User login
Vitamin D: Recent findings and implications for clinical practice
This transcript has been edited for clarity.
Hello. This is Dr JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. As a director of the Vitamin D and Omega-3 trial (VITAL), the largest randomized clinical trial in the world, I’m often asked, “How much vitamin D do we need, and should I take a vitamin D supplement?” I want to review the findings from recent randomized clinical trials and the implications for practice.
For a long time, vitamin D has been perceived as a magic bullet, a panacea, and a cure-all for many chronic health conditions such as cancer, cardiovascular disease, diabetes, bone fractures, cognitive decline, and depression. Many of the findings, though, have been from observational studies where a higher blood level of 25-hydroxy vitamin D has been linked to a lower risk for these health conditions.
We know in epidemiology that correlation doesn’t prove causation. Other factors could be involved; for example, people who have higher blood levels of vitamin D may have healthier diets, or they may be spending more time outdoors, being physically active and exposed to the sun. Some of these other factors could be lowering their risk.
When the randomized trials began to emerge, in many of these large-scale trials, the findings were generally neutral or null for cardiovascular disease, total cancer, diabetes, cognitive decline, depression, and many other health outcomes, including fracture. So, the question was asked, does this mean that vitamin D is not important to health?
To the contrary, these findings suggest that vitamin D is so essential to health that we need only small to moderate amounts of vitamin D. Vitamin D is very tightly regulated in the body – the metabolism and function of vitamin D. Even small to moderate amounts will meet the requirements for vitamin D and bone health and many other outcomes.
This is what the National Academy of Medicine, U.S. Preventive Services Task Force, and many other professional organizations have advised, that widespread screening for vitamin D deficiency and blanket universal supplementation with vitamin D would not be indicated.
The randomized trials of vitamin D, including the VITAL study, have generally not shown reductions in the major health outcomes. We found two exceptions in VITAL. We saw promising signals, including a 22% reduction in autoimmune conditions (rheumatoid arthritis and psoriasis) and a 17% reduction in advanced (metastatic or fatal) cancers. In meta-analyses of other large-scale randomized trials, the findings were a signal for a reduction in advanced cancers, even with very small doses of vitamin D (400-800 IUs daily). We tested 2,000 IUs daily in VITAL.
Overall, it’s recommended that small to moderate amounts of vitamin D are adequate, and among the healthy population, most people do not need screening or supplements.
The reduction in autoimmune diseases suggests that vitamin D may play a role in tamping down inflammation. The question has been raised about whether vitamin D is beneficial in reducing the severity of COVID illness, the need for hospitalization, and long COVID. We are looking at this question in a separate trial called VIVID (Vitamin D for COVID Trial) which tests a higher dose (> 3,000 IUs daily) of vitamin D. Those results will be available at the end of this year or early next year.
In other randomized trials of COVID and vitamin D, the results have been mixed and inconsistent, with no clear answer. During the COVID pandemic, I have generally advised that it’s reasonable to take 1,000-2,000 IUs of vitamin D daily as a form of insurance. This dose is known to be very safe. Over 5.3 years in the VITAL trial we saw that a dose of 2,000 IUs was very safe.
But it’s not essential to take a supplement. And overall, aside from some high-risk groups, most people do not need a supplement. The high-risk groups include patients in nursing homes who may have restricted diets and limited time out of doors. For people with malabsorption conditions such as Crohn’s disease, celiac disease, post–gastric bypass surgery, and those with osteoporosis who are on medications for osteoporosis, it’s still quite reasonable to prescribe calcium and vitamin D.
Recommendations for vitamin D in the generally healthy population really should focus on a healthy diet. The United States has a fortified food supply. Vitamin D is added to many foods, dairy products, and cereals, as well as beverages. Natural sources of vitamin D include fatty fish and wild mushrooms.
We should be looking at food labels (which now include vitamin D content) and try to get adequate vitamin D from our diet, and also do our best to spend time outdoors, being physically active, because it is of great benefit to our health. The general principle is that a dietary supplement will never be a substitute for a healthy diet or healthy lifestyle. And those other behaviors really should be the focus at this time.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine at Brigham and Women’s Hospital, both in Boston. She has received infrastructure support from Mars Symbioscience for the COSMOS trial.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. This is Dr JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. As a director of the Vitamin D and Omega-3 trial (VITAL), the largest randomized clinical trial in the world, I’m often asked, “How much vitamin D do we need, and should I take a vitamin D supplement?” I want to review the findings from recent randomized clinical trials and the implications for practice.
For a long time, vitamin D has been perceived as a magic bullet, a panacea, and a cure-all for many chronic health conditions such as cancer, cardiovascular disease, diabetes, bone fractures, cognitive decline, and depression. Many of the findings, though, have been from observational studies where a higher blood level of 25-hydroxy vitamin D has been linked to a lower risk for these health conditions.
We know in epidemiology that correlation doesn’t prove causation. Other factors could be involved; for example, people who have higher blood levels of vitamin D may have healthier diets, or they may be spending more time outdoors, being physically active and exposed to the sun. Some of these other factors could be lowering their risk.
When the randomized trials began to emerge, in many of these large-scale trials, the findings were generally neutral or null for cardiovascular disease, total cancer, diabetes, cognitive decline, depression, and many other health outcomes, including fracture. So, the question was asked, does this mean that vitamin D is not important to health?
To the contrary, these findings suggest that vitamin D is so essential to health that we need only small to moderate amounts of vitamin D. Vitamin D is very tightly regulated in the body – the metabolism and function of vitamin D. Even small to moderate amounts will meet the requirements for vitamin D and bone health and many other outcomes.
This is what the National Academy of Medicine, U.S. Preventive Services Task Force, and many other professional organizations have advised, that widespread screening for vitamin D deficiency and blanket universal supplementation with vitamin D would not be indicated.
The randomized trials of vitamin D, including the VITAL study, have generally not shown reductions in the major health outcomes. We found two exceptions in VITAL. We saw promising signals, including a 22% reduction in autoimmune conditions (rheumatoid arthritis and psoriasis) and a 17% reduction in advanced (metastatic or fatal) cancers. In meta-analyses of other large-scale randomized trials, the findings were a signal for a reduction in advanced cancers, even with very small doses of vitamin D (400-800 IUs daily). We tested 2,000 IUs daily in VITAL.
Overall, it’s recommended that small to moderate amounts of vitamin D are adequate, and among the healthy population, most people do not need screening or supplements.
The reduction in autoimmune diseases suggests that vitamin D may play a role in tamping down inflammation. The question has been raised about whether vitamin D is beneficial in reducing the severity of COVID illness, the need for hospitalization, and long COVID. We are looking at this question in a separate trial called VIVID (Vitamin D for COVID Trial) which tests a higher dose (> 3,000 IUs daily) of vitamin D. Those results will be available at the end of this year or early next year.
In other randomized trials of COVID and vitamin D, the results have been mixed and inconsistent, with no clear answer. During the COVID pandemic, I have generally advised that it’s reasonable to take 1,000-2,000 IUs of vitamin D daily as a form of insurance. This dose is known to be very safe. Over 5.3 years in the VITAL trial we saw that a dose of 2,000 IUs was very safe.
But it’s not essential to take a supplement. And overall, aside from some high-risk groups, most people do not need a supplement. The high-risk groups include patients in nursing homes who may have restricted diets and limited time out of doors. For people with malabsorption conditions such as Crohn’s disease, celiac disease, post–gastric bypass surgery, and those with osteoporosis who are on medications for osteoporosis, it’s still quite reasonable to prescribe calcium and vitamin D.
Recommendations for vitamin D in the generally healthy population really should focus on a healthy diet. The United States has a fortified food supply. Vitamin D is added to many foods, dairy products, and cereals, as well as beverages. Natural sources of vitamin D include fatty fish and wild mushrooms.
We should be looking at food labels (which now include vitamin D content) and try to get adequate vitamin D from our diet, and also do our best to spend time outdoors, being physically active, because it is of great benefit to our health. The general principle is that a dietary supplement will never be a substitute for a healthy diet or healthy lifestyle. And those other behaviors really should be the focus at this time.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine at Brigham and Women’s Hospital, both in Boston. She has received infrastructure support from Mars Symbioscience for the COSMOS trial.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. This is Dr JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. As a director of the Vitamin D and Omega-3 trial (VITAL), the largest randomized clinical trial in the world, I’m often asked, “How much vitamin D do we need, and should I take a vitamin D supplement?” I want to review the findings from recent randomized clinical trials and the implications for practice.
For a long time, vitamin D has been perceived as a magic bullet, a panacea, and a cure-all for many chronic health conditions such as cancer, cardiovascular disease, diabetes, bone fractures, cognitive decline, and depression. Many of the findings, though, have been from observational studies where a higher blood level of 25-hydroxy vitamin D has been linked to a lower risk for these health conditions.
We know in epidemiology that correlation doesn’t prove causation. Other factors could be involved; for example, people who have higher blood levels of vitamin D may have healthier diets, or they may be spending more time outdoors, being physically active and exposed to the sun. Some of these other factors could be lowering their risk.
When the randomized trials began to emerge, in many of these large-scale trials, the findings were generally neutral or null for cardiovascular disease, total cancer, diabetes, cognitive decline, depression, and many other health outcomes, including fracture. So, the question was asked, does this mean that vitamin D is not important to health?
To the contrary, these findings suggest that vitamin D is so essential to health that we need only small to moderate amounts of vitamin D. Vitamin D is very tightly regulated in the body – the metabolism and function of vitamin D. Even small to moderate amounts will meet the requirements for vitamin D and bone health and many other outcomes.
This is what the National Academy of Medicine, U.S. Preventive Services Task Force, and many other professional organizations have advised, that widespread screening for vitamin D deficiency and blanket universal supplementation with vitamin D would not be indicated.
The randomized trials of vitamin D, including the VITAL study, have generally not shown reductions in the major health outcomes. We found two exceptions in VITAL. We saw promising signals, including a 22% reduction in autoimmune conditions (rheumatoid arthritis and psoriasis) and a 17% reduction in advanced (metastatic or fatal) cancers. In meta-analyses of other large-scale randomized trials, the findings were a signal for a reduction in advanced cancers, even with very small doses of vitamin D (400-800 IUs daily). We tested 2,000 IUs daily in VITAL.
Overall, it’s recommended that small to moderate amounts of vitamin D are adequate, and among the healthy population, most people do not need screening or supplements.
The reduction in autoimmune diseases suggests that vitamin D may play a role in tamping down inflammation. The question has been raised about whether vitamin D is beneficial in reducing the severity of COVID illness, the need for hospitalization, and long COVID. We are looking at this question in a separate trial called VIVID (Vitamin D for COVID Trial) which tests a higher dose (> 3,000 IUs daily) of vitamin D. Those results will be available at the end of this year or early next year.
In other randomized trials of COVID and vitamin D, the results have been mixed and inconsistent, with no clear answer. During the COVID pandemic, I have generally advised that it’s reasonable to take 1,000-2,000 IUs of vitamin D daily as a form of insurance. This dose is known to be very safe. Over 5.3 years in the VITAL trial we saw that a dose of 2,000 IUs was very safe.
But it’s not essential to take a supplement. And overall, aside from some high-risk groups, most people do not need a supplement. The high-risk groups include patients in nursing homes who may have restricted diets and limited time out of doors. For people with malabsorption conditions such as Crohn’s disease, celiac disease, post–gastric bypass surgery, and those with osteoporosis who are on medications for osteoporosis, it’s still quite reasonable to prescribe calcium and vitamin D.
Recommendations for vitamin D in the generally healthy population really should focus on a healthy diet. The United States has a fortified food supply. Vitamin D is added to many foods, dairy products, and cereals, as well as beverages. Natural sources of vitamin D include fatty fish and wild mushrooms.
We should be looking at food labels (which now include vitamin D content) and try to get adequate vitamin D from our diet, and also do our best to spend time outdoors, being physically active, because it is of great benefit to our health. The general principle is that a dietary supplement will never be a substitute for a healthy diet or healthy lifestyle. And those other behaviors really should be the focus at this time.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine at Brigham and Women’s Hospital, both in Boston. She has received infrastructure support from Mars Symbioscience for the COSMOS trial.
