User login
Pelvic fracture pattern predicts the need for hemorrhage control
WAIKOLOA, HAWAII – Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma. They identified three main force patterns, including lateral compression, anterior posterior compression, and vertical shear (Radiology. 1986 Aug;160 [2]:445-51).
In a recently published study, Dr. Costantini and his associates found wide variability in the use of various pelvic hemorrhage control methods (J Trauma Acute Care Surg. 2016 May;80 [5]:717-25). “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level I trauma centers over a two year period. Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over the age of 18, blunt mechanism of injury, and shock on admission, which was defined as an admission systolic blood pressure of less than 90 mm Hg, or heart rate greater than 120, or base deficit greater than 5. Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr. Costantini said. Next, they used univariate and multivariate logistic regression analysis to analyze predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the two year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic blood pressure was 93 mm Hg, their mean heart rate was 117 beats per minute, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian versus auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage control intervention, those who did received more transfusion of packed red blood cells (13 vs. 7 units, respectively; P less than .01) and fresh frozen plasma (10 vs. 5 units; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs. 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (odds ratio, 109.43; P less than .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important, “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
Dr. Costantini reported having no financial disclosures.
WAIKOLOA, HAWAII – Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma. They identified three main force patterns, including lateral compression, anterior posterior compression, and vertical shear (Radiology. 1986 Aug;160 [2]:445-51).
In a recently published study, Dr. Costantini and his associates found wide variability in the use of various pelvic hemorrhage control methods (J Trauma Acute Care Surg. 2016 May;80 [5]:717-25). “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level I trauma centers over a two year period. Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over the age of 18, blunt mechanism of injury, and shock on admission, which was defined as an admission systolic blood pressure of less than 90 mm Hg, or heart rate greater than 120, or base deficit greater than 5. Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr. Costantini said. Next, they used univariate and multivariate logistic regression analysis to analyze predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the two year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic blood pressure was 93 mm Hg, their mean heart rate was 117 beats per minute, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian versus auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage control intervention, those who did received more transfusion of packed red blood cells (13 vs. 7 units, respectively; P less than .01) and fresh frozen plasma (10 vs. 5 units; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs. 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (odds ratio, 109.43; P less than .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important, “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
Dr. Costantini reported having no financial disclosures.
WAIKOLOA, HAWAII – Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma. They identified three main force patterns, including lateral compression, anterior posterior compression, and vertical shear (Radiology. 1986 Aug;160 [2]:445-51).
In a recently published study, Dr. Costantini and his associates found wide variability in the use of various pelvic hemorrhage control methods (J Trauma Acute Care Surg. 2016 May;80 [5]:717-25). “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level I trauma centers over a two year period. Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over the age of 18, blunt mechanism of injury, and shock on admission, which was defined as an admission systolic blood pressure of less than 90 mm Hg, or heart rate greater than 120, or base deficit greater than 5. Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr. Costantini said. Next, they used univariate and multivariate logistic regression analysis to analyze predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the two year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic blood pressure was 93 mm Hg, their mean heart rate was 117 beats per minute, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian versus auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage control intervention, those who did received more transfusion of packed red blood cells (13 vs. 7 units, respectively; P less than .01) and fresh frozen plasma (10 vs. 5 units; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs. 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (odds ratio, 109.43; P less than .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important, “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
Dr. Costantini reported having no financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point:
Major finding: On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (odds ratio, 109.43; P less than .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Data source: A prospective evaluation of 163 patients with pelvic fracture who were admitted to 11 Level I trauma centers over a two-year period.
Disclosures: Dr. Costantini reported having no financial disclosures.
The Highs and Lows of Medical Marijuana
Marijuana has been used medicinally worldwide for thousands of years.1,2 In the early 1990s, the discovery of cannabinoid receptors in the central and peripheral nervous systems began to propagate interest in other potential therapeutic values of marijuana.3 Since then, marijuana has been used by patients experiencing chemotherapy-induced anorexia, nausea and vomiting, pain, and forms of spasticity. Use among patients with glaucoma and HIV/AIDS has also been widely reported.
In light of this—and of increasing efforts to legalize medical marijuana use across the United States—clinicians should be cognizant of the substance’s negative effects, as well as its potential health benefits. Marijuana has significant systemic effects and associated risks of which patients and health care providers should be aware. Questions remain regarding the safety, efficacy, and long-term impact of use. Use of marijuana for medical purposes requires a careful examination of the risks and benefits.
PHARMACOKINETICS
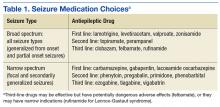
Marijuana contains approximately 60 cannabinoids, two of which have been specifically identified as primary components. The first, delta-9 tetrahydrocannabinol (THC), is believed to be the most psychoactive.4,5 THC was identified in 1964 and is responsible for the well-documented symptoms of euphoria, appetite stimulation, impaired memory and cognition, and analgesia. The THC content in marijuana products varies widely and has increased over time, complicating research on the long-term effects of marijuana use.5,6
The second compound, cannabidiol (CBD), is a serotonin receptor agonist that lacks psychoactive effects. Potential benefits of CBD include antiemetic and anxiolytic properties, as well as anti-inflammatory effects. There is some evidence to suggest that CBD might also have antipsychotic properties.1,4
AVAILABLE FORMULATIONS
Two synthetic forms of THC have been approved by the FDA since 1985 for medicinal use: nabilone (categorized as a Schedule II drug) and dronabinol (Schedule III). Both are cannabinoid receptor agonists approved for treating chemotherapy-induced nausea and vomiting. They are recommended for use after failure of standard therapies, such as 5-HT3 receptor antagonists, but overall interest has decreased since the advent of agents such as ondansetron.2,4
Nabiximols, an oral buccal spray, is a combination of THC and CBD. It was approved in Canada in 2005 for pain management in cancer patients and for multiple sclerosis–related pain and spasticity. It is not currently available in the US.2,4
Marijuana use is currently legal in 25 states and the District of Columbia.7,8 However, state laws regarding the criteria for medical use are vague and varied. For example, not all states require that clinicians review risks and benefits of marijuana use with patients. Even for those that do, the lack of clinical trials on the safety and efficacy of marijuana make it difficult for clinicians to properly educate themselves and their patients.9
LIMITATIONS OF RESEARCH
Why the lack of data? In 1937, a federal tax restricted marijuana prescription in the US, and in 1942, marijuana was removed from the US Pharmacopeia.2,4 The Controlled Substances Act in 1970 designated marijuana as a Schedule I drug, a categorization for drugs with high potential for abuse and no currently accepted medical use.9 Following this designation, research on marijuana was nearly halted in the US. Several medical organizations have subsequently called for reclassification to Schedule II in order to facilitate scientific research into marijuana’s medicinal benefits and risks.
Research is also limited due to the comorbid use of tobacco and other drugs in study subjects, the variation of cannabinoid levels among products, and differences in the route of administration—particularly smoking versus oral or buccal routes.5 Conducting marijuana research in a fashion similar to pharmaceuticals would not only serve the medical community but also the legislative faction.
Despite these obstacles, there is some available evidence on medical use of marijuana. A review of the associated risks and potential uses for the substance follows.
RISKS ASSOCIATED WITH MARIJUANA USE
Acute effects
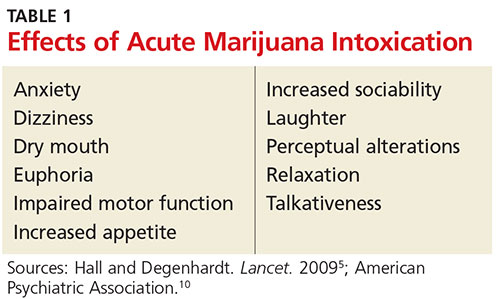
Most symptoms of marijuana intoxication are attributed to the THC component and occur due to the presence of cannabinoid receptors in the central nervous system (see Table 1).5,10 Additional objective signs of acute or chronic intoxication include conjunctival injection, tachycardia, cannabis odor, yellowing of fingertips (from smoking), cough, and food cravings.10
A more recently identified effect of long-term marijuana use is a paradoxical hyperemesis syndrome, in which individuals experience nausea, vomiting, and abdominal pain. They obtain relief with hot showers or baths.6,8
Since there is a near absence of cannabinoid receptors in the brain stem, marijuana does not stimulate the autonomic nervous system. It is therefore believed that marijuana use cannot be fatal. Corroborating this theory, no deaths have been reported from marijuana overdose.2,11
Withdrawal symptoms
Approximately 10% of regular marijuana users become physically and psychologically dependent on the substance. Once tolerance develops, withdrawal symptoms occur with cessation of use (see Table 2).2,5,10 Symptoms peak within the first week following cessation and may last up to two weeks. Sleep disturbances may occur for more than one month.10

Unlike with other substances of abuse, there are no pharmaceutical agents to treat marijuana withdrawal; rather, treatment is supportive. Marijuana users often resume use following a period of cessation in order to avoid withdrawal.
Chronic effects
Dental/oral. Smoking marijuana is associated with an increased risk for dental caries, periodontal disease, and oral infections.1 Premalignant oral lesions, such as leukoplakia and erythroplakia, have also been reported. Patient education on the risks and need for proper oral hygiene is vital, as are regular dental examinations.
Respiratory. There are several known pulmonary implications of smoking marijuana, and therefore, this route of administration is not recommended for medicinal use. Respiratory effects of marijuana smoke are similar to those seen with tobacco: cough, dyspnea, sputum production, wheezing, bronchitis, pharyngitis, and hoarseness.4 Increased rates of pneumonia and other respiratory infections have also been identified.6 Research on long-term marijuana smoking has revealed hyperinflation and airway resistance.6 At this time, evidence is inconclusive as to whether smoking marijuana leads to chronic obstructive pulmonary disease.1
Studies have compared the chemical content of tobacco and marijuana and found similar components, including carcinogens, but data regarding concentrations of these chemicals are conflicting.1,4 It is unknown whether vaping (a trending practice in which a device is used to heat the substance prior to inhalation) reduces this risk.4
Unfortunately, data regarding the carcinogenic effects of long-term marijuana smoking are inconclusive; some studies have shown potential protective effects.4-6 Other evidence suggests that the risk is lower in comparison to tobacco smoking.6
Cardiovascular. The effects of marijuana on the cardiovascular system are not fully understood. Known symptoms include tachycardia, peripheral vasodilation, hypotension, and syncope.4 There is some evidence that marijuana use carries an increased risk for angina in patients with previously established heart disease.5 Patients, especially those with known cardiovascular disease, should be educated about these risks.
Reproductive. There are several identified reproductive consequences of marijuana use. Research has found decreased sperm count and gynecomastia in men and impaired ovulation in women.4 Studies on marijuana use in pregnancy consistently reveal low birth weight—this effect is, however, less than that seen with tobacco smoking.5 Other complications or developmental abnormalities may occur, but there is currently a lack of evidence to support further conclusions.
Neurologic. The use of marijuana results in short-term memory loss and other cognitive impairments. There is conflicting evidence as to whether long-term effects remain after cessation.5,6 Because acute intoxication impairs motor skills, it is associated with increased rates of motor vehicle accidents.6 Driving while under the influence of marijuana should be cautioned against.
Psychiatric. Marijuana use is associated with the onset and exacerbation of acute psychosis. However, its role as a causal factor in schizophrenia has not been established.4,10 There is some evidence to suggest that CBD has antipsychotic properties, warranting further research. An amotivational syndrome has also been affiliated with chronic marijuana use; affected individuals exhibit a lack of goal-directed behavior, which may result in work or school dysfunction.10 Several studies have supported an association between marijuana use and risk for depression and anxiety. Due to the extensive risk factors for these disorders, including genetic and environmental, causality has yet to be established.5,6
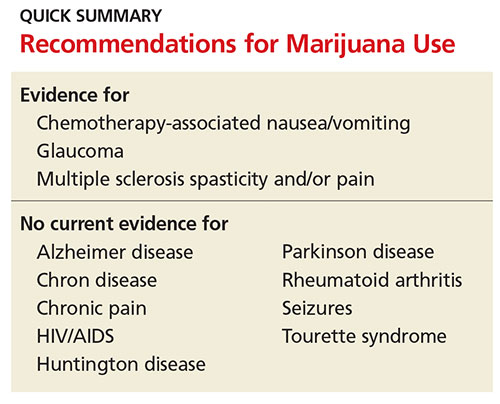
Conditions for Which Marijuana May Offer Therapeutic Benefits
Glaucoma
Research has demonstrated that marijuana decreases intraocular pressure, and many patients with glaucoma use marijuana. However, it is not recommended as firstline treatment.
The beneficial effects of smoked marijuana are short-lived, requiring patients to dose repeatedly throughout the day. Use is also often discontinued due to adverse effects including dry mouth, dizziness, confusion, and anxiety.8
Topical preparations of THC have not been successfully developed due to the low water solubility of cannabis and minimal penetration through the cornea to the intraocular space.8 Standard treatments available for glaucoma are more effective and without obvious psychoactive effects.6
Nausea
One of the first medical uses of marijuana was for nausea. Due to the presence of cannabinoid receptors that govern food intake, marijuana is known to stimulate appetite, making its use in reducing chemotherapy-associated nausea and vomiting widespread.2,6 Despite the variation in state laws regarding medical use of marijuana, cancer is included as a qualifying illness in every state that allows it.8 Cannabis-based medications may be useful for treating refractory nausea secondary to chemotherapy; however, dronabinol and nabilone are not recommended as firstline therapies.12
HIV/AIDS
Short-term evidence suggests that patients with HIV and/or AIDS benefit from marijuana use through improved appetite, weight gain, lessened pain, and improved quality of life.6,13 Studies with small sample sizes have been conducted using smoked marijuana and dronabinol.8 Long-term studies are needed to compare the use of marijuana with other nutritional and caloric supplements. Overall, reliable research regarding the therapeutic value of marijuana in these patients is inconclusive, and therefore no recommendations for incorporating marijuana into the treatment regimen have been made.8
Multiple sclerosis
For centuries, marijuana has been used for pain relief. The discovery of cannabinoid receptors in high concentrations throughout pain pathways of the brain supports the notion that marijuana plays a role in analgesia. While response to acute pain is poor, there is evidence to suggest that various cannabis formulations relieve chronic neuropathic pain and spasticity, as seen in multiple sclerosis.3,6
Subjective improvements in pain and spasticity were seen with the use of oral cannabis extract, THC, and nabiximols.11 Smoked marijuana is of uncertain efficacy and is not recommended for use in this patient population; it has been shown to potentially worsen cognition.8,11
Seizures
Research into the role of marijuana in decreasing seizure frequency is inconclusive.11 Large studies with human subjects are lacking, and most data thus far have come from animals and case studies.8 Some case reports have suggested a decrease in seizures with marijuana use, but further investigation is needed.6
At this time, it is not appropriate to recommend marijuana for patients with seizure disorders, but the use of cannabidiol might be more promising. Studies are ongoing.14
Alzheimer disease
Alzheimer disease is the most common cause of dementia.8 Despite known adverse effects on memory and cognition with acute use, studies have shown that marijuana might inhibit the development of amyloid beta plaques in Alzheimer disease.4 Further research on dronabinol has not provided sufficient data to support its use, and no studies utilizing smoked marijuana have been performed.8 Therefore, no recommendations exist for the use of marijuana in this patient population, and further research is warranted.
Ongoing research
There are some additional areas of potential therapeutic use of marijuana. Limited evidence has revealed that marijuana has anti-inflammatory properties, leading researchers to examine its use for autoimmune diseases, such as rheumatoid arthritis and Crohn disease. Studies investigating marijuana’s potential ability to inhibit cancer growth and metastasis are ongoing.
Unfortunately, research in patients with Parkinson disease has not shown improvement in dyskinesias.11 Studies on other movement disorders, such as Tourette syndrome and Huntington disease, have not shown symptom improvement with marijuana use. Research on these conditions and others is ongoing.
CONCLUSION
Marijuana use has negative effects on a variety of body systems, but it also may provide therapeutic benefit in certain patient populations. Clinicians and patients are currently hampered by the dearth of reliable information on its safety and efficacy (resulting from federal restrictions and other factors). Comparative studies between marijuana and established standards of care are needed, as is additional research to identify therapeutic effects that could be maximized and ways to minimize or eliminate negative sequelae.
1. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Cannabis: effective and safe analgesic? J Pain Manage. 2014;7(3):209-233.
2. Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172-186.
3. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409-2438.
4. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81.
5. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391.
6. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
7. National Conference of State Legislatures. State medical marijuana laws (updated 7/20/2016). www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed September 7, 2016.
8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10(1):1-10.
9. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362(16):1453-1457.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
11. Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology. 2014; 82(17):1556-1563.
12. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
14. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;(6):CD009270.
Marijuana has been used medicinally worldwide for thousands of years.1,2 In the early 1990s, the discovery of cannabinoid receptors in the central and peripheral nervous systems began to propagate interest in other potential therapeutic values of marijuana.3 Since then, marijuana has been used by patients experiencing chemotherapy-induced anorexia, nausea and vomiting, pain, and forms of spasticity. Use among patients with glaucoma and HIV/AIDS has also been widely reported.
In light of this—and of increasing efforts to legalize medical marijuana use across the United States—clinicians should be cognizant of the substance’s negative effects, as well as its potential health benefits. Marijuana has significant systemic effects and associated risks of which patients and health care providers should be aware. Questions remain regarding the safety, efficacy, and long-term impact of use. Use of marijuana for medical purposes requires a careful examination of the risks and benefits.

PHARMACOKINETICS
Marijuana contains approximately 60 cannabinoids, two of which have been specifically identified as primary components. The first, delta-9 tetrahydrocannabinol (THC), is believed to be the most psychoactive.4,5 THC was identified in 1964 and is responsible for the well-documented symptoms of euphoria, appetite stimulation, impaired memory and cognition, and analgesia. The THC content in marijuana products varies widely and has increased over time, complicating research on the long-term effects of marijuana use.5,6
The second compound, cannabidiol (CBD), is a serotonin receptor agonist that lacks psychoactive effects. Potential benefits of CBD include antiemetic and anxiolytic properties, as well as anti-inflammatory effects. There is some evidence to suggest that CBD might also have antipsychotic properties.1,4
AVAILABLE FORMULATIONS
Two synthetic forms of THC have been approved by the FDA since 1985 for medicinal use: nabilone (categorized as a Schedule II drug) and dronabinol (Schedule III). Both are cannabinoid receptor agonists approved for treating chemotherapy-induced nausea and vomiting. They are recommended for use after failure of standard therapies, such as 5-HT3 receptor antagonists, but overall interest has decreased since the advent of agents such as ondansetron.2,4
Nabiximols, an oral buccal spray, is a combination of THC and CBD. It was approved in Canada in 2005 for pain management in cancer patients and for multiple sclerosis–related pain and spasticity. It is not currently available in the US.2,4
Marijuana use is currently legal in 25 states and the District of Columbia.7,8 However, state laws regarding the criteria for medical use are vague and varied. For example, not all states require that clinicians review risks and benefits of marijuana use with patients. Even for those that do, the lack of clinical trials on the safety and efficacy of marijuana make it difficult for clinicians to properly educate themselves and their patients.9
LIMITATIONS OF RESEARCH
Why the lack of data? In 1937, a federal tax restricted marijuana prescription in the US, and in 1942, marijuana was removed from the US Pharmacopeia.2,4 The Controlled Substances Act in 1970 designated marijuana as a Schedule I drug, a categorization for drugs with high potential for abuse and no currently accepted medical use.9 Following this designation, research on marijuana was nearly halted in the US. Several medical organizations have subsequently called for reclassification to Schedule II in order to facilitate scientific research into marijuana’s medicinal benefits and risks.
Research is also limited due to the comorbid use of tobacco and other drugs in study subjects, the variation of cannabinoid levels among products, and differences in the route of administration—particularly smoking versus oral or buccal routes.5 Conducting marijuana research in a fashion similar to pharmaceuticals would not only serve the medical community but also the legislative faction.
Despite these obstacles, there is some available evidence on medical use of marijuana. A review of the associated risks and potential uses for the substance follows.
RISKS ASSOCIATED WITH MARIJUANA USE
Acute effects
Most symptoms of marijuana intoxication are attributed to the THC component and occur due to the presence of cannabinoid receptors in the central nervous system (see Table 1).5,10 Additional objective signs of acute or chronic intoxication include conjunctival injection, tachycardia, cannabis odor, yellowing of fingertips (from smoking), cough, and food cravings.10

A more recently identified effect of long-term marijuana use is a paradoxical hyperemesis syndrome, in which individuals experience nausea, vomiting, and abdominal pain. They obtain relief with hot showers or baths.6,8
Since there is a near absence of cannabinoid receptors in the brain stem, marijuana does not stimulate the autonomic nervous system. It is therefore believed that marijuana use cannot be fatal. Corroborating this theory, no deaths have been reported from marijuana overdose.2,11
Withdrawal symptoms
Approximately 10% of regular marijuana users become physically and psychologically dependent on the substance. Once tolerance develops, withdrawal symptoms occur with cessation of use (see Table 2).2,5,10 Symptoms peak within the first week following cessation and may last up to two weeks. Sleep disturbances may occur for more than one month.10

Unlike with other substances of abuse, there are no pharmaceutical agents to treat marijuana withdrawal; rather, treatment is supportive. Marijuana users often resume use following a period of cessation in order to avoid withdrawal.
Chronic effects
Dental/oral. Smoking marijuana is associated with an increased risk for dental caries, periodontal disease, and oral infections.1 Premalignant oral lesions, such as leukoplakia and erythroplakia, have also been reported. Patient education on the risks and need for proper oral hygiene is vital, as are regular dental examinations.
Respiratory. There are several known pulmonary implications of smoking marijuana, and therefore, this route of administration is not recommended for medicinal use. Respiratory effects of marijuana smoke are similar to those seen with tobacco: cough, dyspnea, sputum production, wheezing, bronchitis, pharyngitis, and hoarseness.4 Increased rates of pneumonia and other respiratory infections have also been identified.6 Research on long-term marijuana smoking has revealed hyperinflation and airway resistance.6 At this time, evidence is inconclusive as to whether smoking marijuana leads to chronic obstructive pulmonary disease.1
Studies have compared the chemical content of tobacco and marijuana and found similar components, including carcinogens, but data regarding concentrations of these chemicals are conflicting.1,4 It is unknown whether vaping (a trending practice in which a device is used to heat the substance prior to inhalation) reduces this risk.4
Unfortunately, data regarding the carcinogenic effects of long-term marijuana smoking are inconclusive; some studies have shown potential protective effects.4-6 Other evidence suggests that the risk is lower in comparison to tobacco smoking.6
Cardiovascular. The effects of marijuana on the cardiovascular system are not fully understood. Known symptoms include tachycardia, peripheral vasodilation, hypotension, and syncope.4 There is some evidence that marijuana use carries an increased risk for angina in patients with previously established heart disease.5 Patients, especially those with known cardiovascular disease, should be educated about these risks.
Reproductive. There are several identified reproductive consequences of marijuana use. Research has found decreased sperm count and gynecomastia in men and impaired ovulation in women.4 Studies on marijuana use in pregnancy consistently reveal low birth weight—this effect is, however, less than that seen with tobacco smoking.5 Other complications or developmental abnormalities may occur, but there is currently a lack of evidence to support further conclusions.
Neurologic. The use of marijuana results in short-term memory loss and other cognitive impairments. There is conflicting evidence as to whether long-term effects remain after cessation.5,6 Because acute intoxication impairs motor skills, it is associated with increased rates of motor vehicle accidents.6 Driving while under the influence of marijuana should be cautioned against.
Psychiatric. Marijuana use is associated with the onset and exacerbation of acute psychosis. However, its role as a causal factor in schizophrenia has not been established.4,10 There is some evidence to suggest that CBD has antipsychotic properties, warranting further research. An amotivational syndrome has also been affiliated with chronic marijuana use; affected individuals exhibit a lack of goal-directed behavior, which may result in work or school dysfunction.10 Several studies have supported an association between marijuana use and risk for depression and anxiety. Due to the extensive risk factors for these disorders, including genetic and environmental, causality has yet to be established.5,6
Conditions for Which Marijuana May Offer Therapeutic Benefits
Glaucoma
Research has demonstrated that marijuana decreases intraocular pressure, and many patients with glaucoma use marijuana. However, it is not recommended as firstline treatment.
The beneficial effects of smoked marijuana are short-lived, requiring patients to dose repeatedly throughout the day. Use is also often discontinued due to adverse effects including dry mouth, dizziness, confusion, and anxiety.8
Topical preparations of THC have not been successfully developed due to the low water solubility of cannabis and minimal penetration through the cornea to the intraocular space.8 Standard treatments available for glaucoma are more effective and without obvious psychoactive effects.6
Nausea
One of the first medical uses of marijuana was for nausea. Due to the presence of cannabinoid receptors that govern food intake, marijuana is known to stimulate appetite, making its use in reducing chemotherapy-associated nausea and vomiting widespread.2,6 Despite the variation in state laws regarding medical use of marijuana, cancer is included as a qualifying illness in every state that allows it.8 Cannabis-based medications may be useful for treating refractory nausea secondary to chemotherapy; however, dronabinol and nabilone are not recommended as firstline therapies.12
HIV/AIDS
Short-term evidence suggests that patients with HIV and/or AIDS benefit from marijuana use through improved appetite, weight gain, lessened pain, and improved quality of life.6,13 Studies with small sample sizes have been conducted using smoked marijuana and dronabinol.8 Long-term studies are needed to compare the use of marijuana with other nutritional and caloric supplements. Overall, reliable research regarding the therapeutic value of marijuana in these patients is inconclusive, and therefore no recommendations for incorporating marijuana into the treatment regimen have been made.8
Multiple sclerosis
For centuries, marijuana has been used for pain relief. The discovery of cannabinoid receptors in high concentrations throughout pain pathways of the brain supports the notion that marijuana plays a role in analgesia. While response to acute pain is poor, there is evidence to suggest that various cannabis formulations relieve chronic neuropathic pain and spasticity, as seen in multiple sclerosis.3,6
Subjective improvements in pain and spasticity were seen with the use of oral cannabis extract, THC, and nabiximols.11 Smoked marijuana is of uncertain efficacy and is not recommended for use in this patient population; it has been shown to potentially worsen cognition.8,11
Seizures
Research into the role of marijuana in decreasing seizure frequency is inconclusive.11 Large studies with human subjects are lacking, and most data thus far have come from animals and case studies.8 Some case reports have suggested a decrease in seizures with marijuana use, but further investigation is needed.6
At this time, it is not appropriate to recommend marijuana for patients with seizure disorders, but the use of cannabidiol might be more promising. Studies are ongoing.14
Alzheimer disease
Alzheimer disease is the most common cause of dementia.8 Despite known adverse effects on memory and cognition with acute use, studies have shown that marijuana might inhibit the development of amyloid beta plaques in Alzheimer disease.4 Further research on dronabinol has not provided sufficient data to support its use, and no studies utilizing smoked marijuana have been performed.8 Therefore, no recommendations exist for the use of marijuana in this patient population, and further research is warranted.
Ongoing research
There are some additional areas of potential therapeutic use of marijuana. Limited evidence has revealed that marijuana has anti-inflammatory properties, leading researchers to examine its use for autoimmune diseases, such as rheumatoid arthritis and Crohn disease. Studies investigating marijuana’s potential ability to inhibit cancer growth and metastasis are ongoing.
Unfortunately, research in patients with Parkinson disease has not shown improvement in dyskinesias.11 Studies on other movement disorders, such as Tourette syndrome and Huntington disease, have not shown symptom improvement with marijuana use. Research on these conditions and others is ongoing.
CONCLUSION
Marijuana use has negative effects on a variety of body systems, but it also may provide therapeutic benefit in certain patient populations. Clinicians and patients are currently hampered by the dearth of reliable information on its safety and efficacy (resulting from federal restrictions and other factors). Comparative studies between marijuana and established standards of care are needed, as is additional research to identify therapeutic effects that could be maximized and ways to minimize or eliminate negative sequelae.
Marijuana has been used medicinally worldwide for thousands of years.1,2 In the early 1990s, the discovery of cannabinoid receptors in the central and peripheral nervous systems began to propagate interest in other potential therapeutic values of marijuana.3 Since then, marijuana has been used by patients experiencing chemotherapy-induced anorexia, nausea and vomiting, pain, and forms of spasticity. Use among patients with glaucoma and HIV/AIDS has also been widely reported.
In light of this—and of increasing efforts to legalize medical marijuana use across the United States—clinicians should be cognizant of the substance’s negative effects, as well as its potential health benefits. Marijuana has significant systemic effects and associated risks of which patients and health care providers should be aware. Questions remain regarding the safety, efficacy, and long-term impact of use. Use of marijuana for medical purposes requires a careful examination of the risks and benefits.

PHARMACOKINETICS
Marijuana contains approximately 60 cannabinoids, two of which have been specifically identified as primary components. The first, delta-9 tetrahydrocannabinol (THC), is believed to be the most psychoactive.4,5 THC was identified in 1964 and is responsible for the well-documented symptoms of euphoria, appetite stimulation, impaired memory and cognition, and analgesia. The THC content in marijuana products varies widely and has increased over time, complicating research on the long-term effects of marijuana use.5,6
The second compound, cannabidiol (CBD), is a serotonin receptor agonist that lacks psychoactive effects. Potential benefits of CBD include antiemetic and anxiolytic properties, as well as anti-inflammatory effects. There is some evidence to suggest that CBD might also have antipsychotic properties.1,4
AVAILABLE FORMULATIONS
Two synthetic forms of THC have been approved by the FDA since 1985 for medicinal use: nabilone (categorized as a Schedule II drug) and dronabinol (Schedule III). Both are cannabinoid receptor agonists approved for treating chemotherapy-induced nausea and vomiting. They are recommended for use after failure of standard therapies, such as 5-HT3 receptor antagonists, but overall interest has decreased since the advent of agents such as ondansetron.2,4
Nabiximols, an oral buccal spray, is a combination of THC and CBD. It was approved in Canada in 2005 for pain management in cancer patients and for multiple sclerosis–related pain and spasticity. It is not currently available in the US.2,4
Marijuana use is currently legal in 25 states and the District of Columbia.7,8 However, state laws regarding the criteria for medical use are vague and varied. For example, not all states require that clinicians review risks and benefits of marijuana use with patients. Even for those that do, the lack of clinical trials on the safety and efficacy of marijuana make it difficult for clinicians to properly educate themselves and their patients.9
LIMITATIONS OF RESEARCH
Why the lack of data? In 1937, a federal tax restricted marijuana prescription in the US, and in 1942, marijuana was removed from the US Pharmacopeia.2,4 The Controlled Substances Act in 1970 designated marijuana as a Schedule I drug, a categorization for drugs with high potential for abuse and no currently accepted medical use.9 Following this designation, research on marijuana was nearly halted in the US. Several medical organizations have subsequently called for reclassification to Schedule II in order to facilitate scientific research into marijuana’s medicinal benefits and risks.
Research is also limited due to the comorbid use of tobacco and other drugs in study subjects, the variation of cannabinoid levels among products, and differences in the route of administration—particularly smoking versus oral or buccal routes.5 Conducting marijuana research in a fashion similar to pharmaceuticals would not only serve the medical community but also the legislative faction.
Despite these obstacles, there is some available evidence on medical use of marijuana. A review of the associated risks and potential uses for the substance follows.
RISKS ASSOCIATED WITH MARIJUANA USE
Acute effects
Most symptoms of marijuana intoxication are attributed to the THC component and occur due to the presence of cannabinoid receptors in the central nervous system (see Table 1).5,10 Additional objective signs of acute or chronic intoxication include conjunctival injection, tachycardia, cannabis odor, yellowing of fingertips (from smoking), cough, and food cravings.10

A more recently identified effect of long-term marijuana use is a paradoxical hyperemesis syndrome, in which individuals experience nausea, vomiting, and abdominal pain. They obtain relief with hot showers or baths.6,8
Since there is a near absence of cannabinoid receptors in the brain stem, marijuana does not stimulate the autonomic nervous system. It is therefore believed that marijuana use cannot be fatal. Corroborating this theory, no deaths have been reported from marijuana overdose.2,11
Withdrawal symptoms
Approximately 10% of regular marijuana users become physically and psychologically dependent on the substance. Once tolerance develops, withdrawal symptoms occur with cessation of use (see Table 2).2,5,10 Symptoms peak within the first week following cessation and may last up to two weeks. Sleep disturbances may occur for more than one month.10

Unlike with other substances of abuse, there are no pharmaceutical agents to treat marijuana withdrawal; rather, treatment is supportive. Marijuana users often resume use following a period of cessation in order to avoid withdrawal.
