User login
The dark side of online mom groups
I have assumed that being a parent has always been an anxiety-producing experience. Even back when the neonatal mortality rate was orders of magnitude greater than we are experiencing now, I suspect that each birth was still accompanied by a period of angst. However, as families no longer felt the need to produce more children to replace those lost to illness, each surviving child fell under the glare of an ever brightening spotlight.
Raising a child no longer became just something that came naturally, learned from one’s parents. Philosophers and eventually physicians felt obligated to advise parents on the best practices. My parents turned to Dr. Benjamin Spock’s classic work when they had a question, but I never got the feeling that they took his words as gospel.
By the time I started in practice the condition of being a parent was morphing into a verb. Books on “parenting” were beginning to fill the shelves of libraries and bookstores. Frustrated by what I saw as poorly conceived instruction manuals I succumbed to the temptation to spread my “better” advice for anxiety-tormented parents by writing books on how to feed picky eaters, or how to get erratic sleepers to sleep, or how to get a misbehaving child to understand the simple concept of “No!”
Back in the pre-Internet days I was competing for the attention of anxiety-driven parents not just with other self-described experts sitting at word processors, but with grandmothers, aunts, and the ladies next door. The book publishing market has cooled but the demand for advice on how to be the best parent has heated up. Into the void, enabled by the Internet, has erupted the phenomenon of social-media mom groups.
The lady next door and the mothers with strollers meeting informally at the playground are a tiny blip on the radar screen compared with the abundance of other mothers eager to listen and comment on social media–based mom groups unlimited by either geographic or temporal time restraints.
Unfortunately, as a recent article in the Wall Street Journal suggests, these support groups can often have a dark side. Researchers from Pepperdine University found in a small survey of a homogenous population of women that stress, as measured by saliva cortisol levels, increased with increasing use of “mom-centric social media” sites.
Citing anecdotal observations by mothers who did not participate in the study, the WSJ article describes episodes of shaming over topics such as steroid use in eczema and vaccine hesitancy. One mother described how she found group discussions about breastfeeding “particularly anxiety-producing.”
I have limited experience with online support groups but I have been surprised by how rude and condescending some of the contributors can be to what I could consider to be emotionally neutral subjects such as outboard motor oil pressure. I can imagine that when it comes to subjects in which there is no one best answer, the relative anonymity of the Internet provides cover for language that can be hurtful and stress inducing for someone already feeling isolated and anxious about being a parent.
Although this Pepperdine study is small, I suspect that a larger study would support the authors’ observations. For us as providers, it suggests that we need to find where parents are getting their information when we are trying to help those who seem particularly distressed. We should caution them that, while sharing information with peers can be reassuring and helpful at times, mom groups can be toxic as well. It also means that we should be careful in recommending social media sites – even those for which we have had good feedback.
And, most importantly, we must continue to work hard to make ourselves available to provide sensible and sensitive answers to those questions that are anxiety-producing for new parents.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I have assumed that being a parent has always been an anxiety-producing experience. Even back when the neonatal mortality rate was orders of magnitude greater than we are experiencing now, I suspect that each birth was still accompanied by a period of angst. However, as families no longer felt the need to produce more children to replace those lost to illness, each surviving child fell under the glare of an ever brightening spotlight.
Raising a child no longer became just something that came naturally, learned from one’s parents. Philosophers and eventually physicians felt obligated to advise parents on the best practices. My parents turned to Dr. Benjamin Spock’s classic work when they had a question, but I never got the feeling that they took his words as gospel.
By the time I started in practice the condition of being a parent was morphing into a verb. Books on “parenting” were beginning to fill the shelves of libraries and bookstores. Frustrated by what I saw as poorly conceived instruction manuals I succumbed to the temptation to spread my “better” advice for anxiety-tormented parents by writing books on how to feed picky eaters, or how to get erratic sleepers to sleep, or how to get a misbehaving child to understand the simple concept of “No!”
Back in the pre-Internet days I was competing for the attention of anxiety-driven parents not just with other self-described experts sitting at word processors, but with grandmothers, aunts, and the ladies next door. The book publishing market has cooled but the demand for advice on how to be the best parent has heated up. Into the void, enabled by the Internet, has erupted the phenomenon of social-media mom groups.
The lady next door and the mothers with strollers meeting informally at the playground are a tiny blip on the radar screen compared with the abundance of other mothers eager to listen and comment on social media–based mom groups unlimited by either geographic or temporal time restraints.
Unfortunately, as a recent article in the Wall Street Journal suggests, these support groups can often have a dark side. Researchers from Pepperdine University found in a small survey of a homogenous population of women that stress, as measured by saliva cortisol levels, increased with increasing use of “mom-centric social media” sites.
Citing anecdotal observations by mothers who did not participate in the study, the WSJ article describes episodes of shaming over topics such as steroid use in eczema and vaccine hesitancy. One mother described how she found group discussions about breastfeeding “particularly anxiety-producing.”
I have limited experience with online support groups but I have been surprised by how rude and condescending some of the contributors can be to what I could consider to be emotionally neutral subjects such as outboard motor oil pressure. I can imagine that when it comes to subjects in which there is no one best answer, the relative anonymity of the Internet provides cover for language that can be hurtful and stress inducing for someone already feeling isolated and anxious about being a parent.
Although this Pepperdine study is small, I suspect that a larger study would support the authors’ observations. For us as providers, it suggests that we need to find where parents are getting their information when we are trying to help those who seem particularly distressed. We should caution them that, while sharing information with peers can be reassuring and helpful at times, mom groups can be toxic as well. It also means that we should be careful in recommending social media sites – even those for which we have had good feedback.
And, most importantly, we must continue to work hard to make ourselves available to provide sensible and sensitive answers to those questions that are anxiety-producing for new parents.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I have assumed that being a parent has always been an anxiety-producing experience. Even back when the neonatal mortality rate was orders of magnitude greater than we are experiencing now, I suspect that each birth was still accompanied by a period of angst. However, as families no longer felt the need to produce more children to replace those lost to illness, each surviving child fell under the glare of an ever brightening spotlight.
Raising a child no longer became just something that came naturally, learned from one’s parents. Philosophers and eventually physicians felt obligated to advise parents on the best practices. My parents turned to Dr. Benjamin Spock’s classic work when they had a question, but I never got the feeling that they took his words as gospel.
By the time I started in practice the condition of being a parent was morphing into a verb. Books on “parenting” were beginning to fill the shelves of libraries and bookstores. Frustrated by what I saw as poorly conceived instruction manuals I succumbed to the temptation to spread my “better” advice for anxiety-tormented parents by writing books on how to feed picky eaters, or how to get erratic sleepers to sleep, or how to get a misbehaving child to understand the simple concept of “No!”
Back in the pre-Internet days I was competing for the attention of anxiety-driven parents not just with other self-described experts sitting at word processors, but with grandmothers, aunts, and the ladies next door. The book publishing market has cooled but the demand for advice on how to be the best parent has heated up. Into the void, enabled by the Internet, has erupted the phenomenon of social-media mom groups.
The lady next door and the mothers with strollers meeting informally at the playground are a tiny blip on the radar screen compared with the abundance of other mothers eager to listen and comment on social media–based mom groups unlimited by either geographic or temporal time restraints.
Unfortunately, as a recent article in the Wall Street Journal suggests, these support groups can often have a dark side. Researchers from Pepperdine University found in a small survey of a homogenous population of women that stress, as measured by saliva cortisol levels, increased with increasing use of “mom-centric social media” sites.
Citing anecdotal observations by mothers who did not participate in the study, the WSJ article describes episodes of shaming over topics such as steroid use in eczema and vaccine hesitancy. One mother described how she found group discussions about breastfeeding “particularly anxiety-producing.”
I have limited experience with online support groups but I have been surprised by how rude and condescending some of the contributors can be to what I could consider to be emotionally neutral subjects such as outboard motor oil pressure. I can imagine that when it comes to subjects in which there is no one best answer, the relative anonymity of the Internet provides cover for language that can be hurtful and stress inducing for someone already feeling isolated and anxious about being a parent.
Although this Pepperdine study is small, I suspect that a larger study would support the authors’ observations. For us as providers, it suggests that we need to find where parents are getting their information when we are trying to help those who seem particularly distressed. We should caution them that, while sharing information with peers can be reassuring and helpful at times, mom groups can be toxic as well. It also means that we should be careful in recommending social media sites – even those for which we have had good feedback.
And, most importantly, we must continue to work hard to make ourselves available to provide sensible and sensitive answers to those questions that are anxiety-producing for new parents.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Debating the clinical trial upending colonoscopy practices
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: Hello, and thank you for joining us today for what promises to be a lively discussion about screening for colon cancer.
My name is Perry Wilson. I’m an associate professor of medicine and director of the Clinical and Translational Research Accelerator at the Yale School of Medicine. My new book, “How Medicine Works and When It Doesn’t: Learning Who to Trust to Get and Stay Healthy,” is available for pre-order now anywhere that books are sold.
I’m joined by two wonderful experts. Dr. David Johnson is a professor of medicine and the chief of gastroenterology at the Eastern Virginia School of Medicine. He is the past president of the American College of Gastroenterology. And I’m very encouraged to see that he’s won a Distinguished Educator Award for his efforts in gastroenterology.
I’m also joined by Dr Kenny Lin. He’s a frequent contributor to Medscape and WebMD. He’s a family physician and public health consultant from Lancaster, Pa., and deputy editor of the American Family Physician journal. He’s also a teacher of residents and students at Lancaster General Health and the Penn Medicine Family Medicine Residency program.
So, we have two great educators with us today to hopefully help teach us something about colon cancer and colon cancer screening. Thank you for joining me today.
David A. Johnson, MD: Thanks for having us.
Kenneth W. Lin, MD, MPH: Good to be here.
Dr. Wilson: Colon cancer is the second leading cause of cancer mortality in the United States. A little over 50,000 people die every year in the United States due to colon cancer.
A month ago, I would have said that there was a pretty broad consensus, at least from my perspective, that people should be getting colonoscopies. That’s certainly what we tell our patients.
Then a paper came out in the New England Journal of Medicine, a very prestigious journal, that has caused a lot of consternation online and led to my receiving a lot questions from patients and their family members. Today,
Dr Johnson, can you give us a brief overview of what this trial was about?
Dr. Johnson: This was a randomized trial looking at screening colonoscopy versus no screening test whatsoever. They looked at the outcomes of prevention of cancer and the prevention of colon cancer–related death.
The short answer was that it was disappointing as it relates to colonoscopy. The study looked at patients from four European countries, with data from three of them (Norway, Poland, and Sweden) ultimately analyzed in this report in NEJM. It got a lot of attention because it surprised a lot of people by saying maybe colonoscopy wasn’t quite as good as we thought it was.
They tried to correct that by only looking at the numbers of patients who got their colonoscopy screening, which still showed value, but it was less than that we’ve seen before. There’s lots of reasons for that, which we’ll discuss shortly.
An invitation to a screening
Dr. Wilson: This was a bit of an interesting trial design. I think I’m correct, Dr Lin, that this was the first randomized trial of screening colonoscopy. But they didn’t really randomize people to get a colonoscopy versus not get a colonoscopy. Can you tell us why this differed from that study design, which I’d have thought would be simpler way of assessing this?
Dr. Lin: It’s definitely an important point to highlight about the study. What investigators did was randomize patients to receive an invitation to get a screening colonoscopy. When the trial was set up, they randomized people before they were asked whether they wanted to participate in the study. If you did it the other way around, by first asking them whether they wanted to be in the study and then randomizing them, you would have been assured that more of them probably would have gotten the colonoscopy.
But in this case, they were more interested in figuring out the real-life results of having a national program that invited patients to receive screening colonoscopy. Because we know that everyone that you recommend to get a colonoscopy doesn’t necessarily want to do that, forgets to do it, or something happens that prevents their actually getting it.
When it comes to measuring the effectiveness of the colonoscopy, it perhaps wasn’t the greatest type of study to do that. But I think it did provide some information about what would happen if you invited people to get colonoscopy, in terms of how many would do it and the results overall for that population.
Lower participation numbers than expected
Dr. Wilson: Dr. Johnson, the data show that 42% of people who were in that invitation arm followed through and got their colonoscopy. You’re a gastroenterologist. Does that seem low or about right? Do about half of people who should get a colonoscopy end up getting one?
Dr. Johnson: No, it’s low. In the United States, those numbers are probably in the 70% range. Certainly, the test doesn’t work for people who don’t get the test performed. So, if 42% of those randomized to receive an invitation to get the colonoscopy got one, that really means the majority of patients never got the test.
Dr. Wilson: Certainly, we wouldn’t expect impressive results if they don’t get the test. But on the other hand, I imagine that people who choose to get the test when they’re invited are sort of a different breed. Perhaps they’re more health conscious or living in other healthy ways. Is that something we should worry about when we look at these results?
Dr. Johnson: I don’t think you can stratify based on this study. Factors like ethnicities and diet weren’t really explained. The key element that will hopefully have the major take-home impact is quality. It’s not just the test. It’s how the test is done.
The key results
Dr. Wilson: Let’s start with the big picture. This was a study looking at everyone invited; not the subgroup of people who got the colonoscopy, but the real randomized study population.
Dr. Lin, the study did show that the invited group had a lower risk of colon cancer over the next 10 years. That’s a good thing, I imagine.
Dr. Lin: I think that’s a significant benefit. Initially in the first few years, they had more colon cancers diagnosed. But that’s probably because those were cancers that were already existing and couldn’t be prevented by the test.
But then over the years the curves crossed, and by the end of the average follow-up of 10 years, there was a significantly lower rate of colon cancers being detected. That’s as you would expect, because you’re finding polyps and removing them before they became colon cancer.
Dr. Wilson: Dr. Johnson, is that the natural history of colon cancer? It starts out as a polyp that maybe can be easily removed and doesn’t require more therapy. Is that why screening colonoscopy is helpful?
Dr. Johnson: The ultimate goal of screening is prevention of cancer, rather than detection of cancer. That occurs by identification and complete removal of the polyps that we find that are precancerous. The key is, first, detection, and second, resection. Adequate resection comes down to some very significant issues of quality, which are questions that I’d raised about this study, and we can talk about momentarily.
Dr. Wilson: Absolutely. Let me first go through the two other big findings in this study.
The fact that there were fewer cases of colon cancer over 10 years seems good. But colon cancer mortality was not significantly different in the two groups. Now, of course, we know that not everyone got a colonoscopy. I would have expected though, if you had less colon cancer, you’d have less death from colon cancer.
Dr. Lin, what might explain this disconnect?
Dr. Lin: I think there are a couple of possible explanations.
One explanation is that they just didn’t follow the people long enough. Colon cancer takes a long time to go from an adenoma to cancer, and from cancer to something that would cause the patient’s death. You may need to follow them for longer than the 10 years that most of these patients were followed to see that benefit. I think there probably will be benefit after a while, because if you are removing colon cancers that otherwise would have progressed and metastasized, you often see a benefit.
We also have to consider the other possibility that not all the polyps removed necessarily were going to progress to advanced cancer. Therefore, you weren’t seeing the death benefit because not every polyp that was removed was necessarily going to cause health consequences.
In colonoscopy, quality is key to success
Dr. Wilson: You’re removing things and have no way of knowing in advance which are the bad ones and which aren’t.
Dr. Johnson, you’ve mentioned several times now that the quality of colonoscopy matters here. So, I’m intuiting that it’s not one-size-fits-all, that it’s not all the same. What do you mean by quality of colonoscopy, and what was it in the NEJM study?
Dr. Johnson: Quality colonoscopy is the quality of the whole process. It starts with the warm-up, if you will, and the clean out for the procedure. That allows the colonoscopist to be able to identify precancerous polyps, which we call adenomas (there are other precancerous polyps called sessile serrated lesions).
The identification of adenomas is extremely important. Even a small increase in the detection of those precancerous polyps has benefits. Well-performed studies looking at large databases show that a small, 1% increase in the adenoma detection leads to a 3% decrease in colon cancer and a 5% decrease in colon cancer–related death. There’s a huge array of effect when we talk about small increases in the adenoma detection rate.
Now, let’s go back to this study in NEJM.
If we base quality on the physician performing the colonoscopy, and say that the colonoscopy is achieving the act of getting all the way around the colon, but not all physicians in the study were able to do that, it starts to raise the question about quality, because adenoma detection is so important. Earlier reports from this group [Nordic-European Initiative on Colorectal Cancer Study Group] have shown that the adenoma detection rates have been way below the national thresholds. So, this raises the question of whether they found the polyp, and then whether they resected the polyp. They also don’t tell us where these cancers were. It is about the colonoscopy quality. It’s not the instrument. It’s the process.
An overview of other screening tools
Dr. Wilson: Dr. Lin, colonoscopy, which requires prep and anesthesia, is not the only colon cancer screening method we have. In fact, there are a bunch. I think we’re on board saying it’s probably better to detect colon cancer early than not detect it. But what are our other options aside from colonoscopy that can allow for early detection of colon cancer?
Dr. Lin: For most of my career, there were three options that I presented patients with. The first was the fecal test, which used to be in the form of initial hemoccult tests. These have been mostly replaced by fecal immunochemical testing. But they’re both just basically looking for the presence of blood in the stool. Anyone who has a positive test would be referred for a diagnostic colonoscopy.
The other test besides colonoscopy, which has been largely phased out in the United States, although it is still very much used in Canada and much of Europe, is flexible sigmoidoscopy. Until this study, the tests supported by randomized controlled trials were the fecal tests and flexible sigmoidoscopy.
Interestingly, there was a recent systematic review of flexible sigmoidoscopy looking at four trials and their effects over 15 years. They showed not only a reduction in colon cancer, but also a reduction in colon cancer mortality, and even a small reduction in all-cause mortality.
I believe three out of the four trials were done where the patients were consented and then randomized, so they had a higher uptake of the procedure.
But when you compare this with the colonoscopy trial, it really isn’t that impressive. You would expect a much larger benefit, because obviously you’re looking at the entire colon. But you really didn’t see that. It was, at best, maybe equivalent to sigmoidoscopy, but not a whole lot better.
Dr. Johnson: Perry, you mentioned sedation. It’s important to understand that this particular cohort of patients are from Norway, Sweden, and Poland, where it’s very much the norm to not get sedation for your colonoscopy. Any of the [audience] who have had colonoscopy will tell you that they are not ones to say, “Don’t give me sedation.” The rate of sedation is around 11% in Norway, maybe 23% in Sweden, and around 45% in Poland. So, the examiner and the patient were never really super comfortable.
I’ve done 50,000 colonoscopies in my career, and many nonsedated. We know that taking time increases the finding of polyps and the adequate identification and resection. So, that ability to perform at a high quality is very much impacted when the patients aren’t comfortable.
Dr. Wilson: Dr. Johnson, we brought up flexible sigmoidoscopy. For the patients watching whose doctors are talking to them about screening colonoscopy, what’s the difference?
Dr. Johnson: Flexible sigmoidoscopy is just a short scope examination, in which you see about one-third of the colon. I’ve been in the field for 45 years, and during that time we’ve seen that there’s a progressive increase in the development of cancers above that bottom third of the colon to the higher end, the two-thirds of the colon that you would miss without doing a full colonoscopy. Also, flexible sigmoidoscopy typically does not get covered for sedation.
Again, if you do the exam and find something, then you’re going to have to come back and do an adequate resection with a colonoscopy. So, one-stop-shopping colon cancer screening is not about detection of cancer, it’s about prevention of cancer, and that’s what colonoscopy does.
Patients want convenience, but at what cost?
Dr. Wilson: Dr. Lin, how are your patients in your family practice handling this study? Have conversations changed around colon cancer screening? What are people asking about these days?
Dr. Lin: I don’t think the conversations have changed in my practice that much. When patients ask about this study, we do discuss the limitations, that it wasn’t designed to assess the maximum benefit of getting a colonoscopy because the majority of people assigned to that group didn’t get colonoscopy.
But I think it is an opportunity in primary care to consider the way we present the options to patients. Because I would guess that a majority of primary care physicians, when they present the options, would say colonoscopy is the gold standard and recommend their patients get it. And they only offer fecal testing to patients who don’t want the colonoscopy or really refuse.
That hasn’t been my practice. I’m usually more agnostic, because there are both harms and benefits. If you get a fecal test, the chance of you having a complication from colonoscopy is automatically lower because most of those people will not get colonoscopy. Now obviously, the complications with colonoscopy are pretty rare and usually self-limited, but they do exist. If you’re doing lots and lots of these, eventually you’ll see them. Probably all primary care physicians have patients who’ve had a complication from colonoscopy and may or may not have regretted it depending on how information was presented.
But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. Is your priority finding every single cancer? Do you want to know exactly what the benefit is? I think with colonoscopy, we’re still trying to figure out exactly what the benefit is. Whereas we can say it pretty confidently for fecal tests because we have those randomized trials.
Dr. Wilson: Dr. Johnson, I think patients who are watching need to know, first of all, that if they do the fecal test route, a positive fecal test does lead to colonoscopy. In some sense, all roads lead to colonoscopy once you have a positive screening test. So, I can certainly see the value of just sort of skipping to that point. But what about this risk-versus-benefit relationship? Colonoscopy, albeit a relatively safe procedure, is still a procedure. There is some risk associated with it. If we can get the same benefit from yearly fecal immunochemical testing, is that a better choice potentially, at least for patients at average risk?
Dr. Johnson: The stool-based testing is really more effective for detection of cancer. That’s not screening, where the entire goal is the prevention of cancer. The fecal-based testing, including the stool-based DNA testing, misses the majority of precancerous polyps. And the fecal immunochemical tests, which Dr. Lin just mentioned, misses virtually all of them. We really want to get to the prevention of cancer, meaning identification and removal of polyps, not just screening for cancer.
Dr. Wilson: Do you see anything on the horizon that could unseat colonoscopy as, to quote Dr. Lin, the potential gold standard for screening for colon cancer?
Dr. Johnson: I think not on the horizon for identification and removal of polyps. That’s really the gold standard. Technology continues to advance. We’ll see what happens. But on the short and intermediate horizon, colonoscopy is going to be needed.
We are finding that some patients are starting to acquiesce to stool-based testing because they can do it at home. Maybe they don’t have to do a prep. We’re talking about screening only here, not about the follow-up of patients who have a family history, patients who have colitis, patients who have had colon polyps, or other reasons. Stool-based testing is not an option for the follow-up of those patients.
Convenience testing, in the face of COVID, also has thrown a wrench into things. Patients may have wanted to stay home and do these tests. Again, we need to be proactive, not reactive. We want to prevent cancer, not detect it.
Changing advice in the face of younger screening thresholds
Dr. Wilson: Dr. Lin, I’m 42 years old. I don’t believe I’m at any increased risk of colon cancer based on my family history or other risk factors. I’m 3 years away from when the U.S. Preventive Services Task Force tells me I should potentially consider starting to screen for colon cancer. That recommendation has recently been moved down from 50 years old to 45 years old. So, it’s on my mind as I approach that age. What do you advise younger patients approaching 45 right now in terms of screening for colon cancer?
Dr. Lin: For patients with the risk factors that Dr. Johnson mentioned, I would recommend screening colonoscopy as the initial test.
Assuming you don’t have those risk factors, I present it as we have a couple of different fecal tests. There’s the traditional one that just looks for blood. Then there’s the newer one that also adds DNA, which is more sensitive for colorectal cancer, but a little less specific, which is a problem just because there are more false positives.
But you need to compare that with colonoscopy, which you only need to get done ideally every 10 years if there are no findings. That is more complete. And theoretically, as we’ve been talking about, it would also prevent as well as detect early cancers.
So, I think it’s really down to your preference in terms of how the various factors that come into play, such as convenience of the test and your level of concern about cancer. I do tell patients that family history of cancer is not terribly predictive of whether you get it or not. A lot of people unfortunately who develop colorectal cancer have no previous family history. Diet will come into play to some extent. There are some things that point to increased risk for colorectal cancer if you have a diet high in red meat and things like that. But ultimately, it really is up to the patient. I lay out the options, and whatever they choose, I’m happy to pursue.
But the most important thing is that they do some test, because doing no test is not going to help anyone. I do agree with the notion that the best test is the test that gets done.
Dr. Wilson: Absolutely. I think the NEJM study supports that, even when we’re talking about colonoscopy.
Dr. Johnson, you’ve had some criticisms about the NEJM study, and I think they make sense. At the same time, as this is the first randomized trial of colonoscopy, it’s kind of the only data we have. Are we going to get better data? Are there other studies going on out there that might help shed some light on what’s turning out to be a complicated issue?
Dr. Johnson: Yes, there are ongoing studies. They’re not taking place within the United States, because you couldn’t get through a no-screening option trial. There are comparative studies that are probably still 5 years away looking at stool-based testing.
But again, we have to recognize that if you do these alternative tests that were eloquently discussed by Dr. Lin, and not the colonoscopy, which would be every 10 years with high-quality performance, that you have to annualize or do them in sequence. It’s important that you follow up on those with regularity. It’s not just a one-time test every 10 years for these individual tests.
And any of the time that those tests are ordered, the patient should be instructed that if it’s positive you need a colonoscopy. We’re seeing a lot of slippage on that front for the stool-based testing. Convenience is not the answer. It’s getting the job done.
Dr. Wilson: Would you agree, Dr. Johnson, that for patients that really don’t want to do the colonoscopy for one reason or another, and you’ve done your best in explaining what you think the risks and benefits are, that you’d rather have them get something than nothing?
Dr. Johnson: Absolutely. It comes down to what I recommend and then what you decide. But I still make the point explicit: If we’ve gone through those checkpoints and it’s positive, we agree that you understand that colonoscopy is the next step.
Final take-home messages
Dr. Wilson: Dr. Lin, I’ll turn the last word over to you, as the person who is probably discussing the choice of screening modalities more than any of us, before someone would get referred to someone like Dr. Johnson. What’s your final take-home message about the NEJM study and the state of colon cancer screening in the United States?
Dr. Lin: My take-home points about the study are that there were some limitations, but it is good to finally have a randomized trial of colonoscopy screening 2 decades after we really started doing that in the United States. It won’t immediately change – nor do I think it should – the way we practice and discuss different options. I think that some of Dr. Johnson’s points about making sure that whoever’s doing the colonoscopies for your practices is doing it in a high-quality way are really important. Just as it’s important, if you’re doing the fecal tests, to make sure that all patients who have positives get expeditiously referred for colonoscopy.
Dr. Johnson: Perry, I’d like to make one concluding comment as the gastroenterology expert in this discussion. I’ve had countless questions about this study from my patients and my peers. I tell them the following: Don’t let the headlines mislead you.
When you look at this study, the instrument is not so much the question. We know that getting the test is the first step in colon cancer screening. But we also know that getting the test done, with the highest-quality providers and the best-quality performance, is really the key to optimizing the true value of colonoscopy for colon cancer prevention.
So please don’t lose sight of this when reading the headlines in the media around this study. We really need to analyze the true characteristics of what we call a quality performance, because that’s what drives success and that’s what prevents colon cancer.
Dr. Wilson: Dr. Johnson and Dr. Lin, thank you very much. I appreciate you spending time with me here today and wish you all the best.
I guess I’ll sum up by saying that if you’re getting a colonoscopy, make sure it’s a good one. But do get screened.
This video originally appeared on WebMD. A transcript appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: Hello, and thank you for joining us today for what promises to be a lively discussion about screening for colon cancer.
My name is Perry Wilson. I’m an associate professor of medicine and director of the Clinical and Translational Research Accelerator at the Yale School of Medicine. My new book, “How Medicine Works and When It Doesn’t: Learning Who to Trust to Get and Stay Healthy,” is available for pre-order now anywhere that books are sold.
I’m joined by two wonderful experts. Dr. David Johnson is a professor of medicine and the chief of gastroenterology at the Eastern Virginia School of Medicine. He is the past president of the American College of Gastroenterology. And I’m very encouraged to see that he’s won a Distinguished Educator Award for his efforts in gastroenterology.
I’m also joined by Dr Kenny Lin. He’s a frequent contributor to Medscape and WebMD. He’s a family physician and public health consultant from Lancaster, Pa., and deputy editor of the American Family Physician journal. He’s also a teacher of residents and students at Lancaster General Health and the Penn Medicine Family Medicine Residency program.
So, we have two great educators with us today to hopefully help teach us something about colon cancer and colon cancer screening. Thank you for joining me today.
David A. Johnson, MD: Thanks for having us.
Kenneth W. Lin, MD, MPH: Good to be here.
Dr. Wilson: Colon cancer is the second leading cause of cancer mortality in the United States. A little over 50,000 people die every year in the United States due to colon cancer.
A month ago, I would have said that there was a pretty broad consensus, at least from my perspective, that people should be getting colonoscopies. That’s certainly what we tell our patients.
Then a paper came out in the New England Journal of Medicine, a very prestigious journal, that has caused a lot of consternation online and led to my receiving a lot questions from patients and their family members. Today,
Dr Johnson, can you give us a brief overview of what this trial was about?
Dr. Johnson: This was a randomized trial looking at screening colonoscopy versus no screening test whatsoever. They looked at the outcomes of prevention of cancer and the prevention of colon cancer–related death.
The short answer was that it was disappointing as it relates to colonoscopy. The study looked at patients from four European countries, with data from three of them (Norway, Poland, and Sweden) ultimately analyzed in this report in NEJM. It got a lot of attention because it surprised a lot of people by saying maybe colonoscopy wasn’t quite as good as we thought it was.
They tried to correct that by only looking at the numbers of patients who got their colonoscopy screening, which still showed value, but it was less than that we’ve seen before. There’s lots of reasons for that, which we’ll discuss shortly.
An invitation to a screening
Dr. Wilson: This was a bit of an interesting trial design. I think I’m correct, Dr Lin, that this was the first randomized trial of screening colonoscopy. But they didn’t really randomize people to get a colonoscopy versus not get a colonoscopy. Can you tell us why this differed from that study design, which I’d have thought would be simpler way of assessing this?
Dr. Lin: It’s definitely an important point to highlight about the study. What investigators did was randomize patients to receive an invitation to get a screening colonoscopy. When the trial was set up, they randomized people before they were asked whether they wanted to participate in the study. If you did it the other way around, by first asking them whether they wanted to be in the study and then randomizing them, you would have been assured that more of them probably would have gotten the colonoscopy.
But in this case, they were more interested in figuring out the real-life results of having a national program that invited patients to receive screening colonoscopy. Because we know that everyone that you recommend to get a colonoscopy doesn’t necessarily want to do that, forgets to do it, or something happens that prevents their actually getting it.
When it comes to measuring the effectiveness of the colonoscopy, it perhaps wasn’t the greatest type of study to do that. But I think it did provide some information about what would happen if you invited people to get colonoscopy, in terms of how many would do it and the results overall for that population.
Lower participation numbers than expected
Dr. Wilson: Dr. Johnson, the data show that 42% of people who were in that invitation arm followed through and got their colonoscopy. You’re a gastroenterologist. Does that seem low or about right? Do about half of people who should get a colonoscopy end up getting one?
Dr. Johnson: No, it’s low. In the United States, those numbers are probably in the 70% range. Certainly, the test doesn’t work for people who don’t get the test performed. So, if 42% of those randomized to receive an invitation to get the colonoscopy got one, that really means the majority of patients never got the test.
Dr. Wilson: Certainly, we wouldn’t expect impressive results if they don’t get the test. But on the other hand, I imagine that people who choose to get the test when they’re invited are sort of a different breed. Perhaps they’re more health conscious or living in other healthy ways. Is that something we should worry about when we look at these results?
Dr. Johnson: I don’t think you can stratify based on this study. Factors like ethnicities and diet weren’t really explained. The key element that will hopefully have the major take-home impact is quality. It’s not just the test. It’s how the test is done.
The key results
Dr. Wilson: Let’s start with the big picture. This was a study looking at everyone invited; not the subgroup of people who got the colonoscopy, but the real randomized study population.
Dr. Lin, the study did show that the invited group had a lower risk of colon cancer over the next 10 years. That’s a good thing, I imagine.
Dr. Lin: I think that’s a significant benefit. Initially in the first few years, they had more colon cancers diagnosed. But that’s probably because those were cancers that were already existing and couldn’t be prevented by the test.
But then over the years the curves crossed, and by the end of the average follow-up of 10 years, there was a significantly lower rate of colon cancers being detected. That’s as you would expect, because you’re finding polyps and removing them before they became colon cancer.
Dr. Wilson: Dr. Johnson, is that the natural history of colon cancer? It starts out as a polyp that maybe can be easily removed and doesn’t require more therapy. Is that why screening colonoscopy is helpful?
Dr. Johnson: The ultimate goal of screening is prevention of cancer, rather than detection of cancer. That occurs by identification and complete removal of the polyps that we find that are precancerous. The key is, first, detection, and second, resection. Adequate resection comes down to some very significant issues of quality, which are questions that I’d raised about this study, and we can talk about momentarily.
Dr. Wilson: Absolutely. Let me first go through the two other big findings in this study.
The fact that there were fewer cases of colon cancer over 10 years seems good. But colon cancer mortality was not significantly different in the two groups. Now, of course, we know that not everyone got a colonoscopy. I would have expected though, if you had less colon cancer, you’d have less death from colon cancer.
Dr. Lin, what might explain this disconnect?
Dr. Lin: I think there are a couple of possible explanations.
One explanation is that they just didn’t follow the people long enough. Colon cancer takes a long time to go from an adenoma to cancer, and from cancer to something that would cause the patient’s death. You may need to follow them for longer than the 10 years that most of these patients were followed to see that benefit. I think there probably will be benefit after a while, because if you are removing colon cancers that otherwise would have progressed and metastasized, you often see a benefit.
We also have to consider the other possibility that not all the polyps removed necessarily were going to progress to advanced cancer. Therefore, you weren’t seeing the death benefit because not every polyp that was removed was necessarily going to cause health consequences.
In colonoscopy, quality is key to success
Dr. Wilson: You’re removing things and have no way of knowing in advance which are the bad ones and which aren’t.
Dr. Johnson, you’ve mentioned several times now that the quality of colonoscopy matters here. So, I’m intuiting that it’s not one-size-fits-all, that it’s not all the same. What do you mean by quality of colonoscopy, and what was it in the NEJM study?
Dr. Johnson: Quality colonoscopy is the quality of the whole process. It starts with the warm-up, if you will, and the clean out for the procedure. That allows the colonoscopist to be able to identify precancerous polyps, which we call adenomas (there are other precancerous polyps called sessile serrated lesions).
The identification of adenomas is extremely important. Even a small increase in the detection of those precancerous polyps has benefits. Well-performed studies looking at large databases show that a small, 1% increase in the adenoma detection leads to a 3% decrease in colon cancer and a 5% decrease in colon cancer–related death. There’s a huge array of effect when we talk about small increases in the adenoma detection rate.
Now, let’s go back to this study in NEJM.
If we base quality on the physician performing the colonoscopy, and say that the colonoscopy is achieving the act of getting all the way around the colon, but not all physicians in the study were able to do that, it starts to raise the question about quality, because adenoma detection is so important. Earlier reports from this group [Nordic-European Initiative on Colorectal Cancer Study Group] have shown that the adenoma detection rates have been way below the national thresholds. So, this raises the question of whether they found the polyp, and then whether they resected the polyp. They also don’t tell us where these cancers were. It is about the colonoscopy quality. It’s not the instrument. It’s the process.
An overview of other screening tools
Dr. Wilson: Dr. Lin, colonoscopy, which requires prep and anesthesia, is not the only colon cancer screening method we have. In fact, there are a bunch. I think we’re on board saying it’s probably better to detect colon cancer early than not detect it. But what are our other options aside from colonoscopy that can allow for early detection of colon cancer?
Dr. Lin: For most of my career, there were three options that I presented patients with. The first was the fecal test, which used to be in the form of initial hemoccult tests. These have been mostly replaced by fecal immunochemical testing. But they’re both just basically looking for the presence of blood in the stool. Anyone who has a positive test would be referred for a diagnostic colonoscopy.
The other test besides colonoscopy, which has been largely phased out in the United States, although it is still very much used in Canada and much of Europe, is flexible sigmoidoscopy. Until this study, the tests supported by randomized controlled trials were the fecal tests and flexible sigmoidoscopy.
Interestingly, there was a recent systematic review of flexible sigmoidoscopy looking at four trials and their effects over 15 years. They showed not only a reduction in colon cancer, but also a reduction in colon cancer mortality, and even a small reduction in all-cause mortality.
I believe three out of the four trials were done where the patients were consented and then randomized, so they had a higher uptake of the procedure.
But when you compare this with the colonoscopy trial, it really isn’t that impressive. You would expect a much larger benefit, because obviously you’re looking at the entire colon. But you really didn’t see that. It was, at best, maybe equivalent to sigmoidoscopy, but not a whole lot better.
Dr. Johnson: Perry, you mentioned sedation. It’s important to understand that this particular cohort of patients are from Norway, Sweden, and Poland, where it’s very much the norm to not get sedation for your colonoscopy. Any of the [audience] who have had colonoscopy will tell you that they are not ones to say, “Don’t give me sedation.” The rate of sedation is around 11% in Norway, maybe 23% in Sweden, and around 45% in Poland. So, the examiner and the patient were never really super comfortable.
I’ve done 50,000 colonoscopies in my career, and many nonsedated. We know that taking time increases the finding of polyps and the adequate identification and resection. So, that ability to perform at a high quality is very much impacted when the patients aren’t comfortable.
Dr. Wilson: Dr. Johnson, we brought up flexible sigmoidoscopy. For the patients watching whose doctors are talking to them about screening colonoscopy, what’s the difference?
Dr. Johnson: Flexible sigmoidoscopy is just a short scope examination, in which you see about one-third of the colon. I’ve been in the field for 45 years, and during that time we’ve seen that there’s a progressive increase in the development of cancers above that bottom third of the colon to the higher end, the two-thirds of the colon that you would miss without doing a full colonoscopy. Also, flexible sigmoidoscopy typically does not get covered for sedation.
Again, if you do the exam and find something, then you’re going to have to come back and do an adequate resection with a colonoscopy. So, one-stop-shopping colon cancer screening is not about detection of cancer, it’s about prevention of cancer, and that’s what colonoscopy does.
Patients want convenience, but at what cost?
Dr. Wilson: Dr. Lin, how are your patients in your family practice handling this study? Have conversations changed around colon cancer screening? What are people asking about these days?
Dr. Lin: I don’t think the conversations have changed in my practice that much. When patients ask about this study, we do discuss the limitations, that it wasn’t designed to assess the maximum benefit of getting a colonoscopy because the majority of people assigned to that group didn’t get colonoscopy.
But I think it is an opportunity in primary care to consider the way we present the options to patients. Because I would guess that a majority of primary care physicians, when they present the options, would say colonoscopy is the gold standard and recommend their patients get it. And they only offer fecal testing to patients who don’t want the colonoscopy or really refuse.
That hasn’t been my practice. I’m usually more agnostic, because there are both harms and benefits. If you get a fecal test, the chance of you having a complication from colonoscopy is automatically lower because most of those people will not get colonoscopy. Now obviously, the complications with colonoscopy are pretty rare and usually self-limited, but they do exist. If you’re doing lots and lots of these, eventually you’ll see them. Probably all primary care physicians have patients who’ve had a complication from colonoscopy and may or may not have regretted it depending on how information was presented.
But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. Is your priority finding every single cancer? Do you want to know exactly what the benefit is? I think with colonoscopy, we’re still trying to figure out exactly what the benefit is. Whereas we can say it pretty confidently for fecal tests because we have those randomized trials.
Dr. Wilson: Dr. Johnson, I think patients who are watching need to know, first of all, that if they do the fecal test route, a positive fecal test does lead to colonoscopy. In some sense, all roads lead to colonoscopy once you have a positive screening test. So, I can certainly see the value of just sort of skipping to that point. But what about this risk-versus-benefit relationship? Colonoscopy, albeit a relatively safe procedure, is still a procedure. There is some risk associated with it. If we can get the same benefit from yearly fecal immunochemical testing, is that a better choice potentially, at least for patients at average risk?
Dr. Johnson: The stool-based testing is really more effective for detection of cancer. That’s not screening, where the entire goal is the prevention of cancer. The fecal-based testing, including the stool-based DNA testing, misses the majority of precancerous polyps. And the fecal immunochemical tests, which Dr. Lin just mentioned, misses virtually all of them. We really want to get to the prevention of cancer, meaning identification and removal of polyps, not just screening for cancer.
Dr. Wilson: Do you see anything on the horizon that could unseat colonoscopy as, to quote Dr. Lin, the potential gold standard for screening for colon cancer?
Dr. Johnson: I think not on the horizon for identification and removal of polyps. That’s really the gold standard. Technology continues to advance. We’ll see what happens. But on the short and intermediate horizon, colonoscopy is going to be needed.
We are finding that some patients are starting to acquiesce to stool-based testing because they can do it at home. Maybe they don’t have to do a prep. We’re talking about screening only here, not about the follow-up of patients who have a family history, patients who have colitis, patients who have had colon polyps, or other reasons. Stool-based testing is not an option for the follow-up of those patients.
Convenience testing, in the face of COVID, also has thrown a wrench into things. Patients may have wanted to stay home and do these tests. Again, we need to be proactive, not reactive. We want to prevent cancer, not detect it.
Changing advice in the face of younger screening thresholds
Dr. Wilson: Dr. Lin, I’m 42 years old. I don’t believe I’m at any increased risk of colon cancer based on my family history or other risk factors. I’m 3 years away from when the U.S. Preventive Services Task Force tells me I should potentially consider starting to screen for colon cancer. That recommendation has recently been moved down from 50 years old to 45 years old. So, it’s on my mind as I approach that age. What do you advise younger patients approaching 45 right now in terms of screening for colon cancer?
Dr. Lin: For patients with the risk factors that Dr. Johnson mentioned, I would recommend screening colonoscopy as the initial test.
Assuming you don’t have those risk factors, I present it as we have a couple of different fecal tests. There’s the traditional one that just looks for blood. Then there’s the newer one that also adds DNA, which is more sensitive for colorectal cancer, but a little less specific, which is a problem just because there are more false positives.
But you need to compare that with colonoscopy, which you only need to get done ideally every 10 years if there are no findings. That is more complete. And theoretically, as we’ve been talking about, it would also prevent as well as detect early cancers.
So, I think it’s really down to your preference in terms of how the various factors that come into play, such as convenience of the test and your level of concern about cancer. I do tell patients that family history of cancer is not terribly predictive of whether you get it or not. A lot of people unfortunately who develop colorectal cancer have no previous family history. Diet will come into play to some extent. There are some things that point to increased risk for colorectal cancer if you have a diet high in red meat and things like that. But ultimately, it really is up to the patient. I lay out the options, and whatever they choose, I’m happy to pursue.
But the most important thing is that they do some test, because doing no test is not going to help anyone. I do agree with the notion that the best test is the test that gets done.
Dr. Wilson: Absolutely. I think the NEJM study supports that, even when we’re talking about colonoscopy.
Dr. Johnson, you’ve had some criticisms about the NEJM study, and I think they make sense. At the same time, as this is the first randomized trial of colonoscopy, it’s kind of the only data we have. Are we going to get better data? Are there other studies going on out there that might help shed some light on what’s turning out to be a complicated issue?
Dr. Johnson: Yes, there are ongoing studies. They’re not taking place within the United States, because you couldn’t get through a no-screening option trial. There are comparative studies that are probably still 5 years away looking at stool-based testing.
But again, we have to recognize that if you do these alternative tests that were eloquently discussed by Dr. Lin, and not the colonoscopy, which would be every 10 years with high-quality performance, that you have to annualize or do them in sequence. It’s important that you follow up on those with regularity. It’s not just a one-time test every 10 years for these individual tests.
And any of the time that those tests are ordered, the patient should be instructed that if it’s positive you need a colonoscopy. We’re seeing a lot of slippage on that front for the stool-based testing. Convenience is not the answer. It’s getting the job done.
Dr. Wilson: Would you agree, Dr. Johnson, that for patients that really don’t want to do the colonoscopy for one reason or another, and you’ve done your best in explaining what you think the risks and benefits are, that you’d rather have them get something than nothing?
Dr. Johnson: Absolutely. It comes down to what I recommend and then what you decide. But I still make the point explicit: If we’ve gone through those checkpoints and it’s positive, we agree that you understand that colonoscopy is the next step.
Final take-home messages
Dr. Wilson: Dr. Lin, I’ll turn the last word over to you, as the person who is probably discussing the choice of screening modalities more than any of us, before someone would get referred to someone like Dr. Johnson. What’s your final take-home message about the NEJM study and the state of colon cancer screening in the United States?
Dr. Lin: My take-home points about the study are that there were some limitations, but it is good to finally have a randomized trial of colonoscopy screening 2 decades after we really started doing that in the United States. It won’t immediately change – nor do I think it should – the way we practice and discuss different options. I think that some of Dr. Johnson’s points about making sure that whoever’s doing the colonoscopies for your practices is doing it in a high-quality way are really important. Just as it’s important, if you’re doing the fecal tests, to make sure that all patients who have positives get expeditiously referred for colonoscopy.
Dr. Johnson: Perry, I’d like to make one concluding comment as the gastroenterology expert in this discussion. I’ve had countless questions about this study from my patients and my peers. I tell them the following: Don’t let the headlines mislead you.
When you look at this study, the instrument is not so much the question. We know that getting the test is the first step in colon cancer screening. But we also know that getting the test done, with the highest-quality providers and the best-quality performance, is really the key to optimizing the true value of colonoscopy for colon cancer prevention.
So please don’t lose sight of this when reading the headlines in the media around this study. We really need to analyze the true characteristics of what we call a quality performance, because that’s what drives success and that’s what prevents colon cancer.
Dr. Wilson: Dr. Johnson and Dr. Lin, thank you very much. I appreciate you spending time with me here today and wish you all the best.
I guess I’ll sum up by saying that if you’re getting a colonoscopy, make sure it’s a good one. But do get screened.
This video originally appeared on WebMD. A transcript appeared on Medscape.com.
This transcript has been edited for clarity.
F. Perry Wilson, MD, MSCE: Hello, and thank you for joining us today for what promises to be a lively discussion about screening for colon cancer.
My name is Perry Wilson. I’m an associate professor of medicine and director of the Clinical and Translational Research Accelerator at the Yale School of Medicine. My new book, “How Medicine Works and When It Doesn’t: Learning Who to Trust to Get and Stay Healthy,” is available for pre-order now anywhere that books are sold.
I’m joined by two wonderful experts. Dr. David Johnson is a professor of medicine and the chief of gastroenterology at the Eastern Virginia School of Medicine. He is the past president of the American College of Gastroenterology. And I’m very encouraged to see that he’s won a Distinguished Educator Award for his efforts in gastroenterology.
I’m also joined by Dr Kenny Lin. He’s a frequent contributor to Medscape and WebMD. He’s a family physician and public health consultant from Lancaster, Pa., and deputy editor of the American Family Physician journal. He’s also a teacher of residents and students at Lancaster General Health and the Penn Medicine Family Medicine Residency program.
So, we have two great educators with us today to hopefully help teach us something about colon cancer and colon cancer screening. Thank you for joining me today.
David A. Johnson, MD: Thanks for having us.
Kenneth W. Lin, MD, MPH: Good to be here.
Dr. Wilson: Colon cancer is the second leading cause of cancer mortality in the United States. A little over 50,000 people die every year in the United States due to colon cancer.
A month ago, I would have said that there was a pretty broad consensus, at least from my perspective, that people should be getting colonoscopies. That’s certainly what we tell our patients.
Then a paper came out in the New England Journal of Medicine, a very prestigious journal, that has caused a lot of consternation online and led to my receiving a lot questions from patients and their family members. Today,
Dr Johnson, can you give us a brief overview of what this trial was about?
Dr. Johnson: This was a randomized trial looking at screening colonoscopy versus no screening test whatsoever. They looked at the outcomes of prevention of cancer and the prevention of colon cancer–related death.
The short answer was that it was disappointing as it relates to colonoscopy. The study looked at patients from four European countries, with data from three of them (Norway, Poland, and Sweden) ultimately analyzed in this report in NEJM. It got a lot of attention because it surprised a lot of people by saying maybe colonoscopy wasn’t quite as good as we thought it was.
They tried to correct that by only looking at the numbers of patients who got their colonoscopy screening, which still showed value, but it was less than that we’ve seen before. There’s lots of reasons for that, which we’ll discuss shortly.
An invitation to a screening
Dr. Wilson: This was a bit of an interesting trial design. I think I’m correct, Dr Lin, that this was the first randomized trial of screening colonoscopy. But they didn’t really randomize people to get a colonoscopy versus not get a colonoscopy. Can you tell us why this differed from that study design, which I’d have thought would be simpler way of assessing this?
Dr. Lin: It’s definitely an important point to highlight about the study. What investigators did was randomize patients to receive an invitation to get a screening colonoscopy. When the trial was set up, they randomized people before they were asked whether they wanted to participate in the study. If you did it the other way around, by first asking them whether they wanted to be in the study and then randomizing them, you would have been assured that more of them probably would have gotten the colonoscopy.
But in this case, they were more interested in figuring out the real-life results of having a national program that invited patients to receive screening colonoscopy. Because we know that everyone that you recommend to get a colonoscopy doesn’t necessarily want to do that, forgets to do it, or something happens that prevents their actually getting it.
When it comes to measuring the effectiveness of the colonoscopy, it perhaps wasn’t the greatest type of study to do that. But I think it did provide some information about what would happen if you invited people to get colonoscopy, in terms of how many would do it and the results overall for that population.
Lower participation numbers than expected
Dr. Wilson: Dr. Johnson, the data show that 42% of people who were in that invitation arm followed through and got their colonoscopy. You’re a gastroenterologist. Does that seem low or about right? Do about half of people who should get a colonoscopy end up getting one?
Dr. Johnson: No, it’s low. In the United States, those numbers are probably in the 70% range. Certainly, the test doesn’t work for people who don’t get the test performed. So, if 42% of those randomized to receive an invitation to get the colonoscopy got one, that really means the majority of patients never got the test.
Dr. Wilson: Certainly, we wouldn’t expect impressive results if they don’t get the test. But on the other hand, I imagine that people who choose to get the test when they’re invited are sort of a different breed. Perhaps they’re more health conscious or living in other healthy ways. Is that something we should worry about when we look at these results?
Dr. Johnson: I don’t think you can stratify based on this study. Factors like ethnicities and diet weren’t really explained. The key element that will hopefully have the major take-home impact is quality. It’s not just the test. It’s how the test is done.
The key results
Dr. Wilson: Let’s start with the big picture. This was a study looking at everyone invited; not the subgroup of people who got the colonoscopy, but the real randomized study population.
Dr. Lin, the study did show that the invited group had a lower risk of colon cancer over the next 10 years. That’s a good thing, I imagine.
Dr. Lin: I think that’s a significant benefit. Initially in the first few years, they had more colon cancers diagnosed. But that’s probably because those were cancers that were already existing and couldn’t be prevented by the test.
But then over the years the curves crossed, and by the end of the average follow-up of 10 years, there was a significantly lower rate of colon cancers being detected. That’s as you would expect, because you’re finding polyps and removing them before they became colon cancer.
Dr. Wilson: Dr. Johnson, is that the natural history of colon cancer? It starts out as a polyp that maybe can be easily removed and doesn’t require more therapy. Is that why screening colonoscopy is helpful?
Dr. Johnson: The ultimate goal of screening is prevention of cancer, rather than detection of cancer. That occurs by identification and complete removal of the polyps that we find that are precancerous. The key is, first, detection, and second, resection. Adequate resection comes down to some very significant issues of quality, which are questions that I’d raised about this study, and we can talk about momentarily.
Dr. Wilson: Absolutely. Let me first go through the two other big findings in this study.
The fact that there were fewer cases of colon cancer over 10 years seems good. But colon cancer mortality was not significantly different in the two groups. Now, of course, we know that not everyone got a colonoscopy. I would have expected though, if you had less colon cancer, you’d have less death from colon cancer.
Dr. Lin, what might explain this disconnect?
Dr. Lin: I think there are a couple of possible explanations.
One explanation is that they just didn’t follow the people long enough. Colon cancer takes a long time to go from an adenoma to cancer, and from cancer to something that would cause the patient’s death. You may need to follow them for longer than the 10 years that most of these patients were followed to see that benefit. I think there probably will be benefit after a while, because if you are removing colon cancers that otherwise would have progressed and metastasized, you often see a benefit.
We also have to consider the other possibility that not all the polyps removed necessarily were going to progress to advanced cancer. Therefore, you weren’t seeing the death benefit because not every polyp that was removed was necessarily going to cause health consequences.
In colonoscopy, quality is key to success
Dr. Wilson: You’re removing things and have no way of knowing in advance which are the bad ones and which aren’t.
Dr. Johnson, you’ve mentioned several times now that the quality of colonoscopy matters here. So, I’m intuiting that it’s not one-size-fits-all, that it’s not all the same. What do you mean by quality of colonoscopy, and what was it in the NEJM study?
Dr. Johnson: Quality colonoscopy is the quality of the whole process. It starts with the warm-up, if you will, and the clean out for the procedure. That allows the colonoscopist to be able to identify precancerous polyps, which we call adenomas (there are other precancerous polyps called sessile serrated lesions).
The identification of adenomas is extremely important. Even a small increase in the detection of those precancerous polyps has benefits. Well-performed studies looking at large databases show that a small, 1% increase in the adenoma detection leads to a 3% decrease in colon cancer and a 5% decrease in colon cancer–related death. There’s a huge array of effect when we talk about small increases in the adenoma detection rate.
Now, let’s go back to this study in NEJM.
If we base quality on the physician performing the colonoscopy, and say that the colonoscopy is achieving the act of getting all the way around the colon, but not all physicians in the study were able to do that, it starts to raise the question about quality, because adenoma detection is so important. Earlier reports from this group [Nordic-European Initiative on Colorectal Cancer Study Group] have shown that the adenoma detection rates have been way below the national thresholds. So, this raises the question of whether they found the polyp, and then whether they resected the polyp. They also don’t tell us where these cancers were. It is about the colonoscopy quality. It’s not the instrument. It’s the process.
An overview of other screening tools
Dr. Wilson: Dr. Lin, colonoscopy, which requires prep and anesthesia, is not the only colon cancer screening method we have. In fact, there are a bunch. I think we’re on board saying it’s probably better to detect colon cancer early than not detect it. But what are our other options aside from colonoscopy that can allow for early detection of colon cancer?
Dr. Lin: For most of my career, there were three options that I presented patients with. The first was the fecal test, which used to be in the form of initial hemoccult tests. These have been mostly replaced by fecal immunochemical testing. But they’re both just basically looking for the presence of blood in the stool. Anyone who has a positive test would be referred for a diagnostic colonoscopy.
The other test besides colonoscopy, which has been largely phased out in the United States, although it is still very much used in Canada and much of Europe, is flexible sigmoidoscopy. Until this study, the tests supported by randomized controlled trials were the fecal tests and flexible sigmoidoscopy.
Interestingly, there was a recent systematic review of flexible sigmoidoscopy looking at four trials and their effects over 15 years. They showed not only a reduction in colon cancer, but also a reduction in colon cancer mortality, and even a small reduction in all-cause mortality.
I believe three out of the four trials were done where the patients were consented and then randomized, so they had a higher uptake of the procedure.
But when you compare this with the colonoscopy trial, it really isn’t that impressive. You would expect a much larger benefit, because obviously you’re looking at the entire colon. But you really didn’t see that. It was, at best, maybe equivalent to sigmoidoscopy, but not a whole lot better.
Dr. Johnson: Perry, you mentioned sedation. It’s important to understand that this particular cohort of patients are from Norway, Sweden, and Poland, where it’s very much the norm to not get sedation for your colonoscopy. Any of the [audience] who have had colonoscopy will tell you that they are not ones to say, “Don’t give me sedation.” The rate of sedation is around 11% in Norway, maybe 23% in Sweden, and around 45% in Poland. So, the examiner and the patient were never really super comfortable.
I’ve done 50,000 colonoscopies in my career, and many nonsedated. We know that taking time increases the finding of polyps and the adequate identification and resection. So, that ability to perform at a high quality is very much impacted when the patients aren’t comfortable.
Dr. Wilson: Dr. Johnson, we brought up flexible sigmoidoscopy. For the patients watching whose doctors are talking to them about screening colonoscopy, what’s the difference?
Dr. Johnson: Flexible sigmoidoscopy is just a short scope examination, in which you see about one-third of the colon. I’ve been in the field for 45 years, and during that time we’ve seen that there’s a progressive increase in the development of cancers above that bottom third of the colon to the higher end, the two-thirds of the colon that you would miss without doing a full colonoscopy. Also, flexible sigmoidoscopy typically does not get covered for sedation.
Again, if you do the exam and find something, then you’re going to have to come back and do an adequate resection with a colonoscopy. So, one-stop-shopping colon cancer screening is not about detection of cancer, it’s about prevention of cancer, and that’s what colonoscopy does.
Patients want convenience, but at what cost?
Dr. Wilson: Dr. Lin, how are your patients in your family practice handling this study? Have conversations changed around colon cancer screening? What are people asking about these days?
Dr. Lin: I don’t think the conversations have changed in my practice that much. When patients ask about this study, we do discuss the limitations, that it wasn’t designed to assess the maximum benefit of getting a colonoscopy because the majority of people assigned to that group didn’t get colonoscopy.
But I think it is an opportunity in primary care to consider the way we present the options to patients. Because I would guess that a majority of primary care physicians, when they present the options, would say colonoscopy is the gold standard and recommend their patients get it. And they only offer fecal testing to patients who don’t want the colonoscopy or really refuse.
That hasn’t been my practice. I’m usually more agnostic, because there are both harms and benefits. If you get a fecal test, the chance of you having a complication from colonoscopy is automatically lower because most of those people will not get colonoscopy. Now obviously, the complications with colonoscopy are pretty rare and usually self-limited, but they do exist. If you’re doing lots and lots of these, eventually you’ll see them. Probably all primary care physicians have patients who’ve had a complication from colonoscopy and may or may not have regretted it depending on how information was presented.
But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. But I feel like this study reinforces my feeling that we ought to be presenting these, and not saying one is superior or inferior to the other. Instead, I’d base it on what the patient’s priorities are. Is your priority finding every single cancer? Do you want to know exactly what the benefit is? I think with colonoscopy, we’re still trying to figure out exactly what the benefit is. Whereas we can say it pretty confidently for fecal tests because we have those randomized trials.
Dr. Wilson: Dr. Johnson, I think patients who are watching need to know, first of all, that if they do the fecal test route, a positive fecal test does lead to colonoscopy. In some sense, all roads lead to colonoscopy once you have a positive screening test. So, I can certainly see the value of just sort of skipping to that point. But what about this risk-versus-benefit relationship? Colonoscopy, albeit a relatively safe procedure, is still a procedure. There is some risk associated with it. If we can get the same benefit from yearly fecal immunochemical testing, is that a better choice potentially, at least for patients at average risk?
Dr. Johnson: The stool-based testing is really more effective for detection of cancer. That’s not screening, where the entire goal is the prevention of cancer. The fecal-based testing, including the stool-based DNA testing, misses the majority of precancerous polyps. And the fecal immunochemical tests, which Dr. Lin just mentioned, misses virtually all of them. We really want to get to the prevention of cancer, meaning identification and removal of polyps, not just screening for cancer.
Dr. Wilson: Do you see anything on the horizon that could unseat colonoscopy as, to quote Dr. Lin, the potential gold standard for screening for colon cancer?
Dr. Johnson: I think not on the horizon for identification and removal of polyps. That’s really the gold standard. Technology continues to advance. We’ll see what happens. But on the short and intermediate horizon, colonoscopy is going to be needed.
We are finding that some patients are starting to acquiesce to stool-based testing because they can do it at home. Maybe they don’t have to do a prep. We’re talking about screening only here, not about the follow-up of patients who have a family history, patients who have colitis, patients who have had colon polyps, or other reasons. Stool-based testing is not an option for the follow-up of those patients.
Convenience testing, in the face of COVID, also has thrown a wrench into things. Patients may have wanted to stay home and do these tests. Again, we need to be proactive, not reactive. We want to prevent cancer, not detect it.
Changing advice in the face of younger screening thresholds
Dr. Wilson: Dr. Lin, I’m 42 years old. I don’t believe I’m at any increased risk of colon cancer based on my family history or other risk factors. I’m 3 years away from when the U.S. Preventive Services Task Force tells me I should potentially consider starting to screen for colon cancer. That recommendation has recently been moved down from 50 years old to 45 years old. So, it’s on my mind as I approach that age. What do you advise younger patients approaching 45 right now in terms of screening for colon cancer?
Dr. Lin: For patients with the risk factors that Dr. Johnson mentioned, I would recommend screening colonoscopy as the initial test.
Assuming you don’t have those risk factors, I present it as we have a couple of different fecal tests. There’s the traditional one that just looks for blood. Then there’s the newer one that also adds DNA, which is more sensitive for colorectal cancer, but a little less specific, which is a problem just because there are more false positives.
But you need to compare that with colonoscopy, which you only need to get done ideally every 10 years if there are no findings. That is more complete. And theoretically, as we’ve been talking about, it would also prevent as well as detect early cancers.
So, I think it’s really down to your preference in terms of how the various factors that come into play, such as convenience of the test and your level of concern about cancer. I do tell patients that family history of cancer is not terribly predictive of whether you get it or not. A lot of people unfortunately who develop colorectal cancer have no previous family history. Diet will come into play to some extent. There are some things that point to increased risk for colorectal cancer if you have a diet high in red meat and things like that. But ultimately, it really is up to the patient. I lay out the options, and whatever they choose, I’m happy to pursue.
But the most important thing is that they do some test, because doing no test is not going to help anyone. I do agree with the notion that the best test is the test that gets done.
Dr. Wilson: Absolutely. I think the NEJM study supports that, even when we’re talking about colonoscopy.
Dr. Johnson, you’ve had some criticisms about the NEJM study, and I think they make sense. At the same time, as this is the first randomized trial of colonoscopy, it’s kind of the only data we have. Are we going to get better data? Are there other studies going on out there that might help shed some light on what’s turning out to be a complicated issue?
Dr. Johnson: Yes, there are ongoing studies. They’re not taking place within the United States, because you couldn’t get through a no-screening option trial. There are comparative studies that are probably still 5 years away looking at stool-based testing.
But again, we have to recognize that if you do these alternative tests that were eloquently discussed by Dr. Lin, and not the colonoscopy, which would be every 10 years with high-quality performance, that you have to annualize or do them in sequence. It’s important that you follow up on those with regularity. It’s not just a one-time test every 10 years for these individual tests.
And any of the time that those tests are ordered, the patient should be instructed that if it’s positive you need a colonoscopy. We’re seeing a lot of slippage on that front for the stool-based testing. Convenience is not the answer. It’s getting the job done.
Dr. Wilson: Would you agree, Dr. Johnson, that for patients that really don’t want to do the colonoscopy for one reason or another, and you’ve done your best in explaining what you think the risks and benefits are, that you’d rather have them get something than nothing?
Dr. Johnson: Absolutely. It comes down to what I recommend and then what you decide. But I still make the point explicit: If we’ve gone through those checkpoints and it’s positive, we agree that you understand that colonoscopy is the next step.
Final take-home messages
Dr. Wilson: Dr. Lin, I’ll turn the last word over to you, as the person who is probably discussing the choice of screening modalities more than any of us, before someone would get referred to someone like Dr. Johnson. What’s your final take-home message about the NEJM study and the state of colon cancer screening in the United States?
Dr. Lin: My take-home points about the study are that there were some limitations, but it is good to finally have a randomized trial of colonoscopy screening 2 decades after we really started doing that in the United States. It won’t immediately change – nor do I think it should – the way we practice and discuss different options. I think that some of Dr. Johnson’s points about making sure that whoever’s doing the colonoscopies for your practices is doing it in a high-quality way are really important. Just as it’s important, if you’re doing the fecal tests, to make sure that all patients who have positives get expeditiously referred for colonoscopy.
Dr. Johnson: Perry, I’d like to make one concluding comment as the gastroenterology expert in this discussion. I’ve had countless questions about this study from my patients and my peers. I tell them the following: Don’t let the headlines mislead you.
When you look at this study, the instrument is not so much the question. We know that getting the test is the first step in colon cancer screening. But we also know that getting the test done, with the highest-quality providers and the best-quality performance, is really the key to optimizing the true value of colonoscopy for colon cancer prevention.
So please don’t lose sight of this when reading the headlines in the media around this study. We really need to analyze the true characteristics of what we call a quality performance, because that’s what drives success and that’s what prevents colon cancer.
Dr. Wilson: Dr. Johnson and Dr. Lin, thank you very much. I appreciate you spending time with me here today and wish you all the best.
I guess I’ll sum up by saying that if you’re getting a colonoscopy, make sure it’s a good one. But do get screened.
This video originally appeared on WebMD. A transcript appeared on Medscape.com.
Is there hope in the fight against aging?
For many years, it has been believed that the aging process is inevitable and that age-related diseases cannot be prevented or reversed. For example, the U.S. Food and Drug Administration does not recognize aging as an indication for drug approval because there are no markers to determine whether possible treatments have a significant impact on the hallmarks of aging.
The field of geroscience aims to find ways to change this by delaying the onset of age-related diseases or by extending the life span. Those mechanisms contribute to the vulnerability of older adults. The presentations focused on identifying biomarkers of aging and on the search for interventions to prevent and treat age-related diseases.
Perspectives from this meeting were published in a report.
An abridged glossary
- Senescent cells: These are old cells with irreversibly damaged DNA; they strongly resist apoptosis. Thus, they are not eliminated and continue to secrete pathogenic proinflammatory molecules.
- Senolytics: This is a class of compounds that promote the removal of senescent cells from the body.
- Autophagy: This is a process that promotes protein degradation, which is attenuated with aging and that impedes the aggregation of proteins harmful to cell function, particularly those of the central nervous system.
- Proteostasis: This is the dynamic regulation of protein homeostasis.
- Epigenetics: This is the field of biology that studies phenotype changes that are not caused by changes in DNA sequencing and that continue to affect cellular division.
- Metabolome: This refers to small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles to genes and proteins.
- Translational research: This involves applying primary research results to clinical research and vice versa.
Possible research topics
Senescence not only occurs with age but also drives aging. At the meeting, evidence was provided that senescent cells may exacerbate the clinical course of older adults in cases of infections (for example, COVID-19) as they lead to cytokine storms.
Experiments on old mice that have undergone genetic modification of senescent cells or the administration of “senolytic cocktails” composed of dasatinib plus quercetin protected the animals from the effects of viral infections. This finding corroborates the idea that factors involved in biological aging increase vulnerability and could be modified through treatment.
Alzheimer’s disease is an example of the effects of cellular senescence. Senescent cells develop a senescence-associated secretory phenotype that can be toxic to neighboring healthy cells and can allow senescence to propagate within tissues. This effect makes Alzheimer’s disease an essential focal point when studying the use of senolytics. In addition, agents that stimulate autophagy may be of interest for treating degenerative diseases.
Assessing therapeutic effects
It may be possible to assess the therapeutic effects of drug candidates using the following biomarkers.
- Growth hormone and type 1 insulin-like growth factor (IGF-1): Older adults are often prescribed growth hormone. However, recent data suggest that doing so is not advantageous to this patient population, because it antagonizes proteostasis and other cell maintenance mechanisms in older age. Experimental studies and studies conducted on centenarians suggest that low growth hormone and IGF-1 levels contribute to longevity and may be therapeutic biomarkers.
- Epigenetics: DNA methylation is a method that offers an “epigenetic clock” to compare biological age with chronologic age. Higher epigenetic age was associated with increased mortality risk, breast cancer, and nonalcoholic fatty liver disease. Therefore, it could also be a therapeutic biomarker.
- Metabolomics: Studying metabolomes facilitates the identification of the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% of longevity variations.
- New translational strategy: It is common practice to treat each age-related disease individually. An alternative strategy would be to target the hallmarks of biological aging to prevent these diseases from developing. The rate of biological aging correlates with the speed of damage accumulation at the macromolecular, organelle, and cellular levels. It also affects the capacity of the body to repair this damage. The assessment of biomarkers would make possibile research into the effects of short- and long-term treatments that minimize damage and enhance resilience related to diseases common with aging.
New translational research
The report highlights two translational research models: the in-depth study of centenarians and the analysis of how immune aging makes older adults vulnerable to COVID-19. The impact of impaired immunity on aging became particularly evident during the pandemic. However, to home in on immunity as a therapeutic target and to better understand immune resilience, the specific nature of immune and biological deficits still need to be defined.
Metformin is among the therapeutic agents under investigation in cutting-edge clinical research. Its effect on aging will be studied in the Targeting Aging with Metformin (TAME) clinical trial. This trial is the first to study aging outcomes. The goal is to create a regulatory framework that future therapies can follow to achieve FDA approval.
There are three promising therapeutic platforms among the cutting-edge research studies. The first aims to produce adenosine triphosphate, levels of which decline dramatically with aging. The second aims to promote autophagy to remove cellular waste to treat neurodegenerative diseases. The third reprograms the epigenome to a younger state.
Research on mitochondrial dysfunction is relevant because it is highly involved in age-related diseases. Mitochondrial-derived peptides could potentially serve as biomarkers of mitochondrial function in aging studies and become promising therapeutic targets in age-related diseases. One of these peptides, humanin, has been demonstrated to exert protective effects on the heart, brain, and liver. Researchers observed that mitochondrial proteins are age-dependent and are suppressed by growth hormone and IGF-1. They also found that humanin levels are correlated with endothelial function. Data from animal studies have shown that sustained humanin levels are positively linked to longevity; these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.
The formation of a Translational Geroscience Network composed of several scientists from various institutions should accelerate the application of this understanding. Despite the ongoing investigational and clinical studies, senolytics should not be regarded as extending life span or treating certain conditions, because their full safety profiles have not yet been elucidated.
Conclusion
Geroscience faces challenges in dealing with age-related problems. It is hoped that these challenges will be overcome through investigational and clinical studies on the mechanisms involved in aging. In-depth study of the interactions of underlying mechanisms of aging are needed to answer the following questions:
- Is there a hierarchical relationship among these mechanisms?
- Are there organ or cell-type differences in the interactions among these mechanisms?
- Is it possible to achieve a synergistic effect through combined interventions targeting several of the processes that drive aging?
It is complicated, but researchers are starting to see the light at the end of the tunnel.
This article was translated from the Medscape Portuguese edition and a version appeared on Medscape.com.
For many years, it has been believed that the aging process is inevitable and that age-related diseases cannot be prevented or reversed. For example, the U.S. Food and Drug Administration does not recognize aging as an indication for drug approval because there are no markers to determine whether possible treatments have a significant impact on the hallmarks of aging.
The field of geroscience aims to find ways to change this by delaying the onset of age-related diseases or by extending the life span. Those mechanisms contribute to the vulnerability of older adults. The presentations focused on identifying biomarkers of aging and on the search for interventions to prevent and treat age-related diseases.
Perspectives from this meeting were published in a report.
An abridged glossary
- Senescent cells: These are old cells with irreversibly damaged DNA; they strongly resist apoptosis. Thus, they are not eliminated and continue to secrete pathogenic proinflammatory molecules.
- Senolytics: This is a class of compounds that promote the removal of senescent cells from the body.
- Autophagy: This is a process that promotes protein degradation, which is attenuated with aging and that impedes the aggregation of proteins harmful to cell function, particularly those of the central nervous system.
- Proteostasis: This is the dynamic regulation of protein homeostasis.
- Epigenetics: This is the field of biology that studies phenotype changes that are not caused by changes in DNA sequencing and that continue to affect cellular division.
- Metabolome: This refers to small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles to genes and proteins.
- Translational research: This involves applying primary research results to clinical research and vice versa.
Possible research topics
Senescence not only occurs with age but also drives aging. At the meeting, evidence was provided that senescent cells may exacerbate the clinical course of older adults in cases of infections (for example, COVID-19) as they lead to cytokine storms.
Experiments on old mice that have undergone genetic modification of senescent cells or the administration of “senolytic cocktails” composed of dasatinib plus quercetin protected the animals from the effects of viral infections. This finding corroborates the idea that factors involved in biological aging increase vulnerability and could be modified through treatment.
Alzheimer’s disease is an example of the effects of cellular senescence. Senescent cells develop a senescence-associated secretory phenotype that can be toxic to neighboring healthy cells and can allow senescence to propagate within tissues. This effect makes Alzheimer’s disease an essential focal point when studying the use of senolytics. In addition, agents that stimulate autophagy may be of interest for treating degenerative diseases.
Assessing therapeutic effects
It may be possible to assess the therapeutic effects of drug candidates using the following biomarkers.
- Growth hormone and type 1 insulin-like growth factor (IGF-1): Older adults are often prescribed growth hormone. However, recent data suggest that doing so is not advantageous to this patient population, because it antagonizes proteostasis and other cell maintenance mechanisms in older age. Experimental studies and studies conducted on centenarians suggest that low growth hormone and IGF-1 levels contribute to longevity and may be therapeutic biomarkers.
- Epigenetics: DNA methylation is a method that offers an “epigenetic clock” to compare biological age with chronologic age. Higher epigenetic age was associated with increased mortality risk, breast cancer, and nonalcoholic fatty liver disease. Therefore, it could also be a therapeutic biomarker.
- Metabolomics: Studying metabolomes facilitates the identification of the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% of longevity variations.
- New translational strategy: It is common practice to treat each age-related disease individually. An alternative strategy would be to target the hallmarks of biological aging to prevent these diseases from developing. The rate of biological aging correlates with the speed of damage accumulation at the macromolecular, organelle, and cellular levels. It also affects the capacity of the body to repair this damage. The assessment of biomarkers would make possibile research into the effects of short- and long-term treatments that minimize damage and enhance resilience related to diseases common with aging.
New translational research
The report highlights two translational research models: the in-depth study of centenarians and the analysis of how immune aging makes older adults vulnerable to COVID-19. The impact of impaired immunity on aging became particularly evident during the pandemic. However, to home in on immunity as a therapeutic target and to better understand immune resilience, the specific nature of immune and biological deficits still need to be defined.
Metformin is among the therapeutic agents under investigation in cutting-edge clinical research. Its effect on aging will be studied in the Targeting Aging with Metformin (TAME) clinical trial. This trial is the first to study aging outcomes. The goal is to create a regulatory framework that future therapies can follow to achieve FDA approval.
There are three promising therapeutic platforms among the cutting-edge research studies. The first aims to produce adenosine triphosphate, levels of which decline dramatically with aging. The second aims to promote autophagy to remove cellular waste to treat neurodegenerative diseases. The third reprograms the epigenome to a younger state.
Research on mitochondrial dysfunction is relevant because it is highly involved in age-related diseases. Mitochondrial-derived peptides could potentially serve as biomarkers of mitochondrial function in aging studies and become promising therapeutic targets in age-related diseases. One of these peptides, humanin, has been demonstrated to exert protective effects on the heart, brain, and liver. Researchers observed that mitochondrial proteins are age-dependent and are suppressed by growth hormone and IGF-1. They also found that humanin levels are correlated with endothelial function. Data from animal studies have shown that sustained humanin levels are positively linked to longevity; these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.
The formation of a Translational Geroscience Network composed of several scientists from various institutions should accelerate the application of this understanding. Despite the ongoing investigational and clinical studies, senolytics should not be regarded as extending life span or treating certain conditions, because their full safety profiles have not yet been elucidated.
Conclusion
Geroscience faces challenges in dealing with age-related problems. It is hoped that these challenges will be overcome through investigational and clinical studies on the mechanisms involved in aging. In-depth study of the interactions of underlying mechanisms of aging are needed to answer the following questions:
- Is there a hierarchical relationship among these mechanisms?
- Are there organ or cell-type differences in the interactions among these mechanisms?
- Is it possible to achieve a synergistic effect through combined interventions targeting several of the processes that drive aging?
It is complicated, but researchers are starting to see the light at the end of the tunnel.
This article was translated from the Medscape Portuguese edition and a version appeared on Medscape.com.
For many years, it has been believed that the aging process is inevitable and that age-related diseases cannot be prevented or reversed. For example, the U.S. Food and Drug Administration does not recognize aging as an indication for drug approval because there are no markers to determine whether possible treatments have a significant impact on the hallmarks of aging.
The field of geroscience aims to find ways to change this by delaying the onset of age-related diseases or by extending the life span. Those mechanisms contribute to the vulnerability of older adults. The presentations focused on identifying biomarkers of aging and on the search for interventions to prevent and treat age-related diseases.
Perspectives from this meeting were published in a report.
An abridged glossary
- Senescent cells: These are old cells with irreversibly damaged DNA; they strongly resist apoptosis. Thus, they are not eliminated and continue to secrete pathogenic proinflammatory molecules.
- Senolytics: This is a class of compounds that promote the removal of senescent cells from the body.
- Autophagy: This is a process that promotes protein degradation, which is attenuated with aging and that impedes the aggregation of proteins harmful to cell function, particularly those of the central nervous system.
- Proteostasis: This is the dynamic regulation of protein homeostasis.
- Epigenetics: This is the field of biology that studies phenotype changes that are not caused by changes in DNA sequencing and that continue to affect cellular division.
- Metabolome: This refers to small molecules that make up the building blocks of all organismal features, from cell membranes to metabolic cycles to genes and proteins.
- Translational research: This involves applying primary research results to clinical research and vice versa.
Possible research topics
Senescence not only occurs with age but also drives aging. At the meeting, evidence was provided that senescent cells may exacerbate the clinical course of older adults in cases of infections (for example, COVID-19) as they lead to cytokine storms.
Experiments on old mice that have undergone genetic modification of senescent cells or the administration of “senolytic cocktails” composed of dasatinib plus quercetin protected the animals from the effects of viral infections. This finding corroborates the idea that factors involved in biological aging increase vulnerability and could be modified through treatment.
Alzheimer’s disease is an example of the effects of cellular senescence. Senescent cells develop a senescence-associated secretory phenotype that can be toxic to neighboring healthy cells and can allow senescence to propagate within tissues. This effect makes Alzheimer’s disease an essential focal point when studying the use of senolytics. In addition, agents that stimulate autophagy may be of interest for treating degenerative diseases.
Assessing therapeutic effects
It may be possible to assess the therapeutic effects of drug candidates using the following biomarkers.
- Growth hormone and type 1 insulin-like growth factor (IGF-1): Older adults are often prescribed growth hormone. However, recent data suggest that doing so is not advantageous to this patient population, because it antagonizes proteostasis and other cell maintenance mechanisms in older age. Experimental studies and studies conducted on centenarians suggest that low growth hormone and IGF-1 levels contribute to longevity and may be therapeutic biomarkers.
- Epigenetics: DNA methylation is a method that offers an “epigenetic clock” to compare biological age with chronologic age. Higher epigenetic age was associated with increased mortality risk, breast cancer, and nonalcoholic fatty liver disease. Therefore, it could also be a therapeutic biomarker.
- Metabolomics: Studying metabolomes facilitates the identification of the link between genetic polymorphisms and longevity, as most polymorphisms explain less than 0.5% of longevity variations.
- New translational strategy: It is common practice to treat each age-related disease individually. An alternative strategy would be to target the hallmarks of biological aging to prevent these diseases from developing. The rate of biological aging correlates with the speed of damage accumulation at the macromolecular, organelle, and cellular levels. It also affects the capacity of the body to repair this damage. The assessment of biomarkers would make possibile research into the effects of short- and long-term treatments that minimize damage and enhance resilience related to diseases common with aging.
New translational research
The report highlights two translational research models: the in-depth study of centenarians and the analysis of how immune aging makes older adults vulnerable to COVID-19. The impact of impaired immunity on aging became particularly evident during the pandemic. However, to home in on immunity as a therapeutic target and to better understand immune resilience, the specific nature of immune and biological deficits still need to be defined.
Metformin is among the therapeutic agents under investigation in cutting-edge clinical research. Its effect on aging will be studied in the Targeting Aging with Metformin (TAME) clinical trial. This trial is the first to study aging outcomes. The goal is to create a regulatory framework that future therapies can follow to achieve FDA approval.
There are three promising therapeutic platforms among the cutting-edge research studies. The first aims to produce adenosine triphosphate, levels of which decline dramatically with aging. The second aims to promote autophagy to remove cellular waste to treat neurodegenerative diseases. The third reprograms the epigenome to a younger state.
Research on mitochondrial dysfunction is relevant because it is highly involved in age-related diseases. Mitochondrial-derived peptides could potentially serve as biomarkers of mitochondrial function in aging studies and become promising therapeutic targets in age-related diseases. One of these peptides, humanin, has been demonstrated to exert protective effects on the heart, brain, and liver. Researchers observed that mitochondrial proteins are age-dependent and are suppressed by growth hormone and IGF-1. They also found that humanin levels are correlated with endothelial function. Data from animal studies have shown that sustained humanin levels are positively linked to longevity; these findings are mirrored in data from centenarians and their offspring, who have higher levels of humanin.
The formation of a Translational Geroscience Network composed of several scientists from various institutions should accelerate the application of this understanding. Despite the ongoing investigational and clinical studies, senolytics should not be regarded as extending life span or treating certain conditions, because their full safety profiles have not yet been elucidated.
Conclusion
Geroscience faces challenges in dealing with age-related problems. It is hoped that these challenges will be overcome through investigational and clinical studies on the mechanisms involved in aging. In-depth study of the interactions of underlying mechanisms of aging are needed to answer the following questions:
- Is there a hierarchical relationship among these mechanisms?
- Are there organ or cell-type differences in the interactions among these mechanisms?
- Is it possible to achieve a synergistic effect through combined interventions targeting several of the processes that drive aging?
It is complicated, but researchers are starting to see the light at the end of the tunnel.
This article was translated from the Medscape Portuguese edition and a version appeared on Medscape.com.
Hair supplements
in JAMA Dermatology in November 2022.
Drake and colleagues evaluated the safety and efficacy of nutritional supplements for treating hair loss. In a systematic database review from inception to Oct. 20, 2021, they evaluated and compiled the findings of all dietary and nutritional interventions for treatment of hair loss among individuals without a known baseline nutritional deficiency. Thirty articles were included, including 17 randomized clinical trials, 11 clinical trials, and 2 case series.
They found the highest-quality evidence showing the most potential benefit were for 12 of the 20 nutritional interventions in their review: Pumpkin seed oil capsules, omega-3 and -6 combined with antioxidants, tocotrienol, Pantogar, capsaicin and isoflavone, Viviscal (multiple formulations), Nourkrin, Nutrafol, apple nutraceutical, Lambdapil, total glucosides of paeony and compound glycyrrhizin tablets, and zinc. Vitamin D3, kimchi and cheonggukjang, and Forti5 had lower-quality evidence for disease course improvement. Adverse effects associated with the supplements were described as mild and rare.
In practice, for patients with nonscarring alopecia, I typically check screening labs for hair loss, in addition to the clinical exam, before starting treatment (including supplements), as addressing the underlying reason, if found, is always paramount. These labs are best performed when the patient is not taking biotin, as biotin has been shown numerous times to potentially be associated with endocrine lab abnormalities, most commonly thyroid-stimulating hormone, especially at higher doses, as well as troponin levels. Some over-the-counter hair supplements will contain much higher doses than the recommended 30 micrograms per day.
Separately, if ferritin levels are within normal range, but below 50 mcg/L, supplementation with Slow Fe or another slow-release iron supplement may also result in improved hair growth. Ferritin levels are typically rechecked 6 months after supplementation to see if levels of 50 mcg/L or above have been achieved.
Another point to consider before beginning supplementation is to educate patients about potential effects of supplementation, including increased hair growth in other areas besides the scalp. For some patients who are self-conscious about potential hirsutism, this could be an issue, whereas for others, this risk does not outweigh the benefit. Unwanted hair growth, should it occur, may also be addressed with hair removal methods including shaving, waxing, plucking, threading, depilatories, prescription eflornithine cream (Vaniqa), or laser hair removal if desired.
Our armamentarium for treating hair loss includes: addressing underlying systemic causes; topical treatments including topical minoxidil; oral supplements; platelet-rich plasma injections; prescription oral medications including finasteride in men or postmenopausal women or off-label oral minoxidil; and hair transplant surgery if warranted. Having this thorough review of the most common hair supplements currently available is extremely helpful and valuable in our specialty.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at [email protected]. This month’s column is by Dr. Wesley. She had no relevant disclosures.
in JAMA Dermatology in November 2022.
Drake and colleagues evaluated the safety and efficacy of nutritional supplements for treating hair loss. In a systematic database review from inception to Oct. 20, 2021, they evaluated and compiled the findings of all dietary and nutritional interventions for treatment of hair loss among individuals without a known baseline nutritional deficiency. Thirty articles were included, including 17 randomized clinical trials, 11 clinical trials, and 2 case series.
They found the highest-quality evidence showing the most potential benefit were for 12 of the 20 nutritional interventions in their review: Pumpkin seed oil capsules, omega-3 and -6 combined with antioxidants, tocotrienol, Pantogar, capsaicin and isoflavone, Viviscal (multiple formulations), Nourkrin, Nutrafol, apple nutraceutical, Lambdapil, total glucosides of paeony and compound glycyrrhizin tablets, and zinc. Vitamin D3, kimchi and cheonggukjang, and Forti5 had lower-quality evidence for disease course improvement. Adverse effects associated with the supplements were described as mild and rare.
In practice, for patients with nonscarring alopecia, I typically check screening labs for hair loss, in addition to the clinical exam, before starting treatment (including supplements), as addressing the underlying reason, if found, is always paramount. These labs are best performed when the patient is not taking biotin, as biotin has been shown numerous times to potentially be associated with endocrine lab abnormalities, most commonly thyroid-stimulating hormone, especially at higher doses, as well as troponin levels. Some over-the-counter hair supplements will contain much higher doses than the recommended 30 micrograms per day.
Separately, if ferritin levels are within normal range, but below 50 mcg/L, supplementation with Slow Fe or another slow-release iron supplement may also result in improved hair growth. Ferritin levels are typically rechecked 6 months after supplementation to see if levels of 50 mcg/L or above have been achieved.
Another point to consider before beginning supplementation is to educate patients about potential effects of supplementation, including increased hair growth in other areas besides the scalp. For some patients who are self-conscious about potential hirsutism, this could be an issue, whereas for others, this risk does not outweigh the benefit. Unwanted hair growth, should it occur, may also be addressed with hair removal methods including shaving, waxing, plucking, threading, depilatories, prescription eflornithine cream (Vaniqa), or laser hair removal if desired.
Our armamentarium for treating hair loss includes: addressing underlying systemic causes; topical treatments including topical minoxidil; oral supplements; platelet-rich plasma injections; prescription oral medications including finasteride in men or postmenopausal women or off-label oral minoxidil; and hair transplant surgery if warranted. Having this thorough review of the most common hair supplements currently available is extremely helpful and valuable in our specialty.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at [email protected]. This month’s column is by Dr. Wesley. She had no relevant disclosures.
in JAMA Dermatology in November 2022.
Drake and colleagues evaluated the safety and efficacy of nutritional supplements for treating hair loss. In a systematic database review from inception to Oct. 20, 2021, they evaluated and compiled the findings of all dietary and nutritional interventions for treatment of hair loss among individuals without a known baseline nutritional deficiency. Thirty articles were included, including 17 randomized clinical trials, 11 clinical trials, and 2 case series.
They found the highest-quality evidence showing the most potential benefit were for 12 of the 20 nutritional interventions in their review: Pumpkin seed oil capsules, omega-3 and -6 combined with antioxidants, tocotrienol, Pantogar, capsaicin and isoflavone, Viviscal (multiple formulations), Nourkrin, Nutrafol, apple nutraceutical, Lambdapil, total glucosides of paeony and compound glycyrrhizin tablets, and zinc. Vitamin D3, kimchi and cheonggukjang, and Forti5 had lower-quality evidence for disease course improvement. Adverse effects associated with the supplements were described as mild and rare.
In practice, for patients with nonscarring alopecia, I typically check screening labs for hair loss, in addition to the clinical exam, before starting treatment (including supplements), as addressing the underlying reason, if found, is always paramount. These labs are best performed when the patient is not taking biotin, as biotin has been shown numerous times to potentially be associated with endocrine lab abnormalities, most commonly thyroid-stimulating hormone, especially at higher doses, as well as troponin levels. Some over-the-counter hair supplements will contain much higher doses than the recommended 30 micrograms per day.
Separately, if ferritin levels are within normal range, but below 50 mcg/L, supplementation with Slow Fe or another slow-release iron supplement may also result in improved hair growth. Ferritin levels are typically rechecked 6 months after supplementation to see if levels of 50 mcg/L or above have been achieved.
Another point to consider before beginning supplementation is to educate patients about potential effects of supplementation, including increased hair growth in other areas besides the scalp. For some patients who are self-conscious about potential hirsutism, this could be an issue, whereas for others, this risk does not outweigh the benefit. Unwanted hair growth, should it occur, may also be addressed with hair removal methods including shaving, waxing, plucking, threading, depilatories, prescription eflornithine cream (Vaniqa), or laser hair removal if desired.
Our armamentarium for treating hair loss includes: addressing underlying systemic causes; topical treatments including topical minoxidil; oral supplements; platelet-rich plasma injections; prescription oral medications including finasteride in men or postmenopausal women or off-label oral minoxidil; and hair transplant surgery if warranted. Having this thorough review of the most common hair supplements currently available is extremely helpful and valuable in our specialty.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at [email protected]. This month’s column is by Dr. Wesley. She had no relevant disclosures.
Mindfulness, exercise strike out in memory trial
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.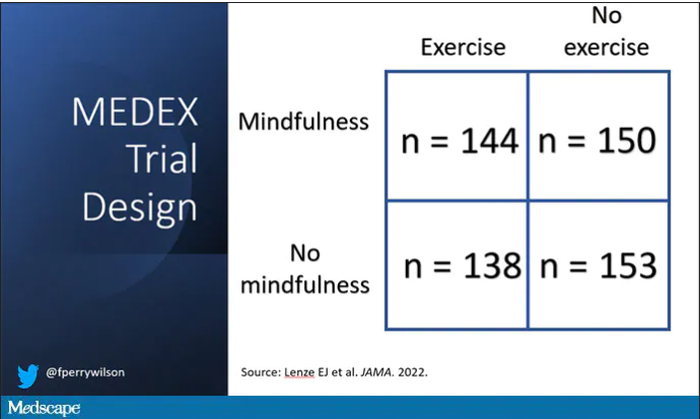
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Nitroglycerin’s safety and value examined
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
The new obesity breakthrough drugs
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.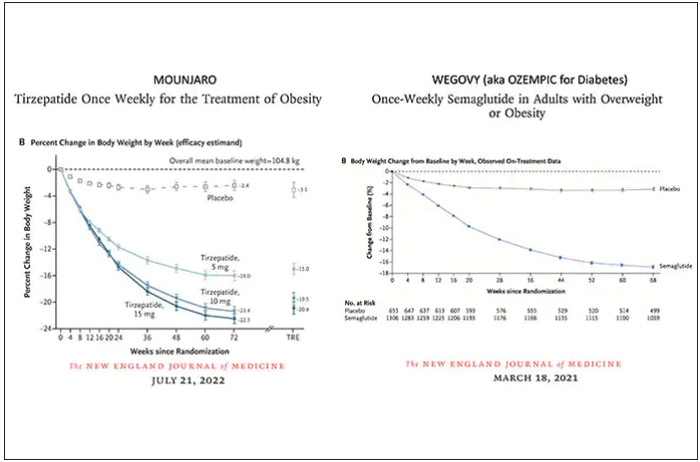
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction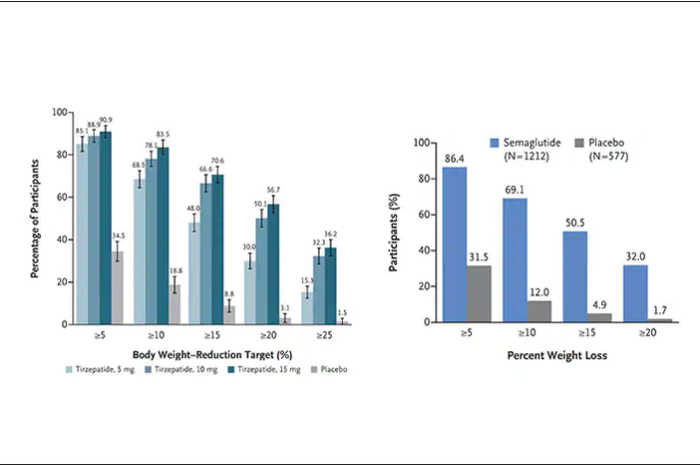
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.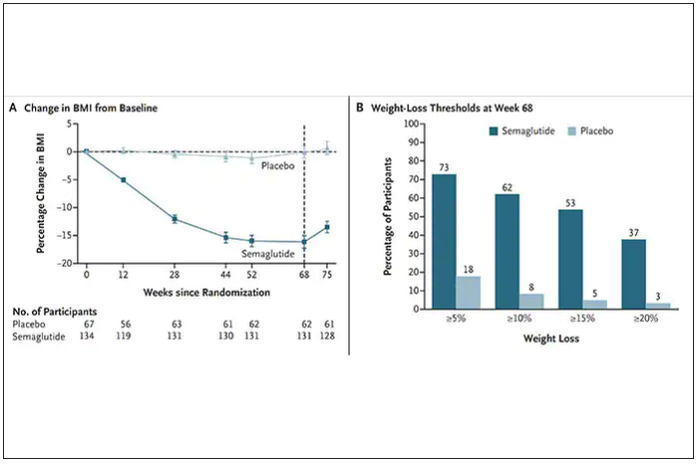
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.
These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.
Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.

Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.

While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
Can a Mediterranean diet ease depression in young men?
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Drew Ramsey, MD: Welcome back, everyone. I’m Dr. Drew Ramsey. I’m on the editorial board with Medscape Psychiatry and I’m an assistant clinical professor of psychiatry at Columbia University. We have a special guest today.
I’m here with nutritionist Jessica Bayes, who’s at the University of Technology Sydney, and she’s the lead author of the AMMEND trial. [Editor’s note: Since completing her PhD, Bayes is now at Southern Cross University.]
Jessica, welcome to Medscape.
Jessica Bayes, PhD: Thank you for having me.
The AMMEND Trial
Dr. Ramsey: Thank you for coming on board and helping all of us as clinicians understand some of your research and some of what is suggested by your research – that young men can change their diet and it helped their depression. Tell us a little bit about the AMMEND trial.
Dr. Bayes: The AMMEND trial was a 12-week randomized controlled trial in young men, 18-25 years old, who had diagnosed moderate to severe clinical depression. They had a poor baseline diet and we got them to eat a healthy Mediterranean diet, which improved their symptoms of depression.
Dr. Ramsey: It was a remarkable trial. Jessica, if I recall, you helped individuals improve the Mediterranean dietary pattern score by 8 points on a 14-point scale. That led to a 20-point reduction in their Beck Depression Inventory. Tell us what that looked like on the ground.
Dr. Bayes: It’s a huge improvement. Obviously, they were feeling much better in the end in terms of their depressive symptoms, but we also measured their energy, sleep, and quality of life. Many of them at the end were at a score cutoff that suggests no depression or in remission.
Dr. Ramsey: There were 72 people in your total trial, so 36% in your intervention arm went into full remission.
Dr. Bayes: Which is just amazing.
Dr. Ramsey: It also follows up the SMILES trial, which was a little bit of a different trial. You had two nutritional counseling sessions and the SMILES trial had seven, but in the SMILES trial, 32.3% of the patients went into full remission when they adopted a Mediterranean-style diet.
Jessica, what is the secret that you and your team know? I think many clinicians, especially clinicians who are parents and have teens, are kind of shaking their heads in disbelief. They’ve been telling their kids to eat healthy. What do you guys know about how to help young men change their diet?
How to Aid Adherence to Mediterranean Diet
Dr. Bayes: Prior to starting this, when I would say this idea to people, everyone would say, “Great idea. There’s no way you’re going to get depressed young men to change their diet. Not going to happen.” We went to them and we asked them. We said, “We’re going to do this study. What do you want from us? What resources would you need? How many appointments would you like? What’s too little or too many?”
We really got their feedback on board when we designed the study, and that obviously paid off. We had a personalized approach and we met them where they were at. We gave them the skills, resources, recipes, meal ideas – all those things – so we could really set them up to succeed.
Dr. Ramsey: You were telling me earlier about a few of the dietary changes that you felt made a big difference for these young men. What were those?
Dr. Bayes: Increasing the vegetables, olive oil, and legumes are probably the big ones that most of them were really not doing beforehand. They were really able to take that on board and make significant improvements in those areas.
Dr. Ramsey: These are really some of the top food categories in nutritional psychiatry as we think about how we help our efforts to improve mental health by thinking about nutrition, nutritional quality, and nutritional density. Certainly, those food categories – nuts and legumes, plants, and olive oil – are really what help get us there.
You also gave the students a food hamper. If you were going to be in charge of mental health in Australia and America and you got to give every college freshman a little box with a note, what would be in that box?
Dr. Bayes: I’d want to put everything in that box! It would be full of brightly colored fruits and vegetables, different nuts and seeds, and legumes. It would be full of recipes and ideas of how to cook things and how to prepare really delicious things. It would be full of different herbs and spices and all of those things to get people really excited about food.
Dr. Ramsey: Did the young men pick up on your enthusiasm and excitement around food? Did they begin to adopt some of that, shifting their view of how they saw the food and how they saw that it is related to their depression?
Dr. Bayes: Hopefully. I do think energy is infectious. I’m sure that played a role somewhat, but trying to get them excited about food can be really quite daunting, thinking, I’ve got to change my entire diet and I’ve got to learn to cook and go out and buy groceries. I don’t even know what to do with a piece of salmon. Trying to get them curious, interested, and just reminding them that it’s not all-or-nothing. Make small changes, give it a go, and have fun.
Dr. Ramsey: You also have a unique aspect of your research that you’re interested in male mental health, and that’s not something that’s been widely researched. Can you tell us a little bit about what these men were like in terms of coming into your trial as depressed young men?
Dr. Bayes: In the context of the COVID-19 pandemic, mental health was at the forefront of many people’s minds. They joined the study saying, “I’ve never seen anything like this before. I’ve never seen myself represented in research. I wanted to contribute. I want to add to that conversation because I feel like we are overlooked.”
Dr. Ramsey: I love hearing this notion that maybe young men aren’t quite who we think they are. They are wanting to be seen around their mental health. They can learn to use olive oil and to cook, and they can engage in mental health interventions that work. We just need to ask, give them some food, encourage them, and it makes a big difference.
Jessica Bayes, thank you so much for joining us and sharing some of your research. Everyone, it’s the AMMEND trial. We will drop a link to the trial below so you can take a peek and tell us what you think.
Please, in the comments, let us know what you think about this notion of helping young men with depression through nutritional interventions. Take a peek at the great work that Jessica and Professor Sibbritt from the University of Technology Sydney have published and put out into the scientific literature for us all.
Thanks so much, Jessica. I look forward to seeing you soon.
Dr. Bayes: Thank you.
Dr. Ramsey is assistant clinical professor, department of psychiatry, Columbia University, New York. He has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for InterContinental Hotels Group; National Kale Day 501(c)3. Received income in an amount equal to or greater than $250 from: Sharecare. Dr. Bayes is a postdoctoral research fellow; clinical nutritionist, Southern Cross University, National Center for Naturopathic Medicine, Lismore, New South Wales, Australia. She has disclosed the following relevant financial relationships: Received research grant from Endeavour College. A version of this article first appeared on Medscape.com.
Doctors of Virtue and Vice: The Best and Worst of Federal Practice in 2023
Regular readers of Federal Practitioner may recall that I have had a tradition of dedicating the last column of the year to an ethics rendition of the popular trope of the annual best and worst. This year we will examine the stories of 2 military physicians through the lens of virtue ethics. Aristotle (384-322
Virtue ethics is among the oldest of ethical theories, and Aristotle articulates this school of thought in his work Nicomachean Ethics.2 It is a good fit for Federal Practitioner as it has been constructively applied to the moral development of both military3 and medical professionals.4
Here is a Reader’s Digest version of virtue theory with apologizes to all the real philosophers out there. There are different ways to categorize ethical theories. One approach is to distinguish them based on the aspects of primary interest. Consequentialist ethics theories are concerned with the outcomes of actions. Deontologic theories emphasize the intention of the moral agent. In contrast, virtue ethics theories focus on the character of a person. The virtuous individual is one who has practiced the habits of moral excellence and embodies the good life. They are honored as heroes and revered as saints; they are the exemplars we imitate in our aspirations.3
The epigraph sums up one of Aristotle’s central philosophical doctrines: the close relationship of ethics and politics.1 Personal virtue is intelligible only in the context of community and aim, and the goal of virtue is to contribute to human happiness.5 War, whether in ancient Greece or modern Europe, is among the forces most inimical to human flourishing. The current war in Ukraine that has united much of the Western world in opposition to tyranny has divided the 2 physicians in our story along the normative lines of virtue ethics.
The doctor of virtue: Michael Siclari, MD. A 71-year-old US Department of Veterans Affairs physician, Siclari had previously served in the military as a National Guard physician during Operation Enduring Freedom (2001-2014) in Afghanistan. He decided to serve again in Ukraine. Siclari expressed his reasons for going to Ukraine in the language of what Aristotle thought was among the highest virtues: justice. “In retrospect, as I think about why I wanted to go to Ukraine, I think it’s more of a sense that I thought an injustice was happening.”7
Echoing the great Rabbi Hillel, Siclari saw the Russian invasion of Ukraine as a personal call to use his experience and training as a trauma and emergency medicine physician to help the Ukrainian people. “If not me, then who?” Siclari demonstrated another virtue: generosity in taking 10 days of personal leave in August 2022 to make the trip to Ukraine, hoping to work in a combat zone tending to wounded soldiers as he had in Afghanistan. When due to logistics he instead was assigned to care for refugees and assist with evacuations from the battlefield, he humbly and compassionately cared for those in his charge. Even now, back home, he speaks to audiences of health care professionals encouraging them to consider similar acts of altruism.5
Virtue for Aristotle is technically defined as the mean between 2 extremes of disposition or temperament. The virtue of courage is found in the moral middle ground between the deficiency of bravery that is cowardice and the vice of excess of reckless abandon. The former person fears too much and the latter too little and both thus exhibit vicious behavior.
The doctor of vice: James Lee Henry. Henry is a major and internal medicine physician in the United States Army stationed at Fort Bragg, headquarters of the US Army Special Operations Command. Along with his wife Anna Gabrielian, a civilian anesthesiologist, he was charged in September with conspiring to divulge the protected health information of American military and government employees to the Russian government.8 According to the Grand Jury indictment, Henry delivered into the hands of an undercover Federal Bureau of Investigation (FBI) agent, the medical records of a US Army officer, Department of Defense employee, and the spouses of 3 Army veterans, 2 of whom were deceased.9 In a gross twisting of virtue language, Gabrielian explained her motivation for the couple’s espionage in terms of sacrifice and loyalty. In an antipode of Siclari’s service, Henry purportedly wanted to join the Russian army but did not have the requisite combat experience. For his part, Henry’s abysmal defense of his betrayal of his country and his oath speaks for itself, if the United States were to declare war on Russia, Henry told the FBI agent, “at that point, I’ll have some ethical issues I have to work through.”8
We become virtuous people through imitating the example of those who have perfected the habits of moral excellence. During 2022, 2 federal practitioners responded to the challenge of war: one displayed the zenith of virtue, the other exhibited the nadir of vice. Seldom does a single year present us with such clear choices of who and how we want to be in 2023. American culture has so trivialized New Year’s resolutions that they are no longer substantive enough for the weight of the profound question of what constitutes the good life. Rather let us make a commitment in keeping with such morally serious matters. All of us live as mixed creatures, drawn to virtue and prone to vice. May we all strive this coming year to help each other meet the high bar another great man of virtue Abraham Lincoln set in his first inaugural address, to be the “better angels of our natures.”10
1. Aristotle. Politics. Book I, 1253.a31.
2. The Ethics of Aristotle. Aristotle. The Nicomachean Ethics. Thompson JAK, trans. Penguin Books; 1953.
3. Schonfeld TL, Hester DM. Brief introduction to ethics and ethical theory. In: Schonfeld TL, Hester DM, eds. Guidance for Healthcare Ethics Committees. 2nd ed. Cambridge University Press; 2022:11-19.
4. Olsthoorn P. Military Ethics and Virtues: An Interdisciplinary Approach for the 21st Century. Routledge; 2010.
5. Pellegrino ED, Thomasma DC. The Virtues in Medical Practice. Oxford University Press; 1993.
6. Edward Clayton. Aristotle Politics. In: Internet Encyclopedia of Philosophy. Accessed November 28, 2022. https://iep.utm.edu/aristotle-politics
7. Tippets R. A VA doctor’s calling to help in Ukraine. VA News. October 23, 2022. Accessed November 28, 2022. https://news.va.gov/109957/a-va-doctors-calling-to-help-in-ukraine
8. Lybrand H. US Army doctor and anesthesiologist charged with conspiring to US military records to the Russian government. CNN Politics, September 29, 2022. Accessed November 28, 2022 https://www.cnn.com/2022/09/29/politics/us-army-doctor-anesthesiologist-russian-government-medical-records
9. United States v Anna Gabrielian and James Lee Henry, (SD Md 2022). Accessed November 28, 2022. https://www.documentcloud.org/documents/23106067-gabrielian-and-henry-indictment
10. Lincoln A. First Inaugural Address of Abraham Lincoln. Accessed November 28, 2022. https://avalon.law.yale.edu/19th_century/lincoln1.asp
Regular readers of Federal Practitioner may recall that I have had a tradition of dedicating the last column of the year to an ethics rendition of the popular trope of the annual best and worst. This year we will examine the stories of 2 military physicians through the lens of virtue ethics. Aristotle (384-322
Virtue ethics is among the oldest of ethical theories, and Aristotle articulates this school of thought in his work Nicomachean Ethics.2 It is a good fit for Federal Practitioner as it has been constructively applied to the moral development of both military3 and medical professionals.4
Here is a Reader’s Digest version of virtue theory with apologizes to all the real philosophers out there. There are different ways to categorize ethical theories. One approach is to distinguish them based on the aspects of primary interest. Consequentialist ethics theories are concerned with the outcomes of actions. Deontologic theories emphasize the intention of the moral agent. In contrast, virtue ethics theories focus on the character of a person. The virtuous individual is one who has practiced the habits of moral excellence and embodies the good life. They are honored as heroes and revered as saints; they are the exemplars we imitate in our aspirations.3
The epigraph sums up one of Aristotle’s central philosophical doctrines: the close relationship of ethics and politics.1 Personal virtue is intelligible only in the context of community and aim, and the goal of virtue is to contribute to human happiness.5 War, whether in ancient Greece or modern Europe, is among the forces most inimical to human flourishing. The current war in Ukraine that has united much of the Western world in opposition to tyranny has divided the 2 physicians in our story along the normative lines of virtue ethics.
The doctor of virtue: Michael Siclari, MD. A 71-year-old US Department of Veterans Affairs physician, Siclari had previously served in the military as a National Guard physician during Operation Enduring Freedom (2001-2014) in Afghanistan. He decided to serve again in Ukraine. Siclari expressed his reasons for going to Ukraine in the language of what Aristotle thought was among the highest virtues: justice. “In retrospect, as I think about why I wanted to go to Ukraine, I think it’s more of a sense that I thought an injustice was happening.”7
Echoing the great Rabbi Hillel, Siclari saw the Russian invasion of Ukraine as a personal call to use his experience and training as a trauma and emergency medicine physician to help the Ukrainian people. “If not me, then who?” Siclari demonstrated another virtue: generosity in taking 10 days of personal leave in August 2022 to make the trip to Ukraine, hoping to work in a combat zone tending to wounded soldiers as he had in Afghanistan. When due to logistics he instead was assigned to care for refugees and assist with evacuations from the battlefield, he humbly and compassionately cared for those in his charge. Even now, back home, he speaks to audiences of health care professionals encouraging them to consider similar acts of altruism.5
Virtue for Aristotle is technically defined as the mean between 2 extremes of disposition or temperament. The virtue of courage is found in the moral middle ground between the deficiency of bravery that is cowardice and the vice of excess of reckless abandon. The former person fears too much and the latter too little and both thus exhibit vicious behavior.
The doctor of vice: James Lee Henry. Henry is a major and internal medicine physician in the United States Army stationed at Fort Bragg, headquarters of the US Army Special Operations Command. Along with his wife Anna Gabrielian, a civilian anesthesiologist, he was charged in September with conspiring to divulge the protected health information of American military and government employees to the Russian government.8 According to the Grand Jury indictment, Henry delivered into the hands of an undercover Federal Bureau of Investigation (FBI) agent, the medical records of a US Army officer, Department of Defense employee, and the spouses of 3 Army veterans, 2 of whom were deceased.9 In a gross twisting of virtue language, Gabrielian explained her motivation for the couple’s espionage in terms of sacrifice and loyalty. In an antipode of Siclari’s service, Henry purportedly wanted to join the Russian army but did not have the requisite combat experience. For his part, Henry’s abysmal defense of his betrayal of his country and his oath speaks for itself, if the United States were to declare war on Russia, Henry told the FBI agent, “at that point, I’ll have some ethical issues I have to work through.”8
We become virtuous people through imitating the example of those who have perfected the habits of moral excellence. During 2022, 2 federal practitioners responded to the challenge of war: one displayed the zenith of virtue, the other exhibited the nadir of vice. Seldom does a single year present us with such clear choices of who and how we want to be in 2023. American culture has so trivialized New Year’s resolutions that they are no longer substantive enough for the weight of the profound question of what constitutes the good life. Rather let us make a commitment in keeping with such morally serious matters. All of us live as mixed creatures, drawn to virtue and prone to vice. May we all strive this coming year to help each other meet the high bar another great man of virtue Abraham Lincoln set in his first inaugural address, to be the “better angels of our natures.”10
Regular readers of Federal Practitioner may recall that I have had a tradition of dedicating the last column of the year to an ethics rendition of the popular trope of the annual best and worst. This year we will examine the stories of 2 military physicians through the lens of virtue ethics. Aristotle (384-322
Virtue ethics is among the oldest of ethical theories, and Aristotle articulates this school of thought in his work Nicomachean Ethics.2 It is a good fit for Federal Practitioner as it has been constructively applied to the moral development of both military3 and medical professionals.4
Here is a Reader’s Digest version of virtue theory with apologizes to all the real philosophers out there. There are different ways to categorize ethical theories. One approach is to distinguish them based on the aspects of primary interest. Consequentialist ethics theories are concerned with the outcomes of actions. Deontologic theories emphasize the intention of the moral agent. In contrast, virtue ethics theories focus on the character of a person. The virtuous individual is one who has practiced the habits of moral excellence and embodies the good life. They are honored as heroes and revered as saints; they are the exemplars we imitate in our aspirations.3
The epigraph sums up one of Aristotle’s central philosophical doctrines: the close relationship of ethics and politics.1 Personal virtue is intelligible only in the context of community and aim, and the goal of virtue is to contribute to human happiness.5 War, whether in ancient Greece or modern Europe, is among the forces most inimical to human flourishing. The current war in Ukraine that has united much of the Western world in opposition to tyranny has divided the 2 physicians in our story along the normative lines of virtue ethics.
The doctor of virtue: Michael Siclari, MD. A 71-year-old US Department of Veterans Affairs physician, Siclari had previously served in the military as a National Guard physician during Operation Enduring Freedom (2001-2014) in Afghanistan. He decided to serve again in Ukraine. Siclari expressed his reasons for going to Ukraine in the language of what Aristotle thought was among the highest virtues: justice. “In retrospect, as I think about why I wanted to go to Ukraine, I think it’s more of a sense that I thought an injustice was happening.”7
Echoing the great Rabbi Hillel, Siclari saw the Russian invasion of Ukraine as a personal call to use his experience and training as a trauma and emergency medicine physician to help the Ukrainian people. “If not me, then who?” Siclari demonstrated another virtue: generosity in taking 10 days of personal leave in August 2022 to make the trip to Ukraine, hoping to work in a combat zone tending to wounded soldiers as he had in Afghanistan. When due to logistics he instead was assigned to care for refugees and assist with evacuations from the battlefield, he humbly and compassionately cared for those in his charge. Even now, back home, he speaks to audiences of health care professionals encouraging them to consider similar acts of altruism.5
Virtue for Aristotle is technically defined as the mean between 2 extremes of disposition or temperament. The virtue of courage is found in the moral middle ground between the deficiency of bravery that is cowardice and the vice of excess of reckless abandon. The former person fears too much and the latter too little and both thus exhibit vicious behavior.
The doctor of vice: James Lee Henry. Henry is a major and internal medicine physician in the United States Army stationed at Fort Bragg, headquarters of the US Army Special Operations Command. Along with his wife Anna Gabrielian, a civilian anesthesiologist, he was charged in September with conspiring to divulge the protected health information of American military and government employees to the Russian government.8 According to the Grand Jury indictment, Henry delivered into the hands of an undercover Federal Bureau of Investigation (FBI) agent, the medical records of a US Army officer, Department of Defense employee, and the spouses of 3 Army veterans, 2 of whom were deceased.9 In a gross twisting of virtue language, Gabrielian explained her motivation for the couple’s espionage in terms of sacrifice and loyalty. In an antipode of Siclari’s service, Henry purportedly wanted to join the Russian army but did not have the requisite combat experience. For his part, Henry’s abysmal defense of his betrayal of his country and his oath speaks for itself, if the United States were to declare war on Russia, Henry told the FBI agent, “at that point, I’ll have some ethical issues I have to work through.”8
We become virtuous people through imitating the example of those who have perfected the habits of moral excellence. During 2022, 2 federal practitioners responded to the challenge of war: one displayed the zenith of virtue, the other exhibited the nadir of vice. Seldom does a single year present us with such clear choices of who and how we want to be in 2023. American culture has so trivialized New Year’s resolutions that they are no longer substantive enough for the weight of the profound question of what constitutes the good life. Rather let us make a commitment in keeping with such morally serious matters. All of us live as mixed creatures, drawn to virtue and prone to vice. May we all strive this coming year to help each other meet the high bar another great man of virtue Abraham Lincoln set in his first inaugural address, to be the “better angels of our natures.”10
1. Aristotle. Politics. Book I, 1253.a31.
2. The Ethics of Aristotle. Aristotle. The Nicomachean Ethics. Thompson JAK, trans. Penguin Books; 1953.
3. Schonfeld TL, Hester DM. Brief introduction to ethics and ethical theory. In: Schonfeld TL, Hester DM, eds. Guidance for Healthcare Ethics Committees. 2nd ed. Cambridge University Press; 2022:11-19.
4. Olsthoorn P. Military Ethics and Virtues: An Interdisciplinary Approach for the 21st Century. Routledge; 2010.
5. Pellegrino ED, Thomasma DC. The Virtues in Medical Practice. Oxford University Press; 1993.
6. Edward Clayton. Aristotle Politics. In: Internet Encyclopedia of Philosophy. Accessed November 28, 2022. https://iep.utm.edu/aristotle-politics
7. Tippets R. A VA doctor’s calling to help in Ukraine. VA News. October 23, 2022. Accessed November 28, 2022. https://news.va.gov/109957/a-va-doctors-calling-to-help-in-ukraine
8. Lybrand H. US Army doctor and anesthesiologist charged with conspiring to US military records to the Russian government. CNN Politics, September 29, 2022. Accessed November 28, 2022 https://www.cnn.com/2022/09/29/politics/us-army-doctor-anesthesiologist-russian-government-medical-records
9. United States v Anna Gabrielian and James Lee Henry, (SD Md 2022). Accessed November 28, 2022. https://www.documentcloud.org/documents/23106067-gabrielian-and-henry-indictment
10. Lincoln A. First Inaugural Address of Abraham Lincoln. Accessed November 28, 2022. https://avalon.law.yale.edu/19th_century/lincoln1.asp
1. Aristotle. Politics. Book I, 1253.a31.
2. The Ethics of Aristotle. Aristotle. The Nicomachean Ethics. Thompson JAK, trans. Penguin Books; 1953.
3. Schonfeld TL, Hester DM. Brief introduction to ethics and ethical theory. In: Schonfeld TL, Hester DM, eds. Guidance for Healthcare Ethics Committees. 2nd ed. Cambridge University Press; 2022:11-19.
4. Olsthoorn P. Military Ethics and Virtues: An Interdisciplinary Approach for the 21st Century. Routledge; 2010.
5. Pellegrino ED, Thomasma DC. The Virtues in Medical Practice. Oxford University Press; 1993.
6. Edward Clayton. Aristotle Politics. In: Internet Encyclopedia of Philosophy. Accessed November 28, 2022. https://iep.utm.edu/aristotle-politics
7. Tippets R. A VA doctor’s calling to help in Ukraine. VA News. October 23, 2022. Accessed November 28, 2022. https://news.va.gov/109957/a-va-doctors-calling-to-help-in-ukraine
8. Lybrand H. US Army doctor and anesthesiologist charged with conspiring to US military records to the Russian government. CNN Politics, September 29, 2022. Accessed November 28, 2022 https://www.cnn.com/2022/09/29/politics/us-army-doctor-anesthesiologist-russian-government-medical-records
9. United States v Anna Gabrielian and James Lee Henry, (SD Md 2022). Accessed November 28, 2022. https://www.documentcloud.org/documents/23106067-gabrielian-and-henry-indictment
10. Lincoln A. First Inaugural Address of Abraham Lincoln. Accessed November 28, 2022. https://avalon.law.yale.edu/19th_century/lincoln1.asp