A version of this article first appeared on Medscape.com.
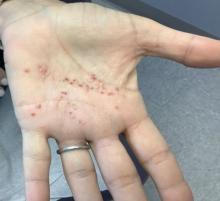
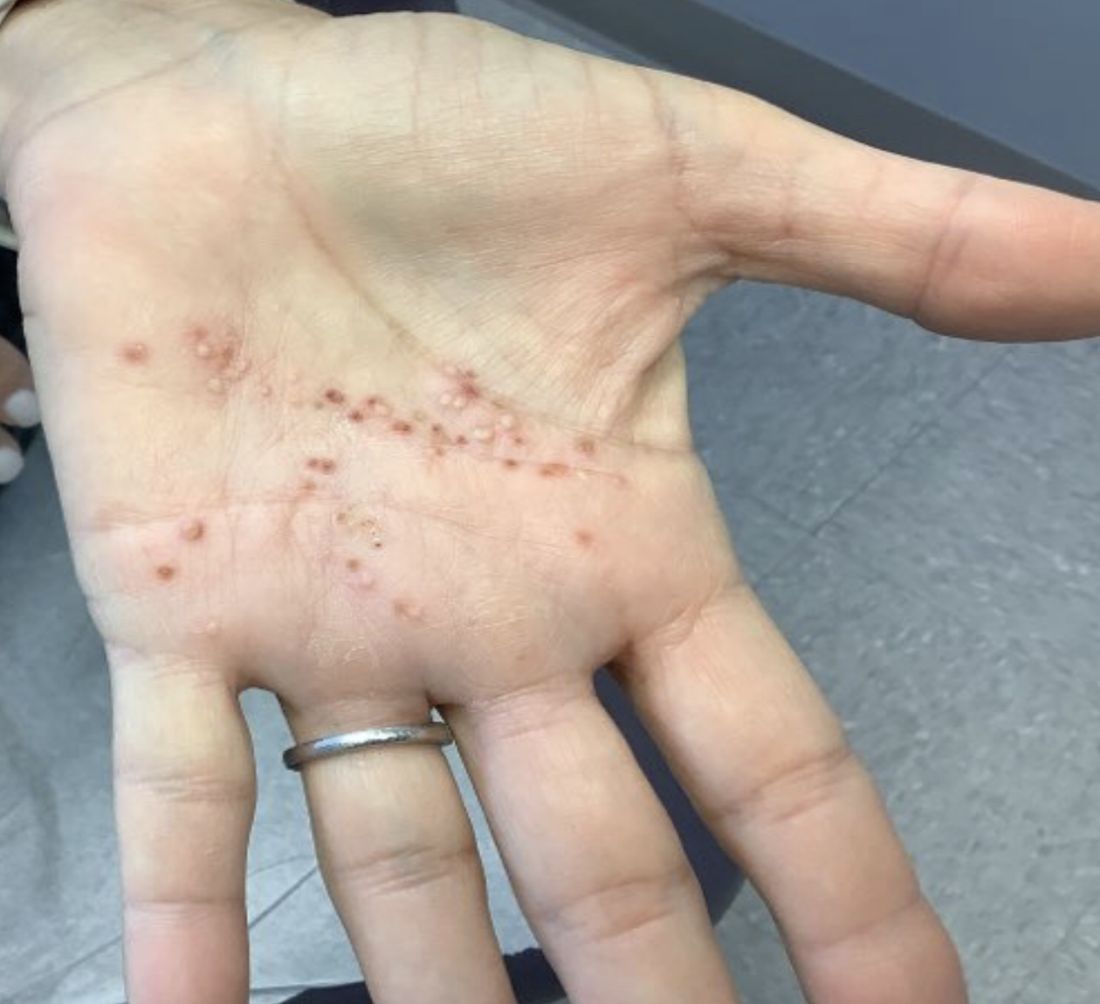
A White female presented with pustules and erythematous macules on the left palm
Psoriasis is an immune-mediated chronic inflammatory disease characterized by well-demarcated, scaly, erythematous plaques. Those who present with the condition often have a family history, which supports recent research uncovering various genes implicated in its pathogenesis. The disease is also associated with other systemic complications, most notably cardiovascular disease.
This condition is found in a small percentage of patients with psoriasis and presentation varies from hyperkeratotic plaques to pustular lesions. The pustular form is known as palmoplantar pustulosis and is within the spectrum of palmoplantar psoriasis.
Psoriasis is typically a clinical diagnosis and its severity can be measured using the Psoriasis Area and Severity Index. If biopsy is performed, the histology demonstrates parakeratosis, orthokeratosis, loss of the stratum granulosum, and dilated vasculature with an inflammatory cell infiltrate. The keratinocytes present with abnormal differentiation and hyperplasia, and the presence of foci of neutrophils known as “Munro’s microabscesses” in the stratum corneum serve as the hallmark of histological diagnosis. However, it is important to note that appearance can vary based on the stage of the lesion and the subtype of psoriasis present.
Palmoplantar psoriasis can be especially limiting and difficult to treat because of its distribution. Topical steroids, topical vitamin D analogues, and narrow band ultraviolet light therapy can be effective for less severe cases. Methotrexate, biologic treatments, and apremilast can be used for more extensive disease.
This patient is HLA-B27 positive and has uveitis. The presence of the HLA-B27 allele has been associated with inflammatory bowel disease, uveitis, psoriatic arthritis, and reactive arthritis. It has also been reported to be associated with pustular psoriasis. She responded well to topical steroids and vitamin D analogues.
This case and photo were submitted by Mr. Shapiro at Nova Southeastern University College of Osteopathic Medicine, Davie, Fla., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Psoriasis: Overview and Diagnosis, in “Evidence-Based Psoriasis. Updates in Clinical Dermatology.” (Cham, Switzerland: Springer International, 2018).
2. Merola JF et al. Dermatol Ther. 2018 May;31(3):e12589.
3. Chung J et al. J Am Acad Dermatol. 2014 Oct;71(4):623-32.
Psoriasis is an immune-mediated chronic inflammatory disease characterized by well-demarcated, scaly, erythematous plaques. Those who present with the condition often have a family history, which supports recent research uncovering various genes implicated in its pathogenesis. The disease is also associated with other systemic complications, most notably cardiovascular disease.
This condition is found in a small percentage of patients with psoriasis and presentation varies from hyperkeratotic plaques to pustular lesions. The pustular form is known as palmoplantar pustulosis and is within the spectrum of palmoplantar psoriasis.
Psoriasis is typically a clinical diagnosis and its severity can be measured using the Psoriasis Area and Severity Index. If biopsy is performed, the histology demonstrates parakeratosis, orthokeratosis, loss of the stratum granulosum, and dilated vasculature with an inflammatory cell infiltrate. The keratinocytes present with abnormal differentiation and hyperplasia, and the presence of foci of neutrophils known as “Munro’s microabscesses” in the stratum corneum serve as the hallmark of histological diagnosis. However, it is important to note that appearance can vary based on the stage of the lesion and the subtype of psoriasis present.
Palmoplantar psoriasis can be especially limiting and difficult to treat because of its distribution. Topical steroids, topical vitamin D analogues, and narrow band ultraviolet light therapy can be effective for less severe cases. Methotrexate, biologic treatments, and apremilast can be used for more extensive disease.
This patient is HLA-B27 positive and has uveitis. The presence of the HLA-B27 allele has been associated with inflammatory bowel disease, uveitis, psoriatic arthritis, and reactive arthritis. It has also been reported to be associated with pustular psoriasis. She responded well to topical steroids and vitamin D analogues.
This case and photo were submitted by Mr. Shapiro at Nova Southeastern University College of Osteopathic Medicine, Davie, Fla., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Psoriasis: Overview and Diagnosis, in “Evidence-Based Psoriasis. Updates in Clinical Dermatology.” (Cham, Switzerland: Springer International, 2018).
2. Merola JF et al. Dermatol Ther. 2018 May;31(3):e12589.
3. Chung J et al. J Am Acad Dermatol. 2014 Oct;71(4):623-32.
Psoriasis is an immune-mediated chronic inflammatory disease characterized by well-demarcated, scaly, erythematous plaques. Those who present with the condition often have a family history, which supports recent research uncovering various genes implicated in its pathogenesis. The disease is also associated with other systemic complications, most notably cardiovascular disease.
This condition is found in a small percentage of patients with psoriasis and presentation varies from hyperkeratotic plaques to pustular lesions. The pustular form is known as palmoplantar pustulosis and is within the spectrum of palmoplantar psoriasis.
Psoriasis is typically a clinical diagnosis and its severity can be measured using the Psoriasis Area and Severity Index. If biopsy is performed, the histology demonstrates parakeratosis, orthokeratosis, loss of the stratum granulosum, and dilated vasculature with an inflammatory cell infiltrate. The keratinocytes present with abnormal differentiation and hyperplasia, and the presence of foci of neutrophils known as “Munro’s microabscesses” in the stratum corneum serve as the hallmark of histological diagnosis. However, it is important to note that appearance can vary based on the stage of the lesion and the subtype of psoriasis present.
Palmoplantar psoriasis can be especially limiting and difficult to treat because of its distribution. Topical steroids, topical vitamin D analogues, and narrow band ultraviolet light therapy can be effective for less severe cases. Methotrexate, biologic treatments, and apremilast can be used for more extensive disease.
This patient is HLA-B27 positive and has uveitis. The presence of the HLA-B27 allele has been associated with inflammatory bowel disease, uveitis, psoriatic arthritis, and reactive arthritis. It has also been reported to be associated with pustular psoriasis. She responded well to topical steroids and vitamin D analogues.
This case and photo were submitted by Mr. Shapiro at Nova Southeastern University College of Osteopathic Medicine, Davie, Fla., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Psoriasis: Overview and Diagnosis, in “Evidence-Based Psoriasis. Updates in Clinical Dermatology.” (Cham, Switzerland: Springer International, 2018).
2. Merola JF et al. Dermatol Ther. 2018 May;31(3):e12589.
3. Chung J et al. J Am Acad Dermatol. 2014 Oct;71(4):623-32.
Loan forgiveness and med school debt: What about me?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I run the division of medical ethics at New York University Grossman School of Medicine.
Many of you know that President Biden created a loan forgiveness program, forgiving up to $10,000 against federal student loans, including graduate and undergraduate education. The Department of Education is supposed to provide up to $20,000 in debt cancellation to Pell Grant recipients who have loans that are held by the Department of Education. Borrowers can get this relief if their income is less than $125,000 for an individual or $250,000 for married couples.
Many people have looked at this and said, “Hey, wait a minute. I paid off my loans. I didn’t get any reimbursement. That isn’t fair.”
who often still have huge amounts of debt, and either because of the income limits or because they don’t qualify because this debt was accrued long in the past, they’re saying, “What about me? Don’t you want to give any relief to me?”
This is a topic near and dear to my heart because I happen to be at a medical school, NYU, that has decided for the two medical schools it runs – our main campus, NYU in Manhattan and NYU Langone out on Long Island – that we’re going to go tuition free. We’ve done it for a couple of years.
We did it because I think all the administrators and faculty understood the tremendous burden that debt poses on people who both carry forward their undergraduate debt and then have medical school debt. This really leads to very difficult situations – which we have great empathy for – about what specialty you’re going to go into, whether you have to moonlight, and how you’re going to manage a huge burden of debt.
Many people don’t have sympathy out in the public. They say doctors make a large amount of money and they live a nice lifestyle, so we’re not going to relieve their debt. The reality is that, whoever you are, short of Bill Gates or Elon Musk, having hundreds of thousands of dollars of debt is no easy task to live with and to work off.
Still, when we created free tuition at NYU for our medical school, there were many people who paid high tuition fees in the past. Some of them said to us, “What about me?” We decided not to try to do anything retrospectively. The plan was to build up enough money so that we could handle no-cost tuition going forward. We didn’t really have it in our pocketbook to help people who’d already paid their debts or were saddled with NYU debt. Is it fair? No, it’s probably not fair, but it’s an improvement.
That’s what I want people to think about who are saying, “What about my medical school debt? What about my undergraduate plus medical school debt?” I think we should be grateful when efforts are being made to reduce very burdensome student loans that people have. It’s good to give that benefit and move it forward.