Chronic effects
Dental/oral. Smoking marijuana is associated with an increased risk for dental caries, periodontal disease, and oral infections.1 Premalignant oral lesions, such as leukoplakia and erythroplakia, have also been reported. Patient education on the risks and need for proper oral hygiene is vital, as are regular dental examinations.
Respiratory. There are several known pulmonary implications of smoking marijuana, and therefore, this route of administration is not recommended for medicinal use. Respiratory effects of marijuana smoke are similar to those seen with tobacco: cough, dyspnea, sputum production, wheezing, bronchitis, pharyngitis, and hoarseness.4 Increased rates of pneumonia and other respiratory infections have also been identified.6 Research on long-term marijuana smoking has revealed hyperinflation and airway resistance.6 At this time, evidence is inconclusive as to whether smoking marijuana leads to chronic obstructive pulmonary disease.1
Studies have compared the chemical content of tobacco and marijuana and found similar components, including carcinogens, but data regarding concentrations of these chemicals are conflicting.1,4 It is unknown whether vaping (a trending practice in which a device is used to heat the substance prior to inhalation) reduces this risk.4
Unfortunately, data regarding the carcinogenic effects of long-term marijuana smoking are inconclusive; some studies have shown potential protective effects.4-6 Other evidence suggests that the risk is lower in comparison to tobacco smoking.6
Cardiovascular. The effects of marijuana on the cardiovascular system are not fully understood. Known symptoms include tachycardia, peripheral vasodilation, hypotension, and syncope.4 There is some evidence that marijuana use carries an increased risk for angina in patients with previously established heart disease.5 Patients, especially those with known cardiovascular disease, should be educated about these risks.
Reproductive. There are several identified reproductive consequences of marijuana use. Research has found decreased sperm count and gynecomastia in men and impaired ovulation in women.4 Studies on marijuana use in pregnancy consistently reveal low birth weight—this effect is, however, less than that seen with tobacco smoking.5 Other complications or developmental abnormalities may occur, but there is currently a lack of evidence to support further conclusions.
Neurologic. The use of marijuana results in short-term memory loss and other cognitive impairments. There is conflicting evidence as to whether long-term effects remain after cessation.5,6 Because acute intoxication impairs motor skills, it is associated with increased rates of motor vehicle accidents.6 Driving while under the influence of marijuana should be cautioned against.
Psychiatric. Marijuana use is associated with the onset and exacerbation of acute psychosis. However, its role as a causal factor in schizophrenia has not been established.4,10 There is some evidence to suggest that CBD has antipsychotic properties, warranting further research. An amotivational syndrome has also been affiliated with chronic marijuana use; affected individuals exhibit a lack of goal-directed behavior, which may result in work or school dysfunction.10 Several studies have supported an association between marijuana use and risk for depression and anxiety. Due to the extensive risk factors for these disorders, including genetic and environmental, causality has yet to be established.5,6
Conditions for Which Marijuana May Offer Therapeutic Benefits
Glaucoma
Research has demonstrated that marijuana decreases intraocular pressure, and many patients with glaucoma use marijuana. However, it is not recommended as firstline treatment.
The beneficial effects of smoked marijuana are short-lived, requiring patients to dose repeatedly throughout the day. Use is also often discontinued due to adverse effects including dry mouth, dizziness, confusion, and anxiety.8
Topical preparations of THC have not been successfully developed due to the low water solubility of cannabis and minimal penetration through the cornea to the intraocular space.8 Standard treatments available for glaucoma are more effective and without obvious psychoactive effects.6
Nausea
One of the first medical uses of marijuana was for nausea. Due to the presence of cannabinoid receptors that govern food intake, marijuana is known to stimulate appetite, making its use in reducing chemotherapy-associated nausea and vomiting widespread.2,6 Despite the variation in state laws regarding medical use of marijuana, cancer is included as a qualifying illness in every state that allows it.8 Cannabis-based medications may be useful for treating refractory nausea secondary to chemotherapy; however, dronabinol and nabilone are not recommended as firstline therapies.12
HIV/AIDS
Short-term evidence suggests that patients with HIV and/or AIDS benefit from marijuana use through improved appetite, weight gain, lessened pain, and improved quality of life.6,13 Studies with small sample sizes have been conducted using smoked marijuana and dronabinol.8 Long-term studies are needed to compare the use of marijuana with other nutritional and caloric supplements. Overall, reliable research regarding the therapeutic value of marijuana in these patients is inconclusive, and therefore no recommendations for incorporating marijuana into the treatment regimen have been made.8
Multiple sclerosis
For centuries, marijuana has been used for pain relief. The discovery of cannabinoid receptors in high concentrations throughout pain pathways of the brain supports the notion that marijuana plays a role in analgesia. While response to acute pain is poor, there is evidence to suggest that various cannabis formulations relieve chronic neuropathic pain and spasticity, as seen in multiple sclerosis.3,6
Subjective improvements in pain and spasticity were seen with the use of oral cannabis extract, THC, and nabiximols.11 Smoked marijuana is of uncertain efficacy and is not recommended for use in this patient population; it has been shown to potentially worsen cognition.8,11
Seizures
Research into the role of marijuana in decreasing seizure frequency is inconclusive.11 Large studies with human subjects are lacking, and most data thus far have come from animals and case studies.8 Some case reports have suggested a decrease in seizures with marijuana use, but further investigation is needed.6
At this time, it is not appropriate to recommend marijuana for patients with seizure disorders, but the use of cannabidiol might be more promising. Studies are ongoing.14
Alzheimer disease
Alzheimer disease is the most common cause of dementia.8 Despite known adverse effects on memory and cognition with acute use, studies have shown that marijuana might inhibit the development of amyloid beta plaques in Alzheimer disease.4 Further research on dronabinol has not provided sufficient data to support its use, and no studies utilizing smoked marijuana have been performed.8 Therefore, no recommendations exist for the use of marijuana in this patient population, and further research is warranted.
Ongoing research
There are some additional areas of potential therapeutic use of marijuana. Limited evidence has revealed that marijuana has anti-inflammatory properties, leading researchers to examine its use for autoimmune diseases, such as rheumatoid arthritis and Crohn disease. Studies investigating marijuana’s potential ability to inhibit cancer growth and metastasis are ongoing.
Unfortunately, research in patients with Parkinson disease has not shown improvement in dyskinesias.11 Studies on other movement disorders, such as Tourette syndrome and Huntington disease, have not shown symptom improvement with marijuana use. Research on these conditions and others is ongoing.
CONCLUSION
Marijuana use has negative effects on a variety of body systems, but it also may provide therapeutic benefit in certain patient populations. Clinicians and patients are currently hampered by the dearth of reliable information on its safety and efficacy (resulting from federal restrictions and other factors). Comparative studies between marijuana and established standards of care are needed, as is additional research to identify therapeutic effects that could be maximized and ways to minimize or eliminate negative sequelae.
1. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Cannabis: effective and safe analgesic? J Pain Manage. 2014;7(3):209-233.
2. Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172-186.
3. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409-2438.
4. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81.
5. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391.
6. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
7. National Conference of State Legislatures. State medical marijuana laws (updated 7/20/2016). www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed September 7, 2016.
8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10(1):1-10.
9. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362(16):1453-1457.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
11. Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology. 2014; 82(17):1556-1563.
12. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
14. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;(6):CD009270.
1. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Cannabis: effective and safe analgesic? J Pain Manage. 2014;7(3):209-233.
2. Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172-186.
3. Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70(18):2409-2438.
4. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46(1):65-81.
5. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391.
6. Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227.
7. National Conference of State Legislatures. State medical marijuana laws (updated 7/20/2016). www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed September 7, 2016.
8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10(1):1-10.
9. Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362(16):1453-1457.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
11. Koppel B, Brust J, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology. 2014; 82(17):1556-1563.
12. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;(11):CD009464.
13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
14. Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;(6):CD009270.
FDA reaffirms rivaroxaban’s atrial fib efficacy in ROCKET AF
The Food and Drug Administration reaffirmed its confidence in the data supporting the claim that rivaroxaban (Xarelto) is a safe and effective alternative to warfarin for preventing strokes and blood clots in patients with nonvalvular atrial fibrillation.
“The FDA concludes that Xarelto is a safe and effective alternative to warfarin in patients with atrial fibrillation,” the agency said in a statement released on Oct. 11.
In response to these events the FDA “completed a variety of analyses to assess the impact that this faulty monitoring device had on the ROCKET AF study results. The agency has determined that effects on strokes or bleeding, including bleeding in the head, were minimal,” the agency said in its statement.
Researchers associated with ROCKET AF published their own analysis of the impact of the faulty device on bleeding rates among patients treated with warfarin in the trial and concluded that device malfunction did not appear to influence the results (N Engl J Med. 2016 Feb 25;374[8]:785-8).
Rivaroxaban is one of four new oral anticoagulants (NOACs) on the U.S. market that are alternatives to warfarin for stroke and clot prevention in patients with nonvalvular atrial fibrillation. An analysis of 2014 data on U.S. office-based prescriptions for NOACs in atrial fibrillation patients showed that rivaroxaban was by far the most commonly prescribed drug in the class, prescribed for patients during 48% of physician office visits that led to a NOAC prescription (Am J Med. 2015 Dec;128[12]:1300-5).
[email protected]
On Twitter @mitchelzoler
The Food and Drug Administration reaffirmed its confidence in the data supporting the claim that rivaroxaban (Xarelto) is a safe and effective alternative to warfarin for preventing strokes and blood clots in patients with nonvalvular atrial fibrillation.
“The FDA concludes that Xarelto is a safe and effective alternative to warfarin in patients with atrial fibrillation,” the agency said in a statement released on Oct. 11.
In response to these events the FDA “completed a variety of analyses to assess the impact that this faulty monitoring device had on the ROCKET AF study results. The agency has determined that effects on strokes or bleeding, including bleeding in the head, were minimal,” the agency said in its statement.
Researchers associated with ROCKET AF published their own analysis of the impact of the faulty device on bleeding rates among patients treated with warfarin in the trial and concluded that device malfunction did not appear to influence the results (N Engl J Med. 2016 Feb 25;374[8]:785-8).
Rivaroxaban is one of four new oral anticoagulants (NOACs) on the U.S. market that are alternatives to warfarin for stroke and clot prevention in patients with nonvalvular atrial fibrillation. An analysis of 2014 data on U.S. office-based prescriptions for NOACs in atrial fibrillation patients showed that rivaroxaban was by far the most commonly prescribed drug in the class, prescribed for patients during 48% of physician office visits that led to a NOAC prescription (Am J Med. 2015 Dec;128[12]:1300-5).
[email protected]
On Twitter @mitchelzoler
The Food and Drug Administration reaffirmed its confidence in the data supporting the claim that rivaroxaban (Xarelto) is a safe and effective alternative to warfarin for preventing strokes and blood clots in patients with nonvalvular atrial fibrillation.
“The FDA concludes that Xarelto is a safe and effective alternative to warfarin in patients with atrial fibrillation,” the agency said in a statement released on Oct. 11.
In response to these events the FDA “completed a variety of analyses to assess the impact that this faulty monitoring device had on the ROCKET AF study results. The agency has determined that effects on strokes or bleeding, including bleeding in the head, were minimal,” the agency said in its statement.
Researchers associated with ROCKET AF published their own analysis of the impact of the faulty device on bleeding rates among patients treated with warfarin in the trial and concluded that device malfunction did not appear to influence the results (N Engl J Med. 2016 Feb 25;374[8]:785-8).
Rivaroxaban is one of four new oral anticoagulants (NOACs) on the U.S. market that are alternatives to warfarin for stroke and clot prevention in patients with nonvalvular atrial fibrillation. An analysis of 2014 data on U.S. office-based prescriptions for NOACs in atrial fibrillation patients showed that rivaroxaban was by far the most commonly prescribed drug in the class, prescribed for patients during 48% of physician office visits that led to a NOAC prescription (Am J Med. 2015 Dec;128[12]:1300-5).
[email protected]
On Twitter @mitchelzoler
Emergency Imaging: Acute abdominal pain
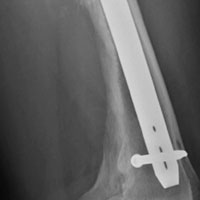
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
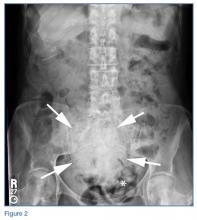
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
Hypertension in the ED
Case Scenarios
Case 1
You had just started your shift, and your first patient presented with symptoms of headache and dizziness, and a blood pressure (BP) of 240/130 mm Hg, without any vomiting or visual symptoms. Physical examination revealed an alert, pleasant 65-year-old black man whose ocular, neurological, and cardiovascular (CV) examinations were normal. The patient reported a history of borderline hypertension, but had never taken any medications for it.
After placing some initial orders, including an electrocardiogram (ECG), basic metabolic panel (BMP), and head computed tomography (CT) scan, and ordering 10 mg intravenous (IV) prochlorperazine and 25 mg IV diphenhydramine to treat the patient’s headache, you are left asking yourself what steps you should take next.
Case 2
Your next patient was a 90-year-old white woman who had been referred to the ED by her primary care physician (PCP) for “hypertensive urgency.” She had no complaints to report. Similar to the first patient, this patient’s physical examination was also normal, with the exception of a persistently elevated BP of 220/140 mm Hg. Her history was significant for congestive heart failure (CHF), but she exhibited no current CV signs or symptoms. The patient had been taking furosemide but was not on any other antihypertensive medications.
Case 3
In the room next to your 90-year-old patient is a 32-year-old uninsured hypertensive white woman. During the history taking, the patient stated that she was trying to become pregnant and was not currently using any form of contraception. Similar to the second patient, she had no complaints to report. Regarding her reason for presentation, the patient stated that when she had her BP checked at a pharmacy earlier that day, the reading was “too high,” and the pharmacist had advised her to go to the ED. She seemed anxious but otherwise well. Her initial BP at presentation was 240/100 mm Hg, but her physical examination was otherwise normal.
Hypertensive Emergencies
As emergency physicians (EPs), we see hypertensive patients every day. According to the US Centers for Disease Control and Prevention, 33% of American adults have hypertension, which is defined by a BP of ≥140/90 mm Hg (Table 1).
Almost 25% of total annual US adult ED visits are directly or indirectly related to hypertension, and about 1% of all ED visits are due solely to elevated BP.3 In an ambulatory care survey for 2007, moderate or severe hypertensive BP readings were found to be more common in patients presenting to the ED (43.5%) than to primary care clinics (27%).4 Patients presenting to the ED with hypertensive BP readings disproportionately represented patients who were older, male, non-Hispanic black, Medicare beneficiaries, or uninsured. Certainly, some patients presenting to the ED have hypertensive BP readings due to pain or anxiety, but multiple studies have suggested that 50% to 70% of ED patients who have hypertensive BP readings will be diagnosed with hypertension on office follow-up.5,6 While a minority of these patients present to the ED with hypertensive emergencies, the majority present either without symptoms of hypertension or with only mild headache. Given the disease burden of hypertension combined with the benefits of treatment, it is worthwhile for the practicing EP to review the most up-to-date guidelines on outpatient management of hypertension.
When a patient presents to the ED with a hypertensive BP reading, the initial priority of the EP is to exclude hypertensive emergency. Hypertensive emergencies are defined by the presence of hypertension (generally grade 3/severe hypertension with BP ≥180/110 mm Hg; see Table 1) in conjunction with evidence of target organ damage.
Target Organ Manifestations
The acuity and/or presence of target organ damage are not always clear on initial ED evaluation. For instance, when a patient who has no history of primary care presents to the ED with severe hypertension, laboratory evaluation may demonstrate protein and blood in his or her urine and an elevated serum creatinine level. In the absence of values from past laboratory studies, it is difficult to determine whether these test results represent normal laboratory parameters for this patient due to longstanding hypertensive kidney disease (ie, hypertensive nephrosclerosis) or if they represent a true hypertensive emergency, (ie, hypertensive emergency-related nephropathy).7 In patients with severe hypertension and possibly new acute kidney injury, it is probably safest to either assume hypertensive emergency-related nephropathy and to treat accordingly or consult with nephrology services. The picture of hypertensive emergency-related nephropathy often only becomes clear after renal biopsy results and improvement in renal parameters with BP control.
The ocular manifestations of hypertensive emergency require detailed fundoscopy, which at times can be challenging in the ED. In assessing for cardiac target organ damage, at our institution, we typically ask patients if they have experienced symptoms of dyspnea and chest pain or pressure. Generally, we also evaluate cardiac enzymes, B-type natriuretic peptide, and order ECG and chest X-ray studies when suggested by history or physical examination. Alarmingly, a study of 161 ED hypertensive (average BP of 183/109 mm Hg), asymptomatic, predominantly black patients found that 146 (90.7%) had subclinical hypertensive heart disease on point-of-care echocardiogram.8
Neurological/Hypertensive Encephalopathy
Hypertensive encephalopathy is a diagnosis of exclusion as alternate causes of confusion and headache, such as intracranial hemorrhage, are excluded and mental status improves with titrated BP control. Nonetheless, it is difficult to confidently state from the literature that patients who present with headache but have a normal mental status in the presence of severe hypertension are not on an early spectrum of hypertensive encephalopathy. Therefore, it is likely that the degree of symptoms should define whether target organ damage exists, though there is certainly a spectrum of hypertensive emergency—the strict definition of which is not always clear.
When a hypertensive emergency is diagnosed, management typically involves the use of antihypertensive IV medication in the intensive care unit. While such management is outside the scope of this paper, Adebayo and Rogers9 have published an excellent review of the care of hypertensive emergencies.
Asymptomatic Hypertension
The American College of Emergency Physicians (ACEP) has developed two clinical policies on the evaluation and management of asymptomatic hypertension in the ED. The original, published in 2006, advised that initially high BP readings of ED patients should be repeated: two separate high readings are adequate for screening, and those patients with hypertension should be referred for follow-up. Furthermore, ACEP policies note that initiating treatment in the ED is not necessary when patients are referred for follow-up. If treatment for hypertension is initiated in the ED, ACEP recommends that such management should attempt only to gradually lower BP, and not to normalize it during the initial ED visit.10
The 2013 update to ACEP’s clinical policy on managing asymptomatic hypertension expanded on the original policy. The updated policy advised against routine testing for target organ damage in patients who have asymptomatic severe hypertension. However, ACEP policy notes that evaluating serum creatinine in these patients with poor follow-up may influence patient disposition.11
The 2013 policy further stated that medical intervention is not required in ED patients who have asymptomatic severe hypertension, but may be considered in patients with poor follow-up. The policies emphasize that all asymptomatic hypertensive patients should be referred for follow-up. The literature cited for the recommendation that ED patients with asymptomatic severe hypertension do not require routine investigation stems from two observational studies. These studies found that screening asymptomatic ED patients who presented with severe hypertension revealed serum creatinine abnormalities in approximately 6%, which impacted patient disposition, though it was not clear from the study results whether admission correlated to meaningful patient outcomes.12,13
Patient Disposition
Since ACEP’s 2013 clinical policy, a study from the Cleveland Clinic has been published. This retrospective cohort study reviewed 6 years of data looking at all patients in its system with a BP of ≥180/110 mm Hg, and compared those office patients discharged to home to those referred to the ED or directly admitted to the inpatient hospital solely on the basis of severe hypertension.14 The study found that 0.5% of 387 patients referred to the ED by primary care clinics for asymptomatic severe hypertension had confirmed acute kidney injury on BMP.14 The Cleveland Clinic study also found that 2.1% of patients had evidence of target organ damage and 5.5% had any abnormal results.14 In addition, referral to the ED from the clinic for hypertension was associated with a slightly higher rate of major adverse CV events at 7 days (2 of 426 [0.5%] versus 61 of 58,109 [0.1%]; P = .02).14
The results of the Cleveland Clinic study confirm that in the absence of target organ damage, hypertension is probably best managed in the outpatient setting. The European Task Force hypertensive guidelines state “hospitalization for hypertension is regarded as inappropriate in most European countries.”15 However, from 2006 to 2012, 26% of US ED patients with primary diagnoses of hypertension were admitted to the hospital.3 In Canada’s most populous province of Ontario, from 2002 to 2011, approximately 8% of hypertensive patients were admitted.16 Part of this discrepancy may be due to the sometimes ambiguous nature of the presentation of patients with hypertension, making it unclear whether a true hypertensive emergency exists. Many patients perceive visual symptoms, headache, dizziness, and even chest pressure as the result of their elevated BP—without clear findings on fundoscopy, ECG, or cardiac marker testing. Perhaps more of these patients would be discharged if EPs felt comfortable initiating appropriate initial antihypertensive treatment.
Management
Initiating Antihypertensive Treatment
Some EPs may feel that an accurate diagnosis of hypertension requires repeat BP testing in the primary care office setting, and for this reason are reluctant to initiate antihypertensive treatment in the ED. The most recent guidelines by the Joint National Committee (JNC 8) do not address how many BP readings are necessary to diagnose hypertension, but JNC 7 suggested that diagnosis of hypertension requires two separate office visits.17 Evidence cited in ACEP’s first clinical policy states that two separate BP measurements in the ED are adequate for screening—but not necessarily for initiating treatment.10 However, European and British outpatient clinical recommendations advocate initiation of antihypertensive medication for a single visit in patients who have an elevated BP categorized as grade 3/severe hypertension (BP of ≥180/110 mm Hg).15,18 Furthermore, for patients with severe hypertension seen in the ED, as many as 97% are likely to have true hypertension at office follow-up.6 Those ED patients presenting with severe hypertension are very likely to have a true diagnosis of hypertension.
A recent retrospective analysis of a group of hypertensive ED patients by Brody et al19 found that patients prescribed BP medications by an EP were more likely to have improved BP control at follow-up 2 weeks later. In their study, the decision to prescribe antihypertensive medications were at the discretion of the EP. Seventy-six patients were given one or more prescriptions for antihypertensive therapy, compared to a control group of 141 patients who were not given a prescription. On follow-up at 2 weeks, there was an 11 mm Hg greater reduction of BP in the group who received prescriptions compared to the control group. None of the patients in either group on follow-up had experienced any new neurological deficits, ischemic events, life-threatening anaphylactic reactions, or clinically significant hypotension.
The Cleveland Clinic study14 also reported on those patents given who received new prescriptions from the ED. Similar to the study by Brody et al,19 none of the 82 patients discharged to home from the ED with a new antihypertensive prescription had any major adverse event at 30-day follow-up.14
Pharmacological Treatment Recommendations
When choosing to treat patients with new prescriptions for antihypertensives, it is important to follow the most current outpatient treatment recommendations. In 2014, JNC 8 released new guidelines for the outpatient management of adults with hypertension.20 The panel issued recommendations based on its systematic review of randomized controlled trials on antihypertensive treatments. The key recommendations are as follows:
- In patients aged 60 years or older, initiate pharmacological treatment at a BP of ≥150/90 mm Hg.
- In patients aged 18 to 59 years, initiate pharmacological treatment at a BP of ≥140/90 mm Hg.
- In the general nonblack population, initial antihypertensive treatment should include a thiazide-type diuretic, a calcium channel blocker (CCB), an angiotensin-converting enzyme inhibitor (ACE-I), or an angiotensin receptor blocker (ARB).
- In the general black population, initial treatment should include a thiazide-type diuretic or a CCB.
- In patients with chronic kidney disease (CKD) (including black patients), initial (or add-on) antihypertensive treatment should include an ACE-I or ARB to improve kidney outcomes—but not both.
- If goal BP is not reached within 1 month of initial treatment, increase the dose of the initial drug or add a second agent (eg, thiazide-type diuretic, CCB, ACE-I, or ARB). If goal is not reached with two drugs, use the third drug from that list if no contraindications exist, but do not use both an ACE-I and an ARB together in the same patient.
Of note, JNC 8, in departure from JNC 7, no longer recommends beta-blockers as first-line therapy for isolated hypertension (there may be compelling alternate indications, such as atrial fibrillation or postmyocardial infarction (MI), such that a beta-blocker would still be the first medication considered). The reason for this stems from a single randomized controlled trial of 9,193 patients that found that despite equivalent BP reduction, use of a beta-blocker in comparison to an ARB resulted in a higher rate of a composite outcome of death, MI, or stroke.21 The main difference was a 25% relative risk reduction for stroke with use of an ARB (losartan) in comparison to a beta-blocker (atenolol). The most recent European guidelines still include beta-blockers among its first-line recommended BP medications, but do acknowledge that they are not as effective in reducing stroke incidence as other alternative medications.15 The European guidelines otherwise include the same list of first-line agents. The British guidelines mirror JNC 8 in terms of first-line antihypertensive medication choices.18
Since the release of JNC 8, the Systolic Blood Pressure Intervention Trial (SPRINT) has been published, and will likely impact future national recommendations on BP management. The SPRINT study was a randomized controlled trial enrolling over 9,000 hypertensive nondiabetic patients older than age 50 years that treated individuals to a standard BP goal (systolic BP of 140 mm Hg) versus an intensive BP goal (systolic BP of 120 mm Hg) over a 3.5-year period. The trial was stopped early for safety as a 25% mortality reduction was observed in the intensive treatment group (1.65 vs 2.19 deaths/y).22 This was in contrast to previous trials that had mostly failed to show this sort of benefit, though previous trials were smaller in number or included only diabetic patients.23 While it is likely that this trial may influence lowering treatment thresholds from the office, it is not likely to impact care from the ED.
The recommendations of JNC 8 do not necessarily coincide with current US EP practice. In the study by Brody et al,19 of patients provided ED antihypertensive prescriptions, 54% received thiazide-type diuretics, 26% ACE-I, 10% CCBs, and 6% beta-blockers. This is noteworthy because 96% of those in the study were black patients who would benefit most from either a thiazide or a CCB. Another recent study of ED patients showed that of patients who were both treated in the ED and discharged with antihypertensive medications, 34% received a diuretic prescription, 32% clonidine, 15% a beta-blocker, 19% an ARB or ACE-I, 12% a CCB, and 2% hydrazine.24 These results are important because according to many published guidelines, including JNC 8, clonidine is only considered one of several fourth-line options for severe resistant hypertension.15,18,20 Since clonidine use can be complicated by rebound hypertension, it is not an ideal agent to be prescribed de novo to patients in the ED. This is particularly true if these patients are not already on maximum doses of the three most recommended agents previously noted, or if there are concerns over patient compliance.
Of the drug classes recommended by JNC 8, Table 3 lists the absolute and relative contraindications.
In clinical trials, amlodipine is among the most effective BP medications and is considered first-line therapy for all groups of patients with hypertension.15,18,20 A simplistic approach for most patients presenting with severe asymptomatic hypertension (BP of ≥180/110 mm Hg) not currently on treatment would be to recheck the BP and assure it remains elevated over the period of the ED visit.
Conclusion
Hypertension is among the most common medical conditions for which emergency patients seek care. The ACEP clinical policies provide guidance on the appropriate work-up and treatment of these patients. Given the occasional lack of clarity on whether a patient’s presentation is on the spectrum of more acute/serious, EPs may feel more comfortable in discharging patients with poor follow-up if they are able to safely prescribe antihypertensive treatment. Prior to prescribing treatment, EPs should refer to the JNC 8 guidelines to appropriately start antihypertensive treatment in select patient groups in the ED. The guidelines of JNC 8 are therefore worth referring to in order to appropriately start treatment in select patient groups from the ED.
Case Scenarios Continued
Case 1
[The 65-year-old black man who presented with headache and dizziness, and had an initial BP of 240/130 mm Hg.]
After treating the patient with prochlorperazine and diphenhydramine, his headache resolved. His BP improved but remained elevated at 190/120 mm Hg. On further questioning, the patient reported a history of similar headaches and wondered whether it was related to his BP. The head CT scan was negative for any acute hemorrhage, infarct, or mass; the ECG only showed evidence of left ventricular hypertrophy; and the BMP showed normal renal function.
After a long discussion with the patient, you agreed to start him on amlodipine 5 mg/d and referred him for follow-up with a local PCP.
Case 2
[The 90-year-old white woman with a history of CHF and an initial BP of 220/140 mm Hg at presentation.]
The BMP evaluation showed a baseline creatinine level of 1.3 mg/dL. Given this patient’s history of CHF, amlodipine would not be the ideal next agent to prescribe. After discussion with her PCP, you elected to start her on losartan at 25 mg/d, and instructed her to follow-up with her PCP within 1 week.
Case 3
[The 32-year-old white woman who presented at the advice of a pharmacist and had an initial BP of 240/100 mm Hg.]While reviewing the patient’s work-up and history, you noted her plans to become pregnant, and recalled a recent review on BP management, noting the contraindications associated with ARB or ACE-I in pregnancy. Based on the patient’s uninsured status and poor follow-up, you considered prescribing amlodipine. Prior to issuing the prescription, you performed a repeat BP check and noted that the patient’s BP had decreased to 130/85 mm Hg. Given the marked improvement in the patient’s BP during her ED course, you were not convinced that she truly had hypertension.
Instead of prescribing an antihypertensive agent, which may not ultimately benefit this patient, you advised her to seek follow-up care at an outpatient clinic to have her BP rechecked. The patient agreed, and you referred her to a local free clinic.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
2. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2013;362(9395):1527-1535.
3. McNaughton CD, Self WH, Zhu Y, Janke AT, Storrow AB, Levy P. Incidence of hypertension-related emergency department visits in the United States, 2006 to 2012. Am J Cardiol. 2015;116(11):1717-1723. doi: 10.1016/j.amjcard.2015.09.007.
4. Niska RW. Blood pressure measurements at emergency department visits by adults: United States, 2007-2008. NCHS Data Brief. 2011;(72):1-8.
5. Chernow SM, Iserson KV, Criss E. Use of the emergency department for hypertension screening: a prospective study. Ann Emerg Med. 1987;16(2):180-182.
6. Backer HD, Decker L, Ackerson L. Reproducibility of increased blood pressure during an emergency department or urgent care visit. Ann Emerg Med. 2003.41(4):507-512.
7. Nonaka K, Ubara Y, Sumida K, et al. Clinical and pathological evaluation of hypertensive emergency-related nephropathy. Intern Med. 2013;52(1):45-53.
8. Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467-474.e1. doi: 10.1016/j.annemergmed.2012.03.030.
9. Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539-551. doi: 10.1016/j.emc.2015.04.005.
10. Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-234. doi: 10.1016/j.annemergmed.2005.10.003
11. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68. doi: 10.1016/j.annemergmed.2013.05.012.
12. Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med. 2006;47(3):230-236.
13. Nishijima DK, Paladino L, Sinert R. Routine testing in patients with asymptomatic elevated blood pressure in the ED. Am J Emerg Med. 2010;28(2):235-242. doi: 10.1016/j.ajem.2008.11.015.
14. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509.
15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151.
16. Masood S, Austin PC, Atzema CL. A population-based analysis of outcomes in patients with a primary diagnosis of hypertension in the emergency department. Ann Emerg Med. 2016;68(3):258-267.e5. doi: 10.1016/j.annemergmed.2016.04.060.
17. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. Erratum in: JAMA. 2003;290(2):197.
18. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891.
19. Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632-635. doi: 10.1111/acem.12660.
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014;311(17):1809.
21. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
22. PRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939.
23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286.
24. Levy PD, Mahn JJ, Miller J, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33(9):1219-1224. doi: 10.1016/j.ajem.2015.05.036.
25. Leung AA, Wright A, Pazo V, Karson A, Bates DW. Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med. 2011;124(11):1064-1072. doi: 10.1016/j.amjmed.2011.06.031.
