User login
How to remain apolitical with patients
It is assumed that psychiatrists in general, but particularly in academia, are progressive liberals. There is evidence to support this idea, with a survey finding that more than three-quarters of U.S. psychiatrists are registered Democrats.1
Other corroborating factors to our field’s progressive tendency include the publication of pseudo-political books like “The Dangerous Case of Donald Trump: 27 Psychiatrists and Mental Health Experts Assess a President” – without a well-known equivalent on the other side.
Additionally, psychiatry has in the recent past, rightfully spent significant effort examining the disproportional trauma faced by patients with underprivileged backgrounds, which is often seen as a political position. The American Psychiatric Association has itself taken a stance on the national debate about abortion to warn against the psychiatric consequences of the Dobbs v. Jackson Supreme Court decision despite the clear political statement it makes.
We understand a likely rationale for psychiatry’s liberal tendency. Most psychiatrists support political objectives that provide resources for the treatment of the severely mentally ill. In general, the psychosocial consequences of mental illness place a downward economic pressure on our patients that leads to poverty and its associated traumas that then tend to feedback to worsen the severity of the illness itself. It is thus natural for psychiatry to promote political causes such as progressivism that focus on the needs of economically and socially struggling communities. If one posits a natural role for psychiatry in promoting the interests of patients, then it is a short leap to psychiatry promoting the political causes of the underprivileged, often in the form of endorsing the Democratic party.
As a result, a proportion of patients come into psychiatric treatment with expectations that their providers will negatively judge them and possibly punish their conservative beliefs or Republican political affiliation. Herein lies a question – “Is psychiatry willing to make 46.9% of Americans uncomfortable?” How should psychiatry address the 46.9% of Americans who voted Republican during the 2020 presidential election? In our desire to support the disadvantaged, how political are we willing to get and at what cost? While we cannot speak for the field as a whole, it is our concern that a vast percentage of Americans feel alienated from talking to us, which is particularly problematic in a field based on mutual trust and understanding.
This problem may be particularly palpable to us, as we are psychiatrists in a large metropolitan area of California who often treat specialty populations like veterans and law enforcement. In one study, law enforcement officers were found to be twice as likely to be Republicans as civilians.2 Michael McHale, the president of the National Association of Police Organizations, spoke at the 2020 Republican Party’s national convention as documented in an article titled “Union leader tells Republican convention why cops back Trump.”3 Similarly, about 60% of veterans identify as Republicans.4
Within the first few sessions, when patients are most vulnerable and sensitive to the perception of being judged, we commonly get asked questions to test our political beliefs. Some patients will display clothing that suggests a political affiliation; those wardrobe arrangements are, at times, an attempt at testing our knowledge of their in-group. While a bright-red cap with a reminder to keep the United States “great” in capital letters may be an overt invitation to address the topic, other patients may have a small symbol of a rattlesnake to test our ability to recognize the “Don’t Tread on Me” Gadsden flag.
Alternatively, other patients will ask our opinion, or bring up news topics, to share their concerns and/or examine our response and reactions. We remember, in particular, a patient who subtly asked if they needed to be vaccinated to attend therapy visits in person as a leading statement into their conservative political beliefs. It is a reminder that many patients fear how we will judge them or where we will draw the line – “Is there something I, the patient, can say that will make him dislike me?”
While the concept of making all patients comfortable may feel abstract or trivial to some, the consequences can be very real. We remember a patient with severe depression and occasional suicidality, who required many months of treatment for him to reveal that he owned a gun. His conservative beliefs made him very resistant to discuss gun ownership with someone who is presumably liberal and has the power to restrict such ownership. However, after a frank discussion that our concerns about his gun were not constitutional or political but medical, the patient agreed to relinquish his gun, at least temporarily, a likely more important intervention than many in psychiatry.
The ramifications are also wider than most imagine. In California, a particularly liberal state, many consistently and reliably liberal patients have some conservative beliefs. Those beliefs are often closeted: a Democratic mother who doesn’t think her 3-year-old daughter should wear a mask in school; a Democratic woman who questioned the veracity of Amber Heard during the Johnny Depp defamation trial and feels guilty about her prior dedication to the #MeToo movement.
Patients may feel torn about those beliefs and may be apprehensive to discuss them despite a nagging need to express or examine them in a place without judgment.
that we attempted to highlight in this article. In particular, a vast proportion of Americans may feel alienated from treatment or may refuse to divulge clinically relevant information, and a large number of patients may enter psychiatric treatment with concerns that they will be judged.
Psychiatry is founded on the honest exchange of thoughts and feelings between patients and providers without the fear of harsh judgment and intellectual retaliation. Psychiatrists would be wise to consider those factors and their repercussions when choosing to take political positions and setting a frame of care with their patients.
Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Sanger-Katz M. Your surgeon is probably a Republican, your psychiatrist probably a Democrat. New York Times. 2016 Oct 6.
2. Ba B et al. Who are the police? Descriptive representation in the coercive arm of government. 2022 Mar 21.
3. Rainey J. Union leader tells Republican convention why cops back Trump. Los Angeles Times. 2020 Aug 26.
4. Igielnik R et al. Trump draws stronger support from veterans than from the public on leadership of U.S. military. Pew Research Center. 2019 July 10.
It is assumed that psychiatrists in general, but particularly in academia, are progressive liberals. There is evidence to support this idea, with a survey finding that more than three-quarters of U.S. psychiatrists are registered Democrats.1
Other corroborating factors to our field’s progressive tendency include the publication of pseudo-political books like “The Dangerous Case of Donald Trump: 27 Psychiatrists and Mental Health Experts Assess a President” – without a well-known equivalent on the other side.
Additionally, psychiatry has in the recent past, rightfully spent significant effort examining the disproportional trauma faced by patients with underprivileged backgrounds, which is often seen as a political position. The American Psychiatric Association has itself taken a stance on the national debate about abortion to warn against the psychiatric consequences of the Dobbs v. Jackson Supreme Court decision despite the clear political statement it makes.
We understand a likely rationale for psychiatry’s liberal tendency. Most psychiatrists support political objectives that provide resources for the treatment of the severely mentally ill. In general, the psychosocial consequences of mental illness place a downward economic pressure on our patients that leads to poverty and its associated traumas that then tend to feedback to worsen the severity of the illness itself. It is thus natural for psychiatry to promote political causes such as progressivism that focus on the needs of economically and socially struggling communities. If one posits a natural role for psychiatry in promoting the interests of patients, then it is a short leap to psychiatry promoting the political causes of the underprivileged, often in the form of endorsing the Democratic party.
As a result, a proportion of patients come into psychiatric treatment with expectations that their providers will negatively judge them and possibly punish their conservative beliefs or Republican political affiliation. Herein lies a question – “Is psychiatry willing to make 46.9% of Americans uncomfortable?” How should psychiatry address the 46.9% of Americans who voted Republican during the 2020 presidential election? In our desire to support the disadvantaged, how political are we willing to get and at what cost? While we cannot speak for the field as a whole, it is our concern that a vast percentage of Americans feel alienated from talking to us, which is particularly problematic in a field based on mutual trust and understanding.
This problem may be particularly palpable to us, as we are psychiatrists in a large metropolitan area of California who often treat specialty populations like veterans and law enforcement. In one study, law enforcement officers were found to be twice as likely to be Republicans as civilians.2 Michael McHale, the president of the National Association of Police Organizations, spoke at the 2020 Republican Party’s national convention as documented in an article titled “Union leader tells Republican convention why cops back Trump.”3 Similarly, about 60% of veterans identify as Republicans.4
Within the first few sessions, when patients are most vulnerable and sensitive to the perception of being judged, we commonly get asked questions to test our political beliefs. Some patients will display clothing that suggests a political affiliation; those wardrobe arrangements are, at times, an attempt at testing our knowledge of their in-group. While a bright-red cap with a reminder to keep the United States “great” in capital letters may be an overt invitation to address the topic, other patients may have a small symbol of a rattlesnake to test our ability to recognize the “Don’t Tread on Me” Gadsden flag.
Alternatively, other patients will ask our opinion, or bring up news topics, to share their concerns and/or examine our response and reactions. We remember, in particular, a patient who subtly asked if they needed to be vaccinated to attend therapy visits in person as a leading statement into their conservative political beliefs. It is a reminder that many patients fear how we will judge them or where we will draw the line – “Is there something I, the patient, can say that will make him dislike me?”
While the concept of making all patients comfortable may feel abstract or trivial to some, the consequences can be very real. We remember a patient with severe depression and occasional suicidality, who required many months of treatment for him to reveal that he owned a gun. His conservative beliefs made him very resistant to discuss gun ownership with someone who is presumably liberal and has the power to restrict such ownership. However, after a frank discussion that our concerns about his gun were not constitutional or political but medical, the patient agreed to relinquish his gun, at least temporarily, a likely more important intervention than many in psychiatry.
The ramifications are also wider than most imagine. In California, a particularly liberal state, many consistently and reliably liberal patients have some conservative beliefs. Those beliefs are often closeted: a Democratic mother who doesn’t think her 3-year-old daughter should wear a mask in school; a Democratic woman who questioned the veracity of Amber Heard during the Johnny Depp defamation trial and feels guilty about her prior dedication to the #MeToo movement.
Patients may feel torn about those beliefs and may be apprehensive to discuss them despite a nagging need to express or examine them in a place without judgment.
that we attempted to highlight in this article. In particular, a vast proportion of Americans may feel alienated from treatment or may refuse to divulge clinically relevant information, and a large number of patients may enter psychiatric treatment with concerns that they will be judged.
Psychiatry is founded on the honest exchange of thoughts and feelings between patients and providers without the fear of harsh judgment and intellectual retaliation. Psychiatrists would be wise to consider those factors and their repercussions when choosing to take political positions and setting a frame of care with their patients.
Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Sanger-Katz M. Your surgeon is probably a Republican, your psychiatrist probably a Democrat. New York Times. 2016 Oct 6.
2. Ba B et al. Who are the police? Descriptive representation in the coercive arm of government. 2022 Mar 21.
3. Rainey J. Union leader tells Republican convention why cops back Trump. Los Angeles Times. 2020 Aug 26.
4. Igielnik R et al. Trump draws stronger support from veterans than from the public on leadership of U.S. military. Pew Research Center. 2019 July 10.
It is assumed that psychiatrists in general, but particularly in academia, are progressive liberals. There is evidence to support this idea, with a survey finding that more than three-quarters of U.S. psychiatrists are registered Democrats.1
Other corroborating factors to our field’s progressive tendency include the publication of pseudo-political books like “The Dangerous Case of Donald Trump: 27 Psychiatrists and Mental Health Experts Assess a President” – without a well-known equivalent on the other side.
Additionally, psychiatry has in the recent past, rightfully spent significant effort examining the disproportional trauma faced by patients with underprivileged backgrounds, which is often seen as a political position. The American Psychiatric Association has itself taken a stance on the national debate about abortion to warn against the psychiatric consequences of the Dobbs v. Jackson Supreme Court decision despite the clear political statement it makes.
We understand a likely rationale for psychiatry’s liberal tendency. Most psychiatrists support political objectives that provide resources for the treatment of the severely mentally ill. In general, the psychosocial consequences of mental illness place a downward economic pressure on our patients that leads to poverty and its associated traumas that then tend to feedback to worsen the severity of the illness itself. It is thus natural for psychiatry to promote political causes such as progressivism that focus on the needs of economically and socially struggling communities. If one posits a natural role for psychiatry in promoting the interests of patients, then it is a short leap to psychiatry promoting the political causes of the underprivileged, often in the form of endorsing the Democratic party.
As a result, a proportion of patients come into psychiatric treatment with expectations that their providers will negatively judge them and possibly punish their conservative beliefs or Republican political affiliation. Herein lies a question – “Is psychiatry willing to make 46.9% of Americans uncomfortable?” How should psychiatry address the 46.9% of Americans who voted Republican during the 2020 presidential election? In our desire to support the disadvantaged, how political are we willing to get and at what cost? While we cannot speak for the field as a whole, it is our concern that a vast percentage of Americans feel alienated from talking to us, which is particularly problematic in a field based on mutual trust and understanding.
This problem may be particularly palpable to us, as we are psychiatrists in a large metropolitan area of California who often treat specialty populations like veterans and law enforcement. In one study, law enforcement officers were found to be twice as likely to be Republicans as civilians.2 Michael McHale, the president of the National Association of Police Organizations, spoke at the 2020 Republican Party’s national convention as documented in an article titled “Union leader tells Republican convention why cops back Trump.”3 Similarly, about 60% of veterans identify as Republicans.4
Within the first few sessions, when patients are most vulnerable and sensitive to the perception of being judged, we commonly get asked questions to test our political beliefs. Some patients will display clothing that suggests a political affiliation; those wardrobe arrangements are, at times, an attempt at testing our knowledge of their in-group. While a bright-red cap with a reminder to keep the United States “great” in capital letters may be an overt invitation to address the topic, other patients may have a small symbol of a rattlesnake to test our ability to recognize the “Don’t Tread on Me” Gadsden flag.
Alternatively, other patients will ask our opinion, or bring up news topics, to share their concerns and/or examine our response and reactions. We remember, in particular, a patient who subtly asked if they needed to be vaccinated to attend therapy visits in person as a leading statement into their conservative political beliefs. It is a reminder that many patients fear how we will judge them or where we will draw the line – “Is there something I, the patient, can say that will make him dislike me?”
While the concept of making all patients comfortable may feel abstract or trivial to some, the consequences can be very real. We remember a patient with severe depression and occasional suicidality, who required many months of treatment for him to reveal that he owned a gun. His conservative beliefs made him very resistant to discuss gun ownership with someone who is presumably liberal and has the power to restrict such ownership. However, after a frank discussion that our concerns about his gun were not constitutional or political but medical, the patient agreed to relinquish his gun, at least temporarily, a likely more important intervention than many in psychiatry.
The ramifications are also wider than most imagine. In California, a particularly liberal state, many consistently and reliably liberal patients have some conservative beliefs. Those beliefs are often closeted: a Democratic mother who doesn’t think her 3-year-old daughter should wear a mask in school; a Democratic woman who questioned the veracity of Amber Heard during the Johnny Depp defamation trial and feels guilty about her prior dedication to the #MeToo movement.
Patients may feel torn about those beliefs and may be apprehensive to discuss them despite a nagging need to express or examine them in a place without judgment.
that we attempted to highlight in this article. In particular, a vast proportion of Americans may feel alienated from treatment or may refuse to divulge clinically relevant information, and a large number of patients may enter psychiatric treatment with concerns that they will be judged.