Does that mean no one should get anything unless everyone with any kind of debt from school is covered? I don’t think so. I don’t think that’s fair either.
It is possible that we could continue to agitate politically and say, let’s go after some of the health care debt. Let’s go after some of the things that are still driving people to have to work more than they would or to choose specialties that they really don’t want to be in because they have to make up that debt.
It doesn’t mean the last word has been said about the politics of debt relief or, for that matter, the price of going to medical school in the first place and trying to see whether that can be driven down.
I don’t think it’s right to say, “If I can’t benefit, given the huge burden that I’m carrying, then I’m not going to try to give relief to others.” I think we’re relieving debt to the extent that we can do it. The nation can afford it. Going forward is a good thing. It’s wrong to create those gigantic debts in the first place.
What are we going to do about the past? We may decide that we need some sort of forgiveness or reparations for loans that were built up for others going backwards. I wouldn’t hold hostage the future and our children to what was probably a very poor, unethical practice about saddling doctors and others in the past with huge debt.
I’m Art Caplan at the division of medical ethics at New York University Grossman School of Medicine. Thank you for watching.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I run the division of medical ethics at New York University Grossman School of Medicine.
Many of you know that President Biden created a loan forgiveness program, forgiving up to $10,000 against federal student loans, including graduate and undergraduate education. The Department of Education is supposed to provide up to $20,000 in debt cancellation to Pell Grant recipients who have loans that are held by the Department of Education. Borrowers can get this relief if their income is less than $125,000 for an individual or $250,000 for married couples.
Many people have looked at this and said, “Hey, wait a minute. I paid off my loans. I didn’t get any reimbursement. That isn’t fair.”
who often still have huge amounts of debt, and either because of the income limits or because they don’t qualify because this debt was accrued long in the past, they’re saying, “What about me? Don’t you want to give any relief to me?”
This is a topic near and dear to my heart because I happen to be at a medical school, NYU, that has decided for the two medical schools it runs – our main campus, NYU in Manhattan and NYU Langone out on Long Island – that we’re going to go tuition free. We’ve done it for a couple of years.
We did it because I think all the administrators and faculty understood the tremendous burden that debt poses on people who both carry forward their undergraduate debt and then have medical school debt. This really leads to very difficult situations – which we have great empathy for – about what specialty you’re going to go into, whether you have to moonlight, and how you’re going to manage a huge burden of debt.
Many people don’t have sympathy out in the public. They say doctors make a large amount of money and they live a nice lifestyle, so we’re not going to relieve their debt. The reality is that, whoever you are, short of Bill Gates or Elon Musk, having hundreds of thousands of dollars of debt is no easy task to live with and to work off.
Still, when we created free tuition at NYU for our medical school, there were many people who paid high tuition fees in the past. Some of them said to us, “What about me?” We decided not to try to do anything retrospectively. The plan was to build up enough money so that we could handle no-cost tuition going forward. We didn’t really have it in our pocketbook to help people who’d already paid their debts or were saddled with NYU debt. Is it fair? No, it’s probably not fair, but it’s an improvement.
That’s what I want people to think about who are saying, “What about my medical school debt? What about my undergraduate plus medical school debt?” I think we should be grateful when efforts are being made to reduce very burdensome student loans that people have. It’s good to give that benefit and move it forward.
Does that mean no one should get anything unless everyone with any kind of debt from school is covered? I don’t think so. I don’t think that’s fair either.
It is possible that we could continue to agitate politically and say, let’s go after some of the health care debt. Let’s go after some of the things that are still driving people to have to work more than they would or to choose specialties that they really don’t want to be in because they have to make up that debt.
It doesn’t mean the last word has been said about the politics of debt relief or, for that matter, the price of going to medical school in the first place and trying to see whether that can be driven down.
I don’t think it’s right to say, “If I can’t benefit, given the huge burden that I’m carrying, then I’m not going to try to give relief to others.” I think we’re relieving debt to the extent that we can do it. The nation can afford it. Going forward is a good thing. It’s wrong to create those gigantic debts in the first place.
What are we going to do about the past? We may decide that we need some sort of forgiveness or reparations for loans that were built up for others going backwards. I wouldn’t hold hostage the future and our children to what was probably a very poor, unethical practice about saddling doctors and others in the past with huge debt.
I’m Art Caplan at the division of medical ethics at New York University Grossman School of Medicine. Thank you for watching.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I run the division of medical ethics at New York University Grossman School of Medicine.
Many of you know that President Biden created a loan forgiveness program, forgiving up to $10,000 against federal student loans, including graduate and undergraduate education. The Department of Education is supposed to provide up to $20,000 in debt cancellation to Pell Grant recipients who have loans that are held by the Department of Education. Borrowers can get this relief if their income is less than $125,000 for an individual or $250,000 for married couples.
Many people have looked at this and said, “Hey, wait a minute. I paid off my loans. I didn’t get any reimbursement. That isn’t fair.”
who often still have huge amounts of debt, and either because of the income limits or because they don’t qualify because this debt was accrued long in the past, they’re saying, “What about me? Don’t you want to give any relief to me?”
This is a topic near and dear to my heart because I happen to be at a medical school, NYU, that has decided for the two medical schools it runs – our main campus, NYU in Manhattan and NYU Langone out on Long Island – that we’re going to go tuition free. We’ve done it for a couple of years.
We did it because I think all the administrators and faculty understood the tremendous burden that debt poses on people who both carry forward their undergraduate debt and then have medical school debt. This really leads to very difficult situations – which we have great empathy for – about what specialty you’re going to go into, whether you have to moonlight, and how you’re going to manage a huge burden of debt.
Many people don’t have sympathy out in the public. They say doctors make a large amount of money and they live a nice lifestyle, so we’re not going to relieve their debt. The reality is that, whoever you are, short of Bill Gates or Elon Musk, having hundreds of thousands of dollars of debt is no easy task to live with and to work off.
Still, when we created free tuition at NYU for our medical school, there were many people who paid high tuition fees in the past. Some of them said to us, “What about me?” We decided not to try to do anything retrospectively. The plan was to build up enough money so that we could handle no-cost tuition going forward. We didn’t really have it in our pocketbook to help people who’d already paid their debts or were saddled with NYU debt. Is it fair? No, it’s probably not fair, but it’s an improvement.
That’s what I want people to think about who are saying, “What about my medical school debt? What about my undergraduate plus medical school debt?” I think we should be grateful when efforts are being made to reduce very burdensome student loans that people have. It’s good to give that benefit and move it forward.
Does that mean no one should get anything unless everyone with any kind of debt from school is covered? I don’t think so. I don’t think that’s fair either.
It is possible that we could continue to agitate politically and say, let’s go after some of the health care debt. Let’s go after some of the things that are still driving people to have to work more than they would or to choose specialties that they really don’t want to be in because they have to make up that debt.
It doesn’t mean the last word has been said about the politics of debt relief or, for that matter, the price of going to medical school in the first place and trying to see whether that can be driven down.
I don’t think it’s right to say, “If I can’t benefit, given the huge burden that I’m carrying, then I’m not going to try to give relief to others.” I think we’re relieving debt to the extent that we can do it. The nation can afford it. Going forward is a good thing. It’s wrong to create those gigantic debts in the first place.
What are we going to do about the past? We may decide that we need some sort of forgiveness or reparations for loans that were built up for others going backwards. I wouldn’t hold hostage the future and our children to what was probably a very poor, unethical practice about saddling doctors and others in the past with huge debt.
I’m Art Caplan at the division of medical ethics at New York University Grossman School of Medicine. Thank you for watching.
A version of this article first appeared on Medscape.com.
The marked contrast in pandemic outcomes between Japan and the United States
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
Playing the fat shame game in medicine: It needs to stop
I will remember that there is art to medicine as well as science, and that warmth, sympathy, and understanding may outweigh the surgeon’s knife or the chemist’s drug.
Upon finishing medical school, many of us recited this passage from a modernized version of the Hippocratic Oath. Though there has been controversy regarding the current relevancy of this oath, it can still serve as a reminder of the promises we made on behalf of our patients: To treat them ethically, with empathy and respect, and without pretension. Though I hadn’t thought about the Hippocratic Oath in ages, it came to mind recently after I read an article about weight trends in adults during the COVID pandemic.
No surprise – we gained weight during the initial surge at a rate of roughly a pound and a half per month following the initial shelter-in-place period. For some of us, that trend in weight gain worsened as the pandemic persisted. A survey conducted in February 2021 suggested that over 40% of adults who experienced undesired weight changes since the start of the pandemic gained an average of 29 pounds (significantly more than the typical gain of 15 pounds, often referred to as the “Quarantine 15” or “COVID-15”).
Updated data, obtained via a review of electronic health records for over 15 million patients, shows that 39% of patients gained weight during the pandemic (10% of them gained more than 12.5 pounds, while 2% gained over 27.5 pounds). Though these recent numbers may be lower than previously reported, they still aren’t reassuring.
Research has already confirmed that sizeism has a negative impact on both a patient’s physical health and psychological well-being, and as medical providers, we’re part of the problem. We cause distress in our patients through disrespectful treatment and medical fat shaming, which can lead to cycles of disordered eating, reduced physical activity, and more weight gain. We discriminate based on weight, causing our patients to delay health care visits and other provider interactions, resulting in increased risks for morbidity and even mortality. We make assumptions that a patient’s presenting complaints are due to weight rather than other causes, resulting in missed diagnoses. And we recommend different treatments for obese patients with the same condition as nonobese patients simply because of their weight.
One study has suggested that over 40% of adults in the United States have suffered from weight stigma, and physicians and coworkers are listed as some of the most common sources. Another study suggests that nearly 70% of overweight or obese patients report feeling stigmatized by physicians, whether through expressed biases or purposeful avoidance (patients have previously reported that their providers addressed weight loss in fewer than 20% of their examinations).
As health care providers, we need to do better. We should all be willing to consider our own biases about body size, and there are self-assessments to help with this, including the Implicit Associations Test: Weight Bias. By becoming more self-aware, hopefully we can change the doctor-patient conversation about weight management.
Studies have shown that meaningful conversations with physicians can have a significant impact on patients’ attempts to change behaviors related to weight. Yet, many medical providers are not trained in how to counsel patients on nutrition, weight loss, and physical activity (if we bring it up at all). We need to better educate ourselves about weight science and treatments.
In the meantime, we can work on how we interact with our patients:
- Make sure that your practice space is accommodating and nondiscriminatory, with appropriately sized furniture in the waiting and exam rooms, large blood pressure cuffs and gowns, and size-inclusive reading materials.
- Ensure that your workplace has an antiharassment policy that includes sizeism.
- Be an ally and speak up against weight discrimination.
- Educate your office staff about weight stigma and ensure that they avoid commenting on the weight or body size of others (being recognized only for losing weight isn’t a compliment, and sharing “fat jokes” isn’t funny).
- Remember that a person’s body size tells you nothing about that person’s health behaviors. Stop assuming that larger body sizes are related to laziness, overeating, or a lack of motivation.
- Ask your overweight or obese patients if they are willing to talk about their weight before jumping into the topic.
- Practice (patients are more likely to report changing their exercise routine and attempting to lose weight with these techniques).
- Be mindful of your word choices; for example, it can be more helpful to focus on comorbidities (such as high blood pressure or prediabetes) rather than body weight, nutrition rather than dieting, and physical activity rather than specific exercises.
Regardless of how you feel about reciting the Hippocratic Oath, our patients, no matter their body size, deserve to be treated with respect and dignity, as others have said in more eloquent ways than I. Let’s stop playing the fat shame game and help fight weight bias in medicine.
Dr. Devlin is president, Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I will remember that there is art to medicine as well as science, and that warmth, sympathy, and understanding may outweigh the surgeon’s knife or the chemist’s drug.
Upon finishing medical school, many of us recited this passage from a modernized version of the Hippocratic Oath. Though there has been controversy regarding the current relevancy of this oath, it can still serve as a reminder of the promises we made on behalf of our patients: To treat them ethically, with empathy and respect, and without pretension. Though I hadn’t thought about the Hippocratic Oath in ages, it came to mind recently after I read an article about weight trends in adults during the COVID pandemic.