26. Mann JFE, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. http://www.uptodate.com/contents/renin-angiotensin-system-inhibition-in-the-treatment-of-hypertension. Accessed September 21, 2016.
Case Scenarios
Case 1
You had just started your shift, and your first patient presented with symptoms of headache and dizziness, and a blood pressure (BP) of 240/130 mm Hg, without any vomiting or visual symptoms. Physical examination revealed an alert, pleasant 65-year-old black man whose ocular, neurological, and cardiovascular (CV) examinations were normal. The patient reported a history of borderline hypertension, but had never taken any medications for it.
After placing some initial orders, including an electrocardiogram (ECG), basic metabolic panel (BMP), and head computed tomography (CT) scan, and ordering 10 mg intravenous (IV) prochlorperazine and 25 mg IV diphenhydramine to treat the patient’s headache, you are left asking yourself what steps you should take next.
Case 2
Your next patient was a 90-year-old white woman who had been referred to the ED by her primary care physician (PCP) for “hypertensive urgency.” She had no complaints to report. Similar to the first patient, this patient’s physical examination was also normal, with the exception of a persistently elevated BP of 220/140 mm Hg. Her history was significant for congestive heart failure (CHF), but she exhibited no current CV signs or symptoms. The patient had been taking furosemide but was not on any other antihypertensive medications.
Case 3
In the room next to your 90-year-old patient is a 32-year-old uninsured hypertensive white woman. During the history taking, the patient stated that she was trying to become pregnant and was not currently using any form of contraception. Similar to the second patient, she had no complaints to report. Regarding her reason for presentation, the patient stated that when she had her BP checked at a pharmacy earlier that day, the reading was “too high,” and the pharmacist had advised her to go to the ED. She seemed anxious but otherwise well. Her initial BP at presentation was 240/100 mm Hg, but her physical examination was otherwise normal.
Hypertensive Emergencies
As emergency physicians (EPs), we see hypertensive patients every day. According to the US Centers for Disease Control and Prevention, 33% of American adults have hypertension, which is defined by a BP of ≥140/90 mm Hg (Table 1).
Almost 25% of total annual US adult ED visits are directly or indirectly related to hypertension, and about 1% of all ED visits are due solely to elevated BP.3 In an ambulatory care survey for 2007, moderate or severe hypertensive BP readings were found to be more common in patients presenting to the ED (43.5%) than to primary care clinics (27%).4 Patients presenting to the ED with hypertensive BP readings disproportionately represented patients who were older, male, non-Hispanic black, Medicare beneficiaries, or uninsured. Certainly, some patients presenting to the ED have hypertensive BP readings due to pain or anxiety, but multiple studies have suggested that 50% to 70% of ED patients who have hypertensive BP readings will be diagnosed with hypertension on office follow-up.5,6 While a minority of these patients present to the ED with hypertensive emergencies, the majority present either without symptoms of hypertension or with only mild headache. Given the disease burden of hypertension combined with the benefits of treatment, it is worthwhile for the practicing EP to review the most up-to-date guidelines on outpatient management of hypertension.
When a patient presents to the ED with a hypertensive BP reading, the initial priority of the EP is to exclude hypertensive emergency. Hypertensive emergencies are defined by the presence of hypertension (generally grade 3/severe hypertension with BP ≥180/110 mm Hg; see Table 1) in conjunction with evidence of target organ damage.
Target Organ Manifestations
The acuity and/or presence of target organ damage are not always clear on initial ED evaluation. For instance, when a patient who has no history of primary care presents to the ED with severe hypertension, laboratory evaluation may demonstrate protein and blood in his or her urine and an elevated serum creatinine level. In the absence of values from past laboratory studies, it is difficult to determine whether these test results represent normal laboratory parameters for this patient due to longstanding hypertensive kidney disease (ie, hypertensive nephrosclerosis) or if they represent a true hypertensive emergency, (ie, hypertensive emergency-related nephropathy).7 In patients with severe hypertension and possibly new acute kidney injury, it is probably safest to either assume hypertensive emergency-related nephropathy and to treat accordingly or consult with nephrology services. The picture of hypertensive emergency-related nephropathy often only becomes clear after renal biopsy results and improvement in renal parameters with BP control.
The ocular manifestations of hypertensive emergency require detailed fundoscopy, which at times can be challenging in the ED. In assessing for cardiac target organ damage, at our institution, we typically ask patients if they have experienced symptoms of dyspnea and chest pain or pressure. Generally, we also evaluate cardiac enzymes, B-type natriuretic peptide, and order ECG and chest X-ray studies when suggested by history or physical examination. Alarmingly, a study of 161 ED hypertensive (average BP of 183/109 mm Hg), asymptomatic, predominantly black patients found that 146 (90.7%) had subclinical hypertensive heart disease on point-of-care echocardiogram.8
Neurological/Hypertensive Encephalopathy
Hypertensive encephalopathy is a diagnosis of exclusion as alternate causes of confusion and headache, such as intracranial hemorrhage, are excluded and mental status improves with titrated BP control. Nonetheless, it is difficult to confidently state from the literature that patients who present with headache but have a normal mental status in the presence of severe hypertension are not on an early spectrum of hypertensive encephalopathy. Therefore, it is likely that the degree of symptoms should define whether target organ damage exists, though there is certainly a spectrum of hypertensive emergency—the strict definition of which is not always clear.
When a hypertensive emergency is diagnosed, management typically involves the use of antihypertensive IV medication in the intensive care unit. While such management is outside the scope of this paper, Adebayo and Rogers9 have published an excellent review of the care of hypertensive emergencies.
Asymptomatic Hypertension
The American College of Emergency Physicians (ACEP) has developed two clinical policies on the evaluation and management of asymptomatic hypertension in the ED. The original, published in 2006, advised that initially high BP readings of ED patients should be repeated: two separate high readings are adequate for screening, and those patients with hypertension should be referred for follow-up. Furthermore, ACEP policies note that initiating treatment in the ED is not necessary when patients are referred for follow-up. If treatment for hypertension is initiated in the ED, ACEP recommends that such management should attempt only to gradually lower BP, and not to normalize it during the initial ED visit.10
The 2013 update to ACEP’s clinical policy on managing asymptomatic hypertension expanded on the original policy. The updated policy advised against routine testing for target organ damage in patients who have asymptomatic severe hypertension. However, ACEP policy notes that evaluating serum creatinine in these patients with poor follow-up may influence patient disposition.11
The 2013 policy further stated that medical intervention is not required in ED patients who have asymptomatic severe hypertension, but may be considered in patients with poor follow-up. The policies emphasize that all asymptomatic hypertensive patients should be referred for follow-up. The literature cited for the recommendation that ED patients with asymptomatic severe hypertension do not require routine investigation stems from two observational studies. These studies found that screening asymptomatic ED patients who presented with severe hypertension revealed serum creatinine abnormalities in approximately 6%, which impacted patient disposition, though it was not clear from the study results whether admission correlated to meaningful patient outcomes.12,13
Patient Disposition
Since ACEP’s 2013 clinical policy, a study from the Cleveland Clinic has been published. This retrospective cohort study reviewed 6 years of data looking at all patients in its system with a BP of ≥180/110 mm Hg, and compared those office patients discharged to home to those referred to the ED or directly admitted to the inpatient hospital solely on the basis of severe hypertension.14 The study found that 0.5% of 387 patients referred to the ED by primary care clinics for asymptomatic severe hypertension had confirmed acute kidney injury on BMP.14 The Cleveland Clinic study also found that 2.1% of patients had evidence of target organ damage and 5.5% had any abnormal results.14 In addition, referral to the ED from the clinic for hypertension was associated with a slightly higher rate of major adverse CV events at 7 days (2 of 426 [0.5%] versus 61 of 58,109 [0.1%]; P = .02).14
The results of the Cleveland Clinic study confirm that in the absence of target organ damage, hypertension is probably best managed in the outpatient setting. The European Task Force hypertensive guidelines state “hospitalization for hypertension is regarded as inappropriate in most European countries.”15 However, from 2006 to 2012, 26% of US ED patients with primary diagnoses of hypertension were admitted to the hospital.3 In Canada’s most populous province of Ontario, from 2002 to 2011, approximately 8% of hypertensive patients were admitted.16 Part of this discrepancy may be due to the sometimes ambiguous nature of the presentation of patients with hypertension, making it unclear whether a true hypertensive emergency exists. Many patients perceive visual symptoms, headache, dizziness, and even chest pressure as the result of their elevated BP—without clear findings on fundoscopy, ECG, or cardiac marker testing. Perhaps more of these patients would be discharged if EPs felt comfortable initiating appropriate initial antihypertensive treatment.
Management
Initiating Antihypertensive Treatment
Some EPs may feel that an accurate diagnosis of hypertension requires repeat BP testing in the primary care office setting, and for this reason are reluctant to initiate antihypertensive treatment in the ED. The most recent guidelines by the Joint National Committee (JNC 8) do not address how many BP readings are necessary to diagnose hypertension, but JNC 7 suggested that diagnosis of hypertension requires two separate office visits.17 Evidence cited in ACEP’s first clinical policy states that two separate BP measurements in the ED are adequate for screening—but not necessarily for initiating treatment.10 However, European and British outpatient clinical recommendations advocate initiation of antihypertensive medication for a single visit in patients who have an elevated BP categorized as grade 3/severe hypertension (BP of ≥180/110 mm Hg).15,18 Furthermore, for patients with severe hypertension seen in the ED, as many as 97% are likely to have true hypertension at office follow-up.6 Those ED patients presenting with severe hypertension are very likely to have a true diagnosis of hypertension.
A recent retrospective analysis of a group of hypertensive ED patients by Brody et al19 found that patients prescribed BP medications by an EP were more likely to have improved BP control at follow-up 2 weeks later. In their study, the decision to prescribe antihypertensive medications were at the discretion of the EP. Seventy-six patients were given one or more prescriptions for antihypertensive therapy, compared to a control group of 141 patients who were not given a prescription. On follow-up at 2 weeks, there was an 11 mm Hg greater reduction of BP in the group who received prescriptions compared to the control group. None of the patients in either group on follow-up had experienced any new neurological deficits, ischemic events, life-threatening anaphylactic reactions, or clinically significant hypotension.
The Cleveland Clinic study14 also reported on those patents given who received new prescriptions from the ED. Similar to the study by Brody et al,19 none of the 82 patients discharged to home from the ED with a new antihypertensive prescription had any major adverse event at 30-day follow-up.14
Pharmacological Treatment Recommendations
When choosing to treat patients with new prescriptions for antihypertensives, it is important to follow the most current outpatient treatment recommendations. In 2014, JNC 8 released new guidelines for the outpatient management of adults with hypertension.20 The panel issued recommendations based on its systematic review of randomized controlled trials on antihypertensive treatments. The key recommendations are as follows:
- In patients aged 60 years or older, initiate pharmacological treatment at a BP of ≥150/90 mm Hg.
- In patients aged 18 to 59 years, initiate pharmacological treatment at a BP of ≥140/90 mm Hg.
- In the general nonblack population, initial antihypertensive treatment should include a thiazide-type diuretic, a calcium channel blocker (CCB), an angiotensin-converting enzyme inhibitor (ACE-I), or an angiotensin receptor blocker (ARB).
- In the general black population, initial treatment should include a thiazide-type diuretic or a CCB.
- In patients with chronic kidney disease (CKD) (including black patients), initial (or add-on) antihypertensive treatment should include an ACE-I or ARB to improve kidney outcomes—but not both.
- If goal BP is not reached within 1 month of initial treatment, increase the dose of the initial drug or add a second agent (eg, thiazide-type diuretic, CCB, ACE-I, or ARB). If goal is not reached with two drugs, use the third drug from that list if no contraindications exist, but do not use both an ACE-I and an ARB together in the same patient.
Of note, JNC 8, in departure from JNC 7, no longer recommends beta-blockers as first-line therapy for isolated hypertension (there may be compelling alternate indications, such as atrial fibrillation or postmyocardial infarction (MI), such that a beta-blocker would still be the first medication considered). The reason for this stems from a single randomized controlled trial of 9,193 patients that found that despite equivalent BP reduction, use of a beta-blocker in comparison to an ARB resulted in a higher rate of a composite outcome of death, MI, or stroke.21 The main difference was a 25% relative risk reduction for stroke with use of an ARB (losartan) in comparison to a beta-blocker (atenolol). The most recent European guidelines still include beta-blockers among its first-line recommended BP medications, but do acknowledge that they are not as effective in reducing stroke incidence as other alternative medications.15 The European guidelines otherwise include the same list of first-line agents. The British guidelines mirror JNC 8 in terms of first-line antihypertensive medication choices.18
Since the release of JNC 8, the Systolic Blood Pressure Intervention Trial (SPRINT) has been published, and will likely impact future national recommendations on BP management. The SPRINT study was a randomized controlled trial enrolling over 9,000 hypertensive nondiabetic patients older than age 50 years that treated individuals to a standard BP goal (systolic BP of 140 mm Hg) versus an intensive BP goal (systolic BP of 120 mm Hg) over a 3.5-year period. The trial was stopped early for safety as a 25% mortality reduction was observed in the intensive treatment group (1.65 vs 2.19 deaths/y).22 This was in contrast to previous trials that had mostly failed to show this sort of benefit, though previous trials were smaller in number or included only diabetic patients.23 While it is likely that this trial may influence lowering treatment thresholds from the office, it is not likely to impact care from the ED.
The recommendations of JNC 8 do not necessarily coincide with current US EP practice. In the study by Brody et al,19 of patients provided ED antihypertensive prescriptions, 54% received thiazide-type diuretics, 26% ACE-I, 10% CCBs, and 6% beta-blockers. This is noteworthy because 96% of those in the study were black patients who would benefit most from either a thiazide or a CCB. Another recent study of ED patients showed that of patients who were both treated in the ED and discharged with antihypertensive medications, 34% received a diuretic prescription, 32% clonidine, 15% a beta-blocker, 19% an ARB or ACE-I, 12% a CCB, and 2% hydrazine.24 These results are important because according to many published guidelines, including JNC 8, clonidine is only considered one of several fourth-line options for severe resistant hypertension.15,18,20 Since clonidine use can be complicated by rebound hypertension, it is not an ideal agent to be prescribed de novo to patients in the ED. This is particularly true if these patients are not already on maximum doses of the three most recommended agents previously noted, or if there are concerns over patient compliance.
Of the drug classes recommended by JNC 8, Table 3 lists the absolute and relative contraindications.
In clinical trials, amlodipine is among the most effective BP medications and is considered first-line therapy for all groups of patients with hypertension.15,18,20 A simplistic approach for most patients presenting with severe asymptomatic hypertension (BP of ≥180/110 mm Hg) not currently on treatment would be to recheck the BP and assure it remains elevated over the period of the ED visit.
Conclusion
Hypertension is among the most common medical conditions for which emergency patients seek care. The ACEP clinical policies provide guidance on the appropriate work-up and treatment of these patients. Given the occasional lack of clarity on whether a patient’s presentation is on the spectrum of more acute/serious, EPs may feel more comfortable in discharging patients with poor follow-up if they are able to safely prescribe antihypertensive treatment. Prior to prescribing treatment, EPs should refer to the JNC 8 guidelines to appropriately start antihypertensive treatment in select patient groups in the ED. The guidelines of JNC 8 are therefore worth referring to in order to appropriately start treatment in select patient groups from the ED.
Case Scenarios Continued
Case 1
[The 65-year-old black man who presented with headache and dizziness, and had an initial BP of 240/130 mm Hg.]
After treating the patient with prochlorperazine and diphenhydramine, his headache resolved. His BP improved but remained elevated at 190/120 mm Hg. On further questioning, the patient reported a history of similar headaches and wondered whether it was related to his BP. The head CT scan was negative for any acute hemorrhage, infarct, or mass; the ECG only showed evidence of left ventricular hypertrophy; and the BMP showed normal renal function.
After a long discussion with the patient, you agreed to start him on amlodipine 5 mg/d and referred him for follow-up with a local PCP.
Case 2
[The 90-year-old white woman with a history of CHF and an initial BP of 220/140 mm Hg at presentation.]
The BMP evaluation showed a baseline creatinine level of 1.3 mg/dL. Given this patient’s history of CHF, amlodipine would not be the ideal next agent to prescribe. After discussion with her PCP, you elected to start her on losartan at 25 mg/d, and instructed her to follow-up with her PCP within 1 week.
Case 3
[The 32-year-old white woman who presented at the advice of a pharmacist and had an initial BP of 240/100 mm Hg.]While reviewing the patient’s work-up and history, you noted her plans to become pregnant, and recalled a recent review on BP management, noting the contraindications associated with ARB or ACE-I in pregnancy. Based on the patient’s uninsured status and poor follow-up, you considered prescribing amlodipine. Prior to issuing the prescription, you performed a repeat BP check and noted that the patient’s BP had decreased to 130/85 mm Hg. Given the marked improvement in the patient’s BP during her ED course, you were not convinced that she truly had hypertension.
Instead of prescribing an antihypertensive agent, which may not ultimately benefit this patient, you advised her to seek follow-up care at an outpatient clinic to have her BP rechecked. The patient agreed, and you referred her to a local free clinic.
Case Scenarios
Case 1
You had just started your shift, and your first patient presented with symptoms of headache and dizziness, and a blood pressure (BP) of 240/130 mm Hg, without any vomiting or visual symptoms. Physical examination revealed an alert, pleasant 65-year-old black man whose ocular, neurological, and cardiovascular (CV) examinations were normal. The patient reported a history of borderline hypertension, but had never taken any medications for it.
After placing some initial orders, including an electrocardiogram (ECG), basic metabolic panel (BMP), and head computed tomography (CT) scan, and ordering 10 mg intravenous (IV) prochlorperazine and 25 mg IV diphenhydramine to treat the patient’s headache, you are left asking yourself what steps you should take next.
Case 2
Your next patient was a 90-year-old white woman who had been referred to the ED by her primary care physician (PCP) for “hypertensive urgency.” She had no complaints to report. Similar to the first patient, this patient’s physical examination was also normal, with the exception of a persistently elevated BP of 220/140 mm Hg. Her history was significant for congestive heart failure (CHF), but she exhibited no current CV signs or symptoms. The patient had been taking furosemide but was not on any other antihypertensive medications.
Case 3
In the room next to your 90-year-old patient is a 32-year-old uninsured hypertensive white woman. During the history taking, the patient stated that she was trying to become pregnant and was not currently using any form of contraception. Similar to the second patient, she had no complaints to report. Regarding her reason for presentation, the patient stated that when she had her BP checked at a pharmacy earlier that day, the reading was “too high,” and the pharmacist had advised her to go to the ED. She seemed anxious but otherwise well. Her initial BP at presentation was 240/100 mm Hg, but her physical examination was otherwise normal.
Hypertensive Emergencies
As emergency physicians (EPs), we see hypertensive patients every day. According to the US Centers for Disease Control and Prevention, 33% of American adults have hypertension, which is defined by a BP of ≥140/90 mm Hg (Table 1).
Almost 25% of total annual US adult ED visits are directly or indirectly related to hypertension, and about 1% of all ED visits are due solely to elevated BP.3 In an ambulatory care survey for 2007, moderate or severe hypertensive BP readings were found to be more common in patients presenting to the ED (43.5%) than to primary care clinics (27%).4 Patients presenting to the ED with hypertensive BP readings disproportionately represented patients who were older, male, non-Hispanic black, Medicare beneficiaries, or uninsured. Certainly, some patients presenting to the ED have hypertensive BP readings due to pain or anxiety, but multiple studies have suggested that 50% to 70% of ED patients who have hypertensive BP readings will be diagnosed with hypertension on office follow-up.5,6 While a minority of these patients present to the ED with hypertensive emergencies, the majority present either without symptoms of hypertension or with only mild headache. Given the disease burden of hypertension combined with the benefits of treatment, it is worthwhile for the practicing EP to review the most up-to-date guidelines on outpatient management of hypertension.
When a patient presents to the ED with a hypertensive BP reading, the initial priority of the EP is to exclude hypertensive emergency. Hypertensive emergencies are defined by the presence of hypertension (generally grade 3/severe hypertension with BP ≥180/110 mm Hg; see Table 1) in conjunction with evidence of target organ damage.
Target Organ Manifestations
The acuity and/or presence of target organ damage are not always clear on initial ED evaluation. For instance, when a patient who has no history of primary care presents to the ED with severe hypertension, laboratory evaluation may demonstrate protein and blood in his or her urine and an elevated serum creatinine level. In the absence of values from past laboratory studies, it is difficult to determine whether these test results represent normal laboratory parameters for this patient due to longstanding hypertensive kidney disease (ie, hypertensive nephrosclerosis) or if they represent a true hypertensive emergency, (ie, hypertensive emergency-related nephropathy).7 In patients with severe hypertension and possibly new acute kidney injury, it is probably safest to either assume hypertensive emergency-related nephropathy and to treat accordingly or consult with nephrology services. The picture of hypertensive emergency-related nephropathy often only becomes clear after renal biopsy results and improvement in renal parameters with BP control.
The ocular manifestations of hypertensive emergency require detailed fundoscopy, which at times can be challenging in the ED. In assessing for cardiac target organ damage, at our institution, we typically ask patients if they have experienced symptoms of dyspnea and chest pain or pressure. Generally, we also evaluate cardiac enzymes, B-type natriuretic peptide, and order ECG and chest X-ray studies when suggested by history or physical examination. Alarmingly, a study of 161 ED hypertensive (average BP of 183/109 mm Hg), asymptomatic, predominantly black patients found that 146 (90.7%) had subclinical hypertensive heart disease on point-of-care echocardiogram.8
Neurological/Hypertensive Encephalopathy
Hypertensive encephalopathy is a diagnosis of exclusion as alternate causes of confusion and headache, such as intracranial hemorrhage, are excluded and mental status improves with titrated BP control. Nonetheless, it is difficult to confidently state from the literature that patients who present with headache but have a normal mental status in the presence of severe hypertension are not on an early spectrum of hypertensive encephalopathy. Therefore, it is likely that the degree of symptoms should define whether target organ damage exists, though there is certainly a spectrum of hypertensive emergency—the strict definition of which is not always clear.
When a hypertensive emergency is diagnosed, management typically involves the use of antihypertensive IV medication in the intensive care unit. While such management is outside the scope of this paper, Adebayo and Rogers9 have published an excellent review of the care of hypertensive emergencies.
Asymptomatic Hypertension
The American College of Emergency Physicians (ACEP) has developed two clinical policies on the evaluation and management of asymptomatic hypertension in the ED. The original, published in 2006, advised that initially high BP readings of ED patients should be repeated: two separate high readings are adequate for screening, and those patients with hypertension should be referred for follow-up. Furthermore, ACEP policies note that initiating treatment in the ED is not necessary when patients are referred for follow-up. If treatment for hypertension is initiated in the ED, ACEP recommends that such management should attempt only to gradually lower BP, and not to normalize it during the initial ED visit.10
The 2013 update to ACEP’s clinical policy on managing asymptomatic hypertension expanded on the original policy. The updated policy advised against routine testing for target organ damage in patients who have asymptomatic severe hypertension. However, ACEP policy notes that evaluating serum creatinine in these patients with poor follow-up may influence patient disposition.11
The 2013 policy further stated that medical intervention is not required in ED patients who have asymptomatic severe hypertension, but may be considered in patients with poor follow-up. The policies emphasize that all asymptomatic hypertensive patients should be referred for follow-up. The literature cited for the recommendation that ED patients with asymptomatic severe hypertension do not require routine investigation stems from two observational studies. These studies found that screening asymptomatic ED patients who presented with severe hypertension revealed serum creatinine abnormalities in approximately 6%, which impacted patient disposition, though it was not clear from the study results whether admission correlated to meaningful patient outcomes.12,13
Patient Disposition
Since ACEP’s 2013 clinical policy, a study from the Cleveland Clinic has been published. This retrospective cohort study reviewed 6 years of data looking at all patients in its system with a BP of ≥180/110 mm Hg, and compared those office patients discharged to home to those referred to the ED or directly admitted to the inpatient hospital solely on the basis of severe hypertension.14 The study found that 0.5% of 387 patients referred to the ED by primary care clinics for asymptomatic severe hypertension had confirmed acute kidney injury on BMP.14 The Cleveland Clinic study also found that 2.1% of patients had evidence of target organ damage and 5.5% had any abnormal results.14 In addition, referral to the ED from the clinic for hypertension was associated with a slightly higher rate of major adverse CV events at 7 days (2 of 426 [0.5%] versus 61 of 58,109 [0.1%]; P = .02).14
The results of the Cleveland Clinic study confirm that in the absence of target organ damage, hypertension is probably best managed in the outpatient setting. The European Task Force hypertensive guidelines state “hospitalization for hypertension is regarded as inappropriate in most European countries.”15 However, from 2006 to 2012, 26% of US ED patients with primary diagnoses of hypertension were admitted to the hospital.3 In Canada’s most populous province of Ontario, from 2002 to 2011, approximately 8% of hypertensive patients were admitted.16 Part of this discrepancy may be due to the sometimes ambiguous nature of the presentation of patients with hypertension, making it unclear whether a true hypertensive emergency exists. Many patients perceive visual symptoms, headache, dizziness, and even chest pressure as the result of their elevated BP—without clear findings on fundoscopy, ECG, or cardiac marker testing. Perhaps more of these patients would be discharged if EPs felt comfortable initiating appropriate initial antihypertensive treatment.
Management
Initiating Antihypertensive Treatment
Some EPs may feel that an accurate diagnosis of hypertension requires repeat BP testing in the primary care office setting, and for this reason are reluctant to initiate antihypertensive treatment in the ED. The most recent guidelines by the Joint National Committee (JNC 8) do not address how many BP readings are necessary to diagnose hypertension, but JNC 7 suggested that diagnosis of hypertension requires two separate office visits.17 Evidence cited in ACEP’s first clinical policy states that two separate BP measurements in the ED are adequate for screening—but not necessarily for initiating treatment.10 However, European and British outpatient clinical recommendations advocate initiation of antihypertensive medication for a single visit in patients who have an elevated BP categorized as grade 3/severe hypertension (BP of ≥180/110 mm Hg).15,18 Furthermore, for patients with severe hypertension seen in the ED, as many as 97% are likely to have true hypertension at office follow-up.6 Those ED patients presenting with severe hypertension are very likely to have a true diagnosis of hypertension.
A recent retrospective analysis of a group of hypertensive ED patients by Brody et al19 found that patients prescribed BP medications by an EP were more likely to have improved BP control at follow-up 2 weeks later. In their study, the decision to prescribe antihypertensive medications were at the discretion of the EP. Seventy-six patients were given one or more prescriptions for antihypertensive therapy, compared to a control group of 141 patients who were not given a prescription. On follow-up at 2 weeks, there was an 11 mm Hg greater reduction of BP in the group who received prescriptions compared to the control group. None of the patients in either group on follow-up had experienced any new neurological deficits, ischemic events, life-threatening anaphylactic reactions, or clinically significant hypotension.
The Cleveland Clinic study14 also reported on those patents given who received new prescriptions from the ED. Similar to the study by Brody et al,19 none of the 82 patients discharged to home from the ED with a new antihypertensive prescription had any major adverse event at 30-day follow-up.14
Pharmacological Treatment Recommendations
When choosing to treat patients with new prescriptions for antihypertensives, it is important to follow the most current outpatient treatment recommendations. In 2014, JNC 8 released new guidelines for the outpatient management of adults with hypertension.20 The panel issued recommendations based on its systematic review of randomized controlled trials on antihypertensive treatments. The key recommendations are as follows:
- In patients aged 60 years or older, initiate pharmacological treatment at a BP of ≥150/90 mm Hg.
- In patients aged 18 to 59 years, initiate pharmacological treatment at a BP of ≥140/90 mm Hg.
- In the general nonblack population, initial antihypertensive treatment should include a thiazide-type diuretic, a calcium channel blocker (CCB), an angiotensin-converting enzyme inhibitor (ACE-I), or an angiotensin receptor blocker (ARB).
- In the general black population, initial treatment should include a thiazide-type diuretic or a CCB.
- In patients with chronic kidney disease (CKD) (including black patients), initial (or add-on) antihypertensive treatment should include an ACE-I or ARB to improve kidney outcomes—but not both.
- If goal BP is not reached within 1 month of initial treatment, increase the dose of the initial drug or add a second agent (eg, thiazide-type diuretic, CCB, ACE-I, or ARB). If goal is not reached with two drugs, use the third drug from that list if no contraindications exist, but do not use both an ACE-I and an ARB together in the same patient.
Of note, JNC 8, in departure from JNC 7, no longer recommends beta-blockers as first-line therapy for isolated hypertension (there may be compelling alternate indications, such as atrial fibrillation or postmyocardial infarction (MI), such that a beta-blocker would still be the first medication considered). The reason for this stems from a single randomized controlled trial of 9,193 patients that found that despite equivalent BP reduction, use of a beta-blocker in comparison to an ARB resulted in a higher rate of a composite outcome of death, MI, or stroke.21 The main difference was a 25% relative risk reduction for stroke with use of an ARB (losartan) in comparison to a beta-blocker (atenolol). The most recent European guidelines still include beta-blockers among its first-line recommended BP medications, but do acknowledge that they are not as effective in reducing stroke incidence as other alternative medications.15 The European guidelines otherwise include the same list of first-line agents. The British guidelines mirror JNC 8 in terms of first-line antihypertensive medication choices.18
Since the release of JNC 8, the Systolic Blood Pressure Intervention Trial (SPRINT) has been published, and will likely impact future national recommendations on BP management. The SPRINT study was a randomized controlled trial enrolling over 9,000 hypertensive nondiabetic patients older than age 50 years that treated individuals to a standard BP goal (systolic BP of 140 mm Hg) versus an intensive BP goal (systolic BP of 120 mm Hg) over a 3.5-year period. The trial was stopped early for safety as a 25% mortality reduction was observed in the intensive treatment group (1.65 vs 2.19 deaths/y).22 This was in contrast to previous trials that had mostly failed to show this sort of benefit, though previous trials were smaller in number or included only diabetic patients.23 While it is likely that this trial may influence lowering treatment thresholds from the office, it is not likely to impact care from the ED.
The recommendations of JNC 8 do not necessarily coincide with current US EP practice. In the study by Brody et al,19 of patients provided ED antihypertensive prescriptions, 54% received thiazide-type diuretics, 26% ACE-I, 10% CCBs, and 6% beta-blockers. This is noteworthy because 96% of those in the study were black patients who would benefit most from either a thiazide or a CCB. Another recent study of ED patients showed that of patients who were both treated in the ED and discharged with antihypertensive medications, 34% received a diuretic prescription, 32% clonidine, 15% a beta-blocker, 19% an ARB or ACE-I, 12% a CCB, and 2% hydrazine.24 These results are important because according to many published guidelines, including JNC 8, clonidine is only considered one of several fourth-line options for severe resistant hypertension.15,18,20 Since clonidine use can be complicated by rebound hypertension, it is not an ideal agent to be prescribed de novo to patients in the ED. This is particularly true if these patients are not already on maximum doses of the three most recommended agents previously noted, or if there are concerns over patient compliance.
Of the drug classes recommended by JNC 8, Table 3 lists the absolute and relative contraindications.
In clinical trials, amlodipine is among the most effective BP medications and is considered first-line therapy for all groups of patients with hypertension.15,18,20 A simplistic approach for most patients presenting with severe asymptomatic hypertension (BP of ≥180/110 mm Hg) not currently on treatment would be to recheck the BP and assure it remains elevated over the period of the ED visit.
Conclusion
Hypertension is among the most common medical conditions for which emergency patients seek care. The ACEP clinical policies provide guidance on the appropriate work-up and treatment of these patients. Given the occasional lack of clarity on whether a patient’s presentation is on the spectrum of more acute/serious, EPs may feel more comfortable in discharging patients with poor follow-up if they are able to safely prescribe antihypertensive treatment. Prior to prescribing treatment, EPs should refer to the JNC 8 guidelines to appropriately start antihypertensive treatment in select patient groups in the ED. The guidelines of JNC 8 are therefore worth referring to in order to appropriately start treatment in select patient groups from the ED.