Psychiatry is founded on the honest exchange of thoughts and feelings between patients and providers without the fear of harsh judgment and intellectual retaliation. Psychiatrists would be wise to consider those factors and their repercussions when choosing to take political positions and setting a frame of care with their patients.
Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Sanger-Katz M. Your surgeon is probably a Republican, your psychiatrist probably a Democrat. New York Times. 2016 Oct 6.
2. Ba B et al. Who are the police? Descriptive representation in the coercive arm of government. 2022 Mar 21.
3. Rainey J. Union leader tells Republican convention why cops back Trump. Los Angeles Times. 2020 Aug 26.
4. Igielnik R et al. Trump draws stronger support from veterans than from the public on leadership of U.S. military. Pew Research Center. 2019 July 10.
Vision loss may be a risk with PRP facial injections
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
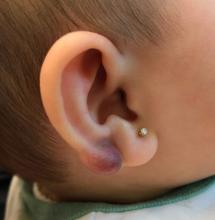
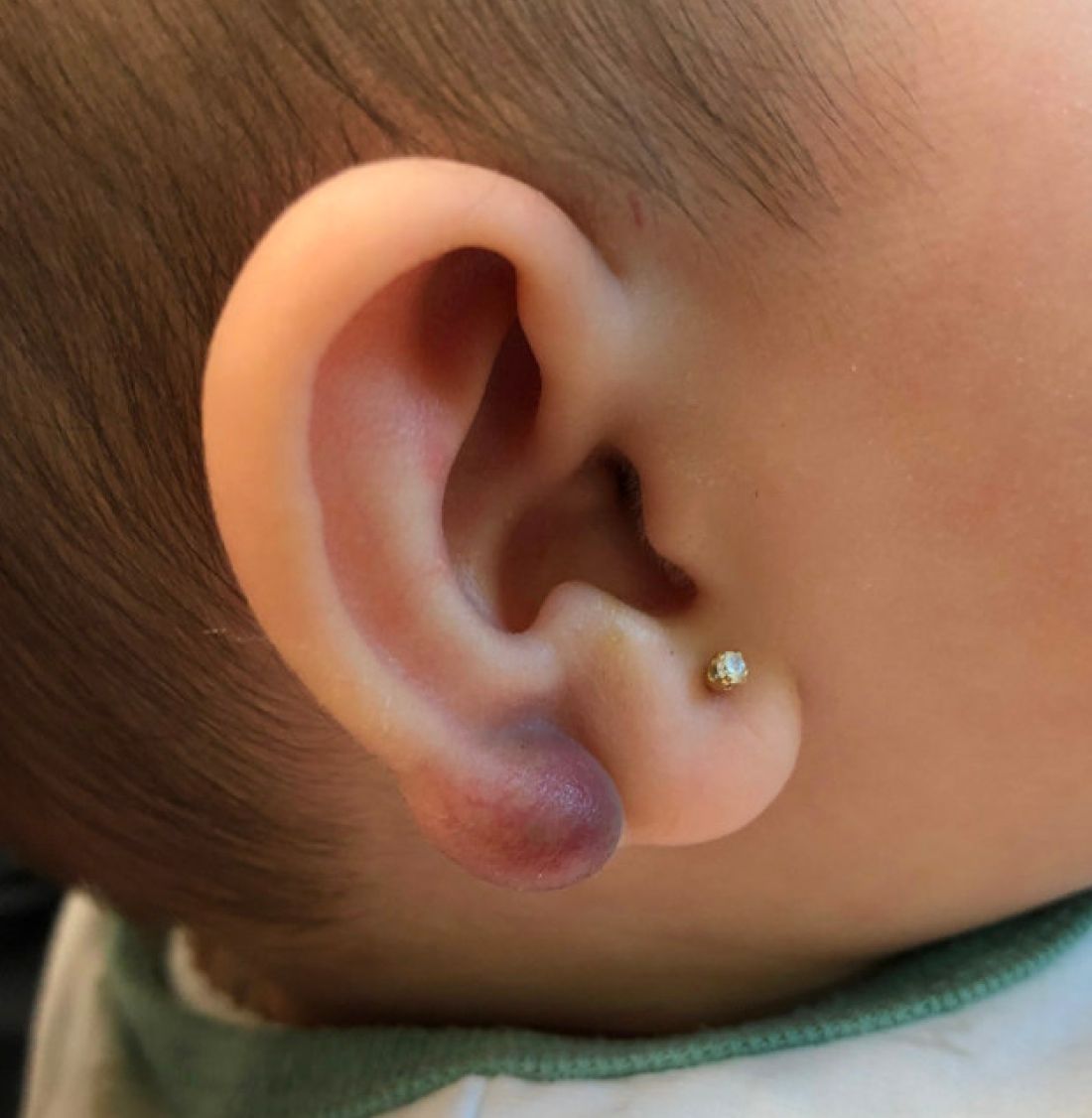
An infant with a tender bump on her ear
A biopsy of the lesion was performed that showed a well-defined nodulocystic tumor composed of nests of basaloid cells that are undergoing trichilemmal keratinization. Shadow cells are seen as well as small areas of calcification. There is also a histiocytic infiltrate with multinucleated giant cells. The histologic diagnosis is of a pilomatrixoma.
Pilomatrixoma, also known as calcifying epithelioma of Malherbe, was first described in 1880, as a tumor of sebaceous gland origin. Later, in 1961, Robert Forbis Jr, MD, and Elson B. Helwig, MD, coined the term pilomatrixoma to describe the hair follicle matrix as the source of the tumor. Pilomatrixomas are commonly seen in the pediatric population, usually in children between 8 and 13 years of age. Our patient is one of the youngest described. The lesions are commonly seen on the face and neck in about 70% of the cases followed by the upper extremities, back, and legs. Clinically, the lesions appear as a firm dermal papule or nodule, which moves freely and may have associated erythema on the skin surface or a blueish gray hue on the underlying skin.
Most pilomatrixomas that have been studied have shown a mutation in Exon 3 of the beta-catenin gene (CTNNB1). The beta-catenin molecule is a subunit of the cadherin protein, which is part of an important pathway in the terminal hair follicle differentiation. Beta-catenin also plays an important role in the Wnt pathway, which regulates cell fate as well as early embryonic patterning. Beta-catenin is responsible for forming adhesion junctions among cells. There have also been immunohistochemical studies that have shown a BCL2 proto-oncogene overexpression to pilomatrixoma.
There are several genetic syndromes that have been associated with the presence of pilomatrixomas: Turner syndrome (XO chromosome abnormality associated with short stature and cardiac defects), Gardner syndrome (polyposis coli and colon and rectal cancer), myotonic dystrophy, Rubinstein-Taybi syndrome (characterized by broad thumbs and toes, short stature, distinctive facial features, and varying degrees of intellectual disability), and trisomy 9. On physical examination our patient didn’t present with any of the typical features or history that could suggest any of these syndromes. A close follow-up and evaluation by a geneticist was recommended because after the initial visit she developed a second lesion on the forehead.
The differential diagnosis for this lesion includes other cysts that may occur on the ear such as epidermal inclusion cyst or dermoid cysts, though these lesions do not tend to be as firm as pilomatrixomas are, which can help with the diagnosis. Dermoid cysts are made of dermal and epidermal components. They are usually present at birth and are commonly seen on the scalp and the periorbital face.
Keloids are rubbery nodules of scar tissue that can form on sites of trauma, and although the lesion occurred after she had her ears pierced, the consistency and rapid growth of the lesion as well as the pathological description made this benign fibrous growth less likely.
When pilomatrixomas are inflamed they can be confused with vascular growths: in this particular case, a hemangioma or another vascular tumor such as a tufted angioma or kaposiform hemangioendothelioma. An ultrasound of the lesion could have helped in the differential diagnosis of the lesion.
Pilomatrixomas can grow significantly and in some cases get inflamed or infected. Surgical management of pilomatrixomas is often required because the lesions do not regress spontaneously.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Forbis R Jr and Helwig EB. Arch Dermatol 1961;83:606-18.
Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016 Jun;85:148-53.
A biopsy of the lesion was performed that showed a well-defined nodulocystic tumor composed of nests of basaloid cells that are undergoing trichilemmal keratinization. Shadow cells are seen as well as small areas of calcification. There is also a histiocytic infiltrate with multinucleated giant cells. The histologic diagnosis is of a pilomatrixoma.
Pilomatrixoma, also known as calcifying epithelioma of Malherbe, was first described in 1880, as a tumor of sebaceous gland origin. Later, in 1961, Robert Forbis Jr, MD, and Elson B. Helwig, MD, coined the term pilomatrixoma to describe the hair follicle matrix as the source of the tumor. Pilomatrixomas are commonly seen in the pediatric population, usually in children between 8 and 13 years of age. Our patient is one of the youngest described. The lesions are commonly seen on the face and neck in about 70% of the cases followed by the upper extremities, back, and legs. Clinically, the lesions appear as a firm dermal papule or nodule, which moves freely and may have associated erythema on the skin surface or a blueish gray hue on the underlying skin.
Most pilomatrixomas that have been studied have shown a mutation in Exon 3 of the beta-catenin gene (CTNNB1). The beta-catenin molecule is a subunit of the cadherin protein, which is part of an important pathway in the terminal hair follicle differentiation. Beta-catenin also plays an important role in the Wnt pathway, which regulates cell fate as well as early embryonic patterning. Beta-catenin is responsible for forming adhesion junctions among cells. There have also been immunohistochemical studies that have shown a BCL2 proto-oncogene overexpression to pilomatrixoma.
There are several genetic syndromes that have been associated with the presence of pilomatrixomas: Turner syndrome (XO chromosome abnormality associated with short stature and cardiac defects), Gardner syndrome (polyposis coli and colon and rectal cancer), myotonic dystrophy, Rubinstein-Taybi syndrome (characterized by broad thumbs and toes, short stature, distinctive facial features, and varying degrees of intellectual disability), and trisomy 9. On physical examination our patient didn’t present with any of the typical features or history that could suggest any of these syndromes. A close follow-up and evaluation by a geneticist was recommended because after the initial visit she developed a second lesion on the forehead.
The differential diagnosis for this lesion includes other cysts that may occur on the ear such as epidermal inclusion cyst or dermoid cysts, though these lesions do not tend to be as firm as pilomatrixomas are, which can help with the diagnosis. Dermoid cysts are made of dermal and epidermal components. They are usually present at birth and are commonly seen on the scalp and the periorbital face.
Keloids are rubbery nodules of scar tissue that can form on sites of trauma, and although the lesion occurred after she had her ears pierced, the consistency and rapid growth of the lesion as well as the pathological description made this benign fibrous growth less likely.
When pilomatrixomas are inflamed they can be confused with vascular growths: in this particular case, a hemangioma or another vascular tumor such as a tufted angioma or kaposiform hemangioendothelioma. An ultrasound of the lesion could have helped in the differential diagnosis of the lesion.
Pilomatrixomas can grow significantly and in some cases get inflamed or infected. Surgical management of pilomatrixomas is often required because the lesions do not regress spontaneously.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Forbis R Jr and Helwig EB. Arch Dermatol 1961;83:606-18.
Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016 Jun;85:148-53.
A biopsy of the lesion was performed that showed a well-defined nodulocystic tumor composed of nests of basaloid cells that are undergoing trichilemmal keratinization. Shadow cells are seen as well as small areas of calcification. There is also a histiocytic infiltrate with multinucleated giant cells. The histologic diagnosis is of a pilomatrixoma.
Pilomatrixoma, also known as calcifying epithelioma of Malherbe, was first described in 1880, as a tumor of sebaceous gland origin. Later, in 1961, Robert Forbis Jr, MD, and Elson B. Helwig, MD, coined the term pilomatrixoma to describe the hair follicle matrix as the source of the tumor. Pilomatrixomas are commonly seen in the pediatric population, usually in children between 8 and 13 years of age. Our patient is one of the youngest described. The lesions are commonly seen on the face and neck in about 70% of the cases followed by the upper extremities, back, and legs. Clinically, the lesions appear as a firm dermal papule or nodule, which moves freely and may have associated erythema on the skin surface or a blueish gray hue on the underlying skin.
Most pilomatrixomas that have been studied have shown a mutation in Exon 3 of the beta-catenin gene (CTNNB1). The beta-catenin molecule is a subunit of the cadherin protein, which is part of an important pathway in the terminal hair follicle differentiation. Beta-catenin also plays an important role in the Wnt pathway, which regulates cell fate as well as early embryonic patterning. Beta-catenin is responsible for forming adhesion junctions among cells. There have also been immunohistochemical studies that have shown a BCL2 proto-oncogene overexpression to pilomatrixoma.
There are several genetic syndromes that have been associated with the presence of pilomatrixomas: Turner syndrome (XO chromosome abnormality associated with short stature and cardiac defects), Gardner syndrome (polyposis coli and colon and rectal cancer), myotonic dystrophy, Rubinstein-Taybi syndrome (characterized by broad thumbs and toes, short stature, distinctive facial features, and varying degrees of intellectual disability), and trisomy 9. On physical examination our patient didn’t present with any of the typical features or history that could suggest any of these syndromes. A close follow-up and evaluation by a geneticist was recommended because after the initial visit she developed a second lesion on the forehead.
The differential diagnosis for this lesion includes other cysts that may occur on the ear such as epidermal inclusion cyst or dermoid cysts, though these lesions do not tend to be as firm as pilomatrixomas are, which can help with the diagnosis. Dermoid cysts are made of dermal and epidermal components. They are usually present at birth and are commonly seen on the scalp and the periorbital face.
Keloids are rubbery nodules of scar tissue that can form on sites of trauma, and although the lesion occurred after she had her ears pierced, the consistency and rapid growth of the lesion as well as the pathological description made this benign fibrous growth less likely.
When pilomatrixomas are inflamed they can be confused with vascular growths: in this particular case, a hemangioma or another vascular tumor such as a tufted angioma or kaposiform hemangioendothelioma. An ultrasound of the lesion could have helped in the differential diagnosis of the lesion.
Pilomatrixomas can grow significantly and in some cases get inflamed or infected. Surgical management of pilomatrixomas is often required because the lesions do not regress spontaneously.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Forbis R Jr and Helwig EB. Arch Dermatol 1961;83:606-18.
Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016 Jun;85:148-53.
A 4-month-old female was referred to our clinic for evaluation of a bump on the right ear. The lesion was first noted at 2 months of age as a little pimple. She was evaluated by her pediatrician and was treated with topical and oral antibiotics without resolution of the lesion. The bump continued to grow and seemed tender to palpation, so she was referred to dermatology for evaluation.
