User login
Multiple Myeloma: New Treatments Aid Patient Subgroups
“The introduction of treatments such as elranatamab (Elrexfio) is allowing patients with multiple myeloma, which is still incurable for now, to have different options and achieve long periods of remission, thus improving their survival,” she added. “This therapeutic innovation is highly effective and well tolerated in patients with relapse or refractory multiple myeloma.” The overall response rate is “up to 61%, early, deep, and long-lasting.”
In an interview with El Médico Interactivo, Dr. Mateos explained the new approaches to multiple myeloma. She highlighted the effectiveness of new treatments and reviewed the latest data on this disease, which were presented at the recent European Hematology Association Congress.
What is the incidence rate of multiple myeloma in the Spanish population?
Multiple myeloma has an incidence of approximately 4-5 new cases per 100,000 inhabitants per year. This means that around 3000 new cases are diagnosed each year in Spain. As with most tumors, multiple myeloma is generally slightly more common in males than females. It is the third most frequent hematologic cancer in men (1757 new cases) and women (1325 new cases), behind lymphoma and leukemias.
At what age is it most often diagnosed?
It affects older people, with recent reports indicating around 68-69 years as the median age. Although more young people are being diagnosed with multiple myeloma, analyses of how this hematologic cancer affects the general population show that it generally impacts patients over age 65 years.
What is the typical survival prognosis?
Thanks to research and therapeutic innovation, the prognosis has changed significantly over the past 20-25 years. Today, if a patient with multiple myeloma receives a diagnosis and does not exhibit poor prognostic characteristics (and this description fits approximately 70%-80% of patients with multiple myeloma), it is realistic to expect a survival exceeding 10 years. A few years ago, this outcome was unimaginable, but a significant amount of therapeutic innovation has made it possible. That’s why I emphasize that it is realistic to provide these data with such a positive outlook.
Is multiple myeloma a refractory type of cancer?
It was a refractory type of cancer. Twenty years ago, there were no treatment options, and therefore survival was around 2-3 years, because treatment mainly consisted of using alkylating agents and corticosteroids. This is what made it refractory.
With the emergence of new therapeutic innovations, patients have been responding better and their responses are lasting longer. Although there is still a group of patients, about 10%-15%, with a poor prognosis and refractory disease, those with standard risk are responding better to different therapies.
Although most patients will eventually exhaust the treatments, which until now were primarily triple-drug regimens (such as proteasome inhibitors, immunomodulators, and antiCD38 antibodies), the introduction of new therapies is extending the duration of responses.
Is the risk for relapse high?
It is very high, in the sense that almost all patients with multiple myeloma eventually relapse. However, we hope that there soon will be some patients who do not relapse.
What are the typical pathologic manifestations of this cancer? Does it affect everyone equally, or in specific ways in each person?
In multiple myeloma, we often say there are multiple myelomas. Clinically, the disease presents in most patients, around 80%, with two clinical manifestations: anemia and bone lesions. Less frequently, patients may also have kidney failure, hypercalcemia, and a higher tendency toward infection. Behind this rather common symptomatology, from a molecular and genetic perspective, each myeloma is practically unique, adding complexity to its treatment. Therefore, ultimately, myelomas end up being refractory.
Elranatamab is a new therapeutic tool. For which patients is it recommended?
It is a bispecific monoclonal antibody that corresponds to the new monotherapy strategies we have for treating patients with multiple myeloma. On the one hand, it targets damaged plasma cells, which are the patient’s tumor cells, and on the other, it binds the patient’s T cells and redirects them to the tumor niche. When this happens, the T cell activates and destroys the tumor cell.
This medication has been approved for patients with relapsed myeloma who have received traditional drugs for their treatment. We know well that patients who have already received proteasome inhibitors, immunomodulators, and anti-CD38 antibodies typically need something new after treatment. Before, there were no other options, and we would reuse what had been previously used. Now we have elranatamab, a bispecific monoclonal antibody targeting a new receptor that has shown significant responses as monotherapy.
More than 60% of patients respond, and more than 30% achieve complete remission. The key is the response duration and progression-free survival of almost a year and a half. This is the longest progression-free survival we have seen to date in previous lines. Therefore, it fills the needs we had for these relapsed or refractory myeloma patients.
What advantages does this new treatment offer?
It represents a therapeutic innovation because, as mentioned, it achieves a response in more than 60% of patients, and around 35% achieve complete remission. The median response duration has not been reached yet. Progression-free survival is 17.2 months, almost a year and a half, and overall survival is almost two years.
Furthermore, it is administered as subcutaneous monotherapy weekly for the first six cycles and then every 15 days. It has a good safety profile, although some adverse events are known, so we have strategies to combat or mitigate them, making the treatment generally well tolerated.
What side effects are being observed?
They are manageable. When the drug is first administered, patients may experience what we call a cytokine release syndrome, which is a result of the treatment’s mechanism. However, we can predict very well when it occurs, usually 2 days after the first doses, and we have strategies to mitigate it.
The second most common adverse event we need to be cautious about is infection. Nowadays, before starting treatment, patients update their vaccination schedule, receive antiviral prophylaxis, and receive prophylaxis against certain germs, resulting in reduced infections. However, infections are probably the adverse events we need to be most careful about when treating the patient.
We must ensure that prophylaxis is performed, and if fever occurs and an infection is suspected, cultures and all kinds of studies must be done to identify and treat it properly.
How does elranatamab change the treatment of an incurable disease? Does it bring us closer to a cure or to making multiple myeloma a manageable chronic disease?
With the already approved elranatamab, the most important aspect is that it adds another treatment option for patients with myeloma. With the progression-free survival data I indicated, life expectancy is increased, with a good quality of life and acceptable safety.
Obviously, elranatamab is still under study and development, even in early lines, including in patients with newly diagnosed myeloma. When we are choosing first-line therapy, we select the best patients by combining traditional drugs with these new immunotherapies, such as elranatamab, it is likely that we are much closer to offering a cure to specific subgroups.
Although it won’t happen in all cases, I believe it will be applicable to a significant subgroup of patients, making chronicity of the disease a reality we are already approaching. Each day, we encounter more patients receiving different lines of treatment and ultimately meeting their life expectancy with myeloma. Even though some may die, it is often due to causes not related to myeloma. This is the most important contribution of these innovations, such as elranatamab.
Dr. Mateos reported receiving honoraria from Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Stemline, Oncopeptides, and Kite for delivering lectures and for participating in advisory boards.
This story was translated from El Médico Interactivo, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
“The introduction of treatments such as elranatamab (Elrexfio) is allowing patients with multiple myeloma, which is still incurable for now, to have different options and achieve long periods of remission, thus improving their survival,” she added. “This therapeutic innovation is highly effective and well tolerated in patients with relapse or refractory multiple myeloma.” The overall response rate is “up to 61%, early, deep, and long-lasting.”
In an interview with El Médico Interactivo, Dr. Mateos explained the new approaches to multiple myeloma. She highlighted the effectiveness of new treatments and reviewed the latest data on this disease, which were presented at the recent European Hematology Association Congress.
What is the incidence rate of multiple myeloma in the Spanish population?
Multiple myeloma has an incidence of approximately 4-5 new cases per 100,000 inhabitants per year. This means that around 3000 new cases are diagnosed each year in Spain. As with most tumors, multiple myeloma is generally slightly more common in males than females. It is the third most frequent hematologic cancer in men (1757 new cases) and women (1325 new cases), behind lymphoma and leukemias.
At what age is it most often diagnosed?
It affects older people, with recent reports indicating around 68-69 years as the median age. Although more young people are being diagnosed with multiple myeloma, analyses of how this hematologic cancer affects the general population show that it generally impacts patients over age 65 years.
What is the typical survival prognosis?
Thanks to research and therapeutic innovation, the prognosis has changed significantly over the past 20-25 years. Today, if a patient with multiple myeloma receives a diagnosis and does not exhibit poor prognostic characteristics (and this description fits approximately 70%-80% of patients with multiple myeloma), it is realistic to expect a survival exceeding 10 years. A few years ago, this outcome was unimaginable, but a significant amount of therapeutic innovation has made it possible. That’s why I emphasize that it is realistic to provide these data with such a positive outlook.
Is multiple myeloma a refractory type of cancer?
It was a refractory type of cancer. Twenty years ago, there were no treatment options, and therefore survival was around 2-3 years, because treatment mainly consisted of using alkylating agents and corticosteroids. This is what made it refractory.
With the emergence of new therapeutic innovations, patients have been responding better and their responses are lasting longer. Although there is still a group of patients, about 10%-15%, with a poor prognosis and refractory disease, those with standard risk are responding better to different therapies.
Although most patients will eventually exhaust the treatments, which until now were primarily triple-drug regimens (such as proteasome inhibitors, immunomodulators, and antiCD38 antibodies), the introduction of new therapies is extending the duration of responses.
Is the risk for relapse high?
It is very high, in the sense that almost all patients with multiple myeloma eventually relapse. However, we hope that there soon will be some patients who do not relapse.
What are the typical pathologic manifestations of this cancer? Does it affect everyone equally, or in specific ways in each person?
In multiple myeloma, we often say there are multiple myelomas. Clinically, the disease presents in most patients, around 80%, with two clinical manifestations: anemia and bone lesions. Less frequently, patients may also have kidney failure, hypercalcemia, and a higher tendency toward infection. Behind this rather common symptomatology, from a molecular and genetic perspective, each myeloma is practically unique, adding complexity to its treatment. Therefore, ultimately, myelomas end up being refractory.
Elranatamab is a new therapeutic tool. For which patients is it recommended?
It is a bispecific monoclonal antibody that corresponds to the new monotherapy strategies we have for treating patients with multiple myeloma. On the one hand, it targets damaged plasma cells, which are the patient’s tumor cells, and on the other, it binds the patient’s T cells and redirects them to the tumor niche. When this happens, the T cell activates and destroys the tumor cell.
This medication has been approved for patients with relapsed myeloma who have received traditional drugs for their treatment. We know well that patients who have already received proteasome inhibitors, immunomodulators, and anti-CD38 antibodies typically need something new after treatment. Before, there were no other options, and we would reuse what had been previously used. Now we have elranatamab, a bispecific monoclonal antibody targeting a new receptor that has shown significant responses as monotherapy.
More than 60% of patients respond, and more than 30% achieve complete remission. The key is the response duration and progression-free survival of almost a year and a half. This is the longest progression-free survival we have seen to date in previous lines. Therefore, it fills the needs we had for these relapsed or refractory myeloma patients.
What advantages does this new treatment offer?
It represents a therapeutic innovation because, as mentioned, it achieves a response in more than 60% of patients, and around 35% achieve complete remission. The median response duration has not been reached yet. Progression-free survival is 17.2 months, almost a year and a half, and overall survival is almost two years.
Furthermore, it is administered as subcutaneous monotherapy weekly for the first six cycles and then every 15 days. It has a good safety profile, although some adverse events are known, so we have strategies to combat or mitigate them, making the treatment generally well tolerated.
What side effects are being observed?
They are manageable. When the drug is first administered, patients may experience what we call a cytokine release syndrome, which is a result of the treatment’s mechanism. However, we can predict very well when it occurs, usually 2 days after the first doses, and we have strategies to mitigate it.
The second most common adverse event we need to be cautious about is infection. Nowadays, before starting treatment, patients update their vaccination schedule, receive antiviral prophylaxis, and receive prophylaxis against certain germs, resulting in reduced infections. However, infections are probably the adverse events we need to be most careful about when treating the patient.
We must ensure that prophylaxis is performed, and if fever occurs and an infection is suspected, cultures and all kinds of studies must be done to identify and treat it properly.
How does elranatamab change the treatment of an incurable disease? Does it bring us closer to a cure or to making multiple myeloma a manageable chronic disease?
With the already approved elranatamab, the most important aspect is that it adds another treatment option for patients with myeloma. With the progression-free survival data I indicated, life expectancy is increased, with a good quality of life and acceptable safety.
Obviously, elranatamab is still under study and development, even in early lines, including in patients with newly diagnosed myeloma. When we are choosing first-line therapy, we select the best patients by combining traditional drugs with these new immunotherapies, such as elranatamab, it is likely that we are much closer to offering a cure to specific subgroups.
Although it won’t happen in all cases, I believe it will be applicable to a significant subgroup of patients, making chronicity of the disease a reality we are already approaching. Each day, we encounter more patients receiving different lines of treatment and ultimately meeting their life expectancy with myeloma. Even though some may die, it is often due to causes not related to myeloma. This is the most important contribution of these innovations, such as elranatamab.
Dr. Mateos reported receiving honoraria from Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Stemline, Oncopeptides, and Kite for delivering lectures and for participating in advisory boards.
