User login
What is HIPEC?
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Grind it out
“And five more, four more, three more, two more, one more, and done!” Just when you thought you could not stand the searing pain any longer, it ends. Your spin instructor is not only helping you be fit, she is also teaching you an important lesson for life: Sometimes you just need to grind it out.
. College basketball teams need to simply grind it out to advance in the NCAA championship tournament. How might Tiger Woods recover from a disastrous few holes at the Masters? “He’ll just have to grind it out on the back nine.” How will you finally finish your PhD thesis? You’ll have to grind it out this month. It’s how I’m writing this column, how I got my taxes in on time, and, sometimes, how I get through clinic.
The phrase is used to describe something which needs to be done that is tedious, laborious, or joyless. Although the outcome of grinding it out is always pleasant, the task is often considered arduous.
In my dermatology practice, patient demand came in like a lion this March, and to meet our awesome access goals, we needed to add clinics on Saturdays, early mornings, and even a few nights. We met our goal, with supply to spare, and felt proud of our accomplishments. Physician wellness gurus (this author not included) say that, to avoid burnout from such excess work, you must find meaning in your work. Be grateful to help that 24-year-old with acne at 8:15 p.m. Think about how lucky you are to serve that lawyer with hand dermatitis at 8:45 p.m. Celebrate the mom’s cancer-free skin screening at 9:00 p.m. By finding meaning in our work, we’re told, we can achieve clinic nirvana. Except it doesn’t always work, and sometimes it serves us badly.
For the long days that ended in night clinic last month, I found myself counting down those last few patients – “four more, three more, two more, and last one.” I love my work and care about my patients, but sometimes I just have to grind it out. I’m proud of what I’ve accomplished.
Now it’s on to spin class.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“And five more, four more, three more, two more, one more, and done!” Just when you thought you could not stand the searing pain any longer, it ends. Your spin instructor is not only helping you be fit, she is also teaching you an important lesson for life: Sometimes you just need to grind it out.
. College basketball teams need to simply grind it out to advance in the NCAA championship tournament. How might Tiger Woods recover from a disastrous few holes at the Masters? “He’ll just have to grind it out on the back nine.” How will you finally finish your PhD thesis? You’ll have to grind it out this month. It’s how I’m writing this column, how I got my taxes in on time, and, sometimes, how I get through clinic.
The phrase is used to describe something which needs to be done that is tedious, laborious, or joyless. Although the outcome of grinding it out is always pleasant, the task is often considered arduous.
In my dermatology practice, patient demand came in like a lion this March, and to meet our awesome access goals, we needed to add clinics on Saturdays, early mornings, and even a few nights. We met our goal, with supply to spare, and felt proud of our accomplishments. Physician wellness gurus (this author not included) say that, to avoid burnout from such excess work, you must find meaning in your work. Be grateful to help that 24-year-old with acne at 8:15 p.m. Think about how lucky you are to serve that lawyer with hand dermatitis at 8:45 p.m. Celebrate the mom’s cancer-free skin screening at 9:00 p.m. By finding meaning in our work, we’re told, we can achieve clinic nirvana. Except it doesn’t always work, and sometimes it serves us badly.
For the long days that ended in night clinic last month, I found myself counting down those last few patients – “four more, three more, two more, and last one.” I love my work and care about my patients, but sometimes I just have to grind it out. I’m proud of what I’ve accomplished.
Now it’s on to spin class.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“And five more, four more, three more, two more, one more, and done!” Just when you thought you could not stand the searing pain any longer, it ends. Your spin instructor is not only helping you be fit, she is also teaching you an important lesson for life: Sometimes you just need to grind it out.
. College basketball teams need to simply grind it out to advance in the NCAA championship tournament. How might Tiger Woods recover from a disastrous few holes at the Masters? “He’ll just have to grind it out on the back nine.” How will you finally finish your PhD thesis? You’ll have to grind it out this month. It’s how I’m writing this column, how I got my taxes in on time, and, sometimes, how I get through clinic.
The phrase is used to describe something which needs to be done that is tedious, laborious, or joyless. Although the outcome of grinding it out is always pleasant, the task is often considered arduous.
In my dermatology practice, patient demand came in like a lion this March, and to meet our awesome access goals, we needed to add clinics on Saturdays, early mornings, and even a few nights. We met our goal, with supply to spare, and felt proud of our accomplishments. Physician wellness gurus (this author not included) say that, to avoid burnout from such excess work, you must find meaning in your work. Be grateful to help that 24-year-old with acne at 8:15 p.m. Think about how lucky you are to serve that lawyer with hand dermatitis at 8:45 p.m. Celebrate the mom’s cancer-free skin screening at 9:00 p.m. By finding meaning in our work, we’re told, we can achieve clinic nirvana. Except it doesn’t always work, and sometimes it serves us badly.
For the long days that ended in night clinic last month, I found myself counting down those last few patients – “four more, three more, two more, and last one.” I love my work and care about my patients, but sometimes I just have to grind it out. I’m proud of what I’ve accomplished.
Now it’s on to spin class.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Distrust
The odds are that you are an employee. In 2016, for the first time ever, fewer than half of physicians in this country owned their own practice. There are numerous explanations for this shift away from independent ownership. But the bottom line is that more physicians are employees than owners (“For the first time, physician practice owners are not the majority,” By Brendan Murphy, AMA Wire, May 31, 2017). The transition to employee status doesn’t always go well.
While an increasing number of physicians are uninterested in or maybe even intimidated by the challenges of practice ownership, they seem to be even less interested in accepting the uncomfortable realities that can be associated with being an employee.
Practice ownership comes with a host of worries including cash flow, staffing, and overhead. On the other hand, an employee has only one critical concern: Can she trust her employer? You may not have considered your relationship with your employer in terms of trust. But I urge you to look at a recent commentary in Clinician Reviews by Randy D. Danielson, PhD, PA, DAAPA, titled, “Do You Trust Your Employer? (2018 Apr;28[4]:6-8). Dr. Danielson relates the experiences of a colleague who complains that the organization for which he worked completely lacked transparency of its goals and failed to provide accurate financial data. This combination of deficiencies prevented “providers from making a positive impact on cost containment.” The colleague added that the organization’s complex compensation formulas did “not account for the vagaries and complexities of health care.”
Do any of these complaints sound familiar to you? Do you share the same lack of trust in your employer that this provider has voiced? The remainder of Dr. Danielson’s commentary is a discussion of the concept of organizational trust and includes this unsurprising observation: “Lack of trust, particularly between management and employers, creates a hostile work environment in which stress levels are high and productivity is reduced.” It makes one wonder how much of the burnout epidemic among physicians and other providers might be the result of organizational distrust.
At what point in your career did you begin to lose trust in your employer? In retrospect, should you have been more diligent in researching its financial history? How did its acquisitions and reorganizations affect its employees? Did they reflect a pattern that is consistent with your philosophy about how and to whom health care should be delivered?
How carefully did you interview the organization’s employees? Did you sense any distrust? This kind of information doesn’t usually seep out in a 1-day visit and meetings with handpicked employees. Did employees feel that there was sufficient transparency? It is likely that they sat on committees. But did those committees have a voice that was heard and acted upon?
If you were going to purchase a practice you would have done hours, days, and weeks of due diligence before signing a purchase and sales agreement. Deciding whether or not to sign a contract with an employer demands an equivalent amount of research and investigation. You already may have discovered that being trapped by a noncompete clause with an organization you don’t trust can put you on the fast track to burnout.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The odds are that you are an employee. In 2016, for the first time ever, fewer than half of physicians in this country owned their own practice. There are numerous explanations for this shift away from independent ownership. But the bottom line is that more physicians are employees than owners (“For the first time, physician practice owners are not the majority,” By Brendan Murphy, AMA Wire, May 31, 2017). The transition to employee status doesn’t always go well.
While an increasing number of physicians are uninterested in or maybe even intimidated by the challenges of practice ownership, they seem to be even less interested in accepting the uncomfortable realities that can be associated with being an employee.
Practice ownership comes with a host of worries including cash flow, staffing, and overhead. On the other hand, an employee has only one critical concern: Can she trust her employer? You may not have considered your relationship with your employer in terms of trust. But I urge you to look at a recent commentary in Clinician Reviews by Randy D. Danielson, PhD, PA, DAAPA, titled, “Do You Trust Your Employer? (2018 Apr;28[4]:6-8). Dr. Danielson relates the experiences of a colleague who complains that the organization for which he worked completely lacked transparency of its goals and failed to provide accurate financial data. This combination of deficiencies prevented “providers from making a positive impact on cost containment.” The colleague added that the organization’s complex compensation formulas did “not account for the vagaries and complexities of health care.”