No surprise – we gained weight during the initial surge at a rate of roughly a pound and a half per month following the initial shelter-in-place period. For some of us, that trend in weight gain worsened as the pandemic persisted. A survey conducted in February 2021 suggested that over 40% of adults who experienced undesired weight changes since the start of the pandemic gained an average of 29 pounds (significantly more than the typical gain of 15 pounds, often referred to as the “Quarantine 15” or “COVID-15”).
Updated data, obtained via a review of electronic health records for over 15 million patients, shows that 39% of patients gained weight during the pandemic (10% of them gained more than 12.5 pounds, while 2% gained over 27.5 pounds). Though these recent numbers may be lower than previously reported, they still aren’t reassuring.
Research has already confirmed that sizeism has a negative impact on both a patient’s physical health and psychological well-being, and as medical providers, we’re part of the problem. We cause distress in our patients through disrespectful treatment and medical fat shaming, which can lead to cycles of disordered eating, reduced physical activity, and more weight gain. We discriminate based on weight, causing our patients to delay health care visits and other provider interactions, resulting in increased risks for morbidity and even mortality. We make assumptions that a patient’s presenting complaints are due to weight rather than other causes, resulting in missed diagnoses. And we recommend different treatments for obese patients with the same condition as nonobese patients simply because of their weight.
One study has suggested that over 40% of adults in the United States have suffered from weight stigma, and physicians and coworkers are listed as some of the most common sources. Another study suggests that nearly 70% of overweight or obese patients report feeling stigmatized by physicians, whether through expressed biases or purposeful avoidance (patients have previously reported that their providers addressed weight loss in fewer than 20% of their examinations).
As health care providers, we need to do better. We should all be willing to consider our own biases about body size, and there are self-assessments to help with this, including the Implicit Associations Test: Weight Bias. By becoming more self-aware, hopefully we can change the doctor-patient conversation about weight management.
Studies have shown that meaningful conversations with physicians can have a significant impact on patients’ attempts to change behaviors related to weight. Yet, many medical providers are not trained in how to counsel patients on nutrition, weight loss, and physical activity (if we bring it up at all). We need to better educate ourselves about weight science and treatments.
In the meantime, we can work on how we interact with our patients:
- Make sure that your practice space is accommodating and nondiscriminatory, with appropriately sized furniture in the waiting and exam rooms, large blood pressure cuffs and gowns, and size-inclusive reading materials.
- Ensure that your workplace has an antiharassment policy that includes sizeism.
- Be an ally and speak up against weight discrimination.
- Educate your office staff about weight stigma and ensure that they avoid commenting on the weight or body size of others (being recognized only for losing weight isn’t a compliment, and sharing “fat jokes” isn’t funny).
- Remember that a person’s body size tells you nothing about that person’s health behaviors. Stop assuming that larger body sizes are related to laziness, overeating, or a lack of motivation.
- Ask your overweight or obese patients if they are willing to talk about their weight before jumping into the topic.
- Practice (patients are more likely to report changing their exercise routine and attempting to lose weight with these techniques).
- Be mindful of your word choices; for example, it can be more helpful to focus on comorbidities (such as high blood pressure or prediabetes) rather than body weight, nutrition rather than dieting, and physical activity rather than specific exercises.
Regardless of how you feel about reciting the Hippocratic Oath, our patients, no matter their body size, deserve to be treated with respect and dignity, as others have said in more eloquent ways than I. Let’s stop playing the fat shame game and help fight weight bias in medicine.
Dr. Devlin is president, Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I will remember that there is art to medicine as well as science, and that warmth, sympathy, and understanding may outweigh the surgeon’s knife or the chemist’s drug.
Upon finishing medical school, many of us recited this passage from a modernized version of the Hippocratic Oath. Though there has been controversy regarding the current relevancy of this oath, it can still serve as a reminder of the promises we made on behalf of our patients: To treat them ethically, with empathy and respect, and without pretension. Though I hadn’t thought about the Hippocratic Oath in ages, it came to mind recently after I read an article about weight trends in adults during the COVID pandemic.
No surprise – we gained weight during the initial surge at a rate of roughly a pound and a half per month following the initial shelter-in-place period. For some of us, that trend in weight gain worsened as the pandemic persisted. A survey conducted in February 2021 suggested that over 40% of adults who experienced undesired weight changes since the start of the pandemic gained an average of 29 pounds (significantly more than the typical gain of 15 pounds, often referred to as the “Quarantine 15” or “COVID-15”).
Updated data, obtained via a review of electronic health records for over 15 million patients, shows that 39% of patients gained weight during the pandemic (10% of them gained more than 12.5 pounds, while 2% gained over 27.5 pounds). Though these recent numbers may be lower than previously reported, they still aren’t reassuring.
Research has already confirmed that sizeism has a negative impact on both a patient’s physical health and psychological well-being, and as medical providers, we’re part of the problem. We cause distress in our patients through disrespectful treatment and medical fat shaming, which can lead to cycles of disordered eating, reduced physical activity, and more weight gain. We discriminate based on weight, causing our patients to delay health care visits and other provider interactions, resulting in increased risks for morbidity and even mortality. We make assumptions that a patient’s presenting complaints are due to weight rather than other causes, resulting in missed diagnoses. And we recommend different treatments for obese patients with the same condition as nonobese patients simply because of their weight.
One study has suggested that over 40% of adults in the United States have suffered from weight stigma, and physicians and coworkers are listed as some of the most common sources. Another study suggests that nearly 70% of overweight or obese patients report feeling stigmatized by physicians, whether through expressed biases or purposeful avoidance (patients have previously reported that their providers addressed weight loss in fewer than 20% of their examinations).
As health care providers, we need to do better. We should all be willing to consider our own biases about body size, and there are self-assessments to help with this, including the Implicit Associations Test: Weight Bias. By becoming more self-aware, hopefully we can change the doctor-patient conversation about weight management.
Studies have shown that meaningful conversations with physicians can have a significant impact on patients’ attempts to change behaviors related to weight. Yet, many medical providers are not trained in how to counsel patients on nutrition, weight loss, and physical activity (if we bring it up at all). We need to better educate ourselves about weight science and treatments.
In the meantime, we can work on how we interact with our patients:
- Make sure that your practice space is accommodating and nondiscriminatory, with appropriately sized furniture in the waiting and exam rooms, large blood pressure cuffs and gowns, and size-inclusive reading materials.
- Ensure that your workplace has an antiharassment policy that includes sizeism.
- Be an ally and speak up against weight discrimination.
- Educate your office staff about weight stigma and ensure that they avoid commenting on the weight or body size of others (being recognized only for losing weight isn’t a compliment, and sharing “fat jokes” isn’t funny).
- Remember that a person’s body size tells you nothing about that person’s health behaviors. Stop assuming that larger body sizes are related to laziness, overeating, or a lack of motivation.
- Ask your overweight or obese patients if they are willing to talk about their weight before jumping into the topic.
- Practice (patients are more likely to report changing their exercise routine and attempting to lose weight with these techniques).
- Be mindful of your word choices; for example, it can be more helpful to focus on comorbidities (such as high blood pressure or prediabetes) rather than body weight, nutrition rather than dieting, and physical activity rather than specific exercises.
Regardless of how you feel about reciting the Hippocratic Oath, our patients, no matter their body size, deserve to be treated with respect and dignity, as others have said in more eloquent ways than I. Let’s stop playing the fat shame game and help fight weight bias in medicine.
Dr. Devlin is president, Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Support for Policy Changes for Therapy Related to Homefront Missions
Recent natural disasters, civil disorder, and the COVID-19 pandemic response created an unprecedented demand for the US National Guard and Reserve components as well as active-duty personnel to serve on homefront missions critical to our nation. At times, those serving in these capacities are front and center to the most tragic events confronting our nation, and they frequently encounter tremendous suffering.
Recognizing the potential for these missions to create psychological sequela for those who serve on them, the authority for the Veterans Health Administration (VHA) vet centers to provide readjustment counseling services was broadened on December 30, 2021. Vet centers are community-based counseling centers that have traditionally served combat veterans, and broadening services reflects a major change in mission. Revised VHA Directive 1500(2) specifies that those who “served on active duty in response to a national emergency or major disaster declared by the President” or “served on active duty in the National Guard of a State under orders of the chief executive of that State in response to a disaster or civil disorder in such State” may now receive therapy at vet centers.1,2
As a result of this recent policy change, National Guard and active-duty Reserve service members now have parity with combat veterans to obtain therapy for symptoms arising as a result of their activation for service on homefront missions. As they seek care, we need to be ready so that these service members can obtain the best therapy services possible. Soldiers who served on homefront missions comprise a new cohort of service members now eligible for vet center therapy. Soldiers who served on homefront missions may present with issues that differ from those of combat veterans and veterans who have experienced military sexual trauma (MST), the populations treated by vet centers and other VHA mental health care clinics prior to this broadened authority. This article highlights some suggestions for service delivery to best meet the needs of this population.
Discussion
Available evidence-based therapies to treat posttraumatic stress disorder (PTSD) are effective regardless of whether the trauma occurred in combat, on the homefront, or in a civilian setting. The vet centers and VHA mental health services already have staff trained to deliver these therapy modalities and, in this sense, are ready to provide trauma-focused therapy treatment to soldiers with PTSD who served on homefront missions.
The broadened authority for the vet centers to provide readjustment services is necessary, as it corrects for a critical gap in services, but the importance of ensuring adequate staffing to meet the expected increased demand for services cannot be underscored. According to clinical practice guidelines for the treatment of PTSD, developed by the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD), the therapies with the strongest evidence-based backing are prolonged exposure-based therapy (PE), cognitive processing therapy (CPT), and eye movement and desensitization reprocessing (EMDR).3 These therapy modalities, based on findings from clinical trials, are predicated on seeing a client for a sufficient number of sessions. Attendance at these sessions is recommended at least weekly to ensure adequate intensity of service delivery.4-7 According to the National Center for PTSD, PE typically involves 8 to 15 weekly or twice weekly sessions; CPT requires 8 to 14 or more weekly sessions, and EMDR is usually 4 to 12 weekly sessions.4-7
Ensuring adequate staffing is critical to offer these therapies at least weekly as the efficacies of these therapies are otherwise not proven if return session visits are stretched out over multiple weeks or months. The most recent clinical research has demonstrated that PTSD recovery can be expedited and there are lower patient dropout rates when sessions are massed or compressed so that multiple sessions are administered over 1 week.8-12 Providing these therapies in a massed format has shown to be as effective as when these therapies are provided weekly.
As the authority to treat soldiers serving on homefront missions is new, epidemiologic data do not yet exist to estimate the proportion of this population who will need treatment or present with PTSD, depression, anxiety, a substance use disorder, and/or comorbid conditions. Those with PTSD can benefit from PTSD evidence-based therapies already available for treatment. Others may benefit from treatments that are proven effective for their mental health diagnoses.
Therapists with experience primarily treating patients with PTSD related to combat or MST will need to be sensitive to the unique experiences of the National Guard and Reserve service members. For example, this component of soldiers served on COVID-19–related missions that provided food service support to nursing homes residents who were locked down from family members. As a result, they developed bonds with residents who later died. This may have been the first time that these soldiers witnessed death. If such a soldier is assessed and does not have PTSD but is nonetheless distressed, then the soldier may need alternate therapies, such as grief counseling. This need may be more pronounced for those soldiers who lost loved ones to COVID-19 while they served on these missions.
New Jersey Army National Guard soldiers provided food service support at the Woodland Behavioral and Nursing Center in Andover, New Jersey. These soldiers witnessed the unfortunate conditions in this facility, which included stacked bodies in a makeshift morgue during the height of the pandemic; however, they did not have the ability to make changes. The facility is under investigation for abuse and neglect of its residents.13
New Jersey National Guard soldiers supporting that facility and similar ones may have experienced moral injury, defined as “…perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”14 Importantly, when these soldiers present for therapy and express moral injury, their therapists need to be open to spiritual discourse. However, vet centers do not have chaplains on staff, so therapists must refer patients to chaplaincy services.