She was born via normal vaginal delivery at 40 weeks. Her mother has no medical conditions and the pregnancy was uneventful. She has been growing and developing well. She takes vitamin D and is currently breast fed.
There have been no other family members with similar lesions. She had her ears pierced at a month of age without any complications.
On skin examination she has a firm red nodule on the right ear that appears slightly tender to touch. She has no other skin lesions of concern. She has normal muscle tone and there are no other abnormalities noted on the physical exam. She has no hepatomegaly, splenomegaly, or lymphadenopathy.
Vaccinium myrtillus (bilberry seed oil) extract
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
The ‘root cause’ visit
“How did we miss out on that?” “What?” my physician friend replied as we stood in line at the coffee cart. “Root cause. I mean, we invented this idea and now all these naturopaths and functional medicine quacks are gettin’ rich off it.” “Take it easy,” he says. “Just order a coffee.”
It’s hard not to be indignant. I had a morning clinic with three patients insisting I find the “root cause” of their problem. Now, if one had flagellate dermatitis after eating Asian mushroom soup, I’d have said “Root cause? Shiitake mushrooms!” and walked out like Costanza in Seinfeld, “All right, that’s it for me! Be good everybody!”
Alas no. They had perioral dermatitis, alopecia areata, eczema – no satisfying “roots” for walk-off answers.
There is a universal desire to find the proximal cause for problems. Patients often want to know it so that we address the root of their trouble and not just cut off the branches. This is deeply gratifying for those who want not only to know why, but also to have agency in how to control their disease. For example, if they believe the root cause of perioral dermatitis was excess yeast, then eating a “candida diet’’ should do the trick! Food sensitivities, hormones, and heavy metals round out the top suspects that root cause patients want to talk about.
Of course, patients have been asking about this for a long time, but lately, the root cause visit seems to be on trend. Check out any hip primary care start-up such as One Medical or any hot direct-to-consumer virtual offering such as ParsleyHealth and you will see root-cause everywhere. Our patients are expecting us to address it, or it seems they will find someone cooler who will.
Yet, it wasn’t the slick marketing team at ParsleyHeath who invented the “root cause doctor visit.” We did. It’s an idea that started with our Greek physician ancestors. Breaking from the diviners and priests, we were the first “naturalists” positing that there was a natural, not a divine cause for illness. The cardinal concept in the Hippocratic Corpus was that health was an equilibrium and illness an imbalance. They didn’t have dehydroepiandrosterone tests or mercury levels, but did have bodily fluids. Yellow bile, black bile, blood, and phlegm, were the root of all root causes. A physician simply had to identify which was in excess or deficient and fix that to cure the disease. Interestingly, the word “diagnosis” appears only once in the Corpus. The word “Diagignoskein” appears occasionally but this describes studying thoroughly, not naming a diagnosis as we understand it.
Advances in chemistry in the 17th century meant physicians could add new theories, and new root causes. Now alkaline or other chemical elixirs were added to cure at the source. Since there was no verifiable evidence to prove causes, theories were adopted to provide some rational direction to treatment. In the 18th century, physicians such as Dr. Benjamin Rush, one of the original faculty at the University of Pennsylvania school of medicine, taught that spasms of the arteries were the root cause of illnesses. “Heroic” treatments such as extreme bloodletting were the cure. (Note, those patients who survived us kept coming back to us for more).
Scientific knowledge and diagnostic technologies led to more and more complex and abstruse causes. Yet, as we became more precise and effective, our explanations became less satisfying to our patients. I can diagnose and readily treat perioral dermatitis, yet I’m hard pressed to give an answer to its root cause. “Root cause? Yes. Just apply this pimecrolimus cream for a couple of weeks and it’ll be better! All right, that’s it for me! Be good everybody!”
You’ll have to do better, George.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
“How did we miss out on that?” “What?” my physician friend replied as we stood in line at the coffee cart. “Root cause. I mean, we invented this idea and now all these naturopaths and functional medicine quacks are gettin’ rich off it.” “Take it easy,” he says. “Just order a coffee.”
It’s hard not to be indignant. I had a morning clinic with three patients insisting I find the “root cause” of their problem. Now, if one had flagellate dermatitis after eating Asian mushroom soup, I’d have said “Root cause? Shiitake mushrooms!” and walked out like Costanza in Seinfeld, “All right, that’s it for me! Be good everybody!”
Alas no. They had perioral dermatitis, alopecia areata, eczema – no satisfying “roots” for walk-off answers.
There is a universal desire to find the proximal cause for problems. Patients often want to know it so that we address the root of their trouble and not just cut off the branches. This is deeply gratifying for those who want not only to know why, but also to have agency in how to control their disease. For example, if they believe the root cause of perioral dermatitis was excess yeast, then eating a “candida diet’’ should do the trick! Food sensitivities, hormones, and heavy metals round out the top suspects that root cause patients want to talk about.
Of course, patients have been asking about this for a long time, but lately, the root cause visit seems to be on trend. Check out any hip primary care start-up such as One Medical or any hot direct-to-consumer virtual offering such as ParsleyHealth and you will see root-cause everywhere. Our patients are expecting us to address it, or it seems they will find someone cooler who will.
Yet, it wasn’t the slick marketing team at ParsleyHeath who invented the “root cause doctor visit.” We did. It’s an idea that started with our Greek physician ancestors. Breaking from the diviners and priests, we were the first “naturalists” positing that there was a natural, not a divine cause for illness. The cardinal concept in the Hippocratic Corpus was that health was an equilibrium and illness an imbalance. They didn’t have dehydroepiandrosterone tests or mercury levels, but did have bodily fluids. Yellow bile, black bile, blood, and phlegm, were the root of all root causes. A physician simply had to identify which was in excess or deficient and fix that to cure the disease. Interestingly, the word “diagnosis” appears only once in the Corpus. The word “Diagignoskein” appears occasionally but this describes studying thoroughly, not naming a diagnosis as we understand it.
Advances in chemistry in the 17th century meant physicians could add new theories, and new root causes. Now alkaline or other chemical elixirs were added to cure at the source. Since there was no verifiable evidence to prove causes, theories were adopted to provide some rational direction to treatment. In the 18th century, physicians such as Dr. Benjamin Rush, one of the original faculty at the University of Pennsylvania school of medicine, taught that spasms of the arteries were the root cause of illnesses. “Heroic” treatments such as extreme bloodletting were the cure. (Note, those patients who survived us kept coming back to us for more).
Scientific knowledge and diagnostic technologies led to more and more complex and abstruse causes. Yet, as we became more precise and effective, our explanations became less satisfying to our patients. I can diagnose and readily treat perioral dermatitis, yet I’m hard pressed to give an answer to its root cause. “Root cause? Yes. Just apply this pimecrolimus cream for a couple of weeks and it’ll be better! All right, that’s it for me! Be good everybody!”
You’ll have to do better, George.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
“How did we miss out on that?” “What?” my physician friend replied as we stood in line at the coffee cart. “Root cause. I mean, we invented this idea and now all these naturopaths and functional medicine quacks are gettin’ rich off it.” “Take it easy,” he says. “Just order a coffee.”
It’s hard not to be indignant. I had a morning clinic with three patients insisting I find the “root cause” of their problem. Now, if one had flagellate dermatitis after eating Asian mushroom soup, I’d have said “Root cause? Shiitake mushrooms!” and walked out like Costanza in Seinfeld, “All right, that’s it for me! Be good everybody!”
Alas no. They had perioral dermatitis, alopecia areata, eczema – no satisfying “roots” for walk-off answers.
There is a universal desire to find the proximal cause for problems. Patients often want to know it so that we address the root of their trouble and not just cut off the branches. This is deeply gratifying for those who want not only to know why, but also to have agency in how to control their disease. For example, if they believe the root cause of perioral dermatitis was excess yeast, then eating a “candida diet’’ should do the trick! Food sensitivities, hormones, and heavy metals round out the top suspects that root cause patients want to talk about.
Of course, patients have been asking about this for a long time, but lately, the root cause visit seems to be on trend. Check out any hip primary care start-up such as One Medical or any hot direct-to-consumer virtual offering such as ParsleyHealth and you will see root-cause everywhere. Our patients are expecting us to address it, or it seems they will find someone cooler who will.
Yet, it wasn’t the slick marketing team at ParsleyHeath who invented the “root cause doctor visit.” We did. It’s an idea that started with our Greek physician ancestors. Breaking from the diviners and priests, we were the first “naturalists” positing that there was a natural, not a divine cause for illness. The cardinal concept in the Hippocratic Corpus was that health was an equilibrium and illness an imbalance. They didn’t have dehydroepiandrosterone tests or mercury levels, but did have bodily fluids. Yellow bile, black bile, blood, and phlegm, were the root of all root causes. A physician simply had to identify which was in excess or deficient and fix that to cure the disease. Interestingly, the word “diagnosis” appears only once in the Corpus. The word “Diagignoskein” appears occasionally but this describes studying thoroughly, not naming a diagnosis as we understand it.
Advances in chemistry in the 17th century meant physicians could add new theories, and new root causes. Now alkaline or other chemical elixirs were added to cure at the source. Since there was no verifiable evidence to prove causes, theories were adopted to provide some rational direction to treatment. In the 18th century, physicians such as Dr. Benjamin Rush, one of the original faculty at the University of Pennsylvania school of medicine, taught that spasms of the arteries were the root cause of illnesses. “Heroic” treatments such as extreme bloodletting were the cure. (Note, those patients who survived us kept coming back to us for more).
Scientific knowledge and diagnostic technologies led to more and more complex and abstruse causes. Yet, as we became more precise and effective, our explanations became less satisfying to our patients. I can diagnose and readily treat perioral dermatitis, yet I’m hard pressed to give an answer to its root cause. “Root cause? Yes. Just apply this pimecrolimus cream for a couple of weeks and it’ll be better! All right, that’s it for me! Be good everybody!”
You’ll have to do better, George.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Since when does providing pediatric care require courage?
I have been noticing “jokes” lately about doctors. Magazine cartoons depict doctors as conveying a bad prognosis in abrupt, indirect, vague ways. I remember, from medical school, the joke about pediatricians being doctors for patients “from the waist up” – wimps about tough topics such as sexuality. As an inherently shy person, I have appreciated the structure of the contractual relationship with families that both gives me permission and requires me to be direct about topics that would not be socially acceptable to discuss in other relationships.
Examples include our asking about bowel movements, genital symptoms, marital conflict, past abortions, food insecurity, adherence to medication, history of trauma or discrimination, substance use, illegal conduct, and suicidal ideation, among others. By bringing up these topics nonjudgmentally and with skill, we are demonstrating openness and making it safe for the patient/parent to ask questions about their own concerns.
Since these may be topics for which we lack knowledge or have very little power to help, it is far easier to not bring them up. Yet failing to have the courage to elicit sensitive information may delay the correct diagnosis, result in inappropriate tests or treatments, or miss factors critical in either the cause or solution for the patient’s problems.
Historically, being a physician has conveyed a promise of confidentiality and always trying to do what is best for the patient. The fact that we had to swear an oath to do so may also indicate that these things are not easy to do.
Yes, we need a lot of knowledge to know what is truly in the patient’s best interest. But we need to take personal risks to do it as well. There have been times when a parent has shouted “That is none of your business” at me or stormed out. Although only one patient has ever connected when striking out at me, other clinicians have not been as lucky (or had such small patients); some have even been killed.
For those of us in private practice, upsetting a patient with our well-intentioned words may mean losing them from our income stream or having them post negative comments online, which may affect our reputation in the community. Patients may not return for needed follow-up if a conversation was too uncomfortable for them. Current political divisions make this even trickier.
These days anxiety about getting behind in seeing the next patient may be a covert reason for avoiding difficult conversations as tears or anger take extra time. Certainly, fears of these outcomes can make us hold back from talking about important but potentially upsetting topics.
Of course, courage does not just mean being direct with questions, stating your observations, or giving advice. Courage requires thoughtfulness about possible adverse outcomes and their effect on others. It is not just “stupid bravery,” to proceed even when sensing danger. Courage is thus best paired with skill. It is:
- Setting up potentially difficult discussions with privacy (from the child or parent), seating, and enough time to listen.
- Normalizing questions by saying “I ask all my patients about ...” so patients do not feel singled out.
- Asking the patient or family first what they think is going on and how their own culture might regard the issue.
- Using simple language and arranging a translator when needed.
- Not just stating facts but checking “to be sure I explained well enough” rather than setting a patient up to appear ignorant for not understanding.
- Offering to contact the patient or other family member/support soon to review what you said and answer more questions.
- Offering a second opinion option.
- Promising to get more information when you do not know.
- Always leaving room for hope and sharing in that hope with them.
And it is crucial to have a way to keep notes about past trauma or difficult topics for a patient so neither you nor subsequent clinicians unnecessarily ask about sensitive topics.
Courage includes facing difficult situations without undue delay. Making that call about an abnormal test result right away, even when you are tired and upset, takes courage. Each time you overcome your own reluctance it takes moral strength but tends to make future courageous acts easier. Speaking up to a specialist on rounds when you think he or she is incorrect takes courage to serve the patient’s best interest. Being willing to try a new workflow in your office takes the courage to risk looking awkward or being judged by your team, but can be essential to progress. Asking for help or an opinion, sometimes from a medical assistant or student, can take courage but may reduce status barriers and improve relationships. Standing up for your values when they are not popular may take courage in some organizations. It takes courage to admit a mistake, even when your mistake may not otherwise be noticed.
How can we grow in courage? T. Berry Brazelton, MD, was a model of courage for me during my training – able and willing to tell about a child’s delays or ask about a parent’s well-being with empathy and by giving hope. Dr. Brazelton, pediatrician, developer of the Neonatal Behavioral Assessment Scale, professor at Harvard Medical School and founder of its Child Development Unit, was a world-renowned educator about the development of children and founder of the Touchpoints program. Our goal should be to promise to partner with the family in dealing with the problem, no matter how difficult or tender. I hope you had role models who not only said what to do but also demonstrated it with patients.
We had the privilege of hearing stories of difficult situations from hundreds of pediatricians in group sessions over the years in the Collaborative Office Rounds program. What group members often said that they valued most from these sessions was hearing examples of words they might say in these cases, either modeled by the coleaders or suggested by their pediatrician peers. Opportunities to share the tough times with trusted empathic peers is an important resource rarer and thus even more worth securing for yourself.
