User login
Diagnosis of Prostate Cancer and Prostate-specific Antigen Level on Initial Prostate Biopsy: Does Race Matter?
Objective
To determine whether Black Veterans are at higher risk for prostate cancer diagnosis on their first prostate biopsy compared to non-Hispanic White (White) Veterans.
Background
Prostate-specific antigen (PSA) testing is widely used to screen for prostate cancer. Although men of African ancestry display an increased incidence of prostate cancer and more aggressive disease, specific PSA thresholds for biopsy referral have yet to be proposed for this population.
Methods
We used the VHA’s electronic medical record data to collect Veterans’ demographic and clinical characteristics including self-identified race/ethnicity, age, date of first prostate biopsy, PSA results, and prostate cancer diagnosis. Veterans’ ZIP code of residence was used to determine urban/rural status, income, and education. We estimated multivariable logistic regression models to predict the likelihood of prostate cancer diagnosis on the first biopsy using race, baseline PSA, age at first PSA test, age at initial biopsy, smoking status, use of statins, and socioeconomic factors as predictors. We calculated adjusted predicted probabilities of cancer detection on the first prostate biopsy from the logistic models at different PSA levels.
Results
We identified 246,056 White and 71,653 Black Veterans who underwent their first prostate biopsy through February 28, 2020 and who had no previous prostate cancer diagnosis or treatment prior to that biopsy. Black Veterans appeared to receive their first PSA test four years earlier and undergo their first prostate biopsy two years earlier than their White counterparts (median age of 57 vs. 61 and 63 vs. 65, respectively). After controlling for selected covariates, we found that Black Veterans were 52% more likely to be diagnosed with prostate cancer on their first prostate biopsy compared to White Veterans (OR 1.52, 95% CI 1.49-1.55). Our model indicated that a Black Veteran with a PSA of 4.0 ng/ml has an equivalent risk of prostate cancer detection as a White Veteran with a PSA of 9.7 ng/ml.
Implications
Our findings suggested that developing a risk-based PSA threshold for referral to prostate biopsy may lead to earlier diagnosis of clinically significant prostate cancer in a population of Veterans known to have an increased incidence and risk of aggressive disease.
Objective
To determine whether Black Veterans are at higher risk for prostate cancer diagnosis on their first prostate biopsy compared to non-Hispanic White (White) Veterans.
Background
Prostate-specific antigen (PSA) testing is widely used to screen for prostate cancer. Although men of African ancestry display an increased incidence of prostate cancer and more aggressive disease, specific PSA thresholds for biopsy referral have yet to be proposed for this population.
Methods
We used the VHA’s electronic medical record data to collect Veterans’ demographic and clinical characteristics including self-identified race/ethnicity, age, date of first prostate biopsy, PSA results, and prostate cancer diagnosis. Veterans’ ZIP code of residence was used to determine urban/rural status, income, and education. We estimated multivariable logistic regression models to predict the likelihood of prostate cancer diagnosis on the first biopsy using race, baseline PSA, age at first PSA test, age at initial biopsy, smoking status, use of statins, and socioeconomic factors as predictors. We calculated adjusted predicted probabilities of cancer detection on the first prostate biopsy from the logistic models at different PSA levels.
Results
We identified 246,056 White and 71,653 Black Veterans who underwent their first prostate biopsy through February 28, 2020 and who had no previous prostate cancer diagnosis or treatment prior to that biopsy. Black Veterans appeared to receive their first PSA test four years earlier and undergo their first prostate biopsy two years earlier than their White counterparts (median age of 57 vs. 61 and 63 vs. 65, respectively). After controlling for selected covariates, we found that Black Veterans were 52% more likely to be diagnosed with prostate cancer on their first prostate biopsy compared to White Veterans (OR 1.52, 95% CI 1.49-1.55). Our model indicated that a Black Veteran with a PSA of 4.0 ng/ml has an equivalent risk of prostate cancer detection as a White Veteran with a PSA of 9.7 ng/ml.
Implications
Our findings suggested that developing a risk-based PSA threshold for referral to prostate biopsy may lead to earlier diagnosis of clinically significant prostate cancer in a population of Veterans known to have an increased incidence and risk of aggressive disease.
Objective
To determine whether Black Veterans are at higher risk for prostate cancer diagnosis on their first prostate biopsy compared to non-Hispanic White (White) Veterans.
Background
Prostate-specific antigen (PSA) testing is widely used to screen for prostate cancer. Although men of African ancestry display an increased incidence of prostate cancer and more aggressive disease, specific PSA thresholds for biopsy referral have yet to be proposed for this population.
Methods
We used the VHA’s electronic medical record data to collect Veterans’ demographic and clinical characteristics including self-identified race/ethnicity, age, date of first prostate biopsy, PSA results, and prostate cancer diagnosis. Veterans’ ZIP code of residence was used to determine urban/rural status, income, and education. We estimated multivariable logistic regression models to predict the likelihood of prostate cancer diagnosis on the first biopsy using race, baseline PSA, age at first PSA test, age at initial biopsy, smoking status, use of statins, and socioeconomic factors as predictors. We calculated adjusted predicted probabilities of cancer detection on the first prostate biopsy from the logistic models at different PSA levels.
Results
We identified 246,056 White and 71,653 Black Veterans who underwent their first prostate biopsy through February 28, 2020 and who had no previous prostate cancer diagnosis or treatment prior to that biopsy. Black Veterans appeared to receive their first PSA test four years earlier and undergo their first prostate biopsy two years earlier than their White counterparts (median age of 57 vs. 61 and 63 vs. 65, respectively). After controlling for selected covariates, we found that Black Veterans were 52% more likely to be diagnosed with prostate cancer on their first prostate biopsy compared to White Veterans (OR 1.52, 95% CI 1.49-1.55). Our model indicated that a Black Veteran with a PSA of 4.0 ng/ml has an equivalent risk of prostate cancer detection as a White Veteran with a PSA of 9.7 ng/ml.
Implications
Our findings suggested that developing a risk-based PSA threshold for referral to prostate biopsy may lead to earlier diagnosis of clinically significant prostate cancer in a population of Veterans known to have an increased incidence and risk of aggressive disease.
Racial Disparities in Treatment and Survival for Early-Stage Non-Small Cell Lung Cancer: Is Equal Access Health Care System the Answer?
Background
Survival for early-stage non-small cell lung cancer (NSCLC) has dramatically improved with advancement in surgical and radiation techniques over last two decades but there exists a disparity for African Americans (AA) having worse overall survival (OS) in recent studies on the general US population. We studied this racial disparity in Veteran population.
Methods
Data for 2589 AA and 14184 Caucasian Veterans diagnosed with early-stage (I, II) NSCLC between 2011-2017 was obtained from the Cancer Cube Registry (VACCR). IRB approval was obtained.
Results
The distribution of newly diagnosed cases of Stage I (73.92% AA vs 74.71% Caucasians) and Stage II (26.07% vs 25.29%) between the two races was comparable (p = .41). More Caucasians were diagnosed above the age of 60 compared to AA (92.22% vs 84.51%, p < .05). More AA were diagnosed with adenocarcinoma at diagnosis (56.01% vs 45.88% Caucasians, p < .05) for both Stage I and II disease. For the limited number of Veterans with reported performance status (PS), similar proportion of patients had a good PS defined as ECOG 0-2 among the two races (93.70% AA vs 93.97% Caucasians, p = .73). There was no statistically significant difference between 5-year OS for AA and Caucasians (69.81% vs 70.78%, p = .33) for both Stage I and II NSCLC. Both groups had similar rate of receipt of surgery as first line treatment or in combination with other treatments (58.90% AA vs 59.07% Caucasians, p = .90). Similarly, the rate of receiving radiation therapy was comparable between AA and Caucasians (42.4% vs 42.3%, p = .96). Although both races showed improved 5-year OS after surgery, there was no statistical difference in survival benefit between AA and Caucasians (69.8% vs 70.8%, p = .33).
Conclusion
In contrast to the studies assessing general US population trends, there was no racial disparity for 5-year OS in early-stage NSCLC for the Veteran population. This points to the inequities in access to treatment and preventive healthcare services as a possible contributing cause to the increased mortality in AA in general US population and a more equitable healthcare delivery within the VHA system.
Background
Survival for early-stage non-small cell lung cancer (NSCLC) has dramatically improved with advancement in surgical and radiation techniques over last two decades but there exists a disparity for African Americans (AA) having worse overall survival (OS) in recent studies on the general US population. We studied this racial disparity in Veteran population.
Methods
Data for 2589 AA and 14184 Caucasian Veterans diagnosed with early-stage (I, II) NSCLC between 2011-2017 was obtained from the Cancer Cube Registry (VACCR). IRB approval was obtained.
Results
The distribution of newly diagnosed cases of Stage I (73.92% AA vs 74.71% Caucasians) and Stage II (26.07% vs 25.29%) between the two races was comparable (p = .41). More Caucasians were diagnosed above the age of 60 compared to AA (92.22% vs 84.51%, p < .05). More AA were diagnosed with adenocarcinoma at diagnosis (56.01% vs 45.88% Caucasians, p < .05) for both Stage I and II disease. For the limited number of Veterans with reported performance status (PS), similar proportion of patients had a good PS defined as ECOG 0-2 among the two races (93.70% AA vs 93.97% Caucasians, p = .73). There was no statistically significant difference between 5-year OS for AA and Caucasians (69.81% vs 70.78%, p = .33) for both Stage I and II NSCLC. Both groups had similar rate of receipt of surgery as first line treatment or in combination with other treatments (58.90% AA vs 59.07% Caucasians, p = .90). Similarly, the rate of receiving radiation therapy was comparable between AA and Caucasians (42.4% vs 42.3%, p = .96). Although both races showed improved 5-year OS after surgery, there was no statistical difference in survival benefit between AA and Caucasians (69.8% vs 70.8%, p = .33).
Conclusion
In contrast to the studies assessing general US population trends, there was no racial disparity for 5-year OS in early-stage NSCLC for the Veteran population. This points to the inequities in access to treatment and preventive healthcare services as a possible contributing cause to the increased mortality in AA in general US population and a more equitable healthcare delivery within the VHA system.
Background
Survival for early-stage non-small cell lung cancer (NSCLC) has dramatically improved with advancement in surgical and radiation techniques over last two decades but there exists a disparity for African Americans (AA) having worse overall survival (OS) in recent studies on the general US population. We studied this racial disparity in Veteran population.
Methods
Data for 2589 AA and 14184 Caucasian Veterans diagnosed with early-stage (I, II) NSCLC between 2011-2017 was obtained from the Cancer Cube Registry (VACCR). IRB approval was obtained.
Results
The distribution of newly diagnosed cases of Stage I (73.92% AA vs 74.71% Caucasians) and Stage II (26.07% vs 25.29%) between the two races was comparable (p = .41). More Caucasians were diagnosed above the age of 60 compared to AA (92.22% vs 84.51%, p < .05). More AA were diagnosed with adenocarcinoma at diagnosis (56.01% vs 45.88% Caucasians, p < .05) for both Stage I and II disease. For the limited number of Veterans with reported performance status (PS), similar proportion of patients had a good PS defined as ECOG 0-2 among the two races (93.70% AA vs 93.97% Caucasians, p = .73). There was no statistically significant difference between 5-year OS for AA and Caucasians (69.81% vs 70.78%, p = .33) for both Stage I and II NSCLC. Both groups had similar rate of receipt of surgery as first line treatment or in combination with other treatments (58.90% AA vs 59.07% Caucasians, p = .90). Similarly, the rate of receiving radiation therapy was comparable between AA and Caucasians (42.4% vs 42.3%, p = .96). Although both races showed improved 5-year OS after surgery, there was no statistical difference in survival benefit between AA and Caucasians (69.8% vs 70.8%, p = .33).
