User login
Testing the limits of medical technology
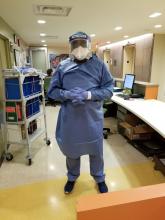
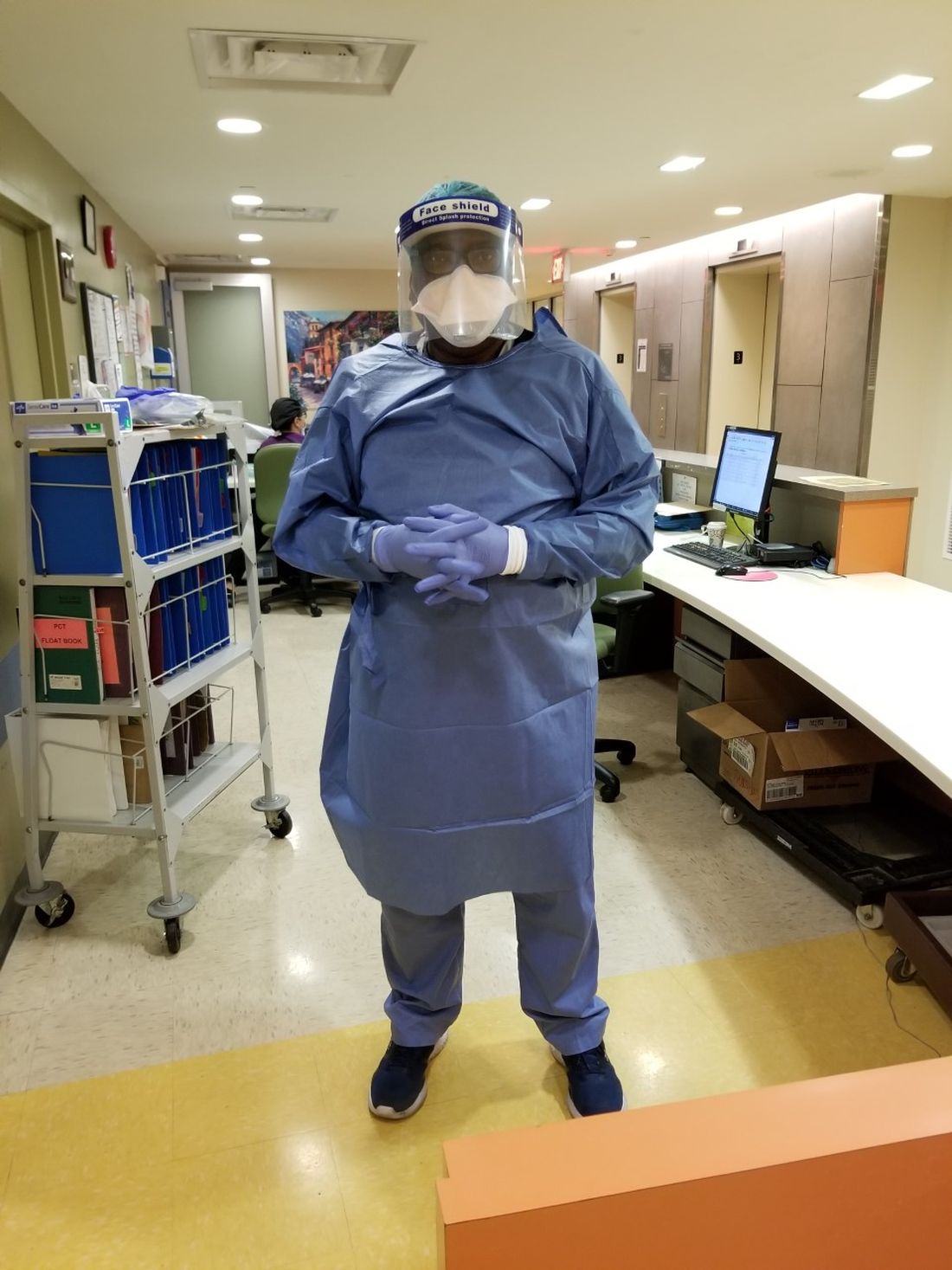
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Improving care for women who have experienced stillbirth
Think of the current standard of care and do the opposite
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a [email protected].
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
Think of the current standard of care and do the opposite
Think of the current standard of care and do the opposite
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a [email protected].
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a [email protected].
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
New York City inpatient detox unit keeps running: Here’s how
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Should all patients with advanced ovarian cancer receive frontline maintenance therapy?
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
Consider ketamine and psychotherapy combo
Preliminary data show intervention helps patients with SUDs
As an addiction psychiatrist specializing in the use of ketamine-assisted psychotherapy, both in patients with mood disorders and substance use disorders, I would like to offer some perspective about limits and possibilities of ketamine and esketamine.
Single infusions of ketamine targeting unipolar mood symptoms indeed yield initial 24-hour response rates of about 60%-70%, though those rates fall precipitously with time.1 Where single treatments fall short in terms of durability of benefit, a series of multiple treatments – modeled around electroconvulsive therapy and pending a noninferiority study to compare the two2 – provide for more robust and durable results.3
Esketamine nasal spray, recently approved by the Food and Drug Administration for treatment-resistant major depressive disorder, consists of one of the component stereoisomers of ketamine and is administered at first twice weekly and then less frequently with time. It now, like off-label ketamine,4 sees clinical use as monotherapy for MDD, as an alternative for patients who have intolerance or lack of response to first-line treatments such as SSRIs.
