User login
Intervention may improve genetic testing for HBOC
Researchers from general obstetrics and gynecology (ob.gyn.) practices in New York and Connecticut have shown that (HBOC).
Genetic screening and testing can reduce the morbidity and mortality from breast, ovarian, and endometrial cancers through prevention and early detection. Mark S. DeFrancesco, MD, of Westwood Women’s Health, Waterbury, Conn., and his colleagues reported that, in spite of the American College of Obstetricians and Gynecologists’ recommendation for ob.gyns. to regularly screen, counsel, and refer accordingly for HBOC (Obstet Gynecol. 2015;125:153843), the “incorporation of hereditary cancer risk assessment and testing remains underutilized in the [ob.gyn.] setting.” The authors have addressed this issue in their own practice with promising results and important caveats (Obstet Gynecol 2018;132:1121-9).
The intervention included a process evaluation, improvements to patient work flow, and training of providers by genetic counselors and engineering personnel from the testing laboratory (Myriad Genetics), which provided support for the study. Patients in the study completed a family history questionnaire and, those meeting National Comprehensive Cancer Center Network criteria for genetic testing, were given pretest counseling and offered testing on the same day or referral for testing within 2 weeks.
Of the 3,811 women who completed the questionnaire, 24% (906) met NCCN criteria, 90% of whom were offered testing. However, only 52% (165) of patients who agreed to testing underwent genetic evaluation. This included 70% of patients who were offered same-day testing and 35% of patients who were offered a referral appointment for testing.
Conversations about HBOC and genetic testing can be complicated and may not be a patient’s initial priority. The authors should be commended for identifying the vast majority of high-risk patients. However, only half of patients meeting criteria completed testing and 10% who should have been offered testing were not – numbers still well below target.
Incorporation of family history questionnaires should become commonplace in the generalist’s office and optimizing EHRs may be an opportunity for rapid risk interpretation. As the success of same-day genetic testing was striking, opportunities for partnerships with insurance companies and private laboratories are likely needed to make this a more feasible option. Lastly, assessing women’s knowledge and attitudes around genetic testing could help to more specifically address barriers to testing in future interventions.
Improving genetic screening and testing completion rates requires a coordinated effort. Using tools and applications to optimize convenience (same-day testing, telemedicine) and simplification (electronic screening platforms), we can strive to appropriately detect all women at high risk for hereditary breast and ovarian cancers.
Michelle Lightfoot is a gynecologic oncology fellow at the Ohio State University in Columbus. She has no relevant financial disclosures. Email her at [email protected].
Researchers from general obstetrics and gynecology (ob.gyn.) practices in New York and Connecticut have shown that (HBOC).
Genetic screening and testing can reduce the morbidity and mortality from breast, ovarian, and endometrial cancers through prevention and early detection. Mark S. DeFrancesco, MD, of Westwood Women’s Health, Waterbury, Conn., and his colleagues reported that, in spite of the American College of Obstetricians and Gynecologists’ recommendation for ob.gyns. to regularly screen, counsel, and refer accordingly for HBOC (Obstet Gynecol. 2015;125:153843), the “incorporation of hereditary cancer risk assessment and testing remains underutilized in the [ob.gyn.] setting.” The authors have addressed this issue in their own practice with promising results and important caveats (Obstet Gynecol 2018;132:1121-9).
The intervention included a process evaluation, improvements to patient work flow, and training of providers by genetic counselors and engineering personnel from the testing laboratory (Myriad Genetics), which provided support for the study. Patients in the study completed a family history questionnaire and, those meeting National Comprehensive Cancer Center Network criteria for genetic testing, were given pretest counseling and offered testing on the same day or referral for testing within 2 weeks.
Of the 3,811 women who completed the questionnaire, 24% (906) met NCCN criteria, 90% of whom were offered testing. However, only 52% (165) of patients who agreed to testing underwent genetic evaluation. This included 70% of patients who were offered same-day testing and 35% of patients who were offered a referral appointment for testing.
Conversations about HBOC and genetic testing can be complicated and may not be a patient’s initial priority. The authors should be commended for identifying the vast majority of high-risk patients. However, only half of patients meeting criteria completed testing and 10% who should have been offered testing were not – numbers still well below target.
Incorporation of family history questionnaires should become commonplace in the generalist’s office and optimizing EHRs may be an opportunity for rapid risk interpretation. As the success of same-day genetic testing was striking, opportunities for partnerships with insurance companies and private laboratories are likely needed to make this a more feasible option. Lastly, assessing women’s knowledge and attitudes around genetic testing could help to more specifically address barriers to testing in future interventions.
Improving genetic screening and testing completion rates requires a coordinated effort. Using tools and applications to optimize convenience (same-day testing, telemedicine) and simplification (electronic screening platforms), we can strive to appropriately detect all women at high risk for hereditary breast and ovarian cancers.
Michelle Lightfoot is a gynecologic oncology fellow at the Ohio State University in Columbus. She has no relevant financial disclosures. Email her at [email protected].
Researchers from general obstetrics and gynecology (ob.gyn.) practices in New York and Connecticut have shown that (HBOC).
Genetic screening and testing can reduce the morbidity and mortality from breast, ovarian, and endometrial cancers through prevention and early detection. Mark S. DeFrancesco, MD, of Westwood Women’s Health, Waterbury, Conn., and his colleagues reported that, in spite of the American College of Obstetricians and Gynecologists’ recommendation for ob.gyns. to regularly screen, counsel, and refer accordingly for HBOC (Obstet Gynecol. 2015;125:153843), the “incorporation of hereditary cancer risk assessment and testing remains underutilized in the [ob.gyn.] setting.” The authors have addressed this issue in their own practice with promising results and important caveats (Obstet Gynecol 2018;132:1121-9).
The intervention included a process evaluation, improvements to patient work flow, and training of providers by genetic counselors and engineering personnel from the testing laboratory (Myriad Genetics), which provided support for the study. Patients in the study completed a family history questionnaire and, those meeting National Comprehensive Cancer Center Network criteria for genetic testing, were given pretest counseling and offered testing on the same day or referral for testing within 2 weeks.
Of the 3,811 women who completed the questionnaire, 24% (906) met NCCN criteria, 90% of whom were offered testing. However, only 52% (165) of patients who agreed to testing underwent genetic evaluation. This included 70% of patients who were offered same-day testing and 35% of patients who were offered a referral appointment for testing.
Conversations about HBOC and genetic testing can be complicated and may not be a patient’s initial priority. The authors should be commended for identifying the vast majority of high-risk patients. However, only half of patients meeting criteria completed testing and 10% who should have been offered testing were not – numbers still well below target.
Incorporation of family history questionnaires should become commonplace in the generalist’s office and optimizing EHRs may be an opportunity for rapid risk interpretation. As the success of same-day genetic testing was striking, opportunities for partnerships with insurance companies and private laboratories are likely needed to make this a more feasible option. Lastly, assessing women’s knowledge and attitudes around genetic testing could help to more specifically address barriers to testing in future interventions.
Improving genetic screening and testing completion rates requires a coordinated effort. Using tools and applications to optimize convenience (same-day testing, telemedicine) and simplification (electronic screening platforms), we can strive to appropriately detect all women at high risk for hereditary breast and ovarian cancers.
Michelle Lightfoot is a gynecologic oncology fellow at the Ohio State University in Columbus. She has no relevant financial disclosures. Email her at [email protected].
Surgical quality: How do we measure something so difficult to define?
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Keeping the sample closet out of medication decisions
When I first began practice the COX-2 inhibitors had first come to market. My sample closet was awash with Celebrex and Vioxx.
I was young and naive. These drugs were allegedly safer than NSAIDs, so shouldn’t I be using them? They were new, and therefore had to be better, than plain old naproxen and ibuprofen. And hey, the samples were free.
As a result, I handed them out for pretty much all musculoskeletal stuff. “Here, try this ... ”
Of course, that came to a crashing halt when I encountered the realities of payers and drug coverage. No history of GI issues, no previous tries/fails ... Why on earth are you prescribing this? Obviously, the answer “because the samples were free” wasn’t going to pass muster.
Granted, history wasn’t particularly kind to the COX-2 drugs. Out of the three that made it to market, two were withdrawn and Celebrex’s star faded with them. But the lesson is still there.
Today, 20 years later, I use more generics. Maybe it’s because I’m familiar with them (many came to market during my career). Maybe it’s because years of calls from patients, pharmacies, and insurance companies have taught me to try them first. Probably a mixture of both.
This isn’t to say I don’t use branded drugs. I prescribe my share. There are plenty of times a generic isn’t appropriate, or a new approach is needed after a treatment failure.
But I’ve also learned that .
We learn a lot about the many different medications available in medical school and residency. But learning facts about dosing, side effects, and mechanisms of action (while quite important) is quite different from the practical aspect of learning what is more likely to be covered and affordable. Only the experience of everyday practice will teach that.
It sure taught me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
When I first began practice the COX-2 inhibitors had first come to market. My sample closet was awash with Celebrex and Vioxx.
I was young and naive. These drugs were allegedly safer than NSAIDs, so shouldn’t I be using them? They were new, and therefore had to be better, than plain old naproxen and ibuprofen. And hey, the samples were free.
As a result, I handed them out for pretty much all musculoskeletal stuff. “Here, try this ... ”
Of course, that came to a crashing halt when I encountered the realities of payers and drug coverage. No history of GI issues, no previous tries/fails ... Why on earth are you prescribing this? Obviously, the answer “because the samples were free” wasn’t going to pass muster.
Granted, history wasn’t particularly kind to the COX-2 drugs. Out of the three that made it to market, two were withdrawn and Celebrex’s star faded with them. But the lesson is still there.
Today, 20 years later, I use more generics. Maybe it’s because I’m familiar with them (many came to market during my career). Maybe it’s because years of calls from patients, pharmacies, and insurance companies have taught me to try them first. Probably a mixture of both.
This isn’t to say I don’t use branded drugs. I prescribe my share. There are plenty of times a generic isn’t appropriate, or a new approach is needed after a treatment failure.
But I’ve also learned that .