Among therapists with existing cultural competency for treating members of the military, some nuances exist for National Guard and Reserve service members. National Guard and Reserve component personnel already may feel that their problems are less important than those experienced by active-duty service members. Now that these soldiers have the eligibility to receive therapy, therapists may have to make extra efforts to both reassure this population that they are welcomed and to validate their need for services.
Special outreach efforts to those who served on historical National Guard and active-duty Reserve missions are a way to show good faith in serving these soldiers because they may have untreated PTSD or other undiagnosed mental health disorders related to earlier deployments, such as hurricane recovery missions. A study of disaster survivors found that the prevalence rate of severe and very severe psychological impact after a natural disaster was about 34%.15 Another epidemiologic study found that the prevalence rate of PTSD was 10% to 20% among disaster rescue workers.16 Specific data about the psychological problems of National Guard and Reserve components serving in disaster recovery are unavailable but is an area for future research.
Therapists who have treated active-duty service members and veterans who worked in mortuary services in a combat zone are used to hearing graphic details of horrifying scenes, but homefront experiences are different. Soldiers on homefront mortuary-based missions frequently reported being unable to forget the faces or the smell of dead bodies as they were stacked up and overwhelming the systems. Experienced vet center therapists should be prepared for the challenges in treating this new cohort of patients.
Conclusions
Now that National Guard and Reserve component soldiers who have responded to national and local emergencies are eligible for therapy, we need to be prepared to provide these services. In addition to addressing systemic staffing concerns, therapists need to be aware of the unique challenges faced by those who have served on homefront missions. These homefront missions have the potential to hit home for therapists.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1550(2): readjustment counseling service. January 26, 2021. Accessed September 1, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
2. US Department of Veterans Affairs. Vet centers (readjustment counseling: vet center eligibility. Updated January 3, 2022. Accessed September 1, 2022. https://www.vetcenter.va.gov/eligibility.asp
3. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction, version 3.0, 2017. Accessed September 1, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
4. US Department of Veterans Affairs, National Center for PTSD. Prolonged exposure (PE) therapy. Updated August 10, 2022. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/prolonged_exposure.asp
5. US Department of Veterans Affairs, National Center for PTSD. Cognitive processing therapy (CPT) for PTSD: how to help your loved one during treatment. Accessed September 1, 2022. https://www.ptsd.va.gov/publications/print/CPT_familyhandout.pdf
6. US Department of Veterans Affairs, National Center for PTSD. A provider’s guide to brief cognitive behavioral therapy. Accessed September 1, 2022. https://www.mirecc.va.gov/visn16/docs/Therapists_Guide_to_Brief_CBTManual.pdf
7. US Department of Veterans Affairs, National Center for PTSD. Eye movement desensitization and reprocessing (EMDR) for PTSD. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/emdr.asp
8. Wachen JS, Dondanville KA, Evans WR, Morris K, Cole A. Adjusting the timeframe of evidence-based therapies for PTSD-massed treatments. Curr Treat Options Psych. 2019;6(2):107-118. doi:10.1007/s40501-019-00169-9
9. Dell L, Sbisa AM, Forbes A, et al. Effect of massed v. standard prolonged exposure therapy on PTSD in military personnel and veterans: a non-inferiority randomised controlled trial [published online ahead of print, 2022 Apr 20]. Psychol Med. 2022;1-8. doi:10.1017/S0033291722000927
10. Held P, Kovacevic M, Petrey K, et al. Treating posttraumatic stress disorder at home in a single week using 1-week virtual massed cognitive processing therapy. J Trauma Stress. 2022;35(4):1215-1225. doi:10.1002/jts.22831
11. Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration [published online ahead of print, 2022 Mar 7]. Psychol Serv. 2022;10.1037/ser0000628. doi:10.1037/ser0000628
12. Galovski TE, Werner KB, Weaver TL, et al. Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 2022;14(5):769-779. doi:10.1037/tra0001100
13. Fallon S. NJ to send monitors into troubled nursing home that stacked bodies in makeshift morgue. Updated March 10, 2022. Accessed September 1, 2022. https://www.northjersey.com/story/news/health/2022/03/09/sussex-county-nj-nursing-home-monitors-covid-morgue/9447243002/
14. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003009
15. Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207-239. doi:10.1521/psyc.65.3.207.20173
16. Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78-91. doi:10.1093/epirev/mxi003
Recent natural disasters, civil disorder, and the COVID-19 pandemic response created an unprecedented demand for the US National Guard and Reserve components as well as active-duty personnel to serve on homefront missions critical to our nation. At times, those serving in these capacities are front and center to the most tragic events confronting our nation, and they frequently encounter tremendous suffering.
Recognizing the potential for these missions to create psychological sequela for those who serve on them, the authority for the Veterans Health Administration (VHA) vet centers to provide readjustment counseling services was broadened on December 30, 2021. Vet centers are community-based counseling centers that have traditionally served combat veterans, and broadening services reflects a major change in mission. Revised VHA Directive 1500(2) specifies that those who “served on active duty in response to a national emergency or major disaster declared by the President” or “served on active duty in the National Guard of a State under orders of the chief executive of that State in response to a disaster or civil disorder in such State” may now receive therapy at vet centers.1,2
As a result of this recent policy change, National Guard and active-duty Reserve service members now have parity with combat veterans to obtain therapy for symptoms arising as a result of their activation for service on homefront missions. As they seek care, we need to be ready so that these service members can obtain the best therapy services possible. Soldiers who served on homefront missions comprise a new cohort of service members now eligible for vet center therapy. Soldiers who served on homefront missions may present with issues that differ from those of combat veterans and veterans who have experienced military sexual trauma (MST), the populations treated by vet centers and other VHA mental health care clinics prior to this broadened authority. This article highlights some suggestions for service delivery to best meet the needs of this population.
Discussion
Available evidence-based therapies to treat posttraumatic stress disorder (PTSD) are effective regardless of whether the trauma occurred in combat, on the homefront, or in a civilian setting. The vet centers and VHA mental health services already have staff trained to deliver these therapy modalities and, in this sense, are ready to provide trauma-focused therapy treatment to soldiers with PTSD who served on homefront missions.
The broadened authority for the vet centers to provide readjustment services is necessary, as it corrects for a critical gap in services, but the importance of ensuring adequate staffing to meet the expected increased demand for services cannot be underscored. According to clinical practice guidelines for the treatment of PTSD, developed by the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD), the therapies with the strongest evidence-based backing are prolonged exposure-based therapy (PE), cognitive processing therapy (CPT), and eye movement and desensitization reprocessing (EMDR).3 These therapy modalities, based on findings from clinical trials, are predicated on seeing a client for a sufficient number of sessions. Attendance at these sessions is recommended at least weekly to ensure adequate intensity of service delivery.4-7 According to the National Center for PTSD, PE typically involves 8 to 15 weekly or twice weekly sessions; CPT requires 8 to 14 or more weekly sessions, and EMDR is usually 4 to 12 weekly sessions.4-7
Ensuring adequate staffing is critical to offer these therapies at least weekly as the efficacies of these therapies are otherwise not proven if return session visits are stretched out over multiple weeks or months. The most recent clinical research has demonstrated that PTSD recovery can be expedited and there are lower patient dropout rates when sessions are massed or compressed so that multiple sessions are administered over 1 week.8-12 Providing these therapies in a massed format has shown to be as effective as when these therapies are provided weekly.
As the authority to treat soldiers serving on homefront missions is new, epidemiologic data do not yet exist to estimate the proportion of this population who will need treatment or present with PTSD, depression, anxiety, a substance use disorder, and/or comorbid conditions. Those with PTSD can benefit from PTSD evidence-based therapies already available for treatment. Others may benefit from treatments that are proven effective for their mental health diagnoses.
Therapists with experience primarily treating patients with PTSD related to combat or MST will need to be sensitive to the unique experiences of the National Guard and Reserve service members. For example, this component of soldiers served on COVID-19–related missions that provided food service support to nursing homes residents who were locked down from family members. As a result, they developed bonds with residents who later died. This may have been the first time that these soldiers witnessed death. If such a soldier is assessed and does not have PTSD but is nonetheless distressed, then the soldier may need alternate therapies, such as grief counseling. This need may be more pronounced for those soldiers who lost loved ones to COVID-19 while they served on these missions.
New Jersey Army National Guard soldiers provided food service support at the Woodland Behavioral and Nursing Center in Andover, New Jersey. These soldiers witnessed the unfortunate conditions in this facility, which included stacked bodies in a makeshift morgue during the height of the pandemic; however, they did not have the ability to make changes. The facility is under investigation for abuse and neglect of its residents.13
New Jersey National Guard soldiers supporting that facility and similar ones may have experienced moral injury, defined as “…perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”14 Importantly, when these soldiers present for therapy and express moral injury, their therapists need to be open to spiritual discourse. However, vet centers do not have chaplains on staff, so therapists must refer patients to chaplaincy services.
Among therapists with existing cultural competency for treating members of the military, some nuances exist for National Guard and Reserve service members. National Guard and Reserve component personnel already may feel that their problems are less important than those experienced by active-duty service members. Now that these soldiers have the eligibility to receive therapy, therapists may have to make extra efforts to both reassure this population that they are welcomed and to validate their need for services.
Special outreach efforts to those who served on historical National Guard and active-duty Reserve missions are a way to show good faith in serving these soldiers because they may have untreated PTSD or other undiagnosed mental health disorders related to earlier deployments, such as hurricane recovery missions. A study of disaster survivors found that the prevalence rate of severe and very severe psychological impact after a natural disaster was about 34%.15 Another epidemiologic study found that the prevalence rate of PTSD was 10% to 20% among disaster rescue workers.16 Specific data about the psychological problems of National Guard and Reserve components serving in disaster recovery are unavailable but is an area for future research.
Therapists who have treated active-duty service members and veterans who worked in mortuary services in a combat zone are used to hearing graphic details of horrifying scenes, but homefront experiences are different. Soldiers on homefront mortuary-based missions frequently reported being unable to forget the faces or the smell of dead bodies as they were stacked up and overwhelming the systems. Experienced vet center therapists should be prepared for the challenges in treating this new cohort of patients.
Conclusions
Now that National Guard and Reserve component soldiers who have responded to national and local emergencies are eligible for therapy, we need to be prepared to provide these services. In addition to addressing systemic staffing concerns, therapists need to be aware of the unique challenges faced by those who have served on homefront missions. These homefront missions have the potential to hit home for therapists.
Recent natural disasters, civil disorder, and the COVID-19 pandemic response created an unprecedented demand for the US National Guard and Reserve components as well as active-duty personnel to serve on homefront missions critical to our nation. At times, those serving in these capacities are front and center to the most tragic events confronting our nation, and they frequently encounter tremendous suffering.
Recognizing the potential for these missions to create psychological sequela for those who serve on them, the authority for the Veterans Health Administration (VHA) vet centers to provide readjustment counseling services was broadened on December 30, 2021. Vet centers are community-based counseling centers that have traditionally served combat veterans, and broadening services reflects a major change in mission. Revised VHA Directive 1500(2) specifies that those who “served on active duty in response to a national emergency or major disaster declared by the President” or “served on active duty in the National Guard of a State under orders of the chief executive of that State in response to a disaster or civil disorder in such State” may now receive therapy at vet centers.1,2
As a result of this recent policy change, National Guard and active-duty Reserve service members now have parity with combat veterans to obtain therapy for symptoms arising as a result of their activation for service on homefront missions. As they seek care, we need to be ready so that these service members can obtain the best therapy services possible. Soldiers who served on homefront missions comprise a new cohort of service members now eligible for vet center therapy. Soldiers who served on homefront missions may present with issues that differ from those of combat veterans and veterans who have experienced military sexual trauma (MST), the populations treated by vet centers and other VHA mental health care clinics prior to this broadened authority. This article highlights some suggestions for service delivery to best meet the needs of this population.
Discussion
Available evidence-based therapies to treat posttraumatic stress disorder (PTSD) are effective regardless of whether the trauma occurred in combat, on the homefront, or in a civilian setting. The vet centers and VHA mental health services already have staff trained to deliver these therapy modalities and, in this sense, are ready to provide trauma-focused therapy treatment to soldiers with PTSD who served on homefront missions.