Ketamine, while perhaps less directly validated and more stigmatized for psychiatric use, recently has been demonstrated in a rigorous trial as noninferior in terms of antidepressant benefit at 24 hours,5 and a multitude of published case studies document maintenance of benefit with repeat doses over a period of months.6 Ketamine notably enjoys several advantages over esketamine as a treatment option: a cost one to two orders of magnitude lower7 (esketamine nasal spray sees a wholesale price of $600-$900 per dose), greater versatility in dose, and lack of a restrictive REMS program.8 The $1-$2 cost of a dose of ketamine means that the clinical barrier of prior authorizations is largely a nonissue and may in and of itself vastly improve access to this novel and efficacious treatment.
My clinical experience involves providing ketamine as an intramuscular bolus along with contemporaneous psychotherapy; such combination of medication and psychotherapy intervention may be more effective than ketamine alone9 and has seen impressive initial results in the treatment of alcohol use disorder, termed ketamine psychedelic therapy.10 I can affirm these hopeful initial findings in the treatment of both mood and substance use disorders, and have observed maintained response from mood symptoms for a period of 1-4 years in several patients, with such sessions provided approximately monthly.11
I hope these preliminary data inform more rigorous study of long-term ketamine as a treatment for psychiatric indications.
Dr. Ryan is a board-certified psychiatrist and addiction psychiatrist who practices in Los Angeles. He has written several articles and a book chapter on ketamine. Dr. Ryan has no disclosures.
References
1. Murrough JW et al. Am J Psychiatry. 2013;170(10):1134-42.
2. Mathew SJ et al. Contemp Clin Trials. 2019;77:19-26.
3. Singh JB et al. Am J Psychiatry. 2016;173(8):816-26.
4. Calabrese L. Int J Psychiatr Res. 2019; 2(5):1-12.
5. Correia-Melo FS et al. J Affect Disord. 2020;264:527-34.
6. Ryan WC, Marta CJ, Koek RJ. Ketamine and depression, in “The Ketamine Papers: Science, Therapy, and Transformation.” Santa Cruz, Calif.: Multidisciplinary Association for Psychedelic Studies, 2016.
7. Institute for Clinical and Economic Review. “Esketamine for the Treatment of Treatment-Resistant Depression: Effectiveness and Value.” Final report. 2019 Jun 20.
8. Spravato package insert. Titusville, N.J.: Janssen Pharmaceuticals.
9. Dore J et al. J Psychoactive Drugs. 2019;51(2):189-98.
10. Krupitsky EM and Grinenko AY. J Psychoactive Drugs. 1997;29(2):165-83.
11. Ryan WC. Ketamine-assisted psychotherapy: Theory and chart review. KRIYA Ketamine Research Institute Conference. Hillsborough, Calif. 2019. Nov 9.
Preliminary data show intervention helps patients with SUDs
Preliminary data show intervention helps patients with SUDs
As an addiction psychiatrist specializing in the use of ketamine-assisted psychotherapy, both in patients with mood disorders and substance use disorders, I would like to offer some perspective about limits and possibilities of ketamine and esketamine.
Single infusions of ketamine targeting unipolar mood symptoms indeed yield initial 24-hour response rates of about 60%-70%, though those rates fall precipitously with time.1 Where single treatments fall short in terms of durability of benefit, a series of multiple treatments – modeled around electroconvulsive therapy and pending a noninferiority study to compare the two2 – provide for more robust and durable results.3
Esketamine nasal spray, recently approved by the Food and Drug Administration for treatment-resistant major depressive disorder, consists of one of the component stereoisomers of ketamine and is administered at first twice weekly and then less frequently with time. It now, like off-label ketamine,4 sees clinical use as monotherapy for MDD, as an alternative for patients who have intolerance or lack of response to first-line treatments such as SSRIs.
Ketamine, while perhaps less directly validated and more stigmatized for psychiatric use, recently has been demonstrated in a rigorous trial as noninferior in terms of antidepressant benefit at 24 hours,5 and a multitude of published case studies document maintenance of benefit with repeat doses over a period of months.6 Ketamine notably enjoys several advantages over esketamine as a treatment option: a cost one to two orders of magnitude lower7 (esketamine nasal spray sees a wholesale price of $600-$900 per dose), greater versatility in dose, and lack of a restrictive REMS program.8 The $1-$2 cost of a dose of ketamine means that the clinical barrier of prior authorizations is largely a nonissue and may in and of itself vastly improve access to this novel and efficacious treatment.
My clinical experience involves providing ketamine as an intramuscular bolus along with contemporaneous psychotherapy; such combination of medication and psychotherapy intervention may be more effective than ketamine alone9 and has seen impressive initial results in the treatment of alcohol use disorder, termed ketamine psychedelic therapy.10 I can affirm these hopeful initial findings in the treatment of both mood and substance use disorders, and have observed maintained response from mood symptoms for a period of 1-4 years in several patients, with such sessions provided approximately monthly.11
I hope these preliminary data inform more rigorous study of long-term ketamine as a treatment for psychiatric indications.
