or specialized surgeon. Patient’s preferences and adherence to follow-up visits should also be a consideration.
The long-term benefits of endoscopic intervention versus surgical intervention are not well established. Esophagectomy is no longer a preferred method of treatment due to high morbidity and mortality associated with the procedure when compared to endoscopic interventions. However, it is still a preferred choice amongst a select group of patients unwilling to follow-up. A cost-effective analysis found that endoscopic ablation provided the longest quality adjusted life expectancy for Barrett’s esophagus with HGD [79,80].
Endoscopic Therapy in Barrett’s Esophagus
Endoscopic Ablative Therapies
With the advancement of endoscopic intervention, we now have multiple tools to ablate abnormal epithelium in Barrett’s esophagus. Examples of ablation techniques include thermal, photochemical, and mechanical techniques [81,82]. RFA is the treatment of choice for ablation [83]. However, non-contact ablative therapy, such as cryoablation, may be prefered if topography of the esophagus doesn’t allow contact ablation.
Radiofrequency Ablation (Figure 2). RFA is a procedure in which heat is generated from medium frequency alternating current and leads to thermal injury [84]. In Barrett’s esophagus, RFA uses radiofrequency energy delivered by a balloon that has a series of closely spaced electrodes in a
Most patients will require multiple sessions of RFA to achieve eradication. It is very rarely a one-time procedure. In a meta-analysis of 18 studies including 3082 patients, the most common adverse effects of RFA were stricture in 5% [76,98], bleeding in 1%, and pain in 3% of patients [99].
It is crucial for successful RFA to continue medical treatment for acid suppression, in order to allow healthy regeneration of the squamous cell lining. It is suggested to use PPI twice a day with sucralfate and ranitidine after the intervention [100,101]. Adhering to a liquid diet for 24 hours is needed, followed by a soft diet to allow faster regeneration of the epithelium.
The caveat with RFA is that new evidence shows a higher rate of recurrence than previously thought. In one study of 246 patients, recurrence of dysplasia occurred in 25% of patients at 48 months after eradication in 80% of the patients, and metaplasia occurred in 50% at 60 months [102]. The other risk is buried Barrett’s, a condition occurring after incomplete ablation, in which squamous cell epithelium covers patches of incompletely destroyed intestinal lining, leading to possible progression of the disease to adenocarcinoma under the surface [103].
It has been reported that patients who underwent RFA had remarkable improvement in quality of life even if RFA did not achieve eradication. Patients reported less depression, less stress and better quality of life [104].
Based on a survey of experts, follow-up at 3 months, 6 months, and then annually is recommended after ablation [1,105]. Biopsies should be taken distal to neosquamous epithelium and from suspicious areas [97,106].
Endoscopic Spray Cryotherapy. This technique involves application of liquid nitrogen or carbon dioxide gas by endoscope on the tissue to freeze it off. Although it has been shown to eliminate HGD in over 95% of the cases and all dysplasia in over 85% of the cases, it was effective in eradicating intestinal metaplasia in only 55% of patients [103,108,109]. Thus, RFA as ablation therapy is still superior to cryotherapy and is still the first-line treatment for dysplastic Barrett’s esophagus. In comparison to cryotherapy, RFA efficacy has been studied extensively with well documented outcomes. However, there is a role for cryotherapy over RFA in certain clinical situations (such as severe chest pain from RFA or lack of efficacy in eradicating intestinal metaplasia or dysplasia by RFA).
Similar to RFA, on occasions of partial ablation, the remaining metaplastic tissue may get buried beneath a layer of squamous epithelium and can possibly progress to adenocarcinoma [110].
Photodynamic Therapy (PDT). This technique works by producing cytotoxicity at the cellular level by exposure to light at a specific wavelength in the presence of a chemical agent known as photosensitize [107]. Although superior to omeprazole, PDT has a significant rate of complications, mainly stricture, and a high occurrence of esophageal cancer during follow-up. For this reason, it is less favorable compared to RFA [107] and mentioned here as a historical therapy.
Endoscopic Resection Techniques: Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD)
Unlike flat mucosa in Barrett’s esophagus, which respond to ablative techniques such as RFA or cryotherapy, nodular Barrett’s esophagus is hard to treat and requires endoscopic resection prior to ablation. Endoscopic mucosal resection (EMR) is the most widely used technique and it is available in most tertiary referral centers. Another technique named endoscopic submucosal dissection (ESD) allows the removal of large nodular areas of Barrett’s esophagus in one piece to ensure complete removal of nodular dysplasia. ESD is technically challenging and it is only available in a handful of centers in the US. Endoscopic resection techniques are the preferred interventions for nodular dysplasia due to their ability to provide valuable information for staging the lesion [111–113]. Endoscopic resection techniques are safer and more effective with similar or better results when compared with other approaches [114].
EMR is completed by the excision of esophageal mucosa down to the submucosa and submitting a large tissue specimen to the pathologist. It additionally serves as a therapeutic measure in cases of no submucosal extension. Another advantage of EMR is the ability to predict lymph node metastasis. The rationale is based on the fact that the most important predictor of lymph node metastasis is the depth of the tumor; hence, invasive tumors would likely be associated with lymph node metastasis [115,116].
In a systematic review of 11 studies, complete EMR was as equally effective in the short-term treatment of dysplastic Barrett’s esophagus when compared to RFA, but adverse event rates were greater with complete EMR (mainly strictures). Strictures are more likely to occur in patients undergoing extensive EMR. In another meta-analysis of 22 studies comparing the efficacy of EMR to RFA, both techniques were effective in eradicating dysplasia (95% in EMR group and 92% in RFA group). However, extensive EMR was associated with higher complication rates suggesting that a combined endoscopic approach of focal EMR followed by RFA is preferred over extensive EMR alone [86].
It should be noted that EMR and ESD information were derived from highly specialized center and these results may not be duplicated in community settings [113,117].
Efficacy of Endoscopic Resection. Endoscopic resection has a success rate comparable to surgical esophagectomy with fewer complications [113,114,118–121] in patients with HGD and early stages of esophageal cancer [122]. Complete remission can be as high as 89%. Recurrence occurred in 6% to 30% of patients [114,118,119], which was attributed to incomplete removal, large lesions, failure to use adjunct therapy, or lack of follow-up [123]. Even when recurrence occurred, it was successfully managed by endoscopic intervention [124].
In a large cohort study of 1000 patients with early mucosal adenocarcinoma who were treated with endoscopic resection, long-term complete remission occurred in 94% of patients. There was no mortality and less than 2% of patients had major complications. Infrequent complications include bleeding, perforations, and strictures [123,125,126]. The rate of complications is lower in highly specialized centers [127–129].
Surgery was necessary in 12 patients (3.7%) after endoscopic therapy failed [123]. Post-resection care and follow-up is similar to the post-RFA care discussed above.
Management of Invasive Esophageal Adenocarcinoma
Patients diagnosed with an invasive adenocarcinoma need to be referred to an oncologist for staging and to discuss treatment options. A select number of patients may be referred by oncology for endoscopic resection, yet the need for a multidisciplinary approach in these situations is absolutely necessary [1].
Esophagectomy
Esophagectomy offers the complete removal of the HGD along with any adenocarcinoma in the regional lymph nodes. However, mortality rates are as high as 12% immediately after the procedure [130]. The multitude of short- and long-term morbidity has significant effects on quality of life. Short-term morbidity is as high as 30%. Patients may develop serious postoperative complications such as myocardial infarction, hospital associated pneumonia, or anatomic leak [131].
Examples of long-term morbidity include dysphagia, transection of vagal nerve, and dumping syndrome. Recent development in minimally invasive surgeries for esophagectomy has not reduced postoperative morbidity rates [132].
Advocates of esophagectomy illustrate the advantage of eradication of occult lymph node metastasis. The counter argument has been established by a systemic review in which occult lymph node metastasis occurred in less than 2% of patients with HGD and intramucosal carcinoma; whereas the mortality rate after esophagectomy is substantially higher with no guarantee of curing metastatic disease [133].
Prevention of Barrett’s Esophagus
Since Barrett’s esophagus precedes most of the cases of EAC if not all [1,134], methods that aim at decreasing the incidence of Barrett’s esophagus could help in prevention. The modifiable risk factors listed by the AGA include BMI, GERD, and hiatal hernia management. Along with diet and exercise, the advent of new therapies to help patients manage their weight could in return help in avoiding a plethora of medical conditions including Barrett’s esophagus. Hiatal hernia management could lower the risk of Barrett’s by restoring normal anatomy. Lastly, proper management of GERD would lower the risk of developing Barrett’s esophagus as discussed in this article [1,9].
It is worth noting that a large trial on the efficacy and safety of aspirin for prevention of adenocarcinoma progression in Barrett’s esophagus is ongoing in the UK (AspECT trial). The AspECT trial examines the efficacy of low dose vs. high dose PPI with or without aspirin for the chemoprevention of esophageal adenocarcinoma. The theory behind the study is the inhibition of COX 2 receptors in Barrett’s cells can decrease tissue progression to cancer. This chempreventive effect of nonsteroidal anti-inflammatory drugs was shown to be augmented when combined with statin intake [56,135–138].
Conclusion
Barrett’s esophagus is usually diagnosed during routine endoscopic examination. The initial symptoms are those associated with GERD, like heartburn, dyspepsia, and regurgitation. Specialized columnar epithelium is the hallmark of histopathological diagnosis. Recommendations of the ACG and AGA suggest treatment based on biopsy results. The intervention would vary on a wide spectrum starting from acid suppression, radiofrequency ablation, endoscopic resection therapy, and rarely, esophagectomy.
Corresponding author: Mohamed O. Othman, MD, Gastroenterology and Hepatology Section, Baylor College of Medicine, 7200 Cambridge St., Suite 8C, Houston, TX 77030, [email protected].
Financial disclosures: Dr. Othman has received grant support from Abbvie and has served as a consultant for Olympus.
1. Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51.
2. Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2.
3. Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8.
4. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836–45.
5. Spechler SJ, Sharma P, Souza RF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91.
6. Sharma P, McQuaid K, Dent J, et al; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127:310–30.
7. ASGE Standards of Practice Committee, Evans JA, Early DS; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94.
8. Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low–grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835.
9. American Gastroenterological, A., et al., American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology, 2011. 140(3): p. 1084–91. [Same as #5]
10. Van Eyken P. Definition of Barrett’s oesophagus. Acta Gastroenterol Belg 2000;63:10–2.
11. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 1999;116(2):277–85.
12. Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar–lined (Barrett’s) esophagus. Comparison of population–based clinical and autopsy findings. Gastroenterology 1990;99:918–22.
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7.
14. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989;30:14–8.
15. Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24.
16. Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220–7;quiz e26.
17. Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7.
18. Rex DK, Cummings OW, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7.
19. Rubenstein JH, Taylor JB. Meta–analysis: the association of oesophageal adenocarcinoma with symptoms of gastro–oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
20. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
21. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology, 2005;129:1825–31.
22. Sampliner RE. A population prevalence of Barrett’s esophagus––finally. Gastroenterology 2005; 129:2101–3.
23. Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30–4.
24. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community–based study, 1994–2006. Gut 2009;58:182–8.
25. Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta–analysis. Ann Thorac Surg 2009;87:655–62.
26. Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett’s oesophagus in women. Gut 2009;58:1460–6.
27. Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410–9.
28. Sharma N, Ho KY. Risk Factors for Barrett’s oesophagus. Gastrointest Tumors 2016;3:103–8.
29. Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis 1996;7:51–60.
30. Ronkainen J, Talley NJ, Storskrubb T. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community–based endoscopic follow–up study. Am J Gastroenterol 2011;106:1946–52.
31. Kim SL, Wo JM, Hunter JG. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53–5.
32. Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med 2002;346:836–42.
33. Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink––is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588–94.
34. Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus––the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033–6.
35. Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43.
36. Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612–20.
37. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.
38. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population–based study. J Natl Cancer Inst 2011;103:1049–57.
39. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577–80.
40. Canto MI, Setrakian S, Willis J, et al. Methylene blue–directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560–8.
41. Scotiniotis IA, Kochman ML, Lewis JD. Accuracy of EUS in the evaluation of Barrett’s esophagus and high–grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 2001;54:689–96.
42. Kobayashi K, Izatt JA, Kulkarni MD, et al. High–resolution cross–sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515–23.
43. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light–scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001. 120: 1620–9.
44. Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe–based confocal laser endomicroscopy. Gastrointest Endosc 2010;72:19–24.
45. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta–analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70.e1–2.–
46. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434–42.
47. Inadomi JM, Sampliner R, Lagergren J. Screening and surveillance for Barrett esophagus in high–risk groups: a cost–utility analysis. Ann Intern Med 2003;138:176–86.
48. Conio M, Blanchi S, Lapertosa G, et al. Long–term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931–9.
49. Rastogi A, Puli S, El–Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high–grade dysplasia: a meta–analysis. Gastrointest Endosc 2008;67:394–8.
50. Kadri SR, Lao–Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non–endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372.
51. Sharma, P., et al., A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology, 2004. 127(1): p. 310–30. [Same as #6]
52. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
53. Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945–9.
54. Kastelein F, Spaander MC, Steyerberg EW, et al; ProBar Study Group. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2013;11:382–8.
55. El–Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877–83.
56. Nguyen DM, El–Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
57. Singh S, Garg SK, Singh PP, et al. Acid–suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta–analysis. Gut 2014;63:1229–37.
58. Spechler SJ, Lee E, Ahnen D, et al. Long–term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow–up of a randomized controlled trial. JAMA 2001;285:2331–8.
59. Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489–94.
60. Ouatu–Lascar R, Triadafilopoulos G. –Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1998;93:711–6.
61. Maret–Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta–analysis. Ann Surg 2016;263:251–7.
62. Evans, J.A., et al., The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc, 2012. 76(6): p. 1087–94. [Same as #7]
63. ASGE Standards of Practice Committee, Evans JA, Early DS,et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
64. Bennett C, Moayyedi P, Corley DA, et al; BOB CAT Consortium. BOB CAT: A large–scale review and delphi consensus for ,anagement of Barrett’s esophagus with no dysplasia, indefinite for, or low–grade dysplasia. Am J Gastroenterol 2015;110:662–82; quiz 683.
65. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta–analysis. Clin Gastroenterol Hepatol 2010;8:235–44; quiz e32.
66. Old O, Moayyedi P, Love S, et al; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64.
67. Weston AP, Sharma P, Topalovski M, et al. Long–term follow–up of Barrett’s high–grade dysplasia. Am J Gastroenterol 2000;95:1888–93.
68. Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80.
69. Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high–grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64.
70. Allison H, Banchs MA, Bonis PA, Guelrud M. Long–term remission of nondysplastic Barrett’s esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow–up. Gastrointest Endosc 2011;73:651–8.
71. Corley DA, Levin TR, Habel LA, Weiss NS, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population–based study. Gastroenterology 2002;122:633–40.
72. Wong T, Tian J, Nagar AB. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med 2010;123:462–7.
73. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003.
74. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population–based cohort study. Am J Gastroenterol 2014;109:1215–22.
75. Kastelein F, van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 2016;65:548–54.
76. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low–grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17.
77. Wang WL, Chang IW, Chen CC, et al. Radiofrequency ablation versus endoscopic submucosal dissection in treating large early esophageal squamous cell neoplasia. Medicine (Baltimore) 2015;94:e2240.
78. Lim CH, Treanor D, Dixon MF, Axon AT. Low–grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy 2007;39:581–7.
79. Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut 2004;53:1736–44.
80. Vij R, Triadafilopoulos G, Owens DK, et al. Cost–effectiveness of photodynamic therapy for high–grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;60:739–56.
81. van den Boogert J, v an Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett’s esophagus with high–grade dysplasia: a review. Am J Gastroenterol 1999;94:1153–60.
82. Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc 2004;59:66–9.
83. Sharma VK, Wang KK, Overholt BF, et al. Balloon–based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1–year follow–up of 100 patients. Gastrointest Endosc 2007;65:185–95.
84. Hanlon CR. Textbook of surgery: The biological basis of modern surgical practice, 14th edition. Ann Surg 1992;216:94.
85. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007;246:1016–20.
86. Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718–31.e3.
87. Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370–9.
88. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9.
89. Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733–9.
90. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5–year follow–up. Gastrointest Endosc 2008;68:867–76.
91. Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310–7.
92. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high–grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc 2008;68:35–40.
93. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1.
94. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8.
95. Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1.
96. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5–year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9.
97. Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61:515–21.
98. Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8.
99. Orman ES, Li N, Shaheen NJ.Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta–analysis. Clin Gastroenterol Hepatol 2013;11:1245–55.
100. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–81.
101. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra–esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625–32.
102. Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high–grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc 2015;81:1158–66.e1–4.
103. Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high–grade dysplasia. Gastrointest Endosc 2010;71:680–5.
104. Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 2010;42:790–9.
105. Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow–up practices after successful endoscopic eradication therapy for Barrett’s esophagus with high–grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc 2013;78:696–701.
106. Sampliner RE, Camargo E, Prasad AR. Prasad, Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277–9.
107. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long–term results. Gastrointest Endosc 2003;58:183–8.
108. Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high–grade dysplasia: long–term results. Gastrointest Endosc 2013;78:260–5.
109. Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:582–91.
110. Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re–epithelialisation of Barrett’s oesophagus. Gut 2000;46:574–7.
111. Pech O, May A, Gossner L, et al. Management of pre–malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004;18:61–76.
112. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
113. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high–grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670–7.
114. Pech O, Behrens A, May A, et al. Long–term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high–grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6.
115. Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 2004;36:776–81.
116. Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high–grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10.
117. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high–grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 2000;52:328–32.
118. Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41–5.
119. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3–10.
120. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high–grade dysplasia and intramucosal carcinoma––an American single–center experience. Am J Gastroenterol 2009;104:2684–92.
121. Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high–volume centers. Ann Surg 2011;254:67–72.
122. Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta–analysis. Gastrointest Endosc 2014;79:233–241.e2.
123. Pech O, May A, Manner H, et al. Long–term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–660.e1.
124. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
125. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high–grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute–phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14(10):1085–91.
126. Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761–71.
127. Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753–60.
128. Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc 2005;62:860–5.
129. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853–8.
130. van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8.
131. Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635–43.
132. Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg 2000;70:1651–5.
133. Dunbar KB, Spechler SJ. The risk of lymph–node metastases in patients with high–grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol 2012;107:850–62; quiz 863.
134. Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 2002;360:1587–9.
135. Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012;10:722–7.
136. Abnet CC, Freedman ND, Kamangar F, et al. Non–steroidal anti–inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta–analysis. Br J Cancer 2009;100:551–7.
137. Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti–inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011;141:2000–8; quiz e13–4.
138. Zhang S, Zhang XQ, Ding XW, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta–analysis. Br J Cancer 2014;110: 2378–88.
1. Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51.
2. Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2.
3. Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8.
4. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836–45.
5. Spechler SJ, Sharma P, Souza RF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91.
6. Sharma P, McQuaid K, Dent J, et al; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004;127:310–30.
7. ASGE Standards of Practice Committee, Evans JA, Early DS; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94.
8. Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low–grade dysplasia in Barrett’s esophagus: Expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology 2016;151:822–835.
9. American Gastroenterological, A., et al., American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology, 2011. 140(3): p. 1084–91. [Same as #5]
10. Van Eyken P. Definition of Barrett’s oesophagus. Acta Gastroenterol Belg 2000;63:10–2.
11. Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 1999;116(2):277–85.
12. Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar–lined (Barrett’s) esophagus. Comparison of population–based clinical and autopsy findings. Gastroenterology 1990;99:918–22.
13. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7.
14. Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut 1989;30:14–8.
15. Winters C Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987;92:118–24.
16. Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220–7;quiz e26.
17. Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101:12–7.
18. Rex DK, Cummings OW, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670–7.
19. Rubenstein JH, Taylor JB. Meta–analysis: the association of oesophageal adenocarcinoma with symptoms of gastro–oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
20. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
21. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology, 2005;129:1825–31.
22. Sampliner RE. A population prevalence of Barrett’s esophagus––finally. Gastroenterology 2005; 129:2101–3.
23. Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6:30–4.
24. Corley DA, Kubo A, Levin TR, et al. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community–based study, 1994–2006. Gut 2009;58:182–8.
25. Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta–analysis. Ann Thorac Surg 2009;87:655–62.
26. Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett’s oesophagus in women. Gut 2009;58:1460–6.
27. Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410–9.
28. Sharma N, Ho KY. Risk Factors for Barrett’s oesophagus. Gastrointest Tumors 2016;3:103–8.
29. Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis 1996;7:51–60.
30. Ronkainen J, Talley NJ, Storskrubb T. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community–based endoscopic follow–up study. Am J Gastroenterol 2011;106:1946–52.
31. Kim SL, Wo JM, Hunter JG. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53–5.
32. Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med 2002;346:836–42.
33. Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink––is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588–94.
34. Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus––the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033–6.
35. Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937–43.
36. Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612–20.
37. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.
38. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population–based study. J Natl Cancer Inst 2011;103:1049–57.
39. Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577–80.
40. Canto MI, Setrakian S, Willis J, et al. Methylene blue–directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest Endosc 2000;51:560–8.
41. Scotiniotis IA, Kochman ML, Lewis JD. Accuracy of EUS in the evaluation of Barrett’s esophagus and high–grade dysplasia or intramucosal carcinoma. Gastrointest Endosc 2001;54:689–96.
42. Kobayashi K, Izatt JA, Kulkarni MD, et al. High–resolution cross–sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515–23.
43. Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light–scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001. 120: 1620–9.
44. Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe–based confocal laser endomicroscopy. Gastrointest Endosc 2010;72:19–24.
45. Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta–analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70.e1–2.–
46. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434–42.
47. Inadomi JM, Sampliner R, Lagergren J. Screening and surveillance for Barrett esophagus in high–risk groups: a cost–utility analysis. Ann Intern Med 2003;138:176–86.
48. Conio M, Blanchi S, Lapertosa G, et al. Long–term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol 2003;98:1931–9.
49. Rastogi A, Puli S, El–Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high–grade dysplasia: a meta–analysis. Gastrointest Endosc 2008;67:394–8.
50. Kadri SR, Lao–Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non–endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341:c4372.
