User login
ID Blog: The waters of death
Diseases of the American Civil War, Part I
If cleanliness is next to godliness, then the average soldier in the American Civil War lived just down the street from hell. In a land at war, before the formal tenets of germ theory had spread beyond the confines of Louis Pasteur’s laboratory in France, the lack of basic hygiene, both cultural and situational, coupled to an almost complete lack of curative therapies created an appalling death toll. Waterborne diseases in particular spared neither general nor private, and neither doctor nor nurse.

“Of all the adversities that Union and Confederate soldiers confronted, none was more deadly or more prevalent than contaminated water,” according to Jeffrey S. Sartin, MD, in his survey of Civil War diseases.1
The Union Army records list 1,765,000 cases of diarrhea or dysentery with 45,000 deaths and 149,000 cases of typhoid fever with 35,000 deaths. Add to these the 1,316,000 cases of malaria (borne by mosquitoes breeding in the waters) with its 10,000 deaths, and it is easy to see how the battlefield itself took second place in service to the grim reaper. (Overall, there were roughly two deaths from disease for every one from wounds.)
The chief waterborne plague, infectious diarrhea – including bacterial, amoebic, and other parasites – as well as cholera and typhoid, was an all-year-long problem, and, with the typical wry humor of soldiers, these maladies were given popular names, including the “Tennessee trots” and the “Virginia quick-step.”
Unsanitary conditions in the camps were primarily to blame, and this problem of sanitation was obvious to many observers at the time.
Despite a lack of knowledge of germ theory, doctors were fully aware of the relationship of unsanitary conditions to disease, even if they ascribed the link to miasmas or particles of filth.
Hospitals, which were under more strict control than the regular army camps, were meticulous about the placement of latrines and about keeping high standards of cleanliness among the patients, including routine washing. However, this was insufficient for complete protection, because what passed for clean in the absence of the knowledge of bacterial contamination was often totally ineffective. As one Civil War surgeon stated: “We operated in old bloodstained and often pus-stained coats, we used undisinfected instruments from undisinfected plush-lined cases. If a sponge (if they had sponges) or instrument fell on the floor it was washed and squeezed in a basin of water and used as if it was clean.”2
Overall, efforts at what passed for sanitation remained a constant goal and constant struggle in the field.
After the First Battle of Bull Run, Women’s Central Association of Relief President Henry W. Bellows met with Secretary of War Simon Cameron to discuss the abysmal sanitary conditions witnessed by WCAR volunteers. This meeting led to the creation of what would become the U.S. Sanitary Commission, which was approved by President Abraham Lincoln on June 13, 1861.

The U.S. Sanitary Commission served as a means for funneling civilian assistance to the military, with volunteers providing assistance in the organization of military hospitals and camps and aiding in the transportation of the wounded. However, despite these efforts, the setup of army camps and the behavior of the soldiers were not often directed toward proper sanitation. “The principal causes of disease, however, in our camps were the same that we have always to deplore and find it so difficult to remedy, simply because citizens suddenly called to the field cannot comprehend that men in masses require the attention of their officers to enforce certain hygienic conditions without which health cannot be preserved.”3
Breaches of sanitation were common in the confines of the camps, despite regulations designed to protect the soldiers. According to one U.S. Army surgeon of the time: “Especially [needed] was policing of the latrines. The trench is generally too shallow, the daily covering ... with dirt is entirely neglected. Large numbers of the men will not use the sinks [latrines], ... but instead every clump of bushes, every fence border in the vicinity.” Another pointed out that, after the Battle of Seven Pines, “the only water was infiltrated with the decaying animal matter of the battlefield.” Commenting on the placement of latrines in one encampment, another surgeon described how “the sink [latrine] is the ground in the vicinity, which slopes down to the stream, from which all water from the camp is obtained.”4
Treatment for diarrhea and dysentery was varied. Opiates were one of the most common treatments for diarrhea, whether in an alcohol solution as laudanum or in pill form, with belladonna being used to treat intestinal cramps, according to Glenna R. Schroeder-Lein in her book “The Encyclopedia of Civil War Medicine.” However, useless or damaging treatments were also prescribed, including the use of calomel (a mercury compound), turpentine, castor oil, and quinine.5
Acute diarrhea and dysentery illnesses occurred in at least 641/1,000 troops per year in the Union army. And even though the death rate was comparatively low (20/1,000 cases), it frequently led to chronic diarrhea, which was responsible for 288 deaths per 1,000 cases, and was the third highest cause of medical discharge after gunshot wounds and tuberculosis, according to Ms. Schroeder-Lein.
Although the American Civil War was the last major conflict before the spread of the knowledge of germ theory, the struggle to prevent the spread of waterborne diseases under wartime conditions remains ongoing. Hygiene is difficult under conditions of abject poverty and especially under conditions of armed conflict, and until the era of curative antibiotics there was no recourse.
Antibiotics are not the final solution for antibiotic resistance in intestinal disease pathogens, as outlined in a recent CDC report, is an increasing problem.6 For example, nontyphoidal Salmonella causes an estimated 1.35 million infections, 26,500 hospitalizations, and 420 deaths each year in the United States, with 16% of strains being resistant to at least one essential antibiotic. On a global scale, according to the World Health Organization, poor sanitation causes up to 432,000 diarrheal deaths annually and is linked to the transmission of other diseases like cholera, dysentery, typhoid, hepatitis A, and polio.7
With regard to actual epidemics, the world is only a hygienic crisis away from a major outbreak of dysentery (the last occurring between 1969 and 1972, when 20,000 people in Central America died), according to researchers who have detected antibiotic resistance in all but 1% of circulating Shigella dysenteriae strains surveyed since the 1990s. “This bacterium is still in circulation, and could be responsible for future epidemics if conditions should prove favorable – such as a large gathering of people without access to drinking water or treatment of human waste,” wrote François-Xavier Weill of the Pasteur Institute’s Enteric Bacterial Pathogens Unit.8
References
1. Sartin JS. Clin Infec Dis. 1993;16:580-4. (Correction published in 2002).
2. Civil War Battlefield Surgery. eHistory. The Ohio State University.
3. “Myths About Antiseptics and Camp Life – George Wunderlich,” published online Oct. 11, 2011. http://civilwarscholars.com/2011/10/myths-about-antiseptics-and-camp-life-george-wunderlich/
4. Dorwart BB. “Death is in the Breeze: Disease during the American Civil War” (The National Museum of the American Civil War Press, 2009).
5. Glenna R, Schroeder-Lein GR. “The Encyclopedia of Civil War Medicine” (New York: M. E. Sharpe, 2008).
6. “Antibiotic resistance threats in the United States 2019” Centers for Disease Control and Prevention.
7. “New report exposes horror of working conditions for millions of sanitation workers in the developing world,” World Health Organization. 2019 Nov 14.
8. Grant B. Origins of Dysentery. The Scientist. Published online March 22, 2016.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Diseases of the American Civil War, Part I
Diseases of the American Civil War, Part I
If cleanliness is next to godliness, then the average soldier in the American Civil War lived just down the street from hell. In a land at war, before the formal tenets of germ theory had spread beyond the confines of Louis Pasteur’s laboratory in France, the lack of basic hygiene, both cultural and situational, coupled to an almost complete lack of curative therapies created an appalling death toll. Waterborne diseases in particular spared neither general nor private, and neither doctor nor nurse.

“Of all the adversities that Union and Confederate soldiers confronted, none was more deadly or more prevalent than contaminated water,” according to Jeffrey S. Sartin, MD, in his survey of Civil War diseases.1
The Union Army records list 1,765,000 cases of diarrhea or dysentery with 45,000 deaths and 149,000 cases of typhoid fever with 35,000 deaths. Add to these the 1,316,000 cases of malaria (borne by mosquitoes breeding in the waters) with its 10,000 deaths, and it is easy to see how the battlefield itself took second place in service to the grim reaper. (Overall, there were roughly two deaths from disease for every one from wounds.)
The chief waterborne plague, infectious diarrhea – including bacterial, amoebic, and other parasites – as well as cholera and typhoid, was an all-year-long problem, and, with the typical wry humor of soldiers, these maladies were given popular names, including the “Tennessee trots” and the “Virginia quick-step.”
Unsanitary conditions in the camps were primarily to blame, and this problem of sanitation was obvious to many observers at the time.
Despite a lack of knowledge of germ theory, doctors were fully aware of the relationship of unsanitary conditions to disease, even if they ascribed the link to miasmas or particles of filth.
Hospitals, which were under more strict control than the regular army camps, were meticulous about the placement of latrines and about keeping high standards of cleanliness among the patients, including routine washing. However, this was insufficient for complete protection, because what passed for clean in the absence of the knowledge of bacterial contamination was often totally ineffective. As one Civil War surgeon stated: “We operated in old bloodstained and often pus-stained coats, we used undisinfected instruments from undisinfected plush-lined cases. If a sponge (if they had sponges) or instrument fell on the floor it was washed and squeezed in a basin of water and used as if it was clean.”2
Overall, efforts at what passed for sanitation remained a constant goal and constant struggle in the field.
After the First Battle of Bull Run, Women’s Central Association of Relief President Henry W. Bellows met with Secretary of War Simon Cameron to discuss the abysmal sanitary conditions witnessed by WCAR volunteers. This meeting led to the creation of what would become the U.S. Sanitary Commission, which was approved by President Abraham Lincoln on June 13, 1861.

The U.S. Sanitary Commission served as a means for funneling civilian assistance to the military, with volunteers providing assistance in the organization of military hospitals and camps and aiding in the transportation of the wounded. However, despite these efforts, the setup of army camps and the behavior of the soldiers were not often directed toward proper sanitation. “The principal causes of disease, however, in our camps were the same that we have always to deplore and find it so difficult to remedy, simply because citizens suddenly called to the field cannot comprehend that men in masses require the attention of their officers to enforce certain hygienic conditions without which health cannot be preserved.”3
Breaches of sanitation were common in the confines of the camps, despite regulations designed to protect the soldiers. According to one U.S. Army surgeon of the time: “Especially [needed] was policing of the latrines. The trench is generally too shallow, the daily covering ... with dirt is entirely neglected. Large numbers of the men will not use the sinks [latrines], ... but instead every clump of bushes, every fence border in the vicinity.” Another pointed out that, after the Battle of Seven Pines, “the only water was infiltrated with the decaying animal matter of the battlefield.” Commenting on the placement of latrines in one encampment, another surgeon described how “the sink [latrine] is the ground in the vicinity, which slopes down to the stream, from which all water from the camp is obtained.”4
Treatment for diarrhea and dysentery was varied. Opiates were one of the most common treatments for diarrhea, whether in an alcohol solution as laudanum or in pill form, with belladonna being used to treat intestinal cramps, according to Glenna R. Schroeder-Lein in her book “The Encyclopedia of Civil War Medicine.” However, useless or damaging treatments were also prescribed, including the use of calomel (a mercury compound), turpentine, castor oil, and quinine.5
Acute diarrhea and dysentery illnesses occurred in at least 641/1,000 troops per year in the Union army. And even though the death rate was comparatively low (20/1,000 cases), it frequently led to chronic diarrhea, which was responsible for 288 deaths per 1,000 cases, and was the third highest cause of medical discharge after gunshot wounds and tuberculosis, according to Ms. Schroeder-Lein.
Although the American Civil War was the last major conflict before the spread of the knowledge of germ theory, the struggle to prevent the spread of waterborne diseases under wartime conditions remains ongoing. Hygiene is difficult under conditions of abject poverty and especially under conditions of armed conflict, and until the era of curative antibiotics there was no recourse.
Antibiotics are not the final solution for antibiotic resistance in intestinal disease pathogens, as outlined in a recent CDC report, is an increasing problem.6 For example, nontyphoidal Salmonella causes an estimated 1.35 million infections, 26,500 hospitalizations, and 420 deaths each year in the United States, with 16% of strains being resistant to at least one essential antibiotic. On a global scale, according to the World Health Organization, poor sanitation causes up to 432,000 diarrheal deaths annually and is linked to the transmission of other diseases like cholera, dysentery, typhoid, hepatitis A, and polio.7
With regard to actual epidemics, the world is only a hygienic crisis away from a major outbreak of dysentery (the last occurring between 1969 and 1972, when 20,000 people in Central America died), according to researchers who have detected antibiotic resistance in all but 1% of circulating Shigella dysenteriae strains surveyed since the 1990s. “This bacterium is still in circulation, and could be responsible for future epidemics if conditions should prove favorable – such as a large gathering of people without access to drinking water or treatment of human waste,” wrote François-Xavier Weill of the Pasteur Institute’s Enteric Bacterial Pathogens Unit.8
References
1. Sartin JS. Clin Infec Dis. 1993;16:580-4. (Correction published in 2002).
2. Civil War Battlefield Surgery. eHistory. The Ohio State University.
3. “Myths About Antiseptics and Camp Life – George Wunderlich,” published online Oct. 11, 2011. http://civilwarscholars.com/2011/10/myths-about-antiseptics-and-camp-life-george-wunderlich/
4. Dorwart BB. “Death is in the Breeze: Disease during the American Civil War” (The National Museum of the American Civil War Press, 2009).
5. Glenna R, Schroeder-Lein GR. “The Encyclopedia of Civil War Medicine” (New York: M. E. Sharpe, 2008).
6. “Antibiotic resistance threats in the United States 2019” Centers for Disease Control and Prevention.
7. “New report exposes horror of working conditions for millions of sanitation workers in the developing world,” World Health Organization. 2019 Nov 14.