Do any of these complaints sound familiar to you? Do you share the same lack of trust in your employer that this provider has voiced? The remainder of Dr. Danielson’s commentary is a discussion of the concept of organizational trust and includes this unsurprising observation: “Lack of trust, particularly between management and employers, creates a hostile work environment in which stress levels are high and productivity is reduced.” It makes one wonder how much of the burnout epidemic among physicians and other providers might be the result of organizational distrust.
At what point in your career did you begin to lose trust in your employer? In retrospect, should you have been more diligent in researching its financial history? How did its acquisitions and reorganizations affect its employees? Did they reflect a pattern that is consistent with your philosophy about how and to whom health care should be delivered?
How carefully did you interview the organization’s employees? Did you sense any distrust? This kind of information doesn’t usually seep out in a 1-day visit and meetings with handpicked employees. Did employees feel that there was sufficient transparency? It is likely that they sat on committees. But did those committees have a voice that was heard and acted upon?
If you were going to purchase a practice you would have done hours, days, and weeks of due diligence before signing a purchase and sales agreement. Deciding whether or not to sign a contract with an employer demands an equivalent amount of research and investigation. You already may have discovered that being trapped by a noncompete clause with an organization you don’t trust can put you on the fast track to burnout.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The odds are that you are an employee. In 2016, for the first time ever, fewer than half of physicians in this country owned their own practice. There are numerous explanations for this shift away from independent ownership. But the bottom line is that more physicians are employees than owners (“For the first time, physician practice owners are not the majority,” By Brendan Murphy, AMA Wire, May 31, 2017). The transition to employee status doesn’t always go well.
While an increasing number of physicians are uninterested in or maybe even intimidated by the challenges of practice ownership, they seem to be even less interested in accepting the uncomfortable realities that can be associated with being an employee.
Practice ownership comes with a host of worries including cash flow, staffing, and overhead. On the other hand, an employee has only one critical concern: Can she trust her employer? You may not have considered your relationship with your employer in terms of trust. But I urge you to look at a recent commentary in Clinician Reviews by Randy D. Danielson, PhD, PA, DAAPA, titled, “Do You Trust Your Employer? (2018 Apr;28[4]:6-8). Dr. Danielson relates the experiences of a colleague who complains that the organization for which he worked completely lacked transparency of its goals and failed to provide accurate financial data. This combination of deficiencies prevented “providers from making a positive impact on cost containment.” The colleague added that the organization’s complex compensation formulas did “not account for the vagaries and complexities of health care.”
Do any of these complaints sound familiar to you? Do you share the same lack of trust in your employer that this provider has voiced? The remainder of Dr. Danielson’s commentary is a discussion of the concept of organizational trust and includes this unsurprising observation: “Lack of trust, particularly between management and employers, creates a hostile work environment in which stress levels are high and productivity is reduced.” It makes one wonder how much of the burnout epidemic among physicians and other providers might be the result of organizational distrust.
At what point in your career did you begin to lose trust in your employer? In retrospect, should you have been more diligent in researching its financial history? How did its acquisitions and reorganizations affect its employees? Did they reflect a pattern that is consistent with your philosophy about how and to whom health care should be delivered?
How carefully did you interview the organization’s employees? Did you sense any distrust? This kind of information doesn’t usually seep out in a 1-day visit and meetings with handpicked employees. Did employees feel that there was sufficient transparency? It is likely that they sat on committees. But did those committees have a voice that was heard and acted upon?
If you were going to purchase a practice you would have done hours, days, and weeks of due diligence before signing a purchase and sales agreement. Deciding whether or not to sign a contract with an employer demands an equivalent amount of research and investigation. You already may have discovered that being trapped by a noncompete clause with an organization you don’t trust can put you on the fast track to burnout.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Advanced practice nurses and physician assistants are not the same
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at [email protected]
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at [email protected]
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at [email protected]
Make the Diagnosis - May 2018
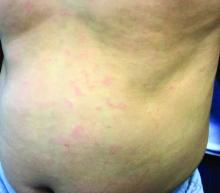
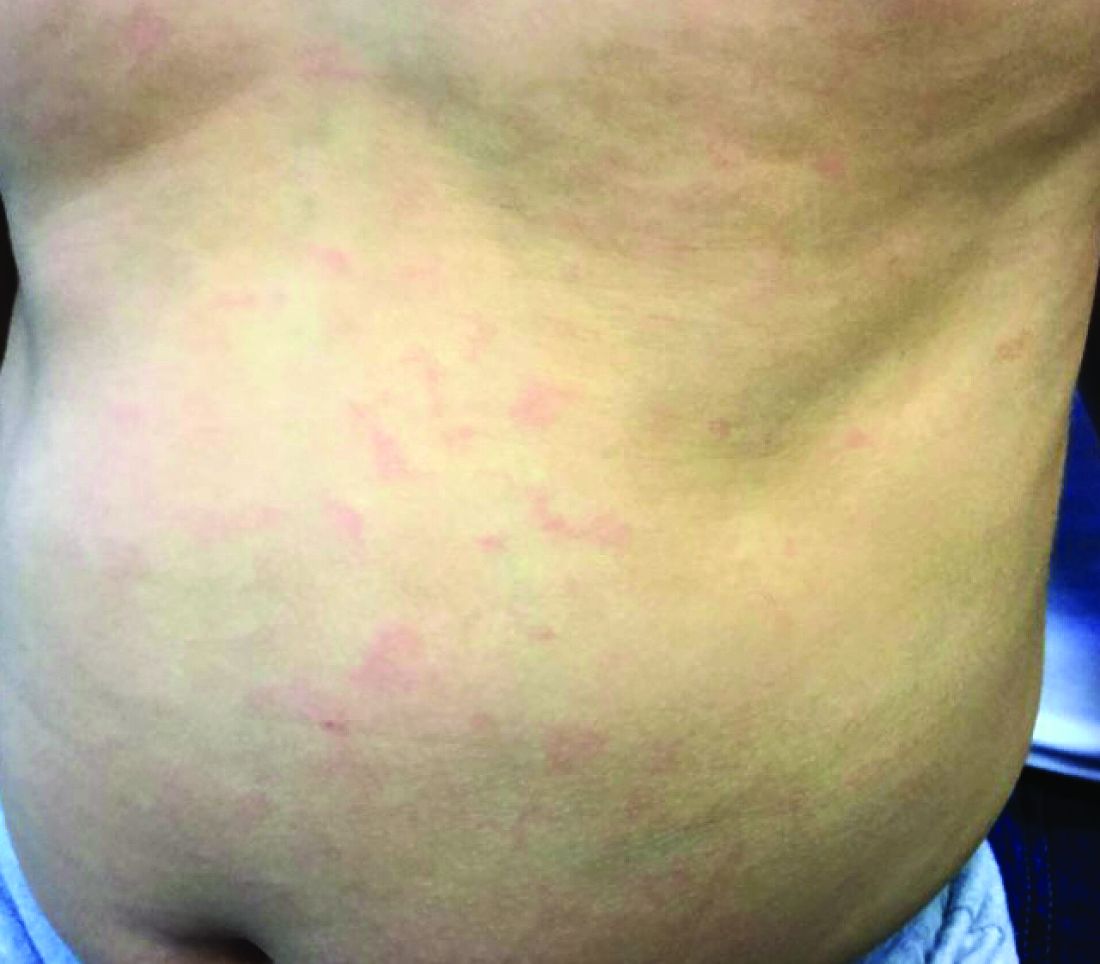
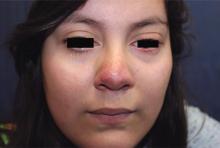
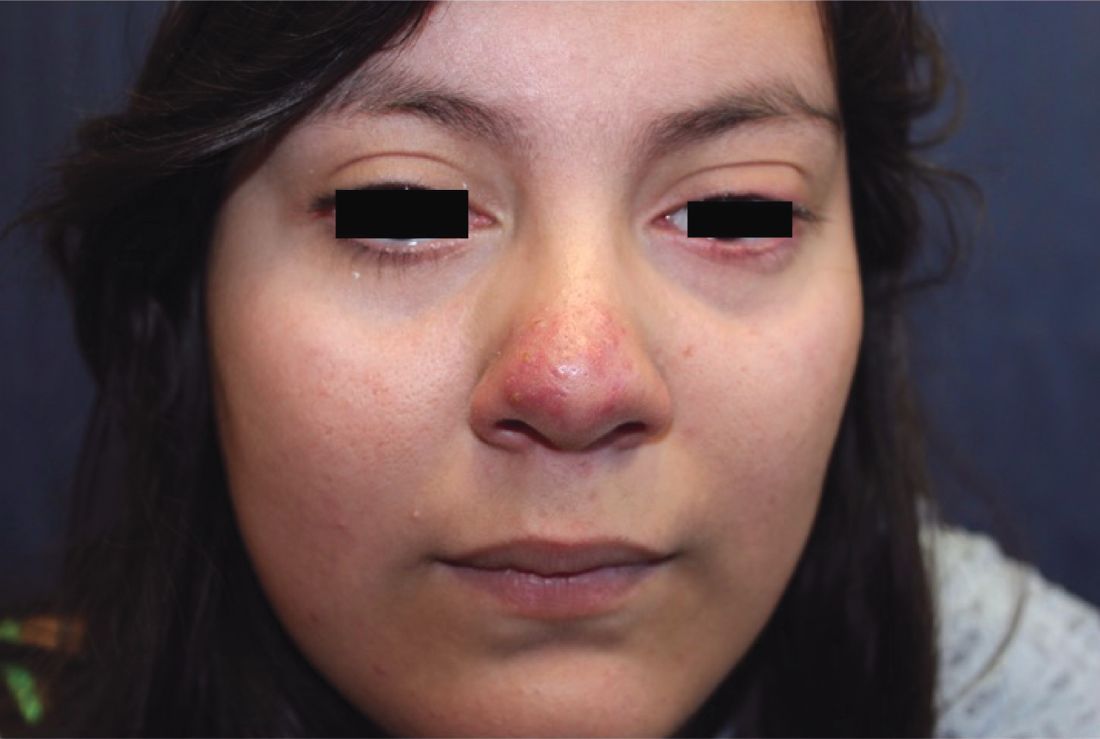
Generally, school-aged children are most often affected. Infections are more likely in late winter and early spring. The virus is spread via respiratory secretions, blood products, and transmission from mother to fetus. The cutaneous findings occur about 10 days after exposure to the virus. By that time, the risk of being contagious is low.
Healthy individuals have no sequelae from fifth disease and require no treatment. However, in patients with hemoglobinopathies, such as sickle cell disease, an aplastic crisis can be triggered. In patients with deficient immune systems, parvovirus B19 may cause infection and anemia, requiring hospitalization. Pregnant women exposed to parvovirus B19 are at risk for hydrops fetalis and rarely, fetal malformations or fetal demise. Other uncommon associations include hepatitis, vasculitides, and neurologic disease.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected]. This case and photo were submitted by Dr. Bilu Martin.
Generally, school-aged children are most often affected. Infections are more likely in late winter and early spring. The virus is spread via respiratory secretions, blood products, and transmission from mother to fetus. The cutaneous findings occur about 10 days after exposure to the virus. By that time, the risk of being contagious is low.
Healthy individuals have no sequelae from fifth disease and require no treatment. However, in patients with hemoglobinopathies, such as sickle cell disease, an aplastic crisis can be triggered. In patients with deficient immune systems, parvovirus B19 may cause infection and anemia, requiring hospitalization. Pregnant women exposed to parvovirus B19 are at risk for hydrops fetalis and rarely, fetal malformations or fetal demise. Other uncommon associations include hepatitis, vasculitides, and neurologic disease.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected]. This case and photo were submitted by Dr. Bilu Martin.
Generally, school-aged children are most often affected. Infections are more likely in late winter and early spring. The virus is spread via respiratory secretions, blood products, and transmission from mother to fetus. The cutaneous findings occur about 10 days after exposure to the virus. By that time, the risk of being contagious is low.
Healthy individuals have no sequelae from fifth disease and require no treatment. However, in patients with hemoglobinopathies, such as sickle cell disease, an aplastic crisis can be triggered. In patients with deficient immune systems, parvovirus B19 may cause infection and anemia, requiring hospitalization. Pregnant women exposed to parvovirus B19 are at risk for hydrops fetalis and rarely, fetal malformations or fetal demise. Other uncommon associations include hepatitis, vasculitides, and neurologic disease.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected]. This case and photo were submitted by Dr. Bilu Martin.
Homework
How do you feel about homework? Do you think your school-age patients are given too much homework? Would they be better off spending their after-school time at home in free play or exploring nonacademic interests? Or, do you feel the school day is too short to adequately cover what a well-educated child needs to know? Doesn’t homework foster good independent work habits and discipline?
Do you have fond memories of doing homework? Are you glad those days of bringing home an hour or 3 of extra work are behind you? Maybe they aren’t behind you. Are you still spending an hour or more getting stuff done at home you didn’t get done in the office?
Primary care pediatrics has never promised its practitioners that they will arrive at home at the end of the workday free of unfinished business. If you have after-hours call responsibilities, there always have been phone calls, decisions to make, and trips to EDs and delivery rooms. Even if you are fortunate enough to not have after-hours call responsibilities, there are certainly evenings when you are nagged by second thoughts and worries about troublesome patients you have seen during the day. Did you make the correct diagnosis or forget to order a critical lab test?
This kind of homework is expected. It’s what you signed up for. But with experience, you learn how to provide better anticipatory guidance that can decrease the number of after-hours calls. You can minimize, but never eliminate, second-guessing by learning to make wiser diagnostic and therapeutic decisions.
However, arriving home with a laptop or notebook loaded with unfinished electronic health records and work-related emails is not what you thought primary care pediatrics was about ... and it didn’t used to be. For the first 35 years of practice, when I saw my last patient, my office work was over. If I wasn’t on call, I could enjoy the entire evening with my family uninterrupted.
But change happens. Coincident with the launch of a new computer system, my workday became an hour longer so that I could complete my electronic office notes before I went home. For some of my colleagues, this unwelcome addition ran more than an hour and a half or 2 hours, and many of them leapt at the practice administrator’s offer to link their home computers with our new office EHR. Buried in what sounded like a good deal to them, I could hear the creaky opening of a Pandora’s box.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How do you feel about homework? Do you think your school-age patients are given too much homework? Would they be better off spending their after-school time at home in free play or exploring nonacademic interests? Or, do you feel the school day is too short to adequately cover what a well-educated child needs to know? Doesn’t homework foster good independent work habits and discipline?
Do you have fond memories of doing homework? Are you glad those days of bringing home an hour or 3 of extra work are behind you? Maybe they aren’t behind you. Are you still spending an hour or more getting stuff done at home you didn’t get done in the office?
Primary care pediatrics has never promised its practitioners that they will arrive at home at the end of the workday free of unfinished business. If you have after-hours call responsibilities, there always have been phone calls, decisions to make, and trips to EDs and delivery rooms. Even if you are fortunate enough to not have after-hours call responsibilities, there are certainly evenings when you are nagged by second thoughts and worries about troublesome patients you have seen during the day. Did you make the correct diagnosis or forget to order a critical lab test?
This kind of homework is expected. It’s what you signed up for. But with experience, you learn how to provide better anticipatory guidance that can decrease the number of after-hours calls. You can minimize, but never eliminate, second-guessing by learning to make wiser diagnostic and therapeutic decisions.
However, arriving home with a laptop or notebook loaded with unfinished electronic health records and work-related emails is not what you thought primary care pediatrics was about ... and it didn’t used to be. For the first 35 years of practice, when I saw my last patient, my office work was over. If I wasn’t on call, I could enjoy the entire evening with my family uninterrupted.
But change happens. Coincident with the launch of a new computer system, my workday became an hour longer so that I could complete my electronic office notes before I went home. For some of my colleagues, this unwelcome addition ran more than an hour and a half or 2 hours, and many of them leapt at the practice administrator’s offer to link their home computers with our new office EHR. Buried in what sounded like a good deal to them, I could hear the creaky opening of a Pandora’s box.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How do you feel about homework? Do you think your school-age patients are given too much homework? Would they be better off spending their after-school time at home in free play or exploring nonacademic interests? Or, do you feel the school day is too short to adequately cover what a well-educated child needs to know? Doesn’t homework foster good independent work habits and discipline?
Do you have fond memories of doing homework? Are you glad those days of bringing home an hour or 3 of extra work are behind you? Maybe they aren’t behind you. Are you still spending an hour or more getting stuff done at home you didn’t get done in the office?
Primary care pediatrics has never promised its practitioners that they will arrive at home at the end of the workday free of unfinished business. If you have after-hours call responsibilities, there always have been phone calls, decisions to make, and trips to EDs and delivery rooms. Even if you are fortunate enough to not have after-hours call responsibilities, there are certainly evenings when you are nagged by second thoughts and worries about troublesome patients you have seen during the day. Did you make the correct diagnosis or forget to order a critical lab test?
This kind of homework is expected. It’s what you signed up for. But with experience, you learn how to provide better anticipatory guidance that can decrease the number of after-hours calls. You can minimize, but never eliminate, second-guessing by learning to make wiser diagnostic and therapeutic decisions.
However, arriving home with a laptop or notebook loaded with unfinished electronic health records and work-related emails is not what you thought primary care pediatrics was about ... and it didn’t used to be. For the first 35 years of practice, when I saw my last patient, my office work was over. If I wasn’t on call, I could enjoy the entire evening with my family uninterrupted.