51. Sharma, P., et al., A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology, 2004. 127(1): p. 310–30. [Same as #6]
52. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
53. Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945–9.
54. Kastelein F, Spaander MC, Steyerberg EW, et al; ProBar Study Group. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2013;11:382–8.
55. El–Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol 2004;99:1877–83.
56. Nguyen DM, El–Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
57. Singh S, Garg SK, Singh PP, et al. Acid–suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta–analysis. Gut 2014;63:1229–37.
58. Spechler SJ, Lee E, Ahnen D, et al. Long–term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow–up of a randomized controlled trial. JAMA 2001;285:2331–8.
59. Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut 1999;45:489–94.
60. Ouatu–Lascar R, Triadafilopoulos G. –Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1998;93:711–6.
61. Maret–Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux surgery and risk of esophageal adenocarcinoma: A systematic review and meta–analysis. Ann Surg 2016;263:251–7.
62. Evans, J.A., et al., The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc, 2012. 76(6): p. 1087–94. [Same as #7]
63. ASGE Standards of Practice Committee, Evans JA, Early DS,et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
64. Bennett C, Moayyedi P, Corley DA, et al; BOB CAT Consortium. BOB CAT: A large–scale review and delphi consensus for ,anagement of Barrett’s esophagus with no dysplasia, indefinite for, or low–grade dysplasia. Am J Gastroenterol 2015;110:662–82; quiz 683.
65. Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta–analysis. Clin Gastroenterol Hepatol 2010;8:235–44; quiz e32.
66. Old O, Moayyedi P, Love S, et al; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22:158–64.
67. Weston AP, Sharma P, Topalovski M, et al. Long–term follow–up of Barrett’s high–grade dysplasia. Am J Gastroenterol 2000;95:1888–93.
68. Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976;295:476–80.
69. Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high–grade dysplasia in Barrett’s esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64.
70. Allison H, Banchs MA, Bonis PA, Guelrud M. Long–term remission of nondysplastic Barrett’s esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow–up. Gastrointest Endosc 2011;73:651–8.
71. Corley DA, Levin TR, Habel LA, Weiss NS, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population–based study. Gastroenterology 2002;122:633–40.
72. Wong T, Tian J, Nagar AB. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med 2010;123:462–7.
73. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003.
74. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population–based cohort study. Am J Gastroenterol 2014;109:1215–22.
75. Kastelein F, van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut 2016;65:548–54.
76. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low–grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17.
77. Wang WL, Chang IW, Chen CC, et al. Radiofrequency ablation versus endoscopic submucosal dissection in treating large early esophageal squamous cell neoplasia. Medicine (Baltimore) 2015;94:e2240.
78. Lim CH, Treanor D, Dixon MF, Axon AT. Low–grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy 2007;39:581–7.
79. Shaheen NJ, Inadomi JM, Overholt BF, Sharma P. What is the best management strategy for high grade dysplasia in Barrett’s oesophagus? A cost effectiveness analysis. Gut 2004;53:1736–44.
80. Vij R, Triadafilopoulos G, Owens DK, et al. Cost–effectiveness of photodynamic therapy for high–grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;60:739–56.
81. van den Boogert J, v an Hillegersberg R, Siersema PD, et al. Endoscopic ablation therapy for Barrett’s esophagus with high–grade dysplasia: a review. Am J Gastroenterol 1999;94:1153–60.
82. Sampliner RE. Endoscopic ablative therapy for Barrett’s esophagus: current status. Gastrointest Endosc 2004;59:66–9.
83. Sharma VK, Wang KK, Overholt BF, et al. Balloon–based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1–year follow–up of 100 patients. Gastrointest Endosc 2007;65:185–95.
84. Hanlon CR. Textbook of surgery: The biological basis of modern surgical practice, 14th edition. Ann Surg 1992;216:94.
85. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007;246:1016–20.
86. Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718–31.e3.
87. Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008;40:370–9.
88. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23–9.
89. Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc 2012;76:733–9.
90. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s esophagus: a multicenter study with 2.5–year follow–up. Gastrointest Endosc 2008;68:867–76.
91. Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310–7.
92. Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high–grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc 2008;68:35–40.
93. Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1.
94. Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy 2010;42:272–8.
95. Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12:1840–7.e1.
96. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5–year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9.
97. Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett’s esophagus ablation. Gastrointest Endosc 2005;61:515–21.
98. Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy 2009;41:2–8.
99. Orman ES, Li N, Shaheen NJ.Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta–analysis. Clin Gastroenterol Hepatol 2013;11:1245–55.
100. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576–81.
101. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra–esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012;57:2625–32.
102. Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high–grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc 2015;81:1158–66.e1–4.
103. Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high–grade dysplasia. Gastrointest Endosc 2010;71:680–5.
104. Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 2010;42:790–9.
105. Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow–up practices after successful endoscopic eradication therapy for Barrett’s esophagus with high–grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc 2013;78:696–701.
106. Sampliner RE, Camargo E, Prasad AR. Prasad, Association of ablation of Barrett’s esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus 2006;19:277–9.
107. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long–term results. Gastrointest Endosc 2003;58:183–8.
108. Gosain S, Mercer K, Twaddell WS, et al. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high–grade dysplasia: long–term results. Gastrointest Endosc 2013;78:260–5.
109. Canto MI, Shin EJ, Khashab MA, et al. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett’s esophagus. Endoscopy 2015;47:582–91.
110. Van Laethem JL, Peny MO, Salmon I, et al. Intramucosal adenocarcinoma arising under squamous re–epithelialisation of Barrett’s oesophagus. Gut 2000;46:574–7.
111. Pech O, May A, Gossner L, et al. Management of pre–malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004;18:61–76.
112. Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003;57:567–79.
113. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high–grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670–7.
114. Pech O, Behrens A, May A, et al. Long–term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high–grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200–6.
115. Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett’s esophagus and early neoplasia. Endoscopy 2004;36:776–81.
116. Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high–grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10.
117. Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high–grade dysplasia within Barrett’s esophagus. Gastrointest Endosc 2000;52:328–32.
118. Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy 2007;39:41–5.
119. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3–10.
120. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high–grade dysplasia and intramucosal carcinoma––an American single–center experience. Am J Gastroenterol 2009;104:2684–92.
121. Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high–volume centers. Ann Surg 2011;254:67–72.
122. Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta–analysis. Gastrointest Endosc 2014;79:233–241.e2.
123. Pech O, May A, Manner H, et al. Long–term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–660.e1.
124. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
125. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high–grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute–phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14(10):1085–91.
126. Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett’s esophagus: experience in 41 patients. Gastrointest Endosc 2011;74:761–71.
127. Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753–60.
128. Choi IJ, Kim CG, Chang HJ, et al. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc 2005;62:860–5.
129. Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010;42:853–8.
130. van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8.
131. Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635–43.
132. Young MM, Deschamps C, Trastek VF, et al. Esophageal reconstruction for benign disease: early morbidity, mortality, and functional results. Ann Thorac Surg 2000;70:1651–5.
133. Dunbar KB, Spechler SJ. The risk of lymph–node metastases in patients with high–grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol 2012;107:850–62; quiz 863.
134. Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett’s oesophagus. Lancet 2002;360:1587–9.
135. Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012;10:722–7.
136. Abnet CC, Freedman ND, Kamangar F, et al. Non–steroidal anti–inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta–analysis. Br J Cancer 2009;100:551–7.
137. Kastelein F, Spaander MC, Biermann K, et al. Nonsteroidal anti–inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011;141:2000–8; quiz e13–4.
138. Zhang S, Zhang XQ, Ding XW, et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta–analysis. Br J Cancer 2014;110: 2378–88.
Current Guidelines for Psoriasis Treatment: A Work in Progress
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10
Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Practice Points
- Guidelines and consensus statements for psoriasis treatment are generally but not always consistent.
- As guidelines evolve, individual patient preferences, disease severity, and comorbid conditions remain important considerations when selecting treatment agents for psoriasis.
- More frequent updates to psoriasis treatment guidelines are becoming increasingly important given the rapid changes in the field.
Emerging Therapies In Psoriasis: A Systematic Review
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.