Case Scenarios Continued
Case 1
[The 65-year-old black man who presented with headache and dizziness, and had an initial BP of 240/130 mm Hg.]
After treating the patient with prochlorperazine and diphenhydramine, his headache resolved. His BP improved but remained elevated at 190/120 mm Hg. On further questioning, the patient reported a history of similar headaches and wondered whether it was related to his BP. The head CT scan was negative for any acute hemorrhage, infarct, or mass; the ECG only showed evidence of left ventricular hypertrophy; and the BMP showed normal renal function.
After a long discussion with the patient, you agreed to start him on amlodipine 5 mg/d and referred him for follow-up with a local PCP.
Case 2
[The 90-year-old white woman with a history of CHF and an initial BP of 220/140 mm Hg at presentation.]
The BMP evaluation showed a baseline creatinine level of 1.3 mg/dL. Given this patient’s history of CHF, amlodipine would not be the ideal next agent to prescribe. After discussion with her PCP, you elected to start her on losartan at 25 mg/d, and instructed her to follow-up with her PCP within 1 week.
Case 3
[The 32-year-old white woman who presented at the advice of a pharmacist and had an initial BP of 240/100 mm Hg.]While reviewing the patient’s work-up and history, you noted her plans to become pregnant, and recalled a recent review on BP management, noting the contraindications associated with ARB or ACE-I in pregnancy. Based on the patient’s uninsured status and poor follow-up, you considered prescribing amlodipine. Prior to issuing the prescription, you performed a repeat BP check and noted that the patient’s BP had decreased to 130/85 mm Hg. Given the marked improvement in the patient’s BP during her ED course, you were not convinced that she truly had hypertension.
Instead of prescribing an antihypertensive agent, which may not ultimately benefit this patient, you advised her to seek follow-up care at an outpatient clinic to have her BP rechecked. The patient agreed, and you referred her to a local free clinic.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
2. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2013;362(9395):1527-1535.
3. McNaughton CD, Self WH, Zhu Y, Janke AT, Storrow AB, Levy P. Incidence of hypertension-related emergency department visits in the United States, 2006 to 2012. Am J Cardiol. 2015;116(11):1717-1723. doi: 10.1016/j.amjcard.2015.09.007.
4. Niska RW. Blood pressure measurements at emergency department visits by adults: United States, 2007-2008. NCHS Data Brief. 2011;(72):1-8.
5. Chernow SM, Iserson KV, Criss E. Use of the emergency department for hypertension screening: a prospective study. Ann Emerg Med. 1987;16(2):180-182.
6. Backer HD, Decker L, Ackerson L. Reproducibility of increased blood pressure during an emergency department or urgent care visit. Ann Emerg Med. 2003.41(4):507-512.
7. Nonaka K, Ubara Y, Sumida K, et al. Clinical and pathological evaluation of hypertensive emergency-related nephropathy. Intern Med. 2013;52(1):45-53.
8. Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467-474.e1. doi: 10.1016/j.annemergmed.2012.03.030.
9. Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539-551. doi: 10.1016/j.emc.2015.04.005.
10. Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-234. doi: 10.1016/j.annemergmed.2005.10.003
11. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68. doi: 10.1016/j.annemergmed.2013.05.012.
12. Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med. 2006;47(3):230-236.
13. Nishijima DK, Paladino L, Sinert R. Routine testing in patients with asymptomatic elevated blood pressure in the ED. Am J Emerg Med. 2010;28(2):235-242. doi: 10.1016/j.ajem.2008.11.015.
14. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509.
15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151.
16. Masood S, Austin PC, Atzema CL. A population-based analysis of outcomes in patients with a primary diagnosis of hypertension in the emergency department. Ann Emerg Med. 2016;68(3):258-267.e5. doi: 10.1016/j.annemergmed.2016.04.060.
17. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. Erratum in: JAMA. 2003;290(2):197.
18. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891.
19. Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632-635. doi: 10.1111/acem.12660.
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014;311(17):1809.
21. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
22. PRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939.
23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286.
24. Levy PD, Mahn JJ, Miller J, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33(9):1219-1224. doi: 10.1016/j.ajem.2015.05.036.
25. Leung AA, Wright A, Pazo V, Karson A, Bates DW. Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med. 2011;124(11):1064-1072. doi: 10.1016/j.amjmed.2011.06.031.
26. Mann JFE, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. http://www.uptodate.com/contents/renin-angiotensin-system-inhibition-in-the-treatment-of-hypertension. Accessed September 21, 2016.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
2. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2013;362(9395):1527-1535.
3. McNaughton CD, Self WH, Zhu Y, Janke AT, Storrow AB, Levy P. Incidence of hypertension-related emergency department visits in the United States, 2006 to 2012. Am J Cardiol. 2015;116(11):1717-1723. doi: 10.1016/j.amjcard.2015.09.007.
4. Niska RW. Blood pressure measurements at emergency department visits by adults: United States, 2007-2008. NCHS Data Brief. 2011;(72):1-8.
5. Chernow SM, Iserson KV, Criss E. Use of the emergency department for hypertension screening: a prospective study. Ann Emerg Med. 1987;16(2):180-182.
6. Backer HD, Decker L, Ackerson L. Reproducibility of increased blood pressure during an emergency department or urgent care visit. Ann Emerg Med. 2003.41(4):507-512.
7. Nonaka K, Ubara Y, Sumida K, et al. Clinical and pathological evaluation of hypertensive emergency-related nephropathy. Intern Med. 2013;52(1):45-53.
8. Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467-474.e1. doi: 10.1016/j.annemergmed.2012.03.030.
9. Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539-551. doi: 10.1016/j.emc.2015.04.005.
10. Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-234. doi: 10.1016/j.annemergmed.2005.10.003
11. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68. doi: 10.1016/j.annemergmed.2013.05.012.
12. Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med. 2006;47(3):230-236.
13. Nishijima DK, Paladino L, Sinert R. Routine testing in patients with asymptomatic elevated blood pressure in the ED. Am J Emerg Med. 2010;28(2):235-242. doi: 10.1016/j.ajem.2008.11.015.
14. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509.
15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151.
16. Masood S, Austin PC, Atzema CL. A population-based analysis of outcomes in patients with a primary diagnosis of hypertension in the emergency department. Ann Emerg Med. 2016;68(3):258-267.e5. doi: 10.1016/j.annemergmed.2016.04.060.
17. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. Erratum in: JAMA. 2003;290(2):197.
18. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891.
19. Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632-635. doi: 10.1111/acem.12660.
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014;311(17):1809.
21. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
22. PRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939.
23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286.
24. Levy PD, Mahn JJ, Miller J, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33(9):1219-1224. doi: 10.1016/j.ajem.2015.05.036.
25. Leung AA, Wright A, Pazo V, Karson A, Bates DW. Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med. 2011;124(11):1064-1072. doi: 10.1016/j.amjmed.2011.06.031.
26. Mann JFE, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. http://www.uptodate.com/contents/renin-angiotensin-system-inhibition-in-the-treatment-of-hypertension. Accessed September 21, 2016.
First EDition: Zika—Not the Only Mosquito-Borne Virus to Worry About
BY DOUG BRUNK
FRONTLINE MEDICAL NEWS
As the spread of the Zika virus continues to garner attention in the national spotlight, two other mosquito-borne viral infections pose a potential threat to the United States: dengue fever and chikungunya.
At the annual meeting of the Pacific Dermatologic Association, Iris Z. Ahronowitz, MD, shared tips on how to spot and diagnose patients with these viral infections.
“You really need to use all the data at your disposal, including a thorough symptom history, a thorough exposure history, and of course, our most important tool in all of this: our eyes,” said Dr Ahronowitz, a dermatologist at the University of Southern California, Los Angeles. Reaching a diagnosis involves asking about epidemiologic exposure, symptoms, morphology, and performing confirmatory testing by polymerase chain reaction (PCR) and/or enzyme-linked immunosorbent assay (ELISA). “Unfortunately we are not getting these results very quickly,” she said. “Sometimes the turnaround time can be 3 weeks or longer.”
She discussed the case of a 32-year-old woman who had returned from travel to Central Mexico. Two days later, the patient developed fever, fatigue, and retro-orbital headache, as well as flushing macular erythema over the chest. Three days later, she developed a generalized morbilliform eruption. Her white blood cell count was 1.5 x 109/L, platelet count was 37 x 109/L, aspartate aminotransferase (AST) was 124 U/L, and alanine aminotransferase (ALT) was 87 U/L.
The differential diagnosis for morbilliform eruption plus fever in a returning traveler is extensive, Dr Ahronowitz said. It includes measles, chikungunya, West Nile virus, O’nyong-nyong virus, Mayaro virus, Sindbis virus, Ross river disease, Ebola/Marburg, dengue, and Zika. Bacterial/rickettsial possibilities include typhoid fever, typhus, and leptospirosis.
The patient was ultimately diagnosed with dengue virus, a mosquito-borne flavivirus. Five serotypes have been identified, the most recent in 2013. According to Dr Ahronowitz, dengue ranks as the most common febrile illness in travelers returning from the Caribbean, South America, and Southeast Asia. “There are up to 100 million cases every year, 40% of the world population is at risk, and an estimated 80% of people are asymptomatic carriers, which is facilitating the spread of this disease,” she said. The most common vector is Aedes aegypti, a daytime biting mosquito that is endemic to the tropics and subtropics. But a new vector is emerging, Aedes albopictus, which is common in temperate areas. “Both types of mosquitoes are in the United States, and they’re spreading rapidly,” she said. “This is probably due to a combination of climate change and international travel.”
Dengue classically presents with sudden onset of fever, headache, retro-orbital pain, and severe myalgia; 50% to 82% of cases develop a distinctive rash. “While most viruses have nonspecific lab abnormalities, one that can be very helpful to you with suspected dengue is thrombocytopenia,” she said. “The incubation period ranges from 3 to 14 days.”
Rashes associated with dengue are classically biphasic and sequential. The initial rash occurs within 24 to 48 hours of symptom onset and is often mistaken for sunburn, with a flushing erythema of the face, neck, and chest. Three to 5 days later, a subsequent rash develops that starts out as a generalized morbilliform eruption but becomes confluent with petechiae and islands of sparing. “It’s been described as ‘white islands in a sea of red,’” Dr Ahronowitz said.
A more severe form of the disease, dengue hemorrhagic fever, is characterized by extensive purpura and bleeding from mucosa, gastrointestinal tract, and injection sites. “The patients who get this have prior immunity to a different serotype,” she said. “This is thought to be due to a phenomenon called antibody-dependent enhancement, whereby the presence of preexisting antibodies facilitates entry of the virus and produces a more robust inflammatory response. Most of these patients, even the ones with severe dengue, recover fully. The most common long-term sequela we’re seeing is chronic fatigue.”
The diagnosis is made with viral PCR from serum less than 7 days from onset of symptoms, or immunoglobulin M (IgM)ELISA more than 4 days from onset of symptoms. The treatment is supportive care with fluid resuscitation and analgesia; there is no specific treatment. “Do not give nonsteroidal anti-inflammatory drugs (NSAIDs) which can potentiate hemorrhage; give acetaminophen for pain and fevers,” she advised. “A tetravalent vaccine is now available for dengue. Prevention is so important because there is no treatment.”
Next, Dr Ahronowitz discussed the case of a 38-year-old man who returned from travel to Bangladesh. Two days after returning he developed fever to 104˚F, headache, and cervical lymphadenopathy. Three days after returning, he developed severe pain in the wrist, knees, and ankles, and a rash. “This rash was not specific; it was a morbilliform eruption primarily on the chest,” she said.
The patient was ultimately diagnosed with chikungunya, a single-strand RNA mosquito-borne virus with the same vectors as dengue. “This has been wreaking havoc across the Caribbean in the past few years,” Dr Ahronowitz said. “Chikungunya was first identified in the Americas in 2013, and there have been hundreds of thousands of cases in the Caribbean.” The first case acquired in the United States occurred in Florida in the summer of 2014. As of January 2016 there were 679 imported cases of the infection in the United States. “Fortunately, this most recent epidemic is slowing down a bit, but it’s important to be aware of,” she said.
Clinical presentation of chikungunya includes an incubation period of 3 to 7 days, acute onset of high fevers, chills, and myalgia. Nonspecific exanthem around 3 days occurs in 40% to 75% of cases, and symmetric polyarthralgias are common in the fingers, wrists, and ankles. Labs may reveal lymphopenia, acute kidney injury, and elevated AST and ALT levels. Acute symptoms resolve within 7 to 10 days.
Besides the rash, other cutaneous signs of the disease include aphthous-like ulcers and anogenital ulcers, particularly around the scrotum. Other patients may present with facial hyperpigmentation, also known as “brownie nose,” that appears with the rash. In babies, bullous lesions can occur. More than 20% of patients who acquire chikungunya still have severe joint pain 1 year after initial presentation. “This can be really debilitating,” she said. “A subset of patients will develop an inflammatory seronegative rheumatoid-like arthritis. It’s generally not a fatal condition except in the extremes of age and in people with a lot of comorbidities. Most people recover fully.”
As in dengue, clinicians can diagnose chikungunya by viral culture in the first 3 days of illness, and by reverse transcription PCR in the first 8 days of illness. On serology, IgM is positive by 5 days of symptom onset.
“If testing is not available locally, contact the Centers for Disease Control and Prevention,” Dr Ahronowitz said. “Treatment is supportive. Evaluate for and treat potential coinfections, including dengue, malaria, and bacterial infections. If dengue is in the differential diagnosis, avoid NSAIDs.” A new vaccine for chikungunya is currently in phase II trials.
BY DOUG BRUNK
FRONTLINE MEDICAL NEWS
As the spread of the Zika virus continues to garner attention in the national spotlight, two other mosquito-borne viral infections pose a potential threat to the United States: dengue fever and chikungunya.
At the annual meeting of the Pacific Dermatologic Association, Iris Z. Ahronowitz, MD, shared tips on how to spot and diagnose patients with these viral infections.
“You really need to use all the data at your disposal, including a thorough symptom history, a thorough exposure history, and of course, our most important tool in all of this: our eyes,” said Dr Ahronowitz, a dermatologist at the University of Southern California, Los Angeles. Reaching a diagnosis involves asking about epidemiologic exposure, symptoms, morphology, and performing confirmatory testing by polymerase chain reaction (PCR) and/or enzyme-linked immunosorbent assay (ELISA). “Unfortunately we are not getting these results very quickly,” she said. “Sometimes the turnaround time can be 3 weeks or longer.”
She discussed the case of a 32-year-old woman who had returned from travel to Central Mexico. Two days later, the patient developed fever, fatigue, and retro-orbital headache, as well as flushing macular erythema over the chest. Three days later, she developed a generalized morbilliform eruption. Her white blood cell count was 1.5 x 109/L, platelet count was 37 x 109/L, aspartate aminotransferase (AST) was 124 U/L, and alanine aminotransferase (ALT) was 87 U/L.
The differential diagnosis for morbilliform eruption plus fever in a returning traveler is extensive, Dr Ahronowitz said. It includes measles, chikungunya, West Nile virus, O’nyong-nyong virus, Mayaro virus, Sindbis virus, Ross river disease, Ebola/Marburg, dengue, and Zika. Bacterial/rickettsial possibilities include typhoid fever, typhus, and leptospirosis.
The patient was ultimately diagnosed with dengue virus, a mosquito-borne flavivirus. Five serotypes have been identified, the most recent in 2013. According to Dr Ahronowitz, dengue ranks as the most common febrile illness in travelers returning from the Caribbean, South America, and Southeast Asia. “There are up to 100 million cases every year, 40% of the world population is at risk, and an estimated 80% of people are asymptomatic carriers, which is facilitating the spread of this disease,” she said. The most common vector is Aedes aegypti, a daytime biting mosquito that is endemic to the tropics and subtropics. But a new vector is emerging, Aedes albopictus, which is common in temperate areas. “Both types of mosquitoes are in the United States, and they’re spreading rapidly,” she said. “This is probably due to a combination of climate change and international travel.”
Dengue classically presents with sudden onset of fever, headache, retro-orbital pain, and severe myalgia; 50% to 82% of cases develop a distinctive rash. “While most viruses have nonspecific lab abnormalities, one that can be very helpful to you with suspected dengue is thrombocytopenia,” she said. “The incubation period ranges from 3 to 14 days.”
Rashes associated with dengue are classically biphasic and sequential. The initial rash occurs within 24 to 48 hours of symptom onset and is often mistaken for sunburn, with a flushing erythema of the face, neck, and chest. Three to 5 days later, a subsequent rash develops that starts out as a generalized morbilliform eruption but becomes confluent with petechiae and islands of sparing. “It’s been described as ‘white islands in a sea of red,’” Dr Ahronowitz said.
A more severe form of the disease, dengue hemorrhagic fever, is characterized by extensive purpura and bleeding from mucosa, gastrointestinal tract, and injection sites. “The patients who get this have prior immunity to a different serotype,” she said. “This is thought to be due to a phenomenon called antibody-dependent enhancement, whereby the presence of preexisting antibodies facilitates entry of the virus and produces a more robust inflammatory response. Most of these patients, even the ones with severe dengue, recover fully. The most common long-term sequela we’re seeing is chronic fatigue.”
The diagnosis is made with viral PCR from serum less than 7 days from onset of symptoms, or immunoglobulin M (IgM)ELISA more than 4 days from onset of symptoms. The treatment is supportive care with fluid resuscitation and analgesia; there is no specific treatment. “Do not give nonsteroidal anti-inflammatory drugs (NSAIDs) which can potentiate hemorrhage; give acetaminophen for pain and fevers,” she advised. “A tetravalent vaccine is now available for dengue. Prevention is so important because there is no treatment.”
Next, Dr Ahronowitz discussed the case of a 38-year-old man who returned from travel to Bangladesh. Two days after returning he developed fever to 104˚F, headache, and cervical lymphadenopathy. Three days after returning, he developed severe pain in the wrist, knees, and ankles, and a rash. “This rash was not specific; it was a morbilliform eruption primarily on the chest,” she said.
The patient was ultimately diagnosed with chikungunya, a single-strand RNA mosquito-borne virus with the same vectors as dengue. “This has been wreaking havoc across the Caribbean in the past few years,” Dr Ahronowitz said. “Chikungunya was first identified in the Americas in 2013, and there have been hundreds of thousands of cases in the Caribbean.” The first case acquired in the United States occurred in Florida in the summer of 2014. As of January 2016 there were 679 imported cases of the infection in the United States. “Fortunately, this most recent epidemic is slowing down a bit, but it’s important to be aware of,” she said.
Clinical presentation of chikungunya includes an incubation period of 3 to 7 days, acute onset of high fevers, chills, and myalgia. Nonspecific exanthem around 3 days occurs in 40% to 75% of cases, and symmetric polyarthralgias are common in the fingers, wrists, and ankles. Labs may reveal lymphopenia, acute kidney injury, and elevated AST and ALT levels. Acute symptoms resolve within 7 to 10 days.
Besides the rash, other cutaneous signs of the disease include aphthous-like ulcers and anogenital ulcers, particularly around the scrotum. Other patients may present with facial hyperpigmentation, also known as “brownie nose,” that appears with the rash. In babies, bullous lesions can occur. More than 20% of patients who acquire chikungunya still have severe joint pain 1 year after initial presentation. “This can be really debilitating,” she said. “A subset of patients will develop an inflammatory seronegative rheumatoid-like arthritis. It’s generally not a fatal condition except in the extremes of age and in people with a lot of comorbidities. Most people recover fully.”
As in dengue, clinicians can diagnose chikungunya by viral culture in the first 3 days of illness, and by reverse transcription PCR in the first 8 days of illness. On serology, IgM is positive by 5 days of symptom onset.
“If testing is not available locally, contact the Centers for Disease Control and Prevention,” Dr Ahronowitz said. “Treatment is supportive. Evaluate for and treat potential coinfections, including dengue, malaria, and bacterial infections. If dengue is in the differential diagnosis, avoid NSAIDs.” A new vaccine for chikungunya is currently in phase II trials.
BY DOUG BRUNK
FRONTLINE MEDICAL NEWS
As the spread of the Zika virus continues to garner attention in the national spotlight, two other mosquito-borne viral infections pose a potential threat to the United States: dengue fever and chikungunya.
At the annual meeting of the Pacific Dermatologic Association, Iris Z. Ahronowitz, MD, shared tips on how to spot and diagnose patients with these viral infections.
“You really need to use all the data at your disposal, including a thorough symptom history, a thorough exposure history, and of course, our most important tool in all of this: our eyes,” said Dr Ahronowitz, a dermatologist at the University of Southern California, Los Angeles. Reaching a diagnosis involves asking about epidemiologic exposure, symptoms, morphology, and performing confirmatory testing by polymerase chain reaction (PCR) and/or enzyme-linked immunosorbent assay (ELISA). “Unfortunately we are not getting these results very quickly,” she said. “Sometimes the turnaround time can be 3 weeks or longer.”
She discussed the case of a 32-year-old woman who had returned from travel to Central Mexico. Two days later, the patient developed fever, fatigue, and retro-orbital headache, as well as flushing macular erythema over the chest. Three days later, she developed a generalized morbilliform eruption. Her white blood cell count was 1.5 x 109/L, platelet count was 37 x 109/L, aspartate aminotransferase (AST) was 124 U/L, and alanine aminotransferase (ALT) was 87 U/L.
The differential diagnosis for morbilliform eruption plus fever in a returning traveler is extensive, Dr Ahronowitz said. It includes measles, chikungunya, West Nile virus, O’nyong-nyong virus, Mayaro virus, Sindbis virus, Ross river disease, Ebola/Marburg, dengue, and Zika. Bacterial/rickettsial possibilities include typhoid fever, typhus, and leptospirosis.
The patient was ultimately diagnosed with dengue virus, a mosquito-borne flavivirus. Five serotypes have been identified, the most recent in 2013. According to Dr Ahronowitz, dengue ranks as the most common febrile illness in travelers returning from the Caribbean, South America, and Southeast Asia. “There are up to 100 million cases every year, 40% of the world population is at risk, and an estimated 80% of people are asymptomatic carriers, which is facilitating the spread of this disease,” she said. The most common vector is Aedes aegypti, a daytime biting mosquito that is endemic to the tropics and subtropics. But a new vector is emerging, Aedes albopictus, which is common in temperate areas. “Both types of mosquitoes are in the United States, and they’re spreading rapidly,” she said. “This is probably due to a combination of climate change and international travel.”
Dengue classically presents with sudden onset of fever, headache, retro-orbital pain, and severe myalgia; 50% to 82% of cases develop a distinctive rash. “While most viruses have nonspecific lab abnormalities, one that can be very helpful to you with suspected dengue is thrombocytopenia,” she said. “The incubation period ranges from 3 to 14 days.”
Rashes associated with dengue are classically biphasic and sequential. The initial rash occurs within 24 to 48 hours of symptom onset and is often mistaken for sunburn, with a flushing erythema of the face, neck, and chest. Three to 5 days later, a subsequent rash develops that starts out as a generalized morbilliform eruption but becomes confluent with petechiae and islands of sparing. “It’s been described as ‘white islands in a sea of red,’” Dr Ahronowitz said.
A more severe form of the disease, dengue hemorrhagic fever, is characterized by extensive purpura and bleeding from mucosa, gastrointestinal tract, and injection sites. “The patients who get this have prior immunity to a different serotype,” she said. “This is thought to be due to a phenomenon called antibody-dependent enhancement, whereby the presence of preexisting antibodies facilitates entry of the virus and produces a more robust inflammatory response. Most of these patients, even the ones with severe dengue, recover fully. The most common long-term sequela we’re seeing is chronic fatigue.”
The diagnosis is made with viral PCR from serum less than 7 days from onset of symptoms, or immunoglobulin M (IgM)ELISA more than 4 days from onset of symptoms. The treatment is supportive care with fluid resuscitation and analgesia; there is no specific treatment. “Do not give nonsteroidal anti-inflammatory drugs (NSAIDs) which can potentiate hemorrhage; give acetaminophen for pain and fevers,” she advised. “A tetravalent vaccine is now available for dengue. Prevention is so important because there is no treatment.”
Next, Dr Ahronowitz discussed the case of a 38-year-old man who returned from travel to Bangladesh. Two days after returning he developed fever to 104˚F, headache, and cervical lymphadenopathy. Three days after returning, he developed severe pain in the wrist, knees, and ankles, and a rash. “This rash was not specific; it was a morbilliform eruption primarily on the chest,” she said.
The patient was ultimately diagnosed with chikungunya, a single-strand RNA mosquito-borne virus with the same vectors as dengue. “This has been wreaking havoc across the Caribbean in the past few years,” Dr Ahronowitz said. “Chikungunya was first identified in the Americas in 2013, and there have been hundreds of thousands of cases in the Caribbean.” The first case acquired in the United States occurred in Florida in the summer of 2014. As of January 2016 there were 679 imported cases of the infection in the United States. “Fortunately, this most recent epidemic is slowing down a bit, but it’s important to be aware of,” she said.
Clinical presentation of chikungunya includes an incubation period of 3 to 7 days, acute onset of high fevers, chills, and myalgia. Nonspecific exanthem around 3 days occurs in 40% to 75% of cases, and symmetric polyarthralgias are common in the fingers, wrists, and ankles. Labs may reveal lymphopenia, acute kidney injury, and elevated AST and ALT levels. Acute symptoms resolve within 7 to 10 days.
Besides the rash, other cutaneous signs of the disease include aphthous-like ulcers and anogenital ulcers, particularly around the scrotum. Other patients may present with facial hyperpigmentation, also known as “brownie nose,” that appears with the rash. In babies, bullous lesions can occur. More than 20% of patients who acquire chikungunya still have severe joint pain 1 year after initial presentation. “This can be really debilitating,” she said. “A subset of patients will develop an inflammatory seronegative rheumatoid-like arthritis. It’s generally not a fatal condition except in the extremes of age and in people with a lot of comorbidities. Most people recover fully.”
As in dengue, clinicians can diagnose chikungunya by viral culture in the first 3 days of illness, and by reverse transcription PCR in the first 8 days of illness. On serology, IgM is positive by 5 days of symptom onset.
“If testing is not available locally, contact the Centers for Disease Control and Prevention,” Dr Ahronowitz said. “Treatment is supportive. Evaluate for and treat potential coinfections, including dengue, malaria, and bacterial infections. If dengue is in the differential diagnosis, avoid NSAIDs.” A new vaccine for chikungunya is currently in phase II trials.
Modern Indications, Results, and Global Trends in the Use of Unicompartmental Knee Arthroplasty and High Tibial Osteotomy in the Treatment of Isolated Medial Compartment Osteoarthritis
An increasingly number of patients with symptomatic isolated medial unicompartmental knee osteoarthritis (OA) are too young and too functionally active to be ideal candidates for total knee arthroplasty (TKA). Isolated medial compartment OA occurs in 10% to 29.5% of all cases, whereas the isolated lateral variant is less common, with a reported incidence of 1% to 7%.1,2 In 1961, Jackson and Waugh3 introduced the high tibial osteotomy (HTO) as a surgical treatment for single-compartment OA. This procedure is designed to increase the life span of articular cartilage by unloading and redistributing the mechanical forces over the nonaffected compartment. Unicompartmental knee arthroplasty (UKA) was introduced in the 1970s as an alternative to TKA or HTO for single-compartment OA.
Since the introduction of these methods, there has been debate about which patients are appropriate candidates for each procedure. Improved surgical techniques and implant designs have led surgeons to reexamine the selection criteria and contraindications for these procedures. Furthermore, given the increasing popularity and use of UKA, the question arises as to whether HTO still has a role in clinical practice in the surgical treatment of medial OA of the knee.
To clarify current ambiguities, we review the modern indications, subjective outcome scores, and survivorship results of UKA and HTO in the treatment of isolated medial compartment degeneration of the knee. In addition, in a thorough review of the literature, we evaluate global trends in the use of both methods.
High Tibial Osteotomy for Medial Compartment OA
Indications
Before the introduction of TKA and UKA for single-compartment OA, surgical management consisted of HTO. When the mechanical axis is slightly overcorrected, the medial compartment is decompressed, ensuring tissue viability and delaying progressive compartment degeneration.
Traditionally, HTO is indicated for young (age <60 years), normal-weight, active patients with radiographic single-compartment OA.6 The knee should be stable and have good range of motion (ROM; flexion >120°), and pain should be localized to the tibiofemoral joint line.
Over the past few decades, numerous authors have reported similar inclusion criteria, clarifying their definition. This definition should be further refined in order to optimize survivorship and clinical outcomes.
Confirming age as an inclusion criterion for HTO, Trieb and colleagues7 found that the risk of failure was significantly (P = .046) higher for HTO patients older than 65 years than for those younger than 65 years (relative risk, 1.5). This finding agrees with findings of other studies, which suggests that, in particular, young patients benefit from HTO.8-11
Moreover, there is a clear relation between HTO survival and obesity. In a study of 159 CWHTOs, Akizuki and colleagues12 reported that preoperative body mass index (BMI) higher than 27.5 kg/m2 was a significant risk factor for early failure. Using BMI higher than 30 kg/m2 as a threshold, Howells and colleagues9 found significantly inferior Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) results for the obese group 5 years after HTO.
Radiographic evidence of severe preoperative compartment degeneration has been associated with early conversion to TKA. Flecher and colleagues11 and van Raaij and colleagues13 both concluded the best long-term survival grades are achieved in HTO patients with mild compartment OA (Ahlbäck14 grade I). The question then becomes whether these patients should be treated nonoperatively instead.15,16The literature supports strict adherence to inclusion criteria in the selection of a potential HTO candidate. Age, BMI, and the preoperative state of OA should be taken into account in order to optimize clinical outcome and survivorship results in patients about to undergo HTO.
Outcomes
Multiple authors have described or compared the midterm or long-term results of the various surgical HTO techniques. Howells and colleagues9 noted overall survival rates of 87% (5 years after CWHTO) and 79% (10 years after CWHTO). Over the 10-year postoperative period, there was significant deterioration in clinical outcome scores and survivorship. Others authors have had similar findings.17-19 van Raaij and colleagues13 found that the 10-year probability of survival after CWHTO was 75%. In 455 patients who underwent lateral CWHTO, Hui and colleagues8 found that 5-year probability of survival was 95%, 10-year probability was 79%, and 15-year probability was 56%. Niinimäki and colleagues10 used the Finnish Arthroplasty Register to report HTO survivorship at a national level. Using conversion to TKA as a cutoff, they noted 5-year survivorship of 89% and 10-year survivorship of 73%. To our knowledge, 2 groups, both in Japan, have reported substantially higher 15-year survival rates: 90%12 and 93%.20 The authors acknowledged that their results were significantly better than in other countries and that Japanese lifestyle, culture, and body habitus therefore require further investigation. At this time, it is not possible to compare their results with Western results.
In an attempt to compare the different survival rates of the various HTO techniques, Schallberger and colleagues21 conducted a retrospective study of OWHTOs and CWHTOs. At median follow-up of 16.5 years, comparative survival rates showed a trend of deterioration. Although data were limited, there were no significant differences in survival or functional outcome between the 2 techniques. In a recent randomized clinical trial, Duivenvoorden and colleagues5 compared these techniques’ midterm results (mean follow-up, 6 years). Clinical outcomes were not significantly different. There were more complications in the OWHTO group and more conversions to TKA in the CWHTO group. Considering these results, the authors suggested OWHTO without autologous bone graft is the best HTO treatment strategy for medial gonarthritis with varus malalignment of <12°.
The HTO results noted in these studies show a similar deteriorating trend; expected 10-year survivorship is 75%. Although modern implants and surgical techniques are being used, evidence supporting use of one surgical HTO method over another is lacking.