This story was translated from El Médico Interactivo, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
“The introduction of treatments such as elranatamab (Elrexfio) is allowing patients with multiple myeloma, which is still incurable for now, to have different options and achieve long periods of remission, thus improving their survival,” she added. “This therapeutic innovation is highly effective and well tolerated in patients with relapse or refractory multiple myeloma.” The overall response rate is “up to 61%, early, deep, and long-lasting.”
In an interview with El Médico Interactivo, Dr. Mateos explained the new approaches to multiple myeloma. She highlighted the effectiveness of new treatments and reviewed the latest data on this disease, which were presented at the recent European Hematology Association Congress.
What is the incidence rate of multiple myeloma in the Spanish population?
Multiple myeloma has an incidence of approximately 4-5 new cases per 100,000 inhabitants per year. This means that around 3000 new cases are diagnosed each year in Spain. As with most tumors, multiple myeloma is generally slightly more common in males than females. It is the third most frequent hematologic cancer in men (1757 new cases) and women (1325 new cases), behind lymphoma and leukemias.
At what age is it most often diagnosed?
It affects older people, with recent reports indicating around 68-69 years as the median age. Although more young people are being diagnosed with multiple myeloma, analyses of how this hematologic cancer affects the general population show that it generally impacts patients over age 65 years.
What is the typical survival prognosis?
Thanks to research and therapeutic innovation, the prognosis has changed significantly over the past 20-25 years. Today, if a patient with multiple myeloma receives a diagnosis and does not exhibit poor prognostic characteristics (and this description fits approximately 70%-80% of patients with multiple myeloma), it is realistic to expect a survival exceeding 10 years. A few years ago, this outcome was unimaginable, but a significant amount of therapeutic innovation has made it possible. That’s why I emphasize that it is realistic to provide these data with such a positive outlook.
Is multiple myeloma a refractory type of cancer?
It was a refractory type of cancer. Twenty years ago, there were no treatment options, and therefore survival was around 2-3 years, because treatment mainly consisted of using alkylating agents and corticosteroids. This is what made it refractory.
With the emergence of new therapeutic innovations, patients have been responding better and their responses are lasting longer. Although there is still a group of patients, about 10%-15%, with a poor prognosis and refractory disease, those with standard risk are responding better to different therapies.
Although most patients will eventually exhaust the treatments, which until now were primarily triple-drug regimens (such as proteasome inhibitors, immunomodulators, and antiCD38 antibodies), the introduction of new therapies is extending the duration of responses.
Is the risk for relapse high?
It is very high, in the sense that almost all patients with multiple myeloma eventually relapse. However, we hope that there soon will be some patients who do not relapse.
What are the typical pathologic manifestations of this cancer? Does it affect everyone equally, or in specific ways in each person?
In multiple myeloma, we often say there are multiple myelomas. Clinically, the disease presents in most patients, around 80%, with two clinical manifestations: anemia and bone lesions. Less frequently, patients may also have kidney failure, hypercalcemia, and a higher tendency toward infection. Behind this rather common symptomatology, from a molecular and genetic perspective, each myeloma is practically unique, adding complexity to its treatment. Therefore, ultimately, myelomas end up being refractory.
Elranatamab is a new therapeutic tool. For which patients is it recommended?
It is a bispecific monoclonal antibody that corresponds to the new monotherapy strategies we have for treating patients with multiple myeloma. On the one hand, it targets damaged plasma cells, which are the patient’s tumor cells, and on the other, it binds the patient’s T cells and redirects them to the tumor niche. When this happens, the T cell activates and destroys the tumor cell.
This medication has been approved for patients with relapsed myeloma who have received traditional drugs for their treatment. We know well that patients who have already received proteasome inhibitors, immunomodulators, and anti-CD38 antibodies typically need something new after treatment. Before, there were no other options, and we would reuse what had been previously used. Now we have elranatamab, a bispecific monoclonal antibody targeting a new receptor that has shown significant responses as monotherapy.
More than 60% of patients respond, and more than 30% achieve complete remission. The key is the response duration and progression-free survival of almost a year and a half. This is the longest progression-free survival we have seen to date in previous lines. Therefore, it fills the needs we had for these relapsed or refractory myeloma patients.
What advantages does this new treatment offer?
It represents a therapeutic innovation because, as mentioned, it achieves a response in more than 60% of patients, and around 35% achieve complete remission. The median response duration has not been reached yet. Progression-free survival is 17.2 months, almost a year and a half, and overall survival is almost two years.
Furthermore, it is administered as subcutaneous monotherapy weekly for the first six cycles and then every 15 days. It has a good safety profile, although some adverse events are known, so we have strategies to combat or mitigate them, making the treatment generally well tolerated.
What side effects are being observed?
They are manageable. When the drug is first administered, patients may experience what we call a cytokine release syndrome, which is a result of the treatment’s mechanism. However, we can predict very well when it occurs, usually 2 days after the first doses, and we have strategies to mitigate it.
The second most common adverse event we need to be cautious about is infection. Nowadays, before starting treatment, patients update their vaccination schedule, receive antiviral prophylaxis, and receive prophylaxis against certain germs, resulting in reduced infections. However, infections are probably the adverse events we need to be most careful about when treating the patient.
We must ensure that prophylaxis is performed, and if fever occurs and an infection is suspected, cultures and all kinds of studies must be done to identify and treat it properly.
How does elranatamab change the treatment of an incurable disease? Does it bring us closer to a cure or to making multiple myeloma a manageable chronic disease?
With the already approved elranatamab, the most important aspect is that it adds another treatment option for patients with myeloma. With the progression-free survival data I indicated, life expectancy is increased, with a good quality of life and acceptable safety.
Obviously, elranatamab is still under study and development, even in early lines, including in patients with newly diagnosed myeloma. When we are choosing first-line therapy, we select the best patients by combining traditional drugs with these new immunotherapies, such as elranatamab, it is likely that we are much closer to offering a cure to specific subgroups.
Although it won’t happen in all cases, I believe it will be applicable to a significant subgroup of patients, making chronicity of the disease a reality we are already approaching. Each day, we encounter more patients receiving different lines of treatment and ultimately meeting their life expectancy with myeloma. Even though some may die, it is often due to causes not related to myeloma. This is the most important contribution of these innovations, such as elranatamab.
Dr. Mateos reported receiving honoraria from Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Stemline, Oncopeptides, and Kite for delivering lectures and for participating in advisory boards.
This story was translated from El Médico Interactivo, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
A 62-year-old Black female presented with an epidermal inclusion cyst on her left upper back
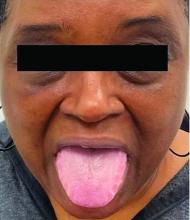
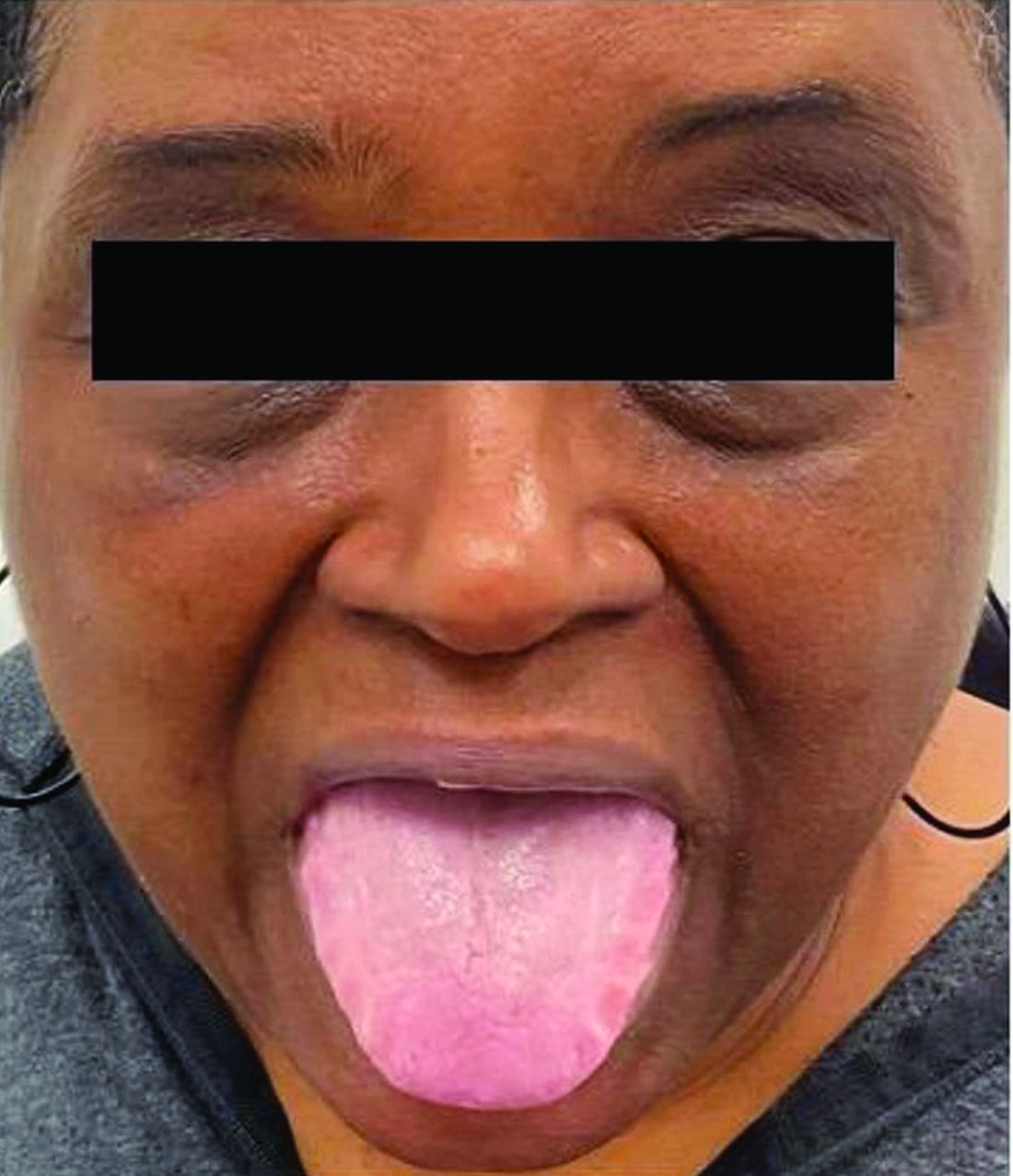
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
This heterogeneous disorder can present with a wide range of clinical manifestations, including dermatological symptoms that may be the first or predominant feature. Systemic amyloidosis is characterized by macroglossia, periorbital purpura, and waxy skin plaques. Lateral scalloping of the tongue may be seen due to impingement of the teeth. Cutaneous amyloidosis occurs when amyloid is deposited in the skin, without internal organ involvement. Variants of cutaneous amyloidosis include macular, lichen, nodular and biphasic.
This condition requires a thorough diagnostic workup, including serum and urine protein electrophoresis and biopsy of the affected tissue. Biopsy of a cutaneous amyloidosis lesion will show fractured, amorphous, eosinophilic material in the dermis. Pigment and epidermal changes are often found with cutaneous amyloidosis, including hyperkeratosis, acanthosis, hypergranulosis, parakeratosis, and epidermal atrophy. Stains that may be used include Congo red showing apple-green birefringence, thioflavin T, and crystal violet.
If untreated, the prognosis is generally poor, related to the extent of organ involvement. Cardiac involvement, a common feature of systemic amyloidosis, can lead to restrictive cardiomyopathy, heart failure, and arrhythmias. Management strategies include steroids, chemotherapy, and stem cell transplantation. Medications include dexamethasone, cyclophosphamide, bortezomib, and melphalan.
This patient went undiagnosed for several years until she began experiencing cardiac issues, including syncope, angina, and restrictive cardiomyopathy with heart failure. A cardiac biopsy confirmed the diagnosis of systemic amyloidosis. This patient is currently awaiting a heart transplant. Early diagnosis of amyloidosis is vital, as it can help prevent severe complications such as heart involvement, significantly impacting the patient’s prognosis and quality of life. When amyloidosis is suspected based on dermatological findings, it is essential to distinguish it from other conditions, such as chronic cutaneous lupus erythematosus, dermatomyositis, scleromyxedema, and lipoid proteinosis. Early identification of characteristic skin lesions and systemic features can lead to timely interventions, more favorable outcomes, and reduction in the risk of advanced organ damage.
The case and photo were submitted by Ms. Cael Aoki and Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Davie, Florida, and Dr. Bartos, of Imperial Dermatology, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Brunt EM, Tiniakos DG. Clin Liver Dis. 2004 Nov;8(4):915-30, x. doi: 10.1016/j.cld.2004.06.009.
