User login
Optimizing Topical Therapy for Onychomycosis: The Importance of Patient Education
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
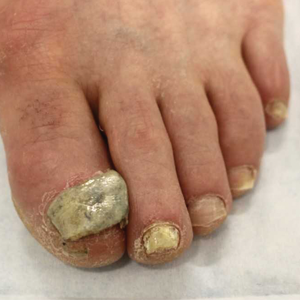
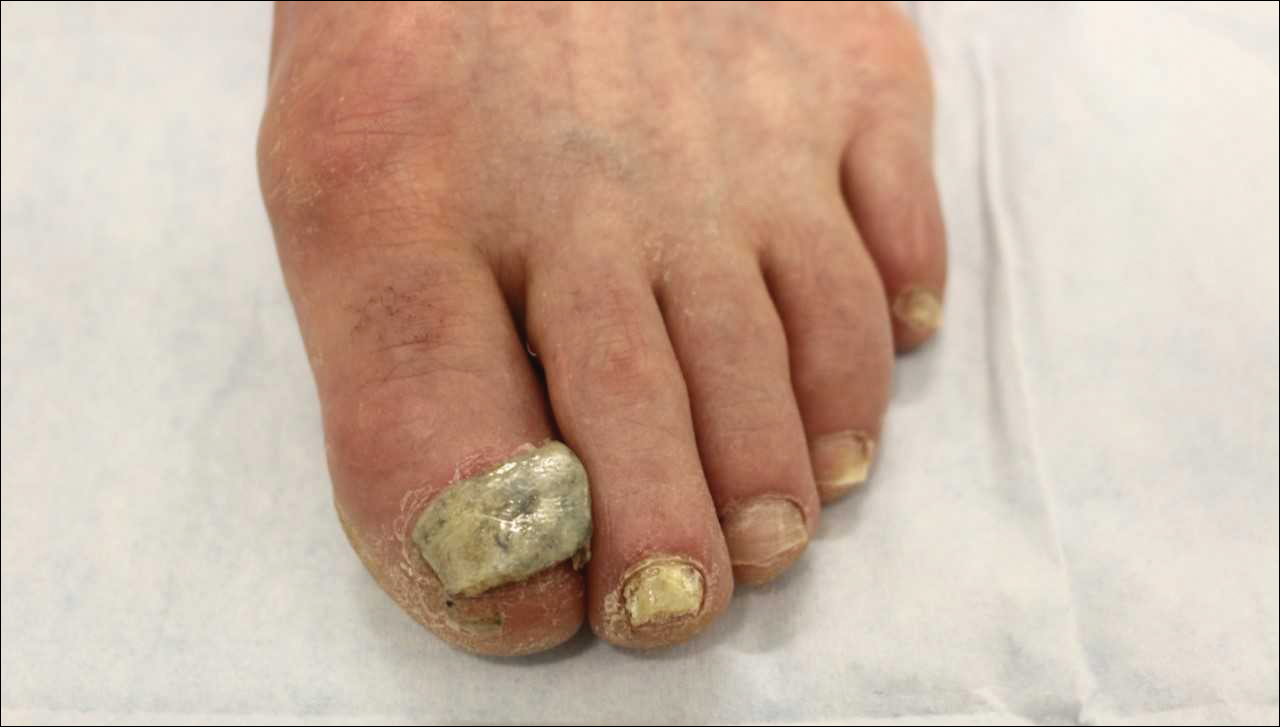
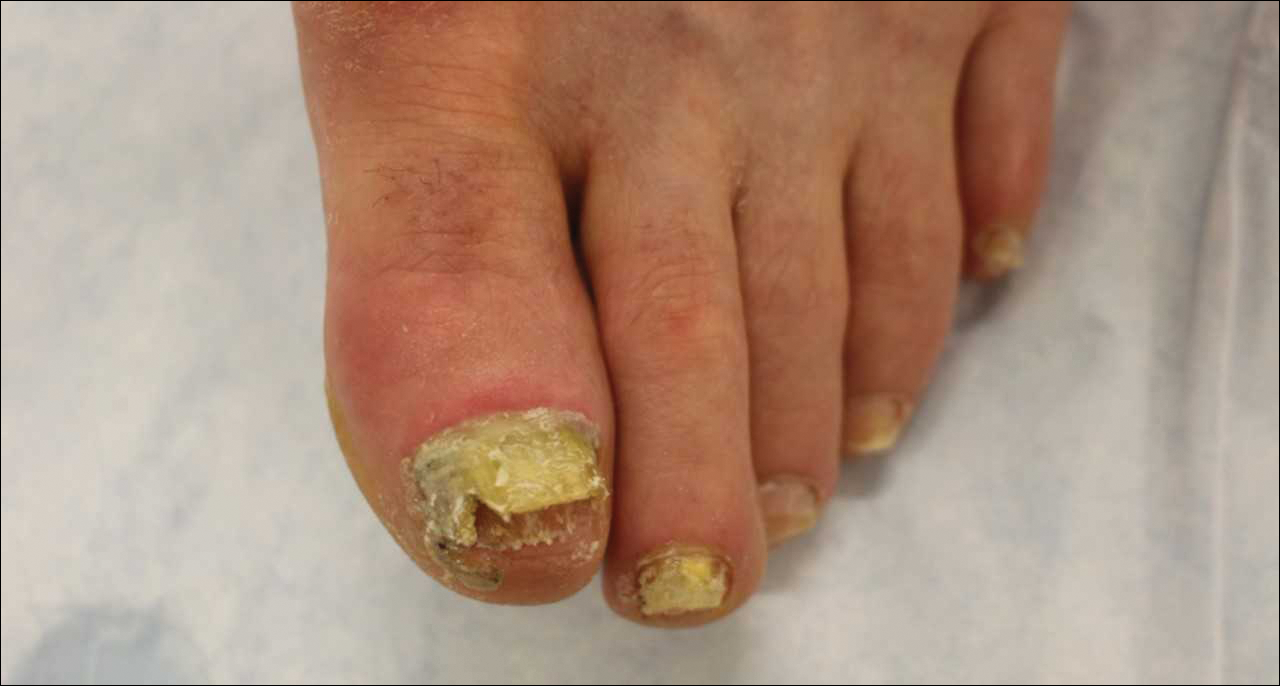
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15
Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15


Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15


Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
Strategies to Reduce Youth Indoor Tanning Injuries
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
‘Phenomenal’ REDUCE-IT establishes triglyceride theory
CHICAGO – REDUCE-IT is a phenomenal trial and a game changer because it has shown for the first time that triglyceride reduction with an appropriate therapy – in this case icosapent ethyl – when used in appropriate doses can make a significant difference.
That’s according to Prakash C. Deedwania, MD, chief of the cardiology division at the Veterans Affairs Medical Center/University of California San Francisco Program in Fresno, who joined MDedge reporter Richard Mark Kirkner for a video interview at the American Heart Association scientific sessions.
In the large, placebo-controlled REDUCE-IT trial in patients with or at high risk for cardiovascular disease received who received 2 g of icosapent ethyl (Vascepa) twice daily or placebo saw a 25% lower risk of cardiovascular death or an ischemic event, compared with placebo.