UKA for Medial Compartment OA
Indications
Since it was first introduced in the 1970s, use of UKA for single-compartment OA has been a subject of debate. The high failure rates reported at the time raised skepticism about the new treatment.22 Kozinn and Scott23 defined classic indications and contraindications. Indications included isolated medial or lateral compartment OA or osteonecrosis of the knee, age over 60 years, and weight under 82 kg. In addition, the angular deformity of the affected lower extremity had to be <15° and passively correctable to neutral at time of surgery. Last, the flexion contracture had to be <5°, and ideal ROM was 90°. Contraindications included high activity, age under 60 years, and inflammatory arthritis. Strict adherence led to improved implant survival and lower revision rates. Because of improved surgical techniques, modern implant designs, and accumulating experience with the procedure, the surgical indications for UKA have expanded. Exact thresholds for UKA inclusion, however, remain unclear.
The modern literature is overturning the traditional idea that UKA is not indicated for patients under age 60 years.23 Using KSS, Thompson and colleagues24 found that younger patients did better than older patients 2 years after UKA using various types of implants. Analyzing survivorship results, Heyse and colleagues25 concluded that UKA can be successful in patients under age 60 years and reported a 15-year survivorship rate of 85.6% and excellent outcome scores. Other authors have had similar findings.26-28
Evaluating the influence of weight, Thompson and colleagues24 found obese patients did not have a higher revision rate but did have slower progression of improvement 2 years after UKA. Cavaignac and colleagues29 concluded that, at minimum follow-up of 7 years (range, 7-22 years), weight did not influence UKA survivorship. Other authors30-33 have found no significant influence of BMI on survival.
Reports on preoperative radiographic parameters that can potentially influence UKA results are limited. In 113 medial UKAs studied by Niinimäki and colleagues,34 mild medial compartment degeneration, seen on preoperative radiographs, was associated with significantly higher failure rates. The authors concluded that other treatment options should be favored in the absence of severe isolated compartment OA.
Although the classic indications defined by Kozinn and Scott23 have yielded good to excellent UKA results, improvements in implants and surgical techniques35-38 have extended the criteria. The modern literature demonstrates that age and BMI should not be used as criteria for excluding UKA candidates. Radiographically, there should be significant isolated compartment degeneration in order to optimize patient-reported outcome and survivorship.
Outcomes
Improved implant designs and modern minimally invasive techniques have effected a change in outcome results and a renewed interest in implants. Over the past decade, multiple authors have described the various modern UKA implants and their survivorship. Reports published since UKA was introduced in the 1970s show a continual increase in implant survival. Koskinen and colleagues,39 using Finnish Arthroplasty Register data on 1819 UKAs performed between 1985 and 2003, found 10-year survival rates of 81% for Oxford implants (Zimmer Biomet), 79% for Miller-Galante II (Zimmer Biomet), 78% for Duracon (Howmedica), and 53% for PCA unicompartmental knee (Howmedica). Heyse and colleagues25 reported 10- and 15-year survivorship data (93.5% and 86.3%, respectively) for 223 patients under age 60 years at the time of their index surgery (Genesis Unicondylar implant, Smith & Nephew), performed between 1993 and 2005. KSS was good to excellent. Similar numbers in cohorts under age 60 years were reported by Schai and colleagues26 using the PFC system (Johnson & Johnson) and by Price and colleagues27 using the medial Oxford UKA. Both groups reported excellent survivorship rates: 93% at 2- to 6-year follow-up and 91% at 10-year follow-up. The outcome in older patients seems satisfactory as well. In another multicenter report, by Price and colleagues,40 medial Oxford UKAs had a 15-year survival rate of 93%. Berger and colleagues41 reported similar numbers for the Miller-Galante prosthesis. Survival rates were 98% (10 years) and 95.7% (13 years), and 92% of patients had good to excellent Hospital for Special Surgery knee scores.
Although various modern implants have had good to excellent results, the historical question of what type of UKA to use (mobile or fixed-bearing) remains unanswered. To try to address it, Peersman and colleagues42 performed a systematic review of 44 papers (9463 knees). The 2 implant types had comparable revision rates. Another recent retrospective study tried to determine what is crucial for implant survival: implant design or surgeon experience.43 The authors concluded that prosthetic component positioning is key. Other authors have reported high-volume centers are crucial for satisfactory UKA results and lower revision rates.44-46
Results of these studies indicate that, where UKAs are being performed in volume, 10-year survivorship rates higher than 90% and good to excellent outcomes can be expected.
UKA vs HTO
Cohort studies that have directly compared the 2 treatment modalities are scarce, and most have been retrospective. In a prospective study, Stukenborg-Colsman and colleagues47 randomized patients with medial compartment OA to undergo either CWHTO (32 patients) with a technique reported by Coventry48 or UKA (28 patients) with the unicondylar knee sliding prosthesis, Tübingen pattern (Aesculap), between 1988 and 1991. Patients were assessed 2.5, 4.5, and 7.5 years after surgery. More postoperative complications were noted in the HTO group. At 7- to 10-year follow-up, 71% of the HTO group and 65% of the UKA group had excellent KSS. Mean ROM was 103° after UKA (range, 35°-140°) and 117° after HTO (range, 85°-135°) during the same assessment. Although differences were not significant, Kaplan-Meier survival analysis was 60% for HTO and 77% for UKA at 10 years. Results were not promising for the implants used, compared with other implants, but the authors concluded that, because of improvements in implant designs and image-guided techniques, better long-term success can be expected with UKA than with HTO.
In another prospective study, Börjesson and colleagues49 evaluated pain during walking, ROM, British Orthopaedic Association (BOA) scores, and gait variables at 1- and 5-year follow-up. Patients with moderate medial OA (Ahlbäck14 grade I-III) were randomly selected to undergo CWHTO or UKA (Brigham, DePuy). There were no significant differences in BOA scores, ROM, or pain during walking between the 2 groups at 3 months, 1 year, and 5 years after surgery. Gait analysis showed a significant difference in favor of UKA only at 3 months after surgery. At 1- and 5-year follow-up, no significant differences were noted.
To clarify current ambiguities, Fu and colleagues50 performed a systematic review of all (11) comparative studies. These studies had a total of 5840 (5081 UKA, 759 HTO) patients. Although ROM was significantly better for the HTO group than the UKA group, the UKA group had significantly better functional results. Walking after surgery was significantly faster for the UKA group. The authors suggested the difference might be attributed to the different postoperative regimens—HTO patients wore a whole-leg plaster cast for 6 weeks, and UKA patients were allowed immediate postoperative weight-bearing. Regarding rates of survival and complications, pooled data showed no significant differences. Despite these results, the authors acknowledged the limitation of available randomized clinical trials and the multiple techniques and implants used. We share their assertion that larger prospective controlled trials are needed. These are crucial to getting a definitive answer regarding which of the 2 treatment strategies should be used for isolated compartment OA.
Current Trends in Use of UKA and HTO
Evaluation of national registries and recent reports showed a global shift in use of both HTO and UKA. Despite the lack of national HTO registries, a few reports have described use of TKA, UKA, and HTO in Western populations over the past 2 decades. Using 1998-2007 data from the Swedish Knee Arthroplasty Register, W-Dahl and colleagues51 found a 3-fold increase in UKA use, whereas HTO use was halved over the same period. Niinimäki and colleagues52 reported similar findings with the Finnish National Hospital Discharge Register. They noted a steady 6.8% annual decrease in osteotomies, whereas UKA use increased sharply after the Oxford UKA was introduced (Phase 3; Biomet). These findings are consistent with several reports from North America. In their epidemiologic analysis covering the period 1985-1990, Wright and colleagues53 found an 11% to 14% annual decrease in osteotomies among the elderly, compared with an annual decrease of only 3% to 4% among patients younger than 65 years. Nwachukwu and colleagues54 recently compared UKA and HTO practice patterns between 2007 and 2011, using data from a large US private payer insurance database. They noted an annual growth rate of 4.7% in UKA use, compared with an annual 3.9% decrease in HTO use. Furthermore, based on their subgroup analysis, they speculated there was a demographic shift toward UKA, as opposed to TKA, particularly in older women. Bolognesi and colleagues55 investigated further. Evaluating all Medicare beneficiaries who underwent knee arthroplasty in the United States between 2000 and 2009, they noted a 1.7-fold increase in TKA use and a 6.2-fold increase in UKA use. As there were no substantial changes in patient characteristics over that period, the authors hypothesized that a possible broadening of inclusion criteria may have led to the increased use of UKA.
There is a possible multifactorial explanation for the current global shift in favor of UKA. First, UKA was once a technically demanding procedure, but improved surgical techniques, image guidance, and robot assistance56 have made it relatively less difficult. Second, UKA surgery is associated with lower reported perioperative morbidities.57 We think these factors have contributed to the global trend of less HTO use and more UKA use in the treatment of unicompartmental OA.
Conclusion
The modern literature suggests the inclusion criteria for HTO have been well investigated and defined; the UKA criteria remain a matter of debate but seem to be expanding. Long-term survival results seem to favor UKA, though patient satisfaction with both procedures is good to excellent. The broadening range of inclusion criteria and consistent reports of durable outcomes, coupled with excellent patient satisfaction, likely explain the shift toward UKA in the treatment of isolated compartment degeneration.
Am J Orthop. 2016;45(6):E355-E361. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7): 520-526.
2. Wise BL, Niu J, Yang M, et al; Multicenter Osteoarthritis (MOST) Group. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 2012;64(6): 847-852.
3. Jackson JP, Waugh W. Tibial osteotomy for osteoarthritis of the knee. J Bone Joint Surg Br. 1961;43:746-751.
4. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88(11):1454-1459.
5. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425-1432.
6. Hutchison CR, Cho B, Wong N, Agnidis Z, Gross AE. Proximal valgus tibial osteotomy for osteoarthritis of the knee. Instr Course Lect. 1999;48:131-134.
7. Trieb K, Grohs J, Hanslik-Schnabel B, Stulnig T, Panotopoulos J, Wanivenhaus A. Age predicts outcome of high-tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):149-152.
8. Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64-70.
9. Howells NR, Salmon L, Waller A, Scanelli J, Pinczewski LA. The outcome at ten years of lateral closing-wedge high tibial osteotomy: determinants of survival and functional outcome. Bone Joint J Br. 2014;96(11):1491-1497.
10. Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J. Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: Finnish registry-based study of 3195 knees. J Bone Joint Surg Br. 2012;94(11):1517-1521.
11. Flecher X, Parratte S, Aubaniac JM, Argenson JN. A 12-28-year followup study of closing wedge high tibial osteotomy. Clin Orthop Relat Res. 2006;(452):91-96.
12. Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90(5):592-596.
13. van Raaij T, Reijman M, Brouwer RW, Jakma TS, Verhaar JN. Survival of closing-wedge high tibial osteotomy: good outcome in men with low-grade osteoarthritis after 10-16 years. Acta Orthop. 2008;79:230-234.
14. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
15. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-1711.
16. Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;(390):173-181.
17. Naudie D, Bourne RB, Rorabeck CH, Bourne TJ. The Install Award. Survivorship of the high tibial valgus osteotomy. A 10- to -22-year followup study. Clin Orthop Relat Res. 1999;(367):18-27.
18. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Br. 2003;85(3):469-474.
19. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
20. Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11(6):439-444.
21. Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):122-127.
22. Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62(8):1329-1337.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
24. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
25. Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19(5):585-591.
26. Schai PA, Suh JT, Thornhill TS, Scott RD. Unicompartmental knee arthroplasty in middle-aged patients: a 2- to 6-year follow-up evaluation. J Arthroplasty. 1998;13(4):365-372.
27. Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87(11):1488-1492.
28. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2003;85(10):1968-1973.
29. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J Br. 2013;95(8):1064-1068.
30. Tabor OB Jr, Tabor OB, Bernard M, Wan JY. Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv. 2005;14(2):59-63.
31. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(5 suppl):19-23.
32. Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25(5):369-373.
33. Plate JF, Augart MA, Seyler TM, et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty [published online April 12, 2015]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3597-5.
34. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
35. Carlsson LV, Albrektsson BE, Regnér LR. Minimally invasive surgery vs conventional exposure using the Miller-Galante unicompartmental knee arthroplasty: a randomized radiostereometric study. J Arthroplasty. 2006;21(2):151-156.
36. Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282-286.
37. Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54-60.
38. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
39. Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78(1):128-135.
40. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
41. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
42. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3296-3305.
43. Zambianchi F, Digennaro V, Giorgini A, et al. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2074-2080.
44. Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45-49.
45. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-525.
46. Robertsson O, Lidgren L. The short-term results of 3 common UKA implants during different periods in Sweden. J Arthroplasty. 2008;23(6):801-807.
47. Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7-10-year follow-up prospective randomised study. Knee. 2001;8(3):187-194.
48. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55(1):23-48.
49. Börjesson M, Weidenhielm L, Mattsson E, Olsson E. Gait and clinical measurements in patients with knee osteoarthritis after surgery: a prospective 5-year follow-up study. Knee. 2005;12(2):121-127.
50. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013;28(5):759-765.
51. W-Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2010;81(2):161-164.
52. Niinimäki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399-1402.
53. Wright J, Heck D, Hawker G, et al. Rates of tibial osteotomies in Canada and the United States. Clin Orthop Relat Res. 1995;(319):266-275.
54. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
55. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
56. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
57. Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8 suppl):86-90.
An increasingly number of patients with symptomatic isolated medial unicompartmental knee osteoarthritis (OA) are too young and too functionally active to be ideal candidates for total knee arthroplasty (TKA). Isolated medial compartment OA occurs in 10% to 29.5% of all cases, whereas the isolated lateral variant is less common, with a reported incidence of 1% to 7%.1,2 In 1961, Jackson and Waugh3 introduced the high tibial osteotomy (HTO) as a surgical treatment for single-compartment OA. This procedure is designed to increase the life span of articular cartilage by unloading and redistributing the mechanical forces over the nonaffected compartment. Unicompartmental knee arthroplasty (UKA) was introduced in the 1970s as an alternative to TKA or HTO for single-compartment OA.
Since the introduction of these methods, there has been debate about which patients are appropriate candidates for each procedure. Improved surgical techniques and implant designs have led surgeons to reexamine the selection criteria and contraindications for these procedures. Furthermore, given the increasing popularity and use of UKA, the question arises as to whether HTO still has a role in clinical practice in the surgical treatment of medial OA of the knee.
To clarify current ambiguities, we review the modern indications, subjective outcome scores, and survivorship results of UKA and HTO in the treatment of isolated medial compartment degeneration of the knee. In addition, in a thorough review of the literature, we evaluate global trends in the use of both methods.
High Tibial Osteotomy for Medial Compartment OA
Indications
Before the introduction of TKA and UKA for single-compartment OA, surgical management consisted of HTO. When the mechanical axis is slightly overcorrected, the medial compartment is decompressed, ensuring tissue viability and delaying progressive compartment degeneration.
Traditionally, HTO is indicated for young (age <60 years), normal-weight, active patients with radiographic single-compartment OA.6 The knee should be stable and have good range of motion (ROM; flexion >120°), and pain should be localized to the tibiofemoral joint line.
Over the past few decades, numerous authors have reported similar inclusion criteria, clarifying their definition. This definition should be further refined in order to optimize survivorship and clinical outcomes.
Confirming age as an inclusion criterion for HTO, Trieb and colleagues7 found that the risk of failure was significantly (P = .046) higher for HTO patients older than 65 years than for those younger than 65 years (relative risk, 1.5). This finding agrees with findings of other studies, which suggests that, in particular, young patients benefit from HTO.8-11
Moreover, there is a clear relation between HTO survival and obesity. In a study of 159 CWHTOs, Akizuki and colleagues12 reported that preoperative body mass index (BMI) higher than 27.5 kg/m2 was a significant risk factor for early failure. Using BMI higher than 30 kg/m2 as a threshold, Howells and colleagues9 found significantly inferior Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) results for the obese group 5 years after HTO.
Radiographic evidence of severe preoperative compartment degeneration has been associated with early conversion to TKA. Flecher and colleagues11 and van Raaij and colleagues13 both concluded the best long-term survival grades are achieved in HTO patients with mild compartment OA (Ahlbäck14 grade I). The question then becomes whether these patients should be treated nonoperatively instead.15,16The literature supports strict adherence to inclusion criteria in the selection of a potential HTO candidate. Age, BMI, and the preoperative state of OA should be taken into account in order to optimize clinical outcome and survivorship results in patients about to undergo HTO.
Outcomes
Multiple authors have described or compared the midterm or long-term results of the various surgical HTO techniques. Howells and colleagues9 noted overall survival rates of 87% (5 years after CWHTO) and 79% (10 years after CWHTO). Over the 10-year postoperative period, there was significant deterioration in clinical outcome scores and survivorship. Others authors have had similar findings.17-19 van Raaij and colleagues13 found that the 10-year probability of survival after CWHTO was 75%. In 455 patients who underwent lateral CWHTO, Hui and colleagues8 found that 5-year probability of survival was 95%, 10-year probability was 79%, and 15-year probability was 56%. Niinimäki and colleagues10 used the Finnish Arthroplasty Register to report HTO survivorship at a national level. Using conversion to TKA as a cutoff, they noted 5-year survivorship of 89% and 10-year survivorship of 73%. To our knowledge, 2 groups, both in Japan, have reported substantially higher 15-year survival rates: 90%12 and 93%.20 The authors acknowledged that their results were significantly better than in other countries and that Japanese lifestyle, culture, and body habitus therefore require further investigation. At this time, it is not possible to compare their results with Western results.
In an attempt to compare the different survival rates of the various HTO techniques, Schallberger and colleagues21 conducted a retrospective study of OWHTOs and CWHTOs. At median follow-up of 16.5 years, comparative survival rates showed a trend of deterioration. Although data were limited, there were no significant differences in survival or functional outcome between the 2 techniques. In a recent randomized clinical trial, Duivenvoorden and colleagues5 compared these techniques’ midterm results (mean follow-up, 6 years). Clinical outcomes were not significantly different. There were more complications in the OWHTO group and more conversions to TKA in the CWHTO group. Considering these results, the authors suggested OWHTO without autologous bone graft is the best HTO treatment strategy for medial gonarthritis with varus malalignment of <12°.
The HTO results noted in these studies show a similar deteriorating trend; expected 10-year survivorship is 75%. Although modern implants and surgical techniques are being used, evidence supporting use of one surgical HTO method over another is lacking.
UKA for Medial Compartment OA
Indications
Since it was first introduced in the 1970s, use of UKA for single-compartment OA has been a subject of debate. The high failure rates reported at the time raised skepticism about the new treatment.22 Kozinn and Scott23 defined classic indications and contraindications. Indications included isolated medial or lateral compartment OA or osteonecrosis of the knee, age over 60 years, and weight under 82 kg. In addition, the angular deformity of the affected lower extremity had to be <15° and passively correctable to neutral at time of surgery. Last, the flexion contracture had to be <5°, and ideal ROM was 90°. Contraindications included high activity, age under 60 years, and inflammatory arthritis. Strict adherence led to improved implant survival and lower revision rates. Because of improved surgical techniques, modern implant designs, and accumulating experience with the procedure, the surgical indications for UKA have expanded. Exact thresholds for UKA inclusion, however, remain unclear.
The modern literature is overturning the traditional idea that UKA is not indicated for patients under age 60 years.23 Using KSS, Thompson and colleagues24 found that younger patients did better than older patients 2 years after UKA using various types of implants. Analyzing survivorship results, Heyse and colleagues25 concluded that UKA can be successful in patients under age 60 years and reported a 15-year survivorship rate of 85.6% and excellent outcome scores. Other authors have had similar findings.26-28
Evaluating the influence of weight, Thompson and colleagues24 found obese patients did not have a higher revision rate but did have slower progression of improvement 2 years after UKA. Cavaignac and colleagues29 concluded that, at minimum follow-up of 7 years (range, 7-22 years), weight did not influence UKA survivorship. Other authors30-33 have found no significant influence of BMI on survival.
Reports on preoperative radiographic parameters that can potentially influence UKA results are limited. In 113 medial UKAs studied by Niinimäki and colleagues,34 mild medial compartment degeneration, seen on preoperative radiographs, was associated with significantly higher failure rates. The authors concluded that other treatment options should be favored in the absence of severe isolated compartment OA.
Although the classic indications defined by Kozinn and Scott23 have yielded good to excellent UKA results, improvements in implants and surgical techniques35-38 have extended the criteria. The modern literature demonstrates that age and BMI should not be used as criteria for excluding UKA candidates. Radiographically, there should be significant isolated compartment degeneration in order to optimize patient-reported outcome and survivorship.
Outcomes
Improved implant designs and modern minimally invasive techniques have effected a change in outcome results and a renewed interest in implants. Over the past decade, multiple authors have described the various modern UKA implants and their survivorship. Reports published since UKA was introduced in the 1970s show a continual increase in implant survival. Koskinen and colleagues,39 using Finnish Arthroplasty Register data on 1819 UKAs performed between 1985 and 2003, found 10-year survival rates of 81% for Oxford implants (Zimmer Biomet), 79% for Miller-Galante II (Zimmer Biomet), 78% for Duracon (Howmedica), and 53% for PCA unicompartmental knee (Howmedica). Heyse and colleagues25 reported 10- and 15-year survivorship data (93.5% and 86.3%, respectively) for 223 patients under age 60 years at the time of their index surgery (Genesis Unicondylar implant, Smith & Nephew), performed between 1993 and 2005. KSS was good to excellent. Similar numbers in cohorts under age 60 years were reported by Schai and colleagues26 using the PFC system (Johnson & Johnson) and by Price and colleagues27 using the medial Oxford UKA. Both groups reported excellent survivorship rates: 93% at 2- to 6-year follow-up and 91% at 10-year follow-up. The outcome in older patients seems satisfactory as well. In another multicenter report, by Price and colleagues,40 medial Oxford UKAs had a 15-year survival rate of 93%. Berger and colleagues41 reported similar numbers for the Miller-Galante prosthesis. Survival rates were 98% (10 years) and 95.7% (13 years), and 92% of patients had good to excellent Hospital for Special Surgery knee scores.
Although various modern implants have had good to excellent results, the historical question of what type of UKA to use (mobile or fixed-bearing) remains unanswered. To try to address it, Peersman and colleagues42 performed a systematic review of 44 papers (9463 knees). The 2 implant types had comparable revision rates. Another recent retrospective study tried to determine what is crucial for implant survival: implant design or surgeon experience.43 The authors concluded that prosthetic component positioning is key. Other authors have reported high-volume centers are crucial for satisfactory UKA results and lower revision rates.44-46
Results of these studies indicate that, where UKAs are being performed in volume, 10-year survivorship rates higher than 90% and good to excellent outcomes can be expected.
UKA vs HTO
Cohort studies that have directly compared the 2 treatment modalities are scarce, and most have been retrospective. In a prospective study, Stukenborg-Colsman and colleagues47 randomized patients with medial compartment OA to undergo either CWHTO (32 patients) with a technique reported by Coventry48 or UKA (28 patients) with the unicondylar knee sliding prosthesis, Tübingen pattern (Aesculap), between 1988 and 1991. Patients were assessed 2.5, 4.5, and 7.5 years after surgery. More postoperative complications were noted in the HTO group. At 7- to 10-year follow-up, 71% of the HTO group and 65% of the UKA group had excellent KSS. Mean ROM was 103° after UKA (range, 35°-140°) and 117° after HTO (range, 85°-135°) during the same assessment. Although differences were not significant, Kaplan-Meier survival analysis was 60% for HTO and 77% for UKA at 10 years. Results were not promising for the implants used, compared with other implants, but the authors concluded that, because of improvements in implant designs and image-guided techniques, better long-term success can be expected with UKA than with HTO.
In another prospective study, Börjesson and colleagues49 evaluated pain during walking, ROM, British Orthopaedic Association (BOA) scores, and gait variables at 1- and 5-year follow-up. Patients with moderate medial OA (Ahlbäck14 grade I-III) were randomly selected to undergo CWHTO or UKA (Brigham, DePuy). There were no significant differences in BOA scores, ROM, or pain during walking between the 2 groups at 3 months, 1 year, and 5 years after surgery. Gait analysis showed a significant difference in favor of UKA only at 3 months after surgery. At 1- and 5-year follow-up, no significant differences were noted.
To clarify current ambiguities, Fu and colleagues50 performed a systematic review of all (11) comparative studies. These studies had a total of 5840 (5081 UKA, 759 HTO) patients. Although ROM was significantly better for the HTO group than the UKA group, the UKA group had significantly better functional results. Walking after surgery was significantly faster for the UKA group. The authors suggested the difference might be attributed to the different postoperative regimens—HTO patients wore a whole-leg plaster cast for 6 weeks, and UKA patients were allowed immediate postoperative weight-bearing. Regarding rates of survival and complications, pooled data showed no significant differences. Despite these results, the authors acknowledged the limitation of available randomized clinical trials and the multiple techniques and implants used. We share their assertion that larger prospective controlled trials are needed. These are crucial to getting a definitive answer regarding which of the 2 treatment strategies should be used for isolated compartment OA.
Current Trends in Use of UKA and HTO
Evaluation of national registries and recent reports showed a global shift in use of both HTO and UKA. Despite the lack of national HTO registries, a few reports have described use of TKA, UKA, and HTO in Western populations over the past 2 decades. Using 1998-2007 data from the Swedish Knee Arthroplasty Register, W-Dahl and colleagues51 found a 3-fold increase in UKA use, whereas HTO use was halved over the same period. Niinimäki and colleagues52 reported similar findings with the Finnish National Hospital Discharge Register. They noted a steady 6.8% annual decrease in osteotomies, whereas UKA use increased sharply after the Oxford UKA was introduced (Phase 3; Biomet). These findings are consistent with several reports from North America. In their epidemiologic analysis covering the period 1985-1990, Wright and colleagues53 found an 11% to 14% annual decrease in osteotomies among the elderly, compared with an annual decrease of only 3% to 4% among patients younger than 65 years. Nwachukwu and colleagues54 recently compared UKA and HTO practice patterns between 2007 and 2011, using data from a large US private payer insurance database. They noted an annual growth rate of 4.7% in UKA use, compared with an annual 3.9% decrease in HTO use. Furthermore, based on their subgroup analysis, they speculated there was a demographic shift toward UKA, as opposed to TKA, particularly in older women. Bolognesi and colleagues55 investigated further. Evaluating all Medicare beneficiaries who underwent knee arthroplasty in the United States between 2000 and 2009, they noted a 1.7-fold increase in TKA use and a 6.2-fold increase in UKA use. As there were no substantial changes in patient characteristics over that period, the authors hypothesized that a possible broadening of inclusion criteria may have led to the increased use of UKA.
There is a possible multifactorial explanation for the current global shift in favor of UKA. First, UKA was once a technically demanding procedure, but improved surgical techniques, image guidance, and robot assistance56 have made it relatively less difficult. Second, UKA surgery is associated with lower reported perioperative morbidities.57 We think these factors have contributed to the global trend of less HTO use and more UKA use in the treatment of unicompartmental OA.
Conclusion
The modern literature suggests the inclusion criteria for HTO have been well investigated and defined; the UKA criteria remain a matter of debate but seem to be expanding. Long-term survival results seem to favor UKA, though patient satisfaction with both procedures is good to excellent. The broadening range of inclusion criteria and consistent reports of durable outcomes, coupled with excellent patient satisfaction, likely explain the shift toward UKA in the treatment of isolated compartment degeneration.
Am J Orthop. 2016;45(6):E355-E361. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
An increasingly number of patients with symptomatic isolated medial unicompartmental knee osteoarthritis (OA) are too young and too functionally active to be ideal candidates for total knee arthroplasty (TKA). Isolated medial compartment OA occurs in 10% to 29.5% of all cases, whereas the isolated lateral variant is less common, with a reported incidence of 1% to 7%.1,2 In 1961, Jackson and Waugh3 introduced the high tibial osteotomy (HTO) as a surgical treatment for single-compartment OA. This procedure is designed to increase the life span of articular cartilage by unloading and redistributing the mechanical forces over the nonaffected compartment. Unicompartmental knee arthroplasty (UKA) was introduced in the 1970s as an alternative to TKA or HTO for single-compartment OA.
Since the introduction of these methods, there has been debate about which patients are appropriate candidates for each procedure. Improved surgical techniques and implant designs have led surgeons to reexamine the selection criteria and contraindications for these procedures. Furthermore, given the increasing popularity and use of UKA, the question arises as to whether HTO still has a role in clinical practice in the surgical treatment of medial OA of the knee.
To clarify current ambiguities, we review the modern indications, subjective outcome scores, and survivorship results of UKA and HTO in the treatment of isolated medial compartment degeneration of the knee. In addition, in a thorough review of the literature, we evaluate global trends in the use of both methods.
High Tibial Osteotomy for Medial Compartment OA
Indications
Before the introduction of TKA and UKA for single-compartment OA, surgical management consisted of HTO. When the mechanical axis is slightly overcorrected, the medial compartment is decompressed, ensuring tissue viability and delaying progressive compartment degeneration.
Traditionally, HTO is indicated for young (age <60 years), normal-weight, active patients with radiographic single-compartment OA.6 The knee should be stable and have good range of motion (ROM; flexion >120°), and pain should be localized to the tibiofemoral joint line.
Over the past few decades, numerous authors have reported similar inclusion criteria, clarifying their definition. This definition should be further refined in order to optimize survivorship and clinical outcomes.
Confirming age as an inclusion criterion for HTO, Trieb and colleagues7 found that the risk of failure was significantly (P = .046) higher for HTO patients older than 65 years than for those younger than 65 years (relative risk, 1.5). This finding agrees with findings of other studies, which suggests that, in particular, young patients benefit from HTO.8-11
Moreover, there is a clear relation between HTO survival and obesity. In a study of 159 CWHTOs, Akizuki and colleagues12 reported that preoperative body mass index (BMI) higher than 27.5 kg/m2 was a significant risk factor for early failure. Using BMI higher than 30 kg/m2 as a threshold, Howells and colleagues9 found significantly inferior Knee Society Score (KSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) results for the obese group 5 years after HTO.
Radiographic evidence of severe preoperative compartment degeneration has been associated with early conversion to TKA. Flecher and colleagues11 and van Raaij and colleagues13 both concluded the best long-term survival grades are achieved in HTO patients with mild compartment OA (Ahlbäck14 grade I). The question then becomes whether these patients should be treated nonoperatively instead.15,16The literature supports strict adherence to inclusion criteria in the selection of a potential HTO candidate. Age, BMI, and the preoperative state of OA should be taken into account in order to optimize clinical outcome and survivorship results in patients about to undergo HTO.
Outcomes
Multiple authors have described or compared the midterm or long-term results of the various surgical HTO techniques. Howells and colleagues9 noted overall survival rates of 87% (5 years after CWHTO) and 79% (10 years after CWHTO). Over the 10-year postoperative period, there was significant deterioration in clinical outcome scores and survivorship. Others authors have had similar findings.17-19 van Raaij and colleagues13 found that the 10-year probability of survival after CWHTO was 75%. In 455 patients who underwent lateral CWHTO, Hui and colleagues8 found that 5-year probability of survival was 95%, 10-year probability was 79%, and 15-year probability was 56%. Niinimäki and colleagues10 used the Finnish Arthroplasty Register to report HTO survivorship at a national level. Using conversion to TKA as a cutoff, they noted 5-year survivorship of 89% and 10-year survivorship of 73%. To our knowledge, 2 groups, both in Japan, have reported substantially higher 15-year survival rates: 90%12 and 93%.20 The authors acknowledged that their results were significantly better than in other countries and that Japanese lifestyle, culture, and body habitus therefore require further investigation. At this time, it is not possible to compare their results with Western results.
In an attempt to compare the different survival rates of the various HTO techniques, Schallberger and colleagues21 conducted a retrospective study of OWHTOs and CWHTOs. At median follow-up of 16.5 years, comparative survival rates showed a trend of deterioration. Although data were limited, there were no significant differences in survival or functional outcome between the 2 techniques. In a recent randomized clinical trial, Duivenvoorden and colleagues5 compared these techniques’ midterm results (mean follow-up, 6 years). Clinical outcomes were not significantly different. There were more complications in the OWHTO group and more conversions to TKA in the CWHTO group. Considering these results, the authors suggested OWHTO without autologous bone graft is the best HTO treatment strategy for medial gonarthritis with varus malalignment of <12°.