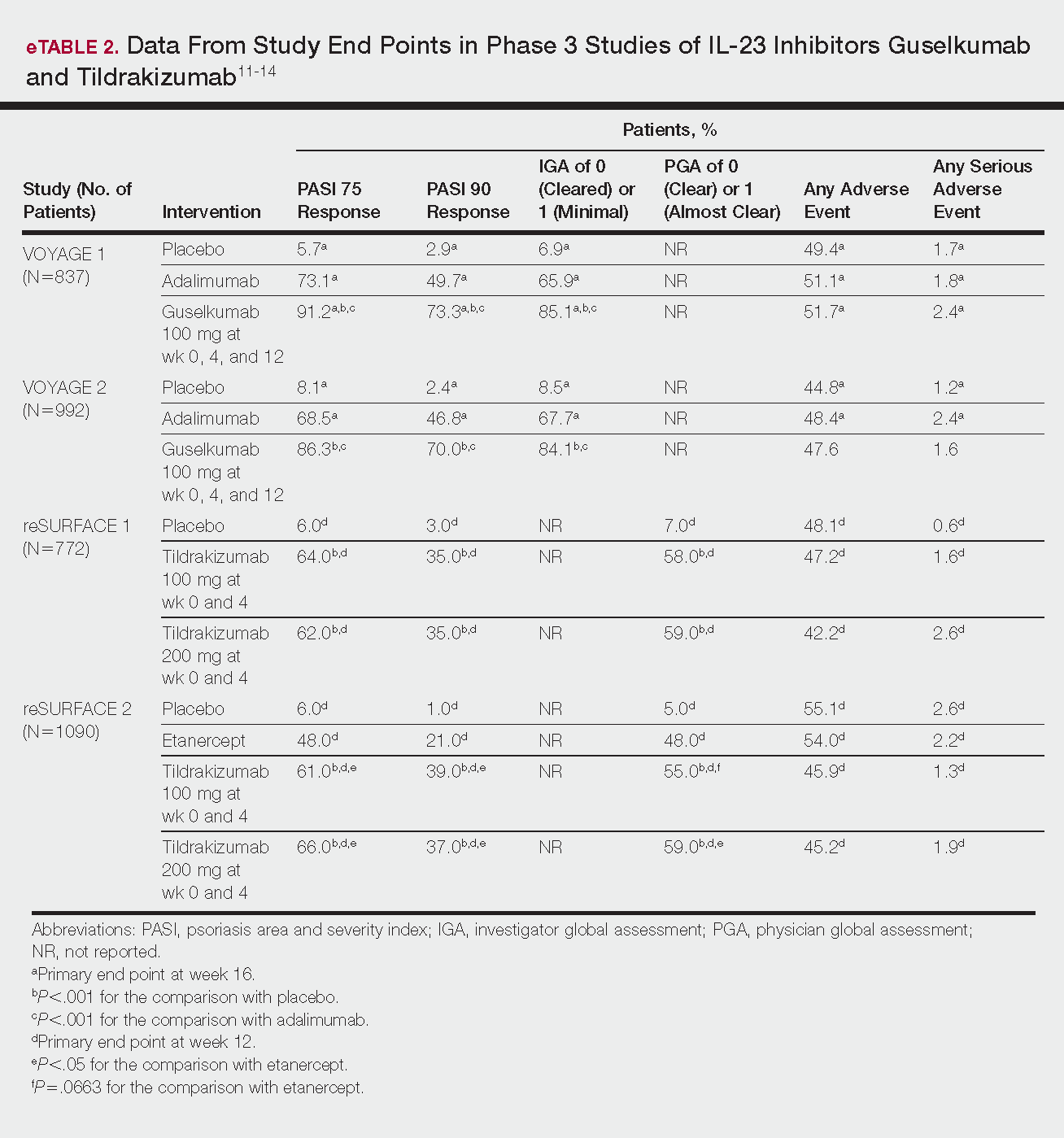
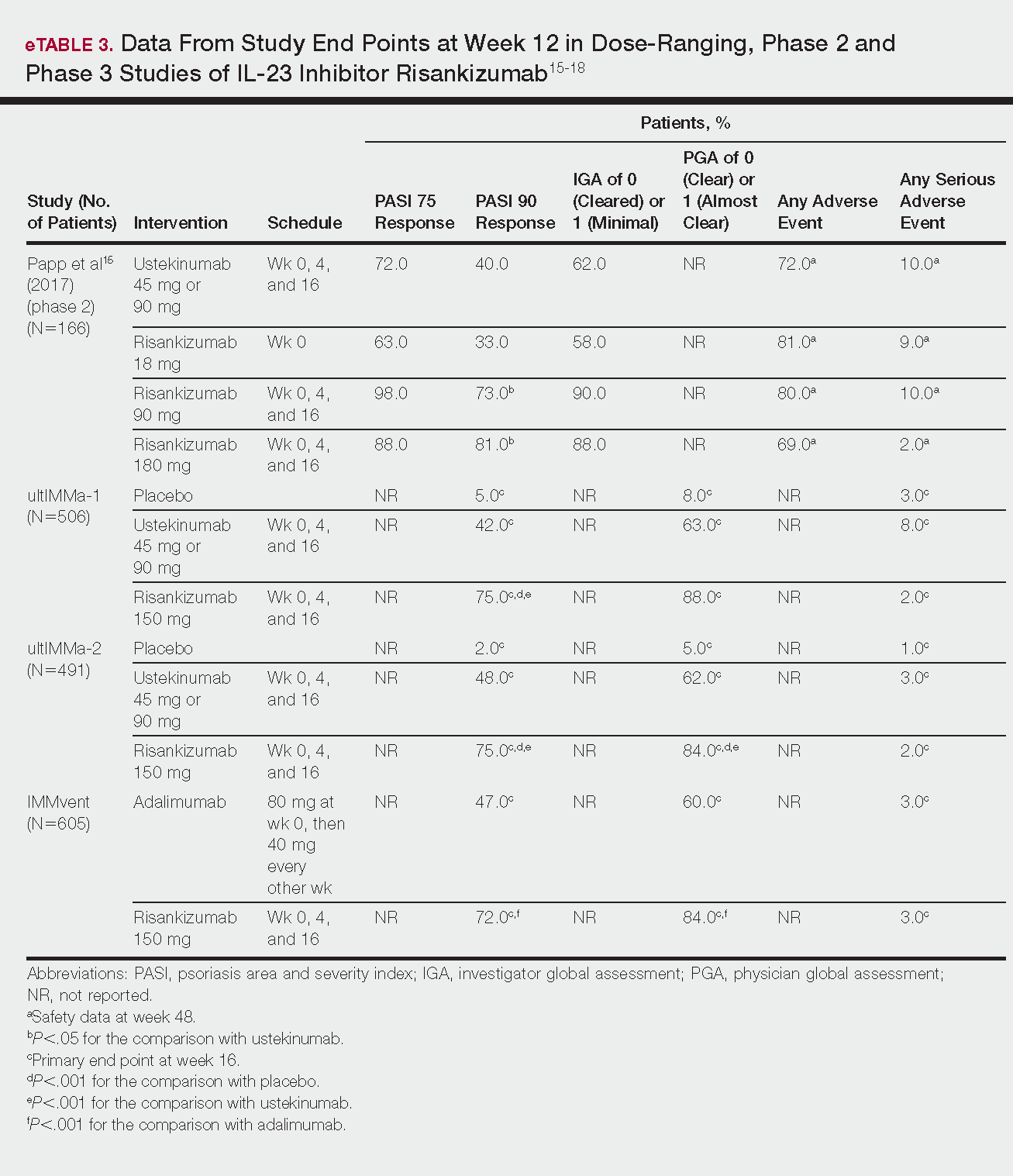
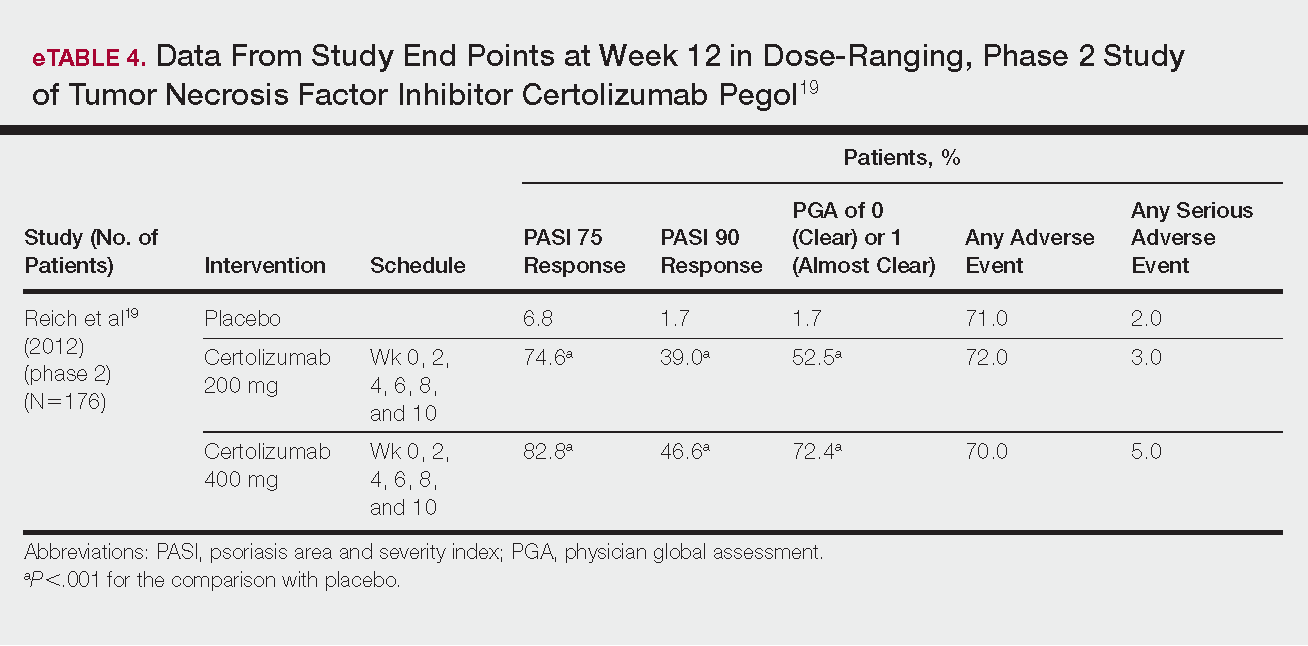
IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Practice Points
- Tumor necrosis factor α, IL-23, and IL-17A are key targets for psoriasis therapy based on an understanding of the key role that these cytokines play in the pathophysiology of disease.
- The biologic agents secukinumab and ixekizumab are approved for use in the management of psoriasis. Other biologics—brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol—have been (and some continue to be) the focus of phase 2 and phase 3 clinical trials.
- Findings of several of those trials support the idea that therapies targeting IL-23, specifically its p19 subunit, but that spare IL-12 are effective against psoriasis.
- Longer-term studies are needed to determine whether the agents reviewed here, including those approved for clinical use, are suitable for prolonged administration.
Deepithelialized Flaps and Grafts: Applications in Dermatologic Surgery
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15
Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).
Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Practice Points
- Deepithelialized flaps should be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.
- Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and reconstruction in areas of high mechanical tension.
Do Psoriasis Patients Engage In Vigorous Physical Activity?
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Practice Points
- Psoriasis is associated with comorbid disease conditions, including cardiovascular disease.
- Regular physical activity is known to decrease the risk of developing cardiovascular disease.
- Patients with psoriasis would likely benefit from regular participation in vigorous physical activity to help reduce the risk of developing cardiovascular disease.
Enhanced recovery after surgery for the patient with chronic pain
CASE Chronic pelvic pain from endometriosis
A 40-year-old woman (G0) has a 20-year history of chronic pelvic pain. Stage III endometriosis is diagnosed on laparoscopic excision of endometriotic tissue. Postoperative pain symptoms include dysmenorrhea and deep dyspareunia, and the patient is feeling anxious. Physical examination reveals a retroverted uterus, right adnexal fullness and tenderness, and tenderness on palpation of the right levator ani and right obturator internus; rectovaginal examination findings are unremarkable. The patient, though now engaged in a pelvic floor physical therapy program, has yet to achieve the pain control she desires. After reviewing the treatment strategies for endometriosis with the patient, she elects definitive surgical management with minimally invasive hysterectomy and salpingo-oophorectomy. What pre-, intra-, and postoperative pain management plan do you devise for this patient?
Chronic pelvic pain presents a unique clinical challenge, as pain typically is multifactorial, and several peripheral pain generators may be involved. Although surgery can be performed to manage anatomically based disease processes, it does not address pain from musculoskeletal or neuropathic sources. A complete medical history and a physical examination are of utmost importance in developing a comprehensive multimodal management plan that may include surgery as treatment for the pain.
The standard of care for surgery is a minimally invasive approach (vaginal, laparoscopic, or robot-assisted laparoscopic), as it causes the least amount of trauma. Benefits of minimally invasive surgery include shorter hospitalization and faster recovery, likely owing to improved perioperative pain control, decreased blood loss, and fewer infections. Although this approach minimizes surgical trauma and thereby helps decrease the surgical stress response, the patient experience can be optimized with use of enhanced recovery pathways (ERPs), a multimodal approach to perioperative care.
ERPs were initially proposed as a means of reducing the degree of surgical injury and the subsequent physiologic stress response.1 This multimodal approach begins in the outpatient setting, includes preoperative and intraoperative modalities, and continues postoperatively. In patients with chronic pain, ERPs are even more important. Assigning “prehabilitation” and setting expectations for surgery goals are the first step in improving the patient experience. Intraoperative use of opioid-sparing anesthetics or regional anesthesia can improve recovery. After surgery, patients with chronic pain and/or opioid dependence receive medications on a schedule, along with short-interval follow-up. Ultimately, reducing acute postoperative pain may lower the risk of developing chronic pain.
In this article on patients with chronic pelvic pain, we highlight elements of ERPs within the framework of enhanced recovery after surgery. Many of the interventions proposed here also can be used to improve the surgical experience of patients without chronic pain.