Being courageous may not be natural part of your personality but Aristotle said, “We become what we repeatedly do.” Even if you do not consider yourself so now, with practice you can become courageous and reap its benefits for your patients and yourself.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
I have been noticing “jokes” lately about doctors. Magazine cartoons depict doctors as conveying a bad prognosis in abrupt, indirect, vague ways. I remember, from medical school, the joke about pediatricians being doctors for patients “from the waist up” – wimps about tough topics such as sexuality. As an inherently shy person, I have appreciated the structure of the contractual relationship with families that both gives me permission and requires me to be direct about topics that would not be socially acceptable to discuss in other relationships.
Examples include our asking about bowel movements, genital symptoms, marital conflict, past abortions, food insecurity, adherence to medication, history of trauma or discrimination, substance use, illegal conduct, and suicidal ideation, among others. By bringing up these topics nonjudgmentally and with skill, we are demonstrating openness and making it safe for the patient/parent to ask questions about their own concerns.
Since these may be topics for which we lack knowledge or have very little power to help, it is far easier to not bring them up. Yet failing to have the courage to elicit sensitive information may delay the correct diagnosis, result in inappropriate tests or treatments, or miss factors critical in either the cause or solution for the patient’s problems.
Historically, being a physician has conveyed a promise of confidentiality and always trying to do what is best for the patient. The fact that we had to swear an oath to do so may also indicate that these things are not easy to do.
Yes, we need a lot of knowledge to know what is truly in the patient’s best interest. But we need to take personal risks to do it as well. There have been times when a parent has shouted “That is none of your business” at me or stormed out. Although only one patient has ever connected when striking out at me, other clinicians have not been as lucky (or had such small patients); some have even been killed.
For those of us in private practice, upsetting a patient with our well-intentioned words may mean losing them from our income stream or having them post negative comments online, which may affect our reputation in the community. Patients may not return for needed follow-up if a conversation was too uncomfortable for them. Current political divisions make this even trickier.
These days anxiety about getting behind in seeing the next patient may be a covert reason for avoiding difficult conversations as tears or anger take extra time. Certainly, fears of these outcomes can make us hold back from talking about important but potentially upsetting topics.
Of course, courage does not just mean being direct with questions, stating your observations, or giving advice. Courage requires thoughtfulness about possible adverse outcomes and their effect on others. It is not just “stupid bravery,” to proceed even when sensing danger. Courage is thus best paired with skill. It is:
- Setting up potentially difficult discussions with privacy (from the child or parent), seating, and enough time to listen.
- Normalizing questions by saying “I ask all my patients about ...” so patients do not feel singled out.
- Asking the patient or family first what they think is going on and how their own culture might regard the issue.
- Using simple language and arranging a translator when needed.
- Not just stating facts but checking “to be sure I explained well enough” rather than setting a patient up to appear ignorant for not understanding.
- Offering to contact the patient or other family member/support soon to review what you said and answer more questions.
- Offering a second opinion option.
- Promising to get more information when you do not know.
- Always leaving room for hope and sharing in that hope with them.
And it is crucial to have a way to keep notes about past trauma or difficult topics for a patient so neither you nor subsequent clinicians unnecessarily ask about sensitive topics.
Courage includes facing difficult situations without undue delay. Making that call about an abnormal test result right away, even when you are tired and upset, takes courage. Each time you overcome your own reluctance it takes moral strength but tends to make future courageous acts easier. Speaking up to a specialist on rounds when you think he or she is incorrect takes courage to serve the patient’s best interest. Being willing to try a new workflow in your office takes the courage to risk looking awkward or being judged by your team, but can be essential to progress. Asking for help or an opinion, sometimes from a medical assistant or student, can take courage but may reduce status barriers and improve relationships. Standing up for your values when they are not popular may take courage in some organizations. It takes courage to admit a mistake, even when your mistake may not otherwise be noticed.
How can we grow in courage? T. Berry Brazelton, MD, was a model of courage for me during my training – able and willing to tell about a child’s delays or ask about a parent’s well-being with empathy and by giving hope. Dr. Brazelton, pediatrician, developer of the Neonatal Behavioral Assessment Scale, professor at Harvard Medical School and founder of its Child Development Unit, was a world-renowned educator about the development of children and founder of the Touchpoints program. Our goal should be to promise to partner with the family in dealing with the problem, no matter how difficult or tender. I hope you had role models who not only said what to do but also demonstrated it with patients.
We had the privilege of hearing stories of difficult situations from hundreds of pediatricians in group sessions over the years in the Collaborative Office Rounds program. What group members often said that they valued most from these sessions was hearing examples of words they might say in these cases, either modeled by the coleaders or suggested by their pediatrician peers. Opportunities to share the tough times with trusted empathic peers is an important resource rarer and thus even more worth securing for yourself.
Being courageous may not be natural part of your personality but Aristotle said, “We become what we repeatedly do.” Even if you do not consider yourself so now, with practice you can become courageous and reap its benefits for your patients and yourself.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
I have been noticing “jokes” lately about doctors. Magazine cartoons depict doctors as conveying a bad prognosis in abrupt, indirect, vague ways. I remember, from medical school, the joke about pediatricians being doctors for patients “from the waist up” – wimps about tough topics such as sexuality. As an inherently shy person, I have appreciated the structure of the contractual relationship with families that both gives me permission and requires me to be direct about topics that would not be socially acceptable to discuss in other relationships.
Examples include our asking about bowel movements, genital symptoms, marital conflict, past abortions, food insecurity, adherence to medication, history of trauma or discrimination, substance use, illegal conduct, and suicidal ideation, among others. By bringing up these topics nonjudgmentally and with skill, we are demonstrating openness and making it safe for the patient/parent to ask questions about their own concerns.
Since these may be topics for which we lack knowledge or have very little power to help, it is far easier to not bring them up. Yet failing to have the courage to elicit sensitive information may delay the correct diagnosis, result in inappropriate tests or treatments, or miss factors critical in either the cause or solution for the patient’s problems.
Historically, being a physician has conveyed a promise of confidentiality and always trying to do what is best for the patient. The fact that we had to swear an oath to do so may also indicate that these things are not easy to do.
Yes, we need a lot of knowledge to know what is truly in the patient’s best interest. But we need to take personal risks to do it as well. There have been times when a parent has shouted “That is none of your business” at me or stormed out. Although only one patient has ever connected when striking out at me, other clinicians have not been as lucky (or had such small patients); some have even been killed.
For those of us in private practice, upsetting a patient with our well-intentioned words may mean losing them from our income stream or having them post negative comments online, which may affect our reputation in the community. Patients may not return for needed follow-up if a conversation was too uncomfortable for them. Current political divisions make this even trickier.
These days anxiety about getting behind in seeing the next patient may be a covert reason for avoiding difficult conversations as tears or anger take extra time. Certainly, fears of these outcomes can make us hold back from talking about important but potentially upsetting topics.
Of course, courage does not just mean being direct with questions, stating your observations, or giving advice. Courage requires thoughtfulness about possible adverse outcomes and their effect on others. It is not just “stupid bravery,” to proceed even when sensing danger. Courage is thus best paired with skill. It is:
- Setting up potentially difficult discussions with privacy (from the child or parent), seating, and enough time to listen.
- Normalizing questions by saying “I ask all my patients about ...” so patients do not feel singled out.
- Asking the patient or family first what they think is going on and how their own culture might regard the issue.
- Using simple language and arranging a translator when needed.
- Not just stating facts but checking “to be sure I explained well enough” rather than setting a patient up to appear ignorant for not understanding.
- Offering to contact the patient or other family member/support soon to review what you said and answer more questions.
- Offering a second opinion option.
- Promising to get more information when you do not know.
- Always leaving room for hope and sharing in that hope with them.
And it is crucial to have a way to keep notes about past trauma or difficult topics for a patient so neither you nor subsequent clinicians unnecessarily ask about sensitive topics.
Courage includes facing difficult situations without undue delay. Making that call about an abnormal test result right away, even when you are tired and upset, takes courage. Each time you overcome your own reluctance it takes moral strength but tends to make future courageous acts easier. Speaking up to a specialist on rounds when you think he or she is incorrect takes courage to serve the patient’s best interest. Being willing to try a new workflow in your office takes the courage to risk looking awkward or being judged by your team, but can be essential to progress. Asking for help or an opinion, sometimes from a medical assistant or student, can take courage but may reduce status barriers and improve relationships. Standing up for your values when they are not popular may take courage in some organizations. It takes courage to admit a mistake, even when your mistake may not otherwise be noticed.
How can we grow in courage? T. Berry Brazelton, MD, was a model of courage for me during my training – able and willing to tell about a child’s delays or ask about a parent’s well-being with empathy and by giving hope. Dr. Brazelton, pediatrician, developer of the Neonatal Behavioral Assessment Scale, professor at Harvard Medical School and founder of its Child Development Unit, was a world-renowned educator about the development of children and founder of the Touchpoints program. Our goal should be to promise to partner with the family in dealing with the problem, no matter how difficult or tender. I hope you had role models who not only said what to do but also demonstrated it with patients.
We had the privilege of hearing stories of difficult situations from hundreds of pediatricians in group sessions over the years in the Collaborative Office Rounds program. What group members often said that they valued most from these sessions was hearing examples of words they might say in these cases, either modeled by the coleaders or suggested by their pediatrician peers. Opportunities to share the tough times with trusted empathic peers is an important resource rarer and thus even more worth securing for yourself.
Being courageous may not be natural part of your personality but Aristotle said, “We become what we repeatedly do.” Even if you do not consider yourself so now, with practice you can become courageous and reap its benefits for your patients and yourself.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Starting a blog
. Health information is one of the most popular topics people search for online. Starting a physician blog can provide your practice with promotional and marketing benefits that you may have a difficult time finding elsewhere. A blog can be an effective way to drive traffic to your website, establish yourself as an authority or expert in a particular area, and stay on the radar with your patients. However, there are a few things you should think about before you start.
Start by determining what you want to accomplish. Do you want to reach quantitative milestones, like a certain number of followers, or are you looking to increase your website traffic from potential patients? One goal will probably be to augment the health knowledge of your patients. Decide early on what your benchmarks will be and how you will track them.
Next, determine who your potential readers are. Initially, most will probably be local (your existing patient base and their family and friends), but your audience may expand geographically as your blog gains in popularity.
By now, you probably realize that blogging will require a significant commitment, over and above the time needed to write the content. Decide whether you have the time and energy to take this on yourself, or whether help will be needed. Ideally, you should have one person in charge of all your social media efforts, so that everything is consistent and has the same voice. That person can be in-house, or you can outsource to any of the many companies that administer blogs and other media functions. (As always, I have no financial interest in any company or service mentioned in this column.)
The advantage of hiring an outside administrator is that a professionally designed blog will be far more attractive and polished than anything you could build yourself. Furthermore, an experienced designer will employ “search engine optimization” (SEO), meaning that content will be created using key words and phrases that will make it readily visible to search engine users.
You can leave design and SEO to the pros, but don’t delegate the content itself; as captain of the ship you are responsible for all the facts and opinions on your blog. You may not be up to writing everything yourself, but anything you don’t write personally needs to be scrutinized by you personally to make sure that it is factually accurate and reflects your personal view. And remember that, once it’s online, it’s online forever; consider the ramifications of anything you post on any site – yours or others – before hitting the “send” button. “The most damaging item about you,” one consultant told me, “could well be something you post yourself.” Just ask any of several prominent politicians who have famously sabotaged their own careers online.
That said, don’t be shy about creating content. Patients appreciate factual information, but they value your opinions too. Give people content that will be of interest or benefit to them. This can include health-related tips, reminders, suggestions, whatever. If they are interested in it, they will keep reading and may even share it with others. You should also write about subjects – medical and otherwise – that interest you personally. If you have expertise in a particular field, be sure to write about that.
Your practice is a local business, so localize your blog to attract people from your area. Be sure to include local city keywords in your writing. You may also want to post about local events in which your practice is involved.
Try to avoid political diatribes. While most physicians have strong political opinions, and some are not shy about expressing them, there are many venues that are more appropriate for those discussions than medical blogs. Also avoid outright sales pitches. It’s fine to describe procedures that you offer, but aggressive solicitation will only turn readers off.
Keep any medical advice in general terms; don’t use any specific examples that might make a patient identifiable and generate a HIPAA violation.
If you are having trouble growing your readership, use your practice’s Facebook page to push blog updates into patients’ feeds. Additionally, track Twitter hashtags that are relevant to your practice, and use them to find existing online communities with an interest in your blog’s topics.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
*This article was updated 10/17/2022.
. Health information is one of the most popular topics people search for online. Starting a physician blog can provide your practice with promotional and marketing benefits that you may have a difficult time finding elsewhere. A blog can be an effective way to drive traffic to your website, establish yourself as an authority or expert in a particular area, and stay on the radar with your patients. However, there are a few things you should think about before you start.
Start by determining what you want to accomplish. Do you want to reach quantitative milestones, like a certain number of followers, or are you looking to increase your website traffic from potential patients? One goal will probably be to augment the health knowledge of your patients. Decide early on what your benchmarks will be and how you will track them.
Next, determine who your potential readers are. Initially, most will probably be local (your existing patient base and their family and friends), but your audience may expand geographically as your blog gains in popularity.
By now, you probably realize that blogging will require a significant commitment, over and above the time needed to write the content. Decide whether you have the time and energy to take this on yourself, or whether help will be needed. Ideally, you should have one person in charge of all your social media efforts, so that everything is consistent and has the same voice. That person can be in-house, or you can outsource to any of the many companies that administer blogs and other media functions. (As always, I have no financial interest in any company or service mentioned in this column.)
The advantage of hiring an outside administrator is that a professionally designed blog will be far more attractive and polished than anything you could build yourself. Furthermore, an experienced designer will employ “search engine optimization” (SEO), meaning that content will be created using key words and phrases that will make it readily visible to search engine users.
You can leave design and SEO to the pros, but don’t delegate the content itself; as captain of the ship you are responsible for all the facts and opinions on your blog. You may not be up to writing everything yourself, but anything you don’t write personally needs to be scrutinized by you personally to make sure that it is factually accurate and reflects your personal view. And remember that, once it’s online, it’s online forever; consider the ramifications of anything you post on any site – yours or others – before hitting the “send” button. “The most damaging item about you,” one consultant told me, “could well be something you post yourself.” Just ask any of several prominent politicians who have famously sabotaged their own careers online.