CHICAGO – REDUCE-IT is a phenomenal trial and a game changer because it has shown for the first time that triglyceride reduction with an appropriate therapy – in this case icosapent ethyl – when used in appropriate doses can make a significant difference.
That’s according to Prakash C. Deedwania, MD, chief of the cardiology division at the Veterans Affairs Medical Center/University of California San Francisco Program in Fresno, who joined MDedge reporter Richard Mark Kirkner for a video interview at the American Heart Association scientific sessions.
In the large, placebo-controlled REDUCE-IT trial in patients with or at high risk for cardiovascular disease received who received 2 g of icosapent ethyl (Vascepa) twice daily or placebo saw a 25% lower risk of cardiovascular death or an ischemic event, compared with placebo.
CHICAGO – REDUCE-IT is a phenomenal trial and a game changer because it has shown for the first time that triglyceride reduction with an appropriate therapy – in this case icosapent ethyl – when used in appropriate doses can make a significant difference.
That’s according to Prakash C. Deedwania, MD, chief of the cardiology division at the Veterans Affairs Medical Center/University of California San Francisco Program in Fresno, who joined MDedge reporter Richard Mark Kirkner for a video interview at the American Heart Association scientific sessions.
In the large, placebo-controlled REDUCE-IT trial in patients with or at high risk for cardiovascular disease received who received 2 g of icosapent ethyl (Vascepa) twice daily or placebo saw a 25% lower risk of cardiovascular death or an ischemic event, compared with placebo.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Lemons into lemonade: The 2019 Medicare physician fee schedule
Of course, there is a new final rule every year, so it really isn’t very final. I know this is confusing to many of you, I was dazed for several days by this year’s proposed final rule.

Each year, the Centers for Medicare & Medicaid Services receives input from innumerable sources and formulates its payment for physicians. These responses are often in response to requests by CMS itself, which wants to make sure reimbursements are accurate. Generally, input comes from the American Medical Association’s RVS Update Committee (RUC), which values new and existing CPT codes, as well as Congress, the Health & Human Services Office of Inspector General, lobbyists, specialty society organizations, public advocacy groups, and anyone who can wrangle an appointment at or write a letter to CMS headquarters in Baltimore. This conflicted brew is hashed over, and published in late July as a proposed rule. Public comments are then solicited (all letters and emails are considered, dermatologists sent 1,500 responses to this one!) and a final rule is published in the fall. I have constructed a flowchart of this process.
This year’s proposed rule was particularly disturbing because of major changes in reimbursement proposed by CMS. As you may recall, officials proposed to collapse all the evaluation and management (E/M) codes into two levels and pay bonuses to certain specialists (but not dermatologists). This might have been agreeable, except Medicare reimbursements are a zero-sum game. If someone is paid more, someone else will be paid less. Of course, you could always let the increase come out of the general pool, but that would decrease the conversion factor, and some health care professionals (usually primary care) might not see an overall increase. So, the proposed rule was going to “pay” for this increase by way of eliminating the 25 modifier, the CPT modifier that allows you to be paid for the evaluation and management (E/M) service on the same day as a procedure. This has been averted, at least for two years.
The final rule also makes a real effort to eliminate some meaningless documentation. Effective Jan. 1, 2019, for established patients, practitioners can focus their notes on patient changes. With new and established patients, they need not personally reenter the chief complaint and history already recorded by staff or the patient, other than simply indicate that they reviewed and verified the information in the medical record. In addition, teaching physicians do not have to duplicate notations by residents. CMS also included practice expense for additional skin biopsies.
CMS is also going to pay for services using communication technology. These include:
- Brief communication technology-based service, e.g. virtual check-in (HCPCS code G2012). This is provided by a physician or other qualified health care professional who can report E/M services for an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion. It is important to note that CMS is allowing for this code to include audio-only, real-time telephone interactions, in addition to synchronous, two-way audio interactions that are enhanced with video or other kinds of data transmission.
- Remote evaluation of recorded video and/or images submitted by an established patient (HCPCS code G2010). This is remote evaluation of recorded video and/or images submitted by an established patient (e.g., store and forward), including interpretation with follow-up with the patient within 24 business hours, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment). The code can be reported effective Jan. 1, 2019, for established patients only.
You can use G2012 to decide if an office visit is needed. Similarly, the service of remote evaluation of recorded video and/or images submitted by an established patient would allow health care professionals to be paid separately for reviewing patient-transmitted photo or video information whether or not a visit is needed. The encounter must be synchronous (real-time), two-way audio interactions enhanced with video or other kinds of data transmission.
It appears that these would only be practical for established patients, and don’t forget, your Internet and text responses to patients’ messages are not secure, unless they are on a secure portal, although their messages to you are HIPAA compliant. However, the telephone, some Internet portals, and your electronic medical record portals are secure. It is intriguing to me that I might get paid for all those bad pictures patients send me, at least if it is not in a global period.
It also appears that Rural Health Clinics and Federally Qualified Health Centers will be able to bill for new and established patient visits via communication technology.
This is all great news to physicians. Kudos to dermatologists Jack Resneck Jr., MD, American Medical Association trustee; and George Hruza, MD, the American Academy of Dermatology president-elect; and Sabra Sullivan, MD, PhD, chair of the AAD’s Council on Government Affairs and Health Policy Government, who organized this lemonade-making effort. And once again, the AAD’s Washington office has shown its great value. This also aptly demonstrates why you write letters to CMS.
In 2021, levels 2-4 will be collapsed into one code (levels 5 will remain, but remember, very few dermatologists use level 5) and you will have to document only at level 2 code levels. Special add-on codes will be added for exceptionally difficult cases for primary care and all specialist physicians, including dermatology. What is not clear is how this new reimbursement schemata will be funded. CMS is still suspicious that there is overlapping work when procedures are performed on the same day as an E/M (evaluation and management code). We may end up fighting this battle all over again.
Currently CMS is conducting a survey, sent to 1,500 dermatologists, on follow-up visits. CMS has stated that they will evaluate the public comments received and consider whether to propose action at a future date. CMS plans to send a letter describing the requirements, once again, to health care professionals in nine affected states, who are required to report the global period encounter. If you are one of these practitioners, please do fill this out and contact Faith McNicholas at AAD ([email protected]) if you have questions. The decision to eliminate global periods (disastrous) will be based on this survey.
This is why you need to stay engaged, write letters, join the AMA, donate to SkinPAC, and attend the legislative fly in, the AAD’s legislative conference held every year in Washington. We are a small specialty. If we do not speak up and stay engaged, we will become the lemons for the next pitcher of lemonade.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Of course, there is a new final rule every year, so it really isn’t very final. I know this is confusing to many of you, I was dazed for several days by this year’s proposed final rule.

Each year, the Centers for Medicare & Medicaid Services receives input from innumerable sources and formulates its payment for physicians. These responses are often in response to requests by CMS itself, which wants to make sure reimbursements are accurate. Generally, input comes from the American Medical Association’s RVS Update Committee (RUC), which values new and existing CPT codes, as well as Congress, the Health & Human Services Office of Inspector General, lobbyists, specialty society organizations, public advocacy groups, and anyone who can wrangle an appointment at or write a letter to CMS headquarters in Baltimore. This conflicted brew is hashed over, and published in late July as a proposed rule. Public comments are then solicited (all letters and emails are considered, dermatologists sent 1,500 responses to this one!) and a final rule is published in the fall. I have constructed a flowchart of this process.