Preadmission education, expectations, and optimization
Preoperative counseling for elective procedures generally occurs in the outpatient setting. Although discussion traditionally has covered the type of procedure and its associated risks, benefits, and alternatives, new guidelines suggest a more mindful and comprehensive approach is warranted. Individualized patient-centered education programs have a positive impact on the perioperative course, effecting reductions in preoperative anxiety, opioid requirements, and hospital length of stay.2 From a pain management perspective, the clinician can take some time during preoperative counseling to inform the patient about the pain to be expected from surgery, the ways the pain will be managed intraoperatively and postoperatively, and the multimodal strategies that will be used throughout the patient’s stay2 and that may allow for early discharge. Although preadmission counseling still should address expectations for the surgery, it also presents an opportunity both to assess the patient’s ability to cope with the physical and psychological stress of surgery and to offer the patient appropriate need-based interventions, such as prehabilitation and cognitive-behavioral therapy (CBT).
Prehabilitation is the process of increasing functional capacity before surgery in order to mitigate the stress of the surgery. Prehabilitation may involve aerobic exercise, strength training, or functional task training. The gynecologic surgery literature lacks prehabilitation data, but data in the colorectal literature support use of a prehabilitation program for patients having a scheduled colectomy, with improved postoperative recovery.3 Although the colectomy cohort predominantly included older men, the principle that guides program implementation is the same: improve recovery after the stress of abdominal surgery. Indeed, a patient who opts for an elective surgery may have to wait several weeks before undergoing the procedure, and during this period behavioral interventions can take effect. With postoperative complications occurring more often in patients with reduced functional capacity, the data support using prehabilitation to decrease the incidence of postoperative complications, particularly among the most vulnerable patients.4 However, a definitive recommendation on use of pelvic floor exercises as an adjunct to prehabilitation cannot be made.4 Successful prehabilitation takes at least 4 weeks and should be part of a multimodal program that addresses other behavioral risk factors that may negatively affect recovery.5 For example, current tobacco users have compromised pulmonary status and wound healing immediately after surgery, and use more opioids.6 Conversely, smoking cessation for as little as 4 weeks before surgery is associated with fewer complications.7 In addition, given that alcohol abuse may compromise the surgical stress response and increase the risk of opioid misuse, addressing alcohol abuse preoperatively may improve postoperative recovery.8
Treating mood disorders that coexist with chronic pain disorders is an important part of outpatient multimodal management—psychological intervention is a useful adjunct to prehabilitation in reducing perioperative anxiety and improving postoperative functional capacity.9 For patients who have chronic pain and are undergoing surgery, it is important to address any anxiety, depression, or poor coping skills (eg, pain catastrophizing) to try to reduce the postoperative pain experience and decrease the risk of chronic postsurgical pain (CPSP).10,11
Before surgery, patients with chronic pain syndromes should be evaluated for emotional distress and pain coping ability. When possible, they should be referred to a pain psychologist, who can initiate CBT and other interventions. In addition, pain coping skills can be developed or reinforced to address preoperative anxiety and pain catastrophizing. These interventions, which may include use of visual imagery, breathing exercises, and other relaxation techniques, are applicable to the management of postoperative anxiety as well.
Read about preoperative multimodal analgesia and intra- and postoperative management.
Preoperative multimodal analgesia
Multimodal analgesia has several benefits. Simultaneous effects can be generated on multiple pain-related neurotransmitters, and a synergistic effect (eg, of acetaminophen and a nonsteroidal anti-inflammatory drug [NSAID]) can improve pain management. In addition, small doses of multiple medications can be given, instead of a large dose of a single medication. Of course, this strategy must be modified in elderly and patients with impaired renal function, who are at high risk for polypharmacy.
Preoperative administration of 3 medications—a selective cyclooxygenase 2 (COX-2) inhibitor, acetaminophen, and a gabapentinoid—is increasingly accepted as part of multimodal analgesia. The selective COX-2 inhibitor targets inflammatory prostaglandins and has anti-inflammatory and analgesic effects; acetaminophen, an effective analgesic with an unclear mechanism of action, can reduce postoperative opioid consumption12 and works synergistically with NSAIDs13; and the gabapentinoid gabapentin has an analgesic effect likely contributing to decreased movement-related pain and subsequent improved functional recovery (data are mixed on whether continuing gabapentin after surgery prevents CPSP).14−16
Although serotonin and norepinephrine reuptake inhibitors (SNRIs) are commonly used in outpatient management of chronic pelvic pain, data suggest that their role in perioperative pain management is evolving. As SNRIs may reduce central nervous system (CNS) sensitization,17 their analgesic effect is thought to result from increased descending inhibitory tone in the CNS, which makes this class of medication ideal for patients with chronic neuropathic pain.15
Limited data also suggest a role for SNRIs in decreasing immediate postoperative pain and CPSP in high-risk patients. Studies of duloxetine use in the immediate perioperative period have found reduced postoperative acute pain and opioid use.18,19 In addition, a short course of low-dose (37.5 mg) venlafaxine both before and after surgery has demonstrated a reduction in postoperative opioid use and a reduction in movement-related pain 6 months after surgery.20
Intraoperative management
The surgical and anesthesia teams share the goal of optimizing both pain control and postoperative recovery. Surgical team members, who want longer-acting anesthetics for infiltration of incision sites, discuss with the anesthesiologist the appropriateness of using peripheral nerve blocks or neuraxial anesthesia, given the patient’s history and planned procedure. Anesthesia team members can improve anesthesia and minimize intraoperative opioid use through several methods, including total intravenous anesthesia,21 dexamethasone,22 ketorolac,23 and intravenous ketamine. Ketamine, in particular, has a wide range of surgical applications and has been found to reduce postoperative pain, postoperative pain medication use, and the risk of CPSP.2
Incision sites should be infiltrated before and after surgery. Lidocaine traditionally is used for its rapid onset of action in reducing surgical site pain, but its short half-life may limit its applicability to postoperative pain. Recently, bupivacaine (half-life, 3.5 hours) and liposomal bupivacaine (24–34 hours) have gained more attention. Both of these medications appear to be as effective as lidocaine in reducing surgical site pain.24
Transversus abdominis plane (TAP) blocks have been used as an adjunct in pain management during abdominopelvic surgery. Although initial data on postoperative pain and opioid use reductions with TAP blocks were inconclusive,25 more recent data showed a role for TAP blocks in a multimodal approach for reducing opioid use during laparoscopic and open surgery.26,27 Given the small number of studies on using liposomal bupivacaine for peripheral nerve blocks (eg, TAP blocks) in postoperative pain management, current data are inconclusive.28
Postoperative management
The ERP approach calls for continuing multimodal analgesia after surgery—in most cases, scheduling early use of oral acetaminophen and ibuprofen, and providing short-acting, low-dose opioid analgesia as needed. All patients should be given a bowel regimen. Similar to undergoing prehabilitation for surgery, patients should prepare themselves for recovery. They should be encouraged to engage in early ambulation and oral intake and, when clinically appropriate, be given same-day discharge for minimally invasive surgical procedures.
Patients with chronic pain before surgery are at increased risk for suboptimal postoperative pain management, and those who are dependent on opioids require additional perioperative measures for adequate postoperative pain control. In these complicated cases, it is appropriate to enlist a pain specialist, potentially before surgery, to help plan perioperative and postoperative pain management.2 Postoperative pain management for opioid-dependent patients should include pharmacologic and nonpharmacologic interventions, such as use of nonopioid medications (eg, gabapentin) and continuation of CBT. Patients with chronic pain should be closely followed up for assessment of postoperative pain control and recovery.
CASE Resolved
Surgical management is one aspect of the longer term multimodal pain management strategy for this patient. After preoperative pelvic floor physical therapy, she is receptive to starting a trial of an SNRI for her pain and mood symptoms. Both interventions allow for optimization of her preoperative physical and psychological status. Expectations are set that she will be discharged the day of surgery and that the surgery is but one component of her multimodal treatment plan. In addition, before surgery, she takes oral acetaminophen, gabapentin, and celecoxib—previously having had no contraindications to these medications. During surgery, bupivacaine is used for infiltration of all incision sites, and the anesthesia team administers ketamine and a TAP block. After surgery, the patient is prepared for same-day discharge and given the NSAIDs and acetaminophen she is scheduled to take over the next 72 hours. She is also given a limited prescription for oxycodone for breakthrough pain. An office visit 1 to 2 weeks after surgery is scheduled.
ERP strategies for surgical management of endometriosis have not only improved this patient’s postoperative recovery but also reduced her surgical stress response and subsequent transition to chronic postoperative pain. Many of the strategies used in this case are applicable to patients without chronic pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606−617.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131−157.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505−514.
- Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189−1201.
- Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery [published online ahead of print January 13, 2018]. Anaesthesia. doi:10.1111/anae.14177.
- Chiang HL, Chia YY, Lin HS, Chen CH. The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res Manag. 2016;2016:9432493.
- Mastracci TM, Carli F, Finley RJ, Muccio S, Warner DO; Members of the Evidence-Based Reviews in Surgery Group. Effect of preoperative smoking cessation interventions on postoperative complications. J Am Coll Surg. 2011;212(6):1094−1096.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869−874.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937−947.
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122−131.
- Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045−1057.
- Moon YE, Lee YK, Lee J, Moon DE. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284(6):1455−1460.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170−1179.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428−442.
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456−472.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2−S15.
- Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: a prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122(1):98−104.
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865−869.
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;(2):CD011419.
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;(12):CD007705.
- Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis [published online ahead of print January 30, 2018]. Colorectal Dis. doi:10.1111/codi.14037.
- Staker JJ, Liu D, Church R, et al. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section [published online ahead of print January 29, 2018]. Anaesthesia. doi:10.1111/anae.14222.
- Hamilton TW, Athanassoglou V, Trivella M, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;(8):CD011476.
CASE Chronic pelvic pain from endometriosis
A 40-year-old woman (G0) has a 20-year history of chronic pelvic pain. Stage III endometriosis is diagnosed on laparoscopic excision of endometriotic tissue. Postoperative pain symptoms include dysmenorrhea and deep dyspareunia, and the patient is feeling anxious. Physical examination reveals a retroverted uterus, right adnexal fullness and tenderness, and tenderness on palpation of the right levator ani and right obturator internus; rectovaginal examination findings are unremarkable. The patient, though now engaged in a pelvic floor physical therapy program, has yet to achieve the pain control she desires. After reviewing the treatment strategies for endometriosis with the patient, she elects definitive surgical management with minimally invasive hysterectomy and salpingo-oophorectomy. What pre-, intra-, and postoperative pain management plan do you devise for this patient?
Chronic pelvic pain presents a unique clinical challenge, as pain typically is multifactorial, and several peripheral pain generators may be involved. Although surgery can be performed to manage anatomically based disease processes, it does not address pain from musculoskeletal or neuropathic sources. A complete medical history and a physical examination are of utmost importance in developing a comprehensive multimodal management plan that may include surgery as treatment for the pain.
The standard of care for surgery is a minimally invasive approach (vaginal, laparoscopic, or robot-assisted laparoscopic), as it causes the least amount of trauma. Benefits of minimally invasive surgery include shorter hospitalization and faster recovery, likely owing to improved perioperative pain control, decreased blood loss, and fewer infections. Although this approach minimizes surgical trauma and thereby helps decrease the surgical stress response, the patient experience can be optimized with use of enhanced recovery pathways (ERPs), a multimodal approach to perioperative care.
ERPs were initially proposed as a means of reducing the degree of surgical injury and the subsequent physiologic stress response.1 This multimodal approach begins in the outpatient setting, includes preoperative and intraoperative modalities, and continues postoperatively. In patients with chronic pain, ERPs are even more important. Assigning “prehabilitation” and setting expectations for surgery goals are the first step in improving the patient experience. Intraoperative use of opioid-sparing anesthetics or regional anesthesia can improve recovery. After surgery, patients with chronic pain and/or opioid dependence receive medications on a schedule, along with short-interval follow-up. Ultimately, reducing acute postoperative pain may lower the risk of developing chronic pain.
In this article on patients with chronic pelvic pain, we highlight elements of ERPs within the framework of enhanced recovery after surgery. Many of the interventions proposed here also can be used to improve the surgical experience of patients without chronic pain.

Preadmission education, expectations, and optimization
Preoperative counseling for elective procedures generally occurs in the outpatient setting. Although discussion traditionally has covered the type of procedure and its associated risks, benefits, and alternatives, new guidelines suggest a more mindful and comprehensive approach is warranted. Individualized patient-centered education programs have a positive impact on the perioperative course, effecting reductions in preoperative anxiety, opioid requirements, and hospital length of stay.2 From a pain management perspective, the clinician can take some time during preoperative counseling to inform the patient about the pain to be expected from surgery, the ways the pain will be managed intraoperatively and postoperatively, and the multimodal strategies that will be used throughout the patient’s stay2 and that may allow for early discharge. Although preadmission counseling still should address expectations for the surgery, it also presents an opportunity both to assess the patient’s ability to cope with the physical and psychological stress of surgery and to offer the patient appropriate need-based interventions, such as prehabilitation and cognitive-behavioral therapy (CBT).
Prehabilitation is the process of increasing functional capacity before surgery in order to mitigate the stress of the surgery. Prehabilitation may involve aerobic exercise, strength training, or functional task training. The gynecologic surgery literature lacks prehabilitation data, but data in the colorectal literature support use of a prehabilitation program for patients having a scheduled colectomy, with improved postoperative recovery.3 Although the colectomy cohort predominantly included older men, the principle that guides program implementation is the same: improve recovery after the stress of abdominal surgery. Indeed, a patient who opts for an elective surgery may have to wait several weeks before undergoing the procedure, and during this period behavioral interventions can take effect. With postoperative complications occurring more often in patients with reduced functional capacity, the data support using prehabilitation to decrease the incidence of postoperative complications, particularly among the most vulnerable patients.4 However, a definitive recommendation on use of pelvic floor exercises as an adjunct to prehabilitation cannot be made.4 Successful prehabilitation takes at least 4 weeks and should be part of a multimodal program that addresses other behavioral risk factors that may negatively affect recovery.5 For example, current tobacco users have compromised pulmonary status and wound healing immediately after surgery, and use more opioids.6 Conversely, smoking cessation for as little as 4 weeks before surgery is associated with fewer complications.7 In addition, given that alcohol abuse may compromise the surgical stress response and increase the risk of opioid misuse, addressing alcohol abuse preoperatively may improve postoperative recovery.8
Treating mood disorders that coexist with chronic pain disorders is an important part of outpatient multimodal management—psychological intervention is a useful adjunct to prehabilitation in reducing perioperative anxiety and improving postoperative functional capacity.9 For patients who have chronic pain and are undergoing surgery, it is important to address any anxiety, depression, or poor coping skills (eg, pain catastrophizing) to try to reduce the postoperative pain experience and decrease the risk of chronic postsurgical pain (CPSP).10,11
Before surgery, patients with chronic pain syndromes should be evaluated for emotional distress and pain coping ability. When possible, they should be referred to a pain psychologist, who can initiate CBT and other interventions. In addition, pain coping skills can be developed or reinforced to address preoperative anxiety and pain catastrophizing. These interventions, which may include use of visual imagery, breathing exercises, and other relaxation techniques, are applicable to the management of postoperative anxiety as well.
Read about preoperative multimodal analgesia and intra- and postoperative management.
Preoperative multimodal analgesia
Multimodal analgesia has several benefits. Simultaneous effects can be generated on multiple pain-related neurotransmitters, and a synergistic effect (eg, of acetaminophen and a nonsteroidal anti-inflammatory drug [NSAID]) can improve pain management. In addition, small doses of multiple medications can be given, instead of a large dose of a single medication. Of course, this strategy must be modified in elderly and patients with impaired renal function, who are at high risk for polypharmacy.
Preoperative administration of 3 medications—a selective cyclooxygenase 2 (COX-2) inhibitor, acetaminophen, and a gabapentinoid—is increasingly accepted as part of multimodal analgesia. The selective COX-2 inhibitor targets inflammatory prostaglandins and has anti-inflammatory and analgesic effects; acetaminophen, an effective analgesic with an unclear mechanism of action, can reduce postoperative opioid consumption12 and works synergistically with NSAIDs13; and the gabapentinoid gabapentin has an analgesic effect likely contributing to decreased movement-related pain and subsequent improved functional recovery (data are mixed on whether continuing gabapentin after surgery prevents CPSP).14−16
Although serotonin and norepinephrine reuptake inhibitors (SNRIs) are commonly used in outpatient management of chronic pelvic pain, data suggest that their role in perioperative pain management is evolving. As SNRIs may reduce central nervous system (CNS) sensitization,17 their analgesic effect is thought to result from increased descending inhibitory tone in the CNS, which makes this class of medication ideal for patients with chronic neuropathic pain.15
Limited data also suggest a role for SNRIs in decreasing immediate postoperative pain and CPSP in high-risk patients. Studies of duloxetine use in the immediate perioperative period have found reduced postoperative acute pain and opioid use.18,19 In addition, a short course of low-dose (37.5 mg) venlafaxine both before and after surgery has demonstrated a reduction in postoperative opioid use and a reduction in movement-related pain 6 months after surgery.20
Intraoperative management
The surgical and anesthesia teams share the goal of optimizing both pain control and postoperative recovery. Surgical team members, who want longer-acting anesthetics for infiltration of incision sites, discuss with the anesthesiologist the appropriateness of using peripheral nerve blocks or neuraxial anesthesia, given the patient’s history and planned procedure. Anesthesia team members can improve anesthesia and minimize intraoperative opioid use through several methods, including total intravenous anesthesia,21 dexamethasone,22 ketorolac,23 and intravenous ketamine. Ketamine, in particular, has a wide range of surgical applications and has been found to reduce postoperative pain, postoperative pain medication use, and the risk of CPSP.2
Incision sites should be infiltrated before and after surgery. Lidocaine traditionally is used for its rapid onset of action in reducing surgical site pain, but its short half-life may limit its applicability to postoperative pain. Recently, bupivacaine (half-life, 3.5 hours) and liposomal bupivacaine (24–34 hours) have gained more attention. Both of these medications appear to be as effective as lidocaine in reducing surgical site pain.24
Transversus abdominis plane (TAP) blocks have been used as an adjunct in pain management during abdominopelvic surgery. Although initial data on postoperative pain and opioid use reductions with TAP blocks were inconclusive,25 more recent data showed a role for TAP blocks in a multimodal approach for reducing opioid use during laparoscopic and open surgery.26,27 Given the small number of studies on using liposomal bupivacaine for peripheral nerve blocks (eg, TAP blocks) in postoperative pain management, current data are inconclusive.28
Postoperative management
The ERP approach calls for continuing multimodal analgesia after surgery—in most cases, scheduling early use of oral acetaminophen and ibuprofen, and providing short-acting, low-dose opioid analgesia as needed. All patients should be given a bowel regimen. Similar to undergoing prehabilitation for surgery, patients should prepare themselves for recovery. They should be encouraged to engage in early ambulation and oral intake and, when clinically appropriate, be given same-day discharge for minimally invasive surgical procedures.
Patients with chronic pain before surgery are at increased risk for suboptimal postoperative pain management, and those who are dependent on opioids require additional perioperative measures for adequate postoperative pain control. In these complicated cases, it is appropriate to enlist a pain specialist, potentially before surgery, to help plan perioperative and postoperative pain management.2 Postoperative pain management for opioid-dependent patients should include pharmacologic and nonpharmacologic interventions, such as use of nonopioid medications (eg, gabapentin) and continuation of CBT. Patients with chronic pain should be closely followed up for assessment of postoperative pain control and recovery.
CASE Resolved
Surgical management is one aspect of the longer term multimodal pain management strategy for this patient. After preoperative pelvic floor physical therapy, she is receptive to starting a trial of an SNRI for her pain and mood symptoms. Both interventions allow for optimization of her preoperative physical and psychological status. Expectations are set that she will be discharged the day of surgery and that the surgery is but one component of her multimodal treatment plan. In addition, before surgery, she takes oral acetaminophen, gabapentin, and celecoxib—previously having had no contraindications to these medications. During surgery, bupivacaine is used for infiltration of all incision sites, and the anesthesia team administers ketamine and a TAP block. After surgery, the patient is prepared for same-day discharge and given the NSAIDs and acetaminophen she is scheduled to take over the next 72 hours. She is also given a limited prescription for oxycodone for breakthrough pain. An office visit 1 to 2 weeks after surgery is scheduled.
ERP strategies for surgical management of endometriosis have not only improved this patient’s postoperative recovery but also reduced her surgical stress response and subsequent transition to chronic postoperative pain. Many of the strategies used in this case are applicable to patients without chronic pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Chronic pelvic pain from endometriosis
A 40-year-old woman (G0) has a 20-year history of chronic pelvic pain. Stage III endometriosis is diagnosed on laparoscopic excision of endometriotic tissue. Postoperative pain symptoms include dysmenorrhea and deep dyspareunia, and the patient is feeling anxious. Physical examination reveals a retroverted uterus, right adnexal fullness and tenderness, and tenderness on palpation of the right levator ani and right obturator internus; rectovaginal examination findings are unremarkable. The patient, though now engaged in a pelvic floor physical therapy program, has yet to achieve the pain control she desires. After reviewing the treatment strategies for endometriosis with the patient, she elects definitive surgical management with minimally invasive hysterectomy and salpingo-oophorectomy. What pre-, intra-, and postoperative pain management plan do you devise for this patient?
Chronic pelvic pain presents a unique clinical challenge, as pain typically is multifactorial, and several peripheral pain generators may be involved. Although surgery can be performed to manage anatomically based disease processes, it does not address pain from musculoskeletal or neuropathic sources. A complete medical history and a physical examination are of utmost importance in developing a comprehensive multimodal management plan that may include surgery as treatment for the pain.
The standard of care for surgery is a minimally invasive approach (vaginal, laparoscopic, or robot-assisted laparoscopic), as it causes the least amount of trauma. Benefits of minimally invasive surgery include shorter hospitalization and faster recovery, likely owing to improved perioperative pain control, decreased blood loss, and fewer infections. Although this approach minimizes surgical trauma and thereby helps decrease the surgical stress response, the patient experience can be optimized with use of enhanced recovery pathways (ERPs), a multimodal approach to perioperative care.