But change happens. Coincident with the launch of a new computer system, my workday became an hour longer so that I could complete my electronic office notes before I went home. For some of my colleagues, this unwelcome addition ran more than an hour and a half or 2 hours, and many of them leapt at the practice administrator’s offer to link their home computers with our new office EHR. Buried in what sounded like a good deal to them, I could hear the creaky opening of a Pandora’s box.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Commentary—Could Prazosin Play a Role in Treating Chronic Posttraumatic Headache?
Headache is a common symptom after any severity traumatic brain injury in the civilian and military populations. Currently, there is no evidence-based treatment protocol for posttraumatic headache, and management largely is based on therapies used in the primary headache disorders.
There is a complex interaction between mood disorders, posttraumatic stress disorder (PTSD), sleep disorders, and headache. Depression and PTSD are frequently seen in civilian and military populations accompanying chronic posttraumatic headache. In civilians, about one-third of patients with posttraumatic headache meet criteria for depression and PTSD. A longitudinal study of Iraq and Afghanistan veterans followed over three years found that co-occurrence of depression, PTSD, or both would increase the risk of chronic posttraumatic headache more than TBI alone. Another meta-analysis of civilian and military TBI found that, though PTSD could affect intensity and severity of chronic posttraumatic headache, TBI was an independent risk factor for chronic posttraumatic headache. PTSD and depression can cause sleep disruption and intensify pain syndromes, including headache.
Though prazosin had been shown to be effective in decreasing nightmares, improving sleep, or decreasing daytime sleepiness in many prior studies, the PACT trial, a randomized, double-blind controlled trial of 304 participants at Veterans Affairs medical centers, did not meet its primary end points of less frequent and less intense trauma-related nightmares, greater improvement in sleep quality, and overall clinical status among veterans assigned to prazosin, compared with veterans assigned to placebo. While disappointing, and surprising given the results of the preceding studies, do these results predict a similar failure in the use of prazosin for treatment of posttraumatic headache?
In an observational study of 126 veterans with blast-related mild TBI during Operation Iraqi Freedom or Operation Enduring Freedom, 82% of participants had co-occurring conditions, including frequent, severe headache, neurologic exam abnormalities, or cognitive disorders. This pilot study found that treatment with prazosin and sleep hygiene counseling improved sleep, but also decreased headache pain and frequency, as well as improved cognitive function over nine weeks. Improvements were maintained for six months. Though difficult to determine the interplay of sleep, posttraumatic headache, and depression, could prazosin independently reduce the burden of headache? Currently, a double-blind, randomized, controlled trial in veterans is examining the effectiveness of prazosin as a preventive agent in treating combat-related posttraumatic headache. This study was scheduled to enroll its last patient at the end of 2017, and results may be out soon.
There may be specific pharmacologic properties that make prazosin a useful drug for headache treatment. Prazosin is a very potent, selective alpha 1-adrenergic antagonist that passes through the blood–brain barrier. It is highly protein bound (97%), so absolute amounts in the CNS are likely to be low. Its use in the treatment of hypertension is based on decreased peripheral vascular resistance as a result of arteriolar and venous receptor blockade. It also can act in the CNS to decrease sympathetic outflow. While an effect on headache could be central, peripheral, or both, other drugs with alpha-adrenergic blocking effects have been used in the treatment of migraine for decades. The ergots, for example, were the first alpha-adrenergic agents to be discovered acting as partial agonists or antagonists at adrenergic, tryptaminergic, and dopaminergic receptors. The hydrogenated ergot alkaloids are among the most potent alpha-adrenergic blocking agents, but adverse effects prevent doses that can cause more than minimal blockade. Chlorpromazine and other dopamine (D2) receptor antagonists, which are highly effective in acute treatment of migraine, particularly with parenteral delivery, also produce significant alpha-adrenergic receptor blockade, while trazodone, amitriptyline, and the atypical antipsychotics, with various levels of alpha-adrenergic antagonism, have found some success in migraine prevention.
Clinical experience has shown that there is wide response variability to acute and chronic medication for migraine. Genetic studies of patients with migraine, though at an early stage, have identified genes involved with vascular and neuronal function. It is likely that clinical observation will be borne out by individual responses to drug classes based on individual genetic profiles, so that subtypes of patients in clinical trial populations may show efficacy based on these profiles. It is likely that prazosin will be useful in certain patient subtypes for the treatment of headache. Posttraumatic headache, which may share some similar pathways of headache physiology with primary headache disorders, adds another layer of response complexity.
—Sylvia Lucas, MD, PhD
Clinical Professor of Neurology and Neurological Surgery
University of Washington
Seattle
Suggested Reading
Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711-719.
Peterlin BL, Nijjar SS, Tietjen GE. Post-traumatic stress disorder and migraine: epidemiology, sex differences, and potential mechanisms. Headache. 2011;51(6):860-868.
Ruff RL, Riechers RG, Wang XF, et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
Headache is a common symptom after any severity traumatic brain injury in the civilian and military populations. Currently, there is no evidence-based treatment protocol for posttraumatic headache, and management largely is based on therapies used in the primary headache disorders.
There is a complex interaction between mood disorders, posttraumatic stress disorder (PTSD), sleep disorders, and headache. Depression and PTSD are frequently seen in civilian and military populations accompanying chronic posttraumatic headache. In civilians, about one-third of patients with posttraumatic headache meet criteria for depression and PTSD. A longitudinal study of Iraq and Afghanistan veterans followed over three years found that co-occurrence of depression, PTSD, or both would increase the risk of chronic posttraumatic headache more than TBI alone. Another meta-analysis of civilian and military TBI found that, though PTSD could affect intensity and severity of chronic posttraumatic headache, TBI was an independent risk factor for chronic posttraumatic headache. PTSD and depression can cause sleep disruption and intensify pain syndromes, including headache.
Though prazosin had been shown to be effective in decreasing nightmares, improving sleep, or decreasing daytime sleepiness in many prior studies, the PACT trial, a randomized, double-blind controlled trial of 304 participants at Veterans Affairs medical centers, did not meet its primary end points of less frequent and less intense trauma-related nightmares, greater improvement in sleep quality, and overall clinical status among veterans assigned to prazosin, compared with veterans assigned to placebo. While disappointing, and surprising given the results of the preceding studies, do these results predict a similar failure in the use of prazosin for treatment of posttraumatic headache?
In an observational study of 126 veterans with blast-related mild TBI during Operation Iraqi Freedom or Operation Enduring Freedom, 82% of participants had co-occurring conditions, including frequent, severe headache, neurologic exam abnormalities, or cognitive disorders. This pilot study found that treatment with prazosin and sleep hygiene counseling improved sleep, but also decreased headache pain and frequency, as well as improved cognitive function over nine weeks. Improvements were maintained for six months. Though difficult to determine the interplay of sleep, posttraumatic headache, and depression, could prazosin independently reduce the burden of headache? Currently, a double-blind, randomized, controlled trial in veterans is examining the effectiveness of prazosin as a preventive agent in treating combat-related posttraumatic headache. This study was scheduled to enroll its last patient at the end of 2017, and results may be out soon.
There may be specific pharmacologic properties that make prazosin a useful drug for headache treatment. Prazosin is a very potent, selective alpha 1-adrenergic antagonist that passes through the blood–brain barrier. It is highly protein bound (97%), so absolute amounts in the CNS are likely to be low. Its use in the treatment of hypertension is based on decreased peripheral vascular resistance as a result of arteriolar and venous receptor blockade. It also can act in the CNS to decrease sympathetic outflow. While an effect on headache could be central, peripheral, or both, other drugs with alpha-adrenergic blocking effects have been used in the treatment of migraine for decades. The ergots, for example, were the first alpha-adrenergic agents to be discovered acting as partial agonists or antagonists at adrenergic, tryptaminergic, and dopaminergic receptors. The hydrogenated ergot alkaloids are among the most potent alpha-adrenergic blocking agents, but adverse effects prevent doses that can cause more than minimal blockade. Chlorpromazine and other dopamine (D2) receptor antagonists, which are highly effective in acute treatment of migraine, particularly with parenteral delivery, also produce significant alpha-adrenergic receptor blockade, while trazodone, amitriptyline, and the atypical antipsychotics, with various levels of alpha-adrenergic antagonism, have found some success in migraine prevention.
Clinical experience has shown that there is wide response variability to acute and chronic medication for migraine. Genetic studies of patients with migraine, though at an early stage, have identified genes involved with vascular and neuronal function. It is likely that clinical observation will be borne out by individual responses to drug classes based on individual genetic profiles, so that subtypes of patients in clinical trial populations may show efficacy based on these profiles. It is likely that prazosin will be useful in certain patient subtypes for the treatment of headache. Posttraumatic headache, which may share some similar pathways of headache physiology with primary headache disorders, adds another layer of response complexity.