The broadened authority for the vet centers to provide readjustment services is necessary, as it corrects for a critical gap in services, but the importance of ensuring adequate staffing to meet the expected increased demand for services cannot be underscored. According to clinical practice guidelines for the treatment of PTSD, developed by the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD), the therapies with the strongest evidence-based backing are prolonged exposure-based therapy (PE), cognitive processing therapy (CPT), and eye movement and desensitization reprocessing (EMDR).3 These therapy modalities, based on findings from clinical trials, are predicated on seeing a client for a sufficient number of sessions. Attendance at these sessions is recommended at least weekly to ensure adequate intensity of service delivery.4-7 According to the National Center for PTSD, PE typically involves 8 to 15 weekly or twice weekly sessions; CPT requires 8 to 14 or more weekly sessions, and EMDR is usually 4 to 12 weekly sessions.4-7
Ensuring adequate staffing is critical to offer these therapies at least weekly as the efficacies of these therapies are otherwise not proven if return session visits are stretched out over multiple weeks or months. The most recent clinical research has demonstrated that PTSD recovery can be expedited and there are lower patient dropout rates when sessions are massed or compressed so that multiple sessions are administered over 1 week.8-12 Providing these therapies in a massed format has shown to be as effective as when these therapies are provided weekly.
As the authority to treat soldiers serving on homefront missions is new, epidemiologic data do not yet exist to estimate the proportion of this population who will need treatment or present with PTSD, depression, anxiety, a substance use disorder, and/or comorbid conditions. Those with PTSD can benefit from PTSD evidence-based therapies already available for treatment. Others may benefit from treatments that are proven effective for their mental health diagnoses.
Therapists with experience primarily treating patients with PTSD related to combat or MST will need to be sensitive to the unique experiences of the National Guard and Reserve service members. For example, this component of soldiers served on COVID-19–related missions that provided food service support to nursing homes residents who were locked down from family members. As a result, they developed bonds with residents who later died. This may have been the first time that these soldiers witnessed death. If such a soldier is assessed and does not have PTSD but is nonetheless distressed, then the soldier may need alternate therapies, such as grief counseling. This need may be more pronounced for those soldiers who lost loved ones to COVID-19 while they served on these missions.
New Jersey Army National Guard soldiers provided food service support at the Woodland Behavioral and Nursing Center in Andover, New Jersey. These soldiers witnessed the unfortunate conditions in this facility, which included stacked bodies in a makeshift morgue during the height of the pandemic; however, they did not have the ability to make changes. The facility is under investigation for abuse and neglect of its residents.13
New Jersey National Guard soldiers supporting that facility and similar ones may have experienced moral injury, defined as “…perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”14 Importantly, when these soldiers present for therapy and express moral injury, their therapists need to be open to spiritual discourse. However, vet centers do not have chaplains on staff, so therapists must refer patients to chaplaincy services.
Among therapists with existing cultural competency for treating members of the military, some nuances exist for National Guard and Reserve service members. National Guard and Reserve component personnel already may feel that their problems are less important than those experienced by active-duty service members. Now that these soldiers have the eligibility to receive therapy, therapists may have to make extra efforts to both reassure this population that they are welcomed and to validate their need for services.
Special outreach efforts to those who served on historical National Guard and active-duty Reserve missions are a way to show good faith in serving these soldiers because they may have untreated PTSD or other undiagnosed mental health disorders related to earlier deployments, such as hurricane recovery missions. A study of disaster survivors found that the prevalence rate of severe and very severe psychological impact after a natural disaster was about 34%.15 Another epidemiologic study found that the prevalence rate of PTSD was 10% to 20% among disaster rescue workers.16 Specific data about the psychological problems of National Guard and Reserve components serving in disaster recovery are unavailable but is an area for future research.
Therapists who have treated active-duty service members and veterans who worked in mortuary services in a combat zone are used to hearing graphic details of horrifying scenes, but homefront experiences are different. Soldiers on homefront mortuary-based missions frequently reported being unable to forget the faces or the smell of dead bodies as they were stacked up and overwhelming the systems. Experienced vet center therapists should be prepared for the challenges in treating this new cohort of patients.
Conclusions
Now that National Guard and Reserve component soldiers who have responded to national and local emergencies are eligible for therapy, we need to be prepared to provide these services. In addition to addressing systemic staffing concerns, therapists need to be aware of the unique challenges faced by those who have served on homefront missions. These homefront missions have the potential to hit home for therapists.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1550(2): readjustment counseling service. January 26, 2021. Accessed September 1, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
2. US Department of Veterans Affairs. Vet centers (readjustment counseling: vet center eligibility. Updated January 3, 2022. Accessed September 1, 2022. https://www.vetcenter.va.gov/eligibility.asp
3. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction, version 3.0, 2017. Accessed September 1, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
4. US Department of Veterans Affairs, National Center for PTSD. Prolonged exposure (PE) therapy. Updated August 10, 2022. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/prolonged_exposure.asp
5. US Department of Veterans Affairs, National Center for PTSD. Cognitive processing therapy (CPT) for PTSD: how to help your loved one during treatment. Accessed September 1, 2022. https://www.ptsd.va.gov/publications/print/CPT_familyhandout.pdf
6. US Department of Veterans Affairs, National Center for PTSD. A provider’s guide to brief cognitive behavioral therapy. Accessed September 1, 2022. https://www.mirecc.va.gov/visn16/docs/Therapists_Guide_to_Brief_CBTManual.pdf
7. US Department of Veterans Affairs, National Center for PTSD. Eye movement desensitization and reprocessing (EMDR) for PTSD. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/emdr.asp
8. Wachen JS, Dondanville KA, Evans WR, Morris K, Cole A. Adjusting the timeframe of evidence-based therapies for PTSD-massed treatments. Curr Treat Options Psych. 2019;6(2):107-118. doi:10.1007/s40501-019-00169-9
9. Dell L, Sbisa AM, Forbes A, et al. Effect of massed v. standard prolonged exposure therapy on PTSD in military personnel and veterans: a non-inferiority randomised controlled trial [published online ahead of print, 2022 Apr 20]. Psychol Med. 2022;1-8. doi:10.1017/S0033291722000927
10. Held P, Kovacevic M, Petrey K, et al. Treating posttraumatic stress disorder at home in a single week using 1-week virtual massed cognitive processing therapy. J Trauma Stress. 2022;35(4):1215-1225. doi:10.1002/jts.22831
11. Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration [published online ahead of print, 2022 Mar 7]. Psychol Serv. 2022;10.1037/ser0000628. doi:10.1037/ser0000628
12. Galovski TE, Werner KB, Weaver TL, et al. Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 2022;14(5):769-779. doi:10.1037/tra0001100
13. Fallon S. NJ to send monitors into troubled nursing home that stacked bodies in makeshift morgue. Updated March 10, 2022. Accessed September 1, 2022. https://www.northjersey.com/story/news/health/2022/03/09/sussex-county-nj-nursing-home-monitors-covid-morgue/9447243002/
14. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003009
15. Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207-239. doi:10.1521/psyc.65.3.207.20173
16. Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78-91. doi:10.1093/epirev/mxi003
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1550(2): readjustment counseling service. January 26, 2021. Accessed September 1, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
2. US Department of Veterans Affairs. Vet centers (readjustment counseling: vet center eligibility. Updated January 3, 2022. Accessed September 1, 2022. https://www.vetcenter.va.gov/eligibility.asp
3. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction, version 3.0, 2017. Accessed September 1, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
4. US Department of Veterans Affairs, National Center for PTSD. Prolonged exposure (PE) therapy. Updated August 10, 2022. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/prolonged_exposure.asp
5. US Department of Veterans Affairs, National Center for PTSD. Cognitive processing therapy (CPT) for PTSD: how to help your loved one during treatment. Accessed September 1, 2022. https://www.ptsd.va.gov/publications/print/CPT_familyhandout.pdf
6. US Department of Veterans Affairs, National Center for PTSD. A provider’s guide to brief cognitive behavioral therapy. Accessed September 1, 2022. https://www.mirecc.va.gov/visn16/docs/Therapists_Guide_to_Brief_CBTManual.pdf
7. US Department of Veterans Affairs, National Center for PTSD. Eye movement desensitization and reprocessing (EMDR) for PTSD. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/emdr.asp
8. Wachen JS, Dondanville KA, Evans WR, Morris K, Cole A. Adjusting the timeframe of evidence-based therapies for PTSD-massed treatments. Curr Treat Options Psych. 2019;6(2):107-118. doi:10.1007/s40501-019-00169-9
9. Dell L, Sbisa AM, Forbes A, et al. Effect of massed v. standard prolonged exposure therapy on PTSD in military personnel and veterans: a non-inferiority randomised controlled trial [published online ahead of print, 2022 Apr 20]. Psychol Med. 2022;1-8. doi:10.1017/S0033291722000927
10. Held P, Kovacevic M, Petrey K, et al. Treating posttraumatic stress disorder at home in a single week using 1-week virtual massed cognitive processing therapy. J Trauma Stress. 2022;35(4):1215-1225. doi:10.1002/jts.22831
11. Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration [published online ahead of print, 2022 Mar 7]. Psychol Serv. 2022;10.1037/ser0000628. doi:10.1037/ser0000628
12. Galovski TE, Werner KB, Weaver TL, et al. Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 2022;14(5):769-779. doi:10.1037/tra0001100
13. Fallon S. NJ to send monitors into troubled nursing home that stacked bodies in makeshift morgue. Updated March 10, 2022. Accessed September 1, 2022. https://www.northjersey.com/story/news/health/2022/03/09/sussex-county-nj-nursing-home-monitors-covid-morgue/9447243002/
14. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003009
15. Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207-239. doi:10.1521/psyc.65.3.207.20173
16. Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78-91. doi:10.1093/epirev/mxi003
Congenital syphilis: It’s still a significant public health problem
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
You’re rounding in the nursery and informed of the following about one of your new patients: He’s a 38-week-old infant delivered to a mother diagnosed with syphilis at 12 weeks’ gestation at her initial prenatal visit. Her rapid plasma reagin (RPR) was 1:64 and the fluorescent treponemal antibody–absorption (FTA-ABS) test was positive. By report she was appropriately treated. Maternal RPRs obtained at 18 and 28 weeks’ gestation were 1:16 and 1:4, respectively. Maternal RPR at delivery and the infant’s RPR obtained shortly after birth were both 1:4. The mother wants to know if her baby is infected.
One result of syphilis during pregnancy is intrauterine infection and resultant congenital disease in the infant. Before you answer this mother, let’s discuss syphilis.
Congenital syphilis is a significant public health problem. In 2021, there were a total of 2,677 cases reported for a rate of 74.1 per 100,000 live births. Between 2020 and 2021, the number of cases of congenital syphilis increased 24.1% (2,158-2,677 cases), concurrent with a 45.8% increase (10.7-15.6 per 100,000) in the rate of primary and secondary syphilis in women aged 15-44 years. Between 2012 and 2021, the number of cases of congenital syphilis increased 701.5% (334-2,677 cases) and the increase in rates of primary and secondary syphilis in women aged 15-44 was 642.9% over the same period.
Why are the rates of congenital syphilis increasing? Most cases result from a lack of prenatal care and thus no testing for syphilis. The next most common cause is inadequate maternal treatment.
Congenital syphilis usually is acquired through transplacental transmission of spirochetes in the maternal bloodstream. Occasionally, it occurs at delivery via direct contact with maternal lesions. It is not transmitted in breast milk. Transmission of syphilis:
- Can occur any time during pregnancy.
- Is more likely to occur in women with untreated primary or secondary disease (60%-100%).
- Is approximately 40% in those with early latent syphilis and less than 8% in mothers with late latent syphilis.
- Is higher in women coinfected with HIV since they more frequently receive no prenatal care and their disease is inadequately treated.
Coinfection with syphilis may also increase the rate of mother-to-child transmission of HIV.