8. Grant B. Origins of Dysentery. The Scientist. Published online March 22, 2016.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
If cleanliness is next to godliness, then the average soldier in the American Civil War lived just down the street from hell. In a land at war, before the formal tenets of germ theory had spread beyond the confines of Louis Pasteur’s laboratory in France, the lack of basic hygiene, both cultural and situational, coupled to an almost complete lack of curative therapies created an appalling death toll. Waterborne diseases in particular spared neither general nor private, and neither doctor nor nurse.

“Of all the adversities that Union and Confederate soldiers confronted, none was more deadly or more prevalent than contaminated water,” according to Jeffrey S. Sartin, MD, in his survey of Civil War diseases.1
The Union Army records list 1,765,000 cases of diarrhea or dysentery with 45,000 deaths and 149,000 cases of typhoid fever with 35,000 deaths. Add to these the 1,316,000 cases of malaria (borne by mosquitoes breeding in the waters) with its 10,000 deaths, and it is easy to see how the battlefield itself took second place in service to the grim reaper. (Overall, there were roughly two deaths from disease for every one from wounds.)
The chief waterborne plague, infectious diarrhea – including bacterial, amoebic, and other parasites – as well as cholera and typhoid, was an all-year-long problem, and, with the typical wry humor of soldiers, these maladies were given popular names, including the “Tennessee trots” and the “Virginia quick-step.”
Unsanitary conditions in the camps were primarily to blame, and this problem of sanitation was obvious to many observers at the time.
Despite a lack of knowledge of germ theory, doctors were fully aware of the relationship of unsanitary conditions to disease, even if they ascribed the link to miasmas or particles of filth.
Hospitals, which were under more strict control than the regular army camps, were meticulous about the placement of latrines and about keeping high standards of cleanliness among the patients, including routine washing. However, this was insufficient for complete protection, because what passed for clean in the absence of the knowledge of bacterial contamination was often totally ineffective. As one Civil War surgeon stated: “We operated in old bloodstained and often pus-stained coats, we used undisinfected instruments from undisinfected plush-lined cases. If a sponge (if they had sponges) or instrument fell on the floor it was washed and squeezed in a basin of water and used as if it was clean.”2
Overall, efforts at what passed for sanitation remained a constant goal and constant struggle in the field.
After the First Battle of Bull Run, Women’s Central Association of Relief President Henry W. Bellows met with Secretary of War Simon Cameron to discuss the abysmal sanitary conditions witnessed by WCAR volunteers. This meeting led to the creation of what would become the U.S. Sanitary Commission, which was approved by President Abraham Lincoln on June 13, 1861.

The U.S. Sanitary Commission served as a means for funneling civilian assistance to the military, with volunteers providing assistance in the organization of military hospitals and camps and aiding in the transportation of the wounded. However, despite these efforts, the setup of army camps and the behavior of the soldiers were not often directed toward proper sanitation. “The principal causes of disease, however, in our camps were the same that we have always to deplore and find it so difficult to remedy, simply because citizens suddenly called to the field cannot comprehend that men in masses require the attention of their officers to enforce certain hygienic conditions without which health cannot be preserved.”3
Breaches of sanitation were common in the confines of the camps, despite regulations designed to protect the soldiers. According to one U.S. Army surgeon of the time: “Especially [needed] was policing of the latrines. The trench is generally too shallow, the daily covering ... with dirt is entirely neglected. Large numbers of the men will not use the sinks [latrines], ... but instead every clump of bushes, every fence border in the vicinity.” Another pointed out that, after the Battle of Seven Pines, “the only water was infiltrated with the decaying animal matter of the battlefield.” Commenting on the placement of latrines in one encampment, another surgeon described how “the sink [latrine] is the ground in the vicinity, which slopes down to the stream, from which all water from the camp is obtained.”4
Treatment for diarrhea and dysentery was varied. Opiates were one of the most common treatments for diarrhea, whether in an alcohol solution as laudanum or in pill form, with belladonna being used to treat intestinal cramps, according to Glenna R. Schroeder-Lein in her book “The Encyclopedia of Civil War Medicine.” However, useless or damaging treatments were also prescribed, including the use of calomel (a mercury compound), turpentine, castor oil, and quinine.5
Acute diarrhea and dysentery illnesses occurred in at least 641/1,000 troops per year in the Union army. And even though the death rate was comparatively low (20/1,000 cases), it frequently led to chronic diarrhea, which was responsible for 288 deaths per 1,000 cases, and was the third highest cause of medical discharge after gunshot wounds and tuberculosis, according to Ms. Schroeder-Lein.
Although the American Civil War was the last major conflict before the spread of the knowledge of germ theory, the struggle to prevent the spread of waterborne diseases under wartime conditions remains ongoing. Hygiene is difficult under conditions of abject poverty and especially under conditions of armed conflict, and until the era of curative antibiotics there was no recourse.
Antibiotics are not the final solution for antibiotic resistance in intestinal disease pathogens, as outlined in a recent CDC report, is an increasing problem.6 For example, nontyphoidal Salmonella causes an estimated 1.35 million infections, 26,500 hospitalizations, and 420 deaths each year in the United States, with 16% of strains being resistant to at least one essential antibiotic. On a global scale, according to the World Health Organization, poor sanitation causes up to 432,000 diarrheal deaths annually and is linked to the transmission of other diseases like cholera, dysentery, typhoid, hepatitis A, and polio.7
With regard to actual epidemics, the world is only a hygienic crisis away from a major outbreak of dysentery (the last occurring between 1969 and 1972, when 20,000 people in Central America died), according to researchers who have detected antibiotic resistance in all but 1% of circulating Shigella dysenteriae strains surveyed since the 1990s. “This bacterium is still in circulation, and could be responsible for future epidemics if conditions should prove favorable – such as a large gathering of people without access to drinking water or treatment of human waste,” wrote François-Xavier Weill of the Pasteur Institute’s Enteric Bacterial Pathogens Unit.8
References
1. Sartin JS. Clin Infec Dis. 1993;16:580-4. (Correction published in 2002).
2. Civil War Battlefield Surgery. eHistory. The Ohio State University.
3. “Myths About Antiseptics and Camp Life – George Wunderlich,” published online Oct. 11, 2011. http://civilwarscholars.com/2011/10/myths-about-antiseptics-and-camp-life-george-wunderlich/
4. Dorwart BB. “Death is in the Breeze: Disease during the American Civil War” (The National Museum of the American Civil War Press, 2009).
5. Glenna R, Schroeder-Lein GR. “The Encyclopedia of Civil War Medicine” (New York: M. E. Sharpe, 2008).
6. “Antibiotic resistance threats in the United States 2019” Centers for Disease Control and Prevention.
7. “New report exposes horror of working conditions for millions of sanitation workers in the developing world,” World Health Organization. 2019 Nov 14.
8. Grant B. Origins of Dysentery. The Scientist. Published online March 22, 2016.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
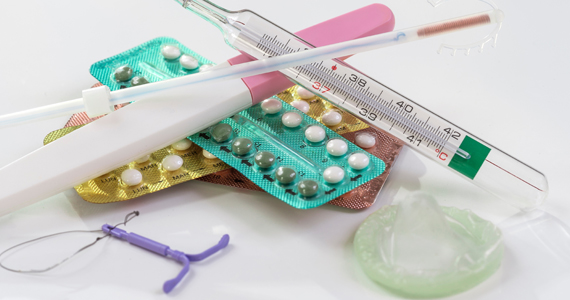
OTC hormonal contraception: An important goal in the fight for reproductive justice
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
Newer IL-17 inhibitors make their case in phase 3 nonradiographic axial spondyloarthritis trials
A major gap in interleukin-17 inhibitor (IL-17i) therapy for axial spondyloarthritis (axSpA) was evidence of efficacy in nonradiographic axSpA. At ACR 2019, we saw two different IL-17i studies showing efficacy in nr-axSpA patients. Now we know that both secukinumab and ixekizumab are effective in the full spectrum of axSpA patients (ankylosing spondylitis [AS] and nr-axSpA).
The majority of clinicians would consider both AS and nr-axSpA to be driven by common processes and so drugs that are effective on one should have the same effect in the other as well. Hence the results are not a big surprise. In certain places, an approved indication for use may be important especially for reimbursement purposes. These results are likely to have maximal impact there.
The COAST-X study on ixekizumab was designed in a way similar to that of the C-axSpAnd study with certolizumab pegol. There was an extended 52-week placebo arm to study the natural history of nr-axSpA patients who are not actively treated with biologics. This design was necessary to respond to the Food and Drug Administration’s concern that, in the absence of this prolonged observation on placebo, we cannot be sure that nr-axSpA patients are not spontaneously remitting (not due to biologics).
However, the results here did surprise me. Unlike in the C-axSpAnd trial where only 13% of actively treated patients (on certolizumab pegol) switched to open-label treatment, in the COAST-X study 40% of patients on both doses of ixekizumab opted for open-label treatment. The number of patients moving out of the placebo arm was around 60% (similar in both studies). There are no straightforward factors evidently explaining this discrepancy. Between 15% and 25% of patients who switched had achieved the primary endpoint of ASAS40. Does this reflect that ASAS40 is not acceptable to patients? As the results show responses plateaued after week 16, it could be that the patients who switched might have done so well into the 52-week observation period.
Patients in the COAST-X study had slightly longer disease duration and marginally lower HLA-B27 prevalence (both factors may indicate lower chance of treatment response).
The primary endpoint of ASAS40 was met at weeks 16 and 52 with significantly higher rates seen with ixekizumab than with placebo. Again the response seems to plateau around 16 weeks with minimal gain up to week 52.
The results from the secukinumab PREVENT study are very similar to those of the COAST-X study showing the superiority of secukinumab over placebo in treating nr-axSpA patients. Interestingly, if we do not use the loading dose for secukinumab, there does not seem to be any difference from standard treatment with loading. This may have economic and administrative implications on the decision to use loading doses of secukinumab. We should carefully consider the MEASURE 4 trial results before making decisions on the utility of loading doses. In the MEASURE 4 study on AS patients, although there was no difference between load and no load arms of secukinumab (around 60% ASAS20 response in both arms), there was no significant gain above placebo with both doses. (The primary endpoint was not met.) This is likely due to the high placebo response (47% ASAS20 response). Similarly, we see a high placebo response in the COAST-X study as well, with an ASAS40 response rate of about 40% in active secukinumab arms vs. 30% in the placebo arm.
The number of patients dropping out over the 52-week follow-up period was not discussed in the PREVENT trial presentation.
There is not much here to favor one IL-17i over the other.
Dr. Haroon is codirector of the Spondylitis Program at University Health Network and associate professor of medicine and rheumatology at the University of Toronto. He is chair of the scientific committee of the Spondyloarthritis Research and Treatment Network. He disclosed serving as a consultant for Amgen, AbbVie, Janssen, Lilly, Novartis, and UCB.
A major gap in interleukin-17 inhibitor (IL-17i) therapy for axial spondyloarthritis (axSpA) was evidence of efficacy in nonradiographic axSpA. At ACR 2019, we saw two different IL-17i studies showing efficacy in nr-axSpA patients. Now we know that both secukinumab and ixekizumab are effective in the full spectrum of axSpA patients (ankylosing spondylitis [AS] and nr-axSpA).
The majority of clinicians would consider both AS and nr-axSpA to be driven by common processes and so drugs that are effective on one should have the same effect in the other as well. Hence the results are not a big surprise. In certain places, an approved indication for use may be important especially for reimbursement purposes. These results are likely to have maximal impact there.
The COAST-X study on ixekizumab was designed in a way similar to that of the C-axSpAnd study with certolizumab pegol. There was an extended 52-week placebo arm to study the natural history of nr-axSpA patients who are not actively treated with biologics. This design was necessary to respond to the Food and Drug Administration’s concern that, in the absence of this prolonged observation on placebo, we cannot be sure that nr-axSpA patients are not spontaneously remitting (not due to biologics).
However, the results here did surprise me. Unlike in the C-axSpAnd trial where only 13% of actively treated patients (on certolizumab pegol) switched to open-label treatment, in the COAST-X study 40% of patients on both doses of ixekizumab opted for open-label treatment. The number of patients moving out of the placebo arm was around 60% (similar in both studies). There are no straightforward factors evidently explaining this discrepancy. Between 15% and 25% of patients who switched had achieved the primary endpoint of ASAS40. Does this reflect that ASAS40 is not acceptable to patients? As the results show responses plateaued after week 16, it could be that the patients who switched might have done so well into the 52-week observation period.
Patients in the COAST-X study had slightly longer disease duration and marginally lower HLA-B27 prevalence (both factors may indicate lower chance of treatment response).
The primary endpoint of ASAS40 was met at weeks 16 and 52 with significantly higher rates seen with ixekizumab than with placebo. Again the response seems to plateau around 16 weeks with minimal gain up to week 52.
The results from the secukinumab PREVENT study are very similar to those of the COAST-X study showing the superiority of secukinumab over placebo in treating nr-axSpA patients. Interestingly, if we do not use the loading dose for secukinumab, there does not seem to be any difference from standard treatment with loading. This may have economic and administrative implications on the decision to use loading doses of secukinumab. We should carefully consider the MEASURE 4 trial results before making decisions on the utility of loading doses. In the MEASURE 4 study on AS patients, although there was no difference between load and no load arms of secukinumab (around 60% ASAS20 response in both arms), there was no significant gain above placebo with both doses. (The primary endpoint was not met.) This is likely due to the high placebo response (47% ASAS20 response). Similarly, we see a high placebo response in the COAST-X study as well, with an ASAS40 response rate of about 40% in active secukinumab arms vs. 30% in the placebo arm.