2. Bolognia JL et al. (2017). Dermatology E-Book. Elsevier Health Sciences.
3. Mehrotra K et al. J Clin Diagn Res. 2017 Aug;11(8):WC01-WC05. doi: 10.7860/JCDR/2017/24273.10334.
4. Banypersad SM et al. J Am Heart Assoc. 2012 Apr;1(2):e000364. doi: 10.1161/JAHA.111.000364.
5. Bustamante JG, Zaidi SRH. Amyloidosis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
It’s Never Too Late to Convince Patients to Quit Smoking
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is There a Role for GLP-1s in Neurology and Psychiatry?
This transcript has been edited for clarity.
I usually report five or six studies in the field of neurology that were published in the last months, but July was a vacation month.
I decided to cover another topic, which is the role of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists beyond diabetes and obesity, and in particular, for the field of neurology and psychiatry. Until a few years ago, the treatment of diabetes with traditional antidiabetic drugs was frustrating for vascular neurologists.
These drugs would lower glucose and had an impact on small-vessel disease, but they had no impact on large-vessel disease, stroke, and vascular mortality. This changed with the sodium-glucose cotransporter 2 antagonists because these drugs were not only effective for diabetes, but they also lowered cardiac mortality, in particular, in patients with cardiac failure.
The next generation of antidiabetic drugs were the GLP-1 receptor agonists and the combined GIP/GLP-1 receptor agonists. These two polypeptides and their receptors play a very important role in diabetes and in obesity. The receptors are found not only in the pancreas but also in the intestinal system, the liver, and the central nervous system.
We have a number of preclinical models, mostly in transgenic mice, which show that these drugs are not effective only in diabetes and obesity, but also in liver disease, kidney failure, and neurodegenerative diseases. GLP-1 receptor agonists also have powerful anti-inflammatory properties. These drugs reduce body weight, and they have positive effects on blood pressure and lipid metabolism.
In the studies on the use of GLP-1 receptor agonists in diabetes, a meta-analysis with more than 58,000 patients showed a significant risk reduction for stroke compared with placebo, and this risk reduction was in the range of 80%.
Stroke, Smoking, and Alcohol
A meta-analysis on the use of GLP-1 receptor agonists in over 30,000 nondiabetic patients with obesity found a significant reduction in blood pressure, mortality, and the risk of myocardial infarction. There was no significant decrease in the risk of stroke, but most probably this is due to the fact that strokes are much less frequent in obesity than in diabetes.
You all know that obesity is also a major risk factor for sleep apnea syndrome. Recently, two large studies with the GIP/GLP-1 receptor agonist tirzepatide found a significant improvement in sleep apnea syndrome compared to placebo, regardless of whether patients needed continuous positive airway pressure therapy or not.
In the therapy studies on diabetes and obesity, there were indications that some smokers in the studies stopped their nicotine consumption. A small pilot study with exenatide in 84 overweight patients who were smokers showed that 46% of patients on exenatide stopped smoking compared with 27% in the placebo group. This could be an indication that GLP-1 receptor agonists have activity on the reward system in the brain. Currently, there are a number of larger placebo-controlled trials ongoing.
Another aspect is alcohol consumption. An epidemiologic study in Denmark using data from the National Health Registry showed that the incidence of alcohol-related events decreased significantly in almost 40,000 patients with diabetes when they were treated with GLP-1 receptor agonists compared with other antidiabetic drugs.
A retrospective cohort study from the United States with over 80,000 patients with obesity showed that treatment with GLP-1 receptor agonists was associated with a 50%-60% lower risk for occurrence or recurrence of high alcohol consumption. There is only one small study with exenatide, which was not really informative.
There are a number of studies underway for GLP-1 receptor agonists compared with placebo in patients with alcohol dependence or alcohol consumption. Preclinical models also indicate that these drugs might be effective in cocaine abuse, and there is one placebo-controlled study ongoing.
Parkinson’s Disease
Let’s come to neurology. Preclinical models of Parkinson’s disease have shown neuroprotective activities of GLP-1. Until now, we have three randomized placebo-controlled trials with exenatide, NLY01, and lixisenatide. Two of these studies were positive, showing that the symptoms of Parkinson’s disease were stable over time and deteriorated with placebo. One study was neutral. This means we need more large-scale placebo-controlled studies in the early phases of Parkinson’s disease.
Another potential use of GIP/GLP-1 receptor agonists is in dementia. These substances, as you know, have positive effects on high blood pressure and vascular risk factors.
A working group in China analyzed 27 studies on the treatment of diabetes. A small number of randomized studies and a large number of cohort studies showed that modern antidiabetic drugs reduce the risk for dementia. The risk reduction for dementia for the GLP-1 receptor agonists was 75%. At the moment, there are only small prospective studies and they are not conclusive. Again, we need large-scale placebo-controlled studies.
The most important limitation at the moment beyond the cost is the other adverse drug reactions with the GLP-1 receptor agonists; these include nausea, vomiting, diarrhea, and constipation. There might be a slightly increased risk for pancreatitis. The US Food and Drug Administration recently reported there is no increased risk for suicide. Another potential adverse drug reaction is nonatherosclerotic anterior optic neuropathy.
These drugs, GLP-1 receptor agonists and GIP agonists, are also investigated in a variety of other non-neurologic diseases. The focus here is on metabolic liver disease, such as fatty liver and kidney diseases. Smaller, positive studies have been conducted in this area, and large placebo-controlled trials for both indications are currently underway.
If these diverse therapeutic properties would turn out to be really the case with GLP-1 receptor agonists, this would lead to a significant expansion of the range of indications. If we consider cost, this would be the end of our healthcare systems because we cannot afford this. In addition, the new antidiabetic drugs and the treatment of obesity are available only to a limited extent.
Finally, at least for neurology, it’s unclear whether the impact of these diseases is in the brain or whether it’s indirect, due to the effectiveness on vascular risk factors and concomitant diseases.
Dr. Diener is Professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany; he has disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I usually report five or six studies in the field of neurology that were published in the last months, but July was a vacation month.
I decided to cover another topic, which is the role of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists beyond diabetes and obesity, and in particular, for the field of neurology and psychiatry. Until a few years ago, the treatment of diabetes with traditional antidiabetic drugs was frustrating for vascular neurologists.
These drugs would lower glucose and had an impact on small-vessel disease, but they had no impact on large-vessel disease, stroke, and vascular mortality. This changed with the sodium-glucose cotransporter 2 antagonists because these drugs were not only effective for diabetes, but they also lowered cardiac mortality, in particular, in patients with cardiac failure.
The next generation of antidiabetic drugs were the GLP-1 receptor agonists and the combined GIP/GLP-1 receptor agonists. These two polypeptides and their receptors play a very important role in diabetes and in obesity. The receptors are found not only in the pancreas but also in the intestinal system, the liver, and the central nervous system.
We have a number of preclinical models, mostly in transgenic mice, which show that these drugs are not effective only in diabetes and obesity, but also in liver disease, kidney failure, and neurodegenerative diseases. GLP-1 receptor agonists also have powerful anti-inflammatory properties. These drugs reduce body weight, and they have positive effects on blood pressure and lipid metabolism.
In the studies on the use of GLP-1 receptor agonists in diabetes, a meta-analysis with more than 58,000 patients showed a significant risk reduction for stroke compared with placebo, and this risk reduction was in the range of 80%.
Stroke, Smoking, and Alcohol
A meta-analysis on the use of GLP-1 receptor agonists in over 30,000 nondiabetic patients with obesity found a significant reduction in blood pressure, mortality, and the risk of myocardial infarction. There was no significant decrease in the risk of stroke, but most probably this is due to the fact that strokes are much less frequent in obesity than in diabetes.
You all know that obesity is also a major risk factor for sleep apnea syndrome. Recently, two large studies with the GIP/GLP-1 receptor agonist tirzepatide found a significant improvement in sleep apnea syndrome compared to placebo, regardless of whether patients needed continuous positive airway pressure therapy or not.
In the therapy studies on diabetes and obesity, there were indications that some smokers in the studies stopped their nicotine consumption. A small pilot study with exenatide in 84 overweight patients who were smokers showed that 46% of patients on exenatide stopped smoking compared with 27% in the placebo group. This could be an indication that GLP-1 receptor agonists have activity on the reward system in the brain. Currently, there are a number of larger placebo-controlled trials ongoing.
Another aspect is alcohol consumption. An epidemiologic study in Denmark using data from the National Health Registry showed that the incidence of alcohol-related events decreased significantly in almost 40,000 patients with diabetes when they were treated with GLP-1 receptor agonists compared with other antidiabetic drugs.
A retrospective cohort study from the United States with over 80,000 patients with obesity showed that treatment with GLP-1 receptor agonists was associated with a 50%-60% lower risk for occurrence or recurrence of high alcohol consumption. There is only one small study with exenatide, which was not really informative.
There are a number of studies underway for GLP-1 receptor agonists compared with placebo in patients with alcohol dependence or alcohol consumption. Preclinical models also indicate that these drugs might be effective in cocaine abuse, and there is one placebo-controlled study ongoing.
Parkinson’s Disease
Let’s come to neurology. Preclinical models of Parkinson’s disease have shown neuroprotective activities of GLP-1. Until now, we have three randomized placebo-controlled trials with exenatide, NLY01, and lixisenatide. Two of these studies were positive, showing that the symptoms of Parkinson’s disease were stable over time and deteriorated with placebo. One study was neutral. This means we need more large-scale placebo-controlled studies in the early phases of Parkinson’s disease.
Another potential use of GIP/GLP-1 receptor agonists is in dementia. These substances, as you know, have positive effects on high blood pressure and vascular risk factors.
A working group in China analyzed 27 studies on the treatment of diabetes. A small number of randomized studies and a large number of cohort studies showed that modern antidiabetic drugs reduce the risk for dementia. The risk reduction for dementia for the GLP-1 receptor agonists was 75%. At the moment, there are only small prospective studies and they are not conclusive. Again, we need large-scale placebo-controlled studies.
The most important limitation at the moment beyond the cost is the other adverse drug reactions with the GLP-1 receptor agonists; these include nausea, vomiting, diarrhea, and constipation. There might be a slightly increased risk for pancreatitis. The US Food and Drug Administration recently reported there is no increased risk for suicide. Another potential adverse drug reaction is nonatherosclerotic anterior optic neuropathy.
These drugs, GLP-1 receptor agonists and GIP agonists, are also investigated in a variety of other non-neurologic diseases. The focus here is on metabolic liver disease, such as fatty liver and kidney diseases. Smaller, positive studies have been conducted in this area, and large placebo-controlled trials for both indications are currently underway.
If these diverse therapeutic properties would turn out to be really the case with GLP-1 receptor agonists, this would lead to a significant expansion of the range of indications. If we consider cost, this would be the end of our healthcare systems because we cannot afford this. In addition, the new antidiabetic drugs and the treatment of obesity are available only to a limited extent.
Finally, at least for neurology, it’s unclear whether the impact of these diseases is in the brain or whether it’s indirect, due to the effectiveness on vascular risk factors and concomitant diseases.
Dr. Diener is Professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany; he has disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I usually report five or six studies in the field of neurology that were published in the last months, but July was a vacation month.
I decided to cover another topic, which is the role of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists beyond diabetes and obesity, and in particular, for the field of neurology and psychiatry. Until a few years ago, the treatment of diabetes with traditional antidiabetic drugs was frustrating for vascular neurologists.
These drugs would lower glucose and had an impact on small-vessel disease, but they had no impact on large-vessel disease, stroke, and vascular mortality. This changed with the sodium-glucose cotransporter 2 antagonists because these drugs were not only effective for diabetes, but they also lowered cardiac mortality, in particular, in patients with cardiac failure.
The next generation of antidiabetic drugs were the GLP-1 receptor agonists and the combined GIP/GLP-1 receptor agonists. These two polypeptides and their receptors play a very important role in diabetes and in obesity. The receptors are found not only in the pancreas but also in the intestinal system, the liver, and the central nervous system.
We have a number of preclinical models, mostly in transgenic mice, which show that these drugs are not effective only in diabetes and obesity, but also in liver disease, kidney failure, and neurodegenerative diseases. GLP-1 receptor agonists also have powerful anti-inflammatory properties. These drugs reduce body weight, and they have positive effects on blood pressure and lipid metabolism.
In the studies on the use of GLP-1 receptor agonists in diabetes, a meta-analysis with more than 58,000 patients showed a significant risk reduction for stroke compared with placebo, and this risk reduction was in the range of 80%.
Stroke, Smoking, and Alcohol
A meta-analysis on the use of GLP-1 receptor agonists in over 30,000 nondiabetic patients with obesity found a significant reduction in blood pressure, mortality, and the risk of myocardial infarction. There was no significant decrease in the risk of stroke, but most probably this is due to the fact that strokes are much less frequent in obesity than in diabetes.