Dr. Ryan is a board-certified psychiatrist and addiction psychiatrist who practices in Los Angeles. He has written several articles and a book chapter on ketamine. Dr. Ryan has no disclosures.
References
1. Murrough JW et al. Am J Psychiatry. 2013;170(10):1134-42.
2. Mathew SJ et al. Contemp Clin Trials. 2019;77:19-26.
3. Singh JB et al. Am J Psychiatry. 2016;173(8):816-26.
4. Calabrese L. Int J Psychiatr Res. 2019; 2(5):1-12.
5. Correia-Melo FS et al. J Affect Disord. 2020;264:527-34.
6. Ryan WC, Marta CJ, Koek RJ. Ketamine and depression, in “The Ketamine Papers: Science, Therapy, and Transformation.” Santa Cruz, Calif.: Multidisciplinary Association for Psychedelic Studies, 2016.
7. Institute for Clinical and Economic Review. “Esketamine for the Treatment of Treatment-Resistant Depression: Effectiveness and Value.” Final report. 2019 Jun 20.
8. Spravato package insert. Titusville, N.J.: Janssen Pharmaceuticals.
9. Dore J et al. J Psychoactive Drugs. 2019;51(2):189-98.
10. Krupitsky EM and Grinenko AY. J Psychoactive Drugs. 1997;29(2):165-83.
11. Ryan WC. Ketamine-assisted psychotherapy: Theory and chart review. KRIYA Ketamine Research Institute Conference. Hillsborough, Calif. 2019. Nov 9.
As an addiction psychiatrist specializing in the use of ketamine-assisted psychotherapy, both in patients with mood disorders and substance use disorders, I would like to offer some perspective about limits and possibilities of ketamine and esketamine.
Single infusions of ketamine targeting unipolar mood symptoms indeed yield initial 24-hour response rates of about 60%-70%, though those rates fall precipitously with time.1 Where single treatments fall short in terms of durability of benefit, a series of multiple treatments – modeled around electroconvulsive therapy and pending a noninferiority study to compare the two2 – provide for more robust and durable results.3
Esketamine nasal spray, recently approved by the Food and Drug Administration for treatment-resistant major depressive disorder, consists of one of the component stereoisomers of ketamine and is administered at first twice weekly and then less frequently with time. It now, like off-label ketamine,4 sees clinical use as monotherapy for MDD, as an alternative for patients who have intolerance or lack of response to first-line treatments such as SSRIs.
Ketamine, while perhaps less directly validated and more stigmatized for psychiatric use, recently has been demonstrated in a rigorous trial as noninferior in terms of antidepressant benefit at 24 hours,5 and a multitude of published case studies document maintenance of benefit with repeat doses over a period of months.6 Ketamine notably enjoys several advantages over esketamine as a treatment option: a cost one to two orders of magnitude lower7 (esketamine nasal spray sees a wholesale price of $600-$900 per dose), greater versatility in dose, and lack of a restrictive REMS program.8 The $1-$2 cost of a dose of ketamine means that the clinical barrier of prior authorizations is largely a nonissue and may in and of itself vastly improve access to this novel and efficacious treatment.
My clinical experience involves providing ketamine as an intramuscular bolus along with contemporaneous psychotherapy; such combination of medication and psychotherapy intervention may be more effective than ketamine alone9 and has seen impressive initial results in the treatment of alcohol use disorder, termed ketamine psychedelic therapy.10 I can affirm these hopeful initial findings in the treatment of both mood and substance use disorders, and have observed maintained response from mood symptoms for a period of 1-4 years in several patients, with such sessions provided approximately monthly.11
I hope these preliminary data inform more rigorous study of long-term ketamine as a treatment for psychiatric indications.
Dr. Ryan is a board-certified psychiatrist and addiction psychiatrist who practices in Los Angeles. He has written several articles and a book chapter on ketamine. Dr. Ryan has no disclosures.
References
1. Murrough JW et al. Am J Psychiatry. 2013;170(10):1134-42.
2. Mathew SJ et al. Contemp Clin Trials. 2019;77:19-26.
3. Singh JB et al. Am J Psychiatry. 2016;173(8):816-26.
4. Calabrese L. Int J Psychiatr Res. 2019; 2(5):1-12.
5. Correia-Melo FS et al. J Affect Disord. 2020;264:527-34.
6. Ryan WC, Marta CJ, Koek RJ. Ketamine and depression, in “The Ketamine Papers: Science, Therapy, and Transformation.” Santa Cruz, Calif.: Multidisciplinary Association for Psychedelic Studies, 2016.
7. Institute for Clinical and Economic Review. “Esketamine for the Treatment of Treatment-Resistant Depression: Effectiveness and Value.” Final report. 2019 Jun 20.
8. Spravato package insert. Titusville, N.J.: Janssen Pharmaceuticals.
9. Dore J et al. J Psychoactive Drugs. 2019;51(2):189-98.
10. Krupitsky EM and Grinenko AY. J Psychoactive Drugs. 1997;29(2):165-83.
11. Ryan WC. Ketamine-assisted psychotherapy: Theory and chart review. KRIYA Ketamine Research Institute Conference. Hillsborough, Calif. 2019. Nov 9.
Is HIPAA critical?