Untreated early syphilis during pregnancy results in spontaneous abortion, stillbirth, or perinatal death in up to 40% of cases. Infected newborns with early congenital syphilis can be asymptomatic or have evidence of hepatosplenomegaly, generalized lymphadenopathy, nasal discharge that is occasionally bloody, rash, and skeletal abnormalities (osteochondritis and periostitis). Other manifestations include edema, hemolytic anemia, jaundice, pneumonia, pseudoparalysis, and thrombocytopenia. Asymptomatic infants may have abnormal cerebrospinal fluid findings including elevated CSF white cell count, elevated protein, and a reactive venereal disease research laboratory test.
Late congenital syphilis, defined as the onset of symptoms after 2 years of age is secondary to scarring or persistent inflammation and gumma formation in a variety of tissues. It occurs in up to 40% of cases of untreated maternal disease. Most cases can be prevented by maternal treatment and treatment of the infant within the first 3 months of life. Common clinical manifestations include interstitial keratitis, sensorineural hearing loss, frontal bossing, saddle nose, Hutchinson teeth, mulberry molars, perforation of the hard palate, anterior bowing of the tibia (saber shins), and other skeletal abnormalities.
Diagnostic tests. Maternal diagnosis is dependent upon knowing the results of both a nontreponemal (RPR, VDRL) and a confirmatory treponemal test (TP-PA, TP-EIA, TP-CIA, FTA-ABS,) before or at delivery. TP-PA is the preferred test. When maternal disease is confirmed, the newborn should have the same quantitative nontreponemal test as the mother. A confirmatory treponemal test is not required
Evaluation and treatment. It’s imperative that children born to mothers with a reactive test, regardless of their treatment status, have a thorough exam performed before hospital discharge. The provider must determine what additional interventions should be performed.
The American Academy of Pediatrics and the Centers for Disease Control and Prevention (www.cdc.gov/std/treatment-guidelines/congenital-syphilis.htm) have developed standard algorithms for the diagnostic approach and treatment of infants born to mothers with reactive serologic tests for syphilis. It is available in the Red Book for AAP members (https://publications.aap.org/redbook). Recommendations based on various scenarios for neonates up to 1 month of age include proven or highly probable congenital syphilis, possible congenital syphilis, congenital syphilis less likely, and congenital syphilis unlikely. It is beyond the scope of this article to list the criteria and evaluation for each scenario. The reader is referred to the algorithm.
If syphilis is suspected in infants or children older than 1 month, the challenge is to determine if it is untreated congenital syphilis or acquired syphilis. Maternal syphilis status should be determined. Evaluation for congenital syphilis in this age group includes CSF analysis for VDRL, cell count and protein, CBC with differential and platelets, hepatic panel, abdominal ultrasound, long-bone radiographs, chest radiograph, neuroimaging, auditory brain stem response, and HIV testing.
Let’s go back to your patient. The mother was diagnosed with syphilis during pregnancy. You confirm that she was treated with benzathine penicillin G, and the course was completed at least 4 weeks before delivery. Treatment with any other drug during pregnancy is not appropriate. The RPR has declined, and the infant’s titer is equal to or less than four times the maternal titer. The exam is significant for generalized adenopathy and slightly bloody nasal discharge. This infant has two findings consistent with congenital syphilis regardless of RPR titer or treatment status. This places him in the proven or highly probable congenital syphilis group. Management includes CSF analysis (VDRL, cell count, and protein), CBC with differential and platelet count, and treatment with penicillin G for 10 days. Additional tests as clinically indicated include: long-bone radiograph, chest radiography, aspartate aminotranferase and alanine aminotransferase levels, neuroimaging, ophthalmologic exam, and auditory brain stem response. Despite maternal treatment, this newborn has congenital syphilis. The same nontreponemal test should be obtained every 2-3 months until it is nonreactive. It should be nonreactive by 6 months. If the infection persists to 6-12 months post treatment, reevaluation including CSF analysis and retreatment may be indicated.
Congenital syphilis can be prevented by maternal screening, diagnosis, and treatment. When that fails it is up to us to diagnosis and adequately treat our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Why people lie about COVID
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
Psychedelics and the Military: What a Long, Strange Trip It’s Been
In 2019 the Defense Advanced Research Projects Agency invested $27 million in the Focused Pharma program to develop new, more efficacious, rapid-acting drugs, including hallucinogens.1 While Focused Pharma does not include human studies, the Veterans Health Administration’s (VHA) newly launched psychedelics program research does include clinical trials.2 When I read of these ambitious projects, I recalled 2 prescient memories from my youth.
The first memory was of a dinner table conversation between my father, then chief of pediatrics at a military hospital, and one of my older brothers, a burgeoning hippie. My father mentioned that the military was doing research on lysergic acid diethylamide (LSD), and my brother asked whether he could bring some home for my brother to try. My father looked up from the dinner table with incredulity and in an ironic monotone replied, “No you would not qualify for the research, you are not in the Army.”
The second was about 10 years later, when I visited the state psychiatric hospital where my father directed the adolescent ward. I saw a group of young adults watching test patterns on an old-fashioned television set. When I asked my father what was wrong with them, he shook his head and said, “Too much LSD.”
Albert Hoffman was a Sandoz chemist when in 1938 he serendipitously developed LSD while working on a fungus that grew on grain. LSD’s psychoactive properties were not discovered until 1943. About a decade later, as the Cold War chilled international relations, the Central Intelligence Agency (CIA) began conducting experiments on military personnel in the MKUltra program using LSD, electroshock, hypnosis, and other techniques to develop a mind control program before its rivals did.3
Beginning in the 1950s, the US government collaborated with pharmaceutical companies and research universities to develop LSD as part of a campaign of psychological warfare. Though planned to be used against enemies, the program instead exploited US service members to develop hallucinogens as a form of chemical warfare that could render enemy troops mentally incapacitated. That psychiatrists, who then (as now) led much of this research, raised a host of ethical concerns about dual roles, disclosure, and duty.4
Government investigations and academic studies have shown that even soldiers who volunteered for the research were not given adequate information about the nature of the experiments and the potential adverse effects, such as persisting flashbacks. The military’s research on LSD ended in 1963, not because of the unethical aspects of the research, but because the effects of LSD were so unpredictable that the drug could not be effectively weaponized. Like Tuskegee and other research abuses of the time, when the MKUltra program was exposed, there were congressional investigations.5 Later studies found that many of the active-duty research subjects experienced a plethora of lasting and serious psychiatric symptoms. VHA practitioners had to put back together many of these broken service members. This program was rife with violations of research ethics and human rights, and those abuses tainted the field of hallucinogenic research in US Department of Defense (DoD) and VHA circles for decades.5 These research abuses, in part, have led to hallucinogens being categorized as Schedule I controlled substances, effectively blocking federal funding for research until recently.
LSD, Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), and 3,4-methylenedioxy-methamphetamine (MDMA), popularly known as psychedelics, are again receiving attention. However, the current investigations into psychedelics are vastly different—scientifically and ethically. The most important difference is that the context and leadership of these studies is not national security—it is health care.
The goal of this new wave of psychedelic research is not mind control or brain alteration, but liberation of the mind from cycles of rumination and trauma and empowerment to change patterns of self-destruction to affirmation of life. The impetus for this research is not international espionage but to find better treatments for chronic posttraumatic stress disorder, severe substance use disorders, and treatment-resistant depression that contribute to unquantifiable mental pain, psychosocial dysfunction, and an epidemic of suicide among military service members and veterans.6 Though we have some effective treatments for these often combat-inflicted maladies—primarily evidence-based psychotherapies—yet these treatments are not tolerable or safe, fast-acting, or long-lasting enough to succor each and every troubled soul. The success of ketamine, a dissociative drug, in relieving the most distressing service-connected psychiatric diagnoses has provided a proof of concept to reinvigorate the moribund hallucinogenic research idea.7
This dark chapter in US military research is a cautionary tale. The often quoted and more often ignored advice of the Spanish American philosopher George Santayana, “Those who cannot remember the past are condemned to repeat it,” should serve as the guiding principle of the new hallucinogenic research.8 Human subjects’ protections have exponentially improved since the days of the secret LSD project even for active-duty personnel. The Common Rule governs that all research participants are given adequate information that includes whatever is known about the risks and benefits of the research.10 Participants must provide full and free informed consent to enroll in these clinical trials, a consent that encompasses the right to withdraw from the research at any time without jeopardizing their careers, benefits, or ongoing health care.10
These rules, though, can be bent, broken, avoided, or worked around. Only the moral integrity of study personnel, administrators, oversight agencies, research compliance officers, and most important, principal investigators can assure that the rules are upheld and the rights they guarantee are respected.9 It would be a tragic shame if the promised hope for the relief of psychic pain went unrealized due to media hype, shared desperation of clinicians and patients, and conflicts of interests that today are more likely to come from profit-driven pharmaceutical companies than national security agencies. And for all of us in federal practice, remembering the sordid past forays with LSD can redeem the present research so future service members and veterans and the clinicians who care for them have better balms to heal the wounds of war.
1. US Department of Defense, Defense Advanced Research Projects Agency. Structure-guided drug design could yield fast-acting remedies for complex neuropsychiatric conditions. Accessed September 12, 2022. https://www.darpa.mil/news-events/2019-09-11#
2. Londono E. After six-decade hiatus, experimental psychedelic therapy returns to the VA. https://www.nytimes.com/2022/06/24/us/politics/psychedelic-therapy-veterans.html
3. Disbennett B. ‘This is the happy warrior, this is he:’ an analysis of CIA and military testing of LSD on non-consenting U.S. service-members and recovery through the VA disability system. Tennessee J Race, Gender, Social Justice. 2015;3(2):1-32. doi:10.2139/ssrn.2416478
4. Smith H. James Ketchum, who conducted mind-altering experiments on soldiers dies at 87. Accessed September 12, 2022. https://www.washingtonpost.com/local/obituaries/james-ketchum-who-conducted-mind-altering-experiments-on-soldiers-dies-at-87/2019/06/04/7b5ad322-86cc-11e9-a491-25df61c78dc4_story.html
5. Ross CA. LSD experiments by the United States Army. Hist Psychiatry. 2017;28(4):427-442. doi:10.1177/0957154X17717678
6. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions of comorbid posttraumatic stress disorder and treatment resistant depression. Clin Psychiatry. 2018;79(3): 17m11634. doi:10.4088/JCP.17m11634
7. Shawler IC, Jordan CH, Jackson CA. Veteran and military mental health issues. Stat Pearls. Updated May 23, 2022. Accessed September 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK572092/#_NBK572092_pubdet_
8. Santayana G. The Life of Reason. 1905. Accessed September 12, 2022. https://www.gutenberg.org/files/15000/15000-h/15000-h.htm
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1200.05(2). Requirements for the protection of human subjects in research. Amended January 8, 2021. Accessed September 12, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8171
10. US Department of Defense, Military Health System. Research protections. Accessed September 12, 2022. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Engineering/Research-Protections
In 2019 the Defense Advanced Research Projects Agency invested $27 million in the Focused Pharma program to develop new, more efficacious, rapid-acting drugs, including hallucinogens.1 While Focused Pharma does not include human studies, the Veterans Health Administration’s (VHA) newly launched psychedelics program research does include clinical trials.2 When I read of these ambitious projects, I recalled 2 prescient memories from my youth.
The first memory was of a dinner table conversation between my father, then chief of pediatrics at a military hospital, and one of my older brothers, a burgeoning hippie. My father mentioned that the military was doing research on lysergic acid diethylamide (LSD), and my brother asked whether he could bring some home for my brother to try. My father looked up from the dinner table with incredulity and in an ironic monotone replied, “No you would not qualify for the research, you are not in the Army.”
The second was about 10 years later, when I visited the state psychiatric hospital where my father directed the adolescent ward. I saw a group of young adults watching test patterns on an old-fashioned television set. When I asked my father what was wrong with them, he shook his head and said, “Too much LSD.”
Albert Hoffman was a Sandoz chemist when in 1938 he serendipitously developed LSD while working on a fungus that grew on grain. LSD’s psychoactive properties were not discovered until 1943. About a decade later, as the Cold War chilled international relations, the Central Intelligence Agency (CIA) began conducting experiments on military personnel in the MKUltra program using LSD, electroshock, hypnosis, and other techniques to develop a mind control program before its rivals did.3
Beginning in the 1950s, the US government collaborated with pharmaceutical companies and research universities to develop LSD as part of a campaign of psychological warfare. Though planned to be used against enemies, the program instead exploited US service members to develop hallucinogens as a form of chemical warfare that could render enemy troops mentally incapacitated. That psychiatrists, who then (as now) led much of this research, raised a host of ethical concerns about dual roles, disclosure, and duty.4
Government investigations and academic studies have shown that even soldiers who volunteered for the research were not given adequate information about the nature of the experiments and the potential adverse effects, such as persisting flashbacks. The military’s research on LSD ended in 1963, not because of the unethical aspects of the research, but because the effects of LSD were so unpredictable that the drug could not be effectively weaponized. Like Tuskegee and other research abuses of the time, when the MKUltra program was exposed, there were congressional investigations.5 Later studies found that many of the active-duty research subjects experienced a plethora of lasting and serious psychiatric symptoms. VHA practitioners had to put back together many of these broken service members. This program was rife with violations of research ethics and human rights, and those abuses tainted the field of hallucinogenic research in US Department of Defense (DoD) and VHA circles for decades.5 These research abuses, in part, have led to hallucinogens being categorized as Schedule I controlled substances, effectively blocking federal funding for research until recently.
LSD, Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), and 3,4-methylenedioxy-methamphetamine (MDMA), popularly known as psychedelics, are again receiving attention. However, the current investigations into psychedelics are vastly different—scientifically and ethically. The most important difference is that the context and leadership of these studies is not national security—it is health care.
The goal of this new wave of psychedelic research is not mind control or brain alteration, but liberation of the mind from cycles of rumination and trauma and empowerment to change patterns of self-destruction to affirmation of life. The impetus for this research is not international espionage but to find better treatments for chronic posttraumatic stress disorder, severe substance use disorders, and treatment-resistant depression that contribute to unquantifiable mental pain, psychosocial dysfunction, and an epidemic of suicide among military service members and veterans.6 Though we have some effective treatments for these often combat-inflicted maladies—primarily evidence-based psychotherapies—yet these treatments are not tolerable or safe, fast-acting, or long-lasting enough to succor each and every troubled soul. The success of ketamine, a dissociative drug, in relieving the most distressing service-connected psychiatric diagnoses has provided a proof of concept to reinvigorate the moribund hallucinogenic research idea.7
This dark chapter in US military research is a cautionary tale. The often quoted and more often ignored advice of the Spanish American philosopher George Santayana, “Those who cannot remember the past are condemned to repeat it,” should serve as the guiding principle of the new hallucinogenic research.8 Human subjects’ protections have exponentially improved since the days of the secret LSD project even for active-duty personnel. The Common Rule governs that all research participants are given adequate information that includes whatever is known about the risks and benefits of the research.10 Participants must provide full and free informed consent to enroll in these clinical trials, a consent that encompasses the right to withdraw from the research at any time without jeopardizing their careers, benefits, or ongoing health care.10
These rules, though, can be bent, broken, avoided, or worked around. Only the moral integrity of study personnel, administrators, oversight agencies, research compliance officers, and most important, principal investigators can assure that the rules are upheld and the rights they guarantee are respected.9 It would be a tragic shame if the promised hope for the relief of psychic pain went unrealized due to media hype, shared desperation of clinicians and patients, and conflicts of interests that today are more likely to come from profit-driven pharmaceutical companies than national security agencies. And for all of us in federal practice, remembering the sordid past forays with LSD can redeem the present research so future service members and veterans and the clinicians who care for them have better balms to heal the wounds of war.
In 2019 the Defense Advanced Research Projects Agency invested $27 million in the Focused Pharma program to develop new, more efficacious, rapid-acting drugs, including hallucinogens.1 While Focused Pharma does not include human studies, the Veterans Health Administration’s (VHA) newly launched psychedelics program research does include clinical trials.2 When I read of these ambitious projects, I recalled 2 prescient memories from my youth.
The first memory was of a dinner table conversation between my father, then chief of pediatrics at a military hospital, and one of my older brothers, a burgeoning hippie. My father mentioned that the military was doing research on lysergic acid diethylamide (LSD), and my brother asked whether he could bring some home for my brother to try. My father looked up from the dinner table with incredulity and in an ironic monotone replied, “No you would not qualify for the research, you are not in the Army.”
The second was about 10 years later, when I visited the state psychiatric hospital where my father directed the adolescent ward. I saw a group of young adults watching test patterns on an old-fashioned television set. When I asked my father what was wrong with them, he shook his head and said, “Too much LSD.”
Albert Hoffman was a Sandoz chemist when in 1938 he serendipitously developed LSD while working on a fungus that grew on grain. LSD’s psychoactive properties were not discovered until 1943. About a decade later, as the Cold War chilled international relations, the Central Intelligence Agency (CIA) began conducting experiments on military personnel in the MKUltra program using LSD, electroshock, hypnosis, and other techniques to develop a mind control program before its rivals did.3
Beginning in the 1950s, the US government collaborated with pharmaceutical companies and research universities to develop LSD as part of a campaign of psychological warfare. Though planned to be used against enemies, the program instead exploited US service members to develop hallucinogens as a form of chemical warfare that could render enemy troops mentally incapacitated. That psychiatrists, who then (as now) led much of this research, raised a host of ethical concerns about dual roles, disclosure, and duty.4
Government investigations and academic studies have shown that even soldiers who volunteered for the research were not given adequate information about the nature of the experiments and the potential adverse effects, such as persisting flashbacks. The military’s research on LSD ended in 1963, not because of the unethical aspects of the research, but because the effects of LSD were so unpredictable that the drug could not be effectively weaponized. Like Tuskegee and other research abuses of the time, when the MKUltra program was exposed, there were congressional investigations.5 Later studies found that many of the active-duty research subjects experienced a plethora of lasting and serious psychiatric symptoms. VHA practitioners had to put back together many of these broken service members. This program was rife with violations of research ethics and human rights, and those abuses tainted the field of hallucinogenic research in US Department of Defense (DoD) and VHA circles for decades.5 These research abuses, in part, have led to hallucinogens being categorized as Schedule I controlled substances, effectively blocking federal funding for research until recently.
LSD, Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), and 3,4-methylenedioxy-methamphetamine (MDMA), popularly known as psychedelics, are again receiving attention. However, the current investigations into psychedelics are vastly different—scientifically and ethically. The most important difference is that the context and leadership of these studies is not national security—it is health care.
The goal of this new wave of psychedelic research is not mind control or brain alteration, but liberation of the mind from cycles of rumination and trauma and empowerment to change patterns of self-destruction to affirmation of life. The impetus for this research is not international espionage but to find better treatments for chronic posttraumatic stress disorder, severe substance use disorders, and treatment-resistant depression that contribute to unquantifiable mental pain, psychosocial dysfunction, and an epidemic of suicide among military service members and veterans.6 Though we have some effective treatments for these often combat-inflicted maladies—primarily evidence-based psychotherapies—yet these treatments are not tolerable or safe, fast-acting, or long-lasting enough to succor each and every troubled soul. The success of ketamine, a dissociative drug, in relieving the most distressing service-connected psychiatric diagnoses has provided a proof of concept to reinvigorate the moribund hallucinogenic research idea.7
This dark chapter in US military research is a cautionary tale. The often quoted and more often ignored advice of the Spanish American philosopher George Santayana, “Those who cannot remember the past are condemned to repeat it,” should serve as the guiding principle of the new hallucinogenic research.8 Human subjects’ protections have exponentially improved since the days of the secret LSD project even for active-duty personnel. The Common Rule governs that all research participants are given adequate information that includes whatever is known about the risks and benefits of the research.10 Participants must provide full and free informed consent to enroll in these clinical trials, a consent that encompasses the right to withdraw from the research at any time without jeopardizing their careers, benefits, or ongoing health care.10
These rules, though, can be bent, broken, avoided, or worked around. Only the moral integrity of study personnel, administrators, oversight agencies, research compliance officers, and most important, principal investigators can assure that the rules are upheld and the rights they guarantee are respected.9 It would be a tragic shame if the promised hope for the relief of psychic pain went unrealized due to media hype, shared desperation of clinicians and patients, and conflicts of interests that today are more likely to come from profit-driven pharmaceutical companies than national security agencies. And for all of us in federal practice, remembering the sordid past forays with LSD can redeem the present research so future service members and veterans and the clinicians who care for them have better balms to heal the wounds of war.
1. US Department of Defense, Defense Advanced Research Projects Agency. Structure-guided drug design could yield fast-acting remedies for complex neuropsychiatric conditions. Accessed September 12, 2022. https://www.darpa.mil/news-events/2019-09-11#
2. Londono E. After six-decade hiatus, experimental psychedelic therapy returns to the VA. https://www.nytimes.com/2022/06/24/us/politics/psychedelic-therapy-veterans.html
3. Disbennett B. ‘This is the happy warrior, this is he:’ an analysis of CIA and military testing of LSD on non-consenting U.S. service-members and recovery through the VA disability system. Tennessee J Race, Gender, Social Justice. 2015;3(2):1-32. doi:10.2139/ssrn.2416478
4. Smith H. James Ketchum, who conducted mind-altering experiments on soldiers dies at 87. Accessed September 12, 2022. https://www.washingtonpost.com/local/obituaries/james-ketchum-who-conducted-mind-altering-experiments-on-soldiers-dies-at-87/2019/06/04/7b5ad322-86cc-11e9-a491-25df61c78dc4_story.html
5. Ross CA. LSD experiments by the United States Army. Hist Psychiatry. 2017;28(4):427-442. doi:10.1177/0957154X17717678
6. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions of comorbid posttraumatic stress disorder and treatment resistant depression. Clin Psychiatry. 2018;79(3): 17m11634. doi:10.4088/JCP.17m11634
7. Shawler IC, Jordan CH, Jackson CA. Veteran and military mental health issues. Stat Pearls. Updated May 23, 2022. Accessed September 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK572092/#_NBK572092_pubdet_
8. Santayana G. The Life of Reason. 1905. Accessed September 12, 2022. https://www.gutenberg.org/files/15000/15000-h/15000-h.htm
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1200.05(2). Requirements for the protection of human subjects in research. Amended January 8, 2021. Accessed September 12, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8171
10. US Department of Defense, Military Health System. Research protections. Accessed September 12, 2022. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Engineering/Research-Protections
1. US Department of Defense, Defense Advanced Research Projects Agency. Structure-guided drug design could yield fast-acting remedies for complex neuropsychiatric conditions. Accessed September 12, 2022. https://www.darpa.mil/news-events/2019-09-11#
2. Londono E. After six-decade hiatus, experimental psychedelic therapy returns to the VA. https://www.nytimes.com/2022/06/24/us/politics/psychedelic-therapy-veterans.html
3. Disbennett B. ‘This is the happy warrior, this is he:’ an analysis of CIA and military testing of LSD on non-consenting U.S. service-members and recovery through the VA disability system. Tennessee J Race, Gender, Social Justice. 2015;3(2):1-32. doi:10.2139/ssrn.2416478
4. Smith H. James Ketchum, who conducted mind-altering experiments on soldiers dies at 87. Accessed September 12, 2022. https://www.washingtonpost.com/local/obituaries/james-ketchum-who-conducted-mind-altering-experiments-on-soldiers-dies-at-87/2019/06/04/7b5ad322-86cc-11e9-a491-25df61c78dc4_story.html
5. Ross CA. LSD experiments by the United States Army. Hist Psychiatry. 2017;28(4):427-442. doi:10.1177/0957154X17717678
6. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions of comorbid posttraumatic stress disorder and treatment resistant depression. Clin Psychiatry. 2018;79(3): 17m11634. doi:10.4088/JCP.17m11634
7. Shawler IC, Jordan CH, Jackson CA. Veteran and military mental health issues. Stat Pearls. Updated May 23, 2022. Accessed September 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK572092/#_NBK572092_pubdet_
8. Santayana G. The Life of Reason. 1905. Accessed September 12, 2022. https://www.gutenberg.org/files/15000/15000-h/15000-h.htm
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1200.05(2). Requirements for the protection of human subjects in research. Amended January 8, 2021. Accessed September 12, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8171
10. US Department of Defense, Military Health System. Research protections. Accessed September 12, 2022. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Engineering/Research-Protections