You all know that obesity is also a major risk factor for sleep apnea syndrome. Recently, two large studies with the GIP/GLP-1 receptor agonist tirzepatide found a significant improvement in sleep apnea syndrome compared to placebo, regardless of whether patients needed continuous positive airway pressure therapy or not.
In the therapy studies on diabetes and obesity, there were indications that some smokers in the studies stopped their nicotine consumption. A small pilot study with exenatide in 84 overweight patients who were smokers showed that 46% of patients on exenatide stopped smoking compared with 27% in the placebo group. This could be an indication that GLP-1 receptor agonists have activity on the reward system in the brain. Currently, there are a number of larger placebo-controlled trials ongoing.
Another aspect is alcohol consumption. An epidemiologic study in Denmark using data from the National Health Registry showed that the incidence of alcohol-related events decreased significantly in almost 40,000 patients with diabetes when they were treated with GLP-1 receptor agonists compared with other antidiabetic drugs.
A retrospective cohort study from the United States with over 80,000 patients with obesity showed that treatment with GLP-1 receptor agonists was associated with a 50%-60% lower risk for occurrence or recurrence of high alcohol consumption. There is only one small study with exenatide, which was not really informative.
There are a number of studies underway for GLP-1 receptor agonists compared with placebo in patients with alcohol dependence or alcohol consumption. Preclinical models also indicate that these drugs might be effective in cocaine abuse, and there is one placebo-controlled study ongoing.
Parkinson’s Disease
Let’s come to neurology. Preclinical models of Parkinson’s disease have shown neuroprotective activities of GLP-1. Until now, we have three randomized placebo-controlled trials with exenatide, NLY01, and lixisenatide. Two of these studies were positive, showing that the symptoms of Parkinson’s disease were stable over time and deteriorated with placebo. One study was neutral. This means we need more large-scale placebo-controlled studies in the early phases of Parkinson’s disease.
Another potential use of GIP/GLP-1 receptor agonists is in dementia. These substances, as you know, have positive effects on high blood pressure and vascular risk factors.
A working group in China analyzed 27 studies on the treatment of diabetes. A small number of randomized studies and a large number of cohort studies showed that modern antidiabetic drugs reduce the risk for dementia. The risk reduction for dementia for the GLP-1 receptor agonists was 75%. At the moment, there are only small prospective studies and they are not conclusive. Again, we need large-scale placebo-controlled studies.
The most important limitation at the moment beyond the cost is the other adverse drug reactions with the GLP-1 receptor agonists; these include nausea, vomiting, diarrhea, and constipation. There might be a slightly increased risk for pancreatitis. The US Food and Drug Administration recently reported there is no increased risk for suicide. Another potential adverse drug reaction is nonatherosclerotic anterior optic neuropathy.
These drugs, GLP-1 receptor agonists and GIP agonists, are also investigated in a variety of other non-neurologic diseases. The focus here is on metabolic liver disease, such as fatty liver and kidney diseases. Smaller, positive studies have been conducted in this area, and large placebo-controlled trials for both indications are currently underway.
If these diverse therapeutic properties would turn out to be really the case with GLP-1 receptor agonists, this would lead to a significant expansion of the range of indications. If we consider cost, this would be the end of our healthcare systems because we cannot afford this. In addition, the new antidiabetic drugs and the treatment of obesity are available only to a limited extent.
Finally, at least for neurology, it’s unclear whether the impact of these diseases is in the brain or whether it’s indirect, due to the effectiveness on vascular risk factors and concomitant diseases.
Dr. Diener is Professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany; he has disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
What Every Provider Should Know About Type 1 Diabetes
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
In July 2024, a 33-year-old woman with type 1 diabetes was boating on a hot day when her insulin delivery device slipped off. By the time she was able to exit the river, she was clearly ill, and an ambulance was called. The hospital was at capacity. Lying in the hallway, she was treated with fluids but not insulin, despite her boyfriend repeatedly telling the staff she had diabetes. She was released while still vomiting. The next morning, her boyfriend found her dead.
This story was shared by a friend of the woman in a Facebook group for people with type 1 diabetes and later confirmed by the boyfriend in a separate heartbreaking post. While it may be an extreme case,
In my 50+ years of living with the condition, I’ve lost track of the number of times I’ve had to speak up for myself, correct errors, raise issues that haven’t been considered, and educate nonspecialist healthcare professionals about even some of the basics.
Type 1 diabetes is an autoimmune condition in which the insulin-producing cells in the pancreas are destroyed, necessitating lifelong insulin treatment. Type 2, in contrast, arises from a combination of insulin resistance and decreased insulin production. Type 1 accounts for just 5% of all people with diabetes, but at a prevalence of about 1 in 200, it’s not rare. And that’s not even counting the adults who have been misdiagnosed as having type 2 but who actually have type 1.
As a general rule, people with type 1 diabetes are more insulin sensitive than those with type 2 and more prone to both hyper- and hypoglycemia. Blood sugar levels tend to be more labile and less predictable, even under normal circumstances. Recent advances in hybrid closed-loop technology have been extremely helpful in reducing the swings, but the systems aren’t foolproof yet. They still require user input (ie, guesswork), so there’s still room for error.
Managing type 1 diabetes is challenging even for endocrinologists. But here are some very important basics that every healthcare provider should know.
We Need Insulin 24/7
Never, ever withhold insulin from a person with type 1 diabetes, for any reason. Even when not eating — or when vomiting — we still need basal (background) insulin, either via long-acting analog or a pump infusion. The dose may need to be lowered to avoid hypoglycemia, but if insulin is stopped, diabetic ketoacidosis will result. And if that continues, death will follow.
This should be basic knowledge, but I’ve read and heard far too many stories of insulin being withheld from people with type 1 in various settings, including emergency departments, psychiatric facilities, and jails. On Facebook, people with type 1 diabetes often report being told not to take their insulin the morning before a procedure, while more than one has described “sneaking” their own insulin while hospitalized because they weren’t receiving any or not receiving enough.
On the flip side, although insulin needs are very individual, the amount needed for someone with type 1 is typically considerably less than for a person with type 2. Too much can result in severe hypoglycemia. There are lots of stories from people with type 1 diabetes who had to battle with hospital staff who tried to give them much higher doses than they knew they needed.
The American Diabetes Association recommends that people with type 1 diabetes who are hospitalized be allowed to wear their devices and self-manage to the degree possible. And please, listen to us when we tell you what we know about our own condition.
Fasting Is Fraught
I cringe every time I’m told to fast for a test or procedure. Fasting poses a risk for hypoglycemia in people with type 1 diabetes, even when using state-of-the-art technology. Fasting should not be required unless absolutely necessary, especially for routine lab tests.
Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University, East Lansing, Michigan, has published several papers on a phenomenon he calls “Fasting-Evoked En Route Hypoglycemia in Diabetes,” in which patients who fast overnight and skip breakfast experience hypoglycemia on the way to the lab.
“Patients continue taking their diabetes medication but don’t eat anything, resulting in low blood sugar levels that cause them to have a hypoglycemic event while driving to or from the lab, putting themselves and others at risk,” Dr. Aldasouqi explained, adding that fasting often isn’t necessary for routine lipid panels.
If fasting is necessary, as for a surgical procedure that involves anesthesia, the need for insulin adjustment — NOT withholding — should be discussed with the patient to determine whether they can do it themselves or whether their diabetes provider should be consulted.
But again, this is tricky even for endocrinologists. True story: When I had my second carpal tunnel surgery in July 2019, my hand surgeon wisely scheduled me for his first procedure in the morning to minimize the length of time I’d have to fast. (He has type 1 diabetes himself, which helped.) My endocrinologist had advised me, per guidelines, to cut back my basal insulin infusion on my pump by 20% before going to bed.
But at bedtime, my continuous glucose monitor (CGM) showed that I was in the 170 mg/dL’s and rising, not entirely surprising since I’d cut back on my predinner insulin dose knowing I wouldn’t be able to eat if I dropped low later. I didn’t cut back the basal.
When I woke up, my glucose level was over 300 mg/dL. This time, stress was the likely cause. (That’s happened before.) Despite giving myself several small insulin boluses that morning without eating, my blood sugar was still about 345 mg/dL when I arrived at the hospital. The nurse told me that if it had been over 375 mg/dL, they would have had to cancel the surgery, but it wasn’t, so they went ahead. I have no idea how they came up with that cutoff.
Anyway, thankfully, everything went fine; I brought my blood sugar back in target range afterward and healed normally. Point being, type 1 diabetes management is a crazy balancing act, and guidelines only go so far.
We Don’t React Well to Steroids
If it’s absolutely necessary to give steroids to a person with type 1 diabetes for any reason, plans must be made in advance for the inevitable glucose spike. If the person doesn’t know how to adjust their insulin for it, please have them consult their diabetes provider. In my experience with locally injected corticosteroids, the spike is always higher and longer than I expected. Thankfully, I haven’t had to deal with systemic steroids, but my guess is they’re probably worse.
Procedures Can Be Pesky
People who wear insulin pumps and/or CGMs must remove them for MRI and certain other imaging procedures. In some cases — as with CGMs and the Omnipod insulin delivery device that can’t be put back on after removal — this necessitates advance planning to bring along replacement equipment for immediately after the procedure.
Diabetes devices can stay in place for other imaging studies, such as x-rays, most CT scans, ECGs, and ultrasounds. For heaven’s sake, don’t ask us to remove our devices if it isn’t totally necessary.
In general, surprises that affect blood sugar are a bad idea. I recently underwent a gastric emptying study. I knew the test would involve eating radioactive eggs, but I didn’t find out there’s also a jelly sandwich with two slices of white bread until the technician handed it to me and told me to eat it. I had to quickly give myself insulin, and of course my blood sugar spiked later. Had I been forewarned, I could have at least “pre-bolused” 15-20 minutes in advance to give the insulin more time to start working.
Another anecdote: Prior to a dental appointment that involved numbing my gums for an in-depth cleaning, my longtime dental hygienist told me “be sure to eat before you come.” I do appreciate her thinking of my diabetes. However, while that advice would have made sense long ago when treatment involved two daily insulin injections without dose adjustments, now it’s more complicated.
Today, when we eat foods containing carbohydrates, we typically take short-acting insulin, which can lead to hypoglycemia if the dose given exceeds the amount needed for the carbs, regardless of how much is eaten. Better to not eat at all (assuming the basal insulin dose is correct) or just eat protein. And for the provider, best to just tell the patient about the eating limitations and make sure they know how to handle them.
Duh, We Already Have Diabetes
I’ve heard of at least four instances in which pregnant women with type 1 diabetes have been ordered to undergo an oral glucose tolerance test to screen for gestational diabetes. In two cases, it was a “can you believe it?!” post on Facebook, with the women rightly refusing to take the test.
But in May 2024, a pregnant woman reported she actually drank the liquid, her blood sugar skyrocketed, she was vomiting, and she was in the midst of trying to bring her glucose level down with insulin on her own at home. She hadn’t objected to taking the test because “my ob.gyn. knows I have diabetes,” so she figured it was appropriate.
I don’t work in a healthcare setting, but here’s my guess: The ob.gyn. hadn’t actually ordered the test but had neglected to UN-order a routine test for a pregnant patient who already had diabetes and obviously should NOT be forced to drink a high-sugar liquid for no reason. If this is happening in pregnancies with type 1 diabetes, it most certainly could be as well for those with pre-existing type 2 diabetes. Clearly, something should be done to prevent this unnecessary and potentially harmful scenario.
In summary, I think I speak for everyone living with type 1 diabetes in saying that we would like to have confidence that healthcare providers in all settings can provide care for whatever brought us to them without adding to the daily burden we already carry. Let’s work together.
Reviewed by Saleh Aldasouqi, MD, chief of endocrinology at Michigan State University. A version of this article first appeared on Medscape.com.
New Tourniquet: The AED for Bleeding?
This discussion was recorded on July 12, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. I recently met an innovative young woman named Hannah Herbst while attending the annual Eagles EMS Conference in Fort Lauderdale, Florida.
Hannah Herbst is a graduate of Florida Atlantic University, selected for Forbes 30 Under 30, and founder of a company called Golden Hour Medical. She has a background in IT and developed an automated pneumatic tourniquet known as AutoTQ, which we’re going to discuss at length here.
Also joining us is Dr. Peter Antevy, a pediatric emergency physician and medical director for Davie Fire Rescue as well as Coral Springs Parkland Fire Rescue. Peter is a member of EMS Eagles Global Alliance and is highly involved in high-quality research in prehospital emergency care and is quite well known in Florida and nationally.