ERPs were initially proposed as a means of reducing the degree of surgical injury and the subsequent physiologic stress response.1 This multimodal approach begins in the outpatient setting, includes preoperative and intraoperative modalities, and continues postoperatively. In patients with chronic pain, ERPs are even more important. Assigning “prehabilitation” and setting expectations for surgery goals are the first step in improving the patient experience. Intraoperative use of opioid-sparing anesthetics or regional anesthesia can improve recovery. After surgery, patients with chronic pain and/or opioid dependence receive medications on a schedule, along with short-interval follow-up. Ultimately, reducing acute postoperative pain may lower the risk of developing chronic pain.
In this article on patients with chronic pelvic pain, we highlight elements of ERPs within the framework of enhanced recovery after surgery. Many of the interventions proposed here also can be used to improve the surgical experience of patients without chronic pain.

Preadmission education, expectations, and optimization
Preoperative counseling for elective procedures generally occurs in the outpatient setting. Although discussion traditionally has covered the type of procedure and its associated risks, benefits, and alternatives, new guidelines suggest a more mindful and comprehensive approach is warranted. Individualized patient-centered education programs have a positive impact on the perioperative course, effecting reductions in preoperative anxiety, opioid requirements, and hospital length of stay.2 From a pain management perspective, the clinician can take some time during preoperative counseling to inform the patient about the pain to be expected from surgery, the ways the pain will be managed intraoperatively and postoperatively, and the multimodal strategies that will be used throughout the patient’s stay2 and that may allow for early discharge. Although preadmission counseling still should address expectations for the surgery, it also presents an opportunity both to assess the patient’s ability to cope with the physical and psychological stress of surgery and to offer the patient appropriate need-based interventions, such as prehabilitation and cognitive-behavioral therapy (CBT).
Prehabilitation is the process of increasing functional capacity before surgery in order to mitigate the stress of the surgery. Prehabilitation may involve aerobic exercise, strength training, or functional task training. The gynecologic surgery literature lacks prehabilitation data, but data in the colorectal literature support use of a prehabilitation program for patients having a scheduled colectomy, with improved postoperative recovery.3 Although the colectomy cohort predominantly included older men, the principle that guides program implementation is the same: improve recovery after the stress of abdominal surgery. Indeed, a patient who opts for an elective surgery may have to wait several weeks before undergoing the procedure, and during this period behavioral interventions can take effect. With postoperative complications occurring more often in patients with reduced functional capacity, the data support using prehabilitation to decrease the incidence of postoperative complications, particularly among the most vulnerable patients.4 However, a definitive recommendation on use of pelvic floor exercises as an adjunct to prehabilitation cannot be made.4 Successful prehabilitation takes at least 4 weeks and should be part of a multimodal program that addresses other behavioral risk factors that may negatively affect recovery.5 For example, current tobacco users have compromised pulmonary status and wound healing immediately after surgery, and use more opioids.6 Conversely, smoking cessation for as little as 4 weeks before surgery is associated with fewer complications.7 In addition, given that alcohol abuse may compromise the surgical stress response and increase the risk of opioid misuse, addressing alcohol abuse preoperatively may improve postoperative recovery.8
Treating mood disorders that coexist with chronic pain disorders is an important part of outpatient multimodal management—psychological intervention is a useful adjunct to prehabilitation in reducing perioperative anxiety and improving postoperative functional capacity.9 For patients who have chronic pain and are undergoing surgery, it is important to address any anxiety, depression, or poor coping skills (eg, pain catastrophizing) to try to reduce the postoperative pain experience and decrease the risk of chronic postsurgical pain (CPSP).10,11
Before surgery, patients with chronic pain syndromes should be evaluated for emotional distress and pain coping ability. When possible, they should be referred to a pain psychologist, who can initiate CBT and other interventions. In addition, pain coping skills can be developed or reinforced to address preoperative anxiety and pain catastrophizing. These interventions, which may include use of visual imagery, breathing exercises, and other relaxation techniques, are applicable to the management of postoperative anxiety as well.
Read about preoperative multimodal analgesia and intra- and postoperative management.
Preoperative multimodal analgesia
Multimodal analgesia has several benefits. Simultaneous effects can be generated on multiple pain-related neurotransmitters, and a synergistic effect (eg, of acetaminophen and a nonsteroidal anti-inflammatory drug [NSAID]) can improve pain management. In addition, small doses of multiple medications can be given, instead of a large dose of a single medication. Of course, this strategy must be modified in elderly and patients with impaired renal function, who are at high risk for polypharmacy.
Preoperative administration of 3 medications—a selective cyclooxygenase 2 (COX-2) inhibitor, acetaminophen, and a gabapentinoid—is increasingly accepted as part of multimodal analgesia. The selective COX-2 inhibitor targets inflammatory prostaglandins and has anti-inflammatory and analgesic effects; acetaminophen, an effective analgesic with an unclear mechanism of action, can reduce postoperative opioid consumption12 and works synergistically with NSAIDs13; and the gabapentinoid gabapentin has an analgesic effect likely contributing to decreased movement-related pain and subsequent improved functional recovery (data are mixed on whether continuing gabapentin after surgery prevents CPSP).14−16
Although serotonin and norepinephrine reuptake inhibitors (SNRIs) are commonly used in outpatient management of chronic pelvic pain, data suggest that their role in perioperative pain management is evolving. As SNRIs may reduce central nervous system (CNS) sensitization,17 their analgesic effect is thought to result from increased descending inhibitory tone in the CNS, which makes this class of medication ideal for patients with chronic neuropathic pain.15
Limited data also suggest a role for SNRIs in decreasing immediate postoperative pain and CPSP in high-risk patients. Studies of duloxetine use in the immediate perioperative period have found reduced postoperative acute pain and opioid use.18,19 In addition, a short course of low-dose (37.5 mg) venlafaxine both before and after surgery has demonstrated a reduction in postoperative opioid use and a reduction in movement-related pain 6 months after surgery.20
Intraoperative management
The surgical and anesthesia teams share the goal of optimizing both pain control and postoperative recovery. Surgical team members, who want longer-acting anesthetics for infiltration of incision sites, discuss with the anesthesiologist the appropriateness of using peripheral nerve blocks or neuraxial anesthesia, given the patient’s history and planned procedure. Anesthesia team members can improve anesthesia and minimize intraoperative opioid use through several methods, including total intravenous anesthesia,21 dexamethasone,22 ketorolac,23 and intravenous ketamine. Ketamine, in particular, has a wide range of surgical applications and has been found to reduce postoperative pain, postoperative pain medication use, and the risk of CPSP.2
Incision sites should be infiltrated before and after surgery. Lidocaine traditionally is used for its rapid onset of action in reducing surgical site pain, but its short half-life may limit its applicability to postoperative pain. Recently, bupivacaine (half-life, 3.5 hours) and liposomal bupivacaine (24–34 hours) have gained more attention. Both of these medications appear to be as effective as lidocaine in reducing surgical site pain.24
Transversus abdominis plane (TAP) blocks have been used as an adjunct in pain management during abdominopelvic surgery. Although initial data on postoperative pain and opioid use reductions with TAP blocks were inconclusive,25 more recent data showed a role for TAP blocks in a multimodal approach for reducing opioid use during laparoscopic and open surgery.26,27 Given the small number of studies on using liposomal bupivacaine for peripheral nerve blocks (eg, TAP blocks) in postoperative pain management, current data are inconclusive.28
Postoperative management
The ERP approach calls for continuing multimodal analgesia after surgery—in most cases, scheduling early use of oral acetaminophen and ibuprofen, and providing short-acting, low-dose opioid analgesia as needed. All patients should be given a bowel regimen. Similar to undergoing prehabilitation for surgery, patients should prepare themselves for recovery. They should be encouraged to engage in early ambulation and oral intake and, when clinically appropriate, be given same-day discharge for minimally invasive surgical procedures.
Patients with chronic pain before surgery are at increased risk for suboptimal postoperative pain management, and those who are dependent on opioids require additional perioperative measures for adequate postoperative pain control. In these complicated cases, it is appropriate to enlist a pain specialist, potentially before surgery, to help plan perioperative and postoperative pain management.2 Postoperative pain management for opioid-dependent patients should include pharmacologic and nonpharmacologic interventions, such as use of nonopioid medications (eg, gabapentin) and continuation of CBT. Patients with chronic pain should be closely followed up for assessment of postoperative pain control and recovery.
CASE Resolved
Surgical management is one aspect of the longer term multimodal pain management strategy for this patient. After preoperative pelvic floor physical therapy, she is receptive to starting a trial of an SNRI for her pain and mood symptoms. Both interventions allow for optimization of her preoperative physical and psychological status. Expectations are set that she will be discharged the day of surgery and that the surgery is but one component of her multimodal treatment plan. In addition, before surgery, she takes oral acetaminophen, gabapentin, and celecoxib—previously having had no contraindications to these medications. During surgery, bupivacaine is used for infiltration of all incision sites, and the anesthesia team administers ketamine and a TAP block. After surgery, the patient is prepared for same-day discharge and given the NSAIDs and acetaminophen she is scheduled to take over the next 72 hours. She is also given a limited prescription for oxycodone for breakthrough pain. An office visit 1 to 2 weeks after surgery is scheduled.
ERP strategies for surgical management of endometriosis have not only improved this patient’s postoperative recovery but also reduced her surgical stress response and subsequent transition to chronic postoperative pain. Many of the strategies used in this case are applicable to patients without chronic pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606−617.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131−157.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505−514.
- Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189−1201.
- Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery [published online ahead of print January 13, 2018]. Anaesthesia. doi:10.1111/anae.14177.
- Chiang HL, Chia YY, Lin HS, Chen CH. The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res Manag. 2016;2016:9432493.
- Mastracci TM, Carli F, Finley RJ, Muccio S, Warner DO; Members of the Evidence-Based Reviews in Surgery Group. Effect of preoperative smoking cessation interventions on postoperative complications. J Am Coll Surg. 2011;212(6):1094−1096.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869−874.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937−947.
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122−131.
- Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045−1057.
- Moon YE, Lee YK, Lee J, Moon DE. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284(6):1455−1460.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170−1179.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428−442.
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456−472.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2−S15.
- Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: a prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122(1):98−104.
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865−869.
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;(2):CD011419.
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;(12):CD007705.
- Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis [published online ahead of print January 30, 2018]. Colorectal Dis. doi:10.1111/codi.14037.
- Staker JJ, Liu D, Church R, et al. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section [published online ahead of print January 29, 2018]. Anaesthesia. doi:10.1111/anae.14222.
- Hamilton TW, Athanassoglou V, Trivella M, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;(8):CD011476.
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606−617.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131−157.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505−514.
- Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189−1201.
- Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery [published online ahead of print January 13, 2018]. Anaesthesia. doi:10.1111/anae.14177.
- Chiang HL, Chia YY, Lin HS, Chen CH. The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res Manag. 2016;2016:9432493.
- Mastracci TM, Carli F, Finley RJ, Muccio S, Warner DO; Members of the Evidence-Based Reviews in Surgery Group. Effect of preoperative smoking cessation interventions on postoperative complications. J Am Coll Surg. 2011;212(6):1094−1096.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869−874.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937−947.
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122−131.
- Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045−1057.
- Moon YE, Lee YK, Lee J, Moon DE. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284(6):1455−1460.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170−1179.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428−442.