The number of patients dropping out over the 52-week follow-up period was not discussed in the PREVENT trial presentation.
There is not much here to favor one IL-17i over the other.
Dr. Haroon is codirector of the Spondylitis Program at University Health Network and associate professor of medicine and rheumatology at the University of Toronto. He is chair of the scientific committee of the Spondyloarthritis Research and Treatment Network. He disclosed serving as a consultant for Amgen, AbbVie, Janssen, Lilly, Novartis, and UCB.
A major gap in interleukin-17 inhibitor (IL-17i) therapy for axial spondyloarthritis (axSpA) was evidence of efficacy in nonradiographic axSpA. At ACR 2019, we saw two different IL-17i studies showing efficacy in nr-axSpA patients. Now we know that both secukinumab and ixekizumab are effective in the full spectrum of axSpA patients (ankylosing spondylitis [AS] and nr-axSpA).
The majority of clinicians would consider both AS and nr-axSpA to be driven by common processes and so drugs that are effective on one should have the same effect in the other as well. Hence the results are not a big surprise. In certain places, an approved indication for use may be important especially for reimbursement purposes. These results are likely to have maximal impact there.
The COAST-X study on ixekizumab was designed in a way similar to that of the C-axSpAnd study with certolizumab pegol. There was an extended 52-week placebo arm to study the natural history of nr-axSpA patients who are not actively treated with biologics. This design was necessary to respond to the Food and Drug Administration’s concern that, in the absence of this prolonged observation on placebo, we cannot be sure that nr-axSpA patients are not spontaneously remitting (not due to biologics).
However, the results here did surprise me. Unlike in the C-axSpAnd trial where only 13% of actively treated patients (on certolizumab pegol) switched to open-label treatment, in the COAST-X study 40% of patients on both doses of ixekizumab opted for open-label treatment. The number of patients moving out of the placebo arm was around 60% (similar in both studies). There are no straightforward factors evidently explaining this discrepancy. Between 15% and 25% of patients who switched had achieved the primary endpoint of ASAS40. Does this reflect that ASAS40 is not acceptable to patients? As the results show responses plateaued after week 16, it could be that the patients who switched might have done so well into the 52-week observation period.
Patients in the COAST-X study had slightly longer disease duration and marginally lower HLA-B27 prevalence (both factors may indicate lower chance of treatment response).
The primary endpoint of ASAS40 was met at weeks 16 and 52 with significantly higher rates seen with ixekizumab than with placebo. Again the response seems to plateau around 16 weeks with minimal gain up to week 52.
The results from the secukinumab PREVENT study are very similar to those of the COAST-X study showing the superiority of secukinumab over placebo in treating nr-axSpA patients. Interestingly, if we do not use the loading dose for secukinumab, there does not seem to be any difference from standard treatment with loading. This may have economic and administrative implications on the decision to use loading doses of secukinumab. We should carefully consider the MEASURE 4 trial results before making decisions on the utility of loading doses. In the MEASURE 4 study on AS patients, although there was no difference between load and no load arms of secukinumab (around 60% ASAS20 response in both arms), there was no significant gain above placebo with both doses. (The primary endpoint was not met.) This is likely due to the high placebo response (47% ASAS20 response). Similarly, we see a high placebo response in the COAST-X study as well, with an ASAS40 response rate of about 40% in active secukinumab arms vs. 30% in the placebo arm.
The number of patients dropping out over the 52-week follow-up period was not discussed in the PREVENT trial presentation.
There is not much here to favor one IL-17i over the other.
Dr. Haroon is codirector of the Spondylitis Program at University Health Network and associate professor of medicine and rheumatology at the University of Toronto. He is chair of the scientific committee of the Spondyloarthritis Research and Treatment Network. He disclosed serving as a consultant for Amgen, AbbVie, Janssen, Lilly, Novartis, and UCB.
An alarming number of bipolar disorder diagnoses or something else?
During a particularly busy day in my inpatient and outpatient practice, I realized that nearly every one of the patients had been given the diagnosis of bipolar disorder at one point or another. The interesting thing is this wasn’t an unusual day.
Nearly all of my patients and their family members have been given the diagnosis of bipolar disorder. Because prevalence of bipolar affective disorders is a little over 2%, this seemed a little odd. Could there be an epidemic of bipolar disorder in the area? Should someone sound the alarm on this unique cluster and get Julia Roberts ready? Unfortunately, the story behind this mystery is a little less sexy but nevertheless interesting.
When I probe more into what symptoms might have led to the diagnosis of bipolar disorder, I most often get some sort of answer about being easily angered (“I’m fine 1 minute and the next minute I’m yelling at my mom”) or mood changing from 1 minute to the next. Rarely do they tell me about sleeping less, increased energy, change in mood (elation, anger, irritability), increase in activity level, and increased pleasurable though dangerous activities all happening around the same time(s). So what is going on?
Beginning in the 1990s, a debate about the phenotypic presentation of pediatric bipolar disorder polarized the field. It was theorized that mania could present with severe nonepisodic irritability with extended periods of very rapid mood cycling within the day as opposed to discrete episodic mood cycles in children and adolescents. With this broader conceptualization in the United States, the rate of bipolar diagnosis increased by over 40 times in less than a decade.1 Similarly, the use of mood stabilizers and atypical antipsychotics in children also rose substantially.2
To help assess if severe nonepisodic irritability belongs in the spectrum of bipolar disorders, the National Institutes of Mental Health proposed a syndrome called “Severe Mood Dysregulation” or SMD, to promote the study of children with this phenotype. In longitudinal studies, Stringaris et al. compared rates of manic episodes in youth with SMD versus bipolar disorder over 2 years and found only one youth (1%) with SMD who presented with manic, hypomanic, or mixed episodes, compared with 58 (62%) with bipolar disorder.3 Leibenluft et al.showed that chronic irritability during early adolescence predicted ADHD at late adolescence and major depressive disorder in early adulthood whereas episodic irritability predicted mania.4 Twenty-year follow-up of the same sample showed chronic irritability in adolescence predicted dysthymia, generalized anxiety disorders, and major depressive disorder.5 Other longitudinal studies essentially have shown the same results.6
At this point, the question of whether chronic irritability is a part of the bipolar spectrum disorder is largely resolved – 7 The diagnosis emphasizes the episodic nature of the illness, and that irritability would wax and wane with other manic symptoms such as changes in energy and sleep. And the ultrarapid mood changes (mood changes within the day) appear to describe mood fluctuations within a manic episode as opposed to each change being a separate episode.
So, most likely, my patients were caught in a time of uncertainty before data were able to clarify their phenotype.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Biol Psychiatry. 2007 Jul 15;62(2):107–14.
2. JAMA Psychiatry. 2015 Sep;72(9):859-60.
3. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397-405.
4. J Child Adolesc Psychopharmacol 2006;16(4):456-66.
5. Am J Psychiatry. 2009 Sep;166(9):1048-54.
6. Biol Psychiatry. 2006 Nov 1;60(9):991-7.
7. Bipolar Disord. 2017 Nov;19(7):524-43.
During a particularly busy day in my inpatient and outpatient practice, I realized that nearly every one of the patients had been given the diagnosis of bipolar disorder at one point or another. The interesting thing is this wasn’t an unusual day.
Nearly all of my patients and their family members have been given the diagnosis of bipolar disorder. Because prevalence of bipolar affective disorders is a little over 2%, this seemed a little odd. Could there be an epidemic of bipolar disorder in the area? Should someone sound the alarm on this unique cluster and get Julia Roberts ready? Unfortunately, the story behind this mystery is a little less sexy but nevertheless interesting.
When I probe more into what symptoms might have led to the diagnosis of bipolar disorder, I most often get some sort of answer about being easily angered (“I’m fine 1 minute and the next minute I’m yelling at my mom”) or mood changing from 1 minute to the next. Rarely do they tell me about sleeping less, increased energy, change in mood (elation, anger, irritability), increase in activity level, and increased pleasurable though dangerous activities all happening around the same time(s). So what is going on?
Beginning in the 1990s, a debate about the phenotypic presentation of pediatric bipolar disorder polarized the field. It was theorized that mania could present with severe nonepisodic irritability with extended periods of very rapid mood cycling within the day as opposed to discrete episodic mood cycles in children and adolescents. With this broader conceptualization in the United States, the rate of bipolar diagnosis increased by over 40 times in less than a decade.1 Similarly, the use of mood stabilizers and atypical antipsychotics in children also rose substantially.2
To help assess if severe nonepisodic irritability belongs in the spectrum of bipolar disorders, the National Institutes of Mental Health proposed a syndrome called “Severe Mood Dysregulation” or SMD, to promote the study of children with this phenotype. In longitudinal studies, Stringaris et al. compared rates of manic episodes in youth with SMD versus bipolar disorder over 2 years and found only one youth (1%) with SMD who presented with manic, hypomanic, or mixed episodes, compared with 58 (62%) with bipolar disorder.3 Leibenluft et al.showed that chronic irritability during early adolescence predicted ADHD at late adolescence and major depressive disorder in early adulthood whereas episodic irritability predicted mania.4 Twenty-year follow-up of the same sample showed chronic irritability in adolescence predicted dysthymia, generalized anxiety disorders, and major depressive disorder.5 Other longitudinal studies essentially have shown the same results.6
At this point, the question of whether chronic irritability is a part of the bipolar spectrum disorder is largely resolved – 7 The diagnosis emphasizes the episodic nature of the illness, and that irritability would wax and wane with other manic symptoms such as changes in energy and sleep. And the ultrarapid mood changes (mood changes within the day) appear to describe mood fluctuations within a manic episode as opposed to each change being a separate episode.
So, most likely, my patients were caught in a time of uncertainty before data were able to clarify their phenotype.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Biol Psychiatry. 2007 Jul 15;62(2):107–14.
2. JAMA Psychiatry. 2015 Sep;72(9):859-60.
3. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397-405.
4. J Child Adolesc Psychopharmacol 2006;16(4):456-66.
5. Am J Psychiatry. 2009 Sep;166(9):1048-54.
6. Biol Psychiatry. 2006 Nov 1;60(9):991-7.
7. Bipolar Disord. 2017 Nov;19(7):524-43.
During a particularly busy day in my inpatient and outpatient practice, I realized that nearly every one of the patients had been given the diagnosis of bipolar disorder at one point or another. The interesting thing is this wasn’t an unusual day.
Nearly all of my patients and their family members have been given the diagnosis of bipolar disorder. Because prevalence of bipolar affective disorders is a little over 2%, this seemed a little odd. Could there be an epidemic of bipolar disorder in the area? Should someone sound the alarm on this unique cluster and get Julia Roberts ready? Unfortunately, the story behind this mystery is a little less sexy but nevertheless interesting.
When I probe more into what symptoms might have led to the diagnosis of bipolar disorder, I most often get some sort of answer about being easily angered (“I’m fine 1 minute and the next minute I’m yelling at my mom”) or mood changing from 1 minute to the next. Rarely do they tell me about sleeping less, increased energy, change in mood (elation, anger, irritability), increase in activity level, and increased pleasurable though dangerous activities all happening around the same time(s). So what is going on?
Beginning in the 1990s, a debate about the phenotypic presentation of pediatric bipolar disorder polarized the field. It was theorized that mania could present with severe nonepisodic irritability with extended periods of very rapid mood cycling within the day as opposed to discrete episodic mood cycles in children and adolescents. With this broader conceptualization in the United States, the rate of bipolar diagnosis increased by over 40 times in less than a decade.1 Similarly, the use of mood stabilizers and atypical antipsychotics in children also rose substantially.2
To help assess if severe nonepisodic irritability belongs in the spectrum of bipolar disorders, the National Institutes of Mental Health proposed a syndrome called “Severe Mood Dysregulation” or SMD, to promote the study of children with this phenotype. In longitudinal studies, Stringaris et al. compared rates of manic episodes in youth with SMD versus bipolar disorder over 2 years and found only one youth (1%) with SMD who presented with manic, hypomanic, or mixed episodes, compared with 58 (62%) with bipolar disorder.3 Leibenluft et al.showed that chronic irritability during early adolescence predicted ADHD at late adolescence and major depressive disorder in early adulthood whereas episodic irritability predicted mania.4 Twenty-year follow-up of the same sample showed chronic irritability in adolescence predicted dysthymia, generalized anxiety disorders, and major depressive disorder.5 Other longitudinal studies essentially have shown the same results.6
At this point, the question of whether chronic irritability is a part of the bipolar spectrum disorder is largely resolved – 7 The diagnosis emphasizes the episodic nature of the illness, and that irritability would wax and wane with other manic symptoms such as changes in energy and sleep. And the ultrarapid mood changes (mood changes within the day) appear to describe mood fluctuations within a manic episode as opposed to each change being a separate episode.
So, most likely, my patients were caught in a time of uncertainty before data were able to clarify their phenotype.
Dr. Chung is a child and adolescent psychiatrist at the University of Vermont Medical Center, Burlington, and practices at Champlain Valley Physician’s Hospital in Plattsburgh, N.Y. Email him at [email protected].
References
1. Biol Psychiatry. 2007 Jul 15;62(2):107–14.
2. JAMA Psychiatry. 2015 Sep;72(9):859-60.
3. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):397-405.