The HTO results noted in these studies show a similar deteriorating trend; expected 10-year survivorship is 75%. Although modern implants and surgical techniques are being used, evidence supporting use of one surgical HTO method over another is lacking.
UKA for Medial Compartment OA
Indications
Since it was first introduced in the 1970s, use of UKA for single-compartment OA has been a subject of debate. The high failure rates reported at the time raised skepticism about the new treatment.22 Kozinn and Scott23 defined classic indications and contraindications. Indications included isolated medial or lateral compartment OA or osteonecrosis of the knee, age over 60 years, and weight under 82 kg. In addition, the angular deformity of the affected lower extremity had to be <15° and passively correctable to neutral at time of surgery. Last, the flexion contracture had to be <5°, and ideal ROM was 90°. Contraindications included high activity, age under 60 years, and inflammatory arthritis. Strict adherence led to improved implant survival and lower revision rates. Because of improved surgical techniques, modern implant designs, and accumulating experience with the procedure, the surgical indications for UKA have expanded. Exact thresholds for UKA inclusion, however, remain unclear.
The modern literature is overturning the traditional idea that UKA is not indicated for patients under age 60 years.23 Using KSS, Thompson and colleagues24 found that younger patients did better than older patients 2 years after UKA using various types of implants. Analyzing survivorship results, Heyse and colleagues25 concluded that UKA can be successful in patients under age 60 years and reported a 15-year survivorship rate of 85.6% and excellent outcome scores. Other authors have had similar findings.26-28
Evaluating the influence of weight, Thompson and colleagues24 found obese patients did not have a higher revision rate but did have slower progression of improvement 2 years after UKA. Cavaignac and colleagues29 concluded that, at minimum follow-up of 7 years (range, 7-22 years), weight did not influence UKA survivorship. Other authors30-33 have found no significant influence of BMI on survival.
Reports on preoperative radiographic parameters that can potentially influence UKA results are limited. In 113 medial UKAs studied by Niinimäki and colleagues,34 mild medial compartment degeneration, seen on preoperative radiographs, was associated with significantly higher failure rates. The authors concluded that other treatment options should be favored in the absence of severe isolated compartment OA.
Although the classic indications defined by Kozinn and Scott23 have yielded good to excellent UKA results, improvements in implants and surgical techniques35-38 have extended the criteria. The modern literature demonstrates that age and BMI should not be used as criteria for excluding UKA candidates. Radiographically, there should be significant isolated compartment degeneration in order to optimize patient-reported outcome and survivorship.
Outcomes
Improved implant designs and modern minimally invasive techniques have effected a change in outcome results and a renewed interest in implants. Over the past decade, multiple authors have described the various modern UKA implants and their survivorship. Reports published since UKA was introduced in the 1970s show a continual increase in implant survival. Koskinen and colleagues,39 using Finnish Arthroplasty Register data on 1819 UKAs performed between 1985 and 2003, found 10-year survival rates of 81% for Oxford implants (Zimmer Biomet), 79% for Miller-Galante II (Zimmer Biomet), 78% for Duracon (Howmedica), and 53% for PCA unicompartmental knee (Howmedica). Heyse and colleagues25 reported 10- and 15-year survivorship data (93.5% and 86.3%, respectively) for 223 patients under age 60 years at the time of their index surgery (Genesis Unicondylar implant, Smith & Nephew), performed between 1993 and 2005. KSS was good to excellent. Similar numbers in cohorts under age 60 years were reported by Schai and colleagues26 using the PFC system (Johnson & Johnson) and by Price and colleagues27 using the medial Oxford UKA. Both groups reported excellent survivorship rates: 93% at 2- to 6-year follow-up and 91% at 10-year follow-up. The outcome in older patients seems satisfactory as well. In another multicenter report, by Price and colleagues,40 medial Oxford UKAs had a 15-year survival rate of 93%. Berger and colleagues41 reported similar numbers for the Miller-Galante prosthesis. Survival rates were 98% (10 years) and 95.7% (13 years), and 92% of patients had good to excellent Hospital for Special Surgery knee scores.
Although various modern implants have had good to excellent results, the historical question of what type of UKA to use (mobile or fixed-bearing) remains unanswered. To try to address it, Peersman and colleagues42 performed a systematic review of 44 papers (9463 knees). The 2 implant types had comparable revision rates. Another recent retrospective study tried to determine what is crucial for implant survival: implant design or surgeon experience.43 The authors concluded that prosthetic component positioning is key. Other authors have reported high-volume centers are crucial for satisfactory UKA results and lower revision rates.44-46
Results of these studies indicate that, where UKAs are being performed in volume, 10-year survivorship rates higher than 90% and good to excellent outcomes can be expected.
UKA vs HTO
Cohort studies that have directly compared the 2 treatment modalities are scarce, and most have been retrospective. In a prospective study, Stukenborg-Colsman and colleagues47 randomized patients with medial compartment OA to undergo either CWHTO (32 patients) with a technique reported by Coventry48 or UKA (28 patients) with the unicondylar knee sliding prosthesis, Tübingen pattern (Aesculap), between 1988 and 1991. Patients were assessed 2.5, 4.5, and 7.5 years after surgery. More postoperative complications were noted in the HTO group. At 7- to 10-year follow-up, 71% of the HTO group and 65% of the UKA group had excellent KSS. Mean ROM was 103° after UKA (range, 35°-140°) and 117° after HTO (range, 85°-135°) during the same assessment. Although differences were not significant, Kaplan-Meier survival analysis was 60% for HTO and 77% for UKA at 10 years. Results were not promising for the implants used, compared with other implants, but the authors concluded that, because of improvements in implant designs and image-guided techniques, better long-term success can be expected with UKA than with HTO.
In another prospective study, Börjesson and colleagues49 evaluated pain during walking, ROM, British Orthopaedic Association (BOA) scores, and gait variables at 1- and 5-year follow-up. Patients with moderate medial OA (Ahlbäck14 grade I-III) were randomly selected to undergo CWHTO or UKA (Brigham, DePuy). There were no significant differences in BOA scores, ROM, or pain during walking between the 2 groups at 3 months, 1 year, and 5 years after surgery. Gait analysis showed a significant difference in favor of UKA only at 3 months after surgery. At 1- and 5-year follow-up, no significant differences were noted.
To clarify current ambiguities, Fu and colleagues50 performed a systematic review of all (11) comparative studies. These studies had a total of 5840 (5081 UKA, 759 HTO) patients. Although ROM was significantly better for the HTO group than the UKA group, the UKA group had significantly better functional results. Walking after surgery was significantly faster for the UKA group. The authors suggested the difference might be attributed to the different postoperative regimens—HTO patients wore a whole-leg plaster cast for 6 weeks, and UKA patients were allowed immediate postoperative weight-bearing. Regarding rates of survival and complications, pooled data showed no significant differences. Despite these results, the authors acknowledged the limitation of available randomized clinical trials and the multiple techniques and implants used. We share their assertion that larger prospective controlled trials are needed. These are crucial to getting a definitive answer regarding which of the 2 treatment strategies should be used for isolated compartment OA.
Current Trends in Use of UKA and HTO
Evaluation of national registries and recent reports showed a global shift in use of both HTO and UKA. Despite the lack of national HTO registries, a few reports have described use of TKA, UKA, and HTO in Western populations over the past 2 decades. Using 1998-2007 data from the Swedish Knee Arthroplasty Register, W-Dahl and colleagues51 found a 3-fold increase in UKA use, whereas HTO use was halved over the same period. Niinimäki and colleagues52 reported similar findings with the Finnish National Hospital Discharge Register. They noted a steady 6.8% annual decrease in osteotomies, whereas UKA use increased sharply after the Oxford UKA was introduced (Phase 3; Biomet). These findings are consistent with several reports from North America. In their epidemiologic analysis covering the period 1985-1990, Wright and colleagues53 found an 11% to 14% annual decrease in osteotomies among the elderly, compared with an annual decrease of only 3% to 4% among patients younger than 65 years. Nwachukwu and colleagues54 recently compared UKA and HTO practice patterns between 2007 and 2011, using data from a large US private payer insurance database. They noted an annual growth rate of 4.7% in UKA use, compared with an annual 3.9% decrease in HTO use. Furthermore, based on their subgroup analysis, they speculated there was a demographic shift toward UKA, as opposed to TKA, particularly in older women. Bolognesi and colleagues55 investigated further. Evaluating all Medicare beneficiaries who underwent knee arthroplasty in the United States between 2000 and 2009, they noted a 1.7-fold increase in TKA use and a 6.2-fold increase in UKA use. As there were no substantial changes in patient characteristics over that period, the authors hypothesized that a possible broadening of inclusion criteria may have led to the increased use of UKA.
There is a possible multifactorial explanation for the current global shift in favor of UKA. First, UKA was once a technically demanding procedure, but improved surgical techniques, image guidance, and robot assistance56 have made it relatively less difficult. Second, UKA surgery is associated with lower reported perioperative morbidities.57 We think these factors have contributed to the global trend of less HTO use and more UKA use in the treatment of unicompartmental OA.
Conclusion
The modern literature suggests the inclusion criteria for HTO have been well investigated and defined; the UKA criteria remain a matter of debate but seem to be expanding. Long-term survival results seem to favor UKA, though patient satisfaction with both procedures is good to excellent. The broadening range of inclusion criteria and consistent reports of durable outcomes, coupled with excellent patient satisfaction, likely explain the shift toward UKA in the treatment of isolated compartment degeneration.
Am J Orthop. 2016;45(6):E355-E361. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7): 520-526.
2. Wise BL, Niu J, Yang M, et al; Multicenter Osteoarthritis (MOST) Group. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 2012;64(6): 847-852.
3. Jackson JP, Waugh W. Tibial osteotomy for osteoarthritis of the knee. J Bone Joint Surg Br. 1961;43:746-751.
4. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88(11):1454-1459.
5. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425-1432.
6. Hutchison CR, Cho B, Wong N, Agnidis Z, Gross AE. Proximal valgus tibial osteotomy for osteoarthritis of the knee. Instr Course Lect. 1999;48:131-134.
7. Trieb K, Grohs J, Hanslik-Schnabel B, Stulnig T, Panotopoulos J, Wanivenhaus A. Age predicts outcome of high-tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):149-152.
8. Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64-70.
9. Howells NR, Salmon L, Waller A, Scanelli J, Pinczewski LA. The outcome at ten years of lateral closing-wedge high tibial osteotomy: determinants of survival and functional outcome. Bone Joint J Br. 2014;96(11):1491-1497.
10. Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J. Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: Finnish registry-based study of 3195 knees. J Bone Joint Surg Br. 2012;94(11):1517-1521.
11. Flecher X, Parratte S, Aubaniac JM, Argenson JN. A 12-28-year followup study of closing wedge high tibial osteotomy. Clin Orthop Relat Res. 2006;(452):91-96.
12. Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90(5):592-596.
13. van Raaij T, Reijman M, Brouwer RW, Jakma TS, Verhaar JN. Survival of closing-wedge high tibial osteotomy: good outcome in men with low-grade osteoarthritis after 10-16 years. Acta Orthop. 2008;79:230-234.
14. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
15. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-1711.
16. Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;(390):173-181.
17. Naudie D, Bourne RB, Rorabeck CH, Bourne TJ. The Install Award. Survivorship of the high tibial valgus osteotomy. A 10- to -22-year followup study. Clin Orthop Relat Res. 1999;(367):18-27.
18. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Br. 2003;85(3):469-474.
19. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
20. Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11(6):439-444.
21. Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):122-127.
22. Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62(8):1329-1337.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
24. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
25. Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19(5):585-591.
26. Schai PA, Suh JT, Thornhill TS, Scott RD. Unicompartmental knee arthroplasty in middle-aged patients: a 2- to 6-year follow-up evaluation. J Arthroplasty. 1998;13(4):365-372.
27. Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87(11):1488-1492.
28. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2003;85(10):1968-1973.
29. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J Br. 2013;95(8):1064-1068.
30. Tabor OB Jr, Tabor OB, Bernard M, Wan JY. Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv. 2005;14(2):59-63.
31. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(5 suppl):19-23.
32. Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25(5):369-373.
33. Plate JF, Augart MA, Seyler TM, et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty [published online April 12, 2015]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3597-5.
34. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
35. Carlsson LV, Albrektsson BE, Regnér LR. Minimally invasive surgery vs conventional exposure using the Miller-Galante unicompartmental knee arthroplasty: a randomized radiostereometric study. J Arthroplasty. 2006;21(2):151-156.
36. Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282-286.
37. Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54-60.
38. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
39. Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78(1):128-135.
40. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
41. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
42. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3296-3305.
43. Zambianchi F, Digennaro V, Giorgini A, et al. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2074-2080.
44. Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45-49.
45. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-525.
46. Robertsson O, Lidgren L. The short-term results of 3 common UKA implants during different periods in Sweden. J Arthroplasty. 2008;23(6):801-807.
47. Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7-10-year follow-up prospective randomised study. Knee. 2001;8(3):187-194.
48. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55(1):23-48.
49. Börjesson M, Weidenhielm L, Mattsson E, Olsson E. Gait and clinical measurements in patients with knee osteoarthritis after surgery: a prospective 5-year follow-up study. Knee. 2005;12(2):121-127.
50. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013;28(5):759-765.
51. W-Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2010;81(2):161-164.
52. Niinimäki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399-1402.
53. Wright J, Heck D, Hawker G, et al. Rates of tibial osteotomies in Canada and the United States. Clin Orthop Relat Res. 1995;(319):266-275.
54. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
55. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
56. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
57. Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8 suppl):86-90.
1. Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52(7): 520-526.
2. Wise BL, Niu J, Yang M, et al; Multicenter Osteoarthritis (MOST) Group. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 2012;64(6): 847-852.
3. Jackson JP, Waugh W. Tibial osteotomy for osteoarthritis of the knee. J Bone Joint Surg Br. 1961;43:746-751.
4. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88(11):1454-1459.
5. Duivenvoorden T, Brouwer RW, Baan A, et al. Comparison of closing-wedge and opening-wedge high tibial osteotomy for medial compartment osteoarthritis of the knee: a randomized controlled trial with a six-year follow-up. J Bone Joint Surg Am. 2014;96(17):1425-1432.
6. Hutchison CR, Cho B, Wong N, Agnidis Z, Gross AE. Proximal valgus tibial osteotomy for osteoarthritis of the knee. Instr Course Lect. 1999;48:131-134.
7. Trieb K, Grohs J, Hanslik-Schnabel B, Stulnig T, Panotopoulos J, Wanivenhaus A. Age predicts outcome of high-tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):149-152.
8. Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64-70.
9. Howells NR, Salmon L, Waller A, Scanelli J, Pinczewski LA. The outcome at ten years of lateral closing-wedge high tibial osteotomy: determinants of survival and functional outcome. Bone Joint J Br. 2014;96(11):1491-1497.
10. Niinimäki TT, Eskelinen A, Mann BS, Junnila M, Ohtonen P, Leppilahti J. Survivorship of high tibial osteotomy in the treatment of osteoarthritis of the knee: Finnish registry-based study of 3195 knees. J Bone Joint Surg Br. 2012;94(11):1517-1521.
11. Flecher X, Parratte S, Aubaniac JM, Argenson JN. A 12-28-year followup study of closing wedge high tibial osteotomy. Clin Orthop Relat Res. 2006;(452):91-96.
12. Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90(5):592-596.
13. van Raaij T, Reijman M, Brouwer RW, Jakma TS, Verhaar JN. Survival of closing-wedge high tibial osteotomy: good outcome in men with low-grade osteoarthritis after 10-16 years. Acta Orthop. 2008;79:230-234.
14. Ahlbäck S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
15. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-1711.
16. Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;(390):173-181.
17. Naudie D, Bourne RB, Rorabeck CH, Bourne TJ. The Install Award. Survivorship of the high tibial valgus osteotomy. A 10- to -22-year followup study. Clin Orthop Relat Res. 1999;(367):18-27.
18. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Br. 2003;85(3):469-474.
19. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
20. Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11(6):439-444.
21. Schallberger A, Jacobi M, Wahl P, Maestretti G, Jakob RP. High tibial valgus osteotomy in unicompartmental medial osteoarthritis of the knee: a retrospective follow-up study over 13-21 years. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):122-127.
22. Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am. 1980;62(8):1329-1337.
23. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
24. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
25. Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19(5):585-591.
26. Schai PA, Suh JT, Thornhill TS, Scott RD. Unicompartmental knee arthroplasty in middle-aged patients: a 2- to 6-year follow-up evaluation. J Arthroplasty. 1998;13(4):365-372.
27. Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg Br. 2005;87(11):1488-1492.
28. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2003;85(10):1968-1973.
29. Cavaignac E, Lafontan V, Reina N, et al. Obesity has no adverse effect on the outcome of unicompartmental knee replacement at a minimum follow-up of seven years. Bone Joint J Br. 2013;95(8):1064-1068.
30. Tabor OB Jr, Tabor OB, Bernard M, Wan JY. Unicompartmental knee arthroplasty: long-term success in middle-age and obese patients. J Surg Orthop Adv. 2005;14(2):59-63.
31. Berend KR, Lombardi AV Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(5 suppl):19-23.
32. Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25(5):369-373.
33. Plate JF, Augart MA, Seyler TM, et al. Obesity has no effect on outcomes following unicompartmental knee arthroplasty [published online April 12, 2015]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3597-5.
34. Niinimäki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
35. Carlsson LV, Albrektsson BE, Regnér LR. Minimally invasive surgery vs conventional exposure using the Miller-Galante unicompartmental knee arthroplasty: a randomized radiostereometric study. J Arthroplasty. 2006;21(2):151-156.
36. Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282-286.
37. Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88(1):54-60.
38. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
39. Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop. 2007;78(1):128-135.
40. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
41. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
42. Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P. Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3296-3305.
43. Zambianchi F, Digennaro V, Giorgini A, et al. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2074-2080.
44. Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83(1):45-49.
45. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg Am. 2007;89(3):519-525.
46. Robertsson O, Lidgren L. The short-term results of 3 common UKA implants during different periods in Sweden. J Arthroplasty. 2008;23(6):801-807.
47. Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7-10-year follow-up prospective randomised study. Knee. 2001;8(3):187-194.
48. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55(1):23-48.
49. Börjesson M, Weidenhielm L, Mattsson E, Olsson E. Gait and clinical measurements in patients with knee osteoarthritis after surgery: a prospective 5-year follow-up study. Knee. 2005;12(2):121-127.
50. Fu D, Li G, Chen K, Zhao Y, Hua Y, Cai Z. Comparison of high tibial osteotomy and unicompartmental knee arthroplasty in the treatment of unicompartmental osteoarthritis: a meta-analysis. J Arthroplasty. 2013;28(5):759-765.
51. W-Dahl A, Robertsson O, Lidgren L. Surgery for knee osteoarthritis in younger patients. Acta Orthop. 2010;81(2):161-164.
52. Niinimäki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399-1402.
53. Wright J, Heck D, Hawker G, et al. Rates of tibial osteotomies in Canada and the United States. Clin Orthop Relat Res. 1995;(319):266-275.
54. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
55. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
56. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
57. Brown NM, Sheth NP, Davis K, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8 suppl):86-90.
Diagnosis and Management of Vestibular Migraine
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, [email protected].
Financial disclosures: None.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, [email protected].
Financial disclosures: None.
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, [email protected].
Financial disclosures: None.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
2016 Update on cancer
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Each year approximately 60,000 women are diagnosed with endometrial cancer. The majority of the identified tumors will be low grade—cancer found at an early stage that may be treated with surgery alone. Unfortunately, however, too many of the 60,000 patients will have poor prognostic features, such as serous or clear cell histology (high-grade cancer), lymphovascular space invasion, or positive lymph node status.
Advances in technology and the state of science have come a long way since the dichotomy of Type I (endometrioid) and Type II (serous and clear cell) tumors were
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations high (serous tumors)
- somatic copy number alterations low (endometrioid cancer).
In 2016, we are now understanding the molecular basis of disease and how it affects survival; these 4 categories have different survival. But why? Perhaps the answer lies within the endogenous immune system. Tumor-infiltrating lymphocytes are associated with improved survival in multiple types of cancer, including endometrial. Whether these lymphocytes are regulatory or cytotoxic T-cells convolutes the matter further.4 To understand these intricacies we need to further categorize how a tumor’s genetic mutations affect antigen exposure to the immune system, quantitate the clinical impact of the findings, and selectively target patients with novel therapeutics.
In this Update, we look at data on POLE mutations, exploring 2 studies that help us to better understand why these types of mutations have uniquely positive prognostic implications (when they logically should not have good survival rates). In addition, we discuss 2 studies that examined mismatch repair defects, in endometrial cancer specifically, and the programmed death (PD)-1 pathway in both endometrial and other cancer types. Are these molecular entities of tumors associated with better or worse prognosis, and why?
Molecular profiling: Prognostic implications of POLE mutations
Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2014;107(1):402.
van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE Proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347 - 3355.
The TCGA identified a subgroup of endometrial carcinomas with mutations of the DNA polymerase POLE. These mutants have a high rate of proofreading error and frequent base pair substitutions. This POLE subgroup (6% to 12% of endometrial tumors) is associated with endometrioid histology and high-grade tumors. Patients with these tumors would be expected to have an aggressive course with poor survival, but often these patients survive without a recurrence. We need more understanding of why.
POLE mutations and prognosis
In a secondary analysis by Church and colleagues of the PORTEC-1 and -2 studies (2 large, randomized controlled trials evaluating postoperative external beam radiation therapy [EBRT] or vaginal brachytherapy), tumors were tested for mutations in POLE (POLE-mutant and POLE wild-type). POLE mutations were detected in 6.1% of tumors overall. Despite their high grade, POLE-mutant tumors resulted in fewer recurrences (6.2% vs 14.1%) and fewer deaths (2.3% vs 9.7%) than POLE wild-type tumors. In grade 3 tumors, 0 of 15 POLE-mutant tumors recurred.
These results indicate that, even with having poor prognostic features, endometrial cancers with mutations in POLE have an excellent prognosis.5
POLE mutations and the immune response
To explain the discrepancy in the results by Church and colleagues, van Gool and colleagues analyzed endometrial cancer specimens from PORTEC-1, -2, and the TCGA studies. Endometrial cancers were categorized as POLE-mutants, POLE wild-type, or microsatellite stable (MSS) tumors. They found that POLE-mutant endometrial cancers have an increased lymphocytic infiltrate (present in 22 of 47 POLE-mutant specimens) as compared with POLE wild-type or MSS tumors.
Also, POLE-mutants had an increased density of cytotoxic T-cells (CD8+) at the tumor center and margin that significantly exceeded that of POLE wild-type or MSS tumors. The proportion of tumors with CD8+ cells exceeding the median were also higher in POLE-mutant (60%) compared with POLE wild-type (31.3%) and MSS (7.2%) tumors. Markers LAG3, TIM-3, TIGI, as well as T-cell inhibitors PD1 and CTLA-4, confirmed evidence of T-cell exhaustion--all of which correlated with CD8 expression.
These findings suggest that POLE mutations lead to hundreds of thousands of DNA fragments stimulating the immune system through prolonged antigenic exposure.6 This immune response is so powerful that even these tumors with poor prognostic features will have excellent clinical outcomes.
POLE-mutant endometrial cancers have mutations that stimulate the immune system with tremendous amounts of antigenic neopeptides. This robust immune response is demonstrated by tumor infiltrating lymphocytes that enhance antitumor effects and host killing in spite of traditional poor prognostic features.
Mismatch repair and immunology: Targeted therapy for targeted patients
McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2016;34(25):3062-3068.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
The most frequent genetic mutation in endometrial cancer is mismatch repair (MMR) deficiency. Loss of this pathway leads to a failure of repairing replication errors and gives rise to small repeated sequences of DNA, known as MSI. Germline mutations in MMR (Lynch syndrome) occur in only 3% to 5% of endometrial cancers. Somatic mutations in MMR give rise to 10% to 20% of colorectal cancers and upwards of 20% to 40% of endometrial cancers.
Given this high frequency, universal screening utilizing immunohistochemistry of proteins MLH1, MSH2, MSH6, and PMS2 has become the standard of care in tumors to identify MMR deficiency. MMR-deficient endometrial tumors are associated with higher grade and lymphovascular space invasion. The actual clinical prognosis of these tumors, however, has not been well described.7 McMeekin and colleagues set out to examine prognosis.
Details of the study by McMeekin and colleagues
In the collaborative study, researchers assessed 1,024 tumors for MMR and categorized them into 1 of 4 groups: normal(62.4%), epigenetic MMR-defective (25.78%),MMR-probable mutation (9.67%), or MSI-low (2.15%). The researchers found that the pathologic features were associatedwith MMR status. For instance, MMR-defective tumors were more likely thanMMR-normal tumors to be Grade 2 (50% vs 40.7%, respectively). Lymphovascular space invasion also occurred more frequently in MMR-defective than in MMR-normal tumors (32.7% vs 17.13%, respectively). Approximately 22% of patients with MMR-defective tumors had stage III or IV disease, while only 13% to 14% of the other groups presented with such advanced stage.
On univariate analysis, an MMR-defective tumor was associated with worsened progression-free survival (hazard ratio [HR], 1.37). On subsequent multivariate analysis, no difference in survival in MMR-defective vs MMR-normal tumors was found. The authors concluded that MMR status is predictive of response to adjuvant therapy.
An intriguing biologic explanation of how MMR status affects response to adjuvant therapy is that MMR-defective tumors contain lymphocytic infiltrates, consistent with an increased immunologic response.8 Similar to the previously discussed POLE mutations, MMR-defective tumors have a tremendous increase in somatic mutations that are on the order of 10 to 100 times that of MMR-proficient tumors. These MMR-defective tumors likely give rise to increased antigen exposure to the immune system.
These immune infiltrates will show signs of exhaustion and upregulate negative feedback systems, which is the point at which the PD-1 pathway becomes critically important. The PD-1 receptor is expressed predominately on T-cells and its ligands regulate the immune system by inhibition of self-reactive T-cells.9
MMR deficiency and anti-programmed death receptor 1
The study by McMeekin and colleagues shows MMR-defective tumors have poor prognostic features but the same survival as those with MMR proficiency or good prognostic features. Why is this the case? A recent study by Le and colleagues analyzed this question.
Details of the study by Le and colleagues
The investigators performed a phase 2 trial evaluating pembrolizumab (10 mg/kg IV every 14 days), an anti-PD 1 immune checkpoint inhibitor in patients with tumors demonstrating MMR-deficiency. The 3 cohorts included: MMR-defective colorectal cancer (n = 10), MMR-proficient colorectal cancer (n = 18), and MMR-defective noncolorectal cancer (n = 7, including 2 endometrial cancers). Objective response rates were 40%, 0%, and 71% for each group, respectively.
MMR-defective tumors had a striking HR of disease progression or death of 0.04 (95% confidence interval, 0.01-0.21; P<.001). Genomic analysis was performed and identified 578 potential mutation- associated neoantigens in the MMR-defective groups (compared with only 21 in the MMR-proficient tumors). These findings promote the concept of a mutation-associated antigen component to the endogenous immune response.10
These studies support the growing evidence that molecular events have a powerful clinical impact that has the potential to supplant traditional histopathologic staging.
Conclusion
The above-stated mutations of mismatch repair and POLE are changing our perspective of endometrial cancer and shedding light on the complexities of tumor biology. As future research increasingly incorporates genomic profiling, we anticipate clinical trials may build evidence that adjuvant therapy will be directed by molecular staging, as opposed to traditional surgical or even histologic staging, as these mutations are the root cause of the tumor phenotype.
Key for readers to take away from this Update is that genomic profiling and enrollment in clinical trials is critical to understanding the implications of these mutations and how to best treat our patients. In addition, we should encourage our patients with endometrial cancer to see genetic counselors and have appropriate screening of MMR-deficiency. This will continue to advance our understanding as well as to provide patients with valuable information regarding their diagnosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10-17.
- Kuroki LM, Mutch DG. Endometrial cancer update: the move toward personalized cancer care. OBG Manag. 2013;25(10):25-32.
- Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- De Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105-110.
- Church DN, Steloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402.
- Van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347-3355.
- Lancaster JM, Powell CB, Chen L-M, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3-7. Erratum in Gynecol. Oncol. 2015;138(3):765.
- McMeekin DS, Tritchler DL, Cohn DE, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(25):3062-3068.
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145-152.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520.
In this Article
- The prognostic significance of tumors with POLE mutations
- Can the immune system kill MMR-deficient endometrial cancer?
Providing Quality Epilepsy Care for Veterans
Epilepsy is a common and complex neurologic condition marked by recurrent seizures. It has been diagnosed in more than 87,000 veterans enrolled in the VA health care system, 16% of whom have comorbid traumatic brain injury (TBI), and nearly 25% also have posttraumatic stress disorder (PTSD).1 These comorbidities were even more common in Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans: TBI in 52.6% and PTSD in 70.4%. With 25 drugs for seizures and 2 approved devices, treatment of epilepsy can prove challenging to providers whose goal is to balance seizure control and adverse effects (AEs).
Despite the therapeutic armamentarium, about one-third of people with epilepsy have poorly controlled seizures, and an untold number may experience delays in referral to higher levels of epilepsy care or undergo troubling antiepileptic medication AEs and comorbid psychiatric disorders that have profound impacts on quality of life (QOL).
Quality generally has been defined as “providing the right care to the right patient at the right time and in the right way to achieve the best possible results.”2 Much work has been done over the past 2 decades to identify “the right care” for epilepsy patients.3
The American Academy of Neurology (AAN) has developed evidence-based, clinically focused guidelines on numerous topics, including antiepileptic drugs and women’s health, and has developed quality measure sets.4,5 More broadly, the Institute of Medicine (IOM) proposed 13 recommendations, including improving quality of care, establishing epilepsy centers and an epilepsy care network, educating health professionals about epilepsy, and providing education for people with epilepsy and their families.6
Within the VA, health care for veterans with epilepsy is changing in part by the Epilepsy Centers of Excellence (ECoC), established by federal law. The ECoE’s primary missions are to improve quality of and access to epilepsy specialty care to improve the health and well-being of veteran patients with epilepsy and other seizure disorders through integration of clinical care, outreach, research, and education to VA providers and patients.7
The goal of this article is to outline the key elements of quality epilepsy care and make recommendations for providing quality care in the VA health care system.
Diagnosis and Seizure Types
Quality care for veterans with epilepsy begins with the provider reviewing pertinent history and establishing the clinical characteristics of the patient’s seizures and epilepsy. The provider should ask about the first signs of the seizure or warning (aura), the seizure (ictal period), and the period after the seizure (postictal period). Seizure histories from the patient and observers are critical.
The first step is to define whether the patient’s seizures are generalized, that is, start all over the brain at once, or focal, starting in one area of the brain. The patient’s initial sensation at the onset of a seizure (aura) may help localize onset and define focal seizures. For example, déjà vu sensations often point to seizure onset in the mesial temporal lobe and hippocampus. Focal seizures can spread and cause cognitive dysfunction, including aphasia and amnesia, or evolve into a generalized convulsion (tonic-clonic seizure). Many patients present with a generalized tonic-clonic seizure and have had brief focal seizures that were not considered seizures by the patient or by other providers. This seizure type should be clarified by asking specifically about paroxysmal symptoms. For example, brief periods of confusion that are episodic may be focal seizures. In general, focal seizures are stereotyped and may have a feature that helps in establishing the diagnosis. Many temporal lobe seizures are associated with lip smacking behaviors (oral buccal automatisms).
Tonic-clonic seizures may begin without an aura and are generalized from onset. Patients with this type of seizure may have electroencephalogram (EEG) findings that define a generalized abnormality, which consist of frontocentral spike and wave discharges in the EEG. In the VA population, the first generalized tonic-clonic seizure may occur while in the military. Some of these patients have juvenile myoclonic epilepsy, and a history of brief jerks on waking (myoclonus) may have been occurring but not recognized as seizures. The treatment of seizures, in part, depends on whether they begin focally or are generalized at onset.
Often people with epilepsy have multiple seizure types. The types of seizures should be documented and, if possible, corroborated by a witness. Epileptic seizures tend to be stereotyped and of relatively brief duration, usually < 2 minutes. The period after a seizure may be followed by a more prolonged period of neurologic dysfunction that includes confusion and fatigue. These symptoms may be the only indication that the patient has had a seizure.