We learn a lot about the many different medications available in medical school and residency. But learning facts about dosing, side effects, and mechanisms of action (while quite important) is quite different from the practical aspect of learning what is more likely to be covered and affordable. Only the experience of everyday practice will teach that.
It sure taught me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
When I first began practice the COX-2 inhibitors had first come to market. My sample closet was awash with Celebrex and Vioxx.
I was young and naive. These drugs were allegedly safer than NSAIDs, so shouldn’t I be using them? They were new, and therefore had to be better, than plain old naproxen and ibuprofen. And hey, the samples were free.
As a result, I handed them out for pretty much all musculoskeletal stuff. “Here, try this ... ”
Of course, that came to a crashing halt when I encountered the realities of payers and drug coverage. No history of GI issues, no previous tries/fails ... Why on earth are you prescribing this? Obviously, the answer “because the samples were free” wasn’t going to pass muster.
Granted, history wasn’t particularly kind to the COX-2 drugs. Out of the three that made it to market, two were withdrawn and Celebrex’s star faded with them. But the lesson is still there.
Today, 20 years later, I use more generics. Maybe it’s because I’m familiar with them (many came to market during my career). Maybe it’s because years of calls from patients, pharmacies, and insurance companies have taught me to try them first. Probably a mixture of both.
This isn’t to say I don’t use branded drugs. I prescribe my share. There are plenty of times a generic isn’t appropriate, or a new approach is needed after a treatment failure.
But I’ve also learned that .
We learn a lot about the many different medications available in medical school and residency. But learning facts about dosing, side effects, and mechanisms of action (while quite important) is quite different from the practical aspect of learning what is more likely to be covered and affordable. Only the experience of everyday practice will teach that.
It sure taught me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Severe chronic malnutrition: What it is and how to diagnose it
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
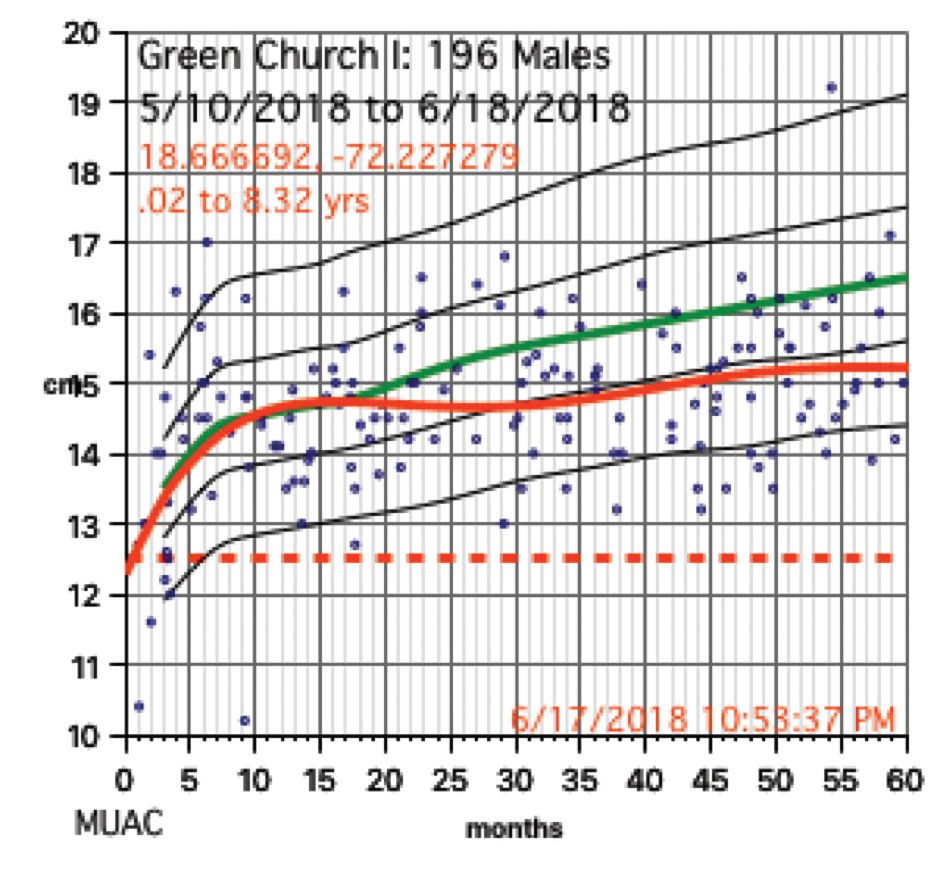
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.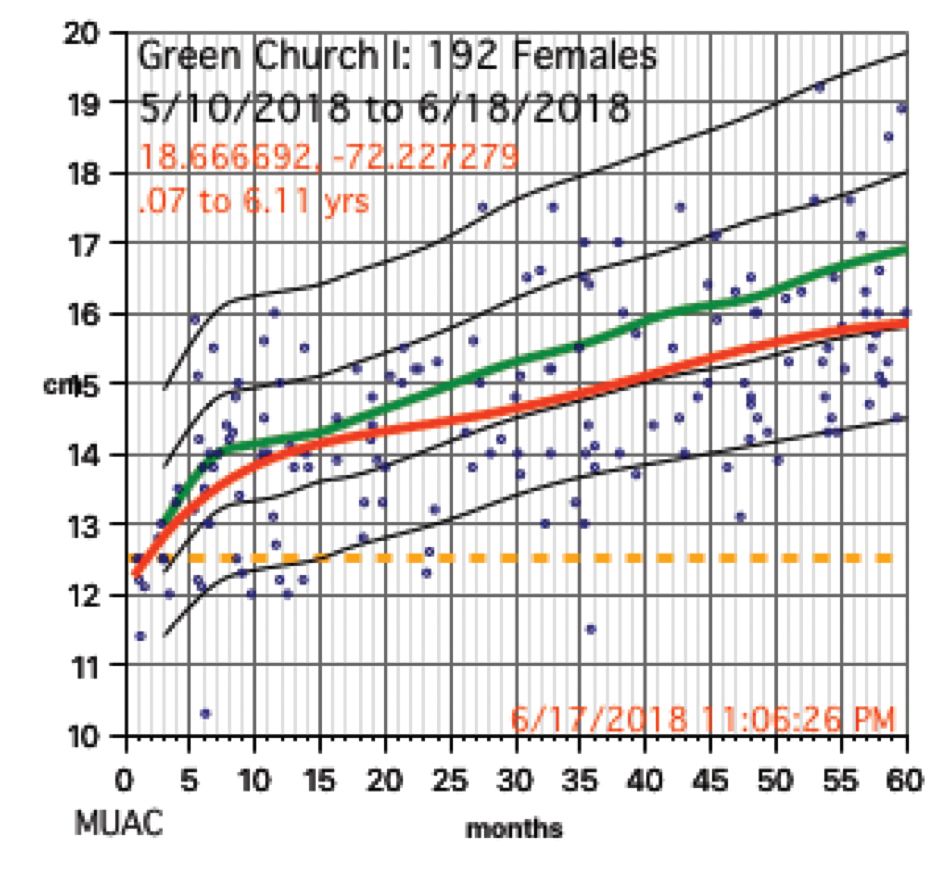
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.
The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 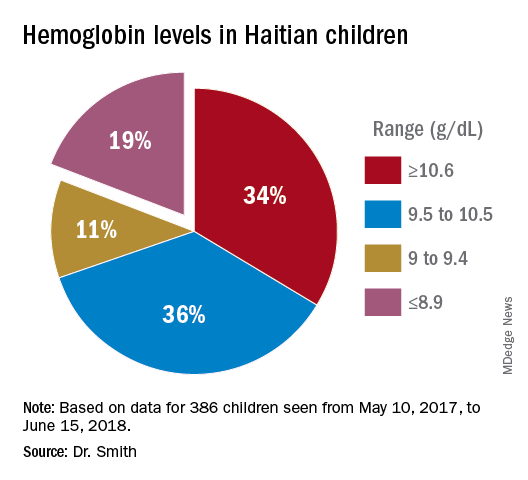
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?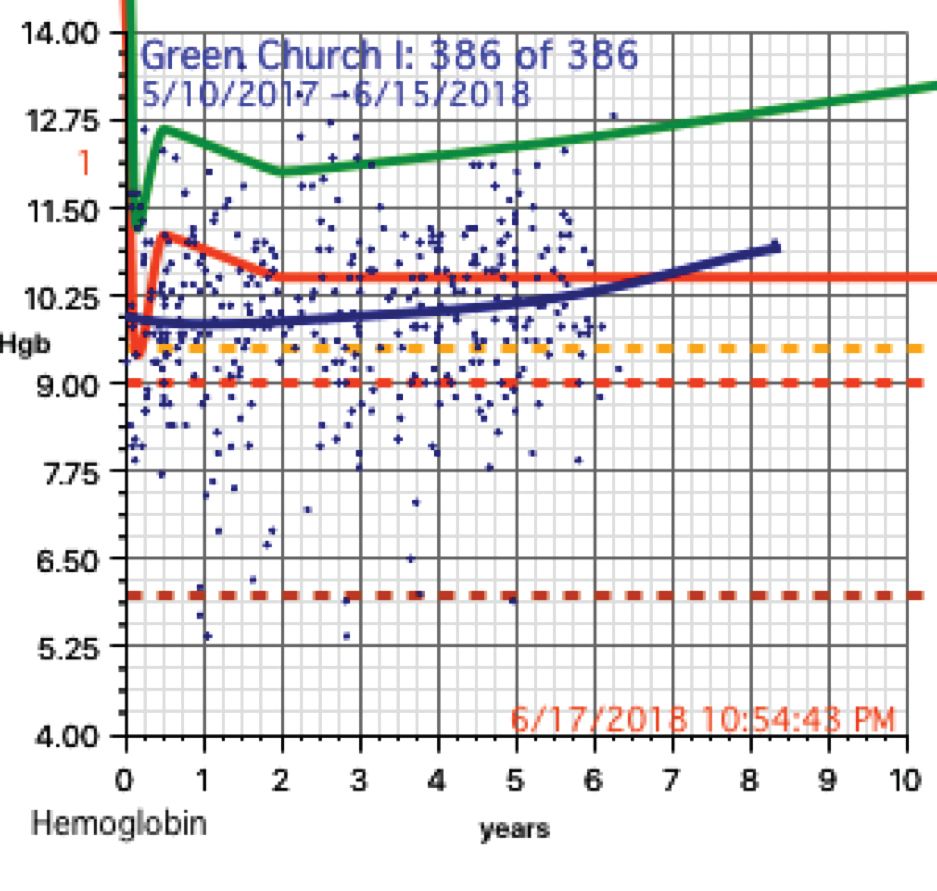
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.

The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
My wife and I have traveled a number of times as far east as Kyrgyzstan and as far south as Paraguay to participate in short 1- to 2-week medical clinics. When I participated in a week-long medical clinic in Haiti in early 2017, the CEO of the hosting U.S. organization asked, “I wonder if we are doing any good here?” His organization had been to Onaville, Haiti for the last 4-5 years.
So my wife Stacy, a retired licensed practical nurse, and I, a general pediatrician with an interest in severe acute malnutrition, went on a 3-month medical sabbatical to Onaville. We were self-funded, with the exception of our home church in Senoia, Ga., paying the cost of our lodging during that time.
Prior to the 2010 Haiti earthquake, the government planned Onaville to be a retirement community area, with a population of only about 1,500, I was told. After the devastating temblor, it became one of several areas where the government sent people displaced from Port-au-Prince. The population today is possibly 250,000 or more.
The poverty in this area has “newer” flavor than areas such as Cité Soleil, which has been there for decades. What we found in Onaville – and probably all of Haiti – is an appalling lack of understanding and appreciation about the nature of malnutrition.
Methods and materials for study
The 1981 World Health Organization’s last printed monograph about severe acute nutrition remains essentially today’s cookbook recipe for treatment. Little seems to have changed since then in the literature I’ve reviewed. It didn’t take long after we started seeing the children in Onaville to shift that interest to something much more serious and widespread.
I wanted to start with basic health assessments in the Onaville children around 5 years and under. These children rarely see a physician, and only about half or so get any vaccine. Most parents do not have any immunization records in their possession to even review.
We decided to measure head size, mid-upper arm circumference, height, weight, and hemoglobin levels. Date of birth was recorded, if known or could at best be closely estimated. Vaccination was recorded as a yes or no response. All children also were examined for evidence of things like swelling, marasmic appearance (wasting, loss of body fat and muscle), yellowed hair, eye findings of vitamin A deficiency, etc. I wanted to get some impression about the health of these children in the same way that most mobile medical clinics do in Haiti.
Being a database programmer since I bought my first computer in 1985, and having written and deployed my office’s current EMR system in 2000, I decided before ever arriving in Haiti to write the software needed for this task. Unlike regular office EMRs, there were some special considerations.
Growth charts needed not only to be generated for individuals, but in aggregate. Hemoglobins levels, too, needed charting. While in the United States, I use Centers for Disease Control and Prevention growth chart data, but for Haiti I used WHO growth data. I was able to procure hemoglobin charting data as well. Aggregate data turned out to be key to our conclusions.
We used a regular consumer quality digital bathroom scale for weights. A sewing tape attached with duct tape to a wall or pillar was used to measure height. Standard head circumference tapes were use to measure heads and arms.

The hemoglobin was measured with a HemoCue Hb 201+ instrument. Size, ruggedness, and cost dictated all our choices because, except for food, we had to carry everything with us. The cost of a new HemoCue was under $400 and each microcuvette test was about $1.50.
Severe anemia
In total, we saw about 386 children, mostly 5 years and under, in Onaville. Toward the end of the 3 months, we were seeing some of those back as follow-ups. One of the first hemoglobins was 4.9 g/dL, with a 5.4 g/dL on repeat. This stunned us. In the first few days, we were seeing what we saw consistently throughout the course of 3 months.
About 19% of these children had hemoglobins from below 9.0 g/dL to below 6 g/dL. More importantly, there was little on physical exam that would trigger one to do a hemoglobin. Low hemoglobins were not associated with yellow-orange hair. No cases of the swelling of kwashiorkor or pencil-like frames of marasmus were seen. 
Severe chronic malnutrition
The scatter charts are very telling and the hemoglobin graphs are explosive. What is demonstrated is that this recent population is slowly starving to death. How can the hemoglobins be so very low in comparison to the only slightly lowered mean averages (the solid red line)?
In over 3 decades of pediatric medicine, I rarely have seen children in the United States with hemoglobins below 9.5 g/dL. Often they have other illnesses that clearly point to the cause. Could the 19% of children with severely lowered hemoglobins (below 9.0 g/dL) be caused by sickle cell disease or something else in these Haitian children?
A search for articles where sickle cell was studied revealed a study done at St. Damien Pediatrics Hospital in Port-au-Prince (Blood. 2012;120:4235). The overall incidence of sickle cell disease was this: “Of the 2,258 samples tested, 247 had HbS, fifty-seven had HbC, ten had HbSS, and three had HbSC.” Only 0.57% of these children had sickle or sickle-C disease where one could expect hemoglobins to be as low as in the children of Onaville. Applying that percentage to the 386 children we saw would account for about only 2 children who might have sickling anemia. Yet we had 73 children in our study with severely lowered hemoglobins below 9.0 g/dL. If you estimate that half of the 250,000 people in Onaville are children, that extrapolates to over 47,000 with severe anemia! I think that a study larger than ours needs to be done to better assess that, however.
My best thought is that these children who have little external evidence of abnormality and mildly lowered growth data represent a type of malnutrition that has not been defined, much less addressed. I call this severe chronic malnutrition. The very low hemoglobins indicate to me that this is not simply a lack of iron – although certainly that is a factor – but rather that these children are in a state of chronic protein deprivation. They represent a large pool of children who exist between those with normal nutritional states and those with the kwashiorkor or marasmus of severe acute malnutrition.
A search of the 69,823 ICD-10 codes in my database for “malnutrition” only turns up the ill-defined terms, “Unspecified severe protein-calorie malnutrition,” “Moderate, and Mild protein-calorie malnutrition,” “Unspecified protein-calorie malnutrition,” and “Sequelae of protein-calorie malnutrition.” Whatever each of those means is purely subjective in my opinion.
Without a clear understanding or definition of what is severe chronic malnutrition, we are like the Titanic trying to avoid icebergs on a moonless night. I think we must define severe chronic malnutrition before we really can understand the pathophysiology and treatment of severe acute malnutrition.
The WHO published its last printed monograph, “The treatment and management of severe acute protein-energy malnutrition,” in 1981. This publication is essentially a cookbook approach for what to do, with no clear presentation of the chemical processes and medicine involved. The primary focus for the WHO is mid-upper arm circumference and weight for height. Reading this document might lead one to believe that all malnutrition is acutely severe. It is most certainly not.
Conclusion
The answer to why some children show the swelling of kwashiorkor and some show marasmus probably will not be found in the study of severe acute malnutrition or refeeding syndrome alone. We must go far beyond the WHO’s cookbook recipe.
I think we must start with the study, definition, and treatment of severe chronic malnutrition.
While in Haiti, we shared these data with three organization that are working to provide nutrition in a starving nation. Together, the Baptist Haiti Mission, Mission of Hope Haiti, and Trinity Hope may well be supplying 175,000 meals a day through school lunches and other avenues throughout the country. Their response was telling. Those at Baptist Haiti Mission, an organization with a presence of almost 80 years there, told us that this information was a “big deal.”
The issue for them is the answer to the question, “How can we tell if we are doing any good in our feeding programs?” A lot of money is being thrown into nutrition without tangible ways to assess impact. Clearly parameters such as mid-upper arm circumference and weight for height that WHO advocates is not adequate, as our plots revealed.
We think that a simple, cheap, hemoglobin finger stick can tell us who is falling through the cracks into severe chronic malnutrition and those at risk for severe acute malnutrition. I am an advocate for instituting hemoglobin surveillance as part of all feeding programs. Then we can come up with the cheapest and most effective in-country mechanisms to treat these children.
Indeed that is our next step in working in Haiti.
Dr. Smith is a board certified pediatrician working in McDonough, Ga., with an interest in malnutrition among the children of Haiti. Email him at [email protected].
Book Review: DuPont’s approach to addiction is tough, yet compassionate
What do Queen Silvia of Sweden, Pope Francis, and the first director of the National Institute on Drug Abuse (NIDA) have in common? They all share a deep and sobering commitment to fighting the global disease of addiction.
Robert L. DuPont, MD, has written a beautiful and surprisingly spiritual guide into the American addiction epidemic. He is the author of “The Selfish Brain: Learning From Addiction” (Center City, Minn.: Hazelden, 2000) and with his newest publication, “Chemical Slavery: Understanding Addiction and Stopping the Drug Epidemic” (Institute for Behavior and Health, 2018), he writes a clear-eyed tome detailing the history of drug and alcohol use within the United States and the current state of America’s drug epidemic.
Dr. DuPont is well known within the American addiction community as NIDA’s first director and as the second drug czar, under two presidents, Richard M. Nixon and Gerald R. Ford. His breadth of experience, spanning 50-plus years, dates from his early career with the District of Columbia Department of Corrections, into his work in public policy on drugs and alcohol. This experience infuses his book with the hard science of addiction and the common-sense compassion required to shepherd people into recovery. One of us has worked with and been influenced by him since the 1970s. The other, also an addiction psychiatrist, finished this book both invigorated and compelled to say thank you to Dr. DuPont and his life’s work! He remains relevant, insightful, and always optimistic about the future of addiction treatment.
Harm reduction explored
The book begins with a cogent history of drug and alcohol use in America. Dr. DuPont details this as well as the public policies that have evolved to address them. He weaves into our national history reasoning behind why, as a “mass consumer” culture, we are more prone to addiction than ever before. The loss of cultural and societal pressures has a role to play in the rise along with genetics and environmental stress. The adolescent brain is prominently discussed throughout this first section as a highly vulnerable organ that can lead to lifelong addiction if primed early by addictive chemicals.
Dr. DuPont addresses the biology of addiction, delving into both the biological mechanisms within the brain, making those details accessible and understandable – to a practicing physician as well as a family member or patient struggling with addiction.
He also addresses harm reduction, a fairly new concept within the field. Harm reduction has taken on more prominence with localities across the country providing people with addictions with safe places and clean needles to continue their substance use without risks of serious or life-threatening diseases and crime. He challenges the idea that harm reduction is active recovery from substance addiction. Instead, he opines that harm reduction must be tethered to and must lead to real recovery work or it risks becoming an organizational enabling of the addict’s behavior.
Dr. DuPont pulls no punches with his language. He uses words such as “fatal,” “addict,” and “alcoholic.” He addresses the concerns by some in the field that those kinds of words are harsh, derogatory, and prejudicial by calling out addiction as a disease hallmarked primarily by loss of control and by dishonesty. To shun those words is to perpetuate the disease, delaying life-saving treatment.
Compassion is a theme throughout. He says we must stigmatize the addiction but not the addict. He advocates for real consequences to addictive behaviors as a key to getting addicted physicians and others with this disease into recovery. Treatment works, but we also have studied specific approaches that work and why.1 His writing conveys a genuine empathy for his patients and argues that treatment is delayed when serious and negative consequences for patients are removed.
Focus on prevention
A clear passion for prevention is evident within his chapters on youth addiction. For adolescents, Dr. DuPont presents a “One Choice approach,” which requires complete abstinence from alcohol, tobacco, and marijuana. He also presents science showing that patients younger than age 21 will have a greater risk of developing lifelong and debilitating addictions if they use these chemicals prior to this age. His emphasis for the One Choice approach carries ramifications throughout the primary care, pediatric, and family practice communities.
Dr. DuPont is nothing if not an optimist. Though he clearly defines addiction as an often-fatal disease, he remains positive about the future of addiction treatment, both with changes in public policy and with advancements in the medicine of addiction. He makes a compelling argument regarding Sweden’s approach to the drug problem, citing Queen Silvia’s lifelong commitment to prevent, control, and treat drug and alcohol addiction. Sweden’s model is, indeed, intriguing, and that country’s outcomes present a strong argument for the marriage of the criminal justice system with medical intervention – an approach that the United States has adopted only in a patchwork fashion.
The book describes controversies throughout, such as Dr. DuPont’s furtherance of our work on how best to treat dual disorders.2 He is not impressed with the self-medication hypothesis as well as the dive into the U.S. national medical marijuana experiment. We had looked at college students having new-onset memory or attention-deficit/hyperactivity problems, only to find that it was likely psychostimulant seeking to reverse marijuana effects.3 Physicians, families, and patients would be well served to review his arguments on the clear definition of “medicine” with regard to marijuana and the risks taken when we medicalize a known addictive chemical. He tackles the push for legalization as well, and the risks of increased societal acceptance and commercialization power that comes with this. He has led the field in thinking about the role of early drug exposure, brain training, and hijacking in the addictive process. This gateway hypothesis can occur whether the first teen drugs are cannabis or tobacco4 or alcohol.
Dr. DuPont finishes his work with a detailed biography, in which he addresses his family history of addiction, and his own overuse of alcohol in his late teens and early 20s as well as a confession of onetime use of marijuana during medical school. He always has led the field in trying to explain the disease of addiction, intervention, treatment, and recovery. But this self-reflection and brutal honesty is refreshing and in step with themes in his book, which promote the idea of recovery as an embrace of honesty, in mind, body, and spirit.
Dr. Jorandby trained in addiction psychiatry at Yale University, New Haven, Conn., and works as an addiction psychiatrist with Amen Clinics in Washington. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
References
1. J Subst Abuse Treat. 2009 Mar;36(2):159-71.
2. J Addict Dis. 2007;26 Suppl 1:13-23.
3. Am J Psychiatry. 2007 Jun;164(6):973.
4. J Addict Dis. 2003;22(3):51-62.
What do Queen Silvia of Sweden, Pope Francis, and the first director of the National Institute on Drug Abuse (NIDA) have in common? They all share a deep and sobering commitment to fighting the global disease of addiction.
Robert L. DuPont, MD, has written a beautiful and surprisingly spiritual guide into the American addiction epidemic. He is the author of “The Selfish Brain: Learning From Addiction” (Center City, Minn.: Hazelden, 2000) and with his newest publication, “Chemical Slavery: Understanding Addiction and Stopping the Drug Epidemic” (Institute for Behavior and Health, 2018), he writes a clear-eyed tome detailing the history of drug and alcohol use within the United States and the current state of America’s drug epidemic.
Dr. DuPont is well known within the American addiction community as NIDA’s first director and as the second drug czar, under two presidents, Richard M. Nixon and Gerald R. Ford. His breadth of experience, spanning 50-plus years, dates from his early career with the District of Columbia Department of Corrections, into his work in public policy on drugs and alcohol. This experience infuses his book with the hard science of addiction and the common-sense compassion required to shepherd people into recovery. One of us has worked with and been influenced by him since the 1970s. The other, also an addiction psychiatrist, finished this book both invigorated and compelled to say thank you to Dr. DuPont and his life’s work! He remains relevant, insightful, and always optimistic about the future of addiction treatment.
Harm reduction explored
The book begins with a cogent history of drug and alcohol use in America. Dr. DuPont details this as well as the public policies that have evolved to address them. He weaves into our national history reasoning behind why, as a “mass consumer” culture, we are more prone to addiction than ever before. The loss of cultural and societal pressures has a role to play in the rise along with genetics and environmental stress. The adolescent brain is prominently discussed throughout this first section as a highly vulnerable organ that can lead to lifelong addiction if primed early by addictive chemicals.
Dr. DuPont addresses the biology of addiction, delving into both the biological mechanisms within the brain, making those details accessible and understandable – to a practicing physician as well as a family member or patient struggling with addiction.
He also addresses harm reduction, a fairly new concept within the field. Harm reduction has taken on more prominence with localities across the country providing people with addictions with safe places and clean needles to continue their substance use without risks of serious or life-threatening diseases and crime. He challenges the idea that harm reduction is active recovery from substance addiction. Instead, he opines that harm reduction must be tethered to and must lead to real recovery work or it risks becoming an organizational enabling of the addict’s behavior.
Dr. DuPont pulls no punches with his language. He uses words such as “fatal,” “addict,” and “alcoholic.” He addresses the concerns by some in the field that those kinds of words are harsh, derogatory, and prejudicial by calling out addiction as a disease hallmarked primarily by loss of control and by dishonesty. To shun those words is to perpetuate the disease, delaying life-saving treatment.
Compassion is a theme throughout. He says we must stigmatize the addiction but not the addict. He advocates for real consequences to addictive behaviors as a key to getting addicted physicians and others with this disease into recovery. Treatment works, but we also have studied specific approaches that work and why.1 His writing conveys a genuine empathy for his patients and argues that treatment is delayed when serious and negative consequences for patients are removed.
Focus on prevention
A clear passion for prevention is evident within his chapters on youth addiction. For adolescents, Dr. DuPont presents a “One Choice approach,” which requires complete abstinence from alcohol, tobacco, and marijuana. He also presents science showing that patients younger than age 21 will have a greater risk of developing lifelong and debilitating addictions if they use these chemicals prior to this age. His emphasis for the One Choice approach carries ramifications throughout the primary care, pediatric, and family practice communities.
Dr. DuPont is nothing if not an optimist. Though he clearly defines addiction as an often-fatal disease, he remains positive about the future of addiction treatment, both with changes in public policy and with advancements in the medicine of addiction. He makes a compelling argument regarding Sweden’s approach to the drug problem, citing Queen Silvia’s lifelong commitment to prevent, control, and treat drug and alcohol addiction. Sweden’s model is, indeed, intriguing, and that country’s outcomes present a strong argument for the marriage of the criminal justice system with medical intervention – an approach that the United States has adopted only in a patchwork fashion.
The book describes controversies throughout, such as Dr. DuPont’s furtherance of our work on how best to treat dual disorders.2 He is not impressed with the self-medication hypothesis as well as the dive into the U.S. national medical marijuana experiment. We had looked at college students having new-onset memory or attention-deficit/hyperactivity problems, only to find that it was likely psychostimulant seeking to reverse marijuana effects.3 Physicians, families, and patients would be well served to review his arguments on the clear definition of “medicine” with regard to marijuana and the risks taken when we medicalize a known addictive chemical. He tackles the push for legalization as well, and the risks of increased societal acceptance and commercialization power that comes with this. He has led the field in thinking about the role of early drug exposure, brain training, and hijacking in the addictive process. This gateway hypothesis can occur whether the first teen drugs are cannabis or tobacco4 or alcohol.
Dr. DuPont finishes his work with a detailed biography, in which he addresses his family history of addiction, and his own overuse of alcohol in his late teens and early 20s as well as a confession of onetime use of marijuana during medical school. He always has led the field in trying to explain the disease of addiction, intervention, treatment, and recovery. But this self-reflection and brutal honesty is refreshing and in step with themes in his book, which promote the idea of recovery as an embrace of honesty, in mind, body, and spirit.
Dr. Jorandby trained in addiction psychiatry at Yale University, New Haven, Conn., and works as an addiction psychiatrist with Amen Clinics in Washington. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
References
1. J Subst Abuse Treat. 2009 Mar;36(2):159-71.
2. J Addict Dis. 2007;26 Suppl 1:13-23.
3. Am J Psychiatry. 2007 Jun;164(6):973.
4. J Addict Dis. 2003;22(3):51-62.
What do Queen Silvia of Sweden, Pope Francis, and the first director of the National Institute on Drug Abuse (NIDA) have in common? They all share a deep and sobering commitment to fighting the global disease of addiction.
Robert L. DuPont, MD, has written a beautiful and surprisingly spiritual guide into the American addiction epidemic. He is the author of “The Selfish Brain: Learning From Addiction” (Center City, Minn.: Hazelden, 2000) and with his newest publication, “Chemical Slavery: Understanding Addiction and Stopping the Drug Epidemic” (Institute for Behavior and Health, 2018), he writes a clear-eyed tome detailing the history of drug and alcohol use within the United States and the current state of America’s drug epidemic.
Dr. DuPont is well known within the American addiction community as NIDA’s first director and as the second drug czar, under two presidents, Richard M. Nixon and Gerald R. Ford. His breadth of experience, spanning 50-plus years, dates from his early career with the District of Columbia Department of Corrections, into his work in public policy on drugs and alcohol. This experience infuses his book with the hard science of addiction and the common-sense compassion required to shepherd people into recovery. One of us has worked with and been influenced by him since the 1970s. The other, also an addiction psychiatrist, finished this book both invigorated and compelled to say thank you to Dr. DuPont and his life’s work! He remains relevant, insightful, and always optimistic about the future of addiction treatment.
Harm reduction explored
The book begins with a cogent history of drug and alcohol use in America. Dr. DuPont details this as well as the public policies that have evolved to address them. He weaves into our national history reasoning behind why, as a “mass consumer” culture, we are more prone to addiction than ever before. The loss of cultural and societal pressures has a role to play in the rise along with genetics and environmental stress. The adolescent brain is prominently discussed throughout this first section as a highly vulnerable organ that can lead to lifelong addiction if primed early by addictive chemicals.
Dr. DuPont addresses the biology of addiction, delving into both the biological mechanisms within the brain, making those details accessible and understandable – to a practicing physician as well as a family member or patient struggling with addiction.
He also addresses harm reduction, a fairly new concept within the field. Harm reduction has taken on more prominence with localities across the country providing people with addictions with safe places and clean needles to continue their substance use without risks of serious or life-threatening diseases and crime. He challenges the idea that harm reduction is active recovery from substance addiction. Instead, he opines that harm reduction must be tethered to and must lead to real recovery work or it risks becoming an organizational enabling of the addict’s behavior.
Dr. DuPont pulls no punches with his language. He uses words such as “fatal,” “addict,” and “alcoholic.” He addresses the concerns by some in the field that those kinds of words are harsh, derogatory, and prejudicial by calling out addiction as a disease hallmarked primarily by loss of control and by dishonesty. To shun those words is to perpetuate the disease, delaying life-saving treatment.
Compassion is a theme throughout. He says we must stigmatize the addiction but not the addict. He advocates for real consequences to addictive behaviors as a key to getting addicted physicians and others with this disease into recovery. Treatment works, but we also have studied specific approaches that work and why.1 His writing conveys a genuine empathy for his patients and argues that treatment is delayed when serious and negative consequences for patients are removed.
Focus on prevention
A clear passion for prevention is evident within his chapters on youth addiction. For adolescents, Dr. DuPont presents a “One Choice approach,” which requires complete abstinence from alcohol, tobacco, and marijuana. He also presents science showing that patients younger than age 21 will have a greater risk of developing lifelong and debilitating addictions if they use these chemicals prior to this age. His emphasis for the One Choice approach carries ramifications throughout the primary care, pediatric, and family practice communities.
Dr. DuPont is nothing if not an optimist. Though he clearly defines addiction as an often-fatal disease, he remains positive about the future of addiction treatment, both with changes in public policy and with advancements in the medicine of addiction. He makes a compelling argument regarding Sweden’s approach to the drug problem, citing Queen Silvia’s lifelong commitment to prevent, control, and treat drug and alcohol addiction. Sweden’s model is, indeed, intriguing, and that country’s outcomes present a strong argument for the marriage of the criminal justice system with medical intervention – an approach that the United States has adopted only in a patchwork fashion.
The book describes controversies throughout, such as Dr. DuPont’s furtherance of our work on how best to treat dual disorders.2 He is not impressed with the self-medication hypothesis as well as the dive into the U.S. national medical marijuana experiment. We had looked at college students having new-onset memory or attention-deficit/hyperactivity problems, only to find that it was likely psychostimulant seeking to reverse marijuana effects.3 Physicians, families, and patients would be well served to review his arguments on the clear definition of “medicine” with regard to marijuana and the risks taken when we medicalize a known addictive chemical. He tackles the push for legalization as well, and the risks of increased societal acceptance and commercialization power that comes with this. He has led the field in thinking about the role of early drug exposure, brain training, and hijacking in the addictive process. This gateway hypothesis can occur whether the first teen drugs are cannabis or tobacco4 or alcohol.
Dr. DuPont finishes his work with a detailed biography, in which he addresses his family history of addiction, and his own overuse of alcohol in his late teens and early 20s as well as a confession of onetime use of marijuana during medical school. He always has led the field in trying to explain the disease of addiction, intervention, treatment, and recovery. But this self-reflection and brutal honesty is refreshing and in step with themes in his book, which promote the idea of recovery as an embrace of honesty, in mind, body, and spirit.
Dr. Jorandby trained in addiction psychiatry at Yale University, New Haven, Conn., and works as an addiction psychiatrist with Amen Clinics in Washington. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
References
1. J Subst Abuse Treat. 2009 Mar;36(2):159-71.
2. J Addict Dis. 2007;26 Suppl 1:13-23.
3. Am J Psychiatry. 2007 Jun;164(6):973.
4. J Addict Dis. 2003;22(3):51-62.
Superheroes
Who’s your favorite superhero? I realize this might be impossible to answer – Marvel and DC Comics alone have thousands of heroes from which to choose. I recently visited the Seattle Museum of Pop Culture, known as MoPOP, where they have an awesome exhibit on the history of Marvel. I left understanding why superheroes are perennially popular and why we need them. I also felt a little more powerful myself.
The Avengers might seem like just a marketing scheme created to take your movie money. They’re more than that. Superheroes like Thor and Black Widow appear in all cultures and throughout time. There are short and tall, black and white, young and old, gay and straight, Muslim and Jewish, European, Asian, and African superheroes. The characters in The Iliad were superheroes to the ancients. In India today, you can buy comics featuring Lord Shiva.
Superheroes change with time, often reflecting our struggles and values. Captain America was created in 1941 to allay our fear of the then-metastasizing Nazis. The most popular Marvel hero at the MoPOP right now is Black Panther. Next year Captain Marvel will be released. Also known as Carol Danvers, Captain Marvel is one of Marvel Comics’ strongest women, a female Air Force officer with superhuman strength and speed.
Heroes change with the times and are metaphors for the real-life challenges we face and our abilities to overcome them. Superhero stories are our own stories.
When I was a kid, Spider-Man was my favorite. I watched him every afternoon at 3 o’clock when I got home from school. Spidey is a nerdy, little kid who can perform amazing feats to keep people safe and to right societal wrongs. Being a little kid who similarly loved science, he seemed like a good role model at the time. Interestingly, Spidey might have helped me. A couple of studies have shown that kids who pretend to be superheroes, like Batman for example, perform better on tasks, compared with those who aren’t pretending. In some ways, this strategy of imagining to have superpowers is an antidote to the impostor syndrome, a common experience of feeling powerless and undeserving of your position or role. By imagining that they have superpowers, children behave commensurately with these beliefs, which can help them develop self-efficacy at a critical period of development.
This strategy can work for adults too. Military men and women will adopt heroes like Punisher for their battalions, surgeons will don Superman scrub caps, and athletes will take nicknames like Batman for their professional personas. It is a strategy our ancient ancestors deployed, imagining they had the power of Hercules going into battle. No doubt, the energizing, empowering emotion we feel when we think of superheroes is why they are still so popular today. It is why you walk with a bit more swagger when you exit the theater of a good hero flick.
So indulge in a little Wonder Woman and Daredevil and Jessica Jones, even after Halloween has passed. When you do, remember they are here because they are us. and one that we need.
Nowadays, I probably relate most to Captain America: Lead a team, help make each team member better. And, yet, looking at Chris Evans, the actor who plays Captain America, it’s clear I need a lot more time at the gym. Or maybe I could just try to get bitten by a spider.
“Can he swing from a thread? Take a look overhead. Hey, there, there goes the Spider-Man!”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Who’s your favorite superhero? I realize this might be impossible to answer – Marvel and DC Comics alone have thousands of heroes from which to choose. I recently visited the Seattle Museum of Pop Culture, known as MoPOP, where they have an awesome exhibit on the history of Marvel. I left understanding why superheroes are perennially popular and why we need them. I also felt a little more powerful myself.
The Avengers might seem like just a marketing scheme created to take your movie money. They’re more than that. Superheroes like Thor and Black Widow appear in all cultures and throughout time. There are short and tall, black and white, young and old, gay and straight, Muslim and Jewish, European, Asian, and African superheroes. The characters in The Iliad were superheroes to the ancients. In India today, you can buy comics featuring Lord Shiva.
Superheroes change with time, often reflecting our struggles and values. Captain America was created in 1941 to allay our fear of the then-metastasizing Nazis. The most popular Marvel hero at the MoPOP right now is Black Panther. Next year Captain Marvel will be released. Also known as Carol Danvers, Captain Marvel is one of Marvel Comics’ strongest women, a female Air Force officer with superhuman strength and speed.
Heroes change with the times and are metaphors for the real-life challenges we face and our abilities to overcome them. Superhero stories are our own stories.
When I was a kid, Spider-Man was my favorite. I watched him every afternoon at 3 o’clock when I got home from school. Spidey is a nerdy, little kid who can perform amazing feats to keep people safe and to right societal wrongs. Being a little kid who similarly loved science, he seemed like a good role model at the time. Interestingly, Spidey might have helped me. A couple of studies have shown that kids who pretend to be superheroes, like Batman for example, perform better on tasks, compared with those who aren’t pretending. In some ways, this strategy of imagining to have superpowers is an antidote to the impostor syndrome, a common experience of feeling powerless and undeserving of your position or role. By imagining that they have superpowers, children behave commensurately with these beliefs, which can help them develop self-efficacy at a critical period of development.
This strategy can work for adults too. Military men and women will adopt heroes like Punisher for their battalions, surgeons will don Superman scrub caps, and athletes will take nicknames like Batman for their professional personas. It is a strategy our ancient ancestors deployed, imagining they had the power of Hercules going into battle. No doubt, the energizing, empowering emotion we feel when we think of superheroes is why they are still so popular today. It is why you walk with a bit more swagger when you exit the theater of a good hero flick.
So indulge in a little Wonder Woman and Daredevil and Jessica Jones, even after Halloween has passed. When you do, remember they are here because they are us. and one that we need.
Nowadays, I probably relate most to Captain America: Lead a team, help make each team member better. And, yet, looking at Chris Evans, the actor who plays Captain America, it’s clear I need a lot more time at the gym. Or maybe I could just try to get bitten by a spider.
“Can he swing from a thread? Take a look overhead. Hey, there, there goes the Spider-Man!”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Who’s your favorite superhero? I realize this might be impossible to answer – Marvel and DC Comics alone have thousands of heroes from which to choose. I recently visited the Seattle Museum of Pop Culture, known as MoPOP, where they have an awesome exhibit on the history of Marvel. I left understanding why superheroes are perennially popular and why we need them. I also felt a little more powerful myself.
The Avengers might seem like just a marketing scheme created to take your movie money. They’re more than that. Superheroes like Thor and Black Widow appear in all cultures and throughout time. There are short and tall, black and white, young and old, gay and straight, Muslim and Jewish, European, Asian, and African superheroes. The characters in The Iliad were superheroes to the ancients. In India today, you can buy comics featuring Lord Shiva.
Superheroes change with time, often reflecting our struggles and values. Captain America was created in 1941 to allay our fear of the then-metastasizing Nazis. The most popular Marvel hero at the MoPOP right now is Black Panther. Next year Captain Marvel will be released. Also known as Carol Danvers, Captain Marvel is one of Marvel Comics’ strongest women, a female Air Force officer with superhuman strength and speed.
Heroes change with the times and are metaphors for the real-life challenges we face and our abilities to overcome them. Superhero stories are our own stories.
When I was a kid, Spider-Man was my favorite. I watched him every afternoon at 3 o’clock when I got home from school. Spidey is a nerdy, little kid who can perform amazing feats to keep people safe and to right societal wrongs. Being a little kid who similarly loved science, he seemed like a good role model at the time. Interestingly, Spidey might have helped me. A couple of studies have shown that kids who pretend to be superheroes, like Batman for example, perform better on tasks, compared with those who aren’t pretending. In some ways, this strategy of imagining to have superpowers is an antidote to the impostor syndrome, a common experience of feeling powerless and undeserving of your position or role. By imagining that they have superpowers, children behave commensurately with these beliefs, which can help them develop self-efficacy at a critical period of development.
This strategy can work for adults too. Military men and women will adopt heroes like Punisher for their battalions, surgeons will don Superman scrub caps, and athletes will take nicknames like Batman for their professional personas. It is a strategy our ancient ancestors deployed, imagining they had the power of Hercules going into battle. No doubt, the energizing, empowering emotion we feel when we think of superheroes is why they are still so popular today. It is why you walk with a bit more swagger when you exit the theater of a good hero flick.
So indulge in a little Wonder Woman and Daredevil and Jessica Jones, even after Halloween has passed. When you do, remember they are here because they are us. and one that we need.
Nowadays, I probably relate most to Captain America: Lead a team, help make each team member better. And, yet, looking at Chris Evans, the actor who plays Captain America, it’s clear I need a lot more time at the gym. Or maybe I could just try to get bitten by a spider.
“Can he swing from a thread? Take a look overhead. Hey, there, there goes the Spider-Man!”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
How much more proof do you need?
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
The Sunshine Act, 5 years hence
You may recall that . The intent was to make relationships between pharmaceutical manufacturers and health care providers more transparent, by requiring the manufacturers to report to the Centers for Medicare & Medicaid Services all payments and other “transfers of value” provided to physicians and teaching hospitals.
Since the CMS has been collecting this information (and publishing it online each September) for 5 years now, I thought I would have a look at what has been learned to date, and what may have changed as a result.
Not much, apparently. In 2014, I predicted that attorneys, activists, and the occasional investigative reporter might peruse the data for their own purposes, but the general public would have little curiosity or use for the information. That appears to be the case thus far; there is no evidence that significant numbers of ordinary citizens have looked at the data or drawn any conclusions from it, perhaps because of the difficulty in accessing it (the website was widely panned when it debuted, although improvements have since been made); or perhaps because neither the CMS nor anyone else has offered the public any assistance in interpreting the raw data. Whether patients think less of doctors who accept an occasional industry-sponsored lunch for their employees, or think more (or less) of those who educate other providers or conduct clinical research, remain open questions.
One measurable – and probably unintended – consequence has been the increasing reluctance of physicians to provide legitimate feedback, or otherwise interact at all with industry, probably out of fear that they might one day have to explain a payment that could be construed by someone with an ax to grind as a conflict of interest. This is a shame, since there is no better way to develop new therapies, or to design solutions to the huge problems facing modern health care, than to actively involve doctors.
Furthermore, it is not clear how well the industry has complied with the law, or how effectively the government is enforcing it. The law authorizes fines of up to $150,000 annually, rising to $1 million for intentional violations; and while Vermont announced in late 2013 that it had levied 25 fines totaling $61,250 for violations of its somewhat stricter version of the statute, I could find no evidence of any similar enforcement by the CMS or any of the other states with standalone conflict of interest laws.*
All of that said, the law’s questionable impact and apparent lack of enforcement do not mean you can ignore it. Increased transparency and scrutiny of physician financial interests apparently are here to stay. The data are still being collected and displayed for anyone to see, so you still want to be certain that what is reported about you is accurate. This means keeping your own records of any money, food, or supplies that you receive from any pharmaceutical company, and making certain that it is in fact your information – and not someone else’s – that is published. (The CMS initially released a free smartphone application to facilitate that independent record-keeping process, but the app apparently is no longer available.)
Since all data must be reported to the CMS by March 31 annually, you need to set aside some time each April or May to review this information. If you have many (or complex) industry relationships, you should probably contact each manufacturer in January or February and ask to see the information before it is submitted. Then, review it again after the CMS gets it, to be sure that nothing has changed. You do have 2 years after the data go live to pursue corrections, but in the interim, the incorrect information remains online; so it’s best to fix it in advance of publication.
If you don’t see drug reps, accept office lunches, attend industry dinners, or give sponsored talks, don’t assume that you are not included in the database. Check anyway; you might be indirectly involved in a compensation that you were not aware of, or you may have been reported in error.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
*California, Colorado, Maine, Massachusetts, Minnesota, Vermont, West Virginia, and the District of Columbia had their own laws in place addressing industry relationships with providers before the ACA was enacted. Maine repealed its law in 2011.
You may recall that . The intent was to make relationships between pharmaceutical manufacturers and health care providers more transparent, by requiring the manufacturers to report to the Centers for Medicare & Medicaid Services all payments and other “transfers of value” provided to physicians and teaching hospitals.
Since the CMS has been collecting this information (and publishing it online each September) for 5 years now, I thought I would have a look at what has been learned to date, and what may have changed as a result.
Not much, apparently. In 2014, I predicted that attorneys, activists, and the occasional investigative reporter might peruse the data for their own purposes, but the general public would have little curiosity or use for the information. That appears to be the case thus far; there is no evidence that significant numbers of ordinary citizens have looked at the data or drawn any conclusions from it, perhaps because of the difficulty in accessing it (the website was widely panned when it debuted, although improvements have since been made); or perhaps because neither the CMS nor anyone else has offered the public any assistance in interpreting the raw data. Whether patients think less of doctors who accept an occasional industry-sponsored lunch for their employees, or think more (or less) of those who educate other providers or conduct clinical research, remain open questions.
One measurable – and probably unintended – consequence has been the increasing reluctance of physicians to provide legitimate feedback, or otherwise interact at all with industry, probably out of fear that they might one day have to explain a payment that could be construed by someone with an ax to grind as a conflict of interest. This is a shame, since there is no better way to develop new therapies, or to design solutions to the huge problems facing modern health care, than to actively involve doctors.
Furthermore, it is not clear how well the industry has complied with the law, or how effectively the government is enforcing it. The law authorizes fines of up to $150,000 annually, rising to $1 million for intentional violations; and while Vermont announced in late 2013 that it had levied 25 fines totaling $61,250 for violations of its somewhat stricter version of the statute, I could find no evidence of any similar enforcement by the CMS or any of the other states with standalone conflict of interest laws.*
All of that said, the law’s questionable impact and apparent lack of enforcement do not mean you can ignore it. Increased transparency and scrutiny of physician financial interests apparently are here to stay. The data are still being collected and displayed for anyone to see, so you still want to be certain that what is reported about you is accurate. This means keeping your own records of any money, food, or supplies that you receive from any pharmaceutical company, and making certain that it is in fact your information – and not someone else’s – that is published. (The CMS initially released a free smartphone application to facilitate that independent record-keeping process, but the app apparently is no longer available.)
Since all data must be reported to the CMS by March 31 annually, you need to set aside some time each April or May to review this information. If you have many (or complex) industry relationships, you should probably contact each manufacturer in January or February and ask to see the information before it is submitted. Then, review it again after the CMS gets it, to be sure that nothing has changed. You do have 2 years after the data go live to pursue corrections, but in the interim, the incorrect information remains online; so it’s best to fix it in advance of publication.
If you don’t see drug reps, accept office lunches, attend industry dinners, or give sponsored talks, don’t assume that you are not included in the database. Check anyway; you might be indirectly involved in a compensation that you were not aware of, or you may have been reported in error.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
*California, Colorado, Maine, Massachusetts, Minnesota, Vermont, West Virginia, and the District of Columbia had their own laws in place addressing industry relationships with providers before the ACA was enacted. Maine repealed its law in 2011.
You may recall that . The intent was to make relationships between pharmaceutical manufacturers and health care providers more transparent, by requiring the manufacturers to report to the Centers for Medicare & Medicaid Services all payments and other “transfers of value” provided to physicians and teaching hospitals.
Since the CMS has been collecting this information (and publishing it online each September) for 5 years now, I thought I would have a look at what has been learned to date, and what may have changed as a result.
Not much, apparently. In 2014, I predicted that attorneys, activists, and the occasional investigative reporter might peruse the data for their own purposes, but the general public would have little curiosity or use for the information. That appears to be the case thus far; there is no evidence that significant numbers of ordinary citizens have looked at the data or drawn any conclusions from it, perhaps because of the difficulty in accessing it (the website was widely panned when it debuted, although improvements have since been made); or perhaps because neither the CMS nor anyone else has offered the public any assistance in interpreting the raw data. Whether patients think less of doctors who accept an occasional industry-sponsored lunch for their employees, or think more (or less) of those who educate other providers or conduct clinical research, remain open questions.
One measurable – and probably unintended – consequence has been the increasing reluctance of physicians to provide legitimate feedback, or otherwise interact at all with industry, probably out of fear that they might one day have to explain a payment that could be construed by someone with an ax to grind as a conflict of interest. This is a shame, since there is no better way to develop new therapies, or to design solutions to the huge problems facing modern health care, than to actively involve doctors.
Furthermore, it is not clear how well the industry has complied with the law, or how effectively the government is enforcing it. The law authorizes fines of up to $150,000 annually, rising to $1 million for intentional violations; and while Vermont announced in late 2013 that it had levied 25 fines totaling $61,250 for violations of its somewhat stricter version of the statute, I could find no evidence of any similar enforcement by the CMS or any of the other states with standalone conflict of interest laws.*
All of that said, the law’s questionable impact and apparent lack of enforcement do not mean you can ignore it. Increased transparency and scrutiny of physician financial interests apparently are here to stay. The data are still being collected and displayed for anyone to see, so you still want to be certain that what is reported about you is accurate. This means keeping your own records of any money, food, or supplies that you receive from any pharmaceutical company, and making certain that it is in fact your information – and not someone else’s – that is published. (The CMS initially released a free smartphone application to facilitate that independent record-keeping process, but the app apparently is no longer available.)
Since all data must be reported to the CMS by March 31 annually, you need to set aside some time each April or May to review this information. If you have many (or complex) industry relationships, you should probably contact each manufacturer in January or February and ask to see the information before it is submitted. Then, review it again after the CMS gets it, to be sure that nothing has changed. You do have 2 years after the data go live to pursue corrections, but in the interim, the incorrect information remains online; so it’s best to fix it in advance of publication.
If you don’t see drug reps, accept office lunches, attend industry dinners, or give sponsored talks, don’t assume that you are not included in the database. Check anyway; you might be indirectly involved in a compensation that you were not aware of, or you may have been reported in error.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
*California, Colorado, Maine, Massachusetts, Minnesota, Vermont, West Virginia, and the District of Columbia had their own laws in place addressing industry relationships with providers before the ACA was enacted. Maine repealed its law in 2011.
For most veterans with PTSD, helping others is a lifeline
I am a former military psychiatrist who has published extensively about posttraumatic stress disorder and other psychological effects of war. Thus, I got sent the news clips many times about a potential candidate for mayor of Kansas City leaving the race to care for himself, his depression, and posttraumatic stress disorder symptoms.
Like many of our readers who are physicians, I have a very mixed response to the former candidate’s news.
On the one hand, kudos to him that he has decided to 1) get the help he says he needs, and 2) go public. On the other hand, I really wish that he did not have to drop out of the race to do so.
There are some parallels with leaving for severe physical illness, such as getting chemotherapy for cancer. However, for example, when Gov. Larry Hogan of Maryland received treatment for his cancer, he stayed in office.
Why can you stay in a race or office with cancer or heart disease but not with the very common psychiatric and treatable condition of PTSD?
I certainly do not know all the reasons the candidate for Kansas City mayor made this decision. He said he is encouraging other veterans to follow his example and get treatment for PTSD. He also alluded to suicidal ideation.
This got me thinking about the concept of needing to leave work to take care of yourself – a decision that is often lauded as both noble and wise. I will not opine much on nobility, other than saying it is always noble to help fellow veterans. Maybe his decision to go public will help other veterans. Hard to say. But I can on opine on wisdom, based on many years of working with veterans with PTSD. I almost always advise them to keep their jobs, if at all possible.
Taking time off from a job you care for actually might increase suicidal thoughts. That is due to less structure in the day, less socialization, and fewer feelings of self-worth. A consequent lack of funds might not help. I have long called holding a good job one of the best mental health interventions, superior to medicine and therapy alone (OK – I am being doctrinaire; there are no placebo-controlled, double blind trials on the topic. But I am also serious.)
In general, Why is it work or saving oneself? In my opinion, work helps to save oneself. Helping others, for most veterans, is a lifeline.
I wonder why he should have to drop out of work to receive treatment. Perhaps he was placed in a residential Veterans Affairs program, which often are 30-60 days long. It is notoriously hard to maintain a job during such treatment.
I believe that we should structure our PTSD therapy so that one can both work and receive appropriate treatment. We need war veterans, with or without PTSD, to run for office. And win.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
I am a former military psychiatrist who has published extensively about posttraumatic stress disorder and other psychological effects of war. Thus, I got sent the news clips many times about a potential candidate for mayor of Kansas City leaving the race to care for himself, his depression, and posttraumatic stress disorder symptoms.
Like many of our readers who are physicians, I have a very mixed response to the former candidate’s news.
On the one hand, kudos to him that he has decided to 1) get the help he says he needs, and 2) go public. On the other hand, I really wish that he did not have to drop out of the race to do so.
There are some parallels with leaving for severe physical illness, such as getting chemotherapy for cancer. However, for example, when Gov. Larry Hogan of Maryland received treatment for his cancer, he stayed in office.
Why can you stay in a race or office with cancer or heart disease but not with the very common psychiatric and treatable condition of PTSD?
I certainly do not know all the reasons the candidate for Kansas City mayor made this decision. He said he is encouraging other veterans to follow his example and get treatment for PTSD. He also alluded to suicidal ideation.
This got me thinking about the concept of needing to leave work to take care of yourself – a decision that is often lauded as both noble and wise. I will not opine much on nobility, other than saying it is always noble to help fellow veterans. Maybe his decision to go public will help other veterans. Hard to say. But I can on opine on wisdom, based on many years of working with veterans with PTSD. I almost always advise them to keep their jobs, if at all possible.
Taking time off from a job you care for actually might increase suicidal thoughts. That is due to less structure in the day, less socialization, and fewer feelings of self-worth. A consequent lack of funds might not help. I have long called holding a good job one of the best mental health interventions, superior to medicine and therapy alone (OK – I am being doctrinaire; there are no placebo-controlled, double blind trials on the topic. But I am also serious.)
In general, Why is it work or saving oneself? In my opinion, work helps to save oneself. Helping others, for most veterans, is a lifeline.
I wonder why he should have to drop out of work to receive treatment. Perhaps he was placed in a residential Veterans Affairs program, which often are 30-60 days long. It is notoriously hard to maintain a job during such treatment.
I believe that we should structure our PTSD therapy so that one can both work and receive appropriate treatment. We need war veterans, with or without PTSD, to run for office. And win.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
I am a former military psychiatrist who has published extensively about posttraumatic stress disorder and other psychological effects of war. Thus, I got sent the news clips many times about a potential candidate for mayor of Kansas City leaving the race to care for himself, his depression, and posttraumatic stress disorder symptoms.
Like many of our readers who are physicians, I have a very mixed response to the former candidate’s news.
On the one hand, kudos to him that he has decided to 1) get the help he says he needs, and 2) go public. On the other hand, I really wish that he did not have to drop out of the race to do so.
There are some parallels with leaving for severe physical illness, such as getting chemotherapy for cancer. However, for example, when Gov. Larry Hogan of Maryland received treatment for his cancer, he stayed in office.
Why can you stay in a race or office with cancer or heart disease but not with the very common psychiatric and treatable condition of PTSD?
I certainly do not know all the reasons the candidate for Kansas City mayor made this decision. He said he is encouraging other veterans to follow his example and get treatment for PTSD. He also alluded to suicidal ideation.
This got me thinking about the concept of needing to leave work to take care of yourself – a decision that is often lauded as both noble and wise. I will not opine much on nobility, other than saying it is always noble to help fellow veterans. Maybe his decision to go public will help other veterans. Hard to say. But I can on opine on wisdom, based on many years of working with veterans with PTSD. I almost always advise them to keep their jobs, if at all possible.
Taking time off from a job you care for actually might increase suicidal thoughts. That is due to less structure in the day, less socialization, and fewer feelings of self-worth. A consequent lack of funds might not help. I have long called holding a good job one of the best mental health interventions, superior to medicine and therapy alone (OK – I am being doctrinaire; there are no placebo-controlled, double blind trials on the topic. But I am also serious.)
In general, Why is it work or saving oneself? In my opinion, work helps to save oneself. Helping others, for most veterans, is a lifeline.
I wonder why he should have to drop out of work to receive treatment. Perhaps he was placed in a residential Veterans Affairs program, which often are 30-60 days long. It is notoriously hard to maintain a job during such treatment.
I believe that we should structure our PTSD therapy so that one can both work and receive appropriate treatment. We need war veterans, with or without PTSD, to run for office. And win.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
Real-world data, machine learning, and the reemergence of humanism
As we relentlessly enter information into our EHRs, we typically perceive that we are just recording information about our patients to provide continuity of care and have an accurate representation of what was done. While that is true, the information we record is now increasingly being examined for many additional purposes. A whole new area of study has emerged over the last few years known as “real-world data,” and innovators are beginning to explore how machine learning (currently employed in other areas by such companies as Amazon and Google) may be used to improve the care of patients. The information we are putting into our EHRs is being translated into discrete data and is then combined with data from labs, pharmacies, and claims databases to examine how medications actually work when used in the wide and wild world of practice.
Let’s first talk about why real-world data are important. Traditionally, the evidence we rely upon in medicine has come from randomized trials to give us an unbiased assessment about the safety and the efficacy of the medications that we use. The Achilles’ heel of randomized trials is that, by their nature, they employ a carefully defined group of patients – with specific inclusion and exclusion criteria – who may not be like the patients in our practices. Randomized trials are also conducted in sites that are different than most of our offices. The clinics where randomized trials are conducted have dedicated personnel to follow up on patients, to make sure that patients take their medications, and ensure that patients remember their follow up visits. What this means is that the results in of those studies might not reflect the results seen in the real world.
A nice example of this was reported recently in the area of diabetes management. Randomized trials have shown that the glucagonlike peptide–1 (GLP-1) class of medications have about twice the effectiveness in lowering hemoglobin A1c as do the dipeptidyl peptidase–4 (DPP-4) inhibitor class of medications, but that difference in efficacy is not seen in practice. When looked at in real-world studies, the two classes of medications have about the same glucose-lowering efficacy. Why might that be? In reality, it might be that compliance with GLP-1s is less than that of DPP-4s because of the side effects of nausea and GI intolerance. When patients miss more doses of their GLP-1, they do not achieve the HbA1c lowering seen in trials in which compliance is far better.1
This exploration of real-world outcomes is just a first step in using the information documented in our charts. The exciting next step will be machine learning, also called deep learning.2 In this process, computers look at an enormous number of data points and find relationships that would otherwise not be detected. Imagine a supercomputer analyzing every blood pressure after any medication is changed across thousands, or even millions, of patients, and linking the outcome of that medication choice with the next blood pressure.3 Then imagine the computer meshing millions of data points that include all patients’ weights, ages, sexes, family histories of cardiovascular disease, renal function, etc. and matching those parameters with the specific medication and follow-up blood pressures. While much has been discussed about using genetics to advance personalized medicine, one can imagine these machine-based algorithms discovering connections about which medications work best for individuals with specific characteristics – without the need for additional testing. When the final loop of this cascade is connected, the computer could present recommendations to the clinician about which medication is optimal for the patient and then refine these recommendations, based on outcomes, to optimize safety and efficacy.
Some have argued that there is no way a computer will be able to perform as well as an experienced clinician who utilizes a combination of data and intuition to choose the best medication for his or her patient. This argument is similar to the controversy over autonomous driving cars. Many have asked how you can be assured that the cars will never have an accident. That is, of course, the wrong question. The correct question, as articulated very nicely by one of the innovators in that field, George Holtz, is how we can make a car that is safer than the way that cars are currently being driven (which means fewer deaths than the 15,000 that occur annually with humans behind the wheel).4
Our current method of providing care often leaves patients without appropriate guideline-recommended medications, and many don’t reach their HbA1c, blood pressure, cholesterol, and asthma-control goals. The era of machine learning with machine-generated algorithms may be much closer than we think, which will allow us to spend more time talking with patients, educating them about their disease, and supporting them in their efforts to remain healthy – an attractive future for both us and our patients.
References
1. Carls GS et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017 Nov;40(11):1469-78.
2. Naylor CD. On the prospects for a (deep) learning health care system. JAMA. 2018 Sep 18;320(11):1099-100.
3. Wang YR et al. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007 Jan 22;167(2):141-7.
4. Super Hacker George Hotz: “I can make your car drive itself for under $1,000.”
As we relentlessly enter information into our EHRs, we typically perceive that we are just recording information about our patients to provide continuity of care and have an accurate representation of what was done. While that is true, the information we record is now increasingly being examined for many additional purposes. A whole new area of study has emerged over the last few years known as “real-world data,” and innovators are beginning to explore how machine learning (currently employed in other areas by such companies as Amazon and Google) may be used to improve the care of patients. The information we are putting into our EHRs is being translated into discrete data and is then combined with data from labs, pharmacies, and claims databases to examine how medications actually work when used in the wide and wild world of practice.
Let’s first talk about why real-world data are important. Traditionally, the evidence we rely upon in medicine has come from randomized trials to give us an unbiased assessment about the safety and the efficacy of the medications that we use. The Achilles’ heel of randomized trials is that, by their nature, they employ a carefully defined group of patients – with specific inclusion and exclusion criteria – who may not be like the patients in our practices. Randomized trials are also conducted in sites that are different than most of our offices. The clinics where randomized trials are conducted have dedicated personnel to follow up on patients, to make sure that patients take their medications, and ensure that patients remember their follow up visits. What this means is that the results in of those studies might not reflect the results seen in the real world.
A nice example of this was reported recently in the area of diabetes management. Randomized trials have shown that the glucagonlike peptide–1 (GLP-1) class of medications have about twice the effectiveness in lowering hemoglobin A1c as do the dipeptidyl peptidase–4 (DPP-4) inhibitor class of medications, but that difference in efficacy is not seen in practice. When looked at in real-world studies, the two classes of medications have about the same glucose-lowering efficacy. Why might that be? In reality, it might be that compliance with GLP-1s is less than that of DPP-4s because of the side effects of nausea and GI intolerance. When patients miss more doses of their GLP-1, they do not achieve the HbA1c lowering seen in trials in which compliance is far better.1
This exploration of real-world outcomes is just a first step in using the information documented in our charts. The exciting next step will be machine learning, also called deep learning.2 In this process, computers look at an enormous number of data points and find relationships that would otherwise not be detected. Imagine a supercomputer analyzing every blood pressure after any medication is changed across thousands, or even millions, of patients, and linking the outcome of that medication choice with the next blood pressure.3 Then imagine the computer meshing millions of data points that include all patients’ weights, ages, sexes, family histories of cardiovascular disease, renal function, etc. and matching those parameters with the specific medication and follow-up blood pressures. While much has been discussed about using genetics to advance personalized medicine, one can imagine these machine-based algorithms discovering connections about which medications work best for individuals with specific characteristics – without the need for additional testing. When the final loop of this cascade is connected, the computer could present recommendations to the clinician about which medication is optimal for the patient and then refine these recommendations, based on outcomes, to optimize safety and efficacy.
Some have argued that there is no way a computer will be able to perform as well as an experienced clinician who utilizes a combination of data and intuition to choose the best medication for his or her patient. This argument is similar to the controversy over autonomous driving cars. Many have asked how you can be assured that the cars will never have an accident. That is, of course, the wrong question. The correct question, as articulated very nicely by one of the innovators in that field, George Holtz, is how we can make a car that is safer than the way that cars are currently being driven (which means fewer deaths than the 15,000 that occur annually with humans behind the wheel).4
Our current method of providing care often leaves patients without appropriate guideline-recommended medications, and many don’t reach their HbA1c, blood pressure, cholesterol, and asthma-control goals. The era of machine learning with machine-generated algorithms may be much closer than we think, which will allow us to spend more time talking with patients, educating them about their disease, and supporting them in their efforts to remain healthy – an attractive future for both us and our patients.
References
1. Carls GS et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017 Nov;40(11):1469-78.
2. Naylor CD. On the prospects for a (deep) learning health care system. JAMA. 2018 Sep 18;320(11):1099-100.
3. Wang YR et al. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007 Jan 22;167(2):141-7.
4. Super Hacker George Hotz: “I can make your car drive itself for under $1,000.”
As we relentlessly enter information into our EHRs, we typically perceive that we are just recording information about our patients to provide continuity of care and have an accurate representation of what was done. While that is true, the information we record is now increasingly being examined for many additional purposes. A whole new area of study has emerged over the last few years known as “real-world data,” and innovators are beginning to explore how machine learning (currently employed in other areas by such companies as Amazon and Google) may be used to improve the care of patients. The information we are putting into our EHRs is being translated into discrete data and is then combined with data from labs, pharmacies, and claims databases to examine how medications actually work when used in the wide and wild world of practice.
Let’s first talk about why real-world data are important. Traditionally, the evidence we rely upon in medicine has come from randomized trials to give us an unbiased assessment about the safety and the efficacy of the medications that we use. The Achilles’ heel of randomized trials is that, by their nature, they employ a carefully defined group of patients – with specific inclusion and exclusion criteria – who may not be like the patients in our practices. Randomized trials are also conducted in sites that are different than most of our offices. The clinics where randomized trials are conducted have dedicated personnel to follow up on patients, to make sure that patients take their medications, and ensure that patients remember their follow up visits. What this means is that the results in of those studies might not reflect the results seen in the real world.
A nice example of this was reported recently in the area of diabetes management. Randomized trials have shown that the glucagonlike peptide–1 (GLP-1) class of medications have about twice the effectiveness in lowering hemoglobin A1c as do the dipeptidyl peptidase–4 (DPP-4) inhibitor class of medications, but that difference in efficacy is not seen in practice. When looked at in real-world studies, the two classes of medications have about the same glucose-lowering efficacy. Why might that be? In reality, it might be that compliance with GLP-1s is less than that of DPP-4s because of the side effects of nausea and GI intolerance. When patients miss more doses of their GLP-1, they do not achieve the HbA1c lowering seen in trials in which compliance is far better.1
This exploration of real-world outcomes is just a first step in using the information documented in our charts. The exciting next step will be machine learning, also called deep learning.2 In this process, computers look at an enormous number of data points and find relationships that would otherwise not be detected. Imagine a supercomputer analyzing every blood pressure after any medication is changed across thousands, or even millions, of patients, and linking the outcome of that medication choice with the next blood pressure.3 Then imagine the computer meshing millions of data points that include all patients’ weights, ages, sexes, family histories of cardiovascular disease, renal function, etc. and matching those parameters with the specific medication and follow-up blood pressures. While much has been discussed about using genetics to advance personalized medicine, one can imagine these machine-based algorithms discovering connections about which medications work best for individuals with specific characteristics – without the need for additional testing. When the final loop of this cascade is connected, the computer could present recommendations to the clinician about which medication is optimal for the patient and then refine these recommendations, based on outcomes, to optimize safety and efficacy.
Some have argued that there is no way a computer will be able to perform as well as an experienced clinician who utilizes a combination of data and intuition to choose the best medication for his or her patient. This argument is similar to the controversy over autonomous driving cars. Many have asked how you can be assured that the cars will never have an accident. That is, of course, the wrong question. The correct question, as articulated very nicely by one of the innovators in that field, George Holtz, is how we can make a car that is safer than the way that cars are currently being driven (which means fewer deaths than the 15,000 that occur annually with humans behind the wheel).4
Our current method of providing care often leaves patients without appropriate guideline-recommended medications, and many don’t reach their HbA1c, blood pressure, cholesterol, and asthma-control goals. The era of machine learning with machine-generated algorithms may be much closer than we think, which will allow us to spend more time talking with patients, educating them about their disease, and supporting them in their efforts to remain healthy – an attractive future for both us and our patients.
References
1. Carls GS et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017 Nov;40(11):1469-78.
2. Naylor CD. On the prospects for a (deep) learning health care system. JAMA. 2018 Sep 18;320(11):1099-100.
3. Wang YR et al. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007 Jan 22;167(2):141-7.
4. Super Hacker George Hotz: “I can make your car drive itself for under $1,000.”