That said, don’t be shy about creating content. Patients appreciate factual information, but they value your opinions too. Give people content that will be of interest or benefit to them. This can include health-related tips, reminders, suggestions, whatever. If they are interested in it, they will keep reading and may even share it with others. You should also write about subjects – medical and otherwise – that interest you personally. If you have expertise in a particular field, be sure to write about that.
Your practice is a local business, so localize your blog to attract people from your area. Be sure to include local city keywords in your writing. You may also want to post about local events in which your practice is involved.
Try to avoid political diatribes. While most physicians have strong political opinions, and some are not shy about expressing them, there are many venues that are more appropriate for those discussions than medical blogs. Also avoid outright sales pitches. It’s fine to describe procedures that you offer, but aggressive solicitation will only turn readers off.
Keep any medical advice in general terms; don’t use any specific examples that might make a patient identifiable and generate a HIPAA violation.
If you are having trouble growing your readership, use your practice’s Facebook page to push blog updates into patients’ feeds. Additionally, track Twitter hashtags that are relevant to your practice, and use them to find existing online communities with an interest in your blog’s topics.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
*This article was updated 10/17/2022.
. Health information is one of the most popular topics people search for online. Starting a physician blog can provide your practice with promotional and marketing benefits that you may have a difficult time finding elsewhere. A blog can be an effective way to drive traffic to your website, establish yourself as an authority or expert in a particular area, and stay on the radar with your patients. However, there are a few things you should think about before you start.
Start by determining what you want to accomplish. Do you want to reach quantitative milestones, like a certain number of followers, or are you looking to increase your website traffic from potential patients? One goal will probably be to augment the health knowledge of your patients. Decide early on what your benchmarks will be and how you will track them.
Next, determine who your potential readers are. Initially, most will probably be local (your existing patient base and their family and friends), but your audience may expand geographically as your blog gains in popularity.
By now, you probably realize that blogging will require a significant commitment, over and above the time needed to write the content. Decide whether you have the time and energy to take this on yourself, or whether help will be needed. Ideally, you should have one person in charge of all your social media efforts, so that everything is consistent and has the same voice. That person can be in-house, or you can outsource to any of the many companies that administer blogs and other media functions. (As always, I have no financial interest in any company or service mentioned in this column.)
The advantage of hiring an outside administrator is that a professionally designed blog will be far more attractive and polished than anything you could build yourself. Furthermore, an experienced designer will employ “search engine optimization” (SEO), meaning that content will be created using key words and phrases that will make it readily visible to search engine users.
You can leave design and SEO to the pros, but don’t delegate the content itself; as captain of the ship you are responsible for all the facts and opinions on your blog. You may not be up to writing everything yourself, but anything you don’t write personally needs to be scrutinized by you personally to make sure that it is factually accurate and reflects your personal view. And remember that, once it’s online, it’s online forever; consider the ramifications of anything you post on any site – yours or others – before hitting the “send” button. “The most damaging item about you,” one consultant told me, “could well be something you post yourself.” Just ask any of several prominent politicians who have famously sabotaged their own careers online.
That said, don’t be shy about creating content. Patients appreciate factual information, but they value your opinions too. Give people content that will be of interest or benefit to them. This can include health-related tips, reminders, suggestions, whatever. If they are interested in it, they will keep reading and may even share it with others. You should also write about subjects – medical and otherwise – that interest you personally. If you have expertise in a particular field, be sure to write about that.
Your practice is a local business, so localize your blog to attract people from your area. Be sure to include local city keywords in your writing. You may also want to post about local events in which your practice is involved.
Try to avoid political diatribes. While most physicians have strong political opinions, and some are not shy about expressing them, there are many venues that are more appropriate for those discussions than medical blogs. Also avoid outright sales pitches. It’s fine to describe procedures that you offer, but aggressive solicitation will only turn readers off.
Keep any medical advice in general terms; don’t use any specific examples that might make a patient identifiable and generate a HIPAA violation.
If you are having trouble growing your readership, use your practice’s Facebook page to push blog updates into patients’ feeds. Additionally, track Twitter hashtags that are relevant to your practice, and use them to find existing online communities with an interest in your blog’s topics.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
*This article was updated 10/17/2022.
Tourette syndrome: Diagnosis is key for best care
Tourette syndrome, attention-deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and autism spectrum disorder (ASD) share significant overlap in symptomatology, and it can be challenging at times to distinguish between these conditions. Being able to do so, however, can help guide more targeted interventions and accommodations to optimize a patient’s level of functioning.
Case example
A healthy, bright 6-year-old boy is referred by his family doctor to an academic medical center for a full team evaluation because of suspicion of ASD, after having already been diagnosed with ADHD at the age of 5. His difficulties with inattention, impulsivity, and hyperactivity, as well as his behavioral rigidities and sensory avoidant and sensory seeking behaviors have caused functional impairments for him in his kindergarten classroom. He has been penalized with removal of recess on more than one occasion. A low dose of a stimulant had been tried but resulted in a perceived increase in disruptive behaviors.
The boy, while hyperkinetic and often paying poor attention, is quite capable of high-quality and well-modulated eye contact paired with typical social referencing and reciprocity when actively engaging with the examiner and his parents. He does have a reported history of serial fixated interests and some repetitive behaviors but is also noted to be flexible in his interpersonal style, maintains other varied and typical interests, easily directs affect, utilizes a wide array of fluid gestures paired naturally with verbal communication, and shares enjoyment with smoothly coordinated gaze. He has mild articulation errors but uses pronouns appropriately and has no scripted speech or echolalia, though does engage in some whispered palilalia intermittently.
He is generally quite cooperative and redirectable when focused and has a completely normal physical and neurologic examination. During the visit, the doctor notices the boy making an intermittent honking sound, which parents report as an attention-seeking strategy during times of stress. Further physician-guided information gathering around other repetitive noises and movements elicits a history of engagement in repetitive hand-to-groin movements, some exaggerated blinking, and a number of other waxing and waning subtle motor and phonic tics with onset in preschool. These noises and movements have generally been identified as “fidgeting” and “misbehaving” by well-meaning caregivers in the home and school environments.
Both Tourette syndrome and ASD are more common in males, with stereotyped patterns of movements and behaviors; anxious, obsessive, and compulsive behaviors resulting in behavioral rigidities; sensory sensitivities; and increased rates of hyperkinesis with decreased impulse control which result in increased sensory-seeking behaviors. Diagnostic criteria for Tourette syndrome are met when a child has had multiple motor tics and at least one phonic tic present for at least 1 year, with tic-free intervals lasting no longer than 3 months, and with onset before the age of 18. Typically, tics emerge in late preschool and early grade school, and some children even develop repetitive movements as early as toddlerhood. Tics tend to worsen around the peripubertal era, then often generally improve in the teen years. Tic types, frequency, and severity general fluctuate over time.
Forty percent of children with Tourette syndrome also meet criteria for OCD, with many more having OCD traits, and about 65% of children with Tourette syndrome also meet criteria for ADHD, with many more having ADHD traits. OCD can lead to more rigid and directive social interactions in children as well as obsessive interests, just as ADHD can lead to less socially attuned and less cooperative behaviors, even in children who do not meet criteria for ASD.
For example, a child with OCD in the absence of ASD may still “police” other kids in class and be overly focused on the rules of a game, which may become a social liability. Likewise, a child with ADHD in the absence of ASD may be so distractible that focusing on what other kids are saying and their paired facial expressions is compromised, leading to poor-quality social reciprocity during interactions with peers. Given the remarkable overlap in shared symptoms, it is essential for pediatric providers to consider Tourette syndrome in the differential for any child with repetitive movements and behaviors in addition to ASD and a wide array of other neurodevelopment differences, including global developmental delays and intellectual disabilities. This is of particular importance as the diagnosis of Tourette syndrome can be used to gain access to developmental disability services if the condition has resulted in true adaptive impairments.
It is determined that the boy does in fact meet criteria for ADHD, but also for OCD and Tourette syndrome. Both his Autism Diagnostic Observation Schedule and DSM-5–influenced autism interview are found to be in the nonclinical ranges, given his quality of communication, social engagement, imaginative play, and varied interests. A diagnosis of ASD is not felt to be an appropriate conceptualization of his neurodevelopmental differences. He is started on a low dose of guanfacine, which induces a decline in tics, impulsivity, and hyperkinesis. He is given a 504 plan in school that includes scheduled “tic breaks,” sensory fidgets for use in the classroom, extra movement opportunities as needed, and utilization of a gentle cueing system between him and his teacher for low-key redirection of disruptive behaviors. He is no longer penalized for inattention or tics, and his 504 plan protects him from the use of recess removal as a behavioral modification strategy.
His parents enroll him in the community swim program for extra exercise, focus on decreasing screen time, and give him an earlier bedtime to help decrease his tics and rigidities, while improving his ability to self-regulate. Eventually, a low dose of a newer-generation stimulant is added to his guanfacine, with excellent results and only a mild increase in tolerable tics.
The child in the vignette did well with a 504 plan based on his medical diagnoses, though if related learning difficulties had persisted, eligibility under Other Health Impaired could be used to provide eligibility for an Individualized Education Plan. Alpha-agonists can be helpful for symptom control in those with Tourette syndrome by simultaneously treating tics, hyperkinesis, and impulsivity, while decreasing the risk of tic exacerbation with use of stimulants. Overall, understanding the neurodiversity related to Tourette syndrome can help providers advocate for home and community-based supports to optimize general functioning and quality of life.
Dr. Roth is a developmental and behavioral pediatrician in Eugene, Ore. She has no conflicts of interest.
References
Darrow S et al. J Am Acad Child Adolescent Psych. 2017;56(7):610-7.
AAP Section on Developmental and Behavioral Pediatrics. Developmental and Behavioral Pediatrics. Voigt RG et al, eds. 2018: American Academy of Pediatrics.
Tourette syndrome, attention-deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and autism spectrum disorder (ASD) share significant overlap in symptomatology, and it can be challenging at times to distinguish between these conditions. Being able to do so, however, can help guide more targeted interventions and accommodations to optimize a patient’s level of functioning.
Case example
A healthy, bright 6-year-old boy is referred by his family doctor to an academic medical center for a full team evaluation because of suspicion of ASD, after having already been diagnosed with ADHD at the age of 5. His difficulties with inattention, impulsivity, and hyperactivity, as well as his behavioral rigidities and sensory avoidant and sensory seeking behaviors have caused functional impairments for him in his kindergarten classroom. He has been penalized with removal of recess on more than one occasion. A low dose of a stimulant had been tried but resulted in a perceived increase in disruptive behaviors.
The boy, while hyperkinetic and often paying poor attention, is quite capable of high-quality and well-modulated eye contact paired with typical social referencing and reciprocity when actively engaging with the examiner and his parents. He does have a reported history of serial fixated interests and some repetitive behaviors but is also noted to be flexible in his interpersonal style, maintains other varied and typical interests, easily directs affect, utilizes a wide array of fluid gestures paired naturally with verbal communication, and shares enjoyment with smoothly coordinated gaze. He has mild articulation errors but uses pronouns appropriately and has no scripted speech or echolalia, though does engage in some whispered palilalia intermittently.
He is generally quite cooperative and redirectable when focused and has a completely normal physical and neurologic examination. During the visit, the doctor notices the boy making an intermittent honking sound, which parents report as an attention-seeking strategy during times of stress. Further physician-guided information gathering around other repetitive noises and movements elicits a history of engagement in repetitive hand-to-groin movements, some exaggerated blinking, and a number of other waxing and waning subtle motor and phonic tics with onset in preschool. These noises and movements have generally been identified as “fidgeting” and “misbehaving” by well-meaning caregivers in the home and school environments.
Both Tourette syndrome and ASD are more common in males, with stereotyped patterns of movements and behaviors; anxious, obsessive, and compulsive behaviors resulting in behavioral rigidities; sensory sensitivities; and increased rates of hyperkinesis with decreased impulse control which result in increased sensory-seeking behaviors. Diagnostic criteria for Tourette syndrome are met when a child has had multiple motor tics and at least one phonic tic present for at least 1 year, with tic-free intervals lasting no longer than 3 months, and with onset before the age of 18. Typically, tics emerge in late preschool and early grade school, and some children even develop repetitive movements as early as toddlerhood. Tics tend to worsen around the peripubertal era, then often generally improve in the teen years. Tic types, frequency, and severity general fluctuate over time.
Forty percent of children with Tourette syndrome also meet criteria for OCD, with many more having OCD traits, and about 65% of children with Tourette syndrome also meet criteria for ADHD, with many more having ADHD traits. OCD can lead to more rigid and directive social interactions in children as well as obsessive interests, just as ADHD can lead to less socially attuned and less cooperative behaviors, even in children who do not meet criteria for ASD.
For example, a child with OCD in the absence of ASD may still “police” other kids in class and be overly focused on the rules of a game, which may become a social liability. Likewise, a child with ADHD in the absence of ASD may be so distractible that focusing on what other kids are saying and their paired facial expressions is compromised, leading to poor-quality social reciprocity during interactions with peers. Given the remarkable overlap in shared symptoms, it is essential for pediatric providers to consider Tourette syndrome in the differential for any child with repetitive movements and behaviors in addition to ASD and a wide array of other neurodevelopment differences, including global developmental delays and intellectual disabilities. This is of particular importance as the diagnosis of Tourette syndrome can be used to gain access to developmental disability services if the condition has resulted in true adaptive impairments.
It is determined that the boy does in fact meet criteria for ADHD, but also for OCD and Tourette syndrome. Both his Autism Diagnostic Observation Schedule and DSM-5–influenced autism interview are found to be in the nonclinical ranges, given his quality of communication, social engagement, imaginative play, and varied interests. A diagnosis of ASD is not felt to be an appropriate conceptualization of his neurodevelopmental differences. He is started on a low dose of guanfacine, which induces a decline in tics, impulsivity, and hyperkinesis. He is given a 504 plan in school that includes scheduled “tic breaks,” sensory fidgets for use in the classroom, extra movement opportunities as needed, and utilization of a gentle cueing system between him and his teacher for low-key redirection of disruptive behaviors. He is no longer penalized for inattention or tics, and his 504 plan protects him from the use of recess removal as a behavioral modification strategy.
His parents enroll him in the community swim program for extra exercise, focus on decreasing screen time, and give him an earlier bedtime to help decrease his tics and rigidities, while improving his ability to self-regulate. Eventually, a low dose of a newer-generation stimulant is added to his guanfacine, with excellent results and only a mild increase in tolerable tics.