This year’s proposed rule was particularly disturbing because of major changes in reimbursement proposed by CMS. As you may recall, officials proposed to collapse all the evaluation and management (E/M) codes into two levels and pay bonuses to certain specialists (but not dermatologists). This might have been agreeable, except Medicare reimbursements are a zero-sum game. If someone is paid more, someone else will be paid less. Of course, you could always let the increase come out of the general pool, but that would decrease the conversion factor, and some health care professionals (usually primary care) might not see an overall increase. So, the proposed rule was going to “pay” for this increase by way of eliminating the 25 modifier, the CPT modifier that allows you to be paid for the evaluation and management (E/M) service on the same day as a procedure. This has been averted, at least for two years.
The final rule also makes a real effort to eliminate some meaningless documentation. Effective Jan. 1, 2019, for established patients, practitioners can focus their notes on patient changes. With new and established patients, they need not personally reenter the chief complaint and history already recorded by staff or the patient, other than simply indicate that they reviewed and verified the information in the medical record. In addition, teaching physicians do not have to duplicate notations by residents. CMS also included practice expense for additional skin biopsies.
CMS is also going to pay for services using communication technology. These include:
- Brief communication technology-based service, e.g. virtual check-in (HCPCS code G2012). This is provided by a physician or other qualified health care professional who can report E/M services for an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion. It is important to note that CMS is allowing for this code to include audio-only, real-time telephone interactions, in addition to synchronous, two-way audio interactions that are enhanced with video or other kinds of data transmission.
- Remote evaluation of recorded video and/or images submitted by an established patient (HCPCS code G2010). This is remote evaluation of recorded video and/or images submitted by an established patient (e.g., store and forward), including interpretation with follow-up with the patient within 24 business hours, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment). The code can be reported effective Jan. 1, 2019, for established patients only.
You can use G2012 to decide if an office visit is needed. Similarly, the service of remote evaluation of recorded video and/or images submitted by an established patient would allow health care professionals to be paid separately for reviewing patient-transmitted photo or video information whether or not a visit is needed. The encounter must be synchronous (real-time), two-way audio interactions enhanced with video or other kinds of data transmission.
It appears that these would only be practical for established patients, and don’t forget, your Internet and text responses to patients’ messages are not secure, unless they are on a secure portal, although their messages to you are HIPAA compliant. However, the telephone, some Internet portals, and your electronic medical record portals are secure. It is intriguing to me that I might get paid for all those bad pictures patients send me, at least if it is not in a global period.
It also appears that Rural Health Clinics and Federally Qualified Health Centers will be able to bill for new and established patient visits via communication technology.
This is all great news to physicians. Kudos to dermatologists Jack Resneck Jr., MD, American Medical Association trustee; and George Hruza, MD, the American Academy of Dermatology president-elect; and Sabra Sullivan, MD, PhD, chair of the AAD’s Council on Government Affairs and Health Policy Government, who organized this lemonade-making effort. And once again, the AAD’s Washington office has shown its great value. This also aptly demonstrates why you write letters to CMS.
In 2021, levels 2-4 will be collapsed into one code (levels 5 will remain, but remember, very few dermatologists use level 5) and you will have to document only at level 2 code levels. Special add-on codes will be added for exceptionally difficult cases for primary care and all specialist physicians, including dermatology. What is not clear is how this new reimbursement schemata will be funded. CMS is still suspicious that there is overlapping work when procedures are performed on the same day as an E/M (evaluation and management code). We may end up fighting this battle all over again.
Currently CMS is conducting a survey, sent to 1,500 dermatologists, on follow-up visits. CMS has stated that they will evaluate the public comments received and consider whether to propose action at a future date. CMS plans to send a letter describing the requirements, once again, to health care professionals in nine affected states, who are required to report the global period encounter. If you are one of these practitioners, please do fill this out and contact Faith McNicholas at AAD ([email protected]) if you have questions. The decision to eliminate global periods (disastrous) will be based on this survey.
This is why you need to stay engaged, write letters, join the AMA, donate to SkinPAC, and attend the legislative fly in, the AAD’s legislative conference held every year in Washington. We are a small specialty. If we do not speak up and stay engaged, we will become the lemons for the next pitcher of lemonade.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Of course, there is a new final rule every year, so it really isn’t very final. I know this is confusing to many of you, I was dazed for several days by this year’s proposed final rule.

Each year, the Centers for Medicare & Medicaid Services receives input from innumerable sources and formulates its payment for physicians. These responses are often in response to requests by CMS itself, which wants to make sure reimbursements are accurate. Generally, input comes from the American Medical Association’s RVS Update Committee (RUC), which values new and existing CPT codes, as well as Congress, the Health & Human Services Office of Inspector General, lobbyists, specialty society organizations, public advocacy groups, and anyone who can wrangle an appointment at or write a letter to CMS headquarters in Baltimore. This conflicted brew is hashed over, and published in late July as a proposed rule. Public comments are then solicited (all letters and emails are considered, dermatologists sent 1,500 responses to this one!) and a final rule is published in the fall. I have constructed a flowchart of this process.
This year’s proposed rule was particularly disturbing because of major changes in reimbursement proposed by CMS. As you may recall, officials proposed to collapse all the evaluation and management (E/M) codes into two levels and pay bonuses to certain specialists (but not dermatologists). This might have been agreeable, except Medicare reimbursements are a zero-sum game. If someone is paid more, someone else will be paid less. Of course, you could always let the increase come out of the general pool, but that would decrease the conversion factor, and some health care professionals (usually primary care) might not see an overall increase. So, the proposed rule was going to “pay” for this increase by way of eliminating the 25 modifier, the CPT modifier that allows you to be paid for the evaluation and management (E/M) service on the same day as a procedure. This has been averted, at least for two years.
The final rule also makes a real effort to eliminate some meaningless documentation. Effective Jan. 1, 2019, for established patients, practitioners can focus their notes on patient changes. With new and established patients, they need not personally reenter the chief complaint and history already recorded by staff or the patient, other than simply indicate that they reviewed and verified the information in the medical record. In addition, teaching physicians do not have to duplicate notations by residents. CMS also included practice expense for additional skin biopsies.
CMS is also going to pay for services using communication technology. These include:
- Brief communication technology-based service, e.g. virtual check-in (HCPCS code G2012). This is provided by a physician or other qualified health care professional who can report E/M services for an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion. It is important to note that CMS is allowing for this code to include audio-only, real-time telephone interactions, in addition to synchronous, two-way audio interactions that are enhanced with video or other kinds of data transmission.
- Remote evaluation of recorded video and/or images submitted by an established patient (HCPCS code G2010). This is remote evaluation of recorded video and/or images submitted by an established patient (e.g., store and forward), including interpretation with follow-up with the patient within 24 business hours, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment). The code can be reported effective Jan. 1, 2019, for established patients only.
You can use G2012 to decide if an office visit is needed. Similarly, the service of remote evaluation of recorded video and/or images submitted by an established patient would allow health care professionals to be paid separately for reviewing patient-transmitted photo or video information whether or not a visit is needed. The encounter must be synchronous (real-time), two-way audio interactions enhanced with video or other kinds of data transmission.
It appears that these would only be practical for established patients, and don’t forget, your Internet and text responses to patients’ messages are not secure, unless they are on a secure portal, although their messages to you are HIPAA compliant. However, the telephone, some Internet portals, and your electronic medical record portals are secure. It is intriguing to me that I might get paid for all those bad pictures patients send me, at least if it is not in a global period.
It also appears that Rural Health Clinics and Federally Qualified Health Centers will be able to bill for new and established patient visits via communication technology.
This is all great news to physicians. Kudos to dermatologists Jack Resneck Jr., MD, American Medical Association trustee; and George Hruza, MD, the American Academy of Dermatology president-elect; and Sabra Sullivan, MD, PhD, chair of the AAD’s Council on Government Affairs and Health Policy Government, who organized this lemonade-making effort. And once again, the AAD’s Washington office has shown its great value. This also aptly demonstrates why you write letters to CMS.
In 2021, levels 2-4 will be collapsed into one code (levels 5 will remain, but remember, very few dermatologists use level 5) and you will have to document only at level 2 code levels. Special add-on codes will be added for exceptionally difficult cases for primary care and all specialist physicians, including dermatology. What is not clear is how this new reimbursement schemata will be funded. CMS is still suspicious that there is overlapping work when procedures are performed on the same day as an E/M (evaluation and management code). We may end up fighting this battle all over again.
Currently CMS is conducting a survey, sent to 1,500 dermatologists, on follow-up visits. CMS has stated that they will evaluate the public comments received and consider whether to propose action at a future date. CMS plans to send a letter describing the requirements, once again, to health care professionals in nine affected states, who are required to report the global period encounter. If you are one of these practitioners, please do fill this out and contact Faith McNicholas at AAD ([email protected]) if you have questions. The decision to eliminate global periods (disastrous) will be based on this survey.
This is why you need to stay engaged, write letters, join the AMA, donate to SkinPAC, and attend the legislative fly in, the AAD’s legislative conference held every year in Washington. We are a small specialty. If we do not speak up and stay engaged, we will become the lemons for the next pitcher of lemonade.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Free advice seems to be history
The 2 short years in the cocoon of residency ended, and I was flying solo on the picturesque but sparsely populated coast of Maine. My colleagues and I were fulfilling our 2-year military obligations at the Brunswick Naval Air Station, hundreds of miles from the support systems our tertiary care centers had provided. As the only pediatrician on the dispensary staff, I felt particularly vulnerable.
At least I was in the same time zone in which I had trained. I still knew most of the beeper numbers or could remember the extensions and the first names of the department secretaries. Usually within minutes, the familiar and calming voice of one of my favorite subspecialists or pediatric mentors would set me on the path to the correct diagnosis and management plan. Of course, I could have asked one of the pediatricians in town for advice, and eventually, I did. But, in the beginning, I was embarrassed to reveal my soft underbelly to the townies.
Within a few months, I was moonlighting for a local pediatrician and, after 2 years, I joined him as a partner. However, it took another several years to wean myself off my dependency on the subspecialists at the big-city medical center where I had trained. To some extent, this was because in the 1970s and 1980s, Maine had few pediatric subspecialists.
Eventually, I developed my own list of favorite local consultants. While the quality of advice was the prime determinant in my choice of a consultant, availability was a close second. How easy was it to get the specialist on the phone? If the clinical situation was not terribly time sensitive and I knew the consultant always was painfully overbooked, I would ask my question in a short typed note, and even include a S.A.S.E. (self-addressed stamped envelope). This – of course – was before email had been invented.
How likely was the consultant going to provide the answer I wanted to hear? If I thought the patient needed P-E (pressure equalizer) tubes, I could choose any ENT specialist. If, on the other hand, I felt that watching and waiting was the better option, I would choose the physician I knew was least likely to advise surgery.
I am unaware that any of the physicians I consulted ever charged the patient for my phone calls or notes. In some cases, their reward came in the opportunity to perform surgery. I rarely received small gifts during the holidays from consultants who were trying to build their practices. And I never gave tokens of appreciation to consultants or their staff to secure their timely response to my pleas for help. However, I always included a personal thank-you note with the self-designed holiday cards I sent to my favorites. And when a consultant had bailed me out of a particularly challenging situation, I often sent a handwritten note in follow-up.
But, for me, the care and grooming of a good stable of consultants began with acknowledging that their time was at least as valuable as mine. The more carefully I crafted my question and the more complete the history I could provide, the more efficiently the consultant could provide me with the answer I needed.
Regrettably, but predictably, those days of free advice are fading away. The new revision of the CPT codes includes at least twelve codes for “interprofessional consultation.” Time is – and has always been – money. As everyone’s time is increasingly being gobbled up by electronic advancements that promised to save us time, no one seems to have time to make or answer that call that begins, “Can I ask you a quick question about a patient?”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The 2 short years in the cocoon of residency ended, and I was flying solo on the picturesque but sparsely populated coast of Maine. My colleagues and I were fulfilling our 2-year military obligations at the Brunswick Naval Air Station, hundreds of miles from the support systems our tertiary care centers had provided. As the only pediatrician on the dispensary staff, I felt particularly vulnerable.
At least I was in the same time zone in which I had trained. I still knew most of the beeper numbers or could remember the extensions and the first names of the department secretaries. Usually within minutes, the familiar and calming voice of one of my favorite subspecialists or pediatric mentors would set me on the path to the correct diagnosis and management plan. Of course, I could have asked one of the pediatricians in town for advice, and eventually, I did. But, in the beginning, I was embarrassed to reveal my soft underbelly to the townies.
Within a few months, I was moonlighting for a local pediatrician and, after 2 years, I joined him as a partner. However, it took another several years to wean myself off my dependency on the subspecialists at the big-city medical center where I had trained. To some extent, this was because in the 1970s and 1980s, Maine had few pediatric subspecialists.
Eventually, I developed my own list of favorite local consultants. While the quality of advice was the prime determinant in my choice of a consultant, availability was a close second. How easy was it to get the specialist on the phone? If the clinical situation was not terribly time sensitive and I knew the consultant always was painfully overbooked, I would ask my question in a short typed note, and even include a S.A.S.E. (self-addressed stamped envelope). This – of course – was before email had been invented.
How likely was the consultant going to provide the answer I wanted to hear? If I thought the patient needed P-E (pressure equalizer) tubes, I could choose any ENT specialist. If, on the other hand, I felt that watching and waiting was the better option, I would choose the physician I knew was least likely to advise surgery.
I am unaware that any of the physicians I consulted ever charged the patient for my phone calls or notes. In some cases, their reward came in the opportunity to perform surgery. I rarely received small gifts during the holidays from consultants who were trying to build their practices. And I never gave tokens of appreciation to consultants or their staff to secure their timely response to my pleas for help. However, I always included a personal thank-you note with the self-designed holiday cards I sent to my favorites. And when a consultant had bailed me out of a particularly challenging situation, I often sent a handwritten note in follow-up.
But, for me, the care and grooming of a good stable of consultants began with acknowledging that their time was at least as valuable as mine. The more carefully I crafted my question and the more complete the history I could provide, the more efficiently the consultant could provide me with the answer I needed.
Regrettably, but predictably, those days of free advice are fading away. The new revision of the CPT codes includes at least twelve codes for “interprofessional consultation.” Time is – and has always been – money. As everyone’s time is increasingly being gobbled up by electronic advancements that promised to save us time, no one seems to have time to make or answer that call that begins, “Can I ask you a quick question about a patient?”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The 2 short years in the cocoon of residency ended, and I was flying solo on the picturesque but sparsely populated coast of Maine. My colleagues and I were fulfilling our 2-year military obligations at the Brunswick Naval Air Station, hundreds of miles from the support systems our tertiary care centers had provided. As the only pediatrician on the dispensary staff, I felt particularly vulnerable.
At least I was in the same time zone in which I had trained. I still knew most of the beeper numbers or could remember the extensions and the first names of the department secretaries. Usually within minutes, the familiar and calming voice of one of my favorite subspecialists or pediatric mentors would set me on the path to the correct diagnosis and management plan. Of course, I could have asked one of the pediatricians in town for advice, and eventually, I did. But, in the beginning, I was embarrassed to reveal my soft underbelly to the townies.
Within a few months, I was moonlighting for a local pediatrician and, after 2 years, I joined him as a partner. However, it took another several years to wean myself off my dependency on the subspecialists at the big-city medical center where I had trained. To some extent, this was because in the 1970s and 1980s, Maine had few pediatric subspecialists.
Eventually, I developed my own list of favorite local consultants. While the quality of advice was the prime determinant in my choice of a consultant, availability was a close second. How easy was it to get the specialist on the phone? If the clinical situation was not terribly time sensitive and I knew the consultant always was painfully overbooked, I would ask my question in a short typed note, and even include a S.A.S.E. (self-addressed stamped envelope). This – of course – was before email had been invented.
How likely was the consultant going to provide the answer I wanted to hear? If I thought the patient needed P-E (pressure equalizer) tubes, I could choose any ENT specialist. If, on the other hand, I felt that watching and waiting was the better option, I would choose the physician I knew was least likely to advise surgery.
I am unaware that any of the physicians I consulted ever charged the patient for my phone calls or notes. In some cases, their reward came in the opportunity to perform surgery. I rarely received small gifts during the holidays from consultants who were trying to build their practices. And I never gave tokens of appreciation to consultants or their staff to secure their timely response to my pleas for help. However, I always included a personal thank-you note with the self-designed holiday cards I sent to my favorites. And when a consultant had bailed me out of a particularly challenging situation, I often sent a handwritten note in follow-up.
But, for me, the care and grooming of a good stable of consultants began with acknowledging that their time was at least as valuable as mine. The more carefully I crafted my question and the more complete the history I could provide, the more efficiently the consultant could provide me with the answer I needed.
Regrettably, but predictably, those days of free advice are fading away. The new revision of the CPT codes includes at least twelve codes for “interprofessional consultation.” Time is – and has always been – money. As everyone’s time is increasingly being gobbled up by electronic advancements that promised to save us time, no one seems to have time to make or answer that call that begins, “Can I ask you a quick question about a patient?”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How well are your phones being answered?
We have several new, young employees in my front office, and it had been quite awhile since I had followed my own advice of “eavesdropping” on their telephone conversations with patients. You would think that Millennials, with all the time they spend on phones, would have little to learn in that department – until you remember that Instagram and Snapchat do not require interpersonal skills.
So I. If you want to adapt it for your own use, be my guest:
• The first impression a new patient has of our office is usually made by our receptionists. Even now, in the era of texting and e-mail, the telephone remains our primary point of contact with new and long-time patients. The way we answer it determines, to a significant extent, how the community thinks of us, as people and as health care providers.
• Everyone in the office needs to know how to answer the phone professionally. If you notice that a phone is ringing and the receptionists are unable to answer it, please pick up the phone; an incoming call must never go unanswered.
• Answer all incoming calls before the third ring.• Answer warmly, enthusiastically, and professionally. Since the caller cannot see you, your voice is the only impression of our office a first-time caller will get.
• Identify yourself and our office immediately. “Good morning, Doctor Eastern’s office. This is __________, how may I help you?” (No one should ever have to ask what office they have reached, or to whom they are speaking.)
• Speak like a professional. Don’t use slang or buzzwords. Instead of “totally” or “for sure,” for example, say “certainly” or “of course.” If you tend to use fillers (“uh huh,” “um,” “like,” “you know,” etc.), train yourself not to use them in the office.
• Adopt a positive vocabulary – one that focuses on helping people. For example, rather than saying, “I don’t know,” say, “Let me find out for you,” or “I’ll make sure someone gets back to you on that.”
• Offer to take a message if the caller has a question or issue you cannot address. Assure the patient that the appropriate staffer will call back later that day. That way, office work flow is not interrupted, and the patient still receives a prompt (and correct) answer.
• All messages left overnight with the answering service must be returned as early as possible the very next business day. This is a top priority each morning. Few things annoy callers trying to reach their doctors more than unreturned calls. If the office will be closed for a holiday, or a response will be delayed for any other reason, make sure the service knows, and passes it on to patients.
• If the phone rings while you are dealing with a patient in person, that patient is your first priority. Put the caller on hold, but always ask permission before doing so, and wait for an answer. Never leave a caller on hold for more than a minute or two unless absolutely unavoidable.
• Never answer with, “Doctor’s office, please hold.” To a patient, that is even worse than not answering at all. No matter how often your hold message tells callers how important they are, they know they are being ignored. Such encounters never end well: Those who wait will be grumpy and rude when you get back to them; those who hang up will be even more grumpy and rude when they call back. Worst of all are those who don’t call back and seek care elsewhere – often leaving a nasty comment on social media besides.
• Maintaining patient confidentiality is a top priority. It makes patients feel secure about being treated in our office, and it is also the law. Be cautious about all information that is given over the phone. Don’t disclose any personal information unless you are absolutely certain you are talking to the correct patient. If the caller is not the patient, never discuss personal information without the patient’s permission.
Keep in mind that patients and others in the office may be able to overhear your phone conversations. Keep your voice down; never use the phone’s hands-free “speaker” function.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
We have several new, young employees in my front office, and it had been quite awhile since I had followed my own advice of “eavesdropping” on their telephone conversations with patients. You would think that Millennials, with all the time they spend on phones, would have little to learn in that department – until you remember that Instagram and Snapchat do not require interpersonal skills.
So I. If you want to adapt it for your own use, be my guest:
• The first impression a new patient has of our office is usually made by our receptionists. Even now, in the era of texting and e-mail, the telephone remains our primary point of contact with new and long-time patients. The way we answer it determines, to a significant extent, how the community thinks of us, as people and as health care providers.
• Everyone in the office needs to know how to answer the phone professionally. If you notice that a phone is ringing and the receptionists are unable to answer it, please pick up the phone; an incoming call must never go unanswered.
• Answer all incoming calls before the third ring.• Answer warmly, enthusiastically, and professionally. Since the caller cannot see you, your voice is the only impression of our office a first-time caller will get.
• Identify yourself and our office immediately. “Good morning, Doctor Eastern’s office. This is __________, how may I help you?” (No one should ever have to ask what office they have reached, or to whom they are speaking.)
• Speak like a professional. Don’t use slang or buzzwords. Instead of “totally” or “for sure,” for example, say “certainly” or “of course.” If you tend to use fillers (“uh huh,” “um,” “like,” “you know,” etc.), train yourself not to use them in the office.
• Adopt a positive vocabulary – one that focuses on helping people. For example, rather than saying, “I don’t know,” say, “Let me find out for you,” or “I’ll make sure someone gets back to you on that.”
• Offer to take a message if the caller has a question or issue you cannot address. Assure the patient that the appropriate staffer will call back later that day. That way, office work flow is not interrupted, and the patient still receives a prompt (and correct) answer.
• All messages left overnight with the answering service must be returned as early as possible the very next business day. This is a top priority each morning. Few things annoy callers trying to reach their doctors more than unreturned calls. If the office will be closed for a holiday, or a response will be delayed for any other reason, make sure the service knows, and passes it on to patients.
• If the phone rings while you are dealing with a patient in person, that patient is your first priority. Put the caller on hold, but always ask permission before doing so, and wait for an answer. Never leave a caller on hold for more than a minute or two unless absolutely unavoidable.
• Never answer with, “Doctor’s office, please hold.” To a patient, that is even worse than not answering at all. No matter how often your hold message tells callers how important they are, they know they are being ignored. Such encounters never end well: Those who wait will be grumpy and rude when you get back to them; those who hang up will be even more grumpy and rude when they call back. Worst of all are those who don’t call back and seek care elsewhere – often leaving a nasty comment on social media besides.
• Maintaining patient confidentiality is a top priority. It makes patients feel secure about being treated in our office, and it is also the law. Be cautious about all information that is given over the phone. Don’t disclose any personal information unless you are absolutely certain you are talking to the correct patient. If the caller is not the patient, never discuss personal information without the patient’s permission.
Keep in mind that patients and others in the office may be able to overhear your phone conversations. Keep your voice down; never use the phone’s hands-free “speaker” function.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
We have several new, young employees in my front office, and it had been quite awhile since I had followed my own advice of “eavesdropping” on their telephone conversations with patients. You would think that Millennials, with all the time they spend on phones, would have little to learn in that department – until you remember that Instagram and Snapchat do not require interpersonal skills.
So I. If you want to adapt it for your own use, be my guest:
• The first impression a new patient has of our office is usually made by our receptionists. Even now, in the era of texting and e-mail, the telephone remains our primary point of contact with new and long-time patients. The way we answer it determines, to a significant extent, how the community thinks of us, as people and as health care providers.
• Everyone in the office needs to know how to answer the phone professionally. If you notice that a phone is ringing and the receptionists are unable to answer it, please pick up the phone; an incoming call must never go unanswered.
• Answer all incoming calls before the third ring.• Answer warmly, enthusiastically, and professionally. Since the caller cannot see you, your voice is the only impression of our office a first-time caller will get.
• Identify yourself and our office immediately. “Good morning, Doctor Eastern’s office. This is __________, how may I help you?” (No one should ever have to ask what office they have reached, or to whom they are speaking.)
• Speak like a professional. Don’t use slang or buzzwords. Instead of “totally” or “for sure,” for example, say “certainly” or “of course.” If you tend to use fillers (“uh huh,” “um,” “like,” “you know,” etc.), train yourself not to use them in the office.
• Adopt a positive vocabulary – one that focuses on helping people. For example, rather than saying, “I don’t know,” say, “Let me find out for you,” or “I’ll make sure someone gets back to you on that.”
• Offer to take a message if the caller has a question or issue you cannot address. Assure the patient that the appropriate staffer will call back later that day. That way, office work flow is not interrupted, and the patient still receives a prompt (and correct) answer.
• All messages left overnight with the answering service must be returned as early as possible the very next business day. This is a top priority each morning. Few things annoy callers trying to reach their doctors more than unreturned calls. If the office will be closed for a holiday, or a response will be delayed for any other reason, make sure the service knows, and passes it on to patients.
• If the phone rings while you are dealing with a patient in person, that patient is your first priority. Put the caller on hold, but always ask permission before doing so, and wait for an answer. Never leave a caller on hold for more than a minute or two unless absolutely unavoidable.
• Never answer with, “Doctor’s office, please hold.” To a patient, that is even worse than not answering at all. No matter how often your hold message tells callers how important they are, they know they are being ignored. Such encounters never end well: Those who wait will be grumpy and rude when you get back to them; those who hang up will be even more grumpy and rude when they call back. Worst of all are those who don’t call back and seek care elsewhere – often leaving a nasty comment on social media besides.
• Maintaining patient confidentiality is a top priority. It makes patients feel secure about being treated in our office, and it is also the law. Be cautious about all information that is given over the phone. Don’t disclose any personal information unless you are absolutely certain you are talking to the correct patient. If the caller is not the patient, never discuss personal information without the patient’s permission.
Keep in mind that patients and others in the office may be able to overhear your phone conversations. Keep your voice down; never use the phone’s hands-free “speaker” function.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
MIS for cervical cancer: Is it not for anyone or not for everyone?
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Veterans are not ‘ticking time bombs’
Like all of us, I was very troubled by the recent mass shooting in Thousand Oaks, Calif. This shooting was on top of the massacre at Pittsburgh’s Tree of Life synagogue, the shootings in a yoga studio ... the sickening list goes on and on.
As both a veteran and a psychiatrist with expertise in posttraumatic stress disorder, I was especially dismayed by the assumption that the Thousand Oaks shooter, who had served in the Marine Corps, had PTSD, and that the PTSD had led to the shooting.
The overall effect of these assumptions is to reinforce the stigma against veterans as “ticking time bombs.”
No question, there are plenty of other stereotypes to go around, especially those of Muslims as terrorists. In reality, as reports from the GAO and independent news sources show, most “terrorist” attacks in the United States have been carried out by right-wing extremists, mainly white, and born in this country.
Back to veterans. It is true that there have been several mass shootings by service members and veterans, including the massacre at Fort Hood, Tex., in 2009 by an Army major, the 2017 shooting up of a church in Texas by someone who had served in the Air Force, and this most recent one by a former Marine.
But there have been many other shootings and acts of political violence by numerous others, including those for whom “life is going down the toilet.” When you look at these situations, the driving factors are usually anger, irritability, and a sense of being wronged. Often, delusions and paranoia emerge.
It is true that there are many barriers to treatment for both veterans and nonveterans, including stigma, lack of insurance, and the dearth of mental health providers.
Those factors have nothing to do with being a veteran, who are normally very proud of both their country and their military service.
Let us celebrate those who have given so much to this country, America’s sons and daughters.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
Like all of us, I was very troubled by the recent mass shooting in Thousand Oaks, Calif. This shooting was on top of the massacre at Pittsburgh’s Tree of Life synagogue, the shootings in a yoga studio ... the sickening list goes on and on.
As both a veteran and a psychiatrist with expertise in posttraumatic stress disorder, I was especially dismayed by the assumption that the Thousand Oaks shooter, who had served in the Marine Corps, had PTSD, and that the PTSD had led to the shooting.
The overall effect of these assumptions is to reinforce the stigma against veterans as “ticking time bombs.”
No question, there are plenty of other stereotypes to go around, especially those of Muslims as terrorists. In reality, as reports from the GAO and independent news sources show, most “terrorist” attacks in the United States have been carried out by right-wing extremists, mainly white, and born in this country.
Back to veterans. It is true that there have been several mass shootings by service members and veterans, including the massacre at Fort Hood, Tex., in 2009 by an Army major, the 2017 shooting up of a church in Texas by someone who had served in the Air Force, and this most recent one by a former Marine.
But there have been many other shootings and acts of political violence by numerous others, including those for whom “life is going down the toilet.” When you look at these situations, the driving factors are usually anger, irritability, and a sense of being wronged. Often, delusions and paranoia emerge.
It is true that there are many barriers to treatment for both veterans and nonveterans, including stigma, lack of insurance, and the dearth of mental health providers.
Those factors have nothing to do with being a veteran, who are normally very proud of both their country and their military service.
Let us celebrate those who have given so much to this country, America’s sons and daughters.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
Like all of us, I was very troubled by the recent mass shooting in Thousand Oaks, Calif. This shooting was on top of the massacre at Pittsburgh’s Tree of Life synagogue, the shootings in a yoga studio ... the sickening list goes on and on.
As both a veteran and a psychiatrist with expertise in posttraumatic stress disorder, I was especially dismayed by the assumption that the Thousand Oaks shooter, who had served in the Marine Corps, had PTSD, and that the PTSD had led to the shooting.
The overall effect of these assumptions is to reinforce the stigma against veterans as “ticking time bombs.”
No question, there are plenty of other stereotypes to go around, especially those of Muslims as terrorists. In reality, as reports from the GAO and independent news sources show, most “terrorist” attacks in the United States have been carried out by right-wing extremists, mainly white, and born in this country.
Back to veterans. It is true that there have been several mass shootings by service members and veterans, including the massacre at Fort Hood, Tex., in 2009 by an Army major, the 2017 shooting up of a church in Texas by someone who had served in the Air Force, and this most recent one by a former Marine.
But there have been many other shootings and acts of political violence by numerous others, including those for whom “life is going down the toilet.” When you look at these situations, the driving factors are usually anger, irritability, and a sense of being wronged. Often, delusions and paranoia emerge.
It is true that there are many barriers to treatment for both veterans and nonveterans, including stigma, lack of insurance, and the dearth of mental health providers.
Those factors have nothing to do with being a veteran, who are normally very proud of both their country and their military service.
Let us celebrate those who have given so much to this country, America’s sons and daughters.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
Why is loxapine overlooked, underprescribed for psychosis?
I have always tried to practice common sense psychiatry, however, sometimes it seems I am alone in this pursuit. My best example is the minimal prescribing of loxapine (Adasuve) for treating the problem of psychosis, most notably schizophrenia.
Mind you, neither I nor anyone in family own stock in any pharmaceutical companies. I don’t lecture for them, so I have no conflicts in writing about this observation – which I hope will improve patient care, thereby saving lives and making a difference.
Everyone should be familiar with the evolution of atypical antipsychotics and how these medications are touted as “second-generation” classes of medication advertised as superior to the older, first-generation antipsychotics. However, as we get more experience with the second-generation atypical antipsychotics, we are learning that they have problematic side effects of their own. For example, they are associated with metabolic syndrome, so they cause weight gain, hyperglycemia, increased risk of stroke, sudden cardiac death, blood clots, and diabetes. Maybe these problems are so endemic in the low-income, African American population I treat that I am overly sensitive to trying to prevent these medical disorders while treating a patient’s mental illness. However, my public health leanings have long caused me to think that low-income African Americans are the canary in America’s health status coal mine, as it seems that what hits this group first eventually will hit the majority population. Accordingly, it seems to me that it is well advised to pay attention to this group’s well-being, physical health, and mental health challenges.
Everyone also should be aware that clozapine (Clozaril) had been dubbed the first atypical antipsychotic. But, in some regard, that designation might be given to thioridazine – although some maintain that the ratio of serotonergic to dopamine effects is not strong enough to earn that title. Unfortunately, both thioridazine and clozapine have serious side effects. Thioridazine is associated with severe cardiac arrhythmias, and clozapine has been associated with the aforementioned side effects of atypical antipsychotics but also can cause life-threatening agranulocytosis, necessitating regular white blood cell counts to monitor for this possibility.
, which belongs class of medication known as dibenzodiazepines – a class that is extraordinarily similar to dibenzoxazepine. The late William Glazer, MD, a distinguished psychopharmacologist long affiliated with Yale University, New Haven, Conn., even suggested that loxapine might behave as an atypical antipsychotic (J Clin Psychiatry. 1999;60 Suppl 10:42-6). Extensive clinical experience with loxapine suggests the same but with some key differences from the standard atypical antipsychotics regarding its side-effect profile.
First, loxapine, despite being chemically related to clozapine, does not cause agranulocytosis, so the need for white blood cell monitoring is not necessary. Second, I have not seen the problematic metabolic syndrome caused by standard atypical antipsychotic medication. It amazes me when I see patients on aripiprazole, clozapine, olanzapine, quetiapine, risperidone, or ziprasidone who also have diabetes and are on metformin – especially when the development of the patients’ diabetes can be traced back to when they were put on an atypical antipsychotic. I often find myself taking patients off their atypical antipsychotic and putting them on loxapine, resulting in gradual weight loss while maintaining the patients’ stable mental status and absence of psychotic symptoms.
It seems to me that if clozapine and loxapine are so similar (they both bind to serotonin and dopamine receptors), loxapine should be the first drug of choice for the treatment of psychotic symptoms. It acts like an atypical but without the problems of weight gain, hyperglycemia, increased risk of stroke, sudden cardiac death, blood clots, and diabetes that the atypicals may cause. Most of the hundreds of patients with psychotic symptoms I have treated over the past 40 years are on the low dose of loxapine 25 mg at bedtime (although the prescribing information on loxapine says it has to be given at least twice a day, as the half life of the medication is only 4 hours). In some rare instances, I prescribe a total of 50 mg at bedtime.
So, not prescribing loxapine does not make sense to me – other than the medication is generic and so it is not being marketed aggressively by people who make money from prescribing medication and are practicing money, not medicine. The other possibility is that most psychiatrists might not know the connection between clozapine and loxapine, so I thought I should use my influence (what little I have) to inform.
Dr. Bell is staff psychiatrist at Jackson Park Hospital’s surgical-medical/psychiatric inpatient unit in Chicago; and chairman of the department of psychiatry at Windsor University, St. Kitts. He also is clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (the birthplace of child psychiatry), all in Chicago.
I have always tried to practice common sense psychiatry, however, sometimes it seems I am alone in this pursuit. My best example is the minimal prescribing of loxapine (Adasuve) for treating the problem of psychosis, most notably schizophrenia.
Mind you, neither I nor anyone in family own stock in any pharmaceutical companies. I don’t lecture for them, so I have no conflicts in writing about this observation – which I hope will improve patient care, thereby saving lives and making a difference.
Everyone should be familiar with the evolution of atypical antipsychotics and how these medications are touted as “second-generation” classes of medication advertised as superior to the older, first-generation antipsychotics. However, as we get more experience with the second-generation atypical antipsychotics, we are learning that they have problematic side effects of their own. For example, they are associated with metabolic syndrome, so they cause weight gain, hyperglycemia, increased risk of stroke, sudden cardiac death, blood clots, and diabetes. Maybe these problems are so endemic in the low-income, African American population I treat that I am overly sensitive to trying to prevent these medical disorders while treating a patient’s mental illness. However, my public health leanings have long caused me to think that low-income African Americans are the canary in America’s health status coal mine, as it seems that what hits this group first eventually will hit the majority population. Accordingly, it seems to me that it is well advised to pay attention to this group’s well-being, physical health, and mental health challenges.
Everyone also should be aware that clozapine (Clozaril) had been dubbed the first atypical antipsychotic. But, in some regard, that designation might be given to thioridazine – although some maintain that the ratio of serotonergic to dopamine effects is not strong enough to earn that title. Unfortunately, both thioridazine and clozapine have serious side effects. Thioridazine is associated with severe cardiac arrhythmias, and clozapine has been associated with the aforementioned side effects of atypical antipsychotics but also can cause life-threatening agranulocytosis, necessitating regular white blood cell counts to monitor for this possibility.
, which belongs class of medication known as dibenzodiazepines – a class that is extraordinarily similar to dibenzoxazepine. The late William Glazer, MD, a distinguished psychopharmacologist long affiliated with Yale University, New Haven, Conn., even suggested that loxapine might behave as an atypical antipsychotic (J Clin Psychiatry. 1999;60 Suppl 10:42-6). Extensive clinical experience with loxapine suggests the same but with some key differences from the standard atypical antipsychotics regarding its side-effect profile.
First, loxapine, despite being chemically related to clozapine, does not cause agranulocytosis, so the need for white blood cell monitoring is not necessary. Second, I have not seen the problematic metabolic syndrome caused by standard atypical antipsychotic medication. It amazes me when I see patients on aripiprazole, clozapine, olanzapine, quetiapine, risperidone, or ziprasidone who also have diabetes and are on metformin – especially when the development of the patients’ diabetes can be traced back to when they were put on an atypical antipsychotic. I often find myself taking patients off their atypical antipsychotic and putting them on loxapine, resulting in gradual weight loss while maintaining the patients’ stable mental status and absence of psychotic symptoms.
It seems to me that if clozapine and loxapine are so similar (they both bind to serotonin and dopamine receptors), loxapine should be the first drug of choice for the treatment of psychotic symptoms. It acts like an atypical but without the problems of weight gain, hyperglycemia, increased risk of stroke, sudden cardiac death, blood clots, and diabetes that the atypicals may cause. Most of the hundreds of patients with psychotic symptoms I have treated over the past 40 years are on the low dose of loxapine 25 mg at bedtime (although the prescribing information on loxapine says it has to be given at least twice a day, as the half life of the medication is only 4 hours). In some rare instances, I prescribe a total of 50 mg at bedtime.
So, not prescribing loxapine does not make sense to me – other than the medication is generic and so it is not being marketed aggressively by people who make money from prescribing medication and are practicing money, not medicine. The other possibility is that most psychiatrists might not know the connection between clozapine and loxapine, so I thought I should use my influence (what little I have) to inform.
Dr. Bell is staff psychiatrist at Jackson Park Hospital’s surgical-medical/psychiatric inpatient unit in Chicago; and chairman of the department of psychiatry at Windsor University, St. Kitts. He also is clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (the birthplace of child psychiatry), all in Chicago.
I have always tried to practice common sense psychiatry, however, sometimes it seems I am alone in this pursuit. My best example is the minimal prescribing of loxapine (Adasuve) for treating the problem of psychosis, most notably schizophrenia.
Mind you, neither I nor anyone in family own stock in any pharmaceutical companies. I don’t lecture for them, so I have no conflicts in writing about this observation – which I hope will improve patient care, thereby saving lives and making a difference.
Everyone should be familiar with the evolution of atypical antipsychotics and how these medications are touted as “second-generation” classes of medication advertised as superior to the older, first-generation antipsychotics. However, as we get more experience with the second-generation atypical antipsychotics, we are learning that they have problematic side effects of their own. For example, they are associated with metabolic syndrome, so they cause weight gain, hyperglycemia, increased risk of stroke, sudden cardiac death, blood clots, and diabetes. Maybe these problems are so endemic in the low-income, African American population I treat that I am overly sensitive to trying to prevent these medical disorders while treating a patient’s mental illness. However, my public health leanings have long caused me to think that low-income African Americans are the canary in America’s health status coal mine, as it seems that what hits this group first eventually will hit the majority population. Accordingly, it seems to me that it is well advised to pay attention to this group’s well-being, physical health, and mental health challenges.
Everyone also should be aware that clozapine (Clozaril) had been dubbed the first atypical antipsychotic. But, in some regard, that designation might be given to thioridazine – although some maintain that the ratio of serotonergic to dopamine effects is not strong enough to earn that title. Unfortunately, both thioridazine and clozapine have serious side effects. Thioridazine is associated with severe cardiac arrhythmias, and clozapine has been associated with the aforementioned side effects of atypical antipsychotics but also can cause life-threatening agranulocytosis, necessitating regular white blood cell counts to monitor for this possibility.
, which belongs class of medication known as dibenzodiazepines – a class that is extraordinarily similar to dibenzoxazepine. The late William Glazer, MD, a distinguished psychopharmacologist long affiliated with Yale University, New Haven, Conn., even suggested that loxapine might behave as an atypical antipsychotic (J Clin Psychiatry. 1999;60 Suppl 10:42-6). Extensive clinical experience with loxapine suggests the same but with some key differences from the standard atypical antipsychotics regarding its side-effect profile.
First, loxapine, despite being chemically related to clozapine, does not cause agranulocytosis, so the need for white blood cell monitoring is not necessary. Second, I have not seen the problematic metabolic syndrome caused by standard atypical antipsychotic medication. It amazes me when I see patients on aripiprazole, clozapine, olanzapine, quetiapine, risperidone, or ziprasidone who also have diabetes and are on metformin – especially when the development of the patients’ diabetes can be traced back to when they were put on an atypical antipsychotic. I often find myself taking patients off their atypical antipsychotic and putting them on loxapine, resulting in gradual weight loss while maintaining the patients’ stable mental status and absence of psychotic symptoms.
It seems to me that if clozapine and loxapine are so similar (they both bind to serotonin and dopamine receptors), loxapine should be the first drug of choice for the treatment of psychotic symptoms. It acts like an atypical but without the problems of weight gain, hyperglycemia, increased risk of stroke, sudden cardiac death, blood clots, and diabetes that the atypicals may cause. Most of the hundreds of patients with psychotic symptoms I have treated over the past 40 years are on the low dose of loxapine 25 mg at bedtime (although the prescribing information on loxapine says it has to be given at least twice a day, as the half life of the medication is only 4 hours). In some rare instances, I prescribe a total of 50 mg at bedtime.
So, not prescribing loxapine does not make sense to me – other than the medication is generic and so it is not being marketed aggressively by people who make money from prescribing medication and are practicing money, not medicine. The other possibility is that most psychiatrists might not know the connection between clozapine and loxapine, so I thought I should use my influence (what little I have) to inform.
Dr. Bell is staff psychiatrist at Jackson Park Hospital’s surgical-medical/psychiatric inpatient unit in Chicago; and chairman of the department of psychiatry at Windsor University, St. Kitts. He also is clinical professor emeritus, department of psychiatry, University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (the birthplace of child psychiatry), all in Chicago.