Ignorance may be bliss for some. But as I sit here in my scenic social isolation on the Maine coast I find that, like most people, what I don’t know unsettles me. How is the COVID-19 virus spread? Does my wife’s wipe down of the doorknobs after I return from the grocery store really make us any less likely to contract the virus? Is wearing my homemade bandana face mask doing anything to protect me? I suspect not, but I wear it as a statement of courtesy and solidarity to my fellow community members.
Does the 6-foot rule make any sense? I’ve read that it is based on a study dating back to the 1930s. I’ve seen images of the 25-foot droplet plume blasting out from a sneeze and understand that, as a bicyclist, I may be generating a shower of droplets in my wake. But, are those droplets a threat to anyone I pedal by if I am symptom free? What does being a carrier mean when we are talking about COVID-19?
What makes me more vulnerable to this particular virus as an apparently healthy septuagenarian? What collection of misfortunes have fallen on those younger victims of the pandemic? How often was it genetic?
Of course, none of us has the information yet that can provide us answers. This vacuum has attracted scores of “experts” bold enough or careless enough to venture an opinion. They may have also issued a caveat, but how often have the media failed to include it in the report or buried it in the fine print at the end of the story?
My discomfort with this information void has left me and you and everyone else to our imaginations to craft our own explanations. So, I try to piece together a construct based on what I can glean from what I read and see in the news because like most people I fortunately have no first-hand information about even a single case. The number of deaths is horrifying, but may not have hit close to home and given most of us a real personal sense of the illness and its character.
Maine is a small state with just over a million inhabitants, and most of us have some connection to one another. It may be that a person is the second cousin of someone who used to live 2 miles down the road. But, there is some feeling of familiarity. We have had deaths related to COVID-19, but very scanty information other than the county about where they occurred and whether the victim was a resident of an extended care facility. We are told very little if any details about exposure as officials invoke HIPAA regulations that leave us in the dark. Other than one vague reference to a “traveling salesman” who may have introduced the virus to several nursing homes, there has been very little information about how the virus may have been spread here in Maine. Even national reports of the deaths of high-profile entertainers and retired athletes are usually draped in the same haze of privacy.
Most of us don’t need to know the names and street addresses of the victims but a few anonymous narratives that include some general information on how epidemiologists believe clusters began and propagated would help us understand our risks with just a glimmer of clarity.
Of course the epidemiologists may not have the answers we are seeking because they too are struggling to untangle connections hampered by concerns of privacy. There is no question that privacy must remain an important part of the physician-patient relationship. But a pandemic has thrown us into a situation where common sense demands that HIPAA be interpreted with an emphasis on the greater good. Finding that balance between privacy and public knowledge will continue to be one of our greatest challenges.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Ignorance may be bliss for some. But as I sit here in my scenic social isolation on the Maine coast I find that, like most people, what I don’t know unsettles me. How is the COVID-19 virus spread? Does my wife’s wipe down of the doorknobs after I return from the grocery store really make us any less likely to contract the virus? Is wearing my homemade bandana face mask doing anything to protect me? I suspect not, but I wear it as a statement of courtesy and solidarity to my fellow community members.
Does the 6-foot rule make any sense? I’ve read that it is based on a study dating back to the 1930s. I’ve seen images of the 25-foot droplet plume blasting out from a sneeze and understand that, as a bicyclist, I may be generating a shower of droplets in my wake. But, are those droplets a threat to anyone I pedal by if I am symptom free? What does being a carrier mean when we are talking about COVID-19?
What makes me more vulnerable to this particular virus as an apparently healthy septuagenarian? What collection of misfortunes have fallen on those younger victims of the pandemic? How often was it genetic?
Of course, none of us has the information yet that can provide us answers. This vacuum has attracted scores of “experts” bold enough or careless enough to venture an opinion. They may have also issued a caveat, but how often have the media failed to include it in the report or buried it in the fine print at the end of the story?
My discomfort with this information void has left me and you and everyone else to our imaginations to craft our own explanations. So, I try to piece together a construct based on what I can glean from what I read and see in the news because like most people I fortunately have no first-hand information about even a single case. The number of deaths is horrifying, but may not have hit close to home and given most of us a real personal sense of the illness and its character.
Maine is a small state with just over a million inhabitants, and most of us have some connection to one another. It may be that a person is the second cousin of someone who used to live 2 miles down the road. But, there is some feeling of familiarity. We have had deaths related to COVID-19, but very scanty information other than the county about where they occurred and whether the victim was a resident of an extended care facility. We are told very little if any details about exposure as officials invoke HIPAA regulations that leave us in the dark. Other than one vague reference to a “traveling salesman” who may have introduced the virus to several nursing homes, there has been very little information about how the virus may have been spread here in Maine. Even national reports of the deaths of high-profile entertainers and retired athletes are usually draped in the same haze of privacy.
Most of us don’t need to know the names and street addresses of the victims but a few anonymous narratives that include some general information on how epidemiologists believe clusters began and propagated would help us understand our risks with just a glimmer of clarity.