Conclusion
In contrast to the studies assessing general US population trends, there was no racial disparity for 5-year OS in early-stage NSCLC for the Veteran population. This points to the inequities in access to treatment and preventive healthcare services as a possible contributing cause to the increased mortality in AA in general US population and a more equitable healthcare delivery within the VHA system.
Survival Analysis of Untreated Early-Stage Non-Small Cell Lung Cancer (NSCLC) in a Veteran Population
Introduction
Veterans with early-stage NSCLC who do not receive any form of treatment have been shown to have a worse overall survival compared to those who receive treatment. Factors that may influence the decision to administer treatment including age, performance status (PS), comorbidities, and racial disparity have not been assessed on a national level in recent years.
Methods
Data for 31,966 veterans diagnosed with early-stage (0, I) NSCLC between 2003-2017 was obtained from the Cancer cube registry (VACCR). IRB approval was obtained.
Results
Patients were divided into treatment (26,833/31,966, 83.16%) and no-treatment group (3096/31966, 9.68%). Of the no-treatment group, 3004 patients were stage I and 92 were stage 0 whereas in the treatment group, the distribution was 26,584 and 249 respectively. Gender, race, and histology distribution were comparable between the two. Patients with poor PS (defined as ECOG III and IV) received less treatment with any modality compared to those with good PS (ECOG I and II) (15.07% in no treatment group vs 4.03% in treatment group, p<0.05). The treatment group had a better 5-year overall survival (OS) as compared to no-treatment group (43.1% vs 14.7%, p<0.05). Regardless of treatment, patients above the age of 60 (41% vs 13.4%, p<0.05) and those with poor PS (19.6% vs 5.8%, p<0.05) had worse 5-year survival, with the effect being greater in the treatment group. Adenocarcinoma had a better 5-year survival compared to squamous cell carcinoma (SCC) in both groups (49.56% vs 39.1% p<0.05). There was no clinically significant OS difference in terms of race (Caucasian or African American) or tumor location (upper, middle, or lower lobe) in between the two groups. Our study was limited by lack of patient- level data including smoking status or reason why no treatment was given.
Conclusion
Patients with early-stage NSCLC who receive no treatment based on poor PS have a worse overall survival compared to the patients that receive treatment. Further investigation is required to assess what other criteria are used to decide treatment eligibility and whether these patients would be candidates for immunotherapy or targeted therapy in the future.
Introduction
Veterans with early-stage NSCLC who do not receive any form of treatment have been shown to have a worse overall survival compared to those who receive treatment. Factors that may influence the decision to administer treatment including age, performance status (PS), comorbidities, and racial disparity have not been assessed on a national level in recent years.
Methods
Data for 31,966 veterans diagnosed with early-stage (0, I) NSCLC between 2003-2017 was obtained from the Cancer cube registry (VACCR). IRB approval was obtained.
Results
Patients were divided into treatment (26,833/31,966, 83.16%) and no-treatment group (3096/31966, 9.68%). Of the no-treatment group, 3004 patients were stage I and 92 were stage 0 whereas in the treatment group, the distribution was 26,584 and 249 respectively. Gender, race, and histology distribution were comparable between the two. Patients with poor PS (defined as ECOG III and IV) received less treatment with any modality compared to those with good PS (ECOG I and II) (15.07% in no treatment group vs 4.03% in treatment group, p<0.05). The treatment group had a better 5-year overall survival (OS) as compared to no-treatment group (43.1% vs 14.7%, p<0.05). Regardless of treatment, patients above the age of 60 (41% vs 13.4%, p<0.05) and those with poor PS (19.6% vs 5.8%, p<0.05) had worse 5-year survival, with the effect being greater in the treatment group. Adenocarcinoma had a better 5-year survival compared to squamous cell carcinoma (SCC) in both groups (49.56% vs 39.1% p<0.05). There was no clinically significant OS difference in terms of race (Caucasian or African American) or tumor location (upper, middle, or lower lobe) in between the two groups. Our study was limited by lack of patient- level data including smoking status or reason why no treatment was given.
Conclusion
Patients with early-stage NSCLC who receive no treatment based on poor PS have a worse overall survival compared to the patients that receive treatment. Further investigation is required to assess what other criteria are used to decide treatment eligibility and whether these patients would be candidates for immunotherapy or targeted therapy in the future.
Introduction
Veterans with early-stage NSCLC who do not receive any form of treatment have been shown to have a worse overall survival compared to those who receive treatment. Factors that may influence the decision to administer treatment including age, performance status (PS), comorbidities, and racial disparity have not been assessed on a national level in recent years.
Methods
Data for 31,966 veterans diagnosed with early-stage (0, I) NSCLC between 2003-2017 was obtained from the Cancer cube registry (VACCR). IRB approval was obtained.
Results
Patients were divided into treatment (26,833/31,966, 83.16%) and no-treatment group (3096/31966, 9.68%). Of the no-treatment group, 3004 patients were stage I and 92 were stage 0 whereas in the treatment group, the distribution was 26,584 and 249 respectively. Gender, race, and histology distribution were comparable between the two. Patients with poor PS (defined as ECOG III and IV) received less treatment with any modality compared to those with good PS (ECOG I and II) (15.07% in no treatment group vs 4.03% in treatment group, p<0.05). The treatment group had a better 5-year overall survival (OS) as compared to no-treatment group (43.1% vs 14.7%, p<0.05). Regardless of treatment, patients above the age of 60 (41% vs 13.4%, p<0.05) and those with poor PS (19.6% vs 5.8%, p<0.05) had worse 5-year survival, with the effect being greater in the treatment group. Adenocarcinoma had a better 5-year survival compared to squamous cell carcinoma (SCC) in both groups (49.56% vs 39.1% p<0.05). There was no clinically significant OS difference in terms of race (Caucasian or African American) or tumor location (upper, middle, or lower lobe) in between the two groups. Our study was limited by lack of patient- level data including smoking status or reason why no treatment was given.
Conclusion
Patients with early-stage NSCLC who receive no treatment based on poor PS have a worse overall survival compared to the patients that receive treatment. Further investigation is required to assess what other criteria are used to decide treatment eligibility and whether these patients would be candidates for immunotherapy or targeted therapy in the future.
Hemophagocytic Lymphohistiocytosis: Early Treatment Leading to an Excellent Outcome
HLH is a rare and deadly disease increasingly more present in adults, but following treatment protocol may yield favorable results.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and deadly disease in which unregulated proliferation of histiocytes and T-cell infiltration takes place. It is known as a pediatric disease in which gene defects result in impaired cytotoxic NK- and T-cell function. It has been associated with autosomal recessive inheritance pattern. Without therapy, survival for these patients with active familial HLH is approximately 2 months.
Recognition of the disease has increased over the years, and as a result the diagnosis of HLH in adults also has increased. An acquired form can be triggered by viruses like Epstein-Barr virus, influenza, HIV, lymphoid malignancies, rheumatologic disorders, or immunodeficiency disorders. Survival rates for untreated HLH have been reported at < 5%.1 Despite early recognition and adequate treatment, HLH carries an overall mortality of 50% in the initial presentation, 90% die in the first 8 weeks of treatment due to uncontrolled disease.2
Case Presentation
A 56-year-old man with no active medical issues except for a remote history of non-Hodgkin lymphoma treated with chemotherapy and splenectomy in 1990 presented to the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico. He was admitted to the medicine ward due to community acquired pneumonia. Three days into admission his clinical status deteriorated, and the patient was transferred to the intensive care unit (ICU) due to acute respiratory failure and sepsis secondary to worsening pneumonia. Chest imaging demonstrated rapidly progressing diffuse bilateral infiltrates. Due to the severity of the chest imaging, a diagnostic bronchoscopy was performed.
The patient’s antibiotics regimen was empirically escalated to vancomycin 1500 mg IV every 12 hours and meropenem 2 g IV every 8 hours. Despite optimization of therapy, the patient did not show clinical signs of improvement. Febrile episodes persisted, pulmonary infiltrates and hypoxemia worsened, and the patient required a neuromuscular blockade. Since the bronchoscopy was nondiagnostic and deterioration persistent, the differential diagnosis was broadened. This led to the ordering of inflammatory markers. Laboratory testing showed ferritin levels > 16,000 ng/mL, pointing to HLH as a possible diagnosis. Further workup was remarkable for triglycerides of 1234 mg/dL and a fibrinogen of 0.77 g/L. In the setting of bicytopenia and persistent fever, HLH-94 regimen was started with dexamethasone 40 mg daily and etoposide 100 mg/m2. CD25 levels of 154,701 pg/mL were demonstrated as well as a decreased immunoglobulin (Ig) G levels with absent IgM and IgA. Bone marrow biopsy was consistent with hemophagocytosis. The patient eventually was extubated and sent to the oncology ward to continue chemotherapy.
Discussion
A high clinical suspicion is warranted for rapid diagnosis and treatment as HLH evolves in most cases to multiorgan failure and death. The diagnostic criteria for HLH was developed by the Histiocyte Society in 1991 and then restructured in 2004.3,4 In the first diagnostic tool developed in 1991, diagnosis was based on 5 criteria (fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis). Three additional laboratory findings were also described as part of HLH diagnosis since 2004: low or absent NK-cell-activity, hyperferritinemia of > 500 ng/dL, and high-soluble interleukin-2-receptor levels (CD25) > 2400 U/mL. Overall, 5 of 8 criteria are needed for the HLH diagnosis.
Despite the common use of these diagnostic criteria, they were developed for the pediatric population but have not been validated for adult patients.5 For adult patients, the HScore was developed in 2014. It has 9 variables: 3 are based on clinical findings (known underlying immunosuppression, high temperature, and organomegaly; 5 are based on laboratory values (ferritin, serum glutamic oxaloacetic transaminase, cytopenia, triglycerides, and fibrinogen levels); the last variable uses cytologic findings in the bone marrow. In the initial study, probability of having HLH ranged from < 1% with an HScore of ≤ 90% to > 99% with an HScore of ≥ 250 in noncritically ill adults.5 A recently published retrospective study demonstrated the diagnostic reliability of both the HLH-2004 criteria and HScore in critically ill adult patients. This study concluded that the best prediction accuracy of HLH diagnosis for a cutoff of 4 fulfilled HLH-2004 criteria had a 95.0% sensitivity and 93.6% specificity and HScore cutoff of 168 reached a 100% sensitivity and 94.1% specificity.6
The early negative bronchoscopy lowered the possibility of an infection as the etiology of the clinical presentation and narrowed the hyperferritinemia differential diagnosis. Hyperferritinemia has a sensitivity and specificity of > 90% for diagnosis when above 10,000 ng/dL in the pediatric population.7 This is not the case in adults. Hyperferritinemia is a marker of different inflammatory responses, such as histoplasmosis infection, malignancy, or iron overload rather than an isolated diagnostic tool for HLH.8 It has been reported that CD25 levels less than the diagnostic threshold of 2400 U/mL have a 100% sensitivity for the diagnosis and therefore can rule out the diagnosis. When this is taken into consideration, it can be concluded that CD25 level is a better diagnostic tool when compared with ferritin, but its main limitation is its lack of widespread availability.9 Still, there is a limited number of pathologies that are associated with marked hyperferritinemia, specifically using thresholds of more than 6000 ng/dL.10 Taking into consideration the high mortality of untreated HLH, isolated hyperferritinemia still warrants HLH workup to aggressively pursue the diagnosis and improve outcomes.
The goal of therapy in HLH is prompt inactivation of the dysregulated inflammation with aggressive immunosuppression. In our deteriorating patient, the treatment was started with only 4 of the 8 HLH-2004 diagnostic criteria being met. As per the 2018 Histiocyte Society consensus statement, the decision to start the HLH-94 treatment relies on not only the HLH-2004 diagnostic criteria, but also the patient’s clinical evolution.11 In 1994 the Histiocyte Society also published a treatment protocol termed HLH-94. A Korean retrospective study demonstrated that this protocol led to a 5-year survival rate of 60 to 80% depending on the HLH trigger and response to initial treatment.12 The protocol consists of etoposide at 150 mg/m2, 2 weekly doses in the first 2 weeks and then 1 dose weekly for the next 6 weeks. Dexamethasone is the steroid of choice as it readily crosses the blood-brain barrier. Its dosage consists of 10 mg/m2 for the first 2 weeks and then it is halved every 2 weeks until the eighth week of treatment. A slow taper follows to avoid adrenal insufficiency. Once 8 weeks of treatment have been completed, cyclosporine is added to a goal trough of 200 mcg/dL. If there is central nervous system (CNS) involvement, early aggressive treatment with intrathecal methotrexate is indicated if no improvement is noted during initial therapy.11
In 2004 the Histiocyte Society restructured the HLH-94 treatment protocol with the aim of presenting a more aggressive treatment strategy. The protocol added cyclosporine to the initial induction therapy, rather than later in the ninth week as HLH-94. Neither the use of cyclosporine nor the HLH-2004 have been demonstrated to be superior to the use of etoposide and dexamethasone alone or in the HLH-94 protocol, respectively.13 Cyclosporine is associated with adverse effects (AEs) and may have many contraindications in the acute phase of the disease. Therefore, the HLH-94 protocol is still the recommended regimen.11
To assess adequate clinical response, several clinical and laboratory parameters are followed. Clinically, resolution of fever, improvement in hepatosplenomegaly, lymphadenopathy, and mental status can be useful. Laboratories can be used to assess improvement from organ specific damage such as hepatic involvement or cytopenia. The limitation of these diagnostic studies is that they could falsely suggest an inadequate response to treatment due to concomitant infection or medication AEs. Other markers such as ferritin levels, CD25, and NK cell activity levels are more specific to HLH. Out of them, a decreasing ferritin level has the needed specificity and widespread availability for repeated assessment. On the other hand, both CD25 and NK cell activity are readily available only in specialized centers. An initial high ferritin level is a marker for a poor prognosis, and the rate of decline correlates with mortality. Studies have demonstrated that persistently elevated ferritin levels after treatment initiation are associated with worse outcomes.14,15
Several salvage treatments have been identified in recalcitrant or relapsing disease. In general, chemotherapy needs to be intensified, either by returning to the initial high dosage if recurrence occurs in the weaning phase of treatment or adding other agents if no response was initially achieved. Emapalumab, an interferon γ antibody, was approved by the US Food and Drug Administration for the treatment of intractable HLH after it demonstrated that when added to dexamethasone, it lead to treatment response in 17 out of 27 pediatric patients, with a relatively safe AE profile.16 The goal of intensifying chemotherapy is to have the patient tolerate allogenic stem cell transplant, which is clinically indicated in familial HLH, malignancy induced HLH, and recalcitrant cases. In patients who undergo hematopoietic cell transplantation (HCT) there is a tendency to increase survival to 66% at 5 years.12
Conclusions
HLH is a rare and deadly disease increasingly more present in adults. Our patient who initially presented with a sepsis diagnosis was suspected of having a hematologic etiology for his clinical findings due to markedly elevated ferritin levels. In our patient, the HLH-94 treatment protocol was used, yielding favorable results. Given the lack of specific scientific data backing updated protocols such as HLH-2004 and a comparatively favorable safety profile, current guidelines still recommend using the HLH-94 treatment protocol. Decreasing ferritin levels may be used in conjunction with clinical improvement to demonstrate therapeutic response. Persistence of disease despite standard treatment may warrant novel therapies, such as emapalumab or HCT. Physicians need to be wary of an HLH diagnosis as early identification and treatment may improve its otherwise grim prognosis.
1. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120(1, pt 1):172-179. doi:10.1016/j.jfma.2020.03.025
2. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. doi:10.1182/blood-2002-01-0172
3. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18(1):29-33.
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
5. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. Published 2020 May 24. doi:10.1186/s13054-020-02941-3
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. doi:10.1002/art.38690
7. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
8. Schaffner M, Rosenstein L, Ballas Z, Suneja M. Significance of Hyperferritinemia in Hospitalized Adults. Am J Med Sci. 2017;354(2):152-158. doi:10.1016/j.amjms.2017.04.016
9. Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534. Published 2017 Dec 6. doi:10.1182/bloodadvances.2017012310
10. Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199-202. Published 2020 Jan 28. doi:10.4081/reumatismo.2019.1221
11. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508-1517. doi:10.1016/j.jaip.2018.05.031
12. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269-276. doi:10.3324/haematol.2018.198655
13. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349
14. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56(1):154-155. doi:10.1002/pbc.22774
15. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71. Published 2020 Mar 12. doi:10.1186/s13023-020-1336-616. Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Presented at: American Society of Hematology Annual Meeting, November 29, 2018. Blood. 2018;132(suppl 1):LBA-6. doi:10.1182/blood-2018-120810
HLH is a rare and deadly disease increasingly more present in adults, but following treatment protocol may yield favorable results.
HLH is a rare and deadly disease increasingly more present in adults, but following treatment protocol may yield favorable results.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and deadly disease in which unregulated proliferation of histiocytes and T-cell infiltration takes place. It is known as a pediatric disease in which gene defects result in impaired cytotoxic NK- and T-cell function. It has been associated with autosomal recessive inheritance pattern. Without therapy, survival for these patients with active familial HLH is approximately 2 months.
Recognition of the disease has increased over the years, and as a result the diagnosis of HLH in adults also has increased. An acquired form can be triggered by viruses like Epstein-Barr virus, influenza, HIV, lymphoid malignancies, rheumatologic disorders, or immunodeficiency disorders. Survival rates for untreated HLH have been reported at < 5%.1 Despite early recognition and adequate treatment, HLH carries an overall mortality of 50% in the initial presentation, 90% die in the first 8 weeks of treatment due to uncontrolled disease.2
Case Presentation
A 56-year-old man with no active medical issues except for a remote history of non-Hodgkin lymphoma treated with chemotherapy and splenectomy in 1990 presented to the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico. He was admitted to the medicine ward due to community acquired pneumonia. Three days into admission his clinical status deteriorated, and the patient was transferred to the intensive care unit (ICU) due to acute respiratory failure and sepsis secondary to worsening pneumonia. Chest imaging demonstrated rapidly progressing diffuse bilateral infiltrates. Due to the severity of the chest imaging, a diagnostic bronchoscopy was performed.
The patient’s antibiotics regimen was empirically escalated to vancomycin 1500 mg IV every 12 hours and meropenem 2 g IV every 8 hours. Despite optimization of therapy, the patient did not show clinical signs of improvement. Febrile episodes persisted, pulmonary infiltrates and hypoxemia worsened, and the patient required a neuromuscular blockade. Since the bronchoscopy was nondiagnostic and deterioration persistent, the differential diagnosis was broadened. This led to the ordering of inflammatory markers. Laboratory testing showed ferritin levels > 16,000 ng/mL, pointing to HLH as a possible diagnosis. Further workup was remarkable for triglycerides of 1234 mg/dL and a fibrinogen of 0.77 g/L. In the setting of bicytopenia and persistent fever, HLH-94 regimen was started with dexamethasone 40 mg daily and etoposide 100 mg/m2. CD25 levels of 154,701 pg/mL were demonstrated as well as a decreased immunoglobulin (Ig) G levels with absent IgM and IgA. Bone marrow biopsy was consistent with hemophagocytosis. The patient eventually was extubated and sent to the oncology ward to continue chemotherapy.
Discussion
A high clinical suspicion is warranted for rapid diagnosis and treatment as HLH evolves in most cases to multiorgan failure and death. The diagnostic criteria for HLH was developed by the Histiocyte Society in 1991 and then restructured in 2004.3,4 In the first diagnostic tool developed in 1991, diagnosis was based on 5 criteria (fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis). Three additional laboratory findings were also described as part of HLH diagnosis since 2004: low or absent NK-cell-activity, hyperferritinemia of > 500 ng/dL, and high-soluble interleukin-2-receptor levels (CD25) > 2400 U/mL. Overall, 5 of 8 criteria are needed for the HLH diagnosis.
Despite the common use of these diagnostic criteria, they were developed for the pediatric population but have not been validated for adult patients.5 For adult patients, the HScore was developed in 2014. It has 9 variables: 3 are based on clinical findings (known underlying immunosuppression, high temperature, and organomegaly; 5 are based on laboratory values (ferritin, serum glutamic oxaloacetic transaminase, cytopenia, triglycerides, and fibrinogen levels); the last variable uses cytologic findings in the bone marrow. In the initial study, probability of having HLH ranged from < 1% with an HScore of ≤ 90% to > 99% with an HScore of ≥ 250 in noncritically ill adults.5 A recently published retrospective study demonstrated the diagnostic reliability of both the HLH-2004 criteria and HScore in critically ill adult patients. This study concluded that the best prediction accuracy of HLH diagnosis for a cutoff of 4 fulfilled HLH-2004 criteria had a 95.0% sensitivity and 93.6% specificity and HScore cutoff of 168 reached a 100% sensitivity and 94.1% specificity.6
The early negative bronchoscopy lowered the possibility of an infection as the etiology of the clinical presentation and narrowed the hyperferritinemia differential diagnosis. Hyperferritinemia has a sensitivity and specificity of > 90% for diagnosis when above 10,000 ng/dL in the pediatric population.7 This is not the case in adults. Hyperferritinemia is a marker of different inflammatory responses, such as histoplasmosis infection, malignancy, or iron overload rather than an isolated diagnostic tool for HLH.8 It has been reported that CD25 levels less than the diagnostic threshold of 2400 U/mL have a 100% sensitivity for the diagnosis and therefore can rule out the diagnosis. When this is taken into consideration, it can be concluded that CD25 level is a better diagnostic tool when compared with ferritin, but its main limitation is its lack of widespread availability.9 Still, there is a limited number of pathologies that are associated with marked hyperferritinemia, specifically using thresholds of more than 6000 ng/dL.10 Taking into consideration the high mortality of untreated HLH, isolated hyperferritinemia still warrants HLH workup to aggressively pursue the diagnosis and improve outcomes.
The goal of therapy in HLH is prompt inactivation of the dysregulated inflammation with aggressive immunosuppression. In our deteriorating patient, the treatment was started with only 4 of the 8 HLH-2004 diagnostic criteria being met. As per the 2018 Histiocyte Society consensus statement, the decision to start the HLH-94 treatment relies on not only the HLH-2004 diagnostic criteria, but also the patient’s clinical evolution.11 In 1994 the Histiocyte Society also published a treatment protocol termed HLH-94. A Korean retrospective study demonstrated that this protocol led to a 5-year survival rate of 60 to 80% depending on the HLH trigger and response to initial treatment.12 The protocol consists of etoposide at 150 mg/m2, 2 weekly doses in the first 2 weeks and then 1 dose weekly for the next 6 weeks. Dexamethasone is the steroid of choice as it readily crosses the blood-brain barrier. Its dosage consists of 10 mg/m2 for the first 2 weeks and then it is halved every 2 weeks until the eighth week of treatment. A slow taper follows to avoid adrenal insufficiency. Once 8 weeks of treatment have been completed, cyclosporine is added to a goal trough of 200 mcg/dL. If there is central nervous system (CNS) involvement, early aggressive treatment with intrathecal methotrexate is indicated if no improvement is noted during initial therapy.11
In 2004 the Histiocyte Society restructured the HLH-94 treatment protocol with the aim of presenting a more aggressive treatment strategy. The protocol added cyclosporine to the initial induction therapy, rather than later in the ninth week as HLH-94. Neither the use of cyclosporine nor the HLH-2004 have been demonstrated to be superior to the use of etoposide and dexamethasone alone or in the HLH-94 protocol, respectively.13 Cyclosporine is associated with adverse effects (AEs) and may have many contraindications in the acute phase of the disease. Therefore, the HLH-94 protocol is still the recommended regimen.11
To assess adequate clinical response, several clinical and laboratory parameters are followed. Clinically, resolution of fever, improvement in hepatosplenomegaly, lymphadenopathy, and mental status can be useful. Laboratories can be used to assess improvement from organ specific damage such as hepatic involvement or cytopenia. The limitation of these diagnostic studies is that they could falsely suggest an inadequate response to treatment due to concomitant infection or medication AEs. Other markers such as ferritin levels, CD25, and NK cell activity levels are more specific to HLH. Out of them, a decreasing ferritin level has the needed specificity and widespread availability for repeated assessment. On the other hand, both CD25 and NK cell activity are readily available only in specialized centers. An initial high ferritin level is a marker for a poor prognosis, and the rate of decline correlates with mortality. Studies have demonstrated that persistently elevated ferritin levels after treatment initiation are associated with worse outcomes.14,15
Several salvage treatments have been identified in recalcitrant or relapsing disease. In general, chemotherapy needs to be intensified, either by returning to the initial high dosage if recurrence occurs in the weaning phase of treatment or adding other agents if no response was initially achieved. Emapalumab, an interferon γ antibody, was approved by the US Food and Drug Administration for the treatment of intractable HLH after it demonstrated that when added to dexamethasone, it lead to treatment response in 17 out of 27 pediatric patients, with a relatively safe AE profile.16 The goal of intensifying chemotherapy is to have the patient tolerate allogenic stem cell transplant, which is clinically indicated in familial HLH, malignancy induced HLH, and recalcitrant cases. In patients who undergo hematopoietic cell transplantation (HCT) there is a tendency to increase survival to 66% at 5 years.12
Conclusions
HLH is a rare and deadly disease increasingly more present in adults. Our patient who initially presented with a sepsis diagnosis was suspected of having a hematologic etiology for his clinical findings due to markedly elevated ferritin levels. In our patient, the HLH-94 treatment protocol was used, yielding favorable results. Given the lack of specific scientific data backing updated protocols such as HLH-2004 and a comparatively favorable safety profile, current guidelines still recommend using the HLH-94 treatment protocol. Decreasing ferritin levels may be used in conjunction with clinical improvement to demonstrate therapeutic response. Persistence of disease despite standard treatment may warrant novel therapies, such as emapalumab or HCT. Physicians need to be wary of an HLH diagnosis as early identification and treatment may improve its otherwise grim prognosis.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and deadly disease in which unregulated proliferation of histiocytes and T-cell infiltration takes place. It is known as a pediatric disease in which gene defects result in impaired cytotoxic NK- and T-cell function. It has been associated with autosomal recessive inheritance pattern. Without therapy, survival for these patients with active familial HLH is approximately 2 months.
Recognition of the disease has increased over the years, and as a result the diagnosis of HLH in adults also has increased. An acquired form can be triggered by viruses like Epstein-Barr virus, influenza, HIV, lymphoid malignancies, rheumatologic disorders, or immunodeficiency disorders. Survival rates for untreated HLH have been reported at < 5%.1 Despite early recognition and adequate treatment, HLH carries an overall mortality of 50% in the initial presentation, 90% die in the first 8 weeks of treatment due to uncontrolled disease.2
Case Presentation
A 56-year-old man with no active medical issues except for a remote history of non-Hodgkin lymphoma treated with chemotherapy and splenectomy in 1990 presented to the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico. He was admitted to the medicine ward due to community acquired pneumonia. Three days into admission his clinical status deteriorated, and the patient was transferred to the intensive care unit (ICU) due to acute respiratory failure and sepsis secondary to worsening pneumonia. Chest imaging demonstrated rapidly progressing diffuse bilateral infiltrates. Due to the severity of the chest imaging, a diagnostic bronchoscopy was performed.
The patient’s antibiotics regimen was empirically escalated to vancomycin 1500 mg IV every 12 hours and meropenem 2 g IV every 8 hours. Despite optimization of therapy, the patient did not show clinical signs of improvement. Febrile episodes persisted, pulmonary infiltrates and hypoxemia worsened, and the patient required a neuromuscular blockade. Since the bronchoscopy was nondiagnostic and deterioration persistent, the differential diagnosis was broadened. This led to the ordering of inflammatory markers. Laboratory testing showed ferritin levels > 16,000 ng/mL, pointing to HLH as a possible diagnosis. Further workup was remarkable for triglycerides of 1234 mg/dL and a fibrinogen of 0.77 g/L. In the setting of bicytopenia and persistent fever, HLH-94 regimen was started with dexamethasone 40 mg daily and etoposide 100 mg/m2. CD25 levels of 154,701 pg/mL were demonstrated as well as a decreased immunoglobulin (Ig) G levels with absent IgM and IgA. Bone marrow biopsy was consistent with hemophagocytosis. The patient eventually was extubated and sent to the oncology ward to continue chemotherapy.
Discussion
A high clinical suspicion is warranted for rapid diagnosis and treatment as HLH evolves in most cases to multiorgan failure and death. The diagnostic criteria for HLH was developed by the Histiocyte Society in 1991 and then restructured in 2004.3,4 In the first diagnostic tool developed in 1991, diagnosis was based on 5 criteria (fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis). Three additional laboratory findings were also described as part of HLH diagnosis since 2004: low or absent NK-cell-activity, hyperferritinemia of > 500 ng/dL, and high-soluble interleukin-2-receptor levels (CD25) > 2400 U/mL. Overall, 5 of 8 criteria are needed for the HLH diagnosis.
Despite the common use of these diagnostic criteria, they were developed for the pediatric population but have not been validated for adult patients.5 For adult patients, the HScore was developed in 2014. It has 9 variables: 3 are based on clinical findings (known underlying immunosuppression, high temperature, and organomegaly; 5 are based on laboratory values (ferritin, serum glutamic oxaloacetic transaminase, cytopenia, triglycerides, and fibrinogen levels); the last variable uses cytologic findings in the bone marrow. In the initial study, probability of having HLH ranged from < 1% with an HScore of ≤ 90% to > 99% with an HScore of ≥ 250 in noncritically ill adults.5 A recently published retrospective study demonstrated the diagnostic reliability of both the HLH-2004 criteria and HScore in critically ill adult patients. This study concluded that the best prediction accuracy of HLH diagnosis for a cutoff of 4 fulfilled HLH-2004 criteria had a 95.0% sensitivity and 93.6% specificity and HScore cutoff of 168 reached a 100% sensitivity and 94.1% specificity.6
The early negative bronchoscopy lowered the possibility of an infection as the etiology of the clinical presentation and narrowed the hyperferritinemia differential diagnosis. Hyperferritinemia has a sensitivity and specificity of > 90% for diagnosis when above 10,000 ng/dL in the pediatric population.7 This is not the case in adults. Hyperferritinemia is a marker of different inflammatory responses, such as histoplasmosis infection, malignancy, or iron overload rather than an isolated diagnostic tool for HLH.8 It has been reported that CD25 levels less than the diagnostic threshold of 2400 U/mL have a 100% sensitivity for the diagnosis and therefore can rule out the diagnosis. When this is taken into consideration, it can be concluded that CD25 level is a better diagnostic tool when compared with ferritin, but its main limitation is its lack of widespread availability.9 Still, there is a limited number of pathologies that are associated with marked hyperferritinemia, specifically using thresholds of more than 6000 ng/dL.10 Taking into consideration the high mortality of untreated HLH, isolated hyperferritinemia still warrants HLH workup to aggressively pursue the diagnosis and improve outcomes.
The goal of therapy in HLH is prompt inactivation of the dysregulated inflammation with aggressive immunosuppression. In our deteriorating patient, the treatment was started with only 4 of the 8 HLH-2004 diagnostic criteria being met. As per the 2018 Histiocyte Society consensus statement, the decision to start the HLH-94 treatment relies on not only the HLH-2004 diagnostic criteria, but also the patient’s clinical evolution.11 In 1994 the Histiocyte Society also published a treatment protocol termed HLH-94. A Korean retrospective study demonstrated that this protocol led to a 5-year survival rate of 60 to 80% depending on the HLH trigger and response to initial treatment.12 The protocol consists of etoposide at 150 mg/m2, 2 weekly doses in the first 2 weeks and then 1 dose weekly for the next 6 weeks. Dexamethasone is the steroid of choice as it readily crosses the blood-brain barrier. Its dosage consists of 10 mg/m2 for the first 2 weeks and then it is halved every 2 weeks until the eighth week of treatment. A slow taper follows to avoid adrenal insufficiency. Once 8 weeks of treatment have been completed, cyclosporine is added to a goal trough of 200 mcg/dL. If there is central nervous system (CNS) involvement, early aggressive treatment with intrathecal methotrexate is indicated if no improvement is noted during initial therapy.11
In 2004 the Histiocyte Society restructured the HLH-94 treatment protocol with the aim of presenting a more aggressive treatment strategy. The protocol added cyclosporine to the initial induction therapy, rather than later in the ninth week as HLH-94. Neither the use of cyclosporine nor the HLH-2004 have been demonstrated to be superior to the use of etoposide and dexamethasone alone or in the HLH-94 protocol, respectively.13 Cyclosporine is associated with adverse effects (AEs) and may have many contraindications in the acute phase of the disease. Therefore, the HLH-94 protocol is still the recommended regimen.11
To assess adequate clinical response, several clinical and laboratory parameters are followed. Clinically, resolution of fever, improvement in hepatosplenomegaly, lymphadenopathy, and mental status can be useful. Laboratories can be used to assess improvement from organ specific damage such as hepatic involvement or cytopenia. The limitation of these diagnostic studies is that they could falsely suggest an inadequate response to treatment due to concomitant infection or medication AEs. Other markers such as ferritin levels, CD25, and NK cell activity levels are more specific to HLH. Out of them, a decreasing ferritin level has the needed specificity and widespread availability for repeated assessment. On the other hand, both CD25 and NK cell activity are readily available only in specialized centers. An initial high ferritin level is a marker for a poor prognosis, and the rate of decline correlates with mortality. Studies have demonstrated that persistently elevated ferritin levels after treatment initiation are associated with worse outcomes.14,15
Several salvage treatments have been identified in recalcitrant or relapsing disease. In general, chemotherapy needs to be intensified, either by returning to the initial high dosage if recurrence occurs in the weaning phase of treatment or adding other agents if no response was initially achieved. Emapalumab, an interferon γ antibody, was approved by the US Food and Drug Administration for the treatment of intractable HLH after it demonstrated that when added to dexamethasone, it lead to treatment response in 17 out of 27 pediatric patients, with a relatively safe AE profile.16 The goal of intensifying chemotherapy is to have the patient tolerate allogenic stem cell transplant, which is clinically indicated in familial HLH, malignancy induced HLH, and recalcitrant cases. In patients who undergo hematopoietic cell transplantation (HCT) there is a tendency to increase survival to 66% at 5 years.12
Conclusions
HLH is a rare and deadly disease increasingly more present in adults. Our patient who initially presented with a sepsis diagnosis was suspected of having a hematologic etiology for his clinical findings due to markedly elevated ferritin levels. In our patient, the HLH-94 treatment protocol was used, yielding favorable results. Given the lack of specific scientific data backing updated protocols such as HLH-2004 and a comparatively favorable safety profile, current guidelines still recommend using the HLH-94 treatment protocol. Decreasing ferritin levels may be used in conjunction with clinical improvement to demonstrate therapeutic response. Persistence of disease despite standard treatment may warrant novel therapies, such as emapalumab or HCT. Physicians need to be wary of an HLH diagnosis as early identification and treatment may improve its otherwise grim prognosis.
1. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120(1, pt 1):172-179. doi:10.1016/j.jfma.2020.03.025
2. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. doi:10.1182/blood-2002-01-0172
3. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18(1):29-33.
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
5. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. Published 2020 May 24. doi:10.1186/s13054-020-02941-3
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. doi:10.1002/art.38690
7. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
8. Schaffner M, Rosenstein L, Ballas Z, Suneja M. Significance of Hyperferritinemia in Hospitalized Adults. Am J Med Sci. 2017;354(2):152-158. doi:10.1016/j.amjms.2017.04.016
9. Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534. Published 2017 Dec 6. doi:10.1182/bloodadvances.2017012310
10. Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199-202. Published 2020 Jan 28. doi:10.4081/reumatismo.2019.1221
11. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508-1517. doi:10.1016/j.jaip.2018.05.031
12. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269-276. doi:10.3324/haematol.2018.198655
13. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349
14. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56(1):154-155. doi:10.1002/pbc.22774
15. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71. Published 2020 Mar 12. doi:10.1186/s13023-020-1336-616. Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Presented at: American Society of Hematology Annual Meeting, November 29, 2018. Blood. 2018;132(suppl 1):LBA-6. doi:10.1182/blood-2018-120810
1. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120(1, pt 1):172-179. doi:10.1016/j.jfma.2020.03.025
2. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. doi:10.1182/blood-2002-01-0172
3. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18(1):29-33.
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
5. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. Published 2020 May 24. doi:10.1186/s13054-020-02941-3
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. doi:10.1002/art.38690
7. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
8. Schaffner M, Rosenstein L, Ballas Z, Suneja M. Significance of Hyperferritinemia in Hospitalized Adults. Am J Med Sci. 2017;354(2):152-158. doi:10.1016/j.amjms.2017.04.016
9. Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534. Published 2017 Dec 6. doi:10.1182/bloodadvances.2017012310
10. Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199-202. Published 2020 Jan 28. doi:10.4081/reumatismo.2019.1221
11. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508-1517. doi:10.1016/j.jaip.2018.05.031
12. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269-276. doi:10.3324/haematol.2018.198655
13. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349
14. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56(1):154-155. doi:10.1002/pbc.22774
15. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71. Published 2020 Mar 12. doi:10.1186/s13023-020-1336-616. Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Presented at: American Society of Hematology Annual Meeting, November 29, 2018. Blood. 2018;132(suppl 1):LBA-6. doi:10.1182/blood-2018-120810
A Rapidly Progressive Thoracic Tumor
Introduction
SMARCA4-deficient thoracic sarcomas are a rare entity, first described in 2015 in a study of 19 patients with a median age of 41 years who presented with large compressive masses with frequent infiltration into surrounding tissues [1]. This malignancy is more frequent in younger males (median 41-59 years) with an extensive smoking history and has an aggressive course with a median overall survival of 4-7 months [1-3]. There is currently no established treatment, but case reports show promise for immunotherapy and immuno- chemotherapy [4-8].
Case Report
We present the case of a 62 year old male with a 44 pack year smoking history who first presented to the emergency department (ED) with left shoulder pain in December 2020. He was initially treated with muscle relaxers but returned to the ED ten days later with hemoptysis and rapid weight loss. X-ray showed a 14.2 X 11.7 cm mass with rightward deviation of the trachea. PET scan showed extensive central necrosis with a surrounding pleural effusion and local pleural and nodal metastasis but no distant disease. He underwent thoracentesis which was negative for malignant cells. He underwent CT-guided biopsy in 1/2021, which showed predominantly discohesive small blue cells with pleomorphic cell contour and slightly plasmacytoid features. Extensive pathology review led to a diagnosis of SMARCA4 deficient thoracic sarcoma. On presentation to oncology clinic in 2/2021 his functional status had markedly deteriorated. He was started on ipilimumab/ nivolumab (ipi/nivo) and 1 week after his first cycle was admitted for severe left arm swelling and pain. Imaging showed significant progression of disease and new adrenal metastasis. He received cycle two of ipi/ nivo and was able to be discharged home on oxygen. By his follow-up appointment for cycle three of ipi/nivo in 3/2021, the patient was wheelchair bound with severe dyspnea. X-ray showed the mass now occupied the majority of the left hemi-thorax with worsening tracheal deviation. After discussion, the patient went home on hospice and died 8 days later. As demonstrated by this case, SMARCA4-deficient sarcoma requires high clinical suspicion with prompt diagnosis and treatment given its remarkably rapid progression and poor outcomes.
Introduction
SMARCA4-deficient thoracic sarcomas are a rare entity, first described in 2015 in a study of 19 patients with a median age of 41 years who presented with large compressive masses with frequent infiltration into surrounding tissues [1]. This malignancy is more frequent in younger males (median 41-59 years) with an extensive smoking history and has an aggressive course with a median overall survival of 4-7 months [1-3]. There is currently no established treatment, but case reports show promise for immunotherapy and immuno- chemotherapy [4-8].
Case Report
We present the case of a 62 year old male with a 44 pack year smoking history who first presented to the emergency department (ED) with left shoulder pain in December 2020. He was initially treated with muscle relaxers but returned to the ED ten days later with hemoptysis and rapid weight loss. X-ray showed a 14.2 X 11.7 cm mass with rightward deviation of the trachea. PET scan showed extensive central necrosis with a surrounding pleural effusion and local pleural and nodal metastasis but no distant disease. He underwent thoracentesis which was negative for malignant cells. He underwent CT-guided biopsy in 1/2021, which showed predominantly discohesive small blue cells with pleomorphic cell contour and slightly plasmacytoid features. Extensive pathology review led to a diagnosis of SMARCA4 deficient thoracic sarcoma. On presentation to oncology clinic in 2/2021 his functional status had markedly deteriorated. He was started on ipilimumab/ nivolumab (ipi/nivo) and 1 week after his first cycle was admitted for severe left arm swelling and pain. Imaging showed significant progression of disease and new adrenal metastasis. He received cycle two of ipi/ nivo and was able to be discharged home on oxygen. By his follow-up appointment for cycle three of ipi/nivo in 3/2021, the patient was wheelchair bound with severe dyspnea. X-ray showed the mass now occupied the majority of the left hemi-thorax with worsening tracheal deviation. After discussion, the patient went home on hospice and died 8 days later. As demonstrated by this case, SMARCA4-deficient sarcoma requires high clinical suspicion with prompt diagnosis and treatment given its remarkably rapid progression and poor outcomes.
Introduction
SMARCA4-deficient thoracic sarcomas are a rare entity, first described in 2015 in a study of 19 patients with a median age of 41 years who presented with large compressive masses with frequent infiltration into surrounding tissues [1]. This malignancy is more frequent in younger males (median 41-59 years) with an extensive smoking history and has an aggressive course with a median overall survival of 4-7 months [1-3]. There is currently no established treatment, but case reports show promise for immunotherapy and immuno- chemotherapy [4-8].
Case Report
We present the case of a 62 year old male with a 44 pack year smoking history who first presented to the emergency department (ED) with left shoulder pain in December 2020. He was initially treated with muscle relaxers but returned to the ED ten days later with hemoptysis and rapid weight loss. X-ray showed a 14.2 X 11.7 cm mass with rightward deviation of the trachea. PET scan showed extensive central necrosis with a surrounding pleural effusion and local pleural and nodal metastasis but no distant disease. He underwent thoracentesis which was negative for malignant cells. He underwent CT-guided biopsy in 1/2021, which showed predominantly discohesive small blue cells with pleomorphic cell contour and slightly plasmacytoid features. Extensive pathology review led to a diagnosis of SMARCA4 deficient thoracic sarcoma. On presentation to oncology clinic in 2/2021 his functional status had markedly deteriorated. He was started on ipilimumab/ nivolumab (ipi/nivo) and 1 week after his first cycle was admitted for severe left arm swelling and pain. Imaging showed significant progression of disease and new adrenal metastasis. He received cycle two of ipi/ nivo and was able to be discharged home on oxygen. By his follow-up appointment for cycle three of ipi/nivo in 3/2021, the patient was wheelchair bound with severe dyspnea. X-ray showed the mass now occupied the majority of the left hemi-thorax with worsening tracheal deviation. After discussion, the patient went home on hospice and died 8 days later. As demonstrated by this case, SMARCA4-deficient sarcoma requires high clinical suspicion with prompt diagnosis and treatment given its remarkably rapid progression and poor outcomes.
Antiviral Therapy Improves Hepatocellular Cancer Survival
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
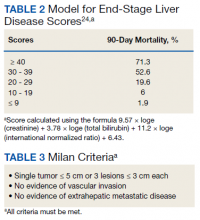
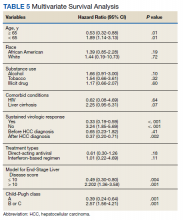
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
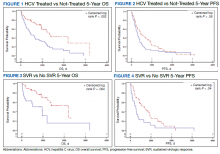
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
MS plus depression can increase risk of death, vascular disease
, a new study has found. “The effects of depression and MS on all-cause mortality are synergistic,” wrote lead author Raffaele Palladino, MD, PhD, research associate, faculty of medicine, Imperial College London.
The study was published in Neurology.
To assess the association between depression, vascular disease, and death in patients with MS, the researchers launched a population-based retrospective cohort study that reviewed English medical records from January 1987 to December 2018 and matched people with and without MS. Ultimately, 12,251 people with MS were matched with 72,572 controls. At baseline, 21% of the MS group (n = 2,535) and 9% of the controls (n = 6,278) had depression. Women were the majority in both cohorts and were more likely than men to be depressed.
People with both MS and depression had an all-cause mortality rate of 10.3 cases per 100,000 person-years (95% confidence interval, 9.17-11.57), compared with 10.6 for people with MS without depression (95% CI, 9.99-11.21), 3.6 for people with depression but not MS (95% CI, 3.18-4.05), and 2.5 for people with neither condition (95% CI, 2.42-2.64). Compared with controls without depression, the 10-year hazard of all-cause mortality was increasingly greater in controls with depression (hazard ratio, 1.75; 95% CI, 1.59-1.91), people with MS but not depression (HR, 3.88; 95% CI, 3.66-4.10), and people with MS and depression (HR, 5.43; 95% CI, 4.88-5.96). Overall, 14% of the observed effect on mortality was attributable to the interaction between MS status and depression.
As for vascular diseases, people with MS had an increased risk regardless of their depression status. That said, people with MS and depression (HR, 3.30; 95% CI, 2.37-4.23) had a notably higher risk than people with MS and no depression (HR, 1.48; 95% CI, 1.23-1.74). Women with MS and depression also had a greater risk of vascular disease than women with MS and no depression, while men with MS did not have significantly different risks of acute coronary syndrome or composite macrovascular disease than those in the control group who did not suffer from depression.
Does treating depression decrease the likelihood of vascular disease?
“The take-home message for me is the importance of treating depression in this population, in which we see it with great regularity,” Joseph Berger, MD, professor of neurology and associate chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia, said in an interview. “The question that I have is: If you treat depression in an individual with MS or an individual who is simply depressed and thus at risk for the subsequent development of vascular disease, does it decrease the likelihood of their subsequent development of vascular disease in comparison to had you not?
“I presume it does,” he added, noting that “the theories underlying why depression would increase one’s risk of subsequent vascular disease are enumerated by the authors, including such things as increased inflammation. Now, the inflammation may be contributing to the depression, or the depression may be contributing to the inflammation; it may be one of those chicken-and-egg scenarios. But if you decrease the depression, do you thereby decrease the inflammation, which has a pernicious effect on endothelial cells and increases one’s vascular risk?
“Alternatively, lifestyle in depressed patients is also altered,” he said. “They’re far less likely to engage in exercise, healthy habits, and healthy diets, and more likely perhaps to smoke. These all need to be addressed, but this study certainly gives you a greater impetus as a MS neurologist to address the issue of depression, realizing that there is also this comorbidity of vascular disease.”
Evaluating the biological interaction between MS and depression
Based on this and other studies, the joint effect of MS and depression on all-cause mortality may qualify as a biological interaction, Amber Salter, PhD, of the University of Texas Southwestern Medical Center, Dallas, wrote in an accompanying editorial.
“Biological interactions consider whether the joint effect of two factors follow an additive pattern, or the joint effect of two factors is greater than the sum of the individual effects for each factor alone,” she wrote. And though the interaction was not found to be present for vascular disease and cardiovascular mortality, it was for all-cause mortality.
“When warranted, the evaluation of biological interactions in future studies should be considered to provide insight on target subpopulations for interventions or test for potential mechanistic forms of interaction,” she added.
Dr. Salter highlighted the study’s strengths, including a large sample size and six controls matched to each MS patient. She also stated that the researchers’ inability to control for risk factors like body mass index and physical activity means the 14% increase in mortality “may not be a large absolute increase in mortality when other covariates cannot be considered.” In addition, their lack of data on suicide – and its association with depression – offers up the possibility that increases in mortality could be tied to a “potentially modifiable risk” as opposed to a biologically increased one.
In acknowledging their study’s limitations, the authors stated that body mass index, though an important vascular risk factor, has a “modest” association with mortality, and that the average annual suicide rate in the MS population – though higher than in the non-MS population – is still “relatively low.”
Two of the authors disclosed receiving support, including grants and research funding, from various institutions and organizations in the United Kingdom, the United States, and Canada, as well as several pharmaceutical companies. Dr. Salter reported no relevant disclosures.
, a new study has found. “The effects of depression and MS on all-cause mortality are synergistic,” wrote lead author Raffaele Palladino, MD, PhD, research associate, faculty of medicine, Imperial College London.
The study was published in Neurology.
To assess the association between depression, vascular disease, and death in patients with MS, the researchers launched a population-based retrospective cohort study that reviewed English medical records from January 1987 to December 2018 and matched people with and without MS. Ultimately, 12,251 people with MS were matched with 72,572 controls. At baseline, 21% of the MS group (n = 2,535) and 9% of the controls (n = 6,278) had depression. Women were the majority in both cohorts and were more likely than men to be depressed.
People with both MS and depression had an all-cause mortality rate of 10.3 cases per 100,000 person-years (95% confidence interval, 9.17-11.57), compared with 10.6 for people with MS without depression (95% CI, 9.99-11.21), 3.6 for people with depression but not MS (95% CI, 3.18-4.05), and 2.5 for people with neither condition (95% CI, 2.42-2.64). Compared with controls without depression, the 10-year hazard of all-cause mortality was increasingly greater in controls with depression (hazard ratio, 1.75; 95% CI, 1.59-1.91), people with MS but not depression (HR, 3.88; 95% CI, 3.66-4.10), and people with MS and depression (HR, 5.43; 95% CI, 4.88-5.96). Overall, 14% of the observed effect on mortality was attributable to the interaction between MS status and depression.
As for vascular diseases, people with MS had an increased risk regardless of their depression status. That said, people with MS and depression (HR, 3.30; 95% CI, 2.37-4.23) had a notably higher risk than people with MS and no depression (HR, 1.48; 95% CI, 1.23-1.74). Women with MS and depression also had a greater risk of vascular disease than women with MS and no depression, while men with MS did not have significantly different risks of acute coronary syndrome or composite macrovascular disease than those in the control group who did not suffer from depression.
Does treating depression decrease the likelihood of vascular disease?
“The take-home message for me is the importance of treating depression in this population, in which we see it with great regularity,” Joseph Berger, MD, professor of neurology and associate chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia, said in an interview. “The question that I have is: If you treat depression in an individual with MS or an individual who is simply depressed and thus at risk for the subsequent development of vascular disease, does it decrease the likelihood of their subsequent development of vascular disease in comparison to had you not?
“I presume it does,” he added, noting that “the theories underlying why depression would increase one’s risk of subsequent vascular disease are enumerated by the authors, including such things as increased inflammation. Now, the inflammation may be contributing to the depression, or the depression may be contributing to the inflammation; it may be one of those chicken-and-egg scenarios. But if you decrease the depression, do you thereby decrease the inflammation, which has a pernicious effect on endothelial cells and increases one’s vascular risk?
“Alternatively, lifestyle in depressed patients is also altered,” he said. “They’re far less likely to engage in exercise, healthy habits, and healthy diets, and more likely perhaps to smoke. These all need to be addressed, but this study certainly gives you a greater impetus as a MS neurologist to address the issue of depression, realizing that there is also this comorbidity of vascular disease.”
Evaluating the biological interaction between MS and depression
Based on this and other studies, the joint effect of MS and depression on all-cause mortality may qualify as a biological interaction, Amber Salter, PhD, of the University of Texas Southwestern Medical Center, Dallas, wrote in an accompanying editorial.
“Biological interactions consider whether the joint effect of two factors follow an additive pattern, or the joint effect of two factors is greater than the sum of the individual effects for each factor alone,” she wrote. And though the interaction was not found to be present for vascular disease and cardiovascular mortality, it was for all-cause mortality.
“When warranted, the evaluation of biological interactions in future studies should be considered to provide insight on target subpopulations for interventions or test for potential mechanistic forms of interaction,” she added.
Dr. Salter highlighted the study’s strengths, including a large sample size and six controls matched to each MS patient. She also stated that the researchers’ inability to control for risk factors like body mass index and physical activity means the 14% increase in mortality “may not be a large absolute increase in mortality when other covariates cannot be considered.” In addition, their lack of data on suicide – and its association with depression – offers up the possibility that increases in mortality could be tied to a “potentially modifiable risk” as opposed to a biologically increased one.
In acknowledging their study’s limitations, the authors stated that body mass index, though an important vascular risk factor, has a “modest” association with mortality, and that the average annual suicide rate in the MS population – though higher than in the non-MS population – is still “relatively low.”
Two of the authors disclosed receiving support, including grants and research funding, from various institutions and organizations in the United Kingdom, the United States, and Canada, as well as several pharmaceutical companies. Dr. Salter reported no relevant disclosures.
, a new study has found. “The effects of depression and MS on all-cause mortality are synergistic,” wrote lead author Raffaele Palladino, MD, PhD, research associate, faculty of medicine, Imperial College London.
The study was published in Neurology.
To assess the association between depression, vascular disease, and death in patients with MS, the researchers launched a population-based retrospective cohort study that reviewed English medical records from January 1987 to December 2018 and matched people with and without MS. Ultimately, 12,251 people with MS were matched with 72,572 controls. At baseline, 21% of the MS group (n = 2,535) and 9% of the controls (n = 6,278) had depression. Women were the majority in both cohorts and were more likely than men to be depressed.
People with both MS and depression had an all-cause mortality rate of 10.3 cases per 100,000 person-years (95% confidence interval, 9.17-11.57), compared with 10.6 for people with MS without depression (95% CI, 9.99-11.21), 3.6 for people with depression but not MS (95% CI, 3.18-4.05), and 2.5 for people with neither condition (95% CI, 2.42-2.64). Compared with controls without depression, the 10-year hazard of all-cause mortality was increasingly greater in controls with depression (hazard ratio, 1.75; 95% CI, 1.59-1.91), people with MS but not depression (HR, 3.88; 95% CI, 3.66-4.10), and people with MS and depression (HR, 5.43; 95% CI, 4.88-5.96). Overall, 14% of the observed effect on mortality was attributable to the interaction between MS status and depression.
As for vascular diseases, people with MS had an increased risk regardless of their depression status. That said, people with MS and depression (HR, 3.30; 95% CI, 2.37-4.23) had a notably higher risk than people with MS and no depression (HR, 1.48; 95% CI, 1.23-1.74). Women with MS and depression also had a greater risk of vascular disease than women with MS and no depression, while men with MS did not have significantly different risks of acute coronary syndrome or composite macrovascular disease than those in the control group who did not suffer from depression.
Does treating depression decrease the likelihood of vascular disease?
“The take-home message for me is the importance of treating depression in this population, in which we see it with great regularity,” Joseph Berger, MD, professor of neurology and associate chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia, said in an interview. “The question that I have is: If you treat depression in an individual with MS or an individual who is simply depressed and thus at risk for the subsequent development of vascular disease, does it decrease the likelihood of their subsequent development of vascular disease in comparison to had you not?
“I presume it does,” he added, noting that “the theories underlying why depression would increase one’s risk of subsequent vascular disease are enumerated by the authors, including such things as increased inflammation. Now, the inflammation may be contributing to the depression, or the depression may be contributing to the inflammation; it may be one of those chicken-and-egg scenarios. But if you decrease the depression, do you thereby decrease the inflammation, which has a pernicious effect on endothelial cells and increases one’s vascular risk?
“Alternatively, lifestyle in depressed patients is also altered,” he said. “They’re far less likely to engage in exercise, healthy habits, and healthy diets, and more likely perhaps to smoke. These all need to be addressed, but this study certainly gives you a greater impetus as a MS neurologist to address the issue of depression, realizing that there is also this comorbidity of vascular disease.”
Evaluating the biological interaction between MS and depression
Based on this and other studies, the joint effect of MS and depression on all-cause mortality may qualify as a biological interaction, Amber Salter, PhD, of the University of Texas Southwestern Medical Center, Dallas, wrote in an accompanying editorial.
“Biological interactions consider whether the joint effect of two factors follow an additive pattern, or the joint effect of two factors is greater than the sum of the individual effects for each factor alone,” she wrote. And though the interaction was not found to be present for vascular disease and cardiovascular mortality, it was for all-cause mortality.
“When warranted, the evaluation of biological interactions in future studies should be considered to provide insight on target subpopulations for interventions or test for potential mechanistic forms of interaction,” she added.
Dr. Salter highlighted the study’s strengths, including a large sample size and six controls matched to each MS patient. She also stated that the researchers’ inability to control for risk factors like body mass index and physical activity means the 14% increase in mortality “may not be a large absolute increase in mortality when other covariates cannot be considered.” In addition, their lack of data on suicide – and its association with depression – offers up the possibility that increases in mortality could be tied to a “potentially modifiable risk” as opposed to a biologically increased one.
In acknowledging their study’s limitations, the authors stated that body mass index, though an important vascular risk factor, has a “modest” association with mortality, and that the average annual suicide rate in the MS population – though higher than in the non-MS population – is still “relatively low.”
Two of the authors disclosed receiving support, including grants and research funding, from various institutions and organizations in the United Kingdom, the United States, and Canada, as well as several pharmaceutical companies. Dr. Salter reported no relevant disclosures.
FROM NEUROLOGY
FDA OKs IV Briviact for seizures in kids as young as 1 month
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
MRI is a poor disability predictor in secondary progressive MS
, new research suggests. Analysis from the phase 3 ASCEND trial of nearly 900 patients showed that MRI measures were not associated with worsening of scores on the Expanded Disability Status Scale (EDSS), the most widely used physical outcome measure.
The few associations that were shown between MRI measures and clinical outcomes “were with the newer and possibly more sensitive outcomes” – the Timed 25-Foot Walk (T25FW) and Nine-Hole Peg Test (NHPT), wrote the investigators, led by Marcus W. Koch, MD, PhD, associate professor of neurology in the MS program at the University of Calgary, Canada.
However, “it is unclear if these associations are clinically meaningful,” they added.
Worsening on the NHPT at 48 weeks was associated with a 0.86% loss in normalized brain volume; worsening at 96 weeks was associated with a 1.47% loss.
The findings were published online July 26 in the Multiple Sclerosis Journal.
ASCEND data analysis
Although brain volume loss occurs in all forms of MS, it is believed to be particularly relevant in SPMS. Clinical trials often use MRI measures of brain volume as endpoints, likely on the assumption that these measures indicate worsening disability.
However, brain volume loss proceeds slowly. Changes that occur during the typical 2-year study period may not be associated with significant physical or cognitive disability.
In the current study, investigators examined data from the ASCEND trial, which assessed the use of natalizumab for patients with SPMS, to examine these potential associations. Eligible participants in ASCEND were between ages 18 and 58 years, had had SPMS for 2 or more years, had had disability progression during the previous year, and had an EDSS score between 3.0 and 6.5 at baseline.
Participants underwent gadolinium-enhanced cranial MRI at screening and at 24, 48, 72, and 96 weeks. MRI outcomes included normalized brain volume, normalized cortical gray matter volume, and normalized whole gray matter volume. The ASCEND investigators also examined the number and volume of T2 and contrast-enhancing lesions.
The study’s clinical outcomes included scores on the EDSS, T25FW, and NHPT, which were administered at baseline and every 12 weeks thereafter. Participants also underwent the Symbol Digit Modalities Test (SDMT), which is a cognitive assessment, at baseline and every 4 weeks thereafter. In addition, 3-month confirmed disability progression was measured every 12 weeks.
Few significant associations
The investigators’ analysis included 889 patients (61.9% women; median age, 48 years). The median EDSS score at screening was 6.
Brain volume measures decreased consistently during follow-up. Mean volume loss at 96 weeks was about 1%. In contrast, T2 lesion volume changed little during follow-up. The cumulative number of contrast-enhancing lesions and the cumulative number of new or newly enlarging T2 lesions increased steadily during follow-up.
For an increasing number of participants, scores on the EDSS, NHPT, and T25FW worsened significantly during follow-up. Performance on SDMT, however, changed little. Of all the clinical measures, the NHPT was most consistently associated with MRI measures.
Among patients whose NHPT score worsened at 48 weeks, there was greater loss of normalized brain volume (0.86%, P = .02), normalized cortical gray matter volume (1.15%, P = .03), and normalized whole gray matter volume (1.08%, P = .03) than among those whose NHPT score did not worsen.
Among patients whose NHPT score worsened at 96 weeks, there was greater normalized brain volume loss (1.47%, P = .002), greater increase in T2 lesion volume (4.68%, P = .02), and a greater number of cumulative new or newly enlarging T2 lesions (7.81, P = .03) than those whose NHPT score did not worsen.
After adjusting the data for covariables, the investigators found few significant associations between MRI measures and clinical outcomes. Worsening on the EDSS and SDMT was not associated with any MRI outcome.
Important disability contributors missed
The odds ratio of 3-month confirmed worsening on the T25FW at 96 weeks was 2.25 for patients with more than 10 cumulative new or newly enlarging T2 lesions (P = .03). The OR of 3-month confirmed worsening on the NHPT at 96 weeks was 3.04 for patients with more than 10 such lesions (P = .03).
Greater normalized brain volume loss at 48 weeks was associated with a greater risk for worsening disability on the NHPT at 48 and 96 weeks. For patients with a volume loss greater than 1.5%, the OR of worsening NHPT at 96 weeks was 4.69 (P = .05).
Although previous cross-sectional studies have shown correlations between brain volume and cognitive dysfunction, the current investigators found no association between change in SDMT performance and MRI measures.
From the ASCEND dataset, they found that performance on the SDMT unexpectedly improved with time, perhaps because of a practice effect.
“The SDMT may therefore not adequately reflect the steady cognitive decline that people with SPMS experience,” the investigators wrote.
The lack of association between MRI measures and clinical outcomes may indicate that traditional MRI does not measure important contributors to disability, they noted.
“Although the investigated volume measures in this study are currently the most commonly used in clinical trials, newer MRI metrics such as thalamic or corpus callosum atrophy may have a closer relation to clinical outcome,” they added.
‘Interesting and provocative’
Commenting on the findings, E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children, Toronto, called the study “interesting and provocative.”
“Other studies previously have shown associations between disability and progression, but many have been cross-sectional,” said Dr. Yeh, who was not involved with the research.
The current study is longitudinal and analyzes carefully documented follow-up data from a clinical trial, she noted. However, the 2-year follow-up period was short, considering the pace at which whole brain volume change occurs, Dr. Yeh said.
Some patients with MS have greater brain volume loss than others. Because of this variability, researchers often examine a population’s average brain volume loss. “When you look at averages, it makes it more difficult to understand if the larger brain volume losses are actually associated with change,” said Dr. Yeh.
She noted that because the study population had high EDSS scores at baseline, it is not surprising that the NHPT and the T25FW were more strongly associated with change in brain volume than the EDSS was. Large changes in EDSS score probably did not occur during follow-up, she added.
“We’ll continue to use the EDSS, because it’s what we have,” said Dr. Yeh. However, newer measures, such as the NHPT and the T25FW, may provide better information, she said. Similarly, composite measures of cognition, such as the Brief International Cognitive Assessment for MS, may be superior to the SDMT but take longer to administer.
“We need to look more deeply at which MRI measures are the best for predicting outcome and that correlate well in a short period of time,” said Dr. Yeh.
These measures could include specific regional brain volumes “and more advanced measures that look at axonal injury or axonal loss.” Studies with longer follow-up are also necessary, she concluded.
The investigators and Dr. Yeh have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Analysis from the phase 3 ASCEND trial of nearly 900 patients showed that MRI measures were not associated with worsening of scores on the Expanded Disability Status Scale (EDSS), the most widely used physical outcome measure.
The few associations that were shown between MRI measures and clinical outcomes “were with the newer and possibly more sensitive outcomes” – the Timed 25-Foot Walk (T25FW) and Nine-Hole Peg Test (NHPT), wrote the investigators, led by Marcus W. Koch, MD, PhD, associate professor of neurology in the MS program at the University of Calgary, Canada.
However, “it is unclear if these associations are clinically meaningful,” they added.
Worsening on the NHPT at 48 weeks was associated with a 0.86% loss in normalized brain volume; worsening at 96 weeks was associated with a 1.47% loss.
The findings were published online July 26 in the Multiple Sclerosis Journal.
ASCEND data analysis
Although brain volume loss occurs in all forms of MS, it is believed to be particularly relevant in SPMS. Clinical trials often use MRI measures of brain volume as endpoints, likely on the assumption that these measures indicate worsening disability.
However, brain volume loss proceeds slowly. Changes that occur during the typical 2-year study period may not be associated with significant physical or cognitive disability.
In the current study, investigators examined data from the ASCEND trial, which assessed the use of natalizumab for patients with SPMS, to examine these potential associations. Eligible participants in ASCEND were between ages 18 and 58 years, had had SPMS for 2 or more years, had had disability progression during the previous year, and had an EDSS score between 3.0 and 6.5 at baseline.
Participants underwent gadolinium-enhanced cranial MRI at screening and at 24, 48, 72, and 96 weeks. MRI outcomes included normalized brain volume, normalized cortical gray matter volume, and normalized whole gray matter volume. The ASCEND investigators also examined the number and volume of T2 and contrast-enhancing lesions.
The study’s clinical outcomes included scores on the EDSS, T25FW, and NHPT, which were administered at baseline and every 12 weeks thereafter. Participants also underwent the Symbol Digit Modalities Test (SDMT), which is a cognitive assessment, at baseline and every 4 weeks thereafter. In addition, 3-month confirmed disability progression was measured every 12 weeks.
Few significant associations
The investigators’ analysis included 889 patients (61.9% women; median age, 48 years). The median EDSS score at screening was 6.
Brain volume measures decreased consistently during follow-up. Mean volume loss at 96 weeks was about 1%. In contrast, T2 lesion volume changed little during follow-up. The cumulative number of contrast-enhancing lesions and the cumulative number of new or newly enlarging T2 lesions increased steadily during follow-up.
For an increasing number of participants, scores on the EDSS, NHPT, and T25FW worsened significantly during follow-up. Performance on SDMT, however, changed little. Of all the clinical measures, the NHPT was most consistently associated with MRI measures.
Among patients whose NHPT score worsened at 48 weeks, there was greater loss of normalized brain volume (0.86%, P = .02), normalized cortical gray matter volume (1.15%, P = .03), and normalized whole gray matter volume (1.08%, P = .03) than among those whose NHPT score did not worsen.
Among patients whose NHPT score worsened at 96 weeks, there was greater normalized brain volume loss (1.47%, P = .002), greater increase in T2 lesion volume (4.68%, P = .02), and a greater number of cumulative new or newly enlarging T2 lesions (7.81, P = .03) than those whose NHPT score did not worsen.
After adjusting the data for covariables, the investigators found few significant associations between MRI measures and clinical outcomes. Worsening on the EDSS and SDMT was not associated with any MRI outcome.
Important disability contributors missed
The odds ratio of 3-month confirmed worsening on the T25FW at 96 weeks was 2.25 for patients with more than 10 cumulative new or newly enlarging T2 lesions (P = .03). The OR of 3-month confirmed worsening on the NHPT at 96 weeks was 3.04 for patients with more than 10 such lesions (P = .03).
Greater normalized brain volume loss at 48 weeks was associated with a greater risk for worsening disability on the NHPT at 48 and 96 weeks. For patients with a volume loss greater than 1.5%, the OR of worsening NHPT at 96 weeks was 4.69 (P = .05).
Although previous cross-sectional studies have shown correlations between brain volume and cognitive dysfunction, the current investigators found no association between change in SDMT performance and MRI measures.
From the ASCEND dataset, they found that performance on the SDMT unexpectedly improved with time, perhaps because of a practice effect.
“The SDMT may therefore not adequately reflect the steady cognitive decline that people with SPMS experience,” the investigators wrote.
The lack of association between MRI measures and clinical outcomes may indicate that traditional MRI does not measure important contributors to disability, they noted.
“Although the investigated volume measures in this study are currently the most commonly used in clinical trials, newer MRI metrics such as thalamic or corpus callosum atrophy may have a closer relation to clinical outcome,” they added.
‘Interesting and provocative’
Commenting on the findings, E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children, Toronto, called the study “interesting and provocative.”
“Other studies previously have shown associations between disability and progression, but many have been cross-sectional,” said Dr. Yeh, who was not involved with the research.
The current study is longitudinal and analyzes carefully documented follow-up data from a clinical trial, she noted. However, the 2-year follow-up period was short, considering the pace at which whole brain volume change occurs, Dr. Yeh said.
Some patients with MS have greater brain volume loss than others. Because of this variability, researchers often examine a population’s average brain volume loss. “When you look at averages, it makes it more difficult to understand if the larger brain volume losses are actually associated with change,” said Dr. Yeh.
She noted that because the study population had high EDSS scores at baseline, it is not surprising that the NHPT and the T25FW were more strongly associated with change in brain volume than the EDSS was. Large changes in EDSS score probably did not occur during follow-up, she added.
“We’ll continue to use the EDSS, because it’s what we have,” said Dr. Yeh. However, newer measures, such as the NHPT and the T25FW, may provide better information, she said. Similarly, composite measures of cognition, such as the Brief International Cognitive Assessment for MS, may be superior to the SDMT but take longer to administer.
“We need to look more deeply at which MRI measures are the best for predicting outcome and that correlate well in a short period of time,” said Dr. Yeh.
These measures could include specific regional brain volumes “and more advanced measures that look at axonal injury or axonal loss.” Studies with longer follow-up are also necessary, she concluded.
The investigators and Dr. Yeh have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Analysis from the phase 3 ASCEND trial of nearly 900 patients showed that MRI measures were not associated with worsening of scores on the Expanded Disability Status Scale (EDSS), the most widely used physical outcome measure.
The few associations that were shown between MRI measures and clinical outcomes “were with the newer and possibly more sensitive outcomes” – the Timed 25-Foot Walk (T25FW) and Nine-Hole Peg Test (NHPT), wrote the investigators, led by Marcus W. Koch, MD, PhD, associate professor of neurology in the MS program at the University of Calgary, Canada.
However, “it is unclear if these associations are clinically meaningful,” they added.
Worsening on the NHPT at 48 weeks was associated with a 0.86% loss in normalized brain volume; worsening at 96 weeks was associated with a 1.47% loss.
The findings were published online July 26 in the Multiple Sclerosis Journal.
ASCEND data analysis
Although brain volume loss occurs in all forms of MS, it is believed to be particularly relevant in SPMS. Clinical trials often use MRI measures of brain volume as endpoints, likely on the assumption that these measures indicate worsening disability.
However, brain volume loss proceeds slowly. Changes that occur during the typical 2-year study period may not be associated with significant physical or cognitive disability.
In the current study, investigators examined data from the ASCEND trial, which assessed the use of natalizumab for patients with SPMS, to examine these potential associations. Eligible participants in ASCEND were between ages 18 and 58 years, had had SPMS for 2 or more years, had had disability progression during the previous year, and had an EDSS score between 3.0 and 6.5 at baseline.
Participants underwent gadolinium-enhanced cranial MRI at screening and at 24, 48, 72, and 96 weeks. MRI outcomes included normalized brain volume, normalized cortical gray matter volume, and normalized whole gray matter volume. The ASCEND investigators also examined the number and volume of T2 and contrast-enhancing lesions.
The study’s clinical outcomes included scores on the EDSS, T25FW, and NHPT, which were administered at baseline and every 12 weeks thereafter. Participants also underwent the Symbol Digit Modalities Test (SDMT), which is a cognitive assessment, at baseline and every 4 weeks thereafter. In addition, 3-month confirmed disability progression was measured every 12 weeks.
Few significant associations
The investigators’ analysis included 889 patients (61.9% women; median age, 48 years). The median EDSS score at screening was 6.
Brain volume measures decreased consistently during follow-up. Mean volume loss at 96 weeks was about 1%. In contrast, T2 lesion volume changed little during follow-up. The cumulative number of contrast-enhancing lesions and the cumulative number of new or newly enlarging T2 lesions increased steadily during follow-up.
For an increasing number of participants, scores on the EDSS, NHPT, and T25FW worsened significantly during follow-up. Performance on SDMT, however, changed little. Of all the clinical measures, the NHPT was most consistently associated with MRI measures.
Among patients whose NHPT score worsened at 48 weeks, there was greater loss of normalized brain volume (0.86%, P = .02), normalized cortical gray matter volume (1.15%, P = .03), and normalized whole gray matter volume (1.08%, P = .03) than among those whose NHPT score did not worsen.
Among patients whose NHPT score worsened at 96 weeks, there was greater normalized brain volume loss (1.47%, P = .002), greater increase in T2 lesion volume (4.68%, P = .02), and a greater number of cumulative new or newly enlarging T2 lesions (7.81, P = .03) than those whose NHPT score did not worsen.
After adjusting the data for covariables, the investigators found few significant associations between MRI measures and clinical outcomes. Worsening on the EDSS and SDMT was not associated with any MRI outcome.
Important disability contributors missed
The odds ratio of 3-month confirmed worsening on the T25FW at 96 weeks was 2.25 for patients with more than 10 cumulative new or newly enlarging T2 lesions (P = .03). The OR of 3-month confirmed worsening on the NHPT at 96 weeks was 3.04 for patients with more than 10 such lesions (P = .03).
Greater normalized brain volume loss at 48 weeks was associated with a greater risk for worsening disability on the NHPT at 48 and 96 weeks. For patients with a volume loss greater than 1.5%, the OR of worsening NHPT at 96 weeks was 4.69 (P = .05).
Although previous cross-sectional studies have shown correlations between brain volume and cognitive dysfunction, the current investigators found no association between change in SDMT performance and MRI measures.
From the ASCEND dataset, they found that performance on the SDMT unexpectedly improved with time, perhaps because of a practice effect.
“The SDMT may therefore not adequately reflect the steady cognitive decline that people with SPMS experience,” the investigators wrote.
The lack of association between MRI measures and clinical outcomes may indicate that traditional MRI does not measure important contributors to disability, they noted.
“Although the investigated volume measures in this study are currently the most commonly used in clinical trials, newer MRI metrics such as thalamic or corpus callosum atrophy may have a closer relation to clinical outcome,” they added.
‘Interesting and provocative’
Commenting on the findings, E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children, Toronto, called the study “interesting and provocative.”
“Other studies previously have shown associations between disability and progression, but many have been cross-sectional,” said Dr. Yeh, who was not involved with the research.
The current study is longitudinal and analyzes carefully documented follow-up data from a clinical trial, she noted. However, the 2-year follow-up period was short, considering the pace at which whole brain volume change occurs, Dr. Yeh said.
Some patients with MS have greater brain volume loss than others. Because of this variability, researchers often examine a population’s average brain volume loss. “When you look at averages, it makes it more difficult to understand if the larger brain volume losses are actually associated with change,” said Dr. Yeh.
She noted that because the study population had high EDSS scores at baseline, it is not surprising that the NHPT and the T25FW were more strongly associated with change in brain volume than the EDSS was. Large changes in EDSS score probably did not occur during follow-up, she added.
“We’ll continue to use the EDSS, because it’s what we have,” said Dr. Yeh. However, newer measures, such as the NHPT and the T25FW, may provide better information, she said. Similarly, composite measures of cognition, such as the Brief International Cognitive Assessment for MS, may be superior to the SDMT but take longer to administer.
“We need to look more deeply at which MRI measures are the best for predicting outcome and that correlate well in a short period of time,” said Dr. Yeh.
These measures could include specific regional brain volumes “and more advanced measures that look at axonal injury or axonal loss.” Studies with longer follow-up are also necessary, she concluded.
The investigators and Dr. Yeh have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From Multiple Sclerosis Journal
FDA warns clinicians to stop using Eco-Med products because of contamination concerns
Earlier this month, the Centers for Disease Control and Prevention and the FDA announced an outbreak of at least 15 Bcc infections associated with contaminated ultrasound gel, and, according to the FDA, Eco-Med ultrasound gels have now been linked to at least 59 infections, 48 of which were blood infections.
On Aug. 4, the Canadian pharmaceutical company, based in Etobicoke, Ont., initiated a voluntary recall of certain lots of EcoGel 200 Ultrasound gel because of contamination with Bcc, but now the FDA warns that all Eco-Med’s ultrasound gels and lotions are at risk.
“The FDA’s determination is based on concerns that the company did not complete its investigation of the issues, the root cause and extent of bacterial contamination was not identified, and multiple products could be affected by manufacturing issues associated with the company’s ultrasound gel (such as inappropriate testing of finished product, inadequate testing of raw materials, and a lack of environmental controls),” the FDA said in a letter to health care providers published Aug. 18.
The letter lists 25 products manufactured by Eco-Med that are sold by distributors in 10 different countries, including the United States and Canada. The list may not be completely comprehensive, the organization notes.
Eco-Med has ceased all operations and is no longer manufacturing or distributing products, according to the FDA statement. Both phone numbers listed for the company were not in operation at the time of reporting.
Beyond stopping use of and discarding Eco-Med products, the FDA recommends that health care providers and facilities stop purchases of Eco-Med products, contact distributors with product disposal questions, and follow professional society guidelines and CDC guidelines for ultrasound use and cleaning products. Providers are encouraged to report adverse events related to Eco-Med ultrasound gels or lotions through MedWatch: The FDA Safety Information and Adverse Event Reporting program.
Though Eco-Med is listed as one of the “prominent players in the ultrasound gel market,” according to a June 2020 report by Grand View Research, the announcement will likely not cause many issues, Lauren Golding, MD, chair of the American College of Radiology Commission on Ultrasound, said in an interview.
“Fortunately, several companies produce ultrasound gel. Barring unforeseen circumstances, we do not expect this FDA action to have a widespread impact on patients’ access to ultrasound exams in the United States,” she said.
A version of this article first appeared on Medscape.com.
Earlier this month, the Centers for Disease Control and Prevention and the FDA announced an outbreak of at least 15 Bcc infections associated with contaminated ultrasound gel, and, according to the FDA, Eco-Med ultrasound gels have now been linked to at least 59 infections, 48 of which were blood infections.
On Aug. 4, the Canadian pharmaceutical company, based in Etobicoke, Ont., initiated a voluntary recall of certain lots of EcoGel 200 Ultrasound gel because of contamination with Bcc, but now the FDA warns that all Eco-Med’s ultrasound gels and lotions are at risk.
“The FDA’s determination is based on concerns that the company did not complete its investigation of the issues, the root cause and extent of bacterial contamination was not identified, and multiple products could be affected by manufacturing issues associated with the company’s ultrasound gel (such as inappropriate testing of finished product, inadequate testing of raw materials, and a lack of environmental controls),” the FDA said in a letter to health care providers published Aug. 18.
The letter lists 25 products manufactured by Eco-Med that are sold by distributors in 10 different countries, including the United States and Canada. The list may not be completely comprehensive, the organization notes.
Eco-Med has ceased all operations and is no longer manufacturing or distributing products, according to the FDA statement. Both phone numbers listed for the company were not in operation at the time of reporting.
Beyond stopping use of and discarding Eco-Med products, the FDA recommends that health care providers and facilities stop purchases of Eco-Med products, contact distributors with product disposal questions, and follow professional society guidelines and CDC guidelines for ultrasound use and cleaning products. Providers are encouraged to report adverse events related to Eco-Med ultrasound gels or lotions through MedWatch: The FDA Safety Information and Adverse Event Reporting program.
Though Eco-Med is listed as one of the “prominent players in the ultrasound gel market,” according to a June 2020 report by Grand View Research, the announcement will likely not cause many issues, Lauren Golding, MD, chair of the American College of Radiology Commission on Ultrasound, said in an interview.
“Fortunately, several companies produce ultrasound gel. Barring unforeseen circumstances, we do not expect this FDA action to have a widespread impact on patients’ access to ultrasound exams in the United States,” she said.
A version of this article first appeared on Medscape.com.
Earlier this month, the Centers for Disease Control and Prevention and the FDA announced an outbreak of at least 15 Bcc infections associated with contaminated ultrasound gel, and, according to the FDA, Eco-Med ultrasound gels have now been linked to at least 59 infections, 48 of which were blood infections.
On Aug. 4, the Canadian pharmaceutical company, based in Etobicoke, Ont., initiated a voluntary recall of certain lots of EcoGel 200 Ultrasound gel because of contamination with Bcc, but now the FDA warns that all Eco-Med’s ultrasound gels and lotions are at risk.
“The FDA’s determination is based on concerns that the company did not complete its investigation of the issues, the root cause and extent of bacterial contamination was not identified, and multiple products could be affected by manufacturing issues associated with the company’s ultrasound gel (such as inappropriate testing of finished product, inadequate testing of raw materials, and a lack of environmental controls),” the FDA said in a letter to health care providers published Aug. 18.
The letter lists 25 products manufactured by Eco-Med that are sold by distributors in 10 different countries, including the United States and Canada. The list may not be completely comprehensive, the organization notes.
Eco-Med has ceased all operations and is no longer manufacturing or distributing products, according to the FDA statement. Both phone numbers listed for the company were not in operation at the time of reporting.
Beyond stopping use of and discarding Eco-Med products, the FDA recommends that health care providers and facilities stop purchases of Eco-Med products, contact distributors with product disposal questions, and follow professional society guidelines and CDC guidelines for ultrasound use and cleaning products. Providers are encouraged to report adverse events related to Eco-Med ultrasound gels or lotions through MedWatch: The FDA Safety Information and Adverse Event Reporting program.
Though Eco-Med is listed as one of the “prominent players in the ultrasound gel market,” according to a June 2020 report by Grand View Research, the announcement will likely not cause many issues, Lauren Golding, MD, chair of the American College of Radiology Commission on Ultrasound, said in an interview.
“Fortunately, several companies produce ultrasound gel. Barring unforeseen circumstances, we do not expect this FDA action to have a widespread impact on patients’ access to ultrasound exams in the United States,” she said.
A version of this article first appeared on Medscape.com.