—Sylvia Lucas, MD, PhD
Clinical Professor of Neurology and Neurological Surgery
University of Washington
Seattle
Suggested Reading
Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711-719.
Peterlin BL, Nijjar SS, Tietjen GE. Post-traumatic stress disorder and migraine: epidemiology, sex differences, and potential mechanisms. Headache. 2011;51(6):860-868.
Ruff RL, Riechers RG, Wang XF, et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
Headache is a common symptom after any severity traumatic brain injury in the civilian and military populations. Currently, there is no evidence-based treatment protocol for posttraumatic headache, and management largely is based on therapies used in the primary headache disorders.
There is a complex interaction between mood disorders, posttraumatic stress disorder (PTSD), sleep disorders, and headache. Depression and PTSD are frequently seen in civilian and military populations accompanying chronic posttraumatic headache. In civilians, about one-third of patients with posttraumatic headache meet criteria for depression and PTSD. A longitudinal study of Iraq and Afghanistan veterans followed over three years found that co-occurrence of depression, PTSD, or both would increase the risk of chronic posttraumatic headache more than TBI alone. Another meta-analysis of civilian and military TBI found that, though PTSD could affect intensity and severity of chronic posttraumatic headache, TBI was an independent risk factor for chronic posttraumatic headache. PTSD and depression can cause sleep disruption and intensify pain syndromes, including headache.
Though prazosin had been shown to be effective in decreasing nightmares, improving sleep, or decreasing daytime sleepiness in many prior studies, the PACT trial, a randomized, double-blind controlled trial of 304 participants at Veterans Affairs medical centers, did not meet its primary end points of less frequent and less intense trauma-related nightmares, greater improvement in sleep quality, and overall clinical status among veterans assigned to prazosin, compared with veterans assigned to placebo. While disappointing, and surprising given the results of the preceding studies, do these results predict a similar failure in the use of prazosin for treatment of posttraumatic headache?
In an observational study of 126 veterans with blast-related mild TBI during Operation Iraqi Freedom or Operation Enduring Freedom, 82% of participants had co-occurring conditions, including frequent, severe headache, neurologic exam abnormalities, or cognitive disorders. This pilot study found that treatment with prazosin and sleep hygiene counseling improved sleep, but also decreased headache pain and frequency, as well as improved cognitive function over nine weeks. Improvements were maintained for six months. Though difficult to determine the interplay of sleep, posttraumatic headache, and depression, could prazosin independently reduce the burden of headache? Currently, a double-blind, randomized, controlled trial in veterans is examining the effectiveness of prazosin as a preventive agent in treating combat-related posttraumatic headache. This study was scheduled to enroll its last patient at the end of 2017, and results may be out soon.
There may be specific pharmacologic properties that make prazosin a useful drug for headache treatment. Prazosin is a very potent, selective alpha 1-adrenergic antagonist that passes through the blood–brain barrier. It is highly protein bound (97%), so absolute amounts in the CNS are likely to be low. Its use in the treatment of hypertension is based on decreased peripheral vascular resistance as a result of arteriolar and venous receptor blockade. It also can act in the CNS to decrease sympathetic outflow. While an effect on headache could be central, peripheral, or both, other drugs with alpha-adrenergic blocking effects have been used in the treatment of migraine for decades. The ergots, for example, were the first alpha-adrenergic agents to be discovered acting as partial agonists or antagonists at adrenergic, tryptaminergic, and dopaminergic receptors. The hydrogenated ergot alkaloids are among the most potent alpha-adrenergic blocking agents, but adverse effects prevent doses that can cause more than minimal blockade. Chlorpromazine and other dopamine (D2) receptor antagonists, which are highly effective in acute treatment of migraine, particularly with parenteral delivery, also produce significant alpha-adrenergic receptor blockade, while trazodone, amitriptyline, and the atypical antipsychotics, with various levels of alpha-adrenergic antagonism, have found some success in migraine prevention.
Clinical experience has shown that there is wide response variability to acute and chronic medication for migraine. Genetic studies of patients with migraine, though at an early stage, have identified genes involved with vascular and neuronal function. It is likely that clinical observation will be borne out by individual responses to drug classes based on individual genetic profiles, so that subtypes of patients in clinical trial populations may show efficacy based on these profiles. It is likely that prazosin will be useful in certain patient subtypes for the treatment of headache. Posttraumatic headache, which may share some similar pathways of headache physiology with primary headache disorders, adds another layer of response complexity.
—Sylvia Lucas, MD, PhD
Clinical Professor of Neurology and Neurological Surgery
University of Washington
Seattle
Suggested Reading
Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711-719.
Peterlin BL, Nijjar SS, Tietjen GE. Post-traumatic stress disorder and migraine: epidemiology, sex differences, and potential mechanisms. Headache. 2011;51(6):860-868.
Ruff RL, Riechers RG, Wang XF, et al. For veterans with mild traumatic brain injury, improved posttraumatic stress disorder severity and sleep correlated with symptomatic improvement. J Rehabil Res Dev. 2012;49(9):1305-1320.
Cutting Edge Technology in Dermatology: Virtual Reality and Artificial Intelligence
The clinical practice of dermatology is changing at a rapid pace. Advances in technology and new inventions in rapid diagnostics are revolutionizing how physicians approach medical care.
Given these advances, how does today’s dermatologist integrate into the future of the specialty? If leveraged properly, current technologies such as teledermatology and patient portals integrated with electronic medical records can be beneficial to dermatology practices by improving access to care, facilitating triage of patients, and improving communication between patients and health care team members. Herein, we discuss some of the emerging technologies that have the potential to shape clinical dermatology practice and remove barriers to care.
Virtual Reality
Teledermatology can be practiced through live video or, more commonly, via a store-and-forward method in which dermatologists review clinical photographs and the patient's history asynchronously with the in-office visit.6 Virtual reality has the potential to augment teledermatology services by enabling a live, interactive visit that more closely models the traditional face-to-face visit. Virtual reality already is available for patients at home with the use of a commercially marketed headset and a smartphone, and the marriage of virtual reailty and telemedicine has the potential to transform health care.
Virtual reality also can be used to deliver an essential component of the physical examination of a patient: sensory information from palpation. Haptic feedback, also known as haptics, is used to relay force and tactile information to the user of a device (eg, a haptic glove).7 In dermatology, this information pertains to the skin texture, skin profile, and physical properties (eg, stiffness, temperature).8 Assessing the texture of the skin surface can help when distinguishing epidermal processes such as psoriasis versus atopic dermatitis or when evaluating edema, induration, and depth of a leg ulcer.9
One model for conducting a teledermatology encounter that captures sensory information would consist of a haptic probe located at a referring medical provider’s office for examining patients and a master robot that controls the probe located at the consulting dermatologist’s facility.8 Another model converts 2-dimensional images taken from traditional full-body optical imaging systems into virtual 3-dimensional (3D) images that can be felt using a haptic device.10,11 In this method, the user is able to both visualize and touch the skin surface at the same time. Currently, 3D imaging of skin lesions is available in the form of a specialized handheld imager that allows the dermatologist to appreciate the texture and elevation of single lesions when viewing clinical photographs. Additionally, full-body 3D mapping of the skin surface is available for monitoring pigmented lesions or other diseases of the skin.12,13
Artificial Intelligence and Machine Learning
Computer algorithms can be helpful in assisting physicians with disease diagnosis. Machine learning is a subfield of artificial intelligence (AI) in which computer programs learn automatically from experience without explicit programming instructions. A machine learning algorithm uses a labeled data set known as a training set to create a function that can make predictions about new inputs in the future.14 The algorithm can successively compare its predicted outputs with the correct outputs and modify its function as errors are found; for example, a database of images of healthy skin as well as the skin of psoriasis patients can be fed into a machine learning algorithm that picks up features such as color and skin texture from the labeled photographs, allowing it to learn how to diagnose psoriasis.15
In one instance, researchers at Stanford University (Stanford, California) used approximately 130,000 images representing over 2000 different skin diseases in order to train their machine learning algorithm to recognize benign and malignant skin lesions.4 Although the algorithm was able to match the performance of experienced dermatologists in many diagnostic categories, further testing in a real-world clinical setting still needs to be done. In the future, nondermatologists may have the option to consult with decision-support systems that include image analysis software, such as the one developed at Stanford University, for making decisions in triage or diagnosis, which may be critical in areas where access to a dermatologist is limited.4 Future AI systems also may provide supplemental assistance in managing patients to dermatology trainees until they have the experience of a more established dermatologist.
Currently, AI cannot match general human intelligence and life experience; therefore, physicians will continue to make the final decisions when it comes to diagnosis and treatment. In the future, AI algorithms may integrate into clinical dermatology practice, leading to more accurate triage of lesions, potentially streamlined referral to dermatologists for skin conditions that require prompt consultation, and improved quality of care.
Summary
In conclusion, emerging technologies have the power to augment and revolutionize dermatology practice. Savvy dermatologists may incorporate new tools in a way that works for their practice, leading to increased efficiency and improved patient outcomes. Eventually, the technology that is most beneficial to clinical practice will likely be adopted by and integrated into mainstream dermatologic care, making it available for the majority of clinicians to use.
- Health Information Technology for Economic and Clinical Health (HITECH) Act, Pub L No. 111-5, 123 Stat 226 (2009).
- Holmgren AJ, Adler-Milstein J. Health information exchange in US hospitals: the current landscape and a path to improved information sharing. J Hosp Med. 2017;12:193-198.
- The IMLC. Interstate Medical Licensure Compact website. http://www.imlcc.org. Accessed March 21, 2018.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The Associated Press. Microsoft eyes buffer zone in TV airwaves for rural internet. ABC News website. http://abcnews.go.com/amp/Technology/wireStory/microsoft-announces-rural-broadband-initiative-48562282. Published July 11, 2017. Accessed March 21, 2018.
- Practice guidelines for dermatology. American Telemedicine Association website. https://higherlogicdownload.s3.amazonaws.com/AMERICANTELEMED/3c09839a-fffd-46f7-916c-692c11d78933/UploadedImages/SIGs/Teledermatology.Final.pdf. Accessed March 27, 2018. Published April 28, 2016.
- Lee O, Lee K, Oh C, et al. Prototype tactile feedback system for examination by skin touch. Skin Res Technol. 2014;20:307-314.
- Waldron KJ, Enedah C, Gladstone H. Stiffness and texture perception for teledermatology. Stud Health Technol Inform. 2005;111:579-585.
- Cox NH. A literally blinded trial of palpation in dermatologic diagnosis. J Am Acad Dermatol. 2007;56:949-951.
- Kim K. Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol. 2016;22:334-340.
- Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol. 2015;21:164-174.
- How it works. 3Derm website. https://www.3derm.com. Accessed March 21, 2018.
- Vectra 3D. Canfield Scientific website. http://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system. Accessed March 21, 2018.
- Alpaydin E. Introduction to Machine Learning. Cambridge, MA: MIT Press; 2014.
- Shrivastava VK, Londhe ND, Sonawane RS, et al. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
The clinical practice of dermatology is changing at a rapid pace. Advances in technology and new inventions in rapid diagnostics are revolutionizing how physicians approach medical care.
Given these advances, how does today’s dermatologist integrate into the future of the specialty? If leveraged properly, current technologies such as teledermatology and patient portals integrated with electronic medical records can be beneficial to dermatology practices by improving access to care, facilitating triage of patients, and improving communication between patients and health care team members. Herein, we discuss some of the emerging technologies that have the potential to shape clinical dermatology practice and remove barriers to care.
Virtual Reality
Teledermatology can be practiced through live video or, more commonly, via a store-and-forward method in which dermatologists review clinical photographs and the patient's history asynchronously with the in-office visit.6 Virtual reality has the potential to augment teledermatology services by enabling a live, interactive visit that more closely models the traditional face-to-face visit. Virtual reality already is available for patients at home with the use of a commercially marketed headset and a smartphone, and the marriage of virtual reailty and telemedicine has the potential to transform health care.
Virtual reality also can be used to deliver an essential component of the physical examination of a patient: sensory information from palpation. Haptic feedback, also known as haptics, is used to relay force and tactile information to the user of a device (eg, a haptic glove).7 In dermatology, this information pertains to the skin texture, skin profile, and physical properties (eg, stiffness, temperature).8 Assessing the texture of the skin surface can help when distinguishing epidermal processes such as psoriasis versus atopic dermatitis or when evaluating edema, induration, and depth of a leg ulcer.9
One model for conducting a teledermatology encounter that captures sensory information would consist of a haptic probe located at a referring medical provider’s office for examining patients and a master robot that controls the probe located at the consulting dermatologist’s facility.8 Another model converts 2-dimensional images taken from traditional full-body optical imaging systems into virtual 3-dimensional (3D) images that can be felt using a haptic device.10,11 In this method, the user is able to both visualize and touch the skin surface at the same time. Currently, 3D imaging of skin lesions is available in the form of a specialized handheld imager that allows the dermatologist to appreciate the texture and elevation of single lesions when viewing clinical photographs. Additionally, full-body 3D mapping of the skin surface is available for monitoring pigmented lesions or other diseases of the skin.12,13
Artificial Intelligence and Machine Learning
Computer algorithms can be helpful in assisting physicians with disease diagnosis. Machine learning is a subfield of artificial intelligence (AI) in which computer programs learn automatically from experience without explicit programming instructions. A machine learning algorithm uses a labeled data set known as a training set to create a function that can make predictions about new inputs in the future.14 The algorithm can successively compare its predicted outputs with the correct outputs and modify its function as errors are found; for example, a database of images of healthy skin as well as the skin of psoriasis patients can be fed into a machine learning algorithm that picks up features such as color and skin texture from the labeled photographs, allowing it to learn how to diagnose psoriasis.15
In one instance, researchers at Stanford University (Stanford, California) used approximately 130,000 images representing over 2000 different skin diseases in order to train their machine learning algorithm to recognize benign and malignant skin lesions.4 Although the algorithm was able to match the performance of experienced dermatologists in many diagnostic categories, further testing in a real-world clinical setting still needs to be done. In the future, nondermatologists may have the option to consult with decision-support systems that include image analysis software, such as the one developed at Stanford University, for making decisions in triage or diagnosis, which may be critical in areas where access to a dermatologist is limited.4 Future AI systems also may provide supplemental assistance in managing patients to dermatology trainees until they have the experience of a more established dermatologist.
Currently, AI cannot match general human intelligence and life experience; therefore, physicians will continue to make the final decisions when it comes to diagnosis and treatment. In the future, AI algorithms may integrate into clinical dermatology practice, leading to more accurate triage of lesions, potentially streamlined referral to dermatologists for skin conditions that require prompt consultation, and improved quality of care.
Summary
In conclusion, emerging technologies have the power to augment and revolutionize dermatology practice. Savvy dermatologists may incorporate new tools in a way that works for their practice, leading to increased efficiency and improved patient outcomes. Eventually, the technology that is most beneficial to clinical practice will likely be adopted by and integrated into mainstream dermatologic care, making it available for the majority of clinicians to use.
The clinical practice of dermatology is changing at a rapid pace. Advances in technology and new inventions in rapid diagnostics are revolutionizing how physicians approach medical care.
Given these advances, how does today’s dermatologist integrate into the future of the specialty? If leveraged properly, current technologies such as teledermatology and patient portals integrated with electronic medical records can be beneficial to dermatology practices by improving access to care, facilitating triage of patients, and improving communication between patients and health care team members. Herein, we discuss some of the emerging technologies that have the potential to shape clinical dermatology practice and remove barriers to care.
Virtual Reality
Teledermatology can be practiced through live video or, more commonly, via a store-and-forward method in which dermatologists review clinical photographs and the patient's history asynchronously with the in-office visit.6 Virtual reality has the potential to augment teledermatology services by enabling a live, interactive visit that more closely models the traditional face-to-face visit. Virtual reality already is available for patients at home with the use of a commercially marketed headset and a smartphone, and the marriage of virtual reailty and telemedicine has the potential to transform health care.
Virtual reality also can be used to deliver an essential component of the physical examination of a patient: sensory information from palpation. Haptic feedback, also known as haptics, is used to relay force and tactile information to the user of a device (eg, a haptic glove).7 In dermatology, this information pertains to the skin texture, skin profile, and physical properties (eg, stiffness, temperature).8 Assessing the texture of the skin surface can help when distinguishing epidermal processes such as psoriasis versus atopic dermatitis or when evaluating edema, induration, and depth of a leg ulcer.9
One model for conducting a teledermatology encounter that captures sensory information would consist of a haptic probe located at a referring medical provider’s office for examining patients and a master robot that controls the probe located at the consulting dermatologist’s facility.8 Another model converts 2-dimensional images taken from traditional full-body optical imaging systems into virtual 3-dimensional (3D) images that can be felt using a haptic device.10,11 In this method, the user is able to both visualize and touch the skin surface at the same time. Currently, 3D imaging of skin lesions is available in the form of a specialized handheld imager that allows the dermatologist to appreciate the texture and elevation of single lesions when viewing clinical photographs. Additionally, full-body 3D mapping of the skin surface is available for monitoring pigmented lesions or other diseases of the skin.12,13
Artificial Intelligence and Machine Learning
Computer algorithms can be helpful in assisting physicians with disease diagnosis. Machine learning is a subfield of artificial intelligence (AI) in which computer programs learn automatically from experience without explicit programming instructions. A machine learning algorithm uses a labeled data set known as a training set to create a function that can make predictions about new inputs in the future.14 The algorithm can successively compare its predicted outputs with the correct outputs and modify its function as errors are found; for example, a database of images of healthy skin as well as the skin of psoriasis patients can be fed into a machine learning algorithm that picks up features such as color and skin texture from the labeled photographs, allowing it to learn how to diagnose psoriasis.15
In one instance, researchers at Stanford University (Stanford, California) used approximately 130,000 images representing over 2000 different skin diseases in order to train their machine learning algorithm to recognize benign and malignant skin lesions.4 Although the algorithm was able to match the performance of experienced dermatologists in many diagnostic categories, further testing in a real-world clinical setting still needs to be done. In the future, nondermatologists may have the option to consult with decision-support systems that include image analysis software, such as the one developed at Stanford University, for making decisions in triage or diagnosis, which may be critical in areas where access to a dermatologist is limited.4 Future AI systems also may provide supplemental assistance in managing patients to dermatology trainees until they have the experience of a more established dermatologist.
Currently, AI cannot match general human intelligence and life experience; therefore, physicians will continue to make the final decisions when it comes to diagnosis and treatment. In the future, AI algorithms may integrate into clinical dermatology practice, leading to more accurate triage of lesions, potentially streamlined referral to dermatologists for skin conditions that require prompt consultation, and improved quality of care.
Summary
In conclusion, emerging technologies have the power to augment and revolutionize dermatology practice. Savvy dermatologists may incorporate new tools in a way that works for their practice, leading to increased efficiency and improved patient outcomes. Eventually, the technology that is most beneficial to clinical practice will likely be adopted by and integrated into mainstream dermatologic care, making it available for the majority of clinicians to use.
- Health Information Technology for Economic and Clinical Health (HITECH) Act, Pub L No. 111-5, 123 Stat 226 (2009).
- Holmgren AJ, Adler-Milstein J. Health information exchange in US hospitals: the current landscape and a path to improved information sharing. J Hosp Med. 2017;12:193-198.
- The IMLC. Interstate Medical Licensure Compact website. http://www.imlcc.org. Accessed March 21, 2018.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The Associated Press. Microsoft eyes buffer zone in TV airwaves for rural internet. ABC News website. http://abcnews.go.com/amp/Technology/wireStory/microsoft-announces-rural-broadband-initiative-48562282. Published July 11, 2017. Accessed March 21, 2018.
- Practice guidelines for dermatology. American Telemedicine Association website. https://higherlogicdownload.s3.amazonaws.com/AMERICANTELEMED/3c09839a-fffd-46f7-916c-692c11d78933/UploadedImages/SIGs/Teledermatology.Final.pdf. Accessed March 27, 2018. Published April 28, 2016.
- Lee O, Lee K, Oh C, et al. Prototype tactile feedback system for examination by skin touch. Skin Res Technol. 2014;20:307-314.
- Waldron KJ, Enedah C, Gladstone H. Stiffness and texture perception for teledermatology. Stud Health Technol Inform. 2005;111:579-585.
- Cox NH. A literally blinded trial of palpation in dermatologic diagnosis. J Am Acad Dermatol. 2007;56:949-951.
- Kim K. Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol. 2016;22:334-340.
- Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol. 2015;21:164-174.
- How it works. 3Derm website. https://www.3derm.com. Accessed March 21, 2018.
- Vectra 3D. Canfield Scientific website. http://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system. Accessed March 21, 2018.
- Alpaydin E. Introduction to Machine Learning. Cambridge, MA: MIT Press; 2014.
- Shrivastava VK, Londhe ND, Sonawane RS, et al. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
- Health Information Technology for Economic and Clinical Health (HITECH) Act, Pub L No. 111-5, 123 Stat 226 (2009).
- Holmgren AJ, Adler-Milstein J. Health information exchange in US hospitals: the current landscape and a path to improved information sharing. J Hosp Med. 2017;12:193-198.
- The IMLC. Interstate Medical Licensure Compact website. http://www.imlcc.org. Accessed March 21, 2018.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The Associated Press. Microsoft eyes buffer zone in TV airwaves for rural internet. ABC News website. http://abcnews.go.com/amp/Technology/wireStory/microsoft-announces-rural-broadband-initiative-48562282. Published July 11, 2017. Accessed March 21, 2018.
- Practice guidelines for dermatology. American Telemedicine Association website. https://higherlogicdownload.s3.amazonaws.com/AMERICANTELEMED/3c09839a-fffd-46f7-916c-692c11d78933/UploadedImages/SIGs/Teledermatology.Final.pdf. Accessed March 27, 2018. Published April 28, 2016.
- Lee O, Lee K, Oh C, et al. Prototype tactile feedback system for examination by skin touch. Skin Res Technol. 2014;20:307-314.
- Waldron KJ, Enedah C, Gladstone H. Stiffness and texture perception for teledermatology. Stud Health Technol Inform. 2005;111:579-585.
- Cox NH. A literally blinded trial of palpation in dermatologic diagnosis. J Am Acad Dermatol. 2007;56:949-951.
- Kim K. Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol. 2016;22:334-340.
- Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol. 2015;21:164-174.
- How it works. 3Derm website. https://www.3derm.com. Accessed March 21, 2018.
- Vectra 3D. Canfield Scientific website. http://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system. Accessed March 21, 2018.
- Alpaydin E. Introduction to Machine Learning. Cambridge, MA: MIT Press; 2014.
- Shrivastava VK, Londhe ND, Sonawane RS, et al. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
Neuro-Ophthalmology: No Longer Diagnose and Adios
Deborah I. Friedman, MD, MPH
Dr. Friedman is a Professor in the Departments of Neurology and Ophthalmology at the University of Texas Southwestern Medical Center in Dallas.
Neuro-ophthalmology is a small subspecialty that had its origins in ophthalmology but has always included neurologists. National neuro-ophthalmology meetings in North America began with the Rocky Mountain Neuro-Ophthalmology Course in 1975, which led to the formation of the Rocky Mountain Neuro-Ophthalmology Society in 1980. By 1986, it had 240 members and changed its name to the North American Neuro-Ophthalmology Society (NANOS). Already existing was the 1.5-day annual Frank Walsh Society meeting with its clinical-pathological correlation format, which originated at Johns Hopkins Medical School. The two societies merged in 1992. Currently, NANOS has grown to more than 650 members and is the premier organization for neuro-ophthalmology worldwide. The society’s journal, the Journal of Neuro-Ophthalmology, commenced publication in 1994, one year after the inaugural issue of Neurology Reviews.
Overcoming an early reputation for “admiring” disease, neuro-ophthalmology has evolved considerably over the past 25 years, with advances in neuroimaging, genomics, proteomics, office-based technologies, and immunology. The first multicenter, randomized trial in neuro-ophthalmology—the Optic Neuritis Treatment Trial (ONTT),1 published 25 years ago—showed that intravenous methylprednisolone 250 mg QID for three days followed by an 11-day prednisone taper was superior to either oral prednisone (1 mg/kg for 14 days) or oral placebo (14 days), speeding the recovery of vision with slightly better vision at six months. There was a higher rate of relapse in the group assigned to receive oral prednisone. The ONTT was a pivotal event for neuro-ophthalmology, heralding a paradigm shift from observational reporting to randomized clinical trials.
Three years later, the Ischemic Optic Neuropathy Decompression Trial (IONDT)2 showed that optic nerve sheath fenestration surgery, performed within 14 days of symptom onset for acute non-arteritic ischemic optic neuropathy or within 30 days in participants with a progressive course, did not improve visual acuity or visual field compared to careful follow-up and medical management at six months. Enrollees randomized to undergo surgery had a lower rate of improvement and were more likely to lose three or more lines of vision than the control group.
With this momentum, the Neuro-Ophthalmology Research Disease Investigators Consortium was formed to create infrastructure for future clinical trials of neuro-ophthalmic disorders. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT)3 studied patients with idiopathic intracranial hypertension and mild visual field loss. IIHTT confirmed that acetazolamide (500 mg BID, increasing to a maximum dose of 4 grams daily) was superior to placebo treatment when combined with a supervised weight loss program, resulting in improved visual field loss, papilledema grade, weight reduction, lumbar puncture opening pressure, and quality of life. Ongoing research efforts are investigating treatments for Leber hereditary optic neuropathy, non-arteritic ischemic optic neuropathy, thyroid eye disease, rehabilitation for poststroke homonymous hemianopia and giant cell arteritis in adults, and idiopathic intracranial hypertension and optic nerve gliomas in children.
Attendance at the NANOS annual meeting continues to grow, emphasizing scientific as well as clinical neuro-ophthalmic topics. Vibrant and active neuro-ophthalmology societies in other parts of the world (eg, Europe, Japan, Australia, British Isles) contribute to the camaraderie, collegiality, collaboration, and discovery that advance our field.
Happy 25th anniversary to Neurology Reviews! The next 25 years will likely transform the field of neuro-ophthalmology as we better understand the molecular and genetic basis of neuro-ophthalmic disorders. No longer “diagnose and adios,” we anticipate significant therapeutic advancements—and perhaps cures—for individuals with severe, sight-impairing, neuro-ophthalmic conditions.
References
2. The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273(8):625-632.
3. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss. JAMA. 2014;311(16):1641-1651.
Deborah I. Friedman, MD, MPH
Dr. Friedman is a Professor in the Departments of Neurology and Ophthalmology at the University of Texas Southwestern Medical Center in Dallas.
Neuro-ophthalmology is a small subspecialty that had its origins in ophthalmology but has always included neurologists. National neuro-ophthalmology meetings in North America began with the Rocky Mountain Neuro-Ophthalmology Course in 1975, which led to the formation of the Rocky Mountain Neuro-Ophthalmology Society in 1980. By 1986, it had 240 members and changed its name to the North American Neuro-Ophthalmology Society (NANOS). Already existing was the 1.5-day annual Frank Walsh Society meeting with its clinical-pathological correlation format, which originated at Johns Hopkins Medical School. The two societies merged in 1992. Currently, NANOS has grown to more than 650 members and is the premier organization for neuro-ophthalmology worldwide. The society’s journal, the Journal of Neuro-Ophthalmology, commenced publication in 1994, one year after the inaugural issue of Neurology Reviews.
Overcoming an early reputation for “admiring” disease, neuro-ophthalmology has evolved considerably over the past 25 years, with advances in neuroimaging, genomics, proteomics, office-based technologies, and immunology. The first multicenter, randomized trial in neuro-ophthalmology—the Optic Neuritis Treatment Trial (ONTT),1 published 25 years ago—showed that intravenous methylprednisolone 250 mg QID for three days followed by an 11-day prednisone taper was superior to either oral prednisone (1 mg/kg for 14 days) or oral placebo (14 days), speeding the recovery of vision with slightly better vision at six months. There was a higher rate of relapse in the group assigned to receive oral prednisone. The ONTT was a pivotal event for neuro-ophthalmology, heralding a paradigm shift from observational reporting to randomized clinical trials.
Three years later, the Ischemic Optic Neuropathy Decompression Trial (IONDT)2 showed that optic nerve sheath fenestration surgery, performed within 14 days of symptom onset for acute non-arteritic ischemic optic neuropathy or within 30 days in participants with a progressive course, did not improve visual acuity or visual field compared to careful follow-up and medical management at six months. Enrollees randomized to undergo surgery had a lower rate of improvement and were more likely to lose three or more lines of vision than the control group.
With this momentum, the Neuro-Ophthalmology Research Disease Investigators Consortium was formed to create infrastructure for future clinical trials of neuro-ophthalmic disorders. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT)3 studied patients with idiopathic intracranial hypertension and mild visual field loss. IIHTT confirmed that acetazolamide (500 mg BID, increasing to a maximum dose of 4 grams daily) was superior to placebo treatment when combined with a supervised weight loss program, resulting in improved visual field loss, papilledema grade, weight reduction, lumbar puncture opening pressure, and quality of life. Ongoing research efforts are investigating treatments for Leber hereditary optic neuropathy, non-arteritic ischemic optic neuropathy, thyroid eye disease, rehabilitation for poststroke homonymous hemianopia and giant cell arteritis in adults, and idiopathic intracranial hypertension and optic nerve gliomas in children.
Attendance at the NANOS annual meeting continues to grow, emphasizing scientific as well as clinical neuro-ophthalmic topics. Vibrant and active neuro-ophthalmology societies in other parts of the world (eg, Europe, Japan, Australia, British Isles) contribute to the camaraderie, collegiality, collaboration, and discovery that advance our field.
Happy 25th anniversary to Neurology Reviews! The next 25 years will likely transform the field of neuro-ophthalmology as we better understand the molecular and genetic basis of neuro-ophthalmic disorders. No longer “diagnose and adios,” we anticipate significant therapeutic advancements—and perhaps cures—for individuals with severe, sight-impairing, neuro-ophthalmic conditions.
References
2. The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273(8):625-632.
3. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss. JAMA. 2014;311(16):1641-1651.
Deborah I. Friedman, MD, MPH
Dr. Friedman is a Professor in the Departments of Neurology and Ophthalmology at the University of Texas Southwestern Medical Center in Dallas.
Neuro-ophthalmology is a small subspecialty that had its origins in ophthalmology but has always included neurologists. National neuro-ophthalmology meetings in North America began with the Rocky Mountain Neuro-Ophthalmology Course in 1975, which led to the formation of the Rocky Mountain Neuro-Ophthalmology Society in 1980. By 1986, it had 240 members and changed its name to the North American Neuro-Ophthalmology Society (NANOS). Already existing was the 1.5-day annual Frank Walsh Society meeting with its clinical-pathological correlation format, which originated at Johns Hopkins Medical School. The two societies merged in 1992. Currently, NANOS has grown to more than 650 members and is the premier organization for neuro-ophthalmology worldwide. The society’s journal, the Journal of Neuro-Ophthalmology, commenced publication in 1994, one year after the inaugural issue of Neurology Reviews.
Overcoming an early reputation for “admiring” disease, neuro-ophthalmology has evolved considerably over the past 25 years, with advances in neuroimaging, genomics, proteomics, office-based technologies, and immunology. The first multicenter, randomized trial in neuro-ophthalmology—the Optic Neuritis Treatment Trial (ONTT),1 published 25 years ago—showed that intravenous methylprednisolone 250 mg QID for three days followed by an 11-day prednisone taper was superior to either oral prednisone (1 mg/kg for 14 days) or oral placebo (14 days), speeding the recovery of vision with slightly better vision at six months. There was a higher rate of relapse in the group assigned to receive oral prednisone. The ONTT was a pivotal event for neuro-ophthalmology, heralding a paradigm shift from observational reporting to randomized clinical trials.
Three years later, the Ischemic Optic Neuropathy Decompression Trial (IONDT)2 showed that optic nerve sheath fenestration surgery, performed within 14 days of symptom onset for acute non-arteritic ischemic optic neuropathy or within 30 days in participants with a progressive course, did not improve visual acuity or visual field compared to careful follow-up and medical management at six months. Enrollees randomized to undergo surgery had a lower rate of improvement and were more likely to lose three or more lines of vision than the control group.
With this momentum, the Neuro-Ophthalmology Research Disease Investigators Consortium was formed to create infrastructure for future clinical trials of neuro-ophthalmic disorders. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT)3 studied patients with idiopathic intracranial hypertension and mild visual field loss. IIHTT confirmed that acetazolamide (500 mg BID, increasing to a maximum dose of 4 grams daily) was superior to placebo treatment when combined with a supervised weight loss program, resulting in improved visual field loss, papilledema grade, weight reduction, lumbar puncture opening pressure, and quality of life. Ongoing research efforts are investigating treatments for Leber hereditary optic neuropathy, non-arteritic ischemic optic neuropathy, thyroid eye disease, rehabilitation for poststroke homonymous hemianopia and giant cell arteritis in adults, and idiopathic intracranial hypertension and optic nerve gliomas in children.
Attendance at the NANOS annual meeting continues to grow, emphasizing scientific as well as clinical neuro-ophthalmic topics. Vibrant and active neuro-ophthalmology societies in other parts of the world (eg, Europe, Japan, Australia, British Isles) contribute to the camaraderie, collegiality, collaboration, and discovery that advance our field.
Happy 25th anniversary to Neurology Reviews! The next 25 years will likely transform the field of neuro-ophthalmology as we better understand the molecular and genetic basis of neuro-ophthalmic disorders. No longer “diagnose and adios,” we anticipate significant therapeutic advancements—and perhaps cures—for individuals with severe, sight-impairing, neuro-ophthalmic conditions.
References
2. The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273(8):625-632.
3. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss. JAMA. 2014;311(16):1641-1651.
Pediatric Dermatology Consult - March 2018
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
A 16-year-old girl presented with a 6-month history of an erythematous eruption of small papules and pustules around the cheeks and nose. She states the erythema had started first, with periods of feeling flushed that became worse with sun exposure. She saw her primary care physician who prescribed topical steroids. After using the steroids, the rash became worse, and she developed papules and pustules.