The child in the vignette did well with a 504 plan based on his medical diagnoses, though if related learning difficulties had persisted, eligibility under Other Health Impaired could be used to provide eligibility for an Individualized Education Plan. Alpha-agonists can be helpful for symptom control in those with Tourette syndrome by simultaneously treating tics, hyperkinesis, and impulsivity, while decreasing the risk of tic exacerbation with use of stimulants. Overall, understanding the neurodiversity related to Tourette syndrome can help providers advocate for home and community-based supports to optimize general functioning and quality of life.
Dr. Roth is a developmental and behavioral pediatrician in Eugene, Ore. She has no conflicts of interest.
References
Darrow S et al. J Am Acad Child Adolescent Psych. 2017;56(7):610-7.
AAP Section on Developmental and Behavioral Pediatrics. Developmental and Behavioral Pediatrics. Voigt RG et al, eds. 2018: American Academy of Pediatrics.
Tourette syndrome, attention-deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and autism spectrum disorder (ASD) share significant overlap in symptomatology, and it can be challenging at times to distinguish between these conditions. Being able to do so, however, can help guide more targeted interventions and accommodations to optimize a patient’s level of functioning.
Case example
A healthy, bright 6-year-old boy is referred by his family doctor to an academic medical center for a full team evaluation because of suspicion of ASD, after having already been diagnosed with ADHD at the age of 5. His difficulties with inattention, impulsivity, and hyperactivity, as well as his behavioral rigidities and sensory avoidant and sensory seeking behaviors have caused functional impairments for him in his kindergarten classroom. He has been penalized with removal of recess on more than one occasion. A low dose of a stimulant had been tried but resulted in a perceived increase in disruptive behaviors.
The boy, while hyperkinetic and often paying poor attention, is quite capable of high-quality and well-modulated eye contact paired with typical social referencing and reciprocity when actively engaging with the examiner and his parents. He does have a reported history of serial fixated interests and some repetitive behaviors but is also noted to be flexible in his interpersonal style, maintains other varied and typical interests, easily directs affect, utilizes a wide array of fluid gestures paired naturally with verbal communication, and shares enjoyment with smoothly coordinated gaze. He has mild articulation errors but uses pronouns appropriately and has no scripted speech or echolalia, though does engage in some whispered palilalia intermittently.
He is generally quite cooperative and redirectable when focused and has a completely normal physical and neurologic examination. During the visit, the doctor notices the boy making an intermittent honking sound, which parents report as an attention-seeking strategy during times of stress. Further physician-guided information gathering around other repetitive noises and movements elicits a history of engagement in repetitive hand-to-groin movements, some exaggerated blinking, and a number of other waxing and waning subtle motor and phonic tics with onset in preschool. These noises and movements have generally been identified as “fidgeting” and “misbehaving” by well-meaning caregivers in the home and school environments.
Both Tourette syndrome and ASD are more common in males, with stereotyped patterns of movements and behaviors; anxious, obsessive, and compulsive behaviors resulting in behavioral rigidities; sensory sensitivities; and increased rates of hyperkinesis with decreased impulse control which result in increased sensory-seeking behaviors. Diagnostic criteria for Tourette syndrome are met when a child has had multiple motor tics and at least one phonic tic present for at least 1 year, with tic-free intervals lasting no longer than 3 months, and with onset before the age of 18. Typically, tics emerge in late preschool and early grade school, and some children even develop repetitive movements as early as toddlerhood. Tics tend to worsen around the peripubertal era, then often generally improve in the teen years. Tic types, frequency, and severity general fluctuate over time.
Forty percent of children with Tourette syndrome also meet criteria for OCD, with many more having OCD traits, and about 65% of children with Tourette syndrome also meet criteria for ADHD, with many more having ADHD traits. OCD can lead to more rigid and directive social interactions in children as well as obsessive interests, just as ADHD can lead to less socially attuned and less cooperative behaviors, even in children who do not meet criteria for ASD.
For example, a child with OCD in the absence of ASD may still “police” other kids in class and be overly focused on the rules of a game, which may become a social liability. Likewise, a child with ADHD in the absence of ASD may be so distractible that focusing on what other kids are saying and their paired facial expressions is compromised, leading to poor-quality social reciprocity during interactions with peers. Given the remarkable overlap in shared symptoms, it is essential for pediatric providers to consider Tourette syndrome in the differential for any child with repetitive movements and behaviors in addition to ASD and a wide array of other neurodevelopment differences, including global developmental delays and intellectual disabilities. This is of particular importance as the diagnosis of Tourette syndrome can be used to gain access to developmental disability services if the condition has resulted in true adaptive impairments.
It is determined that the boy does in fact meet criteria for ADHD, but also for OCD and Tourette syndrome. Both his Autism Diagnostic Observation Schedule and DSM-5–influenced autism interview are found to be in the nonclinical ranges, given his quality of communication, social engagement, imaginative play, and varied interests. A diagnosis of ASD is not felt to be an appropriate conceptualization of his neurodevelopmental differences. He is started on a low dose of guanfacine, which induces a decline in tics, impulsivity, and hyperkinesis. He is given a 504 plan in school that includes scheduled “tic breaks,” sensory fidgets for use in the classroom, extra movement opportunities as needed, and utilization of a gentle cueing system between him and his teacher for low-key redirection of disruptive behaviors. He is no longer penalized for inattention or tics, and his 504 plan protects him from the use of recess removal as a behavioral modification strategy.
His parents enroll him in the community swim program for extra exercise, focus on decreasing screen time, and give him an earlier bedtime to help decrease his tics and rigidities, while improving his ability to self-regulate. Eventually, a low dose of a newer-generation stimulant is added to his guanfacine, with excellent results and only a mild increase in tolerable tics.
The child in the vignette did well with a 504 plan based on his medical diagnoses, though if related learning difficulties had persisted, eligibility under Other Health Impaired could be used to provide eligibility for an Individualized Education Plan. Alpha-agonists can be helpful for symptom control in those with Tourette syndrome by simultaneously treating tics, hyperkinesis, and impulsivity, while decreasing the risk of tic exacerbation with use of stimulants. Overall, understanding the neurodiversity related to Tourette syndrome can help providers advocate for home and community-based supports to optimize general functioning and quality of life.
Dr. Roth is a developmental and behavioral pediatrician in Eugene, Ore. She has no conflicts of interest.
References
Darrow S et al. J Am Acad Child Adolescent Psych. 2017;56(7):610-7.
AAP Section on Developmental and Behavioral Pediatrics. Developmental and Behavioral Pediatrics. Voigt RG et al, eds. 2018: American Academy of Pediatrics.
The WPATH guidelines for treatment of adolescents with gender dysphoria have changed
The World Professional Association for Transgender Health (WPATH) is an interdisciplinary professional and educational organization devoted to transgender health. One of their activities is to produce the Standards of Care (SOC) for treatment of individuals with gender dysphoria. According to WPATH, the SOC “articulate a professional consensus about the psychiatric, psychological, medical, and surgical management of gender dysphoria and help professionals understand the parameters within which they may offer assistance to those with these conditions.” Many clinicians around the world use these guidelines to help them care for patients with gender dysphoria and diverse gender expressions.
The most recent SOC, version 8, were released on Sept. 15, 2022, after a 2-year postponement because of the pandemic. These new standards represent the first update to the SOC since version 7, which was released in 2012. Given how recent this update is, this column will attempt to summarize the changes in the new guidelines that affect children and adolescents.
One of the major differences between SOC versions 7 and 8 is that version 8 now includes a chapter specifically dedicated to the care of adolescents. Version 7 lumped children and adolescents together into one chapter. This is an important distinction for SOC 8, as it highlights that care for prepubertal youth is simply social in nature and distinct from that of pubertal adolescents. Social transition includes things such as using an affirmed name/pronouns and changing hair style and clothes. It does not include medications of any kind. Allowing these youth the time and space to explore the natural gender diversity of childhood leads to improved psychological outcomes over time and reduces adversity. Psychological support, where indicated, should be offered to gender-diverse children and their families to explore the persistence, consistence, and insistence of that child’s gender identity.
Once a child reaches puberty, medications may come into play as part of an adolescent’s transition. SOC 7 had established a minimum age of 16 before any partially reversible medications (testosterone, estrogen) were started as part of a patient’s medical transition. Starting with SOC 8, a minimum age has been removed for the initiation of gender-affirming hormone therapy. However, a patient must still have begun their natal puberty before any medication is started. A specific age was removed to acknowledge that maturity in adolescents occurs on a continuum and at different ages. SOC 8 guidelines continue to recommend that the individual’s emotional, cognitive, and psychosocial development be taken into account when determining their ability to provide consent for treatment. These individuals should still undergo a comprehensive assessment, as described below.
Similar to SOC 7, SOC 8 continues to stress the importance of a comprehensive, multidisciplinary evaluation of those adolescents who seek medical therapy as part of their transition. This allows for the exploration of additional coexisting causes of gender dysphoria, such as anxiety, depression, or other mental health conditions. If these exist, then they must be appropriately treated before any gender-affirming medical treatment is initiated. Assessments should be performed by clinicians who have training and expertise with the developmental trajectory of adolescents, as well as with common mental health conditions. These assessments are also critical, as SOC 8 acknowledges a rise in the number of adolescents who may not have had gender-diverse expression in childhood.
SOC 8 and the Endocrine Society Guidelines (see references) provide physicians and other health care professionals with a road map for addressing the needs of transgender and gender-diverse persons. By referencing these guidelines when taking care of these patients, physicians and other health care professionals will know that they are providing the most up-to-date, evidence-based care.
Dr. M. Brett Cooper is an assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
SOC 8: https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
SOC 7: https://www.wpath.org/media/cms/Documents/SOC%20v7/SOC%20V7_English2012.pdf?_t=1613669341
Endocrine Society Gender Affirming Care Guidelines: https://academic.oup.com/jcem/article/102/11/3869/4157558?login=false
The World Professional Association for Transgender Health (WPATH) is an interdisciplinary professional and educational organization devoted to transgender health. One of their activities is to produce the Standards of Care (SOC) for treatment of individuals with gender dysphoria. According to WPATH, the SOC “articulate a professional consensus about the psychiatric, psychological, medical, and surgical management of gender dysphoria and help professionals understand the parameters within which they may offer assistance to those with these conditions.” Many clinicians around the world use these guidelines to help them care for patients with gender dysphoria and diverse gender expressions.
The most recent SOC, version 8, were released on Sept. 15, 2022, after a 2-year postponement because of the pandemic. These new standards represent the first update to the SOC since version 7, which was released in 2012. Given how recent this update is, this column will attempt to summarize the changes in the new guidelines that affect children and adolescents.
One of the major differences between SOC versions 7 and 8 is that version 8 now includes a chapter specifically dedicated to the care of adolescents. Version 7 lumped children and adolescents together into one chapter. This is an important distinction for SOC 8, as it highlights that care for prepubertal youth is simply social in nature and distinct from that of pubertal adolescents. Social transition includes things such as using an affirmed name/pronouns and changing hair style and clothes. It does not include medications of any kind. Allowing these youth the time and space to explore the natural gender diversity of childhood leads to improved psychological outcomes over time and reduces adversity. Psychological support, where indicated, should be offered to gender-diverse children and their families to explore the persistence, consistence, and insistence of that child’s gender identity.
Once a child reaches puberty, medications may come into play as part of an adolescent’s transition. SOC 7 had established a minimum age of 16 before any partially reversible medications (testosterone, estrogen) were started as part of a patient’s medical transition. Starting with SOC 8, a minimum age has been removed for the initiation of gender-affirming hormone therapy. However, a patient must still have begun their natal puberty before any medication is started. A specific age was removed to acknowledge that maturity in adolescents occurs on a continuum and at different ages. SOC 8 guidelines continue to recommend that the individual’s emotional, cognitive, and psychosocial development be taken into account when determining their ability to provide consent for treatment. These individuals should still undergo a comprehensive assessment, as described below.
Similar to SOC 7, SOC 8 continues to stress the importance of a comprehensive, multidisciplinary evaluation of those adolescents who seek medical therapy as part of their transition. This allows for the exploration of additional coexisting causes of gender dysphoria, such as anxiety, depression, or other mental health conditions. If these exist, then they must be appropriately treated before any gender-affirming medical treatment is initiated. Assessments should be performed by clinicians who have training and expertise with the developmental trajectory of adolescents, as well as with common mental health conditions. These assessments are also critical, as SOC 8 acknowledges a rise in the number of adolescents who may not have had gender-diverse expression in childhood.
SOC 8 and the Endocrine Society Guidelines (see references) provide physicians and other health care professionals with a road map for addressing the needs of transgender and gender-diverse persons. By referencing these guidelines when taking care of these patients, physicians and other health care professionals will know that they are providing the most up-to-date, evidence-based care.
Dr. M. Brett Cooper is an assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
SOC 8: https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
SOC 7: https://www.wpath.org/media/cms/Documents/SOC%20v7/SOC%20V7_English2012.pdf?_t=1613669341
Endocrine Society Gender Affirming Care Guidelines: https://academic.oup.com/jcem/article/102/11/3869/4157558?login=false
The World Professional Association for Transgender Health (WPATH) is an interdisciplinary professional and educational organization devoted to transgender health. One of their activities is to produce the Standards of Care (SOC) for treatment of individuals with gender dysphoria. According to WPATH, the SOC “articulate a professional consensus about the psychiatric, psychological, medical, and surgical management of gender dysphoria and help professionals understand the parameters within which they may offer assistance to those with these conditions.” Many clinicians around the world use these guidelines to help them care for patients with gender dysphoria and diverse gender expressions.
The most recent SOC, version 8, were released on Sept. 15, 2022, after a 2-year postponement because of the pandemic. These new standards represent the first update to the SOC since version 7, which was released in 2012. Given how recent this update is, this column will attempt to summarize the changes in the new guidelines that affect children and adolescents.
One of the major differences between SOC versions 7 and 8 is that version 8 now includes a chapter specifically dedicated to the care of adolescents. Version 7 lumped children and adolescents together into one chapter. This is an important distinction for SOC 8, as it highlights that care for prepubertal youth is simply social in nature and distinct from that of pubertal adolescents. Social transition includes things such as using an affirmed name/pronouns and changing hair style and clothes. It does not include medications of any kind. Allowing these youth the time and space to explore the natural gender diversity of childhood leads to improved psychological outcomes over time and reduces adversity. Psychological support, where indicated, should be offered to gender-diverse children and their families to explore the persistence, consistence, and insistence of that child’s gender identity.