Of course the epidemiologists may not have the answers we are seeking because they too are struggling to untangle connections hampered by concerns of privacy. There is no question that privacy must remain an important part of the physician-patient relationship. But a pandemic has thrown us into a situation where common sense demands that HIPAA be interpreted with an emphasis on the greater good. Finding that balance between privacy and public knowledge will continue to be one of our greatest challenges.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Ignorance may be bliss for some. But as I sit here in my scenic social isolation on the Maine coast I find that, like most people, what I don’t know unsettles me. How is the COVID-19 virus spread? Does my wife’s wipe down of the doorknobs after I return from the grocery store really make us any less likely to contract the virus? Is wearing my homemade bandana face mask doing anything to protect me? I suspect not, but I wear it as a statement of courtesy and solidarity to my fellow community members.
Does the 6-foot rule make any sense? I’ve read that it is based on a study dating back to the 1930s. I’ve seen images of the 25-foot droplet plume blasting out from a sneeze and understand that, as a bicyclist, I may be generating a shower of droplets in my wake. But, are those droplets a threat to anyone I pedal by if I am symptom free? What does being a carrier mean when we are talking about COVID-19?
What makes me more vulnerable to this particular virus as an apparently healthy septuagenarian? What collection of misfortunes have fallen on those younger victims of the pandemic? How often was it genetic?
Of course, none of us has the information yet that can provide us answers. This vacuum has attracted scores of “experts” bold enough or careless enough to venture an opinion. They may have also issued a caveat, but how often have the media failed to include it in the report or buried it in the fine print at the end of the story?
My discomfort with this information void has left me and you and everyone else to our imaginations to craft our own explanations. So, I try to piece together a construct based on what I can glean from what I read and see in the news because like most people I fortunately have no first-hand information about even a single case. The number of deaths is horrifying, but may not have hit close to home and given most of us a real personal sense of the illness and its character.
Maine is a small state with just over a million inhabitants, and most of us have some connection to one another. It may be that a person is the second cousin of someone who used to live 2 miles down the road. But, there is some feeling of familiarity. We have had deaths related to COVID-19, but very scanty information other than the county about where they occurred and whether the victim was a resident of an extended care facility. We are told very little if any details about exposure as officials invoke HIPAA regulations that leave us in the dark. Other than one vague reference to a “traveling salesman” who may have introduced the virus to several nursing homes, there has been very little information about how the virus may have been spread here in Maine. Even national reports of the deaths of high-profile entertainers and retired athletes are usually draped in the same haze of privacy.
Most of us don’t need to know the names and street addresses of the victims but a few anonymous narratives that include some general information on how epidemiologists believe clusters began and propagated would help us understand our risks with just a glimmer of clarity.
Of course the epidemiologists may not have the answers we are seeking because they too are struggling to untangle connections hampered by concerns of privacy. There is no question that privacy must remain an important part of the physician-patient relationship. But a pandemic has thrown us into a situation where common sense demands that HIPAA be interpreted with an emphasis on the greater good. Finding that balance between privacy and public knowledge will continue to be one of our greatest challenges.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Changes in patient behavior during COVID-19: What I’ve observed
Unprecedented circumstances, extraordinary times, continental shift, life-altering experience—the descriptions of the coronavirus disease 2019 (COVID-19) pandemic have been endless, and accurate. Every clinician who has cared for patients during these trying times has noticed new patterns in patient behavior. Psychiatrists are acutely aware of the emotional, behavioral, and cognitive methods that patients are using to protect themselves from the chaos around them, and the ways in which they process a societal catastrophe such as COVID-19 (Figure). Here are some new patterns I have noticed among my own patients.
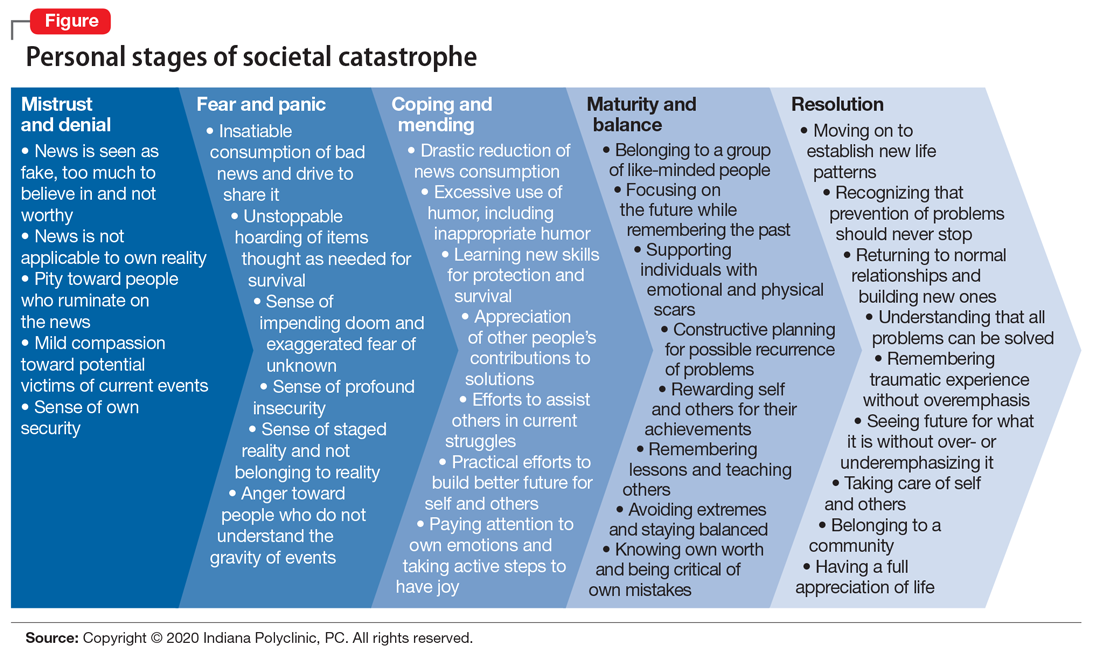
Physical and emotional separation
I first noticed the changes in my patients’ behavior at the front desk, where they now spend less time talking with the staff. They bring their own pens for filling out the paperwork, avoid touching items around them, and try to keep social interactions brief and to the point. Patients have been more cooperative about scheduling and rescheduling their appointments. They have generally been nicer to the staff, frequently thanking us for the work we do, and verbalizing their support for health care professionals in general.