Welcome to both of you.
Hannah Herbst: Thank you very much. Very grateful to be here.
Dr. Glatter: Hannah, I’ll let you start by explaining what AutoTQ is and then compare that to a standard Combat Application Tourniquet (CAT).
Ms. Herbst: Thank you. Unfortunately, blood loss is a leading cause of preventable death and trauma. When there’s blood loss occurring from an arm or a leg, the easiest way to stop it is by applying a tourniquet, which is this compression type of device that you place above the site of bleeding, and it then applies a high amount of pressure to stop blood flow through the limb.
Currently, tourniquets on the market have failure rates as high as 84%. This became very real to me back in 2018, when I became aware of mass casualty incidents when I was a student. I became interested in how we can reimagine the conventional tourniquet and try to make it something that’s very user-friendly, much like an automated external defibrillator (AED).
My team and I developed AutoTQ, which is an automated tourniquet. which is a leading cause of tourniquet failure and being able to effectively administer treatment to a patient that may bleed out.
Tourniquet Failure Rates
Dr. Glatter: In terms of tourniquet failure, how often do standard tourniquets fail, like the CAT combat-type tourniquet?
Ms. Herbst: Unfortunately, they fail very frequently. There are several studies that have been conducted to evaluate this. Many of them occur immediately after training. They found failure rates between 80% and 90% for the current conventional CAT tourniquet immediately after training, which is very concerning.
Dr. Glatter: In terms of failure, was it the windlass aspect of the tourniquet that failed? Or was it something related to the actual strap? Was that in any way detailed?
Ms. Herbst: There are usually a few different failure points that have been found in the literature. One is placement. Many times, when you’re panicked, you don’t remember exactly how to place it. It should be placed high and tight above the bleed and not over a joint.
The second problem is inadequate tightness. For a CAT tourniquet to be effective, you have to get it extremely tight on that first pull before the windlass is activated, and many times people don’t remember that in the stress of the moment.
Dr. Glatter: Peter, in terms of tourniquet application by your medics in the field, certainly the CAT-type device has been in existence for quite a while. Hannah’s proposing a new iteration of how to do this, which is automated and simple. What is your take on such a device? And how did you learn about Hannah’s device?
Peter M. Antevy, MD: We’ve been training on tourniquets ever since the military data showed that there was an extreme benefit in using them. We’ve been doing training for many years, including our police officers. What we’ve noticed is that every time we gather everyone together to show them how to place a tourniquet — and we have to do one-on-one sessions with them — it’s not a device that they can easily put on. These are police officers who had the training last year.
Like Hannah said, most of the time they have a problem unraveling it and understanding how to actually place it. It’s easier on the arm than it is on the leg. You can imagine it would be harder to place it on your own leg, especially if you had an injury. Then, they don’t tighten it well enough, as Hannah just mentioned. In order for a tourniquet to really be placed properly, it’s going to hurt that person. Many people have that tendency not to want to tighten it as much as they can.
Having said that, how I got into all of this is because I’m the medical director for Coral Springs and Parkland, and unfortunately, we had the 2018 Valentine’s Day murders that happened where we lost 17 adults and kids. However, 17 people were saved that day, and the credit goes to our police officers who had tourniquets or chest seals on before those patients were brought out to EMS. Many lives were saved by the tourniquet.
If you look at the Boston Marathon massacre and many other events that have happened, I believe — and I’ve always believed — that tourniquets should be in the glove box of every citizen. It should be in every school room. They should be in buildings along with the AED.
In my town of Davie, we were the first in the country to add an ordinance that required a Stop the Bleed kit in the AED cabinet, and those were required by buildings of certain sizes. In order to get this lifesaving device everywhere, I think it has to be put into local ordinance and supported by states and by the national folks, which they are doing.
Trials Are Underway
Dr. Glatter: In terms of adoption of such a device, it certainly has to go through rigorous testing and maybe some trials. Hannah, where are you at with vetting this in terms of any type of trial? Has it been compared head to head with standard tourniquets?
Ms. Herbst: Yes, we’re currently doing large amounts of field testing. We’re doing testing on emergency vehicles and in the surgical setting with different customers. In addition, we’re running pilot studies at different universities and with different organizations, including the military, to make sure that this device is effective. We’re evaluating cognitive offloading of people. We’re hoping to start that study later this year. We’re excited to be doing this in a variety of settings.
We’re also testing the quality of it in different environmental conditions and under different atmospheric pressure. We’re doing everything we can to ensure the device is safe and effective. We’re excited to scale and fill our preorders and be able to develop this and deliver it to many people.
Dr. Glatter: I was wondering if you could describe the actual device. There’s a brain part of it and then, obviously, the strap aspect of it. I was curious about contamination and reusability issues.
Ms. Herbst: That’s a great question. One of the limitations of conventional tourniquets on the market is that they are single use, and often, it requires two tourniquets to stop a bleed, both of which have to be disposed of.
With AutoTQ, we have a reusable component and a disposable component. I actually have one here that I can show you. We have a cover on it that says: Stop bleed, slide up and power on. You just pull this cover off and then you have a few simple commands. You have powering the device on. I’ll just click this button: Tighten strap above bleeding, then press inflate. It delivers audible instructions telling you exactly how to use the device. Then, you tighten it above your bleed on the limb, and you press the inflate button. Then it administers air into the cuff and stops the patient’s bleed.
Tourniquet Conversion and Limb Salvage
Dr. Glatter: In terms of ischemia time, how can a device like this make it easier for us to know when to let the tourniquet down and allow some blood flow? Certainly, limb salvage is important, and we don’t want to have necrosis and so forth.
Dr. Antevy: That’s a great question. The limb salvage rate when tourniquets have been used is 85%. When used correctly, you can really improve the outcomes for many patients.
On the flip side of that, there’s something called tourniquet conversion. That’s exactly what you mentioned. It’s making sure that the tourniquet doesn’t stay on for too long of a time. If you can imagine a patient going to an outlying hospital where there’s no trauma center, and then that patient then has to be moved a couple hours to the trauma center, could you potentially have a tourniquet on for too long that then ends up causing the patient a bad outcome? The answer is yes.
I just had someone on my webinar recently describing the appropriate conversion techniques of tourniquets. You don’t find too much of that in the literature, but you really have to ensure that as you’re taking the tourniquet down, the bleeding is actually stopped. It’s not really recommended to take a tourniquet down if the patient was just acutely bleeding.
However, imagine a situation where a tourniquet was put on incorrectly. Let’s say a patient got nervous and they just put it on a patient who didn’t really need it. You really have to understand how to evaluate that wound to be sure that, as you’re taking the tourniquet down slowly, the patient doesn’t rebleed again.
There are two sides of the question, Rob. One is making sure it’s not on inappropriately. The second one is making sure it’s not on for too long, which ends up causing ischemia to that limb.
Dr. Glatter: Hannah, does your device collect data on the number of hours or minutes that the tourniquet has been up and then automatically deflate it in some sense to allow for that improvement in limb salvage?
Ms. Herbst: That’s a great question, and I really appreciate your answer as well, Dr Antevy. Ischemia time is a very important and critical component of tourniquet use. This is something, when we were designing AutoTQ, that we took into high consideration.
We found, when we evaluated AutoTQ vs a CAT tourniquet in a mannequin model, that AutoTQ can achieve cessation of hemorrhage at around 400 mm Hg of mercury, whereas CAT requires 700-800 mm Hg. Already our ischemia time is slightly extended just based on existing literature with pneumatic tourniquets because it can stop the bleed at a lower pressure, which causes less complications with the patient’s limb.
There are different features that we build out for different customers, so depending on what people want, it is possible to deflate the tourniquet. However, typically, you’re at the hospital within 30 minutes. It’s quick to get them there, and then the physician can treat and take that tourniquet down in a supervised and controlled setting.
Dr. Glatter: In terms of patients with obesity, do you have adjustable straps that will accommodate for that aspect?
Ms. Herbst: Yes, we have different cuff sizes to accommodate different limbs.
Will AutoTQ Be Available to the Public?
Dr. Glatter: Peter, in terms of usability in the prehospital setting, where do you think this is going in the next 3-5 years?
Dr. Antevy: I’ll start with the public safety sector of the United States, which is the one that is actually first on scene. Whether you’re talking about police officers or EMS, it would behoove us to have tourniquets everywhere. On all of my ambulances, across all of my agencies that I manage, we have quite a number of tourniquets.
Obviously, cost is a factor, and I know that Hannah has done a great job of making that brain reusable. All we have to do is purchase the straps, which are effectively the same cost, I understand, as a typical tourniquet you would purchase.
Moving forward though, however, I think that this has wide scalability to the public market, whether it be schools, office buildings, the glove box, and so on. It’s really impossible to teach somebody how to do this the right way, if you have to teach them how to put the strap on, tighten it correctly, and so on. If there was an easy way, like Hannah developed, of just putting it on and pushing a button, then I think that the outcomes and the scalability are much further beyond what we can do in EMS. I think there’s great value in both markets.
The ‘AED of Bleeding’: Rechargeable and Reusable
Dr. Glatter: This is the AED of bleeding. You have a device here that has wide-scale interest, certainly from the public and private sector.
Hannah, in terms of battery decay, how would that work out if it was in someone’s garage? Let’s just say someone purchased it and they hadn’t used it in 3 or 4 months. What type of decay are we looking at and can they rely on it?
Ms. Herbst: AutoTQ is rechargeable by a USB-C port, and our battery lasts for a year. Once a year, you’ll get an email reminder that says: “Hey, please charge your AutoTQ and make sure it’s up to the battery level.” We do everything in our power to make sure that our consumers are checking their batteries and that they’re ready to go.
Dr. Glatter: Is it heat and fire resistant? What, in terms of durability, does your device have?
Ms. Herbst: Just like any other medical device, we come with manufacturer recommendations for the upper and lower bounds of temperature and different storage recommendations. All of that is in our instructions for use.
Dr. Glatter: Peter, getting back to logistics. In terms of adoption, do you feel that, in the long term, this device will be something that we’re going to be seeing widely adopted just going forward?
Dr. Antevy: I do, and I’ll tell you why. When you look at AED use in this country, the odds of someone actually getting an AED and using it correctly are still very low. Part of that is because it’s complicated for many people to do. Getting tourniquets everywhere is step No. 1, and I think the federal government and the Stop the Bleed program is really making that happen.
We talked about ordinances, but ease of use, I think, is really the key. You have people who oftentimes have their child in cardiac arrest in front of them, and they won’t put two hands on their chest because they just are afraid of doing it.
When you have a device that’s a tourniquet, that’s a single-button turn on and single-button inflate, I think that would make it much more likely that a person will use that device when they’re passing the scene of an accident, as an example.
We’ve had many non–mass casualty incident events that have had tourniquets. We’ve had some media stories on them, where they’re just happening because someone got into a motor vehicle accident. It doesn’t have to be a school shooting. I think the tourniquets should be everywhere and should be easily used by everybody.
Managing Pain
Dr. Glatter: Regarding sedation, is there a need because of the pain involved with the application? How would you sedate a patient, pediatric or adult, who needs a tourniquet?
Dr. Antevy: We always evaluate people’s pain. If the patient is an extremist, we’re just going to be managing and trying to get them back to life. Once somebody is stabilized and is exhibiting pain of any sort, even, for example, after we intubate somebody, we have to sedate them and provide them pain control because they have a piece of plastic in their trachea.
It’s the same thing here for a tourniquet. These are painful, and we do have the appropriate medications on our vehicles to address that pain. Again, just simply the trauma itself is very painful. Yes, we do address that in EMS, and I would say most public agencies across this country would address pain appropriately.
Training on Tourniquet Use
Dr. Glatter: Hannah, can you talk a little bit about public training types of approaches? How would you train a consumer who purchases this type of device?
Ms. Herbst: A huge part of our mission is making blood loss prevention and control training accessible to a wide variety of people. One way that we’re able to do that is through our online training platform. When you purchase an AutoTQ kit, you plug it into your computer, and it walks you through the process of using it. It lets you practice on your own limb and on your buddy’s limb, just to be able to effectively apply it. We think this will have huge impacts in making sure that people are prepared and ready to stop the bleed with AutoTQ.
Dr. Glatter: Do you recommend people training once a month, in general, just to keep their skills up to use this? In the throes of a trauma and very chaotic situation, people sometimes lose their ability to think clearly and straightly.
Ms. Herbst: One of the studies we’re conducting is a learning curve study to try to figure out how quickly these skills degrade over time. We know that with the windlass tourniquet, it degrades within moments of training. With AutoTQ, we think the learning curve will last much longer. That’s something we’re evaluating, but we recommend people train as often as they can.