Once a child reaches puberty, medications may come into play as part of an adolescent’s transition. SOC 7 had established a minimum age of 16 before any partially reversible medications (testosterone, estrogen) were started as part of a patient’s medical transition. Starting with SOC 8, a minimum age has been removed for the initiation of gender-affirming hormone therapy. However, a patient must still have begun their natal puberty before any medication is started. A specific age was removed to acknowledge that maturity in adolescents occurs on a continuum and at different ages. SOC 8 guidelines continue to recommend that the individual’s emotional, cognitive, and psychosocial development be taken into account when determining their ability to provide consent for treatment. These individuals should still undergo a comprehensive assessment, as described below.
Similar to SOC 7, SOC 8 continues to stress the importance of a comprehensive, multidisciplinary evaluation of those adolescents who seek medical therapy as part of their transition. This allows for the exploration of additional coexisting causes of gender dysphoria, such as anxiety, depression, or other mental health conditions. If these exist, then they must be appropriately treated before any gender-affirming medical treatment is initiated. Assessments should be performed by clinicians who have training and expertise with the developmental trajectory of adolescents, as well as with common mental health conditions. These assessments are also critical, as SOC 8 acknowledges a rise in the number of adolescents who may not have had gender-diverse expression in childhood.
SOC 8 and the Endocrine Society Guidelines (see references) provide physicians and other health care professionals with a road map for addressing the needs of transgender and gender-diverse persons. By referencing these guidelines when taking care of these patients, physicians and other health care professionals will know that they are providing the most up-to-date, evidence-based care.
Dr. M. Brett Cooper is an assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
SOC 8: https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
SOC 7: https://www.wpath.org/media/cms/Documents/SOC%20v7/SOC%20V7_English2012.pdf?_t=1613669341
Endocrine Society Gender Affirming Care Guidelines: https://academic.oup.com/jcem/article/102/11/3869/4157558?login=false
Health care providers should have higher suspicion for rare diseases
The number of cataloged rare diseases continues to grow every day. According to the National Human Genome Research Institute, more than 6,800 rare diseases have been identified and between 25 million and 30 million Americans are living with rare diseases today.
Rare diseases have collectively emerged as a unique field of medicine with an “entirely new generation of conditions,” said Marshall L. Summar, MD, chief of the division of genetics and metabolism at Children’s National Hospital in Washington, DC. He places the number of rare diseases closer to 8,000, and said it is “growing by a rate of 10 to 12 a week.”
Although the field has made significant advancements in health care providers’ ability to diagnose rare diseases, it has also highlighted what isn’t known as well, said Dr. Summar, who is also past president and a former scientific advisory board member with the National Organization for Rare Disorders (NORD).
Keeping up to date on the latest rare diseases may seem like a daunting task to the average health care professional. However, while rare diseases remain the domain of the subspecialists, the generalist “can make a tremendous impact for their patients” early in the process by having a higher suspicion for rare diseases in their practice, said Dr. Summar.
Thinking of rare diseases in categories
Many patients with undiagnosed rare diseases undergo what’s commonly referred to as a “diagnostic odyssey,” moving from one provider to another to try to find an explanation for a condition they may or may not know is rare. For some patients, this process can take many years or even decades. From the patient’s perspective, the main challenges are recognizing they have a problem that doesn’t fit a common disease model. Once they recognize they have a potential rare disease, working with a provider to find the right diagnosis among the “vast number of disease diagnoses and designations, and actually sifting through it to find the one that’s right for that patient” is the next challenge, said Dr. Summar.
However, knowledge of rare diseases among health care professionals is low, according to a 2019 paper published in the Orphanet Journal of Rare Diseases. In a survey from that paper asking general practitioners, pediatricians, specialists caring for adults, and specialists caring for children to evaluate their own knowledge of rare diseases, 42% of general practitioners said they had poor knowledge and 44% said they had a substandard understanding of rare diseases.
From a clinician’s standpoint, diagnosing rare diseases in their patients can be challenging, with the potential for overreferral or overdiagnosis. However, it is also easy to underdiagnose rare diseases by missing them, noted Dr. Summar. This issue can vary based on the experience of the provider, he said, because while general practitioners who recently began practicing may have had more exposure to rare diseases, for health care professionals who have been practicing for decades, “this is a new arrival in their field.”
During a busy day finding that extra time in an appointment to stop and question whether a patient might have a rare disease is another problem generalists face. “It is really tough for those general practitioners, because if you see 40 or 50 patients per day, how do you know which one of those [patients] were the ones that had something that wasn’t quite fitting or wasn’t quite ordinary?” he said.
When it comes to considering rare diseases in their patients, health care professionals in general practice should think in categories, rather than a particular rare disease, according to Dr. Summar. As the generalist is typically on the front lines of patient care, they don’t necessarily need to know everything about the rare disease they suspect a patient of having to help them. “You don’t need to know the specific gene and the specific mutation to make the diagnosis, or to even move the patient forward in the process,” he said.
The first steps a clinician can take include noticing when something with a patient is amiss, thinking about the disease category, and then creating a plan to move forward, such as referring the patient to a subspecialist. Learning to recognize when a cluster of symptoms doesn’t fit a pattern is important, as patients and their providers tend to gravitate toward diagnoses they are used to seeing, rather than suspecting a disease outside a usual pattern.
The framing of rare diseases as categories is a change in thinking over the last decade, said Dr. Summar. Whereas rare disease diagnoses previously focused on fitting certain criteria, the development of more refined genetic sequencing has allowed specialists to focus on categories and genes that affect rare diseases. “Getting at a diagnosis has really been turned up on its head, so that by employing both next-generation sequencing, newborn screening, and other [tools], we can actually get to diagnoses faster than we could before,” he said.
In terms of assessing for symptoms, health care professionals should be aware that “common” symptoms can be a sign of rare disease. What to look out for are the uncommon symptoms that create an “aha moment.” Having a “good filter” for sensing when something isn’t quite right with a patient is key. “It’s like any time when you’re screening things: You want high sensitivity, but you also have to have high specificity,” he said.
Another clinical pearl is that good communication between patient and provider is paramount. “We’re not always good listeners. Sometimes we hear what we expect to hear,” said Dr. Summar.
Rare disease warning signs
Within the context of rare neurological diseases, Dr. Summar noted one major category is delays in neurological development, which is typically identified in children or adolescents. As the most complex organ in the body, “the brain probably expresses more genes than any other tissue on a regular basis, both in its formation and its function,” said Dr. Summar. He said the single disease that rare disease specialists see most often is Down syndrome.
Another separate but overlapping major category is autism, identified in younger children through trouble with social interaction, lack of eye contact, and delays in speech and communication skills. A third major category is physical manifestations of neurological problems, such as in patients who have epilepsy.
A telltale sign in identifying a child with a potential rare neurological disease is when they are “not thriving in their development or not doing the things on track that you would expect, and you don’t have a really good answer for it,” said Dr. Summar. Generalists are normally on watch for developmental delays in newborns born premature or with a rough course in the nursery, but they should also be aware of delays in children born under otherwise typical circumstances. “If I have a patient who had normal pregnancy, normal labor and delivery, no real illnesses or anything like that, and yet wasn’t meeting those milestones, that’s a patient I would pay attention to,” he said.
Another clue general practitioners can use for suspecting rare diseases is when a patient is much sicker than usual during a routine illness like a cold or flu. “Those are patients we should be paying attention to because it may be there’s an underlying biochemical disorder or some disorder in how they’re responding to stress that’s just not quite right,” said Dr. Summar. How a patient responds to stressful situations can be a warning sign “because that can often unmask more severe symptoms in that rare disease and make it a little more apparent,” he said.
Learning more about rare diseases
Dr. Summar said he and his colleagues in the rare disease field have spent a lot of time working with medical schools to teach this mindset in their curricula. The concept is introduced in basic medical science courses and then reinforced in clinical rotations in the third or fourth year, he explained.
“One of the best places is during the pediatrics rotations in medical school,” he said. “Remember, kids are basically healthy. If a child has a chronic illness or a chronic disease, more often than not, it is probably a rare disease.”
For medical professionals not in pediatric practice, the concept is applied the same way for adult medicine. “You just want to make sure everyone takes a second when they have a patient and try not to assume. Don’t assume it’s exactly what it seems. Look at it carefully and make sure there’s not something else going on,” he said.
Health care professionals in general practice looking to learn more about rare diseases can increasingly find rare disease topics in their CME programs. Rare disease topics in CME programs are “one of the best places” to learn about the latest developments in the field, said Dr. Summar.
Will rare disease screening tools come to primary care?
Asking more doctors to refer out to rare disease specialists raises an issue: There simply aren’t enough rare disease specialists in the field to go around.
Dr. Summar said partnering testing – where a general practitioner contacts a specialist to begin the process of testing based on the suspected condition – is a good stopgap solution. Telemedicine, which rose in popularity during the COVID-19 pandemic, can also play an important role in connecting patients and their providers with rare disease specialists, especially for generalists in remote communities. Dr. Summar noted he continues to see approximately 30% of his patients this way today. Telemedicine appointments can take place in the patient’s home or at the provider’s office.
“It actually provides access to folks who otherwise might not be able to either take off from work for a day – particularly some of our single parent households – or have a child who just doesn’t travel well, or can’t really get there, even if it’s the patient themselves,” he explained. “We can see patients that historically would have had trouble or difficulty coming in, so for me, that’s been a good thing.”
Telemedicine also helps give access to care for more medically fragile patients, many of whom have rare diseases, he added. While some aspects of care need to occur in person, “it’s a good 80% or 90% solution for a lot of these things,” he said.
Sharing educational videos is another way for health care providers in general practice to inform patients and their families about rare diseases. Children’s National Medical Center has created a collection of these videos in a free app called GeneClips, which is available on major smartphone app stores. However, Dr. Summar emphasized that genetic counseling should still be performed by a rare disease specialist prior to testing.
“We’re still at the point where I think having genetic counseling for a family before they’re going into testing is really advisable, since a lot of the results have a probability assigned to them,” he said. “I don’t think we’re really at the level where a practitioner is going to, first of all, have the time to do those, and I don’t think there’s enough general public awareness of what these things mean.”
Although primary care providers may one day be able to perform more generalized sequencing in their own practice, that time has not yet come – but it is closer than you think. “The technology is there, and actually the cost has come down a lot,” said Dr. Summar.
One potential issue this would create is an additional discussion to manage expectations of test results with family when the results are unclear, which “actually takes more time than counseling about a yes or no, or even an outcome that is unexpected,” explained Dr. Summar.
“[W]e’re in a midlife period right now where we’re bringing forward this new technology, but we’ve got to continually prepare the field for it first,” he said. “I think in the future we’ll see that it has much greater utility in the general setting,” he said.
Jeff Craven is a freelance journalist specializing in medicine and health.
Suggested reading
Vandeborne L et al. Information needs of physicians regarding the diagnosis of rare diseases: A questionnaire-based study in Belgium. Orphanet J Rare Dis. 2019;14(1):99.
The number of cataloged rare diseases continues to grow every day. According to the National Human Genome Research Institute, more than 6,800 rare diseases have been identified and between 25 million and 30 million Americans are living with rare diseases today.
Rare diseases have collectively emerged as a unique field of medicine with an “entirely new generation of conditions,” said Marshall L. Summar, MD, chief of the division of genetics and metabolism at Children’s National Hospital in Washington, DC. He places the number of rare diseases closer to 8,000, and said it is “growing by a rate of 10 to 12 a week.”
Although the field has made significant advancements in health care providers’ ability to diagnose rare diseases, it has also highlighted what isn’t known as well, said Dr. Summar, who is also past president and a former scientific advisory board member with the National Organization for Rare Disorders (NORD).
Keeping up to date on the latest rare diseases may seem like a daunting task to the average health care professional. However, while rare diseases remain the domain of the subspecialists, the generalist “can make a tremendous impact for their patients” early in the process by having a higher suspicion for rare diseases in their practice, said Dr. Summar.
Thinking of rare diseases in categories
Many patients with undiagnosed rare diseases undergo what’s commonly referred to as a “diagnostic odyssey,” moving from one provider to another to try to find an explanation for a condition they may or may not know is rare. For some patients, this process can take many years or even decades. From the patient’s perspective, the main challenges are recognizing they have a problem that doesn’t fit a common disease model. Once they recognize they have a potential rare disease, working with a provider to find the right diagnosis among the “vast number of disease diagnoses and designations, and actually sifting through it to find the one that’s right for that patient” is the next challenge, said Dr. Summar.
However, knowledge of rare diseases among health care professionals is low, according to a 2019 paper published in the Orphanet Journal of Rare Diseases. In a survey from that paper asking general practitioners, pediatricians, specialists caring for adults, and specialists caring for children to evaluate their own knowledge of rare diseases, 42% of general practitioners said they had poor knowledge and 44% said they had a substandard understanding of rare diseases.
From a clinician’s standpoint, diagnosing rare diseases in their patients can be challenging, with the potential for overreferral or overdiagnosis. However, it is also easy to underdiagnose rare diseases by missing them, noted Dr. Summar. This issue can vary based on the experience of the provider, he said, because while general practitioners who recently began practicing may have had more exposure to rare diseases, for health care professionals who have been practicing for decades, “this is a new arrival in their field.”
During a busy day finding that extra time in an appointment to stop and question whether a patient might have a rare disease is another problem generalists face. “It is really tough for those general practitioners, because if you see 40 or 50 patients per day, how do you know which one of those [patients] were the ones that had something that wasn’t quite fitting or wasn’t quite ordinary?” he said.
When it comes to considering rare diseases in their patients, health care professionals in general practice should think in categories, rather than a particular rare disease, according to Dr. Summar. As the generalist is typically on the front lines of patient care, they don’t necessarily need to know everything about the rare disease they suspect a patient of having to help them. “You don’t need to know the specific gene and the specific mutation to make the diagnosis, or to even move the patient forward in the process,” he said.
The first steps a clinician can take include noticing when something with a patient is amiss, thinking about the disease category, and then creating a plan to move forward, such as referring the patient to a subspecialist. Learning to recognize when a cluster of symptoms doesn’t fit a pattern is important, as patients and their providers tend to gravitate toward diagnoses they are used to seeing, rather than suspecting a disease outside a usual pattern.
The framing of rare diseases as categories is a change in thinking over the last decade, said Dr. Summar. Whereas rare disease diagnoses previously focused on fitting certain criteria, the development of more refined genetic sequencing has allowed specialists to focus on categories and genes that affect rare diseases. “Getting at a diagnosis has really been turned up on its head, so that by employing both next-generation sequencing, newborn screening, and other [tools], we can actually get to diagnoses faster than we could before,” he said.