Patients have been more supportive of their family members and other patients in the clinic, with some noticeable exceptions, such as maintaining social distancing for their own comfort and safety. Some patients wear face masks not just for safety but also to separate themselves and hide their emotions from the world. This allows them to feel more emotionally secure when interacting with other people.
The use of telehealth has given many patients the security of not having to leave their home, and the decreased need for travel adds to their comfort.
Changes I didn’t expect
The COVID-19 pandemic has resulted in some unexpected changes in my patients. Only a minority of my patients have expressed increased anxiety, while most have become less anxious overall on issues other than the pandemic. Many of my patients who have stressful jobs, especially teachers, say they feel more comfortable working from home and have less anxiety and depression because they are removed from their daily stressors. There also has been an increase in patients’ use of humor, including inappropriate humor, to defend against their fear of COVID-19.
Our clinic is a multidisciplinary facility that specializes in integrating mental and physical health treatments for pain, and for some patients, increased anxiety is clearly associated with an increase in pain. However, during the COVID-19 pandemic, patients have recognized this connection and verbalized their concerns. Some somatic patients have had a decrease in their physical symptoms, including chronic pain, because they see that the whole world is not well, which somehow helps to validate their concerns.
The changes in our patients’ psychological well-being will likely continue to morph as we enter a more stable period. The eventual resolution of the pandemic will bring further changes to our patients’ emotional lives. As we go through these times together, we will continue to uncover new ways that our patients will use to defend themselves against stress and adversities.
Unprecedented circumstances, extraordinary times, continental shift, life-altering experience—the descriptions of the coronavirus disease 2019 (COVID-19) pandemic have been endless, and accurate. Every clinician who has cared for patients during these trying times has noticed new patterns in patient behavior. Psychiatrists are acutely aware of the emotional, behavioral, and cognitive methods that patients are using to protect themselves from the chaos around them, and the ways in which they process a societal catastrophe such as COVID-19 (Figure). Here are some new patterns I have noticed among my own patients.

Physical and emotional separation
I first noticed the changes in my patients’ behavior at the front desk, where they now spend less time talking with the staff. They bring their own pens for filling out the paperwork, avoid touching items around them, and try to keep social interactions brief and to the point. Patients have been more cooperative about scheduling and rescheduling their appointments. They have generally been nicer to the staff, frequently thanking us for the work we do, and verbalizing their support for health care professionals in general.
Patients have been more supportive of their family members and other patients in the clinic, with some noticeable exceptions, such as maintaining social distancing for their own comfort and safety. Some patients wear face masks not just for safety but also to separate themselves and hide their emotions from the world. This allows them to feel more emotionally secure when interacting with other people.
The use of telehealth has given many patients the security of not having to leave their home, and the decreased need for travel adds to their comfort.
Changes I didn’t expect
The COVID-19 pandemic has resulted in some unexpected changes in my patients. Only a minority of my patients have expressed increased anxiety, while most have become less anxious overall on issues other than the pandemic. Many of my patients who have stressful jobs, especially teachers, say they feel more comfortable working from home and have less anxiety and depression because they are removed from their daily stressors. There also has been an increase in patients’ use of humor, including inappropriate humor, to defend against their fear of COVID-19.
Our clinic is a multidisciplinary facility that specializes in integrating mental and physical health treatments for pain, and for some patients, increased anxiety is clearly associated with an increase in pain. However, during the COVID-19 pandemic, patients have recognized this connection and verbalized their concerns. Some somatic patients have had a decrease in their physical symptoms, including chronic pain, because they see that the whole world is not well, which somehow helps to validate their concerns.
The changes in our patients’ psychological well-being will likely continue to morph as we enter a more stable period. The eventual resolution of the pandemic will bring further changes to our patients’ emotional lives. As we go through these times together, we will continue to uncover new ways that our patients will use to defend themselves against stress and adversities.
Unprecedented circumstances, extraordinary times, continental shift, life-altering experience—the descriptions of the coronavirus disease 2019 (COVID-19) pandemic have been endless, and accurate. Every clinician who has cared for patients during these trying times has noticed new patterns in patient behavior. Psychiatrists are acutely aware of the emotional, behavioral, and cognitive methods that patients are using to protect themselves from the chaos around them, and the ways in which they process a societal catastrophe such as COVID-19 (Figure). Here are some new patterns I have noticed among my own patients.