Dr. Antevy: Rob, if I can mention that there is a concept of just-in-time training. I think that with having the expectation that people are going to be training frequently, unfortunately, as many of us know, even with the AED as a perfect example, people don’t do that.
Yes. I would agree that you have to train at least once a year, is what I would say. At my office, we have a 2-hour training that goes over all these different items once a year.
The device itself should have the ability to allow you to figure out how to use it just in time, whether via video, or like Hannah’s device, by audio. I think that having both those things would make it more likely that the device be used when needed.
People panic, and if they have a device that can talk to them or walk them through it, they will be much more likely to use it at that time.
Final Takeaways
Dr. Glatter: Any other final thoughts or a few pearls for listeners to take away? Hannah, I’ll start with you.
Ms. Herbst: I’m very grateful for your time, and I’m very excited about the potential for AutoTQ. To me, it’s so exciting to see people preordering the device now. We’ve had people from school bus companies and small sports teams. I think, just like Dr Antevy said, tourniquets aren’t limited to mass casualty situations. Blood loss can happen anywhere and to anyone.
Being able to equip people and serve them to better prepare them for this happening to themselves, their friends, or their family is just the honor of a lifetime. Thank you very much for covering the device and for having me today.
Dr. Glatter: Of course, my pleasure. Peter?
Dr. Antevy: The citizens of this country, and everyone who lives across the world, has started to understand that there are things that we expect from our people, from the community. We expect them to do CPR for cardiac arrest. We expect them to know how to use an EpiPen. We expect them to know how to use an AED, and we also expect them to know how to stop bleeding with a tourniquet.
The American public has gotten to understand that these devices are very important. Having a device that’s easily used, that I can teach you in 10 seconds, that speaks to you — these are all things that make this product have great potential. I do look forward to the studies, not just the cadaver studies, but the real human studies.
I know Hannah is really a phenom and has been doing all these things so that this product can be on the shelves of Walmart and CVS one day. I commend you, Hannah, for everything you’re doing and wishing you the best of luck. We’re here for you.
Dr. Glatter: Same here. Congratulations on your innovative capability and what you’ve done to change the outcomes of bleeding related to penetrating trauma. Thank you so much.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. Hannah D. Herbst, BS, is a graduate of Florida Atlantic University, was selected for Forbes 30 Under 30, and is the founder/CEO of Golden Hour Medical. Peter M. Antevy, MD, is a pediatric emergency medicine physician and medical director for Davie Fire Rescue and Coral Springs–Parkland Fire Department in Florida. He is also a member of the EMS Eagles Global Alliance.
A version of this article first appeared on Medscape.com.
This discussion was recorded on July 12, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. I recently met an innovative young woman named Hannah Herbst while attending the annual Eagles EMS Conference in Fort Lauderdale, Florida.
Hannah Herbst is a graduate of Florida Atlantic University, selected for Forbes 30 Under 30, and founder of a company called Golden Hour Medical. She has a background in IT and developed an automated pneumatic tourniquet known as AutoTQ, which we’re going to discuss at length here.
Also joining us is Dr. Peter Antevy, a pediatric emergency physician and medical director for Davie Fire Rescue as well as Coral Springs Parkland Fire Rescue. Peter is a member of EMS Eagles Global Alliance and is highly involved in high-quality research in prehospital emergency care and is quite well known in Florida and nationally.
Welcome to both of you.
Hannah Herbst: Thank you very much. Very grateful to be here.
Dr. Glatter: Hannah, I’ll let you start by explaining what AutoTQ is and then compare that to a standard Combat Application Tourniquet (CAT).
Ms. Herbst: Thank you. Unfortunately, blood loss is a leading cause of preventable death and trauma. When there’s blood loss occurring from an arm or a leg, the easiest way to stop it is by applying a tourniquet, which is this compression type of device that you place above the site of bleeding, and it then applies a high amount of pressure to stop blood flow through the limb.
Currently, tourniquets on the market have failure rates as high as 84%. This became very real to me back in 2018, when I became aware of mass casualty incidents when I was a student. I became interested in how we can reimagine the conventional tourniquet and try to make it something that’s very user-friendly, much like an automated external defibrillator (AED).
My team and I developed AutoTQ, which is an automated tourniquet. which is a leading cause of tourniquet failure and being able to effectively administer treatment to a patient that may bleed out.
Tourniquet Failure Rates
Dr. Glatter: In terms of tourniquet failure, how often do standard tourniquets fail, like the CAT combat-type tourniquet?
Ms. Herbst: Unfortunately, they fail very frequently. There are several studies that have been conducted to evaluate this. Many of them occur immediately after training. They found failure rates between 80% and 90% for the current conventional CAT tourniquet immediately after training, which is very concerning.
Dr. Glatter: In terms of failure, was it the windlass aspect of the tourniquet that failed? Or was it something related to the actual strap? Was that in any way detailed?
Ms. Herbst: There are usually a few different failure points that have been found in the literature. One is placement. Many times, when you’re panicked, you don’t remember exactly how to place it. It should be placed high and tight above the bleed and not over a joint.
The second problem is inadequate tightness. For a CAT tourniquet to be effective, you have to get it extremely tight on that first pull before the windlass is activated, and many times people don’t remember that in the stress of the moment.
Dr. Glatter: Peter, in terms of tourniquet application by your medics in the field, certainly the CAT-type device has been in existence for quite a while. Hannah’s proposing a new iteration of how to do this, which is automated and simple. What is your take on such a device? And how did you learn about Hannah’s device?
Peter M. Antevy, MD: We’ve been training on tourniquets ever since the military data showed that there was an extreme benefit in using them. We’ve been doing training for many years, including our police officers. What we’ve noticed is that every time we gather everyone together to show them how to place a tourniquet — and we have to do one-on-one sessions with them — it’s not a device that they can easily put on. These are police officers who had the training last year.
Like Hannah said, most of the time they have a problem unraveling it and understanding how to actually place it. It’s easier on the arm than it is on the leg. You can imagine it would be harder to place it on your own leg, especially if you had an injury. Then, they don’t tighten it well enough, as Hannah just mentioned. In order for a tourniquet to really be placed properly, it’s going to hurt that person. Many people have that tendency not to want to tighten it as much as they can.
Having said that, how I got into all of this is because I’m the medical director for Coral Springs and Parkland, and unfortunately, we had the 2018 Valentine’s Day murders that happened where we lost 17 adults and kids. However, 17 people were saved that day, and the credit goes to our police officers who had tourniquets or chest seals on before those patients were brought out to EMS. Many lives were saved by the tourniquet.
If you look at the Boston Marathon massacre and many other events that have happened, I believe — and I’ve always believed — that tourniquets should be in the glove box of every citizen. It should be in every school room. They should be in buildings along with the AED.
In my town of Davie, we were the first in the country to add an ordinance that required a Stop the Bleed kit in the AED cabinet, and those were required by buildings of certain sizes. In order to get this lifesaving device everywhere, I think it has to be put into local ordinance and supported by states and by the national folks, which they are doing.
Trials Are Underway
Dr. Glatter: In terms of adoption of such a device, it certainly has to go through rigorous testing and maybe some trials. Hannah, where are you at with vetting this in terms of any type of trial? Has it been compared head to head with standard tourniquets?
Ms. Herbst: Yes, we’re currently doing large amounts of field testing. We’re doing testing on emergency vehicles and in the surgical setting with different customers. In addition, we’re running pilot studies at different universities and with different organizations, including the military, to make sure that this device is effective. We’re evaluating cognitive offloading of people. We’re hoping to start that study later this year. We’re excited to be doing this in a variety of settings.
We’re also testing the quality of it in different environmental conditions and under different atmospheric pressure. We’re doing everything we can to ensure the device is safe and effective. We’re excited to scale and fill our preorders and be able to develop this and deliver it to many people.
Dr. Glatter: I was wondering if you could describe the actual device. There’s a brain part of it and then, obviously, the strap aspect of it. I was curious about contamination and reusability issues.
Ms. Herbst: That’s a great question. One of the limitations of conventional tourniquets on the market is that they are single use, and often, it requires two tourniquets to stop a bleed, both of which have to be disposed of.
With AutoTQ, we have a reusable component and a disposable component. I actually have one here that I can show you. We have a cover on it that says: Stop bleed, slide up and power on. You just pull this cover off and then you have a few simple commands. You have powering the device on. I’ll just click this button: Tighten strap above bleeding, then press inflate. It delivers audible instructions telling you exactly how to use the device. Then, you tighten it above your bleed on the limb, and you press the inflate button. Then it administers air into the cuff and stops the patient’s bleed.
Tourniquet Conversion and Limb Salvage
Dr. Glatter: In terms of ischemia time, how can a device like this make it easier for us to know when to let the tourniquet down and allow some blood flow? Certainly, limb salvage is important, and we don’t want to have necrosis and so forth.
Dr. Antevy: That’s a great question. The limb salvage rate when tourniquets have been used is 85%. When used correctly, you can really improve the outcomes for many patients.
On the flip side of that, there’s something called tourniquet conversion. That’s exactly what you mentioned. It’s making sure that the tourniquet doesn’t stay on for too long of a time. If you can imagine a patient going to an outlying hospital where there’s no trauma center, and then that patient then has to be moved a couple hours to the trauma center, could you potentially have a tourniquet on for too long that then ends up causing the patient a bad outcome? The answer is yes.
I just had someone on my webinar recently describing the appropriate conversion techniques of tourniquets. You don’t find too much of that in the literature, but you really have to ensure that as you’re taking the tourniquet down, the bleeding is actually stopped. It’s not really recommended to take a tourniquet down if the patient was just acutely bleeding.
However, imagine a situation where a tourniquet was put on incorrectly. Let’s say a patient got nervous and they just put it on a patient who didn’t really need it. You really have to understand how to evaluate that wound to be sure that, as you’re taking the tourniquet down slowly, the patient doesn’t rebleed again.
There are two sides of the question, Rob. One is making sure it’s not on inappropriately. The second one is making sure it’s not on for too long, which ends up causing ischemia to that limb.
Dr. Glatter: Hannah, does your device collect data on the number of hours or minutes that the tourniquet has been up and then automatically deflate it in some sense to allow for that improvement in limb salvage?
Ms. Herbst: That’s a great question, and I really appreciate your answer as well, Dr Antevy. Ischemia time is a very important and critical component of tourniquet use. This is something, when we were designing AutoTQ, that we took into high consideration.
We found, when we evaluated AutoTQ vs a CAT tourniquet in a mannequin model, that AutoTQ can achieve cessation of hemorrhage at around 400 mm Hg of mercury, whereas CAT requires 700-800 mm Hg. Already our ischemia time is slightly extended just based on existing literature with pneumatic tourniquets because it can stop the bleed at a lower pressure, which causes less complications with the patient’s limb.
There are different features that we build out for different customers, so depending on what people want, it is possible to deflate the tourniquet. However, typically, you’re at the hospital within 30 minutes. It’s quick to get them there, and then the physician can treat and take that tourniquet down in a supervised and controlled setting.
Dr. Glatter: In terms of patients with obesity, do you have adjustable straps that will accommodate for that aspect?
Ms. Herbst: Yes, we have different cuff sizes to accommodate different limbs.
Will AutoTQ Be Available to the Public?
Dr. Glatter: Peter, in terms of usability in the prehospital setting, where do you think this is going in the next 3-5 years?
Dr. Antevy: I’ll start with the public safety sector of the United States, which is the one that is actually first on scene. Whether you’re talking about police officers or EMS, it would behoove us to have tourniquets everywhere. On all of my ambulances, across all of my agencies that I manage, we have quite a number of tourniquets.
Obviously, cost is a factor, and I know that Hannah has done a great job of making that brain reusable. All we have to do is purchase the straps, which are effectively the same cost, I understand, as a typical tourniquet you would purchase.
Moving forward though, however, I think that this has wide scalability to the public market, whether it be schools, office buildings, the glove box, and so on. It’s really impossible to teach somebody how to do this the right way, if you have to teach them how to put the strap on, tighten it correctly, and so on. If there was an easy way, like Hannah developed, of just putting it on and pushing a button, then I think that the outcomes and the scalability are much further beyond what we can do in EMS. I think there’s great value in both markets.
The ‘AED of Bleeding’: Rechargeable and Reusable
Dr. Glatter: This is the AED of bleeding. You have a device here that has wide-scale interest, certainly from the public and private sector.
Hannah, in terms of battery decay, how would that work out if it was in someone’s garage? Let’s just say someone purchased it and they hadn’t used it in 3 or 4 months. What type of decay are we looking at and can they rely on it?
Ms. Herbst: AutoTQ is rechargeable by a USB-C port, and our battery lasts for a year. Once a year, you’ll get an email reminder that says: “Hey, please charge your AutoTQ and make sure it’s up to the battery level.” We do everything in our power to make sure that our consumers are checking their batteries and that they’re ready to go.