4. J Child Adolesc Psychopharmacol 2006;16(4):456-66.
5. Am J Psychiatry. 2009 Sep;166(9):1048-54.
6. Biol Psychiatry. 2006 Nov 1;60(9):991-7.
7. Bipolar Disord. 2017 Nov;19(7):524-43.
Proposed RESPONSE Act targets potential shooters
As I’m writing, my Twitter feed announces yet another public shooting, this one at a Walmart in Oklahoma. It’s a problem that gets worse as it gets more attention and the argument over how to approach the issue of mass shootings still continues down two separate and distinct pathways: Is this the result of too-easy access to firearms or is it one of untreated mental illness?
Sen. John Cornyn (R-Tex.) spoke on the Senate floor on Oct. 23, 2019, about new legislation he is cosponsoring in the aftermath of two mass shootings in Texas this past August. The Restoring, Enhancing, Strengthening, and Promoting Our Nation’s Safety Efforts Act of 2019 (S. 2690), or the RESPONSE Act, is designed to “reduce mass violence, strengthen mental health collaboration in communities, improve school safety, and for other purposes.” Sen. Cornyn notes that in the aftermath of those shootings he met with his constituents and he heard a common refrain: Please do something.
“Unfortunately, there is no quick fix, no simple answer, instead we are left to look at the factors that led to these attacks and to try to do something to prevent the sequence of events from playing out again in the future,” Sen. Cornyn said.
“While mental illness is not the prevailing cause of mass violence, enhanced mental health resources are critical to saving lives,” he said, adding that most gun deaths are from suicide. In his speech, he outlined the issues it would address – and despite his statement that mental illness is not the cause of mass violence – he went on to elaborate on the issues that the bill would address.
“First, this legislation takes aim at unlicensed firearms dealers who are breaking the law,” he said. This legislation would create a task force to prosecute those who buy and sell firearms through unlicensed dealers, and he notes that one of the Texas shooters was denied a gun by a licensed firearms dealer before purchasing one from an unlicensed dealer. That Sen. Cornyn’s proposed legislation would not create any new gun legislation is not a surprise: he has an A+ rating from the National Rifle Association and his website’s fun facts include the statement: “Sen. Cornyn owns several firearms and hunts as often as he can.”
The rest of the RESPONSE Act takes aim at those who have or might have psychiatric disorders or a tendency toward violence. Sen. Cornyn noted that the act would expand assisted outpatient treatment (AOT, or outpatient civil commitment). He referenced this as a way for families to get care for their loved ones in the community rather than in a hospital and did not allude to the involuntary nature of the treatment.
Marvin Swartz, MD, is professor of psychiatry at Duke University, Durham, N.C., and lead investigator on outcome studies following the implementation of outpatient civil commitment legislation.
“AOT may be justified in improving treatment adherence and service provision,” Dr. Swartz noted, “but there is no direct line to serious violence. The violence we documented as reduced were mainly minor acts of interpersonal violence – pushing and shoving – what we call minor acts of violence. There is no evidence that AOT is a remedy to serious acts of violence – mass shootings included.”
In addition, Sen. Cornyn noted there would be expanded crisis intervention teams and increased coordination between mental health providers and law enforcement. Furthermore, the bill would make schools safer by identifying students whose behavior indicated a threat of violence and providing those students with the services they need. This would be done “by promoting best practices within our schools and promoting Internet safety.”
Finally, Sen. Cornyn talked about using social media as a means to identify those who might be a danger. “Because so often these shooters advertise on social media ... this legislation includes provisions to [ensure] that law enforcement can receive timely information about threats made online.”
The bill already has garnered both support and opposition. It has been supported by the National Council for Behavioral Health, the National Alliance on Mental Illness (NAMI), and the Treatment Advocacy Center. Those opposed to the legislation include the National Disability Rights Network, the American Association of People with Disabilities, the National Council on Independent Living, the Disability Rights Education & Defense Fund, the Bazelon Center for Mental Health Law, and the Autistic Self Advocacy Network. The American Psychiatric Association has not made a statement on the proposed legislation as of this writing.
The National Council for Behavioral Health posted an endorsement on its website. It notes: “The RESPONSE Act authorizes up to $10 million of existing funds in the Department of Justice for partnership between law enforcement and mental health providers to increase access to long-acting medically assisted treatment. Additionally, it requires the Department of Health and Human Services (HHS) to develop and disseminate guidance for states to fund mental health programs and crisis intervention teams through Medicaid as well as to issue a report to Congress on best practices to expand the mental health workforce. These provisions aim to divert more individuals from incarceration and will create more opportunities for community-based treatment and recovery.”
There is no question that psychiatric treatment for those with mental illness is underfunded and often inaccessible. But while it is true that some individuals become violent when they are ill, most do not, and targeting those one in five Americans who suffer from a psychiatric disorder each year in an effort to identify, then thwart, the rare mass murderer among us makes no sense.
Acts of mass violence remain rare. In 2018, the year we had a record-breaking number of mass shootings, there were 12 mass murders in the United States, according to the criteria used by Mother Jones, and 27 active shooter incidents using the FBI’s criteria. Approximately half of all mass shooters showed signs of mental illness prior to the shooting and of those, some had never come to the attention of mental health professionals in a way that would have predicted violence. While linking mass violence to mental illness may seem reasonable, the numbers just don’t make sense and targeting this presumed link between mental illness and mass violence is stigmatizing.
The text of the RESPONSE Act reveals proposed legislation that is perhaps more thoughtful than Sen. Cornyn’s speech suggested; the bill starts with funding services for those with psychiatric disorders who are being released from the correctional system, a population that may be at higher risk for acts of violence. The funding for outpatient civil commitment is worded in such a way that it is hard to know exactly what is required. The bill starts by mandating that each state must use 10% of the funding it gets from this bill for court-ordered treatment (AOT), but then lists alternative ways states may use that 10%, including “otherwise support evidence-based programs that address the needs of eligible patients.” In all, the proposed legislation is long and complex and attempts to address issues related to terrorism, the Internet, mental health, and the educational system. It’s an ambitious use of $10 million a year for our entire country.
At a time when mental health care is desperately underfunded and many are unable to access treatment, it is tempting to endorse any legislation that improves funding. But does it serve society to endorse legislation that suggests psychiatrists can prevent mass shootings? Does that ultimately serve our patients?
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
As I’m writing, my Twitter feed announces yet another public shooting, this one at a Walmart in Oklahoma. It’s a problem that gets worse as it gets more attention and the argument over how to approach the issue of mass shootings still continues down two separate and distinct pathways: Is this the result of too-easy access to firearms or is it one of untreated mental illness?
Sen. John Cornyn (R-Tex.) spoke on the Senate floor on Oct. 23, 2019, about new legislation he is cosponsoring in the aftermath of two mass shootings in Texas this past August. The Restoring, Enhancing, Strengthening, and Promoting Our Nation’s Safety Efforts Act of 2019 (S. 2690), or the RESPONSE Act, is designed to “reduce mass violence, strengthen mental health collaboration in communities, improve school safety, and for other purposes.” Sen. Cornyn notes that in the aftermath of those shootings he met with his constituents and he heard a common refrain: Please do something.
“Unfortunately, there is no quick fix, no simple answer, instead we are left to look at the factors that led to these attacks and to try to do something to prevent the sequence of events from playing out again in the future,” Sen. Cornyn said.
“While mental illness is not the prevailing cause of mass violence, enhanced mental health resources are critical to saving lives,” he said, adding that most gun deaths are from suicide. In his speech, he outlined the issues it would address – and despite his statement that mental illness is not the cause of mass violence – he went on to elaborate on the issues that the bill would address.
“First, this legislation takes aim at unlicensed firearms dealers who are breaking the law,” he said. This legislation would create a task force to prosecute those who buy and sell firearms through unlicensed dealers, and he notes that one of the Texas shooters was denied a gun by a licensed firearms dealer before purchasing one from an unlicensed dealer. That Sen. Cornyn’s proposed legislation would not create any new gun legislation is not a surprise: he has an A+ rating from the National Rifle Association and his website’s fun facts include the statement: “Sen. Cornyn owns several firearms and hunts as often as he can.”
The rest of the RESPONSE Act takes aim at those who have or might have psychiatric disorders or a tendency toward violence. Sen. Cornyn noted that the act would expand assisted outpatient treatment (AOT, or outpatient civil commitment). He referenced this as a way for families to get care for their loved ones in the community rather than in a hospital and did not allude to the involuntary nature of the treatment.
Marvin Swartz, MD, is professor of psychiatry at Duke University, Durham, N.C., and lead investigator on outcome studies following the implementation of outpatient civil commitment legislation.
“AOT may be justified in improving treatment adherence and service provision,” Dr. Swartz noted, “but there is no direct line to serious violence. The violence we documented as reduced were mainly minor acts of interpersonal violence – pushing and shoving – what we call minor acts of violence. There is no evidence that AOT is a remedy to serious acts of violence – mass shootings included.”
In addition, Sen. Cornyn noted there would be expanded crisis intervention teams and increased coordination between mental health providers and law enforcement. Furthermore, the bill would make schools safer by identifying students whose behavior indicated a threat of violence and providing those students with the services they need. This would be done “by promoting best practices within our schools and promoting Internet safety.”
Finally, Sen. Cornyn talked about using social media as a means to identify those who might be a danger. “Because so often these shooters advertise on social media ... this legislation includes provisions to [ensure] that law enforcement can receive timely information about threats made online.”
The bill already has garnered both support and opposition. It has been supported by the National Council for Behavioral Health, the National Alliance on Mental Illness (NAMI), and the Treatment Advocacy Center. Those opposed to the legislation include the National Disability Rights Network, the American Association of People with Disabilities, the National Council on Independent Living, the Disability Rights Education & Defense Fund, the Bazelon Center for Mental Health Law, and the Autistic Self Advocacy Network. The American Psychiatric Association has not made a statement on the proposed legislation as of this writing.
The National Council for Behavioral Health posted an endorsement on its website. It notes: “The RESPONSE Act authorizes up to $10 million of existing funds in the Department of Justice for partnership between law enforcement and mental health providers to increase access to long-acting medically assisted treatment. Additionally, it requires the Department of Health and Human Services (HHS) to develop and disseminate guidance for states to fund mental health programs and crisis intervention teams through Medicaid as well as to issue a report to Congress on best practices to expand the mental health workforce. These provisions aim to divert more individuals from incarceration and will create more opportunities for community-based treatment and recovery.”
There is no question that psychiatric treatment for those with mental illness is underfunded and often inaccessible. But while it is true that some individuals become violent when they are ill, most do not, and targeting those one in five Americans who suffer from a psychiatric disorder each year in an effort to identify, then thwart, the rare mass murderer among us makes no sense.
Acts of mass violence remain rare. In 2018, the year we had a record-breaking number of mass shootings, there were 12 mass murders in the United States, according to the criteria used by Mother Jones, and 27 active shooter incidents using the FBI’s criteria. Approximately half of all mass shooters showed signs of mental illness prior to the shooting and of those, some had never come to the attention of mental health professionals in a way that would have predicted violence. While linking mass violence to mental illness may seem reasonable, the numbers just don’t make sense and targeting this presumed link between mental illness and mass violence is stigmatizing.
The text of the RESPONSE Act reveals proposed legislation that is perhaps more thoughtful than Sen. Cornyn’s speech suggested; the bill starts with funding services for those with psychiatric disorders who are being released from the correctional system, a population that may be at higher risk for acts of violence. The funding for outpatient civil commitment is worded in such a way that it is hard to know exactly what is required. The bill starts by mandating that each state must use 10% of the funding it gets from this bill for court-ordered treatment (AOT), but then lists alternative ways states may use that 10%, including “otherwise support evidence-based programs that address the needs of eligible patients.” In all, the proposed legislation is long and complex and attempts to address issues related to terrorism, the Internet, mental health, and the educational system. It’s an ambitious use of $10 million a year for our entire country.
At a time when mental health care is desperately underfunded and many are unable to access treatment, it is tempting to endorse any legislation that improves funding. But does it serve society to endorse legislation that suggests psychiatrists can prevent mass shootings? Does that ultimately serve our patients?
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
As I’m writing, my Twitter feed announces yet another public shooting, this one at a Walmart in Oklahoma. It’s a problem that gets worse as it gets more attention and the argument over how to approach the issue of mass shootings still continues down two separate and distinct pathways: Is this the result of too-easy access to firearms or is it one of untreated mental illness?
Sen. John Cornyn (R-Tex.) spoke on the Senate floor on Oct. 23, 2019, about new legislation he is cosponsoring in the aftermath of two mass shootings in Texas this past August. The Restoring, Enhancing, Strengthening, and Promoting Our Nation’s Safety Efforts Act of 2019 (S. 2690), or the RESPONSE Act, is designed to “reduce mass violence, strengthen mental health collaboration in communities, improve school safety, and for other purposes.” Sen. Cornyn notes that in the aftermath of those shootings he met with his constituents and he heard a common refrain: Please do something.
“Unfortunately, there is no quick fix, no simple answer, instead we are left to look at the factors that led to these attacks and to try to do something to prevent the sequence of events from playing out again in the future,” Sen. Cornyn said.
“While mental illness is not the prevailing cause of mass violence, enhanced mental health resources are critical to saving lives,” he said, adding that most gun deaths are from suicide. In his speech, he outlined the issues it would address – and despite his statement that mental illness is not the cause of mass violence – he went on to elaborate on the issues that the bill would address.
“First, this legislation takes aim at unlicensed firearms dealers who are breaking the law,” he said. This legislation would create a task force to prosecute those who buy and sell firearms through unlicensed dealers, and he notes that one of the Texas shooters was denied a gun by a licensed firearms dealer before purchasing one from an unlicensed dealer. That Sen. Cornyn’s proposed legislation would not create any new gun legislation is not a surprise: he has an A+ rating from the National Rifle Association and his website’s fun facts include the statement: “Sen. Cornyn owns several firearms and hunts as often as he can.”