Physical and emotional separation
I first noticed the changes in my patients’ behavior at the front desk, where they now spend less time talking with the staff. They bring their own pens for filling out the paperwork, avoid touching items around them, and try to keep social interactions brief and to the point. Patients have been more cooperative about scheduling and rescheduling their appointments. They have generally been nicer to the staff, frequently thanking us for the work we do, and verbalizing their support for health care professionals in general.
Patients have been more supportive of their family members and other patients in the clinic, with some noticeable exceptions, such as maintaining social distancing for their own comfort and safety. Some patients wear face masks not just for safety but also to separate themselves and hide their emotions from the world. This allows them to feel more emotionally secure when interacting with other people.
The use of telehealth has given many patients the security of not having to leave their home, and the decreased need for travel adds to their comfort.
Changes I didn’t expect
The COVID-19 pandemic has resulted in some unexpected changes in my patients. Only a minority of my patients have expressed increased anxiety, while most have become less anxious overall on issues other than the pandemic. Many of my patients who have stressful jobs, especially teachers, say they feel more comfortable working from home and have less anxiety and depression because they are removed from their daily stressors. There also has been an increase in patients’ use of humor, including inappropriate humor, to defend against their fear of COVID-19.
Our clinic is a multidisciplinary facility that specializes in integrating mental and physical health treatments for pain, and for some patients, increased anxiety is clearly associated with an increase in pain. However, during the COVID-19 pandemic, patients have recognized this connection and verbalized their concerns. Some somatic patients have had a decrease in their physical symptoms, including chronic pain, because they see that the whole world is not well, which somehow helps to validate their concerns.
The changes in our patients’ psychological well-being will likely continue to morph as we enter a more stable period. The eventual resolution of the pandemic will bring further changes to our patients’ emotional lives. As we go through these times together, we will continue to uncover new ways that our patients will use to defend themselves against stress and adversities.
Tool-less but not clueless
There is apparently some debate about which of our ancestors was the first to use tools. It probably was Homo habilis, the “handy man.” But it could have been a relative of Lucy, of the Australopithecus afarensis tribe. Regardless of which pile of chipped rocks looks more tool-like to you, it is generally agreed that our ability to make and use tools is one of the key ingredients to our evolutionary success.
I have always enjoyed the feel of good quality knife when I am woodcarving, and the tool collection hanging on the wall over my work bench is one of my most prized possessions. But when I was practicing general pediatrics, I could never really warm up to the screening tools that were being touted as must-haves for detecting developmental delays.
It turns out I was not alone. A recent study published in Pediatrics found that the number of pediatricians who reported using developmental screening tools increased from 21% to 63% between 2002 and 2016. (Pediatrics. 2020 Apr. doi: 10.1542/peds.2019-0851). However, this means that, despite a significant increase in usage, more than a third of pediatricians still are not employing screening tools. Does this suggest that one out of every three pediatricians, including me and maybe you, is a knuckle-dragging pre–Homo sapiens practicing in blissful and clueless ignorance?
Mei Elansary MD, MPhil, and Michael Silverstein, MD, MPH, who wrote a companion commentary in the same journal, suggested that maybe those of us who have resisted the call to be tool users aren’t prehistoric ignoramuses (Pediatrics. 2020 Apr. doi: 10.1542/peds.2020-0164). They observed that, regardless of whether the pediatricians were using screening tools, more than 40% of the those surveyed did not refer patients for early intervention.
The commentators pointed out that the decision of when, whom, and how to screen must be viewed as part of a “complicated web of changing epidemiology, time and reimbursement constraints, and service availability.” They observe that pediatricians facing this landscape in upheaval “default to what they know best: clinical judgment.” Citing one study of the management of febrile infants, the authors point out that relying on guidelines doesn’t always result in improved clinical care.
My decision of when to refer a patient for early intervention was based on what I had observed over a series of visits and whether I thought that the early intervention resources available in my community would have a significant benefit for any particular child. Because I crafted my practice around a model that put a strong emphasis on continuity, my patients almost never saw another provider for a health maintenance visit and usually saw me for their sick visits, including ear rechecks.
I guess you could argue that there are situations in which seeing a variety of providers, each with a slightly different perspective, might benefit the patient. But when we are talking about a domain like development that is defined by change, or lack of change, over time, multiple observations by a single observer usually can be more valuable.
If I were practicing in a situation in which I didn’t have the luxury of continuity, I think I would be more likely to use a screening tool. Although I have found screening guidelines can be helpful as mnemonics in some situations, they aren’t equally applicable in all clinical settings.
While I may be asking for trouble by questioning anything even remotely related to the concept of early intervention, I must say that I wholeheartedly agree with Dr. Elansary and Dr. Silverstein when they wrote “the pediatrics community may have something to learn from the significant minority of pediatricians who do not practice formalized screening.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
There is apparently some debate about which of our ancestors was the first to use tools. It probably was Homo habilis, the “handy man.” But it could have been a relative of Lucy, of the Australopithecus afarensis tribe. Regardless of which pile of chipped rocks looks more tool-like to you, it is generally agreed that our ability to make and use tools is one of the key ingredients to our evolutionary success.
I have always enjoyed the feel of good quality knife when I am woodcarving, and the tool collection hanging on the wall over my work bench is one of my most prized possessions. But when I was practicing general pediatrics, I could never really warm up to the screening tools that were being touted as must-haves for detecting developmental delays.