Dr. Glatter: Is it heat and fire resistant? What, in terms of durability, does your device have?
Ms. Herbst: Just like any other medical device, we come with manufacturer recommendations for the upper and lower bounds of temperature and different storage recommendations. All of that is in our instructions for use.
Dr. Glatter: Peter, getting back to logistics. In terms of adoption, do you feel that, in the long term, this device will be something that we’re going to be seeing widely adopted just going forward?
Dr. Antevy: I do, and I’ll tell you why. When you look at AED use in this country, the odds of someone actually getting an AED and using it correctly are still very low. Part of that is because it’s complicated for many people to do. Getting tourniquets everywhere is step No. 1, and I think the federal government and the Stop the Bleed program is really making that happen.
We talked about ordinances, but ease of use, I think, is really the key. You have people who oftentimes have their child in cardiac arrest in front of them, and they won’t put two hands on their chest because they just are afraid of doing it.
When you have a device that’s a tourniquet, that’s a single-button turn on and single-button inflate, I think that would make it much more likely that a person will use that device when they’re passing the scene of an accident, as an example.
We’ve had many non–mass casualty incident events that have had tourniquets. We’ve had some media stories on them, where they’re just happening because someone got into a motor vehicle accident. It doesn’t have to be a school shooting. I think the tourniquets should be everywhere and should be easily used by everybody.
Managing Pain
Dr. Glatter: Regarding sedation, is there a need because of the pain involved with the application? How would you sedate a patient, pediatric or adult, who needs a tourniquet?
Dr. Antevy: We always evaluate people’s pain. If the patient is an extremist, we’re just going to be managing and trying to get them back to life. Once somebody is stabilized and is exhibiting pain of any sort, even, for example, after we intubate somebody, we have to sedate them and provide them pain control because they have a piece of plastic in their trachea.
It’s the same thing here for a tourniquet. These are painful, and we do have the appropriate medications on our vehicles to address that pain. Again, just simply the trauma itself is very painful. Yes, we do address that in EMS, and I would say most public agencies across this country would address pain appropriately.
Training on Tourniquet Use
Dr. Glatter: Hannah, can you talk a little bit about public training types of approaches? How would you train a consumer who purchases this type of device?
Ms. Herbst: A huge part of our mission is making blood loss prevention and control training accessible to a wide variety of people. One way that we’re able to do that is through our online training platform. When you purchase an AutoTQ kit, you plug it into your computer, and it walks you through the process of using it. It lets you practice on your own limb and on your buddy’s limb, just to be able to effectively apply it. We think this will have huge impacts in making sure that people are prepared and ready to stop the bleed with AutoTQ.
Dr. Glatter: Do you recommend people training once a month, in general, just to keep their skills up to use this? In the throes of a trauma and very chaotic situation, people sometimes lose their ability to think clearly and straightly.
Ms. Herbst: One of the studies we’re conducting is a learning curve study to try to figure out how quickly these skills degrade over time. We know that with the windlass tourniquet, it degrades within moments of training. With AutoTQ, we think the learning curve will last much longer. That’s something we’re evaluating, but we recommend people train as often as they can.
Dr. Antevy: Rob, if I can mention that there is a concept of just-in-time training. I think that with having the expectation that people are going to be training frequently, unfortunately, as many of us know, even with the AED as a perfect example, people don’t do that.
Yes. I would agree that you have to train at least once a year, is what I would say. At my office, we have a 2-hour training that goes over all these different items once a year.
The device itself should have the ability to allow you to figure out how to use it just in time, whether via video, or like Hannah’s device, by audio. I think that having both those things would make it more likely that the device be used when needed.
People panic, and if they have a device that can talk to them or walk them through it, they will be much more likely to use it at that time.
Final Takeaways
Dr. Glatter: Any other final thoughts or a few pearls for listeners to take away? Hannah, I’ll start with you.
Ms. Herbst: I’m very grateful for your time, and I’m very excited about the potential for AutoTQ. To me, it’s so exciting to see people preordering the device now. We’ve had people from school bus companies and small sports teams. I think, just like Dr Antevy said, tourniquets aren’t limited to mass casualty situations. Blood loss can happen anywhere and to anyone.
Being able to equip people and serve them to better prepare them for this happening to themselves, their friends, or their family is just the honor of a lifetime. Thank you very much for covering the device and for having me today.
Dr. Glatter: Of course, my pleasure. Peter?
Dr. Antevy: The citizens of this country, and everyone who lives across the world, has started to understand that there are things that we expect from our people, from the community. We expect them to do CPR for cardiac arrest. We expect them to know how to use an EpiPen. We expect them to know how to use an AED, and we also expect them to know how to stop bleeding with a tourniquet.
The American public has gotten to understand that these devices are very important. Having a device that’s easily used, that I can teach you in 10 seconds, that speaks to you — these are all things that make this product have great potential. I do look forward to the studies, not just the cadaver studies, but the real human studies.
I know Hannah is really a phenom and has been doing all these things so that this product can be on the shelves of Walmart and CVS one day. I commend you, Hannah, for everything you’re doing and wishing you the best of luck. We’re here for you.
Dr. Glatter: Same here. Congratulations on your innovative capability and what you’ve done to change the outcomes of bleeding related to penetrating trauma. Thank you so much.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. Hannah D. Herbst, BS, is a graduate of Florida Atlantic University, was selected for Forbes 30 Under 30, and is the founder/CEO of Golden Hour Medical. Peter M. Antevy, MD, is a pediatric emergency medicine physician and medical director for Davie Fire Rescue and Coral Springs–Parkland Fire Department in Florida. He is also a member of the EMS Eagles Global Alliance.
A version of this article first appeared on Medscape.com.
This discussion was recorded on July 12, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. I recently met an innovative young woman named Hannah Herbst while attending the annual Eagles EMS Conference in Fort Lauderdale, Florida.
Hannah Herbst is a graduate of Florida Atlantic University, selected for Forbes 30 Under 30, and founder of a company called Golden Hour Medical. She has a background in IT and developed an automated pneumatic tourniquet known as AutoTQ, which we’re going to discuss at length here.
Also joining us is Dr. Peter Antevy, a pediatric emergency physician and medical director for Davie Fire Rescue as well as Coral Springs Parkland Fire Rescue. Peter is a member of EMS Eagles Global Alliance and is highly involved in high-quality research in prehospital emergency care and is quite well known in Florida and nationally.
Welcome to both of you.
Hannah Herbst: Thank you very much. Very grateful to be here.
Dr. Glatter: Hannah, I’ll let you start by explaining what AutoTQ is and then compare that to a standard Combat Application Tourniquet (CAT).
Ms. Herbst: Thank you. Unfortunately, blood loss is a leading cause of preventable death and trauma. When there’s blood loss occurring from an arm or a leg, the easiest way to stop it is by applying a tourniquet, which is this compression type of device that you place above the site of bleeding, and it then applies a high amount of pressure to stop blood flow through the limb.
Currently, tourniquets on the market have failure rates as high as 84%. This became very real to me back in 2018, when I became aware of mass casualty incidents when I was a student. I became interested in how we can reimagine the conventional tourniquet and try to make it something that’s very user-friendly, much like an automated external defibrillator (AED).
My team and I developed AutoTQ, which is an automated tourniquet. which is a leading cause of tourniquet failure and being able to effectively administer treatment to a patient that may bleed out.
Tourniquet Failure Rates
Dr. Glatter: In terms of tourniquet failure, how often do standard tourniquets fail, like the CAT combat-type tourniquet?
Ms. Herbst: Unfortunately, they fail very frequently. There are several studies that have been conducted to evaluate this. Many of them occur immediately after training. They found failure rates between 80% and 90% for the current conventional CAT tourniquet immediately after training, which is very concerning.
Dr. Glatter: In terms of failure, was it the windlass aspect of the tourniquet that failed? Or was it something related to the actual strap? Was that in any way detailed?
Ms. Herbst: There are usually a few different failure points that have been found in the literature. One is placement. Many times, when you’re panicked, you don’t remember exactly how to place it. It should be placed high and tight above the bleed and not over a joint.
The second problem is inadequate tightness. For a CAT tourniquet to be effective, you have to get it extremely tight on that first pull before the windlass is activated, and many times people don’t remember that in the stress of the moment.
Dr. Glatter: Peter, in terms of tourniquet application by your medics in the field, certainly the CAT-type device has been in existence for quite a while. Hannah’s proposing a new iteration of how to do this, which is automated and simple. What is your take on such a device? And how did you learn about Hannah’s device?
Peter M. Antevy, MD: We’ve been training on tourniquets ever since the military data showed that there was an extreme benefit in using them. We’ve been doing training for many years, including our police officers. What we’ve noticed is that every time we gather everyone together to show them how to place a tourniquet — and we have to do one-on-one sessions with them — it’s not a device that they can easily put on. These are police officers who had the training last year.
Like Hannah said, most of the time they have a problem unraveling it and understanding how to actually place it. It’s easier on the arm than it is on the leg. You can imagine it would be harder to place it on your own leg, especially if you had an injury. Then, they don’t tighten it well enough, as Hannah just mentioned. In order for a tourniquet to really be placed properly, it’s going to hurt that person. Many people have that tendency not to want to tighten it as much as they can.
Having said that, how I got into all of this is because I’m the medical director for Coral Springs and Parkland, and unfortunately, we had the 2018 Valentine’s Day murders that happened where we lost 17 adults and kids. However, 17 people were saved that day, and the credit goes to our police officers who had tourniquets or chest seals on before those patients were brought out to EMS. Many lives were saved by the tourniquet.
If you look at the Boston Marathon massacre and many other events that have happened, I believe — and I’ve always believed — that tourniquets should be in the glove box of every citizen. It should be in every school room. They should be in buildings along with the AED.
In my town of Davie, we were the first in the country to add an ordinance that required a Stop the Bleed kit in the AED cabinet, and those were required by buildings of certain sizes. In order to get this lifesaving device everywhere, I think it has to be put into local ordinance and supported by states and by the national folks, which they are doing.
Trials Are Underway
Dr. Glatter: In terms of adoption of such a device, it certainly has to go through rigorous testing and maybe some trials. Hannah, where are you at with vetting this in terms of any type of trial? Has it been compared head to head with standard tourniquets?
Ms. Herbst: Yes, we’re currently doing large amounts of field testing. We’re doing testing on emergency vehicles and in the surgical setting with different customers. In addition, we’re running pilot studies at different universities and with different organizations, including the military, to make sure that this device is effective. We’re evaluating cognitive offloading of people. We’re hoping to start that study later this year. We’re excited to be doing this in a variety of settings.
We’re also testing the quality of it in different environmental conditions and under different atmospheric pressure. We’re doing everything we can to ensure the device is safe and effective. We’re excited to scale and fill our preorders and be able to develop this and deliver it to many people.
Dr. Glatter: I was wondering if you could describe the actual device. There’s a brain part of it and then, obviously, the strap aspect of it. I was curious about contamination and reusability issues.
Ms. Herbst: That’s a great question. One of the limitations of conventional tourniquets on the market is that they are single use, and often, it requires two tourniquets to stop a bleed, both of which have to be disposed of.
With AutoTQ, we have a reusable component and a disposable component. I actually have one here that I can show you. We have a cover on it that says: Stop bleed, slide up and power on. You just pull this cover off and then you have a few simple commands. You have powering the device on. I’ll just click this button: Tighten strap above bleeding, then press inflate. It delivers audible instructions telling you exactly how to use the device. Then, you tighten it above your bleed on the limb, and you press the inflate button. Then it administers air into the cuff and stops the patient’s bleed.
Tourniquet Conversion and Limb Salvage
Dr. Glatter: In terms of ischemia time, how can a device like this make it easier for us to know when to let the tourniquet down and allow some blood flow? Certainly, limb salvage is important, and we don’t want to have necrosis and so forth.
Dr. Antevy: That’s a great question. The limb salvage rate when tourniquets have been used is 85%. When used correctly, you can really improve the outcomes for many patients.
On the flip side of that, there’s something called tourniquet conversion. That’s exactly what you mentioned. It’s making sure that the tourniquet doesn’t stay on for too long of a time. If you can imagine a patient going to an outlying hospital where there’s no trauma center, and then that patient then has to be moved a couple hours to the trauma center, could you potentially have a tourniquet on for too long that then ends up causing the patient a bad outcome? The answer is yes.
I just had someone on my webinar recently describing the appropriate conversion techniques of tourniquets. You don’t find too much of that in the literature, but you really have to ensure that as you’re taking the tourniquet down, the bleeding is actually stopped. It’s not really recommended to take a tourniquet down if the patient was just acutely bleeding.