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456−472.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2−S15.
- Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: a prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122(1):98−104.
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865−869.
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;(2):CD011419.
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;(12):CD007705.
- Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis [published online ahead of print January 30, 2018]. Colorectal Dis. doi:10.1111/codi.14037.
- Staker JJ, Liu D, Church R, et al. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section [published online ahead of print January 29, 2018]. Anaesthesia. doi:10.1111/anae.14222.
- Hamilton TW, Athanassoglou V, Trivella M, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;(8):CD011476.
Endometriosis: Expert perspectives on medical and surgical management
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.

Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.

Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Take-home points
- Endometriosis management involves fluidity of care. Treatment approaches will change throughout a patient's reproductive life, depending on the patient's presenting symptoms and reproductive goals.
- Inform the patient of the disease process and how it may affect her menstrual pain symptoms and family planning.
- Educate patients so they may effectively participate in the management discussion. Hear the voice of the patient to make a tailored plan of care for each individual.
- Endometriosis can be a complex medical problem. Use a comprehensive multidisciplinary approach when appropriate.
Watch: Video roundtable–Endometriosis: Expert perspectives on medical and surgical management
2018 Update on gynecologic cancer
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.

The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.

The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
Antibiotic Overprescribing: Still a Major Concern

Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections (CDI), allergic reactions, and increased health care costs (see Table 1).1-6 And yet, clinicians continue to overprescribe this class of medication.

A 2016 report from the CDC estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a postantibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program (now known as “Be Antibiotics Aware”), focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) in 2015 developed a five-year strategic framework for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
WHEN AND WHERE ARE ANTIBIOTICS MOST OFTEN INAPPROPRIATELY PRESCRIBED?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 Refer to national clinical guidelines, which delineate when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, there are conflicting findings as to whether antibiotic prescribing differs in office-based versus emergency department (ED) settings.
- One study found a higher rate of antibiotic prescribing during ED visits than office visits (21% vs 9%), even though, between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- In a cross-sectional study using data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS), more than half of patients with uncomplicated acute rhinosinusitis received a prescription for antibiotics, but there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs did (51%).12
STICK TO NARROW-SPECTRUM AGENTS WHEN POSSIBLE
Using broad-spectrum antibiotics, such as quinolones or imipenem, firstline, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which clinicians prescribe narrow-spectrum, firstline antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that providers used firstline agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate firstline antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non-firstline antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), clinicians should prescribe a narrow-spectrum antibiotic first.
ANTIBIOTIC OVERPRESCIBING AFFECTS THE GUT AND BEYOND
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by CDI. CDI is now the most common health care-related infection, accounting for about a half-million health care facility infections per year.22 It extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early HIV infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in two-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn disease, type 2 diabetes, and obesity. (For a discussion of the relationship between the gut microbiome and diabetes, see Endocrine Consult: The Gut Microbiome in Type 2 Diabetes.)
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children younger than 24 months of age increases the risk for childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis, resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiomes of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.
WHAT CAN WE DO RIGHT NOW?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why clinicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and fear of losing patients are major reasons providers cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis, clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (see Table 2).34-36 
One example comes from a 1996-1998 study of four primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated multiple elements, including office-based and household patient educational materials and a clinician intervention involving education, practice profiling, and academic detailing. Clinicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
Employing EMRs. A more recent study focused on use of electronic medical records (EMRs) and communications to modify clinician antibiotic prescribing.35 By sending clinicians monthly emails comparing their prescribing patterns to those of peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified providers’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the clinician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged providers to follow prescribing guidelines by capitalizing on their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics.
Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19%, compared with about a 9% reduction in the control groups.36,37
DOES PRESCRIBING ANTIBIOTICS AFFECT PATIENT SATISFACTION?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with clinicians who explained why antibiotics were not indicated versus those who simply prescribed them—and that such explanations do not need to take a lot of time (see Table 3 for patient care tips).37,38
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
REDUCING ANTIBIOTIC PRESCRIBING REDUCES RESISTANCE
There is also strong evidence that when clinicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Multiple studies have since demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis of 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (by 51%), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of CDI (32%).43
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A; the High Value Care Task Force of the American College of Physicians and the CDC. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315: 1864-1873.
9. CDC. Antibiotic prescribing and use. www.cdc.gov/antibiotic-use/index.html. Accessed January 16, 2018.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed January 16, 2018.
11. World Health Organization. Global action plan on antimicrobial resistance (2015). www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed January 16, 2018.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;126:2439-2444.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014; 38:1-12.31. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract . 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA . 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA . 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA . 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics . 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H, et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract . 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae . Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007; 57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.

Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections (CDI), allergic reactions, and increased health care costs (see Table 1).1-6 And yet, clinicians continue to overprescribe this class of medication.

A 2016 report from the CDC estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a postantibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program (now known as “Be Antibiotics Aware”), focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) in 2015 developed a five-year strategic framework for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
WHEN AND WHERE ARE ANTIBIOTICS MOST OFTEN INAPPROPRIATELY PRESCRIBED?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 Refer to national clinical guidelines, which delineate when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, there are conflicting findings as to whether antibiotic prescribing differs in office-based versus emergency department (ED) settings.
- One study found a higher rate of antibiotic prescribing during ED visits than office visits (21% vs 9%), even though, between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- In a cross-sectional study using data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS), more than half of patients with uncomplicated acute rhinosinusitis received a prescription for antibiotics, but there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs did (51%).12
STICK TO NARROW-SPECTRUM AGENTS WHEN POSSIBLE
Using broad-spectrum antibiotics, such as quinolones or imipenem, firstline, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which clinicians prescribe narrow-spectrum, firstline antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that providers used firstline agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate firstline antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non-firstline antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), clinicians should prescribe a narrow-spectrum antibiotic first.
ANTIBIOTIC OVERPRESCIBING AFFECTS THE GUT AND BEYOND
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by CDI. CDI is now the most common health care-related infection, accounting for about a half-million health care facility infections per year.22 It extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early HIV infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in two-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn disease, type 2 diabetes, and obesity. (For a discussion of the relationship between the gut microbiome and diabetes, see Endocrine Consult: The Gut Microbiome in Type 2 Diabetes.)
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children younger than 24 months of age increases the risk for childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis, resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiomes of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.
WHAT CAN WE DO RIGHT NOW?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why clinicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and fear of losing patients are major reasons providers cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis, clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (see Table 2).34-36 
One example comes from a 1996-1998 study of four primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated multiple elements, including office-based and household patient educational materials and a clinician intervention involving education, practice profiling, and academic detailing. Clinicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
Employing EMRs. A more recent study focused on use of electronic medical records (EMRs) and communications to modify clinician antibiotic prescribing.35 By sending clinicians monthly emails comparing their prescribing patterns to those of peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified providers’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the clinician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged providers to follow prescribing guidelines by capitalizing on their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics.
Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19%, compared with about a 9% reduction in the control groups.36,37
DOES PRESCRIBING ANTIBIOTICS AFFECT PATIENT SATISFACTION?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with clinicians who explained why antibiotics were not indicated versus those who simply prescribed them—and that such explanations do not need to take a lot of time (see Table 3 for patient care tips).37,38
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
REDUCING ANTIBIOTIC PRESCRIBING REDUCES RESISTANCE
There is also strong evidence that when clinicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Multiple studies have since demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis of 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (by 51%), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of CDI (32%).43

Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections (CDI), allergic reactions, and increased health care costs (see Table 1).1-6 And yet, clinicians continue to overprescribe this class of medication.

A 2016 report from the CDC estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a postantibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program (now known as “Be Antibiotics Aware”), focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) in 2015 developed a five-year strategic framework for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
WHEN AND WHERE ARE ANTIBIOTICS MOST OFTEN INAPPROPRIATELY PRESCRIBED?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 Refer to national clinical guidelines, which delineate when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, there are conflicting findings as to whether antibiotic prescribing differs in office-based versus emergency department (ED) settings.
- One study found a higher rate of antibiotic prescribing during ED visits than office visits (21% vs 9%), even though, between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- In a cross-sectional study using data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS), more than half of patients with uncomplicated acute rhinosinusitis received a prescription for antibiotics, but there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs did (51%).12
STICK TO NARROW-SPECTRUM AGENTS WHEN POSSIBLE
Using broad-spectrum antibiotics, such as quinolones or imipenem, firstline, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which clinicians prescribe narrow-spectrum, firstline antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that providers used firstline agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate firstline antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non-firstline antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), clinicians should prescribe a narrow-spectrum antibiotic first.
ANTIBIOTIC OVERPRESCIBING AFFECTS THE GUT AND BEYOND
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by CDI. CDI is now the most common health care-related infection, accounting for about a half-million health care facility infections per year.22 It extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early HIV infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in two-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn disease, type 2 diabetes, and obesity. (For a discussion of the relationship between the gut microbiome and diabetes, see Endocrine Consult: The Gut Microbiome in Type 2 Diabetes.)
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children younger than 24 months of age increases the risk for childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis, resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiomes of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.
WHAT CAN WE DO RIGHT NOW?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why clinicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and fear of losing patients are major reasons providers cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis, clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (see Table 2).34-36 
One example comes from a 1996-1998 study of four primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated multiple elements, including office-based and household patient educational materials and a clinician intervention involving education, practice profiling, and academic detailing. Clinicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
Employing EMRs. A more recent study focused on use of electronic medical records (EMRs) and communications to modify clinician antibiotic prescribing.35 By sending clinicians monthly emails comparing their prescribing patterns to those of peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified providers’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the clinician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged providers to follow prescribing guidelines by capitalizing on their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics.
Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19%, compared with about a 9% reduction in the control groups.36,37
DOES PRESCRIBING ANTIBIOTICS AFFECT PATIENT SATISFACTION?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with clinicians who explained why antibiotics were not indicated versus those who simply prescribed them—and that such explanations do not need to take a lot of time (see Table 3 for patient care tips).37,38
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
REDUCING ANTIBIOTIC PRESCRIBING REDUCES RESISTANCE
There is also strong evidence that when clinicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Multiple studies have since demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis of 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (by 51%), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of CDI (32%).43
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A; the High Value Care Task Force of the American College of Physicians and the CDC. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315: 1864-1873.
9. CDC. Antibiotic prescribing and use. www.cdc.gov/antibiotic-use/index.html. Accessed January 16, 2018.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed January 16, 2018.
11. World Health Organization. Global action plan on antimicrobial resistance (2015). www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed January 16, 2018.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;126:2439-2444.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014; 38:1-12.31. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract . 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA . 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA . 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA . 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics . 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H, et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract . 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae . Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007; 57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A; the High Value Care Task Force of the American College of Physicians and the CDC. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315: 1864-1873.
9. CDC. Antibiotic prescribing and use. www.cdc.gov/antibiotic-use/index.html. Accessed January 16, 2018.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed January 16, 2018.
11. World Health Organization. Global action plan on antimicrobial resistance (2015). www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed January 16, 2018.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;126:2439-2444.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014; 38:1-12.31. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract . 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA . 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA . 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA . 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics . 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H, et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract . 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae . Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007; 57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.