It turns out I was not alone. A recent study published in Pediatrics found that the number of pediatricians who reported using developmental screening tools increased from 21% to 63% between 2002 and 2016. (Pediatrics. 2020 Apr. doi: 10.1542/peds.2019-0851). However, this means that, despite a significant increase in usage, more than a third of pediatricians still are not employing screening tools. Does this suggest that one out of every three pediatricians, including me and maybe you, is a knuckle-dragging pre–Homo sapiens practicing in blissful and clueless ignorance?
Mei Elansary MD, MPhil, and Michael Silverstein, MD, MPH, who wrote a companion commentary in the same journal, suggested that maybe those of us who have resisted the call to be tool users aren’t prehistoric ignoramuses (Pediatrics. 2020 Apr. doi: 10.1542/peds.2020-0164). They observed that, regardless of whether the pediatricians were using screening tools, more than 40% of the those surveyed did not refer patients for early intervention.
The commentators pointed out that the decision of when, whom, and how to screen must be viewed as part of a “complicated web of changing epidemiology, time and reimbursement constraints, and service availability.” They observe that pediatricians facing this landscape in upheaval “default to what they know best: clinical judgment.” Citing one study of the management of febrile infants, the authors point out that relying on guidelines doesn’t always result in improved clinical care.
My decision of when to refer a patient for early intervention was based on what I had observed over a series of visits and whether I thought that the early intervention resources available in my community would have a significant benefit for any particular child. Because I crafted my practice around a model that put a strong emphasis on continuity, my patients almost never saw another provider for a health maintenance visit and usually saw me for their sick visits, including ear rechecks.
I guess you could argue that there are situations in which seeing a variety of providers, each with a slightly different perspective, might benefit the patient. But when we are talking about a domain like development that is defined by change, or lack of change, over time, multiple observations by a single observer usually can be more valuable.
If I were practicing in a situation in which I didn’t have the luxury of continuity, I think I would be more likely to use a screening tool. Although I have found screening guidelines can be helpful as mnemonics in some situations, they aren’t equally applicable in all clinical settings.
While I may be asking for trouble by questioning anything even remotely related to the concept of early intervention, I must say that I wholeheartedly agree with Dr. Elansary and Dr. Silverstein when they wrote “the pediatrics community may have something to learn from the significant minority of pediatricians who do not practice formalized screening.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
There is apparently some debate about which of our ancestors was the first to use tools. It probably was Homo habilis, the “handy man.” But it could have been a relative of Lucy, of the Australopithecus afarensis tribe. Regardless of which pile of chipped rocks looks more tool-like to you, it is generally agreed that our ability to make and use tools is one of the key ingredients to our evolutionary success.
I have always enjoyed the feel of good quality knife when I am woodcarving, and the tool collection hanging on the wall over my work bench is one of my most prized possessions. But when I was practicing general pediatrics, I could never really warm up to the screening tools that were being touted as must-haves for detecting developmental delays.
It turns out I was not alone. A recent study published in Pediatrics found that the number of pediatricians who reported using developmental screening tools increased from 21% to 63% between 2002 and 2016. (Pediatrics. 2020 Apr. doi: 10.1542/peds.2019-0851). However, this means that, despite a significant increase in usage, more than a third of pediatricians still are not employing screening tools. Does this suggest that one out of every three pediatricians, including me and maybe you, is a knuckle-dragging pre–Homo sapiens practicing in blissful and clueless ignorance?
Mei Elansary MD, MPhil, and Michael Silverstein, MD, MPH, who wrote a companion commentary in the same journal, suggested that maybe those of us who have resisted the call to be tool users aren’t prehistoric ignoramuses (Pediatrics. 2020 Apr. doi: 10.1542/peds.2020-0164). They observed that, regardless of whether the pediatricians were using screening tools, more than 40% of the those surveyed did not refer patients for early intervention.
The commentators pointed out that the decision of when, whom, and how to screen must be viewed as part of a “complicated web of changing epidemiology, time and reimbursement constraints, and service availability.” They observe that pediatricians facing this landscape in upheaval “default to what they know best: clinical judgment.” Citing one study of the management of febrile infants, the authors point out that relying on guidelines doesn’t always result in improved clinical care.
My decision of when to refer a patient for early intervention was based on what I had observed over a series of visits and whether I thought that the early intervention resources available in my community would have a significant benefit for any particular child. Because I crafted my practice around a model that put a strong emphasis on continuity, my patients almost never saw another provider for a health maintenance visit and usually saw me for their sick visits, including ear rechecks.
I guess you could argue that there are situations in which seeing a variety of providers, each with a slightly different perspective, might benefit the patient. But when we are talking about a domain like development that is defined by change, or lack of change, over time, multiple observations by a single observer usually can be more valuable.
If I were practicing in a situation in which I didn’t have the luxury of continuity, I think I would be more likely to use a screening tool. Although I have found screening guidelines can be helpful as mnemonics in some situations, they aren’t equally applicable in all clinical settings.
While I may be asking for trouble by questioning anything even remotely related to the concept of early intervention, I must say that I wholeheartedly agree with Dr. Elansary and Dr. Silverstein when they wrote “the pediatrics community may have something to learn from the significant minority of pediatricians who do not practice formalized screening.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Stop calling it ‘behavioral health’: Psychiatry is much more
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
The injustice of pre-authorization
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri