User login
What Neglected Tropical Diseases Teach Us About Stigma
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
The VA Ketamine Controversies
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
When providing contraceptive counseling to women with migraine headaches, how do you identify migraine with aura?
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
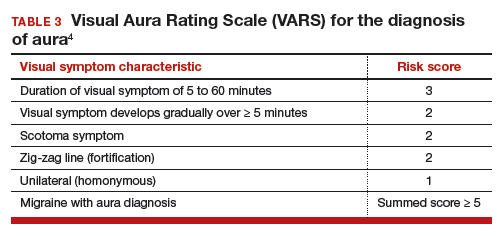
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4
Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Can we discern optimal long-term osteoporosis treatment for women?
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
Ovarian tumor markers: What to draw and when
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.
So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Premature mortality across most psychiatric disorders
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
Food as therapy and toxin
I return to write the Editor’s comments after missing last month because I joined over 700,000 Americans who, this year, will undergo knee replacement surgery.
This month, we feature a couple articles from the 2019 James W. Freston Conference (an annual AGA event that highlights cutting-edge science). Jim was the 89th AGA President (1995) and this conference is a fitting legacy. This year’s topic was “Food at the intersection of gut health and disease.” As usual, the Freston Conference attracted international experts and interested clinicians who want to understand how current research will alter our clinical care in the near future.
Our front-page articles are fascinating. One highlights new advances in the management of celiac disease. Although the only current treatment that reverses intestinal immunological damage is adoption of a gluten-free diet, there is demand for alternative treatments including medical therapies targeting specific steps in the celiac damage pathway. While none are ready for wide-spread adoption, research will continue. Patient self-management with gluten detection-devices were also discussed.
Advances in the genetics of Crohn’s disease are being published at an accelerating rate. This month we highlight an article about how gene expression analysis can predict response to a Crohn’s flare. Evidence-based therapy for inflammatory bowel disease is complex, so clinicians need to stay current. Each year, the premier IBD educational venue is co-produced by the AGA and the Crohn’s & Colitis Foundation. The 2020 Crohn’s and Colitis Congress will be held in Austin, Texas January 23-25. Learn more at: https://www.crohnscolitiscongress.org.
Finally, I want to highlight an article about the risk of venous thromboembolism (VTE) during and after an IBD flare. This risk is underappreciated by many treating physicians but it is real and can be life-threatening. Gastroenterologists must be knowledgeable about current guidelines for VTE in IBD patients (see Gastroenterology 2014;146:835-48).
John I. Allen, MD, MBA, AGAF
Editor in Chief
I return to write the Editor’s comments after missing last month because I joined over 700,000 Americans who, this year, will undergo knee replacement surgery.
This month, we feature a couple articles from the 2019 James W. Freston Conference (an annual AGA event that highlights cutting-edge science). Jim was the 89th AGA President (1995) and this conference is a fitting legacy. This year’s topic was “Food at the intersection of gut health and disease.” As usual, the Freston Conference attracted international experts and interested clinicians who want to understand how current research will alter our clinical care in the near future.
Our front-page articles are fascinating. One highlights new advances in the management of celiac disease. Although the only current treatment that reverses intestinal immunological damage is adoption of a gluten-free diet, there is demand for alternative treatments including medical therapies targeting specific steps in the celiac damage pathway. While none are ready for wide-spread adoption, research will continue. Patient self-management with gluten detection-devices were also discussed.
Advances in the genetics of Crohn’s disease are being published at an accelerating rate. This month we highlight an article about how gene expression analysis can predict response to a Crohn’s flare. Evidence-based therapy for inflammatory bowel disease is complex, so clinicians need to stay current. Each year, the premier IBD educational venue is co-produced by the AGA and the Crohn’s & Colitis Foundation. The 2020 Crohn’s and Colitis Congress will be held in Austin, Texas January 23-25. Learn more at: https://www.crohnscolitiscongress.org.
Finally, I want to highlight an article about the risk of venous thromboembolism (VTE) during and after an IBD flare. This risk is underappreciated by many treating physicians but it is real and can be life-threatening. Gastroenterologists must be knowledgeable about current guidelines for VTE in IBD patients (see Gastroenterology 2014;146:835-48).
John I. Allen, MD, MBA, AGAF
Editor in Chief
I return to write the Editor’s comments after missing last month because I joined over 700,000 Americans who, this year, will undergo knee replacement surgery.
This month, we feature a couple articles from the 2019 James W. Freston Conference (an annual AGA event that highlights cutting-edge science). Jim was the 89th AGA President (1995) and this conference is a fitting legacy. This year’s topic was “Food at the intersection of gut health and disease.” As usual, the Freston Conference attracted international experts and interested clinicians who want to understand how current research will alter our clinical care in the near future.
Our front-page articles are fascinating. One highlights new advances in the management of celiac disease. Although the only current treatment that reverses intestinal immunological damage is adoption of a gluten-free diet, there is demand for alternative treatments including medical therapies targeting specific steps in the celiac damage pathway. While none are ready for wide-spread adoption, research will continue. Patient self-management with gluten detection-devices were also discussed.
Advances in the genetics of Crohn’s disease are being published at an accelerating rate. This month we highlight an article about how gene expression analysis can predict response to a Crohn’s flare. Evidence-based therapy for inflammatory bowel disease is complex, so clinicians need to stay current. Each year, the premier IBD educational venue is co-produced by the AGA and the Crohn’s & Colitis Foundation. The 2020 Crohn’s and Colitis Congress will be held in Austin, Texas January 23-25. Learn more at: https://www.crohnscolitiscongress.org.
Finally, I want to highlight an article about the risk of venous thromboembolism (VTE) during and after an IBD flare. This risk is underappreciated by many treating physicians but it is real and can be life-threatening. Gastroenterologists must be knowledgeable about current guidelines for VTE in IBD patients (see Gastroenterology 2014;146:835-48).
John I. Allen, MD, MBA, AGAF
Editor in Chief
Preemptive pacifier promotion
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
Labeling of medication warnings
Question: Which one of the following statements regarding medication warnings is incorrect?
A. The drug package “insert” or “label” contains, among other things, a drug’s pharmacology, indications, contraindications, risks and warnings.
B. The Physicians’ Desk Reference (PDR) is an annually updated drug compendium, which can be admitted into evidence as a learned treatise.
C. Drug labeling is a dual responsibility of the manufacturer and the Food and Drug Administration.
D. The FDA is solely responsible for a drug’s warnings and sets the absolute standard of care regarding side effects and complications.
E. State law can impose liability for negligent failure to warn even if the FDA has not included the warning in the drug’s label.
Answer: D. In medical products liability, injured plaintiffs frequently claim a failure to warn of known risks. An example is the cardiovascular deaths caused by Vioxx, a nonsteroidal, anti-inflammatory drug that was withdrawn in 2004. Other examples alleging failure to warn are Actos-associated bladder cancer and Baycol-related rhabdomyolysis. At the time of product approval, the FDA sets out the labeling that goes with each drug, and then makes periodic changes to reflect new indications, warnings and risks. The manufacturer has the prime responsibility for submitting all updated information, especially of augmented risks that come with field experience. In 2012, for example, the FDA mandated the revision of the labeling of Lipitor and other statins to warn of the increased risk of diabetes.
The drug manufacturer stands in the unique position as having the most detailed and up-to-date data and bears a serious responsibility to submit its full findings to the FDA, including its request for label change. Litigation over failure to warn of risks frequently turns on whether the drug manufacturer knew or should have known, had failed to inform the FDA, or whether the FDA itself had declined to make the changes, e.g., because of incomplete or premature data. Notwithstanding the FDA’s overarching federal status, a plaintiff may still attempt to use state tort law to hold a manufacturer liable should the federally approved labeling be silent on the matter.
Two U.S. Supreme Court cases sought to clarify the rules under which a drug manufacturer, when sued for failure to warn, may seek protection under its FDA-approved labeling. The first case involved Diana Levine, a Vermont musician and migraine sufferer, who lost her arm after the drug Phenergan, given by intravenous push, accidentally entered an artery and caused gangrene. Although the intravenous use of Phenergan is approved by the FDA and the risk of such use is clearly stated in the drug’s package insert, the lawsuit alleged that under state law, such a warning was inadequate and should have been strengthened to prohibit this mode of administration. A Vermont jury awarded damages of $6.7 million. On appeal, Wyeth, the defendant pharmaceutical company, maintained that its warning was appropriate, as it had been approved by the federal government through the FDA. It further argued that the drug’s package insert could not be unilaterally altered or modified without running afoul of federal regulations.
In a 6-3 decision,1 the U.S. Supreme Court ruled that the manufacturer was in fact at liberty to issue a more stringent warning, and FDA approval does not bar lawsuits. The Court opined that “Federal law does not pre-empt Levine’s claim that Phenergan’s label did not contain an adequate warning about the IV-push method of administration.” Wyeth had argued that it was impossible for the company to provide additional warnings, since it was the FDA that made the sole determination of the nature and scope of a drug’s label. However, the court held that Wyeth never attempted to change the label to warn of the risk and failed to provide “clear” evidence that the FDA would have prevented it from changing its label. Without defining what constituted “clear” evidence, it rejected Wyeth’s broad assertion that unilaterally changing the Phenergan label would have violated federal law, which was based on the fundamental misunderstanding that the FDA, rather than the manufacturer, bears primary responsibility for drug labeling.
In 2019, the landmark case of Merck Sharp & Dohme Corp v. Albrecht et al.2 reached the U.S. Supreme Court. This class-action suit involved more than 500 individuals who took Fosamax, an effective anti-resorptive drug for treating osteoporosis, and suffered atypical femoral fractures between 1999 and 2010. When the FDA first approved of the manufacture and sale of Fosamax in 1995, the Fosamax label did not warn of the then-speculative risk of atypical femoral fractures. But stronger evidence connecting Fosamax to atypical fractures developed after 1995, prompting the FDA to add a warning in 2011. Merck argued that plaintiffs’ state-law failure-to-warn claims should be dismissed as preempted by federal law. It conceded that the FDA regulations would have permitted Merck to try to change the label to add a warning before 2010 but believed the FDA would have rejected that attempt. In particular, it claimed that the FDA’s rejection of Merck’s 2008 attempt to warn of a risk of “stress fractures” showed that the FDA would also have rejected any attempt by Merck to warn of the risk of atypical femoral fractures. In short, Merck was relying on the legal doctrine of “impossibility preemption,” i.e., it was impossible to comply with both state law (adequate label warning of atypical fractures) and federal law (FDA control of warning labels). The plaintiffs’ position was that Merck’s proposed warning to the FDA had minimized the seriousness of the femoral fracture risk, characterizing them only as “stress fractures.”3
The Court’s earlier Levine decision had held that a state-law failure-to-warn claim is preempted where there is “clear” evidence the FDA would not have approved a label change. In the Albrecht decision, which also sided with the plaintiffs, the court indicated that “Clear evidence is evidence that shows the court that the drug manufacturer fully informed the FDA of the justifications for the warning required by state law and that the FDA, in turn, informed the drug manufacturer that the FDA would not approve a change to the drug’s label to include that warning.” The court also held that issues relating to presumption of impossibility are law-based, and thus it remains for the judge, not the jury, to make that determination.
Issuing timely warnings regarding medical products promotes patient safety, and the law appears to place the major onus on the manufacturer. Still, striking the proper balance is important. During oral arguments in Albrecht, Associate Justice Neil Gorsuch is said to have cautioned against “ ... incentives for companies to submit weakly supported label changes to the agency, knowing that when those label changes are rejected the companies will be free of further liability.” And as pointed out in the earlier cited Johnston article: “ ... a system that creates incentives for manufacturers to over-warn physicians and patients could harm patients by listing the important warnings of adverse effects among numerous less important warnings, which may discourage physicians and patients from choosing potentially useful drugs. On the other hand, a shift of responsibility for labeling to the FDA raises questions about whether the agency, which has resources that are dwarfed by the combined resources of industry, is necessarily capable to serve in this role ...”
Finally, this issue is more complex for devices because of the Medical Device Amendments Act of 1976 (MDA), which may preempt state-based lawsuits. In a claim brought after a Medtronic catheter ruptured in a patient’s coronary artery during heart surgery, the plaintiff alleged that the device was designed, labeled, and manufactured in a manner that violated New York common law. The case was appealed to the U.S. Supreme Court. The court held that the MDA preempted petitioner’s common-law claims challenging the safety or effectiveness of a medical device marketed in a form that received premarket approval from the FDA.4 The court ruled that MDA created a scheme of federal safety oversight for medical devices while sweeping back state oversight schemes.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Wyeth v. Levine, 555 U.S. 2 (2009).
2. Merck, Sharp & Dohme Corp. v. Albrecht et al., 587 U. S. ____ (2019).
3. Johnston MC et al., A new Supreme Court ruling on drug liability. JAMA 2019;322(7):607-8.
4. Riegel v. Medtronic, 128 S. Ct. 999 (2008).
Question: Which one of the following statements regarding medication warnings is incorrect?
A. The drug package “insert” or “label” contains, among other things, a drug’s pharmacology, indications, contraindications, risks and warnings.
B. The Physicians’ Desk Reference (PDR) is an annually updated drug compendium, which can be admitted into evidence as a learned treatise.
C. Drug labeling is a dual responsibility of the manufacturer and the Food and Drug Administration.
D. The FDA is solely responsible for a drug’s warnings and sets the absolute standard of care regarding side effects and complications.
E. State law can impose liability for negligent failure to warn even if the FDA has not included the warning in the drug’s label.
Answer: D. In medical products liability, injured plaintiffs frequently claim a failure to warn of known risks. An example is the cardiovascular deaths caused by Vioxx, a nonsteroidal, anti-inflammatory drug that was withdrawn in 2004. Other examples alleging failure to warn are Actos-associated bladder cancer and Baycol-related rhabdomyolysis. At the time of product approval, the FDA sets out the labeling that goes with each drug, and then makes periodic changes to reflect new indications, warnings and risks. The manufacturer has the prime responsibility for submitting all updated information, especially of augmented risks that come with field experience. In 2012, for example, the FDA mandated the revision of the labeling of Lipitor and other statins to warn of the increased risk of diabetes.
The drug manufacturer stands in the unique position as having the most detailed and up-to-date data and bears a serious responsibility to submit its full findings to the FDA, including its request for label change. Litigation over failure to warn of risks frequently turns on whether the drug manufacturer knew or should have known, had failed to inform the FDA, or whether the FDA itself had declined to make the changes, e.g., because of incomplete or premature data. Notwithstanding the FDA’s overarching federal status, a plaintiff may still attempt to use state tort law to hold a manufacturer liable should the federally approved labeling be silent on the matter.
Two U.S. Supreme Court cases sought to clarify the rules under which a drug manufacturer, when sued for failure to warn, may seek protection under its FDA-approved labeling. The first case involved Diana Levine, a Vermont musician and migraine sufferer, who lost her arm after the drug Phenergan, given by intravenous push, accidentally entered an artery and caused gangrene. Although the intravenous use of Phenergan is approved by the FDA and the risk of such use is clearly stated in the drug’s package insert, the lawsuit alleged that under state law, such a warning was inadequate and should have been strengthened to prohibit this mode of administration. A Vermont jury awarded damages of $6.7 million. On appeal, Wyeth, the defendant pharmaceutical company, maintained that its warning was appropriate, as it had been approved by the federal government through the FDA. It further argued that the drug’s package insert could not be unilaterally altered or modified without running afoul of federal regulations.
In a 6-3 decision,1 the U.S. Supreme Court ruled that the manufacturer was in fact at liberty to issue a more stringent warning, and FDA approval does not bar lawsuits. The Court opined that “Federal law does not pre-empt Levine’s claim that Phenergan’s label did not contain an adequate warning about the IV-push method of administration.” Wyeth had argued that it was impossible for the company to provide additional warnings, since it was the FDA that made the sole determination of the nature and scope of a drug’s label. However, the court held that Wyeth never attempted to change the label to warn of the risk and failed to provide “clear” evidence that the FDA would have prevented it from changing its label. Without defining what constituted “clear” evidence, it rejected Wyeth’s broad assertion that unilaterally changing the Phenergan label would have violated federal law, which was based on the fundamental misunderstanding that the FDA, rather than the manufacturer, bears primary responsibility for drug labeling.
In 2019, the landmark case of Merck Sharp & Dohme Corp v. Albrecht et al.2 reached the U.S. Supreme Court. This class-action suit involved more than 500 individuals who took Fosamax, an effective anti-resorptive drug for treating osteoporosis, and suffered atypical femoral fractures between 1999 and 2010. When the FDA first approved of the manufacture and sale of Fosamax in 1995, the Fosamax label did not warn of the then-speculative risk of atypical femoral fractures. But stronger evidence connecting Fosamax to atypical fractures developed after 1995, prompting the FDA to add a warning in 2011. Merck argued that plaintiffs’ state-law failure-to-warn claims should be dismissed as preempted by federal law. It conceded that the FDA regulations would have permitted Merck to try to change the label to add a warning before 2010 but believed the FDA would have rejected that attempt. In particular, it claimed that the FDA’s rejection of Merck’s 2008 attempt to warn of a risk of “stress fractures” showed that the FDA would also have rejected any attempt by Merck to warn of the risk of atypical femoral fractures. In short, Merck was relying on the legal doctrine of “impossibility preemption,” i.e., it was impossible to comply with both state law (adequate label warning of atypical fractures) and federal law (FDA control of warning labels). The plaintiffs’ position was that Merck’s proposed warning to the FDA had minimized the seriousness of the femoral fracture risk, characterizing them only as “stress fractures.”3
The Court’s earlier Levine decision had held that a state-law failure-to-warn claim is preempted where there is “clear” evidence the FDA would not have approved a label change. In the Albrecht decision, which also sided with the plaintiffs, the court indicated that “Clear evidence is evidence that shows the court that the drug manufacturer fully informed the FDA of the justifications for the warning required by state law and that the FDA, in turn, informed the drug manufacturer that the FDA would not approve a change to the drug’s label to include that warning.” The court also held that issues relating to presumption of impossibility are law-based, and thus it remains for the judge, not the jury, to make that determination.
Issuing timely warnings regarding medical products promotes patient safety, and the law appears to place the major onus on the manufacturer. Still, striking the proper balance is important. During oral arguments in Albrecht, Associate Justice Neil Gorsuch is said to have cautioned against “ ... incentives for companies to submit weakly supported label changes to the agency, knowing that when those label changes are rejected the companies will be free of further liability.” And as pointed out in the earlier cited Johnston article: “ ... a system that creates incentives for manufacturers to over-warn physicians and patients could harm patients by listing the important warnings of adverse effects among numerous less important warnings, which may discourage physicians and patients from choosing potentially useful drugs. On the other hand, a shift of responsibility for labeling to the FDA raises questions about whether the agency, which has resources that are dwarfed by the combined resources of industry, is necessarily capable to serve in this role ...”
Finally, this issue is more complex for devices because of the Medical Device Amendments Act of 1976 (MDA), which may preempt state-based lawsuits. In a claim brought after a Medtronic catheter ruptured in a patient’s coronary artery during heart surgery, the plaintiff alleged that the device was designed, labeled, and manufactured in a manner that violated New York common law. The case was appealed to the U.S. Supreme Court. The court held that the MDA preempted petitioner’s common-law claims challenging the safety or effectiveness of a medical device marketed in a form that received premarket approval from the FDA.4 The court ruled that MDA created a scheme of federal safety oversight for medical devices while sweeping back state oversight schemes.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Wyeth v. Levine, 555 U.S. 2 (2009).
2. Merck, Sharp & Dohme Corp. v. Albrecht et al., 587 U. S. ____ (2019).
3. Johnston MC et al., A new Supreme Court ruling on drug liability. JAMA 2019;322(7):607-8.
4. Riegel v. Medtronic, 128 S. Ct. 999 (2008).
Question: Which one of the following statements regarding medication warnings is incorrect?
A. The drug package “insert” or “label” contains, among other things, a drug’s pharmacology, indications, contraindications, risks and warnings.
B. The Physicians’ Desk Reference (PDR) is an annually updated drug compendium, which can be admitted into evidence as a learned treatise.
C. Drug labeling is a dual responsibility of the manufacturer and the Food and Drug Administration.
D. The FDA is solely responsible for a drug’s warnings and sets the absolute standard of care regarding side effects and complications.
E. State law can impose liability for negligent failure to warn even if the FDA has not included the warning in the drug’s label.
Answer: D. In medical products liability, injured plaintiffs frequently claim a failure to warn of known risks. An example is the cardiovascular deaths caused by Vioxx, a nonsteroidal, anti-inflammatory drug that was withdrawn in 2004. Other examples alleging failure to warn are Actos-associated bladder cancer and Baycol-related rhabdomyolysis. At the time of product approval, the FDA sets out the labeling that goes with each drug, and then makes periodic changes to reflect new indications, warnings and risks. The manufacturer has the prime responsibility for submitting all updated information, especially of augmented risks that come with field experience. In 2012, for example, the FDA mandated the revision of the labeling of Lipitor and other statins to warn of the increased risk of diabetes.
The drug manufacturer stands in the unique position as having the most detailed and up-to-date data and bears a serious responsibility to submit its full findings to the FDA, including its request for label change. Litigation over failure to warn of risks frequently turns on whether the drug manufacturer knew or should have known, had failed to inform the FDA, or whether the FDA itself had declined to make the changes, e.g., because of incomplete or premature data. Notwithstanding the FDA’s overarching federal status, a plaintiff may still attempt to use state tort law to hold a manufacturer liable should the federally approved labeling be silent on the matter.
Two U.S. Supreme Court cases sought to clarify the rules under which a drug manufacturer, when sued for failure to warn, may seek protection under its FDA-approved labeling. The first case involved Diana Levine, a Vermont musician and migraine sufferer, who lost her arm after the drug Phenergan, given by intravenous push, accidentally entered an artery and caused gangrene. Although the intravenous use of Phenergan is approved by the FDA and the risk of such use is clearly stated in the drug’s package insert, the lawsuit alleged that under state law, such a warning was inadequate and should have been strengthened to prohibit this mode of administration. A Vermont jury awarded damages of $6.7 million. On appeal, Wyeth, the defendant pharmaceutical company, maintained that its warning was appropriate, as it had been approved by the federal government through the FDA. It further argued that the drug’s package insert could not be unilaterally altered or modified without running afoul of federal regulations.
In a 6-3 decision,1 the U.S. Supreme Court ruled that the manufacturer was in fact at liberty to issue a more stringent warning, and FDA approval does not bar lawsuits. The Court opined that “Federal law does not pre-empt Levine’s claim that Phenergan’s label did not contain an adequate warning about the IV-push method of administration.” Wyeth had argued that it was impossible for the company to provide additional warnings, since it was the FDA that made the sole determination of the nature and scope of a drug’s label. However, the court held that Wyeth never attempted to change the label to warn of the risk and failed to provide “clear” evidence that the FDA would have prevented it from changing its label. Without defining what constituted “clear” evidence, it rejected Wyeth’s broad assertion that unilaterally changing the Phenergan label would have violated federal law, which was based on the fundamental misunderstanding that the FDA, rather than the manufacturer, bears primary responsibility for drug labeling.
In 2019, the landmark case of Merck Sharp & Dohme Corp v. Albrecht et al.2 reached the U.S. Supreme Court. This class-action suit involved more than 500 individuals who took Fosamax, an effective anti-resorptive drug for treating osteoporosis, and suffered atypical femoral fractures between 1999 and 2010. When the FDA first approved of the manufacture and sale of Fosamax in 1995, the Fosamax label did not warn of the then-speculative risk of atypical femoral fractures. But stronger evidence connecting Fosamax to atypical fractures developed after 1995, prompting the FDA to add a warning in 2011. Merck argued that plaintiffs’ state-law failure-to-warn claims should be dismissed as preempted by federal law. It conceded that the FDA regulations would have permitted Merck to try to change the label to add a warning before 2010 but believed the FDA would have rejected that attempt. In particular, it claimed that the FDA’s rejection of Merck’s 2008 attempt to warn of a risk of “stress fractures” showed that the FDA would also have rejected any attempt by Merck to warn of the risk of atypical femoral fractures. In short, Merck was relying on the legal doctrine of “impossibility preemption,” i.e., it was impossible to comply with both state law (adequate label warning of atypical fractures) and federal law (FDA control of warning labels). The plaintiffs’ position was that Merck’s proposed warning to the FDA had minimized the seriousness of the femoral fracture risk, characterizing them only as “stress fractures.”3
The Court’s earlier Levine decision had held that a state-law failure-to-warn claim is preempted where there is “clear” evidence the FDA would not have approved a label change. In the Albrecht decision, which also sided with the plaintiffs, the court indicated that “Clear evidence is evidence that shows the court that the drug manufacturer fully informed the FDA of the justifications for the warning required by state law and that the FDA, in turn, informed the drug manufacturer that the FDA would not approve a change to the drug’s label to include that warning.” The court also held that issues relating to presumption of impossibility are law-based, and thus it remains for the judge, not the jury, to make that determination.
Issuing timely warnings regarding medical products promotes patient safety, and the law appears to place the major onus on the manufacturer. Still, striking the proper balance is important. During oral arguments in Albrecht, Associate Justice Neil Gorsuch is said to have cautioned against “ ... incentives for companies to submit weakly supported label changes to the agency, knowing that when those label changes are rejected the companies will be free of further liability.” And as pointed out in the earlier cited Johnston article: “ ... a system that creates incentives for manufacturers to over-warn physicians and patients could harm patients by listing the important warnings of adverse effects among numerous less important warnings, which may discourage physicians and patients from choosing potentially useful drugs. On the other hand, a shift of responsibility for labeling to the FDA raises questions about whether the agency, which has resources that are dwarfed by the combined resources of industry, is necessarily capable to serve in this role ...”
Finally, this issue is more complex for devices because of the Medical Device Amendments Act of 1976 (MDA), which may preempt state-based lawsuits. In a claim brought after a Medtronic catheter ruptured in a patient’s coronary artery during heart surgery, the plaintiff alleged that the device was designed, labeled, and manufactured in a manner that violated New York common law. The case was appealed to the U.S. Supreme Court. The court held that the MDA preempted petitioner’s common-law claims challenging the safety or effectiveness of a medical device marketed in a form that received premarket approval from the FDA.4 The court ruled that MDA created a scheme of federal safety oversight for medical devices while sweeping back state oversight schemes.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Wyeth v. Levine, 555 U.S. 2 (2009).
2. Merck, Sharp & Dohme Corp. v. Albrecht et al., 587 U. S. ____ (2019).
3. Johnston MC et al., A new Supreme Court ruling on drug liability. JAMA 2019;322(7):607-8.
4. Riegel v. Medtronic, 128 S. Ct. 999 (2008).
Meeting the obstetrical needs of trans and gender nonconforming patients
Like their cisgender counterparts, transgender and gender nonconforming patients (trans patients) may reach a point in their lives where they want to build their own families. This may be achieved through adoption, alternative insemination with donor sperm, or assisted reproductive treatment with donor sperm or egg, cryopreserved sperm or egg, or surrogacy.1 Obstetricians can provide more equitable care to trans individuals by acknowledging these needs and providing gender-inclusive counseling and guidance.
The American Society for Reproductive Medicine recommends that medical providers counsel patients about the potential effects of medical transitioning on their fertility prior to the initiation of hormonal or surgical therapies.2 Patients should be educated about options for fertility preservation and reproduction since exogenous hormones and gonadectomy impact fertility.3 A referral to a fertility specialist should be placed for patients interested in oocyte or sperm cryopreservation, embryo cryopreservation, or ovarian tissue cryopreservation.2
If a trans patient presents to the obstetrician/gynecologist for preconception counseling after undergoing medical gender transition, they should be offered evidence-based guidance based on an organ inventory (surgical history with documentation of natal sex organs still in situ). A biologic pregnancy may be a fertility option for a patient who has a vagina, uterus, fallopian tubes, and ovaries and is not currently using testosterone. Gender-affirming testosterone therapy suppresses ovulation and causes amenorrhea in most patients, although this is often reversible once the exogenous hormone is discontinued.2 When the patient is ovulating on their own or undergoes ovulation induction, conception may be achieved via the same methods used with cisgender couples: Sperm is obtained from a partner or donor, followed by intercourse if the patient is comfortable with this, intrauterine insemination (IUI), or in vitro fertilization (IVF).
Conversely, a trans patient with a penis and testicles who has already undergone medical gender affirmation with estrogen should be counseled that prior exposure to estrogen may have caused irreversible testicular damage, making assisted reproductive treatment more challenging if sperm had not been cryopreserved prior to starting gender-affirming hormone therapy.2 If spermatogenesis is successful or sperm was previously cryopreserved, the next step in reproductive counseling for these patients centers on finding gestational carriers and egg donors if the patient does not already have a partner who is willing or able to carry the child. At this point in time, uterine transplantation has not been attempted in a trans patient and therefore is not considered a viable fertility option.
The trans patient who becomes pregnant will encounter physical changes that may trigger underlying gender dysphoria. One study found that transgender men who experience pregnancy exhibited varying degrees of gender dysphoria.4 Obstetrician/gynecologists should have an awareness about the possibility of heightened gender dysphoria and sensitively approach prenatal visits by avoiding triggering language or using inappropriate pronouns. Simply asking a trans patient about preferred pronouns and terminology for body parts can be the difference between a negative and positive pregnancy experience. For example, a transman may prefer a different term for vagina/vulva/cervix. This is especially important at the time of delivery, when exams may become more frequent for the patient. However, inclusive prenatal care starts from the first prenatal visit when the patient checks in and continues all the way through the doctor/patient experience. All office staff should be trained to use preferred names and pronouns and gender-neutral restrooms should be easily accessible. Likewise, waiting rooms should include visible support for the LGBTQ (lesbian, gay, bisexual, transgender and queer or questioning) patient population.
The anatomy ultrasound and “gender reveal” during the pregnancy and at the time of delivery can understandably also be a sensitive subject for a pregnant trans patient. Previous cultural practice has been to describe the gender of the fetus at the anatomy ultrasound, when in fact, gender can only be self-determined by an individual many years after birth. What the anatomy ultrasound does convey is the appearance of external genitalia to help predict the assigned sex. As obstetrician/gynecologists who practice evidence-based medicine, we are encouraged to challenge the cultural norm of announcing the gender of the baby at time of ultrasound and at time of birth. We should focus instead on conveying what objective information we do know. After the infant is born, we know the sex they are assigned based on the what external reproductive organs are seen.
In the postpartum period, trans patients who successfully carried a pregnancy may choose to feed their infant with their own human milk. For some trans patients, breastfeeding may be referred to as chestfeeding, since this terminology is more gender neutral. Having prior chest masculinization surgery does not exclude a transmasculine patient from lactating, although milk production may vary. Patients should be counseled that there is limited data on the safety of testosterone use while lactating.1 We found only one case report of induced lactation in a nonpuerperal transfeminine patient.5 In addition to addressing infant feeding concerns, obstetrician/gynecologists should counsel postpartum trans patients about contraceptive options and screen for perinatal mood disorders, especially those patients with a history of mood disorders before pregnancy.
Ultimately, trans patients seeking fertility options and obstetrical care have a right to obtain reliable information and access gender-inclusive treatment from their obstetrician/gynecologists. Each family makeup is unique and should be respected by all health care professionals taking care of the patient. As obstetrician/gynecologists, it is our duty to coordinate and advocate for the equitable care of our trans patients who want to grow their families.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Katie Riddle is an ob.gyn. in Connecticut who is passionate about LGBTQ health care. She recently completed her residency in Ann Arbor, Mich. Dr. Riddle identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joyner and Dr. Riddle said they had no financial disclosures. Email them at [email protected].
References
1. Viloria RP. “Reproductive Health and Obstetric Care in Transgender Patients.” Fenway Health.
2. Amato P. “Fertility options for transgender persons.” University of California, San Francisco Transgender Care and Treatment Guidelines. 2016 Jun 17.
3. Fertil Steril. 2015 Nov. doi: 10.1016/j.fertnstert.2015.08.021.
4. Obstet Gynecol. 2014. doi: 10.1097/AOG.0000000000000540.
5. Transgend Health. 2018 Jan 1. doi: 10.1089/trgh.2017.0044.
Like their cisgender counterparts, transgender and gender nonconforming patients (trans patients) may reach a point in their lives where they want to build their own families. This may be achieved through adoption, alternative insemination with donor sperm, or assisted reproductive treatment with donor sperm or egg, cryopreserved sperm or egg, or surrogacy.1 Obstetricians can provide more equitable care to trans individuals by acknowledging these needs and providing gender-inclusive counseling and guidance.
The American Society for Reproductive Medicine recommends that medical providers counsel patients about the potential effects of medical transitioning on their fertility prior to the initiation of hormonal or surgical therapies.2 Patients should be educated about options for fertility preservation and reproduction since exogenous hormones and gonadectomy impact fertility.3 A referral to a fertility specialist should be placed for patients interested in oocyte or sperm cryopreservation, embryo cryopreservation, or ovarian tissue cryopreservation.2
If a trans patient presents to the obstetrician/gynecologist for preconception counseling after undergoing medical gender transition, they should be offered evidence-based guidance based on an organ inventory (surgical history with documentation of natal sex organs still in situ). A biologic pregnancy may be a fertility option for a patient who has a vagina, uterus, fallopian tubes, and ovaries and is not currently using testosterone. Gender-affirming testosterone therapy suppresses ovulation and causes amenorrhea in most patients, although this is often reversible once the exogenous hormone is discontinued.2 When the patient is ovulating on their own or undergoes ovulation induction, conception may be achieved via the same methods used with cisgender couples: Sperm is obtained from a partner or donor, followed by intercourse if the patient is comfortable with this, intrauterine insemination (IUI), or in vitro fertilization (IVF).
Conversely, a trans patient with a penis and testicles who has already undergone medical gender affirmation with estrogen should be counseled that prior exposure to estrogen may have caused irreversible testicular damage, making assisted reproductive treatment more challenging if sperm had not been cryopreserved prior to starting gender-affirming hormone therapy.2 If spermatogenesis is successful or sperm was previously cryopreserved, the next step in reproductive counseling for these patients centers on finding gestational carriers and egg donors if the patient does not already have a partner who is willing or able to carry the child. At this point in time, uterine transplantation has not been attempted in a trans patient and therefore is not considered a viable fertility option.
The trans patient who becomes pregnant will encounter physical changes that may trigger underlying gender dysphoria. One study found that transgender men who experience pregnancy exhibited varying degrees of gender dysphoria.4 Obstetrician/gynecologists should have an awareness about the possibility of heightened gender dysphoria and sensitively approach prenatal visits by avoiding triggering language or using inappropriate pronouns. Simply asking a trans patient about preferred pronouns and terminology for body parts can be the difference between a negative and positive pregnancy experience. For example, a transman may prefer a different term for vagina/vulva/cervix. This is especially important at the time of delivery, when exams may become more frequent for the patient. However, inclusive prenatal care starts from the first prenatal visit when the patient checks in and continues all the way through the doctor/patient experience. All office staff should be trained to use preferred names and pronouns and gender-neutral restrooms should be easily accessible. Likewise, waiting rooms should include visible support for the LGBTQ (lesbian, gay, bisexual, transgender and queer or questioning) patient population.
The anatomy ultrasound and “gender reveal” during the pregnancy and at the time of delivery can understandably also be a sensitive subject for a pregnant trans patient. Previous cultural practice has been to describe the gender of the fetus at the anatomy ultrasound, when in fact, gender can only be self-determined by an individual many years after birth. What the anatomy ultrasound does convey is the appearance of external genitalia to help predict the assigned sex. As obstetrician/gynecologists who practice evidence-based medicine, we are encouraged to challenge the cultural norm of announcing the gender of the baby at time of ultrasound and at time of birth. We should focus instead on conveying what objective information we do know. After the infant is born, we know the sex they are assigned based on the what external reproductive organs are seen.
In the postpartum period, trans patients who successfully carried a pregnancy may choose to feed their infant with their own human milk. For some trans patients, breastfeeding may be referred to as chestfeeding, since this terminology is more gender neutral. Having prior chest masculinization surgery does not exclude a transmasculine patient from lactating, although milk production may vary. Patients should be counseled that there is limited data on the safety of testosterone use while lactating.1 We found only one case report of induced lactation in a nonpuerperal transfeminine patient.5 In addition to addressing infant feeding concerns, obstetrician/gynecologists should counsel postpartum trans patients about contraceptive options and screen for perinatal mood disorders, especially those patients with a history of mood disorders before pregnancy.
Ultimately, trans patients seeking fertility options and obstetrical care have a right to obtain reliable information and access gender-inclusive treatment from their obstetrician/gynecologists. Each family makeup is unique and should be respected by all health care professionals taking care of the patient. As obstetrician/gynecologists, it is our duty to coordinate and advocate for the equitable care of our trans patients who want to grow their families.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Katie Riddle is an ob.gyn. in Connecticut who is passionate about LGBTQ health care. She recently completed her residency in Ann Arbor, Mich. Dr. Riddle identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joyner and Dr. Riddle said they had no financial disclosures. Email them at [email protected].
References
1. Viloria RP. “Reproductive Health and Obstetric Care in Transgender Patients.” Fenway Health.
2. Amato P. “Fertility options for transgender persons.” University of California, San Francisco Transgender Care and Treatment Guidelines. 2016 Jun 17.
3. Fertil Steril. 2015 Nov. doi: 10.1016/j.fertnstert.2015.08.021.
4. Obstet Gynecol. 2014. doi: 10.1097/AOG.0000000000000540.
5. Transgend Health. 2018 Jan 1. doi: 10.1089/trgh.2017.0044.
Like their cisgender counterparts, transgender and gender nonconforming patients (trans patients) may reach a point in their lives where they want to build their own families. This may be achieved through adoption, alternative insemination with donor sperm, or assisted reproductive treatment with donor sperm or egg, cryopreserved sperm or egg, or surrogacy.1 Obstetricians can provide more equitable care to trans individuals by acknowledging these needs and providing gender-inclusive counseling and guidance.
The American Society for Reproductive Medicine recommends that medical providers counsel patients about the potential effects of medical transitioning on their fertility prior to the initiation of hormonal or surgical therapies.2 Patients should be educated about options for fertility preservation and reproduction since exogenous hormones and gonadectomy impact fertility.3 A referral to a fertility specialist should be placed for patients interested in oocyte or sperm cryopreservation, embryo cryopreservation, or ovarian tissue cryopreservation.2
If a trans patient presents to the obstetrician/gynecologist for preconception counseling after undergoing medical gender transition, they should be offered evidence-based guidance based on an organ inventory (surgical history with documentation of natal sex organs still in situ). A biologic pregnancy may be a fertility option for a patient who has a vagina, uterus, fallopian tubes, and ovaries and is not currently using testosterone. Gender-affirming testosterone therapy suppresses ovulation and causes amenorrhea in most patients, although this is often reversible once the exogenous hormone is discontinued.2 When the patient is ovulating on their own or undergoes ovulation induction, conception may be achieved via the same methods used with cisgender couples: Sperm is obtained from a partner or donor, followed by intercourse if the patient is comfortable with this, intrauterine insemination (IUI), or in vitro fertilization (IVF).
Conversely, a trans patient with a penis and testicles who has already undergone medical gender affirmation with estrogen should be counseled that prior exposure to estrogen may have caused irreversible testicular damage, making assisted reproductive treatment more challenging if sperm had not been cryopreserved prior to starting gender-affirming hormone therapy.2 If spermatogenesis is successful or sperm was previously cryopreserved, the next step in reproductive counseling for these patients centers on finding gestational carriers and egg donors if the patient does not already have a partner who is willing or able to carry the child. At this point in time, uterine transplantation has not been attempted in a trans patient and therefore is not considered a viable fertility option.
The trans patient who becomes pregnant will encounter physical changes that may trigger underlying gender dysphoria. One study found that transgender men who experience pregnancy exhibited varying degrees of gender dysphoria.4 Obstetrician/gynecologists should have an awareness about the possibility of heightened gender dysphoria and sensitively approach prenatal visits by avoiding triggering language or using inappropriate pronouns. Simply asking a trans patient about preferred pronouns and terminology for body parts can be the difference between a negative and positive pregnancy experience. For example, a transman may prefer a different term for vagina/vulva/cervix. This is especially important at the time of delivery, when exams may become more frequent for the patient. However, inclusive prenatal care starts from the first prenatal visit when the patient checks in and continues all the way through the doctor/patient experience. All office staff should be trained to use preferred names and pronouns and gender-neutral restrooms should be easily accessible. Likewise, waiting rooms should include visible support for the LGBTQ (lesbian, gay, bisexual, transgender and queer or questioning) patient population.
The anatomy ultrasound and “gender reveal” during the pregnancy and at the time of delivery can understandably also be a sensitive subject for a pregnant trans patient. Previous cultural practice has been to describe the gender of the fetus at the anatomy ultrasound, when in fact, gender can only be self-determined by an individual many years after birth. What the anatomy ultrasound does convey is the appearance of external genitalia to help predict the assigned sex. As obstetrician/gynecologists who practice evidence-based medicine, we are encouraged to challenge the cultural norm of announcing the gender of the baby at time of ultrasound and at time of birth. We should focus instead on conveying what objective information we do know. After the infant is born, we know the sex they are assigned based on the what external reproductive organs are seen.
In the postpartum period, trans patients who successfully carried a pregnancy may choose to feed their infant with their own human milk. For some trans patients, breastfeeding may be referred to as chestfeeding, since this terminology is more gender neutral. Having prior chest masculinization surgery does not exclude a transmasculine patient from lactating, although milk production may vary. Patients should be counseled that there is limited data on the safety of testosterone use while lactating.1 We found only one case report of induced lactation in a nonpuerperal transfeminine patient.5 In addition to addressing infant feeding concerns, obstetrician/gynecologists should counsel postpartum trans patients about contraceptive options and screen for perinatal mood disorders, especially those patients with a history of mood disorders before pregnancy.
Ultimately, trans patients seeking fertility options and obstetrical care have a right to obtain reliable information and access gender-inclusive treatment from their obstetrician/gynecologists. Each family makeup is unique and should be respected by all health care professionals taking care of the patient. As obstetrician/gynecologists, it is our duty to coordinate and advocate for the equitable care of our trans patients who want to grow their families.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Katie Riddle is an ob.gyn. in Connecticut who is passionate about LGBTQ health care. She recently completed her residency in Ann Arbor, Mich. Dr. Riddle identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joyner and Dr. Riddle said they had no financial disclosures. Email them at [email protected].
References
1. Viloria RP. “Reproductive Health and Obstetric Care in Transgender Patients.” Fenway Health.
2. Amato P. “Fertility options for transgender persons.” University of California, San Francisco Transgender Care and Treatment Guidelines. 2016 Jun 17.
3. Fertil Steril. 2015 Nov. doi: 10.1016/j.fertnstert.2015.08.021.
4. Obstet Gynecol. 2014. doi: 10.1097/AOG.0000000000000540.
5. Transgend Health. 2018 Jan 1. doi: 10.1089/trgh.2017.0044.