In terms of assessing for symptoms, health care professionals should be aware that “common” symptoms can be a sign of rare disease. What to look out for are the uncommon symptoms that create an “aha moment.” Having a “good filter” for sensing when something isn’t quite right with a patient is key. “It’s like any time when you’re screening things: You want high sensitivity, but you also have to have high specificity,” he said.
Another clinical pearl is that good communication between patient and provider is paramount. “We’re not always good listeners. Sometimes we hear what we expect to hear,” said Dr. Summar.
Rare disease warning signs
Within the context of rare neurological diseases, Dr. Summar noted one major category is delays in neurological development, which is typically identified in children or adolescents. As the most complex organ in the body, “the brain probably expresses more genes than any other tissue on a regular basis, both in its formation and its function,” said Dr. Summar. He said the single disease that rare disease specialists see most often is Down syndrome.
Another separate but overlapping major category is autism, identified in younger children through trouble with social interaction, lack of eye contact, and delays in speech and communication skills. A third major category is physical manifestations of neurological problems, such as in patients who have epilepsy.
A telltale sign in identifying a child with a potential rare neurological disease is when they are “not thriving in their development or not doing the things on track that you would expect, and you don’t have a really good answer for it,” said Dr. Summar. Generalists are normally on watch for developmental delays in newborns born premature or with a rough course in the nursery, but they should also be aware of delays in children born under otherwise typical circumstances. “If I have a patient who had normal pregnancy, normal labor and delivery, no real illnesses or anything like that, and yet wasn’t meeting those milestones, that’s a patient I would pay attention to,” he said.
Another clue general practitioners can use for suspecting rare diseases is when a patient is much sicker than usual during a routine illness like a cold or flu. “Those are patients we should be paying attention to because it may be there’s an underlying biochemical disorder or some disorder in how they’re responding to stress that’s just not quite right,” said Dr. Summar. How a patient responds to stressful situations can be a warning sign “because that can often unmask more severe symptoms in that rare disease and make it a little more apparent,” he said.
Learning more about rare diseases
Dr. Summar said he and his colleagues in the rare disease field have spent a lot of time working with medical schools to teach this mindset in their curricula. The concept is introduced in basic medical science courses and then reinforced in clinical rotations in the third or fourth year, he explained.
“One of the best places is during the pediatrics rotations in medical school,” he said. “Remember, kids are basically healthy. If a child has a chronic illness or a chronic disease, more often than not, it is probably a rare disease.”
For medical professionals not in pediatric practice, the concept is applied the same way for adult medicine. “You just want to make sure everyone takes a second when they have a patient and try not to assume. Don’t assume it’s exactly what it seems. Look at it carefully and make sure there’s not something else going on,” he said.
Health care professionals in general practice looking to learn more about rare diseases can increasingly find rare disease topics in their CME programs. Rare disease topics in CME programs are “one of the best places” to learn about the latest developments in the field, said Dr. Summar.
Will rare disease screening tools come to primary care?
Asking more doctors to refer out to rare disease specialists raises an issue: There simply aren’t enough rare disease specialists in the field to go around.
Dr. Summar said partnering testing – where a general practitioner contacts a specialist to begin the process of testing based on the suspected condition – is a good stopgap solution. Telemedicine, which rose in popularity during the COVID-19 pandemic, can also play an important role in connecting patients and their providers with rare disease specialists, especially for generalists in remote communities. Dr. Summar noted he continues to see approximately 30% of his patients this way today. Telemedicine appointments can take place in the patient’s home or at the provider’s office.
“It actually provides access to folks who otherwise might not be able to either take off from work for a day – particularly some of our single parent households – or have a child who just doesn’t travel well, or can’t really get there, even if it’s the patient themselves,” he explained. “We can see patients that historically would have had trouble or difficulty coming in, so for me, that’s been a good thing.”
Telemedicine also helps give access to care for more medically fragile patients, many of whom have rare diseases, he added. While some aspects of care need to occur in person, “it’s a good 80% or 90% solution for a lot of these things,” he said.
Sharing educational videos is another way for health care providers in general practice to inform patients and their families about rare diseases. Children’s National Medical Center has created a collection of these videos in a free app called GeneClips, which is available on major smartphone app stores. However, Dr. Summar emphasized that genetic counseling should still be performed by a rare disease specialist prior to testing.
“We’re still at the point where I think having genetic counseling for a family before they’re going into testing is really advisable, since a lot of the results have a probability assigned to them,” he said. “I don’t think we’re really at the level where a practitioner is going to, first of all, have the time to do those, and I don’t think there’s enough general public awareness of what these things mean.”
Although primary care providers may one day be able to perform more generalized sequencing in their own practice, that time has not yet come – but it is closer than you think. “The technology is there, and actually the cost has come down a lot,” said Dr. Summar.
One potential issue this would create is an additional discussion to manage expectations of test results with family when the results are unclear, which “actually takes more time than counseling about a yes or no, or even an outcome that is unexpected,” explained Dr. Summar.
“[W]e’re in a midlife period right now where we’re bringing forward this new technology, but we’ve got to continually prepare the field for it first,” he said. “I think in the future we’ll see that it has much greater utility in the general setting,” he said.
Jeff Craven is a freelance journalist specializing in medicine and health.
Suggested reading
Vandeborne L et al. Information needs of physicians regarding the diagnosis of rare diseases: A questionnaire-based study in Belgium. Orphanet J Rare Dis. 2019;14(1):99.
The number of cataloged rare diseases continues to grow every day. According to the National Human Genome Research Institute, more than 6,800 rare diseases have been identified and between 25 million and 30 million Americans are living with rare diseases today.
Rare diseases have collectively emerged as a unique field of medicine with an “entirely new generation of conditions,” said Marshall L. Summar, MD, chief of the division of genetics and metabolism at Children’s National Hospital in Washington, DC. He places the number of rare diseases closer to 8,000, and said it is “growing by a rate of 10 to 12 a week.”
Although the field has made significant advancements in health care providers’ ability to diagnose rare diseases, it has also highlighted what isn’t known as well, said Dr. Summar, who is also past president and a former scientific advisory board member with the National Organization for Rare Disorders (NORD).
Keeping up to date on the latest rare diseases may seem like a daunting task to the average health care professional. However, while rare diseases remain the domain of the subspecialists, the generalist “can make a tremendous impact for their patients” early in the process by having a higher suspicion for rare diseases in their practice, said Dr. Summar.
Thinking of rare diseases in categories
Many patients with undiagnosed rare diseases undergo what’s commonly referred to as a “diagnostic odyssey,” moving from one provider to another to try to find an explanation for a condition they may or may not know is rare. For some patients, this process can take many years or even decades. From the patient’s perspective, the main challenges are recognizing they have a problem that doesn’t fit a common disease model. Once they recognize they have a potential rare disease, working with a provider to find the right diagnosis among the “vast number of disease diagnoses and designations, and actually sifting through it to find the one that’s right for that patient” is the next challenge, said Dr. Summar.
However, knowledge of rare diseases among health care professionals is low, according to a 2019 paper published in the Orphanet Journal of Rare Diseases. In a survey from that paper asking general practitioners, pediatricians, specialists caring for adults, and specialists caring for children to evaluate their own knowledge of rare diseases, 42% of general practitioners said they had poor knowledge and 44% said they had a substandard understanding of rare diseases.
From a clinician’s standpoint, diagnosing rare diseases in their patients can be challenging, with the potential for overreferral or overdiagnosis. However, it is also easy to underdiagnose rare diseases by missing them, noted Dr. Summar. This issue can vary based on the experience of the provider, he said, because while general practitioners who recently began practicing may have had more exposure to rare diseases, for health care professionals who have been practicing for decades, “this is a new arrival in their field.”
During a busy day finding that extra time in an appointment to stop and question whether a patient might have a rare disease is another problem generalists face. “It is really tough for those general practitioners, because if you see 40 or 50 patients per day, how do you know which one of those [patients] were the ones that had something that wasn’t quite fitting or wasn’t quite ordinary?” he said.
When it comes to considering rare diseases in their patients, health care professionals in general practice should think in categories, rather than a particular rare disease, according to Dr. Summar. As the generalist is typically on the front lines of patient care, they don’t necessarily need to know everything about the rare disease they suspect a patient of having to help them. “You don’t need to know the specific gene and the specific mutation to make the diagnosis, or to even move the patient forward in the process,” he said.
The first steps a clinician can take include noticing when something with a patient is amiss, thinking about the disease category, and then creating a plan to move forward, such as referring the patient to a subspecialist. Learning to recognize when a cluster of symptoms doesn’t fit a pattern is important, as patients and their providers tend to gravitate toward diagnoses they are used to seeing, rather than suspecting a disease outside a usual pattern.
The framing of rare diseases as categories is a change in thinking over the last decade, said Dr. Summar. Whereas rare disease diagnoses previously focused on fitting certain criteria, the development of more refined genetic sequencing has allowed specialists to focus on categories and genes that affect rare diseases. “Getting at a diagnosis has really been turned up on its head, so that by employing both next-generation sequencing, newborn screening, and other [tools], we can actually get to diagnoses faster than we could before,” he said.
In terms of assessing for symptoms, health care professionals should be aware that “common” symptoms can be a sign of rare disease. What to look out for are the uncommon symptoms that create an “aha moment.” Having a “good filter” for sensing when something isn’t quite right with a patient is key. “It’s like any time when you’re screening things: You want high sensitivity, but you also have to have high specificity,” he said.
Another clinical pearl is that good communication between patient and provider is paramount. “We’re not always good listeners. Sometimes we hear what we expect to hear,” said Dr. Summar.
Rare disease warning signs
Within the context of rare neurological diseases, Dr. Summar noted one major category is delays in neurological development, which is typically identified in children or adolescents. As the most complex organ in the body, “the brain probably expresses more genes than any other tissue on a regular basis, both in its formation and its function,” said Dr. Summar. He said the single disease that rare disease specialists see most often is Down syndrome.
Another separate but overlapping major category is autism, identified in younger children through trouble with social interaction, lack of eye contact, and delays in speech and communication skills. A third major category is physical manifestations of neurological problems, such as in patients who have epilepsy.
A telltale sign in identifying a child with a potential rare neurological disease is when they are “not thriving in their development or not doing the things on track that you would expect, and you don’t have a really good answer for it,” said Dr. Summar. Generalists are normally on watch for developmental delays in newborns born premature or with a rough course in the nursery, but they should also be aware of delays in children born under otherwise typical circumstances. “If I have a patient who had normal pregnancy, normal labor and delivery, no real illnesses or anything like that, and yet wasn’t meeting those milestones, that’s a patient I would pay attention to,” he said.
Another clue general practitioners can use for suspecting rare diseases is when a patient is much sicker than usual during a routine illness like a cold or flu. “Those are patients we should be paying attention to because it may be there’s an underlying biochemical disorder or some disorder in how they’re responding to stress that’s just not quite right,” said Dr. Summar. How a patient responds to stressful situations can be a warning sign “because that can often unmask more severe symptoms in that rare disease and make it a little more apparent,” he said.
Learning more about rare diseases
Dr. Summar said he and his colleagues in the rare disease field have spent a lot of time working with medical schools to teach this mindset in their curricula. The concept is introduced in basic medical science courses and then reinforced in clinical rotations in the third or fourth year, he explained.
“One of the best places is during the pediatrics rotations in medical school,” he said. “Remember, kids are basically healthy. If a child has a chronic illness or a chronic disease, more often than not, it is probably a rare disease.”
For medical professionals not in pediatric practice, the concept is applied the same way for adult medicine. “You just want to make sure everyone takes a second when they have a patient and try not to assume. Don’t assume it’s exactly what it seems. Look at it carefully and make sure there’s not something else going on,” he said.
Health care professionals in general practice looking to learn more about rare diseases can increasingly find rare disease topics in their CME programs. Rare disease topics in CME programs are “one of the best places” to learn about the latest developments in the field, said Dr. Summar.
Will rare disease screening tools come to primary care?
Asking more doctors to refer out to rare disease specialists raises an issue: There simply aren’t enough rare disease specialists in the field to go around.
Dr. Summar said partnering testing – where a general practitioner contacts a specialist to begin the process of testing based on the suspected condition – is a good stopgap solution. Telemedicine, which rose in popularity during the COVID-19 pandemic, can also play an important role in connecting patients and their providers with rare disease specialists, especially for generalists in remote communities. Dr. Summar noted he continues to see approximately 30% of his patients this way today. Telemedicine appointments can take place in the patient’s home or at the provider’s office.
“It actually provides access to folks who otherwise might not be able to either take off from work for a day – particularly some of our single parent households – or have a child who just doesn’t travel well, or can’t really get there, even if it’s the patient themselves,” he explained. “We can see patients that historically would have had trouble or difficulty coming in, so for me, that’s been a good thing.”
Telemedicine also helps give access to care for more medically fragile patients, many of whom have rare diseases, he added. While some aspects of care need to occur in person, “it’s a good 80% or 90% solution for a lot of these things,” he said.
Sharing educational videos is another way for health care providers in general practice to inform patients and their families about rare diseases. Children’s National Medical Center has created a collection of these videos in a free app called GeneClips, which is available on major smartphone app stores. However, Dr. Summar emphasized that genetic counseling should still be performed by a rare disease specialist prior to testing.
“We’re still at the point where I think having genetic counseling for a family before they’re going into testing is really advisable, since a lot of the results have a probability assigned to them,” he said. “I don’t think we’re really at the level where a practitioner is going to, first of all, have the time to do those, and I don’t think there’s enough general public awareness of what these things mean.”
Although primary care providers may one day be able to perform more generalized sequencing in their own practice, that time has not yet come – but it is closer than you think. “The technology is there, and actually the cost has come down a lot,” said Dr. Summar.
One potential issue this would create is an additional discussion to manage expectations of test results with family when the results are unclear, which “actually takes more time than counseling about a yes or no, or even an outcome that is unexpected,” explained Dr. Summar.
“[W]e’re in a midlife period right now where we’re bringing forward this new technology, but we’ve got to continually prepare the field for it first,” he said. “I think in the future we’ll see that it has much greater utility in the general setting,” he said.
Jeff Craven is a freelance journalist specializing in medicine and health.
Suggested reading
Vandeborne L et al. Information needs of physicians regarding the diagnosis of rare diseases: A questionnaire-based study in Belgium. Orphanet J Rare Dis. 2019;14(1):99.