However, imagine a situation where a tourniquet was put on incorrectly. Let’s say a patient got nervous and they just put it on a patient who didn’t really need it. You really have to understand how to evaluate that wound to be sure that, as you’re taking the tourniquet down slowly, the patient doesn’t rebleed again.
There are two sides of the question, Rob. One is making sure it’s not on inappropriately. The second one is making sure it’s not on for too long, which ends up causing ischemia to that limb.
Dr. Glatter: Hannah, does your device collect data on the number of hours or minutes that the tourniquet has been up and then automatically deflate it in some sense to allow for that improvement in limb salvage?
Ms. Herbst: That’s a great question, and I really appreciate your answer as well, Dr Antevy. Ischemia time is a very important and critical component of tourniquet use. This is something, when we were designing AutoTQ, that we took into high consideration.
We found, when we evaluated AutoTQ vs a CAT tourniquet in a mannequin model, that AutoTQ can achieve cessation of hemorrhage at around 400 mm Hg of mercury, whereas CAT requires 700-800 mm Hg. Already our ischemia time is slightly extended just based on existing literature with pneumatic tourniquets because it can stop the bleed at a lower pressure, which causes less complications with the patient’s limb.
There are different features that we build out for different customers, so depending on what people want, it is possible to deflate the tourniquet. However, typically, you’re at the hospital within 30 minutes. It’s quick to get them there, and then the physician can treat and take that tourniquet down in a supervised and controlled setting.
Dr. Glatter: In terms of patients with obesity, do you have adjustable straps that will accommodate for that aspect?
Ms. Herbst: Yes, we have different cuff sizes to accommodate different limbs.
Will AutoTQ Be Available to the Public?
Dr. Glatter: Peter, in terms of usability in the prehospital setting, where do you think this is going in the next 3-5 years?
Dr. Antevy: I’ll start with the public safety sector of the United States, which is the one that is actually first on scene. Whether you’re talking about police officers or EMS, it would behoove us to have tourniquets everywhere. On all of my ambulances, across all of my agencies that I manage, we have quite a number of tourniquets.
Obviously, cost is a factor, and I know that Hannah has done a great job of making that brain reusable. All we have to do is purchase the straps, which are effectively the same cost, I understand, as a typical tourniquet you would purchase.
Moving forward though, however, I think that this has wide scalability to the public market, whether it be schools, office buildings, the glove box, and so on. It’s really impossible to teach somebody how to do this the right way, if you have to teach them how to put the strap on, tighten it correctly, and so on. If there was an easy way, like Hannah developed, of just putting it on and pushing a button, then I think that the outcomes and the scalability are much further beyond what we can do in EMS. I think there’s great value in both markets.
The ‘AED of Bleeding’: Rechargeable and Reusable
Dr. Glatter: This is the AED of bleeding. You have a device here that has wide-scale interest, certainly from the public and private sector.
Hannah, in terms of battery decay, how would that work out if it was in someone’s garage? Let’s just say someone purchased it and they hadn’t used it in 3 or 4 months. What type of decay are we looking at and can they rely on it?
Ms. Herbst: AutoTQ is rechargeable by a USB-C port, and our battery lasts for a year. Once a year, you’ll get an email reminder that says: “Hey, please charge your AutoTQ and make sure it’s up to the battery level.” We do everything in our power to make sure that our consumers are checking their batteries and that they’re ready to go.
Dr. Glatter: Is it heat and fire resistant? What, in terms of durability, does your device have?
Ms. Herbst: Just like any other medical device, we come with manufacturer recommendations for the upper and lower bounds of temperature and different storage recommendations. All of that is in our instructions for use.
Dr. Glatter: Peter, getting back to logistics. In terms of adoption, do you feel that, in the long term, this device will be something that we’re going to be seeing widely adopted just going forward?
Dr. Antevy: I do, and I’ll tell you why. When you look at AED use in this country, the odds of someone actually getting an AED and using it correctly are still very low. Part of that is because it’s complicated for many people to do. Getting tourniquets everywhere is step No. 1, and I think the federal government and the Stop the Bleed program is really making that happen.
We talked about ordinances, but ease of use, I think, is really the key. You have people who oftentimes have their child in cardiac arrest in front of them, and they won’t put two hands on their chest because they just are afraid of doing it.
When you have a device that’s a tourniquet, that’s a single-button turn on and single-button inflate, I think that would make it much more likely that a person will use that device when they’re passing the scene of an accident, as an example.
We’ve had many non–mass casualty incident events that have had tourniquets. We’ve had some media stories on them, where they’re just happening because someone got into a motor vehicle accident. It doesn’t have to be a school shooting. I think the tourniquets should be everywhere and should be easily used by everybody.
Managing Pain
Dr. Glatter: Regarding sedation, is there a need because of the pain involved with the application? How would you sedate a patient, pediatric or adult, who needs a tourniquet?
Dr. Antevy: We always evaluate people’s pain. If the patient is an extremist, we’re just going to be managing and trying to get them back to life. Once somebody is stabilized and is exhibiting pain of any sort, even, for example, after we intubate somebody, we have to sedate them and provide them pain control because they have a piece of plastic in their trachea.
It’s the same thing here for a tourniquet. These are painful, and we do have the appropriate medications on our vehicles to address that pain. Again, just simply the trauma itself is very painful. Yes, we do address that in EMS, and I would say most public agencies across this country would address pain appropriately.
Training on Tourniquet Use
Dr. Glatter: Hannah, can you talk a little bit about public training types of approaches? How would you train a consumer who purchases this type of device?
Ms. Herbst: A huge part of our mission is making blood loss prevention and control training accessible to a wide variety of people. One way that we’re able to do that is through our online training platform. When you purchase an AutoTQ kit, you plug it into your computer, and it walks you through the process of using it. It lets you practice on your own limb and on your buddy’s limb, just to be able to effectively apply it. We think this will have huge impacts in making sure that people are prepared and ready to stop the bleed with AutoTQ.
Dr. Glatter: Do you recommend people training once a month, in general, just to keep their skills up to use this? In the throes of a trauma and very chaotic situation, people sometimes lose their ability to think clearly and straightly.
Ms. Herbst: One of the studies we’re conducting is a learning curve study to try to figure out how quickly these skills degrade over time. We know that with the windlass tourniquet, it degrades within moments of training. With AutoTQ, we think the learning curve will last much longer. That’s something we’re evaluating, but we recommend people train as often as they can.
Dr. Antevy: Rob, if I can mention that there is a concept of just-in-time training. I think that with having the expectation that people are going to be training frequently, unfortunately, as many of us know, even with the AED as a perfect example, people don’t do that.
Yes. I would agree that you have to train at least once a year, is what I would say. At my office, we have a 2-hour training that goes over all these different items once a year.
The device itself should have the ability to allow you to figure out how to use it just in time, whether via video, or like Hannah’s device, by audio. I think that having both those things would make it more likely that the device be used when needed.
People panic, and if they have a device that can talk to them or walk them through it, they will be much more likely to use it at that time.
Final Takeaways
Dr. Glatter: Any other final thoughts or a few pearls for listeners to take away? Hannah, I’ll start with you.
Ms. Herbst: I’m very grateful for your time, and I’m very excited about the potential for AutoTQ. To me, it’s so exciting to see people preordering the device now. We’ve had people from school bus companies and small sports teams. I think, just like Dr Antevy said, tourniquets aren’t limited to mass casualty situations. Blood loss can happen anywhere and to anyone.
Being able to equip people and serve them to better prepare them for this happening to themselves, their friends, or their family is just the honor of a lifetime. Thank you very much for covering the device and for having me today.
Dr. Glatter: Of course, my pleasure. Peter?
Dr. Antevy: The citizens of this country, and everyone who lives across the world, has started to understand that there are things that we expect from our people, from the community. We expect them to do CPR for cardiac arrest. We expect them to know how to use an EpiPen. We expect them to know how to use an AED, and we also expect them to know how to stop bleeding with a tourniquet.
The American public has gotten to understand that these devices are very important. Having a device that’s easily used, that I can teach you in 10 seconds, that speaks to you — these are all things that make this product have great potential. I do look forward to the studies, not just the cadaver studies, but the real human studies.
I know Hannah is really a phenom and has been doing all these things so that this product can be on the shelves of Walmart and CVS one day. I commend you, Hannah, for everything you’re doing and wishing you the best of luck. We’re here for you.
Dr. Glatter: Same here. Congratulations on your innovative capability and what you’ve done to change the outcomes of bleeding related to penetrating trauma. Thank you so much.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. Hannah D. Herbst, BS, is a graduate of Florida Atlantic University, was selected for Forbes 30 Under 30, and is the founder/CEO of Golden Hour Medical. Peter M. Antevy, MD, is a pediatric emergency medicine physician and medical director for Davie Fire Rescue and Coral Springs–Parkland Fire Department in Florida. He is also a member of the EMS Eagles Global Alliance.
A version of this article first appeared on Medscape.com.
Dementia Deemed Highly Preventable: Here’s How
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A Checklist for Compounded Semaglutide or Tirzepatide
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
Consider this: If you’re taking your children to the beach, how do you protect them from drowning? You don’t tell them, “Don’t go into the ocean.” You teach them how to swim; you give them floaties; and you accompany them in the water and go in only when a lifeguard is present. In other words, you give them all the tools to protect themselves because you know they will go into the ocean anyway.
Patients are diving into the ocean. Patients with obesity, who know that a treatment for their disease exists but is inaccessible, are diving into the ocean. Unfortunately, they are diving in without floaties or a lifeguard, and well-meaning bystanders are simply telling them to not go.
Compounded peptides are an ocean of alternative therapies. Even though compounding pharmacists need specialized training, facilities and equipment need to be properly certified, and final dosage forms need extensive testing, pharmacies are not equal when it comes to sterile compounding. Regulatory agencies such as the US Food and Drug Administration (FDA) have expressed caution around compounded semaglutide. Professional societies such as the Obesity Medicine Association (OMA) advise against compounded peptides because they lack clinical trials that prove their safety and efficacy. Ask any individual doctor and you are likely to receive a range of opinions.
As an endocrinologist specializing in obesity, I practice evidence-based medicine as much as possible. However, I also recognize how today’s dysfunctional medical system compels patients to dive into that ocean in search of an alternative solution.
With the help of pharmacists, compounding pharmacists, researchers, and clinicians, here is a checklist for patients who seek compounded semaglutide or tirzepatide:
- Check the state licensing board website to see if there have been any complaints or disciplinary actions made against the pharmacy facility. These government-maintained websites vary in searchability and user-friendliness, but you are specifically looking for whether the FDA ever issued a 483 form.
- Ask for the pharmacy’s state board inspection report. There should be at least one of these reports, issued at the pharmacy’s founding, and there may be more depending on individual state regulations on frequencies of inspections.
- Ask if the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation is an extra optional step that some compounding pharmacies take to be legitimized by a third party.
- Ask if the pharmacy follows Current Good Manufacturing Practice (CGMP). CGMP is not required of 503a pharmacies, which are pharmacies that provide semaglutide or tirzepatide directly to patients, but following CGMP means an extra level of quality assurance. The bare minimum for anyone doing sterile compounding in the United States is to meet the standards found in the US Pharmacopeia, chapter <797>, Sterile Compounding.
- Ask your compounding pharmacy where they source the medication’s active pharmaceutical ingredient (API).
- Find out if this supplier is registered with the FDA by searching here or here.
- Confirm that semaglutide base, not semaglutide salt, is used in the compounding process.
- Request a certificate of analysis (COA) of the active pharmaceutical ingredient, which should be semaglutide base. This shows you whether the medication has impurities or byproducts due to its manufacturing process.
- Ask if they have third-party confirmation of potency, stability, and sterility testing of the final product.
In generating this guidance, I’m not endorsing compounded peptides, and in fact, I recognize that there is nothing keeping small-time compounding pharmacies from skirting some of these quality measures, falsifying records, and flying under the radar. However, I hope this checklist serves as a starting point for education and risk mitigation. If a compounder is unwilling or unable to answer these questions, consider it a red flag and look elsewhere.
In an ideal world, the state regulators or the FDA would proactively supervise instead of reactively monitor; trusted compounding pharmacies would be systematically activated to ease medication shortages; and patients with obesity would have access to safe and efficacious treatments for their disease. Until then, we as providers can acknowledge the disappointments of our healthcare system while still developing realistic and individualized solutions that prioritize patient care and safety.
Dr. Tchang is assistant professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine, and a physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York. She is an adviser for Novo Nordisk, which manufactures Wegovy, and an adviser for Ro, a telehealth company that offers compounded semaglutide, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk.
A version of this article first appeared on Medscape.com.
What You Need to Know About Oropouche Virus Disease
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.