At each clinic visit, the characteristics of the patient’s seizures should be reviewed and the frequency of seizures documented. A calendar to track seizure frequency is helpful to understand precipitating factors and response to treatment.
The health care provider (HCP) should look for the cause of a patient’s epilepsy. It is important to ask the patient about family history, age of first seizure, occurrence of febrile seizures, developmental history, past history of meningitis or encephalitis, history of childhood seizures or spells, and history of brain lesions, including tumors, strokes, or TBI. Most patients with epilepsy do not have a clear cause for their epilepsy, but the cause may be clarified with EEG and magnetic resonance imaging (MRI) testing.
EEG and Brain Imaging
All patients with epilepsy should be evaluated with an EEG, and for those with focal epilepsy or undefined epilepsy, with an imaging study of the brain, preferably an MRI. These results should be reviewed at each visit. The EEG may show focal features that are related to neurophysiologic dysfunction, such as slowing that is not definitely epileptiform in character, or show focal spike or sharp waves that are epileptiform in character. Generalized abnormalities may include generalized slowing that is not an epileptiform feature or frontocentral spike wave patterns that are epileptiform in character. The EEG cannot rule out epilepsy, but can rule in the likelihood of epilepsy when definite epileptiform features are present.
Brain imaging can define many conditions that can cause focal epilepsy, and an MRI is more sensitive for defining a number of these conditions (cavernous angiomas, hippocampal sclerosis, developmental migration disorders, and low-grade neoplasms). Significant trauma with signal abnormalities to suggest prior bleeding predispose to epilepsy. When patients are refractory to medical therapy and have imaging findings concordant with EEG onset of seizures, then surgery can be a better treatment.
Adverse Effects
Broad-spectrum drug treatments are efficacious for either generalized or focal seizures, whereas narrow-spectrum treatments are most efficacious for focal seizures (Table 1). The choice of a seizure medication is based on the patient’s seizure type(s) and other comorbid conditions.7 For example, a patient with epilepsy and migraines may do better with a seizure medication that also is used for migraine prophylaxis (valproate or topiramate). In general, seizure control is unlikely to be achieved if patients fail the first 2 medications tried.8 Treating with > 1 medication may improve seizure control but may increase AEs. A review of current seizure medications and their AEs can be found on the ECoE website (http://www.epilepsy.va.gov/Provider_Education.asp).
In VA cooperative studies that evaluated seizure medications, the most common reason for discontinuing a drug was the combination of ineffectiveness and AEs.9-11 Addressing AEs is a quality measure for the care of patients with epilepsy. Adverse effects may be dose dependent or idiosyncratic (rashes). Drug levels may help in determining dose-dependent AEs; for example, diplopia with carbamazepine levels above 10 μg/mL. Each patient may have susceptibility to medication AEs that do not exactly match therapeutic levels. When patients have AEs, a reduction in dose or trial of an alternative medication is advised.
Uncontrollable Epilepsy
About one-third of people with epilepsy have uncontrolled seizures, known as medically intractable epilepsy, which may be identified early in their clinical course by failure of the first 2 tolerated medications.8 Patients should be referred to an epilepsy center so their epilepsy can be defined by video EEG monitoring to capture seizures. Unfortunately, in the VA system, this route is often delayed, and patients may not be diagnosed appropriately for years.12 Some of these patients may be considered treatment failures because the right medications were not tried (eg, generalized epilepsy that is treated with narrow-spectrum seizure medications). Juvenile myoclonic epilepsy often may not be controlled by phenytoin or carbamazepine, but valproate, lamotrigine, levetiracetam, and zonisamide may be more effective.
Other patients may not have epilepsy but have psychogenic nonepileptic seizures (PNES). These behavioral seizures do not have an EEG epileptiform correlate. About 25% of patients who undergo prolonged video EEG monitoring have PNES, and seizure medications do not treat these events.12 A smaller percentage of patients have both epileptic and nonepileptic seizures (5%-15%). Psychogenic nonepileptic seizures often occur within the context of traumatic exposure(s) or previous physical or sexual abuse.
In the VA population, PNES is more often associated with PTSD or head trauma history than in patients with epilepsy.13,14 To confirm the diagnosis of PNES, video-EEG capture of the patient’s seizures is required. Because of the increased number of combat veterans with TBI and PTSD, the diagnosis of epilepsy may be difficult without video-EEG monitoring. Management consists of addressing the underlying conversion disorder and recognition and treatment of comorbidities, such as mood, anxiety, personality, or PTSD. Recently, cognitive behavioral-informed psychotherapy (CB-ip) has been shown to be effective in patients with PNES and is available through the VA national telemental health center and at some ECoE sites.15
If a patient with uncontrolled epilepsy has focal seizures, surgical therapy is more likely to result in seizure control than will medical therapy.16,17 This is especially true when other testing, including MRI, positron emission tomography, and neuropsychiatric evaluation, point to a concordance of localization. These patients should be evaluated in a center that can provide surgical therapy and if necessary also record seizures with invasive techniques using electrodes placed directly over the cortex or into the brain to sample deeper structures like the hippocampus or amygdala. Patients who are refractory should be considered for reevaluation every 2 years by a comprehensive epilepsy center.
Unfortunately, some patients have seizures that begin in eloquent cortex, which if removed, leads to undesirable neurologic loss or multifocal seizure onset. In these patients, seizure frequency can be reduced by vagus nerve stimulation or intracranial responsive neurostimulation.18,19
Safety
Epilepsy has inherent risks for injury. Patients and their families often need to be informed about risks and risky behaviors to avoid. A frank discussion about safety is prudent. What to do for the patient during a seizure should be addressed. For convulsive seizures: Protect the patient from injury by placing something soft between the patient’s head and the floor, keep the patient on his or her side; do not restrain the patient or put anything in the mouth; stay calm and time the seizure; as the patient gains consciousness, talk to the patient and be reassuring. For nonconvulsive seizures: Stay with the patient; time the seizure; gently guide the patient away from dangerous situations like streets or stairs; stay with the patient until he or she is back to normal, and reassure the patient.
Driving
People with epilepsy identify driving as one of their major concerns; therefore, it is important for HCPs to properly counsel patients with seizure disorders and their families about driving (Figure).20 In general people with controlled seizures are permitted to drive in every state in the U.S., but people with uncontrolled seizures are restricted from licensure. Despite the desire and necessity to drive for many individuals with epilepsy, seizures while driving pose risks for crashes, which may result in property damage, injuries, and death.21 Factors, such as duration of seizure freedom, help predict the risk for crashes. The legal rules for determining control and administering restrictions are a complex mix of federal and state laws, regulations, and local practices, which vary widely across the country.21,22 The standards also change over time; updated information is available from local state authorities and on good informational sites, such as those of the Epilepsy Foundation.
The key standard for determining accident risks is the seizure free interval, which is the duration of time a person with epilepsy has been seizure-free.21-23 In the U.S., the accepted period for seizure freedom varies from about 3 months to 12 months, depending on individual state rules.24
California, Delaware, Nevada, New Jersey, Oregon, and Pennsylvania require mandatory reporting. Generally physician groups in the U.S. and elsewhere oppose such mandatory reporting, because of the concern that their patients will not report their seizures, and thus may not receive appropriate treatment. Indeed, patients with epilepsy often do not tell physicians about their seizures, fearing loss of driving privileges and other social consequences.21,23 Providers should make an effort to determine seizure frequency and whether the patient is being truthful. This information then provides a background for the provider to discuss driving issues.
Injury
People with epilepsy are susceptible to injury during a seizure and need to be counseled regarding safety, particularly when seizures are not well controlled. Hazardous situations include being near stoves or cooking, bathing alone, swimming alone, working at heights without a safety harness, and using power tools.26
Sudden Unexplained Death
Patients with recurrent seizures have an increased risk for accidental fatality and for sudden unexplained death in epilepsy (SUDEP), which accounts for up to 17% of all deaths in people with epilepsy. The risk for sudden death from recurrent seizures increases 2.3 times compared with the risk in the general population.25 A SUDEP is an unexpected death in a person who has epilepsy with no other obvious cause of death.26 Because increased seizure frequency, the presence of tonic-clonic seizures, and other accidental risks of seizures are associated with SUDEP, the subject should be discussed with patients and their families, to encourage adherence to treatment. Epileptologists also discuss these risks with patients and their families when surgical interventions are being considered. The potential risks for injury or SUDEP may offset the surgical risks when pursuing a potentially curative epilepsy procedure.
Women of Childbearing Age
In January 2015, the ECoE started a women veterans epilepsy workgroup with the goal of improving clinical care within the VAHCS to provide education to patients, family members, and VA health care providers about the care of women with epilepsy.
Providers need to be aware that seizure medications that induce certain hepatic enzymes can lead to hormonal contraceptive failure (Table 2).27 Preconception folic acid supplementation (with at least 0.4 mg) should be considered, because it may reduce the risk of major congenital malformations.28 The goal of epilepsy management prior to conception is to maximize seizure control with the optimal seizure medication to avoid the need to make changes during the pregnancy.
During pregnancy, the volume of distribution increases and seizure medication metabolism may change requiring dose adjustment. The best predictor of seizure frequency during pregnancy is a woman’s epilepsy pattern prior to conception. Seizure freedom for 9 months prior to conception is associated with a 84% to 92% likelihood of seizure freedom throughout the pregnancy.29
International seizure medication pregnancy registries have provided valuable information regarding the risk of major congenital malformation (MCM) of development, which seems to be a consequence of seizure medication therapy and not epilepsy itself. The risk of MCM associated with seizure medication therapy is about 4% to 5% compared with 1.5% to 3% in the general population.30,31 A seizure medication table that supplements the existing VA ECoE information specifically addresses women’s issues with the recognition that recent revisions to the teratogenicity classification have been made by the FDA (Table 2).32 If possible, valproate should be avoided during pregnancy due to its higher rate of MCM and impact on neurocognitive function.33 Obstetrical input is essential in arranging routine prenatal fetal testing. Although women with epilepsy do not have a substantially increased risk of undergoing a cesarean section, delivery in a hospital obstetric unit is advised.
Postpartum women veterans with epilepsy should be encouraged to breast feed since the potential benefits seem to outweigh any established risk of seizure medication exposure to the infant. No relative impact on cognition was found in breastfed infants exposed to a variety of seizure medications.34 Following delivery, vigilance is needed to monitor for sleep deprivation, postpartum depression, and the safe care of the infant.35 Care of women with epilepsy does not end with pregnancy planning, additional important topics include psychiatric comorbidities, catamenial epilepsy, and bone health, which are unique to women veterans with epilepsy.
Identifying Psychiatric Conditions
People with epilepsy have a number of psychiatric comorbidities. Suicide and suicide attempts are 6 to 25 times more common in patients with temporal lobe epilepsy compared with those in the general population.36-38 Although the FDA identified all seizure medications as potential contributors to suicide risk, a recent longitudinal study of suicidal ideation and attempt found that those who received seizure medications were more likely to have suicidal ideation and attempt than those who did not received seizure medications, suggesting that medication may relate to baseline depression or suicidal ideation.39 When seeing patients with epilepsy, screening for suicidal ideation is good practice.
Depression and anxiety disorders are the most common psychiatric comorbidities in people with epilepsy.40,41 About half of people with epilepsy have symptoms of depression, and 40% have anxiety.42 Depression often precedes the diagnosis of epilepsy, and anxiety often is present and related to the fear of having seizures and of social embarrassment.43 People with epilepsy may not self-report these symptoms if not asked directly. Identification of comorbid depression and anxiety should lead to appropriate treatment. The CB-ip being used for PNES also is being used for treatment of epilepsy and its comorbidities.44
Mild traumatic brain injury (mTBI) has a small increased risk of epilepsy.45 Veterans with mTBI that occurs in the context of blasts are set up for the development of PTSD. These veterans may have other mild cognitive symptoms that can be confused with seizures. Furthermore, mTBI and PNES often occur together, more so than do mTBI and epileptic seizures.14 Video-EEG monitoring may be warranted for these patients.
Education and Self-Management
The IOM report on epilepsy identified patient and family education as essential for better epilepsy care.6 Providers should help educate patients about their epilepsy and refer them to resources available online (Table 3). A continuing exchange about their condition and treatment with seizure medications should occur with each visit. People with epilepsy should also receive guidance regarding how to manage their epilepsy and day-to-day issues. Referring, patients to social workers, psychologists, vocational rehabilitation services, and support groups can enhance a patient’s QOL.3,6 The stigma of epilepsy is another burden that can be diminished by attending support groups. Recently, being a part of an online patient community of veterans was found to improve self-management.46
Conclusion
People with epilepsy have many issues that are unique to the condition and, in part, are related to the unpredictable occurrence of seizures and loss of function. Ideally, seizure control provides a normal lifestyle; however, some mood and anxiety comorbidities may persist despite seizure control. Care in the VA system includes access to 16 sites that have programs dedicated to treating veterans with epilepsy and many more consortium sites that interact with the ECoE to provide high-quality patient care (http:\\www.epilepsy.va.gov). The ECoE also provides a readily available resource to optimally manage veterans with epilepsy. Attention to the issues addressed in this article will promote quality care for veterans with epilepsy.
1. Rehman R, Kelly P, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762.
2. National Committee for Quality Assurance (NCQA). The essential guide to health care quality. https://www.ncqa.org/Portals/0/Publications/Resource%20Library/NCQA_Primer_web.pdf. Accessed August 9, 2016.
3. Pugh MJ, Berlowitz DR, Montouris GB, et al. What constitutes high quality of care for adults with epilepsy? Neurology. 2007;69(21):2020-2027.
4. Fountain NB, Van Ness PC, Swain-Eng R, Tonn S, Bever CT Jr; American Academy of Neurology Epilepsy Measure Development Panel and the American Medical Association-Convened Physician Consortium for Performance Improvement Independent Measure Development Process. Quality improvement in neurology: AAN epilepsy quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology. 2011;76(1):94-99.
5. Fountain NB, Van Ness PC, Bennett A, et al. Quality improvement in neurology: epilepsy update quality measurement set. Neurology. 2015;84(14):1483-1487.
6. England MJ, Liverman CT, Schultz AM, Strawbridge LM, eds; Committee on the Public Health Dimensions of the Epilepsies, Board on Health Sciences Policy, Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012.
7. Tortorice K, Rutecki P. Principles of Treatment. In: Hussain, AM, Tran TT, eds. Department of Veterans Affairs Epilepsy Manual. San Francisco, CA: Epilepsy Centers of Excellence, Department of Veteran Affairs; 2014:120-127.
8. Kwan P, Brodie MJ. Early Identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
9. Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Eng J Med. 1985;313(3):145-151.
10. Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Eng J Med. 1992;327(11):765-771.
11. Rowan AJ, Ramsay RE, Collins JF, et al; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868-1873.
12. Salinsky M, Spencer D, Boudreau E, Ferguson F. Psychogenic nonepileptic seizures in US veterans. Neurology. 2011;77(10):945-950.
13. Salinsky M, Evrard C, Storzbach D, Pugh MJ. Psychiatric comorbidity in veterans with psychogenic seizures. Epilepsy Behav. 2012;25(3):345-349.
14. Salinsky M, Storzbach D, Goy E, Evrard C. Traumatic brain injury and psychogenic seizures in veterans. J Head Trauma Rehabil. 2015;30(1):E65-E70.
15. LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
16. Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, control trial for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318.
17. Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003;44(6):741-751.
18. Morris GL III, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy. report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453-1459.
19. Morrell M; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304.
20. Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia. 1997;38(2):233-236.
21. Krumholz A. Driving issues in epilepsy: past, present, and future. Epilepsy Curr. 2009;9(2):31-35.
22. Krauss GL, Ampaw L, Krumholz A. Individual state driving restrictions for people with epilepsy in the US. Neurology. 2001;57(10):1780-1785.
23. Krauss GL, Krumholz A, Carter RC, Kaplan P. Risk factors for seizure-related motor vehicle crashes in patients with epilepsy. Neurology. 1999;52(7):1324-1329.
24. Consensus statements, sample statutory provisions, and model regulations regarding driver licensing and epilepsy. American Academy of Neurology. American Epilepsy Society, Epilepsy Foundation of America. Epilepsia. 1994:35(3):696-705.
25. Cavazos, JE. SUDEP and Other Risks of Seizures. In: Husain AM, Tran, TT, eds. VA Epilepsy Manual. San Francisco, CA: Epilepsy Centers of Excellence, Department of Veteran Affairs; 2014:206-209.
26. Tolstykh GP, Cavazos JE. Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav. 2013;26(3):410-414.
27. Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception. 2011;83(1):16-29.
28. Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2009;73(2):142-149.
29. Harden CL, Hopp J, Ting TY, et al; American Academy of Neurology; American Epilepsy Society. Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): 1. Obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1229-1236.
30. Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64(11):1874-1878.
31. Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77(2):193-198.
32. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Published December 3, 2014. Accessed June 27, 2016.
33. Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
34. Meador KH, Baker GA, Browning N, et al; NEAD Study Group. Effects of breastfeeding in children of women taking antiepileptic drugs. Neurology. 2010;75(22):1954-1960.
35. Klein A. The postpartum period in women with epilepsy. Neurol Clin. 2012;30(3):867-875.
36. Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
37. Jones JE, Hermann BP, Barry JJ, Gilliam FG, Kanner AM, Meador KJ. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2013;4(suppl 3):S31-S38.
38. Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol. 2007;6(8):693-698.
39. Pugh MJ, Hesdorffer D, Wang CP, et al. Temporal trends in new exposure to antiepileptic drug monotherapy and suicide-related behavior. Neurology. 2013;81(22):1900-1906.
40. Barry JJ, Ettinger AB, Friel P, et al; Advisory Group of the Epilepsy Foundation as part of its Mood Disorder. Consensus statement: the evaluation and treatment of people with epilepsy and affective disorders. Epilepsy Behav. 2008;13(suppl 1):S1-S29.
41. Ottman R, Lipton RB, Ettinger AB, et al. Comorbidities of epilepsy: results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011;52(2):308-315.
42. Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanism, and treatment. Biol Psychiatry. 2003;54(3):388-398.
43. Kanner AM. The treatment of depressive disorders in epilepsy: what all neurologists should know. Epilepsia. 2013;54(suppl 1):3-12.
44. Reiter JM, Andrews DJ. A neurobehavioral approach for treatment of complex partial epilepsy: efficacy. Seizure. 2000;9(3):198-203.
45. Pugh MJ, Orman JA, Jaramillo CA, et al. The prevalence of epilepsy and association with traumatic brain Injury in Veterans of the Afghanistan and Iraq Wars. J Head Trauma Rehabil. 2015;30(1):29-37.
46. Hixson JD, Barnes D, Parko K, et al. Patients optimizing epilepsy management via an online community: the POEM Study. Neurology. 2015;85(2):129-136.
47. Winterfeld U, Merlob P, Baud D, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology. 2016;86(24):2251-2257.
Epilepsy is a common and complex neurologic condition marked by recurrent seizures. It has been diagnosed in more than 87,000 veterans enrolled in the VA health care system, 16% of whom have comorbid traumatic brain injury (TBI), and nearly 25% also have posttraumatic stress disorder (PTSD).1 These comorbidities were even more common in Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans: TBI in 52.6% and PTSD in 70.4%. With 25 drugs for seizures and 2 approved devices, treatment of epilepsy can prove challenging to providers whose goal is to balance seizure control and adverse effects (AEs).
Despite the therapeutic armamentarium, about one-third of people with epilepsy have poorly controlled seizures, and an untold number may experience delays in referral to higher levels of epilepsy care or undergo troubling antiepileptic medication AEs and comorbid psychiatric disorders that have profound impacts on quality of life (QOL).
Quality generally has been defined as “providing the right care to the right patient at the right time and in the right way to achieve the best possible results.”2 Much work has been done over the past 2 decades to identify “the right care” for epilepsy patients.3
The American Academy of Neurology (AAN) has developed evidence-based, clinically focused guidelines on numerous topics, including antiepileptic drugs and women’s health, and has developed quality measure sets.4,5 More broadly, the Institute of Medicine (IOM) proposed 13 recommendations, including improving quality of care, establishing epilepsy centers and an epilepsy care network, educating health professionals about epilepsy, and providing education for people with epilepsy and their families.6
Within the VA, health care for veterans with epilepsy is changing in part by the Epilepsy Centers of Excellence (ECoC), established by federal law. The ECoE’s primary missions are to improve quality of and access to epilepsy specialty care to improve the health and well-being of veteran patients with epilepsy and other seizure disorders through integration of clinical care, outreach, research, and education to VA providers and patients.7
The goal of this article is to outline the key elements of quality epilepsy care and make recommendations for providing quality care in the VA health care system.
Diagnosis and Seizure Types
Quality care for veterans with epilepsy begins with the provider reviewing pertinent history and establishing the clinical characteristics of the patient’s seizures and epilepsy. The provider should ask about the first signs of the seizure or warning (aura), the seizure (ictal period), and the period after the seizure (postictal period). Seizure histories from the patient and observers are critical.
The first step is to define whether the patient’s seizures are generalized, that is, start all over the brain at once, or focal, starting in one area of the brain. The patient’s initial sensation at the onset of a seizure (aura) may help localize onset and define focal seizures. For example, déjà vu sensations often point to seizure onset in the mesial temporal lobe and hippocampus. Focal seizures can spread and cause cognitive dysfunction, including aphasia and amnesia, or evolve into a generalized convulsion (tonic-clonic seizure). Many patients present with a generalized tonic-clonic seizure and have had brief focal seizures that were not considered seizures by the patient or by other providers. This seizure type should be clarified by asking specifically about paroxysmal symptoms. For example, brief periods of confusion that are episodic may be focal seizures. In general, focal seizures are stereotyped and may have a feature that helps in establishing the diagnosis. Many temporal lobe seizures are associated with lip smacking behaviors (oral buccal automatisms).
Tonic-clonic seizures may begin without an aura and are generalized from onset. Patients with this type of seizure may have electroencephalogram (EEG) findings that define a generalized abnormality, which consist of frontocentral spike and wave discharges in the EEG. In the VA population, the first generalized tonic-clonic seizure may occur while in the military. Some of these patients have juvenile myoclonic epilepsy, and a history of brief jerks on waking (myoclonus) may have been occurring but not recognized as seizures. The treatment of seizures, in part, depends on whether they begin focally or are generalized at onset.
Often people with epilepsy have multiple seizure types. The types of seizures should be documented and, if possible, corroborated by a witness. Epileptic seizures tend to be stereotyped and of relatively brief duration, usually < 2 minutes. The period after a seizure may be followed by a more prolonged period of neurologic dysfunction that includes confusion and fatigue. These symptoms may be the only indication that the patient has had a seizure.
At each clinic visit, the characteristics of the patient’s seizures should be reviewed and the frequency of seizures documented. A calendar to track seizure frequency is helpful to understand precipitating factors and response to treatment.
The health care provider (HCP) should look for the cause of a patient’s epilepsy. It is important to ask the patient about family history, age of first seizure, occurrence of febrile seizures, developmental history, past history of meningitis or encephalitis, history of childhood seizures or spells, and history of brain lesions, including tumors, strokes, or TBI. Most patients with epilepsy do not have a clear cause for their epilepsy, but the cause may be clarified with EEG and magnetic resonance imaging (MRI) testing.
EEG and Brain Imaging
All patients with epilepsy should be evaluated with an EEG, and for those with focal epilepsy or undefined epilepsy, with an imaging study of the brain, preferably an MRI. These results should be reviewed at each visit. The EEG may show focal features that are related to neurophysiologic dysfunction, such as slowing that is not definitely epileptiform in character, or show focal spike or sharp waves that are epileptiform in character. Generalized abnormalities may include generalized slowing that is not an epileptiform feature or frontocentral spike wave patterns that are epileptiform in character. The EEG cannot rule out epilepsy, but can rule in the likelihood of epilepsy when definite epileptiform features are present.
Brain imaging can define many conditions that can cause focal epilepsy, and an MRI is more sensitive for defining a number of these conditions (cavernous angiomas, hippocampal sclerosis, developmental migration disorders, and low-grade neoplasms). Significant trauma with signal abnormalities to suggest prior bleeding predispose to epilepsy. When patients are refractory to medical therapy and have imaging findings concordant with EEG onset of seizures, then surgery can be a better treatment.
Adverse Effects
Broad-spectrum drug treatments are efficacious for either generalized or focal seizures, whereas narrow-spectrum treatments are most efficacious for focal seizures (Table 1). The choice of a seizure medication is based on the patient’s seizure type(s) and other comorbid conditions.7 For example, a patient with epilepsy and migraines may do better with a seizure medication that also is used for migraine prophylaxis (valproate or topiramate). In general, seizure control is unlikely to be achieved if patients fail the first 2 medications tried.8 Treating with > 1 medication may improve seizure control but may increase AEs. A review of current seizure medications and their AEs can be found on the ECoE website (http://www.epilepsy.va.gov/Provider_Education.asp).
In VA cooperative studies that evaluated seizure medications, the most common reason for discontinuing a drug was the combination of ineffectiveness and AEs.9-11 Addressing AEs is a quality measure for the care of patients with epilepsy. Adverse effects may be dose dependent or idiosyncratic (rashes). Drug levels may help in determining dose-dependent AEs; for example, diplopia with carbamazepine levels above 10 μg/mL. Each patient may have susceptibility to medication AEs that do not exactly match therapeutic levels. When patients have AEs, a reduction in dose or trial of an alternative medication is advised.
Uncontrollable Epilepsy
About one-third of people with epilepsy have uncontrolled seizures, known as medically intractable epilepsy, which may be identified early in their clinical course by failure of the first 2 tolerated medications.8 Patients should be referred to an epilepsy center so their epilepsy can be defined by video EEG monitoring to capture seizures. Unfortunately, in the VA system, this route is often delayed, and patients may not be diagnosed appropriately for years.12 Some of these patients may be considered treatment failures because the right medications were not tried (eg, generalized epilepsy that is treated with narrow-spectrum seizure medications). Juvenile myoclonic epilepsy often may not be controlled by phenytoin or carbamazepine, but valproate, lamotrigine, levetiracetam, and zonisamide may be more effective.
Other patients may not have epilepsy but have psychogenic nonepileptic seizures (PNES). These behavioral seizures do not have an EEG epileptiform correlate. About 25% of patients who undergo prolonged video EEG monitoring have PNES, and seizure medications do not treat these events.12 A smaller percentage of patients have both epileptic and nonepileptic seizures (5%-15%). Psychogenic nonepileptic seizures often occur within the context of traumatic exposure(s) or previous physical or sexual abuse.
In the VA population, PNES is more often associated with PTSD or head trauma history than in patients with epilepsy.13,14 To confirm the diagnosis of PNES, video-EEG capture of the patient’s seizures is required. Because of the increased number of combat veterans with TBI and PTSD, the diagnosis of epilepsy may be difficult without video-EEG monitoring. Management consists of addressing the underlying conversion disorder and recognition and treatment of comorbidities, such as mood, anxiety, personality, or PTSD. Recently, cognitive behavioral-informed psychotherapy (CB-ip) has been shown to be effective in patients with PNES and is available through the VA national telemental health center and at some ECoE sites.15
If a patient with uncontrolled epilepsy has focal seizures, surgical therapy is more likely to result in seizure control than will medical therapy.16,17 This is especially true when other testing, including MRI, positron emission tomography, and neuropsychiatric evaluation, point to a concordance of localization. These patients should be evaluated in a center that can provide surgical therapy and if necessary also record seizures with invasive techniques using electrodes placed directly over the cortex or into the brain to sample deeper structures like the hippocampus or amygdala. Patients who are refractory should be considered for reevaluation every 2 years by a comprehensive epilepsy center.
Unfortunately, some patients have seizures that begin in eloquent cortex, which if removed, leads to undesirable neurologic loss or multifocal seizure onset. In these patients, seizure frequency can be reduced by vagus nerve stimulation or intracranial responsive neurostimulation.18,19
Safety
Epilepsy has inherent risks for injury. Patients and their families often need to be informed about risks and risky behaviors to avoid. A frank discussion about safety is prudent. What to do for the patient during a seizure should be addressed. For convulsive seizures: Protect the patient from injury by placing something soft between the patient’s head and the floor, keep the patient on his or her side; do not restrain the patient or put anything in the mouth; stay calm and time the seizure; as the patient gains consciousness, talk to the patient and be reassuring. For nonconvulsive seizures: Stay with the patient; time the seizure; gently guide the patient away from dangerous situations like streets or stairs; stay with the patient until he or she is back to normal, and reassure the patient.
Driving
People with epilepsy identify driving as one of their major concerns; therefore, it is important for HCPs to properly counsel patients with seizure disorders and their families about driving (Figure).20 In general people with controlled seizures are permitted to drive in every state in the U.S., but people with uncontrolled seizures are restricted from licensure. Despite the desire and necessity to drive for many individuals with epilepsy, seizures while driving pose risks for crashes, which may result in property damage, injuries, and death.21 Factors, such as duration of seizure freedom, help predict the risk for crashes. The legal rules for determining control and administering restrictions are a complex mix of federal and state laws, regulations, and local practices, which vary widely across the country.21,22 The standards also change over time; updated information is available from local state authorities and on good informational sites, such as those of the Epilepsy Foundation.
The key standard for determining accident risks is the seizure free interval, which is the duration of time a person with epilepsy has been seizure-free.21-23 In the U.S., the accepted period for seizure freedom varies from about 3 months to 12 months, depending on individual state rules.24
California, Delaware, Nevada, New Jersey, Oregon, and Pennsylvania require mandatory reporting. Generally physician groups in the U.S. and elsewhere oppose such mandatory reporting, because of the concern that their patients will not report their seizures, and thus may not receive appropriate treatment. Indeed, patients with epilepsy often do not tell physicians about their seizures, fearing loss of driving privileges and other social consequences.21,23 Providers should make an effort to determine seizure frequency and whether the patient is being truthful. This information then provides a background for the provider to discuss driving issues.
Injury
People with epilepsy are susceptible to injury during a seizure and need to be counseled regarding safety, particularly when seizures are not well controlled. Hazardous situations include being near stoves or cooking, bathing alone, swimming alone, working at heights without a safety harness, and using power tools.26
Sudden Unexplained Death
Patients with recurrent seizures have an increased risk for accidental fatality and for sudden unexplained death in epilepsy (SUDEP), which accounts for up to 17% of all deaths in people with epilepsy. The risk for sudden death from recurrent seizures increases 2.3 times compared with the risk in the general population.25 A SUDEP is an unexpected death in a person who has epilepsy with no other obvious cause of death.26 Because increased seizure frequency, the presence of tonic-clonic seizures, and other accidental risks of seizures are associated with SUDEP, the subject should be discussed with patients and their families, to encourage adherence to treatment. Epileptologists also discuss these risks with patients and their families when surgical interventions are being considered. The potential risks for injury or SUDEP may offset the surgical risks when pursuing a potentially curative epilepsy procedure.
Women of Childbearing Age
In January 2015, the ECoE started a women veterans epilepsy workgroup with the goal of improving clinical care within the VAHCS to provide education to patients, family members, and VA health care providers about the care of women with epilepsy.
Providers need to be aware that seizure medications that induce certain hepatic enzymes can lead to hormonal contraceptive failure (Table 2).27 Preconception folic acid supplementation (with at least 0.4 mg) should be considered, because it may reduce the risk of major congenital malformations.28 The goal of epilepsy management prior to conception is to maximize seizure control with the optimal seizure medication to avoid the need to make changes during the pregnancy.