The rest of the RESPONSE Act takes aim at those who have or might have psychiatric disorders or a tendency toward violence. Sen. Cornyn noted that the act would expand assisted outpatient treatment (AOT, or outpatient civil commitment). He referenced this as a way for families to get care for their loved ones in the community rather than in a hospital and did not allude to the involuntary nature of the treatment.
Marvin Swartz, MD, is professor of psychiatry at Duke University, Durham, N.C., and lead investigator on outcome studies following the implementation of outpatient civil commitment legislation.
“AOT may be justified in improving treatment adherence and service provision,” Dr. Swartz noted, “but there is no direct line to serious violence. The violence we documented as reduced were mainly minor acts of interpersonal violence – pushing and shoving – what we call minor acts of violence. There is no evidence that AOT is a remedy to serious acts of violence – mass shootings included.”
In addition, Sen. Cornyn noted there would be expanded crisis intervention teams and increased coordination between mental health providers and law enforcement. Furthermore, the bill would make schools safer by identifying students whose behavior indicated a threat of violence and providing those students with the services they need. This would be done “by promoting best practices within our schools and promoting Internet safety.”
Finally, Sen. Cornyn talked about using social media as a means to identify those who might be a danger. “Because so often these shooters advertise on social media ... this legislation includes provisions to [ensure] that law enforcement can receive timely information about threats made online.”
The bill already has garnered both support and opposition. It has been supported by the National Council for Behavioral Health, the National Alliance on Mental Illness (NAMI), and the Treatment Advocacy Center. Those opposed to the legislation include the National Disability Rights Network, the American Association of People with Disabilities, the National Council on Independent Living, the Disability Rights Education & Defense Fund, the Bazelon Center for Mental Health Law, and the Autistic Self Advocacy Network. The American Psychiatric Association has not made a statement on the proposed legislation as of this writing.
The National Council for Behavioral Health posted an endorsement on its website. It notes: “The RESPONSE Act authorizes up to $10 million of existing funds in the Department of Justice for partnership between law enforcement and mental health providers to increase access to long-acting medically assisted treatment. Additionally, it requires the Department of Health and Human Services (HHS) to develop and disseminate guidance for states to fund mental health programs and crisis intervention teams through Medicaid as well as to issue a report to Congress on best practices to expand the mental health workforce. These provisions aim to divert more individuals from incarceration and will create more opportunities for community-based treatment and recovery.”
There is no question that psychiatric treatment for those with mental illness is underfunded and often inaccessible. But while it is true that some individuals become violent when they are ill, most do not, and targeting those one in five Americans who suffer from a psychiatric disorder each year in an effort to identify, then thwart, the rare mass murderer among us makes no sense.
Acts of mass violence remain rare. In 2018, the year we had a record-breaking number of mass shootings, there were 12 mass murders in the United States, according to the criteria used by Mother Jones, and 27 active shooter incidents using the FBI’s criteria. Approximately half of all mass shooters showed signs of mental illness prior to the shooting and of those, some had never come to the attention of mental health professionals in a way that would have predicted violence. While linking mass violence to mental illness may seem reasonable, the numbers just don’t make sense and targeting this presumed link between mental illness and mass violence is stigmatizing.
The text of the RESPONSE Act reveals proposed legislation that is perhaps more thoughtful than Sen. Cornyn’s speech suggested; the bill starts with funding services for those with psychiatric disorders who are being released from the correctional system, a population that may be at higher risk for acts of violence. The funding for outpatient civil commitment is worded in such a way that it is hard to know exactly what is required. The bill starts by mandating that each state must use 10% of the funding it gets from this bill for court-ordered treatment (AOT), but then lists alternative ways states may use that 10%, including “otherwise support evidence-based programs that address the needs of eligible patients.” In all, the proposed legislation is long and complex and attempts to address issues related to terrorism, the Internet, mental health, and the educational system. It’s an ambitious use of $10 million a year for our entire country.
At a time when mental health care is desperately underfunded and many are unable to access treatment, it is tempting to endorse any legislation that improves funding. But does it serve society to endorse legislation that suggests psychiatrists can prevent mass shootings? Does that ultimately serve our patients?
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
Health policy Q&A: Oncology Care Model
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
The Oncology Care Model is a value-based payment approach aimed at encouraging coordinated cancer care through targeted bonus payments to practices. The payment experiment was launched by the Centers for Medicare & Medicaid Services in 2016 and now includes 175 practices and 10 payers. It is set to end in 2021. As agency officials consider whether to continue the program, Stephen S. Grubbs, MD, vice president for clinical affairs at the American Society of Clinical Oncology, weighs in on the model’s track record and its future.
Question: How would you rate the Oncology Care Model in helping to drive practice transformation?
Dr. Grubbs: Participants in the Oncology Care Model (OCM) have demonstrated improved care coordination, psychosocial support, use of risk assessment tools, and other strategies to lower costs and adverse events. Over the past 2 years, ASCO has accepted numerous posters, articles, and abstracts from OCM participants on their outstanding work to advance cancer care delivery.
Question: Should the model be extended beyond 2021?
Dr. Grubbs: Changes to the model are necessary prior to a significant extension or expansion. Some have suggested that CMS extend OCM for an additional year with current participants. This would give CMS time to consider input from all stakeholders on its eventual replacement.
Question: What additional resources or payments do oncology practices need to be more successful in meeting the goals of the Oncology Care Model?
Dr. Grubbs: OCM has shown that by providing oncologists with payment for care management – OCM participants receive $160 per patient, per month – the results are better care coordination and reduced hospital and emergency department visits. If CMS chooses to expand payments to all oncology providers, we could expect to see improved care for cancer patients.
Question: ASCO has advanced its own Patient-Centered Oncology Payment model. What are the main elements of this strategy and how does it differ from the Oncology Care Model?
Dr. Grubbs: The Patient-Centered Oncology Payment (PCOP) model is the result of input from a wide group of stakeholders, including providers, employers, and managed care organizations. In the coming months, ASCO will publish an updated copy of the PCOP model.
Our review of OCM is that the included prediction model and two-sided risk options place small, rural, and certain other practices at considerable peril because of imprecise and inconsistent cost predictions. PCOP takes a different approach. Rather than requiring that practices take on actuarial risk for total cost of care, PCOP includes a three-part performance methodology. Practices are measured on adherence to clinical treatment pathways; electronically capturable quality measures; and select, targeted cost-of-care measures. Practices that perform well in PCOP’s performance methodology receive increased incentive payments to fund further advancements in care.
Question: The PCOP model includes payments to oncology practices for participation in clinical trials. How might that drive a change in behavior in a typical practice?
Dr. Grubbs: Practices that enroll patients in clinical trials have the same or greater storage and handling requirements as those treated with standard treatments, yet forgo revenue associated with the Medicare Part B average sales price methodology. PCOP ensures that such practices are not disadvantaged for supporting clinical research.
Question: Are there other areas – such as tumor biomarker tests – in which a tailored payment approach would improve the quality of care?
Dr. Grubbs: Recent studies have shown that not all patients receive the appropriate genomic profiling and other tests necessary to ensure that they benefit from personalized therapies. Clinical treatment pathways have the ability to inform and measure diagnostic completeness to improve the quality of care.
Question: What are the barriers that are keeping oncology practices from participating in alternative payment models designed to improve care?
Dr. Grubbs: Some alternative payment models, such as OCM, place a high administrative burden on their participants. Manual reporting of measures and clinical data, complicated billing requirements, and lack of support from electronic health record vendors create barriers for expanded participation. Practices are also concerned about the financial risks placed upon participants; it is impractical to expect that physicians hire actuaries in order to participate in a Medicare program.
ASCO has offered support for OCM practices through its PracticeNET benchmarking program, but we have also proposed PCOP as an appropriate alternative, applicable to practices of all types and sizes.
Dr. Grubbs joined ASCO in 2015 as the vice president of the newly launched clinical affairs department. Before joining ASCO, Dr. Grubbs worked as a community oncologist and managing partner at Medical Oncology Hematology Consultants in Newark, Del. Dr. Grubbs is a volunteer and the principal investigator of the Delaware Christiana Care National Cancer Institute Community Oncology Research Program. Dr. Grubbs reported having no financial disclosures.
Football for the young
A few weeks ago I was at a Friday-night football game, but not to watch the game. I’ve been there and done that too many times when I used to be the team physician. I was there to listen to my granddaughter drumming in the pep band. And there was a lot of drumming because her high school’s team is having a hot year and outscoring opponents by three and four touchdowns every week.
At half time, the field was swarmed by 45-50 early grade schoolers looking like bobblehead dolls in their oversize helmets and surprisingly professional-appearing miniature football outfits. Under the lights, on the local college’s turf field, they were in football heaven. The pep band got into it and there was more drumming as the few kids who had a clue what football was about were scampering over and around their teammates and opponents who were roughhousing with each other, rolling around on the turf having a grand time, blissfully unimpressed by such trivial concepts as the line of scrimmage or the difference between blocking and tackling or even offense and defense.
Despite all the alarming articles both lay and professional that you and I see, this was an evening on which no one seemed particularly concerned about sports-related concussions. This is class B football in Maine, not a state well known as an incubator of Division I college football players. While there were a few scrawny kids with some speed,
Watching 4- and 5-year-olds in their football uniforms seemed to me to be a rather harmless exercise and certainly a more positive investment in their time on a Friday night than sitting on the couch with an electronic device clutched in their little hands. A recent report in JAMA Pediatrics suggests that my lack of concern has some validity (“Consensus statement on sports-related concussions in youth sports using a modified delphi approach.” JAMA Pediatr. 2019 Nov 11. doi: 10.1001/jamapediatrics.2019.4006). Eleven experts in sports-related injuries were surveyed with multiple rounds of questionnaires. Their anonymous responses were aggregated and shared with the group after each round until a consensus could be arrived on for each of seven broad questions about sports-related concussions. It is a paper worth reading and like most good literature surveys determined that in many situations more study needs to be done.
Among the many findings that impressed me was the group’s failure to find an “association between repetitive head impact exposure in youth and long-term neurocognitive outcomes.” In addition, “there is little evidence that age at first exposure repetitive head impacts in sports is independently associated with neurodegenerative changes.” The experts also could find “no evidence that growth or development affect the risk of sports-related concussions.”
The problem with youth football is that it is the portal that can lead to college and professional football, in which large bodies are allowed to collide after accelerating at speeds we mortals only can achieve behind the wheel of our motor vehicles. Rules to minimize those collisions do exist, but lax enforcement has failed to prevent their cumulative damage.
Whether the culture of big-time football is going to change to a point at which a conscientious parent could encourage his or her child to play after adolescence remains to be seen. However, the evidence seems to suggest that allowing young children to bang themselves around imitating the big guys seems to be reasonably safe. At least as safe as what kids used to do to each other before we adults invented television and video games.
When my son was 3 or 4 years old, he played on a hockey team he thought was called the Toronto Make-Believes (Maple Leafs). Maybe we should be telling parents it’s safe for their children to play make-believe contact sports. The challenge comes after those kids reach puberty and want to start playing the real thing.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I was at a Friday-night football game, but not to watch the game. I’ve been there and done that too many times when I used to be the team physician. I was there to listen to my granddaughter drumming in the pep band. And there was a lot of drumming because her high school’s team is having a hot year and outscoring opponents by three and four touchdowns every week.
At half time, the field was swarmed by 45-50 early grade schoolers looking like bobblehead dolls in their oversize helmets and surprisingly professional-appearing miniature football outfits. Under the lights, on the local college’s turf field, they were in football heaven. The pep band got into it and there was more drumming as the few kids who had a clue what football was about were scampering over and around their teammates and opponents who were roughhousing with each other, rolling around on the turf having a grand time, blissfully unimpressed by such trivial concepts as the line of scrimmage or the difference between blocking and tackling or even offense and defense.
Despite all the alarming articles both lay and professional that you and I see, this was an evening on which no one seemed particularly concerned about sports-related concussions. This is class B football in Maine, not a state well known as an incubator of Division I college football players. While there were a few scrawny kids with some speed,
Watching 4- and 5-year-olds in their football uniforms seemed to me to be a rather harmless exercise and certainly a more positive investment in their time on a Friday night than sitting on the couch with an electronic device clutched in their little hands. A recent report in JAMA Pediatrics suggests that my lack of concern has some validity (“Consensus statement on sports-related concussions in youth sports using a modified delphi approach.” JAMA Pediatr. 2019 Nov 11. doi: 10.1001/jamapediatrics.2019.4006). Eleven experts in sports-related injuries were surveyed with multiple rounds of questionnaires. Their anonymous responses were aggregated and shared with the group after each round until a consensus could be arrived on for each of seven broad questions about sports-related concussions. It is a paper worth reading and like most good literature surveys determined that in many situations more study needs to be done.
Among the many findings that impressed me was the group’s failure to find an “association between repetitive head impact exposure in youth and long-term neurocognitive outcomes.” In addition, “there is little evidence that age at first exposure repetitive head impacts in sports is independently associated with neurodegenerative changes.” The experts also could find “no evidence that growth or development affect the risk of sports-related concussions.”
The problem with youth football is that it is the portal that can lead to college and professional football, in which large bodies are allowed to collide after accelerating at speeds we mortals only can achieve behind the wheel of our motor vehicles. Rules to minimize those collisions do exist, but lax enforcement has failed to prevent their cumulative damage.
Whether the culture of big-time football is going to change to a point at which a conscientious parent could encourage his or her child to play after adolescence remains to be seen. However, the evidence seems to suggest that allowing young children to bang themselves around imitating the big guys seems to be reasonably safe. At least as safe as what kids used to do to each other before we adults invented television and video games.
When my son was 3 or 4 years old, he played on a hockey team he thought was called the Toronto Make-Believes (Maple Leafs). Maybe we should be telling parents it’s safe for their children to play make-believe contact sports. The challenge comes after those kids reach puberty and want to start playing the real thing.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I was at a Friday-night football game, but not to watch the game. I’ve been there and done that too many times when I used to be the team physician. I was there to listen to my granddaughter drumming in the pep band. And there was a lot of drumming because her high school’s team is having a hot year and outscoring opponents by three and four touchdowns every week.
At half time, the field was swarmed by 45-50 early grade schoolers looking like bobblehead dolls in their oversize helmets and surprisingly professional-appearing miniature football outfits. Under the lights, on the local college’s turf field, they were in football heaven. The pep band got into it and there was more drumming as the few kids who had a clue what football was about were scampering over and around their teammates and opponents who were roughhousing with each other, rolling around on the turf having a grand time, blissfully unimpressed by such trivial concepts as the line of scrimmage or the difference between blocking and tackling or even offense and defense.
Despite all the alarming articles both lay and professional that you and I see, this was an evening on which no one seemed particularly concerned about sports-related concussions. This is class B football in Maine, not a state well known as an incubator of Division I college football players. While there were a few scrawny kids with some speed,
Watching 4- and 5-year-olds in their football uniforms seemed to me to be a rather harmless exercise and certainly a more positive investment in their time on a Friday night than sitting on the couch with an electronic device clutched in their little hands. A recent report in JAMA Pediatrics suggests that my lack of concern has some validity (“Consensus statement on sports-related concussions in youth sports using a modified delphi approach.” JAMA Pediatr. 2019 Nov 11. doi: 10.1001/jamapediatrics.2019.4006). Eleven experts in sports-related injuries were surveyed with multiple rounds of questionnaires. Their anonymous responses were aggregated and shared with the group after each round until a consensus could be arrived on for each of seven broad questions about sports-related concussions. It is a paper worth reading and like most good literature surveys determined that in many situations more study needs to be done.
Among the many findings that impressed me was the group’s failure to find an “association between repetitive head impact exposure in youth and long-term neurocognitive outcomes.” In addition, “there is little evidence that age at first exposure repetitive head impacts in sports is independently associated with neurodegenerative changes.” The experts also could find “no evidence that growth or development affect the risk of sports-related concussions.”
The problem with youth football is that it is the portal that can lead to college and professional football, in which large bodies are allowed to collide after accelerating at speeds we mortals only can achieve behind the wheel of our motor vehicles. Rules to minimize those collisions do exist, but lax enforcement has failed to prevent their cumulative damage.
Whether the culture of big-time football is going to change to a point at which a conscientious parent could encourage his or her child to play after adolescence remains to be seen. However, the evidence seems to suggest that allowing young children to bang themselves around imitating the big guys seems to be reasonably safe. At least as safe as what kids used to do to each other before we adults invented television and video games.
When my son was 3 or 4 years old, he played on a hockey team he thought was called the Toronto Make-Believes (Maple Leafs). Maybe we should be telling parents it’s safe for their children to play make-believe contact sports. The challenge comes after those kids reach puberty and want to start playing the real thing.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
‘Tis the season to reflect and take stock
‘Tis the season to reflect and take stock: Maybe you had a baby, or learned a new procedure, or bought a Tesla? Of course, you made (loads of?) money and treated many patients. Imagine if I asked you this in person, what would you reply? And what made you most proud? I’d tell you this story.
Last week I saw a 50-something-year-old woman for her annual skin screening. She asked if I remembered her mother, who was also my patient. Squinting through my dermatoscope at the nevi on her back, I tried to recall. “Yes, I think so.” (Actually, I was unsure.)
“Well she passed away last week from breast cancer,” she said.
“Oh, I’m sorry to hear that,” I replied.
She added: “Yes, yet she lived much longer than we thought. I want you to know we believe it was in large part because of you.”
I stopped and wheeled around to face her. How could that possibly be true? I had only treated her for a simple skin cancer. She explained that I had seen her mom about a year ago and cut out a skin cancer on her face. Her mom was afraid of needles and of surgery. Apparently when she asked me if it would hurt, I replied: “Well, most patients, yes, but not you.” Pausing, I added: “Because you’re a tough old bird.” She laughed. Apparently that warmth I conveyed and display of confidence in her was just what she needed at that moment. She didn’t flinch.
Not long after, she was diagnosed with breast cancer. When given the news with her children present, she replied, “well, I’ll just fight it. I’m a tough old bird.” It was just what they needed in that moment. “I’m a tough old bird” became their rally cry. Apparently with each stage, surgery, radiation, chemo, they fell back on it. Her son had “Tough Old Bird” made into a magnet and prominently posted on the refrigerator door where she would see it every day.
Sadly, she ultimately succumbed to her disease, but did so later than had been expected and having fought all the way. My patient teared up and asked if she could give me a hug on behalf of her mom. “Thank you, Dr. Benabio. We won’t forget what you did for her.”
I did recall her now, remembering even what exam room she was in when I said it. Yet, I had no idea what I had done. I wonder how many others there were. Of the many things you accomplished this year, try to recall these achievements. Not the psoriasis cleared, or tumor extirpated, or new homes bought. But the comfort and care you brought to the mother with worry, the father with anguish, the daughter with anxieties, or the son with misdeeds.
It is a beautiful, hard, and joyous life we have as physicians, for our “happiness lies in the absorption in some vocation which satisfies the soul; that we are here to add what we can to, not get what we can from life.”* How fortunate are we. Take stock.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Reference
*William Osler, “Doctor and Nurse.” Address given at Training School for Nurses at Johns Hopkins Hospital, June 4, 1891
‘Tis the season to reflect and take stock: Maybe you had a baby, or learned a new procedure, or bought a Tesla? Of course, you made (loads of?) money and treated many patients. Imagine if I asked you this in person, what would you reply? And what made you most proud? I’d tell you this story.
Last week I saw a 50-something-year-old woman for her annual skin screening. She asked if I remembered her mother, who was also my patient. Squinting through my dermatoscope at the nevi on her back, I tried to recall. “Yes, I think so.” (Actually, I was unsure.)
“Well she passed away last week from breast cancer,” she said.
“Oh, I’m sorry to hear that,” I replied.
She added: “Yes, yet she lived much longer than we thought. I want you to know we believe it was in large part because of you.”
I stopped and wheeled around to face her. How could that possibly be true? I had only treated her for a simple skin cancer. She explained that I had seen her mom about a year ago and cut out a skin cancer on her face. Her mom was afraid of needles and of surgery. Apparently when she asked me if it would hurt, I replied: “Well, most patients, yes, but not you.” Pausing, I added: “Because you’re a tough old bird.” She laughed. Apparently that warmth I conveyed and display of confidence in her was just what she needed at that moment. She didn’t flinch.
Not long after, she was diagnosed with breast cancer. When given the news with her children present, she replied, “well, I’ll just fight it. I’m a tough old bird.” It was just what they needed in that moment. “I’m a tough old bird” became their rally cry. Apparently with each stage, surgery, radiation, chemo, they fell back on it. Her son had “Tough Old Bird” made into a magnet and prominently posted on the refrigerator door where she would see it every day.
Sadly, she ultimately succumbed to her disease, but did so later than had been expected and having fought all the way. My patient teared up and asked if she could give me a hug on behalf of her mom. “Thank you, Dr. Benabio. We won’t forget what you did for her.”
I did recall her now, remembering even what exam room she was in when I said it. Yet, I had no idea what I had done. I wonder how many others there were. Of the many things you accomplished this year, try to recall these achievements. Not the psoriasis cleared, or tumor extirpated, or new homes bought. But the comfort and care you brought to the mother with worry, the father with anguish, the daughter with anxieties, or the son with misdeeds.
It is a beautiful, hard, and joyous life we have as physicians, for our “happiness lies in the absorption in some vocation which satisfies the soul; that we are here to add what we can to, not get what we can from life.”* How fortunate are we. Take stock.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Reference
*William Osler, “Doctor and Nurse.” Address given at Training School for Nurses at Johns Hopkins Hospital, June 4, 1891
‘Tis the season to reflect and take stock: Maybe you had a baby, or learned a new procedure, or bought a Tesla? Of course, you made (loads of?) money and treated many patients. Imagine if I asked you this in person, what would you reply? And what made you most proud? I’d tell you this story.
Last week I saw a 50-something-year-old woman for her annual skin screening. She asked if I remembered her mother, who was also my patient. Squinting through my dermatoscope at the nevi on her back, I tried to recall. “Yes, I think so.” (Actually, I was unsure.)
“Well she passed away last week from breast cancer,” she said.
“Oh, I’m sorry to hear that,” I replied.
She added: “Yes, yet she lived much longer than we thought. I want you to know we believe it was in large part because of you.”
I stopped and wheeled around to face her. How could that possibly be true? I had only treated her for a simple skin cancer. She explained that I had seen her mom about a year ago and cut out a skin cancer on her face. Her mom was afraid of needles and of surgery. Apparently when she asked me if it would hurt, I replied: “Well, most patients, yes, but not you.” Pausing, I added: “Because you’re a tough old bird.” She laughed. Apparently that warmth I conveyed and display of confidence in her was just what she needed at that moment. She didn’t flinch.
Not long after, she was diagnosed with breast cancer. When given the news with her children present, she replied, “well, I’ll just fight it. I’m a tough old bird.” It was just what they needed in that moment. “I’m a tough old bird” became their rally cry. Apparently with each stage, surgery, radiation, chemo, they fell back on it. Her son had “Tough Old Bird” made into a magnet and prominently posted on the refrigerator door where she would see it every day.
Sadly, she ultimately succumbed to her disease, but did so later than had been expected and having fought all the way. My patient teared up and asked if she could give me a hug on behalf of her mom. “Thank you, Dr. Benabio. We won’t forget what you did for her.”
I did recall her now, remembering even what exam room she was in when I said it. Yet, I had no idea what I had done. I wonder how many others there were. Of the many things you accomplished this year, try to recall these achievements. Not the psoriasis cleared, or tumor extirpated, or new homes bought. But the comfort and care you brought to the mother with worry, the father with anguish, the daughter with anxieties, or the son with misdeeds.
It is a beautiful, hard, and joyous life we have as physicians, for our “happiness lies in the absorption in some vocation which satisfies the soul; that we are here to add what we can to, not get what we can from life.”* How fortunate are we. Take stock.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Reference
*William Osler, “Doctor and Nurse.” Address given at Training School for Nurses at Johns Hopkins Hospital, June 4, 1891
Office of Inspector General
Question: Which one of the following statements is incorrect?
A. Office of Inspector General (OIG) is a federal agency of Department of Health & Human Services (HHS) that investigates statutory violations of health care fraud and abuse.
B. The three main legal minefields for physicians are false claims, kickbacks, and self-referrals.
C. Jail terms are part of the penalties provided by law.
D. OIG is also responsible for excluding violators from participating in Medicare/Medicaid programs, as well as curtailing a physician’s license to practice.
E. A private citizen can file a qui tam lawsuit against an errant practitioner or health care entity for fraud and abuse.
Answer: D. Health care fraud, waste, and abuse consume some 10% of federal health expenditures despite well-established laws that attempt to prevent and reduce such losses. The Department of Health & Human Services (HHS) Office of Inspector General, as well as the Department of Justice and the Centers for Medicare & Medicaid Services are charged with enforcing these and other laws like the Emergency Medical Treatment and Labor Act. Their web pages, referenced throughout this article, contain a wealth of information for the practitioner.
The term “Office of Inspector General” (OIG) refers to the oversight division of a federal or state agency charged with identifying and investigating fraud, waste, abuse, and mismanagement within that department or agency. There are currently 73 separate federal offices of inspectors general, which employ armed and unarmed criminal investigators, auditors, forensic auditors called “audigators,” and a variety of other specialists. An Act of Congress in 1976 established the first OIG under HHS. Besides being the first, HHS-OIG is also the largest, with a staff of approximately 1,600. A majority of resources goes toward the oversight of Medicare and Medicaid, as well as programs under other HHS institutions such as the Centers for Disease Control and Prevention, National Institutes of Health, and the Food and Drug Administration.1
HHS-OIG has the authority to seek civil monetary penalties, assessments, and exclusion against an individual or entity based on a wide variety of prohibited conduct affecting federal health care programs. Stiff penalties are regularly assessed against violators, and jail terms are not uncommon; however, it has no direct jurisdictions over physician licensure or nonfederal programs. The government maintains a pictorial list of its most wanted health care fugitives2 and provides an excellent set of Physician Education Training Materials on its website.3
False Claims Act (FCA)
In the health care arena, violation of FCA (31 U.S.C. §§3729-3733) is the foremost infraction. False claims by physicians can include billing for noncovered services such as experimental treatments, double billing, billing the government as the primary payer, or regularly waiving deductibles and copayments, as well as quality of care issues and unnecessary services. Wrongdoing also includes knowingly using another patient’s name for purposes of, say, federal drug coverage, billing for no-shows, and misrepresenting the diagnosis to justify services, as well as other claims. In the modern doctor’s office, the EMR enables easy check-offs on a preprinted form as documentation of actual work done. However, fraud is implicated if the information is deliberately misleading such as for purposes of upcoding. Importantly, physicians are liable for the actions of their office staff, so it is prudent to oversee and supervise all such activities. Naturally, one should document all claims that are sent and know the rules for allowable and excluded services.
FCA is an old law that was first enacted in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false. Intent to defraud is not a required element but knowing or showing reckless disregard of the truth is. However, an error that is negligently committed is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim, as well as possible imprisonment and criminal fines. A so-called whistle-blower may file a lawsuit on behalf of the government and is entitled to a percentage of any recoveries. Whistle-blowers may be current or ex-business partners, hospital or office staff, patients, or competitors. The fact that a claim results from a kickback or is made in violation of the Stark law (discussed below) may also render it fraudulent, thus creating additional liability under FCA.
HHS-OIG, as well as the Department of Justice, discloses named cases of statutory violations on their websites. A few random 2019 examples include a New York licensed doctor was convicted of nine counts in connection with Oxycodone and Fentanyl diversion scheme; a Newton, Mass., geriatrician agreed to pay $680,000 to resolve allegations that he violated the False Claims Act by submitting inflated claims to Medicare and the Massachusetts Medicaid program (MassHealth) for care rendered to nursing home patients; and two Clermont, Fla., ophthalmologists agreed to pay a combined total of $157,312.32 to resolve allegations that they violated FCA by knowingly billing the government for mutually exclusive eyelid repair surgeries.
Anti-Kickback Statute (AKS)
AKS (42 U.S.C. § 1320a-7b[b]) is a criminal law that prohibits the knowing and willful payment of “remuneration” to induce or reward patient referrals or the generation of business involving any item or service payable by federal health care programs. Remuneration includes anything of value and can take many forms besides cash, such as free rent, lavish travel, and excessive compensation for medical directorships or consultancies. Rewarding a referral source may be acceptable in some industries, but is a crime in federal health care programs.
Moreover, the statute covers both the payers of kickbacks (those who offer or pay remuneration) and the recipients of kickbacks. Each party’s intent is a key element of their liability under AKS. Physicians who pay or accept kickbacks face penalties of up to $50,000 per kickback plus three times the amount of the remuneration and criminal prosecution. As an example, a Tulsa, Okla., doctor earlier this year agreed to pay the government $84,666.42 for allegedly accepting illegal kickback payments from a pharmacy, and in another case, a marketer agreed to pay nearly $340,000 for receiving kickbacks in exchange for prescription referrals.
A physician is an attractive target for kickback schemes. The kickback prohibition applies to all sources, even patients. For example, where the Medicare and Medicaid programs require patients to be responsible for copays for services, the health care provider is generally required to collect that money from the patients. Advertising the forgiveness of copayments or routinely waiving these copays would violate AKS. However, one may waive a copayment when a patient cannot afford to pay one or is uninsured. AKS also imposes civil monetary penalties on physicians who offer remuneration to Medicare and Medicaid beneficiaries to influence them to use their services. Note that the government does not need to prove patient harm or financial loss to show that a violation has occurred, and physicians can be guilty even if they rendered services that are medically necessary.
There are so-called safe harbors that protect certain payment and business practices from running afoul of AKS. The rules and requirements are complex, and require full understanding and strict adherence.4
Physician Self-Referral Law
The Physician Self-Referral Law (42 U.S.C. § 1395nn), commonly referred to as the Stark law, prohibits physicians from referring patients to receive “designated health services” payable by Medicare or Medicaid from entities with which the physician or an immediate family member has a financial relationship, unless an exception applies. Financial relationships include both ownership/investment interests and compensation arrangements. A partial list of “designated health services” includes services related to clinical laboratory, physical therapy, radiology, parenteral and enteral supplies, prosthetic devices and supplies, home health care outpatient prescription drugs, and inpatient and outpatient hospital services. This list is not meant to be an exhaustive one.
Stark is a strict liability statute, which means proof of specific intent to violate the law is not required. The law prohibits the submission, or causing the submission, of claims in violation of the law’s restrictions on referrals. Penalties for physicians who violate the Stark law include fines, as well as exclusion from participation in federal health care programs. Like AKS, Stark law features its own safe harbors.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some materials may have been discussed in earlier columns. For additional information, readers may contact the author at [email protected].
References
1. HHS Office of Inspector General. About OIG. https://oig.hhs.gov/about-oig/about-us/index.asp
2. HHS Office of Inspector General. OIG most wanted fugitives.
3. HHS Office of Inspector General. “Physician education training materials.” A roadmap for new physicians: Avoiding Medicare and Medicaid fraud and abuse.
4. HHS Office of Inspector General. Safe harbor regulations.
Question: Which one of the following statements is incorrect?
A. Office of Inspector General (OIG) is a federal agency of Department of Health & Human Services (HHS) that investigates statutory violations of health care fraud and abuse.
B. The three main legal minefields for physicians are false claims, kickbacks, and self-referrals.
C. Jail terms are part of the penalties provided by law.
D. OIG is also responsible for excluding violators from participating in Medicare/Medicaid programs, as well as curtailing a physician’s license to practice.
E. A private citizen can file a qui tam lawsuit against an errant practitioner or health care entity for fraud and abuse.
Answer: D. Health care fraud, waste, and abuse consume some 10% of federal health expenditures despite well-established laws that attempt to prevent and reduce such losses. The Department of Health & Human Services (HHS) Office of Inspector General, as well as the Department of Justice and the Centers for Medicare & Medicaid Services are charged with enforcing these and other laws like the Emergency Medical Treatment and Labor Act. Their web pages, referenced throughout this article, contain a wealth of information for the practitioner.
The term “Office of Inspector General” (OIG) refers to the oversight division of a federal or state agency charged with identifying and investigating fraud, waste, abuse, and mismanagement within that department or agency. There are currently 73 separate federal offices of inspectors general, which employ armed and unarmed criminal investigators, auditors, forensic auditors called “audigators,” and a variety of other specialists. An Act of Congress in 1976 established the first OIG under HHS. Besides being the first, HHS-OIG is also the largest, with a staff of approximately 1,600. A majority of resources goes toward the oversight of Medicare and Medicaid, as well as programs under other HHS institutions such as the Centers for Disease Control and Prevention, National Institutes of Health, and the Food and Drug Administration.1
HHS-OIG has the authority to seek civil monetary penalties, assessments, and exclusion against an individual or entity based on a wide variety of prohibited conduct affecting federal health care programs. Stiff penalties are regularly assessed against violators, and jail terms are not uncommon; however, it has no direct jurisdictions over physician licensure or nonfederal programs. The government maintains a pictorial list of its most wanted health care fugitives2 and provides an excellent set of Physician Education Training Materials on its website.3
False Claims Act (FCA)
In the health care arena, violation of FCA (31 U.S.C. §§3729-3733) is the foremost infraction. False claims by physicians can include billing for noncovered services such as experimental treatments, double billing, billing the government as the primary payer, or regularly waiving deductibles and copayments, as well as quality of care issues and unnecessary services. Wrongdoing also includes knowingly using another patient’s name for purposes of, say, federal drug coverage, billing for no-shows, and misrepresenting the diagnosis to justify services, as well as other claims. In the modern doctor’s office, the EMR enables easy check-offs on a preprinted form as documentation of actual work done. However, fraud is implicated if the information is deliberately misleading such as for purposes of upcoding. Importantly, physicians are liable for the actions of their office staff, so it is prudent to oversee and supervise all such activities. Naturally, one should document all claims that are sent and know the rules for allowable and excluded services.
FCA is an old law that was first enacted in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false. Intent to defraud is not a required element but knowing or showing reckless disregard of the truth is. However, an error that is negligently committed is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim, as well as possible imprisonment and criminal fines. A so-called whistle-blower may file a lawsuit on behalf of the government and is entitled to a percentage of any recoveries. Whistle-blowers may be current or ex-business partners, hospital or office staff, patients, or competitors. The fact that a claim results from a kickback or is made in violation of the Stark law (discussed below) may also render it fraudulent, thus creating additional liability under FCA.
HHS-OIG, as well as the Department of Justice, discloses named cases of statutory violations on their websites. A few random 2019 examples include a New York licensed doctor was convicted of nine counts in connection with Oxycodone and Fentanyl diversion scheme; a Newton, Mass., geriatrician agreed to pay $680,000 to resolve allegations that he violated the False Claims Act by submitting inflated claims to Medicare and the Massachusetts Medicaid program (MassHealth) for care rendered to nursing home patients; and two Clermont, Fla., ophthalmologists agreed to pay a combined total of $157,312.32 to resolve allegations that they violated FCA by knowingly billing the government for mutually exclusive eyelid repair surgeries.
Anti-Kickback Statute (AKS)
AKS (42 U.S.C. § 1320a-7b[b]) is a criminal law that prohibits the knowing and willful payment of “remuneration” to induce or reward patient referrals or the generation of business involving any item or service payable by federal health care programs. Remuneration includes anything of value and can take many forms besides cash, such as free rent, lavish travel, and excessive compensation for medical directorships or consultancies. Rewarding a referral source may be acceptable in some industries, but is a crime in federal health care programs.
Moreover, the statute covers both the payers of kickbacks (those who offer or pay remuneration) and the recipients of kickbacks. Each party’s intent is a key element of their liability under AKS. Physicians who pay or accept kickbacks face penalties of up to $50,000 per kickback plus three times the amount of the remuneration and criminal prosecution. As an example, a Tulsa, Okla., doctor earlier this year agreed to pay the government $84,666.42 for allegedly accepting illegal kickback payments from a pharmacy, and in another case, a marketer agreed to pay nearly $340,000 for receiving kickbacks in exchange for prescription referrals.
A physician is an attractive target for kickback schemes. The kickback prohibition applies to all sources, even patients. For example, where the Medicare and Medicaid programs require patients to be responsible for copays for services, the health care provider is generally required to collect that money from the patients. Advertising the forgiveness of copayments or routinely waiving these copays would violate AKS. However, one may waive a copayment when a patient cannot afford to pay one or is uninsured. AKS also imposes civil monetary penalties on physicians who offer remuneration to Medicare and Medicaid beneficiaries to influence them to use their services. Note that the government does not need to prove patient harm or financial loss to show that a violation has occurred, and physicians can be guilty even if they rendered services that are medically necessary.
There are so-called safe harbors that protect certain payment and business practices from running afoul of AKS. The rules and requirements are complex, and require full understanding and strict adherence.4
Physician Self-Referral Law
The Physician Self-Referral Law (42 U.S.C. § 1395nn), commonly referred to as the Stark law, prohibits physicians from referring patients to receive “designated health services” payable by Medicare or Medicaid from entities with which the physician or an immediate family member has a financial relationship, unless an exception applies. Financial relationships include both ownership/investment interests and compensation arrangements. A partial list of “designated health services” includes services related to clinical laboratory, physical therapy, radiology, parenteral and enteral supplies, prosthetic devices and supplies, home health care outpatient prescription drugs, and inpatient and outpatient hospital services. This list is not meant to be an exhaustive one.
Stark is a strict liability statute, which means proof of specific intent to violate the law is not required. The law prohibits the submission, or causing the submission, of claims in violation of the law’s restrictions on referrals. Penalties for physicians who violate the Stark law include fines, as well as exclusion from participation in federal health care programs. Like AKS, Stark law features its own safe harbors.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some materials may have been discussed in earlier columns. For additional information, readers may contact the author at [email protected].
References
1. HHS Office of Inspector General. About OIG. https://oig.hhs.gov/about-oig/about-us/index.asp
2. HHS Office of Inspector General. OIG most wanted fugitives.
3. HHS Office of Inspector General. “Physician education training materials.” A roadmap for new physicians: Avoiding Medicare and Medicaid fraud and abuse.
4. HHS Office of Inspector General. Safe harbor regulations.
Question: Which one of the following statements is incorrect?
A. Office of Inspector General (OIG) is a federal agency of Department of Health & Human Services (HHS) that investigates statutory violations of health care fraud and abuse.
B. The three main legal minefields for physicians are false claims, kickbacks, and self-referrals.
C. Jail terms are part of the penalties provided by law.
D. OIG is also responsible for excluding violators from participating in Medicare/Medicaid programs, as well as curtailing a physician’s license to practice.
E. A private citizen can file a qui tam lawsuit against an errant practitioner or health care entity for fraud and abuse.
Answer: D. Health care fraud, waste, and abuse consume some 10% of federal health expenditures despite well-established laws that attempt to prevent and reduce such losses. The Department of Health & Human Services (HHS) Office of Inspector General, as well as the Department of Justice and the Centers for Medicare & Medicaid Services are charged with enforcing these and other laws like the Emergency Medical Treatment and Labor Act. Their web pages, referenced throughout this article, contain a wealth of information for the practitioner.
The term “Office of Inspector General” (OIG) refers to the oversight division of a federal or state agency charged with identifying and investigating fraud, waste, abuse, and mismanagement within that department or agency. There are currently 73 separate federal offices of inspectors general, which employ armed and unarmed criminal investigators, auditors, forensic auditors called “audigators,” and a variety of other specialists. An Act of Congress in 1976 established the first OIG under HHS. Besides being the first, HHS-OIG is also the largest, with a staff of approximately 1,600. A majority of resources goes toward the oversight of Medicare and Medicaid, as well as programs under other HHS institutions such as the Centers for Disease Control and Prevention, National Institutes of Health, and the Food and Drug Administration.1
HHS-OIG has the authority to seek civil monetary penalties, assessments, and exclusion against an individual or entity based on a wide variety of prohibited conduct affecting federal health care programs. Stiff penalties are regularly assessed against violators, and jail terms are not uncommon; however, it has no direct jurisdictions over physician licensure or nonfederal programs. The government maintains a pictorial list of its most wanted health care fugitives2 and provides an excellent set of Physician Education Training Materials on its website.3
False Claims Act (FCA)
In the health care arena, violation of FCA (31 U.S.C. §§3729-3733) is the foremost infraction. False claims by physicians can include billing for noncovered services such as experimental treatments, double billing, billing the government as the primary payer, or regularly waiving deductibles and copayments, as well as quality of care issues and unnecessary services. Wrongdoing also includes knowingly using another patient’s name for purposes of, say, federal drug coverage, billing for no-shows, and misrepresenting the diagnosis to justify services, as well as other claims. In the modern doctor’s office, the EMR enables easy check-offs on a preprinted form as documentation of actual work done. However, fraud is implicated if the information is deliberately misleading such as for purposes of upcoding. Importantly, physicians are liable for the actions of their office staff, so it is prudent to oversee and supervise all such activities. Naturally, one should document all claims that are sent and know the rules for allowable and excluded services.
FCA is an old law that was first enacted in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false. Intent to defraud is not a required element but knowing or showing reckless disregard of the truth is. However, an error that is negligently committed is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim, as well as possible imprisonment and criminal fines. A so-called whistle-blower may file a lawsuit on behalf of the government and is entitled to a percentage of any recoveries. Whistle-blowers may be current or ex-business partners, hospital or office staff, patients, or competitors. The fact that a claim results from a kickback or is made in violation of the Stark law (discussed below) may also render it fraudulent, thus creating additional liability under FCA.
HHS-OIG, as well as the Department of Justice, discloses named cases of statutory violations on their websites. A few random 2019 examples include a New York licensed doctor was convicted of nine counts in connection with Oxycodone and Fentanyl diversion scheme; a Newton, Mass., geriatrician agreed to pay $680,000 to resolve allegations that he violated the False Claims Act by submitting inflated claims to Medicare and the Massachusetts Medicaid program (MassHealth) for care rendered to nursing home patients; and two Clermont, Fla., ophthalmologists agreed to pay a combined total of $157,312.32 to resolve allegations that they violated FCA by knowingly billing the government for mutually exclusive eyelid repair surgeries.
Anti-Kickback Statute (AKS)
AKS (42 U.S.C. § 1320a-7b[b]) is a criminal law that prohibits the knowing and willful payment of “remuneration” to induce or reward patient referrals or the generation of business involving any item or service payable by federal health care programs. Remuneration includes anything of value and can take many forms besides cash, such as free rent, lavish travel, and excessive compensation for medical directorships or consultancies. Rewarding a referral source may be acceptable in some industries, but is a crime in federal health care programs.
Moreover, the statute covers both the payers of kickbacks (those who offer or pay remuneration) and the recipients of kickbacks. Each party’s intent is a key element of their liability under AKS. Physicians who pay or accept kickbacks face penalties of up to $50,000 per kickback plus three times the amount of the remuneration and criminal prosecution. As an example, a Tulsa, Okla., doctor earlier this year agreed to pay the government $84,666.42 for allegedly accepting illegal kickback payments from a pharmacy, and in another case, a marketer agreed to pay nearly $340,000 for receiving kickbacks in exchange for prescription referrals.
A physician is an attractive target for kickback schemes. The kickback prohibition applies to all sources, even patients. For example, where the Medicare and Medicaid programs require patients to be responsible for copays for services, the health care provider is generally required to collect that money from the patients. Advertising the forgiveness of copayments or routinely waiving these copays would violate AKS. However, one may waive a copayment when a patient cannot afford to pay one or is uninsured. AKS also imposes civil monetary penalties on physicians who offer remuneration to Medicare and Medicaid beneficiaries to influence them to use their services. Note that the government does not need to prove patient harm or financial loss to show that a violation has occurred, and physicians can be guilty even if they rendered services that are medically necessary.
There are so-called safe harbors that protect certain payment and business practices from running afoul of AKS. The rules and requirements are complex, and require full understanding and strict adherence.4
Physician Self-Referral Law
The Physician Self-Referral Law (42 U.S.C. § 1395nn), commonly referred to as the Stark law, prohibits physicians from referring patients to receive “designated health services” payable by Medicare or Medicaid from entities with which the physician or an immediate family member has a financial relationship, unless an exception applies. Financial relationships include both ownership/investment interests and compensation arrangements. A partial list of “designated health services” includes services related to clinical laboratory, physical therapy, radiology, parenteral and enteral supplies, prosthetic devices and supplies, home health care outpatient prescription drugs, and inpatient and outpatient hospital services. This list is not meant to be an exhaustive one.
Stark is a strict liability statute, which means proof of specific intent to violate the law is not required. The law prohibits the submission, or causing the submission, of claims in violation of the law’s restrictions on referrals. Penalties for physicians who violate the Stark law include fines, as well as exclusion from participation in federal health care programs. Like AKS, Stark law features its own safe harbors.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some materials may have been discussed in earlier columns. For additional information, readers may contact the author at [email protected].
References
1. HHS Office of Inspector General. About OIG. https://oig.hhs.gov/about-oig/about-us/index.asp
2. HHS Office of Inspector General. OIG most wanted fugitives.
3. HHS Office of Inspector General. “Physician education training materials.” A roadmap for new physicians: Avoiding Medicare and Medicaid fraud and abuse.
4. HHS Office of Inspector General. Safe harbor regulations.
Letters From Maine: An albatross or your identity?
The last time I saw her she was coiled up like a garter snake resting comfortable in the old toiletries travel case that was my “black bag” for more than 40 years. Joining her in peaceful solitude were a couple of ear curettes, an insufflator, and a dead pocket flashlight. The Kermit the Frog sticker on her diaphragm was faded to a barely recognizable blur. The chest piece was frozen in the diaphragm position as it had been for several decades. I never felt comfortable using the bell side.
She was the gift from a drug company back when medical students were more interested in freebies than making a statement about conflicts of interest. I have had to change her tubing several times when cracks at the bifurcation would allow me to hear my own breath sounds better than the patient’s. The ear pieces were the originals that I modified to fit my auditory canals more comfortably.
I suspect that many of you have developed a close relationship with your stethoscope, as I did. We were always close. She was either her coiled up in my pants’ pocket or clasped around my neck where she wore through collars at a costly clip. Her chest piece was kept tucked in my shirt to keep it warm for the patients. I never hung her over my shoulders the way physicians do in publicity photos. I always found that practice pretentious and impractical.
If I decided tomorrow to leave the challenges of retirement behind and reopen my practice would it make any sense to go down to the basement and roust out my old stethoscope from her slumber? There are better ways evaluate hearts and lungs and many of them will fit in my pocket just as well as that old stethoscope. Paul Wallach, MD, an executive associate dean at the Indiana University, Indianapolis, predicts that within a decade hand-held ultrasound devices with become part of a routine part of the physical exam (Lindsey Tanner. “Is the stethoscope dying? High-tech rivals pose a challenge.” Associated Press. 2019 Oct 23). Instruction in the use of these devices has already become part of the curriculum in some medical schools.
There have been several studies demonstrating that chest auscultation is a skill that some of us have lost and many others never successfully mastered. As much as I treasure my old stethoscope, is it time to get rid of those albatrosses hanging around our necks? They do bang against desks with a deafening ring. Cute infants and toddlers yank on them while we are trying to listen to their chests. If there are better ways to auscultate chests that will fit in our pockets shouldn’t we be using them?
Well, there is the cost for one thing. But, inevitably the price will come down and portability will go up. If we allow our stethoscopes to become nothing more than nostalgic museum pieces to sit along with the head mirror, What will photographers drape over our shoulders? With very few of us in office practice wearing white coats or scrub suits, we run the risk of losing our identity.
Sadly, I fear we will have to accept the disappearance of the stethoscope as a natural consequence of the technological march. But, it also is an unfortunate reflection of the fact that the art of doing a physical exam is fading. With auscultation and palpation disappearing from our diagnostic tool kit we must be careful to preserve and improve the one skill that is indispensable to the practice of medicine.
And, that is listening to what the patient has to tell us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The last time I saw her she was coiled up like a garter snake resting comfortable in the old toiletries travel case that was my “black bag” for more than 40 years. Joining her in peaceful solitude were a couple of ear curettes, an insufflator, and a dead pocket flashlight. The Kermit the Frog sticker on her diaphragm was faded to a barely recognizable blur. The chest piece was frozen in the diaphragm position as it had been for several decades. I never felt comfortable using the bell side.
She was the gift from a drug company back when medical students were more interested in freebies than making a statement about conflicts of interest. I have had to change her tubing several times when cracks at the bifurcation would allow me to hear my own breath sounds better than the patient’s. The ear pieces were the originals that I modified to fit my auditory canals more comfortably.
I suspect that many of you have developed a close relationship with your stethoscope, as I did. We were always close. She was either her coiled up in my pants’ pocket or clasped around my neck where she wore through collars at a costly clip. Her chest piece was kept tucked in my shirt to keep it warm for the patients. I never hung her over my shoulders the way physicians do in publicity photos. I always found that practice pretentious and impractical.
If I decided tomorrow to leave the challenges of retirement behind and reopen my practice would it make any sense to go down to the basement and roust out my old stethoscope from her slumber? There are better ways evaluate hearts and lungs and many of them will fit in my pocket just as well as that old stethoscope. Paul Wallach, MD, an executive associate dean at the Indiana University, Indianapolis, predicts that within a decade hand-held ultrasound devices with become part of a routine part of the physical exam (Lindsey Tanner. “Is the stethoscope dying? High-tech rivals pose a challenge.” Associated Press. 2019 Oct 23). Instruction in the use of these devices has already become part of the curriculum in some medical schools.
There have been several studies demonstrating that chest auscultation is a skill that some of us have lost and many others never successfully mastered. As much as I treasure my old stethoscope, is it time to get rid of those albatrosses hanging around our necks? They do bang against desks with a deafening ring. Cute infants and toddlers yank on them while we are trying to listen to their chests. If there are better ways to auscultate chests that will fit in our pockets shouldn’t we be using them?
Well, there is the cost for one thing. But, inevitably the price will come down and portability will go up. If we allow our stethoscopes to become nothing more than nostalgic museum pieces to sit along with the head mirror, What will photographers drape over our shoulders? With very few of us in office practice wearing white coats or scrub suits, we run the risk of losing our identity.
Sadly, I fear we will have to accept the disappearance of the stethoscope as a natural consequence of the technological march. But, it also is an unfortunate reflection of the fact that the art of doing a physical exam is fading. With auscultation and palpation disappearing from our diagnostic tool kit we must be careful to preserve and improve the one skill that is indispensable to the practice of medicine.
And, that is listening to what the patient has to tell us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The last time I saw her she was coiled up like a garter snake resting comfortable in the old toiletries travel case that was my “black bag” for more than 40 years. Joining her in peaceful solitude were a couple of ear curettes, an insufflator, and a dead pocket flashlight. The Kermit the Frog sticker on her diaphragm was faded to a barely recognizable blur. The chest piece was frozen in the diaphragm position as it had been for several decades. I never felt comfortable using the bell side.
She was the gift from a drug company back when medical students were more interested in freebies than making a statement about conflicts of interest. I have had to change her tubing several times when cracks at the bifurcation would allow me to hear my own breath sounds better than the patient’s. The ear pieces were the originals that I modified to fit my auditory canals more comfortably.
I suspect that many of you have developed a close relationship with your stethoscope, as I did. We were always close. She was either her coiled up in my pants’ pocket or clasped around my neck where she wore through collars at a costly clip. Her chest piece was kept tucked in my shirt to keep it warm for the patients. I never hung her over my shoulders the way physicians do in publicity photos. I always found that practice pretentious and impractical.
If I decided tomorrow to leave the challenges of retirement behind and reopen my practice would it make any sense to go down to the basement and roust out my old stethoscope from her slumber? There are better ways evaluate hearts and lungs and many of them will fit in my pocket just as well as that old stethoscope. Paul Wallach, MD, an executive associate dean at the Indiana University, Indianapolis, predicts that within a decade hand-held ultrasound devices with become part of a routine part of the physical exam (Lindsey Tanner. “Is the stethoscope dying? High-tech rivals pose a challenge.” Associated Press. 2019 Oct 23). Instruction in the use of these devices has already become part of the curriculum in some medical schools.
There have been several studies demonstrating that chest auscultation is a skill that some of us have lost and many others never successfully mastered. As much as I treasure my old stethoscope, is it time to get rid of those albatrosses hanging around our necks? They do bang against desks with a deafening ring. Cute infants and toddlers yank on them while we are trying to listen to their chests. If there are better ways to auscultate chests that will fit in our pockets shouldn’t we be using them?
Well, there is the cost for one thing. But, inevitably the price will come down and portability will go up. If we allow our stethoscopes to become nothing more than nostalgic museum pieces to sit along with the head mirror, What will photographers drape over our shoulders? With very few of us in office practice wearing white coats or scrub suits, we run the risk of losing our identity.
Sadly, I fear we will have to accept the disappearance of the stethoscope as a natural consequence of the technological march. But, it also is an unfortunate reflection of the fact that the art of doing a physical exam is fading. With auscultation and palpation disappearing from our diagnostic tool kit we must be careful to preserve and improve the one skill that is indispensable to the practice of medicine.
And, that is listening to what the patient has to tell us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].