During pregnancy, the volume of distribution increases and seizure medication metabolism may change requiring dose adjustment. The best predictor of seizure frequency during pregnancy is a woman’s epilepsy pattern prior to conception. Seizure freedom for 9 months prior to conception is associated with a 84% to 92% likelihood of seizure freedom throughout the pregnancy.29
International seizure medication pregnancy registries have provided valuable information regarding the risk of major congenital malformation (MCM) of development, which seems to be a consequence of seizure medication therapy and not epilepsy itself. The risk of MCM associated with seizure medication therapy is about 4% to 5% compared with 1.5% to 3% in the general population.30,31 A seizure medication table that supplements the existing VA ECoE information specifically addresses women’s issues with the recognition that recent revisions to the teratogenicity classification have been made by the FDA (Table 2).32 If possible, valproate should be avoided during pregnancy due to its higher rate of MCM and impact on neurocognitive function.33 Obstetrical input is essential in arranging routine prenatal fetal testing. Although women with epilepsy do not have a substantially increased risk of undergoing a cesarean section, delivery in a hospital obstetric unit is advised.
Postpartum women veterans with epilepsy should be encouraged to breast feed since the potential benefits seem to outweigh any established risk of seizure medication exposure to the infant. No relative impact on cognition was found in breastfed infants exposed to a variety of seizure medications.34 Following delivery, vigilance is needed to monitor for sleep deprivation, postpartum depression, and the safe care of the infant.35 Care of women with epilepsy does not end with pregnancy planning, additional important topics include psychiatric comorbidities, catamenial epilepsy, and bone health, which are unique to women veterans with epilepsy.
Identifying Psychiatric Conditions
People with epilepsy have a number of psychiatric comorbidities. Suicide and suicide attempts are 6 to 25 times more common in patients with temporal lobe epilepsy compared with those in the general population.36-38 Although the FDA identified all seizure medications as potential contributors to suicide risk, a recent longitudinal study of suicidal ideation and attempt found that those who received seizure medications were more likely to have suicidal ideation and attempt than those who did not received seizure medications, suggesting that medication may relate to baseline depression or suicidal ideation.39 When seeing patients with epilepsy, screening for suicidal ideation is good practice.
Depression and anxiety disorders are the most common psychiatric comorbidities in people with epilepsy.40,41 About half of people with epilepsy have symptoms of depression, and 40% have anxiety.42 Depression often precedes the diagnosis of epilepsy, and anxiety often is present and related to the fear of having seizures and of social embarrassment.43 People with epilepsy may not self-report these symptoms if not asked directly. Identification of comorbid depression and anxiety should lead to appropriate treatment. The CB-ip being used for PNES also is being used for treatment of epilepsy and its comorbidities.44
Mild traumatic brain injury (mTBI) has a small increased risk of epilepsy.45 Veterans with mTBI that occurs in the context of blasts are set up for the development of PTSD. These veterans may have other mild cognitive symptoms that can be confused with seizures. Furthermore, mTBI and PNES often occur together, more so than do mTBI and epileptic seizures.14 Video-EEG monitoring may be warranted for these patients.
Education and Self-Management
The IOM report on epilepsy identified patient and family education as essential for better epilepsy care.6 Providers should help educate patients about their epilepsy and refer them to resources available online (Table 3). A continuing exchange about their condition and treatment with seizure medications should occur with each visit. People with epilepsy should also receive guidance regarding how to manage their epilepsy and day-to-day issues. Referring, patients to social workers, psychologists, vocational rehabilitation services, and support groups can enhance a patient’s QOL.3,6 The stigma of epilepsy is another burden that can be diminished by attending support groups. Recently, being a part of an online patient community of veterans was found to improve self-management.46
Conclusion
People with epilepsy have many issues that are unique to the condition and, in part, are related to the unpredictable occurrence of seizures and loss of function. Ideally, seizure control provides a normal lifestyle; however, some mood and anxiety comorbidities may persist despite seizure control. Care in the VA system includes access to 16 sites that have programs dedicated to treating veterans with epilepsy and many more consortium sites that interact with the ECoE to provide high-quality patient care (http:\\www.epilepsy.va.gov). The ECoE also provides a readily available resource to optimally manage veterans with epilepsy. Attention to the issues addressed in this article will promote quality care for veterans with epilepsy.
Epilepsy is a common and complex neurologic condition marked by recurrent seizures. It has been diagnosed in more than 87,000 veterans enrolled in the VA health care system, 16% of whom have comorbid traumatic brain injury (TBI), and nearly 25% also have posttraumatic stress disorder (PTSD).1 These comorbidities were even more common in Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans: TBI in 52.6% and PTSD in 70.4%. With 25 drugs for seizures and 2 approved devices, treatment of epilepsy can prove challenging to providers whose goal is to balance seizure control and adverse effects (AEs).
Despite the therapeutic armamentarium, about one-third of people with epilepsy have poorly controlled seizures, and an untold number may experience delays in referral to higher levels of epilepsy care or undergo troubling antiepileptic medication AEs and comorbid psychiatric disorders that have profound impacts on quality of life (QOL).
Quality generally has been defined as “providing the right care to the right patient at the right time and in the right way to achieve the best possible results.”2 Much work has been done over the past 2 decades to identify “the right care” for epilepsy patients.3
The American Academy of Neurology (AAN) has developed evidence-based, clinically focused guidelines on numerous topics, including antiepileptic drugs and women’s health, and has developed quality measure sets.4,5 More broadly, the Institute of Medicine (IOM) proposed 13 recommendations, including improving quality of care, establishing epilepsy centers and an epilepsy care network, educating health professionals about epilepsy, and providing education for people with epilepsy and their families.6
Within the VA, health care for veterans with epilepsy is changing in part by the Epilepsy Centers of Excellence (ECoC), established by federal law. The ECoE’s primary missions are to improve quality of and access to epilepsy specialty care to improve the health and well-being of veteran patients with epilepsy and other seizure disorders through integration of clinical care, outreach, research, and education to VA providers and patients.7
The goal of this article is to outline the key elements of quality epilepsy care and make recommendations for providing quality care in the VA health care system.
Diagnosis and Seizure Types
Quality care for veterans with epilepsy begins with the provider reviewing pertinent history and establishing the clinical characteristics of the patient’s seizures and epilepsy. The provider should ask about the first signs of the seizure or warning (aura), the seizure (ictal period), and the period after the seizure (postictal period). Seizure histories from the patient and observers are critical.
The first step is to define whether the patient’s seizures are generalized, that is, start all over the brain at once, or focal, starting in one area of the brain. The patient’s initial sensation at the onset of a seizure (aura) may help localize onset and define focal seizures. For example, déjà vu sensations often point to seizure onset in the mesial temporal lobe and hippocampus. Focal seizures can spread and cause cognitive dysfunction, including aphasia and amnesia, or evolve into a generalized convulsion (tonic-clonic seizure). Many patients present with a generalized tonic-clonic seizure and have had brief focal seizures that were not considered seizures by the patient or by other providers. This seizure type should be clarified by asking specifically about paroxysmal symptoms. For example, brief periods of confusion that are episodic may be focal seizures. In general, focal seizures are stereotyped and may have a feature that helps in establishing the diagnosis. Many temporal lobe seizures are associated with lip smacking behaviors (oral buccal automatisms).
Tonic-clonic seizures may begin without an aura and are generalized from onset. Patients with this type of seizure may have electroencephalogram (EEG) findings that define a generalized abnormality, which consist of frontocentral spike and wave discharges in the EEG. In the VA population, the first generalized tonic-clonic seizure may occur while in the military. Some of these patients have juvenile myoclonic epilepsy, and a history of brief jerks on waking (myoclonus) may have been occurring but not recognized as seizures. The treatment of seizures, in part, depends on whether they begin focally or are generalized at onset.
Often people with epilepsy have multiple seizure types. The types of seizures should be documented and, if possible, corroborated by a witness. Epileptic seizures tend to be stereotyped and of relatively brief duration, usually < 2 minutes. The period after a seizure may be followed by a more prolonged period of neurologic dysfunction that includes confusion and fatigue. These symptoms may be the only indication that the patient has had a seizure.
At each clinic visit, the characteristics of the patient’s seizures should be reviewed and the frequency of seizures documented. A calendar to track seizure frequency is helpful to understand precipitating factors and response to treatment.
The health care provider (HCP) should look for the cause of a patient’s epilepsy. It is important to ask the patient about family history, age of first seizure, occurrence of febrile seizures, developmental history, past history of meningitis or encephalitis, history of childhood seizures or spells, and history of brain lesions, including tumors, strokes, or TBI. Most patients with epilepsy do not have a clear cause for their epilepsy, but the cause may be clarified with EEG and magnetic resonance imaging (MRI) testing.
EEG and Brain Imaging
All patients with epilepsy should be evaluated with an EEG, and for those with focal epilepsy or undefined epilepsy, with an imaging study of the brain, preferably an MRI. These results should be reviewed at each visit. The EEG may show focal features that are related to neurophysiologic dysfunction, such as slowing that is not definitely epileptiform in character, or show focal spike or sharp waves that are epileptiform in character. Generalized abnormalities may include generalized slowing that is not an epileptiform feature or frontocentral spike wave patterns that are epileptiform in character. The EEG cannot rule out epilepsy, but can rule in the likelihood of epilepsy when definite epileptiform features are present.
Brain imaging can define many conditions that can cause focal epilepsy, and an MRI is more sensitive for defining a number of these conditions (cavernous angiomas, hippocampal sclerosis, developmental migration disorders, and low-grade neoplasms). Significant trauma with signal abnormalities to suggest prior bleeding predispose to epilepsy. When patients are refractory to medical therapy and have imaging findings concordant with EEG onset of seizures, then surgery can be a better treatment.
Adverse Effects
Broad-spectrum drug treatments are efficacious for either generalized or focal seizures, whereas narrow-spectrum treatments are most efficacious for focal seizures (Table 1). The choice of a seizure medication is based on the patient’s seizure type(s) and other comorbid conditions.7 For example, a patient with epilepsy and migraines may do better with a seizure medication that also is used for migraine prophylaxis (valproate or topiramate). In general, seizure control is unlikely to be achieved if patients fail the first 2 medications tried.8 Treating with > 1 medication may improve seizure control but may increase AEs. A review of current seizure medications and their AEs can be found on the ECoE website (http://www.epilepsy.va.gov/Provider_Education.asp).
In VA cooperative studies that evaluated seizure medications, the most common reason for discontinuing a drug was the combination of ineffectiveness and AEs.9-11 Addressing AEs is a quality measure for the care of patients with epilepsy. Adverse effects may be dose dependent or idiosyncratic (rashes). Drug levels may help in determining dose-dependent AEs; for example, diplopia with carbamazepine levels above 10 μg/mL. Each patient may have susceptibility to medication AEs that do not exactly match therapeutic levels. When patients have AEs, a reduction in dose or trial of an alternative medication is advised.
Uncontrollable Epilepsy
About one-third of people with epilepsy have uncontrolled seizures, known as medically intractable epilepsy, which may be identified early in their clinical course by failure of the first 2 tolerated medications.8 Patients should be referred to an epilepsy center so their epilepsy can be defined by video EEG monitoring to capture seizures. Unfortunately, in the VA system, this route is often delayed, and patients may not be diagnosed appropriately for years.12 Some of these patients may be considered treatment failures because the right medications were not tried (eg, generalized epilepsy that is treated with narrow-spectrum seizure medications). Juvenile myoclonic epilepsy often may not be controlled by phenytoin or carbamazepine, but valproate, lamotrigine, levetiracetam, and zonisamide may be more effective.
Other patients may not have epilepsy but have psychogenic nonepileptic seizures (PNES). These behavioral seizures do not have an EEG epileptiform correlate. About 25% of patients who undergo prolonged video EEG monitoring have PNES, and seizure medications do not treat these events.12 A smaller percentage of patients have both epileptic and nonepileptic seizures (5%-15%). Psychogenic nonepileptic seizures often occur within the context of traumatic exposure(s) or previous physical or sexual abuse.
In the VA population, PNES is more often associated with PTSD or head trauma history than in patients with epilepsy.13,14 To confirm the diagnosis of PNES, video-EEG capture of the patient’s seizures is required. Because of the increased number of combat veterans with TBI and PTSD, the diagnosis of epilepsy may be difficult without video-EEG monitoring. Management consists of addressing the underlying conversion disorder and recognition and treatment of comorbidities, such as mood, anxiety, personality, or PTSD. Recently, cognitive behavioral-informed psychotherapy (CB-ip) has been shown to be effective in patients with PNES and is available through the VA national telemental health center and at some ECoE sites.15
If a patient with uncontrolled epilepsy has focal seizures, surgical therapy is more likely to result in seizure control than will medical therapy.16,17 This is especially true when other testing, including MRI, positron emission tomography, and neuropsychiatric evaluation, point to a concordance of localization. These patients should be evaluated in a center that can provide surgical therapy and if necessary also record seizures with invasive techniques using electrodes placed directly over the cortex or into the brain to sample deeper structures like the hippocampus or amygdala. Patients who are refractory should be considered for reevaluation every 2 years by a comprehensive epilepsy center.
Unfortunately, some patients have seizures that begin in eloquent cortex, which if removed, leads to undesirable neurologic loss or multifocal seizure onset. In these patients, seizure frequency can be reduced by vagus nerve stimulation or intracranial responsive neurostimulation.18,19
Safety
Epilepsy has inherent risks for injury. Patients and their families often need to be informed about risks and risky behaviors to avoid. A frank discussion about safety is prudent. What to do for the patient during a seizure should be addressed. For convulsive seizures: Protect the patient from injury by placing something soft between the patient’s head and the floor, keep the patient on his or her side; do not restrain the patient or put anything in the mouth; stay calm and time the seizure; as the patient gains consciousness, talk to the patient and be reassuring. For nonconvulsive seizures: Stay with the patient; time the seizure; gently guide the patient away from dangerous situations like streets or stairs; stay with the patient until he or she is back to normal, and reassure the patient.
Driving
People with epilepsy identify driving as one of their major concerns; therefore, it is important for HCPs to properly counsel patients with seizure disorders and their families about driving (Figure).20 In general people with controlled seizures are permitted to drive in every state in the U.S., but people with uncontrolled seizures are restricted from licensure. Despite the desire and necessity to drive for many individuals with epilepsy, seizures while driving pose risks for crashes, which may result in property damage, injuries, and death.21 Factors, such as duration of seizure freedom, help predict the risk for crashes. The legal rules for determining control and administering restrictions are a complex mix of federal and state laws, regulations, and local practices, which vary widely across the country.21,22 The standards also change over time; updated information is available from local state authorities and on good informational sites, such as those of the Epilepsy Foundation.
The key standard for determining accident risks is the seizure free interval, which is the duration of time a person with epilepsy has been seizure-free.21-23 In the U.S., the accepted period for seizure freedom varies from about 3 months to 12 months, depending on individual state rules.24
California, Delaware, Nevada, New Jersey, Oregon, and Pennsylvania require mandatory reporting. Generally physician groups in the U.S. and elsewhere oppose such mandatory reporting, because of the concern that their patients will not report their seizures, and thus may not receive appropriate treatment. Indeed, patients with epilepsy often do not tell physicians about their seizures, fearing loss of driving privileges and other social consequences.21,23 Providers should make an effort to determine seizure frequency and whether the patient is being truthful. This information then provides a background for the provider to discuss driving issues.
Injury
People with epilepsy are susceptible to injury during a seizure and need to be counseled regarding safety, particularly when seizures are not well controlled. Hazardous situations include being near stoves or cooking, bathing alone, swimming alone, working at heights without a safety harness, and using power tools.26
Sudden Unexplained Death
Patients with recurrent seizures have an increased risk for accidental fatality and for sudden unexplained death in epilepsy (SUDEP), which accounts for up to 17% of all deaths in people with epilepsy. The risk for sudden death from recurrent seizures increases 2.3 times compared with the risk in the general population.25 A SUDEP is an unexpected death in a person who has epilepsy with no other obvious cause of death.26 Because increased seizure frequency, the presence of tonic-clonic seizures, and other accidental risks of seizures are associated with SUDEP, the subject should be discussed with patients and their families, to encourage adherence to treatment. Epileptologists also discuss these risks with patients and their families when surgical interventions are being considered. The potential risks for injury or SUDEP may offset the surgical risks when pursuing a potentially curative epilepsy procedure.
Women of Childbearing Age
In January 2015, the ECoE started a women veterans epilepsy workgroup with the goal of improving clinical care within the VAHCS to provide education to patients, family members, and VA health care providers about the care of women with epilepsy.
Providers need to be aware that seizure medications that induce certain hepatic enzymes can lead to hormonal contraceptive failure (Table 2).27 Preconception folic acid supplementation (with at least 0.4 mg) should be considered, because it may reduce the risk of major congenital malformations.28 The goal of epilepsy management prior to conception is to maximize seizure control with the optimal seizure medication to avoid the need to make changes during the pregnancy.
During pregnancy, the volume of distribution increases and seizure medication metabolism may change requiring dose adjustment. The best predictor of seizure frequency during pregnancy is a woman’s epilepsy pattern prior to conception. Seizure freedom for 9 months prior to conception is associated with a 84% to 92% likelihood of seizure freedom throughout the pregnancy.29
International seizure medication pregnancy registries have provided valuable information regarding the risk of major congenital malformation (MCM) of development, which seems to be a consequence of seizure medication therapy and not epilepsy itself. The risk of MCM associated with seizure medication therapy is about 4% to 5% compared with 1.5% to 3% in the general population.30,31 A seizure medication table that supplements the existing VA ECoE information specifically addresses women’s issues with the recognition that recent revisions to the teratogenicity classification have been made by the FDA (Table 2).32 If possible, valproate should be avoided during pregnancy due to its higher rate of MCM and impact on neurocognitive function.33 Obstetrical input is essential in arranging routine prenatal fetal testing. Although women with epilepsy do not have a substantially increased risk of undergoing a cesarean section, delivery in a hospital obstetric unit is advised.
Postpartum women veterans with epilepsy should be encouraged to breast feed since the potential benefits seem to outweigh any established risk of seizure medication exposure to the infant. No relative impact on cognition was found in breastfed infants exposed to a variety of seizure medications.34 Following delivery, vigilance is needed to monitor for sleep deprivation, postpartum depression, and the safe care of the infant.35 Care of women with epilepsy does not end with pregnancy planning, additional important topics include psychiatric comorbidities, catamenial epilepsy, and bone health, which are unique to women veterans with epilepsy.
Identifying Psychiatric Conditions
People with epilepsy have a number of psychiatric comorbidities. Suicide and suicide attempts are 6 to 25 times more common in patients with temporal lobe epilepsy compared with those in the general population.36-38 Although the FDA identified all seizure medications as potential contributors to suicide risk, a recent longitudinal study of suicidal ideation and attempt found that those who received seizure medications were more likely to have suicidal ideation and attempt than those who did not received seizure medications, suggesting that medication may relate to baseline depression or suicidal ideation.39 When seeing patients with epilepsy, screening for suicidal ideation is good practice.
Depression and anxiety disorders are the most common psychiatric comorbidities in people with epilepsy.40,41 About half of people with epilepsy have symptoms of depression, and 40% have anxiety.42 Depression often precedes the diagnosis of epilepsy, and anxiety often is present and related to the fear of having seizures and of social embarrassment.43 People with epilepsy may not self-report these symptoms if not asked directly. Identification of comorbid depression and anxiety should lead to appropriate treatment. The CB-ip being used for PNES also is being used for treatment of epilepsy and its comorbidities.44
Mild traumatic brain injury (mTBI) has a small increased risk of epilepsy.45 Veterans with mTBI that occurs in the context of blasts are set up for the development of PTSD. These veterans may have other mild cognitive symptoms that can be confused with seizures. Furthermore, mTBI and PNES often occur together, more so than do mTBI and epileptic seizures.14 Video-EEG monitoring may be warranted for these patients.
Education and Self-Management
The IOM report on epilepsy identified patient and family education as essential for better epilepsy care.6 Providers should help educate patients about their epilepsy and refer them to resources available online (Table 3). A continuing exchange about their condition and treatment with seizure medications should occur with each visit. People with epilepsy should also receive guidance regarding how to manage their epilepsy and day-to-day issues. Referring, patients to social workers, psychologists, vocational rehabilitation services, and support groups can enhance a patient’s QOL.3,6 The stigma of epilepsy is another burden that can be diminished by attending support groups. Recently, being a part of an online patient community of veterans was found to improve self-management.46
Conclusion
People with epilepsy have many issues that are unique to the condition and, in part, are related to the unpredictable occurrence of seizures and loss of function. Ideally, seizure control provides a normal lifestyle; however, some mood and anxiety comorbidities may persist despite seizure control. Care in the VA system includes access to 16 sites that have programs dedicated to treating veterans with epilepsy and many more consortium sites that interact with the ECoE to provide high-quality patient care (http:\\www.epilepsy.va.gov). The ECoE also provides a readily available resource to optimally manage veterans with epilepsy. Attention to the issues addressed in this article will promote quality care for veterans with epilepsy.
1. Rehman R, Kelly P, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762.
2. National Committee for Quality Assurance (NCQA). The essential guide to health care quality. https://www.ncqa.org/Portals/0/Publications/Resource%20Library/NCQA_Primer_web.pdf. Accessed August 9, 2016.
3. Pugh MJ, Berlowitz DR, Montouris GB, et al. What constitutes high quality of care for adults with epilepsy? Neurology. 2007;69(21):2020-2027.
4. Fountain NB, Van Ness PC, Swain-Eng R, Tonn S, Bever CT Jr; American Academy of Neurology Epilepsy Measure Development Panel and the American Medical Association-Convened Physician Consortium for Performance Improvement Independent Measure Development Process. Quality improvement in neurology: AAN epilepsy quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology. 2011;76(1):94-99.
5. Fountain NB, Van Ness PC, Bennett A, et al. Quality improvement in neurology: epilepsy update quality measurement set. Neurology. 2015;84(14):1483-1487.
6. England MJ, Liverman CT, Schultz AM, Strawbridge LM, eds; Committee on the Public Health Dimensions of the Epilepsies, Board on Health Sciences Policy, Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012.
7. Tortorice K, Rutecki P. Principles of Treatment. In: Hussain, AM, Tran TT, eds. Department of Veterans Affairs Epilepsy Manual. San Francisco, CA: Epilepsy Centers of Excellence, Department of Veteran Affairs; 2014:120-127.
8. Kwan P, Brodie MJ. Early Identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
9. Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Eng J Med. 1985;313(3):145-151.
10. Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Eng J Med. 1992;327(11):765-771.
11. Rowan AJ, Ramsay RE, Collins JF, et al; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868-1873.
12. Salinsky M, Spencer D, Boudreau E, Ferguson F. Psychogenic nonepileptic seizures in US veterans. Neurology. 2011;77(10):945-950.
13. Salinsky M, Evrard C, Storzbach D, Pugh MJ. Psychiatric comorbidity in veterans with psychogenic seizures. Epilepsy Behav. 2012;25(3):345-349.
14. Salinsky M, Storzbach D, Goy E, Evrard C. Traumatic brain injury and psychogenic seizures in veterans. J Head Trauma Rehabil. 2015;30(1):E65-E70.
15. LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
16. Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, control trial for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318.
17. Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003;44(6):741-751.
18. Morris GL III, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy. report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453-1459.
19. Morrell M; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304.
20. Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia. 1997;38(2):233-236.
21. Krumholz A. Driving issues in epilepsy: past, present, and future. Epilepsy Curr. 2009;9(2):31-35.
22. Krauss GL, Ampaw L, Krumholz A. Individual state driving restrictions for people with epilepsy in the US. Neurology. 2001;57(10):1780-1785.
23. Krauss GL, Krumholz A, Carter RC, Kaplan P. Risk factors for seizure-related motor vehicle crashes in patients with epilepsy. Neurology. 1999;52(7):1324-1329.
24. Consensus statements, sample statutory provisions, and model regulations regarding driver licensing and epilepsy. American Academy of Neurology. American Epilepsy Society, Epilepsy Foundation of America. Epilepsia. 1994:35(3):696-705.
25. Cavazos, JE. SUDEP and Other Risks of Seizures. In: Husain AM, Tran, TT, eds. VA Epilepsy Manual. San Francisco, CA: Epilepsy Centers of Excellence, Department of Veteran Affairs; 2014:206-209.
26. Tolstykh GP, Cavazos JE. Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav. 2013;26(3):410-414.
27. Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception. 2011;83(1):16-29.
28. Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2009;73(2):142-149.
29. Harden CL, Hopp J, Ting TY, et al; American Academy of Neurology; American Epilepsy Society. Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): 1. Obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1229-1236.
30. Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64(11):1874-1878.
31. Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77(2):193-198.
32. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Published December 3, 2014. Accessed June 27, 2016.
33. Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
34. Meador KH, Baker GA, Browning N, et al; NEAD Study Group. Effects of breastfeeding in children of women taking antiepileptic drugs. Neurology. 2010;75(22):1954-1960.
35. Klein A. The postpartum period in women with epilepsy. Neurol Clin. 2012;30(3):867-875.
36. Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
37. Jones JE, Hermann BP, Barry JJ, Gilliam FG, Kanner AM, Meador KJ. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2013;4(suppl 3):S31-S38.
38. Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol. 2007;6(8):693-698.
39. Pugh MJ, Hesdorffer D, Wang CP, et al. Temporal trends in new exposure to antiepileptic drug monotherapy and suicide-related behavior. Neurology. 2013;81(22):1900-1906.
40. Barry JJ, Ettinger AB, Friel P, et al; Advisory Group of the Epilepsy Foundation as part of its Mood Disorder. Consensus statement: the evaluation and treatment of people with epilepsy and affective disorders. Epilepsy Behav. 2008;13(suppl 1):S1-S29.
41. Ottman R, Lipton RB, Ettinger AB, et al. Comorbidities of epilepsy: results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011;52(2):308-315.
42. Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanism, and treatment. Biol Psychiatry. 2003;54(3):388-398.
43. Kanner AM. The treatment of depressive disorders in epilepsy: what all neurologists should know. Epilepsia. 2013;54(suppl 1):3-12.
44. Reiter JM, Andrews DJ. A neurobehavioral approach for treatment of complex partial epilepsy: efficacy. Seizure. 2000;9(3):198-203.
45. Pugh MJ, Orman JA, Jaramillo CA, et al. The prevalence of epilepsy and association with traumatic brain Injury in Veterans of the Afghanistan and Iraq Wars. J Head Trauma Rehabil. 2015;30(1):29-37.
46. Hixson JD, Barnes D, Parko K, et al. Patients optimizing epilepsy management via an online community: the POEM Study. Neurology. 2015;85(2):129-136.
47. Winterfeld U, Merlob P, Baud D, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology. 2016;86(24):2251-2257.
1. Rehman R, Kelly P, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762.
2. National Committee for Quality Assurance (NCQA). The essential guide to health care quality. https://www.ncqa.org/Portals/0/Publications/Resource%20Library/NCQA_Primer_web.pdf. Accessed August 9, 2016.
3. Pugh MJ, Berlowitz DR, Montouris GB, et al. What constitutes high quality of care for adults with epilepsy? Neurology. 2007;69(21):2020-2027.
4. Fountain NB, Van Ness PC, Swain-Eng R, Tonn S, Bever CT Jr; American Academy of Neurology Epilepsy Measure Development Panel and the American Medical Association-Convened Physician Consortium for Performance Improvement Independent Measure Development Process. Quality improvement in neurology: AAN epilepsy quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology. 2011;76(1):94-99.
5. Fountain NB, Van Ness PC, Bennett A, et al. Quality improvement in neurology: epilepsy update quality measurement set. Neurology. 2015;84(14):1483-1487.
6. England MJ, Liverman CT, Schultz AM, Strawbridge LM, eds; Committee on the Public Health Dimensions of the Epilepsies, Board on Health Sciences Policy, Institute of Medicine. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012.
7. Tortorice K, Rutecki P. Principles of Treatment. In: Hussain, AM, Tran TT, eds. Department of Veterans Affairs Epilepsy Manual. San Francisco, CA: Epilepsy Centers of Excellence, Department of Veteran Affairs; 2014:120-127.
8. Kwan P, Brodie MJ. Early Identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319.
9. Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Eng J Med. 1985;313(3):145-151.
10. Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Eng J Med. 1992;327(11):765-771.
11. Rowan AJ, Ramsay RE, Collins JF, et al; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868-1873.
12. Salinsky M, Spencer D, Boudreau E, Ferguson F. Psychogenic nonepileptic seizures in US veterans. Neurology. 2011;77(10):945-950.
13. Salinsky M, Evrard C, Storzbach D, Pugh MJ. Psychiatric comorbidity in veterans with psychogenic seizures. Epilepsy Behav. 2012;25(3):345-349.
14. Salinsky M, Storzbach D, Goy E, Evrard C. Traumatic brain injury and psychogenic seizures in veterans. J Head Trauma Rehabil. 2015;30(1):E65-E70.
15. LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
16. Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, control trial for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318.
17. Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003;44(6):741-751.
18. Morris GL III, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy. report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453-1459.
19. Morrell M; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304.
20. Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia. 1997;38(2):233-236.
21. Krumholz A. Driving issues in epilepsy: past, present, and future. Epilepsy Curr. 2009;9(2):31-35.
22. Krauss GL, Ampaw L, Krumholz A. Individual state driving restrictions for people with epilepsy in the US. Neurology. 2001;57(10):1780-1785.
23. Krauss GL, Krumholz A, Carter RC, Kaplan P. Risk factors for seizure-related motor vehicle crashes in patients with epilepsy. Neurology. 1999;52(7):1324-1329.
24. Consensus statements, sample statutory provisions, and model regulations regarding driver licensing and epilepsy. American Academy of Neurology. American Epilepsy Society, Epilepsy Foundation of America. Epilepsia. 1994:35(3):696-705.
25. Cavazos, JE. SUDEP and Other Risks of Seizures. In: Husain AM, Tran, TT, eds. VA Epilepsy Manual. San Francisco, CA: Epilepsy Centers of Excellence, Department of Veteran Affairs; 2014:206-209.
26. Tolstykh GP, Cavazos JE. Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav. 2013;26(3):410-414.
27. Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception. 2011;83(1):16-29.
28. Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology; American Epilepsy Society. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2009;73(2):142-149.
29. Harden CL, Hopp J, Ting TY, et al; American Academy of Neurology; American Epilepsy Society. Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): 1. Obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1229-1236.
30. Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64(11):1874-1878.
31. Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77(2):193-198.
32. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm. Published December 3, 2014. Accessed June 27, 2016.
33. Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605.
34. Meador KH, Baker GA, Browning N, et al; NEAD Study Group. Effects of breastfeeding in children of women taking antiepileptic drugs. Neurology. 2010;75(22):1954-1960.
35. Klein A. The postpartum period in women with epilepsy. Neurol Clin. 2012;30(3):867-875.
36. Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205-228.
37. Jones JE, Hermann BP, Barry JJ, Gilliam FG, Kanner AM, Meador KJ. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2013;4(suppl 3):S31-S38.
38. Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol. 2007;6(8):693-698.
39. Pugh MJ, Hesdorffer D, Wang CP, et al. Temporal trends in new exposure to antiepileptic drug monotherapy and suicide-related behavior. Neurology. 2013;81(22):1900-1906.
40. Barry JJ, Ettinger AB, Friel P, et al; Advisory Group of the Epilepsy Foundation as part of its Mood Disorder. Consensus statement: the evaluation and treatment of people with epilepsy and affective disorders. Epilepsy Behav. 2008;13(suppl 1):S1-S29.
41. Ottman R, Lipton RB, Ettinger AB, et al. Comorbidities of epilepsy: results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011;52(2):308-315.
42. Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanism, and treatment. Biol Psychiatry. 2003;54(3):388-398.
43. Kanner AM. The treatment of depressive disorders in epilepsy: what all neurologists should know. Epilepsia. 2013;54(suppl 1):3-12.
44. Reiter JM, Andrews DJ. A neurobehavioral approach for treatment of complex partial epilepsy: efficacy. Seizure. 2000;9(3):198-203.
45. Pugh MJ, Orman JA, Jaramillo CA, et al. The prevalence of epilepsy and association with traumatic brain Injury in Veterans of the Afghanistan and Iraq Wars. J Head Trauma Rehabil. 2015;30(1):29-37.
46. Hixson JD, Barnes D, Parko K, et al. Patients optimizing epilepsy management via an online community: the POEM Study. Neurology. 2015;85(2):129-136.
47. Winterfeld U, Merlob P, Baud D, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology. 2016;86(24):2251-2257.